Abstract
Pancreatic beta cell damage caused by pro-inflammatory cytokines interleukin-1β (IL-1β), interferon-γ (IFNγ) and tumor necrosis factor-α (TNFα) is a key event in the pathogenesis of type 1 diabetes. The suppressor of cytokine signaling-1 (SOCS-1) blocks IFNγ-induced signaling and prevents diabetes in the non-obese diabetic mouse. Here, we investigated if SOCS-1 overexpression in primary beta cells provides protection from cytokine-induced islet cell dysfunction and death. We demonstrate that SOCS-1 does not prevent increase in NO production and decrease in glucose-stimulated insulin secretion in the presence of IL-1β, IFNγ, TNFα. However, it decreases the activation of caspase-3, -8 and -9, and thereby, promotes a robust protection from cytokine-induced beta cell death. Our data suggest that SOCS-1 overexpression may not be sufficient in preventing all the biological activities of IFNγ in beta cells. In summary, we show that interference with IFNγ signal transduction pathways by SOCS-1 inhibits cytokine-stimulated pancreatic beta cell death.
Keywords: Apoptosis, Islets of Langerhans, Pancreatic beta-cell, Cytokine, Caspase, Interferon-gamma
Introduction
Type 1 diabetes is an autoimmune disease characterized by a selective destruction of the insulin-secreting pancreatic beta cells [1, 2]. Despite intensive insulin therapy, the majority of patients with type 1 diabetes eventually develop complications that decrease quality of life and reduce life expectancy. Several observations have led to the suggestion that pro-inflammatory cytokines secreted by macrophages and T lymphocytes infiltrating the pancreatic islets may contribute to the development of type 1 diabetes. Recent studies by us and others have provided further support for the hypothesis that cytokines contribute to type 1 diabetes by acting directly on the beta cells [3–6].
Added in combination, pro-inflammatory cytokines such as IL-1β, IFNγ and TNFα induce beta cell dysfunction and death [7–10]. Cytokines activate intricate networks of intracellular signal-transduction pathways, but those that lead to beta cell damage are incompletely understood. Cytokine-induced expression of the inducible nitric oxide synthase (iNOS) and elevated intracellular NO concentration contribute to decreased glucose-stimulated insulin secretion [9] and induction of beta cell death [11, 12] in cultured rodent islets. However, NO-independent pathways contributing to beta cell death have been described [10]. Many cytokine-induced signal-transduction pathways converge at the level of caspase activation, and we and others have shown the importance of caspase-3 activation for cytokine-induced apoptosis in islets and beta cell lines [12–15]. Caspase-3 is an effector caspase that is activated by initiator caspases [16], such as caspase-8 and caspase-9. Caspase-8 is shown to be activated by TNFα in insulin-producing cell lines [17, 18], and caspase-9 by the combination of IL-1β, IFNγ, and TNFα in human pancreatic islets [19].
In vitro, IFNγ itself does not induce cell death [20]; however, it potentiates the detrimental effects of IL-1β or TNFα on islet cell function and survival [7, 20, 21]. Moreover, TNFα induces apoptosis of primary beta cells only when added in combination with IFNγ [22]. These data suggest that IFNγ-induced signal-transduction pathways control the pathways activated by other cytokines leading to primary beta cell damage. IFNγ signals via a receptor associated with janus-activated kinase (JAK) and, upon IFNγ binding to the receptor, JAK becomes activated. Activated JAK phosphorylates the IFNγ receptor allowing signal transducer and activator of transcription (STAT-1) to bind. The subsequent phosphorylation of STAT-1 is a crucial step in the formation of STAT-1-STAT-1 homodimers, which translocate to the nucleus and initiate the transcription of IFNγ-activated genes [23, 24]. Besides its participation in IFNγ triggered signaling, STAT-1 is a component of, for example, the TNFα and IL-1β receptor complexes, and IFNγ can induce interaction between TRADD, a component of TNFα receptor complex, and STAT-1 [25–27].
Activation of the JAK/STAT pathway is prevented by the suppressor of cytokine signaling-1 (SOCS-1), which binds to phosphorylated JAK and one of the phosphotyrosine residues on the IFNγ receptor. The consequence of this interaction is inhibition of JAK and prevention of downstream STAT-1 activation [28, 29]. Besides this, SOCS-1 has been shown to accelerate the ubiquitination and degradation of phosphorylated JAK [30], and SOCS-1 also interferes with Toll-like receptor (TLR) signaling [31–33], a receptor family to which the IL-1β receptor belongs.
We have recently demonstrated that beta cell expression of SOCS-1 protects NOD mice from developing diabetes [4]. Others have shown that SOCS-1 deficient beta cells are hypersensitive to TNFα-induced NO production and TNFα-induced cell death [20]. However, how signaling via the JAK/STAT pathway controls beta cell dysfunction and death induced by other cytokines is not entirely clear. To further evaluate this we have investigated whether beta cell expression of SOCS-1 affects islet cell function and death induced by a combination of pro-inflammatory cytokines (IL-1β, IFNγ, and TNFα). Specifically, we have investigated the effects of SOCS-1 overexpression on insulin release, cytokine-induced NO production, caspase activation and islet cell death.
Materials and methods
Materials
RPMI-1640 medium and fetal calf serum were obtained from Gibco (Middlesex, UK). Bovine serum albumin (BSA), extravidin fluorescein isothiocyanate (FITC) conjugate, propidium iodide (PI) and recombinant murine TNFα were purchased from Sigma (St. Louis, MO, USA). Recombinant human IL-1β was received from Calbiochem (San Diego, CA, USA). Recombinant murine IFNγ was purchased from Life Technologies (Gaithersburg, MD, USA). The kit for TdT-mediated X-dUTP was purchased from Roche (Basel, Switzerland). All other reagents were of analytical grade and were obtained from VWR International (West Chester, PA, USA).
Animals
C57BL/6 (here denoted B6) and SOCS-1-Tg mice on the B6 background [34, 35] originally obtained from The Scripps Research Institute (La Jolla, CA), were bred and maintained at a specific pathogen free environment at Karolinska Institutet (Stockholm, Sweden). Heterozygote SOCS-1-Tg B6 mice were bred with non-transgenic B6 mice and the littermates were genotyped by PCR analysis of tail DNA [34]. All experiments were conducted in accordance with institutional guidelines and approved by the local animal ethics committee at Karolinska Institutet (Stockholm, Sweden).
Isolation of islets and islet cells
Islets of Langerhans from 2- to 6-month-old B6 mice or SOCS-1-Tg B6 mice were isolated by collagenase digestion and hand-picked as previously described [36]. The islets were cultured in RPMI-1640 medium supplemented with 11 mM glucose, 10% (vol/vol) fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (standard medium) with or without a mixture of IL-1β and TNFα (IL-1β, 25 U/ml; TNFα, 100 U/ml) or a mixture of IL-1β, TNFα, and IFNγ (IL-1β, 25 U/ml; IFNγ, 100 U/ml; TNFα, 100 U/ml) at 37°C for 40 h.
