Abstract
The vertebrate olfactory system recognizes and discriminates between thousands of structurally diverse odorants. Detection of odorants in mammals is mediated by olfactory receptors (ORs), which comprise the largest superfamily of G protein-coupled receptors (GPCRs). Upon odorant binding, ORs couple to G proteins, resulting in an increase in intracellular cAMP levels and subsequent receptor signaling. In this review, we will discuss recently published studies outlining the molecular basis of odor discrimination, focusing on pharmacology, G protein activation, and desensitization of ORs. A greater understanding of the molecular mechanisms underlying OR activity may help in the discovery of agonists and antagonists of ORs, and of GPCRs with potential therapeutic applications.
Keywords: Odorant, Olfaction, Olfactory receptor, G protein, Signal transduction, Desensitization
Introduction
The detection of odorants is essential for the survival of both individual animals and species in general. Olfactory function enables animals to locate food and suitable mating partners, and to avoid potential predators or toxic substances. The olfactory system is able to detect and discriminate between thousands of structurally diverse odorants [1, 2]. Detection of odorants in mammals is mediated via several hundred olfactory receptors (ORs) that are expressed on olfactory sensory neurons (OSNs). Genes encoding ORs were first discovered by Buck and Axel in 1991 [3]. Relying on degenerate polymerase chain reaction (PCR), a novel experimental strategy at that time, Buck and Axel identified a diverse family of approximately 1,000 genes in the rat olfactory epithelium. Subsequent studies have led to the identification of the OR gene family in numerous additional species, including human [4–7], mouse [4, 6, 8–10], fish [11, 12], nematode [13], fruit fly [14–17], mosquito [18], red flour beetle [19, 20], honeybee [21], and platypus [22]. Vertebrate OR genes comprise the largest family of G protein-coupled receptors (GPCRs) [23]. Of the 1,282 GPCR genes in humans, 387 are known to encode for ORs, while 1,035 of the 2,288 mouse GPCR genes encode for ORs [6]. Invertebrate OR genes do not appear to exhibit any significant sequence similarity to vertebrate OR genes, and possess a distinct membrane topology [24]. These findings suggest the occurrence of independent evolutionary processes in OR gene development between vertebrate and invertebrate species. Indeed, our group has recently shown that insect ORs comprise heteromeric ligand-gated ion channels [25]. Hansson and his colleagues [26] have demonstrated the same fast ionotropic responses of insect ORs, but have also indicated the presence of a G protein-dependent signal cascade. Further investigation is required to elucidate the mechanisms underlying odorant recognition of insect ORs.
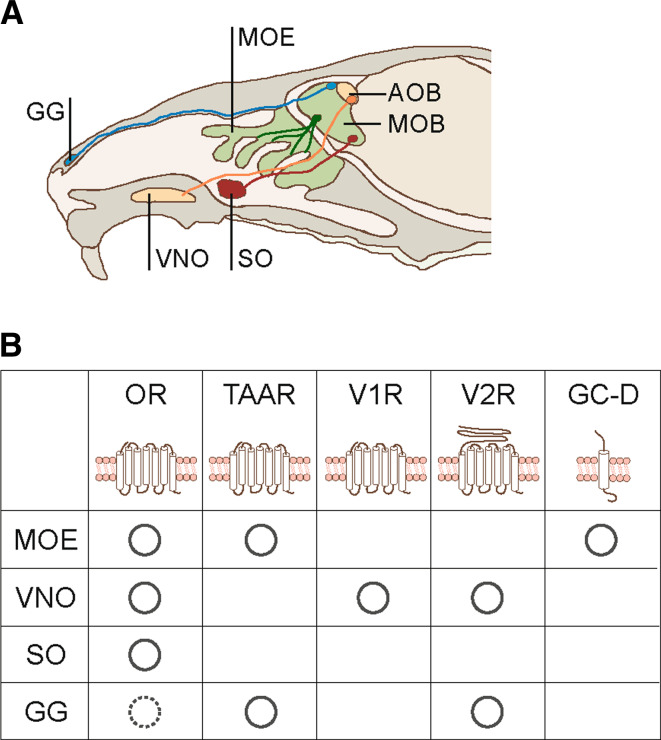
Olfactory receptors are predominantly expressed in OSNs located in the main olfactory epithelium (MOE) of the nasal cavity. Thus, ORs expressed in the MOE play a major role in olfaction (Fig. 1). However, recent studies have demonstrated that the chemosensory system is much more complex [27, 28]; for example, trace amine-associated receptors (TAARs) are also expressed in the MOE. TAARs were originally thought to be involved in psychiatric disorders [29]; however, it now appears that they function as a second class of chemosensory receptors in the MOE [30]. Some murine TAARs recognize volatile amines present in urine, and it has been hypothesized that TAARs may function in the detection of ligands associated with social cues [30]. A population of OSNs is also known to express guanylyl cyclase D (GC-D), but not ORs or TAARs. These OSNs project their axons into the dorsal part of the olfactory bulb (OB), called the ‘neckless glomeruli’ [31, 32]. GC-D neurons have been shown to respond to peptide hormones [33], CO2 [31], and even various pheromones [34]. In addition to the MOE, 50–80 ORs have also been shown to be expressed in the septal organ (SO). The SO comprises an island of sensory tissue located on either side of the nasal septum and is positioned at the nasoplatine duct [35, 36]. Furthermore, olfactory marker protein, which is expressed in mature OSNs, is expressed in the Grueneberg ganglion (GG). Neurons in the GG project their axons to defined glomeruli within the OB [37, 38]. GG neurons appear to express various types of chemosensory receptors [39–41] that may play a role in detecting alarm pheromones [42]. In combination, these reports suggest that the olfactory system comprises a variety of morphological, molecular, and functional subsystems that exhibit defined axon projection patterns [27, 28]. In the current review, we focus our discussion on GPCR-type ORs expressed in the MOE, and outline recent published studies reporting the molecular mechanisms underlying odor discrimination and signal transduction.
Fig. 1.
Anatomical structure of the mammalian olfactory system and chemosensory receptors. a Schematic diagram of the internal structure of the murine olfactory system. Sensory neuron axons of the main olfactory epithelium (MOE) project to the main olfactory bulb (MOB) (green), while sensory neuron axons of the vomeronasal organ (VNO) project to the accessory olfactory bulb (AOB) (orange). The septal organ (SO) is an island of olfactory sensory neurons (OSNs) that project axons to glomeruli located in the ventral olfactory bulb (red). The Grueneberg ganglion (GG) is located in the anterior tip of the nasal cavity and projects axons to specific glomeruli that surround the anterior AOB (blue). b The expression pattern and typical membrane topology of chemosensory receptors. Olfactory receptors (OR) are expressed in adult MOE [3], VNO [110] and SO [35, 36]. It has also been reported that one OR, mOR256-17, is expressed in several cells in GG during the pre- and peri-natal stages (hashed line) [39]. Trace amine-associated receptors (TAAR) are expressed in the MOE [30] and GG [40, 41]. V1R genes are expressed in the apical layer of the VNO [67, 111]. V2R genes are expressed in the basal region of the VNO [67, 112–114] and the GG [39, 41]. Guanyly cyclase D (GC-D) is expressed in a subset of OSNs located in the MOE [76, 115, 116]
Olfactory receptor pharmacology
Ligand specificity of olfactory receptors
Functional evidence supporting the hypothesis that ORs mediate specific odorant response was reported many years after the initial discovery of receptors. This was largely due to difficulties in achieving functional expression of ORs in heterologous cell systems. The first evidence reported the use of the adenovirus-mediated gene transfer of ORI7 in vivo in rat OSNs [43]. Zhao et al. [43] demonstrated elevated responses in the infected epithelium when treated with octyl aldehyde and other short-chain aliphatic aldehyde. In an independent study, given that each OSN is known to express only one functional OR gene [44], the functional single cell RT-PCR approach was successfully used in the investigation of the murine OR MOR23, and recapitulated the odorant response of a cell from which the receptor gene was functionally cloned [45]. Although the adenovirus-mediated approach using an OSN as the homologous expression system proved a useful investigative tool, it has since been replaced with heterologous expression systems that provide faster assays for the determination of OR function [46]. However, the functional expression of an OR in heterologous cells has still been a challenging problem. In addition, odorant responsiveness of an OR obtained from a heterologous expression system may not necessarily correspond to in vivo response of the OR [47], and also sometimes depends on the G protein employed [48], and thus the results must be interpreted carefully.
