Abstract
Since bone morphogenetic proteins (BMPs) play an important role in melanoma progression, we aimed to determine the molecular mechanisms leading to overexpression of BMP4 in melanoma cells compared to normal melanocytes. With our experimental approach we revealed that loss of expression of a microRNA represents the starting point for a signaling cascade finally resulting in overexpression of BMP4 in melanoma cells. In detail, strongly reduced expression of the microRNA miR-196a in melanoma cells compared to healthy melanocytes leads to enhanced HOX-B7 mRNA and protein levels, which subsequently raise Ets-1 activity by inducing basic fibroblast growth factor (bFGF). Ets-1 finally accounts for induction of BMP4 expression. We were furthermore able to demonstrate that bFGF-mediated induction of migration is achieved via activation of BMP4, thus determining BMP4 as major modulator of migration in melanoma. In summary, our study provides insights into the early steps of melanoma progression and might thereby harbor therapeutic relevance.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-010-0394-7) contains supplementary material, which is available to authorized users.
Keywords: Malignant melanoma, Bone morphogenetic proteins, MicroRNA, HOX genes, Fibroblast growth factor, Migration
Introduction
Bone morphogenetic proteins (BMPs) belong to the transforming growth factor β (TGF-β) protein superfamily of growth factors. BMPs control a broad range of biological activities in many cell types such as epithelial, mesenchymal, or neuronal cells [1, 2] by affecting cellular processes like proliferation, differentiation, chemotaxis, motility, or cell death [3]. Furthermore, they are involved in early embryonic development and control formation and patterning of various tissues, left–right asymmetry, and morphogenesis of a number of organs [4, 5]. Besides their function in embryonic development and normal cellular processes, BMPs are also linked to tumor formation and progression. Several groups were able to show that BMP transcripts are expressed in various carcinoma cell lines from gastric, ovarian, prostate, pancreatic, and breast origin [6–11]. In previous studies, we as well as others could demonstrate an overexpression of BMPs in malignant melanoma [12, 13]. BMPs contribute to cell invasion and migration by exerting autocrine effects on the tumor cells themselves and paracrine effects on tumor surrounding fibroblasts [14]. In recent studies, we were able to show an increase of BMP7 expression in malignant melanoma compared to benign nevi and thereby determined BMP7 as a potential novel prognostic marker for melanoma progression [15]. Furthermore, we identified the transcription factor AP-2 as a negative regulator of BMP4 expression, whereas Ets-1 acts as an activator of BMP4 expression in melanoma cells [12].
It is known that growth factors of the fibroblast growth factor family interact with BMP signaling in different cell types [16, 17]. In detail, Nakamura et al. [17] demonstrated induced phosphorylation of Smad1 via BMP2 signaling after treatment with low doses of recombinant basic FGF protein in muscle-derived primary culture cells. Basic fibroblast growth factor (bFGF, FGF-2) is crucial for the survival of melanocytes. However, melanocytes are not able to produce bFGF by themselves. In vivo, dermal keratinocytes and fibroblasts secrete substantial amounts of bFGF and thereby stabilize melanocytes in the skin [18, 19]. In contrast, melanoma cells commonly acquire the ability to express bFGF [20]. This is thought to be an early event in melanoma progression because high bFGF expression can already be detected in dysplastic nevi [21]. Several groups analyzed the role of bFGF in melanoma cells and revealed that bFGF has an autocrine effect on melanoma cells [22, 23]. Cells treated with antisense oligonucleotides targeting bFGF displayed reduced proliferation in vitro and in vivo [22], whereas transduction of melanocytes with bFGF-expressing adenoviruses led to increased migration [23]. Care et al. [24] previously identified bFGF as the main downstream target of the transcription factor HOX-B7 in melanoma. In their study, they further showed that HOX-B7 binds and transactivates the bFGF promoter in several melanoma cell lines, whereas inhibition of HOX-B7 by antisense oligomers diminished expression of bFGF and inhibited cell proliferation. Products of homeobox (HOX) genes control cellular differentiation during development by either activating or repressing transcription of a variety of target genes [25]. In addition, altered HOX gene expression is associated with different neoplasia, e.g., aberrant expression of HOX-A1 and HOX-A5 was detected in lung cancer [26] and cervical cancer samples showed abnormal HOX-B2 expression compared to healthy tissue [27].
In previous studies, it was shown that several HOX genes are targeted by microRNAs (miRNAs) [28–31]. MiRNAs are small non-coding RNA molecules that are able to induce posttranscriptional repression of a defined set of target genes by binding to specific sequences in the 3′ untranslated regions (3′UTRs) of the corresponding mRNAs with only incomplete complementarity [32, 33]. The silencing of gene expression by miRNAs can be mediated by destabilization of the target mRNA and/or by repression of translation [34–37]. There is a bulk of data accumulating that microRNAs are involved in initiation as well as in progression of a variety of human cancers, such as glioma, breast, colon, and lung cancer [38, 39]. Regarding melanoma, our group was already able to identify subsets of miRNAs associated with formation and progression of this type of skin tumor and to show the involvement of a particular miRNA, namely let-7a, in regulating migration and invasion of melanoma cells [40, 41]. We recently summarized the impact of specific miRNAs on melanomagenesis and their potential to serve as diagnostic markers or therapeutic targets in malignant melanoma [42].
In this study, we link expression of the microRNA miR-196a to BMP expression in melanoma cells by investigating the interaction of this miRNA with the homeobox transcription factor HOX-B7 leading to overexpression of BMPs via the transcription factor Ets-1 and bFGF.
Materials and methods
Cell culture
Melanoma cell lines Mel Im, Mel Ei, Mel Wei, Mel Ho, Mel Juso, Mel Ju, SkMel 28, and SkMel 3 were described previously [43]. Cell lines Mel Ei, Mel Wei, Mel Ho, and Mel Juso were derived from primary cutaneous melanomas; Mel Im, Mel Ju, SkMel 28, and SkMel 3 were derived from metastases of malignant melanomas. Cells were maintained in DMEM supplemented with penicillin (400 U/ml), streptomycin (50 μg/ml), l-glutamine (300 μg/ml), and 10% FCS (Sigma, Deisenhofen, Germany) and split at a 1:5 ratio every 3 days. Normal human epidermal melanocytes (NHEMs) were derived from neonatal skin. Isolation and cultivation of NHEMs were described previously [44]. Melanocytes of three different donors were used in the experiments and mean values were displayed as one graph bar.
Combined miRNA/total RNA isolation and reverse transcription
Enriched small RNA species containing miRNAs and total cellular RNA were isolated from cultured cells using the mirVana™ miRNA Isolation Kit (Ambion, Austin, TX, USA). MiRNAs were reverse transcribed using the RT reaction setup of a mirVana™ qRT-PCR miRNA Detection Kit (25 ng small RNA input) together with mirVana™ RT primers specific for hsa-miR-196a and 5S rRNA (Ambion), respectively. For details, see [41]. Total cellular RNA was isolated from cultured cells using an RNeasy Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. cDNAs of total RNA fractions (500 ng total RNA input) were generated using SuperScript II Reverse Transcriptase Kit (Invitrogen, Groningen, the Netherlands).
Analysis of gene and miR-196a expression by quantitative PCR
Quantitative real-time-PCR (qRT-PCR) was performed on a Lightcycler (Roche, Mannheim, Germany). cDNA template (1 μl), 0.5 μl (20 μM) of forward and reverse primers (Table 1) and 10 μl of SYBR Premix Ex Taq (TaKaRa, Shiga, Japan) in a total of 20 μl were applied to the following PCR program: 30 s 95°C (initial denaturation); 20°C/s temperature transition rate up to 95°C for 15 s, 10 s annealing, 20 s 72°C, fluorescence measurement at optimized melting temperature (acquisition mode single), repeated 40 times (amplification). Annealing and melting temperatures were optimized for each primer set (Table 1). β-Actin was used for normalization.
Table 1.
Oligonucleotide sequences and qRT-PCR conditions
| Gene | Primer sequences (fwd/rev) | Ta (°C) | Tm (°C) |
|---|---|---|---|
| β-Actin | 5′-CTA CGT CGC CCT GGA CTT CGA GC-3′ | 60–68 | 80 |
| 5′-GAT GGA GCC GCC GAT CCA CAC GG-3′ | |||
| bFGF | 5′-ACG TAG GAG ACA CAG CGG TTC GAG-3′ | 67 | 81 |
| 5′-AGC CTA GCA ACT CTG CTG GTG ATG G-3′ | |||
| BMP4 | 5′-GCC GGA GGG CCA AGC GTA GCC CTA AG-3′ | 68 | 87 |
| 5′-CTG CCT GAT CTC AGC GGC ACC CAC ATC-3′ | |||
| HOX-B7 | 5′-CAC TAC AAT CGC TAC CTG ACG-3′ | 62 | 80 |
| 5′-TCC TCT TCC TCC TCT GCT TC-3′ | |||
| HOX-B8 | 5′-AGC AGC CGC CGG ACG CAG GCG-3′ | 60 | 85 |
| 5′-GCT GGG GAA CTT GTC TTT GTT-3′ | |||
| Id1 | 5′-TGT TAC TCA CGC CTC AAG GAG-3′ | 62 | 86 |
| 5′-TTC AGC GAC ACA AGA TGC G-3′ |
Quantitative real-time PCR of reverse transcribed miRNAs was performed as described previously [41]. Briefly, cDNA template (1 μl), 1 μl miRNA-specific PCR primers (Ambion) and 10 μl of SYBR Premix Ex Taq (TaKaRa) were added in a total of 20 μl. The following PCR program was used for hsa-miR-196a amplification: 30 s 95°C (initial denaturation); 20°C/s temperature transition rate up to 95°C for 3 s, 5 s 58°C, 16 s 72°C, fluorescence measurement at 78°C (acquisition mode single), repeated 50 times (amplification). 5S rRNA was used for normalization.
