Abstract
Cytokines and growth factors play a crucial role in the maintenance of haematopoietic homeostasis. They transduce signals that regulate the competing commitments of haematopoietic stem cells, quiescence or proliferation, retention of stem cell pluripotency or differentiation, and survival or demise. When the balance between these commitments and the requirements of the organisms is disturbed, particularly when it favours survival and proliferation, cancer may result. Cell death provoked by loss of growth factor signalling is regulated by the Bcl-2 family of apoptosis regulators, and thus survival messages transduced by growth factors must regulate the activity of these proteins. Many aspects of direct interactions between cytokine signalling and regulation of apoptosis remain elusive. In this review, we explore the mechanisms by which cytokines, in particular Interleukin-3 and granulocyte–macrophage colony-stimulating factor, promote cell survival and suppress apoptosis as models of how cytokine signalling and apoptotic pathways intersect.
Keywords: Cytokines, Growth factors, Apoptosis, Haematopoiesis, Bcl-2 family, Tumorigenesis
Introduction
The average lifespan of a red blood cell in humans is 3–4 months, platelets live 3–5 days, and granulocytes 1 or 2 days, perhaps even less. Some lymphocytes may live many years, others only a few days. Yet, for the most part, in healthy individuals, the number of cells remains fairly constant. At times of stress, for example in response to infection or depletion of granulocytes and lymphocytes after cancer chemotherapy, vast numbers of new cells need to be generated. When infections are resolved or cells are adequately replaced, cells that are no longer required are removed and the rate of replacement scaled back. During these processes, haematopoietic stem cells (HSC) face decisions about self-renewal, to proliferate and to differentiate into various types of mature, functional blood cells, such as granulocytes, erythrocytes, megakaryocytes, monocytes and lymphocytes. Haematopoietic growth factors are a critical part of the mechanisms that maintain the HSC population and that regulate this homeostasis. For example, erythropoietin is required for normal development of red blood cell progenitors and increased levels are observed in response to anaemia (or in professional cyclists) to raise red cell numbers [1–3]. In contrast, studies in which the gene for granulocyte–macrophage colony-stimulating factor (GM-CSF) has been deleted suggest that this cytokine has a minor or redundant role in haematopoiesis under normal conditions [4, 5,] but does promote survival and self-renewal of granulocyte progenitors, enhances survival of mature neutrophils and induces proliferation and increases numbers of neutrophils, eosinophils and monocytes in response to stress [6–8].
Programmed cell death or apoptosis is one of the normal fates of HSC. HSC and their progeny rely on signals from growth factors for survival, and when those signals are lost, an endogenous cell death program is activated and cells rapidly die and are engulfed by phagocytes. Failure to die has a profound effect on normal homeostasis and may contribute to oncogenic transformation. Perhaps one of the most striking examples of this is when B lymphocytes acquire the t(14:18) translocation. As a result, cells overexpress Bcl-2, which blocks cell death in these lymphocytes. The outcome is the development of follicular lymphoma [9]. The identification of Bcl-2 as a transforming oncogene in follicular lymphoma, and subsequent demonstrations that Bcl-2 functions as an apoptotic inhibitor, established the paradigm that inhibition of cell death is part of the pathway to malignant transformation.
Growth factors such as GM-CSF and Interleukin-3 (IL-3) promote survival of haematopoietic cells, and depriving dependent cells of these signals results in apoptosis that can be blocked by Bcl-2. The clear implication is that GM-CSF and IL-3 signalling must, in some way, regulate the activity of Bcl-2 and related family members. As we shall discuss, there is substantial evidence to support this hypothesis but there also are suggestions that growth factor survival signalling exerts its effects independently of the Bcl-2 family.
Cytokines: IL-3, IL-5 and GM-CSF
IL-3, GM-CSF and interleukin-5 (IL-5) are related cytokines because their heteromeric receptors share a common ß chain. They are produced by several cell types, including activated T cells and mast cells, and have important roles in maintenance of stem cell populations, and immune and inflammatory responses. For example, IL-3 stimulates differentiation and proliferation of pluripotent stem cells and myeloid progenitor cells (which in turn may differentiate into erythrocytes, thrombocytes, granulocytes, monocytes and dendritic cells) and may influence growth and differentiation of T cells in immune responses [10–12]. GM-CSF induces the production of granulocytes (neutrophils, eosinophils and basophils) and monocytes that are capable of maturing into macrophages. IL-5 is primarily involved in the eosinophilic response observed following some infections [13–15].
The receptors for IL-3, IL-5 and GM-CSF consist of a heterodimeric complex of a specific ligand-binding alpha chain (αc) and a common beta chain (βc) [16, 17]. Activation of the receptor by GM-CSF is now understood at a structural level and currently serves as the model for signal activation for IL-5 and IL-3 [18, 19]. Following association of the ligand with α-chain, there is subsequent interaction between the ligand bound α-chain and the βc subunit leading ultimately to the assembly of a higher order, dodecameric signalling complex. Unlike other tyrosine kinase receptors, the βc has no intrinsic tyrosine kinase activity. Instead, each βc subunit is bound to a member of the Janus kinase family, JAK2. As the active signalling complex is assembled, the JAK2 molecules are brought into close proximity, allowing transphosphorylation of JAK2 and activation of signalling [20, 21].
The physiological roles of this family of cytokines have been established both by deleting the gene for the βc chain of the receptor in mice and by specific deletions of ligands. Deletion of βc had little if any impact on haematopoietic cell numbers but there were clear defects in mature cell function. The most prominent phenotype was delayed clearance of surfactant from the lungs resulting in an alveolar proteinosis, as a result of abnormal phagocyte function [22, 23]. When irradiated mice were transplanted with marrow from animals lacking βc, they were significantly slower to restore granulocyte population. In addition, GM-CSF and IL-5 were important in normal response to certain infections [4, 14, 24]. However, it is the established clinical utility of GM-CSF, and more recently G-CSF, which are used to treat significant granulocytopaenia, for example following chemotherapy [25], that underpins the importance of these signalling pathways.
GM-CSF, IL-3 and malignancy
In their much-cited paper in 2000, Hanahan and Weinberg suggested that most cancers have acquired, by mechanisms that vary from one cancer to another, a common set of functional capabilities [26]. The constitutive activation of growth factor signalling or abnormal expression of growth factors, such as IL-3 or GM-CSF, may contribute to the acquisition of some of these capabilities, namely proliferative signalling and protection against apoptosis. This is perhaps particularly so in haematopoietic malignancies. The link between GM-CSF and IL-3 signalling and cancer are highlighted in the following examples.
Approximately one-third of acute myeloid leukaemia (AML) and a lesser number of B-cell acute lymphoblastic leukaemia (B-ALL) overexpress the alpha subunit of the IL-3 receptor (IL-3Rα). Although a clear correlation between IL-3Rα expression and blast cells numbers exists, a correlation that has prognostic significance [27], less clear are the signalling consequences of IL-3Rα expression [28]. Constitutive IL-3 signalling alone is sufficient to induce lethal myeloproliferative disease in mice but does not result in a transplantable tumour [29]. An interesting clinical correlate of this experimental observation is juvenile myelomonocytic leukaemia (JMML). This disease is more properly classified as a myeloproliferative disorder, and one characteristic feature is a hypersensitivity to GM-CSF, meaning that monocytic cells can proliferate ex vivo in limiting doses of GM-CSF [30, 31]. However, activating mutations in GM-CSF are not found in this disease. Instead, JMML is typically associated with activating mutations of Ras or inactivating mutations of NF1 or PTPN11. PTPN11 mutations appear to induce hyperactivation of Ras and contribute to hypersensitivity of haematopoietic progenitors to GM-CSF [32].
It appears that oncogenic mutations that result in a phenotype of constitutive activation of growth factor signalling are most commonly identified in downstream signalling molecules. In spite of this, it is the case that IL-3 and GM-CSF receptors might still influence the behaviour of these cells. In Philadelphia chromosome positive (Ph+ or Bcr-Abl+) chronic myelogenous leukaemia (CML), elevated levels of GM-CSF and IL-3 and autocrine signalling in CD34+ population can be inhibited by anti-IL-3, anti-GM-CSF or anti-IL3Rα antibodies [33]. Myeloproliferation in tissue culture of JMML can also be blocked by anti-GM-CSF antibodies [34].
