Abstract
Nanos is known as an evolutionarily conserved RNA-binding protein, the function of which is implicated in germ cell development. This includes the maintenance of both the primordial germ cells (PGCs) and germline stem cells. In mice, Nanos2 exhibits a unique feature in which its expression is induced only in the germ cells within the sexually determined male gonad. Nanos2 promotes male germ cell differentiation, while simultaneously suppressing a female program. In addition, Nanos2 is also expressed in the spermatogonial stem cells and functions as an intrinsic factor to maintain the stem cell population during spermatogenesis. Detailed cytological and biochemical analyses in embryonic male gonads in the mouse have revealed that Nanos2 localizes to the P-bodies, a center of RNA processing. It has also been shown that the Nanos2 interacts with protein components of the deadenylation complex involved in the initial step of the RNA degradation pathway.
Keywords: Primordial germ cell (PGC), Sexual differentiation, Retinoic acid, Meiosis, Spermatogenesis, P-body
Introduction
The Nanos gene encodes an RNA binding protein containing a zinc-finger motif and was first identified as a maternal effect gene in Drosophila required for abdomen formation [1]. It was later found that Nanos mRNA and protein are incorporated into germline progenitor cells and are necessary for functional germ cell formation [2]. The function of Drosophila Nanos is not only confined to the early development of germ cells during the embryonic stage. The zygotic product has been shown to be involved in the maintenance of germline stem cells by preventing the differentiation of stem cells in the ovary [3]. Drosophila Nanos is also known to work with Pumilio as a translational repressor through its binding to a Nanos-response element (NRE) located in the 3’ untranslated region (UTR) of its target mRNAs [4]. However, Nanos is not implicated in the sexual differentiation of germ cells or in regulation of meiosis in Drosophila.
Nanos-related genes have been studied in several other invertebrates including leech Helobdella robusta (Hro-nos) [5], sea urchin, C. elegans (nos-1, nos-2 and nos-3) [6] and Hydra magnipapillata (Cnnos1 and Cnnos2) [7] and in vertebrates such as Xenopus laevis (Xcat-2) [8], zebrafish Danio rerio (nos1 and nos2) [9], medaka (nanos1a, 1b and nanos2) [10] and human (Nanos1–3) [11]. It is notable that all three Nanos genes in C. elegans work redundantly for the survival of germ cells at the larval stage, whereas NOS-3 is involved in the sperm-oocyte switch mechanism in hermaphrodites via its direct interaction with FBF, a Pumilio-family protein [12].
The mouse genome contains three Nanos genes, Nanos1–3. Nanos1 is expressed in both embryonic and adult brains and also in adult testes. However, Nanos1 knockout mice show no abnormalities and are fertile, indicating that this gene is dispensable for germ cell development [13]. In contrast, Nanos2 and Nanos3 are expressed in the embryonic germ cells, and a deficiency in these genes results in the loss of germ cells. Nanos3 is activated in the early PGCs [14], and although these cells develop and are capable of migrating to the genital ridge in Nanos3-null mice, their number is greatly reduced in the knockout animals. Hence, the gonads of the homozygous mice show defects in both the ovary and testis [14]. In contrast to Nanos3, the expression of Nanos2 is restricted to the male germ cells after their colonization of the gonads and plays a key role in the sexual differentiation of germ cells by promoting the male fate but suppressing the female fate [15]. Nanos2 protein expression is maintained in all male gonocytes during embryogenesis, but becomes confined to a small population of the spermatogonia after birth [16]. The current genetic evidence indicates that Nanos2 is required for the maintenance of spermatogonial stem cells [17].
In this review, the function of Nanos2 in the sexual differentiation of germ cells during embryogenesis and in the maintenance of spermatogonial stem cells after birth will be discussed. Once the germ cells enter either the male or female genital ridges during embryogenesis, they adopt completely different genetic programs. It is well known that the sexual determination of germ cells is primarily based on the sex of the somatic cells surrounding them. Indeed, no intrinsic genetic factors that guide germ cell sexual differentiation have been reported. Our recent findings indicate that Nanos2 is one of the male germ cell determinants capable of promoting the male differentiation pathway. In addition, Nanos2 is required for the maintenance of the spermatogonial stem cell population. The possible molecular mechanisms underlying the functions of this protein are also discussed based on recent findings in our laboratory.
