Abstract
Crossing biological barriers represents a major limitation for clinical applications of biomolecules such as nucleic acids, peptides or proteins. Cell penetrating peptides (CPP), also named protein transduction domains, comprise short and usually basic amino acids-rich peptides originating from proteins able to cross biological barriers, such as the viral Tat protein, or are rationally designed. They have emerged as a new class of non-viral vectors allowing the delivery of various biomolecules across biological barriers from low molecular weight drugs to nanosized particles. Encouraging data with CPP-conjugated oligonucleotides have been obtained both in vitro and in vivo in animal models of diseases such as Duchenne muscular dystrophy. Whether CPP-cargo conjugates enter cells by direct translocation across the plasma membrane or by endocytosis remains controversial. In many instances, however, endosomal escape appears as a major limitation of this new delivery strategy.
Keywords: Cell penetrating peptides, Nucleic acids delivery, Drug delivery
Introduction
Crossing biological membranes is essentially limited to low molecular mass uncharged drugs and to biomolecules having the appropriate membrane transporter or receptor. The clinical potential of many biomolecules has therefore remained poorly exploited despite a crucial need for new drugs with increased specificity and lower side effects than current low molecular mass drugs for the treatment of devastating diseases such as cancers or infectious diseases. For example, nucleic acids- and peptide-based drugs have led to few clinical applications, despite their enormous potential and now highly effective enzymatic and chemical synthesis methods.
The concept of cell penetrating peptides (CPP) has emerged from serendipitous observations by virologists on the HIV-1 Tat trans-activating factor [1] and by neurobiologists on the Drosophila Antennapedia transcription factor [2]. In both cases, the purified protein was able to promote its biological effects when added to cell cultures, thus implying an unexpected ability to be delivered intact within cells. The group of Prochiantz for the Antennapedia protein [3] and our own group for the Tat protein [4] were able to assign membrane translocation to short basic amino acids-rich peptides, also named protein transduction domains (PTD). Interest in CPPs rapidly increased when it became evident that they were able to act as vectors for the delivery of chemically conjugated biomolecules such as peptides or oligonucleotides (ON). In addition, initial experiments made on both CPPs led to the proposal of an unusual and yet to be fully explained mechanism of receptor- and energy-independent translocation across the plasma membrane. The long awaited non-viral vector thus seemed available to allow delivery of biomolecules in nearly all cell types without relying on endocytosis and its associated problems. However, the reality turned out to be more complicated than initially expected and CPP mechanisms of internalization are still controversial.
Somewhat surprisingly, interest in the physiological relevance of these phenomena, e.g., the ability of a protein to be shuttled into cells, has received less attention than its applications in the drug delivery area. Recent studies by the group of Prochiantz have proposed a role for homeoproteins in signaling during the development of the central nervous system [5].
As expected, the search for other CPPs has been exploding and is still going on either by screening natural proteins for PTD domains or by rational design, and a few representative examples of the most widely used CPPs are given in Table 1. Besides Penetratin or Tat 48–60, which can be considered as bona fide PTDs, chimeric CPPs such as MPG [6] or Transportan (Tp) [7] have been proposed in which domains isolated from different proteins have been joined together. For example, Divita and his colleagues have proposed a series of MPG peptides in which a hydrophilic domain (e.g., the nuclear localization signal of SV40 large T antigen) has been appended to the hydrophobic fusion domain of the HIV-1 gp41 protein, thus leading to an amphipathic peptide [8]. Other peptidic vectors have been designed from structure–activity studies (SAR) on already known CPPs. For example, extensive SAR studies by Wender and his colleagues have pointed to the key role played by arginine side-chains in Tat 48–60, thus corroborating and extending our own data. Interestingly, the replacement of arginine residues by lysines was detrimental, thus pointing to special properties linked to the guanidinium side chains of arginines. These observations led these authors to propose oligoarginines as alternatives to Tat 48–60. This same group has also proposed completely synthetic transporters named peptoids in which guanidinium groups were grafted onto non-peptidic backbones [9].
Table 1.
A few classical CPPs
| Name | Origin/design | Sequence | References |
|---|---|---|---|
| Protein transduction domains | |||
| Penetratin (pAntp) | Antennapedia Drosophila melanogaster | RQIKIWFQNRRMKWK | [3] |
| Tat 48–60 | HIV-1 transactivator (Tat) | GRKKRRQRRRPPQ | [4] |
| Chimeric CPPs | |||
| Transportan | Galanin + Mastoparan | GWTLNSAGYLLGKINLKALAALAKKIL | [7] |
| MPG | HIV-1 gp 41 + NLS SV40 | GALFLGFLGAAGSTMGAWSQPKKKRKV | [6] |
| Synthetic CPPs | |||
| Oligoarginine | From the key role of Arg residues in Tat | (R)n, 6 < n <12 |
[78] [79] |
| MAP | Model amphipatic peptides | KLALKLALKALKAALKA | [80] |
| Second generation CPPs from SAR studies | |||
| (R-Ahx-R)4 | From oligoarg SAR | RAhxR RAhxR RAhxR RAhxRAhxB | [36] |
| Pip2b | Penetratin derivatives | (R-Ahx-R)3-IHILFQN-dRRMKWHKBC* | [41] |
| Stearylated-TP10 | Transportan derivatives | Stearyl-AGYLLGKINLKALAALAKKIL | [43] |
* dR denote D-isomer of arginine
Altogether, many CPPs have now been proposed and are still being suggested. It should be noted that most CPPs described so far were designed to be covalently conjugated to the cargo to be transported, except the MPG family. Nevertheless, Tat 48–60, Penetratin, oligoArg and Tp10 (a Transportan derivative) remain the most widely studied CPPs.
The following sections will overview the large range of cargoes delivered by CPPs with emphasis on ON delivery and a detailed description of promising pre-clinical data gathered recently in murine models of Duchenne muscular dystrophy (DMD). The last section will summarize the still controversial issues of CPP cellular internalization.
CPPs as versatile delivery vectors for biomolecules
The crucial need for non-viral delivery vectors able to improve the bioavailability of biomolecules, and the apparent receptor-independent mechanism of cellular internalization of CPPs, quickly led to substantial interest in CPPs amongst the scientific community. Hundreds of publications have explored the potential of CPPs to promote the internalization of biomolecules from low molecular weight drugs to nanosized particles (Table 2). Initial publications describing Penetratin [3] and Tat 48–60 [4] already pointed to the possible use of CPPs as vectors for the delivery of chemically conjugated antisense ON and peptides. CPP conjugation or complexation now appears as one of the most promising strategies for the delivery of antisense ON and siRNAs, as will be reviewed in detail in the following sections.
Table 2.
Examples of intracellular delivery of different cargoes by CPPs
| CPP | Cargo | References |
|---|---|---|
| Large size cargoes | ||
| Proteins | ||
| Tat | Heterologous proteins | [16] |
| Tat | Tumor associated proteins and peptides | [81] |
| Nucleic acids | ||
| Tat | Gene markers encapsulated in λ phage | [82] |
| Various CPP | Potential for CPP to deliver DNA | [83] |
| Imaging agents | ||
| CPP for in vivo molecular imaging applications | [84] | |
| Tat | Paramagnetic labels | [85] |
| Tat | Technetium and rhenium | [86] |
| Nanocarriers | ||
| Tat | Pharmaceutical nanocarriers | [87] |
| Solid lipid nanoparticles | [88] | |
| Gold nanoparticles | [89] | |
| Polymeric micelles | [90] | |
| Liposomes | ||
| Tat, penetratin | Liposomes | [87, 91] |
| Tat | Liposomes | [92] |
| Small size cargoes | ||
| Oligo arg | Taxol | [93] |
| Oligo arg | Cyclosporine A | [94] |
| Oligoguanidinium | Various small molecules | [22] |
| Oligonucleotides | ||
| Arg rich peptides | PMO, PNA |
[42] [95] |
| Various CPP | PMO, PNA | [96] |
| Peptides | ||
| Tat, transportan, MAP, penetratin | Pentapeptide | [97] |
| Tat | Peptidic inhibitor of Leishmania GP 63 | [98] |
| Tat | Von Hippel Linau tumor suppressor peptide | [99] |
CPPs have also been used frequently for the delivery of bioactive peptides in cell-based assays and in murine models of human diseases such as cancers or ischemic stroke. Many studies have been devoted to the regulation of apoptotic cascades to counteract apoptosis-inhibitory pathways which limit the efficacy of anticancer drugs or, in contrast, to prevent caspase activation as a strategy to limit ischemic injuries in various tissues [10]. As an example, several groups took advantage of the 16 amino acids BH4 peptide which is shared by several anti-apoptotic proteins of the Bcl-2 family. Tat-BH4 constructs were found to be effective to down-regulate apoptosis in cellular assays [11] and to reduce injuries after ischemia–reperfusion in the Langendorff perfused heart model [12]. Likewise, Tat CPP-conjugated PKCδ peptidic inhibitors reduced infarct size after local or systemic administration in rat [13] or porcine [14] models of acute myocardial infarction. These encouraging data in pre-clinical studies have prompted the development (KAI Pharmaceuticals) of PKCδ-based inhibitors as drug candidates for the treatment of myocardial infarction in patients, and clinical trials have been started.
