Abstract
The microtubule-system organizes the cytoplasm during interphase and segregates condensed chromosomes during mitosis. Four unrelated conserved proteins, XMAP215/Dis1/TOGp, MCAK, MAP4 and Op18/stathmin, have all been implicated as predominant regulators of tubulin monomer–polymer partitioning in animal cells. However, while studies employing the Xenopus egg extract model system indicate that the partitioning is largely governed by the counteractive activities of XMAP215 and MCAK, studies of human cell lines indicate that MAP4 and Op18 are the predominant regulators of the interphase microtubule-array. Here, we review functional interplay of these proteins during interphase and mitosis in various cell model systems. We also review the evidence that MAP4 and Op18 have interphase-specific, counteractive and phosphorylation-inactivated activities that govern tubulin subunit partitioning in many mammalian cell types. Finally, we discuss evidence indicating that partitioning regulation by MAP4 and Op18 may be of significance to establish cell polarity.
Keywords: Microtubules, Oncoprotein 18, Microtubule-associated proteins, PAR1, MARK, XKCM1, Calmodulin, CaM-dependent kinase
Introduction
The microtubule cytoskeleton is built from α/β-tubulin heterodimer subunits, which assemble into stiff polar hollow tubes (reviewed in [1]). Microtubule formation is facilitated by templated nucleation mediated by γ-tubulin ring complexes at the microtubule organizing center, known as the centrosome in animal cells (reviewed in [2]). In most cells, the interphase microtubule-array is organized with the minus ends anchored within the matrix of the centrosome and the plus ends extending out towards the cell periphery (reviewed in [3]). The interphase microtubule cytoskeleton transforms during mitosis into the bipolar spindle (Fig. 1a). During mitosis, the microtubules of the mitotic spindle organize the duplicated chromosomes onto the cellular equator and thereafter segregate them so that each daughter cell receives a complete set of chromosomes.
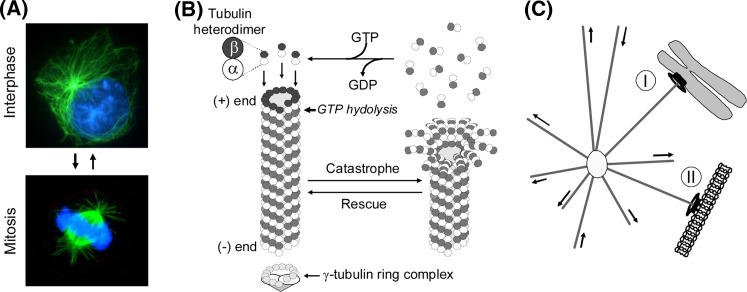
Fig. 1.
a Immunofluorescence images showing DNA (blue) and microtubules (green) in an interphase and a mitotic human leukemia cell. b Dynamic instability of microtubules and the role of GTP hydrolysis for this process are illustrated. c Depiction of how dynamic instability of microtubules facilitates exploration of the intracellular space for stabilizing cues on either chromosomes (I) or the plasma membrane (II). Selective stabilization of dynamic microtubules at stabilizing cues plays a key role during both formation of the mitotic spindle and during morphogenesis
The major functions of the interphase microtubule system are to organize the cytoplasm and to facilitate cellular morphogenesis and polarity, which to a large extent involve transport of various cargos along microtubules. This transport depends on motor proteins such as dynein and kinesins (reviewed in [4]). In this way, microtubules organize cellular organelles and facilitate vesicle transport, e.g., in the secretory- and endocytotic pathways (reviewed in [5]). Moreover, polarized intracellular transport of cargos such as membrane vesicles or actin regulatory proteins is important during cellular migration, which also involves dynein-dependent re-orientation of the centrosome of the cell (reviewed in [6]).
Microtubules exhibit non-equilibrium dynamics, characterized by periods of polymerization and depolymerization, with stochastic transitions between these two states (Fig. 1b) [7, 8]. The transition between polymerization and depolymerization is termed a catastrophe and the opposite event a rescue. The non-equilibrium dynamics of microtubules provide the basis for the “search-and-capture” model of microtubule function (Fig. 1c) [9], which provides the cell with a system to probe its interior for either stabilizing cues on condensed chromosomes during mitosis or membrane proximal stabilization cues during interphase of the cell cycle.
The driving force for the dynamic behavior of microtubules is provided by GTP hydrolysis within the microtubule lattice [10]. Both α- and β-tubulin of the heterodimer have a GTP-binding site but only the E-site (Exchangeable) located on β-tubulin is of regulatory significance. As depicted in Fig. 1b, the E-site bound GTP is exposed at the plus end and becomes buried at the newly formed heterodimer interface during polymerization. This simultaneously prevents GTP-exchange and exposes GTP to a catalytic loop on α-tubulin that promotes its hydrolysis to GDP [11]. Hydrolysis to GDP in turn induces a straight-to-curved conformational change in the tubulin heterodimer (reviewed in [12]). The classical GTP-cap model postulates that the conformational change that results from GTP-hydrolysis at the plus end generates an energetically unstable microtubule lattice that requires a layer of straight GTP loaded heterodimers to prevent depolymerization. The stochastic losses of the GTP-cap, due to either hydrolysis or heterodimer disassociation, allows the GDP-containing protofilaments to relax into a more energetically favorable curved conformation, which disrupts the stabilizing lateral interactions between adjacent protofilaments. The individual protofilaments will consequently rapidly depolymerize since longitudinal interactions alone are not sufficient to maintain the polymer.
The parameters that govern the dynamics of the microtubule-system are the rates of polymerization/depolymerization and frequencies of catastrophe/rescue events (Fig. 1b; reviewed in [13]). The intrinsic microtubule dynamics are modified in the cell by interaction with an array of proteins that stabilize or destabilize microtubules. Cell cycle-specific regulation of such proteins has been reported to mediate an increased frequency of catastrophes during mitosis [14]. A recent proteomic search in Drosophila embryos identified 270 microtubule-interacting proteins [15], out of which about 100 were not previously known to bind microtubules. The task of determining which of these proteins are important for regulation of various aspects of microtubule function represents a major challenge.
Regulation of microtubule dynamics together with regulation of the nucleating activity at the centrosome governs monomer–polymer partitioning of tubulin dimers [16]. Four proteins, XMAP215/Dis1/TOGp, MCAK, MAP4 and Op18/Stathmin, have all been proposed to be the predominant regulators of tubulin subunit partitioning. We here review the importance of these proteins for regulation of tubulin subunit partitioning in various model systems. We also review recent reports suggesting that the phosphorylation-responsive and counteractive activities of Op18 and MAP4 may have a role in establishing cell polarity.
Microtubule regulatory proteins
Many of the evolutionary conserved proteins that regulate the microtubule system can be grouped in two broad functional categories, namely proteins that stabilize or destabilize microtubules. The relative levels and activities of these microtubule stabilizing/destabilizing proteins determine tubulin monomer–polymer partitioning during interphase and mitosis.
The so-called classical microtubule-associated proteins (MAPs) are most abundant and best characterized in neural tissues. These proteins promote tubulin polymerization and stabilize microtubules by cross-linking adjacent protofilaments [17]. Classical MAPs are all composed of an N-terminal projection domain, which protrudes from the microtubule surface, and a C-terminal domain that contains various numbers of microtubule binding repeats. This protein family includes the neuronal members MAP2 and tau, and the ubiquitously expressed MAP4.
A diverse and more recently described family of proteins that interacts with microtubules is termed +TIP-proteins due to their propensity to bind at the plus ends (reviewed in [18]). Members of the +TIP family include XMAP215/Dis1/TOGp, APC, CLASP, CLIP170 and EB1. Some of these proteins have been shown to facilitate stabilization of dynamic microtubules at specific cellular locations, such as the kinetochores of the mitotic chromosomes and polarized sites in the plasma membrane (Fig. 1c). Some +TIP-proteins have also been shown to bind alongside microtubules and thereby serve a stabilizing function, e.g., XMAP215/Dis1/TOGp family members.
Microtubules can be disrupted/destabilized by several distinct mechanisms. The best characterized examples include (1) breakage within the lattice and consequent microtubule severing (reviewed in [19]), (2) catastrophe promotion by interactions with protofilaments at microtubule ends (reviewed in [20]), and (3) sequestering of tubulin heterodimers from the polymerization process (reviewed in [21]). MCAK and Op18/stathmin are examples of microtubule-destabilizing proteins, both of which have been proposed to exert predominant and globally acting destabilizing activities.
The evolutionary conserved XMAP215/Dis1/TOGp +Tip-protein family
Members of the XMAP215/Dis1/TOGp family are found in all eukaryotes and appear to be multi-functional regulators of the microtubule-system (reviewed in [20]). Their most conserved feature is the tubulin binding TOG domain [22], which is repeated between two and five times depending on the organism (reviewed in [23]). Electron microscopy studies have revealed an elongated shape for both Xenopus XMAP215 [24] and the S. cerevisiae family member Stu2p [25]. The crystal structure of a TOG-domain of the C. elegans family member Zyg-9 reveals a flat and paddle-shaped structure [22].
XMAP215 increases the plus-end polymerization rate about sevenfold during assembly of purified tubulin [26]. Two mutually exclusive mechanistic models have been proposed to explain the accelerated polymerization rate, namely (1) XMAP215 serves as a template for tubulin oligomers and thereby facilitates delivery of preassembled protofilaments to the plus-end of microtubules [27, 28], and (2) XMAP215 binds processively to the plus end of the microtubule and facilitates repeated rounds of addition of tubulin heterodimers into polymerizing protofilaments [29], possibly through the recruitment of tubulin heterodimers by high affinity binding.
The MCAK family of catastrophe promoting proteins
The MCAK protein is conserved among vertebrates and belongs to a unique group of the kinesin motor proteins, the kinesin-13 family (previously known as KIN I for their internal motor domain), which depolymerize microtubules rather than translocate along them (reviewed in [30]). Human MCAK is preferentially expressed in proliferative cells [31] and appears frequently overexpressed in solid tumors [32, 33]. The catastrophe-promoting activity of MCAK was originally described by studies of the Xenopus ortholog previously termed XKCM1 [34].
