Abstract
Periostin, also called osteoblast-specific factor 2 (OSF-2), is a member of the fasciclin family and a disulfide-linked cell adhesion protein that has been shown to be expressed preferentially in the periosteum and periodontal ligaments, where it acts as a critical regulator of bone and tooth formation and maintenance. Furthermore, periostin plays an important role in cardiac development. Recent clinical evidence has also revealed that periostin is involved in the development of various tumors, such as breast, lung, colon, pancreatic, and ovarian cancers. Periostin interacts with multiple cell-surface receptors, most notably integrins, and signals mainly via the PI3-K/Akt and other pathways to promote cancer cell survival, epithelial–mesenchymal transition (EMT), invasion, and metastasis. In this review, aspects related to the function of periostin in tumorigenesis are summarized.
Keywords: Periostin, Cancer, Tumorigenesis, Extracellular matrix (ECM), Epithelial–mesenchymal transition (EMT), Metastasis
Introduction
Periostin, originally designated osteoblast-specific factor 2 (OSF-2), was first identified in a mouse osteoblastic cell line as a cell adhesion protein for pre-osteoblasts [1, 2]. Thereafter, orthologous genes in human, rat, and zebrafish were also cloned [3–5]. Most current studies of periostin’s role in development have focused on its expression and function in bones, teeth, and the heart. Periostin has been shown to be highly expressed in early osteoblastic cells in vitro and in periosteum and periodontal ligaments in vivo and to play a potential role in formation and structural maintenance of bones and teeth [2]. During their development, periostin is expressed in teeth and surrounding tissues. Mice lacking periostin exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype [6]. As an important developmental factor and a secreted adhesion molecule, periostin has also been shown to be involved in the development of heart valves and other tissues [7–9]. Periostin is also re-expressed in adults after myocardial [7, 10, 11], vascular [12], and skeletal muscle [13] injuries or bone fracture [14]. In addition, recent studies have reported that periostin is frequently found to be highly expressed in various types of human cancer cell lines in vitro and human cancer tissues in vivo [15–18].
In this review, we will focus on findings related to the current understanding of the role of periostin in tumorigenesis and summarize the molecular mechanisms of periostin in these activities.
Structure of periostin
The murine periostin gene is located on chromosome 3, while human periostin is found on chromosome 13q [19]. The length of mouse periostin cDNA is 3,187 bp, with an 18-bp 5′ untranslated region, a 733-bp 3′ untranslated region, and a 2,436-bp open-reading frame, which encodes a protein of 811 amino acids with a MW of 90.2 kDa [1]. Currently, five different forms of human periostin have been isolated, due to multiple splicing events that can occur within the C-terminal domain. Alternative splicing of the C-terminus gives rise to periostin isoforms [1]. Isoforms lacking the entire carboxyl domain have been shown to inhibit cell motility and migration [20]. The first two isolated human periostin cDNAs were screened from placental and osteosarcoma cDNA libraries, using mouse periostin cDNA as a probe. The human placental periostin open-reading frame encodes a protein of 779 amino acids, with a MW of 87.0 kDa, while the human osteosarcoma periostin open-reading frame encodes a protein of 836 amino acids with a MW of 93.3 kDa. Homology analysis shows that periostin is highly conserved between mouse and human. The amino acid identity between the two species is 89.2% for the entire protein and 90.1% for the mature form. However, as compared to other regions within the mature periostin protein, the C-terminal region shows slightly less conservation, with 85.5% identity.
Periostin is a unique, evolutionarily conserved extracellular matrix (ECM) protein that shares high homology with the insect axon guidance protein fasciclin 1 (FAS1). In Drosophila, the ancestral fasciclin domain functions as an adhesion molecule linked to axonal guidance, migration, and differentiation [1, 19, 21]. FAS1-like domains exist in many proteins from various species, including bacteria and plants, suggesting that this domain represents an evolutionarily ancient adhesion domain. Therefore, periostin has been assigned to the fasciclin family, which includes βig-h3 (TGF-β-induced gene clone 3), stabling I and II, MBP-70, algal-CAM, and periostin-like factor (PLF) [22]. These proteins all contain repeats of the FAS1 domain, each consisting of 150 amino acids. Periostin protein contains a typical N-terminal secretory signal sequence but lacks a typical transmembrane domain. Adjacent to the signal sequence is a cysteine-rich domain, followed by four internal homologous repeat regions which precede the C-terminal hydrophilic domain [1, 2]. The four internal homologous repeats in periostin are homologous to FAS1 and are thought to be important for periostin’s adhesive activity.
Periostin has been shown to interact with other ECM proteins, such as fibronectin, tenascin-C, collagen V, and periostin itself [23, 24]. Periostin co-localizes with fibronectin, tenascin-C, and collagens, known components of subepithelial fibrosis of bronchial asthma, indicating that periostin forms a reticular structure by binding to these ECM proteins [24]. Functionally, periostin interacts with integrins to support cell adhesion and the spreading of chondrocytes, fibroblasts, and cancer cells.
Expression of periostin in clinical cancers
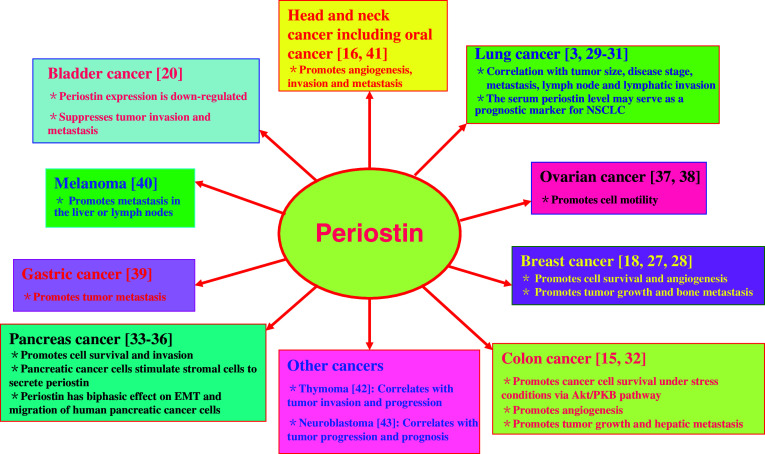
Periostin is a unique ECM protein found in collagen-rich connective tissues and is highly expressed in the embryonic periosteum, cardiac valves, placenta, and periodontal ligaments, as well as in many adult tissues, and its deposition is augmented by an increase in mechanical pressure [6, 25, 26]. Recently, periostin was found to be overexpressed in various types of human cancer, such as non-small-cell lung carcinoma, breast cancer, colon cancer, head and neck cancer, ovarian cancer, and pancreatic ductal adenocarcinoma. Here, we focus on the expression of periostin in five prevalent human cancers: breast, lung, colon, pancreatic, and ovarian cancers (Fig. 1).
Fig. 1.
Expression and function of periostin in human cancers
Breast cancer
High levels of periostin expression are associated with human breast cancers [18, 27]. The level of periostin expression is undetectable in normal breast tissues or in an immortalized cell line derived from normal mammary epithelial cells. However, the expression of periostin is readily detectable in the vast majority of breast tumor samples, with an average level of periostin expression 20-fold higher than the baseline expression, defined by gene array data obtained from normal breast tissues [27]. Another report shows that serum periostin levels are elevated in breast cancer patients with bone metastases from breast, but not lung cancer [28].
