Abstract
The pathomechanism of antibody-mediated tissue damage in autoimmune diseases can be best studied in experimental models by passively transferring specific autoantibodies into animals. The reproduction of the disease in animals depends on several factors, including the cross-reactivity of patient autoantibodies with the animal tissue. Here, we show that autoantibodies from patients with epidermolysis bullosa acquisita (EBA), a subepidermal autoimmune blistering disease, recognize multiple epitopes on murine collagen VII. Indirect immunofluorescence microscopy revealed that EBA patients’ IgG cross-reacts with mouse skin. Overlapping, recombinant fragments of murine collagen VII were used to characterize the reactivity of EBA sera and to map the epitopes on the murine antigen by ELISA and immunoblotting. The patients’ autoantibody binding to murine collagen VII triggered pathogenic events as demonstrated by a complement fixing and an ex vivo granulocyte-dependent dermal–epidermal separation assay. These findings should greatly facilitate the development of improved disease models and novel therapeutic strategies.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-009-0256-3) contains supplementary material, which is available to authorized users.
Keywords: Autoimmunity, Autoantigen, Basement membrane zone, Collagen, Extracellular matrix
Introduction
Autoantibodies cause tissue damage in a group of autoimmune diseases. The demonstration of autoantibody pathogenicity and the characterization of the underlying mechanisms require the reproduction of the disease by the passive transfer of specific antibodies into laboratory animals. Thus, the pathogenic potential of autoantibodies in several autoimmune diseases has been established by the passive transfer of the IgG serum fraction purified from patients into mice or rats [1–7].
Epidermolysis bullosa acquisita (EBA), a severe chronic subepidermal blistering disease of skin and mucous membranes, is characterized by tissue-bound and circulating IgG antibodies to the dermal–epidermal junction [8]. Patients’ serum autoantibodies bind to the 290-kDa collagen VII, the major component of anchoring fibrils [8, 9]. Autoantibodies in the majority of EBA patients recognize different epitopes within the noncollagenous (NC) 1 and 2 domains of collagen VII [8–11]. The pathogenic relevance of antibodies against collagen VII is supported by compelling evidence: (1) EBA autoantibodies were shown to recruit and activate leucocytes ex vivo, resulting in dermal–epidermal separation in cryosections of human skin [12]; (2) antibodies against collagen VII induce subepidermal blisters when passively transferred into mice [5, 13]; and (3) immunization with recombinant autologous collagen VII induces an autoimmune response to this protein, resulting in a blistering phenotype closely resembling human EBA [14].
The replication of the major clinical, histo- and immunopathological features of human EBA by the passive transfer of antibodies specific to collagen VII into mice greatly facilitated the dissection of the pathophysiology of blister formation in this disease [15–17]. The existing mouse models reproducing the blister formation in experimental EBA use the passive transfer of IgG antibodies from rabbits immunized against murine collagen VII or EBA patients’ IgG into animals. While these studies suggest that antibodies directed against different epitopes of the NC1 domain of collagen VII are pathogenic, further pathogenic epitopes may exist, and their relative contribution for blistering in EBA has not yet been addressed. Using patients’ autoantibodies for the passive transfer experiments in mice has several advantages over rabbit antibodies, especially the fact that patient autoantibodies already possess the “ideal” specificity and effector functions. Major disadvantages of using patients’ autoantibodies in mice include the limited availability of patient autoantibodies as well as the fact that the degree of cross-reactivity may be low and the epitope recognition pattern may differ substantially for the murine compared with the human antigen [18].
Autoantibodies from a few EBA patients were shown to cross-react with murine skin [5, 13]. However, autoantibodies from other patients bound poorly or not at all to mouse skin [5]. In addition, the epitopes on the murine antigen recognized by EBA autoantibodies have not yet been characterized. This information is highly relevant for the development of standardized reagents such as blister-inducing human monoclonal antibodies, which could replace EBA patient autoantibodies. Therefore, the aim of the present study was to systematically analyze the degree of cross-reactivity of autoantibodies with murine collagen VII in a large number of patients. For this purpose, we analyzed the reactivity of patients’ autoantibodies with murine skin by indirect immunofluorescence (IF) microscopy. Subsequently, recombinant forms of murine collagen VII covering the NC1 and NC2 domains of the protein were generated. The reactivity of EBA sera with murine collagen VII was characterized by ELISA using these recombinantly expressed protein fragments. Further, the recognition pattern of murine collagen VII epitopes by patients’ autoantibodies was characterized by immunoblotting and ELISA. Finally, the pathogenic potential of patients’ autoantibodies binding to murine skin has been assessed by a complement-fixation test and by the ex vivo autoantibody-induced dermal–epidermal separation assay.
Materials and methods
Human sera
Serum samples were obtained from patients with EBA (n = 35) prior to the initiation of treatment. Patients were characterized by: (1) blisters on the skin; (2) linear deposits of IgG at the DEJ, as shown by direct IF microscopy; (3) circulating IgG autoantibodies binding to the dermal side of 1 mol/l of NaCl-split human skin, as shown by indirect IF microscopy; and (4) immunoblot reactivity with dermal and/or recombinant collagen VII. The distribution of IgG subclasses in serum of EBA patients (n = 19) was examined by indirect immunofluorescnce microscopy, using human salt-split skin sections. The results of this analysis are summarized in the electronic supplementary material, ESM, Table 2. EBA patients’ autoantibodies binding to the dermal side of the salt-split skin belonged to all four IgG subclasses. As expected, the predominant subclass was IgG4, followed by IgG1 and IgG2, while IgG3 autoantibodies were detected in few EBA patients. For the experiments conducted, we obtained approval from the Ethics Committee of the Medical Faculty of the University of Freiburg, Freiburg, Germany (Institutional Board Projects no. 318/07 and 407/08). We obtained informed consent from patients whose material was used in the study, in adherence to the Helsinki principles.