Growth factors and Bcl-2 family members: the link for cell survival
It is self-evident that because GM-CSF and IL-3 maintain the viability and proliferation of some haematopoietic cell populations and cells default to apoptosis when growth factor is removed, the signals transduced by these cytokines suppress activation of apoptosis pathways. The main question is how. It is worth considering this question in a historical context as it throws some light onto the subsequent progress of research in this area. Bcl-2, the founding member of the Bcl-2 family of apoptosis regulators, was cloned at the breakpoint of the t(14:18) translocation associated with follicular lymphoma and demonstrated to be the oncogene responsible for this tumour [9, 35]. However, it was unclear how Bcl-2 functioned. Another line of research, directed to the identification and understanding of growth factors’ characteristics, used haematopoietic progenitors derived from mouse bone marrow, serially passaged in conditioned media derived from the WEHI3B tumour line (which produces high amounts of IL-3). Several haematopoietic cell lines were derived, including FDCP-1 cells, and were dependent on conditioned media (IL-3) for proliferation and survival [36]. FDCP-1 cells proved a useful tool in assessing potential oncogenes. For example, overexpression of Bcr-Abl permitted these cells to survive and proliferate in the absence of IL-3 [37]. When Bcl-2 was overexpressed in FDCP-1 cells, it was evident that they were not able to proliferate in the absence of IL-3. However, strikingly, neither did these cells die when deprived of growth factor. Once IL-3 signalling was restored to these cells, proliferation recommenced. Bcl-2 thus maintained cell viability and regulated the cell death response to growth factor deprivation [38, 39]. It has since been demonstrated several times that Bcl-2 and related genes such as Bcl-xL can block apoptosis in response to virtually all models of growth factor (or serum) deprivation. Direct regulation of the activity of the Bcl-2 family would be one obvious mechanism by which growth factors could regulate apoptosis (Fig. 1).
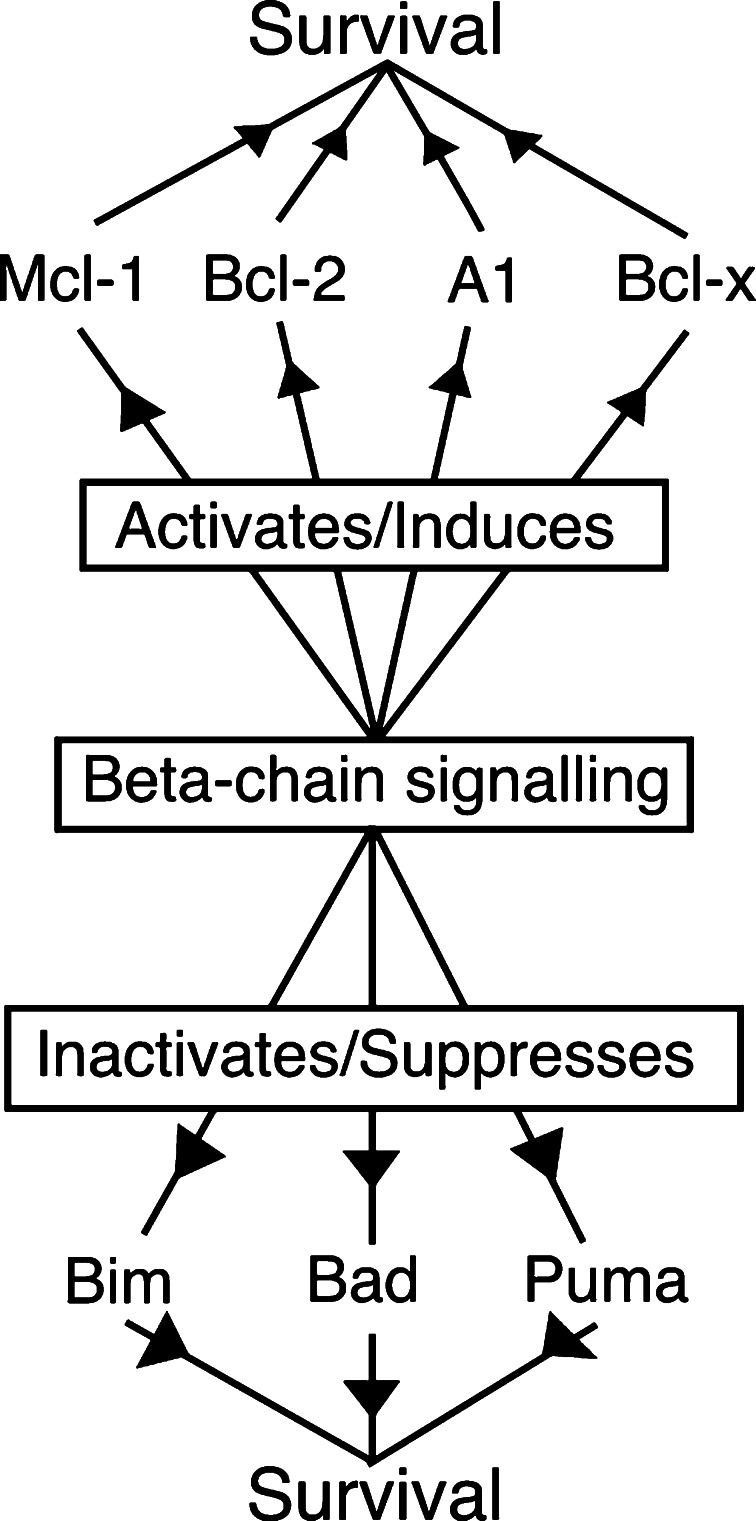
Fig. 1.
Cytokine signalling and Bcl-2 family members. One of the mechanisms by which cytokine signalling promotes cell survival is through inhibition of apoptotic pathways. Bcl-2 family members are crucial for the regulation of cell death signalling. Activation of beta-chain signalling can suppress expression of BH3-only pro-apoptotic proteins, in particular, Bim, Puma and Bad. Induction and maintenance of anti-apoptotic proteins Mcl-1, Bcl-2, A1 and Bcl-x during activation of cytokine signalling is also crucial to prevent cell death induced by cytokine deprivation
Bcl-2 family
Death of cells promoted by cytokine withdrawal is characterised by mitochondria membrane permeabilisation followed by release of cytochrome c, Smac/Diablo and other factors from the mitochondria to the cytoplasm and finally execution of the cells by activated caspases [40]. The Bcl-2 family of proteins function as central regulators of apoptosis by promoting or blocking changes in mitochondrial membrane permeability and, consequently, the release of pro-apoptotic factors and activation of caspases. The Bcl-2 family are so classified by the presence of one or more Bcl-2 homology (BH) domains, and members are divided into two major groups, the inhibitors of apoptosis and the inducers of cell death. The pro-apoptotic proteins required for initiation and execution of apoptosis includes the Bax death family and the BH3-only proteins [41]. The BH3-only members are activated by intracellular stresses that constitute a death stimulus and then function to directly suppress anti-apoptotic Bcl-2 proteins. The Bax-like proteins, in particular Bax and Bak, are essential to induce mitochondria disruption and apoptosis and do so by undergoing conformational changes [42–45]. Activation of these proteins is initiated by BH3-only proteins in two major ways, through neutralisation of anti-apoptotic Bcl-2 proteins and by direct activation of Bax and Bak [46, 47].
Expression and activation of Bax-like proteins play a central role in apoptosis mediated by growth factor deprivation. Withdrawal of IL-3 in IL-3-dependent cells results in translocation of Bax from the cytosol to the mitochondria, conformational change in Bax and induction of apoptosis [48]. However, even though IL-3-dependent cells from Bax-null mice fail to activate caspases in the first 24 h after IL-3 removal, apoptosis induced by IL-3 deprivation was only delayed in these cells, indicating that cell death mediated by growth factor withdrawal is not absolutely dependent on the expression and activation of Bax, although it is sufficient to induce apoptosis [49]. This is also true for IL-3-dependent cells lacking only Bak. However, in cytokine-dependent cells lacking both Bax and Bak, cell death mediated by growth factor withdrawal is completely abolished. Importantly, surviving cells retain the ability to proliferate and form colonies when IL-3 is restored, in much the same way as cells overexpressing anti-apoptotic Bcl-2 protein. Activation of Bax or Bak is therefore the key step in the commitment to apoptosis mediated by cytokine withdrawal [50, 51]. Thus, regulation of conformational changes and activation of Bax/Bak is the major checkpoint in governing whether cells live or die, which is determined by the interaction with other Bcl-2 family members, the Bax-like proteins and the BH3-only proteins.
