Abstract
Enterococci are commensal organisms in the alimentary tract. However, they can cause a variety of life-threatening infections, especially in nosocomial settings. We hypothesized that induction of cell death might enable these facultative pathogenic bacteria to evade the innate immune response and to cause infections of their host. We demonstrate that E. faecium when exposed to lysozyme induces cell death in macrophages in vitro and in vivo. Flow cytometric analyses of J774A.1 macrophages infected with E. faecium revealed loss of cell membrane integrity indicated by uptake of propidium iodide and decrease of the inner mitochondrial transmembrane potential ΔΨm. Inhibition of caspases, treatment of macrophages with cytochalasin D, or rifampicin did not prevent cells from dying, suggesting cell death mechanisms that are independent of caspase activation, bacterial uptake, and intracellular bacterial replication. Characteristics of necrotic cell death were demonstrated by both lack of procaspase 3 activation and cell shrinkage, electron microscopy, and release of lactate dehydrogenase. Pretreatment of E. faecium with lysozyme and subsequently with broad spectrum protease considerably reduced cell death, suggesting that a bacterial surface protein is causative for cell death induction. Moreover, in a mouse peritonitis model we demonstrated that E. faecium induces cell death of peritoneal macrophages in vivo. Altogether, our results show that enterococci, under specific conditions such as exposure to lysozyme, induce necrotic cell death in macrophages, which might contribute to disseminated infections by these facultative pathogenic bacteria.
Keywords: Enterococcus faecium, Enterococci, Lysozyme, Necrosis, Mouse macrophage, Toll-like receptors, Peritonitis model
Introduction
Enterococci are human commensals colonizing the oral cavity, the gastrointestinal tract, the vagina, and the skin [1]. Under physiological conditions, Enterococcus spp. do not cause infections in otherwise healthy persons [2]. However, enterococci can cause serious and life-threatening systemic infections, especially in immunocompromised patients, i.e., infections of the urinary tract, the bloodstream, endocardium, abdomen, biliary tract, and burn wounds [3, 4]. Most of the infections (80–90%) are caused by E. faecalis, the remaining 10–20% are caused by E. faecium. Infections with other Enterococcus species, i.e., E. avium, E. casseliflavus, E. durans, E. gallinarum, or E. hirae are very rare [1]. Altogether, infections with enterococci are the third leading cause of bacteremia emanating from intravenous lines, abscesses, urinary tract and intestinal tract infections, and the fourth leading cause of hospital-acquired infections in the US [5]. Outbreaks of infections with enterococci resistant to second-line antibiotics such as glycopeptides are an increasing problem since infections with vancomycin-resistant enterococci (VRE) are associated with a higher mortality compared to infections with vancomycin-susceptible enterococci [6].
The course of infection with enterococci typically includes the adherence to host cells and extracellular matrix, tissue invasion and abscess formation, the modulation of host inflammatory responses, and toxin-mediated tissue damage [1]. Several virulence factors contribute to the ability of enterococci to invade tissues and cause infections. Most of them have been described in E. faecalis but not in E. faecium strains. Cytolysin is produced by most E. faecalis strains isolated from human infections. These strains are bacteriolytic towards a couple of Gram-positive bacteria and exhibit cytolytic activity towards selected eukaryotic cells such as human and mouse erythrocytes. However, this factor is very rare in clinical isolates of E. faecium [1]. Further virulence factors of enterococci, mainly found in E. faecalis, are aggregation substance mediating adherence to eukaryotic cells, collagen-hydrolyzing gelatinase, and lipoteichoic acids anchored to the cell membrane, which contribute to the formation of biofilms and the adherence to host cells [1]. However, the analysis of different clinical E. faecalis isolates revealed that the aforementioned virulence genes were detected more frequently among colonizing isolates than among invasive isolates. Hence, the presence of virulence genes is not necessarily linked to pathogenicity and rather other unknown bacterial factors might contribute to infections caused by enterococci [7].
Macrophages located in the subepithelial lamina propria and mesenteric lymph nodes are the first cells that fight intraperitoneal bacteria. An essential role of macrophages for the prevention of E. faecalis-translocation and the development of sepsis has been shown in an oral infection model using thermally injured mice [8]. In this study, mice depleted of polymorphonuclear neutrophils (PMN) were resistant to oral E. faecalis infection, whereas mice depleted of PMNs and macrophages were highly susceptible.
Little is known about the mechanisms by which enterococci induce cell death in eukaryotic cells. Analyzing cells of the innate and the adaptive immune system, E. faecalis has been previously shown to induce cell death in macrophages, PMNs, and lymphocytes [9–11], which suggests an immunosuppressive potential of E. faecalis.
The present study was performed to identify the underlying mechanisms of macrophage cell death induced by enterococci, in particular by E. faecium.
Materials and methods
Cultivation and infection of macrophages
J774A.1 macrophages (ATCC TIB67) were cultivated in RPMI 1640 medium (Biochrom, Berlin, Germany) supplemented with 10% (v/v) fetal calf serum (Sigma), 2 mM glutamine (Invitrogen, Karlsruhe, Germany), 50 μM 2-mercaptoethanol (Sigma), 1% (v/v) non-essential amino acids, 1 mM sodium pyruvate (both from Biochrom), and 100 U/ml penicillin and streptomycin (Invitrogen) in 94-mm-diameter tissue-culture dishes (Greiner Bio-One, Frickenhausen, Germany). For the infection experiments, cells were washed with HANK’s salt solution (Biochrom), detached by incubation with 1 ml accutase (PAA, Egelsbach, Germany) at 37°C for 15 min, washed with cell culture medium without antibiotics, and seeded at a density of 5 × 105 per well in uncoated 48-well cell culture plates (BD Falcon, Heidelberg, Germany).
Bacteria were grown overnight in 3–5 ml Brain Heart Infusion broth at 37°C while gently shaking, washed with PBS, and then the optical density was measured at 600 nm. Bacterial suspensions were diluted with PBS to a concentration of 3 × 108 bacteria/ml checked by plating serial dilutions and counting the colony forming units (CFU).
J774A.1 cells were infected with enterococci or S. aureus in a PBS volume of 33 μl at a multiplicity of infection (MOI) 2, 20, or 200. The Enterococcus and Staphylococcus strains used are listed in Table 1. Then, 2.5 h after infection, extracellular enterococci or staphylococci were killed by the addition of lysozyme (10 mg/ml) (from chicken egg white; Sigma, L6876) and lysostaphin (40 μg/ml) (Sigma, L0761), respectively, and cells were incubated further for the indicated time.
Table 1.
Enterococcus and Staphylococcus strains used in this study
| Strain/isolate | Description/reference |
|---|---|
| ATCC 6057 | E. faecium isolated from cheese |
| ATCC 29212 | E. faecalis isolated from urine |
| ATCC 14025 | E. avium isolated from human feces |
| ATCC 8043 | E. hirae isolated from intestine/gut |
| ATCC43076 | E. saccharolyticus |
| ST4014 | E. faecium isolated from stool, vancomycin-susceptible (colonized patient from our clinic) |
| S269esp + | Vancomycin-resistant E. faecium, patient’s isolate (outbreak 2005/06 at our clinic), positive for the esp (extracellular surface protein)-gene [39] |
| S261esp− | Vancomycin-resistant E. faecium, patient’s isolate (outbreak 2005/06 at our clinic), negative for the esp-gene [39] |
| ATCC 29213 | S. aureus isolated from wound |
As a control, silica beads (1 μm, Kisker-Biotech, Steinfurt, Germany), after washing three times with PBS, were added at MOI 20.
In order to inhibit caspase activation, cells were incubated for 1 h with the pancaspase inhibitor zVAD-fmk (100 μM) (Bachem, Heidelberg, Germany) prior to infection. For the inhibition of bacterial uptake, cells were incubated for 30 min with cytochalasin D (2 μg/ml) (Calbiochem, Darmstadt, Germany) prior to infection. To inhibit intracellular replication of bacteria, rifampicin (20 μg/ml) (Sigma) was added 2.5 h after infection.
In order to separately analyze bacterial pellets and supernatants for their cell death-inducing capacities, enterococci were treated with or without enzymes active towards different structures on the enterococcal surface prior to infection of macrophages. 1 × 108 viable E. faecium ATCC 6057 were resuspended in 30 μl PBS with or without the glycosidases lysozyme (10 mg/ml) or mutanolysin (400 U/ml) or with or without the endopeptidases lysostaphin (40 μg/ml) or achromopeptidase (4,000 U/ml) (all from Sigma) and incubated for 1 h at 37°C. Thereafter, bacterial suspensions were centrifuged (4,000 × g, 5 min), supernatants were discarded and 30 μl PBS with or without broad spectrum protease proteinase K (300 or 100 μg/ml), or phospholipase C (2 U/ml) (both from Sigma) were added. After incubation for 1 h at 37°C, bacterial suspensions were centrifuged (4,000 × g, 5 min), supernatants were discarded and, after resuspension in 30 μl PBS, pellets were added to 5 × 105 J774A.1 macrophages (MOI 200). Infected macrophages were centrifuged at 400 × g for 5 min and incubated for 6 h. After staining with PI, macrophages were analyzed by flow cytometry.
