Abstract
The G protein-coupled receptors (GPCRs) are a superfamily of transmembrane receptors that have a broad distribution and can collectively recognise a diverse array of ligands. Activation or inhibition of GPCR signalling can affect many (patho)physiological processes, and consequently they are a major target for existing and emerging drug therapies. A common observation has been that the pharmacological, signalling and regulatory properties of GPCRs can differ in a cell- and tissue-specific manner. Such “phenotypic” diversity might be attributable to post-translational modifications and/or association of GPCRs with accessory proteins, however, post-transcriptional mechanisms are also likely to contribute. Although approximately 50% of GPCR genes are intronless, those that possess introns can undergo alternative splicing, generating GPCR subtype isoforms that may differ in their pharmacological, signalling and regulatory properties. In this review we shall highlight recent research into GPCR splice variation and discuss the potential consequences this might have for GPCR function in health and disease.
Keywords: G protein-coupled receptor (GPCR), Alternative splicing, Exon, Isoform, Signalling properties, Pathophysiology
Introduction
Contrary to estimates made in the “pre-genomic” era that there would be more than 100,000 human genes, a surprising outcome of the human genome project [1] was the finding that we are apparently less complex than we had imagined, with perhaps only 30,000–40,000 genes being sufficient to make us who and what we are. This discrepancy can be partially explained by the fact that a single gene can potentially encode a number of protein products due to alternative mRNA splicing. Alternative mRNA splicing is an important mechanism for generating protein diversity. Indeed, recent microarray analyses suggest that around 40% of Drosophila genes [2] and >90% of human genes undergo alternative splicing [3].
Alternative splicing is the process by which exons (or even introns) can be either included or excluded from precursor-mRNA (pre-mRNA) resulting in multiple, mature mRNA variants. This process is carried out by a large complex, the spliceosome, which is a supramolecular assembly consisting of five small nuclear ribonucleoprotein particles (snRNPs) [4]. The process is governed by (1) various cis-acting elements encoded in the pre-mRNA sequence itself [the 5′ and 3′ splice sites, branch points, exonic and intronic enhancer/silencer sites (ESE/ESS, ISE/ISS)], (2) trans-acting elements, specific proteins that recognise particular ESE/ESS or ISE/ISS and bind to them [serine/arginine-rich (SR) proteins and heterogeneous ribonucleoproteins (hnRNPs)] and (3) cell-type-specific regulatory mechanisms. The final outcome of alternative splicing is translation of isoformic variants of proteins, encoded by the same gene, but differing in sequence and therefore potentially in their biomolecular and cellular properties. In addition, if alternative splicing of mRNA leads to the introduction of a premature stop codon, some of these mRNAs are not translated to proteins but undergo nonsense-mediated decay (NMD) [5]. Proteins affected by alternative splicing might have unaltered function, altered function, or no function at all. Splicing that generates non-functional isoforms might have a significant impact on susceptibility to and development of a range of illnesses [e.g. muscular dystrophy, cystic fibrosis, asthma, insulin-dependent (type I) diabetes, cancer [6]]. In addition, mutations that lead to alterations in the splicing of various proteins, including G protein-coupled receptors (GPCRs), have been identified in a variety of cancers, including melanomas, breast, ovarian, prostate, liver and gastrointestinal cancers [7–9].
GPCRs constitute the largest family of membrane-associated receptors. A key feature of the GPCR family is that different receptor subtypes have evolved that can specifically detect very small (e.g. H+, Ca2+) or very large (e.g. thyroid-stimulating hormone) ligands and everything in between. In addition, these receptors, through G protein-dependent and G protein-independent mechanisms, can link to an array of intracellular signalling pathways that can in turn regulate a plethora of physiological functions. Not surprisingly therefore, GPCRs are almost universally expressed in cells/tissues, and specific manipulation of their activity is the primary target of approximately 50% of presently available drugs [10–12]. It is also becoming increasingly apparent that GPCRs sometimes exhibit cell- and tissue-specific signalling, regulatory and/or pharmacological properties [13]. Genetic variation (single nucleotide polymorphisms), post-translational modification (including phosphorylation, glycosylation and lipidation) as well as association with accessory proteins may contribute to such “phenotypic” diversity [11, 13]. However, much accruing evidence also indicates that alternative splicing of GPCRs can profoundly affect signalling, regulatory and/or pharmacological properties. In addition, it is worth noting that alternatively spliced GPCR isoforms can differ in their abilities to undergo post-translational modification and/or to interact with accessory proteins. For example, a splice-variant form of the parathyroid hormone (PTH)/PTH-related peptide receptor expressed only in kidney is not post-translationally glycosylated [14].
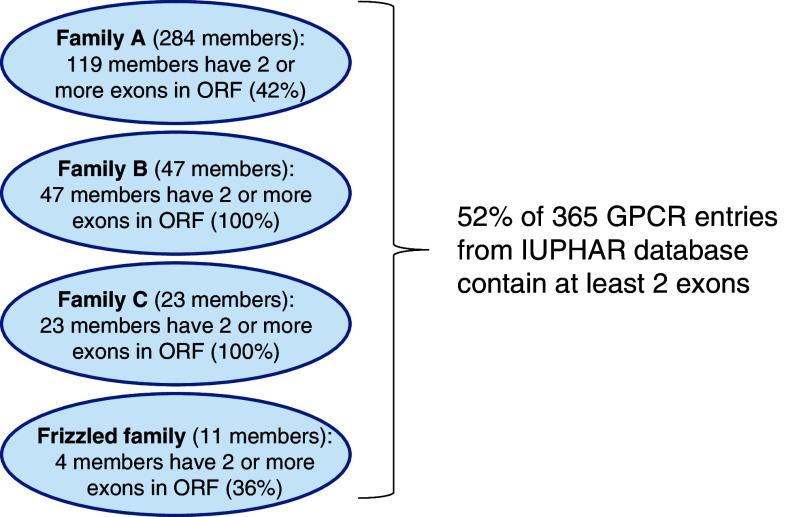
For a long time it was assumed that human GPCRs that undergo alternative splicing to form different mRNA variants and protein isoforms were rare. In 1999, based on data available from GeneBank, Gentles and Karlin [15] estimated that more than 90% of human GPCRs are intronless. This estimation was based on 120 mammalian GPCRs and 82 primate GPCRs. Since then, many more GPCR entries have been created. An interrogation of 365 human GPCR entries from the IUPHAR database (http://www.iuphar-db.org/GPCR/ReceptorListForward) using the CCDS sequence data available from the NCBI web site (http://www.ncbi.nlm.nih.gov/CCDS/CcdsBrowse.cgi) showed that 52% of these GPCRs contain at least two exons in their open reading frame (ORF), suggesting the existence of an intron (see Fig. 1). All members of family B (secretin and adhesion) and family C (glutamate) GPCRs have multiple exons; while only 58% of rhodopsin (family A) (N.B. olfactory receptors were not included) and 64% of frizzled family GPCRs are intronless. Thus, the assumption that more than 90% of human GPCRs are intronless needs to be revised [15]. An important role in postulating the existence of novel splice variants is played by bioinformatics studies. This approach sets in motion fast identification of novel splice variants as demonstrated for adhesion GPCRs [16].
Fig. 1.
Overview of GPCRs encoded by more than one exon. In the “pre-human genome era” it was assumed that more than 90% of human GPCRs were intronless [15]. However, analysis from various databases and bioinformatic studies indicate that the number of intron-containing GPCRs has been under-estimated. Using a list of over 350 GPCRs from the IUPHAR database and examining their ORF sequences for the presence of alternate exons (using CCDS data from NCBI), it is obvious that a large number of GPCRs contain more than one exon (52%). This does not mean that all of them can be expressed as alternatively spliced variants (mRNA) or isoforms (proteins), but that they are not intronless. These data are also likely to be an under-estimate because if the CCDS data are not available for a gene, we would assign the GPCR as intronless
Although a large proportion (~50%) of GPCRs are intronless (see Fig. 1), those encoded by more than a single exon can potentially undergo alternative mRNA splicing. Almost a decade ago, Kilpatrick et al. [17] and Minneman [18] thoroughly reviewed GPCR splice variants known at the time and their (altered) signalling and functional characteristics; thus in this manuscript we will focus on what has happened subsequently in this area. A list of newly identified GPCR variants/isoforms is given in Table 1. However, we cannot move forward without first mentioning two classic examples. The D2 dopamine receptor was one of the first GPCRs identified as being expressed in two variant forms generated by alternative splicing [36]. The isoforms, termed short (D2S) and long (D2L), differ by 29 amino acids in the third intracellular (i3) loop, and this change in amino acid sequence results in isoforms that are differentially expressed in the CNS and have subtly distinct signalling properties [37]. Thus, the D2L receptor is highly expressed post-synaptically in the regions that receive dopaminergic input, while the D2S receptor isoform is more abundant in regions of the hypothalamus and mesencephalon known to synthesize and release dopamine [36, 37]. Another well-established example of a GPCR that is expressed as multiple isoforms is the rat type 1 pituitary adenylate cyclase-activating peptide receptor (PAC1 receptor). This receptor has been reported to be expressed in five splice variant forms with the variation existing within the i3 loop (variants termed “hip”, “hop1”, “hop2”, “hip-hop1”, and null). In this case, protein isoforms differ with respect to their regulation of adenylate cyclase and phospholipase C activities [85].
Table 1.
