Abstract
The ATP-binding cassette family is one of the largest groupings of membrane proteins, moving allocrites across lipid membranes, using energy from ATP. In bacteria, they reside in the inner membrane and are involved in both uptake and export. In eukaryotes, these transporters reside in the cell’s internal membranes as well as in the plasma membrane and are unidirectional—out of the cytoplasm. The range of substances that these proteins can transport is huge, which makes them interesting for structure–function studies. Moreover, their abundance in nature has made them targets for structural proteomics consortia. There are eight independent structures for ATP-binding cassette transporters, making this one of the best characterised membrane protein families. Our understanding of the mechanism of transport across membranes and membrane protein structure in general has been enhanced by recent developments for this family.
Keywords: ATP-binding cassette, Membrane protein, Structure, Transporter, Lipid bilayer
Introduction
ATP-binding cassette (ABC) transporters play a crucial role in eukaryotes, bacteria and archaea. These proteins constitute a very ancient family of transporters with phylogenetic evidence supporting the idea that the ABC transporter family diversified before bacteria, archaea and eukaryotes diverged on separate evolutionary paths [1]. ABC systems can be divided into three main functional categories which include importers mediating the uptake of nutrients; exporters involved in secretion; and those which are not involved with transport but in other cellular processes such as in ion flux, translation of mRNA and in DNA repair. For the purpose of this review, the focus will be on the ABC transporters, which are integral membrane proteins that actively transport molecules across the lipid membrane against a concentration gradient, hydrolysing ATP to ADP to generate energy to complete the transport process.
In the mid-1980s, the ABC-transporter superfamily was identified when sequence homology was detected between a eukaryotic multidrug efflux pump and a prokaryotic binding-protein-dependent importer [2, 3]. Defects in ABC transporters are responsible for a number of human diseases including cystic fibrosis and Tangier disease [4]. They have also been implicated in multidrug resistance in both human cancer cells and bacteria, as they pump diverse anti-cancer drugs and antibiotics into the extracellular space [5]. Genome sequencing has given us an indication of the importance of ABC transporters in normal cellular processes and ~52 ABC transporters have been identified in the human genome [4] and ~70–90 in that of different Escherichia coli strains [6–8] (see http://www1.pasteur.fr/recherche/unites/pmtg/abc/database.iphtml).
Basic structure of ABC transporters: the TMDs and NBDs
All ABC transporters share a common basic structure which consists of four domains: two transmembrane domains (TMDs) and two nucleotide-binding domains (NBDs). The four domains may be organised in several ways (Fig. 1). In many eukaryotic transporters, the four domains are contained within a single polypeptide, while in bacteria the four domains are typically found as four separate polypeptides.
Fig. 1.
Topology of ABC transporters, which consist of two transmembrane domains (TMDs), typically with six transmembrane spans per domain, and two cytoplasmic nucleotide-binding domains (NBDs) which catalyse nucleotide hydrolysis. The figure shows a version of this arrangement where all four domains are fused together as a single polypeptide, which is typical for eukaryotic ABC proteins. However, apart from the basic requirement for two TMDs and two NBDs, almost any arrangement of these can be found. In bacteria, some ABC transporters are encoded as a single polypeptide, where a TMD and an NBD are fused together, and a homodimer of the protein is the active form. In other cases, bacterial ABC transporters are encoded by two polypeptides—the TMD and NBD subunits—and a homodimer of heterodimers is formed. More complex versions also exist where either the TMD or NBD subunits are non-identical, i.e. three separate polypeptides are expressed to form the active transporter. Similarly, fused TMDs and NBDs are also possible. The sequence of N-terminus–TMD–NBD–C-terminus is the most common for transporters with fused TMD and NBD, but in some cases the sequence is reversed. The topology appears to be universally held, however, with the NBDs located in the cytoplasm. The membrane in which the ABC proteins are found is usually the plasma membrane (inner membrane in bacteria), but in eukaryotes some ABC transporters are found in organellar membranes, in which case the NBDs are still situated on the cytoplasmic side of the organelle membrane. Finally, ABC proteins have evolved with various additions and adaptations. Bacterial transporters in this family often have regulatory domains associated with the NBDs, whilst the TMDs may have several additional transmembrane spans. Fewer than six spans are also predicted for some prokaryotic TMDs, but so far no structures of this type have been solved with fewer than five transmembrane spans per TMD
The TMDs of the ABC transporter form the pathway across the membrane, through which substrate (allocrite) is transported. Low sequence similarities have been found between TMDs of various transporters reflecting the large diversity of substrates transported. For the eight ABC proteins for which crystal structures have been obtained, three distinct folds can be identified (types I, II, III), a surprising structural plasticity (Fig. 2). A recent study of the structural data and phylogenetic classification available on TMDs by Rees et al. [5], would also suggest that there may be many uncharacterised folds still to be revealed. In comparison, photosynthetic reaction centre proteins, which rival the ABC transporters in terms of available structural information, and out-do them in terms of the antiquity of their pedigree, all display a similar core fold [9]. Hence the ABC transporters could be a surprisingly rich source of novel transmembrane folds. The TMDs of ABC exporters are thought to require at minimum two TMDs with five to six transmembrane helices each, although TMDs with fewer than five transmembrane helices are predicted, and TMDs with more than six membrane-spanning helices have been characterised in structural terms (Fig. 2: MalF of the MalFGK2 importer [10], BtuC [11], the metal chelate transporter [12] and by biochemical topological studies). A conserved characteristic of TMDs found in importers and not exporters is the EAA motif or L-loop (see Fig. 4), which provides an interaction site between the NBD domain and the TMD [11, 13].
Fig. 2.
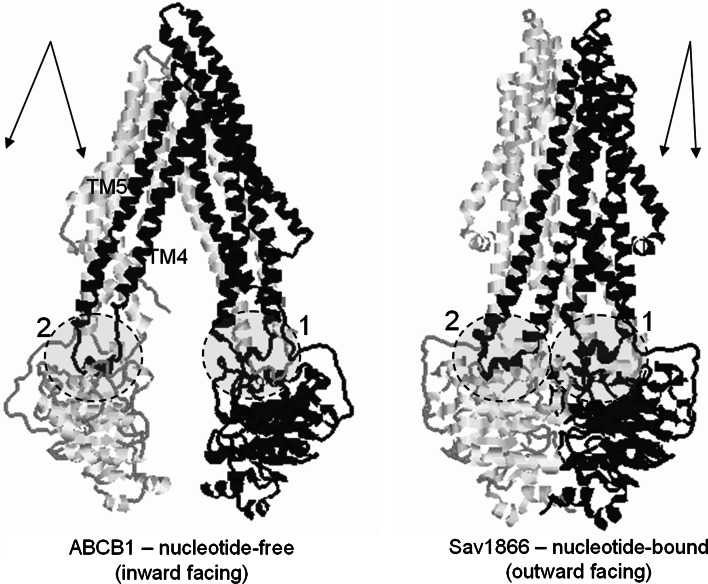
Family portrait: structures of ABC transporters. a Sav1866 homodimer [27]. b MalFGK2 with the periplasmic maltose-binding protein (cyan) [10]. c ModBC, with the periplasmic-binding protein ModA (purple) [28]. d BtuCD [11]. e Putative metal chelate transporter H10796 [12]. f MetNI methionine transporter [29]. g–i MsbA from S. typhimurium, V. cholera and E. coli. respectively [43]. j Murine P-glycoprotein a.k.a. ABCB1 [30]. The approximate location of the lipid bilayer is indicated by the pink band, as estimated by the boundaries of the outward-facing hydrophobic amino acids. Structures a and g–j are thought to be exporters, b–g are thought to be importers. a and g–i are homodimers with one TMD and one NBD fused together. Structures b–e consist of four polypeptide chains, forming the basic domain structure, whilst some have an accessory periplamic substrate-binding protein (b, c). In j, all the domains are fused as a single polypeptide, with the N-terminal half coloured green. The nucleotide-binding domain structures are strongly conserved (with the exception of h and i). Some structures (b, c, f) have an additional extension of the NBD, probably involved in regulation of transport activity. In contrast, the transmembrane domain structures are variable. At least three separate folds are apparent, and primary structure is hardly conserved at all. The unusual structure shown in i displays limited contacts between the two halves of the unit, which may be a product of the purification or crystallisation process. Some of these structures were determined in the presence of ATP (or another nucleotide), some in the absence of nucleotide. However, only two structures so far (b, j) show the allocrite (transported molecule) bound. All are ‘static’ structures that may need to be considered together to gain insights into the transport mechanism
Fig. 4.
Secreted substrates: buried co-factors and allocrites in ABC transporters. a The Sav1866 transporter (blue and yellow atoms representing the two polypeptides) in the presence of nucleotide (green atoms, AMP-PNP). The nucleotide-binding site is almost buried in the structure, as revealed when the front half of the structure is removed [91]. There is no pathway large enough to allow entrance/exit of nucleotide without significant conformational movements allowing the opening up of the NBD dimer. Note how the two polypeptides in Sav1866 both contribute to each nucleotide-binding pocket, a characteristic feature of the nucleotide-binding domains of ABC proteins. b The maltose transporter (MalFGK2) is the sole ABC protein structure where maltose, the allocrite (red atoms), is present. The maltose-binding pocket in the transmembrane region, formed by the F (blue) and G (yellow) TMD polypeptides, is completely occluded by the periplasmic maltose-binding protein (MBP, orange), and there is no obvious pathway to the cytoplasmic region, as revealed when the front half of the structure is removed [91]. In this view of the MalK dimer, there is a glimpse of the ATP-binding pocket from the outside, but this window is too narrow to allow exit/entrance of co-factor. The impression (perhaps false, see Table 1) gained from such images is of a very tight dimer of NBDs that concertedly bind the nucleotides
The NBDs of ABC transporters have highly conserved characteristic ‘cassettes’ or short defining sequences within a ~200 amino acid residue region (Fig. 3). All ABC-NBDs have a two-domain architecture which consists of a RecA-like catalytic domain [14] and a second, smaller helical domain. The overall general appearance of the NBD subunit is an L-shape. Both structural and biochemical evidence has led to proposals concerning the function of the conserved ABC motifs in ATP hydrolysis and the mechanism by which ATP hydrolysis is coupled to substrate transport. The signature motif, linker peptide, or LSSGQ motif is used to identify ABC transporters and is found between the Walker A and B motifs [15]. In the well-conserved NBD 3D structures, the signature sequence is located some distance from the ATP-binding site formed by the Walker A and B motifs, an observation of considerable significance for structure–function considerations, since it is only when a NBD dimer is formed that the signature sequence comes into play, interacting with the ATP bound by the opposing NBD Walker A and B motifs. This so-called sandwich dimer of NBDs gives a structural explanation for the highly conserved signature motif and implies that the dimeric structure of the ABC proteins probably emerged at a very early stage in the evolution of these proteins. In contrast to the signature motif, the Walker A motif is found in a huge variety of ATP- and GTP-hydrolysing proteins and makes contact with the phosphate moieties [16]. The A-loop located approximately 25 residues N-terminal to the Walker A motif contains a relatively well-conserved tyrosine residue involved in interactions with the adenine moiety of ATP [17, 18]. The Walker B motif also interacts with ATP and is involved in contacts with both the γ-phosphate of ATP and the Mg2+ cofactor through water molecules. A glutamate residue directly following the Walker B motif has also been suggested to act as the general base in the ATP hydrolysis reaction [19]. However, in another proposed mechanism for this reaction, ATP hydrolysis may be assisted by substrate-assisted catalysis [20]. In this case, the conserved histidine in the H-loop is thought to be important.
