Abstract
The apolipoprotein B mRNA-editing enzyme catalytic polypeptide (APOBEC) family of cytidine deaminases has emerged as an intensively studied field as a result of their important biological functions. These enzymes are involved in lipid metabolism, antibody diversification, and the inhibition of retrotransposons, retroviruses, and some DNA viruses. The APOBEC proteins function in these roles by deaminating single-stranded (ss) DNA or RNA. There are two high-resolution crystal structures available for the APOBEC family, Apo2 and the C-terminal catalytic domain (CD2) of Apo3G or Apo3G-CD2 [Holden et al. (Nature 456:121–124, 2008); Prochnow et al. (Nature 445:447–451, 2007)]. Additionally, the structure of Apo3G-CD2 has also been determined using NMR [Chen et al. (Nature 452:116–119, 2008); Furukawa et al. (EMBO J 28:440–451, 2009); Harjes et al. (J Mol Biol, 2009)]. A detailed structural analysis of the APOBEC proteins and a comparison to other zinc-coordinating deaminases can facilitate our understanding of how APOBEC proteins bind nucleic acids, recognize substrates, and form oligomers. Here, we review the recent development of structural and functional studies that apply to Apo3G as well as the APOBEC deaminase family.
Keywords: APOBEC, Cytidine deaminase, Viral infectivity factor (Vif), Human immunodeficiency virus (HIV), DNA deamination, RNA editing
Introduction
The apolipoprotein B mRNA-editing enzyme catalytic polypeptide (APOBEC) family of cytidine deaminases consists of 11 members: APOBEC-1 (Apo1), APOBEC-2 (Apo2), activation-induced cytidine deaminase (AID), APOBEC-3A, -3B, -3C, -3DE, -3F, -3G, -3H (Apo3A–H), and APOBEC-4 (Apo4). These enzymes catalyze deamination of cytidine to uracil on single-stranded (ss) DNA or RNA. The APOBEC enzymes have a catalytic domain defined by a conserved cytidine deamination sequence motif (H-X-E-X23–28-P–C-X-C) [6]. The residues within the motif coordinate a Zn atom, which carries out the nucleophilic attack in the cytidine deamination reaction. Four APOBEC enzymes, Apo3G, Apo3F, Apo3B, and Apo3DE, are composed of two catalytic domains containing the residues necessary for catalysis. Current data indicate that the N-terminal domains (CD1) of Apo3G and Apo3F are enzymatically inactive and that the C-terminal catalytic domains (CD2) are active [7–11]. Both domains of Apo3B are enzymatically active [12]. The reason why some APOBEC domains are inactive is not understood. For all of the APOBEC proteins, excluding Apo2 and Apo4, catalytic activity has been observed and biological roles have been determined.
Overview of the biological roles of the APOBEC family
The founding member of the APOBEC family, Apo1, has a well-characterized role in lipid metabolism [13–15]. Apo1 deaminates the 6,666 cytidine in the apolipoprotein B (apoB) mRNA, thereby creating two isoforms of the apoB protein. The apoB100 (full-length) and apoB48 (truncated) are used to transport cholesterol and triglyceride, respectively, in the blood [15]. Although Apo1 can deaminate ssDNA, the apoB mRNA is its only known substrate in vivo [16]. Apo1 is the only APOBEC protein known to deaminate a RNA substrate. Further, Apo1 RNA deamination requires a cellular co-factor [17].
Another APOBEC protein, AID, is required for antibody affinity maturation [18]. In activated germinal center B cells, AID presumably initiates somatic hypermutation (SHM) by introducing dC → dU mutations in the VDJ region of the immunoglobulin (Ig) gene [19]. The Ig gene hypermutation enhances the ability of the antibody to bind and neutralizes the antigen. The biochemical characterization of AID deamination activity demonstrates that AID specifically acts on ssDNA substrates and targets cytidines in SHM hotspot sequence motifs (WRCY) observed in vivo [20–22]. Transcription of the Ig gene provides a ssDNA substrate for AID [23]. AID also initiates class switch recombination (CSR), which enables the expression of different antibody isotypes (for example, IgM or IgA, etc.) by a rearrangement of the constant regions of the Ig gene [18]. AID introduces dC → dU deaminations in the transcribed switch regions located upstream of the constant regions [19, 24]. AID-induced deamination events lead to the DNA double-strand breaks that are required for switching the constant regions [25]. Individuals who lack a functional AID protein develop a rare immunodeficiency disease called Hyper-IgM-2 (HIGM-2) syndrome [26]. Immuno-compromised HIGM-2 patients suffer with severe and recurrent inflammatory and autoimmune disorders [26–28].
While AID is important in the adaptive immune response, the Apo3A-H deaminases have a role in the innate immune response. Apo3G (Apo3G, previously named CEM15) inhibits the replication of an HIV-1 strain that is deficient for the viral infectivity factor (Vif) protein [29]. The HIV-1 Vif protein counters the Apo3G replication block primarily by targeting Apo3G for polyubiquitylation and proteasomal degradation [30–34]. The action of Vif significantly depletes the cell of Apo3G and prevents the incorporation of Apo3G into HIV-1 virions [35]. In the absence of Vif, Apo3G multimers are packaged into budding HIV-1 virions [36]. Virion recruitment of Apo3G depends on its binding to the nucleocapsid component of the Gag polyprotein, the viral genomic RNA, or both [37–42]. When these virions fuse with new target cells and the viral genomic RNA is reverse transcribed into cDNA, Apo3G introduces multiple cytidine deaminations on the HIV-1 minus strand cDNA [43–48]. Apo3G hypermutation of the HIV-1 cDNA may inactivate the provirus or induce degradation of the viral cDNA by cellular repair enzymes. Apo3G can also impair the integration of the provirus [49, 50]. Additionally, a non-enzymatic mode of Apo3G HIV-1 inhibition [7, 8] involves disruptions of HIV-1 reverse transcription [49, 51–55].
Both domains of Apo3G are important for HIV-1 inhibition. The catalytically inactive CD1 domain of Apo3G is required for binding RNA, interactions with HIV-1 Gag and incorporation into HIV-1 virions [7, 8, 12, 38, 39, 56, 57]. Deamination activity of the catalytically active CD2 domain is also required for restriction of HIV-1 [58, 59]. The APOBEC enzymes with double catalytic domains, Apo3G, Apo3F, Apo3B, and Apo3DE, most efficiently inhibit HIV-1 replication, while the single domain Apo3 proteins, Apo3A and Apo3C, are weakly active against HIV-1[11, 56, 60–62].
Apo3G, Apo3F, Apo3B, and Apo3C can also disrupt HBV replication because the HBV RNA packaged within virions must undergo a reverse transcription step to form a double-stranded DNA genome [63–67]. The adeno-associated virus, which replicates as ssDNA, is inhibited by the action of Apo3A [68]. Even the human papillomavirus (HPV) is susceptible to editing by APOBEC proteins, Apo3A, Apo3C, and Apo3H, expressed in keratinocytes [69]. Finally, Apo3A, Apo3B, Apo3C, Apo3F, and Apo3G are capable of restricting various types of retrotranspositions (reviewed in [70]).
The APOBEC proteins are powerful DNA mutators that play critical roles in many important biological processes. However, their mutator activities outside their designated roles can be detrimental as they are implicated in various types of diseases, including cancers and immune disorders [71–77]. Thus, understanding the detailed molecular mechanisms of APOBEC function is important for understanding their biological processes and for developing new therapeutics for related diseases. The availability of new APOBEC structures is an important advance toward understanding APOBEC biology and creating strategies that take advantage of the intrinsic anti-viral properties of the APOBEC proteins.
