Abstract
Mitochondria control whether a cell lives or dies. The role mitochondria play in deciding the fate of a cell was first identified in the mid-1990s, because mitochondria-enriched fractions were found to be necessary for activation of death proteases, the caspases, in a cell-free model of apoptotic cell death. Mitochondrial involvement in apoptosis was subsequently shown to be regulated by Bcl-2, a protein that was known to contribute to cancer in specific circumstances. The important role of mitochondria in promoting caspase activation has therefore been a major focus of apoptosis research; however, it is also clear that mitochondria contribute to cell death by caspase-independent mechanisms. In this review, we will highlight recent findings and discuss the mechanism underlying the mitochondrial control of apoptosis and caspase-independent cell death.
Keywords: Mitochondria, Bcl-2 family, Cell death, Apoptosis, Caspase-independent cell death, Mitochondrial outer membrane permeabilization, Cancer, BH3 mimetics
Introduction
Cell death is a physiological process needed to remove unwanted or damaged cells. A considerable body of evidence demonstrates that mitochondria are the major organelles involved in signal transduction and execution of cell death. The mitochondria-dependent (or intrinsic) pathway to death is activated by diverse stimuli including growth factor withdrawal, DNA damage, heat shock, UV, gamma radiation, and chemotherapeutic drugs. The signaling pathways activated by these stressors culminate in permeabilization of the mitochondrial outer membrane and release of soluble proteins from the mitochondrial inter-membrane space (IMS).
Some proteins released from the IMS have been shown to trigger death by apoptosis, including cytochrome c, which complexes with Apaf-1 and caspase-9 to form the apoptosome. This results in activation of caspase-9, which activates downstream or executioner caspases, which orchestrate apoptotic cell death by cleaving a subset of cellular proteins. Other IMS proteins have also been suggested to play a role in caspase activation and in promoting other forms of cell death. To delineate these processes, we refer to caspase-dependent death as apoptosis and to caspase-independent cell death (CICD) as any other type of death, excluding necrosis, that may occur as a consequence of failed apoptosis. We review recent evidence that implicate mitochondria and mitochondrial proteins as key players in both of these processes. Interestingly enough, in a separate review in this issue, Autret and Martin extensively discuss the mechanisms regulating mitochondrial fusion/fission and possible roles in apoptosis.
How is mitochondrial outer membrane permeabilization controlled upon cell death induction?
Mitochondrial outer membrane permeabilization (MOMP) is regulated by Bcl-2 family members. Anti-apoptotic (or pro-survival) members preserve the integrity of the outer mitochondrial membrane (OMM) whereas pro-apoptotic members promote permeabilization, allowing efflux of cytochrome c. The Bcl-2 family members have generally been grouped into three classes depending on their activities and the particular Bcl-2 Homology (BH) domains conserved within the protein. The first class is anti-apoptotic, and includes molecules that contain BH domains 1, 2, 3, and 4, such as Bcl-2, Bcl-XL, and Mcl-1. The second class promotes apoptosis, and only contains BH domains 1, 2, and 3; these include Bax and Bak. The last class also displays a pro-apoptotic activity, but they only contain the third BH domain, and are often referred to as BH3-only proteins. These include Bad, Bik, Bid, Bim, Noxa, and Puma (for review, see [1]).
The importance of Bcl-2 in regulating cell death became evident from studies which showed that overexpression of Bcl-2 contributed to myc-induced lymphoma by promoting the survival of B cell precursors [2]. Bcl-2 was then shown to be homologous to Ced-9, one of only three genes shown to regulate the execution phase of apoptosis in C. elegans [3]. The importance of Bax and Bak in regulating cell death was less evident at first, because knocking out either protein did not affect viability of the mice or protect cells from various pro-apoptotic stimuli. However, intercrossing these mice did result in embryonic lethality, and embryonic fibroblasts from these animals were resistant to diverse death stimuli because cytochrome c was not released from the mitochondria [4]. The exact mechanism by which Bax and Bak permeabilize the mitochondrial outer membrane is still the topic of much debate, and various models have been proposed. Some models predict that MOMP is the result of pores or tears in the outer membrane, or because of matrix swelling which forces the larger inner membrane to burst through the outer membrane. (reviewed in [5])
Regulation of Bax/Bak mediated activation
Although there appears to be redundancy between Bax and Bak, this is not simply due to their similarity, because they require different steps for activation. Bax exists as an inactive monomer in the cytosol, and its activation requires several steps including conformational change, mitochondrial translocation, and oligomerization. So far, most of the studies on Bax have investigated the α isoform; however, a new β isoform of Bax, resulting from the unspliced mRNA of intron 5 of bax, has been found to be exclusively expressed in human cells. Baxβ is constitutively active because it lacks the crucial domain that maintains Baxα in an inactive conformation [6]; however, it acquires a unique C-terminus domain that targets it for proteasome-mediated degradation. Baxβ is therefore highly unstable, but may accumulate in a cell, either if the proteasome is blocked or if it is stabilized by a binding partner.
In contrast, Bak is normally located in the OMM and, recently, the group of Dr. Kluck described a novel mechanism for its activation and oligomerization [7]. Bak oligomerization is mediated by the exposure of the BH3 domain in an early step of its activation leading to the insertion of the BH3 of one molecule of Bak into the groove of another Bak molecule. This interaction results in an unexpected symmetric dimer that is essential for oligomerization and its pro-apoptotic function. These scientists therefore proposed a new model for Bak homo-oligomerization in the OMM, in which multiple symmetric dimers produce the higher Bak multimer required for MOMP.
