Abstract
It has been believed that the immunoglobulin (Ig) found abundantly in the colostrum of lactating mammalian is derived from serum or secreted by plasma cells present in the mammary gland. The recent finding of Ig gene rearrangements in breast cancer cells and benign hyperplastic breast epithelial cells suggests that it is likely that hyperplastic mammary gland epithelial cells during lactation can also produce Ig. In this study, we have demonstrated the presence of abundant amounts of Ig heavy and light chain transcripts in sorted cytokeratin 18-positive mammary gland epithelial cells of lactating mice. Interestingly, we found two specific Igκ variable region sequences (VCW9Jκ1 and VBV9Jκ1) that were dominantly expressed in different strains of mice. Our data demonstrate that IgG is expressed by mammary gland epithelial cells of lactating mice, and suggest that the IgG found in murine colostrum is at least partially produced by the mammary gland epithelial cells.
Keywords: Immunoglobulin, Colostrum, Mammary gland epithelial cells
Introduction
Mammary glands are modified sweat (sudoriferous) glands that secrete milk and serve as accessories to the reproductive system. In several mammals, including humans and mice, the mammary gland secretes abundant amounts of immunoglobulins (Igs) upon lactation, with the Ig fractions in colostrum and milk mainly belonging to the IgA and IgG classes [1, 2]. IgA and IgG in colostrum are important in protecting the newborns’ gastrointestinal tract against infections [3] and support the development and maturation of the infants’ own immune system [4].
It is well known that specific IgG and IgA antibodies present in the milk guard against pathogenic organisms that enter the body via mucosa, e.g., in the gut and the respiratory tract [5]. The current theories suggest that the IgG and IgA present in colostrum and milk are derived from circulating blood and/or produced by plasma cells present in the mammary gland. The Ig could be transferred into the colostrum and milk through the neonatal Fc receptors (FcRn), which play important roles in transporting IgG across epithelial cell barriers and protecting IgG from degradation [6–9], or the poly-Ig receptors, which play a major role in transporting IgA across epithelial cell barriers on the membranes of mammary gland epithelial cells [10].
Recently, Ig genes have been found to be expressed in almost all kinds of epithelial cancer cells, including breast cancer cells and actively proliferating mammary gland cells [11–14]. Furthermore, activation-induced cytidine deaminase (AID), a B cell-specific enzyme with DNA-editing activity that plays an important role in the Ig V(D)J gene somatic hypermutation and Ig heavy chain (IgH) class switching recombination (CSR) [15, 16], has been found to be expressed in breast epithelial cancer cell lines [13]. The mammary gland epithelial cells of lactating mice are likewise actively proliferating, raising an interesting question of whether IgG transcripts are also produced in the hyperplastic mammary gland epithelia of lactating mice.
In this study, we first demonstrated the existence of IgG in the epithelial cells of mammary glands from pregnant and lactating mice by immunohistochemistry. We then identified IgG1 mRNA by northern blot analysis and in situ hybridization in the mammary gland epithelial cells of lactating mice. To avoid contamination by other cells, in particularly B lymphocytes and plasma cells, we purified lactating mouse epithelial cells by flow cytometry cell sorting with antibodies against the luminal epithelial cell, cytokeratin 18 (CK18) [17], and confirmed the presence of CK18 transcripts, IgG1 heavy chain transcripts, and γ1 germline transcripts, as well as Igκ transcripts in the cDNA libraries of CK18-positive cells. However, transcripts of B cell markers (CD19 and CD20), common lymphocyte antigen (CD45), and plasma cell marker (CD138) were not detected. These data suggest that mammary gland epithelial cells can express IgG in the lactating state.
Materials and methods
Animals and tissue sources
All procedures were carried out in accordance with the guidelines of the People’s Republic of China Ministry of Health and approved by the Animal Care and Use Committee of Peking University Health Science Center. The BALB/c and C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA, USA). The Chinese Kung Ming (KM) mice, an outbreed strain widely used in pharmaceutical and genetic studies in China, were supplied by the Department of Laboratory Animal Science of Peking University Health Science Center. For immunohistochemistry and in situ hybridization, four virgin (8 weeks old), three pregnant (day 17 of pregnancy) and five lactating (day 1 of lactation) BALB/c mice were sacrificed by quick and humane cervical dislocation, and the mammary gland, lymph node, and spleen tissues were dissected and fixed in 10% formalin. For northern blot, the mammary gland, spleen, and kidney tissues of lactating BALB/c mice (day 1 of lactation) were dissected and stored at −80°C. For mammary gland epithelial cell sorting, we used mammary glands from three KM mice, three BALB/c mice, and three C57BL/6 mice. These mice were sacrificed on day 1 of lactation, and mammary gland tissues were dissected from the underarm area and excised free of lymph nodes, fat pads, and skin.
Immunohistochemical staining
The mammary gland, lymph node, and spleen tissues were fixed in 10% formalin, embedded in paraffin, and sectioned. After deparaffinization, antigen retrieval was performed twice in 0.01 M citrate buffer (pH 6.0) at 90°C for 5 min in a microwave oven. Endogenous peroxidase activity was blocked with 3% H2O2 in water for 10 min followed by a wash in phosphate-buffered saline (PBS). The sections were then immersed in 10% normal goat serum in PBS for 10 min, covered with primary biotinylated goat anti-mouse IgG (KPL, Gaithersburg, MD, USA) (1:100 dilutions in PBS), and incubated at 37°C for 60 min. This was followed by a wash with PBS and incubation with horseradish peroxidase (HRP)-conjugated streptavidin (Vector, Burlingame, CA, USA) at 37°C for 25 min. After washing with PBS, 3,3′-diaminobenzidine (DAB) was applied for 10–15 min, and the sections were examined microscopically. Sections treated without the primary antibody were used as negative controls.