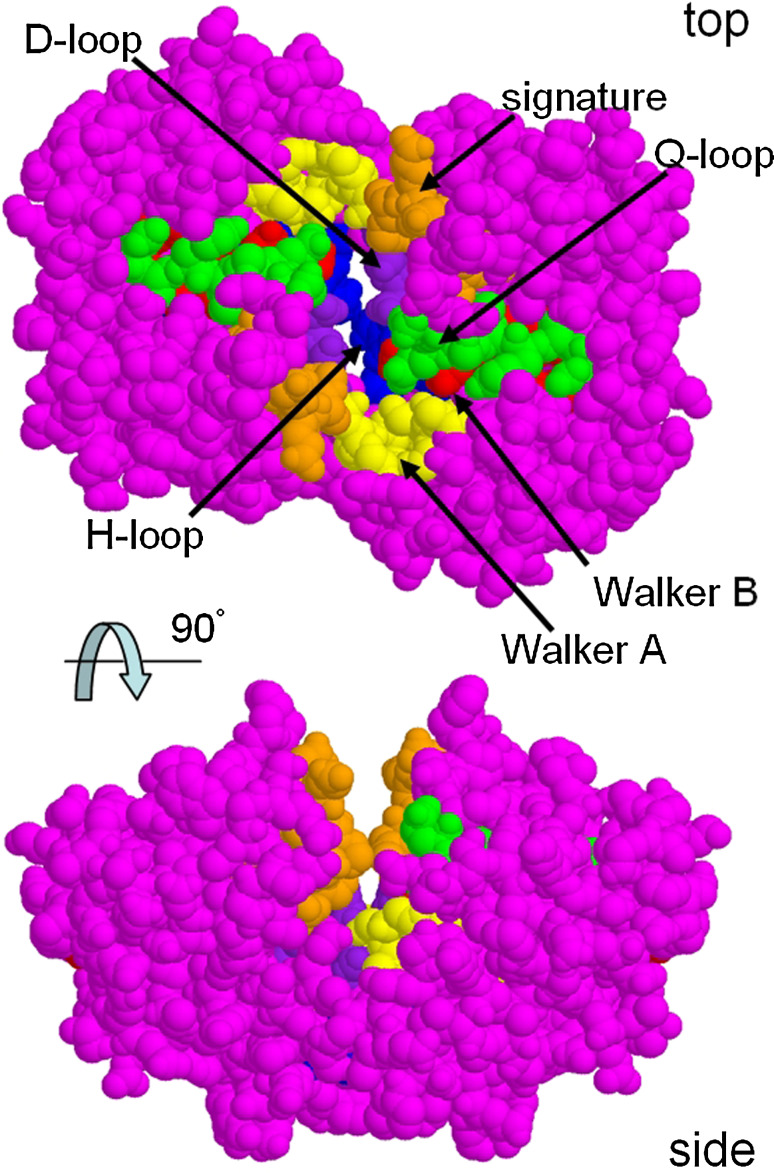
Fig. 3.
Conserved regions of the nucleotide-binding domains viewed from the transmembrane region (top) or from the side (parallel to the membrane plane). The signature region (orange atoms) and the Walker A and B regions (yellow and red atoms) all form the ATP-binding pockets, whilst the Q-loop (green atoms), D-loop (purple atoms) and H-loop (blue atoms) also contribute to the binding of two ATP molecules. Residues around the Q-loop may be involved in transmission of conformational changes to and from loops extending down from the TMDs and are placed appropriately for this interaction (top view). The BtuD NBD dimer is shown [11]
The D-loop contains the consensus sequence SALD and is located on the C-terminal side of the Walker B motif. The D-loop was found to contact residues from both the cis and trans NBD monomers in the ATP-binding sites. It may therefore function in communication between the two ATP-binding sites in the NBD dimer [20–22]. The Q-loop contains a single conserved residue, a glutamine, which like the Walker B motif contacts the γ-phosphate of ATP [23, 24].
Between the Q-loop and the ABC signature sequence, a structurally diverse region exists and is thought to target the NBDs to the cognate TMDs [25]. In bacteria where there are many ABC proteins (for example cyanobacteria, with >100 ABC proteins) and where many have their TMD and NBD subunits expressed as separate polypeptides, docking to the correct TMD by freshly synthesised NBD may represent a challenging molecular recognition problem. Hence diversity in the primary and tertiary structures of this region will be important for recognition. Conformational changes in the NBDs are thought to be transmitted to or from the TMDs via non-covalent interactions at this shared recognition interface through amino acid side chains around the Q-loop (Fig. 3). Despite the differences between TMDs of various ABC transporters, all so far are thought to feature ‘coupling helices’ which dock into cavities on the interfacial surface of the NBDs [26]. Differences between the contacts of these helices do, however, exist between importers and exporters, as exemplified in the eight ABC transporters for which high-resolution structural data have been produced to date. Exporter structures (thus far) display two intracellular loops from each TMD that dock into cavities in the NBDs, whilst importers appear to mainly interact via one loop (L-loop) per TMD containing the ‘EAA’ motif. Moreover in exporters such as Sav1866, the rough equivalent of the coupling helix found in the importer structures is swapped over, such that it interacts in a pocket of the NBD that is part of the opposing polypeptide [27].
In the crystal structures of the importers ModABC [28], BtuCD [11], HI1470/71 [12], MalFGK2 [10] and MetNI [29], there is no obvious equivalent of this swapping-over of contacts, but in all these cases, the NBDs are expressed as separate polypeptides, so the judgement of ‘swapping’ is somewhat arbitrary (see Table 1 below). Data from mutagenesis and cross-linking of eukaryotic ABC proteins have recently provided evidence that the same swap-over of TMD intracellular loops may exist, recently confirmed by the crystal structure of a eukaryotic ABC transporter [30].
Table 1.
Does the tail wag the dog? Theoretical free-energy change upon dissociation for various domains in ABC transporters, calculated using the PISA server of the European Bioinformatics Institute
| ATP-binding cassette protein complex or PDB ID | Co-factors bound | ΔG dissociation (kcal/mol) TMD–TMDa | ΔG dissociation (kcal/mol) NBD–NBDa |
|---|---|---|---|
| MalBP–MalFGK2 | ATP, Mal | 84.9 | 13.0 |
| MalF′GK2 | – | 62.1/62.0 | 3.2/2.8 |
| ModABC | WO4/PO4 | 80.6, 79.4 | 11.6, 11.5 |
| ModBC | WO4 | 83.5 | 8.8 |
| Sav1866b | ADP | 86.8 | 22.3 |
| Sav1866b | AMP-PNP | 84.4 | 14.8 |
| MsbA (S. typhimurium)b | AMP-PNP | 90.6 | 11.6 |
| BtuCD | VO4 | 20.1 | −0.3 |
| BtuCDF | SO4/PO4 | 41.4 | 3.2 |
| MetNI | – | 22.8, 24.8 | 3.0, 3.8 |
| Putative metal chelate transporter | – | 26.7 | 17.8 |
| ABCB1 (P-glycoprotein)b | – | 124.4 | No contacts |
| ABCB1 (P-glycoprotein)b | QZ59-RRR | 110.1 | No contacts |
| ABCB1 (P-glycoprotein)b | QZ59-SSS | 111.6 | No contacts |
| HlyB | ATP | 14.4,13.7 | |
| Rad50 | – | 28.4 | |
| UvrA | ADP | 6.7 | |
| CFTR–NBD1c | ATP | 5.5 | |
| TAPc | ATP | 6.2,3.5 | |
| MalK | ADP | 0.7, −0.6 | |
| MalK | – | 6.9 | |
| Multi-sugar transporter 1vci | ATP | 0.8 | |
| MJ0796 | ATP | 3.5, 3.6 | |
| Unknown function 2ihy | SO4 | 4.9 | |
| ArtP 2q0h | ADP | 0.1 | |
| Average ΔG dissociation | 69.7 | 8.0 |
The large positive values for the TMDs imply that these represent dimeric structures with high thermodynamic stability. In contrast, the generally low values for the NBDs suggest that NBD–NBD interactions are only weak (in one case a negative free energy, or favoured dissociation, is predicted). Unconventional hypotheses emerge when considering these theoretical calculations
aWhen two values are shown, there are more than one non-equivalent dimer in the crystallographic asymmetric unit
bSeparate coordinate files for the TMDs and NBDs were generated
cNon-physiological NBD–NBD homodimeric complex
NBD structures
The NBDs are considered to be the motor or energy-utilising domains of the ABC transporters. The first high-resolution structural data that emerged for the ABC family was for these more soluble domains. The first complete NBD subunit to be resolved at atomic resolution was that of the histidine transporter HisP [31]. HisP was first described as a dimer in which hydrophobic interactions between each monomer formed the dimer interface. An alternative model for the HisP dimer was later proposed [32]. In this new model, two bound ATP molecules were coordinated by residues from each monomer. From one monomer the ATP was in contact with the Walker A region and from the other monomer, it would have contact with the LSGGQ signature motif. This organisation is also commonly known as the ‘head-to-tail’ conformation. The structure of the catalytic domain of Rad50, a member of the ABC superfamily involved in double-strand DNA break repair, provided additional support for the second HisP dimer model [23]. The structure of Rad50 was solved in the presence and absence of ATP. Dimerisation was postulated to be ATP-dependent, and residues from each monomer were found to coordinate each of the two bound ATP molecules.
The next structure of a NBD to be reported was that of MalK, the maltose importer of Thermococcus litoralis [33]. Only the position of the pyrophosphate moiety with residues from the Walker A motif was seen in the MalK monomer, even though it had been crystallised in the presence of ADP. A dimer conformation for MalK was selected from a number of possible dimers based on the largest buried surface area at the dimer interface. This dimer showed a different organisation than both those previously suggested for HisP and Rad50. This surprising diversity in the quaternary organisation of the first NBD structures led to some confusion in their interpretation in terms of the hydrolysis mechanism and their potential interaction sites with the TMDs. We will return later in this review to consider the promiscuity of NBD–NBD interactions, and what this may tell us about the transport mechanism in these proteins.