Studies on purified MCAK reveal disruption of both minus and plus ends of microtubules [35], and it has been proposed that ATP-independent diffusion along the sides of microtubules facilitates rapid targeting of MCAK to the microtubule ends [36]. The mechanism behind catastrophe promotion involves MCAK-mediated curling of protofilaments at microtubule ends, with the energy from ATP hydrolysis being utilized to release the protein from the curling protofilament for subsequent recycling of MCAK [35].
Immunofluorescence analyses of cultured cells have shown that MCAK concentrates to kinetochores and spindle poles during mitosis [37]. Inactivation of MCAK, by either microinjection of a dominant negative mutant [38, 39] or RNA interference-mediated protein knockdown [40–42], has relatively modest effects during mitotic spindle assembly. Significantly, however, an increase of lagging chromosomes during both prometaphase and anaphase has been documented [38, 39, 43–45]. There is currently good evidence for a model implying that MCAK is specifically recruited to kinetochores with incorrect microtubule attachments, and subsequently depolymerizes microtubules at these faulty attachments (reviewed in [46]). The regulation of recruitment and activation of MCAK during this process is complex and involves the Aurora B kinase (reviewed in [46]).
XMAP215/TOGp and MCAK exert predominant and antagonistic regulation of tubulin subunit partitioning in Xenopus egg extracts but not in human cell model systems
A partial immunodepletion (~60%) of XMAP215 is sufficient for a pronounced decrease in microtubule length in Xenopus egg extracts, which could be attributed to a general increase in the catastrophe frequency (Fig. 2a) [14]. The authors also noted that, when depletion of XMAP215 was close to 90%, no microtubule asters were detected. Such a dependence of XMAP215 for microtubule formation in egg extracts was also indicated by an earlier report [47]. Given the high degree of conservation between XMAP215 and TOGp (reviewed in [48]), it has been assumed that the globally acting microtubule polymerizing/stabilizing activity of this protein observed in Xenopus egg cells applies for all animal cell systems.
Fig. 2.
a Depiction of the experimental evidence based on aster sizes that the balance between MCAK and XMAP215 in Xenopus egg extracts determines tubulin monomer–polymer partitioning during interphase and mitosis (adapted from [48, 143]). b A model of the demonstrated antagonistic regulation of microtubule dynamics by XMAP215 and MCAK in Xenopus egg extract (the concept adopted from [48]). The consequences of these balancing activities on tubulin monomer–polymer partitioning are also illustrated
Depletion of the catastrophe-promoting MCAK protein in Xenopus egg extracts results in the opposite effect to depletion of XMAP215, namely exceedingly long microtubules due to a general decrease in the catastrophe frequency [34]. Significantly, it was subsequently shown that inhibition of MCAK (by inactivating antibodies) combined with a partial (~60%) immunodepletion of the XMAP215 protein results in microtubules with similar length and catastrophe frequency as observed in control extracts (Fig. 2a) [14]. These experiments provided the foundation for the prevailing concept that MCAK and XMAP215 exert antagonistic activities that determine the partitioning of tubulin subunits and the dynamic behavior of microtubules during both interphase and mitosis in the Xenopus egg extract system (Fig. 2b) [14].
Based on antagonistic activities of MCAK and XMAP215 in Xenopus egg extracts, a general model has been proposed that would explain differences in microtubule length and dynamics during interphase and mitosis [48]. Accordingly, the dominant microtubule stabilizing activity of XMAP215 is proposed to be essential for the long and less dynamic microtubules observed during interphase. At the entry into mitosis, an apparent decrease of XMAP215 activity is thought to relieve inhibition of a globally acting catastrophe-promoting activity by MCAK, which would explain how MCAK in Xenopus egg extracts mediates formation of short and highly dynamic microtubules during mitosis, but not during interphase. Phosphorylation-inactivation of XMAP215 during mitosis is thought to be mediated by the cyclin-dependent kinase CDK1 (Fig. 2b) [49]. This model, outlined in Fig. 2b, has formed the current paradigm on how the microtubule-system is regulated during interphase and mitosis by predominant and antagonistic activities of XMAP215 and MCAK family members (see influential text books such as [50, 51].
Depletion of the human homologue of XMAP215, TOGp, reveals an essential function during assembly of the bipolar spindle during mitosis [52], and this phenotype is suppressed by co-depletion of MCAK [40, 41]. However, not even extensive TOGp-depletion has detectable effects on tubulin subunit partitioning or the appearance of the interphase microtubule-array of human cells [41, 52]. These interphase and mitotic phenotypes in human cells are clearly different to the effects of immunodepletion of Xenopus egg extracts of the highly homologous XMAP215 protein.
Similar to the situation for TOGp, the endogenous levels of the catastrophe-promoting MCAK protein are not of significance for global regulation of tubulin subunit partitioning of the interphase-array of human cell lines [41]. Moreover, extensive MCAK depletion has a comparably modest effect on spindle formation in mammalian cell lines, which appears limited to modest increase in the frequency of mitotic aberrancies such as monopolar spindles and lagging chromosomes [53]. These aberrancies are not associated with detectable global changes of microtubule content [41], which is consistent with the demonstrated local action of MCAK in mammalian cells to depolymerize microtubules at faulty kinetochore attachments (reviewed in [46]).
Purified XMAP215 and the budding yeast homolog Stu2p exert a plus end-specific microtubule-destabilizing activity, which argued against the view of this protein family as a general microtubule stabilizer [54, 55]. Consistent with the potential to destabilize microtubules, overexpressed TOGp causes a partial destabilization of interphase microtubules in human cells, a phenomenon that depends on the presence of the endogenous MCAK protein [41]. Moreover, simultaneous overexpression of MCAK and TOGp has an additive destabilizing effect on the interphase-array of microtubules. While TOGp-mediated destabilization of microtubules may represent non-physiological consequences of overexpression, these results still refute the simple model of XMAP215/TOGp and MCAK interplay depicted in Fig. 2.
In summary, although the functions of TOGp and MCAK are interdependent during spindle assembly in cultured human cells, their functional interplay appears non-reciprocal and altogether very different as compared with the function of the corresponding proteins in the Xenopus egg model system. It also seems clear that the endogenous levels of TOGp and MCAK do not exert significant antagonistic and/or global effects on tubulin subunit partitioning of human cells. These differences between species and cell model systems (i.e., egg cells vs somatic cells) may be due to differential abundance and/or differences in the composition of other microtubule regulatory proteins.
MAP4 is a predominant regulator of tubulin subunit partitioning of the interphase microtubule-array in mammalian cells
The classical microtubule-associated proteins (MAPs), MAP2, MAP4 and tau, are evolutionary related and have a common structural organization (reviewed in [56]). However, only MAP4 appears ubiquitously expressed, while the others are abundant in neuronal tissues. MAP4 is conserved among mammals, and Xenopus cells express a protein with similar overall structure but with weak sequence homology (~30% identity) (reviewed in [57]). MAP4 is encoded by a single gene but, due to alternative RNA splicing, exists as several isoforms that have between three and five repeats of the conserved microtubule binding region. The five-repeat isomer is predominant in most tissues and cell lines but mRNAs encoding the shorter isoforms are developmentally regulated in certain tissues, e.g., heart [58].
The DNA binding p53 tumor suppressor protein has been reported to transcriptionally repress the MAP4 promoter and thereby decrease MAP4 expression [59], which suggests that MAP4 may be upregulated in p53-deficient tumor cells. To our knowledge, however, a clear-cut tumor associated upregulation of MAP4 protein levels or an association between the p53 status and MAP4 protein content has not been reported. Hence, based on the current literature, it appears that the MAP4 protein is present at relatively constant levels in most tissues and cell lines.
Studies on the dynamic behavior of microtubules assembled from purified tubulin show that MAP4 has the potential to increase the rescue frequency, i.e., the transition from depolymerization to polymerization [60]. Moreover, MAP4 binding to microtubules also counteracts the microtubule severing activity of katanin in Xenopus egg extracts [61]. Overexpressed MAP4 has also been reported to act as “road-bumps” that inhibit the microtubule-dependent motility of organelles in the mouse Ltk-cell line [62] and specific mRNA containing ribonucleoprotein particles in rat cardiocytes [63]. However, it remains to be established whether MAP4 at endogenous levels is sufficient to slow down these microtubule-dependent transport functions.
The physiological relevance of microtubule stabilization by MAP4 (then termed XMAP230) was first demonstrated in Xenopus oocytes and eggs in which MAP4 activity was inhibited by microinjected antibodies [64]. Immunodepletion also revealed that MAP4 exerts a significant stabilizing activity on spindle microtubules in the Xenopus egg extract model system [65]. However, antibody-mediated inhibition of microtubule binding by MAP4 in human fibroblasts or monkey epithelial cells did not reveal detectable alterations of microtubule stability or dynamics and cells progressed to mitosis with apparently normal spindles [66]. Still, partial downregulation of MAP4 (~35% decrease) by stable expression of anti-sense RNA in HeLa cells has been reported to be sufficient to cause an approximately twofold decrease in the fraction of polymerized tubulin subunits in interphase cells, i.e., altered tubulin subunit partitioning [67]. The significance of endogenous MAP4 levels for tubulin subunit partitioning in interphase cells was later confirmed by stable expression of interfering short hairpin RNA in human leukemia/lymphoma cell lines, which revealed that ~85% MAP4 depletion causes an approximately twofold decrease in the fraction of polymeric tubulin in interphase cells without detectable effects on the density or function of the mitotic spindle [68].
Post-translational regulation of the microtubule stabilizing activity of MAP4 during interphase and mitosis
Members of the PAR1/MARK family of kinases are essential for epithelial cell polarity (reviewed in [69]) and phosphorylate conserved KXGS motifs that are present in all classical MAPs, which results in their detachment from microtubules (reviewed in [70]). Studies on the cellular effects of MARK-mediated phosphorylation in mammalian cells have centered on neuronal systems, which has been prompted by Alzheimer’s disease-associated phosphorylation of the neural MAP tau at the MARK-specific Ser-262 target site.
