Abstract
Here, we report the DNA sequence of the rhodopsin gene in the alga Cyanophora paradoxa (Glaucophyta). The primers were designed according to the conserved regions of prokaryotic and eukaryotic rhodopsin-like proteins deposited in the GenBank. The sequence consists of 1,272 bp comprised of 5 introns. The correspondent protein, named Cyanophopsin, showed high identity to rhodopsin-like proteins of Archea, Bacteria, Fungi, and Algae. At the N-terminal, the protein is characterized by a region with no transmembrane α-helices (80 aa), followed by a region with 7α-helices (219 aa) and a shorter 35-aa C-terminal region. The DNA sequence of the N-terminal region was expressed in E. coli and the recombinant purified peptide was used as antigen in hens to obtain polyclonal antibodies. Indirect immunofluorescence in C. paradoxa cells showed a marked labeling of the muroplast (aka cyanelle) membrane.
Keywords: Rhodopsin-like protein, Cyanophora paradoxa, Muroplast, Cyanelle, Antibody labeling
Introduction
Glaucophyta are single-cell freshwater algae that became important in the past few years as model systems to study plastid evolution. They are considered as living fossils [1] because their photosynthetic organelles retained a peptidoglycan (murein) wall, a clear indication of their origin from cyanobacterial endosymbionts. These organelles are termed muroplasts [2, 3] and are the only members of the so-called “glaucophyte” lineage of plastids [4]. They are bound by a double membrane with a vestige of a peptidoglycan prokaryotic cell wall between the two membranes. Internally, muroplasts have unstacked, concentric thylakoid membranes bearing many phycobilisomes and contain a carboxysome [5]; all of these characters, together with the sensitivity of muroplasts to lysozyme, are very cyanobacterial and mark the muroplasts as being the most primitive of all plastids [1].
One of the best known and most well-studied genera (and species) in this group is Cyanophora paradoxa Korschikov, for which considerable information exists on its structure, biochemistry, and molecular biology. This oval-shaped alga possesses two flagella inserted subapically, one directed anteriorly and the other posteriorly [6]. The cytoplasm contains mitochondria with flattened cristae and starch grains [7]. One or two typical muroplasts surrounded by a thin cell wall remnant are present inside the cells [8]. The size of the muroplast genome of C. paradoxa is 135 kbp [9], which is in the same range of genome size of other algal or land plant chloroplast genomes and less than 1/20th of cyanobacterial genomes [10]. Thus, muroplasts in fact represent the plastids of Cyanophora, and are not a cyanobacterial symbiont anymore.
Despite the numerous investigations on this glaucophyte, especially focused upon its phylogenetic role and position, almost no data exist on the presence and localization of photoreceptive proteins in C. paradoxa. Old data [11] hypothesized a photoreceptive role for the phycobiliproteins located in muroplast phycobilisomes. Over the past 10 years, genome sequencing and sequence comparison tools have revealed that genes encoding rhodopsin-like photoreceptive proteins are shared among distant taxa in all three domains of life: Archaea, Eubacteria, and Eukarya [12]. As far as algae are concerned, the earlier predictions based on photobiology and biochemistry are in many cases corroborated by contemporary genomic data on Type I rhodopsin sequences in different taxa [13, 14]. Spectroscopical and biochemical evidence of rhodopsin-based photoreceptor is available for algae belonging to Euglenophyta [15–17] and Heterokontophyta [18, 19]. Genes encoding functional protein probably related to photoreception have been detected both in prokaryotic (Cyanophyta) [20] and eukaryotic genera (Dinophyta, Cryptophyta, Chlorophyta) [21–24], though examples of transporters (proton or chloride transport) are also present [25].
These reports provide examples of the broad distribution of type I rhodopsin-based photoreceptors among different algal division and allow us to assume that these proteins are also present in Glaucophyta. The peculiar nature of Cyanophora, one of the three eukaryotic descendants, which have acquired photosynthesis by primary endosymbiosis, gave us the possibility of also investigating whether the photoreceptive proteins belong to the host or to the symbiont. We report here the full sequence of a rhodopsin gene in the alga C. paradoxa (Glaucophyta), together with an analysis of the structure of the gene, and the intracellular immunolocalization of the protein.
Materials and methods
Algal cultures
Cyanophora paradoxa Korshikov strain 29.80 M was obtained from the Culture Collection of Algae of the University of Gottingen (SAG, Sammlung von Algenkulturen der Universität Göttingen, Germany). Cells were grown in medium CY-II [6] at 23°C under continuous light (15 μmol photons m−2 s−1).