Cell culture
PAM 212 murine keratinocytes [19] were cultured in DMEM (CCPro) supplemented with 10% fetal calf serum, 4 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (all from Biochrom).
Heterologous expression of recombinant forms of collagen VII in bacteria
The cDNA sequences coding for six overlapping fragments of the NC1 and one fragment corresponding to the NC2 domain of collagen VII were cloned into prokaryotic expression vectors and expressed in Escherichia coli following protocols described previously [12, 20]. Briefly, DNA sequence data for murine collagen VII was retrieved from GenBank using the accession number NM_007738 [21]. Primers for polymerase chain reactions (PCR) were synthesized by MWG Biotech (Ebersberg, Germany; Table 1). The cDNA fragments were obtained by PCR on a cDNA pool generated by reverse transcription of mRNA extracted from cultured PAM212 cells or on synthetic DNA encoding for fragments of collagen VII provided by GenScript (Piscataway, NJ, USA). Restriction sites for BamHI and SalI were introduced by primers (Table 1). Collagen VII cDNA fragments were cloned into linearized pGEX-6P-1 (Amersham Biosciences, Freiburg, Germany), resulting in the recombinant vectors pGEX-mCOL7-1, pGEX-mCOL7-2, pGEX-mCOL7-3, pGEX-mCOL7-4, pGEX-mCOL7-5, pGEX-mCOL7Cr, and pGEX-mCOL7-Z. For the generation of recombinant murine collagen VII with a His-tag, the DNA fragments of collagen VII were subcloned in pQE41 (Amersham Biosciences), resulting in the recombinant vectors pQE41-mCOL7-1, pQE41-mCOL7-2, pQE41-mCOL7-3, pQE41-mCOL7-4, pQE41-mCOL7-5, pQE41-mCOL7Cr, and pQE41-mCOL7-Z. Correct ligation and in-frame insertion of the various DNA fragments were confirmed by DNA sequence analysis. Recombinant GST fusion and His-tagged proteins were expressed in E. coli TOP 10 and XL1-Blue and purified by gluthatione agarose and metallochelate affinity chromatography, respectively as described [22, 23].
Table 1.
Primer sequences for PCR amplification of cDNA fragments of murine collagen VII
| Fragment | Size (bp) | Primer sequences (5′–3′) |
|---|---|---|
| mCVII-1 | 851 |
F: GATCGGATCCCAGCCCAGAGATAGAGTGACCTGCAC R: GATCGTCGACTCATGGCCGGAGACCCTGGAG |
| mCVII-2 | 957 |
F: GATCGGATCCCGACAGGAGGTGAACATC R: GATCGTCGACTCATCTGTGGATCGTTAGGATG |
| mCVII-3 | 972 |
F: GATCGGATCCTTTGACTTAGATGATGTTCG R: GATCGTCGACTCAGCTCTGCACAACTTGAAG |
| mCVII-4 | 788 |
F: GATCGGATCCCTGGAGACTCTTCAAGTTG R: GATCGTCGACTCAGATGTCACGGATCTTTCG |
| mCVII-5 | 671 |
F: GATCGGATCCCCACTGAATAGTTCCCATG R: GATCGTCGACTCACTTAATGCCAGGAGACCC |
| mCVIICr | 698 |
F: GATCGGATCCACCCACGTAGCTGGTGTGGATG R: GATCGTCGACTCACACTGGGGTCCAGGTCAAAG |
| mCOL-Z | 491 |
F: GATCGGATCCTGCCAGGGCCAGTTTATTG R: GATCGTCGACATCATCCGGGCCTCAGTCCTAG |
F Forward primer, R reverse primer
IF microscopy and immunoblot analysis
Detection of serum autoantibodies followed published protocols with minor modifications [22, 24]. Briefly, after incubating with diluted serum, the frozen sections of murine and human skin were treated with 100-fold diluted AlexaFluor 488 conjugated Abs to human IgG (Invitrogen). The rabbit polyclonal antibody SA6310 was produced as described previously [5], against the recombinant GST-fusion protein containing a sequence of murine collagen VII (GST-mCVIICr) generated for this study. IgG subclass detection in the sera of patients was done following previously described protocols [14]. Briefly, frozen sections of human salt-split skin were incubated in a first step with 10-fold diluted EBA sera, and in a second step with 500-fold diluted, biotin-conjugated monoclonal mouse Abs specific to human IgG1, IgG2, IgG3 and IgG4 (clones HP6070, HP6014, HP6047, HP6023; Invitrogen). As a tertiary detecting reagent, we have used an AlexaFluor 488-conjugated Streptavidin. Extracts of murine dermis were prepared as described [5]. Recombinant proteins were fractionated by 12% SDS-PAGE, transferred to nitrocellulose and analyzed by immunoblotting [5].
Measurement of autoantibody levels by ELISA
ELISA using recombinant murine collagen VII was performed at room temperature in 96-well microtiter plates. The optimal working conditions of the assay were defined by chessboard titrations with dilutions of Ag and secondary Ab, as described [14]. The optimized ELISA was run under the following conditions: wells were coated with equimolar amounts of purified His-mCVII fragments in 0.1 M bicarbonate buffer (pH 9.6). After blocking, wells were incubated for 1 h with a 100-fold dilution of patients’ sera previously preadsorbed with cleared cell-lysate of bacteria transformed with pQE41 wildtype vector. Bound Abs were detected using a 5,000-fold dilution of an HRP-labeled rabbit anti-mouse IgG Ab (Abcam) and orthophenylene diamine (Dako).
