Abstract
Cellular communication between the bone component cells osteoblasts, osteocytes and (pre-)osteoclasts is essential for bone remodeling which maintains bone integrity. As in the remodeling of other organs, cell death is a trigger for remodeling of bone. During the systematic process of bone remodeling, direct or indirect cell–cell communication is indispensable. Thus, osteoblasts induce migration and differentiation of preosteoclasts, which is followed by bone resorption (by mature multinuclear osteoclasts). After completion of bone resorption, apoptosis of mature osteoclasts and differentiation of osteoblasts are initiated. At this time, the osteoblasts do not support osteoclast differentiation but do support bone formation. Finally, osteoblasts differentiate to osteocytes in bone or to bone lining cells on bone surfaces. In this way, old bone areas are regenerated as new bone. In this review the role of cell–cell communication in bone remodeling is discussed.
Keywords: Osteoblast, Osteocyte, Osteoclast, Gap junction, Cell communication, Adhesion molecule, cAMP
Introduction
Bone consists mainly of osteoblasts, osteocytes and osteoclasts. Osteocytes (which differentiate from osteoblasts) are embedded in the bone matrix and are the most abundant cells in bone. Osteocytes are reported to sense gravity and unloading [1–3]. It has recently been suggested that osteocytes also give a signal to osteoblasts to induce bone remodeling [4–6]. Bone remodeling is precisely regulated by the balance between bone formation and bone resorption throughout life. This balance is reflected by the fact that osteoblasts exhibit two opposite phenotypes. One is the osteogenic phenotype, which secretes bone matrix at the bone resorption site, and the other is the osteoclastogenic phenotype, which supports osteoclast differentiation in the old bone area. How do osteoclast precursors recognize the area of old bone, and how are they regulated? In this review, we discuss these questions from the point of view of cell–cell communication.
Both direct and indirect cell communications are involved in the process of osteoclastogenesis (Fig. 1). In the bone, osteocyte cell death is mainly induced by fatigue or by cell senescence. As is the case with other organs, the area containing dead cells is regenerated as new tissue. Initially, osteoblasts will sense osteocyte cell death, and will attract osteoclast precursors. Next, the osteoblasts will change their function to the support osteoclastogenesis. During this process, direct cell–cell communication occurs, not only between osteocytes, but also between osteocytes and osteoblasts via gap junctions [7]. Indeed, osteoblasts may sense osteocyte cell death via gap junctional intercellular communication (GJIC). The osteoblasts will then express adhesion molecules on the cell surface in order to make specific contact with the osteoclast precursors. These adhesion molecules may also play an important role in the migration of osteoclast precursors under the osteoblast layer. At the same time, osteoblasts will express differentiation factors such as receptor activator of NF-κB ligand (RANKL) that induce osteoclastogenesis (osteoclastogenic phenotype). Following exposure of the osteoclast precursors to these differentiation factors, preosteoclasts (pre-OCs) proliferate and fuse to each other in order to resorb the old bone. During this process, prior to the fusion of these cells, there is cell–cell communication among the pre-OCs. After the old bone area is resorbed, osteoclasts will die by apoptosis. Lastly, the resorbed area of bone is refilled with new bone that is generated by differentiated osteoblasts (osteogenic phenotype). What is the trigger of phenotypic change in osteoblasts during bone remodeling? This review highlights cell–cell communications involved in the mechanism of bone remodeling, and proposes a novel theory regarding the mechanism by which osteoclast precursors sense the old bone area.
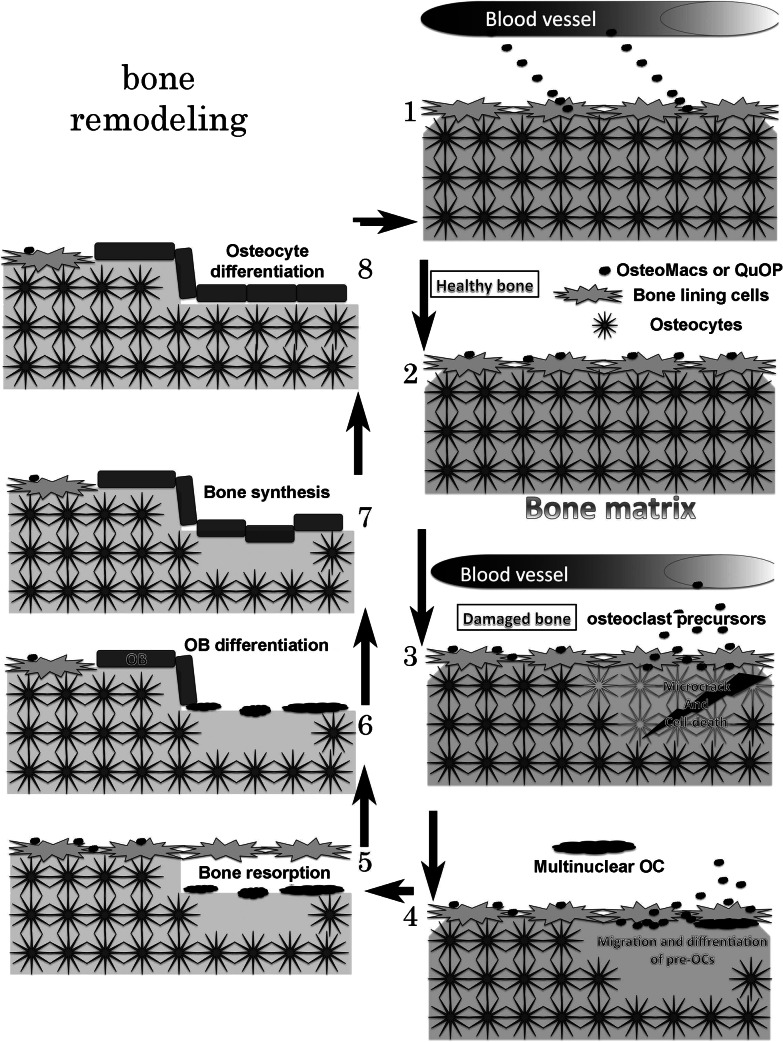
Fig. 1.
