Abstract
Plant pathogenic bacteria inject about 30 virulence effector proteins into the host cell using a specialized secretion apparatus. Bacteria which are unable to do this elicit host immunity and cannot grow inside living plant tissue. Thus, the primary function of the effectors is to suppress host immunity. The identity of individual effectors within each complement varies even between closely related bacterial strains, and effectors themselves act redundantly and are apparently interchangeable. Many effectors are known to target components of plant defense pathways, but it is difficult to study their role in molecular terms. For some of them, there is controversy about their mode of action. We propose that effectors act promiscuously by targeting host molecules with low specificity and affinity.
Keywords: Type III secretion system, Effector proteins, MAMPs, Innate immunity, Virulence factors
Introduction
Plant pathogenic bacteria, in contrast to many obligate eukaryotic pathogens, can choose whether or not to deploy their arsenals of virulence weaponry. Normally resident in soil as saprophytes, or on leaves as epiphytes, bacteria move opportunistically into the leaf in response to unknown cues [1]. The attraction of this niche is the access to host nutrients which can be exploited to drive bacterial replication to an extent that is impossible in the other niches. The drawback is the enforced proximity to the host immune system, which responds to the bacterial presence through both extracellular and intracellular perception mechanisms [2]. Given the right conditions, bacteria replicate exponentially to high levels and cause the typical spots, wilts, and canker symptoms of infection. The ability to opt out of the pathogenic lifestyle, and the generalist nature of bacterial infectivity, means that hosts can be relieved of selective pressure from pathogens. Similarly, selection pressures on the virulence components of bacteria can be modified by infection of alternate hosts, or released from selection pressure entirely during non-pathogenic phases. This is a remarkably different selection framework when compared to eukaryotic pathogens which are obligate and frequently restricted to a single host species, and hence the forces which shape evolution of their respective virulence machineries may vary significantly.
The most important strategy for virulence of plant pathogenic bacteria is deployment of proteinaceous ‘effectors’ within the host cell. On entry to the leaf, bacteria form tight associations with the host cell wall [3]. This sets into play a chain of host perception events that must be avoided or suppressed by the pathogen, in a relationship that is often characterized as a molecular arms race [2]. Thus, bacteria are betrayed by the presence of characteristic elicitors, which are detected at the cell surface by specific receptor proteins that activate immunity. Immunity triggered by elicitor recognition is suppressed by bacterial effector proteins, but these can themselves act as elicitors after recognition by cytoplasmic receptors of the nucleotide-binding-leucine rich repeats (NB-LRR) class. Predictably, some effectors suppress NB-LRR signaling; more surprisingly, it was shown recently that a host defense protein can modify a pathogen effector to ensure its cytoplasmic detection [4]. Although the molecular activities and host targets of most effectors have not been defined, there is an emerging theme of degradative activities and weak specificities, congruent with the idea of bacteria as generalist, non-obligate pathogens. This complicates the identification of effector targets, because it is difficult to discriminate between false and true positives. However, everything that is worthwhile in life is also difficult, as the Beatles remind us in “The Long and Winding Road”; this review takes their works as themes to explore current topics in effector biology.
Anti-bacterial immunity: “A beginning”
In the initial steps of an infection, plants perceive microbes through detection of conserved elicitors known as PAMPs or MAMPs (for pathogen- or microbe-associated molecular patterns) [5]. Recognition of bacterial MAMPs occurs through specific pattern recognition receptors (PRRs) [6]. The PRRs that respond to bacterial MAMPs so far as is known are receptor kinase proteins, many of which form a complex with a second transmembrane kinase called BAK1 (for BRI1-associated receptor kinase 1) immediately after elicitation [7–9]. Upon formation of the recognition complex, a cascade of responses is mounted which includes an increase in cytosolic calcium from external stores, the generation of a burst of reactive oxygen species, activation of a mitogen-activated protein kinase (MAPK) cascade, induction of defense-associated genes, and deposition of callose into cell walls at the site of infection [10, 11]. There are some dependency relationships amongst these responses, but it is not yet clear whether they follow a linear pathway or comprise a signaling network. The outcome of PRR activation is PAMP-triggered immunity (PTI), although the specific mechanisms that curtail bacterial growth are unknown [5, 12]. To counteract PTI, pathogens of all kinds deposit so-called effector molecules into the plant cell, which suppress immunity through various strategies, most of which are unknown. As noted previously, effectors can also act as elicitors due to direct or indirect recognition by NB-LRR proteins. This review concentrates on the virulence activities of bacterial effectors with a focus on the model pathogen Pseudomonas syringae pv. tomato DC3000 (Pto DC3000), which is pathogenic on tomato and the model plant Arabidopsis thaliana.
Effector secretion by pathogenic bacteria: “All things must pass”
Phytopathogenic bacteria remain extracellular throughout the life cycle. They enter the intercellular spaces called the apoplast through stomatal openings or wounds. Once inside the leaf, they adhere to the plant cell wall and form dense colonies [13]. Early insights with non-pathogenic hrp mutants (hypersensitive response and pathogenicity) outlined the fundamental characteristics of bacterial pathogenicity [14]. The hypersensitive response (HR) is a rapid localized cell death response at the infection site that is associated with immunity. Bacteria mutated in hrp genes do not induce the HR on resistant hosts, and behave as non-pathogens (i.e., do not replicate or cause symptoms) on susceptible hosts. Thus, there is a link between bacterial pathogenicity and recognition by the host immune system leading to the HR [15]. A highly conserved subset of hrp genes is present in all major gram-negative bacterial phytopathogens [e.g., Pseudomonas syringae, Xanthomonas spp., Ralstonia solanacearum, and Pectobacterium (formerly Erwinia) spp.]. Those conserved with animal pathogens are called hrc genes (hypersensitive response and conserved). Hrc genes encode structural components of the Type-III secretion system (TTSS), a supramolecular, filamentous needle-like structure that is the conduit for effector delivery [16, 17]. They are regulated collectively by the transcription factors hrpR/S and hrpL which bind to the hrp box motif in the respective promoter regions [18–20]. The Type-III pilus is composed of hrpA building blocks and grows progressively from its tip [16, 17]. It extends across both bacterial cell membranes and the plant cell wall, and apparently penetrates the host plasma membrane. The pilus injects around 30 virulence effector proteins directly into the host cytoplasm, the expression of which are also regulated by hrpL [21–23]. Injected effector proteins localize to various cellular compartments where they manipulate the host in favor of pathogen growth.