Muroplast preparation
For muroplast isolation, cells harvested by centrifugation at 2,000g for 5 min were washed once in HEMS buffer (50 mM HEPES–NaOH, pH 7.5, 2 mM EGTA, 1 mM MgCl2, 0.5 M sucrose), and suspended in the same buffer. Harvested cells were disrupted osmotically by dilution of the suspension with HEM buffer (50 mM HEPES–NaOH, pH 7.5, 2 mM EGTA, 1 mM MgCl2), and cell fragments and muroplasts collected by centrifugation at 2,500g for 5 min at 4°C. The pellet was suspended in HEMS buffer, placed on the same medium containing 40% Percoll, and centrifuged at 10,000g for 10 min. The intact muroplasts were recovered in the precipitate. Isolated muroplasts were incubated with 300 μg ml−1 lysozyme for 30 min at room temperature in the dark resulting in peptidoglycan wall digestion. The sample was then sonicated for 2 min, pelletted at 30,000g for 20 min at 4°C, and processed for SDS-PAGE according to Laemnly [26].
Preparation of genomic DNA and cDNA
Cyanophora cells were harvested by centrifugation at 4,000g for 5 min at 4°C; genomic DNA and total RNA were extracted from 100 mg wet weight of pellet using TriPure Isolation Reagent (Roche, USA). cDNA was synthesized from oligo (dT)12–18 primers (Invitrogen, USA) and 2 μg of total RNA in reverse transcription PCR, using SuperScriptTM II RNase H-RT (Invitrogen) according to the manufacturer’s suggested protocol.
cDNA cloning and sequencing
cDNA was subjected to PCR amplification, using degenerate oligonucleotides based on homologous regions of microbial opsins (according to the conserved regions of rhodopsin like proteins of Archea, Bacteria, Fungi, and Algae in the GenBank) (Fw_Helix-C:5′-TACGCVCGHTACRTYGAYTGG-3; Rev_Helix-G:5′-AWGAKCCAGAYRATBGGRTA-3′). The amplified product, a 332-bp band, was purified using NucleoSpin extract kit (Macherey-Nagel, Germany) and cloned in the pGem®-T Vector System I (Promega, Madison, WI, USA). The inserts were sequenced by MWG Biotech (Germany). Based on the obtained sequences, gene-specific primers were designed and used in combination with the anchor primers at the 3′- and 5′-terminus in 3′- and 5′-RACE-reactions. The PCR conditions were: 15 min at 95°C, 35 cycles of 1 min at 94°C, 1 min at 55°C, 1 min at 72°C. Finally, 3′ and 5′ RACE products were cloned and sequenced as described above.
Amplification of genomic DNA
Gene-specific primers (Fw:5′-CATATGTCCCCCACCTTCGCCCGGTGG-3′; Rev:5′-GAATTCCTACGCCTTGGAGAGCACACC-3′) were used for amplification of rhodopsin-coding gene from genomic DNA. A 1,272-bp product was produced, which was cloned into the pGem-T vector sistem I (Promega) and sequenced from both sides using T7 and Sp6 primers (MWG Biotech).
Plasmid construction pCPR
A 240-bp fragment at the N-terminal side of cDNA sequence was chosen to construct a plasmid for antigen production. The PCR amplification was performed with primers Fw:5′-CATATGTCCCCCACCTTCGCCCGGTGG-3′ introducing an NdeI restriction site; and Rev:5′-GAATTCCTAGCCGCGCTCGGCGGCGTC-3′ introducing an EcoRI restriction site. The amplified DNA fragment was digested with NdeI and EcoRI and cloned into pET5b, yielding pCPR.
Expression and purification of recombinant peptide
E. coli BL21(DE3) harboring pCPR was grown at 37°C in LB medium supplemented with ampicillin (100 μg/ml). When the absorbance at 600 nm of the cultures reached 0.7, isopropyl-β-d-thiogalactopyranoside (IPTG) (Promega) was added to a final concentration of 0.4 mM for induction of the gene expression. After an additional incubation of 2 h, cells were harvested, sonicated on ice and centrifuged at 12,000g for 20 min at 4°C. The precipitate was collected and washed three times with Tris–HCl 50 mM, pH 7.4. The peptide was purified by gel filtration on Superose 12 (GE Healthcare, UK). Five fractions were collected and pooled.
Immunization of hens
The generation of chicken egg yolk antibodies (IgY) was performed according to the method of Song et al. [27]. One ml of a column fraction highly enriched in the expressed peptide was emulsified with 1 ml of incomplete Freund’s adjuvant (Sigma). The suspension was divided into 4 aliquots, one 800-μl aliquot for the initial injection and three 400-μl aliquots for the booster injections. The 400-μl aliquots were further emulsified with an equal volume of incomplete Freund’s adjuvant. The suspension was injected into the pectoral muscle at four different sites on three 20-week-old white Leghorn laying hens. Booster shots were given after 10, 20, and 30 days. Eggs were collected daily for 3 months, labeled and stored at 4°C for antibody isolation.