Splice variation and potential physiological roles of receptor isoforms
| Receptor | Event description | Potential physiological relevance | References |
|---|---|---|---|
| 5-HT receptors | Several | Various | Reviewed in [19] and [20] |
| Muscarinic acetylcholine receptor | Novel intron/exon | Unknown | [21] |
| Adenosine receptors | 5′-UTR splicing; novel exons | Differential translation | [21, 22] |
| α1-Adrenoceptors | Several | Various | Reviewed in [23] |
| Angiotensin-II receptor | Exon inclusion/exclusion | Distinct signalling properties | [24, 25] |
| Calcitonin receptor | Exon exclusion-headless variant | Abolished ligand binding/dominant-negative effect | [26, 27] |
| Cannabinoid receptor type 1 | Alternative donor/acceptor site | Altered pharmacology | [28, 29] |
| Chemokine receptor (CXCR3) | Novel acceptor site-distinct N-terminus | Distinct signalling properties | [30] |
| Cholecystokinin receptor (CCK-B) | Intron inclusion | Constitutively active, ligand–independent Src activation | [8, 31, 32] |
| Corticotropin-releasing hormone receptors (type 1 and 2) | Several | Various | [33–35] |
| Dopamine receptor (D2) | Exon inclusion/exclusion | Distinct signalling properties and cellular localisation | [36, 37] |
| Dopamine receptor (D3) | Premature stop codon-lacks TM6 and TM7 | Distinct cellular localisation | [38] |
| Endothelin B receptor | Exon exclusion | Not transcribed | [39] |
| Epidermal growth factor-seven TM receptor | Trans-splicing | Diversification of ligand repertoire | [40] |
| Follicle-stimulating hormone receptor | Exon exclusion and used of additional exon | Not GPCR, but growth factor receptor | [41] |
| GABAB receptor | Several | Various | Reviewed in [42] |
| Gastric inhibitory polypeptide receptor | Intron retention-premature stop codon | Impaired signalling; dominant-negative effect | [43] |
| Growth hormone-releasing hormone receptor | Exon exclusion, intron retention | Impaired signalling; dominant-negative effect | [44–49] |
| Histamin receptors (H3 and H4) | Several | Various | [50–52] |
| Leukotriene B4 receptor | Alternative donor/acceptor sites, novel exons/introns | Unknown | [21] |
| Luteinizing hormone receptor | Mutation at the intron 10/exon 11, resulting in 8 aa deletion | Abolished reproduction | [53, 54] |
| Melanocortin 2 receptor | 5′-UTR splicing | Adipogenesis? | [55] |
| Metabotropic glutamate receptors | Alternative C-terminal exons | Distinct signalling; role in development | [56–58] |
| Neurokinin receptor 1 | Exon exclusion- truncation | Distinct signalling; role in immunomodulation | [59–64] |
| Neurokinin receptor 2 | Exon exclusion | Impaired ligand binding and signalling | [65, 66] |
| Neuropeptide S receptor (GPRA) | Alternative C-terminal exons, truncation | Intracellular localisation of truncated isoform | [67] |
| Opioid receptors | Several | Various | Reviewed in [68] |
| Parathyroid hormone (-related peptide) receptor | 5′-UTR; exon exclusion | Lack of post-translational glycosylation | [14, 69] |
| Prostaglandin receptors | Several | Various | [70–72] |
| Relaxin receptors | Several | Various | [73–75] |
| Secretin receptor | Exon exclusion | Unknown—cancer biology? | [76, 77] |
| Somatostatin receptor (sst2) | Exon exclusion-truncation | Altered desensitisation | [78], reviewed in [79] |
| Thromboxane receptors | Alternative C-terminal exons | Distinct internalisation/interaction with arrestins | [80, 81] |
| Vasopressin V2 receptor | Alternative splice site | Dominant-negative effect | [82, 83] |
| VIP and PACAP receptors | Several | Various | Reviewed in [84] |
Numerous splicing events that affect the expression of GPCRs mRNA and protein have been described since 2001 (for splice variants known before 2001, see [17, 18]). Over-expression studies provide valuable insights into potential physiological relevance of these events, although in many cases the real physiological roles in endogenous systems are unknown
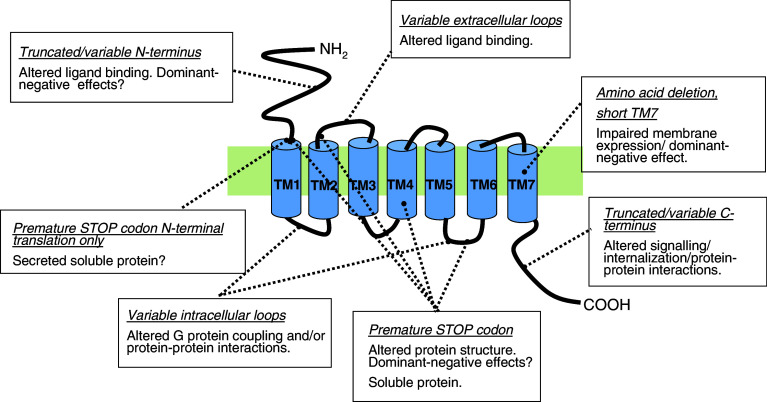
In general, alternative splicing of GPCRs can lead to the expression of proteins with the following (see Fig. 2):
Isoforms in which the N-terminus differs due to distinct promoters [e.g. corticotropin-releasing hormone receptor type 2 (CRH-R2), PTH-R], or deletion of exons [e.g. calcitonin receptor (CT-R), corticotropin-releasing hormone receptor type 1 (CRH-R1), secretin receptor]. In most cases these isoforms have impaired or abolished ligand binding properties.
Isoforms in which the C-terminus differs in length [e.g. prostaglandin EP3 receptor, prostaglandin F2α receptor, α1A-adrenoceptor, γ-aminobutyric acid type B (GABAB) receptor, metabotropic glutamate (mGlu) receptors, μ-opioid receptor, 5-hydroxytryptamine (5HT) receptors, somatostatin receptors, thyrotropin-releasing hormone (TRH) receptor type 1, neurokinin-1 receptor (NK1)] may have distinct signalling and regulatory (desensitisation/internalisation) properties. In some cases retention of an intron or deletion of an exon can introduce a premature stop-codon, leading to expression of a truncated receptor. In a structural sense, these receptors can be missing the original C-terminus, parts of the seventh transmembrane domain (TM7) and even several transmembrane regions. Some of these receptor variants can exhibit a “dominant-negative” effect, acting to nullify signalling by the wild-type receptor (e.g. for the gonadotropin-releasing hormone receptor [86]). A unique example where alternative splicing changes the nature of a receptor is follicle-stimulating hormone (FSH) receptor. A full-length isoform of FSH receptor is a GPCR which couples to the Gαs protein; in contrast, a truncated isoform of FSH receptor (generated by exclusion of exons 9 and 10 and use of additional exon 11) belongs to the superfamily of growth factor receptors [41].
Isoforms in which an intracellular loop differs in size [e.g. CRH-R1, D2 dopamine, H3 histamine, cholecystokinin-B (CCKB) and PAC1 receptors]. Alternative splicing within the i3 (and perhaps also i1/i2) loops can affect G protein coupling preference and/or coupling can be altered or even abolished.
Isoforms in which TM7 is shortened by 14 amino acids (e.g. CRH-R1, CT-R, PTH-R, VPAC2 receptor) can result in poor receptor expression at the plasma membrane and potential dominant-negative effects on wild-type receptor function. To date, these isoforms have been reported only for secretin (B1 family) GPCRs [27, 69, 87–89]; however, some members of the newly classified family of adhesion GPCRs which have exon-intron organisation similar to the secretin family [90] could potentially give rise to similar splice variants.
Isoforms with insertions or deletions within the extracellular loops of GPCRs are much less common compared to intracellular loop variants and to date only a small number have been reported (e.g. CT-R, D3 dopamine, orphanin FQ/nociceptin receptors [17]). In the case of the CT-R, an insertion of 37 amino acids within the first extracellular (e1) loop alters ligand specificity and binding kinetics, while wild-type receptor signal transduction properties appear to be preserved [91].
Soluble isoforms of GPCRs are severely truncated spliced forms usually composed only of all or part of the extracellular N-terminal domain (e.g. luteinizing hormone receptor-LHR [54], mGluRs [58], CRH-Rs [92]). In many cases these proteins can bind their ligand but do not transduce a signal, and thus behave as “decoy” receptors.
Fig. 2.
Schematic representation of GPCR structure summarising potential splice variants. The 7TM domains are shown as blue cylinders; the N-terminus is facing the extracellular side of the plasma membrane, while the C-terminus is intracellular. The events that lead to translation of alternatively spliced isoforms are outlined in the boxes
Although many members of the rhodopsin (family A) and frizzled/taste2 (frizzled and family C) sub-families are expressed as multiple isoforms, all members of the secretin (family B), adhesion (family B) and glutamate (family C) sub-families studied to date appear to undergo extensive alternative splicing due to their unique exon-intron organisation [58, 89, 90]. A fascinating example of the importance of splicing in GPCR biology and hormone actions is the CRH-R1 receptor, which orchestrates mammalian responses to stressful stimuli [33]. In humans the CRH-R1 gene spans over 50 kb and contains 14 exons, and at least 14 alternatively spliced mRNAs for CRH-R1 variants have been detected to date [33, 34]. The complete gene product incorporating all 14 exons encodes a 444-amino acid GPCR, termed CRH-R1β, which displays weak agonist binding and impaired signalling properties [93–95]. Omission of exon 6 from the mature mRNA results in expression of a 415-amino acid GPCR, termed CRH-R1α, which encodes the major functional CRH-R1 isoform. Deletion of exon 3 results in CRH-R1c, which lacks 40 amino acids within the N-terminus and no longer binds CRH [96]. Various exon deletions or insertions of “cryptic” exons (exons that are absent in the normal form but occur in an alternative form) result in frame-shifts and the potential introduction of a premature termination codon, leading to the expression of soluble or severely truncated membrane proteins (termed CRH-R1e-h) [34, 97]. Another splice variant, termed CRH-R1d, contains a 42-base-pair exon deletion resulting in a 14-amino acid deletion from TM7 [87]. It is interesting to note that at least three other members of the secretin family of GPCRs (CT-R, PTH-R and VPAC2 receptor) can undergo similar splicing to produce isoforms with shortened TM7 domains [27, 69, 88, 89]; and in each case this sequence modification causes signalling impairment, at least partly due to endoplasmic reticulum retention and poor plasma membrane expression of the spliced isoform [27, 98, 99]. At present, the biological relevance of expression of these different splice variants is unknown; however, a recent study suggested that over-expression of CRH-R1d might lead to impairment of type 2 CRH-R signalling [98, 100], and similar results have been obtained for the calcitonin receptor variant CTR∆13 [27, 99] and the VPAC2 receptor variant SD-VPAC2 [101].
Mechanisms of alternative splicing of GPCRs
Although what governs GPCR alternative splicing remains largely unknown, there are a number of indications that changing (patho)physiological conditions can influence the way in which GPCR pre-mRNAs are processed. Thus, it has been reported that activation of protein kinases (PKA and PKC) and environmental insults (UV irradiation) can lead to modulation of CRH-R1 mRNA expression in keratinocytes and melanoma cells [34]. In addition, physiological changes, such as the onset of labour, can alter the relative expression of CRH-R1 variants in the uterus [102]. An increasing body of evidence points towards steroid hormones as potential modulators of alternative splicing. Oestrogen can bring about the up-regulation of important regulators for splicing, such as SR-protein (SC35) and hnRNPA1 [103] and can regulate transcription and mRNA splicing directly though CAPER molecules [104]. Oestrogen and other steroid hormones can also affect the ratio of D2S and D2L dopamine receptor variants in various target cells both in vitro and in vivo without affecting total receptor mRNA expression, suggesting that steroid sex hormones exert their effects, at least in part, at the level of alternative splicing [105, 106]. In addition, in myometrial smooth muscle, progesterone treatment can regulate the ratio of CRH-R1α/β mRNA expression [33].
During normal development the expression profile of some of GPCR splice variants changes. A study published recently by Bovolin et al. [57] showed that mice embryonic mitral cells express mainly the mGlu1b receptor variant, but by 1 week of age, this splice variant has become a minor component and mRNA for the mGlu1a receptor variant predominates. The authors speculate that the two splice variants, which have different trafficking properties and sub-cellular localisations [56], might encode proteins that have distinct roles in embryonic mitral cell maturation (mGlu1b receptor) and post partum in synaptic refinement (mGlu1a receptor) [57]. The differential expression patterns of the wild-type gonadotropin-releasing hormone receptor and its splice variant forms in neonatal and adult tissues also suggest that different isoforms of this receptor are involved in early development of ovary and testis [107]. In many cancers, proteins involved in regulation of alternative splicing are down-regulated resulting in expression of differently spliced GPCR isoforms; such is a case with CCK-B and secretin receptors in gastrointestinal, colonic and pancreatic cancers [31, 76].
All of the examples of GPCR isoforms described so far are products of cis-splicing, that is, a single molecule of pre-mRNA is processed to generate mRNA. However, trans-splicing, alternative splicing of the 5′-untranslated region (5′-UTR) and single nucleotide polymorphisms (SNP) have been also identified as important mechanisms in alternative splicing of GPCRs.
