Abstract
Although HCV is an enveloped virus, naked nucleocapsids have been reported in the serum of infected patients. The HCV core particle serves as a protective capsid shell for the viral genome and recombinant in vitro assembled HCV core particles induce strong specific immunity. We investigated the post-binding mechanism of recombinant core particle uptake and its intracellular fate. In hepatic cells, these particles are internalized, most likely in a clathrin-dependent pathway, reaching early to late endosomes and finally lysosomes. The endocytic acidic milieu is implicated in trafficking process. Using specific phosphoantibodies, signaling pathway inhibitors and chemical agents, ERK1/2 was found to be activated in a sustained way after endocytosis, followed by downstream immediate early genes (c-fos and egr-1) modulation. We propose that the intriguing properties of cellular internalization of HCV non-enveloped particles can induce specific ERK1/2–MAPKs events that could be important in HCV life cycle and pathogenesis of HCV infection.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-010-0351-5) contains supplementary material, which is available to authorized users.
Keywords: Hepatitis C virus, Non-enveloped particles, Endocytosis, ERK1/2, c-fos, egr-1
Introduction
Hepatitis C virus (HCV) is a major cause of chronic hepatitis worldwide and often leads to chronic liver disease and hepatocellular carcinoma [1]. The virus is classified as a member of the Flaviviridae family and produces a precursor polyprotein that is processed by proteases in order to yield structural and non-structural proteins. Core protein forms the capsid, which is surrounded by a lipid bilayer containing the glycoproteins, E1 and E2. However, different forms of HCV particles may exist in the circulation of infected individuals, one form being naked capsids [2]. HCV nucleocapsid devoid of enveloped proteins in the bloodstream of HCV-infected patients is a good indicator of the circulating viral load [3]. The overproduction and release of non-enveloped HCV nucleocapsids into the bloodstream and accumulation of the core protein (or core particles) in liver cells during an early phase of infection may be unconventional means by which HCV circumvents the host immune response and ensures its survival in the infected host [4]. Moreover non-enveloped core protein has been found in the serum of most HCV chronic carriers with active liver disease and in almost half of those with inactive disease [5], and it has been detected in cryoprecipitable immune complexes suggesting a role in the pathogenesis of cryoglobulin-related damage [6]. Furthermore, novel HCV subgenomes with in-frame deletions of both envelope proteins (E1 and E2), were identified in the liver, as well as in the serum of HCV-infected individuals [7–9].
When expressed in various heterologous systems, HCV core efficiently self-assembles into capsid-like core particles indistinguishable from native non-enveloped capsid shells [10, 11]. Baumert et al. [12] reported that some of the virus-like particles produced in insect cells reacted with anti-core antibodies and stimulated anti-core antibody responses. We previously reported not only the generation of recombinant non-enveloped HCV core particles in the absence of other HCV proteins but also, more importantly, demonstrated that these naked capsids can be uptaken by cells and induce cell-signaling phenomena [13, 14].
Recent studies have revealed a surprising variety of endocytic routes for enveloped as well as non-enveloped viral capsids [15, 16]. Entry via some of these pathways proceeds through endosomes, and the decreasing pH of these vesicular compartments often assists further viral trafficking [17]. This is also the case for HCV enveloped particles. For entry, cell surface molecules are required (CD81, SR-B1, Claudin-1, occludin, and glycosaminoglycans), reviewed in [18, 19], and are internalized in a clathrin-mediated pathway into endosomes, where pH triggers fusion between viral envelope and endosomal membrane [20–23]. The early endosome marker, Rab5, a small GTPase which is a key regulator of early endosomal function including internalization and signaling, is involved in this process [23]. The endocytosis pathway and endosomal pH modifications are also used by certain non-enveloped virus families, like Picornaviridae [24, 25], Adenoviridae [26], and Parvoviridae [27]. This critical process is highly regulated and/or coordinated by a number of cellular and viral factors [28–30]. It occurs either after binding to a surface receptor or from endocytic vesicles.
In this study, we attempt to elucidate the post-binding entry mechanism of recombinant HCV non-enveloped capsid-like core particles (designated HCVne particles) and their relationship to different events. We provide evidence that following entry in cells of hepatic origin, HCVne are processed through early to late endosomes and finally lysosomes via the clathrin-dependent pathway. The acidic milieu of endosomes and different host phosphorylated proteins are essential for this process. We also demonstrate that HCVne particles modulate the mitogen-activated protein kinase (MAPK) cascade during their entry and, more specifically, activate the extracellular signal-regulated kinase (ERK1/2) pathway. A sustained activation was observed that leads to ERK1/2 nuclear translocation and induction of c-fos and egr-1 immediate early genes.
Materials and methods
Cells, plasmids, antibodies and reagents
HepG2 (human hepatocellular liver carcinoma cell line) and Spodoptera frugiperda Sf9 cells were purchased from ATCC. Huh-7 (human hepatoma) cells were kindly provided by Dr. R. Bartenshlager (University of Heidelberg, Germany). Plasmids mRFP-Rab5 and m-RFP-Rab7 [31] (plasmids 14437 and 14436, respectively) as well as plasmid pFos WT-GL3 [32] (plasmid 11983) were all obtained from Addgene. GFP-Rab5WT, GFP-Rab5S34N, and GFP-Rab5Q79L were a gift from Dr. Juan S. Bonifacino [33]. pEgr1.2-luc plasmid was kindly provided by Dr. Gerald Thiel [34].
The following antibodies were used: mouse anti-EE autoantigen 1 (EEA1) (BD Biosciences), mouse anti-Lysosome associated membrane protein 2 (Lamp2) (Santa Cruz), mouse anti-actin (Chemicon), rabbit anti-phospho-p44/42, rabbit anti-phospho p38, mouse anti-p38, rabbit anti-c-fos (all from Cell Signaling), rabbit anti-ERK1 (K-23) (Santa Cruz) which detects both ERK1 and ERK2, mouse anti-α-tubulin (Molecular Probes), and rabbit anti-core [13]. As secondary antibodies for immunostaining, we used rabbit and mouse Alexa-488, -546, and -647 (Molecular Probes). MEK inhibitors UO126 and PD98059 were from Cell Signaling, recombinant human EGF from R&D, Alexa fluor 546-conjugated transferrin was from Molecular Probes, and Alexa Fluor 546-phalloidin from Invitrogen. All other reagents were from Sigma.
Cell culture, transient transfection and western blotting
Cells were grown in low glucose DMEM supplemented with 10% (v/v) FCS 100 U/ml penicillin and 100 μg/ml streptomycin 2.5 × 105. HepG2 cells were transiently transfected with JetPEI reagent (PolyPlus) according to the manufacturer’s protocol.
For western blotting analysis, cells were lysed in ice-cold lysis buffer [1% (v/v) Triton X-100, 50 mM KCl, 10 mM Tris pH 7.5, 1 mM DTT, 2 mM MgCl2, complete-mini protease inhibitor cocktail tablets (Roche), 1 mM PMSF, 2 mM sodium orthovanadate], electrophoretically separated on 8–10% (w/v) SDS-gels, transferred onto nitrocellulose membranes, incubated with appropriate antibodies, and detected by enhanced chemiluminescence (Pierce). Preparation of nuclear and cytoplasmic extracts has been described elsewhere [35]. Software Quantity One 4.4.1 (Bio-Rad) was used for densitometric analysis of gels.
Production of HCV-non enveloped capsid-like particles
The capsid-like particles were isolated from cell lysates, as described in [13]. Fractions (650 μl each) were collected from the top of the gradient, the density was determined by refractometry, and HCV antigen was analyzed both with the Ortho HCV core antigen ELISA test system (dilution 1:1,000 in PBS), and by SDS–PAGE followed by immunoblotting.
Immunofluorescence staining
Following addition of HCVne, corresponding to 10 ng of core protein, at the indicated times, cells grown on coverslips were washed three times with phosphate-buffered saline (PBS), fixed with 4% (w/v) paraformaldehyde in PBS (30 min), and the remaining reactive groups were blocked with 100 mM glycine. Cells were permeabilized with 0.02% (v/v) Triton X-100, and incubated with the appropriate primary and secondary antibodies, respectively, for 45 min. Slides were examined using a Leica TCS-SPS confocal microscope. Sequential scanning between channels was used to separate fluorescence emission from different fluorochromes and to completely eliminate bleed-through between channels. Typically, 9–15 confocal sections from three to five independent experiments, were quantified with Image-Pro Plus software (Media Cybernetics).
HCV non-enveloped particles tracking
For live microscopy, mRFP-Rab5- and mRFP-Rab7-transfected cells were grown in 35-mm glass-bottomed culture dishes (MatTek, Ashland, MA, USA). Internalization of HCVne into the host cells and cytoplasmic transport at 37°C were monitored at the indicated times by time-lapse live imaging microscope. An inverted time-lapse Olumpus X181 Cell-R microscope equipped with a Hamamatsu CCD ORCA/AG camera and ×60 or ×100 objective lenses. Cells were maintained at 37°C. Procession of movies was made with the Cell-R software (Olympus).
Luciferase assay
HepG2 cells (2.5 × 105) were transfected as already described. Twenty-four hours after transfection, cells were starved by incubation with DMEM without serum for 12 h and HCVne, as well as controls, were added for 18 h. Cell extracts were prepared and luciferase assays were performed using the Luciferase Assay System (Promega). In brief, 20 μl of the room temperature cell extract was mixed with 100 μl of room temperature luciferase assay reagent containing the substrate. The reaction was performed and measured in a Luminometer GloMax™ 20/20 (Promega). Protein concentration was determined using a protein assay kit (Bio-Rad) and was used for normalization of the luciferase assays. All experiments were repeated at least five times in duplicate wells.
mRNA expression analysis
Total cellular RNA was extracted from HepG2 cells using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. The purity and integrity of RNA samples were assessed by Nanodrop spectrophotometer measurement and gel analysis. Next, 1 μg of RNA was reversely transcribed using MMLV reverse transcriptase (Promega) and the resulting cDNA was subjected to semi-quantitative PCR analysis. Primers used were for: c-fos [36], egr-1 [37], 28S rRNA (same for human and mouse) as internal control in the PCR reaction [38]. All PCR conditions were in the exponential phase of amplification and, therefore, provided a direct correlation between the amount of products and RNA template abundance in the samples. The PCR products were analyzed on a 2% (w/v) agarose gel and the Quantity-One software (BioRad) was employed for densitometric analysis of the gels.
Statistical analysis
Statistical analysis of significance between control and treated samples from the confocal microscopy quantifications was performed by ANOVA and Student’s t test. Transfection assays results were analyzed with an unpaired Student’s t test. The levels of significance were set as P < 0.05, P < 0.01, and P < 0.001.