Sexual differentiation of germ cells in the mouse gonads
Factors involved in the sex determination of germ cells
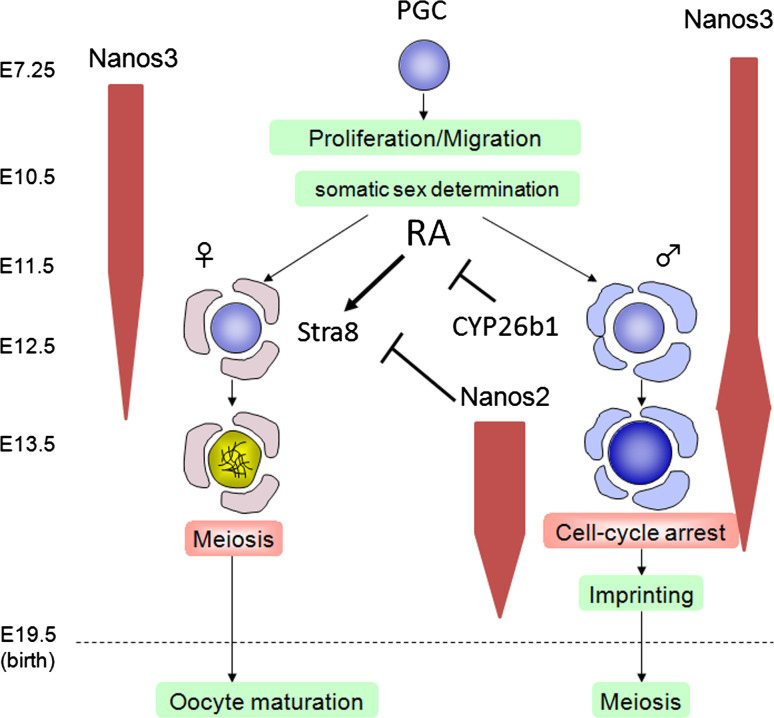
Germ cells are sexually bi-potential at the migrating stage, and their sex-specific differentiation begins after their colonization in the gonads at around E10.5 (Fig. 1). The germ cells of both sexes still retain the potency to differentiate into both sexes up to E11.5, but male germ cells have been found in co-culture fate conversion experiments to commit to spermatogenesis by E12.5, 1 day earlier than female germ cells [18]. Once committed to the normal developmental process, female germ cells enter meiosis at E13.5, whereas male germ cells undergo cell cycle arrest at G1/G0 and never enter meiosis during the embryonic stages of development [19]. It is known that the microenvironment is important for the sexual differentiation of germ cells and that the sex of the germ cells follows the sex of the somatic cells that surround them [20]. SRY expression in somatic cells triggers the sexual differentiation of male gonads. However, the factors required for the sexual differentiation of germ cells have long been unidentified. Three years ago, several reports strongly indicated that retinoic acid (RA) is the meiosis-inducing factor required for the embryonic female germ cells [21, 22]. In addition, the function of Dazl, known as a translational regulator containing an RNA binding motif, has been implicated in the mechanism by which germ cells acquire the competence to enter meiosis as RA signaling has no effect on meiosis in PGCs if Dazl function is compromised [23]. However, the molecular mechanism underlying the action of Dazl remains unknown. In male gonads, it has been shown that the failure of germ cells to enter meiosis is attributable to the function of Cyp26b1, which is expressed in the male gonad and functions to destroy RA [21, 24]. This indicates that Cyp26b1 is a meiosis-inhibiting factor (Fig. 1).
Fig. 1.
Developmental pathways involved in the sexual differentiation of germ cells. Once PGCs are formed, they proliferate and migrate toward the future gonads in which somatic cells commence sexual differentiation by expressing either male or female promoting factors. Retinoic acid (RA) is produced in both male and female gonads, but is destroyed by CYP26b1, which is specifically expressed in the male gonad. RA induces Stra8 expression in female germ cells, which then enter meiosis. In contrast, Stra8 expression is suppressed by RA degradation via Cyp26b1 and later by Nanos2 expression in germ cells. Male germ cells do not enter meiosis during embryogenesis. The expression of Nanos3 and Nanos2 is schematically indicated
Nanos2 and the sexual differentiation of germ cells
In the RA signaling pathway, an RA-responsive gene Stra8 has been implicated in premeiotic DNA replication, and female germ cells lacking this gene function fail to enter meiosis [22]. Inversely, in male germ cells lacking Cyp26b1, Stra8 is up-regulated, and this results in the induction of meiosis. These two knockout mouse studies thus established an important functional link between RA signaling and Stra8 expression, i.e., as regulators of meiotic initiation. A similar phenotype is observed in Nanos2-null male germ cells in which Stra8 expression is up-regulated, and as a result, meiosis is initiated [15]. However, in Cyp26b1-null testes, Stra8 induction occurs at E13.5, similar to the stage at which it is induced in embryonic ovaries, but this does not occur until E14.5 in Nanos2-null testes. This difference appears to be due to the presence of Cys26b1 expression in Nanos2-null gonads, which is normally higher at E12.5–13.5, but becomes gradually reduced after E13.5, similar to the situation in the wild-type. The reduction of Cyp26b1 might facilitate the upregulation of RA, which may in turn lead to Stra8 induction. In the wild-type mouse, Nanos2 begins to be expressed from E13.5 when Cyp26b1 is downregulated, and as a result, no Stra8 expression is observed. These observations strongly indicate that Nanos2 is required to suppress Stra8 expression, which is otherwise caused by the up-regulation of RA signaling, to prevent male germ cells from entering meiosis.
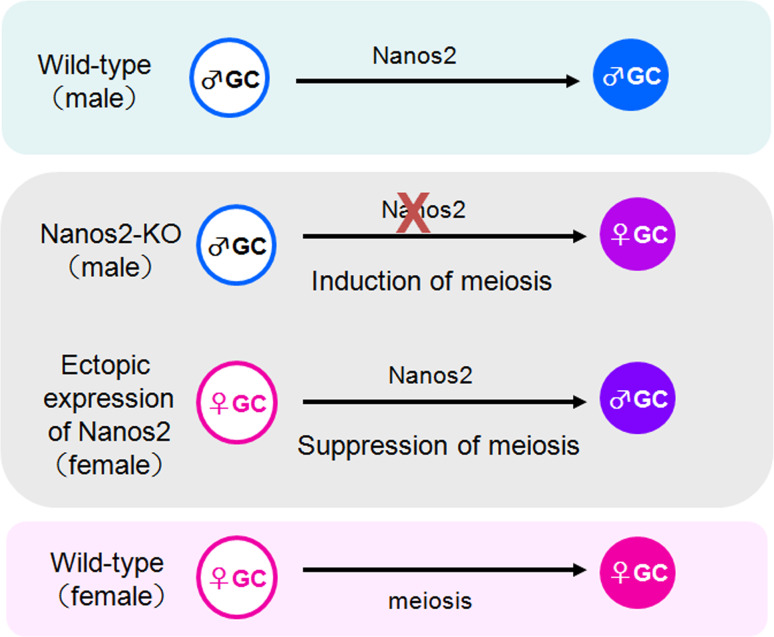
This idea is further supported by analyses of female germ cells that ectopically express Nanos2 (Fig. 2). The suppression of Stra8 is observed in these cells, and this eventually leads to the suppression of meiosis progression. Surprisingly, the effects of Nanos2 expression are not limited to the suppression of meiosis, but also show promoting effects in the male program. There are several indicators of this, which include the up-regulation of male germ cell-specific genes, such as Dnmt3L [25] and Tdrd1 [26], and the induction of a male-type epigenetic state involving specific histone modifications. Hence, Nanos2 acts as a cell-intrinsic factor that induces male-promoting programs and in turn shows suppressive functions for female programs (Fig. 2). The results of Gene-Chip analyses of Nanos2-null germ cells further support this idea as they revealed that many transcripts involved in meiosis and oogenesis are upregulated, whereas transcripts involved in promoting male characteristics are downregulated. The molecular mechanisms leading to such changes in the transcriptome are still unclear, but are in part mediated via the destabilization of RNAs interacting with Nanos2, as described further in a later section.