Northern blot analysis
Total RNA was extracted from mouse mammary gland, spleen, and kidney tissues using TRIzol reagent (Gibco BRL, Grand Island, NY, USA) according to the manufacturer’s instructions. For each specimen, a total of 30 μg RNA was separated in 1% agarose/formaldehyde gel and transferred onto a nitrocellulose membrane (Hybond-C; Amersham, Buckinghamshire, UK). We used a [α]-32P-dCTP-labeled chain-specific cDNA probe, IgG1, a 381-bp fragment containing an upstream portion of the constant region of the IgG1 gene. The nitrocellulose membrane was prehybridized in a sealed bag at 42°C for 4–5 h in hybridization buffer (Gibco BRL), and hybridized in the same solution containing 106 dpm/mL denatured probes at 68°C for 18 h. The membrane was then washed in 2× SSC (1× SSC: 150 mM NaCl, 15 mM Na-citrate) at room temperature for 15 min, in 2× SSC containing 0.1% SDS at 50°C twice at 30 min each, and in 0.1× SSC at room temperature for 30 min. Autoradiography was performed by exposure to a Super Resolution phosphor screen (Packard Instrument, Downers Grove, IL, USA) for 1–3 h.
In situ hybridization
In situ hybridization was performed on 5-μm serial sections of paraffin-embedded tissue sections. We used the same specific cDNA probe for IgG1 as described for northern blot analysis. The probe was labeled with digoxigenin (DIG). Plasmids with inserted antisense and sense (negative control) probes for constant region cDNA fragments of the IgG1 were linearized and transcribed by T7 RNA polymerase (for the antisense probe) or SP6 RNA polymerase (for the sense probe) (Boehringer Mannheim, Mannheim, Germany) as described previously [11] to produce cRNA probes. In situ hybridization was performed as described previously [11]. Briefly, deparaffinized sections were treated with Proteinase K, fixed with paraformaldehyde, prehybridized at 42°C for 4 h, and hybridized with the DIG-labeled cRNA probe (2 μg/mL) at 45°C overnight. After hybridization, the sections were washed in 2× SSC and 0.2× SSC at 65°C, respectively, and then treated with RNase. The samples were incubated with alkaline phosphatase-conjugated antidigoxigenin antibody (1:500; Roche Diagnostics, Rotkreuz, Switzerland). 5-Bromo-4-chloro-3-indolyl phosphate and nitro-blue-tetrazolium (Sigma, Saint Louis, MO, USA) were used for the color reaction. Slides incubated with RNase or with the corresponding sense probe were used as controls.
Flow cytometry cell sorting of CK18+CD19− cells
Mammary glands were dissected from three BALB/c, three KM, and three C57BL/6 mice that were on day 1 of lactation. After mechanical homogenization, the tissue was placed in culture medium (DMEM with 1 mM glutamine) supplemented with 5% bovine calf serum, 300 U/mL collagenase (Sigma) and 100 U/mL hyaluronidase (Sigma), and digested at 37°C for 2 h. The suspension was subsequently resuspended in 0.25% trypsin-EGTA for 1–2 min, 0.1 mg/mL DNase (Worthington, OH, USA) for 5 min, and 0.64% NH4Cl for 3 min before filtration through a 40-mm mesh. The cells were then fixed in chilled acetone for 1 min, permeablized in 0.1% Tween/PBS on ice for 5 min, and treated with 2% bovine serum in PBS for 30 min to block non-specific binding. These were followed by incubation with antibody against CK18 (a mouse mammary epithelial cell marker; Santa Cruz, CA, USA) at 4°C for 30 min, and after washing with PBS, the cells were incubated with FITC-conjugated antibody against rabbit IgG (1:100) (Santa Cruz Biotechnology) and anti-mouse CD19-PE (a pan B cell marker; Becton Dickinson, San Jose, CA, USA) at 4°C for 30 min. After washing with PBS, cell sorting was carried out on a FACS Vantage cell sorter (Becton Dickinson). Cells that were positive for CK18 and negative for CD19-PE were collected. The purity and viability of sorted populations was generally greater than 95%. To confirm the IgG present in sorted CK18+ mouse mammary gland epithelial cells, the sorted CK18+CD19− cells were incubated with anti-mouse IgG-PE (1:100) (Santa Cruz Biotechnology). After washing with PBS, cells were analyzed by FACS.
Nested reverse transcription-polymerase chain reaction
Total RNA of the sorted CK18+CD19− mammary epithelial cells and unsorted mammary gland cells (input, as a control) were extracted using the RNeasy Micro kit (Qiangen, Hilden, Germany), and reverse transcribed and amplified using the ThermoscriptT reverse transcription-polymerase chain reaction (RT-PCR) System (Invitrogen, Grand Island, NY, USA) according to the manufacturer’s instructions. Nested PCR primers were used to amplify CK18 (a mammary epithelial cell marker), as well as CD19 and CD20 (two pan B cell markers). Half-nested PCR primers were used to amplify CD45 (leukocyte common antigen) and CD138 (plasma cell marker). We also performed half-nested PCR for the constant and variable regions of IgG1, the γ1 germline transcript, and the variable region of the Igκ chain, as well as for AID and recombination activating gene (RAG1/RAG2), the water, or the total RNA reverse transcribed without reverse transcriptase to replace the cDNA of CK18+CD19− cells as negative control. The sequences of the above primers are listed in Table 1. All the forward and reverse primers mentioned above were located in the exons, which makes it possible to distinguish cDNA and genomic DNA. The PCR products were separated on 2% agarose gel by electrophoresis, purified, and ligated into the pGEM-T Easy vector (Promega, Madison, WI, USA), and the sequences of the PCR products confirmed by DNA sequencing (ABI 3730; Foster City, CA, USA).
Table 1.