Heat-kill was performed by heating bacteria for 10 min at 95°C.
Assessment of cell death by flow cytometry
The inner mitochondrial transmembrane potential ΔΨm was determined by staining of cells with 40 nM tetramethyl-rhodamine ethyl ester (TMRE) (Molecular Probes, Leiden, Netherlands) for 15 min at 37°C in cell culture medium. Ten minutes prior to the analysis by flow cytometry (FACSCalibur, BD Biosciences, Heidelberg, Germany) using MDI version 2.8 software (J. Trotter, The Scripps Institute, La Jolla, CA, USA), 50 ng/ml propidium iodide (PI) (Calbiochem, Bad Soden, Germany) was added to analyze PI-uptake via disrupted cellular membranes.
Incubation of cells with staurosporine (4 μM) (Sigma) for 6 h to induce apoptosis or FCCP (Carbonyl cyanide 4-trifluoro-methoxy-phenylhydrazone, 100 μM) (Sigma) for 30 min to depolarize the inner mitochondrial transmembrane potential was used as control. For the induction of necrotic cell death, cells were heated for 10 min at 60°C.
Transmission electron microscopy
A total of 1 × 107 J774A.1 macrophages were seeded in uncoated six-well plates (BD Falcon), cautiously scraped off 6 h after infection and transferred into 1.5-ml tubes. After washing with warmed PBS, cells were fixed with warmed Karnovsky’s fixative for 10 min at 37°C and stored at 4°C. For electron microscopic analyses, the cell pellets were embedded in 3.5% agarose at 37°C, coagulated at room temperature, and fixed again in Karnovsky’s solution. Post-fixation was based on 1.0% osmium tetroxide containing 1.5% K-ferrocyanide in aqua bidest for 2 h. After following standard methods, blocks were embedded in glycide ether and cut using an ultra microtome (Ultracut, Reichert, Vienna, Austria). Ultra-thin sections (30 nm) were mounted on copper grids and analyzed using a Zeiss LIBRA 120 transmission electron microscope (Carl Zeiss, Oberkochen, Germany) operating at 80 kV.
Immunoblotting for caspase 3 and caspase 1
4 × 106 J774A.1 cells and bone marrow-derived macrophages, respectively, were seeded in six-well plates, cautiously scraped off at the indicated time points after infection, washed with PBS, and lysed by 40 μl buffer containing 50 mM Tris pH7.4, 50 mM NaCl, 1 mM EDTA, and 0.5% Nonidet-P40. After the addition of 10 μl 5 × Laemmli buffer [500 mM Tris–HCl pH 6.8, 1 M DTT, 50% (v/v) glycerol, 20% (v/v) SDS, 0.5% (v/v) bromophenol blue] to the lysates and heating at 95°C for 10 min, 9 μl each of the cell lysates were loaded, separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis, and electrotransferred to nitrocellulose membranes (Schleicher & Schüll, Dassel, Germany). After blocking for 2 h with 5% skim milk, the membranes were incubated overnight with anti-caspase 3 (8G10, 1:1000) (New England Biolabs, Frankfurt, Germany) or anti-caspase 1 antibodies (sc-514, 1:500) (Santa Cruz Biotechnology, Heidelberg, Germany). Immunoreactive bands were visualized by peroxidase-conjugated secondary antibodies (anti-rabbit, 1:1,000, 1 h, from Dako, Hamburg, Germany) using enhanced chemiluminescence reagents (ECL, Amersham Biosciences, Freiburg, Germany).
Membranes were stripped thereafter by washing steps with aqua bidest for 10 min, 50 mM NaOH for 15 min, and aqua bidest for 10 min, and blocked for 2 h with 5% skim milk prior to overnight incubation with antibodies to β-actin (1: 100,000, AC-15, Sigma). Anti-mouse peroxidase-conjugated secondary antibodies (1:1,000, 1 h) used were from Dako.
LDH-release assay
In uncoated 24-well plates, 2 × 106 J774A.1 cells were seeded in 1 ml cell culture medium without FCS and antibiotics and were infected for 6 h. Then, 950 μl cell culture medium was collected in 1.5-ml tubes, centrifuged, and 10 μl of the supernatant was analyzed for LDH content using the LDH-P mono kit (Biocon, Vöhl, Germany) according to the manufacturer’s instructions as described [12].
Bone marrow-derived macrophages (BMDM)
BMDM were obtained from C57BL/6x129 Sv, TLR2 −/−, TLR4 −/−, and TLR2 −/− × TLR4 −/− mice (all obtained from the C57BL/6x129Sv background and kindly provided by Carsten J. Kirschning) as described previously [13, 14]. Briefly, bone marrow-derived macrophages were generated by flushing cells from femurs and tibias of mice, seeding and cultivating them in 145-mm-diameter tissue-culture dishes (Greiner Bio-One) in 40 ml of DMEM (Invitrogen) supplemented with 10% FCS, 5% horse serum, 2 mM glutamine, 1 mM sodium pyruvate, and 20% of supernatants from L929 cells containing macrophage-colony stimulating factor. After 8 days, medium and non-adherent cells were withdrawn by suction, adherent macrophages were detached by addition of ice-cold PBS and incubation for 15 min on ice, and thereafter suspended in DMEM supplemented with 10% FCS, 2 mM glutamine, and 1 mM sodium pyruvate.
BMDM were characterized by double staining of 3 × 105 cells (in 100 μl PBS) with PE-conjugated antibodies to CD11b (1 μl; BD Pharmingen 553311, Heidelberg, Germany) and APC-conjugated antibodies to F4/80 (1 μl; serotec MCA 497APC, Düsseldorf, Germany) for 30 min on ice. After washing with PBS, cells were analyzed by flow cytometry showing a purity of >97% macrophages (CD11b- and F4/80-double-positive cells). Isotypes (rat IgG2b) used were from Pharmingen.
Mouse peritonitis experiments
Ten- to fourteen-week-old female C57BL/6x129Sv mice were injected intraperitoneally (i.p.) with 200 μl PBS or 1 × 108 CFU of E. faecium ATCC 6057 in 200 μl PBS. Two hundred microliters of lysozyme solution (10 mg/ml PBS) was administered 2.5 h later by a second i.p. injection. The mice were killed 24 and 48 h after infection and peritoneal lavages were performed with 10 ml ice-cold PBS each. Bloody lavages were excluded from further analyses.
In order to determine total leucocyte numbers and cell death rates from the peritoneal lavage samples, cells were centrifuged and resuspended in 350 μl PBS. Total leucocyte numbers (live and dead cells) were counted after diluting an aliquot of the peritoneal cell suspension with Trypan Blue solution (Sigma) using a hematocytometer. In order to perform cell death analyses, 100 μl each of the cell suspensions were centrifuged and resuspended in 500 μl RPMI 1640 medium supplemented with 10% (v/v) fetal calf serum (Sigma), 2 mM glutamine (Invitrogen), 50 μM 2-mercaptoethanol (Sigma), 1% (v/v) non-essential amino acids, and 1 mM sodium pyruvate (both from Biochrom) prior to triple staining of cells. The inner mitochondrial transmembrane potential was determined by staining with TMRE as described above. Thereafter, cells were centrifuged, resuspended in 100 μl PBS, and stained with APC-conjugated antibodies to F4/80 (1 μl per sample, serotec) for 30 min on ice. After washing with PBS, cells were resuspended in 400 μl PBS and propidium iodide was added as described above prior to flow cytometric analysis. By gating on F4/80+ cells, the macrophage subpopulation of each peritoneal fluid was analyzed for cell death and 5 × 104 F4/80+ cells per sample were analyzed for breakdown of the mitochondrial transmembrane potential (TMRE-negative cells) and leakage of cellular membranes (PI-positive cells).
Statistical analysis
The unpaired two-tailed Student’s t test was used to evaluate the difference in means between two groups. For the in vivo experiments, statistical analyses were performed using the analysis of variance one-way test (ANOVA) followed by the Bonferroni post-test using Graph Pad Prism version 4 Software (GraphPad Software, La Jolla, CA, USA).
P values were considered statistically significant if p < 0.05 (*), p < 0.01 (**), or p < 0.001 (***).