The model represented by the Rad50 dimer is now generally accepted as the correct model for the physiological NBD dimer. Structural as well as biochemical data from other NBDs support this model, as well as the multiple structures for intact ABC transporters. A catalytically inactive mutant of MJ0796 from Methanococcus jannaschii showed a stable dimer when two bound molecules of ATP were coordinated between the Walker A motif of one monomer and the signature sequence of the other monomer, presenting a sandwich dimer [22]. Biochemical studies of vanadate-trapped MalK, demonstrated that both the Walker A and signature motifs were located adjacent to the ATP molecule during hydrolysis [34]—a result that was consistent with the sandwich dimer model. The transition state structure/conformation is thought to be trapped with vanadate, which mimics the γ-phosphate of ATP after cleavage. Following UV irradiation, specific cleavage of the residues adjacent to the trapped vanadate ion occurs [35–37]. Biochemical evidence for the location of ATP within the NBD dimer was also obtained for the eukaryotic multidrug exporter P-glycoprotein using oxidative cross-linking of engineered cysteine residues [38]. Since then, many biophysical and biochemical studies have confirmed the validity of the sandwich dimer configuration proposed by Jones and George.
It is accepted that the structures of NBDs are highly conserved between various ABC transporters and that they comprise the ‘engine’ of the transport process, binding and hydrolysing ATP, whilst the Walker A and Walker B motifs, H-loop and signature motifs are responsible for the binding and hydrolysis of ATP [39]. The Q- and D-loops are thought to couple the allocrite-binding sites within the TMDs to the ATP-binding sites of the NBDs, as well as having a role in NBD–NBD interactions [39, 40]. The free-energy change brought about by the hydrolysis of ATP evidently drives substrate translocation, but there is still considerable debate about whether ATP is needed to initiate transport events or whether its role is merely to re-set the transporter to a resting state. Whether the NBDs operate at the start (initiation) or end (re-setting) of the transport cycle, is inconsequential from a thermodynamic, hence evolutionary perspective, but in either scenario, the coupling of the NBDs’ activity to TMD configuration is key. Hence the NBDs can be considered as ATP-regulated switches that can be coupled to a variety of different functions, and not necessarily restricted to energy-requiring roles such as the transport of substances against a concentration gradient.
Structures of intact ATP-binding cassette transporters: models of the transport mechanism
To date, structures for eight intact ABC transporters are currently available. These include three exporters, Sav1866, MsbA and P-glycoprotein (ABCB1), and five importers, BtuCD, HI1470/HI1471, ModABC, MalFGK2 and MetNI [10–12, 27–30, 41–44]. In addition, there are lower-resolution structural studies of ABC proteins using transmission electron microscopy [45–54] and small-angle X-ray scattering [55].
The high-resolution structures of intact ABC transporters reveal different interactions between the TMD proteins and the NBD proteins. However, the structures do not immediately suggest a substrate-specific channel formed by the helices in the TMD proteins. All the structures are closed off on either the cytoplasmic or extracytoplasmic side of the transporter, implying that significant conformational reorganisation is required for transport to occur (see Figs. 2, 4). Associations of the NBD monomers in the complete transporter structures range from the tight sandwich dimer to a configuration where almost no NBD–NBD contacts exist. Although the ATP-binding sites are clearly exposed in the view from the transmembrane region of the NBD sandwich dimer (Fig. 3), when the TMDs are present, the sites are almost entirely buried, as illustrated in Fig. 4a and b. In the Sav1866 structure, the nucleotide molecules are almost invisible from the outside when a space-filling model of the structure is employed (Fig. 4a). It is only when the front half of the structure is stripped away that we can observe the buried nucleotides. This view through the centre of the structure illustrates the closing off of the TMDs on the cytoplasmic side. There is no hint of a channel through which allocrite might move. For the MalFGK2 transporter (Fig. 4b), the situation is similar. In the sandwich NBD dimer, the ATP molecules are almost buried. The view with the front half of the complex stripped away reveals a tightly packed TMD region with no visible channel for allocrite (maltose). This is the only ABC importer structure (so far) with the allocrite bound. The maltose is bound in a small pocket on the extracellular side of the TMDs (Fig. 4b) that is almost completely occluded from the exterior by the maltose-binding protein that sits on top of the MalF and G subunits like a lid. The only exporter structure with allocrite bound is the recently released murine P-glycoprotein (ABCB1) multi-drug transporter, which was generated in the absence of nucleotide [30]. This crystallographic study shows drugs bound at separate sites within a large internal cavity. This cavity is produced by an opening up of the structure and a separation of the two NBDs (see later sections).
The general conclusion from these structures is that major conformational changes must occur during the transport cycle. In order for ATP to bind and for ADP to be released in the Sav1866 and MalFGK2 protein complexes, the NBDs must move apart to create a route into and out of the binding sites. The extent of this movement is the subject of debate, but we know that the NBDs in the ModABC and particularly MetNI and P-glycoprotein structures are sufficiently far apart to allow easy access to the ATP-binding sites. For these transporters, the reverse is the case: significant conformational changes have to be invoked in order to bring the NBDs close together to form a catalytically active NBD dimer. Any NBD–NBD interactions in P-glycoprotein over such a signifcant distance must be long-range charge–charge interactions, as suggested by recent mutagenesis studies of the TAP NBDs [56]. Significant, though probably more local, conformational changes have to be hypothesised for the TMDs. In some fashion, the transport of allocrites will require the creation of a transient pathway through the TMDs (the MalFGK2 structure argues against the possibility that allocrite is passed around the outside of the TMDs). However, the creation of such a path must not lead to channel-like properties (which would lead to uncoupling of the membrane), implying that allocrite could be important in the control of this process, as well as acting as a molecular ‘bung’. Moreover, ABC transporters are active, not passive transporters, usually transporting substances against a concentration gradient. Thus the conformational changes generating the transport pathway in the TMDs must be tightly unidirectional, by being coupled to the hydrolysis of ATP.
Differences may also exist between the mechanisms of transport based on the fact that there are transporters that serve as importers and those that serve as exporters. A recent review of the structures of ABC transporters available, categorised the structures as belonging to three groups: small importers having TMDs with a five-helix core, large importers with 20 membrane-spanning helices, and exporters [57]. Most of the information that is available is for the structures of the small importers which include MalFGK2. The structure of the nucleotide-free maltose transporter in conjunction with the crystal structure of the transporter in two different conformations provides supporting evidence for a alternating access model of transport [58]. More information on how exporters work, which includes how they would bind substrate is required before a general transport mechanism can be applied to all ABC transporters.
Based on biochemical and structural information, models have been proposed for transport by ABC proteins [26, 59–61]. The models differ in a number of aspects, including the number of ATP molecules hydrolysed per transport event, when the ATP molecules are hydrolysed, and the point in the transport cycle at which substrate is translocated. The models suggest mechanisms for the coupling of conformational changes to the transport of allocrites, all stressing the importance of two discrete states of the transporter: the inward-facing and outward-facing configurations of the TMDs. Examples of these configurations were suspected to exist in the structural database of the ABC transporters (e.g. Sav1866 = outward facing, ModABC = inward facing), but the folds of the TMDs from these proteins were entirely different, preventing an unambiguous interpretation.
It was, finally, the generation of the MalFGK2 structure, which appeared to be in an outward-facing configuration, that allowed further insight into the mechanism of transport. Davidson and her co-workers noticed that MalFGK2 shared the same basic fold in the TMD region as ModABC (and more recently, MetNI) but that the ModB TMDs were noticeably more open towards the inner, cytoplasmic face (see Fig. 5). Aligning together one TMD from each transporter using the EAA loop (which interacts with the Q-loop region of the NBD, Fig. 3), it became clear that, in ModABC, the first membrane-spanning helix subtends a much larger angle versus the rest of the TMD than in the maltose transporter structure (arrows, Fig. 5). This helix reaches across to interact with the bundle of five helices from the opposing TMD, suggesting that such conformational shifts in one helix would be coupled to global shifts in the TMD configuration.
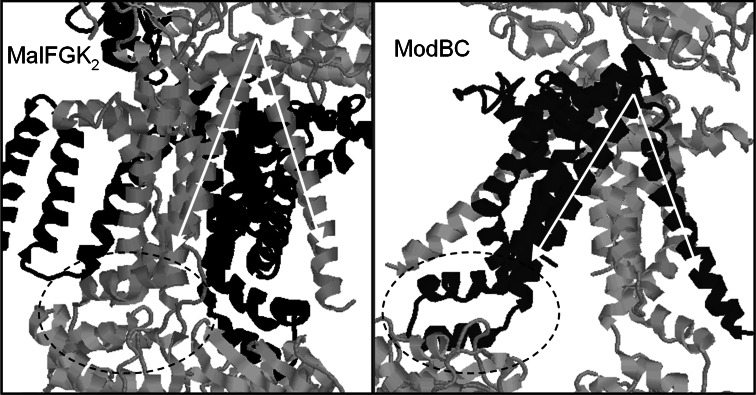
Fig. 5.
Dance of the TMDs. Comparison of the transmembrane regions of the MalFGK2 (left) and the ModBC [91] transporters, which share the same basic topological fold. Alignment of MalG (grey) and ModB (black) using the ‘EAA’ loop (dashed ellipse) which connects to the NBDs shows that the ModBC structure is more open to the cytoplasmic side due to an increased tilting of two alpha helices indicated by the white arrows which splay out the TMDs. Since the MalFGK2 structure is for the nucleotide-bound state, whilst the ModBC structure lacks nucleotide, this has led to the suggestion [92] that these structures could represent outward and inward facing conformations, i.e. snapshots of crucial stages in the transport cycle of ABC transporters [57]
Although the TMD configuration in the ModABC structure appears very open on the inward-face, the opposite is not particularly apparent for MalFGK2—in this case the TMDs do not appear very open on the external face. However, the presence of maltose in an external-facing pocket in the MalFGK2 structure is strongly persuasive, suggesting that this does indeed represent an outward-facing configuration for this protein complex. Whether the MalFGK2 structure represents an intermediate state or the most overtly outward-facing state possible for this transporter is debatable. The Sav1866 structure, for example, shows a much more starkly outward-facing profile than MalFGK2. Similarly, the Hl.1470/1471 structure [12], which has an inward-facing cavity in the TMDs, is not as starkly inward-facing in configuration as that seen for the ModABC, MetNI and P-glycoprotein structures. Any lingering questions about the validity of Davidson’s comparison of the outward-facing MalFGK2 with the inward-facing ModABC structure were answered recently by the publication of the structure of MalFGK2 in the absence of both nucleotide- and periplasmic-binding protein [58]. This structure is very similar to the ModABC structure, with a distinctive inward-facing configuration with a central cavity open to the cytoplasmic side of the protein, but closed off on the periplasmic side.