MARK family kinases have also been shown to phosphorylation-inactivate MAP4 both in vitro and in co-transfected cells that overexpress both MARK kinases and MAP4 [71]. Consistent with these results, overexpression of MARK family members in cell lines results in a dramatic destabilization of the interphase microtubule-array [72]. Interestingly, the MARK kinases have been shown to be activated by the LKB1 kinase [73], which is a tumor suppressor gene product implicated in the regulation of both epithelial polarity and energy utilization (reviewed in [74]). However, regulation of LKB1 activity is complex and LKB1 has potential to control the activity of at least 13 downstream kinases known to have diverse roles in the regulation of cellular polarity and metabolism [73]. Thus, the significance of the LKB1 kinase-mediated regulation of MARK kinase family members and consequent phosphorylation-inactivation of MAP4 remains unclear.
Phosphorylation of MAP4 by CDK1 decreases its affinity for microtubules [75]. The significance of this mitotic phosphorylation event has been investigated in Xenopus cells by overexpressing a kinase-target site-deficient mutant of MAP4, which was shown to interfere with chromosome movement during anaphase [76]. Hence, phosphorylation inactivation of MAP4 during mitosis seems essential for spindle function.
Septins are conserved proteins found among all eukaryotes except plants and have been described to have essential functions during cell division, cytoskeletal organization and membrane-remodeling events (reviewed in [77]). Interestingly, MAP4 has also been shown to be subject to negative regulation by members of the septin family. Thus, a heterotrimer of septins 2, 6 and 7 has been reported to bind MAP4 and thereby inhibit MAP4 association to microtubules both in vitro and in intact HeLa and MDCK cells [78, 79]. However, the functional roles of septins in animal cells are still poorly described, and further work is required to evaluate the general significance of septin-MAP4 interactions.
Inactivation of MAP4 by injection of specific antibodies [66] or essentially complete depletion of the MAP4 protein by stable expression of interfering RNA [68, 80] does not cause detectable effects on the function, morphology or density of the mitotic spindle. These results appear consistent with phosphorylation-inactivation of MAP4 by CDK1 at mitotic entry. Thus, the function of MAP4 appears to be restricted to stabilize the interphase array of microtubules, and this function has been reported to be post-translationally regulated by multiple mechanisms (Fig. 3).
Fig. 3.
Proposed mechanisms of post-translational regulation of MAP4 during the cell cycle
Op18/stathmin is a predominant interphase-regulator of tubulin subunit partitioning in cell types in which it is abundantly expressed
The cytosolic protein Op18/stathmin (Op18) was initially identified due to phosphorylation in response to a diverse array of signaling events and upregulation in various malignancies (reviewed in [81]). Op18 appears ubiquitous but is still developmentally regulated with expression levels up to 50-fold higher in some neonatal tissues as compared with the corresponding adult tissue [82]. Op18 also seems highly expressed in most post-mitotic neuronal tissues. In T-lymphocytes, Op18 expression is dramatically increased by stimulation with poly-clonally activating mitogens. The Op18 protein is encoded by a single highly conserved gene among vertebrates, and the Drosophila ortholog shares 31–35% amino acid identity [83].
Op18 was identified as a microtubule-destabilizing protein in Xenopus egg extracts [84] and in intact human cells [85]. By in vitro analysis, it was shown that Op18 exerts specific catastrophe-promoting activity [84] and that Op18 forms a ternary complex with two tubulin heterodimers [86]. Subsequent structural analysis revealed that the Op18–tubulin complex consists of two tubulin heterodimers arranged head-to-tail [87] with each of the two tandem helical repeats of Op18 contacting one heterodimer [88].
The mechanism behind Op18-mediated microtubule destabilization was originally proposed to involve specific catastrophe promotion [84]. This view was subsequently challenged by reports claiming that Op18 solely acts by tubulin sequestering and that catastrophe promotion is an indirect effect of decreased free tubulin concentration [86, 89]. However, subsequent reports have confirmed tubulin sequestering-independent catastrophe-promoting properties of Op18 [90–92]. The controversy concerning a catastrophe-promoting activity in vitro can at least in part be explained by different pH in assay buffers since Op18 binds tubulin with lower affinity at pH > 7, which decreases the otherwise dominant tubulin sequestering activity, as compared to the standard pH 6.8 assay conditions (reviewed in [93]). In addition, the catastrophe-promoting activity requires the N-terminal part of Op18, while tubulin sequestering-like activity requires an intact C-terminal part of Op18 [90].
The physiological relevance of Op18 was initially indicated by immunodepletion of Xenopus egg extracts [84], which caused a large increase in the amount of polymerized tubulin in mitotic asters, and antibody-mediated inactivation of Op18 in newt lung cells [94], which in interphase cells revealed reduced catastrophe promotion that was associated with a dramatic increase of microtubule polymer content. Analyses of human leukemia/lymphoma cell model systems, representing cell types with either normal or very high Op18 content, showed that depletion of Op18 by RNA interference results in variable but in all cases pronounced over-polymerization of the interphase microtubule system [68]. These results establish Op18 as a major regulator of tubulin subunit partitioning in various cell types.
Op18 may govern tubulin subunit partitioning by either setting the number of microtubules or influencing their length. A recent study of mouse embryonic fibroblasts from an Op18 knockout mouse has indicated that at least part of the mechanism by which Op18 regulates tubulin subunit partitioning involves an inhibitory effect on centrosomal nucleation [95]. Consistent with these results, it was also reported that a modest increase of Op18 protein levels in the pig kidney epithelial LLCPK cell line decreased centrosomal nucleation. It is notable that the low endogenous Op18 levels in the fibroblast cell model system (~25-fold molar excess of tubulin dimers over Op18) still had a very substantial effect on tubulin subunit partitioning in interphase cells without detectable alterations of dynamics of individual microtubules [95]. This is consistent with Op18 having the potential to regulate the microtubule-system independently of modulation of the free tubulin subunit concentration by tubulin sequestering, and further suggests that Op18 may modulate the interphase microtubule-content by several means.
A posttranscriptional autoregulatory mechanism has been identified that responds to the action of microtubule polymerizing/depolymerizing drugs and involves changes in the stability of the tubulin polysomal mRNA (reviewed in [96]). Interestingly, recent evidence based on Op18-deficient Drosophila embryos [97] and a human leukemia cell line depleted of Op18 by RNA-interference [98], which in both cases represent cell systems with abundant Op18 expression, indicate that Op18 regulates tubulin synthesis through this autoregulatory mechanism. Thus, the function of Op18 involves both regulation of tubulin subunit partitioning and the concentration of tubulin subunits.
Op18 has been implicated in cell migration during Drosophila development [83, 99]. It was subsequently established that Op18 is required for specific microtubule-dependent processes during early Drosophila development and that the mechanism involves maintenance of adequate tubulin pools [97]. However, mice with the Op18 gene inactivated by gene targeting develop normally [100], which suggests some redundancy in mammals of the essential function of Op18 in Drosophila. Nevertheless, Op18-deficient mice still show some neuronal phenotypes such as age-dependent axonopathy of both the central and peripheral nervous systems [101] and impaired function of the basolateral amygdala [102]. The microtubule regulatory properties of Op18 have also been proposed to be of significance for the invasive behavior and metastasis of solid tumor cells [103, 104] and the migratory properties of both transformed and normal cells [105–108].
Op18 is the prototype member of the Op18/stathmin family that is defined by a highly conserved tandem-repeat region that forms a complex with two head-to-tail arranged tubulin heterodimers [109]. However, while Op18 is located in the cytosol and expressed at variable levels in diverse cell types, the other members of the Op18/stathmin family have a hydrophobic N-terminal membrane targeting region, and are expressed mainly in the nervous system (reviewed in [110]). It is notable that all three membrane-localized Op18/stathmin family members, RB3, SCG10, and SCLIP, are expressed at much lower levels than Op18 even in the cell types in which they are most abundant [111]. Thus, it seems likely that the function of these membrane-localized proteins will differ from the frequently abundant cytosolic Op18 protein.
Differential degrees of phosphorylation-inactivation of Op18 during mitosis of mammalian cell lines and in mitotic Xenopus egg extracts
Op18 is phosphorylated at four Ser residues (Ser-16, -25, -38, and -63) in response to activation of several signal transducing and cell cycle regulating kinase-systems (Fig. 4a) [93]. Phosphorylation of these Ser-residues results in various degrees of Op18 inactivation (Fig. 4b). The spindle disruptive activities of kinase-target site deficient mutants of Op18 reveal that spindle formation in human cell lines depends on phosphorylation of the cyclin-dependent kinase sites Ser-25 and Ser-38 at the onset of mitosis [85, 112, 113]. Subsequent studies in mitotic Xenopus egg extracts have revealed phosphorylation of Ser-16 by Aurora B and the polo-like kinase Plx1, which is also essential for spindle assembly [114, 115].
Fig. 4.
a Op18 is phosphorylated by multiple signal transducing kinases during interphase and mitotically activated kinase systems during mitotic entry. The identity of the kinase “X” responsible for Ser-63 phosphorylation during mitosis remains to be identified. b Depiction of how phosphorylation-inactivation of Op18 in response to signal transducing kinase systems regulates tubulin monomer–polymer partitioning in interphase cells. During mitosis of animal cell lines, the Op18 protein is phosphorylated to completion on Ser-25 and Ser-38, and to a substantial degree on Ser-16 and Ser-63, which in most cases appears to result in essentially complete inactivation
In a mitotic population derived from human leukemia cells or HeLa cells, Op18 is phosphorylated to completion on Ser-25 and Ser-38 and to high stoichiometry on Ser-16 and Ser-63 [112, 116]. This suggest that Op18 is essentially inactive during mitosis of human cell lines; a scenario that is consistent with depletion of Op18 by RNA interference causing a pronounced increase in interphase microtubule polymers, but unaltered density and function of the mitotic spindle [68, 117]. However, Op18 expressed at the abnormally high levels observed in some cases of human malignancies still exerts a residual spindle-destabilizing activity during mitosis [117]. Thus, at these excessive levels, Op18 has the potential to perturb spindle formation and thereby bring about the chromosomal instability phenotype that is frequent among malignant tumors.