Fig. 2.
Scheme of the functions of Nanos2 during the sexual differentiation of germ cells. In wild-type male germ cells, Nanos2 promotes male-type differentiation, whereas the absence of Nanos2 in these cells (Nanos2-KO) results in the abnormal induction of meiosis. The forced expression of Nanos2 in female germ cells causes the suppression of meiosis
Function of Nanos2 after birth
The expression of Nanos2 during spermatogenesis
In the mouse testes, spermatogenesis is initiated from a small subset of stem cells belonging to undifferentiated spermatogonia. The type Asingle (As, isolated single cells), Apaired (Apr, chains of 2 cells), and Aaligned (Aal, chains of 4, 8, 16 or occasionally 32 cells) spermatogonia are the most primitive sets of germ cells observed in mature testes and are collectively described as undifferentiated spermatogonia [27]. The Nanos2 expression pattern observed in the embryonic stages declines after E16.5, although the protein is retained after the mRNA expression levels drop [28]. After birth, spermatogonia regain their proliferative capacity and establish the stem cell system that gives rise to a large number of differentiating spermatogenic cell populations. In adult testes, Nanos2 expression is restricted to a portion of the undifferentiated spermatogonia, particularly the As and Apr types, and its expression is gradually reduced in clusters of more than four cells [29]. This expression pattern is quite similar to that of GFRa1, a co-receptor for GDNF, and a factor known to be required for the maintenance of the spermatogonial stem cell population [30]. However, this raises the question of whether Nanos2 has any function in the maintenance of spermatogonial stem cell population.
Nanos2 is required for the maintenance of spermatogonial stem cells
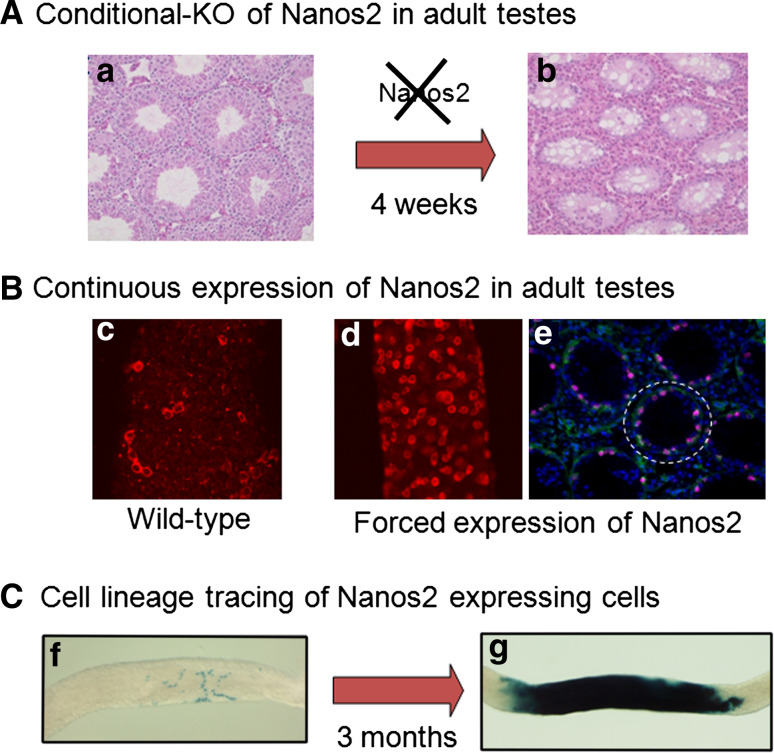
To elucidate the in vivo function of Nanos2 during mouse spermatogenesis, a Cre-lox conditional strategy was employed in our laboratory since Nanos2-null germ cells die via apoptosis before birth. The conditional loss of Nanos2 was induced in 4-week-old male mice by the addition of tamoxifen. Histological analysis of testis sections from the postnatal Nanos2-deficient mice revealed a progressive loss of germ cells with age (Fig. 3A). In the 8-week old mice (4 weeks after the induction of the Nanos2 knockout), a clear reduction in the sizes of the seminiferous tubules was evident, and by 12 weeks after birth (8 weeks after the knockout), most of these tubules were devoid of any germ cells. These observations indicated that a Nanos2-deficiency causes a germ cell-loss phenotype within a few cycles of spermatogenesis. The lack of germ cells was clearly revealed by immunostaining with TRA98 [31], a marker of these cells, and by reactivity with an anti-Plzf antibody, which detects undifferentiated spermatogonia [32]. A probable cause of this reduction is apoptotic cell death since Nanos2 is implicated in the maintenance of embryonic male gonocytes [14]. However, subsequent quantitative analysis of apoptotic cells using poly ADP-ribose polymerase (PARP) cleavage failed to show significant differences between the control and Nanos2-deficient testes. Hence, apoptosis may not be the underlying cause of the germ cell loss in Nanos2-deficient testes.