The sequences of PCR primers used in this study
| Gene name | Primer sequence 5′-3′ | Product size, bp | |
|---|---|---|---|
| CK18 | External senses primer | TGCCGCCGATGACTTTAG | 454 |
| External antisense primer | CCTCTGCCCGAGTTTGTG | ||
| Internal sense primer | TCCGCAAGGTGGTAGATGAC | ||
| Internal antisense primer | CTCCATCTGTGCTTGTATCG | ||
| CD19 | External senses primer | AAGGAAGCGAATGACTGACCC | 344 |
| External antisense primer | GGAAGTCCATCATCCTGCCA | ||
| Internal sense primer | GGAAGCGAATGACTGACC | ||
| Internal antisense primer | GGGGTTCTCATAGCCACTC | ||
| CD20 | The same sense primer | TTCAAACTTCCAAGCCGTATG | 241 |
| External antisense primer | GAGTTTAAGGAGCGATCTC | ||
| Internal antisense primer | GACAGCAGAACCACATTAGAT | ||
| CD45 | The same sense primer | GCACACCAAAAGAAAAGGCTAATA | 277 |
| External antisense primer | CCATTGACATAGGCAAGTAGG | ||
| Internal antisense primer | GGAATCCCCAAATCTGCTGC | ||
| CD138 | External senses primer | ACCAGCAGCAACACCGAGAC | 606 |
| Internal sense primer | TTCACTGCTCGGGACAAGG | ||
| The same antisense primer | TCCCCATCAGGCGTAGAAC | ||
| IGHG1 constant | External senses primer | CCTGGTCAAGGGCTATTTCC | 381 |
| Internal sense primer | TCTACACTCTGAGCAGCTCAG | ||
| The same antisense primer | GGTGCATGATGGGAAGTTCAC | ||
| IGHG1 variable | External senses primer | AGGTCCAGCTQCTCGAGTCWGG (Q = G/T; W = A/T) | 394 |
| Internal sense primer | CGGTACCAAGAASAMCCTGTWCCTGCAAATGASC (S = C/G; M = A/C; W = A/T) | ||
| The same antisense primer | TCTTGTCCACCTTGGTGCTGCTG | ||
| IGHG1 germline transcript | External senses primer | AGCTGCAAGAAGAGGCCAT | 429 |
| Internal sense primer | GGCCCTTCCAGATCTTTGAG | ||
| The same antisense primer | GGATCCAGAGTTCCAGGTCACT | ||
| Igκ variable | The same sense primer | GACATTCAGCTGACCCAGTCTCCA | 311 |
| External antisense primer | GTCCTGATCAGTCCAACTGTTCA | ||
| Internal antisense primer | AGNTTGGTTCCACCRCCGAACG (N = C/T; R = T/C) | ||
| AID | The same sense primer | AGGGAGTCAAGAAAGTCACGC | 347 |
| External antisense primer | GCCTTGCGGTCTTCACAG | ||
| Internal antisense primer | GCTGAGGTTAGGGTTCCATC | ||
| RAG1 | The same sense primer | CCAAGCTGCAGACATTCTAGCACTC | 379 |
| External antisense primer | CTGGATCCGGAAAATCCTGGCAATG | ||
| Internal antisense primer | GCCTTGGCTTGGTGAACTGCTT | ||
| RAG2 | The same sense primer | CACATCCACAAGCAGGAAGTACAC | 330 |
| External antisense primer | GGTTCAGGGACATCTCCTACTAAG | ||
| Internal antisense primer | GCTTGTCAGAGTCTATGCTGCCTTTGT | ||
Results
IgG exists in the mammary gland epithelium of pregnant and lactating BALB/c mice
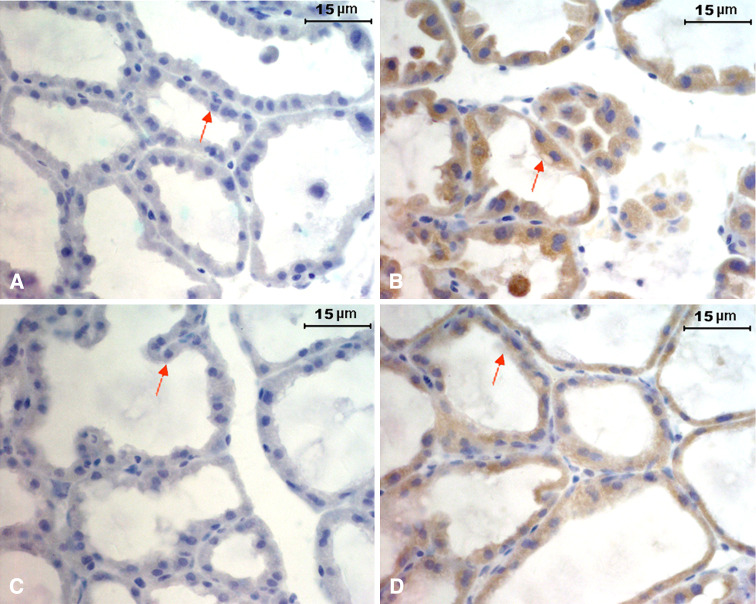
We performed immunohistochemical studies for IgG on sections of mammary glands from virgin (n = 4), pregnant (n = 3), and lactating mice (n = 5). No positive staining for IgG was found in the mammary gland epithelium of the virgin mice (data not shown). However, significant IgG immunoreactivity was demonstrated in most of the mammary gland epithelial cells and the milk itself of pregnant (day 17) and lactating (day 1) mice (Fig. 1b, d). A few stromal plasma cells and B lymphocytes were also found in the mammary gland tissues during lactation (data not shown). No immunoreactivity to the isotype control of anti-mouse IgG was observed (Fig. 1a, c).
Fig. 1.
Expression of IgG in mammary gland epithelial cells. Immunohistochemical analysis using biotin-conjugated goat anti-mouse IgG and HRP-conjugated streptavidin showing positive staining in mammary gland epithelium of pregnant mice (day 17, b) and lactating mice (day 1, d). No staining in mammary gland epithelium of pregnant (a) and lactating mice (c) when using the goat IgG as isotype control. Scale bar 15 μm
Transcripts of IgG1 were found in mammary gland tissues of lactating BALB/c mice by northern blot and in situ hybridization
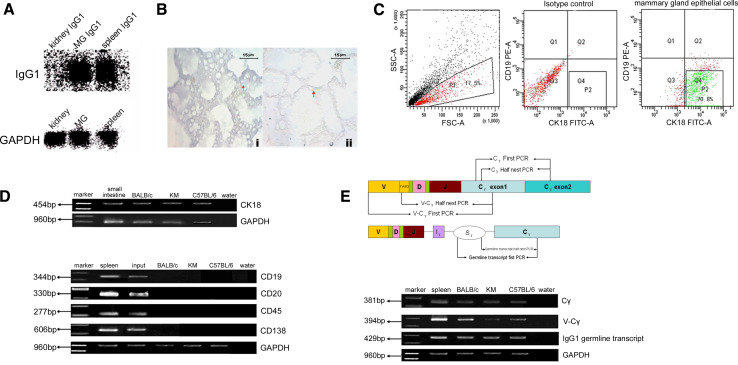
To discover whether IgG is expressed in the BALB/c mammary gland epithelium of lactating mice, we performed northern blot analysis using cDNA probe to IgG1 to detect whether IgG1 transcripts were present in the mammary gland. The lactating mouse mammary gland, spleen, and kidney tissues were immediately obtained after the mice were sacrificed, and total RNA was extracted. Significantly levels of IgG1 mRNA were detected in the mammary gland and spleen (but not the kidney) tissues of lactating mice (Fig. 2a).
