Abstract
T cell memory is a crucial feature of the adaptive immune system in the defense against pathogens. During the last years, numerous studies have focused their efforts on uncovering the signals, inflammatory cues, and extracellular factors that support memory differentiation. This research is beginning to decipher the complex gene network that controls memory programming. However, how the different signals, that a T cell receives during the process of differentiation, interplay to trigger memory programming is still poorly defined. In this review, we focus on the most recent advances in the field and discuss how T cell receptor signaling and inflammation control CD8 memory differentiation.
Keywords: T cell memory, Signaling, Inflammation, Infection, T cell receptor, CD8 T cell, CD4 T cell
Introduction
Successful protection against infectious diseases depends on the induction of immunological memory. This is the main purpose of vaccination. The great majority of current vaccines are based on the generation and maintenance of high levels of neutralizing antibodies and memory B cells. However, protective immune responses and vaccine regimes also require the differentiation of memory T cells to release cytokines that inhibit the pathogen and eliminate infected cells. This may be especially crucial for diseases such as HIV and TB where an effective vaccine has not yet been developed.
Upon infection or vaccination, naive T cells use their T cell receptors (TCRs) to recognize antigenic pathogen peptides associated with MHC molecules (p-MHC) on the surface of antigen presenting cells (APCs). As a consequence, naive T cells get activated in the lymph nodes and undergo clonal expansion and differentiation. This results in: (1) an enormous expansion of the small population of the pathogen-specific naive T cells; (2) the allowance of these cells to leave lymph nodes and patrol non-lymphoid tissues in search of infected cells and (3) acquisition of effector functions, such as cytokine production and cytotoxicity, which facilitate the elimination of the pathogen.
Once the pathogen has been cleared, most of the antigen-specific effector T cells die. Only about 1–5% of these T cells survive to establish the memory pool that will offer protection against re-infection. Memory T cells are characterized by their long-term survival, their rapidity at proliferating, and efficiency at differentiating into effector cells upon re-challenge. At the end of the secondary responses, memory T cell numbers increase. It is still unclear, however, whether these secondary memory cells maintain the same quality and longevity as primary and tertiary T cells [1–3]. This aspect is especially critical in the context of re-boosting vaccine regimes.
During the last years, considerable effort has been put into determining how memory T cells are generated, which factors regulate their homeostasis, and which mechanisms support their function. These studies have helped to identify factors that are involved in T cell memory survival [4] and extracellular cues that are required for memory differentiation [5]. They have also shown the complex interrelationship that is required between CD4, B and CD8 T cells to induce protective immunity [6–8]. In addition, they have provided evidence as to how diverse T cell memory populations are [9] and how important it is to identify and avoid artifacts caused by non-physiological experimental conditions [10, 11]. Together, these advances introduce new insight as to how the creation of better experimental models will lead to the generation of more clinically relevant data. This also suggests the necessity of studying T cell memory from an intracellular level. This review focuses on T cell memory differentiation with a special emphasis on the signals and intracellular mechanisms that regulate their generation, survival, and secondary responses.
CD4 T cell memory
CD4 T cells are important in combating viruses, intracellular and extracellular bacteria, parasites, and fungi. T helper (TH) cells work as cell mediators that secrete cytokines and chemokines or provide help to other lymphocytes. Different subsets of CD4 T cells act on several target cells such as B cells (TFH), macrophages (TH1), eosinophils(TH2), neotrophils (TH17), and keratinocytes (TH22) to limit infections (review in [12]). While numerous studies have determined how different subsets of effector CD4 T cells arise, it is still unclear whether the different CD4 Th subsets maintain their lineage commitment as memory T cells. More importantly, the role that memory CD4 T cells have in protective immunity is still up for debate (reviewed in [13]).
CD4 T cell memory has remained elusive for several reasons. First, CD4 T cell immune responses are less robust than CD8 T cells. Second, the experimental models that have been used have introduced artifacts that preclude reproducibility and clarity in the results. The transfer of transgenic T cells at non-physiologically high precursor frequencies alters CD4 T cell memory survival [11, 14]. Furthermore, transgenic T cells have been transferred into lymphopenic hosts where they acquire a memory phenotype. Thus, the conclusions from these studies may not necessarily apply to what happens upon infection, considering that the inflammatory, costimulatory, and TCR/p-MHC component are very different. Finally, in humans, it is difficult to determine the specificity of memory populations or to follow the immune responses that originate them. The use of MHC-Class II tetramers to examine CD4 T cell responses may help in this respect.
Another added difficulty to the interpretation of the data is the immense phenotypic diversity of CD4 memory T cells. Based on human studies, CD4 memory T cell, as well as CD8 T cells, can be divided into central memory (TCM) and effector-memory (TEM). TCM cells express CD62L and CCR7, home to secondary lymphoid organs, have little immediate effector function but can rapidly proliferate, secrete interleukin-2 (IL-2) and differentiate into effectors upon re-challenge. TEM cells, on the other hand, exhibit low levels of CD62L and CCR7, home to peripheral tissues and have immediate effector function [15, 16]. Presumably within the TCM and TEM subsets, it is necessary to delineate memory subtypes that originate from the various effector T helper subsets predecessors. This becomes even more complex when considering the flexibility of the different subtypes and that some T cells can secrete cytokines that are not exclusive to their subtype [17, 18]. Additionally, upon re-challenge, memory CD4 T cells can differentiate into an effector TH subset different than the one they were originally committed. This raises a question as to how definitive the mechanisms that drive the different subsets are, since apparently some of the polarizing cytokines are mutually exclusive (Ex. Interferonγ –IFNγ- and IL-4). It also suggests that the epigenetic changes that regulate the TH lineage are more flexible than originally thought.
While CD4 and CD8 memory T cells share some common characteristics, they are essentially different in their mechanism of differentiation and survival. Both cell types require specific TCR signals, costimulation and cytokines. However, CD4 T cells require a more sustained/prolonged period of antigenic stimulation. The idea that CD4 T cells have a higher TCR signaling threshold becomes apparent in in vitro cultures of effector differentiation and in studies that show that high precursor frequencies or low TCR avidity have a detrimental effect on the generation of memory CD4 T cells and their survival [14, 19]. This seems to correlate with the duration of dendritic cell (DC)-T cell interaction required to commit naive CD4 T cells to proliferate and express cytokines [20], much longer than for CD8 T cells [21].
It has also become clear that neither CD4 nor CD8 memory T cells require MHC class II or I protein interaction for their survival. In contrast, their homeostasis depends on the cytokines IL-7 and IL-15 [4]. Both cytokines are essential for memory CD4 survival, although IL-7 seems to have a predominant role [4]. This may be because of the low levels of IL-15 receptor expressed on CD4 T cells when compared to CD8 or natural killer (NK) cells [22].
Homeostatic proliferating T cell memory (HP-memory)
Naive T cells undergoing homeostatic proliferation (HP) in lymphopenic conditions (such us exposure to cytotoxic drugs, irradiation, or infection with certain viruses) acquire a memory phenotype similar to true-memory cells generated under conditions of acute infection [23]. This includes high levels of memory phenotypic markers (CD44, CD122, CD127 and Ly6C); a gene expression profile similar to antigen-experienced memory cells [24] and the ability to perform immediate effector functions upon stimulation. CD8 HP-memory cells are also able to confer protection against bacterial infection, provided CD4 help is present [25]. Interestingly, CD4 help is not antigen-specific but rather depends on CD40-CD40L interactions [26]. Altogether, these data suggest that HP-memory cells could be an excellent surrogate for true-memory cells. However, recently it has been shown that CD8 HP-memory T cells cannot compete with true-memory cells during infection. When similar numbers of HP-memory and true-memory cells were co-transferred in mice that were subsequently infected with Listeria monocytogenes, HP-memory T cells contracted more dramatically and generated fewer secondary memory T cells [27].
Unlike true-memory T cells, HP-memory T cells don’t seem to pass by an effector phase. This raises the question as to whether both memory differentiation pathways utilize the same signals and intracellular intermediates to regulate gene expression profiles that do not seem to differ very much between both cell types [24]. In addition, what are the mechanisms that provide HP-memory with memory function if they don’t pass by an effector phase or express effector genes [24]? HP-memory cells are generated in response to low-affinity self-antigens and the cytokines IL-7 and IL-15 [4]. They don’t require conventional costimulation [28], although recently B- and T-lymphocyte attenuator (BTLA) [29] and CD24 [30] have been reported to be required for homeostatic expansion. True-memory T cells, on the other hand, respond to TCR signals upon recognition of high-affinity foreign antigens, costimulatory molecules, and inflammatory cytokines (IL-2, typeI IFN) (reviewed in [5]). IL-7 and IL-15 [4, 31] are required for CD8 memory T cell homeostasis/survival. However, none of them seem to be required at the time of priming to drive memory generation. IL-7 seems to promote the survival of memory precursors while, IL-15 is important for short live effectors survival [32].
HP-memory T cells generated in TCR or rag-deficient lymphopenic hosts differentiate into a memory phenotype but are not able to acquire memory functionality. In contrast, memory functionality is acquired when HP-memory cells are produced in irradiated hosts. This is due to the inflammatory component that is derived from sub-lethal irradiation in these mice [26]. Interestingly, IL-12 can also substitute for CD4 help in inducing protective HP-memory CD8 T cells [26]. Thus, it is tempting to suggest that, as in true-memory T cells, inflammation is also needed to acquire effector functions in HP-memory T cells.