As evidenced by the electrophoretic mobility of Op18 phospho-isomers in mitotic Xenopus egg extracts, it appears that Op18 in this model system is sub-stoichiometrically phosphorylated on all sites [118]. Consistent with only partial phosphorylation-inactivation of Op18 in mitotic Xenopus egg extracts, immunodepletion of Op18 caused an approximately sixfold increase of polymerized tubulin in mitotic asters [84]. Based on an increase in Op18 phosphorylation in response to mitotic chromatin added to Xenopus egg extracts, a model has been proposed in which Op18 is predominantly phosphorylation-inactivated in the vicinity of condensed chromosomes, which would imply a local protection against the microtubule-destabilizing activity of Op18 during spindle assembly [118, 119]. Subsequent development of a method to analyze Op18–tubulin interactions within intact cells, by means of a fluorescence resonance energy transfer biosensor, has provided direct evidence for such a phosphorylation-gradient around condensed chromosomes in Xenopus cells [120].
The significance of the result from Xenopus cell systems outlined above for spindle formation in mammalian cells remains to be established; in particular since mitosis and meiosis appear unaffected in Op18 mouse knockouts, as evidenced by unperturbed fertility [100], and that Op18 depletion has no detectable consequences for spindle assembly in human cell model systems [117]. Nevertheless, the demonstrated local phosphorylation-inactivation of Op18 in the Xenopus system still provides a proof-of-principle for the existence of this type of local regulation of the microtubule system.
Phosphorylation-inactivation of Op18 may be of significance for establishment of cell polarity in response to cell surface receptor stimulation
Diverse intracellular signaling events have been shown to be associated with increased phosphorylation of Op18 on single or dual Ser-residues (Fig. 4a). There is good evidence that the Ca2+- and calmodulin-dependent protein kinase IV (CaMKIV) and MAP kinase family members phosphorylate Op18 on Ser-16 and Ser-25, respectively (reviewed in [121]). Phosphorylation-inactivation of overexpressed Op18 during interphase has been studied by conditional co-expression of the cognate signal transducing kinases and a series of kinase target site-deficient mutants of Op18 [122, 123], which shows that single site phosphorylation on Ser-16 is sufficient to severely decrease Op18 activity. Given that the Ser-25 phosphorylation-site is absent in chicken Op18 [124], the MAPK target site may be of less significance. This indeed seems to be the case because stoichiometric phosphorylation of Ser-25 alone (by ectopic expression of the MAPK activator MEK1) does not significantly alter the microtubule-destabilizing activity of Op18 in intact cells (our unpublished data).
The actin regulatory Rac protein regulates the interphase microtubule system, which is associated with activation of downstream kinase systems that phosphorylate Op18 on Ser-16 [91, 125]. The significance of Rac-mediated regulation of Op18 activity is indicated by the demonstration that the Rac activator DOCK7 regulates local phosphorylation on Ser-16 [126]. Based on the effect of removing the Ser-16 phosphorylation site of ectopic Op18, it was proposed that this signaling event has a role in regulating neuronal polarity and axon development [126]. Moreover, the lymphocyte-specific Rac activator Dock2 has also been shown to be important for T cell receptor-mediated phosphorylation of Ser-16 [127]. Evidence was presented implying that phosphorylation-inactivation of Op18 could be of significance for microtubule-dependent delivery of internalized IL-4 receptors to the lysosome. Thus, there is ample evidence that Rac-activated kinases cause local or global inactivation of Op18 by phosphorylation on Ser-16, which may be of significance for any Rac-regulated microtubule-dependent process, such as cell migration (reviewed in [128]).
We have recently evaluated the physiological significance of site-specific Op18 phosphorylation in response to antigen receptor activation of human T cells [68]. To provide a system for inducible gene product replacement, replicating vectors directing stable expression of shRNAs in combination with regulatable ectopic expression were employed. Using this strategy, it was established in two distinct human cell types that site-specific phosphorylation of Op18 is indeed the cause of the increased microtubule content observed in response to induced expression of a constitutively active CaMKIV derivative. Importantly, by inducible gene product replacement in the Jurkat model system for T cell signaling, it was shown that Op18 phosphorylation at Ser-16 and Ser-25, previously shown to be CaMKIV and MAPK target sites, respectively [129, 130], is the direct cause of the observed increase in tubulin polymerization in response to antigen receptor activation [68]. Since the fraction of polymerized tubulin dimers in activated Jurkat T cells rapidly increased from ~40 to almost 70% of the total pool of tubulin dimers, phosphorylation-inactivation of Op18 clearly has a major effect on the microtubule-system in this cell model system.
Stimulation of the T cell antigen receptor has been shown to activate a multitude of signaling pathways (reviewed in [131]). Nevertheless, the study outlined above revealed that phosphorylation of Op18 is both sufficient and necessary for the increase in microtubule content that occurs after stimulation of this receptor complex. Physiological stimulation of the T cell receptor by an antigen-presenting cell results in formation of an immunological synapse at the region of cell-to-cell contact (reviewed in [132]), which is associated with T cell polarization as defined by relocalization of the centrosome (reviewed in [133]). Using the Jurkat T cell model system, it has been shown that the minus end-directed molecular motor dynein is recruited to the immunological synapse and generates the microtubule-dependent pulling forces required for relocalization of the centrosome [134]. This appears to involve the same general Cdc42- and dynein-dependent mechanism as described for polarization of astrocytes [135, 136]. Based on these arguments, we propose that phosphorylation-mediated inactivation of Op18 activity and consequent increased polymerization of tubulin increases the probability of dynein to encounter and productively engage microtubules at the immunological synapse, which would predictably be of significance for the efficiency of T cell polarization as illustrated in Fig. 5.
Fig. 5.
A model of how phosphorylation-inactivation of Op18 in response to T cell antigen receptor triggering may facilitate polarization of the microtubule-array towards the antigen-presenting cell. a Op18 is predominantly non-phosphorylated, i.e., active, in unstimulated cells and its microtubule-destabilizing activity regulates subunit partitioning such that less than 50% of all tubulin dimers are polymerized into microtubules. b Stimulation of the T cell antigen receptor by an antigen-presenting cell results in high stoichiometry of Op18 phosphorylation by CaMKIV and MAPK and consequent Op18 inactivation, which causes a pronounced increase in the amount of microtubules. c The minus end-directed molecular motor dynein, which is recruited to the immunological synapse of T cells (dotted circle), generates the microtubule-dependent pulling forces required for T cell polarization, i.e., relocalization of the centrosome towards the antigen-presenting cell. A global increase of microtubule content via phosphorylation-inactivation of Op18 would be predicted to increase the likelihood of microtubule capture by dynein, and thus the efficiency of cell polarization
Op18 levels are known to vary greatly between cell lines and tissues, with particularly high protein levels in neural and embryonic tissues and in diverse diagnostic groups of tumors (reviewed in [137]). Thus, the extent of increased tubulin polymerization in response to Op18 phosphorylation will obviously vary between cell types, depending on the levels of Op18 and the actual stoichiometry of phosphorylation. It follows that the relative importance of the mechanism proposed in Fig. 5 will vary between cell types.
MAP4 and Op18 mediate counteractive phosphorylation-responsive regulation of tubulin subunit partitioning in human cell model systems
As outlined above, both MAP4 and Op18 have been reported to be regulators of tubulin subunit partitioning in diverse cell model systems. By stable expression of interfering shRNA for extensive depletion of MAP4 and/or Op18 for extended periods, we have found that depletion of either of these proteins alone in human leukemia/lymphoma cell model systems results in profound and opposite shifts in tubulin subunit partitioning in interphase cells, and that simultaneous depletion results in close to the normal tubulin subunit partitioning [68]. Thus, MAP4 and Op18 are clearly both predominant and counteractive regulators of tubulin subunit partitioning in these cell types. As discussed above, although both MAP4 and Op18 are ubiquitous proteins, their expression levels certainly vary between tissues and Op18 in particular is renowned for its variable protein levels. It follows that various cell types will differ in their MAP4:Op18 ratio, and the significance of their counteractive activities will consequently be variable.
Analysis of MARK- and CaMKIV-dependent regulation of tubulin subunit partitioning indicates that these kinases depend entirely on MAP4 and Op18, respectively, for their microtubule regulatory activities in leukemia/lymphoma cell model systems [68]. In the case of MARK, which has the potential to phosphorylation-inactivate all classical MAPs [71], this is consistent with MAP4 being the only classical MAP expressed at significant levels in cells of non-neural origin. As predicted by the dependence of the MAP4 and Op18 phosphorylation-targets, co-expression of MARK and CaMKIV also exerts reciprocally counteractive effects on tubulin subunit partitioning. Finally, alterations of the microtubule-system in response to excessive MARK or CaMKIV activities were only evident in interphase cells [68].
The presence of kinases with reciprocally counteractive functions, which operate by inactivation of either MAP4 or Op18, raises the question whether signal transducing kinases may alter tubulin subunit partitioning by phosphorylation of either of these substrates. As outlined above, there is ample evidence of signal transduction events that mediate phosphorylation-inactivation of Op18. However, there is no evidence for rapid MAP4 phosphorylation in response to signal transduction events, and a study on MAP4 phosphorylation after growth factor stimulation reveals only a very slow (12–24 h) and modest phosphorylation response [138].
