Abstract
Although the glycation of Tau that is involved in paired helical filament formation in Alzheimer’s disease has been widely studied, little attention has been paid to the role of d-ribose in the glycation of Tau. Here, we show that Tau is rapidly glycated in the presence of d-ribose, resulting in oligomerization and polymerization. Glycated derivatives appeared after 24 h incubation. Western blotting indicated the formation of advanced glycation end-products (AGEs) during initial stages of glycation. Thioflavin T-positive (ThT-positive) aggregations that appeared from day 4 indicated the globular-like features. Atomic force microscopy revealed that the surface morphology of ribosylated Tau40 was globular-like. Kinetic studies suggested that d-ribosylated Tau is slowly oligomerized and rapidly polymerized with ThT-positive features. Moreover, d-ribosylated Tau aggregates were highly toxic to SHSY5Y cells and resulted in both apoptosis and necrosis. This work has demonstrated that d-ribose reacted with Tau protein rapidly, producing ThT-positive aggregations which had high cytotoxicity.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-009-0058-7) contains supplementary material, which is available to authorized users.
Keywords: d-Ribose, Tau protein, Glycation, Aggregation, Cytotoxicity
Introduction
Tau is a major microtubule-binding protein that is important for the assembly and stabilization of microtubules [1], which are required for axonal transport and morphogenesis [2, 3]. In a normal neuron, Tau is localized in the axons, neuronal soma [4–6] and nuclei [7], and its function is regulated by phosphorylation [8]. The discovery that Tau is the major protein subunit of paired helical filaments (PHFs)/neurofibrillary tangles in Alzheimer’s disease (AD) has markedly stimulated interest in understanding the structure and function of this protein [9–11]. Furthermore, PHF-Tau is not only related to hyperphosphorylation but also to other post-translational modifications such as glycation [12–14].
Glycated Tau protein has been found in PHFs from the brain tissues of Alzheimer’s patients [15]. In AD, non-enzymatically glycated Tau induces neuronal oxidant stress resulting in cytokine gene expression and release of amyloid beta-peptide [16]. Kuhla and coworkers have observed the promotive effects of reactive carbonyl compounds (RCCs) on Tau aggregation and filament formation [17]. As an in vitro study shows, glucose-glycated Tau has been found to promote fibrillization by shifting the equilibrium toward the fibrillized state, but does not promote filament nucleation [18]. However, it is still unclear whether glycation causes Tau to form amyloid-like aggregates. Thus, the relationship between glycation and Tau misfolding is worth studying to clarify the role of glycation in protein aggregation and cytotoxicity.
So far, most work has focused on the role of d-glucose in the glycation of Tau [18–21]. d-Glucose exists in solution as an intramolecular hemiacetal in which the free hydroxyl group at C-5 reacts with the aldehydic C-1, rendering the latter asymmetric and producing a stereoisomer. The six-membered aldopyranose ring is much more stable than the aldofuranose five-membered ring because the pentose ring of d-ribose is not planar but occurs in one of a variety of conformations generally described as “puckered” [22]. The unstable aldofuranose ring is vulnerable to reaction with an amino group. Therefore, d-glucose is not as efficient in glycating a protein as reducing furanoses such as d-ribose.
d-Ribose is a naturally occurring pentose monosaccharide present in all living cells and is an essential component for energy production in the body. It is used to synthesize nucleotides, nucleic acids, glycogen, and other important metabolic products. d-Ribose is also formed in the body from conversion of d-glucose via the pentose phosphate pathway. Thus, d-ribose is continually present both intracellularly and extracellularly, and has opportunities to react with proteins and produce glycated derivatives. For this reason, glycation of Tau protein with d-ribose needs to be addressed and investigated.
Glycation of a protein causes fluorescence at 410 nm (or 450 nm) [23–26] that is thought to indicate the formation of advanced glycation end-products (AGEs). However, few authors have studied the relationship between the formation of the fluorescence and Tau protein aggregation, that is, whether the formation of the fluorescence is positively related to the polymerization of a protein, especially the appearance of ThT-positive aggregations during glycation. Clarification of this problem will be helpful for using fluorescence to study the structure–function relationship of a protein during glycation.
