Abstract
In the few years since their discovery, T helper 17 cells (TH17) have been shown to play an important role in host defense against infections, and in tissue inflammation during autoimmunity. TH17 cells produce IL-17, IL-21, IL-10, and IL-22 cytokines, and thus have broad effects on a variety of tissues. Notably, the requirement for the immunosuppressive cytokine TGF-β along with the pro-inflammatory cytokine IL-6 for TH17 differentiation supports the intimate relationship between the TH17 subset and FOXP3+ regulatory T cells. Here, we discuss current knowledge on effector functions and differentiation of the TH17 lineage. Furthermore, we now know of a physiological stimulus for TH17 differentiation: innate immune recognition of cells undergoing apoptosis as a direct result of infection induces unique development of this subset. As our knowledge of TH17 and T regulatory cells grows, we are building on a new framework for the understanding of effector T cell differentiation and the biology of CD4+ T cell adaptive immune responses.
Keywords: TH17, Effector T cells, Regulatory T cells, Innate immunity, Apoptosis, Tolerance, Autoimmunity, Host defense
Introduction
CD4+ T helper (TH) cells are a sub-category of T lymphocytes that regulate both innate and adaptive immune processes and help to determine what type of immune response is mounted against a particular pathogen. CD4+ effector TH cells express T cell receptors (TCR) that recognize antigens bound to major histocompatibility complex (MHC) class II molecules when presented by dendritic cells (DC) or other antigen presenting cells (APC). TCR activation along with co-stimulatory signaling activates the CD4+ T cell and allows it to undergo proliferation and differentiation. Naïve CD4+ T cells, when activated, can differentiate into effector TH cells or regulatory T cells, or Treg cells. Effector TH cells can be further subdivided into the T helper 1 (TH1) and T helper 2 (TH2) subsets, where each subset has distinct pro-inflammatory functions to orchestrate the immune response to particular pathogens via production of different cytokines. For example, TH1 cells produce interferon-γ (IFN-γ) and stimulate clearance of intracellular infections, while TH2 cells produce interleukin-4 (IL-4) and IL-5 and promote clearance of extracellular parasitic infections [1]. Treg cells synthesize anti-inflammatory cytokines such as TGF-β, and these cells serve to limit and control inflammation. Effector TH cells have also been implicated in pathological responses: TH1 cells can participate in autoimmune inflammation, while TH2 cells mediate allergic inflammation.
The process by which an uncommitted, or naïve, CD4+ T cell develops into a mature TH1 or TH2 cell is one of developmentally regulated gene expression, influenced by many factors. In general, APC direct differentiation of naïve T cells by translating molecular features on pathogens they encounter into the production of cytokines, which in turn instruct naïve CD4+ T cells towards a particular lineage. IL-12 and IL-4, acting through signal transducer and activator of transcription 4 (STAT4) and STAT6 transcription factors, respectively, are important cytokines in differentiation decisions towards the TH1 or TH2 lineage [2]. Subsequent activation of specific transcription factors T-bet (for TH1) or GATA3 (for TH2) also plays a key role in induction of these subsets. More recent studies have indicated that activation of the Notch pathway by specific ligands expressed on APC can also instruct TH cell differentiation in the absence of IL-4 or IL-12 [3].
TH17 cells
Recent advances in the understanding of the development of a subset of CD4+ effector T cells that produce another cytokine, IL-17, have led to substantial modifications in the original TH cell subset paradigm. In 1986, two landmark papers by Mosmann and Coffman provided a foundation for understanding T cell biology when they proposed the TH1–TH2 hypothesis [4, 5]. The theory was based on the observation that subsets of CD4+ TH cells had distinct cytokine expression profiles that defined their function: TH1 cells induced cell-mediated inflammatory responses, and TH2 cells provided B cell help. Coffman also predicted a cross-regulatory element of TH cell differentiation, where the effector cytokines produced by one subset of cells would regulate the development and activity of the other. This idea has been validated over the past 20 years by molecular and genetic studies demonstrating a complex network of cross-regulatory signaling pathways and transcriptional regulators that co-ordinate genetic and activated programming of the differentiating T cells. On the basis of these kinds of studies [6], it became possible to define the IL-17-producing CD4+ T cells as a distinct subset of TH cells, TH17 cells. This distinction was made according to the same general criteria by which TH cell lineages had been defined: naïve CD4+ T cells differentiate independently into each lineage in vitro and in vivo, and each lineage has a distinct, heritable gene-expression signature.
Even before the definition of TH17 cells as a separate subset, IL-17 cytokine produced by CD4+ T cells has been associated with host defense against infectious pathogens as well as with autoimmune diseases. However, it was not clear how IL-17 production was regulated. At the turn of the century, Infante-Duarte et al. [7] showed that a population of murine or human CD4+ T cells primed in the presence of Borrelia burgdorferi or mycobacterial lysates express IL-17 and do not co-express IFN-γ. Subsequent studies of mice deficient for IL-23, an IL-12 family cytokine that will be discussed presently, in autoimmune disease models showed that although this cytokine was not important for expression of IFN-γ, it was required for expression of IL-17, indicating that IL-17 and IFN-γ are differentially regulated [8]. IL-23 was subsequently shown to selectively induce proliferation of in vivo-primed IL-17-expressing CD4+ T cells [9]. T cells induced to proliferate with IL-23 express a distinctive set of genes: they do not produce IFN-γ or IL-4 but instead express IL-23 receptor (IL-23R) and IL-17. Following this work, two groups independently showed that naïve CD4+ T cells can differentiate into IL-17-expressing T cells in vitro and in vivo, distinct from TH1 or TH2 development [10, 11]. Since then, TH17 cells have been recognized as a separate lineage of TH cells that plays a crucial role in T cell-mediated adaptive immunity. This idea has been supported in the last 4 years with many studies describing their unique cytokine profile and transcriptional regulation, both in human and mouse systems. TH17 cells are not only distinct from other TH cell subsets in terms of gene expression and regulation, but also in their biological function. TH17 cells are generally thought to be pro-inflammatory, especially through the production of IL-17 [12]. They have been shown to participate in development of autoimmunity, and also to play an important role in host defense against infection, by recruiting neutrophils and macrophages to infected tissues, promoting abscess formation [13], and inducing expression of antimicrobial peptides [14].
Differentiation of TH17 cells: transcriptional regulation
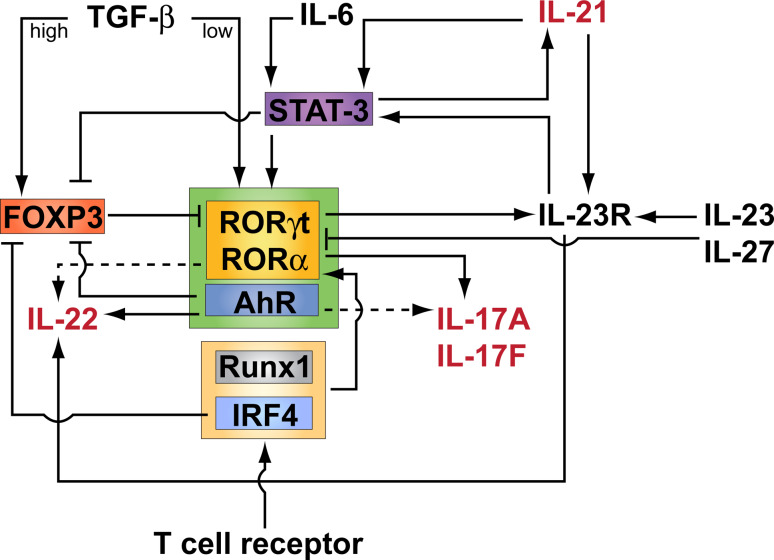
After the observation that TH17 cells expressed a distinct subset of cytokines and chemokines as compared to TH1 and TH2 cells, several groups performed experiments to look for a TH17-specific transcription factor. A retinoid orphan nuclear receptor, RORγt (encoded by Rorc), was shown to be specifically expressed by mouse and human TH17 cells [15, 16]. Expression of RORγt in naïve CD4+ T cells was necessary and sufficient to induce IL-17 expression. A related nuclear receptor, RORα, was shown to synergize with RORγt to promote differentiation of TH17 cells [17]. Binding of a Runx family transcription factor, Runx1, to Il17 promoter and enhancer regions along with RORγt was shown to be required for optimal IL-17 expression in CD4+ T cells [18]. In vitro, Runx1 could also upregulate expression of RORγt directly in TH17 polarizing conditions [18]. Interleukin regulatory factor 4 (IRF4), a transcription factor originally reported to be important in TH2 development [19], was recently shown to be critical for TH17 induction as well. IRF4-deficient T cells were impaired in TH17 polarization, and IRF4-deficient mice were resistant to TH17-mediated induction of experimental autoimmune encephalomyelitis (EAE) [20], a mouse model of multiple sclerosis. TH17 cell development and function is also critically dependent on the transcription factor STAT3. Upregulation of RORγt is STAT3-dependent [21], and selective deletion of STAT3 in T cells abrogates TH17 development partly because expression of RORα and RORγt are also abrogated [17]. STAT3 is activated by IL-6 and IL-23 (reviewed in [22]), and STAT3 can also directly regulate the expression of IL-21 and IL-17 [23]. Thus, TH17 cell differentiation fate, effector functions, and maintenance are all in some part regulated by STAT3 via various cytokine mediators. The regulatory network of transcriptional regulation of TH17 cells is shown in Fig. 1.