Results
Enterococci induce cell death in J774A.1 mouse macrophages
Previous studies revealed that macrophages underwent cell death when incubated with supernatants of hemolysin-producing E. faecalis strains or infected with viable E. faecalis [9, 10].
To analyze the cell death-inducing capacities of E. faecium in macrophages, J774A.1 cells were infected with E. faecium ATCC 6057 at different MOI (Fig. 1). Lysozyme was added to the cell culture after 2.5 h to kill extracellular enterococci and cells were incubated further for 3.5 h. Six hours after infection, cells were double-stained with TMRE and PI in order to analyze the inner mitochondrial transmembrane potential (ΔΨm) and the uptake of PI via disrupted cellular membranes by flow cytometry. As shown in Fig. 1a (bottom row) and Fig. 1b, E. faecium induced cell death in an MOI-dependent manner indicated by ΔΨm low/PI−, ΔΨm low/PI+, and ΔΨm high/PI+ cells, respectively. Even at low MOI 2 E. faecium induced cell death in 56.3% of the cells (Fig. 1b). At low MOI (2, 20), a substantial percentage of dead cells was ΔΨm high/PI+ (30.7 and 32.7%, respectively) indicating leakage of cellular membranes but intact mitochondrial transmembrane potential. By contrast, after infection with E. faecium at high MOI (200), cells with disrupted cell membranes and low inner mitochondrial transmembrane potential prevailed (87.3% ΔΨm low/PI+ cells). The efficacy of lysozyme (10 mg/ml) to kill E. faecium was 100% after incubation of 107 bacteria (equivalent to MOI 20 for the infection of 5 × 105 cells) for 2, 4, or 6 h in J774A.1 medium. This was determined by serial dilution, plating on agar plates, and counting of CFU (data not shown).
Fig. 1.
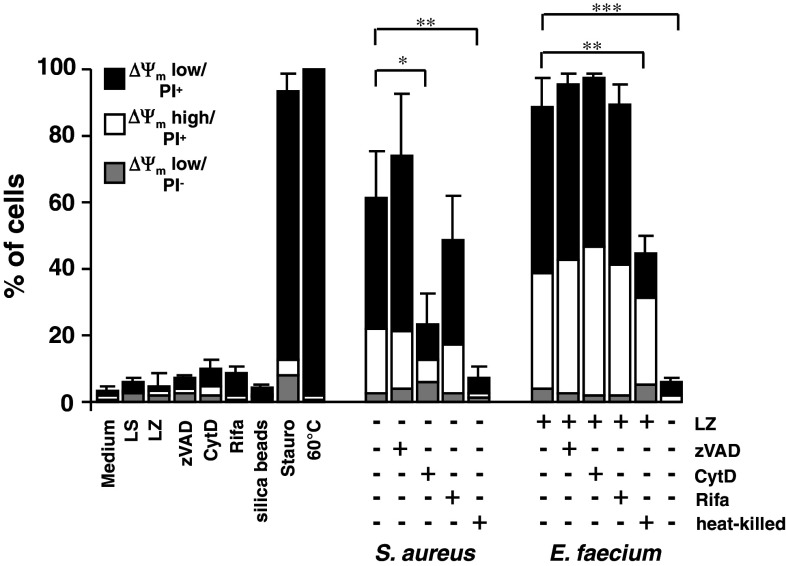
Cell death induction by enterococci in J774A.1 macrophages. J774A.1 macrophages were infected at different MOI with E. faecium ATCC 6057 and S. aureus ATCC 29213, respectively. Extracellular bacteria were killed 2.5 h after infection by the addition of lysozyme (10 mg/ml) or lysostaphin (40 μg/ml) and incubated further for 3.5 h. Six hours after infection, cells were stained with TMRE and propidium iodide (PI) and analyzed by flow cytometry. Lysostaphin (LS)-, lysozyme (LZ)-, FCCP (100 μM)-, staurosporine (Stauro, 4 μM)-treated cells, and cells heated for 10 min at 60°C were used as controls. Data shown in a are representative of three independent experiments and data shown in b are mean ± standard deviations of three independent experiments
As a control, cells were infected with S. aureus ATCC 29213, which is known to induce both necrotic and apoptotic cell death [15] (Fig. 1a, third row, and Fig. 1b). Extracellular S. aureus bacteria were killed by the addition of lysostaphin 2.5 h after infection as described [16]. Cells infected with S. aureus at MOI 20 or MOI 200 exhibited predominantly (late) apoptotic/necrotic features 6 h after infection indicated by 61.3 and 71.1% ΔΨm low/PI+ cells, respectively (Fig. 1b). By contrast, the percentages of ΔΨm high/PI+ cells were low after infection with S. aureus (max. 11.1% at MOI 20).
Addition of lysostaphin or lysozyme alone did not induce cell death in J774A.1 cells (Fig. 1a, upper row, and Fig. 1b), indicating that bacteria are essential for cell death induction.
Positive controls used were FCCP to cause break-down of mitochondrial transmembrane potential, staurosporine (4 μM) to induce (late) apoptotic cell death and cells heated at 60°C to induce necrotic cell death (Fig. 1a, second row, and Fig. 2b).
Fig. 2.
Mechanisms of cell death induced by E. faecium. J774A.1 macrophages were incubated with zVAD-fmk (zVAD, 100 μM), cytochalasin D (CytD, 2 μg/ml) for 1 h and then infected at MOI 20 with E. faecium ATCC 6057 and S. aureus ATCC 29213, respectively; 2.5 h after infection, bacteria were either not killed or killed by the addition of lysozyme (LZ) (10 mg/ml), rifampicin (Rifa, 20 μg/ml) or lysostaphin (LS) (40 μg/ml, all S. aureus conditions) and incubated further for 3.5 h. Cells were stained with TMRE and propidium iodide (PI) 6 h after infection and analyzed by flow cytometry. Uninfected, lysostaphin (LS)-, lysozyme (LZ)-, zVAD-, cytochalasin D-, rifampicin (20 μg/ml) and staurosporine (Stauro, 4 μM)-treated cells, cells heated for 10 min at 60°C, and cells incubated with silica beads (1 μm, MOI 20) to provide an antigen of similar size compared to that of enterococci were used as controls. Data shown are mean ± standard deviations of three independent experiments
When compared to E. faecium, other Enterococcus species (E. faecalis, E. avium, E. hirae, E. saccharolyticus, see Table 1) induced cell death in J774A.1 macrophages to a similar extent (data not shown) indicating that cell death induction by enterococci in the presence of lysozyme is independent of species.
Altogether, enterococci independent of species induced cell death in J774A.1 macrophages even at low MOI 2. Strikingly, macrophages with leaky cellular membranes, but still intact inner transmitochondrial potential, predominated at low MOI, suggesting a possible direct interaction of enterococci with cellular membranes. This was in contrast to S. aureus-infected cells exhibiting classical late apoptotic/necrotic features.
E. faecium-induced cell death is independent of caspase activation and intracellular replication of bacteria
S. aureus involves both caspase-dependent and caspase-independent pathways [17]. To analyze if caspase activation is involved in E. faecium-induced cell death, J774A.1 macrophages were preincubated for 1 h with the pancaspase inhibitor zVAD.fmk to inhibit caspase activation prior to infection with E. faecium and S. aureus, respectively. As shown in Fig. 2, zVAD.fmk did not prevent cell death, which indicates that classical apoptotic cell death mediated by activation of caspases is neither involved in E. faecium- nor in S. aureus-induced macrophage cell death.
Inhibition of bacterial uptake by preincubation of cells with cytochalasin D or inhibition of intracellular replication by addition of the intracellularly active antibiotic rifampicin 2.5 h after infection partially inhibited cell death induced by S. aureus, confirming the results of other groups [16, 17]. By contrast, cell death induced by E. faecium was neither affected by cytochalasin D nor by rifampicin used at a concentration tenfold higher than MIC. Hence, we conclude that neither uptake of bacteria nor intracellular replication of E. faecium contribute to cell death induction by enterococci.
However, the percentage of dead cells was reduced by half when cells were infected with heat-killed enterococci, and to control level when cells were infected with enterococci, not exposed to lysozyme. Infection of J774A.1 cells with different E. faecium strains (VRE/esp−, VRE/esp+, E. faecium clinical isolate from stool, see Table 1) revealed similar results (data not shown), indicating that E. faecium of different sources are capable of cell death induction and that the extracellular surface protein does not contribute to cell death induction by E. faecium.
Altogether, we conclude that the addition of lysozyme is necessary to facilitate cell death induction by E. faecium. We speculate that a cell wall component, which is uncovered only in the presence of lysozyme and is at least partially susceptible to heat, might be responsible for cell death induction by E. faecium. Accordingly, treatment of macrophages with lysozyme followed by infection in the absence of the enzyme did not cause cell death (data not shown).
