Abstract
The small heat shock protein Hsp27 or its murine homologue Hsp25 acts as an ATP-independent chaperone in protein folding, but is also implicated in architecture of the cytoskeleton, cell migration, metabolism, cell survival, growth/differentiation, mRNA stabilization, and tumor progression. A variety of stimuli induce phosphorylation of serine residues 15, 78, and 82 in Hsp27 and serines 15 and 86 in Hsp25. This post-translational modification affects some of the cellular functions of Hsp25/27. As a consequence of the functional importance of Hsp25/27 phosphorylation, aberrant Hsp27 phosphorylation has been linked to several clinical conditions. This review focuses on the different Hsp25/27 kinases and phosphatases that regulate the phosphorylation pattern of Hsp25/27, and discusses the recent findings of the biological implications of these phosphorylation events in physiological and pathological processes. Novel therapeutic strategies aimed at restoring anomalous Hsp27 phosphorylation in human diseases will be presented.
Keywords: Hsp27, Kinase, Phosphatase, Disease, Therapy
Introduction
Heat shock protein Hsp27 (or HSPB1) or the mouse homologue Hsp25 belongs to the family of small heat shock proteins (sHsp), which to date includes nine other isoforms designated HSPB2-9 [1–4]. All these proteins contain a highly conserved region referred to as the α-crystallin domain, which contains β-sheets (Fig. 1; [1–3]). Somewhat less conserved is the N-terminal WDPF domain, owing its name to the presence of the amino acid residues W (tryptophan), D (aspartic acid), P (proline), and F (phenylalanine). The amino-terminal part of the protein also contains the partially conserved sequence PSRLFDQXFGEXLL, while the carboxy-terminal region consists of a flexible region. The WDPF region of Hsp27 is crucial for oligomerization, but other sequences in the N-terminal region are required [2]. The α-crystallin domain is essential for oligomerization of Hsp27, while the flexible region has been suggested to participate in the interaction with target proteins, to be involved in oligomerization, and to be important for solubility (reviewed in [1, 3]).The role of the partially conserved sequence remains unknown [1].
Fig. 1.
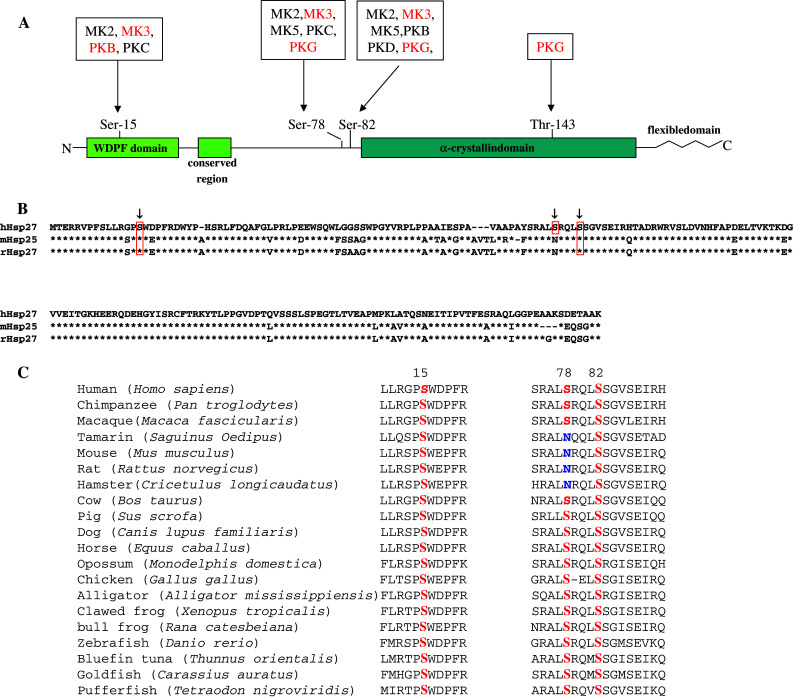
Structural properties of human Hsp27. a Schematic diagram of human Hsp27 showing the WDPF domain, the conserved N-terminal region, and the α-crystallin domain. The zigzag line corresponds to the flexible domain in the C-terminal part of the protein. The numbers refer to the amino acid residues. The phosphorylation sites Ser-15, Ser-78, and Ser-82 are indicated. The protein kinases shown to phosphorylate Hsp27 at these sites in vivo are indicated in black, while the protein kinases that were reported to phosphorylate these sites in vitro are depicted in red. b Alignment of the amino acid sequences of human (h) and rat (r) heat shock protein 27, and the mouse (m) homologue Hsp25. *Indicates an identical corresponding residue. The phosphoacceptor sites Ser-15, Ser-78, and Ser-82 (resp. Ser-86) are shown in red boxes. The one-letter amino acid code is used. c Comparison of the regions encompassing the phosphoacceptor sitesSer-15, Ser-78, and Ser-82 in human Hsp27 with the corresponding regions in Hsp27 homologous of other animals
Hsp25/27 can be detected in most cells examined, although the expression levels seem to vary with some cells expressing undetectable or relatively low levels, while other cells express Hsp25/27 abundantly [5–8]. Synthesis of Hsp25/27 can be induced by different conditions, including heat shock and other stress conditions, oestrogens, nerve injury, and differentiation [5, 9–12]. Hsp25/27 can oligomerize into large aggregates up to 800 kDa, but it can also form heteromeric structures with other sHsp family members (e.g., Hsp20), and complexes with additional proteins such as p38 mitogen-activated protein kinase mitogen-activated protein kinase (MAPK)-activated protein kinase 2 (MK2) and Akt (see further). Oligomerization is regulated by phosphorylation [4, 13, 14]. Unphosphorylated Hsp27 forms large multimers, while phosphorylation results in conformational changes, alteration of the direct interaction with other Hsp and dimer interaction with actin [15, 16].
Hsp25/27 is a multifunctional protein that participates in several processes in the cells. As a high molecular mass complex, Hsp25/27 acts as ATP-independent molecular chaperone [17–20]. Phosphorylation of Hsp25/27 has been shown to result in complex dissociation and the subsequent loss of chaperoning activity [13, 21, 22]. In addition to its chaperone function, Hsp25/27 also seems to be an important regulator of structural integrity and membrane stability, actin polymerization and intermediate filament cytoskeleton formation, cell migration, epithelial cell-cell adhesion, cell cycle progression, proinflammatory gene expression, muscle contraction, signal transduction pathways, mRNA stabilization, presentation of oxidized proteins to the proteasome, differentiation, and apoptosis [4, 13, 15, 23–36].
There are numerous reports that sustain a role for Hsp27 in human pathogenesis [2, 10, 18, 22, 24, 34–44]. Many cancer cells have markedly increased Hsp27 levels, and Hsp27 expression contributes to the malignant properties of these cells, including increased tumorigenicity and treatment resistance, and apoptosis inhibition [21, 25, 37–47]. Aberrant expression of Hsp27 can also be associated with the pathogenesis of cataracts, neurodegenerative disorders, and cardiovascular disease, while mutations in the gene encoding Hsp27 have been reported in inherited peripheral neuropathy (for reviews see, e.g., [4, 13, 27, 48–51]).
This review summarizes the identified protein kinases and protein phosphatases that control the reversible phosphorylation of Hsp25/27 and focuses on novel functional implications of Hsp25/27 phosphorylation. In addition, pathogenic conditions linked to aberrant Hsp25/27 phosphorylation, and potential therapeutic strategies aimed at modulating Hsp25/27 phosphorylation, will be briefly discussed.
Hsp27 kinases
A plethora of stimuli, including heat shock, mitogens, cytokines, lipopolysaccharides, phorbol esters, thrombin, serotonin, angiotensin, vasopressin, endothelin, carbachol, ceramide, glucose, metals, cAMP-elevating agents, vitamin D3 and retinoic acid, can induce phosphorylation of Hsp27 [4, 12, 22, 29, 52–60]. Analyzing the primary sequence of Hsp27 divulges 10.2% serine residues, 6.8% threonine residues, and 2.4% tyrosine residues, suggesting multiple potential phosphoacceptor sites [61]. Mapping the phosphorylation sites of native Hsp25 purified from Ehrlich ascites tumor cells showed that Ser-15 and Ser-86 were genuine phosphoacceptor sites [62], while phosphopeptide mapping and mutagenesis studies with Hsp27 identified Ser-15, Ser-82 (corresponds to Ser-86 in Hsp25), and in addition Ser-78 as in vivo phosphoacceptor sites (Fig. 1 and Sections “MK2” to “Apoptosis signal-regulating kinase” for references). Although proteins that are phosphorylated at multiple sites often become phosphorylated in a sequential fashion [63], this does not seem to apply for Hsp27 as the group of Landry showed that phosphorylation of Hsp27 does not occur in any strict obligate order [37]. Phosphorylation not only affects the function but also the quaternary structure of Hsp27 [1, 3]. This latter event is beyond the scope of this review (see, e.g., [4] and references therein for this topic). Known Hsp27 kinases and their target sites are discussed below (Fig. 2).
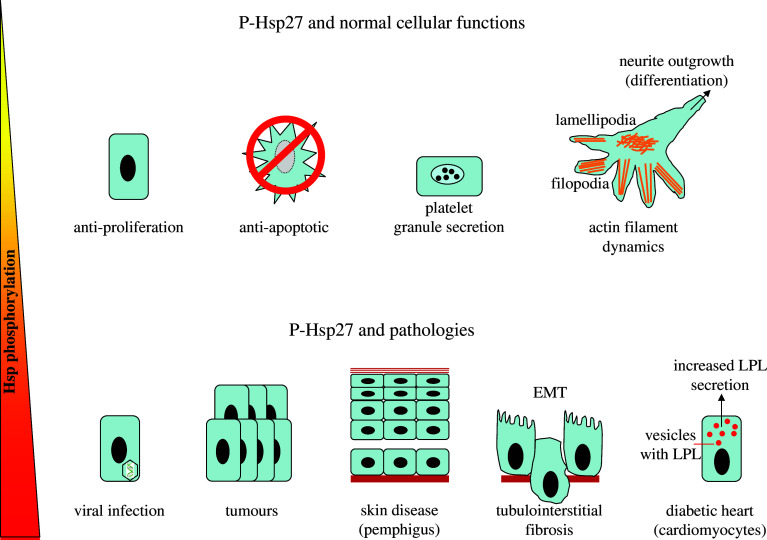
Fig. 2.
Schematic presentation of the functions linked to phosphorylated Hsp27. Top panel Under normal conditions, phosphorylated Hsp27 exerts anti-proliferative and anti-apoptotic effects, and is involved in platelet granule secretion and actin filament dynamics. Bottom panel Anomalous Hsp27 phosphorylation levels (indicated by the triangle) has been correlated to pathologies such as viral infections, specific tumor cells, autoimmune diseases of the skin (pemphigius vulgaris and pemphigius foliaceus, kidney diseases (e.g., epithelial-to-mesenchymal transition; EMT), and increased lipoprotein lipase (LPL) secretion in diabetic cardiomyocytes. See text for details
MK2
In an early study, Benndorf and his colleagues set out to identify the Hsp25 kinase activity in Ehrlich ascites tumor cells [64]. Inhibitors of cAMP-dependent protein kinase or protein kinase A (PKA), protein kinase C (PKC), cGMP-dependent protein kinase (PKG), and Ca2+/camodulin-dependent protein kinase (CaMK) could not abrogate phosphorylation of Hsp25, indicating that (an)other protein kinase(s) could phosphorylate Hsp25 [64]. In the same and following years, in vitro studies showed that Hsp25/27 is a very good substrate for MK2 [54, 65–70]. Moreover, 45 kDa/54 kDa polypeptides from Chinese hamster CCL39 cells, which were antigenic identical with MK2, possessed Hsp27 kinase activity [71]. Studies using, e.g., knockout cells, siRNA-mediated depletion of target proteins, dominant negative mutants, and specific p38 MAPK inhibitors have led to the general accepted concept that Hsp27 is phosphorylated through the p38 MAPK/MK2 module and that this pathway is important in modulating microfilament dynamics [12, 70, 72–77]. The involvement of MK2 in stimulus-induced Hsp27 phosphorylation may be overestimated because Hsp27 kinase activity towards Hsp27 in vitro was determined in several studies after immunoprecipitation with a rabbit polyclonal antibody raised against a GST fusion protein containing the 223 C-terminal amino acids of Chinese hamster MK2. This antibody immunoprecipitates both the p45 and p54 isoforms of human MK2, one of which may correspond to the recently described MAPK-activated protein kinase 3 (MK3) [78]. Between MK2 and MK3, MK2 comprises the major Hsp27 kinase activity because MK2 expression levels and activity are higher than MK3 in the cells tested, and MK2 seems to be preferentially activated by stimuli that induce Hsp27 phosphorylation [55, 79].
MAPK-activated protein kinase-3 (MK3)
MK3 was originally isolated in a yeast two-hybrid assay using p38 MAPK as bait, and subsequently shown to phosphorylate Hsp27 in vitro [80]. Similar studies performed by Zakowski et al. [81] confirmed that Hsp27 is a good MK3 substrate, at least in vitro. MK3 phosphorylates Hsp27 at three sites in vitro, with phosphorylation of Ser-82 more vividly than Ser-78 and Ser-15 [55]. The in vivo contribution of MK3 in Hsp25/27 phosphorylation has been silently ignored probably because MK3’s expression and activity is lower than MK2’s [79], and no Hsp25 phosphorylation could be detected in MK2−/− mouse embryonic fibroblasts after p38 MAPK activation [76]. The lack of MK3 deficient cells may have hampered studies aimed at scrutinizing its bona fide function as Hsp25/27 kinase, but RNA interference and dominant negative MK3 mutants could operate as valuable alternative tools for such studies.
