Abstract
When they recognize a target cell, natural killer (NK) cells mount an attack to kill the target by exerting their cytotoxicity via the exocytosis of cytotoxic granules. Although the details of this process (which includes the movement of cytotoxic granules in the immune synapse and their fusion with the plasma membrane, releasing granzymes and perforin into the synaptic cleft) are relatively better understood, the post-exocytosis regulation of the process is still largely unknown. Here we show that a clathrin-dependent endocytosis stimulated by target cell occurs in NK92 cell line, which is closely correlated with granzyme B recovery. Inhibition of the endocytosis significantly attenuates the cytotoxicity of NK92 cells. The NK cell recovery of its released effector molecules, in turn, suggests that endocytosis may well play a key role in the post exocytosis regulation of immune cells.
Keywords: Endocytosis, Exocytosis, Natural killer cells, Granzyme B, Clathrin
Introduction
Cytotoxic lymphocytes mainly include cytotoxic T lymphocyte (CTL) and natural killer (NK) cells. They are critical in the defense against viral infections and tumors [1], exerting their cytotoxicity through the exocytosis of cytotoxic granules, which serves both as the secretory granule and as the lysosome called secretory lysosome [2–4]. When a cytotoxic lymphocyte recognizes a target cell, cytotoxic granules move to the immune synapse where the cytotoxic granules fuse with the plasma membrane and release effector molecules into the synaptic cleft [5–7]. In NK cells, this process is regulated by the balance between inhibitory receptors and activating receptors. Activation receptors recognize and bind to their specific ligands, triggering a series of responses that result in secretory lysosome release that kill the intended target cell [8]. Activation takes place in a special contact zone between NK cells and target cells known as the activating NK cell immunological synapses (aNKIS). The formation of the aNKIS can be divided into several discrete stages: an initiation stage including contact, adhesion and initial signaling; an effector stage including tight conjugation, signal amplification, microtubule organization center (MTOC) polarization and secretory lysosome polarization and release; and finally a termination stage marked by relative inactivity and immunological synapse (IS) disassembly [8, 9]. Granzymes and perforin are the main effective molecules released from cytotoxic granules. When it comes into contact with a target cell, granzyme B binds to the target cell membrane by electrostatic interactions or by specific receptors, such as the cation-independent mannose-6-phosphate receptor (MPR) [10]. Perforin, on the other hand, makes microscopic holes in the plasma membrane of target cells, causing an influx of calcium and triggering a rapid endocytosis of granzymes bound to the cell surface [11]. Granzyme B is a caspase-like proteinase which induces target cell apoptosis by activating the caspases or by directly cleaving the key caspase substrates. On the other side of the synapse, cytotoxic lymphocytes have to deal with the released effector molecules to avoid self-destruction [12]. The protective mechanism against perforin autoinjury is the same as that found in CTL. It is provided by the surface-bound cathepsin B, which is minimally expressed on the surface of resting CTL, but rapidly detectable therein after degranulation. Surface cathepsin B is enzymatically active and able to cleave perforin, protecting the activated CTL from damage by any released perforin [13]. Although an intracellular inhibitor of granzyme B called nucleocytoplasmic serpin, proteinase inhibitor-9 (PI-9) was found in cytotoxic lymphocytes, whose primary role appears to be the inactivation of leaked or misdirected granzyme B from the cytotoxic granules [14], the mechanism of how cytotoxic lymphocytes deal with released granzyme B is still largely unknown. This study has demonstrated the subsequent recovery, via clathrin-dependent endocytosis, of the NK granzyme B released in response to target cell stimulation. This recovery, in turn, is closely correlated with the cytotoxicity and self-cytotoxin resistance of NK cells.
Materials and methods
Cell lines
The human malignant non-Hodgkin’s lymphoma NK cells, NK92, were cultured in Alpha-Minimum Essential Medium (Invitrogen, Carlsbad, CA, USA) supplemented with 12.5% fetal calf serum (Invitrogen), 12.5% horse serum (Invitrogen) and 100 units/ml recombinant human IL-2. Primary porcine endothelial cells (PEC) were cultured in M199 medium supplemented with 10% fetal calf serum. Human erythroleukemia cell line K562 was used as a target cell and grown in RPMI 1640 supplemented with 10% fetal bovine serum. All cell lines were maintained at 37°C in a humidified incubator gassed with 5% CO2.
Antibodies
The antibodies used were as follows: mouse anti-EEA1 monoclonal antibody (clone 14/EEA-1, BD Bioscience); rabbit anti-granzyme B polyclonal antibody (Protein Tech Group, Inc. Chicago, USA); mouse anti-LAMP-1 monoclonal antibody (clone H4A3, Abcam); mouse anti-clathrinHC monoclonal antibody (TD.1) and mouse anti-CD63 monoclonal antibody (NK1/C3) (Santa Cruz Biotechnology). FITC-conjugated goat anti-mouse IgG, TRITC-conjugated goat anti-rabbit IgG and HRP-conjugated goat anti-rabbit IgG were from Pierce.
Reagents
FM1-43FX, Hoechst33342 and Alexa Fluor-488-conjugated transferrin (Tfr) were purchased from Molecular Probes (Invitrogen). Carboxyfluorescein succinimidyl ester (CFSE), propidium iodide, chlorpromazine hydrochloride (CPZ), sucrose, and crystal violet and leupeptin were purchased from Sigma Company (Sigma, St. Louis, MO, USA). Human granzyme B ELISA kit was from Bender Medsystems (Bender Medsystems, Vienna, Austria).
Flash-photolysis and [Ca2+]i measurement
Flashes of UV light and fluorescence excitation light were generated as described by Liu et al. [3, 15]. Briefly, [Ca2+]i of individual cells was monitored with a photomultiplier-based system by using a monochromatic light source (TILL Photonics, Grafelting, Germany) tuned to excite 2-[2-(5-carboxy)oxazole]-5-hydroxy-6-aminobenzofuran-N,N,O-triacetic acid fluorescence at 345 and 385 nm. [Ca2+]i was calculated from the fluorescence ratio R according to Grynkiewicz et al. [16]. To obtain stepwise [Ca2+]i increases, short flashes of UV light from a xenon arc flash lamp (Rapp Opto-Electronics, Hamburg, Germany) were applied to the whole cell loaded with caged Ca2+.
Membrane capacitance measurement
Capacitance of membrane was measured with the method described by Liu et al. [3]. Briefly, conventional whole-cell patch-clamp measurements were performed by using an EPC-9 patch-clamp amplifier and PULSE software (HEKA, Lambrecht, Germany). Capacitance measurements were performed by using the ‘‘sinedc’’ software lock-in amplifier method implemented in the PULSE software. A sinusoid voltage stimulation (1,000 Hz with an amplitude of 40 mV) was superimposed on a holding potential of 60 mV.
Visualization of membrane endocytosis stimulated by target cells
2 × 105 PEC cells (target cell) were preseeded on each well of 6-well culture plates. 2 × 106 NK 92 cells (effector cell) were incubated with 2 ml of FM1-43FX solution (5 μg/ml in HBSS solution) for 5 min on ice. FM1-43FX labeled NK cells were added into PEC culture plates to yield an effector/target (E/T) ratio of 10:1. For the observation of endocytosis, both NK and PEC cells were kept in low concentration of FM1-43 solution (2 μg/ml). After being incubated for 30 min at 4°C, the cells were further incubated for 1 h at 37°C and then washed twice with ice-cold phosphate-buffered saline (PBS) to eliminate non-internalized cell surface FM1-43FX. For visualization of inhibitory effect of clathrin-mediated endocytosis, 5 × 106 NK92 cells were harvested by centrifugation at 1,200 rpm for 5 min and washed with PBS three times. Cells were incubated in 2 ml of serum-free medium containing 10 μg/ml chlorpromazine hydrochloride for 30 min at 37°C and were labeled with FM1-43FX and mixed with PEC, then endocytosis were visualized as described above.