Structural insights for APOBEC deaminases
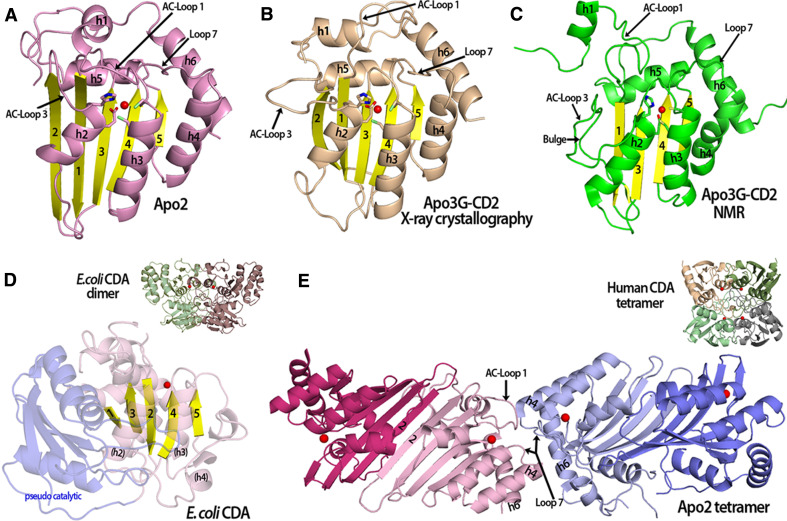
The only high-resolution APOBEC crystal structures are that of Apo2 and Apo3G-CD2 [1, 2]. The structures of an Apo2 monomer (amino acids 41–224) and Apo3G-CD2 (amino acids 197–380) have a common β-sheet core consisting of five β-strands surrounded by six α-helices (Fig. 1a, b). A zinc molecule is coordinated by active site residues that reside on helices 2 and 3 (Fig. 1a, b). The positions of two active center (AC) loops 1 and 3 are important for substrate access to the active site (Fig. 1a, b). The core structures of Apo3G-CD2 and Apo2 monomers are strikingly similar with the obvious differences found in the position and lengths of the AC-loops [1]. Also, the three NMR structures of Apo3G-CD2 are similar to the crystal structure except for the length of the β2 strand, where a short β2 strand is followed by a large bulge [3–5] (Fig. 1b, c). Among all of the Apo3G-CD2 structures, including the NMR and the X-ray structures, there are significant differences in the positions of helix 1 (h1) and the AC-loops 1 and 3 (Fig. 1b, c). The structural differences between the Apo3G-CD2 structures are a source of controversy concerning how the two domains of Apo3G are predicted to fold together in the full-length protein as discussed later in the text.
Fig. 1.
Comparison of APOBEC and fntCDA structures. a An Apo2 monomer (PDB 2nyt, chain A-closed loop conformation) showing the catalytic residues and a zinc molecule (red sphere) in the active center. b The crystal structure of an Apo3G-CD2 monomer (PDB 3e1u) contains 5 beta strands similar to Apo2 and other deaminases. c An Apo3G-CD2 structure solved by NMR (PDB 2KBO) which is very similar to the other APOBEC structures (see a and b). In contrast to the Apo3G-CD2 crystal structure, the NMR structure has a discontinuous beta 2 strand that contains a bulge. Also, AC-Loop 1 and h1 are positioned differently in comparison to the Apo3G-CD2 structure in (b). d The structure of an ECDA monomer containing a pseudo-catalytic domain (light blue) connected to the catalytic domain (light purple with yellow beta strands) via a long flexible loop. The active center zinc is represented as a red sphere. Unlike the APOBEC structures that contain a long h4 and h6, the equivalent ECDA h4 forms a long flexible loop and small 310 helices that connect to the pseudo-catalytic domain (inset). The square-shaped E. coli CDA dimer (PDB 1ALN) with the monomers shown in green and purple, each containing an active center zinc (red sphere). e The Apo2 tetramer (PDB 2nyt) displays a novel type of oligomerization forming dimers through a head–head interaction. H4 and h6 allow for the extended tetramer formation with interactions occurring though residues from Loop 7, AC-Loop 1, h4 and h6. The closed AC-Loop 1 conformations found in the inner monomers (light pink and light blue) at the tetramer interface may block substrate access to these active centers. Each monomer is colored differently with a zinc ion in the active center (red sphere) (inset). The human CDA square-shaped tetramer (PDB 1mq0) with the monomers shown in different colors and the zinc ion represented as a red sphere
The Apo2 and Apo3G-CD2 structures show similar core structural features with other cytidine deaminases within the superfamily of zinc-coordinating deaminases [1, 78]. All high-resolution structures of cytidine deaminases have a typical core β-sheet consisting of five β-strands and an active site conformation with a zinc atom coordinated by three residues (two Cys and a His/Cys) from the second and the third α-helices (see supplementary in [1]) (Fig. 1a–d). The number and position of surrounding helices differentiate the APOBEC structures from other known zinc-deaminase structures [1]. The long h4 and h6 of Apo3G-CD2 and Apo2 are unique structural features that are absent from the other cytidine deaminases (Fig. 1a–d). For example, in the Escherchia coli cytidine deaminase (ECDA), h6 is completely absent [79] (Fig. 1d). Additionally, the equivalent ECDA h4, also known as the ECDA linker region, forms a long flexible loop with one or two small 310 helices that connects with the smaller pseudo-catalytic domain at the C-terminus (Fig. 1d). Originally, APOBEC catalytic domains were modeled to have the same structural organization as the ECDA with one catalytic active site region, a linker region and a pseudoactive site region [80, 81] (Fig. 1d). However, the recent APOBEC structures clearly show the inaccuracies of such models. Additionally, the structures reveal important differences in how these deaminases oligomerize.
The crystal structure of the Apo2 tetramer reveals a novel type of cytidine deaminase oligomerization [2]. An Apo2 dimer is formed by two Apo2 monomers pairing their β2 strands, thereby doubling of the width of the beta-sheet (Fig. 1e). This type of dimerization has not been observed in any other CDA structures. The Apo2 tetramer is formed by two Apo2 dimers forming head-to-head interactions via h6, AC-loop 1 and a flexible loop (Loop 7) connected to h4 on two inner Apo2 monomers (Fig. 1e). The position of the h4 and h6 prevent the formation of the square-shaped dimers and tetramers formed by free nucleotide cytidine deaminases (fnt CDAs) (Fig. 1d, e, insets). In contrast to the square-shaped fnt CDA tetramers (Fig. 1e, inset), the elongated Apo2 tetramer (126.9 Å) has accessible active sites that can accommodate large DNA or RNA substrates (Fig. 1e). However, the active sites of the inner monomers that form the Apo2 tetramer do not appear to be accessible to nucleic acid substrates as the AC-loop 1 is collapsed over the active sites (Fig. 1e). Therefore, Apo2 oligomerization could possibly inactivate deamination activity of the Apo2 monomers in the tetrameric interface. Deamination activity for Apo2 has not yet been observed.
The APOBEC active site and DNA binding
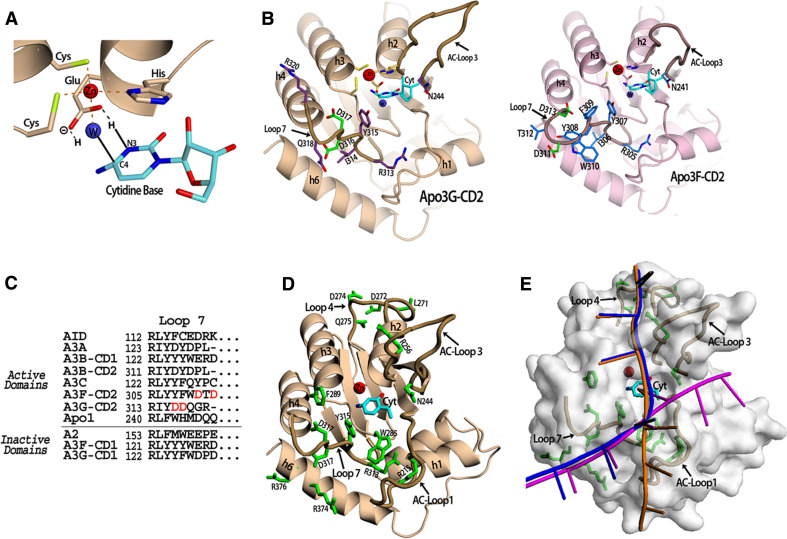
The active sites of Apo2 and Apo3G-CD2 involve a canonical type of zinc coordination where the active center Zn atom is coordinated by three residues (His, Cys and Cys) (Fig. 2a). Structural superposition of the active centers of Apo3G-CD2 and the mouse cytidine deaminase in complex with a free cytidine substrate allow for the creation of a model of an APOBEC protein with a target cytidine base positioned in the active center [82] (Fig. 2a). The closely positioned water molecule serves as a hydrogen donor during deamination [78, 83], and the conserved glutamate residue (E100 in Apo2 and E259 in Apo3G-CD2) facilitates catalysis by functioning as a proton shuffler during the hydrolytic reaction (Fig. 2a). The proposed APOBEC cytidine deamination reaction is derived from the mechanism of the fntCDAs [78, 83].