MAPK-activated protein kinase-5 (MK5)
Two Hsp27 kinase activities were described in hamster CCL39 and human endothelial cells; one associated with a 45-kDa protein, identified as MK2, and one with an unidentified 54-kDa protein [71, 78]. In 1998, two independent research groups isolated a novel p38 MAPK-activated protein kinase of ~54 kDa designated as MAPK-activated protein kinase-5 (MAPKAPK5 or MK5) in mouse and p38-regulated/activated kinase (PRAK) in human [82–84]. It was shown that recombinant PRAK phosphorylated Hsp25 and Hsp27 in vitro with similar stoichiometry as recombinant MK2 [82]. Tryptic phosphopeptide mapping revealed that PRAK phosphorylated Hsp27 at Ser-15, Ser-78, and Ser-82 [82]. The same group also reported that depletion of PRAK in HeLa cell lysates with anti-PRAK antibodies specifically removed the Hsp27 kinase activity associated with the ~54-kDa protein [82]. Another group demonstrated that stimulation of platelets with thrombin resulted in activation of MK2 and PRAK and phosphorylation of Hsp27 [85]. Recent results from ourselves and others impose a role upon MK5 as a genuine Hsp27 kinase, because MK5 and Hsp27 (both endogenous as well as exogenous human Hsp27) can form complexes in the cell and MK5 induce phosphorylation of ectopically-expressed human Hsp27 Ser-78 and Ser-82 in vivo [86–88]. These results support a role for MK5/PRAK as an in vivo Hsp27 kinase. However, lysates of arsenite-treated MK5−/− mouse embryonic fibroblasts (MEF) failed to phosphorylate recombinant Hsp25, while no Hsp25 kinase activity was detected in immunoprecipitates with anti-PRAK antibodies from treated wild-type and MK5−/− MEFs [76]. Moreover, phosphoHsp25 was detected by mass spectrometry in arsenite-treated wild-type and MK5-deficient cells, but not in cells lacking MK2 [76]. The discrepancy between these results was explained by the source of the PRAK antibody. Shi et al. [76] showed that the PRAK antibody used in the study by New et al. [82] could immunoprecipitate Hsp27 kinase activity from MK5 deficient cells, but not from MK2−/− MEFs. This antibody may thus cross-react with an Hsp27 kinase different from MK2. Alternatively, MK5 does not act as an Hsp27 kinase in these cells, or may induce Hsp27 upon activation by other pathways. Indeed, while MK2 seems to be the kinase to phosphorylate Hsp27 in response to stress (see, e.g., [66, 68, 70, 71, 75]), we demonstrated that MK5 mediated Hsp27 phosphorylation upon activation of the cAMP/PKA pathway [86, 88].
A role for MK2 in PKA-induced Hsp27 phosphorylation can most probably be excluded because we were unable to find an interaction between MK2 and PKA in PC12 cells, and PKA could not phosphorylate MK2 in vitro, nor did p38 MAPK become activated in forskolin-treated cells [86, 88]. On the other hand, Gaestel and his group showed that forskolin induced MK2 expression levels and activity in PC12 cells in a PKA- and p38 MAPK-dependent, but ERK-independent, manner [60]. Kinetic studies revealed that p38 MAPK became phosphorylated within 15 min, reaching a maximum after 4 h, and that the kinetics of MK2 activation was very similar to that of p38 MAPK activation [60]. Their results indicate that the PKA might cause Hsp25 phosphorylation through MK2. At the moment, we cannot explain the discrepancy between our results and the results of Gaestel and his group. The use of specific inhibitors for MK2, studies with siRNA directed against MK2, and identical PC12 subclones are required to unequivocally establish a possible contribution of MK2 in forskolin-induced Hsp25/27 phosphorylation. We have not addressed a possible role for MK3 in PKA-triggered Hsp27 phosphorylation, but MK3 is expressed at very low/undetectable levels in PC12 cells [55, 80, 89], making it unlikely that MK3 is a major Hsp27 kinase in these cells.
Protein kinase A or cAMP-dependent protein kinase
Several independent studies report that protein kinase A (PKA) can phosphorylate Hsp25/27 in vitro [62, 90–92]. Cairns and colleagues tested the protein kinases PKA, ERK1, p34cdc2, and casein kinase and found that PKA was the most effective kinase in phosphorylating Hsp27, while none of the other kinases catalyzed a significant phosphorylation in vitro [90]. Gaestel et al. [62] identified PKA as being capable of phosphorylation Hsp25 in vitro at Ser-15 and Ser-86. Afterwards, the same group demonstrated, by applying recombinant wild-type and Hsp27 mutants, that the catalytic subunit of PKA phosphorylated Hsp27 at Ser-78, Ser-82, and Thr-143, but probably not at Ser-15 [91]. By using an in vitro kinase approach with rat uterus homogenates, Huang and co-workers reported that the purified catalytic subunit of PKA phosphorylated Hsp27 at Ser-5, Ser-15, Ser-86, Ser-102, and Tyr-147 [92]. However, Stokoe and colleagues and Landry’s group reported that PKA could not phosphorylate Hsp25/27 in vitro [38, 65]. These data, and the observation that cAMP elevating agents did not stimulate phosphorylation of Hsp27 in human embryonal MRC5 fibroblasts and in aortic smooth muscle A10 cells, suggest that Hsp27 is not a physiological substrate for PKA [93, 94]. On the other hand, micro-injection of rat embryonic fibroblast REF-52 cells with the catalytic subunit of PKA caused phosphorylation of Hsp27, indicating that PKA may mediate phosphorylation of Hsp27 in cells [95]. The phosphorylation sites were not mapped, and it cannot be excluded that the phosphorylation of Hsp27 by PKA occurs indirectly. In conclusion, Hsp25/27 is probably not a genuine substrate for PKA and the ability of PKA to cause Hsp27 phosphorylation may depend on an indirect mechanism [e.g., involving MK2 or/and MK5 as discussed in Section “MAPK-activated protein kinase-5 (MK5”)] and be cell-specific. The reason why forskolin can induce phosphorylation of Hsp27 in PC12 cells, but not in MRC5 cells, may be explained by the different expression patterns of MK5 in these cells. MK5 is readily detected in PC12 cells and neural tissues [86, 96], while MK5 transcripts were undetectable in embryonic lung tissue [96]. As MRC5 cells are human embryonic fibroblasts, they may lack or modestly express MK5. Alternatively, differences in Hsp27 phosphorylation response upon PKA activation may depend on subcellular compartmentalization of PKA through interaction with A-kinase anchoring proteins (AKAPs), which allows the controlling of signal transduction events at specific sites within the cell and accurate substrate selection [97].
Akt/protein kinase B (PKB)
Several observations point to a role for Akt as Hsp25/27 kinase [94, 98–102]. First, the Akt1, Akt2, and Akt3 isoforms can bind directly to Hsp27 and can be found in a complex with p38 MAPK, MK2, and Hsp27 [98–100]. Second, Rane and colleagues showed that Akt could phosphorylate Hsp27 at Ser-82, but not Ser-15 or Ser-78, in vitro, while co-expression of an active Akt mutant and Hsp27 in HEK cells resulted in Hsp27 phosphorylation at the same residue [100]. Third, comparative proteomic analysis of the gastric carcinoma cell line NU-GC-3 with Epstein–Barr virus (EBV)-infected cells revealed increased phosphorylation of Hsp27 at Ser-82 in an Akt-dependent, but MEK/ERK- and p38 MAPK-independent, manner [101]. Total protein levels of phosphoAkt, but not Akt, MK2 or phosphoMK2, were increased, suggesting that phosphorylated (activated) Akt controls EBV-induced Hsp27 phosphorylation. Phosphorylation of Ser-15 and Ser-78 was not detected, but could be due to technical limitations of mass spectrometry. Furthermore, phosphorylation of Hsp25 at Ser-86 in epidermis, and Hsp25 levels, were reduced in single Akt 1 and Akt 2 null mice [102]. Finally, the group of Kozawa reported that treatment of rat aortic smooth muscle A10 cells with the antidiuretic hormone arginine vasopressin or the serine protease thrombin resulted in Hsp27 phosphorylation. The authors showed that this phosphorylation was partially mediated by the phosphatidylinositol 3-kinase (PI3K)/Akt pathway independent of p38 MAPK [58, 94].
Results from in vitro kinase studies suggest that Akt is probably not a major Hsp27 kinase because recombinant MK2 readily phosphorylated purified Hsp27 at Ser-82, and moderate or weak phosphorylated Ser-78 and Ser-15, while 10-fold higher concentrations of Akt gave only weak phosphorylation of Ser-15 [58]. Serine residue 9 and threonine residue 143 in Hsp27 form putative Akt phosphoacceptor sites [58], but it was not investigated whether Akt could actually phosphorylate these sites. However, overexpression of the phosphomimicking Hsp27-3D mutant in which the serines 15, 78, and 82 are replaced by aspartate disrupted the binding with Akt [58]. This may suggest that Ser-9 and Thr-143 are not involved in Akt-Hsp27 interaction and therefore are not phosphorylated by Akt. Heat shock treatment of PC12 cells resulted in a fast, but transient, activation of Akt with phosphorylation of Akt after 5 min, but no phosphorylated Akt was detected after 1 h. Hsp27 phosphorylation levels, however, only increased after 3 h. At this time point, phospho-p38 MAPK levels were also enhanced, while the activity of MK2 was not tested. These results indicate that p38 MAPK pathway, rather than Akt, is the Hsp27 kinase [103]. In this respect, it is intriguing that MK2 can phosphorylate Akt on Ser-473 in vitro thereby enhancing the kinase activity of Akt [104]. Whether MK2-mediated Hsp25/27 phosphorylation can be (partially) mediated through Akt remains to be established.
The biological implications of Akt-mediated phosphorylation of Hsp27 are not fully understood. A role in subcellular redistribution of Hsp27, apoptosis of neutrophils, and establishing of a malignant phenotype in EBV-infected gastric carcinomas has been proposed ([101] and references therein). Alternatively, the Akt:Hsp27 interaction is not an enzyme:substrate interaction, but Hsp27 acts as a chaperone to protect Akt from dephosphorylation and destabilization, and to secure Akt’s natural conformation and enzymatic activity [11, 58].
Protein kinase C
In 1983, Feuerstein and Cooper reported that treatment of HL-60 cells with the phorbol ester phorbol-12-myristate-13-acetate (PMA) resulted in increased phosphorylation of a protein with apparent molecular mass of 27 kDa [105]. This finding was confirmed and extended to other cell lines and other PKC activating agents such as thrombin, histamine, vascular endothelial growth factor 1 (VEGF-1), and interleukin-1 (IL-1) ([22, 106, 107], and references therein). Moreover, depletion of PKC, as well as PKC inhibitors prevented phosphorylation of Hsp27 triggered by PKC activators ([22, 106, 107], and references therein). Evans and co-workers mapped VEGF-1-induced Hsp27 phosphorylation to Ser-15 and Ser-78, and this phosphorylation was sensitive to PKC inhibition [107]. In vitro kinase studies with recombinant PKC isoforms and Hsp27 revealed that PKCδ was most efficient in phosphorylating Hsp27, while PKCα had 30–50% Hsp27 kinase activity compared to PKCδ. PKCβ1, β2, and ζ isoforms were poor Hsp27 kinases. PKCδ phosphorylated Hsp27 at Ser-15 and Ser-78 in vitro [108]. Gaestel et al. reported in vitro phosphorylation of Hsp25 by PKC on Ser-15 and Ser-86 [62]. An in vivo role for PKC as Hsp25/27 kinase can be derived from the study by Lee and colleagues who showed a direct interaction between Hsp25 and PKCδ, which resulted in phosphorylation of Ser-15 and Ser-86 [43]. However, the role of PKC as genuine Hsp25/27 kinase remains controversial. PMA failed to induce Hsp27 phosphorylation in K562 cells [22], while Zhou et al. [38] could not detect Hsp27 phosphorylation in the presence of PKC purified from rat brain. Gaestel’s group found that PMA activated the MEK/ERK and p38 MAPK pathways, and triggered phosphorylation of Hsp27, but blocking the PKC isoforms α, β, and ε by the inhibitor RO31-8220 abrogated ERK2, p38 MAPK, and MK2 activation, as well as Hsp27 phosphorylation [109]. PMA-induced phosphorylation was, however, not affected by the MEK1/2 inhibitor PD098059, but was reduced by the p38 MAPK inhibitor SB203580. These results indicate that PKC indirectly phosphorylates Hsp27 through activation of the p38 MAPK/MK2 pathway [109]. This was later confirmed by the work of Takai et al. [110] who showed that PMA induced Hsp27 phosphorylation in human HCC-derived HuH7 cells. Knockdown of PKCδ suppressed Hsp27 phosphorylation as well as p38 MAPK phosphorylation. SB203580 inhibited PMA-induced Hsp27 phosphorylation, indicating that PKCδ mediates Hsp27 phosphorylation through p38 MAPK.
Protein kinase D
The protein kinase D (PKD) family consists of three closely related members: PKD1, PKD2, and PKD3, and is grouped into the calcium- and calmodulin-dependent kinases family of protein kinases [111]. The first indication that Hsp27 is a substrate for PKD was provided by Döppler et al. [112], who generated an antibody directed against the optimal consensus phosphomotif of PKD and used this antibody to detect immunoreactive phosphoproteins in lysates of cells treated with PKD agonists. The authors discovered a strong immunoreactive band of ~27 kDa. Because Hsp27 contains two putative PKD consensus phosphorylation motifs, one at Ser-15 and one at Ser-82, the authors examined whether Hsp27 is a bona fide PKD substrate. Depletion of Hsp27 or PKD1 and PKD2 by siRNA reduced the immunoreactivity of the ~27 kDa band, while in vitro kinase assay with GST-Hsp27 fusion proteins demonstrated that PKD directly phosphorylated Hsp27 at Ser-82, but not at Ser-15. PKD-mediated phosphorylation of Ser-82 was confirmed in cell culture studies [112]. Another research team used PKD-deficient cells to address the role of PKD as Hsp27 kinase [113]. DT40 B cells express the PKD isoforms PKD1 and PKD3. Studies in PKD1−/−, PDK3−/− and double knockout DT40 B cells showed that PKD activating stimuli were able to induce phosphorylation of Hsp27 at Ser-82 in the single knockout cells, but not in the double knockout. Hence, PKD1 as well as PKD3 can mediate phosphorylation of Hsp27 at Ser-82 in vivo [113].
Previous studies have shown that VEGF triggered Hsp27 phosphorylation at Ser-82 via the p38 MAPK/MK2 pathway [114]. In a recent study, it was proven that VEGF triggered phosphorylation of Hsp27 at Ser-15, -78, and -82 in human umbilical vein endothelial cells [107]. Inhibition of the p38 MAP/MK2 pathway by SB203580 or by siRNA-mediated knockdown of p38α or MK2 blocked VEGF-provoked phosphorylation of Ser-15 and -78, but not Ser-82. Preincubation with PKC/PKD inhibitors or depletion of PKD1 and PKD2 attenuated VEGF-induced Ser-82 phosphorylation, while the additional inhibition of p38 MAPK completely prevented Ser-82 phosphorylation. Knockdown of PKD3 had no effect on VEGF-provoked Hsp27 phosphorylation. Moreover, recombinant PKD1 or immunoprecipitated PKD2 from VEGF-stimulated cells could directly phosphorylate Hsp27 at Ser-82 in vitro. These observations combined with previous results suggest that Ser-82 is an in vivo phosphoacceptor site for the p38 MAPK/MK2 [114, 115], while PDK mediates phosphorylation of Hsp27 in response to VEGF [104]. Both these pathways also seem to be involved in mediating neurotensin-induced Hsp27 Ser-82 phosphorylation in the human ductual pancreatic adenocarcinoma PANC-1 cells [116]. Indeed, p38 MAPK, as well as PKC inhibitors, blunted phosphoSer-82 Hsp27 levels caused by neurotensin. Depletion of PKD1 or PKD2 reduced, while overexpression of PKD1 potentiated Hsp27 phosphorylation by neurotensin [116]. As the PKD phosphorylation motif LxRxxS/T also acts as a good substrate for other Ser/Thr kinases such as MK2 [113], it is possible that PKD may directly or indirectly activate MK2, which in turn then phosphorylates Hsp27. To unequivocally elucidate whether PKD can directly phosphorylate Hsp27, co-immunoprecipitation studies to identify cellular PKD-Hsp27 complexes and studies with, e.g., MK2 inhibitors, MK2 depletion, and MK2−/− cells are required.