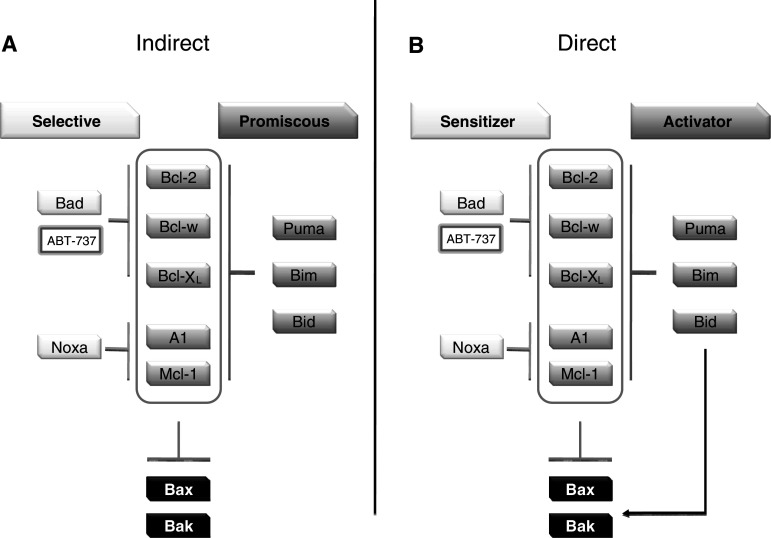
Anti-apoptotic members (Bcl-2, Bcl-XL, and Mcl-1) regulate MOMP by inhibiting Bax and Bak activities; however, both appear to be present in cells in an inactive state. It has therefore been puzzling how Bax and Bak are activated. The interaction model between individual members of the anti-apoptotic class of Bcl-2 family has been well documented and occurs in the hydrophobic BH3 binding pocket formed by BH-1 and -3 domains. These studies highlighted the importance of these domains in regulating protein–protein interactions. It is now generally accepted that the BH3-only members of the Bcl-2 family play an important role in Bax and Bak activation. How this occurs is still controversial [8], but two conflicting models have been presented (Fig. 1). The indirect or de-repressor model predicts that BH3 family members simply de-repress the intrinsic activities of Bax and Bak. The generation of Bim-Bid double-deficient animals supported this model, which indicates that BH3-only proteins de-repress Bax and Bak by binding and inhibiting anti-apoptotic Bcl-2 family members [9]. In this model, all classes of pro-survival proteins must be inhibited to induce apoptosis. Some “promiscuous” BH3-only proteins are able to induce apoptosis by themselves, because they inhibite the entire anti-apoptotic repertoire. By contrast, BH3-only proteins, which have more restricted inhibition profile, named “selective” group, are less potent activators. Indeed, a combination of “selective” BH3-only proteins with complementary binding profile induces apoptosis as well as a single “promiscuous” member (Fig. 1).
Fig. 1.
Indirect (a) and direct (b) model of Bax/Bak activation by BH3-only proteins (see text for details)
The direct or activator model predicts that most of the BH3 family members act as de-repressors, but some can bind and activate Bax and Bax directly. In support of this, Lovell et al. [10] used liposome reconstitution to show that tBid insertion in the membrane is an active step that initiates recruitment and activation of Bax in the bilayer. They also show that Bcl-XL can sequester tBid at the membrane, and that Bad, a “sensitizing” BH3-only protein, is able to displace tBid, which becomes free to activate Bax. It has also been shown previously that non Bcl-2 related proteins could also directly activate Bax and Bak. For instance, the tumor suppressor p53 has a transcription-independent pro-apoptotic function, and it can bind and activate both Bax [11] and Bak [12].
In addition to these dogmatic models, the group of Dr. Walensky investigated the model of Bax direct activation by an amphiphilic BH3 peptide derived from Bim [13]. They show the first structural evidence of a new site for protein interaction located near Bax N-terminus, distinct from the canonical hydrophobic groove on the opposite face. This new BH3 binding site appears to be crucial for apoptotic functions of Bax and will have a particular interest as a pharmacological target and for therapeutic modulation of apoptosis.
Regulation of Bcl-2 family members
The BH3-only proteins relay or integrate upstream death signal with mitochondria, but these family members are present in the cell in an inactive form, or are not expressed at a concentration that is sufficient to cause MOMP. Activation of these family members appears to be diverse. For example, activation of Bid requires cleavage by specific proteases to generate the active or the truncated form (tBid). Other BH3 family members are under transcriptional regulation, and are only made when signaling pathways are activated. These include Puma and Noxa, production of which are under the control of p53 [14, 15] and Bim which is controlled by FOXO3A [16]. Bim is also upregulated upon activation of ER stress pathways and activation of Bim in this model requires (1) a dephosphorylation by phosphatase 2A preventing its ubiquitination and degradation, and (2) an induction of transcription by CHOP-C/EBPa [17].
Anti-apoptotic proteins are also tightly regulated. Among these, Mcl-1 is of particular interest. Several signaling pathways can modulate its transcription, including MAPK, JAK, PI3 K, or microRNAs [18]. Mcl-1 is also regulated at translational level by the initiation factor eIF4E [19] and at post-translational level by GSK–3 phosphorylation [20]. Recently, regulation of Bcl-2 at the translational level was described: Bcl-2 mRNA contains an internal ribosome entry site (IRES) [21] allowing a selective increase of the mRNA through overexpression of translational regulatory protein 4E-BP1 and initiation factor eIF4G [22]. In this line, it was established that inhibition of glucose metabolism (by glucose removal or by using the glucose analog 2-deoxyglucose, 2DG) leads to a decrease in Mcl-1 protein levels through an inhibition of its translation via AMP-activated protein kinase (AMPK) activation and mammalian target of rapamycin (mTOR) inhibition [23].
