Abstract
Together with the estrogen receptor (ER) alpha, estrogen receptor beta (ERβ) mediates many of the physiological effects of estrogens. As ERβ is crucially involved in a variety of important physiological processes, its activity should be tightly regulated. ERβ regulation is achieved by hormone binding as well as by posttranslational modifications of the receptor. Furthermore, ERβ expression levels are under circadian control and can be regulated by DNA methylation of the ERβ promoter region. There are also a number of factors that can interfere with ERβ activity, such as phytoestrogens, endocrine disruptive chemicals, and growth factors. In this article, we outline different mechanisms of ERβ regulation and how they are implicated in various diseases. We also discuss how these insights might help to specifically target ERβ in drug design.
Keywords: Estrogen receptor beta, Phytoestrogens, Endocrine disruption, Circadian regulation, DNA methylation, Cancer, Diabetes type 2
Introduction
Many of the physiological actions of estrogens are mediated by the two estrogen receptor (ER) subtypes, alpha and beta (ERα and ERβ) that belong to the nuclear receptor (NR) superfamily. The ERs are not only indispensable for development and function of the female reproductive organs, but also play important roles in, e.g., male reproduction and maintenance of bone mass, as well as in the cardiovascular, central nervous, and immune systems [1, 2]. The ERs are transcribed from different genes located on separate chromosomes, and display discrete expression patterns as well as distinct ligand specificities. It has become clear that ERβ has functions that are distinct from those of ERα. This is, for example, illustrated by the fact that mice lacking ERβ (βERKO) display a very different phenotype than those devoid of ERα (ERKO). Analyses of these mice have shown that ERα is the main player in mediating female reproductive functions whereas ERβ is more important in non-classical target tissues, such as prostate, colon, and cardiovascular and central nervous systems. βERKO mice develop hypertension and malignancies in colon and prostate, and show increased anxiety [1].
Involvement of ERβ in these processes implies that its activity should be tightly regulated. This is achieved on several levels. First of all, ERβ acts as a classical ligand-induced transcription factor. Like ERα, it is activated by estrogens, of which 17-β-estradiol (E2) is the main form in humans. Many exogenous compounds are known to compete with estrogen binding, including plant-derived substances (phytoestrogens), man-made chemicals, and drugs targeting the ERs. These compounds can either act as agonists or antagonists, thus activating or inhibiting ER function. Additionally, a number of chemicals interfere with ER signaling without direct binding. Furthermore, ERβ function is regulated at the transcriptional as well as at the posttranslational level. Posttranslational modifications have been shown to fine tune ERβ activity, both in the absence and presence of E2. This is an important mechanism by which other signals, such as growth factors, can modulate ER transcriptional activation. Finally, ERβ expression has been shown to be regulated both by members of the circadian machinery and by DNA methylation of its promoter.
In this article, we will outline the different mechanisms by which ERβ activity is regulated and will discuss how they are implicated in common disorders and diseases. In the first four sections, we will illustrate how ERβ activity is affected by endogenous and exogenous signals; in the last two sections, we will discuss how ERβ protein levels can be regulated.
The estrogen receptor beta: structure and signaling upon hormone induction
ERβ structure
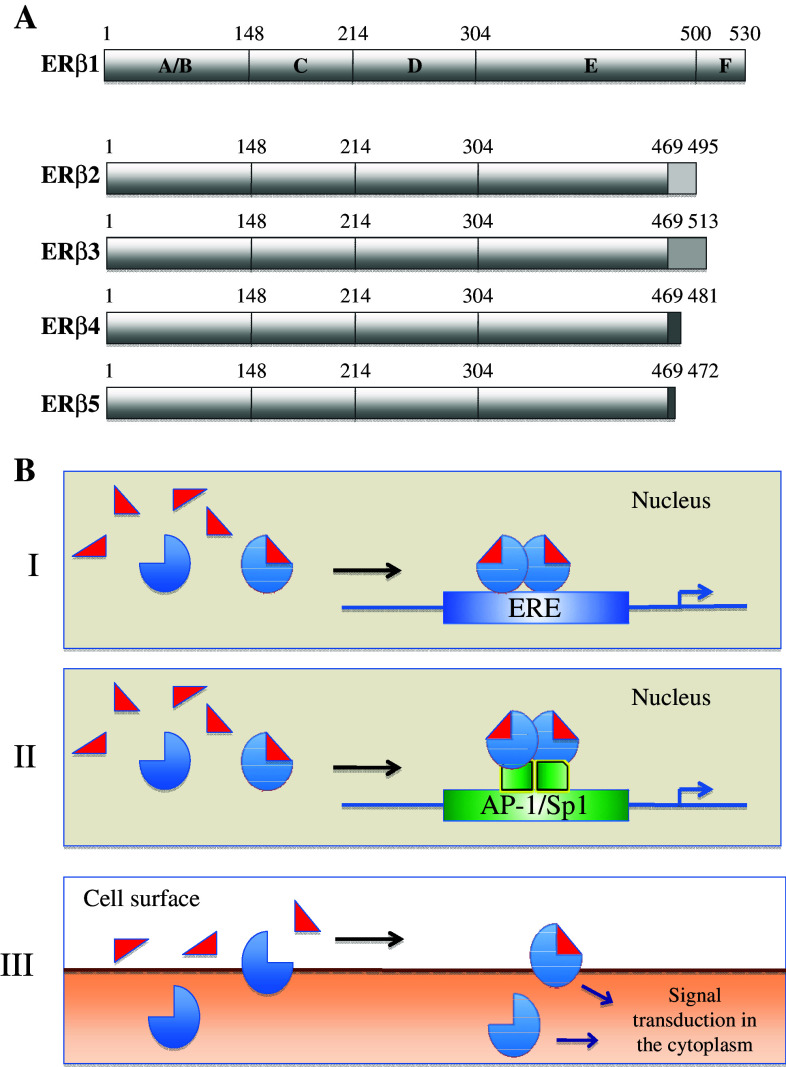
Like all the NR family members, ERβ displays a modular structure consisting of six functional domains labeled A–F (Fig. 1a). The A/B domain that is most variable between the NR family members is located in the amino terminal part of the protein. This domain mediates transcriptional activation through recruitment of coactivator proteins. ERβ was long suspected to lack a functional transcriptional activation function; however, recent experiments have demonstrated that indeed the A/B domain of ERβ does interact with certain transcriptional coactivators [3, 4]. Adjacent to the A/B domain lies the highly conserved C-domain containing the DNA-binding function, followed by the flexible D-domain, which acts as a “hinge” between the C and E domains and also comprises a nuclear localization signal. The E-domain mediates ligand binding and contains a dimerization interface and an additional nuclear localization signal. Although the E domains of ERα and ERβ are only 56% identical at the amino acid level [3], both ERs bind estradiol with high affinity [5]. However, the existing differences provide a basis for distinct responses to certain compounds, i.e., subtype-selective ligands [6]. Finally, the F domain resides in the carboxy terminus of the ER, which seems to have a complex regulatory role [2]. Both ERs display two activation functions, AF-1 in the A/B domains and AF-2 in the E domain, and synergy between these two functions leads to full transcriptional activity [7].
Fig. 1.
ERβ structure and signaling mechanisms. a Domain structure of full-length ERβ (ERβ1) and its isoforms ERβ2–5. b I “Classical” ER activity through direct binding to estrogen response elements (EREs); II activated ERs signal through protein–protein interactions with other transcription factors, such as AP-1 or Sp1; III non-genomic activity involves other signal transducers and causes rapid responses
To date, five full-length ERβ splice variants have been described in human, ERβ1–5 [8, 9], reviewed in, e.g., [2]. They arise from differential splicing of the last exon (Fig. 1a). ERβ1 (mostly referred to as ERβ) is the full-length receptor consisting of 530 amino acids coded by exons 1–8. ERβ2–5 share exons 1–7 with ERβ1 but display unique sequences instead of exon 8. These differences in the C-terminal part of ERβ2–5 result in a truncation of the LBD and ablation of the AF2. Thus, ERβ1 is the only isoform that is able to bind ligand. In contrast to ERβ1, ERβ2, 4, and 5 cannot form functional homodimers [10]. Nevertheless, they have been shown to heterodimerize with both ERβ1 and ERα, the latter resulting in inhibition of ERα function [2]. ERβ3 expression is thought to be restricted to testis and functional studies using this isoform are lacking. Interestingly, changed ratio of these splice variants have been found in several cancers, e.g., in breast (reviewed in [11]) and in ovary [12]. However, results regarding the correlation of ERβ isoform expression and parameters such as tumor progression, success of treatment, and relapse have been contradictory as yet.
ERβ signaling
ER signaling in response to hormone can be divided into two discrete modes of action, often referred to as genomic and non-genomic activity (reviewed in [13]). The classical, genomic ER signaling occurs through direct binding of ligand-bound ER dimers to ERE sequences in the regulatory regions of estrogen responsive genes (Fig. 1b I). The ERE consensus sequence consists of two half-sites and two 5-base pair inverted repeats, separated by any three base pairs, GGTCAnnnTGACC. EREs can be located both at the proximal promoter regions [14] and far away from the transcriptional start site [15]. Another type of genomic activity occurs through an indirect association with promoters by protein–protein interactions with other transcription factors, such as AP-1 or Sp1 [16] (Fig. 1b II). Both ERα and ERβ are able to modulate gene expression by either classical ERE-mediated signaling, or by interacting with other transcription factors like AP-1 and Sp1.
Upon ligand binding, the LBD undergoes a structural change to provide a binding surface for cofactors such as p160 coactivators [17]. Crystallographic studies have revealed that the LBDs of both ERs have a similar structural design where the AF-2 interaction surfaces are exposed when ER agonists are present [17]. Both in the absence and presence of ligand, ERs exist as multiprotein complexes with coregulator molecules. The coregulators can either stimulate or inhibit transcription; hence, they are referred to as coactivators (e.g., SRC1, TIF2, AIB1) or corepressors (e.g., NCoR, SMRT), respectively. For ERα, it has been shown that recruitment of the receptor as well as complexes of coactivators to EREs occurs in cycles [18]. Furthermore, chromatin remodeling events as well as rapid DNA methylation and demethylation accompanies the cycling of productive transcriptional complexes on active ERα-regulated promoters [18–20].
The second mode of action, referred to as non-genomic activity, has been shown to involve ER interactions with cytoplasmatic signal transduction proteins, such as MAPK, Stats, and members of the Src family of tyrosine kinases [21–23] (Fig. 1b III). This activity is characterized by rapid effects in response to E2, and membrane-localized ERs have been reported to mediate this signal transduction [24, 25].
Modulation of ERβ activity by phytoestrogens
Phytoestrogens are plant-derived di- or poly-phenolic compounds that are structurally similar to estrogens and thus induce estrogenic responses in mammals. The main classes of phytoestrogens are the flavonoids, lignans, and coumestans. Within the flavonoid class, the two major subgroups are the flavones and isoflavones. The flavone group consists of flavones (apigenin: found in celery and parsley), flavonols (quercetin and kaempferol: found in onion and leafy green vegetables), and flavanones (naringenin and liquiritigenin: found in grapefruit and liquorice). The isoflavones consist of genistein, daidzein, and glycitein, which are found in soybeans and soy products, while formononetin (plant precursor of daidzein) and biochanin A (plant precursor of genistein) are abundant in clover. In addition to the parent compounds, isoflavone metabolites have also demonstrated estrogenic properties including equol and O-desmethylangolensin (O-DMA), gut microbe-derived metabolites of daidzein, and also the glyceollins and phytoalexins synthesized in the plant in response to stress. Among the flavonoids, the isoflavone genistein has been studied most extensively for its potential role in modulating ER signaling and human health. The lignans consist of secoisolariciresinol, matairesinol, pinoresinol, sesamin, and lariciresinol, to name a few, are abundant in cereals and oilseeds, as well as in various fruits and vegetables. These compounds, are converted by the gut microflora to the mammalian lignans, enterolactone and enterodiol, which have demonstrated weak estrogenic properties. Within the coumestan class, coumestrol is the most potent phytoestrogen and it is found in alfalfa, clover, sprouts, and legumes.
Phytoestrogen binding and activation of ERβ
It was reported more than 10 years ago that phytoestrogens can bind and activate the ER. Of particular interest was the finding that many phytoestrogens bind with a higher affinity to ERβ compared to ERα, suggesting that they may induce physiological effects through this ER subtype [26]. However, because of their chemical structural differences, not all phytoestrogens have equal binding affinities for ERβ. Since there are many different experimental models used to assess binding affinity, as well as the use of different phytoestrogens and their metabolites, it is difficult to compare the relative potencies of different phytoestrogens for ERβ. In one study using radioligand binding assay, the order of the relative binding affinity for ERβ was coumestrol > genistein > apigenin > kaempferol > daidzein > naringenin > quercetin > formononetin = biochanin A [26]. In another study using fluorescence polarization technology, the order of potencies for binding to ERβ was coumestrol > genistein > equol > and enterolactone (ENL) [27]. Using both binding assays, phytoestrogens bound with a greater affinity to ERβ, with the exception of ENL which, albeit weak, had a higher affinity for ERα [27, 28]. Importantly, it has been demonstrated that at least ENL requires cellular transformation to become a high affinity ERα ligand; however, the molecular mechanisms and factors involved in this conversion are still not known [28].
Although ER ligand binding may be the first step in the activation of the signaling pathway, the degree to which a compound binds to the ER may not necessarily reflect its ability to activate ER signaling. For example, using human 293 embryonic kidney cells transiently transfected with ERα or ERβ, as well as an ERE-luciferase reporter gene, the order of the relative transcriptional potencies of the phytoestrogens through ERβ was genistein > coumestrol > daidzein > biochanin A = kaempferol > apigenin > naringenin > formononetin [26]. Here, it can be noted that while biochanin A had the weakest affinity for binding to ERβ, it was a stronger inducer of ERβ transcriptional activation compared to other phytoestrogens with greater binding affinities, highlighting that binding affinity alone does not necessarily predict the potency of a phytoestrogen to activate ERβ signaling. Furthermore, it should be noted that the phytoestrogen concentrations required to activate transcription through both ERα and ERβ are generally less than 1 μM, which are concentrations physiologically achievable in humans after consumption of phytoestrogen-rich foods or supplements. This indicates that while these compounds can activate ERβ signaling at lower concentrations than required to activate ERα, in tissues expressing both ER subtypes, activation of both ERα and ERβ may occur.
Factors contributing to the effects of phytoestrogens on ERβ
There are many factors which could modulate the ability of phytoestrogens to induce ERβ-mediated effects, including the expression of coregulatory proteins in various tissues, the gene and promoter context, the expression levels of ERα and ERβ, the formation of homo- and heterodimers, and the hormonal milieu of the tissue. The differences in chemical structures of phytoestrogens result in differences in the ER tertiary structure induced upon ligand binding [29]. Since the conformational change results in the exposure of ER regions capable of binding various coregulatory proteins, different phytoestrogens may result in the recruitment of different coregulatory proteins [30]. For example, genistein has been shown to induce a conformational change to ERβ that differs from that of E2 [29]. Genistein also causes the recruitment of different coregulatory proteins depending on the ER subtype expressed [27, 30–32]. For example, in cells only expressing ERα, low dose genistein (6 nM) does not recruit RIP140; however, it does when cells coexpress ERα and ERβ [31]. Liquiritigenin, a flavanone found in liquorice plants, also recruits coactivator proteins in an ER subtype-specific manner. Even though this phytoestrogen can bind with similar affinity to both ER subtypes, it selectively activates transcription through ERβ and specifically recruits SRC-2 to ERβ. Additionally, genistein can induce ERα and ERβ homo- and heterodimers, while liquiritigenin can induce ERβ homodimers and ERα/β heterodimers but not ERα homodimers [33]. Thus, depending on the phytoestrogen and the level of ERα and ERβ coexpression, the effects induced by these phytoestrogens may differ. Another potential modulator of phytoestrogen action through ERβ may be the level of ERβ isoforms (ERβ1–5) expressed in various tissues. It has been shown that, while ERβ1 is fully functional, the other isoforms must heterodimerize with ERβ1 in order to activate transcription [10]. While E2 is capable of inducing ERβ isoform heterodimers, the phytoestrogens, genistein and apigenin, do not. Genistein and apigenin were shown only to induce ERβ1 homodimers. Therefore, depending on the expression of the ERβ isoforms, different physiological effects may occur upon stimulation by phytoestrogens.