Fig. 3.
Genetic experiments that demonstrate the function of the Nanos2 in spermatogonial stem cells in the adult mouse testes. A The conditional knockout of the Nanos2 gene was induced by TM injection of 4-week-old male mice containing a floxed Nanos2 and ERT2Cre (a). After 4 weeks, almost all of the germ cells were depleted from the seminiferous tubules (b). B The continuous expression of Nanos2 was achieved by crossing CAG-floxed CAT-Nanos2 transgenic and Nanos3-cre mice. Nanos2 expression is maintained continuously in all germ cells of the progeny from the embryonic stages. In the wild-type male testes, the number of Nanos2-positive cells (red) is low (c). However, these cells are markedly increased and occupy the entire tubular surface in the induced testes (d). The forced expression of Nanos2 (green) results in the upregulation of PLZF (magenta), a marker of undifferentiated spermatogonia, including stem cells (e). C A cell lineage tracing experiment conducted by crossing a Nanos2-MCM [MerCreMer, a tamoxifen (TM) inducible Cre] mouse with a Rosa26 reporter mouse. Following the transient administration of TM in the 6-week-old male progeny, β-gal activity was detectable at 7 weeks (f) and 18 weeks (g). The presence of β-gal-positive patches that contained spermatogenic germ cells at all stages of development indicates that Nanos2 is expressed in the stem cell population
Nanos2 maintains stem cell properties by suppressing differentiation
A Nanos2 deficiency causes germ cell depletion because of the loss of the spermatogonial cell population, indicating its requirement for spermatogonial stem cell maintenance in a cell-autonomous manner. To further address this issue, we conducted a gain of function study (Fig. 3B). To achieve the continuous expression of Nanos2, the CAG-floxed-CAT-3 × Flag-Nanos2 transgenic mouse was crossed with the Nanos3-Cre mouse [33]. The resulting progeny show Nanos2 expression from the early embryonic stages in most germ cells. The Nanos2-overexpressing male mice are infertile, and the testis weights are reduced such that most tubules retain cells only on the basement membrane where spermatogonia usually reside. Marker analysis also clearly revealed that Scp3-positive meiotic cells were decreased in these Nanos2-overexpressing testes, indicating that Nanos2 expression prevents the entry of spermatogonia into meiosis. In addition, C-Kit expression, a hallmark of differentiating spermatogonia [34], was rarely observed in the tubules of Nanos2-overexpressing testes. In contrast, Plzf-positive undifferentiated spermatogonia were found to be increased in Nanos2-overexpressing mice. Furthermore, these cells had lower proliferation rates and normal levels of apoptosis. These data suggest that Nanos2-overexpressing cells show characteristics of undifferentiated spermatogonia and accumulate as a result of blocked differentiation rather than hyper-proliferation or a reduction in the levels of apoptosis.
Nanos2-expressing undifferentiated spermatogonia exhibit properties of “actual stem cells”
In previous pulse-chase experiments using the Ngn3 gene promoter, Nakagawa et al., proposed that two types of spermatogonial stem cell populations existed, i.e., actual and potential stem cell compartments, among which Ngn3 might be expressed more highly in potential stem cells than actual stem cells [35]. To examine the properties of endogenous Nanos2-expressing cells and thereby ascertain whether they exhibit the characteristics of actual or potential stem cells, a similar pulse-labeling experiment was conducted in our laboratory. A transgenic mouse that expresses MCM (MER-Cre-MER), a TM-inducible version of Cre recombinase [36], under the control of the Nanos2 enhancer was crossed with reporter mice to delineate the fate of all progeny of spermatogonia that express Nanos2 (Fig. 3C). The 6-week-old mice were subjected to a TM pulse for 5 days, and were killed at 7, 10, 18, 22 and 26 weeks (see below) of age. Distinct differences in the cell fates between the Nanos2-lineage and the previously reported Ngn3-lineage were observed even over a short tracing period. At 10 weeks, some labeled cells were observed in transit-amplifying cells, and this number was far lower than that of the Ngn3-lineage cells. Namely, in the case of the Ngn3-lineage, the majority of the labeled cells were observed as differentiating spermatids, whereas in the Nanos2-lineage, greater numbers of undifferentiated spermatogonia were observed [17].
Differences between the two lineages were also observed at 18 weeks (3 months after labeling) in the patches containing all stages of spermatogenic germ cells, indicating the existence of stem cells. In the Ngn3-lineage, the average number of patches was reported to be 6.1 ± 0.7 per testis at 3 months, while in the Nanos2-lineage, this number was 86.5 ± 8.1 per testis. These numbers are markedly higher than those of the Ngn3-lineage. Taken together, these results support the notion that the majority of Ngn3-expressing cells are transit-amplifying cells (potential stem cells), whereas Nanos2-expressing cells contain a large number of actual stem cells rather than transit-amplifying cells.