Bcl-2 family Inhibitors
Inhibition of cell death is a key event allowing survival of cancer cells [24], therefore application of the knowledge of Bcl-2 family members has resulted in the generation of potential anti-cancer therapies based on neutralizing Bcl-2. These include ABT-737, Gossypol, and derivates, Chelerythrine and BH3 l-1, which are mimetics of the BH3 domains of pro-apoptotic Bcl-2 family members. The major difference between the various inhibitors is their selectivity and affinity for different Bcl-2 members, and the most potent and specific of these inhibitors appears to be aminobenzotriazole (ABT)-737.
ABT-737 acts similarly to peptides of the BH3 domain of Bad and inhibits Bcl-2, Bcl-XL, and Bcl-w, but not Mcl-1 or Bcl-2-A1, and it inhibits those Bcl-2 members by binding to the hydrophobic groove. It is effective at sub-nanomolar concentrations, with a dissociation constant below 1 nM. This compound is widely used for research purposes to uncover mechanisms of Bcl-2-mediated control of apoptosis, but has also shown some promise as a single agent therapy for various cancers [25]. Other cancers are resistant to ABT-737 as a single agent, and it has been proposed that sensitivity/resistance of cancer cells may depend on the amount of Mcl-1 present in those tumors [26]. Other studies have suggested that Mcl-1 levels alone are not sufficient to confer the sensitivity to ABT-737, and it may depend on the kind of defect in apoptosis signaling that has contributed to that cell becoming cancerous. These defects may include BH3-only deletion, Bax/Bak downregulation, or overexpression of anti-apoptotic proteins other than Bcl-2 [27]. Interestingly, ABT-737 does not produce major toxicity in normal cells except for a reversible platelet-depletion in which survival depends on Bcl-XL/Bak interaction. This suggests that normal cells do not depend on Bcl-2 for survival, and that ABT-737 will only be successful if overexpression of Bcl-2 was required for the cell to become cancerous, and is still required to maintain survival of these cancer cells.
Other mechanisms of MOMP induction
It has been reported that mitochondria can be permeabilized by other mechanisms, such as opening of the voltage-dependent anion channel (VDAC), opening of the mitochondrial permeability transition pore (PTP), or opening of ceramide channels. VDAC is the most abundant protein located in the OMM, and is responsible for transporting ATP and ADP in and out of the mitochondria (in conjunction with the adenine nucleotide transporter on the inner membrane). Its implication in cytochrome c release was suggested because it can interact with Bax, Bcl-2, and Bcl-XL [5]. VDAC has also been proposed to promote MOMP as part of a supramolecular pore known as the permeability transition pore (PTP), which engages proteins located both in the outer membrane, the inner membrane (IMM), and the matrix including VDAC, ANT, and cyclophilin D. In support of this model, a recent study shows the role of Bad on the sensitization of PTP openings [28]. Under ceramide or staurosporine stimulation, protein phosphatase 2A inhibition and/or protein kinase A/C activation leads to Bad dephosphorylation, therefore displacing the interaction between Bcl-XL and VDAC, leading to PTP sensitization to Ca2+. This mechanism of MOMP seems to be independent of Bax/Bak activation but mediated by VDAC [28]. The implication of VDAC in MOMP remains controversial because Baines et al. [29] established in 2007 that cytochrome c release was unaffected in MEFs lacking all isoforms of VDAC, thus indicating that VDAC is dispensable for MOMP.
Consequences of MOMP for cell viability?
Apoptosis is the primary form of cell death that occurs following MOMP, and is coordinated by caspases. As discussed previously, cytochrome c can promote caspase activation when it is released from mitochondria; however, another protein, second mitochondria-derived activator of caspase/direct IAP-binding protein with low pI (Smac/DIABLO) also contributes to caspase activation by neutralizing inhibitor of apoptosis proteins (IAPs); endogenous proteins that impair apoptosome-mediated activation of caspases [30, 31]. This particular function led to the will to create some Smac-mimetic in order to induce cancer cell death. These mimetics were engineered based on the AVPI binding motif, the four amino acid residues known to constitute the IAP interacting domain of Smac/DIABLO. These compounds have been shown to sensitize cells to death receptors or chemotherapeutic agents-induced death [32] and to be efficient in combination therapy on pre-clinical cancer mouse model [33]. Surprisingly, as recently reviewed by Wu et al. [34], it appears that Smac mimetics, in addition to targeting XIAP to relieve caspase-9 inhibition, can bind cIAPs to stimulate their degradation, leading to NF-κB activation and TNFα secretion, inducing thereby a caspase-8-dependent cell death.
Verhagen et al., who identified DIABLO, recently found some new potential IAP antagonists released from the mitochondria. Glutamate dehydrogenase, Nipsnap 3 and 4, CLPX, leucine-rich pentatricopeptide repeat motif-containing protein, and 3-hydroxyisobutyrate dehydrogenase are able to interact with the BIR2 domain of XIAP, cIAP1, and cIAP2, and to antagonize XIAP inhibition of caspase-3 in an in vitro essay [35]. They seemed to have a different specificity than Smac/DIABLO or Omi, which are able to interact with the BIR3 domain and therefore efficiently inhibit the caspase-9-XIAP binding.