A schematic picture of the inward- and outward-facing configurations of the ABC importers is displayed in Fig. 6. What is not resolved in such schemes is the question of dynamics of the allocrite. We see two before-and-after snapshots of the process, but what is the nature of the pathway between these two states? There are no clearly conserved residues in the centre of the TMD helices that might point to a gate-like role. We could postulate that the allocrite is an integral part of the global conformational changes in the TMDs rather than acting as a relatively passive passenger molecule. TMDs of many ABC transporters, including P-glycoprotein and MsbA have been mapped using fluorescence spectroscopy showing substrate-binding pockets located in the TMD component [62–65]. Binding studies with MsbA with both lipid A (thought to be its native substrate), and daunorubicin, a chemotherapy drug, show separate binding sites within the TMD which were able to communicate with one another as well as the NBDs.
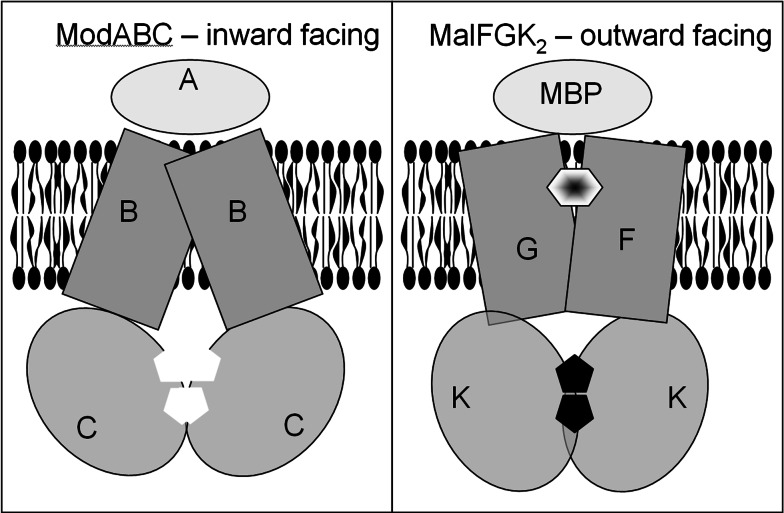
Fig. 6.
Working model: schematic representation of the inward- and outward-facing conformations proposed for the transport mechanism of ABC proteins. The left panel presents the inward-facing conformation, typified by the ModABC structure. The NBDs (red) have moved apart in the nucleotide-free state, and the TMDs (blue) are open to the cytoplasm. The right panel shows the outward-facing configuration typified by the structure of MalFGK2. The NBDs have moved together and concertedly bind ATP (black pentagons). The TMDs are more open to the extracellular medium, and a channel communicating between the periplasmic-binding protein (MBP) and the TMDs is partly occupied by the transported allocrite (maltose, hexagon)
Accessory domains: how do they help the transport process?
It is also important to mention that the specificity and regulation of the transport process can be complicated by accessory domains present in many importers as well as some exporters. These accessory domains can be divided into four groups: extracytoplasmic domains, membrane-embedded domains, cytoplasmic regulatory domains and cytoplasmic catalytic domains [66]. MalFGK2 has some of the best characterised accessory domains, one of which is a periplasmic substrate-binding domain. The periplasmic substrate-binding protein involved in maltose uptake is known as the maltose-binding protein (MBP). The MBP is the main determinant of substrate specificity of the maltose uptake system and confers a high affinity on the transport process. Recent evidence suggests that the periplasmic maltose-binding protein is required to stimulate ATPase activity in the MalFGK2 transporter [67]. In the absence of the MBP, ATP hydrolysis is prevented as both binding of ATP and the MBP are required to initiate a concerted conformational change that opens the MBP as MalK closes to hydrolyse the bound ATP. In order for the necessary conformational changes to occur in both the MBP and the transporter (MalFGK2), this complex must be relatively unstable [68]. A similar mechanism for the BtuCD transporter involved in vitamin B12 uptake has also been described where the substrate-binding protein, BtuF, is required for ATP hydrolysis [69].
A second accessory domain of the MalFGK2 is located C-terminal to the NBD. This domain interacts with MalT, the transcriptional regulator (activator) of the mal operon [70]. MalT is sequestered by MalK in the absence of the transporter substrate, maltotriose. In this resting state, the MalK subunits of the transporter are bound to ATP leaving the MalK subunits in an open conformation and bound to MalT, resulting in only basal level expression of the mal genes [71]. The interactions of the two proteins are thought to occur between two domains of MalT (DT1 and DT3) and the nucelotide-binding domain and regulatory domain of MalK [72–74]. MalK is also found to bind to an enzyme participating in the transport and phosphorylation of glucose, GlcIIA, which results in the inhibition of maltose transport when glucose is readily available [75, 76].
Specificity of the ABC transporters has also been characterised for exporters such as that involved in the secretion of haemolysin A by the ABC transporter HlyB. Using surface plasmon resonance, it has been demonstrated that the NBD of HlyB interacts with the C terminus of HlyA, the transporter substrate [77]. Another export system which has been characterised is the O-polysacchairde exporter of Escherichia coli O9a. The NBDs in this case (Wzt polypeptides) have an extended C-terminal region which has been crystallised and shown to be specific in binding the completed O-antigen [78]. The structure of the C-terminal domain (C-Wzt) showed a fold which is found in a number of carbohydrate-binding molecules. It is thought that this may serve as some type of accessory domain aiding in the transport of the mannose polysaccharide. Mutational analysis of C-Wzt suggests that the proximity of the C-terminal domain to the more conserved structure of the N-terminal portion of the NBD may allow for interaction of the substrate with this portion of Wzt as well [78]. In the polysaccharide export system an unresolved question remains as to whether the TMD Wzm has any role in substrate recognition as is seen in many of the eukaryotic exporters.
Another question that is of interest when looking at these transporters is how large molecules such as polysaccharides are transported. How does a concerted mechanism moving from an inward-facing to an outward facing conformation account for the activity of ABC transporters which transport large molecules and polymers where the substrate must be slowly threaded through the transporter? So far there is no information on structures of the transmembrane domains of such transporters in the ABC family. The NBD structures available for such transporters (e.g. for HlyB and TAP1) do not provide any clues about how large allocrites are handled in the ABC transport mechanism.
Wasteful hydrolysis of ATP
A further question that remains is why ATP hydrolysis does not run amok in cells containing ABC transporters. In multi-drug-resistant cancer cells which express high levels of ABC exporters such as ABCB1, ABCC1 and ABCG2, it is surprising that these cells can proliferate at all given the potential of the ABC proteins for ATP hydrolysis. Indeed, the same quandary could be argued to apply for the majority of cells, since ABC transporters are abundant and their Km for ATP is usually well below the cellular ATP concentration. Hence in the absence of a controlling factor, most ABC transporters would spend most of the time in the nucleotide-bound, sandwich dimer state and be hydrolysing ATP at significant basal rates. According to the models of the transport cycle, this would imply that the allocrite-binding site would be exposed on the external side of the cell membrane (outward-facing configuration) for most of the time. For importer ABC proteins such as MalFGK2, this could be a desirable state of affairs, but for exporters such as Sav1866, the configuration would be entirely wrong. In order for an ABC exporter to work, bound ATP would have to be hydrolysed and released, restoring the transporter to a high affinity/inward-facing configuration. Then allocrite would have to bind very rapidly before ATP could re-bind to the NBDs and convert the transporter back to its unproductive configuration. Since the allocrite is generally at a much lower activity than ATP in the cell, one would have to postulate many unproductive cycles of ATP binding and hydrolysis before a single translocation event would, by chance, occur. Data for the stoichiometry of ATP hydrolysis versus allocrite translocation are not abundant, but there is no indication from the available data that very high ratios of ATP per transported substrate are required for export [79]. Hence there are consequences of the current structure–function model that are inconsistent with the experimental data as well as being somewhat counter-intuitive for a protein family that has been subject to evolutionary pressure for perhaps billions of years.
In consequence, one of the models for transport which is becoming more generally accepted is the Switch Model, which divides transport into four different steps [40, 60, 80]. Substrate-binding sites in the TMDs are empty and the NBDs are in an open-dimer conformation in the basal state. When substrate binds the TMDs, transport is initiated. This leads to a conformational change in the NBDs, which in turn allows for ATP binding and closed dimer formation. Formation of the closed dimer results in a conformational change in the TMDs to facilitate substrate translocation. ATP is then hydrolysed, initiating the transition back to the open-dimer conformation in the NBDs. ADP and phosphate are released, resulting in the resetting of the transporter.
The Alternating Catalytic Site Model may also provide some solutions to the problem. This model suggests that the ATP binding at two different sites is coordinated with the binding of the substrate and essentially leads to the conformational changes required for substrate translocation and ATP hydrolysis [61]. However, isolated NBDs generally show high rates of ATPase activity, implying that tight sandwich dimer formation is not a pre-requisite for ATP hydrolysis. Hence some further regulatory mechanism separate from sandwich dimer formation is required in order to understand the mechanism of ABC transporters.
TMD versus NBD: Dog (NBD) wagging tail (TMD) or tail wagging dog?
These various concerns about the models of transport in ABC proteins suggest that an alternative conceptualisation is required that is still consistent with the structures observed by crystallography. One train of argument that could be considered arises from the structural database itself.
There are now many structures for bacterial ABC proteins in the protein databank (PDB), the large majority being for isolated NBD subunits of the proteins. Moreover there are a few structures for isolated NBD domains of eukaryotic ABC proteins, where the NBD has been separated from the TMD region by genetic engineering. A quick examination of the structural database for NBDs of ABC proteins shows that the majority of these structures are for monomeric NBDs. Of course, some of these NBD ‘monomer’ structures do show protein–protein interactions as part of the crystallographic packing, but these interactions are different from the NBD dimer configuration determined for intact ABC proteins, as we have discussed previously. This lack of correct NBD dimer structures in the database is somewhat surprising if one considers the formation of the sandwich dimer with ATP bound as an energetically favoured state, i.e. a state that drives translocation of allocrite across the membrane. It is reasonable to suppose that the crystal trials that were set up for the isolated NBDs will have included various nucleotides, and indeed some of the monomer structures are with nucleotide bound. An example of this behaviour is HisP, the first ABC protein polypeptide for which a structure was determined [31]. Showing non-physiological protein–protein contacts, this protein also had nucleotide bound in the crystal structure. Hence the presence of nucleotide does not necessarily favour the so-called sandwich dimer configuration of the NBDs, at least under the conditions of crystallisation of HisP and various other NBD subunits.
