Abstract
Dengue virus (DENV 1-4) represents a major emerging arthropod-borne pathogen. All four DENV serotypes are prevalent in the (sub) tropical regions of the world and infect 50–100 million individuals annually. Whereas the majority of DENV infections proceed asymptomatically or result in self-limited dengue fever, an increasing number of patients present more severe manifestations, such as dengue hemorrhagic fever and dengue shock syndrome. In this review we will give an overview of the infectious life cycle of DENV and will discuss the viral and host factors that are important in controlling DENV infection.
Keywords: Dengue, Flavivirus, Life cycle, Immune response, Pathogenesis, Host factors, Virulence, Antibody-dependent enhancement
Introduction
Dengue is the most common arthropod-borne viral infection in the world [1, 2]. The disease is endemic in more than 100 countries throughout Africa, the Americas, the Eastern Mediterranean, South-East Asia, and the Western Pacific. There are four distinct serotypes of dengue virus (DENV) and each of these serotypes can cause disease symptoms ranging from self-limited febrile illness called dengue fever (DF) to dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) [2–4]. Infection with one serotype confers protective immunity against that serotype but not against other serotypes. In fact, several retrospective and prospective studies have revealed that secondary infection with a heterologous serotype is a risk factor for developing DHF/DSS [4–8]. Also, infants born to dengue-immune mothers are at risk to develop more severe dengue during a primary infection [9, 10]. This suggests that antibodies play an important role in controlling the outcome of an infection. It is believed that antibodies specifically direct the virus particles to cells carrying Fc-receptors (FcR), such as monocytes, macrophages, and dendritic cells, which—as the natural targets for the virus—are permissive for DENV infection. This leads to enhanced infection of these cells and thus, to high viral loads, resulting in extensive T cell activation early in the infection process. As a consequence, high amounts of cytokines and chemical mediators are released, which may lead to endothelial cell damage and subsequent plasma leakage. Other factors that are implicated in disease pathogenesis include viral virulence, the ethnic background and age of the individual, and specific epidemiological conditions [11–15]. In this review we will give a general overview of the infectious life cycle of DENV, and describe the viral and host factors that may influence disease outcome.
Dengue virus life cycle
Virion structure
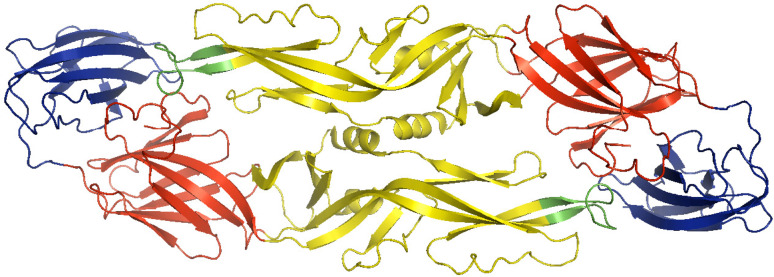
DENV is an enveloped positive-strand RNA virus belonging to the Flaviviridae family [16, 17]. Mature virions contain three structural proteins, the capsid protein C, membrane protein M, and the envelope protein E. Multiple copies of the C protein (11 kDa) encapsulate the RNA genome to form the viral nucleocapsid [18–20]. The nucleocapsid is surrounded by a host-cell-derived lipid bilayer, in which 180 copies of M and E are anchored. The M protein is a small (approx. 8 kDa) proteolytic fragment of its precursor form prM (approx. 21 kDa). The E protein is 53 kDa and has three distinct structural domains (Fig. 1) [21–24]. Domain I is structurally positioned between domain II, the homodimerization domain, and the immunoglobulin-like domain III.
Fig. 1.
Dengue E protein dimer with three defined domains within each monomer: domain I in red, domain II in yellow with fusion loop in green, and domain III in blue (23). The image was prepared using the program PyMol Molecular Graphics System
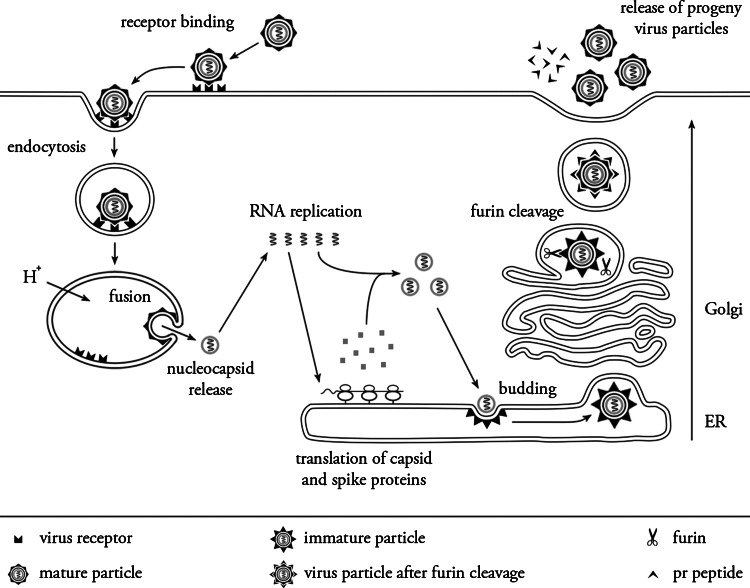
Structural analysis of mature DENV virions revealed that the virus possesses an icosahedral envelope organization and a spherical nucleocapsid core [25]. In mature virions, E is organized as 90 head-to-tail orientated homodimers, which lie in sets of three nearly parallel to each other and to the viral surface, forming a smooth “herringbone” configuration. As a result, DENV virions lack a true T = 3 symmetry, which means that the three E monomers present in each icosahedral asymmetric unit exist in three chemically distinct environments and may therefore play a distinct role in different stages of the infection [25–27]. The infectious life cycle is depicted in Fig. 2 and will be discussed in detail below.
Fig. 2.
Life cycle of Dengue virus. See text for details. Adapted from H. M. van der Schaar
Replication and assembly of dengue virus particles
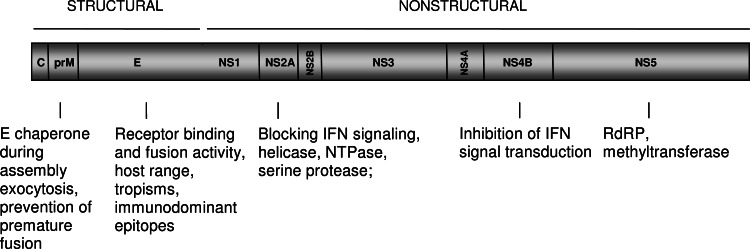
A schematic representation of the DENV genomic RNA and the translation of the viral proteins are depicted in Fig. 3. After virus cell entry (see below) and uncoating of the nucleocapsid, the RNA molecule is translated as a single polyprotein (see [28], for a great review on this topic). During this process, the signal- and stop-transfer sequences of the polyprotein direct its back-and-forth translocation across the endoplasmic reticulum (ER) membrane. The polyprotein is processed co- and post-translationally by cellular and virus-derived proteases into three structural proteins (C, prM, and E) and seven nonstructural (NS) proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). The E protein is glycosylated at amino acid residue Asn67 and Asn153 to assure proper folding of the protein [23, 29]. Other potential N-linked glycosylation sites are located in prM at position 7, 31, and 52 and within NS1 at position 130 and 207 [30, 31]. Upon protein translation and folding of the individual proteins, the NS proteins initiate replication of the viral genome [28]. The newly synthesized RNA is subsequently packaged by the C protein to form a nucleocapsid. The prM and E proteins form heterodimers that are oriented into the lumen of the ER. Then, the prM/E heterodimers associate into trimers and these oligomeric interactions are believed to induce a curved surface lattice, which guides virion budding [25, 26]. It is unclear how this is synchronized with the engulfment of the nucleocapsid since no specific interactions between C and prM/E proteins have been identified yet [32, 33]. Interestingly, encapsulation of the nucleocapsid during virus assembly is not crucial as the formation and release of capsidless subviral particles has been often documented [34–37].
Fig. 3.
Schematic representation of Dengue virus genome. See text for details
Structural analysis of newly assembled immature virions revealed that a single virion contains 180 prM/E heterodimers that project vertically outward from the viral surface as 60 trimeric spikes [32, 33, 38]. The immature particles formed in the ER mature as they travel through the secretory pathway. The slightly acidic pH (~5.8–6.0) of the trans-Golgi network (TGN) triggers dissociation of the prM/E heterodimers, which leads to the formation of 90 dimers that lie flat on the surface of the particle, with prM capping the fusion peptide of the E protein. This global structural reorganization of the glycoproteins enables the cellular endoprotease furin to cleave prM [39–41]. Furin cleavage occurs at a Arg-X-(Lys/Arg)-Arg (where X is any amino acid) recognition sequence and leads to the generation of membrane-associated M and a “pr” peptide. A recent study has shown that the pr peptide remains associated with the virion until the virus is secreted to the extracellular milieu [40]. Both the prM protein as well as the pr peptide are believed to act as chaperones stabilizing the E protein during transit through the secretory pathway, thereby preventing premature conformational changes of the E protein that would lead to membrane fusion. Upon dissociation of the pr peptide, mature virions are formed that are able to infect new cells.
Receptor interaction and viral entry
During natural infection, cells of the mononuclear phagocyte lineage [monocytes (MO), macrophages (MØ), and dendritic cells (DCs)], including the skin-resident Langerhans cells, are primary targets for DENV infection [42, 43]. In insects, DENV was found to initially infect the midgut from where it spreads and replicates in many body compartments and organs [44–47]. Also, DENV has been shown to infect numerous cell lines, including human (K562, U937, THP-1, HepG2, HUVEC, ECV304, Raji, HSB-2, Jurkat, LoVo, KU812), mosquito (C6/36), monkey (Vero, BS-C-1, CV-1, LLC-MK2), hamster (BHK), as well as murine MØ (Raw, P388D1, J774) cell lineages [39, 48–61]. The wide range of DENV-permissive cells indicates that the virus must bind to an ubiquitous cell-surface molecule, or exploit multiple receptors to mediate infection. Over the last decade, several candidate receptors and/or attachment factors have been identified, which suggests that DENV is capable of utilizing multiple molecules to enter the cell. In mosquito cells, DENV has been shown to interact with heat-shock protein 70 (Hsp70) [62], R80, R67 [63], and a 45-kDa protein [64]. Heparan sulfate [65–67], Hsp90 [62], CD14 [68], GRP78/BiP [69], and a 37/67-kDa high-affinity laminin receptor [70] have been identified as receptors on mammalian cells. C-type lectin receptors (CLR) are involved in the interaction of DENV particles with human myeloid cells [71]. These include DC-specific intracellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN, CD209) [72–74], mannose receptor (MR) [75] and C-type lectin domain family 5, member A (CLEC5, MDL-1) [52].
DENV [76–78] as well as other flaviviruses [79, 80] use clathrin-mediated endocytosis for cell entry. Using a single-particle tracking approach, we have revealed that DENV-2 strain S1 particles land on the cell surface and migrate in a diffusive manner toward a pre-existing clathrin-coated pit [77]. This suggests that DENV particles move along the cell surface by rolling over different receptors, or migrate as virus–receptor complexes. Upon internalization, the particles are delivered to Rab5-positive early endosomes, which mature into Rab7-positive late endosomes, where membrane fusion primarily occurs [77]. A recent report also demonstrated that DENV, depending on the serotype and/or the target cell type used, is able to utilize an alternative internalization pathway, independent of clathrin, caveolae, and lipid rafts [81]. The subcellular organelle from which membrane fusion occurs is most likely dependent on the pH-dependent membrane fusion properties of the virus and may therefore vary between individual DENV strains [77, 78].