Extraction and purification of chicken antibodies
Following the protocol of Polson [28], egg yolks were carefully separated from the white by washing with deionized water and collected in a graduated cylinder. The yolks were homogenized with two volumes of 100 mM phosphate buffer (pH 7.6). Equal volume of yolk homogenate and chloroform were distributed in capped centrifuge tubes, the mixture shaken twice to obtain a thick emulsion, and the tubes spun at 1,000g for 30 min. Three phases separated, a lower orange-colored solution of lecithin in chlorophorm, a middle semi-solid emulsion of the yolk substances in chlorophorm, and an upper watery phase of chicken serum proteins. The watery phase was decanted, its volume measured, and 12% w/v pulverized PEG 6000 dissolved in the fluid. The precipitated solution was centrifuged at 15,700g for 10 min, and the pellet containing the IgY dissolved in 100 mM phosphate buffer of volume equivalent to 1/6th of the non-homogenized yolk. The IgY solution was purified by means of a HiTrap IgY Purification HP (Pharmacia), divided in 100-μl aliquots and stored at −80°C until use.
Western blot
Solubilized muroplast preparation and the purified peptide expressed in E. coli were subjected to SDS-PAGE and transferred to nitrocellulose membrane. The membrane was blocked with 5% skim milk in PBS (pH 7.4) and incubated overnight with the primary antibody at a concentration of 0.07 mg/ml in PBS (pH 7.4). After washing in PBS, the membrane was incubated with the secondary antibody (rabbit anti-chicken IgG alkaline phosphatase conjugate, Sigma) diluted 1:1,000 in PBS. The development was achieved by BCIP/NBT liquid substrate system (Sigma).
Immunofluorescence microscopy
Immunolocalization of the protein was performed on both intact cells and isolated muroplasts. Both cells and muroplasts were fixed in methanol at −20°C for 20 min. Fixed samples were incubated in growth medium with 0.1% Triton X-100 at 4°C for 30 min, washed twice with the same medium, and kept for 1 h at 4°C in growth medium containing 5% NGS (Normal Goat Serum) and 0.1% Triton X-100. Samples were then incubated overnight at 4°C in the primary anti-rhodopsin antibody diluted 1:100 in 5% NGS and 0.1% Triton X-100 in growth medium. After rinsing in growth medium, samples were incubated for 2 h at room temperature in secondary antibody (rabbit anti-chicken IgG FITC conjugate; Sigma, USA) diluted 1:500 in 5% NGS in growth medium. Samples were rinsed and examined with a Zeiss Axioplan microscope (Zeiss, Germany), equipped with an epifluorescence system, a ×100 planapochromatic objective, and a 100-W mercury lamp. Fluorescence images were acquired with a blue-violet filter set (8 nm band-pass excitation filter, 436 nm; chromatic beam splitter, 460 nm; barrier filter, 470 nm).
Photography
Fluorescence photographs were recorded with an Olympus Camedia C-30303 digital camera (Olympus, Japan) mounted on the Zeiss Axioplan microscope.
Multiple alignments and bioinformatic analysis
Comparative and bioinformatic analysis of sequences were carried out online at http://workbench.sdsc.edu and http://ncbi.nlm.nih.gov.
Results and discussion
The complete coding sequence of Cyanophora opsin DNA is deposited as GenBank accession GQ402542. Before the completion of this work, sequences encoding proteins homologous to type I opsins in C. paradoxa appeared in an EST (expressed sequence tag) database (http://amoebidia.bcm.umontreal.ca/pepdb/). The sequence identified in the cluster CDL00000525 partly overlaps our sequence.
The rhodopsin-like protein of C. paradoxa, which we refer to as Cyanophopsin, can be defined as an integral membrane protein. It is comprised of an extracellular N-terminal 80 residues and a shorter 35-aa cytoplasmic C-terminal region that have no similarity to known protein families. The core region consists of a 219-aa residue, for which the predicted topology reveals seven-transmembrane helices typical of other retinyldene proteins (not shown). Each of these regions consists of a α-helix of 23 residues, embedded in the membrane and loop regions connecting the transmembrane segments with the following length from helix A to helix G: 8 aa, 19 aa, 6 aa, 4 aa, 12 aa, 9 aa.