Results
HCVne particles colocalize with early endosomes
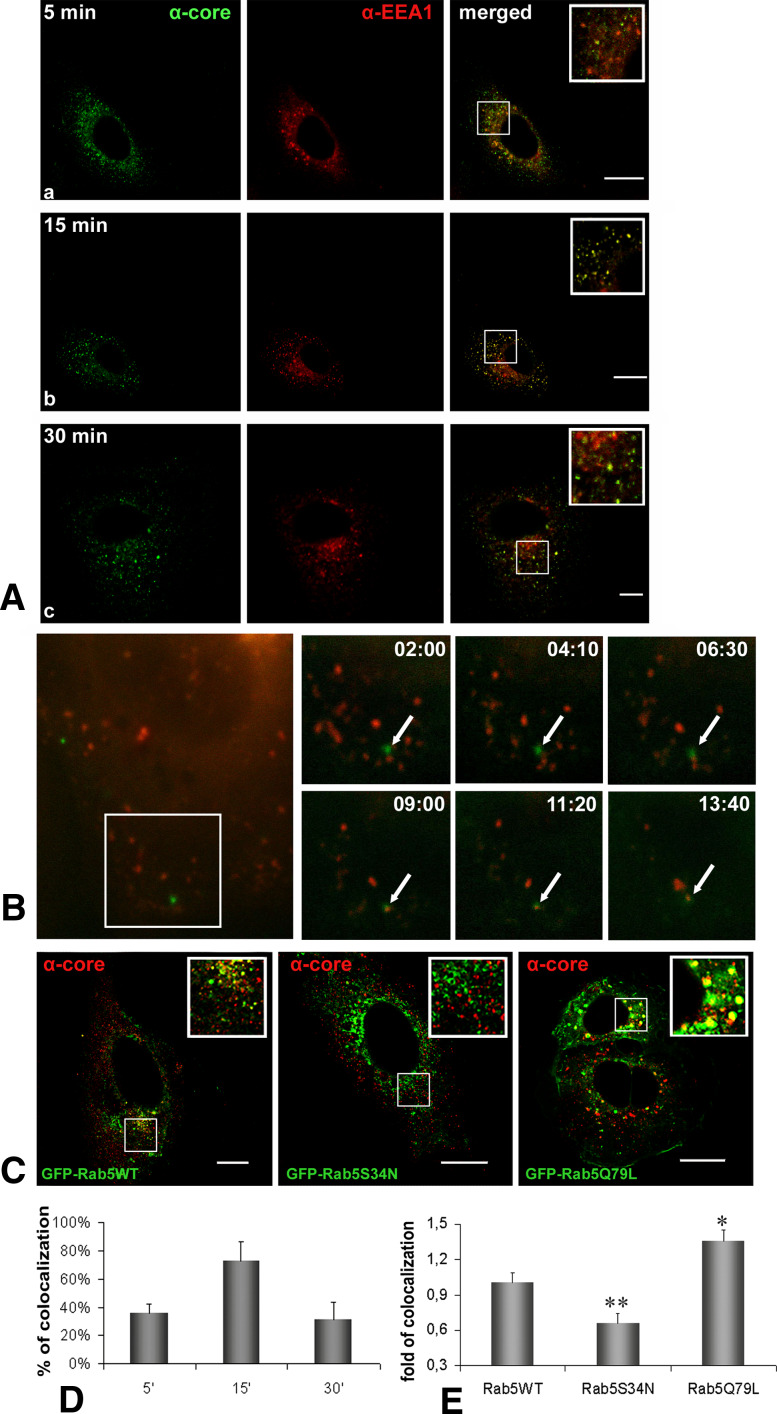
Internalization of HCVne particles in Huh7 cells was tested by studying their colocalization with EEA1, a specific marker for early endosomes. Fluorescence confocal microscopy visualisation revealed extensive colocalization at 15 min post-incubation at 37°C which decreased after 30 min (Fig. 1A, D). Specificity of immunostaining was verified with the use of secondary antibodies Alexa 488 (green) and Alexa 647 (blue), sequentially scanned to avoid any overlaps in the emission spectra (data not shown). Similar results concerning early endosomal localization of particles were obtained in live microscopy, using green fluorescent protein (GFP)-tagged non-enveloped particles (described in [14]) together with transiently expressed red fluorescent protein (mRFP)-tagged Rab5 protein. Observed colocalization started approximately 9 min after HCVne particles were added (Fig. 1B; Electronic supplementary material, ESM, Movie 1).
Fig. 1.
HCVne particles enter early endosomes. A Confocal microscopy of immunolabeled Huh7 cells incubated with HCVne particles using anti-core (green) or anti-EEA1 (red) antibodies at 5 (a), 15 (b), or 30 (c) min. Colocalization is shown in yellow in the merged images and inset. Βars a , b 20 μm, c 8 μm. B Selected images obtained from time lapse videos (Movie S1), representing time trajectories of movement of GFP fluorescent HCVne particles [14] to early endosomes in mRFP-Rab5 transfected cells. Arrows indicate traffic of fluorescent spot (min:s). C Huh7 transfected cells with GFP-Rab5WT, GFP-Rab5S34N, or GFP-Rab5Q79L plasmids treated with HCVne particles for 15 min. Immunofluorescence staining with anti-core (red) antibody. Bars a 20 μm, b,c 16 μm. D,E Image-Pro Plus quantification of colocalization for HCVne with EEA1 from (A) and with GFP-Rab5 WT and mutants from (C) (the colocalization level measured in GFP-Rab5 WT was used as the basis of comparison). *P < 0.05, **P < 0.01
To test the possible involvement of Rab5 in the HCVne particle uptake and trafficking, different Rab5 mutants were used. Rab5S34N is a dominant negative GDP-binding mutant shown to reduce endocytosis of different ligands, and Rab5Q79L is a GTPase-deficient, constitutively active mutant which increases ligand endocytosis [39]. Expression of WT (wild-type) Rab5 did not affect HCVne particles endocytosis, as colocalization with HCVne could be observed. As expected, an increased colocalization rate in cells transfected with Rab5Q79L was observed, in addition to the morphology alterations of the early endosomes previously reported [39]. In contrast, Rab5S34N produced a drop of the colocalization signal (Fig. 1C, E). Levels of expression of WT and mutated Rab5 proteins after transfection were similar. Additional confirmation of this result was obtained by using EEA1 antibody (data not shown). Overall, these results suggested that between 9 and 15 min of incubation at 37°C, HCVne particles were transported to early endosomes, and that Rab5 was involved in this transport.
HCVne particles ‘travel’ from endosomes to lysosomes in a microtubule-dependent way
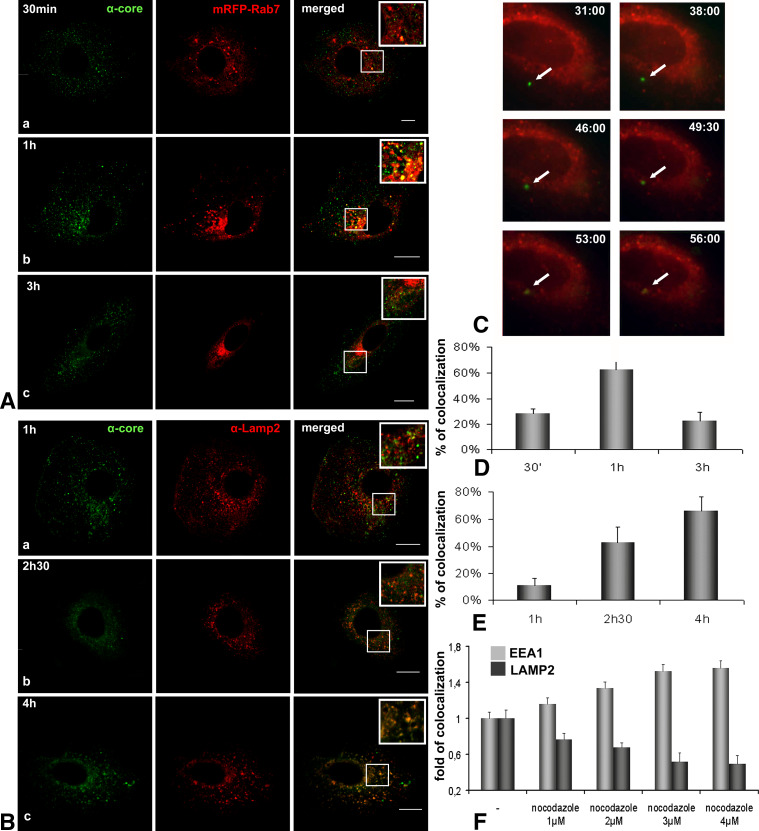
A punctuate perinuclear localisation of HCVne particles has been repeatedly observed [13, 14]; therefore, an eventual colocalization with late endosomes and lysosomes was investigated. As shown in Fig. 2A, D, within 1 h of incubation at 37°C, 62.5% of HCVne particles were present in compartments positive for Rab7, a late endosomal marker, and this percentage decreased over time. This was further confirmed by live microscopy using mRFP-Rab7 plasmid (Fig. 2C; ESM, Movie 2), where time-lapse series displayed a Rab7–HCVne colocalization starting at 53 min after particle binding. At 4 h post-incubation at 37°C, 66% of HCVne particles showed colocalization with a well-known lysosomal marker, Lamp2 (Fig. 2B, E). Similar results were observed with HepG2, another hepatic derived cell line (ESM, Figure S1).
Fig. 2.
Presence of HCVne particles in late endosomes and lysosomes. A Confocal microscopy using anti-core (green) antibody and mRFP-Rab7 plasmid (red) for 30 min (a), 1 (b), and 3 (c) h of HCVne particles incubation. Colocalization is observed in yellow in the merged images and inset. Bars a 8 μm, b 16 μm, c 20 μm. B Following HCVne particles addition, Huh7 cells, were co-labelled with anti-core (green) and anti-Lamp2 (red) antibodies at 1 (a), 2.5 (b), or 4 (c) h of incubation at 37°C. Insets show high magnification regions of the merged images. Bars 16 μm. C Selected images obtained from time lapse videos (ESM, Movie 2), representing time trajectories of movement of GFP fluorescent HCVne particles [14] to late endosomes in mRFP-Rab7 expressing cells. Arrows indicate traffic of fluorescent HCVne particles (min:s). D,E Colocalization’s quantification for HCVne particles with Rab7 and Lamp-2, respectively. F Disruption of microtubules with increasing concentrations of nocodazole. Cells were pretreated for 2 h, HCVne were added for 20 min, and cells were fixed 4 h later. EEA1-HCVne colocalization is shown in light grey bars and Lamp2-HCVne in dark grey bars
The host cytoskeleton, namely the actin filaments and microtubules, has been involved in the trafficking of endocytic compartments. Actin filaments are required for the initial uptake whereas microtubules are involved in maintaining the endosomal traffic between peripheral early and late endosomes [40, 41]. Our labeling results were in agreement with an actin filament and microtubules-mediated transport of particles inside endosomes (Figure S2). Nocodazole disrupts microtubules dynamics and, thus, transport from the early endosomes to lysosomes [42]. Increasing concentration of nocodazole increased the colocalization of HCVne particles with early endosomes and decreased their colocalization with lysosomes (Fig. 2F). Taken together, these results provided evidence that HCVne particles reached late endosomes after approximately 1 h of incubation and arrived at lysosomes 3 h later, in a microtubule-dependent way.