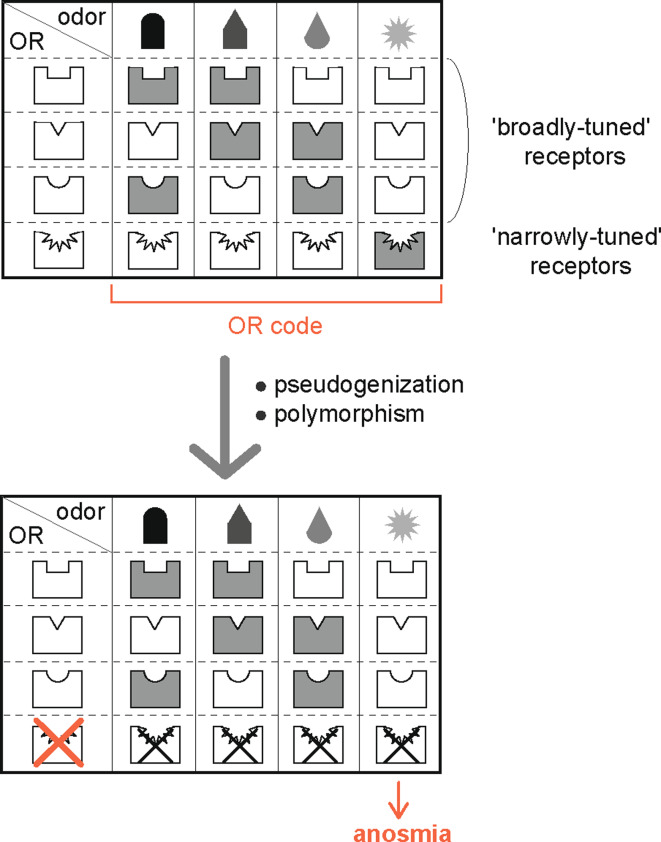
Several efforts to determine the ligand specificity of ORs have revealed that each individual OR not only demonstrates a high specificity for some functional groups and molecular features, but also has high tolerance in some aspects [43, 46, 48–56]. That is, ORs appear to discriminate between and recognize a wide variety of structurally similar odorants. For example, the ligand specificity of the mouse OR mOR-EG has been systematically analyzed in heterologous [50, 57] and in vivo systems using transgenic mice [47]. In these systems, it was revealed that mOR-EG recognized at least 25 different odorants that shared certain molecular features with distinct EC50 values [57]. Similarly, some ORs, including I7, human OR1D2 (also known as hOR17-4), and the MOR42 subfamily, have been reported to be responsive to approximately 20 different ligands [49, 54, 58, 59]. Thus, the general consensus for ligand specificity of ORs is that each OR has a distinct ligand spectrum, and that each odorant can be detected by a combination of ORs (Fig. 2).
Fig. 2.
Combinatorial receptor codes for odors. It is well established that a single odorant is recognized by multiple receptors (shaded boxes) and that a single OR recognizes multiple odorants (upper panel). It has been recently reported that some ‘narrowly tuned’ ORs such as OR7D4 (the androstenone receptor) are selectively activated by a limited number of odorant molecules [60, 61]. Genetic variation such as polymorphism resulting in amino acid substitutions or pseudogenes for these ORs affects odorant perception in vivo (lower panel)
While ORs, including mOR-EG, hOR17-4, and MOR42, appear to be broadly tuned and can recognize a large number of odorants, some other ORs are narrowly tuned to only a small number of closely related odorants [60]. It has been reported that the human OR OR7D4 is selectively activated by androstenone and androstadienone, and that single nucleotide polymorphisms resulted in a reduced sensitivity to these odorants in vivo [61]. These findings suggested that odorant perceptual variability may be due to genetic variability in specific ORs (Fig. 2). In contrast, some ORs, including the mouse OR MOR139-3, possess a non-specific ligand spectrum and thus recognize various functional groups with different structures, including aldehydes, alcohols, and ketones [62]. Pharmacological experiments have revealed that odorant perception appears to be based on a complex interaction between odorants and ORs.
As with other GPCRs, OR ligands are able to serve as both agonists and antagonists [47–49, 59, 63]. For example, the eugenol response of mOR-EG has been shown to be inhibited by structurally similar odorants including methyl-isoeugenol and isosafrole [64, 65]. In MOR42-3, co-application of C12 dicarboxylic acid or dodecanedioic acid reduces the response to nonanedioic acid (C9) [63]. The response of I7 to octanal is inhibited by C5–C6 aldehydes and cyclic octanal analogs [55]. The identification of OR antagonists has provided significant insight into the molecular basis underlying agonist-induced conformational changes in ORs, as an antagonist-bound form will likely represent the inactive state.
Ligand binding modes of olfactory receptors
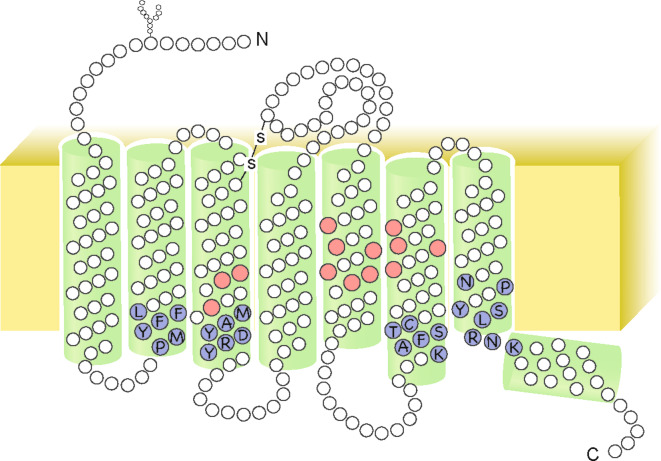
What determines the differences in ligand specificity among ORs has been the subject of intense investigation. Mammalian OR proteins belong to the GPCR family with a seven hydrophobic transmembrane (TM) domain structure. The conserved sequences and residues in the OR family, including the KAFSTC motif, are predicted to reside within the cytoplasmic side of the TM domains (Fig. 3). These conserved motifs appear to contribute to the common functions of the OR family, such as the normal folding and activation of OR. In contrast, the amino acid residues residing in the extracellular side of the TM domains appear to be relatively variable, suggesting numerous possible recognition sites for a large variety of odorant molecules [57]. Recently, a combination of computational and experimental approaches has led to a prediction that the odorant-binding site is located in a pocket formed by nine amino acids in TM3, TM5, and TM6 of mOR-EG. Based on these results, a structural model that accommodates a broad spectrum of odorants was proposed [57] (Fig. 3). A similar study investigating MOR42-3 also suggested the essential amino acid residues required for ligand-binding in TM3, TM5 and TM6 [63].
Fig. 3.
Molecular structure of the olfactory receptor. Mammalian OR proteins possess seven hydrophobic transmembrane (TM) domains. Amino acid residues involved in ligand binding in mOR-EG shown as red. The conserved sequences in the OR family shown as blue, and labeled with the single-letter amino acid code: A Ala, C Cys, D Asp, F Phe, K Lys, L Leu, M Met, N Asn, P Pro, R Arg, S Ser, T Thr, Y Tyr
Numerous molecular modeling and mutagenesis studies investigating GPCRs have revealed that the vast majority of the Family 1 GPCRs share a similar ligand-binding site located deep within the TM regions of TM3, TM5, TM6, and TM7 [66]. The spatial location of the odorant-binding pocket was found to be similar to that of biogenic GPCRs, including the β2-adrenergic receptor (β2AR), while the molecular environment of the odorant-binding site in ORs is somewhat distinct. Typical GPCRs such as the β2AR have been shown to interact with their ligand via the formation of ionic bonds, including hydrogen bonds and electorostatic interactions. In contrast, the hydrophobic environment of the odorant-binding site appears to play a critical role in odorant recognition, indicating a weaker association of the hydrophobic interactions between odorant molecules and ORs [57, 66]. The recognition of odorants by ORs also appears to be selective for the shape, size, and length of a ligand. This selectivity is determined by the environment of the binding site of each OR.