Caspase activation is not the only consequence of MOMP. Firstly, cytochrome c transports electrons from complex III to complex IV of the electron transport chain to help generate an electrical potential across the mitochondrial inner membrane (ΔΨm). This potential is used by complex V to generate ATP from ADP and free phosphate by aerobic respiration, and is also required for protein import and mitochondrial biogenesis. Loss of cytochrome c from the IMS causes a transient loss of this potential; however, this event alone is not sufficient to result in complete impairment of oxidative phosphorylation or ATP production [36]. When caspases are activated following MOMP, they cleave the p75 subunit of complex I [37]. This event results in sustained loss of ΔΨm and impaired production ATP. Because ATP is the primary form of cellular energy and is essential for living cells, disruption of ATP production is likely to prevent long-term survival. Further, MOMP causes increased ROS production and loss of mitochondrial structural integrity. One consequence of MOMP may therefore be to prevent cell survival by blocking mitochondrial repair and to prevent several mitochondrial functions that are essential for cell viability. Because complex I is located on the mitochondrial inner membrane, caspase cleavage of p75 cannot occur in the absence of MOMP.
Contribution of MOMP to caspase-independent cell death
Consistent with the essential contribution of mitochondrial function to cell survival, it became apparent early that, although inhibition of caspase activity blocked the phenotypic appearance of apoptosis, it did not preserve cell survival. This was first observed in culture models which showed that the pan caspase inhibitor was not able to prevent cell death induced by a wide variety of pro-apoptotic stimuli. Similarly, other studies showed that genetic modification of cells to overexpress the caspase inhibitors, XIAP, Crm-A, or p35, did not permit long-term survival of cells treated with pro-apoptotic stimuli [38–40]. Some argued that caspase inhibition was not total in those settings, and that the death was in fact orchestrated by residual caspase activity. A more final approach investigated the impact of genetic invalidation of key components of the intrinsic apoptotic pathway such as Apaf-1, cytochrome c, or caspase-9 [41–43]. In these knockout models, the authors investigated loss of the interdigital web, a paradigm of cell death during development. Removal of these webs is a well-defined and characteristic apoptosis-dependent process, which occurs during development upon engagement of the intrinsic apoptosis pathway. The authors found that depletion of Apaf-1 delayed, but did not prevent loss of interdigital web [44]. Similarly, another study established that an in vivo caspase inhibition (using a pan caspase-inhibitor) was not able to prevent the loss of this web [45]. These studies also demonstrated that CICD can be found in vivo and therefore does not only represent a cell culture artifact. We will therefore refer to it as caspase-independent cell death (CICD). This generic name is far from being satisfying, but until someone comes up with a molecular definition of it, we can only define it in a default manner. We know what it is not, but we do not know what it is. Finally, it is very likely that this CICD regroups different types of cell death (see [46]).
As described earlier, cells from mice deficient in Bax and Bak were resistant to death and did not undergo MOMP following treatment with diverse pro-apoptotic stimuli. Importantly, the interdigital web was maintained during development in these mice highlighting the essential contribution of MOMP to CICD. Altogether, these studies have led to the idea that many cells can become committed to die prior to the point of caspase activation but following MOMP (see Fig. 2).
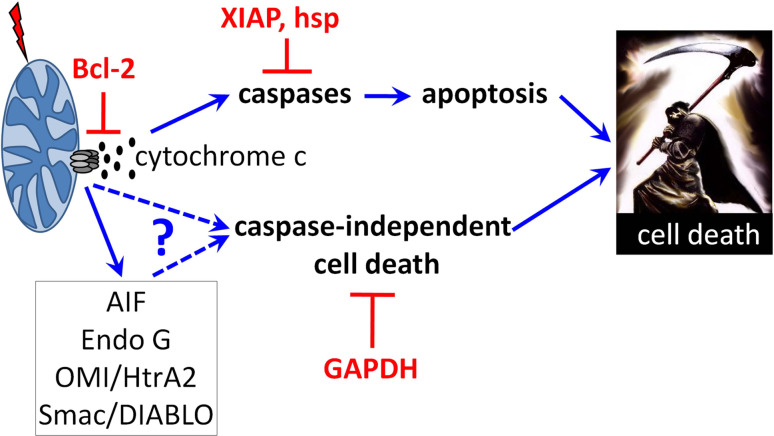
Fig. 2.
Summary of mitochondrial control of apoptosis and CICD. Upon stress, pro-apoptotic members of the Bcl-2 family will lead to the mitochondrial outer membrane permeabilization and subsequent release of inter membrane space proteins. Once free in the cytosol, those proteins will induce several death pathways including apoptosis and caspase-independent cell death. Specific inhibitors are indicated in red
Cytochrome c is not the only protein that is released from mitochondria following MOMP (Fig. 2). Among the other proteins released, EndoG was identified as a mitochondrial endonuclease that could relocate to the nucleus and induce caspase-independent nucleosomal DNA fragmentation [47]. Although one group reported that mice deficient in EndoG were embryonic lethal [48], two others found no specific phenotype, no mitochondrial defects, nor any difference in cell death susceptibility [49, 50]. The importance of EndoG in caspase-independent cell death is therefore unclear, and elucidation of this process may only become clear when EndoG−/− mice are crossed with mice that contain defects in caspase-dependent apoptosis pathways. These may include crosses between EndoG and Apaf null mice.