Trans-splicing
Numerous members of the adhesion GPCR sub-family are subject to undergoing alternative splicing to generate functionally unique isoforms [16, 108]. Although in mammals the process of trans-splicing (occurring between two separate mRNAs [109]) is rare compared to cis-splicing, adhesion GPCRs undergo this type of event. The epidermal growth factor-seven transmembrane spanning (EGF-TM7) receptors, which are mostly restricted to leukocytes, undergo extensive cis- and trans-splicing in order to expand the range of functional adhesion GPCRs [40], including diversification of ligand repertoire and cellular functions.
5′-UTR alternative splicing
Even in the case of GPCRs that have only one intron or are intronless in the ORF, alternative splicing of the 5′-UTR could play a crucial role in their post-transcriptional regulation, including the modulation of translational efficiency, message stability and subcellular localisation. For example, Kreth et al. [22] have reported that alternative splicing of the 5′-UTR of the A2A adenosine receptor gene can alter receptor protein expression, and this could be of potential importance in how these receptors contribute to the physiological response to sepsis. It is established that adenosine, via activation of A2A adenosine receptor, reduces phagocytosis, increases secretion of anti-inflammatory cytokines and induces lymphocyte apoptosis [110]. Thus, the reduced expression of the receptor might be beneficial in the treatment of infection and sepsis [110].
Single nucleotide polymorphisms (SNPs)
SNPs, as providers of molecular diversity, are responsible for approximately 90% of human DNA variation. The SNPs in the coding regions of genes can be synonymous (silent) or non-synonymous, with the latter causing an amino acid change leading to potential phenotypic variation [111]. However, SNPs in the non-coding regions of genes can have an effect on gene expression and splicing. Recently, it has been reported that several SNPs within the D2 dopamine receptor gene can affect the receptor variant expression profile [112]. One of these SNPs is an upstream promoter polymorphism, and two intronic SNPs are able to affect D2 receptor splicing by generating a novel transcription factor binding site and new cis-acting sites to which splicing factors (e.g. SC35 and SRp40) could bind [112]. A physiological consequence of these SNPs is that the genetically driven changes in D2 dopamine receptor splicing appear to affect behaviour and working memory in humans [112].
Aberrant transcription versus physiologically relevant processing
The majority of GPCR splice variants have initially been identified at the mRNA level, and the physiological relevance of alternative mRNA splicing to generate GPCR isoforms might be questioned or even dismissed as the result of aberrant or “leaky” transcription. A general lack of isoform-specific antibodies has often made it very difficult to demonstrate that mRNA variants are translated into mature proteins in endogenous systems. However, in recent years increasing numbers of reports suggest potential physiological consequences of GPCR alternative splicing. For example, it has been established that alternatively spliced GPCRs can have distinct (and perhaps physiologically desirable) signalling characteristics (e.g. PAC1 receptor [85, 113], mGlu1 and mGlu5 receptors [114–116], NK1 receptor [63]), impaired signalling properties and/or dominant-negative effects on wild-type receptors (as described above for CT-R and CRH-R1) and constitutive activity [e.g. mGlu1, prostaglandin EP3 and 5HT4 receptors (reviewed in [18])]. Perhaps more importantly, hormone responsiveness can be fine-tuned by regulating the relative expression of GPCR isoforms [e.g. angiotensin II type 1 (AT1) [24, 25] and CRH [102] receptors]. A recent study by Einstein et al. [21] has shown that the GPCR complement within human airway smooth muscle (ASM) undergoes frequent alternative splicing, resulting in unexpected receptor isoforms which might be of potential significance in the development of future drugs for respiratory diseases.
Alternative splicing of GPCRs in cancer
A large number of molecular changes, including genetic instability, insufficient DNA mismatch repair, increased oncogene expression and defects in several splicing mechanisms, have been described in cancer cells relative to their healthy counterparts [7, 117]. Alternatively spliced mRNAs of many GPCRs have been detected in various cancers. However, whether this is simply a consequence of cancer cell biology, or the cancer-associated GPCR isoforms have an effect on cancer growth and metastasis is poorly understood. Nevertheless, the role of GPCRs and their ligands in cancer progression has been quite widely investigated.
Proliferation of tumour cells is promoted by neuropeptides and growth factors, which are either synthesized locally by the cancerous cells or derived from the systemic circulation. One such peptide is growth hormone-releasing hormone (GHRH). Many cancers, including ovarian, endometrial, prostate, small-cell lung carcinoma and various sarcoma cell lines have the ability to produce GHRH, which might serve as an autocrine growth factor for these cancers [44, 118]. Until recently [44], full-length GHRH-receptor (pGHRH-R), which is abundant in the anterior pituitary gland, was not detected in many GHRH-responsive cancers and human cancer cell-lines; instead four alternatively spliced, truncated mRNAs for GHRH-R have been detected [45, 46]. Among these truncated isoforms of the GHRH receptor, SV1, which is primarily detected in a majority of GHRH-responsive tumours, shows the greatest similarity to full-length pGHRH-R. This isoform differs only in the first three exons, encoding a part of the extracellular domain of the receptor. In the SV1 isoform this region is replaced by a fragment of intron 3, which has a new putative in-frame start codon [46]. It has been demonstrated that when expressed in MCF-7 breast cancer cells that do not possess either pGHRH-R or SV1, SV1 is more potent than pGHRH-R in inducing ligand-dependent cell proliferation [47]. Additionally, MCF-7 cells transfected with SV1 proliferate more quickly than non-transfected counterparts, even in the absence of GHRH, suggesting the existence of ligand-independent SV1 activity [47]. Moreover, in vitro studies utilising human oestrogen-independent breast cancer cell lines (MDA-MB-468 and MDA-MB-435), as well as mouse mammary carcinomas, showed that GHRH antagonists can directly inhibit cell proliferation [119, 120]. Thus, an antagonist of GHRH-R might be effective in the treatment of some oestrogen-independent cancers, and it might be desirable to selectively target the SV1 isoform, potentially sparing normal GHRH-R function [48, 49, 121]. In 2005, Havt et al. [44] employed a sensitive real-time PCR technique, combined with Western blotting and a receptor ligand-binding assay to show that malignant and non-malignant human tissues express the full-length pGHRH-R and its splice variants. These findings demonstrated for the first time the co-existence of pGHRH-R and its splice variants in human tumours, but further studies are needed to dissect any (patho)physiological significance of this co-expression.
Thromboxane synthesis has been reported to be elevated in bladder cancers and inhibition of the synthesis or action of these prostanoid mediators can decrease rates of cell proliferation and migration [122]. In the late 1990s it was demonstrated that two isoforms of the thromboxane receptor (TP-α and TP-β), alternatively spliced at the C-terminus, have distinct agonist-induced internalisation properties and abilities to bind β-arrestins [80], with only the TP-β isoform able to internalise in a ligand-dependent manner. Thromboxane receptor splicing could, as a consequence of each variant giving rise to distinct cellular responses, differentially affect key processes, such as mitogenesis. In this context, it is interesting to note that the TP-β isoform has been shown to be over-expressed in some patients with bladder cancer, and this expression is associated with a poorer prognosis. Moreover, this isoform alone is able to transform SV-HUC (immortalised human uroepithelial cells) into highly proliferative and motile cells and is highly expressed in various cancer-derived cell-lines, including prostate, breast, colon and renal cell carcinoma [81], suggesting an important role of this isoform in determining the progression of the disease.
Another group of GPCRs that has been heavily implicated in cancer pathophysiology are the gastrointestinal peptide hormone receptors, including the gastrin/cholecystokinin-B (CCK-B) [31], secretin receptors [76, 77] and somatostatin [78]. An alternatively spliced isoform of the CCK-B receptor that retains the fourth intron might play an important role in the development and progression of colon and pancreatic carcinoma [8, 31]. Thus, it has been demonstrated that in a pancreatic carcinoma cell line (MIA PaCa-2) the low expression of U2AF35 (a small unit of the U2AF complex that specifically recognises the 3′-end dinucleotide AG which serves as an important splicing signal for pre-mRNA processing) is responsible for the retention and incorporation of the fourth intron into the mature CCK-B receptor protein [31], and this isoform plays a role in stimulating tumour growth [8].
Detecting the expression and understanding the significance of the various GPCR isoforms up-regulated in cancer could be of pivotal importance for future in vivo targeting of tumours both diagnostically and therapeutically.
Alternative splicing of GPCRs in reproduction
Much effort has been made to identify genes that are up-regulated or down-regulated in pregnancy and especially in pathophysiological conditions, such as pre-term labour and pre-eclampsia. Here too, alternative pre-mRNA splicing has been shown to play a major role in smooth-muscle myogenesis and contractility. The role of myometrial processes in regulating the activity of the uterus during gestation and parturition are linked to the differential expression and function of specific genes including cyclooxygenase-2, oxytocin receptors, progesterone receptors, specific prostaglandin receptor subtypes, CRH-R1 and Gαs protein [123]. Most of these, and many other proteins, are expressed as distinct isoforms generated from alternate pre-mRNA splicing. These isoforms have a potentially dominant role in the normal progression of pregnancy.
CRH and CRH-related peptides are expressed in placental and intrauterine tissues during pregnancy and labour. However, the precise biological function of CRH during human pregnancy is still an enigma [124]. There is a wealth of evidence, including myometrial contractility studies, suggesting that CRH plays a “protective” role for the pregnant uterus, by regulating intracellular signalling pathways that can maintain the uterus in a state of relaxation and prevent contraction [124]. On the other hand, increased maternal plasma CRH levels are a predictor of the onset of labour [125]. It is evident that human pregnancy is linked with changes in CRH-R isoformic expression in reproductive tissues [102, 126]. Two CRH-R1 mRNA variants, R1α and R1c, have been identified in syncytiotrophoblasts (the outermost syncytial layer of the foetal component of the placenta) and amniotic epithelial cells [127]. Additionally, the presence of CRH-R1d mRNA has been found in foetal membranes (chorion and amnion tissues) [87]. Seven mRNA splice variants (R1α, R1β, R1c, R1d, R2α, R2β and R2γ) of the CRH receptors have also been reported in the human pregnant myometrium immediately prior to onset of labour, whereas only three isoforms (R1α, R1β and R2β) are routinely detected in the non-pregnant myometrium [87, 128]. These findings suggest that CRH, acting via different receptor variants, may be able to exert distinct actions on the human myometrium in pregnant versus non-pregnant states [129]. More recent evidence indicates that as pregnancy progresses towards labour (either term or pre-term) transcription of the CRH-R1 gene and its alternative splicing are increased [102]. The precise mechanism underlying this event is unknown, but in vitro studies of myometrial cells originating from pregnant subjects identified interleukin-1β as a potential regulator of CRH-R1 gene transcription and splicing [102]. However, due to a lack of CRH-R isoform-specific antibodies, it has not been possible to establish which of these alternatively spliced mRNAs are translated to mature proteins. Therefore, the physiological importance of these alternative splicing events is presently unclear. However, it is tempting to speculate that the expression of various CRH-R isoforms might allow activation of alternative signalling cascades (or dampen wild-type receptor activity), facilitating the transition from relaxed to contractile uterine states [102, 129].
Another hormone involved in reproductive physiology is relaxin, which binds to the relaxin family peptide receptors (RXFP1 and RXFP2) [130]. Several splice variants of RXFP1 [formerly known as LGR7 (leucine-rich-repeat-containing G protein-coupled receptor 7)] have been identified. Some of them produce a soluble, truncated and secreted protein which binds to relaxin and antagonises its actions in vivo [73]. Several splice variants, cloned from human foetal membranes [74, 75], human uterus and brain [130], encode RXFP1 isoforms with different lengths of the extracellular domain. These N-terminally truncated isoforms can exert dominant-negative effects on the wild-type receptor, which could be of potential functional significance in dampening the responsiveness of reproductive tissues to relaxin, especially since animal studies have indicated that these truncated RXFP1 isoforms are prevalent in pregnancy [75].