For determination of NO production and for measurements of caspase activity, islets were disrupted into a suspension of single cells with dispase followed by centrifugation in BSA as previously described [37]. Cell preparations were plated in microtiter plates and cultured in the standard medium with or without the cytokine mixtures at 37°C for 40 h.
Measurement of islet cell apoptosis
The terminal transferase-mediated dUTP nick end labeling (TUNEL) technique was used to detect DNA strand breaks in situ as previously described [38, 39]. Islets were double-stained with FITC and PI and fixed on glass slides with 80% glycerol in phosphate-buffered saline. Fluorescence was monitored with Leica TCS-NT laser scanning confocal microscope (Leica Microsystems, Wetzlar, Germany) with excitation from the 488-nm line of an argon/krypton laser. Fluorescence emission was detected with a band-pass filter (Chroma Technology, Rockingham, VT, USA) centered at 530 nm for FITC and above 590 nm for PI. Several confocal images were used for counting the number of apoptotic cells in each islet.
Measurement of insulin release
Static incubations of isolated islets and measurements of insulin release were performed as previously described [40].
Nitrite determination
Islet cell nitrite production was determined with Griess reaction. Culture medium was withdrawn and centrifuged for 2 min at 1,500g, and 100 μl samples of supernatant were transferred to a 96-well plate and mixed with 50 μl of Griess reagent (Alexis, Carlsbad, CA, USA) as previously described [12, 38]. The reaction was carried out for 15 min at room temperature. Nitrite production was determined by 540 nm absorbance with reference at 620 nm in a 96-well plate reader. For calibration of data, a standard curve for NaNO2 in RPMI 1640 medium was established in each assay. The results were expressed as μM of NO2 − per μg of total protein.
Caspase activity measurement
Measurement of caspase activity was performed using fluorometric Caspase-3 Activity Assay, Caspase-8 Activity Assay (Oncogene, Darmstadt, Germany) and Caspase-9 Fluorometric Assay (R&D Systems, Minneapolis, MN, USA) according to the supplier’s instructions. The assays are based on the cleavage of a caspase-specific substrate labelled with a fluorescent molecule, 7-amino-4-trifluoromethyl coumarin (AFC). Reaction was monitored by a blue to green shift in fluorescence upon cleavage of the AFC fluorophore. Fluorescence measurements were performed using a fluorescence plate reader, with excitation and emission wavelengths of 400 and 505 nm, respectively, and bypass filter 430 nm. Results were quantified as relative units of caspase activity and expressed as percentage of the activity in unstimulated B6 islet cells.
Statistical analysis
The difference of means was estimated with unpaired Student’s t test using Sigma Plot 2001 for Windows (Jandel, USA).
Results
No effects of SOCS-1 on cytokine-induced inhibition of glucose-stimulated insulin secretion
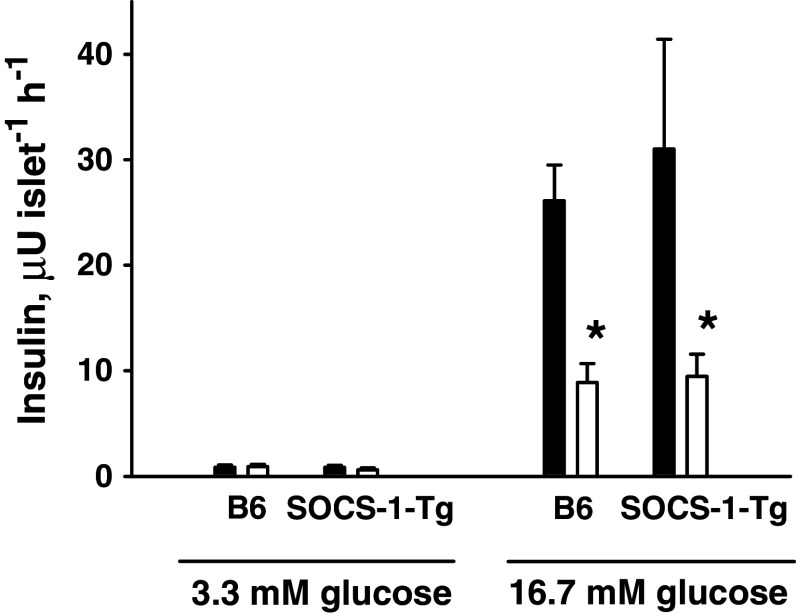
A well-known deleterious effect of pro-inflammatory cytokines on islet cell function is the reduction of glucose-induced insulin release. Previous studies have indicated that this effect is dependent upon an IFNγ-driven signal transduction pathway [41, 42]. Therefore, we first evaluated whether beta cell expression of SOCS-1 affects the inhibition of glucose-stimulated insulin secretion induced by cytokines. To this end, islets isolated from B6 and SOCS-1-Tg B6 mice were incubated in the presence or absence of the mixture of IL-1β, TNFα, and IFNγ for 40 h. Following incubation, glucose-stimulated insulin release was determined. The results depicted in Fig. 1 demonstrate that overexpression of SOCS-1 in beta cells did not affect glucose-induced insulin secretion under normal conditions. As expected, incubation with cytokines impaired glucose-induced insulin release in B6 islets (Fig. 1). Similarly, cytokine-treated islets from SOCS-1-Tg mice demonstrated an impaired glucose response (Fig. 1), suggesting that overexpression of SOCS-1 does not protect pancreatic beta cells from cytokine-induced decrease in glucose-stimulated insulin secretion.
Fig. 1.