While it remains unclear how PAR1/MARK kinase activity may be regulated in response to external signals, it is well established that members of this kinase family are essential to establish epithelial cell polarity [139, 140]. The general importance of these kinases in regulating cell polarity gains additional support from the finding that the bacterial Helicobacter pylori CagA protein disrupts the polarity of gastric epithelial cells through inactivation of MARK family members [141]. Given the evidence that the PAR1/MARK family of kinases mediates their microtubule regulatory function in non-neural cell types through phosphorylation-inactivation of MAP4, it seems still reasonable to assume that MARK-dependent regulation of epithelial cell polarity involves MAP4 phosphorylation. This is also consistent with the apparent MAP4 abundance in most types of tissues including epithelial cells [142]. We have indeed found that MAP4 is approximately fourfold more abundant in the epithelial HeLa cell line as compared to human leukemia/lymphoma cell lines, in which we observed a dramatic effect of MARK phosphorylation of endogenous MAP4 (our unpublished data).
Concluding remarks
The function of the XMAP215/Dis1/TOGp, MCAK, MAP4 and Op18 proteins were initially characterized in Xenopus egg extracts. Immunodepletion revealed that each one of these proteins significantly modulates the amount of polymerized tubulin within mitotic asters. However, the prevailing concept of counteractive regulators has been solely founded on experiments involving immunodepletion of XMAP215 and/or MCAK. These two proteins clearly function as the predominant regulators of tubulin subunit partitioning during both interphase and mitosis of the Xenopus egg extract model system (Fig. 2). However, analysis of the corresponding proteins, TOGp and MCAK, in mammalian model systems does not reveal any significance of these proteins for global regulation of tubulin subunit partitioning and their essential roles seem limited to local actions during spindle formation in mitosis.
In human cell lines, MAP4 and Op18 appear to exert the predominant reciprocally counteractive activities that regulate tubulin subunit partitioning during interphase of the cell cycle. The functions of MAP4 and Op18 are regulated by cognate kinase systems that mediate phosphorylation-inactivation, which allows for rapid alterations of tubulin subunit partitioning in any cell type in which these proteins are sufficiently abundant. These alterations in tubulin subunit partitioning may be either global or spatially restricted. As illustrated in Fig. 5, a global increase in microtubule polymers may be of significance for the efficiency by which cell polarity is established in response to external cues.
Acknowledgments
The authors are supported by the Swedish Research Council.
References
- 1.Nogales E. Structural insights into microtubule function. Annu Rev Biochem. 2000;69:277–302. doi: 10.1146/annurev.biochem.69.1.277. [DOI] [PubMed] [Google Scholar]
- 2.Raynaud-Messina B, Merdes A. Gamma–tubulin complexes and microtubule organization. Curr Opin Cell Biol. 2007;19:24–30. doi: 10.1016/j.ceb.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Wiese C, Zheng Y. Microtubule nucleation: gamma-tubulin and beyond. J Cell Sci. 2006;119:4143–4153. doi: 10.1242/jcs.03226. [DOI] [PubMed] [Google Scholar]
- 4.Caviston JP, Holzbaur EL. Microtubule motors at the intersection of trafficking and transport. Trends Cell Biol. 2006;16:530–537. doi: 10.1016/j.tcb.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Mellman I, Nelson WJ. Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol. 2008;9:833–845. doi: 10.1038/nrm2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li R, Gundersen GG. Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nat Rev Mol Cell Biol. 2008;9:860–873. doi: 10.1038/nrm2522. [DOI] [PubMed] [Google Scholar]
- 7.Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- 8.Horio T, Hotani H. Visualization of the dynamic instability of individual microtubules by dark-field microscopy. Nature. 1986;321:605–607. doi: 10.1038/321605a0. [DOI] [PubMed] [Google Scholar]
- 9.Kirschner M, Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986;45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- 10.Hyman AA, Salser S, Drechsel DN, Unwin N, Mitchison TJ. Role of GTP hydrolysis in microtubule dynamics: information from a slowly hydrolyzable analogue, GMPCPP. Mol Biol Cell. 1992;3:1155–1167. doi: 10.1091/mbc.3.10.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nogales E, Whittaker M, Milligan RA, Downing KH. High-resolution model of the microtubule. Cell. 1999;96:79–88. doi: 10.1016/S0092-8674(00)80961-7. [DOI] [PubMed] [Google Scholar]
- 12.Nogales E, Wang HW. Structural intermediates in microtubule assembly and disassembly: how and why? Curr Opin Cell Biol. 2006;18:179–184. doi: 10.1016/j.ceb.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- 14.Tournebize R, Popov A, Kinoshita K, Ashford AJ, Rybina S, Pozniakovsky A, Mayer TU, Walczak CE, Karsenti E, Hyman AA. Control of microtubule dynamics by the antagonistic activities of XMAP215 and XKCM1 in Xenopus egg extracts. Nat Cell Biol. 2000;2:13–19. doi: 10.1038/71330. [DOI] [PubMed] [Google Scholar]
- 15.Hughes JR, Meireles AM, Fisher KH, Garcia A, Antrobus PR, Wainman A, Zitzmann N, Deane C, Ohkura H, Wakefield JG (2008) A microtubule interactome: complexes with roles in cell cycle and mitosis. PLoS Biol 6 e98 [DOI] [PMC free article] [PubMed]
- 16.Mitchison TJ, Kirschner MW. Some thoughts on the partitioning of tubulin between monomer and polymer under conditions of dynamic instability. Cell Biophys. 1987;11:35–55. doi: 10.1007/BF02797111. [DOI] [PubMed] [Google Scholar]
- 17.Gustke N, Trinczek B, Biernat J, Mandelkow EM, Mandelkow E. Domains of tau protein and interactions with microtubules. Biochemistry. 1994;33:9511–9522. doi: 10.1021/bi00198a017. [DOI] [PubMed] [Google Scholar]
- 18.Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9:309–322. doi: 10.1038/nrm2369. [DOI] [PubMed] [Google Scholar]
- 19.Quarmby L. Cellular samurai: katanin and the severing of microtubules. J Cell Sci. 2000;113(Pt 16):2821–2827. doi: 10.1242/jcs.113.16.2821. [DOI] [PubMed] [Google Scholar]
- 20.Howard J, Hyman AA. Microtubule polymerases and depolymerases. Curr Opin Cell Biol. 2007;19:31–35. doi: 10.1016/j.ceb.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Walczak CE. Microtubule dynamics and tubulin interacting proteins. Curr Opin Cell Biol. 2000;12:52–56. doi: 10.1016/S0955-0674(99)00056-3. [DOI] [PubMed] [Google Scholar]
- 22.Al-Bassam J, Larsen NA, Hyman AA, Harrison SC. Crystal structure of a TOG domain: conserved features of XMAP215/Dis1-family TOG domains and implications for tubulin binding. Structure. 2007;15:355–362. doi: 10.1016/j.str.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Ohkura H, Garcia MA, Toda T. Dis1/TOG universal microtubule adaptors: one MAP for all? J Cell Sci. 2001;114:3805–3812. doi: 10.1242/jcs.114.21.3805. [DOI] [PubMed] [Google Scholar]
- 24.Cassimeris L, Gard D, Tran PT, Erickson HP. XMAP215 is a long thin molecule that does not increase microtubule stiffness. J Cell Sci. 2001;114:3025–3033. doi: 10.1242/jcs.114.16.3025. [DOI] [PubMed] [Google Scholar]
- 25.Al-Bassam J, van Breugel M, Harrison SC, Hyman A. Stu2p binds tubulin and undergoes an open-to-closed conformational change. J Cell Biol. 2006;172:1009–1022. doi: 10.1083/jcb.200511010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gard DL, Kirschner MW. A microtubule-associated protein from Xenopus eggs that specifically promotes assembly at the plus-end. J Cell Biol. 1987;105:2203–2215. doi: 10.1083/jcb.105.5.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerssemakers JW, Munteanu EL, Laan L, Noetzel TL, Janson ME, Dogterom M. Assembly dynamics of microtubules at molecular resolution. Nature. 2006;442:709–712. doi: 10.1038/nature04928. [DOI] [PubMed] [Google Scholar]
- 28.Slep KC, Vale RD. Structural basis of microtubule plus end tracking by XMAP215, CLIP-170, and EB1. Mol Cell. 2007;27:976–991. doi: 10.1016/j.molcel.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brouhard GJ, Stear JH, Noetzel TL, Al-Bassam J, Kinoshita K, Harrison SC, Howard J, Hyman AA. XMAP215 is a processive microtubule polymerase. Cell. 2008;132:79–88. doi: 10.1016/j.cell.2007.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinoshita K, Noetzel TL, Arnal I, Drechsel DN, Hyman AA. Global and local control of microtubule destabilization promoted by a catastrophe kinesin MCAK/XKCM1. J Muscle Res Cell Motil. 2006;27:107–114. doi: 10.1007/s10974-005-9045-2. [DOI] [PubMed] [Google Scholar]