Growth factors and anti-apoptotic Bcl-2 family members: partners in life
The anti-apoptotic Bcl-2 family members include Bcl-2, Bcl-xL, Mcl-1, A1 and Bcl-w. Studies in knock-out mice emphasise the crucial role that anti-apoptotic Bcl-2 family members play in normal development. Bcl-2 is required for survival of mature lymphocytes and melanocytes, and mice lacking Bcl-2 develop severe kidney disease [52]. Bcl-xL is important for neuronal and erythroid cells, and mice lacking Bcl-xL die during embryonic development [53, 54]. Mcl-1-deficient mice suffer a similar fate but even earlier in development, since Mcl-1 is essential for early haematopoiesis and later stages of granulocyte differentiation [55]. A1 is required for the survival of mature B cells and neutrophils whereas Bcl-w is necessary for the normal development of sperm progenitors in adult mice [56, 57].
What evidence is there that cytokine signalling regulates the levels or function of anti-apoptotic Bcl-2 family members? Overexpression of Bcl-2 family members in cytokine-dependent cells can take one only so far in considering this question as induced expression of the anti-apoptotic Bcl-2 family members will block apoptosis. But this does not indicate that growth factors block apoptosis by upregulating the levels of Bcl-2 family members [58, 59]. However, in the case of at least one Bcl-2 family member, Mcl-1, there is substantial evidence to suggest that growth factors, in particular GM-CSF, critically regulate Mcl-1 levels particularly in haematopoietic cells.
GM-CSF signalling pathways tightly control Mcl-1 expression in TF-1 myeloid progenitor cells. Upon deprivation of GM-CSF from these cells, Mcl-1 protein levels dramatically decline as a result of proteosomal degradation followed by loss of cell survival. Restimulation of TF-1 cells with GM-CSF immediately induced Mcl-1 mRNA followed by resynthesis of the protein. Downregulation of Mcl-1 by antisense constructs antagonised, in part at least, cell survival signalling transduced through the GM-CSF receptor. Moreover, truncation mutants of the GM-CSF receptor revealed that a region between amino acids 573 and 755 of the receptor β chain was required for Mcl-1 induction [60]. Other studies have shown that addition of GM-CSF to neutrophils is able to delay apoptosis of these cells by stabilising and increasing intracellular levels of Mcl-1 [61, 62]. Together, these analyses suggested that Mcl-1 is an immediate-early gene activated by the GM-CSF signalling pathway and may therefore be one of the major components of the mechanism by which GM-CSF and similar cytokines maintain survival.
Clearly, there is a transcriptional upregulation of Mcl-1 by growth factors but it remains unclear what transcription factors are required. Another remaining question is how does loss of GM-CSF result in Mcl-1 degradation? Mcl-1 is phosphorylated by GSK-3, leading to Mcl-1 ubquitinylation and degradation. In the presence of GM-CSF or IL-3 signalling, GSK-3 activity is suppressed by PI3K/AKT. Once the signal is lost, GSK becomes activated and capable of phosphorylating Mcl-1 which is the target for degradation [63]. It seems likely that, once GM-CSF signalling is lost, several parallel pathways rapidly lead to Mcl-1 inactivation and degradation. Other studies suggests that inhibition of Mcl-1 activity may be independent of its degradation. Antagonism by BH3-only proteins and disruption of Mcl-1 binding to Bak or Bax may be sufficient to inactivate Mcl-1 [64, 65] and induce cell death. Degradation of Mcl-1 is not absolutely required for its inactivation [65].
Bcl-2 and Bcl-xL may also be regulated by phosphorylation mediated by kinases activated by cytokine signalling or its loss. Phosphorylation of Bcl-2 by PKC-α or ERK1/2 appears to be required for survival mediated by IL-3 signalling [66–68]. However, the functional significance of phosphorylation or dephosphorylation of Bcl-2 and Bcl-xL in the control of apoptosis responses remains controversial. We will discuss the kinases and their role in growth factor-mediated survival and death more specifically later in the review.
BH3-only: opposing survival factors signalling
To date, at least ten mammalian BH3-only proteins have been described, among them, Bad, Bid, Bim, Bmf, Noxa, Puma and Hrk. Using knockout mice and cell lines derived from these mice, roles for each in a range of apoptotic stimuli, including cytokine deprivation, antigen receptor signalling, oncogenes activation and chemotherapeutic drugs, have been described [51, 69–72]. From these studies, it has been established that the BH3-only proteins Bad, Bim and Puma are involved in cell death mediated by loss of growth factor signals, particularly in haematopoietic cells.
The BH3-only protein Bad is able to bind and inhibit the function of Bcl-xL, Bcl-2 and Bcl-w but not of Mcl-1 or A1 [73]. In studies using IL-3-dependent cells overexpressing Bad, it was evident that the ability of Bad to bind and neutralise Bcl-xL was being regulated during growth factor signalling. Bad was phosphorylated at two critical serine residues, in a manner that could be blocked by PI3K inhibitors, and when phosphorylated formed a complex with the chaperone protein 14-3-3 in the cytosol [74, 75]. Bad bound to 14-3-3 was unable to bind to Bcl-xL. The sequestering of Bad by 14-3-3 was reversed in the absence of IL-3, as a consequence of PI3K inactivation, Bad was no longer phosphorylated and was thus free to bind and suppress Bcl-xL. Surprisingly, IL-3-dependent cells derived from Bad null mice remained susceptible to apoptosis induced by IL-3 deprivation indicating that Bad was redundant for IL-3 deprivation-induced apoptosis, at least in this model [51]. Even though deletion of Bad has been associated with malignant transformation, resistance to cytokine deprivation does not seem to be the mechanism by which tumour development occurs.
Bim-mediated apoptosis plays an important role in the regulation of haematopoetic cell numbers since Bim-deficient mice accumulate abnormally high numbers of lymphoid and myeloid cells [72]. These animals are particularly susceptible to lymphoid tumours in the presence of another oncogenic stimuli, such as the overexpression of c-myc [76]. In vitro, several compelling lines of experimental evidence suggest an important role for Bim in apoptosis mediated by cytokine deprivation in different cell types. Bim expression is necessary for apoptosis induced by IL-2 deprivation in human T cell lymphoblasts, since downmodulation of Bim expression by siRNA in these cells increased cell survival in the absence of IL-2 [77]. In mice, Bim levels increased in activated T cells deprived of IL-2 and apoptosis is delayed and partially inhibited in activated T cells derived from bim −/− mice [78]. IL-3-dependent mast cells derived from bone marrow of Bim-deficient mice also survive IL-3 deprivation [79]. However, the protective effect of Bim deletion is not observed in all models of IL-3 deprivation [51].
There are several potential mechanisms by which Bim is activated when growth factor signalling is lost. In sympathetic neurones deprived of nerve growth factor (NGF), Bim expression increased and could be suppressed by a dominant negative of c-Jun. This suggests a possible pathway in which c-Jun, activated by NGF signalling, transcriptionally represses Bim expression [80]. In haematopoietic cells, Bim may be transcriptionally regulated by the forkhead transcription factors (FoxO) [81]. In another models, lack of cytokine signalling promoted dephosphorylation of FoxO3a, as a result, loss of PI3K/AKT activity, leading to FoxO3a translocation to the nucleus and transcriptional upregulation of Bim and Puma [81, 82]. Deletion of FoxO3a did not completely abolish expression levels of these BH3-only proteins but partially prevented apoptosis induced by growth factor withdrawal [83]. Clearly, other transcription factors regulate Bim expression in response to cytokine deprivation and other apoptotic stimuli, and transcriptional regulation of Bim is more complex than our current understanding.
Bim is also regulated post-translationally by sequestration to cytoskeletal structure of the cells. In normal conditions, Bim isoforms BimEL and BimL are sequestered into dynein motor complex through a direct interaction with LC8. In IL-3 deprivation, the localisation of Bim to microtubules by virtue of the interaction with LC8 is disrupted by cytoskeletal changes that occur early (prior to caspase activation) in cytokine deprivation [84]. This mechanism is not confined to IL-3 deprivation and appears to be a more general mechanism by which Bim senses cytoskeletal alteration as an early indicator that a cell is sufficiently compromised and should now activate the apoptotic program.
Bim protein levels and function can be regulated by post-translational modification, in particular phosphorylation [85, 86]. Serum stimulation induces a MAPK-dependent (ERK1/2) phosphorylation of serine residues in exon 2, in particular serine 65 targets Bim for degradation but does not substantially alter Bim interactions with anti-apoptotic Bcl-2 family members. JNK-dependent phosphorylation at threonine 112 (T112), in response to UV irradiation for example, appears to be important for Bim-Bcl-2 binding, and mutation of this residue to alanine diminishes the proapoptotic activity of Bim [86]. This may be because Bim phosphorylated at T112 is no longer bound to the dynein motor complex. Cytokine deprivation results in phosphorylation of Bim at T112 as part of the mechanism of Bim activation. The presence of a survival signal activates kinases that phosphorylate BimEL as a means of reducing the total input of Bim in the cell (Fig. 2). It remains to be determined how true this is in the context of IL-3 and GM-CSF signalling.
