Abstract
Hypoxia-inducible factor-1α (HIF-1α) protein is degraded under normoxia by its association to von Hippel-Lindau protein (pVHL) and further proteasomal digestion. However, human renal cells HK-2 treated with 15-deoxy-Δ12,14-prostaglandin-J2 (15d-PGJ2) accumulate HIF-1α in normoxic conditions. Thus, we aimed to investigate the mechanism involved in this accumulation. We found that 15d-PGJ2 induced an over-accumulation of HIF-1α in RCC4 cells, which lack pVHL and in HK-2 cells treated with inhibitors of the pVHL-proteasome pathway. These results indicated that pVHL-proteasome-independent mechanisms are involved, and therefore we aimed to ascertain them. We have identified a new lysosomal-dependent mechanism of HIF-1α degradation as a target for 15d-PGJ2 based on: (1) HIF-1α colocalized with the specific lysosomal marker Lamp-2a, (2) 15d-PGJ2 inhibited the activity of cathepsin B, a lysosomal protease, and (3) inhibition of lysosomal activity did not result in over-accumulation of HIF-1α in 15d-PGJ2-treated cells. Therefore, expression of HIF-1α is also modulated by lysosomal degradation.
Keywords: Hypoxia inducible factor; 15-Deoxy-Δ12,14-prostaglandin-J2; Proximal tubular cells; Calpain; Lysosome; Heat shock protein-90
Introduction
Transcriptional adaptive responses to hypoxia are controlled primarily through the nuclear accumulation of hypoxia-inducible factor (HIF-1). HIF transcription factors are heterodimers consisting of a constitutively expressed β subunit and an oxygen-regulated α subunit, which is mainly regulated at the protein level. In normoxia, HIF-α is hydroxylated at specific proline residues by iron-dependent HIF prolyl-hydroxylases (PHDs) [1–5]. This posttranslational modification is an absolute requirement for its recognition by the protein pVHL, the substrate recognition component of an E3 ubiquitin ligase complex that targets HIFα for proteasomal degradation [1, 2]. In hypoxia, this interaction is suppressed; HIFα is thereby stabilized, and together with the β subunit and transcriptional coactivators, it binds to hypoxia-responsive elements (HRE) in target genes [6].
The activation of HIF is found in a large number of human pathologies as a consequence of the hypoxic environment. However, in other pathologies, like in renal pathologies, induction of HIF-1α may have a therapeutic interest. For instance, pre-treatment of rats with a HIF-specific PHD inhibitor to increase HIF activity, or cobalt chloride, a hypoxia-mimetic agent known to stabilize HIF-1α, resulted in improved renal clearance after clamping of the renal pedicle [7, 8]. Administration of 15-deoxy-Δ12,14-prostaglandin-J2 (15d-PGJ2), an agonist of the peroxisome proliferator-activator-γ (PPAR γ), produces a significant reduction of renal dysfunction and injury caused by ischemia/reperfusion of the kidney in an in vivo model in rodents [9]. Therefore, it is possible that the protective effect of 15d-PGJ2 is due to 15d-PGJ2-induced stabilization of HIF-1α protein, which we have recently described in several types of cultured renal cells [10]. Accumulation of transcriptionally active HIF-1α by 15d-PGJ2 is achieved in normoxia, in a PPARγ-independent manner, without affecting HIF-1α mRNA levels or proteasome activity. This stabilization is dependent on the thiol-antioxidant-sensitive, electrophilic reactivity of the α,β-unsaturated carbonyl present in the 15d-PGJ2 molecule [10]. However, the mechanism for this stabilization has not yet been described.
Multiple lines of evidence indicate that stabilization of HIF-1α is also possible through pVHL-independent mechanisms. Thus, under normoxic conditions, HIF-1α expression is modulated by glycogen synthase kinase-3 [11] receptor of activated protein kinase C-1 [12], histone deacetylases [13], heat shock protein 90 [14], calcium-calpain [15] and cathepsin B [16]. Our aim here was to determine the particular mechanism of 15d-PGJ2-mediated accumulation of HIF-1α. Our results demonstrated that pVHL-proteasome-independent mechanisms were involved in 15d-PGJ2-induced over-accumulation of HIF-1α. Most importantly, we have identified a new lysosomal-dependent mechanism of HIF-1α degradation which may be acting simultaneously or not with other degradation pathways.
Materials and methods
Materials
15-deoxy-Δ12,14prostaglandin-J2 (15d-PGJ2), was purchased from Cayman Chemical Company (Ann Arbor, MI). Proteasome inhibitor MG132, calpain substrate S-LLY-AMC, calpain inhibitors PD150606 (a cell-permeable, non-competitive, selective non-peptide calpain inhibitor directed towards the calcium binding sites of calpain) and calpastatin (a 27-residue peptide specific calpain inhibitor), cathepsin B inhibitor CA-074 Me and protease inhibitors were from Calbiochem (San Diego, CA). Nitrocellulose membrane was from Bio-rad (Hercules, CA). Enhanced chemiluminescence ECL detection system was from Amersham Biosciences (Airlington Heights, IL). Protein A/G Plus was from Santa Cruz Biotechnology (Santa Cruz, CA). Unless otherwise stated, all the biochemical reagents used in this study were purchased from Sigma (St. Louis, MO).
Anti-human protein antibodies were obtained from the following sources and used at the indicated dilutions: monoclonal HIF-1α (1:1,000) and Hsp-90 (1:500) (Transduction Laboratories, BD Biosciences, Palo Alto, CA), anti-rabbit horseradish peroxidase antibody (1:2,000, Chemicon, CA) and cathepsin B antibody (1:2,000) were from Santa Cruz Biotechnology), and calpain antibody was from Biomol International (Hamburg, Germany). Polyclonal α-actin (1:3,000) and horseradish peroxidase-coupled sheep anti-mouse antibodies (1:3,000) were obtained from Sigma. Lysosome-associated membrane protein type 2a (Lamp2a) antibody (1:100) and polyclonal HIF-1α were from Abcam (Cambridge, UK).
Cell Culture and treatment
Human kidney HK-2 cells were purchased from American Type Culture Collection (Rockville, MD). Cells were maintained in DMEM supplemented with hydrocortisone, Insulin-Transferrine-Selenium, non-essential amino acids, 100 units/ml penicillin, 100 μg/ml streptomycin and 10% heat-inactivated fetal bovine serum. Except for antibiotics and serum, which were obtained from Invitrogen (Carlsbad, CA), all the other products were purchased from Sigma. Parental VHL-negative RCC4 cells (herein described as RCC4-) and the corresponding VHL stable transfectants (RCC4+) were maintained in RPMI 1640 with Glutamax-I (Life Technologies). For RCC4+, G418 sulphate (100 μg/ml; Promega) was added to culture media. In all cases, culture media were supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin and 10% fetal bovine serum.
Cells were routinely cultured in 95% air, 5% CO2 (normoxic conditions) at 37°C. To expose the cells to hypoxia, they were placed in an airtight chamber with inflow and outflow valves (Billups–Rothenberg, Del Mar, CA) that were infused with a mixture of 1% O2, 5% CO2, 94% N2 (S.E. Carburos-Metálicos, Madrid, Spain). In all experiments, cells were plated at 70–90% confluence and, when completely attached, they were treated for 6 h (unless otherwise indicated) with prostaglandin or exposed to hypoxia or desferroxamine in serum free media.
Western blot
Immediately after treatments, cells were washed with ice-cold phosphate-buffered saline (PBS) and lysed in 80 μl of lysis buffer (50 mM Trizma, pH 8.0, 150 mM NaCl, 0.1% Triton X-100, 10 mM EDTA, 0.25% sodium deoxycholate and protease inhibitors). Cell lysates were sonicated and proteins were resolved onto 8–10% SDS-polyacrylamide gels. The proteins were then transferred to a nitrocellulose membrane, blocked with 5% non-fat dry milk in PBS-T (50 mM Trizma, pH 7.6, 150 mM NaCl, 0.1% Tween 20) and incubated overnight at 4°C with the corresponding antibodies. Immunolabeling was detected by enhanced chemiluminescence.
