Abstract
O6-methylguanine-DNA methyltransferase (MGMT) repairs the cancer chemotherapy-relevant DNA adducts, O6-methylguanine and O6-chloroethylguanine, induced by methylating and chloroethylating anticancer drugs, respectively. These adducts are cytotoxic, and given the overwhelming evidence that MGMT is a key factor in resistance, strategies for inactivating MGMT have been pursued. A number of drugs have been shown to inactivate MGMT in cells, human tumour models and cancer patients, and O6-benzylguanine and O6-[4-bromothenyl]guanine have been used in clinical trials. While these agents show no side effects per se, they also inactivate MGMT in normal tissues and hence exacerbate the toxic side effects of the alkylating drugs, requiring dose reduction. This might explain why, in any of the reported trials, the outcome has not been improved by their inclusion. It is, however, anticipated that, with the availability of tumour targeting strategies and hematopoetic stem cell protection, MGMT inactivators hold promise for enhancing the effectiveness of alkylating agent chemotherapy.
Keywords: MGMT, Alkyltransferase, Glioblastoma, Melanoma, Drug resistance, Repair inhibitors, Inhibitor targeting, O6-benzylguanine
Introduction
DNA alkylating agents have been used in cancer therapy for almost 30 years. Table 1 lists the two categories of these agents, which as a group are frequently referred to as “O 6-alkylating agents”. The methylating nitrosamide, N-methyl-N-nitrosourea (MNU), which is a highly neurotropic carcinogen [1], was paradoxically one of the first anticancer drugs used in brain tumour therapy [2]. Later, streptozotocin, which is a glucose derivative of MNU, was introduced in the clinic and is still in use for the therapy of islet-cell carcinomas [2]. MNU is quite unstable and, at neutral and alkaline pH, decomposes spontaneously reacting immediately with cellular nucleophils. The agent was replaced by procarbazine and dacarbazine (DTIC), which are much more stable needing metabolic activation by cytochrome P450 enzymes to generate alkylating species. The newest generation drug is temozolomide (Temodal, Temodar®). This is a triazene derivative that does not need metabolic activation, decomposing spontaneously into the active form, methyltriazenoimidazole carboxamide (MITC), which releases carbonium ions that alkylate DNA.
Table 1.
Alkylating anticancer drugs
| Clinical application | |
|---|---|
| Methylating anticancer drugs | |
| Streptozotocine (zanosar) | Metastatic cancer of the pancreatic islet cells |
| Procarbazine (natulan) | Hodgkin’s Lymphoma (MOPP regimen) malignant gliomas (PCV regimen) |
| Dacarbacine (DTIC) | Metastatic melanoma (ABVD regimen) Hodgkin’s lymphoma, sarcoma (MAID regimen) |
| Temozolomide (temodal) | Malignant gliomas, melanoma |
| Chloroethylating anticancer drugs | |
| ACNU (nimustine) | Malignant gliomas (PCV regimen) |
| BCNU (carmustine) | Malignant gliomas (PCV regimen), medulloblastoma, astrocytoma, melanoma, multiple myeloma, malignant lymphoma (Hodgkin’s and non-Hodgkin) |
| CCNU (lomustine) | Malignant gliomas (PCV regimen), Hodgkin’s lymphoma, melanoma, bronchial carcinoma |
| MeCCNU (semustine) | Malignant gliomas (PCV regimen), lymphomas, colorectal cancer, stomach cancer. |
| HeCNU (elmustine) | Malignant gliomas (PCV regimen) |
| Estramustine | Prostate cancer |
| Fotemustine | Melanoma |
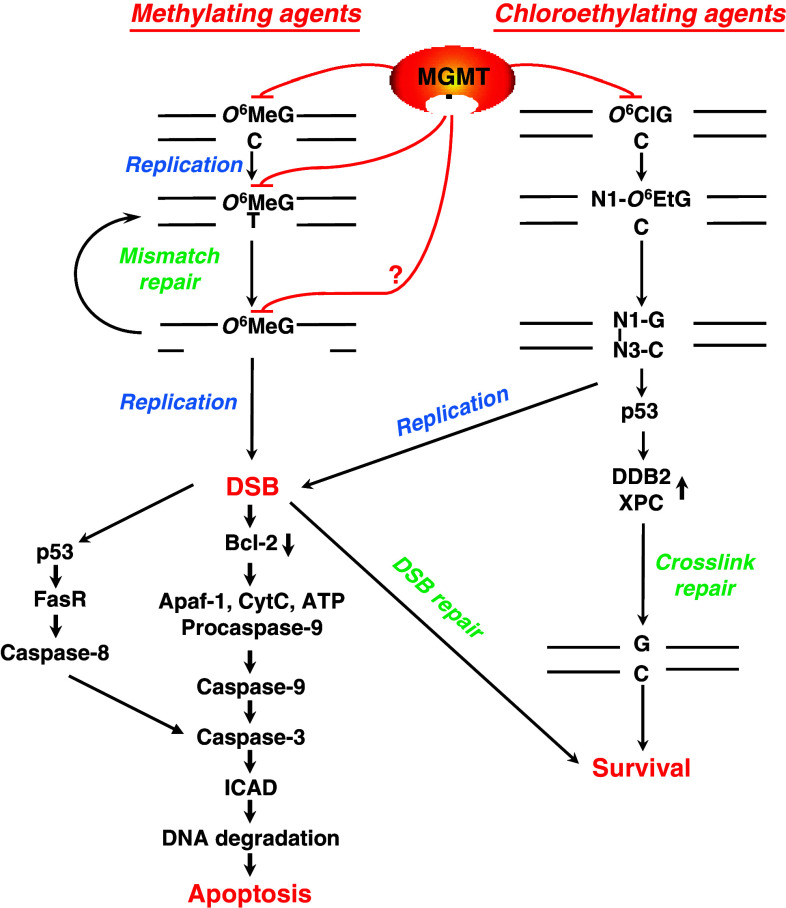
The chloroethylating agents encompass carmustine (BCNU), nimustine (ACNU), semustine (methyl-CCNU), lomustine (CCNU) and the second generation drug fotemustine. These monofunctional nitrosourea derivatives generate, among other lesions, O 6-chloroethylguanine in DNA. Within several hours after formation, this unstable adduct undergoes intramolecular rearrangement, forming the N1-O 6-ethenoguanine adduct and subsequently a N1-guanine-N3-cytosine interstrand DNA crosslink [3]. These crosslinks are highly toxic (see Fig. 1), activating the apoptotic pathway, as do crosslinks induced by bifunctional drugs such as cyclophosphamide [4].
Fig. 1.
O6-MeG and O6-chloroethylguanine driven cell death pathways, and protection by MGMT. During DNA replication, O6-MeG mispairs with thymine forming O6-MeG-thymine [142]. Mismatch repair removes thymine from O6-MeG-T mispairs. Due to the mispairing properties of O6-MeG, thymine is again inserted, which results in a futile repair cycle. This may result in single-strand DNA repair patches that block replication. In a subsequent round of replication this eventually results in DNA double-strand breaks [143] that are potent activators of the apoptotic pathway [144]. O6-chloroethylguanine in DNA is an unstable adduct undergoing a slow intramolecular rearrangement, forming the cyclic etheno adduct and subsequently a N1-guanine-N3-cytosine interstrand crosslink. If not repaired by the crosslink repair system, which involves the p53 regulated proteins DDB2 and XPC [145], these crosslinks are highly toxic, activating the apoptotic pathway [4]. MGMT repairs the initially formed O6-MeG as well as O6-MeG mispaired with thymine [20]. Therefore, its resynthesis exerts protection even some time after the primary lesion O6-MeG was induced. MGMT also repairs the O6-chloroethylguanine adduct by transferring the chloroethyl group to its own cysteine
Both methylating and chloroethylating agents damage cellular macromolecules via a unimolecular nucleophilic substitution reaction (SN1 reaction), and they thus have a strong electrophilic affinity towards oxygen atoms in DNA. Among these, the O 6 position of guanine is biologically very likely the most important. O 6-alkylguanine is repaired by the suicide enzyme O 6-methylguanine-DNA methyltransferase (MGMT), which protects against a substantial portion of the toxic and mutagenic effects of methylating and chloroethylating agents (Fig. 1). Although there is some controversy about whether or not MGMT is also able to protect against cyclophosphamide toxicity [5–7], MGMT inactivating agents are effective only with the O 6-alkylating agents. Therefore, this review refers solely to this group of anticancer drugs and the preclinical development and clinical application of MGMT-inactivating agents.
Cytotoxicity mechanisms of the O6-alkylating agents
Although methylating agents generate 13 adducts in DNA [8], it has been shown that, under most circumstances, the minor product O 6-MeG, amounting to less than 8% of total alkylations, is the major toxic lesion. For the chloroethylating agents, O 6-chloroethylguanine is also a minor lesion to which most of the toxicity is attributed. The most compelling evidence supporting the mechanisms of the toxic effects of O 6-alkylating agents is that, in the vast majority of situations, repair by MGMT (see below) almost completely abolishes cell killing, particularly in the lower dose range of the agents. In vitro experiments indicated that, at high dose levels other repair pathways, e.g. base excision repair removing other lesions, e.g. N-alkyl purines, may become saturated and hence these lesions may contribute to cytotoxicity. In this case, protection by MGMT is less important for determining overall toxicity. For methylating and chloroethylating agents, the pathways leading to cell death, which is executed mainly by apoptosis in both melanoma [9] and glioblastoma cells [10], are outlined in Fig. 1.
It is important to note that chloroethylating agents induce apoptosis in the post-treatment cell cycle following the passage of cells through S-phase. This process is mismatch repair (MMR)-independent, whereas methylating agents require two cell cycles, with MMR acting after the first, in order to generate DNA double-strand breaks (DSBs) that trigger apoptosis [11].
The DNA repair protein MGMT
The DNA repair protein MGMT protects cells against the toxic effects of methylating and chloroethylating agents. It belongs to the group of enzymes that repair DNA by damage reversal. Human cells contain just one MGMT species, and the MGMT gene consists of one non-coding and four coding exons, is about 145 kb long and is located at chromosomal position 10q26. The gene encodes an mRNA of 866 nucleotides that codes for a protein containing 207 amino acids with a molecular weight of 24 kDa. MGMT is a relatively stable protein, having a half-life of >24 h [12]. There are reports that phosphorylation of MGMT affects its activity [13, 14].
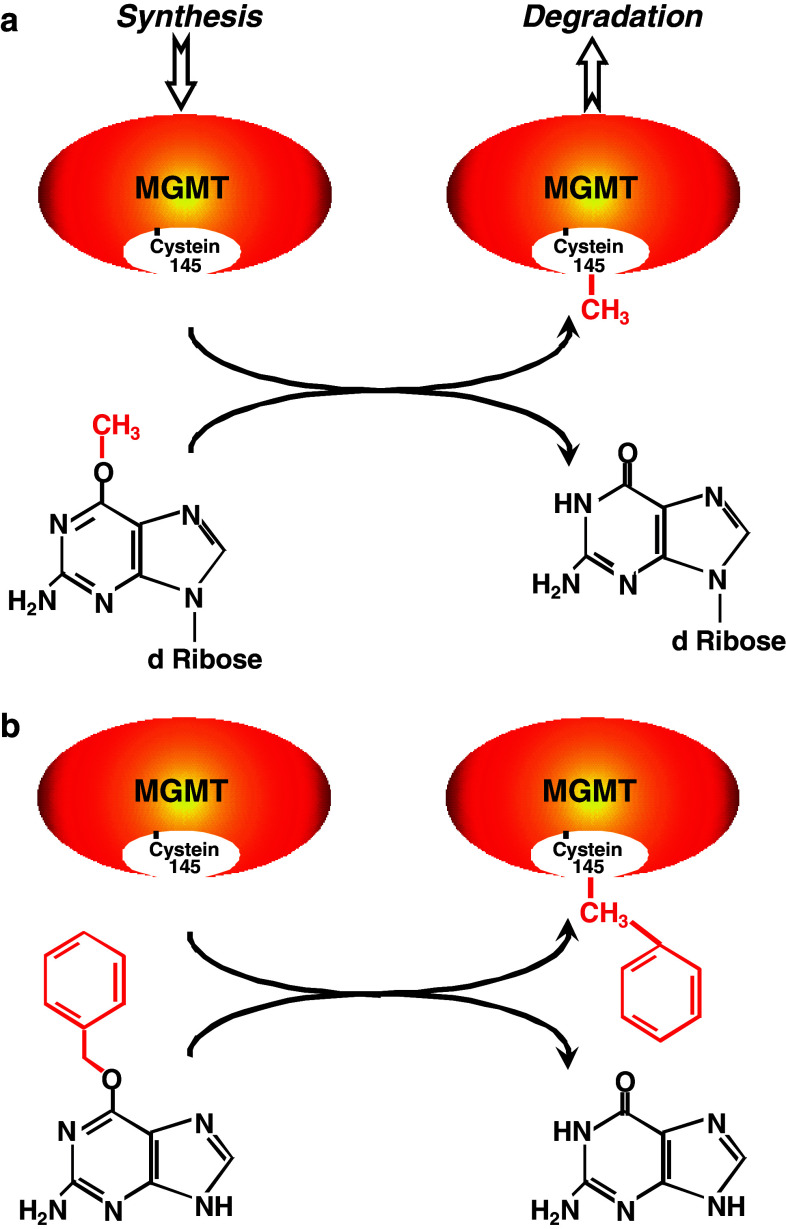
Repair occurs in a one-step reaction that does not involve excision of the alkylated base from DNA, instead the methyl or chloroethyl group at the O 6 position of guanine is transfered to the cysteine residue (Cys145) in the active centre of the MGMT molecule (Fig. 2a) [15, 16]. This results in restoration of guanine in the DNA and irreversible inactivation of MGMT. Therefore, MGMT is often referred to as a “suicide enzyme”.
Fig. 2.