Expression of SOCS-1 does not affect cytokine-induced inhibition of glucose-stimulated insulin secretion. B6 or SOCS-1-Tg mouse islets were incubated with or without a mixture of IL-1β, TNFα and IFNγ for 40 h. Insulin release was determined by radioimmunoassay using rat insulin as standard. Black bars control islets, white bars cytokine-treated islets. Results are means ± SEM of five separate experiments. *P < 0.05 relative to untreated control islets of the same genotype
Overexpression of SOCS-1 protects from islet cell death induced by cytokines
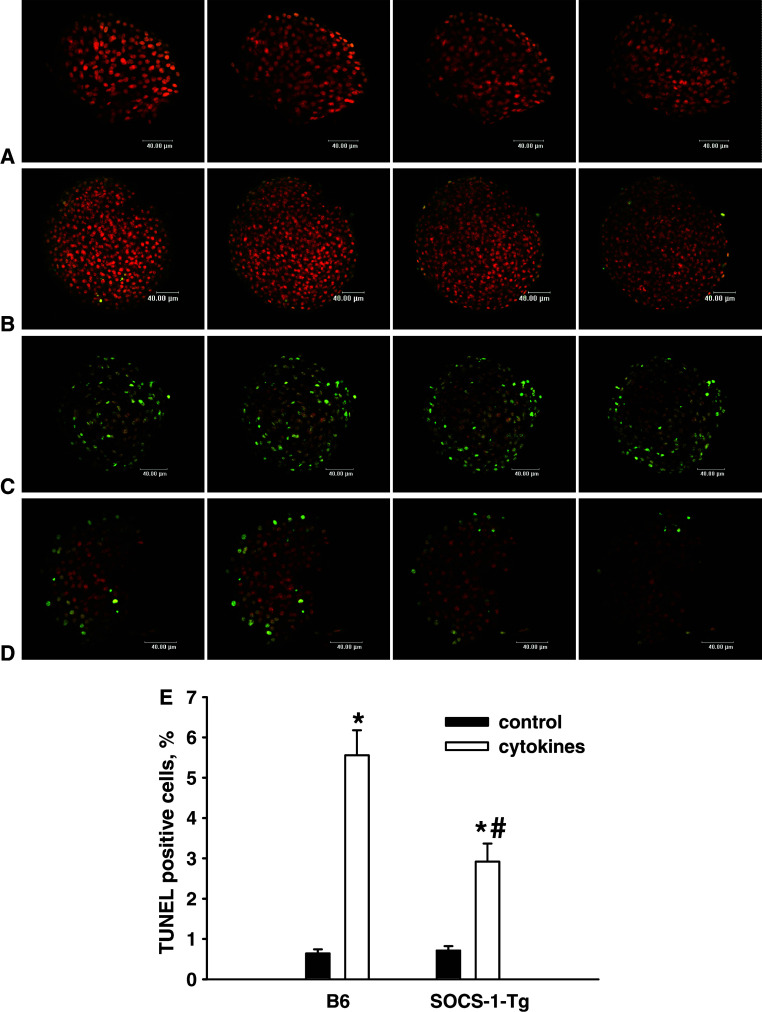
NOD mice harboring beta cells expressing SOCS-1 have a markedly reduced incidence of diabetes [4], indicating that SOCS-1 may protect against beta cell death. Previous studies have demonstrated that cytokines are potent inducers of beta cell apoptosis [10]. To evaluate whether SOCS-1 expression protects against cytokine-induced cell death, apoptosis was determined in islets stimulated with the mixture of IL-1β, TNFα, and IFNγ by TUNEL technique. As shown in Fig. 2, SOCS-1 overexpression did not affect the level of apoptosis in untreated islets. A pronounced increase in apoptotic cell death was observed in cytokine-treated B6 islets. However, a markedly reduced apoptosis was observed in cytokine-treated SOCS-1-Tg islets. These observations indicate that beta cell expression of SOCS-1 provides substantial protection against cytokine-induced cell death.
Fig. 2.
Expression of SOCS-1 protects islet cells from cytokine-induced cell death. B6 or SOCS-1-Tg mouse islets were incubated with or without the mixture of IL-1β, TNFα and IFNγ for 40 h. Confocal images of B6 control islet (a), SOCS-1-Tg control islet (b), B6 islet incubated with cytokines (c), SOCS-1-Tg islet incubated with cytokines (d), after nick translation labelling of DNA strand breaks (TUNEL) and double staining with FITC/PI. Apoptotic cells have a green colour. Bar width 40 μm. Several confocal images of each islet are represented. e Percentage of TUNEL positive cells detected by confocal microscopy. Black bars control islets; white bars cytokine-treated islets. Results are means ± SEM of 74–103 islets from five B6 and six SOCS-1-Tg mice. *P < 0.0005 relative to untreated control islets of the same genotype, # P < 0.05 relative to B6 islets treated with cytokines
SOCS-1 does not affect cytokine-induced NO formation
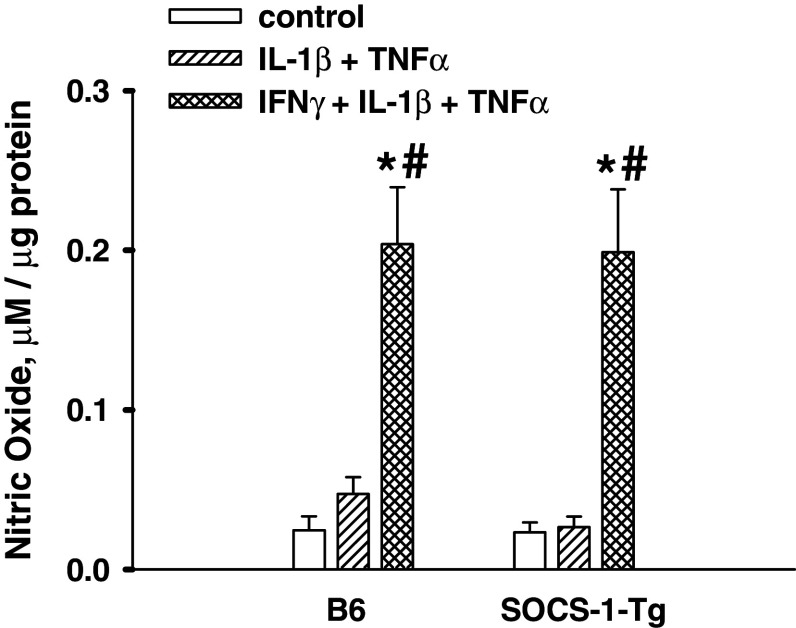
It has been shown that NO produced upon treatment with cytokines plays an important role in pancreatic beta cell death [9, 12, 43]. In order to study the mechanisms underlying the protective effect of SOCS-1, we next investigated whether SOCS-1 affects NO production induced by the mixture of IL-1β, TNFα, and IFNγ. To this end, NO was measured in media harvested from B6 mice and SOCS-1-Tg B6 mouse islet cell preparations incubated in the presence or absence of the cytokine mixture using the Griess reaction. The results shown in Fig. 3 demonstrate that the cytokine mixture induced production of NO in B6 mouse islet cells. Moreover, SOCS-1 overexpression did not prevent NO formation. The data obtained suggest that changes in NO production cannot be responsible for the protective effect of SOCS-1 overexpression in beta cells.
Fig. 3.