Analysis of the available structures for intact ABC transporters as well as for NBD–NBD dimers gives rise to some unexpected predictions. Using the Proteins Interfaces Surfaces and Assemblies (PISA) server of the European Bioinformatics Institute [81], theoretical free energies for dissociation of ABC subunits can be calculated (Table 1). TMD–TMD interactions are robust, with ΔG for dissociation averaging +70 kcal/mol over the available structures (range +124.4 to +20.1 kcal/mol). In contrast, NBD–NBD interactions are predicted to be much weaker, with ΔG for dissociation averaging +8 kcal/mol over the 29 structures analysed (range +28.4 to −0.3 kcal/mol). Indeed, the highest value for NBD–NBD interactions is for the Rad50 dimer [23], which, along with UvrA [82], has distinct function–structure relationships compared to the rest of the NBD–NBD dimers listed. These two proteins are not involved in transport processes. The theoretical calculations in Table 1 provide no support for the hypothesis that a NBD–NBD dimer is stabilised by the presence of nucleotide. For NBD–NBD dimers as a whole, there appears to be no obvious relationship between the presence of nucleotide and the predicted stability of the dimer. For example, MalK dimers are predicted to be slightly more stable in the absence rather than the presence of nucleotide.
It seems more likely therefore from these structural databases, that it is the TMDs that hold the complex together and dictate the movement of the NBDs towards the formation of the sandwich dimer configuration. This alternative conceptualisation leads to hypotheses about the mechanism of transport that emphasises the crucial coupling role of the TMDs over the NBDs: in this hypothesis, the TMDs in the absence of allocrite display an inward-facing configuration and prevent, or disfavour, formation of a sandwich dimer. It is only upon allocrite binding and a change in the TMDs to an outward-facing configuration that the formation of the NBD–NBD dimer and concerted nucleotide binding is allowed. Controlled hydrolysis of ATP and the release of products can occur, re-setting the inward-facing configuration of the TMDs. The coupling of conformational changes in the NBDs to the TMD region occurs only at the end of the cycle where ATP hydrolysis and release of ADP and phosphate re-set the transporter to an inward-facing configuration.
This model differs only marginally from the Switch model but emphasises the role of the TMDs and the allocrite in driving the conformational changes in the ABC transporters. How unproductive hydrolysis of ATP is prevented is, however, not explained by this conjecture. Single NBD subunits can hydrolyse ATP at significant rates, hence we must propose that in vivo, the TMDs in the allocrite-free ABC transporter not only separate the NBDs but also change their conformation into an inactive state for ATP hydrolysis.
Eukaryotic ABC transporters
Most of this review has been concerned with data obtained from prokaryotic members of the ABC transporter family. Eukaryotic ABC transporters are also very widespread, although it appears, so far, as though only exporters are present in these higher organisms [83]. Whilst structural data for eukaryotic ABC proteins are restricted, what has been obtained until now appears to be consistent with the data from prokaryotes. The NBDs of eukaryotic ABC transporters are highly similar to their bacterial counterparts [84–86], and a medium resolution structure for a eukaryotic multi-drug transporter (ABCB1) in the presence of nucleotide bears a strong resemblance to the Sav1866 structure [50, 55] (see Fig. 7). Very recently, the X-ray crystal structure of the same protein (ABCB1), but in the nucleotide-free state, has been published [30]. Hence for the first time, we are able to compare the structures of an export ABC transporter in both inward-facing and outward-facing conformations. Figures 7 and 8 imply that large conformational changes are associated with the transition from nucleotide-free to nucleotide-bound states.
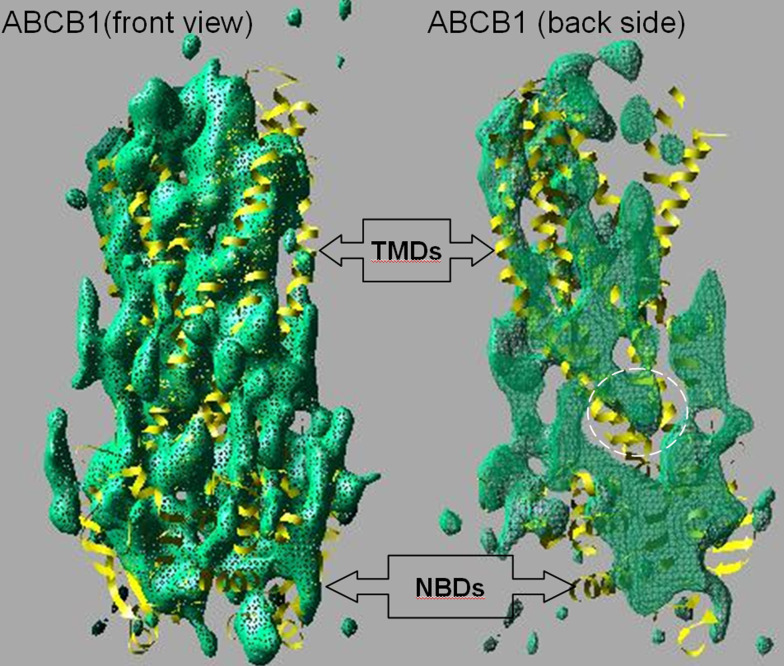
Fig. 7.
Family resemblances. Green netting shows the Coulomb density map of the eukaryotic ABC transporter P-glycoprotein (ABCB1) obtained in the presence of nucleotide using ~8-Å resolution electron crystallography data which have recently been combined with small-angle X-ray scattering data [50, 55]. The front of the map is removed in the right panel, and the netting has been made semi-transparent, allowing the interior and back of the map to be observed. When compared with the closest current homologous structure—that of Sav1866 ([27], yellow ribbon trace)—there is a striking similarity in overall shape and size as well as in the opposing tilts of the helices on the front and back sides of the map. The interior view (right panel) also shows some density matching with the intracytoplasmic loop II of Sav1866 that crosses over from one TMD to the opposing NBD (as indicated by the white dashed ellipse). These structural data therefore imply that ABCB1 probably possesses a similar overall architecture to the Sav1866 bacterial ABC exporter with ‘domain-swapping’ within the TMDs and NBDs. Tracing the polypeptide chain is not possible at the current resolution of the ABCB1 map
Fig. 8.
Dance of the TMDs, part 2. Conformational changes associated with allocrite-binding and nucleotide-binding, as suggested by comparison of the structures of ABCB1 (P-glycoprotein) in the absence of nucleotide and Sav1866 in the presence of nucleotide [91]. One half of each transporter is highlighted in black for clarity. The positions and identities of the intracytoplasmic loops of the TMDs are indicated by the dashed ellipses and numbers. The approximate orientations of transmembrane helices 4 and 5 (TM4, TM5) versus the rest of the TMD in each transporter are indicated by the double arrows. As in Fig. 5, nucleotide binding is apparently giving rise to a change in the angle between transmembrane α-helices in the TMD. TM helices 4 and 5, which are linked via intracytoplasmic loop 2, subtend a wider angle with the rest of the TMD in the nucleotide-free state. Because the intracytoplasmic loop 2 crosses over to connect to the opposing NBD, the change in angle of the transmembrane helices readily suggests a mechanism for controlling the formation of the sandwich dimer of NBDs as well as linking this to the generation of an outward-facing conformation of the transporter
Closer examination of the comparison, as well as extension of the exercise to the apparently outward-facing conformation of the Sav1866 structure, as shown in Fig. 8, implies that the closing up of the TMDs and NBDs is triggered by a change in the angle of the transmembrane helices, akin to that proposed by Davidson [57] for the ABC importers (see also Fig. 5). In this case, it is helices 4 and 5 of the Sav1866 structure (equivalent to helices 4, 5 and 10, 11 in ABCB1) that change their angle relative to the rest of the TMD. The hinge or hinges for this change of angle appear to be on the extracellular side of the TMD region, just as for the motion proposed for the ABC importers. The importance of TM helices 4 and 5 (and in ABCB1, the pseudo-symmetry related TM helices 10 and 11) in manifesting, or perhaps coordinating, the conformational change is paramount in exporter ABC proteins. It is these TM helices that extend down to connect on the cytoplasmic side via an intracytoplasmic loop that crosses over to contact the opposing NBD. Hence the structural data for ABCB1 and Sav1866 go a long way to explaining the exquisite coupling between the TMDs and NBDs in the transport cycle of the ABC proteins. Data from biochemical, biophysical and low-resolution structural studies had previously indicated that major conformational changes are present during the transport cycle of the eukaryotic ABC transporters [46, 48, 49], but the details of this change could only be guessed at. With the publication of the nucleotide-free structure of ABCB1 [30], we now have a useful framework describing the precise nature of the large conformational changes. This framework can now be tested by mutagenesis and other biochemical and biophysical approaches where systems closer to the in vivo state can be probed.
Influence of the lipid bilayer
All the structural data discussed so far for the ABC transporters were from detergent-solubilised complexes that have been crystallised at high protein and solute concentrations, rapidly frozen and then subjected to structural analysis using X-ray diffraction at temperatures close to 77 K. It is reasonable therefore to wonder whether structural insights from such a non-physiological set of conditions can be of any use to us. The answer to this, for proteins in general at least, is almost certainly ‘yes’, since the correspondence between the structures of proteins obtained in this fashion and information about their biological activity (both in vitro and in vivo) is exquisitely correlated. However, for membrane proteins, we must acknowledge that the conditions required for their crystallisation are even less physiological than for soluble proteins, and moreover that the measurement of full biological activity of the purified membrane protein is often impossible. More often than not, the activity of a membrane protein can only be manifested once it is present in a membrane—either a sealed vesicle across which concentration gradients can be studied—or in a membrane patch forming an electrical barrier where electrophysiology measurements can be made. So far no one has succeeded in forming 3D crystals of such a complex system in the absence of detergent, but two-dimensional crystals of membrane proteins embedded in lipid bilayers and vesicles have been studied for several decades using electron microscopy. Where comparisons are possible between structures derived from lipid-embedded and detergent-bound proteins, few differences have been noted [87–89], but the database for such comparisons is very small and is biased towards very stable and readily crystallisable membrane proteins. Hence, it will continue to be important for structural models of membrane proteins derived from X-ray crystallography to be validated and tested using separate biochemical and biophysical methods.
