Abstract
Dopamine is an important neurotransmitter that regulates several key functions in the brain, such as motor output, motivation and reward, learning and memory, and endocrine regulation. Dopamine does not mediate fast synaptic transmission, but rather modulates it by triggering slow-acting effects through the activation of dopamine receptors, which belong to the G-protein-coupled receptor superfamily. Besides activating different effectors through G-protein coupling, dopamine receptors also signal through interaction with a variety of proteins, collectively termed dopamine receptor-interacting proteins. We focus on the dopamine D4 receptor, which contains an important polymorphism in its third intracellular loop. This polymorphism has been the subject of numerous studies investigating links with several brain disorders, such as attention-deficit hyperactivity disorder and schizophrenia. We provide an overview of the structure, signalling properties and regulation of dopamine D4 receptors, and briefly discuss their physiological and pathophysiological role in the brain.
Keywords: GPCR, Dopamine, D4 receptor, Variable number of tandem repeats (VNTR), Dimerization, Internalization, Dopamine receptor-interacting protein (DRIP), Attention-deficit hyperactivity disorder (ADHD)
Dopaminergic neurons in the brain
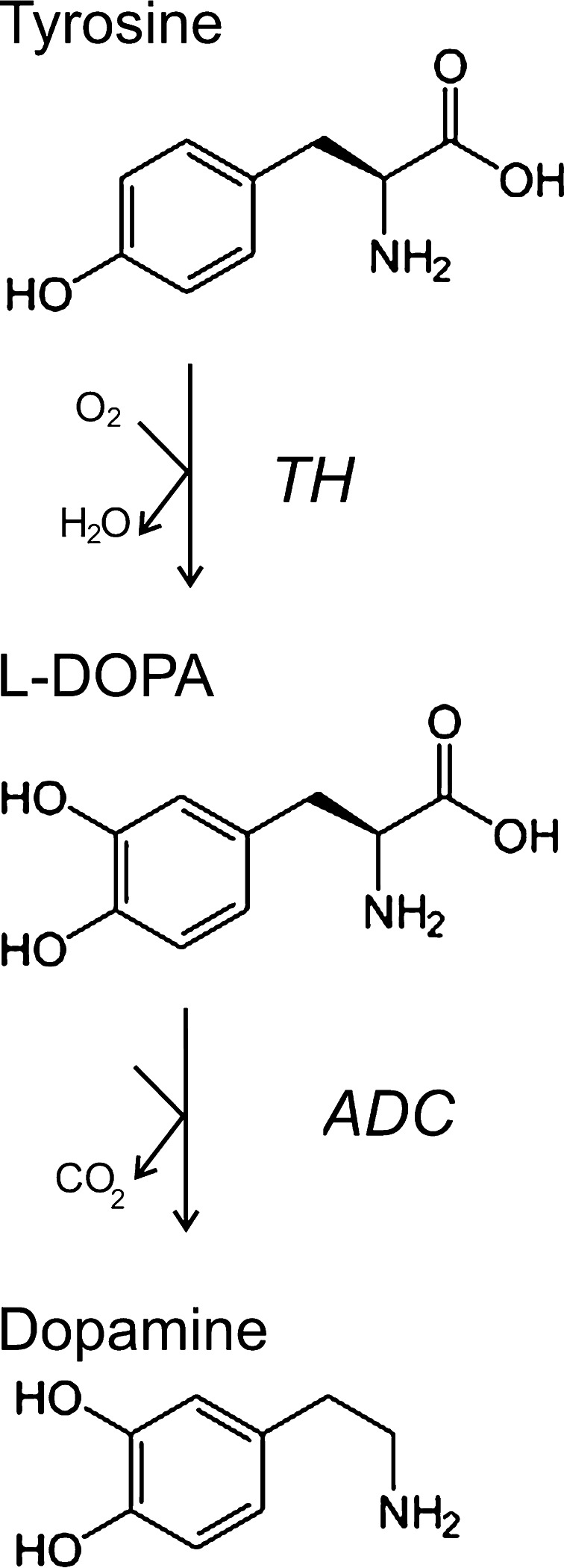
Dopamine is a neurotransmitter that belongs to the group of catecholamines. These contain a nucleus of catechol (benzene ring with two adjacent hydroxyl groups) and a side chain of ethylamine or one of its derivatives. Dopamine is synthesized from the amino acid tyrosine in a two-step enzymatic process (Fig. 1). The first, rate-limiting, reaction involves the conversion of tyrosine into l-3,4-dihydroxyphenylalanine (also referred to as l-DOPA or levodopa), catalysed by tyrosine hydroxylase (TH). The second step is carried out by aromatic l-amino acid decarboxylase (AADC), and produces dopamine by decarboxylation of DOPA. Interestingly, exogenously applied levodopa has been used for a long time to alleviate the symptoms of Parkinson’s disease on the basis of its conversion to dopamine (reviewed in references [1] and [2]). In other neurons, or in the adrenal medulla, dopamine can further be transformed into noradrenaline/norepinephrine and adrenaline/epinephrine.
Fig. 1.
Synthesis of dopamine. In the first rate-determining step, tyrosine is converted into l-DOPA by the enzyme TH, in the presence of tetrahydrobiopterin as a cofactor. In the second step, l-DOPA is converted into dopamine by the enzyme AADC
Although dopaminergic neurons are rare (<1/100,000 brain neurons), they regulate several important aspects of basic brain function. They are necessary for diverse tasks of the brain regions they innervate, such as motor output, motivation, memory and endocrine regulation. Dopamine also plays an important role in the brain reward system, that controls and stimulates the learning of many behaviours [3]. Dysregulation of dopaminergic signalling is linked to several pathological conditions, such as Parkinson’s disease, schizophrenia, and attention-deficit hyperactivity disorder (ADHD). Artificial increase in dopamine transmission seems to be the common mechanism of action of drugs of abuse that lead to addiction. Therefore, understanding how dopamine works is an important subject of neuroscience research.
Brain areas that synthesize dopamine have projections that give rise to four axonal pathways, namely nigrostriatal, mesolimbic, mesocortical and tuberoinfundibular (reviewed in reference [1]). The nigrostriatal pathway is formed by projections that arise from dopamine-synthesizing neurons of the midbrain nucleus, the substantia nigra compacta, which innervates the dorsal striatum (caudate putamen). This pathway is involved in unconditioned and conditioned (learned) behaviour [4–6]. Degeneration of nigrostriatal neurons causes Parkinson’s disease. The mesolimbic pathway originates from the midbrain ventral tegmental area and innervates the olfactory tubercle, the ventral striatum (nucleus accumbens) and parts of the limbic system. This pathway is involved in sensitivity to rewarding stimuli and reward-based, associative (pavlovian) learning [7], and averse stimuli. It is also implicated in the psychomotor effects generated by drugs of abuse including cocaine and alcohol [8, 9]. The mesocortical pathway arises from the ventral tegmental area and innervates different regions of the frontal cortex and both D1 and D2 receptors are involved in learning and memory [10–13]. Finally, the tuberoinfundibular pathway arises from cells of the periventricular and arcuate nuclei of the hypothalamus. Projections reach the median eminence of the hypothalamus, where they release dopamine in the hypothalamic-hypophyseal portal system. Consequently, dopamine is transported to the anterior pituitary, where it has an inhibitory effect on prolactin release by lactotrophs.
Dopamine is not a simple excitatory or inhibitory neurotransmitter, but rather a neuromodulator that alters the responses of target neurons to other neurotransmitters, and that can alter synaptic plasticity. In this regard, dopamine (as do other monoamines) does not ‘mediate’ fast synaptic transmission, but ‘modulates’ it by triggering slow-acting effects through signalling cascades. This complex feature of dopamine makes it difficult to study its physiological role, although recent progress in many neuroscience areas has helped elucidate the function of dopamine and neuropsychiatric illnesses.
Dopamine exerts its effects in the brain through different types of dopamine receptors (D1–D5). In this article we focus on the D4 receptor. Due to its potential role in mediating the effects of atypical antipsychotics, the D4 receptor has been the subject of a vast number of studies during the past two decades. Despite these efforts, the specific function of this receptor in the brain, and particularly the role of its remarkable VNTR polymorphism, remain far from being understood. However, since the link of seven repeat alleles with ADHD was recently confirmed [14], and since it has become clear that these variants have been subject to positive selection during recent evolution, elucidating the role of this polymorphism, as well as the regulation and signalling properties of the D4 receptor, is even more challenging. This review gives a comprehensive overview of the general properties of the D4 receptor and many of the molecular studies that have been performed since its discovery. We also include the most recent data indicating that this receptor shows specific signalling properties and regulation mechanisms that differ from those of other dopamine receptor subtypes. We stress the importance of D4 receptor-interacting proteins (DRIPs) that place this receptor in multiprotein complexes, allowing specific regulation, fast, coordinated and pleiotropic signalling and extensive crosstalk.
Dopamine D4 receptor
Characterization, structure and the VNTR polymorphism
Biochemical studies (almost four decades ago) showed that dopamine is able to stimulate adenylyl cyclase (AC), thereby suggesting the existence of dopamine receptors [15]. Further research demonstrated that these receptors belong to the GPCR superfamily and the homology cloning approach rapidly resulted in the identification of five dopamine receptor types [16, 17]. Further structural, biochemical and pharmacological studies demonstrated the existence of two categories of dopamine receptors. D1 and D5 receptors (previously sometimes denoted as D1A and D1B, respectively) are classified as D1-like, whereas D2, D3 and D4 receptors are characterized as D2-like.
The genomic organization of dopamine receptors indicates that they are derived from the divergence of two gene families. In contrast to D1-like receptor genes, those encoding D2-like receptors are interrupted by introns, which allow the generation of receptor variants. Both the human D4 receptor gene and rat or mouse homologous genes contain four exons [17–19]. The D4 receptor gene, located on chromosome 11 (11p15.5), contains a transcription initiation site at 400–500 bp upstream, whereas promoter sequences are located further upstream of the transcription initiation site [20]. The D4 gene contains quite a large number of polymorphisms in its coding sequence. The most extensive polymorphism is found in exon 3 in a region that codes for the third intracellular loop (IC3) of the receptor [19]. This polymorphism consists of a variable number of tandem repeats (VNTR), in which a 48-bp sequence exists as a 2- to 11-fold repeat giving rise to polymorphic D4 receptor variants, denoted as D4.2 to D4.11 [18, 21]. Thus, the length of the polymorphism varies from two (2×16 amino acids) to eleven (11×16 amino acids) repeats. Several of the repeat units vary in sequence from each other. At least 18 different repeat units (different nucleotide sequence) have been described and found in various positions [19, 22, 23]. Interestingly, a similar VNTR is also present in various nonhuman primate species, but is not seen in rodents [19, 24].
The allele frequencies of the different human polymorphic receptor gene variants are very heterologous [23, 25]. The four-repeat alleles have been found to be the most frequent (global frequency 64%), followed by the seven-repeat alleles (21%) and the two-repeat alleles (8%). However, it became evident that there are considerable differences in allele frequencies among the different populations. For example, D4.7 alleles only appear occasionally (<1%) in several Asian populations, whereas the D4.2 allele is more frequent (up to 18%) in Asia than globally (8%). Interestingly, there is evidence that the seven-repeat alleles are at least five to ten times younger than the common four-repeat allele, but nevertheless have increased in frequency in human populations by positive selection [21].
Analysis of the dopamine receptor structure has revealed considerable homology between members of the same family. The D2 and D3 receptors share 75% identity in their transmembrane (TM) domain, and the D2 and D4 receptors 53% [26]. The N-terminal end has a similar length in all receptor subtypes and contains a variable number of consensus N-glycosylation sites. Biochemical and pharmacological studies show that the D4 receptor is able to undergo N-linked glycosylation on a single conserved site (Asn 3), and that this glycosylation is not involved in ligand binding or receptor trafficking to the plasma membrane [27–29] (see Fig. 2). Regarding the C-terminal end of the receptors, differences are more obvious between the two subfamilies, as the C-terminal tail is about seven times longer for D1-like receptors than for D2-like receptors. They all contain a cysteine residue (conserved in GPCRs) that has been demonstrated to be palmitoylated to anchor the cytoplasmic tail of several GPCRs, such as β-adrenergic receptors and rhodopsin, to the membrane [30–33]. Two other cysteine residues, located in extracellular loops 2 and 3 of all dopamine receptors (and GPCRs), are also of interest, as they have been suggested to form an intramolecular disulphide bridge to stabilize the receptor structure [34, 35]. Finally, D2-like receptors have a long IC3, whereas D1-like receptors contain a short IC3, a common feature for Gi- and Gs-coupled receptors, respectively. Other studies revealed that, besides an important role for the IC3, also the IC2 of dopamine receptors is involved in G-protein coupling [36–39].
Fig. 2.
