Abstract
Malignant melanoma is one of the most aggressive human neoplasms which develop from the malignant transformation of normal epithelial melanocytes and share the lineage with retinal cells. cGMP-phosphodiesterase 6 (PDE6) is one of the cancer-retina antigens newly identified in melanoma cells. Normally, PDE6 hydrolyzes the photoreceptor second messenger cGMP allowing the visual signal transduction in photoreceptor cells. cGMP also play an important signaling role in stimulating melanogenesis in human melanocytes. Here, we present evidence that PDE6 is a key enzyme regulating the cGMP metabolism in melanoma cells. Decrease in intracellular cGMP leads to calcium accumulation in melanoma cells. In these cells, cGMP-phosphodiesterase 6 can be activated by another cancer-retina antigen, transducin, through Wnt5a–Frizzled-2 cascade, which leads to a lowering of cGMP and an increase in intracellular calcium mobilization. Thus, the aberrant expression of PDE6 may control cGMP metabolism and calcium homeostasis in melanoma cells.
Keywords: cGMP-phosphodiesterase 6, Transducin, Cancer-retina antigens, Wnt, Frizzled, Melanoma
Introduction
Malignant melanoma is one of the most aggressive human neoplasms which progresses through radial and vertical growth phases and finally metastasizes [1]. The incidence of this cancer increased worldwide over a quarter of a century [2]. Having a very poor prognosis and being resistant to conventional therapy like chemotherapy [3], melanoma nevertheless responds eventually to a variety of immunotherapeutic approaches [4]. Sun exposure and genetic susceptibility have been proposed as major etiological and predisposing factors for melanoma development [5]. Melanoma cells presumably develop from the malignant transformation of normal epithelial melanocytes, thus sharing the lineage with retinal cells [6].
Recently, we have shown that the key photoreceptor proteins, that are normally restricted to retinal cells and the pineal gland, function as cancer-retina antigens in melanoma cells [7]. The following photoreceptor proteins were classified as cancer-retina antigens: rhodopsin, transducin, cGMP-phosphodiesterase 6 (PDE6), cGMP-dependent channels, guanylyl cyclase, rhodopsin kinase, recoverin, and arrestin. Among these, PDE6 is expressed at a high frequency. Such expression of cancer-retina antigens could generate an autoimmune response [8], which in turn might lead to development of a paraneoplastic neurological syndrome, melanoma-associated retinopathy [9]. Furthermore, we demonstrated that expression of cancer-retina antigens can be modulated by light as it proceeds in photoreceptor cells [10]. Whereas the biochemistry and physiology of visual transduction have been extensively studied, the role of the photoreceptor proteins in melanoma cells still remains to be resolved.
Photoreceptor PDE6 consists of two types of catalytic subunits (α and β) and an inhibitory γ subunit. In the retina, PDE6 hydrolyzes the photoreceptor secondary messenger, cGMP, allowing the closure of cGMP-gated channels located in the plasma membrane of photoreceptors [11]. cGMP effector proteins also include cGMP-dependent protein kinase (PKG) and phosphodiesterases themselves [12]. In melanocytes, cGMP plays an important signaling role and stimulates melanogenesis through the ultraviolet B radiation [13].
In addition to photoreceptor and melanoma cells, PDE6 is expressed in zebrafish embryos and mouse F9 embryonic teratocarcinoma cells [14]. There, PDE6 can be activated through the cascade Wnt5a–Frizzled-2. Such activation of PDE6 leads to a decrease of intracellular cGMP that inhibits PKG and stimulates the intracellular calcium mobilization [14]. This chain of events involves PKG which is inhibited at a low cGMP concentration [15]. As a consequence, a high calcium concentration activates the phosphoprotein phosphatase calcineurin and calcium/calmodulin protein kinase II, and finally stimulates the gene expression through NF-AT [16].
Here, we present evidence that PDE6 is a key enzyme regulating the cGMP metabolism in melanoma cells. Decrease in the intracellular cGMP as a result of its hydrolysis by PDE6 leads to an accumulation of intracellular calcium. In melanoma cells, PDE6 can be activated through Wnt5a–Frizzled-2 by another cancer-retina antigen transducin, leading to a decrease of intracellular cGMP concentration and an increase of intracellular calcium mobilization.
Materials and methods
Materials
Pertussis toxin, zaprinast, Fura-2/AM, and polyclonal (monospecific) rabbit antibodies against PDE6β and Frizzled-2 were purchased from Sigma-Aldrich (Schnelldorf, Germany). Sildenafil (Viagra) was obtained from Pfizer Pharma (Karsruhe, Germany). Wnt5a purified protein was purchased from Abnova (Heidelberg, Germany). The Apo-BrdU-IHC™ Kit was from Biovision (Mountain View, CA, USA). SureSilencing™ shRNA plasmids for α, β, and γ subunits of human PDE6 were purchased from SABiosciences (Frederic, MD, USA). A polyclonal (monospecific) rabbit antibody against transducin was obtained as described in [7]. A polyclonal (monospecific) rabbit antibody against PDE6γ was purchased from Affinity BioReagents (Golden, CO, USA), a polyclonal (monospecific) rabbit antibody against Wnt5a was obtained from Cell Signaling Technology (Danvers, MA), and a monoclonal antibody against poly(ADP-ribose) was purchased from Alexis (Axxora, LCC San Diego, CA, USA).
Cell cultures
Established melanoma cell lines (Ma-Mel-04, -05, -11, -12, -17, -21, UKRV-Mel-02, -06, -14a, -21, -31, MeWo, and SK-Mel-023) from the Skin Cancer Unit at the German Cancer Research Center, Heidelberg, Germany were cultivated in RPMI-1640 medium. The keratinocyte cell line HaCaT [17] was cultivated in DMEM High Glucose (4.5 g/l) medium. Melanocytes were derived from foreskin and are from a commercial source (batches 4121602, 0100402.1, and 6030102.2; Promocell, Heidelberg, Germany). Normal epithelial melanocytes (NEM) were cultivated in MEM Alpha Medium with ribo- and deoxyribonucleosides (Promocell). All media were supplemented with 10% fetal calf serum, l-glutamine (2 mM) and penicillin/streptomycin solution (5 U/ml), and were purchased from PAA Laboratories (Coelbe, Germany). Cell lines were cultivated at 37°C and 5% CO2. For drug treatments, cells (1 × 105 per Petri dish (35 mm)) were cultivated in complete medium for 24 h and then supplemented with 0.3 μM zaprinast (ZAP) or 500 ng/ml pertussis toxin and cultivated for 24 h. Subsequently, cells were washed, counted, and equal amounts of cells were used for PDE activity, cGMP, and calcium measurement.
shRNA knockdown of PDE6 subunit expression
Plasmid-based RNA interference was performed as described by the manufacturer. Briefly, competent E. coli cells were transformed with the provided plasmids for shRNA for PDE6-α, PDE6-β, and PDE6-γ following the manufacturer’s protocol. Subsequently, plasmids were isolated, purified, and transfected separately to melanoma cell lines with replicates. Transfected cells were enriched by selection for neomycin resistance. Knock-down effects were checked by western blotting using specific antibodies against PDE6-α, PDE6-β, and PDE6-γ.