Being able to deliver peptides intracellularly in vitro in often difficult-to-transfect primary cells such as β-pancreatic islets or in vivo in animal models has numerous applications for the validation of intracellular targets, to unravel details of the complex network of protein interactions and ultimately to develop peptide-based drugs. Much knowledge has still to be acquired, however, in terms of CPP pharmacokinetics, ADME and toxicology [15].
Importantly, CPPs also allow the cellular internalization of otherwise non-permeant macromolecules such as proteins. It was first demonstrated [16, 17] that fused constructs between the Tat CPP and β-galactosidase could be delivered inside cells both in vitro and in vivo after intraperitoneal injection in mice. This landmark publication opened the way to the protein transduction technology, thus widely broadening potential clinical applications of proteins to intracellular targets. CPP-based delivery of proteins (with Tat 48–60 as CPP in most cases) has now been exploited by many groups for both fundamental studies or for pre-clinical studies aimed at fighting neurodegenerative diseases, cancers or ischemic strokes to cite just a few (see [18, 19] for reviews). Applications are also envisaged to improve the bioavailability of therapeutic proteins such as insulin which have to be administered intravenously or sub-cutaneously. A series of reports demonstrate the potential of CPPs in improving the intestinal bioavailability of insulin [20].
As often the case, however, biologists rediscovered and exploited a phenomenon that nature had already been using to transfer information between cells. Pioneering work by the group of Prochiantz on a model of axonal guidance has indeed revealed that many homeoproteins include a PTD motif (as in the Antennapedia Drosophila protein) which allows their transduction from cell to cell and provides an entirely new protein-based network of regulation [21].
Aside from nucleic acid- and peptide-based biomolecules, CPPs have improved the pharmacological properties of several low molecular weight drugs. For example, oligoArg conjugates of cyclosporine A were shown to be transported across the striatum corneum and to foster a local anti-inflammatory response upon topical application, thus providing a more selective effect than oral administration [22]. In the anti-tumoral drug field, CPP conjugation of Taxol was found beneficial in increasing the aqueous solubility of the drug and, importantly, overcoming multidrug resistance which is a frequent cause of resistance in cancer therapeutics [23].
To close this brief panorama of the variety of transported cargoes, CPP-derivatized nanosized particles have been proposed for both imaging and therapeutic applications [24]. Dextran-coated superparamagnetic iron oxide particles like cross-linked iron oxide (CLIO) were taken up more efficiently when derivatized with Tat CPPs than the unmodified particles [25]. Applications in magnetic resonance imaging and in magnetic separation of cells have been proposed. Along the same lines, grafting CPPs to liposomes or lipoplexes was shown to improve the delivery of liposome-encapsulated drugs or plasmid DNA [26] .
CPP delivery of oligonucleotides
An early publication by Langel and coworkers [27] has demonstrated the in vitro and in vivo delivery of a 21-mer antisense peptide nucleic acid (PNA) targeting the type 1 galanin receptor mRNA when conjugated to Transportan through a reducible disulfide bridge. Remarkably, the expected phenotypic responses were elicited in the central nervous system after systemic administration, thus attesting to passage across the blood brain barrier. Somewhat surprisingly, very few publications have been recorded since then in the antisense ON area, although delivery had been identified as a major roadblock for most clinical applications [28] and our own efforts towards this goal remained unsuccessful for a long time.
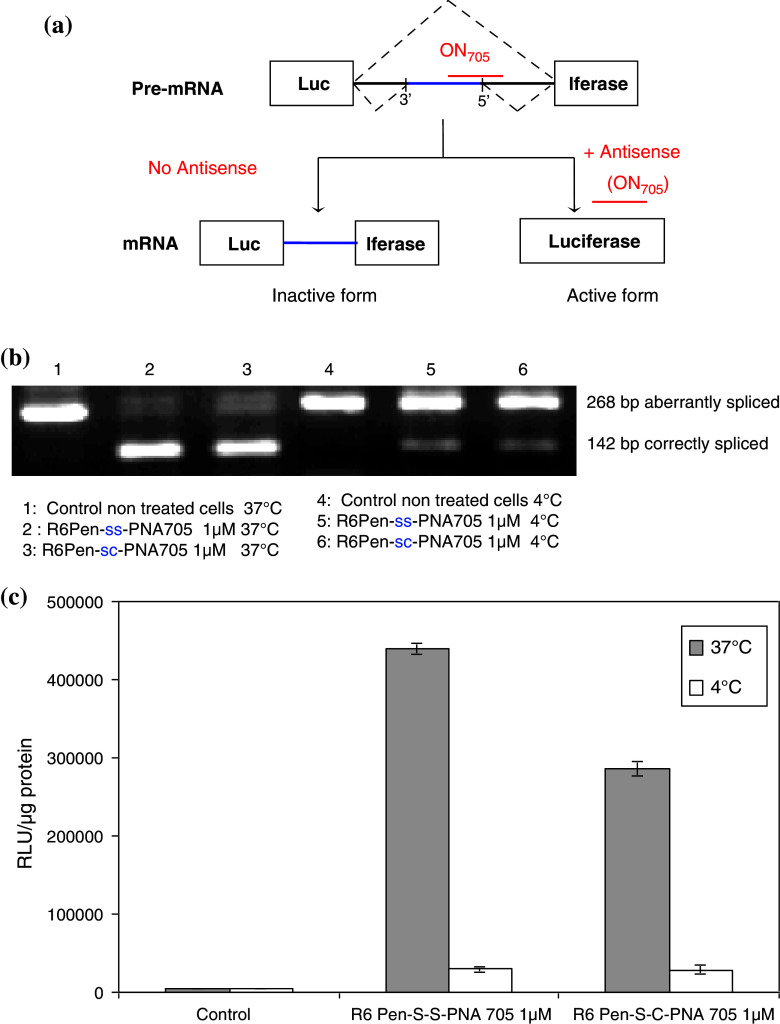
Most groups are now using the splicing redirection assay described by Kole and coworkers [29] (Fig. 1) to assess the nuclear delivery of steric block ONs. The assay is easy to implement, sensitive, sequence-specific and, importantly, provides a positive readout over a low background with a large dynamic response range. Since the chemical conjugation of negatively charged ON analogs to basic amino acids-rich and therefore cationic CPPs turned out to be difficult, most recent work has focused on neutral PNA or phosphorodiamidate morpholino oligomers (PMO). These DNA mimics have favorable pharmacological properties including poor sensitivity to nuclease degradation and high affinity for the targeted RNA sequence. Since they cannot recruit RNase H, they only act as steric-block agents, in contrast to RNase H-competent ON such as unmodified DNA or phophorothioate (PS) ON.
Fig. 1.