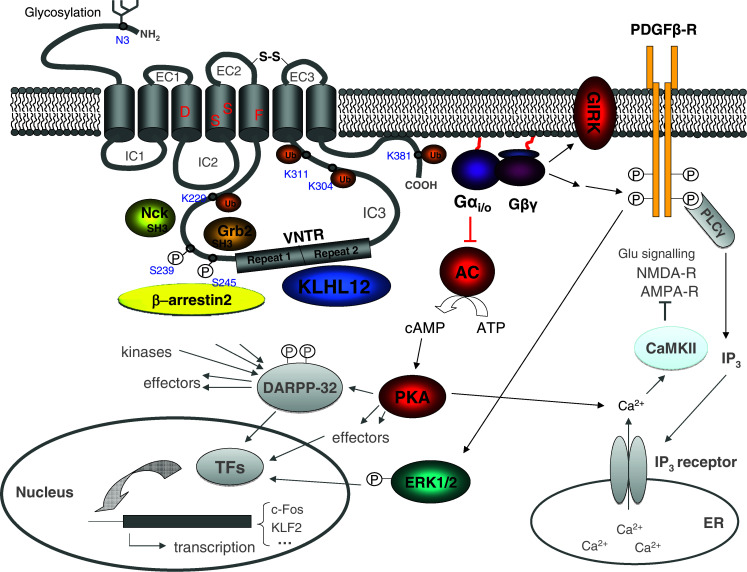
Schematic illustration of the D4.2 receptor and its interacting proteins. Residues that are important for dopamine binding are indicated in red. The N-linked glycosylation site is located on the extracellular N-terminal end of the receptor, at N3. The two repeats (16 amino acids each) of the VNTR polymorphism are indicated in the third intracellular loop (IC3) of the receptor. More information about the role of the interacting proteins can be found in the text
Signalling by D4 receptors
Before discussing several dopamine receptor-induced signalling pathways, it should be mentioned that in studies using heterologous expression of dopamine receptors in several cellular systems (e.g. HEK293T, CHO, etc.), the receptors may be expressed in an environment that could contain different G-proteins, effectors and other molecules from those found in vivo. As a result, heterologous expression systems sometimes lead to apparently conflicting results. Nevertheless, these systems have the advantage of allowing the study of a single type of receptor, and are of great help in unravelling different signalling properties of dopamine receptors. On the other hand, the historical lack of truly selective agonists (see below) which discriminate between the various receptor subtypes, and specific and sensitive antibodies against the D4 receptor, has added another level of difficulty for studies on D4 receptor signalling in brain cells.
Multiple G-protein coupling
As demonstrated for many GPCRs, each dopamine receptor subtype is able to interact with more than one G-protein, allowing a multiplicity of signalling responses [38]. Furthermore, interaction with multiple G-proteins allows receptors to elicit effects which can act to enhance and subsequently suppress the original receptor response, and to activate distinct signalling pathways. For example, if the initial receptor-mediated response is Gs-coupled, this response could be attenuated by a switch in receptor coupling specificity to the inhibitory Gi-protein, leading to termination of the initial signal, even in the persistent presence of agonist. Such a system enables the receptor to modulate its own functional response, in a feedback-type inhibition.
Adenylyl cyclase and cAMP
Many studies have demonstrated that activation of AC (which catalyses the formation of cAMP from ATP), resulting in cAMP production, is a general property of D1-like receptors, through Gs or Golf proteins in several brain tissues [40], whereas inhibition of AC (inhibition of cAMP production) through Gi/o-proteins characterizes D2-like receptors [41]. Regarding the VNTR polymorphism in the IC3 of the D4 receptor, studies have suggested that this repeat region might be important in coupling to AC and even to G-proteins [42, 43]. Comparison of various D4 polymorphic variants has shown that the D4.7 receptor has a two- to threefold lower potency for dopamine-mediated coupling to AC than the D4.2 and D4.4 receptors [44]. However, the D4.10 receptor was even two- to threefold more potent in AC coupling than the D4.2 receptor [45]. cAMP, in turn, is an important and ubiquitous second messenger for many signalling pathways and can influence various effectors, such as protein kinase A (PKA) and DARPP-32 [46]. As DARPP-32 is an intermediate in many signalling pathways, activated by various neurotransmitters, it plays a role in integrating their actions in neurons, and provides a link to other effectors and transcription factors (reviewed in reference [47]). Further downstream, activation of the D4 receptor has been demonstrated to activate NFκB, an important transcription factor that plays a role in inflammation [48], to induce Kruppel-like factor-2 (KLF2), a critical regulator of quiescence in T-lymphocytes [49] and c-Fos expression [50], which upon dimerization with c-jun forms the transcription factor AP-1 that activates transcription of genes involved in proliferation, differentiation, defence against invasion and cell damage.
Calcium
Dopamine D4 receptors have been reported to influence intracellular calcium levels through a variety of different mechanisms, depending on the cell type, e.g. (1) inhibition of calcium current in GH4C1 cells [38] and in AtT20 cells [25], due to signalling to plasma membrane-expressed calcium channels, and (2) stimulation of calcium current in HEK293 cells [25], mediated by IP3 receptors in the endoplasmic reticulum membrane. More complex mechanisms have been described in which the D4 receptor transactivates the platelet-derived growth factor (PDGF) β receptor in CA1 hippocampal neurons, leading to PLCγ activation and subsequent IP3-dependent Ca2+ release from the endoplasmic reticulum [36]. Calcium induction by D4 receptors can result in several downstream effects: in cultured prefrontal cortical neurons, D4 activation results in synaptic translocation and activation of CaMKII, which in turn regulates other targets such as AMPA receptors, that play a crucial role in glutamatergic transmission in the brain [39, 40]. Besides influencing AMPA receptors via calcium signalling, activation of the D4 receptor in prefrontal cortex (PFC) interneurons also causes a persistent suppression of AMPA receptor-mediated synaptic transmission, by regulation of actin dynamics and AMPA receptor trafficking [41]. Although the regulation of calcium levels in the cell by dopamine receptors is firmly established, the recent identification of many calcium-sensing DRIPs (see below; [42]) indicates that cellular calcium can in turn modify dopamine receptor signalling properties, indicating possible crosstalk and feedback mechanisms and additional levels of control on dopamine receptor function.
Potassium channels
D2-like receptors can influence different types of potassium channel. Several experiments have shown that D2 and D4 receptors interact with GIRK (G-protein-coupled inwardly rectifying potassium channel; Kir3), an important regulator of cellular excitability [51–53], the opening of which reduces the firing rate of neurons. In a recent study, dopamine was demonstrated to stimulate D4.2, D4.4 and D4.7 receptors and modulate GIRK currents in Xenopus through oocyte Gi/o-proteins [54]. Furthermore, dopamine was five-fold more potent on D4.2 and D4.7 than on D4.4, suggesting that the actions of dopamine and therapeutic drugs on D4 receptors might vary among individuals.
However, the D4 receptor has also been reported to influence another type of potassium channel than GIRK, more specifically the voltage-dependent outward potassium current, which induces cell hyperpolarization by increasing outward potassium currents via G-proteins [55, 56].
Arachidonic acid and Na+/H+ exchange
D4 receptors can also induce arachidonic acid release, probably via G-proteins and PKC activation [28, 57, 58] and affect the activity of Na+/H+ exchangers, which regulate intracellular pH, extracellular acidification and cell volume [59, 60].
MAPK signalling
Several studies have shown a positive effect of D2-like receptors on mitogenesis via classical mitogen-activated protein kinases (MAPKs, more specifically the extracellular signal-regulated kinase ERK1 and 2), whereas other studies have shown an inhibiting effect by the same receptors in other cell lines [61, 62]. The specific pathway used to activate ERK upon D4 receptor stimulation depends on the specific cell type [63, 64]. First, it was demonstrated that D4 receptors can activate the ERK cascade in CHO cells and this is dependent on transactivating the PDGFβ receptor, a receptor tyrosine kinase (RTK) [63]. Very recently it was shown that intracellular PDGFβ receptors can also be transactivated by D4 receptors [65]. Finally, it is noteworthy that no differences were observed in the magnitude or duration of MAPK activation comparing D4.2, D4.4 and D4.7 receptor-mediated signalling in CHO cells [63]. In vivo studies have shown that this transactivation results in depression of excitatory transmission, mediated by NMDA receptors [63, 66]. Although the precise mechanisms by which the D4 receptor transactivates PDGFβ receptors are not completely clear, these findings indicate an important role for RTKs in the regulation and communication of dopamine and glutamate signalling in the CNS. Interestingly, D4 receptors and reduced glutamate signalling have been implicated in neurological disorders that affect cognition and attention, such as schizophrenia and ADHD [67].
Effects on GABAA signalling
The PFC, which shows a high expression of D4 receptors [74], is associated with cognitive and emotional processes, attention, and both working and long-term memory [68–70]. The synchronization of pyramidal neuron activity is controlled by GABAergic interneurons, mediated by ligand-gated ion channels, GABAA receptors [71, 72]. Previous studies have revealed that GABAA receptors are subject to D4 receptor regulation in PFC pyramidal neurons [75] and that D4 receptor activation decreases functional GABAA receptor levels at the plasma membrane by decreasing its transport through actin depolymerization [76].
Dopamine receptor-interacting proteins
Recent data support the model that GPCRs are not randomly distributed in the plasma membrane, but are rather concentrated in specialized distinct microdomains, such as lipid rafts and caveolae. In addition to classic G-proteins, these structures contain a variety of signalling molecules, such as AC, PKC and many other proteins. By recruiting different signalling components, these microdomains can enhance the speed and specificity of GPCR signalling. These observations have led to the concept of signalsomes or signalplexes, that underscore the importance of multiprotein complexes in the regulation and signalling properties of GPCRs. In accordance with this model, GPCRs have been demonstrated to interact with a wide variety of intracellular proteins [77–80], forming dynamic complexes that contribute to the fine-tuning of downstream signalling complexes. These insights have stimulated the search for novel receptor-interacting proteins to provide possible clues to the regulation and signalling properties of GPCRs. Also for dopamine receptors, more and more DRIPs have been characterized. The finding that D1-like and D2-like receptors interact with a different set of DRIPs supports the theory that these interactions determine many of the functional properties that are different between the two subtypes [81]. Whereas most of the studies on DRIPs are based on the D2 receptor, serving as the model receptor for D2-like receptors, only a few DRIPs have been characterized so far (Fig. 2).
The proline-rich sequences of the D4 receptor, mainly located in the polymorphic region of the IC3, can interact with SH3 domain-containing proteins. Strong interactions have been detected with Grb2 and Nck [43], two adapter proteins without any known catalytic activity but capable of recruiting multiprotein complexes to the receptor and influencing cell proliferation, cell movement, axon guidance, organization of the actin cytoskeleton, etc. Removal of all these putative binding sites in the receptor results in a mutant receptor that can still bind dopamine and G-protein, but fails to couple with AC. This receptor furthermore shows strong constitutive internalization, suggesting that the SH3-binding sites of the receptor are involved in the control of receptor internalization. Another type of D4 receptor-interacting protein is GIRK (see higher), which modulates ion passage in response to D4 stimulation. Additionally, we have recently discovered another protein that specifically interacts with the D4 receptor but not with the other dopamine receptor subtypes, i.e. the BTB-Kelch protein KLHL12, that specifically binds to the polymorphic region of the D4 receptor and functions as a substrate-specific adaptor in a Cul3-based E3 ubiquitin ligase complex for subsequent ubiquitination of the D4 receptor [82]. This type of DRIP thus alters the structure of the D4 receptor through secondary modification. For a detailed and recently updated list of confirmed DRIPs of all dopamine receptor subtypes, and their functional implications, we further refer to Yao et al. [83].
Dimerization/oligomerization of dopamine D4 receptors
For the dopamine receptors, D1, D2, D3 and D5, it has been shown that they are able to associate with themselves, as well as with other receptors to form multi-receptor networks that may have unique functional properties. These networks could contribute to several aspects of dopamine receptor signalling, including cross-talk with other receptor systems. Concerning the D4 receptor in particular, no data have been reported yet, although we have unpublished results indicating that this receptor could indeed form oligomers and that dimerization plays a role in receptor biogenesis.
Regulation
As for other GPCRs, dopamine receptor functionality is regulated by a variety of systems, among which agonist-induced desensitization and internalization are important. Previous studies on dopamine receptors have revealed a great variability of agonist-induced desensitization subtypes. Most studies on regulation of D2-type receptors have shown that continuous agonist application results in phosphorylation of the D2 receptor, leading to uncoupling of G-proteins and subsequent arrestin recruitment and internalization. The process of internalization of these receptors seems to be dependent on a rather common dynamin-dependent mechanism of endocytosis [84–87].
