Abstract
Acclimatization to long-term hypoxia takes place at high altitude and allows gradual improvement of the ability to tolerate the hypoxic environment. An important component of this process is the hypoxic ventilatory acclimatization (HVA) that develops over several days. HVA reveals profound cellular and neurochemical re-organization occurring both in the peripheral chemoreceptors and in the central nervous system (in brainstem respiratory groups). These changes lead to an enhanced activity of peripheral chemoreceptor and re-inforce the central translation of peripheral inputs to efficient respiratory motor activity under the steady low O2 pressure. We will review the cellular processes underlying these changes with a particular emphasis on changes of neurotransmitter function and ion channel properties in peripheral chemoreceptors, and present evidence that low O2 level acts directly on brainstem nuclei to induce cellular changes contributing to maintain a high tonic respiratory drive under chronic hypoxia. (This study is part of a multi-author review.)
Keywords: Chronic hypoxia, Hypoxic ventilatory acclimatization, Peripheral chemoreceptors, Brainstem, Nucleus tractus solitarius
Introduction
Physiological responses induced by high altitude exposure aim at increasing O2 delivery to the tissues. The reduced PaO2 at high altitude is sensed by peripheral chemoreceptors in the carotid body at the sinus of the common carotid artery, which sends afferent projections onto brainstem respiratory nuclei to induce hyperventilation. During a long-term (i.e., weeks or years) sojourn at altitude, the global O2 sensitivity of this pathway increases gradually, leading to hypoxic ventilatory acclimatization (HVA), defined as a gradual elevation of ventilation despite a continuously increasing arterial PO2, i.e., a decrease of the initial stimulus [1]. Early studies carried out in sheep showed that HVA is not achieved after transsection of peripheral chemosensory innervation [2], but can be induced by perfusion of hypoxic normocapnic blood restricted to the carotid bodies [3]. Accordingly, peripheral chemoreceptors are considered a key component of HVA, and several functional alterations in peripheral chemoreceptors underlie HVA. On the other hand, it is also becoming increasingly clear that central structures are important to modulate the integration of carotid chemo-afferent inputs within specific nuclei involved in the integration of stimuli to shape the final respiratory motor output.
Importance of HVA at altitude
The extent of ventilatory acclimatization determines the overall ability to cope with hypoxia, and is therefore of critical importance for travelers and residents at high altitude. For climbers, the maximum altitude that an individual can reach on Mt Everest is related to the hypoxic ventilatory response measured at sea level and during the ascension [4]. In permanent residents at altitude (>2,500 m), 10–15% of young and more than 30% of aged (above 50 years) are at risk to develop chronic mountain sickness [5, 6], a form of “de-acclimatization” whose most important feature is an excessive erythrocytosis induced by poor arterial oxygen saturation associated to a reduced sensitivity of peripheral chemoreceptors [7–9]. In rats permanently living at 3,600 m above sea level (Bolivian Institute for Altitude Biology—IBBA, La Paz, Bolivia), bilateral transsection of the carotid sinus nerve, which suppresses the stimulatory effect of the peripheral chemoreceptors on breathing, induces a drastic elevation of the hematocrit from a baseline level around 50% to more than 70% within a few weeks, and numerous animals died during this period. These results highlight the function of peripheral chemoreceptors at high altitude: they maintain a constant neural drive for lung ventilation and O2 delivery within vital boundaries.
The carotid bodies under chronic hypoxia
Neurotransmission between glomic cells and carotid sinus nerve endings
As mentioned above, carotid bodies are required for the establishment of HVA, and consistently with this idea, several studies have reported that chronic hypoxia increases hypoxic sensitivity of carotid bodies, evaluated as enhanced hypoxic ventilatory response in vivo [10], increased carotid sinus nerve (CSN) response to hypoxia in vitro [11] and increased excitability of chemosensitive cells in the carotid body [12]. One of the striking changes affecting the carotid body during chronic hypoxia is a marked hypertrophy due to an increased number and volume of chemosensitive cells [13–15], an increased number of fibroblasts located in the conjunctive walls surrounding the carotid body [16] and an enhanced vascularization due to both vasodilatation and VEGF-dependent (vascular endothelial growth factor) ingrowth of new blood vessels [17], which may contribute to sustaining the high level of sensory activity (and related metabolic demand) in chemoreceptors during chronic hypoxia. Glomic cells are submitted to ultrastructural changes in chronic hypoxia including an increased volume density of mitochondria and enlargement of dense-core vesicles that store neurotransmitters and modulators [15]. These changes would suggest that HVA is mainly due to enhanced sensory activity and associated release of excitatory neurotransmitters from chemosensitive cells.
Acetylcholine (ACh) is one of the neurotransmitter between glomic cells and sensory CSN endings in the carotid bodies [18]; it is then logical to speculate that cholinergic transmission is specifically enhanced during chronic hypoxic exposure. A series of studies has consistently demonstrated that this is indeed the case: the excitatory effect of bolus ACh application on CSN activity recorded in vitro is drastically higher following chronic hypoxic exposure in rats [11]. This response is consistent with data showing enhanced expression of α3- and α7-nicotinic receptor sub-units on petrosal ganglion neurons [19], in which are located the CSN neuronal cell bodies, i.e., the postsynaptic sensory element in the carotid body. Consistently with current models of neurochemical transmission between chemosensitive type I cells and CSN terminals, the nicotinic antagonist mecamylamine effectively reduces the stimulatory effect of ACh on CSN activity both in normoxic controls and following chronic hypoxia [11]. Mecamylamine also reduces hypoxic-induced carotid sinus nerve response in carotid bodies dissected from control animals, but it does not affect hypoxia-induced CSN response following chronic hypoxia. A similar response was observed for the muscarinic receptor antagonist atropine or the selective α7-nicotinic receptor antagonist methyllycaconitine. These paradoxical findings show that cholinergic neurotransmission is drastically affected under chronic hypoxia, but also highlight the well-acknowledged complexity and numerous paradoxical findings of carotid body neurochemistry.
ATP is also considered as a key element in carotid body neurotransmission. ATP is released during hypoxic stimulation [20] and acts as a co-transmitter with ACh to stimulate CSN activity through P2X2 purinergic receptors [18, 21]. On an in vitro preparation of carotid body/CSN recordings in rats, purinergic antagonists are able to block about 65–75% of hypoxia-evoked increase of CSN activity under normal conditions [22]. However, following chronic hypoxia, this antagonistic effect is less important (around 50%). But if expressed as absolute values (i.e., impulses/seconds, rather than % changes vs. baseline), the blocking effect of purinergic receptor antagonists was much higher following chronic hypoxia versus normoxic control, and the purinergic antagonists suppressed the higher level of basal (i.e., normoxic) CSN activity recorded following chronic hypoxic exposure compared to controls. Accordingly, and unlike cholinergic mechanism, the stimulatory effect of ATP on CSN activity appears to be enhanced following chronic hypoxia, but still purinergic mechanisms cannot completely explain the enhanced hypoxic sensitivity of carotid bodies following chronic hypoxic exposure. Consistently a full explanation of changes affecting carotid body function during chronic hypoxia must include further mechanisms.