Olfactory signal transduction
Canonical pathway for olfactory signal transduction
Once an OR has bound an odorant molecule, a signal transduction cascade that transforms the chemical information into a neural signal is initiated [67]. Odorant-bound ORs activate a protein called Gαolf, which in turn activates adenylyl cyclase type III (ACIII), leading to cAMP production [68]. cAMP then binds to and opens cyclic nucleotide-gated (CNG) channels comprising CNGA2, CNGA4, and CNGB1b subunits [69]. Calcium influx through the CNG channel then results in the opening of calcium-gated chloride channels [70–72] (Fig. 4). Together, the calcium influx and chloride efflux allow the depolarization of membrane potentials in OSNs. The anosmic phenotypes of mouse knockouts for Gαolf, ACIII, and CNGA2 indicate that the cAMP pathway plays a major role in olfactory signal transduction [68, 73, 74].
Fig. 4.
Canonical pathway of olfactory signal transduction in OSNs. The heteromeric G protein, adenylyl cyclase III (ACIII), cyclic nucleotide-gated cation channel (CNG channel), and the calcium-gated chloride (Cl −) channels are thought to be common elements among the OSN signaling pathway (upper panel). Odorant-bound olfactory receptors (OR) activate Gαolf, which in turn activates ACIII, leading to cAMP production [68]. cAMP then binds to and opens CNG channels comprising CNGA2, CNGA4, and CNGB1b subunits [69]. Calcium influx through the CNG channel then results in the opening of calcium-gated chloride channels [70, 117]
Noncanonical pathway for olfactory signaling
Although investigation of odorant response in Gαolf, ACIII, and CNGA2 knockout mice strongly suggested that cAMP played a crucial role in olfactory signal transduction [68, 73, 74], CNGA2-deficient mice retained a limited electro-olfactogram (EOG) response to some odorants [34]. Such residual responses were thought to be contributed by the atypical OSNs that express TRPM5 [75] or GC-D [76], or from the microvillar cells called Jourdan cells [77]. TRPM5 is a calcium channel located in cells expressing taste receptors, and is also expressed in a subset of OSNs that express CNGA2 [75]. It is well established that these OSNs project their axons to the ventral region of the OB. In contrast, GC-D neurons are known to project their axons to the dorsal region of the OB called the neckless glomeruli. GC-D neurons respond to peptide hormones [33], CO2 [31], and even some pheromones [34], but do not express the elements required for canonical olfactory signaling via the cAMP pathway. Odorant-responsive microvillar cells appear to utilize the phospholipase C/inositol-1,4,5-triphosphate (PLC/IP3) pathway [77]. As these cells are not thought to extend their axons to the OB [77], it is possible that they do not function as sensory neurons.
G protein activation by olfactory receptors
G protein coupling of olfactory receptors
While the precise mechanisms underlying olfactory signal transduction and the interaction between ORs and odorants have been extensively studied, less is known about the coupling of an OR with G proteins. It has been revealed that truncation of the OR C-terminal sequence impairs odorant responsiveness, suggesting that the C-terminal domain is crucial for G protein coupling activity [57]. However, the specific mechanism by which odorant-bound ORs couple with G proteins has not been fully characterized. A recent study has demonstrated the molecular mechanism underlying G protein coupling of ORs [78].
Based on sequence alignment of mOR-EG, bovine rhodopsin, and β2AR, it was suggested that the C-terminal domain of mOR-EG contains a region called the eighth intracellular helical domain (Helix8), as well as rhodopsin and β2AR. Lys296, Lys299, and Lys303 in mOR-EG were predicted to face the cytoplasm and to be located within the putative Helix8. In order to determine whether these amino acid residues were actually involved in G protein coupling, these were targeted for site-directed mutagenesis, and the odor responsiveness in the mutants was measured using two functional assays [78]. The first assay monitored intracellular cAMP levels in HEK293 cells, which reflects OR coupling to endogenous Gαs. The second assay involved the detection of calcium via imaging after co-transfection with the non-specific G protein α subunit Gα15, which signals via the PLC/IP3 pathway [46].
The mutations at these residues resulted in a significant decrease in cAMP levels; however, a similar EG-response as measured by calcium imaging was retained [78]. The interaction with Gα15 and subsequent calcium cascade appeared to be unaffected by the mutations, suggesting that ligand binding was unchanged [78]. These results suggest that mutations in Lys296, Lys299, and Lys303 in mOR-EG significantly affected coupling to Gαs. Thus, it appears likely that these residues are involved in the interaction with Gαs. Interestingly, the introduction of Pro residues into Helix8 resulted in the complete loss of EG-response in both the cAMP and calcium assays, suggesting that the helical structure of the C-terminal domain was crucial for the interaction between ORs and G proteins [78]. A similar strategy revealed that Ile222, Leu227, and Ser231 in the third intracellular loop (IC3) were involved in coupling to Gαs/olf subunit [78] (Fig. 5). Odorant response in the presence of mutations of these residues were also affected, even in the presence of Gαolf, with the exception that the K299R mutant showed similar responsiveness to that of wild-type mOR-EG [78]. These findings indicate that ORs couple with Gα proteins and Gαolf in a similar but not identical manner.
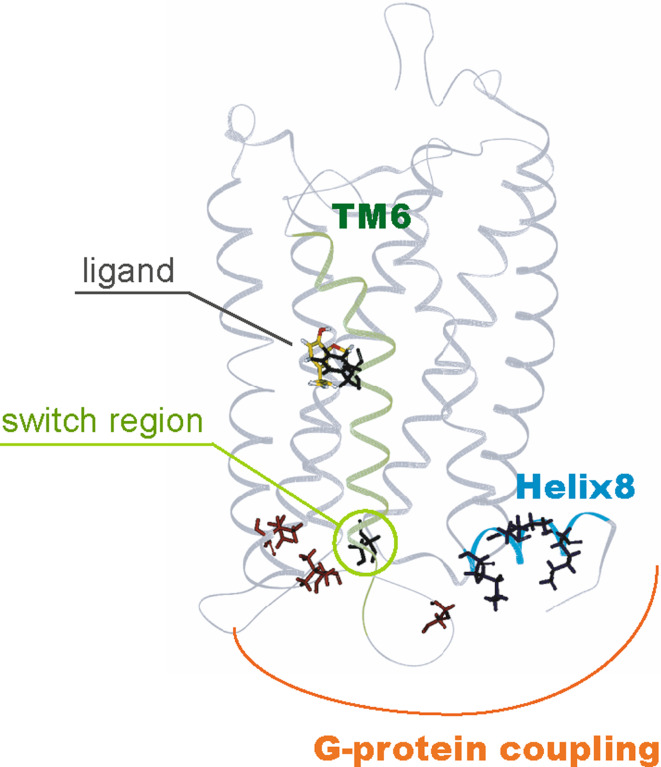
Fig. 5.
Three-dimensional model of G protein activation of a mouse olfactory receptor, mOR-EG. The amino acid residues involved in Gαs coupling in the IC3 and Helix8 are shown in red and blue, respectively [78]. The intracellular Helix8 and TM6 regions are highlighted in light blue and light green, respectively. The residues involved in conformational changes including Phe252 and Ser240 are shown as green sticks [78]. Phe252 also interacts with ligand (yellow) [57]
For non-olfactory GPCRs, there is ample evidence showing that G protein-interacting domains are located in the intracellular loops and in the C-terminus region [79–82]. Indeed, for rhodopsin and β2AR, Helix 8 has been identified as a G protein-interacting domain [83–85]. Lys311 in rhodopsin, which corresponds with Lys296 in mOR-EG, was shown to be located within close proximity to transducin [85]. Furthermore, the D382L mutant form of the human β1-adrenergic receptor (β1AR) has been shown to uncouple the receptor from Gαs [86]. In rhodopsin and β2AR, IC3 has also been identified as a G protein coupling site [81, 87]. These results suggest that ORs belonging to the GPCR family are structurally similar, and that IC3 and the C-terminal helix play a similar role in the interactions with Gαs-type G proteins.
Conformational dynamics of olfactory receptors
As the GPCR family is a major target of therapeutic agents, a great deal of research has focused on further understanding the molecular mechanisms underlying ligand recognition. However, the design of specific agonists and antagonists has proven difficult, as receptor activation appears to involve complex conformational alterations.