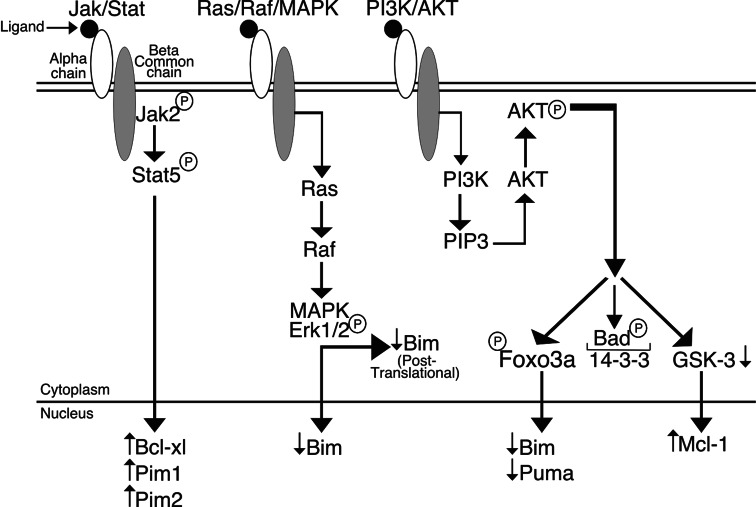
Fig. 2.
Downstream signalling events after receptor activation. Cytokine stimulation results in initiation of several signalling cascades influencing the transcription and post-translational modification of certain downstream targets. Three well-examined signalling pathways that are activated by IL-3 or GM-CSF via beta-chain activation are briefly illustrated here. Jak/Stat pathway: receptor activation leads to JAK2 phosphorylation, in turn phosphorylating the Stat family proteins which translocates to the nucleus to control genes responsible for survival and proliferation, such as Bcl-xL, Pim1 and Pim2. Ras/Raf/MAPK pathway: receptor activation induces phosphorylation and activation of Ras, which in turn activates Raf and the MAP kinases. Post-translational as well as possible transcriptional regulation of Bim is controlled in part by the Ras/Raf/MAPK pathway. PI3K/AKT pathway: activation of PI3K through cytokine signalling induces activation and phosphorylation of AKT that in turn phosphorylates and inactivates Bad by binding to 14-3-3. FoxO3a is also phosphorylated, in turn negatively regulating the BH3 only proteins, Bim and Puma. Phosphorylated AKT also regulates GSK-3, indirectly maintaining Mcl-1 levels
Puma/Bbc3 was identified as a BH3-only protein transcriptionally regulated by p53. This BH3-only protein is essential for hematopoietic cell death triggered by several stimuli such as ionising radiation (IR), deregulated c-Myc expression and cytokine withdrawal [87, 88]. Puma-deficient hematopoietic cells derived in a variety of ways resist growth factor deprivation both in short-term and clonogenic assays, indicating Puma functions to ensure cells commit to apoptosis in the absence of cytokines [51, 87, 88]. Inhibition of Puma expression by treatment of cells with shRNA attenuated cell death mediated by IL-3 withdrawal [89]. Puma, like Bim (but unlike Bad), can bind and inhibit all anti-apoptotic Bcl-2 family members, and Puma and Bim may cooperate to induce apoptosis in some instances, since puma −/− or double knockout puma −/− bim −/− mast cells or activated T cells appear to be more resistant than bim −/− to cytokine withdrawal. On the other hand, in a model of IL-3 deprivation, puma −/− cells were clonogenically protected from IL-3 deprivation whereas bim −/− cells behaved like wild-type cells [90].
Most evidence suggests that Puma is transcriptionally regulated in response to cytokine deprivation. Protein and mRNA levels increase in myeloid progenitors after IL-3 deprivation [90]. Several transcription factors appear to be involved in Puma regulation in response to different death stimuli. p53 and the SNAIL family member Slug regulate Puma expression following gamma irradiation. In response to cytokine deprivation, Puma is regulated, in part, by FoxO3a, which may also regulate Bim expression. Whilst cytokine deprivation is not typically thought of as a p53-dependent death stimulus, experimental evidence indicates that p53−/− myeloid progenitor cells have increased survival in limiting cytokine concentration [91] and that shRNA knockdown of p53 increases survival and decreases Puma expression in IL-3-starved FL5.12 cells [89]. It remains unknown whether Puma, like Bim, is also subject to post-translational regulation. It is clear however, that unlike Bim, Puma is always localised to mitochondria [92].
Overall, the withdrawal of IL-3 or GM-CSF from dependent cell lines has the net effect of diminishing levels of at least one anti-apoptotic Bcl-2 protein whilst increasing levels and activation of pro-apoptotic family members, in particular the BH3-only proteins. The balance is tipped in favour of activation of Bax and Bak and cell death.
Tracing the pathway back: from Bcl-2 family members to the receptor
At the level of Bcl-2 family members, there is now a relatively detailed understanding of interactions between family members and how the nature of these interactions can favour cell survival or cell death. Although several mechanisms that might regulate Bcl-2 family members in response to cytokine signalling or cytokine deprivation have been described, it is this connection between growth factor signalling and regulation of apoptotic pathways that remains poorly understood. Whether there is a specific signalling kinase or pathway activation during GM-CSF or IL-3 signalling that regulates apoptosis or whether suppression of cell death is the net effect of many signalling pathways activated by GM-CSF and IL-3 remains to be determined. However, the bulk of evidence would suggest that multiple signalling pathways contribute to interaction between cytokine and apoptosis signalling (Fig. 2). We will discuss the evidence supporting a role for some of these kinases below.
In contrast to other tyrosine receptor kinases, IL-3 and GM-CSF receptors do not have intrinsic tyrosine kinase activity and are dependent on the Janus kinase JAK2 to activate and initiate the multiplicity of signalling cascades that occur after the ligand binds the receptor. Following GM-CSF stimulation and multimerization of the receptor, JAK2 is activated by transphosphorylation and in turn may phosphorylate other tyrosine residues on βc resulting in the recruitment of signalling molecules to the βc and activation of several signalling cascades [21, 93, 94]. Activation of JAK2 leads to βc phosphorylation of several tyrosine residues some of which act as docking sites for signal transduction and activators of transcription (STATs). Phosphorylation of STATs by JAK results in its dissociation from the receptor and translocation to the nucleus to activate gene transcription. In the case of IL-3 and GM-CSF signalling, STAT5 activation appears important since constitutively active mutants of βc result in constitutive STAT5 activation [95]. Several extensive mutational analyses of βc chain have defined regions and residues required for JAK2 activation and downstream STAT activation [96–98]. It is evident that JAK2 activation is required for all signalling outcomes transduced by βc, including proliferation, differentiation and survival. Disruption of STAT5 phosphorylation, by mutation of various tyrosine residues in βc chain, also diminishes proliferative, differentiation and survival signals, although the nature and magnitude of these effects vary with the model used [99, 100]. Likewise, increased apoptotic cell death of haematopoietic cells is observed in STAT5-deficient mice [101]. Several transcriptional targets of STAT5 key to survival signalling have been suggested, including c-fos, pim-1 and Bcl-xL [102–104].
The Pim serine/threonine kinases, Pim-1 and Pim-2, are important mediators of cytokine signalling and may contribute to the development of solid tumours and certain types of leukaemia [105, 106]. Pim-2 protein levels rapidly decline following IL-3 withdrawal in FL5.12 cells and are induced again after IL-3 restoration. Constitutive expression of Pim-2 maintains haematopoietic cell survival in the absence of IL-3 at a similar level to constitutively active AKT but importantly independent of AKT [107, 108]. This indicates that AKT activation and Pim-2 activation after IL-3 signalling transduce independent survival signals. Exactly how Pim-2 promotes cell survival is unclear because, even though exogenously expressed Pim-2 can phosphorylate the BH3-only protein Bad [107, 108], Bad is redundant for IL-3 withdrawal-induced cell death. Another possible mechanism is suggested by the data that demonstrates NF-κB activation by Pim-2 [109]. Canonical NF-κB signals cell survival through several mechanisms, including regulation of Bcl-2 members [110].