Numerous functional and structural studies have been undertaken to unravel the molecular mechanisms involved in the membrane fusion process of the virus [27, 82–85]. It is postulated that the acidic pH in endosomes triggers dissociation of E homodimers, which then leads to the outward projection of domain II and exposure of the hydrophobic fusion peptide to the target membrane [86]. Subsequently, the hydrophobic residues in the fusion loop would insert into the outer leaflet of the target membrane, triggering the assembly of E trimers. Next, domain III is assumed to shift and fold back toward the fusion peptide into a hairpin-like conformation. This folding-back mechanism would force the target membrane and the viral membrane to bend towards each other and eventually to fuse, releasing the nucleocapsid into the cell cytosol.
Immune response to DENV: a crucial determinant in the outcome of infection
Innate immunity
The first line of defense against invading DENV is the production of interferons (IFNs). Upon a mosquito bite, DENV first infects interstitial DCs which, within hours of infection, initiate production of type-I IFNs. Not only DCs but the majority of DENV-infected cells produce IFNs. Both type I (α, β) and type II (γ) IFNs have been found to be crucial for protection against DENV infection in vivo and in vitro [52, 87–90]. Furthermore, early activation of natural killer (NK) cells, the main producers of IFNγ, appears to be important in clearing DENV infection [91, 92].
The production of IFN is initiated upon virus interaction with pathogen-recognition receptors (PRRs), e.g., C-type lectins [71] and toll-like receptors (TLRs) that are expressed on the myeloid cells [93, 94]. C-type lectins such as DC-SIGN, MR, and CLEC5, but also TLR3 and TLR 7, have been reported to participate in the induction of an innate response upon DENV infection [52, 95, 96]. Activated PRRs convey their signal through several transcription factors, which ultimately induce the expression of IFN. Secreted IFN binds to IFN receptors present on the same cells as well as on neighboring cells. This activates the JAK/STAT pathway leading to the expression of more than 100 effector proteins [97–100]. Both STAT-1-dependent and STAT-1-independent pathways have been implicated in the IFN-mediated response to DENV infection [51, 52, 101, 102]. IFN-mediated responses induce an antiviral state and initiate a variety of processes including metabolic control to limit virus infection. Moreover, these responses promote the adaptive immune response through stimulation of DC maturation and by direct activation of B and T cells [103].
While the importance of the innate immune response in controlling DENV infections is clear, several studies have demonstrated that DENV is able to inhibit the IFNα-mediated innate antiviral response [88, 104–106]. In particular, viral NS2A, NS4A, NS4B, and NS5 are thought to block IFN signaling by reducing STAT activation [88, 104, 106]. Interestingly, the ability of DENV to suppress type I IFN response has been shown to be strain-dependent, as within each serotype, both non-suppressive and suppressive strains that block STAT1/STAT2 pathways could be found [107]. Reduction of the IFNα-mediated response does not however correlate with disease progression to DHF, as no significant differences were found in IFNα levels between DENV isolates associated with DF and those associated with DHF [108].
Antibody response
Human humoral immunity develops approximately 6 days after a bite from a DENV-infected mosquito. The antibody response is mainly directed against the E and prM glycoproteins present on the surface of the virus [54, 109, 110]. NS1 antibodies are also generated, as this protein is expressed on the surface of infected cells and is secreted from these cells as a soluble factor [31, 110–112]. Antibodies against NS1 have been shown to activate complement-mediated lysis of DENV-infected cells and protect mice from DENV challenge [113–116]. Antibodies directed against E and prM antibodies were observed to directly influence the infectious properties of DENV particles. Antibodies can both neutralize and enhance DENV infectivity in vivo and in vitro and thus appear to play a dual role in controlling DENV virus infection [55, 56, 117–119].
Antibody-mediated neutralization of infection
Neutralization of infection occurs via a multiple ‘hit’ phenomenon. This means that virus inactivation occurs only when the number of antibodies docked on the virion exceeds a certain threshold [54, 120]. In case of strongly neutralizing antibodies, binding of only a small fraction of the accessible epitopes is required for neutralization. In contrast, weakly neutralizing antibodies that bind to poorly accessible sites on the virion may require full occupation to achieve neutralization. For DENV as well as other flaviviruses, the most potent neutralizing antibodies are strain-specific and directed against domain III of the E protein [121–123]. Cross-reactive and weaker neutralizing antibodies were observed to predominantly localize to domain II near or within the fusion peptide [110, 124]. Notably, the human antibody response is dominated by antibodies directed against domains I and II.
In vitro studies have shown that neutralizing antibodies block attachment of the virus to its natural receptor on the cell surface and/or inhibit subsequent steps in the entry process of DENV [125–127]. However, inhibition of a virus binding to a cellular receptor will not result in neutralization of infection per se as virus-immune complexes can be internalized by cells carrying Fc receptors (FcR), including dendritic cells and macrophages. This process of FcR-mediated uptake may thus result in delivery of the virus into acidic compartments of the endosomal/phagosomal pathway, in a manner very similar to uptake of DENV after interaction with its natural receptor. Therefore, we believe that potent neutralizing antibodies should act downstream of virus–receptor interaction at the cell surface. A detailed structural and functional analysis of the potent neutralizing WNV antibody E16 has indeed shown that infection is blocked at the stage of membrane fusion presumably by inhibiting the conformational changes of the E protein required for membrane fusion [126].
Antibody-mediated enhancement of infection
Epidemiological studies have demonstrated that secondary infection with a heterologous serotype or primary infection of infants born to dengue immune mothers significantly increases the risk to develop severe disease. These clinical observations have led to the widely accepted hypothesis of antibody-dependent enhancement (ADE) of disease [4, 128, 129]. As indicated above, it is believed that antibodies specifically direct the virus particles to cells carrying FcR, such as monocytes, macrophages and dendritic cells, which—as the natural targets for the virus—are permissive for DENV infection, resulting in a higher virus burden and eventually enhancement of disease.
Studies with E-specific antibodies suggest that when virion opsonization occurs at the occupancy that does not exceed the threshold required for virus neutralization, these antibodies may enhance the efficiency of virus attachment to the cell surface and facilitate entry of virions via FcR-mediated endocytosis [120, 129]. The molecular mechanism underlying the ADE phenomenon remains to be identified [127]. Uptake of DENV-immune complexes via FcR-mediated entry may not only lead to a higher number of infected cells, it can also influence the number of virus particles produced per infected cell. A recent study showed that infection of THP-1 cells with DENV-immune complexes resulted in downregulation of production of IL-12, IFN-γ, TNF-α, and NO, and enhanced expression of IL-6 and IL-10, indicating that FcR-mediated entry suppresses the antiviral immune response, thereby promoting virus particle production [51].
Not only E antibodies but also prM antibodies have been implicated in the phenomenon of ADE [56, 119, 130, 131]. Remarkably, we recently observed that prM antibodies have the capacity to render essentially non-infectious fully immature particles nearly as infectious as wild-type virus [119]. We found that prM antibodies enhance the infectivity of immature virions but have no promoting effect on the number of progeny virions produced per infected cell. The potential role of immature DENV particles in disease pathogenesis is further discussed in the section about virus virulence.
T cell response
Little is known about the role of CD8+ T cells in protection or enhancement of disease. Both serotype-specific and cross-reactive CD8+ T cells that are cytolytic and produce IFN-γ and TNF-α have been detected in DENV-immune individuals [132–136]. Studies on the role of CD8+ T cells in protection against disease have been hampered by the lack of good animal models. However, a recent study revealed that CD8+ T cells are important in the host defense against DENV [137]. On the other hand, activation of memory CD8+ T cells during heterologous secondary DENV infection is believed to result in a massive production of cytokines and immune modulators characteristic of DHF/DSS [138].
Cytokine storm
The hallmark of the pathogenesis of DHF/DSS is the loss of endothelial integrity, which is assumed to be the result of an abnormal immune response against the virus. Clinical studies have shown that the levels of cytokines and immune mediators such as TNF- α, IL-1β, IL-2, IL-4, IL-6, IL-7, IL-8, IL-10, IL-13, IL-18, MCP-1, and IFN-γ and IFN-α are significantly increased in patients suffering from DHF/DSS [139–148]. The role of cytokines in increased vascular permeability has been substantiated in murine model systems [147, 149].
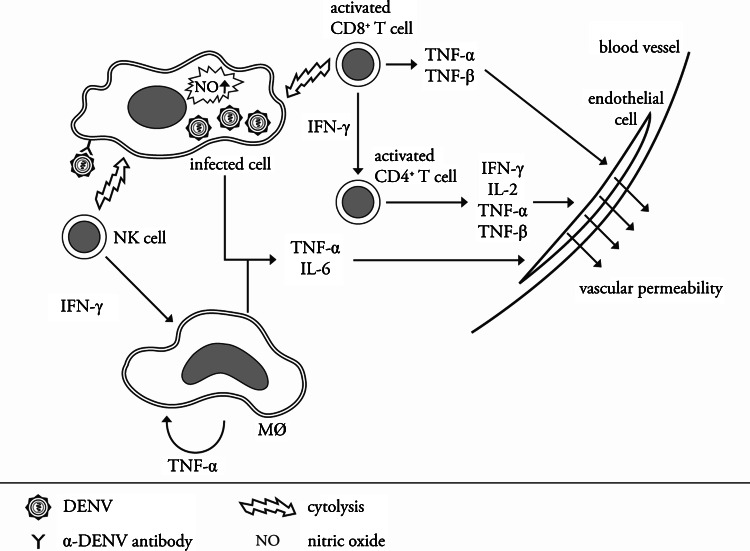
Many scientists believe that the cytokine storm is induced by the activation of a high number of cross-reactive low-avidity T cells through a process referred to as ‘original antigenic sin’. These low-avidity T cells have been shown to exhibit suboptimal degranulation, altered cytokine production and cytolytic activity, through which not only are they unable to efficiently clear the infection but they cause a massive immune activation [132, 134, 150, 151]. However, aberrant T-cell responses during secondary infections cannot explain the observations of DHF/DSS in infants born to dengue-immune mothers [9, 10, 152, 153]. Interestingly, a recent study showed that antibody-mediated DENV infection of mature DCs also leads to increased levels of TNF-α and IL-6, indicating that ADE of infection may alter cytokine responses [154, 155]. The hypotheses are not mutually exclusive, and most probably both antibodies and T-cells play an important role in the progression of dengue disease to DHF during secondary infection. An integrated view of the immunological processes that contribute to DHF is shown in Fig. 4.
Fig. 4.
Immunopathogenesis of severe dengue—an integrated model
Other viral and host factors controlling DENV infection
Virus virulence
Genotype differences
Low-fidelity replication together with natural-selection processes have led to the development of multiple genotypes within each DENV serotype (see [156] for a great review on this topic). Interestingly, several studies have indicated that certain DENV-2 and DENV-3 genotypes are more often associated with DHF [157–165]. For example, the first outbreak of DHF in the Americas coincided with the introduction of the DENV-2 Southeast Asia genotype. This suggested that the Southeast Asian genotype is more virulent than the co-circulating American DENV-2 genotype in that region, which almost exclusively caused DF [164]. Detailed genomic sequence analysis of both genotypes have revealed nucleotide differences in prM, E, NS4B, and NS5 genes as well as in the 5′ and 3′ UTRs [166]. Nucleotide variation at amino acid position 390 of the E protein may be of major significance, as this region has been linked to host-range specificity and virulence [166]. Furthermore, it was shown that the Asian DENV-2 genotype replicates to higher titers in human monocyte-derived macrophages and DCs compared to the American genotype [167, 168]. In addition, analysis of the American and Asian lineages for their ability to infect several populations of A. aegypti demonstrated that the overall infection rates were higher for the Southeast Asian genotypes.