Studies on the desensitization properties of the D4 receptor are rare and also involved in vitro experiments, using heterologous expression systems in different cell lines, and it is not yet completely clear how D4 expression levels are regulated. We have shown using biochemical and immunofluorescence microscopy in several cell lines and in rat hippocampal primary neurons that D4 receptors neither undergo agonist-promoted downregulation nor internalization, in contrast to β2-adrenergic and D2 receptors [29, 88, 89]. We showed a blunted response to agonist-induced β-arrestin1/2 recruitment and D4 receptor phosphorylation [88]. Furthermore, tandem mass spectrometry of D4 receptor peptides proved the constitutive phosphorylation of Ser 239 and Ser 245 (Fig. 2) [88]. Experiments in HEK293T cells also suggest that the D4 receptor is not strongly regulated through desensitization mechanisms compared with for example β2-adrenergic receptors [18, 90]. Rather than the IC3 polymorphism, the SH3-binding domains of the receptor would be responsible for β-arrestin2/3 recruitment. Previously, Oldenhof et al. demonstrated that by deleting all putative SH3-binding sites, a mutant receptor is generated that shows strong constitutive internalization but can still bind dopamine [43].
Another very important mechanism to control D4 receptor function is the regulation of gene expression. The mechanisms regulating D4 receptor expression levels, however, are far from being understood. A few in vivo studies have been performed in the context of schizophrenia and antipsychotic drug response. Some studies have suggested the upregulation of D4 receptors in the striatum of schizophrenic patients, although it can be debated whether these data reflect true D4 receptor sites [18, 91]. Furthermore, both increases and decreases in D4 mRNA in the frontal cortex of schizophrenics have been found in different studies. Moreover, it is unclear how and to what extent antipsychotic medication can alter D4 receptor expression. Furthermore, these changes can be region-specific. Additionally, it is not clear to what extent changes in mRNA levels affect the expression levels of functional D4 receptors. Regarding biosynthesis of the receptor, we have demonstrated that folding efficiency is rate-limiting in biogenesis of the receptor [29] and that antipsychotics can function as pharmacological chaperones upregulating receptor expression by stabilizing it in the ER [92]. Also dopamine has been shown to be a potent chaperone upon entering the cell through dopamine transporters.
Finally, we stress the importance of DRIPs in the regulation of dopamine receptors, as they act on different levels of receptor activity control, from biosynthesis to desensitization. Some proteins act as chaperones for the proper folding or posttranslational modifications of dopamine receptors and their subsequent export from the ER, such as the ER chaperone calnexin that regulates the export of D1 and D2 receptors to the Golgi complex. Some DRIPs even mediate postendocytic sorting and downregulation; for example, the protein GASP targets internalized D2 receptors to lysosomes [93], and expression of the protein ZIP binds to D2 receptors and has been demonstrated to promote lysosomal degradation of D2 and also D4 receptors [94].
Expression profile
D1- and D2-type receptors are present in all targets of dopamine in the CNS of vertebrates [95]. Although D1 and D2 receptors can be expressed in the same cells, most neurons do not simultaneously express D1 and D2 receptors, or only at very different levels, suggesting that transcription is regulated differently in the two subtypes of receptors. For a detailed summary of tissue distribution of the various subtypes, we refer to Callier et al. [95]. It seems, however, that D4 receptors are much less abundant in the brain than D2 receptors. Moreover, the distribution of D4 receptors shows a significant overlap with that of D2 receptors, suggesting a large degree of redundancy between the two receptor subtypes. Historically, the detection and localization of D4 receptors have been difficult due to the similarity of their pharmacological properties, and the absence of true selective ligands for specific D4 receptor binding (see also below). Furthermore, antibodies against the D4 receptor are often found to be insufficiently selective and sensitive. However, in general, expression levels of D4 receptors seem to be significantly lower than those of D2 receptors. The presence of D4 receptors in the cerebral cortex, amygdala, hippocampus and the striatum has been demonstrated by Northern blot and RT-PCR [17, 96], in situ hybridization [97–99] and ligand binding [100–103]. These findings were mainly confirmed by immunohistochemistry, which showed localization to GABAergic neurons in the cerebral cortex, hippocampus, substantia nigra pars reticulata, globus pallidus, and a subset of cortical pyramidal neurons [74, 100, 104–108]. D4 mRNA has also been detected at high levels in the human retina [96]. Interestingly, the expression of D4 receptors is not exclusive for the CNS. Significant expression levels have been detected in the cardiac atrium, lymphocytes and kidney [73, 109–111].
Pharmacology
Agonist binding probably occurs within a narrow pocket formed by highly conserved residues in the hydrophobic TM domains (Fig. 2). An aspartate residue in TM3 would bind the amine group of catecholamines, whereas two serine residues in TM5 would function as hydrogen bond donors for the catecholamine hydroxyl groups. Finally, a highly conserved phenylalanine residue in TM6 could stabilize the interaction with the aromatic ring of the ligand [112–115].
The pharmacological profile of the D4 receptor is very comparable to that of the D2 and D3 receptors, although specific differences have been detected (see Table 1). The most important feature distinguishing the D4 receptor from D2 and D3 receptors is a higher affinity for clozapine. As clozapine is an important antipsychotic, this observation led to the hypothesis that the D4 receptor may mediate (some) effects of antipsychotics [116]. Raclopride, on the other hand, exhibits much lower affinity for the D4 receptor than for D2 and D3 receptors. Interestingly, the D4 receptor can also be activated by (nor)epinephrine [45, 117, 118]. Although the affinity of the D4 receptor is about tenfold lower for (nor)epinephrine than for dopamine, these compounds may still be relevant for receptor activation under certain conditions [18]. Finally, the pharmacological profiles of the polymorphic variants are not significantly different [22]. In fact, the repeat sequence can be deleted from the receptor without changing its pharmacological profile [22, 42]. These results strongly suggest that there is no direct linear relationship between the length of the polymorphism and functional (see “Signalling by D4 receptors”) or pharmacological activity.
Table 1.
Pharmacological properties of dopamine receptors (Ki values in nanomolar)
| Receptor/ligand | D1 | D5 | D2 | D3 | D4 |
|---|---|---|---|---|---|
| Dopamine | ~2,500 [163] | ~225 [163] | ~500 [164, 165] | ~20–100 [164–166] | Canine brain ~28; D4.2 receptor ~180–400 [175]; D4.4 receptor ~43 [176] |
| Norepinephrine |
~50,000 [163] |
~12,000 [163] |
>10,000 [165] |
n.d. |
Canine striatum ~1,750 [17] |
| Quinpirole |
>10,000 [167] |
>10,000 [167] |
~600–1200 |
~15–45 |
~30–45 |
| Haloperidol |
~25–350 |
~50–175 |
~0.4–2.5 |
~2–10 |
~0.8–20 |
| Raclopride | n.d. | n.d. |
~0.5–2.5 |
~1–2 |
~600 |
| Clozapine |
~150–500 |
~250 |
~40–400 |
~100–500 |
~1.6–55 |
n.d. no data found.
For all D2-like receptors the existence of a G-protein-coupled and uncoupled states (that is a high- and a low-affinity state) have been demonstrated [44, 58, 119].
There are specific compounds that can differentiate D2-like receptors from D1-like receptors, such as quinpirole, N-0437 and PHNO (agonists), and domperidone, nemonapride and (−)-sulpiride (antagonists). Initially, very few specific ligands were available to sufficiently distinguish the D4 receptor from other D2-like receptors. Over the years, however, various D4-selective ligands have been characterized and synthesized, mainly due to the potential role of this receptor as an antipsychotic target (see Table 2). For example L-745,870 is a highly D4 receptor-selective antagonist (about 2,000-fold) that has been used extensively both in vivo and in vitro [52, 120–122]. Some other D4-selective compounds and their main uses and applications are shown in Table 2. For a more general pharmacological profile of all dopamine receptor subtypes, we refer to Missale et al. [28], Oak et al. [18], and Vallone et al. [123].
Table 2.
Selective ligands for the dopamine D4 receptor
| Ligand | Affinity for D4 (nM) | Specific purposes, uses, applications |
|---|---|---|
| L-745,870 | 0.43–0.51 | In vivo administration in rodents for functional, behavioural and drug-dependence studies [177–179]; development of PET radioligands [180]; in D4 receptor-overexpressing cell systems [52, 181]; tests for antipsychotic activity [120, 182] |
| L-741,742 | 3.5 | In vivo administration to mice for anxiety testing [183] |
| ABT 724 | ~45–65 | In vivo administration to rats to test potential treatment of erectile dysfunction [184, 185] |
| WAY 100635 | 16.4 | D4 receptor agonist and 5HT1A antagonist; produces discriminative stimulus effects in rats [186, 187] |
| PD 168077 | 8.7 | Selective D4 agonist, proerectile effect in rats [188]; tested in mice for memory consolidation studies [189] |
| PNU 96415E | 3 | Tested for antipsychotic potential [190] |
| Ro 10-5824 | 5.2 | Selective D4 agonist, increases novel exploration in mice [191] |
| NGD 94-1 | 3 | In vivo administration to monkey for cognitive and memory tests [192]; in vitro autoradiography studies [193, 194] |
| NRA 0160 | 0.5 | Tested for antipsychotic activity in animal models [195, 196] |
| CI 1030 | 4.3 | Tested for antipsychotic activity in animal models [195, 196] |
| A-412997 | 7.9 | D4 receptor agonist; improves cognitive performance and stimulates motor activity in rats [197] |
Physiological role of the D4 receptor in the brain
The precise subcellular localization of a receptor within a cell is very important for the function of the receptor in the cell, especially in highly polarized cells such as neurons. D2 receptors are located both presynaptically (predominantly D2S), where they can inhibit dopamine release by decreasing calcium activity, and postsynaptically (predominantly D2L), where they can activate potassium channels [39, 124–128]. Immunohistochemical studies have demonstrated that the D4 receptor is found mainly (postsynaptically) in dendritic shafts and spines of mammalian striatum [108] and, by projecting back to the substantia nigra, may control dopaminergic transmission.
In an accepted model for dopamine signalling states dopamine is considered not to be a simple excitatory or inhibitory neurotransmitter, and not to mediate fast synaptic transmission in the CNS, but rather to modulate it. Therefore, dopamine is referred to as a mediator of ‘slow synaptic transmission’ that alters the responses of target neurons to other neurotransmitters, thus altering synaptic plasticity. Dopamine controls the activity of glutamate receptors (two major subtypes are NMDA and AMPA receptors) that mediate corticostriatal neurotransmission, for example through binding the D4 receptor subtype. Furthermore, dopamine regulates the activity of several voltage-gated ion channels and transcription factors, which in turn activate transcription of early and late genes that are essential for the long-lasting effects of dopamine on synaptic transmission. Very recent data also suggest that the D2-like receptors themselves are voltage-dependent, demonstrating that the membrane potential could influence the potency with which dopamine is able to activate the receptor [129, 130]. These observations suggest a function for D2-like receptor in activity-dependent regulation of synaptic strength. It has recently been suggested that the D4 receptor has the unique ability to carry out phospholipid methylation that can affect the kinetics of ion channels [131, 132]. This mechanism may be important for modulation of neuronal firing activity, where impaired methylation can contribute to disorders of attention.
Another tool for studying the in vivo role of dopamine receptors is the use of genetically modified mice and, although the deletion of one particular gene may indicate redundancy, a hint of the functional role of the deleted gene can be obtained. In D4 receptor mutant mice, locomotion [28, 123, 133] and behavioural response to novelty [134, 135] are reduced. These findings are of great interest, as previous studies suggested an association between the D4 VNTR polymorphism (the seven-repeat alleles) and novelty-seeking behaviour [136, 137]. However, other studies failed to replicate these findings and also a recently performed meta-analysis did not find a link between the VNTR of the D4 receptor and novelty seeking [138]. Nevertheless, the same study demonstrated an association with the C-521T polymorphism of the D4 receptor. Finally, D4 receptor mutant mice show enhanced activity of cortical pyramidal neurons, and experiments with D4-selective pharmacological agents indicated that the receptor plays an inhibitory role in frontal cortex glutamatergic activity [139], which was confirmed in later studies showing reduced NMDA and AMPA signalling upon D4 receptor activation (see above).
Several drugs of abuse, including cocaine, opiates, amphetamines and alcohol, generate psychomotor effects and activate reward mechanisms in the brain, in which the mesolimbic dopaminergic system plays an important role (see above). Cocaine and amphetamines, for example, increase dopamine levels in the synaptic cleft by blocking the activity of the dopamine transporter. It has been demonstrated that by inhibiting dopamine receptors (via antagonists), hyperlocomotion and the reward effects of cocaine and amphetamines in rats and mice can be attenuated. On the contrary, D2-like agonists mimic the effects of these drugs of abuse. Experiments with dopamine receptor null mice have revealed some more interesting observations. D4 null mice, for example, appear to become more sensitive to the stimulation of locomotor activity induced by ethanol, cocaine and methamphetamine. These results are surprising in view of the hypoactive locomotor phenotype of these mice [140]. The underlying molecular mechanisms of the physiological response to drugs of abuse are not completely clear. It is, however, appreciated that different drugs increase dopamine release in the nucleus accumbens, leading to an overstimulation of dopamine receptors. The challenging task that lies ahead is thus the identification of the genes whose expression is modulated by dopamine receptors in response to drugs of abuse.