Quantification of internalized FM1-43FX
NK92 cells pretreated with chlorpromazine for indicated time were harvested and incubated with PBS containing 2% FBS for 20 min on ice followed by incubation with 5 μg/ml FM1-43FX solution on ice. After incubation with target cells at 37°C as described above, the cells were washed twice with ice-cold PBS to eliminate non-internalized cell surface FM1-43FX. The mean fluorescence intensity (MFI) of FM1-43FX was measured by flow cytometry.
Cytotoxicity assay
In the NK92 cell cytotoxicity assay, two cell lines were used as target cells. NK92 cells were pretreated with two kinds of clathrin-mediated endocytosis inhibitors, 0.45 M sucrose or 10 μg/ml chlorpromazine for 30 min at 37°C, respectively. For porcine endothelial cells (PEC) as a target cell, cytotoxicity assay based on crystal violet described by Krizhanovsky et al. [17] with slight modifications. Briefly, 1 × 104 of PEC (target cells) were seeded per well in a 96-well plate. NK92 cells (effector cells) with or without inhibitor treatment were added to yield indicated effector-to-target cell (E/T) ratios. After the mixture was incubated for 3 h, culture medium and NK92 cells were discarded and PEC cells were fixed with 50 μl of 2.5% glutaraldehyde per well for 30 min, then stained with 30 μl of 0.1% crystal violet for 20 min. After being washed three times and dried for 20 min, crystal violet staining PEC was dissolved with 100 μl of 10% acetic acid. The absorbance value was measured at 570 nm. The specific cytotoxicity was calculated by the following formula: [(ODcontrol PEC − ODassayed PEC)/ODcontrol PEC] × 100%. For K562 as a target cell, assessment of the specific cytotoxicity of NK92 cells was done by a flow cytometric assay based on a procedure described by Piriou et al. [18] with slight modifications. Briefly, 3 × 106 K562 (target cells) in 1 ml culture medium were incubated with 1 μl of CFSE (450 μM) for 10 min at 37°C and washed with PBS three times. NK92 (effector cells) were pretreated with inhibitors and mixed with 4 × 104 labeled K562 (target cells) in a 24-well plate with indicated effector-to-target cell (E/T) ratios. The mixture was centrifuged to enhance cell contact and incubated for 3 h. For the last 30 min of incubation, 10 μl of 200 μg/ml propidium iodide (PI) was added to the cells. Samples were analyzed by FCM (BD LSR-II) immediately. Each sample was prepared and analyzed in triplicate. The specific killing percentage was calculated by the following formula:
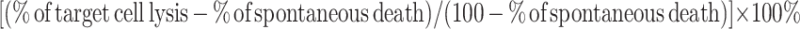 |
Immunofluorescence and image collection
NK cells were treated and harvested at indicated times and fixed with 4% polyformaldehyde at room temperature for 30 min and washed twice with ice-cold PBS. The cells were spread on glass slides and permeated with PBS containing 0.2% TritonX-100 for 10 min. Non-specific binding sites were blocked with 5 mg/ml BSA for 2 h at 37°C. Cells were incubated with primary antibody anti-granzyme B, anti-EEA-1, or anti-LAMP-1 or anti-CD63 and secondary antibody (FITC-conjugated, 1:200; TRITC-conjugated, 1:200) solution supplemented with Hoechst dye 33342 (1 μg/ml). Cells were viewed under a confocal laser scanning biological microscope (FV500, Olympus Inc., Japan) with 60 × (NA = 1.40) oil objective. Images were acquired using Fluoview software that came with the confocal microscope and analyzed with ImageJ 1.40 and Photoshop 7.0.
siRNA transfection
The clathrin heavy chain targeting siRNAs CHC (UCUGAAGAGUUUUCCCAGCTT) and control (scrambled) siRNA oligonucleotides were synthesized by GenePharma (Shanghai, China) and 300 nM of indicated siRNAs were transfected to 5 × 106 NK92 cells in a 12-well plate by the Amaxa Cell line Nucleofector kit R, program A-024. Knockdown efficiencies were tested by immunoblotting after 48 h of siRNA transfection.
Western blot
A total of 5 × 106 NK92 cells transfected with siRNA or stimulated with PEC for indicated time were harvested and washed once in ice-cold PBS. Cell pellets were lysed on ice by non-denaturing NP40 lysis buffer (1% NP-40, 50 mM Tris–Cl (pH 8.0), 150 mM NaCl) or denaturing SDS lysis buffer (50 mM Tris–Cl pH 6.8, 2% SDS, 10% glycerol) in the presence of protease inhibitors cocktail (100 μg/ml PMSF, 1 μg/ml aprotinin, and 1 μg/ml leupeptin) for 30 min. Cell lysates were centrifuged at 1,200 rpm for 10 min and the supernatants were subjected to 12% SDS-PAGE and then transferred to nitrocellulose membranes. Membranes were blocked overnight at 4°C in blocking buffer (5% non-fat dried milk in PBS, 0.1% Tween-20) and immunoblotted with primary antibody anti-clathrin (1:200) or anti-granzyme B (1:200) for 2 h at 37°C. Horseradish peroxidase-conjugated (HRP) anti-IgG diluted at 1:5,000 was used to label the membrane-bound antibodies. The β-actin was used as a loading control. The enhanced chemiluminescence system (Pierce) was used for detection.
ELISA
Released granzyme B was measured using ELISA. Briefly, NK92 cells with or without CPZ treatment were incubated with PEC or K562 at an E/T ratio of 10:1. At the indicated time point (1, 3, 5 h), the supernatants were collected for released granzyme B measurement. Each sample was analyzed in triplicate with ELISA according to the kit manual. The concentration of released granzyme B was calculated according to standard curve. NK92 cells were centrifuged and lysed with non-denaturing NP40 lysis buffer for NK cells associated granzyme B detection by Western blot.
Statistic analysis
Results were analyzed by using Student’s t test. All values were expressed as means ± SD. Differences were considered to be statistically significant when p < 0.05.