Fig. 2.
APOBEC DNA Binding. a The APOBEC active site containing a cytidine base. The Apo3G-CD2 crystal structure was superimposed with the mouse CDA co-crystal structure with cytidine in the active site (PDB 2fr6) in order to place the cytidine base in this position (shown in cyan). Three residues (2 cysteines and 1 histidine) coordinate the zinc ion (red sphere) along with a water molecule (blue sphere). The conserved glutamate shuffles the protons from the water molecule to the cytidine during the hydrolytic deamination. The proposed mechanism occurs via the glutamate acting to protonate N3 of the cytidine ring by transferring a hydrogen molecule from the water. Next, the activated water molecule (a Zn-hydroxide) attacks the C4 of the cytidine ring resulting in the release of ammonia. b The Apo3G-CD2 structure (PDB 3e1u) which shows the residues on Loop 7 which influence APOBEC substrate specificity. Residues previously shown to be important for Apo3G-CD2 substrate specificity (D316 and D317) are shown in green. Other residues on Loop 7 (shown in purple) may contact the residues neighboring the target cytidine through electrostatic, hydrophobic or base stacking interactions. N244 on AC-Loop 3 is conserved throughout the zinc-dependent deaminase family and has been shown to contact the target base directly (inset). The model of Apo3F-CD2 shows the predicted Loop 7 residues. The SWISS-MODEL homology modeling program was used to generate the model using the Apo3F sequence and the Apo3G-CD2 crystal structure (PDB 3e1u) as a template [106]. Residues D311 and D313 shown in green on Loop 7 have previously been shown to be important in substrate specificity. The conserved N241 which may directly contact the target cytidine is shown on AC-Loop 3. c An alignment of APOBEC proteins showing residues predicted to be in Loop 7. The domains are categorized according to whether they have been shown to be active for deamination activity. d Apo3G-CD2 residues previously reported to be important for DNA binding. The cytidine base is colored cyan and DNA binding residues are colored green. e The proposed Apo3G-CD2 DNA binding models. The target cytidine (cyan) is shown in its proposed position in the active center (see a). The DNA binding model proposed by Holden et al. [1] is shown in magenta. The model proposed by the Chen et al. and Furukawa et al. is shown in orange [3, 4]. A combination of both models is shown in blue
The APOBEC enzymes deaminate cytidines within specific trinucleotide sequence motifs. For example, Apo3G has a very strong preference for a CCC motif whereby it deaminates the third cytidine of the triplet on ssDNA and seldom deaminates other cytidines outside that motif [85]. AID favors cytidines in the hot spot motif of WRC (R = purine; W = A or T), but also often deaminates cytidines outside that motif [22]. Apo3F prefers cytidines in a TTC motif [10]. Recently, a report utilizing nucleoside analog interference mapping demonstrates that the pyrimidine rings of the two cytidines at positions-1 and -2 in the CCC motif are important for Apo3G specificity and activity [89]. Residues located on Loop 7 between the β4 strand and h4 of catalytically active APOBEC domains play an important role in DNA substrate specificity [1, 78, 90] (Fig. 2b). The Apo3G mutant D316R/D317R has an altered preference for the second cytidine in the CCC motif [1] (Fig. 2b). Also, mutating aspartic acid residues on A3F within the same loop significantly alters substrate specificity [21] (Fig. 2b, inset). The A3F D311A mutant has an increased preference for G or C in the −1 position of the tri-nucleotide motif (TG/CC) instead of the wildtype A3F preference for TTC [21] (Fig. 2b, inset). The A3F D313A mutant has an altered preference for G in the −2 position (GTC) [21] (Fig. 2b, inset). A sequence alignment analysis of APOBEC proteins reveals differences in amino acids within this Loop 7 region that may account for APOBEC preferences for cytidines in the context of different sequence motifs (Fig. 2c). The residues on Loop 7 may interact with DNA bases through electrostatic, hydrophobic, or base stacking interactions.
Two interesting properties of Apo3G deamination activity, processivity and polarity, have been observed on purified ssDNA substrates and HIV-1 cDNA [47, 84–86]. Apo3G, as well as AID, can deaminate multiple cytidine residues on a single ssDNA target molecule before acting on another substrate [22, 85, 86]. Publications from Goodman’s group [86, 87] define this type of deamination activity as “processive” and propose that this mechanism can involve jumping and sliding on the DNA as well as the intersegmental transfer mechanism proposed by Nowarski et al. [88]. Additionally, Apo3G deaminates cytidines with a 3′ → 5′ polarity bias, whereby deamination events preferentially occur near the 5′-end of the ssDNA substrate [86]. How Apo3G interacts with DNA to produce these effects is not well understood and remains controversial [87].
Data from structure guided mutagenesis studies and NMR chemical shift perturbation experiments identify Apo3G residues near the active center that likely interact with the target ssDNA substrate [1, 3, 4]. Chemical shift perturbations were observed for the active site residues (E259, H257, C291, and C288) and for positively charged residues capable of interacting with the negatively charged phosphate backbone of ssDNA (R215, R256, and R313) [3, 4] (Fig. 2d). Additionally, hydrophobic and negatively charged residues (Y285, Y315, F289, D316, and D317) are also proposed to make contact with the ssDNA based on chemical perturbation shifts and mutagenesis results [1, 3, 4] (Fig. 2d). Many of these residues are located on the AC-loops 1 and 3 of Apo3G-CD2 (Fig. 2d). Residues R374 and R376 on helix 6 may also contact the ssDNA [1], as well as other residues (L271, D272, D274 and Q275) located on the loop between h2 and the β3 strand [4] (Fig. 2d). An asparagine, N244, which is conserved throughout the zinc-dependent deaminase family, is likely to contact the target substrate base [1] (Fig. 2b, c). This contact has been shown in other structural studies with other deaminases, such as TadA and hCDA [83, 91]. Mutation of N244 on Apo3G and N51 of AID to alanine completely disrupts deamination activity of both enzymes [1, 92].
DNA binding models have been proposed based on the current structures of Apo3G-CD2 [1, 3, 4]. A surface representation of the Apo3G-CD2 crystal structure reveals a substrate groove that may accommodate single-stranded (ss) DNA or a RNA substrate [1] (Fig. 2d, magenta line). In this representation, the DNA could be positioned between the AC-loops 1 and 3, then sink into the deep pocket of the active site, and extend out across Loop 7 and over h6. DNA binding studies of Apo3G mutants suggest that the DNA interacts with residues on Loop 7 and h6 [1]. Furukawa et al. and Chen et al. propose that the DNA positions near h2 and h3, then into the active site, and out above h5 [3, 4] (Fig. 2d, orange line). In support of this model, Furukawa et al. observed chemical shift perturbations on residues located along the proposed path near the active site and on the loop between h2 with the β3 strand (Loop 4) [3, 4]. The substrate groove of the Apo3G-CD2 crystal structure would allow for this possibility of DNA binding near h2 and h3; however, the location of the AC-loop 1 and h1 in the crystal structure would block the proposed path of DNA across h5 (Fig. 2d). The position of the AC-loop 1 and h1 is different in all of the Apo3G-CD2 structures, and the exact positioning of this region may differ in the full-length Apo3G molecule. Also, the DNA in the model based on the NMR structures is not positioned near the residues on Loop 7 that are important for target sequence specificity (Fig. 2d, orange line). Perhaps a combination of both models would be a possibility where the DNA binds near h2 and h3, the target cytidine binds deep into the active center, and passes over Loop 7 and h6 (Fig. 2d, blue line). Although the current data are not sufficient to determine a definitive position of DNA binding in the full-length Apo3G active site, these models suggest new possibilities for consideration and further investigation. A co-crystal structure containing the protein and DNA substrate will help resolve these detailed interactions.