Glycosylation of E and NS1 proteins
The addition of carbohydrates to viral proteins is important for viral virulence [29, 169–171]. For example, glycosylation at position E Asn153 has been suggested to play a role in stabilizing dimer interactions between E monomers by partially occluding the fusion peptide [21]. Furthermore, the addition of carbohydrates to E Asn67 have been observed to be critical for virus particle production in mammalian and mosquito cell lines [29]. Also, this carbohydrate group has been implicated to mediate binding of the virus particle to DC-SIGN on DCs [172].
In addition to the E protein, several studies have suggested that glycosylation of the NS1 protein is important for viral virulence [30, 170, 173]. While complete ablation of DENV-2 16681 NS1 glycosylation results in a genetically unstable virus, removal of only one glycosylation site allows replication of the mutated virus albeit with a less virulent phenotype [30]. Furthermore, it was shown that NS1 protein glycosylation is required for efficient secretion of the protein from infected cells [31, 169]. This, combined with the observation that patients experiencing DHF have high levels of NS1 protein in the blood, suggests that NS1 protein glycosylation is important in disease pathogenesis [112, 174].
Maturation state of the virus
Dengue virus maturation appears to be inefficient as dengue-infected mosquito and mammalian cells have been shown to secrete large numbers (up to 30%) of prM-containing particles [39, 48, 130, 175–184]. Moreover, the presence of prM-specific antibodies in sera of DENV-positive patients suggests that immature particles are also formed during a natural infection [109, 110, 185–187]. Incomplete cleavage of DENV has been linked to the existence of an acidic residue at position P3 within the 13-amino-acid sequence proximal to the prM cleavage site [188, 189].
Numerous studies have demonstrated that fully immature particles lack the ability to infect cells and therefore these particles are generally believed to be of minor importance in DENV pathogenesis [39–41, 190]. Remarkably, however, we recently showed that prM antibodies render essentially non-infectious fully immature particles nearly as infectious as wild-type virus [119]. We observed that prM antibodies facilitate efficient binding and internalization of virus-immune complexes in cells after which furin within the target cell cleaves prM to M thereby activating the membrane fusion potential of the E protein. Enhancement of infection was also observed using DENV-immune sera, which indicates that prM-containing particles may be important in disease pathogenesis. This notion is supported by a recent report which showed that the rates of prM antibody responses are higher in patients experiencing a secondary infection [110].
Antibodies may react differently to immature, partially mature and fully mature particles given the large structural differences between these particles [124, 191]. Antibodies specifically recognizing (partially) immature virions may be detrimental for disease protection. For example, it is tempting to speculate that prM antibodies may not be able to neutralize viral infection of partially immature particles as the mature aspect of the virion would be able to mediate infection. Indeed, Nelson and colleagues showed that neutralization of WNV infection by E antibodies is significantly influenced by the maturation state of the virus. Increased virus maturation resulted in the reduction of neutralizing potency of many E antibodies that bind to epitopes which are predicted to be poorly accessible in mature virions [124]. Taken together, the maturation status of the virus particle may be important in determining the ability of an antibody to neutralize or enhance viral infection.
Host genetic factors
Differences in disease symptoms are seen at the individual level but also within certain human populations. For example, no apparent DHF/DSS was documented in a Haitian population while there was hyperendemic transmission of several DENV serotypes. This study, together with the observation that black people less frequently develop severe dengue than whites has led to the notion that gene polymorphisms and gene mutations may contribute to variable susceptibility among humans [192–194]. A number of studies have identified candidate genes variants that may predispose or protect an individual to develop DHF/DSS. These include specific human leukocyte antigens (HLAs) alleles and non-HLA gene polymorphisms. Several human HLA class I alleles (A*01, A*0207, A*24, B*07, B*46, B*51) and class II alleles (DQ*1, DR*1, DR*4) were found to be associated with severe disease susceptibility whereas individuals expressing HLA-B*13, HLA-B*14, and HLA-*29 were found to be protected [195–200]. Out of the non-HLA polymorphic alleles, the FcRII, vitamin D receptor [201], tumor necrosis factor alpha (TNF-α) [202], CTLA-4 [203], and transforming growth factor β (TGF-β) [203] have been linked to the development of more severe dengue. Moreover, analysis of three independent cohorts from Thai hospitals by the group of Sakuntabhai revealed that a promoter variant in the DC-SIGN1-336 gene is involved in disease progression to DHF [204]. Other host factors like glucose 6-phosphate dehydrogenase (G6PD) deficiency may also predispose individuals to develop DHF, as DENV has been shown to replicate to high titers in monocytes derived from these individuals [205]. Furthermore, individuals with chronic diseases such as diabetes mellitus (DM) are more susceptible to develop severe dengue [206–209]. It has been speculated that increased production of cytokines in type 2 DM patients might predispose them to vascular leakage, a hallmark of DHF.
Concluding remarks and future directions
Significant progress has been made in recent years with regard to our understanding of the structure of DENV particles, life cycle, and disease pathogenesis. However, despite the discovery of many host and viral factors that predispose or protect to severe disease, the complex nature of their mutual interactions during natural infection as well as the lack of a suitable animal model makes it difficult to fully explain the pathogenesis of DHF/DSS. The low percentage of DHF/DSS cases during secondary DENV infections suggests that host factors are critical determinants of disease progression. Therefore, more research is required to probe the role of host gene polymorphism (for example immunological pathways) in predisposition to DHF. Furthermore, it will be important to further investigate the role of genetic variations between circulating DENV strains in predisposition to DHF. It will be of interest to determine whether virus virulence is correlated with ADE of infection and aberrant T-cell responses. The observation that infants born to dengue-immune mothers develop DHF during primary infections demonstrates that antibodies play an important role in disease pathogenesis. Future research should investigate by which pathway DENV-immune complexes are internalized in cells, and whether this entry mechanism activates signaling pathways that may prime aberrant immune responses. Given our recent results on the infectious properties of immature DENV-immune complexes, it will be of particular interest to determine if antibody-dependent enhancement of infection by immature DENV particles correlates with disease presentation.
Acknowledgments
We thank R. van Tongeren and B. Moesker for help with the graphics. This work was supported by the Pediatric Dengue Vaccine Initiative.
References
- 1.Gubler DJ. Dengue/dengue haemorrhagic fever: history and current status. Novartis Found Symp. 2006;277:3–16. doi: 10.1002/0470058005.ch2. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO report on global surveillance of epidemic-prone infectious diseases—dengue and dengue haemorrhagic fever. World Health Organization. http://www.who.int/csr/resources/publications/dengue/CSR_ISR_2000_1/en/. Accessed 9 March 2010
- 3.Burke DS, Monath TP. Flaviviruses. In: Knipe DM, Howley PM, editors. Fields virology. Philadelphia: Lippincott Williams and Wilkins; 2001. pp. 1043–1125. [Google Scholar]
- 4.Halstead SB. Observations related to pathogenesis of dengue hemorrhagic fever. VI. Hypotheses and discussion. Yale J Biol Med. 1970;42:350–362. [PMC free article] [PubMed] [Google Scholar]
- 5.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 6.Guzman MG, Kouri G, Valdes L, Bravo J, Vazquez S, Halstead SB. Enhanced severity of secondary dengue-2 infections: death rates in 1981 and 1997 Cuban outbreaks. Rev Panam Salud Publica. 2002;11:223–227. doi: 10.1590/S1020-49892002000400003. [DOI] [PubMed] [Google Scholar]
- 7.Recker M, Blyuss KB, Simmons CP, Hien TT, Wills B, Farrar J, Gupta S. Immunological serotype interactions and their effect on the epidemiological pattern of dengue. Proc Biol Sci. 2009;276:2541–2548. doi: 10.1098/rspb.2009.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thein S, Aung MM, Shwe TN, Aye M, Zaw A, Aye K, Aye KM, Aaskov J. Risk factors in dengue shock syndrome. Am J Trop Med Hyg. 1997;56:566–572. doi: 10.4269/ajtmh.1997.56.566. [DOI] [PubMed] [Google Scholar]
- 9.Halstead SB, Lan NT, Myint TT, Shwe TN, Nisalak A, Kalyanarooj S, Nimmannitya S, Soegijanto S, Vaughn DW, Endy TP. Dengue hemorrhagic fever in infants: research opportunities ignored. Emerg Infect Dis. 2002;8:1474–1479. doi: 10.3201/eid0812.020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988;38:411–419. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- 11.Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10:100–103. doi: 10.1016/S0966-842X(01)02288-0. [DOI] [PubMed] [Google Scholar]
- 12.Guzman MG, Kouri G, Bravo J, Valdes L, Vazquez S, Halstead SB. Effect of age on outcome of secondary dengue 2 infections. Int J Infect Dis. 2002;6:118–124. doi: 10.1016/S1201-9712(02)90072-X. [DOI] [PubMed] [Google Scholar]
- 13.Sierra B, Alegre R, Perez AB, Garcia G, Sturn-Ramirez K, Obasanjo O, Aguirre E, Alvarez M, Rodriguez-Roche R, Valdes L, Kanki P, Guzman MG. HLA-A, -B, -C, and -DRB1 allele frequencies in Cuban individuals with antecedents of dengue 2 disease: advantages of the Cuban population for HLA studies of dengue virus infection. Hum Immunol. 2007;68:531–540. doi: 10.1016/j.humimm.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Thisyakorn U, Nimmannitya S. Nutritional status of children with dengue hemorrhagic fever. Clin Infect Dis. 1993;16:295–297. doi: 10.1093/clind/16.2.295. [DOI] [PubMed] [Google Scholar]