Dealkylation of O 6-methylguanosine and the pseudosubstrate O 6-benzylguanine by MGMT. a Following DNA methylation, the methyl group is transferred from O 6-methylguanosine to the active centre of MGMT. The same happens if the free base O 6-MeG is used as a pseudo-substrate. b In case of O 6-BG, the benzyl group is transferred to Cys 145 of MGMT. Alkyl group transfer to MGMT inactivates the protein and subjects it to degradation via ubiquitination
Because of the stoichiometry of the repair reaction, repair capacity is determined initially by the number of active MGMT molecules in a cell and, if damage levels exceed the levels of pre-existing protein molecules, the rate of de novo synthesis following their inactivation. Protection against cell killing is a linear function of MGMT activity up to a level of MGMT expression of about 200,000 molecules per cell [17], above which the toxicity of other lesions probably becomes dominant. There is some evidence that, after alkyl group transfer, MGMT undergoes ubiquitination and proteasome-mediated degradation [18]. De novo synthesis rates were first studied in rat liver [19]. In glioma cells in vitro, the MGMT activity is recovered within 1–2 cell cycle following a single temozolomide treatment (Kaina, unpublished data), but this effect will likely be dose- and cell line-dependent.
The important consideration is that, because of the stoichiometry and autoinactivation mechanism, cells can be depleted of MGMT and consequently can be more susceptible to killing by O 6-alkylating agents. Since O 6-MeG can be repaired if either cytosine or thymine is the opposite base [20] and cell killing by methylating agents requires two rounds of DNA synthesis, it would be predicted that sustained inactivation of MGMT is essential for enhancing toxicity. Recently, it has been shown that apoptosis occurs not only in the 2nd, but also the 3rd and 4th cell cycle following treatment with temozolomide [11]. It remains to be established if MGMT is able to attenuate the killing effect if it becomes replenished several cell cycles after treatment. For the chloroethylating agents, MGMT ablation needs only to extend until the formation of the interstrand crosslink, which cannot be repaired by MGMT, but may be processed by other pathways (see Fig. 1). There is one report that MGMT can become covalently bound to the intermediate 1-O 6-ethenoguanine in DNA [21], but the physiological significance of this, if any, has yet to be established.
The action of MGMT is not restricted to O 6-alkylguanine lesions in DNA; free bases can also be substrates and can therefore also inactivate the protein—a characteristic that has allowed the development of MGMT-inactivating drugs (Fig. 2). Indeed, as is discussed below, an enormous range of O 6-alkylguanine bases and adducts in synthetic oligonucleotides are known to completely or partially inactivate (or inhibit) MGMT.
No other functions of MGMT have been described. Thus, in adherent cells, no effect on cell growth is seen whether they express low or very high MGMT levels, indicating that MGMT itself is not linked to regulation of proliferation. In addition, although MGMT is frequently upregulated in cells in more aggressively growing tumours [22], there is no evidence that MGMT has a direct stimulatory or inhibitory effect on tumour growth, although there is one report that the methylated (inactivated) MGMT can bind to the oestrogen receptor and affects growth rate [23]. It has also been shown that, in CD34+ stem cells, which have low MGMT activity [24], very high level expression of a mutant form of MGMT that is inactivator-resistant inhibits stem cell proliferation, but the basis of this has not been established [25].
Variable expression of MGMT in tumours and normal tissue
The activity of MGMT has been determined in various human normal and tumor tissues including brain, colon, ovary, testis and breast [26, 27]. Expression was highly variable, particularly in the tumour tissue [28]. There were high levels of MGMT activity in colon cancer, pancreatic carcinoma and lung cancer, while brain tumours and malignant melanoma generally express low levels. MGMT activity in tumour was often higher than that in surrounding normal tissue [27]. For ovarian cancer, MGMT expression correlated with grading and staging [22] and, in glioblastomas, MGMT activity was shown to increase in recurrences [29].
In normal tissues, the expression of MGMT is tissue- and cell-type-regulated. Thus, in rat and human, liver expresses the highest MGMT level, followed by lung and kidney. The MGMT activity also differs between individuals and in the same individual as a function of time. Thus, a long-term study of peripheral blood mononuclear cells (PBMC) from healthy individuals revealed high inter-individual (7.6-fold) and intra-individual (1.4- to 3.5-fold over a 42-day period) variation of MGMT expression [30]. A comparison of the inter-individual MGMT activity in normal lung and colon samples also revealed variation [28].
MGMT and resistance to chemotherapy
The mechanism of cell killing by O 6-alkylguanine suggests that, while high levels of MGMT activity will result in resistance to O 6-alkylating drugs, low levels do not necessarily cause sensitivity. This reflects the fact that several downstream events are involved in converting O 6-alkylguanine into a killing event (see Fig. 1), all of which might be modulated to attenuate cell kill signalling and execution. Nevertheless, there is overwhelming evidence that MGMT is clearly a very important marker of O 6-alkylating drug resistance in cultured rodent and human cells, in animal models, including transgenic and knockout mice, and in a wide range of human tumour xenografts.
There is also evidence for the protective effect of MGMT in patients treated with O 6-alkylating drugs. Thus, low MGMT expression levels, assessed by immunostaining, correlated with DTIC response in malignant melanoma [31], but this was not the case with temozolomide in another study [32]. Silencing of MGMT by promoter hypermethylation of CpG islands can occur early in human tumorigenesis, and this has frequently been observed in several tumour types including colon, brain, lung, head and neck cancer, and lymphomas [33]. In human glioblastoma, MGMT promoter methylation is related to a better therapeutic response of the patients [34–36]. The same is true for MGMT activity with high MGMT-expressing gliomas (>30 fmol/mg protein) responding poorly to O 6-alkylating drug-based therapy [29]. Since low MGMT activity in gliomas (asctocytomas WHO III and glioblastomas) is considered to correlate with promoter methylation [37], and several trials showed MGMT promoter methylation correlates with better outcome of therapy (for review, see [38]), the methylation status of the MGMT promoter is currently being used to predict those patients who are likely to have successful temozolomide or combined temozolomide/CCNU/ACNU chemotherapy. It should be noted that, in current glioma therapy, temozolomide is administered concomitantly with radiotherapy (RT), the outcome of which also correlates with MGMT promoter methylation [35]. It should be further noted that in some studies on glioma [39] and melanoma [40] MGMT expression did not correlate with MGMT promoter methylation status.
Myelosuppression is the dose-limiting toxicity of temozolomide and indeed the other O 6-alkylating drugs. Thus, it was shown that 7% of patients had grade 3 or 4 haematological toxic effects following concomitant radiotherapy and temozolomide, and 14% following adjuvant temozolomide [41]. One study showed that there were lower levels of MGMT activity in the PBMC of patients who suffered the highest degree of bone marrow toxicity [42] suggesting that this parameter may be used to identify patients best likely to tolerate dose intensification.
MGMT inhibitors
The finding that MGMT activity has a major impact on the sensitivity of tumour cells to O 6-alkylating agents stimulated the search for strategies to inhibit MGMT activity in the tumour tissue in order to increase the response to these agents. The concept of MGMT inhibition was established more than 25 years ago and was first achieved by pretreatment with O 6-alkylating agents themselves. Thus, pretreatment of colon carcinoma cells or normal human fibroblasts with the model methylating agent N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) increased carmustine-induced toxicity. This was explained by the ability of MNNG to generate O 6-MeG in DNA and this resulted in MGMT depletion so that more DNA crosslinks were generated when the carmustine “challenge” dose was applied [43–45]. In further studies, it was shown that O 6-MeG added as a free base to the cell culture medium was capable of inhibiting MGMT in HeLa cells [46], in human fibroblasts and tumour cells [47], and in nude mice HT29 xenografts following intraperitoneal administration. [48]. This was consistent with the fact that O 6-MeG acts as a weak substrate for MGMT [46].
In the following years, a huge number of O 6-guanine derivatives and related compounds have been described and used for inactivating MGMT in a variety of experimental settings. The most potent of these agents, as demonstrated in numerous in vitro and in vivo studies, was O 6-benzylguanine (O 6-BG, Figs. 2b, 3), which is more than 2,000-times more effective than O 6-MeG. In vitro, complete inactivation of MGMT activity was observed as soon as 15 min after addition of O 6-BG to the culture medium, and this resulted in a substantial increase in the cytotoxicity of CCNU [7, 49]. Also, in rodent liver and kidney, O 6-BG was shown to almost completely (>95%) inactivate MGMT. In further experiments, a series of O 6-substituted purine and S6-substitute thiopurine derivatives were synthesised, along with many other compounds that were considered to be suitable candidates for effective MGMT inactivation. The order of potency of some of the most effective “pseudosubstrate” agents for MGMT inactivation were O 6-(p-Y-benzyl)-guanine (where Y is H, F, Cl, or CH3) > O 6-benzyl-2′-deoxyguanosine > O 6-(p-Y-benzyl)guanosine (where Y is H, Cl, or CH3) ≥ several 9-substituted O 6-benzylguanine derivatives ≥ O 6-allylguanine > O 6-benzylhypoxanthine > O 6-methylguanine [50]. The 7-substituted benzylguanine derivatives (2-amino-6-(p-Y-benzylthio)purine (where Y is H or CH3), 2-amino-6-[(p-nitrobenzyl)thio]-9-beta-d-ribofuranosylpurine, and 7-benzylguanine were inactive.
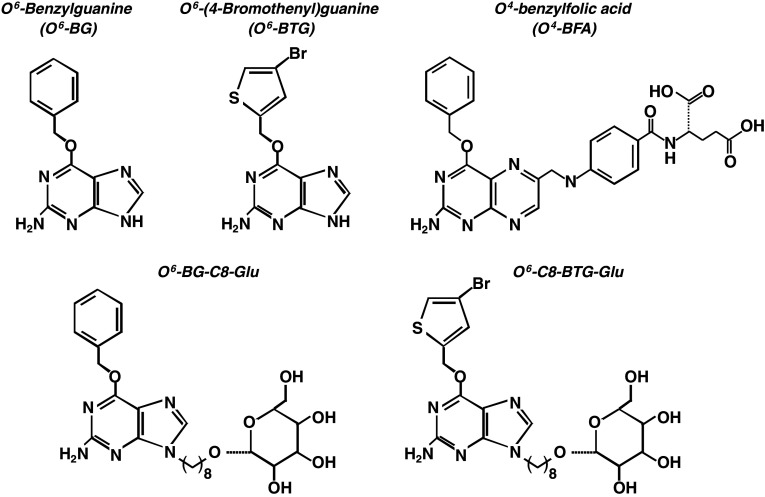
Fig. 3.
Chemical structure of O 6-BG and O 6-BTG and the corresponding folate and glucose derivatives
Where this has been assessed, O 6-MeG analogues inactivate MGMT by alkyl group transfer to Cys 145 in the active centre of the protein (Fig. 2b) [16, 51], and it is presumed that all such agents act in this way. It should be noted that it has been shown for very few compounds that the O 6-alkyl group, or its equivalent, is actually transferred to the MGMT active site cysteine residue, so it is formally possible that a portion of the agents are strong competitive inhibitors of MGMT rather than inactivators. MGMT is a DNA binding protein that, on recognizing substrates in alkylated DNA, an arginine “finger” flips the base out of the minor groove and into the active site pocket, so it is intriguing to consider precisely how this mechanism acts on free bases.
While considerable effort has been invested in developing and assessing novel MGMT inactivating agents, O 6-BG has remained the most extensively used agent for experimental purposes and in pre-clinical and clinical trials. The only other inactivator that has entered clinical trials is O 6-(4-bromothenyl) guanine (O 6-BTG, Lomeguatrib, previously called PaTrin-2; see Fig. 3). This is about 10 times more potent than O 6-BG with an IC50 of 3.4 nM compared to 180 nM for O 6-BG [52]; in vivo, an IC50 for O 6-BTG of 4 nM and for O 6-BG of 35 nM was reported for HeLa S3 cells [53]. These agents are discussed extensively below.
Pharmacokinetic parameters of O6-BG and O6-BTG
The metabolism, distribution between plasma and cerebrospinal fluid and elimination of O 6-BG has been analysed in a number of studies. O 6-BG is metabolised in the liver to O 6-benzyl-8-oxoguanine (8-oxo-BG), which is mediated by CYP1A2 and, to a 200-fold lesser extent, CYP3A4 [54]. 8-oxo-BG, which also inactivates MGMT, is further metabolised by de-benzylation into 8-oxoguanine, which is also accomplished by CYP1A2 [55]. The half-life, clearance and the area under curve (AUC) of O 6-BG and 8-oxo-BG have been analysed in rats [56], non-human primates [57, 58], adult humans [59] and children [60]. The data are compiled in Table 2. In addition, the penetration O 6-BG into the cerebrospinal fluid was analysed. In non-human primates, O 6-benzyl-8-oxoguanine (8-oxo-BG) showed a very high CSF:plasma ratio (36%) compared to O 6-BG (4.3%) [57]. This more extensive penetration of 8-oxo-BG into the cerebrospinal fluid was verified in another study [61]. 8-oxo-BG and O 6-BG showed comparable MGMT inactivation in kidney and brain of mice, while O 6-BG was more effective than 8-oxo-BG in MGMT inactivation in D456 human brain tumor xenografts [62]. For O 6-BTG, only one study evaluating the pharmacokinetic parameters in patients with advanced solid tumors is available [63].
Table 2.