The biological role of PKD-regulated Hsp27 phosphorylation is not fully understood, but because PKD, as well as phosphorylation of Hsp27, play a role in protecting cells from oxidative stress [117, 118], it is tempting to speculate that PKD-mediated phosphorylation of Hsp27 may be pivotal in this response. On the other hand, Liu and co-workers showed that PKD1 and PKD3 were dispensable for oxidative stress survival responses in DT40 B cells [113]. The PKC inhibitors, Gö6983 and GF109203X, or knockdown of PDK or Hsp27, inhibited VEGF-induced human umbilical vein endothelial cell migration and tubulogenesis [107]. In contrast, pharmacological inhibition of p38 MAPK or depletion of MK2 had no effect on these processes. Thus, PKD-mediated Hsp27 phosphorylation at Ser-82 may play a selective role in cellular processes such as protection against stress and angiogenic responses to VEGF [107].
cGMP-dependent protein kinase
The cGMP/cGMP-dependent protein kinase (PKG) pathway can inhibit platelet activation, but the molecular mechanisms are only partially understood [119]. Butt and co-workers demonstrated that the PKG activator 8-pCTP-cGMP caused phosphorylation of Hsp27 in human platelets in a p38 MAPK- and MK2-independent manner [91]. Purified PKG isoforms Iα, Iβ, and II all caused incorporation of phosphate in recombinant Hsp27 at Ser-78, Ser-82, and Thr-143, but not Ser-15. The phosphomimicking triple mutant Hsp27 S15D/S78D/S82D enhanced G-actin polymerization in vitro, while Hsp27 T143E had no effect on actin polymerization. Surprisingly, Hsp27 S15D/S78D/S82D/T143E reduced G-actin polymerization compared to the triple mutant [91]. These studies indicate that Hsp27 is a genuine substrate for PKG and that PKG may mediate inhibition of platelet aggregation through phosphorylation of Hsp27 and subsequent prevent of actin polymerization [91].
Huang et al. [120] used mass spectrometry to determine the degree and location of Hsp27 phosphorylation in extracts of pregnant rat uteri exposed to 8-bromo-cGMP analogue. They found 20 and 8% increased phosphate incorporation at Ser-15 and Ser-82, respectively, compared to untreated animals. Densitometry of western blot signals obtained with phosphoSer-15 specific antibodies confirmed ~20% increase of Hsp27 phosphorylation at this residue after treatment with 8-bromo-cGMP [120]. These findings corroborate that PKG may mediate in vivo phosphorylation of Hsp27 and that Ser-15 may be the major phosphoacceptor site. Phosphorylation of Ser-86 and Thr-143 was not detected by this method. The observation by Huang et al. demonstrating that exposure of rat uteri to cGMP analogues triggered Hsp27 phosphorylation at Ser-15 [120] is in contrast with the in vitro kinase studies with purified PKG that failed to detect Ser-15 phosphorylation [91]. One explanation may be that in vivo PKG may mediate Hsp27 indirectly through activating an Hsp27 kinase with activity towards Ser-15 and Ser-82. In support of this assumption is the finding that the Hsp27 kinase MK2 can be activated in a cGMP-dependent manner [121]. Alternatively, the source of PKG used for the in vitro studies was different from rat uteri, and PKG from different species may have different substrate specificities.
Ribosomal protein S6 kinase II (p70RSK)
Ser-15, Ser-78, and Ser-82 in Hsp27 (Ser-15 and Ser-86 in Hsp25) are part of the RXXS motif, a known recognition site for p70RSK [37, 62]. Stimuli that activate p70RSK also caused phosphorylation of Hsp27 at Ser-78 and Ser-82 [37]. This indicates that p70RSK may function as an Hsp27 kinase. Indeed, p70RSK weakly phosphorylated Hsp25 in vitro, but the phosphorylation sites were not determined [64]. However, a later report by the group of Landry, jeopardized the role of p70RSK as an Hsp27 kinase, as they were unable to detect p70RSK-mediated Hsp27 phosphorylation [38]. The use of the p70RSK inhibitor rapamycin or siRNA-mediated depletion of p70RSK may help to clarify whether p70RSK is a genuine Hsp25/27 kinase.
Ca2±/calmodulin-dependent kinases
Because Ser-15 and Ser-86 are part of the CaMKII RXXS motif, Hsp25/27 is a presumed substrate for CaMKII [64]. However, treatment of Ehrlich ascites tumor cells with CaMK inhibitors did not prevent Hsp25 phosphorylation, suggesting that CaMK II is not a genuine Hsp25 kinase [64]. CaMKII may indirectly modulate Hsp27 phosphorylation through activation of the p38 MAPK pathway (reviewed in [122]).
Apoptosis signal-regulating kinase
The N-terminal region of Hsp27, which contains the critical phosphorylation sites for functional regulation, can physically interact with apoptosis signal-regulating kinase (ASK1) in cells [35]. Whether phosphorylation of Hsp27 affects its interaction and whether the phosphorylation status of Hsp27 is altered upon interaction with ASK1 was not investigated. However, Hsp27 binds to the kinase domain of ASK1 and inhibits its enzymatic activity, making it unlikely that Hsp27 is an ASK1 substrate [35]. Moreover, the molecular mass of ASK1 (approximately 165 kDa) does not correspond with any of the described Hsp27 kinases.
Hsp27 phosphatases
Time course studies revealed a transient increase of Hsp27 phosphorylation in response to several stimuli (e.g., [41, 43, 58, 70, 103, 116]). This indicates that the phosphorylation of Hsp27 is reversible and controlled by protein phosphatases. Several studies with protein phosphatase inhibitors have shown that protein phosphatase 2A (PP2A) is involved in dephosphorylation of Hsp27 [90, 123–126]. Cairns and co-workers showed that purified PP2A readily (90%), purified protein phosphatase 2B (PP2B) moderately (15%), and purified protein phosphatase 1 (PP1) weakly (<6%) dephosphorylated immunoprecipitated phosphoHsp27 in vitro [90]. Studies in MCR-5 fibroblasts with okadaic acid concentrations that inhibit PP2A, but not PP1, and with the PP2B inhibitor cyclosporin proved that only PP2A could dephosphorylate Hsp27 in vivo [90]. The group of Gaestel also found that, by using cell lysates of Ehrlich ascites cells, PP2B dephosphorylated Hsp25 in vitro [127]. The failure of PP2B to dephosphorylate Hsp27 in vivo disagrees with the in vitro observations. Cairns et al. [90] explained this by proposing that Hsp27 and PP2B are not in the same cellular compartment. In a recent study, it was demonstrated that Hsp27 and PP2A could form complexes in cells [126], while incubation of in vitro phosphorylated recombinant Hsp27 with PP1 did not diminish the phosphorylation state of Hsp27. The same group reported that Hsp27 dephosphorylation was signal regulated because platelet-derived growth factor (PDGF) caused an increase in PP2A activity which was concomitant with Hsp27 dephosphorylation [126]. Hence, Hsp27 phosphorylation levels may not only be regulated by signals that activate pathways engaging protein kinases discussed in Section “Hsp27 kinases”, but may also involve activation of protein phosphatases that dephosphorylate Hsp27. Taken together, all findings so far indicate that PP2A is implicated in Hsp25/27 dephosphorylation in vivo, but the action of other protein phosphatases cannot be excluded. Interestingly, as PP2A has been reported to inactivate MK2 [41, 128], PP2A may also affect Hsp27 dephosphorylation by an indirect mechanism, i.e., by preventing active MK2 from phosphorylation of Hsp27.
Hsp27 phosphorylation and function
Several cellular functions associated with Hsp25/27 phosphorylation will be discussed in this section and are depicted in Fig. 2.
Actin filament dynamics
The regulatory role of phosphorylated Hsp27 in actin filament dynamics and linked processes such as cell migration, muscle contraction, cell division, differentiation, and pinocytosis represents probably one of the major functions of phosphoHsp27, and has been excellently described before (e.g., [8, 69–71, 75, 78, 88, 129–136]). While most of these studies have focused on the role of MK2 as Hsp27 kinase, we found that MK5 may mediate cAMP/PKA-induced F-actin rearrangement through phosphorylation of Hsp27 [86, 88]. Another group confirmed that MK5 is involved in cytoskeleton organization and cell migration through phosphorylation of Hsp27 [87].
Cell survival
A role of phosphoHsp27 in cellular protection and survival against a variety of stresses is also well documented. For example, exposure of cells to heat shock stimulates the expression of Hsp25/27 and induces a thermoresistant state, while ectopic overexpression of Hsp27 also conferred a thermoresistant phenotype. These findings suggest that enhanced levels of Hsp27 protect the cells against increased temperature. However, agents that stimulated Hsp27 phosphorylation without inducing its synthesis also caused a transient state of elevated thermoresistance [41]. PKD-mediated phosphorylation of Hsp27 at Ser-82 may play a selective role in stress response because PKD, as well as phosphorylation of Hsp27, play a role in protecting cells from oxidative stress [117, 118]. On the other hand, Liu and co-workers showed that PKD1 and PKD3 were dispensable for oxidative stress survival responses in DT40 B cells [113]. Studies with wild-type, phosphomimicking and non-phosphorylatable Hsp27 variants have underscored the importance of Hsp27 phosphorylation in its anti-apoptotic action. It is beyond the scope of this review to discuss the molecular basis for the anti-apoptotic effects of phospho-Hsp27, but several mechanisms have been described, including blocking Daxx-mediated apoptosis by interaction of phosphoHsp27 with Daxx and disruption of the PKB-p38-MK2-Hsp27 complex which plays an important role in controlling stress-induced apoptosis, and phosphorylation-induced nuclear import of Hsp25/27 and subsequent protection of nuclear breakdown [21, 25, 30, 32, 37–47, 52, 101].
Anti-proliferation
Increased accumulation and phosphorylation of Hsp27 have been observed as cells reached growth saturation [137, 138], while overexpression of Hsp25/27 can inhibit cell proliferation, and this property seems to be associated with the phosphorylation and aggregation state of Hsp25/27 [139, 140]. This finding was extended to several cell lines derived from different species by showing that ectopic expression of Hsp27 in which the phosphorylation sites Ser-15, -78,- and -82 had been substituted by glycine or mutants in which the C-terminal aggregation domain was deleted had no effect on cell growth [6]. The molecular basis for Hsp27-induced growth arrest is not known, but phosphoryation of Hsp25 resulted in nuclear import [41], which may allow phosphoHsp25 to interfere with nuclear processes or nuclear proteins involved in cell cycle regulation. A recent study may shed light upon the molecular mechanism by which phosphoHsp27 inhibits cell proliferation. Stable overexpression of phosphomimicking Hsp27 (Hsp-3D), but not non-phosphorylatable Hsp27 (Hsp-3A), delayed TNFα-induced cell growth of human hepatocarcinoma HuH7 cells [141]. The authors showed that phosphoHsp27 inhibited the MEK/ERK signalling pathway by a dual mechanism involving attenuation of c-Raf activity and stimulation of MAPK phosphatase-1 (MKP1) through p38 MAPK. This blockage of the MEK/ERK pathway resulted in a significant reduction in cyclin D1 levels, which may explain cell cycle arrest. Interestingly, the levels of phosphoSer-15 Hsp27 were inversely correlated with ERK activity in human hepatocellular carcinoma, while no correlation was found with phosphoSer-78 and phosphoSer-82 Hsp27 levels [141]. This may suggest that modulation of Ser-15 phosphorylation is important for repressing cell proliferation [141]. Another study has shown that Hsp27 can directly interact with p53 and regulate its transcriptional activity [142]. This results in, e.g., enhanced transcription of the cyclin-dependent protein kinase inhibitor p21Cip−1/Waf−1 and G2/M cell cycle arrest of cardiac H9c2 cells [142]. The p53-Hsp27 interaction was more pronounced in heat shock-treated cells than in control cells, but whether this was because of the increased levels of total Hsp27 protein and/or due to enhanced phosphorylation of Hsp27 was not tested. In line with this, injection of Hsp27-transfected L929 cells exhibited delayed tumor growth compared to control mice injected with non-transfected L929 cells. On the other hand, ectopical expression of Chinese hamster Hsp27 in murine NIH 3T3 fibroblasts had no effect on cell proliferation [130]. O’Callaghan-Sunol et al. [143] found that depletion of Hsp27 led to growth arrest of HCT116 colon carcinoma through the activation of the p53/p21Cip−1/Waf−1 pathway. Thus the effect of overexpression and/or phosphorylation of Hsp25/27 on cell proliferation may be cell-specific. Identifying nuclear target proteins for phospoHsp27 may increase our understanding how phosphoHsp27 may participate in cell cycle regulation.
Intracellular virus transport
Hsp25/27 may also be involved in intracellular transport of virus particles to the site of replication. Adenovirus infection resulted in activation of PKA and p38 MAPK, and inhibiting PKA and p38 MAPK, or infection of MK2−/− cells significantly reduced, while activation of PKA and p38 MAPK increased, nuclear targeting of adenovirus [144]. These results indicate that PKA and the p38 MAPK/MK2/Hsp27 phosphorylation cascade are involved in adenovirus transversing the cytoplasm to the nucleus. The exact mechanism by which MK2/Hsp27 stimulates nuclear targeting of incoming adenovirus is not known, but the authors suggested that phosphoHsp27 may affect protein trafficking through modulating microfilament dynamics. Overexpression of Hsp27 or the phosphomimicking Hsp27-3D mutant in MK2−/− cells avoided accumulation of virus particles near the cell periphery, indicating that enhanced Hsp27 or/and phosphoHsp27 levels can promote nuclear transport of adenovirus in MK2-deficient cells [144]. The effect of non-phosphorylatable Hsp27-3A on nuclear targeting should be tested to unequivocally establish the necessity of phosphoHsp27 in this process.
Cell differentiation
Because PMA-induced differentiation of HL-60 cells was correlated with increased expression and phosphorylation of Hsp27, it was speculated that phosphorylation of Hsp27 is required for differentiation [145]. However, the p38 MAPK inhibitor SB203580 could prevent PMA-induced Hsp27 phosphorylation, but did not abrogate differentiation, making it unlikely that Hsp27 phosphorylation is absolutely required for PMA-induced HL-60 cell differentiation [145]. Other studies have reported altered Hsp27 protein levels in differentiated cells [23, 24, 47, 102], but few have addressed the correlation with changed Hsp27 phosphorylation [10, 136]. The role of phosphoHsp27 in differentiation remains incompletely understood, but changes in F-actin remodeling may contribute to morphological changes of the differentiated cell.