Since all of the signals mentioned above regulate gene expression through the intracellular activation of different signaling cascades, an important question is, what is the role of TCR or cytokine signaling in HP-memory? Leukocyte-specific protein tyrosine kinase (Lck) and Fyn have been reported to be important for naive homeostasis. In lymphopenic conditions, TCR-driven proliferation seems to be dependent exclusively on Lck. However, the role of IL-7R signaling in HP is independent of both Src family kinases [33]. IL-7R seems to be regulating survival predominantly through Janus protein tyrosine kinase 3/Signal Transducers and Activator of Transcription 5 (JAK3/STAT5) and by inducing the expression of B cell lymphoma-2 (Bcl-2) and myeloid cell leukemia sequence 1 (Mcl-1) (review in [4]). Nevertheless, it has been proposed that increased IL-7 signaling amplifies the weak TCR signaling triggered by self-p-MHC ligands [34]. However, how this cooperation takes place intracellularly remains to be solved.
Different studies have suggested a role for the classical and alternative nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathways in T cell memory and HP-memory [35–39]. IκB kinase 2 (IKK2)-deficient CD4 and CD8 T cells fail to proliferate in lymphopenic hosts [35]. In contrast, homeostatic proliferation in naive CD4 T cells deficient in NIK (NF-κB inducing kinase in the NF-κB2 pathway that regulates the generation of p52) is enhanced while in memory T cells is impaired [37]. Most recently, two reports have suggested a specific role for Mammalian mitogen-activated protein kinase kinase kinase 3 (MEKK3) and ribosomal s6 kinase 2 (Rsk2) in HP. MEKK3-deficient T cells are able to proliferate in response to antigen but are defective in lymphopenic induced proliferation and HP-memory differentiation. MEKK3 exerts its role through ERK1/2. In addition, it is exclusively involved in the TCR signaling component required for HP but dispensable for the IL-7 signal [40]. Similarly, Rsk2 is not involved in IL-7 signaling. However, its activation is induced in response to IL-15 and TCR stimulation. Rsk2-deficient T cells are impaired in homeostatic proliferation and this defect cannot be rescued by exogenous administration of IL-15, suggesting again an important role downstream of the TCR [41]. Interestingly, both MEKK3[42]and Rsk2 [43] are able to regulate NF-κB activity. Hence, it is tempting to speculate that TCR signal regulates memory phenotype through the induction of NF-κB. With regards to other TCR signaling intermediates such us nuclear factor of activated T cells (NF-AT), c-Jun N-terminal kinase (JNK) or phosphoinositide 3-kinase (PI3 K). It is not clear whether these play a role in HP-memory.
CD8 T cell memory
T cell memory differentiation has received significant attention in the last years. Much of what the field knows about the mechanisms and external factors required for T cell memory generation, survival, and function come from studies performed in CD8 T cells. For that reason, we will dedicate the rest of the review to the discussion of the most recent advances and challenges that the field still faces to fully understand the complex molecular network that regulates T cell memory.
CD8 T cells are necessary to combat intracellular pathogens. They are characterized by their cytotoxic functions that allow them to eliminate infected cells upon recognition of pathogen-derived peptides bound to MHC-class I molecules. They produce large amounts of cytokines such as IFNγ and tumor necrosis factor α (TNF-α), although their ability to secrete IL-2 is poor.
In the face of an acute infection, the CD8 T cell immune response is triggered and can be divided into different phases. In the early phase of priming, naive CD8 T cells receive three signals upon encounter with dendritic cells. Signal 1 or TCR signal that results from the recognition of pathogen-derived p-MHC class I complexes on the surface of the APC; Signal 2 provided by costimulation and Signal 3 or inflammatory signals derived from IL-12, TypeI IFNs or Toll-like receptor (TLR) ligands (reviewed in [5]). The priming phase is followed by an expansion phase where antigen-specific T cell clones acquire an effector phenotype as they divide vigorously and increase their frequency. Effector cells are able to migrate to all tissues and clear the infection by secreting cytokines and killing infected cells. In general, by the peak of the immune response the pathogen has been cleared. At this point, both effector and memory precursors are present although the latter in very low numbers compared to effectors. Most cells at the peak of the response die by apoptosis (90–95%) in the contraction phase and the small percentage that survives establishes the memory pool. How memory T cells arise and are able to survive the contraction phase still remains obscure. Different hypotheses have been proposed. However, the great diversity of memory T cells and the lack of definitive markers make it difficult to settle on an absolute model of memory ontogeny (discussed later).
The requirements for differentiating memory T cells differ depending on the infection. For example, B cells play an important role in CD8 responses to Listeria monocytogenes (L.m.) and chronic lymphocytic choriomeningitis virus (LCMV) infections. However, CD8 T cell responses don’t require B cells in other infections [44]. The absence of B cells in Listeria infections leads to enhanced contractions and lower T cell memory [8]. In the case of CD4 T cells, B cell help seems to be necessary for CD4 T cell memory in an antibody independent manner, at least in LCMV infections [7]. Nevertheless, it remains to be seen whether this is the case in all infections.
CD4 T cell help is also required for CD8 memory in different models [45–48]. CD4 T cells are especially necessary for the survival of CD8 memory T cells and their function, as shown in CD8 memory T cells generated or maintained in CD4-deficient hosts [6]. CD4 T cell help is thought to be provided by CD40-CD40L interactions on APCs and required in situations where APCs are not directly activated by the pathogen [46, 49]. Alternatively, IL-2 secretion by CD4 T cells may also be important, since CD8 memory T cells deficient in IL-2Rα (CD25) are also impaired in memory function [50]. Most recently, these data have been challenged by the finding that CD8 memory T cells generated in CD25−/− hosts exhibit normal secondary responses, in L.m. infections [51]. Hence, further studies will be needed to explain the mechanisms that regulate the requirement for CD4 help during CD8 memory development.
With regards to memory CD8 homeostasis, it is now clear that the survival of memory CD8 T cells is not dependent on self-MHC class I interactions [52]. Memory CD8 T cells express high levels of IL-7R and IL-15R. It is their interaction with IL-7 and IL-15 what triggers the signals that support memory CD8 T cell homeostasis. IL-7 signaling is responsible for cell viability, while IL-15 induces basic homeostatic proliferation. Thus, CD8 T cells where IL-7R signaling is impaired and cannot activate JAK3/STAT5 to induce Bcl-2 expression are able to differentiate into memory T cells but gradually disappear unless Bcl-2 is overexpressed. IL-15 is secreted by non-T cells in response to inflammatory cytokines. IL-15Rβ, on the other hand, is upregulated upon priming of naive CD8 T cells and its expression depends on the transcription factors T-bet and Eomes. In the absence of IL-15, IL-15Rβ or in T cells where T-bet and Eomes are not functional, memory CD8 T cells are depleted (reviewed in [4]). Recently, two unrevealed roles of IL-7 and IL-15 have also been reported. During the contraction phase, IL-15 and IL-12 promote the survival of short-lived effector/memory cells. However, IL-7 seems to favor the proliferation of long-lived memory precursors [32]. Along with this, secondary responses in IL-15-deficient mice fail to generate a subset of CD8 effector memory cells [3]. In addition, in the absence of IL-15, secondary memory T cells survival and basal proliferation are also affected [3].
Memory programming
CD8 T cell memory differentiation does not require the continuous presence of antigen. Several studies have concluded that the signals that naive CD8 T cells receive during the first days (1–3 days) of an infection are sufficient to support the entire process of memory differentiation [53, 54]. These early signals imprint, on the naive T cell, a program of epigenetic changes that are inherited by the daughter cells and regulate clonal expansion, acquisition of effector functions, contraction, and differentiation into memory [5, 55]. The realization that memory T cells are not a singular phenotype ,but on the contrary, are a diverse collection of phenotypically and functionally different subsets of cells, has raised a question about the flexibility of the program and how much of it is determined at the priming phase versus how much of the program can be further influenced by other factors such as location or inflammation. Accordingly, the fact that memory precursors can already be detected very early in the response suggests that the commitment to memory might occur during the priming phase [56–58]. Nevertheless, this does not discard the possibility that other signals may be needed later in the response for a full consummation of the memory program. What seems now less likely is that memory T cells could arise from effector T cells as a product of stochastic survival during the contraction phase.
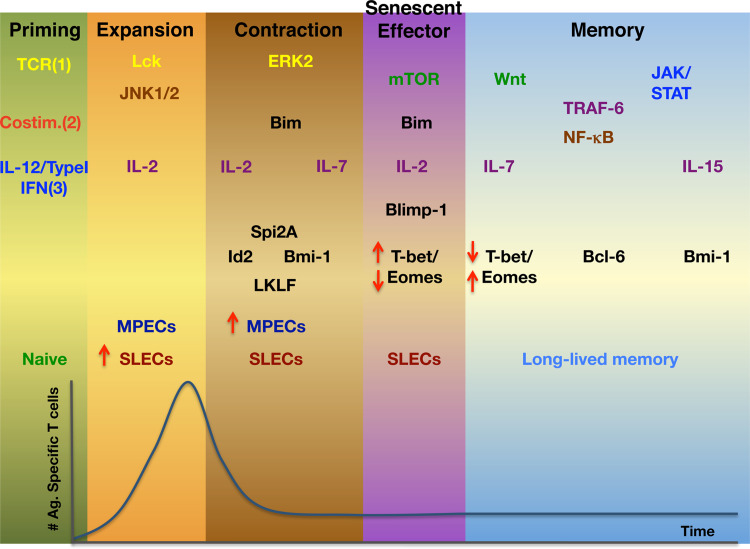
Memory programming includes diverse epigenetic changes that ultimately lead to the regulation of gene expression (Fig. 1). In the most recent years, several studies have shed light into some of the transcription factors that may control CD8 memory programming. T-bet is a T box transcription factor that is a master regulator for Th1 differentiation [59]. T-bet and the homologous transcription factor eomesodermin (Eomes) have been linked to effector and memory T cell differentiation [60]. In mice with compound mutations in the genes that encode both transcription factors, T cells were unable to up-regulate IL-2Rβ/CD122 or induce the expression of effector genes such as Granzyme B, IFNγ and perforin [61]. Due to the lack of CD122/IL-15 stimulation, these mutant mice also showed a lack in CD8 T cell memory [61]. Interestingly, IL-12 inversely regulates T-bet and Eomes expression during CD8 T cell differentiation. Thus, IL-12 promotes T-bet induction and effector differentiation while it represses Eomes. In memory T cells, T-bet expression is reduced but Eomes, in turn, is highly expressed [62, 63]. This seems to correlate with the finding that IL-12-deficient mice show an increased memory to effector T cell ratio upon L.m. infection [64]. Furthermore, the level of inflammation, as measured by the expression of T-bet, determines the ratio of memory precursor effector cells (MPECS)-KLRG1lo IL-7Rhi- to short-lived effector cells (SLECs)-KLRGhi IL-7Rlo- generated at the peak of the response [65]. Early in the response, an increasing gradient of T-bet is triggered upon infection. Cells with higher T-bet expression differentiate into effectors and cells with lower T-bet expression become memory T cells. Although these studies clearly demonstrate a role of inflammation on the effector/memory decision via T-bet and Eomes, further research is needed to characterize how they are regulated by other signals such as TCR and costimulation.