Pharmacokinetic parameters for O 6-BG, O 6-BTG and 8-oxo-BG
| Organism | Substance | T1/2 (h) | AUC (µM h) | C L | C max | V D | Reference |
|---|---|---|---|---|---|---|---|
| Rat | O6-BG i.v. | 1.6 | 160 ml/h/kg | 405 ml/kg | [56] | ||
| 8-oxo-BG | 1.2 | 312 ml/h/kg | 507 ml/kg | [56] | |||
| Nonhuman primates | O6-BG 200 mg/m2 i.v. | 1.6 | 212 | 68 ml/min/m2 | [57] | ||
| 8-oxo-BG | 14 | 2420 | 6.4 ml/min/m2 | [57] | |||
| Nonhuman primates (cerebrospinal fluid) | O6-BG (1 mg intraventricular) | 0.52 | 319 | 0.22 ml/min | 412 µM | [58] | |
| 8-oxo-BG | 0.76 | 5 | 1.9 µM | [58] | |||
| Adult humans | O6-BG 10–80 mg/m2 i.v. | 0.078–0.1 | 0.28–2.3 | 35.9–37.7 l/h/m2 | 0.22–1.82 µg/ml | [59] | |
| 8-oxo-BG | 2.8–9.2 | 3.45–65.65 | 0.42–4.33 µg/ml | [59] | |||
| O6-BTG (10-40 mg/m2 i.v.) | Initial 0.43 terminal 2.97 | 259.9–499.2 | 85.2–131.8 l/min (C L/F) | [63] | |||
| O6-BTG (10 mg/m2 oral) | 52.7–70.6 | ||||||
| Children | O6-BG 120 mg/m2 i.v. | 1.42 | 13.25 | 760 ml/min/m2 | 11 µM | [60] | |
| 8-oxo-BG | 6 | 378.3 | 35 µM | [60] |
T1/2 Half-life, AUC area under the curve, CL clearance, Cmax peak concentration in plama or cerebrospinal fluid, VD volume of distribution, F bioavailability
Preclinical studies: human tumour xenograft models
O6-benzylguanine
The effectiveness of O 6-BG in sensitising cancer cells to the therapeutic effects of O 6-alkylating agents has been clearly demonstrated in a large number of human tumour xenograft models (Table 3). Initial experiments showed that pretreatment with O 6-BG increased carmustine sensitivity in athymic mice bearing either human medulloblastoma (D341 Med) or human glioblastoma (D-456 MG) xenografts, leading to regression of 18/20 xenografts [64]. Pretreatment with O 6-BG (35 mg/kg) completely depleted MGMT activity in mice bearing human melanoma (A375P) xenografts, and the combination of temozolomide (40 mg/kg) given together with O 6-BG on 5 consecutive days produced a significant tumour growth delay in comparison to temozolomide alone. This, however, was not the case upon combination of a single O 6-BG pretreatment and a single dose of 200 mg/kg temozolomide [65]. In MGMT-proficient human gastric adenocarcinoma xenografts, O 6-BG completely suppressed MGMT activity for up to 12 h and O 6-BG given at a single dose of 90 mg/kg i.p. 2 h before carmustine (25 mg/kg) produced a significant growth delay [66]. Similar results were obtained using human glioma SF767 xenografts to test different schedules of O6-BG administration. Treatment with O 6-BG (80 mg/kg) completely ablated xenograft MGMT activity for 12 h, which recovered to 40% of control activity after 24 h. Combined treatment with O 6-BG and 15 mg/kg carmustine resulted in significant inhibition of tumour growth compared to carmustine alone [67]. An even stronger sensitising effect was observed after combining the O6-BG bolus treatment (80 mg/kg) with additional low-dose O6-BG pre- and post-treatment (8 mg/kg for 24 h), which led to ablation of MGMT activity for 24 h [68].
Table 3.
Preclinical studies with O 6-BG
| Tumor xenograft model | Anticancer drug | Readout | Reference |
|---|---|---|---|
| Medulloblastoma (D341 Med) | Carmustine | Tumour regression | [64] |
| Glioblastoma multiforme (D-456 MG) | |||
| Melanoma (A375P) | TMZ | Tumour growth delay | [65] |
| Gastric adenocarcinoma (BGC-823) | Carmustine | Tumour growth delay | [66] |
| Glioma (SF767) | Carmustine | Tumour growth delay | [67] |
| Glioma (SF767) | Carmustine | Tumour growth delay | [68] |
| Medulloblastoma (D341MED) glioma (D-245 MG) | Carmustine | Tumour growth delay | [69] |
| Pancreatic tumours (MIA PaCa-2, CFPAC-1, PANC-1, CAPAN-2 and BxPC-3) | TMZ or carmustine | Tumour growth delay | [70] |
| Malignant glioma (D-456 MG) | Cyclophosphamide plus carmustine TMZ plus irinotecan | Tumour growth delay | [71] |
| Metastatic neuroblastoma | TMZ plus irinotecan | Prolonged survival | [72] |
| Malignant glioma (F98) | Carmustin | Prolonged survival | [73] |
| Medulloblastoma (Daoy) | Carmustine | Tumour growth delay | [74] |
| Malignant glioma (U87MG) | Carmustine | Tumour growth delay | [75] |
| CNS tumor (D-54M/D-245 MG) | Carmustine | Tumour growth delay | [76] |
| Intracranial malignant glioma (D-456 MG) | Carmustine i.a versus i.p | Prolonged survival | [77] |
| Melanoma | TMZ | Tumour growth delay | [78] |
| Medulloblastoma (Daoy) | Carmustine | Tumour growth delay | [79] |
In MGMT-proficient medulloblastoma xenografts, O 6-BG produced tumour growth delays following carmustine treatment [69]. The same was found in MGMT-proficient pancreatic xenografts using carmustine or temozolomide as the therapeutic agent [70]. Addition of O 6-BG also enhanced the response of xenografts to combined chemotherapy. Thus, in MGMT-proficient malignant glioma xenografts (D-456 MG), O 6-BG produced growth delay upon combined treatment with either cyclophosphamide plus carmustine or temozolomide plus irinotecan [71]. Also, in the case of metastatic neuroblastoma xenografts, the survival of mice after combined temozolomide/irinotecan therapy was enhanced by O 6-BG from 10 to 56% [72].
O6-BG also enhanced the response of xenografts in the rat brain. Thus, in MGMT-proficient malignant glioma xenografts (F98), O6-BG (50 mg/kg) prolonged the median survival (to 34 days) compared to O6-BG alone (22 days), carmustine alone (25 days) or the non-treated control group (23.5 days) [73], demonstrating that O6-BG can effectively abrogate carmustine resistance. In addition to O6-BG, its derivative O6-benzyl-2′-deoxyguanosine was analysed in a MGMT-positive medulloblastoma xenograft (Daoy), and this agent showed a greater enhancing effect compared to O6-BG. Thus, O6-benzyl-2′-deoxyguanosine (134 mg/m2) given i.p. 1 h before BCNU (25 mg/m2) produced increased growth delay and survival compared to an equimolar dose of O6-BG (90 mg/m2) combined with BCNU or treatment with BCNU alone [74].
Interestingly, O 6-BG was also found to sensitise xenografts expressing very low MGMT activity. Thus, 1-h pretreatment of athymic mice with O 6-BG (40 mg/kg) reduced MGMT activity in subcutaneous human glioma (U87MG) xenografts from 4.3 to 0.9 fmol/mg protein, and produced tumour growth delays of 23.3 days upon temozolomide (35 mg/kg) and 11.8 days upon carmustine (10 mg/kg) treatment [75]. Similarly, O 6-BG produced substantial tumour growth delays in MGMT-deficient glioma xenografts (D-54 MG and D-245 MG) upon carmustine treatment [76]. The data suggest that even very low levels of MGMT are already adequate for protecting cells against killing by O 6-alkylating drugs. If so, complete and protracted inactivation of MGMT prior to and following alkylating agent administration are likely to be necessary in order to achieve maximal cytotoxicity. However, even in these circumstances, alternative resistance mechanisms may override the lack of MGMT activity in a chronic administration setting.
A problem encountered in the systemic application of O 6-BG is that MGMT depletion in normal tissue cells also sensitises them to killing by the cytotoxins. To counter this exacerbated dose-limiting toxicity, the dose of the O 6-alkylating agent needs to be reduced even though this may impact on the therapeutic effectiveness of the agent. Routes of administration that limit the exposure of normal tissues to the MGMT inactivator and/or the cytotoxin might circumvent this problem. Indeed, in athymic rats carrying intracranial human malignant glioma (D-456 MG) xenografts, intraarterial (i.a.) administration of O 6-BG was superior to intraperitoneal (i.p.) application. O 6-BG depleted MGMT activity in the xenografts to a similar level with a dose of 2.5 mg/kg (i.a.) or 10 mg/kg (i.p.). In addition, i.a. application allowed the use of higher carmustine doses (25 mg/kg vs 6.25 mg/kg), resulting in longer median survival (59–61 days) compared with i.p. application (37 days) [77]. In human melanoma xenografts in nude rats, it was shown that 3.5 mg/kg O 6-BG depleted the tumor MGMT by 93.5%, and when combined with regional administered temozolomide, a significant reduction in tumour growth was achieved [78]. In addition to O 6-BG, 9-substituted derivatives of BG (O 6-benzyl-2′-deoxyguanosine and O 6-benzylguanosine) showed additional tumour growth delay in human medulloblastoma (Daoy) tumour xenografts upon carmustine treatment, but this was not the case with O 6-benzyl-9-cyanomethylguanine. The effectiveness of these guanosine derivatives results from their efficient cellular uptake and catabolism into O 6-BG [79].
O6-bromothenylguanine (lomeguatrib, PaTrin-2)
The effect of O 6-BTG on MGMT activity and growth inhibition following O 6-alkylating drug administration has been analysed in several human tumour xenografts (Table 4). O 6-BTG (20 mg/kg) depleted MGMT in human melanoma (A375M) xenografts 2 h after i.p administration [80]. Combining O 6-BTG (20 mg/kg i.p. daily) and temozolomide increased the tumour quintupling time by 8.7 days. Interestingly, the toxicity of this combination was less than that of combined temozolomide and O 6-BG [80].
Table 4.
Preclinical studies with O 6-BTG
| Tumour xenograft model | Anticancer drug | Readout | Reference |
|---|---|---|---|
| Human melanoma (A375M) | TMZ (1×) | Depletion of MGMT tumour quintupling time | [80] |
| Human melanoma (A375M) | TMZ (5×, spaced 4 h) | Tumour quintupling time higher after 5× then 1× | [81] |
| Human melanoma (A375M) | TMZ (5×, spaced 0, 4, 12, 24 h) | Tumour quintupling time independent of spacing | [82] |
| Breast carcinosarcoma (MCF-7) | TMZ | Depletion of MGMT tumour quintupling time | [83] |
| Primary leukaemia blasts | TMZ | Increased sensitivity | [84] |
Shorter periods of temozolomide treatment (every 4 h) increased tumour growth delay to 33.6 days versus 23.2 days using the same total dose as a single administration, but with increased systemic toxicity [81]. In a third study, nude mice bearing human melanoma (A375M) xenografts were treated a total of 5 times with temozolomide (100 mg/kg i.p.) at intervals of 4, 12 or 24 h, and this resulted in tumour quintupling times of 16.8, 5.9 and 6.2 days, respectively. Combining these schedules with O 6-BTG (20 mg/kg i.p.) given 1 h prior to temozolmide resulted in quintupling times of 22.1, 21.3 and 22.3 days, respectively. It was concluded that MGMT inactivation by O 6-BTG is more promising in enhancing the activity of temozolomide than compressed temozolomide scheduling [82]. A further study showed that human breast carcinoma (MCF-7) xenografts expressed very high levels of MGMT and were completely resistant to temozolomide given daily at 200 mg/kg for 5 days. O 6-BTG (20 mg/kg i.p.) completely inactivated tumour MGMT and in combination with this schedule temozolomide substantially increased tumour quintupling time by 22 days without significant increase in toxicity [83]. O 6-BTG was also shown to enhance the toxicity of temozolomide in long-term cultured cells obtained from patients with acute lymphoblastic leukaemia and acute myeloblastic leukaemia. This suggests that O 6-BTG may benefit leukemia patients treated with temozolomide or dacarbazine [84].
Clinical studies
O6-benzylguanine
Phase I trials
A large number of phase I and II clinical trials involving O 6-BG are listed (http://www.clinicaltrials.gov). Thus, from 31 trials, 16 are already completed, 2 are recruiting, 9 are active but not recruiting, and 4 have been suspended or terminated (as of April 2010).
Initial phase I trials determined the dose of O 6-BG needed for complete depletion of tumour MGMT activity and defined the maximum tolerated dose of the anticancer drug when combined with O 6-BG (Table 5). In adult patients, O 6-BG doses of 100 mg/m2 [85, 86] to 120 mg/m2 [87, 88] are necessary for complete inactivation of MGMT. Interestingly, depletion of MGMT activity occurred in PBMCs at lower doses and did not correlate with tumour MGMT [87]. PBMC should thus not be considered a surrogate for events in tumour tissue, except perhaps to confirm that the patient did receive the inactivator. Another study showed that a bolus of 120 mg/m2 O 6-BG followed 1 h later by a continuous infusion of 30 mg/m2/day for 48 h completely depleted MGMT activity in progressive malignant glioma [89]. Using an O 6-BG dose of 100 mg/m2 [86] or 120 mg/m2 [90], the maximal tolerated dose of carmustine was 40 mg/m2, which is considerably lower than the dose of 120 mg/m2 without the MGMT inhibitor. Using bolus infusion of O 6-BG (120 mg/m2,1 h) on days 1, 3, and 5, combined with a continuous infusion of O 6-BG at 30 mg/m2/day, the maximal tolerated dose of temozolomide was 200 mg/m2 on day 1 and 50 mg/m2/day on days 2–5 [91]. The total dose (400 mg/m2) was only slightly less than the maximum tolerated dose of a single temozolomide application without MGMT inhibitor, which was 472 mg/m2 [89]. O 6-BG was also administered (continuous infusion of 30 mg/m2/day) together with carmustine wafers (Gliadel), which allow topical application of BCNU [92]. The results of more extensive clinical trials using these strategies are awaited with interest.
Table 5.
Clinical studies with O 6-BG (phase I)
| O6-BG dose | Anticancer drug | Readout | Reference |
|---|---|---|---|
| 100 mg/m2 | – | Complete depletion of MGMT for 18 h | [85] |
| 100 mg/m2 | Carmustine (40 mg/m2) | Maximal tolerated dose | [86] |
| 120 mg/m2 | – | Complete depletion of MGMT | [87] |
| 120 mg/m2 | – | Complete depletion of MGMT after 6 but not 18 h | [88] |
| Bolus of 120 mg/m2 + continuous infusion of 30 mg/m2/day | TMZ (472 mg/m2) | Complete depletion of MGMT maximal tolerated dose | [89] |
| 120 mg/m2 | Carmustine (40 mg/m2) | Maximal tolerated dose | [90] |
| Bolus of 120 mg/m2 + continuous infusion of 30 mg/m2/day | TMZ (200 mg/m2 on day 1 + 50 mg/m2/day on days 2–5) | Maximal tolerated dose | [91] |
| Continuous infusion of 30 mg/m2/day | Carmustine (8 wafers) | Maximal tolerated dose | [92] |
| 120 mg/m2/day | TMZ (>55 mg/m2/day) | Antitumour activity | [93] |
| 100 mg/m2/day | Maximal tolerated dose (in children) | ||
| Bolus of 120 mg/m2 + continuous infusion of 30 mg/m2/day | TMZ (200 mg/m2), irinotecan (80 mg/m2) | Maximal tolerated dose | [94] |
| Bolus of 120 mg/m2 + continuous infusion of 30 mg/m2/day | TMZ (407–562 mg/m2) | Maximal tolerated dose (in children) | [95] |
| O6-BG (120 mg/m2) | Carmustine (58 mg/m2) | Maximal tolerated dose (in children) | [96] |
In all cases, dose limitation was myelosuppression manifested as neutropenia, leukopenia, and thrombocytopenia [86, 89, 91, 93, 94]. That O 6-BG exacerbated this toxicity provides strong circumstantial evidence for MGMT being a major resistance mechanism, at least in the corresponding normal human bone marrow stem cells. Strategies to overcome this problem have been proposed and some have been used in clinical trials (see below).