Luteinizing hormone (LH) and its receptor (LHR) play a pivotal role in female and male gonadal function, pregnancy and foetal sex differentiation. Mutations that lead to loss-of-function in the male are linked with severe phenotypes, such as female external genitalia, micropenis and oligospermia, while in women primary and secondary sexual characteristics develop normally, but infertility is common [131, 132]. Bruysters et al. [53] have recently described a family that carries a homozygous mutation G → A at position −1 at the intron 10/exon 11 boundary of the LHR gene, resulting in alternative splicing of LHR and an eight amino acid deletion. In vitro studies have shown that receptor expression is not affected, but the potency of LH (but not human chorionic gonadotropin) is reduced; thus, this LHR isoform shows impaired hormone responsiveness [53]. A male patient and his three sisters showed reproductive impairment. The male patient had delayed puberty, micropenis and oligospermia, while two of his sisters were infertile and the third sister had three spontaneous miscarriages [53]. Another splice variant of LHR that results in a frame-shift in the reading frame and creates a truncated receptor composed of the N-terminal ectodomain (responsible for high-affinity ligand binding) causes an alteration in pituitary-gonadal function and morphological changes in the adrenals and kidneys of transgenic mice [54]. This variant also appears to control the cell surface expression of the wild-type receptor by misrouting newly synthesized receptors in the ER [54].
Alternative splicing of GPCR in other pathophysiological conditions
Obesity
Insulin secretion from pancreatic β-cells is potentiated by binding of “incretin” peptides, including gastric inhibitory polypeptide (GIP), to their receptors. The 460-amino acid wild-type GIP receptor, a GPCR of the secretin family, is expressed in mouse β-cells along with a GIP receptor splice variant that retains intron 8, which introduces a premature stop-codon resulting in a truncated C-terminus [43]. GIP activation of this latter isoform does not lead to production of cyclic AMP and inhibits the ability of the wild-type receptor to activate this pathway on agonist addition. This is achieved through interactions between truncated and wild-type GIP receptors in the ER, which reduce trafficking of the wild-type GIP receptor from the ER to the cell-surface [43]. Mice fed a high-fat diet exhibit a relative reduction in the expression of the truncated isoform, suggesting that loss of this isoform might be involved in the hypersensitivity to GIP and hyperinsulinaemia evident in this diet-induced model of obesity [43].
Immuno-modulation
The neurokinin-1 (NK1) receptor, which binds substance P, an important neuropeptide involved in pain perception and the modulation of both inflammatory and immune responses, occurs as two variants: a full-length 407-amino acid receptor, and a truncated 311-amino acid isoform [60–62, 133]. These isoforms have distinct signalling properties, with only the wild-type receptor being able to increase intracellular Ca2+ concentration and activate the transcription factor NFκB, leading to increased expression of mRNA for interleukin-8 [59]. In addition, compared to the wild-type receptor, the truncated NK1 receptor variant may have an increased ability to interact with different G proteins, other GPCRs or G protein-independent signalling pathways [59, 63]. This could be of considerable importance in cells and tissues that have distinct expressions of the NK1 receptor isoforms. For example, in undifferentiated THP-1 cells (a human monocytic cell-line), only the truncated NK1 receptor isoform is expressed. However, differentiation of these cells towards a macrophage phenotype with a phorbol ester leads to the expression of both isoforms [64], which could modulate cellular activity. Different brain regions appear to exhibit different patterns of NK1 receptor pre-mRNA alternative splicing, with expression of the truncated NK1 receptor often being greater than that of the full-length variant [64], which could result in effects on pain perception.
Histamine receptor-associated pathophysiological states
Drugs that target different histamine receptors have been widely used to treat various conditions, including allergies, gastric-related disorders and migraine. In contrast to the H1 and H2 histamine receptor genes, the genes encoding H3 and H4 histamine receptors include several intronic sequences. This can result in expression of alternatively spliced isoforms and several H3 histamine receptor-spliced variants have been described (see [50] for review). For example, novel isoforms lacking TM7 and containing an alternate C-terminus (6TM-H3 isoforms) have been isolated and characterised from rat brain [51]. When recombinantly expressed, these isoforms are retained within intracellular compartments, and thus they are unlikely to bind ligand or couple to signalling pathways [51]. However, co-expression studies have demonstrated that 6TM-H3 can selectively interfere with the membrane expression of the wild-type H3 receptor and affect its ability to signal normally; thus, 6TM-H3 acts as a dominant-negative isoform. It is therefore again tempting to speculate that functional (and constitutive) activity of the H3 receptor can be “fine-tuned” through expression of both 6TM-H3 and wild-type 7TM isoforms in a given system [51].
Recently, van Rijn et al. [52] have identified two novel, alternatively spliced H4 histamine receptor isoforms from CD34+ cord blood-cell-derived eosinophils and mast cells. One splice variant encoded a 302-amino acid protein that contained a deletion of 88 amino acids between predicted TM2 and TM4 domains, while a second splice variant encoded only the first 67 amino acids of the whole receptor. Both of the receptor variants, when over-expressed in HEK293 cells, had primarily an intracellular localisation and failed to bind ligand. However, when co-expressed with the wild-type H4 histamine receptor, both isoforms had a dominant-negative effect via hetero-oligomerisation [52]. Again, more research is needed to assess whether splice variations in H4 receptor expression are relevant to any pathophysiological aspects associated with the normal functioning of this receptor subtype in vivo.
Respiratory diseases
Susceptibility to asthma and related respiratory diseases has, in many cases, a genetic component. A genome-wide scan suggested that one of the contributory factors to asthma and other IgE-mediated diseases could be GPRA (also known as GPR154 and the neuropeptide S receptor) [134]. Several splice variants of the receptor have been described [67]. Two splice variants that encode GPRA isoforms with distinct C-termini (termed A and B isoforms) are full-length isoforms that traffic to the plasma membrane. Interestingly, another isoform, the short one that is expressed in intracellular compartments, does not affect the cell surface expression of the full-length receptors when co-expressed [67]. Immunohistochemical studies showed that the A isoform is primarily expressed in smooth muscle cells, while the B isoform is principally detected in epithelial cells. When these authors compared ASM of asthmatic and healthy individuals, they found that the B isoform was more strongly expressed in samples from asthmatic patients [134], suggesting that the neuropeptide S receptor could be a strong candidate for involvement in the pathophysiology of asthma and respiratory atopy in Caucasian populations. However, pharmacological studies did not find any substantial differences in the binding and signalling properties of the two GPRA isoforms [135]. Further work on knockout GPRA−/− mouse model failed to detect differences in the development of allergic lung disease in these animals, despite the demonstrable loss of functional GPRA [136]. Additionally, studies conducted in a Mexican population showed that in contrast to Caucasian and Asian populations, GPRA variants do not appear to be important contributors to childhood asthma susceptibility in Mexicans [137].
Work by Einstein et al. [21] highlighted the complexity of GPCR expression and alternative splicing in human ASM. The authors hypothesized that sometimes complex and contradictory pharmacological responses of ASM GPCRs might be due to variable GPCR expression and the frequent alternative splicing of the receptors leading to a “highly diversified receptor milieu”. This study highlighted the fact that potentially 353 different GPCRs are expressed in airways smooth muscle, of which 192 GPCRs have on average five alternatively spliced variants. One GPCR expressed in multiple splice variants is the leukotriene B4 (LTB4) receptor, a receptor known to contribute to abnormal responsiveness in lung disease [21]. High-affinity LTB4 receptors, expressed on neutrophils, eosinophils and T-lymphocytes, upon activation by its endogenous agonists facilitate leukocyte migration to the lung [138]. Moreover, expression of the LTB4 receptor in ASM could contribute to bronchoconstriction, due to coupling of the receptor to Gq and Gi proteins [139]. The work from Einstein et al. [21] raises a possibility that discrepant bronchoconstrictive effects of LTB4 receptor antagonists might be due to the presence of different receptor isoforms in ASM.
Neuronal function
The mammalian CNS shows the greatest diversity of GPCR expression of any organ in the body. Neuronal GPCRs are presently targeted directly (GPCR agonists and antagonists) or indirectly (through the use of drugs that alter endogenous neurotransmitter levels) to treat a variety of neurological and psychiatric disorders. As already stated, an array of important neurotransmitter/neuromodulator GPCRs are expressed in a number of alternatively spliced variant isoforms, including dopamine, opioid, 5HT4 and 5HT7, V2 vasopressin, metabotropic glutamate (mGlu) and GABAB receptors [17, 19, 20, 68, 83, 140, 141]. Although some of the better characterised GPCR splice variants (e.g. D2S and D2L dopamine receptors) exhibit only quite subtle differences in their signalling properties, data are beginning to appear that at least hint at how such splice variation might affect GPCR function both physiologically and pathophysiologically.
For example, C-terminal μ-opioid receptor variants differ in their ability to be recycled to the plasma membrane (“resensitised”) following agonist-induced internalisation [142]. As described above, mGlu1 receptor isoforms (a and b) are differentially expressed during neuronal development and differentiation, and the mGlu1a and 1b isoforms appear to have distinct cellular localisations and constitutive activities, as well as exhibiting potency and/or efficacy differences with respect to agonists [56, 57, 140]. Similarly, alternative splicing of the D3 dopamine receptor can produce seven variants, including a non-dopamine binding D3nf isoform [38, 143]. Quaternary interaction of D3nf with full-length D3 (or D1) dopamine receptors to form dimers can reduce trafficking of the dopamine-binding isoform to the plasma membrane [143]. Altered splicing efficiency with respect to the D3 dopamine receptor and the consequent alteration in the proportion of full-length and truncated forms generated in neuronal populations has been hypothesized to be linked to schizophrenia [18, 38, 143].
Closing remarks
In this short review we have not sought to cover all GPCRs that are expressed from alternative splicing of pre-mRNAs, but we have attempted to provide an overview of GPCR alternative splicing with an emphasis on newly emerging aspects. In Table 1 we provide an additional summary of some recent findings not mentioned above (and see [17, 18] for the developments before 2001). Additionally, we have aimed to show that many more GPCRs possess introns than was initially thought (or perhaps has become a widely accepted misconception)(Fig. 1). This does not mean that all multi-exon-containing GPCRs will undergo alternative pre-mRNA splicing. For GPCRs that are encoded by only two exons (e.g. some members of the rhodopsin and frizzled families), it is very likely that alternatively spliced mRNA would be subject to NMD, and the message is never translated into a protein product.
The interest in alternative splicing of GPCRs is increasing, and novel variants are being identified on a regular basis. In 2005, two companies launched GPCR SpliceArray for the identification of alternatively spliced GPCR mRNAs (http://www.exonhit.com). Other manufacturers, including Affimetrix and Jivan Company, also provide GPCR arrays. The arrays can be custom-made in order to investigate particular (sometimes rare) splicing events within a sub-group of GPCRs, but also broader arrays can be designed to study the usage of alternative splice donor/acceptor sites, insertion of novel exons, exon skipping, intron retention, or a partial internal exon deletion in a broad number of GPCRs. This new high-throughput approach based on microarray technology uses several probes (usually six) that are specific for an exon and span over the exon junctions allowing robust and specific detection of the two possible mRNAs resulting from each splicing event. The data obtained from these arrays have to be validated using traditional experimental methods (usually RT-PCR and western blotting) which confirm the presence of particular alternatively spliced mRNAs and protein isoforms. Bioinformatics studies that predict the existence of mRNA variants and analyse whether potentially transcribed protein isoforms would be functional are of great importance in this task [16].