Although many studies have been conducted to determine the potential effects of various phytoestrogens on ERβ-mediated responses in vitro, in vivo the reality is that these compounds, once consumed, are rapidly metabolized and biotransformed. The resulting metabolites (hydroxylated and reduced forms, and sulfuric and glucuronic acid conjugates) may induce different effects in vivo compared to their phytoestrogen parent compounds. One study showed, for example, that daidzein, its gut metabolites (equol and O-DMA) and sulfinated conjugates (daidzein-7-sulfate, daidzein-4-sulfate, diadzein-4,7-disulfate) all have different abilities to activate ERβ-mediated transcription [34]. Daidzein was the strongest inhibitor of ERβ-transfected Hela cell growth, followed by equol, then daidzein-7-sulfate, while O-DMA, daidzein-4-sulfate, and diadzein-4,7-disulfate did not have an effect on cell growth in these cells. It is now well known that only 30% of the population bears the specific gut microflora capable of converting daidzein to equol [35]. Thus, there may be a wide range of daidzein metabolites and conjugates circulating in different humans. Similarly, differential binding affinities were observed for genistein and its conjugates with genistein-4-sulfate having a stronger affinity for ER binding than genistein, followed by genistein-7-sulfate [36]. Thus, depending on how daidzein and genistein are metabolized in vivo, the effects on ERα- and ERβ-mediated responses, and resulting physiological effects, may differ.
Another important factor which can determine the physiological effects of phytoestrogens is the enantiomeric state of the compound. Equol, for example, can exist as S- and R-stereoisomers. It has been shown that the R-enantiomer has a higher binding affinity and is a selective transcriptional activator of ERα, while the S-enantiomer has a higher binding affinity for ERβ, but equally transactivates ERα and ERβ [37]. In mice administered a racemic mixture of R- and S-equol, significant uterotropic and vaginal epithelium growth were observed, which are indicative of an ERα response [38]. On the other hand, a clear increase in vaginal mucification was induced by equol, which has been suggested to demonstrate an ERβ-mediated effect [39]. Since only the S-equol isomer has been detected in human serum after consumption of a soy-based food [40], experimental studies utilizing racemic mixtures, or the R-enantiomer of equol, may demonstrate physiological effects that do not reflect those that could occur in humans.
Although it is quite clear that individual purified phytoestrogens and their metabolites can bind and activate ERβ, human exposures to phytoestrogens do not represent single compounds, but a mixture of different phytoestrogens and metabolites. These mixtures may induce different physiological effects compared to a single phytoestrogenic compound. With regards to ERβ signaling, it was shown that when genistein, daidzein, and equol were combined (a natural mixture of phytoestrogens after consumption of soy-based foods) the selectivity for ERβ binding increased, compared to genistein alone [41]. It has also been shown that by combining different phytoestrogens (coumestrol and enterolactone) the number of regulated genes in MCF-7 breast cancer cells dramatically increased compared to the same concentration of either phytoestrogen alone [42]. Since MCF-7 cells contain primarily ERα, the effects of such combinations in cells coexpressing ERα and ERβ may also display differential gene expression patterns when exposed to mixtures of different phytoestrogens. To more accurately reflect human exposures to phytoestrogens, further studies are required to determine the role of phytoestrogen mixtures on ER signaling.
Phytoestrogens, ERβ, and cancer
Colon cancer
The role of ERβ in colon carcinogenesis has received much attention in recent years. This is because, in the normal colon mucosa, ERβ is the prevalent ER subtype expressed, while in colon tumors, ERβ expression is considerably reduced [43, 44]. Thus, it is possible that compounds that can promote the expression and activation of ERβ signaling in the colon may help to reduce the incidence of colon cancer. In vitro, a mixture (1:1:0.2) of soy isoflavones (genistein, daidzein, and glycetin) was found to reduce DLD-1 colon cancer cell growth, which was also accompanied by an increase in ERβ gene and protein expression [45]. Treatment of HT29, Colo320, Lovo, and SW480 colon cancer cells (all of which express ERβ) with genistein also resulted in a reduction of cell growth in all cell lines, with the exception of SW480 [46]. Furthermore, rats exposed to low (40 mg/kg) or high (1,000 mg/kg) dietary soy isoflavones throughout life (in utero and postnatally) and subsequently exposed to the colon carcinogen azoxymethane (AOM) in adulthood, had reduced colon tumor size and burden and enhanced ERβ expression [45]. This demonstrates the potential for isoflavones to reduce colon carcinogenesis, which may potentially be through the modulation of ERβ signaling. Similar effects were observed in an earlier study in which rodents were fed soy protein throughout life; however, when exposure was limited to in utero, a significant increase in tumor multiplicity occurred [47]. This may potentially demonstrate the importance of timing of exposure to phytoestrogens in the effects induced on colon carcinogenesis.
Breast cancer
With regards to the treatment of breast cancer, the role of phytoestrogens has been the topic of considerable debate [48]. It is well known that ER-positive breast cancers are stimulated by estrogens; thus, exposure to phytoestrogens may also enhance tumor growth (as summarized in Table 1). In vitro experiments, in which ER-positive human breast cancer cells are treated with various phytoestrogens, including isoflavones genistein [49], daidzein, and equol [50], flavone apigenin [51–53], and mammalian lignan enterolactone [54], all enhance cell growth through ER-mediated mechanisms, at physiological relevant doses. In vivo, this phenomenon has also been observed for the isoflavone genistein. In mice bearing MCF-7 breast tumors, genistein, as well as isoflavone-rich soy protein, enhances tumor growth, and interferes with the tumor inhibitory effects of the antiestrogen, tamoxifen [55–57]. Furthermore, a soy supplement (containing 45 mg isoflavones) given to women for 2 weeks enhanced nipple aspirate pS2 expression, which is indicative of an estrogenic response in the breast, demonstrating estrogenic effects in humans as well as rodents [58]. These findings have raised concern about the consumption of phytoestrogens by breast cancer patients, especially postmenopausal women, in which endogenous estrogens are low and thus tumor growth could be stimulated by phytoestrogens. On the other hand, using similar in vivo mouse models, isoflavones daidzein and equol [50], the mammalian lignans [59], and the flavanone liquiritigenin [60] induced either no or very weak tumor stimulatory effects, demonstrating that the ER modulating effects observed in vitro do not always translate to the effects observed in vivo.
Table 1.
Summary of the effects of selected phytoestrogens on hormone sensitive human breast cancer growth in vitro and in vivo
| Phytoestrogen | ER binding preference | In Vitro cell-based models | In Vivo effects |
|---|---|---|---|
| Isoflavones and metabolites | |||
| Genistein | ERβ [26] | Stimulates MCF-7 and T47D cell growth [49, 52, 61]; Reduces the growth of T47D cells overexpressing ERβ [64] | Stimulates MCF-7 tumor growth in athymic nude mice [55] |
| Daidzein | ERβ [26] | Stimulates MCF-7 cell growth [36] | Weak stimulator of MCF-7 tumor growth in athymic nude mice [50] |
| (±)Equol | ERβ [37] | Stimulates MCF-7 cell growth [36] | Does not stimulate the growth of MCF-7 tumors [50] |
| Flavones | |||
| Apigenin | ERβ [5] | Stimulates MCF-7 [53], T47D cell growth [52]; Reduces MDA-MB-231 cell growth through ERβ [51] | No studies conducted |
| Quercetin | ERβ [26] | Stimulates MCF-7 and T47D cell growth [61] and reduces growth of MDA-MB-231; Reduces the growth of T47D cells overexpressing ERβ [61] | No studies conducted |
| Naringenin | ERβ [26] | Stimulates MCF-7 growth [53] | No studies conducted |
| Liquiritigenin | Similar for ERα and ERβ; ERβ activator only [60] | No studies conducted | Does not stimulate the growth of MCF-7 tumors [60] |
| Lignan metabolite | |||
| Enterolactone | ERα [27, 28] | Stimulates MCF-7 cell growth [54] | Does not stimulate the growth of MCF-7 tumors [59] |
The tumor growth stimulatory effects of phytoestrogens in breast cancer cells and genistein in breast tumors have been attributed to the activation of ERα. MCF-7 and T47D cells have much higher ERα expression compared to ERβ, and thus the physiological effects of phytoestrogens acting through ERβ may not be demonstrated in these breast cancer models. Since phytoestrogens can bind and activate both ER subtypes in a concentration-dependent manner, it has been hypothesized that, depending on the tissue expression levels of ERα and ERβ, different physiological effects may result upon exposure to different phytoestrogens. In stably transfected T47D cells, in which ERβ expression is regulated by tetracycline, the effects of phytoestrogens genistein and quercetin differs depending on the level of ER expression [61]. When ERβ expression is low, the phytoestrogens stimulate cell growth; however, when ERβ expression is high, the growth stimulatory effects were negated, with quercetin inducing the greatest effect. This study, as well as others [62–64], demonstrates the ability of ERβ to modulate the proliferative effects induced by ERα. Thus, phytoestrogens may have more beneficial effects in breast cancer patients in which tumors express ERβ, more so than those which express predominantly ERα.
Endocrine disruptive chemicals interfering with ERβ activity
Some environmental contaminants have the potential to interfere with the endocrine system, a scenario referred to as endocrine disruption. This phenomenon has raised worldwide concern during the last decades. Endocrine disruptive chemicals (EDCs) pose a documented risk to wildlife and ecosystems. In addition, EDCs have been shown to exert numerous hormone disruptive activities in vitro and in experimental animals. These factors, in combination with the increased incidence in certain endocrine-related human diseases, have led to the assumption that EDCs are also potential health threats in humans [65].
EDCs targeting ERβ
The ligand-binding cavity of the ERs can interact with a wide variety of structurally diverse compounds, which leads to susceptibility for EDC action. There are several known examples of xenoestrogens that are widespread in the environment and have been found in human samples. Plasticizers, detergents, herbicides, and pesticides are classes of chemicals that have been shown to act disruptively on estrogen signaling in vivo, including methoxychlor, chlordecone, octylphenol, bisphenol A and B, nonylphenol, and ethynyl estradiol. In a recent review, the effects of several EDCs on the female reproductive system in mammals were summarized and discussed [66]. The author concludes that many EDCs disturb ovarian and uterine functions by targeting steroidogenesis pathways, but the effects are highly variable and both ER-dependent and -independent. For most in vivo data of EDCs affecting estrogenic signaling, the molecular mechanisms remain largely unknown. In particular, the involvement of ERβ is unexplored. In many cases where the effects seem to be receptor-mediated, the focus has either been on ERα, or the ERα- and ERβ-mediated activities have not been assessed separately. However, many in vitro studies (ligand-binding assays, reporter gene assays, etc.) have demonstrated that several xenoestrogens act more potently via ERβ than via ERα. For instance, in a screening of xenoestrogenic and phytoestrogenic substances, Kuiper et al. [26] reported relatively high binding affinities of several of these compounds for ERβ, in comparison with ERα. Also, Balaguer and coworkers [67–70] have performed multiple screens of xenoestrogens in reporter cell lines, where the ERβ-mediated activities have been assessed. In the following section, we will discuss selected EDCs acting on the estrogenic signaling in mammals and where ERβ involvement has been suggested (summarized in Table 2).
Table 2.
Effect of EDCs on ERβ function
| EDC | Effects on ERβ | References |
|---|---|---|
| PCBs | Estrogenic in vitro | [30, 78–81, 94] |
| PCB3 | Porcine ovary: ERβ protein ↓ | |
| Aroclor in utero (rat) | Brain (AVPV): ERβ protein ↓ | |
| Biphenyls | Breast cancer cells: ERβ mRNA ↑ | |
| TCDD | Anti-estrogenic in vitro | [4, 84] |
| PBDE-99 in utero (rat) | Uterus (offspring): ERβ mRNA ↓ | [88, 89] |
| PBDEs in fish liver oil | Isolated pig follicles: ERβ protein ↓ | |
| BPA | ERβ binding in vitro | [94–97, 99, 100] |
| BPA in utero (rat) | Uterus (offspring): ERβ protein ↓ | |
| BPA neonatally (rat) | Behavioral effects in adults (via ERβ) | |
| MXC in puberty (female monkeys) | Antagonism of ERβ → impaired cognitive functions | [105, 106] |
| MXC in utero (rat) | Follicles in adults: ERβ protein ↓ | |
| Phthalates | Antagonists in vitro | [107, 109] |
| DBP (rat) | Testis: ERβ inhibited |
EDC Endocrine disruptive chemical, PCB polychlorinated biphenyls, TCDD 2,3,7,8-tetrachlorodibenzo-p-dioxin, PBDE polybrominated diphenylethers, BPA bisphenol A, MXC methoxychlor, DBP di(n-butyl) phthalate
Polychlorinated biphenyls
Polychlorinated biphenyls (PCBs) are environmental contaminants that have been found at high levels in human samples, like adipose tissue, breast milk, and ovarian follicle fluid [71–73]. Depending on their chemical structure, PCBs may act disruptive on estrogen, androgen, or thyroid hormone receptors and have been demonstrated to exert numerous adverse effects on reproduction, the immune system, and development [74–77].
In porcine ovaries, PCB3 was found to downregulate expression of ERβ while no effect was seen on ERα levels [78]. This suggests that PCB3 may act as an agonist of ERβ, which is predominant in the ovary. This report is in line with data showing that certain xenoestrogens are more potent on ERβ than on ERα, measured by coactivator recruitment and transcriptional activity [30]. Cappelletti et al. [79] reported that ERβ mRNA levels were upregulated in breast cancer cells in response to several xenoestrogens (e.g., biphenyls) while ERα expression did not change. The ERβ increase was dependent on the presence of ERα. These results support a role for ERβ in mediating xenoestrogenic effects and in controlling ERα transcriptional activities, also observed by others [62, 63].
Aroclor 1221 is a commercial PCB mixture and has been shown to exert mainly estrogenic activities [80]. Salama et al. [81] investigated the neurobiological effects in rats prenatally exposed to Aroclor 1221. The effects on ERβ in the anteroventral periventricular nucleus (AVPV), a sexually dimorphic region in the brain that controls reproduction, were assessed. In Aroclor-treated rats, the expression of ERβ in AVPV was strongly inhibited, resulting in a masculinizing effect. These results indicate that low-dose exposure to Aroclor and related compounds, via ERβ, has the potential to disturb reproductive success.