Models of full-length Apo3G
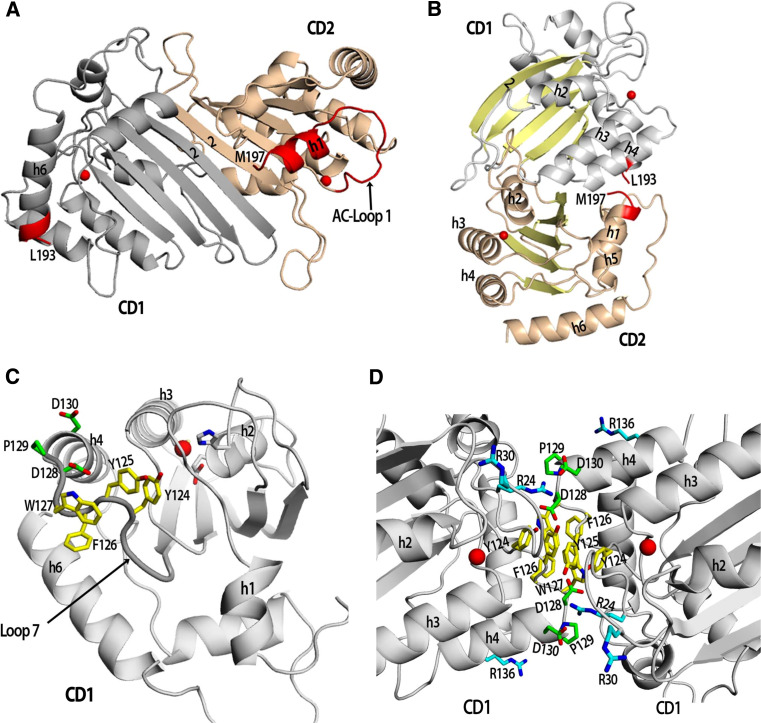
Two different full-length Apo3G structures containing CD1 and CD2 have been modeled based on the current APOBEC structures [5, 57, 93] (Fig. 3a, b). The close similarity of the Apo3G-CD2 structure with Apo2 and a high sequence similarity between Apo3G-CD1 and Apo3G-CD2 suggest that the structure of Apo3G-CD1 would be similar to that of Apo3G-CD2 as well as of Apo2. In the full-length Apo3G model proposed by Hutthoff et al. and Zhang et al., CD1 and CD2, interact with each other by pairing their β2 strands to form a double domain structure, analogous to two Apo2 monomers within a dimer [57, 93] (Figs. 1e, 3a). In this model, h6 of CD1 would need to connect with h1 of CD2 (Fig. 3a). The amino acids necessary (three not shown on the model) to link CD1 h6 to CD2 h1 cannot physically make that connection unless one or both of those helices are somehow repositioned. The long flexible CD2 AC-loop 1 could stretch and reposition in the full-length structure to make a connection with h6 on CD1. Whether the CD2 AC-loop 1 can stretch to CD1 h6 without disrupting deamination activity in the CD2 domain is uncertain. Alternatively, h6 on CD1 may reposition to connect with the h1 on CD2. An altered position of h6 on CD1 could explain why CD1 is enzymatically inactive.
Fig. 3.
Models of Apo3G monomers and dimers. a A model of an Apo3G monomer whereby the two domains fold similar to an Apo2 dimer by pairing of the β2 strands. The inter domain connection between Apo3G-CD1 and Apo3G-CD2 would have to be made through an additional three amino acids (194–196) that could connect Apo3G-CD1 h6 (L193) to h1 of Apo3G-CD2 (M197) (connection to be made is highlighted in red). The SWISS-MODEL protein homology modeling program was used to generate the Apo3G-CD1 model using an Apo2 monomer in a closed conformation as a template [106]. PyMOL was used to generate the full-length Apo3G monomer using an Apo2 dimer as a template (PDB 2nyt): the Apo3G-CD1 model was aligned with one Apo2 monomer and the structure of Apo3G-CD2 (PDB 3e1u) with the other Apo2 monomer [107]. Apo3G-CD1 is colored grey, Apo3G-CD2 is colored wheat and the two zinc molecules are represented by red spheres. b A different model of an Apo3G monomer adapted from Harjes et al. where the two active center domains of Apo3G-CD1 and Apo3G-CD2 lie on opposite faces (zinc ion represented by red spheres). The Apo3G-CD2 structure (3e1u) was aligned with the Apo3G-CD1 model according to Harjes et al. [5]. c A model of Apo3G-CD1 displaying the residues on Loop 7 important for dimerization (yellow residues) and Vif binding (green residues). d A model of the proposed Apo3G dimer CD1–CD1 (head–head) interface based off the Apo2 tetramer interface (PDB 2nyt). Yellow colored residues (Y124, Y125 and W127) have been proposed to be important for dimerization and virion incorporation. The residues (D128, P129 and D130) that interact with Vif are colored green. Residues important for binding RNA, R24, R30 and R136, are colored cyan
Recently, Harjes et al. proposed a different type of fold for the Apo3G monomer based on structural differences within the NMR structure in contrast to the crystal structure of Apo3G-CD2 [5] (Fig. 3b). Harjes et al. claim that a bulge observed in the β2 strand of the NMR structure would preclude interaction of Apo3G domains by pairing of their β2 strands and contend that the CD2 h1 cannot be repositioned to connect with the CD1 domain without disrupting deamination activity in the CD2 domain. In contrast to the first model, the active centers are located on opposite faces of the monomer [5] (Fig. 3b). Although very thought provoking, all the current full-length Apo3G models are speculative and await verification by a high-resolution structure of a full-length Apo3G structure determined experimentally.
Structural insights for the inactive Apo3G CD1 and Apo3G oligomerization
Residues on the inactive CD1 of Apo3G are important for interactions with HIV-1 Vif, RNA binding and dimerization. A single amino acid residue on Apo3G CD1, D128, determines species-specific interactions with Vif proteins from HIV-1 or simian immunodeficiency virus (SIV) of the African green monkey (AGM) [45, 94–96]. The corresponding Apo3G residue in the AGM is K128. Mutant human Apo3G D128K is resistant to HIV-1 Vif and sensitive to SIVagm Vif. Conversely, mutant Apo3Gagm K128D is resistant to SIVagm and sensitive to HIV-1 Vif [45, 94–96]. In a mutagenesis study, Huthoff et al. further characterize this HIV-1 Vif binding region on the human Apo3G CD1 domain and show that electrostatic interactions occur between Vif and the D128 and D130 residues [97]. Additionally, the mutation of P129 to an alanine or glycine severely disrupts interactions with Vif suggesting a structural requirement for the interaction [97]. This DPD128–130 Vif binding region is located on the predicted CD1 Loop 7, the same loop on CD2 that influences substrate specificity (Figs. 2b, 3c). While residues in the Apo3G CD1 domain are necessary for Vif binding, residues extending into the CD2 domain (157–245) are required for Vif-mediated proteasomal degradation [98].
Not all the APOBEC proteins interact with Vif in the same manner. Vif does not bind to Apo3F in the same region as Vif binds to Apo3G [99–102], and an E127K mutation does not render Apo3F resistant to Vif degradation as does the corresponding D128K mutation on Apo3G [62, 101]. Additionally, Vif can bind Apo3B and Apo3C but cannot induce proteasomal degradation of these APOBEC proteins [103]. Vif does not bind wild-type Apo3A but does bind an Apo3A mutant with residues YDYD125–128 mutated to match residues (YYFW124–127) on an analogous region of Apo3G CD1 [56]. However, Vif does not induce proteasomal degradation of this Apo3A mutant. These residues on the Apo3A mutant and Apo3G are also important for their interactions with the HIV-1 Gag protein and virion incorporation [56]. This region on Apo3G is further discussed below.
Adjacent to the Apo3G DPD128–130 Vif binding region, a cluster of hydrophobic residues (YYFW124–127) are necessary for HIV-1 Gag protein interactions, virion incorporation, and Apo3G dimerization [56, 57, 97] (Figs. 2c, 3c and d). These residues are located on the predicted CD1 Loop 7. Of these residues, the Y124 and W127 are most critical for virion incorporation and Apo3G dimerization, since mutating either one of these residues severely disrupts both functions [57]. The Apo3G double mutant (F126D/W127Y) fails to interact with the HIV-1 Gag protein suggesting that Gag only recognizes Apo3G dimers or multimers [56]. These results are congruous with a previous report that shows Apo3G multimers are incorporated into HIV-1 virions [36]. The model structure of Apo3G-CD1 shows that Vif binds very close to this Apo3G dimerization interface (YYFW124–127). Given that the Y124A and W127A Apo3G mutants remain sensitive to Vif degradation, Vif must be able to recognize an Apo3G monomer as well as a dimer [57]. Gooch et al. propose that Vif may compete with Gag for two different binding sites that partially overlap. This proposal accounts for the ability of Vif to inhibit Apo3G virion incorporation in the absence of Apo3G proteasomal degradation [56]. Finally, the data clearly demonstrate that Apo3G dimerizes via head-to-head interactions of two Apo3G-CD1 domains (Fig. 3d).