More often than not, isoforms of a particular GPCR have distinct pharmacological and signalling properties. In order to start understanding the link between these diverse pharmacological and signalling characteristics on one side and physiological consequences of the activation of various GPCR isoforms on the other side, comprehensive studies that dissect the regulatory mechanisms at the molecular level of GPCRs alternative mRNA splicing are crucial. These studies could be of pivotal importance in understanding and treating pathophysiological conditions that might favour expression of isoforms with altered signalling.
Interestingly, signalling cascades, including the Ras/extracellular signal-regulated kinase and Ca2+/calmodulin-dependent protein kinase pathways, which are often activated as a consequence of ligand binding to various GPCRs, have been implicated in regulation of alternative splicing of CD44, a cell-surface receptor that is also involved in cell adhesion and migration [144] and the large conductance, voltage- and Ca2+-activated (SLO) K+ channel pre-mRNA splicing [145]. Moreover, signalling via D1 dopamine receptors activates a pathway that results in the phosphorylation of key transcriptional and splicing factors, including Ania-6 (human homologue = cyclin-L), RNA polymerase II, SC35 and CDKp110 [146], and could potentially regulate the alternative splicing of an array of proteins. It is well established that the majority of GPCRs are desensitised, internalised and down-regulated by constant or repeated exposure to agonist. Is it possible that expression of spliced isoforms allows fine-tuning of this process? Is it possible that activation of a GPCR “auto-regulates” expression of its own spliced isoforms or regulates the expression of other GPCR isoforms? These and many other questions remain to be answered.
Acknowledgements
We would like to thank Drs. Carl Nelson and Gary Willars (University of Leicester) for helpful comments and suggestions on earlier drafts of this review.
References
- 1.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann N, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, Gibbs RA, Muzny DM, Scherer SE, Bouck JB, Sodergren EJ, Worley KC, Rives CM, Gorrell JH, Metzker ML, Naylor SL, Kucherlapati RS, Nelson DL, Weinstock GM, Sakaki Y, Fujiyama A, Hattori M, Yada T, Toyoda A, Itoh T, Kawagoe C, Watanabe H, Totoki Y, Taylor T, Weissenbach J, Heilig R, Saurin W, Artiguenave F, Brottier P, Bruls T, Pelletier E, Robert C, Wincker P, Smith DR, Doucette-Stamm L, Rubenfield M, Weinstock K, Lee HM, Dubois J, Rosenthal A, Platzer M, Nyakatura G, Taudien S, Rump A, Yang H, Yu J, Wang J, Huang G, Gu J, Hood L, Rowen L, Madan A, Qin S, Davis RW, Federspiel NA, Abola AP, Proctor MJ, Myers RM, Schmutz J, Dickson M, Grimwood J, Cox DR, Olson MV, Kaul R, Raymond C, Shimizu N, Kawasaki K, Minoshima S, Evans GA, Athanasiou M, Schultz R, Roe BA, Chen F, Pan H, Ramser J, Lehrach H, Reinhardt R, McCombie WR, de la Bastide M, Dedhia N, Blocker H, Hornischer K, Nordsiek G, Agarwala R, Aravind L, Bailey JA, Bateman A, Batzoglou S, Birney E, Bork P, Brown DG, Burge CB, Cerutti L, Chen HC, Church D, Clamp M, Copley RR, Doerks T, Eddy SR, Eichler EE, Furey TS, Galagan J, Gilbert JG, Harmon C, Hayashizaki Y, Haussler D, Hermjakob H, Hokamp K, Jang W, Johnson LS, Jones TA, Kasif S, Kaspryzk A, Kennedy S, Kent WJ, Kitts P, Koonin EV, Korf I, Kulp D, Lancet D, Lowe TM, McLysaght A, Mikkelsen T, Peterson J, Felsenfeld A, Wetterstrand KA, Patrinos A, Catanese JJ, Osoegawa K, Shizuya H, Choi S. Moran JV, Mulder N, Pollara VJ, Ponting CP, Schuler G, Schultz J, Slater G, Smit AF, Stupka E, Szustakowski J, Thierry-Mieg D, Thierry-Mieg J, Wagner L, Wallis J, Wheeler R, Williams A, Wolf YI, Wolfe KH, Yang SP. Nature. 2001;409:860–921. [Google Scholar]
- 2.Stolc V, Gauhar Z, Mason C, Halasz G, van Batenburg MF, Rifkin SA, Hua S, Herreman T, Tongprasit W, Barbano PE, Bussemaker HJ, White KP. A gene expression map for the euchromatic genome of Drosophila melanogaster . Science. 2004;306:655–660. doi: 10.1126/science.1101312. [DOI] [PubMed] [Google Scholar]
- 3.Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wahl MC, Will CL, Luhrmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 5.McGlincy NJ, Smith CW. Alternative splicing resulting in nonsense-mediated mRNA decay: what is the meaning of nonsense? Trends Biochem Sci. 2008;33:385–393. doi: 10.1016/j.tibs.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Wang GS, Cooper TA. Splicing in disease: disruption of the splicing code and the decoding machinery. Nat Rev Genet. 2007;8:749–761. doi: 10.1038/nrg2164. [DOI] [PubMed] [Google Scholar]
- 7.Srebrow A, Kornblihtt AR. The connection between splicing and cancer. J Cell Sci. 2006;119:2635–2641. doi: 10.1242/jcs.03053. [DOI] [PubMed] [Google Scholar]
- 8.Hellmich MR, Rui XL, Hellmich HL, Fleming RYD, Evers BM, Townsend CM. Human colorectal cancers express a constitutively active cholecystokinin-B/gastrin receptor that stimulates cell growth. J Biol Chem. 2000;275:32122–32128. doi: 10.1074/jbc.M005754200. [DOI] [PubMed] [Google Scholar]
- 9.Lee HJ, Wall B, Chen S. G-protein-coupled receptors and melanoma. Pigment Cell Melanoma Res. 2008;21:415–428. doi: 10.1111/j.1755-148X.2008.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bond RA, Ijzerman AP. Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends Pharmacol Sci. 2006;27:92–96. doi: 10.1016/j.tips.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Insel PA, Tang CM, Hahntow I, Michel MC. Impact of GPCRs in clinical medicine: monogenic diseases, genetic variants and drug targets. Biochim Biophys Acta. 2007;1768:994–1005. doi: 10.1016/j.bbamem.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagerstrom MC, Schioth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- 13.Nelson CP, Challiss RAJ. “Phenotypic” pharmacology: the influence of cellular environment on G protein-coupled receptor antagonist and inverse agonist pharmacology. Biochem Pharmacol. 2007;73:737–751. doi: 10.1016/j.bcp.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Joun H, Lanske B, Karperien M, Qian F, Defize L, Abou-Samra A. Tissue-specific transcription start sites and alternative splicing of the parathyroid hormone (PTH)/PTH-related peptide (PTHrP) receptor gene: a new PTH/PTHrP receptor splice variant that lacks the signal peptide. Endocrinology. 1997;138:1742–1749. doi: 10.1210/endo.138.4.5085. [DOI] [PubMed] [Google Scholar]
- 15.Gentles AJ, Karlin S. Why are human G-protein-coupled receptors predominantly intronless? Trends Genet. 1999;15:47–49. doi: 10.1016/s0168-9525(98)01648-5. [DOI] [PubMed] [Google Scholar]
- 16.Bjarnadottir TK, Geirardsdóttir K, Ingemansson M, Mirza MAI, Fredrikssonn R, Schioth HB. Identification of novel splice variants of adhesion G protein-coupled receptors. Gene. 2007;387:38–48. doi: 10.1016/j.gene.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 17.Kilpatrick GJ, Dautzenberg FM, Martin GR, Eglen RM. 7TM receptors: the splicing on the cake. Trends Pharmacol Sci. 1999;20:294–301. doi: 10.1016/s0165-6147(99)01355-3. [DOI] [PubMed] [Google Scholar]
- 18.Minneman KP. Splice variants of G protein-coupled receptors. Mol Interv. 2001;1:108–116. [PubMed] [Google Scholar]
- 19.Bockaert J, Claeysen S, Becamel C, Dumuis A, Marin P. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006;326:553–572. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- 20.Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Einstein R, Jordan H, Zhou W, Brenner M, Moses EG, Liggett SB. Alternative splicing of the G protein-coupled receptor superfamily in human airway smooth muscle diversifies the complement of receptors. Proc Natl Acad Sci USA. 2008;105:5230–5235. doi: 10.1073/pnas.0801319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kreth S, Ledderose C, Kaufmann I, Groeger G, Thiel M. Differential expression of 5′-UTR splice variants of the adenosine A2A receptor gene in human granulocytes: identification, characterization, and functional impact on activation. FASEB J. 2008;22:3276–3286. doi: 10.1096/fj.07-101097. [DOI] [PubMed] [Google Scholar]
- 23.Hawrylyshyn KA, Michelotti GA, Cogé F, Guénin SP, Schwinn DA. Update on human α1-adrenoceptor subtype signaling and genomic organization. Trends Pharmacol Sci. 2004;25:449–455. doi: 10.1016/j.tips.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Martin MM, Willardson BM, Burton GF, White CR, McLaughlin JN, Bray SM, Ogilvie JW, Jr, Elton TS. Human angiotensin II type 1 receptor isoforms encoded by messenger RNA splice variants are functionally distinct. Mol Endocrinol. 2001;15:281–293. doi: 10.1210/mend.15.2.0598. [DOI] [PubMed] [Google Scholar]
- 25.Martin MM, Buckenberger JA, Knoell DL, Strauch AR, Elton TS. TGF-β1 regulation of human AT1 receptor mRNA splice variants harboring exon-2. Mol Cell Endocrinol. 2006;249:21–31. doi: 10.1016/j.mce.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Nag K, Sultana N, Kato A, Hirose S. Headless splice variant acting as dominant negative calcitonin receptor. Biochem Biophys Res Commun. 2007;362:1037–1043. doi: 10.1016/j.bbrc.2007.08.107. [DOI] [PubMed] [Google Scholar]
- 27.Seck T, Baron R, Horne WC. The alternatively spliced delta-e13 transcript of the rabbit calcitonin receptor dimerizes with the C1a isoform and inhibits its surface expression. J Biol Chem. 2003;278:23085–23093. doi: 10.1074/jbc.M211280200. [DOI] [PubMed] [Google Scholar]
- 28.Shire D, Carillon C, Kaghad M, Calandra B, Rinaldi-Carmona M, Le Fur G, Caput D, Ferrara A. An amino-terminal variant of the central cannabinoid receptor resulting from alternative splicing. J Biol Chem. 1995;270:3726–3731. doi: 10.1074/jbc.270.8.3726. [DOI] [PubMed] [Google Scholar]
- 29.Ryberg E, Vu HK, Larsson N, Groblewski T, Hjorth S, Elebring T, Sjogren S, Greasley PJ. Identification and characterisation of a novel splice variant of the human CB1 receptor. FEBS Lett. 2005;579:259–264. doi: 10.1016/j.febslet.2004.11.085. [DOI] [PubMed] [Google Scholar]