Polychlorinated dibenzo-p-dioxins
Polychlorinated dibenzo-p-dioxins (PCDDs) and dibenzofurans (PCDFs) are byproducts from various industrial processes and incineration reactions. These pollutants are omnipresent in the environment and ingested primarily through contaminated food. The most toxic of the PCDD congeners, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), has been found in human blood, adipose tissue, and breast milk. TCDD acts through the aryl hydrocarbon receptor (AhR) and has anti-estrogenic effects in vitro and in vivo, suggesting that a complex interplay between AhR-ER pathways exists (reviewed in [82]). Intriguingly, results from our group show that the anti-estrogenic effects of TCDD are much stronger on ERβ- than on ERα-mediated activities, indicating subtype-selective mechanisms are involved [4, 83]. In addition, Kietz and colleagues [84] reported that TCDD inhibits ERα-mediated upregulation of ERβ mRNA in breast cancer cells, suggesting again that TCDD affects ERβ activity more severely than that of ERα. However, the physiological outcome of TCDD’s interference with ERβ is not clear as most studies have studied the effects of TCDD on ERα.
Polybrominated diphenylethers
Polybrominated diphenylethers (PBDEs) are used as flame retardants in plastics, textiles, and electronic devices. PBDEs tend to bioaccumulate and have been found to increase exponentially in human breast milk and cord blood during the last 25 years [85, 86]. PBDEs are considered as EDCs, since they disrupt thyroid homeostasis, and for some PBDE congeners, estrogenic activities have been reported (reviewed in [87]).
Ceccatelli et al. [88] investigated the effects of prenatal exposure to PBDE-99, one major congener in human breast milk, and the commercial PCB mixture Aroclor 1254, on the reproductive functions in rats. In female rat offspring, the uterine expression of estrogen target genes (progesterone receptor, ERα, ERβ, IGF-1) was significantly altered following in utero exposure to these substances.
The predominance of ERβ in the ovarian follicles was the basis for a study investigating the endocrine disruptive activities of two different fish liver oils [89]. These natural mixtures have been shown to contain PBDEs and PCBs [90], and the effects were studied in isolated pig ovarian follicles. High levels of PBDEs was found to downregulate ERβ protein levels, while the PCB-containing mixture did not, suggesting ERβ-mediated effects of PBDEs in the ovaries.
Bisphenol A
One recently debated example of an EDC is the xenoestrogen bisphenol A (BPA). It is used in its polymeric form in food and beverage containers, baby bottles, and dental fillings. BPA has been shown to leak from the plastic [91], and it has been found in human plasma, urine, and breast milk (reviewed in e.g., [92]). Recently, an epidemiological report describes a link between high urinary levels of BPA and cardiovascular disease, liver abnormalities, and diabetes in a US adult population cross-sectional study, establishing a correlation between high BPA exposure and disease in humans [93].
BPA exhibits estrogenic agonist activities both on ERα and ERβ in vitro [94, 95]. In binding studies, BPA has been shown to display relatively high ERβ selectivity [96, 97], although there are also data indicating that it may be both agonist and antagonist of ERα depending on cell type [98]. However, when assessed in vivo, BPA did not exert estrogenic effects in the uterus, bone, and vagina of exposed rats [96].
The effects of in utero exposure to BPA in rats were investigated by Schönfelder et al. [99]. Data from this study showed disturbances in the uterus of exposed offspring. Moreover, a significant increase of ERα expression and, in contrast, downregulation of ERβ were seen indicating that the observed morphological uterine changes may be due to BPA-induced dysregulation of ERα/ERβ levels.
Data from ER knockout mice suggest that ERα activation enhances aggression while activation of ERβ has the opposite effect. In male rats neonatally exposed to BPA, the ERβ agonist diarylproprionitrile (DPN), or the daidzein metabolite equol (EQ), behavioral effects like anxiety and increased aggressiveness could be seen in the adult rats. This indicates that exposure to ERβ agonistic compounds at neonatal stages may influence the behavior later in life [100]. In contrast, other reports have shown that exposure during adulthood with DPN reduces anxiety in rodents, indicating that the timing of exposure is crucial [101, 102].
Methoxychlor
Many chlorinated insecticides have endocrine disruptive properties. One prominent example is 1,1,1-trichloro-2,2-bis[chlorophenyl]ethane (DDT), which is spread in certain parts of the world in the fight against malaria, although its use is now heavily restricted. Methoxychlor (1,1,1-trichloro-2,2-bis[4-methoxyphenyl]ethane; MXC) is structurally similar to DDT, and is estrogenic in vivo but also acts on AR [103, 104].
Exogenous estrogens diethylstilbestrol (DES) and MXC were administered to female monkeys during puberty. Since it is not known what brain functions are influenced by estrogen in pubertal girls, some cognitive functions that are known to be gender-differentiated and sensitive to estrogens in postmenopausal women were tested. No disruption of behavior in the DES group could be observed, while MXC exposure significantly impaired cognitive functions, possibly due to differential interactions with ERα/ERβ [105].
In rats, exposure to MXC during pregnancy caused adverse effects on several reproductive parameters in the adult animals, in particular in the ovary [106]. ERβ expression was reduced in follicles, which may contribute to reduced responsiveness to gonadotropins and to subsequent decrease in female fertility.
Phthalates
Phthalates are plasticizers used in high quantities and found ubiquitously in the environment. Some types of phthalates are suspected EDCs. Takeuchi and colleagues [107] investigated phthalates in ER reporter gene assays and showed that several of the tested compounds displayed ERβ-antagonism while they acted estrogenic via ERα. In vivo, chronic exposure to di(n-butyl) phthalate (DBP), a phthalate found in humans [108], was demonstrated to inhibit ERβ and AR in rat testis, suggesting a role in the observed dysregulation of steroidogenesis [109].
EDCs and human health
Biomonitoring data indicate that the general human population is continuously exposed to low doses of mixtures of EDCs, primarily through ingestion of contaminated food. This background exposure makes it difficult to establish a direct causal association between a particular EDC and adverse health outcomes. In the global assessment report by WHO, several adverse human effects are discussed such as reproductive effects, cancers, and immune function. It is concluded that there are strong indications of human health problems caused by EDCs, which makes this area a high research priority [65]. The fact that numerous xenoestrogens and phytoestrogens show stronger binding affinities to ERβ calls for more studies on ER subtype-specific effects and in ERβ-expressing tissues.
Interestingly, ERβ is identified as a negative regulator of the lipid-sensing peroxisome proliferator-activated receptor PPARγ, which is a key player in insulin, glucose, and fatty acid metabolism [110]. ERβ attenuates ligand-induced PPARγ transcriptional activity and PPARγ-regulated adipogenesis in vitro. Additionally, ERβ knockout mice display improved insulin response when kept on high fat diet compared to wild-type mice. PPARγ is targeted by organotins, environmental pollutants with endocrine disruptive properties [111, 112]. Organotins and similar compounds have been suggested to act as obesogens, leading to dysregulation of adipogenesis and contributing to the Western world epidemic of obesity (reviewed in [113]). The finding that ERβ is an important regulator of PPARγ suggests that EDCs may influence adipogenesis by acting either on ERβ, and/or acting directly on PPARγ. Hence, the adipogenesis pathway may be a system particularly vulnerable for EDCs adverse effects.
Modulation of ERβ activity by posttranslational modifications
A considerable amount of evidence has accumulated that there is crosstalk between growth factor and ER signaling, particularly for the ERα isoform (reviewed in, e.g., [114–116]). Peptide growth factors are important regulators of cell proliferation. They act via their membrane-bound receptors (receptor tyrosine kinases) and induce mitogen-activated protein kinase (MAPK) signaling. Growth factors such as epidermal growth factors (EGFs) and insulin-like growth factors (IGFs) have been shown to change the phosphorylation status and thus the activity of ERα. More recently, it has become clear that ERβ function can also be modulated by phosphorylation in its N-terminal region, linking ERβ activity to growth factor signaling.
Posttranslational modifications of ERβ
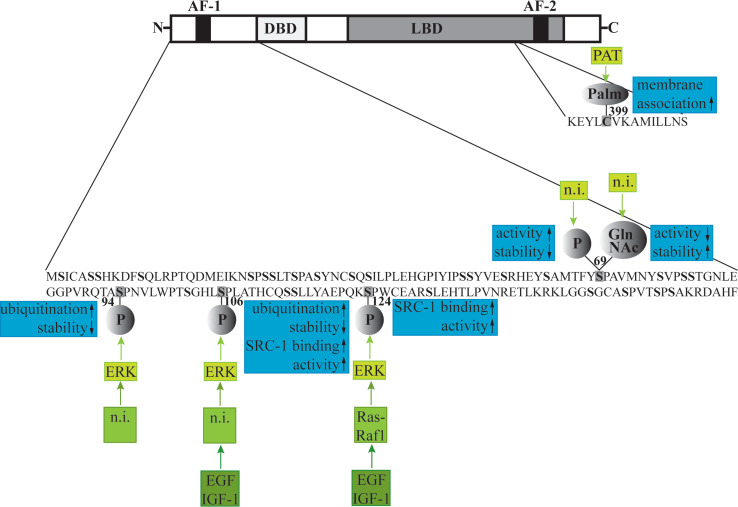
Upon cloning of the mouse ERβ, analysis of its function revealed that ligand-induced ERβ activity is potentiated by inducing the Ras-Raf1-MAPK pathway, which lies downstream of the growth factor receptors [117]. This potentiating effect was abolished by mutation of the serine residue at position 124 to an alanine, i.e., by preventing ERβ to be phosphorylated at this residue. This first indication that ERβ can be regulated by phosphorylation was consolidated by a study showing that the transcriptional coactivator SRC-1 ligand independently interacts with ERβ AF-1, more specifically with the region containing Ser124 and another serine residue, Ser106, when these serines are phosphorylated [118] (Fig. 2). This interaction resulted in enhanced ligand-independent transcriptional activity of ERβ. Furthermore, SRC-1 recruitment was inhibited by a MAPK inhibitor and enhanced by EGF and IGF-1, indicating that MAPK signaling is involved in ligand-independent activation of ERβ by SRC-1. Moreover, the MAPK extracellular signal-regulated 2 (ERK2) readily phosphorylated the A/B domain of ERβ containing AF-1 in vitro [119].
Fig. 2.
Posttranslational modifications of ERβ: the signaling cascades leading to the respective modification (green), and the effects of the modification on ERβ (blue). AF Activation function, DBD DNA binding domain, LBD ligand binding domain, Palm palmitoylation, PAT palmitoyltransferase, P phosphorylation, GlnNAc 2-amino-2-deoxyglucosylation, n.i. not investigated, ERK extracellular signal-regulated, EGF epidermal growth factor, IGF insulin-like growth factor
The MAPK p38 has also been shown to regulate ERβ activity (Fig. 2). However, in contrast to ERK2, it is not clear where the p38 phosphorylation sites are in the receptor. Moreover, different stimuli result in opposite effects on ERβ activity: whereas p38 activation by expression of the proto-oncogen Brx enhances ligand-induced ERβ function [120], hormone-dependent ERβ activity is repressed by the growth factors heregulins via p38 [121]. It is thus likely that p38 does not only phosphorylate ERβ directly but regulates other factors that are important for ERβ signaling. For example, it has been shown that the ER coregulators, AIB1 [122] and GRIP1 [123], are phosphorylated by p38.
Phosphorylation of the ERβ AF-1 also regulates other posttranslational modifications of the receptor, which in turn control its activity. Phosphorylation of serine 94 and 106 has been suggested to enhance ubiquitination of ERβ by increased recruitment of the ubiquitin ligase E6-AP [124] (Fig. 2). Ubiquitination directs proteins to the 26S proteasome where they are degraded. Interestingly, 26S proteasome function is necessary for transcriptional activity of both ER isoforms as its inhibition leads to abolished response to hormone [124, 125]. Proteasome inhibition leads to diminished ER mobility within the nucleus, which is thought to be necessary for its transcriptional activity [126]. Picard et al. [124] showed that serine to alanine substitution at residues 94 and 106 results in increased ERβ stability, whereas activation of ERK MAPK decreased ERβ half-life. These findings suggest that phosphorylation also regulates ERβ transcriptional activity by dictating protein stability.
Serine residue 69 has been shown to be alternatively modified by phosphorylation or 2-amino-2-deoxyglucosylation (GlcNAcylation) [127] (Fig. 2). GlcNAcylation is a common posttranslational modification and is responsive to cell cycle, extracellular signals, glucose metabolism, and the growth state of a cell. As it occurs on the same hydroxyl moiety as phosphorylation, these two modifications are in many cases mutually exclusive [128]. Interestingly, in a follow-up study, Cheng and colleagues [129] showed that serine 69 phosphorylation results in higher ERβ activity but decreased stability, whereas GlcNAcylation at this residue had the opposite effect. Peptides containing the unmodified, phosphorylated, or GlcNAcylated ERβ sequence were shown to adopt different secondary structures, which could explain the differences in ERβ stability induced by these modifications [130]. However, it is also possible that phosphorylation at residue 69 additionally affects ubiquitination and thus proteosomal degradation of ERβ. This has not been investigated and would contribute to the understanding of how posttranscriptional modifications regulate ERβ activity.
Like ERα, ERβ has recently been shown to be S-palmitoylated [131]. S-palmitoylation occurs upon attachment of palmitate to a non-N-terminal Cys residue through a thioester linkage and is catalyzed by palmitoyltransferase (PAT) [132]. This modification renders the protein more hydrophilic and facilitates membrane association. In the case of ERβ, palmitoylation occurs on residue Cys399 and has been suggested to be necessary for the localization of the receptor at the plasma membrane and thus for rapid, non-genomic effects mediated by membrane-associated ERβ. Additionally, inhibition of ERβ-palmitoylation weakly decreased ERE-dependent transcriptional activation [131].
Physiological implications
The physiological implications of ERβ modulation by posttranslational modifications remain an open question. For ERα, it is well documented that crosstalk with growth factors play an important role in cancer progression and, in particular, in drug resistance in breast cancer patients (reviewed in, e.g., [115]). As the role of ERβ in tumor cells has not been completely solved yet, the importance of its regulation by growth factors is not easy to evaluate. As there is evidence that ERβ acts as tumor suppressor, it might counteract the proliferative effects of growth factors. However, this has to be specifically addressed. In colon cancer, pro-apoptotic effects of ERβ have been suggested to be mediated by membrane-associated receptors, activating the p38/MAPK pathway, which in turn leads to downstream apoptotic events [133]. Inhibition of ERβ palmitoylation in colon cancer cells prevented the induction of this pro-apoptotic cascade [131]. Thus, palmitoylation of ERβ seems to be important for its role as tumor suppressor.
Interesting is the finding that ERβ can be GlcNAcylated. GlcNAcylated proteins increase in insulin resistance induced by diabetes type 2 [134]. Recently, ERβ has been suggested to act as diabetogenic factor [135]; thus, it would be exciting to further investigate the role of GlcNAcylation of ERβ in the context of glucose metabolism.
Circadian regulation of ERβ expression
Although ERβ is widely distributed throughout the body, the expression levels vary significantly among different tissues and cell types. Even within a single tissue, there are time-dependent changes in ERβ expression, both on a long-term scale, such as in fetal and postnatal development, and short-term during the circadian cycle.
The abundance of ERβ in different tissues varies during development, via unknown regulatory mechanisms, suggesting an important role of ERβ-mediated estrogen action in the maturation of fetal and postnatal tissues [136–138]. In the mouse brain, for instance, ERβ protein expression has been shown to appear in distinct regions between embryonic day 12.5 and 16.5. At day 18.5, there is a significant increase in ERβ levels in most parts of the brain [139]. High ERβ levels are maintained throughout birth and the first two postnatal weeks, whereas they drop significantly between postnatal day 14 and 35. Four months after birth, however, they increase again [138].