Dopamine-related diseases
A profound overview of this topic is beyond the scope of this review, so we only briefly mention some diseases in which D4 receptors may play a role.
ADHD is predominantly a condition of childhood, and is characterized by symptoms such as inattention, hyperactivity and distraction. ADHD is thought to affect up to 6% of children [141], and evidence from family data and twin studies suggests that ADHD is familial and heritable [141–143]. It is commonly accepted that dysfunction of the dopamine system lies at the basis of the disorder, although norepinephrine and serotonin systems have also been suggested to play a role [123]. Conventional treatment of ADHD generally involves the administration of psychostimulant compounds, such as methylphenidate (Ritalin) or d-amphetamine. Whereas methylphenidate blocks the reuptake of dopamine by the dopamine transporter and releases dopamine from vesicle stores, d-amphetamine causes dopamine release by reversal of the dopamine transporter function. Although these drugs stimulate dopaminergic activity in normal individuals, they exert a calming effect in ADHD patients [123]. A great deal of interest in the human dopamine D4 receptor was generated by several studies that found an association between ADHD and D4 receptor gene polymorphism, more specifically the seven-repeat allele [144–147]. In contrast, other studies did not find an association between D4 receptor polymorphism and ADHD [148]. Later, a meta-analysis obtained strong evidence that the seven-repeat allele confers an increased risk for the development of ADHD, whereas the four-allele is protective [14]. Besides its association with ADHD, the seven-repeat allele has been associated with other complex behaviours including Tourette’s syndrome and the personality trait of novelty seeking [136, 137]. Other studies, however, failed to confirm this association [149–151] and a recent meta-analysis also did not support it [138]. Nevertheless, the meta-analysis revealed an association between novelty seeking and another polymorphism, specifically C-521T, a single nucleotide polymorphism in the promoter region of the D4 receptor. Interestingly, the T allele is associated with a significant reduction in transcription levels compared with the C allele [152, 153].
The D4 receptor has also been linked with schizophrenia, a complex neuropsychiatric disorder, associated with alterations in cognitive and emotional functioning, and involves positive symptoms (psychosis, hallucinations, delusions and paranoia) and negative symptoms (loss of energy and motivation, slowed speech) [116]. Although several dopamine-related genes have been implicated as risk factors for the development of schizophrenia [154], there is accumulating evidence for several other candidates, including dysbindin, neuregulin 1, dystrobrevin-binding protein 1 (DNTBP1) (reviewed in references [155] and [156]). The observation that D2 receptors could be blocked (antagonized) by classical neuroleptic compounds led to the theory that dopamine plays an essential role in the pathogenesis of schizophrenia. A series of promising reports generated a great deal of interest in the potential role of the D4 receptor in schizophrenia and in the development of new therapeutics: (1) elevated D4 receptor density in post-mortem brains from schizophrenic patients [157] (although other studies failed to confirm this finding), and (2) the antipsychotic clozapine exhibits higher affinity for D4 receptors than for D2 or D3 receptors [17, 19, 22]. However, it was proved that the antipsychotic activity of clozapine cannot be merely explained by its combined serotonin/D4 receptor antagonist properties. Furthermore, many searches for possible associations between schizophrenia and the D4 gene polymorphism have not led to strong links [151, 158, 159], and genetic studies too have failed to demonstrate a correlation between D4 polymorphism and the clozapine response [160, 161]. However, a lot of evidence supports the hypothesis that a reduction in positive symptoms following treatment with antipsychotics is mediated through blockade of D2 receptors. Finally, although exclusive blockade at the D4 receptor may not be sufficient for antipsychotic action, it might result in an improved symptomatic profile in combination with D2 receptor blockade [116, 162].
Conclusion
Our understanding of the biochemical and signalling properties of the dopamine D4 receptor has advanced over the past several years. More insight has been obtained into receptor signalling, regulation, internalization, dimerization, and posttranslational modifications. A major step forward was the identification of interacting proteins that modulate the activity of specific processes.
Many genetic studies have shown the involvement of the VNTR polymorphism of the dopamine D4 receptor in the development of ADHD although pharmacological studies have not revealed a profound difference between the polymorphic D4 receptor variants. Physiological and biochemical comparison of the variants need to be further clarified in the future and this will also lead to a better understanding of the signalling properties of the dopamine D4 receptor.
Acknowledgments
The dopamine D4 receptor work was financially supported by FWO (Fonds voor Wetenschappelijk Onderzoek) Vlaanderen (project no. G010909N). Kathleen Van Craenenbroeck has a post-doctoral FWO fellowship.
References
- 1.Iversen SD, Iversen LL. Dopamine: 50 years in perspective. Trends Neurosci. 2007;30:188–193. doi: 10.1016/j.tins.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Olanow CW, Lees A, Obeso J. Levodopa therapy for Parkinson’s disease: challenges and future prospects. Mov Disord. 2008;23(Suppl 3):S495–S496. doi: 10.1002/mds.22048. [DOI] [PubMed] [Google Scholar]
- 3.Girault JA, Greengard P. The neurobiology of dopamine signaling. Arch Neurol. 2004;61:641–644. doi: 10.1001/archneur.61.5.641. [DOI] [PubMed] [Google Scholar]
- 4.Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–1161. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
- 5.Dobrossy MD, Dunnett SB. The influence of environment and experience on neural grafts. Nat Rev Neurosci. 2001;2:871–879. doi: 10.1038/35104055. [DOI] [PubMed] [Google Scholar]
- 6.Faure A, Haberland U, Conde F, El Massioui N. Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation. J Neurosci. 2005;25:2771–2780. doi: 10.1523/JNEUROSCI.3894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002;417:282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- 8.Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-J. [DOI] [PubMed] [Google Scholar]
- 9.Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/S0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- 10.Sawaguchi T, Goldmanrakic PS. D1 dopamine-receptors in prefrontal cortex – involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- 11.Sawaguchi T, Goldmanrakic PS. The role of D1-dopamine receptor in working-memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus-monkeys performing an oculomotor delayed-response task. J Neurophysiol. 1994;71:515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- 12.Wilkerson A, Levin ED. Ventral hippocampal dopamine D-1 and D-2 systems and spatial working memory in rats. Neuroscience. 1999;89:743–749. doi: 10.1016/S0306-4522(98)00346-7. [DOI] [PubMed] [Google Scholar]
- 13.Williams GV, Goldmanrakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 14.Li D, Sham PC, Owen MJ, He L. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD) Hum Mol Genet. 2006;15:2276–2284. doi: 10.1093/hmg/ddl152. [DOI] [PubMed] [Google Scholar]
- 15.Kebabian JW, Greengard P. Dopamine-sensitive adenyl cyclase: possible role in synaptic transmission. Science. 1971;174:1346–1349. doi: 10.1126/science.174.4016.1346. [DOI] [PubMed] [Google Scholar]
- 16.Bunzow JR, Van Tol HH, Grandy DK, Albert P, Salon J, Christie M, Machida CA, Neve KA, Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988;336:783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- 17.Van Tol HH, Bunzow JR, Guan HC, Sunahara RK, Seeman P, Niznik HB, Civelli O. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991;350:610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- 18.Oak JN, Oldenhof J, Van Tol HH. The dopamine D(4) receptor: one decade of research. Eur J Pharmacol. 2000;405:303–327. doi: 10.1016/S0014-2999(00)00562-8. [DOI] [PubMed] [Google Scholar]
- 19.Van Tol HH, Wu CM, Guan HC, Ohara K, Bunzow JR, Civelli O, Kennedy J, Seeman P, Niznik HB, Jovanovic V. Multiple dopamine D4 receptor variants in the human population. Nature. 1992;358:149–152. doi: 10.1038/358149a0. [DOI] [PubMed] [Google Scholar]
- 20.Kamakura S, Iwaki A, Matsumoto M, Fukumaki Y. Cloning and characterization of the 5′-flanking region of the human dopamine D4 receptor gene. Biochem Biophys Res Commun. 1997;235:321–326. doi: 10.1006/bbrc.1997.6770. [DOI] [PubMed] [Google Scholar]
- 21.Ding YC, Chi HC, Grady DL, Morishima A, Kidd JR, Kidd KK, Flodman P, Spence MA, Schuck S, Swanson JM, Zhang YP, Moyzis RK. Evidence of positive selection acting at the human dopamine receptor D4 gene locus. Proc Natl Acad Sci USA. 2002;99:309–314. doi: 10.1073/pnas.012464099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asghari V, Schoots O, van Kats S, Ohara K, Jovanovic V, Guan HC, Bunzow JR, Petronis A, Van Tol HH. Dopamine D4 receptor repeat: analysis of different native and mutant forms of the human and rat genes. Mol Pharmacol. 1994;46:364–373. [PubMed] [Google Scholar]
- 23.Lichter JB, Barr CL, Kennedy JL, Van Tol HH, Kidd KK, Livak KJ. A hypervariable segment in the human dopamine receptor D4 (DRD4) gene. Hum Mol Genet. 1993;2:767–773. doi: 10.1093/hmg/2.6.767. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Rogers J, Lichter JB. Variability of dopamine D4 receptor (DRD4) gene sequence within and among nonhuman primate species. Proc Natl Acad Sci USA. 1995;92:427–431. doi: 10.1073/pnas.92.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang FM, Kidd JR, Livak KJ, Pakstis AJ, Kidd KK. The world-wide distribution of allele frequencies at the human dopamine D4 receptor locus. Hum Genet. 1996;98:91–101. doi: 10.1007/s004390050166. [DOI] [PubMed] [Google Scholar]
- 26.Civelli O, Bunzow JR, Grandy DK. Molecular diversity of the dopamine receptors. Annu Rev Pharmacol Toxicol. 1993;33:281–307. doi: 10.1146/annurev.pa.33.040193.001433. [DOI] [PubMed] [Google Scholar]
- 27.Lanau F, Brockhaus M, Pink JR, Franchet C, Wildt-Perinic D, Goepfert C, Probst A, Hartman DS. Development and characterization of antibodies against the N terminus of the human dopamine D4 receptor. J Neurochem. 1997;69:2169–2178. doi: 10.1046/j.1471-4159.1997.69052169.x. [DOI] [PubMed] [Google Scholar]
- 28.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 29.Van Craenenbroeck K, Clark SD, Cox MJ, Oak JN, Liu F, Van Tol HH. Folding efficiency is rate-limiting in dopamine D4 receptor biogenesis. J Biol Chem. 2005;280:19350–19357. doi: 10.1074/jbc.M414043200. [DOI] [PubMed] [Google Scholar]