PCR reactions were evaluated by melting-curve analysis and checking the PCR products on 1.5% agarose gels (HOX-B7, HOX-B8, bFGF, BMP4, Id1) and on 6% polyacrylamide gels (hsa-miR-196a), respectively.
Generating miR-196a expression constructs
Hsa-miR-196a-1 and hsa-miR-196a-2 pre-miR sequences were subcloned into a pCRII-TOPO vector (TOPO TA cloning Kit Dual Promoter, Invitrogen) using the following primer sequences: hsa-miR-196a-1_fwd: 5′-TCT TCC GAT GTG TTG TTT AGT AG-3′; hsa-miR-196a-1_rev: 5′-GAC ACT TCC CAG ATC TCT TCT-3′; hsa-miR-196a-2_fwd: 5′-TGC TCG CTC AGC TGA TCT GT-3′; hsa-miR-196a-2_rev: 5′-GCC CTC GAC GAA AAC CGA C-3′. TOPO_miR-196a-1 and TOPO_miR-196a-2 as well as empty pcDNA3 expression vector (Invitrogen) were double-digested with BamHI and XbaI (NEB, Frankfurt a.M., Germany). Fragments were purified by gel extraction (QIAquick gel extraction kit, Qiagen). Digested vector was dephosphorylated using antarctic phosphatase (NEB). Ligation was performed at 16°C overnight using T4-Ligase (NEB) to obtain hsa-miR-196a expression plasmids pcDNA3_miR-196a-1 and pcDNA3_miR-196a-2. DNA sequencing was performed to assure correctness of the sequences cloned (Entelechon, Regensburg, Germany).
Generation of stable miR-196a cell clones
A panel of Mel Im cell clones re-expressing miR-196a was established by stable transfection with miR-196a-1 and miR-196a-2 expression plasmids, respectively. Related control clones were transfected with empty pcDNA3 vector. Transfections were performed using the Lipofectamine Plus (Invitrogen) method. One day after transfection, cells were placed into selection medium containing 50 μg/ml G418 (Sigma-Aldrich). G418 concentration in the medium was raised stepwise to a final concentration of 2.5 mg G418/ml medium. After 25 days of selection, individual G418-resistant colonies were subcloned. Control cell clones were generated the same way by transfection with empty pcDNA3 vector (Invitrogen). MiR-196a expression levels in the stable cell clones were determined by qRT-PCR analysis.
Cloning of the HOX-B7 3′UTR into a reporter plasmid and mutation of the miR-196a target sequence
A 570-bp fragment of the HOX-B7 3′UTR containing the predicted miR-196a target sequence was PCR amplified using primers HOX-B7_3′UTR_fwd (5′-CAG TCT AGA AGG GCA GAG GAA GAG ACA TGA-3′) and HOX-B7_3′UTR_rev (5′-CAG TCT AGA ATC CTT TAG ATA CAC ACA CAG AAT GT-3′) containing XbaI restriction sites. PCR fragment and pGL3 control vector (Promega) were digested with XbaI (NEB) and purified by gel-extraction (QIAquick gel extraction kit, Qiagen). Digested vector was dephosphorylated using antarctic phosphatase (NEB). Ligation was performed at 16°C overnight using T4-Ligase (NEB) to obtain HOX-B7_3′UTR_wt reporter vector. To delete 10 bp of the miR-196a target sequence site-directed mutagenesis by overlap extension (SOE)-PCR was performed as described in [45] using additional primers HOX-B7_3′UTR_SOE_fwd (5′-CAG ACT TTC CTA TTT TTC AGT GTT G-3′) and HOX-B7_3′UTR_SOE_rev (5′-CAA CAC TGA AAA ATA GGA AAG TCT G-3′). The resulting 560-bp fragment was cloned into the pGL3 control vector as described above to obtain HOX-B7_3′UTR_mut reporter plasmid. DNA sequencing was performed to assure correctness of the sequences cloned (Entelechon, Regensburg, Germany).
Transfection and luciferase assay
For siRNA transfections, 1.5 μg of a mixture of two HOX-B7 siRNAs (Qiagen HOX-B7 siRNA #1 and HOX-B7 siRNA #4) or of a bFGF siRNA (Qiagen FGF2 siRNA #7) and the corresponding negative control siRNA, respectively, were transfected using 18 μl HiPerFect transfection reagent (Qiagen) per well of a six-well culture plate. After incubation for 48 h in the case of HOX-B7 and 24 h in the case of bFGF total RNA was isolated, transcribed into cDNA, and qRT-PCR was performed. In the anti-miR experiments, NHEMs were transfected with 1.5 μg of LNA against miR-196a or a scramble-miR (Exiqon, Denmark), respectively, for 48 h using HiPerFect transfection reagent as described above.
BMP4 promoter reporter constructs were described previously [12]. The Ets-1 construct containing three times the Ets-1 binding sites cloned into PTKFLUC vector (afterwards named Ets-1 construct) was a kind gift of Prof. Soncin, France [44]. Co-transfection was performed using a bFGF expression plasmid, kindly provided by Dr. Madry, Germany [46].
Cells (2 × 105 per well) were seeded into six-well plates and transfected with 0.5 μg of reporter constructs using Lipofectamine Plus (Invitrogen). The cells were lysed 24 h after transfection and the luciferase activity was measured. To normalize transfection efficiency, 0.2 μg of a pRL-TK plasmid (Promega, Mannheim, Germany) was co-transfected and renilla luciferase activity was measured by a luminometric assay (Dual-Luciferase Reporter Assay; Promega). All transfection experiments were repeated three times.
Transduction of Mel Im cells or NHEMs with a bFGF overexpressing adenovirus
Cells were seeded into T25 flasks containing DMEM without FCS and transduced with EGFP or bFGF overexpressing adenoviruses (generated using standard protocols described previously [47]), respectively. After incubation for 24 h, cells were washed to remove the adenoviral vector and DMEM containing FCS was added. Forty-eight hours after transfection, RNA was isolated, transcribed into cDNA, and qRT-PCR was performed.
Quantification of BMP4 and bFGF protein levels
Protein expression of BMP4 and bFGF, respectively, was determined by enzyme-linked immunosorbent assay (ELISA) using protein-specific Quantikine ELISA kits (R&D Systems, Abingdon, UK) according to the manufacturer’s protocols. All experiments were repeated for a total of three times.
Immunofluorescence
Immunofluorescence assays were performed as described previously [48]. In brief, Mel Im cells were transfected with HOX-B7 siRNA or control siRNA using HiPerFect transfection reagent. After incubation for 24 h, cells were seeded in four-well chamber slides and grown further for another 24 h. Melanoma cell lines Mel Wei, Mel Juso, Mel Ju, Mel Im, and normal melanocytes were seeded in eight-well chamber slides and incubated for 24 h. Thereafter, cells were washed with PBS, fixed with 4% paraformaldehyde for 10 min, permeabilized using 0.1% Triton X-100 for 5 min, washed again, and blocked for 1 h with 1% BSA/PBS. Subsequently, cells were incubated with anti-HOX-B7 antibody (H00003217-D01P, Abnova, Taipei, Taiwan; dilution 1:100) overnight at 4°C. After washing, cells were incubated with secondary antibody (FITC-conjugated polyclonal swine anti-rabbit immunoglobulins, DAKO, Hamburg, Germany; dilution 1:40) for 2 h followed by rinsing with PBS and mounting with Vectashield Hard Set Mounting Medium with DAPI H-1500 (Vector Laboratories, Burlingame, USA). Images were collected by immunofluorescence microscopy using an Axio Imager Zeiss Z1 fluorescence microscope (Axiovision Rel. 4.6.3; Carl Zeiss AG, Oberkochen, Germany).
Migration assay
Migration assays were performed using Boyden chambers, as described previously [12]. Filters were coated with gelatine (5 mg/l) to improve cell attachment. The lower chamber compartment was filled with fibroblast-conditioned medium used as a chemoattractant. Mel Im cells were treated with 100 ng/ml BMP4 (R&D Systems, Richmond, CA) or 2 μg/ml anti-bFGF antibody (R&D Systems), respectively, or a combination of both. After incubation for 24 h the cells were harvested, resuspended in DMEM without FCS to a density of 3 × 104 cells/ml, and placed in the upper compartment. After incubation at 37°C for 4 h, the filters were collected and cells adhering to the lower surface were fixed, stained, and counted. Experiments were carried out in triplicate and were repeated three times with consistent results.