- 31.Kim IG, Jun DY, Sohn U, Kim YH. Cloning and expression of human mitotic centromere-associated kinesin gene. Biochim Biophys Acta. 1997;1359:181–186. doi: 10.1016/S0167-4889(97)00103-1. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura Y, Tanaka F, Haraguchi N, Mimori K, Matsumoto T, Inoue H, Yanaga K, Mori M. Clinicopathological and biological significance of mitotic centromere-associated kinesin overexpression in human gastric cancer. Br J Cancer. 2007;97:543–549. doi: 10.1038/sj.bjc.6603905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimo A, Tanikawa C, Nishidate T, Lin ML, Matsuda K, Park JH, Ueki T, Ohta T, Hirata K, Fukuda M, Nakamura Y, Katagiri T. Involvement of kinesin family member 2C/mitotic centromere-associated kinesin overexpression in mammary carcinogenesis. Cancer Sci. 2008;99:62–70. doi: 10.1111/j.1349-7006.2007.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walczak CE, Mitchison TJ, Desai A. XKCM1: a Xenopus kinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell. 1996;84:37–47. doi: 10.1016/S0092-8674(00)80991-5. [DOI] [PubMed] [Google Scholar]
- 35.Desai A, Verma S, Mitchison TJ, Walczak CE. Kin I kinesins are microtubule-destabilizing enzymes. Cell. 1999;96:69–78. doi: 10.1016/S0092-8674(00)80960-5. [DOI] [PubMed] [Google Scholar]
- 36.Helenius J, Brouhard G, Kalaidzidis Y, Diez S, Howard J. The depolymerizing kinesin MCAK uses lattice diffusion to rapidly target microtubule ends. Nature. 2006;441:115–119. doi: 10.1038/nature04736. [DOI] [PubMed] [Google Scholar]
- 37.Wordeman L, Mitchison TJ. Identification and partial characterization of mitotic centromere-associated kinesin, a kinesin-related protein that associates with centromeres during mitosis. J Cell Biol. 1995;128:95–104. doi: 10.1083/jcb.128.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maney T, Hunter AW, Wagenbach M, Wordeman L. Mitotic centromere-associated kinesin is important for anaphase chromosome segregation. J Cell Biol. 1998;142:787–801. doi: 10.1083/jcb.142.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kline-Smith SL, Khodjakov A, Hergert P, Walczak CE. Depletion of centromeric MCAK leads to chromosome congression and segregation defects due to improper kinetochore attachments. Mol Biol Cell. 2004;15:1146–1159. doi: 10.1091/mbc.E03-08-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cassimeris L, Morabito J. TOGp, the human homolog of XMAP215/Dis1, is required for centrosome integrity, spindle pole organization, and bipolar spindle assembly. Mol Biol Cell. 2004;15:1580–1590. doi: 10.1091/mbc.E03-07-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmfeldt P, Stenmark S, Gullberg M. Differential functional interplay of TOGp/XMAP215 and the KinI kinesin MCAK during interphase and mitosis. EMBO J. 2004;23:627–637. doi: 10.1038/sj.emboj.7600076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ganem NJ, Upton K, Compton DA. Efficient mitosis in human cells lacking poleward microtubule flux. Curr Biol. 2005;15:1827–1832. doi: 10.1016/j.cub.2005.08.065. [DOI] [PubMed] [Google Scholar]
- 43.Walczak CE, Gan EC, Desai A, Mitchison TJ, Kline-Smith SL. The microtubule-destabilizing kinesin XKCM1 is required for chromosome positioning during spindle assembly. Curr Biol. 2002;12:1885–1889. doi: 10.1016/S0960-9822(02)01227-7. [DOI] [PubMed] [Google Scholar]
- 44.Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR. Aurora B regulates MCAK at the mitotic centromere. Dev Cell. 2004;6:253–268. doi: 10.1016/S1534-5807(04)00025-5. [DOI] [PubMed] [Google Scholar]
- 45.Lan W, Zhang X, Kline-Smith SL, Rosasco SE, Barrett-Wilt GA, Shabanowitz J, Hunt DF, Walczak CE, Stukenberg PT. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr Biol. 2004;14:273–286. doi: 10.1016/j.cub.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 46.Walczak CE, Heald R. Mechanisms of mitotic spindle assembly and function. Int Rev Cytol. 2008;265:111–158. doi: 10.1016/S0074-7696(07)65003-7. [DOI] [PubMed] [Google Scholar]
- 47.Wilde A, Zheng Y. Stimulation of microtubule aster formation and spindle assembly by the small GTPase Ran. Science. 1999;284:1359–1362. doi: 10.1126/science.284.5418.1359. [DOI] [PubMed] [Google Scholar]
- 48.Kinoshita K, Habermann B, Hyman AA. XMAP215: a key component of the dynamic microtubule cytoskeleton. Trends Cell Biol. 2002;12:267–273. doi: 10.1016/S0962-8924(02)02295-X. [DOI] [PubMed] [Google Scholar]
- 49.Vasquez RJ, Gard DL, Cassimeris L. Phosphorylation by CDK1 regulates XMAP215 function in vitro. Cell Motil Cytoskeleton. 1999;43:310–321. doi: 10.1002/(SICI)1097-0169(1999)43:4<310::AID-CM4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 50.Alberts B. Molecular biology of the cell. 5. New York: Garland Science; 2008. [Google Scholar]
- 51.Lodish HF. Molecular cell biology. 6. New York: Freeman; 2008. [Google Scholar]
- 52.Gergely F, Draviam VM, Raff JW. The ch-TOG/XMAP215 protein is essential for spindle pole organization in human somatic cells. Genes Dev. 2003;17:336–341. doi: 10.1101/gad.245603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kline-Smith SL, Walczak CE. The microtubule-destabilizing kinesin XKCM1 regulates microtubule dynamic instability in cells. Mol Biol Cell. 2002;13:2718–2731. doi: 10.1091/mbc.E01-12-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shirasu-Hiza M, Coughlin P, Mitchison T. Identification of XMAP215 as a microtubule-destabilizing factor in Xenopus egg extract by biochemical purification. J Cell Biol. 2003;161:349–358. doi: 10.1083/jcb.200211095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Breugel M, Drechsel D, Hyman A. Stu2p, the budding yeast member of the conserved Dis1/XMAP215 family of microtubule-associated proteins is a plus end-binding microtubule destabilizer. J Cell Biol. 2003;161:359–369. doi: 10.1083/jcb.200211097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mandelkow E, Mandelkow EM. Microtubules and microtubule-associated proteins. Curr Opin Cell Biol. 1995;7:72–81. doi: 10.1016/0955-0674(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 57.Andersen SS. Spindle assembly and the art of regulating microtubule dynamics by MAPs and Stathmin/Op18. Trends Cell Biol. 2000;10:261–267. doi: 10.1016/S0962-8924(00)01786-4. [DOI] [PubMed] [Google Scholar]
- 58.Chapin SJ, Lue CM, Yu MT, Bulinski JC. Differential expression of alternatively spliced forms of MAP4: a repertoire of structurally different microtubule-binding domains. Biochemistry. 1995;34:2289–2301. doi: 10.1021/bi00007a025. [DOI] [PubMed] [Google Scholar]
- 59.Murphy M, Hinman A, Levine AJ. Wild-type p53 negatively regulates the expression of a microtubule-associated protein. Genes Dev. 1996;10:2971–2980. doi: 10.1101/gad.10.23.2971. [DOI] [PubMed] [Google Scholar]
- 60.Ookata K, Hisanaga S, Bulinski JC, Murofushi H, Aizawa H, Itoh TJ, Hotani H, Okumura E, Tachibana K, Kishimoto T. Cyclin B interaction with microtubule-associated protein 4 (MAP4) targets p34cdc2 kinase to microtubules and is a potential regulator of M-phase microtubule dynamics. J Cell Biol. 1995;128:849–862. doi: 10.1083/jcb.128.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McNally KP, Buster D, McNally FJ. Katanin-mediated microtubule severing can be regulated by multiple mechanisms. Cell Motil Cytoskeleton. 2002;53:337–349. doi: 10.1002/cm.10080. [DOI] [PubMed] [Google Scholar]
- 62.Bulinski JC, McGraw TE, Gruber D, Nguyen HL, Sheetz MP. Overexpression of MAP4 inhibits organelle motility and trafficking in vivo. J Cell Sci. 1997;110(Pt 24):3055–3064. doi: 10.1242/jcs.110.24.3055. [DOI] [PubMed] [Google Scholar]
- 63.Scholz D, McDermott P, Garnovskaya M, Gallien TN, Huettelmaier S, DeRienzo C, Cooper Gt. Microtubule-associated protein-4 (MAP-4) inhibits microtubule-dependent distribution of mRNA in isolated neonatal cardiocytes. Cardiovasc Res. 2006;71:506–516. doi: 10.1016/j.cardiores.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 64.Cha BJ, Error B, Gard DL. XMAP230 is required for the assembly and organization of acetylated microtubules and spindles in Xenopus oocytes and eggs. J Cell Sci. 1998;111(Pt 16):2315–2327. doi: 10.1242/jcs.111.16.2315. [DOI] [PubMed] [Google Scholar]
- 65.Cha B, Cassimeris L, Gard DL. XMAP230 is required for normal spindle assembly in vivo and in vitro. J Cell Sci. 1999;112(Pt 23):4337–4346. doi: 10.1242/jcs.112.23.4337. [DOI] [PubMed] [Google Scholar]
- 66.Wang XM, Peloquin JG, Zhai Y, Bulinski JC, Borisy GG. Removal of MAP4 from microtubules in vivo produces no observable phenotype at the cellular level. J Cell Biol. 1996;132:345–357. doi: 10.1083/jcb.132.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nguyen HL, Gruber D, Bulinski JC. Microtubule-associated protein 4 (MAP4) regulates assembly, protomer-polymer partitioning and synthesis of tubulin in cultured cells. J Cell Sci. 1999;112(Pt 12):1813–1824. doi: 10.1242/jcs.112.12.1813. [DOI] [PubMed] [Google Scholar]
- 68.Holmfeldt P, Stenmark S, Gullberg M. Interphase-specific phosphorylation-mediated regulation of tubulin dimer partitioning in human cells. Mol Biol Cell. 2007;18:1909–1917. doi: 10.1091/mbc.E07-01-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hurov J, Piwnica-Worms H. The Par-1/MARK family of protein kinases: from polarity to metabolism. Cell Cycle. 2007;6:1966–1969. doi: 10.4161/cc.6.16.4576. [DOI] [PubMed] [Google Scholar]