There are conflicting reports on whether Apo3G dimerization is dependent upon RNA binding. Crosslinking studies of Apo3G in the cell extracts reveal monomers and dimers that are RNA-dependent [57]. However, highly purified Apo3G from insect cells, is observed as monomers, dimers and higher ordered oligomers by AFM analysis in the absence RNA [86]. The AFM study shows that salt concentration alone, as well as the addition of ssDNA, can alter the oligomerization state of Apo3G [86]. In another report, SAXS data reveal that purified recombinant Apo3G, in the absence of RNase treatment, forms an elongated oligomer that is likely to be two Apo3G dimers [104]. When Apo3G is pretreated with RNase, the SAXS data reveal an elongated structure with dimensions and a molecular weight that suggests an Apo3G dimer [104]. Although RNA may promote the formation of Apo3G dimers, tetramers or higher oligomers, there is evidence that Apo3G can dimerize in the absence of RNA [86, 104]. The conflicting results of RNA dependence on Apo3G dimerization may be due to differences of salt concentrations in the experimental conditions.
How Apo3G monomers interact with each during dimerization also remains controversial. Bennett et al. propose that Apo3G forms dimers via interactions of the CD2 domain that are independent of RNA [105]. Based on Fret and co-immunoprecipitation experiments, Bennett et al. show that the truncated Apo3G-CD1 proteins do not self associate, while the truncated Apo3G-CD2 proteins do [105]. However, these results remain controversial as two other reports demonstrate that Apo3G-CD2 protein exists as a monomer [1, 3]. Hutthoff et al. propose a model of an Apo3G dimer based on their mutagenesis data and the Apo2 tetramer structure [2, 57] (Fig. 3d). Similar to Apo2, the Apo3G dimer forms by the head-to-head interactions of the CD1 domains. In this model, the active sites of the CD1 domains would not be accessible to ssDNA substrates, which may explain why the CD1 domains are inactive (Fig. 3d). The residues that are critical for Apo3G dimerization are predicted to form hydrophobic interactions within the dimeric interface, similar to the Apo2 tetramer interface. Positively charged residues are clustered around the surface of the dimeric interface and are predicted to bind RNA. Mutagenesis data indirectly suggest that R24, R30 and R136 bind RNA (Fig. 3d, cyan residues) as mutation of these residues to alanines disrupts Apo3G RNA-dependent oligomerization [57]. Also in this model, the HIV-1 Gag protein could not interact directly with residues F126 and W127 since these residues are buried (Fig. 3d, yellow residues). Therefore, Gag may be recognizing the Apo3G dimeric interface, which would certainly overlap with the Vif binding region (Fig. 3d, green residues). Therefore, a drug designed to inhibit Apo3G and Vif interactions would need to be carefully crafted so as not to disrupt interactions with Gag or RNA.
Concluding remarks
Rapid advancement has been made in the studies of the biological functions of APOBEC deaminase enzymes in recent years. The structural and functional studies of Apo2, AID, and Apo3G have provided a great deal of information for understanding the mechanisms by which these three enzymes and other APOBEC enzymes function. However, there are still many questions that remain unanswered. For example, how do the two domains in the double deaminase APOBEC proteins such as Apo3G fold together in a full-length molecule? Why are some APOBEC catalytic domains inactive even though they contain the residues necessary for catalysis? How do APOBEC enzymes bind DNA versus RNA? Precisely, how do APOBEC enzymes oligomerize? Most importantly, how can we exploit the potent anti-viral activity of Apo3G and Apo3F to prevent and treat HIV infections? These questions will be the subject of future investigations in the exciting field of the APOBEC deaminases.
Acknowledgment
This work was supported by the National Institutes of Health grant R01GM087986.
References
- 1.Holden LG, Prochnow C, Chang PY, Bransteitter R, Chelico L, Sen U, Stevens RC, Goodman MF, Chen XS. Crystal structure of the anti-viral APOBEC3G catalytic domain and functional implications. Nature. 2008;456:121–124. doi: 10.1038/nature07357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prochnow C, Bransteitter R, Klein MG, Goodman MF, Chen XS. The APOBEC-2 crystal structure and functional implications for the deaminase AID. Nature. 2007;445:447–451. doi: 10.1038/nature05492. [DOI] [PubMed] [Google Scholar]
- 3.Chen KM, Harjes E, Gross PJ, Fahmy A, Lu Y, Shindo K, Harris RS, Matsuo H. Structure of the DNA deaminase domain of the HIV-1 restriction factor APOBEC3G. Nature. 2008;452:116–119. doi: 10.1038/nature06638. [DOI] [PubMed] [Google Scholar]
- 4.Furukawa A, Nagata T, Matsugami A, Habu Y, Sugiyama R, Hayashi F, Kobayashi N, Yokoyama S, Takaku H, Katahira M. Structure, interaction and real-time monitoring of the enzymatic reaction of wild-type APOBEC3G. EMBO J. 2009;28:440–451. doi: 10.1038/emboj.2008.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harjes E, Gross PJ, Chen KM, Lu Y, Shindo K, Nowarski R, Gross JD, Kotler M, Harris RS, Matsuo H (2009) An extended structure of the APOBEC3G catalytic domain suggests a unique holoenzyme model. J Mol Biol (in press) [DOI] [PMC free article] [PubMed]
- 6.Jarmuz A, Clhester A, Bayliss J, Gisbourne J, Dunham I, Scott J, Navaratnam N. An anthropoid-specific locus of orphan C to U RNA-editing enzymes on chromosome 22. Genomics. 2002;79:285–296. doi: 10.1006/geno.2002.6718. [DOI] [PubMed] [Google Scholar]
- 7.Navarro F, Bollman B, Chen H, Konig R, Yu Q, Chiles K, Landau NR. Complementary function of the two catalytic domains of APOBEC3G. Virology. 2005;333:374–386. doi: 10.1016/j.virol.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Newman EN, Holmes RK, Craig HM, Klein KC, Lingappa JR, Malim MH, Sheehy AM. Antiviral function of APOBEC3G can be dissociated from cytidine deaminase activity. Curr Biol. 2005;15:166–170. doi: 10.1016/j.cub.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 9.Iwatani Y, Takeuchi H, Strebel K, Levin JG. Biochemical activities of highly purified, catalytically active human APOBEC3G: correlation with antiviral effect. J Virol. 2006;80:5992–6002. doi: 10.1128/JVI.02680-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liddament MT, Brown WL, Schumacher AJ, Harris RS. APOBEC3F properties and hypermutation preferences indicate activity against HIV-1 in vivo. Curr Biol. 2004;14:1385–1391. doi: 10.1016/j.cub.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 11.Bishop KN, Holmes RK, Sheehy AM, Davidson NO, Cho S, Malim MH. Cytidine deamination of retroviral DNA by diverse APOBEC proteins. Curr Biol. 2004;14:1392–1396. doi: 10.1016/j.cub.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 12.Bogerd HP, Wiegand HL, Doehle BP, Cullen BR. The intrinsic antiretroviral factor APOBEC3B contains two enzymatically active cytidine deaminase domains. Virology. 2007;364:486–493. doi: 10.1016/j.virol.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navaratnam N, Morrison JR, Bhattacharya S, Patel D, Funahashi T, Giannoni F, Teng B, Davidson NO, Scott J. The p27 catalytic subunit of the apolipoprotein B mRNA editing enzyme is a cytidine deaminase. J Biol Chem. 1993;28:20709–20712. [PubMed] [Google Scholar]
- 14.Teng B, Burant CF, Davidson NO. Molecular cloning of an apolipoprotein B messenger RNA editing protein. Science. 1993;260:1816–1819. doi: 10.1126/science.8511591. [DOI] [PubMed] [Google Scholar]