Immunoprecipitation assay
HK-2 cells were seeded in 10-cm dishes 1 day before experiments. After the different treatments, cells were lysed in 500 μl lysis buffer (50 mM Tris, 150 mM NaCl, 5 mM EDTA, 0.5% NP-40, 100 μM Orthovanadate, 100 μM PMSF and protease inhibitor cocktail, pH 8) and collected. Samples were centrifuged (12,000g for 20 min) and supernatants (500 μg protein) were transferred into fresh tubes to pre-clear them with 20 μl of Protein A/G Plus at 4°C for 1 h. Then, 2 μg of antibodies anti-Hsp-90, anti-HIF-1α or anti-Calpain-1 were added and incubated overnight at 4°C. Thereafter, 20 μl of Protein A/G Plus were added and incubated at 4°C for an additional 3 h. Beads were collected, washed three times with 500 μl lysis buffer and finally supplemented with 30 μl 2× SDS-PAGE sample buffer and boiled at 95°C for 5 min. Beads were removed by centrifugation and supernatants were loaded onto 8% SDS-PAGE. Western blot analysis was performed using calpain-1, anti-HIF-1α, anti-Lamp-2a or anti-Hsp-90 antibodies.
Calpain activity assay
Cells were resuspended in lysis buffer (100 mM Tris–HCl, pH 7.3, 145 mM NaCl, 10 mM EDTA). CaCl2 (10 mM final concentration) was added to cell lysates, and then centrifuged for 10 min at 10,000g. CaCl2 was substituted by EDTA in blanks. Calpain activity was measured in the supernatant fraction using the fluorogenic substrate (80 μM) S-LLY-AMC (Calbiochem) for 30 min at 37°C [17]. Equal fractions of the supernatants were pre-incubated with the calpain inhibitor PD150606 (50 nM), for 30 min at 37°C before addition of the substrate. The fluorescence was determined by a Luminescence spectrophotometer LS50 (Perkin Elmer, Wellesley, MA) with an excitation wavelength of 380 nm and an emission wavelength of 460 nm. These enzyme activities were described as arbitrary unit/(min/mg) protein and they were expressed as percentage of the activity found in control cells. Calpain activity was determined as the difference between fluorescence measured without and with PD150606 and expressed as percentage of control.
Cathepsin B activity assay
After different cell treatments, HK-2 cells were lisated in lysis buffer (20 mM Tris pH 7; 0.5% NP-40, 250 mM NaCl, 3 mM EDTA, 2 mM DTT, 3 mM EGTA, 0,5 mM PMSF, 20 mM glycerol phosphate, cocktail protease inhibitor) and then centrifuged for 20 min at 10,000g. Supernatant fractions were recovered and Cathepsin B activity was measured as described elsewhere [18]. Briefly, equal fractions of the cell supernatants were pre-incubated with the cathepsin B inhibitor CA-074 Me (10 μM) and a fluorogenic substrate Z-RR-AMC (50 μM) (Bachem, Bubendorf, Switzerland) for 60 min at 37°C. The fluorescence was analyzed by a Luminescence spectrophotometer LS50 (Perkin Elmer) with an excitation wavelength of 355 nm and an emission wavelength of 440 nm. Cathepsin B activity was determined as the difference between fluorescence measured with or without CA-074 Me, represented as percentage of control.
Immunofluorescence analysis
Cells were fixed with 4% paraformaldehyde in PBS, 10 min at RT, rinsed, and permeabilized with 0.5% Triton X-100 in PBS (10 min). Cells were then incubated for 1 h with 5% donkey serum in PBS to block nonspecific binding. Afterwards cells were incubated overnight at 4°C with anti-HIF-1α antibody (1:100) and anti-Lamp-2a (1:100) and then rinsed with PBS. Finally, cells were incubated with FITC-labelled anti-mouse IgG and Texas red (anti-rabbit), both diluted 1:500, for 1 h in the darkness. Slides were then washed and mounted with FluorSave™ Reagent (Calbiochem). Dual-color detection was performed by confocal laser scan microscopy LEICA TCS-SL (Heidelberg, Germany).
Statistical analysis
Each experiment was repeated at least three times. Results are expressed as the mean ± SEM. Statistical analysis was performed by either nonparametric Kruskal–Wallis test or Mann–Whitney test. A value of P < 0.05 was considered statistically significant.
Results
pVHL-independent mechanisms are involved in 15d-PGJ2-induced HIF-1α stabilization
It has been widely accepted that oxygen-induced HIF-1α degradation mainly occurs through the canonic PHD-pVHL-ubiquitin dependent proteasome pathway. Therefore, accumulation of HIF-1α in normoxia is easily achieved when inhibiting this pathway. However, multiple lines of evidence indicate that stabilization of HIF-1α is also possible through pVHL-independent mechanisms [11–13, 15, 19]. According to this, we have previously shown that 15d-PGJ2 induces normoxic stabilization of HIF-1α protein, without affecting HIF-1α mRNA levels or chymotrypsin-like proteasome activity [10]. These studies suggested that 15d-PGJ2 might also inhibit HIF-1α degradation by other mechanisms. Although these results might also be compatible with a 15d-PGJ2-mediated inhibition of HIF-1α degradation by the known pathway, we asked whether other mechanisms than the canonic pathway were relevant for the 15d-PGJ2-mediated accumulation of HIF-1α.
To confirm this hypothesis, we first studied the effect of 15d-PGJ2 on HIF-1α accumulation in HK-2 cells in which we had previously inhibited the PHD-pVHL-ubiquitin-dependent proteasome pathway by hypoxia, desferroxamine (DFX) or lactacystine. Our results showed that under such conditions 15d-PGJ2 induced an over-accumulation of HIF-1α in all instances (Fig. 1a, b). Most importantly, this was also confirmed in RCC4-cells (Fig. 1c), which lack VHL protein and therefore are unable to degrade HIF-1α by the VHL-dependent pathway. This would not be possible if 15d-PGJ2 were only an inhibitor of the canonic pathway of HIF-1α degradation. Next, we studied the turnover of HIF-1α in HK-2 cells that had been treated with either DFX or 15d-PGJ2 for 6 h, followed by 30–120 min incubation with the protein translation inhibitor cycloheximide (CHX). Our results indicated that in 15d-PGJ2-treated cells HIF-1α had a longer half-life compared to DFX-treated ones (Fig. 1d). Taken together, these results suggested that PHD-pVHL-independent mechanisms contribute to 15d-PGJ2-mediated increase on HIF-1α half-life. Therefore, the aim of our present study is to identify those mechanisms.
Fig. 1.
pVHL-independent mechanisms are involved in 15d-PGJ2-induced HIF-1α stabilization. a HK-2 cells were incubated for 6 h in normoxia or hypoxia (H, 1% O2) and treated with or without 2 μM 15d-PGJ2 or in combination with 380 μM DFX, or b 10 μM lactacystin. c RCC4 cells and their counterparts expressing pVHL [VHL(−) or VHL(+), respectively], were incubated for 6 h in the absence or presence of (2–5) μM 15d-PGJ2 or with 380 μM DFX. Cells from a, b and c were lysed and HIF-1α levels were determined by Western blot analysis. As a loading control, blots were reprobed with anti-α-actin. d Stability of HIF-1α was determined in cells treated with DFX or with 15d-PGJ2. Cells were incubated for 6 h with or without 380 μM DFX or 2 μM 15d-PGJ2. Thereafter, the protein translation inhibitor cycloheximide (CHX, 50 μg/ml) was added and incubation continued for up 2 h. Each experiment was performed at least three times and representative data are shown
Stabilization of HIF-1α by 15d-PGJ2 does not require interaction between Hsp-90 and HIF-1α
It has been recently found that Hsp-90 is a target for modification by 15d-PGJ2 in renal mesangial cells [20]. On the other hand, HIF-1α degradation is regulated in an oxygen-independent manner by Hsp-90 [14]. These authors show that geldanamycin (GA), an Hsp-90 antagonist, promotes efficient ubiquitination and proteasome-mediated degradation of HIF-1α in RCC4 cells lacking functional VHL. Taking this into consideration, we hypothesized that Hsp-90 could be involved in the 15d-PGJ2-mediated accumulation of HIF-1α.
In order to assess our hypothesis, we examined the effect of GA on cells incubated with DFX or 15d-PGJ2 (Fig. 2). Whereas incubation with 250 nM GA resulted in a dramatic decrease in HIF-1α expression in DFX-treated cells (Fig. 2a), 15d-PGJ2-induced HIF-1α accumulation was further increased by GA (Fig. 2b). However, co-immunoprecipitations using an anti-Hsp-90 antibody followed by detection with a HIF-1α antibody indicated that GA had dissociated the HIF-1α-Hsp-90 chaperone complex in 15d-PGJ2-treated cells (Fig. 2c). Therefore, we can conclude from these results that interaction between Hsp-90 and HIF-1α is not necessary for 15d-PGJ2-induced stabilization of HIF-1α.
Fig. 2.