Schematic illustration of bone remodeling. 1 Bone marrow-derived mononuclear cells (BMMs, osteoclast precursors) migrate on osteoblast. 2 In healthy bone, osteoclast precursors stay on the osteoblast layer and are named osteal macrophages (OsteoMacs) or cell cycle-arrested quiescent osteoclast precursors (QuOP). 3 A microcrack in the bone induces osteocyte apoptosis following which, osteal (or QuOP) and BM-derived macrophages migrate under the osteoblast layer. The macrophages may differentiate into preosteoclast (pre-OC). 4 The pre-OCs fuse with each other under the osteoblast layer. 5 Multinuclear osteoclasts erode the bone matrix containing dead osteocytes. 6 Osteogenic osteoblasts proliferate and cover the bone resorption area. 7 Bone is synthesized. 8 Some osteoblasts differentiate into osteocytes
Distribution of bone cells
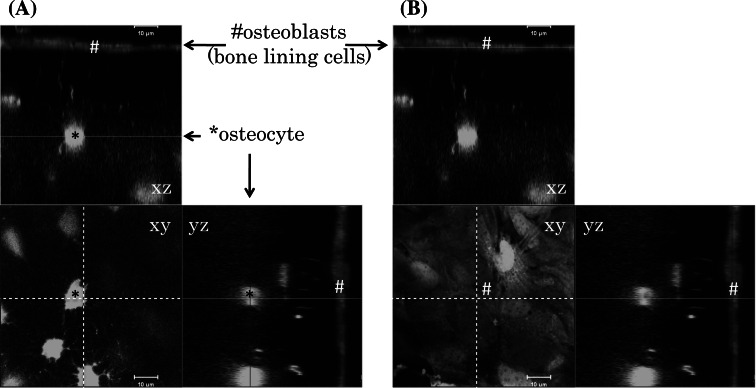
The spatial distribution of bone cells is shown in Fig. 2, which shows living cells in the calvaria of an adult, CAG promoter-driven green fluorescent protein transgenic mouse (unpublished data). Osteocytes (Fig. 2, asterisks) have many cellular processes which form a network within bone. As shown in previous reports, a large number of osteocytes are embedded in bone and they directly communicate with each other via gap junctions [7, 8]. Furthermore, osteocyte processes also make contact with osteoblasts (Fig. 2, hashes) [9]. A scanning electron microscope study has shown that the bone lining cell layer forms a complete and contiguous layer on the bone matrix [10]. Osteoblasts (bone-lining cells) express cadherins [11–14], claudins [15, 16] and connexin [17, 18], and are thought to be polarized cells [19]. These reports indicate not only that the adherence, tight and gap junctions are formed between the osteoblasts but also that the polarized nature of osteoblasts may play an important role in bone remodeling. It has recently been reported that resident tissue macrophages termed ‘OsteoMacs’ or cell cycle-arrested quiescent osteoclast precursors (QuOPs) are present on osteoblasts [20, 21]. The QuOPs, mononuclear cells (MNC) expressing c-Fms (a receptor for macrophage-colony stimulating factor, M-CSF) and RANK (a receptor for RANKL) but not Ki67, are detected along bone surface [22]. However, it is unclear whether OsteoMacs differentiate into osteoclasts or not. The QuOPs may be identical to OsteoMacs, in terms of their localization. These resident osteoclast precursors are one of the origins of osteoclasts, in addition to bone marrow-derived osteoclast precursors.
Fig. 2.
Confocal microscopic images of the calvaria of an adult, CAG promoter-driven green fluorescence protein transgenic mouse. Optical sections (x, y) are shown with additional xz and yz projections. Osteocyte–osteocyte and osteoblast–osteocyte communications were found. Bar 10 μm. a Image focused on an osteocyte (asterisks). b Image focused on the osteoblast layer (hashes)
Extravasation of osteoclast precursors (endothelial cell–osteoclast precursor communication, chemotactic factors)
The trigger for bone remodeling is osteocyte apoptosis in fatigue and unloading [23–25]. Recently, it has also been reported that osteocyte apoptosis-triggered osteoporosis induced by ovariectomy is suppressed by treatment with caspase inhibitor [26]. Resorption of old bone is carried out by multinuclear osteoclasts, which differentiate from MNCs (osteoclast progenitor or osteoclast precursor) in peripheral blood and bone marrow [27–29]. In their journey to the bone, it is first necessary that osteoclast precursors migrate from the bloodstream into the extravascular space through the endothelial cell layer. In this respect it is interesting that recent studies have shown that there are two routes of leukocyte migration under the endothelial cell layer [30, 31] (as shown in Fig. 3). One route is paracellular migration during which the leukocytes migrate via cell junctions. The other route is transcellular migration during which the leukocytes migrate through the cell body of the endothelial cells. The expression of intercellular adhesion molecule-1 (ICAM-1, CD54) is involved in migration via the both routes [32, 33]. Kindle et al. [34] reported that the cell surface adhesion molecules ICAM-1 and CD44 partially contribute to the direct communication of CD14-positive osteoclast precursors and endothelial cells in vitro. Although they did not examine the migratory routes (paracellular or transcellular), there would be two routes for migration of osteoclast precursors as mentioned above (Fig. 3). The soluble factors for involvement in extravasation of osteoclast precursors are sphingosine-1 phosphate (S1P) and stromal cell-derived factor-1 (SDF-1, CXCL12). Osteoclast precursors exhibit positive chemotaxis along an S1P gradient. Because S1P concentration in blood is usually high in comparison with that in tissues, osteoclast precursors in the marrow space re-enter the blood circulation in the absence of stimulation by the differentiation factor, RANKL, which is produced by osteoblasts. When expressed, RANKL stimulates downregulation of the expression of S1P1, a receptor of S1P, in osteoclast precursors. Therefore, the RANKL-stimulated osteoclast precursor is retained in the tissue where it differentiates into a mature osteoclast [35]. A chemoattractant receptor (CXCR4), which is highly expressed in murine osteoclast precursors, mediates SDF-1-induced chemoattraction, collagen transmigration, and MMP-9 expression. SDF-1 is secreted by endothelial and stromal cells, and this secretion induces osteoclast precursor migration from blood to tissue [36]. Recently, so-called ‘find-me’ signals of apoptotic cells have been defined as ATP and UTP, and function to recruit monocytes to apoptotic cells [37]. P2Y2, the receptor of the find-me signals, is expressed in human osteoclasts [38, 39]. Thus, these humoral factors may also contribute to the migration of osteoclast precursors by their diffusion.
Fig. 3.