Fig. 1.
Schematic representation of the transcriptional regulation of TH17 cell differentiation. Activation of CD4+ T cells through the T cell receptor (TCR) in the presence of a T cell costimulatory signal results in activation and differentiation of CD4 T cells into different fates depending on the cytokine milieu. Activation of the TCR in the presence of high concentrations of TGF-β induces expression of both RORγt and FOXP3, but FOXP3 antagonizes RORγt functions leading to naïve CD4+ T cell differentiation into regulatory T cells (Treg). When the pro-inflammatory cytokine IL-6 is present together with low concentrations of TGF-β, the TH17 specific transcription factors RORγt and RORα are induced in a STAT-3-dependent manner leading to the transcriptional activation of IL-17, IL-22, and IL-23 receptor (IL-23R). Through STAT-3, IL-6 induces IL-21, which acts in a positive autocrine loop in order to amplify TH17 cell differentiation. Indeed, IL-21 induces IL-23R expression, which imparts responsiveness to IL-23, a cytokine that maintains and expands TH17 cells. IL-23 is also important in inducing the expression of IL-22 by TH17 cells. Although IL-6 is dominant in vivo, in its absence and when Treg cells are experimentally depleted, IL-21 together with TGF-β can induce TH17 cell differentiation. Many transcription factors cooperate with RORγt and RORα in inducing maximal IL-17 and IL-22 expression. These include the aryl hydrocarbon receptor (AhR), which is also induced during TH17 cell differentiation and inhibits FOXP3 expression, TCR-induced interferon regulatory factor IRF-4 which under TH17 polarizing conditions upregulates RORγt expression and plays a role in IL-6 mediated downregulation of FOXP3 expression, and TCR-induced Runx1 which also upregulates RORγt expression and, together with RORγt, directs Il17 transcription. IL-27 counters the effects of TGF-β and IL-6 on differentiating CD4+ T cells, effectively blocking TH17 differentiation in a STAT-1-dependent manner, and inhibiting expression of RORγt and IL-17 production. Cytokines shown in red are induced upon the initiation of TH17 cell differentiation and are thus produced by TH17 cells
Another factor in the regulation of transcription of TH17 cytokines is the aryl hydrocarbon receptor (AhR), a ligand-dependent transcription factor that mediates effects of environmental toxins such as dioxin or FICZ (a UV photoproduct of tryptophan) [24]. Experiments with AhR-deficient cells suggested that it is required for expression of IL-22, and to a lesser extent IL-17, in TH17 polarizing conditions and in the presence of either dioxin or FICZ [25, 26]. However, the requirement for AhR in IL-17 expression remains controversial [27]: while one group found opposing effects on TH17 and Treg differentiation in response to different AhR ligands (dioxin vs FICZ) [26], another saw no difference between the individual ligands [27]. More recently, an AP-1 transcription factor, BATF [28], was shown to also play a role in TH17 differentiation. Batf −/− mice had a defect specifically in differentiation of TH17 cells, and were resistant to EAE [29]. This effect is thought to occur via BATF binding to the Il17a, Il21, and Il22 promoters; Batf −/− T cells are also deficient in induction of the TH17-specific transcription factor RORγt [29].
Differentiation of TH17 cells: cytokines
Meanwhile, with the establishment of TH17 cells as an independent subset of TH cells, studies were also under way to determine the specific cytokine factors that promote differentiation of these cells. In 2006, three independent studies found that a combination of the prototypical, pleiotropic pro-inflammatory cytokine IL-6 along with the immunoregulatory cytokine TGF-β were required to induce IL-17 expression in naïve CD4+ T cells [30–32]. Because the synergistic effect of two cytokines with supposedly opposing consequences was required to activate differentiation of this cell subset, signaling molecules and transcription factors downstream of the TGF-β and IL-6 receptors were thought to work together to induce TH17 differentiation. Two collaborating groups performed experiments using a 2D2 TCR transgenic mouse, which has T cell receptor specificity to myelin oligodendroycte glycoprotein (MOG), crossed to a TGF-β transgenic mouse that overexpresses TGF-β under the IL-2 promoter (2D2/TGF-β Tg mouse). When they injected the mice with MOG peptide alone, the T cells from the 2D2/TGF-β transgenic mice were able to suppress EAE on adoptive transfer. This was because T cells activated in the presence of TGF-β alone (which was constitutively expressed by the T cells, under the IL-2 promoter) differentiated into anti-inflammatory Treg cells [33]. When the mice were immunized with both peptide and complete Freund’s adjuvant (CFA), however, the splenocytes from these mice made high levels of IL-6, and the T cells were much more potent at induction of EAE as compared to T cells from immunized 2D2 mice that did not overexpress TGF-β [31]. Although some early reports suggested that TH17 differentiation in human cells could occur in the absence of TGF-β, these results were revealed to be artifactual by several studies showing the absolute requirement of TGF-β for TH17 differentiation in human as well as mouse cells [34–36].
Because of this finding, the groups predicted that the T cell repertoire of IL-6-deficient mice would be dominated by Treg cells, and that Il6 −/− animals would lack the ability to generate TH17 cells and be resistant to development of EAE. This proved to be the case. In fact, although there were increased numbers of Treg cells in the Il6 −/− mice [37], these mice were still susceptible to EAE via a pathogenic TH17 response when the Treg population was depleted, suggesting an IL-6-independent pathway for TH17 differentiation. By screening for cytokines that might substitute for the effect of IL-6, they identified a cytokine that could do so, the IL-2 family member IL-21[37], a cytokine originally identified as a factor that enhances T cell proliferation and drives differentiation of NK cells [38]. Other groups were able to corroborate the finding that IL-21 could promote TH17 differentiation, solidifying the notion that IL-21 can pair with TGF-β for induction of TH17 responses [39, 40]. Several groups also found that IL-21 was equally as effective at induction of RORγt transcription factor and the IL-23 receptor as was IL-6. In further examination of the differences in effect of IL-6 and IL-21, however, it became clear that compared with IL-6-deficient T cells, cells deficient in IL-21 were markedly impaired in induction of IL-23R, RORγt, and IL-17 [40]. IL-21 therefore not only enhances TH17 cell differentiation by inducing RORγt, but it is also important in helping TH17 cells to attain a fully inflammatory phenotype by upregulating IL-23R [40]. Expression of IL-21 is not dependent on RORγt, as RORγt-deficient mice express normal levels of IL-21 [40], and IL-21 produced by differentiating TH17 cells therefore likely acts in a positive feedback loop, amplifying the TH17 response.