Fig. 1.
Signals, signaling pathways, signaling intermediates, cytokines, and transcription factors involved in the differentiation of short-lived (senescent) effector cells and long-lived memory T cells and their positive involvement in the phases of the immune response. Colors are related to the Signal 1, 2, and 3 that initiate T cell differentiation in the priming phase, yellow, red, and blue, respectively. Brown applies to molecules activated by Signal 1 and 2. Green corresponds to activation via Signals 1, 2, and 3
Transcriptional repressor B-lymphocyte-induced maturation protein 1(Blimp-1) and Bcl-6 are transcriptional repressors with well-documented roles in B cell differentiation (Fig. 1). It has recently been shown that both contribute to the CD8 effector/memory decision. Blimp-1 is expressed in both effector and memory T cells [66]. Blimp-1 is essential in the terminal differentiation of effector CD8 T cells. Lack of Blimp-1 impedes CD8 effector T cell differentiation and diverts T cells into memory precursors with a central memory phenotype [67, 68]. Additionally, memory immune responses are compromised [67]. In agreement with this, downstream of Blimp-1, X-box-binding protein 1(XBP-1) promotes the generation of effector CD8 T cells. XBP-1 is important for the secretory machinery, which may partially explain the defects in effector function in Blimp-1-deficient T cells [69]. Bcl-6, on the other hand, is essentially required for memory differentiation. Over-expression of Bcl-6 leads to an increased generation of memory T cells [70]. In contrast, lack of Bcl-6 affects the generation and survival of memory T cells [71]. Bcl-6b, a homologue of Bcl-6, is also required for secondary memory responses [72]. It is known that Bcl-6 and Blimp-1 can block each other’s expression. Indeed, Blimp-1-deficient T cells show decreased T-bet expression and high Bcl-6 and Eomes expression. Thus, it is tempting to speculate that both Bcl-6 and Blimp-1 regulate the effector/memory decision by regulating each other’s levels and may also be regulating the expression of T-bet and Eomes. How Bcl-6 and Blimp-1 levels are modulated by the early signals that programmed memory differentiation still remains to be elucidated.
DNA-binding protein inhibitor 2 (Id2), Bmi-1, serine protease inhibitor 2A (Spi-2A) and Lung Krüppel-like factor (LKLF), have also been linked to the effector/memory decision (Fig. 1). Id2 is an inhibitor of the E-protein family of transcription factors. In the absence of Id2, expanding effector cells are lost due to increased apoptosis (high levels of Bim together with low levels of Bcl-2 and Spi6) [73]. As a consequence, less memory CD8 T cells are generated and they are skewed toward a central memory phenotype. However, it is unlikely that Id-2 has a role in determining CD8 T cell memory subsets, since both TCM and TEM have equal Id2 expression [74]. TCR-induced Bmi-1 expression is maintained in memory precursors, but inhibited in terminal effector T cells [75]. Furthermore, the absence of Bmi-1 in CD4 T cells leads to impairment in Th1 memory generation due to the expression of proteins involved in cell cycle arrest (Ink4a) and apoptosis (Arf and Noxa) [76]. Serine protease inhibitor 2A (Spi-2A) is a protein involved in CD8 survival that has been suggested to promote memory differentiation [77]. Spi2A is also a NF-κB dependent inhibitor of lysosomal proteases that protects CD8 T cells from apoptosis during the contraction phase. Overexpression of Spi-2A in bone marrow chimeras infected with LCMV leads to the generation of more memory T cells. The opposite effect occurs when an anti-sense Spi2A mRNA is expressed. Finally, LKLF is known for its role in naive T cell survival. Its expression is down-regulated upon TCR stimulation. Interestingly, LKLF is induced by IL-2 and IL-7 but not by IL-12. Its re-expression coincides with long-term survival and development of memory T cells, where it is highly expressed, thus, suggesting that LKLF may have also a role in memory T cell survival [78].
TCR and costimulatory signals induce the expression of the transcription factor ThPOK, which promotes the expansion and function of memory T cells [79]. IL-7R signaling is not essential for memory differentiation [80]. However, IL-7R expression is crucial in survival of memory T cells and its expression levels, together with killer-cell lectin-like receptor subfamily G member 1(KLRG1) and CD62L expression, are currently used to identify effectors and memory precursors. IL-7R expression is regulated by the balance of the transcriptional repressor growth factor independence 1(Gfi-1) and the transcription activator GA-binding protein α (GABPα). Gfi-1 maintains hypoacetylation of histones, leading to a stable repression of IL-7R expression in late effector CD8 T cells. Conversely, GABPα promotes histone acetylation and IL-7R expression in memory precursors [81]. It is been shown that histone acetylation of Eomes and the genes that encode for perforin and granzyme B is significantly higher in memory than in naive T cells, providing with a mechanism that poise T cells to rapidly respond to stimulation [82]. Thus, histone acetylation is an epigenetic change that may play an important role in programming memory cells for increased function. Furthermore, histone methylation has also been suggested to regulate memory CD8 function [83].
Signals required for CD8 T cell memory differentiation
Three signals are required during the priming phase to initiate the process of CD8 T cell memory differentiation. TCR signal or Signal 1 is triggered in naive T cells upon recognition of p-MHC molecules on DCs. This signal provides specificity to the immune response. Features of the TCR-pMHC interaction are antigen dose, duration, affinity and efficiency of signaling. Studies evaluating the role of antigen dose/signal strength [84, 85] and the duration of the antigenic stimulation [86, 87] in the generation of T cell memory, have suggested that memory differentiation could follow a “Goldilocks” model (reviewed in [88, 89] where early in the infection, only optimal antigen stimulation (not too weak or too strong) would support memory (Fig. 2). Thus, shortening antigenic stimulation only affects the expansion of effector T cells without perturbing the generation of memory and memory function [87, 90]. Conversely, prolonged TCR stimulation or a high dose of TCR signal supports the generation of exhausted long-lived T cells [55, 91, 92].
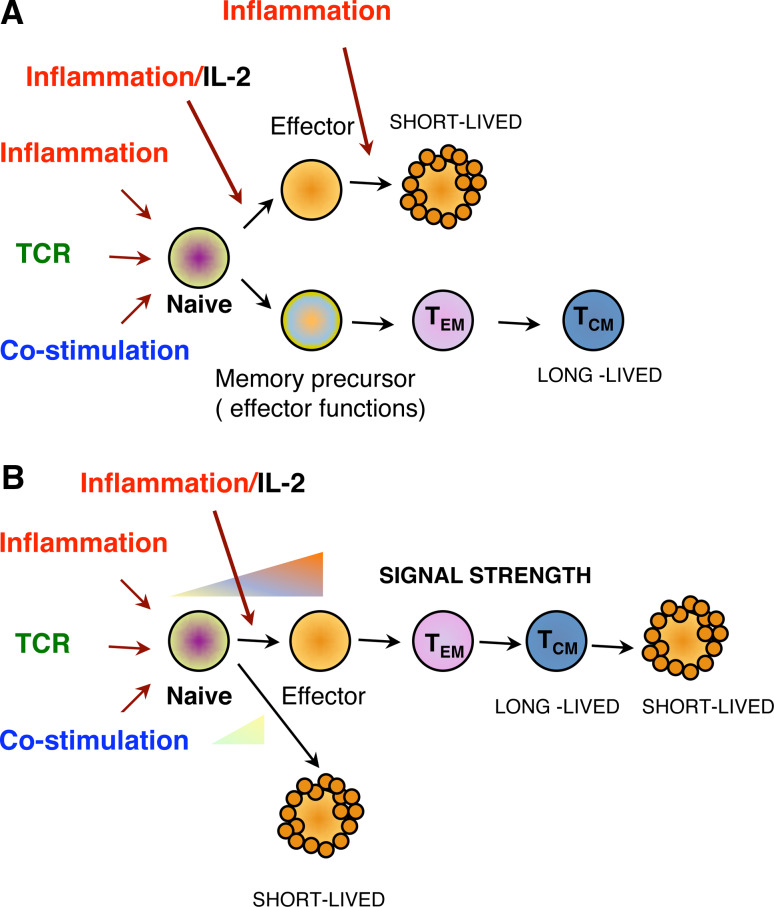
Fig. 2.