Splicing redirection assay. a A mutated β-globin gene intron carrying a cryptic splice site has been inserted in the coding sequence of a luciferase reporter gene and the construct has been stably transfected in HeLa pLuc 705 cells. Abnormal splicing does not allow the complete removal of the intron and no functional luciferase is produced. The nuclear delivery of a steric block ON analogue (ON 705) and its binding to the mutated site redirects the cellular splicing machinery towards complete elimination of this intron, leading to the production of the correctly spliced luciferase mRNA and to the production of active luciferase protein. b Luciferase up-regulation by PNA conjugates at low and high temperatures. HeLa pLuc 705 cells were preincubated for 30 min at 4 or 37°C before treatment. Cells were then incubated with 1 μM R6Pen-ss-PNA or R6Pen-sc-PNA in OptiMEM for 1 h at low or high temperatures and washed twice with PBS buffer. Luciferase quantification was processed after 23 h supplementary incubation in DMEM at 37°C. These two PNA conjugates differ in the nature of the linker between the peptidic and the oligonucleotidic parts of the conjugates (reducible disulfide—ss—or stable thioester—sc—covalent bond). c RT–PCR analysis of splicing redirection. Total RNA was purified from the same cellular lysates and amplified by RT–PCR. The correctly spliced mRNA can be distinguished from the aberrant one for each conjugate at the two temperatures
Several groups reported splicing redirection by PNA oligomers appended with a short oligo Lys tail [30]. In contrast to these encouraging data, we found no such activity with PNAs conjugated to oligo Lys unless endosomolytic agents such as chloroquine were added [31]. Similar data were reported for Tat- or Penetratin-PNA conjugates, in keeping with a segregation in endocytotic vesicles [32]. Discrepancies between these two sets of data might have been partly due to differences in CPP–PNA concentrations. Indeed, luciferase expression was observed at 5–10 μM CPP–ON concentrations, but these high concentrations did lead to cell membrane permeabilization and to the uptake of ON–CPP conjugates by a non-endocytotic mechanism [30].
In keeping with a strong limitation due to endosomal sequestration, various endosomolytic drugs and endosome-disruptive peptides (such as influenza hemagglutinin-derived peptides) had a positive impact on CPP delivery of nucleic acids. Unfortunately, however, most of the existing endosomolytic treatments are too toxic to be envisaged in vivo.
A notable exception is photochemical internalization (or PCI) which capitalizes on the production of reactive oxygen species upon irradiation. Both PNA–CPP-based gene silencing [33] and splicing redirection [34] have been described in vitro with this promising strategy.
In a search for more active CPPs than Tat, oligoArg or Pen, we capitalized on a RXR family of synthetic CPPs first proposed by P.A. Wender and his colleagues. In these Arg-rich CPPs, arginine residues have been interspersed with non-natural linkers of various lengths based on the assumption that not all guanidinium side chains would be required for heparan sulfate binding at the cellular membrane[35]. Their extensive structure–activity (SAR) studies concluded that 6-aminohexanoic acid (Ahx) is the optimal spacer in terms of cellular uptake. Interestingly, the inclusion of non-natural uncharged amino acids in the structure should increase CPP metabolic stability and reduce their net charge, thus improving their pharmacological properties. Both (R-Ahx-R)4-PMO [36] and -PNA [37] lead to potent and sequence-specific splicing redirection activity in HeLa pLuc 705 with EC50 in the micromolar range in the absence of endosomolytic agents.
However, it should be pointed out that a large proportion of the conjugated material remained sequestered in endocytotic vesicles, thus leaving room for improvements. Extensive SAR studies with a series of (R-X-R)n-PMO conjugates differing in terms of charge spacing, in linker hydrophobicity, in stereochemistry, or in the number of (R-X-R) repeats did not lead to more efficient CPPs when this splicing redirection assay was used as end point [38]. In parallel with these in vitro SAR mechanistic studies, (R-Ahx-R)4-PMO conjugates have been taken to pre-clinical studies by H. Moulton and her colleagues (AVI Biopharma) in murine models of severe viral infections ([39] and references therein) and of Duchenne muscular dystrophy.
Similar studies have been carried out on R6Pen CPPs in which Penetratin has been appended at its N-terminus with Arg residues. These R6Pen-PNA conjugates were found to be active at submicromolar concentrations in the splicing redirection assay [37] or in a Tat-dependent trans-activation assay [40] whilst Penetratin itself was poorly efficient. Further optimization with regard to improved resistance to serum proteases has led to new PNA internalization peptides (Pip) [41] now undergoing pre-clinical evaluation.
In conclusion, neutral PNA and PMO can be delivered to cell nuclei when covalently linked to arginine-rich CPPs, while few well-documented examples of successful delivery of negatively charged ON have been reported (reviewed in [42]).
A few promising strategies capitalizing on the non-covalent complexation of negatively charged oligo- or polynucleotides (steric block ON, siRNA, plasmid DNA) with CPPs have been described recently. As an example, Mae et al. [43] have engineered N-terminally stearylated versions of Transportan 10 (TP10) which turned out to be very efficient in the splicing redirection assay. Luciferase expression levels were largely increased when compared to PMO or PNA conjugates described so far and were close to the levels achieved with cationic lipids, such as lipofectamine. Likewise, stearylation largely increases the splicing redirection activity of (RAhxR)4 and allows the delivery of charged ON analogs in a non-covalent strategy (T. Lehto, R. Abes et al., unpublished observations). Ongoing mechanistic studies using a liposome leakage effect are pointing to improved endosomal escape as a plausible explanation for this increased activity (F. Said Hassane et al., unpublished observations).
Along the same lines, Dowdy and coworkers [44] have engineered a peptidic delivery vector allowing the efficient and non-toxic delivery of siRNA in a large collection of cell types including difficult-to-transfect primary cells. Their PTD–DRBD construct comprises a dsRNA binding domain (DRBD) fused to a Tat-based PTD. The DRBD entity binds siRNA with high affinity and masks its negative charges while the fused Tat CPP allows the intracellular delivery of the PTD–DRBD siRNA complex. Along the same lines, the complexation of a MPG derivative called MPG-8 to a siRNA targeting cyclinB at a 20:1 ratio prevented tumor growth in mice after systemic administration [45].
These examples represent encouraging, easy to implement and versatile strategies for the delivery of charged steric block ON and siRNA, respectively. Importantly, they are as efficient as commercially available cationic lipid formulations while being less cytotoxic. Whether they might represent a solution to ON’s poor availability will have to await more extensive in vivo experimentation.
Exon skipping by CPP–PMO conjugates for treatment of Duchenne muscular dystrophy
The first clinical trial involving a CPP–PMO conjugate for the treatment of DMD is planned for 2010 (AVI 5038, www.aviobio.com). DMD is an X-chromosome linked muscle disease caused by mutations in the dystrophin gene. DMD patients suffer from severe and progressive muscle wasting, whereas the milder Becker muscular dystrophy (BMD) is caused by in-frame deletions that result in expression of a shortened but partially functional dystrophin protein. ONs have been shown to induce targeted ‘exon skipping’ to correct the reading frame of mutated dystrophin mRNA such that shorter dystrophin forms are produced with activity similar to that of BMD [46]. Phase I clinical trials in the UK and in the Netherlands involving intramuscular injections of a 30-mer PMO (UK) [47] or a 20-mer 2’-O-methylphosphorothioate ON (Netherlands) [48] are now continuing to intravenous delivery.