Although TH17 cells were first described as a result of experiments that suggested IL-23 as an important directive signal for their induction [9], IL-23 was later shown to be unnecessary for generation of TH17 cells from naïve CD4+ T cells. In fact, the receptor for IL-23 (IL-23R) is expressed on activated or memory T cell populations but not on naïve T cells [41], indicating that IL-23 could not be involved in the initial generation of TH17 cells. Nonetheless, IL-23 is actually essential for the full and sustained differentiation of inflammatory TH17 cells. In the absence of IL-23, TH17 cells demonstrated reduced production of inflammatory cytokines and increased secretion of the immunoregulatory cytokine IL-10, which correlated with an impaired ability to transfer EAE [42]. Thus, IL-23 is required for TH17 cells to “mature” to their fully inflammatory potential. Besides IL-23, there are other pro-inflammatory mediating events that promote differentiation of TH17 cells, including release of IL-1, which, like IL-21, can substitute for IL-6 in induction of TH17 cells [43], and ligation of the tumor necrosis factor receptor family member DR3 by its cognate ligand, TNF family ligand 1A (TL1A, also known as TNFSF15) [44]. A related cytokine, IL-27, counters the effect of TGF-β + IL-6 on differentiating CD4+ T cells, effectively blocking TH17 differentiation via a mechanism dependent on STAT-1, by directly inhibiting expression of RORγt [45, 46]. IL-27 has anti-inflammatory properties, likely due to its ability to promote IL-10 production by T cells [47], and notably the inhibitory effects of IL-27 on expression of RORγt are specific to differentiating TH17 cells rather than committed ones [48].
TH17 cells and cytokine factors
The TH17 lineage was originally defined by production of the cytokine interleukin-17 (IL-17A) [49], a member of a family of IL-17 cytokines. Additional studies have shown that TH17 cells are also characterized by the production of IL-17F (another member of the IL-17 family) and an IL-10 family cytokine, IL-22 [9, 50]. Besides acting in concert with TGF-β to promote TH17 differentiation, IL-21 is also produced by TH17 cells themselves [37]. TH17 cells can produce certain cytokines that are expressed by other TH-cell lineages, including tumor necrosis factor-α (TNFα) and lymphotoxin-β, and the TH17 subset can also be characterized by expression of chemokine receptor CCR6 and the CCR6 ligand, CCL20 [51].
The IL-17 cytokine family has six members, of which IL-17A and IL-17F, both produced by TH17 cells, are the best characterized. IL-17A and IL-17F are closely related, with about 50% amino acid identity [9]. However, they appear to have non-redundant roles in inflammation, as mice deficient in IL-17A or IL-17F respond differently in terms of neutrophil recruitment in response to airway challenge, and in colitis models, despite the high homology between the two proteins [52]. In addition to the production of IL-17A and IL-17F homodimers, TH17 cells can produce IL-17A/F heterodimers, which have biological activity in vitro and in airway neutrophil recruitment in vivo [53]. While IL-17A and IL-17F are clearly associated with inflammation, the precise roles of the different forms of secreted cytokines are yet to be elucidated.
Another cytokine preferentially produced by TH17 cells is the IL-10 family member IL-22 [50], which is expressed both during primary polarization of naïve CD4+ T cells towards the TH17 lineage, as well as during restimulation of TH17 cells, in an IL-23-dependent manner [50]. Although TH17 cells have the capacity to secrete IL-22 together with IL-17, they do not necessarily all co-express both cytokines. Expression of IL-22 by TH17 cells has been shown to be dependent on the AhR transcriptional pathway linking TH17 induction with environmental factors [25].
IL-22 receptor is not expressed on the surface of immune cells [54], so secreted IL-22 acts to regulate tissue inflammation via induction of signal transduction in non-immune cells. Notably, the downstream effects of IL-22 signaling can be anti-inflammatory, protective, or pro-inflammatory depending on the microenvironment. IL-22 can induce expression of acute phase reactant genes in a mouse model of hepatitis, conferring protection from acute inflammation [55], and the cytokine is also protective in a mouse model of ulcerative colitis [56]. Although IL-22 is induced in EAE, depletion of IL-22 does not affect disease progression [57], suggesting that this cytokine does not play a major role in the context of neuron-specific autoimmune inflammation. IL-22 also participates in epithelial barrier immunosurveillance. Two groups recently showed an important role for IL-22 in host defense against mucosal infections [58, 59]. In lung infections with Klebsiella pneumoniae, IL-23 was required for IL-22 expression from T cells, and although IL-22 and IL-17 both contributed to the production of cytokines and anti-microbial products from epithelial cells, IL-22 was ultimately more important than IL-17 for defense against the bacteria [58]. In an infection model of the intestinal mucosa, blocking IL-22 resulted in increased susceptibility to the enteric pathogen Citrobacter rodentium [59], supporting the notion that IL-22 is an important cytokine for bacterial defense at mucosal surfaces. IL-22 also participates in protection by inducing expression of β-defensins and anti-microbial proteins, including the Reg family of anti-microbial proteins (RegIIIβ and RegIIIγ), at epithelial barrier surfaces [59]. Although these studies have delineated a protective role for this cytokine, other studies have shown that skin inflammation in a mouse model of psoriasis is dependent on IL-22 [60], indicating that IL-22 also contributes to pathologic inflammatory processes. Thus, as noted, IL-22, either from TH17 cells or otherwise, can have divergent effects depending on the situation. Two recent papers have identified a newly described population of CD4+ T cells that express IL-22 in the absence of IL-17, and that are distinct from the TH1, TH2, and TH17 subsets [61, 62]. These cells were, like TH17 cells, also induced by AhR agonists, and, consistent with the known roles of IL-22 from TH17 cells, seem to be important in maintenance of skin homeostasis and wound healing.
Another IL-10 family cytokine, IL-10 itself, is expressed by some but not all TH17 cell subsets. IL-10 is an anti-inflammatory cytokine that helps to control TH1- and TH17-mediated inflammatory processes [42], and will be discussed more completely below.
Reciprocal relationship between TH17 and Treg
Polarization of naïve T cells towards the TH17 lineage requires a combination of TCR stimulation as well as the cytokines TGF-β and IL-6. TGF-β is a cytokine produced by various cell types including Treg cells and innate immune cells, with broad inhibitory effects on various elements of the immune system. TGF-β has been thought of primarily as an anti-inflammatory cytokine, partly because it induces the Treg-specific transcription factor forkhead box P3 (FOXP3) and thus in vitro differentiation of FOXP3-expressing Treg [63]. Thus, TGF-β can induce either anti-inflammatory Treg or pro-inflammatory TH17 cells, depending on the presence or absence of IL-6. The common requirement for TGF-β in generation of TH17 and Treg cells was established by Betelli et al. [31], who demonstrated the presence of a reciprocal relationship between the developmental pathways for generation of TH17 effector cells and Treg cells. This reciprocal relationship was further elucidated by Littman and colleagues, who showed that the TH17-specific and Treg-specific transcription factors, RORγt and FOXP3, respectively, are co-expressed in naïve CD4+ T cells exposed to TGF-β, and that the balance between generation of TH17 or Treg cells depends on cytokine-induced interactions between these transcription factors [64]. IL-6, by tipping the balance from FOXP3 towards RORγt expression, acts as the “switch factor” controlling the preferential differentiation of TH17 cells over Treg [64]. Consistent with this concept is the observation that conditional deletion of FOXP3 in adult mice results in the increase of both IL-17 and RORγt expression [65]. TGF-β is actually required for expression of both FOXP3 and RORγt transcription factors [64], although the downstream signaling requirements after TGF-β receptor engagement are likely to differ in each case.