Hsp27 and vascular functions
An involvement of phosphoHsp27 in adenosine diphosphate (ADP)-induced activation of platelets and in thrombogenesis has been proposed by the work of Kato and colleagues who showed that ADP-induced Hsp27 phosphorylation at Ser-15, -78, and -82 via the MEK/ERK and p38 MAPK pathways was sufficient for platelet granule secretion, but not for platelet aggregation [146]. The precise role for phosphoHsp27 was not scrutinized, but these results suggest that drugs aimed at modulating Hsp27 phosphorylation may be clinically applied in thrombotic diseases [146]. Another study showed that the PKC inhibitors, Gö6983 and GF109203X, or knockdown of PDK or Hsp27, inhibited VEGF-induced human umbilical vein endothelial cell migration and tubulogenesis [107]. In contrast, pharmacological inhibition of p38 MAPK or depletion of MK2 had no effect on these processes. Thus, PKD-mediated Hsp27 phosphorylation may be involved in angiogenic responses to VEGF [107]. Altered Hsp27 levels have been monitored in various other vascular diseases compared to healthy conditions, but the differences did not seem to relate to any risk factor. The phosphorylation status of Hsp27 in vascular diseases has not been meticulously examined (reviewed in [4]).
Role of the different phosphorylation sites in Hsp27
An obvious question is what is the function implication of the phosphorylation of a particular serine residue in Hsp27. Studies addressing the contribution of single phosphorylation of Hsp27 at Ser-15, Ser-78, or Ser-82 in biological processes have not been meticulously addressed. Studies have shown that oligomerization of Hsp27 is regulated by phosphorylation of Ser-78 and/or Ser-82 and the WDPF motif, while phosphorylation of Ser-15 seems to induce only a small effect on oligomerization. Complete dissociation of oligomers was observed only after phosphorylation of all three sites [1, 15]. Another question that needs to be solved is whether phosphorylation of Ser-78 is superfluous. The residues corresponding to Ser-15 and Ser-82 in human Hsp27 are conserved throughout the animal kingdom (Fig. 1c). Ser-78, however, is replaced by asparagine in rodents (rat, mouse, hamster) and in the New World monkey tamarin (Fig. 1c) despite higher identity between mouse and rat Hsp25/27 with human Hsp27 (82% identity) than, e.g., between zebrafish Hsp-1 and human Hsp27 (63% identity). This may indicate that Ser-78 is not as crucial as Ser-82, an assumption supported by the work of Landry et al. [37]. The authors demonstrated by tryptic mapping and subsequent reverse phase HPLC phosphoamino acid analysis that Ser-82 was the major site of phosphorylation after treating the human breast cancer cell cline MCF-7 with arsenite or heat shock. Moreover, transfection studies in Chinese hamster CCL39 cells with expression plasmids encoding wild-type Hsp27, Hsp27 S78G, or Hsp27 S82G revealed more intense phosphorylation of the S78G mutant protein as compared to the S82G mutant [37], underscoring the conclusion from the tryptic peptide analysis that S82 is the major phosphorylation site. In addition, in vitro kinase assays with MK2 and Hsp27 showed that Ser-82 is phosphorylated much faster than either Ser-15 or Ser-78 [65].
Hsp27 phosphorylation, human diseases and therapeutic strategies
Hsp27 phosphorylation and cancer
The expression levels of Hsp27 in human cancer have been intensively investigated, and aberrant expression is associated with aggressive tumor behavior, increased resistance to chemotherapy, and poor prognosis for the patient. However, the phosphorylation state compared to healthy tissue has been examined much less (for recent reviews, see [50, 147]). One study reported increased Hsp27 phosphorylation in B cell precursors from common acute lymphoblastic leukaemia (ALL) compared to healthy donors and non-acute lymphoblastic leukaemia patients [148]. The molecular basis for increased Hsp27 phosphorylation in these cancer cells is not known, nor is the precise role in ALL understood, but the authors suggested that phosphorylation of Hsp27 was mediated by MK2 and caused stabilization of actin polymerization and dysregulation of proliferation and differentiation of B cell precursors [148]. Variable Hsp27 phosphorylation at the different phosphoacceptor sites has also been detected in renal cell carcinoma and hepatocellular carcinoma compared to homologous normal tissue [141, 149, 150]. A recent report demonstrated a twofold increased phosphorylation of Hsp27 at Ser-78, but not Ser-15 and Ser-82, in HER-2/neu positive breast cancer samples (n = 10) compared to HER-2/neu negative tumors [151]. Treatment of the HER-2/neu positive BT474 breast cancer cell line with SB203580 reduced pSer-78 levels by 70%, while pSer-82 and pSer-15 levels were only inhibited by 30% or unchanged, respectively. This indicates that phosphorylation of Ser-78 in these cells is predominantly mediated by the p38 MAPK pathway and that inhibition of this pathway may offer a therapeutic strategy for patients with HER-2/neu-positive breast cancer. Moreover, these results suggest that several protein kinases that are not part of the p38 MAPK pathway may also be implicated in Hsp27 phosphorylation in these cells. Interestingly, the transcription factor STAT3, which is frequently constitutively active in breast carcinoma [152], can physically interact with Hsp27 and enhances Hsp27 expression and phosphorylation on residue Ser-78 [151]. The exact role of Hsp27 phosphorylation in the physiology of cancer remains incompletely understood, but phosphoHsp27 may suppress apoptosis [13, 150, 153].
Hsp27 phosphorylation and the autoimmune disease pemphigus
Pemphigus vulgaris and pemphigus foliaceus are autoimmune diseases of the skin characterized by the autoantibodies against desmoglein 3 and 1, respectively, which cause loss of keratinocyte cell-cell adhesion or acantholysis. Untreated, these disorders can be lethal [154]. These autoantibodies induce phosphorylation of Hsp25/27 in cell culture and recent studies showed increased phospho-p38 MAPK and phosphoHsp27 levels in skin biopsies of all pemphigus patients tested compared to healthy controls [154–157]. The exact in vivo role of p38 MAPK/Hsp27 in acantholysis is not known, but studies in cell cultures and animals showed that desmoglein autoantibodies induced reorganization of the actin cytoskeleton and intermediate filament collapse [154–157]. Inhibition of the p38 MAPK/MK2/Hsp27 pathway may be applicable to the therapy of pemphigus patients, as encouraging results illustrated that specific p38 MAPK inhibitors prevented blistering of the skin of mice treated with IgG purified from sera of pemphigus foliaceus patients [156, 157]. However, the time point of drug administration in treatment may be crucial, as a recent study revealed that only pretreatment with p38 MAPK inhibitor before injection of desmoglein-1 autoantibodies blocked cytoskeletal reorganization in human keratinocyte cell cultures or acantholysis in mouse models [154–157]. Another indication that aberrant phosphoHsp27 may be associated with skin disease is suggested by the finding that Akt-mediated phosphorylation of Hsp25/27 plays a role in skin formation [102], such that abnormal Akt activity or Hsp25/27 phosphorylation may lead to defective skin formation. However, so far, no clinical proof for the aberrant involvement of Akt/phosphoHsp27 in dermatological pathologies exists.
Hsp27 phosphorylation in the diabetic kidney and heart
Abnormal Hsp27 phosphorylation is observed in renal cancers, as well as in other kidney diseases [158]. Enhanced Hsp27 phosphorylation compared to control animals was observed in animal models of nephrotic syndrome and diabetic nephropathy [59, 159–161]. Park and co-workers [59] elaborated how Hsp27 phosphorylation might be implicated in diabetic nephropathy. They showed enhanced Hsp25 Ser-86 phosphorylation in wild-type mice in the diabetic state compared to normal wild-type mice. Strongly reduced Hsp25 phosphorylation was monitored in MK2−/− and diabetic MK2−/− mice compared to their MK2+/+ controls. This suggests that phosphorylation of Hsp25 in the diabetic kidney occurs in a MK2-dependent manner, but other protein kinases may also be involved [59]. One candidate is MK3, which is also activated by p38 MAPK [162]. The authors hesitate about this possibility because MK3 is weakly expressed in the kidney and MK3 levels are not compensatory upregulated in MK2-deficient mice [59, 76]. Further studies are necessary to clarify the role of Hsp27 phosphorylation in the pathogenic development of these diseases.
Kim and colleagues [163] unveiled a putative physiological role of augmented Hsp27 phosphorylation in the diabetic heart. Whereas in normal heart muscle ~30% of the energy is provided by carbohydrate and 70% through fatty acids metabolism, fatty acid is the sole energy source in a diabetic cardiomyocytes. This switch in energy supply is achieved by increased lipoprotein lipase (LPL) activity at the coronary lumen. The authors showed that phosphorylation of Hsp27 resulted in increased dissociation of Hsp27-PKCδ complexes in cardiomyocytes. Disruption of Hsp27-PKCδ complexes allowed activation of PKCδ and subsequent phosphorylation/activation of PKD. Activated PKD stimulated LPL activity at the coronary lumen, causing hydrolysis of triglyceride-rich lipoproteins to fatty acids. The increased Hsp27 phosphorylation is mediated through p38 MAPK and, although not proven by the authors, they suggested MK2 [163, 164].
Hsp27 phosphorylation and epithelial-to-mesenchymal transition of tubular epithelial cells into myofibroblasts in kidney fibrosis
Using an animal unilateral ureteral obstruction model of kidney fibrosis and epithelial-to-mesenchymal transition (EMT), an important profibrotic event, Vidyasagar et al. [165] found increased total and phosphoSer-86 Hsp27 levels in the kidneys compared to control kidneys. Similarly, TGFβ1-induced EMT of rat proximal tubular epithelial cells also resulted in increased Hsp27 and phosphoHsp27 levels compared to untreated cells. Overexpression of Hsp27 in these cells caused upregulation of E-cadherin, a biomarker for EMT. The authors therefore concluded that Hsp27 may be implicated in the pathophysiology of kidney tubulointerstitial fibrosis through upregulation of E-cadherin, thereby promoting epithelial-to-mesenchymal transition of tubular epithelial cells into myofibroblasts [165]. The effect of overexpression of phosphomimicking Hsp27 mutant on EMT was not tested and further studies are required to establish whether anomalous phosphoHsp27 levels may possess a causative role in kidney fibrosis.
Hsp27 phosphorylation and viral infection
Phosphorylation of Hsp27 in host cells may facilitate infection by respiratory syncytial virus (RSV) infection [166]. RSV is the major cause of bronchiolitis and pneumonia in infants, but infection with this virus can also lead to severe respiratory infections in elderly and immunocompromised individuals [167]. RSV infection is characterized by accumulation of fluid in the infected lungs due to increased lung permeability. This fluid extravasation can cause alveolar flooding, running nose, middle ear effusions, and increase in bacterial infections [167]. Singh and co-workers demonstrated that RSV infection was concomitant with increased Hsp27 phosphorylation on both Ser-78 and Ser-82 (Ser-15 was not examined), and led to actin microfilament rearrangement, cell shape modification, and change in cell permeability [167].
Hsp27 phosphorylation and other diseases
Mutations in Hsp27 have been detected in patients with hereditary motor neuropathy, but so far no mutations in the phosphoacceptor sites have been reported (see, e.g., [168, 169]).
Therapeutic strategies against anomalous Hsp27 phosphorylation
Because of known pathogenic conditions linked to aberrant Hsp27 phosphorylation, therapeutic strategies aimed at modulating Hsp27 expression and phosphorylation can be designed. Although successfully used in animal models (see, e.g., [170]), clinical proof of the beneficial effect of preventing Hsp27 expression is lacking. Pseudosubstrate inhibitors of Hsp25 have been designed, but they lack specificity [171, 172]. Knockdown of Hsp27 expression or interfering with Hsp27’s functions should be directed towards diseased cells considering the diverse array of functions that Hsp27 exerts in normal cells. Another approach is to impede phosphorylation of Hsp27. Numerous specific inhibitors against some of the Hsp27 kinases have been developed [173–176], but clinical trials in patients suffering from conditions with abnormal Hsp27 phosphorylation are lacking. Cell culture studies with a cell permeable MK2 inhibitor protein showed that preventing phosphorylation of Hsp27 reversed TGF-β1-induced F-actin rearrangements in human keloid fibroblasts, indicating that administration of Hsp27 kinase inhibitors may be a promising strategy in conditions with anomalous Hsp27 phosphorylation [177]. However, the selectivity of this inhibitor against other protein kinases and pharmacokinetic studies remains to be further investigated. Another indication for the therapeutic potential of targeting the p38 MAPK/MK2/Hsp27 pathway is provided by the finding that inhibition of p38 MAPK by SB203580 or MK2 by the inhibitory peptide KKKALNRQLGVAA prevented lung permeability in ventilator-associated lung injury in mice [178]. The synthetic molecule KRIBB3 was shown to specifically bind Hsp27 and to prevent PMA-induced Hsp27 phosphorylation and tumor cell migration at low concentrations (nM range), making KRIBB3 an attractive drug for clinical conditions with anomalous Hsp27 expression or phosphorylation [179]. Potentiating Hsp27 expression may be advantageous under certain circumstances such as stress resistance (anti-apoptotic activity) or inhibition of tumor cell proliferation (see Sections “Cell survival” and “Anti-proliferation”). As such, drugs that promote Hsp27 phosphorylation may possess therapeutic potentials. Quercetin and specific quercetin derivates can induce phosphorylation of Hsp27 at Ser-78 by an unknown mechanism [180]. However, these drugs inhibit expression of Hsp70 and inhibit casein kinase II and CaMK activity [180], making them unsuitable for therapy.