The molecular functions of Nanos2
Gene knockout studies have unequivocally demonstrated the critical functions of Nanos2 both during the embryonic stages and spermatogenesis. Nanos2 is expressed in all embryonic male germ cells and represses the female fate pathways by suppressing meiosis, which in turn results in the promotion of the male fate. In addition, once its expression becomes confined to the most primitive spermatogonial stem cells after birth, Nanos2 appears to maintain the stem cell fate. How Nanos2 regulates such pleiotropic functions is therefore a question of much interest. To address this issue, it will be necessary to fully elucidate the molecular nature of the Nanos2 protein.
Nanos2 localizes to P-bodies and interacts with the deadenylation complex
Nanos2 protein is first detectable at E13.5 in the cytoplasm of male gonocytes, and this signal intensity increases until about E16.5 [28]. Interestingly, this protein was found to be localized in P-bodies, which are known to function as a center of RNA degradation and storage [37], since Nanos2 foci clearly merge with those of DCP2 and XRN, major components of P-bodies. P-bodies are present in both sexes at E12.5, are gradually reduced and eventually lost by E14.5 in female gonocytes, and are specifically observed only in germ cells, but not in somatic cells in E15.5 male gonads. This indicates that Nanos2 is involved in the formation of P-bodies. However, many of these structures are still observed in both Nanos2 +/− and Nanos2 −/− male gonocytes at E13.5, though their number is reduced at the later stages in the absence of Nanos2. This indicates that Nanos2 is not essential for the assembly of P-bodies, but is required for maintaining their normal state.
Nanos2 interacts with the functional CCR4-NOT deadenylation complex and regulates its localization
The key to our understanding of the molecular functions of Nanos2 was provided by searches for interacting proteins. In these analyses, immunoprecipitations were conducted using male gonadal extracts from Nanos2 +/− and Nanos2 −/− embryos using Nanos2 antibodies. The major protein exclusively precipitated from Nanos2 +/− gonads was identified as CNOT1, a component of the CCR4-NOT deadenylation complex [37]. Further immunoprecipitation experiments identified other components of the CCR4-NOT complex (CNOT3, CNOT6L/Ccr4b, CNOT7/Caf1a and CNOT9/Rcd1), indicating that Nanos2 associates with the CCR4-NOT deadenylation complex in vivo. This was also confirmed by histological analyses in which CNOT proteins were found to colocalize with Nanos2 in P-bodies, suggesting that this complex may play a role in the activities of these elements. It became critically important to determine whether the Nanos2-interacting deadenylase complex actually had catalytic activity, and for this purpose we used an in vitro deadenylase assay system. The results of this experiment clearly showed that the cleavage of the poly(A) RNA substrate occurred only with Nanos2 immunoprecipitates [38]. This indicated that Nanos2 promotes the degradation of Nanos2-interacting mRNAs through the deadenylase activity of the CCR4-NOT complex. Interestingly, CNOT3, a component of CCR4-NOT deadenylation complex, was found to clearly localize to P-bodies in Nanos2 +/− male gonads, whereas only weak signals were found at P-bodies in the absence of Nanos2, although the abundance of CNOT3 protein was unchanged. These data suggest that Nanos2 promotes the localization of the CCR4-NOT deadenylation complex to P-bodies.
Nanos2 interacts with specific mRNAs and may promote their degradation
Based on the working hypothesis that Nanos2 promotes the degradation of Nanos2-interacting mRNAs by recruiting the deadenylation complex, it has been speculated that the Nanos2 complex contains specific mRNAs that are degraded via a deadenylation-dependent pathway. In such cases, it is expected that the expression levels of many transcripts would be up-regulated in the absence of Nanos2. These possibilities were first examined by comparative analyses of the transcriptomes of wild-type and Nanos2-null male gonads. Interestingly, many transcripts were found to be up- or down-regulated in the absence of Nanos2. For example, genes that are highly expressed only in male gonocytes, such as Dnmt3l [25], Tdrd1 [26] and Miwi2/Piwi-like 4 [39], are down-regulated in the Nanos2 −/− male gonads, whereas the Figla [40], Lhx8 [41] and Nobox [42] genes, which have been shown to be essential only for oogenesis and not for spermatogenesis, are accumulated in the Nanos2-null male gonads. These results suggest that Nanos2-null male gonocytes cannot enter the male pathways and therefore become feminized via the up-regulation of female-type genes. It is noteworthy in particular that Stra8, a key gene required for premeiotic replication, is strongly up-regulated in the absence of Nanos2, which is consistent with the fact that the Stra8 protein levels are up-regulated under these conditions. Part of the molecular basis for the up-regulation of such transcripts is very likely to be RNA stabilization via the suppression of RNA degradation by the Nanos2-deadenylation complex. This possibility was examined by RT-PCR analysis of RNAs that co-precipitated with Nanos2. Interestingly, transcripts of Sycp3 [43], Stra8, Taf7l [44], Dazl, and Meisetz [45] genes that are implicated in meiosis, were specifically detected in the Nanos2 protein precipitates in spite of their very low expression in male gonads [38]. In contrast, G3pdh, Dnmt3l, and Dnmt3a mRNAs did not show any specific accumulation in the Nanos2 precipitates, although they are all highly expressed in male gonads. These data indicate that the mRNAs involved in meiosis specifically interact with Nanos2 in vivo.