More recent work, also from the Littman group in collaboration with others, has demonstrated that TGF-β-induced FOXP3 inhibits TH17 cell differentiation by directly antagonizing RORγt function, via a mechanism requiring both exon 2 of FOXP3, which binds directly to RORγt, and the FOXP3 Forkhead (FKH) domain, which mediates FOXP3 DNA-binding activity [64]. This work suggests that T cells that encounter TGF-β cytokine have the potential to develop into either the Treg or the TH17 lineage. In the absence of pro-inflammatory cytokines, FOXP3 inhibits the activity of RORγt, and cells do not differentiate into pro-inflammatory TH17 cells. In the presence of pro-inflammatory cytokines, FOXP3 is repressed, and RORγt is concurrently stabilized or upregulated, allowing for differentiation of TH17 cells [64]. The transcription factor Runx1, which is required both for differentiation of TH17 cells as well as for FOXP3 function [18, 66], also participates in the interplay between FOXP3 and RORγt. Binding of Runx1 and RORγt together to the Il17a locus leads to increased expression of IL-17, while FOXP3 can bind to both Runx1 and RORγt to inhibit activity [18]. Runx1, therefore, may be able to bind differentially to RORγt and FOXP3 to mediate activating or repressing activities depending on the cytokine environment.
Plasticity in TH17 and Treg development
It is clear that there are many connections in the development of TH17 and Treg cells, from the shared requirement of TGF-β to the reciprocal properties and interactions of RORγt and FOXP3. There are even some studies that have described populations of cells that express both transcription factors in vitro and in vivo [64, 67], although the function of these dual-expressors remains unclear. Contrastingly, TH17 cells are thought to develop relatively independently from the factors that promote TH1 and TH2 differentiation [11]. However, a notable similarity in pathways does exist. TH1 and TH17 cells differ in their dependence on IL-12 family cytokines IL-12 and IL-23. During differentiation, TH1 cells upregulate IL-12R and not IL-23R downstream of the TH1-specific transcription factor T-bet to enhance responsiveness to IL-12 [68]. Meanwhile, developing TH17 cells upregulate both IL-23R and IL-12R, enabling responsiveness to both IL-23 and IL-12 [69]. The upshot of this nonspecificity of TH17 cells still needs to be elucidated, but this observation helps to explain a curious finding: cells that co-express both IFN-γ and IL-17, especially in inflammatory settings [69]. This raised the idea that CD4+ T cells may not differentiate into rigidly defined TH1, TH2, TH17, and Treg categories as had originally been thought. Subsequently, the potential for reversibility of function in TH cells was addressed experimentally, showing that so-called “master regulator” transcription factors such as T-bet exhibit a broad range of epigenetic states, allowing for activation and plasticity in already differentiated CD4+ cells [70]. Furthermore, a recent study by Lexberg et al. reported that IL-17A-producing cells could be repolarized to either a TH1 or a TH2 phenotype in the presence of IL-12 or IL-4 [71]. However, IL-17+ memory T cells retained TH17 characteristics despite culture with IL-12 or IL-4, indicating that some TH17 cells do maintain phenotypic stability [71]. Other studies have shown TH17 cells to transition to an IFN-γ-producing population in in vivo models. Transfer of TH17-polarized islet-reactive TCR transgenic T cells induced diabetes associated with the transition of the transferred T cells to TH1-type cells, where islet injury was dependent on IFN-γ [72, 73]. Similarly, transfer of ex vivo polarized TH17 cells into immunodeficient hosts induced colitis that was associated with a decrease in IL-17A and IL-17F production, and an induction of IFN-γ in the transferred T cells [69]. Along with TH17 cells, Treg cells also exhibit considerable plasticity of phenotype, especially in the context of inflammatory cytokine production. Treg cells stimulated with IL-6 can downregulate FOXP3 and induce expression of IL-17, suggesting that mature Treg cells can be subverted into the TH17 differentiation programme [74]. This idea is corroborated by the finding that Treg cells can be converted into IL-17-producing cells by DC activated with dectin-1 [75].
These studies illustrating the capability of TH17 and Treg cell subsets to undergo phenotype conversions are very compelling. Importantly, however, the finding of specific roles for both of these subsets in mediating immune tolerance, in host defense, and in autoimmunity indicate that while there may be some plasticity, TH17 and Treg as such are still necessary. It remains possible that these subsets are particularly adept at responding to changing environmental conditions in order to maintain appropriate immune control depending on the presence of microbes, signals from innate immune cells, and the cytokine microenvironment.
Treg cells and controlling the immune response
Regulatory CD4+ T cells, or Treg cells, actively suppress the immune system, maintaining immune system homeostasis and tolerance to self-antigens, and thereby preventing pathological self-reactive inflammation and autoimmunity [76]. There has been interest in a so-called ‘suppressor’ population of T cells that limit inflammation and prevent autoimmunity for at least the last 30 years [77], but the modern field of regulatory T cell biology really began with the identification by Sakaguchi et al. [78] of a population of CD4+ T cells constitutively expressing the interleukin 2 receptor-α (IL-2Rα) chain (CD25) that could prevent autoimmune disease via suppressor activity. These CD4+CD25+ Treg cells thus became prime candidates for a T cell population mediating dominant tolerance to self. Other markers on Treg cells were identified, including: CD5 and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), two negative regulators of TCR signaling and T cell activation; TNF receptor family member GITR; neuropilin-1 (Nrp-1), a neuronal guidance protein; and lymphocyte activation gene 3 [79–83]. However, the presence of these markers on some activated nonregulatory T cells precluded their use as functionally definitive molecules for the Treg population. The need for a specific Treg marker was fulfilled with the identification of the transcription factor FOXP3 [84, 85], a discovery that revolutionized the field and led to many subsequent studies that later conclusively showed the essential role of FOXP3 in both the development and function of Treg cells [86]. There are two types of Treg cells: the naturally occurring Treg cells that originate in the thymus from a dedicated lineage, and the ‘induced’ Treg cells that differentiate de novo in secondary lymphoid tissues from naïve CD4+ T cell precursors in the presence of TGF-β [63]. These ‘induced’ Treg cells produce TGF-β, express FOXP3, and have suppressive activity indistinguishable from that of ‘natural’ Treg cells [87].
Mechanisms by which Treg cells mediate immune suppression are not fully resolved. Expression of IL-10 and TGF-β have been linked to suppression mediated by Treg in in vivo experimental models [88, 89], although blockade of these cytokines does not abrogate suppressive ability in vitro [90]. Treg cells can directly suppress activity via reverse signaling through crosslinking of B7 (CD80 and CD86) on APC or activated T cells with CTLA-4 [91]. The role of CTLA-4 in Treg cells has been recently confirmed in a study where selective ablation of this marker resulted in spontaneous lymphoproliferation and fatal T cell-mediated autoimmune disease due to a loss of function in the Treg cells, particularly in their ability to downregulate CD80 and CD86 on DC [92]. This suggested that Treg may critically require CTLA-4 to suppress immune responses by modulating potency of APC. Other surface molecules on Treg cells can also affect DC activity: recently, a novel Ig family member, TIGIT, which is expressed at high levels on Treg cells, was shown to induce DC to produce immunosuppressive cytokines IL-10 and TGF-β when Treg interacted physically with the DC [93]. Long-lasting physical Treg cell interactions with DC do occur [94], and these interactions are facilitated by Nrp-1, as blockade of Nrp-1 interferes with Treg cell-mediated suppression [95].
Several secreted proteins identified in gene expression studies, including granzyme B, IL-9, IL-10, and IL-35, have also been implicated in Treg cell-mediated suppressor function. Expression of ectoenzymes CD73 and CD39 on the surface of Treg cells can also contribute to immune suppression via a mechanism involving hydrolysis of ATP [96, 97]. Another mechanism by which Treg cells may suppress inflammation is by “soaking up” T cell growth factors such as IL-2 [98].
As many of these suppression mechanisms seem to be at least somewhat redundant, the likelihood is that specific mechanisms of Treg suppression activity may vary depending on the tissue and type of inflammation. For example, in an experimental skin transplantation model, Treg-derived granzyme B and IL-9 contribute to tolerance and long-lived graft survival [99], while controlling inflammation in the colon seems to be largely independent of granzyme B, instead requiring IL-10 and IL-35 secretion by Treg cells [100, 101].