Lung cancer
A recent report showed that periostin was expressed in 42% of 88 patients with non-small-cell lung cancer (NSCLC). Its expression was significantly correlated with tumor size, disease stage, metastasis, and lymph node and lymphatic invasion [29]. Another report also demonstrated that high expression of periostin in either stroma or tumor epithelia, correlated with male gender, higher stage, higher pT category, and larger tumor size; similarly, periostin expression in stroma also correlated with tumor relapse [30]. There also was a significant relationship between periostin expression and density of both circulatory and lymphatic microvessels. NSCLC patients with periostin expression have significantly poorer survival rates than patients showing no periostin expression [3, 29]. In addition, the serum periostin level may serve as a prognostic marker for NSCLC [31]. These data indicate that periostin correlates with increased tumor progression, angiogenesis, and lymphangiogenesis, as well as a worse prognosis for NSCLC patients.
Colon cancer
We have previously found that periostin is differentially overexpressed in more than 80% of human primary colon cancer samples [15]. Quantitative analysis indicates that two-thirds (20 of 29 cases) of examined primary colon carcinomas show a tumor/normal (T/N) ratio of periostin message expression higher than 5-fold, with one-third of these primary tumors having a T/N ratio higher than 10-fold. Furthermore, periostin is overexpressed in all cases of colon metastatic tumors in the liver. Importantly, the expression level of periostin in hepatic metastases, derived mostly from patients with relapses of their colon cancers, is noticeably higher than in matched primary colon tumors from the same patients (eight of nine cases) [15]. Another report also demonstrated that periostin is upregulated in primary colorectal cancers and liver metastases [32]. These results suggest that late-stage metastatic tumors express higher levels of periostin, which may play a role during the metastatic stage of colon cancer progression [15].
Pancreatic cancer
Periostin is overexpressed in a large set of pancreatic cancer tissues. Although the periostin transcript is exclusively expressed in tumor cells, the protein product is only detected in the extracellular matrix adjacent to cancer cells. There are significantly increased levels of periostin in the sera of pancreatic cancer patients, compared to non-cancer controls. Furthermore, periostin promotes the invasiveness of tumor cells and enhances the survival of tumor cells exposed to hypoxic conditions [33]. Several other reports dealing with pancreatic cancer have shown that the periostin protein is secreted from pancreatic stromal cells rather than cancer cells [34–36]. Therefore, the interaction between cancer cells and stromal cells plays a critical role in pancreatic cancer development, and periostin from pancreatic satellite cells might create a tumor-supportive microenvironment in the pancreas [35].
Ovarian cancer
Periostin transcription is up-regulated in epithelial ovarian tumors [37]. While periostin transcripts are expressed in several normal tissues and highly expressed in fetal tissues, they are not found in normal ovaries. Ovarian cancer cells secrete periostin, which can accumulate in malignant ascites in patients with ovarian cancer. There are not any significant changes in the serum levels of periostin in women with ovarian cancer, when compared with controls. However, the majority of ascites from ovarian cancer patients contain high levels of periostin [37, 38].
Other cancers
Periostin is also highly expressed in tumors from patients suffering from other cancers, including melanoma, gastric cancer, head and neck squamous cell carcinoma, oral squamous cell carcinoma, thymoma, and neuroblastoma, although it is not expressed in the patients’ normal tissues [3, 16, 39–43]. However, other studies have reported a down-regulation of periostin transcription in bladder carcinoma [20].
Role of periostin in the hallmarks of cancer
The development of human cancer, ultimately caused by genomic instability, involves a complex series of processes. During tumorigenesis, cancer cells can acquire some special capabilities, such as the ability to overcome the restraints of the microenvironment of nearby normal tissue, self-sufficiency for mitogenic signals, deregulation of the cell cycle, escape from apoptosis, and the potential for unlimited replication [44, 45]. Within a growing tumor mass, the genetic and epigenetic alterations generated also enable cancer cells to gain the ability to induce angiogenesis, invade neighboring tissues, and metastasize to distant organs [15]. Current reports have demonstrated that periostin plays a critical role in the acquisition of most of these hallmarks of cancer cells (Table 1).
Table 1.
Periostin and the hallmarks of cancer
| Hallmarks of cancer | Functions of periostin in hallmarks of cancer | Cancer types or cancer cell lines |
|---|---|---|
| Resistance to anti-proliferation signals and independence from exogenous growth factor signals | Pro-proliferation | Colon cancer, colorectal cancer MIF101 cells |
| Anti-proliferation | 293T, mouse melanoma B16F1 cells, MDA-MB-231 | |
| Evasion of apoptosis | Anti-apoptosis | Colon cancer, pancreatic cancer, breast cancer cells |
| Limitless replicative potential | Regulation of immortalization or senescence | None reported |
| Induction of angiogenesis | Pro-angiogenesis | Colon cancer, breast cancer, NSCLC, oral cancer |
| Evasion of the immune system | Escape from immunosurveillance | None reported |
| Tissue invasion and metastasis | Pro-metastasis | Colon cancer, NSCLC, oral cancer, breast cancer, head and neck cancer, oral cancer, gastric cancer, neuroblastoma, thymoma, pancreatic cancer cells |
| Anti-metastasis | Bladder carcinoma, pancreatic cancer cells | |
| Genomic instability | Genomic instability results in overexpression of periostin | Breast cancer |
Promoting or inhibiting cell proliferation
Cell proliferation is strictly regulated by the concerted actions of both mitogenic growth signals and anti-proliferative signals that converge on regulators of the cell cycle [44, 45]. Periostin has been reported to promote re-entry of differentiated mononucleated cardiomyocytes into the cell cycle, where such cell cycle re-entry required integrins and the PI3-K/Akt pathway [7]. Exposure of MIF101 colorectal cancer cells to periostin-induced a dramatic increase in cell proliferation [32]. To reveal the role of periostin in the progression of tumor development, Shao et al. [27] used three tumor cell lines, 293T, the highly invasive mouse melanoma cell B16F1, and the metastatic human breast cancer cell MDA-MB-231, to engineer stable cell lines that overexpress periostin. Interestingly, the proliferation rate of these periostin-producing cells was noticeably slower than that of the control cells in culture, suggesting that periostin does not promote proliferation of tumor cells in vitro. However, these periostin-overexpressing tumor cell lines showed a phenotype of accelerated growth and angiogenesis when planted as xenografts in immunocompromised SCID-Beige mice [27]. Kudo et al. [41] also demonstrated that periostin overexpression does not promote cell proliferation, but it dramatically enhances the invasiveness of the head and neck cancer cell lines HSC2 and HSC3. Since the activities of this mesenchyme-specific gene product may not be exclusively associated with the promotion of cell proliferation, evaluation of the potential contribution of periostin to the progression of tumorigenesis must be based on an assessment of its ability to promote tumorigenesis in in vivo studies of xenografts or transgenic animal model systems, rather than solely on in vitro studies in cell culture [27].
Evasion of apoptosis
It is well known that tumors grow in an uncontrolled manner, resulting from an imbalance between cell proliferation and death [45]. In contrast to normal cells, cancer cells can break the balance between pro- and anti-apoptotic factors to promote cell survival under conditions of environmental stress [15]. Our previous work has revealed that cancer cells can induce the expression of some secreted ECM proteins, such as periostin and OPN, to prevent apoptosis in the context of a tumor [15, 46–48]. Periostin can dramatically enhance the metastatic growth of colon cancer by promoting survival of both cancer cells and endothelial cells under stress conditions that are commonly associated with metastatic tumors and fast-growing tumor masses, such as hypoxia, nutrient depletion, and loss of adhesion [15]. In addition, periostin promotes the survival of human breast cancer cells under several stress conditions, including hypoxia, serum starvation, and acid conditions (our unpublished data). Periostin can also promote the survival of pancreatic cancer cells under hypoxic conditions [33]. Interestingly, pancreatic stellate cells can secrete periostin to perpetuate fibrogenic activity and support tumor cell growth under serum deprivation and hypoxia [35]. As a result of periostin’s promotion of cell survival, requirements for the establishment of metastatic colonies would be less stringent. Therefore, promoting cell survival or evading apoptosis might be one of the key mechanisms of periostin-enhanced tumor growth.