Protein analysis
Western blot analysis and ELISA were performed as described elsewhere [10].
cGMP measurement
cGMP concentration was measured with the HitHunter cGMP Assay (Amersham Bioscience Europe, Freiburg, Germany) as described by the manufacturer. Briefly, harvested cells were resuspended in PBS at the concentration of 40,000 per 10 μl and seeded on 96 half-well white plates. After incubation with different reagents from the kit at room temperature, the plates were read on a luminescent reader at 1 s/well. Standard concentrations of cGMP (from 10−4 to 10 μM) were simultaneously proceeded to estimate cGMP concentration in the probes of interest. Each cell line was measured in triplicate, and the final concentration of cGMP was determined from at least three independent experiments.
Measurement of PDE-activity
PDE activity was measured with the HitHunter cGMP Assay as a decrease of cGMP concentration after incubation with protein extracts from the cell lines. Briefly, the proteins were extracted with TRIS buffer (10 mM TRIS pH 7.4, containing 50 mM NaCl, 1 mM DTT, and proteinase inhibitor cocktail). For each cell line, the HitHunter cGMP Assay was performed using standard concentrations of cGMP (from 10−4 to 10 μM) with or without protein extract from the cells using different protein concentrations. PDE activity was calculated as a decrease of cGMP concentration during 1 min at 37°C per 1 mg of protein. PDE activity was measured in triplicate, and three cGMP concentration points were used for the activity calculation. The final activity was calculated from three independent experiments with different protein concentrations added.
Calcium measurement
The free intracellular calcium concentration ([Ca2+]i) was measured with fura-2/AM. 1 × 105 cells were incubated with 3 μM of fura-2/AM for 30 min at 37°C in the dark. After sedimentation, cells were resuspended in Krebs/HEPES buffer (143.3 mM Na+, 4.7 mM K+, 2.5 mM Ca2+, 1.3 mM Mg2+, 125.6 mM Cl−, 25 mM HCO3 −, 1.2 mM H2PO4 −, 1.2 mM SO4 2−, 11.7 mM glucose, and 10 mM HEPES, pH 7.4), and then the cells were incubated for 15 min at room temperature in the dark. After washing with the Krebs/HEPES buffer, the fluorescence was measured by 340 and 380 nm excitation, and by 510 nm emission. The [Ca2+]i was calculated according to the Grynkewicz equation [18]:
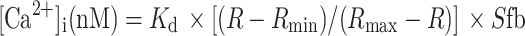 |
where K d (for Ca2+ binding to fura-2 at 37°C) = 225 nM, R = 340/380 ratio, R max = 340/380 ratio under Ca2+-saturating conditions (after adding 0.1% Triton X 100), R min = 340/380 ratio under Ca2+-free conditions (after adding 4.5 mM EGTA), and Sfb = ratio of baseline fluorescence (380 nm) under Ca2+-free and -bound conditions. For measurement of [Ca2+]i changing, fura-2/AM-loaded cells were treated with Wnt5a (20 ng/ml) or buffer alone. Measurement of fluorescence by 340 and 380 nm excitation was performed with TECAN.SPECTRAFluor Plus each 60 s during 15 min, and the ratio 340/380 was calculated.
Statistical analysis
Statistical analysis was performed by Student’s t test with significant differences determined as P < 0.05.
Results
Expression of PDE6 subunits in melanoma cell lines
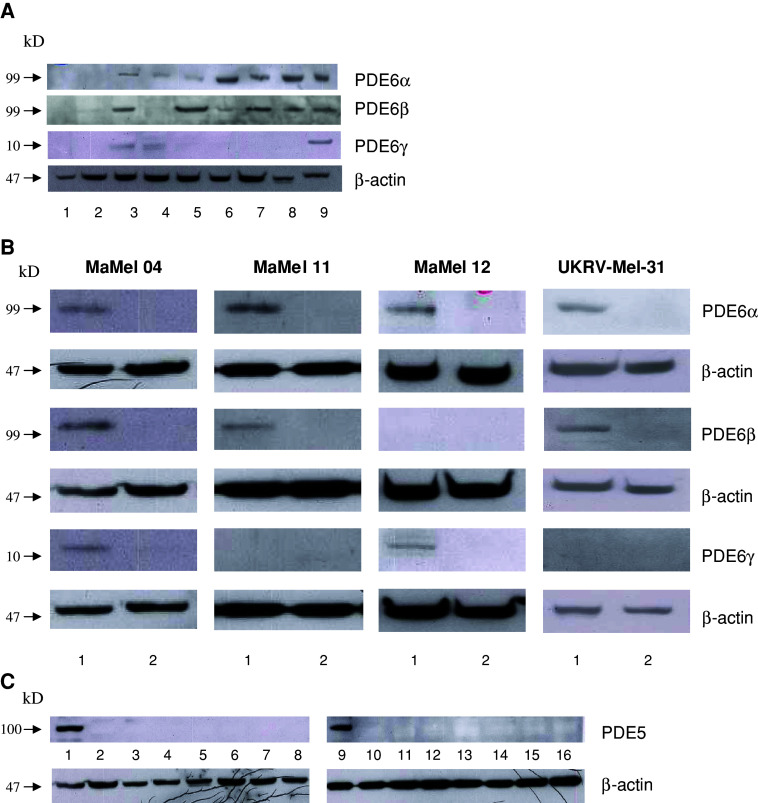
Since the α subunit of PDE6 (PDE6-α) was frequently detected in melanoma cells [7] (Fig. 1a), we wanted to understand whether other PDE6 subunits, namely β and γ (PDE6-β and PDE6-γ, respectively), are also expressed in melanoma cell lines. Western blot analysis revealed the expression of both PDE6-β and -γ in some melanoma cell lines, but not in keratinocytes nor in melanocytes (Fig. 1a; Table 1). To confirm that the positive western blotting signals indeed correspond to subunits of PDE6, we performed stable transfections of four melanoma cell lines (Ma-Mel-04, Ma-Mel-11, Ma-Mel-12, and UKRV-Mel-31) with shRNA plasmids for PDE6-α, PDE6-β, and PDE6-γ. Transfection of the melanoma cell lines with the PDE6-α shRNA plasmid led to the disappearance of the PDE6-α band (Fig. 1b). The same knock-down effects were seen after transfection of Ma-Mel-04, Ma-Mel-11, and UKRV-Mel-31 with the PDE6-β shRNA plasmid, and after transfection of Ma-Mel-04 and Ma-Mel-12 with the PDE6-γ shRNA plasmid (Fig. 1b). Thus, PDE6-α, PDE6-β, and PDE6-γ subunits are indeed expressed in melanoma cell lines.