Phase I trials with O 6-BG were also performed in children suffering from CNS tumours. The maximal tolerated dose of temozolomide given 30 min after infusion of 120 mg/m2/day O6-BG for 5 consecutive days was 100 mg/m2/day [93]. When O 6-BG was given i.v. at a dose of 120 mg/m2 followed by 48 h continuous infusion at 30 mg/m2/day, the maximum tolerated total dose of temozolomide (given 6 h after O 6-BG bolus) was 407–562 mg/m2 [95]. Antitumour activity was observed at 120 mg/m2/day O 6-BG combined with temozolomide doses of >55 mg/m2/day. In another trial, a combination of O 6-BG (120 mg/m2) and carmustine showed a maximal tolerated dose of 58 mg/m2 [96].
In addition to alkylating drugs, O 6-BG has been examined for its effect on irinotecan-induced toxicity, showing a maximal tolerated dose of irinotecan of 80 mg/m2 when combined with 200 mg/m2 TMZ [94].
Phase II trials
Several phase II trials have evaluated the safety and toxicity of O 6-BG in combination with O 6-alkylating agents and these are outlined in Table 6. One study in patients with recurrent glioblastoma multiforme showed that O 6-BG can be coadministered safely with carmustine wafers: the overall survival was 82% after 6 months, 47% after 1 year and 10% after 2 years [97]. A similar study evaluated a combination of temozolomide and O 6-BG in patients with glioblastoma multiforme and anaplastic glioma: only 3% of the former and 16% of the latter responded to therapy, and in 48% of the patients, grade 4 hematologic events were observed [98]. Combined treatment with O 6-BG and carmustine every 6 weeks in 18 patients with CNS tumours also failed to show any impact on clinical outcome [99]. In another study, in 17 patients with multiple myeloma, 1 complete response and 3 partial responses were observed [100]; in 18 patients with chemo-naive advanced melanoma, 1 complete response, 4 stable disease and 13 progressive disease were observed, and in 18 prior-chemotherapy patients, no responses, 3 stable disease and 15 progressive disease were observed [101]. In 12 patients with advanced soft tissue sarcoma, there were also no responders [102]. It should be noted that in all these trials the response of patients receiving O 6-BG with temozolomide or carmustine was not compared with the alkylating drug only group, which makes assessment of the data difficult.
Table 6.
Clinical studies with O 6-BG (phase II)
| tumour | Anticancer drug | Readout | Reference |
|---|---|---|---|
| Glioblastoma multiforme | O6-BG (Bolus of 120 mg/m2 + continuous infusion of 30 mg/m2/day) + carmustine wafers | Safety of application no impact on clinical outcome | [97] |
| Glioblastoma multiforme anaplastic glioma | O6-BG (Bolus of 120 mg/m2 + continuous infusion of 30 mg/m2/day) + TMZ (472 mg/m2) | Safety of application no impact on clinical outcome | [98] |
| CNS tumours | O6-BG (120 mg/m2) + 40 mg/m2 carmustine | No impact on clinical outcome | [99] |
| Multiple myeloma | O6-BG (120 mg/m2) + 40 mg/m2 carmustine | No impact on clinical outcome | [100] |
| Advanced melanoma | O6-BG (120 mg/m2) + 40 mg/m2 carmustine | No impact on clinical outcome | [101] |
| Advanced soft tissue sarcoma | O6-BG (120 mg/m2) + 40 mg/m2 carmustine | No impact on clinical outcome | [102] |
O6-bromothenylguanine
Phase I and II trials
In two phase I studies, the combination of O 6-BTG and temozolomide or irinotecan was analysed in various solid tumours to define the biologically effective (i.e. MGMT-inactivating) dose, the maximum tolerated dose and dose-limiting toxicity of the combination (Table 7). In the first study, O 6-BTG was administered to patients with advanced solid tumours [63]. Within 4 h, depletion of MGMT activity was observed in PBMCs (≥95%) and tumour biopsies (≥92%) at doses of ≥10 mg/m2/day i.v. or ≥20 mg/m2/day orally. The maximal tolerated dose of temozolomide in combination with O 6-BTG was 150 mg/m2 (vs 200 mg/m2 without O 6-BTG) and dose-limiting toxicity was myelosuppression [63]. In another study, O 6-BTG was administered to 24 patients with metastatic colorectal cancer [103], and the maximum tolerated dose was 80 mg/day when combined with 300 mg/m2 irinotecan; dose-limiting toxicity was neutropaenia and diarrhoea. At this dose level, O 6-BTG administration resulted in complete MGMT inactivation in PBMCs [103]. Besides use in therapy of solid tumours, pilot studies were performed to analyse O 6-BTG in refractory acute leukaemia. The data indicate that also in this cancer, O 6-BTG (40 mg/m2/day orally, day 0–10) can suppress MGMT activity and that after combined treatment with temozolomide (150 mg/m2/day orally, day 1–7) six out of eight patients showed partial or complete disappearance of blast cells in peripheral blood and in bone marrow [104].
Table 7.
Clinical studies with O 6-BTG
| tumour model | Anticancer drug | Readout | Reference |
|---|---|---|---|
| Phase I | |||
| Advanced solid tumours | O6-BTG (≥10 mg/m2/day i.v. or ≥20 mg/m2/day) | Depletion of MGMT in PBMCs and tumour biopsies, maximal tolerated dose | [63] |
| TMZ (150 mg/m2) | |||
| Metastatic colorectal cancer | O6-BTG (80 mg/day) Irinotecan (300 mg/m2) | Depletion of MGMT in PBMCs and maximal tolerated dose | [103] |
| Refractory acute leukaemia | O6-BTG (40 mg/m2/day, day 0–10), temozolomide (150 mg/m2/day, day 1–7) | Depletion of MGMT, response rate | [104] |
| Metastatic melanoma | O6-BTG (40 mg) for 10 or 14 day plus TMZ (75–100 mg/m2) day 1–5 | Toxicity, adverse effects, response rate | [107] |
| Colorectal cancer | O6-BTG (120 mg) | Complete MGMT inactivation in the tumour after 12 h | [109] |
| Prostate cancer | O6-BTG (120 mg) | ||
| CNS cancer | O6-BTG (160 mg) | ||
| Phase II | |||
| Metastatic melanoma | O6-BTG (40-80 mg) 5× | Response rate, median time to disease progression | [105] |
| TMZ (125 or 200 mg/m2) 5× | |||
| Metastatic colorectal carcinoma | O6-BTG (40 mg) 5× | Median time to disease progression, pharmacokinetics of TMZ and O 6-BTG depletion of MGMT activity in PBMCs | [106] |
| TMZ (50–200 mg/m2) 5× | |||
| Metastatic melanoma | O6-BTG (40–80 mg) 5× |
Pharmacodynamic analysis for [105] and [107], depletion and recovery of MGMT activity in PBMCs and tumour biopsies |
[108] |
| TMZ (125 or 200 mg/m2) 5× | |||
In one phase II trial, over 100 patients with metastatic melanoma were treated with temozolomide alone or a combination of O 6-BTG and temozolomide on days 1–5 every 28 days for up to 6 cycles [105]. Combination with O 6-BTG did not significantly influence the overall response rate (13.5 vs 17.3%) or the median time to disease progression (65.5 vs 68 days). In another phase II study in 19 patients with stage IV metastatic colorectal carcinoma, O 6-BTG and temozolomide orally for 5 consecutive days resulted in the same outcome as the temozolomide alone group. In both groups, the median time to progression was 50 days and the commonest adverse effects were gastrointestinal and haematologic toxicity [106].
It was suggested that the inability of O 6-BTG to enhance clinical response to temozolomide might be a consequence of scheduling, since tumour biopsies showed recovery of MGMT activity within 24 h [105]. This would have been missed in the previous phase I studies, because MGMT activity was determined in the tumour at early time points after treatment. Based on this, higher daily doses of O 6-BTG and an extended dosing period beyond that of temozolomide was assessed in additional phase I studies. Thirty-two patients with metastatic melanoma were treated orally with O 6-BTG (40 mg) for 10 or 14 days and temozolomide (75–100 mg/m2) on days 1–5. Due to haematologic toxicity, the optimal extended O 6-BTG dosing schedule was O 6-BTG for 10 days combined with temozolomide (75 mg/m2). However, this extended O 6-BTG dosing schedule also showed no advantage compared to temozolomide alone in the treatment of melanoma [107]. This study showed furthermore that, while MGMT activity was completely inactivated in PBMC and tumours biopsied on the last day of treatment with O 6-BTG, it thereafter quickly recovered in tumours, indicating that even more protracted dosing with O 6-BTG would be needed for extensive ablation of MGMT activity [108].
In order to establish if different doses of O 6-BTG would be needed to deplete MGMT activity in prostate, colorectal or brain tumours [109], a total of 32 patients were given a single dose of O 6-BTG orally approximately 12 h before resection of their primary tumour. Complete inactivation of MGMT in prostate and colorectal cancers required a dose of 120 mg, and in CNS tumours, a dose of 160 mg O 6-BTG [109]. This indicates that the doses used in the phase II studies (40–80 mg) may have been lower than optimal.
An additional factor that thwarts attempts to improve the therapeutic index of O 6-alkylating agents in melanoma is very likely the inherent drug resistance of melanomas. Melanoma cells undergo apoptosis in response to methylating and chloroethylating agents, and the upstream pathways have been elucidated [9]. However, cell death execution is inefficient due to silencing of downstream pro-apoptotic pathways (Roos and Kaina, unpublished data). Therefore, MGMT inhibition together with strategies aimed at reactivating apoptotic signaling should be considered in future trials in order to enhance the therapeutic response of DTIC, temozolomide and other O 6-alkylating agents for melanoma therapy.
MGMT inhibitor targeting
As has been described above, potent MGMT inactivating agents have been developed that are without any toxicity per se in animals, inhibit the growth of human tumour xenografts in nude mice when combined with O 6-alkylating drugs and, in patients, inactivate MGMT in tumour, PBMC and other tissues and have no side effects per se. However, in clinical trials, they have not yet resulted in any improvement in the therapeutic efficacy (in terms of overall survival) of methylating or chloroethylating anticancer drugs in glioma and melanoma therapy (see Tables 1, 2, 3 and 4). A likely major reason for this is that dose reduction of the alkylating drug is necessary for patients to tolerate the increased systemic side effects and that this dose reduction effectively decreases tumour cell kill. It would therefore be highly desirable to develop strategies for specifically targeting the MGMT-inactivating agent selectively to the tumour. A simple, but technically sophisticated, approach is to administer the inhibitor locally. This has been done in an individual trial with a patient suffering from glioblastoma multiforme. After dissection of a recurrence that expressed MGMT (175 fmol/mg protein; for comparison, the average MGMT expression level of pretreatment gliomas is 37 fmol/mg protein [29]), an Ommaya reservoir was implanted into the tumour cavity and used to administer O 6-BG directly into the brain prior to systemic temozolomide. No systemic or neuronal toxicity was observed due to intracranial O 6-BG administration, which indicates that this approach is feasible and tolerated by the patient [110]. Currently, treatment of a larger group of patients is awaiting approval for assessing the feasibility, costs and benefit of such treatment.
In addition to topical delivery of O 6-BG two chemical modification strategies have been assessed for targeting MGMT inactivators to tumours. These are (1) conjugation of O 6-BG to folate and (2) conjugation of O 6-BG and O 6-BTG to β-d-glucose. The first approach is based on the observation that tumour cells often exhibit high levels of folate receptors. The O 6-BG-folate conjugates were shown to be effective MGMT inactivators and to predominantly kill cells expressing high folate receptor levels in comparison to low level expressing cells [111]. In addition to O 4-benzylfolic acid (Fig. 3), folate ester derivatives of O 6-benzyl-2′-deoxyguanosine and O 6-[4-(hydroxymethyl)benzyl]guanine were synthesised. The former is a potent MGMT inactivator that effectively sensitised human tumour cells to BCNU [112]. Despite these encouraging findings, no results of in vivo studies with xenografts have been published to date.
The second approach exploits the finding that a common characteristic of tumour cells is increased glucose consumption [113], which is related to elevated glucose uptake via the up-regulation of glucose transporters [114]. On this basis, MGMT inactivators have been conjugated to d-glucose. The in vitro testing of such conjugates showed that a short spacer of 1–4 carbon atoms between d-glucose and the N9 of O 6-BG completely abolished or strongly attenuated MGMT inactivation. One possible explanation is that d-glucose in the immediate vicinity of the O 6-BG moiety prevents access of the free base to the active site in MGMT. Extending the linker to more than six carbon atoms restored most of the activity [115, 116]. A linker of eight carbons appeared to be optimal, since it retained activity and, serendipitously, significantly increased water solubility. It was also shown that for MGMT inactivation, linking the N9 of O 6-BG via a C8 spacer to β-d-glucose was superior to α-d-glucose. The free base inhibitors (see Fig. 3) inactivated MGMT in cell extracts and in living cells in the sequence: O 6-BTG > O 6-I-BG (O 6-iodobenzylguanine) > O 6-BG > O 6-iodothenylguanine, and the same order of potency was seen with the corresponding glucose conjugates. O 6-(2-fluropyridimylmethl)guanine-C8-β-d-glucose was significantly less effective than the other inactivators [53]. The conjugates were not cytotoxic per se in cell culture, penetrated quickly into living cells and depleted MGMT within ~45 min (unpublished data). When given 1 h prior to and after treatment with the alkylating agent, O 6-BTG-C8-β-d-glucose was similar to O 6-BTG in its ability to sensitise MGMT-expressing CHO and HeLa cells to fotemustine and temozolomide toxicity in colony formation experiments [53].