Perspectives and conclusion
Hsp27 is a multifunctional protein with multiple regulatory phosphorylation sites. Recent studies have unveiled distinct roles for (phosphorylated) Hsp27 in cellular processes, but the exact functions of the different phosphorylated forms of Hsp27 need to be further explored. Aberrant phosphorylation of Hsp27 has been reported in several diseases but the casual relationship and molecular mechanisms by which anomalous Hsp27 phosphorylation is implicated in the development of the disease remain to be established. Transgenic mice can be applied to unravel the role and mechanism of phosphoHsp25 in pathogenesis. Indeed, overexpressing Hsp27 or Hsp27-3A and -3D mutants have been successfully used to study the importance of Hsp25/27 phosphorylation in pancreas and in protection against ischemia/reperfusion [181, 182]. Although important studies, these transgenic mice express mutated Hsp25 from a heterologous promoter, which may result in non-physiological concentrations. Moreover, wild-type Hsp25 that is still expressed by these animals can interfere with the functions of the mutant protein. Studies in mice in which the endogenous hsp25 gene has been mutated to encode phosphomimicking Hsp25 S115D/S86D or non-phosphorylatable Hsp25 S15A/S86A may therefore be more suitable to monitor the in vivo contribution of Hsp27 in pathogenic processes. Another aspect in Hsp27 phosphorylation that has often been neglected is the putative contribution of several signaling pathways in mediating stimulus-induced Hsp27 phosphorylation. For example, cholecystokinin (CCK)- and prostaglandin D2 (PDG2)-induced phosphorylation of Hsp27 was partially reduced in the presence of SB203580, indicating the involvement of other kinases than MK2/3 [73, 110]. Because both CCK and PDG2 can activate PKA [73, 183], it is possible that phosphorylation of Hsp27 could also be mediated by PKA, either directly or through MK5 [86–88]. Differences in kinetics of MK2 activation and Hsp27 phosphorylation may also point to the involvement of other Hsp27 kinases. For example, the onset and peak of Hsp27 phosphorylation in bovine endothelial cells after shear stress were delayed compared to the activation of p38 MAPK and MK2 [70]. We have previously shown that stress-induced nuclear export of MK5 is postponed compared to MK2 [184] and that Hsp27 is a genuine substrate for MK5 [88]. Hence, MK5 may also be implicated in shear stress-triggered phosphorylation of Hsp27.
In conclusion, phosphorylation of Hsp27 not only regulates the structural organization of this protein, but also affects its biological functions. Abnormal Hsp27 phosphorylation has been associated with several pathological conditions. A better understanding of the role of phosphoHsp27 in these diseases and the development of specific and efficient drugs that modulate Hsp27 phosphorylation may offer novel strategies for treatment of diseases in which this phosphoprotein is implicated.
References
- 1.Gusev NB, Bogatcheva NV, Marston SB. Structure and properties of small heat shock proteins (sHsp) and their interaction with cytoskeleton proteins. Biochemistry. 2002;67:511–519. doi: 10.1023/a:1015549725819. [DOI] [PubMed] [Google Scholar]
- 2.Kappe G, Franck E, Verschuure P, Boelens WC, Leunissen JA, de Jong WW. The human genome encodes 10 α-crystallin-related small heat shock proteins: HspB1–10. Cell Stress Chaperones. 2003;8:53–61. doi: 10.1379/1466-1268(2003)8<53:thgecs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lelj-Garolla B, Mauk AG. Self-association of a small heat shock protein. J Mol Biol. 2005;345:631–642. doi: 10.1016/j.jmb.2004.10.056. [DOI] [PubMed] [Google Scholar]
- 4.Ferns G, Shams S, Shafi S. Heat shock protein 27: its potential role in vascular disease. Int J Exp Path. 2006;87:253–274. doi: 10.1111/j.1365-2613.2006.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavoie JN, Gingras-Breton G, Tanguay RM, Landry J. Induction of chinese hamster Hsp27 gene expression in mouse cells confers resistance to heat shock. J Biol Chem. 1993;268:3420–3429. [PubMed] [Google Scholar]
- 6.Arata S, Hamaguchi S, Nose K. Inhibition of colony formation of NIH 3T3 cells by the expression of the small molecular weight heat shock protein HSP27: involvement of its phosphorylation and aggregation at the C-terminal region. J Cell Physiol. 1997;170:19–26. doi: 10.1002/(SICI)1097-4652(199701)170:1<19::AID-JCP3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 7.Blackburn RV, Galoforo SS, Berns CM, Armour EP, McEachern D, Corry PM, Lee YJ. Comparison of tumor growth between Hsp25- and Hsp27-transfected murine L929 cells in nude mice. Int J Cancer. 1997;72:871–877. doi: 10.1002/(sici)1097-0215(19970904)72:5<871::aid-ijc26>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 8.Hedges JC, Dechert MA, Yamboliev IA, Martin JL, Hickey E, Weber LA, Gerthoffer WT. A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. J Biol Chem. 1999;274:24211–24219. doi: 10.1074/jbc.274.34.24211. [DOI] [PubMed] [Google Scholar]
- 9.Cooper LF, Uoshima K. Differential estrogenic regulation of small Mr heat shock protein expression in osteoblasts. J Biol Chem. 1994;269:7869–7873. [PubMed] [Google Scholar]
- 10.Spector NL, Mehlen P, Ryan C, Hardly L, Samson W, Levine H, Nadler LM, Fabre N, Arrigo AP. Regulation of the 28 kDa heat shock protein by retinoic acid during differentiation of human leukemic HL-60 cells. FEBS Lett. 1994;337:184–188. doi: 10.1016/0014-5793(94)80270-x. [DOI] [PubMed] [Google Scholar]
- 11.Murashov AK, Ul Haq I, Hill C, Park E, Smith M, Wang X, Wang X, Goldberg DJ, Wolgemuth DJ. Crosstalk between p38, Hsp25 and Akt in spinal motor neurons after sciatic nerve injury. Mol Brain Res. 2001;93:199–208. doi: 10.1016/s0169-328x(01)00212-1. [DOI] [PubMed] [Google Scholar]
- 12.Okada T, Otani H, Wu Y, Kyoi S, Enoki C, Fujiwara H, Sumida T, Hattori R, Imamura H. Role of F-actin organization in p38 MAP kinase-mediated apoptosis and necrosis in neonatal rat cardiomyocytes subjected to stimulated ischemia and reoxygenation. Am J Physiol Heart Circ Physiol. 2005;289:H2310–H2318. doi: 10.1152/ajpheart.00462.2005. [DOI] [PubMed] [Google Scholar]
- 13.Welsh MJ, Gaestel M. Small heat-shock protein family: function in health and disease. Ann N Y Acad Sci. 1997;851:28–35. doi: 10.1111/j.1749-6632.1998.tb08973.x. [DOI] [PubMed] [Google Scholar]
- 14.Bukach OV, Glukhova AE, Seit-Nebi AS, Gusev NB. Heterooligomeric complexes formed by human small heat shock proteins HspB1 (Hsp27) and HspB6 (Hsp20) Biochim Biophys Acta. 2009;1794:486–495. doi: 10.1016/j.bbapap.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Lambert H, Charette SJ, Bernier AF, Guimond A, Landry J. Hsp27 multimerization mediated by phosphorylation-sensitive intermolecular interactions at the amino terminus. J Biol Chem. 1999;274:9378–9385. doi: 10.1074/jbc.274.14.9378. [DOI] [PubMed] [Google Scholar]
- 16.Sun X, Welsh MJ, Benndorf R. Conformational changes resulting from pseudophosphorylation of mammalian small heat shock proteins–a two-hybrid study. Cell Stress Chaperones. 2006;11:61–70. doi: 10.1379/CSC-149R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- 18.Knauf U, Jakob U, Engel K, Buchner J, Gaestel M. Stress- and mitogen-induced phosphorylation of the small heat shock protein Hsp25 by MAPKAP kinase 2 is not essential for chaperone properties and cellular thermoresistance. EMBO J. 1994;13:54–60. doi: 10.1002/j.1460-2075.1994.tb06234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehrnsperger M, Gräber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vos MJ, Hageman J, Carra S, Kampinga H. Structural and functional diversities between members of the human HSPB, HSPH, HSPA, and DNAJ chaperone families. Biochemistry. 2008;47:7001–7011. doi: 10.1021/bi800639z. [DOI] [PubMed] [Google Scholar]
- 21.Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C, Paul C, Wieske M, Arrigo AP, Buchner J, Gaestel M. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor α by phosphorylation. J Biol Chem. 1999;274:18947–18956. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- 22.Kato K, Ito H, Iwamoto I, Iida K, Inaguma Y. Protein kinase inhibitors can suppress stress-induced dissociation of Hsp27. Cell Stress Chaperones. 2001;6:16–20. doi: 10.1379/1466-1268(2001)006<0016:pkicss>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spector NL, Ryan C, Samson W, Levine H, Nadler LM, Arrigo AP. Heat shock protein is a unique marker of growth arrest during macrophage differentiation of HL-60 cells. J Cell Physiol. 1993;156:619–625. doi: 10.1002/jcp.1041560322. [DOI] [PubMed] [Google Scholar]
- 24.Kindas-Mugge I, Trautinger F. Increased expression of the Mr 27, 000 heat shock protein (hsp27) in in vitro differentiated normal human keratiinocytes. Cell Growth Differ. 1994;5:777–781. [PubMed] [Google Scholar]
- 25.Charrette SJ, Lavoie JN, Lambert H, Landry J. Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol. 2000;20:7602–7612. doi: 10.1128/mcb.20.20.7602-7612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lasa M, Mahtani KR, Finch A, Brewer G, Saklatavla J, Clark AR. Regulation of cyclooxygenase 2 mRNA stability by the mitogen-activated protein kinase p38 signaling cascade. Mol Cell Biol. 2000;20:4265–4272. doi: 10.1128/mcb.20.12.4265-4274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Patil SB, Pawar MD, Bitar KN. Phosphorylated HSP27 is essential for acetylcholine-induced association of RhoA with PKCα. Am J Physiol Gastrointest Liver Physiol. 2004;286:G635–G644. doi: 10.1152/ajpgi.00261.2003. [DOI] [PubMed] [Google Scholar]
- 29.Gerthoffer WT. Signal-transduction pathways that regulate visceral smooth muscle function. III. Coupling of muscarinic receptors to signaling kinases and effector proteins in gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol. 2005;288:G849–G853. doi: 10.1152/ajpgi.00530.2004. [DOI] [PubMed] [Google Scholar]
- 30.Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- 31.Alford KA, Glennie S, Rawlinson L, Saklatvala J, Dean JL. Heat shock protein 27 functions in inflammatory gene expression and transforming growth factor-beta-activated kinase-1 (TAK1)-mediated signaling. J Biol Chem. 2007;282:6232–6241. doi: 10.1074/jbc.M610987200. [DOI] [PubMed] [Google Scholar]
- 32.Arrigo AP. The cellular “networking” of mammalian Hsp27 and its functions in the control of protein folding, redox state and apoptosis. Adv Exp Med Biol. 2007;594:14–26. doi: 10.1007/978-0-387-39975-1_2. [DOI] [PubMed] [Google Scholar]
- 33.Wu R, Kausar H, Johnson P, Montoya-Durango DE, Merchant M, Rane MJ. Hsp27 regulates Akt activation and polymporphonuclear leukocyte apoptosis by scaffolding MK2 to Akt signal complex. J Biol Chem. 2007;282:21598–21608. doi: 10.1074/jbc.M611316200. [DOI] [PubMed] [Google Scholar]
- 34.Sinsimer KS, Gratacós FM, Knapinska AM, Lu J, Krause CD, Wierzbowski AV, Mahler LR, Scrudato S, Rivera YM, Gupta S, Turrin DK, De La Cruz MP, Pestka S, Brewer G. Chaperone Hsp27, a novel subunit of AUF1 protein complexes, functions in AU-rich element-mediated mRNA decay. Mol Cell Biol. 2008;28:5223–5237. doi: 10.1128/MCB.00431-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stetler RA, Ca G, Gao Y, Zhang F, Wang S, Wenig Z, Vosler P, Zhang L, Signore A, Graham SH, Chen J. Hsp27 protects against ischemic brain injury via attenuation of a novel stress-response cascade upstream of mitochondrial cell death signaling. J Neurosci. 2008;28:13038–13055. doi: 10.1523/JNEUROSCI.4407-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doshi BM, Hightower LE, Lee J (2009) The role of Hsp27 and actin in the regulation of movement in human cancer cells responding to heat shock. Cell Stress Chaperones. Feb 18 [Epub ahead of print]. doi:10.1007/s12192-008-1 [DOI] [PMC free article] [PubMed]
- 37.Landry J, Lambert H, Zhou M, Lavoie JN, Hickey E, Weber LA, Anderson CW. Human Hsp27 is phosphorylated at serine 78 and 82 by heat shock and mitogen-activated protein kinases that recognize the same amino acid motif as S6 kinase II. J Biol Chem. 1992;267:794–803. [PubMed] [Google Scholar]
- 38.Zhou M, Lambert H, Landry J. Transient activation of a distinct serine protein kinase is responsible for 27-kDa heat shock protein phosphorylation in mirogen-stimulated and heat-shocked cells. J Biol Chem. 1993;268:35–43. [PubMed] [Google Scholar]
- 39.Préville X, Schultz H, Knauf U, Gaestel M, Arrigo AP. Analysis of the role of Hsp25 phosphorylation reveals the importance of the oligomerization state of this small heat shock protein in its protective function against TNFalpha- and hydrogen peroxide-induced cell death. J Cell Biochem. 1998;69:436–453. [PubMed] [Google Scholar]
- 40.Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol. 1995;15:505–516. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geum D, Son GH, Kim K. Phosphorylation-dependent cellular localization and thermoprotective role of heat shock protein 25 in hippocampal progenitor cells. J Biol Chem. 2002;277:19913–19921. doi: 10.1074/jbc.M104396200. [DOI] [PubMed] [Google Scholar]