Results
Endocytosis occurred in NK92 cells stimulated by target cells
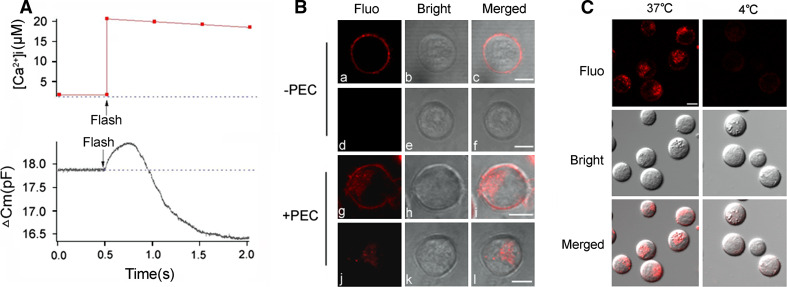
Membrane capacitance (C m) measurement, which measures plasma membrane capacitance and its changes of a cell, is one of the most important quantitative techniques and widely used in the studies of exocytosis and endocytosis [19]. Exocytosis increases surface membrane as well as C m and endocytosis decreases them. As shown in Fig. 1a, following the 0.5 pF increase in Cm, which marks that well-known exocytosis that occurs in response to stimulation by 20 μM of [Ca2+]i, a startling decrease in C m then began, which continued to below the pre-stimulation level, suggesting the existence of a hitherto unknown stage of endocytosis. This appeared to be a Ca2+-triggered post-exocytosis, endocytosis. The cytotoxicity and exocytosis of NK cells is usually triggered by target cell, for example, virus-infected cells, tumor cells, or heterologous cells [1, 3, 20]. In order to confirm whether the endocytosis triggered by target cell occurs, FM1-43FX, well-known to researchers in membrane dynamics [21, 22] as an inducer of fluorescent emissions, in double-molecular layers (not in single), was used to label and show the endocytosis on the surface membrane of the cell. FM1-43FX has the added advantage of being easily removed with PBS from the surface but not from the insides of cell membranes. As shown in Fig. 1b, (a, b, c), NK cells labeled with FM1-43FX but neither stimulated by a target cell (PEC) nor washed, showed no fluorescence on their surface membranes. When washed, the FM1-43FX was removed from the surface membrane completely and no fluorescence was detected (d, e, f), indicating that there was no significant endocytosis in the NK cells without target cell stimulation. In the group of NK cells stimulated with target cell, on the other hand, without washing, fluorescent emissions were observed not only on the surface membrane but also inside the NK cells (g, h, i). Washing with PBS only eliminated the NK cell surface fluorescence without affecting the intracellular fluorescence (j, k, l), suggesting that some of the surface membrane emissions were internalized when the NK cells were stimulated by target cells. As a further confirmation that endocytosis had indeed occurred and was an active process, as expected, no emissions were detected at 4°C when endocytosis-dependent cellular internalization is known not to occur [23]. This is illustrated in Fig. 1c.
Fig. 1.
Membrane internalization occurs in NK cells stimulated by target cells. a Example traces of C m increase and decrease from an NK92 cell patched with 20 µM free Ca2+ in the pipette solution. b Effect of PEC stimulation on surface membrane endocytosis. NK cells were pre-stained with FM1-43 (5 µg/ml) for 5 min and stimulated with PEC for 1 h. a, b, c Neither PEC stimulation nor PBS wash post stimulation; d, e, f without PEC stimulation and with PBS wash; g, h, i with PEC stimulation and without PBS wash; j, k, l with both PEC stimulation and PBS wash. c Effect of incubation at 4°C on surface membrane endocytosis. NK cells were pre-stained with FM1-43 (5 µg/ml) for 5 min and stimulated with PEC at 37 or 4°C for 1 h as well as washed twice with PBS. Data are representative of three independent experiments. Scale bar is 5 µm
Endocytosis stimulated by target cell was clathrin-dependent
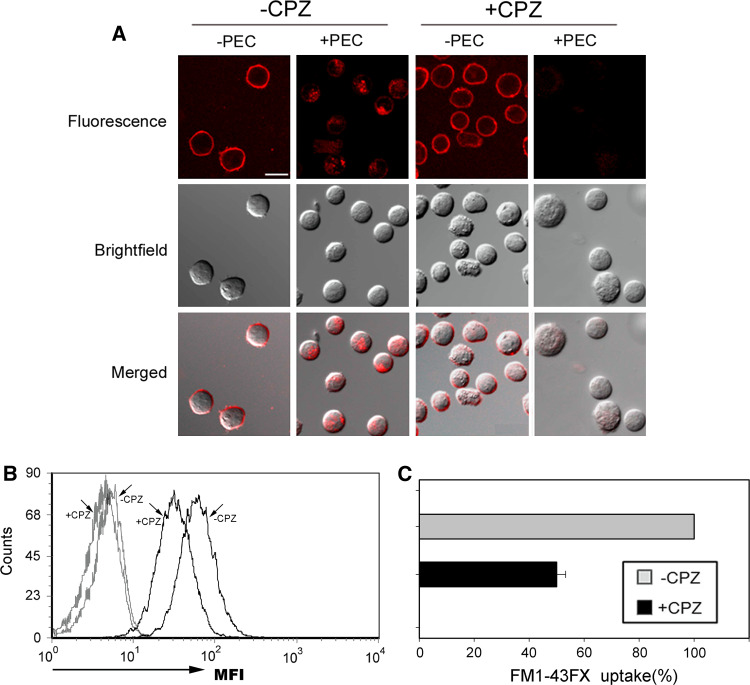
Endocytic pathways are known to at least include both a clathrin-dependent and a clathrin-independent pathway [24]. Clathrin-dependent endocytosis has been best characterized and has been shown to be inhibited specifically by CPZ [25]. In this experiment, CPZ was used to detect whether the endocytosis stimulated by target cells is clathrin-dependent or not. In order to determine the effect of CPZ on NK cells vitality and endocytosis, the concentration of CPZ was titrated carefully. As illustrated in supplementary Figure 1, 10 μg/ml CPZ treatment had the strongest inhibitory effect (inhibiting about 60%) on transferrin endocytosis which is typical clathrin-dependent, but almost no significant effect on NK92 cells vitality. As shown in supplementary Figure 2, the uptake of FM1-43FX (Figure S2a) or transferrin endocytosis (Figure S2b) in NK cells pretreated with CPZ for 30 min was no significant difference with that at 3 h continuous treatment. So the concentration (10 μg/ml) and treatment time (30 min) of CPZ was selected in the following experiments. It was discovered that CPZ treatment significantly decreased the FM1-43FX fluorescence inside stimulated NK cells relative to NK cells without any stimulation (Fig. 2), suggesting strongly that endocytosis stimulated by target cell in NK92 cells is clathrin-dependent.
Fig. 2.
Membrane internalization stimulated by target cells is clathrin-dependent. a Effect of CPZ pretreatment on membrane internalization of NK cells stimulated by PEC. NK cells were pre-treated with 10 µg/ml of CPZ for 30 min and labeled with FM1-43FX (5 µg/ml) for 5 min and stimulated with PEC for 1 h. “−CPZ”: no CPZ pretreatment; “+CPZ”: CPZ pretreatment; “−PEC”: neither PEC stimulation nor PBS wash; “+PEC”: PEC stimulation and PBS wash. b, c Effect of CPZ pretreatment on FM1-43FX uptake of NK cells stimulated by PEC. The mean fluorescence intensities (MFI) showed the uptake of FM1-43FX. Black lines mean that NK cells were stimulated with PEC, and gray lines mean that NK cells were without PEC stimulation. The uptake of FM1-43FX in NK cells without CPZ pretreatment was defined to 100%. Relative uptake of FM1-43FX in CPZ pretreated NK cells was calculated by MFI (+CPZ)/MFI (−CPZ) × 100%. Error bar indicates SD. Scale bar is 10 µm. Data are representative of three independent experiments
Inhibition of clathrin-dependent endocytosis attenuated the cytotoxicity of NK92 cells
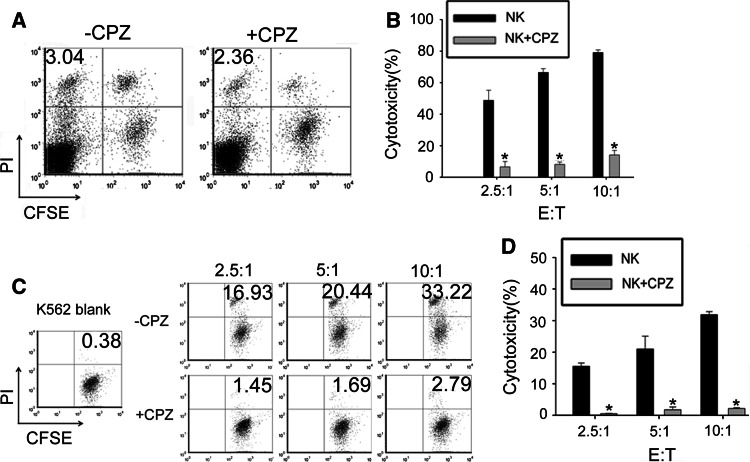
NK cells auto-lysis rate is an important parameter to evaluate whether the activation induced cell death occurs. In order to detect the effect of endocytosis on NK92 cells, the death rate of NK92 cells was tested. The spontaneous death of NK92 cells stimulated by target cell is similar to that of non-stimulated NK92 cells (data not shown). As shown in Fig. 3a, CPZ treatment did not change the death rate of NK92 cells stimulated by target cells. The cytotoxicity of NK92 cells against PEC cells (Fig. 3b) or K562 cells (Fig. 3c, d) in different effector:target cell ratios was significantly attenuated by CPZ pretreatment. Pre-treating NK92 cells with alternative inhibitor 0.45 M sucrose obtained similar results (Figure S3).
