Abstract
The N-myc downstream-regulated gene 2 (NDRG2) is involved in cell differentiation and apoptosis, but its function in the pancreas remains to be established. Herein we examine the expression and function of NDRG2 in the endocrine pancreas. NDRG2 immunoreactivity was localized mainly in the cytoplasm of pancreatic β cells. When β-TC3 cells were exposed chronically to high levels of free fatty acid (FFA), cell viability was impaired, and Akt and NDRG2 phosphorylation were reduced. NDRG2 is a potential substrate of protein kinase Akt. Overexpression of constitutively active Akt enhanced NDRG2 phosphorylation and abolished the apoptosis induced by FFA in β-TC3 cells, whereas NDRG2 knock-down attenuated Akt-mediated protection of β cells against fatty acid-triggered apoptosis. Collectively, these data indicate that NDRG2 acts as a key molecule in pancreatic β cells and is involved in the Akt-mediated protection of β cells against lipotoxicity.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-010-0258-1) contains supplementary material, which is available to authorized users.
Keywords: NDRG2, Pancreatic β cell, Protein kinase Akt, Free fatty acid, Apoptosis
Introduction
The N-myc downstream-regulated gene 2 (NDRG2) is a member of the NDRG gene family, which is involved in cellular differentiation and development [1, 2]. Human NDRG2 was first cloned in our laboratory from a normal human brain cDNA library by subtractive hybridization [3]. Several reports have provided insight into the physiological roles of NDRG2 in the central nervous system. NDRG2 is expressed in astrocytes and inhibits astrocytoma/glioblastoma cell proliferation [4, 5]. NDRG2 also promotes neurite outgrowth of nerve growth factor (NGF)-differentiated pheochromocytoma (PC12) cells [6]. Furthermore, NDRG2 expression is upregulated in Alzheimer’s disease [7]. In addition to its known functions in the brain, NDRG2 is an early aldosterone-induced gene, involved in stimulating amiloride-sensitive Na+ currents in Xenopus laevis oocytes and Fischer rat thyroid cells [8]. NDRG2 gene expression is downregulated during skeletal muscle hypertrophy and upregulated during muscle atrophy. Therefore, NDRG2 acts as a novel regulator of myoblast proliferation and can be regulated by anabolic and catabolic factors [9]. In a previous study, we analyzed NDRG2 expression in mouse embryonic and adult tissue, and discovered NDRG2 immunoreactivity in the islet cells of adult mouse pancreas [10]. However, very little information is available regarding the cellular localization and physiological role of NDRG2 in the pancreas.
Long-term exposure of the endocrine pancreas to free fatty acid (FFA) such as palmitic acid has an adverse effect on β-cell function and survival [11, 12]. However, the mechanisms by which FFA induces β-cell dysfunction and injury are not fully understood. The protein kinase Akt is an important molecule in the insulin signaling pathway and promotes the survival of pancreatic islets [13]; especially Akt activation can prevent β-cell apoptosis induced by FFA [14]. Interestingly, phosphorylation of NDRG2 protein by protein kinase Akt and protein kinase C (PKC) has been described in the insulin signaling pathway [15]. NDRG2 acts as the new substrate of Akt in the insulin signaling pathway. Whether NDRG2 is involved in the Akt-mediated protection of β cells against FFA-triggered damage needs to be further studied.
In this study, we investigate further the expression and cellular localization of NDRG2 in the pancreatic islet, as well as its potential function in pancreatic islet cells. We demonstrate firstly that NDRG2 is highly expressed in the cytoplasm of pancreatic β cells but shows low expression in other pancreatic endocrine cells. In addition, our experiments suggest that the phosphorylation of Akt and its substrate NDRG2 is decreased in β-TC3 cells under lipotoxic conditions. Overexpression of the constitutively active form of Akt can enhance NDRG2 phosphorylation and reduce β-cell apoptosis triggered by high concentrations of FFA, whereas NDRG2 knock-down can attenuate Akt-mediated β-cell protection against lipoapoptosis. Our study suggests that NDRG2 can protect pancreatic β cells against FFA-induced lipotoxicity, and it may represent a novel area for therapeutic intervention in clinical diabetes.
Materials and methods
Plasmids and adenovirus
DNA fragments encoding FLAG-tagged human AKTα and myristoylated-AKTα (myr-AKTα) were PCR-amplified using a human dental pulp cDNA library as template with the following primers: 5′-GCGGATCCATGAGCGACGTGGCTATTGTG-3′ and 5′-GCGGATCCATGGGCTGTGGCTGCAGCTCACACCCGGAAGATGACATGAGCGACGTGGCTATTGTG-3′ for AKTα, and 5′-GCGAATTCTCACTTGTCATCGTCGTCCTTGTAGTCGCCACAGGCCGTGCTGCTG-3′ for myr-AKTα, and then cloned into the BamHI/EcoRI sites of the pcDNA3.1(+) vector (Invitrogen Life Technologies, Carlsbad, CA). The dominant negative form of human AKTα (K179M) was kindly provided by Dr. Han et al. [16]. Expression vectors were transfected into cells with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions.
Recombinant adenoviruses carrying LacZ (Ad-LacZ) or enhanced green fluorescent protein (EGFP; Ad-EGFP) were purchased from Benyuan Zhengyang Gene Technology Company Ltd. (Beijing, China). Recombinant adenoviruses containing myr-AKTα were generated using the AdEasy adenoviral vector system (Stratagene, La Jolla, CA). Recombinant adenovirus particles were used to infect β-TC3 cells. A pilot study with the control virus Ad-EGFP demonstrated that 90–95% of the cells were infected, as detected by fluorescence microscope (Olympus, Tokyo, Japan; Supplemental Fig. 1).
Cell and tissue preparation
Mouse β-TC6 cells were kindly provided by Dr. Hongyang Wang (Second Military Medical University, Shanghai, China). Mouse β-TC3 cells were kindly provided by Dr. Qiuhe Ji (Fourth Military Medical University, Xi’an, China). INS-1 cells and HEK293 cells were obtained from the China Center for Type Culture Collection (CCTCC; Wuhan, China). INS-1 cells, β-TC3, and β-TC6 were cultured in DMEM medium (Invitrogen) containing 5.5 mM glucose, 15% horse serum, 2.5% fetal bovine serum, and 6 mM l-glutamine. HEK293 cells were cultured in DMEM medium supplemented with 10% fetal bovine serum. All cells were maintained in a humidified 5% CO2 atmosphere at 37°C.
C57 BL/6J mice (Fourth Military Medical University Animal Research Center, Xian, China) were anesthetized by intraperitoneal injection of 100 mg kg−1 ketamine and 10 mg kg−1 xylazine before dissection of the pancreas. After removal, the tissues were immediately frozen in liquid nitrogen until analysis. Mice were handled according to animal protocols institutionally approved by the Fourth Military Medical University.