Effect of the Hsp-90 inhibitor geldanamycin (GA) in the 15d-PGJ2-mediated stability of HIF-1α. HK-2 cells were co-incubated for 6 h with 250 nM GA and either 380 μM DFX (a) or 2 μM 15d-PGJ2 (b). Cells were lysed, and HIF-1α and Hsp-90 levels were determined by Western blot analysis. As a loading control, blots were reprobed with anti-α actin. Each experiment was performed at least three times and a representative one is shown. c To assess the effect of GA on 15d-PGJ2-mediated Hsp-90 association with HIF-1α, cells were incubated for 6 h with 15d-PGJ2 in the absence or presence of 250 nM GA. Cells were then collected followed by immunoprecipitation with anti-Hsp-90 antibody as described in “Materials and methods”. Input controls show protein expression in cell lysates. Immunoprecipitates were probed by western analysis using anti-HIF-1α or Hsp-90 antibodies. A representative experiment out of three is shown
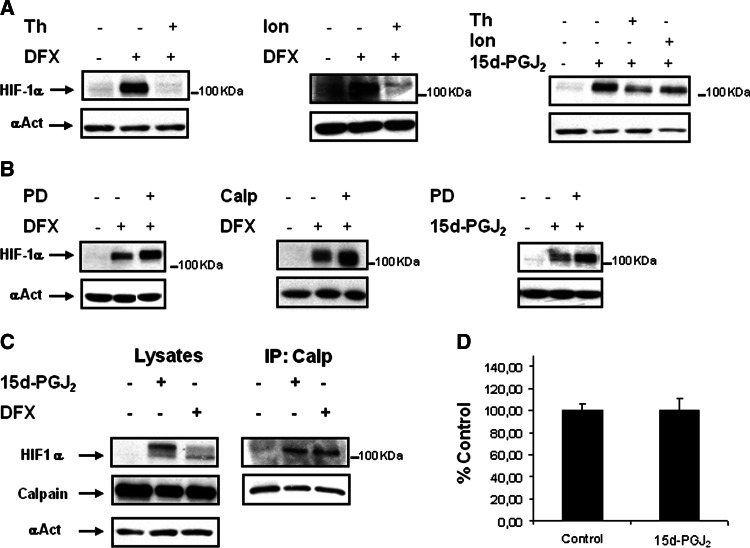
Stabilization of HIF-1α by 15d-PGJ2 is not due to inhibition of calcium/calpain activity
It has been previously shown that calcium/calpain activity mediates a pVHL-independent degradation of HIF-1α [15]. Therefore, the possibility exists that this system could be involved in the pVHL-independent accumulation of HIF-1α by 15d-PGJ2. To ascertain this, we aimed to determine whether inhibition of calcium/calpain-mediated degradation of HIF-1α was inhibited by 15d-PGJ2. To pursue this aim, we first analyzed the effect of ionomycin or thapsigargin, two agents known to promote intracellular calcium, or the effect of calpain inhibitors such as PD150606 and calpastatin on 15d-PGJ2-induced HIF-1α accumulation in HK-2 cells. Incubation of HK-2 cells with ionomycin or thapsigargin resulted in lower DFX- or 15d-PGJ2-induced HIF-1α accumulation (Fig. 3a), thus indicating that calcium-mediated HIF-1α degradation is active in both instances. According to these results, the treatment with calpain inhibitors resulted in over-accumulation of HIF-1α in both DFX-treated and 15d-PGJ2-treated cells (Fig. 3b). This indicated that calpain-mediated degradation of HIF-1α is still operative even after treatment with 15d-PGJ2. To confirm these results, we performed immunoprecipitation assays to determine if there was interaction of calpain with HIF-1α in 15d-PGJ2-treated cells. Our results indicated that 15d-PGJ2 did not inhibit the interaction between HIF-1α and calpain (Fig. 3c). Finally, we assessed the effect of 15d-PGJ2 on calpain activity in HK-2 cells. Our results showed that incubation with 15d-PGJ2 was not proved to induce changes on calpain activity (Fig. 3d). Taken together, these results indicate that stabilization of HIF-1α by 15d-PGJ2 was not due to the inhibition of the calcium/calpain pathway.
Fig. 3.
Degradation of HIF-1α via calcium/calpain in HK-2 cells and effect of 15d-PGJ2. The effect of agents that increase cytosolic calcium or calpain inhibitors on the 15d-PGJ2-mediated stabilization of HIF-1α was determined. Cells were incubated for 6 h with or without 380 μM DFX or 15d-PGJ2 (2 μM) in the presence or absence of the following compounds: a 30 nM thapsigargin (Th), 1 μM ionomycin (Ion), and b calpain inhibitors such as 50 μM PD150606 (PD) or 2 μM calpastatin (Calp). Thereafter, cells were lysed, and HIF-1α levels were determined by Western blot analysis. As a loading control, blots were reprobed with anti-α-actin. Each experiment was performed at least three times and a representative one is shown. c Cells under normoxic conditions were incubated in the presence of 380 μM DFX or 15d-PGJ2 for 6 h. Cells were then collected and immunoprecipitated with an anti-calpain antibody as described in “Materials and methods”. Input controls show protein expression in cell lysates. Immunoprecipitates were probed for HIF-1α and calpain-1 protein levels using anti-HIF-1α or anti-calpain-1 antibodies, respectively. The experiment was performed at least three times and a representative one is shown. d Effect of 15d-PGJ2 on calpain activity. Cells were incubated for 6 h in the absence (control) or with 2 μM 15d-PGJ2 and calpain activity was determined as described in “Materials and methods”. Calpain activity is represented as percentage of control. Values are the mean ± SD of four independent experiments
15d-PGJ2 prevents HIF-1α degradation by lysosomal activity
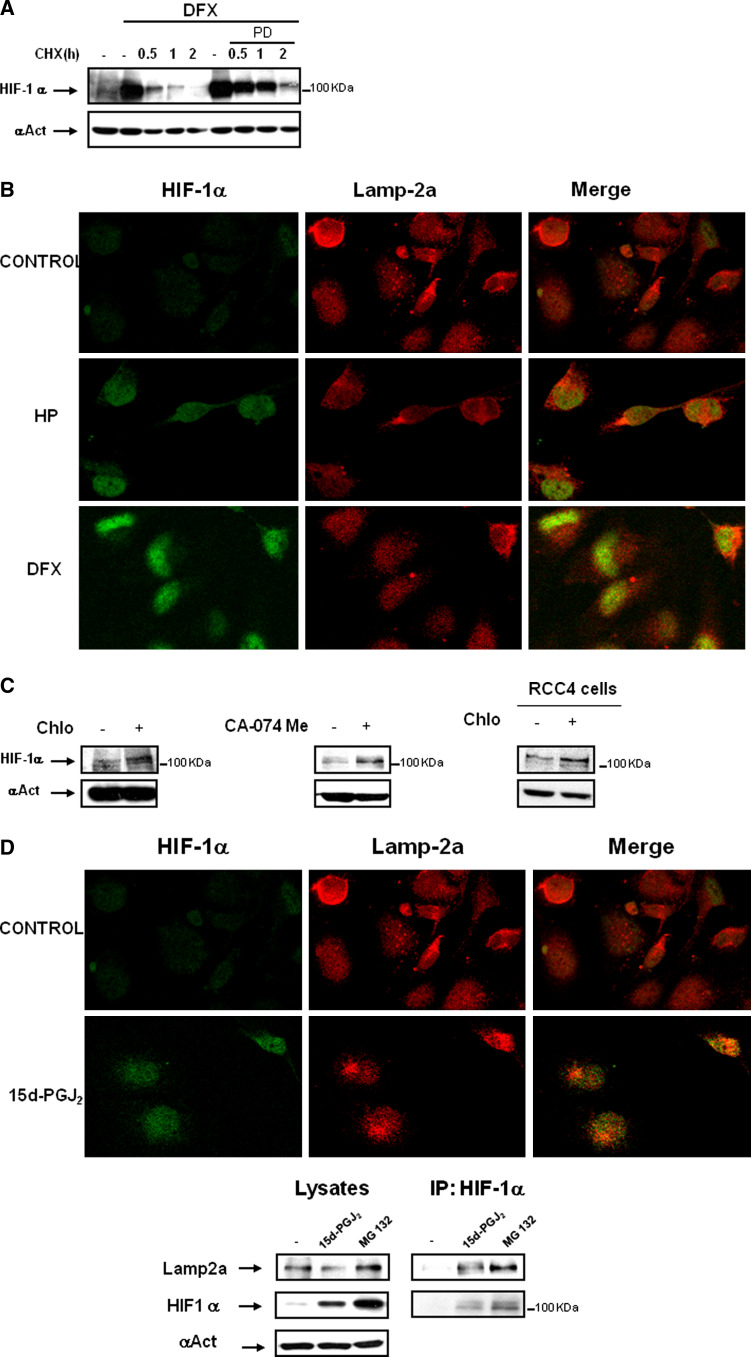
To ascertain whether other enzymatic (proteolytic) systems could be involved in HIF-1α degradation, we determined HIF-1α half-life in HK-2 cells where both proteasome and calcium/calpain pathways were inhibited with DFX and PD150606. In this setting, HIF-1α levels induced by DFX were quickly reduced by ≈50% during the first 30 min, and there was no remaining HIF-1α after incubation for 2 h with CHX. Thus, HIF-1α levels accumulated by DFX and PD had longer half-life than with DFX alone; however, HIF-1α was also degraded after 2 h with CHX (Fig. 4a). This suggested the existence of other VHL-independent and calcium/calpain-independent mechanisms that are also actively degrading HIF-1α.