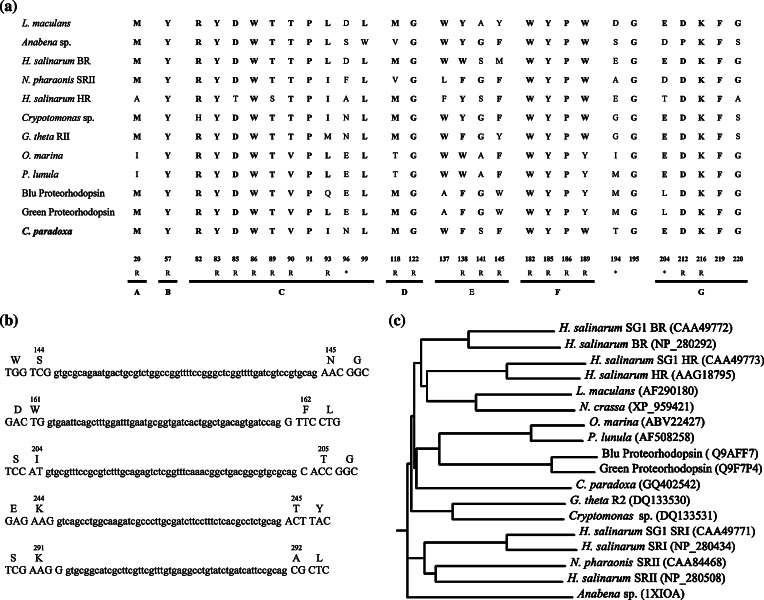
ClustalW alignment of Cyanophopsin and other microbial opsins deposited in GeneBank suggests relative position of the seven helices, and highlights conserved regions. Figure 1a shows the alignment relative to the regions corresponding to helices A, B, C, D, E, F, and G. The amino acid residues are numbered according to the sequence of bacteriorhodopsin. The alignment reveals that the sequence of the core region of Cyanophopsin possesses the amino acid hallmarks of microbial rhodopsins [13, 14], since it harbors the following common motif: M (helix A), Y (helix B), RYxDWxxTx(T/V)Pxx(I/L)xxxxxL (helix C), MxxxG (helix D), Wx(F/W)xx(G/S) (helix E), WxxYPxxW (helix F), G (loop between helix F and helix G), and ExxxxxxxDxxxKxxFG (helix G). These strictly conserved residues are required for structure and function of rhodopsins, in particular the Lys216 (K) present in helix G, which is required for covalent bonding with the retinal molecule. As to the residues identified as necessary for the function (pumping activity, or photosensory function), the sequence of Cyanophopsin shows some peculiarities. In fact, Cyanophopsin conserves only 3 out of the 5 residues revealed to constitute a main proton pathway according to Lanyi [29], i.e. Arg82 (R), Asp85 (D), and Glu204 (E), while uncharged residues are present in both position 96 and position 194. Position 96, i.e. the Schiff base proton donor position, is occupied by a carboxylic acid (D, E) in all BRs and proteorhodopsins, according to their proton pump activity, and by non-carboxylate residues in all sensory rhodopsins, Phe (F) or Tyr (Y), in prokaryotic photomotility receptors such as sensory rhodopsin II, Ser (S) in Anabaena sensory rhodopsin, and His (H) in channelrhodopsin. In the case of Cyanophopsin, Asn (N) is present in position 96. This uncharged, non-aromatic residue cannot serve as a proton donor, and hence does not guarantee proton pumping ability of the Cyanophora protein, and nor can it serve a hypothetical sensory function. Position 194, occupied by a Glu (E), i.e. a charged residue, in proton pumps, is occupied by a Thr (T), i.e. an uncharged residue, in Cyanophosin. A similar situation is present in Cryptomonas sp. and Guillardia theta rhodopsins (GtRII), where there is an Asn in position 96 and a Gly (G) in position 194, and for which a sensory function was hypothesized. Still, the presence of a carbon-concentrating mechanism of presumably carboxysomal type in the muroplast of Cyanophora involving accumulation of HCO3 − inside the organelle [5], together with the localization of Cyanophosin on the muroplast membrane (see later) should be connected to the presence of some sort of cation pumping activity. The pump could function via different funnelling cation pathway consisting of other amino acid residues.
Fig. 1.
a ClustalW alignment of selected high conserved regions of representative microbial rhodopsins. Residues and putative transmembrane helices are numbered according to H. salinarum bacteriorhodopsin sequence (BR). R Amino acid residues contacting retinal, Asterisk amino acid residues necessary for the function. b Position and sequence of the introns of the Cyanophopsin. c Phylogenetic relationship between type I microbial rhodopsins. Accession numbers are indicated for each sequence
The DNA sequence shows five introns (Figure 1b). The intron–exon splice junctions of the five introns conform to the GT/AG rule [30]. The first intron is localized in the loop between helix B and helix C, after Ser144 (numbering according to Cyanophopsin sequence). The second intron interrupts the highly conserved region in helix C and splits codon 161. The third intron splits the codon 204, which is the last of helix D. The fourth intron inserts just before the beginning of helix G, after residue 244. The fifth splits codon 291, just after the Lys290 which links retinal. The position of the introns in C. paradoxa does not correspond to the position of introns in any of the other microbial opsin sequences available.
A phylogenetic tree of type-I opsin was constructed by the neighbor-joining method performed with the computer program ClustalW (Fig. 1c). The topology of the tree indicates that Cyanophopsin is closer to proteorhodopsins and algal opsin than to fungal and haloarchaeal opsins, hence closer to eubacterial opsins.