Expression of ERβ also oscillates following the circadian cycle within one tissue, such as lung or liver [140]. Circadian rhythm is an internal biological clock that regulates a variety of biological processes according to a roughly 24-h period [141]. Although the suprachiasmatic nucleus (SCN) is the main controller of the rhythm, molecular oscillators are also found in individual cells [142]. At the molecular level, the circadian oscillator is driven by a transcription/translation feedback loop comprised of a set of clock genes. Heterodimers formed by helix-loop-helix (bHLH)-Per-ARNT-sim (PAS) proteins CLOCK and BMAL1 (also called ARNT3) generate the transcription of negative circadian regulators, such as PER and CRY, and other clock-controlled genes (CCGs) by binding to the E-box motifs within the promoter regions of these genes. Accumulated PER and CRY proteins form in turn negative regulatory complexes, which interact with the CLOCK-BMAL1 and inhibit transcription of their own genes and other CCGs [143–145].
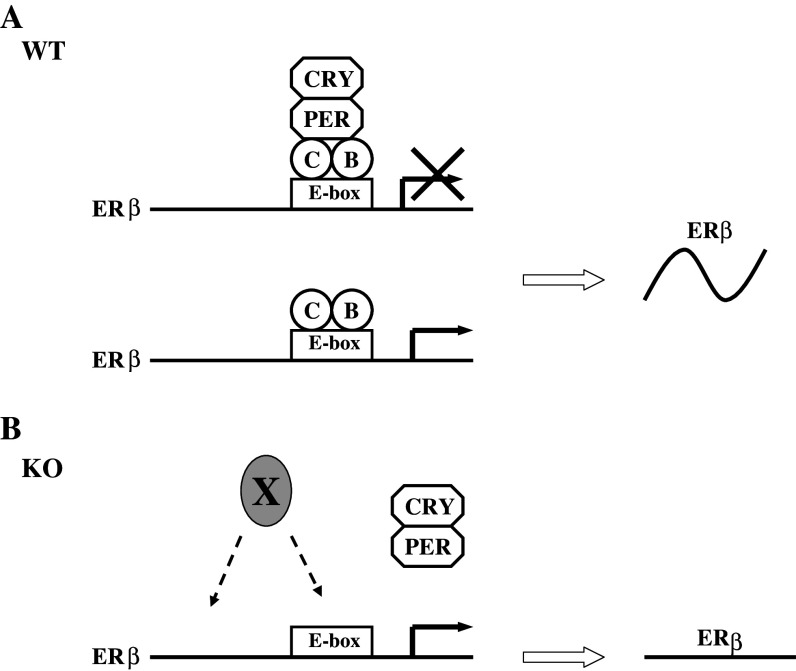
ERβ mRNA displays a circadian expression pattern in both synchronized cultured cells and mouse tissues, but has been found in constantly high levels without any oscillation in BMAL1 knockout mice. Moreover, both positive and negative circadian components are recruited to ERβ promoter in mouse HC11 cells. As is the case for most CCGs, the circadian components bind to an evolutionarily conserved E-box (CACGTG) enhancer in the proximal promoter region of ERβ. The recruitment of PER1 shows a circadian oscillation with the same pattern as ERβ mRNA expression, whereas no rhythmic change is observed in the recruitment of CLOCK. The possible mechanism is depicted in Fig. 3: CLOCK-BMAL1 heterodimers bind to the ERβ promoter and induce ERβ transcription, while negatively regulatory heterodimers PER-CRY interact with CLOCK-BMAL1 to inhibit the transcription. In wild-type mice, constant binding of CLOCK-BMAL1 and circadian recruitment of PER-CRY generate the rhythmic expression of ERβ (Fig. 3a). In BMAL1 knockout animals, on the other hand, there is no recruitment of PER-CRY due to the lack of CLOCK-BMAL1, instead, ERβ is induced by other activating transcriptional factors and remains highly expressed (Fig. 3b). Interestingly, ERα is not regulated by circadian clock [140].
Fig. 3.
Schematic model of circadian regulation of ERβ expression. a In wild-type mice and cells, CLOCK-BMAL1 heterodimers bind constantly to the E-box enhancer in the ERβ promoter and induces ERβ transcription. The negative circadian regulator PER-CRY works as the main driving force of the circadian expression of ERβ. Recruitment of PER-CRY causes an inhibition of ERβ expression, and the release of PER-CRY results in an upregulation of ERβ expression. b In BMAL1 KO mice, the negative regulator PER-CRY cannot be recruited to ERβ promoter. Instead, the expression of ERβ is induced by unknown activating transcription factors (X) and kept at high levels with no oscillation. Figure reproduced with permission from the American Society for Microbiology
Circadian rhythms play critical roles in maintaining optimal physical functioning; disrupted circadian cycles can lead to increased susceptibility of various diseases [146]. Accumulating findings indicate that shiftwork, a typical example of circadian disturbance, is a risk factor for a number of disorders [147]. Among these health problems are reproductive malfunctions in female shift workers [148–150] and increased risk of developing mood disorders [151, 152]. ERβ could be involved in these circadian effects. It is involved in the function of the female reproductive system, and several recent studies have reported an important role of ERβ in mood disorders and an antidepressant and anxiolytic effect of ERβ selective agonists [102, 153, 154]. Moreover, different studies suggest both circadian rhythm and ERβ could function as suppressors in development of tumors [44, 155–157]. A very recent study in colorectal cancer even indicates a correlated downregulation of ERβ and PER1 [158]. However, direct evidence to prove circadian clock regulating biological processes through ERβ in vivo is still lacking. In addition, isoform specific regulation of ERs by the circadian system implies that ERα/ERβ ratio could be subject to the daily light–dark cycle. Given the distinct biological roles of the two ER isoforms, oscillation of the ERα/ERβ ratio could lead to daytime-dependent changes in estrogen action and susceptibility of ER-related diseases.
Regulation of ERβ expression by DNA methylation
DNA methylation is a way to encode epigenetic information and an important mechanism to control gene expression. In vertebrates, methylation occurs on cytosines in CG dinucleotides (annotated CpGs). DNA methylation is catalyzed by DNA methyltransferase (DNMT) 3, the de novo methylation enzyme, and DNMT 1, responsible for maintenance of methylation during replication. It is still not clear, on the other hand, how the process of demethylation is regulated [159].
Throughout the genome, the frequency of CpGs is lower than expected from random distribution. However, there are stretches of DNA, often associated with gene promoters, which are CpG enriched, so-called CpG islands. Generally, unmethylated CpG islands are associated with open chromatin allowing active gene transcription, whereas DNA methylation correlates with a closed chromatin structure and gene silencing [159]. Additionally to DNA methylation, modifications at the N-terminal tails of the core histones and at histone-associated proteins, contribute to the chromatin structure and thus to epigenetic regulation of gene transcription. For example, histone acetylation at histones 3 and 4 are a mark for active gene transcription whereas deacetylation is associated with inactive genes. Histone methylation also correlates with gene activity. However, the correlation is more complex and depends on the combination of methylated lysines as well as on the differentiation state of the cell (reviewed in, e.g., [160, 161]).
Regulation of gene expression by DNA methylation is important in many processes, e.g., in the establishment of cell lineage-specific gene expression during embryonic development, in the regulation of imprinted genes (genes that are transcribed either from the maternal or paternal allele only), and in the inactivation of one of the two female X chromosomes. Additionally, aberrant DNA hypermethylation in the promoter region of tumor suppressor genes has been described for many cancers, leading to silencing of tumor suppressor gene expression. It has become evident that ERβ expression can be regulated by DNA methylation in its promoter region. This is associated with reduced ERβ transcription.
The ERβ promoter as a target for DNA methylation
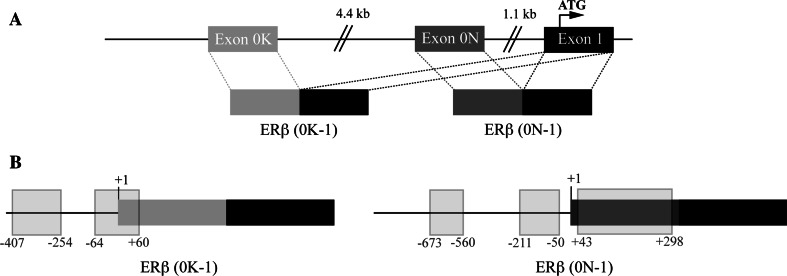
The promoter region of human ERβ was cloned and characterized in 2000 by Li and colleagues [162]. Shortly thereafter, two ERβ isoforms were described that originate from two different untranslated first exons, exon 0K and exon 0N [163]. These exons are spliced to exon 1 and give rise to the two messenger RNAs (0K-1) and (0N-1), respectively (Fig. 4a). The originally described promoter sequence [163] includes the sequence of exon 0N. Exon 0K lies around 50 kb upstream of exon 1 (Fig. 4a) and has not been characterized in detail. Notably, full-length ERβ transcripts have been detected, which contain neither exon 0K nor 0N [8], suggesting the presence of (an) additional, yet to be identified, exon(s) 0. Interestingly, (0K-1) and (0N-1) have a distinct tissue distribution [163], and their ratio changes in tumor cells [12].
Fig. 4.
DNA methylation in the ERβ untranslated region. a Schematic drawing of the ERβ untranslated region and the splicing events leading to the two ERβ mRNAs (0K-1) and (0N-1). b Positioning of the CpG islands (light gray rectangles) in ERβ (0K-1) and ERβ (0N-1). The transcription initiation site is assigned as position +1
Both ERβ promoter regions are GC rich and exhibit several predicted CpG islands (Fig. 4b). It is now clear that ERβ expression can be regulated by DNA methylation in these CpG islands, at least in those around exon 0N. Changed DNA methylation patterns in the ERβ promoters have been described for several forms of cancer and recently also in endometriosis and atherosclerosis and vascular senescence (summarized in Table 3).
Table 3.
Summary of the DNA methylation events in the promoter region of ERβ
| Tissue | Investigated region | Differentially methylated CpGs | Correlation between methylation and ERβ expression | Correlation between methylation and disease | References |
|---|---|---|---|---|---|
| Prostate carcinoma/BPH | Exon 0N (+29 to +301) | All 19 | Yes, inverse | Yes, direct | [175] |
| Cancerous and healthy prostate tissue/prostate cell lines | Exon 1 (+597 to +705) | All 3 | Yes, inverse | Yes, direct | [176] |
| Healthy prostate/PCa grade 1-5/lymph knot and bone metastases | Promoter region/exon 0N (−204 to +315) | −146, −144, −142, −139, −136, −132; +31, +51, +53, +62, +94, +105, +108; +160, +174, +178, +201, +205, +224, +227, +232 | Yes, inverse | Direct for primary tumors, no correlation for metastases | [177] |
| Breast epithelium/breast carcinoma/various breast cancer cell lines | Exon 0N (+29 to +301) and 0K (−137 to +157) | All 19 in exon 0N, none in exon 0K | Yes, inverse | Yes, direct | [186] |
| Cancerous and healthy breast tissue | Exon 0N (+217 to +406) and 0K | All 11 in exon 0N, none in exon 0K | Yes, inverse | Yes, direct | [187] |
| Ovarian cancer tissue and cell lines | Promoter region/Exon 0N (−252 to +393) and 0K (−137 to +157) | All 45 in exon 0N, none in exon 0K | Yes, inverse | [12] | |
| Endometrial and endometriotic tissue and primary cells | 0N Promoter (−202 to −37) | All 13 | Yes, inverse | Yes, inverse | [194] |
| Atherosclerotic and normal tissue | Exon 0 N (+34 to +192) | Partial for all 10 | Not measured | Slightly, direct | [195] |
The transcription initiation site is assigned as position +1
DNA methylation of ERβ promoter in prostate cancer
Aberrant DNA methylation in the promoter region of ERβ was first found in prostate carcinoma [12]. ERβ is the predominant ER form in the human prostate. ERα and ERβ are differentially expressed, with ERβ found in the basal epithelium whereas ERα is expressed in the stroma [164]. It has been suggested that ERβ has antiproliferative functions in basal epithelial cells, which could result in a protective effect against prostate cancer (PCa) [165]. Furthermore, several studies show that ERβ is more effective than ERα in protecting cells from oxidative stress caused by estrogens. Estrogens can be genotoxic both via intermediates that form DNA adducts and by induction of oxidative stress, which results in the formation of reactive oxygen species. ERβ is more efficient than ERα in activating antioxidant enzyme such as quinone reductase and glutathione S-transferases, which clear the organism from these genotoxic compounds [166–168]. In line with these findings, ERβ knockout mice, but not ERα knockouts, develop prostatic hyperplasia in old age [169].
ERβ expression changes during different stages of prostate cancer. In primary tumors, its levels decrease with increasing tumor progression and are lost in high-grade carcinomas [170–172]. On the other hand, high levels of ERβ are observed in the majority of PCa metastases in bone and lymph nodes [173]. The reason for this and the role of ERβ in metastases is unknown. There are speculations that local factors can lead to re-expression of ERβ at these sites. Alternatively, ERβ-expressing tumor cells might have a higher capability to migrate and establish themselves in distant sites [174].
DNA methylation in the 5′ untranslated region of ERβ inversely correlates with its expression in the prostate. This was first shown in a study by Nojima et al. [175] where DNA methylation of 19 CpG between 376 and 117 bp upstream of the ATG in exon 0N were analyzed in human prostate carcinoma and benign prostatic hyperplasia (BPH). They could demonstrate that all 19 CpGs were methylated in the carcinomas, which coincided with lack of ERβ expression. In BPH, on the other hand, these CpGs were not methylated and ERβ was expressed. Furthermore, treatment with the demethylation agent 5-aza-2Δ-deoxycytidine (5-AZAC) of PCa-derived cell lines devoid of ERβ resulted in re-expression of ERβ. These results were confirmed in a similar study by Sasaki et al. [176].
Zhu and colleagues [177] elaborated on these initial findings by including the promoter region upstream of exon 0N into the DNA methylation analysis and by comparing different grades of PCa and metastatic tissue. They concluded that both CpG island 1 in the promoter region and CpG island 2 in exon 0N become higher methylated the more the cancer has progressed. However, in PCa metastases, in which ERβ is normally expressed, DNA methylation in the two CpG islands was comparable with healthy tissue. Three CpG clusters were identified that were heavily methylated when ERβ transcription was silenced: CpGs 3–8 in the promoter region, and CpGs 16–22 and 28–35 in exon 0N. Again, these results were confirmed in PCa cell lines. Furthermore, they could show that PC3 cells, a PCa cell line that expresses ERβ, become more methylated in the ERβ promoter the longer they are kept in culture. Interestingly, increase in methylation starts at the first CpG cluster and “spreads” downstream with increasing passaging number. Additionally, using methylated sense oligonucleotides (MOs) in PC3 cells, sequence-specific methylation of CpG island 1 or 2 was achieved. Interestingly, methylation of CpG island 1 led to suppression of ERβ transcription whereas there was no effect when CpG island 2 was methylated. Thus, the methylation status of CpGs 3–8 seems to be most important for ERβ expression.
A follow-up study by the same research group identified a binding site for the transcription factor activator protein-2 (AP-2) in this region [178]. Using chromatin immunoprecipitation, they could show that AP-2 is recruited to the ERβ promoter and that overexpression or downregulation of AP-2 leads to increased or reduced ERβ transcription, respectively. Similarly to ERβ, AP-2 expression is downregulated in PCa [179]. The authors speculate that loss of AP-2 expression is the initial cause for decreased ERβ expression. Reduced AP-2 recruitment could lead to an increased DNMT recruitment to this site, which in turn results in DNA methylation and long-term silencing of the ERβ gene [178].