HCVne particles entry is pH dependent
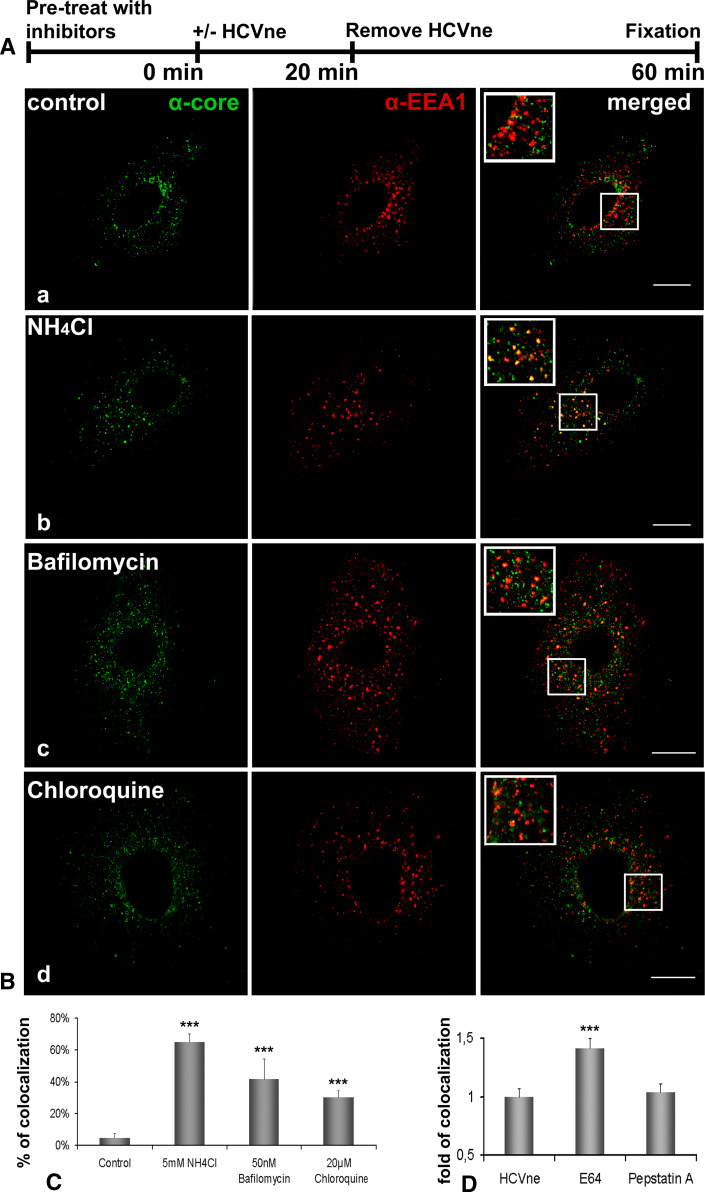
Many viruses that traffic through the endocytic vesicles require the acidic pH of these compartments for proper transport or to otherwise facilitate function or infection [16, 17, 20–27, 43]. We investigated the importance of acidic pH during HCVne particles entry by treating cells with three inhibitors of endosomal acidification, the lysosomotropic agents ammonium chloride (NH4Cl) and chloroquine, as well as bafilomycin A1, which inhibits the vacuolar H+-ATPase [44, 45]. After an adequate pre-treatment of the cells with the inhibitors (30 min for bafilomycin A1 and chloroquine and 15 min for NH4Cl) particles were added in the medium for 20 min. Unbound capsids were washed away, and incubation was extended for additional 40 min (Fig. 3A). EEA1 co-staining demonstrated that the particles remained in early endosomes even at 1 h post-incubation in the drug-treated samples, whereas in the untreated control they moved onto the late endosomes as expected (Fig. 3B, C). Differences in the percentage of colocalization were probably due to the efficacy or mechanism of action of each agent. Minimal cell toxicity was observed in drug-treated cells through the spectra of concentration used in this experiment.
Fig. 3.
HCVne particles require low pH for internalization. A Schematic diagram representation of the experiment. B Huh7 cells untreated (a) or incubated with 5 mM NH4Cl (b), 20 μM chloroquine (c), or 50 nM bafilomycin (d) were added with particles as described in (A) and immunolabeled with anti-core (green) and anti-EEA1 (red) antibodies. Inset images show a higher magnification of the boxed section of the merged pictures. Bars a,b,d 16 μm, c 20 μm. C Image-Pro quantification of colocalization. The control was arbitrarily set at 100% and all other values are a percentage of this. D Quantification of colocalization from cells treated with E64 (50 μm, 2.5 h) and pepstatin A (50 μm, 2.5 h). Cells were pretreated with the inhibitors as described in (A). ***P < 0.001
Cathepsins B and L are E64-sensitive cysteine proteases that are the most abundant proteases present in endosomes and lysosomes and are active at acidic pHs in a broad range of mammalian cells [46]. Incubation of cells with E64 or the aspartic protease inhibitor pepstatin A prior to HCVne addition (Fig. 3D) was followed by an increase in the EEA1 colocalization even 80 min after HCVne addition only with E64, thus providing support that cysteine but not aspartic proteases are implicated. Taken together, these results suggested for a strong involvement of acidic pH in HCVne trafficking.
Clathrin-mediated endocytosis is required for HCVne particles entry
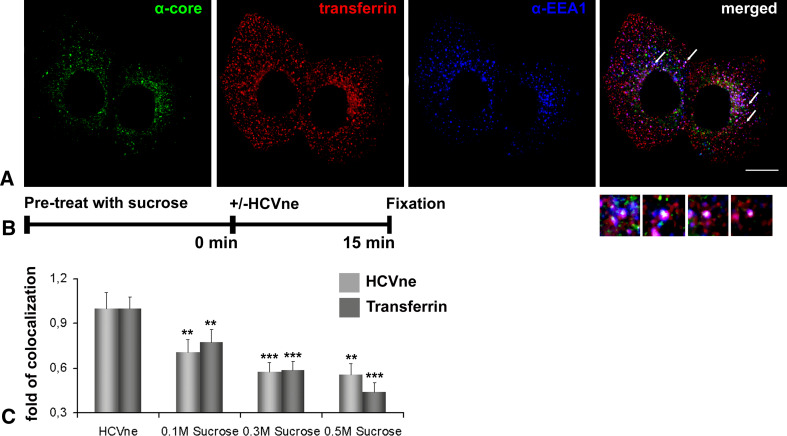
Clathrin-mediated endocytosis constitutes the most exploited low pH-dependent internalization pathway used by viruses. However, other pathways including caveolae, macropinocytosis, and non-caveolin; non-clathrin endocytosis can be used as well [15]. Transferrin is generally accepted as a ligand exclusively internalized via the clathrin-coated-pit pathway [47]. Therefore, we internalized Alexa 546-transferrin together with HCVne particles for 15 min at 37°C and, as shown in Fig. 4A, a triple colocalization (EEA1, transferrin, HCVne) was observed, thereby indicating that this pathway could be used for particle entry. To further investigate, we used sucrose. Indeed, sucrose is a chemical inhibitor of clathrin-mediated endocytosis. Its inhibition involves the dispersion of clathrin lattices on the plasma membrane [48]. In Fig. 4B, C, colocalization of either particles or Alexa 546-transferrin with EEA1, was significantly decreased in a dose-dependent way. These results strongly argue towards the clathrin-mediated entry of the HCVne particles uptake in Huh7 cells. However, the lack of complete Alexa 546-transferrin/HCVne colocalization makes an alternative entry pathway plausible.
Fig. 4.
HCVne particles are internalised via a clathrin-dependent pathway. A HCVne particles and Alexa 546-transferrin (red) in Huh7 cells, processed for immunofluorescence with anti-core (green) and anti-EEA1 (blue) antibodies. Arrows show individual endosomes positive for the two markers (insets). Bar 16 μm. B Schematic diagram representation of the experiment. C Quantification of colocalization of HCVne particles and transferrin with EEA1. Huh7 cells pre-treated with increasing concentrations of sucrose. Following addition of HCVne particles or transferrin, cells were fixed and immunostained either with anti-core/anti-EEA1 or anti-EEA1. **P < 0.01, ***P < 0.001
Endocytosis of HCVne particles mediates activation of MAPK–ERK1/2
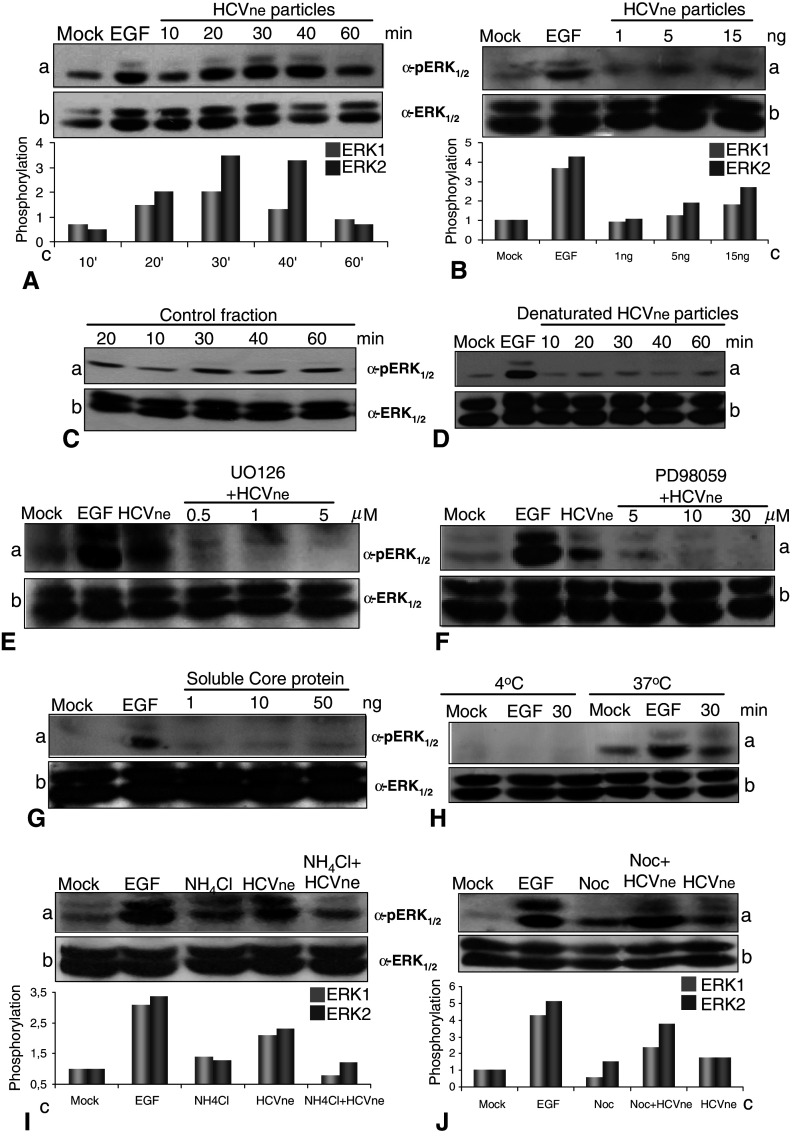
Endocytosis is a sophisticated process which can regulate different signaling pathways [28–30, 49]. The MAPK–ERK1/2 pathway is involved in the control of many fundamental cellular processes and is targeted by different viruses [29, 30]. Moreover, earlier studies from our laboratory showed an HCVne particles dependent delocalization of ERF, a downstream target of ERK1/2, [13, 14]. In the light of this, we tested ERK1/2 phosphorylation in HepG2 cells challenged with HCVne particles. As shown in Fig. 5A, an approximately twofold activation of ERK1 and a 3.5-fold activation of ERK2 were observed 30 min post-incubation at 37°C. Epidermal growth factor (EGF 50 nM), a well-known stimulator of the pathway, was added for 10 min as a positive control. ERK-activation by HCVne particles was found to be dose-dependent, since a rising signal was detected with increasing particle concentrations (Fig. 5B). Similar results where obtained in Huh7 cells (data not shown). As controls, we used either a fraction with the same sucrose density expressing GFP protein instead of core (described in [14]), or the same fraction but heat denaturated (1 h at 100°C). In both cases, no ERK1/2 activation was observed (Fig. 5C, D). In addition, UO126 and PD98059, specific MEK inhibitors [50], were shown to block ERK activation induced by HCVne particles (Fig. 5E, F). Purified bacterial soluble core protein was also tested, and even at high concentrations, no ERK1/2 phosphorylation was observed (Fig. 5G).