- 15.Chester A, Scott J, Anant S, Navaratnam N. RNA editing: cytidine to uridine conversion in apolipoprotein B mRNA. Biochim Biophys Acta. 2000;1494:1–13. doi: 10.1016/s0167-4781(00)00219-0. [DOI] [PubMed] [Google Scholar]
- 16.Petersen-Mahrt SK, Neuberger MS. In vitro deamination of cytosine to uracil in single-stranded DNA by apolipoprotein B editing complex catalytic subunit 1 (APOBEC1) J Biol Chem. 2003;278:19583–19586. doi: 10.1074/jbc.C300114200. [DOI] [PubMed] [Google Scholar]
- 17.Mehta A, Kinter MT, Sherman NE, Driscoll DM. Molecular cloning of apobec-1 complementation factor, a novel RNA-binding protein involved in the editing of apolipoprotein B mRNA. Mol Cell Biol. 2000;20:1846–1854. doi: 10.1128/mcb.20.5.1846-1854.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 19.Neuberger MS, Harris RS, Di Noia JM, Petersen-Mahrt SK. Immunity through DNA deamination. Trends Biochem Sci. 2003;28:305–312. doi: 10.1016/S0968-0004(03)00111-7. [DOI] [PubMed] [Google Scholar]
- 20.Bransteitter R, Pham P, Scharff MD, Goodman MF. Activation-induced cytidine deaminase deaminates deoxycytidine on single-stranded DNA but requires the action of RNase. Proc Natl Acad Sci USA. 2003;100:4102–4107. doi: 10.1073/pnas.0730835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langlois MA, Beale RCL, Conticello SG, Neuberger MS. Mutational comparison of the single-domained APOBEC3C and double-domained APOBEC3F/G anti-retroviral cytidine deaminases providing insight into their DNA target site specificities. Nucleic Acids Res. 2005;33:1913–1923. doi: 10.1093/nar/gki343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pham P, Bransteitter R, Petruska J, Goodman MF. Processive AID-catalysed cytosine deamination on single-stranded DNA simulates somatic hypermutation. Nature. 2003;424:103–107. doi: 10.1038/nature01760. [DOI] [PubMed] [Google Scholar]
- 23.Peters A, Storb U. Somatic hypermutation of immunoglobulin genes is linked to transcription initiation. Immunity. 1996;4:57–65. doi: 10.1016/s1074-7613(00)80298-8. [DOI] [PubMed] [Google Scholar]
- 24.Imai K, Slupphaug G, Lee WI, Revy P, Nonoyama S, Catalan N, Yel L, Forveille M, Kavli B, Krokan HE, Ochs HD, Fischer A, Durandy A. Human uracil–DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nat Immunol. 2003;4:1023–1028. doi: 10.1038/ni974. [DOI] [PubMed] [Google Scholar]
- 25.Schrader CE, Guikema JE, Wu X, Stavnezer J. The roles of APE1, APE2, DNA polymerase beta and mismatch repair in creating S region DNA breaks during antibody class switch. Philos Trans R Soc Lond B. 2009;364:645–652. doi: 10.1098/rstb.2008.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Revy P, Muto T, Levy Y, Greissmann F, Plebani A, Sanal O, Catalan N, Forveille M, Dufourcq-Labelouse R, Gennery A, Tezcan I, Ersoy F, Kayserili H, Ugazio AG, Brousse N, Muramatsu M, Notarangelo LD, Kinoshita K, Honjo T, Fischer A, Durandy A. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 27.Durandy A, Peron S, Fischer A. Hyper-IgM syndromes. Curr Opin Rheumatol. 2006;18:369–376. doi: 10.1097/01.bor.0000231905.12172.b5. [DOI] [PubMed] [Google Scholar]
- 28.Minegishi Y, Lavoie A, Cunningham-Rundles C, Bedard P, Hebert J, Cote L, Dan K, Sedlak D, Buckley RH, Fischer A, Durandy A, Conley ME. Mutations in activation-induced cytidine deaminase in patients with hyper IgM syndrome. Clin Immunol. 2000;97:203–210. doi: 10.1006/clim.2000.4956. [DOI] [PubMed] [Google Scholar]
- 29.Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- 30.Conticello SG, Harris RS, Neuberger MS. The Vif protein of HIV triggers degradation of the human antiretroviral DNA deaminase APOBEC3G. Curr Biol. 2003;13:2009–2013. doi: 10.1016/j.cub.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 31.Marin M, Rose KM, Kozak SL, Kabat D. HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat Med. 2003;9:1398–1403. doi: 10.1038/nm946. [DOI] [PubMed] [Google Scholar]
- 32.Sheehy AM, Gaddis NC, Malim MH. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat Med. 2003;9:1404–1407. doi: 10.1038/nm945. [DOI] [PubMed] [Google Scholar]
- 33.Stopak K, de Noronha C, Yonemoto W, Greene WC. HIV-1 Vif blocks antiviral activity of APOBEC3G by impairing both its translation and intracellular stability. Mol Cell. 2003;12:591–601. doi: 10.1016/s1097-2765(03)00353-8. [DOI] [PubMed] [Google Scholar]
- 34.Yu X, Yu Y, Liu B, Luo K, Kong W, Mao P, Yu XF. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif–Cul5–SCF complex. Science. 2003;302:1056–1060. doi: 10.1126/science.1089591. [DOI] [PubMed] [Google Scholar]
- 35.Opi S, Kao S, Goila-Gaur R, Khan M, Miyagi E, Takeuchi H, Strebel K. Human immunodeficiency virus type 1 Vif inhibits packaging and antiviral activity of a degradation-resistant APOBEC3G variant. J Virol. 2007;81:8236–8246. doi: 10.1128/JVI.02694-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burnett A, Spearman P. APOBEC3G multimers are recruited to the plasma membrane for packaging into human immunodeficiency virus type 1 virus-like particles in an RNA-dependent process requiring the nc basic linker. J Virol. 2007;81:5000–5013. doi: 10.1128/JVI.02237-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alce TM, Popik W. APOBEC3G is incorporated into virus-like particles by a direct interaction with HIV-1 Gag nucleocapsid protein. J Biol Chem. 2004;279:34083–34086. doi: 10.1074/jbc.C400235200. [DOI] [PubMed] [Google Scholar]
- 38.Cen S, Guo F, Niu M, Saadatmand J, Deflassieux J, Kleiman L. The interaction between HIV-1 Gag and APOBEC3G. J Biol Chem. 2004;279:33177–33184. doi: 10.1074/jbc.M402062200. [DOI] [PubMed] [Google Scholar]
- 39.Luo K, Lui B, Xiao Z, Yu Y, Yu X, Gorelick R, Yu XF. Amino-terminal region of the human immunodeficiency virus type 1 nucleo-capsid is required for human APOBEC3G packaging. J Virol. 2004;78:11841–11852. doi: 10.1128/JVI.78.21.11841-11852.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan MA, Kao S, Miyagi E, Takeuchi H, Goila-Gaur R, Opi S, Gipson CL, Parslow TG, Ly H, Strebel K. Viral RNA is required for the association of APOBEC3G with human immunodeficiency virus type 1 nucleoprotein complexes. J Virol. 2005;79:5870–5874. doi: 10.1128/JVI.79.9.5870-5874.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zennou VD, Perez-Caballero D, Gottlinger H, Bieniasz PD. APOBEC3G incorporation into human immunodeficiency virus type 1 particles. J Virol. 2004;78:12058–12061. doi: 10.1128/JVI.78.21.12058-12061.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scharfer A, Bogerd HP, Cullen BR. Specific packaging of APOBEC3G into HIV-1 virions is mediated by the nucleocapsid domain of the gag polyprotein precursor. Virology. 2004;328:163–168. doi: 10.1016/j.virol.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Harris RS, Bishop KN, Sheehy AM, Craig HM, Petersen-Mahrt SK, Watt IN, Neuberger MS, Malim MH. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113:803–809. doi: 10.1016/s0092-8674(03)00423-9. [DOI] [PubMed] [Google Scholar]
- 44.Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- 45.Mariani R, Chen D, Schrofelbauer B, Navarro F, Konig R, Bollman B, Munk C, Nymark-McMahon H, Landau NR. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell. 2003;114:21–31. doi: 10.1016/s0092-8674(03)00515-4. [DOI] [PubMed] [Google Scholar]