Additionally, cell death induced by enterococci was characterized by damage of cellular membranes (prior to mitochondrial depolarization) and was not inhibited by inhibition of caspases, suggesting that enterococci induce necrotic cell death.
Cell death induced by E. faecium exhibits features of necrosis
To further characterize cell death induced by E. faecium in macrophages, immunoblotting for caspase 3 from E. faecium-infected J774A.1 cells was performed (Fig. 3a). Procaspase 3 is an effector caspase, which, upon pro-apoptotic stimuli, is cleaved by initiator caspases like caspase 8 (death receptor pathway) or caspase 9 (mitochondrial death pathway) thereby getting activated. As shown in Fig. 3a, the cleavage products (p17/p19) of procaspase 3 were detected in cells incubated with staurosporine for 2, 3, or 4 h indicating classical apoptosis. However, cleavage of procaspase 3 was not observed within 4 h after infection with E. faecium, indicating that E. faecium-induced cell death is not mediated by activation of caspases and therefore is different from classical apoptosis. Moreover, this result is in accordance with the experiments in Fig. 2, showing that the pancaspase inhibitor zVAD.fmk does not protect from dying. Pyroptosis, a caspase 1-dependent, non-apoptotic cell death pathway was excluded as well since processed caspase 1 was not detected by immunoblotting of E. faecium infected bone marrow-derived macrophages neither in the presence of nor in the absence of lysozyme (data not shown).
Fig. 3.

Characteristics of necrotic cell death in E. faecium-infected macrophages. J774A.1 macrophages were infected at MOI 20 with E. faecium ATCC 6057 if not otherwise depicted. At 2.5 h after infection, extracellular bacteria were either killed or not by the addition of lysozyme (LZ) (10 mg/ml) and then incubated further. a Lack of caspase 3 activation. At different time points after infection, cells were harvested, lysed, and immunoblotting for caspase 3 was performed from cell extracts. Data shown are representative of three independent experiments. Staurosporine (Stauro, 4 μM)-treated cells were used as a positive control. As a loading control, membranes were stripped and incubated with antibodies to β-actin. b Lack of cell shrinkage. The cell size was assessed by analysis of the mean forward scatter (FSC) at different time points after infection (upper panel). Staurosporine (Stauro, 4 μM)-treated cells and cells heated for 10 min at 60°C were used as controls. In parallel, the percentages of PI-positive cells were determined by flow cytometry (lower panel). Data shown are mean ± standard deviations of three independent experiments. c LDH release. At 6 h after infection with E. faecium ATCC 6057 at two different MOI cell culture supernatants were analyzed for their LDH content. All values (% control) are referred to untreated control cells (medium without lysozyme). As a positive control, cells heated for 10 min at 60°C were used. Data shown are mean ± standard deviations of three independent experiments performed in duplicates. d Transmission electron microscopic analysis. Electron microscopic pictures were taken from cells infected for 6 h with E. faecium ATCC 6057. Staurosporine (4 μM)-treated cells and cells heated for 10 min at 60°C were used as controls for apoptosis and necrosis, respectively. Bar, 2 μm each
It has been shown that necrotic cell death is characterized by an intracellular Na+ accumulation and concomitant cell swelling [18]. Concomitant efflux of K+ can slow down cell swelling of necrotic cells and is essential for cell shrinkage during apoptosis [19, 20]. Hence, as a measure for cell size, the mean forward scatter (FSC) was detected at several time points after infection with E. faecium. As shown in Fig. 3b, 2–4 h after infection of J774A.1 with E. faecium no substantial changes of the cell size (upper panel) were detected. However, coincidence of strong cell death induction within that time indicated by the percentage of PI-positive cells was observed (lower panel). This data suggest that E. faecium-infected cells undergo necrotic cell death characterized by large, not shrunk cells. As a positive control for necrosis, cells were heated for 10 min at 60°C. Cells treated in this manner exhibited cell sizes similar to that of cells incubated in medium (Fig. 3b, upper panel). By contrast, cells treated with staurosporine to induce apoptosis underwent continuous shrinking within 2–4 h, indicating classical apoptotic cell death.
A further characteristic of necrotic cell death is the release of cytosolic lactate dehydrogenase (LDH) via disrupted cellular membranes. Therefore, cells were infected with E. faecium for 6 h, and cell culture supernatants were analyzed for LDH. As shown in Fig. 3c, no LDH release was observed when cells were infected at MOI 20. However, the LDH-content of supernatants from cells infected with E. faecium at MOI 200 in the presence of lysozyme was 1.8-fold higher compared to cells infected with E. faecium at MOI 200 in the absence of lysozyme. This indicates that E. faecium, at least at high MOI 200, when treated with lysozyme, induces LDH release in J774A.1 macrophages. As a control, cells were heated for 10 min at 60°C to induce necrosis accompanied by strong LDH release (>sevenfold when compared to cells kept in medium). Compared to the results shown in Figs. 1, 2, and 3, demonstrating that E. faecium induces leakage of cellular membranes indicated by PI-positivity in most of the cells, this result might seemingly be contradictory. However, the molecular weight of LDH is about 170-fold greater than that of PI. Hence, if membrane damages caused by enterococci are relatively small, the differences in molecular size might explain why PI can enter the cells whereas LDH cannot be released from the cells to the same extent.
Finally, J774A.1 cells infected with E. faecium for 6 h were analyzed for morphological features typical for apoptotic and necrotic cell death, respectively (Fig. 3d). Treatment of cells with lysozyme alone did not affect cell morphology (first row). Cell shrinking, chromatin condensation, and nuclear fragmentation (second row) was observed in cells incubated with staurosporine for 6 h to induce apoptotic cell death. By contrast, necrotic cells heated for 10 min at 60°C exhibited vacuolized cytoplasm but no cell shrinking (second row). The morphology of J774A.1 cells infected with E. faecium for 6 h without addition of lysozyme was intact, exhibiting neither characteristics of apoptotic nor necrotic cell death. Additionally, bacteria with intact cell wall were detected intracellularly (in vacuoles) and extracellularly. By contrast, after infection with E. faecium in the presence of lysozyme, cells were characterized by a large cell size and vacuolized cytoplasm, indicating that cells underwent necrotic cell death.
An enterococcal (surface) protein is causative for necrosis induction
Our data indicate that the presence of lysozyme is necessary for cell death induction of macrophages by enterococci. Hence, we speculated whether lysozyme by hydrolysis of peptidoglycans could uncover a so far unknown cell death-inducing factor, either soluble or not, i.e., from the enterococcal cell wall. To verify this hypothesis, viable bacterial cells were incubated with PBS with or without the glycosidases lysozyme or mutanolysin or endopeptidases (staphylolysin, achromopeptidase) prior to infection of J774A.1 macrophages. Subsequently, bacteria were incubated in PBS with or without proteinase K at different concentrations or phospholipase C. Bacterial supernatants of E. faecium treated with lysozyme or mutanolysin alone were separated from pellets by centrifugation (4,000 × g, 5 min) and added to cell cultures. The remaining supernatants were discarded, pellets were resuspended in PBS, and added to cell cultures thereafter. J774A.1 macrophages were analyzed for cell death (PI-positivity) 6 h after addition of supernatants and pellets, respectively. As shown in Fig. 4 (white bars), bacterial pellets of E. faecium treated with lysozyme induced cell death in 31.7% of the macrophages, whereas infection of cells with bacteria incubated in PBS in the absence of lysozyme resulted in cell death of only 13.7% of the cells, confirming that lysozyme activates E. faecium to induce necrosis. Infection of macrophages with bacteria treated with mutanolysin also resulted in cell death induction by E. faecium (18.4% PI-positive cells). These results are consistent as the muramidases lysozyme and mutanolysin both cleave the N-acetylmuramyl-β(1-4)-N-acetylglucosamine linkages of the bacterial cell wall peptidoglycans. In contrast, the endopeptidases lysostaphin and achromopeptidase, which both cleave the cross-links of the peptidoglycan layer, did not trigger cell death induction by E. faecium (data not shown). When compared to macrophages that were directly infected with viable enterococci, the cell death-inducing effect of the bacterial pellets (+lysozyme/mutanolysin) was less pronounced. This was probably due to a loss of bacteria during washing steps and due to a lack of a bacterial replication when lysozyme- or mutanolysin-treated pellets added to cell cultures.
Fig. 4.