- 15.Thomas SJ, Strickman D, Vaughn DW. Dengue epidemiology: virus epidemiology, ecology, and emergence. Adv Virus Res. 2003;61:235–289. doi: 10.1016/S0065-3527(03)61006-7. [DOI] [PubMed] [Google Scholar]
- 16.Harris E, Holden KL, Edgil D, Polacek C, Clyde K. Molecular biology of flaviviruses. Novartis Found Symp. 2006;277:23–39. doi: 10.1002/0470058005.ch3. [DOI] [PubMed] [Google Scholar]
- 17.Lindenbach BD, Rice CM. Molecular biology of flaviviruses. Adv Virus Res. 2003;59:23–61. doi: 10.1016/S0065-3527(03)59002-9. [DOI] [PubMed] [Google Scholar]
- 18.Ma L, Jones CT, Groesch TD, Kuhn RJ, Post CB. Solution structure of dengue virus capsid protein reveals another fold. Proc Natl Acad Sci USA. 2004;101:3414–3419. doi: 10.1073/pnas.0305892101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones CT, Ma L, Burgner JW, Groesch TD, Post CB, Kuhn RJ. Flavivirus capsid is a dimeric alpha-helical protein. J Virol. 2003;77:7143–7149. doi: 10.1128/JVI.77.12.7143-7149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang CJ, Luh HW, Wang SH, Lin HJ, Lee SC, Hu ST. The heterogeneous nuclear ribonucleoprotein K (hnRNP K) interacts with dengue virus core protein. DNA Cell Biol. 2001;20:569–577. doi: 10.1089/104454901317094981. [DOI] [PubMed] [Google Scholar]
- 21.Rey FA, Heinz FX, Mandl C, Kunz C, Harrison SC. The envelope glycoprotein from tick-borne encephalitis virus at 2 Å resolution. Nature. 1995;375:291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- 22.Nybakken GE, Nelson CA, Chen BR, Diamond MS, Fremont DH. Crystal structure of the West Nile virus envelope glycoprotein. J Virol. 2006;80:11467–11474. doi: 10.1128/JVI.01125-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Modis Y, Ogata S, Clements D, Harrison SC. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc Natl Acad Sci USA. 2003;100:6986–6991. doi: 10.1073/pnas.0832193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modis Y, Ogata S, Clements D, Harrison SC. Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. J Virol. 2005;79:1223–1231. doi: 10.1128/JVI.79.2.1223-1231.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, Jones CT, Mukhopadhyay S, Chipman PR, Strauss EG, Baker TS, Strauss JH. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell. 2002;108:717–725. doi: 10.1016/S0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Zhang W, Ogata S, Clements D, Strauss JH, Baker TS, Kuhn RJ, Rossmann MG. Conformational changes of the flavivirus E glycoprotein. Structure. 2004;12:1607–1618. doi: 10.1016/j.str.2004.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukhopadhyay S, Kuhn RJ, Rossmann MG. A structural perspective of the flavivirus life cycle. Nat Rev Microbiol. 2005;3:13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- 28.Clyde K, Kyle JL, Harris E. Recent advances in deciphering viral and host determinants of dengue virus replication and pathogenesis. J Virol. 2006;80:11418–11431. doi: 10.1128/JVI.01257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bryant JE, Calvert AE, Mesesan K, Crabtree MB, Volpe KE, Silengo S, Kinney RM, Huang CY, Miller BR, Roehrig JT. Glycosylation of the dengue 2 virus E protein at N67 is critical for virus growth in vitro but not for growth in intrathoracically inoculated Aedes aegypti mosquitoes. Virology. 2007;366:415–423. doi: 10.1016/j.virol.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Crabtree MB, Kinney RM, Miller BR. Deglycosylation of the NS1 protein of dengue 2 virus, strain 16681: construction and characterization of mutant viruses. Arch Virol. 2005;150:771–786. doi: 10.1007/s00705-004-0430-8. [DOI] [PubMed] [Google Scholar]
- 31.Flamand M, Megret F, Mathieu M, Lepault J, Rey FA, Deubel V. Dengue virus type 1 nonstructural glycoprotein NS1 is secreted from mammalian cells as a soluble hexamer in a glycosylation-dependent fashion. J Virol. 1999;73:6104–6110. doi: 10.1128/jvi.73.7.6104-6110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Corver J, Chipman PR, Zhang W, Pletnev SV, Sedlak D, Baker TS, Strauss JH, Kuhn RJ, Rossmann MG. Structures of immature flavivirus particles. EMBO J. 2003;22:2604–2613. doi: 10.1093/emboj/cdg270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang W, Chipman PR, Corver J, Johnson PR, Zhang Y, Mukhopadhyay S, Baker TS, Strauss JH, Rossmann MG, Kuhn RJ. Visualization of membrane protein domains by cryo-electron microscopy of dengue virus. Nat Struct Biol. 2003;10:907–912. doi: 10.1038/nsb990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allison SL, Stadler K, Mandl CW, Kunz C, Heinz FX. Synthesis and secretion of recombinant tick-borne encephalitis virus protein E in soluble and particulate form. J Virol. 1995;69:5816–5820. doi: 10.1128/jvi.69.9.5816-5820.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fonseca BA, Pincus S, Shope RE, Paoletti E, Mason PW. Recombinant vaccinia viruses co-expressing dengue-1 glycoproteins prM and E induce neutralizing antibodies in mice. Vaccine. 1994;12:279–285. doi: 10.1016/0264-410X(94)90206-2. [DOI] [PubMed] [Google Scholar]
- 36.Hunt AR, Cropp CB, Chang GJ. A recombinant particulate antigen of Japanese encephalitis virus produced in stably-transformed cells is an effective noninfectious antigen and subunit immunogen. J Virol Methods. 2001;97:133–149. doi: 10.1016/S0166-0934(01)00346-9. [DOI] [PubMed] [Google Scholar]
- 37.Konishi E, Fujii A. Dengue type 2 virus subviral extracellular particles produced by a stably transfected mammalian cell line and their evaluation for a subunit vaccine. Vaccine. 2002;20:1058–1067. doi: 10.1016/S0264-410X(01)00446-7. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Kaufmann B, Chipman PR, Kuhn RJ, Rossmann MG. Structure of immature West Nile virus. J Virol. 2007;81:6141–6145. doi: 10.1128/JVI.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zybert IA, dE M, Wilschut J, Smit JM. Functional importance of dengue virus maturation: infectious properties of immature virions. J Gen Virol. 2008;89:3047–3051. doi: 10.1099/vir.0.2008/002535-0. [DOI] [PubMed] [Google Scholar]
- 40.Yu IM, Zhang W, Holdaway HA, Li L, Kostyuchenko VA, Chipman PR, Kuhn RJ, Rossmann MG, Chen J. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science. 2008;319:1834–1837. doi: 10.1126/science.1153264. [DOI] [PubMed] [Google Scholar]
- 41.Stadler K, Allison SL, Schalich J, Heinz FX. Proteolytic activation of tick-borne encephalitis virus by furin. J Virol. 1997;71:8475–8481. doi: 10.1128/jvi.71.11.8475-8481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jessie K, Fong MY, Devi S, Lam SK, Wong KT. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J Infect Dis. 2004;189:1411–1418. doi: 10.1086/383043. [DOI] [PubMed] [Google Scholar]
- 43.Wu SJ, Grouard-Vogel G, Sun W, Mascola JR, Brachtel E, Putvatana R, Louder MK, Filgueira L, Marovich MA, Wong HK, Blauvelt A, Murphy GS, Robb ML, Innes BL, Birx DL, Hayes CG, Frankel SS. Human skin Langerhans cells are targets of dengue virus infection. Nat Med. 2000;6:816–820. doi: 10.1038/77553. [DOI] [PubMed] [Google Scholar]
- 44.Mercado-Curiel RF, Black WC, Munoz ML. A dengue receptor as possible genetic marker of vector competence in Aedes aegypti . BMC Microbiol. 2008;8:118. doi: 10.1186/1471-2180-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molina-Cruz A, Gupta L, Richardson J, Bennett K, Black W, Barillas-Mury C. Effect of mosquito midgut trypsin activity on dengue-2 virus infection and dissemination in Aedes aegypti . Am J Trop Med Hyg. 2005;72:631–637. [PubMed] [Google Scholar]
- 46.Salazar MI, Richardson JH, Sanchez-Vargas I, Olson KE, Beaty BJ. Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 2007;7:9. doi: 10.1186/1471-2180-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sriurairatna S, Bhamarapravati N. Replication of dengue-2 virus in Aedes albopictus mosquitoes. An electron microscopic study. Am J Trop Med Hyg. 1977;26:1199–1205. doi: 10.4269/ajtmh.1977.26.1199. [DOI] [PubMed] [Google Scholar]
- 48.Schaar HM, Rust MJ, Waarts BL, dE M, Kuhn RJ, Wilschut J, Zhuang X, Smit JM. Characterization of the early events in dengue virus cell entry by biochemical assays and single-virus tracking. J Virol. 2007;81:12019–12028. doi: 10.1128/JVI.00300-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Avirutnan P, Malasit P, Seliger B, Bhakdi S, Husmann M. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J Immunol. 1998;161:6338–6346. [PubMed] [Google Scholar]
- 50.Cabrera-Hernandez A, Thepparit C, Suksanpaisan L, Smith DR. Dengue virus entry into liver (HepG2) cells is independent of hsp90 and hsp70. J Med Virol. 2007;79:386–392. doi: 10.1002/jmv.20786. [DOI] [PubMed] [Google Scholar]
- 51.Chareonsirisuthigul T, Kalayanarooj S, Ubol S. Dengue virus (DENV) antibody-dependent enhancement of infection upregulates the production of anti-inflammatory cytokines, but suppresses anti-DENV free radical and pro-inflammatory cytokine production, in THP-1 cells. J Gen Virol. 2007;88:365–375. doi: 10.1099/vir.0.82537-0. [DOI] [PubMed] [Google Scholar]
- 52.Chen ST, Lin YL, Huang MT, Wu MF, Cheng SC, Lei HY, Lee CK, Chiou TW, Wong CH, Hsieh SL. CLEC5A is critical for dengue-virus-induced lethal disease. Nature. 2008;453:672–676. doi: 10.1038/nature07013. [DOI] [PubMed] [Google Scholar]
- 53.Edgil D, Diamond MS, Holden KL, Paranjape SM, Harris E. Translation efficiency determines differences in cellular infection among dengue virus type 2 strains. Virology. 2003;317:275–290. doi: 10.1016/j.virol.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 54.Gromowski GD, Barrett AD. Characterization of an antigenic site that contains a dominant, type-specific neutralization determinant on the envelope protein domain III (ED3) of dengue 2 virus. Virology. 2007;366:349–360. doi: 10.1016/j.virol.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 55.Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res. 2003;60:421–467. doi: 10.1016/S0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- 56.Huang KJ, Yang YC, Lin YS, Huang JH, Liu HS, Yeh TM, Chen SH, Liu CC, Lei HY. The dual-specific binding of dengue virus and target cells for the antibody-dependent enhancement of dengue virus infection. J Immunol. 2006;176:2825–2832. doi: 10.4049/jimmunol.176.5.2825. [DOI] [PubMed] [Google Scholar]