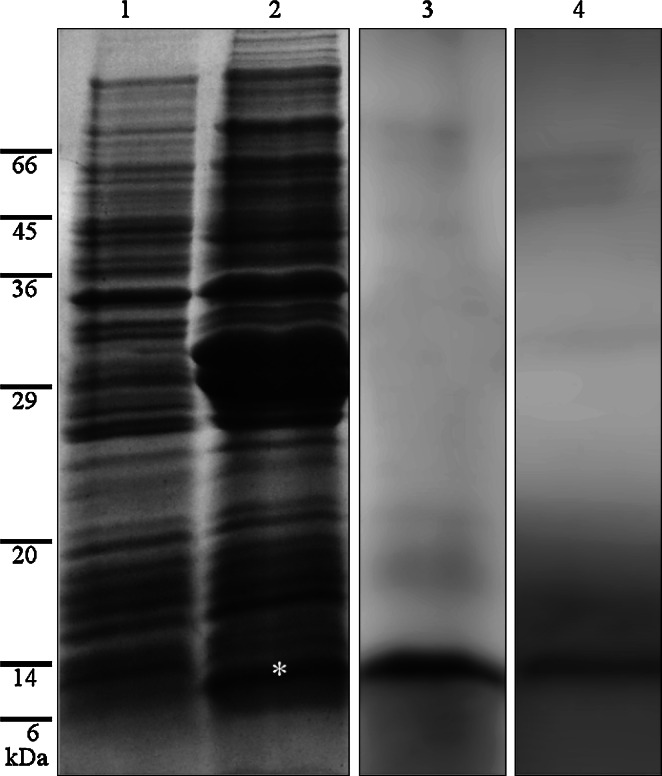
Figure 2 shows the expression of the fragment of the first 80-aa residues of Cyanophopsin obtained in E. coli using the plasmid pCPR. The 8-KDa peptide is clear evident in lane 2 (asterisk) and absent in the control culture non-expressing the sequence (lane 1). Lane 3 shows the purified peptide on SDS gel after Coomassie staining. Polyclonal antibody raised against this peptide stained an 8-KDa band on western blot of the lysate of E. coli expressing the fragment (lane 4).
Fig. 2.
Expression of N-ter 80 residue of Cyanophopsin in E. coli. Lane 1 Control culture without the gene fragment, lane 2 culture expressing the 8-KDa peptide (asterisk), lane 3 SDS gel of the purified peptide, lane 4 anti-peptide antibody staining on western blot of E. coli lysate expressing the peptide
BLAST analysis showed no similarity between the N-ter 80 residue with any protein sequence present in GenBank bacterial and microbial databases. To verify the localization of Cyanophopsin in individual C. paradoxa cells, indirect immunofluorescence analysis was performed using the same antibody (Fig. 3). The staining is evident only on the muroplast profile, and it is completely absent from the Cyanophora membrane (Fig. 3a–c). Immunofluorescence analysis was also performed on isolated muroplasts; the localization of the Cyanophopsin in the muroplast membrane allows the observation of the different stages of the organelle division cycle (Fig. 3d–i). The pictures illustrate that muroplast division is initiated from a kidney-shaped state since the organelle constriction occurs asymmetrically from one side of the division plane (Fig. 3e). As division progresses, the constriction spreads around the muroplast (Fig. 3f), which gradually becomes dumbbell-shaped (Fig. 3g). As the thylakoid segregation occurs, the division plane becomes more evident (Fig. 3h), and finally splits the two daughter muroplasts (Fig. 3i). These images can be considered complementary to the immunofluorescence images by Sato [31, 32]. These authors follow the process of muroplast division to assess the role of the cell division protein FtsZ in the formation of the peptidoglycan septum necessary for organelle cleavage.
Fig. 3.
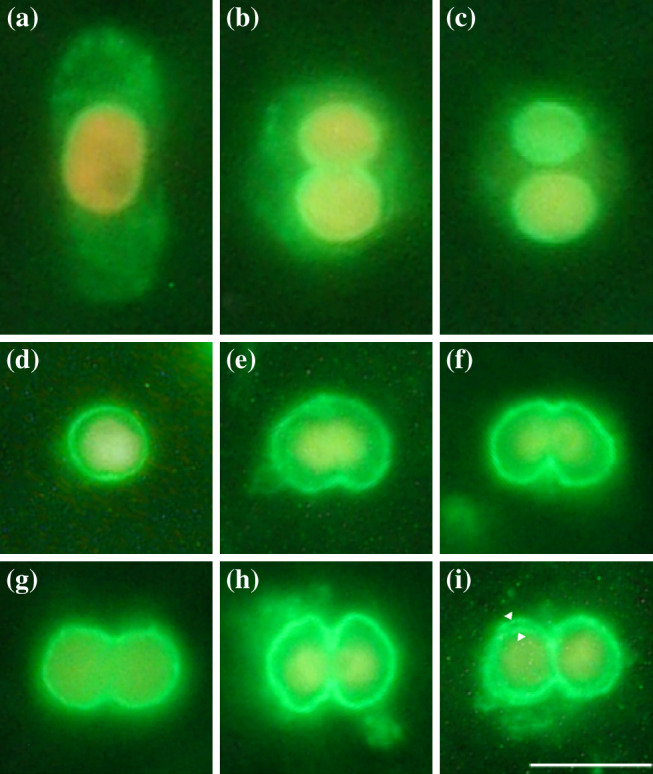
Immunofluorescence analysis of muroplasts performed using the anti-Cyanophopsin antibody. See text for details
To exclude the possibility of cross-reactions between the fluorescent antibody probe and other muroplast membrane proteins, western blot was performed on solubilized muroplast preparation (Fig. 4, lane 1). The antibody reaction decorated a band of approximately 33 kDa, which corresponds to the size calculated for Cyanophopsin (Fig. 4, lane 2).