Immunostaining
The monoclonal antibody FMU-NDRG2.3, which reacts specifically with human and mouse NDRG2 [10, 17], was generated from mice immunized with recombinant His-tagged human NDRG2 protein. Western blotting analysis confirmed that this antibody is specific only to NDRG2 and not to NDRG1, NDRG3, and NDRG4 protein [18]. Human and mouse pancreatic tissues were fixed in a freshly prepared solution of 4% paraformaldehyde, dehydrated through graded solutions of ethanol, and embedded in paraffin. Serial sections (5 μm thick) were cut and mounted on glass slides (Fisher, Pittsburgh, PA). After dewaxing and microwave antigen retrieval, slides were incubated with 10% normal goat serum for 1 h and then overnight at 4°C with FMU-NDRG2.3 (diluted 1:150) or mouse monoclonal antibody to insulin (diluted 1:150; Boster, Wu Han, China). Biotinylated anti-mouse IgG (1:400; Sigma, St. Louis, MO) was applied and detected with a streptavidin-peroxidase complex and 0.1% 3, 3-diaminobenzidine (Sigma) in phosphate-buffered saline (PBS) with 0.05% H2O2 for 5 min at room temperature.
For immunofluorescence histochemistry, nonspecific antibody-binding sites were first blocked with 1% bovine serum albumin (BSA; Sigma) in PBS, after which dewaxed slides were incubated with FMU-NDRG2.3 alone or in combination with guinea-pig anti-insulin (diluted 1:150; Abcam, Cambridge, UK), rabbit anti-glucagon (diluted 1:100; Zhongshan Goldenbridge, Beijing, China), somatostatin (diluted 1:100; Boster), or pancreatic polypeptide (Maxim, Fuzhou, China). The combinations were visualized using a mixture of fluorescein isothiocyanate (FITC)-conjugated anti-mouse (diluted 1:400; Sigma) and Cy3-conjugated anti-rabbit (diluted 1:600; Sigma) or Cy3-conjugated anti-guinea pig (diluted 1:200; Jackson ImmunoResearch, West Grove, PA). Specimens were examined with a FV300 laser scanning microscope (Olympus, Tokyo, Japan).
For NDRG2 subcellular localization, the cells were fixed in a freshly prepared solution of 4% paraformaldehyde, rinsed, and permeabilized with 0.1% Triton X-100 in PBS. Permeabilized cells were then incubated with horse serum in PBS to block nonspecific binding. After thorough rinsing with PBS, the cells were incubated overnight at 4°C with FMU-NDRG2.3 (diluted 1:100), and incubated with fluorescein isothiocyanate (FITC)-conjugated anti-mouse antibody or Cy3-conjugated anti-mouse antibody (diluted 1:600; Sigma). Dual-color detection was performed by confocal laser scan microscopy after treatment with 4′,6-diamidino-2-phenylindole (DAPI) to label nuclear DNA.
Immunoprecipitation and Western blotting analysis
Tissues and cells were collected and lysed as described previously [19]. For immunoprecipitation, cell lysates containing 1.5 mg total protein (quantified by BCA protein assay; Pierce, Rockford, IL) were incubated with anti-c-Myc (9E10) antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 4 h at 4°C, followed by protein A-Sepharose beads (Pierce) for 2 h at 4°C. Lysates and precipitates were resolved by 10% SDS-PAGE, transferred to nitrocellulose membranes (Amersham Biosciences, Uppsala, Sweden) and probed with anti-c-Myc (9E10) and anti-phospho Akt substrate antibody (Cell Signaling Technology, Irvine, CA).
For Western blotting, 50 μg total protein from tissue or cell extracts was subjected to SDS-PAGE, blotted and probed with anti-Akt, anti-phospho-Akt, anti-PTEN (Cell Signaling), anti-NDRG2 (Abnova, Taiwan), anti-phospho-NDRG2 (Kinasource, Scotland), anti-caspase 3 active (Sigma), anti-α-tubulin, or anti-β-actin antibody (Boster), alone or in combination. Secondary antibodies conjugated to IRDye™800 (1:20,000 dilution; Rockland Inc., IL, USA) were detected by an Odyssey infrared imaging system (LI-COR Inc., Lincoln, NE).
Treatment of cells with FFA
Lipid-containing media were prepared by conjugation of saturated palmitic acid (Sigma) as the FFA with BSA using a modification of a previously published method [20]. After preincubation overnight in the presence of 5.5 mM glucose and 10% fetal bovine serum, cells were washed three times with PBS and exposed to FFA (0–1.0 mM) for 72 h to detect insulin secretion by ELISA assay and cell viability by MTT assay. Equivalent amounts of fatty acid-free BSA were added to the control group.
Evaluation of insulin secretion
β-TC3 cells were seeded into 96-well plates and incubated with FFA (0–1.0 mM) before glucose-stimulated insulin secretion (GSIS) studies. The treated cells were washed three times and preincubated for 1 h in glucose-free Krebs-Ringer HEPES buffer [21] in a 37°C humidified incubator. Subsequently, cells were incubated in the same buffer containing 5.5 or 25 mM glucose for 1 h, and the incubation media were collected to measure secreted insulin. The insulin concentration in the supernatant was determined by ELISA using commercially available kits according to the manufacturer’s instructions (Linco, Charles, MO). The number of live cells in each well was estimated using resazurin (Sigma) [22]. The insulin secretion was expressed in terms of the number of live cells in each well at the end of the experiment.
Treatment of cells with small interfering RNA (siRNA) for NDRG2
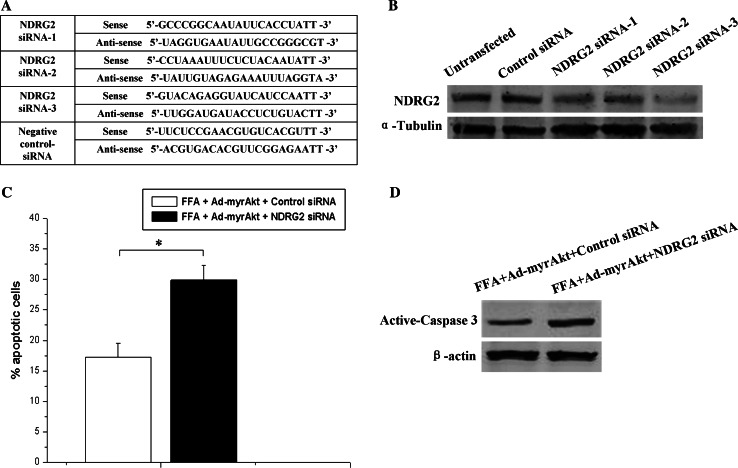
All siRNA oligonucleotides were purchased from GenePharma Company Ltd. (Shanghai, China). The sequences of all specific siRNAs targeted to mouse NDRG2 are shown in Fig. 7a. The siRNAs were transfected into cells with Lipofectamine 2000 (Invitrogen). A pilot study with the control fluorescent dye labeled siRNA demonstrated that 90–95% of the cells were transfected, as detected by confocal fluorescence microscopy (Supplemental Fig. 2).
Fig. 7.