Complement-fixation assay
Complement-fixing activity of EBA patients’ autoantibodies to the dermal–epidermal junction was determined using a modification of published protocols [25]. Briefly, cryosections of human and murine skin were incubated with EBA patient serum or normal human serum obtained from healthy donors at 37°C for 30 min followed by washing with PBS pH 7.2, twice for 10 min. Subsequently, sections were treated with fresh human serum from healthy donors as a source of mammalian complement, diluted 1:5 with Gelatin Veronal Buffer (Sigma), for 30 min at 37°C. Complement deposition was visualized with a monoclonal antibody to human C3 (Acris Antibodies) and an AlexaFluor-488-conjugated anti-mouse IgG antibody (Invitrogen).
Ex vivo cryosection assay
The ability of EBA patients’ autoantibodies to activate human leukocytes was assessed using an ex vivo assay of antibody-induced granulocyte-dependent dermal–epidermal separation in cryosections of normal human and murine skin, respectively, as described previously [25, 26].
Sequence analysis
Protein sequences of murine and human collagen VII were retrieved from Genbank. Sequence alignments were performed using the algorithm developed by Smith and Waterman [27]. The antigenic determinants of collagen VII were analyzed by both MIF Bioinformatic Tools (http://bio.dfci.harvard.edu/Tools/antigenic.html) and the Antigenic routine of the EMBOSS package, which computes an antigenic score based on a prediction algorithm developed by Kolaskar and Tongaonkar [28].
Results
In silico analysis of antigenic epitopes of human and murine collagen VII
The murine and human forms of pro-collagen VII both with a length of 2,944 aa show by analysis using the Smith–Waterman algorithm an identity and similarity of 84.1 and 89.2%, respectively (see detailed results in ESM). B cell epitopes of murine and human collagen VII only partly overlap as predicted by analysis using specialized bioinformatic software (ESM, Table 1).
Analysis of cross-reactivity of EBA sera by IF microscopy on murine skin sections
The reactivity of IgG autoantibodies from serum of 35 patients with EBA was analyzed by IF microscopy using human and mouse skin sections. The results of this analysis are summarized in Table 2 and representative examples are shown in Fig. 1. Twenty-eight of 30 EBA sera, which reacted with the dermal–epidermal junction of the human skin, also recognized the murine epidermal basement membrane zone.
Table 2.
Cross-reactivity of EBA sera with murine collagen VII
| Patient | Age (years) | Reactivity with | CBT IF scoresa | DES | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human skin (IIF)a | Murine skin (IIF)a | mCVII fragments (ELISA/IB) | Human skin | Mouse skin | Human skin | Mouse skin | ||||||||
| 1 | 2 | 3 | 4 | 5 | Cr | Z | ||||||||
| EBA1 | 51 | ++++ | ++++ | X | X | ++++ | ++ | ++++ | ++++ | |||||
| EBA2 | 19 | ++ | ++ | X | X | X | − | − | − | − | ||||
| EBA3 | 20 | ++++ | +++ | X | X | X | ++ | ++ | ++ | ++ | ||||
| EBA4 | 13 | ++ | + | X | n.d. | n.d. | n.d. | n.d. | ||||||
| EBA5 | 46 | ++ | + | X | X | n.d. | n.d. | n.d. | n.d. | |||||
| EBA6 | 67 | + | + | − | − | − | − | − | − | − | n.d. | n.d. | + | − |
| EBA7 | 77 | + | + | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| EBA8 | − | ++ | ++ | X | X | n.d. | n.d. | +++ | − | |||||
| EBA9 | 59 | +++ | +++ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | +++ | + | ++ | − |
| EBA10 | 56 | ++ | ++ | X | X | n.d. | n.d. | +++ | ++ | |||||
| EBA11 | 89 | ++ | ++ | X | X | n.d. | n.d. | ++ | + | |||||
| EBA12 | 49 | + | + | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| EBA13 | − | ++++ | ++++ | − | − | − | − | − | − | − | +++ | − | ++++ | +++ |
| EBA14 | 66 | ++ | ++ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | + | + |
| EBA15 | − | +++ | +++ | X | − | − | ++++ | − | ||||||