Fig. 1.
Protein expression of PDE6 and PDE5. a Expression of PDE6 subunits in NEM (1), HaCaT (2) and melanoma cell lines Ma-Mel-04 (3), Ma-Mel-12 (4), UKRV-Mel-31 (5), Ma-Mel-11 (6), Ma-Mel-21 (7), and MeWo (8), as well as Y79 retinoblastoma cell line as a positive control (9); b expression of PDE6 subunits in untransfected controls (1) or in cell lines transfected with shDNA plasmids (2) as indicated on the right side; c expression of PDE5 in heart as a positive control (1, 9), NEM (2), HaCaT (3), and melanoma cell lines Ma-Mel-04 (4), Ma-Mel-12 (5), UKRV-Mel-31 (6), Ma-Mel-11 (7), Ma-Mel-21 (8), MeWo (10), UKRV-Mel-06 (11), UKRV-Mel-14a (12), UKRV-Mel-21 (13), SK-Mel23 (14), Ma-Mel-05 (15), and Ma-Mel-21 (16)
Table 1.
Expression of PDE6 subunits, PDE activity, and concentrations of cGMP and Ca2+ in melanoma and normal skin cell lines
| Cell line | Expression of PDE6 subunits | Total PDE activity, [U/mg × 10−6]a | [cGMP], [nM]a | [Ca2+]i, [nM]a | ||
|---|---|---|---|---|---|---|
| α | β | γ | ||||
| NEMb | – | – | – | 0.083 ± 0.0098 | 21.2 ± 3.6 | 2.9 ± 1.5 |
| HaCaT | – | – | – | 3.13 ± 1.55 | 8.93 ± 5.05 | 1.9 ± 0.5 |
| Ma-Mel-17 | – | – | – | 0 | 50.3 ± 7.6 | 2.9 ± 0.6 |
| Ma-Mel-37 | – | – | – | 0 | 25.7 ± 6.6 | 7.6 ± 1.8 |
| UKRV-Mel-06 | – | – | + | 0.14 ± 0.11 | 43.4 ± 5.8 | 2.1 ± 0.66 |
| UKRV-Mel-14a | – | – | + | 0.46 ± 0.18 | 163.7 ± 10.1 | 1.4 ± 0.7 |
| Ma-Mel-04 | + | + | + | 3.8 ± 1.9 | 9.3 ± 4.9 | 4.2 ± 0.3 |
| Ma-Mel-12 | + | – | + | 1.56 ± 0.56 | 18.2(± 8.3) | 1.6 ± 0.02 |
| UKRV-Mel-31 | + | + | – | 15.9 ± 1.1 | 10.7 ± 1.8 | 24.5 ± 0.8 |
| Ma-Mel-11 | + | + | – | 24.3 ± 9.45 | 11.9 ± 6.6 | 11.2 ± 2 |
| UKRV-Mel-21 | + | – | – | 23.9 ± 0.66 | 15 ± 0.2 | 31.8 ± 6.3 |
| SK-Mel23 | + | – | – | 35.9 ± 1.7 | 4.5 ± 2.7 | 21.9 ± 5.6 |
| Ma-Mel-05 | + | – | – | 14.4 ± 6.4 | 9.5 ± 2.1 | 34.2 ± 7.6 |
| Ma-Mel-21 | – | + | – | 25.8 ± 0.29 | 5 ± 0.8 | 11.2 ± 1 |
| MeWo | + | – | – | 44.2 ± 15.6 | 2.1 ± 0.2 | 10 ± 2.8 |
aMean of at least three independent experiments
bPooled NEM from three healthy donors
PDE6 is functional in melanoma cell lines
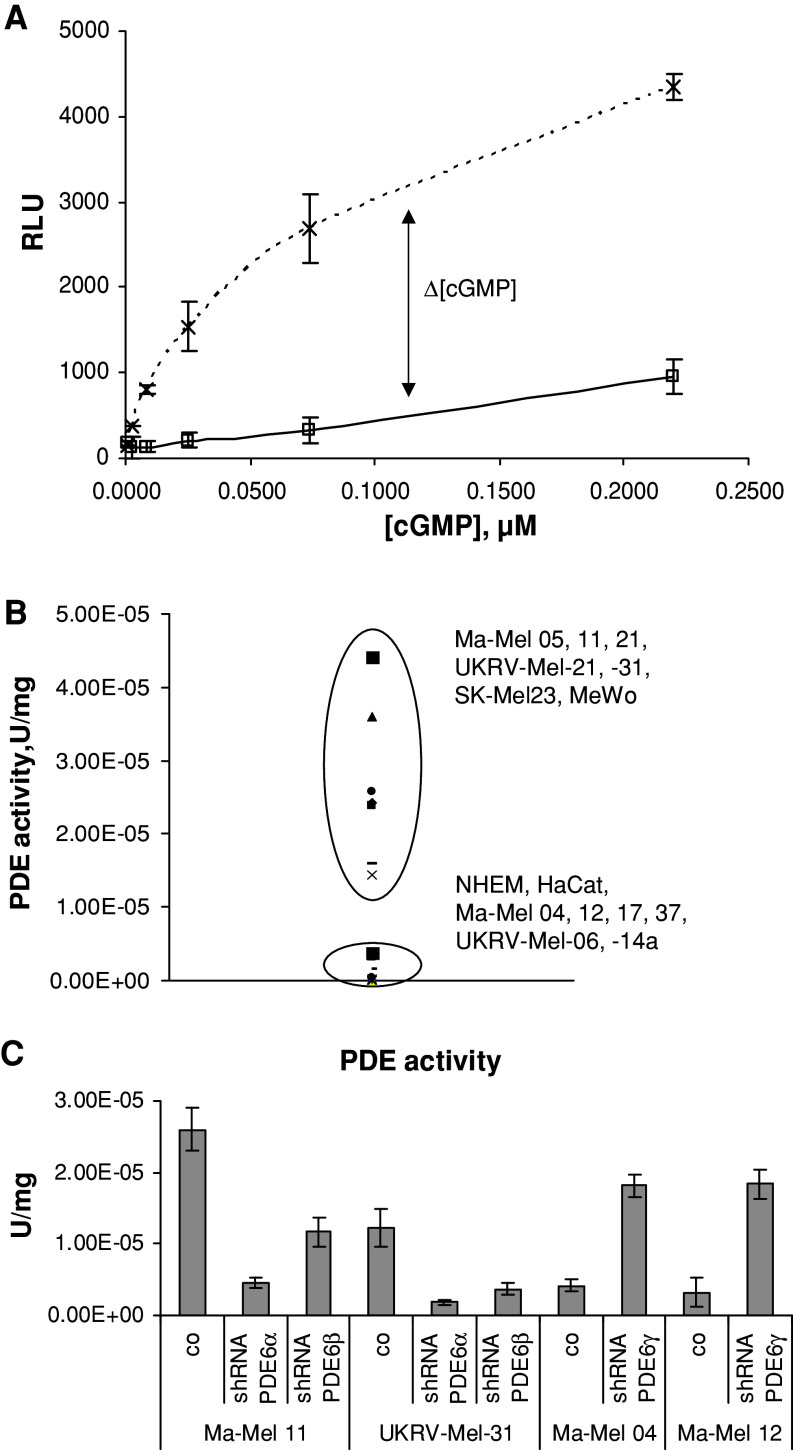
To understand whether PDE6 is functional in melanoma cells, measurement of the general PDE activity in various melanoma cell lines was conducted (Fig. 2a). The analyzed cell lines can be divided in two groups according to their PDE activity (Table 1; Fig. 2b): The first group has a low PDE activity and consists of cell lines which (1) do not express any PDE6 subunit (NEM, HaCaT, Ma-Mel-17 and -37) or (2) possess PDE6-γ (UKRV-Mel-06, -14a, Ma-Mel-04 and 12). The second group (Ma-Mel-05, -11, -21, UKRV-Mel-21, -31, Sk-Mel-231, and MeWo) has a high PDE activity and comprises cell lines, containing PDE6-α and/or PDE6-β but have no PDE6-γ. Thus, the data of western blot analysis of PDE6 subunits correlates well with the PDE activity. We also compare the PDE activity with the level of cGMP in the cell lines tested and detected the nM range of cGMP concentration in those cell lines which show a low PDE activity (Table 1). However, the PDE activity in Ma-Mel-04, Ma-Mel-05, UKRV-Mel-21, and UKRV-Mel-31 cell lines does not show a good correlation with the level of cGMP. This discrepancy could be attributed to the functional activity of guanylyl cyclase 1 which has been earlier found to be expressed in these cell lines [7].
Fig. 2.
PDE activity in melanoma cell lines is attributed to PDE6. a Exemplary determination of PDE activity in melanoma cell line Ma-Mel-05. Luminescence signal (y axis) in relative luminescence unit (RLU) which is proportional to the cGMP concentration was measured at different concentrations of cGMP (x axis) without (broken line) or with (solid line) whole protein extract of the cell line (0.35 mg/ml). For details, see "Materials and methods". b Dispersion of PDE activity in melanoma cell lines, HaCat and NEM, depends on the pattern of PDE6 expression. c PDE activity was measured in melanoma cell lines transfected with shDNA plasmids. Statistically significant differences (P < 0.05) are marked with an asterisk