Pim-1, when exogenously expressed, can maintain the survival of haematopoietic cells in the absence of IL-3 and can phosphorylate Bad at serine 112 [111]. Activation of STAT-3 and -5 by GM-CSF signalling may induce Pim-1 expression in human eosinophils and suppresses apoptosis of these cells [104]. Studies using pharmacological inhibitors of Pim kinases and overexpression of dominant-negative of Pim have demonstrated that Pim kinase activity is required for IL-3-mediated survival of basophils [112]. Once again, it remains to be determined precisely how Pim-1 can mediate factor independent cell survival.
The PI3K and AKT kinase pathway has been the focus of considerable attention with regard to cytokine-mediated survival signalling, and it has been previously mentioned in this review. In the presence of IL-3 or GM-CSF, PI3K is activated and generates PIP3 which recruits AKT to the cell membrane promoting its phosphorylation and activation [113]. Once activated, AKT phosphorylates many different molecules that contribute to the suppression of apoptosis (Fig. 2). The principal line of evidence that support the role of PI3K/AKT survival signals are that PI3K inhibitors cause apoptosis in IL-3 or GM-CSF-dependent cells and overexpression of constitutively activated AKT promotes growth factor-independent cell survival [114]. As previously mentioned, AKT can directly phosphorylate and inactivate Bad, phosphorylate and inactivate FoxO3a (which in turn may regulate Bim and Puma) and suppress GSK-3 activity, and so maintain Mcl-1 levels. It seems likely that all such effects of PI3K/AKT activation, and perhaps many others, contribute to the survival effect of AKT, and much remains to be learned. There are three isoforms of AKT, AKT1, AKT2 and AKT3, and little is known about which isoform phosphorylates which targets to contribute to cell survival. Interestingly, deletion of single isoforms of AKT has no impact on haematopoietic cell survival. PI3K inhibitors and AKT-specific inhibitors may have therapeutic utility in the treatment of several malignancies, including AML. Whilst neither PI3K nor AKT are often the subject of activating mutations in AML, both are often constitutively activated in the disease, even in the absence of mutation [115].
Overexpression of constitutively active AKT can partially mimic the survival effects but not the proliferative effects of IL-3 signalling. Also, like IL-3 signalling, AKT maintains the expression of nutrient transporter proteins and uptake of many essential nutrients into cells, an effect in large part mediated via activation of mTOR [116]. Indeed, in IL-3-dependent FL5.12 cells, AKT-dependent survival in the absence of IL-3 was completely dependent on the presence of glucose in the media [117]. Such observations clearly indicate that AKT regulates survival by maintaining adequate substrates for vital intracellular processes, quite independently of any effect on the expression or activation of Bcl-2 family members or other apoptosis proteins. By extension, the manner in which cytokines maintain cell viability may also be mediated by similar metabolic effects. Equally clear, however, is the fact that apoptosis pathways are activated in the absence of IL-3 or following the deprivation of metabolic substrates such as glucose. Even if the primary effect of cytokine deprivation is diminished intracellular levels of nutrients, this is still a trigger for the activation of apoptotic pathways. Indeed, the elevated Puma expression that follows IL-3 deprivation can be blunted by enforced expression of the glucose transporter Glut1 or the provision of a freely diffusible glucose substrate, methyl-pyruvate [89]. The question then becomes not which survival kinases regulate apoptosis pathways, but how are apoptosis pathways activated by limited metabolic substrates? It is perhaps most likely that cytokine signalling both maintains cellular nutrient uptake and directly regulates the propensity of apoptosis pathways to activate.
In addition to activation of Jak/STAT and PI3K/AKT pathways, IL-3 signalling may also activate the Ras/Raf/MAPK pathway to promote cell survival, as previously mentioned in post-translational modification of Bim. Amplification of ras proto-oncogene and activating mutations leading to expression of constitutively active form of Ras and/or Raf are found in 30% of human cancers, such as melanomas and some leukaemias. These mutations are strongly associated with the ability of cells to proliferate and survive in the absence of growth factors. Overexpression of an active mutant of Ras (Ras G12V) prevented apoptosis mediated by IL-3 withdrawal, although it did not induce a significant IL-3-independent proliferation [118]. Furthermore, inhibition of MAPK activity decreased viability in limiting doses of IL-3 [119]. The mechanisms by which MAPKs mediate cell survival remain to be determined. Although, like other signalling kinases, some evidence supports regulation of expression or activation of Bcl-2 family members such as Bim and Bcl-xL [120].
The activation of several kinases and different signalling pathways by cytokines leads to multiple synchronised change in a cell to control the balance between life and death. The exact mechanisms and signalling pathways that are activated remain controversial. Although Bcl-2 family members appear to be one of the key points for regulation of survival mediated by growth factors, cells lacking Bax and Bak, still succumb eventually to IL-3/substrate deprivation, indicating that activation of apoptotic pathways is critical in situations where rapid and efficient execution of apoptosis is required. However, in the long term, many other signalling pathways may also contribute to the suppression of apoptosis. Understanding the multiple signals and identifying specific protein modifications in cytokine signalling will add to our knowledge of molecular interactions involved in maintenance of haematopoiesis and growing tumours.
References
- 1.Socolovsky M, Fallon AE, Wang S, Brugnara C, Lodish HF. Fetal anemia and apoptosis of red cell progenitors in Stat5a-/-5b-/- mice: a direct role for Stat5 in Bcl-X(L) induction. Cell. 1999;98:181–191. doi: 10.1016/S0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki N, Ohneda O, Takahashi S, Higuchi M, Mukai HY, Nakahata T, Imagawa S, Yamamoto M. Erythroid-specific expression of the erythropoietin receptor rescued its null mutant mice from lethality. Blood. 2002;100:2279–2288. doi: 10.1182/blood-2002-01-0124. [DOI] [PubMed] [Google Scholar]
- 3.Wu H, Klingmuller U, Acurio A, Hsiao JG, Lodish HF. Functional interaction of erythropoietin and stem cell factor receptors is essential for erythroid colony formation. Proc Natl Acad Sci USA. 1997;94:1806–1810. doi: 10.1073/pnas.94.5.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, Maher DW, Cebon J, Sinickas V, Dunn AR. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci USA. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dranoff G, Mulligan RC. Activities of granulocyte-macrophage colony-stimulating factor revealed by gene transfer and gene knockout studies. Stem Cells. 1994;12:173–182. [PubMed] [Google Scholar]
- 6.Metcalf D. Clonal analysis of proliferation and differentiation of paired daughter cells: action of granulocyte-macrophage colony-stimulating factor on granulocyte-macrophage precursors. Proc Natl Acad Sci USA. 1980;77:5327–5330. doi: 10.1073/pnas.77.9.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metcalf D. Transgenic mice as models of hemopoiesis. Cancer. 1991;67:2695–2699. doi: 10.1002/1097-0142(19910515)67:10+<2695::AID-CNCR2820671704>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 8.Nishinakamura R, Miyajima A, Mee PJ, Tybulewicz VL, Murray R. Hematopoiesis in mice lacking the entire granulocyte-macrophage colony-stimulating factor/interleukin-3/interleukin-5 functions. Blood. 1996;88:2458–2464. [PubMed] [Google Scholar]
- 9.Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228:1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- 10.Kindler V, Thorens B, de Kossodo S, Allet B, Eliason JF, Thatcher D, Farber N, Vassalli P. Stimulation of hematopoiesis in vivo by recombinant bacterial murine interleukin 3. Proc Natl Acad Sci USA. 1986;83:1001–1005. doi: 10.1073/pnas.83.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saeland S, Caux C, Favre C, Aubry JP, Mannoni P, Pebusque MJ, Gentilhomme O, Otsuka T, Yokota T, Arai N. Effects of recombinant human interleukin-3 on CD34-enriched normal hematopoietic progenitors and on myeloblastic leukemia cells. Blood. 1988;72:1580–1588. [PubMed] [Google Scholar]