Fig. 5.
HCVne particles activate MEK1/2–ERK1/2 pathway. A Time course of ERK1/2 phosphorylation from lysates of HepG2 cells maintained in serum-free conditions incubated with HCVne particles (5 ng) for 10, 20, 30, 40, and 60 min at 37°C. EGF (50 nM) was used as positive control. Western blot with phospho-ERK1/2 (a), ERK1/2 (b), and quantification of the optical densities of phospho-ERK immunoreactive bands normalized to the optical densities of total ERK1/2 in the same samples (c) are presented. ERK1 is represented in light grey bars and ERK2 in dark grey bars. B HepG2 cells, maintained in serum-free conditions, were incubated for 30 min with increasing concentrations of HCVne particles (expressed in ng of core protein). C HepG2 cells treated with a fraction of equivalent sucrose density from Sf9 cell lysates infected with a control (GFP only producing) baculovirus or D with the HCVne fraction heat denatured (1 h at 100°C). E,F HepG2 cells were pre-treated with MEK inhibitors UO126 or PD98059 at increasing concentrations before being treated with HCVne particles for 30 min. G HepG2 cells treated with soluble bacterial core protein added at increasing concentrations for 30 min. H HepG2 cells were incubated at either 4°C or 37°C followed by 30 min of HCVne particles or 10 min of EGF addition. I,J NH4Cl (5 mM, 15 min) or nocodazole (4 μM, 2 h) treated cells with HCVne for 30 min. All lysates were analyzed by immunoblotting as described in A
To investigate if the observed ERK1/2 signaling occurred during HCVne binding or post-binding processing, endocytosis was inhibited by incubation at 4°C. Binding and inhibition of HCVne particles entry at 4°C has already been demonstrated [14]. As shown in Fig. 5H, no ERK1/2 phosphorylation was observed for HCVne or for the control EGF at 4°C, indicating that the activation was endocytosis-dependent. Next, the previously demonstrated ability of NH4Cl to increase the endosomal pH therefore blocking particles in the endosomes was used. Treatment of HepG2 cells with this chemical agent decreased ERK1/2 phosphorylation produced by HCVne particles (Fig. 5I), granting endosomes an important role in the pathway activation. Additional proof was also provided by the use of nocodazole. It is well known that cytoskeleton stability and signaling are correlated. Nocodazole treatment produces not only a peripheral localization of endosomes but also a sustained ERK1/2 activation [51]. This was also observed in Fig. 5J, indicating the possibility of a link between ERK1/2 signaling and endosomes. Taken together, these results suggested that HCVne particles were capable of activating the MEK1/2–ERK1/2 pathway in a dose-dependent way, and that the particulate form and not the protein itself produced this phenomenon.
Host cell proteins influence uptake and trafficking of HCVne particles
Signal transduction and endocytosis are inseparably linked cell functions. We tested the effects of broad-spectrum phospho-inhibitors in HCVne particles uptake or cellular trafficking by measuring their colocalization with the EEA1 endosomal marker. Cells were pre-treated with the drugs for an adequate time at 37°C, and remained present during incubation time.
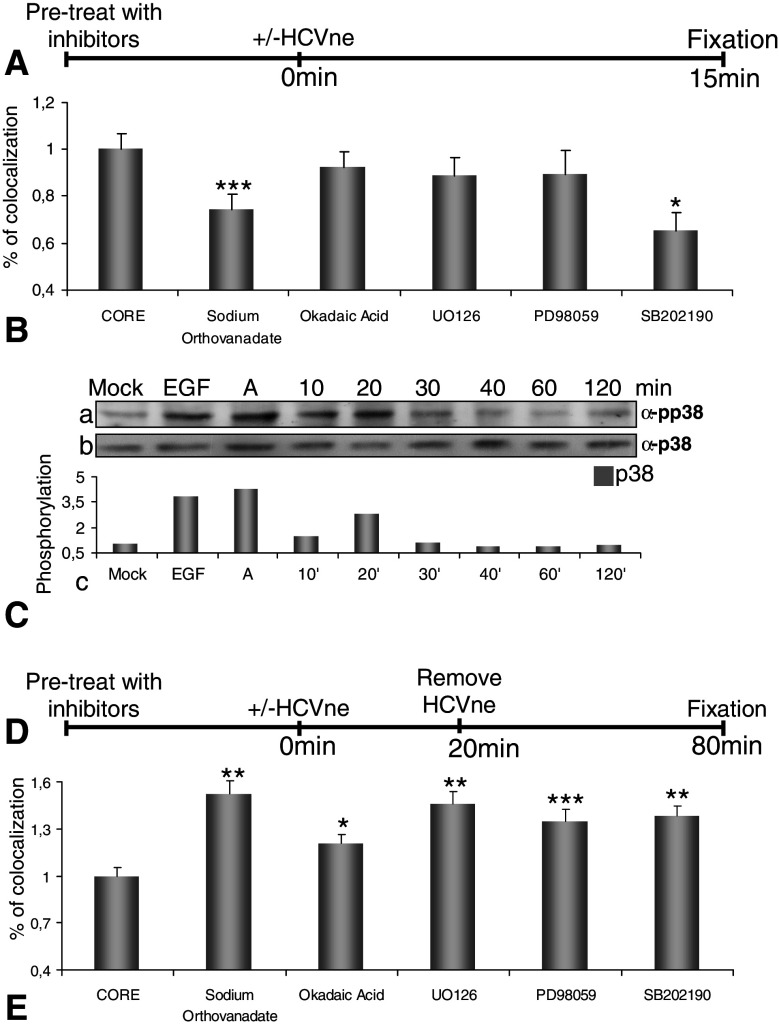
We first tested the influence of these inhibitors in the post-binding entry, by testing the EEA1-HCVne colocalization 15 min post-incubation at 37°C (Fig. 6A). Sodium orthovanadate (5 mM), an inhibitor of tyrosine/alkaline phosphatases and of a number of ATPases, and SB202190 (20 μM), a selective p38-MAPK inhibitor, significantly reduced the transport of HCVne particles to early endosomes by 30 and 35%, respectively (Fig. 6B). Such an effect was not observed in the case of okadaic acid (0.5 μM), a general inhibitor of serine/threonine phosphatases or UO126 (1 μM) and PD98059 (5 μM), well-known MEK inhibitors. To confirm these results, the involvement of MAPK-p38 in the entry process of HCVne particles was tested further with specific phospho-antibodies, where a twofold activation was observed 20 min after the addition of HCVne particles (Fig. 6C).
Fig. 6.
Implication of host cell proteins in HCVne trafficking. A,D Schematic representation of the experiment. HepG2 cells were pre-treated with sodium orthovanadate (5 mM, 30 min), okadaic acid (0.5 μM, 30 min), UO126 (1 μM, 1.5 h), PD98059 (5 mM, 1.5 h), and SB202190 (20 μM, 1.5 h). Particles were added for either 15 min (B) or for 20 min, removed and incubated until 80 min (E). Cells were fixed, immunostained with anti-core (green) and anti-EEA1 (red) and observed with confocal microscopy. Then, 9–15 confocal sections from three to five different experiments were selected and quantified with Image-Pro Plus software. C HCVne particles were added in HepG2 cells for indicated times. EGF (50 nM) and anisomycin labeled (A, 10 μM) were used as positive controls. Lysates were analysed by western blotting with anti-phospho-p38 (a) or anti-p38 (b) antibodies. Densitometric analysis is expressed in arbitrary units (c). *P < 0.05, **P < 0.01, ***P < 0.001
Next, we tested whether cellular phospho-proteins were involved in trafficking of HCVne particles from early to late endosomes. Therefore, following pre-treatment of cells, particles were added for 20 min, washed off, and incubated further for a total of 80 min (Fig. 6D). Little EEA1-particles colocalization was measured in the control (no inhibitors added) however, a significant colocalization was observed in the presence of sodium orthovanadate, okadaic acid, UO126, PD98059 and SB202190 (Fig. 6E). These results suggested that tyrosine phospho-proteins and p38 protein, but not ERK1/2, seem to be important for the transport of particles into early endosomes. In contrast, for the processing of the particles from early to late endosomes and lysosomes, ERK1/2 and p38 phosphorylation, as well as serine/threonine and tyrosine phosphatases, were necessary. It is important to comment that the unexpected high colocalization observed with sodium orthovanadate and SB202190 inhibitors was probably due to a possible previous (Fig. 6B) obstruction of HCVne particles in early endosomes.
HCVne particles confer induction of immediate early gene (IEG) transcription via ERK1/2 nuclear translocation
Upon activation, ERK1/2 proteins can either phosphorylate other proteins in the cytoplasm or translocate to the nucleus and activate different genes [52]. To investigate which possibility is plausible, confocal sections of 100 cells were observed for ERK1/2 localization. As shown in Fig. 7A, B, cells starved or treated with the GFP-expressing and heat-denatured control fractions exhibited almost completely cytoplasmic localisation of ERK1/2. In contrast, there was a 46 and 50% nuclear translocation of ERK1/2 in the EGF or HCVne particle-treated cells, respectively.
Fig. 7.