- 30.Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E, Marra F, Romagnani S, Serio M, Romagnani P. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. 2003;197:1537–1549. doi: 10.1084/jem.20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding WQ, Kuntz SM, Miller LJ. A misspliced form of the cholecystokinin-B/gastrin receptor in pancreatic carcinoma: role of reduced cellular U2AF35 and a suboptimal 3′-splicing site leading to retention of the fourth intron. Cancer Res. 2002;62:947–952. [PubMed] [Google Scholar]
- 32.Olszewska-Pazdrak B, Townsend CM, Jr, Hellmich MR. Agonist-independent activation of Src tyrosine kinase by a cholecystokinin-2 (CCK2) receptor splice variant. J Biol Chem. 2004;279:40400–40404. doi: 10.1074/jbc.C400208200. [DOI] [PubMed] [Google Scholar]
- 33.Grammatopoulos DK, Hillhouse EW. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocrine Rev. 2006;27:260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- 34.Pisarchik A, Slominski AT. Alternative splicing of CRH-R1 receptors in human and mouse skin: identification of new variants and their differential expression. FASEB J. 2001;15:2754–2756. doi: 10.1096/fj.01-0487fje. [DOI] [PubMed] [Google Scholar]
- 35.Sztainberg Y, Kuperman Y, Issler O, Gil S, Vaughan J, Rivier J, Vale W, Chen A. A novel corticotropin-releasing factor receptor splice variant exhibits dominant negative activity: a putative link to stress-induced heart disease. FASEB J. 2009;23:2186–2196. doi: 10.1096/fj.08-128066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monsma FJ, McVittie LD, Gerfen CR, Mahan LC, Sibley DR. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989;342:926–929. doi: 10.1038/342926a0. [DOI] [PubMed] [Google Scholar]
- 37.Khan ZU, Mrzljak L, Gutierrez A, de la Calle A, Goldman-Rakic PS. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci USA. 1998;95:7731–7736. doi: 10.1073/pnas.95.13.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karpa KD, Lin R, Kabbani N, Levenson R. The dopamine D3 receptor interacts with itself and the truncated D3 splice variant d3nf: D3–D3nf interaction causes mislocalization of D3 receptors. Mol Pharmacol. 2000;58:677–683. doi: 10.1124/mol.58.4.677. [DOI] [PubMed] [Google Scholar]
- 39.Tanoue A, Koshimizu TA, Tsuchiya M, Ishii K, Osawa M, Saeki M, Tsujimoto G. Two novel transcripts for human endothelin B receptor produced by RNA editing/alternative splicing from a single gene. J Biol Chem. 2002;277:33205–33212. doi: 10.1074/jbc.M203972200. [DOI] [PubMed] [Google Scholar]
- 40.Chiu PL, Hg BH, Chang GW, Gordon S, Lin HH. Putative alternative trans-splicing of leukocyte adhesion-GPCR pre-mRNA generates functional chimeric receptors. FEBS Lett. 2008;582:792–798. doi: 10.1016/j.febslet.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Babu PS, Krishnamurthy H, Chedrese PJ, Sairam MR. Activation of extracellular-regulated kinase pathways in ovarian granulosa cells by the novel growth factor type 1 follicle-stimulating hormone receptor. Role in hormone signaling and cell proliferation. J Biol Chem. 2000;275:27615–27626. doi: 10.1074/jbc.M003206200. [DOI] [PubMed] [Google Scholar]
- 42.Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, Bonner TI, Enna SJ. International Union of Pharmacology. XXXIII. Mammalian gamma-aminobutyric acid-B receptors: structure and function. Pharmacol Rev. 2002;54:247–264. doi: 10.1124/pr.54.2.247. [DOI] [PubMed] [Google Scholar]
- 43.Harada N, Yamada Y, Tsukiyama K, Yamada C, Nakamura Y, Mukai E, Hamasaki A, Liu X, Toyoda K, Seino Y, Inagaki N. A novel GIP receptor splice variant influences GIP sensitivity of pancreatic β-cells in obese mice. Am J Physiol. 2008;294:E61–E68. doi: 10.1152/ajpendo.00358.2007. [DOI] [PubMed] [Google Scholar]
- 44.Havt A, Schally AV, Halmos G, Varga JL, Toller GL, Horvath JE, Szepeshazi K, Koster F, Kovitz K, Groot K, Zarandi M, Kanashiro CA. The expression of the pituitary growth hormone-releasing hormone receptor and its splice variants in normal and neoplastic human tissues. Proc Natl Acad Sci USA. 2005;102:17424–17429. doi: 10.1073/pnas.0506844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hashimoto K, Koga M, Motomura T, Kasayama S, Kouhara H, Ohnishi T, Arita N, Hayakawa T, Sato B, Kishimoto T. Identification of alternatively spliced messenger ribonucleic acid encoding truncated growth hormone-releasing hormone receptor in human pituitary adenomas. J Clin Endocrinol Metab. 1995;80:2933–2939. doi: 10.1210/jcem.80.10.7559877. [DOI] [PubMed] [Google Scholar]
- 46.Rekasi Z, Czompoly T, Schally AV, Halmos G. Isolation and sequencing of cDNAs for splice variants of growth hormone-releasing hormone receptors from human cancers. Proc Natl Acad Sci USA. 2000;97:10561–10566. doi: 10.1073/pnas.180313297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barabutis N, Tsellou E, Schally AV, Kouloheri S, Kalofoutis A, Kiaris H. Stimulation of proliferation of MCF-7 breast cancer cells by a transfected splice variant of growth hormone-releasing hormone receptor. Proc Natl Acad Sci USA. 2007;104:5575–5579. doi: 10.1073/pnas.0700407104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcia-Fernandez MO, Schally AV, Varga JL, Groot K, Busto R. The expression of growth hormone-releasing hormone (GHRH) and its receptor splice variants in human breast cancer lines; the evaluation of signaling mechanisms in the stimulation of cell proliferation. Breast Cancer Res Treat. 2003;77:15–26. doi: 10.1023/a:1021196504944. [DOI] [PubMed] [Google Scholar]
- 49.Chatzistamou I, Volakaki AA, Schally AV, Kiaris H, Kittas C. Expression of growth hormone-releasing hormone receptor splice variant 1 in primary human melanomas. Reg Peptides. 2008;147:33–36. doi: 10.1016/j.regpep.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 50.Leurs R, Bakker RA, Timmerman H, de Esch IJ. The histamine H3 receptor: from gene cloning to H3 receptor drugs. Nat Rev Drug Discov. 2005;4:107–120. doi: 10.1038/nrd1631. [DOI] [PubMed] [Google Scholar]
- 51.Bakker RA, Lozada AF, van Marle A, Shenton FC, Drutel G, Karlstedt K, Hoffmann M, Lintunen M, Yamamoto Y, van Rijn RM, Chazot PL, Panula P, Leurs R. Discovery of naturally-occurring splice variants of the rat histamine H3 receptor that act as dominant-negative isoforms. Mol Pharmacol. 2006;69:1194–1206. doi: 10.1124/mol.105.019299. [DOI] [PubMed] [Google Scholar]
- 52.van Rijn RM, van Marle A, Chazot PL, Langemeijer E, Qin Y, Shenton FC, Lim HD, Zuiderveld OP, Sansuk K, Dy M, Smit MJ, Tensen CP, Bakker RA, Leurs R. Cloning and characterization of dominant-negative splice variants of the human histamine H4 receptor. Biochem J. 2008;414:121–131. doi: 10.1042/BJ20071583. [DOI] [PubMed] [Google Scholar]
- 53.Bruysters M, Christin-Maitre S, Verhoef-Post M, Sultan C, Auger J, Faugeron I, Larue L, Lumbroso S, Themmen AP, Bouchard P. A new LH receptor splice mutation responsible for male hypogonadism with subnormal sperm production in the propositus, and infertility with regular cycles in an affected sister. Hum Reprod. 2008;23:1917–1923. doi: 10.1093/humrep/den180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Apaja PM, Tuusa JT, Pietilä EM, Rajaniemi HJ, Petaja-Repo UE. Luteinizing hormone receptor ectodomain splice variant misroutes the full-length receptor into a subcompartment of the endoplasmic reticulum. Mol Biol Cell. 2006;17:2243–2255. doi: 10.1091/mbc.E05-09-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noon LA, Bakmanidis A, Clark AJ, O’Shaughnessy PJ, King PJ. Identification of a novel melanocortin 2 receptor splice variant in murine adipocytes: implications for post-transcriptional control of expression during adipogenesis. J Mol Endocrinol. 2006;37:415–420. doi: 10.1677/jme.1.02023. [DOI] [PubMed] [Google Scholar]
- 56.Francesconi A, Duvoisin RM. Alternative splicing unmasks dendritic and axonal targeting signals in metabotropic glutamate receptor 1. J Neurosci. 2002;22:2196–2205. doi: 10.1523/JNEUROSCI.22-06-02196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bovolin P, Bovetti S, Fasolo A, Katarova Z, Szabo G, Shipley MT, Margolis FL, Puche AC. Developmental regulation of metabotropic glutamate receptor 1 splice variants in olfactory bulb mitral cells. J Neurosci Res. 2009;87:369–379. doi: 10.1002/jnr.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- 59.Lai JP, Ho WZ, Kilpatrick LE, Wang X, Tuluc F, Korchak HM, Douglas SD. Full-length and truncated neurokinin-1 receptor expression and function during monocyte/macrophage differentiation. Proc Natl Acad Sci USA. 2006;103:7771–7776. doi: 10.1073/pnas.0602563103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kage R, Leeman SE, Boyd ND. Biochemical characterization of two different forms of the substance P receptor in rat submaxillary gland. J Neurochem. 1993;60:347–351. doi: 10.1111/j.1471-4159.1993.tb05857.x. [DOI] [PubMed] [Google Scholar]
- 61.Duric V, McCarson KE. Neurokinin-1 (NK-1) receptor and brain-derived neurotrophic factor (BDNF) gene expression is differentially modulated in the rat spinal dorsal horn and hippocampus during inflammatory pain. Mol Pain. 2007;3:32. doi: 10.1186/1744-8069-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tulic F, Lai JP, Kilpatrick LE, Evans DL, Douglas SD. Neurokinin 1 receptor isoforms and the control of innate immunity. Trends Immunol. 2009;30:271–276. doi: 10.1016/j.it.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 63.Lai JP, Lai S, Tuluc F, Tansky MF, Kilpatrick LE, Leeman SE, Douglas SD. Differences in the length of the C-terminus mediate functional properties of neurokinin-1 receptor. Proc Natl Acad Sci USA. 2008;105:12605–12610. doi: 10.1073/pnas.0806632105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lai JP, Cnaan A, Zhao H, Douglas SD. Detection of full-length and truncated neurokinin-1 receptor mRNA expression in human brain regions. J Neurosci Methods. 2008;168:127–133. doi: 10.1016/j.jneumeth.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Candenas ML, Cintado CG, Pennefather JN, Pereda MT, Loizaga JM, Maggi CA, Pinto FM. Identification of a tachykinin NK2 receptor splice variant and its expression in human and rat tissues. Life Sci. 2002;72:269–277. doi: 10.1016/s0024-3205(02)02240-3. [DOI] [PubMed] [Google Scholar]
- 66.Bellucci F, Meini S, Catalioto RM, Catalani C, Giuliani S, Quartara L, Giolitti A, Faiella A, Rotondaro L, Candenas ML, Pinto FM, Maggi CA. Pharmacological evaluation of α and β human tachykinin NK2 receptor splice variants expressed in CHO cells. Eur J Pharmacol. 2004;499:229–238. doi: 10.1016/j.ejphar.2004.07.075. [DOI] [PubMed] [Google Scholar]