Endothelin (ET) is a small peptide transmitter that has been involved as an important excitatory transmitter in the carotid body, specifically following chronic hypoxic exposure. In a detailed series of studies, Chen et al. [23] have provided clear evidence that the excitatory effect of ET on carotid sinus nerve activity and hypoxic response gradually increases during 16 days of hypoxic exposure in rats, following a pattern that closely matches the changes of minute ventilation during similar hypoxic exposure as earlier reported by Olson and Dempsey [24]. These functional changes are accompanied by important elevations of protein expression of ET and its receptor (ETA) in chemosensitive type I cells during chronic hypoxia. Similarly, mRNA expression of ET and ETA was also elevated following chronic hypoxia. A prominent feature of the effects of ET on CSN activity is that it is marginally expressed under normoxic control conditions but gradually increases during chronic hypoxia, closely matching the gradual increase of minute ventilation: blockade of ETA receptor depresses hypoxic CSN response by only 10% in normoxic control and by 50% after 16 days of hypoxia [23]. An additional study showed that bosetan, an ET receptor antagonist, selectively abolished the hyperplasia of chemosensitive cells under chronic hypoxia (but not the increased vasculature density) and drastically reduced the increased sensitivity of carotid sinus nerve response to hypoxia induced by chronic hypoxic exposure in rats [25]. Accordingly, under chronic hypoxia, the carotid body recruits new excitatory transmitters that selectively induce chemosensitive cells hyperplasia and act to sustain the elevated sensory activity during chronic hypoxia.
Among the numerous neuroactive factors synthesized by carotid body glomic cells, the role of dopamine has been particularly studied during chronic hypoxia. Dopamine is found at high concentration in the carotid bodies and is a potent inhibitory neuromodulator of carotid body chemotransduction both under acute hypoxia or following chronic hypoxic exposure [26–30]. Chronic hypoxia increases the content, turnover and synthesis of dopamine in the carotid body, mainly by increasing the tyrosine hydroxylase mRNA, protein level and activity. The expression level of D2 dopamine receptors (D2R) that mediate the inhibitory feedback of dopamine on carotid body response to hypoxia also increases during chronic hypoxic exposure. Functional studies indicate that the inhibitory function of dopamine on minute ventilation is slightly affected by chronic hypoxia [13, 27], consequently long-term D2R blockade in peripheral chemoreceptors enhanced HVA in chronically hyoxic rats [31].
Modulation of dopamine signaling in the carotid body is also a major mediator of the stimulatory effect of ovarian steroids (progesterone and estradiol) on respiratory chemoreflex. We have consistently showed that ovarian steroids stimulate breathing by reducing the inhibitory dopaminergic drive in the carotid bodies, this being particularly evident in rats raised at high altitude [29]. Interestingly, in this study, while domperidone (a specific D2R antagonist) enhanced resting minute ventilation in ovariectomized females, it inhibited resting minute ventilation in females following ten daily injections of ovarian steroids. This demonstrates that ovarian steroids are able to modify the dopaminergic component of carotid body chemoresponsiveness, which is determinant at high altitude. Furthermore, these results showed that the resting level of minute ventilation, under strictly normoxic conditions, is submitted to a determinant peripheral dopaminergic drive and that individual differences in resting minute ventilation are directly related to individual differences in the carotid body dopaminergic drive. Thus, up-regulation of carotid body dopaminergic metabolism may explain the de-acclimatization syndrome observed following menopause in permanent high altitude women [32, 33]. This process may also explain individual variability in the amplitude of HVA [31], which is a determinant factor that may lead to the development of chronic mountain sickness in human residents at high altitude [7].
Ion channels function and expression in glomic cells
Release of neuromodulators from glomic cells in hypoxia requires several steps from O2-sensing molecules that are tightly coupled to transmembrane ion channels (K+, Na+, Ca2+) to allow an increase of intracellular calcium concentration in glomic cells. It is evident that a sustained excitatory activity of glomic cells and higher hypoxic sensitivity in chronic hypoxia should require consistent changes of expression and function of channel proteins to maintain a consistent elevated flow of ions through the cellular membrane. Acute hypoxic exposure in glomic cells involves potent inhibition of a K+ current mediated by an acute down-regulation of O2-dependent CO production by hemeoxygenase 2 (HO2) [34], which leads to the activation of a voltage-dependent-Ca2+-channel, Ca2+ influx and exocytosis of neurotransmitters and modulators. Some studies have reported that chronic hypoxia in newborn rats decreases the density of K+ currents in glomus cells [35] and increases calcium influx through voltage-gated channels [36], but since these studies have been performed in newborn rats, and since neonatal hypoxia delays postnatal development of hypoxic sensitivity, these studies are difficult to take into account to discuss the mechanisms underlying HVA in adults. More recently, studies using glomic cells from adult New-Zealand rabbits showed that chronic hypoxia (in vitro) decreases the amplitude of an outward K+ current by down-regulating the expression (protein and mRNA) and function of Kv3.4 channels [37]. Interestingly, this channel is not O2-sensitive, but the down-regulation of this component of whole cell K+ current apparently increased the relative contribution of the O2-sensitive K+ and consequently increased cell excitability after chronic hypoxic exposure. Furthermore, in female rats, chronic hypoxia increases the expression of the voltage sensitive Na+ channel Nav1.1, which selectively contributes to enhance neurotransmitter release from chemosensitive type I cells [38].
The central nervous system under chronic hypoxia
While early HVA is likely to be driven by direct effects of hypoxia on the peripheral chemoreflex [39], changes in the central integration of the carotid body inputs and plasticity of the NTS are further involved in later HVA process [40]. Clearcut evidence for an increased responsiveness of the central nervous system to the peripheral chemosensory inputs was provided by Dwinell and Powell [41] who reported that long-term exposure (7 days) to hypoxia facilitates the translation of arterial chemoreceptor afferent input to ventilatory efferent output through a central mechanism. The chemosensory fibers from the peripheral chemoreceptors terminate caudal to the obex [42] in the nucleus tractus solitarius (NTS), which contains the premotor neurons of the dorsal respiratory group [43]. NTS neurons receiving carotid chemoreceptor inputs project to the ventrolateral medulla [44] containing the premotor neurons of the ventral respiratory group [43]. Accordingly, the specific neurochemistry of these neurons is a potential target underlying the changes affecting the central translation of inputs from peripheral chemoreceptors to respiratory neurons.
Neurotransmitters and neuromodulators
Although a large variety of cell types and neurochemicals is present in the NTS and could be considered as putative neurotransmitters and/or neuromodulators, so far a limited number of these transmitters have been clearly recognized to play a significant role in the HVA.