TH17 cells in autoimmunity
Findings over many years of study in the field of experimental autoimmunity pointed to IL-12 and TH1 cells with specificity for self-antigens as autopathogenic and required for induction of organ-specific autoimmunity. However, the concept of autoimmunity as a TH1-driven condition was challenged when it became clear that IFN-γ- and IFN-γ receptor-deficient mice, as well as mice lacking other molecules involved in TH1 differentiation including IL-12p35, were not protected from EAE, but rather developed more severe disease [102, 103]. This suggested that another subset of T cells, distinct from the TH1 lineage, might be required for induction of EAE and other organ-specific autoimmune diseases.
From a computational sequence screen looking for homologs of the IL-6 cytokine family, a novel cytokine chain, termed p19, was discovered and found to form heterodimers with the p40 chain of IL-12 [104]. The cytokine formed by this heterodimeric pairing was called IL-23. Early experiments on p19 where the cytokine subunit was transgenically expressed in mice showed that expression of p19 resulted in widespread multiorgan inflammation and early death [105]. Because of the participation of the IL-12p40 chain in IL-23, the specificity to IL-12 of many of the experiments that had been performed using polyclonal antibodies to block IL-12 were called into question, as any approaches that targeted IL-12p40 would also have affected IL-23. The studies that needed to be re-examined included ones pointing to the importance of IL-12 in the development of EAE and collagen-induced arthritis (CIA), essentially implicating IL-12 and TH1 cells as being critical for organ-specific autoimmune pathology [106]. In a set of seminal experiments to settle this problem, Cua and colleagues created IL-23p19 knockout mice and compared them with mice deficient in IL-12p35. In this way, they demonstrated that IL-23 and not IL-12 was crucial for the development of EAE [107]. Furthermore, IL-23 expanded an IL-17-producing TH cell population that was effective at induction of EAE when adoptively transferred into naïve mice, and these IL-17-producing T cells were reduced in the central nervous system of IL-23-p19-deficient mice [9].
Since these initial studies, the importance of TH17 cells in the pathogenesis of organ-specific autoimmune inflammation has now been well established, and demonstrated in many different animal models. IL-17 is directly involved in cartilage and bone erosion as observed in an experimental model of autoimmune arthritis [108], and blocking IL-17A prior to disease onset attenuates antigen-induced arthritis in mice [109]. Consistent with these findings, IL-17A-deficient mice develop reduced CIA [110], as well as developing EAE with delayed onset and reduced severity [111]. IL-17 has also been demonstrated to participate in regulation of germinal center formation as well as autoantibody production in autoimmune mice [112]. Consistent with these studies, the loss of a proximal regulator of IL-17, IL-23, is protective in autoimmune arthritis in mouse models, while loss of IL-12 correlates with increased severity of disease as well as increased numbers of IL-17-expressing lymphocytes [8].
Reports describing the presence of TH17 cells in various human autoimmune conditions have also been compelling, and they help to validate mouse studies indicating the importance of the IL-23-IL-17 axis in autoimmune disorders. IL-17A is upregulated in central nervous system lesions of patients with multiple sclerosis (MS) [113]. Also, IL-17-expressing perivascular lymphocytes have been described in brain lesions of patients with active MS, while these IL-17+ cells were reduced in patients with quiescent MS [114]. IL-17 has also been observed in psoriatic skin lesions [115], and has been shown to induce intracellular adhesion molecule-1 (ICAM-1), IL-6, and IL-8 in human keratinocytes [115]. More recent studies have shown that IL-17-expressing cells in human psoriatic skin are in fact TH17 cells, and that these cells express IL-17A and IL-17F, IL-23R, and CCL20 as well as the TH17 transcription factor, RORγt [16]. Transcripts of IL-1β, an important factor for human TH17 differentiation, were also observed to be upregulated in psoriatic skin [16].
TH17 cells are also associated with chronic inflammatory diseases. Patients with the inflammatory bowel diseases, ulcerative colitis and Crohn’s disease, have elevated IL-17 mRNA in the colonic mucosa as compared to corresponding samples from either normal controls or patients with infectious or ischemic colitis [116]. IL-17-producing cells in gut mucosa of humans with Crohn’s disease have been observed. Along with IL-17, these cells also produce IFN-γ and express IL-23R and CCR6 [117]. IL-17 is also implicated in mouse models of inflammatory bowel disease. Blocking IL-23 is sufficient to prevent onset of colitis in IL-10-deficient mice, by a mechanism involving inhibition of IL-17. Also, in a TNBS-induced colitis model, an animal model of inflammatory bowel disease employing the use of 2,4,6-trinitrobenzenesulfonic acid (TNBS) which, when administered intrarectally to SJL/J mice, induces colonic inflammation in these mice that resembles features of human Crohn’s disease, IL-17R-deficient mice show reduced emigration of polymorphonuclear cells into the colon and reduced severity of disease [118].
Overall, the IL-17 pathway plays an essential pathological role in many autoimmune diseases. Phase II studies for the efficacy and safety of a monoclonal anti-IL-17 antibody, AIN457, are presently underway for Crohn’s disease and psoriasis patients that are refractory to current therapies [144]. The results of these trials will likely advance our understanding of the contributions of IL-17 and the IL-17 pathway to these diseases. Specifically, it will be important to elucidate whether anti-IL-17 antibody therapies will have increased benefits as compared to or combined with anti-TNF and anti-IL-23 therapies, especially given the cooperative nature and related aspects of these pathways.
TH17 cells in host defense
The first studies demonstrating a role for IL-17 in host defense against microbial pathogens were performed by Kolls and colleagues comparing susceptibility of IL-17 receptor (IL-17R)-deficient and control mice to infection with experimental Klebsiella pneumoniae pulmonary infection. After intranasal infection, IL-17R-deficient mice had increased numbers of bacteria in the lung, increased dissemination of bacteria into the spleen, and reduced overall survival [119]. This enhanced susceptibility was associated with delayed neutrophil recruitment and defective granulocyte colony stimulating factor (G-CSF) and CXCL-1 expression in the lung in response to the infection [119, 120]. Treatment with exogenous G-CSF does not restore neutrophil recruitment to the pulmonary compartment. This is likely due to the fact that IL-17R-deficient mice, as well as mice treated with IL-17 blocking antibodies, are defective in production of CXC chemokines [120, 121]. IL-23 plays an important role in amplifying the IL-17 response in protection against this pathogen. Similar to IL-17R-deficient mice, IL-23p19-deficient mice are highly susceptible to infection with K. pneumoniae and, importantly, do not show the characteristic upregulation of IL-17 in response to infection seen in resistant control mice [122]. Furthermore, IL-23p19-deficient mice treated with recombinant IL-17 show reduced bacterial burdens and restored chemokine responses [122]. IL-17A-deficient mice are also susceptible to K. pneumoniae infection and show reduced G-CSF and CXC chemokines in the lung following infection [58]. Taken together, these studies demonstrate that IL-23p19 and IL-17 together play an important role in recruitment of neutrophils and other inflammatory cells to provide protective immunity from K. pneumoniae infection.
Since these initial studies, important roles for IL-23 and IL-17 in host defense against many other pathogens have been established. IL-17 released by CD4+ T cells plays a critical role in the orchestration of the formation of intra-abdominal abscesses and neutrophil recruitment in in vivo responses to infection with the gram-negative bacteria Bacteroides fragilis [13]. IL-17R-deficient mice are also highly susceptible to parasitic infection with Toxoplasmosis gondii [123], as well as fungal infection with Candida albicans [124]. IL-17-expressing CD4+ T cells have been shown to be actively induced in response to infection with the mouse enteric pathogen Citrobacter rodentium [32], and early phase defense against this pathogen requires IL-22 as well as IL-23, both TH17-related cytokines [59]. TH17 cytokines also seem to be important in immune responses to infection with Mycoplasma: neutralization of IL-23p19 before Mycoplasma pneumoniae infection impairs IL-17 expression but also impairs effective bacterial clearance [125].