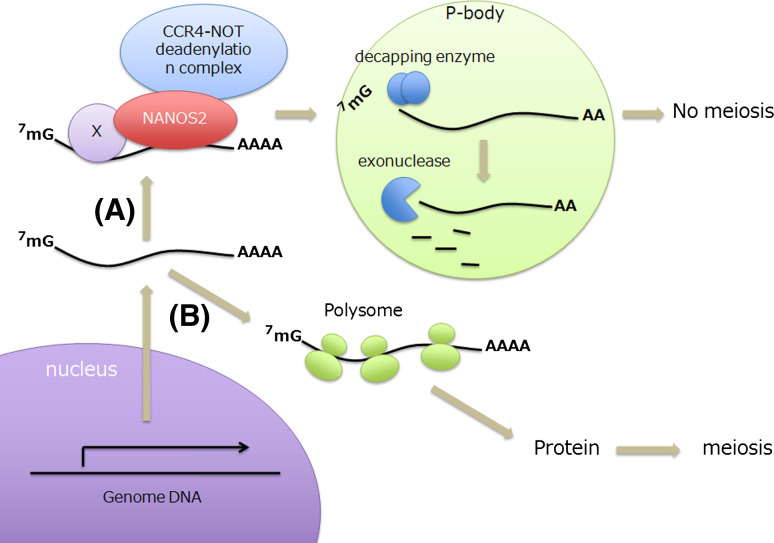
Based upon these results, a working model of the molecular functions of Nanos2 can be proposed (Fig. 4). It is known that Nanos proteins are not directly involved in target RNA recognition [46], and Pumilio is implicated in target selection in other animals [12, 47]. Since the partner protein has not been identified yet in mouse embryos, we have denoted this factor as X in our model. In wild-type male gonocytes, specific mRNAs are recognized by Nanos2 in complex with X and incorporated into the CCR4-NOT deadenylation complex that would be recruited into P-bodies via Nanos2-mediated mechanisms. These substrate mRNAs would then be degraded by the enzymes contained in the P-bodies after shortening of the poly(A) tail by the catalytic subunits of the CCR4-NOT deadenylation complex. However, in the absence of Nanos2, the CCR4-NOT deadenylation complex cannot access and degrade these transcripts, leading to their accumulation in the male gonocyte. We expect that some of these mRNAs may be translated, which results in the manifestation of the Nanos2 −/− phenotype.
Fig. 4.
A proposed model for the molecular functions of Nanos2. In male mouse germ cells, RA may reach and activate the transcription of meiotic genes such as Stra8 after E13.5 when Cyp26b1 expression begins to decrease. However, in wild-type mice, these transcripts would be incorporated into the CCR4-NOT deadenylation complex via a Nanos2-mediated mechanism and loaded onto P-bodies where RNAs are degraded by the enzymes contained therein. This results in the suppression of meiosis (X is an unidentified Nanos2-interacting protein) (a). In contrast, RNAs are not loaded onto P-bodies in the absence of Nanos2 and may thus go to the polysome and be translated into the proteins required for meiosis (b)
Perspectives
The RNA binding protein Nanos2 contains a zinc finger motif that is conserved among other Nanos proteins expressed in many species from fly to human. Although homology outside of the zinc finger domain is low among the Nanos proteins, their functions as germ cell factors are conserved, which includes the suppression of apoptosis, an event that is critically associated with several aspects of germ cell development. This conservation is also quite interesting since each animal species uses a different strategy to specify and maintain its germ cell lineage. However, the maternal Nanos has been shown to be involved in the specification of germ cells by suppressing somatic cell lineage in Drosophila [48], which is not observed in mice, although the anti-apoptotic function of mouse Nanos3 in the early migration stage of germ cells is evident [33]. In contrast, Nanos is not involved in the suppression of meiosis or sexual differentiation of germ cells in Drosophila, where it is implicated in the maintenance of germ line stem cells. The basis for this species difference is likely dependent on the interacting factors, which may help to coordinate Nanos function. In terms of this function, it is interesting to consider that the Nanos proteins themselves are not solely involved in the selection of target RNAs. In Drosophila, Pumilio is implicated as a factor that determines Nanos target specificity [49]. In mouse, Pumilio2 is also indicated as a candidate partner protein for Nanos2 and Nanos3 to select target RNAs [50]. However, as far as we have observed, neither Pumilio1 nor Pumilio2 is a Nanos2-interacting protein during the embryonic stage. The major proteins that were found to co-precipitate with Nanos2 in our experiments are components of the deadenylation complex, and these interactions were RNA-independent. Hence, the major function of Nanos2 in mouse germ cells appears to be the degradation of target RNAs to prevent the initiation of inappropriate genetic programs or pathways. However, we have also found that Nanos2 is required for the progression of the normal male pathways and that most of the transcripts associated with this pathway are down-regulated in the Nanos2-null male gonads. It is currently unknown whether this is also dependent on the degradation of certain RNAs such as those encoding transcriptional repressors. Alternatively, Nanos2 might be involved in other mechanisms that lead to the accumulation of mRNAs by facilitating their storage in P-bodies for future translation when required. However, the mechanisms by which target RNAs are selected by the Nanos2-deadenylation complex, and whether the RNAs are recruited to P-bodies via a Nanos2-dependent mechanism, remain to be elucidated.