- 42.Huot J, Houle F, Spitz DR, Landry J. Hsp27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996;56:273–279. [PubMed] [Google Scholar]
- 43.Lee YJ, Lee DH, Cho CK, Bae S, Jhon GJ, Lee SJ, Soh JW, Lee YS. Hsp25 inhibits protein kinase Cδ-mediated cell death through direct interaction. J Biol Chem. 2005;280:18108–18119. doi: 10.1074/jbc.M501131200. [DOI] [PubMed] [Google Scholar]
- 44.Benn SC, Perrelet D, Kato AC, Scholz J, Decosterd I, Mannion RJ, Bakowska JC, Woolf CJ. Hsp27 upregulation and phosphorylation is required for injury sensory and motor neuron survival. Neuron. 2002;36:45–56. doi: 10.1016/s0896-6273(02)00941-8. [DOI] [PubMed] [Google Scholar]
- 45.Wyttenbach A, Sauvageot O, Carmichael J, Diaz-Latoud C, Arrigo AP, Rubinsztein DC. Heat shock protein 27 prevents cellular polyglutamine toxicity and suppresses the increase of reactive oxygen species caused by huntingtin. Hum Mol Genet. 2002;11:1137–1151. doi: 10.1093/hmg/11.9.1137. [DOI] [PubMed] [Google Scholar]
- 46.de Graauw M, Tijdens I, Cramer R, Corless S, Timms JF, van de Water B. Heat shock protein 27 is the major differentially phosphorylated protein involved in renal epithelial cellular stress response and controls focal adhesion organization and apoptosis. J Biol Chem. 2005;280:29885–29898. doi: 10.1074/jbc.M412708200. [DOI] [PubMed] [Google Scholar]
- 47.Winger QA, Guttormsen J, Gavin H, Bhushan F. Heat shock protein 1 and the mitogen-activated protein kinase 14 pathway are important for mouse trophoblast stem cell differentiation. Biol Reprod. 2007;76:884–891. doi: 10.1095/biolreprod.106.056820. [DOI] [PubMed] [Google Scholar]
- 48.Evgrafov OV, Mersiyanova I, Irobi J, Van Den Bosch L, Dierick I, Leung CL, Schagina O, Verpoorten N, Van Impe K, Fedotov V, Dadali E, Auer-Grumbach M, Windpassinger C, Wagner K, Mitrovic Z, Hilton-Jones D, Talbot K, Martin JJ, Vasserman N, Tverskaya S, Polyakov A, Liem RK, Gettemans J, Robberecht W, De Jonghe P, Timmerman V. Mutant small heat-shock protein 27 causes axonal Charcot-Marie-Tooth disease and distal hereditary motor neuropathy. Nat Genet. 2004;36:602–606. doi: 10.1038/ng1354. [DOI] [PubMed] [Google Scholar]
- 49.Chen S, Brown IR. Neuronal expression of constitutive heat shock proteins: implications for neurodegenerative diseases. Cell Stress Chaperones. 2007;12:51–58. doi: 10.1379/CSC-236R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Calderwood SK, Ciocca DR. Heat shock proteins: stress proteins with Janus-like properties in cancer. Int J Hyperthermia. 2008;24:31–39. doi: 10.1080/02656730701858305. [DOI] [PubMed] [Google Scholar]
- 51.Houlden H, Laura M, Wavrant-De Vrièze F, Blake J, Wood N, Reilly MM. Mutations in the HSP27 (HSPB1) gene cause dominant, recessive, and sporadic distal HMN/CMT type 2. Neurology. 2008;71:1660–1668. doi: 10.1212/01.wnl.0000319696.14225.67. [DOI] [PubMed] [Google Scholar]
- 52.Robaye B, Hepburn A, Lecocq R, Fiers W, Boeynaems JM, Dumont JE. Tumor necrosis factor-alpha induces the phosphorylation of 28kDa stress proteins in endothelial cells: possible role in protection against cytotoxicity? Biochem Biophys Res Commun. 1989;163:301–308. doi: 10.1016/0006-291x(89)92135-9. [DOI] [PubMed] [Google Scholar]
- 53.Arrigo AP, Michel MR. Decreased heat- and tumor necrosis factor-mediated Hsp28 phosphorylation in thermotolerant HeLa cells. FEBS Lett. 1991;282:152–156. doi: 10.1016/0014-5793(91)80466-g. [DOI] [PubMed] [Google Scholar]
- 54.Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 55.Clifton AD, Young PR, Cohen P. A comparison of the substrate specificity of MAPKAP kinase-2 and MAPKAP kinase-3 and their activation by cytokines and cellular stress. FEBS Lett. 1996;392:209–214. doi: 10.1016/0014-5793(96)00816-2. [DOI] [PubMed] [Google Scholar]
- 56.Tilly BC, Gaestel M, Engel K, Edixhoven MJ, de Jonge HR. Hypo-osmotic cell swelling activates the p38 MAP kinase signalling cascade. FEBS Lett. 1996;395:133–136. doi: 10.1016/0014-5793(96)01028-9. [DOI] [PubMed] [Google Scholar]
- 57.Buitrago CG, Ronda AC, de Boland AR, Boland R. MAP kinase p38 and JNK are activated by the steroid hormone 1α, 25(OH)2-vitamin D3 in the C2C12 muscle cell line. J Cell Biochem. 2006;97:698–708. doi: 10.1002/jcb.20639. [DOI] [PubMed] [Google Scholar]
- 58.Takai S, Akamatsu S, Kato K, Oiso Y, Kozawa O. Possible involvement of phosphatidylinositol 3-kinase/Akt signal pathway in vasopressin-induced Hsp27 phosphorylation in aortic smooth muscle A10 cells. Arch Biochem Biophys. 2005;438:137–145. doi: 10.1016/j.abb.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Park JK, Ronkina N, Höft A, Prohl C, Menne J, Gaestel M, Haller H, Meier M. Deletion of MK2 signalling in vivo inhibits small Hsp phosphorylation but not diabetic nephropathy. Nephrol Dial Transplant. 2008;23:1844–1853. doi: 10.1093/ndt/gfm917. [DOI] [PubMed] [Google Scholar]
- 60.Thomas T, Hitti E, Kotlyarov A, Potschka H, Gaestel M. MAP-kinase-activated protein kinase 2 expression and activity is induced after neuronal depolarization. Eur J NeuroSci. 2008;28:642–654. doi: 10.1111/j.1460-9568.2008.06382.x. [DOI] [PubMed] [Google Scholar]
- 61.Hickey E, Brandon SE, Potter R, Stein G. Sequence and organization of genes encoding the human 27 kDa heat shock protein. Nucleic Acids Res. 1986;14:4127–4145. doi: 10.1093/nar/14.10.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gaestel M, Schröder W, Benndorf R, Lippmann C, Buchner K, Hucho F, Erdmann VA, Bielka H. Identification of the phosphorylation sites of the murine small heat shock protein Hsp25. J Biol Chem. 1991;266:14721–14724. [PubMed] [Google Scholar]
- 63.Gardner KH, Montminy M (2005) Can you hear me now? Regulating transcriptional activators by phosphorylation. Sci STKE 301, pe44 [DOI] [PubMed]
- 64.Benndorf R, Hayes K, Stahl J, Bielka H. Cell-free phosphorylation of the murine small heat-shock protein hsp25 by an endogenous kinase from Erhlich ascites tumor cells. Biochim Biophys Acta. 1992;1136:203–207. doi: 10.1016/0167-4889(92)90258-d. [DOI] [PubMed] [Google Scholar]
- 65.Stokoe D, Engel K, Campbell DG, Cohen P, Gaestel M. Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. FEBS Lett. 1992;313:307–313. doi: 10.1016/0014-5793(92)81216-9. [DOI] [PubMed] [Google Scholar]
- 66.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 67.Ahlers A, Belka C, Gaestel M, Lamping N, Sott C, Herrmann F, Brach MA. Interleukin-1-induced intracellular signaling pathways converge in the activation of mitogen-activated protein kinase and mitogen-activated protein kinase-activated protein kinase 2 and the subsequent phosphorylation of the 27-kilodalton heat shock protein in monocytic cells. Mol Pharmacol. 1994;46:1077–1083. [PubMed] [Google Scholar]
- 68.Larsen JK, Yamboliev IA, Weber LA, Gerthoffer WT. Phosphorylation of the 27-kDa heat shock protein via p38 MAP kinase and MAPKAP kinase in smooth muscle. Am J Physiol. 1997;273:L930–L940. doi: 10.1152/ajplung.1997.273.5.L930. [DOI] [PubMed] [Google Scholar]
- 69.Rousseau S, Houle F, Landry J, Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997;15:2169–2177. doi: 10.1038/sj.onc.1201380. [DOI] [PubMed] [Google Scholar]
- 70.Azuma N, Akasaka N, Kito H, Ikeda M, Gahtan V, Sasajima T, Sumpio BE. Role of p38 MAP kinase in endothelial cell alignment induced by fluid shear stress. Am J Physiol Heart Circ Physiol. 2001;280:H189–H197. doi: 10.1152/ajpheart.2001.280.1.H189. [DOI] [PubMed] [Google Scholar]
- 71.Huot J, Lambert H, Lavoie JN, Guimond A, Houle F, Landry J. Characterization of 45-kDa/54-kDa Hsp27 kinase, a stress-sensitive kinase which may activate the phosphorylation-dependent protective function of mammalian 27-kDa heat-shock protein HSP27. Eur J Biochem. 1995;227:416–427. doi: 10.1111/j.1432-1033.1995.tb20404.x. [DOI] [PubMed] [Google Scholar]
- 72.Pietersma A, Tilly BC, Gaeste M, de Jong N, Lee JC, Koster JF, Sluiter W. p38 mitogen activated protein kinase regulates endothelial VCAM-1 expression at the post-transcriptional level. Biochem Biophys Res Comm. 1997;230:44–48. doi: 10.1006/bbrc.1996.5886. [DOI] [PubMed] [Google Scholar]
- 73.Schäfer C, Ross SE, Bragado MJ, Groblewski GE, Ernst SA, Williams JA. A role for the p38 mitogen-activated protein kinase/Hsp27 pathway in cholecystokinin-induced changes in the actin cytoskeleton in rat pancreatic acini. J Biol Chem. 1998;273:24173–24180. doi: 10.1074/jbc.273.37.24173. [DOI] [PubMed] [Google Scholar]
- 74.Chevalier D, Allen BG. Two distinct forms of MAPKAP kinase-2 in adult cardiac ventricular myocytes. Biochemistry. 2000;39:6145–6156. doi: 10.1021/bi9928389. [DOI] [PubMed] [Google Scholar]
- 75.Kayyali US, Pennella CM, Trujillo C, Villa O, Gaestel M, Hassoun PM. Cytoskeletal changes in hypoxia pulmonary endothelial cells are dependent on MAPK-activated protein kinase MK2. J Biol Chem. 2002;277:42596–42602. doi: 10.1074/jbc.M205863200. [DOI] [PubMed] [Google Scholar]
- 76.Shi Y, Kotlyarov A, Laasz K, Gruber AD, Butt E, Marcus K, Meyer HE, Friedrich A, Volk HD, Gaestel M. Elimination of protein kinase MK5/PRAK activity by targeted homologous recombination. Mol Cell Biol. 2003;23:7732–7741. doi: 10.1128/MCB.23.21.7732-7741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rousseau S, Dolado I, Beardmore V, Shpiro N, Marquez R, Nebreda AR, Arthur JS, Case LM, Tessier-Lavigne M, Gaestel M, Cuenda A, Cohen P. CXCL12 and C5a trigger cell migration via a PAK1/2–p38α MAPK-MAPKAP-K2-Hsp27 pathway. Cell Signal. 2006;18:1897–1905. doi: 10.1016/j.cellsig.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 78.Huot J, Houle F, Marceau F, Landry J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ Res. 1997;80:383–392. doi: 10.1161/01.res.80.3.383. [DOI] [PubMed] [Google Scholar]
- 79.Ronkina N, Kotlyarov A, Gaestel M. MK2 and MK3–a pair of isoenzymes? Front Biosci. 2008;13:5511–5521. doi: 10.2741/3095. [DOI] [PubMed] [Google Scholar]
- 80.McLaughlin MM, Kumar S, McDonnell PC, Van Horn S, Lee JC, Livi GP, Young PR. Identification of mitogen-activated protein (MAP) kinase-activated protein kinase-3, a novel substrate of CSBP p38 MAP kinase. J Biol Chem. 1996;271:8488–8492. doi: 10.1074/jbc.271.14.8488. [DOI] [PubMed] [Google Scholar]
- 81.Zakowski V, Keramas G, Kilian K, Rapp UR, Ludwig S. Mitogen-activated 3p kinase is active in the nucleus. Exp Cell Res. 2004;299:101–109. doi: 10.1016/j.yexcr.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 82.New L, Jiang Y, Zhao M, Liu K, Zhu W, Flood LJ, Kato Y, Parry GCN, Han J. PRAK, a novel protein kinase regulated by the p38 MAP kinase. EMBO J. 1998;17:3372–3384. doi: 10.1093/emboj/17.12.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ni H, Wang XS, Diener K, Yao Z. MAPKAPK5, a novel mitogen-activated protein kinase (MAPK)-activated protein kinase, is a substrate of the extracellular-regulated kinase (ERK) and p38 kinase. Biochem Biophys Res Commun. 1998;243:492–496. doi: 10.1006/bbrc.1998.8135. [DOI] [PubMed] [Google Scholar]
- 84.Gaestel M. MAPKAP kinases- MKs–two’s company, three’s a crowd. Nat Rev Mol Cell Biol. 2006;7:120–130. doi: 10.1038/nrm1834. [DOI] [PubMed] [Google Scholar]
- 85.Polanowska-Gabrowska R, Gear AR. Heat-shock proteins and platelet function. Platelets. 2000;11:6–22. doi: 10.1080/09537100075742. [DOI] [PubMed] [Google Scholar]
- 86.Gerits N, Mikalsen T, Kostenko S, Shiryaev A, Johannessen M, Moens U. Modulation of F-actin rearrangement by the cyclic AMP/cAMP-dependent protein kinase (PKA) pathway is mediated by MAPK-activated protein kinase 5 and requires PKA-induced nuclear export of MK5. J Biol Chem. 2007;282:37232–37243. doi: 10.1074/jbc.M704873200. [DOI] [PubMed] [Google Scholar]
- 87.Tak H, Jang E, Kim SB, Park J, Suk J, Yoon YS, Ahn JK, Lee J-H, Joe CO. 14-3-3epsilon inhibits MK5-mediated cell migration by disrupting F-actin polymerization. Cell Signal. 2007;19:2379–2387. doi: 10.1016/j.cellsig.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 88.Kostenko S, Johannessen M, Moens U. PKA-induced F-actin rearrangement requires phosphorylation of Hsp27 by the MAPKAP kinase MK5. Cell Signal. 2009;21:712–718. doi: 10.1016/j.cellsig.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 89.Sithanandam G, Latif F, Duh FM, Bernal R, Smola U, Li H, Kuzmin I, Wixler V, Geil L, Shrestha S. 3pK, a new mitogen-activated protein kinase-activated protein kinase located in the small cell lung cancer tumor suppressor gene region. Mol Cell Biol. 1996;16:868–876. doi: 10.1128/mcb.16.3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cairns J, Qin S, Philp R, Tan YH, Guy GR. Dephosphorylaton of the small heat shock protein Hsp27 in vivo by protein phosphatase 2A. J Biol Chem. 1994;269:9176–9183. [PubMed] [Google Scholar]
- 91.Butt E, Immler D, Meyer HE, Kotlyarov A, Laass K, Gaestel M. Heat shock protein 27 is a substrate of cGMP-dependent protein kinase in intact human platelets. J Biol Chem. 2001;276:7108–7113. doi: 10.1074/jbc.m009234200. [DOI] [PubMed] [Google Scholar]