Fig. 3.
Cytotoxicity of NK cells is attenuated by pretreatment with CPZ. a Spontaneous death of NK92 cells pretreated (+CPZ) or non-pretreated (−CPZ) with 10 µg/ml CPZ and stimulated by K562 cells at NK: K562 (E:T) ratio 10:1 for 3 h. K562 cells were labeled with CFSE. Dead cells were labeled with PI. Upper left quadrant exhibits dead NK cells. The number shown indicates spontaneous death rate of NK cells. b The cytotoxicity of NK92 cells to PEC. After NK cells with or without CPZ pretreatment and PEC cells were cocultured at indicated ratio in the graft for 3 h, NK cells were discarded and PEC survivals were stained with crystal violet. The absorbance value was measured to calculate the cytotoxicity. c, d The cytotoxicity of NK92 cells to K562 cells. After incubation of NK cells with or without CPZ pretreatment and K562 cells pre-labeled with CFSE at indicated ratio in the graft for 3 h, all cells were stained with PI and analyzed by FCM. c The cytotoxicity was calculated with formula described in the methods. d Upper right quadrant shows the K562 death rate. *CPZ pretreatment versus no treatment, p < 0.01. Data are representative of four independent experiments
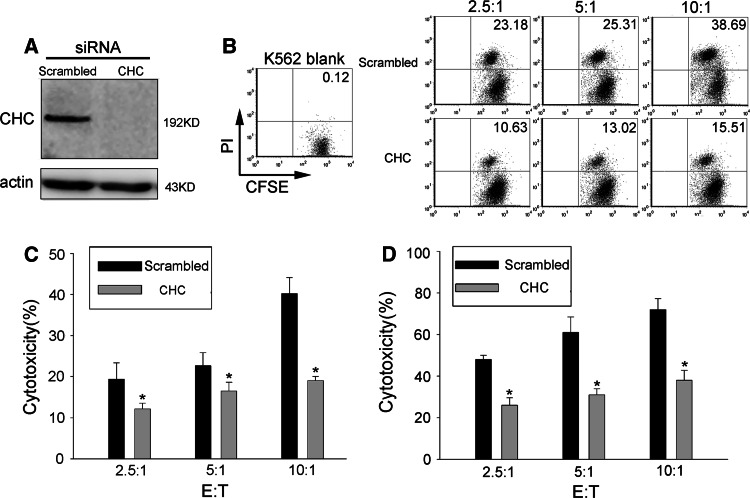
Clathrin heavy chain (CHC) siRNA is a more specific inhibitor of clathrin-mediated endocytosis. After 48 h of transfection, clathrin heavy chain was completely knocked down in CHC siRNA transfected NK92 cells (Fig. 4a). The cytotoxicity of NK92 cells against K562 cells (Fig. 4b, c) or PEC cells (Fig. 4d) was significantly attenuated by CHC siRNA transfection. These results suggest that the target cell-stimulated endocytosis had biological significance.
Fig. 4.
Cytotoxicity of NK cells is hampered by clathrin knockdown. a Clathrin heavy chain was knocked down by siRNA transfection. b–c Cytotoxicity of NK92 cells against K562 cells. NK92 cells transfected with CHC siRNA or scrambled siRNA were incubated with CFSE pre-labeled K562 cells for 3 h. CFSE-positive cells were gated and the percentage of PI-positive cells within this gate is shown. This experiment was performed three times independently, yielding comparable results. d Cytotoxicity of NK92 cells against PEC cells. NK92 cells transfected with CHC siRNA or scrambled siRNA and PEC cells were co-cultured at the indicated ratios for 3 h, NK cells were discarded, and PEC survivals were stained with crystal violet. The absorbance value was measured to calculate the cytotoxicity. *p < 0.05. Data are representative of three independent experiments
Endocytosis stimulated by target cell was associated with the recovery of granzyme B
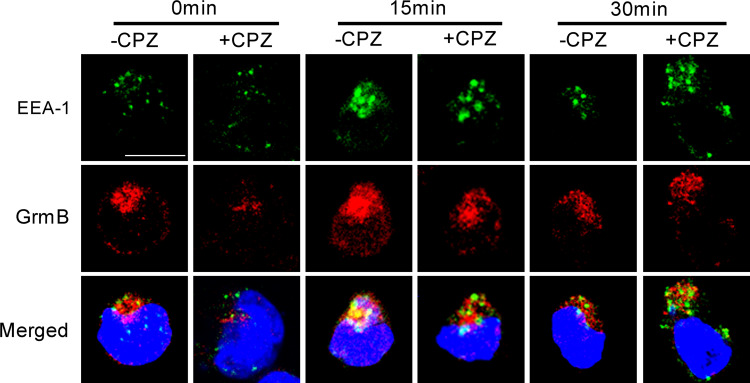
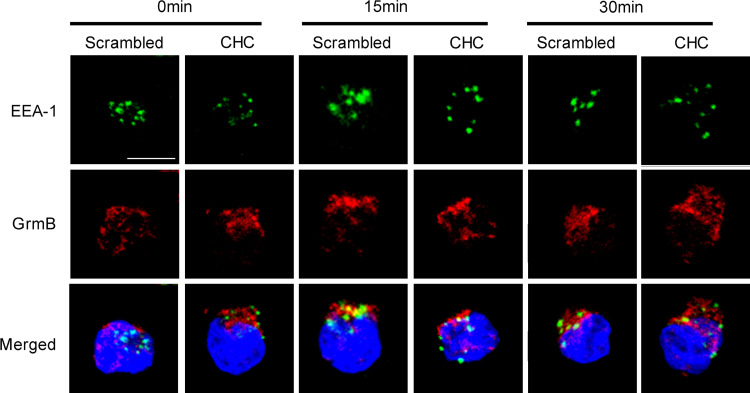
Most internalized cargoes are delivered to the early endosome via vesicular (clathrin- or caveolin-coated vesicles) or tubular intermediates (known as clathrin- and dynamin-independent carriers (CLICs)) that are derived from the plasma membrane [26]. Therefore, the colocalization of cargoes and early endosome is a typical appearance of endocytosis. The experiment here focused upon granzyme B, a kind of effector molecule released from NK cells when stimulated by target cells. Granzyme B and early endosome colocalization appeared prominently in the results as shown in Fig. 5. Without PEC stimulation, an initial endosomal marker EEA-1 (green fluorescence) and granzyme B (red fluorescence) were localized in different compartments of NK cells (0 min, left panel). After 15 min of stimulation with PEC, most of the EEA-1 had colocalized with some of the granzyme B in NK cells (15 min, left panel). At 30 min, colocalization of EEA-1 and granzyme B became less prominent than before at 15 min (30 min, left panel). CPZ pre-treatment inhibited most of EEA-1 and granzyme B colocalization at 15 and 30 min of stimulation. CHC siRNA treatment obtained similar results (Fig. 6) with CPZ treatment. These results indicated that endocytosis stimulated by the target cell was associated with the recovery of granzyme B in NK92 cells.