To date, 62 Type-III effectors of P. syringae have been identified, which fall into 29 families, 10 of which contain multiple members (significant variants), and 16 of which are singletons (http://www.pseudomonas-syringae.org/; [24]). Genome sequencing of three P. syringae strains [pvs tomato DC3000, syringae B728a (Psy B728a), and phaseolicola 1448a (Pph 1448a)] and the draft genome sequence of a fourth [pv. tabaci 11528 (Pta 11528)] has revealed important information on the diversity of effectors encoded by these strains [24–27]. Each strain encodes about 20–30 effectors. Despite very high levels of sequence identity over the genome as a whole, remarkably few effector genes are shared between genomes. For example, Pph 1448a and Pta 11528 share 97% nucleotide identity over the alignable portions of their genomes, but share only 9 effector genes [24]. This scenario is generally true, and despite the concept of “core” and “variable” effector compliments in the literature [28], the four genomes share only four effectors, and it seems likely that this will be diminished by sampling further genome sequences. The inherent variability of effector complements between strains may reflect selective pressure from the host’s immune system, particularly via intracellular recognition. In addition, the lack of overlap between effector complements of strains that share a common host, such as Pto DC3000 and Psy B728a, suggests that effectors are interchangeable.
Effector redundancy: “You won’t see me”
The first bacterial effector proteins were identified based on the ability to cause the HR—a conspicuous immune reaction—on certain host plants [2]. Why should pathogens secrete proteins which curtail their infectivity so spectacularly? Because of the extreme selection pressure active against recognized effectors, it was clear that they must play important roles in the absence of recognition; that is, on susceptible plant hosts. Suppression of immunity by virulent bacteria is easy to demonstrate: bacteria as extracellular pathogens elicit PTI responses, which are suppressed by intact pathogens but not by those lacking a functional TTSS [29]. Demonstration of virulence roles for individual effectors has proven to be more difficult, with only quantitative contributions to bacterial growth conferred by even the strongest effectors. This is likely due to effector redundancy [31, 32]. For example, both AvrPto and AvrPtoB target PRR receptor complexes to abrogate signal transduction [30–32]. Likewise, individual mutations of the conserved effector locus (CEL) comprising the effector genes AvrE and HopM1 did not affect pathogen growth [33]. However, a complete knockout of the CEL locus compromised bacterial growth strongly [34], suggesting that AvrE and HopM1 act redundantly. Overall, individual knockouts tend not to be informative, whereas multiple knockouts can begin to identify redundancy relationships between various effectors [35].
Detecting effector activity on plants: “Within you without you”
It has been difficult to study the physiological and molecular activities of Type III effectors. This is due in part to the quantitative contribution of individual effectors to bacterial virulence [2, 36]. Therefore, methods to study individual effectors in isolation are necessary, although a completely satisfactory method for achieving this is not currently available. Two common approaches are to express effector genes transgenically, either from stably transformed plants expressing the effector gene from an inducible promoter, e.g., in Arabidopsis, or by Agrobacterium-mediated transient transformation of Nicotiana benthamiana leaves [11, 37, 38]. Another popular approach is to transform plant protoplasts with constructs for effector gene expression [39, 40]. These approaches are very powerful, as they allow elicitation of transformed tissue with MAMPs coupled with assays for suppression of defined immune responses by effectors. Transient gene expression methods have the additional benefit of being relatively quick, allowing higher throughput. However, in all cases, the effector is present before the MAMP stimulus—different from how the natural infection is supposed to progress—and super-accumulates to unrealistic levels. Agrobacterium-based transient expression assays have the additional drawback that Agrobacterium has endogenous MAMPs of its own, which might obscure the response to exogenous MAMPs. The use of protoplasts is also controversial, because they are under stress and lack the cell wall which is a hallmark of the plant–bacterial interaction. Nevertheless, all three systems have delivered important data, and results obtained with these systems can be tested independently using genetically altered bacterial strains on intact plants. An alternative strategy for effector delivery is to use P. fluorescens complemented by a TTSS from P. syringae [41], allowing individual delivery of each effector at presumed biological levels. However, this system pairs poorly with most assays of MAMP-triggered immunity. Finally, effectors are not normally delivered individually, and such reductionist experiments might miss informative interactions between effectors.
Virulence targets and search for function: “Here, there, and everywhere”
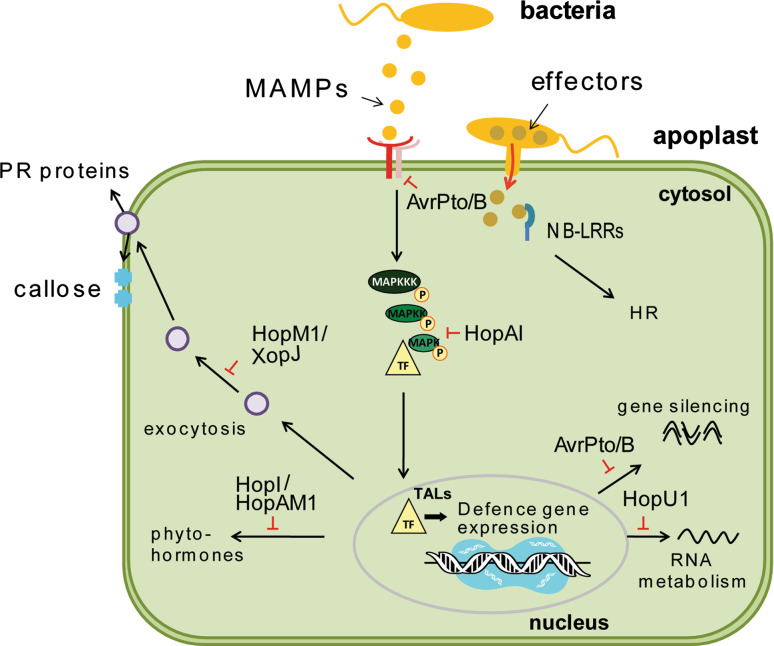
The molecular functions or host virulence targets of most Type-III effectors are poorly defined. We summarize the effectors with known functions and/or targets in Table 1. Probably the best understood effector is AvrPtoB of Pto DC3000 (Fig. 1). This is a multidomain protein with an N-terminal region that is a general kinase-binding domain and a C-terminal ubiquitin ligase domain [42–44]. AvrPtoB suppresses perception of bacterial flagellin and fungal chitin through targeting of their respective receptor kinases FLS2 and CERK1 [30, 45, 46]. Its E3 ligase activity is required for ubiquitination of CERK1 leading to its lysosomal degradation [46]. For suppression of FLS2, the role of the E3 ligase domain is less clear. Several workers showed that the AvrPtoB E3 domain is dispensable for suppression of flagellin responses [11, 47], yet Gohre et al. [45] reported that AvrPtoB ubiquitinates FLS2 for subsequent proteasomal degradation. FLS2 levels were reduced only slightly in the presence of AvrPtoB, whereas CERK1 was completely degraded [45, 46]. In an alternative model, Shan and colleagues reported that AvrPtoB suppresses flagellin signaling by targeting the FLS2 co-factor BAK1, thus preventing complex formation [30]. This model is attractive because it provides an explanation of how AvrPtoB might suppress multiple MAMP-responsive pathways. However, as the FLS2 complex assembles subsequent to MAMP perception but before effector delivery, it is unclear how this model might work in vivo, and additionally, BAK1 levels were unaffected by AvrPtoB expression [45].