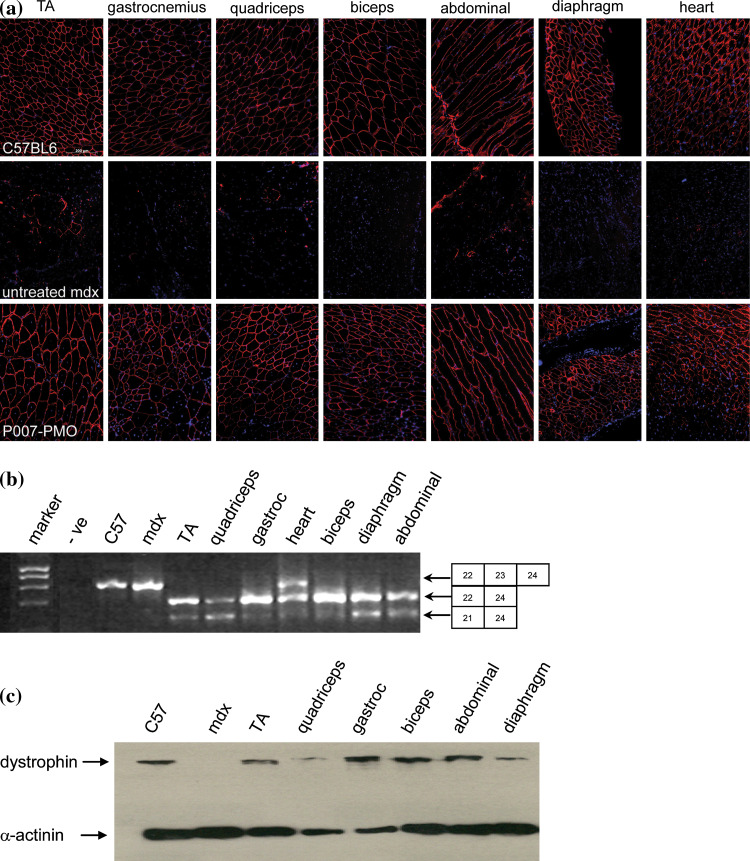
In an mdx mouse model of exon skipping, dystrophin production is assessed following local intramuscular administration or systemic delivery [49–52]. A key therapeutic requirement is for generation of sufficient dystrophin in all muscle types, including heart, following systemic injection. For example, seven i.v. administrations of unmodified 25-mer PMO led to varying levels of dystrophin in a range of muscle types but were unable to generate significant dystrophin in mdx mouse heart, even with repeated dosage of 100 mg/kg [53]. CPP attachment to the PMO has made dramatic improvements to dystrophin production in mdx mice. A single i.p. injection of (R-Ahx-R)4Ahx-βAla-PMO (R-PMO) at 25 mg/kg into neonatal mdx mice resulted in near-normal levels of dystrophin production in diaphragm and lower levels in other skeletal muscles, except heart [54]. More recently, Yin et al. have shown that single i.v. delivery at 25 mg/kg of R-PMO (also known as P007-PMO) in adult mdx mice gave near-normal levels of dystrophin production in a range of muscle types as well as significant production in heart compared to control C57BL6 mice (Fig. 2a) [55]. Exon skipping was almost complete in most muscle types, as shown by RT–PCR analysis, and dystrophin protein was readily detected by western blotting (Fig. 2b, c). Such levels were also sufficient to obtain demonstrated physiological benefit.
Fig. 2.
Restoration of muscle and cardiac dystrophin expression in adult mdx mice following single 25 mg/kg intravenous injection of (R-Ahx-R)4-Ahx-βAla-PMO (reproduced from Fig. 1 from Ref. [54], with permission). a Immunostaining of muscle tissue cross sections to detect dystrophin protein expression and localization in normal (C57BL6) mice (top) untreated mdx mice (middle) and peptide-PMO treated mice (lower) showing near normal levels of dystrophin in the treated mice. TA Tibialis anterior muscle. b RT–PCR to detect exon skipping efficiency at the RNA level demonstrating almost complete exon 23 skipping in the peripheral skeletal muscles and ca. 50% in heart in treated mdx mice. This is shown by the shorter exon-skipped bands (indicated by the box numbered 22–24 for exon 23 skipping). c Western blotting for dystrophin expression in peripheral skeletal muscles showed ca. 100% dystrophin restoration in all skeletal muscles except the diaphragm
The current CPP of choice for clinical studies appears to be a variant of the R-peptide, (R-Ahx-R-R-βAla-R)4Ahx-βAla (B-peptide). Although B-PMO is slightly less effective than R-PMO [55], other studies have shown how multiple doses of B-PMO delivered intravenously into mdx mice are extremely effective in all muscles including heart. For example, 12 mg/kg for 4 days treatment led to high exon skipping in heart, diaphragm and quadriceps that persisted for several weeks [56]. Remarkably high levels of dystrophin production could be obtained with six biweekly injections of B-PMO at 30 mg/kg, and mdx mice treated just twice at this dose showed significant improvement of cardiac function [57]. Encouragingly, there was no sign shown of any immune response to B-PMO or toxicity in treated mdx mice.
Since DMD patients are likely to require long-term and continuous treatment with CPP–PMO, preclinical toxicology, safety and tolerability studies with B-PMO will act as a paradigm for the use of this class of Arg-rich CPPs in a clinical setting. Use of improved CPPs as PMO conjugates may allow further dosage reductions. For example, a hybrid of B peptide with a muscle-specific peptide MSP conjugated to PMO has been shown to be more active in mdx mice than B-PMO [58], and encouraging preliminary results have been obtained with the Pip peptide series (serum-stabilized derivatives based on Arg-extended Penetratin) in conjugation with PNA [59] and with PMO (H. Yin, A. Saleh, M.J. Gait, and M.J.A. Wood, unpublished results). The DMD model is acting as a spearhead for development of CPPs as ON conjugates to enhance ON cell delivery and in vivo activity and justifies development of several ON drugs targeting the most common clusters of DMD mutations as well as other genetic diseases in coming years.
CPP mechanism of cellular internalization
Initial data from most groups have proposed a receptor-free and non-endocytotic mechanism of internalization for CPPs and their cargo conjugates. Arguments in favor of this mechanism included energy independence, absence of effects of endocytosis inhibitors, fast accumulation in nuclei, and absence of labeling of endocytotic vesicles. While for a large part based on experimental artefacts as will be seen later, paradoxically this view has contributed to the enthusiasm raised for CPPs. Indeed, CPPs were thought to provide a strategy avoiding problems known to be associated with non-viral delivery vectors such as entrapment in endocytotic vesicles and cargo degradation by lysosomal enzymes.
Cell fractionation experiments performed in collaboration with the group of P. Courtoy (UCL, Bruxelles) had already complicated the widely accepted view of a direct Tat translocation through the plasma membrane followed by nuclear accumulation. Indeed, a Tat peptide radioactively labeled by an additional iodinated-Tyr residue was found essentially associated with the plasma membrane fraction. No radioactivity could be detected in nuclei and a small proportion only was found associated with endocytotic vesicles (P. Courtoy, E. Vives and B. Lebleu, unpublished observations).
Independent further studies on Penetratin [60] and on Tat 48–60 [61] clearly pointed to artefacts due to the strong basic character of these peptides. First, even supposedly mild fixation procedures used for fluorescence microscopy did lead to a rapid redistribution of membrane-associated material [62]. Secondly, CPPs such as Tat or Penetratin strongly bind to the plasma membrane and cannot be removed by conventional washing protocols used in FACS analysis to discriminate membrane-bound from internalized material. These experimental artefacts could be avoided by fluorescence microscopy analysis on live unfixed cells and by modifying the protocol of cell treatment before FACS analysis. We favor the inclusion of a brief proteolytic (pronase or trypsin) treatment to degrade membrane-bound material [61, 63], but other protocols such as the quenching of membrane-bound fluorochrome have been proposed [64]. Implementing these protocols gave rise to experimental data in line with an active process of cellular internalization involving endocytosis [63]. These data were rapidly corroborated by many groups for basic CPPs and their conjugates with various cargoes. As an example, Tat-conjugated liposomes were first thought to be internalized by direct translocation [65] while more recent data plead for an endocytotic mechanism [66].
However, conflicting data continue to be produced even in conditions which should avoid the artefacts described above. As an example, an endocytosis-independent internalization of Tat peptide using physical (low temperature) or genetic (cell mutants incapable of performing caveolin- or clathrin-mediated endocytosis) tools has been described [67].
A possible explanation for this endless dispute has been provided recently in a comprehensive and careful study by Brock’s group [68]. They propose the possible involvement of both endocytotic and non-endocytotic mechanisms with the former being predominant at low CPP concentrations and the latter at higher ones. Discrepancies between published data would then become explainable since the threshold value (generally above 5 μM) for the non-endocytotic mechanism varies with CPPs, with conjugated cargoes, and with cell types. While intellectually satisfactory, this model still raises questions concerning the mechanism through which an elevated local concentration of basic CPPs causes direct translocation. Whether observations made at high CPP concentration are relevant to drug delivery is also questionable since CPP delivery aims at allowing cargo delivery at the lowest possible concentration.