Further, we analyzed whether the PDE activity revealed in melanoma cells can be attributed to the retinal-specific PDE6, but not to other members of the PDE family. For this aim, we performed a stable transfection of melanoma cell lines with shRNA plasmids for PDE6-α, PDE6-β, and PDE6-γ. Transfection of melanoma cells with shRNA plasmids for PDE6-α or PDE6-β led to inhibition of PDE activity between 55 and 85% (Fig. 2c). Furthermore, transfection of Ma-Mel-04 and Ma-Mel-12 with shRNA plasmids for PDE6-γ led to upregulation of PDE activity in these cell lines (Fig. 2c).
A confirmatory result was obtained in the cell culture system. In this case, melanoma cell lines and keratinocyte cell line HaCaT were cultivated with an inhibitor of PDE6 ZAP (300 nM) for 24 h, and then the amount of cGMP accumulated in the cells was measured (Fig. 3a). Melanoma cell lines positive for PDE6-α and/or PDE6-β and negative for PDE6-γ accumulated intracellular cGMP after cultivation with ZAP (Fig. 3a). However, the inhibitors had no effect upon cGMP accumulation in the cells positive for PDE6-γ or negative for PDE6-α and/or PDE6-β. Cultivation of Ma-Mel-11 and UKRV-Mel-31 transfected with shRNA plasmids for either PDE6-α or PDE6-β led to cGMP accumulation in comparison with non-transfected cell lines (Fig. 3b). In Ma-Mel-04 and Ma-Mel-12 transfected with shRNA plasmids for PDE6-γ, we observed a reduction of cGMP (Fig. 3b).
Fig. 3.

cGMP accumulation and [Ca2+]i mobilization depend on PDE6. Melanoma cell lines with different patterns of PDE6 expression and the HaCaT cell line were cultivated for 24 h with 300 nM ZAP (a) or melanoma cell lines were transfected with shRNA coding plasmids (b) and then the accumulation of cGMP in the cells was measured. c Melanoma cell lines and the HaCaT cell line were cultivated with 300 nM ZAP or transfected with shRNA coding plasmids, and then the [Ca2+]i was measured. Statistically significant differences (P < 0.05) are marked with an asterisk
In total, the data of this section confirm that the PDE activity detected in melanoma cell lines belongs to PDE6 but not to other members of the PDE family.
Changes in calcium mobilization in melanoma cell lines correlates with cGMP levels and PDE6 activity
Recently, it has been shown that reduction of the cGMP concentration through down-regulation of PKG leads to the intracellular calcium ([Ca2+]i) mobilization in the mouse totipotent F9 teratocarcinoma cells [15]. We were curious to know whether the melanoma cell lines with different intracellular cGMP concentrations have various [Ca2+]i concentrations. Indeed, differences in [Ca2+]i dependent on cGMP concentrations were detected: melanoma cell lines with high levels of cGMP concentration had increased (above 10 nM) [Ca2+]i (Table 1).
To further confirm the interrelationship between intracellular [Ca2+]i and cGMP concentration, we cultivated the cell lines with PDE6 inhibitor zaprinast (ZAP) and measured the [Ca2+]i concentration. Cultivation with ZAP led to a decrease of the [Ca2+]i mobilization in the cell lines positive for PDE6 but had no influence on the [Ca2+]i in the cell lines negative for the catalytic subunits of PDE6 (Fig. 3c). These data were confirmed in experiments with stable transfected melanoma cell lines with shRNA plasmids for PDE6-α, PDE6-β, or PDE6-γ. Cultivation of Ma-Mel-11 and UKRV-Mel-31 transfected with shRNA plasmids for either PDE6-α or PDE6-β led to a decrease of the [Ca2+]i mobilization in comparison with non-transfected cell lines (Fig. 3c). In addition, we found an increase of the [Ca2+]i mobilization in Ma-Mel-04 and Ma-Mel-12 transfected with shRNA plasmids for PDE6-γ (Fig. 3c). These results suggest that PDE6 participates in the calcium homeostasis in melanoma cells.