Decreased NDRG2 expression attenuates the Akt-mediated anti-apoptosis effect in β-TC3 cells exposed to a high concentration of palmitic acid. a The siRNA sequences used in this experiment. b β-TC3 cells were transfected with 60 nM of three different siRNAs targeting NDRG2 for 48 h, after which samples were collected for Western blotting with antibodies against NDRG2 and α-tubulin. c β-TC3 cells were infected with Ad-myristoylated Aktα for 48 h and transfected with 60 nM NDRG2 siRNA3 or control siRNA and then incubated with 1.0 mM palmitic acid complexed to 1% BSA for 72 h. The apoptotic cells were quantified by flow cytometry. d Western blotting was performed using antibodies against active caspase 3 and β-actin (*P < 0.05 compared to the control)
MTT assay
Cells were seeded into 96-well plates in triplicate at a starting density of 1 × 104 cells well−1 and treated with FFA. Treated cells were washed and incubated with the tetrazolium salt (MTT, 100 μg ml−1; Sigma) at 37°C for 4 h. The supernatant was removed, and 150 μl dimethyl sulfoxide (DMSO) was added to each well. The absorbance (OD) of the reaction solution at 490 nm was recorded.
Detection of apoptosis
Flow cytometry analysis was used to detect apoptosis. Cells were collected and washed with PBS. Cell death was measured using two-color analysis of fluorescein isothiocyanate-labeled annexin V (Roche Applied Science) binding and propidium iodide (PI) uptake using a Becton–Dickinson fluorescence-activated cell sorter (FACS) apparatus.
Statistical analysis
Statistical analysis was performed with SPSS software (version 10.0; SPSS, Chicago, IL) using the Student’s t test. Results are presented as the mean ± SEM from at least three independent experiments unless otherwise stated. Statistical significance was defined as P < 0.05, and histograms were prepared with Origin 6.0 (Microcal Software, Inc., Northampton, MA).
Results
NDRG2 is highly expressed in the cytoplasm of pancreatic β cells
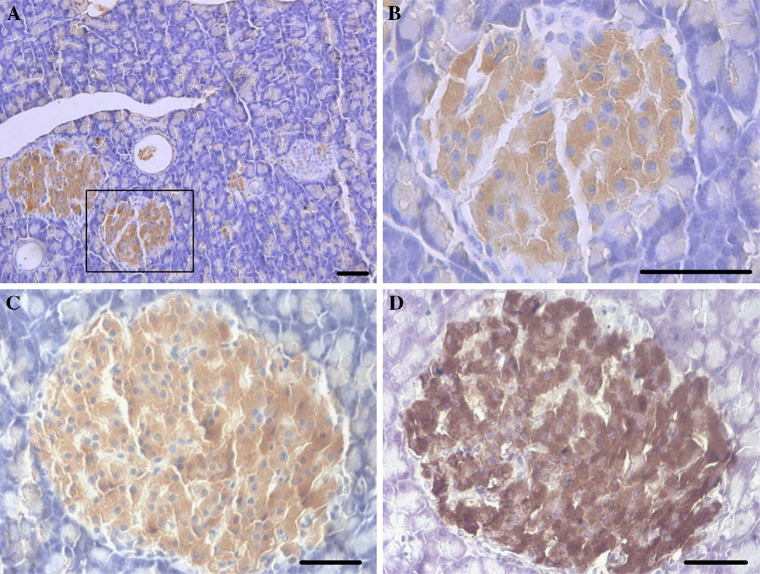
Immunohistochemistry was performed to examine the tissue and cellular distribution of NDRG2 in the adult mouse pancreas. Consistent with our previous study [10], NDRG2 immunoreactivity was confined to pancreatic islets, with a weaker signal in pancreatic acinar tissues (Fig. 1a, b). Furthermore, significant staining of NDRG2 (Fig. 1c) overlapped with insulin staining (Fig. 1d) in the adult mouse pancreas. NDRG2 immunoreactivity was also localized to adult human pancreatic islets and showed strong similarity to insulin immunolocalization (data not shown). A negative control of nonimmune serum in the absence of the primary antibody abolished immunostaining in all tissues examined.
Fig. 1.
NDRG2 is present in pancreatic islets. a and b Immunohistochemistry of mouse pancreatic tissues showing NDRG2 staining; rectangles indicate areas shown at higher magnification. b In the next micrograph (scale bars 50 μm). c and d Immunohistochemical localization of NDRG2 (c) and insulin (d) in adult mouse pancreatic tissues, showing expression in the same cells in pancreatic islet (scale bars 50 μm)
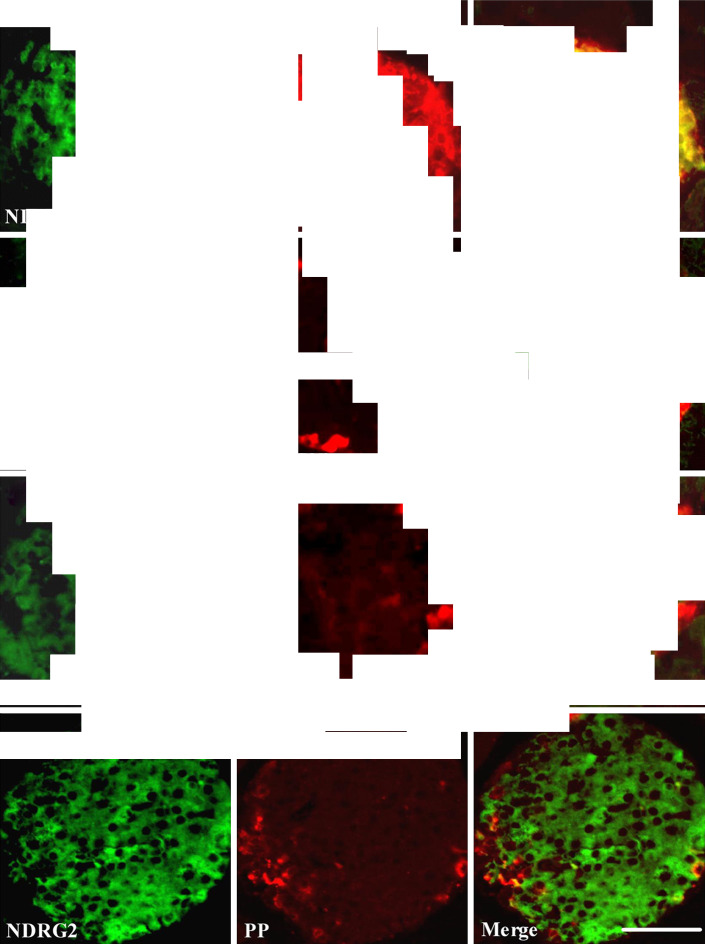
To examine the cellular distribution of NDRG2, we performed double-labeling immunofluorescence histochemistry on mouse pancreatic islets. NDRG2 immunoreactivity was detected in insulin-containing cells (Fig. 2a), but not in glucagon- (Fig. 2b), somatostatin- (Fig. 2c), and pancreatic polypeptide-containing cells (Fig. 2d). Immunostaining of serial sections of the islet with anti-NDRG2, anti-insulin, anti-glucagon, anti-somatostatin, and anti-pancreatic polypeptide antibodies revealed that NDRG2 exhibited high expression in pancreatic β cells, but low expression in pancreatic α cells and δ cells, and moderate expression in PP cells (Fig. 2).