Statistical analysis
In the bar graphs, results are expressed as mean ± SD (range) or percent. Comparison between groups was made using Student’s unpaired t test. A p value < 0.05 was considered as statistically significant (ns: not significant, *p < 0.05, **p < 0.01, ***p < 0.001). All calculations were performed using GraphPad Prism Software (GraphPad Software, Inc., San Diego, USA).
Results
Regulation of BMP4 expression by bFGF via Ets-1
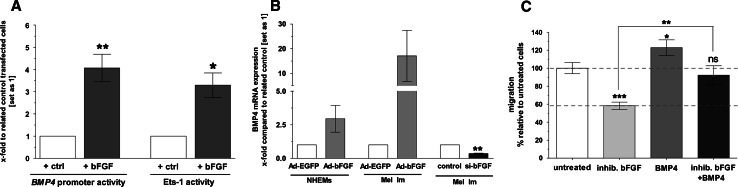
Previously, several studies demonstrated a cooperation between BMPs and members of the fibroblast growth factor family [49, 50]. In order to investigate a potential regulation of BMP4 by bFGF in melanoma, we co-transfected Mel Im melanoma cells with a bFGF expression plasmid together with a BMP4 promoter construct. BMP4 promoter activity was increased to more than fourfold after transfection of the bFGF expression construct (Fig. 1a). Extending previous findings by our group [12], we showed a strong upregulation of the Ets-1 activity in luciferase assays by co-transfection of a bFGF expression plasmid together with an Ets-1 reporter construct (Fig. 1a). In further experiments, we transduced NHEMs and the melanoma cell line Mel Im, respectively, with a bFGF expressing adenovirus for 48 h and observed an induction of BMP4 expression up to three-fold in NHEMs and up to more than 15-fold in Mel Im cells in comparison to an adenovirus expressing EGFP (both in Fig. 1b; overexpression of bFGF in transduced cells was confirmed as shown in Fig. S1). Additionally, transfection of Mel Im cells with a siRNA against bFGF led to a significant downregulation of BMP4 expression compared to cells transfected with a control siRNA consisting of a scrambled siRNA sequence (Fig. 1b).
Fig. 1.
BMP4 expression is indirectly regulated by bFGF and is the main regulator of migration in malignant melanoma. a Transfection with a BMP4 reporter construct or an Ets-1 luciferase construct containing three times the Ets-1 binding sites together with a bFGF expression plasmid into Mel Im cells resulted in strong upregulation of the BMP4 promoter activity and Ets-1 activity compared to control transfected cells. Cells transfected with empty pcDNA3 vector were used as the control and set as 1. b Incubation of NHEMs (normal human epidermal melanocytes) and the melanoma cell line Mel Im, respectively, with a bFGF expressing adenovirus for 48 h induces expression of BMP4 mRNA as measured by qRT-PCR. Cells transfected with an EGFP-expressing adenovirus served as the control and were set as 1. Transfection of Mel Im cells with a bFGF siRNA for 24 h resulted in downregulation of BMP4 expression. Transfection with scrambled control siRNA served as the control and was set as 1. c Mel Im cells were treated for 24 h with recombinant BMP4, an inhibitory anti-bFGF antibody or the combination of both, respectively. Migratory potential was studied using Boyden chambers. Treatment with inhibitory bFGF antibody led to reduction of migration, whereas recombinant BMP4 enhanced the migratory potential. Combined treatment compensated the inhibitory activity of the bFGF antibody. Student’s t test was performed to compare treated cells to control cells; additionally inhibitory bFGF treated melanoma cells were compared to inhibitory bFGF + BMP4 treated cells. Bars show the mean ± SD of three independent experiments and measurements were performed in duplicate (***p < 0.001, **p < 0.01, *p < 0.05)
Since it is known that bFGF strongly contributes to the migration of melanoma cells [51] we investigated whether the effect of bFGF on melanoma cell migration was achieved by induction of BMP4 expression. Therefore, we treated Mel Im cells with an inhibitory bFGF antibody for 24 h. As expected, we observed an approximately 40% reduction in the migratory potential of inhibitory antibody treated cells compared to untreated cells in Boyden chamber assays (Fig. 1c; for a compilation of photographs exemplary depicting filter areas counted in this experiment, see Fig. S2). Additional treatment with recombinant BMP4 protein was sufficient to compensate this effect of the bFGF inhibitory antibody almost completely (Fig. 1c). Further, the addition of recombinant BMP4 to melanocyte cell cultures strongly enhanced the migratory potential of NHEMs (Fig. S3). As discussed below, these data point out that BMP4 acts as an important modulator of migration in malignant melanoma.
Regulation of bFGF expression by HOX-B7
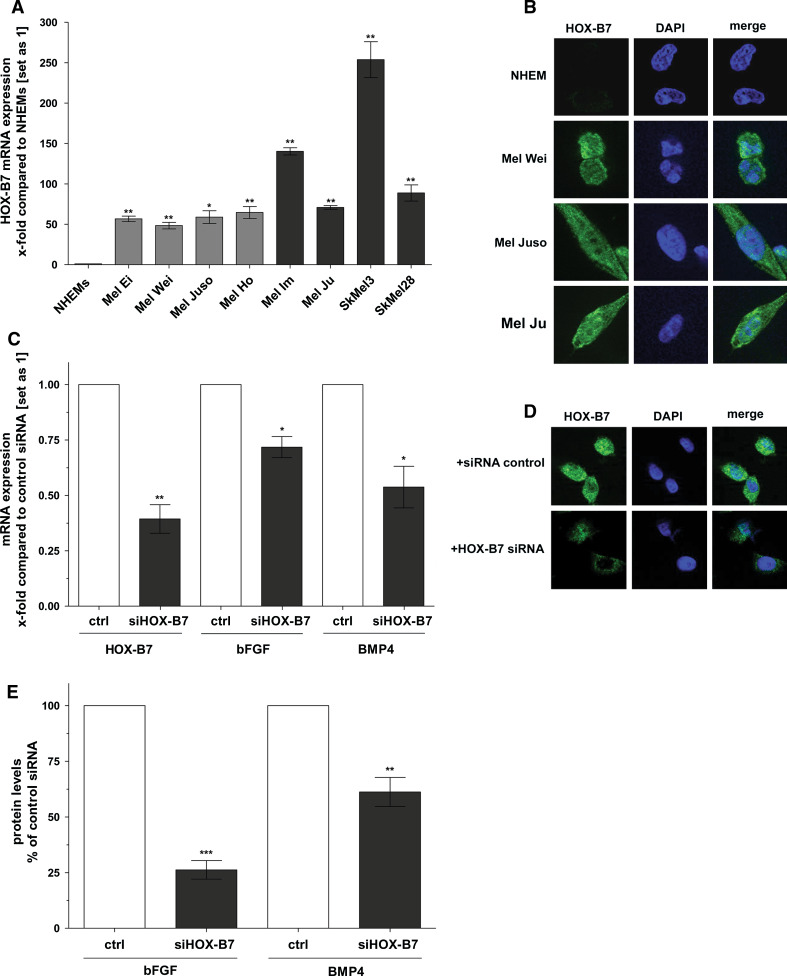
Certainly, bFGF and BMP4 represent important oncogenes [13, 52]. It, therefore, is of major interest to investigate the molecular details leading to induction of expression of these genes to understand early processes in tumor progression. Care et al. [24] showed that the homeobox transcription factor HOX-B7 regulates mRNA expression of bFGF in melanoma cells. Stimulated by these findings, we determined the expression level of HOX-B7 in four cell lines derived from primary cutaneous melanoma as well as in four cell lines derived from metastases of malignant melanoma. We revealed a strong upregulation of HOX-B7 mRNA (between 50-fold and approximately 300-fold) in all melanoma cell lines compared to NHEMs (Fig. 2a). For the first time, we were able to confirm enhanced HOX-B7 expression on the protein level, performing immunofluorescence analysis on four melanoma cell lines derived from two primary melanomas and two melanoma metastases, respectively (Figs. 2b, 3e). Whereas NHEMs showed no detectable HOX-B7 protein expression, melanoma cells gave a strong fluorescence signal after incubation with an anti-HOX-B7 antibody. We additionally determined bFGF and BMP4 protein expression in the specific melanoma cell lines used for these experiments by ELISA to assure correlation between HOX-B7, bFGF, and BMP4 expression levels (Fig. S4A, B).
Fig. 2.