Models of memory development according to memory programming. a Two-lineage model. Upon priming, the naive T cell is stimulated by TCR, costimulatory and inflammatory signals/IL-2. It commits to become either a short-lived effector or a memory precursor that has acquired effector characteristics but is able to further differentiate into an effector memory T cell (TEM) and a central memory T cell (TCM). b Linear differentiation model/“Goldilocks model”. Signals that are too weak or too strong result in cells that differentiate into a short-lived end-stage effectors. Intermediate signal strengths support TEM and TCM. differentiation passing by an effector phase
When considering the heterogeneity of the memory populations and data suggesting that memory cells gradually differentiate after the peak of the response, T cell memory differentiation would fit better into a decreasing potential model for antigen stimulation. In this model, effector cells would lose memory potential as TCR stimulation increases [85, 93]. Furthermore, it has also been shown for CD4 T cells that there are increasing TCR affinity thresholds that gradually select the T cell clones that will expand, reach the peak of the response and constitute the memory pool [94]. CD8 T cells also undergo TCR affinity maturation for the polyclonal population that constitutes the memory pool at the end of the immune response [94–97]. Strikingly, these data clearly contrast with recent publications where even very weak ligands are able to generate efficient memory cells despite weak expansion phases and with no indication of overt autoimmunity [98]. These latter studies, interestingly, correlate with the generation of cells with memory phenotype in lymphopenic conditions where TCR- self p-MHC interactions are very weak. Taken all together, two potential scenarios for TCR signaling could be proposed for the differential generation of short-lived effector cells and long-lived memory cells. In the first model, naive T cells are selected to become memory precursors depending on the overall strength of the TCR signal and Signals 2 (costimulation) and 3 (inflammation). The stronger the stimulation, the more the T cell differentiates towards end-stage effectors. There is experimental evidence that indicate that in situations of low inflammation or weak TCR signal, memory generation is favored [98–100]. On the other hand, naive T cells may be recruited into the end-stage effector or long-lived memory pool depending on differences in the quality of TCR signals or other signals. This might explain the heterogeneity among effector and memory T cells. There is also experimental data in favor of this hypothesis, naive T cells that are selectively impaired in NF-κB-dependent TCR signaling (but not other TCR signaling pathways) differentiate into short-lived effectors but cannot differentiate into memory T cells [39]. In addition, the fact that some transcription factors are preferentially required for memory differentiation and not effector differentiation and vice versa also supports the idea that different signals are determining their differential expression [62, 67, 71]. If long-lived memory cells and short-lived effectors where only differentiated based on overall signal strength, one would expect a skewing of the TCR repertoire that make up both pools. Conversely, no alteration in the TCR repertoire is observed in the transition from effector to memory [101]. All these findings have increased our knowledge about the factors that regulate memory at the extracellular and nuclear level. However, our understanding of the signaling pathways that are triggered by the TCR, costimulatory, and inflammatory signals; how they are modulated extracellularly and how they are regulating the potential master transcription factors that determine memory is still very limited.
One of the most TCR-proximal signaling events upon TCR stimulation is Lck activation. Lck phosphorylates the immunoreceptor tyrosine-based activation (ITAM) motifs of the CD3 chains of the TCR/CD3 complex and allows the recruitment and activation of zeta-chain-associated protein kinase 70 (ZAP-70) to the membrane. ZAP-70, in turn, phosphorylates different adaptor molecules such as linker for activation of T cells (LAT), SH2 domain-containing leukocyte protein of 76 kDa(SLP-76), and Vav, which promote the recruitment and activation of other adapters and kinases responsible for initiating calcium mobilization, mitogen-activated protein kinases (MAPKs) activation, and NF-κB signaling (reviewed in [102]). During acute infection, Lck is important for clonal expansion and effector function. However, when Lck is conditionally turned off at the memory stage, no affect in memory survival or function is observed [103]. NF-κB signaling is also triggered upon TCR stimulation. NF-κB induction depends on the recruitment and TCR co-localization with the protein Carma-1 and protein kinase C θ (PKCθ) in the plasma membrane. Carma-1 is phosphorylated by PKCθ and recruits the (Carma-1/Bcl-10/Malt-1) complex to the membrane, where Bcl-10, mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT-1) and TNF receptor-associated factor-6 (TRAF-6) mediate the activation of the IKK complex. Finally, the IKK complex promotes the phosphorylation and translocation of NF-κB to the nuclei [104]. Mice genetically engineered to express impaired NF-κB signals are deficient in the generation and function of memory T cells [36]. However, given the role of NF-κB in inflammation, these studies were unable to determine whether TCR and/or inflammatory signals were required for memory. Most recently we have shown that mutant TCR transgenic T cells with a selective impairment in TCR-dependent NF-κB induction are unable to differentiate into memory T cells upon L.m. infection [39]. In agreement with this data, mice deficient in TRAF-6, an ubiquitin ligase that mediates the activation of TGF-beta activated kinase 1(TAK-1) and activation of the IKK complex, are also selectively deficient in memory development, whereas primary immune responses are normal [105]. These studies also showed that the memory defect was related to impairments in fatty acid metabolism. Whether this is the downstream effect of TRAF-6 deficiency through the TCR or through other signals [TRAF-6 is also activated by Toll-like receptors(TLR4)] still remains to be elucidated.
MAPKs, ERK, JNK, and p38 are also activated upon TCR triggering [106, 107]. The role of ERK1 and ERK2 in infection has been studied. ERK1 is dispensable for all the phases of the immune response. ERK2, however, is not required for effector function but is essential for T cell survival dependent on Bim and Bcl-xL [106]. JNK activation requires both TCR and CD28 costimulatory signals. JNK1 and JNK2 seem to play opposite roles on CD8 T cell function. Upon infection with LCMV, JNK2-deficient cells secrete increased levels of IL-2 and their expansion is enhanced. Conversely, JNK1-deficient virus-specific T cells are impaired in their expansion most likely due to a reduced CD25 expression and exhibit increased apoptosis of effector cells [108]. JNK1-deficient T cells also show defects in cytotoxicity and low expression of T-bet, Eomes and perforin caused by impairment in early TCR-mediated IFNγ production [109]. Another stress kinase activated by the TCR is p38MAPK. p38 is involved in apoptosis of CD8 T cells mediated by Bcl-2 and in the production of IFNγ [110]. Nevertheless, its role in CD8 T cell responses upon acute infection still needs to be studied.
Mammalian target of rapamycin (mTOR) is a serine threonine kinase that regulates cell growth, proliferation, motility, survival, as well as protein synthesis and transcription. mTOR nucleates the multi-protein complexes mTORC1 and mTORC2 [111]. Recently, mTORC1 has been linked to CD8 memory development [105, 112]. Inhibition of mTOR with the drug rapamycin, causes an increase in memory precursors during the expansion phase and memory differentiation that results in enhanced numbers of memory T cells with higher memory function upon LCMV and vaccinia infection [112]. Since inflammation can also activate mTORC1 complex, this study could not determine whether the effect of mTOR on memory differentiation was solely dependent on inflammation or also on TCR signaling in the context of the infection. Indeed, TCR and CD28 signaling activate mTOR kinase. Remarkably, IL-12 signaling can enhance antigen-induced mTOR activity via PI3K and STAT4. This leads to T-bet sustained expression and effector differentiation. However, when mTOR is inhibited by rapamycin, T-bet expression is curtailed and Eomes expression is maintained, resulting in the acquisition of memory phenotype [113]. Interestingly, mTOR activity does not seem to have the same effect on CD4 T cell differentiation [113]. This study posits mTOR as a potential central integrator of TCR, costimulatory and inflammatory signals to regulate the T-bet/Eomes expression balance that determines CD8 effector vs. memory differentiation. Taking into account that mTOR is under the control of growth factors, energy status, oxygen levels, aminoacids, and genotoxic stress, it is reasonable to think that they might also be affecting memory programming as they change during the course of the immune response. If this holds true, then when in the immune response are their effects more influential than the priming signals? Additionally, antigen, costimulation and inflammatory factors vary in the context of different infections. It would be interesting to determine whether different doses of each of the signals, change the mTOR regulation of memory.
mTORC1 complex activity can also be modulated by Wnt signaling through glycogen synthase kinase 3 (GSK3) inhibition. CD8 T cells where GSK3 was inhibited, exhibit an arrest in effector differentiation and a generation of T cell memory stem cells with proliferative and antitumor capacities superior to TCM and TEM cells [114]. The experimental approach of this study could not determine if this signaling pathway is, indeed, operating in memory differentiation upon infection. This study could not discard that other signaling pathways under the control of GSK3 could be responsible for the stem cell memory phenotype as well. Most recently, Dong-Mei et al. have solved these issues. Their data shows that in the context of an acute infection, constitutive Wnt signaling through the enforced expression of T-cell factor/lymphoid enhancer factor 1 (TCF-1) and β-catenin, leads to the generation of more CD8 memory T cells with improved function [115]. This study also implies that Wnt signaling may have a distinct way of regulating memory differentiation independent of mTOR.