Cell communication between MNCs and endothelial cells. MNCs migrate from the bloodstream to the extravascular space. Extravasation of MNCs is controlled by adhesion molecules (ICAM-1 and LFA-1, CD44). Two migratory routes through the endothelia have been reported (paracellular and transcellular migration)
Osteoclast precursor migration under the bone lining cell layer (osteoblast–osteoclast precursor communication)
Osteoclast precursors will start to differentiate into osteoclasts following migration onto the bone surface. How do osteoclast precursors bind to the osteoblasts during migration, and how do they migrate under the osteoblast layer? According to in vitro studies, several lines of evidence show that ICAM-1 is one of the candidates for mediation of osteoclast precursor adhesion and migration [40–43]. Considering the similarity between endothelial cells and osteoblast cells as described above (based on the expression of junctional proteins and on cell polarity), it is likely that the osteoclast precursors (MNCs) also migrate through the osteoblast layer using these two routes (Fig. 4). Recently, it has been found during coculture of human osteoblast-like cells and human peripheral blood MNCs that ICAM-1 is basolaterally localized in osteoclast precursors. Furthermore, this study also showed colocalization of clustered ICAM-1 and F-actin filaments in osteoclast precursors and multinuclear osteoclasts. This study strongly suggests that ICAM-1 that is expressed on osteoclast precursors is involved, not only in cell adhesion, but also in cell migration [44]. Interestingly, Koizumi et al. [45] reported that CX3CL1/fractalkine, a membrane-bound chemokine belonging to the CX3C subfamily, is an adhesion and migration factor for osteoclast precursors. CX3CR1, a receptor for CX3CL1 is expressed on osteoblasts and has been suggested to be involved in the migration of CX3CL1-expressing osteoclast precursors. It is still unclear whether the migration of osteoclast precursors under osteoblasts is essential for osteoclastogenesis or not. However, the microenvironment under the osteoblast would be suitable for osteoclastogenesis, because osteoclast differentiation is found only on bone matrix during normal bone remodeling. M-CSF and RANKL, the essential factors for osteoclastogenesis, are mainly provided by osteoblasts. Therefore, these factors may be concentrated on the basolateral surface of osteoblasts. On the other hand, transmigration of mature osteoclasts has been shown in in vitro experiments [46]. Multinuclear osteoclasts may differentiate in other place and migrate onto the bone matrix in pathological conditions.
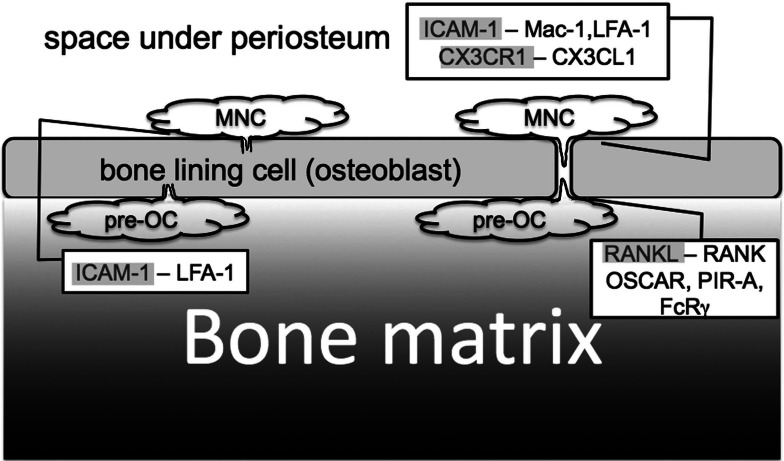
Fig. 4.
Cell communication between osteoclast precursors (MNCs) and osteoblasts. MNCs migrate under osteoblasts. This migration is also mediated by adhesion molecules (ICAM-1-Mac1, ICAM-1-LFA-1, CX3CR1-CX3CL1). Two migratory routes have been proposed (paracellular and transcellular migration). After MNCs have migrated under the osteoblast layer, they differentiate into pre-OCs, which are induced by RANK-RANKL binding. Osteoblasts express ICAM-1, CX3CR1 and RANKL. Pre-OCs express Mac-1, LFA-1, CX3CL1, RANK, OSCAR, FcRγ, and PIR-A
Osteoclast differentiation 1 (pre-osteoclast–osteoblast communication)
Pre-OCs are exposed to the differentiation factors RANKL and M-CSF, which are mainly provided by the osteoblasts under which they migrate, and which induce the pre-OCs to form multinuclear osteoclasts. In addition to these factors, Koga et al. [47] have reported that the costimulatory factors, Fc receptor common γ subunit (FcRγ) or DNAX-activating protein 12 (DAP12), are essential for differentiation of osteoclasts in vivo. These authors showed that both FcRγ and DAP12 double knockout mice exhibit severe osteopetrosis owing to impaired osteoclast differentiation. FcRγ has been reported to interact with osteoclast-associated receptor (OSCAR) or with paired immunoglobulin-like receptor-A (PIR-A), which is expressed by pre-OCs (Fig. 4). OSCAR has been reported to be involved in the interaction of osteoclasts with osteoblasts [48]. The ligand for OSCAR is thought to be expressed on osteoblasts. However, this ligand has not yet been identified. Upon activation of these costimulatory factors (FcRγ or DAP12), spleen tyrosine kinase (Syk) is activated via the phosphorylation of immunoreceptor tyrosine-based activation motif (ITAM) sequences. The details of signaling pathways that mediate osteoclastogenesis are reviewed elsewhere [49–53].
Osteoclast differentiation 2 (pre-osteoclast–pre-osteoclast communication)
Osteoclast precursors differentiate into tartrate-resistant acid phosphatase positive MNCs (TRAP+ pre-OCs) after RANKL stimulation. Thereafter, these TRAP+ pre-OCs fuse with each other and differentiate into multinuclear osteoclasts. For TRAP+ pre-OC fusion to occur, TRAP+ pre-OC–TRAP+ pre-OC interaction is essential so that TRAP+ pre-OCs can identify each other (Fig. 5). The dendritic cell-specific transmembrane protein (DC-STAMP) has also been shown to be essential for this TRAP+ pre-OC cell–cell fusion [54, 55]. Thus, osteoclast fusion is impaired by knockdown or knockout of DC-STAMP, even though TRAP+ mononuclear pre-OCs are differentiated in these mice. However, the ligand for DC-STAMP, and the signaling pathway(s) downstream of DC-STAMP have not yet been identified. TRAP+ cell–cell communication may be essential, not only for cell–cell fusion but also for amplification of the differentiation signal.
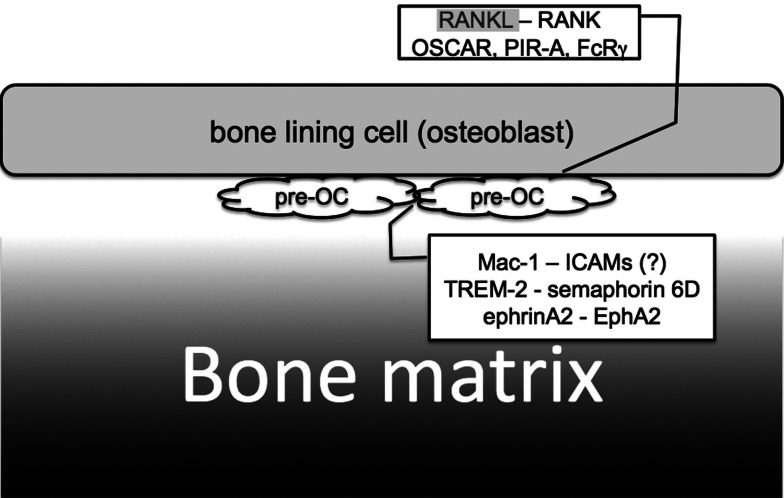
Fig. 5.