| EBA16 | 71 | +++ | +++ | − | − | − | − | − | − | − | − | − | ++ | ++ |
| EBA17 | 60 | +++ | +++ | X | ++ | ++ | +++ | − | ||||||
| EBA18 | 49 | ++++ | +++ | − | − | − | − | − | − | − | ± | − | ++ | − |
| EBA19 | 26 | +++ | ++ | X | X | n.d. | n.d. | +++ | − | |||||
| EBA20 | 68 | + | + | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| EBA21 | 40 | ++++ | ++++ | X | X | X | + | ± | ++++ | − | ||||
| EBA22 | 75 | − | − | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| EBA23 | 61 | + | + | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| EBA24 | 85 | ++ | ++ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | − | − | + | − |
| EBA25 | − | − | − | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| EBA26 | 57 | ++ | ++ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | − | − |
| EBA27 | 49 | + | − | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| EBA28 | − | +++ | +++ | X | X | X | ++ | − | ++ | − | ||||
| EBA29 | − | +++ | ++ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ++++ | ± | +++ | ++ |
| EBA30 | − | ++++ | +++ | − | − | − | − | − | − | − | ± | − | ++ | ++ |
| EBA31 | 69 | ++++ | +++ | X | ± | − | ++ | + | ||||||
| EBA32 | 80 | − | − | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| EBA33 | 22 | ± | − | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| EBA34 | 7 | − | − | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| EBA35 | − | − | − | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| TOTAL | 30/35 | 28/35 | 5/19 | 4/19 | 5/19 | 7/19 | 4/19 | 3/19 | 0/19 | 11/15 | 6/15 | 20/22 | 10/22 | |
aThe fluorescence intensity has been scored as follows: − no staining, + focal faint staining of the basal membrane, ++ faint staining of the basal membrane, +++ medium staining of the basal membrane, ++++ strong staining of the basal membrane
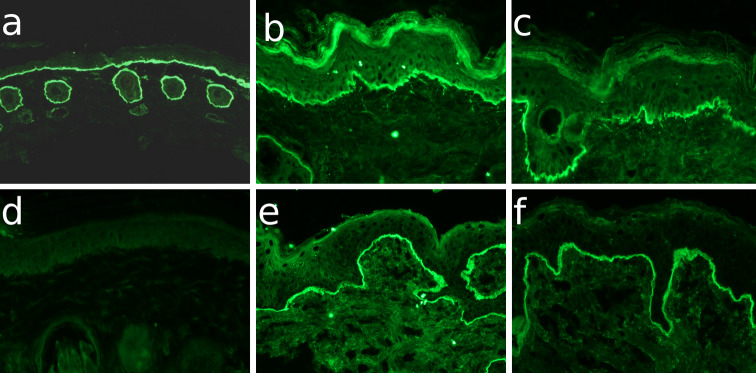
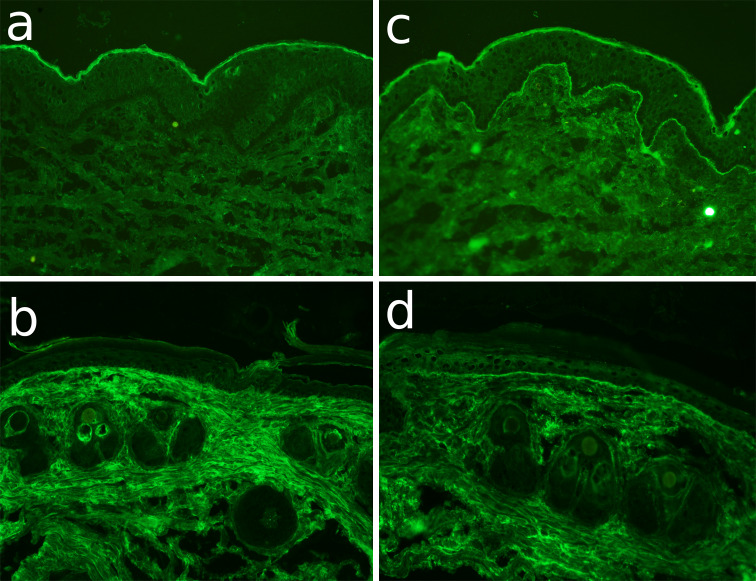
Fig. 1.
Cross-reactivity of EBA patients sera with murine skin by immunofluorescence microscopy. Cryosections of a–d murine and e, f human skin were incubated with a serum from a rabbit immunized with GST-mCVIICr (SA6310), b,e serum from EBA3, c,f serum from EBA1, and d normal human serum (NHS). After washing in PBS, sections were incubated with a anti-rabbit IgG and b–f anti-human IgG both labeled with AlexaFluor 488 (magnifications, all ×200)
Generation of recombinant proteins
The cDNA sequences coding for seven fragments of murine collagen VII were cloned into prokaryotic expression vectors and expressed in Escherichia coli (Table 1; Fig. 2). The GST fusion and His-tagged murine collagen VII fragments were purified by glutathione- and metallochelate-affinity chromatography, respectively. All proteins migrated consistently with their calculated masses when separated by SDS-PAGE (ESM, Fig. 1).
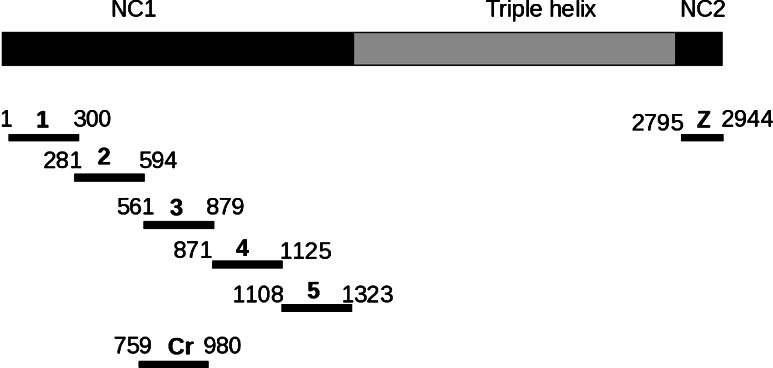
Fig. 2.
Recombinant fragments of collagen VII generated in this study. Collagen VII is composed of three identical α chains, each consisting of a central triple helical collagenous domain, flanked by a large amino-terminal noncollagenous domain (NC1) and a smaller carboxy-terminal noncollagenous domain (NC2). Fragments of murine collagen VII cDNA corresponding to the NC1 and NC2 domains were cloned into pGEX-6P-1 and pQE41 and expressed in E. coli. Amino acid residue numbers are shown above the fragments
Molecular analysis of cross-reactivity of EBA sera with murine collagen VII
To characterize the molecular target(s) of EBA autoantibodies in murine skin, the reactivity of EBA sera was analyzed with the recombinant His-tagged fragments of murine collagen VII by ELISA and immunoblotting. The results of these analyses are summarized in Table 2 and representative examples are shown in Figs. 3 and 4. IgG autoantibodies from 14 of 19 EBA patients recognized recombinant murine collagen VII by immunoblotting.