Apoptosis-inducing factor (AIF) was one of the first proteins shown to be released from mitochondria during apoptosis. AIF was first identified as a factor that caused DNA fragmentation when added to isolated nuclei. It is now known to be a flavoprotein that is anchored to the mitochondrial inner-membrane [51]. How AIF is released is still controversial; however, it has been proposed that, once it is released, it translocates to the nucleus where it cooperates with EndoG and/or cyclophilin A in a DNA degradation complex [52, 53]. The role of AIF in cell death was supported by data from knockout animals which died at the embryo stage, and exhibited an associated increase in cell death. However, this particular phenotype may reflect a major role of AIF in essential mitochondrial function. Consistent with this, AIF is required for maintenance of the mitochondrial respiratory chain [54]. In order to dissociate the two potential functions of AIF, Cheung et al. [55] used a membrane-anchored AIF that cannot be released from the mitochondria during apoptosis. They established that AIF is required to maintain the mitochondria structure and that it plays an important role in the death process, once relocated in the nucleus. After some controversies, it appears that AIF, as most mitochondrial proteins, displays a dual role, one in aerobic respiration and the other in promoting cell death, depending on its localization.
Omi/HtrA2 is a mitochondrial serine protease with an IAP-binding motif which is able, like Smac, to target IAP members. Antisense and RNAi-mediated knockdown of Omi increases the resistance of multiple cell lines against death stimuli (for review, see [56]). However, Omi-deficient mice, or cells derived from them, show no evidence of reduced rates of cell death. Their development was not obviously perturbed but they present an early neurodegenerative disorder with a parkinsonian phenotype [57]. As for some other IMS proteins, Omi seems to have at least two distinct roles, a physiological anti-apoptotic role via mitochondria protection under some stresses, [58, 59] and a death effector role, via the inhibition of IAPs, and also, maybe, via the cleavage of other substrates [56].
The contribution of IMS proteins to CICD remains controversial, because recent studies have shown that CICD can be prevented even when these IMS proteins and nucleases were released from the mitochondria. Using a retroviral screen, we established that overexpression of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) could effectively protect cells from CICD downstream of MOMP [60]. This GAPDH protection was generated in part by the glycolytic function of the enzyme but also through its nuclear function, which promoted induction autophagy. As it was not tested in this study, it still remains possible that GAPDH-mediated protection occurs via interaction with some IMS proteins [61].
Caspase-independent cell death and cancer
The role of caspases in apoptosis, and the contribution of defects in caspase activation to cancer progression, have been largely described. Indeed, reduced expression of Apaf-1 was first described in metastatic melanomas and correlated with poor prognosis [62]. Apaf-1 reduction has also described in acute myeloid leukemia, colorectal cancers, and cervical carcinomas [63–65]. Altered expression of XIAP, which blocks caspase activity, has been found in various tumors [66–68], and overexpression of Bcr-Abl, which is characteristic of chronic myeloid leukemia (CML), can prevent apoptosis downstream of mitochondrial cytochrome c release by perturbing caspase-9 recruitment to Apaf-1 [69, 70]. Also, apoptosome-induced caspase-3 activation was found to be deficient in several CML patients presenting a resistance to imatinib treatment [71].
The contribution of defects in caspase activation to cancer progression has been controversial, because most apoptotic stimuli and chemotherapeutic drugs trigger MOMP by a process that is independent of caspase activity. MOMP promotes apoptosis and CICD at the same time albeit with different kinetics (Fig. 2), and blocking caspase activity does not permit long-term survival of cells, suggesting that CICD ensures death of a tumor when caspase activation is defective. This also suggests that tumors that are resistant to chemotherapeutic agents must be protected from both apoptosis and CICD. A role for CICD in cancer was recently described in CML models. Imatinib mesylate is widely used for the treatment of patients with CML acting to induce apoptosis by counteracting Bcr-Abl activity. It was shown to induce apoptosis and CICD at the same time [71, 72]. However, 20–25% of patients will develop some resistance to imatinib. This indicates that an imatinib-resistant CML patient has either lost the ability to respond to imatinib (due to Bcr-Abl mutations or over-expression [73]) or the tumors have developed resistance to apoptosis and CICD at the same time. Recently, it was shown that some imatinib-resistant cells present a spontaneous GAPDH overexpression leading to CICD inhibition. Moderate GAPDH knockdown did not affect cellular metabolism but sensitized resistant cells to imatinib [74]. Altogether, this suggested that targeting CICD could be an innovative way to sensitize imatinib-resistant CML patients. In this regard, a recent study suggested that a modulation of GAPDH levels could be a way to modulate prednisolone resistance in acute lymphoblastic leukemia cells [75].
Much has been done to uncover the role of mitochondria in the control of cell death. As underlined in this review, this is still a very hot and controversial area of research. Several aspects will need further clarification and, by doing so, will probably lead to significant improvement of current cancer therapies.
Acknowledgments
We would like to thank Dr. Waterhouse (Mater Medical Research Institute, Brisbane, Australia) for critical reading of this manuscript. This work was supported in part by grants from Association pour la Recherche sur le Cancer (ARC), l’Agence Nationale de la Recherche (ref ANR-09-JCJC-0003-01), la Fondation de France and Région Provence-Alpes-Cote-d’Azur. J-E.R. is a recipient of a contrat d’interface INSERM-CHU de Nice.