- 12.Dan Y, Katakura Y, Ametani A, Kaminogawa S, Asano Y. IL-3 augments TCR-mediated responses of type 2 CD4 T cells. J Immunol. 1996;156:27–34. [PubMed] [Google Scholar]
- 13.Nishinakamura R, Nakayama N, Hirabayashi Y, Inoue T, Aud D, McNeil T, Azuma S, Yoshida S, Toyoda Y, Arai K. Mice deficient for the IL-3/GM-CSF/IL-5 beta c receptor exhibit lung pathology and impaired immune response, while beta IL3 receptor-deficient mice are normal. Immunity. 1995;2:211–222. doi: 10.1016/1074-7613(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 14.Schwarze J, Cieslewicz G, Hamelmann E, Joetham A, Shultz LD, Lamers MC, Gelfand EW. IL-5 and eosinophils are essential for the development of airway hyperresponsiveness following acute respiratory syncytial virus infection. J Immunol. 1999;162:2997–3004. [PubMed] [Google Scholar]
- 15.Yamaguchi Y, Suda T, Suda J, Eguchi M, Miura Y, Harada N, Tominaga A, Takatsu K. Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J Exp Med. 1988;167:43–56. doi: 10.1084/jem.167.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guthridge MA, Stomski FC, Thomas D, Woodcock JM, Bagley CJ, Berndt MC, Lopez AF. Mechanism of activation of the GM-CSF, IL-3, and IL-5 family of receptors. Stem Cells. 1998;16:301–313. doi: 10.1002/stem.160301. [DOI] [PubMed] [Google Scholar]
- 17.Robb L. Cytokine receptors and hematopoietic differentiation. Oncogene. 2007;26:6715–6723. doi: 10.1038/sj.onc.1210756. [DOI] [PubMed] [Google Scholar]
- 18.Hansen G, Hercus TR, McClure BJ, Stomski FC, Dottore M, Powell J, Ramshaw H, Woodcock JM, Xu Y, Guthridge M, McKinstry WJ, Lopez AF, Parker MW. The structure of the GM-CSF receptor complex reveals a distinct mode of cytokine receptor activation. Cell. 2008;134:496–507. doi: 10.1016/j.cell.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 19.Hercus TR, Thomas D, Guthridge MA, Ekert PG, King-Scott J, Parker MW, Lopez AF. The GM-CSF receptor: linking its structure to cell signaling and its role in disease. Blood. 2009;114:1289–1298. doi: 10.1182/blood-2008-12-164004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S, Vanin EF, Bodner S, Colamonici OR, van Deursen JM, Grosveld G, Ihle JN. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/S0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 21.Quelle FW, Sato N, Witthuhn BA, Inhorn RC, Eder M, Miyajima A, Griffin JD, Ihle JN. JAK2 associates with the beta c chain of the receptor for granulocyte-macrophage colony-stimulating factor, and its activation requires the membrane-proximal region. Mol Cell Biol. 1994;14:4335–4341. doi: 10.1128/mcb.14.7.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dirksen U, Nishinakamura R, Groneck P, Hattenhorst U, Nogee L, Murray R, Burdach S. JAK2 associates with the beta c chain of the receptor for granulocyte-macrophage colony-stimulating factor, and its activation requires the membrane-proximal region. J Clin Invest. 1997;100:2211–2217. doi: 10.1172/JCI119758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Moczygemba M, Doan ML, Elidemir O, Fan LL, Cheung SW, Lei JT, Moore JP, Tavana G, Lewis LR, Zhu Y, Muzny DM, Gibbs RA, Huston DP. Pulmonary alveolar proteinosis caused by deletion of the GM-CSFRalpha gene in the X chromosome pseudoautosomal region 1. J Exp Med. 2008;205:2711–2716. doi: 10.1084/jem.20080759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metcalf D, Robb L, Dunn AR, Mifsud S, Di Rago L. Role of granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor in the development of an acute neutrophil inflammatory response in mice. Blood. 1996;88:3755–3764. [PubMed] [Google Scholar]
- 25.Ravandi F. Role of cytokines in the treatment of acute leukemias: a review. Leukemia. 2006;20:563–571. doi: 10.1038/sj.leu.2404152. [DOI] [PubMed] [Google Scholar]
- 26.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 27.Testa U, Riccioni R, Militi S, Coccia E, Stellacci E, Samoggia P, Latagliata R, Mariani G, Rossini A, Battistini A, Lo-Coco F, Peschle C. Elevated expression of IL-3Ralpha in acute myelogenous leukemia is associated with enhanced blast proliferation, increased cellularity, and poor prognosis. Blood. 2002;100:2980–2988. doi: 10.1182/blood-2002-03-0852. [DOI] [PubMed] [Google Scholar]
- 28.Testa U, Riccioni R, Diverio D, Rossini A, Lo Coco F, Peschle C. Interleukin-3 receptor in acute leukemia. Leukemia. 2004;18:219–226. doi: 10.1038/sj.leu.2403224. [DOI] [PubMed] [Google Scholar]
- 29.Perkins A, Kongsuwan K, Visvader J, Adams JM, Cory S. Homeobox gene expression plus autocrine growth factor production elicits myeloid leukemia. Proc Natl Acad Sci USA. 1990;87:8398–8402. doi: 10.1073/pnas.87.21.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasle H. Myelodysplastic and myeloproliferative disorders in children. Curr Opin Pediatr. 2007;19:1–8. doi: 10.1097/MOP.0b013e3280128ce8. [DOI] [PubMed] [Google Scholar]
- 31.Emanuel PD, Bates LJ, Castleberry RP, Gualtieri RJ, Zuckerman KS. Selective hypersensitivity to granulocyte-macrophage colony-stimulating factor by juvenile chronic myeloid leukemia hematopoietic progenitors. Blood. 1991;77:925–929. [PubMed] [Google Scholar]
- 32.Chan RJ, Leedy MB, Munugalavadla V, Voorhorst CS, Li Y, Yu M, Kapur R. Human somatic PTPN11 mutations induce hematopoietic-cell hypersensitivity to granulocyte-macrophage colony-stimulating factor. Blood. 2005;105:3737–3742. doi: 10.1182/blood-2004-10-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang X, Lopez A, Holyoake T, Eaves A, Eaves C. Autocrine production and action of IL-3 and granulocyte colony-stimulating factor in chronic myeloid leukemia. Proc Natl Acad Sci USA. 1999;96:12804–12809. doi: 10.1073/pnas.96.22.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gualtieri RJ, Emanuel PD, Zuckerman KS, Martin G, Clark SC, Shadduck RK, Dracker RA, Akabutu J, Nitschke R, Hetherington ML, Dickerman JD, Hakami N, Castleberry RP. Granulocyte-macrophage colony-stimulating factor is an endogenous regulator of cell proliferation in juvenile chronic myelogenous leukemia. Blood. 1989;74:2360–2367. [PubMed] [Google Scholar]
- 35.Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984;226:1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- 36.Dexter TM, Garland J, Scott D, Scolnick E, Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980;152:1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hariharan IK, Adams JM, Cory S. bcr-abl oncogene renders myeloid cell line factor independent: potential autocrine mechanism in chronic myeloid leukemia. Oncogene Res. 1988;3:387–399. [PubMed] [Google Scholar]
- 38.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 39.Vaux DL, Aguila HL, Weissman IL. Bcl-2 prevents death of factor-deprived cells but fails to prevent apoptosis in targets of cell mediated killing. Int Immunol. 1992;4:821–824. doi: 10.1093/intimm/4.7.821. [DOI] [PubMed] [Google Scholar]
- 40.Chen Q, Takeyama N, Brady G, Watson AJ, Dive C. Blood cells with reduced mitochondrial membrane potential and cytosolic cytochrome C can survive and maintain clonogenicity given appropriate signals to suppress apoptosis. Blood. 1998;92:4545–4553. [PubMed] [Google Scholar]
- 41.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 42.Nechushtan A, Smith CL, Lamensdorf I, Yoon SH, Youle RJ. Bax and Bak coalesce into novel mitochondria-associated clusters during apoptosis. J Cell Biol. 2001;153:1265–1276. doi: 10.1083/jcb.153.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nechushtan A, Smith CL, Hsu YT, Youle RJ. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffiths GJ, Dubrez L, Morgan CP, Jones NA, Whitehouse J, Corfe BM, Dive C, Hickman JA. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J Cell Biol. 1999;144:903–914. doi: 10.1083/jcb.144.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Annis MG, Soucie EL, Dlugosz PJ, Cruz-Aguado JA, Penn LZ, Leber B, Andrews DW. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 2005;24:2096–2103. doi: 10.1038/sj.emboj.7600675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, Andrews DW. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 48.Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Low W, Olmos-Centenera G, Madsen C, Leverrier Y, Collins MK. Role of Bax in apoptosis of IL-3-dependent cells. Oncogene. 2001;20:4476–4483. doi: 10.1038/sj.onc.1204580. [DOI] [PubMed] [Google Scholar]