It is well known that glycation affects the structure and function of proteins such as hemoglobin [27] and albumin [28, 29]. Glycation of hemoglobin results in a marked loss of its function in transportation of oxygen in the blood and in releasing carbon dioxide in the lung [30]. In this laboratory, we have also observed that glycation induced inactivation and conformational change in d-glyceraldehyde-3-phosphate dehydrogenase [31, 32]. Rapid in vitro glycation of α-synuclein with d-glucose (requiring less than 7 days), however, did not result in distinct conformational changes or inactivation of the protein [33]. Furthermore, our unpublished data showed that extracellular ribose was able to induce intracellular proteins including tau to be glycated. We have also found that Tau protein aggregates in the presence of formaldehyde at low concentrations and forms amyloid-like deposits [34]. Here, we show that glycation of Tau protein in the presence of d-ribose generates globular ThT-positive cytotoxic deposits.
Materials and methods
Expression and purification of Tau40
Human wild type tau40 was expressed in Escherichia coli BL21 (DE3) using the pRK172-tau40 plasmid (a kind gift from Dr. Goedert of the University of Cambridge, UK) and purified as described before [35, 36]. The purified Tau40 protein showed a single band on 12% SDS-PAGE gels with a purity of over 95%. The identity of the Tau40 protein band was confirmed by Western blotting using Tau-1 monoclonal antibody (Sigma, USA) (data not shown). The purified Tau protein was lyophilized and stored at −70°C before use.
Preparation of d-ribosylated protein
Tau40 (final concentration 0.2 mM) was freshly dissolved in 20 mM Tris–HCl (pH 7.4), mixed with 1 M d-ribose, and incubated for different time intervals at 37°C. Tau40 in the absence of d-ribose and d-ribose alone were used as controls. All solutions were filtered with 0.22 μm membranes (Millipore, USA). A Bicinchoninic acid protein-assay kit (Pierce Biotechnology, USA) was used to determine protein concentration [37].
NBT colorimetric fructosamine assay
The extent of glycation of individual Tau40 preparations was assessed using the nitroblue tetrazolium (NBT) assay as described previously [29, 38]. This method is based on a color change correlated with the reduction of NBT to monoformazan by Amadori rearrangement products in alkaline buffer [39]. Along with 10 μl of the sample or standard, 200 μl of 0.75 mM NBT (Ameresco, USA) was added to a 96-well microplate. The kinetics of the reduction of NBT by fructosamine groups (0.1 M carbonate buffer, pH 10.35) were measured at 540 nm using an MK3 microplate reader (Thermo, USA) after incubating for 30 min at 37°C. Standard curves were generated by addition of 10 μl of 1-deoxy-1-morpholino-d-fructose (1-DMF; Sigma-Aldrich, USA). Fructosamine formation was monitored by comparison with standard curves (R 2 > 0.99).
Measurement of fluorescence
Intrinsic fluorescence of Tau40 (final concentration 2 μM) was monitored on an F4500 fluorescence spectrophotometer (Hitachi, Japan). The emission spectrum from 290 to 500 nm was recorded by excitation at 280 nm at 25°C. To assess the fluorescence of AGEs derived from glycated protein, we scanned the emission spectrum from 350 to 500 nm (λex = 320 nm) as described previously [40].
Measurement of ThT-binding fluorescence
Tau40 (final concentration 2 μM) and ThT (30 μM; Sigma) were mixed at 25°C, and fluorescence was subsequently measured (λex 450 nm; λem 485 nm) as described [34].
Observation of protein aggregation with atomic force microscopy (AFM)
The conditions for d-ribosylation of Tau were as described above. All of the solutions used were filtered through a 0.22-μm filter. The glycated protein was diluted to the desired concentration using Tris–HCl buffer (pH 7.4). Samples (10 μl) were kept at room temperature for 5 min to allow the protein to absorb onto the mica. Observation under an atomic force microscope (Mutiplemode-I; Digital Instruments, USA) was performed as described previously [41].
Circular Dichroism (CD) measurements
Far-UV CD measurements were taken with a circular dichrograph (Jasco J-720; Japan). Samples in 1-mm quartz cuvettes were maintained at 25°C using a circulating water bath. Spectra of d-ribose-glycated Tau40 (final concentration 20 μM) were measured (195–260 nm) with a step size of 1.0 nm. All measurements were replicated 10 times and averaged. The background of the corresponding buffers in the absence of protein and d-ribose was subtracted for all samples.
Cell culture
SHSY5Y cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, USA) containing 10% fetal bovine serum (Hyclone, USA), 100 IU/ml penicillin and 100 μg/ml streptomycin (Sigma) at 37°C in a humidified 5% CO2 incubator. Cells were grown to 70–80% confluence in 25-mm-diameter dishes and fed every fourth day. The culture was replaced with serum-free medium before addition of glycated protein during experiments. Tau protein was incubated with d-ribose and aliquots were taken at different time intervals to incubate with cells for 8 h. After that, the medium was changed to DMEM with 10% fetal bovine serum.