Uncertainties have also been raised concerning cell surface determinants implicated in CPP-cargo recognition. Here again, arginine-rich CPPs have been the most carefully studied. Our own preliminary Ala scan on Tat 48–60 pointed to the important role of arginine residues in cell uptake [69]. Extensive structure function studies on the Tat peptides convincingly established the key role played by the guanidinium head groups of arginine in cell uptake and gave rise to oligoArg CPPs and to oligoguanidine peptoid derivatives [22]. These guanidinium groups are known to form bidentate hydrogen bonds with anions such as sulfates or phosphate groups. Whether initial binding involves cell surface proteoglycans or membrane lipid polar heads also remains a matter of dispute. Initial studies have favored interaction with lipids, but they essentially relied on lipid model systems whose relevance to the plasma membrane remains uncertain. Recent studies from several groups have pointed to the role of sulfated proteoglycans such as heparan sulfates. Amongst the strongest arguments in favor of their role is the observed reduced uptake of arginine-rich CPPs such as Tat and its conjugates in mutant CHO cells deficient in the expression of cell surface glycosaminoglycans (GAG) [70]. Whether GAG-bound CPPs are shuttled from this primary binding site to other membrane-associated binding sites (polar head groups of lipids for example) remains unknown to our knowledge. Likewise, the fate of GAG-bound CPPs within endocytotic vesicles has not been unraveled. Intriguingly, our recent SAR studies on a series of (R-Ahx-R)n-PMO conjugates do indicate that too high an affinity for heparan sulfates is detrimental to biological activity. Our working hypothesis is that internalized CPP-cargo conjugates need to dissociate from proteoglycans before being shuttled through the membrane of endocytotic vesicles.
Whether endocytosis is involving one or several specific pathways is also a matter of debate. Three major pathways, e.g., macropinocytosis, clathrin-coated pits-mediated endocytosis, and lipid rafts-mediated endocytosis have been proposed. Divergent data remain even considering a single well-characterized CPP such as Tat 48–60 and its cargo conjugates. We have investigated the uptake of fluorochrome-tagged Tat and Tat-PNA conjugates using a panel of pharmacological agents affecting preferentially each endocytotic pathway and specific markers for these routes. Clathrin-dependent endocytosis appeared as the major pathway used for Tat and Tat-PNA conjugates in HeLa cells. Co-localization of Tat and transferrin, a known marker of clathrin-coated pits-mediated endocytosis, in acidic cytoplasmic vesicles was also observed [63]. Similar conclusions have been drawn for Tat by Potocky et al. [71] and importantly for the full size Tat protein [72]. More recently, we capitalized on a functional assay (e.g., the splicing redirection assay) to assess the mechanism of internalization of Tat-PNA conjugates. Similar conclusions concerning the clathrin-dependent endocytosis were reached, thus confirming that the delivery of splice-correcting ONs to the nuclei was mediated by this pathway.
Similar tools (e.g., endocytosis inhibitors and endocytosis markers) have, however, led other groups to different conclusions. A Tat-GFP fusion protein was found to co-localize with caveolin-1 and its uptake was inhibited by cholesterol depletion and cytochalasin D treatment in keeping with a lipid raft/caveolae route of internalization [73]. The involvement of macropinocytosis has been established in a series of recent publications [74, 75] using genetic and pharmacological tools. Importantly, this conclusion was reached when monitoring either Tat-Cre recombinase fusion protein localization or recombination of a reporter gene. Co-localization with dextran beads and inhibition by actin polymerization inhibitors were observed in line with a lipid raft-dependent pathway.
These discrepancies may be attributed in part to the poor specificity of the inhibitors used in these studies and to shared components between endocytotic pathways [19]. Other parameters are probably to be considered, such as the possibility for a CPP construct to use several of these endocytotic pathways. Whatever the case, all studies are consistent with an endocytotic pathway and consequently point to the obstacle of endosomal escape being limiting for delivery. Further complications arise from the fact that endocytosis as a whole as well as its various pathways vary with cell types and with physiological conditions. As an example, organized MDCK (Madine-Darby canine kidney) cells, a well-studied model of epithelial cells, take up Tat poorly in parallel with a reduced endocytotic activity. However, exposure to inflammatory cytokines strongly increases CPP uptake [76]. Such variations might be exploited for a preferential delivery of CPP-conjugated drugs to inflamed tissues.
As pointed out repeatedly in this review, efficient crossing of the endosomal membrane now appears as a major challenge in CPP delivery of drugs. Hopefully, ongoing and future SAR studies will provide a better knowledge of molecular features required for CPP-mediated membrane destabilization and will help in the design of more efficient analogs. Improving translocation across the endosomal membrane without concurrently increasing cytotoxicity might, however, be difficult to achieve.
Targeting CPPs to allow a preferential release of the transported drug is another challenge, but this issue is only starting to being considered. An interesting strategy for tumor-targeting has been proposed by the group of R. Tsien. They have engineered leashed-constructs in which the oligoArg CPP is masked by a negatively charged peptide. Since both peptides are linked by a metalloprotease (MMP)-cleavable linker, they will dissociate preferentially in the vicinity of MMP-secreting tumors [77].
Whatever the limitations, CPPs have already proved to be very useful tools to promote the cellular internalization of biomolecules, which would otherwise not been taken up significantly. They have considerably broadened the spectrum of biomolecule applications in vitro and in vivo in animal models of several diseases. It is too early, however, to speculate on their clinical usefulness.
Acknowledgments
Work in the authors' laboratories has been financed by grants from AFM (Association Française contre les Myopathies), ANR (Agence Nationale de la Recherche) and LNFCC (Ligue Nationale de Lutte contre le Cancer). A.F. Saleh is supported by a Development Gap Fund grant from MRC-Technology and we acknowledge additional funding from Action Duchenne. We thank A.A. Arzumanov, D. Williams and D. Owen for their work in synthesis and activity of PNA-peptide and PMO-peptide conjugates. We also thank H. Yin and M. Wood (University of Oxford) for providing information and figures regarding in vivo mdx mouse studies in DMD.
Contributor Information
F. Said Hassane, Email: fatouma.said-hassane@univ-montp2.fr
Bernard Lebleu, Phone: +33-4-67149203, FAX: +33-4-67149201, Email: bernard.lebleu@univ-montp2.fr.
References
- 1.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 2.Joliot AH, Triller A, Volovitch M, Pernelle C, Prochiantz A. alpha-2, 8-Polysialic acid is the neuronal surface receptor of antennapedia homeobox peptide. New Biol. 1991;3:1121–1134. [PubMed] [Google Scholar]
- 3.Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]
- 4.Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- 5.Joliot A, Prochiantz A. Homeoproteins as natural Penetratin cargoes with signaling properties. Adv Drug Deliv Rev. 2008;60:608–613. doi: 10.1016/j.addr.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Morris MC, Vidal P, Chaloin L, Heitz F, Divita G. A new peptide vector for efficient delivery of oligonucleotides into mammalian cells. Nucleic Acids Res. 1997;25:2730–2736. doi: 10.1093/nar/25.14.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pooga M, Hallbrink M, Zorko M, Langel U. Cell penetration by transportan. FASEB J. 1998;12:67–77. doi: 10.1096/fasebj.12.1.67. [DOI] [PubMed] [Google Scholar]