Fig. 4.
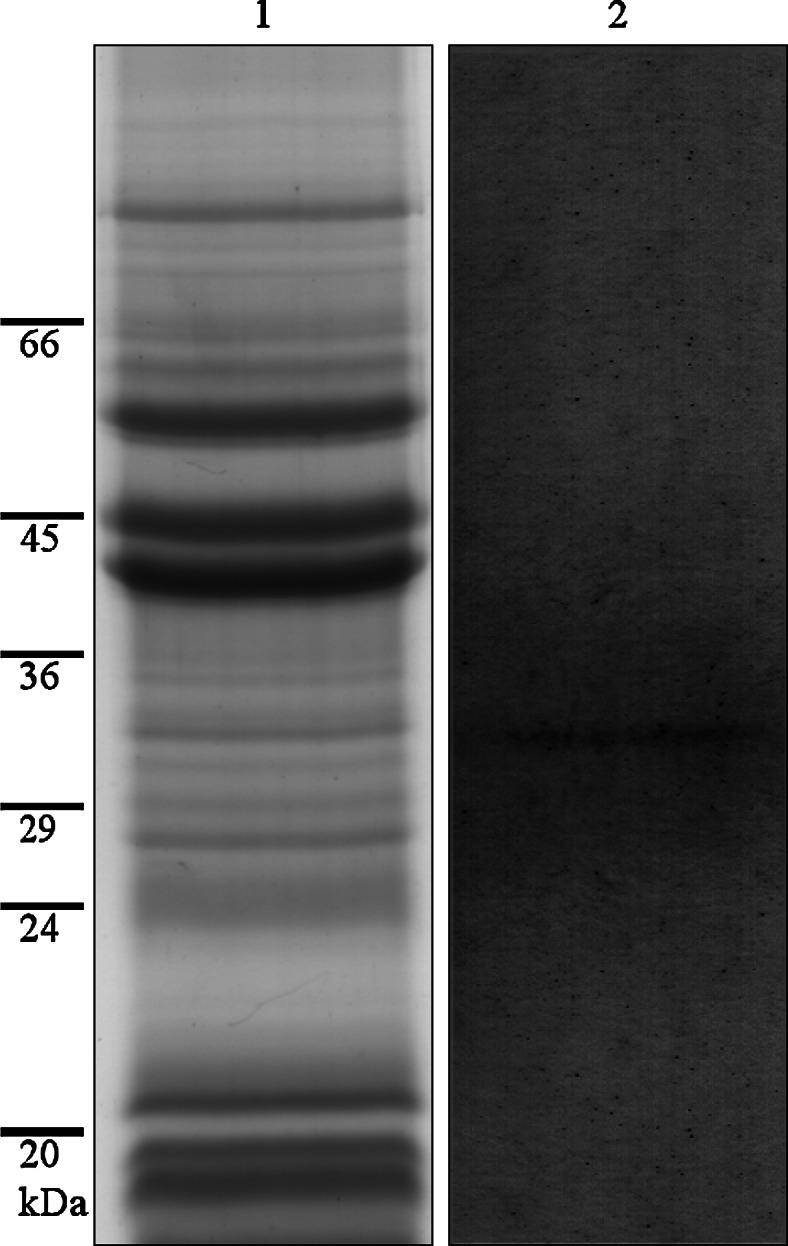
SDS gel of the solubilized muroplast preparation, lane 1; western blot using the anti-Cyanophopsin antibody visualizing an approximately 33-kDa band on the muroplast preparation, lane 2
The even localization of the Cyanophopsin on the entire surface of the muroplast (both on the outer and inner envelope membranes of the organelle (Fig. 3i, arrowheads) resembles more that of bacteriorhodopsin than that of sensory rhodopsin in Halobacterium salinarum. This topology is barely compatible with a phototaxis receptor function, since these proteins are usually concentrated in small structure (e.g., Euglena gracilis) or membrane patches (e.g., Chlamydomonas reinhardtii) in contact with the external environment, [4]. Still, the presence of a carboxysomal-type mechanism of HCO3 − accumulation in the muroplast of Cyanophora allows the hypothesis of cation pumping activity in the membrane. Moreover, because the protein is present on both the muroplast membranes, it can easily manage transport inside the periplasm and then inside the stroma.
The sequence of Cyanophopsin was aligned with the sequence of the complete muroplast genome (Genbank accession no. NC001675) to verify whether the photoreceptive protein is coded inside the organelle or belongs to the genomic pool of the alga. No matching resulted from the alignment, excluding the coding of the rhodopsin-like protein in the muroplast genome. Hence, the Cyanophopsin gene could belong to that group of genes transferred from the endosymbiont genome to the host genome, and the corresponding protein should be imported into muroplasts.