Table 1.
Molecular activities of bacterial Type-III effector proteins
| Bacterial strain(s) | Effector | Function | Action on plant cell | Virulence target(s) | Function of target(s) | Ref |
|---|---|---|---|---|---|---|
| Pto DC3000 | AvrPto | Unknown | Stops initiation of MAMP signaling | BAK1, FLS2, EFR and possibly other PRRs | MAMP perception and signaling | [30, 32] |
| Pto DC3000 | AvrPtoB | E3 protein ligase | Stops initiation of MAMP signaling | BAK1, FLS2, EFR, CERK1 and possibly other PRRs | MAMP perception and signaling | [30, 45, 46] |
| Pto DC3000 | HopAI1 | Phosphothreonine lyase | Suppresses MAPK signaling | MAPKs | Kinase activity/MAMP signaling | [49] |
| X. campestris pv. vesicatoria | XopJ | Unknown | Suppresses exocytosis | Unknown | Probably involved in exocytosis | [52] |
| Pto DC3000 | HopM1 | Unknown but initiates target degradation | Suppression of callose deposition | AtMIN7 and others | ARF-GEF involved in exocytosis | [37] |
| Pto DC3000 | HopU1 | ADP-ribosyl transferase (ADP-RT) | Suppression of flagellin-induced callose deposition and cell death | GRP7 | RNA binding protein | [54] |
| Xanthomonas spp. | TAL effectors | Transcription factors | Interfere with host gene transcription | Diverse host genes | Development and immunity | [55–57] |
| X. campestris pv. vesicatoria | XopN | Unknown | Suppression of defense genes | TARK1 | Atypical receptor kinase of unknown function | [66] |
| Pto DC3000 | HopAA1-1 | Potential GTPase-activating protein (GAP) domain | Involved in speck lesion formation | Unknown | Unknown | [67] |
| P. syringae spp. | HopI1 | Putative J domain | Thylakoid remodelling and suppression of SA | Unknown | Unknown | [73] |
| Pto DC3000 | HopAM1 | Unknown | Suppression of ABA and aids adaptation to drought | Unknown | Unknown | [74] |
Fig. 1.
Suppression of defense signaling through effector proteins at different stages of signaling
The Pto DC3000 effector AvrPto, which is structurally unrelated to AvrPtoB, also suppresses flagellin-dependent responses (Fig. 1). Competing models to explain this suppression exist. Constitutive overexpression of AvrPto in stable transgenic Arabidopsis lines causes dwarfing that phenocopies mutants deficient in perception of the growth hormone brassinosteroid (BR). As BAK1 participates in both pathways as a co-factor of FLS2 or the brassinosteroid receptor BRI1, inhibition of BAK1 by AvrPto was a likely hypothesis [30]. Indeed, Shan et al. [30] showed that AvrPto targets BAK1 leading to disruption of the BAK1-FLS2 complex. However, another study showed that AvrPto interacts directly with FLS2, inhibiting its kinase activity and subsequent signaling [32]. It is not possible at this stage to reconcile these models, and in fact they are not exclusive. Shan et al. [30] also found that AvrPto interacted with FLS2, albeit at a lower affinity compared with BAK1 interaction. BAK1 is a plausible target because all known BAK1-dependent MAMP perception systems are suppressed by AvrPto, and AvrPto also suppressed signaling downstream of BR perception [30]. However, the growth defect conferred by constitutive expression of AvrPto in Arabidopsis plants was far greater than the mild bak1 mutant phenotype [8]. Other hormonal pathways involved in plant growth such as auxin were not affected as shown by analysis of gene expression [30]. Despite the discrepancies between the proposed mechanisms it is clear that both AvrPto and AvrPtoB target receptor complexes [11, 30, 38, 46]. In general, it is likely that both effectors target multiple protein kinases, because they also interact with members of the cytosolic Pto kinase family [48]. This observation is of potentially considerable importance because it introduces the idea that effectors may lack specificity and have many host targets.
Disrupting the initial steps of pathogen detection is one strategy to ensure successful infection. However, bacteria rely not only on disruption of MAMP perception but also interfere with downstream components of the signaling cascade. For example, the Pto DC3000 effector HopAI1 was reported to suppress MAPK activation via its phosphothreonine lyase activity (Fig. 1) [49]. However, He et al. [38] observed neither MAPK suppression nor reductions in defense gene expression in the presence of HopAI1. Further studies are necessary to resolve the activities of this effector.
Vesicle transport to the plasma membrane is associated with plant defense against bacteria [50, 51]. Two effectors, namely the Pto DC3000 effector HopM1 and the Xanthomonas campestris pv. vesicatoria effector XopJ interfere with this pathway (Fig. 1) [37, 52]. While the molecular target(s) of XopJ remain(s) to be identified, HopM1 interacts with an adenosine diphosphate (ADP) ribosylation factor–guanine nucleotide exchange factor (ARF–GEF) protein named AtMIN7 (Arabidopsis thaliana HopM interactor 7). ARF–GEFs are well-known components of vesicle trafficking [37]. The importance of AtMIN7 was underlined by studies on knockout mutants which no longer exhibited callose deposition in response to flagellin and allowed increased growth of the TTSS-deficient strain Pto DC3000 hrcC. In addition, AtMIN7 participates in brefeldin A-dependent vesicle formation and localization of the auxin efflux carrier PIN1 [53]. It is as yet unknown which defense compounds require AtMIN7 for trafficking, and it is conceivable that it plays multiple roles in plant immunity.
Another effector which targets a known host protein is HopU1, an ADP-ribosyl transferase (ADP-RT) which interacts with and modifies the RNA-binding protein GRP7 (Fig. 1) [54]. Pto DC3000 requires HopU1 for cell death suppression on N. tabacum, and this is dependent on the ADP-RT catalytic residues of HopU1. Similarly, suppression of flagellin-dependent callose deposition by HopU1 required enzymatic activity. Consistent with these observations, grp7 mutant Arabidopsis lines were more susceptible to both Pto DC3000 and a non-pathogenic hrcC mutant, and deposited less callose in response to flagellin [54]. GRP7 has characteristics of an RNA binding protein, but what is its role in plant immunity? The question remains open; possible roles include acting as an important post-transcriptional regulator of gene expression through the trafficking, stabilization or processing of specific mRNAs. A second important question is the role of ADP-ribosylation in modulating GRP7 function. A final question is whether HopU1 has other targets in host immunity.