- 57.King CA, Marshall JS, Alshurafa H, Anderson R. Release of vasoactive cytokines by antibody-enhanced dengue virus infection of a human mast cell/basophil line. J Virol. 2000;74:7146–7150. doi: 10.1128/JVI.74.15.7146-7150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kurane I, Meager A, Ennis FA. Dengue virus-specific human T cell clones. Serotype crossreactive proliferation, interferon gamma production, and cytotoxic activity. J Exp Med. 1989;170:763–775. doi: 10.1084/jem.170.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin YL, Liu CC, Chuang JI, Lei HY, Yeh TM, Lin YS, Huang YH, Liu HS. Involvement of oxidative stress, NF-IL-6, and RANTES expression in dengue-2-virus-infected human liver cells. Virology. 2000;276:114–126. doi: 10.1006/viro.2000.0524. [DOI] [PubMed] [Google Scholar]
- 60.Littaua R, Kurane I, Ennis FA. Human IgG Fc receptor II mediates antibody-dependent enhancement of dengue virus infection. J Immunol. 1990;144:3183–3186. [PubMed] [Google Scholar]
- 61.Moreno-Altamirano MM, Sanchez-Garcia FJ, Legorreta-Herrera M, guilar-Carmona I. Susceptibility of mouse macrophage J774 to dengue virus infection. Intervirology. 2007;50:237–239. doi: 10.1159/000100567. [DOI] [PubMed] [Google Scholar]
- 62.Reyes-Del VJ, Chavez-Salinas S, Medina F, Del Angel RM. Heat shock protein 90 and heat shock protein 70 are components of dengue virus receptor complex in human cells. J Virol. 2005;79:4557–4567. doi: 10.1128/JVI.79.8.4557-4567.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mercado-Curiel RF, Esquinca-Aviles HA, Tovar R, az-Badillo A, Camacho-Nuez M, Munoz ML. The four serotypes of dengue recognize the same putative receptors in Aedes aegypti midgut and Ae. albopictus cells. BMC Microbiol. 2006;6:85. doi: 10.1186/1471-2180-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yazi MM, Salas-Benito JS, Lanz-Mendoza H, Hernandez-Martinez S, Del Angel RM. A putative receptor for dengue virus in mosquito tissues: localization of a 45-kDa glycoprotein. Am J Trop Med Hyg. 2002;67:76–84. doi: 10.4269/ajtmh.2002.67.76. [DOI] [PubMed] [Google Scholar]
- 65.Chen Y, Maguire T, Hileman RE, Fromm JR, Esko JD, Linhardt RJ, Marks RM. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med. 1997;3:866–871. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 66.Germi R, Crance JM, Garin D, Guimet J, Lortat-Jacob H, Ruigrok RW, Zarski JP, Drouet E. Heparan sulfate-mediated binding of infectious dengue virus type 2 and yellow fever virus. Virology. 2002;292:162–168. doi: 10.1006/viro.2001.1232. [DOI] [PubMed] [Google Scholar]
- 67.Hilgard P, Stockert R. Heparan sulfate proteoglycans initiate dengue virus infection of hepatocytes. Hepatology. 2000;32:1069–1077. doi: 10.1053/jhep.2000.18713. [DOI] [PubMed] [Google Scholar]
- 68.Chen YC, Wang SY, King CC. Bacterial lipopolysaccharide inhibits dengue virus infection of primary human monocytes/macrophages by blockade of virus entry via a CD14-dependent mechanism. J Virol. 1999;73:2650–2657. doi: 10.1128/jvi.73.4.2650-2657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jindadamrongwech S, Thepparit C, Smith DR. Identification of GRP 78 (BiP) as a liver cell expressed receptor element for dengue virus serotype 2. Arch Virol. 2004;149:915–927. doi: 10.1007/s00705-003-0263-x. [DOI] [PubMed] [Google Scholar]
- 70.Thepparit C, Smith DR. Serotype-specific entry of dengue virus into liver cells: identification of the 37-kilodalton/67-kilodalton high-affinity laminin receptor as a dengue virus serotype 1 receptor. J Virol. 2004;78:12647–12656. doi: 10.1128/JVI.78.22.12647-12656.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fernandez-Garcia MD, Mazzon M, Jacobs M, Amara A. Pathogenesis of flavivirus infections: using and abusing the host cell. Cell Host. Microbe. 2009;5:318–328. doi: 10.1016/j.chom.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 72.Lozach PY, Burleigh L, Staropoli I, Navarro-Sanchez E, Harriague J, Virelizier JL, Rey FA, Despres P, Renzana-Seisdedos F, Amara A. Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN)-mediated enhancement of dengue virus infection is independent of DC-SIGN internalization signals. J Biol Chem. 2005;280:23698–23708. doi: 10.1074/jbc.M504337200. [DOI] [PubMed] [Google Scholar]
- 73.Navarro-Sanchez E, Altmeyer R, Amara A, Schwartz O, Fieschi F, Virelizier JL, renzana-Seisdedos F, Despres P. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 2003;4:723–728. doi: 10.1038/sj.embor.embor866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tassaneetrithep B, Burgess TH, Granelli-Piperno A, Trumpfheller C, Finke J, Sun W, Eller MA, Pattanapanyasat K, Sarasombath S, Birx DL, Steinman RM, Schlesinger S, Marovich MA. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med. 2003;197:823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller JL, de Wet BJ, Martinez-Pomares L, Radcliffe CM, Dwek RA, Rudd PM, Gordon S. The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog. 2008;4:e17. doi: 10.1371/journal.ppat.0040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Acosta EG, Castilla V, Damonte EB. Functional entry of dengue virus into Aedes albopictus mosquito cells is dependent on clathrin-mediated endocytosis. J Gen Virol. 2008;89:474–484. doi: 10.1099/vir.0.83357-0. [DOI] [PubMed] [Google Scholar]
- 77.Schaar HM, Rust MJ, Chen C, dE M, Wilschut J, Zhuang X, Smit JM. Dissecting the cell entry pathway of dengue virus by single-particle tracking in living cells. PLoS Pathog. 2008;4:e1000244. doi: 10.1371/journal.ppat.1000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krishnan MN, Sukumaran B, Pal U, Agaisse H, Murray JL, Hodge TW, Fikrig E. Rab 5 is required for the cellular entry of dengue and West Nile viruses. J Virol. 2007;81:4881–4885. doi: 10.1128/JVI.02210-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chu JJ, Ng ML. Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway. J Virol. 2004;78:10543–10555. doi: 10.1128/JVI.78.19.10543-10555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nawa M. Development of a new cell system for the infectivity assay of dengue viruses: plaque formation and virus growth of prototype and wild-type dengue virus strains in a newly established cell line, GK. Microbiol Immunol. 1984;28:765–776. doi: 10.1111/j.1348-0421.1984.tb00732.x. [DOI] [PubMed] [Google Scholar]
- 81.Acosta EG, Castilla V, Damonte EB. Alternative infectious entry pathways for dengue virus serotypes into mammalian cells. Cell Microbiol. 2009;11:1533–1549. doi: 10.1111/j.1462-5822.2009.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harrison SC. Viral membrane fusion. Nat Struct Mol Biol. 2008;15:690–698. doi: 10.1038/nsmb.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heinz FX, Allison SL. Flavivirus structure and membrane fusion. Adv Virus Res. 2003;59:63–97. doi: 10.1016/S0065-3527(03)59003-0. [DOI] [PubMed] [Google Scholar]
- 84.Kielian M, Rey FA. Virus membrane–fusion proteins: more than one way to make a hairpin. Nat Rev Microbiol. 2006;4:67–76. doi: 10.1038/nrmicro1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Modis Y, Ogata S, Clements D, Harrison SC. Structure of the dengue virus envelope protein after membrane fusion. Nature. 2004;427:313–319. doi: 10.1038/nature02165. [DOI] [PubMed] [Google Scholar]
- 86.Stiasny K, Allison SL, Schalich J, Heinz FX. Membrane interactions of the tick-borne encephalitis virus fusion protein E at low pH. J Virol. 2002;76:3784–3790. doi: 10.1128/JVI.76.8.3784-3790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Diamond MS, Roberts TG, Edgil D, Lu B, Ernst J, Harris E. Modulation of Dengue virus infection in human cells by alpha, beta, and gamma interferons. J Virol. 2000;74:4957–4966. doi: 10.1128/JVI.74.11.4957-4966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ho LJ, Hung LF, Weng CY, Wu WL, Chou P, Lin YL, Chang DM, Tai TY, Lai JH. Dengue virus type 2 antagonizes IFN-alpha but not IFN-gamma antiviral effect via down-regulating Tyk2-STAT signaling in the human dendritic cell. J Immunol. 2005;174:8163–8172. doi: 10.4049/jimmunol.174.12.8163. [DOI] [PubMed] [Google Scholar]
- 89.Johnson AJ, Roehrig JT. New mouse model for dengue virus vaccine testing. J Virol. 1999;73:783–786. doi: 10.1128/jvi.73.1.783-786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shresta S, Kyle JL, Snider HM, Basavapatna M, Beatty PR, Harris E. Interferon-dependent immunity is essential for resistance to primary dengue virus infection in mice, whereas T- and B-cell-dependent immunity are less critical. J Virol. 2004;78:2701–2710. doi: 10.1128/JVI.78.6.2701-2710.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Azeredo EL, De Oliveira-Pinto LM, Zagne SM, Cerqueira DI, Nogueira RM, Kubelka CF. NK cells, displaying early activation, cytotoxicity and adhesion molecules, are associated with mild dengue disease. Clin Exp Immunol. 2006;143:345–356. doi: 10.1111/j.1365-2249.2006.02996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shresta S, Kyle JL, Robert BP, Harris E. Early activation of natural killer and B cells in response to primary dengue virus infection in A/J mice. Virology. 2004;319:262–273. doi: 10.1016/j.virol.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 93.Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- 94.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 95.de K, Setiati TE, Mairuhu AT, Koraka P, Aberson HA, Spek CA, Osterhaus AD, Reitsma PH, Brandjes DP, Soemantri A, van Gorp EC (2008) Differential gene expression changes in children with severe dengue virus infections. PLoS Negl Trop Dis 2:e215 [DOI] [PMC free article] [PubMed]
- 96.Tsai YT, Chang SY, Lee CN, Kao CL. Human TLR3 recognizes dengue virus and modulates viral replication in vitro. Cell Microbiol. 2009;11:604–615. doi: 10.1111/j.1462-5822.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- 97.Aebi M, Fah J, Hurt N, Samuel CE, Thomis D, Bazzigher L, Pavlovic J, Haller O, Staeheli P. cDNA structures and regulation of two interferon-induced human Mx proteins. Mol Cell Biol. 1989;9:5062–5072. doi: 10.1128/mcb.9.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clemens MJ, Williams BR. Inhibition of cell-free protein synthesis by pppA2′p5′A2′p5′A: a novel oligonucleotide synthesized by interferon-treated L cell extracts. Cell. 1978;13:565–572. doi: 10.1016/0092-8674(78)90329-X. [DOI] [PubMed] [Google Scholar]