Wnt5a activates PDE6 through Frizzled-2 and transducin and stimulates calcium mobilization
From the elegant work of Ahumada et al. [14], we learned that in F9 and in Chinese hamster ovary cells the activation of Frizzled-2 (FZ2) receptor after binding of Wnt5a leads to activation of the G protein transducin, which in turn binds to PDE6-γ and thus activates PDE6 [14]. We supposed that in the melanoma cell lines, which also possess PDE6-γ, the sequence of events could be the same.
First, we checked the protein expression of transducin, Wnt5a, and FZ2 in melanoma cell lines, keratinocytes and melanocytes (Table 2 ; Fig. 4). It has been found that transducin is expressed in three melanoma cell lines (Ma-Mel-04, -12 and -37). Wnt5a is detected in half of melanoma cell lines tested and in HaCaT, but not in melanocytes. FZ2 expression is present in all melanoma cell lines, but neither in keratinocytes nor in NEM.
Table 2.
Protein expression of transducin, Wnt5a and Frizzled-2 in melanoma and normal skin cell lines
| Cell lines | Transducin | Wnt5a | Frizzled-2 |
|---|---|---|---|
| NEM | – | – | – |
| HaCaT | – | + | – |
| UKRV-Mel-06 | – | + | + |
| UKRV-Mel-14a | – | + | + |
| UKRV-Mel-21 | – | + | + |
| UKRV-Mel-31 | – | + | + |
| SK-Mel23 | – | + | + |
| Ma-Mel-04 | + | – | + |
| Ma-Mel-05 | – | – | + |
| Ma-Mel-11 | – | – | + |
| Ma-Mel-12 | + | + | + |
| Ma-Mel-17 | – | + | + |
| Ma-Mel-21 | – | – | + |
| Ma-Mel-37 | + | – | + |
| Ma-Mel-52 | – | + | + |
| MeWo | – | + | + |
Fig. 4.
Protein expression of transducin, Wnt5a, and FZ2. Protein expression in Y79 (1), NHEM (2), HaCat (3), UKRV-Mel-06 (4), -14a (5), -21 (6), -31 (7), SK-Mel-23 (8), Ma-Mel-04 (9), -05 (10), -11 (11), -12 (12), -17 (13), -21 (14), -37 (15), -52 (16), and MeWo (17)
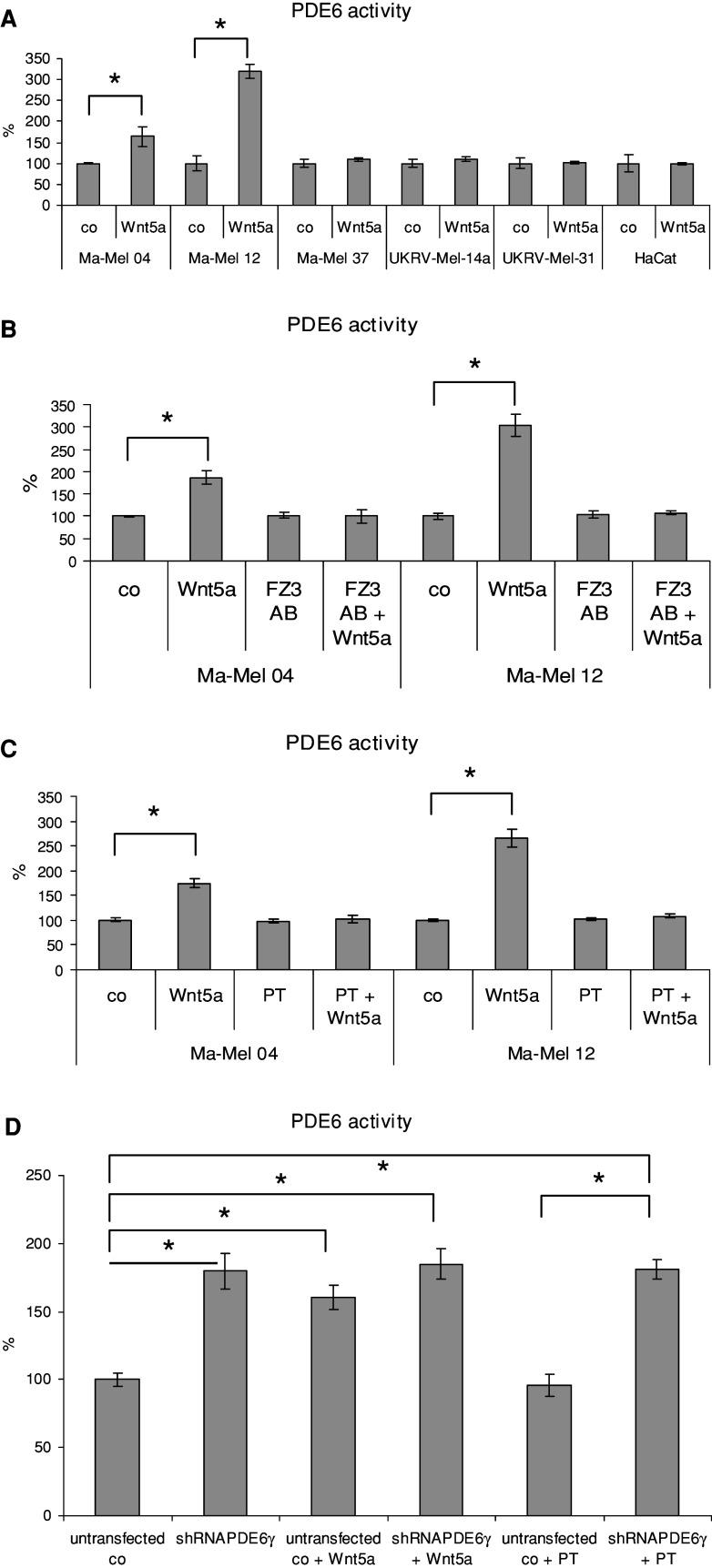
Using two melanoma cell lines (Ma-Mel-04 and -12) positive for PDE6, transducin and FZ2, we studied the effects of Wnt5 on PDE6 activation. Incubation of the cells with the purified recombinant Wnt5a led to an increase of PDE6 activity. Other cell lines with different expression profiles of PDE6, transducin, and FZ2 did not respond to Wnt5a (Fig. 5a). To show that Wnt5a acts through FZ2 receptor, the cells were pretreated with antibody against FZ2 before the incubation with Wnt5a. This pretreatment abolished the effect of Wnt5a on the PDE6 activation (Fig. 5b). Also, this effect was prevented by pertussis toxin, a specific inhibitor of Gi proteins and transducin (Fig. 5c). Thus, Wnt5a activates PDE6 through FZ2 receptor and G protein transducin. We found additional support for the hypothesis that the Wnt5a activates PDE6 through influence on PDE6-γ in experiments with stable transfection of the melanoma cell line Ma-Mel-04 with shRNA plasmids for PDE6-γ (Fig. 5d). Transfection of melanoma cells with shRNA plasmids for PDE6-γ led to the same effect as after incubation with Wnt5a-upregulation of PDE activity in this cell line (Fig. 5d). At the same time, an addition of Wnt5a to the transfected cells did not affect the PDE activity. An incubation of the transfected Ma-Mel-04 with PT did not lead to the abolishment of high PDE activity induced by PDE6-γ transfection (Fig. 5d). This indicates that PDE6 is fully activated and does not need an additional activation by the Wnt5a–FZ2–transducin cascade.