Amino acids and components of the NMDA glutamate receptor pathway
The ventilatory response to hypoxia is influenced by the balance between inhibitory (GABA, glycine and taurine) and excitatory (glutamate and aspartate) brainstem amino acid neurotransmitters. When stimulated by acute hypoxia, the chemosensory nerve endings release glutamate within the caudal NTS, which in turn stimulates breathing [45–47]. Systemic injections or local microinjections of MK-801 into the caudal NTS [46–48] reduce the acute hypoxic ventilatory response. In mice, long-term hypoxia increases the expression of NMDA receptors [49], while in rats acclimatized to hypoxia intraperitoneal MK-801 injection decreased the tidal volume response to hypoxia, but had no effect on tidal volume before acclimatization [48]. Accordingly, NMDA receptor up-regulation contributes to time-dependent changes in ventilation during chronic hypoxia. From the evidence that activation of PDGF-β receptor mediates down-regulation of NMDA receptor currents in rat hippocampal slices, Gozal et al. [50] raised the hypothesis that PDGF-β receptors (which are densely distributed in brainstem nuclei) could be involved in HVA. They found that expression of PDGF-β receptors in the rat NTS is decreased following long-term hypoxia and that this reduced expression correlates with the magnitude of HVA [51].
In the NTS, NO works as a retrograde messenger in an l-glutamate-releasing positive feedback system contributing to the induction and maintenance of hyperventilation produced by acute hypoxia [52–54]. According to the model proposed by Haxhiu et al. [53], the activation of postsynaptic NMDA receptors by glutamate released from CSN terminals leads to the increase of intracellular Ca2+, which stimulates NO synthase (NOS) activity. The produced NO diffuses back to the presynaptic element and increases the cGMP level that in turn potentiates the release of glutamate. Evidence for the contribution of NO to central ventilatory control has been recently extended to HVA. Hypoxia lasting for 2 weeks up-regulates the expression of neuronal NOS and increases NO production in mice brainstems, while the pharmacological inhibition of nNOS reduces minute ventilation in mice acclimatized to hypoxia but not in control mice [49], indicating that the nNOS pathway is recruited during chronic hypoxia to help sustain the high required level of minute ventilation.
Catecholamines
The NTS and the ventrolateral medulla contain the catecholaminergic cell groups, A2C2 and A1C1, respectively, which are adjacent to, or intermingled with, respiratory neurons [55]. Electrical stimulation of the carotid sinus nerve or hypoxic stimulation of the carotid bodies induces c-Fos-like immunoreactivity colocalized with tyrosine hydroxylase (TH), the rate-limiting enzyme of catecholamine biosynthesis, in several cardiorespiratory brainstem areas, i.e., the NTS, the dorsal vagal complex, the ventrolateral medulla oblongata, the locus coeruleus and the A5 noradrenergic cell group in the pons [56]. Respiratory neurons possess adrenergic receptors and receive close appositions from TH-immunoreactive neurons [55]. There is large evidence that the brainstem catecholaminergic areas participate in the control of cardiorespiratory functions under normoxia or hypoxia [43, 57–59] and can display a functional neuroplasticity under environmental challenges such as hypoxia [60, 61].
In response to acute hypoxia, dopamine is released from dopaminergic neurons in the NTS [62]. Dopaminergic D2R have been localized in the NTS [63], and their blockade decreases hypoxic ventilatory response [64], implying that central D2R facilitates the hypoxic ventilatory response. Thus, an enhanced effect of central dopamine on ventilation during chronic exposure to hypoxia may contribute to the increased central gain of the hypoxic ventilatory response, a hallmark of HVA. This point has been demonstrated in rats exposed to 0, 2 and 8 days of hypoxia: following blockade of D2 receptors in the central nervous system, minute ventilation decreased significantly more after 8 days of hypoxia than in normoxia, but did not change significantly after 2 days of hypoxia [27]. Thus, a transitory insensitivity to dopamine appeared during the first days of hypoxia. Contrasting with data on minute ventilation [27], the level of mRNA encoding D2R in the caudal NTS (that receives the CSN terminals) decreases below control levels after 7 days of hypoxia, but protein levels were not assessed in this study [65]. These findings indicate that central dopamine is an important excitatory neuromodulator of HVA, but does not contribute to the early stage of HVA. The implication of dopamine to HVA is also supported by data showing that knock-out mice lacking the D2R gene display abnormal HVA, i.e., no time-dependent increases in baseline ventilation or hypoxic ventilatory response during chronic hypoxia [66].
Central norepinephrine, unlike dopamine, is an inhibitory neuromodulator able to modulate the arterial chemoreflex in the caudal NTS through α2 adreno-receptors located on premotor respiratory neurons [43, 67]. The A2C2 neurons in caudal NTS are activated by long-term hypoxia, as shown by increases in norepinephrine turnover, TH activity and mRNA level [60]. TH activity in A2C2 neurons increased gradually after several days of sustained hypoxia to reach the maximal level after 10–14 days in rats [68]. After 2 weeks of hypoxia the changes in TH protein amount within the NTS correlated with the increases in ventilatory output [68], suggesting that changes in noradrenergic function might act as a fine tuning of the increasing ventilatory output in long-term hypoxic rats.
Evidence for direct O2-dependent re-modeling in brainstem under chronic hypoxia
In vivo and in vitro studies demonstrate that direct central O2-sensing may elicit excitatory and inhibitory effects on ventilation [69]. The central effects of hypoxia in the modulation of respiratory output may be evidenced following bilateral CSN transsection. In these chemodenervated rats, the hypoxic ventilatory response is strongly blunted, and only a weak ventilatory increase in ventilation persists, possibly due to secondary peripheral arterial chemoreceptors. After a few weeks, the chemodenervated rats recover their initial hypoxic ventilatory response, but the pattern of response is strikingly different from that observed in intact rats. While in intact rats the respiratory frequency is a major component of the hypoxic ventilatory response, the post-chemodenervation ventilatory recovery is induced by a selective increase in tidal volume without any change in respiratory frequency [70]. Furthermore, ventilatory acclimatization to hypoxia was altered, but still persisted in chemodenervated rats [71]. Since the caudal NTS is a major site of integration for peripheral chemosensory inputs, it is worth noting that the hypoxia-induced increase in TH mRNA in caudal NTS was not impaired by carotid chemodenervation [71]. The functional remodeling of ventilatory response to hypoxia after irreversible disruption of the chemoafferent pathway associated with persistent upregulation of TH mRNA suggests that NTS neurons involved in ventilatory control may be actively affected by hypoxia [72] and/or can develop O2-chemosensitive properties [69].