Fig. 2.
Expression of IgG1 heavy chain in mammary gland epithelial cells. a Northern blot analysis. Total RNA from lactating mammary gland, spleen (positive control), and kidney (negative control) were electrophoresed, transferred to nylon membrane, and then probed with a probe to IgG1 heavy chain constant region. The blot was striped and rehybridized with a GAPDH probe (MG mammary gland tissue). b In situ hybridization analysis using antisense (i) or sense (ii) cRNA for heavy chain constant region of IgG1 as a probe. Scale bar 15 μm. c Flow cytometry sorting of the lactating mammary gland epithelial cells by antibodies against CK18 and CD19. The background fluorescence was determined using cells incubated with secondary antibody only (without primary antibody). Cells which were positive for CK18 but negative for CD19 as shown in gate P2 were selected for further analysis. d RT-PCR analysis showed expression of CK18 and lack of expression of CD19, CD20, CD45, and CD138 in the sorted CK18+CD19− cells. Input Cells from mammary gland of lactating mouse without sorting. The small intestine cDNA library was used as a positive control for CK18. The spleen cDNA library was also used as a positive control for CD19, CD20, CD45, and CD138. e PCR analysis showed the expression of IgG1 heavy chain constant and variable regions and IgG1 germline transcripts. V-Cγ The variable region and constant regions of IgG1 heavy chain; Cγ the constant region of IgG1 heavy chain
In addition, we performed in situ hybridization analysis using DIG-labeled antisense cRNA of IgG1 constant region as a probe and demonstrated that IgG1 gene transcripts were expressed in mammary gland epithelial cells of lactating mice (Fig. 2b). No specific signal was revealed in the absence of the probe, or when the sections were treated with the sense probe (Fig. 2b).
Pure mammary epithelial cells were obtained from lactating mice by flow cytometry sorting
The mammary gland tissues of day 1 lactating mice were made into single-cell suspensions. To exclude contamination by other cells, in particularly B cells and plasma cells, we performed flow cytometry cell sorting using an epithelial cell marker (CK18) and a pan B cell marker (CD19). We identified 2–5 × 105 CK18+CD19− cells in each specimen (Fig. 2c). The total RNA of CK18+CD19− cells were extracted and reverse transcribed. Using the cDNA as a template, we proved that there was no contamination of the sorted CK18+CD19− cells, but not input, by B cells or plasma cells by the lack of expression of B cell markers (CD19 and CD20), leukocyte common antigen (CD45), and a plasma marker (CD138) (Fig. 2d).
IgG1 heavy chain transcripts were detected in sorted CK18+CD19− mammary epithelial cells of lactating BALB/c, KM, and C57BL/6 mice by RT-PCR
We performed RT-PCR using specific primers for the IgG1 variable region of VH7183–CH1, the IgG1 constant region of CH1–CH2, and the IgG1 germline transcript, respectively. We demonstrated the presence of transcripts of the IgG1 variable and constant regions and IgG1 germline sequence in the CK18+CD19− cell cDNA libraries of lactating BALB/c, KM, and C57BL/6 mice (Fig. 2e), but not in the control using the total RNA reverse transcribed without reverse transcriptase as template (electronic supplementary material, ESM, Fig. 2). The identification of the PCR products was further confirmed by DNA sequencing.
Gene expression of the Igκ variable region in sorted CK18+CD19− mammary epithelial cells in lactating BALB/c, KM, and C57BL/6 mice showed unique transcript sequence
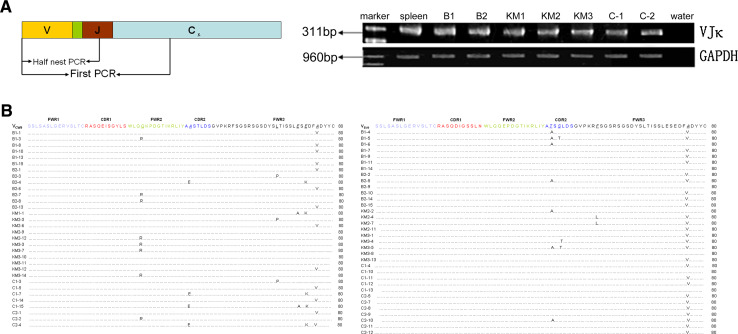
Using specific primers for the Igκ variable region, we detected the presence of the Igκ transcripts in the CK18+CD19− cell cDNA libraries of lactating BALB/c, KM, and C57BL/6 mice (Fig. 3a). We cloned 30 VκJκ recombinants from two BALB/c mice, 29 VκJκ recombinants from three KM mice, and 29 VκJκ recombinants from two C57BL/6 mice (Table 2). By DNA sequencing and alignment, we found that 13 of 30 VκJκ recombinants in BALB/c mice, 11 of 29 VκJκ recombinants in KM mice, and 8 of 29 VκJκ recombinants in C57BL/6 mice originated from the VCW9Jκ1 germline sequences with some mutations (ESM Fig. 3A). Additionally, 13 of 30 VκJκ recombinants in BALB/c mice, 9 of 29 VκJκ recombinants in KM mice, and 12 of 29 VκJκ recombinants in C57BL/6 mice originated from the VBV9Jκ1 germline sequences with some mutations (ESM Fig. 3B). To exclude the possibility that the primers used in this study were limited to amplify only VCW9Jκ1 or VBV9Jκ1 sequences, we used two spleen cDNA libraries from the BALB/c mice (the positive control) as the template and completely sequenced 15 VκJκ recombinants. A distinct diversity was shown in spleen cDNA libraries (Table 2). Interestingly, the VCW9Jκ1 recombinants derived from different strains not only showed identical VκJκ usage, but also showed identical mutation targets, as did the VBV9Jκ1 recombinants derived from different strains. In spite of certain mutation points, there remained a high rate of homology within VCW9 and VBV9 recombinants. These two kinds of recombinants and the amino acid sequences they encoded in BALB/c mice, KM mice, and C57BL/6 mice had nearly the same germline sequence as VCW9 or VBV9 (Fig. 3b, c).