References
- 1.Youle R, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 2.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 3.Hengartner MO, Ellis RE, Horvitz HR. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature. 1992;356:494–499. doi: 10.1038/356494a0. [DOI] [PubMed] [Google Scholar]
- 4.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/S1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 6.Fu N, Sukumaran S, Kerk S, Yu V. Baxβ: a constitutively active human Bax isoform that is under tight regulatory control by the proteasomal degradation mechanism. Mol Cell. 2009;33:15–29. doi: 10.1016/j.molcel.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Dewson G, Kratina T, Sim H, Puthalakath H, Adams J, Colman P, Kluck R. To trigger apoptosis, Bak exposes its BH3 domain and homodimerizes via BH3: groove interactions. Mol Cell. 2008;30:369–380. doi: 10.1016/j.molcel.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Chipuk J, Green D. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, Strasser A, Kluck RM, Adams JM, Huang DC. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 10.Lovell J, Billen L, Bindner S, Shamasdin A, Fradin C, Leber B, Andrews D. Membrane binding by tBid Initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 12.Leu JI, Dumont P, Hafey M, Murphy ME, George DL. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol. 2004;6:443–450. doi: 10.1038/ncb1123. [DOI] [PubMed] [Google Scholar]
- 13.Gavathiotis E, Suzuki M, Davis M, Pitter K, Bird G, Katz S, Tu H, Kim H, Cheng EH, Tjandra N, Walensky L. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 15.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/S1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 16.Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–1204. doi: 10.1016/S0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 17.Puthalakath H, O’reilly L, Gunn P, Lee L, Kelly P, Huntington N, Hughes P, Michalak E, Mckimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 18.Warr MR, Shore GC. Unique biology of Mcl-1: therapeutic opportunities in cancer. Curr Mol Med. 2008;8:138–147. doi: 10.2174/156652408783769580. [DOI] [PubMed] [Google Scholar]
- 19.Wendel H, Silva R, Malina A, Mills J, Zhu H, Ueda T, Watanabe-Fukunaga R, Fukunaga R, Teruya-Feldstein J, Pelletier J, Lowe S. Dissecting eIF4E action in tumorigenesis. Genes Dev. 2007;21:3232–3237. doi: 10.1101/gad.1604407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Sherrill KW, Byrd MP, Van Eden ME, Lloyd RE. BCL-2 translation is mediated via internal ribosome entry during cell stress. J Biol Chem. 2004;279:29066–29074. doi: 10.1074/jbc.M402727200. [DOI] [PubMed] [Google Scholar]
- 22.Braunstein S, Karpisheva K, Pola C, Goldberg J, Hochman T, Yee H, Cangiarella J, Arju R, Formenti SC, Schneider RJ. A hypoxia-controlled cap-dependent to cap-independent translation switch in breast cancer. Mol Cell. 2007;28:501–512. doi: 10.1016/j.molcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 23.Pradelli LA, Beneteau M, Chauvin C, Jacquin MA, Marchetti S, Munoz-Pinedo C, Auberger P, Pende M, Ricci JE (2009) Glycolysis inhibition sensitizes tumor cells to death receptors-induced apoptosis by AMP kinase activation leading to Mcl-1 block in translation. Oncogene (in press) [DOI] [PubMed]
- 24.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 25.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117:112–121. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, Verhaegen M, Soengas M, Ruvolo VR, McQueen T, Schober WD, Watt JC, Jiffar T, Ling X, Marini FC, Harris D, Dietrich M, Estrov Z, McCubrey J, May WS, Reed JC, Andreeff M. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12:171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Roy S, Madesh M, Davies E, Antonsson B, Danial N, Hajnóczky G. Bad targets the permeability transition pore independent of Bax or Bak to switch between Ca2+-dependent cell survival and death. Mol Cell. 2009;33:377–388. doi: 10.1016/j.molcel.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/S0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 31.Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/S0092-8674(00)00009-X. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 33.Chauhan D, Neri P, Velankar M, Podar K, Hideshima T, Fulciniti M, Tassone P, Raje N, Mitsiades C, Mitsiades N, Richardson P, Zawel L, Tran M, Munshi N, Anderson KC. Targeting mitochondrial factor Smac/DIABLO as therapy for multiple myeloma (MM) Blood. 2007;109:1220–1227. doi: 10.1182/blood-2006-04-015149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu H, Tschopp J, Lin SC. Smac mimetics and TNFalpha: a dangerous liaison? Cell. 2007;131:655–658. doi: 10.1016/j.cell.2007.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verhagen AM, Kratina TK, Hawkins CJ, Silke J, Ekert PG, Vaux DL. Identification of mammalian mitochondrial proteins that interact with IAPs via N-terminal IAP binding motifs. Cell Death Differ. 2007;14:348–357. doi: 10.1038/sj.cdd.4402001. [DOI] [PubMed] [Google Scholar]