To test this hypothesis, Pascual et al. [73] investigated the hypoxic responses of NTS neurons in brainstem slices and found that hypoxia elicited two types of responses in vitro: a neuronal population was depolarized by hypoxia, while a second population of neurons was instead hyperpolarized. Furthermore, all hypoxic neuronal responses were reversed by progesterone over 2–3 min, suggesting a non-genomic mechanism of action for this steroid hormone. Thus, the cellular signaling of NTS neurons may be affected by hypoxia independently of the peripheral chemosensory inputs. Therefore, neurons in the NTS appear not only as the site of integration of chemo-afferent inputs, but also as the target for direct modulatory effects of hypoxia, which may play a determinant role on HVA.
Besides these direct effects of hypoxia on the activity of NTS neurons, transcriptional gene regulation is a pivotal mechanism resulting in upregulation of adaptive and neuroprotective genes within the brainstem of animals subjected to long-term hypoxia. The most widespread molecular mechanism for hypoxia-dependent regulation of gene expression is induction via binding of the hypoxia-inducible transcription factor (HIF). HIF-1 is responsible for the activation of a number of genes, including TH, which contribute to the physiological adjustments required by the reduction in oxygen availability [74]. Pascual and colleagues [75] showed that moderate hypoxia (10% O2) sustained for several hours can induce HIF-1α protein expression first in glial cells and later in neurons selectively located in the caudal NTS and in the ventrolateral medulla. The glia surrounding neurons of dorsomedian and ventrolateral respiratory areas showed HIF-1 staining after 1 h hypoxia. After 6 h of tolerable hypoxia, a subset of catecholaminergic neurons co-localized TH and HIF-1α proteins, suggesting that HIF-1 may participate in the in vivo control of hypoxia-induced central expression of TH. This finding suggests that glial cells are the early responsive element of NTS O2-sensing and then translate the stimulus to adjacent neurons located in respiratory areas. In astrocytes, the isoform HIF-2α mediates the transcriptional activation of erythropoietin expression, and this pathway may promote astrocytic paracrine-dependent neuron physiology or survival during tolerable hypoxia or ischemia, respectively [76]. Indeed, under tolerable long-term hypoxia, neuronal erythopoietin is a key factor for HVA, stimulating ventilatory control by alteration of catecholaminergic metabolism in the brainstem respiratory areas [77, 78]. A detailed review on this exciting new topic is presented in this series of mini-reviews (cf. Soliz and Gassmann: Epo and ventilation in hypoxia).
The catabolism of HIF-1α protein needs O2 and Fe2+. Therefore, the level of HIF-1α protein may be upregulated not only by hypoxia, but also pharmacologically by chelating the Fe2+ [79]. An iron chelator, such as desferrioxamine, is a useful tool to accumulate HIF-1α in normoxic conditions and induce overexpression of genes that are targets for the HIF-1 α protein [80]. In order to study the physiological consequences of central up-regulation of HIF-1 α independently of both hypoxia and chemosensory inputs, we treated normoxic chemodenervated rats with desferrioxamine [81]. As expected, the desferrioxamine treatment induced an overexpression of TH after few days in the NTS. The TH overexpression did not modify the basal ventilation, but in contrast was associated with a moderate increase in acute hypoxic ventilatory response [81]. Conversely, Kline et al. [82] reported that mice partially deficient for HIF-1 (Hif1α+/−) subjected to long-term hypoxia did not augment their hypoxic ventilatory or basal ventilation. Altogether, these studies demonstrate that (1) plasticity of brainstem catecholaminergic cells plays a pivotal role in HVA, and (2) HIF-1 participates in the neural remodeling contributing to HVA through the central regulation of two target genes, TH and erythropoietin, involved in integration of chemo-afferent inputs or brainstem O2-sensing.
Mice lacking the immediate early gene fos B are unable to enhance their baseline ventilation or hypoxic ventilatory response following long-term hypoxia, a characteristic feature of HVA observed in the wild-type mice [83]. The inability of mutant mice to develop HVA may be the functional index of the contribution of fos-related gene proteins to cellular adaptations to hypoxia. The products of the c-Fos and c-Jun genes activated by hypoxia can form heterodimers (Fos and Jun family proteins) or homodimers (Jun family proteins) constituting the AP-1 binding complex [84], which regulates the transcription of TH gene [85, 86].
Conclusion
Exposure to long-term hypoxia elicits a number of changes in neurotransmitters present in the carotid body and brainstem respiratory areas. It is meaningful that the changes in the activity of neurotransmission were detected at different levels of the metabolic pattern, i.e., genic and protein expression, neurotransmitter turnover and receptor expression, and are associated with cellular plasticity of the glomic cells and NTS neurons involved in integration of chemo-afferent inputs. From a cellular point of view, hypoxia may be considered as a stress factor that disturbs a pre-established steady state and progressively leads to subsequent re-organization of cellular systems required for sustained activity at a much higher level than under the previous normoxic conditions (see also Fig. 1). A complex series of events ensures that this enhanced activity is maintained throughout the chemosensory pathway, including morphological changes taking place in the carotid body, changes of ion channel protein expression for adequate equilibrium of ion gradients and movements in chemosensitive cells (without alterations of O2-sensing ion channels), sustained excitatory neurotransmitter release from chemosensitive cells, and accompanying increased of post-synaptic receptor expression and function. In the central nervous system, the strategy developed ensures that the message delivered by the sensitive nerve endings of the peripheral chemoreceptor is adequately transferred into enhanced respiratory activity by up-regulating the glutamatergic NMDA receptor system and marked contribution of putative O2 sensitive elements that could directly contribute to enhanced respiratory output characterizing HVA.
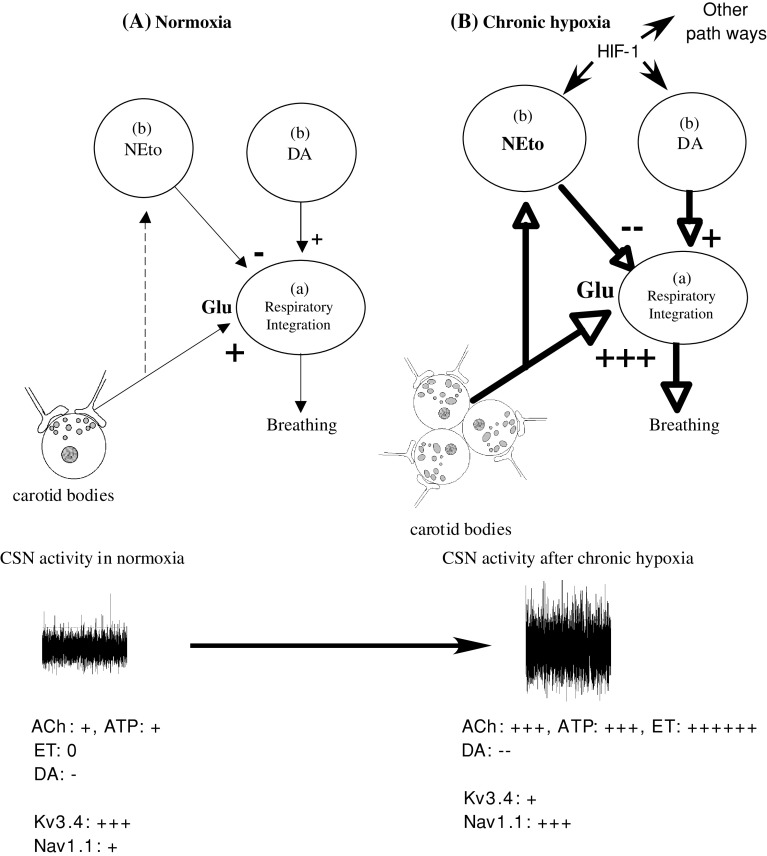
Fig. 1.