SOCS-1 overexpression does not affect cytokine-induced NO production by islet cells. B6 or SOCS-1-Tg mouse islet cells were incubated with the mixture of IL-1β and TNFα or with the mixture of IL-1β, TNFα and IFNγ for 40 h. NO production was measured as release of NO2 − into the medium by using Griess reagent. Results are means ± SEM of 4–11 independent experiments. *P < 0.05 relative to untreated control islet cells of the same genotype, # P < 0.05 relative to islet cells of the same genotype treated with the mixture of IL-1β and TNFα
It is a well-known fact that IFNγ substantially potentiates IL-1β-induced NO production in pancreatic islets [7]. The absence of difference in cytokine-induced NO formation in B6 and SOCS-1-Tg B6 mouse islet cells raised the question whether IFNγ activates signal transduction pathway(s) leading to elevation of NO production in B6 and SOCS-1-Tg B6 mouse islet cells under our experimental conditions. Therefore, we examined whether the mixture of IL-1β and TNFα can induce NO production and whether IFNγ affects NO production induced by the mixture of IL-1β and TNFα in B6 mice and SOCS-1-Tg B6 mouse islet cells. Following stimulation with the mixtures of cytokines, NO was measured in media harvested from B6 mice and SOCS-1-Tg B6 mouse islet cell preparations. The data presented in Fig. 3 demonstrate that the mixture of IL-1β and TNFα at concentrations used did not induce NO formation in B6 mouse islet cells. Neither did the mixture stimulate NO production in SOCS-1-Tg B6 mouse islet cells. However, an addition of IFNγ to the mixture of IL-1β and TNFα produced a strong stimulatory effect on NO formation both in B6 and SOCS-1-Tg B6 mouse islet cells. The obtained results suggest that IFNγ-induced signal-transduction pathway(s) leading to elevation of NO production are active both in B6 and in SOCS-1-Tg B6 mouse islet cells.
Thus, our data clearly demonstrate that the NO formation in SOCS-1-Tg B6 mouse islet cells incubated with the cytokine mixture containing IFNγ is drastically elevated compared to that incubated with the combination of IL-1β and TNFα. Taking this into consideration, we then evaluated whether the presence of IFNγ in cytokine mixture affects also the level of cell death in SOCS-1-Tg B6 islets. Apoptosis was determined in SOCS-1-Tg B6 islets incubated with the combination of IL-1β and TNFα or the mixture of IL-1β, TNFα, and IFNγ by the TUNEL technique. In good correlation with the absence of NO production, the mixture of two cytokines did not enhance SOCS-1-Tg B6 islet cell death (93.8 ± 13.8% of 42 islets from 6 mice) compared to control conditions (100 ± 10.7% of 83 islets from 12 mice). On the contrary, the presence of IFNγ in the mixture produced an increase in cell death in SOCS-1-Tg B6 islets (289.8 ± 35.7% of 51 islets from 12 mice; *P < 0.05 relative to untreated control, # P < 0.05 relative to islets treated with two cytokines), although the level of cell death induced by a combination of IL-1β, TNFα, and IFNγ in SOCS-1-Tg B6 islets is approximately half of that in B6 islets (Fig. 2). The increase in cell death in SOCS-1-Tg B6 islets incubated with the combination of IL-1β, TNFα, and IFNγ is in line with the strong induction of NO formation under this condition (Fig. 3), suggesting that the elevation of cell death in the presence of IFNγ could be attributed to the elevation of NO.
SOCS-1 decreases cytokine-induced caspase activity in islet cells
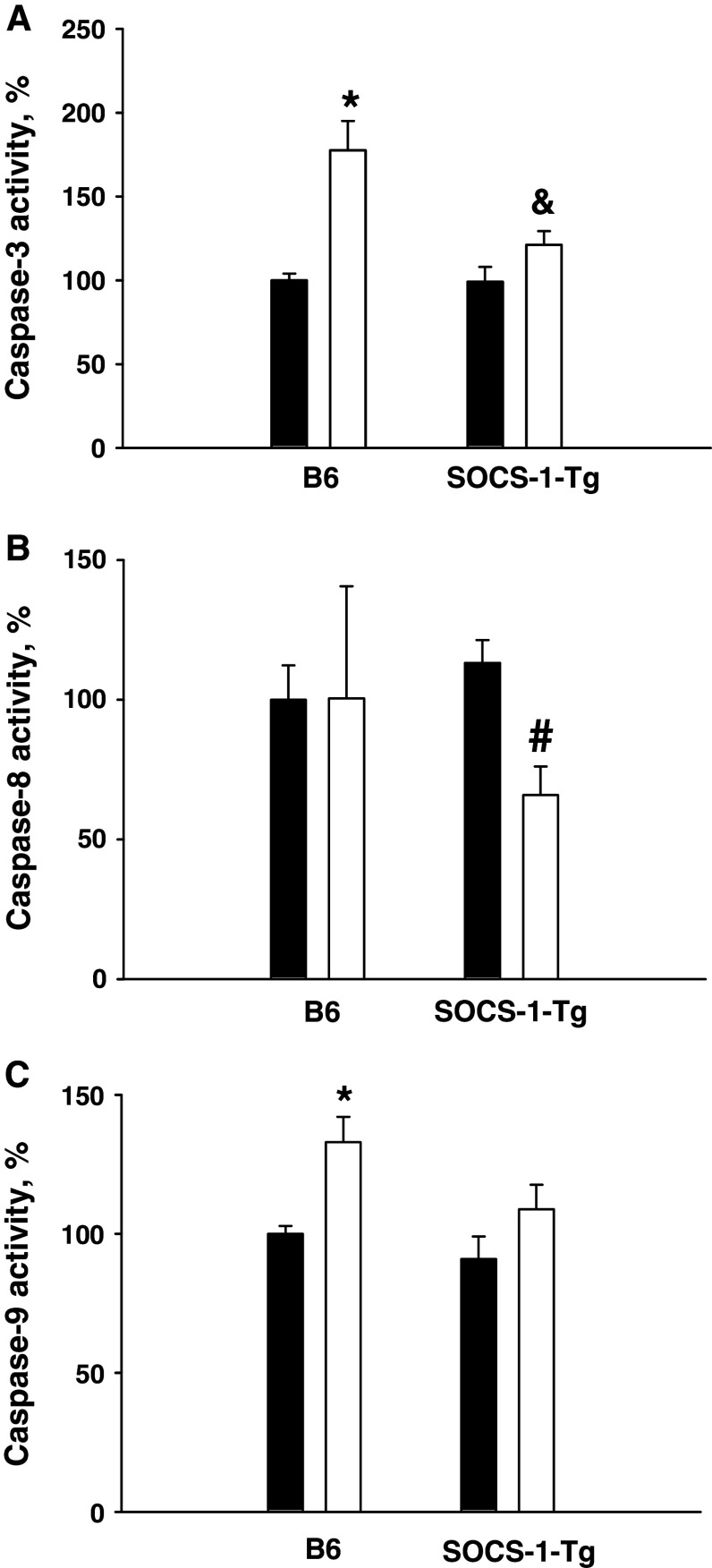
Caspase activation plays a central role in the execution of apoptosis [44]. It has previously been shown that cytokines induce caspase-3 activation in pancreatic beta cells [45], and that activation of this effector caspase is essential for the induction of apoptosis in beta cells [14, 18]. Moreover, SOCS-1 deficiency increases TNFα-mediated caspase activation in pancreatic beta cells [20]. To further investigate the mechanisms involved in the anti-apoptotic effect of SOCS-1, we next evaluated caspase-3 activation induced by cytokines in islet cells from B6 and SOCS-1 mice. To this end, caspase-3 activity was measured in cell lysates from islet cells incubated in the presence or absence of the mixture of IL-1β, TNFα, and IFNγ for 40 h. In good correlation with the enhanced apoptosis (Fig. 2), cytokines increased caspase-3 activation in B6 mouse islet cells (Fig. 4a). In contrast, the cytokine mixture failed to significantly activate caspase-3 in SOCS-1 overexpressing islet cells (Fig. 4a). These results suggest that the protective effect of beta cell expression of SOCS-1 at least in part relies on the inhibition of caspase-3 activation in response to cytokine treatment.