These in vitro studies demonstrate that the glucose conjugates are able to enter cells and inactivate MGMT resulting in substantial potentiation of the killing effect of O 6-alkylating agents. Although it is likely that glucose transporters (sodium–glucose-linked transporters (SGLTs) or glucose transporters (GLUTs)) are involved in the uptake, the transport mechanism still needs to be elucidated. Another issue is the possible expression of these transporters in proliferating haematopoetic progenitor cells and other normal proliferating cells in the body, which could result in additional side effects when using these targeted inhibitors. Our preliminary data show that cells from different tumour types display substantial differences in uptake efficiency of O 6-BG-C8-β-d-glucose. This suggests that the transporter/s involved are expressed to different extents in cancers, and hence that it will be essential to identify the transporter/s involved in order to screen individual tumour biopsies for expression.
Stem cell protection
The myelosuppressive effects of the O 6-alkylating agents are likely due to the low levels of MGMT expressed in haematopoetic stem cells or a proliferating precursor pool in the bone marrow. This is supported by the consistent demonstrations that myelosuppression is more extensive following administration of MGMT-inactivating agents. To attenuate the severity of myelosuppression, gene transfer and high level expression of MGMT in haematopoetic stem cells, in the context of autologous bone marrow transplantation, is a feasible proposition. Such a strategy could additionally involve tumour sensitisation using MGMT-inactivating agents but only if an inactivating agent-resistant MGMT was employed. It is this latter approach that has received most attention in many in vitro and preclinical studies [117]. An additional benefit of this approach would likely be the protection of bone marrow stem cells/hematopoetic precursor cells against the mutagenic and carcinogenic effects of O 6-alkylating agents such as temozolomide, which was suggested by in vivo experiments performed in mice [118] and by case reports of long-time surviving patients [119–121].
Initial studies involved the transfer of mgmt gene into CD34+ stem cells harvested from peripheral blood and this provided haematoprotection against the toxicity of O 6-alkylating agents [122–126]. The simultaneous myeloprotection–tumour sensitisation strategy was investigated using the E. coli alkyltransferase, Ada, which is not inhibited by O 6-BG [127] and versions of the human MGMT cDNA encoding G156A or P140K, which are also resistant to inactivation by O 6-BG [128–130] and, in the case of P140K, O 6-BTG [131]. Resistance to O 6-BG inactivation clearly varies between the wt and both mutant MGMT proteins. Thus, in MGMT-transduced haematopoetic K562 cells, the wt MGMT was already inhibited by 0.1 μM O 6-BG, the G156A mutant had an IC50 of 15 μM and the P140K mutants remained active up to 1 mM O 6-BG [132].
A number of preclinical studies have investigated various strategies to achieve expression of the mutant MGMT in haematopoetic cells and to assess its impact on the combined administration of MGMT inactivator and O 6-alkylating agents. Retroviral transduction of bone marrow cells with MGMT(G156A) and transplantation into mice protects these animals against the myelotoxic effects of O 6-BG-BCNU treatment [133]. It also increased tumour kill in human xenograft models because it allowed administration of increased doses of the cytostatic drug [134]. The same was also observed using transduction of MGMT(P140K) in combination with O 6-BG and BCNU [135, 136], temozolomide [137] or ACNU [138] as well as combined O 6-BTG and temozolomide treatment [131].
One problem with MGMT gene transfer is the low cellular transduction rate of the viruses used. Thus, alternative viral systems like foamy viral [139] or lentiviral vectors [140] carrying MGMT(P140K) have been investigated. In addition to the transduction of mutated MGMT, transduction of MGMT (P140K) together with multi-drug resistance 1 gene (mdr1) have been investigated [141], and significantly higher survival of transduced HL60 cells following treatment with O 6-BG/temozolomide and paclitaxel has been reported. These preclinical studies have been translated into the clinic. Of the three phase I clinical trials of MGMT-based myeloprotective gene therapy described (http://www.clinicaltrials.gov), one has already been suspended, one is ongoing and the third was completed this year with the results yet to be published. The outcome of these studies is awaited with great interest.
Conclusions
The ability of MGMT-inactivating agents to sensitise mammalian cells and human tumour xenografts to the toxic or therapeutic effect of O 6-alkylating drugs has been clearly demonstrated in virtually all experimental systems in which the strategy has been assessed. However, despite all the indications that MGMT is a major resistance factor in human tumours, and also in bone marrow, there is at present no convincing evidence that this strategy (i.e. systemic MGMT depletion) has been able to improve the outcome in cancer patients treated with these agents.
Ongoing studies intended to limit exposure of normal tissues to MGMT inactivators and or O 6-alkylating agents involve alternative delivery routes, topical applications and tumour-targeted inactivators. These hold considerable promise for the success of drugs and drug combinations the efficacy of which is attenuated by MGMT. The alternative strategy of protecting the bone marrow against the collateral toxicity of O 6-alkylating agents, which itself is increased by MGMT inactivators, currently involves the delivery and expression of an inactivator-resistant MGMT variant in bone marrow stem cells. MGMT gene transfer is just beginning to enter the clinic, and several problematic aspects have still to be resolved. These include the regulation of MGMT expression levels by an appropriate vector system, the growth inhibitory side effects of mutant forms of MGMT and the perceived hazard of viral genomic integration. On the other hand, this approach has additional potential benefits: it may allow dose escalation even if MGMT inactivators are not used, and it may attenuate the long-term side effects of O 6-alkylating agents in the bone marrow, which include myeloproliferative disorders and leukemias.
Our increasing understanding of the mechanisms of cell killing by O 6-alkylating agents has indicated the possibility that gliomas and malignant melanomas may be inherently resistant to a wide range of these and other anticancer drugs. Therefore, a concerted strategy might be required in which inactivation of MGMT is combined both with inhibition of other repair pathways involved in protection against temozolomide- and chloroethylation-induced toxicity and with abrogation of downstream drug resistance factors, e.g. by reactivating and enhancing apoptotic pathways.
Acknowledgments
The work of B.K. and M.C. is supported by DFG, Ka724 and Deutsche Krebsstiftung. G.P.M. thanks Cancer Research UK and CHEMORES for support.
References
- 1.Kleihues P, Magee PN. Alkylation of rat brain nucleic acids by N-methyl-N-nitrosourea and methyl methanesulphonate. J Neurochem. 1973;20:595–606. doi: 10.1111/j.1471-4159.1973.tb12158.x. [DOI] [PubMed] [Google Scholar]
- 2.Skipper HE, Schabel FM, Jr, Trader MW, Thomson JR. Experimental evaluation of potential anticancer agents. VI. Anatomical distribution of leukemic cells and failure of chemotherapy. Cancer Res. 1961;21:1154–1164. [PubMed] [Google Scholar]
- 3.Ludlum DB. DNA alkylation by the haloethylnitrosoureas: nature of modifications produced and their enzymatic repair or removal. Mutat Res. 1990;233:117–126. doi: 10.1016/0027-5107(90)90156-x. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein M, Roos WP, Kaina B. Apoptotic death induced by the cyclophosphamide analogue mafosfamide in human lymphoblastoid cells: contribution of DNA replication, transcription inhibition and Chk/p53 signaling. Toxicol Appl Pharmacol. 2008;229:20–32. doi: 10.1016/j.taap.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Preuss I, Thust R, Kaina B. Protective effect of O6-methylguanine-DNA methyltransferase (MGMT) on the cytotoxic and recombinogenic activity of different antineoplastic drugs. Int J Cancer. 1996;65:506–512. doi: 10.1002/(SICI)1097-0215(19960208)65:4<506::AID-IJC19>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Friedman HS, Pegg AE, Johnson SP, Loktionova NA, Dolan ME, Modrich P, Moschel RC, Struck R, Brent TP, Ludeman S, Bullock N, Kilborn C, Keir S, Dong Q, Bigner DD, Colvin OM. Modulation of cyclophosphamide activity by O6-alkylguanine-DNA alkyltransferase. Cancer Chemother Pharmacol. 1999;43:80–85. doi: 10.1007/s002800050866. [DOI] [PubMed] [Google Scholar]
- 7.Dolan ME, Mitchell RB, Mummert C, Moschel RC, Pegg AE. Effect of O6-benzylguanine analogues on sensitivity of human tumor cells to the cytotoxic effects of alkylating agents. Cancer Res. 1991;51:3367–3372. [PubMed] [Google Scholar]
- 8.Kaina B, Christmann M, Naumann S, Roos WP. MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair (Amst) 2007;6:1079–1099. doi: 10.1016/j.dnarep.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Naumann SC, Roos WP, Jost E, Belohlavek C, Lennerz V, Schmidt CW, Christmann M, Kaina B. Temozolomide- and fotemustine-induced apoptosis in human malignant melanoma cells: response related to MGMT, MMR, DSBs, and p53. Br J Cancer. 2009;100:322–333. doi: 10.1038/sj.bjc.6604856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roos WP, Batista LF, Naumann SC, Wick W, Weller M, Menck CF, Kaina B. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene. 2007;26:186–197. doi: 10.1038/sj.onc.1209785. [DOI] [PubMed] [Google Scholar]
- 11.Quiros S, Roos WP, Kaina B. Processing of O6-methylguanine into DNA double-strand breaks requires two rounds of replication whereas apoptosis is also induced in subsequent cell cycles. Cell Cycle. 2010;9:168–178. doi: 10.4161/cc.9.1.10363. [DOI] [PubMed] [Google Scholar]
- 12.Fritz G, Tano K, Mitra S, Kaina B. Inducibility of the DNA repair gene encoding O6-methylguanine-DNA methyltransferase in mammalian cells by DNA-damaging treatments. Mol Cell Biol. 1991;11:4660–4668. doi: 10.1128/mcb.11.9.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srivenugopal KS, Mullapudi SR, Shou J, Hazra TK, Ali-Osman F. Protein phosphorylation is a regulatory mechanism for O6-alkylguanine-DNA alkyltransferase in human brain tumor cells. Cancer Res. 2000;60:282–287. [PubMed] [Google Scholar]
- 14.Mullapudi SR, Ali-Osman F, Shou J, Srivenugopal KS. DNA repair protein O6-alkylguanine-DNA alkyltransferase is phosphorylated by two distinct and novel protein kinases in human brain tumour cells. Biochem J. 2000;351(Pt 2):393–402. [PMC free article] [PubMed] [Google Scholar]
- 15.Hazra TK, Roy R, Biswas T, Grabowski DT, Pegg AE, Mitra S. Specific recognition of O6-methylguanine in DNA by active site mutants of human O6-methylguanine-DNA methyltransferase. Biochemistry. 1997;36:5769–5776. doi: 10.1021/bi963085i. [DOI] [PubMed] [Google Scholar]
- 16.Pegg AE, Dolan ME, Moschel RC. Structure, function, and inhibition of O6-alkylguanine-DNA alkyltransferase. Prog Nucleic Acid Res Mol Biol. 1995;51:167–223. doi: 10.1016/s0079-6603(08)60879-x. [DOI] [PubMed] [Google Scholar]
- 17.Kaina B, Fritz G, Mitra S, Coquerelle T. Transfection and expression of human O6-methylguanine-DNA methyltransferase (MGMT) cDNA in Chinese hamster cells: the role of MGMT in protection against the genotoxic effects of alkylating agents. Carcinogenesis. 1991;12:1857–1867. doi: 10.1093/carcin/12.10.1857. [DOI] [PubMed] [Google Scholar]
- 18.Srivenugopal KS, Yuan XH, Friedman HS, Ali-Osman F. Ubiquitination-dependent proteolysis of O6-methylguanine-DNA methyltransferase in human and murine tumor cells following inactivation with O6-benzylguanine or 1,3-bis(2-chloroethyl)-1-nitrosourea. Biochemistry. 1996;35:1328–1334. doi: 10.1021/bi9518205. [DOI] [PubMed] [Google Scholar]
- 19.Kleihues P, Margison GP. Exhaustion and recovery of repair excision of O6-methylguanine from rat liver DNA. Nature. 1976;259:153–155. doi: 10.1038/259153a0. [DOI] [PubMed] [Google Scholar]