Fig. 3.
Expression of Igκ transcripts in CK18+CD19− mammary gland epithelial cells. a RT-PCR showed the expression of Igκ in CK18+CD19− mammary gland epithelial cells. B BALB/c mice; KM KM mice; C C57BL/6 mice. The spleen cDNA library was used as a positive control. b All Igκ VCW9 amino acid sequence mutation points are shown compared with Igκ VCW9 germline amino acid sequences. c All Igκ VBV9 amino acid sequence mutation points are shown compared with Igκ VBV9 germline amino acid sequences
Table 2.
The sequences of Igκ transcripts detected in the CK18+CD19− cell cDNA libraries of lactating BALB/c, KM, and C57BL/6 mice
| Mouse no. | Clone no. | BALb/c ep | Mouse no. | Clone no. | KM ep | Mouse no. | Clone no. | C57 ep | Mouse no. | Clone no. | Spleen |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | B1-1 | VCW9Jκ1 | 1 | KM1-1 | VCW9Jκ1 | 1 | C1-1 | V 21-10 J κ1 | 1 | 1 | VBV9Jκ2 |
| B1-2 | V BW20 J κ5 | 2 | KM2-1 | V 12-44 J κ2 | C1-2 | V 21-5 J κ1 | 2 | V21-5Jκ5 | |||
| B1-3 | VCW9Jκ1 | KM2-2 | VBV9Jκ1 | C1-3 | VCW9Jκ1 | 3 | V21-10Jκ1 | ||||
| B1-4 | VBV9Jκ1 | KM2-3 | VCW9Jκ1 | C1-4 | VBV9Jκ1 | 4 | VBA9Jκ1 | ||||
| B1-5 | VBV9Jκ1 | KM2-4 | VBV9Jκ1 | C1-5 | VCW9Jκ1 | 5 | V12-44Jκ2 | ||||
| B1-6 | VBV9Jκ1 | KM2-5 | V 21-9 J κ1 | C1-6 | V 21-10 J κ1 | 6 | V4-57JL1 | ||||
| B1-7 | VBV9Jκ1 | KM2-6 | VCW9Jκ1 | C1-7 | VCW9Jκ1 | 7 | VCI12Jκ2 | ||||
| B1-8 | VCW9Jκ1 | KM2-7 | VBV9Jκ1 | C1-8 | V 21-10 J κ1 | 8 | VBR9JL1 | ||||
| B1-9 | VBV9Jκ1 | KM2-8 | V 21-10 J κ1 | C1-9 | V 12-44 J κ2 | 9 | V12-41Jκ5 | ||||
| B1-10 | VCW9Jκ1 | KM2-9 | VCW9Jκ1 | C1-10 | VBV9Jκ1 | 2 | 1 | Vfl12Jκ1 | |||
| B1-11 | VBV9Jκ1 | KM2-10 | V 21-9 J κ1 | C1-11 | VBV9Jκ1 | 2 | V21-9Jκ1 | ||||
| B1-12 | V 21-10 J κ1 | KM2-11 | VBV9Jκ1 | C1-12 | VBV9Jκ1 | 3 | V12-38JL1 | ||||
| B1-13 | VCW9Jκ1 | KM2-12 | VCW9Jκ1 | C1-13 | VBV9Jκ1 | 4 | V12-44Jκ2 | ||||
| B1-14 | VBV9Jκ1 | KM2-13 | V 21-10 J κ1 | C1-14 | VCW9Jκ1 | 5 | Vfr12Jκ1 | ||||
| B1-15 | VCW9Jκ1 | C1-15 | VCW9Jκ1 | 6 | Vcl9Jκ5 | ||||||
| 2 | B2-1 | VCW9Jκ1 | 3 | KM3-1 | VBV9Jκ1 | 2 | C2-1 | VCW9Jκ1 | |||
| B2-2 | VBV9Jκ1 | KM3-2 | V BW20 J κ5 | C2-2 | VCW9Jκ1 | ||||||
| B2-3 | VCW9Jκ1 | KM3-3 | VCW9Jκ1 | C2-3 | V 21-10 J κ1 | ||||||
| B2-4 | VCW9Jκ1 | KM3-4 | VBV9Jκ1 | C2-4 | VCW9Jκ1 | ||||||
| B2-5 | VCW9Jκ1 | KM3-5 | VBV9Jκ1 | C2-5 | VBV9Jκ1 | ||||||
| B2-6 | VCW9Jκ1 | KM3-6 | V 21-10 J κ1 | C2-6 | V BW20 J κ5 | ||||||
| B2-7 | VCW9Jκ1 | KM3-7 | VCW9Jκ1 | C2-7 | VBV9Jκ1 | ||||||
| B2-8 | VCW9Jκ1 | KM3-8 | VBV9Jκ1 | C2-8 | VBV9Jκ1 | ||||||
| B2-9 | VBV9Jκ1 | KM3-9 | V 21-10 J κ1 | C2-9 | VBV9Jκ1 | ||||||
| B2-10 | VBV9Jκ1 | KM3-10 | VCW9Jκ1 | C2-10 | VBV9Jκ1 | ||||||
| B2-11 | V 21-5 J κ5 | KM3-11 | VCW9Jκ1 | C2-11 | VBV9Jκ1 | ||||||
| B2-12 | V 12-44 J κ5 | KM3-12 | VCW9Jκ1 | C2-12 | VBV9Jκ1 | ||||||
| B2-13 | VCW9Jκ1 | KM3-13 | VBV9Jκ1 | C2-13 | V 21-10 J κ1 | ||||||
| B2-14 | VBV9Jκ1 | KM3-14 | VCW9Jκ1 | C2-14 | V 21-10 J κ1 | ||||||
| B2-15 | VBV9Jκ1 | KM3-15 | V fl12 J κ1 | ||||||||
BALB/c ep The sequences of Igκ in BALB/c mice CK18+CD19− mammary gland epithelial cells; KM ep the sequences of Igκ in KM mice CK18+CD19− mammary gland epithelial cells; C57 ep the sequences of Igκ in C57BL/6 mice CK18+CD19− mammary gland epithelial cells; Spleen the sequences of Igκ in BALB/c spleen cDNA library (positive control). The sequences not belonging to the VCW9 or VBV9 sequence are in bold
Expression of AID but not RAG1/2 was detected in mammary epithelial cells during lactation in BALB/c, KM and C57BL/6 mice
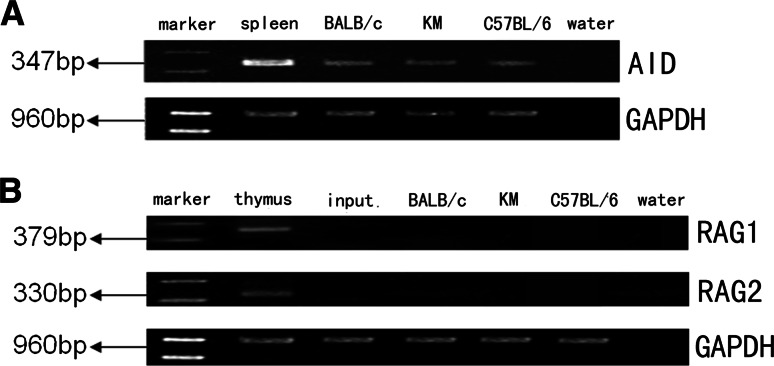
To examine the expression of AID, RAG1, and RAG2, we performed nested RT-PCR to amplify the conserved active site of AID, as well as RAG1 and RAG2. We found that AID was expressed in the mammary epithelial cells during lactation (Fig. 4a). However, expression of RAG1 and RAG2 was not detected (Fig. 4b).
Fig. 4.
Expressions of AID, RAG1, and RAG2 in CK18+CD19− mammary gland epithelial cells. a AID. Spleen cDNA library was used as a positive control. b RAG1/RAG2. Thymus cDNA library was used as a positive control. Input Cells from mammary gland of lactating mouse without sorting
Discussion