Fig. 4.
Cytokines activate caspases in B6 but not in SOCS-1-Tg islet cells. B6 or SOCS-1-Tg mouse islet cells were incubated with or without the mixture of IL-1β, TNFα, and IFNγ for 40 h. Caspase-3 (a), caspase-8 (b), and caspase-9 (c) activities were measured by a fluorometric assay (see “Methods”). Black bars control, white bars cytokine-treated. Results are means ± SEM of 6–14 observations per condition from 3–6 independent experiments. The activity in unstimulated B6 islet cells was defined as 100%. *P < 0.05 relative to unstimulated B6 islet cells, & P < 0.05 relative to B6 islet cells stimulated with cytokines, # P < 0.05 relative to unstimulated SOCS-1-Tg islet cells
The effector caspase-3 is known to be activated by initiator caspases [16], including caspase-8 and caspase-9, that were previously shown to be activated by cytokines in beta cell lines or primary beta cells [18, 19]. To determine which apoptotic pathway leading to caspase-3 activation is blocked by SOCS-1, we evaluated the effects of SOCS-1 overexpression on cytokine-induced caspase-8 and caspase-9 activation. Following stimulation with the mixture of IL-1β, TNFα, and IFNγ for 40 h, caspase-8 and caspase-9 activities were measured in cell lysates from B6 or SOCS-1-Tg B6 mouse islet cells. In contrast to previous reports using beta cell lines [17, 18], we were unable to detect any caspase-8 activation in primary B6 islet cells stimulated with cytokines. However, caspase-9 was activated in islet cells from B6 mice stimulated with the cytokine mixture (Fig. 4b, c). In full agreement with the absence of caspase-3 activation in islet cells overexpressing SOCS-1, cytokine stimulation did not result in the activation of caspase-8 and caspase-9. In fact, SOCS-1 overexpressing beta cells demonstrated a reduced activity of caspase-8 (Fig. 4b, c). Taken together, these findings indicate that SOCS-1 inhibits activation of initiator caspases in primary beta cells.
Discussion
Cytokines contribute to beta cell destruction in type 1 diabetes. IFNγ impairs glucose-stimulated insulin secretion [41] and markedly potentiates the detrimental effects of other pro-inflammatory cytokines on beta cell function and survival [7, 21]. Intracellular signaling by IFNγ and some other cytokines using the JAK/STAT signaling pathway can be prevented by SOCS-1 [30], and overexpression of SOCS-1 in pancreatic beta cells protects from cytokine damage and diabetes development in the NOD mouse [4]. To further understand the mechanisms behind this protection, we investigated how SOCS-1 affects the islet response to multiple pro-inflammatory cytokines. We now demonstrate that IFNγ-induced signal-transduction pathways are activated in B6 mouse islet cells and that overexpression of SOCS-1 does not protect pancreatic islet cells against impairment in glucose-stimulated insulin secretion or prevent islet cell NO production induced by a mixture of IL-1β, TNFα, and IFNγ. However, it provides a robust protection against islet cell apoptosis induced by a combination of IL-1β, TNFα, and IFNγ. This is accomplished by an inhibition of the pathways leading to caspase-9 and subsequently caspase-3 activation. Hence, inhibition of cytokine-induced pancreatic islet cell death triggered by SOCS-1 is based on the inhibition of activation of caspases.
Caspase-3 activation may be a final event in the induction of beta cell apoptosis [13, 18]. Our data support this view since activation of caspase-3 in B6 mouse islet cells is accompanied by an increase in cell death. Initiator caspase-8 and caspase-9 can activate the effector caspase-3 [44], and we and others demonstrated that cytokines activate caspase-8 in beta cell lines [17, 18]. However, our observation that caspase-8 known to be induced after ligation of the TNF receptor [46] is not activated in primary mouse islet cells incubated with a combination of IL-1β, TNFα, and IFNγ suggests that the mechanisms underlying cytokine-induced primary beta cell death may differ from those triggering cell death in beta cell lines. In that way, the intrinsic mitochondrial apoptotic pathway leading to caspase-9 activation, followed by an increased caspase-3 activity, is likely to be important for the induction of cytokine-stimulated primary islet cell death.
Overexpression of SOCS-1 in B6 mouse pancreatic beta cells resulted in a pronounced decrease in cytokine-induced islet cell apoptosis. This was accompanied by the absence of caspase-9 activation and a substantial decrease in caspase-8 activation. Therefore, SOCS-1 may interfere with the pathways activating caspase-8 and caspase-9, respectively. The interference with the caspase-8 activation pathway can be due to a blockage in STAT-1 and TNF receptor interactions [18, 20, 26], or to inhibition of STAT-1-dependent IFNγ-induced increased caspase-8 expression [47]. The former pathway starting from the TNF receptor can activate both caspase-8 and c-jun N-terminal kinase (JNK) [46], and it was previously reported that TNFα potentiates IL-1β-induced JNK activation in pancreatic islets [48]. Activated JNK may initiate apoptosis by interfering with mitochondria, resulting in the release of cytochrome c [49] and subsequent activation of caspase-9. It was previously shown that SOCS-1 inhibits TNFα-induced apoptosis in part by interfering with TNFα-induced JNK activation [50–52]. Hence, it is possible that SOCS-1, by interfering with the TNFα pathway, abolishes the potentiation of IL-1β-induced JNK activation and therefore reduces caspase-9 activation. If so, then it is likely that SOCS-1 inhibits the activation of both caspase-8 and caspase-9 by its interaction with the pathway(s) by which IFNγ regulates the TNFα signaling pathway.
The elevated NO production is discussed to be an important factor for the induction of pancreatic beta cell death [10–12]. The mixture of IL-1β and TNFα does not affect cell death as well as NO formation in the SOCS-1-Tg B6 islets, while the presence of IFNγ in the cytokine mixture induces both the elevation of cell death and rise in NO production. However, despite the fact that NO production is elevated in the SOCS-1-Tg B6 islet cells up to the level observed in B6 mouse islet cells, the cytokine-induced cell death in the SOCS-1-Tg B6 islets is significantly less than in B6 islets. Therefore, the inhibition of cytokine-induced pancreatic islet cell death by SOCS-1 is not mediated by changes in NO production.