- 20.Lips J, Kaina B. Repair of O(6)-methylguanine is not affected by thymine base pairing and the presence of MMR proteins. Mutat Res. 2001;487:59–66. doi: 10.1016/s0921-8777(01)00105-7. [DOI] [PubMed] [Google Scholar]
- 21.Gonzaga PE, Potter PM, Niu TQ, Yu D, Ludlum DB, Rafferty JA, Margison GP, Brent TP. Identification of the cross-link between human O6-methylguanine-DNA methyltransferase and chloroethylnitrosourea-treated DNA. Cancer Res. 1992;52:6052–6058. [PubMed] [Google Scholar]
- 22.Hengstler JG, Tanner B, Moller L, Meinert R, Kaina B. Activity of O(6)-methylguanine-DNA methyltransferase in relation to p53 status and therapeutic response in ovarian cancer. Int J Cancer. 1999;84:388–395. doi: 10.1002/(sici)1097-0215(19990820)84:4<388::aid-ijc10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Teo AK, Oh HK, Ali RB, Li BF. The modified human DNA repair enzyme O(6)-methylguanine-DNA methyltransferase is a negative regulator of estrogen receptor-mediated transcription upon alkylation DNA damage. Mol Cell Biol. 2001;21:7105–7114. doi: 10.1128/MCB.21.20.7105-7114.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerson SL, Phillips W, Kastan M, Dumenco LL, Donovan C. Human CD34 + hematopoietic progenitors have low, cytokine-unresponsive O6-alkylguanine-DNA alkyltransferase and are sensitive to O6-benzylguanine plus BCNU. Blood. 1996;88:1649–1655. [PubMed] [Google Scholar]
- 25.Sorg UR, Kleff V, Fanaei S, Schumann A, Moellmann M, Opalka B, Thomale J, Moritz T. O6-methylguanine-DNA-methyltransferase (MGMT) gene therapy targeting haematopoietic stem cells: studies addressing safety issues. DNA Repair (Amst) 2007;6:1197–1209. doi: 10.1016/j.dnarep.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 26.Citron M, White A, Decker R, Wasserman P, Li B, Randall T, Guerra D, Belanich M, Yarosh D. O6-methylguanine-DNA methyltransferase in human brain tumors detected by activity assay and monoclonal antibodies. Oncol Res. 1995;7:49–55. [PubMed] [Google Scholar]
- 27.Preuss I, Haas S, Eichhorn U, Eberhagen I, Kaufmann M, Beck T, Eibl RH, Dall P, Bauknecht T, Hengstler J, Wittig BM, Dippold W, Kaina B. Activity of the DNA repair protein O6-methylguanine-DNA methyltransferase in human tumor and corresponding normal tissue. Cancer Detect Prev. 1996;20:130–136. [PubMed] [Google Scholar]
- 28.Margison GP, Povey AC, Kaina B, Santibanez Koref MF. Variability and regulation of O(6)-alkylguanine-DNA alkyltransferase. Carcinogenesis. 2003;24:625–635. doi: 10.1093/carcin/bgg005. [DOI] [PubMed] [Google Scholar]
- 29.Wiewrodt D, Nagel G, Dreimuller N, Hundsberger T, Perneczky A, Kaina B. MGMT in primary and recurrent human glioblastomas after radiation and chemotherapy and comparison with p53 status and clinical outcome. Int J Cancer. 2008;122:1391–1399. doi: 10.1002/ijc.23219. [DOI] [PubMed] [Google Scholar]
- 30.Janssen K, Eichhorn-Grombacher U, Schlink K, Nitzsche S, Oesch F, Kaina B. Long-time expression of DNA repair enzymes MGMT and APE in human peripheral blood mononuclear cells. Arch Toxicol. 2001;75:306–312. doi: 10.1007/s002040100226. [DOI] [PubMed] [Google Scholar]
- 31.Egyhazi S, Margison GP, Hansson J, Ringborg U. Immunohistochemical examination of the expression of O6-methylguanine-DNA methyltransferase in human melanoma metastases. Eur J Cancer. 1997;33:129–134. doi: 10.1016/s0959-8049(96)00342-5. [DOI] [PubMed] [Google Scholar]
- 32.Rietschel P, Wolchok JD, Krown S, Gerst S, Jungbluth AA, Busam K, Smith K, Orlow I, Panageas K, Chapman PB. Phase II study of extended-dose temozolomide in patients with melanoma. J Clin Oncol. 2008;26:2299–2304. doi: 10.1200/JCO.2007.14.5292. [DOI] [PubMed] [Google Scholar]
- 33.Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999;59:793–797. [PubMed] [Google Scholar]
- 34.Hegi ME, Diserens AC, Godard S, Dietrich PY, Regli L, Ostermann S, Otten P, Van Melle G, de Tribolet N, Stupp R. Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res. 2004;10:1871–1874. doi: 10.1158/1078-0432.ccr-03-0384. [DOI] [PubMed] [Google Scholar]
- 35.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 36.Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB, Herman JG. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 37.Christmann M, Nagel G, Horn S, Krahn U, Wiewrodt D, Sommer C, Kaina B (2010) MGMT activity, promoter methylation and immunohistochemistry of pre-treatment and recurrent malignant gliomas: a comparative study on astrocytoma and glioblastoma. Int J Cancer (Epub ahead of print) [DOI] [PubMed]
- 38.Weller M, Stupp R, Reifenberger G, Brandes AA, van den Bent MJ, Wick W, Hegi ME. MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol. 2009;6:39–51. doi: 10.1038/nrneurol.2009.197. [DOI] [PubMed] [Google Scholar]
- 39.Spiegl-Kreinecker S, Pirker C, Filipits M, Lotsch D, Buchroithner J, Pichler J, Silye R, Weis S, Micksche M, Fischer J, Berger W. O6-Methylguanine DNA methyltransferase protein expression in tumor cells predicts outcome of temozolomide therapy in glioblastoma patients. Neuro Oncol. 2010;12:28–36. doi: 10.1093/neuonc/nop003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Augustine CK, Yoo JS, Potti A, Yoshimoto Y, Zipfel PA, Friedman HS, Nevins JR, Ali-Osman F, Tyler DS. Genomic and molecular profiling predicts response to temozolomide in melanoma. Clin Cancer Res. 2009;15:502–510. doi: 10.1158/1078-0432.CCR-08-1916. [DOI] [PubMed] [Google Scholar]
- 41.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 42.Sabharwal A, Waters R, Danson S, Clamp A, Lorigan P, Thatcher N, Margison GP, Middleton MR (2009) Predicting the myelotoxicity of chemotherapy: the use of pretreatment O6-methylguanine-DNA methyltransferase determination in peripheral blood mononuclear cells. Melanoma Res (Epub ahead of print) [DOI] [PubMed]
- 43.Zlotogorski C, Erickson LC. Pretreatment of normal human fibroblasts and human colon carcinoma cells with MNNG allows chloroethylnitrosourea to produce DNA interstrand crosslinks not observed in cells treated with chloroethylnitrosourea alone. Carcinogenesis. 1983;4:759–763. doi: 10.1093/carcin/4.6.759. [DOI] [PubMed] [Google Scholar]
- 44.Zlotogorski C, Erickson LC. Pretreatment of human colon tumor cells with DNA methylating agents inhibits their ability to repair chloroethyl monoadducts. Carcinogenesis. 1984;5:83–87. doi: 10.1093/carcin/5.1.83. [DOI] [PubMed] [Google Scholar]
- 45.Gerson SL, Berger NA, Arce C, Petzold SJ, Willson JK. Modulation of nitrosourea resistance in human colon cancer by O6-methylguanine. Biochem Pharmacol. 1992;43:1101–1107. doi: 10.1016/0006-2952(92)90618-s. [DOI] [PubMed] [Google Scholar]
- 46.Dolan ME, Morimoto K, Pegg AE. Reduction of O6-alkylguanine-DNA alkyltransferase activity in HeLa cells treated with O6-alkylguanines. Cancer Res. 1985;45:6413–6417. [PubMed] [Google Scholar]
- 47.Yarosh DB, Hurst-Calderone S, Babich MA, Day RS., 3rd Inactivation of O6-methylguanine-DNA methyltransferase and sensitization of human tumor cells to killing by chloroethylnitrosourea by O6-methylguanine as a free base. Cancer Res. 1986;46:1663–1668. [PubMed] [Google Scholar]
- 48.Dolan ME, Larkin GL, English HF, Pegg AE. Depletion of O6-alkylguanine-DNA alkyltransferase activity in mammalian tissues and human tumor xenografts in nude mice by treatment with O6-methylguanine. Cancer Chemother Pharmacol. 1989;25:103–108. doi: 10.1007/BF00692348. [DOI] [PubMed] [Google Scholar]
- 49.Dolan ME, Moschel RC, Pegg AE. Depletion of mammalian O6-alkylguanine-DNA alkyltransferase activity by O6-benzylguanine provides a means to evaluate the role of this protein in protection against carcinogenic and therapeutic alkylating agents. Proc Natl Acad Sci USA. 1990;87:5368–5372. doi: 10.1073/pnas.87.14.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moschel RC, McDougall MG, Dolan ME, Stine L, Pegg AE. Structural features of substituted purine derivatives compatible with depletion of human O6-alkylguanine-DNA alkyltransferase. J Med Chem. 1992;35:4486–4491. doi: 10.1021/jm00101a028. [DOI] [PubMed] [Google Scholar]
- 51.Moore MH, Gulbis JM, Dodson EJ, Demple B, Moody PC. Crystal structure of a suicidal DNA repair protein: the Ada O6-methylguanine-DNA methyltransferase from E. coli. EMBO J. 1994;13:1495–1501. doi: 10.1002/j.1460-2075.1994.tb06410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shibata T, Glynn N, McMurry TB, McElhinney RS, Margison GP, Williams DM. Novel synthesis of O6-alkylguanine containing oligodeoxyribonucleotides as substrates for the human DNA repair protein, O6-methylguanine DNA methyltransferase (MGMT) Nucleic Acids Res. 2006;34:1884–1891. doi: 10.1093/nar/gkl117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaina B, Muhlhausen U, Piee-Staffa A, Christmann M, Garcia Boy R, Rosch F, Schirrmacher R. Inhibition of O6-methylguanine-DNA methyltransferase by glucose-conjugated inhibitors: comparison with nonconjugated inhibitors and effect on fotemustine and temozolomide-induced cell death. J Pharmacol Exp Ther. 2004;311:585–593. doi: 10.1124/jpet.104.071316. [DOI] [PubMed] [Google Scholar]
- 54.Roy SK, Korzekwa KR, Gonzalez FJ, Moschel RC, Dolan ME. Human liver oxidative metabolism of O6-benzylguanine. Biochem Pharmacol. 1995;50:1385–1389. doi: 10.1016/0006-2952(95)02019-5. [DOI] [PubMed] [Google Scholar]
- 55.Long L, Moschel RC, Dolan ME. Debenzylation of O(6)-benzyl-8-oxoguanine in human liver: implications for O(6)-benzylguanine metabolism. Biochem Pharmacol. 2001;61:721–726. doi: 10.1016/s0006-2952(01)00523-8. [DOI] [PubMed] [Google Scholar]
- 56.Roy SK, Gupta E, Dolan ME. Pharmacokinetics of O6-benzylguanine in rats and its metabolism by rat liver microsomes. Drug Metab Dispos. 1995;23:1394–1399. [PubMed] [Google Scholar]
- 57.Berg SL, Gerson SL, Godwin K, Cole DE, Liu L, Balis FM. Plasma and cerebrospinal fluid pharmacokinetics of O6-benzylguanine and time course of peripheral blood mononuclear cell O6-methylguanine-DNA methyltransferase inhibition in the nonhuman primate. Cancer Res. 1995;55:4606–4610. [PubMed] [Google Scholar]
- 58.Berg SL, Murry DJ, McCully CL, Godwin K, Balis FM. Pharmacokinetics of O6-benzylguanine and its active metabolite 8-oxo-O6-benzylguanine in plasma and cerebrospinal fluid after intrathecal administration of O6-benzylguanine in the nonhuman primate. Clin Cancer Res. 1998;4:2891–2894. [PubMed] [Google Scholar]
- 59.Dolan ME, Roy SK, Fasanmade AA, Paras PR, Schilsky RL, Ratain MJ. O6-benzylguanine in humans: metabolic, pharmacokinetic, and pharmacodynamic findings. J Clin Oncol. 1998;16:1803–1810. doi: 10.1200/JCO.1998.16.5.1803. [DOI] [PubMed] [Google Scholar]
- 60.Neville K, Blaney S, Bernstein M, Thompson P, Adams D, Aleksic A, Berg S. Pharmacokinetics of O(6)-benzylguanine in pediatric patients with central nervous system tumors: a pediatric oncology group study. Clin Cancer Res. 2004;10:5072–5075. doi: 10.1158/1078-0432.CCR-03-0123. [DOI] [PubMed] [Google Scholar]
- 61.Long L, Berg SL, Roy SK, McCully CL, Song-Yoo HW, Moschel RC, Balis FM, Dolan ME. Plasma and cerebrospinal fluid pharmacokinetics of O6-benzylguanine and analogues in nonhuman primates. Clin Cancer Res. 2000;6:3662–3669. [PubMed] [Google Scholar]
- 62.Ewesuedo RB, Wilson LR, Friedman HS, Moschel RC, Dolan ME. Inactivation of O6-alkylguanine-DNA alkyltransferase by 8-substituted O6-benzylguanine analogs in mice. Cancer Chemother Pharmacol. 2001;47:63–69. doi: 10.1007/s002800000202. [DOI] [PubMed] [Google Scholar]
- 63.Ranson M, Middleton MR, Bridgewater J, Lee SM, Dawson M, Jowle D, Halbert G, Waller S, McGrath H, Gumbrell L, McElhinney RS, Donnelly D, McMurry TB, Margison GP. Lomeguatrib, a potent inhibitor of O6-alkylguanine-DNA-alkyltransferase: phase I safety, pharmacodynamic, and pharmacokinetic trial and evaluation in combination with temozolomide in patients with advanced solid tumors. Clin Cancer Res. 2006;12:1577–1584. doi: 10.1158/1078-0432.CCR-05-2198. [DOI] [PubMed] [Google Scholar]
- 64.Friedman HS, Dolan ME, Moschel RC, Pegg AE, Felker GM, Rich J, Bigner DD, Schold SC., Jr Enhancement of nitrosourea activity in medulloblastoma and glioblastoma multiforme. J Natl Cancer Inst. 1992;84:1926–1931. doi: 10.1093/jnci/84.24.1926. [DOI] [PubMed] [Google Scholar]
- 65.Wedge SR, Porteous JK, Newlands ES. Effect of single and multiple administration of an O6-benzylguanine/temozolomide combination: an evaluation in a human melanoma xenograft model. Cancer Chemother Pharmacol. 1997;40:266–272. doi: 10.1007/s002800050657. [DOI] [PubMed] [Google Scholar]