Pre-OC–Pre-OC cell–cell communication. Pre-OCs fuse with each other and differentiate into multinuclear osteoclasts. Pre-OCs express Mac-1, ICAM-1, TREM-2, semaphorin 6D, ephrinA2, EphA2, and DC-STAMP
Our previous study showed that expression of the cell surface receptor macrophage antigen 1 (Mac-1, CD11b/CD18) might accelerate differentiation into osteoclasts via modulation of pre-OC cell–cell communication [56]. We demonstrated a positive relationship between CD11b expression on pre-OCs and osteoclastogenesis, an inhibitory effect of a CD11b neutralizing antibody on osteoclastogenesis, and an inhibitory effect of knockdown of CD11b on osteoclastogenesis using a macrophage cell line (RAW264.7) and bone marrow-derived macrophages stimulated with RANKL. A second candidate costimulatory factor for pre-OC interactions is triggering receptor expressed on myeloid cells 2 (TREM-2) [47], which is expressed by pre-OCs and which is reported to function as a phagocytic receptor for bacteria [57]. TREM-2 interacts with both the transmembrane protein plexin A1 and DAP-12 in the same cell. This interaction is followed by the binding of semaphorin 6D (which is a secreted and membrane-bound protein) to plexinA1, resulting in the activation of DAP12 followed by Syk activation [58, 59]. Genetic analysis of patients with Nasu-Hakola disease has revealed that loss-of-function mutations in either DAP-12 or TREM-2 result in this disease [60–62]. More recently, it has been reported that the ephrin ligand and Eph receptor tyrosine kinase are involved in osteoclastogenesis. Irie et al. [63] showed that the ephrinA2 ligand is rapidly induced by RANKL treatment of pre-OCs and they suggested that ephrinA2–EphA2 interaction facilitates the initiation phase of bone remodeling. As mentioned above, pre-OCs differentiate into multinuclear osteoclasts in the presence of RANKL and costimulatory factors. These multinuclear osteoclasts then resorb bone to remove old bone areas.
Bone regeneration (osteoclast–osteoblast communication)
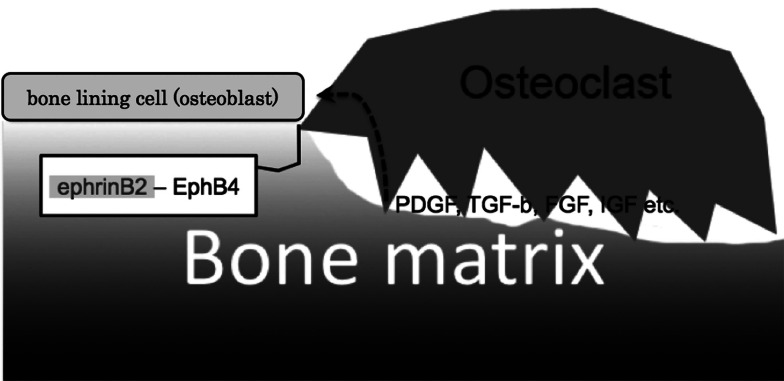
After bone resorption is completed, osteoclasts will die by apoptosis. Osteoclast–osteoblast communication has been reported to play a role in the mechanism by which osteoclasts know that bone resorption is complete [64] (Fig. 6). EphrinB2 expression is under the control of nuclear factor of activated T cells c1 (NFATc1, a master regulator of osteoclastogenesis [65]). Therefore, as the cells differentiate into osteoclasts and the levels of NFATc1 increase, there is a corresponding increase in ephrinB2 expression. On the other hand, EphB4, a receptor of ephrinB2 is expressed in osteoblasts but not in osteoclasts. Using gain- and loss-of-function experiments, Zhao et al. demonstrated that the binding of ephrinB2 (osteoclasts) and EphB4 (osteoblasts) results in attenuation of osteoclastogenesis [64]. Furthermore, they found that this binding also resulted in enhancement of osteoblast differentiation. EphrinB2–EphB4 binding may therefore provide one of the signals that induces osteoblasts to switch their phenotype from osteoclastogenesis to osteogenesis. Both the growth of osteoblasts and their exocytosis of extracellular matrix are necessary for repair of the resorbed bone area.
Fig. 6.
Cell communication between osteoclasts and osteoblasts. After completion of bone resorption, osteoclasts make contact with osteoblasts. Apoptosis of osteoclasts and proliferation of osteoblasts are induced by bidirectional signaling of ephrinB2–EphB4, which are expressed by osteoclasts and osteoblasts, respectively. Osteoblasts proliferate due to the action of growth factors released from the bone matrix
It has been reported that humoral factors embedded in bone matrix are involved in osteoblast growth and differentiation during this repair process [66]. Mesenchymal stem cells in the periosteum are known to differentiate into osteoblasts [67]. Recently, it has been found using the transcriptome assay that signals induced by platelet-derived growth factor (PDGF), transforming growth β factor (TGF-β) and fibroblast growth factor (FGF) are important for growth of mesenchymal stem cells [68]. PDGF and FGF induce osteoblast proliferation [69]. Insulin-like growth factor and TGF-β promote osteoblast differentiation [70–73]. These factors will therefore contribute to bone formation after bone resorption. When does bone formation stop? Recent findings have revealed that the molecule sclerostin plays a significant role in the control of bone volume. Sclerostin is secreted by osteocytes and functions as an antagonist of bone morphogenetic protein [74, 75]. Moreover, secretion of sclerostin is dependent on osteocyte maturation [76]. These findings suggest that sclerostin is essential for the termination of bone remodeling.