Signal 2 or Costimulation is governed by members of the CD28 (CD28, CTLA-4, ICOS, PD-1, BTLA), TNF/TNFR (OX40, CD27, 4-1BB, CD30, GITR, and HVEM) and integrin (LFA-1, VLA-4) families. They impact differently on CD4 or CD8 T cell responses. In addition, differences in the kinetics of expression during the immune response suggest that each one may have distinct roles during the several phases of the immune response and possibly in instructing the ultimate T cell fate. CD28, CD27, and 4-1BB are involved in CD8 T cell responses, especially when TCR stimulation is weak (reviewed in [5]). PD-1 negatively regulates T cells in chronic infections [116] and BTLA and OX40 have been linked to inhibition of CD8 memory [29, 117]. It seems likely that the role of these molecules is redundant since disruption of any of them only modestly affects the immune response. Nevertheless, the role they may play in the improvement of immune responses to weak antigens should not be underestimated, especially when considered with the fact that most of these costimulatory proteins trigger signaling cascades that complement TCR signal transduction. Thus, CD28 signaling synergizes with TCR signals to efficiently activate NF-κB, PI3 K/Akt, and JNK, most likely by regulating the recruitment and colocalization of PKCθ with TCR and CD28 to the immunological synapse [118]. The TNF/TNFR members also regulate NF-κB, PI3 K/Akt, and JNK activities through different TRAF molecules [102]. These signaling pathways are important for activation, clonal expansion, and survival. Elucidating the mechanisms of TCR and costimulatory signal synergism and when they take place in the immune response will greatly benefit the therapeutical targeting of costimulatory molecules for vaccine improvement.
The influence of Signal 3 or Inflammation on T cell differentiation is mediated by Toll-like receptors (TLR) and inflammatory cytokines (IL-12, type I Interferon, IFNγ). The nature of the pathogen or the adjuvant in vaccine formulations determines the cytokine and TLR that will direct memory development. The group of Matthew Mescher was the first to describe that a third signal provided by inflammatory cytokines was crucial to acquire effector functions and memory phenotype [119]. This signal needs to be provided at priming and sustained for at least 3 days to support memory programming [120]. Recent work suggests that the initial gene program is triggered by TCR and costimulatory signals and can only continue when inflammatory signaling is present. IL-12 and IFNα/β increase histone acetylation and consequently release gene repression. This applies to genes like Granzyme B or Eomes [121]. IL-12 signal has been intensively studied in Th1 differentiation. It depends on the induced expression of the IL-12Rβ1 and IL-12Rβ2 chains of the IL-12R [122]. Upon binding, IL-12Rβ2 becomes tyrosine phosphorylated and provides binding sites for kinases, Tyk2 and Jak2. These are important in activating JAK/STAT4 pathway. STAT4 regulates the transcription of several genes including IFNγ. Similar to IL-12, type I interferon (IFNα and IFNβ) also induce JAK/STAT pathway, and regulate the gene transcription trough STAT1 and STAT2. IFNα/β signaling can also be modulated by other signaling pathways such as PI3K, Vav/Rac/p38MAPK, and mTOR [123]. Remarkably, all these signaling pathways are also induced by TCR and CD28 signaling, suggesting another potential early interplay of the three signals at priming. The effects of other signaling pathways in regulating IL-12 signaling are unknown. However, the fact that IL-12 can regulate transcription by promoting histone-mediated chromatin remodeling [121] and that p38MAPK activation mediated by IFNα/β signaling also mediates acetyl transferase activation, indicates that both pro-inflammatory cytokines may be regulating memory/effector programming by sustaining the accessibility to specific genetic loci.
Lastly, IL-2/IL-2R expression has also been linked to effector versus memory differentiation. IL-2 induces Eomes and effector genes at the same time it represses memory gene expression such as Bcl-6 and IL-7Rα. By contrast, inflammation induces the expression of IL-2Rα(CD25) and T-bet as well as delayed expression of Eomes and release of the IL-2 mediated IL-7Rα repression [124]. In another study, Kalia and colleagues confirm the role of IL-2 in the effector/memory decision. They observed that in acute infections, two populations could be identified depending on CD25 expression. The ones that are CD25lo, less sensitive to IL-2 signaling differentiate into long-lived memory T cells. However, the CD25hi cells continue to receive IL-2 signals and differentiate into terminal effectors [125]. Both studies reconcile previous unclear data about the role of CD25 and IL-2 in effector and memory differentiation and propose that together with the TCR, costimulatory and inflammatory signals, IL-2 and IL-15 signals contribute to keep the balance that determines the fate of a T cell upon infection (short-lived effector or long-lived memory) (Fig. 1).
The balance between TCR signals and inflammation
Proinflammatory cytokines affect the rate of effector/memory generation. Thus, under low inflammatory conditions, fewer effector T cells are generated. Pretreatment with antibiotics, DC vaccination or IFNγR signals reduce the generation of effector cells [126]. Similarly, in the absence of IL-12, more memory T cells are generated [64]. Numerous studies have pointed to inflammation as the main signal that drives the generation of memory versus effector T cells. However, the question still remains if the three signals act in a tight balance to regulate memory differentiation or if some of them are more influential than others and thus, they are principally reinforcing a main signal. Several studies suggest that strong inflammation, either through IL-12 directly or through TLR ligands (CpG) appears to favor effector differentiation, while low inflammation enhances memory. In addition, it has also been demonstrated that pro-inflammatory cytokines are required during the first 3 days of priming to ensure memory differentiation [120]. Taken together, one could propose the “3 signals balance” hypothesis as a model to explain how memory or effector differentiation proceeds. During an acute infection, naive T cells encounter mature activated DCs that provide inflammation, costimulation, and antigen. These three signals act in a tight balance, such that when the three of them are equal in strength memory and effector development are ensured. However, if the balance is tilted towards TCR signals, this then leads to memory differentiation. This is supported by DC vaccination studies, where most of the cells at the peak of the response show the phenotype of memory precursors [127]. Other experimental evidence is shown in studies where low TCR avidity affects memory but not effector development [84]. The opposite scenario, where inflammatory signals are stronger than TCR signals, favors effector differentiation. Our experiments also support this. Naive T cells bearing a point mutation in the TCRβ transmembrane domain exhibit a defect in TCR signaling that correlates with a profound defect in memory but not in effector development [39]. Thus, when the TCR signal is impaired, even when inflammation and costimulation are provided at the same level, memory development fails [39]. The three-signal balance model would imply the existence of biochemical sensors able to integrate the different signals and initiate the adequate program. As discussed before, mTOR could serve as one of these sensors that regulates the balance of T-bet, Eomes, Bcl-6, and Blimp-1. To explain the acquisition of memory phenotype in lymphopenic conditions, it would be necessary to evaluate whether the low inflammatory signal provided by irradiation is stronger than TCR-self -p-MHC signal. Alternatively, memory development might be a default pathway governed by TCR stimulation. Thus, as long as TCR signaling is provided, memory development is guaranteed by default. However, when inflammation is present, not only at priming but until the infection is cleared, effector development proceeds. In support of this hypothesis, in DC vaccination experiments only when inflammation is provided at priming and the expansion phase, effector, and memory T cells are generated; otherwise, memory differentiation prevails [128]. There is also experimental evidence showing that low-affinity TCR ligands in the context of an infection poorly induce effector cells but are able to generate memory T cells. These results would be better explained with our second model [98]. Lastly, our results could also fit this second model. Given that TCR signals would be more important for memory, any defect in TCR signaling would preferentially affect memory without altering effector development [39].
Memory ontogeny
Over the last 10 years, immunologists have tried to determine how and when memory T cells arise. Understanding memory ontogeny is important in improving vaccine design against pathogens but also for generating immune protocols that ameliorate chronic infections or cancer and for regulating T cell responses in autoimmunity and organ transplantation. Still today, we don’t have a clear model for describing memory ontogeny. Part of the controversy comes from the difficulty to define what a memory T cell is. The lack of definitive phenotypic markers and the heterogeneity of memory T cells makes it difficult to find a model that fits all possible scenarios. In spite of this, different hypothetical models have been proposed. Several recent reviews have been dedicated to this issue [9, 129–133]. For this reason, we will only briefly discuss the models and present the questions that are still open in the field (Fig. 2).
One of the first models of memory differentiation is the linear differentiation model [134]. This model postulates that all memory T cells arise from effector T cells. Thus, after the peak of the response, the cells that are going to become part of the memory pool either are rescued from death by survival factors or are programmed somehow to dedifferentiate to cells that are long-lived and posses high proliferative and effector potential. This establishes the point of commitment to memory in the contraction phase, and suggests that there is a continuum of differentiation where cells transition form effectors to TEM to TCM. However, most likely, memory cells do not arise because they are rescued from apoptosis by survival factors. Survival cytokines such as IL-7 and IL-15 support the homeostasis of the cells once they are memory but increased expression or signaling of these cytokines does not seem to instruct the generation of more memory T cells [32, 80]. It has been described that in the effector to memory transition, memory precursors slowly acquire memory properties. This suggests that until a point there might be some extent of dedifferentiation, assuming that these memory T cells are descendents of true effector cells [135]. On the other hand, memory cells may not need to pass through a full phase of effector differentiation [65, 99, 136].
A second model is the decreasing potential model. In this model, T cells acquire the memory phenotype in progressive steps of further differentiation. The rounds of differentiation depend on the continuum of stimulation by antigen or other signals. This model predicts that the cells that undergo the maximum steps of differentiation will reach an end-stage and die, while the ones that differentiate the least will increase their survival potential and become memory. This model is supported by experimental data where curtailing the duration of antigenic stimulation (ex. with antibiotic treatment) leads to the early generation of memory T cells [58, 128, 136, 137].
The progressive differentiation model is a third model that tries to explain the heterogeneity of the memory subsets. In this model, the strength of the signal at the priming phase determines the degree of differentiation a naive T cell is going to experience. Thus, different thresholds of signal control the generation of the different memory subsets. Weak signals will favor TCM, intermediate signals TEM, and strong signals short-lived effectors. This model implies the presence of memory T cells or memory precursors very early in the response. Several studies support this [39, 114, 136, 138–140]. Mutant TCR memory T cells deficient in memory and TCR signaling may not achieve the threshold of stimulation that is required to progress from effector to memory [39].
