Abstract
Epstein-Barr virus (EBV) is a human gamma herpes virus that infects B cells and induces their transformation into immortalized lymphoblasts that can grow as cell lines (LCLs) in vitro. EBNA-3 is a member of the EBNA-3-protein family that can regulate transcription of cellular and viral genes. The identification of EBNA-3 cellular partners and a study of its influence on cellular pathways are important for understanding the transforming action of the virus. In this work, we have identified the vitamin D receptor (VDR) protein as a binding partner of EBNA-3. We found that EBNA3 blocks the activation of VDR-dependent genes and protects LCLs against vitamin-D3-induced growth arrest and/or apoptosis. The presented data shed some light on the anti-apoptotic EBV program and the role of the EBNA-3-VDR interaction in the viral strategy.
Keywords: EBV, VDR, Transcription regulation, EBNA, Vitamin D3
Introduction
Epstein-Barr virus (EBV) is a human gamma herpes virus that causes lymphoproliferative disease in patients with immunodeficiencies and is associated with Burkitt’s and Hodgkin’s lymphomas, other B and T cell lymphomas, anaplastic nasopharyngeal carcinoma and some gastric carcinomas [1].
EBV infects B cells and transforms them into immortalized lymphoblasts that grow in vitro as cell lines (LCLs). LCLs express six nuclear proteins (EBNA-1–6) and three membrane proteins (LMP-1, -2A and -2B), out of which five, EBNA-2, EBNA-3, EBNA-5, EBNA-6 and LMP1, are essential for efficient transformation [1].
EBNA-3 is a member of the EBNA-3-protein family that also includes EBNA-4 and EBNA-6. All three proteins do not bind to DNA directly. However, they bind to the major isoform of DNA-binding protein RBP-Jκ, RBP-2N, in B-lymphoblasts, the only host cells where the EBNA-3 family proteins are expressed [2–6]. They can also activate or repress transcription from the LMP-1 promoter in vitro [5], [7, 8]. EBNA-3 may also influence the transcription of cellular genes, such as c-myc, CD21, CD23 [9] and Aryl hydrocarbon receptor (AhR) [10, 11]. Notably, upon EBV infection, many nuclear receptors show an altered expression pattern compared to naïve and mitogen-activated B cells [12, 13]. One of the genes with decreased expression in LCL is the vitamin D receptor (VDR) (GenBank: NP_000367) [13].
We report now that VDR interacts with EBNA-3 in LCL. This binding blocks the transcription of VDR-responsive genes and consequently protects LCLs against vitamin D3-induced growth arrest and/or apoptosis.
Materials and methods
Plasmids
Cloning of the full-length EBNA-3 cDNA was described earlier [14].
Cell lines, transfections and imaging
DG75-EBNA-3, DG75-EBNA-5 (EBV-negative Burkitt’s lymphoma DG75 cell line that expressed EBNA-3 or EBNA-5 constitutively, a kind gift of Lars Rymo, Göteborg, Sweden), MCF7 breast carcinoma cell line and all LCLs were cultured at 37°C in Iscove’s medium that contained 10% fetal bovine serum and an appropriate antibiotic. Periodic staining with Hoechst 33258 (Sigma, St. Louis, MO) monitored the absence of mycoplasma. Prior to the transfection experiments, the MCF7 cells were grown in Petri dishes (diameter 13.5 cm). The cells were transfected with an EBNA-3 construct using Lipofectamine Plus Reagent (Life Technology, Carlsbad, CA) according to the manufacturer’s protocol. Expression of GFP-fusion proteins was shown by Western blotting. Peripheral B-cells were isolated from buffy coat blood (Karolinska Hospital, Stockholm) on Lymphoprep gradients. Two subsequent rounds of E-rosetting removed the T cells. The B95.8 EBV strain was used for B cell infection to establish LCLs.
BK-LCL (wt) and ∆EBNA3-BK-LCL are isogenic lines that were produced by infection of tonsil B cells of one donor (a kind gift of Bettina Kempkes, Munich, Germany, described in detail in [15]).
To measure the proliferation rate, we started from about 10,000 cells in six-well plates. During the growth, cells were transferred sequentially to a 250-ml flask. Cells were counted for 15 days as represented in the log of cell numbers: ×10,000.
1-Alpha,25-dihydroxyvitamin D3 (1,25(OH)2D3), a metabolite of vitamin D3, was purchased from Sigma.
Immunostaining
BK-LCL and ∆EBNA3-BK-LCL cells, untreated and treated with 1,25(OH)2D3, were captured on glass slides using a Cytospin centrifuge. Cells were fixed in a −20°C cold mixture of methanol and acetone (1:1), re-hydrated in PBS and stained with mouse monoclonal anti-VDR (Santa Cruz Biotechnology, Santa Cruz, CA) and anti-PCNA (DAKO, Glostrup, Denmark) antibodies. Secondary rabbit anti-mouse FITC conjugated antibody was purchased from DAKO. The images were captured using a DAS microscope Leitz DM RB with a dual-mode cooled charge-coupled device (CCD) camera C4880 (Hamamatsu, Japan).
Western blotting
We prepared whole cell lysates using NP40 lysis buffer (1% NP40, 150 mM NaCl, 50 mM Tris, pH = 8) with a protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO). Lysates were cleared by centrifugation. All cell lysates contained 0.5% of BSA as a non-specific competitor.
Proteins were separated using sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis (SDS-PAGE). After transfer, the membrane was probed with mouse monoclonal anti-GFP (ABKAM Ltd., Cambridge, UK), anti-EBNA-5 (DAKO), anti-VDR (Santa Cruz Biotechnology), anti-EBNA-6 (a kind gift of Martin Rowe, University of Wales, College of Medicine, Cardiff) antibodies and rabbit polyclonal anti-EBNA-3 antibody [produced against GST-EBNA-3 (C-terminus, residues 773–944) by AsLa Ltd., Riga, Latvia]. Secondary antibodies (anti-rabbit and anti-mouse IgG horseradish-conjugated) were purchased from GE Healthcare Bio-Sciences AB, Uppsala, Sweden.