A recent important discovery made on the transcription activator-like (TAL) effectors of Xanthomonas spp. should also be mentioned here [55–57]. These effectors activate transcription of host genes directly through a central tandem repeat domain which mediates DNA binding (Fig. 1). Some of the genes activated by TAL effectors appear to be involved in plant development and lead to changes in cell division, cell enlargement, and hypertrophy, thus contributing to disease symptoms [57–60]. However, many more target genes of TAL effectors remain to be identified. The striking discovery is that two hypervariable amino acid residues in each repeat domain specify interaction with a characteristic nucleotide within the effector recognition site. Thus, the amino acid sequence of the tandem repeats predicts the nucleotide sequence of the target DNA binding site with complete accuracy. This enables precise modification of gene expression in vivo using biotechnological methods, including turning this system against Xanthomonas spp. by engineering TAL recognition sites upstream of active resistance genes [57].
The examples above are based on classical studies of plant immunity with a conceptual bias toward linear signal perception, transduction and response pathways. Plants also respond to pathogens through an endogenous mechanism called gene silencing [61, 62]. Gene silencing is mediated by short single-stranded RNA molecules which target complimentary sequences within the genome to downregulate gene expression [63]. One prominent subclass of small RNAs are the so-called microRNAs (miRNAs), which are transcribed and subsequently processed from non-coding genes, and can target one or more coding genes in order to suppress their expression [63, 64]. For example, the endogenous miRNA miR393 is MAMP-induced and contributes to bacterial resistance by interference with auxin signaling [61]. Furthermore, Arabidopsis plants mutated for components of the miRNA silencing machinery were found to be hypersusceptible to adapted and unadapted bacterial pathogens [65]. Several effectors from Pto DC3000, including AvrPto, suppressed expression of MAMP induced and even endogenous miRNA at various steps (Fig. 1). In light of what we know about AvrPto, one might suspect that this effect is due solely to suppression of the MAMP signal at the level of perception [65]. However, Navarro et al. also found that the miRNA precursor molecules were not affected, and that miRNAs involved in endogenous pathways not regulated by MAMPs were likewise downregulated. Therefore, the authors argued that AvrPto might target a component of the silencing machinery directly, or that components of PRR complexes such as BAK1 might somehow cross talk with endogenous silencing pathways [65]. The role of RNA silencing in defense against phytopathogenic bacteria is in its infancy and needs further work to be firmly established.
Most effectors have no detected or defined roles in pathogen virulence. One confounding factor could be the timepoint during infection at which some effectors are active. Bacterial growth is typically measured within the first few days of inoculation between lag phase and stationary phase, and what happens after this is largely disregarded. One interesting example is the effector XopN of X. campestris pv. vesicatoria, which conferred growth benefits that could be measured only after 6 days post-inoculation [66]. XopN targets the Solanum lycopersicum atypical receptor kinase (TARK1) at the plasma membrane. Plants silenced for TARK1 were more susceptible to X. campestris pv. vesicatoria infection. The role of TARK1 in immunity is unknown. As MAMP responses are important in the very early stages of infection, it seems likely that the XopN-TARK1 interaction must play some other role in bacterial pathogenesis.
Other scenarios for absent effector phenotypes are possible. For example, the respective effector might be dispensable for infection of the plant species under investigation, or require additional pathogen-derived molecules for function. Others may play roles in disease symptom formation, such as the Pto DC3000 effector HopAA1-1, which promotes the formation of bacterial speck lesions and carries a potential GTPase-activating protein (GAP) domain [67]. In addition, HopAA1-1 suppresses flagellin-induced NHO1 expression and is localized to mitochondria in yeast [34, 68]. Overall, there are many open hypotheses and this field is only beginning to be explored.
Roles for effectors outside of defense suppression: “Magical mystery tour”
A long-standing hypothesis is that effectors may facilitate nutrient release to aid pathogen growth. However, experimental evidence for such a role is still absent. However, some evidence for alterations in metabolic activities comes from transcriptome studies comparing plant responses to virulent and non-pathogenic (hrcC) bacteria indicating that some genes involved in sugar metabolism and transport were induced by the Pto DC3000 effector repertoire [69–71]. However, this is a descriptive approach, and the role of these genes in host susceptibility has not been validated. Furthermore, these experiments compared intact virulent bacteria with mutant strains lacking the TTSS, which prevents insights into the activities of individual effectors. It would be interesting to perform similar microarray analyses on transgenic plants expressing individual effectors with previously unidentified function, with and without prior MAMP treatment. Interestingly, in a proteomic study, it was found that virulent Pto DC3000 specifically induced the secretion of some enzymes of primary metabolism in Arabidopsis cell cultures [72]. These enzymes were not secreted in response to either the non-virulent Pto DC3000 hrcC mutant or Pto DC3000 carrying AvrRpm1 which induces ETI in this system. Therefore, it is tempting to speculate that bacterial Type III effectors manipulate the host’s secretion machinery to release these enzymes into the intercellular space.
Another potential role of effectors might be to aid environmental adaption of plants. Data supporting this have been presented for two effectors, HopI1 and HopAM1 (Fig. 1) [73, 74]. HopI1 is a ubiquitous P. syringae effector which localizes to the chloroplast where it promotes thylakoid remodeling and suppression of salicylic acid production [73]. The C-terminus of HopI1 has J domain activity which is commonly found in heat shock protein 70 (Hsp70) co-chaperones, and was required for alterations in thylakoid structure. Consistently, HopI1-expressing transgenic plants show increased heat tolerance indicating that HopI1 can engage the plant stress-response machinery. Likewise, HopAM1 appears to aid the adaptation of infected plants to drought and salinity stress by manipulation of ABA signaling [74]. In general, effectors are studied under standard laboratory conditions which might obscure some activities associated with specific environments.
Effector delivery and host cell death: “All together now”
A surprising number of effectors suppress the HR associated with effector recognition [75–77]. However, cell death is not always associated with immunity but can be a consequence of disease. For hemibiotrophic pathogens such as P. syringae, it may be important not only to suppress the HR but also to induce disease-associated cell death late in the infection cycle. It therefore seems logical that such pathogens invest significant resources to keep such cell death-inducing effectors in check. For example, the cell death induced by the Pto DC3000 effector HopAI on N. tabacum was suppressed by at least 26 of the 35 Pto DC3000 effectors [76].To fully understand the interplay of these effectors it will be important to investigate the order and speed they are secreted into the host. What is the timing of effector delivery and does it vary for different effectors?