- 50.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ekert PG, Jabbour AM, Manoharan A, Heraud JE, Yu J, Pakusch M, Michalak EM, Kelly PN, Callus B, Kiefer T, Verhagen A, Silke J, Strasser A, Borner C, Vaux DL. Cell death provoked by loss of interleukin-3 signaling is independent of Bad, Bim, and PI3 kinase, but depends in part on Puma. Blood. 2006;108:1461–1468. doi: 10.1182/blood-2006-03-014209. [DOI] [PubMed] [Google Scholar]
- 52.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-M. [DOI] [PubMed] [Google Scholar]
- 53.Gonzalez-Garcia M, Garcia I, Ding L, O’Shea S, Boise LH, Thompson CB, Nunez G. bcl-x is expressed in embryonic and postnatal neural tissues and functions to prevent neuronal cell death. Proc Natl Acad Sci USA. 1995;92:4304–4308. doi: 10.1073/pnas.92.10.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S, Loh DY. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- 55.Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 2000;14:23–27. [PMC free article] [PubMed] [Google Scholar]
- 56.Ross AJ, Waymire KG, Moss JE, Parlow AF, Skinner MK, Russell LD, MacGregor GR. Testicular degeneration in Bclw-deficient mice. Nat Genet. 1998;18:251–256. doi: 10.1038/ng0398-251. [DOI] [PubMed] [Google Scholar]
- 57.Hamasaki A, Sendo F, Nakayama K, Ishida N, Negishi I, Nakayama K, Hatakeyama S. Accelerated neutrophil apoptosis in mice lacking A1-a, a subtype of the bcl-2-related A1 gene. J Exp Med. 1998;188:1985–1992. doi: 10.1084/jem.188.11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klampfer L, Zhang J, Nimer SD. GM-CSF rescues TF-1 cells from growth factor withdrawal-induced, but not differentiation-induced apoptosis: the role of BCL-2 and MCL-1. Cytokine. 1999;11:849–855. doi: 10.1006/cyto.1999.0514. [DOI] [PubMed] [Google Scholar]
- 59.Rinaudo MS, Su K, Falk LA, Halder S, Mufson RA. Human interleukin-3 receptor modulates bcl-2 mRNA and protein levels through protein kinase C in TF-1 cells. Blood. 1995;86:80–88. [PubMed] [Google Scholar]
- 60.Chao JR, Wang JM, Lee SF, Peng HW, Lin YH, Chou CH, Li JC, Huang HM, Chou CK, Kuo ML, Yen JJ, Yang-Yen HF. Mcl-1 is an immediate-early gene activated by the granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling pathway and is one component of the GM-CSF viability response. Mol Cell Biol. 1998;18:4883–4898. doi: 10.1128/mcb.18.8.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moulding DA, Quayle JA, Hart CA, Edwards SW. Mcl-1 expression in human neutrophils: regulation by cytokines and correlation with cell survival. Blood. 1998;92:2495–2502. [PubMed] [Google Scholar]
- 62.Derouet M, Thomas L, Cross A, Moots RJ, Edwards SW. Granulocyte macrophage colony-stimulating factor signaling and proteasome inhibition delay neutrophil apoptosis by increasing the stability of Mcl-1. J Biol Chem. 2004;279:26915–26921. doi: 10.1074/jbc.M313875200. [DOI] [PubMed] [Google Scholar]
- 63.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 64.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee EF, Czabotar PE, van Delft MF, Michalak EM, Boyle MJ, Willis SN, Puthalakath H, Bouillet P, Colman PM, Huang DC, Fairlie WD. A novel BH3 ligand that selectively targets Mcl-1 reveals that apoptosis can proceed without Mcl-1 degradation. J Cell Biol. 2008;180:341–355. doi: 10.1083/jcb.200708096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poommipanit PB, Chen B, Oltvai ZN. Interleukin-3 induces the phosphorylation of a distinct fraction of bcl-2. J Biol Chem. 1999;274:1033–1039. doi: 10.1074/jbc.274.2.1033. [DOI] [PubMed] [Google Scholar]
- 67.Ruvolo PP, Deng X, May WS. Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia. 2001;15:515–522. doi: 10.1038/sj.leu.2402197. [DOI] [PubMed] [Google Scholar]
- 68.Deng X, Ruvolo P, Carr B, May WS., Jr Survival function of ERK1/2 as IL-3-activated, staurosporine-resistant Bcl2 kinases. Proc Natl Acad Sci USA. 2000;97:1578–1583. doi: 10.1073/pnas.97.4.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuroda J, Puthalakath H, Cragg MS, Kelly PN, Bouillet P, Huang DC, Kimura S, Ottmann OG, Druker BJ, Villunger A, Roberts AW, Strasser A. Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proc Natl Acad Sci USA. 2006;103:14907–14912. doi: 10.1073/pnas.0606176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Erlacher M, Michalak EM, Kelly PN, Labi V, Niederegger H, Coultas L, Adams JM, Strasser A, Villunger A. BH3-only proteins Puma and Bim are rate-limiting for gamma-radiation- and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood. 2005;106:4131–4138. doi: 10.1182/blood-2005-04-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaufmann T, Jost PJ, Pellegrini M, Puthalakath H, Gugasyan R, Gerondakis S, Cretney E, Smyth MJ, Silke J, Hakem R, Bouillet P, Mak TW, Dixit VM, Strasser A. Fatal hepatitis mediated by tumor necrosis factor TNFalpha requires caspase-8 and involves the BH3-only proteins Bid and Bim. Immunity. 2009;30:56–66. doi: 10.1016/j.immuni.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 73.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 74.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/S0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 75.Zha J, Harada H, Osipov K, Jockel J, Waksman G, Korsmeyer SJ. BH3 domain of BAD is required for heterodimerization with BCL-XL and pro-apoptotic activity. J Biol Chem. 1997;272:24101–24104. doi: 10.1074/jbc.272.39.24101. [DOI] [PubMed] [Google Scholar]
- 76.Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci USA. 2004;101:6164–6169. doi: 10.1073/pnas.0401471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bosque A, Marzo I, Naval J, Anel A. Apoptosis by IL-2 deprivation in human CD8+ T cell blasts predominates over death receptor ligation, requires Bim expression and is associated with Mcl-1 loss. Mol Immunol. 2007;44:1446–1453. doi: 10.1016/j.molimm.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 78.You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, Villunger A, Mak TW. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203:1657–1663. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alfredsson J, Puthalakath H, Martin H, Strasser A, Nilsson G. Proapoptotic Bcl-2 family member Bim is involved in the control of mast cell survival and is induced together with Bcl-XL upon IgE-receptor activation. Cell Death Differ. 2005;12:136–144. doi: 10.1038/sj.cdd.4401537. [DOI] [PubMed] [Google Scholar]
- 80.Whitfield J, Neame SJ, Paquet L, Bernard O, Ham J. Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron. 2001;29:629–643. doi: 10.1016/S0896-6273(01)00239-2. [DOI] [PubMed] [Google Scholar]
- 81.Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–1204. doi: 10.1016/S0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 82.Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, Medema RH. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168:5024–5031. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- 83.Ekoff M, Kaufmann T, Engstrom M, Motoyama N, Villunger A, Jonsson JI, Strasser A, Nilsson G. The BH3-only protein Puma plays an essential role in cytokine deprivation induced apoptosis of mast cells. Blood. 2007;110:3209–3217. doi: 10.1182/blood-2007-02-073957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Puthalakath H, Huang DC, O’Reilly LA, King SM, Strasser A. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999;3:287–296. doi: 10.1016/S1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- 85.Qi XJ, Wildey GM, Howe PH. Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. J Biol Chem. 2006;281:813–823. doi: 10.1074/jbc.M505546200. [DOI] [PubMed] [Google Scholar]