It has been believed that the IgG in colostrum and milk is derived from circulating blood or produced by local Ig-producing plasma cells. The IgG in mammary gland epithelial cells was thought to be transferred into colostrum and milk from the circulating blood through the FcRn found on the membrane of mammary gland epithelial cells [6–9]. However, whether FcRn mediates the transfer of IgG from plasma to milk still remains speculative. Lu et al. [18] recently generated transgenic mice that overexpressed the bovine FcRn in their lactating mammary glands. They detected significantly increased IgG in the sera and milk from these transgenic mice. However, they found no selective accumulation of endogenous mouse IgG or injected bovine IgG in the milk of the transgenic females. Thus, it is not certain whether FcRn mediates the transfer of IgG from plasma to milk.
Recently, Ig genes have been found to be expressed in a variety of epithelial tumor cells including breast cancer cells [13, 14, 16] as well as normal neuron and germ cells [17, 19]. We hypothesized that the mammary gland proliferating epithelial cells in the lactating state could produce IgG. In this study, we tested whether rearrangement and transcription of the Ig gene also occur in the mammary gland epithelial cells of lactating mice.
We first showed that IgG is present in the mammary gland epithelial cells of pregnant and lactating but not virgin mice by immunohistochemical analysis, suggesting that the proliferating mammary gland cells can produce IgG. We also found IgG immunoreactivity in a few B cells and plasma cells in mammary gland tissues as predicted. We then detected expression of rearranged Ig gene transcript in murine epithelial cells during lactation. Using IgG1 as a probe for northern blot analysis, we unexpectedly found abundant IgG1 transcripts in the mammary gland of lactating BALB/c mice, the quantity nearly equal to that seen in the spleen which served as a positive control. This result suggested that the abundant IgG1 transcripts were unlikely originated from the few B cells or plasma cells present in the mammary glands. Furthermore, using IgG1 probe for in situ hybridization, we found that the IgG1 transcripts were localized in the epithelial cells of lactating BALB/c mice.
However, a pivotal point in this study is how to avoid contamination by B cells and/or plasma cells which could infiltrate into mammary gland tissues and are mainly located in the stroma. We performed flow cytometry cell sorting to select CK18+CD19− epithelial cells to exclude the mammary tissue-infiltrating B lymphocytes and plasma cells using both antibodies for an epithelial cell marker (CK18), and a pan B cell marker (CD19). After confirming no contaminating B lymphocytes or plasma cells in the CK18+CD19− mammary epithelial cells by nested RT-PCR using primers for leukocyte common antigen (CD45), pan B cell marker (CD19) and plasma cell marker (CD138), we detected the presence of IgG1 constant region transcript, rearranged VHDJH-CHγ1 transcript, and germline transcript, as well as rearranged Igκ variable region transcript in sorted CK18+CD19− mammary gland epithelial cells by RT-PCR. Furthermore, to determine the sensitivity of our nested RT-PCR system for B cells, we harvested B cells from the spleens of BALB/c mice using anti-CD19 coupling magnetic beads (Dynal Biotech/Invitrogen, Carlsbad, CA, USA), which have a purity of more than 95% according to the manufacture’s guidelines. We then performed serial dilution by mixing cDNA libraries of 1,000, 96, 48, 24, 12, or 6 B cells into 3 × 105 EMT6 cells (mouse breast cancer cell line; gift from Dr. Ning Fu, Southern University, China). The demonstration of CD19, CD20, and CD45 amplification in each of the above dilutions suggested that our nested RT-PCR system was highly sensitive (ESM Fig. 4), and that it was very unlikely for contamination of the sorted CK18+CD19− epithelial cells by B cells or plasma cells. More importantly, none of the CK18+CD19− epithelial-cell–derived VκJκ sequences was homologous to the VκJκ recombinants from the normal spleen. These overall data support our hypothesis that transcripts of IgG1 and Igκ are derived from the mammary gland epithelial cells rather than contaminating B cells or plasma cells.