Perspective: “All I’ve got to do”
Although investigation of the effectors of plant pathogenic bacteria is at a relatively early stage, enough progress has been made to sketch their strategy in a tentative way. The key characteristics are that effectors are redundant and interchangeable. Individual effectors do not impact pathogenicity significantly except in a negative sense, if they are recognized by the host immune system, but collectively are indispensable for pathogenicity. Pairs of unrelated effectors such as AvrPto and AvrPtoB, or HopM1 and AvrE, work redundantly [77]. There is no evidence that individual effectors provide specificity on a particular host, in fact, the opposite may be true. Thus, Pto DC3000, which is not an efficient pathogen on N. benthamiana, can be converted into one by deletion of the recognized effector HopQ1, and hence, the remaining effectors which are not evolved to suppress N. benthamiana defense responses, can do so efficiently [78]. A second emerging theme is the probable lack of specificity of individual effectors, exemplified by AvrPtoB. This effector currently has five recognized cellular protein kinase targets, and likely more because of the high relatedness of the plant protein kinase family. This makes obvious sense for bacteria as generalist or occasional pathogens—to gain the most advantage from each effector, firstly that they have multiple targets, and moreover, that they have enzyme activity so each effector can process multiple targets. The overall strategy behind effector deployment may be a brute force one—to disable the host proteome, particularly the plasma membrane proteome where the PRRs reside, as comprehensively as possible, with little regard to the identity of individual targets. Of course, evolution will select those effector–target interactions that are most beneficial, but bacteria, unlike obligate pathogens, can release such selective pressures by infecting alternate hosts or eschewing the pathogenic lifestyle. We therefore propose that promiscuous, low affinity interactions will be characteristic of binding events between effectors of plant pathogenic bacteria and their host targets.
What remains to be done? We know so little about effector targets, but these will be hard to detect and validate, especially if the model above holds. But this has much to teach us about immune pathways, of which we know equally little. We need to know more about the pathogenic niche, and how the pathogen manipulates cell death and other environmental variables to its advantage. We know nothing about nutrient acquisition and whether effectors act to manipulate this. Lastly, effector detection systems embodied by NB-LRR proteins and their co-factors, which appear to react to the activity of specific effectors, are truly interesting. For these studies and others which we are yet to foresee, we need to invent new approaches and tool kits. To borrow a final line from the Fab Four, “We can work it out”!
Acknowledgments
The authors thank Thomas Boller and Selena Gimenez-Ibanez for critical reviews of the manuscript, and Thomas Boller for support. J.P.R. is an Australian Research Council Future Fellow (FT0992129). D.R.H. is funded by the Suisse National Science Foundation and the Suisse Initiative in Systems Biology (SystemsX: Plant growth in a changing environment).
References
- 1.Melotto M, Underwood W, He SY. Role of stomata in plant innate immunity and foliar bacterial diseases. Annu Rev Phytopathol. 2008;46:101–122. doi: 10.1146/annurev.phyto.121107.104959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 3.Soylu S, Brown IR, Mansfield JW. Cellular reactions in Arabidopsis following challenge by strains of Pseudomonas syringae: from basal resistance to compatibility. Physiol Mol Plant Pathol. 2005;66:232–243. doi: 10.1016/j.pmpp.2005.08.005. [DOI] [Google Scholar]
- 4.Ntoukakis V, Mucyn TS, Gimenez-Ibanez S, Chapman HC, Gutierrez JR, Balmuth AL, Jones AM, Rathjen JP. Host inhibition of a bacterial virulence effector triggers immunity to infection. Science. 2009;324:784–787. doi: 10.1126/science.1169430. [DOI] [PubMed] [Google Scholar]
- 5.Boller T, He SY. Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science. 2009;324:742–744. doi: 10.1126/science.1171647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boller T, Felix G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. doi: 10.1146/annurev.arplant.57.032905.105346. [DOI] [PubMed] [Google Scholar]
- 7.Heese A, Hann DR, Gimenez-Ibanez S, Jones AM, He K, Li J, Schroeder JI, Peck SC, Rathjen JP. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA. 2007;104:12217–12222. doi: 10.1073/pnas.0705306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Wen JQ, Lease KA, Doke JT, Tax FE, Walker JC. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell. 2002;110:213–222. doi: 10.1016/S0092-8674(02)00812-7. [DOI] [PubMed] [Google Scholar]
- 9.Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nurnberger T, Jones JD, Felix G, Boller T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 2007;448:497–500. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
- 10.Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JDG, Felix G, Boller T. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 2004;428:764–767. doi: 10.1038/nature02485. [DOI] [PubMed] [Google Scholar]
- 11.Hann DR, Rathjen JP. Early events in the pathogenicity of Pseudomonas syringae on Nicotiana benthamiana . Plant J. 2007;49:607–618. doi: 10.1111/j.1365-313X.2006.02981.x. [DOI] [PubMed] [Google Scholar]
- 12.Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: shaping the evolution of the plant immune response. Cell. 2006;124:803–814. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Sequeira L. Surface components involved in bacterial pathogen-plant host recognition. J Cell Sci Suppl. 1985;2:301–316. doi: 10.1242/jcs.1985.supplement_2.16. [DOI] [PubMed] [Google Scholar]