The two lineage model proposes that both short-lived effectors and long-lived memory T cells are committed very early in the response to follow different paths of differentiation. This model is supported by data from Reiner’s group that suggest that asymmetric division promotes the differential segregation of signaling molecules at the level of the membrane among daughter cells [141]. As a consequence, the daughter cells inherit different fates, ones destined to become short-lived effectors and the others doomed to become memory. Our experimental data, where mutant TCR T cells defective in TCR signaling and in the immunological synapse assembly are selectively impaired in memory but not in effector differentiation, also supports this model [39]. This model does not explain, though, how diversity in the memory pool can be achieved or how signals that are present after the priming phase shape memory precursors. However, it does not discard that even when distinct signals instruct effector and memory programs, memory T cells have to pass by an effector phase [142]. It may also be possible that both programs are dependent on extrinsic factors that will act later in the immune response. In addition, recent data has demonstrated that the transfer of a single naive transgenic T cell is able to produce both effector and diverse memory populations [143]. However, it is still unclear if this can be generalized to normal immune responses or there are cases where specific signals contribute to only one of the effector or memory pools.
The last model is the self-renewing effector model where naive T cells can become TCM or effector cells with self-renewal potential. These cells will reside in lymphoid tissues and give rise to TEM that migrate to sites of infection with no self-renewal potential. These senescent terminally differentiated effector cells would be derived from TEM [132, 133].
In summary, more research is required to truly uncover the origin of memory T cells. Strategies in the future will need to take into account the enormous plasticity of T cells as well as their heterogeneity in phenotype, location, and function. Only that way, will the field be able to develop real strategies to improve future vaccines.
Concluding remarks
Over the last 10 years, incredible progress has been made in the understanding of T cell memory. Numerous studies have shed light onto the extracellular cues, survival mechanisms, and phenotypic/functional differences of the T cell memory pool. However, they have also provided evidence for the differences among CD4, CD8, and HP-memory, as well as the great diversity within the memory pool. The field is starting to map the transcriptional network that supports T cell memory differentiation. Yet, new challenges wait ahead to understand how different extracellular cues (TCR signal, costimulation, inflammation) orchestrate the signaling pathways that control the expression of lineage-determining genes such as T-bet, Eomes, Blimp-1, or Bcl-6. Furthermore, it is also important to identify the epigenetic changes that regulate the expression of genes involved in T cell memory differentiation. Answers to these questions are crucial for a rational design of better vaccines.
Acknowledgments
We acknowledge the University of Missouri Mission Enhancement Fund for supporting our work. We thank Karin Knudson, Cody Cunningham, and William Olson for interesting discussions and Dr. Ed Palmer for his support. We apologize to all authors we could not cite because of space restrictions.
Abbreviations
- APC
Antigen presenting cell
- Bcl- or BCL
B-cell lymphoma/leukemia
- Blimp-1
Transcriptional repressor B-lymphocyte-induced maturation protein 1
- BTLA
B- and T-lymphocyte attenuator
- DC
Dendritic cell
- ERK
Extracellular signal-regulated kinases
- GABPα
GA-binding protein α
- Gfi-1
Growth factor independence 1
- GSK3
Glycogen synthase kinase 3
- HP
Homeostatic proliferation
- Id2
DNA-binding protein inhibitor 2
- IFN
Interferon
- IKK
IκB kinase
- IL-
Interleukin
- ITAM
Immunoreceptor tyrosine-based activation motif
- JAK
Janus protein tyrosine kinase
- JNK
c-Jun N-terminal kinase
- KLRG1
Killer cell lectin-like receptor subfamily G member 1
- L.m.
Listeria monocytogenes
- LAT
Linker for activation of T cells
- Lck
Leukocyte-specific protein tyrosine kinase
- LCMV
Lymphocytic choriomeningitis virus
- LKLF
Lung Krüppel-like factor
- MALT-1
Mucosa-associated lymphoid tissue lymphoma translocation protein 1
- MAPK
Mitogen-activated protein kinase
- Mcl-1
Myeloid cell leukemia sequence 1
- MEKK
Mammalian mitogen-activated protein kinase kinase kinase
- MHC
Major histocompatibility complex
- MPECs
Memory precursor effector cells
- mTOR
Mammalian target of rapamycin
- NF-AT
Nuclear factor of activated T cells
- NF-κB
Nuclear factor κ-light-chain-enhancer of activated B cells
- NIK
NF-κB-inducing kinase
- NK cells
Natural killer cells
- p-MHC
Peptide-MHC complex
- PI3K
Phosphoinositide 3-kinase
- PKCθ
Protein kinase C θ
- Rsk
Ribosomal s6 kinase
- SLECs
Short-lived effector cells
- SLP-76
SH2 domain containing leukocyte protein of 76 kDa
- Spi-2A
Serine protease inhibitor 2A
- STAT
Signal transducers and activator of transcription
- TAK-1
TGF-beta activated kinase 1
- TCF-1
T-cell factor/lymphoid enhancer factor 1
- TCM
Central memory T cell
- TCR
T cell receptor
- TEM
Effector memory T cell
- TH
T helper cell
- TLR
Toll-like receptor
- TNF-α
Tumor necrosis factor
- TNFR
Tumor necrosis factor receptor
- TRAF-6
TNF receptor-associated factor-6
- XBP-1
X-box-binding protein 1
- ZAP-70
Zeta-chain-associated protein kinase 70
References
- 1.Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J Exp Med. 2006;203:919–932. doi: 10.1084/jem.20052237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masopust D, Ha S-J, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 3.Sandau MM, Kohlmeier JE, Woodland DL, Jameson SC. IL-15 regulates both quantitative and qualitative features of the memory CD8 T cell pool. J Immunol. 2010;184:35–44. doi: 10.4049/jimmunol.0803355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 6.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitmire JK, Asano MS, Kaech SM, Sarkar S, Hannum LG, Shlomchik MJ, Ahmed R. Requirement of B cells for generating CD4+ T cell memory. J Immunol. 2009;182:1868–1876. doi: 10.4049/jimmunol.0802501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen H, Whitmire JK, Fan X, Shedlock DJ, Kaech SM, Ahmed R. A specific role for B cells in the generation of CD8 T cell memory by recombinant Listeria monocytogenes . J Immunol. 2003;170:1443–1451. doi: 10.4049/jimmunol.170.3.1443. [DOI] [PubMed] [Google Scholar]
- 9.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 12.Sallusto F, Lanzavecchia A. Heterogeneity of CD4+ memory T cells: functional modules for tailored immunity. Eur J Immunol. 2009;39:2076–2082. doi: 10.1002/eji.200939722. [DOI] [PubMed] [Google Scholar]
- 13.Macleod M, Clambey E, Kappler J, Marrack P. CD4 memory T cells: what are they and what can they do? Semin Immunol. 2009;21:53–61. doi: 10.1016/j.smim.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blair DA, Lefrancois L. Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells. Proc Natl Acad Sci USA. 2007;104:15045–15050. doi: 10.1073/pnas.0703767104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 16.Lefrancois L. Development, trafficking, and function of memory T-cell subsets. Immunol Rev. 2006;211:93–103. doi: 10.1111/j.0105-2896.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 17.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 18.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foulds KE, Shen H. Clonal competition inhibits the proliferation and differentiation of adoptively transferred TCR transgenic CD4 T cells in response to infection. J Immunol. 2006;176:3037–3043. doi: 10.4049/jimmunol.176.5.3037. [DOI] [PubMed] [Google Scholar]
- 20.Celli S, Lemaître F, Bousso P. Real-time manipulation of T cell-dendritic cell interactions in vivo reveals the importance of prolonged contacts for CD4+ T cell activation. Immunity. 2007;27:625–634. doi: 10.1016/j.immuni.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 21.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 22.Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204:951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho BK, Rao VP, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldrath AW, Luckey CJ, Park R, Benoist C, Mathis D. The molecular program induced in T cells undergoing homeostatic proliferation. Proc Natl Acad Sci USA. 2004;101:16885–16890. doi: 10.1073/pnas.0407417101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton SE, Jameson SC. The nature of the lymphopenic environment dictates protective function of homeostatic-memory CD8+ T cells. Proc Natl Acad Sci USA. 2008;105:18484–18489. doi: 10.1073/pnas.0806487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung KP, Yang E, Goldrath AW. Memory-like CD8+ T cells generated during homeostatic proliferation defer to antigen-experienced memory cells. J Immunol. 2009;183:3364–3372. doi: 10.4049/jimmunol.0900641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prlic M, Blazar BR, Khoruts A, Zell T, Jameson SC. Homeostatic expansion occurs independently of costimulatory signals. J Immunol. 2001;167:5664–5668. doi: 10.4049/jimmunol.167.10.5664. [DOI] [PubMed] [Google Scholar]