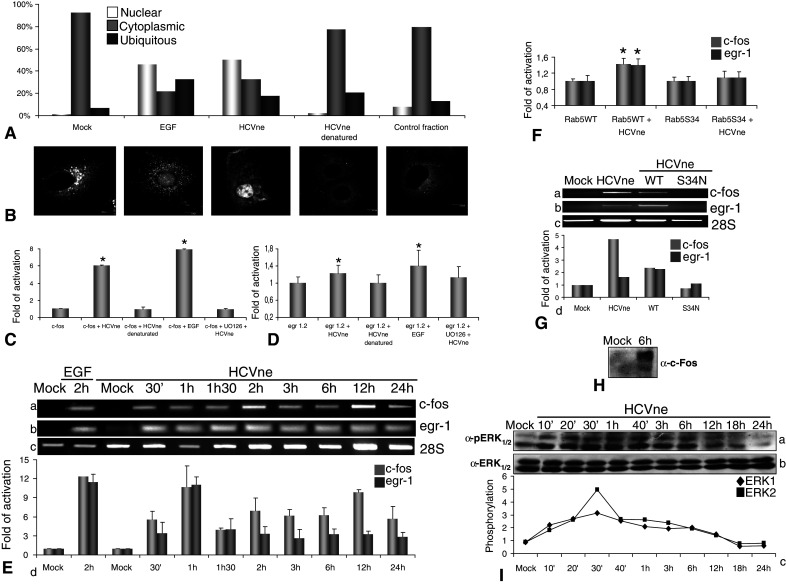
Endocytosis-dependent ERK1/2 nuclear translocation, sustained activation and IEG upregulation. A Untreated starved Huh7 cells or treated with EGF (50nM, 10 min), HCVne particles (30 min), heat denaturated fraction (1 h at 100°C, 30 min), and a fraction of equivalent sucrose density from Sf9 cell lysates infected with a control (GFP producing) baculovirus (30 min) were fixed and immunostained with anti-ERK1/2. Then, 100 cells were counted for nuclear and cytoplasmic ERK localisation. B Representative confocal sections are presented. C–F HepG2 cells were transfected with pFos WT-GL3 (c-fos), Egr1.2-luc (egr-1), and/or GFP-Rab5WT, GFP-Rab5S34N plasmids for 24 h, then serum starved for 8 h, and treated or not with HCVne particles or with controls (same fraction heat denatured) for 18 h. Relative light units were measured and values were normalized to the total protein amount. E,G Total mRNAs from HepG2 cells transfected or not with GFP-Rab5WT, GFPRab5S34N were isolated at indicated time points and cells incubated with HCVne. RT–PCR was performed with specific primers for c-fos (a), egr-1 (b), 28S (c). Densitometric results were normalized against 28S are in arbitrary units (c) (representative experiment of triplicates). H Nuclear extract of HepG2 cells treated for 6 h with HCVne particles and immunostained with c-fos antibody. I Incubation of cells with HCVne particles at various times, immunostained with anti-phospho ERK1/2 (a), ERK1/2 (b), and densitometric analysis of phospho-ERK1 and ERK2 after normalisation against total ERKs is also presented in (c). *P < 0.05
The induction of IEG represents the first major transcriptional response following exposure to extracellular stimuli [53]. Specifically, the MEK1/2–ERK1/2 signaling pathway plays a crucial role in IEG induction by directly activating IEG promoter-bound transcription factors [54, 55]. Thus, we sought to investigate whether the HCVne particles could affect the transcription of two important IEG genes, c-fos and egr-1, through ERK1/2 activation. For this purpose, hepatic cells were transfected with luciferase reporter plasmids containing c-fos or egr-1 promoter sequences. An important upregulation of luciferase activity was observed with c-fos plasmid following incubation of the transfected cells with HCVne particles (Fig. 7C) compared to the heat-denatured ones. The response was utterly abolished by the use of MEK inhibitor UO126, therefore showing that it was ERK1/2-mediated. A significant upregulation, although lower, was also observed in the case of egr-1-luc plasmid as compared to the heat-denatured control (Fig. 7D). In contrast, the use of UO126 incompletely abolished the HCVne particles conferred induction, thereby indicating that there are additional factors regulating egr-1 promoter activity [56]. Furthermore, endogenous mRNA levels of these genes were tested and normalized against 28S (Fig. 7E). Upregulation of both genes was observed by 30 min post-incubation with HCVne particles and was evident throughout the 24 h time-course. Moreover, an important activation was observed in late time-points. As before, the levels of activation of c-fos were higher than egr-1, probably due to the different promoter’s elements. In addition, when HepG2 cells were transfected with GFP-Rab5S34N plasmid, an important downregulation was observed compared to the GFP-Rab5WT transfected cells (Fig. 7F), a phenomenon which was confirmed by endogenous mRNA levels (Fig. 7G), indicating that c-fos and egr-1 activation is related to endocytosis.
Magnitude and duration of ERK1/2 phosphorylation is important for IEG promoter activity as well as the stability of the produced proteins [52, 55]. Because we observed high mRNA levels at late hours, especially for c-fos, ERK1/2 activation was studied. After a peak at 30 min, a stable signal was observed for 12 h (Fig. 7I). To further support this observation, stability of c-Fos protein was investigated. It has been established that c-fos is transcriptionally induced upon stimulation and degraded within 45 min unless phosphorylated by ERK1/2 [57]. High steady-state levels of c-Fos protein in HepG2 nuclear extracts, 6 h post-incubation with HCVne particles, indicated high protein stability, thus implying sustained ERK1/2 activation (Fig. 7H).
Taken together, these results suggest that HCVne endocytosis induced ERK1/2 phosphorylation followed by its translocation to the nucleus and subsequent transcriptional activation of the c-fos and egr-1 genes. Their expression exhibited the same classic ‘immediate early genes’ pattern and was entirely (c-fos) or partially (egr-1) dependent by the ERK1/2 activation and duration.
Discussion
The presence of non-enveloped nucleocapsids for the hepatitis C virus has been previously described [2]; however, their role in HCV infection remains unclear. Clathrin-mediated endocytosis is a commonly exploited entry pathway for members of the Flaviviridae family such as west-nile virus [58], dengue virus [59], bovine viral diarrhea virus [60], and HCV enveloped particles [20, 21, 23]. However, non-enveloped viruses including polyomavirus JC [61], human rhinovirus serotype 2 [24, 62], and HBV core particles [63], also use this pathway. Data presented here suggested that HCVne particles penetrated into hepatic cells via pH-dependent clathrin-mediated endocytosis during which different MAPK pathways were activated. A variety of experimental approaches was utilized to study the detailed entry pathway taken by HCVne particles.
The entry process started with the attachment of HCVne particles at the cell surface which was followed by a clathrin-mediated internalization, and localization of particles to early endosomes. Transferrin, a well-known ligand of clarthin-mediated endocytosis, partially colocalized with HCVne particles strongly implying that this pathway was used for HCVne entry. This result was supported by the observation that, when using sucrose to inhibit clathrin-mediated endocytosis, colocalization of HCVne with early endosomes was decreased. Furthermore, okadaic acid, a general inhibitor of serine/threonine phosphatases that has been reported to increase caveolar internalization [64], did not have a significant effect in the entry process, making caveolin-dependent endocytosis a less plausible entry pathway. Internalization occured relatively fast, with the majority of entering viral particles internalized in early endosomes between 9 and 15 min which correlated with the average time of endosomal dengue virus, foot-and-mouth disease virus, and semliki forest virus particles’ localization [31, 60, 65].
Endocytosis via clathrin is related to low endosomal pH; therefore, we have tested HCVne particles entry in the presence of ammonium chloride, chloroquine, and bafilomycin A1, agents that block endosomal acidification. Particles were obstructed in early endosomes even at late time points, indicating that the acidic milieu of this compartment is important in their trafficking. These results were in agreement with the known impact of the intra-endosomal neutralization which has been shown to block the transport from early to late endosomes [66]. Hepatitis B virus capsid-like core particles follow a similar pathway [63]. On the other hand, non-enveloped viruses use pH to provoke either detachment from a receptor or to undergo conformational changes [16, 24, 67]. Thus, the role of cathepsins B and L was investigated. Results suggested for a putative effect of these acid-dependent proteases in the HCVne particle stability/disassembly. This finding is also supported by the notion that chloroquine could inhibit cathepsins B and L reversibly [68]. It is also important to note that these proteases are abundant in the liver [69], and that other hepatitis viruses as well as non-enveloped viruses require their endocytic presence [46, 63, 67]. Further clarification is needed to determine the exact role of these proteases in HCVne traffic as previous data concerning HCV enveloped virions dependency for cathepsins B and L showed no such requirement for entry and infectivity [22].
Early endosomes are characterized by well-defined and peculiar lipid and protein composition, which typically include the small GTPase Rab5 and EEA1. Rab5 contributes to the compartmental specificity, robustness, and dynamic properties of the early endosome [39, 70] and is shown to be involved in the transport of HCVne particles from the cellular surface to early endosomes, as different mutants provoke changes in the entry rate. This is also established for HBV core particles and adenovirus [63, 71]. It is interesting to note that Rab5-GTP activity is implicated in HCV genome replication [72].
SB202190, a specific p38 pathway inhibitor caused a significant reduction of the HCVne–early endosome colocalization. Activation of p38 increases endocytic rates allowing more efficient internalization of cell surface components [73]. We also observed a very early activation of p38 after cell incubation with HCVne particles; this event may be important for endocytosis and should be further investigated. Tyrosine phospho-proteins are also involved in HCVne transport to early endosomes as is the case for SV40 virus [64]. A large number of receptors are related to tyrosine kinases (RTK) [74]; therefore, it would be tempting to speculate a possible involvement of receptor-mediated endocytosis in the entry of HCVne particles.
After entering early endosomes, HCVne particles moved along from endosomal to lysosomal compartments, with the assistance of the microtubules network. Presented data showed a colocalization of HCVne with late endosomes at 1 h post-incubation reaching maximum colocalization with lysosomes at 4 h. This phenomenon was further investigated with the use of nocodazole, which suppresses the association and dissociation rates of tubulin, thus stabilizing the microtubules dynamics [75]. Our results clearly showed that colocalization with lysosomes, diminished impressively in a dose-dependent manner, and HCVne particles where blocked in early endosomes. Various phosphorylated proteins were showed to be essential during early endosome–lysosome transport, such as serine/threonine and tyrosine phosphoproteins, as well as phospho-ERK1/2 and possibly phospho-p38.
Previous data [13, 14] concerning specific ERF translocation from the nucleus to the cytoplasm after HCVne particles uptake were further investigated and endocytosis-dependent ERK1/2 pathway activation was demonstrated. Maximum activation was observed 30 min after internalization while no effect was observed when using different controls or soluble core protein indicating that the particulate form induces pathway activation.
The endosomal system serves as an intracellular site for the initiation and regulation of signal transduction. The endosome population acts as a platform for the assembly of different signaling effectors [28, 49, 76, 77]. One of them, Rab5, has been involved in ERK1/2 signal transduction [78, 79]. When HCVne particles were blocked into early endosomes, after NH4Cl treatment, ERK1/2 activation decreased. An analogous effect of further upregulation of the sustained ERK activation produced by nocodazole treatment was observed due to HCVne obstruction in endosomes. In addition, the use of MEK inhibitors UO126 and PD98059 blocked HCVne particles specific transport from early to late endosomes, but not from the surface to the early endosomes, thereby associating ERK1/2 and early endosomes. These results, together with the fact that no ERK1/2 activation was observed when endocytosis was blocked at 4°C, put forward the possibility of an early endosome involvement in signaling. However, ERK1/2 activation can also occur from a surface receptor or late endosomes [76, 80]. Further investigation with specific mutants are in progress.
The importance of the ERK1/2 pathway in the viral life cycle is well established since it has been reported to influence viral protein expression and replication, as well as host cell modifications [81–83]. Endocytosis has been proposed to produce a sustained ERK1/2 activation and nuclear accumulation [84]. Both phenomena were observed during HCVne endocytosis. Previous studies reported a sustained ERK1/2 activation in EGF-stimulated core-expressing cells that, together with higher basal ERK1/2 levels, have been suggested to correlate with hepatocellular carcinoma development and progression [85]. Furthermore, activated MEK1/2 and ERK1/2 are frequently observed in different type of tumors [86, 87].
ERK1/2 activation and nuclear localization leads to modulation of IEGs like c-fos and egr-1 [55, 57]. c-fos is a member of the fos family which dimerizes with members of the Jun family to create AP-1 transcription factors that bind to AP-1 binding sites of gene promoters and initiate gene transcription [88]. The c-Fos protein is considered as a ‘sensor’ of the duration of ERK1/2 activity. Sustained activation allows efficient phosphorylation of the C-terminus of newly synthesized c-Fos, stabilizing it for several hours, thus dictating the number and type of genes whose transcriptional regulation is AP-1-mediated [55]. Our experimental data showed that ERK1/2 sustained activation after HCVne endocytosis regulated promoter, mRNA, and protein levels of c-fos. This is an interesting observation as c-fos has been found to be overexpressed with high frequency in aggressive and invasive hepatocarcinomas [89]. In addition, a similar phenomenon was previously observed with latent membrane protein 1 (LMP-1) of Epstein-Barr virus, and has been suggested to contribute in viral tumorigenicity [90].