- 99.Pavlovic J, Zurcher T, Haller O, Staeheli P. Resistance to influenza virus and vesicular stomatitis virus conferred by expression of human MxA protein. J Virol. 1990;64:3370–3375. doi: 10.1128/jvi.64.7.3370-3375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Samuel CE. Molecular mechanisms of interferon action: interferon-mediated phosphorylation of ribosome-associated protein P1 and protein synthesis initiation factor eIF-2. Tex. Rep. Biol. Med. 1981;41:463–470. [PubMed] [Google Scholar]
- 101.Kurane I, Ennis FA. Production of interferon alpha by dengue virus-infected human monocytes. J Gen Virol. 1988;69(Pt 2):445–449. doi: 10.1099/0022-1317-69-2-445. [DOI] [PubMed] [Google Scholar]
- 102.Shresta S, Sharar KL, Prigozhin DM, Snider HM, Beatty PR, Harris E. Critical roles for both STAT1-dependent and STAT1-independent pathways in the control of primary dengue virus infection in mice. J Immunol. 2005;175:3946–3954. doi: 10.4049/jimmunol.175.6.3946. [DOI] [PubMed] [Google Scholar]
- 103.Erickson AK, Gale M., Jr Regulation of interferon production and innate antiviral immunity through translational control of IRF-7. Cell Res. 2008;18:433–435. doi: 10.1038/cr.2008.46. [DOI] [PubMed] [Google Scholar]
- 104.Ashour J, Laurent-Rolle M, Shi PY, Garcia-Sastre A. NS5 of dengue virus mediates STAT2 binding and degradation. J Virol. 2009;83:5408–5418. doi: 10.1128/JVI.02188-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jones M, Davidson A, Hibbert L, Gruenwald P, Schlaak J, Ball S, Foster GR, Jacobs M. Dengue virus inhibits alpha interferon signaling by reducing STAT2 expression. J Virol. 2005;79:5414–5420. doi: 10.1128/JVI.79.9.5414-5420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Munoz-Jordan JL, Sanchez-Burgos GG, Laurent-Rolle M, Garcia-Sastre A. Inhibition of interferon signaling by dengue virus. Proc Natl Acad Sci USA. 2003;100:14333–14338. doi: 10.1073/pnas.2335168100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Umareddy I, Tang KF, Vasudevan SG, Devi S, Hibberd ML, Gu F. Dengue virus regulates type I interferon signalling in a strain-dependent manner in human cell lines. J Gen Virol. 2008;89:3052–3062. doi: 10.1099/vir.0.2008/001594-0. [DOI] [PubMed] [Google Scholar]
- 108.Takhampunya R, Palmer DR, McClain S, Barvir DA, Lynch J, Jarman RG, Thomas S, Gibbons RV, Burgess TH, Sun P, Kamau E, Putnak R, Zhang C. Phenotypic analysis of dengue virus isolates associated with dengue fever and dengue hemorrhagic fever for cellular attachment, replication and interferon signaling ability. Virus Res. 2009;145:31–38. doi: 10.1016/j.virusres.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 109.Cardosa MJ, Wang SM, Sum MS, Tio PH. Antibodies against prM protein distinguish between previous infection with dengue and Japanese encephalitis viruses. BMC Microbiol. 2002;2:9. doi: 10.1186/1471-2180-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lai CY, Tsai WY, Lin SR, Kao CL, Hu HP, King CC, Wu HC, Chang GJ, Wang WK. Antibodies to envelope glycoprotein of dengue virus during the natural course of infection are predominantly cross-reactive and recognize epitopes containing highly conserved residues at the fusion loop of domain II. J Virol. 2008;82:6631–6643. doi: 10.1128/JVI.00316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shu PY, Chen LK, Chang SF, Yueh YY, Chow L, Chien LJ, Chin C, Lin TH, Huang JH. Dengue NS1-specific antibody responses: isotype distribution and serotyping in patients with dengue fever and dengue hemorrhagic fever. J Med Virol. 2000;62:224–232. doi: 10.1002/1096-9071(200010)62:2<224::AID-JMV14>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 112.Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, Green S, Vaughn DW, Nisalak A, Ennis FA, Rothman AL. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis. 2002;186:1165–1168. doi: 10.1086/343813. [DOI] [PubMed] [Google Scholar]
- 113.Costa SM, Freire MS, Alves AM. DNA vaccine against the non-structural 1 protein (NS1) of dengue 2 virus. Vaccine. 2006;24:4562–4564. doi: 10.1016/j.vaccine.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 114.Henchal EA, Henchal LS, Schlesinger JJ. Synergistic interactions of anti-NS1 monoclonal antibodies protect passively immunized mice from lethal challenge with dengue 2 virus. J Gen Virol. 1988;69(Pt 8):2101–2107. doi: 10.1099/0022-1317-69-8-2101. [DOI] [PubMed] [Google Scholar]
- 115.Kurosu T, Chaichana P, Yamate M, Anantapreecha S, Ikuta K. Secreted complement regulatory protein clusterin interacts with dengue virus nonstructural protein 1. Biochem Biophys Res Commun. 2007;362:1051–1056. doi: 10.1016/j.bbrc.2007.08.137. [DOI] [PubMed] [Google Scholar]
- 116.Schlesinger JJ, Brandriss MW, Walsh EE. Protection of mice against dengue 2 virus encephalitis by immunization with the dengue 2 virus non-structural glycoprotein NS1. J Gen Virol. 1987;68(Pt 3):853–857. doi: 10.1099/0022-1317-68-3-853. [DOI] [PubMed] [Google Scholar]
- 117.Kaufman BM, Summers PL, Dubois DR, Eckels KH. Monoclonal antibodies against dengue 2 virus E-glycoprotein protect mice against lethal dengue infection. Am J Trop Med Hyg. 1987;36:427–434. doi: 10.4269/ajtmh.1987.36.427. [DOI] [PubMed] [Google Scholar]
- 118.Kaufman BM, Summers PL, Dubois DR, Cohen WH, Gentry MK, Timchak RL, Burke DS, Eckels KH. Monoclonal antibodies for dengue virus prM glycoprotein protect mice against lethal dengue infection. Am J Trop Med Hyg. 1989;41:576–580. doi: 10.4269/ajtmh.1989.41.576. [DOI] [PubMed] [Google Scholar]
- 119.Rodenhuis-Zybert IA, van der Schaar HM, da Silva Voorham JM, van der Ende-Metselaar H, Lei HY, Wilschut J, Smit JM. Immature dengue virus: a veiled pathogen? PLoS Pathog. 2010;6:e1000718. doi: 10.1371/journal.ppat.1000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pierson TC, Xu Q, Nelson S, Oliphant T, Nybakken GE, Fremont DH, Diamond MS. The stoichiometry of antibody-mediated neutralization and enhancement of West Nile virus infection. Cell Host Microbe. 2007;1:135–145. doi: 10.1016/j.chom.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lisova O, Hardy F, Petit V, Bedouelle H. Mapping to completeness and transplantation of a group-specific, discontinuous, neutralizing epitope in the envelope protein of dengue virus. J Gen Virol. 2007;88:2387–2397. doi: 10.1099/vir.0.83028-0. [DOI] [PubMed] [Google Scholar]
- 122.Oliphant T, Nybakken GE, Engle M, Xu Q, Nelson CA, Sukupolvi-Petty S, Marri A, Lachmi BE, Olshevsky U, Fremont DH, Pierson TC, Diamond MS. Antibody recognition and neutralization determinants on domains I and II of West Nile Virus envelope protein. J Virol. 2006;80:12149–12159. doi: 10.1128/JVI.01732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rajamanonmani R, Nkenfou C, Clancy P, Yau YH, Shochat SG, Sukupolvi-Petty S, Schul W, Diamond MS, Vasudevan SG, Lescar J. On a mouse monoclonal antibody that neutralizes all four dengue virus serotypes. J Gen Virol. 2009;90:799–809. doi: 10.1099/vir.0.006874-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nelson S, Jost CA, Xu Q, Ess J, Martin JE, Oliphant T, Whitehead SS, Durbin AP, Graham BS, Diamond MS, Pierson TC. Maturation of West Nile virus modulates sensitivity to antibody-mediated neutralization. PLoS Pathog. 2008;4:e1000060. doi: 10.1371/journal.ppat.1000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nybakken GE, Oliphant T, Johnson S, Burke S, Diamond MS, Fremont DH. Structural basis of West Nile virus neutralization by a therapeutic antibody. Nature. 2005;437:764–769. doi: 10.1038/nature03956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thompson BS, Moesker B, Smit JM, Wilschut J, Diamond MS, Fremont DH. A therapeutic antibody against west Nile virus neutralizes infection by blocking fusion within endosomes. PLoS Pathog. 2009;5:e1000453. doi: 10.1371/journal.ppat.1000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van der Schaar HM, Wilschut JC, Smit JM (2009) Role of antibodies in controlling dengue virus infection. Immunobiology. doi:10.1016/j.imbio.2008.11.008 [DOI] [PubMed]
- 128.Halstead SB, O’Rourke EJ. Antibody-enhanced dengue virus infection in primate leukocytes. Nature. 1977;265:739–741. doi: 10.1038/265739a0. [DOI] [PubMed] [Google Scholar]
- 129.Halstead SB, O’Rourke EJ. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977;146:201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Randolph VB, Winkler G, Stollar V. Acidotropic amines inhibit proteolytic processing of flavivirus prM protein. Virology. 1990;174:450–458. doi: 10.1016/0042-6822(90)90099-D. [DOI] [PubMed] [Google Scholar]
- 131.Huang KJ, Yang YC, lin YS, liu HS, Yeh TM, Chen SH, Liu CC, Lei HY. Flow cytometric determination for dengue virus-infected cells: its application for antibody-dependent enhancement study. Dengue Bull. 2005;29:142–150. [Google Scholar]
- 132.Mongkolsapaya J, Dejnirattisai W, Xu XN, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, Sawasdivorn S, Duangchinda T, Dong T, Rowland-Jones S, Yenchitsomanus PT, McMichael A, Malasit P, Screaton G. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med. 2003;9:921–927. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- 133.Mongkolsapaya J, Duangchinda T, Dejnirattisai W, Vasanawathana S, Avirutnan P, Jairungsri A, Khemnu N, Tangthawornchaikul N, Chotiyarnwong P, Sae-Jang K, Koch M, Jones Y, McMichael A, Xu X, Malasit P, Screaton G. T cell responses in dengue hemorrhagic fever: are cross-reactive T cells suboptimal? J Immunol. 2006;176:3821–3829. doi: 10.4049/jimmunol.176.6.3821. [DOI] [PubMed] [Google Scholar]
- 134.Rothman AL. T lymphocyte responses to heterologous secondary dengue virus infections. Ann N Y Acad Sci. 2009;1171(suppl 1):E36–E41. doi: 10.1111/j.1749-6632.2009.05055.x. [DOI] [PubMed] [Google Scholar]
- 135.Rothman AL. Cellular immunology of sequential dengue virus infection and its role in disease pathogenesis. Curr Top Microbiol Immunol. 2010;338:83–98. doi: 10.1007/978-3-642-02215-9_7. [DOI] [PubMed] [Google Scholar]
- 136.Screaton G, Mongkolsapaya J. T cell responses and dengue haemorrhagic fever. Novartis Found Symp. 2006;277:164–171. doi: 10.1002/0470058005.ch12. [DOI] [PubMed] [Google Scholar]