Upon stimulation, GPCRs undergo conformational changes that result in G protein activation. The light-induced conformational changes of rhodopsin have been defined through a series of elegant biophysical studies [88, 89], and it has been shown that photo-activation of rhodopsin involves both rotation and tilting of TM6 relative to TM3 [88]. Further evidence suggests that the β2AR undergoes structural changes similar to those observed upon activation of rhodopsin [90]. The Asp–Arg pair located at the cytoplasmic end of TM3 was shown to form part of the highly conserved (D/E)RY motif identified in the rhodopsin-family of GPCRs, whereas the Glu residue located at the cytoplasmic end of TM6 was highly conserved in amine receptors and opsin. The ionic link between the Asp–Arg pair and the Glu residue (also known as the ionic lock) has been proposed to maintain GPCR in the resting state [90, 91], and has been shown to be disrupted following photoactivation of rhodopsin [80, 89].
A previous study investigating mOR-EG activity reported that TM6 was similarly involved in OR activation [57]. Specifically, mutation of the Phe252 residue in TM6 of the mOR-EG resulted in a complete loss of the inhibitory activity of methyl isoeugenol, a potent antagonist of mOR-EG [57]. This finding suggests that Phe252 forms a stable interaction with the antagonist and aids in the maintenance of the inactive conformation of the receptor. To further our understanding of how OR function is related to receptor dynamics, the residues that reside in the cytosolic half of TM6 were targeted for mutation. Mutation at Ser240 exhibited an interesting and significant phenotype. While S240T did not result in any significant differences in a ligand response, S240A and S240V mutations resulted in both a higher sensitivity and efficacy to a ligand in the cAMP assay, and displayed a similar effect on activity when measured using calcium imaging [78]. These mutations did not appear to affect basal cAMP levels, suggesting that these mutants were not constitutively active. In addition, substitution in this Ser residue in other ORs such as M71 and MOR204-34 resulted in an increase in cAMP response [78]. Ser240 in the mOR-EG was found to correspond with the Ser residue located in the KAFSTC motif, which has been reported to be a highly conserved residue among members of the OR family. These results suggest that the Ser residue located in the KAFSTC motif is critical for receptor efficacy, and aids in the regulation of conformational changes from the inactive to active forms of the OR family (Fig. 5).
Olfactory adaptation
Calcium-mediated adaptation in olfactory sensory neurons
Odor perception not only depends on the chemical structure and concentration of odorants, but also on the previous experience of the OSNs, a process termed odor adaptation [92]. In simple terms, odor adaptation may be viewed as a form of neuronal plasticity. In the context of sensory processing, odor adaptation refers to the ability of the olfactory system to adjust its sensitivity at different stimulus intensities, a process that is likely to be essential for the prevention of cellular transduction machinery saturation, thus allowing high sensitivity retention during continuous or repetitive odor stimulation.
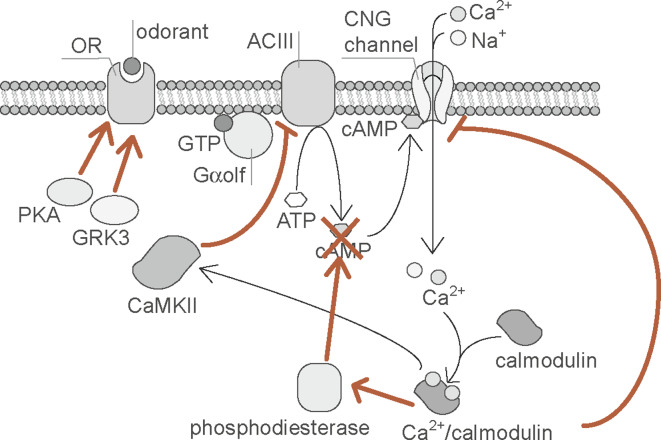
In OSNs, activated signal transduction molecules are targeted for negative feedback regulation (Fig. 6). Calcium rise via CNG channels is shown to mediate inactivation of CNG channels, ACIII and phophodiesterase [92]. Calcium and the calcium-binding protein, calmodulin (CaM), bind to and close the CNG channels, which is thought to be the major negative feedback regulation in OSNs [93–96]. ACIII is also targeted for negative feedback regulation. In this process, calcium/CaM-dependent protein kinase II (CaMKII) phosphorylates ACIII, thereby reducing cAMP production [97]. Furthermore, CaMKII is known to activate phosphodiesterase PDE1C2, which is enriched in the olfactory cilia, resulting in the hydrolysis of cAMP [98].
Fig. 6.
Schematic diagram of the negative feedback regulation of olfactory signal transduction in olfactory sensory neurons. Within the olfactory cilia, calcium entry occurs via the activation of CNG channels. Elevated intracellular calcium accelerates inhibition of CNG channels and ACIII, in addition to activating phosphodiestrase (red); calcium and the calcium-binding protein, calmodulin (CaM), bind to and close the CNG channels [93–96]. Calcium/CaM-dependent protein kinase II (CaMKII) phosphorylates ACIII, thereby reducing cAMP production [97]. CaMKII is also known to activate phosphodiesterase PDE1C2, which is enriched in the olfactory cilia, resulting in the hydrolysis of cAMP [98]. Furthermore, PKA and GRK3 are thought to mediate OR desensitization (red arrows) [108, 109] (Kato et al., unpublished)
Desensitization of olfactory receptors
The vast majority of GPCRs display a rapid loss of responsiveness in the continuing or recurring presence of an agonist or stimulus. This process, termed desensitization, is the consequence of a combination of mechanisms. These mechanisms include uncoupling of receptor from heterotrimeric G proteins in response to receptor phosphorylation, the internalization of cell surface receptors to the cytosol, and down-regulation of the total cellular complement of receptors due to reduced receptor mRNA or protein synthesis, in addition to both the lysosomal and plasma membrane degradation of pre-existing receptors [99]. The period of time over which these processes occur ranges from seconds (phosphorylation) to minutes (endocytosis) and hours (down-regulation). The uncoupling of GPCRs from their associated G protein is mainly mediated via receptor phosphorylation by intracellular kinases. It is generally accepted that both second messenger-dependent protein kinases including cAMP-dependent protein kinase A (PKA) and calcium-dependent protein kinase C (PKC) and GPCR kinases (GRKs) phosphorylate the serine and threonine residues located within the intracellular loops and C-terminal region of GPCRs [99–101]. GRKs selectively phosphorylate agonist-activated receptors, thereby promoting binding of cytosolic co-factor proteins called arrestins, which sterically uncouple the receptor from the G protein [102, 103]. In contrast, second messenger-dependent protein kinases not only phosphorylate activated GPCRs, but also indiscriminately phosphorylate receptors that have not been exposed to agonist [104].
Multiple studies have explored the roles of GRK and arrestins in the regulation of GPCRs. However, the precise activity of the largest subfamily of GPCRs, the ORs, remains elusive. It has been previously reported that the GRK3 member of the GRK family (also known as β-adrenergic receptor kinase 2) and β-arrestin2 are highly enriched in OSNs, and preincubation with antibodies to GRK3 and β-arrestin2 resulted in an elevation of cAMP response in the presence of an odorant [105]. GRK3 has also been shown to be translocated from the cytosol to the cell membrane upon odorant stimulation [106]. Furthermore, it has been demonstrated that cilia preparations derived from GRK3-deficient mice lack the rapid agonist-induced desensitization normally observed following odorant stimulation [107]. These findings indicated that GRK3 and β-arrestin2 mediated the desensitization of ORs. Hatt and colleagues recently demonstrated that the human ORs hOR17-4 and OR2AG1 are targeted for phosphorylation by PKA and β-arrestin-mediated internalization [108, 109].
Our laboratory has attempted to elucidate the molecular mechanisms required for desensitization of mOR-EG, and determined that the signaling activity was attenuated upon odorant binding, suggesting receptor desensitization. Flow cytometric analysis revealed that attenuation of mOR-EG signaling was not associated with receptor internalization, indicating the existence of receptor dephosphorylation mechanisms located on the cell surface. As ORs are expressed in the ciliary architecture in vivo, this result appears to represent a reasonable model for olfaction, which is essential for the detection of constant changes in the odor environment. Furthermore, immunoblot analyses using an anti-phosphoserine antibody strongly supported the hypothesis that GRK3-mediated phosphorylation was involved in mOR-EG desensitization (Kato et al., unpublished). Currently, calcium-mediated inhibition of channel activity has been thought to be the major event to account for adaptation of OSNs, and, thus, the precise mechanisms underlying desensitization of ORs and the significance for negative feedback in vivo remain to be fully explored.