Fig. 4.
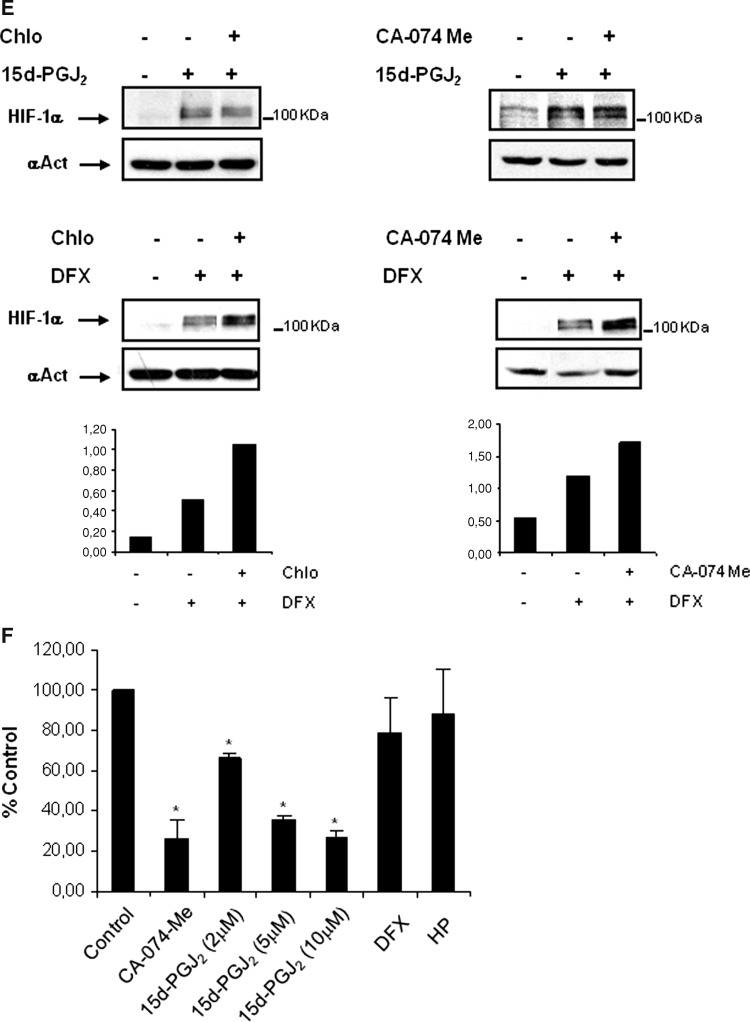
Effect of 15d-PGJ2 on lysosomal activity and its contribution to HIF-1α stabilization. a The effect of PD150606 calpain inhibitor on HIF-1α half-life was determined in HK-2 cells. Cells were incubated for 6 h with 380 μM DFX in the presence or absence of 50 μM PD150606. Thereafter, the protein translation inhibitor cycloheximide (CHX, 50 μg/ml) was added and incubation continued for up to 2 h. HIF-1α protein levels were determined by Western blot. b Immunofluorescence experiments were performed to determine whether HIF-1α, accumulated under several conditions, co-localizes with the lysosomal protein Lamp-2a. Role of lysosomes in HK-2 cells under hypoxia or DFX treated cells versus normoxic conditions. HK-2 cells were incubated for 6 h with 380 μM DFX or incubated under hypoxic conditions (1% O2). Then, cells were fixed and incubated with anti-HIF-1α and anti-Lamp-2a antibodies as described in “Materials and methods”. Immunofluorescence for Lamp-2a (red) and HIF-1α (green) is shown. c Accumulation of HIF-1α by lysosome inhibitors in normoxia. Cells were incubated for 24 h in the presence or absence of 50 μM chloroquine or 10 μM CA-074 Me. After incubation, cells were lysed, and HIF-1α levels were determined by Western blot analysis. d HK-2 cells were incubated for 6 h with 2 μM 15d-PGJ2. Then, cells were fixed and co-incubated with anti-HIF-1α and anti-Lamp-2a antibodies as described in “Materials and methods”. Immunofluorescence for Lamp-2a (red) and HIF-1α (green) is shown. As cells were incubated in parallel with DFX and hypoxia treatment, the control is the same as shown in Fig. 2b. Association of HIF-1α with lysosome marker Lamp-2a was assessed by immunoprecipitating the cells after 6 h in the presence of 15d-PGJ2 (2μM) or MG132 (10μM) as control of HIF-1α accumulation with an anti-HIF-1α antibody as described in “Materials and methods”. Immunoprecipitates were then probed by Western blot analysis using anti-HIF-1α or Lamp2a antibodies. Input controls show protein expression in cell lysates. A representative experiment out of three is shown. e Effect of chloroquine (Chlo) or the cathepsin B inhibitor CA-074 Me on 15d-PGJ2 or DFX-mediated HIF-1α stabilization. Cells were incubated for 24 h with 2 μM 15d-PGJ2 or 380 μM DFX in the presence or absence of 50 μM chloroquine or 10 μM CA-074 Me. HIF-1α expression levels were analyzed by Western Blot. f To determine the effect of 15d-PGJ2 on cathepsin B activity, cells were incubated for 24 h with 10 μM cathepsin B inhibitor CA-074Me, 2-5-10 μM 15d-PGJ2, 380 μM DFX or under hypoxic conditions (1% O2). Cathepsin B activity was measured as described in “Materials and methods”. The experiment was performed three times and each bar is the mean ± SD of the obtained results. *P < 0.01 versus control
It has been previously reported that inhibitors of cathepsin B stabilized HIF-1α in retinal endothelial cells [16]. Although cathepsin B is a protease present in lysosomes, other reports have localized this protease in extralysosomal compartments [21–23]. Therefore, it is not clear from Im et al.’s studies whether these organelles are involved in HIF-1α degradation. Therefore, this can be an interesting pathway that might be involved in 15d-PGJ2-induced HIF-1α stabilization.
Firstly, we analyzed whether HIF-1α could be localized at the lysosomal fraction. Therefore, we incubated HK-2 cells or HeLa cells under hypoxia or treated with DFX versus normoxic conditions. Confocal microscopy analysis showed that a fraction of HIF-1α in cells treated with DFX or under hypoxia co-localized with Lamp-2a, a specific lysosomal marker (Fig. 4b and data not shown). In addition, expression of HIF-1α increased in HK-2 cells treated with chloroquine (an inhibitor of lysosomal activity) or CA-074 Me (an inhibitor of cathepsin B) incubated under normoxia for 24 h. In RCC4- cells (which lack VHL protein) HIF-1α expression was also increased after chloroquine stimulation for 24 h (Fig. 4c). These results indicate that lysosomes participate in the degradation of HIF-1α in normoxia.
Given that degradation of HIF-1α in lysosomes has not previously been described, we investigated the implication of lysosomes in the degradation of HIF-1α in cells treated with 15d-PGJ2. HK-2 cells were incubated for 6 h with 15d-PGJ2, and HIF1-α localization was assessed under confocal microscopy. We found that a fraction of HIF-1α in HK-2 cells co-localized with Lamp-2a. To determine whether there was interaction of HIF-1α with Lamp-2a, we performed immunoprecipitation assays with cells treated with either 15d-PGJ2 or MG132, an inhibitor of proteasome, calpain and most lysosomal enzymes [24, 25]. Our results confirmed that HIF-1α and Lamp-2a not only co-localized in the lysosomes but interacted with each other (Fig. 4d). The next question was to ascertain whether the degradation of HIF-1α inside the lysosomal compartment was inhibited by 15d-PGJ2, thus contributing to HIF-1α stabilization. This issue was addressed by co-incubating HK-2 cells with 15d-PGJ2 and either chloroquine or CA-074 Me. Incubation with these inhibitors did not result in over-accumulation of HIF-1α in 15d-PGJ2-treated cells (Fig. 4e) which suggested that lysosomal degradation of HIF-1α was already inhibited by 15d-PGJ2. In contrast to these results, incubation with DFX and either chloroquine or CA-074 Me resulted in an over-accumulation of HIF-1α (Fig. 4e). Therefore, our results pointed out that 15d-PGJ2-mediated accumulation of HIF-1α might be due to inhibition of lysosomal degradation by this cyclopentenone prostaglandin. To confirm these results, we assessed lysosomal activity in the presence of 15d-PGJ2. We analyzed Cathepsin B activity under different 15d-PGJ2 concentrations, hypoxia or desferroxamine. Our results indicated that increased concentrations of 15d-PGJ2 inhibited cathepsin B activity (34% at 2 μM and 74% at 10 μM vs. control). At 10 μM, the percentage of inhibition was similar to the inhibition levels achieved by CA-074 Me compared to control with no treatment. However, cathepsin B activity was not affected by hypoxia or DFX (Fig. 4f). Therefore, these results indicated that 15d-PGJ2 effect on HIF-1α accumulation was due to inhibition of lysosomal activity.