DNA methylation of ERβ promoter in breast and ovary cancer
ERβ downregulation has been reported both in breast and in ovary [180–183]. In both cancers, ERβ acts as pro-apoptotic factor; thus, it has been suggested that ERβ acts as tumor suppressor gene [184, 185]. As in PCa, ERβ expression has been shown to be regulated by DNA methylation in both breast and ovary tissue. Zhao and colleagues [186] measured ERβ expression and promoter methylation in various cell lines derived from mammary epithelium or breast carcinoma as well as in primary breast cancer tumors. Again, low ERβ expression in tumors and an inverse correlation between DNA methylation of the exon 0N and ERβ expression was found. The authors also investigated the methylation pattern in exon 0K. However, there was no correlation between methylation and ERβ expression; exon 0K was unmethylated both in healthy and in cancerous cells. Like in PCa cell lines, treatment with 5-AZAC led to increased ERβ expression.
Increased ERβ methylation in exon 0N in breast cancer was confirmed in a later study by Rody et al. [187]. Additionally, possible mechanisms for ERβ methylation were investigated. The presence of reverse promoters downstream of exon 0N and 0K led the authors to hypothesize that antisense transcripts are generated from the reverse promoters leading to methylation of the ERβ promoter. This mechanism has been described for epigenetic silencing and imprinting [188–190]. However, no antisense transcripts to the ERβ promoter could be detected. On the other hand, by comparison with other genes known to be regulated by DNA methylation in breast cancer, the authors could identify a short common motive in the promoter region of these genes. A whole genome search identified 45 promoter regions containing this motive, 18 of which had been analyzed before in micro-array studies with breast cancer samples. Strikingly, most of them were indeed downregulated in breast cancer [187].
In a recent study, ERβ methylation was analyzed in ovarian cancer cell lines and tissues [12]. ERβ expression was reduced in cancerous compared to normal tissue, which inversely correlated with DNA methylation in the promoter region and exon 0N. Again, exon 0K was unmethylated both in healthy and in tumor cells, and 5-AZAC treatment led to re-expression of ERβ in ovarian cancer cell lines.
DNA methylation of ERβ promoter in other conditions
Endometriosis is a common gynaecologic condition, in which endometrium-like tissue is present outside the uterine cavity. It causes severe pain in the patients and is associated with infertility [191]. ERβ expression is significantly higher in endometriotic compared to endometrial cells and tissue [192, 193]. Xue and colleagues [194] demonstrated that higher ERβ levels in endometriotic stromal cells were associated with lower DNA methylation of the CpG island in exon 0N compared to endometrial stromal cells. ERβ mRNA could be induced by 5-AZAC treatment in endometrial stromal cells, and in vitro methylation analysis showed that the methylation of the CpG island inhibits transcriptional activity in these cells.
In a study by Kim et al. [195], DNA methylation changes in the ERβ promoter/exon 0N were investigated in atherosclerotic and cardiovascular tissue. The authors describe higher DNA methylation in atherosclerotic compared to vascular tissue. Additionally, DNA methylation was compared in plaque and non-plaque regions from the same blood vessel. Calcium plaque formation is a hallmark of atherosclerosis. ERβ methylation was higher in the plaque than the non-plaque region of the same patient whereby the plaques showing most atherosclerotic changes had the highest increase in DNA methylation. The authors also studied methylation changes in in vitro vascular senescence of smooth muscle cells and endothelial cells. Half the cell lines used showed elevated ERβ methylation with increasing passage numbers. Additionally, some of the senescent cells were treated with the DNA demethylation agent 5-azacytidine, which resulted in increased ERβ expression in these cells. However, no correlation of DNA methylation and ERβ expression levels was done in this study otherwise. Furthermore, it has been described in earlier studies that ERβ expression is increased in atherosclerotic plaque lesions compared to non-plaque regions [196, 197]. Thus, the biological significance of the results by Kim et al. has to be further investigated.
Aberrant DNA methylation of ERβ as a potential therapeutic target
The results summarized in this section clearly show that ERβ silencing by promoter methylation coincides with different forms of cancers and possibly other conditions. In most of the studies, the findings were complemented with 5-AZAC treatment of cell lines, which led to demethylation of the promoter and subsequent ERβ re-expression. DNA demethylation agents, alone or in combination with histone deacetylase (HDAC) inhibitors, are used in cancer therapy, for example for myelodysplastic syndromes, leukemia, and ovarian and prostate cancer (reviewed in, e.g., [198]). In vitro studies also demonstrate that DNA demethylation agents, particularly in combination with HDAC inhibitors, induce apoptosis, cell differentiation and/or growth arrest, e.g., in human lung, breast, colon, and prostate cancer cells [199, 200]. Walton and colleagues [200] correlated the pro-apoptotic effects of DNA demethylation and HDAC inhibition to re-expression of ERβ in prostate cancer cell lines. However, due to the lack of specificity of DNA demethylation agents, it is impossible to causally link ERβ expression and anti-cancerous effects of these drugs. Although ERβ has been suggested to function as tumor suppressor, and mice lacking ERβ develop, e.g., severe epithelial hypoplasia of the prostate [165], further studies are needed to demonstrate a causal role for ERβ silencing in the development of cancer. In particular, the molecular mechanisms leading to ERβ promoter methylation should be addressed, like in the studies by Zhang et al. [178] and Rody et al. [187]. This might not only lead to a better understanding of the role of ERβ in cancer but also to more specific anticancer therapies. Independently of whether ERβ silencing is causal for tumor growth or the result of cell transformation, Rody and colleagues have suggested that its methylation status might serve as a marker for malignancy and prognosis. These authors describe higher ERβ methylation in patients with relapse during follow-up studies, i.e., DNA methylation was inversely correlated to prognosis [187]. However, the data were not statistically significant. Thus, further studies should investigate the interesting possibility that ERβ methylation status could be a prognostic marker for breast cancer patients.
Concluding remarks
In this article, we have tried to summarize different means by which ERβ activity is modulated and regulated. ERβ is an interesting therapeutic target, e.g., in the treatment of some cancer forms, sub-fertility, and depression [2]. However, in order to achieve optimal efficiency with minimal side effects, specificity of a drug, regarding ER subtype and target tissue, is desirable. Understanding its regulation, both by endogenous processes and exogenous factors, opens up the possibility to specifically interfere with ERβ function. For instance, analyzing the subtype selectivity and its underlying mechanisms of certain phytoestrogens can inspire the design of novel ERβ agonistic drugs. Unraveling the effects of posttranslational modifications can open up opportunities for ERβ targeting via other signaling pathways. And understanding the mechanisms leading to ERβ silencing by promoter methylation might offer the possibility to reactivate ERβ expression in tumors. The fact that ERβ expression is regulated by the circadian system implies that even the timing of drug delivery might be crucial for efficiency and specificity. Timing of drug delivery has already been shown to be essential in cancer therapy, particularly in patients with metastatic colorectal cancer [201]. In conclusion, further efforts are needed to delineate the exact mechanisms regulating ERβ activity in order to target it specifically.
Acknowledgment
The authors are supported by the European Commission funded CASCADE Network of Excellence, the European Commission funded CRESCENDO project, the Swiss National Research Foundation, and Agriculture and Agri-food Canada.
Footnotes
E. Swedenborg and K. A. Power contributed equally.
References
- 1.Harris HA. Estrogen receptor-beta: recent lessons from in vivo studies. Mol Endocrinol. 2007;21:1–13. doi: 10.1210/me.2005-0459. [DOI] [PubMed] [Google Scholar]
- 2.Zhao C, Dahlman-Wright K, Gustafsson JA. Estrogen receptor beta: an overview and update. Nucl Recept Signal. 2008;6:e003. doi: 10.1621/nrs.06003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjold M, Gustafsson JA. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997;82:4258–4265. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- 4.Ruegg J, Swedenborg E, Wahlstrom D, Escande A, Balaguer P, Pettersson K, Pongratz I. The transcription factor aryl hydrocarbon receptor nuclear translocator functions as an estrogen receptor beta-selective coactivator, and its recruitment to alternative pathways mediates antiestrogenic effects of dioxin. Mol Endocrinol. 2008;22:304–316. doi: 10.1210/me.2007-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 6.Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S. Differential response of estrogen receptor alpha and estrogen receptor beta to partial estrogen agonists/antagonists. Mol Pharmacol. 1998;54:105–112. doi: 10.1124/mol.54.1.105. [DOI] [PubMed] [Google Scholar]
- 7.Delaunay F, Pettersson K, Tujague M, Gustafsson JA. Functional differences between the amino-terminal domains of estrogen receptors alpha and beta. Mol Pharmacol. 2000;58:584–590. doi: 10.1124/mol.58.3.584. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa S, Inoue S, Watanabe T, Orimo A, Hosoi T, Ouchi Y, Muramatsu M. Molecular cloning and characterization of human estrogen receptor betacx: a potential inhibitor of estrogen action in human. Nucleic Acids Res. 1998;26:3505–3512. doi: 10.1093/nar/26.15.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore JT, McKee DD, Slentz-Kesler K, Moore LB, Jones SA, Horne EL, Su JL, Kliewer SA, Lehmann JM, Willson TM. Cloning and characterization of human estrogen receptor beta isoforms. Biochem Biophys Res Commun. 1998;247:75–78. doi: 10.1006/bbrc.1998.8738. [DOI] [PubMed] [Google Scholar]
- 10.Leung YK, Mak P, Hassan S, Ho SM. Estrogen receptor (ER)-beta isoforms: a key to understanding ER-beta signaling. Proc Natl Acad Sci USA. 2006;103:13162–13167. doi: 10.1073/pnas.0605676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green CA, Peter MB, Speirs V, Shaaban AM. The potential role of ER beta isoforms in the clinical management of breast cancer. Histopathology. 2008;53:374–380. doi: 10.1111/j.1365-2559.2008.02968.x. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki F, Akahira J, Miura I, Suzuki T, Ito K, Hayashi S, Sasano H, Yaegashi N. Loss of estrogen receptor beta isoform expression and its correlation with aberrant DNA methylation of the 5′-untranslated region in human epithelial ovarian carcinoma. Cancer Sci. 2008;99:2365–2372. doi: 10.1111/j.1349-7006.2008.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 14.O’Lone R, Frith MC, Karlsson EK, Hansen U. Genomic targets of nuclear estrogen receptors. Mol Endocrinol. 2004;18:1859–1875. doi: 10.1210/me.2003-0044. [DOI] [PubMed] [Google Scholar]
- 15.Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 16.Jakacka M, Ito M, Weiss J, Chien PY, Gehm BD, Jameson JL. Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J Biol Chem. 2001;276:13615–13621. doi: 10.1074/jbc.M008384200. [DOI] [PubMed] [Google Scholar]
- 17.Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, Ohman L, Greene GL, Gustafsson JA, Carlquist M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 18.Metivier R, Penot G, Hubner MR, Reid G, Brand H, Kos M, Gannon F. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell. 2003;115:751–763. doi: 10.1016/s0092-8674(03)00934-6. [DOI] [PubMed] [Google Scholar]
- 19.Metivier R, Gallais R, Tiffoche C, Le Peron C, Jurkowska RZ, Carmouche RP, Ibberson D, Barath P, Demay F, Reid G, Benes V, Jeltsch A, Gannon F, Salbert G. Cyclical DNA methylation of a transcriptionally active promoter. Nature. 2008;452:45–50. doi: 10.1038/nature06544. [DOI] [PubMed] [Google Scholar]
- 20.Kangaspeska S, Stride B, Metivier R, Polycarpou-Schwarz M, Ibberson D, Carmouche RP, Benes V, Gannon F, Reid G. Transient cyclical methylation of promoter DNA. Nature. 2008;452:112–115. doi: 10.1038/nature06640. [DOI] [PubMed] [Google Scholar]
- 21.Wong CW, McNally C, Nickbarg E, Komm BS, Cheskis BJ. Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc Natl Acad Sci USA. 2002;99:14783–14788. doi: 10.1073/pnas.192569699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Bjornstrom L, Sjoberg M. Signal transducers and activators of transcription as downstream targets of nongenomic estrogen receptor actions. Mol Endocrinol. 2002;16:2202–2214. doi: 10.1210/me.2002-0072. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki T, Yu HP, Hsieh YC, Choudhry MA, Bland KI, Chaudry IH. Mitogen activated protein kinase (MAPK) mediates non-genomic pathway of estrogen on T cell cytokine production following trauma-hemorrhage. Cytokine. 2008;42:32–38. doi: 10.1016/j.cyto.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER. Plasma membrane estrogen receptors exist and functions as dimers. Mol Endocrinol. 2004;18:2854–2865. doi: 10.1210/me.2004-0115. [DOI] [PubMed] [Google Scholar]
- 25.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 26.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 27.Mueller SO, Simon S, Chae K, Metzler M, Korach KS. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol Sci. 2004;80:14–25. doi: 10.1093/toxsci/kfh147. [DOI] [PubMed] [Google Scholar]
- 28.Penttinen P, Jaehrling J, Damdimopoulos AE, Inzunza J, Lemmen JG, van der Saag P, Pettersson K, Gauglitz G, Makela S, Pongratz I. Diet-derived polyphenol metabolite enterolactone is a tissue-specific estrogen receptor activator. Endocrinology. 2007;148:4875–4886. doi: 10.1210/en.2007-0289. [DOI] [PubMed] [Google Scholar]