Cell viability test
To determine cell viability, we used the standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma) test, with the slight modifications suggested by Mayo and Stein [42]. SHSY5Y cells were seeded on a 96-well plate at a concentration of 105 cells per well with or without exposure to glycated Tau40 (3-day incubation) at various concentrations for 8 h. After 24 or 48 h, MTT (final concentration 0.5 mg/ml) was added and plates were incubated at 37°C for 4 h. The reaction was stopped by replacement of the MTT-containing medium with 150 μl dimethysulfoxide, and absorbance at 540 nm was measured on a Multiscan MK3 spectrophotometer (Thermo Electron Corporation, USA).
Flow cytometric analysis
Cells undergoing apoptosis were detected by using double staining with Annexin V-FITC/PI (propidium iodide) in the dark according to the manufacturer’s instructions. Briefly, cells attached to plastic dishes were harvested using 0.25% trypsin and washed twice with cold PBS. Cell pellets were suspended in 1× binding buffer (10 mM HEPES/NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2) at a concentration of 1 × 106 cells/ml. Cells were then incubated with AnnexinV-FITC and PI for 15 min (22–25°C) in the dark. The stained cells were immediately analyzed on a flow cytometer (FAC Svantage SE, USA). Each measurement was replicated at least three times.
Data analysis
All values reported are means ± standard errors (SE), except where otherwise indicated. Data were analyzed by employing Origin 6.0 statistical software. Differences between experimental groups were considered to be significant if the probability was <0.05 in two-tailed tests. Kinetic data were analyzed as described by Tsou [43].
Results
Glycation of Tau40 in the presence of d-ribose
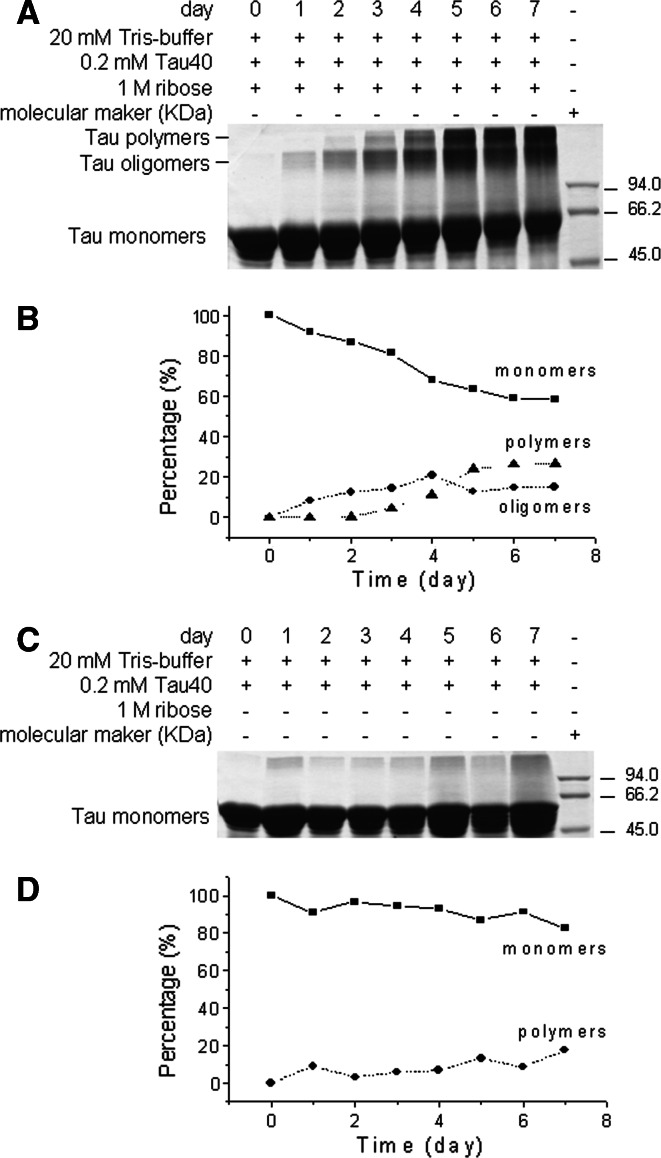
During glycation, Tau40 was incubated with d-ribose and aliquots were taken for 12% SDS-PAGE at different time intervals (Fig. 1, panels A and B). While only one low molecular weight protein band was present in the unglycated Tau40 control, two high molecular weight protein bands appeared in samples containing glycated Tau40, demonstrating Tau protein aggregation. One of the bands was smeared in appearance suggesting that Tau monomers oligomerized to dimers or octamers (oligomers); and the other smearing of the high molecular weight band indicated polymerization to decamers (polymers). Our results thus indicate that Tau began to oligomerize from 1 day after the start of incubation, and polymerization occurred markedly from day 2. The Tau monomer band was retarded in the presence of d-ribose with increasing incubation time, suggesting that the apparent molecular mass of the glycated Tau protein increased with time. However, significant polymerization and band retardation were not detected for Tau alone in the absence of d-ribose (panels C and D, Fig. 1). The difference between the apparent molecular masses suggested that ~51 ribose units bound to one Tau protein.