Although IL-17 is generally thought to be more salient for host defense against extracellular rather than intracellular bacterial pathogens, there is some evidence that IL-17 can also enhance responses to intracellular micro-organisms. In these cases, however, TH17 cells do not seem to be necessary; rather, IL-17 derived from non-CD4+ T cell sources (such as gammadelta- (γδ-) T cells) is playing an important role. For example, although IL-17-deficient mice survive sublethal infections with Salmonella enterica equally as well as control mice, there are consistently higher bacterial burdens in the spleen and liver of mice lacking IL-17 [126]. Similarly, increased bacterial burdens and defects in formation of organized granulomas are found in IL-17-deficient mice infected with Listeria monocytogenes [127].
It is not yet clear what elements of these infections elicit specifically a TH17 versus a TH1 or other immune response in vivo. One thing that many of these pathogens have in common is that infection can result in induction of host cell apoptosis. Some pathogens induce apoptosis in infected or neighboring host cells directly, such as B. fragilis [128] and C. rodentium [129], whereas infection with others, like C. albicans, can cause host cells to initiate apoptosis intrinsically [130]. Similarly, upon infection with L. monocytogenes, macrophages activate caspase-1 and undergo apoptotic or pyroptotic cell death as a mechanism of defense against the infection [131]. Be that as it may, these studies overall suggest that IL-17 and IL-23 expressed by CD4+ T cells are critical for generation of effective, appropriate host immune responses to infection, particularly infections with extracellular and/or gram-negative bacteria.
Self-regulation of TH17 cell pathology
Regulation of robust T cell responses is necessary for preventing host tissue damage and bystander pathology during clearance of infectious pathogens. Unregulated T cell activity can lead to autoimmunity in various ways. The immunoregulatory cytokine IL-10 has long been thought of as important for regulation of TH1 responses, but only more recently has it been described to be produced by TH1 cells themselves, along with their typical production of IFN-γ, as a self-regulatory mechanism [132]. A similar process has been described for TH17 cells. When TH17 cells are generated in the presence of TGF-β + IL-6, the resultant population includes a subset of cells that produce IL-10 along with IL-17 [42]. Continued exposure of TH17 cells to TGF-β and IL-6 results in increased production of regulatory IL-10 cytokine, while restimulation of TH17 cells generated in this manner with IL-23 results in an overall gain of pathogenic function by promoting TH17 cells that do not co-produce IL-10 [42]. IL-23 does not specifically maintain IL-10 production induced by TGF-β + IL-6 but does not directly inhibit its expression. These IL-17+IL-10+ co-producing cells are not thought of as a separate subset; rather, the production of IL-10 by TH17 cells is considered to be a mechanism of self-regulation to limit an otherwise potentially dangerous TH17 immune response. TH17 cells have also been associated with tissue repair functions through their production of the cytokine IL-22 along with IL-10 [133]. The protective roles of IL-22 in infections [59], as well as acute and chronic inflammatory conditions [55, 134], are associated with its functions in maintaining the integrity of epithelial barriers.
Stimuli that promote TH17 cell-inducing dendritic cells
Several studies have been performed delineating the microbial factors and pathways that stimulate the TH17 cell-inducing phenotype in DC. As noted above, IL-17 is important in the control or clearance of various pathogens [12]. These include the extracellular bacteria K. pneumoniae [119] and C. rodentium [32], as well as systemic infection with the fungal pathogen C. albicans [124].
Several adjuvants including bacterial lipopolysaccharide (LPS) and bacterial CpG DNA products promote synthesis of some TH17-promoting cytokines, such as IL-23 and IL-6 by DC, but these also strongly induce IL-12 and therefore promote TH1 cell responses preferentially over IL-17-expressing cells. However, the Saccharomyces cerevisiae cell wall component, zymosan, promotes strong TH17 cell responses in murine cell cultures and in vivo [135]. β-glucan components in zymosan signal through TLR-independent pathways to promote induction of TH17 cells [136]. Fungal β-glucans, including curdlan, bind to dectin-1, a C-type lectin on DC, and trigger IL-23 production. Dectin-1-activated DC also synthesize pro-inflammatory cytokines such as IL-6 and TNFα and upregulate co-stimulatory molecules. These dectin-1-activated DC can preferentially induce TH17 over TH1 cell responses, but only when in the presence of TGF-β-producing Treg cells or exogenous TGF-β [136]. Mycobacterium tuberculosis, a key component in complete freund’s adjuvant (CFA), can promote TH17 responses in murine cell culture and in vivo. CFA is a widely used adjuvant for murine immunization protocols, and while it was long thought to initiate TH1 responses, it is now clear that CFA can also induce TH17 responses. This is likely due to the ability of CFA to induce not only IL-6 and IL-23 but also TGF-β, allowing for differentiation of naïve CD4+ T cells into TH17 without the addition of exogenous TGF-β, as is required for LPS-activated APC-induced TH17 cells [30]. Although APC activated with LPS can induce TH17 in the presence of TGF-β, those activated with TLR3 ligand poly(I:C) preferentially produce IL-12 and IL-27 over IL-6 and TGF-β [137], thereby specifically blocking TH17 differentiation via inhibition of RORγt.
Signaling innate immune cells to drive TH17 differentiation
A question remained as to the nature of a specific, physiological innate immune stimulus that could elicit both TGF-β and IL-6 together from APC in order to promote TH17 differentiation. This was a confounding problem because of the seemingly disparate actions of the two cytokines. TGF-β is an important tolerogenic cytokine. It has been known for some time that phagocytosis of apoptotic cells by macrophages induces synthesis of TGF-β [138, 139], presumably in order to maintain a state of tolerance towards self-antigens present in dead and dying cells that are cleared by phagocytes. Contrastingly, IL-6 is a prototypical pro-inflammatory cytokine synthesized by APC as a direct result of TLR activation by microbial structures [140–142].
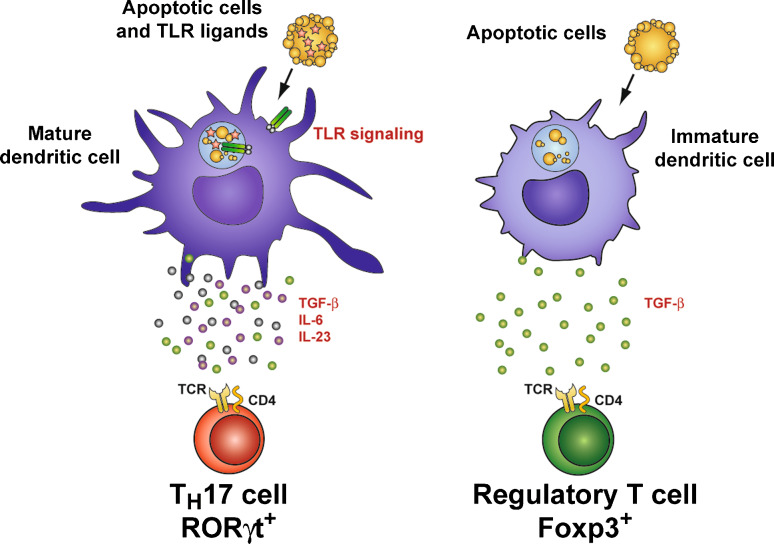
We hypothesized that DC recognition of apoptotic cells dying as a result of infection might induce not only pathways necessary for synthesis of TGF-β, but would also, because of the presence of TLR ligands from the infection, promote synthesis of IL-6. When we investigated our hypothesis, we found that this was in fact the case, and that the TGF-β and IL-6 from DC that recognized apoptotic cells carrying TLR ligands were able to drive differentiation of naïve CD4+ T cells to the TH17 lineage [143]. Furthermore, phagocytosis of apoptotic cells in the absence of TLR ligands induced TGF-β alone, causing DC to direct naïve CD4+ T cells towards the reciprocal Treg lineage [143]. In other words, the presence or absence of TLR ligands in apoptotic cells dictates whether DC that phagocytose those cells instruct generation of TH17 or Treg cells (Fig. 2).