Concluding remarks
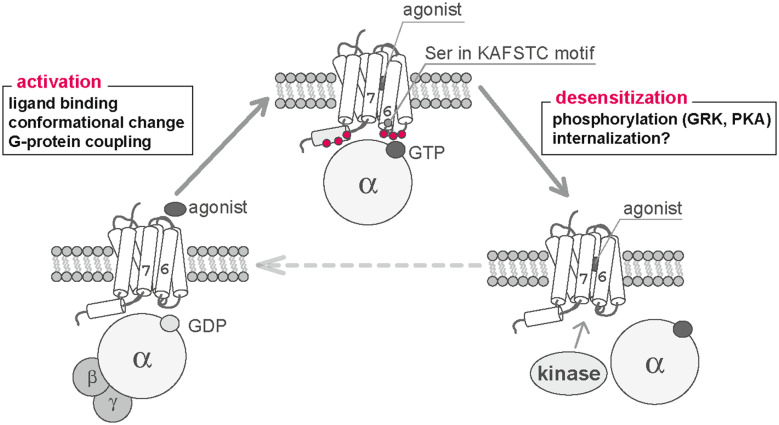
In mammals, ORs represent multifunctional signaling molecules that are involved in odorant recognition, singular OR gene choice, and OSN projection. However, the elucidation of the precise molecular mechanisms underlying odorant recognition by ORs has been complicated by the difficulties associated with cell-surface expression in heterologous systems. As we have discussed in this review, several efforts have been made to define the specific molecular mechanisms underlying activation and desensitization of ORs upon odorant stimulation (Fig. 7). Information regarding the mechanisms required for G protein coupling, conformational dynamics, and desensitization of ORs may provide meaningful information for the pharmacological screening and design of both agonists and antagonists for GPCRs, at which over 50% of pharmaceuticals are currently targeted.
Fig. 7.
Activation and desensitization of olfactory receptors. Upon agonist binding, the location of the TM6 is altered. This movement leads to activation of G proteins via specific interactions with the residues in IC3 and Helix8 (red circle). Agonist-mediated activation may then stimulate receptor desensitization. Recent experimental data suggests that PKA/GRK3-mediated phosphorylation is involved in desensitization of the OR
Abbreviations
- AC
Adenylyl cyclase
- β1AR
β1-Adrenergic receptor
- β2AR
β2-Adrenergic receptor
- CaM
Calmodulin
- CaMKII
Calcium/CaM-dependent protein kinase II
- CNG channel
Cyclic nucleotide-gated channel
- GC
Guanylyl cyclase
- GG
Grueneberg ganglion
- GPCR
G protein-coupled receptor
- GRK
GPCR kinase
- Helix8
Eighth intracellular helical domain
- IC3
Third intracellular loop
- MOE
Main olfactory epithelium
- OB
Olfactory bulb
- OR
Olfactory receptor
- OSN
Olfactory sensory neuron
- PCR
Polymerase chain reaction
- PKA
Protein kinase A
- PKC
Protein kinase C
- PLC/IP3
Phospholipase C/inositol-1,4,5-triphosphate
- SO
Septal organ
- TAAR
Trace amine-associated receptor
- TM
Transmembrane
References
- 1.Spehr M, Munger SD. Olfactory receptors: G protein-coupled receptors and beyond. J Neurochem. 2009;109:1570–1583. doi: 10.1111/j.1471-4159.2009.06085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Touhara K, Vosshall LB. Sensing odorants and pheromones with chemosensory receptors. Annu Rev Physiol. 2009;71:307–332. doi: 10.1146/annurev.physiol.010908.163209. [DOI] [PubMed] [Google Scholar]
- 3.Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-X. [DOI] [PubMed] [Google Scholar]
- 4.Man O, Gilad Y, Lancet D. Prediction of the odorant binding site of olfactory receptor proteins by human-mouse comparisons. Protein Sci. 2004;13:240–254. doi: 10.1110/ps.03296404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malnic B, Godfrey PA, Buck LB. The human olfactory receptor gene family. Proc Natl Acad Sci USA. 2004;101:2584–2589. doi: 10.1073/pnas.0307882100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niimura Y, Nei M (2007) Extensive gains and losses of olfactory receptor genes in mammalian evolution. PLoS ONE 2, e708 [DOI] [PMC free article] [PubMed]
- 7.Zhang X, De la Cruz O, Pinto JM, Nicolae D, Firestein S, Gilad Y. Characterizing the expression of the human olfactory receptor gene family using a novel DNA microarray. Genome Biol. 2007;8:R86. doi: 10.1186/gb-2007-8-5-r86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godfrey PA, Malnic B, Buck LB. The mouse olfactory receptor gene family. Proc Natl Acad Sci USA. 2004;101:2156–2161. doi: 10.1073/pnas.0308051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Firestein S. The olfactory receptor gene superfamily of the mouse. Nat Neurosci. 2002;5:124–133. doi: 10.1038/nn800. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Rodriguez I, Mombaerts P, Firestein S. Odorant and vomeronasal receptor genes in two mouse genome assemblies. Genomics. 2004;83:802–811. doi: 10.1016/j.ygeno.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Alioto TS, Ngai J. The odorant receptor repertoire of teleost fish. BMC Genomics. 2005;6:173. doi: 10.1186/1471-2164-6-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niimura Y, Nei M. Evolutionary dynamics of olfactory receptor genes in fishes and tetrapods. Proc Natl Acad Sci USA. 2005;102:6039–6044. doi: 10.1073/pnas.0501922102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- 14.Robertson HM, Warr CG, Carlson JR. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster . Proc Natl Acad Sci USA. 2003;100(Suppl 2):14537–14542. doi: 10.1073/pnas.2335847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clyne PJ, Warr CG, Freeman MR, Lessing D, Kim J, Carlson JR. A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron. 1999;22:327–338. doi: 10.1016/S0896-6273(00)81093-4. [DOI] [PubMed] [Google Scholar]
- 16.Gao Q, Chess A. Identification of candidate Drosophila olfactory receptors from genomic DNA sequence. Genomics. 1999;60:31–39. doi: 10.1006/geno.1999.5894. [DOI] [PubMed] [Google Scholar]
- 17.Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 1999;96:725–736. doi: 10.1016/S0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- 18.Hill CA, Fox AN, Pitts RJ, Kent LB, Tan PL, Chrystal MA, Cravchik A, Collins FH, Robertson HM, Zwiebel LJ. G protein-coupled receptors in Anopheles gambiae . Science. 2002;298:176–178. doi: 10.1126/science.1076196. [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Latief M. A family of chemoreceptors in Tribolium castaneum (Tenebrionidae: Coleoptera) PLoS ONE. 2007;2:e1319. doi: 10.1371/journal.pone.0001319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engsontia P, Sanderson AP, Cobb M, Walden KK, Robertson HM, Brown S. The red flour beetle’s large nose: an expanded odorant receptor gene family in Tribolium castaneum . Insect Biochem Mol Biol. 2008;38:387–397. doi: 10.1016/j.ibmb.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Robertson HM, Wanner KW. The chemoreceptor superfamily in the honey bee, Apis mellifera: expansion of the odorant, but not gustatory, receptor family. Genome Res. 2006;16:1395–1403. doi: 10.1101/gr.5057506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warren WC, Hillier LW, Marshall Graves JA, Birney E, Ponting CP, Grutzner F, Belov K, Miller W, Clarke L, Chinwalla AT, Yang SP, Heger A, Locke DP, Miethke P, Waters PD, Veyrunes F, Fulton L, Fulton B, Graves T, Wallis J, Puente XS, Lopez-Otin C, Ordonez GR, Eichler EE, Chen L, Cheng Z, Deakin JE, Alsop A, Thompson K, Kirby P, Papenfuss AT, Wakefield MJ, Olender T, Lancet D, Huttley GA, Smit AF, Pask A, Temple-Smith P, Batzer MA, Walker JA, Konkel MK, Harris RS, Whittington CM, Wong ES, Gemmell NJ, Buschiazzo E, Vargas Jentzsch IM, Merkel A, Schmitz J, Zemann A, Churakov G, Kriegs JO, Brosius J, Murchison EP, Sachidanandam R, Smith C, Hannon GJ, Tsend-Ayush E, McMillan D, Attenborough R, Rens W, Ferguson-Smith M, Lefevre CM, Sharp JA, Nicholas KR, Ray DA, Kube M, Reinhardt R, Pringle TH, Taylor J, Jones RC, Nixon B, Dacheux JL, Niwa H, Sekita Y, Huang X, Stark A, Kheradpour P, Kellis M, Flicek P, Chen Y, Webber C, Hardison R, Nelson J, Hallsworth-Pepin K, Delehaunty K, Markovic C, Minx P, Feng Y, Kremitzki C, Mitreva M, Glasscock J, Wylie T, Wohldmann P, Thiru P, Nhan MN, Pohl CS, Smith SM, Hou S, Nefedov M, de Jong PJ, Renfree MB, Mardis ER, Wilson RK. Genome analysis of the platypus reveals unique signatures of evolution. Nature. 2008;455:256. doi: 10.1038/nature07253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nei M, Niimura Y, Nozawa M. The evolution of animal chemosensory receptor gene repertoires: roles of chance and necessity. Nat Rev Genet. 2008;9:951–963. doi: 10.1038/nrg2480. [DOI] [PubMed] [Google Scholar]