Fig. 2.
NDRG2 is expressed specifically in the cytoplasm of pancreatic β cells. Paraffin sections of pancreatic islets were stained with anti-NDRG2, anti-insulin, anti-glucagon, anti-somatostatin, and anti-pancreatic polypeptide antibodies, and visualized using either FITC (green) or Cy3 (red). Confocal images show that NDRG2 immunoreactivity is present in insulin-containing cells (a), but not in glucagon-containing (b), somatostatin-containing (c), or pancreatic polypeptide-containing islet cells (d). Colocalization of NDRG2 immunoreactivity (green) with insulin (red) is indicated by yellow color in the overlay image (scale bars 50 μm)
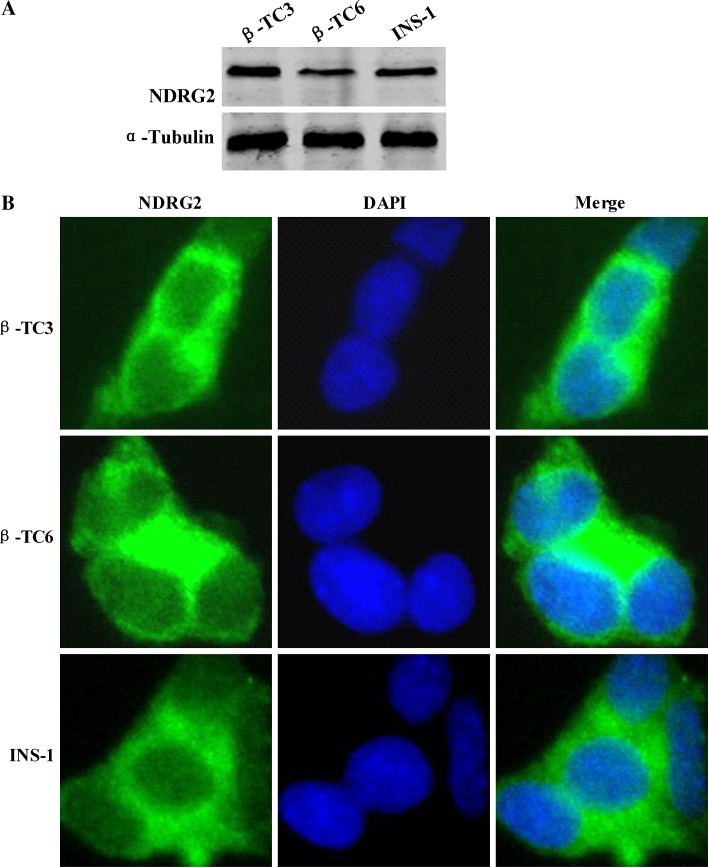
We further examined the expression and distribution of NDRG2 in cultured insulinoma cell lines including mouse β-TC3 cells, mouse β-TC6 cells, and rat INS-1 cells. Western blotting was performed to detect endogenous NDRG2 expression in β-TC3, β-TC6, and INS-1 cells, all of which supported the evidence from the tissue studies showing that NDRG2 is highly expressed in the pancreatic β cell (Fig. 3a). The subcellular localization of NDRG2 was determined by immunofluorescent staining. Dual-color detection of NDRG2 and DAPI demonstrated that NDRG2 was localized mainly in the cytoplasm of pancreatic β cells (Fig. 3b) and remained in the cytoplasm during apoptosis (Supplemental Fig. 3).
Fig. 3.
NDRG2 is highly expressed in the cytoplasm of insulinoma cell lines. a Whole cell protein samples from INS-1, β-TC3, and β-TC6 cells were examined for NDRG2 expression by Western blotting analysis. b Immunocytochemical staining of INS-1, β-TC3, and β-TC6 cells by using NDRG2 antibody and FITC-conjugated secondary antibody shows the localization of NDRG2 (green). DAPI staining (blue) reveals the cell nuclei. Magnification 400×
NDRG2 is a substrate of protein kinase Akt
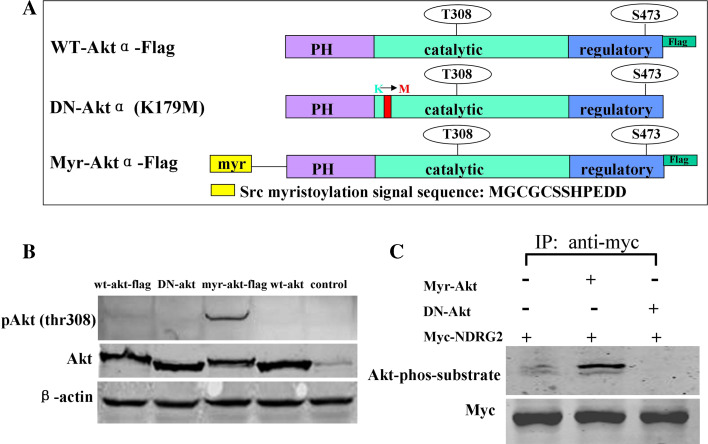
The presence of NDRG2 in pancreatic β-cell and insulinoma cell lines suggests that NDRG2 has an important role in the biological function of these cells. It is reported that Akt mediates insulin-stimulated phosphorylation of NDRG2 [15]. Meanwhile, insulin/IGF/Akt signaling is involved in β-cell survival and ER stress-induced apoptosis [23]. We further identified NDRG2 as a new substrate of Akt and investigated the function of this pathway in β cells. Constitutively active Aktα containing a myristoylation signal (myr-Aktα; Fig. 4a) or a dominant negative Akt (K179M; DN-Akt) was co-overexpressed with Myc-tagged NDRG2 in HEK293 cells. NDRG2 was immunoprecipitated and immunoblotted with Myc antibody and phospho-(Ser/Thr) Akt substrate antibody. Compared with endogenous Aktα, overexpression of constitutively active Aktα increased phosphorylation of NDRG2, whereas overexpression of inactive Akt had little effect (Fig. 4b, c).
Fig. 4.
Constitutive activation of Aktα promotes NDRG2 phosphorylation. a Schematic representation of the various Aktα vectors showing the structures of wild-type Aktα, DN-Aktα, and myr-Aktα. b HEK293 cells were transiently transfected with constructs encoding FLAG-tagged wild-type Aktα, DN-Aktα (K179M), myr-Aktα, and wild-type Aktα. Cell lysates were separated by SDS-PAGE and subjected to Western blotting prior to detection with antibodies against Akt, phospho-Akt (Thr308), or β-actin. c HEK293 cells were transiently transfected with a cDNA construct encoding Myc-tagged NDRG2, with either myr-Aktα or DN-Aktα as indicated. NDRG2 was immunoprecipitated and immunoblotted with phospho-(Ser/Thr) Akt substrate antibody as described in the “Materials and methods” section
Long-term exposure to palmitic acid decreases β-TC3 cells viability and Akt-NDRG2 phosphorylation
It is well known that chronic hyperlipidemia contributes to β-cell apoptosis, reduction in insulin secretory capacity, and strikingly similar gene expression patterns [24]. Furthermore, FFA induced pancreatic β-cell apoptosis by ER stress [25, 26], accompanyied with the reduction of Akt phosphorylation [23]. However, it is not known whether such changes also include NDRG2 expression and phosphorylation, which might be involved in β-cell survival and apoptosis under stress conditions. Therefore, we investigated the effect of FFA on the expression and phosphorylation of Akt and NDRG2 in β-TC3 cells.