Immunoprecipitations
For the immunoprecipiations, 5 μg of the antibodies (rabbit anti-EBNA-3 and mouse anti-VDR) and the normal mouse or rabbit serum as a negative control were coupled to the CN-Br Sepharose 4 Fast Flow according manufacturer protocol (GE Healthcare Bio-Sciences AB), and such supports were used to capture the corresponding proteins from the NP40 cell lysates of LCL (usually 1 × 107 cells were used for one probe). After extensive washing with NP40 lysis buffer and PBS, the protein complexes were eluted by heat (94°C) and separated on the SDS-PAGE.
For the mass spectrometry, a similar procedure was performed using mouse anti-GFP for MCF7 cells transfected with GFP-fusion constructs. The starting number of cells was not less than 5 × 107. The obtained protein complexes were separated on SDS-PAGE (both 12 and 8% gels). Gels were fixed in buffer that contained methanol and acetic acid, and were stained with colloidal Coomassie blue (0.2% Coomassie blue, 7.5% acetic acid, 50% ethanol) overnight. Relevant bands were excised and further analyzed by mass spectrometry.
Mass spectrometry
The samples were treated for in-gel digestion essentially as follows [16]. In brief, the stain was removed by washing with acetonitrile and ammonium bicarbonate buffer, and the gel pieces were then dried with acetonitrile. Modified porcine trypsin, sequence grade (Promega, Madison, WI), was allowed to soak into the substrate in the gel. After overnight incubation at 30°C followed by acidification, the generated peptides were analyzed by peptide mass fingerprinting on a MALDI TOF/TOF MS (Ultraflex TOF/TOF, Bruker Daltonics, Bremen, Germany). The instrument was optimized to measure analytes in the range of 600 to 4,500 Da, and alfa-cyano 4-hydroxycinnamic acid served as the matrix. Each spectrum was internally calibrated using autolytic peptides from the trypsin resulting in a precision of about 20 ppm. The identification of the unknown samples was obtained by scanning sequence databases (NCBI nr, latest version) via the search engine ProFound. For further confirmation of an identity, selected peptides were sequenced by Post Source Decay after sulfonation of the N-termini [17].
Real-time PCR
Total RNA was isolated from cell lines using the Total RNA isolation kit (Sigma). RNA was isolated from cell lines (DG75-EBNA-3, DG75-EBNA-5, LCL, BK-LCL and ∆EBNA3-BK-LCL) at normal conditions and after treatment with 1-alpha,25-dihydroxyvitamin D3 (1,25(OH)2D3) for 24 h at a concentration of 1 × 10−7 M.
Approximately 1 μg of total RNA was used for cDNA synthesis using the First Strand Synthesis Kit (Sigma) according to the manufacturer’s protocol. cDNA obtained was used for real-time PCR studies. Primer concentration was adjusted to a final concentration of 3 μM. Total reaction volume for all real-time PCR experiments was 20 μl. Real-time PCR was performed using SYBR GREEN Master mix on a 7900 machine (Applied Biosystems, Foster City, CA).
Primers for VDR responsive genes, such as CYP24A1 (GenBank: NM_000782), GADD45A (GenBank: NM_001924), CYCLIN C (CCNC, GenBank: NM_005190), P21 (CDKN1A, GenBank: NM_000389) and C-FOS (FOS, GenBank: NM_005252) were the following: C-FOS - F-CAAACGCAGGAACAGTGCTA, R-GAGAGCGCCTTTTTACCCTT; P21 - F-CACCAGACTTCTCTGAGCCC, R-GGAA-GGAGGGAATTGGAGAG; CYCLIN C - F-TTTAAGCAAAGGCCTCAGGA, R-TGTGACAGTGTGGATTCGGT; CYP24A1 - F-TGGTTTCAAGCTCTGTGTGC, R-ATTAATGCACGTAAAGCGGC; GADD45A - F-TGTGGGTGTCAGATGGTTGT, R-GATGGCAGGGAACCAAGTTA.
As an internal control for standardization, we used the expression of a gene encoding TATA-binding protein (TBP, accession no.: GenBank: NM_003194). The following primers were used: F-TTTCTTGCCAGTCTGGAC, R-CACGAACCAC GGCACTGATT.
Flow cytometry
The 7-amino actinomycin D (7AAD) and phycoerythrin (PE)-conjugated Annexin V were purchased from BD Biosciences (Franklin Lakes, NJ). Cells that were untreated and treated with 1,25(OH)2D3 were resuspended after washing in Binding Buffer (BD Biosciences) at a concentration of 1 × 106 cells/ml; 100 μl of solution (1 × 105 cells) was taken from each cell line and stained with the optimal concentrations of PE-conjugated Annexin V and 7AAD-PerCP, and incubated at room temperature for 25–30 min. Cells were then washed twice and analyzed using two-color flow cytometry (FACScan, BD Biosciences). The FACS analysis was performed using FlowJo software (Three Star, Stanford, CA).