Limitless replicative potential
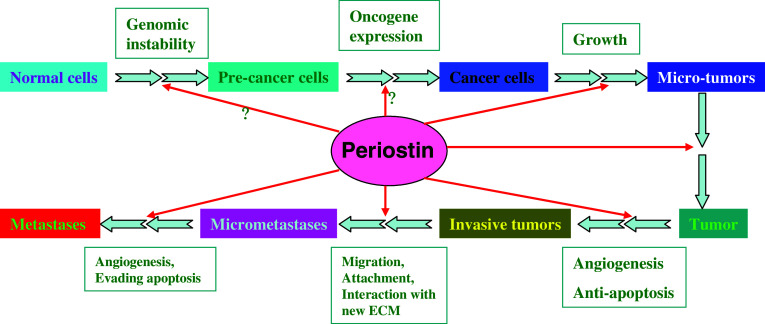
Oncogene-induced senescence is an important mechanism of tumor suppression that restricts the progression of benign tumors in the absence of additional cooperating mutations. It has been demonstrated that many genes trigger oncogene-induced senescence in vitro and in vivo [49]. Periostin has been shown to be highly expressed in immortalized human microvascular endothelial cells [50] and TesPDL cells, derived from miniature swine periodontal ligaments [51], where these cells are transfected with hTERT to prevent cell senescence. However, there are no reports on the direct role of periostin as an oncogene product that results in the unlimited replicative potential of cancer cells. We also do not know whether overexpression of periostin is sufficient to induce senescence in vitro and in vivo. Further studies are required to reveal the role of periostin in cell immortalization and evasion of senescence during tumorigenesis (Fig. 2).
Fig. 2.
Hypothetical illustration of the role of periostin in tumorigenesis. This model depicts the potential role of periostin in regulating the transformation of normal cells into malignant cancer cells and, eventually, metastatic tumors. Periostin, which is highly expressed in various malignancies, interacts with integrins or other receptors to induce a variety of cellular events. Current reports have revealed that periostin contributes to malignancies mainly by preventing apoptosis and promoting angiogenesis, invasion, and metastasis. The roles of periostin in regulating cell proliferation, evasion of senescence and immunosurveillance, and genomic instability of cancer cells during tumorigenesis still require further investigation
Induction of angiogenesis
It is now well established that unrestricted growth of tumors is dependent upon angiogenesis [45, 52]. There is accumulating evidence indicating that many molecules can promote angiogenic signaling cascades in endothelial cells [53]. VEGF, which acts through its membrane tyrosine kinase receptors VEGF receptor 1 (Flt-1) and receptor 2 (Flk-1/KDR) is one of the most potent angiogenic molecules. As a mesenchyme-specific gene product, periostin has also been defined as a novel potent angiogenic factor for tumor growth [27, 50]. One of the mechanisms by which periostin dramatically enhances metastatic growth of colon cancer is through augmentation of human endothelial cell survival, which promotes angiogenesis [15]. Overexpression of periostin in human breast cancers leads to a significant enhancement of angiogenesis. The underlying mechanism of periostin-mediated induction of angiogenesis has been found to derive, in part, from the upregulation of Flk-1/KDR by endothelial cells through an integrin αvβ3-focal adhesion kinase (FAK)-mediated signaling pathway [27]. Thus, although periostin may confer a growth advantage to breast tumors in vivo by altering the microenvironment through promotion of angiogenesis, its overexpression may impose a growth disadvantage when the tumor cells are grown in culture [27]. In oral cancers and NSCLC, periostin is also frequently overexpressed and enhances angiogenesis and invasion [16, 29]. Therefore, epithelial cell-derived tumors may gain the ability to generate more blood vessels, invade, and metastasize during late stages of tumorigenesis via the acquired expression of genes that normally are associated only with mesenchymal cells [27].
Tissue invasion and metastasis
Tumor invasion and metastasis is a multifaceted, controlled, and complicated process in the final phases of tumor development; it includes intravasation, survival in the circulatory system, arrest and extravasation into a new tissue, initiation and maintenance of growth, and reactivation of angiogenesis, in order to successfully establish metastatic colonies in the parenchyma of distant organs [15]. To complete this journey, cancer cells utilize numerous strategies, all of which lead to the same goal: the establishment of secondary sites of tumor growth [54]. In this multistep process, metastasis requires interactions between cancer and stromal cells, as well as between cancer cells and the ECM. Alterations of components in the ECM within the tumor microenvironment exert a considerable impact on the metastatic process [55].
Periostin, a fasciclin-containing adhesive ECM glycoprotein, promotes tumor metastasis not only in breast, lung, and colon cancers, as mentioned above, but also in melanoma, gastric cancer, head and neck squamous cell carcinoma, thymoma, and neuroblastoma. In melanoma samples, the average periostin expression is not increased in primary tumors, whereas periostin overexpression is detected in about 60% of metastatic melanoma tumors in the liver or lymph nodes [40]. Periostin is also overexpressed in gastric cancer and lymph node metastases [39]. Overexpression of periostin is frequently observed in head and neck squamous cell carcinoma and is believed to promote angiogenesis, tumor invasion, and metastasis of oral squamous cell carcinoma cases [16, 41]. In addition, periostin mRNA levels correlate with neuroblastoma tumor progression and prognosis [43]. Serum periostin levels are not significantly different between most thymoma patients and controls; however, the serum periostin level of stage IV thymoma patients is significantly higher than that of controls, suggesting that serum periostin level may indicate tumor invasion and progression of thymoma [42].
One critical step in tumor metastasis is termed epithelial–mesenchymal transition (EMT), which enables epithelial cancer cells to acquire invasive and metastatic potential [56–59]. Periostin has been shown to facilitate the migration and differentiation of cells that have undergone EMT, both during embryogenesis and in pathological conditions [60]. Furthermore, periostin is associated with EMT during cardiac development [19, 61]. Periostin is expressed throughout all stages of murine tooth development, especially in the embryonic sites of epithelial-mesenchymal interaction and in later newborn cells that transdifferentiate from one phenotype to another [62]. Various aggressive tumors are characterized by overexpression of periostin. As a mesenchyme-specific gene product, periostin is a potential contributor to metastasis and EMT in tumor progression. Stable expression of periostin in tumorigenic, but nonmetastatic, 293T cells induces those cells to undergo EMT and promotes cell migration, invasion, and adhesion [63]. High expression of periostin is also noted during EMT of cancer cells in NSCLC [30].
However, periostin may play a role as a suppressor of invasion and metastasis in the progression of human bladder cancers [20, 64]. The induced expression of periostin in pancreatic cancer cells (to levels of 150 ng/ml) can inhibit EMT and reduce cell migration in vitro as well as lead to formation of smaller tumors and suppression of metastasis in vivo. On the other hand, a high concentration of recombinant periostin (1 μg/ml) promotes cell migration with Akt activation [34]. Periostin can bind different integrin receptors, and different cancers express specific integrins. In addition, there are different spliced periostin isoforms in different tissues. These isoforms are not expressed uniformly but are differentially expressed in various cells [1, 2, 64]. Taken together, these results suggest that context influences the function of periostin, as related to tumor invasion and metastasis.