Fig. 5.
CPZ treatment attenuated colocalization of granzyme B and EEA-1 in NK cells stimulated by PEC cells. NK cells pretreated (+CPZ) or non-pretreated (−CPZ) with CPZ were stimulated by PEC for 0, 15, or 30 min and then fixed, permeabilized and non-specific binding sites blocked and incubated with anti-EEA-1 (1:200), anti-granzyme B (1:200) and FITC or TRITC conjugated secondary antibody (1:200), respectively. EEA-1 is shown in green. Granzyme B (GrmB) is shown in red. Nucleus is shown in blue. Scale bar is 10 µm. Data are representative of three independent experiments
Fig. 6.
CHC RNAi treatment attenuated colocalization of granzyme B and EEA-1 in NK cells stimulated by PEC cells. NK cells treated with CHC specific RNAi (CHC) or scrambled RNAi (scrambled) were stimulated by PEC for 0, 15, or 30 min, then fixed, permeabilized, and non-specific binding sites blocked and incubated with anti-EEA-1 (1:200), anti-granzyme B (1:200) and FITC- or TRITC-conjugated secondary antibody (1:200), respectively. EEA-1 is shown in green. Granzyme B (GrmB) is shown in red. Nucleus is shown in blue. Scale bar is 10 µm. Data are representative of three independent experiments
Recovered granzyme B was sorted to LAMP-1-positive lysosome
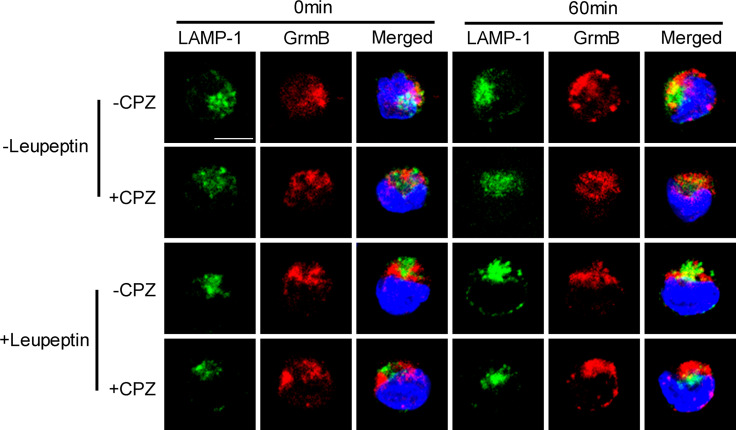
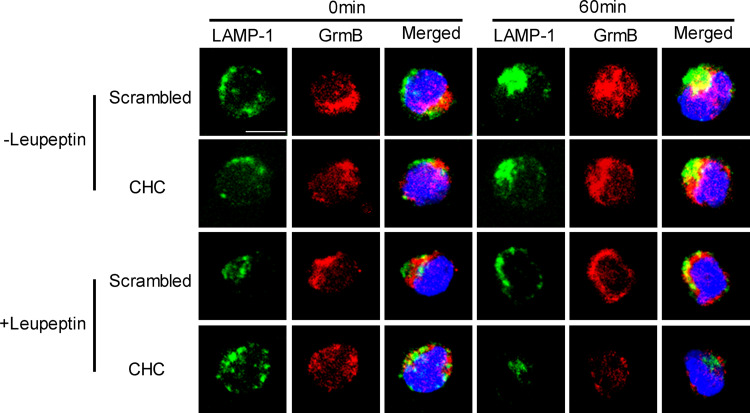
Because the recovered granzyme B does not kill parental NK92 cells, it would appear to be reasonable to conclude that the recovered granzyme B can only have been sorted or re-cycled to the secretory lysosome for use again or degeneration. CTL or NK cells that have secretory lysosomes package both lysosomal and secretory proteins in one compartment [2]. Secretory lysosomes have no specific markers but share similar surface markers with conventional lysosome, such as lysosome-associated membrane protein-1 (LAMP-1) and CD63, so also called LAMP-1-positive lysosome [2, 27]. In this experiment, predominant incidences of overlapping of granzyme B and LAMP-1 in NK cells were observed post-PEC stimulation for 60 min. As shown in Fig. 7, a few of LAMP-1 and granzyme B overlapped in the NK92 cells without stimulation (0 min), which is consistent with the understanding that granzyme B can be constitutively expressed and sorted to secretory lysosomes in resting NK cells [28]. After 60 min of stimulation, the colocalization of LAMP-1 and granzyme B became predominant and was significantly reduced in NK cells pretreated by the endocytosis inhibitor CPZ (Fig. 7 −CPZ vs. +CPZ). The co-localization of granzyme B and LAMP-1 suggested that the recovered granzyme B was sorted to LAMP-1+ lysosome. Proteins sorted for degeneration will be cleaved by lysosomal enzymes and cannot be detected by lysosome surface maker co-localization assay, so the observed co-localization of granzyme B and LAMP-1 means the granzyme B was not recovered for degeneration. To further demonstrate this point, a lysosomal enzyme blocking assay was performed. As shown in Fig. 7, the co-localization of granzyme B and LAMP-1 was not increased significantly by an inhibitor of lysosomal enzymes or leupeptin treatment (+leupeptin-CPZ vs. −leupeptin-CPZ). Treating NK cells with CHC siRNA obtained similar results, as shown in Fig. 8. Using another lysosome surface marker CD63 antibody obtained similar colocalization result with LAMP-1 antibody (Figure S4). Considering the fact that the CPZ pre-treatment or CHC siRNA treatment significantly attenuates the cytotoxicity of NK cells, it would appear reasonable to suggest that most of, if not all, the recovered granzyme B was sorted to LAMP-1+ lysosomes, re-cycled, and used again.
Fig. 7.
CPZ treatment attenuated colocalization of granzyme B and LAMP-1 in NK cells stimulated by PEC cells. NK cells with CPZ pretreatment (+CPZ) or non-treatment (−CPZ) were stimulated by PEC for 0 or 60 min. The following immunostaining was the same as in the Fig. 5 legend. Anti-LAMP-1 antibody was diluted in 1:150. LAMP-1 is shown in green. Granzyme B (GrmB) is shown in red. Nucleus is shown in blue. For leupeptin treatment assay, all cells were pre-cultured for 16 h in the presence or absence of leupeptin (15 µM). Scale bar is 10 µm. Data are representative of three independent experiments
Fig. 8.