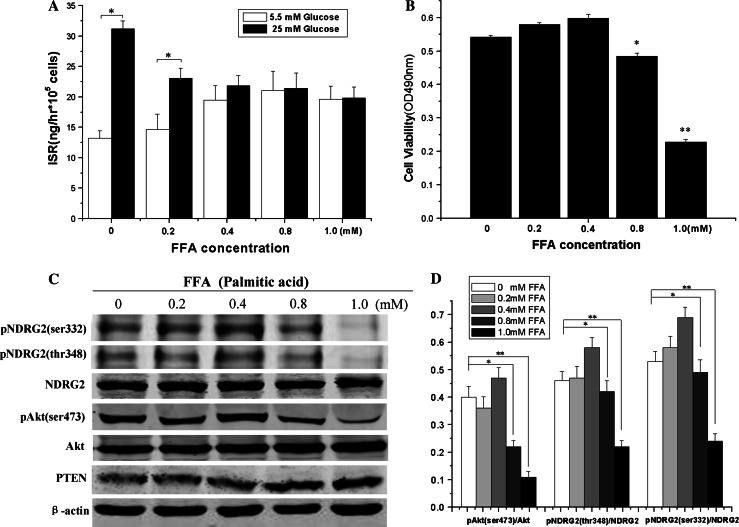
After β-TC3 cells were incubated with FFA (palmitic acid) at the indicated concentration for 72 h, GSIS decreased in a FFA concentration-dependent manner (Fig. 5a). However, cell viability decreased significantly in β-TC3 cells exposed to 0.8 and 1.0 mM palmitic acid (Fig. 5b). Moreover, Western blotting showed that prolonged exposure of β-TC3 cells to palmitic acid at high concentrations (0.8 and 1.0 mM) elevated the expression of PTEN (phosphatase and tensin homolog), and decreased Akt phosphorylation at Ser-473 and NDRG2 phosphorylation at both Ser-332 and Thr-348 (Fig. 5c, d). In addition, the Akt phosphorylation at Ser-473 began to decrease in β-TC3 cells exposed to 0.5 mM palmitic acid concentration (Supplemental Fig. 4). There results suggest that prolonged exposure of β-TC3 cells to high levels of FFA reduces both cell viability and phosphorylation of Akt and NDRG2.
Fig. 5.
Prolonged exposure to high concentrations of palmitic acid induces β-cell dysfunction and decreases Akt–NDRG2 phosphorylation. β-TC3 cells were incubated with either 1% BSA or the indicated concentration of palmitic acid complexed to 1% BSA for 72 h. a and b GSIS was measured by ELISA assay, and cell viability was measured by MTT assay. c Western blot results of the effect on members of the PTEN-Akt-NDRG2 signaling pathway in β-TC3 cells chronically exposed to high levels of palmitic acid. β-TC3 cells were incubated with the indicated concentration palmitic acid complexed to 1% BSA for 72 h, and cell lysates were immunoblotted with antibodies against PTEN, Akt, phospho-Akt (Ser473), NDRG2, phospho-NDRG2 (Ser332), phosphor-NDRG2 (Thr348), and β-actin. d The histograms of the quantitative analysis of Western blot results showing the relative density of phospho-Akt (Ser473) compared to Akt, and either phospho-NDRG2 (Ser332) or phospho-NDRG2 (Thr348) compared to NDRG2 in β-TC3 cells exposed to the indicated concentration of palmitic acid complexed to 1% BSA. Data are shown as the means ± SEM from at least three independent experiments (*P < 0.05, **P < 0.01 compared to the control)
Akt–NDRG2 pathway activation inhibits palmitic acid-induced β-TC3 cell apoptosis
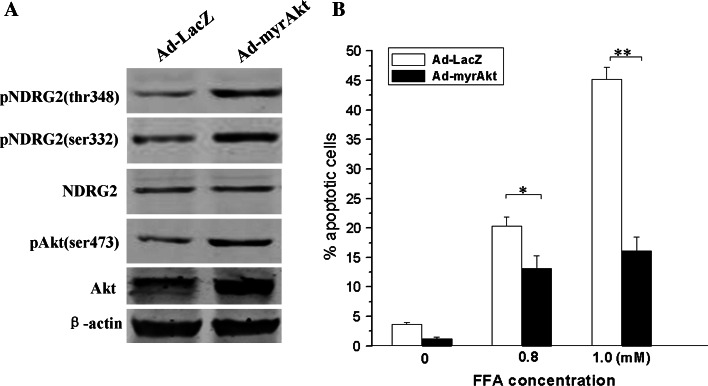
To further investigate the functional role of Akt and NDRG2 in pancreatic β cells under lipotoxic conditions, we examined the effect of β-TC3 cells infected with adenovirus encoding myristoylated Aktα or LacZ control in response to high concentrations of FFA (0.8 and 1.0 mM palmitic acid) for 72 h. In agreement with in vitro experiments, overexpression of constitutively active Aktα using 6.25 multiplicities of infection (MOI) adenovirus enhanced NDRG2 phosphorylation at Ser-332 and Thr-348 in β-TC3 cells (Fig. 6a). Furthermore, cell apoptosis triggered by high concentrations of FFA was markedly attenuated when Akt and NDRG2 were activated by overexpression of myr-Aktα (Fig. 6b).
Fig. 6.
Akt-NDRG2 activation abolishes lipoapoptosis induced by high levels of palmitic acid in β-TC3 cells. a The cells were infected with Ad-myristoylated Aktα or Ad-LacZ for 48 h and samples collected for Western blotting with antibodies against Akt, phospho-Akt (Thr308), NDRG2, phospho-NDRG2 (Ser332), phospho-NDRG2 (Thr348), and β-actin. b The infected cells were incubated with either 1% BSA or indicated concentrations of palmitic acid complexed to 1% BSA for 72 h, then the apoptotic cells were quantified by flow cytometry. Data are shown as the means ± SEM from at least three independent experiments (*P < 0.05, **P < 0.01 compared to the control)
NDRG2 knock-down attenuates Akt-mediated protection of β-TC3 cell against lipoapoptosis induced by palmitic acid
To investigate further whether NDRG2 is involved in Akt-mediated cell protection against lipotoxicity, we examined the anti-apoptotic effect of Akt-protection against lipotoxicity when NDRG2 expression was knocked down. As the half-life of NDRG2 protein in β-TC3 cells was less than 8 h (Supplemental Fig. 5), we examined the effect of NDRG2 expression in β-TC3 cells after transfection with siRNAs against NDRG2 for 48 h (Fig. 7a). Western blotting showed that NDRG2 siRNAs (siRNA1, siRNA2, and siRNA3) suppressed endogenous NDRG2 expression; siRNA3 was especially effective at reducing NDRG2 expression in β-TC3 cells (Fig. 7b). Furthermore, when cells were infected with Ad-myr Akt and treated with 1.0 mM palmitic acid, NDRG2 knock-down increased the apoptosis of β-TC3 cells (Fig. 7c, d; Supplemental Fig. 6).