Influence of the lipid bilayer: ‘Mind the gap’
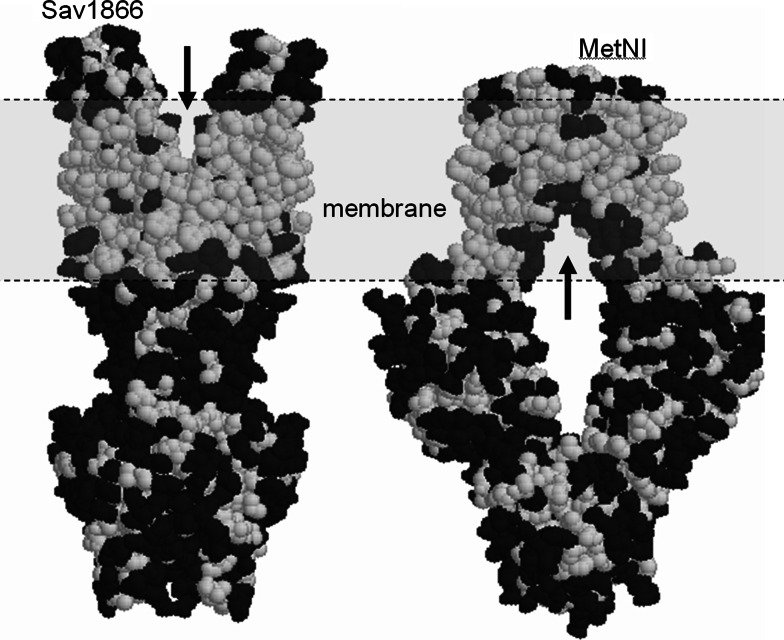
Several of the current structures of ABC transporters display gaps on the sides of the TMDs that would potentially allow access to and from the lipid bilayer (see Fig. 9). The Sav1866 structure and S. typhimurium MsbA structure, for example, display ‘V’-shaped openings to the presumed outer leaflet of the lipid membrane. In contrast, the suspected inward-facing configurations of the recently published MetNI structure, as well as the A. fulgidus ModABC and the trans-inhibited M. acetovirans ModBC structures clearly show inverted ‘V’-shaped gaps that presumably open to the inner leaflet of the membrane. Finally, the recent ABCB1 multi-drug exporter structure, obtained in the absence of nucleotide, also displays two inverted ‘V’-shaped gaps to the presumed inner leaflet of the membrane [30]. Such gaps into the lipid milieu are puzzling, and they are, by no means, commonly occurring features in the transmembrane regions of membrane proteins in general (where structures are available). Hence it is perhaps worth discussing the potential significance of the gaps for the functioning of these transporters.
Fig. 9.
Mind the gap. Structures of Sav1866 (left) and MetNI (right) [91] using space-filling representations for the non-hydrogen protein atoms. Hydrophobic amino acid residues are coloured white and polar residues are coloured black. A clear delineation between the transmembrane region and the extramembraneous regions is evident for each protein. A ‘V’-shaped gap extending towards the putative lipid bilayer (grey region) is present for each protein (arrows). For Sav1866, the gap would contact the outer leaflet of the lipid bilayer, whilst for MetNI [91] it would be the inner leaflet
For ABC transporters that move water-insoluble compounds across membranes, the gaps at first hand seem entirely reasonable. Access of allocrite to the transporter could, for example, be via gaps through to the hydrophobic lipid bilayer since it is likely that hydrophobic compounds will tend to partition mostly into the hydrophobic interiors of cellular membranes. Similarly, many ABC transporters that act as lipid flippases (e.g. MsbA) move the allocrite from one leaflet of the lipid bilayer to the other. Here, a movable gap communicating with the lipid environment would seem to be an essential component of such a system. However, ABC proteins, such as MetNI and ModABC, transport methionine and molybdate, which are water-soluble compounds. Hence there may be some other rationale for the presence of a gap connecting to the lipid bilayer.
If we imagine what might happen at the lipid–gap interface, two possibilities spring to mind: Firstly, flexible lipid molecules might diffuse into the centre of the transporter, filling both the gap and the internal chamber of the transporter molecule. In this way, a simple extension of the lipid leaflet would occur. However, conformational shifts from inward-to-outward facing modes would presumably require the expulsion of these bulky lipid molecules, which could be energetically costly and take significant time periods to occur. Also, in the publications describing the structures, bound lipid or detergent molecules in the ‘gap’ regions were not reported. Alternatively, the internal chamber could be water-filled, and therefore the gap would expose nearby hydrocarbon lipid tails to a polar aqueous environment. This could create an energetic tension in the system, rather like a spring. Here, closing off the gap during a conformational rearrangement would appear to be energetically favoured and could occur rapidly. Closer examination reveals that in Sav1866, ABCB1, MsbA, ModABC and MetNI, the internal cavities are lined with polar groups. This implies that the second scenario is more likely, i.e. a water-filled cavity will exist, contacting lipid chains via the gaps. Whether the gaps have a functional role in ABC transporters in general is therefore a moot point (particularly as the third ABC TMD fold, represented by the BtuCD and the HI1470/71 structures, does not display these gaps, and neither the inward- nor outward-facing structures for MalFGK2 display gaps to either lipid leaflet). These discussions emphasise the need to take into account the role of the lipid membrane for our understanding of the transport mechanism.
‘In-membrano’ structures
For ABC transporters, there are only a few reports of structural studies of 2D crystals generated in a lipid membrane [47, 54, 90], and only projection data or limited 3D information was available from these previous studies. However, a recent study of MsbA in tubular 2D crystals [51] generated 3D information to a resolution of about 1/20 Å−1. Comparison with the available X-ray crystal structure for S. typhimurium MsbA [43] indicated a good agreement—at least at the resolution indicated for the electron microscopy study. The overall conclusion so far is that the X-ray crystallography-derived configurations of ABC transporters in detergent appear to be similar to the corresponding state in a lipid bilayer. The only caveat to this statement is for the E. coli MsbA structure in detergent and in the absence of nucleotide [43], where the NBDs are widely separated and the TMDs form a very open inverted ‘V’ shape [43] with limited contact between the two bundles of six transmembrane helices in each half of the 3D structure (see Fig. 2i). Unfortunately, the nucleotide-free state of MsbA did not generate large enough 2D crystals in the membrane to allow a structure to be determined [51], and hence the unusual X-ray crystal structure remains to be confirmed or ruled out [51].
Conclusions
Structural analyses of the various transporters available from prokaryotes have allowed for the development of various models of the transport process; however, there are still many parts of the process that remain to be explained due to limitations in the methods currently available. Much remains to be characterised about the eukaryotic ABC transporters: the intermediate conformations of the transport process, the hydrolysis of ATP, and the communication process between TMD and NBD remain to be further portrayed. Nevertheless, the structural data available on ABC transporters have greatly advanced our understanding of this protein family and further endeavours should continue to surprise, fascinate and enlighten us.
Acknowledgements
The authors wish to acknowledge the many useful discussions, insights and explanations from the countless people who have contributed directly and indirectly to this review. In particular we would like to thank Richard Callaghan, Ian Kerr, Kenny Linton, Amy Davidson, Susan Cole, Jack Riordan, John Hunt, Geoffrey Chang, Stephen Aller, Andrew Ward, Tony George, Karl Küchler, Jim Naismith and Chris Whitfield for crystallising thoughts (as well as proteins) that have enabled this review to be written.
References
- 1.Fuellen G, Spitzer M, Cullen P, Lorkowski S. Correspondence of function and phylogeny of ABC proteins based on an automated analysis of 20 model protein data sets. Proteins. 2005;61:888–899. doi: 10.1002/prot.20616. [DOI] [PubMed] [Google Scholar]
- 2.Chen CJ, Chin JE, Ueda K, Clark DP, Pastan I, Gottesman MM, Roninson IB. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986;47:381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- 3.Gros P, Croop J, Housman D. Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell. 1986;47:371–380. doi: 10.1016/0092-8674(86)90594-5. [DOI] [PubMed] [Google Scholar]
- 4.Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res. 2001;42:1007–1017. [PubMed] [Google Scholar]
- 5.Rees DC, Johnson E, Lewinson O. ABC transporters: the power to change. Nat Rev Mol Cell Biol. 2009;10:218–227. doi: 10.1038/nrm2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linton KJ, Higgins CF. The Escherichia coli ATP-binding cassette (ABC) proteins. Mol Microbiol. 1998;28:5–13. doi: 10.1046/j.1365-2958.1998.00764.x. [DOI] [PubMed] [Google Scholar]
- 7.Dassa E, Bouige P. The ABC of ABCS: a phylogenetic and functional classification of ABC systems in living organisms. Res Microbiol. 2001;152:211–229. doi: 10.1016/S0923-2508(01)01194-9. [DOI] [PubMed] [Google Scholar]
- 8.Bouige P, Laurent D, Piloyan L, Dassa E. Phylogenetic and functional classification of ATP-binding cassette (ABC) systems. Curr Protein Pept Sci. 2002;3:541–559. doi: 10.2174/1389203023380486. [DOI] [PubMed] [Google Scholar]
- 9.Cogdell RJ, Gardiner AT, Hashimoto H, Brotosudarmo TH. A comparative look at the first few milliseconds of the light reactions of photosynthesis. Photochem Photobiol Sci. 2008;7:1150–1158. doi: 10.1039/b807201a. [DOI] [PubMed] [Google Scholar]
- 10.Oldham ML, Khare D, Quiocho FA, Davidson AL, Chen J. Crystal structure of a catalytic intermediate of the maltose transporter. Nature. 2007;450:515–521. doi: 10.1038/nature06264. [DOI] [PubMed] [Google Scholar]
- 11.Locher KP, Lee AT, Rees DC. The E. coli BtuCD structure: a framework for ABC transporter architecture and mechanism. Science. 2002;296:1091–1098. doi: 10.1126/science.1071142. [DOI] [PubMed] [Google Scholar]
- 12.Pinkett HW, Lee AT, Lum P, Locher KP, Rees DC. An inward-facing conformation of a putative metal-chelate-type ABC transporter. Science. 2007;315:373–377. doi: 10.1126/science.1133488. [DOI] [PubMed] [Google Scholar]
- 13.Mourez M, Hofnung M, Dassa E. Subunit interactions in ABC transporters: a conserved sequence in hydrophobic membrane proteins of periplasmic permeases defines an important site of interaction with the ATPase subunits. EMBO J. 1997;16:3066–3077. doi: 10.1093/emboj/16.11.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Story RM, Steitz TA. Structure of the recA protein-ADP complex. Nature. 1992;355:374–376. doi: 10.1038/355374a0. [DOI] [PubMed] [Google Scholar]