Another IEG, egr-1, was found to be upregulated during HCVne endocytosis in a partly ERK1/2-dependent manner. Even though to a lower extent, a sustained transcriptional activation was observed. Egr-1 upregulation has been described in murine hepatitis virus entry and infection, and correlates with the establishment of viral persistence [91]. It has also been involved in human polyoma JC, rabies, and borna disease viruses life cycles [92, 93]. Additionally, Lee et al. [94] observed that HCV core protein stimulated egr-1 phosphorylation, which indirectly increased insulin-like growth factor II (IGF-II) gene transcription [95]. IGF-II has been implicated in tumor progression of several tumor cell types including prostate, hepatoma, pancreatoma, and breast cancer [96]. It is noteworthy that, in prostate cancer, significantly increased egr-1 levels were recorded in tumors with aggressive morphology [97], making egr-1 an important target protein for antiviral therapy.
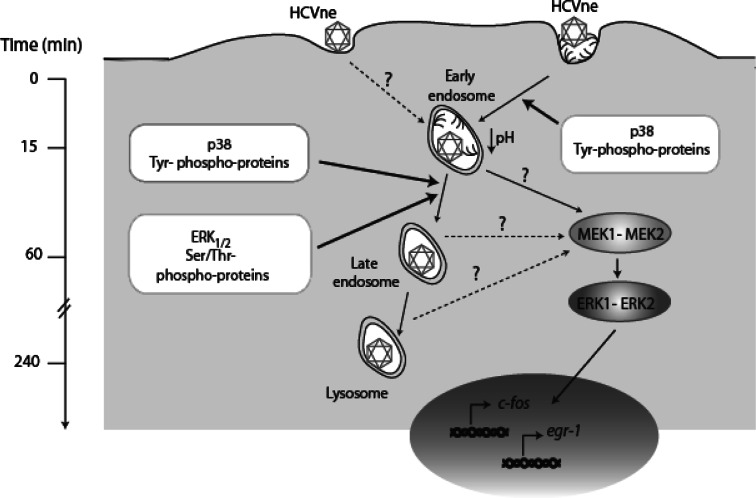
In conclusion, HCV nucleocapsids can be found in significant quantities in serum. HCV propagation, although progressed, is restricted to certain genotypes, and additionaly HCV production of different forms of viral particles in cell culture is still largely limited, begging the question of whether naturally occurring HCVne particles can indeed be infectious. Another interesting question raised by this study was whether HCVne particles, possessing a highly conserved YXXL and distal di-leucine motifs that represent primary endocytosis signals [98], had the potential to be endocytosed and to activate the ERK1/2 pathway as proposed in Fig. 8. Similar endocytic processing was reported for recombinant HBV capsid-like particles [63].
Fig. 8.
Hypothetical model for HCVne particle entry HCVne particles enter the cells most likely by the clathrin-dependent pathway, even though an alternative entry pathway is not excluded, and reach early endosomes. p38 as well as tyrosine phosphatases are involved in this process. At 1 h of incubation, particles are progressing to late endosomes in a ERK1/2 and serine/threonine phosphatases-dependent manner. Finally, particles reach the lysosomes (4 h). During this progression, the MEK1/2–ERK1/2 pathway is activated in a sustainable manner. When phosphorylated, ERKs proteins translocate to the nucleus and activate immediate early genes c-fos and egr-1
Cellular environment modifications produced by signaling events similar to those exerted by the endocytotic properties of HCVne particles could potentially be of major importance for HCV life cycle and severity of the disease during natural HCV infection. Furthermore, virus-like particles (VLPs) are attractive as a recombinant protein vaccine because they have proved to be efficient in generating powerful immune responses [99]. Recent data [100] offered support, for HCV core protein, towards this possibility. Thus, particle uptake and processing during HCV natural infection can possibly be involved in the intricate processes of immune activation and immune evasion by the virus. Data presented in this study can provide evidence for additional important features of particulate HCV core protein, raising new questions concerning its role as regulator of cellular functions leading to HCV disease progression.
Electronic supplementary material
Below is the link to the electronic supplementary material.
HCVne particles in HepG2 cells follow an indentical progression from early to late endosomes and lysosomes. Cells were incubated with HCVne particles for 15 minutes or 4 hours and immunostained with anti-core (green) and anti-EEA1/anti-Lamp2 (red). Cells were also transfected with mRFP-Rab7 (red). 24 hours post-transfection HCVne particles were added for 1 hour, and cells were fixed and immunostained for anti-core (green). Colocalization is observed in yellow. Bars:8μm (MPG 3701 kb)
HCVne particles in endosomes use actin filaments and microtubules for their traffic. A) HCVne particles were added for 15 minutes in Huh7 cells which were fixed and immunolabeled with anti-core (green) / anti-EEA1 (blue) antibodies and counterstained with Alexa 546-phalloidin. B) Huh7 cells where transfected with mRFP-Rab5. 24 hours post-transfection, cells were incubated with HCVne for 15 minutes, immunostained with anti-core (green) and anti-α tubulin (blue). Parts of cells are presented and a triple colocalization (white spot) is observed (MPG 908 kb)
HCVne particles colocalize with early endosomes. Dual-color live fluorescence microscopy experiment recorded in Huh7 cells transfected with mRFP-Rab5 (red) in the presence of green fluorescent GFP-HCVne particles described in (14). One picture every 10 second was recorded with a 100x objective at 37oC for 20 minutes after recombinant HCVne binding (TIFF 459 kb)
HCVne particles colocalize with late endosomes. Live Huh7 cells transfected with mRFP-Rab7 (red) and challenged with green fluorescent GFP-HCVne particles (14). Video was recorded 30 minutes after particles were added with 60x objective at 37oC for 30 minutes (one picture every 30 seconds). Time elapsed from the beginning of recording is noted in each frame of figure 2C (TIFF 1582 kb)
Acknowledgments
We thank P. Foka for useful discussions and Dr. D. Blaas for critical reading. We also thank our colleague Dr. K. Lazaridis (Department of Biochemistry, Hellenic Pasteur Institute) for assisting in statistical analysis. This work was supported by PENED 03EΔ297, co-financed by E.U.-European Social Fund (75%) and the Greek Ministry of Development-GSRT (25%).
References
- 1.But DY, Lai CL, Yuen MF. Natural history of hepatitis-related hepatocellular carcinoma. World J Gastroenterol. 2008;14:1652–1656. doi: 10.3748/wjg.14.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andre P, Perlemuter G, Budkowska A, Brechot C, Lotteau V. Hepatitis C virus particles and lipoprotein metabolism. Semin Liver Dis. 2005;25:93–104. doi: 10.1055/s-2005-864785. [DOI] [PubMed] [Google Scholar]
- 3.Aoyagi K, Ohue C, Iida K, Kimura T, Tanaka E, Kiyosawa K, Yagi S. Development of a simple and highly sensitive enzyme immunoassay for hepatitis C virus core antigen. J Clin Microbiol. 1999;37:1802–1808. doi: 10.1128/jcm.37.6.1802-1808.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maillard P, Krawczynski K, Nitkiewicz J, Bronnert C, Sidorkiewicz M, Gounon P, Dubuisson J, Faure G, Crainic R, Budkowska A. Nonenveloped nucleocapsids of hepatitis C virus in the serum of infected patients. J Virol. 2001;75:8240–8250. doi: 10.1128/JVI.75.17.8240-8250.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouvier-Alias M, Patel K, Dahari H, Beaucourt S, Larderie P, Blatt L, Hezode C, Picchio G, Dhumeaux D, Neumann AU, McHutchison JG, Pawlotsky JM. Clinical utility of total HCV core antigen quantification: a new indirect marker of HCV replication. Hepatology. 2002;36:211–218. doi: 10.1053/jhep.2002.34130. [DOI] [PubMed] [Google Scholar]
- 6.Sansonno D, Tucci F, Ghebrehiwet B, Lauletta G, Peerschke EIB, Condetuca V, Russi S, Gatti P, Sansonno L, Dammacco F. Role of the receptor for the globular domain of C1q protein in the pathogenesis of Hepatitis C virus-related cryoglobulin vascular damage1. J Immunol. 2009;183:6013–6020. doi: 10.4049/jimmunol.0902038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noppornpanth S, Smits SL, Lien TX, Poovorawan Y, Osterhaus AD, Haagmans BL. Characterization of hepatitis C virus deletion mutants circulating in chronically infected patients. J Virol. 2007;81:12496–12503. doi: 10.1128/JVI.01059-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugiyama K, Suzuki K, Nakazawa T, Funami K, Hishiki T, Ogawa K, Saito S, Shimotohno KW, Suzuki T, Shimizu Y, Tobita R, Hijikata M, Takaku H, Shimotohno K. Genetic analysis of hepatitis C virus with defective genome and its infectivity in vitro. J Virol. 2009;83:6922–6928. doi: 10.1128/JVI.02674-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwai A, Marusawa H, Takada Y, Egawa H, Ikeda K, Nabeshima M, Uemoto S, Chiba T. Identification of novel defective HCV clones in liver transplant recipients with recurrent HCV infection. J Viral Hepat. 2006;13:523–531. doi: 10.1111/j.1365-2893.2006.00760.x. [DOI] [PubMed] [Google Scholar]
- 10.Lechmann M, Murata K, Satoi J, Vergalla J, Baumert TF, Liang TJ. Hepatitis C virus-like particles induce virus-specific humoral and cellular immune responses in mice. Hepatology. 2001;34:417–423. doi: 10.1053/jhep.2001.26523. [DOI] [PubMed] [Google Scholar]
- 11.Acosta-Rivero N, Rodriguez A, Musacchio A, Falcon V, Suarez VM, Martinez G, Guerra I, Paz-Lago D, Morera Y, de la Rosa MC, Morales-Grillo J, Duenas-Carrera S. In vitro assembly into virus-like particles is an intrinsic quality of Pichia pastoris derived HCV core protein. Biochem Biophys Res Commun. 2004;325:68–74. doi: 10.1016/j.bbrc.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Baumert TF, Vergalla J, Satoi J, Thomson M, Lechmann M, Herion D, Greenberg HB, Ito S, Liang TJ. Hepatitis C virus-like particles synthesized in insect cells as a potential vaccine candidate. Gastroenterology. 1999;117:1397–1407. doi: 10.1016/S0016-5085(99)70290-8. [DOI] [PubMed] [Google Scholar]
- 13.Tsitoura P, Georgopoulou U, Petres S, Varaklioti A, Karafoulidou A, Vagena D, Politis C, Mavromara P. Evidence for cellular uptake of recombinant hepatitis C virus non-enveloped capsid-like particles. FEBS Lett. 2007;581:4049–4057. doi: 10.1016/j.febslet.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 14.Katsarou K, Serti E, Tsitoura P, Lavdas AA, Varaklioti A, Pickl-Herk AM, Blaas D, Oz-Arslan D, Zhu R, Hinterdorfer P, Mavromara P, Georgopoulou U. Green fluorescent protein—tagged HCV non-enveloped capsid like particles: development of a new tool for tracking HCV core uptake. Biochimie. 2009;91:903–915. doi: 10.1016/j.biochi.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Pelkmans L, Helenius A. Insider information: what viruses tell us about endocytosis. Curr Opin Cell Biol. 2003;15:414–422. doi: 10.1016/S0955-0674(03)00081-4. [DOI] [PubMed] [Google Scholar]
- 16.Tsai B. Penetration of nonenveloped viruses into the cytoplasm. Annu Rev Cell Dev Biol. 2007;23:23–43. doi: 10.1146/annurev.cellbio.23.090506.123454. [DOI] [PubMed] [Google Scholar]
- 17.Smith AE, Helenius A. How viruses enter animal cells. Science. 2004;304:237–242. doi: 10.1126/science.1094823. [DOI] [PubMed] [Google Scholar]
- 18.von Hahn T, Rice CM. Hepatitis C virus entry. J Biol Chem. 2008;283:3689–3693. doi: 10.1074/jbc.R700024200. [DOI] [PubMed] [Google Scholar]