References
- 1.Lehmann R, Nusslein-Volhard C. The maternal gene nanos has a central role in posterior pattern formation of the Drosophila embryo. Development. 1991;112:679–691. doi: 10.1242/dev.112.3.679. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi S, Yamada M, Asaoka M, Kitamura T. Essential role of the posterior morphogen nanos for germline development in Drosophila . Nature. 1996;380:708–711. doi: 10.1038/380708a0. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Lin H. Nanos maintains germline stem cell self-renewal by preventing differentiation. Science. 2004;303:2016–2019. doi: 10.1126/science.1093983. [DOI] [PubMed] [Google Scholar]
- 4.Asaoka M, Sano H, Obara Y, Kobayashi S. Maternal Nanos regulates zygotic gene expression in germline progenitors of Drosophila melanogaster . Mech Dev. 1998;78:153–158. doi: 10.1016/S0925-4773(98)00164-6. [DOI] [PubMed] [Google Scholar]
- 5.Pilon M, Weisblat DA. A nanos homolog in leech. Development. 1997;124:1771–1780. doi: 10.1242/dev.124.9.1771. [DOI] [PubMed] [Google Scholar]
- 6.Subramaniam K, Seydoux G. nos-1 and nos-2, two genes related to Drosophila nanos, regulate primordial germ cell development and survival in Caenorhabditis elegans . Development. 1999;126:4861–4871. doi: 10.1242/dev.126.21.4861. [DOI] [PubMed] [Google Scholar]
- 7.Mochizuki K, Sano H, Kobayashi S, Nishimiya-Fujisawa C, Fujisawa T. Expression and evolutionary conservation of nanos-related genes in Hydra . Dev Genes Evol. 2000;210:591–602. doi: 10.1007/s004270000105. [DOI] [PubMed] [Google Scholar]
- 8.MacArthur H, Bubunenko M, Houston DW, King ML. Xcat2 RNA is a translationally sequestered germ plasm component in Xenopus . Mech Dev. 1999;84:75–88. doi: 10.1016/S0925-4773(99)00075-1. [DOI] [PubMed] [Google Scholar]
- 9.Koprunner M, Thisse C, Thisse B, Raz E. A zebrafish nanos-related gene is essential for the development of primordial germ cells. Genes Dev. 2001;15:2877–2885. doi: 10.1101/gad.212401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aoki Y, Nakamura S, Ishikawa Y, Tanaka M. Expression and syntenic analyses of four nanos genes in medaka. Zoolog Sci. 2009;26:112–118. doi: 10.2108/zsj.26.112. [DOI] [PubMed] [Google Scholar]
- 11.Kusz KM, Tomczyk L, Sajek M, Spik A, Latos-Bielenska A, Jedrzejczak P, Pawelczyk L, Jaruzelska J. The highly conserved NANOS2 protein: testis-specific expression and significance for the human male reproduction. Mol Hum Reprod. 2009;15:165–171. doi: 10.1093/molehr/gap003. [DOI] [PubMed] [Google Scholar]
- 12.Kraemer B, Crittenden S, Gallegos M, Moulder G, Barstead R, Kimble J, Wickens M. NANOS-3 and FBF proteins physically interact to control the sperm-oocyte switch in Caenorhabditis elegans . Curr Biol. 1999;9:1009–1018. doi: 10.1016/S0960-9822(99)80449-7. [DOI] [PubMed] [Google Scholar]
- 13.Haraguchi S, Tsuda M, Kitajima S, Sasaoka Y, Nomura-Kitabayashid A, Kurokawa K, Saga Y. nanos1: a mouse nanos gene expressed in the central nervous system is dispensable for normal development. Mech Dev. 2003;120:721–731. doi: 10.1016/S0925-4773(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 14.Tsuda M, Sasaoka Y, Kiso M, Abe K, Haraguchi S, Kobayashi S, Saga Y. Conserved role of nanos proteins in germ cell development. Science. 2003;301:1239–1241. doi: 10.1126/science.1085222. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki A, Saga Y. Nanos2 suppresses meiosis and promotes male germ cell differentiation. Genes Dev. 2008;22:430–435. doi: 10.1101/gad.1612708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuda M, Kiso M, Saga Y. Implication of nanos2-3′UTR in the expression and function of nanos2. Mech Dev. 2006;123:440–449. doi: 10.1016/j.mod.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Sada A, Suzuki A, Suzuki H, Saga Y. The RNA-binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science. 2009;325:1394–1398. doi: 10.1126/science.1172645. [DOI] [PubMed] [Google Scholar]
- 18.McLaren A, Southee D. Entry of mouse embryonic germ cells into meiosis. Dev Biol. 1997;187:107–113. doi: 10.1006/dbio.1997.8584. [DOI] [PubMed] [Google Scholar]
- 19.McLaren A. Mammalian germ cells: birth, sex, and immortality. Cell Struct Funct. 2001;26:119–122. doi: 10.1247/csf.26.119. [DOI] [PubMed] [Google Scholar]
- 20.Wilhelm D, Palmer S, Koopman P. Sex determination and gonadal development in mammals. Physiol Rev. 2007;87:1–28. doi: 10.1152/physrev.00009.2006. [DOI] [PubMed] [Google Scholar]
- 21.Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- 22.Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci USA. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Y, Gill ME, Koubova J, Page DC. Germ cell-intrinsic and -extrinsic factors govern meiotic initiation in mouse embryos. Science. 2008;322:1685–1687. doi: 10.1126/science.1166340. [DOI] [PubMed] [Google Scholar]
- 24.MacLean G, Li H, Metzger D, Chambon P, Petkovich M. Apoptotic extinction of germ cells in testes of Cyp26b1 knockout mice. Endocrinology. 2007;148:4560–4567. doi: 10.1210/en.2007-0492. [DOI] [PubMed] [Google Scholar]