CHC RNAi treatment attenuated colocalization of granzyme B and LAMP-1 in NK cells stimulated by PEC cells. After NK cells with CHC-specific RNAi pretreatment (CHC) or scrambled RNAi (scrambled) pretreatment were stimulated by PEC for 0 or 60 min, colocalization of LAMP-1 and granzyme B was observed by immunostaining. Anti-LAMP-1 antibody was diluted in 1:150 and anti-granzyme B was diluted in 1:200. LAMP-1 is shown in green. Granzyme B (GrmB) is shown in red. Nucleus is shown in blue. Leupeptin treatment is the same as in Fig. 7
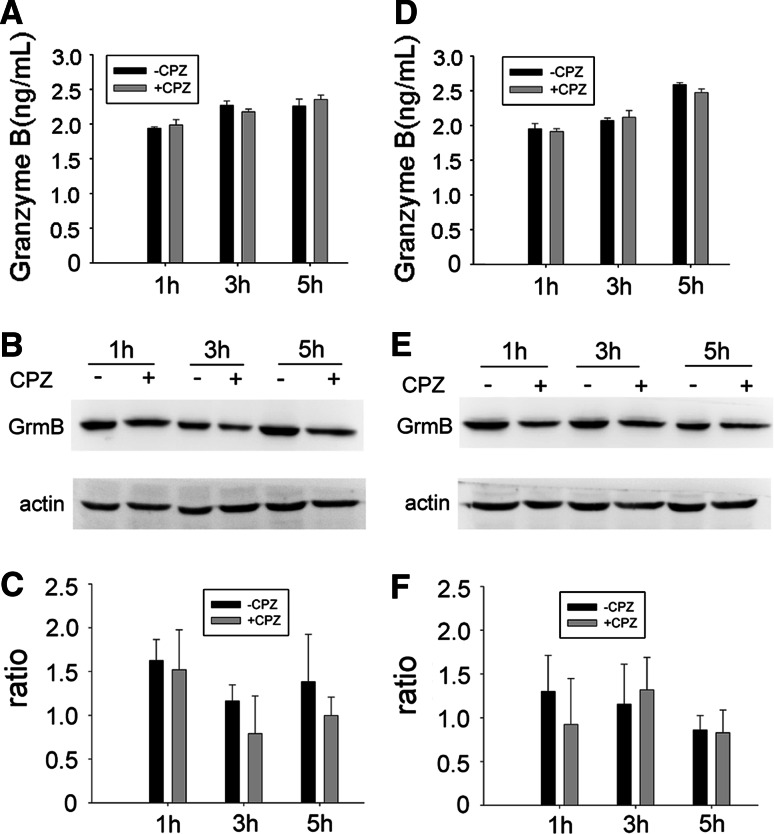
The significant change in the amount of granzyme B was not detected in CPZ-pretreated NK92 cells and corresponding supernatants
In order to determine the effect of CPZ pre-treatment on the amount of internalized granzyme B, ELISA procedures for supernatants and Western-blot procedures for the cells were carried out. As shown in Fig. 9a, granzyme B in the supernatant of NK92 and incubated PEC cells increased in the CPZ pre-treatment group as time went on. It appeared as if the quantity of granzyme B had risen slightly at the 1-h and 5-h junctions. In contrast, CPZ pre-treatment caused a decrease of intracellular granzyme B as shown in Fig. 9b, c. A similar result was observed with alternative target cell K562 cells (Fig. 9d–f). These results suggest that CPZ pre-treatment not only does not significantly affect the release of granzyme B but that the cytotoxicity of NK92 cell is at least partly associated with the recovery of granzyme B.
Fig. 9.
Quantity of granzyme B in the supernatants and NK92 cells. 1 × 106 CPZ pretreated or non-pretreated NK92 cells were stimulated with 1 × 105 PEC (a–c) or K562 cells (d–f) for 1, 3, and 5 h, respectively. The supernatants were collected to test the granzyme B with ELISA (a, d). NK92 cells were harvested to detect the granzyme B by Western blot (b, e). c, f Density ratio of granzyme B versus actin band. Data are representative of three independent experiments
Discussion
Although the mechanism underlying the NK cell release of two major effector molecules, namely granzyme B and perforin, by exocytosis of cytotoxic granules is relatively well understood, the mechanism governing the post-release regulation of these cytotoxic molecules is not clear. Why granzyme B kills target cells but has no destructive effect on the effector cells themselves is still an unanswered question. One possible answer is the presence of cathepsin B, which binds by granular fusion to the killer cell’s surface membrane, inactivating (and thereby protecting) effector cells from returned perforin [13]. The entry of granzyme B into target cells is through the holes made by perforin [11]. Perforin-deficient mice have demonstrated the absolutely essential requirement of this protein for granzyme-dependent killings [29]. However, CTL from cathepsin B-deficient mice survive normally in vitro and in vivo, after encountering and killing target cells [30]. These observations suggest that other mechanisms underlie killer cell self-protection from their own secretions. Another possible mechanism involves the intracellular inhibitor of human granzyme B, namely proteinase inhibitor-9 (PI-9), which is expressed by lymphocytes including NK cells and protects effector, accessory and bystander cells from ectopic granzyme B during an immune response [12, 14]. Here we report a new possible answer that released granzyme B is recovered and used again, so avoiding the attack to NK cells themselves.
Although exocytosis is the most critical and discussed feature of an NK cell’s anti-tumor and anti-viral defense, there are some papers that also report the occurrence of an endocytosis in these NK cells. These reports, in turn, beg the question of what useful function this endocytosis may serve. Rajagopalan et al. have reported that KIR2DL4 is constitutively internalized into intracellular vesicles in resting NK cells. Soluble HLA-G, which is the ligand of KIR2DL4, is also known to be re-cycled by endocytosis into the KIR2DL4-containing compartments, facilitating the re-engagement of KIR2DL4 to induce a unique package of cytokine/chemokine secretions [31]. Furthermore, one of the inhibitory receptors of NK cells, known as CD94/NKG2A utilizes an uncommon means of endocytosis that is associated with rapid recycling into the cell surface [27]. Endocytosis of both KIR2DL4 and CD94/NKG2A receptors are triggered by soluble ligands or antibodies. This endocytosis is a primary endocytosis. However, in physiological or pathological situations, NK cell activity is triggered by contact with a target cell. As mentioned earlier, in such circumstances exocytosis is usually observed. In the present study, a target cell triggered endocytosis of surface membrane was found. This kind of endocytosis cannot be spontaneous, as the labeled membrane is not observed in NK cells without target cell stimulation. Only after stimulation by target cells are some surface membranes re-cycled and internalized back into the cytoplasm. Our previous data showed that target cell recognition induced the increase of membrane capacitance, which immediately indicates exocytosis [3]. In the present study, increased calcium concentration inside the NK cell also induced an exocytosis but one followed by a second and subsequent endocytosis. So the endocytosis observed in the study is not a primary endocytosis, but a secondary endocytosis, which we call post-exocytosis, endocytosis. This endocytosis may be important for the membrane size recovery and the continued ability of NK cells to kill targets. The colocalization of granzyme B and initial endosomes have corroborated the existence of this post-exocytosis, endocytosis. This second endocytosis may well play a role in the mediation, uptake, and successful attack of granzyme B granules on target cells, proffering a new explanation and a new mechanism to post-exocytosis regulation of cytotoxic granules.