The presence of integral membrane proteins as Cyanophopsin in the membrane of the muroplast, the most primitive of all plastids, should indicate that the common primordial rhodopsin existed in pre-eukaryotic cells before the appearance of the first photosynthetic eukaryotic cell about 1.55 × 109 years ago, [33].
Acknowledgments
We wish to thank all the fellows of the “Cooperativa l’Uovo di Colombo” of Viareggio (Italy) for the care they took of our Leghorn hens and Dr. Antonio Barsanti for veterinary assistance.
References
- 1.Fathinejad S, Steiner JM, Reipert S, Marchetti M, Allmaier G, Burey SC, Ohnishi N, Fukuzawa H, Loffelhardt W, Bohnert HJ. A carboxysomal carbon-concentrating mechanism in the cyanelles of the “coelacanth” of the algal world, Cyanophora paradoxa? Physiol Plant. 2008;133:27–32. doi: 10.1111/j.1399-3054.2007.01030.x. [DOI] [PubMed] [Google Scholar]
- 2.Wise RR. The diversity of plastid form and function. In: Wise RR, Hoober JK, editors. The structure and function of plastids. Netherlands: Springer; 2007. pp. 3–26. [Google Scholar]
- 3.Schenk HEA. Cyanophora paradoxa: anagenetic model or missing link of plastid evolution. Endocytobiosis Cell Res. 1994;10:87–106. [Google Scholar]
- 4.Barsanti L, Gualtieri P. Algae—anatomy, biochemistry and biotechnology. Boca Raton: CRC, Taylor & Francis; 2006. [Google Scholar]
- 5.Raven JA. Carboxysomes and peptidoglycan walls of cyanelles: possible physiological function. Eur J Phycol. 2003;38:47–53. doi: 10.1080/0967026031000096245. [DOI] [Google Scholar]
- 6.Trench RK, Pool RR, Logan M, Engelland A. Aspects of the Relation between Cyanophora paradoxa (Korschikoff) and its endosymbiotic cyanelles Cyanocyta korschikoffiana (Hall & Claus). I. Growth, ultrastructure, photosynthesis and the obligate nature of the association. Proc R Soc Lond B. 1978;202:423–443. doi: 10.1098/rspb.1978.0077. [DOI] [Google Scholar]
- 7.Plancke C, Colleoni C, Deschamps P, Dauvillée D, Nakamura Y, Haebel S, Ritte G, Steup M, Buléon A, Putaux JL, Dupeyre D, d’Hulst C, Ral JP, Löffelhardt W, Ball SG. Pathway of cytosolic starch synthesis in the model glaucophyte Cyanophora paradoxa . Eukaryot Cell. 2008;7:247–257. doi: 10.1128/EC.00373-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aitken A, Stanier RY. Characterization of peptidoglycan from the cyanelles of Cyanophora paradoxa . J Gen Microbiol. 1979;112:219–223. [Google Scholar]
- 9.Stirewalt VL, Michalowski CB, Loffelhardt W, Bohnert HJ, Bryant DA. Nucleotide sequence of the cyanelle genome from Cyanophora paradoxa . Plant Mol Biol Rep. 1995;13:327–332. doi: 10.1007/BF02669186. [DOI] [Google Scholar]
- 10.Kaneko T, Nakamura Y, Wolk CP, Kuritz T, Sasamoto S, Watanabe A, Iriguchi M, Ishikawa A, Kawashima K, Kimura T, Kishida Y, Kohara M, Matsumoto M, Matsuno A, Muraki A, Nakazaki N, Shimpo S, Sugimoto M, Takazawa M, Yamada M, Yasuda M, Tabata S. Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 2001;8:205–213. doi: 10.1093/dnares/8.5.205. [DOI] [PubMed] [Google Scholar]
- 11.Hader DP. Photomovement in Cyanophora paradoxa. Arch Microbiol. 1985;143:100–104. doi: 10.1007/BF00414776. [DOI] [Google Scholar]
- 12.Jung KH. The distinct signalling mechanisms of microbial sensory Rhodopsins in Archaea, Eubacteria and Eukarya. Photochem Photobiol. 2007;83:63–69. doi: 10.1562/2006-03-20-IR-853. [DOI] [PubMed] [Google Scholar]
- 13.Spudich JL, Yang CS, Jung KH, Spudich EN. Retinylidene proteins: structures and functions from archaea to humans. Annu Rev Cell Dev Biol. 2000;16:365.392. doi: 10.1146/annurev.cellbio.16.1.365. [DOI] [PubMed] [Google Scholar]
- 14.Spudich JL, Jung KH. Microbial rhodopsins: phylogenetic and functional diversity. In: Briggs WR, Spudich JL, editors. Handbook of photosensory receptors. Weinheim: Wiley; 2005. pp. 1–23. [Google Scholar]