- 66.Wan Y, Wu D, Gao H, Lu H. Potentiation of BCNU anticancer activity by O6-benzylguanine: a study in vitro and in vivo. J Environ Pathol Toxicol Oncol. 2000;19:69–75. [PubMed] [Google Scholar]
- 67.Marathi UK, Dolan ME, Erickson LC. Anti-neoplastic activity of sequenced administration of O6-benzylguanine, streptozotocin, and 1,3-bis(2-chloroethyl)-1-nitrosourea in vitro and in vivo. Biochem Pharmacol. 1994;48:2127–2134. doi: 10.1016/0006-2952(94)90514-2. [DOI] [PubMed] [Google Scholar]
- 68.Kreklau EL, Kurpad C, Williams DA, Erickson LC. Prolonged inhibition of O(6)-methylguanine DNA methyltransferase in human tumor cells by O(6)-benzylguanine in vitro and in vivo. J Pharmacol Exp Ther. 1999;291:1269–1275. [PubMed] [Google Scholar]
- 69.Felker GM, Friedman HS, Dolan ME, Moschel RC, Schold C. Treatment of subcutaneous and intracranial brain tumor xenografts with O6-benzylguanine and 1,3-bis(2-chloroethyl)-1-nitrosourea. Cancer Chemother Pharmacol. 1993;32:471–476. doi: 10.1007/BF00685892. [DOI] [PubMed] [Google Scholar]
- 70.Kokkinakis DM, Ahmed MM, Chendil D, Moschel RC, Pegg AE. Sensitization of pancreatic tumor xenografts to carmustine and temozolomide by inactivation of their O6-methylguanine-DNA methyltransferase with O6-benzylguanine or O6-benzyl-2′-deoxyguanosine. Clin Cancer Res. 2003;9:3801–3807. [PubMed] [Google Scholar]
- 71.Friedman HS, Keir S, Pegg AE, Houghton PJ, Colvin OM, Moschel RC, Bigner DD, Dolan ME. O6-benzylguanine-mediated enhancement of chemotherapy. Mol Cancer Ther. 2002;1:943–948. [PubMed] [Google Scholar]
- 72.Wagner LM, McLendon RE, Yoon KJ, Weiss BD, Billups CA, Danks MK. Targeting methylguanine-DNA methyltransferase in the treatment of neuroblastoma. Clin Cancer Res. 2007;13:5418–5425. doi: 10.1158/1078-0432.CCR-07-0418. [DOI] [PubMed] [Google Scholar]
- 73.Rhines LD, Sampath P, Dolan ME, Tyler BM, Brem H, Weingart J. O6-benzylguanine potentiates the antitumor effect of locally delivered carmustine against an intracranial rat glioma. Cancer Res. 2000;60:6307–6310. [PubMed] [Google Scholar]
- 74.Schold SC, Jr, Kokkinakis DM, Rudy JL, Moschel RC, Pegg AE. Treatment of human brain tumor xenografts with O6-benzyl-2′-deoxyguanosine and BCNU. Cancer Res. 1996;56:2076–2081. [PubMed] [Google Scholar]
- 75.Wedge SR, Newlands ES. O6-benzylguanine enhances the sensitivity of a glioma xenograft with low O6-alkylguanine-DNA alkyltransferase activity to temozolomide and BCNU. Br J Cancer. 1996;73:1049–1052. doi: 10.1038/bjc.1996.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keir ST, Dolan ME, Pegg AE, Lawless A, Moschel RC, Bigner DD, Friedman HS. O6-benzylguanine-mediated enhancement of nitrosourea activity in Mer—central nervous system tumor xenografts—implications for clinical trials. Cancer Chemother Pharmacol. 2000;45:437–440. doi: 10.1007/s002800051016. [DOI] [PubMed] [Google Scholar]
- 77.Kurpad SN, Dolan ME, McLendon RE, Archer GE, Moschel RC, Pegg AE, Bigner DD, Friedman HS. Intraarterial O6-benzylguanine enables the specific therapy of nitrosourea-resistant intracranial human glioma xenografts in athymic rats with 1,3-bis(2-chloroethyl)-1-nitrosourea. Cancer Chemother Pharmacol. 1997;39:307–316. doi: 10.1007/s002800050577. [DOI] [PubMed] [Google Scholar]
- 78.Ueno T, Ko SH, Grubbs E, Yoshimoto Y, Augustine C, Abdel-Wahab Z, Cheng TY, Abdel-Wahab OI, Pruitt SK, Friedman HS, Tyler DS. Modulation of chemotherapy resistance in regional therapy: a novel therapeutic approach to advanced extremity melanoma using intra-arterial temozolomide in combination with systemic O6-benzylguanine. Mol Cancer Ther. 2006;5:732–738. doi: 10.1158/1535-7163.MCT-05-0098. [DOI] [PubMed] [Google Scholar]
- 79.Kokkinakis DM, Moschel RC, Pegg AE, Schold SC. Potentiation of BCNU antitumor efficacy by 9-substituted O6-benzylguanines. Effect of metabolism. Cancer Chemother Pharmacol. 2000;45:69–77. doi: 10.1007/pl00006746. [DOI] [PubMed] [Google Scholar]
- 80.Middleton MR, Kelly J, Thatcher N, Donnelly DJ, McElhinney RS, McMurry TB, McCormick JE, Margison GP. O(6)-(4-bromothenyl)guanine improves the therapeutic index of temozolomide against A375M melanoma xenografts. Int J Cancer. 2000;85:248–252. doi: 10.1002/(sici)1097-0215(20000115)85:2<248::aid-ijc16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 81.Middleton MR, Kelly J, Goodger S, Thatcher N, Margison GP. Four-hourly scheduling of temozolomide improves tumour growth delay but not therapeutic index in A375M melanoma xenografts. Cancer Chemother Pharmacol. 2000;45:15–20. doi: 10.1007/PL00006737. [DOI] [PubMed] [Google Scholar]
- 82.Middleton MR, Thatcher N, McMurry TB, McElhinney RS, Donnelly DJ, Margison GP. Effect of O6-(4-bromothenyl)guanine on different temozolomide schedules in a human melanoma xenograft model. Int J Cancer. 2002;100:615–617. doi: 10.1002/ijc.10532. [DOI] [PubMed] [Google Scholar]
- 83.Clemons M, Kelly J, Watson AJ, Howell A, McElhinney RS, McMurry TB, Margison GP. O6-(4-bromothenyl)guanine reverses temozolomide resistance in human breast tumour MCF-7 cells and xenografts. Br J Cancer. 2005;93:1152–1156. doi: 10.1038/sj.bjc.6602833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Turriziani M, Caporaso P, Bonmassar L, Buccisano F, Amadori S, Venditti A, Cantonetti M, D’Atri S, Bonmassar E. O6-(4-bromothenyl)guanine (PaTrin-2), a novel inhibitor of O6-alkylguanine DNA alkyl-transferase, increases the inhibitory activity of temozolomide against human acute leukaemia cells in vitro. Pharmacol Res. 2006;53:317–323. doi: 10.1016/j.phrs.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 85.Friedman HS, Kokkinakis DM, Pluda J, Friedman AH, Cokgor I, Haglund MM, Ashley DM, Rich J, Dolan ME, Pegg AE, Moschel RC, McLendon RE, Kerby T, Herndon JE, Bigner DD, Schold SC., Jr Phase I trial of O6-benzylguanine for patients undergoing surgery for malignant glioma. J Clin Oncol. 1998;16:3570–3575. doi: 10.1200/JCO.1998.16.11.3570. [DOI] [PubMed] [Google Scholar]
- 86.Friedman HS, Pluda J, Quinn JA, Ewesuedo RB, Long L, Friedman AH, Cokgor I, Colvin OM, Haglund MM, Ashley DM, Rich JN, Sampson J, Pegg AE, Moschel RC, McLendon RE, Provenzale JM, Stewart ES, Tourt-Uhlig S, Garcia-Turner AM, Herndon JE, 2nd, Bigner DD, Dolan ME. Phase I trial of carmustine plus O6-benzylguanine for patients with recurrent or progressive malignant glioma. J Clin Oncol. 2000;18:3522–3528. doi: 10.1200/JCO.2000.18.20.3522. [DOI] [PubMed] [Google Scholar]
- 87.Spiro TP, Gerson SL, Liu L, Majka S, Haaga J, Hoppel CL, Ingalls ST, Pluda JM, Willson JK. O6-benzylguanine: a clinical trial establishing the biochemical modulatory dose in tumor tissue for alkyltransferase-directed DNA repair. Cancer Res. 1999;59:2402–2410. [PubMed] [Google Scholar]
- 88.Schold SC, Jr, Kokkinakis DM, Chang SM, Berger MS, Hess KR, Schiff D, Robins HI, Mehta MP, Fink KL, Davis RL, Prados MD. O6-benzylguanine suppression of O6-alkylguanine-DNA alkyltransferase in anaplastic gliomas. Neuro Oncol. 2004;6:28–32. doi: 10.1215/S115285170300019X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Quinn JA, Desjardins A, Weingart J, Brem H, Dolan ME, Delaney SM, Vredenburgh J, Rich J, Friedman AH, Reardon DA, Sampson JH, Pegg AE, Moschel RC, Birch R, McLendon RE, Provenzale JM, Gururangan S, Dancey JE, Maxwell J, Tourt-Uhlig S, Herndon JE, 2nd, Bigner DD, Friedman HS. Phase I trial of temozolomide plus O6-benzylguanine for patients with recurrent or progressive malignant glioma. J Clin Oncol. 2005;23:7178–7187. doi: 10.1200/JCO.2005.06.502. [DOI] [PubMed] [Google Scholar]
- 90.Schilsky RL, Dolan ME, Bertucci D, Ewesuedo RB, Vogelzang NJ, Mani S, Wilson LR, Ratain MJ. Phase I clinical and pharmacological study of O6-benzylguanine followed by carmustine in patients with advanced cancer. Clin Cancer Res. 2000;6:3025–3031. [PubMed] [Google Scholar]
- 91.Quinn JA, Jiang SX, Reardon DA, Desjardins A, Vredenburgh JJ, Rich JN, Gururangan S, Friedman AH, Bigner DD, Sampson JH, McLendon RE, Herndon JE, Jr, Walker A, Friedman HS. Phase I trial of temozolomide plus O6-benzylguanine 5-day regimen with recurrent malignant glioma. Neuro Oncol. 2009;11:556–561. doi: 10.1215/15228517-2009-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weingart J, Grossman SA, Carson KA, Fisher JD, Delaney SM, Rosenblum ML, Olivi A, Judy K, Tatter SB, Dolan ME. Phase I trial of polifeprosan 20 with carmustine implant plus continuous infusion of intravenous O6-benzylguanine in adults with recurrent malignant glioma: new approaches to brain tumor therapy CNS consortium trial. J Clin Oncol. 2007;25:399–404. doi: 10.1200/JCO.2006.06.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Warren KE, Aikin AA, Libucha M, Widemann BC, Fox E, Packer RJ, Balis FM. Phase I study of O6-benzylguanine and temozolomide administered daily for 5 days to pediatric patients with solid tumors. J Clin Oncol. 2005;23:7646–7653. doi: 10.1200/JCO.2005.02.0024. [DOI] [PubMed] [Google Scholar]
- 94.Quinn JA, Jiang SX, Reardon DA, Desjardins A, Vredenburgh JJ, Gururangan S, Sampson JH, McLendon RE, Herndon JE, 2nd, Friedman HS. Phase 1 trial of temozolomide plus irinotecan plus O6-benzylguanine in adults with recurrent malignant glioma. Cancer. 2009;115:2964–2970. doi: 10.1002/cncr.24336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Broniscer A, Gururangan S, MacDonald TJ, Goldman S, Packer RJ, Stewart CF, Wallace D, Danks MK, Friedman HS, Poussaint TY, Kun LE, Boyett JM, Gajjar A. Phase I trial of single-dose temozolomide and continuous administration of o6-benzylguanine in children with brain tumors: a pediatric brain tumor consortium report. Clin Cancer Res. 2007;13:6712–6718. doi: 10.1158/1078-0432.CCR-07-1016. [DOI] [PubMed] [Google Scholar]
- 96.Adams DM, Zhou T, Berg SL, Bernstein M, Neville K, Blaney SM. Phase 1 trial of O6-benzylguanine and BCNU in children with CNS tumors: a Children’s Oncology Group study. Pediatr Blood Cancer. 2008;50:549–553. doi: 10.1002/pbc.21362. [DOI] [PubMed] [Google Scholar]
- 97.Quinn JA, Jiang SX, Carter J, Reardon DA, Desjardins A, Vredenburgh JJ, Rich JN, Gururangan S, Friedman AH, Bigner DD, Sampson JH, McLendon RE, Herndon JE, 2nd, Threatt S, Friedman HS. Phase II trial of Gliadel plus O6-benzylguanine in adults with recurrent glioblastoma multiforme. Clin Cancer Res. 2009;15:1064–1068. doi: 10.1158/1078-0432.CCR-08-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Quinn JA, Jiang SX, Reardon DA, Desjardins A, Vredenburgh JJ, Rich JN, Gururangan S, Friedman AH, Bigner DD, Sampson JH, McLendon RE, Herndon JE, 2nd, Walker A, Friedman HS. Phase II trial of temozolomide plus o6-benzylguanine in adults with recurrent, temozolomide-resistant malignant glioma. J Clin Oncol. 2009;27:1262–1267. doi: 10.1200/JCO.2008.18.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Quinn JA, Pluda J, Dolan ME, Delaney S, Kaplan R, Rich JN, Friedman AH, Reardon DA, Sampson JH, Colvin OM, Haglund MM, Pegg AE, Moschel RC, McLendon RE, Provenzale JM, Gururangan S, Tourt-Uhlig S, Herndon JE, 2nd, Bigner DD, Friedman HS. Phase II trial of carmustine plus O(6)-benzylguanine for patients with nitrosourea-resistant recurrent or progressive malignant glioma. J Clin Oncol. 2002;20:2277–2283. doi: 10.1200/JCO.2002.09.084. [DOI] [PubMed] [Google Scholar]
- 100.Batts ED, Maisel C, Kane D, Liu L, Fu P, O’Brien T, Remick S, Bahlis N, Gerson SL. O6-benzylguanine and BCNU in multiple myeloma: a phase II trial. Cancer Chemother Pharmacol. 2007;60:415–421. doi: 10.1007/s00280-007-0442-7. [DOI] [PubMed] [Google Scholar]
- 101.Gajewski TF, Sosman J, Gerson SL, Liu L, Dolan E, Lin S, Vokes EE. Phase II trial of the O6-alkylguanine DNA alkyltransferase inhibitor O6-benzylguanine and 1,3-bis(2-chloroethyl)-1-nitrosourea in advanced melanoma. Clin Cancer Res. 2005;11:7861–7865. doi: 10.1158/1078-0432.CCR-05-0060. [DOI] [PubMed] [Google Scholar]
- 102.Ryan CW, Dolan ME, Brockstein BB, McLendon R, Delaney SM, Samuels BL, Agamah ES, Vokes EE. A phase II trial of O6-benzylguanine and carmustine in patients with advanced soft tissue sarcoma. Cancer Chemother Pharmacol. 2006;58:634–639. doi: 10.1007/s00280-006-0210-0. [DOI] [PubMed] [Google Scholar]
- 103.Sabharwal A, Corrie PG, Midgley RS, Palmer C, Brady J, Mortimer P, Watson AJ, Margison GP, Middleton MR (2009) A phase I trial of lomeguatrib and irinotecan in metastatic colorectal cancer. Cancer Chemother Pharmacol (Epub ahead of print) [DOI] [PubMed]