HOX-B7 is overexpressed in melanoma cell lines compared to NHEMs and regulates expression of bFGF and BMP4. a Expression of HOX-B7 was analyzed in NHEMs and malignant melanoma cell lines by qRT-PCR. HOX-B7 mRNA levels were significantly increased in all melanoma cell lines compared to NHEMs (set as 1). b Immunofluorescence on NHEMs and melanoma cell lines confirmed enhanced expression of HOX-B7 on the protein level in melanoma cell lines compared to NHEMs (×60). c Mel Im cells were transfected with HOX-B7 siRNA and negative control siRNA, respectively, for 48 h. HOX-B7, bFGF, and BMP4 mRNA expression was significantly decreased in HOX-B7 siRNA-transfected cells compared to the related control-transfected cells (set as 1). d Transfection of Mel Im cells with HOX-B7 siRNA for 48 h revealed decreased expression of HOX-B7 protein as shown by immunofluorescence (×43). e Mel Im cells were treated with HOX-B7 siRNA for 48 h. As determined by ELISA experiments, HOX-B7 siRNA-treated cells showed strongly reduced bFGF and BMP4 protein expression levels compared to the related controls (set as 100%). Bars show the mean ± SD of three independent experiments, measurements were performed in duplicate (***p < 0.001, **p < 0.01, *p < 0.05)
Fig. 3.
Expression of miR-196a in NHEMs, melanoma cells, and stably transfected cell clones and its regulatory impact on HOX-B7 expression. a Expression level of miR-196a was determined in NHEMs and melanoma cell lines by qRT-PCR analysis. Melanoma cell lines showed significantly downregulated expression of miR-196a compared to NHEMs. b Mel Im cells stably transfected with miR-196a expression plasmids re-expressed miR-196a as determined by qRT-PCR. c HOX-B8 mRNA expression is diminished in miR-196a re-expressing cell clones compared to empty vector controls, thus confirming functionality of ectopically expressed miR-196a molecules (qRT-PCR). d HOX-B7 mRNA expression is reduced by about 50–75% in miR-196a re-expressing melanoma cells as determined by qRT-PCR. e Decreased expression of HOX-B7 in the miR-196a cell clones compared to Mel Im and control transfected cells was confirmed on protein level by immunofluorescence experiments. ×43 (upper three rows) and ×20 (lower row), respectively. Bars show the mean ± SD of three independent experiments, measurements were performed in duplicate (***p < 0.001, **p < 0.01, *p < 0.05)
To investigate a potential regulation of bFGF and its downstream target BMP4 by HOX-B7, we transfected Mel Im melanoma cells with two different siRNAs against HOX-B7 and the related control siRNA representing a scrambled nucleotide sequence. Since mRNA expression of HOX-B7 was significantly reduced in Mel Im cells transfected with each of the HOX-B7 siRNAs (Fig. S5), we performed the following experiments with a mixture of both siRNAs against HOX-B7. Immunofluorescence staining revealed reduced HOX-B7 protein expression in siRNA-transfected cells compared to control cells (Fig. 2d). In addition, 48 h after transfection of HOX-B7 siRNA, a significant downregulation of bFGF and BMP4 expression on mRNA as well as on protein level was detectable by qRT-PCR and ELISA, respectively (Fig. 2c, e; for absolute bFGF and BMP4 protein levels, see exemplary measurement presented in Fig. S6).
MiRNA-196a regulates expression of HOX-B7
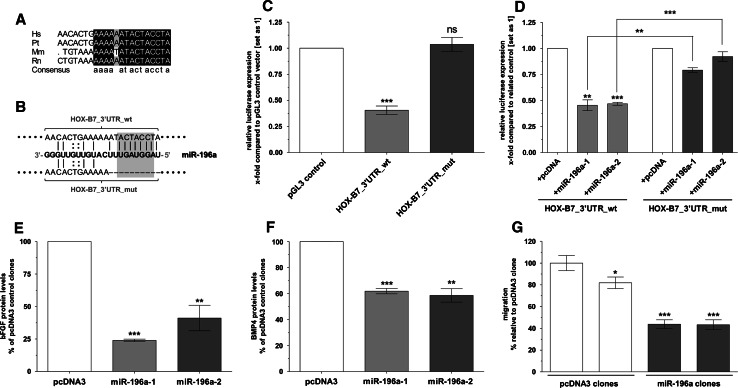
Mansfield et al. [30] described in 2004 that the miR-196 miRNAs are embedded within the four HOX clusters (HOX A to D) of mammalian homeobox transcription factor genes. It was further shown that miR-196a is able to bind to the 3′UTR of the HOX-B8 transcript and inhibits gene expression by direct cleavage of the HOX-B8 mRNA [31]. To examine whether HOX-B7 is also a potential target of miR-196a, we employed three different software attempts (miRanda [53], TargetScan S [54], and PicTar-Vert [55]) and determined a binding site for miR-196a in the HOX-B7 3′UTR (Fig. 4a). In qRT-PCR experiments, we analyzed expression of miR-196a in our cell panel and observed a strong reduction of miR-196a expression in melanoma cell lines compared to NHEMs (Fig. 3a). Thus, HOX-B7 and miR-196a actually showed the reciprocal expression pattern necessary for a possible regulatory relationship between those two molecules.
Fig. 4.
MicroRNA miR-196a directly binds to the HOX-B7 3′UTR and protein levels of bFGF and BMP4 are diminished in miR-196a cell clones. a Schematic representation of the 22-bp conserved miR-196a target sequence located in the HOX-B7 3′UTR (Hs Homo sapiens, Pt Pan troglodytes, Mm Mus musculus, Rn Rattus norvegicus). b Predicted interaction of miR-196a with the wild-type human HOX-B7 3′UTR target sequence (top) cloned into a reporter construct (HOX-B7_3′UTR_wt). In another reporter construct, 10 bp of the target sequence were deleted (HOX-B7_3′UTR_mut; bottom) so that no interaction at the 5′ part of the miRNA was possible. The grey square indicates nucleotides belonging to the seed-sequence of miR-196a (nucleotides 2–8 from the miRNAs 5′ end). c Both HOX-B7 3′UTR reporter constructs were transfected into NHEMs. While luciferase expression was strongly reduced if the reporter construct contained the wild-type target sequence, this effect was abolished after partial deletion of the miR-196 target sequence. d Both HOX-B7 reporter constructs were co-transfected together with either miR-196a expression plasmids or empty pcDNA3 vector into Mel Im melanoma cells. Co-transfection of the wild-type HOX-B7 3′UTR reporter construct together with the miR-196a expression constructs led to strong repression of luciferase activity compared to control cells co-transfected with empty pcDNA3 vector. Luciferase expression was almost unchanged in cells which received the mutated HOX-B7 3′UTR reporter construct independently of co-transfection of either empty pcDNA3 vector or the miR-196a expression plasmids. e, f bFGF and BMP4 protein levels in miR-196a cell clones were analyzed performing ELISA. bFGF and BMP4 protein expression was significantly reduced in miR-196a re-expressing cell clones compared to empty vector control (set as 100%). g Reduced migratory potential of the miR-196a re-expressing cell clones compared to empty pcDNA3 control transfected cells (set as 100%) as determined by Boyden chamber assays. Experiments were performed with three miR-196a-1 and miR-196a-2 cell clones each, shown as one graph bar. Bars show the mean ± SD of three independent experiments, measurements were performed in duplicate (***p < 0.001, **p < 0.01, *p < 0.05)
For subsequent experiments, cell clones stably transfected with miR-196a expression plasmids were generated. Although miR-196a is encoded by two different chromosomal loci (located on chromosome 17 and chromosome 12, respectively) the mature sequence of miR-196a-1 and miR-196a-2 is completely identical. Nevertheless, to exclude that miR-196a-1 and miR-196a-2 exert different regulatory mechanisms, we generated both miR-196a-1 and miR-196a-2 stably transfected cell clones. Successful re-expression of miR-196a-1 and miR-196a-2 in the stable cell clones was confirmed by qRT-PCR (Fig. 3b). In contrast to control-transfected cells (containing empty pcDNA3 vector), cells stably transfected with miR-196a expression plasmids showed about four- to tenfold increased miR-196a expression levels. To ensure functionality of the ectopically expressed miR-196a molecules, we analyzed expression of the already verified miR-196a target HOX-B8 [31] in our stable cell clones. Quantitative RT-PCR analysis revealed that miR-196a re-expressing melanoma cell clones showed an about 50% reduced expression of HOX-B8 mRNA (Fig. 3c). In accordance with our hypothesis, we were also able to demonstrate a downregulation of HOX-B7 gene expression in the miR-196a-1 and miR-196a-2 re-expressing cell clones on RNA as well as on protein level (Fig. 3d, e). In the miR-196a re-expressing cell clones a reduction in HOX-B7 mRNA ranging from 50 to 75% compared to control transfected cells was measured (Fig. 3d) and almost no HOX-B7 protein expression was detectable in these cells by immunofluorescence staining (Fig. 3e).