- 36.Waterhouse NJ, Goldstein JC, von Ahsen O, Schuler M, Newmeyer DD, Green DR. Cytochrome c maintains mitochondrial transmembrane potential and ATP generation after outer mitochondrial membrane permeabilization during the apoptotic process. J Cell Biol. 2001;153:319–328. doi: 10.1083/jcb.153.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ricci JE, Munoz-Pinedo C, Fitzgerald P, Bailly-Maitre B, Perkins GA, Yadava N, Scheffler IE, Ellisman MH, Green DR. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell. 2004;117:773–786. doi: 10.1016/j.cell.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Denmeade SR, Lin XS, Tombal B, Isaacs JT. Inhibition of caspase activity does not prevent the signaling phase of apoptosis in prostate cancer cells. Prostate. 1999;39:269–279. doi: 10.1002/(SICI)1097-0045(19990601)39:4<269::AID-PROS7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 39.Okuno S, Shimizu S, Ito T, Nomura M, Hamada E, Tsujimoto Y, Matsuda H. Bcl-2 prevents caspase-independent cell death. J Biol Chem. 1998;273:34272–34277. doi: 10.1074/jbc.273.51.34272. [DOI] [PubMed] [Google Scholar]
- 40.Wilkinson JC, Cepero E, Boise LH, Duckett CS. Upstream regulatory role for XIAP in receptor-mediated apoptosis. Mol Cell Biol. 2004;24:7003–7014. doi: 10.1128/MCB.24.16.7003-7014.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hakem R, Hakem A, Duncan GS, Henderson JT, Woo M, Soengas MS, Elia A, de la Pompa JL, Kagi D, Khoo W, Potter J, Yoshida R, Kaufman SA, Lowe SW, Penninger JM, Mak TW. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–352. doi: 10.1016/S0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 42.Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, Su MS, Rakic P, Flavell RA. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/S0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 43.Li K, Li Y, Shelton JM, Richardson JA, Spencer E, Chen ZJ, Wang X, Williams RS. Cytochrome c deficiency causes embryonic lethality and attenuates stress-induced apoptosis. Cell. 2000;101:389–399. doi: 10.1016/S0092-8674(00)80849-1. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida H, Kong YY, Yoshida R, Elia AJ, Hakem A, Hakem R, Penninger JM, Mak TW. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell. 1998;94:739–750. doi: 10.1016/S0092-8674(00)81733-X. [DOI] [PubMed] [Google Scholar]
- 45.Chautan M, Chazal G, Cecconi F, Gruss P, Golstein P. Interdigital cell death can occur through a necrotic and caspase- independent pathway. Curr Biol. 1999;9:967–970. doi: 10.1016/S0960-9822(99)80425-4. [DOI] [PubMed] [Google Scholar]
- 46.Tait SW, Green DR. Caspase-independent cell death: leaving the set without the final cut. Oncogene. 2008;27:6452–6461. doi: 10.1038/onc.2008.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J, Dong M, Li L, Fan Y, Pathre P, Dong J, Lou D, Wells JM, Olivares-Villagomez D, Van Kaer L, Wang X, Xu M. Endonuclease G is required for early embryogenesis and normal apoptosis in mice. Proc Natl Acad Sci USA. 2003;100:15782–15787. doi: 10.1073/pnas.2636393100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.David KK, Sasaki M, Yu SW, Dawson TM, Dawson VL. EndoG is dispensable in embryogenesis and apoptosis. Cell Death Differ. 2006;13:1147–1155. doi: 10.1038/sj.cdd.4401787. [DOI] [PubMed] [Google Scholar]
- 50.Irvine RA, Adachi N, Shibata DK, Cassell GD, Yu K, Karanjawala ZE, Hsieh CL, Lieber MR. Generation and characterization of endonuclease G null mice. Mol Cell Biol. 2005;25:294–302. doi: 10.1128/MCB.25.1.294-302.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Susin SA, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med. 1996;184:1331–1341. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niikura Y, Dixit A, Scott R, Perkins G, Kitagawa K. BUB1 mediation of caspase-independent mitotic death determines cell fate. J Cell Biol. 2007;178:283–296. doi: 10.1083/jcb.200702134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cande C, Vahsen N, Kouranti I, Schmitt E, Daugas E, Spahr C, Luban J, Kroemer RT, Giordanetto F, Garrido C, Penninger JM, Kroemer G. AIF and cyclophilin A cooperate in apoptosis-associated chromatinolysis. Oncogene. 2004;23:1514–1521. doi: 10.1038/sj.onc.1207279. [DOI] [PubMed] [Google Scholar]
- 54.Brown D, Yu BD, Joza N, Benit P, Meneses J, Firpo M, Rustin P, Penninger JM, Martin GR. Loss of Aif function causes cell death in the mouse embryo, but the temporal progression of patterning is normal. Proc Natl Acad Sci USA. 2006;103:9918–9923. doi: 10.1073/pnas.0603950103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheung EC, Joza N, Steenaart NA, McClellan KA, Neuspiel M, McNamara S, MacLaurin JG, Rippstein P, Park DS, Shore GC, McBride HM, Penninger JM, Slack RS. Dissociating the dual roles of apoptosis-inducing factor in maintaining mitochondrial structure and apoptosis. EMBO J. 2006;25:4061–4073. doi: 10.1038/sj.emboj.7601276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vande Walle L, Lamkanfi M, Vandenabeele P. The mitochondrial serine protease HtrA2/Omi: an overview. Cell Death Differ. 2008;15:453–460. doi: 10.1038/sj.cdd.4402291. [DOI] [PubMed] [Google Scholar]
- 57.Martins LM, Morrison A, Klupsch K, Fedele V, Moisoi N, Teismann P, Abuin A, Grau E, Geppert M, Livi GP, Creasy CL, Martin A, Hargreaves I, Heales SJ, Okada H, Brandner S, Schulz JB, Mak T, Downward J. Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice. Mol Cell Biol. 2004;24:9848–9862. doi: 10.1128/MCB.24.22.9848-9862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moisoi N, Klupsch K, Fedele V, East P, Sharma S, Renton A, Plun-Favreau H, Edwards RE, Teismann P, Esposti MD, Morrison AD, Wood NW, Downward J, Martins LM. Mitochondrial dysfunction triggered by loss of HtrA2 results in the activation of a brain-specific transcriptional stress response. Cell Death Differ. 2009;16:449–464. doi: 10.1038/cdd.2008.166. [DOI] [PubMed] [Google Scholar]