Model of HVA taking into account re-modeling of peripheral chemoreceptors and central integration of CSN inputs. After several days of hypoxic exposure, the carotid bodies are hypertrophied, changes affecting both neurotransmitter release (and postsynaptic receptors) and activity of specific ion channels in chemosensitive cells are completed (see text). In the NTS, the release of glutamate from CSN terminals is sustained at a high level, and target neurons develop a stronger response to glutamate (see text). Norepinephrine turn-over is increased due to increased TH mRNA and protein expression, which exerts an inhibitory tone on breathing. At the same time, the stimulatory effect of dopamine is reinforced (see text). Direct O2 sensing in brainstem nuclei may contribute to cellular and neurochemical remodeling and contribute to HVA through HIF-1-dependent regulation of gene expression. Relative thickness of the arrows represents the level of activation of the corresponding pathway. Relative excitatory (+) and inhibitory (−) effects of neurotransmitter in the carotid bodies on CSN activity and response to hypoxia are indicated
References
- 1.Weil JV. Control of ventilation in chronic hypoxia: role of peripheral chemoreceptors. In: Lahiri S, Cherniak NS, Fitzgerald RS, editors. Response and adaptation to hypoxia: organ to organelle. New York: Oxford University Press; 1991. pp. 122–132. [Google Scholar]
- 2.Smith C, Bisgard G, Nielsen A, Daristotle L, Kressin N, Forster H, Dempsey J. Carotid bodies are requiered for ventilatory acclimatization to chronic hypoxia. J Appl Physiol. 1986;60:1003–1010. doi: 10.1152/jappl.1986.60.3.1003. [DOI] [PubMed] [Google Scholar]
- 3.Busch MA, Bisgard GE, Forster HV. Ventilatory acclimatization to hypoxia is not dependant on arterial hypoxemia. J Appl Physiol. 1985;58:1874–1880. doi: 10.1152/jappl.1985.58.6.1874. [DOI] [PubMed] [Google Scholar]
- 4.Schoene RB, Lahiri S, Hackett PH, Peters RM, Jr, Milledge JS, Pizzo CJ, Sarnquist FH, Boyer SJ, Graber DJ, Maret KH, West JB. Relationship of hypoxic ventilatory response to exercise performance on Mount Everest. J Appl Physiol. 1984;56:1478–1483. doi: 10.1152/jappl.1984.56.6.1478. [DOI] [PubMed] [Google Scholar]
- 5.León-Velarde F, Arregui A, Monge C, Ruiz Y, Ruiz H. Aging at high altitudes and the risk of Chronic Mountain Sickness. J Wild Med. 1993;4:183–188. [Google Scholar]
- 6.Monge C, Arregui A, León-Velarde F. Pathophysiology and epidemiology of chronic mountain sickness. Int J Sports Med. 1992;13:S79–S81. doi: 10.1055/s-2007-1024603. [DOI] [PubMed] [Google Scholar]
- 7.Moore LG, Niermeyer S, Vargas E. Does chronic mountain sickness (CMS) have perinatal origins? Respir Physiol Neurobiol. 2007;158:180–189. doi: 10.1016/j.resp.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Severinghaus JW, Bainton GR, Carcelen A. Respiratory insensitivity to hypoxia in chronically hypoxic man. Respir Physiol. 1966;1:308–334. doi: 10.1016/0034-5687(66)90049-1. [DOI] [PubMed] [Google Scholar]
- 9.Kryger M, McCullough RE, Collins DD, Scoggin CH, Weil JV, Grover RF. Treatment of excessive polycythemia of high altitude with respiratory stimulant drugs. Am Rev Respir Dis. 1978;117:455–464. doi: 10.1164/arrd.1978.117.3.455. [DOI] [PubMed] [Google Scholar]
- 10.Joseph V, Soliz J, Pequignot J, Sempore B, Cottet-Emard JM, Dalmaz Y, Favier R, Spielvogel H, Pequignot JM. Gender differentiation of the chemoreflex during growth at high altitude: functional and neurochemical studies. Am J Physiol Regul Integr Comp Physiol. 2000;278:R806–R816. doi: 10.1152/ajpregu.2000.278.4.R806. [DOI] [PubMed] [Google Scholar]
- 11.He L, Dinger B, Fidone S. Effect of chronic hypoxia on cholinergic chemotransmission in rat carotid body. J Appl Physiol. 2005;98:614–619. doi: 10.1152/japplphysiol.00714.2004. [DOI] [PubMed] [Google Scholar]
- 12.Stea A, Jackson A, Macintyre L, Nurse CA. Long-term modulation of inward currents in O2 chemoreceptors by chronic hypoxia and cyclic AMP in vitro. J Neurosci. 1995;15:2192–2202. doi: 10.1523/JNEUROSCI.15-03-02192.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bee D, Pallot DJ. Acute hypoxic ventilation, carotid body cell division, and dopamine content during early hypoxia in rats. J Appl Physiol. 1995;79:1504–1511. doi: 10.1152/jappl.1995.79.5.1504. [DOI] [PubMed] [Google Scholar]
- 14.Pallot D, Bee D, Barer G, Jacob S. Some effects of chronic stimulation on the rat carotid body. In: Eyzaguirre C, Fidone S, Fitzgerald R, Lahiri S, McDonald D, editors. Arterial chemoreception. New York: Springer; 1990. pp. 293–301. [Google Scholar]
- 15.Pequignot JM, Hellstrom S, Johansson C. Intact and sympathectomized carotid bodies of long-term hypoxic rats: a morphometric ultrastructural study. J Neurocytol. 1984;13:481–493. doi: 10.1007/BF01148336. [DOI] [PubMed] [Google Scholar]
- 16.Laidler P, Kay J. A quantitative morphological study of the carotid bodies of rats living at a stimulated altitude of 4300 m. J Pathol. 1975;117:183–191. doi: 10.1002/path.1711170308. [DOI] [PubMed] [Google Scholar]
- 17.Pequignot JM, Hellstrom S. Intact and sympathectomized carotid bodies of long-term hypoxic rats. A morphometric light microscopical study. Virchows Arch A Pathol Anat Histopathol. 1983;400:235–243. doi: 10.1007/BF00612185. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, Zhong H, Vollmer C, Nurse CA. Co-release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. J Physiol. 2000;525 Pt 1:143–158. doi: 10.1111/j.1469-7793.2000.t01-1-00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinger B, He L, Chen J, Stensaas L, Fidone S. Mechanisms of morphological and functional plasticity in the chronically hypoxic carotid body. In: Lahiri S, Semenza GL, Prabhakar NR, editors. Oxygen sensing: Responses and adaptation to hypoxia. New York: Marcel Dekker Inc; 2003. pp. 439–465. [Google Scholar]