- 29.Pike AC, Brzozowski AM, Hubbard RE, Bonn T, Thorsell AG, Engstrom O, Ljunggren J, Gustafsson JA, Carlquist M. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. EMBO J. 1999;18:4608–4618. doi: 10.1093/emboj/18.17.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Routledge EJ, White R, Parker MG, Sumpter JP. Differential effects of xenoestrogens on coactivator recruitment by estrogen receptor (ER) alpha and ERbeta. J Biol Chem. 2000;275:35986–35993. doi: 10.1074/jbc.M006777200. [DOI] [PubMed] [Google Scholar]
- 31.An J, Tzagarakis-Foster C, Scharschmidt TC, Lomri N, Leitman DC. Estrogen receptor beta-selective transcriptional activity and recruitment of coregulators by phytoestrogens. J Biol Chem. 2001;276:17808–17814. doi: 10.1074/jbc.M100953200. [DOI] [PubMed] [Google Scholar]
- 32.Chang EC, Charn TH, Park SH, Helferich WG, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen Receptors alpha and beta as determinants of gene expression: influence of ligand, dose, and chromatin binding. Mol Endocrinol. 2008;22:1032–1043. doi: 10.1210/me.2007-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powell E, Xu W. Intermolecular interactions identify ligand-selective activity of estrogen receptor alpha/beta dimers. Proc Natl Acad Sci USA. 2008;105:19012–19017. doi: 10.1073/pnas.0807274105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Totta P, Acconcia F, Virgili F, Cassidy A, Weinberg PD, Rimbach G, Marino M. Daidzein-sulfate metabolites affect transcriptional and antiproliferative activities of estrogen receptor-beta in cultured human cancer cells. J Nutr. 2005;135:2687–2693. doi: 10.1093/jn/135.11.2687. [DOI] [PubMed] [Google Scholar]
- 35.Lampe JW. Isoflavonoid and lignan phytoestrogens as dietary biomarkers. J Nutr. 2003;133(Suppl 3):956S–964S. doi: 10.1093/jn/133.3.956S. [DOI] [PubMed] [Google Scholar]
- 36.Pugazhendhi D, Watson KA, Mills S, Botting N, Pope GS, Darbre PD. Effect of sulphation on the oestrogen agonist activity of the phytoestrogens genistein and daidzein in MCF-7 human breast cancer cells. J Endocrinol. 2008;197:503–515. doi: 10.1677/JOE-07-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muthyala RS, Ju YH, Sheng S, Williams LD, Doerge DR, Katzenellenbogen BS, Helferich WG, Katzenellenbogen JA. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem. 2004;12:1559–1567. doi: 10.1016/j.bmc.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 38.Selvaraj V, Zakroczymski MA, Naaz A, Mukai M, Ju YH, Doerge DR, Katzenellenbogen JA, Helferich WG, Cooke PS. Estrogenicity of the isoflavone metabolite equol on reproductive and non-reproductive organs in mice. Biol Reprod. 2004;71:966–972. doi: 10.1095/biolreprod.104.029512. [DOI] [PubMed] [Google Scholar]
- 39.Wang XN, Simmons HA, Salatto CT, Cosgrove PG, Thompson DD. Lasofoxifene enhances vaginal mucus formation without causing hypertrophy and increases estrogen receptor beta and androgen receptor in rats. Menopause. 2006;13:609–620. doi: 10.1097/01.gme.0000227337.73738.c9. [DOI] [PubMed] [Google Scholar]
- 40.Setchell KD, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe BE, Nechemias-Zimmer L, Brown NM, Lund TD, Handa RJ, Heubi JE. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr. 2005;81:1072–1079. doi: 10.1093/ajcn/81.5.1072. [DOI] [PubMed] [Google Scholar]
- 41.Zhao L, Mao Z, Brinton RD. A select combination of clinically relevant phytoestrogens enhances estrogen receptor beta-binding selectivity and neuroprotective activities in vitro and in vivo. Endocrinology. 2009;150:770–783. doi: 10.1210/en.2008-0715. [DOI] [PubMed] [Google Scholar]
- 42.Dip R, Lenz S, Gmuender H, Naegeli H. Pleiotropic combinatorial transcriptomes of human breast cancer cells exposed to mixtures of dietary phytoestrogens. Food Chem Toxicol. 2009;47:787–795. doi: 10.1016/j.fct.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 43.Di Leo A, Barone M, Maiorano E, Tanzi S, Piscitelli D, Marangi S, Lofano K, Ierardi E, Principi M, Francavilla A. ER-beta expression in large bowel adenomas: implications in colon carcinogenesis. Dig Liver Dis. 2008;40:260–266. doi: 10.1016/j.dld.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 44.Konstantinopoulos PA, Kominea A, Vandoros G, Sykiotis GP, Andricopoulos P, Varakis I, Sotiropoulou-Bonikou G, Papavassiliou AG. Oestrogen receptor beta (ERbeta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour’s dedifferentiation. Eur J Cancer. 2003;39:1251–1258. doi: 10.1016/s0959-8049(03)00239-9. [DOI] [PubMed] [Google Scholar]
- 45.Raju J, Bielecki A, Caldwell D, Lok E, Taylor M, Kapal K, Curran I, Cooke GM, Bird RP, Mehta R. Soy isoflavones modulate azoxymethane-induced rat colon carcinogenesis exposed pre- and postnatally and inhibit growth of DLD-1 human colon adenocarcinoma cells by increasing the expression of estrogen receptor-beta. J Nutr. 2009;139:474–481. doi: 10.3945/jn.108.099200. [DOI] [PubMed] [Google Scholar]
- 46.Arai N, Strom A, Rafter JJ, Gustafsson JA. Estrogen receptor beta mRNA in colon cancer cells: growth effects of estrogen and genistein. Biochem Biophys Res Commun. 2000;270:425–431. doi: 10.1006/bbrc.2000.2444. [DOI] [PubMed] [Google Scholar]
- 47.Xiao R, Hennings LJ, Badger TM, Simmen FA. Fetal programming of colon cancer in adult rats: correlations with altered neonatal growth trajectory, circulating IGF-I and IGF binding proteins, and testosterone. J Endocrinol. 2007;195:79–87. doi: 10.1677/JOE-07-0256. [DOI] [PubMed] [Google Scholar]
- 48.Rice S, Whitehead SA. Phytoestrogens and breast cancer–promoters or protectors? Endocr Relat Cancer. 2006;13:995–1015. doi: 10.1677/erc.1.01159. [DOI] [PubMed] [Google Scholar]
- 49.Hsieh CY, Santell RC, Haslam SZ, Helferich WG. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998;58:3833–3838. [PubMed] [Google Scholar]
- 50.Ju YH, Fultz J, Allred KF, Doerge DR, Helferich WG. Effects of dietary daidzein and its metabolite, equol, at physiological concentrations on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Carcinogenesis. 2006;27:856–863. doi: 10.1093/carcin/bgi320. [DOI] [PubMed] [Google Scholar]
- 51.Mak P, Leung YK, Tang WY, Harwood C, Ho SM. Apigenin suppresses cancer cell growth through ERbeta. Neoplasia. 2006;8:896–904. doi: 10.1593/neo.06538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seo HS, DeNardo DG, Jacquot Y, Laios I, Vidal DS, Zambrana CR, Leclercq G, Brown PH. Stimulatory effect of genistein and apigenin on the growth of breast cancer cells correlates with their ability to activate ER alpha. Breast Cancer Res Treat. 2006;99:121–134. doi: 10.1007/s10549-006-9191-2. [DOI] [PubMed] [Google Scholar]
- 53.van Meeuwen JA, Korthagen N, de Jong PC, Piersma AH, van den Berg M. (Anti) estrogenic effects of phytochemicals on human primary mammary fibroblasts, MCF-7 cells and their co-culture. Toxicol Appl Pharmacol. 2007;221:372–383. doi: 10.1016/j.taap.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 54.Welshons WV, Murphy CS, Koch R, Calaf G, Jordan VC. Stimulation of breast cancer cells in vitro by the environmental estrogen enterolactone and the phytoestrogen equol. Breast Cancer Res Treat. 1987;10:169–175. doi: 10.1007/BF01810580. [DOI] [PubMed] [Google Scholar]
- 55.Allred CD, Allred KF, Ju YH, Virant SM, Helferich WG. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res. 2001;61:5045–5050. [PubMed] [Google Scholar]
- 56.Ju YH, Allred CD, Allred KF, Karko KL, Doerge DR, Helferich WG. Physiological concentrations of dietary genistein dose-dependently stimulate growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in athymic nude mice. J Nutr. 2001;131:2957–2962. doi: 10.1093/jn/131.11.2957. [DOI] [PubMed] [Google Scholar]
- 57.Ju YH, Doerge DR, Allred KF, Allred CD, Helferich WG. Dietary genistein negates the inhibitory effect of tamoxifen on growth of estrogen-dependent human breast cancer (MCF-7) cells implanted in athymic mice. Cancer Res. 2002;62:2474–2477. [PubMed] [Google Scholar]
- 58.Hargreaves DF, Potten CS, Harding C, Shaw LE, Morton MS, Roberts SA, Howell A, Bundred NJ. Two-week dietary soy supplementation has an estrogenic effect on normal premenopausal breast. J Clin Endocrinol Metab. 1999;84:4017–4024. doi: 10.1210/jcem.84.11.6152. [DOI] [PubMed] [Google Scholar]
- 59.Power KA, Saarinen NM, Chen JM, Thompson LU. Mammalian lignans enterolactone and enterodiol, alone and in combination with the isoflavone genistein, do not promote the growth of MCF-7 xenografts in ovariectomized athymic nude mice. Int J Cancer. 2006;118:1316–1320. doi: 10.1002/ijc.21464. [DOI] [PubMed] [Google Scholar]
- 60.Mersereau JE, Levy N, Staub RE, Baggett S, Zogovic T, Chow S, Ricke WA, Tagliaferri M, Cohen I, Bjeldanes LF, Leitman DC. Liquiritigenin is a plant-derived highly selective estrogen receptor beta agonist. Mol Cell Endocrinol. 2008;283:49–57. doi: 10.1016/j.mce.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sotoca AM, Ratman D, van der Saag P, Strom A, Gustafsson JA, Vervoort J, Rietjens IM, Murk AJ. Phytoestrogen-mediated inhibition of proliferation of the human T47D breast cancer cells depends on the ERalpha/ERbeta ratio. J Steroid Biochem Mol Biol. 2008;112:171–178. doi: 10.1016/j.jsbmb.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 63.Pettersson K, Delaunay F, Gustafsson JA. Estrogen receptor beta acts as a dominant regulator of estrogen signaling. Oncogene. 2000;19:4970–4978. doi: 10.1038/sj.onc.1203828. [DOI] [PubMed] [Google Scholar]
- 64.Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 65.(2002) Global assessment on the state of the science of endocrine disruptors. IPCS/WHO
- 66.Tiemann U. In vivo and in vitro effects of the organochlorine pesticides DDT, TCPM, methoxychlor, and lindane on the female reproductive tract of mammals: a review. Reprod Toxicol. 2008;25:316–326. doi: 10.1016/j.reprotox.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 67.Buteau-Lozano H, Velasco G, Cristofari M, Balaguer P, Perrot-Applanat M. Xenoestrogens modulate vascular endothelial growth factor secretion in breast cancer cells through an estrogen receptor-dependent mechanism. J Endocrinol. 2008;196:399–412. doi: 10.1677/JOE-07-0198. [DOI] [PubMed] [Google Scholar]
- 68.Molina-Molina JM, Hillenweck A, Jouanin I, Zalko D, Cravedi JP, Fernandez MF, Pillon A, Nicolas JC, Olea N, Balaguer P. Steroid receptor profiling of vinclozolin and its primary metabolites. Toxicol Appl Pharmacol. 2006;216:44–54. doi: 10.1016/j.taap.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 69.Lemaire G, Mnif W, Mauvais P, Balaguer P, Rahmani R. Activation of alpha- and beta-estrogen receptors by persistent pesticides in reporter cell lines. Life Sci. 2006;79:1160–1169. doi: 10.1016/j.lfs.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 70.Gomez E, Pillon A, Fenet H, Rosain D, Duchesne MJ, Nicolas JC, Balaguer P, Casellas C. Estrogenic activity of cosmetic components in reporter cell lines: parabens, UV screens, and musks. J Toxicol Environ Health A. 2005;68:239–251. doi: 10.1080/15287390590895054. [DOI] [PubMed] [Google Scholar]
- 71.Younglai EV, Foster WG, Hughes EG, Trim K, Jarrell JF. Levels of environmental contaminants in human follicular fluid, serum, and seminal plasma of couples undergoing in vitro fertilization. Arch Environ Contam Toxicol. 2002;43:121–126. doi: 10.1007/s00244-001-0048-8. [DOI] [PubMed] [Google Scholar]
- 72.Kannan N, Tanabe S, Ono M, Tatsukawa R. Critical evaluation of polychlorinated biphenyl toxicity in terrestrial and marine mammals: increasing impact of non-ortho and mono-ortho coplanar polychlorinated biphenyls from land to ocean. Arch Environ Contam Toxicol. 1989;18:850–857. doi: 10.1007/BF01160300. [DOI] [PubMed] [Google Scholar]
- 73.McFarland VA, Clarke JU. Environmental occurrence, abundance, and potential toxicity of polychlorinated biphenyl congeners: considerations for a congener-specific analysis. Environ Health Perspect. 1989;81:225–239. doi: 10.1289/ehp.8981225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carpenter DO. Polychlorinated biphenyls (PCBs): routes of exposure and effects on human health. Rev Environ Health. 2006;21:1–23. doi: 10.1515/reveh.2006.21.1.1. [DOI] [PubMed] [Google Scholar]
- 75.Meerts IA, Lilienthal H, Hoving S, van den Berg JH, Weijers BM, Bergman A, Koeman JH, Brouwer A. Developmental exposure to 4-hydroxy-2, 3, 3′, 4′, 5-pentachlorobiphenyl (4-OH-CB107): long-term effects on brain development, behavior, and brain stem auditory evoked potentials in rats. Toxicol Sci. 2004;82:207–218. doi: 10.1093/toxsci/kfh252. [DOI] [PubMed] [Google Scholar]
- 76.Meerts IA, Hoving S, van den Berg JH, Weijers BM, Swarts HJ, van der Beek EM, Bergman A, Koeman JH, Brouwer A. Effects of in utero exposure to 4-hydroxy-2, 3, 3′, 4′, 5-pentachlorobiphenyl (4-OH-CB107) on developmental landmarks, steroid hormone levels, and female estrous cyclicity in rats. Toxicol Sci. 2004;82:259–267. doi: 10.1093/toxsci/kfh251. [DOI] [PubMed] [Google Scholar]
- 77.Meerts IA, Assink Y, Cenijn PH, Van Den Berg JH, Weijers BM, Bergman A, Koeman JH, Brouwer A. Placental transfer of a hydroxylated polychlorinated biphenyl and effects on fetal and maternal thyroid hormone homeostasis in the rat. Toxicol Sci. 2002;68:361–371. doi: 10.1093/toxsci/68.2.361. [DOI] [PubMed] [Google Scholar]
- 78.Ptak A, Ludewig G, Gregoraszczuk EL. A low halogenated biphenyl (PCB3) increases CYP1A1 expression and activity via the estrogen receptor beta in the porcine ovary. J Physiol Pharmacol. 2008;59:577–588. [PubMed] [Google Scholar]
- 79.Cappelletti V, Saturno G, Miodini P, Korner W, Daidone MG. Selective modulation of ER-beta by estradiol and xenoestrogens in human breast cancer cell lines. Cell Mol Life Sci. 2003;60:567–576. doi: 10.1007/s000180300048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jansen HT, Cooke PS, Porcelli J, Liu TC, Hansen LG. Estrogenic and antiestrogenic actions of PCBs in the female rat: in vitro and in vivo studies. Reprod Toxicol. 1993;7:237–248. doi: 10.1016/0890-6238(93)90230-5. [DOI] [PubMed] [Google Scholar]
- 81.Salama J, Chakraborty TR, Ng L, Gore AC. Effects of polychlorinated biphenyls on estrogen receptor-beta expression in the anteroventral periventricular nucleus. Environ Health Perspect. 2003;111:1278–1282. doi: 10.1289/ehp.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Safe S, Wormke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha cross-talk and mechanisms of action. Chem Res Toxicol. 2003;16:807–816. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- 83.Brunnberg S, Pettersson K, Rydin E, Matthews J, Hanberg A, Pongratz I. The basic helix-loop-helix-PAS protein ARNT functions as a potent coactivator of estrogen receptor-dependent transcription. Proc Natl Acad Sci USA. 2003;100:6517–6522. doi: 10.1073/pnas.1136688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kietz S, Thomsen JS, Matthews J, Pettersson K, Strom A, Gustafsson JA. The Ah receptor inhibits estrogen-induced estrogen receptor beta in breast cancer cells. Biochem Biophys Res Commun. 2004;320:76–82. doi: 10.1016/j.bbrc.2004.05.132. [DOI] [PubMed] [Google Scholar]