- 8.Morris MC, Deshayes S, Heitz F, Divita G. Cell-penetrating peptides: from molecular mechanisms to therapeutics. Biol Cell. 2008;100:201–217. doi: 10.1042/BC20070116. [DOI] [PubMed] [Google Scholar]
- 9.Wender PA, Galliher WC, Goun EA, Jones LR, Pillow TH. The design of guanidinium-rich transporters and their internalization mechanisms. Adv Drug Deliv Rev. 2008;60:452–472. doi: 10.1016/j.addr.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asoh S, Ohta S. PTD-mediated delivery of anti-cell death proteins/peptides and therapeutic enzymes. Adv Drug Deliv Rev. 2008;60:499–516. doi: 10.1016/j.addr.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Cantara S, Thorpe PE, Ziche M, Donnini S. TAT-BH4 counteracts Abeta toxicity on capillary endothelium. FEBS Lett. 2007;581:702–706. doi: 10.1016/j.febslet.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 12.Ono M, Sawa Y, Ryugo M, Alechine AN, Shimizu S, Sugioka R, Tsujimoto Y, Matsuda H. BH4 peptide derivative from Bcl-xL attenuates ischemia/reperfusion injury thorough anti-apoptotic mechanism in rat hearts. Eur J Cardiothorac Surg. 2005;27:117–121. doi: 10.1016/j.ejcts.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Bright R, Raval AP, Dembner JM, Perez-Pinzon MA, Steinberg GK, Yenari MA, Mochly-Rosen D. Protein kinase C delta mediates cerebral reperfusion injury in vivo. J Neurosci. 2004;24:6880–6888. doi: 10.1523/JNEUROSCI.4474-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeno F, Inagaki K, Rezaee M, Mochly-Rosen D. Impaired perfusion after myocardial infarction is due to reperfusion-induced deltaPKC-mediated myocardial damage. Cardiovasc Res. 2007;73:699–709. doi: 10.1016/j.cardiores.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Harrison SD. Cell-penetrating peptides in drug development: enabling intracellular targets. Biochem Soc Trans. 2007;35:821–825. doi: 10.1042/BST0350821. [DOI] [PubMed] [Google Scholar]
- 16.Fawell S, Seery J, Daikh Y, Moore C, Chen LL, Pepinsky B, Barsoum J. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci USA. 1994;91:664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 18.Murriel CL, Dowdy SF. Influence of protein transduction domains on intracellular delivery of macromolecules. Expert Opin Drug Deliv. 2006;3:739–746. doi: 10.1517/17425247.3.6.739. [DOI] [PubMed] [Google Scholar]
- 19.Edenhofer F. Protein transduction revisited: novel insights into the mechanism underlying intracellular delivery of proteins. Curr Pharm Des. 2008;14:3628–3636. doi: 10.2174/138161208786898833. [DOI] [PubMed] [Google Scholar]
- 20.Kamei N, Morishita M, Eda Y, Ida N, Nishio R, Takayama K. Usefulness of cell-penetrating peptides to improve intestinal insulin absorption. J Control Release. 2008;132:21–25. doi: 10.1016/j.jconrel.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Brunet I, Di Nardo AA, Sonnier L, Beurdeley M, Prochiantz A. The topological role of homeoproteins in the developing central nervous system. Trends Neurosci. 2007;30:260–267. doi: 10.1016/j.tins.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Goun EA, Pillow TH, Jones LR, Rothbard JB, Wender PA. Molecular transporters: synthesis of oligoguanidinium transporters and their application to drug delivery and real-time imaging. Chembiochem. 2006;7:1497–1515. doi: 10.1002/cbic.200600171. [DOI] [PubMed] [Google Scholar]
- 23.Dubikovskaya EA, Thorne SH, Pillow TH, Contag CH, Wender PA. Overcoming multidrug resistance of small-molecule therapeutics through conjugation with releasable octaarginine transporters. Proc Natl Acad Sci USA. 2008;105:12128–12133. doi: 10.1073/pnas.0805374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarthy JR, Weissleder R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv Drug Deliv Rev. 2008;60:1241–1251. doi: 10.1016/j.addr.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Josephson L, Tung CH, Moore A, Weissleder R. High-efficiency intracellular magnetic labeling with novel superparamagnetic-Tat peptide conjugates. Bioconjug Chem. 1999;10:186–191. doi: 10.1021/bc980125h. [DOI] [PubMed] [Google Scholar]
- 26.Torchilin VP. Cell penetrating peptide-modified pharmaceutical nanocarriers for intracellular drug and gene delivery. Biopolymers. 2008;90:604–610. doi: 10.1002/bip.20989. [DOI] [PubMed] [Google Scholar]
- 27.Pooga M, Soomets U, Hallbrink M, Valkna A, Saar K, Rezaei K, Kahl U, Hao JX, Xu XJ, Wiesenfeld-Hallin Z, Hokfelt T, Bartfai T, Langel U. Cell penetrating PNA constructs regulate galanin receptor levels and modify pain transmission in vivo. Nat Biotechnol. 1998;16:857–861. doi: 10.1038/nbt0998-857. [DOI] [PubMed] [Google Scholar]
- 28.Juliano R, Bauman J, Kang H, Ming X. Biological barriers to therapy with antisense and siRNA oligonucleotides. Mol Pharm. 2009;6:686–695. doi: 10.1021/mp900093r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang SH, Cho MJ, Kole R. Up-regulation of luciferase gene expression with antisense oligonucleotides: implications and applications in functional assay development. Biochemistry. 1998;37:6235–6239. doi: 10.1021/bi980300h. [DOI] [PubMed] [Google Scholar]
- 30.Sazani P, Kang SH, Maier MA, Wei C, Dillman J, Summerton J, Manoharan M, Kole R. Nuclear antisense effects of neutral, anionic and cationic oligonucleotide analogs. Nucleic Acids Res. 2001;29:3965–3974. doi: 10.1093/nar/29.19.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abes S, Williams D, Prevot P, Thierry A, Gait MJ, Lebleu B. Endosome trapping limits the efficiency of splicing correction by PNA-oligolysine conjugates. J Control Release. 2006;110:595–604. doi: 10.1016/j.jconrel.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 32.Shiraishi T, Pankratova S, Nielsen PE. Calcium ions effectively enhance the effect of antisense peptide nucleic acids conjugated to cationic tat and oligoarginine peptides. Chem Biol. 2005;12:923–929. doi: 10.1016/j.chembiol.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Boe S, Hovig E. Photochemically induced gene silencing using PNA-peptide conjugates. Oligonucleotides. 2006;16:145–157. doi: 10.1089/oli.2006.16.145. [DOI] [PubMed] [Google Scholar]
- 34.Shiraishi T, Nielsen PE. Photochemically enhanced cellular delivery of cell penetrating peptide-PNA conjugates. FEBS Lett. 2006;580:1451–1456. doi: 10.1016/j.febslet.2006.01.077. [DOI] [PubMed] [Google Scholar]
- 35.Rothbard JB, Kreider E, VanDeusen CL, Wright L, Wylie BL, Wender PA. Arginine-rich molecular transporters for drug delivery: role of backbone spacing in cellular uptake. J Med Chem. 2002;45:3612–3618. doi: 10.1021/jm0105676. [DOI] [PubMed] [Google Scholar]