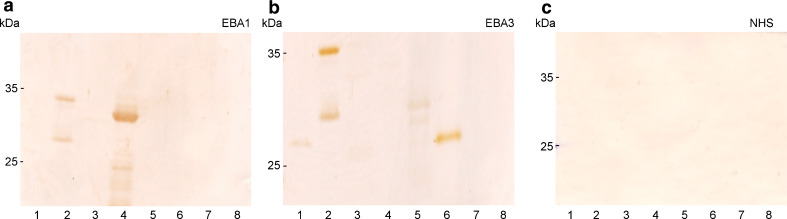
Fig. 3.
Immunoblot analysis of the EBA sera reactivity with mCVII. His-tagged recombinant mCVII fragments 1, 2, 3, 4, 5 and Z (lanes 2–8) as well as His-DHFR (lane 1) were separated by 12% SDS-PAGE and electrophoretically transferred to nitrocellulose. The membranes were immunoblotted with a serum from patients EBA1, b EBA3, and c normal human serum (NHS). Migration positions of molecular weight markers (kDa) are shown on the left
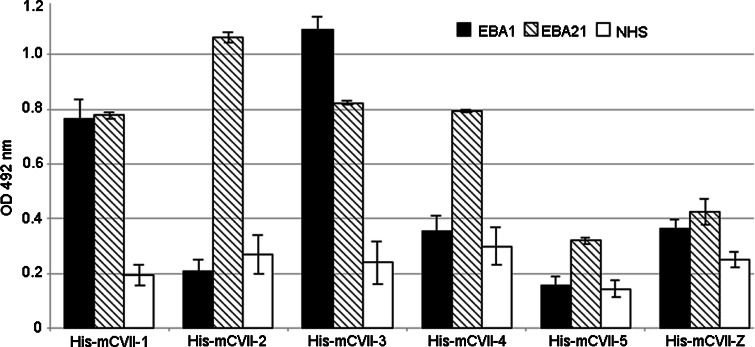
Fig. 4.
Analysis of the reactivity of EBA sera with murine collagen VII by ELISA. Levels of serum IgG autoantibodies were measured in triplicate by ELISA using recombinant fragments of murine collagen VII as described in “Materials and methods”. OD readings of serum samples from patients EBA1, EBA21 and from a healthy donor are given as mean ± SD
Mapping of epitopes on murine collagen VII recognized by EBA sera
Autoantibodies from EBA patients bind to several epitopes within the NC1 and NC2 domains of human collagen VII [10, 11]. The distribution of antigenic determinants recognized by EBA autoantibodies in murine collagen VII molecule was analyzed by immunoblotting and ELISA with different fragments of murine collagen VII (Table 2; Fig. 4). IgG autoantibodies from 5, 4, 5, 7, 4, and none of 19 EBA patients’ sera recognized fragments 1, 2, 3, 4, 5, and Z, respectively.
EBA autoantibodies fix complement to the murine dermal–epidermal junction
To address the question whether EBA autoantibody binding to the dermal–epidermal junction of murine skin can activate complement locally, their complement-binding ability was assessed ex vivo by an immunofluorescent complement-fixation test. The results of this assay are summarized in Fig. 5. In contrast to sera from healthy donors (Fig. 5a, b), autoantibodies from 6 of 11 EBA patients showing complement-binding potential in human skin sections (Fig. 5c), also fixed complement at the dermal–epidermal junction of murine skin (Fig. 5d).
Fig. 5.
EBA patient autoantibodies fix complement to the dermal–epidermal junction of murine skin sections. Frozen sections of human (a,c) and murine (b,d) skin were incubated with serum from a healthy donor (a,b) and from EBA1 patient (c,d). After washing in phosphate-buffered saline, the sections were further incubated with fresh human serum as a source of complement. C3 deposits were visualized by incubation with a monoclonal antibody specific to human C3 followed by an AlexaFluor-488-conjugated anti-mouse IgG antibody. While normal human serum (a,b) did not fix complement in the cryosections, the EBA serum bound complement at the dermal–epidermal junction of both human (c) and murine (d) skin sections
EBA sera induce dermal–epidermal separation of murine skin cryosections
Autoantibodies against collagen VII have been shown to recruit and activate granulocytes to the dermal–epidermal junction and induce subepidermal splits in cryosections of human skin when coincubated with granulocytes from healthy donors [12, 17, 29]. To assess the ability of EBA patient autoantibodies to induce granulocyte-dependent dermal–epidermal separation in murine skin, sera from EBA patients and healthy donors were incubated with murine and human skin cryosections. Representative examples of this assay are shown in Fig. 6. In contrast to the normal human sera (Fig. 6a, b), after the addition of human leukocytes, autoantibodies from 20 and 10 EBA patients induced subepidermal splits in the cryosections of (Fig. 6c) human and (Fig. 6d) murine skin, respectively.
Fig. 6.
EBA patient autoantibodies induce dermal–epidermal separation in sections of murine skin. Frozen sections of human (a,c) and murine (b,d) skin were incubated with serum from a healthy donor (a,b) and from EBA1 patient (c,d). After washing in phosphate-buffered saline, the sections were further incubated with fresh human serum as a source of leucocytes. In contrast to sections incubated with serum from a healthy donor (a,b), sections treated with serum from the EBA patient (c,d) developed dermal–epidermal separation after incubation with normal human granulocytes
Discussion
Several animal models of blister formation by the passive transfer of collagen VII-specific antibodies into mice have been recently established [5, 13, 30]. In addition to demonstrating the autoimmune nature of EBA, these models represent exquisite tools for studying the cellular and molecular aspects of the autoantibody-induced tissue damage [5, 16, 17, 23, 25]. However, among other limitations, the epitopes recognized by patient autoantibodies may not match when murine collagen VII is compared with its human ortholog. Previous studies and our present analysis indeed show a relatively low degree of homology between human and murine collagen VII compared with the homology of other collagen molecules [31]. In addition, a cross-reactivity of autoantibodies with murine skin could not be previously documented in all tested EBA patients [5]. Furthermore, as our present in silico analysis shows, the predicted B cell epitopes on murine and human collagen VII only partly overlap. Despite these possible limitations, patient autoantibodies are in theory ideal reagents in terms of both specificity and effector functions to use for the study of blister formation. Therefore, in the present work, we characterized the epitopes on murine collagen VII recognized by EBA patient autoantibodies.