Fig. 5.
Wnt5a activates PDE6 through the FZ2 and transducin cascade. a PDE6 activity was measured in melanoma cell lines with different pattern of PDE6 expression and in HaCaT cell line incubated with or without 20 ng/ml Wnt5a for 1 h. b PDE6 activity after stimulation with Wnt5a was measured in Ma-Mel-04 and Ma-Mel-12 melanoma cells pretreated with 50 ng/μl antibody against FZ2 for 30 min. c PDE6 activity after stimulation with Wnt5a was measured in melanoma cells cultivated with 50 ng/ml of pertussis toxin for 24 h. d PDE6 activity in untrasfected and transfected with shRNA coding plasmids for PDE6-γ, Ma-Mel-04 with and without additional stimulation/inhibition with Wnt5a or PT. Statistically significant differences (P < 0.05) are marked with an asterisk
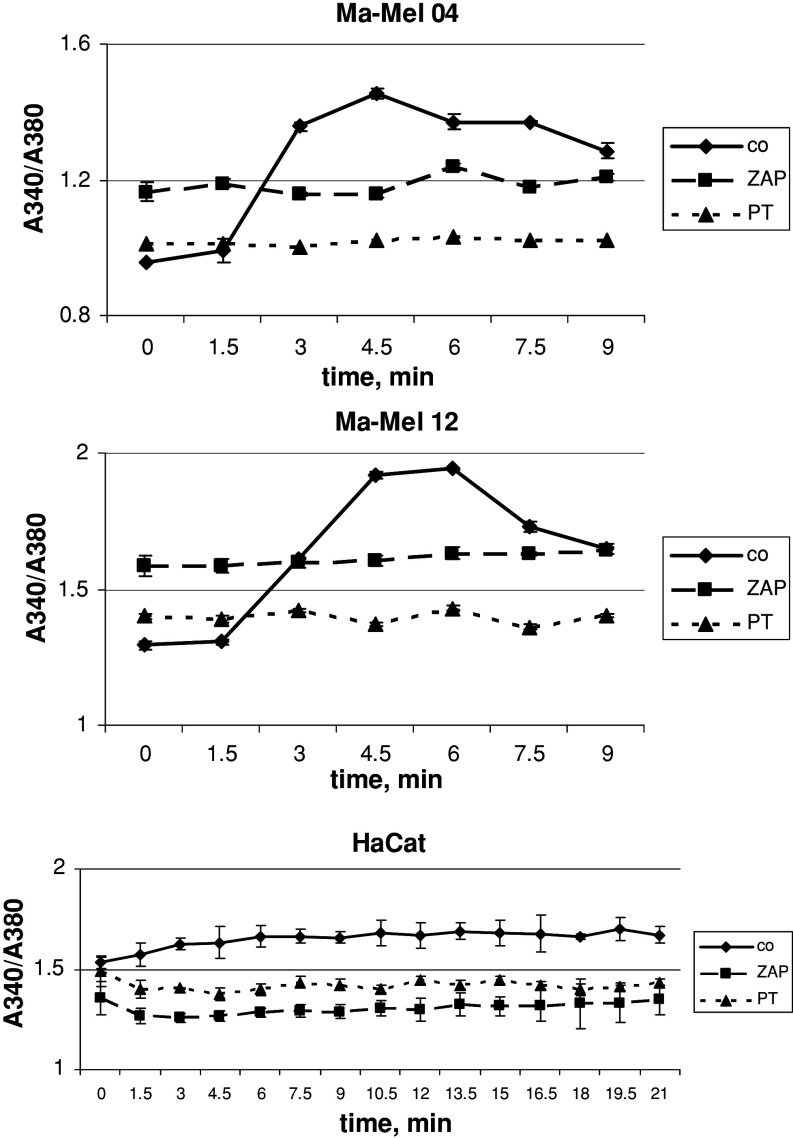
To understand whether PDE6 activation by Wnt5a would lead to an increase of [Ca2+]i mobilization, we measured kinetics of the [Ca2+]i mobilization in Ma-Mel-04 and Ma-Mel-12 after adding of Wnt5a (Fig. 6). Indeed, Wnt5a stimulated the [Ca2+]i mobilization in these melanoma cell lines, with a maximum seen 5 min after incubation, but this stimulation had no effect in the case of keratinocytes even after 20 min of incubation. The Wnt5a-dependent [Ca2+]i mobilization was blocked by the cultivation of the cells with ZAP or pertussis toxin (Fig. 6). Thus, one may conclude that Wnt5a regulates the [Ca2+]i mobilization through the cascade FZ2 receptor–G protein–transducin–PDE6.
Fig. 6.
Wnt5a stimulates the increase of the [Ca2+]i mobilization in Ma-Mel-04 and Ma-Mel-12 melanoma cells, but not in HaCaT cell line. Untreated and ZAP- or pertussis toxin-treated cells were loaded with fura-2/AM and then 20 ng/ml of Wnt5a was added to start the reaction. Measurement of fluorescence by 340 and 380 nm excitation was performed each 60 s during 15 min, and the ratio 340/380 was calculated. For details, see “Materials and methods”
Discussion
The main aim of this study was to investigate whether the aberrantly expressed PDE6 is functional in cancer cells. We have shown that: (1) PDE6 is active and hydrolyses cGMP in melanoma cell lines; (2) a decrease in intracellular cGMP concentration leads to an increase of intracellular calcium mobilization in the cells; and (3) PDE6 can be activated through the cascade Wnt5a–Frizzled-2–transducin–PDE6.