The observation that SOCS-1 overexpression does not affect NO synthesis indicates that the pathways perturbed by SOCS-1 are not essential for cytokine-induced NO production. This finding was somewhat surprising, as IFNγ potentiates IL-1β-induced islet cell NO production ([7] and Fig. 3). It is possible that SOCS-1 does not interfere with the IL-1β driven signal-transduction and the pathway(s) by which IFNγ enhances IL-1β-induced NO production. Indeed, IFNγ activates pathways other than the JAK/STAT-signaling pathway, including the mitogen-activated protein kinase (MAPK) pathway [23], which may contribute to the potentiation of NO production [53, 54]. Likewise, SOCS-1 may not block the signaling pathways that are essential for the decrease in glucose-stimulated insulin secretion observed after stimulation with a combination of IL-1β, TNFα, and IFNγ. Impairment of this important beta cell function is dependent upon NO in murine islets [7, 9, 42]. Our studies show that inhibition of glucose-stimulated insulin secretion induced by a mixture of IL-1β, TNFα, and IFNγ correlates with an increase in NO production. Based upon this and previously published data [7, 9, 42], it may be hypothesized that the cytokine-induced decrease in glucose-stimulated insulin secretion is a consequence of increased NO production driven by IFNγ-dependent signal-transduction pathways other than the JAK/STAT pathway.
Transplantation of pancreatic islets which can accurately sense and respond to the quick changes in blood glucose levels is a desired approach in the treatment of type 1 diabetes. However, transplanted islets are extremely sensitive to host immune responses, and such immune responses play an important role in early islet graft destruction. An inflammatory reaction generated by the transplant itself may result in the release of pro-inflammatory cytokines. Moreover, a primed autoimmune response to beta cells causes activation of beta cell-specific autoimmunity leading to cytokine exposure. Our finding that SOCS-1 expression does not have a major effect on glucose-stimulated insulin secretion, together with the observed protection against cytokine-induced islet cell death supports the possibility to use SOCS-1 overexpression as a means to genetically engineer beta cells with resistance to inflammation.
In summary, our data suggest that protection from type 1 diabetes in NOD mice harboring beta cells that express SOCS-1 may at least in part be explained by reduced ability of pro-inflammatory cytokines to induce cell death. Thus, our data support the notion that a block in IFNγ-driven signal transduction inhibits cytokine-stimulated pancreatic beta cell death [6, 42, 55]. However, an inhibition of the JAK-STAT signaling pathway alone may not be sufficient for the prevention of all the biological activities of IFNγ in beta cells. Therefore, future work may reveal JAK-STAT-independent pathways that are triggered by IFNγ in beta cells and which may be evaluated for their relative importance with regard to beta cell function and viability.
Acknowledgments
This work was supported by Grant N5-2001-34 from the Juvenile Diabetes Foundation International, the Swedish Foundation for Strategic Research, the European Foundation for the Study of Diabetes, the Swedish Research Council, the Swedish Diabetes Association, funds from Karolinska Institutet, the Novo Nordisk Foundation, The Family Erling-Persson Foundation, Berth von Kantzow’s Foundation, and Novo Nordisk A/S. Stella Jacobson is greatly acknowledged for assistance with the genotyping of the mice.
References
- 1.Chandra J, Zhivotovsky B, Zaitsev S, Juntti-Berggren L, Berggren PO, Orrenius S. Role of apoptosis in pancreatic beta-cell death in diabetes. Diabetes. 2001;50(Suppl 1):S44–S47. doi: 10.2337/diabetes.50.2007.S44. [DOI] [PubMed] [Google Scholar]
- 2.Mandrup-Poulsen T. Beta cell death and protection. Ann N Y Acad Sci. 2003;1005:32–42. doi: 10.1196/annals.1288.005. [DOI] [PubMed] [Google Scholar]
- 3.Hultcrantz M, Jacobson S, Hill NJ, Santamaria P, Flodstrom-Tullberg M. The target cell response to cytokines governs the autoreactive T cell repertoire in the pancreas of NOD mice. Diabetologia. 2009;52:299–305. doi: 10.1007/s00125-008-1193-7. [DOI] [PubMed] [Google Scholar]
- 4.Flodstrom-Tullberg M, Yadav D, Hagerkvist R, Tsai D, Secrest P, Stotland A, Sarvetnick N. Target cell expression of suppressor of cytokine signaling-1 prevents diabetes in the NOD mouse. Diabetes. 2003;52:2696–2700. doi: 10.2337/diabetes.52.11.2696. [DOI] [PubMed] [Google Scholar]
- 5.Chong MM, Chen Y, Darwiche R, Dudek NL, Irawaty W, Santamaria P, Allison J, Kay TW, Thomas HE. Suppressor of cytokine signaling-1 overexpression protects pancreatic beta cells from CD8 + T cell-mediated autoimmune destruction. J Immunol. 2004;172:5714–5721. doi: 10.4049/jimmunol.172.9.5714. [DOI] [PubMed] [Google Scholar]
- 6.Barral AM, Thomas HE, Ling EM, Darwiche R, Rodrigo E, Christen U, Ejrnaes M, Wolfe T, Kay TW, von Herrath MG. SOCS-1 protects from virally-induced CD8 T cell mediated type 1 diabetes. J Autoimmun. 2006;27:166–173. doi: 10.1016/j.jaut.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Corbett JA, Sweetland MA, Wang JL, Lancaster JR, Jr, McDaniel ML. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci USA. 1993;90:1731–1735. doi: 10.1073/pnas.90.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Southern C, Schulster D, Green IC. Inhibition of insulin secretion by interleukin-1 beta and tumour necrosis factor-alpha via an L-arginine-dependent nitric oxide generating mechanism. FEBS Lett. 1990;276:42–44. doi: 10.1016/0014-5793(90)80502-A. [DOI] [PubMed] [Google Scholar]
- 9.Hadjivassiliou V, Green MH, James RF, Swift SM, Clayton HA, Green IC. Insulin secretion, DNA damage, and apoptosis in human and rat islets of Langerhans following exposure to nitric oxide, peroxynitrite, and cytokines. Nitric Oxide. 1998;2:429–441. doi: 10.1006/niox.1998.0203. [DOI] [PubMed] [Google Scholar]