Results
Interaction of EBNA3 with vitamin D receptor complex
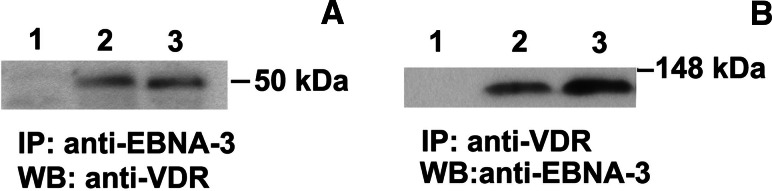
We have found that VDR is expressed at the lower mRNA level in LCL compared to naïve and mitogen-activated B cells [13]. In order to answer the question whether EBNA-3 might bind the vitamin D receptor or not, the immunoprecipitations of LCL lysates were performed using anti-EBNA-3 and anti-VDR antibodies coupled to the surface of CNBr Sepharose beads. Figure 1a shows Western blotting with anti-VDR antibody after immunoprecipitation with rabbit anti-EBNA-3 serum. VDR was detected in immunocomplexes with endogenous EBNA-3 in LCLs. In the converse experiments, the same LCL lysates were immunoprecipitated using anti-VDR antibody on the beads. From the Western blotting with anti-EBNA-3, shown on Fig. 1b, the same conclusion was drawn. Thus, endogenous VDR and EBNA-3 were found in one immunocomplex in LCLs. The negative control (normal mouse or rabbit serum coupled to CNBr Sepharose beads) showed no VDR or EBNA-3 in these experiments (Fig. 1a, b, lane 1). About 5% of the total amount of VDR and EBNA-3 (soluble in NP40 buffer) was found in immunocomplexes.
Fig. 1.
Western blotting after immunoprecipitations from LCL cell lysate. a Immunoprecipitation was performed with the anti-EBNA-3 rabbit serum. Membrane after transfer was probed with mouse anti-VDR antibody. Lane 1 negative control (normal rabbit serum on the CNBr Sepharose beads), lane 2 LCL cell lysate (5% of input), lane 3 anti-EBNA-3 on the surface of CNBr Sepharose beads. b Immunoprecipitation was performed from the same LCL cell lysate with mouse anti-VDR antibody immobilized on the surface of CNBr Sepharose beads. Western blot was probed with anti-EBNA-3 rabbit serum. Lane 1 negative control (normal mouse serum on the CNBr Sepharose beads), lane 2 LCL cell lysate (3% of input), lane 3 anti-VDR on the surface of CNBr Sepharose beads
Mass spectrometry analysis of EBNA-3 interacting proteins
In order to find additional EBNA-3 binding cellular proteins, we performed immuno-capturing assays followed by mass spectrometry.
To achieve a high level of EBNA-3, we transfected MCF7 cells with GFP-EBNA-3 plasmid. The protein complexes that contained GFP-EBNA-3 were obtained by immunoprecipitation, using mouse monoclonal anti-GFP antibody, bound to the surface of the CNBr Sepharose beads. Mock-transfected MCF7 served as a negative control. The precipitated protein complexes were separated by SDS-PAGE (12 and 8% of acrylamide). A few bands absent in the negative control were selected for protein identification by peptide mass fingerprinting (PMF). The bands of ~14, ~16, ~40, ~130 and ~140 kDa were excised and subjected to tryptic digestion, followed by PMF and sequencing by PSD of peptides using mass spectrometry (see Table 1). We could not detect VDR on SDS-PAG by Coomassie blue staining probably because of a high threshold of protein concentration for detection (find a concentration). One of the units of the EBNA-3 immunocomplex was SWI/SNF chromatin remodeling protein (GenBank: NP_620706). Notably, the SWI/SNF protein is a cellular component of the VDR protein complex and plays a regulatory role in the transcription of VDR responsive genes [18].
Table 1.
Results of the analysis of EBNA-3-containing immunocomplexes using mass spectrometry