From the biochemical point of view, PDE6 (as almost all PDEs) is a dimer and composed of two large homologous catalytic subunits PDE6-αβ in rod or PDE6-α in cone, and two small inhibitory subunits PDE6-γ [19]. In this work, we identified different patterns of the PDE6 subunit expression: PDE6-αβγ, PDE6-αα, PDE6-αβ, PDE6-β, and PDE6-γ. Although the dominant catalytic unit of rod PDE6 is a heterodimer PDE6-αβ [19], this does not exclude the existence of functional PDE6-αα and PDE6-ββ [20]. However, the role of dimerization of the PDE6 subunits is not fully understood, neither in photoreceptors nor in cancer cells, and needs future investigations. Another interesting point is the expression of PDE6-γ alone. PDE6-γ belongs to the group of so-called intrinsically disordered proteins which represent a number of natively unfolded or intrinsically unstructed proteins [21]. Such proteins have little or no ordered structure under physiological conditions and, due to their structural plasticity, can bind to different targets and thus fulfill diverse functions [22]. Hence, the expression of PDE6-γ without expression of the catalytic subunits of PDE6 could be explained by different functions and other molecular targets of PDE6-γ in melanoma cells.
Depending on the PDE6 subunits expression and the level of phosphodiesterase activity, the melanoma cell lines tested can be classified into three groups (Fig. 7). The first group consists of melanoma cell lines which do not express PDE6 and have a low, or even no, phosphodiesterase activity (Fig. 7: type I). Type II comprises cells with a high phosphodiesterase activity, these cells express PDE6-α and/or PDE6-β, are negative for PDE6-γ and can be inhibited by phosphodiesterase inhibitors (Fig. 7). Type III represents cells expressing both catalytic (PDE6-α and PDE6-β) and inhibitory (PDE6-γ) subunits, show a low phosphodiesterase activity and express transducin (Fig. 7). The cascade Wnt5a–Frizzled-2–transducin–PDE6 is operational in the melanoma cell lines of type III, consequently these cells posses a high phosphodiesterase activity upon activation (see Fig. 7).
Fig. 7.
A schematic presentation of three types of melanoma cells in regard to PDE6. See text for details
Wnt5a is a secreted regulatory protein serving as a ligand for the FZ-2 receptor [23]. Since the FZ-2 receptor is structurally similar to visual rhodopsin [24], the Wnt5a–FZ-2 downstream cascade in the embryonic development seems to have the same molecular partners as rhodopsin has in photoreceptor cells, i.e. transducin and PDE6 [14]. Thus, the Wnt5a–FZ-2 cascade shares the same signaling pathway as does the visual cascade [25]. In this work, we present evidence that the same signaling paradigm also takes place in melanoma cells (see Fig. 7): Wnt5a binds to FZ-2 receptor which activates G protein transducin. Activated transducin, in turn, binds to the inhibitory γ subunit of the PDE6 holoenzyme and makes PDE6 active. Finally, active PDE6 hydrolyses cGMP.
In zebrafish embryos and mouse F9 embryonic teratocarcinoma cells, signal transduction in the cascade Wnt5a–FZ-2–transducin–PDE6 leads to a decrease of intracellular cGMP, thus stimulating the intracellular calcium mobilization [14, 26]. This chain of events involves PKG which is inhibited at a low cGMP concentration [15]. As a consequence, a high calcium concentration activates the phosphoprotein phosphatase calcineurin and calcium/calmodulin protein kinase II, and finally stimulates the gene expression through NF-AT [16]. Do the same signaling proteins serve as molecular targets for calcium in melanoma cells? This question should be resolved in future investigations.
In zebrafish development, the cascade Wnt5a–FZ-2–transducin–PDE6 can be manifested in gastrulation movements [14, 27]. As in development, tumorigenesis involves morphogenesis, movement, and proliferation. Wnt5a signaling can directly affect cell motility and invasion of metastatic melanoma through the activation of protein kinase C by a high calcium concentration [28]. It has also been shown that the Wnt5a gene expression can be used for separation of highly aggressive melanomas from their less invasive counterparts [29]. The usage of PDE6 inhibitors could simulate a reverse effect of Wnt5a by reduction of PDE6 activity. Serafini et al. [30] have shown that PDE inhibitors (sildenafil, tadalafil, and vardenafil) modulate the antitumor immune response by reducing the myeloid-derived suppressor cell function. The authors discuss a potential use of these inhibitors as supplement to the tumor-specific immune therapy.
Why do not all melanoma cell lines express the components of Wnt5a–FZ–2–transducin–PDE6 cascade (instead of FZ-2)? It is well known that cancer cell lines in comparison to cancer tissues lose the ability to express some proteins after their isolation from the tumors and cultivation in vivo [31]. How frequently are PDE6 subunits and transducin expressed in melanoma tissues? Does this expression correlate with clinical data of melanoma patients? Is the cascade Wnt5a–FZ-2–transducin–PDE6 really involved in motility and invasion of melanoma cells? These questions should be answered in future studies.
Acknowledgments
We are grateful to Mrs. A. Heinzelmann for her excellent technical assistance and Dr. M. Rogers for the English correction. This work was supported by the Intramural Program of DKFZ to A.V.B., and by the Russian Foundation for Basic Research (09-04-00395-a) and Hanse Wissenschaftskolleg, Germany to P.P.P.
Abbreviations
- PDE6
cGMP-phosphodiesterase 6
- NEM
Melanocytes
- FZ2
Frizzled-2
- [Ca2+]i
Intracellular calcium concentration
- ZAP
Zaprinast
- DIP
Dipyridamole
- SIL
Sildenafil
- VAR
Vardenafil
- PKG
cGMP-dependent protein kinase
Footnotes
The Editor-in-Chief has retracted this article [1] due to errors in Figure 1B, Figure 1C and Figure 4.
In Figure 1 a number of panels are replicated between experimental groups:
In Figure 1B, the MaMel 04/β-actin control for PDE6α panel replicates β-actin panel 8 in Figure 1C.
In Figure 1B, the MaMel 04/β-actin control for PDE6β panel replicates β-actin panels 1 and 2 in Figure 1C.
In Figure 1B, the MaMel 11/β-actin control for PDE6β panel replicates β-actin panels 11 and 12 in Figure 1C.
In Figure 1B, the MaMel 11/β-actin control for PDE6γ panel replicates β-actin panels 13 and 14 in Figure 1C.
In Figure 1B, the MaMel 11/PDE6α panel reproduces panels 2 and 3 in the PKGIα/β band from Figure 1 in [2].
In Figure 1C, the β-actin panels 1-7 reproduce panels 1-7 in the GAPDH band from Figure 1 in [2].
In Figure 4, the Transducin and Wnt5a bands appear to be spliced.
Alexandr V. Bazhin, Dirk Schadendorf and Stefan B. Eichmüller agree to this retraction. Vojtech Tambor and Pavel P. Philippov have not responded to any correspondence from the publisher about this retraction. Boyan Dikov could not be contacted.
1. Bazhin AV, Tambor V, Dikov B, Philippov PP, Schadendorf D, Eichmüller SB. cGMP-phosphodiesterase 6, transducin and Wnt5a/Frizzled-2-signaling control cGMP and Ca 2+ homeostasis in melanoma cells. Cellular and molecular life sciences. 2010 Mar 1;67(5):817-28.