- 20.Buttigieg J, Nurse CA. Detection of hypoxia-evoked ATP release from chemoreceptor cells of the rat carotid body. Biochem Biophys Res Commun. 2004;322:82–87. doi: 10.1016/j.bbrc.2004.07.081. [DOI] [PubMed] [Google Scholar]
- 21.Rong W, Gourine AV, Cockayne DA, Xiang Z, Ford AP, Spyer KM, Burnstock G. Pivotal role of nucleotide P2X2 receptor subunit of the ATP-gated ion channel mediating ventilatory responses to hypoxia. J Neurosci. 2003;23:11315–11321. doi: 10.1523/JNEUROSCI.23-36-11315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He L, Chen J, Dinger B, Stensaas L, Fidone S. Effect of chronic hypoxia on purinergic synaptic transmission in rat carotid body. J Appl Physiol. 2006;100:157–162. doi: 10.1152/japplphysiol.00859.2005. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, He L, Dinger B, Stensaas L, Fidone S. Role of endothelin and endothelin A-type receptor in adaptation of the carotid body to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1314–L1323. doi: 10.1152/ajplung.00454.2001. [DOI] [PubMed] [Google Scholar]
- 24.Olson EB, Jr, Dempsey JA. Rat as a model for humanlike ventilatory adaptation to chronic hypoxia. J Appl Physiol. 1978;44:763–769. doi: 10.1152/jappl.1978.44.5.763. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, He L, Liu X, Dinger B, Stensaas L, Fidone S. Effect of the endothelin receptor antagonist bosentan on chronic hypoxia-induced morphological and physiological changes in rat carotid body. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1257–L1262. doi: 10.1152/ajplung.00419.2006. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- 27.Huey KA, Brown IP, Jordan MC, Powell FL. Changes in dopamine D(2)-receptor modulation of the hypoxic ventilatory response with chronic hypoxia. Respir Physiol. 2000;123:177–187. doi: 10.1016/S0034-5687(00)00175-4. [DOI] [PubMed] [Google Scholar]
- 28.Iturriaga R, Larrain C, Zapata P. Effects of dopaminergic blockade upon carotid chemosensory activity and its hypoxia-induced excitation. Brain Res. 1994;663:145–154. doi: 10.1016/0006-8993(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 29.Joseph V, Soliz J, Soria R, Pequignot J, Favier R, Spielvogel H, Pequignot JM. Dopaminergic metabolism in carotid bodies and high altitude acclimatization in female rats. Am J Physiol Regul Integr Comp Physiol. 2002;282:R765–R773. doi: 10.1152/ajpregu.00398.2001. [DOI] [PubMed] [Google Scholar]
- 30.Zapata P, Torrealba F. Blockade of dopamine-induced chemosensory inhibition by domperidone. Neurosci Lett. 1984;51:359–364. doi: 10.1016/0304-3940(84)90403-8. [DOI] [PubMed] [Google Scholar]
- 31.Gamboa J, Macarlupu JL, Rivera-Chira M, Monge CC, Leon-Velarde F. Effect of domperidone on ventilation and polycythemia after 5 weeks of chronic hypoxia in rats. Respir Physiol Neurobiol. 2003;135:1–8. doi: 10.1016/S1569-9048(03)00065-X. [DOI] [PubMed] [Google Scholar]
- 32.León-Velarde F, Ramos MA, Hernández JA, De Idiáquez D, Muñoz LS, Gaffo A, Córdova S, Durand D, Monge C. The role of menopause in the development of chronic mountain sickness. Am J Physiol. 1997;272:R90–R94. doi: 10.1152/ajpregu.1997.272.1.R90. [DOI] [PubMed] [Google Scholar]
- 33.León-Velarde F, Rivera-Chira M, Tapia R, Huicho L, Monge C. Relationship of ovarian hormones to hypoxemia in women residents of 4,300 m. Am J Physiol. 2001;280:R488–R493. doi: 10.1152/ajpregu.2001.280.2.R488. [DOI] [PubMed] [Google Scholar]
- 34.Williams SE, Wootton P, Mason HS, Bould J, Iles DE, Riccardi D, Peers C, Kemp PJ. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science. 2004;306:2093–2097. doi: 10.1126/science.1105010. [DOI] [PubMed] [Google Scholar]
- 35.Wyatt CN, Wright C, Bee D, Peers C. O2-sensitive K+ currents in carotid body chemoreceptor cells from normoxic and chronically hypoxic rats and their roles in hypoxic chemotransduction. Proc Natl Acad Sci USA. 1995;92:295–299. doi: 10.1073/pnas.92.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hempleman SC. Increased calcium current in carotid body glomus cells following in vivo acclimatization to chronic hypoxia. J Neurophysiol. 1996;76:1880–1886. doi: 10.1152/jn.1996.76.3.1880. [DOI] [PubMed] [Google Scholar]
- 37.Kaab S, Miguel-Velado E, Lopez-Lopez JR, Perez-Garcia MT. Down regulation of Kv3.4 channels by chronic hypoxia increases acute oxygen sensitivity in rabbit carotid body. J Physiol. 2005;566:395–408. doi: 10.1113/jphysiol.2005.085837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caceres AI, Obeso A, Gonzalez C, Rocher A. Molecular identification and functional role of voltage-gated sodium channels in rat carotid body chemoreceptor cells. Regulation of expression by chronic hypoxia in vivo. J Neurochem. 2007;102:231–245. doi: 10.1111/j.1471-4159.2007.04465.x. [DOI] [PubMed] [Google Scholar]
- 39.Robbins PA. Role of the peripheral chemoreflex in the early stages of ventilatory acclimatization to altitude. Respir Physiol Neurobiol. 2007;158:237–242. doi: 10.1016/j.resp.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Powell FL, Huey KA, Dwinell MR. Central nervous system mechanisms of ventilatory acclimatization to hypoxia. Respir Physiol. 2000;121:223–236. doi: 10.1016/S0034-5687(00)00130-4. [DOI] [PubMed] [Google Scholar]
- 41.Dwinell MR, Powell FL. Chronic hypoxia enhances the phrenic nerve response to arterial chemoreceptor stimulation in anesthetized rats. J Appl Physiol. 1999;87:817–823. doi: 10.1152/jappl.1999.87.2.817. [DOI] [PubMed] [Google Scholar]