| Number of band | Protein name | Molecular weight (kDa) | Accession number (GenBank) |
|---|---|---|---|
| 1 | H2A, histone 2 A | 14 | NP_542163.1 |
| 2 | H2B, histone 2 B | 16.4 | AAA63192.1 |
| 3 | Heterogeneous nuclear ribonucleoprotein AB isoform A | 36.2 | NP_112556.2 |
| 4 | SWI/SNF1 chromatin remodeling complex | 138 | NP_620706 |
| 5 | DNA helicase II | 142 | CAA71668.1 |
EBNA3 represses the transcription of VDR responsive genes
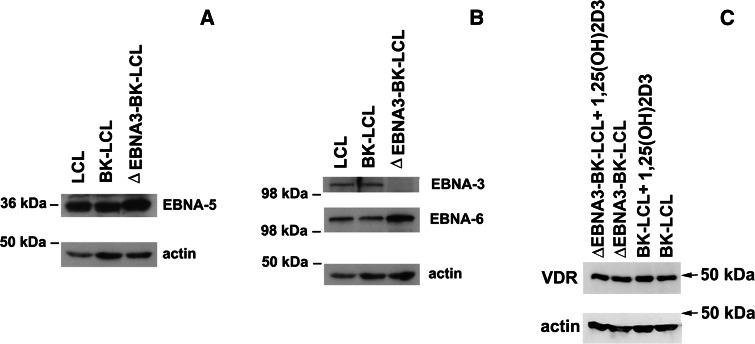
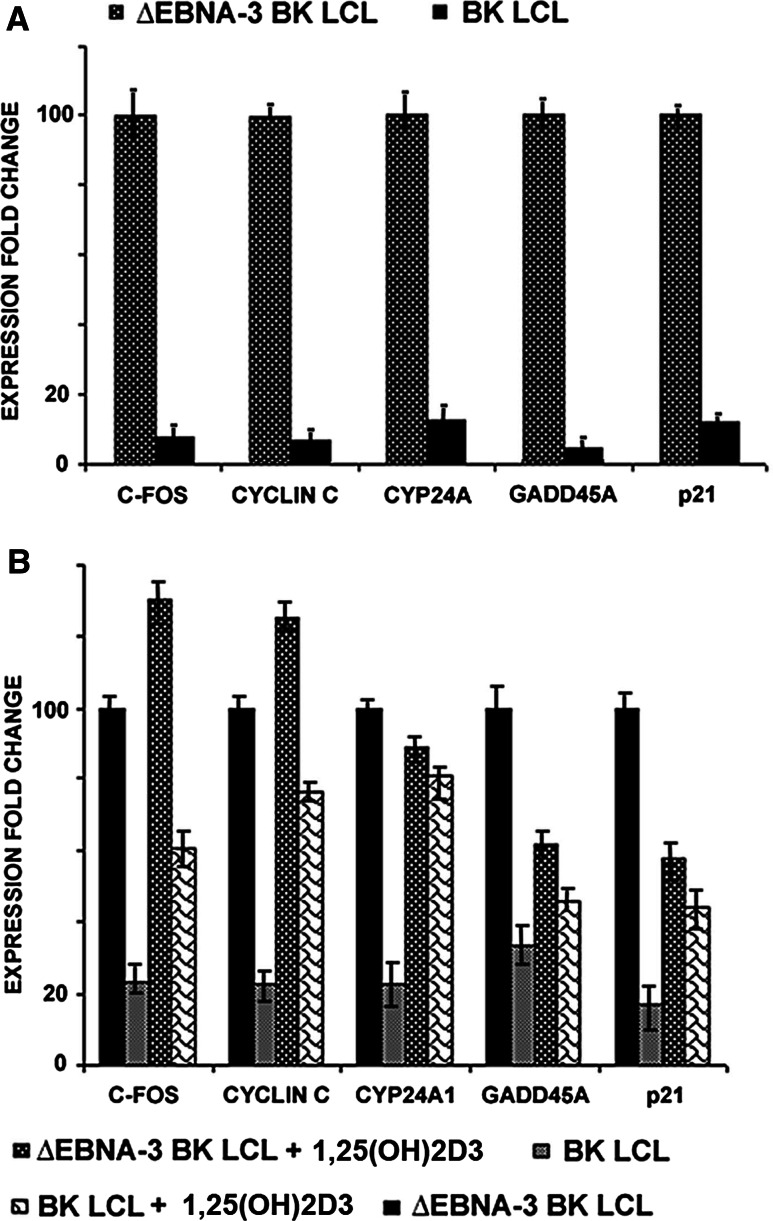
In order to investigate the regulatory role of EBNA3 in the VDR protein complex, transcription of the VDR responsive genes C-FOS, CYCLIN C, CYP24A1, GADD45A and P21 was assayed in the presence and absence of EBNA-3. To do this, the isogenic lymphoblastoid cell lines BK-LCL (wt) and ∆EBNA3-BK-LCL were used. Both lines expressed all EBNA-s, except EBNA-3 in ∆EBNA3-BK-LCL. Expression of the EBNA-3, EBNA-5 and EBNA-6 was assayed by Western blotting (Fig. 2a, b). RNA was isolated from BK-LCL and ∆EBNA3-BK-LCL lines, and quantitative or real-time PCR was performed to measure mRNA levels. The VDR responsive genes (C-FOS, CYCLIN C, CYP24A1, GADD45A, and P21) were expressed at more than five-fold lower levels in BK-LCL than in ∆EBNA3-BK-LCL (Fig. 3a). The mRNA levels of the studied genes were related to the expression of TBP (TATA box binding protein), an endogenous control.
Fig. 2.
Western blotting of LCL cell lysates. a Membrane was probed with mouse monoclonal anti-EBNA-5 antibody. All studied cell lines expressed EBNA-5 protein. b Membrane was probed with mouse anti-EBNA-6 antibody and rabbit anti-EBNA-3 serum. All cell lines expressed EBNA-6. Only control LCL and BK-LCL expressed EBNA-3. ΔEBNA3-BK-LCL, isogenic to BK-LCL line, did not express EBNA-3 protein. c Membrane was probed with anti-VDR and anti-actin antibodies
Fig. 3.
Expression of VDR responsive genes was studied at m-RNA levels using Q-PCR. a Relative fold change of mRNA expression values (log10) of VDR responsive genes in EBNA3 negative LCLs compared to EBNA3 positive LCLs. Notice that all studied genes were downregulated in EBNA-3-positive LCL. b Relative fold change of mRNA expression values (log10) of VDR responsive genes in BK-LCL and ∆EBNA3-BK-LCL untreated and treated with 1,25(OH)2D3. Notice that all studied genes were downregulated in EBNA-3 positive LCL
Similar experiments were performed following the treatment of BK-LCL and ∆EBNA3-BK-LCL with 1-alpha,25-dihydroxyvitamin D3 (1,25(OH)2D3), a metabolite of vitamin D3, the VDR ligand. As we observed earlier (Fig. 3a), all VDR responsive genes were expressed at lower levels in BK-LCL compared to ∆EBNA3-BK-LCL (Fig. 3b). Treatment with 1,25(OH)2D3 led to induction of all responsive genes in BK-LCL. However, the levels of responsive gene expression were lower compared to 1,25(OH)2D3-treated EBNA3-deficient LCL (Fig. 3b).
In order to confirm that the change in expression of the VDR-dependent genes was due to the binding to EBNA-3 and not to differences in VDR expression levels, Western blotting was performed using the whole cell lysates of ∆EBNA3-BK-LCL and BK-LCL. No change in expression of VDR was observed under normal conditions of cell culturing or 1,25(OH)2D3 treatment (Fig. 2c).