- 104.Caporaso P, Turriziani M, Venditti A, Marchesi F, Buccisano F, Tirindelli MC, Alvino E, Garbin A, Tortorelli G, Toppo L, Bonmassar E, D’Atri S, Amadori S. Novel role of triazenes in haematological malignancies: pilot study of temozolomide, lomeguatrib and IL-2 in the chemo-immunotherapy of acute leukaemia. DNA Repair (Amst) 2007;6:1179–1186. doi: 10.1016/j.dnarep.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 105.Ranson M, Hersey P, Thompson D, Beith J, McArthur GA, Haydon A, Davis ID, Kefford RF, Mortimer P, Harris PA, Baka S, Seebaran A, Sabharwal A, Watson AJ, Margison GP, Middleton MR. Randomized trial of the combination of lomeguatrib and temozolomide compared with temozolomide alone in chemotherapy naive patients with metastatic cutaneous melanoma. J Clin Oncol. 2007;25:2540–2545. doi: 10.1200/JCO.2007.10.8217. [DOI] [PubMed] [Google Scholar]
- 106.Khan OA, Ranson M, Michael M, Olver I, Levitt NC, Mortimer P, Watson AJ, Margison GP, Midgley R, Middleton MR. A phase II trial of lomeguatrib and temozolomide in metastatic colorectal cancer. Br J Cancer. 2008;98:1614–1618. doi: 10.1038/sj.bjc.6604366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kefford RF, Thomas NP, Corrie PG, Palmer C, Abdi E, Kotasek D, Beith J, Ranson M, Mortimer P, Watson AJ, Margison GP, Middleton MR. A phase I study of extended dosing with lomeguatrib with temozolomide in patients with advanced melanoma. Br J Cancer. 2009;100:1245–1249. doi: 10.1038/sj.bjc.6605016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Watson AJ, Middleton MR, McGown G, Thorncroft M, Ranson M, Hersey P, McArthur G, Davis ID, Thomson D, Beith J, Haydon A, Kefford R, Lorigan P, Mortimer P, Sabharwal A, Hayward O, Margison GP. O(6)-methylguanine-DNA methyltransferase depletion and DNA damage in patients with melanoma treated with temozolomide alone or with lomeguatrib. Br J Cancer. 2009;100:1250–1256. doi: 10.1038/sj.bjc.6605015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Watson AJ, Sabharwal A, Thorncroft M, McGown G, Kerr R, Bojanic S, Soonawalla Z, King A, Miller A, Waller S, Leung H, Margison GP, Middleton MR. Tumor O(6)-methylguanine-DNA methyltransferase inactivation by oral lomeguatrib. Clin Cancer Res. 2010;16:743–749. doi: 10.1158/1078-0432.CCR-09-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Koch D, Hundsberger T, Boor S, Kaina B. Local intracerebral administration of O(6)-benzylguanine combined with systemic chemotherapy with temozolomide of a patient suffering from a recurrent glioblastoma. J Neurooncol. 2007;82:85–89. doi: 10.1007/s11060-006-9244-8. [DOI] [PubMed] [Google Scholar]
- 111.Nelson ME, Loktionova NA, Pegg AE, Moschel RC. 2-amino-O4-benzylpteridine derivatives: potent inactivators of O6-alkylguanine-DNA alkyltransferase. J Med Chem. 2004;47:3887–3891. doi: 10.1021/jm049758+. [DOI] [PubMed] [Google Scholar]
- 112.Javanmard S, Loktionova NA, Fang Q, Pauly GT, Pegg AE, Moschel RC. Inactivation of O(6)-alkylguanine-DNA alkyltransferase by folate esters of O(6)-benzyl-2′-deoxyguanosine and of O(6)-[4-(hydroxymethyl)benzyl]guanine. J Med Chem. 2007;50:5193–5201. doi: 10.1021/jm0705859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Argiles JM, Lopez-Soriano FJ. Why do cancer cells have such a high glycolytic rate? Med Hypotheses. 1990;32:151–155. doi: 10.1016/0306-9877(90)90039-h. [DOI] [PubMed] [Google Scholar]
- 114.Yamamoto T, Seino Y, Fukumoto H, Koh G, Yano H, Inagaki N, Yamada Y, Inoue K, Manabe T, Imura H. Over-expression of facilitative glucose transporter genes in human cancer. Biochem Biophys Res Commun. 1990;170:223–230. doi: 10.1016/0006-291x(90)91263-r. [DOI] [PubMed] [Google Scholar]
- 115.Reinhard J, Eichhorn U, Wiessler M, Kaina B. Inactivation of O(6)-methylguanine-DNA methyltransferase by glucose-conjugated inhibitors. Int J Cancer. 2001;93:373–379. doi: 10.1002/ijc.1336. [DOI] [PubMed] [Google Scholar]
- 116.Reinhard J, Hull WE, von der Lieth CW, Eichhorn U, Kliem HC, Kaina B, Wiessler M. Monosaccharide-linked inhibitors of O(6)-methylguanine-DNA methyltransferase (MGMT): synthesis, molecular modeling, and structure-activity relationships. J Med Chem. 2001;44:4050–4061. doi: 10.1021/jm010006e. [DOI] [PubMed] [Google Scholar]
- 117.Roth RB, Samson LD. Gene transfer to suppress bone marrow alkylation sensitivity. Mutat Res. 2000;462:107–120. doi: 10.1016/s1383-5742(00)00021-1. [DOI] [PubMed] [Google Scholar]
- 118.Geiger H, Schleimer D, Nattamai KJ, Dannenmann SR, Davies SM, Weiss BD. Mutagenic potential of temozolomide in bone marrow cells in vivo. Blood. 2006;107:3010–3011. doi: 10.1182/blood-2005-09-3649. [DOI] [PubMed] [Google Scholar]
- 119.Kushner BH, Laquaglia MP, Kramer K, Modak S, Cheung NK. Recurrent metastatic neuroblastoma followed by myelodysplastic syndrome: possible leukemogenic role of temozolomide. Pediatr Blood Cancer. 2008;51:552–554. doi: 10.1002/pbc.21658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.De Vita S, De Matteis S, Laurenti L, Chiusolo P, Reddiconto G, Fiorini A, Leone G, Sica S. Secondary Ph + acute lymphoblastic leukemia after temozolomide. Ann Hematol. 2005;84:760–762. doi: 10.1007/s00277-005-1093-6. [DOI] [PubMed] [Google Scholar]
- 121.Su YW, Chang MC, Chiang MF, Hsieh RK. Treatment-related myelodysplastic syndrome after temozolomide for recurrent high-grade glioma. J Neurooncol. 2005;71:315–318. doi: 10.1007/s11060-004-2028-0. [DOI] [PubMed] [Google Scholar]
- 122.Allay JA, Dumenco LL, Koc ON, Liu L, Gerson SL. Retroviral transduction and expression of the human alkyltransferase cDNA provides nitrosourea resistance to hematopoietic cells. Blood. 1995;85:3342–3351. [PubMed] [Google Scholar]
- 123.Moritz T, Mackay W, Glassner BJ, Williams DA, Samson L. Retrovirus-mediated expression of a DNA repair protein in bone marrow protects hematopoietic cells from nitrosourea-induced toxicity in vitro and in vivo. Cancer Res. 1995;55:2608–2614. [PubMed] [Google Scholar]
- 124.Jelinek J, Fairbairn LJ, Dexter TM, Rafferty JA, Stocking C, Ostertag W, Margison GP. Long-term protection of hematopoiesis against the cytotoxic effects of multiple doses of nitrosourea by retrovirus-mediated expression of human O6-alkylguanine-DNA-alkyltransferase. Blood. 1996;87:1957–1961. [PubMed] [Google Scholar]
- 125.Maze R, Carney JP, Kelley MR, Glassner BJ, Williams DA, Samson L. Increasing DNA repair methyltransferase levels via bone marrow stem cell transduction rescues mice from the toxic effects of 1,3-bis(2-chloroethyl)-1-nitrosourea, a chemotherapeutic alkylating agent. Proc Natl Acad Sci U S A. 1996;93:206–210. doi: 10.1073/pnas.93.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Allay JA, Davis BM, Gerson SL. Human alkyltransferase-transduced murine myeloid progenitors are enriched in vivo by BCNU treatment of transplanted mice. Exp Hematol. 1997;25:1069–1076. [PubMed] [Google Scholar]
- 127.Dolan ME, Pegg AE, Dumenco LL, Moschel RC, Gerson SL. Comparison of the inactivation of mammalian and bacterial O6-alkylguanine-DNA alkyltransferases by O6-benzylguanine and O6-methylguanine. Carcinogenesis. 1991;12:2305–2309. doi: 10.1093/carcin/12.12.2305. [DOI] [PubMed] [Google Scholar]
- 128.Loktionova NA, Pegg AE. Point mutations in human O6-alkylguanine-DNA alkyltransferase prevent the sensitization by O6-benzylguanine to killing by N,N′-bis (2-chloroethyl)-N-nitrosourea. Cancer Res. 1996;56:1578–1583. [PubMed] [Google Scholar]
- 129.Crone TM, Goodtzova K, Edara S, Pegg AE. Mutations in human O6-alkylguanine-DNA alkyltransferase imparting resistance to O6-benzylguanine. Cancer Res. 1994;54:6221–6227. [PubMed] [Google Scholar]
- 130.Xu-Welliver M, Kanugula S, Pegg AE. Isolation of human O6-alkylguanine-DNA alkyltransferase mutants highly resistant to inactivation by O6-benzylguanine. Cancer Res. 1998;58:1936–1945. [PubMed] [Google Scholar]
- 131.Woolford LB, Southgate TD, Margison GP, Milsom MD, Fairbairn LJ. The P140K mutant of human O(6)-methylguanine-DNA-methyltransferase (MGMT) confers resistance in vitro and in vivo to temozolomide in combination with the novel MGMT inactivator O(6)-(4-bromothenyl)guanine. J Gene Med. 2006;8:29–34. doi: 10.1002/jgm.816. [DOI] [PubMed] [Google Scholar]
- 132.Davis BM, Roth JC, Liu L, Xu-Welliver M, Pegg AE, Gerson SL. Characterization of the P140K, PVP(138–140)MLK, and G156A O6-methylguanine-DNA methyltransferase mutants: implications for drug resistance gene therapy. Hum Gene Ther. 1999;10:2769–2778. doi: 10.1089/10430349950016500. [DOI] [PubMed] [Google Scholar]
- 133.Davis BM, Reese JS, Koc ON, Lee K, Schupp JE, Gerson SL. Selection for G156A O6-methylguanine DNA methyltransferase gene-transduced hematopoietic progenitors and protection from lethality in mice treated with O6-benzylguanine and 1,3-bis(2-chloroethyl)-1-nitrosourea. Cancer Res. 1997;57:5093–5099. [PubMed] [Google Scholar]
- 134.Koc ON, Reese JS, Davis BM, Liu L, Majczenko KJ, Gerson SL. DeltaMGMT-transduced bone marrow infusion increases tolerance to O6-benzylguanine and 1,3-bis(2-chloroethyl)-1-nitrosourea and allows intensive therapy of 1,3-bis(2-chloroethyl)-1-nitrosourea-resistant human colon cancer xenografts. Hum Gene Ther. 1999;10:1021–1030. doi: 10.1089/10430349950018418. [DOI] [PubMed] [Google Scholar]
- 135.Kreklau EL, Pollok KE, Bailey BJ, Liu N, Hartwell JR, Williams DA, Erickson LC. Hematopoietic expression of O(6)-methylguanine DNA methyltransferase-P140K allows intensive treatment of human glioma xenografts with combination O(6)-benzylguanine and 1,3-bis-(2-chloroethyl)-1-nitrosourea. Mol Cancer Ther. 2003;2:1321–1329. [PubMed] [Google Scholar]
- 136.Reese JS, Davis BM, Liu L, Gerson SL. Simultaneous protection of G156A methylguanine DNA methyltransferase gene-transduced hematopoietic progenitors and sensitization of tumor cells using O6-benzylguanine and temozolomide. Clin Cancer Res. 1999;5:163–169. [PubMed] [Google Scholar]
- 137.Sawai N, Zhou S, Vanin EF, Houghton P, Brent TP, Sorrentino BP. Protection and in vivo selection of hematopoietic stem cells using temozolomide, O6-benzylguanine, and an alkyltransferase-expressing retroviral vector. Mol Ther. 2001;3:78–87. doi: 10.1006/mthe.2000.0223. [DOI] [PubMed] [Google Scholar]
- 138.Jansen M, Sorg UR, Ragg S, Flasshove M, Seeber S, Williams DA, Moritz T. Hematoprotection and enrichment of transduced cells in vivo after gene transfer of MGMT(P140K) into hematopoietic stem cells. Cancer Gene Ther. 2002;9:737–746. doi: 10.1038/sj.cgt.7700490. [DOI] [PubMed] [Google Scholar]
- 139.Cai S, Ernstberger A, Wang H, Bailey BJ, Hartwell JR, Sinn AL, Eckermann O, Linka Y, Goebel WS, Hanenberg H, Pollok KE. In vivo selection of hematopoietic stem cells transduced at a low multiplicity-of-infection with a foamy viral MGMT(P140K) vector. Exp Hematol. 2008;36:283–292. doi: 10.1016/j.exphem.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zielske SP, Gerson SL. Lentiviral transduction of P140K MGMT into human CD34(+) hematopoietic progenitors at low multiplicity of infection confers significant resistance to -BG/BCNU and allows selection in vitro. Mol Ther. 2002;5:381–387. doi: 10.1006/mthe.2002.0571. [DOI] [PubMed] [Google Scholar]
- 141.Maier P, Spier I, Laufs S, Veldwijk MR, Fruehauf S, Wenz F, Zeller WJ. Chemoprotection of human hematopoietic stem cells by simultaneous lentiviral overexpression of multidrug resistance 1 and O(6)-methylguanine-DNA methyltransferase(P140K) Gene Ther. 2010;17:389–399. doi: 10.1038/gt.2009.133. [DOI] [PubMed] [Google Scholar]
- 142.Christmann M, Tomicic MT, Roos WP, Kaina B. Mechanisms of human DNA repair: an update. Toxicology. 2003;193:3–34. doi: 10.1016/s0300-483x(03)00287-7. [DOI] [PubMed] [Google Scholar]
- 143.Kaina B, Christmann M. DNA repair in resistance to alkylating anticancer drugs. Int J Clin Pharmacol Ther. 2002;40:354–367. doi: 10.5414/cpp40354. [DOI] [PubMed] [Google Scholar]
- 144.Lips J, Kaina B. DNA double-strand breaks trigger apoptosis in p53-deficient fibroblasts. Carcinogenesis. 2001;22:579–585. doi: 10.1093/carcin/22.4.579. [DOI] [PubMed] [Google Scholar]
- 145.Batista LF, Roos WP, Christmann M, Menck CF, Kaina B. Differential sensitivity of malignant glioma cells to methylating and chloroethylating anticancer drugs: p53 determines the switch by regulating xpc, ddb2, and DNA double-strand breaks. Cancer Res. 2007;67:11886–11895. doi: 10.1158/0008-5472.CAN-07-2964. [DOI] [PubMed] [Google Scholar]