An enterococcal protein is causative for cell death induction by E. faecium. J774A.1 macrophages were infected at MOI 200 with E. faecium ATCC 6057. Prior to infection of macrophages, viable bacteria were preincubated with PBS with or without the glycosidases lysozyme (LZ, 10 mg/ml) or mutanolysin (Mut, 400 U/ml) for 1 h at 37°C. Thereafter, bacterial suspensions were centrifuged, supernatants were removed, and pellets were incubated in PBS with or without proteinase K (ProtK, 100 or 300 μg/ml) or phospholipase C (PhosphoC, 2 U/ml) for 1 h at 37°C. After centrifugation, supernatants were discarded, and pellets, after resuspension in PBS, were used for infection of macrophages for 6 h. After staining with PI, macrophages were analyzed by flow cytometry. Data shown are mean ± standard deviations of one experiment performed in triplicates representative of four independent experiments. P values result from the comparison with cell death rates of the corresponding PBS-treated controls after treatment with LZ and Mut, respectively
In summary, pretreatment of E. faecium with lysozyme or mutanolysin was necessary for cell death induction in J774A.1 macrophages by E. faecium. These data suggest that the muramidases by hydrolysis of peptidoglycans might uncover an enterococcal surface molecule (i.e., protein or lipid) causative for cell death induction. As shown in Fig. 4, E. faecium incubated with the glycosidases lysozyme or mutanolysin and subsequently with proteinase K, was no more capable of inducing cell death (black bars) even at low concentration of proteinase K. By contrast, subsequent treatment with phospholipase C (striped bars) did not reduce cell death. These results strongly suggest that an enterococcal (surface) protein is causative for cell death induction. Accordingly, supernatants of lysozyme- or mutanolysin-treated E. faecium were not capable of cell death induction (data not shown).
E. faecium induces cell death in mouse bone marrow-derived macrophages independently of TLR2 and TLR4 signaling
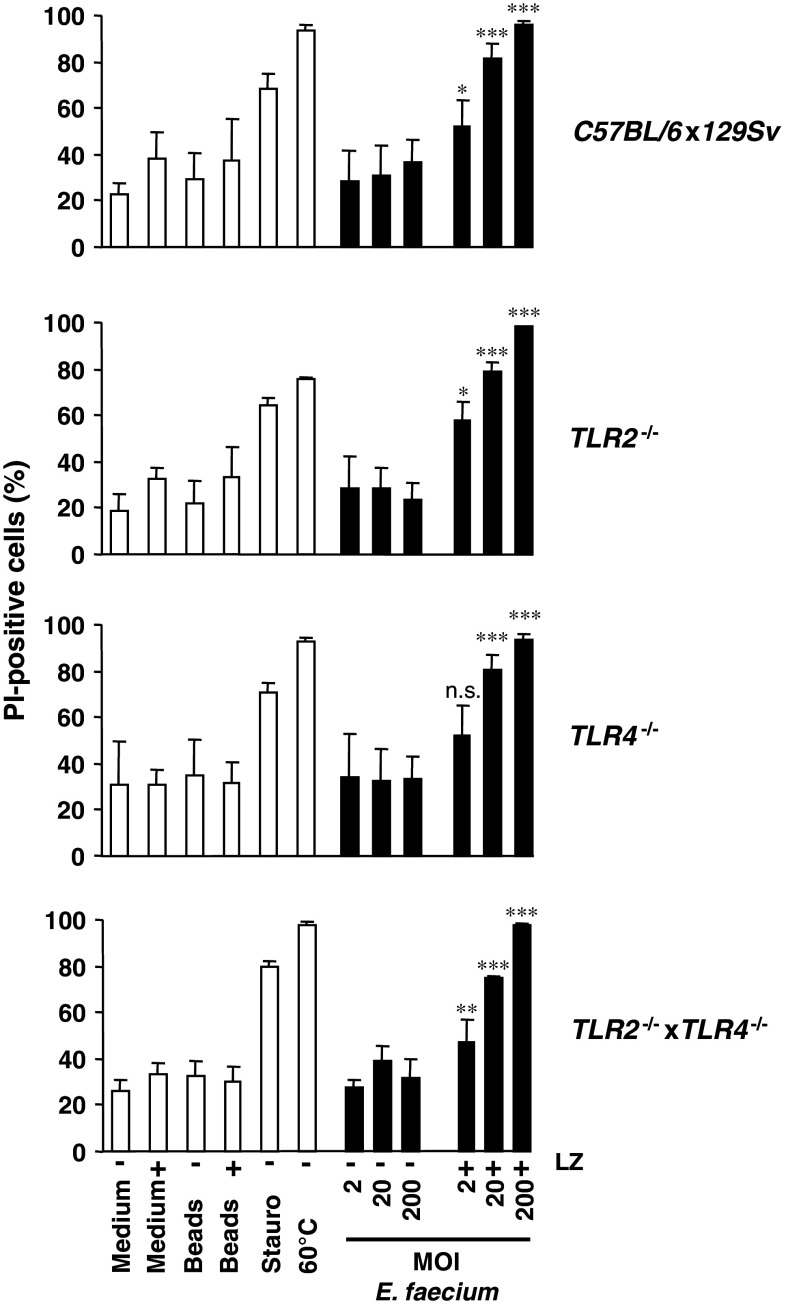
In order to determine whether E. faecium can induce necrosis in primary macrophages as well, bone marrow-derived macrophages (BMDM) from C57BL/6x129Sv mice were infected at different MOI for 6 h and PI-positive cells were assessed by flow cytometry (Fig. 5, upper panel). In the absence of lysozyme, no cell death induction by E. faecium was observed. However, in the presence of lysozyme, BMDM underwent cell death indicated by an increase of PI-positive cells dependent on MOI. These results are in accordance with the results obtained in J774A.1 macrophages and show that E. faecium induces cell death in a lysozyme-dependent manner in primary macrophages as well.
Fig. 5.
E. faecium induces cell death in bone marrow-derived macrophages (BMDM) independently of TLR2 and TLR4 signaling. BMDM obtained from C57BL/6x129Sv, TLR2−/−, TLR4−/−, and TLR2−/− × TLR4−/− mice, respectively, were infected at MOI 20 with E. faecium ATCC 6057. At 2.5 h after infection, extracellular bacteria were killed by the addition of lysozyme (LZ) (10 mg/ml) and incubated for another 3.5 h. At 6 h after infection, cells were stained with TMRE and propidium iodide (PI) and analyzed by flow cytometry. Lysozyme (LZ)-, staurosporine (Stauro, 4 μM)-treated cells, and cells heated for 10 min at 60°C were used as controls. Cells incubated with silica beads (Beads) (1 μm, MOI 20) with or without addition of lysozyme after 2.5 h were used as an additional negative control. Data shown are mean ± standard deviations of four independent experiments. P values result from the comparison of macrophages infected with E. faecium with or without addition of lysozyme
It has recently been shown in a mouse peritonitis model that TLR2 signaling via the TLR adaptor molecule MyD88 contributes to the defense against intraperitoneal infection with a vancomycin-resistant E. faecium strain [21]. In this model, when compared to wild-type mice, MyD88 and TLR2 knockout mice exhibited an impaired immune response to E. faecium peritonitis indicated by higher numbers of CFU in the peritoneum and liver and by an attenuated peritoneal influx of neutrophils 2 h after infection. Therefore, we speculated whether cell death induction of macrophages by E. faecium might also occur in a TLR2/MyD88-dependent manner and thereby contribute to overcome the infection, i.e., by inhibition of an overwhelming immune response. However, as shown in Fig. 5, in comparison to wild-type mice no substantial differences in cell death rates were observed when BMDM cultivated from TLR2 −/−, TLR4 −/−, and TLR2 −/− × TLR4 −/− mice were infected with E. faecium. These data indicate that E. faecium-induced cell death of BMDM is independent of TLR2 and TLR4 signaling. Therefore, cell death induction in macrophages is unlikely to contribute to the TLR2/MyD88-dependent immune response observed in vivo.
Cell death induction by E. faecium in vivo in peritoneal mouse macrophages
We finally analyzed whether E. faecium-induced macrophage cell death does not only occur in vitro but can be observed in vivo as well. E. faecium thereby might evade the immune response of the host, or, alternatively, macrophage cell death induction by enterococci might facilitate a balanced immune response in advantage of the host.