- 137.Yauch LE, Zellweger RM, Kotturi MF, Qutubuddin A, Sidney J, Peters B, Prestwood TR, Sette A, Shresta S. A protective role for dengue virus-specific CD8+ T cells. J Immunol. 2009;182:4865–4873. doi: 10.4049/jimmunol.0801974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mathew A, Rothman AL. Understanding the contribution of cellular immunity to dengue disease pathogenesis. Immunol Rev. 2008;225:300–313. doi: 10.1111/j.1600-065X.2008.00678.x. [DOI] [PubMed] [Google Scholar]
- 139.Azeredo EL, Zagne SM, Santiago MA, Gouvea AS, Santana AA, Neves-Souza PC, Nogueira RM, Miagostovich MP, Kubelka CF. Characterisation of lymphocyte response and cytokine patterns in patients with dengue fever. Immunobiology. 2001;204:494–507. doi: 10.1078/0171-2985-00058. [DOI] [PubMed] [Google Scholar]
- 140.Basu A, Chaturvedi UC. Vascular endothelium: the battlefield of dengue viruses. FEMS Immunol Med Microbiol. 2008;53:287–299. doi: 10.1111/j.1574-695X.2008.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bozza FA, Cruz OG, Zagne SM, Azeredo EL, Nogueira RM, Assis EF, Bozza PT, Kubelka CF. Multiplex cytokine profile from dengue patients: MIP-1beta and IFN-gamma as predictive factors for severity. BMC Infect Dis. 2008;8:86. doi: 10.1186/1471-2334-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chakravarti A, Kumaria R. Circulating levels of tumour necrosis factor-alpha & interferon-gamma in patients with dengue & dengue haemorrhagic fever during an outbreak. Indian J Med Res. 2006;123:25–30. [PubMed] [Google Scholar]
- 143.Dong T, Moran E, Vinh CN, Simmons C, Luhn K, Peng Y, Wills B, Phuong DN, Thi Thu TL, Hien TT, McMichael A, Farrar J, Rowland-Jones S. High pro-inflammatory cytokine secretion and loss of high avidity cross-reactive cytotoxic T-cells during the course of secondary dengue virus infection. PLoS One. 2007;2:e1192. doi: 10.1371/journal.pone.0001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Green S, Vaughn DW, Kalayanarooj S, Nimmannitya S, Suntayakorn S, Nisalak A, Rothman AL, Ennis FA. Elevated plasma interleukin-10 levels in acute dengue correlate with disease severity. J Med Virol. 1999;59:329–334. doi: 10.1002/(SICI)1096-9071(199911)59:3<329::AID-JMV12>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 145.Hober D, Poli L, Roblin B, Gestas P, Chungue E, Granic G, Imbert P, Pecarere JL, Vergez-Pascal R, Wattre P. Serum levels of tumor necrosis factor-alpha (TNF-alpha), interleukin-6 (IL-6), and interleukin-1 beta (IL-1 beta) in dengue-infected patients. Am J Trop Med Hyg. 1993;48:324–331. doi: 10.4269/ajtmh.1993.48.324. [DOI] [PubMed] [Google Scholar]
- 146.Kurane I, Innis BL, Nimmannitya S, Nisalak A, Meager A, Janus J, Ennis FA. Activation of T lymphocytes in dengue virus infections. High levels of soluble interleukin 2 receptor, soluble CD4, soluble CD8, interleukin 2, and interferon-gamma in sera of children with dengue. J Clin Invest. 1991;88:1473–1480. doi: 10.1172/JCI115457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shresta S, Sharar KL, Prigozhin DM, Beatty PR, Harris E. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J Virol. 2006;80:10208–10217. doi: 10.1128/JVI.00062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Suharti C, van Gorp EC, Dolmans WM, Setiati TE, Hack CE, Djokomoeljanto R, van der Meer JW. Cytokine patterns during dengue shock syndrome. Eur Cytokine Netw. 2003;14:172–177. [PubMed] [Google Scholar]
- 149.Atrasheuskaya A, Petzelbauer P, Fredeking TM, Ignatyev G. Anti-TNF antibody treatment reduces mortality in experimental dengue virus infection. FEMS Immunol Med Microbiol. 2003;35:33–42. doi: 10.1111/j.1574-695X.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 150.Aichele P, Brduscha-Riem K, Oehen S, Odermatt B, Zinkernagel RM, Hengartner H, Pircher H. Peptide antigen treatment of naive and virus-immune mice: antigen-specific tolerance versus immunopathology. Immunity. 1997;6:519–529. doi: 10.1016/S1074-7613(00)80340-4. [DOI] [PubMed] [Google Scholar]
- 151.Mangada MM, Rothman AL. Altered cytokine responses of dengue-specific CD4+ T cells to heterologous serotypes. J Immunol. 2005;175:2676–2683. doi: 10.4049/jimmunol.175.4.2676. [DOI] [PubMed] [Google Scholar]
- 152.Nguyen TH, Lei HY, Nguyen TL, Lin YS, Huang KJ, Le BL, Lin CF, Yeh TM, Do QH, Vu TQ, Chen LC, Huang JH, Lam TM, Liu CC, Halstead SB. Dengue hemorrhagic fever in infants: a study of clinical and cytokine profiles. J Infect Dis. 2004;189:221–232. doi: 10.1086/380762. [DOI] [PubMed] [Google Scholar]
- 153.Simmons CP, Chau TN, Thuy TT, Tuan NM, Hoang DM, Thien NT, Lien lB, Quy NT, Hieu NT, Hien TT, McElnea C, Young P, Whitehead S, Hung NT, Farrar J. Maternal antibody and viral factors in the pathogenesis of dengue virus in infants. J Infect Dis. 2007;196:416–424. doi: 10.1086/519170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sun P, Fernandez S, Marovich MA, Palmer DR, Celluzzi CM, Boonnak K, Liang Z, Subramanian H, Porter KR, Sun W, Burgess TH. Functional characterization of ex vivo blood myeloid and plasmacytoid dendritic cells after infection with dengue virus. Virology. 2009;383:207–215. doi: 10.1016/j.virol.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 155.Boonnak K, Slike BM, Burgess TH, Mason RM, Wu SJ, Sun P, Porter K, Rudiman IF, Yuwono D, Puthavathana P, Marovich MA. Role of dendritic cells in antibody-dependent enhancement of dengue virus infection. J Virol. 2008;82:3939–3951. doi: 10.1128/JVI.02484-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Weaver S, Vasilakis N. Molecular evolution of dengue viruses: contributions of phylogenetics to understanding the history and epidemiology of the preeminent arbovial disease. Infect Genet Evol. 2009;9:523–540. doi: 10.1016/j.meegid.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Blok J, Gibbs AJ, McWilliam SM, Vitarana UT. NS 1 gene sequences from eight dengue-2 viruses and their evolutionary relationships with other dengue-2 viruses. Arch Virol. 1991;118:209–223. doi: 10.1007/BF01314031. [DOI] [PubMed] [Google Scholar]
- 158.Kanakaratne N, Wahala WM, Messer WB, Tissera HA, Shahani A, Abeysinghe N, de-Silva AM, Gunasekera M. Severe dengue epidemics in Sri Lanka, 2003–2006. Emerg Infect Dis. 2009;15:192–199. doi: 10.3201/eid1502.080926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mangada MN, Igarashi A. Sequences of terminal non-coding regions from four dengue-2 viruses isolated from patients exhibiting different disease severities. Virus Genes. 1997;14:5–12. doi: 10.1023/A:1007914520454. [DOI] [PubMed] [Google Scholar]
- 160.Mangada MN, Igarashi A. Molecular and in vitro analysis of eight dengue type 2 viruses isolated from patients exhibiting different disease severities. Virology. 1998;244:458–466. doi: 10.1006/viro.1998.9093. [DOI] [PubMed] [Google Scholar]
- 161.Messer WB, Gubler DJ, Harris E, Sivananthan K, de Silva AM. Emergence and global spread of a dengue serotype 3, subtype III virus. Emerg Infect Dis. 2003;9:800–809. doi: 10.3201/eid0907.030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pandey BD, Igarashi A. Severity-related molecular differences among nineteen strains of dengue type 2 viruses. Microbiol Immunol. 2000;44:179–188. doi: 10.1111/j.1348-0421.2000.tb02480.x. [DOI] [PubMed] [Google Scholar]
- 163.Raekiansyah M, Pramesyanti A, Bela B, Kosasih H, Ma’roef CN, Tobing SY, Rudiman PI, Alisjahbana B, Endi TP, Green S, Kalayanarooj S, Rothman AL, Sudiro TM. Genetic variations and relationship among dengue virus type 3 strains isolated from patients with mild or severe form of dengue disease in Indonesia and Thailand. Southeast Asian J Trop Med Public Health. 2005;36:1187–1197. [PubMed] [Google Scholar]
- 164.Rico-Hesse R, Harrison LM, Salas RA, Tovar D, Nisalak A, Ramos C, Boshell J, de Mesa MT, Nogueira RM, da Rosa AT. Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology. 1997;230:244–251. doi: 10.1006/viro.1997.8504. [DOI] [PubMed] [Google Scholar]
- 165.Watts DM, Porter KR, Putvatana P, Vasquez B, Calampa C, Hayes CG, Halstead SB. Failure of secondary infection with American genotype dengue 2 to cause dengue haemorrhagic fever. Lancet. 1999;354:1431–1434. doi: 10.1016/S0140-6736(99)04015-5. [DOI] [PubMed] [Google Scholar]
- 166.Leitmeyer KC, Vaughn DW, Watts DM, Salas R, Villalobos I, de C, Ramos C, Rico-Hesse R. Dengue virus structural differences that correlate with pathogenesis. J Virol. 1999;73:4738–4747. doi: 10.1128/jvi.73.6.4738-4747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cologna R, Armstrong PM, Rico-Hesse R. Selection for virulent dengue viruses occurs in humans and mosquitoes. J Virol. 2005;79:853–859. doi: 10.1128/JVI.79.2.853-859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pryor MJ, Carr JM, Hocking H, Davidson AD, Li P, Wright PJ. Replication of dengue virus type 2 in human monocyte-derived macrophages: comparisons of isolates and recombinant viruses with substitutions at amino acid 390 in the envelope glycoprotein. Am J Trop Med Hyg. 2001;65:427–434. doi: 10.4269/ajtmh.2001.65.427. [DOI] [PubMed] [Google Scholar]
- 169.Pryor MJ, Wright PJ. Glycosylation mutants of dengue virus NS1 protein. J Gen Virol. 1994;75(Pt 5):1183–1187. doi: 10.1099/0022-1317-75-5-1183. [DOI] [PubMed] [Google Scholar]
- 170.Pryor MJ, Gualano RC, Lin B, Davidson AD, Wright PJ. Growth restriction of dengue virus type 2 by site-specific mutagenesis of virus-encoded glycoproteins. J Gen Virol. 1998;79(Pt 11):2631–2639. doi: 10.1099/0022-1317-79-11-2631. [DOI] [PubMed] [Google Scholar]
- 171.Vorndam V, Mathews JH, Barrett AD, Roehrig JT, Trent DW. Molecular and biological characterization of a non-glycosylated isolate of St Louis encephalitis virus. J Gen Virol. 1993;74(Pt 12):2653–2660. doi: 10.1099/0022-1317-74-12-2653. [DOI] [PubMed] [Google Scholar]
- 172.Pokidysheva E, Zhang Y, Battisti AJ, Bator-Kelly CM, Chipman PR, Xiao C, Gregorio GG, Hendrickson WA, Kuhn RJ, Rossmann MG. Cryo-EM reconstruction of dengue virus in complex with the carbohydrate recognition domain of DC-SIGN. Cell. 2006;124:485–493. doi: 10.1016/j.cell.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 173.Tajima S, Takasaki T, Kurane I. Characterization of Asn130-to-Ala mutant of dengue type 1 virus NS1 protein. Virus Genes. 2008;36:323–329. doi: 10.1007/s11262-008-0211-7. [DOI] [PubMed] [Google Scholar]