Genomic instability
It is crucial for cells to maintain their genomic integrity and stability because the DNA contained in every mammalian cell is under constant attack by many stresses and damaging agents [65, 66]. It is well known that genomic instability, one of the hallmarks of human cancer, is responsible for cellular changes that confer progressive transformation on cancer cells [45]. Genetic defects in DNA repair mechanisms and cell cycle checkpoints result in increased genomic instability and cancer predisposition [67, 68]. The genetic instability of tumorigenesis allows cancer cells to frequently bypass these systems. A recent paper has shown that periostin is overexpressed in Brca1 mutant cancer cells [69]. The Brca1 tumor suppressor, a checkpoint protein, plays a role in homologous recombination and may function in DNA repair by serving as a scaffold for ATM and ATR, thereby facilitating phosphorylation of downstream targets [70, 71]. Inherited mutations in Brca1 predispose individuals to breast and ovarian cancer; for carriers, the lifetime risk of breast cancer is 60–80%, and the risk of ovarian cancer is 25–50% [71–74]. Quaresima et al. [69] used microarray analysis to explore the gene expression pattern produced by the cancer-associated Brca1 5083del19 founder mutation. They found that periostin was significantly upregulated in HeLa/(5083del19)Brca1 cells, compared with both HeLa/(pcDNA3.1/empty) and HeLa/(wt)Brca1 cells. This finding was confirmed both in vitro, in breast cancer cell lines harboring mutations in Brca1, and in vivo, in breast cancer specimens bearing the 5083del19 Brca1 mutation as well as sera obtained from patients and healthy carriers of the same mutation [69]. Since cancer is caused by a multistep process of sequential alterations in several oncogenes and tumor suppressor genes, periostin overexpression, together with Brca1 mutations, may be key steps in tumorigenesis of some breast and ovarian cancers.
Periostin activation of intracellular signaling pathways in tumorigenesis
Integrins: versatile integrators of periostin-mediated cell signaling
Integrins are transmembrane, heterodimeric receptors with noncovalently associated α and β subunits and are involved in both cell–cell and cell–ECM interactions [75]. Through integrins, cells can sense dimensionality and other physical and biochemical properties of the glycoproteins in the extracellular matrix, either in basement membranes or the interstitial matrix, or sense other ligands on the surface of neighboring cells [76]. Hence, integrins constitute the majority of receptors for sensing the environment of the cell. In addition to sensing the environment through their extracellular region, integrins are also able to engage several effectors on their cytosolic side. Through coupling to kinases, scaffolding proteins, or small GTPases, integrins modulate intracellular signaling pathways to determine adhesion, migration, polarity, survival, growth, or death of the cell [77].
The expression of integrins is frequently altered in tumors [78]. Cancer cells that express a wide variety of integrins can constitutively activate signaling pathways to promote tumor cell growth, survival, and migration [45, 79]. Current studies have shown that periostin is upregulated in various tumors and enhances cancer cell proliferation, survival, angiogenesis, and metastasis. Identified integrin receptors of periostin that play a role in tumorigenesis include αvβ3, αvβ5, and α6β4. Receptors αvβ3 and/or αvβ5 are thought to regulate adhesion and migration of ovarian, breast, colon, and oral cancer cells [15, 38]. Gillan et al. [38] have found that purified recombinant periostin protein supports the adhesion of ovarian epithelial cells, which can be inhibited by monoclonal antibodies against αvβ3 or αvβ5 integrin, but not by anti-β1 integrin antibody. Furthermore, αvβ3 integrins, but not β1 integrin, co-localize with the focal adhesion plaques formed on periostin. Cells plated on periostin form fewer stress fibers and are more motile than those plated on fibronectin. Therefore, periostin functions as a ligand for αvβ3 and αvβ5 integrins to support the adhesion and migration of ovarian epithelial cells [38].
In determining whether integrins are involved in the activity of periostin to promote survival of human colon cancer cells (CX-1NS) and microvessel endothelial cells (HMVECs), we have found that the activation of the Akt/PKB survival pathway by periostin is mediated primarily through the αvβ3 integrin signaling pathway [15]. The angiogenic activity of periostin has been correlated with the increased expression of the VEGF receptor Flk-1/KDR on endothelial cells via an integrin αvβ3-FAK-mediated signaling pathway [27]. In addition, the α6β4 integrin complex acts as the receptor for periostin in pancreatic cancer cells [33]. At the mechanistic level, it is not surprising that periostin exerts its effect via integrins, because current reports have demonstrated that integrins on the surface of tumor cells and adhesion molecules in the ECM microenvironment are extremely important for tumor cell survival, growth, and migration [15, 80–82]. One recent report has shown that secreted periostin interacts with αvβ5 integrins, and the intracellular signaling activation via cross-talk between integrins, and EGFR promotes the cell to undergo EMT, resulting in tumor invasion and metastasis [63]. Nonetheless, we cannot yet rule out the existence of other receptors that transduce periostin signals in tumorigenesis.
PI3-K/Akt: a critical pathway in regulating periostin-induced tumorigenesis
Signals from the ECM are based on the engagement of integrins with specific matrix proteins to stimulate cell survival signaling molecules, including the extracellular signal-regulated kinases 1 and 2 (ERK1/2) and PI3-K in the MAP kinase cascade [83, 84]. It is now generally accepted that the PI3-K/Akt survival pathway is a central regulator of cell survival and proliferation [48, 85]. Akt functions as a cardinal node for transduction of extracellular and intracellular signals. It is positively regulated by PI3-K and negatively regulated by PTEN. Akt plays a critical role in a variety of cellular events including cell growth, motility, and survival, in both normal and cancer cells. The PI3-K/Akt pathway is also instrumental in EMT and angiogenesis during tumorigenesis. Deregulation of the PI3-K/Akt pathway is one of the most common signaling alterations in human malignancy [85]. Our previous work has revealed that periostin can dramatically enhance metastatic growth of colon cancer both by preventing stress-induced apoptosis in cancer cells and by augmenting endothelial cell survival via the Akt/PKB pathway [15]. Interestingly, OPN and SPARC are other secreted matrixcellular proteins that play important roles in the progression of tumor development [86–88]. Our recent results suggest that the underlying mechanism of OPN-mediated promotion of tumor development is largely associated with Akt activation, which enhances cell survival under stress [15, 46–48]. In addition, periostin can induce Akt phosphorylation via binding to α6β4 integrins and can activate the PI3-K/Akt pathway, rather than the RAF/MEK/ERK pathway, to promote the survival of pancreas cancer cells [33]. In this regard, acquired expression of periostin, OPN, SPARC, and similar types of proteins may enable tumor cells to thrive in a tumor microenvironment.