- 92.Huang SY, Tsai ML, Chen GY, Wu CJ, Chen SH. A systematic MS-based approach for identifying in vitro substrates of PKA and PKG in rat uteri. J Proteome Res. 2007;6:2674–2684. doi: 10.1021/pr070134c. [DOI] [PubMed] [Google Scholar]
- 93.Saklatvala J, Guedson F. Interleukin 1 signal transduction. Agents Actions Suppl. 1991;35:35–40. [PubMed] [Google Scholar]
- 94.Nakajima K, Hirade K, Ishisaki A, Matsuno H, Suga H, Kanno Y, Shu E, Kitajima Y, Katagiri Y, Kozawa O. Akt regulates thrombin-induced Hsp27 phosphorylation in aortic smooth muscle cells: function at a point downstream from p38 MAP kinase. Life Sci. 2005;77:96–107. doi: 10.1016/j.lfs.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 95.Lamb NJC, Fernandez A, Feramisco JR, Welch WJ. Modulation of vimentin containing intermediate filament distribution and phosphorylation in living fibroblasts by the cAMP-dependent protein kinase. J Cell Biol. 1989;108:2409–2422. doi: 10.1083/jcb.108.6.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gerits N, Shiryaev A, Kostenko S, Klenow H, Shiryaeva O, Johannessen M, Moens U (2009) Transcriptional regulation and cell-specific expression of the mitogen-activated protein kinase-activated protein kinase MK5. Cell Mol Biol Lett. May 30. [Epub ahead of print]. doi:10.2478/s11658-009-0020-6 [DOI] [PMC free article] [PubMed]
- 97.Michel JJ, Scott JD. AKAP mediated signal transduction. Annu Rev Pharmacol Toxicol. 2002;42:235–257. doi: 10.1146/annurev.pharmtox.42.083101.135801. [DOI] [PubMed] [Google Scholar]
- 98.Konishi H, Matsuzaki H, Tanaka M, Takemura Y, Kuroda S, Ono Y, Kikkawa U. Activation of protein kinase B (Akt/Rac-protein kinase) by cellular stress and its association with heat shock protein Hsp27. FEBS Lett. 1997;410:493–498. doi: 10.1016/s0014-5793(97)00541-3. [DOI] [PubMed] [Google Scholar]
- 99.Rane MJ, Coxon PY, Powell DW, Webster R, Klein JB, Ping P, Pierce W, McLeish KR. p38 Kinase-dependent MAPKAPK-2 activation functions as 3-phosphoinositide-dependent kinase-2 for Akt in human neutrophils. J Biol Chem. 2001;276:3517–3523. doi: 10.1074/jbc.M005953200. [DOI] [PubMed] [Google Scholar]
- 100.Rane MJ, Pan Y, Singh S, Powell DW, Wu R, Cummins T, Chen Q, McLeish KR, Klein JB. Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem. 2003;278:27826–27835. doi: 10.1074/jbc.M303417200. [DOI] [PubMed] [Google Scholar]
- 101.Fukagawa Y, Nishikawa J, Kuramitsu Y, Iwakiri D, Takada K, Imai S, Satake M, Okamoto T, Fujimoto M, Okita K, Nakamura K, Sakaida I. Epstein-Barr virus upregulates phosphorylated heat shock protein 27 kDa in carcinoma cells using the phosphoinositide 3-kinase/Akt pathway. Electrophoresis. 2008;29:3192–3200. doi: 10.1002/elps.200800086. [DOI] [PubMed] [Google Scholar]
- 102.O’Shaughnessy RFL, Welti JC, Cooke JC, Avillion AA, Monks B, Birnbaum MJ, Byrne C. AKT-dependent HspB1 (Hsp27) activity in epidermal differentiation. J Biol Chem. 2007;282:17297–17305. doi: 10.1074/jbc.M610386200. [DOI] [PubMed] [Google Scholar]
- 103.Mearow KM, Dodge ME, Rahimtula M, Yegappan C. Stress-mediated signaling in PC12 cells—the role of the small heat shock protein, Hsp27, and Akt in protecting cells from heat stress and nerve growth factor withdrawal. J Neurochem. 2002;83:452–462. doi: 10.1046/j.1471-4159.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- 104.Alessi DR, Andjelkovich M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 105.Feuerstein N, Cooper HL. Rapid protein phosphorylation induced by phorbol ester in HL-60 cells. Unique alkali-stable phosphorylation of a 17, 000-dalton protein detected by two-dimensional gel electrophoresis. J Biol Chem. 1983;258:10786–10793. [PubMed] [Google Scholar]
- 106.Santell L, Bartfeld NS, Levin EG. Identification of a protein transiently phosphorylated by activators of endothelial function as the heat-shock protein HSP27. Biochem J. 1992;284:705–710. doi: 10.1042/bj2840705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Evans IM, Britton G, Zachary IC. Vascular endothelial growth factor induces heat shock protein (HSP) 27 serine 82 phosphorylation and endothelial tubulogenesis via protein kinase D and independent of p38 kinase. Cell Signal. 2008;20:1375–1384. doi: 10.1016/j.cellsig.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 108.Maizels ET, Peters CA, Kline M, Cutler RE, Jr, Shanmugam M, Hunzicker-Dunn M. Heat-schock protein-25/27 phosphorylation by the δ isoform of protein kinase C. Biochem J. 1998;332:703–712. doi: 10.1042/bj3320703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schultz H, Engel K, Gaestel M. PMA-induced activation of the p42/44ERK- and p38RK-MAP kinase cascades in HL-60 cells is PKC dependent but not essential for differentiation to the macrophage-like phenotype. J Cell Physiol. 1997;173:310–318. doi: 10.1002/(SICI)1097-4652(199712)173:3<310::AID-JCP2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 110.Takai S, Matsushima-Nishiwaki R, Tokuda H, Yasuda E, Toyoda H, Kaneoka Y, Yamaguchi A, Kumada T, Kozawa O. Protein kinase C delta regulates the phosphorylation of heat shock protein 27 in human hepatocellular carcinoma. Life Sci. 2007;81:585–591. doi: 10.1016/j.lfs.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 111.Van Lint J, Ryckx A, Vantus T, Vandenheede JR. Getting to know protein kinase D. Int J Biochem Cell Biol. 2002;34:577–581. doi: 10.1016/s1357-2725(01)00163-7. [DOI] [PubMed] [Google Scholar]
- 112.Döppler H, Storz P, Li J, Comb MJ, Toker A. A phosphorylatin state-specific antibody recognizes Hsp27, a novel substrate of protein kinase D. J Biol Chem. 2005;280:15013–15019. doi: 10.1074/jbc.C400575200. [DOI] [PubMed] [Google Scholar]
- 113.Liu P, Scharenber AM, Cantrell DA, Matthews SA. Protein kinase D enzymes are dispensable for proliferation, survival and antigen receptor-regulated NFκB activity in vertebrate B-cells. FEBS Lett. 2007;581:1377–1382. doi: 10.1016/j.febslet.2007.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McMullen ME, Bryant PW, Glembotski CC, Vincent PA, Pumiglia KM. Activation of p38 has opposing effects on the proliferation and migration of endothelial cells. J Biol Chem. 2005;280:20995–21003. doi: 10.1074/jbc.M407060200. [DOI] [PubMed] [Google Scholar]
- 115.Kobayashi M, Nishita M, Mishima T, Ohashi K, Mizuno K. MAPKAPK-2-mediated LIM-kinase activation is critical for VEGF-induced actin remodeling and cell migration. EMBO J. 2007;25:713–726. doi: 10.1038/sj.emboj.7600973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yuan J, Rozengurt E. PDK, PDK2, and p38 MAPK mediate Hsp27 serine-82 phosphorylation induced by neurotensin in pancreatic cancer PANC-1 cells. J Cell Biochem. 2008;103:648–662. doi: 10.1002/jcb.21439. [DOI] [PubMed] [Google Scholar]
- 117.Storz P, Stoker A. Protein kinase D mediates a stress-induced NF-kappaB activation and survival pathway. EMBO J. 2003;22:109–120. doi: 10.1093/emboj/cdg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arrigo AP. Hsp27: novel regulator of intracellular redox state. IUMMB. 2001;52:303–307. doi: 10.1080/152165401317291156. [DOI] [PubMed] [Google Scholar]
- 119.Walter U, Gambaryan S. cGMP and cGMP-dependent protein kinase in platelets and blood cells. Handb Exp Pharmacol. 2009;191:533–548. doi: 10.1007/978-3-540-68964-5_23. [DOI] [PubMed] [Google Scholar]
- 120.Huang S-Y, Tsai M-L, Wu C-J, Hsu J-L, Ho S-H, Chen S-H. Quantitation of protein phosphorylation in pregnant rat uteri using stable isotope dimethyl labeling coupled with IMAC. Proteomics. 2006;6:1722–1734. doi: 10.1002/pmic.200500507. [DOI] [PubMed] [Google Scholar]
- 121.Kim SO, Xu Y, Katz S, Pelech S. Cyclic GMP-dependent and -independent regulation of MAP kinases by sodium nitroprusside in isolated cardiomyocytes. Biochim Biophys Acta. 2000;1496:277–284. doi: 10.1016/s0167-4889(00)00026-4. [DOI] [PubMed] [Google Scholar]
- 122.Cai H, Liu D, Garcia JG. CaM Kinase II-dependent pathophysiological signalling in endothelial cells. Cardiovac Res. 2008;77:30–34. doi: 10.1093/cvr/cvm010. [DOI] [PubMed] [Google Scholar]
- 123.Levin EG, Santell L. Phosphorylation of an Mr = 29, 000 protein by IL-1 is susceptible to partial down-regulation after endothelial cell activation. J Immunol. 1991;146:3772–3778. [PubMed] [Google Scholar]
- 124.Loktionova SA, Kabakov AE. Protein phosphatase inhibitors and heat preconditioning prevent Hsp27 dephosphorylation, F-actin disruption and deterioration of morphology in ATP-depleted endothelial cells. FEBS Lett. 1998;433:294–300. doi: 10.1016/s0014-5793(98)00920-x. [DOI] [PubMed] [Google Scholar]
- 125.Tar K, Csortos C, Czikora I, Olah G, Ma SF, Wadgaonkar R, Gergely P, Garcia JG, Verin AD. Role of protein phosphatase 2A in the regulation of endothelial cell cytoskeleton structure. J Cell Biochem. 2006;98:931–953. doi: 10.1002/jcb.20829. [DOI] [PubMed] [Google Scholar]
- 126.Berrou E, Bryckaert M. Recruitment of protein phosphatase 2A to dorsal ruffles by platelet-derived growth factor in smooth muscle cells: dephosphorylation of Hsp27. Exp Cell Res. 2009;315:836–848. doi: 10.1016/j.yexcr.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 127.Gaestel M, Benndorf R, Hayess K, Priemer E, Engel K. Dephosphorylation of the small heat shock protein hsp25 by calcium/calmodulin-dependent (type 2B) protein phosphatase. J Biol Chem. 1992;267:21607–21611. [PubMed] [Google Scholar]
- 128.Stokoe D, Campbell DG, Nakielny S, Hikada H, Leevers SJ, Marshall C, Cohen P. MAPKAP kinase-2; a novel protein kinase activated by mitogen-activated protein kinase. EMBO J. 1992;11:3985–3994. doi: 10.1002/j.1460-2075.1992.tb05492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Miron T, Vancompernollle K, Vandekerckhove J, Wilchek M, Geiger B. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J Cell Biol. 1991;114:255–261. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lavoie JN, Hickey E, Weber LA, Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J Biol Chem. 1993;268:24210–24214. [PubMed] [Google Scholar]
- 131.Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein Hsp25 abolish its actin polymerization-inhibiting activity. J Biol Chem. 1994;269:20780–20784. [PubMed] [Google Scholar]
- 132.Huot J, Houle F, Rousseau S, Deschesnes RG, Shah GM, Landry J. SAPK2/p38-dependent F-actin reorganization regulates early membrane blebbing during stress-induced apoptosis. J Cell Biol. 1998;143:1361–1373. doi: 10.1083/jcb.143.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Piotrowicz RS, Hickey E, Levin EG. Heat shock protein 27 kDa expression and phosphorylation regulates endothelial cell migration. FASEB J. 1998;12:1481–1490. doi: 10.1096/fasebj.12.14.1481. [DOI] [PubMed] [Google Scholar]
- 134.Dalle-Donne I, Rossi R, Milzani A, Di Simplicio P, Colombo R. The actin cytoskeleton response to oxidants: from small heat shock protein phosphorylation to changes in the redox state of actin itself. Free Radic Biol Med. 2001;31:1624–1632. doi: 10.1016/s0891-5849(01)00749-3. [DOI] [PubMed] [Google Scholar]
- 135.Chen HF, Xie LD, Xu CS. Role of heat shock protein 27 phosphorylation in migration of vascular smooth muscle cells. Mol Cell Biochem. 2009;327:1–6. doi: 10.1007/s11010-009-0034-4. [DOI] [PubMed] [Google Scholar]
- 136.Hong Z, Zhang QY, Liu J, Wang ZQ, Zhang Y, Xiao Q, Lu J, Zhou HY, Chen S. Phosphoproteome study reveals Hsp27 as a novel signaling molecule involved in GDNF-induced neurite outgrowth. J Proteome Res. 2009;8:2768–2787. doi: 10.1021/pr801052v. [DOI] [PubMed] [Google Scholar]
- 137.Gaestel M, Gross B, Benndorf R, Strauus M, Schunk WH, Kraft R, Otto A, Böhm H, Stahl J, Drabsch H, Bielka H. Molecular cloning, sequencing and expression in Escherichia coli of the 25-kDa growth-related protein of Ehrlich ascites tumor and its homology to mammalian stress proteins. Eur J Biochem. 1989;179:209–213. doi: 10.1111/j.1432-1033.1989.tb14542.x. [DOI] [PubMed] [Google Scholar]
- 138.Oesterreich S, Benndorf R, Bielka H. The expression of the growth-related 25kDa protein (p25) of Ehrlich ascites tumor cells is increased by hyperthermic treatment (heat shock) Biomed Biochim Acta. 1990;49:219–226. [PubMed] [Google Scholar]
- 139.Shibanuma M, Kuroki T, Nose K. Cell-cycle dependent phosphorylation of HSP28 by TGF beta 1 and H2O2 in normal mouse osteoblastic cells (MC3T3–E1), but not in their ras-transformants. Biochem Bipophys Res Commun. 1992;187:1418–1425. doi: 10.1016/0006-291x(92)90460-3. [DOI] [PubMed] [Google Scholar]
- 140.Sullivan CM, Smith DM, Matsui NM, Andrews LE, Clauser KR, Chapeaurouge A, Burlingame AL, Epstein LB. Identification of constitutive and gamma-interferon- and interleukin 4-regulated proteins in the human renal carcinoma cell line ACHN. Cancer Res. 1997;57:1137–1143. [PubMed] [Google Scholar]