- 42.Finley J, Katz D. The central organization of carotid-body afferents-projections to the brainstem of the rat. Brain Res. 1992;572:108–116. doi: 10.1016/0006-8993(92)90458-L. [DOI] [PubMed] [Google Scholar]
- 43.Bianchi AL, Denavit-Saubié M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- 44.Koshiya N, Guyenet PG. NTS neurons with carotid chemoreceptor inputs arborize in the rostral ventrolateral medulla. Am J Physiol. 1996;270:R1273–R1278. doi: 10.1152/ajpregu.1996.270.6.R1273. [DOI] [PubMed] [Google Scholar]
- 45.Gozal D, Xue YD, Simakajornboon N. Hypoxia induces c-Fos protein expression in NMDA but not AMPA glutamate receptor labeled neurons within the nucleus tractus solitarii of the conscious rat. Neurosci Lett. 1999;262:93–96. doi: 10.1016/S0304-3940(99)00065-8. [DOI] [PubMed] [Google Scholar]
- 46.Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Kurosawa H, Okabe S, Takishima T, Shirato K. In vivo release of glutamate in nucleus tractus solitarii of the rat during hypoxia. J Physiol (Lond) 1994;478:55–65. doi: 10.1113/jphysiol.1994.sp020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohtake PJ, Torres JE, Gozal YM, Graff GR, Gozal D. NMDA receptors mediate peripheral chemoreceptor afferent input in the conscious rat. J Appl Physiol. 1998;84:853–861. doi: 10.1152/jappl.1998.84.3.853. [DOI] [PubMed] [Google Scholar]
- 48.Reid SG, Powell FL. Effects of chronic hypoxia on MK-801-induced changes in the acute hypoxic ventilatory response. J Appl Physiol. 2005;99:2108–2114. doi: 10.1152/japplphysiol.01205.2004. [DOI] [PubMed] [Google Scholar]
- 49.El Hasnaoui-Saadani R, Alayza RC, Launay T, Pichon A, Quidu P, Beaudry M, Leon-Velarde F, Richalet JP, Duvallet A, Favret F. Brain stem NO modulates ventilatory acclimatization to hypoxia in mice. J Appl Physiol. 2007;103:1506–1512. doi: 10.1152/japplphysiol.00486.2007. [DOI] [PubMed] [Google Scholar]
- 50.Gozal D, Simakajornboon N, Czapla MA, Xue YD, Gozal E, Vlasic V, Lasky JA, Liu JY. Brainstem activation of platelet-derived growth factor-beta receptor modulates the late phase of the hypoxic ventilatory response. J Neurochem. 2000;74:310–319. doi: 10.1046/j.1471-4159.2000.0740310.x. [DOI] [PubMed] [Google Scholar]
- 51.Alea OA, Czapla MA, Lasky JA, Simakajornboon N, Gozal E, Gozal D. PDGF-beta receptor expression and ventilatory acclimatization to hypoxia in the rat. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1625–R1633. doi: 10.1152/ajpregu.2000.279.5.R1625. [DOI] [PubMed] [Google Scholar]
- 52.Gozal D, Gozal E, Simakajornboon N. Signaling pathways of the acute hypoxic ventilatory response in the nucleus tractus solitarius. Respir Physiol. 2000;121:209–221. doi: 10.1016/S0034-5687(00)00129-8. [DOI] [PubMed] [Google Scholar]
- 53.Haxhiu MA, Chang CH, Dreshaj IA, Erokwu B, Prabhakar NR, Cherniack NS. Nitric oxide and ventilatory response to hypoxia. Respir Physiol. 1995;101:257–266. doi: 10.1016/0034-5687(95)00020-E. [DOI] [PubMed] [Google Scholar]
- 54.Ogawa H, Mizusawa A, Kikuchi Y, Hida W, Miki H, Shirato K. Nitric oxide as a retrograde messenger in the nucleus tractus solitarii of rats during hypoxia. J Physiol. 1995;486(Pt 2):495–504. doi: 10.1113/jphysiol.1995.sp020828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pilowsky PM, Jiang C, Lipsky J. An intracellular study of respiratory neurons in the rostral ventrolateral medulla of the rat and their relationship to catecholamine-containing neurons. J Comp Neurol. 1990;301:604–617. doi: 10.1002/cne.903010409. [DOI] [PubMed] [Google Scholar]
- 56.Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol. 1994;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- 57.Coles SK, Dick TE. Neurones in the ventrolateral pons are required for post-hypoxic frequency decline in rats. J Physiol. 1996;497:79–94. doi: 10.1113/jphysiol.1996.sp021751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guyenet PG. Neural structures that mediate sympathoexcitation during hypoxia. Respir Physiol. 2000;121:147–162. doi: 10.1016/S0034-5687(00)00125-0. [DOI] [PubMed] [Google Scholar]
- 59.Guyenet PG, Koshiya N, Huangfu D, Verberne AJM, Riley TA. Central respiratory control of A5 and A6 pontine noradrenergic neurons. Am J Physiol. 1993;264:R1035–R1044. doi: 10.1152/ajpregu.1993.264.6.R1035. [DOI] [PubMed] [Google Scholar]
- 60.Dumas S, Pequignot JM, Ghilini G, Mallet J, Denavit-Saubie M. Plasticity of tyrosine hydroxylase gene expression in the rats nucleus tractus solitarius after ventilatory acclimatazation to hypoxia. Brain Res Mol Brain Res. 1996;40:188–194. doi: 10.1016/0169-328X(96)00050-2. [DOI] [PubMed] [Google Scholar]
- 61.Soulier V, Gestreau C, Borghini N, Dalmaz Y, Cottet-Emard JM, Pequignot JM. Peripheral chemosensitivity and central integration: neuroplasticity of catecholaminergic cells under hypoxia. Comp Biochem Physiol A Physiol. 1997;118:1–7. doi: 10.1016/S0300-9629(96)00369-6. [DOI] [PubMed] [Google Scholar]
- 62.Goiny M, Lagercrantz H, Srinivasan M, Ungerstedt U, Yamamoto Y. Hypoxia-mediated in vivo release of dopamine in nucleus tractus solitarii of rabbits. J Appl Physiol. 1991;70:2395–2400. doi: 10.1063/1.349413. [DOI] [PubMed] [Google Scholar]
- 63.Yokoyama C, Okamura H, Nakajima T, Taguchi J, Ibata Y. Autoradiographic distribution of [3H]YM-09151–2, a high-affinity and selective antagonist ligand for the dopamine D2 receptor group, in the rat brain and spinal cord. J Comp Neurol. 1994;344:121–136. doi: 10.1002/cne.903440109. [DOI] [PubMed] [Google Scholar]
- 64.Smatresk NJ, Pokorski M, Lahiri S. Opposing effects of dopamine receptor blockade on ventilation and carotid chemoreceptor activity. J Appl Physiol. 1983;54:1567–1573. doi: 10.1152/jappl.1983.54.6.1567. [DOI] [PubMed] [Google Scholar]