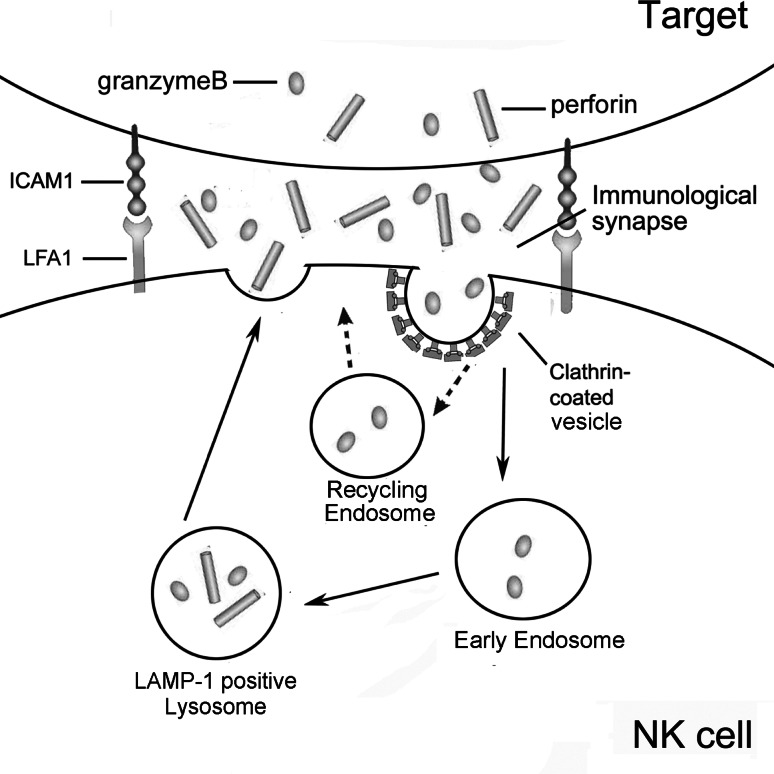
Clathrin-coated vesicle biogenesis is best characterized in nerve terminals where it serves as the major pathway for the recycling of synaptic-vesicle components after release of neurotransmitters in response to action potentials [24]. In contrast to a neural synapse, the immunological synapse was originally defined as the crucial junction between a T cell and an antigen-presenting cell (APC) at which T-cell receptors (TCRs) interact with MHC molecules [9, 32, 33]. Subsequent studies extended immunological synapses between different types of immune cells, as well as between immune cells and non-immune cells [8, 9]. Immunological synapses are actually the orderly re-arrangement of molecules in an immune cell and its interface with another cell. Although an immunological synapse between NK cells and target cells is only a transient affair, the release of effector molecules is based on cytotoxic granules (vesicles) that are not dissimilar to those of neurotransmitters. Given these similarities, the recycling of cytolytic molecules after exocytosis is both plausible and possible. As shown in Fig. 10, granzyme B is released to the immunological synapse between NK cells and target cells when NK cells are stimulated by target cells. Some granzyme B enter and destroy the target cell while others are recovered via clathrin-dependent endocytosis and return to their initial endosomes and LAMP-1+ lysosomes to be reused in the continued battle of the immune system against target cells.
Fig. 10.
Scheme of exocytosis and endocytosis occurring in NK cells stimulated by target cells. Firstly, a NK cell recognizes a target cell and forms immunological synapse (IS). Secondly, secretory lysosome moves to the IS where it fuses with the plasma membrane and releases effector molecules granzyme B and perforin to the synaptic cleft. Thirdly, some granzyme B enters the target cell, and the others are recovered into early endosome then LAMP-1+ lysosome to be used again. Solid line indicates that the pathway is addressed in this study and the broken line indicates that the pathway is not clear
Electronic supplementary material
The files are unfortunately not in the Publisher's archive anymore.
Figure S1. CPZ concentration titration. a,b The effect of chlorpromazine (CPZ) on the vitality of NK92 cells. 1 × 106 NK92 cells were pretreated with different concentrations of CPZ for 30 min at 37°C, respectively. The cells were then washed twice and stained with 10 μg/ml propidium iodide (PI) for 30 min. The death rate of NK92 cells was immediately analyzed by flow cytometry. c,d Effect of CPZ on transferrin (Tfr) internalization. 1 × 106 NK92 cells were pretreated with different concentrations of CPZ for 30 min at 37°C, respectively, and then washed and incubated with Alexa488-transferrin (10 µg/ml) for 30 min on ice for binding, washed, and transferred to 37°C for 30 min for internalization. After that, cells were washed with acid buffer (50 mM glycine, 150 mM NaCl, pH 2.5) to remove uninternalized transferrin, followed by neutralization with RPMI (pH 10). The endocytosis of Alexa488-transferrin is calculated as the fold change in mean fluorescence intensities (MFI). MFI of bound Alexa488-transferrin on the cell surface on ice was named MFI(maximum). MFI of internalized Alexa488-transferrin was named MFI(experiment). The percentage internalization is calculated by the following formula: percentage internalization = MFI(experiment)/MFI(maximum) × 100. Error bar indicates SD. Data are representative of three independent experiments (18_2010_377_MOESM1_ESM.tiff 779 kb)
Figure S2. Internalization of FM1-43FX and transferrin assays. A Effect of CPZ treatment on uptake of FM1-43FX by NK cells with PEC stimulation. a NK92 cells that were labeled with 5 μg/ml FM1-43FX for 5 min and kept always at 4℃ for maximum fluorescence. b NK92 cells that internalized FM1-43FX after stimulation with PEC for 3 h and washed with PBS to remove surface FM1-43FX. c NK92 cells that were pretreated with 10 μg/ml CPZ for 30 min and internalized FM1-43FX after stimulation with PEC. d NK92 cells that were continuously treated with CPZ for 3 h and internalized FM1-43FX with PEC stimulation; e No treatment of NK92 cells were as a control. B: Effect of CPZ treatment on endocytosis of Alexa488-transferrin. a NK92 cells that were labeled with 10 μg/ml Alexa488-transferrin for 30 min and kept always on ice for maximum transferrin fluorescence. b NK92 cells that were labeled with Alexa488-transferrin then transferred to 37°C to allow internalization for 3 h, then washed with acid wash buffer to remove surface ligands. c NK92 cells that were pretreated with 10 ug/ml CPZ for 30 min, and internalized Alexa488-transferrin. d NK92 cells that were continuously treated with CPZ for 3 h and internalized Alexa488-transferrin; e: No treatment of NK92 cells were as a control. Data are representative of three independent experiments. (18_2010_377_MOESM2_ESM.tiff 467 kb)
Figure S3. Cytotoxicity of NK cells is attenuated by pretreatment with sucrose. a, b The cytotoxicity of NK92 cells against K562 cells. After incubation of 0.45 M sucrose pretreated or non-pretreated NK cells and CFSE pre-labeled K562 cells at indicated ratio in the graft for 3 h, all cells were stained with PI and analyzed by FCM. a: Upper right quadrant shows the K562 death rate. b The cytotoxicity was calculated with formula described in the Materials and methods section. c The cytotoxicity of NK92 cells to PEC. After NK cells with or without sucrose pretreatment and PEC cells were cocultured at indicated ratio in the graft for 3 h, NK cells were discarded and PEC survivals were stained with crystal violet. The absorbance value was measured to calculate the cytotoxicity. *: p < 0.05. Data are representative of three independent experiments. (18_2010_377_MOESM3_ESM.tiff 1173 kb)
Figure S4. Colocalization of CD63 and granzyme B. a NK cells with CPZ pretreatment (+CPZ) or non-treatment (−CPZ) were stimulated by PEC for 0 min or 60 min, then fixed, permeabilized, and non-specific binding sites blocked and incubated with anti-CD63 (1:200), anti-granzyme B (1:200) and FITC- or TRITC-conjugated secondary antibody (1:200), respectively. b After NK cells with CHC-specific RNAi pretreatment (CHC) or scrambled RNAi (scrambled) pretreatment were stimulated by PEC for 0 min or 60 min, colocalization of LAMP-1 and granzyme B was observed by immunostaining as described above. CD63 is shown in green. Granzyme B (GrmB) is shown in red. Nucleus is shown in blue. Scale bar is 10 μm. Data are representative of three independent experiments. (18_2010_377_MOESM4_ESM.tiff 721 kb)
Acknowledgements
We thank D. Phil (Oxon) Foad Katirai for his professional English editing and thank Prof. Tao Xu, Ms. Jingze Lu, and Mr. Yinong Zhang for their help with the confocal work. We thank Dr. Zhigang Tian, Dr. Ming Zhu, and Dr. Shi Chen for their help in the work of cell cultures. This work was supported by grants from the National Natural Sciences Foundation of China (No. 30400390) and Major State Basic Research Development Program of China (973 Program) (No. 2007CB512402) and the Ministry of Science and Technology (No. 2009DFA31940).
Contributor Information
Fang Zheng, Email: zhengfangtj@yahoo.com.