- 70.Drewes G, Ebneth A, Mandelkow EM. MAPs, MARKs and microtubule dynamics. Trends Biochem Sci. 1998;23:307–311. doi: 10.1016/S0968-0004(98)01245-6. [DOI] [PubMed] [Google Scholar]
- 71.Drewes G, Ebneth A, Preuss U, Mandelkow EM, Mandelkow E. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89:297–308. doi: 10.1016/S0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- 72.Ebneth A, Drewes G, Mandelkow EM, Mandelkow E. Phosphorylation of MAP2c and MAP4 by MARK kinases leads to the destabilization of microtubules in cells. Cell Motil Cytoskeleton. 1999;44:209–224. doi: 10.1002/(SICI)1097-0169(199911)44:3<209::AID-CM6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 73.Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hezel AF, Bardeesy N. LKB1; linking cell structure and tumor suppression. Oncogene. 2008;27:6908–6919. doi: 10.1038/onc.2008.342. [DOI] [PubMed] [Google Scholar]
- 75.Ookata K, Hisanaga S, Sugita M, Okuyama A, Murofushi H, Kitazawa H, Chari S, Bulinski JC, Kishimoto T. MAP4 is the in vivo substrate for CDC2 kinase in HeLa cells: identification of an M-phase specific and a cell cycle-independent phosphorylation site in MAP4. Biochemistry. 1997;36:15873–15883. doi: 10.1021/bi971251w. [DOI] [PubMed] [Google Scholar]
- 76.Shiina N, Tsukita S. Mutations at phosphorylation sites of Xenopus microtubule-associated protein 4 affect its microtubule-binding ability and chromosome movement during mitosis. Mol Biol Cell. 1999;10:597–608. doi: 10.1091/mbc.10.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weirich CS, Erzberger JP, Barral Y. The septin family of GTPases: architecture and dynamics. Nat Rev Mol Cell Biol. 2008;9:478–489. doi: 10.1038/nrm2407. [DOI] [PubMed] [Google Scholar]
- 78.Kremer BE, Haystead T, Macara IG. Mammalian septins regulate microtubule stability through interaction with the microtubule-binding protein MAP4. Mol Biol Cell. 2005;16:4648–4659. doi: 10.1091/mbc.E05-03-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spiliotis ET, Hunt SJ, Hu Q, Kinoshita M, Nelson WJ. Epithelial polarity requires septin coupling of vesicle transport to polyglutamylated microtubules. J Cell Biol. 2008;180:295–303. doi: 10.1083/jcb.200710039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holmfeldt P, Zhang X, Stenmark S, Walczak CE, Gullberg M. CaMKIIgamma-mediated inactivation of the Kin I kinesin MCAK is essential for bipolar spindle formation. EMBO J. 2005;24:1256–1266. doi: 10.1038/sj.emboj.7600601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sobel A. Stathmin: a relay phosphoprotein for multiple signal transduction? Trends Biochem Sci. 1991;16:301–305. doi: 10.1016/0968-0004(91)90123-D. [DOI] [PubMed] [Google Scholar]
- 82.Koppel J, Boutterin MC, Doye V, Peyro-Saint-Paul H, Sobel A. Developmental tissue expression and phylogenetic conservation of stathmin, a phosphoprotein associated with cell regulations. J Biol Chem. 1990;265:3703–3707. [PubMed] [Google Scholar]
- 83.Ozon S, Guichet A, Gavet O, Roth S, Sobel A. Drosophila stathmin: a microtubule-destabilizing factor involved in nervous system formation. Mol Biol Cell. 2002;13:698–710. doi: 10.1091/mbc.01-07-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Belmont LD, Mitchison TJ. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell. 1996;84:623–631. doi: 10.1016/S0092-8674(00)81037-5. [DOI] [PubMed] [Google Scholar]
- 85.Marklund U, Larsson N, Gradin HM, Brattsand G, Gullberg M. Oncoprotein 18 is a phosphorylation-responsive regulator of microtubule dynamics. EMBO J. 1996;15:5290–5298. [PMC free article] [PubMed] [Google Scholar]
- 86.Jourdain L, Curmi P, Sobel A, Pantaloni D, Carlier MF. Stathmin: a tubulin-sequestering protein which forms a ternary T2S complex with two tubulin molecules. Biochemistry. 1997;36:10817–10821. doi: 10.1021/bi971491b. [DOI] [PubMed] [Google Scholar]
- 87.Steinmetz MO, Kammerer RA, Jahnke W, Goldie KN, Lustig A, van Oostrum J. Op18/stathmin caps a kinked protofilament-like tubulin tetramer. EMBO J. 2000;19:572–580. doi: 10.1093/emboj/19.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gigant B, Curmi PA, Martin-Barbey C, Charbaut E, Lachkar S, Lebeau L, Siavoshian S, Sobel A, Knossow M. The 4 A X-ray structure of a tubulin:stathmin-like domain complex. Cell. 2000;102:809–816. doi: 10.1016/S0092-8674(00)00069-6. [DOI] [PubMed] [Google Scholar]
- 89.Curmi PA, Andersen SS, Lachkar S, Gavet O, Karsenti E, Knossow M, Sobel A. The stathmin/tubulin interaction in vitro. J Biol Chem. 1997;272:25029–25036. doi: 10.1074/jbc.272.40.25029. [DOI] [PubMed] [Google Scholar]
- 90.Howell B, Larsson N, Gullberg M, Cassimeris L. Dissociation of the tubulin-sequestering and microtubule catastrophe-promoting activities of oncoprotein 18/stathmin. Mol Biol Cell. 1999;10:105–118. doi: 10.1091/mbc.10.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wittmann T, Bokoch GM, Waterman-Storer CM. Regulation of microtubule destabilizing activity of Op18/stathmin downstream of Rac1. J Biol Chem. 2004;279:6196–6203. doi: 10.1074/jbc.M307261200. [DOI] [PubMed] [Google Scholar]
- 92.Manna T, Thrower D, Miller HP, Curmi P, Wilson L. Stathmin strongly increases the minus end catastrophe frequency and induces rapid treadmilling of bovine brain microtubules at steady state in vitro. J Biol Chem. 2006;281:2071–2078. doi: 10.1074/jbc.M510661200. [DOI] [PubMed] [Google Scholar]
- 93.Cassimeris L. The oncoprotein 18/stathmin family of microtubule destabilizers. Curr Opin Cell Biol. 2002;14:18–24. doi: 10.1016/S0955-0674(01)00289-7. [DOI] [PubMed] [Google Scholar]
- 94.Howell B, Deacon H, Cassimeris L. Decreasing oncoprotein 18/stathmin levels reduces microtubule catastrophes and increases microtubule polymer in vivo. J Cell Sci. 1999;112(Pt 21):3713–3722. doi: 10.1242/jcs.112.21.3713. [DOI] [PubMed] [Google Scholar]
- 95.Ringhoff DN Cassimeris L (2009) Stathmin regulates centrosomal nucleation of microtubules and tubulin dimer/polymer partitioning. Mol Biol Cell (in press) [DOI] [PMC free article] [PubMed]
- 96.Cleveland DW. Autoregulated instability of tubulin mRNAs: a novel eukaryotic regulatory mechanism. Trends Biochem Sci. 1988;13:339–343. doi: 10.1016/0968-0004(88)90103-X. [DOI] [PubMed] [Google Scholar]
- 97.Fletcher G, Rorth P. Drosophila stathmin is required to maintain tubulin pools. Curr Biol. 2007;17:1067–1071. doi: 10.1016/j.cub.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 98.Sellin ME, Holmfeldt P, Stenmark S, Gullberg M. Global regulation of the interphase microtubule system by abundantly expressed op18/stathmin. Mol Biol Cell. 2008;19:2897–2906. doi: 10.1091/mbc.E08-01-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Borghese L, Fletcher G, Mathieu J, Atzberger A, Eades WC, Cagan RL, Rorth P. Systematic analysis of the transcriptional switch inducing migration of border cells. Dev Cell. 2006;10:497–508. doi: 10.1016/j.devcel.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schubart UK, Yu J, Amat JA, Wang Z, Hoffmann MK, Edelmann W. Normal development of mice lacking metablastin (P19), a phosphoprotein implicated in cell cycle regulation. J Biol Chem. 1996;271:14062–14066. doi: 10.1074/jbc.271.24.14062. [DOI] [PubMed] [Google Scholar]
- 101.Liedtke W, Leman EE, Fyffe RE, Raine CS, Schubart UK. Stathmin-deficient mice develop an age-dependent axonopathy of the central and peripheral nervous systems. Am J Pathol. 2002;160:469–480. doi: 10.1016/S0002-9440(10)64866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shumyatsky GP, Malleret G, Shin RM, Takizawa S, Tully K, Tsvetkov E, Zakharenko SS, Joseph J, Vronskaya S, Yin D, Schubart UK, Kandel ER, Bolshakov VY. Stathmin, a gene enriched in the amygdala, controls both learned and innate fear. Cell. 2005;123:697–709. doi: 10.1016/j.cell.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 103.Baldassarre G, Belletti B, Nicoloso MS, Schiappacassi M, Vecchione A, Spessotto P, Morrione A, Canzonieri V, Colombatti A. p27(Kip1)–stathmin interaction influences sarcoma cell migration and invasion. Cancer Cell. 2005;7:51–63. doi: 10.1016/j.ccr.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 104.Belletti B, Nicoloso MS, Schiappacassi M, Berton S, Lovat F, Wolf K, Canzonieri V, D’Andrea S, Zucchetto A, Friedl P, Colombatti A, Baldassarre G. Stathmin activity influences sarcoma cell shape, motility, and metastatic potential. Mol Biol Cell. 2008;19:2003–2013. doi: 10.1091/mbc.E07-09-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Langenickel TH, Olive M, Boehm M, San H, Crook MF, Nabel EG. KIS protects against adverse vascular remodeling by opposing stathmin-mediated VSMC migration in mice. J Clin Invest. 2008;118:3848–3859. doi: 10.1172/JCI33206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ng DC, Lin BH, Lim CP, Huang G, Zhang T, Poli V, Cao X. Stat3 regulates microtubules by antagonizing the depolymerization activity of stathmin. J Cell Biol. 2006;172:245–257. doi: 10.1083/jcb.200503021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Verma NK, Dourlat J, Davies AM, Long A, Liu WQ, Garbay C, Kelleher D, Volkov Y. STAT3–stathmin interactions control microtubule dynamics in migrating T-cells. J Biol Chem. 2009;284:12349–12362. doi: 10.1074/jbc.M807761200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Singer S, Malz M, Herpel E, Warth A, Bissinger M, Keith M, Muley T, Meister M, Hoffmann H, Penzel R, Gdynia G, Ehemann V, Schnabel PA, Kuner R, Huber P, Schirmacher P, Breuhahn K. Coordinated expression of stathmin family members by far upstream sequence element-binding protein-1 increases motility in non-small cell lung cancer. Cancer Res. 2009;69:2234–2243. doi: 10.1158/0008-5472.CAN-08-3338. [DOI] [PubMed] [Google Scholar]