In a first set of experiments, we analyzed the reactivity of serum autoantibodies with the dermal–epidermal junction in a large group of EBA patients. In line with previous data [5], the results show a cross-reactivity of EBA autoantibodies with murine skin in the majority of our EBA patients. As expected, these EBA sera specifically reacted with recombinant murine collagen VII as demonstrated by immunoblot analysis.
To map the epitopes on murine collagen VII recognized by the EBA sera, we used overlapping recombinant fragments covering the NC1 and NC2 domains of this antigen. The overall pattern of reactivity is matching the one reported for the reactivity with the human collagen VII fragments [10, 11, 32]. We show that autoantibodies in EBA sera react with different epitopes within the NC1, but not NC2, domains of murine collagen VII.
In further experiments, we addressed the pathogenic relevance of autoantibodies from a relatively large number of EBA patients cross-reacting with murine skin. Because of the limited amounts of EBA serum, we used two ex vivo assays to assess the effector functions of autoantibodies. The capacity of autoantibodies to fix complement C3 at the murine dermal–epidermal junction was reduced compared to their ability to activate complement on human skin sections. This finding is compatible with the assumption that EBA autoantibodies recognize fewer (pathogenic) epitopes on murine collagen VII compared to its human ortholog. Importantly, autoantibodies from a subgroup of the EBA sera which induced dermal–epidermal separation in human skin were also pathogenic in the murine skin. In line with our previous observation that complement activation is not required for the granulocyte-dependent dermal–epidermal separation in this model [22], the ability of EBA autoantibodies to induce subepidermal splits did not closely match their complement-fixing properties. In EBA patients, the autoantibodies against collagen VII belong to different IgG subclasses and their complement-fixing ability does not correlate with the inflammatory or classical clinical forms of the disease [33]. However, since results from in vivo models of EBA demonstrate that complement activation is a prerequisite for the antibody-induced blistering and suggest that IgG subclass of autoantibodies is determining their pathogenicity, further investigation of the association of complement-binding capacity and IgG subclass of autoantibodies with disease activity in EBA patients is required [14, 16].
One major disadvantage of using IgG from EBA patients for in vivo studies is their low availability [18]. This issue could be addressed by generating human autoantibodies recombinantly or by classical hybridoma technology in quantities sufficient to sustain long-term research using this model. Our present results provide the rationale and the tools to develop recombinant or monoclonal human autoantibodies specific to collagen VII. The characterization of the pathogenic epitopes recognized by patient autoantibodies is also relevant for addressing further questions, including the epitope-specific affinity purification of patient serum autoantibodies, the development of an improved animal model by peptide immunization, and the induction of immunological tolerance.
In conclusion, our results demonstrate for the first time that blister-inducing EBA autoantibodies recognize multiple epitopes on murine collagen VII. Patient autoantibodies binding to murine collagen VII epitopes retain in part their pathogenic activity. Our results significantly add to the rationale of studying the blister formation in EBA by passively transferring patients’ autoantibodies and suggest the existence of multiple pathogenic epitopes in EBA. In addition, the reagents developed in this work should be useful for pathogenetic studies and the development of more specific therapeutic approaches.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1 Expression of the recombinant GST-fusion and His-tagged fragments of murine collagen VII. (a) Equimolar amounts of GST (lane 2), GST-mCVII-1 (lane 3), GST-mCVII-2 (lane 4), GST-mCVII-3 (lane 5), GST-mCVII-4 (lane 6), GST-mCVII-5 (lane 7), GST-mCVII-Z (lane 8), GST-mCVII-Cr (lane 9) as well as (b) His-DHFR (lane 2), His-mCVII-1 (lane 3), His-mCVII-2 (lane 4), His-mCVII-3 (lane 5), His-mCVII-4 (lane 6), His-mCVII-5 (lane 7), His-mCVII-Z (lane 8) and His-mCVII-Cr (lane 9) were separated by 12% SDS-PAGE and stained with BioSafe Coomassie blue. The GST and His labelled proteins migrated according to their expected molecular weight. (c) The His-DHFR (lane 1) and the His tagged recombinant murine collagen VII fragments (lanes 2-8) were separated on a 12% polyacrylamide gel. Immunoblot analysis with a mouse IgG raised against RGS-His (Qiagen), in a first step, and with a HRP conjugated anti mouse antibody (BioRad) in a second step resulted in detection of all His labelled proteins. (TIF 13.2 kb)
Acknowledgments
The authors acknowledge support by grants from the Deutsche Forschungsgemeinschaft SI-1281/2-1, SI-1281/4-1 and BIOSS-B13 (CS), from the Medical Faculty of the University of Freiburg (C.S.) and by an ERASMUS stipend (V.F.). We thank Dr. Leena Bruckner-Tuderman, Freiburg, Germany, for critical reading of the manuscript and helpful advice.