Fig. 2.
Innate immune recognition of infected apoptotic cells instructs TH17 cell differentiation. When dendritic cells phagocytose apoptotic cells carrying TLR ligands, pathogen associated molecular patterns (PAMPs) associated with the infection engage Toll-like receptors, allowing for DC maturation and production of pro-inflammatory cytokines such as IL-6 and IL-23. The apoptotic cell element of the phagocytic cargo induces DC synthesis of the immunoregulatory cytokine TGF-β through recognition of apoptotic cell-specific molecules such as phosphatidyl serine (not shown in figure). Thus, phagocytosis of apoptotic cells by DC during an infection by DC preferentially induces differentiation of naïve CD4+ T cells into TH17 cells. When DC phagocytose apoptotic cells in the absence of infection or TLR ligands, they synthesize TGF-β without pro-inflammatory cytokines, thereby promoting differentiation of tolerogenic FOXP3+ Treg cells
Using C. rodentium as a model of infection that has been shown to induce TH17 immunity [32], we also found that C. rodentium induction of host cell apoptosis was necessary to induce this response. Thus, phagocytosis of apoptotic cells contributes signals which, in combination with TLR engagement, induce tailored immunity to bacterial infection through development of TH17 cells. Many pathogens that are potent inducers of host cell apoptosis have been implicated as requiring TH17 immunity for host defense as discussed above, including not only C. rodentium but also B. fragilis, C. albicans, and L. monocytogenes. TH17 cells are also associated with tissue repair processes besides inflammatory IL-17 production [59, 133], and thus may be uniquely suited to aid in host response against pathogens that cause significant apoptosis and tissue damage.
Conclusions
Over the last few years since its identification as a distinct lineage of T helper cells, the TH17 subset has been implicated in many immune processes. Much progress has been made in understanding the kinds of immunity mediated by IL-17 and TH17 cells, as well as the mechanisms behind cell differentiation to the subset. TH17 responses are induced by specific pathogens, and effective TH17 responses as well as TH17-related cytokines are necessary for clearance of many infections. Naïve CD4+ T cells need signals from the innate immune system to differentiate into TH17 as well as TH1 and TH2 cells, but it was surprising that TH17 lineage commitment required a combination of pro-inflammatory and anti-inflammatory cytokines for differentiation to occur. The role of TGF-β in the differentiation of both induced Treg cells as well as TH17 cells, along with the documented interactions between RORγt and FoxP3 that influence the two subsets, suggest a system that balances inflammation with tolerance. The finding that apoptotic cells dying during infection can provide the stimulus to innate immune cells to synthesize TGF-β and IL-6 and drive differentiation of TH17 cells supports this notion, further indicating that TH17 cells have a singular role in providing specific, tailored immunity to bacterial infection.
Many questions remain regarding the TH17 subset and its roles in vivo. TH17 cytokines can have varied effects depending on location and context, and TH17 responses clearly play key roles not only in the defense against certain pathogens but also in driving inflammation and autoimmunity. The link of apoptotic cells dying during infection with TH17 differentiation can provide a new direction when considering how to minimize TH17-driven immunopathology while retaining important host defense and tissue repair functions of the subset.
Acknowledgment
We are grateful to Johan Garaude for help with figure preparation.
References
- 1.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Amsen D, Spilianakis CG, Flavell RA. How are T(H)1 and T(H)2 effector cells made? Curr Opin Immunol. 2009;21:153–160. doi: 10.1016/j.coi.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amsen D, Antov A, Flavell RA. The different faces of Notch in T-helper-cell differentiation. Nat Rev Immunol. 2009;9:116–124. doi: 10.1038/nri2488. [DOI] [PubMed] [Google Scholar]
- 4.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 5.Coffman RL, Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J Immunol. 1986;136:949–954. [PubMed] [Google Scholar]
- 6.Glimcher LH, Murphy KM. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 2000;14:1693–1711. [PubMed] [Google Scholar]
- 7.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–6115. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 8.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 12.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung DR, Kasper DL, Panzo RJ, Chitnis T, Grusby MJ, Sayegh MH, Tzianabos AO. CD4+ T cells mediate abscess formation in intra-abdominal sepsis by an IL-17-dependent mechanism. J Immunol. 2003;170:1958–1963. doi: 10.4049/jimmunol.170.4.1958. [DOI] [PubMed] [Google Scholar]
- 14.Kao CY, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW, Wu R. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol. 2004;173:3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 15.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 16.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 17.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat Immunol. 2008;9:1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rengarajan J, Mowen KA, McBride KD, Smith ED, Singh H, Glimcher LH. Interferon regulatory factor 4 (IRF4) interacts with NFATc2 to modulate interleukin 4 gene expression. J Exp Med. 2002;195:1003–1012. doi: 10.1084/jem.20011128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 21.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 22.O’Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei L, Laurence A, Elias KM, O’Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21:102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 26.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 27.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci USA. 2008;105:9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams KL, Nanda I, Lyons GE, Kuo CT, Schmid M, Leiden JM, Kaplan MH, Taparowsky EJ. Characterization of murine BATF: a negative regulator of activator protein-1 activity in the thymus. Eur J Immunol. 2001;31:1620–1627. doi: 10.1002/1521-4141(200105)31:5<1620::AID-IMMU1620>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 29.Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, Sahota G, Sim J, Mukasa R, Cemerski S, Hatton RD, Stormo GD, Weaver CT, Russell JH, Murphy TL, Murphy KM. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460:405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 32.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 33.Carrier Y, Yuan J, Kuchroo VK, Weiner HL. Th3 cells in peripheral tolerance. I. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGF-beta T cell-transgenic mice. J Immunol. 2007;178:179–185. doi: 10.4049/jimmunol.178.1.179. [DOI] [PubMed] [Google Scholar]
- 34.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 37.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly RD, Foster D. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 39.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 40.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 41.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 42.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 43.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pappu BP, Borodovsky A, Zheng TS, Yang X, Wu P, Dong X, Weng S, Browning B, Scott ML, Ma L, Su L, Tian Q, Schneider P, Flavell RA, Dong C, Burkly LC. TL1A-DR3 interaction regulates Th17 cell function and Th17-mediated autoimmune disease. J Exp Med. 2008;205:1049–1062. doi: 10.1084/jem.20071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 46.Stumhofer JS, Silver J, Hunter CA. Negative regulation of Th17 responses. Semin Immunol. 2007;19:394–399. doi: 10.1016/j.smim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 48.El-behi M, Ciric B, Yu S, Zhang GX, Fitzgerald DC, Rostami A. Differential effect of IL-27 on developing versus committed Th17 cells. J Immunol. 2009;183:4957–4967. doi: 10.4049/jimmunol.0900735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 50.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, Sakaguchi N, Sakaguchi S. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z, Dong C. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liang SC, Long AJ, Bennett F, Whitters MJ, Karim R, Collins M, Goldman SJ, Dunussi-Joannopoulos K, Williams CM, Wright JF, Fouser LA. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007;179:7791–7799. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- 54.Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168:5397–5402. doi: 10.4049/jimmunol.168.11.5397. [DOI] [PubMed] [Google Scholar]
- 55.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kreymborg K, Etzensperger R, Dumoutier L, Haak S, Rebollo A, Buch T, Heppner FL, Renauld JC, Becher B. IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J Immunol. 2007;179:8098–8104. doi: 10.4049/jimmunol.179.12.8098. [DOI] [PubMed] [Google Scholar]
- 58.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 60.Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, Senices M, Gill D, Dunussi-Joannopoulos K, Collins M, Nickerson-Nutter C, Fouser LA, Young DA. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H (2009) Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol (in press) [DOI] [PubMed]
- 62.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F (2009) Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol (in press) [DOI] [PubMed]