- 67.Vendelin J, Pulkkinen V, Rehn M, Pirskanen A, Raisanen-Sokolowski A, Laitinen A, Laitinen LA, Kere J, Laitinen T. Characterization of GPRA, a novel G protein-coupled receptor related to asthma. Am J Respir Cell Mol Biol. 2005;33:262–270. doi: 10.1165/rcmb.2004-0405OC. [DOI] [PubMed] [Google Scholar]
- 68.Pasternak GW. Multiple opiate receptors: déjà vu all over again. Neuropharmacology. 2004;47(Suppl 1):312–323. doi: 10.1016/j.neuropharm.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 69.Ding C, Racusen L, Wilson P, Burrow C, Levine MA. Identification of an alternative spliced form of PTH/PTHrP receptor mRNA in immortalized renal tubular cells. J Bone Mineral Res. 1995;10(Suppl 1):S484. [Google Scholar]
- 70.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 71.Vielhauer GA, Fujino H, Regan JW. Cloning and localization of hFP(S): a six-transmembrane mRNA splice variant of the human FP prostanoid receptor. Arch Biochem Biophys. 2004;421:175–185. doi: 10.1016/j.abb.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 72.Kotelevets L, Foudi N, Louedec L, Couvelard A, Chastre E, Norel X. A new mRNA splice variant coding for the human EP3-I receptor isoform. Prostaglandin Leukot Essent Fatty Acid. 2007;77:195–201. doi: 10.1016/j.plefa.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 73.Scott DJ, Layfield S, Yan Y, Sudo S, Hsueh AJ, Tregear GW, Bathgate RA. Characterization of novel splice variants of LGR7 and LGR8 reveals that receptor signaling is mediated by their unique low density lipoprotein class A modules. J Biol Chem. 2006;281:34942–34954. doi: 10.1074/jbc.M602728200. [DOI] [PubMed] [Google Scholar]
- 74.Lowndes K, Amano A, Yamamoto SY, Bryant-Greenwood GD. The human relaxin receptor (LGR7): expression in the fetal membranes and placenta. Placenta. 2006;27:610–618. doi: 10.1016/j.placenta.2005.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kern A, Hubbard D, Amano A, Bryant-Greenwood GD. Cloning, expression, and functional characterization of relaxin receptor (leucine-rich repeat-containing G protein-coupled receptor 7) splice-variants from human fetal membranes. Endocrinology. 2008;149:1277–1294. doi: 10.1210/en.2007-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Korner M, Hayes GM, Rehmann R, Zimmermann A, Friess H, Miller LJ, Reubi JC. Secretin receptors in normal and diseased human pancreas. Am J Pathol. 2005;167:959–968. doi: 10.1016/S0002-9440(10)61186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Korner MU, Hayes GM, Carrigan PE, Rehmann R, Miller LJ, Reubi JC. Wild-type and splice-variant secretin receptors in lung cancer: over-expression in carcinoid tumors and peri-tumoral lung tissue. Mol Pathol. 2008;21:387–395. doi: 10.1038/modpathol.3801005. [DOI] [PubMed] [Google Scholar]
- 78.Taylor JE, Theveniau MA, Bashirzadeh R, Reisine T, Eden PA. Detection of somatostatin receptor subtype 2 (SSTR2) in established tumors and tumor cell lines: evidence for SSTR2 heterogeneity. Peptides. 1994;15:1229–1236. doi: 10.1016/0196-9781(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 79.Moller LN, Stidsen CE, Hartmann B, Holst JJ. Somatostatin receptors. Biochim Biophys Acta. 2003;1616:1–84. doi: 10.1016/s0005-2736(03)00235-9. [DOI] [PubMed] [Google Scholar]
- 80.Parent JL, Labrecque P, Orsini MJ, Benovic JL. Internalization of the TXA2 receptor alpha and beta isoforms. Role of the differentially spliced cooh terminus in agonist-promoted receptor internalization. J Biol Chem. 1999;274:8941–8948. doi: 10.1074/jbc.274.13.8941. [DOI] [PubMed] [Google Scholar]
- 81.Moussa O, Ashton AW, Fraig M, Garrett-Mayer E, Ghoneim MA, Halushka PV, Watson DK. Novel role of thromboxane receptor β-isoform in bladder cancer pathogenesis. Cancer Res. 2008;68:4097–4104. doi: 10.1158/0008-5472.CAN-07-6560. [DOI] [PubMed] [Google Scholar]
- 82.Sarmiento JM, Anazco CC, Campos DM, Prado GN, Navarro J, Gonzalez CB. Novel down-regulatory mechanism of the surface expression of the vasopressin V2 receptor by an alternative splice receptor variant. J Biol Chem. 2004;279:47017–47023. doi: 10.1074/jbc.M410011200. [DOI] [PubMed] [Google Scholar]
- 83.Vargas KJ, Sarmiento JM, Ehrenfeld P, Anazco CC, Villanueva CI, Carmona PL, Brenet M, Navarro J, Muller-Esterl W, Gonzalez CB. Postnatal expression of V2 vasopressin receptor splice variants in the rat cerebellum. Differentiation. 2009;77:377–385. doi: 10.1016/j.diff.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dickson L, Finlayson K. VPAC and PAC receptors: from ligands to function. Pharmacol Ther. 2009;121:294–316. doi: 10.1016/j.pharmthera.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 85.Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- 86.Grosse R, Schoneberg T, Schultz G, Gudermann T. Inhibition of gonadotropin-releasing hormone receptor signalling of a splice variant of the human receptor. Mol Endocrinol. 1997;11:1305–1318. doi: 10.1210/mend.11.9.9966. [DOI] [PubMed] [Google Scholar]
- 87.Grammatopoulos DK, Dai Y, Randeva HS, Levine MA, Karteris E, Easton AJ, Hillhouse EW. A novel spliced variant of the type 1 corticotropin-releasing hormone receptor with a deletion in the seventh transmembrane domain present in the human pregnant term myometrium and fetal membranes. Mol Endocrinol. 1999;13:2189–2202. doi: 10.1210/mend.13.12.0391. [DOI] [PubMed] [Google Scholar]
- 88.Grinninger C, Wang W, Oskoui KB, Voice JK, Goetzl EJ. A natural variant type II G protein-coupled receptor for vasoactive intestinal peptide with altered function. J Biol Chem. 2004;279:40259–40262. doi: 10.1074/jbc.C400332200. [DOI] [PubMed] [Google Scholar]
- 89.Markovic D, Grammatopoulos DK (2009) Focus on the splicing of B1-GPCRs transmembrane-domain 7. Trends Biochem Sci (in press) [DOI] [PubMed]
- 90.Nordstrom KJ, Lagerstrom MC, Waller LM, Fredriksson R, Schioth HB. The secretin GPCRs descended from the family of adhesion GPCRs. Mol Biol Evol. 2009;26:71–84. doi: 10.1093/molbev/msn228. [DOI] [PubMed] [Google Scholar]
- 91.Hossami S, Findlay DM, Brady CL, Myers DE, Martin TJ, Sexton PM. Isoforms of the rat calcitonin receptor: consequences for ligand binding and signal transduction. Endocrinology. 1994;135:183–190. doi: 10.1210/endo.135.1.8013352. [DOI] [PubMed] [Google Scholar]
- 92.Chen AM, Perrin MH, Digruccio MR, Vaughan JM, Brar BK, Arias CM, Lewis KA, Rivier JE, Sawchenko PE, Vale WW. A soluble mouse brain splice variant of type 2alpha corticotropin-releasing factor (CRF) receptor binds ligands and modulates their activity. Proc Natl Acad Sci USA. 2005;102:2620–2625. doi: 10.1073/pnas.0409583102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing factor receptor. Proc Natl Acad Sci USA. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Markovic D, Papadopoulou N, Teli T, Randeva H, Levine MA, Hillhouse EW, Grammatopoulos DK. Differential responses of CRH receptor type 1 variants to PKC phosphorylation. J Pharmacol Exp Ther. 2006;319:1032–1042. doi: 10.1124/jpet.106.107441. [DOI] [PubMed] [Google Scholar]
- 95.Teli T, Markovic D, Hewitt ME, Levine MA, Hillhouse EW, Grammatopoulos DK. Structural domains determining signalling characteristics of the CRH-receptor type 1 variant R1β and response to PKC phosphorylation. Cell Signal. 2008;20:40–49. doi: 10.1016/j.cellsig.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 96.Ross PC, Kostas CM, Ramabhadran TV. A variant of the human corticotropin-releasing factor (CRF) receptor: cloning, expression and pharmacology. Biochem Biophys Res Commun. 1994;205:1836–1842. doi: 10.1006/bbrc.1994.2884. [DOI] [PubMed] [Google Scholar]
- 97.Zmijewski MA, Slominski AT. CRF1 receptor splicing in epidermal keratinocytes: potential biological role and environmental regulations. J Cell Physiol. 2009;218:593–602. doi: 10.1002/jcp.21632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Markovic D, Lehnert H, Levine MA, Grammatopoulos DK. Structural determinants critical for localization and signalling within the 7th transmembrane domain of the type 1 corticotropin releasing hormone receptor: lessons from the variant R1d. Mol Endocrinol. 2008;22:2505–2519. doi: 10.1210/me.2008-0177. [DOI] [PubMed] [Google Scholar]
- 99.Seck T, Pellegrini M, Florea AM, Grignoux V, Baron R, Mierke DF, Horne WC. The delta-e13 isoform of the calcitonin receptor forms a six-transmembrane domain receptor with dominant-negative effects on receptor surface expression and signaling. Mol Endocrinol. 2005;19:2132–2144. doi: 10.1210/me.2004-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Markovic D, Punn A, Lehnert H, Grammatopoulos DK. Intracellular mechanisms regulating CRH-R2β-endocytosis and interaction with ERK1/2 and p38 MAPK signalling cascades. Mol Endocrinol. 2008;22:689–6706. doi: 10.1210/me.2007-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang MC, Miller AL, Wang W, Kong Y, Paul S, Goetzl EJ. Differential signaling of T cell generation of IL-4 by wild-type and short-deletion variant of type 2 G protein-coupled receptor for vasoactive intestinal peptide (VPAC2) J Immunol. 2006;176:6640–6646. doi: 10.4049/jimmunol.176.11.6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Markovic D, Vatish M, Gu M, Slater D, Newton R, Lehnert H, Grammatopoulos DK. The onset of labour alters corticotropin releasing hormone type 1 receptor (CRH-R1) variant expression in human myometrium: putative role of IL-1β. Endocrinology. 2007;148:3205–3213. doi: 10.1210/en.2007-0095. [DOI] [PubMed] [Google Scholar]
- 103.Donev R, Newall A, Thome J, Sheer D. A role for SC35 and hnRNPA1 in the determination of amyloid precursor protein isoforms. Mol Psychiatry. 2007;12:681–690. doi: 10.1038/sj.mp.4001971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dowhan DH, Hong EP, Auboeuf D, Dennis AP, Wilson MM, Berget SM, O’Malley BW. Steroid hormone receptor coactivation and alternative RNA splicing by U2AF65-related proteins CAPERα and CAPERβ. Mol Cell. 2005;17:429–439. doi: 10.1016/j.molcel.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 105.Guivarc’h D, Vernier P, Vincent JD. Sex steroid hormones change the differential distribution of the isoforms of the D2 dopamine receptor messenger RNA in the rat brain. Neuroscience. 1995;69:159–166. doi: 10.1016/0306-4522(95)00228-b. [DOI] [PubMed] [Google Scholar]