Regulators of periostin in tumorigenesis
Periostin expression has previously been shown to be significantly increased by both TGF-β and bone morphogenetic protein (BMP)-2 [2, 89]. BMP-2 induces cell migration and periostin expression during atrioventricular valvulogenesis [90]. Since TGF-β has been revealed to promote EMT and tumor metastasis, it is possible that periostin acts as an effector that mediates the pro-metastatic activity of TGF-β in certain cancers. Besides TGF-β and BMP-2, PDGF-bb, PDGF-aa, FGF-B, and FGF-A are also potent secretagogues for periostin in pancreatic stellate cells [35]. Periostin has also been shown to be regulated by twist, which is a basic helix–loop–helix (bHLH) transcription factor important for cell proliferation, migration, and differentiation in embryonic progenitor cell populations and transformed tumor cells. Twist can bind the periostin promoter in undifferentiated pre-osteoblasts and upregulate periostin expression [91]. The hypoxia-responsive growth factors FGF-1 and angiotensin II enhance periostin expression in pulmonary arterial smooth muscle cells by activation of the PI3-K/Akt/p70S6K, Ras/MEK1/2/ERK1/2, and Ras/p38MAPK signaling pathways, but not the Ras/JNK pathway [92]. We have also found that TGF-α and bFGF upregulate the expression of periostin to promote the survival of A549 lung cancer cells in a hypoxic microenvironment via activation of PI3-K/Akt pathway (our unpublished data). In addition, periostin can be regulated by Wnt-3 in mouse mammary epithelial cells [93] and by IL-4 or IL-13 in lung fibroblasts [24]. However, most of the data about the regulators of periostin have come from studies on embryonic or adult development. Further studies are necessary to identify the regulators of periostin in tumorigenesis.
Concluding remarks
A tumor-supportive microenvironment is critical for tumor cell proliferation, survival, angiogenesis, invasion, and metastasis because tumors require essential growth factors, survival signals, pro-angiogenic factors, and various adhesion molecules [94]. It is well accepted that tumors are composed of several distinct cell types, including cancer cells, immune cells, fibroblasts, and endothelial cells. Tumorigenesis largely depends on alterations in the heterotypic interactions between incipient cancer cells and their normal neighbors [45]. Cancer cells can modify the composition of the adjacent stroma by secreting their own ECM proteins to create a permissive and supportive environment for their growth [33, 95, 96]. However, it is still not well understood how cancer cells manipulate periostin and other ECM proteins to cooperate with other cell types within tumors to promote their own survival and growth under stressful microenvironments. A recent report demonstrated that once stimulated by pancreatic cancer cells, pancreatic stellate cells remain active via an autocrine periostin loop. This process is exacerbated by even radiotherapy, resulting in the production of an excess of ECM proteins, creating a tumor-supportive microenvironment. Therefore, pancreatic stellate cells can secrete excessive amounts of ECM proteins, including periostin, collagen-1, and fibronectin, which promote tumor growth under serum deprivation, hypoxia, and chemotherapeutic pressure [35]. The class of nonstructural ECM proteins, including SPARC, osteopontin, thrombospondin, and tenascin-C, is structurally diverse, but regulates similar biological functions during embryonic development, tissue injury, and tumorigenesis by promoting the adhesion, migration, and survival of cancer cells [33, 88, 97, 98]. In contrast to many defined oncogenes, the normal functions of this type of gene are not often associated with the promotion of cell proliferation. Instead, this group of proteins may exert their influence on tumorigenesis by changing the microenvironment through the regulation or alteration of cell adhesion, composition of the extracellular matrix, and the activities of stromal cells within and surrounding the tumor mass [27]. As an adhesive protein, periostin is highly expressed in the embryonic tissues and in several normal adult tissues, predominantly bone, and is strongly upregulated in some adult tissues after injury [6, 7]. Furthermore, periostin has been found to potently promote adhesive interaction through desmoplastic stroma and to enhance metastatic development of various cancers. Therefore, periostin can be regarded as a new member of the matrixcellular proteins; increased periostin expression may confer a selective advantage to cancer cells during the process of metastasis and reflect a more aggressive tumor phenotype.
It is important to note that the data obtained from reports on βig-h3 may also provide important clues about the role of periostin in development and tumorigenesis. βig-h3 and periostin are both TGF-β-induced ECM proteins, both have FAS1 domains, and both are assigned to the FAS1 family. βig-h3 contains a signal sequence at the N-terminus, an Arg-Gly-Asp (RGD) sequence at the C-terminus, and four FAS1 domains; it shares a significant structural homology with periostin [55, 99]. However, the RGD motif (an integrin recognition site) near the C-terminus in βig-h3 can be deleted without affecting cell adhesion [100]. βig-h3 does not contain any sequence homologous to the C-terminal hydrophilic domain in periostin, while periostin does not contain an RGD motif, suggesting that functional differences may exist between the two proteins during development and tumorigenesis [15, 17, 55]. As a secreted protein, the major function of βig-h3 is to mediate cell spreading, adhesion, proliferation, migration, and the promotion of tumorigenesis. Current studies have revealed that these functions are mediated through interactions between the FAS1 domains and integrin receptors, such as α3β1, αvβ5, αvβ3, and α6β4 [19]. Interestingly, although βig-h3 and periostin share significant sequence and structural homology, these two ECM proteins are involved in different processes in tumorigenesis. βig-h3 appears to promote colon cancer metastasis primarily during extravasation, a critical step in the metastatic dissemination of cancer cells, by inducing the dissociation of VE-cadherin junctions between endothelial cells via activation of the integrin αvβ5-Src signaling pathway [55]. However, as noted earlier, periostin has been shown to promote metastatic development of colon tumors by activation of the Akt/PKB signaling pathway through αvβ3 integrins to increase cell survival [15]. Therefore, the differences in their C-terminal domains may contribute to their differential integrin binding specificities, which, in turn, may result in differing impacts on tumor progression [15, 55].
Considering the key role periostin plays in bone and tooth formation, cardiac development, cardiovascular disease, oncogenesis, and tumor metastasis, this secreted protein could serve as a potential therapeutic target. Further studies will facilitate a deeper understanding of the mechanisms involved in the function, regulation, and biological activities of periostin in embryonic development and tumorigenesis.
Acknowledgments
We apologize to those research groups whose work was not included in this review due to space limitation. We would like to thank the members of the Laboratory of Cancer Cell and Molecular Biology for fruitful discussions and constructive comments. This work was supported by grants from the National Nature Science Foundation of China (No. 30570935, 30871242), NCETXMU, and a Berkeley Scholar Fellowship to G.O.