- 29.Krieg C, Boyman O, Fu Y-X, Kaye J. B and T lymphocyte attenuator regulates CD8+ T cell-intrinsic homeostasis and memory cell generation. Nat Immunol. 2007;8:162–171. doi: 10.1038/ni1418. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Zheng P. CD24: a genetic checkpoint in T cell homeostasis and autoimmune diseases. Trends Immunol. 2007;28:315–320. doi: 10.1016/j.it.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Kieper WC, Tan JT, Bondi-Boyd B, Gapin L, Sprent J, Ceredig R, Surh CD. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J Exp Med. 2002;195:1533–1539. doi: 10.1084/jem.20020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubinstein MP, Lind NA, Purton JF, Filippou P, Best JA, McGhee PA, Surh CD, Goldrath AW. IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood. 2008;112:3704–3712. doi: 10.1182/blood-2008-06-160945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seddon B, Zamoyska R. TCR and IL-7 receptor signals can operate independently or synergize to promote lymphopenia-induced expansion of naive T cells. J Immunol. 2002;169:3752–3759. doi: 10.4049/jimmunol.169.7.3752. [DOI] [PubMed] [Google Scholar]
- 34.Surh CD, Sprent J. Regulation of mature T cell homeostasis. Semin Immunol. 2005;17:183–191. doi: 10.1016/j.smim.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt-Supprian M, Tian J, Ji H, Terhorst C, Bhan AK, Grant EP, Pasparakis M, Casola S, Coyle AJ, Rajewsky K. I kappa B kinase 2 deficiency in T cells leads to defects in priming, B cell help, germinal center reactions, and homeostatic expansion. J Immunol. 2004;173:1612–1619. doi: 10.4049/jimmunol.173.3.1612. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt-Supprian M, Courtois G, Tian J, Coyle AJ, Israël A, Rajewsky K, Pasparakis M. Mature T cells depend on signaling through the IKK complex. Immunity. 2003;19:377–389. doi: 10.1016/S1074-7613(03)00237-1. [DOI] [PubMed] [Google Scholar]
- 37.Ishimaru N, Kishimoto H, Hayashi Y, Sprent J. Regulation of naive T cell function by the NF-kappaB2 pathway. Nat Immunol. 2006;7:763–772. doi: 10.1038/ni1351. [DOI] [PubMed] [Google Scholar]
- 38.Sriskantharajah S, Belich MP, Papoutsopoulou S, Janzen J, Tybulewicz V, Seddon B, Ley SC. Proteolysis of NF-kappaB1 p105 is essential for T cell antigen receptor-induced proliferation. Nat Immunol. 2009;10:38–47. doi: 10.1038/ni.1685. [DOI] [PubMed] [Google Scholar]
- 39.Teixeiro E, Daniels MA, Hamilton SE, Schrum AG, Bragado R, Jameson SC, Palmer E. Different T cell receptor signals determine CD8+ memory versus effector development. Science. 2009;323:502–505. doi: 10.1126/science.1163612. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Chang X, Facchinetti V, Zhuang Y, Su B. MEKK3 is essential for lymphopenia-induced T cell proliferation and survival. J Immunol. 2009;182:3597–3608. doi: 10.4049/jimmunol.0803738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin J-X, Spolski R, Leonard WJ. Critical role for Rsk2 in T-lymphocyte activation. Blood. 2008;111:525–533. doi: 10.1182/blood-2007-02-072207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shinohara H, Yamasaki S, Maeda S, Saito T, Kurosaki T. Regulation of NF-kappaB-dependent T cell activation and development by MEKK3. Int Immunol. 2009;21:393–401. doi: 10.1093/intimm/dxp007. [DOI] [PubMed] [Google Scholar]
- 43.Schouten GJ, Vertegaal AC, Whiteside ST, Israel A, Toebes M, Dorsman JC, van der Eb AJ, Zantema A. IkappaB alpha is a target for the mitogen-activated 90 kDa ribosomal S6 kinase. EMBO J. 1997;16:3133–3144. doi: 10.1093/emboj/16.11.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Epstein MM, Di Rosa F, Jankovic D, Sher A, Matzinger P. Successful T cell priming in B cell-deficient mice. J Exp Med. 1995;182:915–922. doi: 10.1084/jem.182.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 46.Shedlock DJ, Whitmire JK, Tan J, MacDonald AS, Ahmed R, Shen H. Role of CD4 T cell help and costimulation in CD8 T cell responses during Listeria monocytogenes infection. J Immunol. 2003;170:2053–2063. doi: 10.4049/jimmunol.170.4.2053. [DOI] [PubMed] [Google Scholar]
- 47.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun JC, Bevan MJ. Cutting edge: long-lived CD8 memory and protective immunity in the absence of CD40 expression on CD8 T cells. J Immunol. 2004;172:3385–3389. doi: 10.4049/jimmunol.172.6.3385. [DOI] [PubMed] [Google Scholar]
- 50.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Obar JJ, Molloy MJ, Jellison ER, Stoklasek TA, Zhang W, Usherwood EJ, Lefrancois L. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc Natl Acad Sci USA. 2010;107:193–198. doi: 10.1073/pnas.0909945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leignadier J, Hardy M-P, Cloutier M, Rooney J, Labrecque N. Memory T-lymphocyte survival does not require T-cell receptor expression. Proc Natl Acad Sci USA. 2008;105:20440–20445. doi: 10.1073/pnas.0806289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 54.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 55.Masopust D, Kaech SM, Wherry EJ, Ahmed R. The role of programming in memory T-cell development. Curr Opin Immunol. 2004;16:217–225. doi: 10.1016/j.coi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 57.Lauvau G, Vijh S, Kong P, Horng T, Kerksiek K, Serbina N, Tuma RA, Pamer EG. Priming of memory but not effector CD8 T cells by a killed bacterial vaccine. Science. 2001;294:1735–1739. doi: 10.1126/science.1064571. [DOI] [PubMed] [Google Scholar]
- 58.Badovinac VP, Harty JT. Manipulating the rate of memory CD8+ T cell generation after acute infection. J Immunol. 2007;179:53–63. doi: 10.4049/jimmunol.179.1.53. [DOI] [PubMed] [Google Scholar]
- 59.Ho IC, Glimcher LH. Transcription: tantalizing times for T cells. Cell. 2002;109(Suppl):S109–120. doi: 10.1016/s0092-8674(02)00705-5. [DOI] [PubMed] [Google Scholar]
- 60.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao C-A, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 61.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 62.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 63.Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 65.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martins GA, Cimmino L, Shapiro-Shelef M, Szabolcs M, Herron A, Magnusdottir E, Calame K. Transcriptional repressor Blimp-1 regulates T cell homeostasis and function. Nat Immunol. 2006;7:457–465. doi: 10.1038/ni1320. [DOI] [PubMed] [Google Scholar]
- 67.Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 68.Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kamimura D, Bevan MJ. Endoplasmic reticulum stress regulator XBP-1 contributes to effector CD8+ T cell differentiation during acute infection. J Immunol. 2008;181:5433–5441. doi: 10.4049/jimmunol.181.8.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ichii H, Sakamoto A, Kuroda Y, Tokuhisa T. Bcl6 acts as an amplifier for the generation and proliferative capacity of central memory CD8+ T cells. J Immunol. 2004;173:883–891. doi: 10.4049/jimmunol.173.2.883. [DOI] [PubMed] [Google Scholar]
- 71.Ichii H, Sakamoto A, Hatano M, Okada S, Toyama H, Taki S, Arima M, Kuroda Y, Tokuhisa T. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat Immunol. 2002;3:558–563. doi: 10.1038/ni802. [DOI] [PubMed] [Google Scholar]
- 72.Manders PM, Hunter PJ, Telaranta AI, Carr JM, Marshall JL, Carrasco M, Murakami Y, Palmowski MJ, Cerundolo V, Kaech SM, Ahmed R, Fearon DT. BCL6b mediates the enhanced magnitude of the secondary response of memory CD8+ T lymphocytes. Proc Natl Acad Sci USA. 2005;102:7418–7425. doi: 10.1073/pnas.0501585102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cannarile MA, Lind NA, Rivera R, Sheridan AD, Camfield KA, Wu BB, Cheung KP, Ding Z, Goldrath AW. Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nat Immunol. 2006;7:1317–1325. doi: 10.1038/ni1403. [DOI] [PubMed] [Google Scholar]
- 74.D’Cruz LM, Rubinstein MP, Goldrath AW. Surviving the crash: transitioning from effector to memory CD8+ T cell. Semin Immunol. 2009;21:92–98. doi: 10.1016/j.smim.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heffner M, Fearon DT. Loss of T cell receptor-induced Bmi-1 in the KLRG1(+) senescent CD8(+) T lymphocyte. Proc Natl Acad Sci USA. 2007;104:13414–13419. doi: 10.1073/pnas.0706040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakayama T, Yamashita M. Critical role of the Polycomb and Trithorax complexes in the maintenance of CD4 T cell memory. Semin Immunol. 2009;21:78–83. doi: 10.1016/j.smim.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 77.Liu N, Phillips T, Zhang M, Wang Y, Opferman JT, Shah R, Ashton-Rickardt PG. Serine protease inhibitor 2A is a protective factor for memory T cell development. Nat Immunol. 2004;5:919–926. doi: 10.1038/ni1107. [DOI] [PubMed] [Google Scholar]
- 78.Schober SL, Kuo CT, Schluns KS, Lefrancois L, Leiden JM, Jameson SC. Expression of the transcription factor lung Krüppel-like factor is regulated by cytokines and correlates with survival of memory T cells in vitro and in vivo. J Immunol. 1999;163:3662–3667. [PubMed] [Google Scholar]
- 79.Setoguchi R, Taniuchi I, Bevan MJ. ThPOK derepression is required for robust CD8 T cell responses to viral infection. J Immunol. 2009;183:4467–4474. doi: 10.4049/jimmunol.0901428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun JC, Lehar SM, Bevan MJ. Augmented IL-7 signaling during viral infection drives greater expansion of effector T cells but does not enhance memory. J Immunol. 2006;177:4458–4463. doi: 10.4049/jimmunol.177.7.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chandele A, Joshi NS, Zhu J, Paul WE, Leonard WJ, Kaech SM. Formation of IL-7Ralphahigh and IL-7Ralphalow CD8 T cells during infection is regulated by the opposing functions of GABPalpha and Gfi-1. J Immunol. 2008;180:5309–5319. doi: 10.4049/jimmunol.180.8.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Araki Y, Fann M, Wersto R, Weng N. Histone acetylation facilitates rapid and robust memory CD8 T cell response through differential expression of effector molecules (eomesodermin and its targets: perforin and granzyme B) J Immunol. 2008;180:8102–8108. doi: 10.4049/jimmunol.180.12.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Araki Y, Wang Z, Zang C, Wood WH, Schones D, Cui K, Roh T-Y, Lhotsky B, Wersto RP, Peng W, Becker KG, Zhao K, Weng NP. Genome-wide analysis of histone methylation reveals chromatin state-based regulation of gene transcription and function of memory CD8+ T cells. Immunity. 2009;30:912–925. doi: 10.1016/j.immuni.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williams MA, Ravkov EV, Bevan MJ. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–545. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sarkar S, Teichgräber V, Kalia V, Polley A, Masopust D, Harrington LE, Ahmed R, Wherry EJ. Strength of stimulus and clonal competition impact the rate of memory CD8 T cell differentiation. J Immunol. 2007;179:6704–6714. doi: 10.4049/jimmunol.179.10.6704. [DOI] [PubMed] [Google Scholar]