- 19.Burlone ME, Budkowska A. Hepatitis C virus cell entry: role of lipoproteins and cellular receptors. J Gen Virol. 2009;90:1055–1070. doi: 10.1099/vir.0.008300-0. [DOI] [PubMed] [Google Scholar]
- 20.Codran A, Royer C, Jaeck D, Bastien-Valle M, Baumert TF, Kieny MP, Pereira CA, Martin JP. Entry of hepatitis C virus pseudotypes into primary human hepatocytes by clathrin-dependent endocytosis. J Gen Virol. 2006;87:2583–2593. doi: 10.1099/vir.0.81710-0. [DOI] [PubMed] [Google Scholar]
- 21.Blanchard E, Belouzard S, Goueslain L, Wakita T, Dubuisson J, Wychowski C, Rouille Y. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J Virol. 2006;80:6964–6972. doi: 10.1128/JVI.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tscherne DM, Jones CT, Evans MJ, Lindenbach BD, McKeating JA, Rice CM. Time- and temperature-dependent activation of hepatitis C virus for low-pH-triggered entry. J Virol. 2006;80:1734–1741. doi: 10.1128/JVI.80.4.1734-1741.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meertens L, Bertaux C, Dragic T. Hepatitis C virus entry requires a critical postinternalization step and delivery to early endosomes via clathrin-coated vesicles. J Virol. 2006;80:11571–11578. doi: 10.1128/JVI.01717-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayer N, Schober D, Huttinger M, Blaas D, Fuchs R. Inhibition of clathrin-dependent endocytosis has multiple effects on human rhinovirus serotype 2 cell entry. J Biol Chem. 2001;276:3952–3962. doi: 10.1074/jbc.M004722200. [DOI] [PubMed] [Google Scholar]
- 25.Chung SK, Kim JY, Kim IB, Park SI, Paek KH, Nam JH. Internalization and trafficking mechanisms of coxsackievirus B3 in HeLa cells. Virology. 2005;333:31–40. doi: 10.1016/j.virol.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Wang K, Huang S, Kapoor-Munshi A, Nemerow G. Adenovirus internalization and infection require dynamin. J Virol. 1998;72:3455–3458. doi: 10.1128/jvi.72.4.3455-3458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parker JS, Parrish CR. Cellular uptake and infection by canine parvovirus involves rapid dynamin-regulated clathrin-mediated endocytosis, followed by slower intracellular trafficking. J Virol. 2000;74:1919–1930. doi: 10.1128/JVI.74.4.1919-1930.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miaczynska M, Pelkmans L, Zerial M. Not just a sink: endosomes in control of signal transduction. Curr Opin Cell Biol. 2004;16:400–406. doi: 10.1016/j.ceb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 29.McPherson PS, Kay BK, Hussain NK. Signaling on the endocytic pathway. Traffic. 2001;2:375–384. doi: 10.1034/j.1600-0854.2001.002006375.x. [DOI] [PubMed] [Google Scholar]
- 30.Greber UF. Signalling in viral entry. Cell Mol Life Sci. 2002;59:608–626. doi: 10.1007/s00018-002-8453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vonderheit A, Helenius A. Rab7 associates with early endosomes to mediate sorting and transport of Semliki forest virus to late endosomes. PLoS Biol. 2005;3:e233. doi: 10.1371/journal.pbio.0030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cen B, Selvaraj A, Burgess RC, Hitzler JK, Ma Z, Morris SW, Prywes R. Megakaryoblastic leukemia 1, a potent transcriptional coactivator for serum response factor (SRF), is required for serum induction of SRF target genes. Mol Cell Biol. 2003;23:6597–6608. doi: 10.1128/MCB.23.18.6597-6608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattera R, Bonifacino JS. Ubiquitin binding and conjugation regulate the recruitment of Rabex-5 to early endosomes. EMBO J. 2008;27:2484–2494. doi: 10.1038/emboj.2008.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groot M, Boxer LM, Thiel G. Nerve growth factor- and epidermal growth factor-regulated gene transcription in PC12 pheochromocytoma and INS-1 insulinoma cells. Eur J Cell Biol. 2000;79:924–935. doi: 10.1078/0171-9335-00126. [DOI] [PubMed] [Google Scholar]
- 35.Kockar FT, Foka P, Hughes TR, Kousteni S, Ramji DP. Analysis of the Xenopus laevis CCAAT-enhancer binding protein alpha gene promoter demonstrates species-specific differences in the mechanisms for both auto-activation and regulation by Sp1. Nucleic Acids Res. 2001;29:362–372. doi: 10.1093/nar/29.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li YQ, Tao KS, Ren N, Wang YH. Effect of c-fos antisense probe on prostaglandin E2-induced upregulation of vascular endothelial growth factor mRNA in human liver cancer cells. World J Gastroenterol. 2005;11:4427–4430. doi: 10.3748/wjg.v11.i28.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SO, Kwon JI, Jeong YK, Kim GY, Kim ND, Choi YH. Induction of Egr-1 is associated with anti-metastatic and anti-invasive ability of beta-lapachone in human hepatocarcinoma cells. Biosci Biotechnol Biochem. 2007;71:2169–2176. doi: 10.1271/bbb.70103. [DOI] [PubMed] [Google Scholar]
- 38.Kong SE, Hall JC, McCauley RD. Estimation of gene expression within the intestinal mucosa using semiquantitative reverse transcriptase-polymerase chain reaction. Anal Biochem. 1999;271:111–114. doi: 10.1006/abio.1999.4123. [DOI] [PubMed] [Google Scholar]
- 39.Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durrbach A, Louvard D, Coudrier E. Actin filaments facilitate two steps of endocytosis. J Cell Sci. 1996;109:457–465. doi: 10.1242/jcs.109.2.457. [DOI] [PubMed] [Google Scholar]
- 41.Girao H, Geli MI, Idrissi FZ. Actin in the endocytic pathway: from yeast to mammals. FEBS Lett. 2008;582:2112–2119. doi: 10.1016/j.febslet.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Matteoni R, Kreis TE. Translocation and clustering of endosomes and lysosomes depends on microtubules. J Cell Biol. 1987;105:1253–1265. doi: 10.1083/jcb.105.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gruenberg J, van der Goot FG. Mechanisms of pathogen entry through the endosomal compartments. Nat Rev Mol Cell Biol. 2006;7:495–504. doi: 10.1038/nrm1959. [DOI] [PubMed] [Google Scholar]
- 44.Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci USA. 1978;75:3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perez L, Carrasco L. Involvement of the vacuolar H(+)-ATPase in animal virus entry. J Gen Virol. 1994;75:2595–2606. doi: 10.1099/0022-1317-75-10-2595. [DOI] [PubMed] [Google Scholar]
- 46.Qiu Z, Hingley ST, Simmons G, Yu C, Das Sarma J, Bates P, Weiss SR. Endosomal proteolysis by cathepsins is necessary for murine coronavirus mouse hepatitis virus type 2 spike-mediated entry. J Virol. 2006;80:5768–5776. doi: 10.1128/JVI.00442-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willingham MC, Hanover JA, Dickson RB, Pastan I. Morphologic characterization of the pathway of transferrin endocytosis and recycling in human KB cells. Proc Natl Acad Sci USA. 1984;81:175–179. doi: 10.1073/pnas.81.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heuser JE, Anderson RG. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol. 1989;108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teis D, Huber LA. The odd couple: signal transduction and endocytosis. Cell Mol Life Sci. 2003;60:2020–2033. doi: 10.1007/s00018-003-3010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.English JM, Cobb MH. Pharmacological inhibitors of MAPK pathways. Trends Pharmacol Sci. 2002;23:40–45. doi: 10.1016/S0165-6147(00)01865-4. [DOI] [PubMed] [Google Scholar]
- 51.Birukova AA, Birukov KG, Gorshkov B, Liu F, Garcia JG, Verin AD. MAP kinases in lung endothelial permeability induced by microtubule disassembly. Am J Physiol Lung Cell Mol Physiol. 2005;289:L75–L84. doi: 10.1152/ajplung.00447.2004. [DOI] [PubMed] [Google Scholar]
- 52.Ebisuya M, Kondoh K, Nishida E. The duration, magnitude and compartmentalization of ERK MAP kinase activity: mechanisms for providing signaling specificity. J Cell Sci. 2005;118:2997–3002. doi: 10.1242/jcs.02505. [DOI] [PubMed] [Google Scholar]
- 53.Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28:370–490. doi: 10.1016/S0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- 54.Turjanski AG, Vaque JP, Gutkind JS. MAP kinases and the control of nuclear events. Oncogene. 2007;26:3240–3253. doi: 10.1038/sj.onc.1210415. [DOI] [PubMed] [Google Scholar]
- 55.Murphy LO, MacKeigan JP, Blenis J. A network of immediate early gene products propagates subtle differences in mitogen-activated protein kinase signal amplitude and duration. Mol Cell Biol. 2004;24:144–153. doi: 10.1128/MCB.24.1.144-153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chalmers CJ, Balmanno K, Hadfield K, Ley R, Cook SJ. Thrombin inhibits Bim (Bcl-2-interacting mediator of cell death) expression and prevents serum-withdrawal-induced apoptosis via protease-activated receptor 1. Biochem J. 2003;375:99–109. doi: 10.1042/BJ20030346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murphy LO, Smith S, Chen RH, Fingar DC, Blenis J. Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol. 2002;4:556–564. doi: 10.1038/ncb822. [DOI] [PubMed] [Google Scholar]
- 58.Chu JJ, Ng ML. Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway. J Virol. 2004;78:10543–10555. doi: 10.1128/JVI.78.19.10543-10555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Schaar HM, Rust MJ, Chen C, van der Ende-Metselaar H, Wilschut J, Zhuang X, Smit JM. Dissecting the cell entry pathway of dengue virus by single-particle tracking in living cells. PLoS Pathog. 2008;4:e1000244. doi: 10.1371/journal.ppat.1000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lecot S, Belouzard S, Dubuisson J, Rouille Y. Bovine viral diarrhoea virus entry is dependent on clathrin-mediated endocytosis. J Virol. 2005;79:10826–10829. doi: 10.1128/JVI.79.16.10826-10829.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pho MT, Ashok A, Atwood WJ. JC virus enters human glial cells by clathrin-dependent receptor-mediated endocytosis. J Virol. 2000;74:2288–2292. doi: 10.1128/JVI.74.5.2288-2292.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Snyers L, Zwickl H, Blaas D. Human rhinovirus type 2 is internalized by clathrin-mediated endocytosis. J Virol. 2003;77:5360–5369. doi: 10.1128/JVI.77.9.5360-5369.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cooper A, Shaul Y. Clathrin-mediated endocytosis and lysosomal cleavage of hepatitis B virus capsid-like core particles. J Biol Chem. 2006;281:16563–16569. doi: 10.1074/jbc.M601418200. [DOI] [PubMed] [Google Scholar]