Feili Gong, Email: flgong@163.com.
References
- 1.Smyth MJ, Cretney E, Kelly JM, Westwood JA, Street SE, Yagita H, Takeda K, van Dommelen SL, Degli-Esposti MA, Hayakawa Y. Activation of NK cell cytotoxicity. Mol Immunol. 2005;42:501–510. doi: 10.1016/j.molimm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 2.Blott EJ, Griffiths GM. Secretory lysosomes. Nat Rev Mol Cell Biol. 2002;3:122–131. doi: 10.1038/nrm732. [DOI] [PubMed] [Google Scholar]
- 3.Liu D, Xu L, Yang F, Li D, Gong F, Xu T. Rapid biogenesis and sensitization of secretory lysosomes in NK cells mediated by target-cell recognition. Proc Natl Acad Sci USA. 2005;102:123–127. doi: 10.1073/pnas.0405737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark R, Griffiths GM. Lytic granules, secretory lysosomes and disease. Curr Opin Immunol. 2003;15:516–521. doi: 10.1016/S0952-7915(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 5.Bossi G, Griffiths GM. CTL secretory lysosomes: biogenesis and secretion of a harmful organelle. Semin Immunol. 2005;17:87–94. doi: 10.1016/j.smim.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Bossi G, Trambas C, Booth S, Clark R, Stinchcombe J, Griffiths GM. The secretory synapse: the secrets of a serial killer. Immunol Rev. 2002;189:152–160. doi: 10.1034/j.1600-065X.2002.18913.x. [DOI] [PubMed] [Google Scholar]
- 7.Fischer A, Latour S, de Saint Basile G. Genetic defects affecting lymphocyte cytotoxicity. Curr Opin Immunol. 2007;19:348–353. doi: 10.1016/j.coi.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Krzewski K, Strominger JL. The killer’s kiss: the many functions of NK cell immunological synapses. Curr Opin Cell Biol. 2008;20:597–605. doi: 10.1016/j.ceb.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motyka B, Korbutt G, Pinkoski MJ, Heibein JA, Caputo A, Hobman M, Barry M, Shostak I, Sawchuk T, Holmes CF, Gauldie J, Bleackley RC. Mannose 6-phosphate/insulin-like growth factor II receptor is a death receptor for granzyme B during cytotoxic T cell-induced apoptosis. Cell. 2000;103:491–500. doi: 10.1016/S0092-8674(00)00140-9. [DOI] [PubMed] [Google Scholar]
- 11.Keefe D, Shi L, Feske S, Massol R, Navarro F, Kirchhausen T, Lieberman J. Perforin triggers a plasma membrane-repair response that facilitates CTL induction of apoptosis. Immunity. 2005;23:249–262. doi: 10.1016/j.immuni.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Chowdhury D, Lieberman J. Death by a thousand cuts: granzyme pathways of programmed cell death. Annu Rev Immunol. 2008;26:389–420. doi: 10.1146/annurev.immunol.26.021607.090404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balaji KN, Schaschke N, Machleidt W, Catalfamo M, Henkart PA. Surface cathepsin B protects cytotoxic lymphocytes from self-destruction after degranulation. J Exp Med. 2002;196:493–503. doi: 10.1084/jem.20011836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun J, Bird CH, Sutton V, McDonald L, Coughlin PB, De Jong TA, Trapani JA, Bird PI. A cytosolic granzyme B inhibitor related to the viral apoptotic regulator cytokine response modifier A is present in cytotoxic lymphocytes. J Biol Chem. 1996;271:27802–27809. doi: 10.1074/jbc.271.44.27802. [DOI] [PubMed] [Google Scholar]
- 15.Xu T, Naraghi M, Kang H, Neher E. Kinetic studies of Ca2+ binding and Ca2+ clearance in the cytosol of adrenal chromaffin cells. Biophys J. 1997;73:532–545. doi: 10.1016/S0006-3495(97)78091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. doi: 10.1016/S0021-9258(19)83641-4. [DOI] [PubMed] [Google Scholar]
- 17.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piriou L, Chilmonczyk S, Genetet N, Albina E. Design of a flow cytometric assay for the determination of natural killer and cytotoxic T-lymphocyte activity in human and in different animal species. Cytometry. 2000;41:289–297. doi: 10.1002/1097-0320(20001201)41:4<289::AID-CYTO7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 19.Heidelberger R, Heinemann C, Neher E, Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 1994;371:513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- 20.Forte P, Baumann BC, Schneider MK, Seebach JD. HLA-Cw4 expression on porcine endothelial cells reduces cytotoxicity and adhesion mediated by CD158a + human NK cells. Xenotransplantation. 2009;16:19–26. doi: 10.1111/j.1399-3089.2009.00510.x. [DOI] [PubMed] [Google Scholar]
- 21.Xu J, McNeil B, Wu W, Nees D, Bai L, Wu LG. GTP-independent rapid and slow endocytosis at a central synapse. Nat Neurosci. 2008;11:45–53. doi: 10.1038/nn2021. [DOI] [PubMed] [Google Scholar]
- 22.Gaffield MA, Betz WJ. Synaptic vesicle mobility in mouse motor nerve terminals with and without synapsin. J Neurosci. 2007;27:13691–13700. doi: 10.1523/JNEUROSCI.3910-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, Chernomordik LV, Lebleu B. Cell-penetrating peptides A reevaluation of the mechanism of cellular uptake. J Biol Chem. 2003;278:585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- 24.Mousavi SA, Malerod L, Berg T, Kjeken R. Clathrin-dependent endocytosis. Biochem J. 2004;377:1–16. doi: 10.1042/bj20031000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishi K, Saigo K. Cellular internalization of green fluorescent protein fused with herpes simplex virus protein VP22 via a lipid raft-mediated endocytic pathway independent of Caveolae and Rho Family GTPases but dependent on Dynamin and Arf6. J Biol Chem. 2007;282:27503–27517. doi: 10.1074/jbc.M703810200. [DOI] [PubMed] [Google Scholar]
- 26.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PubMed] [Google Scholar]
- 27.Masilamani M, Narayanan S, Prieto M, Borrego F, Coligan JE. Uncommon endocytic and trafficking pathway of the natural killer cell CD94/NKG2A inhibitory receptor. Traffic. 2008;9:1019–1034. doi: 10.1111/j.1600-0854.2008.00738.x. [DOI] [PubMed] [Google Scholar]
- 28.Kummer JA, Kamp AM, Tadema TM, Vos W, Meijer CJ, Hack CE. Localization and identification of granzymes A and B-expressing cells in normal human lymphoid tissue and peripheral blood. Clin Exp Immunol. 1995;100:164–172. doi: 10.1111/j.1365-2249.1995.tb03619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cullen SP, Martin SJ. Mechanisms of granule-dependent killing. Cell Death Differ. 2008;15:251–262. doi: 10.1038/sj.cdd.4402244. [DOI] [PubMed] [Google Scholar]
- 30.Baran K, Ciccone A, Peters C, Yagita H, Bird PI, Villadangos JA, Trapani JA. Cytotoxic T lymphocytes from cathepsin B-deficient mice survive normally in vitro and in vivo after encountering and killing target cells. J Biol Chem. 2006;281:30485–30491. doi: 10.1074/jbc.M602007200. [DOI] [PubMed] [Google Scholar]
- 31.Rajagopalan S, Bryceson YT, Kuppusamy SP, Geraghty DE, van der Meer A, Joosten I, Long EO. Activation of NK cells by an endocytosed receptor for soluble HLA-G. PLoS Biol. 2006;4:e9. doi: 10.1371/journal.pbio.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 33.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]