The inverse expression patterns of miR-196a and HOX-B7 in NHEMs, melanoma cell lines, and miR-196a re-expressing melanoma cell clones strongly pointed out that miR-196a is actually involved in regulation of HOX-B7 expression. To confirm a direct interaction of miR-196a with the predicted target sequence (Fig. 4a) in the HOX-B7 3′UTR, we generated a reporter construct containing 570 bp of the HOX-B7 3′UTR behind the luciferase gene in a pGL3 control vector (HOX-B7_3′UTR_wt; Fig. 4b). Additionally, we created another 3′UTR reporter plasmid in which we deleted 10 bp of the predicted target sequence (containing the region interacting with the miR-196a seed-sequence, HOX-B7_3′UTR_mut; Fig. 4b). Transfection of the wild-type HOX-B7_3′UTR reporter construct into normal human melanocytes (showing high endogenous miR-196a levels) resulted in a significant reduction of luciferase expression compared to melanocytes receiving empty pGL3 control vector. In contrast, NHEMs transfected with the mutated plasmid luciferase activity reached a level similar to cells receiving the pGL3 control vector without the HOX-B7 3′UTR (Fig. 4c). Additionally, co-transfection of either of the 3′UTR reporter constructs together with the miR-196a expression plasmids or the related empty vector was performed in Mel Im melanoma cells. While the wild-type construct responded with a strong decrease in luciferase activity to co-transfection of either of the miR-196a expression plasmids, this effect was almost completely abolished if using the mutated version of the 3′UTR reporter plasmid (Fig. 4d). These data clearly show that miR-196a actually binds directly to the predicted target sequence in the HOX-B7 3′UTR and thus represses further processing of the transcript.
To investigate the downstream effects caused by the regulatory influence of miR-196a on HOX-B7, we analyzed expression of bFGF and BMP4 in miR-196a re-expressing melanoma cells and the related control cells by performing ELISAs. The protein level of bFGF was strongly reduced in miR-196a-1 and miR-196a-2 cell clones in comparison to control-transfected cells (Fig. 4e). In addition, BMP4 protein expression was also significantly diminished in the stable transfected cell clones, as determined by ELISA experiments (Fig. 4f). Moreover, we determined the expression level of Id1, a well-known BMP4 target gene [56] in the cell clones and observed a strong downregulation of Id1 mRNA expression in miR-196a cell clones compared to pcDNA3 clones (Fig. S7). Strikingly, miR-196a cell clones showed an approx. 50% reduced migratory potential compared to the pcDNA3 control clones as determined in Boyden chamber assays (Fig. 4g).
Enhanced target gene expression and increased migratory potential of anti-miR-196a treated melanocytes
In the last set of experiments, we analyzed whether inhibition of miR-196a expression in normal human melanocytes will result in altered expression of the related downstream targets. Therefore, we transfected NHEMs with LNAs (locked nucleic acids) against miR-196a or a scramble-miR control, respectively, for 48 h. mRNA expression of HOX-B7, bFGF, and BMP4 was significantly enhanced in anti-miR-196a-transfected cells (Fig. 5a). Moreover, immunofluorescence staining revealed elevated levels of HOX-B7 protein in anti-miR-196 treated melanocytes compared to scramble-miR transfected control cells (Fig. 5b). Finally, we performed Boyden chamber assays using NHEMs treated with LNAs against miR-196a and observed a strong increase in their migratory potential compared to scramble-miR control-transfected cells (Fig. 5c).
Fig. 5.
Anti-miR-196a treatment results in increased expression of HOX-B7, bFGF, and BMP4 and enhanced migratory potential in NHEMs. a Transfection of NHEMs with LNAs against miR-196a or a scramble-miR, respectively, for 48 h. HOX-B7, bFGF, and BMP4 mRNA expression was strongly increased in anti-miR transfected cells compared to scramble-miR control transfected cells (set as 1). b Immunofluorescence staining revealed elevated levels of HOX-B7 protein in miR-196a anti-miR transfected cells (×43). c Anti-miR-196a-transfected NHEMs showed a significantly enhanced migratory potential of up to 200% when compared to scramble-miR transfected cells (set as 100%). Bars show the mean ± SD of three independent experiments, measurements were performed in duplicates (***p < 0.001, **p < 0.01, *p < 0.05)
Discussion
It is known that BMPs play an important role in melanoma progression. Although plenty of data are available about downstream signaling of BMPs, including functional relevance of BMPs in different cell types, regulation pathways leading to overexpression of BMPs in malignant melanoma are still poorly defined. In this study we therefore investigated signaling upstream of BMPs.
Recently, we were able to show that expression of BMPs is controlled at the transcriptional level by the transcription factor AP-2 acting as negative regulator of BMP4 promoter activity, whereas Ets-1 acts as an enhancer of BMP4 expression [12]. Thus, the elevated expression level of Ets-1 detected in melanoma cells leads to overexpression of BMP4 as previously demonstrated by Rothhammer et al. [12, 44]. Taken together, these data point towards a complex mechanism of BMP regulation, which may include different types of interaction partners and transcription factors. In this study, we analyzed one main aspect of BMP regulation in melanoma cells in detail: the upstream effects on positive regulation of BMP4 expression by transcription factor Ets-1.
It is known that members of the fibroblast growth factor family (FGFs) induce expression of Ets-1 in different cell types. In detail, it was shown by Iwasaka et al. [57] that incubation of endothelial cells with bFGF resulted in increased DNA-Ets complexes. Hence, we investigated a potential interaction between basic FGF, Ets-1, and BMPs in melanoma. Transfection of a bFGF expression plasmid into melanoma cells resulted in enhanced Ets-1 activity and subsequently in an upregulation of BMP4 promoter activity. Along with our previous study demonstrating binding of Ets-1 to the BMP4 promoter [12], we were able to show for the first time a signaling pathway leading to enhanced expression of BMP4 in malignant melanoma due to binding of transcription factor Ets-1, which in turn is activated by bFGF.
Functional studies concerning regulation of migration and proliferation of melanoma cells are still ambiguous: It was shown that antisense oligomers against bFGF inhibit proliferation of melanoma cells [58]. Furthermore, Tsunoda et al. [59] illustrated stimulation of migration by a single injection of bFGF in B16-BL6 mouse melanoma. Other studies by Langenfeld et al. [60] demonstrated increased migration of human lung cancer cell lines after treatment with BMP2. Moreover, our group was able to show that BMP4 promotes migration of melanoma cells [12]. We therefore asked whether BMPs or bFGF control migration capacity of melanoma cells. Treatment of melanoma cells with an inhibitory bFGF antibody resulted in decreased migration, whereas addition of BMP4 counterbalanced this effect. These data indicate that bFGF exerts its main effect on migratory potential by regulation of BMP expression thereby enhancing the migratory potential of melanoma cells.
Care et al. [24] demonstrated that activation of bFGF is mediated by the homeobox transcription factor HOX-B7 in melanoma cell lines. They showed enhanced expression of HOX-B7 on mRNA level in various melanoma cells compared to NHEMs, a fact that is supported by our own studies in which we were further able to confirm increased HOX-B7 expression on the protein level. It is well known that several other cancer cells also show elevated levels of HOX-B7 mRNA, for example ovarian and breast cancer cell lines [61, 62]. Moreover, in breast cancer cells the increased expression of HOX-B7 mRNA is correlated with upregulation of bFGF [62]. In transfection experiments with siRNA against HOX-B7 we were able to show downregulation of bFGF and subsequently decreased expression of BMP4. These observations show a direct functional association between HOX-B7 and bFGF, with HOX-B7 acting upstream of bFGF. We therefore state that in melanoma cells, elevated mRNA levels of HOX-B7 lead to an upregulation of bFGF, which again activate transcriptional activity of Ets-1. This complex regulation pathway results in increased BMP4 expression and thereby contributes to enhanced migratory potential of melanoma cells. Other groups reported a functional link between increased HOX-B7 expression and progression of cancer in different cancer cell types. In their studies, transfection of antisense HOX-B7 led to a significant reduction of invasion in ovarian cancer cell lines compared to parental cells [61]. Furthermore, it was shown in a breast carcinoma cell line, that transduction with a vector carrying HOX-B7 cDNA induced bFGF expression and increased the proliferation rate of SkBr3 cells [63]. Contemplating our results in the context of literature available, therefore, strongly indicates that the effects observed are specific to HOX-B7, although—at this point—we cannot rule out that other members of the HOX-gene family may additionally have an influence on the regulatory pathway described.
To examine upstream regulation of HOX-B7, we employed computer-based methods and detected a potential binding site for microRNA miR-196a in the 3′UTR of HOX-B7. In addition, Yekta et al. [31] were able to show miR-196 mediated repression of another member of the HOX-B cluster, namely HOX-B8. Hence, we investigated the expression level of miR-196a in our melanoma cells and observed strong reduction of this miRNA in different melanoma cell lines compared to NHEMs. Moreover, melanoma cell clones stably re-expressing miR-196a showed decreased expression of HOX-B7 mRNA levels compared to control cells.