- 25.Sakai Y, Suetake I, Shinozaki F, Yamashina S, Tajima S. Co-expression of de novo DNA methyltransferases Dnmt3a2 and Dnmt3L in gonocytes of mouse embryos. Gene Expr Patterns. 2004;5:231–237. doi: 10.1016/j.modgep.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Chuma S, Hosokawa M, Kitamura K, Kasai S, Fujioka M, Hiyoshi M, Takamune K, Noce T, Nakatsuji N. Tdrd1/Mtr-1, a tudor-related gene, is essential for male germ-cell differentiation and nuage/germinal granule formation in mice. Proc Natl Acad Sci USA. 2006;103:15894–15899. doi: 10.1073/pnas.0601878103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Rooij DG. Proliferation and differentiation of spermatogonial stem cells. Reproduction. 2001;121:347–354. doi: 10.1530/rep.0.1210347. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki A, Tsuda M, Saga Y. Functional redundancy among Nanos proteins and a distinct role of Nanos2 during male germ cell development. Development. 2007;134:77–83. doi: 10.1242/dev.02697. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki H, Sada A, Yoshida S, Saga Y. The heterogeneity of spermatogonia is revealed by their topology and expression of marker proteins including the germ cell-specific proteins Nanos2 and Nanos3. Dev Biol. 2009;336:222–231. doi: 10.1016/j.ydbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Buageaw A, Sukhwani M, Ben-Yehudah A, Ehmcke J, Rawe VY, Pholpramool C, Orwig KE, Schlatt S. GDNF family receptor alpha1 phenotype of spermatogonial stem cells in immature mouse testes. Biol Reprod. 2005;73:1011–1016. doi: 10.1095/biolreprod.105.043810. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka H, Pereira LA, Nozaki M, Tsuchida J, Sawada K, Mori H, Nishimune Y. A germ cell-specific nuclear antigen recognized by a monoclonal antibody raised against mouse testicular germ cells. Int J Androl. 1997;20:361–366. doi: 10.1046/j.1365-2605.1998.00080.x. [DOI] [PubMed] [Google Scholar]
- 32.Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki H, Tsuda M, Kiso M, Saga Y. Nanos3 maintains the germ cell lineage in the mouse by suppressing both Bax-dependent and -independent apoptotic pathways. Dev Biol. 2008;318:133–142. doi: 10.1016/j.ydbio.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Rossi P, Sette C, Dolci S, Geremia R. Role of c-kit in mammalian spermatogenesis. J Endocrinol Invest. 2000;23:609–615. doi: 10.1007/BF03343784. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1 + cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parker R, Sheth U. P bodies and the control of mRNA translation and degradation. Mol Cell. 2007;25:635–646. doi: 10.1016/j.molcel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki A, Igarashi K, Aisaki K, Kanno J, Saga Y. NANOS2 interacts with the CCR4-NOT deadenylation complex and leads to suppression of specific RNAs. Proc Natl Acad Sci USA. 2010;107:3594–3599. doi: 10.1073/pnas.0908664107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aravin AA, Sachidanandam R, Bourc’his D, Schaefer C, Pezic D, Toth KF, Bestor T, Hannon GJ. A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Mol Cell. 2008;31:785–799. doi: 10.1016/j.molcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soyal SM, Amleh A, Dean J. FIGalpha, a germ cell-specific transcription factor required for ovarian follicle formation. Development. 2000;127:4645–4654. doi: 10.1242/dev.127.21.4645. [DOI] [PubMed] [Google Scholar]
- 41.Choi Y, Ballow DJ, Xin Y, Rajkovic A. Lim homeobox gene, lhx8, is essential for mouse oocyte differentiation and survival. Biol Reprod. 2008;79:442–449. doi: 10.1095/biolreprod.108.069393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science. 2004;305:1157–1159. doi: 10.1126/science.1099755. [DOI] [PubMed] [Google Scholar]
- 43.Yuan L, Liu JG, Zhao J, Brundell E, Daneholt B, Hoog C. The murine SCP3 gene is required for synaptonemal complex assembly, chromosome synapsis, and male fertility. Mol Cell. 2000;5:73–83. doi: 10.1016/S1097-2765(00)80404-9. [DOI] [PubMed] [Google Scholar]
- 44.Cheng Y, Buffone MG, Kouadio M, Goodheart M, Page DC, Gerton GL, Davidson I, Wang PJ. Abnormal sperm in mice lacking the Taf7l gene. Mol Cell Biol. 2007;27:2582–2589. doi: 10.1128/MCB.01722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayashi K, Yoshida K, Matsui Y. A histone H3 methyltransferase controls epigenetic events required for meiotic prophase. Nature. 2005;438:374–378. doi: 10.1038/nature04112. [DOI] [PubMed] [Google Scholar]
- 46.Curtis D, Treiber DK, Tao F, Zamore PD, Williamson JR, Lehmann R. A CCHC metal-binding domain in Nanos is essential for translational regulation. EMBO J. 1997;16:834–843. doi: 10.1093/emboj/16.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zamore PD, Bartel DP, Lehmann R, Williamson JR. The PUMILIO-RNA interaction: a single RNA-binding domain monomer recognizes a bipartite target sequence. Biochemistry. 1999;38:596–604. doi: 10.1021/bi982264s. [DOI] [PubMed] [Google Scholar]
- 48.Hayashi Y, Hayashi M, Kobayashi S. Nanos suppresses somatic cell fate in Drosophila germ line. Proc Natl Acad Sci USA. 2004;101:10338–10342. doi: 10.1073/pnas.0401647101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muraro NI, Weston AJ, Gerber AP, Luschnig S, Moffat KG, Baines RA. Pumilio binds para mRNA and requires Nanos and Brat to regulate sodium current in Drosophila motoneurons. J Neurosci. 2008;28:2099–2109. doi: 10.1523/JNEUROSCI.5092-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lolicato F, Marino R, Paronetto MP, Pellegrini M, Dolci S, Geremia R, Grimaldi P. Potential role of Nanos3 in maintaining the undifferentiated spermatogonia population. Dev Biol. 2008;313:725–738. doi: 10.1016/j.ydbio.2007.11.011. [DOI] [PubMed] [Google Scholar]