References
- 1.Nishie W, Sawamura D, Goto M, Ito K, Shibaki A, McMillan J, Sakai K, Nakamura H, Olasz E, Yancey K, Akiyama M, Shimizu H. Humanization of autoantigen. Nat Med. 2007;13:378–383. doi: 10.1038/nm1496. [DOI] [PubMed] [Google Scholar]
- 2.Toyka KV, Brachman DB, Pestronk A, Kao I. Myasthenia gravis: passive transfer from man to mouse. Science. 1975;190:397–399. doi: 10.1126/science.1179220. [DOI] [PubMed] [Google Scholar]
- 3.Liu Z, Diaz LA, Troy JL, Taylor AF, Emery DJ, Fairley JA, Giudice GJ. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest. 1993;92:2480–2488. doi: 10.1172/JCI116856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazarova Z, Yee C, Darling T, Briggaman RA, Yancey KB. Passive transfer of anti-laminin 5 antibodies induces subepidermal blisters in neonatal mice. J Clin Invest. 1996;98:1509–1518. doi: 10.1172/JCI118942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sitaru C, Mihai S, Otto C, Chiriac MT, Hausser I, Dotterweich B, Saito H, Rose C, Ishiko A, Zillikens D. Induction of dermal–epidermal separation in mice by passive transfer of antibodies specific to type VII collagen. J Clin Invest. 2005;115:870–878. doi: 10.1172/JCI21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anhalt GJ, Labib RS, Voorhees JJ, Beals TF, Diaz LA. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N Engl J Med. 1982;306:1189–1196. doi: 10.1056/NEJM198205203062001. [DOI] [PubMed] [Google Scholar]
- 7.Sitaru C. Bullous pemphigoid: a prototypical antibody-mediated organ-specific autoimmune disease. J Invest Dermatol. 2009;129:822–824. doi: 10.1038/jid.2009.12. [DOI] [PubMed] [Google Scholar]
- 8.Mihai S, Sitaru C. Immunopathology and molecular diagnosis of autoimmune bullous diseases. J Cell Mol Med. 2007;11:462–481. doi: 10.1111/j.1582-4934.2007.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sitaru C, Zillikens D. Mechanisms of blister induction by autoantibodies. Exp Dermatol. 2005;14:861–875. doi: 10.1111/j.1600-0625.2005.00367.x. [DOI] [PubMed] [Google Scholar]
- 10.Lapiere JC, Woodley DT, Parente MG, Iwasaki T, Wynn KC, Christiano AM, Uitto J. Epitope mapping of type VII collagen. Identification of discrete peptide sequences recognized by sera from patients with acquired epidermolysis bullosa. J Clin Invest. 1993;92:1831–1839. doi: 10.1172/JCI116774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishii N, Yoshida M, Hisamatsu Y, Ishida-Yamamoto A, Nakane H, Iizuka H, Tanaka T, Hashimoto T. Epidermolysis bullosa acquisita sera react with distinct epitopes on the NC1 and NC2 domains of type VII collagen: study using immunoblotting of domain-specific recombinant proteins and postembedding immunoelectron microscopy. Br J Dermatol. 2004;150:843–851. doi: 10.1111/j.1365-2133.2004.05933.x. [DOI] [PubMed] [Google Scholar]
- 12.Sitaru C, Kromminga A, Hashimoto T, Bröcker EB, Zillikens D. Autoantibodies to type VII collagen mediate Fcgamma-dependent neutrophil activation and induce dermal–epidermal separation in cryosections of human skin. Am J Pathol. 2002;161:301–311. doi: 10.1016/s0002-9440(10)64182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodley DT, Ram R, Doostan A, Bandyopadhyay P, Huang Y, Remington J, Hou Y, Keene DR, Liu Z, Chen M. Induction of epidermolysis bullosa acquisita in mice by passive transfer of autoantibodies from patients. J Invest Dermatol. 2006;126:1323–1330. doi: 10.1038/sj.jid.5700254. [DOI] [PubMed] [Google Scholar]
- 14.Sitaru C, Chiriac MT, Mihai S, Büning J, Gebert A, Ishiko A, Zillikens D. Induction of complement-fixing autoantibodies against type VII collagen results in subepidermal blistering in mice. J Immunol. 2006;177:3461–3468. doi: 10.4049/jimmunol.177.5.3461. [DOI] [PubMed] [Google Scholar]
- 15.Chen M, Doostan A, Bandyopadhyay P, Remington J, Wang X, Hou Y, Liu Z, Woodley DT. The cartilage matrix protein subdomain of type VII collagen is pathogenic for epidermolysis bullosa acquisita. Am J Pathol. 2007;170:2009–2018. doi: 10.2353/ajpath.2007.061212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mihai S, Chiriac MT, Takahashi K, Thurman JM, Holers VM, Zillikens D, Botto M, Sitaru C. The alternative pathway of complement activation is critical for blister induction in experimental epidermolysis bullosa acquisita. J Immunol. 2007;178:6514–6521. doi: 10.4049/jimmunol.178.10.6514. [DOI] [PubMed] [Google Scholar]
- 17.Chiriac MT, Roesler J, Sindrilaru A, Scharffetter-Kochanek K, Zillikens D, Sitaru C. NADPH oxidase is required for neutrophil-dependent autoantibody-induced tissue damage. J Pathol. 2007;212:56–65. doi: 10.1002/path.2157. [DOI] [PubMed] [Google Scholar]
- 18.Sitaru C. Experimental models of epidermolysis bullosa acquisita. Exp Dermatol. 2007;16:520–531. doi: 10.1111/j.1600-0625.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- 19.Yuspa SH, Hawley-Nelson P, Koehler B, Stanley JR. A survey of transformation markers in differentiating epidermal cell lines in culture. Cancer Res. 1980;40:4694–4703. [PubMed] [Google Scholar]