- 10.Eizirik DL, Mandrup-Poulsen T. A choice of death—the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia. 2001;44:2115–2133. doi: 10.1007/s001250100021. [DOI] [PubMed] [Google Scholar]
- 11.Cetkovic-Cvrlje M, Eizirik DL. TNF-alpha and IFN-gamma potentiate the deleterious effects of IL-1 beta on mouse pancreatic islets mainly via generation of nitric oxide. Cytokine. 1994;6:399–406. doi: 10.1016/1043-4666(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 12.Zaitsev SV, Appelskog IB, Kapelioukh IL, Yang SN, Kohler M, Efendic S, Berggren PO. Imidazoline compounds protect against interleukin 1beta-induced beta-cell apoptosis. Diabetes. 2001;50(Suppl 1):S70–S76. doi: 10.2337/diabetes.50.2007.S70. [DOI] [PubMed] [Google Scholar]
- 13.Todaro M, Di Gaudio F, Lavitrano M, Stassi G, Papaccio G. Islet beta-cell apoptosis triggered in vivo by interleukin-1beta is not related to the inducible nitric oxide synthase pathway: evidence for mitochondrial function impairment and lipoperoxidation. Endocrinology. 2003;144:4264–4271. doi: 10.1210/en.2003-0385. [DOI] [PubMed] [Google Scholar]
- 14.Kohler M, Zaitsev SV, Zaitseva II, Leibiger B, Leibiger IB, Turunen M, Kapelioukh IL, Bakkman L, Appelskog IB, de Monvel JB, Imreh G, Berggren PO. On-line monitoring of apoptosis in insulin-secreting cells. Diabetes. 2003;52:2943–2950. doi: 10.2337/diabetes.52.12.2943. [DOI] [PubMed] [Google Scholar]
- 15.Augstein P, Bahr J, Wachlin G, Heinke P, Berg S, Salzsieder E, Harrison LC. Cytokines activate caspase-3 in insulinoma cells of diabetes-prone NOD mice directly and via upregulation of Fas. J Autoimmun. 2004;23:301–309. doi: 10.1016/j.jaut.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 17.Zaitseva II, Storling J, Mandrup-Poulsen T, Berggren PO, Zaitsev SV. The imidazoline RX871024 induces death of proliferating insulin-secreting cells by activation of c-jun N-terminal kinase. Cell Mol Life Sci. 2008;65:1248–1255. doi: 10.1007/s00018-008-7564-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suk K, Kim S, Kim YH, Kim KA, Chang I, Yagita H, Shong M, Lee MS. IFN-gamma/TNF-alpha synergism as the final effector in autoimmune diabetes: a key role for STAT1/IFN regulatory factor-1 pathway in pancreatic beta cell death. J Immunol. 2001;166:4481–4489. doi: 10.4049/jimmunol.166.7.4481. [DOI] [PubMed] [Google Scholar]
- 19.Contreras JL, Smyth CA, Bilbao G, Young CJ, Thompson JA, Eckhoff DE. 17beta-Estradiol protects isolated human pancreatic islets against proinflammatory cytokine-induced cell death: molecular mechanisms and islet functionality. Transplantation. 2002;74:1252–1259. doi: 10.1097/00007890-200211150-00010. [DOI] [PubMed] [Google Scholar]
- 20.Chong MMW, Thomas HE, Kay TWH. Suppressor of cytokine signaling-1 regulates the sensitivity of pancreatic beta cells to tumor necrosis factor. J Biol Chem. 2002;277:27945–27952. doi: 10.1074/jbc.M110214200. [DOI] [PubMed] [Google Scholar]
- 21.Hoorens A, Stange G, Pavlovic D, Pipeleers D. Distinction between interleukin-1-induced necrosis and apoptosis of islet cells. Diabetes. 2001;50:551–557. doi: 10.2337/diabetes.50.3.551. [DOI] [PubMed] [Google Scholar]
- 22.Stephens LA, Thomas HE, Ming L, Grell M, Darwiche R, Volodin L, Kay TW. Tumor necrosis factor-alpha-activated cell death pathways in NIT-1 insulinoma cells and primary pancreatic beta cells. Endocrinology. 1999;140:3219–3227. doi: 10.1210/en.140.7.3219. [DOI] [PubMed] [Google Scholar]
- 23.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 24.O’Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109(Suppl):S121–S131. doi: 10.1016/S0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- 25.Wesemann DR, Qin H, Kokorina N, Benveniste EN. TRADD interacts with STAT1-alpha and influences interferon-gamma signaling. Nat Immunol. 2004;5:199–207. doi: 10.1038/ni1025. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Wu TR, Cai S, Welte T, Chin YE. Stat1 as a component of tumor necrosis factor alpha receptor 1-TRADD signaling complex to inhibit NF-kappaB activation. Mol Cell Biol. 2000;20:4505–4512. doi: 10.1128/MCB.20.13.4505-4512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen H, Chatterjee-Kishore M, Jiang Z, Qing Y, Ramana CV, Bayes J, Commane M, Li X, Stark GR. IRAK-dependent phosphorylation of Stat1 on serine 727 in response to interleukin-1 and effects on gene expression. J Interferon Cytokine Res. 2003;23:183–192. doi: 10.1089/107999003765027384. [DOI] [PubMed] [Google Scholar]
- 28.Wormald S, Hilton DJ. Inhibitors of cytokine signal transduction. J Biol Chem. 2004;279:821–824. doi: 10.1074/jbc.R300030200. [DOI] [PubMed] [Google Scholar]
- 29.Qing Y, Costa-Pereira AP, Watling D, Stark GR. Role of tyrosine 441 of interferon-gamma receptor subunit 1 in SOCS-1-mediated attenuation of STAT1 activation. J Biol Chem. 2005;280:1849–1853. doi: 10.1074/jbc.M409863200. [DOI] [PubMed] [Google Scholar]
- 30.Davey GM, Heath WR, Starr R. SOCS1: a potent and multifaceted regulator of cytokines and cell-mediated inflammation. Tissue Antigens. 2006;67:1–9. doi: 10.1111/j.1399-0039.2005.00532.x. [DOI] [PubMed] [Google Scholar]
- 31.Mansell A, Smith R, Doyle SL, Gray P, Fenner JE, Crack PJ, Nicholson SE, Hilton DJ, O’Neill LA, Hertzog PJ. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat Immunol. 2006;7:148–155. doi: 10.1038/ni1299. [DOI] [PubMed] [Google Scholar]
- 32.Nakagawa R, Naka T, Tsutsui H, Fujimoto M, Kimura A, Abe T, Seki E, Sato S, Takeuchi O, Takeda K, Akira S, Yamanishi K, Kawase I, Nakanishi K, Kishimoto T. SOCS-1 participates in negative regulation of LPS responses. Immunity. 2002;17:677–687. doi: 10.1016/S1074-7613(02)00449-1. [DOI] [PubMed] [Google Scholar]
- 33.Kinjyo I, Hanada T, Inagaki-Ohara K, Mori H, Aki D, Ohishi M, Yoshida H, Kubo M, Yoshimura A. SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity. 2002;17:583–591. doi: 10.1016/S1074-7613(02)00446-6. [DOI] [PubMed] [Google Scholar]