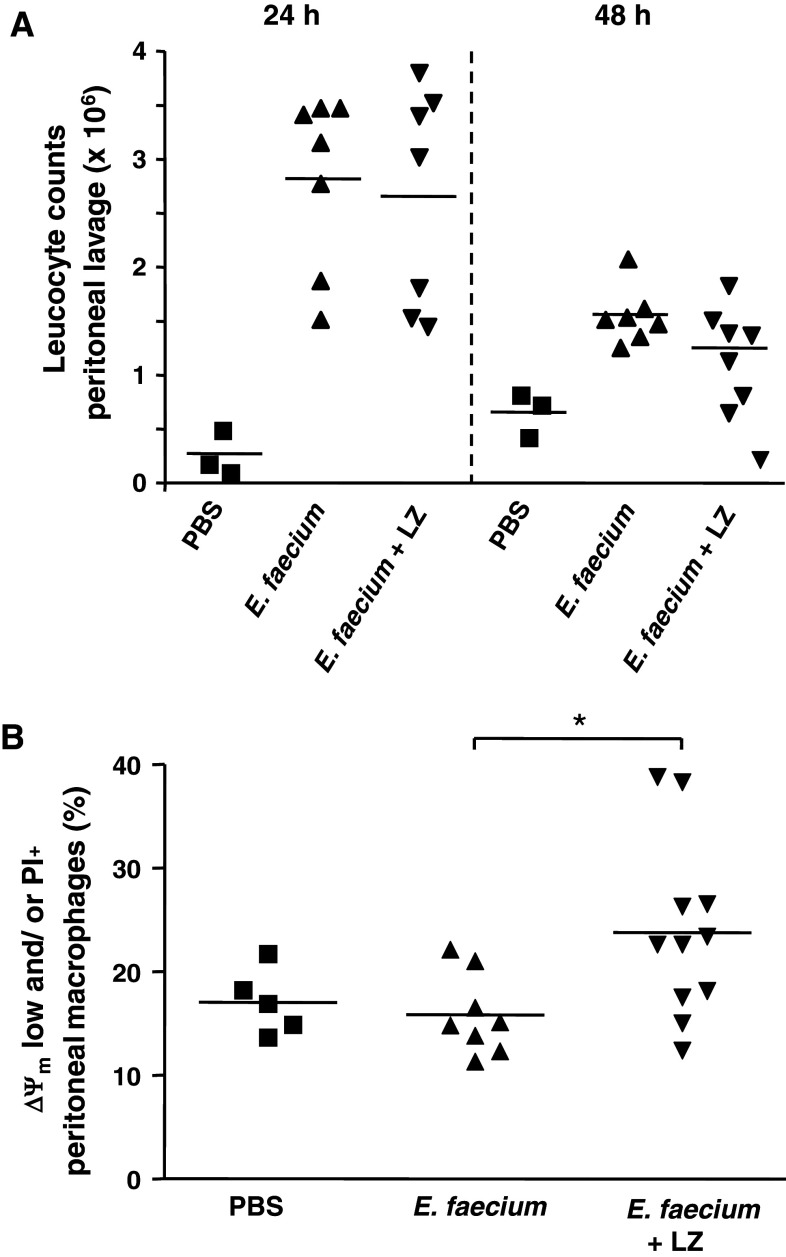
Mice were injected intraperitoneally with 1 × 108 E. faecium ATCC 6057 according to a recently published work reporting a 100% survival rate in mice [21]. Leucocyte counts of the peritoneal fluids were determined 24 h after infection, when mice exhibited symptoms of disease, and 48 h after infection, when mice clinically had overcome the infection. Additionally, the percentages of peritoneal leucocyte subsets and cell death rates of macrophages were determined by flow cytometric analyses 24 and 48 h after infection. According to the in vitro experiments, one group of mice was administered a second i.p. injection with lysozyme 2.5 h after infection with enterococci. Figure 6a shows that, compared to PBS injection, peritoneal leucocyte counts (live and dead cells) strongly increased 24 h and decreased 48 h after infection with E. faecium, reflecting the illness of the mice and confirming the results of a recently published work showing that peritoneal leucocyte counts reached a maximum at 24 h after infection [21]. No substantial differences in the leucocyte counts were observed between mice injected with E. faecium alone and mice injected with E. faecium and lysozyme (Fig. 6a), neither 24 nor 48 h after infection. By contrast, no influx of leucocytes was observed in mice injected with lysozyme alone (data not shown).
Fig. 6.
Cell death induction by E. faecium in peritoneal macrophages in vivo. C57BL/6x129Sv mice were injected intraperitoneally with 108 E. faecium ATCC 6057 or PBS. After 2.5 h, a second i.p. injection with lysozyme (LZ) or PBS was administered contralaterally. The mice were killed 24 and 48 h after infection, peritoneal lavages were performed, and leucocyte counts were determined using a hematocytometer (a). Additionally, 24 h after infection, peritoneal cells were triple stained with TMRE, propidium iodide (PI), and APC-conjugated antibodies to F4/80. F4/80+ macrophages were analyzed for depolarization of the inner mitochondrial transmembrane potential (ΔΨm low cells) and uptake of propidium iodide (PI) (b)
Differentiation of peritoneal leucocytes was performed by staining of several surface markers such as CD11b, F4/80, CD4, CD8, and CD19 and analysis by flow cytometry. The percentages of neutrophils (CD11b+/F4/80−) strongly decreased from 24 to 48 h after infection both in mice infected with E. faecium alone and in mice infected with E. faecium and treated with lysozyme thereafter (37.3 vs. 11.0% and 42.1 vs. 8.7%, respectively, data not shown). By contrast, the percentage of macrophages (F4/80+/CD11b−), and even more of lymphocytes (CD4+, CD8+, CD19+), increased from 24 h to 48 h after infection (macrophages: 30.3 vs. 42.9% and 31.6 vs. 41.6%, lymphocytes: 7.6 vs. 37.9% and 5.1 vs. 26.6%, data not shown) reflecting that mice had overcome the infection.
Analysis of cell death among peritoneal macrophages 24 h after i.p. infection (Fig. 6b) revealed a slight increase of cells which had lost mitochondrial transmembrane potential (ΔΨm low) and/or cell membrane integrity (PI-positive cells) (15.9 vs. 23.8%). To perform these analyses, peritoneal cells were triple stained (F4/80, TMRE, PI) and gated on F4/80-positive macrophages. Injection of lysozyme alone did not affect cell death rates after 24 or 48 h among peritoneal macrophages when compared to PBS injection (data not shown). Moreover, no differences in cell death rates were observed 48 h after infection with E. faecium between animals treated with bacteria alone and mice treated with bacteria and lysozyme.
Altogether, we observed augmented cell death rates among peritoneal macrophages 24 h after intraperitoneal infection with E. faecium and administration of lysozyme. Coincidentally, mice exhibited symptoms of disease and high peritoneal neutrophil counts. Therefore, we hypothesize that cell death of macrophages induced by E. faecium and lysozyme might contribute to the prevention of an overwhelming immune response thereby helping to clear the infection within 48 h accompanied by an intraperitoneal reduction of neutrophils and an increased mono-lymphocytic response.
Discussion
In the present study we demonstrated that enterococci of different species induce cell death in J774A.1 macrophages. Cells infected with E. faecium for 6 h were highly susceptible to cell death induction even at low multiplicity of infection (Fig. 1). Strikingly, the addition of lysozyme to the coculture of cells and bacteria was necessary to induce cell death by E. faecium (Fig. 2). In contrast to E. faecium, E. faecalis induces cell death both in a lysozyme-dependent and a lysozyme-independent manner since the percentages of propidium iodide (PI)-positive cells increased from 49.2 to 80.1% when lysozyme was added to J774A.1 macrophages infected with E. faecalis at MOI 20 for 6 h (data not shown). This is in accordance with a previous study which demonstrates that E. faecalis induces necrosis-like cell death in RAW264.7 macrophages without addition of lysozyme, indicated by about 35% eosin-positive cells 6 h after infection with E. faecalis at unknown MOI [10]. Production of cytolysin by E. faecalis might be causative for the lysozyme-independent cell death, since previous studies have demonstrated that supernatants of hemolysin-producing E. faecalis strains are capable of cell death induction in mouse macrophages [9]. However, our present study has shown that lysozyme activates enterococci to induce necrosis independently of the Enterococcus species used. This suggests that virulence factors, most of which are found in E. faecalis, such as cytolysin, aggregation substance, and gelatinase, play a minor role in the induction of cell death.
Our results indicate that E. faecium involves cell death processes different from apoptosis as we observed both lack of procaspase 3 activation and cell shrinkage of infected macrophages. In addition, inflammasome-mediated cell death processes were excluded since cell death induction by E. faecium was independent of caspase-1 activation (data not shown). Necrosis was confirmed by electron microscopic analyses and release of lactate dehydrogenase by macrophages infected with E. faecium.