A new theory regarding the mechanism by which old bone is sensed (osteoblast–osteocyte communication)
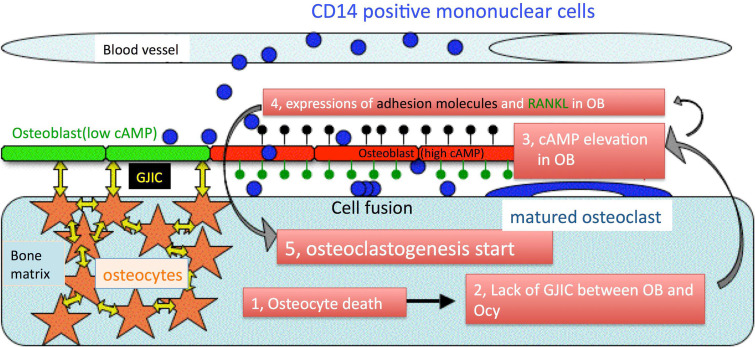
As mentioned above, the mechanism by which pre-OCs differentiate only on old bone is not clearly understood. After careful consideration of this important and interesting problem, we have come up with a simple working hypothesis to explain this mechanism, which, however, remains to be proved (Fig. 7). Our hypothesis is based on three pieces of evidence. Firstly, the trigger of bone remodeling is osteocyte cell apoptosis (discussed above). More recently, Tatsumi et al. [77] reported that osteocyte ablation caused osteoporosis by upregulation of RANKL expression in bone. These authors generated a transgenic mouse that expresses diphtheria toxin receptor under the control of dentin matrix protein 1 promoter, for inducible ablation of osteocytes in vivo. After the induction of osteocyte death by the administration of diphtheria toxin, the mice exhibit a severe osteoporotic phenotype. These results provide direct evidence that osteocyte death is a trigger of osteoclastogenesis in vivo. Secondly, the phenotypic change of osteoblasts from osteogenesis to osteoclastogenesis is due to elevation of the level of cyclic AMP (cAMP) in osteoblasts. Several lines of evidence suggest that agents that elevate the level of cAMP induce RANKL expression in osteoblasts [78–80]. Furthermore, Mak et al. [81] have shown that up-regulation of PTHrP (parathyroid hormone-related peptide) in osteoblasts induces RANKL expression via cAMP-dependent protein kinase A in vivo. Promoter analysis of RANKL has revealed that cAMP and the transcription factor Runx2 play a predominant role in RANKL expression [82]. Interestingly, since Runx2 is an osteoblast-specific transcription factor, Runx2 regulation of RANKL expression by binding to the RANKL promoter may also contribute cell-type specific expression of RANKL. On the other hand, the expression of osteoprotegerin, an osteoblast-secreted decoy receptor that specifically binds to RANKL and inhibits osteoclast maturation, is inhibited via a cAMP/PKA-dependent pathway [83]. Lastly, there is communication between osteoblasts and osteocytes via gap junctions [84]. Osteoblasts continuously express connexin 43 at high levels [85]. Furthermore, parathyroid hormone, prostaglandin E2, or shear stress, upregulate connexin 43 expression and GJIC activity in osteocytes [8, 86, 87].
Fig. 7.
A new theory regarding the mechanism by which old bone is sensed. This theory is based on cAMP-dependent changes in osteoblast phenotype from osteogenic to osteoclastogenic. 1–3 Microcrack-induced osteocyte apoptosis causes the elevation of cAMP in adjacent osteoblasts (red). 4, 5 The osteoblasts express adhesion molecules (for MNC migration, black pins) and RANKL (for osteoclastogenesis, green pins). The elevation of cAMP in osteoblasts is dependent on communication between the osteoblasts and osteocytes via gap junctions (yellow arrows)
The combined evidence led us to hypothesize the following simple story. Thus, cAMP levels in osteoblasts are kept low (not enough to induce RANKL expression) in healthy or new bone. Moreover, the elevation of cAMP levels in osteoblasts is always attenuated by diffusion of the cAMP from osteoblasts to osteocytes via gap junctions. This diffusion would occur even if a transient stimulation was applied to the healthy osteoblasts, because it is known that elevation of cAMP spreads to adjacent cells within seconds via gap junctions [88]. In other words, osteocytes play an important role in the regulation of ostoclastogenesis by cAMP hydrolysis. Conversely, cAMP levels in osteoblasts will increase in the absence of GJIC between osteoblasts and osteocytes, because cAMP in osteoblasts cannot efflux into osteocytes without GJICs. The elevation of cAMP in osteoblasts then leads to the synthesis of adhesion molecules and differentiation factors for osteoclastogenesis [89]. These adhesion molecules will not only retain the osteoclast precursors on the osteoblasts but also induce the migration of osteoclast precursors under the osteoblast layer. While under the osteoblast layer, osteoclast precursors will be exposed to a microenvironment that is suitable for osteoclastogenesis.
Acknowledgment
The original work of the author that is cited here was supported in part by research grants 20390470, 20659306, 20390463, and 21659432 from the Japan Society for the Promotion of Science, Tokyo, Japan.
References
- 1.Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int. 1995;57:344–358. doi: 10.1007/BF00302070. [DOI] [PubMed] [Google Scholar]
- 2.Burger EH, Klein-Nulend J. Mechanotransduction in bone – role of the lacuno-canalicular network. FASEB J. 1999;13(Suppl):S101–S112. [PubMed] [Google Scholar]
- 3.Reijnders CM, Bravenboer N, Holzmann PJ, Bhoelan F, Blankenstein MA, Lips P. In vivo mechanical loading modulates insulin-like growth factor binding protein-2 gene expression in rat osteocytes. Calcif Tissue Int. 2007;80:137–143. doi: 10.1007/s00223-006-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robling AG, Bellido T, Turner CH. Mechanical stimulation in vivo reduces osteocyte expression of sclerostin. J Musculoskelet Neuronal Interact. 2006;6:354. [PubMed] [Google Scholar]
- 5.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 6.Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24:1651–1661. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- 7.Donahue HJ. Gap junctions and biophysical regulation of bone cell differentiation. Bone. 2000;26:417–422. doi: 10.1016/S8756-3282(00)00245-3. [DOI] [PubMed] [Google Scholar]
- 8.Ishihara Y, Kamioka H, Honjo T, Ueda H, Takano-Yamamoto T, Yamashiro T. Hormonal, pH, and calcium regulation of connexin 43-mediated dye transfer in osteocytes in chick calvaria. J Bone Miner Res. 2008;23:350–360. doi: 10.1359/jbmr.071102. [DOI] [PubMed] [Google Scholar]
- 9.Kamioka H, Honjo T, Takano-Yamamoto T. A three-dimensional distribution of osteocyte processes revealed by the combination of confocal laser scanning microscopy and differential interference contrast microscopy. Bone. 2001;28:145–149. doi: 10.1016/S8756-3282(00)00421-X. [DOI] [PubMed] [Google Scholar]