- 15.Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 16.Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases, and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ambudkar SV, Kim IW, Xia D, Sauna ZE. The A-loop, a novel conserved aromatic acid subdomain upstream of the Walker A motif in ABC transporters, is critical for ATP binding. FEBS Lett. 2006;580:1049–1055. doi: 10.1016/j.febslet.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 18.Kim IW, Peng XH, Sauna ZE, FitzGerald PC, Xia D, Muller M, Nandigama K, Ambudkar SV. The conserved tyrosine residues 401 and 1044 in ATP sites of human P-glycoprotein are critical for ATP binding and hydrolysis: evidence for a conserved subdomain, the A-loop in the ATP-binding cassette. Biochemistry. 2006;45:7605–7616. doi: 10.1021/bi060308o. [DOI] [PubMed] [Google Scholar]
- 19.Moody JE, Millen L, Binns D, Hunt JF, Thomas PJ. Cooperative, ATP-dependent association of the nucleotide binding cassettes during the catalytic cycle of ATP-binding cassette transporters. J Biol Chem. 2002;277:21111–21114. doi: 10.1074/jbc.C200228200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaitseva J, Jenewein S, Jumpertz T, Holland IB, Schmitt L. H662 is the linchpin of ATP hydrolysis in the nucleotide-binding domain of the ABC transporter HlyB. EMBO J. 2005;24:1901–1910. doi: 10.1038/sj.emboj.7600657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Lu G, Lin J, Davidson AL, Quiocho FA. A tweezers-like motion of the ATP-binding cassette dimer in an ABC transport cycle. Mol Cell. 2003;12:651–661. doi: 10.1016/j.molcel.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Smith PC, Karpowich N, Millen L, Moody JE, Rosen J, Thomas PJ, Hunt JF. ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Mol Cell. 2002;10:139–149. doi: 10.1016/S1097-2765(02)00576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hopfner KP, Karcher A, Shin DS, Craig L, Arthur LM, Carney JP, Tainer JA. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell. 2000;101:789–800. doi: 10.1016/S0092-8674(00)80890-9. [DOI] [PubMed] [Google Scholar]
- 24.Hopfner KP, Tainer JA. Rad50/SMC proteins and ABC transporters: unifying concepts from high-resolution structures. Curr Opin Struct Biol. 2003;13:249–255. doi: 10.1016/S0959-440X(03)00037-X. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt L, Benabdelhak H, Blight MA, Holland IB, Stubbs MT. Crystal structure of the nucleotide-binding domain of the ABC-transporter haemolysin B: identification of a variable region within ABC helical domains. J Mol Biol. 2003;330:333–342. doi: 10.1016/S0022-2836(03)00592-8. [DOI] [PubMed] [Google Scholar]
- 26.Hollenstein K, Dawson RJ, Locher KP. Structure and mechanism of ABC transporter proteins. Curr Opin Struct Biol. 2007;17:412–418. doi: 10.1016/j.sbi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Dawson RJ, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- 28.Hollenstein K, Frei DC, Locher KP. Structure of an ABC transporter in complex with its binding protein. Nature. 2007;446:213–216. doi: 10.1038/nature05626. [DOI] [PubMed] [Google Scholar]
- 29.Kadaba NS, Kaiser JT, Johnson E, Lee A, Rees DC. The high-affinity E. coli methionine ABC transporter: structure and allosteric regulation. Science. 2008;321:250–253. doi: 10.1126/science.1157987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hung LW, Wang IX, Nikaido K, Liu PQ, Ames GF, Kim SH. Crystal structure of the ATP-binding subunit of an ABC transporter. Nature. 1998;396:703–707. doi: 10.1038/25393. [DOI] [PubMed] [Google Scholar]
- 32.Jones PM, George AM. Subunit interactions in ABC transporters: towards a functional architecture. FEMS Microbiol Lett. 1999;179:187–202. doi: 10.1111/j.1574-6968.1999.tb08727.x. [DOI] [PubMed] [Google Scholar]
- 33.Diederichs K, Diez J, Greller G, Muller C, Breed J, Schnell C, Vonrhein C, Boos W, Welte W. Crystal structure of MalK, the ATPase subunit of the trehalose/maltose ABC transporter of the archaeon Thermococcus litoralis . EMBO J. 2000;19:5951–5961. doi: 10.1093/emboj/19.22.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fetsch EE, Davidson AL. Vanadate-catalyzed photocleavage of the signature motif of an ATP-binding cassette (ABC) transporter. Proc Natl Acad Sci USA. 2002;99:9685–9690. doi: 10.1073/pnas.152204499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cremo CR, Loo JA, Edmonds CG, Hatlelid KM. Vanadate catalyzes photocleavage of adenylate kinase at proline-17 in the phosphate-binding loop. Biochemistry. 1992;31:491–497. doi: 10.1021/bi00117a027. [DOI] [PubMed] [Google Scholar]
- 36.Grammer JC, Loo JA, Edmonds CG, Cremo CR, Yount RG. Chemistry and mechanism of vanadate-promoted photooxidative cleavage of myosin. Biochemistry. 1996;35:15582–15592. doi: 10.1021/bi961901g. [DOI] [PubMed] [Google Scholar]
- 37.Ko YH, Bianchet M, Amzel LM, Pedersen PL. Novel insights into the chemical mechanism of ATP synthase. Evidence that in the transition state the gamma-phosphate of ATP is near the conserved alanine within the P-loop of the beta-subunit. J Biol Chem. 1997;272:18875–18881. doi: 10.1074/jbc.272.30.18875. [DOI] [PubMed] [Google Scholar]
- 38.Loo TW, Clarke DM. Vanadate trapping of nucleotide at the ATP-binding sites of human multidrug resistance P-glycoprotein exposes different residues to the drug-binding site. Proc Natl Acad Sci USA. 2002;99:3511–3516. doi: 10.1073/pnas.022049799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaitseva J, Oswald C, Jumpertz T, Jenewein S, Wiedenmann A, Holland IB, Schmitt L. A structural analysis of asymmetry required for catalytic activity of an ABC-ATPase domain dimer. EMBO J. 2006;25:3432–3443. doi: 10.1038/sj.emboj.7601208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linton KJ, Higgins CF. Structure and function of ABC transporters: the ATP switch provides flexible control. Pflugers Arch. 2007;453:555–567. doi: 10.1007/s00424-006-0126-x. [DOI] [PubMed] [Google Scholar]
- 41.Dawson RJ, Hollenstein K, Locher KP. Uptake or extrusion: crystal structures of full ABC transporters suggest a common mechanism. Mol Microbiol. 2007;65:250–257. doi: 10.1111/j.1365-2958.2007.05792.x. [DOI] [PubMed] [Google Scholar]
- 42.Hvorup RN, Goetz BA, Niederer M, Hollenstein K, Perozo E, Locher KP. Asymmetry in the structure of the ABC transporter-binding protein complex BtuCD-BtuF. Science. 2007;317:1387–1390. doi: 10.1126/science.1145950. [DOI] [PubMed] [Google Scholar]
- 43.Ward A, Reyes CL, Yu J, Roth CB, Chang G. Flexibility in the ABC transporter MsbA: alternating access with a twist. Proc Natl Acad Sci USA. 2007;104:19005–19010. doi: 10.1073/pnas.0709388104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerber S, Comellas-Bigler M, Goetz BA, Locher KP. Structural basis of trans-inhibition in a molybdate/tungstate ABC transporter. Science. 2008;321:246–250. doi: 10.1126/science.1156213. [DOI] [PubMed] [Google Scholar]
- 45.Rosenberg MF, Callaghan R, Ford RC, Higgins CF. Structure of the multidrug resistance P-glycoprotein to 2.5 nm resolution determined by electron microscopy and image analysis. J Biol Chem. 1997;272:10685–10694. doi: 10.1074/jbc.272.3.1665. [DOI] [PubMed] [Google Scholar]
- 46.Rosenberg MF, Velarde G, Ford RC, Martin C, Berridge G, Kerr ID, Callaghan R, Schmidlin A, Wooding C, Linton KJ, Higgins CF. Repacking of the transmembrane domains of P-glycoprotein during the transport ATPase cycle. EMBO J. 2001;20:5615–5625. doi: 10.1093/emboj/20.20.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenberg MF, Mao Q, Holzenburg A, Ford RC, Deeley RG, Cole SP. The structure of the multidrug resistance protein 1 (MRP1/ABCC1). Crystallization and single-particle analysis. J Biol Chem. 2001;276:16076–16082. doi: 10.1074/jbc.M100176200. [DOI] [PubMed] [Google Scholar]
- 48.Rosenberg MF, Kamis AB, Callaghan R, Higgins CF, Ford RC. Three-dimensional structures of the mammalian multidrug resistance P-glycoprotein demonstrate major conformational changes in the transmembrane domains upon nucleotide binding. J Biol Chem. 2003;278:8294–8299. doi: 10.1074/jbc.M211758200. [DOI] [PubMed] [Google Scholar]
- 49.Rosenberg MF, Kamis AB, Aleksandrov LA, Ford RC, Riordan JR. Purification and crystallization of the cystic fibrosis transmembrane conductance regulator (CFTR) J Biol Chem. 2004;279:39051–39057. doi: 10.1074/jbc.M407434200. [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg MF, Callaghan R, Modok S, Higgins CF, Ford RC. Three-dimensional structure of P-glycoprotein: the transmembrane regions adopt an asymmetric configuration in the nucleotide-bound state. J Biol Chem. 2005;280:2857–2862. doi: 10.1074/jbc.M410296200. [DOI] [PubMed] [Google Scholar]
- 51.Ward A, Mulligan S, Carragher B, Chang G, Milligan RA. Nucleotide dependent packing differences in helical crystals of the ABC transporter MsbA. J Struct Biol. 2009;165:169–175. doi: 10.1016/j.jsb.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chami M, Steinfels E, Orelle C, Jault JM, Di Pietro A, Rigaud JL, Marco S. Three-dimensional structure by cryo-electron microscopy of YvcC, an homodimeric ATP-binding cassette transporter from Bacillus subtilis . J Mol Biol. 2002;315:1075–1085. doi: 10.1006/jmbi.2001.5309. [DOI] [PubMed] [Google Scholar]
- 53.Ferreira-Pereira A, Marco S, Decottignies A, Nader J, Goffeau A, Rigaud JL. Three-dimensional reconstruction of the Saccharomyces cerevisiae multidrug resistance protein Pdr5p. J Biol Chem. 2003;278:11995–11999. doi: 10.1074/jbc.M212198200. [DOI] [PubMed] [Google Scholar]