To analyze the Ig repertoire of the Igκ variable region in the lactating mammary epithelial cells, we used a pair of primers suitable for all Vκ genes. Surprisingly, we identified that restricted VκJκ recombinants, in particularly VCW9Jκ1 and VBV9Jκ1, were preferentially selected and expressed in the mammary epithelial cells. Furthermore, the VCW9Jκ1 and the VBV9Jκ1 derived from different individuals, even different strains of mice, not only had the same usage of VκJκ but also had similar mutation targets in the VCW9 and the VBV9 genes. In contrast, using the same primers, the spleen-derived VκJκ recombinants displayed a distinct diversity of VκJκ recombinants. These data suggest that the Ig repertoire in lactating epithelial cells has a distinct usage and plays an unknown function in different individuals and strains of mice.
Activation-induced cytidine deaminase (AID) plays an important role in somatic hypermutation and class switching recombination of B lymphocyte-derived Ig [21]. In this study, we have detected the expression of AID in mammary epithelial cells, suggesting that the AID may also be involved in somatic hypermutation and class switching recombination of mammary gland epithelial cells-derived Ig. However, our initial results showed only few mutation points in the V region of mammary gland epithelial cells-derived VκJκ (Fig. 3b), which does not belong to the somatic hypermutation. The results suggested that AID is not closely related to mammary gland epithelial cells-derived Ig, at least the VκJκ, somatic hypermutation. Additionally, RAG1/RAG2, which play an important role in driving genomic V(D)J rearrangements [20], were not detected in the mammary epithelial cells of lactating mice, suggesting two possibilities: the RAG1/RAG2 was temporarily expressed for V(D)J rearrangement in the mammary epithelial cells before lactation, or the RAG1/RAG2 was not involved in IgG production in mammary gland epithelial cells, unlike in B cells [22].
Our results did not exclude the current hypothesis that mammary gland epithelial cells could transfer IgG into colostrum and milk through the FcRn [6–8]. However, the results in this study suggest that the IgG in colostrum could also be derived from mammary gland epithelial cells themselves. It is possible that, in the special lactating physiological state, the IgG derived from the epithelial cells might cooperate with the IgG derived from the plasma to support the development and maturation of the infant’s own immune system via the milk to protect the newborn’s gastrointestinal tract against infections [10, 11]. It is also likely that the IgG derived from the epithelial cells, with its distinct Ig repertoire, could support the proliferation of lactating epithelial cells themselves.
In summary, we confirmed that functional transcripts of IgG1 and Igκ are expressed in the mammary gland epithelial cells of lactating mice. The preferentially selected expression of certain VκJκ recombinants and the similar mutation targets in the Vκ genes in mammary epithelial cells from different individuals and different strains suggest that the Ig may play an unknown important function in innate immunity or supporting the proliferation of lactating epithelial cells.
Electronic supplementary material
The files are unfortunately not in the Publisher's archive anymore.
SUPPLEMENTARY Fig. 1. IgG is present in sorted CK18+CD19− mouse mammary gland epithelial cells. The mouse mammary gland epithelial cells were first incubated with antibody against CK18, and after washing with PBS, the cells were incubated with FITC-conjugated antibody against rabbit IgG and PE-conjugated anti-mouse CD19. After washing with PBS, cell sorting was carried out on a FACS Vantage cell sorter. The cells that are positive for CK18 and negative for CD19-PE were collected. To confirm if IgG present in sorted CK18+ mouse mammary gland epithelial cells, the sorted CK18+CD19− cells were incubated with PE-conjugated anti-mouse IgG. The FACs analysis proved that the CK18+ cells indeed contained the IgG. (A) Forward scatter (FSC) versus side scatter (SSC). (B) The isotype control. (C) The CK18+ cells in R2 were harvested. (D) The CK18+ cells were stained with PE-conjugated anti-mouse IgG. Supplementary material 1 (18_2009_231_MOESM1_ESM.rar 649 kb)
SUPPLEMENTARY Fig. 2. The rearranged Ig gene variable, constant regions, especially the γ1 germline transcript amplified by RT-PCR in mouse mammary epithelial cells of lactating mouse are derived from Ig transcripts, but not from genomic Ig gene or re-integrated pseudogenes. Total RNA of the sorted CK18+CD19− mammary epithelial cells was extracted using the RNeasy Micro kit, reverse transcribed and amplified using a half-nested PCR for the constant (Cγ) and variable (V-Cγ) regions of IgG1, the γ1 germline transcript, and the variable region of the Igκ chain. The spleen cDNA library was used as a positive control for these genes. Total RNA reverse transcribed without reverse transcriptase to replace the cDNA of CK18+CD19− cells as a negative control. Supplementary material 2 (18_2009_231_MOESM2_ESM.rar 602 kb)
SUPPLEMENTARY Fig. 3. The mutation points of Igκ. (A) All Igκ VCW9 sequence mutation points are shown in comparison with Igκ VCW9 germline gene. (B) All Igκ VBV9 sequence mutation points are shown in comparison with Igκ VBV9 germline gene. Supplementary material 3 (18_2009_231_MOESM3_ESM.rar 317 kb)
SUPPLEMENTARY Fig. 4. The sensitivity of nested RT-PCR system. (A) Expressions of CD19, CD20 and CD45 were detected in the presence of 1000, 96, 48, 24, 12, and 6 B cells. (B) Expressions of CD19, CD20 and CD45 were detected in the presence of 3×105 EMT6 cells mixed with 1000, 96, 48, 24, 12, and 6 B cells. Supplementary material 4 (18_2009_231_MOESM4_ESM.rar 665 kb)
Acknowledgments
We thank Drs. Dalong Ma and Wenling Han (Peking University Center for Human Disease Genomics); Erdan Dong (The Natural Science Foundation of China) and Ning Fu (Department of Immunology, Southern Medical University) for their help. This work was supported by Fundamental Research grants 30572094 and 30772470 from the Natural Sciences Foundation, China.