- 86.Hubner A, Barrett T, Flavell RA, Davis RJ. Multisite phosphorylation regulates Bim stability and apoptotic activity. Mol Cell. 2008;30:415–425. doi: 10.1016/j.molcel.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, MacLean KH, Han J, Chittenden T, Ihle JN, McKinnon PJ, Cleveland JL, Zambetti GP. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–328. doi: 10.1016/S1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 88.Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, Adams JM, Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 89.Zhao Y, Coloff JL, Ferguson EC, Jacobs SR, Cui K, Rathmell JC. Glucose metabolism attenuates p53 and Puma-dependent cell death upon growth factor deprivation. J Biol Chem. 2008;283:36344–36353. doi: 10.1074/jbc.M803580200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jabbour AM, Heraud JE, Daunt CP, Kaufmann T, Sandow J, O’Reilly LA, Callus BA, Lopez A, Strasser A, Vaux DL, Ekert PG. Puma indirectly activates Bax to cause apoptosis in the absence of Bid or Bim. Cell Death Differ. 2009;16:555–563. doi: 10.1038/cdd.2008.179. [DOI] [PubMed] [Google Scholar]
- 91.Lotem J, Sachs L. Cytokine suppression of protease activation in wild-type p53-dependent and p53-independent apoptosis. Proc Natl Acad Sci USA. 1997;94:9349–9353. doi: 10.1073/pnas.94.17.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/S1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 93.Zhao Y, Wagner F, Frank SJ, Kraft AS. The amino-terminal portion of the JAK2 protein kinase is necessary for binding and phosphorylation of the granulocyte-macrophage colony-stimulating factor receptor beta c chain. J Biol Chem. 1995;270:13814–13818. doi: 10.1074/jbc.270.23.13814. [DOI] [PubMed] [Google Scholar]
- 94.Watanabe S, Itoh T, Arai K. JAK2 is essential for activation of c-fos and c-myc promoters and cell proliferation through the human granulocyte-macrophage colony-stimulating factor receptor in BA/F3 cells. J Biol Chem. 1996;271:12681–12686. doi: 10.1074/jbc.271.21.12681. [DOI] [PubMed] [Google Scholar]
- 95.Jenkins BJ, Blake TJ, Gonda TJ. Saturation mutagenesis of the beta subunit of the human granulocyte-macrophage colony-stimulating factor receptor shows clustering of constitutive mutations, activation of ERK MAP kinase and STAT pathways, and differential beta subunit tyrosine phosphorylation. Blood. 1998;92:1989–2002. [PubMed] [Google Scholar]
- 96.Inhorn RC, Carlesso N, Durstin M, Frank DA, Griffin JD. Identification of a viability domain in the granulocyte/macrophage colony-stimulating factor receptor beta-chain involving tyrosine-750. Proc Natl Acad Sci USA. 1995;92:8665–8669. doi: 10.1073/pnas.92.19.8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sakamaki K, Miyajima I, Kitamura T, Miyajima A. Critical cytoplasmic domains of the common beta subunit of the human GM-CSF, IL-3 and IL-5 receptors for growth signal transduction and tyrosine phosphorylation. EMBO J. 1992;11:3541–3549. doi: 10.1002/j.1460-2075.1992.tb05437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Polotskaya A, Zhao Y, Lilly MB, Kraft AS. Mapping the intracytoplasmic regions of the alpha granulocyte-macrophage colony-stimulating factor receptor necessary for cell growth regulation. J Biol Chem. 1994;269:14607–14613. [PubMed] [Google Scholar]
- 99.Brown AL, Peters M, D’Andrea RJ, Gonda TJ. Constitutive mutants of the GM-CSF receptor reveal multiple pathways leading to myeloid cell survival, proliferation, and granulocyte-macrophage differentiation. Blood. 2004;103:507–516. doi: 10.1182/blood-2003-05-1435. [DOI] [PubMed] [Google Scholar]
- 100.Itoh T, Liu R, Yokota T, Arai KI, Watanabe S. Definition of the role of tyrosine residues of the common beta subunit regulating multiple signaling pathways of granulocyte-macrophage colony-stimulating factor receptor. Mol Cell Biol. 1998;18:742–752. doi: 10.1128/mcb.18.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kieslinger M, Woldman I, Moriggl R, Hofmann J, Marine JC, Ihle JN, Beug H, Decker T. Antiapoptotic activity of Stat5 required during terminal stages of myeloid differentiation. Genes Dev. 2000;14:232–244. [PMC free article] [PubMed] [Google Scholar]
- 102.Mui AL, Wakao H, Kinoshita T, Kitamura T, Miyajima A. Suppression of interleukin-3-induced gene expression by a C-terminal truncated Stat5, role of Stat5 in proliferation. EMBO J. 1996;15:2425–2433. [PMC free article] [PubMed] [Google Scholar]
- 103.Dumon S, Santos SC, Debierre-Grockiego F, Gouilleux-Gruart V, Cocault L, Boucheron C, Mollat P, Gisselbrecht S, Gouilleux F. IL-3 dependent regulation of Bcl-xL gene expression by STAT5 in a bone marrow derived cell line. Oncogene. 1999;18:4191–4199. doi: 10.1038/sj.onc.1202796. [DOI] [PubMed] [Google Scholar]
- 104.Stout BA, Bates ME, Liu LY, Farrington NN, Bertics PJ. IL-5 and granulocyte-macrophage colony-stimulating factor activate STAT3 and STAT5 and promote Pim-1 and cyclin D3 protein expression in human eosinophils. J Immunol. 2004;173:6409–6417. doi: 10.4049/jimmunol.173.10.6409. [DOI] [PubMed] [Google Scholar]
- 105.Amson R, Sigaux F, Przedborski S, Flandrin G, Givol D, Telerman A. The human protooncogene product p33pim is expressed during fetal hematopoiesis and in diverse leukemias. Proc Natl Acad Sci USA. 1989;86:8857–8861. doi: 10.1073/pnas.86.22.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Amaravadi R, Thompson CB. The survival kinases Akt and Pim as potential pharmacological targets. J Clin Invest. 2005;115:2618–2624. doi: 10.1172/JCI26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fox CJ, Hammerman PS, Cinalli RM, Master SR, Chodosh LA, Thompson CB. The serine/threonine kinase Pim-2 is a transcriptionally regulated apoptotic inhibitor. Genes Dev. 2003;17:1841–1854. doi: 10.1101/gad.1105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yan B, Zemskova M, Holder S, Chin V, Kraft A, Koskinen PJ, Lilly M. The PIM-2 kinase phosphorylates BAD on serine 112 and reverses BAD-induced cell death. J Biol Chem. 2003;278:45358–45367. doi: 10.1074/jbc.M307933200. [DOI] [PubMed] [Google Scholar]
- 109.Hammerman PS, Fox CJ, Cinalli RM, Xu A, Wagner JD, Lindsten T, Thompson CB. Lymphocyte transformation by Pim-2 is dependent on nuclear factor-kappaB activation. Cancer Res. 2004;64:8341–8348. doi: 10.1158/0008-5472.CAN-04-2284. [DOI] [PubMed] [Google Scholar]
- 110.Banerjee A, Grumont R, Gugasyan R, White C, Strasser A, Gerondakis S. NF-kappaB1 and c-Rel cooperate to promote the survival of TLR4-activated B cells by neutralizing Bim via distinct mechanisms. Blood. 2008;112:5063–5073. doi: 10.1182/blood-2007-10-120832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aho TL, Sandholm J, Peltola KJ, Mankonen HP, Lilly M, Koskinen PJ. Pim-1 kinase promotes inactivation of the pro-apoptotic Bad protein by phosphorylating it on the Ser112 gatekeeper site. FEBS Lett. 2004;571:43–49. doi: 10.1016/j.febslet.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 112.Didichenko SA, Spiegl N, Brunner T, Dahinden CA. IL-3 induces a Pim1-dependent antiapoptotic pathway in primary human basophils. Blood. 2008;112:3949–3958. doi: 10.1182/blood-2008-04-149419. [DOI] [PubMed] [Google Scholar]
- 113.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 114.Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 115.Tamburini J, Elie C, Bardet V, Chapuis N, Park S, Broët P, Cornillet-Lefebvre P, Lioure B, Ugo V, Blanchet O, Ifrah N, Witz F, Dreyfus F, Mayeux P, Lacombe C, Bouscary D. Blood. 2007;110:1025–1028. doi: 10.1182/blood-2006-12-061283. [DOI] [PubMed] [Google Scholar]
- 116.Edinger AL, Thompson CB. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol Biol Cell. 2002;13:2276–2288. doi: 10.1091/mbc.01-12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rathmell JC, Fox CJ, Plas DR, Hammerman PS, Cinalli RM, Thompson CB. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol Cell Biol. 2003;23:7315–7328. doi: 10.1128/MCB.23.20.7315-7328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Terada K, Kaziro Y, Satoh T. Ras is not required for the interleukin 3-induced proliferation of a mouse pro-B cell line, BaF3. J Biol Chem. 1995;270:27880–27886. doi: 10.1074/jbc.270.46.27880. [DOI] [PubMed] [Google Scholar]
- 119.Perkins GR, Marshall CJ, Collins MK. The role of MAP kinase kinase in interleukin-3 stimulation of proliferation. Blood. 1996;87:3669–3675. [PubMed] [Google Scholar]
- 120.Ewings KE, Hadfield-Moorhouse K, Wiggins CM, Wickenden JA, Balmanno K, Gilley R, Degenhardt K, White E, Cook SJ. ERK1/2-dependent phosphorylation of BimEL promotes its rapid dissociation from Mcl-1 and Bcl-xL. EMBO J. 2007;26:2856–2867. doi: 10.1038/sj.emboj.7601723. [DOI] [PMC free article] [PubMed] [Google Scholar]