- 24.Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4:e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato K, Pellegrino M, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- 26.Wicher D, Schafer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hansson BS. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–1011. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- 27.Breer H, Fleischer J, Strotmann J. The sense of smell: multiple olfactory subsystems. Cell Mol Life Sci. 2006;63:1465–1475. doi: 10.1007/s00018-006-6108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munger SD, Leinders-Zufall T, Zufall F. Subsystem organization of the mammalian sense of smell. Annu Rev Physiol. 2008;71:115–140. doi: 10.1146/annurev.physiol.70.113006.100608. [DOI] [PubMed] [Google Scholar]
- 29.Zucchi R, Chiellini G, Scanlan TS, Grandy DK. Trace amine-associated receptors and their ligands. Br J Pharmacol. 2006;149:967–978. doi: 10.1038/sj.bjp.0706948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- 31.Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, Luo M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- 32.Sun L, Wang H, Hu J, Han J, Matsunami H, Luo M. Guanylyl cyclase-D in the olfactory CO2 neurons is activated by bicarbonate. Proc Natl Acad Sci USA. 2009;106:2041–2046. doi: 10.1073/pnas.0812220106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leinders-Zufall T, Cockerham RE, Michalakis S, Biel M, Garbers DL, Reed RR, Zufall F, Munger SD. Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc Natl Acad Sci USA. 2007;104:14507–14512. doi: 10.1073/pnas.0704965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin W, Arellano J, Slotnick B, Restrepo D. Odors detected by mice deficient in cyclic nucleotide-gated channel subunit A2 stimulate the main olfactory system. J Neurosci. 2004;24:3703–3710. doi: 10.1523/JNEUROSCI.0188-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaluza JF, Gussing F, Bohm S, Breer H, Strotmann J. Olfactory receptors in the mouse septal organ. J Neurosci Res. 2004;76:442–452. doi: 10.1002/jnr.20083. [DOI] [PubMed] [Google Scholar]
- 36.Tian H, Ma M. Molecular organization of the olfactory septal organ. J Neurosci. 2004;24:8383–8390. doi: 10.1523/JNEUROSCI.2222-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuss SH, Omura M, Mombaerts P. The Grueneberg ganglion of the mouse projects axons to glomeruli in the olfactory bulb. Eur J Neurosci. 2005;22:2649–2654. doi: 10.1111/j.1460-9568.2005.04468.x. [DOI] [PubMed] [Google Scholar]
- 38.Koos DS, Fraser SE. The Grueneberg ganglion projects to the olfactory bulb. NeuroReport. 2005;16:1929–1932. doi: 10.1097/01.wnr.0000186597.72081.10. [DOI] [PubMed] [Google Scholar]
- 39.Fleischer J, Schwarzenbacher K, Besser S, Hass N, Breer H. Olfactory receptors and signalling elements in the Grueneberg ganglion. J Neurochem. 2006;98:543–554. doi: 10.1111/j.1471-4159.2006.03894.x. [DOI] [PubMed] [Google Scholar]
- 40.Fleischer J, Schwarzenbacher K, Breer H. Expression of trace amine-associated receptors in the Grueneberg ganglion. Chem Senses. 2007;32:623–631. doi: 10.1093/chemse/bjm032. [DOI] [PubMed] [Google Scholar]
- 41.Mamasuew K, Breer H, Fleischer J. Grueneberg ganglion neurons respond to cool ambient temperatures. Eur J Neurosci. 2008;28:1775–1785. doi: 10.1111/j.1460-9568.2008.06465.x. [DOI] [PubMed] [Google Scholar]
- 42.Brechbuhl J, Klaey M, Broillet MC. Grueneberg ganglion cells mediate alarm pheromone detection in mice. Science. 2008;321:1092–1095. doi: 10.1126/science.1160770. [DOI] [PubMed] [Google Scholar]
- 43.Zhao H, Ivic L, Otaki JM, Hashimoto M, Mikoshiba K, Firestein S. Functional expression of a mammalian odorant receptor. Science. 1998;279:237–242. doi: 10.1126/science.279.5348.237. [DOI] [PubMed] [Google Scholar]
- 44.Serizawa S, Miyamichi K, Sakano H. One neuron-one receptor rule in the mouse olfactory system. Trends Genet. 2004;20:648–653. doi: 10.1016/j.tig.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Touhara K, Sengoku S, Inaki K, Tsuboi A, Hirono J, Sato T, Sakano H, Haga T. Functional identification and reconstitution of an odorant receptor in single olfactory neurons. Proc Natl Acad Sci USA. 1999;96:4040–4045. doi: 10.1073/pnas.96.7.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Touhara K. Deorphanizing vertebrate olfactory receptors: recent advances in odorant-response assays. Neurochem Int. 2007;51:132–139. doi: 10.1016/j.neuint.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 47.Oka Y, Katada S, Omura M, Suwa M, Yoshihara Y, Touhara K. Odorant receptor map in the mouse olfactory bulb: in vivo sensitivity and specificity of receptor-defined glomeruli. Neuron. 2006;52:857–869. doi: 10.1016/j.neuron.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 48.Shirokova E, Schmiedeberg K, Bedner P, Niessen H, Willecke K, Raguse JD, Meyerhof W, Krautwurst D. Identification of specific ligands for orphan olfactory receptors. G protein-dependent agonism and antagonism of odorants. J Biol Chem. 2005;280:11807–11815. doi: 10.1074/jbc.M411508200. [DOI] [PubMed] [Google Scholar]
- 49.Araneda RC, Kini AD, Firestein S. The molecular receptive range of an odorant receptor. Nat Neurosci. 2000;3:1248–1255. doi: 10.1038/81774. [DOI] [PubMed] [Google Scholar]
- 50.Kajiya K, Inaki K, Tanaka M, Haga T, Kataoka H, Touhara K. Molecular bases of odor discrimination: reconstitution of olfactory receptors that recognize overlapping sets of odorants. J Neurosci. 2001;21:6018–6025. doi: 10.1523/JNEUROSCI.21-16-06018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wetzel CH, Oles M, Wellerdieck C, Kuczkowiak M, Gisselmann G, Hatt H. Specificity and sensitivity of a human olfactory receptor functionally expressed in human embryonic kidney 293 cells and Xenopus laevis oocytes. J Neurosci. 1999;19:7426–7433. doi: 10.1523/JNEUROSCI.19-17-07426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bozza T, Feinstein P, Zheng C, Mombaerts P. Odorant receptor expression defines functional units in the mouse olfactory system. J Neurosci. 2002;22:3033–3043. doi: 10.1523/JNEUROSCI.22-08-03033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaillard I, Rouquier S, Pin JP, Mollard P, Richard S, Barnabe C, Demaille J, Giorgi D. A single olfactory receptor specifically binds a set of odorant molecules. Eur J Neurosci. 2002;15:409–418. doi: 10.1046/j.0953-816x.2001.01871.x. [DOI] [PubMed] [Google Scholar]