- 10.Menton DN, Simmons DJ, Chang SL, Orr BY. From bone lining cell to osteocyte – an SEM study. Anat Rec. 1984;209:29–39. doi: 10.1002/ar.1092090105. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari SL, Traianedes K, Thorne M, Lafage-Proust MH, Genever P, Cecchini MG, Behar V, Bisello A, Chorev M, Rosenblatt M, Suva LJ. A role for N-cadherin in the development of the differentiated osteoblastic phenotype. J Bone Miner Res. 2000;15:198–208. doi: 10.1359/jbmr.2000.15.2.198. [DOI] [PubMed] [Google Scholar]
- 12.Kawaguchi J, Azuma Y, Hoshi K, Kii I, Takeshita S, Ohta T, Ozawa H, Takeichi M, Chisaka O, Kudo A. Targeted disruption of cadherin-11 leads to a reduction in bone density in calvaria and long bone metaphyses. J Bone Miner Res. 2001;16:1265–1271. doi: 10.1359/jbmr.2001.16.7.1265. [DOI] [PubMed] [Google Scholar]
- 13.Kii I, Amizuka N, Shimomura J, Saga Y, Kudo A. Cell–cell interaction mediated by cadherin-11 directly regulates the differentiation of mesenchymal cells into the cells of the osteo-lineage and the chondro-lineage. J Bone Miner Res. 2004;19:1840–1849. doi: 10.1359/JBMR.040812. [DOI] [PubMed] [Google Scholar]
- 14.Hay E, Laplantine E, Geoffroy V, Frain M, Kohler T, Muller R, Marie PJ. N-cadherin interacts with axin and LRP5 to negatively regulate Wnt/beta-catenin signaling, osteoblast function, and bone formation. Mol Cell Biol. 2009;29:953–964. doi: 10.1128/MCB.00349-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatakeyama N, Kojima T, Iba K, Murata M, Thi MM, Spray DC, Osanai M, Chiba H, Ishiai S, Yamashita T, Sawada N. IGF-I regulates tight-junction protein claudin-1 during differentiation of osteoblast-like MC3T3-E1 cells via a MAP-kinase pathway. Cell Tissue Res. 2008;334:243–254. doi: 10.1007/s00441-008-0690-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wongdee K, Pandaranandaka J, Teerapornpuntakit J, Tudpor K, Thongbunchoo J, Thongon N, Jantarajit W, Krishnamra N, Charoenphandhu N. Osteoblasts express claudins and tight junction-associated proteins. Histochem Cell Biol. 2008;130:79–90. doi: 10.1007/s00418-008-0419-6. [DOI] [PubMed] [Google Scholar]
- 17.Civitelli R, Beyer EC, Warlow PM, Robertson AJ, Geist ST, Steinberg TH. Connexin43 mediates direct intercellular communication in human osteoblastic cell networks. J Clin Invest. 1993;91:1888–1896. doi: 10.1172/JCI116406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donahue HJ, McLeod KJ, Rubin CT, Andersen J, Grine EA, Hertzberg EL, Brink PR. Cell-to-cell communication in osteoblastic networks: cell line-dependent hormonal regulation of gap junction function. J Bone Miner Res. 1995;10:881–889. doi: 10.1002/jbmr.5650100609. [DOI] [PubMed] [Google Scholar]
- 19.Prele CM, Horton MA, Caterina P, Stenbeck G. Identification of the molecular mechanisms contributing to polarized trafficking in osteoblasts. Exp Cell Res. 2003;282:24–34. doi: 10.1006/excr.2002.5668. [DOI] [PubMed] [Google Scholar]
- 20.Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, Maylin ER, Ripoll VM, Hume DA, Pettit AR. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181:1232–1244. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 21.Pettit AR, Chang MK, Hume DA, Raggatt LJ. Osteal macrophages: a new twist on coupling during bone dynamics. Bone. 2008;43:976–982. doi: 10.1016/j.bone.2008.08.128. [DOI] [PubMed] [Google Scholar]
- 22.Mizoguchi T, Muto A, Udagawa N, Arai A, Yamashita T, Hosoya A, Ninomiya T, Nakamura H, Yamamoto Y, Kinugawa S, Nakamura M, Nakamichi Y, Kobayashi Y, Nagasawa S, Oda K, Tanaka H, Tagaya M, Penninger JM, Ito M, Takahashi N. Identification of cell cycle-arrested quiescent osteoclast precursors in vivo. J Cell Biol. 2009;184:541–554. doi: 10.1083/jcb.200806139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verborgt O, Gibson GJ, Schaffler MB. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res. 2000;15:60–67. doi: 10.1359/jbmr.2000.15.1.60. [DOI] [PubMed] [Google Scholar]
- 24.Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, Bellido T. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res. 2006;21:605–615. doi: 10.1359/jbmr.060107. [DOI] [PubMed] [Google Scholar]
- 25.Cardoso L, Herman BC, Verborgt O, Laudier D, Majeska RJ, Schaffler MB. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Miner Res. 2009;24:597–605. doi: 10.1359/jbmr.081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emerton KB, Hu B, Woo AA, Sinofsky A, Hernandez C, Majeska RJ, Jepsen KJ, Schaffler MB. Osteocyte apoptosis and control of bone resorption following ovariectomy in mice. Bone. 2010;46:577–583. doi: 10.1016/j.bone.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholson GC, Malakellis M, Collier FM, Cameron PU, Holloway WR, Gough TJ, Gregorio-King C, Kirkland MA, Myers DE. Induction of osteoclasts from CD14-positive human peripheral blood mononuclear cells by receptor activator of nuclear factor kappaB ligand (RANKL) Clin Sci (Lond) 2000;99:133–140. doi: 10.1042/CS19990355. [DOI] [PubMed] [Google Scholar]
- 28.Quinn JM, Neale S, Fujikawa Y, McGee JO, Athanasou NA. Human osteoclast formation from blood monocytes, peritoneal macrophages, and bone marrow cells. Calcif Tissue Int. 1998;62:527–531. doi: 10.1007/s002239900473. [DOI] [PubMed] [Google Scholar]
- 29.Fujikawa Y, Quinn JM, Sabokbar A, McGee JO, Athanasou NA. The human osteoclast precursor circulates in the monocyte fraction. Endocrinology. 1996;137:4058–4060. doi: 10.1210/en.137.9.4058. [DOI] [PubMed] [Google Scholar]
- 30.Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, Dvorak HF, Dvorak AM, Springer TA. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 2007;26:784–797. doi: 10.1016/j.immuni.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Millan J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol. 2006;8:113–123. doi: 10.1038/ncb1356. [DOI] [PubMed] [Google Scholar]
- 33.Shaw SK, Ma S, Kim MB, Rao RM, Hartman CU, Froio RM, Yang L, Jones T, Liu Y, Nusrat A, Parkos CA, Luscinskas FW. Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompany neutrophil transmigration. J Exp Med. 2004;200:1571–1580. doi: 10.1084/jem.20040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kindle L, Rothe L, Kriss M, Osdoby P, Collin-Osdoby P. Human microvascular endothelial cell activation by IL-1 and TNF-alpha stimulates the adhesion and transendothelial migration of circulating human CD14+ monocytes that develop with RANKL into functional osteoclasts. J Bone Miner Res. 2006;21:193–206. doi: 10.1359/JBMR.051027. [DOI] [PubMed] [Google Scholar]