- 64.Pelkmans L, Puntener D, Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science. 2002;296:535–539. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- 65.O’Donnell V, LaRocco M, Duque H, Baxt B. Analysis of foot-and-mouth disease virus internalization events in cultured cells. J Virol. 2005;79:8506–8518. doi: 10.1128/JVI.79.13.8506-8518.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bayer N, Schober D, Prchla E, Murphy RF, Blaas D, Fuchs R. Effect of bafilomycin A1 and nocodazole on endocytic transport in hela cells: implications for viral uncoating and infection. J Virol. 1998;72:9645–9655. doi: 10.1128/jvi.72.12.9645-9655.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chandran K, Nibert ML. Animal cell invasion by a large nonenveloped virus: reovirus delivers the goods. Trends Microbiol. 2003;11:374–382. doi: 10.1016/S0966-842X(03)00178-1. [DOI] [PubMed] [Google Scholar]
- 68.Otto HH, Schirmeister T. Cysteine proteases and their inhibitors. Chem Rev. 1997;97:133–172. doi: 10.1021/cr950025u. [DOI] [PubMed] [Google Scholar]
- 69.Xing R, Addington AK, Mason RW. Quantification of cathepsins B and L in cells. Biochem J. 1998;332:499–505. doi: 10.1042/bj3320499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwartz SL, Cao C, Pylypenko O, Rak A, Wandinger-Ness A. Rab GTPases at a glance. J Cell Sci. 2007;120:3905–3910. doi: 10.1242/jcs.015909. [DOI] [PubMed] [Google Scholar]
- 71.Rauma T, Tuukkanen J, Bergelson JM, Denning G, Hautala T. rab5 GTPase regulates adenovirus endocytosis. J Virol. 1999;73:9664–9668. doi: 10.1128/jvi.73.11.9664-9668.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stone M, Jia S, Heo WD, Meyer T, Konan KV. Participation of rab5, an early endosome protein, in hepatitis C virus RNA replication machinery. J Virol. 2007;81:4551–4563. doi: 10.1128/JVI.01366-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Felberbaum-Corti M, Cavalli V, Gruenberg J. Capture of the small GTPase Rab5 by GDI: regulation by p38 MAP kinase. Methods Enzymol. 2005;403:367–381. doi: 10.1016/S0076-6879(05)03032-6. [DOI] [PubMed] [Google Scholar]
- 74.Hubbard SR, Miller WT. Receptor tyrosine kinases: mechanisms of activation and signaling. Curr Opin Cell Biol. 2007;19:117–123. doi: 10.1016/j.ceb.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vasquez RJ, Howell B, Yvon AM, Wadsworth P, Cassimeris L. Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. Mol Biol Cell. 1997;8:973–985. doi: 10.1091/mbc.8.6.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sadowski L, Pilecka I, Miaczynska M. Signaling from endosomes: location makes a difference. Exp Cell Res. 2009;315:1601–1609. doi: 10.1016/j.yexcr.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 77.Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barbieri MA, Fernandez-Pol S, Hunker C, Horazdovsky BH, Stahl PD. Role of rab5 in EGF receptor-mediated signal transduction. Eur J Cell Biol. 2004;83:305–314. doi: 10.1078/0171-9335-00381. [DOI] [PubMed] [Google Scholar]
- 79.Hunker CM, Kruk I, Hall J, Giambini H, Veisaga ML, Barbieri MA. Role of Rab5 in insulin receptor-mediated endocytosis and signaling. Arch Biochem Biophys. 2006;449:130–142. doi: 10.1016/j.abb.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 80.Lu A, Tebar F, Alvarez-Moya B, Lopez-Alcala C, Calvo M, Enrich C, Agell N, Nakamura T, Matsuda M, Bachs O. A clathrin-dependent pathway leads to KRas signaling on late endosomes en route to lysosomes. J Cell Biol. 2009;184:863–879. doi: 10.1083/jcb.200807186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharma-Walia N, Krishnan HH, Naranatt PP, Zeng L, Smith MS, Chandran B. ERK1/2 and MEK1/2 induced by Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) early during infection of target cells are essential for expression of viral genes and for establishment of infection. J Virol. 2005;79:10308–10329. doi: 10.1128/JVI.79.16.10308-10329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Panteva M, Korkaya H, Jameel S. Hepatitis viruses and the MAPK pathway: is this a survival strategy? Virus Res. 2003;92:131–140. doi: 10.1016/S0168-1702(02)00356-8. [DOI] [PubMed] [Google Scholar]
- 83.Zheng Y, Li J, Johnson DL, Ou JH. Regulation of hepatitis B virus replication by the ras-mitogen-activated protein kinase signaling pathway. J Virol. 2003;77:7707–7712. doi: 10.1128/JVI.77.14.7707-7712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kermorgant S, Parker PJ. Receptor trafficking controls weak signal delivery: a strategy used by c-Met for STAT3 nuclear accumulation. J Cell Biol. 2008;182:855–863. doi: 10.1083/jcb.200806076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Giambartolomei S, Covone F, Levrero M, Balsano C. Sustained activation of the Raf/MEK/Erk pathway in response to EGF in stable cell lines expressing the Hepatitis C Virus (HCV) core protein. Oncogene. 2001;20:2606–2610. doi: 10.1038/sj.onc.1204372. [DOI] [PubMed] [Google Scholar]
- 86.Voisin L, Julien C, Duhamel S, Gopalbhai K, Claveau I, Saba-El-Leil MK, Rodrigue-Gervais IG, Gaboury L, Lamarre D, Basik M, Meloche S. Activation of MEK1 or MEK2 isoform is sufficient to fully transform intestinal epithelial cells and induce the formation of metastatic tumors. BMC Cancer. 2008;8:337. doi: 10.1186/1471-2407-8-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sebolt-Leopold JS. MEK inhibitors: a therapeutic approach to targeting the Ras-MAP kinase pathway in tumors. Curr Pharm Des. 2004;10:1907–1914. doi: 10.2174/1381612043384439. [DOI] [PubMed] [Google Scholar]
- 88.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 89.Feo F, Frau M, Tomasi ML, Brozzetti S, Pascale RM. Genetic and epigenetic control of molecular alterations in hepatocellular carcinoma. Exp Biol Med (Maywood) 2009;234:726–736. doi: 10.3181/0901-MR-40. [DOI] [PubMed] [Google Scholar]
- 90.Vaysberg M, Hatton O, Lambert SL, Snow AL, Wong B, Krams SM, Martinez OM. Tumor-derived variants of Epstein-Barr virus latent membrane protein 1 induce sustained Erk activation and c-Fos. J Biol Chem. 2008;283:36573–36585. doi: 10.1074/jbc.M802968200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cai Y, Liu Y, Zhang X. Induction of transcription factor Egr-1 gene expression in astrocytoma cells by Murine coronavirus infection. Virology. 2006;355:152–163. doi: 10.1016/j.virol.2006.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fu ZF, Weihe E, Zheng YM, Schafer MK, Sheng H, Corisdeo S, Rauscher FJ, Koprowski H, Dietzschold B. Differential effects of rabies and borna disease viruses on immediate-early- and late-response gene expression in brain tissues. J Virol. 1993;67:6674–6681. doi: 10.1128/jvi.67.11.6674-6681.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Romagnoli L, Sariyer IK, Tung J, Feliciano M, Sawaya BE, Del Valle L, Ferrante P, Khalili K, Safak M, White MK. Early growth response-1 protein is induced by JC virus infection and binds and regulates the JC virus promoter. Virology. 2008;375:331–341. doi: 10.1016/j.virol.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee S, Park U, Lee YI. Hepatitis C virus core protein transactivates insulin-like growth factor II gene transcription through acting concurrently on Egr1 and Sp1 sites. Virology. 2001;283:167–177. doi: 10.1006/viro.2001.0892. [DOI] [PubMed] [Google Scholar]
- 95.Eto K, Kaur V, Thomas MK. Regulation of insulin gene transcription by the immediate-early growth response gene Egr-1. Endocrinology. 2006;147:2923–2935. doi: 10.1210/en.2005-1336. [DOI] [PubMed] [Google Scholar]
- 96.Adamson ED, Mercola D. Egr1 transcription factor: multiple roles in prostate tumor cell growth and survival. Tumour Biol. 2002;23:93–102. doi: 10.1159/000059711. [DOI] [PubMed] [Google Scholar]
- 97.Eid MA, Kumar MV, Iczkowski KA, Bostwick DG, Tindall DJ. Expression of early growth response genes in human prostate cancer. Cancer Res. 1998;58:2461–2468. [PubMed] [Google Scholar]
- 98.Li Y, Marzolo MP, van Kerkhof P, Strous GJ, Bu G. The YXXL motif, but not the two NPXY motifs, serves as the dominant endocytosis signal for low density lipoprotein receptor-related protein. J Biol Chem. 2000;275:17187–17194. doi: 10.1074/jbc.M000490200. [DOI] [PubMed] [Google Scholar]
- 99.Ruedl C, Schwarz K, Jegerlehner A, Storni T, Manolova V, Bachmann MF. Virus-like particles as carriers for T-cell epitopes: limited inhibition of T-cell priming by carrier-specific antibodies. J Virol. 2005;79:717–724. doi: 10.1128/JVI.79.2.717-724.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Acosta-Rivero N, Poutou J, Alvarez-Lajonchere L, Guerra I, Aguilera Y, Musacchio A, Rodriguez A, Aguilar JC, Falcon V, Alvarez-Obregon JC, Soria Y, Torres D, Linares M, Perez A, Morales-Grillo J, Duenas-Carrera S. Recombinant in vitro assembled hepatitis C virus core particles induce strong specific immunity enhanced by formulation with an oil-based adjuvant. Biol Res. 2009;42:41–56. doi: 10.4067/S0716-97602009000100005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HCVne particles in HepG2 cells follow an indentical progression from early to late endosomes and lysosomes. Cells were incubated with HCVne particles for 15 minutes or 4 hours and immunostained with anti-core (green) and anti-EEA1/anti-Lamp2 (red). Cells were also transfected with mRFP-Rab7 (red). 24 hours post-transfection HCVne particles were added for 1 hour, and cells were fixed and immunostained for anti-core (green). Colocalization is observed in yellow. Bars:8μm (MPG 3701 kb)
HCVne particles in endosomes use actin filaments and microtubules for their traffic. A) HCVne particles were added for 15 minutes in Huh7 cells which were fixed and immunolabeled with anti-core (green) / anti-EEA1 (blue) antibodies and counterstained with Alexa 546-phalloidin. B) Huh7 cells where transfected with mRFP-Rab5. 24 hours post-transfection, cells were incubated with HCVne for 15 minutes, immunostained with anti-core (green) and anti-α tubulin (blue). Parts of cells are presented and a triple colocalization (white spot) is observed (MPG 908 kb)
HCVne particles colocalize with early endosomes. Dual-color live fluorescence microscopy experiment recorded in Huh7 cells transfected with mRFP-Rab5 (red) in the presence of green fluorescent GFP-HCVne particles described in (14). One picture every 10 second was recorded with a 100x objective at 37oC for 20 minutes after recombinant HCVne binding (TIFF 459 kb)
HCVne particles colocalize with late endosomes. Live Huh7 cells transfected with mRFP-Rab7 (red) and challenged with green fluorescent GFP-HCVne particles (14). Video was recorded 30 minutes after particles were added with 60x objective at 37oC for 30 minutes (one picture every 30 seconds). Time elapsed from the beginning of recording is noted in each frame of figure 2C (TIFF 1582 kb)