- 14.Fett WF, Osman SF, Dunn MF, Panopoulos NJ. Cell-surface properties of Pseudomonas-Syringae pv. phaseolicola wild-type and Hrp mutants. J Phytopathol Phytopathol Z. 1992;135:135–152. doi: 10.1111/j.1439-0434.1992.tb01260.x. [DOI] [Google Scholar]
- 15.Lindgren PB, Peet RC, Panopoulos NJ. Gene cluster of Pseudomonas syringae pv. “phaseolicola” controls pathogenicity of bean plants and hypersensitivity of nonhost plants. J Bacteriol. 1986;168:512–522. doi: 10.1128/jb.168.2.512-522.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin Q, He SY. Role of the Hrp pilus in type III protein secretion in Pseudomonas syringae . Science. 2001;294:2556–2558. doi: 10.1126/science.1066397. [DOI] [PubMed] [Google Scholar]
- 17.Brown IR, Mansfield JW, Taira S, Roine E, Romantschuk M. Immunocytochemical localization of HrpA and HrpZ supports a role for the Hrp pilus in the transfer of effector proteins from Pseudomonas syringae pv. tomato across the host plant cell wall. Mol Plant Microbe Interact. 2001;14:394–404. doi: 10.1094/MPMI.2001.14.3.394. [DOI] [PubMed] [Google Scholar]
- 18.Huynh TV, Dahlbeck D, Staskawicz BJ. Bacterial blight of soybean: regulation of a pathogen gene determining host cultivar specificity. Science. 1989;245:1374–1377. doi: 10.1126/science.2781284. [DOI] [PubMed] [Google Scholar]
- 19.Innes RW, Bisgrove SR, Smith NM, Bent AF, Staskawicz BJ, Liu YC. Identification of a disease resistance locus in Arabidopsis that is functionally homologous to the RPG1 locus of soybean. Plant J. 1993;4:813–820. doi: 10.1046/j.1365-313X.1993.04050813.x. [DOI] [PubMed] [Google Scholar]
- 20.Jenner C, Hitchin E, Mansfield J, Walters K, Betteridge P, Teverson D, Taylor J. Gene-for-gene interactions between Pseudomonas syringae pv. phaseolicola and Phaseolus . Mol Plant Microbe Interact. 1991;4:553–562. [PubMed] [Google Scholar]
- 21.Lan LF, Deng X, Zhou JM, Tang XY. Genome-wide gene expression analysis of Pseudomonas syringae pv. tomato DC3000 reveals overlapping and distinct pathways regulated by hrpL and hrpRS. Mol Plant Microbe Interact. 2006;19:976–987. doi: 10.1094/MPMI-19-0976. [DOI] [PubMed] [Google Scholar]
- 22.Vencato M, Tian F, Alfano JR, Buell CR, Cartinhour S, DeClerck GA, Guttman DS, Stavrinides J, Joardar V, Lindeberg M, Bronstein PA, Mansfield JW, Myers CR, Collmer A, Schneider DJ. Bioinformatics-enabled identification of the HrpL regulon, type III secretion system effector proteins of Pseudomonas syringae pv. phaseolicola 1448A. Mol Plant Microbe Interact. 2006;19:1193–1206. doi: 10.1094/MPMI-19-1193. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira AO, Myers CR, Gordon JS, Martin GB, Vencato M, Collmer A, Wehling MD, Alfano JR, Moreno-Hagelsieb G, Lamboy WF, DeClerck G, Schneider DJ, Cartinhour SW. Whole-genome expression profiling defines the HrpL regulon of Pseudomonas syringae pv. tomato DC3000, allows de novo reconstruction of the Hrp cis clement, and identifies novel coregulated genes. Mol Plant Microbe Interact. 2006;19:1167–1179. doi: 10.1094/MPMI-19-1167. [DOI] [PubMed] [Google Scholar]
- 24.Studholme DJ, Ibanez SG, MacLean D, Dangl JL, Chang JH, Rathjen JP. A draft genome sequence and functional screen reveals the repertoire of type III secreted proteins of Pseudomonas syringae pathovar tabaci 11528. BMC Genomics. 2009;10:395. doi: 10.1186/1471-2164-10-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feil H, Feil WS, Chain P, Larimer F, DiBartolo G, Copeland A, Lykidis A, Trong S, Nolan M, Goltsman E, Thiel J, Malfatti S, Loper JE, Lapidus A, Detter JC, Land M, Richardson PM, Kyrpides NC, Ivanova N, Lindow SE. Comparison of the complete genome sequences of Pseudomonas syringae pv. syringae B728a and pv. tomato DC3000. Proc Natl Acad Sci USA. 2005;102:11064–11069. doi: 10.1073/pnas.0504930102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farrer RA, Kemen E, Jones JD, Studholme DJ. De novo assembly of the Pseudomonas syringae pv. syringae B728a genome using Illumina/Solexa short sequence reads. FEMS Microbiol Lett. 2009;291:103–111. doi: 10.1111/j.1574-6968.2008.01441.x. [DOI] [PubMed] [Google Scholar]
- 27.Lindeberg M, Cartinhour S, Myers CR, Schechter LM, Schneider DJ, Collmer A. Closing the circle on the discovery of genes encoding Hrp regulon members and type III secretion system effectors in the genomes of three model Pseudomonas syringae strains. Mol Plant Microbe Interact. 2006;19:1151–1158. doi: 10.1094/MPMI-19-1151. [DOI] [PubMed] [Google Scholar]
- 28.Reinhardt JA, Baltrus DA, Nishimura MT, Jeck WR, Jones CD, Dangl JL. De novo assembly using low-coverage short read sequence data from the rice pathogen Pseudomonas syringae pv. oryzae . Genome Res. 2009;19:294–305. doi: 10.1101/gr.083311.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hauck P, Thilmony R, He SY. A Pseudomonas syringae type III effector suppresses cell wall-based extracellular defense in susceptible Arabidopsis plants. Proc Natl Acad Sci USA. 2003;100:8577–8582. doi: 10.1073/pnas.1431173100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shan L, He P, Li J, Heese A, Peck SC, Nurnberger T, Martin GB, Sheen J. Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe. 2008;4:17–27. doi: 10.1016/j.chom.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin NC, Martin GB. An avrPto/avrPtoB mutant of Pseudomonas syringae pv. tomato DC3000 does not elicit Pto-mediated resistance and is less virulent on tomato. Mol Plant Microbe Interact. 2005;18:43–51. doi: 10.1094/MPMI-18-0043. [DOI] [PubMed] [Google Scholar]
- 32.Xiang T, Zong N, Zou Y, Wu Y, Zhang J, Xing W, Li Y, Tang X, Zhu L, Chai J, Zhou JM. Pseudomonas syringae effector AvrPto blocks innate immunity by targeting receptor kinases. Curr Biol. 2008;18:74–80. doi: 10.1016/j.cub.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 33.Badel JL, Nomura K, Bandyopadhyay S, Shimizu R, Collmer A, He SY. Pseudomonas syringae pv. tomato DC3000 HopPtoM (CEL ORF3) is important for lesion formation but not growth in tomato and is secreted and translocated by the Hrp type III secretion system in a chaperone-dependent manner. Mol Microbiol. 2003;49:1239–1251. doi: 10.1046/j.1365-2958.2003.03647.x. [DOI] [PubMed] [Google Scholar]