Footnotes
S. Zhang, Y. Mao and J. Huang contributed equally to this work.
References
- 1.van der Feltz MJ, de Groot N, Bayley JP, Lee SH, Verbeet MP, de Boer HA. Lymphocyte homing and Ig secretion in the murine mammary gland. Scand J Immunol. 2001;54:292–300. doi: 10.1046/j.1365-3083.2001.00933.x. [DOI] [PubMed] [Google Scholar]
- 2.Gapper LW, Copestake DEJ, Otter DE, Indyk HE. Analysis of bovine immunoglobulin G in milk, colostrum and dietary supplements: a review. Anal Bioanal Chem. 2007;389:93–109. doi: 10.1007/s00216-007-1391-z. [DOI] [PubMed] [Google Scholar]
- 3.Løland BF, Baerug AB, Nylander G. Human milk, immune responses and health effects. Tidsskr Nor Laegeforen. 2007;127:2395–2398. [PubMed] [Google Scholar]
- 4.Brandtzaeg P, Farstad IN, Johansen F-E, Morton CH, Norderhaug IN, Yamanaka T. The B-cell system of human mucosae and exocrine glands. Immunol Rev. 1999;171:45–87. doi: 10.1111/j.1600-065X.1999.tb01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrington GM, Besser TE, Davis WC, Gay CC, Reeves JJ, McFadden TB. Expression of immunoglobulin G1 receptors by bovine mammary epithelial cells and mammary leukocytes. J Dairy Sci. 1997;80:86–93. doi: 10.3168/jds.S0022-0302(97)75915-0. [DOI] [PubMed] [Google Scholar]
- 6.Mayer B, Doleschall M, Bender B, Bartyik J, Bosze Z, Frenyo LV, Kacskovics I. Expression of the neonatal Fc receptor (FcRn) in the bovine mammary gland. J Dairy Res. 2005;72:107–112. doi: 10.1017/S0022029905001135. [DOI] [PubMed] [Google Scholar]
- 7.Cianga P, Cianga C, Cozma L, Ward ES, Carasevici E. The MHC class I related Fc receptor, FcRn, is expressed in the epithelial cells of the human mammary gland. Hum Immunol. 2003;64:1152–1159. doi: 10.1016/j.humimm.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 8.Schnulle PM, Hurley WL. Sequence and expression of the FcRn in the porcine mammary gland. Vet Immunol Immunopathol. 2003;91:227–231. doi: 10.1016/S0165-2427(02)00294-5. [DOI] [PubMed] [Google Scholar]
- 9.Mayer B, Zolnai A, Frenyo LV, Jancsik V, Szentirmay Z, Hammarstrom L, Kacskovics I. Localization of the sheep FcRn in the mammary gland. Vet Immunol Immunopathol. 2002;87:327–330. doi: 10.1016/S0165-2427(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 10.Rincheval-Arnold A, Belair L, Cencic A, Djiane J. Up-regulation of polymeric immunoglobulin receptor mRNA in mammary epithelial cells by IFN-gamma. Mol Cell Endocrinol. 2002;194:95–105. doi: 10.1016/S0303-7207(02)00183-1. [DOI] [PubMed] [Google Scholar]
- 11.Qiu X, Zhu X, Zhang L, Mao Y, Zhang J, Hao P, Li G, Lv P, Li Z, Sun X, Wu L, Zheng J, Deng Y, Hou C, Tang P, Zhang S, Zhang Y. Human epithelial cancers secrete immunoglobulin G with unidentified specificity to promote growth and survival of tumor cells. Cancer Res. 2003;63:6488–6495. [PubMed] [Google Scholar]
- 12.Zheng J, Huang J, Mao Y, Liu S, Sun X, Zhu X, Ma T, Zhang L, Ji J, Zhang Y, Yin CC, Qiu X (2009) Immunoglobulin gene transcripts have distinct VHDJH recombination characteristics in human epithelial cancer cells. J Biol Chem (in press) [DOI] [PMC free article] [PubMed]
- 13.Babbage G, Ottensmeier VH, Blaydes J, Stevenson FK, Sahota SS. Immunoglobulin heavy chain locus events and expression of activation-induced cytidine deaminase in epithelial breast cancer cell lines. Cancer Res. 2006;66:3990–4000. doi: 10.1158/0008-5472.CAN-05-3704. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z, Gu J. Immunoglobulin G expression in carcinomas and cancer cell lines. FASEB J. 2007;21:2931–2938. doi: 10.1096/fj.07-8073com. [DOI] [PubMed] [Google Scholar]
- 15.Bransteitter R, Sneeden J, Allen S, Pham P, Goodman MF. First AID is needed to produce high-affinity isotype-switched antibodies. J Biol Chem. 2006;281:16833–16836. doi: 10.1074/jbc.R600006200. [DOI] [PubMed] [Google Scholar]
- 16.Longerich S, Tanaka A, Bozek G, Nicolae D, Storb U. The very 5′ end and the constant region of Ig genes are spared from somatic mutation because AID does not access these regions. J Exp Med. 2005;202:1443–1454. doi: 10.1084/jem.20051604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 18.Lu W, Zhao Z, Zhao Y, Yu S, Zhao Y, Fan B, Kacskovics I, Hammarström L, Li N. Over-expression of the bovine FcRn in the mammary gland results in increased IgG levels in both milk and serum of transgenic mice. Immunology. 2007;122:401–408. doi: 10.1111/j.1365-2567.2007.02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, Sun X, Mao Y, Zhu X, Zhang P, Zhang L, Du J, Qiu X. Expression of immunoglobulin gene with classical V-(D)-J rearrangement in mouse brain neurons. Int J Biochem Cell Biol. 2007;40:1604–1615. doi: 10.1016/j.biocel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Huang J, Zhang L, Ma T, Zhang P, Qiu X. Expression of immunoglobulin gene with classical V-(D)-J rearrangement in mouse testis and epididymis. J Histochem Cytochem. 2009;57:339–349. doi: 10.1369/jhc.2008.951434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selsing E. Ig class switching: targeting the recombinational mechanism. Curr Opin Immunol. 2006;18:249–254. doi: 10.1016/j.coi.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Han S, Zheng B, Schatz DG, Spanopoulou E, Kelsoe G. Neoteny in lymphocytes: Rag1 and Rag2 expression in germinal center B Cells. Science. 1996;274:2094–2097. doi: 10.1126/science.274.5295.2094. [DOI] [PubMed] [Google Scholar]