- 30.O’Dowd BF, Hnatowich M, Caron MG, Lefkowitz RJ, Bouvier M. Palmitoylation of the human beta 2-adrenergic receptor. Mutation of Cys341 in the carboxyl tail leads to an uncoupled nonpalmitoylated form of the receptor. J Biol Chem. 1989;264:7564–7569. [PubMed] [Google Scholar]
- 31.Ovchinnikov YuA, Abdulaev NG, Bogachuk AS. Two adjacent cysteine residues in the C-terminal cytoplasmic fragment of bovine rhodopsin are palmitylated. FEBS Lett. 1988;230:1–5. doi: 10.1016/0014-5793(88)80628-8. [DOI] [PubMed] [Google Scholar]
- 32.Grunewald S, Haase W, Reilander H, Michel H. Glycosylation, palmitoylation, and localization of the human D2S receptor in baculovirus-infected insect cells. Biochemistry. 1996;35:15149–15161. doi: 10.1021/bi9607564. [DOI] [PubMed] [Google Scholar]
- 33.Ng GY, O’Dowd BF, Caron M, Dennis M, Brann MR, George SR. Phosphorylation and palmitoylation of the human D2L dopamine receptor in Sf9 cells. J Neurochem. 1994;63:1589–1595. doi: 10.1046/j.1471-4159.1994.63051589.x. [DOI] [PubMed] [Google Scholar]
- 34.Dohlman HG, Caron MG, DeBlasi A, Frielle T, Lefkowitz RJ. Role of extracellular disulfide-bonded cysteines in the ligand binding function of the beta 2-adrenergic receptor. Biochemistry. 1990;29:2335–2342. doi: 10.1021/bi00461a018. [DOI] [PubMed] [Google Scholar]
- 35.Hwa J, Garriga P, Liu X, Khorana HG. Structure and function in rhodopsin: packing of the helices in the transmembrane domain and folding to a tertiary structure in the intradiscal domain are coupled. Proc Natl Acad Sci USA. 1997;94:10571–10576. doi: 10.1073/pnas.94.20.10571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pangalos MN, Davies CH (2002) Understanding G protein-coupled receptors and their role in the CNS. In: Molecular and cellular neurobiology. Oxford University Press, London
- 37.Helmreich EJ, Hofmann KP. Structure and function of proteins in G-protein-coupled signal transfer. Biochim Biophys Acta. 1996;1286:285–322. doi: 10.1016/s0304-4157(96)00013-5. [DOI] [PubMed] [Google Scholar]
- 38.Sidhu A, Niznik HB. Coupling of dopamine receptor subtypes to multiple and diverse G proteins. Int J Dev Neurosci. 2000;18:669–677. doi: 10.1016/S0736-5748(00)00033-2. [DOI] [PubMed] [Google Scholar]
- 39.Lane JR, Powney B, Wise A, Rees S, Milligan G. G protein coupling and ligand selectivity of the D2L and D3 dopamine receptors. J Pharmacol Exp Ther. 2008;325:319–330. doi: 10.1124/jpet.107.134296. [DOI] [PubMed] [Google Scholar]
- 40.Herve D, Levi-Strauss M, Marey-Semper I, Verney C, Tassin JP, Glowinski J, Girault JA. G(olf) and Gs in rat basal ganglia: possible involvement of G(olf) in the coupling of dopamine D1 receptor with adenylyl cyclase. J Neurosci. 1993;13:2237–2248. doi: 10.1523/JNEUROSCI.13-05-02237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/RRS-200029981. [DOI] [PubMed] [Google Scholar]
- 42.Kazmi MA, Snyder LA, Cypess AM, Graber SG, Sakmar TP. Selective reconstitution of human D4 dopamine receptor variants with Gi alpha subtypes. Biochemistry. 2000;39:3734–3744. doi: 10.1021/bi992354c. [DOI] [PubMed] [Google Scholar]
- 43.Oldenhof J, Vickery R, Anafi M, Oak J, Ray A, Schoots O, Pawson T, von Zastrow M, Van Tol HH. SH3 binding domains in the dopamine D4 receptor. Biochemistry. 1998;37:15726–15736. doi: 10.1021/bi981634+. [DOI] [PubMed] [Google Scholar]
- 44.Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem. 1995;65:1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- 45.Jovanovic V, Guan HC, Van Tol HH. Comparative pharmacological and functional analysis of the human dopamine D4.2 and D4.10 receptor variants. Pharmacogenetics. 1999;9:561–568. doi: 10.1097/00008571-199910000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Hemmings HC, Greengard P, Tung HYL, Cohen P. Darpp-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984;310:503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- 47.Le Novere N, Li L, Girault JA. DARPP-32: molecular integration of phosphorylation potential. Cell Mol Life Sci. 2008;65:2125–2127. doi: 10.1007/s00018-008-8150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhen X, Zhang J, Johnson GP, Friedman E. D(4) dopamine receptor differentially regulates Akt/nuclear factor-kappa b and extracellular signal-regulated kinase pathways in D(4)MN9D cells. Mol Pharmacol. 2001;60:857–864. [PubMed] [Google Scholar]
- 49.Sarkar C, Das S, Chakroborty D, Chowdhury UR, Basu B, Dasgupta PS, Basu S. Cutting edge: stimulation of dopamine D4 receptors induce T cell quiescence by up-regulating Kruppel-like factor-2 expression through inhibition of ERK1/ERK2 phosphorylation. J Immunol. 2006;177:7525–7529. doi: 10.4049/jimmunol.177.11.7525. [DOI] [PubMed] [Google Scholar]
- 50.Bitner RS, Nikkel AL, Otte S, Martino B, Barlow EH, Bhatia P, Stewart AO, Brioni JD, Decker MW, Moreland RB. Dopamine D4 receptor signaling in the rat paraventricular hypothalamic nucleus: evidence of natural coupling involving immediate early gene induction and mitogen activated protein kinase phosphorylation. Neuropharmacology. 2006;50:521–531. doi: 10.1016/j.neuropharm.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 51.Lavine N, Ethier N, Oak JN, Pei L, Liu F, Trieu P, Rebois RV, Bouvier M, Hebert TE, Van Tol HH. G protein-coupled receptors form stable complexes with inwardly rectifying potassium channels and adenylyl cyclase. J Biol Chem. 2002;277:46010–46019. doi: 10.1074/jbc.M205035200. [DOI] [PubMed] [Google Scholar]
- 52.Pillai G, Brown NA, McAllister G, Milligan G, Seabrook GR. Human D2 and D4 dopamine receptors couple through betagamma G-protein subunits to inwardly rectifying K+ channels (GIRK1) in a Xenopus oocyte expression system: selective antagonism by L-741,626 and L-745,870 respectively. Neuropharmacology. 1998;37:983–987. doi: 10.1016/S0028-3908(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 53.Werner P, Hussy N, Buell G, Jones KA, North RA. D2, D3, and D4 dopamine receptors couple to G protein-regulated potassium channels in Xenopus oocytes. Mol Pharmacol. 1996;49:656–661. [PubMed] [Google Scholar]
- 54.Wedemeyer C, Goutman JD, Avale ME, Franchini LF, Rubinstein M, Calvo DJ. Functional activation by central monoamines of human dopamine D(4) receptor polymorphic variants coupled to GIRK channels in Xenopus oocytes. Eur J Pharmacol. 2007;562:165–173. doi: 10.1016/j.ejphar.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 55.Liu LX, Burgess LH, Gonzalez AM, Sibley DR, Chiodo LA. D2S, D2L, D3, and D4 dopamine receptors couple to a voltage-dependent potassium current in N18TG2 x mesencephalon hybrid cell (MES-23.5) via distinct G proteins. Synapse. 1999;31:108–118. doi: 10.1002/(SICI)1098-2396(199902)31:2<108::AID-SYN3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 56.Wilke RA, Hsu SF, Jackson MB. Dopamine D4 receptor mediated inhibition of potassium current in neurohypophysial nerve terminals. J Pharmacol Exp Ther. 1998;284:542–548. [PubMed] [Google Scholar]
- 57.Piomelli D, Pilon C, Giros B, Sokoloff P, Martres MP, Schwartz JC. Dopamine activation of the arachidonic acid cascade as a basis for D1/D2 receptor synergism. Nature. 1991;353:164–167. doi: 10.1038/353164a0. [DOI] [PubMed] [Google Scholar]
- 58.Chio CL, Drong RF, Riley DT, Gill GS, Slightom JL, Huff RM. D4 dopamine receptor-mediated signaling events determined in transfected Chinese hamster ovary cells. J Biol Chem. 1994;269:11813–11819. [PubMed] [Google Scholar]
- 59.Felder CC, Campbell T, Albrecht F, Jose PA. Dopamine inhibits Na(+)–H+ exchanger activity in renal BBMV by stimulation of adenylate cyclase. Am J Physiol. 1990;259:F297–F303. doi: 10.1152/ajprenal.1990.259.2.F297. [DOI] [PubMed] [Google Scholar]
- 60.Coldwell MC, Boyfield I, Brown AM, Stemp G, Middlemiss DN. Pharmacological characterization of extracellular acidification rate responses in human D2(long), D3 and D4.4 receptors expressed in Chinese hamster ovary cells. Br J Pharmacol. 1999;127:1135–1144. doi: 10.1038/sj.bjp.0702657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lajiness ME, Chio CL, Huff RM. D2 dopamine receptor stimulation of mitogenesis in transfected Chinese hamster ovary cells: relationship to dopamine stimulation of tyrosine phosphorylations. J Pharmacol Exp Ther. 1993;267:1573–1581. [PubMed] [Google Scholar]
- 62.Narkar VA, Hussain T, Pedemonte C, Lokhandwala MF. Dopamine D(2) receptor activation causes mitogenesis via p44/42 mitogen-activated protein kinase in opossum kidney cells. J Am Soc Nephrol. 2001;12:1844–1852. doi: 10.1681/ASN.V1291844. [DOI] [PubMed] [Google Scholar]
- 63.Oak JN, Lavine N, Van Tol HH. Dopamine D(4) and D(2L) receptor stimulation of the mitogen-activated protein kinase pathway is dependent on trans-activation of the platelet-derived growth factor receptor. Mol Pharmacol. 2001;60:92–103. doi: 10.1124/mol.60.1.92. [DOI] [PubMed] [Google Scholar]
- 64.Wang C, Buck DC, Yang R, Macey TA, Neve KA. Dopamine D2 receptor stimulation of mitogen-activated protein kinases mediated by cell type-dependent transactivation of receptor tyrosine kinases. J Neurochem. 2005;93:899–909. doi: 10.1111/j.1471-4159.2005.03055.x. [DOI] [PubMed] [Google Scholar]
- 65.Gill RS, Hsiung MS, Sum CS, Lavine N, Clark SD, Van Tol HH. The dopamine D4 receptor activates intracellular platelet-derived growth factor receptor beta to stimulate ERK1/2. Cell Signal. 2010;22:285–290. doi: 10.1016/j.cellsig.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 66.Kotecha SA, Oak JN, Jackson MF, Perez Y, Orser BA, Van Tol HH, MacDonald JF. A D2 class dopamine receptor transactivates a receptor tyrosine kinase to inhibit NMDA receptor transmission. Neuron. 2002;35:1111–1122. doi: 10.1016/S0896-6273(02)00859-0. [DOI] [PubMed] [Google Scholar]
- 67.Ferguson SS. Receptor tyrosine kinase transactivation: fine-tuning synaptic transmission. Trends Neurosci. 2003;26:119–122. doi: 10.1016/S0166-2236(03)00022-5. [DOI] [PubMed] [Google Scholar]
- 68.Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs. 2009;23(Suppl 1):33–41. doi: 10.2165/00023210-200923000-00005. [DOI] [PubMed] [Google Scholar]
- 69.de Almeida J, Palacios JM, Mengod G. Distribution of 5-HT and DA receptors in primate prefrontal cortex: implications for pathophysiology and treatment. Prog Brain Res. 2008;172:101–115. doi: 10.1016/S0079-6123(08)00905-9. [DOI] [PubMed] [Google Scholar]
- 70.Jung MW, Baeg EH, Kim MJ, Kim YB, Kim JJ. Plasticity and memory in the prefrontal cortex. Rev Neurosci. 2008;19:29–46. doi: 10.1515/revneuro.2008.19.1.29. [DOI] [PubMed] [Google Scholar]