- 54.Lee JY, Urbatsch IL, Senior AE, Wilkens S. Projection structure of P-glycoprotein by electron microscopy. Evidence for a closed conformation of the nucleotide binding domains. J Biol Chem. 2002;277:40125–40131. doi: 10.1074/jbc.M206871200. [DOI] [PubMed] [Google Scholar]
- 55.McDevitt CA, Shintre CA, Grossmann JG, Pollock NL, Prince SM, Callaghan R, Ford RC. Structural insights into P-glycoprotein (ABCB1) by small angle X-ray scattering and electron crystallography. FEBS Lett. 2008;582:2950–2956. doi: 10.1016/j.febslet.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 56.Procko E, Gaudet R. Functionally important interactions between the nucleotide-binding domains of an antigenic peptide transporter. Biochemistry. 2008;47:5699–5708. doi: 10.1021/bi7024854. [DOI] [PubMed] [Google Scholar]
- 57.Oldham ML, Davidson AL, Chen J. Structural insights into ABC transporter mechanism. Curr Opin Struct Biol. 2008;18:726–733. doi: 10.1016/j.sbi.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khare D, Oldham ML, Orelle C, Davidson AL, Chen J. Alternating access in maltose transporter mediated by rigid-body rotations. Mol Cell. 2009;33:528–536. doi: 10.1016/j.molcel.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Callaghan R, Ford RC, Kerr ID. The translocation mechanism of P-glycoprotein. FEBS Lett. 2006;580:1056–1063. doi: 10.1016/j.febslet.2005.11.083. [DOI] [PubMed] [Google Scholar]
- 60.Higgins CF, Linton KJ. The ATP switch model for ABC transporters. Nat Struct Mol Biol. 2004;11:918–926. doi: 10.1038/nsmb836. [DOI] [PubMed] [Google Scholar]
- 61.Senior AE, al-Shawi MK, Urbatsch IL. The catalytic cycle of P-glycoprotein. FEBS Lett. 1995;377:285–289. doi: 10.1016/0014-5793(95)01345-8. [DOI] [PubMed] [Google Scholar]
- 62.Lugo MR, Sharom FJ. Interaction of LDS-751 with P-glycoprotein and mapping of the location of the R drug binding site. Biochemistry. 2005;44:643–655. doi: 10.1021/bi0485326. [DOI] [PubMed] [Google Scholar]
- 63.Qu Q, Sharom FJ. Proximity of bound Hoechst 33342 to the ATPase catalytic sites places the drug binding site of P-glycoprotein within the cytoplasmic membrane leaflet. Biochemistry. 2002;41:4744–4752. doi: 10.1021/bi0120897. [DOI] [PubMed] [Google Scholar]
- 64.Siarheyeva A, Sharom FJ. The ABC transporter MsbA interacts with lipid A and amphipathic drugs at different sites. Biochem J. 2009;419:317–328. doi: 10.1042/BJ20081364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smriti Zou P, McHaourab HS. Mapping daunorubicin binding sites in the ABC transporter MsbA using site-specific quenching by spin labels. J Biol Chem. 2009;284:13904–13913. doi: 10.1074/jbc.M900837200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Biemans-Oldehinkel E, Doeven MK, Poolman B. ABC transporter architecture and regulatory roles of accessory domains. FEBS Lett. 2006;580:1023–1035. doi: 10.1016/j.febslet.2005.11.079. [DOI] [PubMed] [Google Scholar]
- 67.Orelle C, Ayvaz T, Everly RM, Klug CS, Davidson AL. Both maltose-binding protein and ATP are required for nucleotide-binding domain closure in the intact maltose ABC transporter. Proc Natl Acad Sci USA. 2008;105:12837–12842. doi: 10.1073/pnas.0803799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shilton BH. The dynamics of the MBP–MalFGK(2) interaction: a prototype for binding protein dependent ABC-transporter systems. Biochim Biophys Acta. 2008;1778:1772–1780. doi: 10.1016/j.bbamem.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 69.Ivetac A, Campbell JD, Sansom MS. Dynamics and function in a bacterial ABC transporter: simulation studies of the BtuCDF system and its components. Biochemistry. 2007;46:2767–2778. doi: 10.1021/bi0622571. [DOI] [PubMed] [Google Scholar]
- 70.Panagiotidis CH, Boos W, Shuman HA. The ATP-binding cassette subunit of the maltose transporter MalK antagonizes MalT, the activator of the Escherichia coli mal regulon. Mol Microbiol. 1998;30:535–546. doi: 10.1046/j.1365-2958.1998.01084.x. [DOI] [PubMed] [Google Scholar]
- 71.Bohm A, Boos W. Gene regulation in prokaryotes by subcellular relocalization of transcription factors. Curr Opin Microbiol. 2004;7:151–156. doi: 10.1016/j.mib.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 72.Richet E, Joly N, Danot O. Two domains of MalT, the activator of the Escherichia coli maltose regulon, bear determinants essential for anti-activation by MalK. J Mol Biol. 2005;347:1–10. doi: 10.1016/j.jmb.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 73.Bohm A, Diez J, Diederichs K, Welte W, Boos W. Structural model of MalK, the ABC subunit of the maltose transporter of Escherichia coli: implications for mal gene regulation, inducer exclusion, and subunit assembly. J Biol Chem. 2002;277:3708–3717. doi: 10.1074/jbc.M107905200. [DOI] [PubMed] [Google Scholar]
- 74.Samanta S, Ayvaz T, Reyes M, Shuman HA, Chen J, Davidson AL. Disulfide cross-linking reveals a site of stable interaction between C-terminal regulatory domains of the two MalK subunits in the maltose transport complex. J Biol Chem. 2003;278:35265–35271. doi: 10.1074/jbc.M301171200. [DOI] [PubMed] [Google Scholar]
- 75.Dean DA, Reizer J, Nikaido H, Saier MH., Jr Regulation of the maltose transport system of Escherichia coli by the glucose-specific enzyme III of the phosphoenolpyruvate-sugar phosphotransferase system. Characterization of inducer exclusion-resistant mutants and reconstitution of inducer exclusion in proteoliposomes. J Biol Chem. 1990;265:21005–21010. [PubMed] [Google Scholar]
- 76.Stein A, Seifert M, Volkmer-Engert R, Siepelmeyer J, Jahreis K, Schneider E. Functional characterization of the maltose ATP-binding-cassette transporter of Salmonella typhimurium by means of monoclonal antibodies directed against the MalK subunit. Eur J Biochem. 2002;269:4074–4085. doi: 10.1046/j.1432-1033.2002.03099.x. [DOI] [PubMed] [Google Scholar]
- 77.Benabdelhak H, Kiontke S, Horn C, Ernst R, Blight MA, Holland IB, Schmitt L. A specific interaction between the NBD of the ABC-transporter HlyB and a C-terminal fragment of its transport substrate haemolysin A. J Mol Biol. 2003;327:1169–1179. doi: 10.1016/S0022-2836(03)00204-3. [DOI] [PubMed] [Google Scholar]
- 78.Cuthbertson L, Kimber MS, Whitfield C. Substrate binding by a bacterial ABC transporter involved in polysaccharide export. Proc Natl Acad Sci USA. 2007;104:19529–19534. doi: 10.1073/pnas.0705709104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patzlaff JS, van der Heide T, Poolman B. The ATP/substrate stoichiometry of the ATP-binding cassette (ABC) transporter OpuA. J Biol Chem. 2003;278:29546–29551. doi: 10.1074/jbc.M304796200. [DOI] [PubMed] [Google Scholar]
- 80.Linton KJ. Structure and function of ABC transporters. Physiology (Bethesda) 2007;22:122–130. doi: 10.1152/physiol.00046.2006. [DOI] [PubMed] [Google Scholar]
- 81.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 82.Pakotiprapha D, Inuzuka Y, Bowman BR, Moolenaar GF, Goosen N, Jeruzalmi D, Verdine GL. Crystal structure of Bacillus stearothermophilus UvrA provides insight into ATP-modulated dimerization, UvrB interaction, and DNA binding. Mol Cell. 2008;29:122–133. doi: 10.1016/j.molcel.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dean M, Annilo T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu Rev Genomics Hum Genet. 2005;6:123–142. doi: 10.1146/annurev.genom.6.080604.162122. [DOI] [PubMed] [Google Scholar]
- 84.Ramaen O, Leulliot N, Sizun C, Ulryck N, Pamlard O, Lallemand JY, Tilbeurgh H, Jacquet E. Structure of the human multidrug resistance protein 1 nucleotide binding domain 1 bound to Mg2+/ATP reveals a non-productive catalytic site. J Mol Biol. 2006;359:940–949. doi: 10.1016/j.jmb.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 85.Gaudet R, Wiley DC. Structure of the ABC ATPase domain of human TAP1, the transporter associated with antigen processing. EMBO J. 2001;20:4964–4972. doi: 10.1093/emboj/20.17.4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lewis HA, Buchanan SG, Burley SK, Conners K, Dickey M, Dorwart M, Fowler R, Gao X, Guggino WB, Hendrickson WA, Hunt JF, Kearins MC, Lorimer D, Maloney PC, Post KW, Rajashankar KR, Rutter ME, Sauder JM, Shriver S, Thibodeau PH, Thomas PJ, Zhang M, Zhao X, Emtage S. Structure of nucleotide-binding domain 1 of the cystic fibrosis transmembrane conductance regulator. EMBO J. 2004;23:282–293. doi: 10.1038/sj.emboj.7600040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gonen T, Cheng Y, Sliz P, Hiroaki Y, Fujiyoshi Y, Harrison SC, Walz T. Lipid–protein interactions in double-layered two-dimensional AQP0 crystals. Nature. 2005;438:633–638. doi: 10.1038/nature04321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harries WE, Akhavan D, Miercke LJ, Khademi S, Stroud RM. The channel architecture of aquaporin 0 at a 2.2-A resolution. Proc Natl Acad Sci USA. 2004;101:14045–14050. doi: 10.1073/pnas.0405274101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Groot BL, Engel A, Grubmuller H. The structure of the aquaporin-1 water channel: a comparison between cryo-electron microscopy and X-ray crystallography. J Mol Biol. 2003;325:485–493. doi: 10.1016/S0022-2836(02)01233-0. [DOI] [PubMed] [Google Scholar]
- 90.Lee JY, Urbatsch IL, Senior AE, Wilkens S. Nucleotide-induced structural changes in P-glycoprotein observed by electron microscopy. J Biol Chem. 2008;283:5769–5779. doi: 10.1074/jbc.M707028200. [DOI] [PubMed] [Google Scholar]
- 91.Eskandari S, Wright EM, Kreman M, Starace DM, Zampighi GA. Structural analysis of cloned plasma membrane proteins by freeze-fracture electron microscopy. Proc Natl Acad Sci USA. 1998;95:11235–11240. doi: 10.1073/pnas.95.19.11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Davidson AL, Dassa E, Orelle C, Chen J. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol Mol Biol Rev. 2008;72:317–364. doi: 10.1128/MMBR.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]