Our present study indicates that NDRG2 acts as the substrate of Akt and is involved in protection of β cells against damage induced by lipotoxicity.
Discussion
NDRG2 plays a variety of roles in cell proliferation and differentiation [27], stress response [28], and p53- and HIF-1-mediated apoptosis [28, 29]. In our earlier observations of NDRG2 expression in mouse adult tissues by immunohistochemistry assay, strong NDRG2 immunoreactions were detected in pancreatic islets [10]. In this study, we expanded earlier observations of the specific expression of NDRG2 in pancreatic β cells and investigated its biological functions. Our results showed that NDRG2 expression was strong in the cytoplasm of pancreatic β cells but weak to moderate in α cells, δ cells, or pancreatic polypeptide (PP) cells. Although pancreatic β cells are only a small fraction of cells in the whole pancreas, strong NDRG2 expression in these cells suggests a potential role in pancreatic β-cell function. The moderate NDRG2 immunostaining in PP cells demonstrates the need for further exploration of the potential biological functions of NDRG2 in PP cells.
Chronic hyperlipidemia presents in some adolescents with type 2 diabetes and exerts deleterious effects on pancreatic β cells [30–32]. Contrary to the acute effects of FFA on β cells (Supplemental Fig. 7), long-term exposure of β cells to high levels of FFA inhibits insulin secretion and induces β-cell apoptosis. However, overexpressing the constitutively active form of Akt attenuates the apoptosis caused by high levels of palmitic acid [14]. We further observed that prolonged exposure to high levels of FFA impaired β-cell viability, accompanying the decrease of Akt and NDRG2 phosphorylation. Furthermore, overexpression of myristoylated Aktα enhanced Akt and NDRG2 phosphorylation and weakened the lipoapoptosis effect caused by prolonged exposure to high levels of FFA, whereas NDRG2 knock-down attenuated Akt-mediated protection of β-TC3 cell against lipoapoptosis induced by palmitic acid. Our study here provides evidence that NDRG2 is an Akt substrate and is probably involved in Akt-mediated protection of β cells against FFA-induced lipoapoptosis.
The mechanisms of FFA-induced lipoapoptosis are still under investigation. Many kinases and their related signal pathways are reported to play important roles in β-cell function and lipoapoptosis. Activation of the ERK-MAPK pathway increased the expression of early growth response gene-1 and reduced β-cell lipoapoptosis [33]. PKC contributed to hepatocyte lipoapoptosis by destabilization of MCA-1, which is an anti-apoptotic member of the Bcl-2 family [34]. Many different proteins are expressed in endocrine β cells, such as pancreatic and duodenal homeobox-1 (PDX-1), sterol regulatory element-binding proteins (SREBP-1), and activin receptor-like kinase 7 [24, 35, 36]. Their biological functions are related to the synthesis of insulin, endoplasmic reticulum stress, and pancreatic β-cell survival. Our experiments suggested that NDRG2 is highly expressed in pancreatic β cells and involved in protein kinase Akt-mediated repression of lipotoxicity possibly through the fatty acid esterification or endoplasmic reticulum stress [23].
NDRG2 is regarded as a putative tumor suppressor in human cancer, and RNAi-mediated inhibition of NDRG2 increases cell proliferation in cancer cell lines [37]. However, knock-down of NDRG2 resulted in reduced myoblast proliferation and an earlier onset of myogenesis [9]. In addition, decreased levels of NDRG1 resulted in the inhibition of trophoblast cell viability and the induction of apoptosis during stress conditions [38]. Therefore, lack of NDRG2 may affect cell apoptosis under specific stress conditions [9]. Consistent with the results in myoblasts, we observed that knock-down of NDRG2 attenuated Akt-mediated protection of β cells against lipotoxicity and affected cell apoptosis under oxidative stress-mediated lipotoxicity.
In summary, this is the first study demonstrating that NDRG2, highly expressed in pancreatic β cells, has a role in their survival. Prolonged exposure to high levels of FFA will decrease insulin release and cell viability of β-TC3 cells, along with the decline of Akt-NDRG2 phosphorylation. Furthermore, overexpression of constitutive Aktα can enhance NDRG2 phosphorylation and abolish apoptosis as the result of lipotoxicity. In addition, NDRG2 knock-down weakens the Akt-dependent protection against lipotoxicity in β-TC3 cells. These findings contribute insights into NDRG2 biological functions in pancreatic β cells.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1. Fluorescence microscopy shows the infection efficiency in β-TC3 cells infected with different MOI of Ad-EGFP for 48 h (JPG 399 kb)
Supplemental Figure 2. Fluorescence microscopy shows the transfection efficiency in β-TC3 cells transfected with fluorescent dye-labeled siRNAs for 12 h (JPG 196 kb)
Supplemental Figure 3. Immunocytochemical staining of apoptotic INS-1, β-TC3 and β-TC6 cells using NDRG2 antibody and Cy3-conjugated secondary antibody shows the localization of NDRG2 (red). DAPI staining (blue) reveals the position and morphology of the cell nuclei. Magnification 400x (JPG 318 kb)
Supplemental Figure 4. Western blotting shows the expression and phosphorylation of Akt in β-TC3 cells chronically exposed to different high palmitic acid concentrations. Cells were incubated with either 1% BSA or the indicated concentration of palmitic acid complexed to 1% BSA for 72 h, and cell lysates separated by SDS-PAGE and subjected to Western blotting prior to detection with antibodies against Akt, phospho-Akt (Ser473) and β-actin. A histogram of the quantitative analysis of Western blot results shows a significant decrease of relative density of phospho-Akt (Ser473) compared to Akt in β-TC3 cells exposed to 0.5 mM palmitic acid complexed to 1% BSA (*P < 0.05 compared to the control) (JPG 216 kb)
Supplemental Figure 5. The half-life of NDRG2 protein in β-TC3 cells treated with cycloheximide (CHX) for 2 h, 4 h, 8 h or 24 h was assessed by Western blotting (JPG 136 kb)
Supplemental Figure 6. The cells were infected with Ad-myristoylated Aktα for 48 h and transfected with 60 nM NDRG2 siRNA or control siRNA and then incubated with 1 mM palmitic acid complexed to 1% BSA for 72 h prior to detection of apoptotic cells by flow cytometry (JPG 306 kb)
Supplemental Figure 7. β-TC3 cells were incubated with 1 mM palmitic acid complexed to 1% BSA for the indicated times and GSIS was measured by ELISA assay (JPG 175 kb)
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (nos. 30600314, 30670303, 30830054, 30801309 and 30170465). We thank members of our laboratory for helpful discussions.