- 54.Krautwurst D, Yau KW, Reed RR. Identification of ligands for olfactory receptors by functional expression of a receptor library. Cell. 1998;95:917–926. doi: 10.1016/S0092-8674(00)81716-X. [DOI] [PubMed] [Google Scholar]
- 55.Peterlin Z, Li Y, Sun G, Shah R, Firestein S, Ryan K. The importance of odorant conformation to the binding and activation of a representative olfactory receptor. Chem Biol. 2008;15:1317–1327. doi: 10.1016/j.chembiol.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Repicky SE, Luetje CW. Molecular receptive range variation among mouse odorant receptors for aliphatic carboxylic acids. J Neurochem. 2009;109:193–202. doi: 10.1111/j.1471-4159.2009.05925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katada S, Hirokawa T, Oka Y, Suwa M, Touhara K. Structural basis for a broad but selective ligand spectrum of a mouse olfactory receptor: mapping the odorant-binding site. J Neurosci. 2005;25:1806–1815. doi: 10.1523/JNEUROSCI.4723-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abaffy T, Matsunami H, Luetje CW. Functional analysis of a mammalian odorant receptor subfamily. J Neurochem. 2006;97:1506–1518. doi: 10.1111/j.1471-4159.2006.03859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spehr M, Gisselmann G, Poplawski A, Riffell JA, Wetzel CH, Zimmer RK, Hatt H. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science. 2003;299:2054–2058. doi: 10.1126/science.1080376. [DOI] [PubMed] [Google Scholar]
- 60.Saito H, Chi Q, Zhuang H, Matsunami H, Mainland JD (2009) Odor coding by a Mammalian receptor repertoire. Sci Signal 2, ra9 [DOI] [PMC free article] [PubMed]
- 61.Keller A, Zhuang H, Chi Q, Vosshall LB, Matsunami H. Genetic variation in a human odorant receptor alters odour perception. Nature. 2007;449:468–472. doi: 10.1038/nature06162. [DOI] [PubMed] [Google Scholar]
- 62.Yoshikawa K, Touhara K. Myr-Ric-8A enhances G(alpha15)-mediated Ca2+ response of vertebrate olfactory receptors. Chem Senses. 2009;34:15–23. doi: 10.1093/chemse/bjn047. [DOI] [PubMed] [Google Scholar]
- 63.Abaffy T, Malhotra A, Luetje CW. The molecular basis for ligand specificity in a mouse olfactory receptor: a network of functionally important residues. J Biol Chem. 2007;282:1216–1224. doi: 10.1074/jbc.M609355200. [DOI] [PubMed] [Google Scholar]
- 64.Oka Y, Nakamura A, Watanabe H, Touhara K. An odorant derivative as an antagonist for an olfactory receptor. Chem Senses. 2004;29:815–822. doi: 10.1093/chemse/bjh247. [DOI] [PubMed] [Google Scholar]
- 65.Oka Y, Omura M, Kataoka H, Touhara K. Olfactory receptor antagonism between odorants. EMBO J. 2004;23:120–126. doi: 10.1038/sj.emboj.7600032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Katada S, Hirokawa T, Touhara K. Exploring the odorant binding site of a G-protein-coupled olfactory receptor. Curr Comput Aided Drug Des. 2008;4:123–131. doi: 10.2174/157340908784533247. [DOI] [Google Scholar]
- 67.Mombaerts P. Genes and ligands for odorant, vomeronasal and taste receptors. Nat Rev Neurosci. 2004;5:263–278. doi: 10.1038/nrn1365. [DOI] [PubMed] [Google Scholar]
- 68.Wong ST, Trinh K, Hacker B, Chan GC, Lowe G, Gaggar A, Xia Z, Gold GH, Storm DR. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron. 2000;27:487–497. doi: 10.1016/S0896-6273(00)00060-X. [DOI] [PubMed] [Google Scholar]
- 69.Zheng J, Zagotta WN. Stoichiometry and assembly of olfactory cyclic nucleotide-gated channels. Neuron. 2004;42:411–421. doi: 10.1016/S0896-6273(04)00253-3. [DOI] [PubMed] [Google Scholar]
- 70.Stephan AB, Shum EY, Hirsh S, Cygnar KD, Reisert J, Zhao H. ANO2 is the cilial calcium-activated chloride channel that may mediate olfactory amplification. Proc Natl Acad Sci USA. 2009;106:11776–11781. doi: 10.1073/pnas.0903304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 73.Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in G(olf) are anosmic. Neuron. 1998;20:69–81. doi: 10.1016/S0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- 74.Brunet LJ, Gold GH, Ngai J. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron. 1996;17:681–693. doi: 10.1016/S0896-6273(00)80200-7. [DOI] [PubMed] [Google Scholar]
- 75.Lin W, Margolskee R, Donnert G, Hell SW, Restrepo D. Olfactory neurons expressing transient receptor potential channel M5 (TRPM5) are involved in sensing semiochemicals. Proc Natl Acad Sci USA. 2007;104:2471–2476. doi: 10.1073/pnas.0610201104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fulle HJ, Vassar R, Foster DC, Yang RB, Axel R, Garbers DL. A receptor guanylyl cyclase expressed specifically in olfactory sensory neurons. Proc Natl Acad Sci USA. 1995;92:3571–3575. doi: 10.1073/pnas.92.8.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elsaesser R, Montani G, Tirindelli R, Paysan J. Phosphatidyl-inositide signalling proteins in a novel class of sensory cells in the mammalian olfactory epithelium. Eur J Neurosci. 2005;21:2692–2700. doi: 10.1111/j.1460-9568.2005.04108.x. [DOI] [PubMed] [Google Scholar]
- 78.Kato A, Katada S, Touhara K. Amino acids involved in conformational dynamics and G protein coupling of an odorant receptor: targeting gain-of-function mutation. J Neurochem. 2008;107:1261–1270. doi: 10.1111/j.1471-4159.2008.05693.x. [DOI] [PubMed] [Google Scholar]
- 79.Eason MG, Liggett SB. Identification of a Gs coupling domain in the amino terminus of the third intracellular loop of the alpha 2A-adrenergic receptor. Evidence for distinct structural determinants that confer Gs versus Gi coupling. J Biol Chem. 1995;270:24753–24760. doi: 10.1074/jbc.270.42.24753. [DOI] [PubMed] [Google Scholar]
- 80.Franke RR, Sakmar TP, Graham RM, Khorana HG. Structure and function in rhodopsin. Studies of the interaction between the rhodopsin cytoplasmic domain and transducin. J Biol Chem. 1992;267:14767–14774. [PubMed] [Google Scholar]
- 81.Liggett SB, Caron MG, Lefkowitz RJ, Hnatowich M. Coupling of a mutated form of the human beta 2-adrenergic receptor to Gi and Gs. Requirement for multiple cytoplasmic domains in the coupling process. J Biol Chem. 1991;266:4816–4821. [PubMed] [Google Scholar]
- 82.Thiagaraj HV, Ortiz TC, Devereaux MC, Jr, Seaver B, Hall B, Parker KK. Regulation of G proteins by human 5-HT1a receptor TM3/i2 and TM5/i3 loop peptides. Neurochem Int. 2007;50:109–118. doi: 10.1016/j.neuint.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 83.Liggett SB. Update on current concepts of the molecular basis of beta2-adrenergic receptor signaling. J Allergy Clin Immunol. 2002;110:S223–S227. doi: 10.1067/mai.2002.129945. [DOI] [PubMed] [Google Scholar]
- 84.Marin EP, Krishna AG, Zvyaga TA, Isele J, Siebert F, Sakmar TP. The amino terminus of the fourth cytoplasmic loop of rhodopsin modulates rhodopsin–transducin interaction. J Biol Chem. 2000;275:1930–1936. doi: 10.1074/jbc.275.3.1930. [DOI] [PubMed] [Google Scholar]
- 85.Nikiforovich GV, Taylor CM, Marshall GR. Modeling of the complex between transducin and photoactivated rhodopsin, a prototypical G-protein-coupled receptor. Biochemistry. 2007;46:4734–4744. doi: 10.1021/bi700185p. [DOI] [PubMed] [Google Scholar]