In conclusion, this set of results indicated that lysosomes degrade HIF-1α and that they are one of the targets through which 15d-PGJ2 accumulates HIF-1α in HK-2 cells. To our knowledge, this is the first report showing lysosomal contribution to the HIF-1α degradation.
Semiquantitative comparison studies on the relative efficiency and kinetics of the three proposed pathways for HIF-degradation
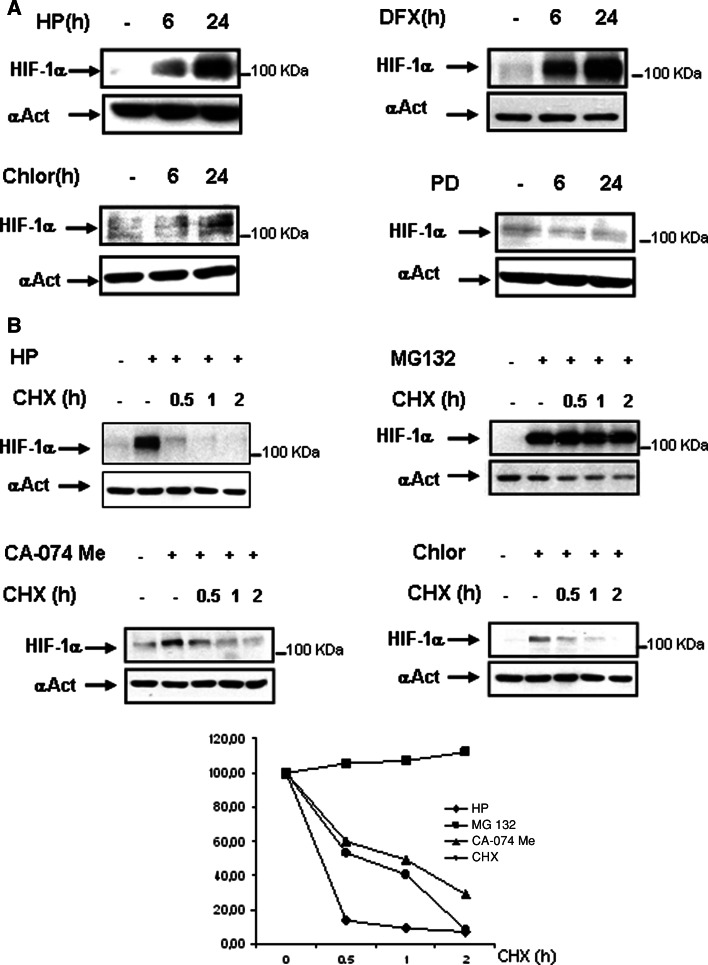
Given that three different pathways—namely proteasome, calpain and lysosomes—are potentially involved in the degradation of HIF-1α in HK-2 cells, we designed experiments to assess the relative efficiency and kinetics of these three pathways. We first studied the time-course of HIF-1α accumulation after inhibiting (1) the proteasome pathway with either hypoxia of desferroxamine, (2) the lysosomal pathway with chloroquine, and (3) the calpain pathway with PD150606. Our results shown in Fig. 5a indicated that (1) inhibition of the proteasome pathway during 6 h resulted in a strong accumulation of HIF-1α, (2) incubation for 24 h with the inhibitor of lysosome chloroquine induced mild accumulation of HIF-1α (while no HIF-1α accumulation was observed at 6 h), and (3) inhibition of the calpain pathway did not result in any accumulation of HIF-1α. In summary, this set of experiments indicated that the proteasome is the quickest and more efficient pathway for HIF-1α degradation, that the lysosomal pathway is slower and less efficient, and that the calpain pathway itself does not contribute significantly to HIF-1α degradation (although, as observed in Fig. 4a, calpain activity modulates the stability of HIF-1α accumulated by simultaneous inhibition of the proteasome pathway).
Fig. 5.
Comparative studies of the relative efficiency and kinetics of the three proposed pathways for HIF-degradation. a HK-2 cells were incubated for 6 or 24 h in the presence of 380 μM DFX; 50 μM calpain inhibitor PD150606 (PD), 50 μM clhoroquine or under hypoxia (1% O2). b Cells were incubated with 10 μM MG132 or under hypoxia (1% O2) for 6 h or with cloroquine (50 μM) or CA-074 Me (10 μM) for 24 h. Thereafter, 50 μg/ml of the protein translation inhibitor cycloheximide (CHX,) was added and incubation continued for up to 2 h. Cells were lysed immediately, and HIF-1α levels were determined by Western blot analysis. As a loading control, blots were reprobed with anti-α-actin. Each experiment was performed at least three times and a representative one is shown. Densitometric analysis is shown below
We next addressed the question of the relative kinetics of HIF-1α degradation after its accumulation by inhibiting the proteasomal or the lysosomal pathways. To this end, cells were incubated for 24 h under hypoxia or treated for 24 h with chloroquine or CA-074Me, and followed by 30–120 min incubation with the protein translation inhibitor cycloheximide (CHX). In order to compare HIF-1α maximal stabilization, cells were incubated with MG132, an inhibitor of proteasome, calpains and lysosomal pathways [24, 25].
In cells under hypoxia, the quick reduction of HIF-1α levels after treatment with CHX (Fig. 5b, left upper panel) indicated that pVHL-proteasome independent pathways of HIF-1α degradation play an important role in the regulation of HIF-1α final levels. This role is clearly indicated by the fact that inhibition of all the potential pathways of HIF-1α degradation by MG132 results in maintained levels of HIF-1α along the 2-h treatment with CHX (Fig. 5b, right upper panel). In CA-074Me- or chloroquine-treated cells, the reduction of HIF-1α levels after treatment with CHX (Fig. 5b) indicated that HIF-1α degradation was slower than that found in cells treated with the inhibitors of the proteasome pathway hypoxia (Fig. 5b) or DFX (Fig. 4a). The slower degradation of HIF-1α probably reflects the fact that HIF-1α is accumulated inside the lysosomes, which makes its degradation by cytosolic pathways such as proteasome and calpains difficult. Altogether, these results indicated that, although the pVHL-dependent degradation pathway is the most efficient for HIF-1α degradation in the cytoplasm, there are also other pathways, like the lysosomal pathway, that contribute to HIF-1α degradation, and they probably become more important when the others do not work.
Discussion
We have previously found that 15d-PGJ2 acts as an inducer of biologically active HIF-1α in renal cells under normoxic conditions. Although the contribution of pVHL-dependent mechanisms cannot be underestimated, here we show that 15d-PGJ2 induced HIF-1α accumulation by a pVHL-proteasome-independent mechanism. Our results have demonstrated that 15d-PGJ2 effect on HIF-1α accumulation was not through calcium/calpain-dependent pathways and did not require interaction between Hsp-90 and HIF-1α, although HIF-1α degradation in HK-2 cell was affected by both. Instead, 15d-PGJ2 inhibited a sub-cellular pathway in the lysosomes, showing this lysosomal degradation of HIF-1α as a new mechanism of HIF-1α stabilization. These results are summarized in Fig. 6.