- 34.Flodstrom M, Maday A, Balakrishna D, Cleary MM, Yoshimura A, Sarvetnick N. Target cell defense prevents the development of diabetes after viral infection. Nat Immunol. 2002;3:373–382. doi: 10.1038/ni771. [DOI] [PubMed] [Google Scholar]
- 35.Solomon M, Flodstrom-Tullberg M, Sarvetnick N. Differences in suppressor of cytokine signaling-1 (SOCS-1) expressing islet allograft destruction in normal BALB/c and spontaneously-diabetic NOD recipient mice. Transplantation. 2005;79:1104–1109. doi: 10.1097/01.TP.0000162979.66954.53. [DOI] [PubMed] [Google Scholar]
- 36.Lernmark A. The preparation of, and studies on, free cell suspensions from mouse pancreatic islets. Diabetologia. 1974;10:431–438. doi: 10.1007/BF01221634. [DOI] [PubMed] [Google Scholar]
- 37.Wollheim CB, Meda P, Halban PA. Isolation of pancreatic islets and primary culture of the intact microorgans or of dispersed islet cells. Methods Enzymol. 1990;192:188–223. doi: 10.1016/0076-6879(90)92071-K. [DOI] [PubMed] [Google Scholar]
- 38.Zaitseva II, Sharoyko V, Storling J, Efendic S, Guerin C, Mandrup-Poulsen T, Nicotera P, Berggren PO, Zaitsev SV. RX871024 reduces NO production but does not protect against pancreatic beta-cell death induced by proinflammatory cytokines. Biochem Biophys Res Commun. 2006;347:1121–1128. doi: 10.1016/j.bbrc.2006.06.197. [DOI] [PubMed] [Google Scholar]
- 39.Efanova IB, Zaitsev SV, Zhivotovsky B, Kohler M, Efendic S, Orrenius S, Berggren PO. Glucose and tolbutamide induce apoptosis in pancreatic beta-cells: A process dependent on intracellular Ca2+ concentration. J Biol Chem. 1998;273:33501–33507. doi: 10.1074/jbc.273.50.33501. [DOI] [PubMed] [Google Scholar]
- 40.Zaitsev SV, Efanov AM, Efanova IB, Larsson O, Ostenson CG, Gold G, Berggren PO, Efendic S. Imidazoline compounds stimulate insulin release by inhibition of K(ATP) channels and interaction with the exocytotic machinery. Diabetes. 1996;45:1610–1618. doi: 10.2337/diabetes.45.11.1610. [DOI] [PubMed] [Google Scholar]
- 41.Laffranchi R, Spinas GA. Interferon-gamma inhibits insulin release and induces cell death in the pancreatic beta-cell line INS-1 independently of nitric oxide production. Exp Cell Res. 1997;237:217–222. doi: 10.1006/excr.1997.3791. [DOI] [PubMed] [Google Scholar]
- 42.Thomas HE, Darwiche R, Corbett JA, Kay TWH. Interleukin-1 plus gamma-interferon-induced pancreatic beta-cell dysfunction is mediated by beta-cell nitric oxide production. Diabetes. 2002;51:311–316. doi: 10.2337/diabetes.51.2.311. [DOI] [PubMed] [Google Scholar]
- 43.Piro S, Lupi R, Dotta F, Patane G, Rabuazzo MA, Marselli L, Santangelo C, Realacci M, Del GS, Purrello F, Marchetti P. Bovine islets are less susceptible than human islets to damage by human cytokines. Transplantation (Baltimore) 2001;71:21–26. doi: 10.1097/00007890-200101150-00004. [DOI] [PubMed] [Google Scholar]
- 44.Reed JC. Mechanisms of apoptosis. Am J Pathol. 2000;157:1415–1430. doi: 10.1016/S0002-9440(10)64779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cattan P, Berney T, Schena S, Molano RD, Pileggi A, Vizzardelli C, Ricordi C, Inverardi L. Early assessment of apoptosis in isolated islets of Langerhans. Transplantation. 2001;71:857–862. doi: 10.1097/00007890-200104150-00006. [DOI] [PubMed] [Google Scholar]
- 46.Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4:139–163. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- 47.Fulda S, Debatin KM. IFNgamma sensitizes for apoptosis by upregulating caspase-8 expression through the Stat1 pathway. Oncogene. 2002;21:2295–2308. doi: 10.1038/sj.onc.1205255. [DOI] [PubMed] [Google Scholar]
- 48.Paraskevas S, Aikin R, Vlaysinger D, Lakey JRT, Cavanagh TJ, Agapitos D, Wang R, Rosenberg L. Modulation of JNK and p38 stress activated protein kinases in isolated islets of Langerhans: Insulin as an autocrine survival signal. Ann Surg. 2001;233:124–133. doi: 10.1097/00000658-200101000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herr I, Debatin KM. Cellular stress response and apoptosis in cancer therapy. Blood. 2001;98:2603–2614. doi: 10.1182/blood.V98.9.2603. [DOI] [PubMed] [Google Scholar]
- 50.He Y, Zhang W, Zhang R, Zhang H, Min W. SOCS1 inhibits tumor necrosis factor-induced activation of ASK1-JNK inflammatory signaling by mediating ASK1 degradation. J Biol Chem. 2006;281:5559–5566. doi: 10.1074/jbc.M512338200. [DOI] [PubMed] [Google Scholar]
- 51.Kimura A, Naka T, Nagata S, Kawase I, Kishimoto T. SOCS-1 suppresses TNF-alpha-induced apoptosis through the regulation of Jak activation. Int Immunol. 2004;16:991–999. doi: 10.1093/intimm/dxh102. [DOI] [PubMed] [Google Scholar]
- 52.Morita Y, Naka T, Kawazoe Y, Fujimoto M, Narazaki M, Nakagawa R, Fukuyama H, Nagata S, Kishimoto T. Signals transducers and activators of transcription (STAT)-induced STAT inhibitor-1 (SSI-1)/suppressor of cytokine signaling-1 (SOCS-1) suppresses tumor necrosis factor alpha-induced cell death in fibroblasts. Proc Natl Acad Sci USA. 2000;97:5405–5410. doi: 10.1073/pnas.090084797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andersen NA, Larsen CM, Mandrup-Poulsen T. TNFalpha and IFNgamma potentiate IL-1beta induced mitogen activated protein kinase activity in rat pancreatic islets of Langerhans. Diabetologia. 2000;43:1389–1396. doi: 10.1007/s001250051544. [DOI] [PubMed] [Google Scholar]
- 54.Kim WH, Lee JW, Gao B, Jung MH. Synergistic activation of JNK/SAPK induced by TNF-alpha and IFN-gamma: apoptosis of pancreatic beta-cells via the p53 and ROS pathway. Cell Signal. 2005;17:1516–1532. doi: 10.1016/j.cellsig.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 55.Gysemans CA, Ladriere L, Callewaert H, Rasschaert J, Flamez D, Levy DE, Matthys P, Eizirik DL, Mathieu C. Disruption of the gamma-interferon signaling pathway at the level of signal transducer and activator of transcription-1 prevents immune destruction of beta-cells. Diabetes. 2005;54:2396–2403. doi: 10.2337/diabetes.54.8.2396. [DOI] [PubMed] [Google Scholar]