- 20.Olaru F, Mihai S, Petrescu I, Zillikens D, Sitaru C. Generation and characterization of monoclonal antibodies against the intracellular domain of human type XVII collagen. Hybridoma. 2006;25:158–162. doi: 10.1089/hyb.2006.25.158. [DOI] [PubMed] [Google Scholar]
- 21.Li K, Tamai K, Tan EM, Uitto J. Cloning of type XVII collagen. Complementary and genomic DNA sequences of mouse 180-kilodalton bullous pemphigoid antigen (BPAG2) predict an interrupted collagenous domain, a transmembrane segment, and unusual features in the 5′-end of the gene and the 3′-untranslated region of the mRNA. J Biol Chem. 1993;268:8825–8834. [PubMed] [Google Scholar]
- 22.Sitaru C, Schmidt E, Petermann S, Munteanu LS, Bröcker E, Zillikens D. Autoantibodies to bullous pemphigoid antigen 180 induce dermal–epidermal separation in cryosections of human skin. J Invest Dermatol. 2002;118:664–671. doi: 10.1046/j.1523-1747.2002.01720.x. [DOI] [PubMed] [Google Scholar]
- 23.Sesarman A, Sitaru AG, Olaru F, Zillikens D, Sitaru C. Neonatal Fc receptor deficiency protects from tissue injury in experimental epidermolysis bullosa acquisita. J Mol Med. 2008;86:951–959. doi: 10.1007/s00109-008-0366-7. [DOI] [PubMed] [Google Scholar]
- 24.Sitaru C, Powell J, Messer G, Bröcker E, Wojnarowska F, Zillikens D. Immunoblotting and enzyme-linked immunosorbent assay for the diagnosis of pemphigoid gestationis. Obstet Gynecol. 2004;103:757–763. doi: 10.1097/01.AOG.0000115506.76104.ad. [DOI] [PubMed] [Google Scholar]
- 25.Sesarman A, Mihai S, Chiriac MT, Olaru F, Sitaru AG, Thurman JM, Zillikens D, Sitaru C. Binding of avian IgY to type VII collagen does not activate complement and leucocytes and fails to induce subepidermal blistering in mice. Br J Dermatol. 2008;158:463–471. doi: 10.1111/j.1365-2133.2007.08388.x. [DOI] [PubMed] [Google Scholar]
- 26.Mihai S, Chiriac MT, Herrero-González JE, Goodall M, Jefferis R, Savage COS, Zillikens D, Sitaru C. IgG4 autoantibodies induce dermal–epidermal separation. J Cell Mol Med. 2007;11:1117–1128. doi: 10.1111/j.1582-4934.2007.00081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith TF, Waterman MS. Identification of common molecular subsequences. J Mol Biol. 1981;147:195–197. doi: 10.1016/0022-2836(81)90087-5. [DOI] [PubMed] [Google Scholar]
- 28.Kolaskar A, Tongaonkar P. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990;276:172–174. doi: 10.1016/0014-5793(90)80535-Q. [DOI] [PubMed] [Google Scholar]
- 29.Shimanovich I, Mihai S, Oostingh GJ, Ilenchuk TT, Bröcker E, Opdenakker G, Zillikens D, Sitaru C. Granulocyte-derived elastase and gelatinase B are required for dermal–epidermal separation induced by autoantibodies from patients with epidermolysis bullosa acquisita and bullous pemphigoid. J Pathol. 2004;204:519–527. doi: 10.1002/path.1674. [DOI] [PubMed] [Google Scholar]
- 30.Woodley DT, Chang C, Saadat P, Ram R, Liu Z, Chen M. Evidence that anti-type VII collagen antibodies are pathogenic and responsible for the clinical, histological, and immunological features of epidermolysis bullosa acquisita. J Invest Dermatol. 2005;124:958–964. doi: 10.1111/j.0022-202X.2005.23702.x. [DOI] [PubMed] [Google Scholar]
- 31.Li K, Christiano AM, Copeland NG, Gilbert DJ, Chu ML, Jenkins NA, Uitto J. cDNA cloning and chromosomal mapping of the mouse type VII collagen gene (Col7a1): evidence for rapid evolutionary divergence of the gene. Genomics. 1993;16:733–739. doi: 10.1006/geno.1993.1255. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka T, Furukawa F, Imamura S. Epitope mapping for epidermolysis bullosa acquisita autoantibody by molecularly cloned cDNA for type VII collagen. J Invest Dermatol. 1994;102:706–709. doi: 10.1111/1523-1747.ep12374333. [DOI] [PubMed] [Google Scholar]
- 33.Gandhi K, Chen M, Aasi S, Lapiere JC, Woodley DT, Chan LS. Autoantibodies to type VII collagen have heterogeneous subclass and light chain compositions and their complement-activating capacities do not correlate with the inflammatory clinical phenotype. J Clin Immunol. 2000;20:416–423. doi: 10.1023/A:1026451530967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 Expression of the recombinant GST-fusion and His-tagged fragments of murine collagen VII. (a) Equimolar amounts of GST (lane 2), GST-mCVII-1 (lane 3), GST-mCVII-2 (lane 4), GST-mCVII-3 (lane 5), GST-mCVII-4 (lane 6), GST-mCVII-5 (lane 7), GST-mCVII-Z (lane 8), GST-mCVII-Cr (lane 9) as well as (b) His-DHFR (lane 2), His-mCVII-1 (lane 3), His-mCVII-2 (lane 4), His-mCVII-3 (lane 5), His-mCVII-4 (lane 6), His-mCVII-5 (lane 7), His-mCVII-Z (lane 8) and His-mCVII-Cr (lane 9) were separated by 12% SDS-PAGE and stained with BioSafe Coomassie blue. The GST and His labelled proteins migrated according to their expected molecular weight. (c) The His-DHFR (lane 1) and the His tagged recombinant murine collagen VII fragments (lanes 2-8) were separated on a 12% polyacrylamide gel. Immunoblot analysis with a mouse IgG raised against RGS-His (Qiagen), in a first step, and with a HRP conjugated anti mouse antibody (BioRad) in a second step resulted in detection of all His labelled proteins. (TIF 13.2 kb)