Footnotes
L. Shen, X. Liu and W. Hou contributed equally to this work.
References
- 1.Lachat P, Shaw P, Gebhard S, van Belzen N, Chaubert P, Bosman FT. Expression of NDRG1, a differentiation-related gene, in human tissues. Histochem Cell Biol. 2002;118:399–408. doi: 10.1007/s00418-002-0460-9. [DOI] [PubMed] [Google Scholar]
- 2.Ohki T, Hongo S, Nakada N, Maeda A, Takeda M. Inhibition of neurite outgrowth by reduced level of NDRG4 protein in antisense transfected PC12 cells. Brain Res Dev Brain Res. 2002;135:55–63. doi: 10.1016/S0165-3806(02)00300-0. [DOI] [PubMed] [Google Scholar]
- 3.Deng YC, Yao LB, Liu XP, Nie XY, Wang JC, Zhang XG, Su CZ. Exploring a new gene containing ACP like domain in human brain and expression it in E. coli . Prog Bichem Biophys. 2001;28:72–76. [Google Scholar]
- 4.Shen L, Zhao ZY, Wang YZ, Ji SP, Liu XW, Che HL, Lin W, Li X, Zhang J, Yao LB. Immunohistochemical detection of Ndrg2 in the mouse nervous system. Neuroreport. 2008;19:927–931. doi: 10.1097/WNR.0b013e32830163d0. [DOI] [PubMed] [Google Scholar]
- 5.Deng Y, Yao L, Chau L, Ng SS, Peng Y, Liu X, Au WS, Wang J, Li F, Ji S, Han H, Nie X, Li Q, Kung HF, Leung SY, Lin MC. N-Myc downstream-regulated gene 2 (NDRG2) inhibits glioblastoma cell proliferation. Int J Cancer. 2003;106:342–347. doi: 10.1002/ijc.11228. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K, Yamada M, Ohata H, Honda K, Yamada M. Ndrg2 promotes neurite outgrowth of NGF-differentiated PC12 cells. Neurosci Lett. 2005;388:157–162. doi: 10.1016/j.neulet.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 7.Mitchelmore C, Buchmann-Moller S, Rask L, West MJ, Troncoso JC, Jensen NA. NDRG2: a novel Alzheimer’s disease associated protein. Neurobiol Dis. 2004;16:48–58. doi: 10.1016/j.nbd.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Wielpütz MO, Lee IH, Dinudom A, Boulkroun S, Farman N, Cook DI, Korbmacher C, Rauh R. (NDRG2) stimulates amiloride-sensitive Na+ currents in Xenopus laevis oocytes and Fisher rat thyroid cells. J Biol Chem. 2007;282:28264–28273. doi: 10.1074/jbc.M702168200. [DOI] [PubMed] [Google Scholar]
- 9.Foletta VC, Prior MJ, Stupka N, Carey K, Segal DH, Jones S, Swinton C, Martin S, Cameron-Smith D, Walder KR. NDRG2, a novel regulator myoblast proliferation, is regulated by anabolic and catabolic factors. J Physiol. 2009;587:1619–1634. doi: 10.1113/jphysiol.2008.167882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu XL, Liu XP, Deng YC, Lin SX, Wu L, Zhang J, Wang LF, Wang XB, Li X, Shen L, Zhang YQ, Yao LB. Expression analysis of the NDRG2 gene in mouse embryonic and adult tissues. Cell Tissue Res. 2006;325:67–76. doi: 10.1007/s00441-005-0137-5. [DOI] [PubMed] [Google Scholar]
- 11.Qader SS, Salehi A, Hakanson R, Lundquist I, Ekelund M. Long-term infusion of nutrients (total parenteral nutrition) suppresses circulating ghrelin in food deprived rats. Regul Pept. 2005;131:82–88. doi: 10.1016/j.regpep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Qader SS, Jimenez-Feltstrom J, Ekelund M, Lundquist I, Salehi A. Expression of islet inducible nitric oxide synthase and inhibition of glucose-stimulated insulin release after long-term lipid infusion in the rat is counteracted by PACAP27. Am J Physiol Endocrinol Metab. 2007;292:E1447–E1455. doi: 10.1152/ajpendo.00172.2006. [DOI] [PubMed] [Google Scholar]
- 13.Aikin R, Hanley S, Maysinger D, Lipsett M, Castellarin M, Paraskevas S, Rosenberg L. Auticrine insulin action activates Akt and increases survival of isolated human islets. Diabetologia. 2006;49:2900–2909. doi: 10.1007/s00125-006-0476-0. [DOI] [PubMed] [Google Scholar]
- 14.Buteau J, El-Assaad W, Rhodes CJ, Rosenberg L, Joly E, Prentki M. Glucagon-like peptide-1 prevents beta cell glucolipotoxicity. Diabetologia. 2004;47:806–815. doi: 10.1007/s00125-004-1379-6. [DOI] [PubMed] [Google Scholar]
- 15.Burchfield JG, Lennard AJ, Narasimhan S, Hughes WE, Wasinger VC, Corthals GL, Okuda T, Kondoh H, Biden TJ, Schmitz-Peiffer C. Akt mediates insulin-stimulated phosphorylation of Ndrg2: evidence for cross-talk with protein kinase C theta. J Biol Chem. 2004;279:18623–18632. doi: 10.1074/jbc.M401504200. [DOI] [PubMed] [Google Scholar]
- 16.Han Z, Hong L, Han Y, Wu K, Han S, Shen H, Li C, Yao L, Qiao T, Fan D. Phospho Akt mediates multidrug resistance of gastric cancer cells through regulation of P-gp, Bcl-2 and Bax. J Exp Clin Cancer Res. 2007;26:261–268. [PubMed] [Google Scholar]
- 17.Hu XL, Yao LB, Zhang YQ, Deng YC, Liu XP. Distribution characteristic of NDRG2 expression in human fetal tissues. Sheng Li Xue Bao. 2006;58:331–336. [PubMed] [Google Scholar]
- 18.Liu X, Hu X, Zhang J, Wang L, Zhang W, Liu X, Li F, Zhang Y, Yao L. Preparation and application of monoclonal antibody against hNDRG2. Appl Biochem Biotechnol. 2009;152:306–315. doi: 10.1007/s12010-008-8267-3. [DOI] [PubMed] [Google Scholar]
- 19.Zhou P, Fernandes N, Dodge IL, Reddi AL, Rao N, Safran H, DiPetrillo TA, Wazer DE, Band V, Band H. ErbB2 degradation mediated by the co-chaperone protein CHIP. J Biol Chem. 2003;278:13829–13837. doi: 10.1074/jbc.M209640200. [DOI] [PubMed] [Google Scholar]
- 20.Wang XL, Zhang L, Youker K, Zhang MX, Wang J, LeMaire SA, Coselli JS, Shen YH. Free fatty acid inhibit insulin signaling-stimulated endothelial nitric oxide synthase activation through upregulating PTEN or inhibiting Akt kinase. Diabetes. 2006;55:2301–2310. doi: 10.2337/db05-1574. [DOI] [PubMed] [Google Scholar]
- 21.Dai FF, Zhang Y, Kang Y, Wang Q, Gaisano HY, Braunewell KH, Chan CB, Wheeler MB. The neuronal Ca2+ sensor protein visinin-like protein-1 is expressed in pancreatic islets and regulates insulin secretion. J Biol Chem. 2006;281:21942–21953. doi: 10.1074/jbc.M512924200. [DOI] [PubMed] [Google Scholar]