References
- 1.Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 1993;294(Pt 1):271–278. doi: 10.1042/bj2940271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 3.Sasaki H, Lo KM, Chen LB, Auclair D, Nakashima Y, Moriyama S, Fukai I, Tam C, Loda M, Fujii Y. Expression of periostin, homologous with an insect cell adhesion molecule, as a prognostic marker in non-small cell lung cancers. Jpn J Cancer Res. 2001;92:869–873. doi: 10.1111/j.1349-7006.2001.tb01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito T, Suzuki A, Imai E, Horimoto N, Ohnishi T, Daikuhara Y, Hori M. Tornado extraction: a method to enrich and purify RNA from the nephrogenic zone of the neonatal rat kidney. Kidney Int. 2002;62:763–769. doi: 10.1046/j.1523-1755.2002.00533.x. [DOI] [PubMed] [Google Scholar]
- 5.Kudo H, Amizuka N, Araki K, Inohaya K, Kudo A. Zebrafish periostin is required for the adhesion of muscle fiber bundles to the myoseptum and for the differentiation of muscle fibers. Dev Biol. 2004;267:473–487. doi: 10.1016/j.ydbio.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Rios H, Koushik SV, Wang H, Wang J, Zhou HM, Lindsley A, Rogers R, Chen Z, Maeda M, Kruzynska-Frejtag A, Feng JQ, Conway SJ. Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol. 2005;25:11131–11144. doi: 10.1128/MCB.25.24.11131-11144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 8.Lie-Venema H, Eralp I, Markwald RR, van den Akker NM, Wijffels MC, Kolditz DP, van der Laarse A, Schalij MJ, Poelmann RE, Bogers AJ, Gittenberger-de Groot AC. Periostin expression by epicardium-derived cells is involved in the development of the atrioventricular valves and fibrous heart skeleton. Differentiation. 2008;76:809–819. doi: 10.1111/j.1432-0436.2007.00262.x. [DOI] [PubMed] [Google Scholar]
- 9.Norris RA, Borg TK, Butcher JT, Baudino TA, Banerjee I, Markwald RR. Neonatal and adult cardiovascular pathophysiological remodeling and repair: developmental role of periostin. Ann NY Acad Sci. 2008;1123:30–40. doi: 10.1196/annals.1420.005. [DOI] [PubMed] [Google Scholar]
- 10.Stanton LW, Garrard LJ, Damm D, Garrick BL, Lam A, Kapoun AM, Zheng Q, Protter AA, Schreiner GF, White RT. Altered patterns of gene expression in response to myocardial infarction. Circ Res. 2000;86:939–945. doi: 10.1161/01.res.86.9.939. [DOI] [PubMed] [Google Scholar]
- 11.Wang D, Oparil S, Feng JA, Li P, Perry G, Chen LB, Dai M, John SW, Chen YF. Effects of pressure overload on extracellular matrix expression in the heart of the atrial natriuretic peptide-null mouse. Hypertension. 2003;42:88–95. doi: 10.1161/01.HYP.0000074905.22908.A6. [DOI] [PubMed] [Google Scholar]
- 12.Lindner V, Wang Q, Conley BA, Friesel RE, Vary CP. Vascular injury induces expression of periostin: implications for vascular cell differentiation and migration. Arterioscler Thromb Vasc Biol. 2005;25:77–83. doi: 10.1161/01.ATV.0000149141.81230.c6. [DOI] [PubMed] [Google Scholar]
- 13.Goetsch SC, Hawke TJ, Gallardo TD, Richardson JA, Garry DJ. Transcriptional profiling and regulation of the extracellular matrix during muscle regeneration. Physiol Genomics. 2003;14:261–271. doi: 10.1152/physiolgenomics.00056.2003. [DOI] [PubMed] [Google Scholar]
- 14.Nakazawa T, Nakajima A, Seki N, Okawa A, Kato M, Moriya H, Amizuka N, Einhorn TA, Yamazaki M. Gene expression of periostin in the early stage of fracture healing detected by cDNA microarray analysis. J Orthop Res. 2004;22:520–525. doi: 10.1016/j.orthres.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, Shao R, Anderson RM, Rich JN, Wang XF. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004;5:329–339. doi: 10.1016/S1535-6108(04)00081-9. [DOI] [PubMed] [Google Scholar]
- 16.Siriwardena BS, Kudo Y, Ogawa I, Kitagawa M, Kitajima S, Hatano H, Tilakaratne WM, Miyauchi M, Takata T. Periostin is frequently overexpressed and enhances invasion and angiogenesis in oral cancer. Br J Cancer. 2006;95:1396–1403. doi: 10.1038/sj.bjc.6603431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kudo Y, Siriwardena BS, Hatano H, Ogawa I, Takata T. Periostin: novel diagnostic and therapeutic target for cancer. Histol Histopathol. 2007;22:1167–1174. doi: 10.14670/HH-22.1167. [DOI] [PubMed] [Google Scholar]
- 18.Puglisi F, Puppin C, Pegolo E, Andreetta C, Pascoletti G, D’Aurizio F, Pandolfi M, Fasola G, Piga A, Damante G, Di Loreto C. Expression of periostin in human breast cancer. J Clin Pathol. 2008;61:494–498. doi: 10.1136/jcp.2007.052506. [DOI] [PubMed] [Google Scholar]
- 19.Litvin J, Zhu S, Norris R, Markwald R. Periostin family of proteins: therapeutic targets for heart disease. Anat Rec A Discov Mol Cell Evol Biol. 2005;287:1205–1212. doi: 10.1002/ar.a.20237. [DOI] [PubMed] [Google Scholar]
- 20.Kim CJ, Yoshioka N, Tambe Y, Kushima R, Okada Y, Inoue H. Periostin is down-regulated in high grade human bladder cancers and suppresses in vitro cell invasiveness and in vivo metastasis of cancer cells. Int J Cancer. 2005;117:51–58. doi: 10.1002/ijc.21120. [DOI] [PubMed] [Google Scholar]
- 21.Hortsch M, Goodman CS. Drosophila fasciclin I, a neural cell adhesion molecule, has a phosphatidylinositol lipid membrane anchor that is developmentally regulated. J Biol Chem. 1990;265:15104–15109. [PubMed] [Google Scholar]
- 22.Litvin J, Selim AH, Montgomery MO, Lehmann K, Rico MC, Devlin H, Bednarik DP, Safadi FF. Expression and function of periostin-isoforms in bone. J Cell Biochem. 2004;92:1044–1061. doi: 10.1002/jcb.20115. [DOI] [PubMed] [Google Scholar]
- 23.Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, Trusk T, Potts JD, Goodwin RL, Davis J, Hoffman S, Wen X, Sugi Y, Kern CB, Mjaatvedt CH, Turner DK, Oka T, Conway SJ, Molkentin JD, Forgacs G, Markwald RR. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem. 2007;101:695–711. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T, Izuhara K. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118:98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 25.Kikuchi Y, Kashima TG, Nishiyama T, Shimazu K, Morishita Y, Shimazaki M, Kii I, Horie H, Nagai H, Kudo A, Fukayama M. Periostin is expressed in pericryptal fibroblasts and cancer-associated fibroblasts in the colon. J Histochem Cytochem. 2008;56:753–764. doi: 10.1369/jhc.2008.951061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton DW. Functional role of periostin in development and wound repair: implications for connective tissue disease. J Cell Commun Signal. 2008;2:9–17. doi: 10.1007/s12079-008-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao R, Bao S, Bai X, Blanchette C, Anderson RM, Dang T, Gishizky ML, Marks JR, Wang XF. Acquired expression of periostin by human breast cancers promotes tumor angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol. 2004;24:3992–4003. doi: 10.1128/MCB.24.9.3992-4003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki H, Yu CY, Dai M, Tam C, Loda M, Auclair D, Chen LB, Elias A. Elevated serum periostin levels in patients with bone metastases from breast but not lung cancer. Breast Cancer Res Treat. 2003;77:245–252. doi: 10.1023/A:1021899904332. [DOI] [PubMed] [Google Scholar]
- 29.Takanami I, Abiko T, Koizumi S. Expression of periostin in patients with non-small cell lung cancer: correlation with angiogenesis and lymphangiogenesis. Int J Biol Markers. 2008;23:182–186. doi: 10.1177/172460080802300308. [DOI] [PubMed] [Google Scholar]