- 109.Ozon S, Maucuer A, Sobel A. The stathmin family–molecular and biological characterization of novel mammalian proteins expressed in the nervous system. Eur J Biochem. 1997;248:794–806. doi: 10.1111/j.1432-1033.1997.t01-2-00794.x. [DOI] [PubMed] [Google Scholar]
- 110.Curmi PA, Gavet O, Charbaut E, Ozon S, Lachkar-Colmerauer S, Manceau V, Siavoshian S, Maucuer A, Sobel A. Stathmin and its phosphoprotein family: general properties, biochemical and functional interaction with tubulin. Cell Struct Funct. 1999;24:345–357. doi: 10.1247/csf.24.345. [DOI] [PubMed] [Google Scholar]
- 111.Bieche I, Maucuer A, Laurendeau I, Lachkar S, Spano AJ, Frankfurter A, Levy P, Manceau V, Sobel A, Vidaud M, Curmi PA. Expression of stathmin family genes in human tissues: non-neural-restricted expression for SCLIP. Genomics. 2003;81:400–410. doi: 10.1016/S0888-7543(03)00031-4. [DOI] [PubMed] [Google Scholar]
- 112.Larsson N, Melander H, Marklund U, Osterman O, Gullberg M. G2/M transition requires multisite phosphorylation of oncoprotein 18 by two distinct protein kinase systems. J Biol Chem. 1995;270:14175–14183. doi: 10.1074/jbc.270.11.5950. [DOI] [PubMed] [Google Scholar]
- 113.Larsson N, Marklund U, Gradin HM, Brattsand G, Gullberg M. Control of microtubule dynamics by oncoprotein 18: dissection of the regulatory role of multisite phosphorylation during mitosis. Mol Cell Biol. 1997;17:5530–5539. doi: 10.1128/mcb.17.9.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gadea BB, Ruderman JV. Aurora B is required for mitotic chromatin-induced phosphorylation of Op18/Stathmin. Proc Natl Acad Sci USA. 2006;103:4493–4498. doi: 10.1073/pnas.0600702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Budde PP, Kumagai A, Dunphy WG, Heald R. Regulation of Op18 during spindle assembly in Xenopus egg extracts. J Cell Biol. 2001;153:149–158. doi: 10.1083/jcb.153.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brattsand G, Marklund U, Nylander K, Roos G, Gullberg M. Cell-cycle-regulated phosphorylation of oncoprotein 18 on Ser16, Ser25 and Ser38. Eur J Biochem. 1994;220:359–368. doi: 10.1111/j.1432-1033.1994.tb18632.x. [DOI] [PubMed] [Google Scholar]
- 117.Holmfeldt P, Brannstrom K, Stenmark S, Gullberg M. Aneugenic activity of Op18/stathmin is potentiated by the somatic Q18–>e mutation in leukemic cells. Mol Biol Cell. 2006;17:2921–2930. doi: 10.1091/mbc.E06-02-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Andersen SS, Ashford AJ, Tournebize R, Gavet O, Sobel A, Hyman AA, Karsenti E. Mitotic chromatin regulates phosphorylation of Stathmin/Op18. Nature. 1997;389:640–643. doi: 10.1038/39382. [DOI] [PubMed] [Google Scholar]
- 119.Tournebize R, Andersen SS, Verde F, Doree M, Karsenti E, Hyman AA. Distinct roles of PP1 and PP2A-like phosphatases in control of microtubule dynamics during mitosis. EMBO J. 1997;16:5537–5549. doi: 10.1093/emboj/16.18.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Niethammer P, Bastiaens P, Karsenti E. Stathmin–tubulin interaction gradients in motile and mitotic cells. Science. 2004;303:1862–1866. doi: 10.1126/science.1094108. [DOI] [PubMed] [Google Scholar]
- 121.Lawler S. Microtubule dynamics: if you need a shrink try stathmin/Op18. Curr Biol. 1998;8:R212–R214. doi: 10.1016/S0960-9822(98)70128-9. [DOI] [PubMed] [Google Scholar]
- 122.Melander Gradin H, Marklund U, Larsson N, Chatila TA, Gullberg M. Regulation of microtubule dynamics by Ca2+/calmodulin-dependent kinase IV/Gr-dependent phosphorylation of oncoprotein 18. Mol Cell Biol. 1997;17:3459–3467. doi: 10.1128/mcb.17.6.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gradin HM, Larsson N, Marklund U, Gullberg M. Regulation of microtubule dynamics by extracellular signals: cAMP-dependent protein kinase switches off the activity of oncoprotein 18 in intact cells. J Cell Biol. 1998;140:131–141. doi: 10.1083/jcb.140.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Antonsson B, Kassel DB, Ruchti E, Grenningloh G. Differences in phosphorylation of human and chicken stathmin by MAP kinase. J Cell Biochem. 2001;80:346–352. doi: 10.1002/1097-4644(20010301)80:3<346::AID-JCB70>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 125.Daub H, Gevaert K, Vandekerckhove J, Sobel A, Hall A. Rac/Cdc42 and p65PAK regulate the microtubule-destabilizing protein stathmin through phosphorylation at serine 16. J Biol Chem. 2001;276:1677–1680. doi: 10.1074/jbc.C000635200. [DOI] [PubMed] [Google Scholar]
- 126.Watabe-Uchida M, John KA, Janas JA, Newey SE, Van Aelst L. The Rac activator DOCK7 regulates neuronal polarity through local phosphorylation of stathmin/Op18. Neuron. 2006;51:727–739. doi: 10.1016/j.neuron.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 127.Tanaka Y, Hamano S, Gotoh K, Murata Y, Kunisaki Y, Nishikimi A, Takii R, Kawaguchi M, Inayoshi A, Masuko S, Himeno K, Sasazuki T, Fukui Y. T helper type 2 differentiation and intracellular trafficking of the interleukin 4 receptor-alpha subunit controlled by the Rac activator Dock2. Nat Immunol. 2007;8:1067–1075. doi: 10.1038/ni1506. [DOI] [PubMed] [Google Scholar]
- 128.Small JV, Rottner K, Kaverina I. Functional design in the actin cytoskeleton. Curr Opin Cell Biol. 1999;11:54–60. doi: 10.1016/S0955-0674(99)80007-6. [DOI] [PubMed] [Google Scholar]
- 129.Marklund U, Brattsand G, Shingler V, Gullberg M. Serine 25 of oncoprotein 18 is a major cytosolic target for the mitogen-activated protein kinase. J Biol Chem. 1993;268:15039–15047. [PubMed] [Google Scholar]
- 130.Marklund U, Larsson N, Brattsand G, Osterman O, Chatila TA, Gullberg M. Serine 16 of oncoprotein 18 is a major cytosolic target for the Ca2+/calmodulin-dependent kinase-Gr. Eur J Biochem. 1994;225:53–60. doi: 10.1111/j.1432-1033.1994.00053.x. [DOI] [PubMed] [Google Scholar]
- 131.Cantrell DA. T-cell antigen receptor signal transduction. Immunology. 2002;105:369–374. doi: 10.1046/j.1365-2567.2002.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gomez TS, Billadeau DD. T cell activation and the cytoskeleton: you can’t have one without the other. Adv Immunol. 2008;97:1–64. doi: 10.1016/S0065-2776(08)00001-1. [DOI] [PubMed] [Google Scholar]
- 133.Huse M, Quann EJ, Davis MM. Shouts, whispers and the kiss of death: directional secretion in T cells. Nat Immunol. 2008;9:1105–1111. doi: 10.1038/ni.f.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Combs J, Kim SJ, Tan S, Ligon LA, Holzbaur EL, Kuhn J, Poenie M. Recruitment of dynein to the Jurkat immunological synapse. Proc Natl Acad Sci USA. 2006;103:14883–14888. doi: 10.1073/pnas.0600914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stowers L, Yelon D, Berg LJ, Chant J. Regulation of the polarization of T cells toward antigen-presenting cells by Ras-related GTPase CDC42. Proc Natl Acad Sci USA. 1995;92:5027–5031. doi: 10.1073/pnas.92.11.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/S0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 137.Mori N, Morii H. SCG10-related neuronal growth-associated proteins in neural development, plasticity, degeneration, and aging. J Neurosci Res. 2002;70:264–273. doi: 10.1002/jnr.10353. [DOI] [PubMed] [Google Scholar]
- 138.Srsen V, Kitazawa H, Sugita M, Murofushi H, Bulinski JC, Kishimoto T, Hisanaga S. Serum-dependent phosphorylation of human MAP4 at Ser696 in cultured mammalian cells. Cell Struct Funct. 1999;24:321–327. doi: 10.1247/csf.24.321. [DOI] [PubMed] [Google Scholar]
- 139.Guo S, Kemphues KJ. par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell. 1995;81:611–620. doi: 10.1016/0092-8674(95)90082-9. [DOI] [PubMed] [Google Scholar]
- 140.Bohm H, Brinkmann V, Drab M, Henske A, Kurzchalia TV. Mammalian homologues of C. elegans PAR-1 are asymmetrically localized in epithelial cells and may influence their polarity. Curr Biol. 1997;7:603–606. doi: 10.1016/S0960-9822(06)00260-0. [DOI] [PubMed] [Google Scholar]
- 141.Saadat I, Higashi H, Obuse C, Umeda M, Murata-Kamiya N, Saito Y, Lu H, Ohnishi N, Azuma T, Suzuki A, Ohno S, Hatakeyama M. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature. 2007;447:330–333. doi: 10.1038/nature05765. [DOI] [PubMed] [Google Scholar]
- 142.Bulinski JC, Borisy GG. Widespread distribution of a 210, 000 mol wt microtubule-associated protein in cells and tissues of primates. J Cell Biol. 1980;87:802–808. doi: 10.1083/jcb.87.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Heald R. A dynamic duo of microtubule modulators. Nat Cell Biol. 2000;2:E11–E12. doi: 10.1038/71394. [DOI] [PubMed] [Google Scholar]