- 15.Mercatelli R, Quercioli F, Barsanti L, Evangelista V, Coltelli P, Passarelli V, Frassanito AM, Gualtieri P. Intramolecular photo-switching and intermolecular energy transfer as primari photoevents in photoreceptive processes: the case of Euglena gracilis . Biochem Biophys Res Comm. 2009;385:176–180. doi: 10.1016/j.bbrc.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 16.Barsanti L, Coltelli P, Evangelista V, Passarelli V, Frassanito AM, Vesentini N, Gualtieri P. Low-resolution characterization of the 3D structure of the Euglena gracilis photoreceptor . Biochem Biophys Res Comm. 2008;375:471–476. doi: 10.1016/j.bbrc.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 17.Barsanti L, Passarelli V, Walne PL, Gualtieri P. The photoreceptor protein of Euglena gracilis . FEBS Lett. 2000;482:247–251. doi: 10.1016/S0014-5793(00)02062-7. [DOI] [PubMed] [Google Scholar]
- 18.Walne PL, Passarelli V, Lenzi P, Barsanti L, Gualtieri P. Isolation of the flagellar swelling and identification of retinal in the phototactic flagellate, Ochromonas danica (Chrysophyceae) J Eukar Microbiol. 1995;42:7–11. doi: 10.1111/j.1550-7408.1995.tb01533.x. [DOI] [Google Scholar]
- 19.Robinson KR, Lorenzi R, Ceccarelli N, Gualtieri P. Retinal identification in Pelvetia fastigiata . Biochem Biophys Res Comm. 1998;243:776–778. doi: 10.1006/bbrc.1998.8176. [DOI] [PubMed] [Google Scholar]
- 20.Jung KH, Trivedi DV, Spudich JL. Demonstration of a sensory rhodopsin in eubacteria. Mol Microbiol. 2003;47:1513–1522. doi: 10.1046/j.1365-2958.2003.03395.x. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto OK, Hastings JW. Novel dinoflagellate clock-related genes identified through microarrays analysis. J Phycol. 2003;39:519–526. doi: 10.1046/j.1529-8817.2003.02170.x. [DOI] [Google Scholar]
- 22.Sineshchekov OA, Jung KH, Spudich JL. Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii . Proc Natl Acad Sci USA. 2002;25:8689–8694. doi: 10.1073/pnas.122243399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sineshchekov OA, Govorunova EG, Jung KH, Zauner S, Maier US, Spudich JL. Rhodopsin-mediated photoreception in cryptophyte flagellates. Biophys J. 2005;89:4310–4319. doi: 10.1529/biophysj.105.070920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsunoda SP, Ewers D, Gazzarrini S, Moroni A, Gradmann D, Hegemann P. H1-pumping rhodopsin from the marine alga Acetabularia . Biophys J. 2006;91:1471–1479. doi: 10.1529/biophysj.106.086421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klare JP, Chizhov I, Engelhard M. Microbial rhodopsins: scaffolds for ion pumps, channels, and sensors. Results Probl Cell Differ. 2008;45:73–122. doi: 10.1007/400_2007_041. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1979;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Song CS, Yu JH, Bai DH, Hester PY, Kim KH. Antibodies to the alpha-subunit of insulin receptor from eggs of immunized hens. J Immunol. 1985;135:3354–3359. [PubMed] [Google Scholar]
- 28.Polson A. Isolation of IgY from the yolk of eggs by a chloroform polyethylene glycol procedure. Immunol Investig. 1990;19:253–258. doi: 10.3109/08820139009041840. [DOI] [PubMed] [Google Scholar]
- 29.Lanyi JK. Mechanism of ion transport across membranes. Bacteriorhodopsin as a prototype for proton pumps. J Biol Chem. 1997;272:31209–31212. doi: 10.1074/jbc.272.50.31209. [DOI] [PubMed] [Google Scholar]
- 30.Wu Q, Krainer AR. AT-AC pre-mRNA splicing mechanisms and conservation of minor introns in voltage-gated ion channel genes. Mol Cell Biol. 1999;19:3225–3236. doi: 10.1128/mcb.19.5.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato M, Nishikawa T, Kajitani H, Kawano S. Conserved relationship between FtsZ and peptidoglycan in the cyanelles of Cyanophora paradoxa similar to that in bacterial division. Planta. 2007;227:177–187. doi: 10.1007/s00425-007-0605-0. [DOI] [PubMed] [Google Scholar]
- 32.Sato M, Mogi Y, Nishikawa T, Miyamura S, Nagumo T, Kawano S. The dynamic surface of dividing cyanelles and ultrastructure of the region directly below the surface in Cyanophora paradoxa . Planta. 2009;229:781–791. doi: 10.1007/s00425-008-0872-4. [DOI] [PubMed] [Google Scholar]
- 33.Yoon SH, Hackett YD, Ciniglia C, Pinto G, Bhattacharya D. A molecular timeline for the origin of photosynthetic eukaryotes. Mol Biol Evol. 2004;21:809–818. doi: 10.1093/molbev/msh075. [DOI] [PubMed] [Google Scholar]