- 30.Soltermann A, Tischler V, Arbogast S, Braun J, Probst-Hensch N, Weder W, Moch H, Kristiansen G. Prognostic significance of epithelial–mesenchymal and mesenchymal–epithelial transition protein expression in non-small cell lung cancer. Clin Cancer Res. 2008;14:7430–7437. doi: 10.1158/1078-0432.CCR-08-0935. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki H, Dai M, Auclair D, Fukai I, Kiriyama M, Yamakawa Y, Fujii Y, Chen LB. Serum level of the periostin, a homologue of an insect cell adhesion molecule, as a prognostic marker in nonsmall cell lung carcinomas. Cancer. 2001;92:843–848. doi: 10.1002/1097-0142(20010815)92:4<843::AID-CNCR1391>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 32.Tai IT, Dai M, Chen LB. Periostin induction in tumor cell line explants and inhibition of in vitro cell growth by anti-periostin antibodies. Carcinogenesis. 2005;26:908–915. doi: 10.1093/carcin/bgi034. [DOI] [PubMed] [Google Scholar]
- 33.Baril P, Gangeswaran R, Mahon PC, Caulee K, Kocher HM, Harada T, Zhu M, Kalthoff H, Crnogorac-Jurcevic T, Lemoine NR. Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia-induced cell death: role of the beta4 integrin and the PI3k pathway. Oncogene. 2007;26:2082–2094. doi: 10.1038/sj.onc.1210009. [DOI] [PubMed] [Google Scholar]
- 34.Kanno A, Satoh K, Masamune A, Hirota M, Kimura K, Umino J, Hamada S, Satoh A, Egawa S, Motoi F, Unno M, Shimosegawa T. Periostin, secreted from stromal cells, has biphasic effect on cell migration and correlates with the epithelial to mesenchymal transition of human pancreatic cancer cells. Int J Cancer. 2008;122:2707–2718. doi: 10.1002/ijc.23332. [DOI] [PubMed] [Google Scholar]
- 35.Erkan M, Kleeff J, Gorbachevski A, Reiser C, Mitkus T, Esposito I, Giese T, Buchler MW, Giese NA, Friess H. Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology. 2007;132:1447–1464. doi: 10.1053/j.gastro.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 36.Fukushima N, Kikuchi Y, Nishiyama T, Kudo A, Fukayama M. Periostin deposition in the stroma of invasive and intraductal neoplasms of the pancreas. Mod Pathol. 2008;21:1044–1053. doi: 10.1038/modpathol.2008.77. [DOI] [PubMed] [Google Scholar]
- 37.Ismail RS, Baldwin RL, Fang J, Browning D, Karlan BY, Gasson JC, Chang DD. Differential gene expression between normal and tumor-derived ovarian epithelial cells. Cancer Res. 2000;60:6744–6749. [PubMed] [Google Scholar]
- 38.Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 2002;62:5358–5364. [PubMed] [Google Scholar]
- 39.Li JS, Sun GW, Wei XY, Tang WH. Expression of periostin and its clinicopathological relevance in gastric cancer. World J Gastroenterol. 2007;13:5261–5266. doi: 10.3748/wjg.v13.i39.5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tilman G, Mattiussi M, Brasseur F, van Baren N, Decottignies A. Human periostin gene expression in normal tissues, tumors and melanoma: evidences for periostin production by both stromal and melanoma cells. Mol Cancer. 2007;6:80. doi: 10.1186/1476-4598-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kudo Y, Ogawa I, Kitajima S, Kitagawa M, Kawai H, Gaffney PM, Miyauchi M, Takata T. Periostin promotes invasion and anchorage-independent growth in the metastatic process of head and neck cancer. Cancer Res. 2006;66:6928–6935. doi: 10.1158/0008-5472.CAN-05-4540. [DOI] [PubMed] [Google Scholar]
- 42.Sasaki H, Dai M, Auclair D, Kaji M, Fukai I, Kiriyama M, Yamakawa Y, Fujii Y, Chen LB. Serum level of the periostin, a homologue of an insect cell adhesion molecule, in thymoma patients. Cancer Lett. 2001;172:37–42. doi: 10.1016/S0304-3835(01)00633-4. [DOI] [PubMed] [Google Scholar]
- 43.Sasaki H, Sato Y, Kondo S, Fukai I, Kiriyama M, Yamakawa Y, Fuji Y. Expression of the periostin mRNA level in neuroblastoma. J Pediatr Surg. 2002;37:1293–1297. doi: 10.1053/jpsu.2002.34985. [DOI] [PubMed] [Google Scholar]
- 44.Elliott RL, Blobe GC. Role of transforming growth factor beta in human cancer. J Clin Oncol. 2005;23:2078–2093. doi: 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 45.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 46.Song G, Cai QF, Mao YB, Ming YL, Bao SD, Ouyang GL. Osteopontin promotes ovarian cancer progression and cell survival and increases HIF-1alpha expression through the PI3-K/Akt pathway. Cancer Sci. 2008;99:1901–1907. doi: 10.1111/j.1349-7006.2008.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song G, Ming Y, Mao Y, Bao S, Ouyang G. Osteopontin prevents curcumin-induced apoptosis and promotes survival through Akt activation via {alpha}v{beta}3 integrins in human gastric cancer cells. Exp Biol Med (Maywood) 2008;233:1537–1545. doi: 10.3181/0805-RM-164. [DOI] [PubMed] [Google Scholar]
- 48.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Courtois-Cox S, Jones SL, Cichowski K. Many roads lead to oncogene-induced senescence. Oncogene. 2008;27:2801–2809. doi: 10.1038/sj.onc.1210950. [DOI] [PubMed] [Google Scholar]
- 50.Shao R, Guo X. Human microvascular endothelial cells immortalized with human telomerase catalytic protein: a model for the study of in vitro angiogenesis. Biochem Biophys Res Commun. 2004;321:788–794. doi: 10.1016/j.bbrc.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 51.Ibi M, Ishisaki A, Yamamoto M, Wada S, Kozakai T, Nakashima A, Iida J, Takao S, Izumi Y, Yokoyama A, Tamura M. Establishment of cell lines that exhibit pluripotency from miniature swine periodontal ligaments. Arch Oral Biol. 2007;52:1002–1008. doi: 10.1016/j.archoralbio.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 52.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 53.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 54.Yilmaz M, Christofori G, Lehembre F. Distinct mechanisms of tumor invasion and metastasis. Trends Mol Med. 2007;13:535–541. doi: 10.1016/j.molmed.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 55.Ma C, Rong Y, Radiloff DR, Datto MB, Centeno B, Bao S, Cheng AW, Lin F, Jiang S, Yeatman TJ, Wang XF. Extracellular matrix protein betaig-h3/TGFBI promotes metastasis of colon cancer by enhancing cell extravasation. Genes Dev. 2008;22:308–321. doi: 10.1101/gad.1632008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 57.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 58.Thiery JP. Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 59.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial–mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 60.Lindsley A, Snider P, Zhou H, Rogers R, Wang J, Olaopa M, Kruzynska-Frejtag A, Koushik SV, Lilly B, Burch JB, Firulli AB, Conway SJ. Identification and characterization of a novel Schwann and outflow tract endocardial cushion lineage-restricted periostin enhancer. Dev Biol. 2007;307:340–355. doi: 10.1016/j.ydbio.2007.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Butcher JT, Norris RA, Hoffman S, Mjaatvedt CH, Markwald RR. Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through Rho/PI 3-kinase. Dev Biol. 2007;302:256–266. doi: 10.1016/j.ydbio.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kruzynska-Frejtag A, Wang J, Maeda M, Rogers R, Krug E, Hoffman S, Markwald RR, Conway SJ. Periostin is expressed within the developing teeth at the sites of epithelial–mesenchymal interaction. Dev Dyn. 2004;229:857–868. doi: 10.1002/dvdy.10453. [DOI] [PubMed] [Google Scholar]