- 59.Chao JR, Parganas E, Boyd K, Hong CY, Opferman JT, Ihle JN. Hax1-mediated processing of HtrA2 by Parl allows survival of lymphocytes and neurons. Nature. 2008;452:98–102. doi: 10.1038/nature06604. [DOI] [PubMed] [Google Scholar]
- 60.Colell A, Ricci JE, Tait S, Milasta S, Maurer U, Bouchier-Hayes L, Fitzgerald P, Guio-Carrion A, Waterhouse NJ, Li CW, Mari B, Barbry P, Newmeyer DD, Beere HM, Green DR. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell. 2007;129:983–997. doi: 10.1016/j.cell.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 61.Colell A, Green DR, Ricci JE. Novel roles for GAPDH in cell death and carcinogenesis. Cell Death Differ. 2009;16:1573–1581. doi: 10.1038/cdd.2009.137. [DOI] [PubMed] [Google Scholar]
- 62.Soengas MS, Capodieci P, Polsky D, Mora J, Esteller M, Opitz-Araya X, McCombie R, Herman JG, Gerald WL, Lazebnik YA, Cordon-Cardo C, Lowe SW. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature. 2001;409:207–211. doi: 10.1038/35051606. [DOI] [PubMed] [Google Scholar]
- 63.Jia L, Srinivasula SM, Liu FT, Newland AC, Fernandes-Alnemri T, Alnemri ES, Kelsey SM. Apaf-1 protein deficiency confers resistance to cytochrome c-dependent apoptosis in human leukemic cells. Blood. 2001;98:414–421. doi: 10.1182/blood.V98.2.414. [DOI] [PubMed] [Google Scholar]
- 64.Leo C, Horn LC, Rauscher C, Hentschel B, Richter CE, Schutz A, Leo CP, Hockel M. Lack of apoptotic protease activating factor-1 expression and resistance to hypoxia-induced apoptosis in cervical cancer. Clin Cancer Res. 2007;13:1149–1153. doi: 10.1158/1078-0432.CCR-06-2371. [DOI] [PubMed] [Google Scholar]
- 65.Zlobec I, Minoo P, Baker K, Haegert D, Khetani K, Tornillo L, Terracciano L, Jass JR, Lugli A. Loss of APAF-1 expression is associated with tumour progression and adverse prognosis in colorectal cancer. Eur J Cancer. 2007;43:1101–1107. doi: 10.1016/j.ejca.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 66.Tamm I, Richter S, Scholz F, Schmelz K, Oltersdorf D, Karawajew L, Schoch C, Haferlach T, Ludwig WD, Wuchter C. XIAP expression correlates with monocytic differentiation in adult de novo AML: impact on prognosis. Hematol J. 2004;5:489–495. doi: 10.1038/sj.thj.6200549. [DOI] [PubMed] [Google Scholar]
- 67.Li M, Song T, Yin ZF, Na YQ. XIAP as a prognostic marker of early recurrence of nonmuscular invasive bladder cancer. Chin Med J (Engl) 2007;120:469–473. [PubMed] [Google Scholar]
- 68.Hong SW, Kim CJ, Park WS, Shin JS, Lee SD, Ko SG, Jung SI, Park IC, An SK, Lee WK, Lee WJ, Jin DH, Lee MS. p34SEI-1 inhibits apoptosis through the stabilization of the X-linked inhibitor of apoptosis protein: p34SEI-1 as a novel target for anti-breast cancer strategies. Cancer Res. 2009;69:741–746. doi: 10.1158/0008-5472.CAN-08-1189. [DOI] [PubMed] [Google Scholar]
- 69.Deming PB, Schafer ZT, Tashker JS, Potts MB, Deshmukh M, Kornbluth S. Bcr-Abl-mediated protection from apoptosis downstream of mitochondrial cytochrome c release. Mol Cell Biol. 2004;24:10289–10299. doi: 10.1128/MCB.24.23.10289-10299.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kurokawa M, Zhao C, Reya T, Kornbluth S. Inhibition of apoptosome formation by suppression of Hsp90beta phosphorylation in tyrosine kinase-induced leukemias. Mol Cell Biol. 2008;28:5494–5506. doi: 10.1128/MCB.00265-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kamitsuji Y, Kuroda J, Kimura S, Toyokuni S, Watanabe K, Ashihara E, Tanaka H, Yui Y, Watanabe M, Matsubara H, Mizushima Y, Hiraumi Y, Kawata E, Yoshikawa T, Maekawa T, Nakahata T, Adachi S. The Bcr-Abl kinase inhibitor INNO-406 induces autophagy and different modes of cell death execution in Bcr-Abl-positive leukemias. Cell Death Differ. 2008;15:1712–1722. doi: 10.1038/cdd.2008.107. [DOI] [PubMed] [Google Scholar]
- 72.Okada M, Adachi S, Imai T, Watanabe K, Toyokuni SY, Ueno M, Zervos AS, Kroemer G, Nakahata T. A novel mechanism for imatinib mesylate-induced cell death of BCR-ABL-positive human leukemic cells: caspase-independent, necrosis-like programmed cell death mediated by serine protease activity. Blood. 2004;103:2299–2307. doi: 10.1182/blood-2003-05-1605. [DOI] [PubMed] [Google Scholar]
- 73.Apperley JF. Part I: mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol. 2007;8:1018–1029. doi: 10.1016/S1470-2045(07)70342-X. [DOI] [PubMed] [Google Scholar]
- 74.Lavallard VJ, Pradelli LA, Paul A, Beneteau M, Jacquel A, Auberger P, Ricci JE. Modulation of caspase-independent cell death leads to resensitization of imatinib mesylate-resistant cells. Cancer Res. 2009;69:3013–3020. doi: 10.1158/0008-5472.CAN-08-2731. [DOI] [PubMed] [Google Scholar]
- 75.Hulleman E, Kazemier KM, Holleman A, VanderWeele DJ, Rudin CM, Broekhuis MJ, Evans WE, Pieters R, Den Boer ML. Inhibition of glycolysis modulates prednisolone resistance in acute lymphoblastic leukemia cells. Blood. 2009;113:2014–2021. doi: 10.1182/blood-2008-05-157842. [DOI] [PMC free article] [PubMed] [Google Scholar]