- 35.Ishii M, Egen JG, Klauschen F, Meier-Schellersheim M, Saeki Y, Vacher J, Proia RL, Germain RN. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458:524–528. doi: 10.1038/nature07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu X, Huang Y, Collin-Osdoby P, Osdoby P. Stromal cell-derived factor-1 (SDF-1) recruits osteoclast precursors by inducing chemotaxis, matrix metalloproteinase-9 (MMP-9) activity, and collagen transmigration. J Bone Miner Res. 2003;18:1404–1418. doi: 10.1359/jbmr.2003.18.8.1404. [DOI] [PubMed] [Google Scholar]
- 37.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowler WB, Littlewood-Evans A, Bilbe G, Gallagher JA, Dixon CJ. P2Y2 receptors are expressed by human osteoclasts of giant cell tumor but do not mediate ATP-induced bone resorption. Bone. 1998;22:195–200. doi: 10.1016/S8756-3282(97)00280-9. [DOI] [PubMed] [Google Scholar]
- 39.Korcok J, Raimundo LN, Du X, Sims SM, Dixon SJ. P2Y6 nucleotide receptors activate NF-kappaB and increase survival of osteoclasts. J Biol Chem. 2005;280:16909–16915. doi: 10.1074/jbc.M410764200. [DOI] [PubMed] [Google Scholar]
- 40.Kurachi T, Morita I, Murota S. Involvement of adhesion molecules LFA-1 and ICAM-1 in osteoclast development. Biochim Biophys Acta. 1993;1178:259–266. doi: 10.1016/0167-4889(93)90202-Z. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka Y, Morimoto I, Nakano Y, Okada Y, Hirota S, Nomura S, Nakamura T, Eto S. Osteoblasts are regulated by the cellular adhesion through ICAM-1 and VCAM-1. J Bone Miner Res. 1995;10:1462–1469. doi: 10.1002/jbmr.5650101006. [DOI] [PubMed] [Google Scholar]
- 42.Kurokouchi K, Kambe F, Yasukawa K, Izumi R, Ishiguro N, Iwata H, Seo H. TNF-alpha increases expression of IL-6 and ICAM-1 genes through activation of NF-kappaB in osteoblast-like ROS17/2.8 cells. J Bone Miner Res. 1998;13:1290–1299. doi: 10.1359/jbmr.1998.13.8.1290. [DOI] [PubMed] [Google Scholar]
- 43.Fujii Y, Fujii K, Nakano K, Tanaka Y. Crosslinking of CD44 on human osteoblastic cells upregulates ICAM-1 and VCAM-1. FEBS Lett. 2003;539:45–50. doi: 10.1016/S0014-5793(03)00182-0. [DOI] [PubMed] [Google Scholar]
- 44.Bloemen V, de Vries TJ, Schoenmaker T, Everts V. Intercellular adhesion molecule-1 clusters during osteoclastogenesis. Biochem Biophys Res Commun. 2009;385:640–645. doi: 10.1016/j.bbrc.2009.05.145. [DOI] [PubMed] [Google Scholar]
- 45.Koizumi K, Saitoh Y, Minami T, Takeno N, Tsuneyama K, Miyahara T, Nakayama T, Sakurai H, Takano Y, Nishimura M, Imai T, Yoshie O, Saiki I. Role of CX3CL1/fractalkine in osteoclast differentiation and bone resorption. J Immunol. 2009;183:7825–7831. doi: 10.4049/jimmunol.0803627. [DOI] [PubMed] [Google Scholar]
- 46.Saltel F, Chabadel A, Zhao Y, Lafage-Proust MH, Clezardin P, Jurdic P, Bonnelye E. Transmigration: a new property of mature multinucleated osteoclasts. J Bone Miner Res. 2006;21:1913–1923. doi: 10.1359/jbmr.060821. [DOI] [PubMed] [Google Scholar]
- 47.Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, Taniguchi T, Takayanagi H, Takai T. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 48.Kim N, Takami M, Rho J, Josien R, Choi Y. A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J Exp Med. 2002;195:201–209. doi: 10.1084/jem.20011681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shinohara M, Takayanagi H. Novel osteoclast signaling mechanisms. Curr Osteoporos Rep. 2007;5:67–72. doi: 10.1007/s11914-007-0005-1. [DOI] [PubMed] [Google Scholar]
- 50.Humphrey MB, Lanier LL, Nakamura MC. Role of ITAM-containing adapter proteins and their receptors in the immune system and bone. Immunol Rev. 2005;208:50–65. doi: 10.1111/j.0105-2896.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 51.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 52.Nakashima T, Takayanagi H. Osteoimmunology: crosstalk between the immune and bone systems. J Clin Immunol. 2009;29:555–567. doi: 10.1007/s10875-009-9316-6. [DOI] [PubMed] [Google Scholar]
- 53.Negishi-Koga T, Takayanagi H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol Rev. 2009;231:241–256. doi: 10.1111/j.1600-065X.2009.00821.x. [DOI] [PubMed] [Google Scholar]
- 54.Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K, Oike Y, Takeya M, Toyama Y, Suda T. DC-STAMP is essential for cell–cell fusion in osteoclasts and foreign body giant cells. J Exp Med. 2005;202:345–351. doi: 10.1084/jem.20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kukita T, Wada N, Kukita A, Kakimoto T, Sandra F, Toh K, Nagata K, Iijima T, Horiuchi M, Matsusaki H, Hieshima K, Yoshie O, Nomiyama H. RANKL-induced DC-STAMP is essential for osteoclastogenesis. J Exp Med. 2004;200:941–946. doi: 10.1084/jem.20040518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayashi H, Nakahama K, Sato T, Tuchiya T, Asakawa Y, Maemura T, Tanaka M, Morita M, Morita I. The role of Mac-1 (CD11b/CD18) in osteoclast differentiation induced by receptor activator of nuclear factor-kappaB ligand. FEBS Lett. 2008;582:3243–3248. doi: 10.1016/j.febslet.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 57.N’Diaye EN, Branda CS, Branda SS, Nevarez L, Colonna M, Lowell C, Hamerman JA, Seaman WE. TREM-2 (triggering receptor expressed on myeloid cells 2) is a phagocytic receptor for bacteria. J Cell Biol. 2009;184:215–223. doi: 10.1083/jcb.200808080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamagnone L, Giordano S. Semaphorin pathways orchestrate osteogenesis. Nat Cell Biol. 2006;8:545–547. doi: 10.1038/ncb0606-545. [DOI] [PubMed] [Google Scholar]
- 59.Takegahara N, Takamatsu H, Toyofuku T, Tsujimura T, Okuno T, Yukawa K, Mizui M, Yamamoto M, Prasad DV, Suzuki K, Ishii M, Terai K, Moriya M, Nakatsuji Y, Sakoda S, Sato S, Akira S, Takeda K, Inui M, Takai T, Ikawa M, Okabe M, Kumanogoh A, Kikutani H. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol. 2006;8:615–622. doi: 10.1038/ncb1416. [DOI] [PubMed] [Google Scholar]
- 60.Paloneva J, Kestila M, Wu J, Salminen A, Bohling T, Ruotsalainen V, Hakola P, Bakker AB, Phillips JH, Pekkarinen P, Lanier LL, Timonen T, Peltonen L. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat Genet. 2000;25:357–361. doi: 10.1038/77153. [DOI] [PubMed] [Google Scholar]