- 46.Zhang H, Yang B, Pomerantz RJ, Zhang C, Arunachalam SC, Gao L. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature. 2003;424:94–98. doi: 10.1038/nature01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu Q, Konig R, Pillai S, Chiles K, Kearney M, Palmer S, Richman D, Coffin JM, Landau NR. Single-strand specificity of APOBEC3G accounts for minus-strand deamination of the HIV genome. Nat Struct Mol Biol. 2004;11:435–442. doi: 10.1038/nsmb758. [DOI] [PubMed] [Google Scholar]
- 48.Suspene R, Sommer P, Henry M, Ferris S, Guetard D, Pochet S, Chester A, Navaratnam N, Wain-Hobson S, Vartanian JP. APOBEC3G is a single-stranded DNA cytidine deaminase and functions independently of HIV reverse transcriptase. Nucleic Acids Res. 2004;32:2421–2429. doi: 10.1093/nar/gkh554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mbisa JL, Barr R, Thomas JA, Vandegraaff N, Dorweiler IJ, Svarovskaia ES, Brown WL, Mansky LM, Gorelick RJ, Harris RS, Engelman A, Pathak VK. Human immunodeficiency virus type 1 cDNAs produced in the presence of APOBEC3G exhibit effects in plus-strand DNA transfer and integration. J Virol. 2007;81:7099–7110. doi: 10.1128/JVI.00272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo K, Wang T, Liu B, Tian C, Xiao Z, Kappes J, Yu X. Cytidine deaminases APOBEC3G and APOBEC3F interact with human immunodeficiency virus type 1 integrase and inhibit proviral DNA formation. J Virol. 2007;81:7238–7248. doi: 10.1128/JVI.02584-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bishop KN, Verma M, Kim EY, Wolinsky SM, Malim MH. APOBEC3G inhibits elongation of HIV-1 reverse transcripts. PLoS Pathog. 2008;4:e1000231. doi: 10.1371/journal.ppat.1000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holmes RK, Koning FA, Bishop KN, Malim MH. APOBEC3F can inhibit the accumulation of HIV-1 reverse transcription products in the absence of hypermutation. Comparisons with APOBEC3G. J Biol Chem. 2007;282:2587–2595. doi: 10.1074/jbc.M607298200. [DOI] [PubMed] [Google Scholar]
- 53.Iwatani Y, Chan DSB, Wang F, Maynard KS, Sugiura W, Gronenborn AM, Rouzina I, Williams MC, Musier-Forsyth K, Levin JG. Deaminase-independent inhibition of HIV-1 reverse transcription by APOBEC3G. Nucleic Acids Res. 2007;35:7096–7108. doi: 10.1093/nar/gkm750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo F, Cen S, Niu M, Saadatmand J, Kleiman L. Inhibition of tRNAlys-primed reverse transcription by human APOBEC3G during human immunodeficiency virus type 1 replication. J Virol. 2006;80:11710–11720. doi: 10.1128/JVI.01038-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li XY, Guo F, Zhang L, Kleiman L, Cen S. APOBEC3G inhibits DNA strand transfer during HIV-1 reverse transcription. J Biol Chem. 2007;282:32065–32074. doi: 10.1074/jbc.M703423200. [DOI] [PubMed] [Google Scholar]
- 56.Gooch BD, Cullen BR. Functional domain organization of human APOBEC3G. Virology. 2008;379:118–124. doi: 10.1016/j.virol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huthoff H, Autore F, Gallois-Montbrun S, Fraternali F, Malim MH. RNA-dependent oligomerization of APOBEC3G is required for restriction of HIV-1. PLoS Pathog. 2009;5:e1000330. doi: 10.1371/journal.ppat.1000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miyagi E, Opi S, Takeuchi H, Khan M, Goila-Gaur R, Kao S, Strebel K. Enzymatically active APOBEC3G is required for efficient inhibition of human immunodeficiency virus type 1. J Virol. 2007;81:13346–13353. doi: 10.1128/JVI.01361-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schumacher AJ, Hache G, Macduff DA, Brown WL, Harris RS. The DNA deaminase activity of human APOBEC3G is required for Ty1, MusD, and human immunodeficiency virus type 1 restriction. J Virol. 2008;82:2652–2660. doi: 10.1128/JVI.02391-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doehle BP, Schafer A, Cullen BR. Human APOBEC3B is a potent inhibitor of HIV-1 infectivity and is resistant to HIV-1 Vif. Virology. 2005;339:281–288. doi: 10.1016/j.virol.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 61.Bourara K, Liegler TJ, Grant RM. Target cell APOBEC3C can induce limited G-to-A mutation in HIV-1. PLoS Pathog. 2007;3:1477–1485. doi: 10.1371/journal.ppat.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dang Y, Wang X, Esselman WJ, Zheng YH. Identification of APOBEC3DE as another antiretroviral factor from the human APOBEC family. J Virol. 2006;80:10522–10533. doi: 10.1128/JVI.01123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Noguchi C, Ishino H, Tsuge M, Fujimoto Y, Imamura M, Takahashi S, Chayama K. G to A hypermutation of hepatitis B virus. Hepatology. 2005;41:626–633. doi: 10.1002/hep.20580. [DOI] [PubMed] [Google Scholar]
- 64.Rosler C, Kock J, Kann M, Malim MH, Blum HE, Baumert TF, von Weizacker F. APOBEC-mediated interference with hepadnavirus production. Hepatology. 2005;42:309–310. doi: 10.1002/hep.20801. [DOI] [PubMed] [Google Scholar]
- 65.Kock J, Blum HE. Hypermutation of hepatitis B virus genomes by APOBEC3G, APOBEC3C and APOBEC3H. J Gen Virol. 2008;89:1184–1191. doi: 10.1099/vir.0.83507-0. [DOI] [PubMed] [Google Scholar]
- 66.Suspene R, Guetard D, Henry M, Sommer P, Wain-Hobson S, Vartanian JP. Extensive editing of both hepatitis B virus DNA strands by APOBEC3 cytidine deaminases in vitro and in vivo. Proc Natl Acad Sci USA. 2005;102:8321–8326. doi: 10.1073/pnas.0408223102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turelli P, Mangeat B, Jost S, Vianin S, Trono D. Inhibition of hepatitis B virus replication by APOBEC3G. Science. 2004;303:1829. doi: 10.1126/science.1092066. [DOI] [PubMed] [Google Scholar]
- 68.Chen H, Lilley CE, Yu Q, Lee DV, Chou J, Narvaiza I, Landau NR, Weitzman MD. APOBEC3A is a potent inhibitor of adeno-associated virus and retrotransposons. Curr Biol. 2006;16:480–485. doi: 10.1016/j.cub.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 69.Vartanian J, Guetard D, Henry M, Wain-Hobson S. Evidence for editing of human papillomavirus DNA by APOBEC3 in benign and precancerous lesions. Science. 2008;320:230–233. doi: 10.1126/science.1153201. [DOI] [PubMed] [Google Scholar]
- 70.Chiu YL, Greene WC. APOBEC3G: an intracellular centurion. Philos Trans R Soc Lond B Biol Sci. 2009;364:689–703. doi: 10.1098/rstb.2008.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okazaki IM, Hiai H, Kakazu N, Yamada S, Muramatsu M, Kinoshita K, Honjo T. Constitutive expression of AID leads to tumorigenesis. J Exp Med. 2003;197:1173–1181. doi: 10.1084/jem.20030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kou T, Marusawa H, Kinoshita K, Endo Y, Okazaki IM, Ueda Y, Kodama Y, Haga H, Ikai I, Chiba T. Expression of activation-induced cytidine deaminase in human hepatocytes during hepatocarcinogenesis. Int J Cancer. 2007;120:469–476. doi: 10.1002/ijc.22292. [DOI] [PubMed] [Google Scholar]
- 73.Komori J, Marusawa H, Machimoto T, Endo Y, Kinoshita K, Kou T, Haga H, Ikai I, Uemoto S, Chiba T. Activation-induced cytidine deaminase links bile duct inflammation to human cholangiocarcinoma. Hepatology. 2008;47:888–896. doi: 10.1002/hep.22125. [DOI] [PubMed] [Google Scholar]
- 74.Longerich S, Orelli BJ, Martin RW, Bishop DK, Storb U. Brca1 in immunoglobulin gene conversion and somatic hypermutation. DNA Repair (Amst) 2008;7:253–266. doi: 10.1016/j.dnarep.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marusawa H. Aberrant AID expression and human cancer development. Int J Biochem Cell Biol. 2008;40:1399–1402. doi: 10.1016/j.biocel.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 76.Matsumoto Y, Marusawa H, Kinoshita K, Endo Y, Kou T, Morisawa T, Azuma T, Okazaki IM, Honjo T, Chiba T. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med. 2007;13:470–476. doi: 10.1038/nm1566. [DOI] [PubMed] [Google Scholar]