- 71.Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-band activity during the delay of a visual short-term memory task in humans. J Neurosci. 1998;18:4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yuen EY, Yan Z. Dopamine D4 receptors regulate AMPA receptor trafficking and glutamatergic transmission in GABAergic interneurons of prefrontal cortex. J Neurosci. 2009;29:550–562. doi: 10.1523/JNEUROSCI.5050-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 74.Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature. 1996;381:245–248. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- 75.Wang X, Zhong P, Yan Z. Dopamine D4 receptors modulate GABAergic signaling in pyramidal neurons of prefrontal cortex. J Neurosci. 2002;22:9185–9193. doi: 10.1523/JNEUROSCI.22-21-09185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Graziane NM, Yuen EY, Yan Z. Dopamine D4 receptors regulate GABAA receptor trafficking via an actin/cofilin/myosin-dependent mechanism. J Biol Chem. 2009;284:8329–8336. doi: 10.1074/jbc.M807387200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Milligan G, White JH. Protein-protein interactions at G-protein-coupled receptors. Trends Pharmacol Sci. 2001;22:513–518. doi: 10.1016/S0165-6147(00)01801-0. [DOI] [PubMed] [Google Scholar]
- 78.Bockaert J, Fagni L, Dumuis A, Marin P. GPCR interacting proteins (GIP) Pharmacol Ther. 2004;103:203–221. doi: 10.1016/j.pharmthera.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 79.Hall RA, Lefkowitz RJ. Regulation of G protein-coupled receptor signaling by scaffold proteins. Circ Res. 2002;91:672–680. doi: 10.1161/01.RES.0000037000.74258.03. [DOI] [PubMed] [Google Scholar]
- 80.Sun Y, McGarrigle D, Huang XY. When a G protein-coupled receptor does not couple to a G protein. Mol Biosyst. 2007;3:849–854. doi: 10.1039/b706343a. [DOI] [PubMed] [Google Scholar]
- 81.Bergson C, Levenson R, Goldman-Rakic PS, Lidow MS. Dopamine receptor-interacting proteins: the Ca(2+) connection in dopamine signaling. Trends Pharmacol Sci. 2003;24:486–492. doi: 10.1016/S0165-6147(03)00232-3. [DOI] [PubMed] [Google Scholar]
- 82.Rondou P, Haegeman G, Vanhoenacker P, Van Craenenbroeck K. BTB protein KLHL12 targets the dopamine D4 receptor for ubiquitination by a Cul3-based E3 ligase. J Biol Chem. 2008;283:11083–11096. doi: 10.1074/jbc.M708473200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yao WD, Spealman RD, Zhang J. Dopaminergic signaling in dendritic spines. Biochem Pharmacol. 2008;75:2055–2069. doi: 10.1016/j.bcp.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- 85.Kim KM, Valenzano KJ, Robinson SR, Yao WD, Barak LS, Caron MG. Differential regulation of the dopamine D2 and D3 receptors by G protein-coupled receptor kinases and beta-arrestins. J Biol Chem. 2001;276:37409–37414. doi: 10.1074/jbc.M106728200. [DOI] [PubMed] [Google Scholar]
- 86.Macey TA, Gurevich VV, Neve KA. Preferential Interaction between the dopamine D2 receptor and Arrestin2 in neostriatal neurons. Mol Pharmacol. 2004;66:1635–1642. doi: 10.1124/mol.104.001495. [DOI] [PubMed] [Google Scholar]
- 87.Vickery RG, von Zastrow M. Distinct dynamin-dependent and -independent mechanisms target structurally homologous dopamine receptors to different endocytic membranes. J Cell Biol. 1999;144:31–43. doi: 10.1083/jcb.144.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Spooren A, Rondou P, Debowska K, Lintermans B, Vermeulen L, Samyn B, Skieterska K, Debyser G, Devreese B, Vanhoenacker P, Wojda U, Haegeman G, Van Craenenbroeck K. Resistance of the dopamine D4 receptor to agonist-induced internalization and degradation. Cell Signal. 2010;22:600–609. doi: 10.1016/j.cellsig.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 89.Cho DI, Beom S, Van Tol HH, Caron MG, Kim KM. Characterization of the desensitization properties of five dopamine receptor subtypes and alternatively spliced variants of dopamine D2 and D4 receptors. Biochem Biophys Res Commun. 2006;350:634–640. doi: 10.1016/j.bbrc.2006.09.090. [DOI] [PubMed] [Google Scholar]
- 90.Watts VJ, Vu MN, Wiens BL, Jovanovic V, Van Tol HH, Neve KA. Short- and long-term heterologous sensitization of adenylate cyclase by D4 dopamine receptors. Psychopharmacology (Berl) 1999;141:83–92. doi: 10.1007/s002130050810. [DOI] [PubMed] [Google Scholar]
- 91.Helmeste DM, Tang SW. Dopamine D4 receptors. Jpn J Pharmacol. 2000;82:1–14. doi: 10.1254/jjp.82.1. [DOI] [PubMed] [Google Scholar]
- 92.Van Craenenbroeck K, Gellynck E, Lintermans B, Leysen JE, Van Tol HH, Haegeman G, Vanhoenacker P. Influence of the antipsychotic drug pipamperone on the expression of the dopamine D4 receptor. Life Sci. 2006;80:74–81. doi: 10.1016/j.lfs.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 93.Bartlett SE, Enquist J, Hopf FW, Lee JH, Gladher F, Kharazia V, Waldhoer M, Mailliard WS, Armstrong R, Bonci A, Whistler JL. Dopamine responsiveness is regulated by targeted sorting of D2 receptors. Proc Natl Acad Sci USA. 2005;102:11521–11526. doi: 10.1073/pnas.0502418102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim OJ, Ariano MA, Namkung Y, Marinec P, Kim E, Han J, Sibley DR. D2 dopamine receptor expression and trafficking is regulated through direct interactions with ZIP. J Neurochem. 2008;106:83–95. doi: 10.1111/j.1471-4159.2008.05348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Callier S, Snapyan M, Le Crom S, Prou D, Vincent JD, Vernier P. Evolution and cell biology of dopamine receptors in vertebrates. Biol Cell. 2003;95:489–502. doi: 10.1016/S0248-4900(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 96.Matsumoto M, Hidaka K, Tada S, Tasaki Y, Yamaguchi T. Full-length cDNA cloning and distribution of human dopamine D4 receptor. Brain Res Mol Brain Res. 1995;29:157–162. doi: 10.1016/0169-328X(94)00245-A. [DOI] [PubMed] [Google Scholar]
- 97.Lidow MS, Wang F, Cao Y, Goldman-Rakic PS. Layer V neurons bear the majority of mRNAs encoding the five distinct dopamine receptor subtypes in the primate prefrontal cortex. Synapse. 1998;28:10–20. doi: 10.1002/(SICI)1098-2396(199801)28:1<10::AID-SYN2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 98.Meador-Woodruff JH, Grandy DK, Van Tol HH, Damask SP, Little KY, Civelli O, Watson SJ., Jr Dopamine receptor gene expression in the human medial temporal lobe. Neuropsychopharmacology. 1994;10:239–248. doi: 10.1038/npp.1994.27. [DOI] [PubMed] [Google Scholar]
- 99.Meador-Woodruff JH, Haroutunian V, Powchik P, Davidson M, Davis KL, Watson SJ. Dopamine receptor transcript expression in striatum and prefrontal and occipital cortex: focal abnormalities in orbitofrontal cortex in schizophrenia. Arch Gen Psychiatry. 1997;54:1089–1095. doi: 10.1001/archpsyc.1997.01830240045007. [DOI] [PubMed] [Google Scholar]
- 100.Defagot MC, Antonelli MC. Autoradiographic localization of the putative D4 dopamine receptor in rat brain. Neurochem Res. 1997;22:401–407. doi: 10.1023/A:1027399408608. [DOI] [PubMed] [Google Scholar]
- 101.Defagot MC, Falzone TL, Low MJ, Grandy DK, Rubinstein M, Antonelli MC. Quantitative analysis of the dopamine D4 receptor in the mouse brain. J Neurosci Res. 2000;59:202–208. doi: 10.1002/(SICI)1097-4547(20000115)59:2<202::AID-JNR6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 102.Primus RJ, Thurkauf A, Xu J, Yevich E, McInerney S, Shaw K, Tallman JF, Gallagher DW. II. Localization and characterization of dopamine D4 binding sites in rat and human brain by use of the novel, D4 receptor-selective ligand [3H]NGD 94-1. J Pharmacol Exp Ther. 1997;282:1020–1027. [PubMed] [Google Scholar]
- 103.Tarazi FI, Kula NS, Baldessarini RJ. Regional distribution of dopamine D4 receptors in rat forebrain. Neuroreport. 1997;8:3423–3426. doi: 10.1097/00001756-199711100-00001. [DOI] [PubMed] [Google Scholar]
- 104.Ariano MA, Wang J, Noblett KL, Larson ER, Sibley DR. Cellular distribution of the rat D4 dopamine receptor protein in the CNS using anti-receptor antisera. Brain Res. 1997;752:26–34. doi: 10.1016/S0006-8993(96)01422-9. [DOI] [PubMed] [Google Scholar]
- 105.Defagot MC, Malchiodi EL, Villar MJ, Antonelli MC. Distribution of D4 dopamine receptor in rat brain with sequence-specific antibodies. Brain Res Mol Brain Res. 1997;45:1–12. doi: 10.1016/S0169-328X(96)00235-5. [DOI] [PubMed] [Google Scholar]
- 106.Khan ZU, Gutierrez A, Martin R, Penafiel A, Rivera A, De La Calle A. Differential regional and cellular distribution of dopamine D2-like receptors: an immunocytochemical study of subtype-specific antibodies in rat and human brain. J Comp Neurol. 1998;402:353–371. doi: 10.1002/(SICI)1096-9861(19981221)402:3<353::AID-CNE5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 107.Mauger C, Sivan B, Brockhaus M, Fuchs S, Civelli O, Monsma F., Jr Development and characterization of antibodies directed against the mouse D4 dopamine receptor. Eur J Neurosci. 1998;10:529–537. doi: 10.1046/j.1460-9568.1998.00056.x. [DOI] [PubMed] [Google Scholar]
- 108.Rivera A, Cuellar B, Giron FJ, Grandy DK, de la Calle A, Moratalla R. Dopamine D4 receptors are heterogeneously distributed in the striosomes/matrix compartments of the striatum. J Neurochem. 2002;80:219–229. doi: 10.1046/j.0022-3042.2001.00702.x. [DOI] [PubMed] [Google Scholar]
- 109.Bondy B, de Jonge S, Pander S, Primbs J, Ackenheil M. Identification of dopamine D4 receptor mRNA in circulating human lymphocytes using nested polymerase chain reaction. J Neuroimmunol. 1996;71:139–144. doi: 10.1016/S0165-5728(96)00148-8. [DOI] [PubMed] [Google Scholar]
- 110.O’Malley KL, Harmon S, Tang L, Todd RD. The rat dopamine D4 receptor: sequence, gene structure, and demonstration of expression in the cardiovascular system. New Biol. 1992;4:137–146. [PubMed] [Google Scholar]
- 111.Sun D, Wilborn TW, Schafer JA. Dopamine D4 receptor isoform mRNA and protein are expressed in the rat cortical collecting duct. Am J Physiol. 1998;275:F742–F751. doi: 10.1152/ajprenal.1998.275.5.F742. [DOI] [PubMed] [Google Scholar]
- 112.Cox BA, Henningsen RA, Spanoyannis A, Neve RL, Neve KA. Contributions of conserved serine residues to the interactions of ligands with dopamine D2 receptors. J Neurochem. 1992;59:627–635. doi: 10.1111/j.1471-4159.1992.tb09416.x. [DOI] [PubMed] [Google Scholar]
- 113.Mansour A, Meng F, Meador-Woodruff JH, Taylor LP, Civelli O, Akil H. Site-directed mutagenesis of the human dopamine D2 receptor. Eur J Pharmacol. 1992;227:205–214. doi: 10.1016/0922-4106(92)90129-J. [DOI] [PubMed] [Google Scholar]
- 114.Neve KA, Cox BA, Henningsen RA, Spanoyannis A, Neve RL. Pivotal role for aspartate-80 in the regulation of dopamine D2 receptor affinity for drugs and inhibition of adenylyl cyclase. Mol Pharmacol. 1991;39:733–739. [PubMed] [Google Scholar]
- 115.Woodward R, Coley C, Daniell S, Naylor LH, Strange PG. Investigation of the role of conserved serine residues in the long form of the rat D2 dopamine receptor using site-directed mutagenesis. J Neurochem. 1996;66:394–402. doi: 10.1046/j.1471-4159.1996.66010394.x. [DOI] [PubMed] [Google Scholar]
- 116.Wilson JM, Sanyal S, Van Tol HH. Dopamine D2 and D4 receptor ligands: relation to antipsychotic action. Eur J Pharmacol. 1998;351:273–286. doi: 10.1016/S0014-2999(98)00312-4. [DOI] [PubMed] [Google Scholar]
- 117.Lanau F, Zenner MT, Civelli O, Hartman DS. Epinephrine and norepinephrine act as potent agonists at the recombinant human dopamine D4 receptor. J Neurochem. 1997;68:804–812. doi: 10.1046/j.1471-4159.1997.68020804.x. [DOI] [PubMed] [Google Scholar]
- 118.Newman-Tancredi A, Audinot-Bouchez V, Gobert A, Millan MJ. Noradrenaline and adrenaline are high affinity agonists at dopamine D4 receptors. Eur J Pharmacol. 1997;319:379–383. doi: 10.1016/S0014-2999(96)00985-5. [DOI] [PubMed] [Google Scholar]