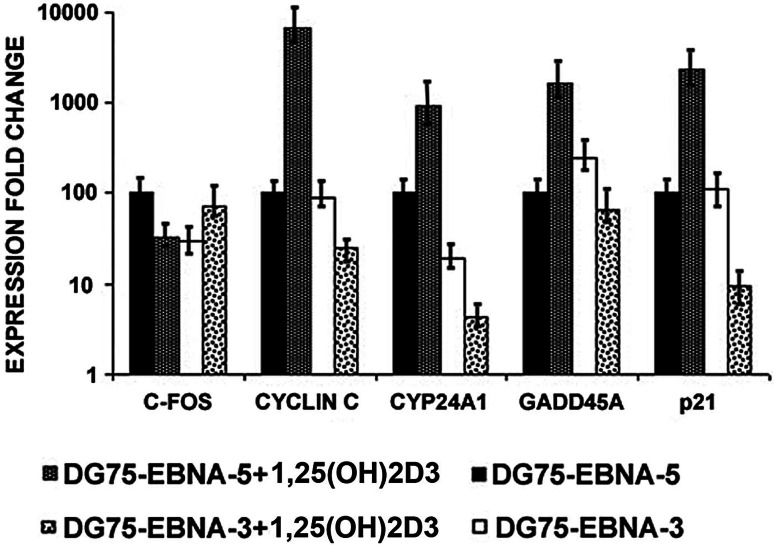
To examine the role of EBNA3 in cells of the different phenotype, EBV-negative Burkitt’s lymphoma line DG75 was used. The expression levels of VDR-responsive genes were studied in DG75 derivatives (DG75-EBNA3 and DG75-EBNA5) that expressed EBNA-3 or EBNA-5 constitutively. CYCLIN C, CYP24A1, GADD45A and P21 genes were upregulated in DG75-EBNA5 cells after 1,25(OH)2D3 treatment (Fig. 4). The expression level of the CYP24A1 gene was lower in DG75-EBNA3 cells at any conditions compared to DG75-EBNA5 (Fig. 4). Differences of expression of VDR responsive genes in DG75 compared to LCL could be explained by different origins of these cells.
Fig. 4.
Expression of VDR responsive genes was studied at m-RNA levels using Q-PCR. Fold change expression values of VDR responsive genes, relatively compared between DG75-EBNA5 and DG75-EBNA3, untreated and treated with 1,25(OH)2D3. VDR responsive genes (except C-FOS) were upregulated in 1,25(OH)2D3 treated DG75-EBNA5 compared to untreated DG75-EBNA5. Notice that 1,25(OH)2D3 treatment did not result in VDR-responsive gene induction in DG75-EBNA-3 cells
EBNA3 counteracts vitamin D3-induced cell death
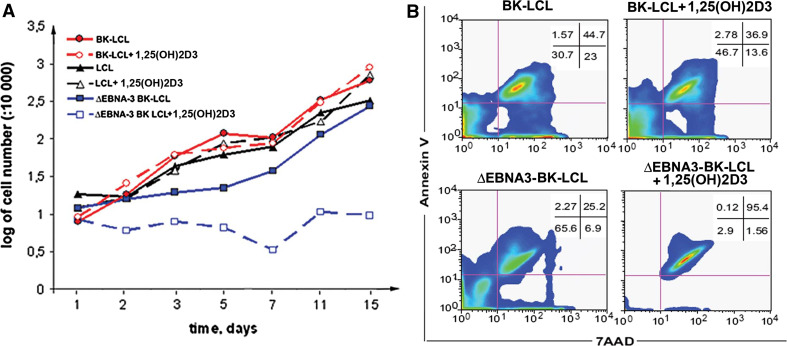
Our experimental data show that the level of various VDR responsive pro-apoptotic genes was strongly repressed by EBNA3, probably due to its binding to VDR protein complex. To relate these findings to the physiological role of EBNA-3, the proliferation rate of freshly established LCL, BK-LCL and ∆EBNA3-BK-LCL were compared in the absence of 1,25(OH)2D3 in the medium and after exposure to 1 × 10−7 M of 1,25(OH)2D3 (Fig. 5a). ∆EBNA3-BK-LCL proliferated more slowly than the two other LCLs. Treatment of ∆EBNA3-BK-LCL with 1,25(OH)2D3 led to growth arrest in contrast to the EBNA-3 expressing LCLs (Fig. 5a).
Fig. 5.
Lymphoblastoid cells are resistant to vitamin D3-induced apoptosis. a Proliferation rate of various cell lines upon the treatment with 1,25(OH)2D3 and in the absence of 1,25(OH)2D3. Notice that ∆EBNA3-BK-LCL proliferates more slowly compared to LCL and BK-LCL. 1,25(OH)2D3 treatment of ∆EBNA3-BK-LCL decreased the proliferation rate significantly in contrast to LCL and BK-LCL. b FACS analysis of the BK-LCL and ∆EBNA3-BK-LCL cultures, growing in the presence of 1,25(OH)2D3 or in its absence. Notice that 1,25(OH)2D3 did not induce apoptosis in BK-LCL cells in contrast to ∆EBNA3-BK-LCL
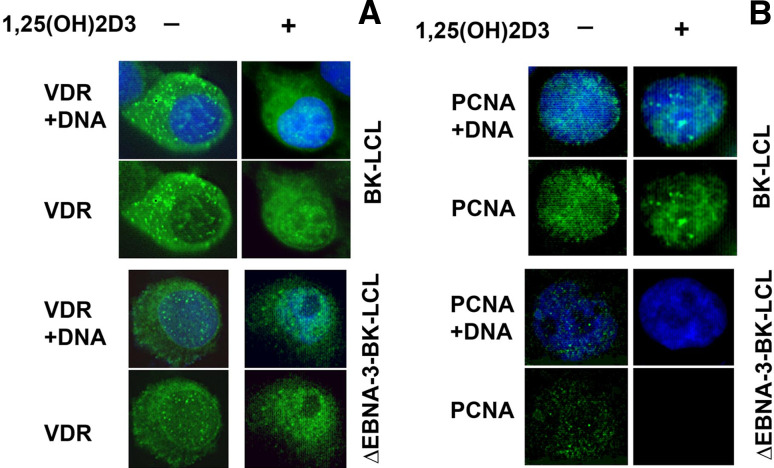
In order to monitor the VDR cellular localization upon treatment of the cells with 1,25(OH)2D3, LCLs were stained with mouse monocloclonal anti-VDR antibody. To assess the cell proliferation, proliferating cell nuclear antigen (PCNA) expression was studied as well. Endogenous VDR was observed mainly in the nucleus upon 1,25(OH)2D3 treatment, regardless of the presence of EBNA-3 (Fig. 6a). Nuclear signal of PCNA was detected in both cell lines at normal conditions. Upon treatment with 1,25(OH)2D3, however, only wild-type LCL showed PCNA staining (Fig. 6b). Thus, immunostaining data allowed us to draw similar conclusions: that ΔEBNA-3-BK-LCL cells are arrested upon treatment with 1,25(OH)2D3.