Hence, mechanisms of cell death induction by E. faecium differ from cell death induced by other Gram-positive bacteria such as S. aureus and streptococci. These bacteria have been described to activate classical apoptotic pathways [22–24]. Additionally, S. aureus-induced cell death exhibits features of necrosis which are dependent on bacterial uptake and intracellular replication [15, 16]. In contrast, necrosis induced by E. faecium was independent of bacterial uptake and intracellular replication as shown by preincubation of macrophages with cytochalasin D and rifampicin, respectively (Fig. 2).
The present study has shown that the presence of lysozyme is necessary for the induction of necrosis by E. faecium in J774A.1 macrophages. Lysozyme as an important enzyme of the innate immune system was found in biological fluids such as saliva and respiratory secretions, tears, and mucus [25]. Moreover, it is one of the major secretory products of macrophages and it is present in cytoplasmic granules of PMNs [26, 27]. Lysozyme concentrations in saliva from healthy persons vary from 2.2 to 79.9 μg/ml dependent on the different assay conditions used for measurement [28]. Under pathological conditions, lysozyme concentrations and leucocyte counts in serum increased simultaneously as shown by a rabbit pneumococcal endocarditis model [29]. Lysis of peptidoglycans by lysozyme results in the release of MurNAc (N-acetylmuramic acid) and N-acetylglucosamine (GlcNAc) from the bacterial cell wall. In Gram-positive bacteria such as staphylococci and enterococci, the lytic response to lysozyme is limited due to ***O-acetylation of peptidoglycans conferring resistance to lysozyme [30, 31]. E. faecalis is resistant to lysozyme even at high concentrations (40 mg/ml) [32]. The higher the extent of O-acetylated peptidoglycans, the higher the resistance to lysozyme and the production of large peptidoglycan fragments [31]. Although more active than hen egg lysozyme, even rat and human lysozyme do not efficiently hydrolyze O-acetylated peptidoglycans [31]. Lysozyme due to its cationic and hydrophobic properties can also kill bacteria by membrane perturbation [33]. Additionally, the adherence of enterococci to host cells might be enhanced in the presence of lysozyme due to its cationic and hydrophobic properties. By microscopical analyses we have observed that immediately after addition of lysozyme to cocultures of macrophages and E. faecium, bacteria disappear from the space between cells, suggesting that they have bound to macrophages (data not shown). Addition of lysozyme thereby seems to increase bacterial adherence, which possibly contributes to the initiation of cell death induction by E. faecium in macrophages. Accordingly, when macrophages were infected at low MOI 2, predominantly cells with leaky cellular membranes but still intact mitochondrial transmembrane potential were observed, suggesting a direct interaction of enterococci with cellular membranes.
Our data suggest that lysozyme by hydrolysis of peptidoglycans might uncover a non-soluble (cell wall) component of E. faecium causative for cell death induction. This hypothesis was confirmed by the fact that antibiotics acting intracellularly by inhibition of bacterial protein synthesis did not enable enterococci to induce cell death (data not shown). Hypothetically, cell wall components of E. faecium such as lipoteichoic acids or proteins might be causative for the induction of cell death. Experiments with heat-killed E. faecium (Fig. 2) point to enterococcal proteins as possible components causative for cell death induction, because the percentage of cell death was reduced by about half when macrophages were infected by heat-killed bacteria compared to viable bacteria. Pretreatment of E. faecium with lysozyme or mutanolysin and subsequently with broad spectrum protease considerably reduced cell death (Fig. 4), strongly suggesting that an enterococcal surface protein is responsible for cell death induction. Further studies will have to identify the protein causative for cell death induction. Possible candidates are the three cell wall-anchored LPXTG surface proteins of E. faecium whereas the enterococcal surface protein (Esp) seems not to play a causative role for cell death induction.
In a nonlethal E. faecium peritonitis model it has been shown that signaling via TLR2 and MyD88 contribute to the attraction of neutrophils to the peritoneal cavity and an early enterococcal clearance [21]. Peritoneal macrophages might account for the attraction of neutrophils since they exhibited a TLR2- and MyD88-dependent production of proinflammatory TNF-α upon infection with E. faecium in vitro [21]. However, our data have shown that neither TLR2 nor TLR4 signaling are involved in macrophage cell death induced by E. faecium in vitro (Fig. 5).
Recently, an essential role of neutrophils for rapid clearance of E. faecium has been demonstrated in mice. Mice depleted of neutrophils before intraperitoneal E. faecium challenge exhibited a severe delay in enterococcal clearance from different organs [34]. Eventually, even in neutropenic mice bacteria were cleared, suggesting that other components of the immune system can compensate the lack of neutrophils. For example, macrophages play a crucial role in host defense against intraperitoneal infection with E. faecium, since mice depleted of peritoneal macrophages exhibited a delay in peritoneal and systemic E. faecium clearance [35]. Of note, in mouse peritonitis models the attraction of macrophages into the peritoneal cavity was diminished despite a greater enterococcal burden [35, 36]. Cell death of peritoneal macrophages caused by enterococci as demonstrated in the present study might be a possible underlying mechanism of this phenomenon. This hypothesis is supported by the fact that survival of mice was enhanced by pretreatment of mice with antiapoptotic protease inhibitors in a mouse peritonitis-sepsis model [37]. In our study, cell death of peritoneal macrophages in mice was observed only 24 h after intraperitoneal infection with E. faecium (Fig. 6b), when mice exhibited symptoms of disease, but not 48 h (data not shown) when mice had overcome their illness. Possibly, cell death of peritoneal macrophages accounts for downregulation of an excessive immune response to E. faecium peritonitis. In peritonitis models, lysozyme from the intestine or produced by peritoneal macrophages [26] might contribute to cell death induction. This is supported by our findings that cell death induction by E. faecium in vivo was dependent on the intraperitoneal application of lysozyme (Fig. 6b).
Cell death induction by enterococci might be a general mechanism to enable these bacteria to traverse natural barriers such as i.e., intestinal or vesical epithelia. For example, it has been described that infection of human urothelial cells with an E. faecalis clinical isolate in a three-dimensional culture system largely caused cell death [38]. Further studies will be necessary to address the role of enterococci for cell death induction in epithelia.
Acknowledgments
The authors thank Birgit Fehrenbacher from the Department of Dermatology and Bettina Hackl from our institute for excellent technical assistance. Additionally, we thank Christopher Weidenmaier from our institute for helpful discussion.