- 86.Delos Santos NM, Gardner LA, White SW, Bahouth SW. Characterization of the residues in helix 8 of the human beta1-adrenergic receptor that are involved in coupling the receptor to G proteins. J Biol Chem. 2006;281:12896–12907. doi: 10.1074/jbc.M508500200. [DOI] [PubMed] [Google Scholar]
- 87.Acharya S, Saad Y, Karnik SS. Transducin-alpha C-terminal peptide binding site consists of C–D and E–F loops of rhodopsin. J Biol Chem. 1997;272:6519–6524. doi: 10.1074/jbc.272.10.6519. [DOI] [PubMed] [Google Scholar]
- 88.Farrens DL, Altenbach C, Yang K, Hubbell WL, Khorana HG. Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science. 1996;274:768–770. doi: 10.1126/science.274.5288.768. [DOI] [PubMed] [Google Scholar]
- 89.Sheikh SP, Zvyaga TA, Lichtarge O, Sakmar TP, Bourne HR. Rhodopsin activation blocked by metal-ion-binding sites linking transmembrane helices C and F. Nature. 1996;383:347–350. doi: 10.1038/383347a0. [DOI] [PubMed] [Google Scholar]
- 90.Ballesteros JA, Jensen AD, Liapakis G, Rasmussen SG, Shi L, Gether U, Javitch JA. Activation of the beta 2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J Biol Chem. 2001;276:29171–29177. doi: 10.1074/jbc.M103747200. [DOI] [PubMed] [Google Scholar]
- 91.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 92.Zufall F, Leinders-Zufall T. The cellular and molecular basis of odor adaptation. Chem Senses. 2000;25:473–481. doi: 10.1093/chemse/25.4.473. [DOI] [PubMed] [Google Scholar]
- 93.Bradley J, Bonigk W, Yau KW, Frings S. Calmodulin permanently associates with rat olfactory CNG channels under native conditions. Nat Neurosci. 2004;7:705–710. doi: 10.1038/nn1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bradley J, Reuter D, Frings S. Facilitation of calmodulin-mediated odor adaptation by cAMP-gated channel subunits. Science. 2001;294:2176–2178. doi: 10.1126/science.1063415. [DOI] [PubMed] [Google Scholar]
- 95.Munger SD, Lane AP, Zhong H, Leinders-Zufall T, Yau KW, Zufall F, Reed RR. Central role of the CNGA4 channel subunit in Ca2+-calmodulin-dependent odor adaptation. Science. 2001;294:2172–2175. doi: 10.1126/science.1063224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Song Y, Cygnar KD, Sagdullaev B, Valley M, Hirsh S, Stephan A, Reisert J, Zhao H. Olfactory CNG channel desensitization by Ca2+/CaM via the B1b subunit affects response termination but not sensitivity to recurring stimulation. Neuron. 2008;58:374–386. doi: 10.1016/j.neuron.2008.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wei J, Zhao AZ, Chan GC, Baker LP, Impey S, Beavo JA, Storm DR. Phosphorylation and inhibition of olfactory adenylyl cyclase by CaM kinase II in Neurons: a mechanism for attenuation of olfactory signals. Neuron. 1998;21:495–504. doi: 10.1016/S0896-6273(00)80561-9. [DOI] [PubMed] [Google Scholar]
- 98.Yan C, Zhao AZ, Bentley JK, Loughney K, Ferguson K, Beavo JA. Molecular cloning and characterization of a calmodulin-dependent phosphodiesterase enriched in olfactory sensory neurons. Proc Natl Acad Sci USA. 1995;92:9677–9681. doi: 10.1073/pnas.92.21.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 100.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 101.Willets JM, Challiss RA, Nahorski SR. Non-visual GRKs: are we seeing the whole picture? Trends Pharmacol Sci. 2003;24:626–633. doi: 10.1016/j.tips.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 102.Benovic JL, Kuhn H, Weyand I, Codina J, Caron MG, Lefkowitz RJ. Functional desensitization of the isolated beta-adrenergic receptor by the beta-adrenergic receptor kinase: potential role of an analog of the retinal protein arrestin (48-kDa protein) Proc Natl Acad Sci USA. 1987;84:8879–8882. doi: 10.1073/pnas.84.24.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 104.Hausdorff WP, Bouvier M, O’Dowd BF, Irons GP, Caron MG, Lefkowitz RJ. Phosphorylation sites on two domains of the beta 2-adrenergic receptor are involved in distinct pathways of receptor desensitization. J Biol Chem. 1989;264:12657–12665. [PubMed] [Google Scholar]
- 105.Dawson TM, Arriza JL, Jaworsky DE, Borisy FF, Attramadal H, Lefkowitz RJ, Ronnett GV. Beta-adrenergic receptor kinase-2 and beta-arrestin-2 as mediators of odorant-induced desensitization. Science. 1993;259:825–829. doi: 10.1126/science.8381559. [DOI] [PubMed] [Google Scholar]
- 106.Boekhoff I, Inglese J, Schleicher S, Koch WJ, Lefkowitz RJ, Breer H. Olfactory desensitization requires membrane targeting of receptor kinase mediated by beta gamma-subunits of heterotrimeric G proteins. J Biol Chem. 1994;269:37–40. [PubMed] [Google Scholar]
- 107.Peppel K, Boekhoff I, McDonald P, Breer H, Caron MG, Lefkowitz RJ. G protein-coupled receptor kinase 3 (GRK3) gene disruption leads to loss of odorant receptor desensitization. J Biol Chem. 1997;272:25425–25428. doi: 10.1074/jbc.272.41.25425. [DOI] [PubMed] [Google Scholar]
- 108.Mashukova A, Spehr M, Hatt H, Neuhaus EM. Beta-arrestin2-mediated internalization of mammalian odorant receptors. J Neurosci. 2006;26:9902–9912. doi: 10.1523/JNEUROSCI.2897-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Neuhaus EM, Mashukova A, Barbour J, Wolters D, Hatt H. Novel function of beta-arrestin2 in the nucleus of mature spermatozoa. J Cell Sci. 2006;119:3047–3056. doi: 10.1242/jcs.03046. [DOI] [PubMed] [Google Scholar]
- 110.Levai O, Feistel T, Breer H, Strotmann J. Cells in the vomeronasal organ express odorant receptors but project to the accessory olfactory bulb. J Comp Neurol. 2006;498:476–490. doi: 10.1002/cne.21067. [DOI] [PubMed] [Google Scholar]
- 111.Dulac C, Axel R. A novel family of genes encoding putative pheromone receptors in mammals. Cell. 1995;83:195–206. doi: 10.1016/0092-8674(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 112.Herrada G, Dulac C. A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell. 1997;90:763–773. doi: 10.1016/S0092-8674(00)80536-X. [DOI] [PubMed] [Google Scholar]
- 113.Matsunami H, Buck LB. A multigene family encoding a diverse array of putative pheromone receptors in mammals. Cell. 1997;90:775–784. doi: 10.1016/S0092-8674(00)80537-1. [DOI] [PubMed] [Google Scholar]
- 114.Ryba NJ, Tirindelli R. A new multigene family of putative pheromone receptors. Neuron. 1997;19:371–379. doi: 10.1016/S0896-6273(00)80946-0. [DOI] [PubMed] [Google Scholar]
- 115.Juilfs DM, Fulle HJ, Zhao AZ, Houslay MD, Garbers DL, Beavo JA. A subset of olfactory neurons that selectively express cGMP-stimulated phosphodiesterase (PDE2) and guanylyl cyclase-D define a unique olfactory signal transduction pathway. Proc Natl Acad Sci USA. 1997;94:3388–3395. doi: 10.1073/pnas.94.7.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meyer MR, Angele A, Kremmer E, Kaupp UB, Muller F. A cGMP-signaling pathway in a subset of olfactory sensory neurons. Proc Natl Acad Sci USA. 2000;97:10595–10600. doi: 10.1073/pnas.97.19.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reisert J, Lai J, Yau KW, Bradley J. Mechanism of the excitatory Cl-response in mouse olfactory receptor neurons. Neuron. 2005;45:553–561. doi: 10.1016/j.neuron.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]