Fig. 6.
Immunostaining of BK-LCL and ∆EBNA3-BK-LCL cells, untreated and treated with 1,25(OH)2D3. a Staining with anti-VDR antibody. Notice that upon treatment with the ligand VDR translocates to the nucleus regardless of the presence of EBNA-3. b Staining for proliferating cell nuclear antigen, PCNA. Notice that upon the ligand treatment there is no PCNA signal in the nucleus of ∆EBNA3-BK-LCL cells, in contrast to EBNA-3 expressing LCL
1-Alpha,25-dihydroxyvitamin D3 induces apoptosis in ∆EBNA3-BK-LCL
The observed absence of PCNA signal and decrease in the proliferation rate of ∆EBNA3-BK-LCL cells treated with 1,25(OH)2D3 could be explained by the 1,25(OH)2D3 induced growth arrest and/or apoptosis. To ascertain this possibility, the annexin assay for apoptosis by flow cytometry was performed. From the Fig. 5b it is clearly seen that the treatment of EBNA3-deficient cells with 1,25(OH)2D3 led to apoptosis, in contrast to wild-type LCL.
Discussion
It is already known that some EBV-encoded proteins expressed in latently infected or growth transformed cells can protect them from apoptosis [19]. The LMP1 protein is a major player in the viral anti-apoptotic strategy (for review, see [1]). However, some of the EBNAs are implicated in this as well. EBNA-2, for example, binds to the nuclear receptor NUR77 (Nr4A1, GenBank: NP_775180) and blocks NUR77-mediated apoptosis [20], [21]. EBNA-2 transactivates the anti-apoptotic gene BFL-1 (BCL2A1, GenBank: NM_004049) in complex with RBP-Jκ [22]. EBNA-2 can also inhibit the pro-apoptotic and anti-proliferative functions of the transforming growth factor beta 1 (TGFB1, GenBank: NP_000651) cytokine, as shown in the EBNA-2 inducible EREB system [23].
The EBNA3 family proteins are also increasingly involved in protection against apoptosis. For example, EBNA-3 can protect Burkitt’s lymphoma cells carrying EBNA-2 deleted virus from apoptosis [24]. Moreover, EBNA-3 and EBNA-6 can repress the transcription of BIM (BCL2L11, GenBank: NM_138621), which also results in the anti-apoptotic effect [25].
We have previously shown that EBNA-3 binds to and regulates the transactivation function of the nuclear receptor AhR. Importantly, EBNA-3 influenced the transcription of AhR-dependent genes at normal conditions and upon an activation of AhR by ligands [xenobiotics, such as 2,3,7,8-tetrachloro-dibenzo-p-dioxin (TCDD)] [10]. The physiological role of the EBNA-3–AhR interaction was illuminated by treating B-cell lines with TCDD. EBNA-3 was found to protect cells from TCDD-induced growth arrest and/or apoptosis.
In the present work, we found that EBNA-3 binds to the VDR protein complex (Fig. 1). VDR is a nuclear receptor that contains the Zn-finger in its N-terminal domain. This part of the protein binds to the general transcription factor II B (TFIIB) [26]. VDR forms heterodimers with the retinoic X receptors alpha and beta to activate transcription of the responsive genes (for review, see [27] and [28]). Expression of VDR-responsive genes may inhibit proliferation and promote cell growth arrest and/or apoptosis (for review, see [29]). The transactivating ability of VDR is enhanced by binding to its ligand, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), a metabolite of vitamin D3 (for review, see [30]).
It was shown earlier that peripheral blood B cells and LCLs express functional VDR [31–33]. The active VDR pathway could inhibit proliferation and enhance the differentiation of leukemic cells [33]. It should be noticed, however, that VDR is expressed at a lower level in LCLs compared to normal tonsil-derived B cells at mRNA and protein levels [13]. Moreover, the expression of VDR-responsive genes was downregulated by EBNA-3 (Figs. 3, 4). EBNA-3 could also inhibit VDR activation even upon cell treatment with its ligand, 1,25(OH)2D3. EBNA-3-deficient cells were arrested and died subsequently by apoptosis. The wild-type LCLs proliferated at an unchanged rate (Fig. 5). The effective dose of 1,25(OH)2D3 required to initiate VDR response is in the range of 1–10 nM [27]. The concentration of 1 × 10−7 M of 1,25(OH)2D3 that was used in our experiments was higher when compared to the physiological level of 1,25(OH)2D3 (for review see [34]). Even at such concentrations of 1,25(OH)2D3, EBNA-3 could protect LCLs from VDR-induced apoptosis.