The inverse correlation between HOX-B7 mRNA levels and miR-196a expression in melanoma cell lines and NHEMs indicated a regulation of HOX-B7 by miR-196a. A potential indirect regulation mechanism was excluded by experiments employing reporter constructs containing either the wild-type HOX-B7 3′UTR or a 3′UTR with partially deleted miR-196a target sequence. While the luciferase expression resulting from transfection of the HOX-B7 wild-type 3′UTR construct was strongly reduced in the presence of high miR-196a levels (i.e., in NHEMs and in melanoma cells co-transfected with miR-196a expression plasmids), this effect was abolished when mutating the miR-196a target sequence in the reporter plasmid. By further analyzing downstream targets of HOX-B7, we determined reduced protein levels of both bFGF and BMP4 in the miR-196a re-expressing cell clones compared to control cells. Moreover, Boyden chamber assays revealed a strong reduction in the migratory potential of miR-196a cell clones, thereby underlining the functional relevance of diminished miR-196a expression in malignant melanoma. Strikingly, this hypothesis is substantiated by our finding that knocking down the high endogenous miR-196a level in NHEMs elicits an increased expression of miR-196a target genes and thus finally results in a strongly enhanced migratory potential.
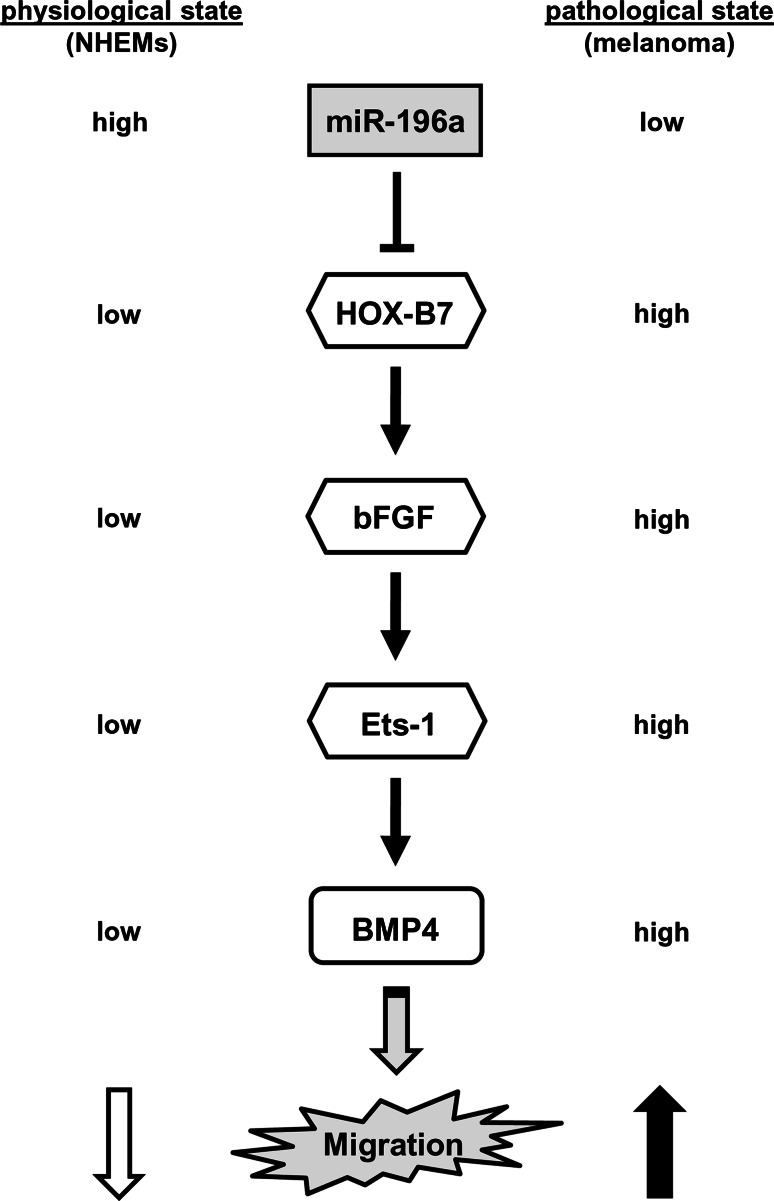
In summary, we were able to determine a complex signaling cascade in melanoma cells starting from microRNA miR-196a via several intermediate steps and finally resulting in enhanced expression of BMPs (a schematic overview on the signaling pathway revealed by our experiments can be found in Fig. 6). Expanding a concept in which bFGF is responsible for the migratory potential of different cancer cell types, we clearly demonstrate that—at least in the case of melanoma cells—bFGF exerts its function as key modulator of tumor cell migration mainly through activating BMP4 expression. We were further able to reveal that an initial step in the upstream signaling cascade leading to overexpression of BMP4 (via HOX-B7, bFGF, and Ets-1) is loss of endogenous miR-196a assigning this miRNA a role as central regulator of BMP4 expression in malignant melanoma cells.
Fig. 6.
Schematic presentation of the signalling pathway described. Via several intermediate steps, the loss of endogenous miR-196a expression results in a strongly enhanced migratory potential of melanoma cells compared to normal human melanocytes
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1 Confirmation of bFGF overexpression in Mel Im melanoma cells transduced with a bFGF expressing adenovirus by qRT-PCR. Bars show the means ± SD of three independent experiments, measurements were performed in duplicates (***, p< 0.001; **, p< 0.01; *, p< 0.5) (JPEG 229 kb)
Fig. S2 Compilation of photographs exemplary for filter areas counted in the Boyden chamber assays described in Fig. 1C (JPEG 2028 kb)
Fig. S3 Enhanced migratory potential of NHEMs in Boyden chamber assays after treatment with recombinant BMP4 protein (100ng/ml) for 24h. Untreated NHEMs served as a control. Bars show the means ± SD of one out of three independent experiments, measurements were performed in triplicates (***, p< 0.001; **, p< 0.01; *, p< 0.5) (TIFF 10503 kb)
Fig. S4 (A) bFGF and (B) BMP4 protein expression in two cell lines derived from primary melanoma lesions and two cell lines derived from metastatic melanomas, respectively. Absolute protein levels were measured by ELISA. Protein levels of bFGF and BMP4 in standard DMEM medium were determined and subsequently subtracted from total values. After subtraction of bFGF and BMP4 levels measured in the melanocyte growth medium used, NHEMs showed no endogenous expression of bFGF and BMP4, respectively. One representative experiment is shown out of three replicates (JPEG 1159 kb)
Fig. S5 HOX-B7 mRNA expression is reduced in the melanoma cell line Mel Im after transfection of either siHOX-B7#1 or siHOX-B7#4 compared to control transfected cells as shown by quantitative RT-PCR. Bars show the means ± SD of three independent experiments, measurements were performed in duplicates (***, p< 0.001; **, p< 0.01; *, p< 0.5) (JPEG 629 kb)
Fig. S6 Absolute bFGF and BMP4 protein levels of Mel Im cells transfected with siRNA against HOX-B7 for 48h. One representative out of three independent experiments is shown (JPEG 615 kb)
Fig. S7 The miR-196a re-expressing cell clones exhibited decreased Id1 mRNA expression compared to pcDNA3 empty vector control cells as shown by quantitative RT-PCR. Bars show the means ± SD of three independent experiments, measurements were performed in duplicates (***, p< 0.001; **, p< 0.01; *, p< 0.5) (JPEG 632 kb)
Acknowledgments
This work was supported by the Deutsche Krebshilfe e.V. (grant number 108777) and the Deutsche Forschungsgemeinschaft (grant number BO1573/16-1). We thank Prof. Soncin (Lille, France) for providing the Ets-1 construct, Dr. Madry (Homburg, Germany) for providing the bFGF expression plasmid; Dr. Moser (Martinsried, Germany) for providing the BMP4 promoter reporter constructs; and Sibylla Lodermeyer for excellent technical assistance.