References
- 1.Jett BD, Huycke MM, Gilmore MS. Virulence of enterococci. Clin Microbiol Rev. 1994;7:462–478. doi: 10.1128/cmr.7.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray BE. The life and times of the Enterococcus . Clin Microbiol Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koch S, Hufnagel M, Theilacker C, Huebner J. Enterococcal infections: host response, therapeutic, and prophylactic possibilities. Vaccine. 2004;22:822–830. doi: 10.1016/j.vaccine.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee I, Iredell JR, Woods M, Lipman J. The implications of enterococci for the intensive care unit. Crit Care Resusc. 2007;9:69–75. [PubMed] [Google Scholar]
- 5.Emori TG, Gaynes RP. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev. 1993;6:428–442. doi: 10.1128/cmr.6.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiazGranados CA, Zimmer SM, Klein M, Jernigan JA. Comparison of mortality associated with vancomycin-resistant and vancomycin-susceptible enterococcal bloodstream infections: a meta-analysis. Clin Infect Dis. 2005;41:327–333. doi: 10.1086/430909. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Garbajosa P, Canton R, Pintado V, Coque TM, Willems R, Baquero F, del Campo R. Genetic and phenotypic differences among Enterococcus faecalis clones from intestinal colonisation and invasive disease. Clin Microbiol Infect. 2006;12:1193–1198. doi: 10.1111/j.1469-0691.2006.01533.x. [DOI] [PubMed] [Google Scholar]
- 8.Tsuda Y, Shigematsu K, Kobayashi M, Herndon DN, Suzuki F. Role of polymorphonuclear neutrophils on infectious complications stemming from Enterococcus faecalis oral infection in thermally injured mice. J Immunol. 2008;180:4133–4138. doi: 10.4049/jimmunol.180.6.4133. [DOI] [PubMed] [Google Scholar]
- 9.Miyazaki S, Ohno A, Kobayashi I, Uji T, Yamaguchi K, Goto S. Cytotoxic effect of hemolytic culture supernatant from Enterococcus faecalis on mouse polymorphonuclear neutrophils and macrophages. Microbiol Immunol. 1993;37:265–270. doi: 10.1111/j.1348-0421.1993.tb03209.x. [DOI] [PubMed] [Google Scholar]
- 10.Kirschnek S, Scheffel J, Heinzmann U, Hacker G. Necrosis-like cell death induced by bacteria in mouse macrophages. Eur J Immunol. 2004;34:1461–1471. doi: 10.1002/eji.200324582. [DOI] [PubMed] [Google Scholar]
- 11.Lee W, Lim S, Son HH, Bae KS. Sonicated extract of Enterococcus faecalis induces irreversible cell cycle arrest in phytohemagglutinin-activated human lymphocytes. J Endod. 2004;30:209–212. doi: 10.1097/00004770-200404000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Armeanu S, Krusch M, Baltz KM, Weiss TS, Smirnow I, Steinle A, Lauer UM, Bitzer M, Salih HR. Direct and natural killer cell-mediated antitumor effects of low-dose bortezomib in hepatocellular carcinoma. Clin Cancer Res. 2008;14:3520–3528. doi: 10.1158/1078-0432.CCR-07-4744. [DOI] [PubMed] [Google Scholar]
- 13.Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD, King KL, Sheehan KC, Yin L, Pennica D, Johnson EM, Jr, Schreiber RD. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93:373–383. doi: 10.1016/S0092-8674(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 14.Celada A, Gray PW, Rinderknecht E, Schreiber RD. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J Exp Med. 1984;160:55–74. doi: 10.1084/jem.160.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Essmann F, Bantel H, Totzke G, Engels IH, Sinha B, Schulze-Osthoff K, Janicke RU. Staphylococcus aureus alpha-toxin-induced cell death: predominant necrosis despite apoptotic caspase activation. Cell Death Differ. 2003;10:1260–1272. doi: 10.1038/sj.cdd.4401301. [DOI] [PubMed] [Google Scholar]
- 16.Krut O, Sommer H, Kronke M. Antibiotic-induced persistence of cytotoxic Staphylococcus aureus in non-phagocytic cells. J Antimicrob Chemother. 2004;53:167–173. doi: 10.1093/jac/dkh076. [DOI] [PubMed] [Google Scholar]
- 17.Haslinger-Loffler B, Kahl BC, Grundmeier M, Strangfeld K, Wagner B, Fischer U, Cheung AL, Peters G, Schulze-Osthoff K, Sinha B. Multiple virulence factors are required for Staphylococcus aureus-induced apoptosis in endothelial cells. Cell Microbiol. 2005;7:1087–1097. doi: 10.1111/j.1462-5822.2005.00533.x. [DOI] [PubMed] [Google Scholar]
- 18.Barros LF, Hermosilla T, Castro J. Necrotic volume increase and the early physiology of necrosis. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:401–409. doi: 10.1016/S1095-6433(01)00438-X. [DOI] [PubMed] [Google Scholar]
- 19.Bortner CD, Hughes FM, Jr, Cidlowski JA. A primary role for K+ and Na+ efflux in the activation of apoptosis. J Biol Chem. 1997;272:32436–32442. doi: 10.1074/jbc.272.51.32436. [DOI] [PubMed] [Google Scholar]
- 20.Carini R, Autelli R, Bellomo G, Albano E. Alterations of cell volume regulation in the development of hepatocyte necrosis. Exp Cell Res. 1999;248:280–293. doi: 10.1006/excr.1999.4408. [DOI] [PubMed] [Google Scholar]
- 21.Leendertse M, Willems RJ, Giebelen IA, van den Pangaart PS, Wiersinga WJ, de Vos AF, Florquin S, Bonten MJ, van der Poll T. TLR2-dependent MyD88 signaling contributes to early host defense in murine Enterococcus faecium peritonitis. J Immunol. 2008;180:4865–4874. doi: 10.4049/jimmunol.180.7.4865. [DOI] [PubMed] [Google Scholar]
- 22.Bantel H, Sinha B, Domschke W, Peters G, Schulze-Osthoff K, Janicke RU. alpha-Toxin is a mediator of Staphylococcus aureus-induced cell death and activates caspases via the intrinsic death pathway independently of death receptor signaling. J Cell Biol. 2001;155:637–648. doi: 10.1083/jcb.200105081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timmer AM, Timmer JC, Pence MA, Hsu LC, Ghochani M, Frey TG, Karin M, Salvesen GS, Nizet V. Streptolysin O promotes group A Streptococcus immune evasion by accelerated macrophage apoptosis. J Biol Chem. 2009;284:862–871. doi: 10.1074/jbc.M804632200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genestier AL, Michallet MC, Prevost G, Bellot G, Chalabreysse L, Peyrol S, Thivolet F, Etienne J, Lina G, Vallette FM, Vandenesch F, Genestier L. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J Clin Invest. 2005;115:3117–3127. doi: 10.1172/JCI22684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jolles P, Jolles J. What’s new in lysozyme research? Always a model system, today as yesterday. Mol Cell Biochem. 1984;63:165–189. doi: 10.1007/BF00285225. [DOI] [PubMed] [Google Scholar]
- 26.Gordon S, Todd J, Cohn ZA. In vitro synthesis and secretion of lysozyme by mononuclear phagocytes. J Exp Med. 1974;139:1228–1248. doi: 10.1084/jem.139.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keshav S, Chung P, Milon G, Gordon S. Lysozyme is an inducible marker of macrophage activation in murine tissues as demonstrated by in situ hybridization. J Exp Med. 1991;174:1049–1058. doi: 10.1084/jem.174.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenzano JW, Hogan SL, Lundblad RL. Factors influencing measurement of human salivary lysozyme in lysoplate and turbidimetric assays. J Clin Microbiol. 1986;24:963–967. doi: 10.1128/jcm.24.6.963-967.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fidelman ES, Averyanova LL. Changes in the blood lysozyme concentration in animals following injections of Streptococcus and of homologous tissue antigen. Bull Exp Biol Med. 1964;58:39–41. [PubMed] [Google Scholar]
- 30.Herbert S, Bera A, Nerz C, Kraus D, Peschel A, Goerke C, Meehl M, Cheung A, Gotz F. Molecular basis of resistance to muramidase and cationic antimicrobial peptide activity of lysozyme in staphylococci. PLoS Pathog. 2007;3:e102. doi: 10.1371/journal.ppat.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfeffer JM, Strating H, Weadge JT, Clarke AJ. Peptidoglycan O acetylation and autolysin profile of Enterococcus faecalis in the viable but nonculturable state. J Bacteriol. 2006;188:902–908. doi: 10.1128/JB.188.3.902-908.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hebert L, Courtin P, Torelli R, Sanguinetti M, Chapot-Chartier MP, Auffray Y, Benachour A. Enterococcus faecalis constitutes an unusual bacterial model in lysozyme resistance. Infect Immun. 2007;75:5390–5398. doi: 10.1128/IAI.00571-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibrahim HR, Aoki T, Pellegrini A. Strategies for new antimicrobial proteins and peptides: lysozyme and aprotinin as model molecules. Curr Pharm Des. 2002;8:671–693. doi: 10.2174/1381612023395349. [DOI] [PubMed] [Google Scholar]
- 34.Leendertse M, Willems RJ, Giebelen IA, Roelofs JJ, Bonten MJ, van der Poll T. Neutrophils are essential for rapid clearance of Enterococcus faecium in mice. Infect Immun. 2009;77:485–491. doi: 10.1128/IAI.00863-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leendertse M, Willems RJ, Giebelen IA, Roelofs JJ, van Rooijen N, Bonten MJ, van der Poll T. Peritoneal macrophages are important for the early containment of Enterococcus faecium peritonitis in mice. Innate Immun. 2009;15:3–12. doi: 10.1177/1753425908100238. [DOI] [PubMed] [Google Scholar]
- 36.LaFleur AM, Lukacs NW, Kunkel SL, Matsukawa A. Role of CC chemokine CCL6/C10 as a monocyte chemoattractant in a murine acute peritonitis. Mediators Inflamm. 2004;13:349–355. doi: 10.1080/09629350400014172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weaver JG, Rouse MS, Steckelberg JM, Badley AD. Improved survival in experimental sepsis with an orally administered inhibitor of apoptosis. FASEB J. 2004;18:1185–1191. doi: 10.1096/fj.03-1230com. [DOI] [PubMed] [Google Scholar]
- 38.Dozmorov MG, Kyker KD, Saban R, Shankar N, Baghdayan AS, Centola MB, Hurst RE. Systems biology approach for mapping the response of human urothelial cells to infection by Enterococcus faecalis . BMC Bioinformatics. 2007;8(Suppl 7):S2. doi: 10.1186/1471-2105-8-S7-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borgmann S, Schulte B, Wolz C, Gruber H, Werner G, Goerke C, Klare I, Beyser K, Heeg P, Autenrieth IB. Discrimination between epidemic and non-epidemic glycopeptide-resistant E. faecium in a post-outbreak situation. J Hosp Infect. 2007;67:49–55. doi: 10.1016/j.jhin.2007.06.002. [DOI] [PubMed] [Google Scholar]