In summary, we have found that EBNA-3 protein is one of the units of the nuclear VDR protein complex. Notably, we have found that EBNA-3 participates in complex formation with other proteins that can regulate transcription by chromatin re-modeling, such as SWI/SNF, DNA helicase II, and histones 2A and 2B (Table 1). We can hypothesize that, when EBNA-3 is bound to VDR, it represses VDR-dependent transcription and protects LCLs from VDR-induced growth arrest and/or apoptosis.
Conclusions
In this study, we found that EBNA-3 and VDR are in the same protein complex. EBNA-3 blocks (or downregulates) transcription of the VDR-dependent genes at the basal level and upon its activation by a ligand, the 1-alpha,25-dihydroxyvitamin D3. Binding between EBNA-3 and VDR prevents LCLs from growth arrest and/or apoptosis induced by vitamin D3.
Acknowledgments
This work was supported by the Swedish Cancer Society, a matching grant from the Concern Foundation (Los Angeles), the Cancer Research Institute (New York), the Swedish Institute, and the Swedish Foundation for Strategic Research.
Conflict of interest statement
None.
References
- 1.Kieff E, Rikinson A. Epstein-Barr virus and its replication. In: Fields BN, editor. Fields Virology, vol II. 2. Philadelphia: Lippincott Williams&Wilkins; 2001. pp. 2511–2574. [Google Scholar]
- 2.Robertson ES, Grossman S, Johannsen E, Miller C, Lin J, Tomkinson B, Kieff E. Epstein-Barr virus nuclear protein 3C modulates transcription through interaction with the sequence-specific DNA-binding protein J kappa. J Virol. 1995;69:3108–3116. doi: 10.1128/jvi.69.5.3108-3116.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robertson ES, Lin J, Kieff E. The amino-terminal domains of Epstein-Barr virus nuclear proteins 3A, 3B, and 3C interact with RBPJ (kappa) J Virol. 1996;70:3068–3074. doi: 10.1128/jvi.70.5.3068-3074.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johannsen E, Miller CL, Grossman SR, Kieff E. EBNA-2 and EBNA-3C extensively and mutually exclusively associate with RBPJkappa in Epstein-Barr virus-transformed B lymphocytes. J Virol. 1996;70:4179–4183. doi: 10.1128/jvi.70.6.4179-4183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao B, Marshall DR, Sample CE. A conserved domain of the Epstein-Barr virus nuclear antigens 3A and 3C binds to a discrete domain of Jkappa. J Virol. 1996;70:4228–4236. doi: 10.1128/jvi.70.7.4228-4236.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krauer K, Kienzle N, Young DB, Sculley TB. Epstein-Barr nuclear antigen-3 and -4 interact with RBP-2N, a major isoform of RBP-J kappa in B lymphocytes. Virology. 1996;226:346–353. doi: 10.1006/viro.1996.0662. [DOI] [PubMed] [Google Scholar]
- 7.Le Roux A, Kerdiles B, Walls D, Dedieu JF, Perricaudet M. The Epstein-Barr virus determined nuclear antigens EBNA-3A, -3B, and -3C repress EBNA-2-mediated transactivation of the viral terminal protein 1 gene promoter. Virology. 1994;205:596–602. doi: 10.1006/viro.1994.1687. [DOI] [PubMed] [Google Scholar]
- 8.Cludts I, Farrell PJ. Multiple functions within the Epstein-Barr virus EBNA-3A protein. J Virol. 1998;72:1862–1869. doi: 10.1128/jvi.72.3.1862-1869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper A, Johannsen E, Maruo S, Cahir-McFarland E, Illanes D, Davidson D, Kieff E. EBNA3A association with RBP-Jkappa down-regulates c-myc and Epstein-Barr virus-transformed lymphoblast growth. J Virol. 2003;77:999–1010. doi: 10.1128/JVI.77.2.999-1010.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kashuba EV, Gradin K, Isaguliants M, Szekely L, Poellinger L, Klein G, Kazlauskas A. Regulation of transactivation function of the aryl hydrocarbon receptor by the Epstein-Barr virus-encoded EBNA-3 protein. J Biol Chem. 2006;281:1215–1223. doi: 10.1074/jbc.M509036200. [DOI] [PubMed] [Google Scholar]
- 11.Kashuba E, Kashuba V, Pokrovskaja K, Klein G, Szekely L. Epstein-Barr virus encoded nuclear protein EBNA-3 binds XAP-2, a protein associated with Hepatitis B virus X antigen. Oncogene. 2000;19:1801–1806. doi: 10.1038/sj.onc.1203501. [DOI] [PubMed] [Google Scholar]
- 12.Yenamandra SP, Klein G, Kashuba E. Nuclear receptors and their role in Epstein-Barr virus induced B cell transformation. Exp Oncol. 2009;31:67–73. [PubMed] [Google Scholar]
- 13.Yenamandra SP, Lundin A, Arulampalam G, Yurchenko M, Pettersson S, Klein G, Kashuba E. Expression profile of nuclear receptors upon Epstein-Barr virus induced B cell transformation. Exp Oncol. 2009;31:92–96. [PubMed] [Google Scholar]
- 14.Buck M, Burgess A, Stirzaker R, Krauer K, Sculley T. Epstein-Barr virus nuclear antigen 3A contains six nuclear-localization signals. J Gen Virol. 2006;87:2879–2884. doi: 10.1099/vir.0.81927-0. [DOI] [PubMed] [Google Scholar]