- 34.Badel JL, Shimizu R, Oh HS, Collmer A. A Pseudomonas syringae pv. tomato avrE1/hopM1 mutant is severely reduced in growth and lesion formation in tomato. Mol Plant Microbe Interact. 2006;19:99–111. doi: 10.1094/MPMI-19-0099. [DOI] [PubMed] [Google Scholar]
- 35.Cunnac S, Lindeberg M, Collmer A. Pseudomonas syringae type III secretion system effectors: repertoires in search of functions. Curr Opin Microbiol. 2009;12:53–60. doi: 10.1016/j.mib.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Almeida NF, Yan S, Lindeberg M, Studholme DJ, Schneider DJ, Condon B, Liu H, Viana CJ, Warren A, Evans C, Kemen E, Maclean D, Angot A, Martin GB, Jones JD, Collmer A, Setubal JC, Vinatzer BA. A draft genome sequence of Pseudomonas syringae pv. tomato T1 reveals a type III effector repertoire significantly divergent from that of Pseudomonas syringae pv. tomato DC3000. Mol Plant Microbe Interact. 2009;22:52–62. doi: 10.1094/MPMI-22-1-0052. [DOI] [PubMed] [Google Scholar]
- 37.Nomura K, Debroy S, Lee YH, Pumplin N, Jones J, He SY. A bacterial virulence protein suppresses host innate immunity to cause plant disease. Science. 2006;313:220–223. doi: 10.1126/science.1129523. [DOI] [PubMed] [Google Scholar]
- 38.He P, Shan L, Lin NC, Martin GB, Kemmerling B, Nurnberger T, Sheen J. Specific bacterial suppressors of MAMP signaling upstream of MAPKKK in Arabidopsis innate immunity. Cell. 2006;125:563–575. doi: 10.1016/j.cell.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 39.He P, Shan L, Sheen J. The use of protoplasts to study innate immune responses. Methods Mol Biol. 2007;354:1–9. doi: 10.1385/1-59259-966-4:1. [DOI] [PubMed] [Google Scholar]
- 40.Yoo SD, Cho YH, Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc. 2007;2:1565–1572. doi: 10.1038/nprot.2007.199. [DOI] [PubMed] [Google Scholar]
- 41.Thomas WJ, Thireault CA, Kimbrel JA, Chang JH. Recombineering and stable integration of the Pseudomonas syringae pv. syringae 61 hrp/hrc cluster into the genome of the soil bacterium Pseudomonas fluorescens Pf0–1. Plant J. 2009;60:919–928. doi: 10.1111/j.1365-313X.2009.03998.x. [DOI] [PubMed] [Google Scholar]
- 42.Dong J, Xiao F, Fan F, Gu L, Cang H, Martin GB, Chai J. Crystal structure of the complex between Pseudomonas effector AvrPtoB and the tomato Pto kinase reveals both a shared and a unique interface compared with AvrPto-Pto. Plant Cell. 2009;21:1846–1859. doi: 10.1105/tpc.109.066878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abramovitch RB, Janjusevic R, Stebbins CE, Martin GB. Type III effector AvrPtoB requires intrinsic E3 ubiquitin ligase activity to suppress plant cell death and immunity. Proc Natl Acad Sci USA. 2006;103:2851–2856. doi: 10.1073/pnas.0507892103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janjusevic R, Abramovitch RB, Martin GB, Stebbins CE. A bacterial inhibitor of host programmed cell death defenses is an E3 ubiquitin ligase. Science. 2006;311:222–226. doi: 10.1126/science.1120131. [DOI] [PubMed] [Google Scholar]
- 45.Gohre V, Spallek T, Haweker H, Mersmann S, Mentzel T, Boller T, de Torres M, Mansfield JW, Robatzek S. Plant pattern-recognition receptor FLS2 is directed for degradation by the bacterial ubiquitin ligase AvrPtoB. Curr Biol. 2008;18:1824–1832. doi: 10.1016/j.cub.2008.10.063. [DOI] [PubMed] [Google Scholar]
- 46.Gimenez-Ibanez S, Hann DR, Ntoukakis V, Petutschnig E, Lipka V, Rathjen JP. AvrPtoB targets the LysM receptor kinase CERK1 to promote bacterial virulence on plants. Curr Biol. 2009;19:423–429. doi: 10.1016/j.cub.2009.01.054. [DOI] [PubMed] [Google Scholar]
- 47.Xiao F, He P, Abramovitch RB, Dawson JE, Nicholson LK, Sheen J, Martin GB. The N-terminal region of Pseudomonas type III effector AvrPtoB elicits Pto-dependent immunity and has two distinct virulence determinants. Plant J. 2007;52:595–614. doi: 10.1111/j.1365-313X.2007.03259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang XY, Frederick RD, Zhou JM, Halterman DA, Jia YL, Martin GB. Initiation of plant disease resistance by physical interaction of AvrPto and Pto kinase. Science. 1996;274:2060–2063. doi: 10.1126/science.274.5295.2060. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Shao F, Li Y, Cui H, Chen L, Li H, Zou Y, Long C, Lan L, Chai J, Chen S, Tang X, Zhou JM. A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell Host Microbe. 2007;1:175–185. doi: 10.1016/j.chom.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Robatzek S. Vesicle trafficking in plant immune responses. Cell Microbiol. 2007;9:1–8. doi: 10.1111/j.1462-5822.2006.00829.x. [DOI] [PubMed] [Google Scholar]
- 51.Lipka V, Panstruga R. Dynamic cellular responses in plant-microbe interactions. Curr Opin Plant Biol. 2005;8:625–631. doi: 10.1016/j.pbi.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 52.Bartetzko V, Sonnewald S, Vogel F, Hartner K, Stadler R, Hammes UZ, Bornke F. The Xanthomonas campestris pv. vesicatoria type III effector protein XopJ inhibits protein secretion: evidence for interference with cell wall-associated defense responses. Mol Plant Microbe Interact. 2009;22:655–664. doi: 10.1094/MPMI-22-6-0655. [DOI] [PubMed] [Google Scholar]
- 53.Kleine-Vehn J, Dhonukshe P, Sauer M, Brewer PB, Wisniewska J, Paciorek T, Benkova E, Friml J. ARF GEF-dependent transcytosis and polar delivery of PIN auxin carriers in Arabidopsis . Curr Biol. 2008;18:526–531. doi: 10.1016/j.cub.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 54.Fu ZQ, Guo M, Jeong BR, Tian F, Elthon TE, Cerny RL, Staiger D, Alfano JR. A type III effector ADP-ribosylates RNA-binding proteins and quells plant immunity. Nature. 2007;447:284–288. doi: 10.1038/nature05737. [DOI] [PubMed] [Google Scholar]
- 55.Romer P, Recht S, Lahaye T. A single plant resistance gene promoter engineered to recognize multiple TAL effectors from disparate pathogens. Proc Natl Acad Sci USA. 2009;106:20526–20531. doi: 10.1073/pnas.0908812106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moscou MJ, Bogdanove AJ. A simple cipher governs DNA recognition by TAL effectors. Science. 2009;326:1501. doi: 10.1126/science.1178817. [DOI] [PubMed] [Google Scholar]