- 63.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 66.Ono M, Yaguchi H, Ohkura N, Kitabayashi I, Nagamura Y, Nomura T, Miyachi Y, Tsukada T, Sakaguchi S. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature. 2007;446:685–689. doi: 10.1038/nature05673. [DOI] [PubMed] [Google Scholar]
- 67.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sinigaglia F, D’Ambrosio D, Panina-Bordignon P, Rogge L. Regulation of the IL-12/IL-12R axis: a critical step in T-helper cell differentiation and effector function. Immunol Rev. 1999;170:65–72. doi: 10.1111/j.1600-065X.1999.tb01329.x. [DOI] [PubMed] [Google Scholar]
- 69.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, Schones DE, Peng W, Sun HW, Paul WE, O’Shea JJ, Zhao K. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lexberg MH, Taubner A, Forster A, Albrecht I, Richter A, Kamradt T, Radbruch A, Chang HD. Th memory for interleukin-17 expression is stable in vivo. Eur J Immunol. 2008;38:2654–2664. doi: 10.1002/eji.200838541. [DOI] [PubMed] [Google Scholar]
- 72.Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A (2009) Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest (in press) [DOI] [PMC free article] [PubMed]
- 73.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25−Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 75.Osorio F, LeibundGut-Landmann S, Lochner M, Lahl K, Sparwasser T, Eberl G, Reis e Sousa C. DC activated via dectin-1 convert Treg into IL-17 producers. Eur J Immunol. 2008;38:3274–3281. doi: 10.1002/eji.200838950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 77.Gershon RK, Kondo K. Infectious immunological tolerance. Immunology. 1971;21:903–914. [PMC free article] [PubMed] [Google Scholar]
- 78.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 79.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 80.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/S1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 81.Bruder D, Probst-Kepper M, Westendorf AM, Geffers R, Beissert S, Loser K, von Boehmer H, Buer J, Hansen W. Neuropilin-1: a surface marker of regulatory T cells. Eur J Immunol. 2004;34:623–630. doi: 10.1002/eji.200324799. [DOI] [PubMed] [Google Scholar]
- 82.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 84.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 85.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 86.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 87.Huter EN, Punkosdy GA, Glass DD, Cheng LI, Ward JM, Shevach EM. TGF-beta-induced Foxp3+ regulatory T cells rescue scurfy mice. Eur J Immunol. 2008;38:1814–1821. doi: 10.1002/eji.200838346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta–TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci USA. 2003;100:10878–10883. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thornton AM, Shevach EM. CD4+CD25 +immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci USA. 2004;101:10398–10403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 93.Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, Eaton D, Grogan JL. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 94.Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, Maraver A, Lafaille JJ, Dustin ML. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sarris M, Andersen KG, Randow F, Mayr L, Betz AG. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity. 2008;28:402–413. doi: 10.1016/j.immuni.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol. 2006;177:6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 97.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barthlott T, Kassiotis G, Stockinger B. T cell regulation as a side effect of homeostasis and competition. J Exp Med. 2003;197:451–460. doi: 10.1084/jem.20021387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gondek DC, Devries V, Nowak EC, Lu LF, Bennett KA, Scott ZA, Noelle RJ. Transplantation survival is maintained by granzyme B+ regulatory cells and adaptive regulatory T cells. J Immunol. 2008;181:4752–4760. doi: 10.4049/jimmunol.181.7.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 101.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 102.Krakowski M, Owens T. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur J Immunol. 1996;26:1641–1646. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- 103.Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, Kamoun M, Rostami A. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169:7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 104.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/S1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 105.Wiekowski MT, Leach MW, Evans EW, Sullivan L, Chen SC, Vassileva G, Bazan JF, Gorman DM, Kastelein RA, Narula S, Lira SA. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J Immunol. 2001;166:7563–7570. doi: 10.4049/jimmunol.166.12.7563. [DOI] [PubMed] [Google Scholar]
- 106.Shevach EM, Chang JT, Segal BM. The critical role of IL-12 and the IL-12R beta 2 subunit in the generation of pathogenic autoreactive Th1 cells. Springer Semin Immunopathol. 1999;21:249–262. doi: 10.1007/BF00812256. [DOI] [PubMed] [Google Scholar]
- 107.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 108.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bush KA, Farmer KM, Walker JS, Kirkham BW. Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion protein. Arthritis Rheum. 2002;46:802–805. doi: 10.1002/art.10173. [DOI] [PubMed] [Google Scholar]
- 110.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 111.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 112.Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL, Le TV, Lorenz RG, Xu H, Kolls JK, Carter RH, Chaplin DD, Williams RW, Mountz JD. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 113.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 114.Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645–649. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- 116.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:382–388. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 119.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 120.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miyamoto M, Prause O, Sjostrand M, Laan M, Lotvall J, Linden A. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J Immunol. 2003;170:4665–4672. doi: 10.4049/jimmunol.170.9.4665. [DOI] [PubMed] [Google Scholar]
- 122.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kelly MN, Kolls JK, Happel K, Schwartzman JD, Schwarzenberger P, Combe C, Moretto M, Khan IA. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect Immun. 2005;73:617–621. doi: 10.1128/IAI.73.1.617-621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 125.Wu Q, Martin RJ, Rino JG, Breed R, Torres RM, Chu HW. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 2007;9:78–86. doi: 10.1016/j.micinf.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schulz SM, Kohler G, Holscher C, Iwakura Y, Alber G. IL-17A is produced by Th17, gammadelta T cells and other CD4- lymphocytes during infection with Salmonella enterica serovar Enteritidis and has a mild effect in bacterial clearance. Int Immunol. 2008;20:1129–1138. doi: 10.1093/intimm/dxn069. [DOI] [PubMed] [Google Scholar]
- 127.Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, Oshiro K, Okamoto Y, Watanabe H, Kawakami K, Roark C, Born WK, O’Brien R, Ikuta K, Ishikawa H, Nakae S, Iwakura Y, Ohta T, Matsuzaki G. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J Immunol. 2008;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sanfilippo L, Li CK, Seth R, Balwin TJ, Menozzi MG, Mahida YR. Bacteroides fragilis enterotoxin induces the expression of IL-8 and transforming growth factor-beta (TGF-beta) by human colonic epithelial cells. Clin Exp Immunol. 2000;119:456–463. doi: 10.1046/j.1365-2249.2000.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vallance BA, Deng W, Jacobson K, Finlay BB. Host susceptibility to the attaching and effacing bacterial pathogen Citrobacter rodentium. Infect Immun. 2003;71:3443–3453. doi: 10.1128/IAI.71.6.3443-3453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Renna MS, Correa SG, Porporatto C, Figueredo CM, Aoki MP, Paraje MG, Sotomayor CE. Hepatocellular apoptosis during Candida albicans colonization: involvement of TNF-alpha and infiltrating Fas-L positive lymphocytes. Int Immunol. 2006;18:1719–1728. doi: 10.1093/intimm/dxl106. [DOI] [PubMed] [Google Scholar]
- 131.Cervantes J, Nagata T, Uchijima M, Shibata K, Koide Y. Intracytosolic Listeria monocytogenes induces cell death through caspase-1 activation in murine macrophages. Cell Microbiol. 2008;10:41–52. doi: 10.1111/j.1462-5822.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- 132.Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, Sher A. Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204:273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 134.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7:1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 136.LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 137.Goriely S, Neurath MF, Goldman M. How microorganisms tip the balance between interleukin-12 family members. Nat Rev Immunol. 2008;8:81–86. doi: 10.1038/nri2225. [DOI] [PubMed] [Google Scholar]
- 138.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 139.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 141.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 142.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 143.Torchinsky MB, Garaude J, Martin AP, Blander JM. Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature. 2009;458:78–82. doi: 10.1038/nature07781. [DOI] [PubMed] [Google Scholar]
- 144.http://clinicaltrials.gov/ct2/show/NCT00584740?term=ain457&rank=12