Footnotes
S. Braig and D. W. Mueller contributed equally to this work.
References
- 1.Mehler MF, Mabie PC, Zhang D, Kessler JA. Bone morphogenetic proteins in the nervous system. Trends Neurosci. 1997;20:309–317. doi: 10.1016/S0166-2236(96)01046-6. [DOI] [PubMed] [Google Scholar]
- 2.Turgeman G, Pittman DD, Muller R, Kurkalli BG, Zhou S, Pelled G, Peyser A, Zilberman Y, Moutsatsos IK, Gazit D. Engineered human mesenchymal stem cells: a novel platform for skeletal cell mediated gene therapy. J Gene Med. 2001;3:240–251. doi: 10.1002/1521-2254(200105/06)3:3<240::AID-JGM181>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 3.Hogan BL. Bone morphogenetic proteins in development. Curr Opin Genet Dev. 1996;6:432–438. doi: 10.1016/S0959-437X(96)80064-5. [DOI] [PubMed] [Google Scholar]
- 4.Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250:231–250. [PubMed] [Google Scholar]
- 5.Yamamoto Y, Oelgeschlager M. Regulation of bone morphogenetic proteins in early embryonic development. Naturwissenschaften. 2004;91:519–534. doi: 10.1007/s00114-004-0575-z. [DOI] [PubMed] [Google Scholar]
- 6.Kleeff J, Maruyama H, Ishiwata T, Sawhney H, Friess H, Buchler MW, Korc M. Bone morphogenetic protein 2 exerts diverse effects on cell growth in vitro and is expressed in human pancreatic cancer in vivo. Gastroenterology. 1999;116:1202–1216. doi: 10.1016/S0016-5085(99)70024-7. [DOI] [PubMed] [Google Scholar]
- 7.Kiyozuka Y, Nakagawa H, Senzaki H, Uemura Y, Adachi S, Teramoto Y, Matsuyama T, Bessho K, Tsubura A. Bone morphogenetic protein-2 and type IV collagen expression in psammoma body forming ovarian cancer. Anticancer Res. 2001;21:1723–1730. [PubMed] [Google Scholar]
- 8.Ye L, Lewis-Russell JM, Kyanaston HG, Jiang WG. Bone morphogenetic proteins and their receptor signaling in prostate cancer. Histol Histopathol. 2007;22:1129–1147. doi: 10.14670/HH-22.1129. [DOI] [PubMed] [Google Scholar]
- 9.Fong YC, Li TM, Wu CM, Hsu SF, Kao ST, Chen RJ, Lin CC, Liu SC, Wu CL, Tang CH. BMP-2 increases migration of human chondrosarcoma cells via PI3K/Akt pathway. J Cell Physiol. 2008;217:846–855. doi: 10.1002/jcp.21568. [DOI] [PubMed] [Google Scholar]
- 10.Hardwick JC, Kodach LL, Offerhaus GJ, van den Brink GR. Bone morphogenetic protein signalling in colorectal cancer. Nat Rev Cancer. 2008;8:806–812. doi: 10.1038/nrc2467. [DOI] [PubMed] [Google Scholar]
- 11.Katsuno Y, Hanyu A, Kanda H, Ishikawa Y, Akiyama F, Iwase T, Ogata E, Ehata S, Miyazono K, Imamura T. Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene. 2008;27:6322–6333. doi: 10.1038/onc.2008.232. [DOI] [PubMed] [Google Scholar]
- 12.Rothhammer T, Poser I, Soncin F, Bataille F, Moser M, Bosserhoff AK. Bone morphogenic proteins are overexpressed in malignant melanoma and promote cell invasion and migration. Cancer Res. 2005;65:448–456. [PubMed] [Google Scholar]
- 13.Hsu MY, Rovinsky S, Penmatcha S, Herlyn M, Muirhead D. Bone morphogenetic proteins in melanoma: angel or devil? Cancer Metastasis Rev. 2005;24:251–263. doi: 10.1007/s10555-005-1575-y. [DOI] [PubMed] [Google Scholar]
- 14.Rothhammer T, Braig S, Bosserhoff AK. Bone morphogenetic proteins induce expression of metalloproteinases in melanoma cells and fibroblasts. Eur J Cancer. 2008;44:2526–2534. doi: 10.1016/j.ejca.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 15.Rothhammer T, Wild PJ, Meyer S, Bataille F, Pauer A, Klinkhammer-Schalke M, Hein R, Hofstaedter F, Bosserhoff AK. Bone morphogenetic protein 7 (BMP7) expression is a potential novel prognostic marker for recurrence in patients with primary melanoma. Cancer Biomark. 2007;3:111–117. doi: 10.3233/cbm-2007-3205. [DOI] [PubMed] [Google Scholar]
- 16.Boswell BA, Lein PJ, Musil LS. Cross-talk between fibroblast growth factor and bone morphogenetic proteins regulates gap junction-mediated intercellular communication in lens cells. Mol Biol Cell. 2008;19:2631–2641. doi: 10.1091/mbc.E08-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura Y, Tensho K, Nakaya H, Nawata M, Okabe T, Wakitani S. Low dose fibroblast growth factor-2 (FGF-2) enhances bone morphogenetic protein-2 (BMP-2)-induced ectopic bone formation in mice. Bone. 2005;36:399–407. doi: 10.1016/j.bone.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Halaban R, Langdon R, Birchall N, Cuono C, Baird A, Scott G, Moellmann G, McGuire J. Paracrine stimulation of melanocytes by keratinocytes through basic fibroblast growth factor. Ann N Y Acad Sci. 1988;548:180–190. doi: 10.1111/j.1749-6632.1988.tb18805.x. [DOI] [PubMed] [Google Scholar]
- 19.Giehl KA, Nagele U, Volkenandt M, Berking C. Protein expression of melanocyte growth factors (bFGF, SCF) and their receptors (FGFR-1, c-kit) in nevi and melanoma. J Cutan Pathol. 2007;34:7–14. doi: 10.1111/j.1600-0560.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- 20.Halaban R, Kwon BS, Ghosh S, Delli Bovi P, Baird A. bFGF as an autocrine growth factor for human melanomas. Oncogene Res. 1988;3:177–186. [PubMed] [Google Scholar]
- 21.Scott G, Stoler M, Sarkar S, Halaban R. Localization of basic fibroblast growth factor mRNA in melanocytic lesions by in situ hybridization. J Invest Dermatol. 1991;96:318–322. doi: 10.1111/1523-1747.ep12465203. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Becker D. Antisense targeting of basic fibroblast growth factor and fibroblast growth factor receptor-1 in human melanomas blocks intratumoral angiogenesis and tumor growth. Nat Med. 1997;3:887–893. doi: 10.1038/nm0897-887. [DOI] [PubMed] [Google Scholar]
- 23.Meier F, Caroli U, Satyamoorthy K, Schittek B, Bauer J, Berking C, Moller H, Maczey E, Rassner G, Herlyn M, Garbe C. Fibroblast growth factor-2 but not Mel-CAM and/or beta3 integrin promotes progression of melanocytes to melanoma. Exp Dermatol. 2003;12:296–306. doi: 10.1034/j.1600-0625.2003.120310.x. [DOI] [PubMed] [Google Scholar]
- 24.Care A, Silvani A, Meccia E, Mattia G, Stoppacciaro A, Parmiani G, Peschle C, Colombo MP. HOXB7 constitutively activates basic fibroblast growth factor in melanomas. Mol Cell Biol. 1996;16:4842–4851. doi: 10.1128/mcb.16.9.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deschamps J, Meijlink F. Mammalian homeobox genes in normal development and neoplasia. Crit Rev Oncog. 1992;3:117–173. [PubMed] [Google Scholar]
- 26.Abe M, Hamada J, Takahashi O, Takahashi Y, Tada M, Miyamoto M, Morikawa T, Kondo S, Moriuchi T. Disordered expression of HOX genes in human non-small cell lung cancer. Oncol Rep. 2006;15:797–802. [PubMed] [Google Scholar]
- 27.Lopez R, Garrido E, Pina P, Hidalgo A, Lazos M, Ochoa R, Salcedo M. HOXB homeobox gene expression in cervical carcinoma. Int J Gynecol Cancer. 2006;16:329–335. doi: 10.1111/j.1525-1438.2006.00350.x. [DOI] [PubMed] [Google Scholar]
- 28.Garzon R, Garofalo M, Martelli MP, Briesewitz R, Wang L, Fernandez-Cymering C, Volinia S, Liu CG, Schnittger S, Haferlach T, Liso A, Diverio D, Mancini M, Meloni G, Foa R, Martelli MF, Mecucci C, Croce CM, Falini B. Distinctive microRNA signature of acute myeloid leukemia bearing cytoplasmic mutated nucleophosmin. Proc Natl Acad Sci USA. 2008;105:3945–3950. doi: 10.1073/pnas.0800135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chopra VS, Mishra RK. “Mir”acles in hox gene regulation. Bioessays. 2006;28:445–448. doi: 10.1002/bies.20401. [DOI] [PubMed] [Google Scholar]
- 30.Mansfield JH, Harfe BD, Nissen R, Obenauer J, Srineel J, Chaudhuri A, Farzan-Kashani R, Zuker M, Pasquinelli AE, Ruvkun G, Sharp PA, Tabin CJ, McManus MT. MicroRNA-responsive ‘sensor’ transgenes uncover Hox-like and other developmentally regulated patterns of vertebrate microRNA expression. Nat Genet. 2004;36:1079–1083. doi: 10.1038/ng1421. [DOI] [PubMed] [Google Scholar]
- 31.Yekta S, Shih IH, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304:594–596. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- 32.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen CB, Shomron N, Sandberg R, Hornstein E, Kitzman J, Burge CB. Determinants of targeting by endogenous and exogenous microRNAs and siRNAs. RNA. 2007;13:1894–1910. doi: 10.1261/rna.768207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Behm-Ansmant I, Rehwinkel J, Doerks T, Stark A, Bork P, Izaurralde E. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 2006;20:1885–1898. doi: 10.1101/gad.1424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu L, Fan J, Belasco JG. MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci USA. 2006;103:4034–4039. doi: 10.1073/pnas.0510928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 2007;17:118–126. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Standart N, Jackson RJ. MicroRNAs repress translation of m7Gppp-capped target mRNAs in vitro by inhibiting initiation and promoting deadenylation. Genes Dev. 2007;21:1975–1982. doi: 10.1101/gad.1591507. [DOI] [PubMed] [Google Scholar]