- 36.Abes S, Moulton HM, Clair P, Prevot P, Youngblood DS, Wu RP, Iversen PL, Lebleu B. Vectorization of morpholino oligomers by the (R-Ahx-R)4 peptide allows efficient splicing correction in the absence of endosomolytic agents. J Control Release. 2006;116:304–313. doi: 10.1016/j.jconrel.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Abes S, Turner JJ, Ivanova GD, Owen D, Williams D, Arzumanov A, Clair P, Gait MJ, Lebleu B. Efficient splicing correction by PNA conjugation to an R6-Penetratin delivery peptide. Nucleic Acids Res. 2007;35:4495–4502. doi: 10.1093/nar/gkm418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abes R, Moulton HM, Clair P, Yang ST, Abes S, Melikov K, Prevot P, Youngblood DS, Iversen PL, Chernomordik LV, Lebleu B. Delivery of steric block morpholino oligomers by (R-X-R)4 peptides: structure-activity studies. Nucleic Acids Res. 2008;36:6343–6354. doi: 10.1093/nar/gkn541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burrer R, Neuman BW, Ting JP, Stein DA, Moulton HM, Iversen PL, Kuhn P, Buchmeier MJ. Antiviral effects of antisense morpholino oligomers in murine coronavirus infection models. J Virol. 2007;81:5637–5648. doi: 10.1128/JVI.02360-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner JJ, Ivanova GD, Verbeure B, Williams D, Arzumanov AA, Abes S, Lebleu B, Gait MJ. Cell-penetrating peptide conjugates of peptide nucleic acids (PNA) as inhibitors of HIV-1 Tat-dependent trans-activation in cells. Nucleic Acids Res. 2005;33:6837–6849. doi: 10.1093/nar/gki991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ivanova GD, Arzumanov A, Abes R, Yin H, Wood MJ, Lebleu B, Gait MJ. Improved cell-penetrating peptide-PNA conjugates for splicing redirection in HeLa cells and exon skipping in mdx mouse muscle. Nucleic Acids Res. 2008;36:6418–6428. doi: 10.1093/nar/gkn671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lebleu B, Moulton HM, Abes R, Ivanova GD, Abes S, Stein DA, Iversen PL, Arzumanov AA, Gait MJ. Cell penetrating peptide conjugates of steric block oligonucleotides. Adv Drug Deliv Rev. 2008;60:517–529. doi: 10.1016/j.addr.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mae M, El Andaloussi S, Lundin P, Oskolkov N, Johansson HJ, Guterstam P, Langel U. A stearylated CPP for delivery of splice correcting oligonucleotides using a non-covalent co-incubation strategy. J Control Release. 2009;134:221–227. doi: 10.1016/j.jconrel.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 44.Eguchi A, Meade BR, Chang YC, Fredrickson CT, Willert K, Puri N, Dowdy SF. Efficient siRNA delivery into primary cells by a peptide transduction domain-dsRNA binding domain fusion protein. Nat Biotechnol. 2009;27:567–571. doi: 10.1038/nbt.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crombez L, Morris MC, Dufort S, Aldrian-Herrada G, Nguyen Q, McMaster G, Coll J-L, Heitz F, Divita G (2009) Targeting cycling B through peptide-based delivery of siRNA prevents tumor growth. Nucleic Acids Res (in press) [DOI] [PMC free article] [PubMed]
- 46.Aartsma-Rus A, van Ommen GB. Antisense-mediated exon skipping: a versatile tool with therapeutic and research applications. RNA. 2007;13:1609–1624. doi: 10.1261/rna.653607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kinali M, Arechavala-Gomeza V, Feng L, Cirak S, Hunt D, Adkin C, Guglieri M, Ashton E, Abbs S, Nihoyanopoulos P, Garraldi EM, Rutherford M, Mcculley C, Popplewell LJ, Graham IR, Dickson G, Wood M, Wells DJ, Wilton SD, Holt T, Kole R, Straub V, Bushby K, Sewry C, Morgan JE, Muntoni F. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–928. doi: 10.1016/S1474-4422(09)70211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Deutekom JC, Janson AA, Ginjaar IB, Frankhuizen WS, Aartsma-Rus A, Bremmer-Bout M, den Dunnen JT, Koop K, van der Kooi AJ, Goemans NM, de Kimpe SJ, Ekhart PF, Venneker EH, Platenburg GJ, Verschuuren JJ, van Ommen GB. Local dystrophin restoration with antisense oligonucleotide PRO051. New England J Med. 2007;357:2677–2686. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
- 49.Dunckley MG, Manoharan M, Villiet P, Eperon IC, Dickson G. Modification of splicing in the dystrophin gene in cultured mdx muscle cells by antisense oligoribonucleotides. Hum Mol Genet. 1998;7:1083–1090. doi: 10.1093/hmg/7.7.1083. [DOI] [PubMed] [Google Scholar]
- 50.Mann CJ, Honeyman K, Cheng AJ, Ly T, Lloyd F, Fletcher S, Morgan JE, Partridge TA, Wilton SD. Antisense-induced exon skipping and synthesis of dystrophin in the mdx mouse. Proc Natl Acad Sci USA. 2001;98:42–47. doi: 10.1073/pnas.011408598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu QL, Rabinowitz A, Chen YC, Yokota T, Yin HF, Alter J, Jadoon A, Bou-Gharios G, Partridge T. Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles. Proc Natl Acad Sci USA. 2005;102:198–203. doi: 10.1073/pnas.0406700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fletcher S, Honeyman K, Fall AM, Harding PL, Johnsen RD, Wilton SD. Dystrophin expression in the mdx mouse after localised and systemic administration of a morpholino antisense oligonucleotide. J Gene Med. 2006;8:207–216. doi: 10.1002/jgm.838. [DOI] [PubMed] [Google Scholar]
- 53.Alter J, Lou F, Rabinowitz A, Yin H, Rosenfeld J, Wilton SD, Partridge TA, Lu QL. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat Med. 2006;12:175–177. doi: 10.1038/nm1345. [DOI] [PubMed] [Google Scholar]
- 54.Fletcher S, Honeyman K, Fall AM, Harding PL, Johnsen RD, Steinhaus JP, Moulton HM, Iversen PL, Wilton SD. Morpholino oligomer-mediated exon skipping averts the onset of dystrophic pathology in the mdx mouse. Mol Ther. 2007;151:587–592. doi: 10.1038/sj.mt.6300245. [DOI] [PubMed] [Google Scholar]
- 55.Yin H, Moulton HM, Seow Y, Boyd C, Boutilier J, Iversen P, Wood MJA. Cell-penetrating peptide-conjugated antisense oligonucleotides restore systemic muscle and cardiac dystrophin expression and function. Hum Mol Genet. 2008;17:3909–3918. doi: 10.1093/hmg/ddn293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jearawiriyapaisarn N, Moulton HM, Buckley B, Roberts J, Sazani P, Fucharoen S, Iversen PL, Kole R. Sustained dystrophin expression induced by peptide-conjugated morpholino oligomers in the muscles of mdx mice. Mol Ther. 2008;16:1624–1629. doi: 10.1038/mt.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu B, Moulton HM, Iversen PL, Juang J, Li J, Spurney CF, Sali A, Guerron AD, Nagaraju K, Doran T, Lu P, Xiao X, Lu QL. Effective rescue of dystrophin improves cardiac function in dystrophin-deficient mice by a modifies morpholino oligomer. Proc Natl Acad Sci USA. 2008;105:14814–14819. doi: 10.1073/pnas.0805676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yin H, Moulton HM, Betts C, Seow Y, Boutilier J, Iversen PL, Wood MJA (2009) A fusion peptide directs enhanced systemic dystrophin exon skipping and functional restoration in dystrophin-deficient mdx mice. Hum Mol Genet (in press) [DOI] [PubMed]
- 59.Ivanova GD, Arzumanov A, Abes R, Yin H, Wood MJA, Lebleu B, Gait MJ. Improved cell-penetrating peptide-PNA conjugates for splicing redirection in HeLa cells and exon skipping in mdx mouse muscle. Nucleic Acids Res. 2008;36:6418–6428. doi: 10.1093/nar/gkn671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drin G, Mazel M, Clair P, Mathieu D, Kaczorek M, Temsamani J. Physico-chemical requirements for cellular uptake of pAntp peptide. Role of lipid-binding affinity. Eur J Biochem. 2001;268:1304–1314. doi: 10.1046/j.1432-1327.2001.01997.x. [DOI] [PubMed] [Google Scholar]
- 61.Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, Chernomordik LV, Lebleu B. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem. 2003;278:585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- 62.Lundberg M, Johansson M. Positively charged DNA-binding proteins cause apparent cell membrane translocation. Biochem Biophys Res Commun. 2002;291:367–371. doi: 10.1006/bbrc.2002.6450. [DOI] [PubMed] [Google Scholar]
- 63.Richard JP, Melikov K, Brooks H, Prevot P, Lebleu B, Chernomordik LV. Cellular uptake of unconjugated TAT peptide involves clathrin-dependent endocytosis and heparan sulfate receptors. J Biol Chem. 2005;280:15300–15306. doi: 10.1074/jbc.M401604200. [DOI] [PubMed] [Google Scholar]
- 64.Drin G, Demene H, Temsamani J, Brasseur R. Translocation of the pAntp peptide and its amphipathic analogue AP-2AL. Biochemistry. 2001;40:1824–1834. doi: 10.1021/bi002019k. [DOI] [PubMed] [Google Scholar]
- 65.Torchilin VP, Rammohan R, Weissig V, Levchenko TS. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc Natl Acad Sci USA. 2001;98:8786–8791. doi: 10.1073/pnas.151247498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fretz MM, Koning GA, Mastrobattista E, Jiskoot W, Storm G. OVCAR-3 cells internalize TAT-peptide modified liposomes by endocytosis. Biochim Biophys Acta. 2004;1665:48–56. doi: 10.1016/j.bbamem.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 67.Ter-Avetisyan G, Tunnemann G, Nowak D, Nitschke M, Herrmann A, Drab M, Cardoso MC. Cell entry of arginine-rich peptides is independent of endocytosis. J Biol Chem. 2009;284:3370–3378. doi: 10.1074/jbc.M805550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duchardt F, Fotin-Mleczek M, Schwarz H, Fischer R, Brock R. A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic. 2007;8:848–866. doi: 10.1111/j.1600-0854.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 69.Vives E, Granier C, Prevot P, Lebleu B. Structure activity relationship study of the plasma membrane translocating potential of a short peptide of HIV-1 Tat protein. Lett Pept Sci. 1997;4:1–8. doi: 10.1023/A:1008850300184. [DOI] [Google Scholar]