- 85.Noren K, Meironyte D. Certain organochlorine and organobromine contaminants in Swedish human milk in perspective of past 20–30 years. Chemosphere. 2000;40:1111–1123. doi: 10.1016/s0045-6535(99)00360-4. [DOI] [PubMed] [Google Scholar]
- 86.Meironyte D, Noren K, Bergman A. Analysis of polybrominated diphenyl ethers in Swedish human milk. A time-related trend study, 1972–1997. J Toxicol Environ Health A. 1999;58:329–341. doi: 10.1080/009841099157197. [DOI] [PubMed] [Google Scholar]
- 87.Darnerud PO. Toxic effects of brominated flame retardants in man and in wildlife. Environ Int. 2003;29:841–853. doi: 10.1016/S0160-4120(03)00107-7. [DOI] [PubMed] [Google Scholar]
- 88.Ceccatelli R, Faass O, Schlumpf M, Lichtensteiger W. Gene expression and estrogen sensitivity in rat uterus after developmental exposure to the polybrominated diphenylether PBDE 99 and PCB. Toxicology. 2006;220:104–116. doi: 10.1016/j.tox.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 89.Gregoraszczuk EL, Ptak A, Skaare JU, Mularz K, Chmielowiec A, Wojtowicz A, Ropstad E. Mechanisms of action of two different natural mixtures of persistent organic pollutants (POPs) in ovarian follicles. Xenobiotica. 2009;39:80–89. doi: 10.1080/00498250802578468. [DOI] [PubMed] [Google Scholar]
- 90.Gregoraszczuk EL, Milczarek K, Wojtowicz AK, Berg V, Skaare JU, Ropstad E. Steroid secretion following exposure of ovarian follicular cells to three different natural mixtures of persistent organic pollutants (POPs) Reprod Toxicol. 2008;25:58–66. doi: 10.1016/j.reprotox.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 91.Le HH, Carlson EM, Chua JP, Belcher SM. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol Lett. 2008;176:149–156. doi: 10.1016/j.toxlet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–S69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 93.Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 94.Paris F, Balaguer P, Terouanne B, Servant N, Lacoste C, Cravedi JP, Nicolas JC, Sultan C. Phenylphenols, biphenols, bisphenol-A and 4-tert-octylphenol exhibit alpha and beta estrogen activities and antiandrogen activity in reporter cell lines. Mol Cell Endocrinol. 2002;193:43–49. doi: 10.1016/s0303-7207(02)00094-1. [DOI] [PubMed] [Google Scholar]
- 95.Gaido KW, Maness SC, McDonnell DP, Dehal SS, Kupfer D, Safe S. Interaction of methoxychlor and related compounds with estrogen receptor alpha and beta, and androgen receptor: structure-activity studies. Mol Pharmacol. 2000;58:852–858. [PubMed] [Google Scholar]
- 96.Seidlova-Wuttke D, Jarry H, Wuttke W. Pure estrogenic effect of benzophenone-2 (BP2) but not of bisphenol A (BPA) and dibutylphtalate (DBP) in uterus, vagina and bone. Toxicology. 2004;205:103–112. doi: 10.1016/j.tox.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 97.Matthews JB, Twomey K, Zacharewski TR. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors alpha and beta. Chem Res Toxicol. 2001;14:149–157. doi: 10.1021/tx0001833. [DOI] [PubMed] [Google Scholar]
- 98.Kurosawa T, Hiroi H, Tsutsumi O, Ishikawa T, Osuga Y, Fujiwara T, Inoue S, Muramatsu M, Momoeda M, Taketani Y. The activity of bisphenol A depends on both the estrogen receptor subtype and the cell type. Endocr J. 2002;49:465–471. doi: 10.1507/endocrj.49.465. [DOI] [PubMed] [Google Scholar]
- 99.Schönfelder G, Friedrich K, Paul M, Chahoud I. Developmental effects of prenatal exposure to bisphenol a on the uterus of rat offspring. Neoplasia. 2004;6:584–594. doi: 10.1593/neo.04217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Patisaul HB, Bateman HL. Neonatal exposure to endocrine active compounds or an ERbeta agonist increases adult anxiety and aggression in gonadally intact male rats. Horm Behav. 2008;53:580–588. doi: 10.1016/j.yhbeh.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 101.Walf AA, Rhodes ME, Frye CA. Antidepressant effects of ERbeta-selective estrogen receptor modulators in the forced swim test. Pharmacol Biochem Behav. 2004;78:523–529. doi: 10.1016/j.pbb.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 102.Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- 103.Chapin RE, Harris MW, Davis BJ, Ward SM, Wilson RE, Mauney MA, Lockhart AC, Smialowicz RJ, Moser VC, Burka LT, Collins BJ. The effects of perinatal/juvenile methoxychlor exposure on adult rat nervous, immune, and reproductive system function. Fundam Appl Toxicol. 1997;40:138–157. doi: 10.1006/faat.1997.2381. [DOI] [PubMed] [Google Scholar]
- 104.Hall DL, Payne LA, Putnam JM, Huet-Hudson YM. Effect of methoxychlor on implantation and embryo development in the mouse. Reprod Toxicol. 1997;11:703–708. doi: 10.1016/s0890-6238(97)00026-9. [DOI] [PubMed] [Google Scholar]
- 105.Golub MS, Germann SL, Hogrefe CE. Endocrine disruption and cognitive function in adolescent female rhesus monkeys. Neurotoxicol Teratol. 2004;26:799–809. doi: 10.1016/j.ntt.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 106.Armenti AE, Zama AM, Passantino L, Uzumcu M. Developmental methoxychlor exposure affects multiple reproductive parameters and ovarian folliculogenesis and gene expression in adult rats. Toxicol Appl Pharmacol. 2008;233:286–296. doi: 10.1016/j.taap.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takeuchi S, Iida M, Kobayashi S, Jin K, Matsuda T, Kojima H. Differential effects of phthalate esters on transcriptional activities via human estrogen receptors alpha and beta, and androgen receptor. Toxicology. 2005;210:223–233. doi: 10.1016/j.tox.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 108.Kohn MC, Parham F, Masten SA, Portier CJ, Shelby MD, Brock JW, Needham LL. Human exposure estimates for phthalates. Environ Health Perspect. 2000;108:A440–A442. doi: 10.1289/ehp.108-a440b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hallmark N, Walker M, McKinnell C, Mahood IK, Scott H, Bayne R, Coutts S, Anderson RA, Greig I, Morris K, Sharpe RM. Effects of monobutyl and di(n-butyl) phthalate in vitro on steroidogenesis and Leydig cell aggregation in fetal testis explants from the rat: comparison with effects in vivo in the fetal rat and neonatal marmoset and in vitro in the human. Environ Health Perspect. 2007;115:390–396. doi: 10.1289/ehp.9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Foryst-Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, Frost N, Krikov M, Bhanot S, Barros R, Morani A, Gustafsson JA, Unger T, Kintscher U. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS Genet. 2008;4:e1000108. doi: 10.1371/journal.pgen.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grun F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147:S50–S55. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- 112.Grun F, Watanabe H, Zamanian Z, Maeda L, Arima K, Cubacha R, Gardiner DM, Kanno J, Iguchi T, Blumberg B. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol Endocrinol. 2006;20:2141–2155. doi: 10.1210/me.2005-0367. [DOI] [PubMed] [Google Scholar]
- 113.Swedenborg E, Ruegg J, Makela S, Pongratz I (2009) Endocrine disruptive chemicals: mechanisms of action and involvement in metabolic disorders. J Mol Endocrinol (in press) [DOI] [PubMed]
- 114.Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor transcription and transactivation: Estrogen receptor alpha and estrogen receptor beta: regulation by selective estrogen receptor modulators and importance in breast cancer. Breast Cancer Res. 2000;2:335–344. doi: 10.1186/bcr78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Osborne CK, Shou J, Massarweh S, Schiff R. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res. 2005;11:865s–870s. [PubMed] [Google Scholar]
- 116.Fagan DH, Yee D. Crosstalk between IGF1R and estrogen receptor signaling in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:423–429. doi: 10.1007/s10911-008-9098-0. [DOI] [PubMed] [Google Scholar]
- 117.Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, Giguere V. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol Endocrinol. 1997;11:353–365. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- 118.Tremblay A, Tremblay GB, Labrie F, Giguere V. Ligand-independent recruitment of SRC-1 to estrogen receptor beta through phosphorylation of activation function AF-1. Mol Cell. 1999;3:513–519. doi: 10.1016/s1097-2765(00)80479-7. [DOI] [PubMed] [Google Scholar]
- 119.Tremblay A, Giguere V. Contribution of steroid receptor coactivator-1 and CREB binding protein in ligand-independent activity of estrogen receptor beta. J Steroid Biochem Mol Biol. 2001;77:19–27. doi: 10.1016/s0960-0760(01)00031-0. [DOI] [PubMed] [Google Scholar]
- 120.Driggers PH, Segars JH, Rubino DM. The proto-oncoprotein Brx activates estrogen receptor beta by a p38 mitogen-activated protein kinase pathway. J Biol Chem. 2001;276:46792–46797. doi: 10.1074/jbc.M106927200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.St-Laurent V, Sanchez M, Charbonneau C, Tremblay A. Selective hormone-dependent repression of estrogen receptor beta by a p38-activated ErbB2/ErbB3 pathway. J Steroid Biochem Mol Biol. 2005;94:23–37. doi: 10.1016/j.jsbmb.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 122.Font de Mora J, Brown M. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol. 2000;20:5041–5047. doi: 10.1128/mcb.20.14.5041-5047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Frigo DE, Basu A, Nierth-Simpson EN, Weldon CB, Dugan CM, Elliott S, Collins-Burow BM, Salvo VA, Zhu Y, Melnik LI, Lopez GN, Kushner PJ, Curiel TJ, Rowan BG, McLachlan JA, Burow ME. p38 mitogen-activated protein kinase stimulates estrogen-mediated transcription and proliferation through the phosphorylation and potentiation of the p160 coactivator glucocorticoid receptor-interacting protein 1. Mol Endocrinol. 2006;20:971–983. doi: 10.1210/me.2004-0075. [DOI] [PubMed] [Google Scholar]
- 124.Picard N, Charbonneau C, Sanchez M, Licznar A, Busson M, Lazennec G, Tremblay A. Phosphorylation of activation function-1 regulates proteasome-dependent nuclear mobility and E6-associated protein ubiquitin ligase recruitment to the estrogen receptor beta. Mol Endocrinol. 2008;22:317–330. doi: 10.1210/me.2007-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lonard DM, Nawaz Z, Smith CL, O’Malley BW. The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-alpha transactivation. Mol Cell. 2000;5:939–948. doi: 10.1016/s1097-2765(00)80259-2. [DOI] [PubMed] [Google Scholar]
- 126.Stenoien DL, Nye AC, Mancini MG, Patel K, Dutertre M, O’Malley BW, Smith CL, Belmont AS, Mancini MA. Ligand-mediated assembly and real-time cellular dynamics of estrogen receptor alpha-coactivator complexes in living cells. Mol Cell Biol. 2001;21:4404–4412. doi: 10.1128/MCB.21.13.4404-4412.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cheng X, Cole RN, Zaia J, Hart GW. Alternative O-glycosylation/O-phosphorylation of the murine estrogen receptor beta. Biochemistry. 2000;39:11609–11620. doi: 10.1021/bi000755i. [DOI] [PubMed] [Google Scholar]
- 128.Kelly WG, Dahmus ME, Hart GW. RNA polymerase II is a glycoprotein. Modification of the COOH-terminal domain by O-GlcNAc. J Biol Chem. 1993;268:10416–10424. [PubMed] [Google Scholar]
- 129.Cheng X, Hart GW. Alternative O-glycosylation/O-phosphorylation of serine-16 in murine estrogen receptor beta: post-translational regulation of turnover and transactivation activity. J Biol Chem. 2001;276:10570–10575. doi: 10.1074/jbc.M010411200. [DOI] [PubMed] [Google Scholar]
- 130.Chen YX, Du JT, Zhou LX, Liu XH, Zhao YF, Nakanishi H, Li YM. Alternative O-GlcNAcylation/O-phosphorylation of Ser16 induce different conformational disturbances to the N terminus of murine estrogen receptor beta. Chem Biol. 2006;13:937–944. doi: 10.1016/j.chembiol.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 131.Galluzzo P, Caiazza F, Moreno S, Marino M. Role of ERbeta palmitoylation in the inhibition of human colon cancer cell proliferation. Endocr Relat Cancer. 2007;14:153–167. doi: 10.1677/ERC-06-0020. [DOI] [PubMed] [Google Scholar]
- 132.Marino M, Ascenzi P. Membrane association of estrogen receptor alpha and beta influences 17beta-estradiol-mediated cancer cell proliferation. Steroids. 2008;73:853–858. doi: 10.1016/j.steroids.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 133.Marino M, Galluzzo P, Leone S, Acconcia F, Ascenzi P. Nitric oxide impairs the 17beta-estradiol-induced apoptosis in human colon adenocarcinoma cells. Endocr Relat Cancer. 2006;13:559–569. doi: 10.1677/erc.1.01106. [DOI] [PubMed] [Google Scholar]
- 134.Copeland RJ, Bullen JW, Hart GW. Cross-talk between GlcNAcylation and phosphorylation: roles in insulin resistance and glucose toxicity. Am J Physiol Endocrinol Metab. 2008;295:E17–E28. doi: 10.1152/ajpendo.90281.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Barros RP, Machado UF, Gustafsson JA. Estrogen receptors: new players in diabetes mellitus. Trends Mol Med. 2006;12:425–431. doi: 10.1016/j.molmed.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 136.Takeyama J, Suzuki T, Inoue S, Kaneko C, Nagura H, Harada N, Sasano H. Expression and cellular localization of estrogen receptors alpha and beta in the human fetus. J Clin Endocrinol Metab. 2001;86:2258–2262. doi: 10.1210/jcem.86.5.7447. [DOI] [PubMed] [Google Scholar]
- 137.Schaub CE, Gersting JA, Keller-Wood M, Wood CE. Development of ER-alpha and ER-beta expression in the developing ovine brain and pituitary. Gene Expr Patterns. 2008;8:457–463. doi: 10.1016/j.gep.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 138.Sugiyama N, Andersson S, Lathe R, Fan X, Alonso-Magdalena P, Schwend T, Nalvarte I, Warner M, Gustafsson JA. Spatiotemporal dynamics of the expression of estrogen receptors in the postnatal mouse brain. Mol Psychiatry. 2009;14:223–232. doi: 10.1038/mp.2008.118. [DOI] [PubMed] [Google Scholar]
- 139.Fan X, Warner M, Gustafsson JA. Estrogen receptor beta expression in the embryonic brain regulates development of calretinin-immunoreactive GABAergic interneurons. Proc Natl Acad Sci USA. 2006;103:19338–19343. doi: 10.1073/pnas.0609663103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cai W, Rambaud J, Teboul M, Masse I, Benoit G, Gustafsson JA, Delaunay F, Laudet V, Pongratz I. Expression levels of estrogen receptor beta are modulated by components of the molecular clock. Mol Cell Biol. 2008;28:784–793. doi: 10.1128/MCB.00233-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ripperger JA, Schibler U. Circadian regulation of gene expression in animals. Curr Opin Cell Biol. 2001;13:357–362. doi: 10.1016/s0955-0674(00)00220-9. [DOI] [PubMed] [Google Scholar]