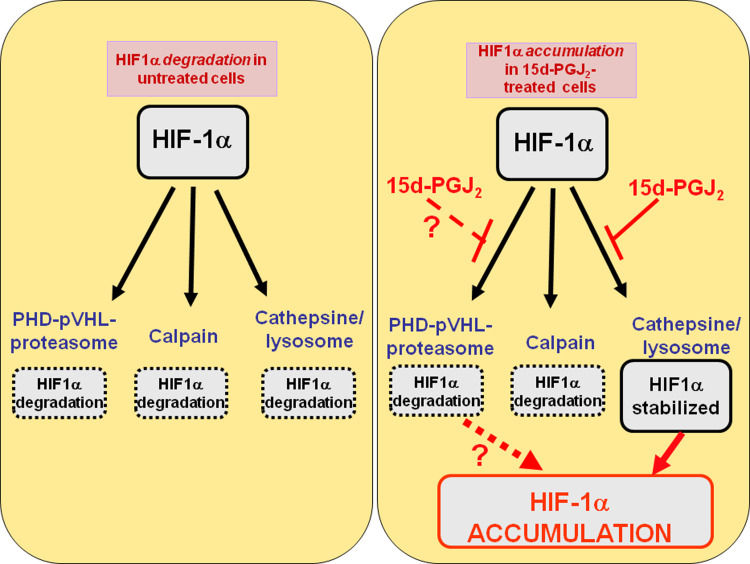
Fig. 6.
Enzymatic systems involved in the degradation of HIF-1α in human proximal tubular renal cells HK-2. Left: there are three operative enzymatic systems for the degradation of HIF-1α in HK-2 cells: proteasome (mainly through the PHD-pVHL-pathway, but also through other PHD-pVHL independent pathways such as this triggered by Hsp-90 inhibitor geldanamycin), calcium/calpain and cathepsin B/lysosome. Right: incubation with 15d-PGJ2 results in HIF-1α stabilization through the inhibition of cathepsin B/Lysosome, although the high degree of stabilization of HIF-1α strongly suggests that 15d-PGJ2 could also inhibit the PHD-pVHL-dependent proteasomal degradation of HIF-1α
There are three different proteolytic activities in mammalian proteasomes: chymotrypsin-like, caspase-like and trypsin-like activities. It is widely assumed that inhibition of the chymotrypsin-like site, the primary target of the proteasome inhibitors used in research and cancer therapy, reflects the degree of inhibition of protein breakdown. Actually, this is far from being rigorously exact: when one type of active site is inactivated, the two others assume increasing importance in cleavage of the protein. Thus, not surprisingly, inhibition of either of the residual sites has a greater impact in reducing protein degradation than when that site alone is blocked [26]. We have previously described that treatment of HK-2 cells with 15d-PGJ2 does not inhibit chymotrypsin-like proteasome activity [10]. However, since 15d-PGJ2 inhibits the protease cathepsin B, we cannot rule out that inhibition of caspase-like and/or trypsin-like proteasomal activities by 15d-PGJ2 might contribute to the accumulation of HIF-1α. Furthermore, there are other ways by which 15d-PGJ2 might affect proteasomal degradation of HIF-1α. For instance, accumulation of HIF-1α by 15d-PGJ2 involves the electrophilic reaction of its α,β-unsaturated cyclopentenone moiety with thiol groups [10], and Δ12-prostaglandin-J2, whose electrophilic reactivity is very similar to 15d-PGJ2, inhibits ubiquitin hydrolase UCH-L1 and elicits ubiquitin-dependent proteasomal degradation of proteins without proteasome inhibition [27]. If this mechanism was also triggered by 15d-PGJ2 (currently under investigation), it would probably contribute to the accumulation of HIF-1α. It is tempting to speculate that 15d-PGJ2 might also inhibit PHD-pVHL-dependent HIF-1α degradation by lowering the availability of Fe(II). This could be achieved either by oxidation of Fe(II) or by the action of 15d-PGJ2 on lysosomes (see below). Nevertheless, it will be necessary to measure the cell levels of Fe(II) to confirm this hypothesis. However, taking into account that the inhibition of lysosomes only needed 24 h to accumulate HIF-1α (Fig. 4c), it seems reasonable to hypothesize that the high degree of HIF-1α stabilization after 6 h incubation with 15d-PGJ2 (Fig. 1d) can only be achieved by the inhibition of the efficient pVHL-dependent pathway and the simultaneous inhibition of other(s) pathways.
In this paper, we have studied the effect of 15d-PGJ2 on non-canonic pathways involved in HIF-1α accumulation. Among the pVHL-independent, proteasome-dependent mechanisms of HIF-1α accumulation which could be potentially affected by 15d-PGJ2, we studied that involving Hsp-90 [14] because this is a previously described target for 15d-PGJ2 [20]. Among the proteasome-independent mechanisms, we studied the effect of 15d-PGJ2 on the calcium/calpain pathway because calpains have a cysteine residue at their active site [28] which might be inactivated by its reaction with 15d-PGJ2, since it is known to bind covalently to cysteines residues in proteins [29–31]. However, according to our results, none of these pathways (Hsp-90 or calcium/calpain) seemed to be involved in 15d-PGJ2-induced HIF-1α accumulation. This led us to study the contribution of other degradative pathways involved in HIF-1α degradation. It has previously been reported that inhibitors of the lysosomal protease cathepsin B stabilize HIF-1α in retinal endothelial cells [16]. In this report, we have demonstrated that lysosomes contribute to the degradation of HIF-1α since: (1) inhibition of lysosomal activity resulted in the accumulation of HIF-1α in normoxic HK-2 cells, (2) incubation with chloroquine or inhibiting cathepsin B resulted in over-accumulation of HIF-1α in DFX-treated cells, and (3) a fraction of HIF-1α, accumulated by hypoxia or by treatment with DFX or 15d-PGJ2, co-localized and immunoprecipitated with the specific lysosomal protein Lamp-2a. The last suggests that lysosomes are a constitutively operative system for HIF-1α degradation. This might help to avoid an excessive increase in the intracellular levels of HIF-1α (which could possibly occur after the inhibition of its degradation through the highly efficient proteasome pathway). In fact, an increase in the number of lysosomes in the adrenal glomerulosa zone in rats under chronic hypoxia [32] as well as in the myocardium of diabetic rats under acute hypoxia has previously been shown [33]. This lysosomal proliferation could enhance the efficiency of the lysosomal-mediated degradation of HIF-1α when hypoxia blocks its proteasomal degradation.
Chaperone-mediated autophagy (CMA) is a generalized form of highly selective autophagy. All the CMA substrate proteins described to date are soluble cytosolic proteins containing a targeting motif biochemically related to the pentapeptide KFERQ (reviewed by [34]). This motif is recognized by the cytosolic chaperone heat shock cognate protein which, after interacting with several cochaperones including Hsp-90 [35], targets the substrate to the lysosomal membrane. In this location it interacts with Lamp-2a, which is responsible of substrate uptake [34]. Interestingly, Hsp-90 is also found in the lysosomal lumen. However, although it has two KFERQ-related motifs, it remains unknown whether it is functional or it is only internalized to be degraded [36]. Although amino acid sequence analysis of HIF-1α revealed the absence of KFERQ motifs (unpublished observations), the interaction between Lamp-2a and HIF-1α-which was indicated by our immunoprecipitation data (Fig. 4d) suggests that CMA could be responsible for the lysosomal degradation of HIF-1α. We hypothesize that Hsp-90, which has KFERQ motifs, acts as a carrier for HIF-1α, targeting it to lysosomes. This proposed mechanism also could contribute to HIF-2α degradation, since HIF-2α also interacts with Hsp-90 [37].
The localization of HIF-1α degradation in the nucleus or in the cytoplasm has been found to be a cell-type characteristic parameter but, whatever the cell type, degradation of HIF-1α in the cytoplasm was always observed [38]. Furthermore, HIF-1α has been found in organelles. For instance, in hepatocytes, hypoxia targeted HIF-1α to the peroxisome, rather than the nucleus, where it co-localized with pVHL and the HIF hydroxylases [39]. It has also been found HIF-1α in endoplasmic reticulum, in normoxia [40]. Therefore, it is likely that part of cytoplasmic HIF-1α may reach other organelles, such as lysosomes, and that it undergoes proteolysis. On the other hand, the inhibition of lysosomal activity might also diminish the Fe(II)-dependent and pVHL-mediated degradation of HIF-1α, since chloroquine, through the inhibition of lysosomal proteolysis of ferritin, causes three-fold more iron to be retained in ferritin in hepatoma cells [41]. The role of this mechanism in 15d-PGJ2-induced accumulation of HIF-1α remains to be confirmed. In addition, the possibility exists that 15d-PGJ2 also increases the rate at which HIF-1α enters into the lysosomal compartment which would help its lysosomal accumulation. Alternatively, cathepsin B may control the level of HIF-1α indirectly, by degrading proteins such as Hsp-90, which are required for stabilizing HIF-1α [14].