- 119.Sokoloff P, Andrieux M, Besancon R, Pilon C, Martres MP, Giros B, Schwartz JC. Pharmacology of human dopamine D3 receptor expressed in a mammalian cell line: comparison with D2 receptor. Eur J Pharmacol. 1992;225:331–337. doi: 10.1016/0922-4106(92)90107-7. [DOI] [PubMed] [Google Scholar]
- 120.Bristow LJ, Kramer MS, Kulagowski J, Patel S, Ragan CI, Seabrook GR. Schizophrenia and L-745,870, a novel dopamine D4 receptor antagonist. Trends Pharmacol Sci. 1997;18:186–188. doi: 10.1016/s0165-6147(97)01066-3. [DOI] [PubMed] [Google Scholar]
- 121.Kulagowski JJ, Broughton HB, Curtis NR, Mawer IM, Ridgill MP, Baker R, Emms F, Freedman SB, Marwood R, Patel S, Patel S, Ragan CI, Leeson PD. 3-((4-(4-Chlorophenyl)piperazin-1-yl)-methyl)-1H-pyrrolo-2,3-b-pyridine: an antagonist with high affinity and selectivity for the human dopamine D4 receptor. J Med Chem. 1996;39:1941–1942. doi: 10.1021/jm9600712. [DOI] [PubMed] [Google Scholar]
- 122.Patel S, Freedman S, Chapman KL, Emms F, Fletcher AE, Knowles M, Marwood R, McAllister G, Myers J, Curtis N, Kulagowski JJ, Leeson PD, Ridgill M, Graham M, Matheson S, Rathbone D, Watt AP, Bristow LJ, Rupniak NM, Baskin E, Lynch JJ, Ragan CI. Biological profile of L-745,870, a selective antagonist with high affinity for the dopamine D4 receptor. J Pharmacol Exp Ther. 1997;283:636–647. [PubMed] [Google Scholar]
- 123.Vallone D, Picetti R, Borrelli E. Structure and function of dopamine receptors. Neurosci Biobehav Rev. 2000;24:125–132. doi: 10.1016/S0149-7634(99)00063-9. [DOI] [PubMed] [Google Scholar]
- 124.Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- 125.Wang Y, Xu R, Sasaoka T, Tonegawa S, Kung MP, Sankoorikal EB. Dopamine D2 long receptor-deficient mice display alterations in striatum-dependent functions. J Neurosci. 2000;20:8305–8314. doi: 10.1523/JNEUROSCI.20-22-08305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lindgren N, Usiello A, Goiny M, Haycock J, Erbs E, Greengard P, Hokfelt T, Borrelli E, Fisone G. Distinct roles of dopamine D2L and D2S receptor isoforms in the regulation of protein phosphorylation at presynaptic and postsynaptic sites. Proc Natl Acad Sci USA. 2003;100:4305–4309. doi: 10.1073/pnas.0730708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fernandez J, Alonso JM, Andres JI, Cid JM, Diaz A, Iturrino L, Gil P, Megens A, Sipido VK, Trabanco AA. Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. J Med Chem. 2005;48:1709–1712. doi: 10.1021/jm049632c. [DOI] [PubMed] [Google Scholar]
- 128.Kongsamut S, Roehr JE, Cai J, Hartman HB, Weissensee P, Kerman LL, Tang L, Sandrasagra A. Iloperidone binding to human and rat dopamine and 5-HT receptors. Eur J Pharmacol. 1996;317:417–423. doi: 10.1016/S0014-2999(96)00840-0. [DOI] [PubMed] [Google Scholar]
- 129.Sahlholm K, Marcellino D, Nilsson J, Fuxe K, Arhem P. Differential voltage-sensitivity of D2-like dopamine receptors. Biochem Biophys Res Commun. 2008;374:496–501. doi: 10.1016/j.bbrc.2008.07.052. [DOI] [PubMed] [Google Scholar]
- 130.Sahlholm K, Nilsson J, Marcellino D, Fuxe K, Arhem P. Voltage-dependence of the human dopamine D2 receptor. Synapse. 2008;62:476–480. doi: 10.1002/syn.20509. [DOI] [PubMed] [Google Scholar]
- 131.Deth R, Muratore C, Benzecry J, Power-Charnitsky VA, Waly M. How environmental and genetic factors combine to cause autism: a redox/methylation hypothesis. Neurotoxicology. 2008;29:190–201. doi: 10.1016/j.neuro.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 132.Kuznetsova AY, Deth RC. A model for modulation of neuronal synchronization by D4 dopamine receptor-mediated phospholipid methylation. J Comput Neurosci. 2008;24:314–329. doi: 10.1007/s10827-007-0057-3. [DOI] [PubMed] [Google Scholar]
- 133.Glickstein SB, Schmauss C. Dopamine receptor functions: lessons from knockout mice [corrected] Pharmacol Ther. 2001;91:63–83. doi: 10.1016/S0163-7258(01)00145-0. [DOI] [PubMed] [Google Scholar]
- 134.Dulawa SC, Grandy DK, Low MJ, Paulus MP, Geyer MA. Dopamine D4 receptor-knock-out mice exhibit reduced exploration of novel stimuli. J Neurosci. 1999;19:9550–9556. doi: 10.1523/JNEUROSCI.19-21-09550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sibley DR. New insights into dopaminergic receptor function using antisense and genetically altered animals. Annu Rev Pharmacol Toxicol. 1999;39:313–341. doi: 10.1146/annurev.pharmtox.39.1.313. [DOI] [PubMed] [Google Scholar]
- 136.Benjamin J, Li L, Patterson C, Greenberg BD, Murphy DL, Hamer DH. Population and familial association between the D4 dopamine receptor gene and measures of novelty seeking. Nat Genet. 1996;12:81–84. doi: 10.1038/ng0196-81. [DOI] [PubMed] [Google Scholar]
- 137.Ebstein RP, Novick O, Umansky R, Priel B, Osher Y, Blaine D, Bennett ER, Nemanov L, Katz M, Belmaker RH. Dopamine D4 receptor (D4DR) exon iii polymorphism associated with the human personality trait of novelty seeking. Nat Genet. 1996;12:78–80. doi: 10.1038/ng0196-78. [DOI] [PubMed] [Google Scholar]
- 138.Munafo MR, Yalcin B, Willis-Owen SA, Flint J. Association of the dopamine D4 receptor (DRD4) gene and approach-related personality traits: meta-analysis and new data. Biol Psychiatry. 2008;63:197–206. doi: 10.1016/j.biopsych.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 139.Rubinstein M, Cepeda C, Hurst RS, Flores-Hernandez J, Ariano MA, Falzone TL, Kozell LB, Meshul CK, Bunzow JR, Low MJ, Levine MS, Grandy DK. Dopamine D4 receptor-deficient mice display cortical hyperexcitability. J Neurosci. 2001;21:3756–3763. doi: 10.1523/JNEUROSCI.21-11-03756.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rubinstein M, Phillips TJ, Bunzow JR, Falzone TL, Dziewczapolski G, Zhang G, Fang Y, Larson JL, McDougall JA, Chester JA, Saez C, Pugsley TA, Gershanik O, Low MJ, Grandy DK. Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell. 1997;90:991–1001. doi: 10.1016/S0092-8674(00)80365-7. [DOI] [PubMed] [Google Scholar]
- 141.Pauls DL. The genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1310–1312. doi: 10.1016/j.biopsych.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 142.Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 143.Shastry BS. Molecular genetics of attention-deficit hyperactivity disorder (ADHD): an update. Neurochem Int. 2004;44:469–474. doi: 10.1016/j.neuint.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 144.LaHoste GJ, Swanson JM, Wigal SB, Glabe C, Wigal T, King N, Kennedy JL. Dopamine D4 receptor gene polymorphism is associated with attention deficit hyperactivity disorder. Mol Psychiatry. 1996;1:121–124. [PubMed] [Google Scholar]
- 145.Smalley SL, Bailey JN, Palmer CG, Cantwell DP, McGough JJ, Del’Homme MA, Asarnow JR, Woodward JA, Ramsey C, Nelson SF. Evidence that the dopamine D4 receptor is a susceptibility gene in attention deficit hyperactivity disorder. Mol Psychiatry. 1998;3:427–430. doi: 10.1038/sj.mp.4000457. [DOI] [PubMed] [Google Scholar]
- 146.Swanson JM, Sunohara GA, Kennedy JL, Regino R, Fineberg E, Wigal T, Lerner M, Williams L, LaHoste GJ, Wigal S. Association of the dopamine receptor D4 (DRD4) gene with a refined phenotype of attention deficit hyperactivity disorder (ADHD): a family-based approach. Mol Psychiatry. 1998;3:38–41. doi: 10.1038/sj.mp.4000354. [DOI] [PubMed] [Google Scholar]
- 147.Faraone SV, Biederman J, Weiffenbach B, Keith T, Chu MP, Weaver A, Spencer TJ, Wilens TE, Frazier J, Cleves M, Sakai J. Dopamine D4 gene 7-repeat allele and attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156:768–770. doi: 10.1176/ajp.156.5.768. [DOI] [PubMed] [Google Scholar]
- 148.Castellanos FX, Lau E, Tayebi N, Lee P, Long RE, Giedd JN, Sharp W, Marsh WL, Walter JM, Hamburger SD, Ginns EI, Rapoport JL, Sidransky E. Lack of an association between a dopamine-4 receptor polymorphism and attention-deficit/hyperactivity disorder: genetic and brain morphometric analyses. Mol Psychiatry. 1998;3:431–434. doi: 10.1038/sj.mp.4000430. [DOI] [PubMed] [Google Scholar]
- 149.Jonsson EG, Nothen MM, Gustavsson JP, Neidt H, Brene S, Tylec A, Propping P, Sedvall GC. Lack of evidence for allelic association between personality traits and the dopamine D4 receptor gene polymorphisms. Am J Psychiatry. 1997;154:697–699. doi: 10.1176/ajp.154.5.697. [DOI] [PubMed] [Google Scholar]
- 150.Malhotra AK, Virkkunen M, Rooney W, Eggert M, Linnoila M, Goldman D. The association between the dopamine D4 receptor (D4DR) 16 amino acid repeat polymorphism and novelty seeking. Mol Psychiatry. 1996;1:388–391. [PubMed] [Google Scholar]
- 151.Wong AH, Buckle CE, Van Tol HH. Polymorphisms in dopamine receptors: what do they tell us? Eur J Pharmacol. 2000;410:183–203. doi: 10.1016/S0014-2999(00)00815-3. [DOI] [PubMed] [Google Scholar]
- 152.Okuyama Y, Ishiguro H, Toru M, Arinami T. A genetic polymorphism in the promoter region of DRD4 associated with expression and schizophrenia. Biochem Biophys Res Commun. 1999;258:292–295. doi: 10.1006/bbrc.1999.0630. [DOI] [PubMed] [Google Scholar]
- 153.Ronai Z, Barta C, Guttman A, Lakatos K, Gervai J, Staub M, Sasvari-Szekely M. Genotyping the -521C/T functional polymorphism in the promoter region of dopamine D4 receptor (DRD4) gene. Electrophoresis. 2001;22:1102–1105. doi: 10.1002/1522-2683()22:6<1102::AID-ELPS1102>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 154.Talkowski ME, Kirov G, Bamne M, Georgieva L, Torres G, Mansour H, Chowdari KV, Milanova V, Wood J, McClain L, Prasad K, Shirts B, Zhang J, O’Donovan MC, Owen MJ, Devlin B, Nimgaonkar VL. A network of dopaminergic gene variations implicated as risk factors for schizophrenia. Hum Mol Genet. 2008;17:747–758. doi: 10.1093/hmg/ddm347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Norton N, Williams HJ, Owen MJ. An update on the genetics of schizophrenia. Curr Opin Psychiatry. 2006;19:158–164. doi: 10.1097/01.yco.0000214341.52249.59. [DOI] [PubMed] [Google Scholar]
- 156.Ross CA, Margolis RL, Reading SAJ, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 157.Seeman P, Guan HC, Van Tol HH. Dopamine D4 receptors elevated in schizophrenia. Nature. 1993;365:441–445. doi: 10.1038/365441a0. [DOI] [PubMed] [Google Scholar]
- 158.Jonsson E, Brene S, Geijer T, Terenius L, Tylec A, Persson ML, Sedvall G. A search for association between schizophrenia and dopamine-related alleles. Eur Arch Psychiatry Clin Neurosci. 1996;246:297–304. doi: 10.1007/BF02189022. [DOI] [PubMed] [Google Scholar]
- 159.Tanaka T, Igarashi S, Onodera O, Tanaka H, Kameda K, Takahashi K, Tsuji S, Ihda S. Lack of association between dopamine D4 receptor gene and schizophrenia. Am J Med Genet. 1995;60:580–582. doi: 10.1002/ajmg.1320600620. [DOI] [PubMed] [Google Scholar]
- 160.Rao PA, Pickar D, Gejman PV, Ram A, Gershon ES, Gelernter J. Allelic variation in the D4 dopamine receptor (DRD4) gene does not predict response to clozapine. Arch Gen Psychiatry. 1994;51:912–917. doi: 10.1001/archpsyc.1994.03950110072009. [DOI] [PubMed] [Google Scholar]