- 142.Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119:693–705. doi: 10.1016/j.cell.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 143.Zhang J, Dong X, Fujimoto Y, Okamura H. Molecular signals of mammalian circadian clock. Kobe J Med Sci. 2004;50:101–109. [PubMed] [Google Scholar]
- 144.Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 145.Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 146.Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- 147.Knutsson A. Health disorders of shift workers. Occup Med (Lond) 2003;53:103–108. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- 148.Labyak S, Lava S, Turek F, Zee P. Effects of shiftwork on sleep and menstrual function in nurses. Health Care Women Int. 2002;23:703–714. doi: 10.1080/07399330290107449. [DOI] [PubMed] [Google Scholar]
- 149.Axelsson G, Ahlborg G, Jr, Bodin L. Shift work, nitrous oxide exposure, and spontaneous abortion among Swedish midwives. Occup Environ Med. 1996;53:374–378. doi: 10.1136/oem.53.6.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu X, Ding M, Li B, Christiani DC. Association of rotating shiftwork with preterm births and low birth weight among never smoking women textile workers in China. Occup Environ Med. 1994;51:470–474. doi: 10.1136/oem.51.7.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Healy D, Minors DS, Waterhouse JM. Shiftwork, helplessness and depression. J Affect Disord. 1993;29:17–25. doi: 10.1016/0165-0327(93)90114-y. [DOI] [PubMed] [Google Scholar]
- 152.Scott AJ, Monk TH, Brink LL. Shiftwork as a risk factor for depression: a pilot study. Int J Occup Environ Health. 1997;3:S2–S9. [PubMed] [Google Scholar]
- 153.Rocha BA, Fleischer R, Schaeffer JM, Rohrer SP, Hickey GJ. 17 Beta-estradiol-induced antidepressant-like effect in the forced swim test is absent in estrogen receptor-beta knockout (BERKO) mice. Psychopharmacology (Berl) 2005;179:637–643. doi: 10.1007/s00213-004-2078-1. [DOI] [PubMed] [Google Scholar]
- 154.Hughes ZA, Liu F, Platt BJ, Dwyer JM, Pulicicchio CM, Zhang G, Schechter LE, Rosenzweig-Lipson S, Day M. WAY-200070, a selective agonist of estrogen receptor beta as a potential novel anxiolytic/antidepressant agent. Neuropharmacology. 2008;54:1136–1142. doi: 10.1016/j.neuropharm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 155.Fu L, Lee CC. The circadian clock: pacemaker and tumour suppressor. Nat Rev Cancer. 2003;3:350–361. doi: 10.1038/nrc1072. [DOI] [PubMed] [Google Scholar]
- 156.Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P. Loss of ERbeta expression as a common step in estrogen-dependent tumor progression. Endocr Relat Cancer. 2004;11:537–551. doi: 10.1677/erc.1.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Campbell-Thompson M, Lynch IJ, Bhardwaj B. Expression of estrogen receptor (ER) subtypes and ERbeta isoforms in colon cancer. Cancer Res. 2001;61:632–640. [PubMed] [Google Scholar]
- 158.Mostafaie N, Kallay E, Sauerzapf E, Bonner E, Kriwanek S, Cross HS, Huber KR, Krugluger W (2009) Correlated downregulation of estrogen receptor beta and the circadian clock gene Per1 in human colorectal cancer. Mol Carcinog (in press) [DOI] [PubMed]
- 159.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 160.Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128:669–681. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 161.Munshi A, Shafi G, Aliya N, Jyothy A. Histone modifications dictate specific biological readouts. J Genet Genomics. 2009;36:75–88. doi: 10.1016/S1673-8527(08)60094-6. [DOI] [PubMed] [Google Scholar]
- 162.Li LC, Yeh CC, Nojima D, Dahiya R. Cloning and characterization of human estrogen receptor beta promoter. Biochem Biophys Res Commun. 2000;275:682–689. doi: 10.1006/bbrc.2000.3363. [DOI] [PubMed] [Google Scholar]
- 163.Hirata S, Shoda T, Kato J, Hoshi K. The multiple untranslated first exons system of the human estrogen receptor beta (ER beta) gene. J Steroid Biochem Mol Biol. 2001;78:33–40. doi: 10.1016/s0960-0760(01)00071-1. [DOI] [PubMed] [Google Scholar]
- 164.Weihua Z, Warner M, Gustafsson JA. Estrogen receptor beta in the prostate. Mol Cell Endocrinol. 2002;193:1–5. doi: 10.1016/s0303-7207(02)00089-8. [DOI] [PubMed] [Google Scholar]
- 165.Weihua Z, Makela S, Andersson LC, Salmi S, Saji S, Webster JI, Jensen EV, Nilsson S, Warner M, Gustafsson JA. A role for estrogen receptor beta in the regulation of growth of the ventral prostate. Proc Natl Acad Sci USA. 2001;98:6330–6335. doi: 10.1073/pnas.111150898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Montano MM, Katzenellenbogen BS. The quinone reductase gene: a unique estrogen receptor-regulated gene that is activated by antiestrogens. Proc Natl Acad Sci USA. 1997;94:2581–2586. doi: 10.1073/pnas.94.6.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Montano MM, Wittmann BM, Bianco NR. Identification and characterization of a novel factor that regulates quinone reductase gene transcriptional activity. J Biol Chem. 2000;275:34306–34313. doi: 10.1074/jbc.M003880200. [DOI] [PubMed] [Google Scholar]
- 168.Montano MM, Deng H, Liu M, Sun X, Singal R. Transcriptional regulation by the estrogen receptor of antioxidative stress enzymes and its functional implications. Oncogene. 2004;23:2442–2453. doi: 10.1038/sj.onc.1207358. [DOI] [PubMed] [Google Scholar]
- 169.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Horvath LG, Henshall SM, Lee CS, Head DR, Quinn DI, Makela S, Delprado W, Golovsky D, Brenner PC, O’Neill G, Kooner R, Stricker PD, Grygiel JJ, Gustafsson JA, Sutherland RL. Frequent loss of estrogen receptor-beta expression in prostate cancer. Cancer Res. 2001;61:5331–5335. [PubMed] [Google Scholar]
- 171.Latil A, Bieche I, Vidaud D, Lidereau R, Berthon P, Cussenot O, Vidaud M. Evaluation of androgen, estrogen (ER alpha and ER beta), and progesterone receptor expression in human prostate cancer by real-time quantitative reverse transcription-polymerase chain reaction assays. Cancer Res. 2001;61:1919–1926. [PubMed] [Google Scholar]
- 172.Pasquali D, Rossi V, Esposito D, Abbondanza C, Puca GA, Bellastella A, Sinisi AA. Loss of estrogen receptor beta expression in malignant human prostate cells in primary cultures and in prostate cancer tissues. J Clin Endocrinol Metab. 2001;86:2051–2055. doi: 10.1210/jcem.86.5.7441. [DOI] [PubMed] [Google Scholar]
- 173.Leav I, Lau KM, Adams JY, McNeal JE, Taplin ME, Wang J, Singh H, Ho SM. Comparative studies of the estrogen receptors beta and alpha and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am J Pathol. 2001;159:79–92. doi: 10.1016/s0002-9440(10)61676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ho SM, Leung YK, Chung I. Estrogens and antiestrogens as etiological factors and therapeutics for prostate cancer. Ann N Y Acad Sci. 2006;1089:177–193. doi: 10.1196/annals.1386.005. [DOI] [PubMed] [Google Scholar]
- 175.Nojima D, Li LC, Dharia A, Perinchery G, Ribeiro-Filho L, Yen TS, Dahiya R. CpG hypermethylation of the promoter region inactivates the estrogen receptor-beta gene in patients with prostate carcinoma. Cancer. 2001;92:2076–2083. doi: 10.1002/1097-0142(20011015)92:8<2076::aid-cncr1548>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 176.Sasaki M, Tanaka Y, Perinchery G, Dharia A, Kotcherguina I, Fujimoto S, Dahiya R. Methylation and inactivation of estrogen, progesterone, and androgen receptors in prostate cancer. J Natl Cancer Inst. 2002;94:384–390. doi: 10.1093/jnci/94.5.384. [DOI] [PubMed] [Google Scholar]
- 177.Zhu X, Leav I, Leung YK, Wu M, Liu Q, Gao Y, McNeal JE, Ho SM. Dynamic regulation of estrogen receptor-beta expression by DNA methylation during prostate cancer development and metastasis. Am J Pathol. 2004;164:2003–2012. doi: 10.1016/s0002-9440(10)63760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang X, Leung YK, Ho SM. AP-2 regulates the transcription of estrogen receptor (ER)-beta by acting through a methylation hotspot of the 0N promoter in prostate cancer cells. Oncogene. 2007;26:7346–7354. doi: 10.1038/sj.onc.1210537. [DOI] [PubMed] [Google Scholar]
- 179.Ruiz M, Troncoso P, Bruns C, Bar-Eli M. Activator protein 2alpha transcription factor expression is associated with luminal differentiation and is lost in prostate cancer. Clin Cancer Res. 2001;7:4086–4095. [PubMed] [Google Scholar]
- 180.Pujol P, Rey JM, Nirde P, Roger P, Gastaldi M, Laffargue F, Rochefort H, Maudelonde T. Differential expression of estrogen receptor-alpha and -beta messenger RNAs as a potential marker of ovarian carcinogenesis. Cancer Res. 1998;58:5367–5373. [PubMed] [Google Scholar]
- 181.Tong D, Schuster E, Seifert M, Czerwenka K, Leodolte S, Zeillinger R. Expression of estrogen receptor beta isoforms in human breast cancer tissues and cell lines. Breast Cancer Res Treat. 2002;71:249–255. doi: 10.1023/a:1014465916473. [DOI] [PubMed] [Google Scholar]
- 182.Brandenberger AW, Tee MK, Jaffe RB. Estrogen receptor alpha (ER-alpha) and beta (ER-beta) mRNAs in normal ovary, ovarian serous cystadenocarcinoma and ovarian cancer cell lines: down-regulation of ER-beta in neoplastic tissues. J Clin Endocrinol Metab. 1998;83:1025–1028. doi: 10.1210/jcem.83.3.4788. [DOI] [PubMed] [Google Scholar]
- 183.Rutherford T, Brown WD, Sapi E, Aschkenazi S, Munoz A, Mor G. Absence of estrogen receptor-beta expression in metastatic ovarian cancer. Obstet Gynecol. 2000;96:417–421. doi: 10.1016/s0029-7844(00)00917-0. [DOI] [PubMed] [Google Scholar]
- 184.Bardin A, Hoffmann P, Boulle N, Katsaros D, Vignon F, Pujol P, Lazennec G. Involvement of estrogen receptor beta in ovarian carcinogenesis. Cancer Res. 2004;64:5861–5869. doi: 10.1158/0008-5472.CAN-04-0552. [DOI] [PubMed] [Google Scholar]
- 185.Hartman J, Lindberg K, Morani A, Inzunza J, Strom A, Gustafsson JA. Estrogen receptor beta inhibits angiogenesis and growth of T47D breast cancer xenografts. Cancer Res. 2006;66:11207–11213. doi: 10.1158/0008-5472.CAN-06-0017. [DOI] [PubMed] [Google Scholar]
- 186.Zhao C, Lam EW, Sunters A, Enmark E, De Bella MT, Coombes RC, Gustafsson JA, Dahlman-Wright K. Expression of estrogen receptor beta isoforms in normal breast epithelial cells and breast cancer: regulation by methylation. Oncogene. 2003;22:7600–7606. doi: 10.1038/sj.onc.1207100. [DOI] [PubMed] [Google Scholar]
- 187.Rody A, Holtrich U, Solbach C, Kourtis K, von Minckwitz G, Engels K, Kissler S, Gatje R, Karn T, Kaufmann M. Methylation of estrogen receptor beta promoter correlates with loss of ER-beta expression in mammary carcinoma and is an early indication marker in premalignant lesions. Endocr Relat Cancer. 2005;12:903–916. doi: 10.1677/erc.1.01088. [DOI] [PubMed] [Google Scholar]
- 188.Ogawa Y, Lee JT. Antisense regulation in X inactivation and autosomal imprinting. Cytogenet Genome Res. 2002;99:59–65. doi: 10.1159/000071575. [DOI] [PubMed] [Google Scholar]
- 189.Tufarelli C, Stanley JA, Garrick D, Sharpe JA, Ayyub H, Wood WG, Higgs DR. Transcription of antisense RNA leading to gene silencing and methylation as a novel cause of human genetic disease. Nat Genet. 2003;34:157–165. doi: 10.1038/ng1157. [DOI] [PubMed] [Google Scholar]
- 190.Tagoh H, Schebesta A, Lefevre P, Wilson N, Hume D, Busslinger M, Bonifer C. Epigenetic silencing of the c-fms locus during B-lymphopoiesis occurs in discrete steps and is reversible. EMBO J. 2004;23:4275–4285. doi: 10.1038/sj.emboj.7600421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wheeler JM. Epidemiology of endometriosis-associated infertility. J Reprod Med. 1989;34:41–46. [PubMed] [Google Scholar]
- 192.Brandenberger AW, Lebovic DI, Tee MK, Ryan IP, Tseng JF, Jaffe RB, Taylor RN. Oestrogen receptor (ER)-alpha and ER-beta isoforms in normal endometrial and endometriosis-derived stromal cells. Mol Hum Reprod. 1999;5:651–655. doi: 10.1093/molehr/5.7.651. [DOI] [PubMed] [Google Scholar]
- 193.Fujimoto J, Hirose R, Sakaguchi H, Tamaya T. Expression of oestrogen receptor-alpha and -beta in ovarian endometriomata. Mol Hum Reprod. 1999;5:742–747. doi: 10.1093/molehr/5.8.742. [DOI] [PubMed] [Google Scholar]
- 194.Xue Q, Lin Z, Cheng YH, Huang CC, Marsh E, Yin P, Milad MP, Confino E, Reierstad S, Innes J, Bulun SE. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod. 2007;77:681–687. doi: 10.1095/biolreprod.107.061804. [DOI] [PubMed] [Google Scholar]
- 195.Kim J, Kim JY, Song KS, Lee YH, Seo JS, Jelinek J, Goldschmidt-Clermont PJ, Issa JP. Epigenetic changes in estrogen receptor beta gene in atherosclerotic cardiovascular tissues and in vitro vascular senescence. Biochim Biophys Acta. 2007;1772:72–80. doi: 10.1016/j.bbadis.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 196.Liu PY, Christian RC, Ruan M, Miller VM, Fitzpatrick LA. Correlating androgen and estrogen steroid receptor expression with coronary calcification and atherosclerosis in men without known coronary artery disease. J Clin Endocrinol Metab. 2005;90:1041–1046. doi: 10.1210/jc.2004-1211. [DOI] [PubMed] [Google Scholar]
- 197.Christian RC, Liu PY, Harrington S, Ruan M, Miller VM, Fitzpatrick LA. Intimal estrogen receptor (ER)beta, but not ERalpha expression, is correlated with coronary calcification and atherosclerosis in pre- and postmenopausal women. J Clin Endocrinol Metab. 2006;91:2713–2720. doi: 10.1210/jc.2005-2672. [DOI] [PubMed] [Google Scholar]
- 198.Kurkjian C, Kummar S, Murgo AJ. DNA methylation: its role in cancer development and therapy. Curr Probl Cancer. 2008;32:187–235. doi: 10.1016/j.currproblcancer.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhu WG, Otterson GA. The interaction of histone deacetylase inhibitors and DNA methyltransferase inhibitors in the treatment of human cancer cells. Curr Med Chem Anticancer Agents. 2003;3:187–199. doi: 10.2174/1568011033482440. [DOI] [PubMed] [Google Scholar]
- 200.Walton TJ, Li G, Seth R, McArdle SE, Bishop MC, Rees RC. DNA demethylation and histone deacetylation inhibition co-operate to re-express estrogen receptor beta and induce apoptosis in prostate cancer cell-lines. Prostate. 2008;68:210–222. doi: 10.1002/pros.20673. [DOI] [PubMed] [Google Scholar]
- 201.Levi F, Focan C, Karaboue A, de la Valette V, Focan-Henrard D, Baron B, Kreutz F, Giacchetti S. Implications of circadian clocks for the rhythmic delivery of cancer therapeutics. Adv Drug Deliv Rev. 2007;59:1015–1035. doi: 10.1016/j.addr.2006.11.001. [DOI] [PubMed] [Google Scholar]