- 77.Morisawa T, Marusawa H, Ueda Y, Iwai A, Okazaki IM, Honjo T, Chiba T. Organ-specific profiles of genetic changes in cancers caused by activation-induced cytidine deaminase expression. Int J Cancer. 2008;123:2735–2740. doi: 10.1002/ijc.23853. [DOI] [PubMed] [Google Scholar]
- 78.Conticello SG, Langlois MA, Yang Z, Neuberger MS. DNA deamination in immunity: AID in the context of its APOBEC relatives. Adv Immunol. 2007;94:37–73. doi: 10.1016/S0065-2776(06)94002-4. [DOI] [PubMed] [Google Scholar]
- 79.Betts L, Xiang S, Short SA, Wolfenden R, Carter CW. Cytidine deaminase. The 2.3 Å crystal structure of an enzyme: transition-state analog complex. Curr Biol. 1994;235:635–656. doi: 10.1006/jmbi.1994.1018. [DOI] [PubMed] [Google Scholar]
- 80.Navaratnam N, Fujino T, Bayliss J, Jarmuz A, How A, Richardson N, Somasekaram A, Bhattacharya S, Carter C, Scott J. Escherichia coli cytidine deaminase provides a molecular model for ApoB RNA editing and a mechanism for RNA substrate recognition. J Mol Biol. 1998;275:695–714. doi: 10.1006/jmbi.1997.1506. [DOI] [PubMed] [Google Scholar]
- 81.Wedekind JE, Dance GS, Sowden MP, Smith HC. Messenger RNA editing in mammals: new members of the APOBEC family seeking roles in the family business. Trends Genet. 2003;19:207–216. doi: 10.1016/S0168-9525(03)00054-4. [DOI] [PubMed] [Google Scholar]
- 82.Teh AH, Kimura M, Yamamoto M, Tanaka N, Yamaguchi I, Kumasaka T. The 1.48 Å resolution crystal structure of the homotetrameric cytidine deaminase from mouse. Biochemistry (Mosc) 2006;45(782):5–7833. doi: 10.1021/bi060345f. [DOI] [PubMed] [Google Scholar]
- 83.Chung SJ, Fromme JC, Verdine GL. Structure of human cytidine deaminase bound to a potent inhibitor. J Med Chem. 2005;48:658–660. doi: 10.1021/jm0496279. [DOI] [PubMed] [Google Scholar]
- 84.Suspene R, Rusniok C, Vartanian JP, Wain-Hobson S. Twin gradients in APOBEC3 edited HIV-1 DNA reflect the dynamics of lentiviral replication. Nucleic Acids Res. 2006;34:4677–4684. doi: 10.1093/nar/gkl555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chelico L, Pham P, Calabrese P, Goodman MF. APOBEC3G DNA deaminase acts processively 3′ → 5′ on single-stranded DNA. Nat Struct Mol Biol. 2006;13:392–399. doi: 10.1038/nsmb1086. [DOI] [PubMed] [Google Scholar]
- 86.Chelico L, Goodman MF. A model for oligomeric regulation of APOBEC3G cytosine deaminase-dependent restriction of HIV. J Biol Chem. 2008;283:13780–13791. doi: 10.1074/jbc.M801004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chelico L, Pham P, Goodman MF. Mechanisms of APOBEC3G-catalyzed processive deamination of deoxycytidine on single-stranded DNA. Nat Struct Mol Biol. 2009;16:454–455. doi: 10.1038/nsmb0509-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nowarski R, Britan-Rosich E, Shiloach T, Kotler M. Hypermutation by intersegmental transfer of APOBEC3G cytidine deaminase. Nat Struct Mol Biol. 2008;15:1059–1066. doi: 10.1038/nsmb.1495. [DOI] [PubMed] [Google Scholar]
- 89.Rausch JW, Chelico L, Goodman MF, Le Grice SF. Dissecting APOBEC3G substrate specificity by nucleoside analog interference. J Biol Chem. 2009;284:7047–7058. doi: 10.1074/jbc.M807258200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Conticello SG, Langlois MA, Neuberger MS. Insights into DNA deaminases. Nat Struct Mol Biol. 2007;14:7–9. doi: 10.1038/nsmb0107-7. [DOI] [PubMed] [Google Scholar]
- 91.Losey HC, Ruthenburg AJ, Verdine GL. Crystal structure of Staphylococcus aureus tRNA adenosine deaminase TadA in complex with RNA. Nat Struct Mol Biol. 2006;13:153–159. doi: 10.1038/nsmb1047. [DOI] [PubMed] [Google Scholar]
- 92.Basu U, Franklin A, Alt FW. Post-translational regulation of activation-induced cytidine deaminase. Philos Trans R Soc Lond B. 2009;364:667–673. doi: 10.1098/rstb.2008.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang KL, Mangeat B, Ortiz M, Zoete V, Trono D, Telenti A, Michielin O. Model structure of human APOBEC3G. PLoS ONE. 2007;2:e378. doi: 10.1371/journal.pone.0000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bogerd HP, Doehle BP, Wiegand HL, Cullen BR. A single amino acid difference in the host APBEC3G protein controls the primate species specificity of HIV type 1 virion infectivity factor. Proc Natl Acad Sci USA. 2004;101:3770–3774. doi: 10.1073/pnas.0307713101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mangeat B, Turelli P, Liao S, Trono D. a single amino acid determinant governs the species-specific sensitivity of APOBEC3G to Vif action. J Biol Chem. 2004;15:14481–14483. doi: 10.1074/jbc.C400060200. [DOI] [PubMed] [Google Scholar]
- 96.Schrofelbauer B, Chen D, Landau NR. A single amino acid of APOBEC3G controls its species-specific interaction with virion infectivity factor (Vif) Proc Natl Acad Sci USA. 2004;101:3927–3932. doi: 10.1073/pnas.0307132101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huthoff H, Malim MH. Identification of amino acid residues in APOBEC3G required for regulation by human immunodeficiency virus type 1 Vif and virion encapsidation. J Virol. 2007;81:3807–3815. doi: 10.1128/JVI.02795-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang L, Saddatmand J, Li X, Guo F, Niu M, Jiang J, Kleiman L, Cen S. Function analysis of sequences in human APOBEC3G involved in Vif-mediated degradation. Virology. 2007;370:113–121. doi: 10.1016/j.virol.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 99.Simon V, Zennou VD, Murray D, Huang Y, Ho DD, Bieniasz PD. Natrual variation in Vif: differential impact on APOBEC3G/3F and a potential role in HIV-1 diversification. PLoS Pathog. 2005;1:e6. doi: 10.1371/journal.ppat.0010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tian C, Yu X, Zhang W, Wang T, Xu R, Yu XF. Differential requirement for conserved tryptophans in human immunodeficiency virus type 1 Vif for the selective suppression of APOBEC3G and APOBEC3F. J Virol. 2006;80:3112–3115. doi: 10.1128/JVI.80.6.3112-3115.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu B, Sarkis PT, Luo K, Yu Y, Yu XF. Regulation of Apobec3F and human immunodeficiency virus type 1 Vif by Vif-Cul5-ElonB/C E3 ubiquitin ligase. J Virol. 2005;79:9579–9587. doi: 10.1128/JVI.79.15.9579-9587.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Russell RA, Pathak VK. Identification of two distinct human immunodeficiency virus type 1 Vif determinants critical for interactions with human APOBEC3G and APOBEC3F. J Virol. 2007;81:8201–8210. doi: 10.1128/JVI.00395-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marin M, Golem S, Rose KM, Kozak SL, Kabat D. Human immunodeficiency virus type 1 Vif functionally interacts with diverse APOBEC3 cytidine deaminases and moves with them between cytoplasmic sites of mRNA metabolism. J Virol. 2008;82:987–998. doi: 10.1128/JVI.01078-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wedekind JE, Gillilan R, Janda A, Krucinska J, Salter JD, Bennett RP, Raina J, Smith HC. Nanostructures of APOBEC3G support a hierarchical assembly model of high molecular ribonucleoprotein particles from dimeric subunits. J Biol Chem. 2006;281:38122–38126. doi: 10.1074/jbc.C600253200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bennett RP, Salter JD, Liu X, Wedekind JE, Smith HC. APOBEC3G subunits self-associate via the C-terminal deaminase domain. J Biol Chem. 2008;283:33329–33336. doi: 10.1074/jbc.M803726200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 107.DeLano WL. The PyMOL molecular graphics system. San Carlos: DeLano; 2002. [Google Scholar]