- 22.O′Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasan S, Ohsugi M, Liu Z, Fatrai S, Bernal-Mizrachi E, Permutt MA. Endoplasmic reticulum stress-induced apoptosis is partly mediated by reduced insulin signaling through phosphatidylinositol 3-kinase/Akt and increased glycogen synthase kinase-3β in mouse insulinoma cells. Diabetes. 2005;54:968–975. doi: 10.2337/diabetes.54.4.968. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Kouri G, Wollheim CB. ER stress and SREBP-1 activation are implicated in β-cell glucolipotoxicity. J Cell Sci. 2005;118:3905–3915. doi: 10.1242/jcs.02513. [DOI] [PubMed] [Google Scholar]
- 25.Kharroubi I, Ladrière L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-κB and endoplasmic reticulum stress. Endocrinology. 2004;145:5087–5096. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- 26.Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752–763. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- 27.Yao L, Zhang J, Liu X. NDRG2: a Myc-repressed gene involved in cancer and cell stress. Acta Biochim Biophys Sin. 2008;40:625–635. doi: 10.1111/j.1745-7270.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Liu N, Yao L, Li F, Zhang J, Deng Y, Liu J, Ji S, Yang A, Han H, Zhang Y, Zhang J, Han W, Liu X. NDRG2 is a new HIF-1 target gene necessary for hypoxia-induced apoptosis in A549 cells. Cell Physiol Biochem. 2008;21:239–250. doi: 10.1159/000113765. [DOI] [PubMed] [Google Scholar]
- 29.Liu N, Wang L, Li X, Yang Q, Liu X, Zhang J, Zhang J, Wu Y, Ji S, Zhang Y, Yang A, Han H, Yao L. N-Myc downstream-regulated gene 2 is involved in p53-mediated apoptosis. Nucleic Acids Res. 2008;36:5335–5349. doi: 10.1093/nar/gkn504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poitout V, Robertson RP. Minireview: secondary beta-cell failure in type 2 diabetes-a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002;143:339–342. doi: 10.1210/en.143.2.339. [DOI] [PubMed] [Google Scholar]
- 31.Jeffrey KD, Alejandro EU, Luciani DS, Kalynyak TB, Hu X, Li H, Lin Y, Townsend RR, Polonsky KS, Johnson JD. Carboxypeptidase E mediates palmitate-induced beta-cell ER stress and apoptosis. Proc Natl Acad Sci USA. 2008;105:8452–8457. doi: 10.1073/pnas.0711232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinhas-Hamiel O, Zeitler P. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet. 2007;369:1823–1831. doi: 10.1016/S0140-6736(07)60821-6. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Xu M, Zhang S, Yan L, Yang G, Lu W, Li Y, Cheng H. The role of G protein-couple receptor 40 in lipoapoptosis in mouse beta-cell line NIT-1. J Mol Endocrinol. 2007;38:651–661. doi: 10.1677/JME-06-0048. [DOI] [PubMed] [Google Scholar]
- 34.Masuoka HC, Mott J, Bronk SF, Werneburg NW, Akazawa Y, Kaufmann SH, Gores GJ. Mcl-1 degradation during hepatocyte lipoapoptosis. J Biol Chem. 2009;284:30039–30048. doi: 10.1074/jbc.M109.039545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melloul D, Marshak S, Cerasi E. Regulation of insulin gene transcription. Diabetologia. 2002;45:309–336. doi: 10.1007/s00125-001-0728-y. [DOI] [PubMed] [Google Scholar]
- 36.Zhang N, Kumar M, Xu G, Ju W, Yoon T, Xu E, Huang X, Gaisano H, Peng C, Wang Q. Activin receptor-like kinase 7 induces apoptosis of pancreatic beta cells and beta cell lines. Diabetologia. 2006;49:506–518. doi: 10.1007/s00125-005-0095-1. [DOI] [PubMed] [Google Scholar]
- 37.Choi SC, Yoon SR, Park YP, Song EY, Kim JW, Kim WH, Yang Y, Lim JS, Lee HG. Expression of NDRG2 is related to tumor progression and survival of gastric cancer patients through Fas-mediated cell death. Exp Mol Med. 2007;39:705–714. doi: 10.1038/emm.2007.77. [DOI] [PubMed] [Google Scholar]
- 38.Chen B, Nelson DM, Sadovsky Y. N-myc down-regulated gene 1 modulates the response of term human trophoblasts to hypoxic injury. J Biol Chem. 2006;281:2764–2772. doi: 10.1074/jbc.M507330200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Fluorescence microscopy shows the infection efficiency in β-TC3 cells infected with different MOI of Ad-EGFP for 48 h (JPG 399 kb)
Supplemental Figure 2. Fluorescence microscopy shows the transfection efficiency in β-TC3 cells transfected with fluorescent dye-labeled siRNAs for 12 h (JPG 196 kb)
Supplemental Figure 3. Immunocytochemical staining of apoptotic INS-1, β-TC3 and β-TC6 cells using NDRG2 antibody and Cy3-conjugated secondary antibody shows the localization of NDRG2 (red). DAPI staining (blue) reveals the position and morphology of the cell nuclei. Magnification 400x (JPG 318 kb)
Supplemental Figure 4. Western blotting shows the expression and phosphorylation of Akt in β-TC3 cells chronically exposed to different high palmitic acid concentrations. Cells were incubated with either 1% BSA or the indicated concentration of palmitic acid complexed to 1% BSA for 72 h, and cell lysates separated by SDS-PAGE and subjected to Western blotting prior to detection with antibodies against Akt, phospho-Akt (Ser473) and β-actin. A histogram of the quantitative analysis of Western blot results shows a significant decrease of relative density of phospho-Akt (Ser473) compared to Akt in β-TC3 cells exposed to 0.5 mM palmitic acid complexed to 1% BSA (*P < 0.05 compared to the control) (JPG 216 kb)
Supplemental Figure 5. The half-life of NDRG2 protein in β-TC3 cells treated with cycloheximide (CHX) for 2 h, 4 h, 8 h or 24 h was assessed by Western blotting (JPG 136 kb)
Supplemental Figure 6. The cells were infected with Ad-myristoylated Aktα for 48 h and transfected with 60 nM NDRG2 siRNA or control siRNA and then incubated with 1 mM palmitic acid complexed to 1% BSA for 72 h prior to detection of apoptotic cells by flow cytometry (JPG 306 kb)
Supplemental Figure 7. β-TC3 cells were incubated with 1 mM palmitic acid complexed to 1% BSA for the indicated times and GSIS was measured by ELISA assay (JPG 175 kb)