The role of Hsp-90 in the physiological degradation of HIF-1α deserves further discussion. Interestingly, this role has been indirectly suggested by the experiments with 15d-PGJ2 and GA. First of all, the fact that 15d-PGJ2-induced HIF-1α accumulation was not reversed by GA (Fig. 3b) suggests that the GA-triggered ubiquitin- and proteasome-dependent pathway of HIF-1α degradation—which is likely responsible for the GA-induced HIF-1α degradation in DFX-treated cells (Fig. 3a)—is inhibited by 15d-PGJ2. As indicated above, electrophilic prostaglandins like 15d-PGJ2 may inhibit ubiquitin-dependent proteasome degradation of proteins [27]. However, the inhibition of this pathway itself does not explain why HIF-1α does actually increase in cells treated with 15d-PGJ2 and GA (Fig. 3b). Let us suppose (1) that there is still another unidentified, low-efficiency pathway for HIF-1α degradation which obligatorily requires that HIF-1α is bound to Hsp-90 (this might be the pathway responsible for lysosomal entry of HIF-1 which we have hypothesized above), and (2) that 15d-PGJ2 also inhibits this pathway. In DFX-treated cells, the disruption by GA of the interaction HIF-1α-Hsp-90 would inhibit the hypothetical Hsp-90 pathway of degradation, but HIF-1α will still decrease through the very efficient pVHL-independent, ubiquitin- and proteasome-dependent pathway. Let us consider the situation in 15d-PGJ2-treated cells: treatment with GA will result, as expected, in the disruption of the interaction HIF-1α-Hsp-90 and the consequent inhibition of the hypothetical Hsp-90-dependent pathway of HIF-1α degradation. However, now the ubiquitin, proteasome-dependent pathway is inhibited by 15d-PGJ2 and, therefore, HIF-1α will over-accumulate after GA treatment.
15d-PGJ2 is able to modify proteins covalently [20] and its binding to free thiol groups of critical cellular proteins, and particularly its effect modifying cysteines, is likely to be in part the cause of its biological effects [29–31, 42–44]. It is conceivable that lysosomal cathepsins B, H, L and S—which are cysteine proteases and have a cysteine residue at their active site [28]—might be inactivated by its reaction with 15d-PGJ2. In this context, the sulfhydryl reagent chloroacetaldehyde, a metabolite of the alkylating agent ifosfamide, has been shown to inhibit the cysteine proteases caspase-3, caspase-8 and cathepsin B in proximal tubule cells in primary culture [45]. In fact, we have previously shown that induction of HIF1-α by 15d-PGJ2 is blocked by thiol-reducing agents N-acetylcysteine and dithiothreitol [10] and we have shown here that the activity of cathepsin B was inhibited by 15d-PGJ2.
In conclusion, lysosomes degrade HIF-1α, and they are one of the targets through which 15d-PGJ2 accumulates HIF-1α in HK-2 cells. The high degree of HIF-1α stability induced by 15d-PGJ2 points out to a potentially powerful effect of agents that induce protein thiol modification to accumulate HIF-1α.
Our work also highlights the potential of pVHL-independent pathways in HIF-1α degradation, particularly when the quickest and more efficient pVHL-dependent pathway has been physiologically or pharmacologically inhibited. For instance, the relatively quick semi-quantitative kinetics of HIF-1α degradation in cells having inhibited the pVHL-pathway by DFX or hypoxia (Figs. 4a, 5b) reflect the important activity of pVHL-independent pathways in the degradation of HIF-1α. However, these pathways are much less efficient than the pVHL-dependent pathway since (1) inhibition of calpain does not affect HIF-1α accumulation (Fig. 5a) unless the pVHL-pathway is also inhibited (Fig. 4a), and (2) inhibition of lysosomal degradation only results in HIF-1α accumulation after 24 h (Fig. 4c). Therefore, the maximal stabilization of HIF-1α will be achieved when the three pathways of degradation—namely pVHL-dependent proteasome, calpain-dependent and lysosome dependent—are simultaneously inhibited, as when MG132 was used (Fig. 5b).
Since HIF-1α is known to upregulate factors that have been shown to be cytoprotective during acute renal injury, inhibition of HIF-1α proteolysis by 15d-PGJ2 might be a useful preventative strategy to improve clinical outcome of ischemia/reperfusion injuries such as those associated with procedures that involve cardiothoracic surgery or renal cadaveric transplantation.
Acknowledgments
This work was supported by grants SAL-0311-2006 from the Comunidad de Madrid, SAF2008-01767 from the Spanish Ministerio de Ciencia e Innovación and CCG08-UAH/BIO-4102 from University of Alcala.
References
- 1.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim AA, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratclife PJ. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 2.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292(5516):464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 3.Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. Independent function of two destruction domains in hypoxia-inducible factor-α chains activated by prolyl hydroxylation. EMBO J. 2001;20:5197–5206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirsila M, Koivunen P, Gunzler V, Kivirikko KI, Myllyharju J. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J Biol Chem. 2003;278(33):30772–30780. doi: 10.1074/jbc.M304982200. [DOI] [PubMed] [Google Scholar]
- 5.Yu F, White SB, Zhao Q, Lee FS. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci USA. 2001;98(17):9630–9635. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain: a hypoxic switch. Science. 2002;295(5556):858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto M, Makino Y, Tanaka T, Tanaka H, Ishizaka N, Noiri E, Fujita T, Nangaku M. Induction of renoprotective gene expression by cobalt ameliorates ischemic injury of the kidney in rats. J Am Soc Nephrol. 2003;14(7):1825–1832. doi: 10.1097/01.ASN.0000074239.22357.06. [DOI] [PubMed] [Google Scholar]
- 8.Guo G. Improvement of kidney function in a rat model of renal ischemia-reperfusion injury by treatment with a novel HIF prolyl-hydroxylase inhibitor. ASN annual meeting, St Louis MO. J Am Soc Nephrol. 2004;15:460A. [Google Scholar]
- 9.Chatterjee PK, Patel NS, Cuzzocrea S, Brown PA, Stewart KN, Mota-Filipe H, Britti D, Eberhardt W, Pfeilschifter J, Thiemermann C. The cyclopentenone prostaglandin 15-deoxy-Δ 12, 14-prostaglandin-J2 ameliorates ischemic acute renal failure. Cardiovasc Res. 2004;61:630–643. doi: 10.1016/j.cardiores.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 10.Olmos G, Conde I, Arenas MI, Del Peso L, Castellanos C, Landazuri MO, Lucio-Cazana FJ. Accumulation of hypoxia-inducible factor-1α through a novel electrophilic, thiol antioxidant-sensitive mechanism. Cell Signal. 2007;19:2098–2105. doi: 10.1016/j.cellsig.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Flügel D, Görlach A, Michiels C, Kietzmann T. Glycogen synthase kinase 3 phosphorylates hypoxia-inducible factor 1α and mediates its destabilization in a VHL-independent manner. Mol Cell Biol. 2007;27:3253–3265. doi: 10.1128/MCB.00015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu YV, Baek JH, Zhang H, Diez R, Cole RN, Semenza GL. RACK1 Competes with HSP90 for Binding to HIF-1α and is Required for O2-independent and HSP90 Inhibitor-induced Degradation of HIF-1α. Mol Cell. 2007;25:207–217. doi: 10.1016/j.molcel.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qian DZ, Kachhap SK, Collis SJ, Verheul HMW, Carducci MA, Atadja P, Pili R. Class II histone deacetylases are associated with VHL-independent regulation of hypoxia-inducible factor 1A. Cancer Res. 2006;66:8814–8821. doi: 10.1158/0008-5472.CAN-05-4598. [DOI] [PubMed] [Google Scholar]