- 15.Hertle ML, Popp C, Peterman S, Maier S, Kremmer E, Lang R, Mages J, Kempkes B. Differential gene expression patterns of EBV infected EBNA-3A positive and negative human B lymphocytes. PLoS Pathog. 2009;5:e1000506. doi: 10.1371/journal.ppat.1000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellman U. Peptide mapping using MALDI-TOFMS. New York: Wiley, Inc; 2002. [Google Scholar]
- 17.Hellman U, Bhikhabhai R. Easy amino acid sequencing of sulfonated peptides using post-source decay on a matrix-assisted laser desorption/ionization time-of-flight mass spectrometer equipped with a variable voltage reflector. Rapid Commun Mass Spectrom. 2002;16:1851–1859. doi: 10.1002/rcm.805. [DOI] [PubMed] [Google Scholar]
- 18.Kitagawa H, Fujiki R, Yoshimura K, Mezaki Y, Uematsu Y, Matsui D, Ogawa S, Unno K, Okubo M, Tokita A, Nakagawa T, Ito T, Ishimi Y, Nagasawa H, Matsumoto T, Yanagisawa J, Kato S. The chromatin-remodeling complex WINAC targets a nuclear receptor to promoters and is impaired in Williams syndrome. Cell. 2003;113:905–917. doi: 10.1016/S0092-8674(03)00436-7. [DOI] [PubMed] [Google Scholar]
- 19.Gregory CD, Dive C, Henderson S, Smith CA, Williams GT, Gordon J, Rickinson AB. Activation of Epstein-Barr virus latent genes protects human B cells from death by apoptosis. Nature. 1991;349:612–614. doi: 10.1038/349612a0. [DOI] [PubMed] [Google Scholar]
- 20.Lee JM, Lee KH, Farrell CJ, Ling PD, Kempkes B, Park JH, Hayward SD. EBNA2 is required for protection of latently Epstein-Barr virus-infected B cells against specific apoptotic stimuli. J Virol. 2004;78:12694–12697. doi: 10.1128/JVI.78.22.12694-12697.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JM, Lee KH, Weidner M, Osborne BA, Hayward SD. Epstein-Barr virus EBNA2 blocks Nur77- mediated apoptosis. Proc Natl Acad Sci USA. 2002;99:11878–11883. doi: 10.1073/pnas.182552499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pegman PM, Smith SM, D’Souza BN, Loughran ST, Maier S, Kempkes B, Cahill PA, Simmons MJ, Gelinas C, Walls D. Epstein-Barr virus nuclear antigen 2 trans-activates the cellular antiapoptotic bfl-1 gene by a CBF1/RBPJ kappa-dependent pathway. J Virol. 2006;80:8133–8144. doi: 10.1128/JVI.00278-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horndasch M, Raschke EE, Bommer G, Schuhmacher M, Dumont E, Kuklik-Roos C, Eick D, Kempkes B. Epstein-Barr virus antagonizes the antiproliferative activity of transforming growth factor-beta but does not abolish its signaling. Int J Cancer. 2002;101:442–447. doi: 10.1002/ijc.10626. [DOI] [PubMed] [Google Scholar]
- 24.Kelly GL, Milner AE, Tierney RJ, Croom-Carter DS, Altmann M, Hammerschmidt W, Bell AI, Rickinson AB. Epstein-Barr virus nuclear antigen 2 (EBNA2) gene deletion is consistently linked with EBNA3A, -3B, and -3C expression in Burkitt’s lymphoma cells and with increased resistance to apoptosis. J Virol. 2005;79:10709–10717. doi: 10.1128/JVI.79.16.10709-10717.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderton E, Yee J, Smith P, Crook T, White RE, Allday MJ. Two Epstein-Barr virus (EBV) oncoproteins cooperate to repress expression of the proapoptotic tumour-suppressor Bim: clues to the pathogenesis of Burkitt’s lymphoma. Oncogene. 2008;27:421–433. doi: 10.1038/sj.onc.1210668. [DOI] [PubMed] [Google Scholar]
- 26.Jurutka PW, Remus LS, Whitfield GK, Thompson PD, Hsieh JC, Zitzer H, Tavakkoli P, Galligan MA, Dang HT, Haussler CA, Haussler MR. The polymorphic N terminus in human vitamin D receptor isoforms influences transcriptional activity by modulating interaction with transcription factor IIB. Mol Endocrinol. 2000;14:401–420. doi: 10.1210/me.14.3.401. [DOI] [PubMed] [Google Scholar]
- 27.Norman AW. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147:5542–5548. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- 28.Gombart AF, Luong QT, Koeffler HP. Epstein-Barr virus antagonizes the antiproliferative activity of transforming growth factor-beta but does not abolish its signaling. Anticancer Res. 2006;26:2531–2542. [Google Scholar]
- 29.Banerjee P, Chatterjee M. Antiproliferative role of vitamin D and its analogs–a brief overview. Mol Cell Biochem. 2003;253:247–254. doi: 10.1023/A:1026072118217. [DOI] [PubMed] [Google Scholar]
- 30.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 31.Morgan JW, Reddy GS, Uskokovic MR, May BK, Omdahl JL, Maizel AL, Sharma S. Functional block for 1 alpha, 25-dihydroxyvitamin D3-mediated gene regulation in human B lymphocytes. J Biol Chem. 1994;269:13437–13443. [PubMed] [Google Scholar]
- 32.Morgan JW, Kouttab N, Ford D, Maizel AL. Vitamin D-mediated gene regulation in phenotypically defined human B cell subpopulations. Endocrinology. 2000;141:3225–3234. doi: 10.1210/en.141.9.3225. [DOI] [PubMed] [Google Scholar]
- 33.Elstner E, Lee YY, Hashiya M, Pakkala S, Binderup L, Norman AW, Okamura WH, Koeffler HP. 1 alpha, 25-Dihydroxy-20-epi-vitamin D3: an extraordinarily potent inhibitor of leukemic cell growth in vitro. Blood. 1994;84:1960–1967. [PubMed] [Google Scholar]
- 34.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]