- 86.Williams M, Bevan M. Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J Immunol. 2004;173:6694. doi: 10.4049/jimmunol.173.11.6694. [DOI] [PubMed] [Google Scholar]
- 87.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gourley TS, Wherry EJ, Masopust D, Ahmed R. Generation and maintenance of immunological memory. Semin Immunol. 2004;16:323–333. doi: 10.1016/j.smim.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 89.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prlic M, Williams MA, Bevan MJ. Requirements for CD8 T-cell priming, memory generation and maintenance. Curr Opin Immunol. 2007;19:315–319. doi: 10.1016/j.coi.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 91.Wherry EJ, Blattman JN, Ahmed R. Low CD8 T-cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J Virol. 2005;79:8960–8968. doi: 10.1128/JVI.79.14.8960-8968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sarkar S, Kalia V, Haining W, Konieczny B, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Malherbe L, Hausl C, Teyton L, McHeyzer-Williams MG. Clonal selection of helper T cells is determined by an affinity threshold with no further skewing of TCR-binding properties. Immunity. 2004;21:669–679. doi: 10.1016/j.immuni.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 95.Savage PA, Boniface JJ, Davis MM. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity. 1999;10:485–492. doi: 10.1016/S1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- 96.Kedzierska K, Day EB, Pi J, Heard SB, Doherty PC, Turner SJ, Perlman S. Quantification of repertoire diversity of influenza-specific epitopes with predominant public or private TCR usage. J Immunol. 2006;177:6705–6712. doi: 10.4049/jimmunol.177.10.6705. [DOI] [PubMed] [Google Scholar]
- 97.Kedzierska K, Venturi V, Field K, Davenport M, Turner SJ, Doherty PC. Early establishment of diverse T cell receptor profiles for influenza-specific CD8(+)CD62L(hi) memory T cells. Proc Natl Acad Sci USA. 2006;103:9184–9189. doi: 10.1073/pnas.0603289103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lauvau G, Vijh S, Kong P, Horng T, Kerksiek K, Serbina N, Tuma RA, Pamer EG. Priming of memory but not effector CD8 T cells by a killed bacterial vaccine. Science. 2001;294:1735–1739. doi: 10.1126/science.1064571. [DOI] [PubMed] [Google Scholar]
- 100.Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 101.Turner SJ, Diaz G, Cross R, Doherty PC. Analysis of clonotype distribution and persistence for an influenza virus-specific CD8+ T cell response. Immunity. 2003;18:549–559. doi: 10.1016/S1074-7613(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 102.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tewari K, Walent J, Svaren J, Zamoyska R, Suresh M. Differential requirement for Lck during primary and memory CD8+ T cell responses. Proc Natl Acad Sci USA. 2006;103:16388–16393. doi: 10.1073/pnas.0602565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hayden M, West A, Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25:6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 105.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.D’Souza WN, Chang C-F, Fischer AM, Li M, Hedrick SM. The Erk2 MAPK regulates CD8 T cell proliferation and survival. J Immunol. 2008;181:7617–7629. doi: 10.4049/jimmunol.181.11.7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rincón M, Davis RJ. Regulation of the immune response by stress-activated protein kinases. Immunol Rev. 2009;228:212–224. doi: 10.1111/j.1600-065X.2008.00744.x. [DOI] [PubMed] [Google Scholar]
- 108.Arbour N, Naniche D, Homann D, Davis RJ, Flavell RA, Oldstone MBA. c-Jun NH(2)-terminal kinase (JNK)1 and JNK2 signaling pathways have divergent roles in CD8(+) T cell-mediated antiviral immunity. J Exp Med. 2002;195:801–810. doi: 10.1084/jem.20011481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gao Y, Tao J, Li MO, Zhang D, Chi H, Henegariu O, Kaech SM, Davis RJ, Flavell RA, Yin Z. JNK1 is essential for CD8+ T cell-mediated tumor immune surveillance. J Immunol. 2005;175:5783–5789. doi: 10.4049/jimmunol.175.9.5783. [DOI] [PubMed] [Google Scholar]
- 110.Merritt C, Enslen H, Diehl N, Conze D, Davis RJ, Rincón M. Activation of p38 mitogen-activated protein kinase in vivo selectively induces apoptosis of CD8(+) but not CD4(+) T cells. Mol Cell Biol. 2000;20:936–946. doi: 10.1128/MCB.20.3.936-946.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gattinoni L, Zhong X-S, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, Paulos CM, Muranski P, Restifo NP. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao D-M, Yu S, Zhou X, Haring JS, Held W, Badovinac VP, Harty JT, Xue H-H. Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. J Immunol. 2010;184:1191–1199. doi: 10.4049/jimmunol.0901199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 117.Mousavi SF, Soroosh P, Takahashi T, Yoshikai Y, Shen H, Lefrançois L, Borst J, Sugamura K, Ishii N. OX40 costimulatory signals potentiate the memory commitment of effector CD8+ T cells. J Immunol. 2008;181:5990–6001. doi: 10.4049/jimmunol.181.9.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yokosuka T, Kobayashi W, Sakata-Sogawa K, Takamatsu M, Hashimoto-Tane A, Dustin ML, Tokunaga M, Saito T. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C theta translocation. Immunity. 2008;29:589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- 120.Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J Immunol. 2009;182:2786–2794. doi: 10.4049/jimmunol.0803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Agarwal P, Raghavan A, Nandiwada SL, Curtsinger JM, Bohjanen PR, Mueller DL, Mescher MF. Gene regulation and chromatin remodeling by IL-12 and type I IFN in programming for CD8 T cell effector function and memory. J Immunol. 2009;183:1695–1704. doi: 10.4049/jimmunol.0900592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schulz E, Mariani L, Radbruch A, Höfer T. Sequential polarization and imprinting of type 1 T helper lymphocytes by interferon-gamma and interleukin-12. Immunity. 2009;30:673–683. doi: 10.1016/j.immuni.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 123.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 124.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8(+) T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 126.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 127.Cui W, Joshi NS, Jiang A, Kaech SM. Effects of signal 3 during CD8 T cell priming: bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine. 2009;27:2177–2187. doi: 10.1016/j.vaccine.2009.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pham NL, Badovinac VP, Harty JT. A default pathway of memory CD8 T cell differentiation after dendritic cell immunization is deflected by encounter with inflammatory cytokines during antigen-driven proliferation. J Immunol. 2009;183:2337–2348. doi: 10.4049/jimmunol.0901203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J Immunol. 2008;180:1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 130.Stemberger C, Neuenhahn M, Gebhardt FE, Schiemann M, Buchholz VR, Busch DH. Stem cell-like plasticity of naive and distinct memory CD8 + T cell subsets. Semin Immunol. 2009;21:62–68. doi: 10.1016/j.smim.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 131.Parish IA, Kaech SM. Diversity in CD8(+) T cell differentiation. Curr Opin Immunol. 2009;21:291–297. doi: 10.1016/j.coi.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bannard O, Kraman M, Fearon D. Pathways of memory CD8+ T-cell development. Eur J Immunol. 2009;39:2083–2087. doi: 10.1002/eji.200939555. [DOI] [PubMed] [Google Scholar]
- 133.Ahmed R, Bevan MJ, Reiner SL, Fearon DT. The precursors of memory: models and controversies. Nat Rev Immunol. 2009;9:662–668. doi: 10.1038/nri2619. [DOI] [PubMed] [Google Scholar]
- 134.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 135.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/S0092-8674(02)01139-X. [DOI] [PubMed] [Google Scholar]
- 136.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 137.D’Souza WN, Hedrick SM. Cutting edge: latecomer CD8 T cells are imprinted with a unique differentiation program. J Immunol. 2006;177:777–781. doi: 10.4049/jimmunol.177.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 139.Wong P, Lara-Tejero M, Ploss A, Leiner I, Pamer EG. Rapid development of T cell memory. J Immunol. 2004;172:7239–7245. doi: 10.4049/jimmunol.172.12.7239. [DOI] [PubMed] [Google Scholar]
- 140.Busch DH, Kerksiek KM, Pamer EG. Differing roles of inflammation and antigen in T cell proliferation and memory generation. J Immunol. 2000;164:4063–4070. doi: 10.4049/jimmunol.164.8.4063. [DOI] [PubMed] [Google Scholar]
- 141.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, Killeen N, Orange JS, Russell SM, Weninger W, Reiner SL. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 142.Bannard O, Kraman M, Fearon DT. Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science. 2009;323:505–509. doi: 10.1126/science.1166831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stemberger C, Huster KM, Koffler M, Anderl F, Schiemann M, Wagner H, Busch DH. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27:985–997. doi: 10.1016/j.immuni.2007.10.012. [DOI] [PubMed] [Google Scholar]