- 61.Paloneva J, Mandelin J, Kiialainen A, Bohling T, Prudlo J, Hakola P, Haltia M, Konttinen YT, Peltonen L. DAP12/TREM2 deficiency results in impaired osteoclast differentiation and osteoporotic features. J Exp Med. 2003;198:669–675. doi: 10.1084/jem.20030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cella M, Buonsanti C, Strader C, Kondo T, Salmaggi A, Colonna M. Impaired differentiation of osteoclasts in TREM-2-deficient individuals. J Exp Med. 2003;198:645–651. doi: 10.1084/jem.20022220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Irie N, Takada Y, Watanabe Y, Matsuzaki Y, Naruse C, Asano M, Iwakura Y, Suda T, Matsuo K. Bidirectional signaling through ephrinA2–EphA2 enhances osteoclastogenesis and suppresses osteoblastogenesis. J Biol Chem. 2009;284:14637–14644. doi: 10.1074/jbc.M807598200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, Suda T, Matsuo K. Bidirectional ephrinB2–EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–121. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 65.Takayanagi H. The role of NFAT in osteoclast formation. Ann N Y Acad Sci. 2007;1116:227–237. doi: 10.1196/annals.1402.071. [DOI] [PubMed] [Google Scholar]
- 66.Hauschka PV, Mavrakos AE, Iafrati MD, Doleman SE, Klagsbrun M. Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin–sepharose. J Biol Chem. 1986;261:12665–12674. [PubMed] [Google Scholar]
- 67.Allen MR, Hock JM, Burr DB. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone. 2004;35:1003–1012. doi: 10.1016/j.bone.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 68.Ng F, Boucher S, Koh S, Sastry KS, Chase L, Lakshmipathy U, Choong C, Yang Z, Vemuri MC, Rao MS, Tanavde V. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112:295–307. doi: 10.1182/blood-2007-07-103697. [DOI] [PubMed] [Google Scholar]
- 69.Kim SJ, Kim SY, Kwon CH, Kim YK. Differential effect of FGF and PDGF on cell proliferation and migration in osteoblastic cells. Growth Factors. 2007;25:77–86. doi: 10.1080/08977190701398977. [DOI] [PubMed] [Google Scholar]
- 70.Joyce ME, Roberts AB, Sporn MB, Bolander ME. Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol. 1990;110:2195–2207. doi: 10.1083/jcb.110.6.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hock JM, Canalis E, Centrella M. Transforming growth factor-beta stimulates bone matrix apposition and bone cell replication in cultured fetal rat calvariae. Endocrinology. 1990;126:421–426. doi: 10.1210/endo-126-1-421. [DOI] [PubMed] [Google Scholar]
- 72.Hock JM, Centrella M, Canalis E. Insulin-like growth factor I has independent effects on bone matrix formation and cell replication. Endocrinology. 1988;122:254–260. doi: 10.1210/endo-122-1-254. [DOI] [PubMed] [Google Scholar]
- 73.Canalis E. Effect of insulinlike growth factor I on DNA and protein synthesis in cultured rat calvaria. J Clin Invest. 1980;66:709–719. doi: 10.1172/JCI109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22:6267–6276. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Lowik CW. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199:805–814. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, Reeve J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842–1844. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- 77.Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 78.Park H, No AL, Lee JM, Chen L, Lee SY, Lee DS, Yim M. PDE4 inhibitor upregulates PTH-induced osteoclast formation via CRE-mediated COX-2 expression in osteoblasts. FEBS Lett. 2010;584:173–180. doi: 10.1016/j.febslet.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 79.Park H, Young Lee S, Lee DS, Yim M. Phosphodiesterase 4 inhibitor regulates the TRANCE/OPG ratio via COX-2 expression in a manner similar to PTH in osteoblasts. Biochem Biophys Res Commun. 2007;354:178–183. doi: 10.1016/j.bbrc.2006.12.174. [DOI] [PubMed] [Google Scholar]
- 80.Takami M, Cho ES, Lee SY, Kamijo R, Yim M. Phosphodiesterase inhibitors stimulate osteoclast formation via TRANCE/RANKL expression in osteoblasts: possible involvement of ERK and p38 MAPK pathways. FEBS Lett. 2005;579:832–838. doi: 10.1016/j.febslet.2004.12.066. [DOI] [PubMed] [Google Scholar]
- 81.Mak KK, Bi YM, Wan C, Chuang PT, Clemens T, Young M, Yang YZ. Hedgehog signaling in mature osteoblasts regulates bone formation and resorption by controlling PTHrP and RANKL expression. Dev Cell. 2008;14:674–688. doi: 10.1016/j.devcel.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 82.Fu Q, Manolagas SC, O’Brien CA. Parathyroid hormone controls receptor activator of NF-kappaB ligand gene expression via a distant transcriptional enhancer. Mol Cell Biol. 2006;26:6453–6468. doi: 10.1128/MCB.00356-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Villa I, Mrak E, Rubinacci A, Ravasi F, Guidobono F. CGRP inhibits osteoprotegerin production in human osteoblast-like cells via cAMP/PKA-dependent pathway. Am J Physiol Cell Physiol. 2006;291:C529–C537. doi: 10.1152/ajpcell.00354.2005. [DOI] [PubMed] [Google Scholar]
- 84.Civitelli R. Cell–cell communication in the osteoblast/osteocyte lineage. Arch Biochem Biophys. 2008;473:188–192. doi: 10.1016/j.abb.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bhattacharjee R, Kaneda M, Nakahama K, Morita I. The steady-state expression of connexin43 is maintained by the PI3K/Akt in osteoblasts. Biochem Biophys Res Commun. 2009;382:440–444. doi: 10.1016/j.bbrc.2009.03.044. [DOI] [PubMed] [Google Scholar]
- 86.Alford AI, Jacobs CR, Donahue HJ. Oscillating fluid flow regulates gap junction communication in osteocytic MLO-Y4 cells by an ERK1/2 MAP kinase-dependent mechanism small star, filled. Bone. 2003;33:64–70. doi: 10.1016/S8756-3282(03)00167-4. [DOI] [PubMed] [Google Scholar]
- 87.Xia X, Batra N, Shi Q, Bonewald LF, Sprague E, Jiang JX. Prostaglandin promotion of osteocyte gap junction function through transcriptional regulation of connexin 43 by glycogen synthase kinase 3/beta-catenin signaling. Mol Cell Biol. 2010;30:206–219. doi: 10.1128/MCB.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ponsioen B, van Zeijl L, Moolenaar WH, Jalink K. Direct measurement of cyclic AMP diffusion and signaling through connexin43 gap junctional channels. Exp Cell Res. 2007;313:415–423. doi: 10.1016/j.yexcr.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 89.Bernot D, Peiretti F, Canault M, Juhan-Vague I, Nalbone G. Upregulation of TNF-alpha-induced ICAM-1 surface expression by adenylate cyclase-dependent pathway in human endothelial cells. J Cell Physiol. 2005;202:434–441. doi: 10.1002/jcp.20134. [DOI] [PubMed] [Google Scholar]