- 70.Console S, Marty C, Garcia-Echeverria C, Schwendener R, Ballmer-Hofer K. Antennapedia and HIV transactivator of transcription (TAT) “protein transduction domains” promote endocytosis of high molecular weight cargo upon binding to cell surface glycosaminoglycans. J Biol Chem. 2003;278:35109–35114. doi: 10.1074/jbc.M301726200. [DOI] [PubMed] [Google Scholar]
- 71.Potocky TB, Menon AK, Gellman SH. Cytoplasmic and nuclear delivery of a TAT-derived peptide and a beta-peptide after endocytic uptake into HeLa cells. J Biol Chem. 2003;278:50188–50194. doi: 10.1074/jbc.M308719200. [DOI] [PubMed] [Google Scholar]
- 72.Vendeville A, Rayne F, Bonhoure A, Bettache N, Montcourrier P, Beaumelle B. HIV-1 Tat enters T cells using coated pits before translocating from acidified endosomes and eliciting biological responses. Mol Biol Cell. 2004;15:2347–2360. doi: 10.1091/mbc.E03-12-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fittipaldi A, Ferrari A, Zoppe M, Arcangeli C, Pellegrini V, Beltram F, Giacca M. Cell membrane lipid rafts mediate caveolar endocytosis of HIV-1 Tat fusion proteins. J Biol Chem. 2003;278:34141–34149. doi: 10.1074/jbc.M303045200. [DOI] [PubMed] [Google Scholar]
- 74.Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 75.Kaplan IM, Wadia JS, Dowdy SF. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J Control Release. 2005;102:247–253. doi: 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 76.Foerg C, Merkle HP. On the biomedical promise of cell penetrating peptides: limits versus prospects. J Pharm Sci. 2008;97:144–162. doi: 10.1002/jps.21117. [DOI] [PubMed] [Google Scholar]
- 77.Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc Natl Acad Sci USA. 2004;101:17867–17872. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Futaki S, Suzuki T, Ohashi W, Yagami T, Tanaka S, Ueda K, Sugiura Y. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J Biol Chem. 2001;276:5836–5840. doi: 10.1074/jbc.M007540200. [DOI] [PubMed] [Google Scholar]
- 79.Mitchell DJ, Kim DT, Steinman L, Fathman CG, Rothbard JB. Polyarginine enters cells more efficiently than other polycationic homopolymers. J Pept Res. 2000;56:318–325. doi: 10.1034/j.1399-3011.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- 80.Oehlke J, Scheller A, Wiesner B, Krause E, Beyermann M, Klauschenz E, Melzig M, Bienert M. Cellular uptake of an alpha-helical amphipathic model peptide with the potential to deliver polar compounds into the cell interior non-endocytically. Biochim Biophys Acta. 1998;1414:127–139. doi: 10.1016/S0005-2736(98)00161-8. [DOI] [PubMed] [Google Scholar]
- 81.Wadia JS, Dowdy SF. Transmembrane delivery of protein and peptide drugs by TAT-mediated transduction in the treatment of cancer. Adv Drug Deliv Rev. 2005;57:579–596. doi: 10.1016/j.addr.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 82.Eguchi A, Akuta T, Okuyama H, Senda T, Yokoi H, Inokuchi H, Fujita S, Hayakawa T, Takeda K, Hasegawa M, Nakanishi M. Protein transduction domain of HIV-1 Tat protein promotes efficient delivery of DNA into mammalian cells. J Biol Chem. 2001;276:26204–26210. doi: 10.1074/jbc.M010625200. [DOI] [PubMed] [Google Scholar]
- 83.Snyder EL, Dowdy SF. Protein/peptide transduction domains: potential to deliver large DNA molecules into cells. Curr Opin Mol Ther. 2001;3:147–152. [PubMed] [Google Scholar]
- 84.Kersemans V, Kersemans K, Cornelissen B. Cell penetrating peptides for in vivo molecular imaging applications. Curr Pharm Des. 2008;14:2415–2447. doi: 10.2174/138161208785777432. [DOI] [PubMed] [Google Scholar]
- 85.Bhorade R, Weissleder R, Nakakoshi T, Moore A, Tung CH. Macrocyclic chelators with paramagnetic cations are internalized into mammalian cells via a HIV-tat derived membrane translocation peptide. Bioconjug Chem. 2000;11:301–305. doi: 10.1021/bc990168d. [DOI] [PubMed] [Google Scholar]
- 86.Polyakov V, Sharma V, Dahlheimer JL, Pica CM, Luker GD, Piwnica-Worms D. Novel Tat-peptide chelates for direct transduction of technetium-99m and rhenium into human cells for imaging and radiotherapy. Bioconjug Chem. 2000;11:762–771. doi: 10.1021/bc000008y. [DOI] [PubMed] [Google Scholar]
- 87.Torchilin VP. Tat peptide-mediated intracellular delivery of pharmaceutical nanocarriers. Adv Drug Deliv Rev. 2008;60:548–558. doi: 10.1016/j.addr.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 88.Rudolph C, Schillinger U, Ortiz A, Tabatt K, Plank C, Muller RH, Rosenecker J. Application of novel solid lipid nanoparticle (SLN)-gene vector formulations based on a dimeric HIV-1 TAT-peptide in vitro and in vivo. Pharm Res. 2004;21:1662–1669. doi: 10.1023/B:PHAM.0000041463.56768.ec. [DOI] [PubMed] [Google Scholar]
- 89.de la Fuente JM, Berry CC. Tat peptide as an efficient molecule to translocate gold nanoparticles into the cell nucleus. Bioconjug Chem. 2005;16:1176–1180. doi: 10.1021/bc050033+. [DOI] [PubMed] [Google Scholar]
- 90.Sethuraman VA, Bae YH. TAT peptide-based micelle system for potential active targeting of anti-cancer agents to acidic solid tumors. J Control Release. 2007;118:216–224. doi: 10.1016/j.jconrel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tseng YL, Liu JJ, Hong RL. Translocation of liposomes into cancer cells by cell-penetrating peptides penetratin and tat: a kinetic and efficacy study. Mol Pharmacol. 2002;62:864–872. doi: 10.1124/mol.62.4.864. [DOI] [PubMed] [Google Scholar]
- 92.Torchilin VP, Levchenko TS. TAT-liposomes: a novel intracellular drug carrier. Curr Protein Pept Sci. 2003;4:133–140. doi: 10.2174/1389203033487298. [DOI] [PubMed] [Google Scholar]
- 93.Kirschberg TA, VanDeusen CL, Rothbard JB, Yang M, Wender PA. Arginine-based molecular transporters: the synthesis and chemical evaluation of releasable taxol-transporter conjugates. Org Lett. 2003;5:3459–3462. doi: 10.1021/ol035234c. [DOI] [PubMed] [Google Scholar]
- 94.Rothbard JB, Garlington S, Lin Q, Kirschberg T, Kreider E, McGrane PL, Wender PA, Khavari PA. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat Med. 2000;6:1253–1257. doi: 10.1038/81359. [DOI] [PubMed] [Google Scholar]
- 95.Abes R, Arzumanov A, Moulton H, Abes S, Ivanova G, Gait MJ, Iversen P, Lebleu B. Arginine-rich cell penetrating peptides: design, structure-activity, and applications to alter pre-mRNA splicing by steric-block oligonucleotides. J Pept Sci. 2008;14:455–460. doi: 10.1002/psc.979. [DOI] [PubMed] [Google Scholar]
- 96.Veldhoen S, Laufer SD, Restle T. Recent developments in peptide-based nucleic acid delivery. Int J Mol Sci. 2008;9:1276–1320. doi: 10.3390/ijms9071276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hallbrink M, Floren A, Elmquist A, Pooga M, Bartfai T, Langel U. Cargo delivery kinetics of cell-penetrating peptides. Biochim Biophys Acta. 2001;1515:101–109. doi: 10.1016/S0005-2736(01)00398-4. [DOI] [PubMed] [Google Scholar]
- 98.Corradin S, Ransijn A, Corradin G, Bouvier J, Delgado MB, Fernandez-Carneado J, Mottram JC, Vergeres G, Mauel J. Novel peptide inhibitors of Leishmania gp63 based on the cleavage site of MARCKS (myristoylated alanine-rich C kinase substrate)-related protein. Biochem J. 2002;367:761–769. doi: 10.1042/BJ20020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Datta K, Sundberg C, Karumanchi SA, Mukhopadhyay D. The 104–123 amino acid sequence of the beta-domain of von Hippel-Lindau gene product is sufficient to inhibit renal tumor growth and invasion. Cancer Res. 2001;61:1768–1775. [PubMed] [Google Scholar]