- 65.Huey KA, Powell FL. Time-dependent changes in dopamine D(2)-receptor mRNA in the arterial chemoreflex pathway with chronic hypoxia. Brain Res Mol Brain Res. 2000;75:264–270. doi: 10.1016/S0169-328X(99)00321-6. [DOI] [PubMed] [Google Scholar]
- 66.Huey KA, Low MJ, Kelly MA, Juarez R, Szewczak JM, Powell FL. Ventilatory responses to acute and chronic hypoxia in mice: effects of dopamine D2 receptors. J Appl Physiol. 2000;89:1142–1150. doi: 10.1152/jappl.2000.89.3.1142. [DOI] [PubMed] [Google Scholar]
- 67.Hayward LF. Evidence for alpha-2 adrenoreceptor modulation of arterial chemoreflexes in the caudal solitary nucleus of the rat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1464–R1473. doi: 10.1152/ajpregu.2001.281.5.R1464. [DOI] [PubMed] [Google Scholar]
- 68.Schmitt P, Soulier V, Pequignot JM, Pujol JF, Denavit-Saubie M. Ventilatory acclimatization to chronic hypoxia: relationship to noradrenaline metabolism in the rat solitary complex. J Physiol (Lond) 1994;477:331–337. doi: 10.1113/jphysiol.1994.sp020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neubauer JA, Sunderram J. Oxygen-sensing neurons in the central nervous system. J Appl Physiol. 2004;96:367–374. doi: 10.1152/japplphysiol.00831.2003. [DOI] [PubMed] [Google Scholar]
- 70.Roux JC, Peyronnet J, Pascual O, Dalmaz Y, Pequignot JM. Ventilatory and central neurochemical reorganisation of O2 chemoreflex after carotid sinus nerve transection in rat. J Physiol (Lond) 2000;522 Pt 3:493–501. doi: 10.1111/j.1469-7793.2000.t01-4-00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roux JC, Pequignot JM, Dumas S, Pascual O, Ghilini G, Pequignot J, Mallet J, Denavit-Saubie M. O2-sensing after carotid chemodenervation: hypoxic ventilatory responsiveness and upregulation of tyrosine hydroxylase mRNA in brainstem catecholaminergic cells. Eur J Neurosci. 2000;12:3181–3190. doi: 10.1046/j.1460-9568.2000.00208.x. [DOI] [PubMed] [Google Scholar]
- 72.Solomon IC. Excitation of phrenic and sympathetic output during acute hypoxia: contribution of medullary oxygen detectors. Respir Physiol. 2000;121:101–117. doi: 10.1016/S0034-5687(00)00122-5. [DOI] [PubMed] [Google Scholar]
- 73.Pascual O, Morin-Surun MP, Barna B, Denavit-Saubie M, Pequignot JM, Champagnat J. Progesterone reverses the neuronal responses to hypoxia in rat nucleus tractus solitarius in vitro. J Physiol. 2002;544:511–520. doi: 10.1113/jphysiol.2002.023994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lahiri S, Roy A, Baby SM, Hoshi T, Semenza GL, Prabhakar NR. Oxygen sensing in the body. Prog Biophys Mol Biol. 2006;91:249–286. doi: 10.1016/j.pbiomolbio.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 75.Pascual O, Denavit-Saubie M, Dumas S, Kietzmann T, Ghilini G, Mallet J, Pequignot JM. selective cardiorespiratory and catecholaminergic areas express the hypoxia-inducible factor-1 a (HIF-1 a) under in vivo hypoxia in rat brainstem. Eur J Neurosci. 2001;14:1981–1991. doi: 10.1046/j.0953-816x.2001.01816.x. [DOI] [PubMed] [Google Scholar]
- 76.Chavez JC, Baranova O, Lin J, Pichiule P. The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J Neurosci. 2006;26:9471–9481. doi: 10.1523/JNEUROSCI.2838-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soliz J, Gassmann M, Joseph V. Soluble erythropoietin receptor is present in the mouse brain and is required for the ventilatory acclimatization to hypoxia. J Physiol. 2007;583:329–336. doi: 10.1113/jphysiol.2007.133454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soliz J, Joseph V, Soulage C, Becskei C, Vogel J, Pequignot JM, Ogunshola O, Gassmann M. Erythropoietin regulates hypoxic ventilation in mice by interacting with brainstem and carotid bodies. J Physiol. 2005;568:559–571. doi: 10.1113/jphysiol.2005.093328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang GL, Semenza GL. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood. 1993;82:3610–3615. [PubMed] [Google Scholar]
- 80.Prass K, Ruscher K, Karsch M, Isaev N, Megow D, Priller J, Scharff A, Dirnagl U, Meisel A. Desferrioxamine induces delayed tolerance against cerebral ischemia in vivo and in vitro. J Cereb Blood Flow Metab. 2002;22:520–525. doi: 10.1097/00004647-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 81.Nguyen MV, Pouvreau S, El Hajjaji FZ, Denavit-Saubie M, Pequignot JM. Desferrioxamine enhances hypoxic ventilatory response and induces tyrosine hydroxylase gene expression in the rat brainstem in vivo. J Neurosci Res. 2007;85:1119–1125. doi: 10.1002/jnr.21202. [DOI] [PubMed] [Google Scholar]
- 82.Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. Proc Natl Acad Sci USA. 2002;99:821–826. doi: 10.1073/pnas.022634199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Malik MT, Peng YJ, Kline DD, Adhikary G, Prabhakar NR. Impaired ventilatory acclimatization to hypoxia in mice lacking the immediate early gene fos B. Respir Physiol Neurobiol. 2005;145:23–31. doi: 10.1016/j.resp.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 84.Rahmsdorf HJ. Jun: transcription factor and oncoprotein. J Mol Med. 1996;74:725–747. doi: 10.1007/s001090050077. [DOI] [PubMed] [Google Scholar]
- 85.Mishra RR, Adhikary G, Simonson MS, Cherniack NS, Prabhakar NR. Role of c-fos in hypoxia-induced AP-1 cis-element activity and tyrosine hydroxylase gene expression. Brain Res Mol Brain Res. 1998;59:74–83. doi: 10.1016/S0169-328X(98)00139-9. [DOI] [PubMed] [Google Scholar]
- 86.Schnell PO, Ignacak ML, Bauer AL, Striet JB, Paulding WR, Czyzyk-Krzeska MF. Regulation of tyrosine hydroxylase promoter activity by the von Hippel-Lindau tumor suppressor protein and hypoxia-inducible transcription factors. J Neurochem. 2003;85:483–491. doi: 10.1046/j.1471-4159.2003.01696.x. [DOI] [PubMed] [Google Scholar]