- 14.Isaacs JS, Jung Y, Mimnaugh EG, Martine ZA, Cuttitta F, Neckers LM. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1α-degradative pathway. J Biol Chem. 2002;277:29936–29944. doi: 10.1074/jbc.M204733200. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Köhl R, Herr B, Frank R, Brüne B. Calpain mediates a von hippel-lindau protein–independent destruction of hypoxia-inducible factor-1α. Mol Cell Biol. 2006;17:1549–1558. doi: 10.1091/mbc.E05-08-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Im E, Venkatakrishan A, Kazlauskas A. Cathepsin B regulates the intrinsic angiogenic threshold of endotelial cells. Mol Biol Cell. 2005;16:3488–3500. doi: 10.1091/mbc.E04-11-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waters SL, Sarang SS, Wang KKW, Schnellmann RG. Calpain mediate calcium and chloride influx during the late phase of cell injury. J Pharmacol Exp Ther. 1997;117:7–1184. [PubMed] [Google Scholar]
- 18.Wu TY, Tan HL, Huang Q, Kim YS, Pan N, Ong WY, Liu ZG, Ong CN, Shen HM. Autophagy plays a protective role during zVAD-induced necrotic cell death. Autophagy. 2008;4:457–466. doi: 10.4161/auto.5662. [DOI] [PubMed] [Google Scholar]
- 19.Kong W, Alvarez-Castelao B, Lin ZJ, Castano JG, Caro J. Constitutive/Hypoxic Degradation of HIF-α Proteins by the Proteasome Is Independent of von Hippel Lindau Protein Ubiquitylation and the Transactivation Activity of the Protein. J Biol Chem. 2007;282:15498–15505. doi: 10.1074/jbc.M700704200. [DOI] [PubMed] [Google Scholar]
- 20.Stamatakis K, Sanchez-Gomez FJ, Perez-Sala D. Identification of novel protein targets for modification by 15-deoxy-Δ12, 14-prostaglandin-J2 in mesangial cells reveals multiple interactions with the cytoskeleton. J Am Soc Nephrol. 2006;17:89–98. doi: 10.1681/ASN.2005030329. [DOI] [PubMed] [Google Scholar]
- 21.Schotte P, Schauvliege R, Janssens S, Beyaert R. The cathepsin B inhibitor z-FA.fmk inhibits cytokine production in macrophages stimulated by lipopolysaccharide. J Biol Chem. 2001;276:21153–21157. doi: 10.1074/jbc.M102239200. [DOI] [PubMed] [Google Scholar]
- 22.Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, Kaufmann SH, Gores G. Cathepsin B contributes to TNFalpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest. 2000;106:1127–1137. doi: 10.1172/JCI9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riccio M, Di Giaimo R, Pianetti S, Palmieri PP, Melli M, Santi S. Nuclear localization of cystatin B, the cathepsin inhibitor implicated in myoclonus epilepsy (EPM1) Exp Cell Res. 2001;262:84–94. doi: 10.1006/excr.2000.5085. [DOI] [PubMed] [Google Scholar]
- 24.Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/S0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- 25.Fuertes G, Martín de Llano JJ, Villarroya A, Rivett A, Knecht E. Changes in the proteolytic activities of proteasomes and lysosomes in human fibroblasts produced by serum withdrawal, amino-acid deprivation and confluent conditions. Biochem J. 2003;375:75–80. doi: 10.1042/BJ20030282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kisselev AF, Callard A, Goldberg AL. Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. J Biol Chem. 2006;281:8582–8590. doi: 10.1074/jbc.M509043200. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Melandri F, Berdo I, Jansen M, Hunter L, Wright S, Valbrun D, Figueiredo-Pereira M. Δ12-prostaglandin J2 elicits ubiquitin-protein aggregation without proteasome inhibition. Biochem Biophys Res Commun. 2004;319:1171–1180. doi: 10.1016/j.bbrc.2004.05.098. [DOI] [PubMed] [Google Scholar]
- 28.Branca D. Calpain-related diseases. Biochem Biophys Res Commun. 2004;322:1098–1104. doi: 10.1016/j.bbrc.2004.07.126. [DOI] [PubMed] [Google Scholar]
- 29.Shibata T, Yamada T, Kondo M, Tanahashi N, Tanaka K, Nakamura H, Masutani H, Yodoi J, Uchida K. An endogenous electrophile that modulates the regulatory mechanism of protein turnover: inhibitory effects of 15-deoxy-Delta12, 14-prostaglandin-J2 on proteasome. Biochemistry. 2003;42(47):13960–13968. doi: 10.1021/bi035215a. [DOI] [PubMed] [Google Scholar]
- 30.Levonen AL, Landar A, Ramachandran A, Ceaser EK, Dickinson DA, Zanoni G, Morrow JD, Darley-Usmar VM. Cellular mechanisms of redox cell signaling: the role of cysteine modification in controlling antioxidant defenses in response to electrophilic lipid oxidation products. Biochem J. 2004;378:373–382. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez-Gomez FJ, Cernuda-Morollon E, Stamatakis K, Perez-Sala D. Protein thiol modification by 15-deoxy-Δ12, 14-prostaglandin J2 addition in mesangial cells: role in the inhibition of pro-inflammatory genes. Mol Pharmacol. 2004;66(5):1349–1358. doi: 10.1124/mol.104.002824. [DOI] [PubMed] [Google Scholar]
- 32.Lorente M, Mirapeix RM, Miguel M, Longmei W, Volk D, Cervós-Navarro J. Chronici hypoxia induced ultrastructural changes in the rat adrenal zona glomerulosa. Histol Histophathol. 2002;17(1):185–190. doi: 10.14670/HH-17.185. [DOI] [PubMed] [Google Scholar]
- 33.Welt K, Fitzl G, Schepper A. Experimental hypoxia of STZ-diabetic rat myocardium and protective effects of Ginkgo biloba extrac. II. Ultrastructura investigation of microvascular endothelium. Exp Toxicol Pathol. 2001;52(6):503–512. doi: 10.1016/S0940-2993(01)80006-3. [DOI] [PubMed] [Google Scholar]
- 34.Massey A, Kiffin R, Cuervo AM. Pathophysiology of chaperone-mediated autophagy. Int J Biochem Cell Biol. 2004;36:2420–2434. doi: 10.1016/j.biocel.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Agarraberes F, Dice J. A molecular chaperone complex at the lysosomal membrane is required for protein translocation. J Cell Sci. 2001;114:2491–2499. doi: 10.1242/jcs.114.13.2491. [DOI] [PubMed] [Google Scholar]
- 36.Kiffin R, Christian C, Knecht E, Cuervo AM. Activation of chaperone-mediated autophagy during oxidative stress. Mol Biol Cell. 2004;15:4829–4840. doi: 10.1091/mbc.E04-06-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katschinsk D, Le L, Schindler S, Thomas T, Voss A, Wenger R. Interaction of the PAS B Domain with HSP90 Accelerates Hypoxia-Inducible Factor-1α Stabilization. Cell Physiol Biochem. 2004;14:4–6. doi: 10.1159/000080345. [DOI] [PubMed] [Google Scholar]
- 38.Zheng X, Ruas JL, Cao R, Salomons FA, Cao Y, Poellinger L, Pereira T. Cell-type-specific regulation of degradation of hypoxia-inducible factor 1 alpha: role of subcellular compartmentalization. Mol Cell Biol. 2006;26:4628–4641. doi: 10.1128/MCB.02236-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan Z, Michalopoulos GK, Stolz DB. Peroxisomal localization of hypoxia-inducible factors and hypoxia inducible factor regulaoty hydroxylases in primary rat hepatocytes exposed to hypoxia-reoxigenation. Am J Pathol. 2006;169(4):1251–1269. doi: 10.2353/ajpath.2006.060360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Q, Berchner-Pfannschmidt U, Moller U, Brecht M, Wotzlaw C, Acker H, Jungermann K, Kietzmann T. Fenton reaction at the endoplasmic reticulum is involved in the redox control of hypoxia-inducible gene expression. Proc Natl Acad Sci USA. 2004;101:4302–4307. doi: 10.1073/pnas.0400265101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kidane TZ, Sauble E, Linder MC. Release of iron from ferritin requires lysosomal activity. Am J Physiol. 2006;291:C445–C455. doi: 10.1152/ajpcell.00505.2005. [DOI] [PubMed] [Google Scholar]
- 42.Cernuda-Morollon E, Pineda-Molina E, Canada FJ, Perez-Sala D. 15-Deoxy-Delta 12, 14-prostaglandin J2 inhibition of NF-kappaB-DNA binding through covalent modification of the p50 subunit. J Biol Chem. 2001;276(38):35530–35536. doi: 10.1074/jbc.M104518200. [DOI] [PubMed] [Google Scholar]
- 43.Strauss DS, Glass CK. Cyclopentenone prostaglandins: new insights on biological activities and cellular targets. Med Res Rev. 2001;21(3):185–210. doi: 10.1002/med.1006. [DOI] [PubMed] [Google Scholar]
- 44.Liu JD, Tsai SH, Lin SY, Ho YS, Hung LF, Pan S, Ho FM, Lin CM, Liang YC. Thiol antioxidant and thiol-reducing agents attenuate 15-deoxy-delta 12, 14-prostaglandin J2-induced heme oxygenase-1 expression. Life Sci. 2004;74:2451–2463. doi: 10.1016/j.lfs.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Benesic A, Schwerdt G, Freudinger R, Mildenberger S, Groezinger F, Wollny B, Kirchhoff A, Gekle M. Chloroacetaldehyde as a sulfhydryl reagent: the role of critical thiol groups in ifosfamide nephropathy. Kidney Blood Press Res. 2006;29:280–293. doi: 10.1159/000096177. [DOI] [PubMed] [Google Scholar]