- 174.Avirutnan P, Punyadee N, Noisakran S, Komoltri C, Thiemmeca S, Auethavornanan K, Jairungsri A, Kanlaya R, Tangthawornchaikul N, Puttikhunt C, Pattanakitsakul SN, Yenchitsomanus PT, Mongkolsapaya J, Kasinrerk W, Sittisombut N, Husmann M, Blettner M, Vasanawathana S, Bhakdi S, Malasit P. Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J Infect Dis. 2006;193:1078–1088. doi: 10.1086/500949. [DOI] [PubMed] [Google Scholar]
- 175.Anderson R, Wang S, Osiowy C, Issekutz AC. Activation of endothelial cells via antibody-enhanced dengue virus infection of peripheral blood monocytes. J Virol. 1997;71:4226–4232. doi: 10.1128/jvi.71.6.4226-4232.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chang GJ, Hunt AR, Holmes DA, Springfield T, Chiueh TS, Roehrig JT, Gubler DJ. Enhancing biosynthesis and secretion of premembrane and envelope proteins by the chimeric plasmid of dengue virus type 2 and Japanese encephalitis virus. Virology. 2003;306:170–180. doi: 10.1016/S0042-6822(02)00028-4. [DOI] [PubMed] [Google Scholar]
- 177.Falconar AK. Identification of an epitope on the dengue virus membrane (M) protein defined by cross-protective monoclonal antibodies: design of an improved epitope sequence based on common determinants present in both envelope (E and M) proteins. Arch Virol. 1999;144:2313–2330. doi: 10.1007/s007050050646. [DOI] [PubMed] [Google Scholar]
- 178.He RT, Innis BL, Nisalak A, Usawattanakul W, Wang S, Kalayanarooj S, Anderson R. Antibodies that block virus attachment to Vero cells are a major component of the human neutralizing antibody response against dengue virus type 2. J Med Virol. 1995;45:451–461. doi: 10.1002/jmv.1890450417. [DOI] [PubMed] [Google Scholar]
- 179.Henchal EA, McCown JM, Burke DS, Seguin MC, Brandt WE. Epitopic analysis of antigenic determinants on the surface of dengue-2 virions using monoclonal antibodies. Am J Trop Med Hyg. 1985;34:162–169. doi: 10.4269/ajtmh.1985.34.162. [DOI] [PubMed] [Google Scholar]
- 180.Murray JM, Aaskov JG, Wright PJ. Processing of the dengue virus type 2 proteins prM and C-prM. J Gen Virol. 1993;74(Pt 2):175–182. doi: 10.1099/0022-1317-74-2-175. [DOI] [PubMed] [Google Scholar]
- 181.Pryor MJ, Azzola L, Wright PJ, Davidson AD. Histidine 39 in the dengue virus type 2M protein has an important role in virus assembly. J Gen Virol. 2004;85:3627–3636. doi: 10.1099/vir.0.80283-0. [DOI] [PubMed] [Google Scholar]
- 182.Purdy DE, Chang GJ. Secretion of noninfectious dengue virus-like particles and identification of amino acids in the stem region involved in intracellular retention of envelope protein. Virology. 2005;333:239–250. doi: 10.1016/j.virol.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 183.Roehrig JT, Bolin RA, Kelly RG. Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica. Virology. 1998;246:317–328. doi: 10.1006/viro.1998.9200. [DOI] [PubMed] [Google Scholar]
- 184.Wang S, He R, Anderson R. PrM- and cell-binding domains of the dengue virus E protein. J Virol. 1999;73:2547–2551. doi: 10.1128/jvi.73.3.2547-2551.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bray M, Lai CJ. Dengue virus premembrane and membrane proteins elicit a protective immune response. Virology. 1991;185:505–508. doi: 10.1016/0042-6822(91)90809-P. [DOI] [PubMed] [Google Scholar]
- 186.Huang KJ, lin YS, Huang JH, Liu HS, Yeh TM, Liu CC, Lei HY. Anti-prM antibody as an autoantibody in Dengue virus infection. Am J Infect Dis. 2008;4:59–67. [Google Scholar]
- 187.Se-Thoe SY, Ng MM, Ling AE. Retrospective study of Western blot profiles in immune sera of natural dengue virus infections. J Med Virol. 1999;57:322–330. doi: 10.1002/(SICI)1096-9071(199903)57:3<322::AID-JMV17>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 188.Junjhon J, Lausumpao M, Supasa S, Noisakran S, Songjaeng A, Saraithong P, Chaichoun K, Utaipat U, Keelapang P, Kanjanahaluethai A, Puttikhunt C, Kasinrerk W, Malasit P, Sittisombut N. Differential modulation of prM cleavage, extracellular particle distribution, and virus infectivity by conserved residues at nonfurin consensus positions of the dengue virus pr-M junction. J Virol. 2008;82:10776–10791. doi: 10.1128/JVI.01180-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Keelapang P, Sriburi R, Supasa S, Panyadee N, Songjaeng A, Jairungsri A, Puttikhunt C, Kasinrerk W, Malasit P, Sittisombut N. Alterations of pr-M cleavage and virus export in pr-M junction chimeric dengue viruses. J Virol. 2004;78:2367–2381. doi: 10.1128/JVI.78.5.2367-2381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Elshuber S, Allison SL, Heinz FX, Mandl CW. Cleavage of protein prM is necessary for infection of BHK-21 cells by tick-borne encephalitis virus. J Gen Virol. 2003;84:183–191. doi: 10.1099/vir.0.18723-0. [DOI] [PubMed] [Google Scholar]
- 191.Cherrier MV, Kaufmann B, Nybakken GE, Lok SM, Warren JT, Chen BR, Nelson CA, Kostyuchenko VA, Holdaway HA, Chipman PR, Kuhn RJ, Diamond MS, Rossmann MG, Fremont DH. Structural basis for the preferential recognition of immature flaviviruses by a fusion-loop antibody. EMBO J. 2009;28:3269–3276. doi: 10.1038/emboj.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Halstead SB, Streit TG, Lafontant JG, Putvatana R, Russell K, Sun W, Kanesa-Thasan N, Hayes CG, Watts DM. Haiti: absence of dengue hemorrhagic fever despite hyperendemic dengue virus transmission. Am J Trop Med Hyg. 2001;65:180–183. doi: 10.4269/ajtmh.2001.65.180. [DOI] [PubMed] [Google Scholar]
- 193.Kouri G, Guzman MG, Valdes L, Carbonel I, del RD, Vazquez S, Laferte J, Delgado J, Cabrera MV (1998) Reemergence of dengue in Cuba: a 1997 epidemic in Santiago de Cuba. Emerg Infect Dis 4:89–92 [DOI] [PMC free article] [PubMed]
- 194.Kouri GP, Guzman MG, Bravo JR, Triana C. Dengue haemorrhagic fever/dengue shock syndrome: lessons from the Cuban epidemic, 1981. Bull World Health Organ. 1989;67:375–380. [PMC free article] [PubMed] [Google Scholar]
- 195.Chiewsilp P, Scott RM, Bhamarapravati N. Histocompatibility antigens and dengue hemorrhagic fever. Am J Trop Med Hyg. 1981;30:1100–1105. doi: 10.4269/ajtmh.1981.30.1100. [DOI] [PubMed] [Google Scholar]
- 196.LaFleur C, Granados J, Vargas-Alarcon G, Ruiz-Morales J, Villarreal-Garza C, Higuera L, Hernandez-Pacheco G, Cutino-Moguel T, Rangel H, Figueroa R, Acosta M, Lazcano E, Ramos C. HLA-DR antigen frequencies in Mexican patients with dengue virus infection: HLA-DR4 as a possible genetic resistance factor for dengue hemorrhagic fever. Hum Immunol. 2002;63:1039–1044. doi: 10.1016/S0198-8859(02)00682-1. [DOI] [PubMed] [Google Scholar]
- 197.Loke H, Bethell DB, Phuong CX, Dung M, Schneider J, White NJ, Day NP, Farrar J, Hill AV. Strong HLA class I-restricted T cell responses in dengue hemorrhagic fever: a double-edged sword? J Infect Dis. 2001;184:1369–1373. doi: 10.1086/324320. [DOI] [PubMed] [Google Scholar]
- 198.Stephens HA, Klaythong R, Sirikong M, Vaughn DW, Green S, Kalayanarooj S, Endy TP, Libraty DH, Nisalak A, Innis BL, Rothman AL, Ennis FA, Chandanayingyong D. HLA-A and -B allele associations with secondary dengue virus infections correlate with disease severity and the infecting viral serotype in ethnic Thais. Tissue Antigens. 2002;60:309–318. doi: 10.1034/j.1399-0039.2002.600405.x. [DOI] [PubMed] [Google Scholar]
- 199.Zivna I, Green S, Vaughn DW, Kalayanarooj S, Stephens HA, Chandanayingyong D, Nisalak A, Ennis FA, Rothman AL. T cell responses to an HLA-B*07-restricted epitope on the dengue NS3 protein correlate with disease severity. J Immunol. 2002;168:5959–5965. doi: 10.4049/jimmunol.168.11.5959. [DOI] [PubMed] [Google Scholar]
- 200.Polizel JR, Bueno D, Visentainer JE, Sell AM, Borelli SD, Tsuneto LT, Dalalio MM, Coimbra MT, Moliterno RA. Association of human leukocyte antigen DQ1 and dengue fever in a white Southern Brazilian population. Mem Inst Oswaldo Cruz. 2004;99:559–562. doi: 10.1590/S0074-02762004000600003. [DOI] [PubMed] [Google Scholar]
- 201.Loke H, Bethell D, Phuong CX, Day N, White N, Farrar J, Hill A. Susceptibility to dengue hemorrhagic fever in Vietnam: evidence of an association with variation in the vitamin d receptor and Fc gamma receptor IIa genes. Am J Trop Med Hyg. 2002;67:102–106. doi: 10.4269/ajtmh.2002.67.102. [DOI] [PubMed] [Google Scholar]
- 202.Fernandez-Mestre MT, Gendzekhadze K, Rivas-Vetencourt P, Layrisse Z. TNF-alpha-308A allele, a possible severity risk factor of hemorrhagic manifestation in dengue fever patients. Tissue Antigens. 2004;64:469–472. doi: 10.1111/j.1399-0039.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- 203.Chen RF, Wang L, Cheng JT, Chuang H, Chang JC, Liu JW, Lin IC, Yang KD. Combination of CTLA-4 and TGFbeta1 gene polymorphisms associated with dengue hemorrhagic fever and virus load in a dengue-2 outbreak. Clin Immunol. 2009;131:404–409. doi: 10.1016/j.clim.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 204.Sakuntabhai A, Turbpaiboon C, Casademont I, Chuansumrit A, Lowhnoo T, Kajaste-Rudnitski A, Kalayanarooj SM, Tangnararatchakit K, Tangthawornchaikul N, Vasanawathana S, Chaiyaratana W, Yenchitsomanus PT, Suriyaphol P, Avirutnan P, Chokephaibulkit K, Matsuda F, Yoksan S, Jacob Y, Lathrop GM, Malasit P, Despres P, Julier C. A variant in the CD209 promoter is associated with severity of dengue disease. Nat Genet. 2005;37:507–513. doi: 10.1038/ng1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chao YC, Huang CS, Lee CN, Chang SY, King CC, Kao CL. Higher infection of dengue virus serotype 2 in human monocytes of patients with G6PD deficiency. PLoS One. 2008;3:e1557. doi: 10.1371/journal.pone.0001557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bravo JR, Guzman MG, Kouri GP. Why dengue haemorrhagic fever in Cuba? 1. Individual risk factors for dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS) Trans R Soc Trop Med Hyg. 1987;81:816–820. doi: 10.1016/0035-9203(87)90041-1. [DOI] [PubMed] [Google Scholar]
- 207.Guzman MG, Kouri G. Dengue: an update. Lancet Infect Dis. 2002;2:33–42. doi: 10.1016/S1473-3099(01)00171-2. [DOI] [PubMed] [Google Scholar]
- 208.Lee MS, Hwang KP, Chen TC, Lu PL, Chen TP. Clinical characteristics of dengue, dengue hemorrhagic fever in a medical center of southern Taiwan during the 2002 epidemic. J Microbiol Immunol Infect. 2006;39:121–129. [PubMed] [Google Scholar]
- 209.Ong A, Sandar M, Chen MI, Sin LY. Fatal dengue hemorrhagic fever in adults during a dengue epidemic in Singapore. Int J Infect Dis. 2007;11:263–267. doi: 10.1016/j.ijid.2006.02.012. [DOI] [PubMed] [Google Scholar]