2. Karakhanova S, Golovastova M, Philippov PP, Werner J, Bazhin AV. Interlude of cGMP and cGMP/protein kinase G type 1 in pancreatic adenocarcinoma cells. Pancreas. 2014 Jul 1;43(5):784-94. doi: 10.1097/MPA.0000000000000104
Change history
1/9/2020
The Editor-in-Chief has retracted this article [1] due to errors in Figs.1b, c and 4.
Change history
1/9/2020
The Editor-in-Chief has retracted this article [1] due to errors in Figs.��1b, c and 4.
References
- 1.Koh HK. Cutaneous melanoma. N Engl J Med. 1991;325:171–182. doi: 10.1056/NEJM199107183250306. [DOI] [PubMed] [Google Scholar]
- 2.Tucker MA, Goldstein AM. Melanoma etiology: where are we? Oncogene. 2003;22:3042–3052. doi: 10.1038/sj.onc.1206444. [DOI] [PubMed] [Google Scholar]
- 3.Geller AC, Miller DR, Annas GD, Demierre MF, Gilchrest BA, Koh HK. Melanoma incidence and mortality among US whites, 1969–1999. JAMA. 2002;288:1719–1720. doi: 10.1001/jama.288.14.1719. [DOI] [PubMed] [Google Scholar]
- 4.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 5.Chudnovsky Y, Khavari PA, Adams AE. Melanoma genetics and the development of rational therapeutics. J Clin Invest. 2005;115:813–824. doi: 10.1172/JCI24808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnheiter H. Evolutionary biology. Eyes viewed from the skin. Nature. 1998;391:632–633. doi: 10.1038/35487. [DOI] [PubMed] [Google Scholar]
- 7.Bazhin AV, Schadendorf D, Willner N, De Smet C, Heinzelmann A, Tikhomirova NK, Umansky V, Philippov PP, Eichmüller SB. Photoreceptor proteins as cancer-retina antigens. Int J Cancer. 2007;120:1268–1276. doi: 10.1002/ijc.22458. [DOI] [PubMed] [Google Scholar]
- 8.Bazhin AV, Schadendorf D, Philippov PP, Eichmüller SB. Recoverin as a cancer-retina antigen. Cancer Immunol Immunother. 2007;56:110–116. doi: 10.1007/s00262-006-0132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bazhin AV, Dalke C, Willner N, Abschütz O, Wildberger HGH, Philippov PP, Dummer R, Graw J, Hrabé de Angelis M, Schadendorf D, Umansky D, Eichmüller SB. Cancer-retina antigens as potential paraneoplastic antigens in melanoma-associated retinopathy. Int J Cancer. 2009;124:140–149. doi: 10.1002/ijc.23909. [DOI] [PubMed] [Google Scholar]
- 10.Bazhin AV, Schadendorf D, Owen RW, Zernii EY, Philippov PP, Eichmüller SB. Visible light modulates the expression of cancer-retina antigens. Mol Cancer Res. 2008;6:110–118. doi: 10.1158/1541-7786.MCR-07-0140. [DOI] [PubMed] [Google Scholar]
- 11.Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- 12.Beavo JA, Brunton LL. Cyclic nucleotide research––still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- 13.Romero-Graillet C, Aberdam E, Biagoli N, Massabni W, Ortonne JP, Ballotti R. Ultraviolet B radiation acts through the nitric oxide and cGMP signal transduction pathway to stimulate melanogenesis in human melanocytes. J Biol Chem. 1996;271:28052–28056. doi: 10.1074/jbc.271.45.28052. [DOI] [PubMed] [Google Scholar]
- 14.Ahumada A, Slusarski DC, Liu X, Moon RT, Malbon CC, Wang HY. Signaling of rat Frizzled-2 through phosphodiesterase and cyclic GMP. Science. 2002;298:2006–2010. doi: 10.1126/science.1073776. [DOI] [PubMed] [Google Scholar]
- 15.Ma L, Wang HY. Suppression of cyclic GMP-dependent protein kinase is essential to the Wnt/cGMP/Ca2+pathway. J Biol Chem. 2006;281:30990–31001. doi: 10.1074/jbc.M603603200. [DOI] [PubMed] [Google Scholar]
- 16.Ma L, Wang HY. Mitogen-activated protein kinase p38 regulates the Wnt/cyclic GMP/Ca2+ non-canonical pathway. J Biol Chem. 2007;282:28980–28990. doi: 10.1074/jbc.M702840200. [DOI] [PubMed] [Google Scholar]
- 17.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 19.Muradov KG, Boyd KK, Martinez SE, Beavo JA, Artemyev NO. The GAFa domains of rod cGMP-phosphodiesterase 6 determine the selectivity of the enzyme dimerization. J Biol Chem. 2003;278:10594–10601. doi: 10.1074/jbc.M208456200. [DOI] [PubMed] [Google Scholar]
- 20.Artemyev NO, Surendran R, Lee JC, Hamm HE. Subunit structure of rod cGMP-phosphodiesterase. J Biol Chem. 1996;271:25382–25388. doi: 10.1074/jbc.271.41.25382. [DOI] [PubMed] [Google Scholar]
- 21.Guo LW, Ruoho AE. The retinal cGMP phosphodiesterase gamma-subunit-a chameleon. Curr Protein Pept Sci. 2008;9:611–625. doi: 10.2174/138920308786733930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 23.Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- 24.Wang HY, Malbon CC. Wnt-frizzled signaling to G-protein-coupled effectors. Cell Mol Life Sci. 2004;61:69–75. doi: 10.1007/s00018-003-3165-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang HY, Malbon CC. Wnt signaling, Ca2+, and cyclic GMP: visualizing Frizzled functions. Science. 2003;300:1529–1530. doi: 10.1126/science.1085259. [DOI] [PubMed] [Google Scholar]
- 26.Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G- protein-linked phosphatidylinositol signalling. Nature. 1997;390:410–413. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- 27.Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, Solnica-Krezel L. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279–288. doi: 10.1016/S1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 29.Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, Radmacher M, Simon R, Yakhini Z, Ben-Dor A, Sampas N, Dougherty E, Wang E, Marincola F, Gooden C, Lueders J, Glatfelter A, Pollock P, Carpten J, Gillanders E, Leja D, Dietrich K, Beaudry C, Berens M, Alberts D, Sondak V. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–540. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- 30.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gazdar AF, Carney DN, Russell EK, Sims HL, Baylin SB, Bunn PA, Jr, Guccion JG, Minna JD. Establishment of continuous, clonable cultures of small-cell carcinoma of lung which have amine precursor uptake and decarboxylation cell properties. Cancer Res. 1980;40:3502–3507. [PubMed] [Google Scholar]