- 106.Guivarc’h D, Vincent JD, Vernier P. Alternative splicing of the D2 dopamine receptor messenger ribonucleic acid is modulated by activated sex steroid receptors in the MMQ prolactin cell line. Endocrinology. 1998;139:4213–4221. doi: 10.1210/endo.139.10.6246. [DOI] [PubMed] [Google Scholar]
- 107.Cheung TC, Hearn JP. Developmental expression and subcellular localization of wallaby gonadotropin-releasing hormone receptor and its splice variants. Gen Comp Endocrinol. 2003;133:88–99. doi: 10.1016/s0016-6480(03)00146-1. [DOI] [PubMed] [Google Scholar]
- 108.Bjarnadottir TK, Fredrikssonn R, Schioth HB. The adhesion GPCRs: a unique family of G-protein-coupled receptors with important roles in both central and peripheral tissues. Cell Mol Life Sci. 2007;64:2104–2119. doi: 10.1007/s00018-007-7067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang Y, Walsh CE. Spliceosome-mediated RNA trans-splicing. Mol Ther. 2005;12:1006–1012. doi: 10.1016/j.ymthe.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 110.Nemeth ZH, Csoka B, Wilmanski J, Xu D, Lu Q, Ledent C, Deitch EA, Pacher P, Spolarics Z, Hasko G. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J Immunol. 2006;176:5616–5626. doi: 10.4049/jimmunol.176.9.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ramensky V, Nurtdinov R, Neverov A, Mironov A, Gelfand M (2006) Human genome polymorphism and alternative splicing. In: Proceedings of 5th international conference on bioinformatics of genome regulation and structure (part 5): comparative and evolutionary genomics and proteomics, Novosibirsk, Russia, pp 211–213
- 112.Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R, Lee ML, Xiao T, Papp A, Wang D, Sadée W. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci USA. 2007;104:20552–20557. doi: 10.1073/pnas.0707106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Journot L, Spengler D, Pantaloni C, Dumuis A, Sebben M, Bockaert J. The PACAP receptor: generation by alternative splicing of functional diversity among G protein-coupled receptors in nerve cells. Semin Cell Biol. 1994;5:263–272. doi: 10.1006/scel.1994.1032. [DOI] [PubMed] [Google Scholar]
- 114.Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- 115.Cai Z, Schools GP, Kimelberg HK. Metabotropic glutamate receptors in acutely isolated hippocampal astrocytes: developmental changes of mGluR5 mRNA and functional expression. Glia. 2000;29:70–80. doi: 10.1002/(sici)1098-1136(20000101)29:1<70::aid-glia7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 116.Hermans E, Challiss RAJ. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem J. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fackenthal JD, Godley LA. Aberrant RNA splicing and its functional consequences in cancer cells. Dis Model Mech. 2008;1:37–42. doi: 10.1242/dmm.000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kiaris H, Schally AV, Varga JL, Groot K, Armatis P. Growth hormone-releasing hormone: an autocrine growth factor for small cell lung carcinoma. Proc Natl Acad Sci USA. 1999;96:14894–14898. doi: 10.1073/pnas.96.26.14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kahan Z, Varga JL, Schally AV, Rekasi Z, Armatis P, Chatzistamou L, Czompoly T, Halmos G. Antagonists of growth hormone-releasing hormone arrest the growth of MDA-MB-468 estrogen-independent human breast cancers in nude mice. Breast Cancer Res Treat. 2000;60:71–79. doi: 10.1023/a:1006363230990. [DOI] [PubMed] [Google Scholar]
- 120.Chatzistamou I, Schally AV, Varga JL, Groot K, Busto R, Armatis P, Halmos G. Inhibition of growth and metastases of MDA-MB-435 human estrogen-independent breast cancers by an antagonist of growth hormone-releasing hormone. Anticancer Drugs. 2001;12:761–768. doi: 10.1097/00001813-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 121.Schally AV, Varga JL. Antagonists of growth hormone-releasing hormone in oncology. Comb Chem High Throughput Screen. 2006;9:163–170. doi: 10.2174/138620706776055449. [DOI] [PubMed] [Google Scholar]
- 122.Moussa O, Yordy JS, Abol-Enein H, Sinha D, Bissada NK, Halushka PV, Ghoneim MA, Watson DK. Prognostic and functional significance of thromboxane synthase gene overexpression in invasive bladder cancer. Cancer Res. 2005;65:11581–11587. doi: 10.1158/0008-5472.CAN-05-1622. [DOI] [PubMed] [Google Scholar]
- 123.Tyson-Capper AJ. Alternative splicing: an important mechanism for myometrial gene regulation that can be manipulated to target specific genes associated with preterm labour. BMC Pregnancy Childbirth. 2007;7(Suppl 1):S13. doi: 10.1186/1471-2393-7-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Grammatopoulos DK. Placental corticotrophin-releasing hormone and its receptors in human pregnancy and labour: still a scientific enigma. J Neuroendocrinol. 2008;20:432–438. doi: 10.1111/j.1365-2826.2008.01660.x. [DOI] [PubMed] [Google Scholar]
- 125.Korebrits C, Ramirez MM, Watson L, Brinkman E, Bocking AD, Challis JR. Maternal corticotropin-releasing hormone is increased with impending preterm birth. J Clin Endocrinol Metab. 1998;83:1585–1591. doi: 10.1210/jcem.83.5.4804. [DOI] [PubMed] [Google Scholar]
- 126.Jin D, He P, You X, Zhu X, Dai L, He Q, Liu C, Hui N, Sha J, Ni X. Expression of corticotropin-releasing hormone receptor type 1 and type 2 in human pregnant myometrium. Reprod Sci. 2007;14:568–577. doi: 10.1177/1933719107307821. [DOI] [PubMed] [Google Scholar]
- 127.Karteris E, Grammatopoulos D, Dai Y, Olah KB, Ghobara TB, Easton A, Hillhouse EW. The human placenta and fetal membranes express the corticotropin-releasing hormone receptor 1α (CRH-1α) and the CRH-C variant receptor. J Clin Endocrinol Metab. 1998;83:1376–1379. doi: 10.1210/jcem.83.4.4705. [DOI] [PubMed] [Google Scholar]
- 128.Grammatopoulos D, Dai Y, Chen J, Karteris E, Papadopoulou N, Easton AJ, Hillhouse EW. Human corticotropin-releasing hormone receptor: differences in subtype expression between pregnant and non-pregnant myometria. J Clin Endocrinol Metab. 1998;83:2539–2544. doi: 10.1210/jcem.83.7.4985. [DOI] [PubMed] [Google Scholar]
- 129.Grammatopoulos DK, Hillhouse EW. Role of corticotropin-releasing hormone in onset of labour. Lancet. 1999;354:1546–1549. doi: 10.1016/S0140-6736(99)03418-2. [DOI] [PubMed] [Google Scholar]
- 130.Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD, Hsueh AJ. Activation of orphan receptors by the hormone relaxin. Science. 2002;295:671–674. doi: 10.1126/science.1065654. [DOI] [PubMed] [Google Scholar]
- 131.Themmen APN, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev. 2000;21:551–583. doi: 10.1210/edrv.21.5.0409. [DOI] [PubMed] [Google Scholar]
- 132.Latronico AC, Arnhold IJ. Inactivating mutations of LH and FSH receptors—from genotype to phenotype. Pediatr Endocrinol Rev. 2006;4:28–31. [PubMed] [Google Scholar]
- 133.McGillis JP, Mitsuhashi M, Payan DG. Immunomodulation by tachykinin neuropeptides. Ann NY Acad Sci. 1990;594:85–94. doi: 10.1111/j.1749-6632.1990.tb40470.x. [DOI] [PubMed] [Google Scholar]
- 134.Laitinen T, Polvi A, Rydman P, Vendelin J, Pulkkinen V, Salmikangas P, Makela S, Rehn M, Pirskanen A, Rautanen A, Zucchelli M, Gullsten H, Leino M, Alenius H, Petays T, Haahtela T, Laitinen A, Laprise C, Hudson TJ, Laitinen LA, Kere J. Characterization of a common susceptibility locus for asthma-related traits. Science. 2004;304:300–304. doi: 10.1126/science.1090010. [DOI] [PubMed] [Google Scholar]
- 135.Reinscheid RK, Xu YL, Okamura N, Zeng J, Chung S, Pai R, Wang Z, Civelli O. Pharmacological characterization of human and murine neuropeptide S receptor variants. J Pharmacol Exp Ther. 2005;315:1338–1345. doi: 10.1124/jpet.105.093427. [DOI] [PubMed] [Google Scholar]
- 136.Allen IC, Pace AJ, Jania LA, Ledford JG, Latour AM, Snouwaert JN, Bernier V, Stocco R, Therien AG, Koller BH. Expression and function of NPSR1/GPRA in the lung before and after induction of asthma-like disease. Am J Physiol. 2006;291:L1005–L1017. doi: 10.1152/ajprenal.00507.2005. [DOI] [PubMed] [Google Scholar]
- 137.Wu H, Romieu I, Sienra-Monge JJ, del Rio-Navarro BE, Burdett L, Yuenger J, Li H, Chanock SJ, London SJ. Lack of association between genetic variation in G-protein-coupled receptor for asthma susceptibility and childhood asthma and atopy. Genes Immun. 2008;9:224–230. doi: 10.1038/gene.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 139.Kuniyeda K, Okuno T, Terawaki K, Miyano M, Yokomizo T, Shimizu T. Identification of the intracellular region of the leukotriene B4 receptor type 1 that is specifically involved in Gi activation. J Biol Chem. 2007;282:3998–4006. doi: 10.1074/jbc.M610540200. [DOI] [PubMed] [Google Scholar]
- 140.Lee CJ, Irizarry K. Alternative splicing in the nervous system: an emerging source of diversity and regulation. Biol Psychiatry. 2003;54:771–776. doi: 10.1016/s0006-3223(03)00375-5. [DOI] [PubMed] [Google Scholar]
- 141.Seifert R, Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- 142.Tanowitz M, Hislop JN, von Zastrow M. Alternative splicing determines the post-endocytic sorting fate of G-protein-coupled receptors. J Biol Chem. 2008;283:35614–35621. doi: 10.1074/jbc.M806588200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Richtand NM. Behavioral sensitization, alternative splicing, and D3 dopamine receptor-mediated inhibitory function. Neuropsychopharmacology. 2006;31:2368–2375. doi: 10.1038/sj.npp.1301163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Konig H, Moll J, Ponta H, Herrlich P. Trans-acting factors regulate the expression of CD44 splice variants. EMBO J. 1996;15:4030–4039. [PMC free article] [PubMed] [Google Scholar]
- 145.Xie J, Black DL. A CaMK-IV responsive RNA element mediates depolarization-induced alternative splicing of ion channels. Nature. 2001;410:936–939. doi: 10.1038/35073593. [DOI] [PubMed] [Google Scholar]
- 146.Berke JD, Sgambato V, Zhu PP, Lavoie B, Vincent M, Krause M, Hyman SE. Dopamine and glutamate induce distinct striatal splice forms of Ania-6, an RNA polymerase II-associated cyclin. Neuron. 2001;32:277–287. doi: 10.1016/s0896-6273(01)00465-2. [DOI] [PubMed] [Google Scholar]