- 63.Yan W, Shao R. Transduction of a mesenchyme-specific gene periostin into 293T cells induces cell invasive activity through epithelial–mesenchymal transformation. J Biol Chem. 2006;281:19700–19708. doi: 10.1074/jbc.M601856200. [DOI] [PubMed] [Google Scholar]
- 64.Yoshioka N, Fuji S, Shimakage M, Kodama K, Hakura A, Yutsudo M, Inoue H, Nojima H. Suppression of anchorage-independent growth of human cancer cell lines by the TRIF52/periostin/OSF-2 gene. Exp Cell Res. 2002;279:91–99. doi: 10.1006/excr.2002.5590. [DOI] [PubMed] [Google Scholar]
- 65.Bao S, Tibbetts RS, Brumbaugh KM, Fang Y, Richardson DA, Ali A, Chen SM, Abraham RT, Wang XF. ATR/ATM-mediated phosphorylation of human Rad17 is required for genotoxic stress responses. Nature. 2001;411:969–974. doi: 10.1038/35082110. [DOI] [PubMed] [Google Scholar]
- 66.Aguilera A, Gomez-Gonzalez B. Genome instability: a mechanistic view of its causes and consequences. Nat Rev Genet. 2008;9:204–217. doi: 10.1038/nrg2268. [DOI] [PubMed] [Google Scholar]
- 67.Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 68.Hartwell L. Defects in a cell cycle checkpoint may be responsible for the genomic instability of cancer cells. Cell. 1992;71:543–546. doi: 10.1016/0092-8674(92)90586-2. [DOI] [PubMed] [Google Scholar]
- 69.Quaresima B, Romeo F, Faniello MC, Di Sanzo M, Liu CG, Lavecchia A, Taccioli C, Gaudio E, Baudi F, Trapasso F, Croce CM, Cuda G, Costanzo F. BRCA1 5083del19 mutant allele selectively up-regulates periostin expression in vitro and in vivo. Clin Cancer Res. 2008;14:6797–6803. doi: 10.1158/1078-0432.CCR-07-5208. [DOI] [PubMed] [Google Scholar]
- 70.Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–182. doi: 10.1016/S0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 71.Deng CX. BRCA1: cell cycle checkpoint, genetic instability, DNA damage response and cancer evolution. Nucleic Acids Res. 2006;34:1416–1426. doi: 10.1093/nar/gkl010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 73.Friedman LS, Ostermeyer EA, Szabo CI, Dowd P, Lynch ED, Rowell SE, King MC. Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat Genet. 1994;8:399–404. doi: 10.1038/ng1294-399. [DOI] [PubMed] [Google Scholar]
- 74.Roskelley CD, Bissell MJ. The dominance of the microenvironment in breast and ovarian cancer. Semin Cancer Biol. 2002;12:97–104. doi: 10.1006/scbi.2001.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-S. [DOI] [PubMed] [Google Scholar]
- 76.Larsen M, Artym VV, Green JA, Yamada KM. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol. 2006;18:463–471. doi: 10.1016/j.ceb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 77.Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol. 2003;19:173–206. doi: 10.1146/annurev.cellbio.19.031103.133334. [DOI] [PubMed] [Google Scholar]
- 78.Varner JA, Cheresh DA. Integrins and cancer. Curr Opin Cell Biol. 1996;8:724–730. doi: 10.1016/S0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- 79.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 80.Jacks T, Weinberg RA. Taking the study of cancer cell survival to a new dimension. Cell. 2002;111:923–925. doi: 10.1016/S0092-8674(02)01229-1. [DOI] [PubMed] [Google Scholar]
- 81.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 82.Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, Werb Z, Bissell MJ. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–216. doi: 10.1016/S1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:E83–E90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- 84.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheng GZ, Park S, Shu S, He L, Kong W, Zhang W, Yuan Z, Wang LH, Cheng JQ. Advances of AKT pathway in human oncogenesis and as a target for anti-cancer drug discovery. Curr Cancer Drug Targets. 2008;8:2–6. doi: 10.2174/156800908783497104. [DOI] [PubMed] [Google Scholar]
- 86.Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16:79–87. doi: 10.1016/j.tcb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 87.Wai PY, Kuo PC. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev. 2008;27:103–118. doi: 10.1007/s10555-007-9104-9. [DOI] [PubMed] [Google Scholar]
- 88.Shi Q, Bao S, Maxwell JA, Reese ED, Friedman HS, Bigner DD, Wang XF, Rich JN. Secreted protein acidic, rich in cysteine (SPARC), mediates cellular survival of gliomas through AKT activation. J Biol Chem. 2004;279:52200–52209. doi: 10.1074/jbc.M409630200. [DOI] [PubMed] [Google Scholar]
- 89.Ji X, Chen D, Xu C, Harris SE, Mundy GR, Yoneda T. Patterns of gene expression associated with BMP-2-induced osteoblast and adipocyte differentiation of mesenchymal progenitor cell 3T3-F442A. J Bone Mineral Metab. 2000;18:132–139. doi: 10.1007/s007740050103. [DOI] [PubMed] [Google Scholar]
- 90.Inai K, Norris RA, Hoffman S, Markwald RR, Sugi Y. BMP-2 induces cell migration and periostin expression during atrioventricular valvulogenesis. Dev Biol. 2008;315:383–396. doi: 10.1016/j.ydbio.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oshima A, Tanabe H, Yan T, Lowe GN, Glackin CA, Kudo A. A novel mechanism for the regulation of osteoblast differentiation: transcription of periostin, a member of the fasciclin I family, is regulated by the bHLH transcription factor, twist. J Cell Biochem. 2002;86:792–804. doi: 10.1002/jcb.10272. [DOI] [PubMed] [Google Scholar]
- 92.Li P, Oparil S, Feng W, Chen YF. Hypoxia-responsive growth factors upregulate periostin and osteopontin expression via distinct signaling pathways in rat pulmonary arterial smooth muscle cells. J Appl Physiol. 2004;97:1550–1558. doi: 10.1152/japplphysiol.01311.2003. [DOI] [PubMed] [Google Scholar]
- 93.Haertel-Wiesmann M, Liang Y, Fantl WJ, Williams LT. Regulation of cyclooxygenase-2 and periostin by Wnt-3 in mouse mammary epithelial cells. J Biol Chem. 2000;275:32046–32051. doi: 10.1074/jbc.M000074200. [DOI] [PubMed] [Google Scholar]
- 94.Ribatti D, Nico B, Vacca A. Importance of the bone marrow microenvironment in inducing the angiogenic response in multiple myeloma. Oncogene. 2006;25:4257–4266. doi: 10.1038/sj.onc.1209456. [DOI] [PubMed] [Google Scholar]
- 95.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bissell MJ, Labarge MA. Context, tissue plasticity, and cancer: are tumor stem cells also regulated by the microenvironment? Cancer Cell. 2005;7:17–23. doi: 10.1016/j.ccr.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608–616. doi: 10.1016/S0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 98.Alford AI, Hankenson KD. Matricellular proteins: extracellular modulators of bone development, remodeling, and regeneration. Bone. 2006;38:749–757. doi: 10.1016/j.bone.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 99.Skonier J, Neubauer M, Madisen L, Bennett K, Plowman GD, Purchio AF. cDNA cloning and sequence analysis of beta ig-h3, a novel gene induced in a human adenocarcinoma cell line after treatment with transforming growth factor-beta. DNA Cell Biol. 1992;11:511–522. doi: 10.1089/dna.1992.11.511. [DOI] [PubMed] [Google Scholar]
- 100.Ohno S, Noshiro M, Makihira S, Kawamoto T, Shen M, Yan W, Kawashima-Ohya Y, Fujimoto K, Tanne K, Kato Y. RGD-CAP ((beta)ig-h3) enhances the spreading of chondrocytes and fibroblasts via integrin alpha(1)beta(1) Biochim Biophys Acta. 1999;1451:196–205. doi: 10.1016/S0167-4889(99)00093-2. [DOI] [PubMed] [Google Scholar]