- 57.Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, Lahaye T, Nickstadt A, Bonas U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326:1509–1512. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- 58.Kay S, Hahn S, Marois E, Hause G, Bonas U. A bacterial effector acts as a plant transcription factor and induces a cell size regulator. Science. 2007;318:648–651. doi: 10.1126/science.1144956. [DOI] [PubMed] [Google Scholar]
- 59.Sugio A, Yang B, Zhu T, White FF. Two type III effector genes of Xanthomonas oryzae pv. oryzae control the induction of the host genes OsTFIIAgamma1 and OsTFX1 during bacterial blight of rice. Proc Natl Acad Sci USA. 2007;104:10720–10725. doi: 10.1073/pnas.0701742104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang B, Sugio A, White FF. Os8N3 is a host disease-susceptibility gene for bacterial blight of rice. Proc Natl Acad Sci USA. 2006;103:10503–10508. doi: 10.1073/pnas.0604088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, Voinnet O, Jones JD. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science. 2006;312:436–439. doi: 10.1126/science.1126088. [DOI] [PubMed] [Google Scholar]
- 62.Ellendorff U, Fradin EF, de Jonge R, Thomma BP. RNA silencing is required for Arabidopsis defence against Verticillium wilt disease. J Exp Bot. 2009;60:591–602. doi: 10.1093/jxb/ern306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baulcombe D. RNA silencing. Trends Biochem Sci. 2005;30:290–293. doi: 10.1016/j.tibs.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 64.Baulcombe D. RNA silencing in plants. Nature. 2004;431:356–363. doi: 10.1038/nature02874. [DOI] [PubMed] [Google Scholar]
- 65.Navarro L, Jay F, Nomura K, He SY, Voinnet O. Suppression of the microRNA pathway by bacterial effector proteins. Science. 2008;321:964–967. doi: 10.1126/science.1159505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim JG, Li X, Roden JA, Taylor KW, Aakre CD, Su B, Lalonde S, Kirik A, Chen Y, Baranage G, McLane H, Martin GB, Mudgett MB. Xanthomonas T3S effector XopN suppresses PAMP-triggered immunity and interacts with a tomato atypical receptor-like kinase and TFT1. Plant Cell. 2009;21:1305–1323. doi: 10.1105/tpc.108.063123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Munkvold KR, Russell AB, Kvitko BH, Collmer A. Pseudomonas syringae pv. tomato DC3000 type III effector HopAA1–1 functions redundantly with chlorosis-promoting factor PSPTO4723 to produce bacterial speck lesions in host tomato. Mol Plant Microbe Interact. 2009;22:1341–1355. doi: 10.1094/MPMI-22-11-1341. [DOI] [PubMed] [Google Scholar]
- 68.Li X, Lin H, Zhang W, Zou Y, Zhang J, Tang X, Zhou JM. Flagellin induces innate immunity in nonhost interactions that is suppressed by Pseudomonas syringae effectors. Proc Natl Acad Sci USA. 2005;102:12990–12995. doi: 10.1073/pnas.0502425102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thilmony R, Underwood W, He SY. Genome-wide transcriptional analysis of the Arabidopsis thaliana interaction with the plant pathogen Pseudomonas syringae pv. tomato DC3000 and the human pathogen Escherichia coli O157:H7. Plant J. 2006;46:34–53. doi: 10.1111/j.1365-313X.2006.02725.x. [DOI] [PubMed] [Google Scholar]
- 70.de Torres-Zabala M, Truman W, Bennett MH, Lafforgue G, Mansfield JW, Egea PR, Bogre L, Grant M. Pseudomonas syringae pv. tomato hijacks the Arabidopsis abscisic acid signalling pathway to cause disease. EMBO J. 2007;26:1434–1443. doi: 10.1038/sj.emboj.7601575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Truman W, de Zabala MT, Grant M. Type III effectors orchestrate a complex interplay between transcriptional networks to modify basal defence responses during pathogenesis and resistance. Plant J. 2006;46:14–33. doi: 10.1111/j.1365-313X.2006.02672.x. [DOI] [PubMed] [Google Scholar]
- 72.Kaffarnik FA, Jones AM, Rathjen JP, Peck SC. Effector proteins of the bacterial pathogen Pseudomonas syringae alter the extracellular proteome of the host plant, Arabidopsis thaliana . Mol Cell Proteomics. 2009;8:145–156. doi: 10.1074/mcp.M800043-MCP200. [DOI] [PubMed] [Google Scholar]
- 73.Jelenska J, Yao N, Vinatzer BA, Wright CM, Brodsky JL, Greenberg JT. A J domain virulence effector of Pseudomonas syringae remodels host chloroplasts and suppresses defenses. Curr Biol. 2007;17:499–508. doi: 10.1016/j.cub.2007.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goel AK, Lundberg D, Torres MA, Matthews R, Akimoto-Tomiyama C, Farmer L, Dangl JL, Grant SR. The Pseudomonas syringae type III effector HopAM1 enhances virulence on water-stressed plants. Mol Plant Microbe Interact. 2008;21:361–370. doi: 10.1094/MPMI-21-3-0361. [DOI] [PubMed] [Google Scholar]
- 75.Jamir Y, Guo M, Oh HS, Petnicki-Ocwieja T, Chen S, Tang X, Dickman MB, Collmer A, Alfano JR. Identification of Pseudomonas syringae type III effectors that can suppress programmed cell death in plants and yeast. Plant J. 2004;37:554–565. doi: 10.1046/j.1365-313X.2003.01982.x. [DOI] [PubMed] [Google Scholar]
- 76.Guo M, Tian F, Wamboldt Y, Alfano JR. The majority of the type III effector inventory of Pseudomonas syringae pv. tomato DC3000 can suppress plant immunity. Mol Plant Microbe Interact. 2009;22:1069–1080. doi: 10.1094/MPMI-22-9-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kvitko BH, Park DH, Velasquez AC, Wei CF, Russell AB, Martin GB, Schneider DJ, Collmer A. Deletions in the repertoire of Pseudomonas syringae pv. tomato DC3000 type III secretion effector genes reveal functional overlap among effectors. PLoS Pathog. 2009;5:e1000388. doi: 10.1371/journal.ppat.1000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei CF, Kvitko BH, Shimizu R, Crabill E, Alfano JR, Lin NC, Martin GB, Huang HC, Collmer A. A Pseudomonas syringae pv. tomato DC3000 mutant lacking the type III effector HopQ1–1 is able to cause disease in the model plant Nicotiana benthamiana . Plant J. 2007;51:32–46. doi: 10.1111/j.1365-313X.2007.03126.x. [DOI] [PubMed] [Google Scholar]