- 38.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schickel R, Boyerinas B, Park SM, Peter ME. MicroRNAs: key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene. 2008;27:5959–5974. doi: 10.1038/onc.2008.274. [DOI] [PubMed] [Google Scholar]
- 40.Mueller DW, Rehli M, Bosserhoff AK. miRNA expression profiling in melanocytes and melanoma cell lines reveals miRNAs associated with formation and progression of malignant melanoma. J Invest Dermatol. 2009;129:1740–1751. doi: 10.1038/jid.2008.452. [DOI] [PubMed] [Google Scholar]
- 41.Muller DW, Bosserhoff AK. Integrin beta 3 expression is regulated by let-7a miRNA in malignant melanoma. Oncogene. 2008;27:6698–6706. doi: 10.1038/onc.2008.282. [DOI] [PubMed] [Google Scholar]
- 42.Mueller DW, Bosserhoff AK. Role of miRNAs in the progression of malignant melanoma. Br J Cancer. 2009;101:551–556. doi: 10.1038/sj.bjc.6605204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacob K, Wach F, Holzapfel U, Hein R, Lengyel E, Buettner R, Bosserhoff AK. In vitro modulation of human melanoma cell invasion and proliferation by all-trans-retinoic acid. Melanoma Res. 1998;8:211–219. doi: 10.1097/00008390-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Rothhammer T, Hahne JC, Florin A, Poser I, Soncin F, Wernert N, Bosserhoff AK. The Ets-1 transcription factor is involved in the development and invasion of malignant melanoma. Cell Mol Life Sci. 2004;61:118–128. doi: 10.1007/s00018-003-3337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 46.Madry H, Emkey G, Zurakowski D, Trippel SB. Overexpression of human fibroblast growth factor 2 stimulates cell proliferation in an ex vivo model of articular chondrocyte transplantation. J Gene Med. 2004;6:238–245. doi: 10.1002/jgm.488. [DOI] [PubMed] [Google Scholar]
- 47.Groitl P, Dobner T. Construction of adenovirus type 5 early region 1 and 4 virus mutants. Methods Mol Med. 2007;130:29–39. doi: 10.1385/1-59745-166-5:29. [DOI] [PubMed] [Google Scholar]
- 48.Arndt S, Poser I, Moser M, Bosserhoff AK. Fussel-15, a novel Ski/Sno homolog protein, antagonizes BMP signaling. Mol Cell Neurosci. 2007;34:603–611. doi: 10.1016/j.mcn.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Montesano R, Sarkozi R, Schramek H. Bone morphogenetic protein-4 strongly potentiates growth factor-induced proliferation of mammary epithelial cells. Biochem Biophys Res Commun. 2008;374:164–168. doi: 10.1016/j.bbrc.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Katoh M. Networking of WNT, FGF, Notch, BMP, and Hedgehog signaling pathways during carcinogenesis. Stem Cell Rev. 2007;3:30–38. doi: 10.1007/s12015-007-0006-6. [DOI] [PubMed] [Google Scholar]
- 51.Meier F, Nesbit M, Hsu MY, Martin B, Van Belle P, Elder DE, Schaumburg-Lever G, Garbe C, Walz TM, Donatien P, Crombleholme TM, Herlyn M. Human melanoma progression in skin reconstructs: biological significance of bFGF. Am J Pathol. 2000;156:193–200. doi: 10.1016/S0002-9440(10)64719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krasagakis K, Garbe C, Zouboulis CC, Orfanos CE. Growth control of melanoma cells and melanocytes by cytokines. Recent Results Cancer Res. 1995;139:169–182. doi: 10.1007/978-3-642-78771-3_12. [DOI] [PubMed] [Google Scholar]
- 53.Griffiths-Jones S. miRBase: the microRNA sequence database. Methods Mol Biol. 2006;342:129–138. doi: 10.1385/1-59745-123-1:129. [DOI] [PubMed] [Google Scholar]
- 54.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/S0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 55.Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 56.Miyazono K, Miyazawa K (2002) Id: a target of BMP signaling. Sci STKE, pe40 [DOI] [PubMed]
- 57.Iwasaka C, Tanaka K, Abe M, Sato Y. Ets-1 regulates angiogenesis by inducing the expression of urokinase-type plasminogen activator and matrix metalloproteinase-1 and the migration of vascular endothelial cells. J Cell Physiol. 1996;169:522–531. doi: 10.1002/(SICI)1097-4652(199612)169:3<522::AID-JCP12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 58.Becker D, Meier CB, Herlyn M. Proliferation of human malignant melanomas is inhibited by antisense oligodeoxynucleotides targeted against basic fibroblast growth factor. EMBO J. 1989;8:3685–3691. doi: 10.1002/j.1460-2075.1989.tb08543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsunoda S, Nakamura T, Sakurai H, Saiki I. Fibroblast growth factor-2-induced host stroma reaction during initial tumor growth promotes progression of mouse melanoma via vascular endothelial growth factor A-dependent neovascularization. Cancer Sci. 2007;98:541–548. doi: 10.1111/j.1349-7006.2007.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Langenfeld EM, Calvano SE, Abou-Nukta F, Lowry SF, Amenta P, Langenfeld J. The mature bone morphogenetic protein-2 is aberrantly expressed in non-small cell lung carcinomas and stimulates tumor growth of A549 cells. Carcinogenesis. 2003;24:1445–1454. doi: 10.1093/carcin/bgg100. [DOI] [PubMed] [Google Scholar]
- 61.Yamashita T, Tazawa S, Yawei Z, Katayama H, Kato Y, Nishiwaki K, Yokohama Y, Ishikawa M. Suppression of invasive characteristics by antisense introduction of overexpressed HOX genes in ovarian cancer cells. Int J Oncol. 2006;28:931–938. [PubMed] [Google Scholar]
- 62.Wu X, Chen H, Parker B, Rubin E, Zhu T, Lee JS, Argani P, Sukumar S. HOXB7, a homeodomain protein, is overexpressed in breast cancer and confers epithelial-mesenchymal transition. Cancer Res. 2006;66:9527–9534. doi: 10.1158/0008-5472.CAN-05-4470. [DOI] [PubMed] [Google Scholar]
- 63.Care A, Silvani A, Meccia E, Mattia G, Peschle C, Colombo MP. Transduction of the SkBr3 breast carcinoma cell line with the HOXB7 gene induces bFGF expression, increases cell proliferation and reduces growth factor dependence. Oncogene. 1998;16:3285–3289. doi: 10.1038/sj.onc.1201875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Confirmation of bFGF overexpression in Mel Im melanoma cells transduced with a bFGF expressing adenovirus by qRT-PCR. Bars show the means ± SD of three independent experiments, measurements were performed in duplicates (***, p< 0.001; **, p< 0.01; *, p< 0.5) (JPEG 229 kb)
Fig. S2 Compilation of photographs exemplary for filter areas counted in the Boyden chamber assays described in Fig. 1C (JPEG 2028 kb)
Fig. S3 Enhanced migratory potential of NHEMs in Boyden chamber assays after treatment with recombinant BMP4 protein (100ng/ml) for 24h. Untreated NHEMs served as a control. Bars show the means ± SD of one out of three independent experiments, measurements were performed in triplicates (***, p< 0.001; **, p< 0.01; *, p< 0.5) (TIFF 10503 kb)
Fig. S4 (A) bFGF and (B) BMP4 protein expression in two cell lines derived from primary melanoma lesions and two cell lines derived from metastatic melanomas, respectively. Absolute protein levels were measured by ELISA. Protein levels of bFGF and BMP4 in standard DMEM medium were determined and subsequently subtracted from total values. After subtraction of bFGF and BMP4 levels measured in the melanocyte growth medium used, NHEMs showed no endogenous expression of bFGF and BMP4, respectively. One representative experiment is shown out of three replicates (JPEG 1159 kb)
Fig. S5 HOX-B7 mRNA expression is reduced in the melanoma cell line Mel Im after transfection of either siHOX-B7#1 or siHOX-B7#4 compared to control transfected cells as shown by quantitative RT-PCR. Bars show the means ± SD of three independent experiments, measurements were performed in duplicates (***, p< 0.001; **, p< 0.01; *, p< 0.5) (JPEG 629 kb)
Fig. S6 Absolute bFGF and BMP4 protein levels of Mel Im cells transfected with siRNA against HOX-B7 for 48h. One representative out of three independent experiments is shown (JPEG 615 kb)
Fig. S7 The miR-196a re-expressing cell clones exhibited decreased Id1 mRNA expression compared to pcDNA3 empty vector control cells as shown by quantitative RT-PCR. Bars show the means ± SD of three independent experiments, measurements were performed in duplicates (***, p< 0.001; **, p< 0.01; *, p< 0.5) (JPEG 632 kb)