- 141.Matsushima-Nishiwaki R, Takai S, Adachi S, Minamitani C, Yasuda E, Noda T, Kato K, Toyoda H, Kaneoka Y, Yamaguchi A, Kumada T, Kozawa O. Phosphorylated heat shock protein 27 represses growth of hepatocellular carcinoma via inhibition of extracellular signal-regulated kinase. J Biol Chem. 2008;283:18852–18860. doi: 10.1074/jbc.M801301200. [DOI] [PubMed] [Google Scholar]
- 142.Venkatakrishnan CD, Dunsmore K, Wong H, Roy S, Sen CK, Wani A, Zweier JL, Ilangovan G. Hsp27 regulates p53 transcriptional activity in doxorubicin treated fibroblasts and cardiac H9c2 cells: upregulation and G2/M phase cell cycle arrest. Am J Physiol Heart Circ Physiol. 2008;294:H1736–H1744. doi: 10.1152/ajpheart.91507.2007. [DOI] [PubMed] [Google Scholar]
- 143.O’Callaghan-Sunol C, Gabai VL, Sherman MY. Hsp27 modulates p53 signaling and suppresses cellular senescence. Cancer Res. 2007;67:11779–11788. doi: 10.1158/0008-5472.CAN-07-2441. [DOI] [PubMed] [Google Scholar]
- 144.Suomalainen M, Nakano MY, Boucke K, Keller S, Greber UF. Adenovirus-activated PKA and p38/MAPK pathways boost microtubule-mediated nuclear targeting of virus. EMBO J. 2001;20:1310–1319. doi: 10.1093/emboj/20.6.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schultz H, Rogalla T, Engel K, Lee JC, Gaestel M. The protein kinase inhibitor SB203580 uncouples PMA-induced differentiation of HL-60 cells from phosphorylation of Hsp27. Cell Stress Chaperones. 1997;2:41–49. doi: 10.1379/1466-1268(1997)002<0041:tpkisu>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kato H, Takai S, Matsushima-Nishiwaki R, Adachi S, Minamitani C, Otsuka T, Tokuda H, Akamatsu S, Doi T, Ogura S, Kozawa O. Hsp27 phosphorylation is correlated with ADP-induced platelet granule secretion. Arch Biochem Biophys. 2008;475:80–86. doi: 10.1016/j.abb.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 147.Arrigo AP, Simon S, Gibert B, Kretiz-Remy C, Nivon M, Czekalla A, Guillet D, Moulin M, Diaz-Latoud C, Vicart P. Hsp27 (HspB1) and αB-crystallin (HspB5) as therapeutic targets. FEBS Lett. 2007;581:3665–3674. doi: 10.1016/j.febslet.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 148.Madsen PS, Hokland P, Clausen N, Ellegaard J, Hokland M. Differential expression of the heat shock protein 27 isoforms in pediatric normal, nonleukemic and common acute lymphoblastic leukemia B-cells precursors. Blood. 1995;85:510–521. [PubMed] [Google Scholar]
- 149.Tremolada L, Magni F, Valsecchi C, Sarto C, Mocarelli P, Perego R, Cordani N, Favini P, Galli Kienle M, Sanchez JC, Hochstrasser DF, Corthals GL. Characterization of heat shock protein 27 phosphorylation sites in renal cell carcinoma. Proteomics. 2005;5:788–795. doi: 10.1002/pmic.200401134. [DOI] [PubMed] [Google Scholar]
- 150.Yasuda E, Kumada T, Takai S, Ishisaki A, Noda T, Matsushima-Nishiwaki R, Yoshimi N, Kato K, Toyoda H, Kaneoka Y, Yamaguchi A, Kozawa O. Attenuated phosphorylation of heat shock protein 27 correlates with tumor progression in patients with hepatocellular carcinoma. Biochem Bipophys Res Commun. 2005;337:337–342. doi: 10.1016/j.bbrc.2005.08.273. [DOI] [PubMed] [Google Scholar]
- 151.Song H, Ethier SP, Dziubinski ML, Lin J. Stat3 modulates heat shock 27 kDa protein expression in breast epithelial cells. Biochem Bipophys Res Commun. 2004;314:143–150. doi: 10.1016/j.bbrc.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 152.Clevenger CV. Roles and regulation of stat family transcription factors in human breast cancer. Am J Pathol. 2004;165:1449–1460. doi: 10.1016/S0002-9440(10)63403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.So A, Hadaschik B, Sowery R, Gleave M. The role of stress proteins in prostate cancer. Curr Genomics. 2007;8:252–261. doi: 10.2174/138920207781386951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Berkowitz P, Hu P, Liu Z, Diaz LA, Enghild JJ, Chua MP, Rubenstein DS. Desmosome signaling. Inhibition of p38MAPK prevents pemphigus vulgaris IgG-induced cytoskeleton reorganization. J Biol Chem. 2005;280:23778–23784. doi: 10.1074/jbc.M501365200. [DOI] [PubMed] [Google Scholar]
- 155.Berkowitz P, Diaz LA, Hall RP, Rubenstein DS. Induction of p38MAPK and Hsp27 phosphorylation in pemphigus patient skin. J Invest Dermatol. 2008;128:738–740. doi: 10.1038/sj.jid.5701080. [DOI] [PubMed] [Google Scholar]
- 156.Berkowitz P, Chua M, Liu Z, Diaz LA, Rubenstein DS. Autoantibodies in the autoimmune disease pemphigus foliaceus induce blistering via p38 mitogen-activated protein kinase-dependent signalling in the skin. Am J Pathol. 2008;173:1628–1636. doi: 10.2353/ajpath.2008.080391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee HE, Berkowitz P, Jolly PS, Diaz LA, Chun MP, Rubenstein DS. Biphasic activation of p38MAPK suggests that apoptosis is a downstream event in pemphigus acantholysis. J Biol Chem. 2009;284:12524–12532. doi: 10.1074/jbc.M808204200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Atkins D, Lichtenfels R, Seliger B. Heat shock proteins in renal cell carcinomas. Contrib Nephrol. 2005;148:35–56. doi: 10.1159/000086042. [DOI] [PubMed] [Google Scholar]
- 159.Smoyer WE, Gupta A, Mundel P, Ballew JD, Welsh MJ. Altered expression of glomerular heat shock protein 27 in experimental nephrotic syndrome. J Clin Invest. 1996;97:2697–2704. doi: 10.1172/JCI118723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dai T, Natarajan R, Nast CC, LaPage J, Chuang P, Sim J, Tong L, Chamberlin M, Wang S, Adler SG. Glucose and diabetes: effects on podocyte and glomerular p38MAPK, heat shock protein 25, and actin cytoskeleton. Kidney Int. 2006;69:806–814. doi: 10.1038/sj.ki.5000033. [DOI] [PubMed] [Google Scholar]
- 161.Barutta F, Pinach S, Giunti S, Vittone F, Forbes JM, Chiarle R, Arnstein M, Perin PC, Camussi G, Copper ME, Gruden G. Heat shock protein expression in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;295:F1817–F1824. doi: 10.1152/ajprenal.90234.2008. [DOI] [PubMed] [Google Scholar]
- 162.Ludwig S, Engel K, Hoffmeyer A, Sithanandam G, Neufeld B, Palm D, Gaestel M, Rapp UR. 3pK, a novel mitogen-activated protein (MAP) kinase-activated protein kinase, is targeted by three MAP kinase pathways. Mol Cell Biol. 1996;16:6687–6697. doi: 10.1128/mcb.16.12.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kim MS, Kewalramani G, Puthanveetil P, Lee V, Kumar U, An D, Abramani A, Rodrigues B. Acute diabetes moderates trafficking of cardiac lipoprotein lipase through p38 mitogen-activated protein kinase-dependent actin cytoskeleton organization. Diabetes. 2008;57:64–76. doi: 10.2337/db07-0832. [DOI] [PubMed] [Google Scholar]
- 164.Kim MS, Wang F, Puthanveetil P, Kewalramani G, Hosseini-Beheshti E, Ng N, Wang Y, Kumar U, Innis S, Proud CG, Abramani A, Rodrigues B. Protein kinase D is a key regulator of cardiomyocyte lipoprotein lipase secretion after diabetes. Circ Res. 2008;103:252–260. doi: 10.1161/CIRCRESAHA.108.178681. [DOI] [PubMed] [Google Scholar]
- 165.Vidyasagar A, Reese S, Acun Z, Hullett D, Djamali A. Hsp27 is involved in the pathogenesis of kidney tubulointerstitial fibrosis. Am J Physiol Renal Physiol. 2008;295:F707–F716. doi: 10.1152/ajprenal.90240.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Singh D, McCann KL, Imani F. MAPK and heat shock protein 27 activation are associated with respiratory syncytial virus induction of human bronchial epithelial monolayer disruption. Am J Physiol Lung Cell Mol Physiol. 2007;293:L436–L445. doi: 10.1152/ajplung.00097.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Collins PL, Crowe JE., Jr . Respiratory syncytial virus and metapneumovirus. In: Knipe DM, Howley PM, editors. Fields virology. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1601–1646. [Google Scholar]
- 168.Gooch C, Shy M. Hereditary motor neuropathy and heat shock proteins: a shocking transformation. Neurology. 2008;71:1656–1657. doi: 10.1212/01.wnl.0000335150.23712.3d. [DOI] [PubMed] [Google Scholar]
- 169.Dierick I, Baets J, Irobi J, Jacobs A, De Vriendt E, Deconinck T, Merlini L, Van den Bergh P, Rasic VM, Robberecht W, Fischer D, Morales RJ, Mitrovic Z, Seeman P, Mazanec R, Kochanski A, Jordanova A, Auer-Grumbach M, Helderman-van den Enden AT, Wokke JH, Nelis E, De Jonghe P, Timmerman V. Relative contribution of mutations in genes for autosomal dominant distal hereditary motor neuropathies: a genotype-phenotype correlation study. Brain. 2008;131:1217–1227. doi: 10.1093/brain/awn029. [DOI] [PubMed] [Google Scholar]
- 170.Kamada M, So A, Muramaki M, Rocchi P, Beraldi E, Gleave M. Hsp27 knockdown using nucleotide-based therapies inhibit tumor growth and enhance chemotherapy in human bladder cancer cells. Mol Cancer Ther. 2007;6:299–308. doi: 10.1158/1535-7163.MCT-06-0417. [DOI] [PubMed] [Google Scholar]
- 171.Zu YL, Ai YX, Huang CK. Characterization of an auto-inhibitory domain in human mitogen-activated protein kinase-activated protein kinase-2. J Biol Chem. 1995;270:202–206. doi: 10.1074/jbc.270.1.202. [DOI] [PubMed] [Google Scholar]
- 172.Hayess K, Benndorf R. Effect of protein kinase inhibitors on activity of mammalian small heat-shock protein (Hsp25) kinase. Biochem Pharmacol. 1997;53:1239–1247. doi: 10.1016/s0006-2952(96)00877-5. [DOI] [PubMed] [Google Scholar]
- 173.Folmer F, Blasius R, Morceau F, Tabudravu J, Dicato M, Jaspars M, Diederich M. Inhibition of TNFα-induced activation of nuclear factor κB by kava (Piper methysticum) derivatives. Biochem Pharmacol. 2006;71:1206–1218. doi: 10.1016/j.bcp.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 174.Mikalsen T, Gerits N, Moens U. Inhibitors of signal transduction protein kinases as targets for cancer therapy. Biotechnol Ann Rev. 2006;12:153–223. doi: 10.1016/S1387-2656(06)12006-2. [DOI] [PubMed] [Google Scholar]
- 175.Anderson DR, Meyers MJ, Vernier WF, Mahoney MW, Kurumbail RG, Caspers N, Poda GI, Schindler JF, Reitz DB, Mourey RJ. Pyrrolopyridine inhibitors of mitogen-activated protein kinase-activated protein kinase 2 (MK-2) J Med Chem. 2007;50:2647–2654. doi: 10.1021/jm0611004. [DOI] [PubMed] [Google Scholar]
- 176.Schlapbach A, Feifel R, Hawtin S, Heng R, Koch G, Moebitz H, Revesz L, Scheufler C, Velcicky J, Waelchli R, Huppertz C. Pyrrolo-pyrimidones: a novel class of MK2 inhibitors with potent cellular activity. Bioorg Med Chem Lett. 2008;18:142–146. doi: 10.1016/j.bmcl.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 177.Lopes LB, Flynn C, Komalavilas P, Panitch A, Brophy CM, Seal BL. Inhibitor of Hsp27 phosphorylation by a cell-permeant MAPKAP kinase 2 inhibitor. Biochem Biophys Res Commun. 2009;382:535–539. doi: 10.1016/j.bbrc.2009.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Damarla M, Hasan E, Boueiz A, Le A, Pae HH, Montouchet C, Kolb T, Simms T, Myers A, Kayyali US, Gaestel M, Peng X, Reddy SP, Damico R, Hassoun PM. Mitogen activated protein kinase activated protein kinase 2 regulates actin polymerization and vascular leak in ventilator associated lung injury. PLoS One. 2009;4:e4600. doi: 10.1371/journal.pone.0004600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shin KD, Lee M-Y, Shin D-S, Lee S, Son K-H, Koh S, Paik Y-Y, Kwon B-M, Han DC. Blocking tumor cell migration and invasion with biphenyl isoxazole derivative KRIBB3, a synthetic molecule that inhibits Hsp27 phosphorylation. J Biol Chem. 2008;280:41439–41448. doi: 10.1074/jbc.M507209200. [DOI] [PubMed] [Google Scholar]
- 180.Wang RE, Kao JL, Hilliard CA, Pandita RK, Roti Roti JL, Hunt CR, Taylor JS. Inhibition of heat shock induction of heat shock protein 70 and enhancement of heat shock protein 27 phosphorylation by quercetin derivatives. J Med Chem. 2009;52:1912–1921. doi: 10.1021/jm801445c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hollander JM, Martin JL, Belke DD, Scott BT, Swanson E, Krishnamoorthy V, Dillmann WH. Overexpression of wild-type heat shock protein 27 and a nonphosphorylatable heat shock 27 mutant protects against ischemia/reperfusion injury in a transgenic mouse model. Circulation. 2004;110:3544–3552. doi: 10.1161/01.CIR.0000148825.99184.50. [DOI] [PubMed] [Google Scholar]
- 182.Kubisch C, Dimagno MJ, Tietz AB, Welsh MJ, Ernst SA, Brandt-Nedelev B, Diebold J, Wagner AC, Göke B, Williams JA, Schäfer C. Overexpression of heat shock protein Hsp27 protects against cerulean-induced pancreatitis. Gastroenterology. 2004;127:275–286. doi: 10.1053/j.gastro.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 183.Malki S, Nef S, Notarnicola C, Thevenet L, Gasca S, Méjean C, Berta P, Poulat F, Boizet-Bonhoure B. Prostaglandin D2 induces nuclear import of the sex-determining factor SOX9 via its cAMP-PKA phosphorylation. EMBO J. 2005;24:1798–1809. doi: 10.1038/sj.emboj.7600660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Seterens OM, Johansen B, Hegge B, Johannessen M, Keyse SM, Moens U. Both binding and activation of p38 mitogen-activated protein kinase (MAPK) play essential roles in regulation of the nucleocytoplasmic distribution of MAPK-activated protein kinase 5 by cell stress. Mol Cell Biol. 2002;22:6931–6945. doi: 10.1128/MCB.22.20.6931-6945.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]