- 161.Shaikh S, Collier D, Kerwin RW, Pilowsky LS, Gill M, Xu WM, Thornton A. Dopamine D4 receptor subtypes and response to clozapine. Lancet. 1993;341:116. doi: 10.1016/0140-6736(93)92594-J. [DOI] [PubMed] [Google Scholar]
- 162.Wong AH, Van Tol HH. The dopamine D4 receptors and mechanisms of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1091–1099. doi: 10.1016/j.pnpbp.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 163.Sunahara RK, Guan HC, O’Dowd BF, Seeman P, Laurier LG, Ng G, George SR, Torchia J, Van Tol HH, Niznik HB. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature. 1991;350:614–619. doi: 10.1038/350614a0. [DOI] [PubMed] [Google Scholar]
- 164.Freedman SB, Patel S, Marwood R, Emms F, Seabrook GR, Knowles MR, McAllister G. Expression and pharmacological characterization of the human D3 dopamine receptor. J Pharmacol Exp Ther. 1994;268:417–426. [PubMed] [Google Scholar]
- 165.Toll L, Berzetei-Gurske IP, Polgar WE, Brandt SR, Adapa ID, Rodriguez L, Schwartz RW, Haggart D, O’Brien A, White A, Kennedy JM, Craymer K, Farrington L, Auh JS. Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Res Monogr. 1998;178:440–466. [PubMed] [Google Scholar]
- 166.Cussac D, Newman-Tancredi A, Sezgin L, Millan MJ. [3H]S33084: a novel, selective and potent radioligand at cloned, human dopamine D3 receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:569–572. doi: 10.1007/s002100000217. [DOI] [PubMed] [Google Scholar]
- 167.Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor, I: a multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J Pharmacol Exp Ther. 2002;303:791–804. doi: 10.1124/jpet.102.039867. [DOI] [PubMed] [Google Scholar]
- 168.Seeman P, Van Tol HH. Dopamine D4 receptors bind inactive (+)-aporphines, suggesting neuroleptic role: sulpiride not stereoselective. Eur J Pharmacol. 1993;233:173–174. doi: 10.1016/0014-2999(93)90365-O. [DOI] [PubMed] [Google Scholar]
- 169.Burstein ES, Ma J, Wong S, Gao Y, Pham E, Knapp AE, Nash NR, Olsson R, Davis RE, Hacksell U, Weiner DM, Brann MR. Intrinsic efficacy of antipsychotics at human D2, D3, and D4 dopamine receptors: identification of the clozapine metabolite N-desmethylclozapine as a D2/D3 partial agonist. J Pharmacol Exp Ther. 2005;315:1278–1287. doi: 10.1124/jpet.105.092155. [DOI] [PubMed] [Google Scholar]
- 170.Seeman P, Tallerico T. Antipsychotic drugs which elicit little or no parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol Psychiatry. 1998;3:123–134. doi: 10.1038/sj.mp.4000336. [DOI] [PubMed] [Google Scholar]
- 171.Vanover KE, Harvey SC, Son T, Bradley SR, Kold H, Makhay M, Veinbergs I, Spalding TA, Weiner DM, Andersson CM, Tolf BR, Brann MR, Hacksell U, Davis RE. Pharmacological characterization of AC-90179 [2-(4-methoxyphenyl)-N-(4-methyl-benzyl)-N-(1-methyl-piperidin-4-yl)-acetamide hydrochloride]: a selective serotonin 2A receptor inverse agonist. J Pharmacol Exp Ther. 2004;310:943–951. doi: 10.1124/jpet.104.066688. [DOI] [PubMed] [Google Scholar]
- 172.Millan MJ, Cussac D, Gobert A, Lejeune F, Rivet JM, Mannoury La Cour C, Newman-Tancredi A, Peglion JL. S32504, a novel naphtoxazine agonist at dopamine D3/D2 receptors, I: cellular, electrophysiological, and neurochemical profile in comparison with ropinirole. J Pharmacol Exp Ther. 2004;309:903–920. doi: 10.1124/jpet.103.062398. [DOI] [PubMed] [Google Scholar]
- 173.Seeman P, Corbett R, Van Tol HH. Atypical neuroleptics have low affinity for dopamine D2 receptors or are selective for D4 receptors. Neuropsychopharmacology. 1997;16:93–110. doi: 10.1016/S0893-133X(96)00187-X. [DOI] [PubMed] [Google Scholar]
- 174.Joyce JN, Janowsky A, Neve KA. Characterization and distribution of [125I]epidepride binding to dopamine D2 receptors in basal ganglia and cortex of human brain. J Pharmacol Exp Ther. 1991;257:1253–1263. [PubMed] [Google Scholar]
- 175.Tallman JF, Primus RJ, Brodbeck R, Cornfield L, Meade R, Woodruff K, Ross P, Thurkauf A, Gallager DW. I. NGD 94-1: identification of a novel, high-affinity antagonist at the human dopamine D4 receptor. J Pharmacol Exp Ther. 1997;282:1011–1019. [PubMed] [Google Scholar]
- 176.Newman-Tancredi A, Audinot V, Chaput C, Verriele L, Millan MJ. [35S]Guanosine-5′-O-(3-thio)triphosphate binding as a measure of efficacy at human recombinant dopamine D4.4 receptors: actions of antiparkinsonian and antipsychotic agents. J Pharmacol Exp Ther. 1997;282:181–191. [PubMed] [Google Scholar]
- 177.Mamiya T, Matsumura T, Ukai M. Effects of L-745,870, a dopamine D4 receptor antagonist, on naloxone-induced morphine dependence in mice. Ann N Y Acad Sci. 2004;1025:424–429. doi: 10.1196/annals.1316.052. [DOI] [PubMed] [Google Scholar]
- 178.Moustgaard A, Hau J, Lind NM. Effects of dopamine D4 receptor antagonist on spontaneous alternation in rats. Behav Brain Funct. 2008;4:49. doi: 10.1186/1744-9081-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tanaka K, Okada Y, Kanno T, Otomo A, Yanagisawa Y, Shouguchi-Miyata J, Suga E, Kohiki E, Onoe K, Osuga H, Aoki M, Hadano S, Itoyama Y, Ikeda JE. A dopamine receptor antagonist L-745,870 suppresses microglia activation in spinal cord and mitigates the progression in ALS model mice. Exp Neurol. 2008;211:378–386. doi: 10.1016/j.expneurol.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 180.Huang Y, Kegeles LS, Bae S, Hwang D, Roth BL, Savage JE, Laruelle M. Synthesis of potent and selective dopamine D(4) antagonists as candidate radioligands. Bioorg Med Chem Lett. 2001;11:1375–1377. doi: 10.1016/S0960-894X(01)00241-4. [DOI] [PubMed] [Google Scholar]
- 181.Gazi L, Bobirnac I, Danzeisen M, Schupbach E, Langenegger D, Sommer B, Hoyer D, Tricklebank M, Schoeffter P. Receptor density as a factor governing the efficacy of the dopamine D4 receptor ligands, L-745,870 and U-101958 at human recombinant D4.4 receptors expressed in CHO cells. Br J Pharmacol. 1999;128:613–620. doi: 10.1038/sj.bjp.0702849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kramer MS, Last B, Getson A, Reines SA. The effects of a selective D4 dopamine receptor antagonist (L-745,870) in acutely psychotic inpatients with schizophrenia: D4 dopamine antagonist group. Arch Gen Psychiatry. 1997;54:567–572. doi: 10.1001/archpsyc.1997.01830180085011. [DOI] [PubMed] [Google Scholar]
- 183.Cao BJ, Rodgers RJ. Dopamine D4 receptor and anxiety: behavioural profiles of clozapine, L-745,870 and L-741,742 in the mouse plus-maze. Eur J Pharmacol. 1997;335:117–125. doi: 10.1016/S0014-2999(97)01234-X. [DOI] [PubMed] [Google Scholar]
- 184.Brioni JD, Moreland RB, Cowart M, Hsieh GC, Stewart AO, Hedlund P, Donnelly-Roberts DL, Nakane M, Lynch JJ, 3rd, Kolasa T, Polakowski JS, Osinski MA, Marsh K, Andersson KE, Sullivan JP. Activation of dopamine D4 receptors by ABT-724 induces penile erection in rats. Proc Natl Acad Sci USA. 2004;101:6758–6763. doi: 10.1073/pnas.0308292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cowart M, Latshaw SP, Bhatia P, Daanen JF, Rohde J, Nelson SL, Patel M, Kolasa T, Nakane M, Uchic ME, Miller LN, Terranova MA, Chang R, Donnelly-Roberts DL, Namovic MT, Hollingsworth PR, Martino BR, Lynch JJ, 3rd, Sullivan JP, Hsieh GC, Moreland RB, Brioni JD, Stewart AO. Discovery of 2-(4-pyridin-2-ylpiperazin-1-ylmethyl)-1H-benzimidazole (ABT-724), a dopaminergic agent with a novel mode of action for the potential treatment of erectile dysfunction. J Med Chem. 2004;47:3853–3864. doi: 10.1021/jm030505a. [DOI] [PubMed] [Google Scholar]
- 186.Chemel BR, Roth BL, Armbruster B, Watts VJ, Nichols DE. WAY-100635 is a potent dopamine D4 receptor agonist. Psychopharmacology (Berl) 2006;188:244–251. doi: 10.1007/s00213-006-0490-4. [DOI] [PubMed] [Google Scholar]
- 187.Marona-Lewicka D, Nichols DE. WAY 100635 produces discriminative stimulus effects in rats mediated by dopamine D(4) receptor activation. Behav Pharmacol. 2009;20:114–118. doi: 10.1097/FBP.0b013e3283242f1a. [DOI] [PubMed] [Google Scholar]
- 188.Melis MR, Succu S, Sanna F, Melis T, Mascia MS, Enguehard-Gueiffier C, Hubner H, Gmeiner P, Gueiffier A, Argiolas A. PIP3EA and PD-168077, two selective dopamine D4 receptor agonists, induce penile erection in male rats: site and mechanism of action in the brain. Eur J Neurosci. 2006;24:2021–2030. doi: 10.1111/j.1460-9568.2006.05043.x. [DOI] [PubMed] [Google Scholar]
- 189.Bernaerts P, Tirelli E. Facilitatory effect of the dopamine D4 receptor agonist PD168,077 on memory consolidation of an inhibitory avoidance learned response in C57BL/6J mice. Behav Brain Res. 2003;142:41–52. doi: 10.1016/S0166-4328(02)00371-6. [DOI] [PubMed] [Google Scholar]
- 190.Tang AH, Franklin SR, Himes CS, Smith MW, Tenbrink RE. PNU-96415E, a potential antipsychotic agent with clozapine-like pharmacological properties. J Pharmacol Exp Ther. 1997;281:440–447. [PubMed] [Google Scholar]
- 191.Powell SB, Paulus MP, Hartman DS, Godel T, Geyer MA. RO-10-5824 is a selective dopamine D4 receptor agonist that increases novel object exploration in C57 mice. Neuropharmacology. 2003;44:473–481. doi: 10.1016/S0028-3908(02)00412-4. [DOI] [PubMed] [Google Scholar]
- 192.Jentsch JD, Taylor JR, Redmond DE, Jr, Elsworth JD, Youngren KD, Roth RH. Dopamine D4 receptor antagonist reversal of subchronic phencyclidine-induced object retrieval/detour deficits in monkeys. Psychopharmacology (Berl) 1999;142:78–84. doi: 10.1007/s002130050865. [DOI] [PubMed] [Google Scholar]
- 193.De La Garza R, Madras BK., 2nd [(3)H]PNU-101958, a D(4) dopamine receptor probe, accumulates in prefrontal cortex and hippocampus of non-human primate brain. Synapse. 2000;37:232–244. doi: 10.1002/1098-2396(20000901)37:3<232::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 194.Langer O, Halldin C, Chou Y, Sandell J, Swahn C, Nagren K, Perrone R, Berardi F, Leopoldo M, Farde L. Carbon-11 pb-12: an attempt to visualize the dopamine d(4) receptor in the primate brain with positron emission tomography. Nucl Med Biol. 2000;27:707–714. doi: 10.1016/S0969-8051(00)00154-2. [DOI] [PubMed] [Google Scholar]
- 195.Belliotti TR, Wustrow DJ, Brink WA, Zoski KT, Shih YH, Whetzel SZ, Georgic LM, Corbin AE, Akunne HC, Heffner TG, Pugsley TA, Wise LD. A series of 6- and 7-piperazinyl- and -piperidinylmethylbenzoxazinones with dopamine D4 antagonist activity: discovery of a potential atypical antipsychotic agent. J Med Chem. 1999;42:5181–5187. doi: 10.1021/jm990277d. [DOI] [PubMed] [Google Scholar]
- 196.Okuyama S, Kawashima N, Chaki S, Yoshikawa R, Funakoshi T, Ogawa SI, Suzuki Y, Ikeda Y, Kumagai T, Nakazato A, Nagamine M, Tomisawa K. A selective dopamine D4 receptor antagonist, NRA0160: a preclinical neuropharmacological profile. Life Sci. 1999;65:2109–2125. doi: 10.1016/S0024-3205(99)00476-2. [DOI] [PubMed] [Google Scholar]
- 197.Woolley ML, Waters KA, Reavill C, Bull S, Lacroix LP, Martyn AJ, Hutcheson DM, Valerio E, Bate S, Jones DN, Dawson LA. Selective dopamine D4 receptor agonist (A-412997) improves cognitive performance and stimulates motor activity without influencing reward-related behaviour in rat. Behav Pharmacol. 2008;19:765–776. doi: 10.1097/FBP.0b013e32831c3b06. [DOI] [PubMed] [Google Scholar]