Fig. 1.
d-Ribosylation of Tau40. Tau40 (final concentration 200 μM) was incubated in the presence of 1 M d-ribose (rib) in 20 mM Tris–HCl buffer (pH 7.4) at 37°C, and aliquots were taken for 12% SDS-PAGE at different time intervals (panel A). Tau40 alone was used as a control (panel C). Grey scanning for Tau in the presence and absence of d-ribose is shown in panels B and D, respectively
In our kinetic studies, the increase in grey density of the bands of oligomers and polymers underwent a relaxation and a biphasic process (slow and fast phases). Density of oligomers and polymers increased from day 1 and 2, respectively, and more rapidly on further incubation. The data were analyzed according to Tsou’s method [43]. The first order rates of the fast phase for oligomers and polymers were five- and three-fold higher than those of the slow phases, respectively (Table 1). Tau monomers likewise decreased in slow and fast phases as oligomers and polymers increased. This suggests that glycation with d-ribose leads to a slow oligomerization of ribosylated Tau protein after a relaxation time, followed by fast polymerization.
Table 1.
The first order rates of changes in grey density, fructosamine and fluorescence during the glycaiton of Tau protein in the presence of d-ribose
| Kinetic measurements | Relaxation time (h) | First phase | Second phase |
|---|---|---|---|
| Grey density | |||
| Monomer | – | 0.81 | 4.60 |
| Oligomer | 24 | 1.25 | 7.84 |
| Polymer | 48 | 1.23 | 4.34 |
| Fructosamine | – | 6.70 | – |
| Fluorescence at 410 nm | 24 | 1.51 | 7.53 |
| Fluorescence at 450 nm | 24 | 1.17 | 9.04 |
| ThT fluorescence | 48 | 1.86 | 5.52 |
Rate constants are in 10−6
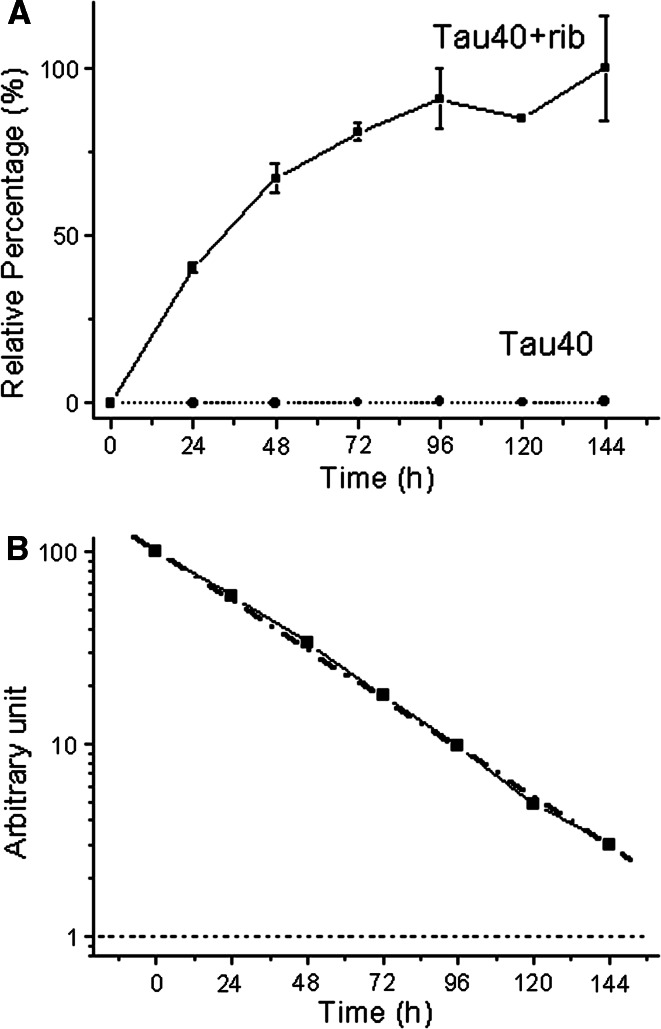
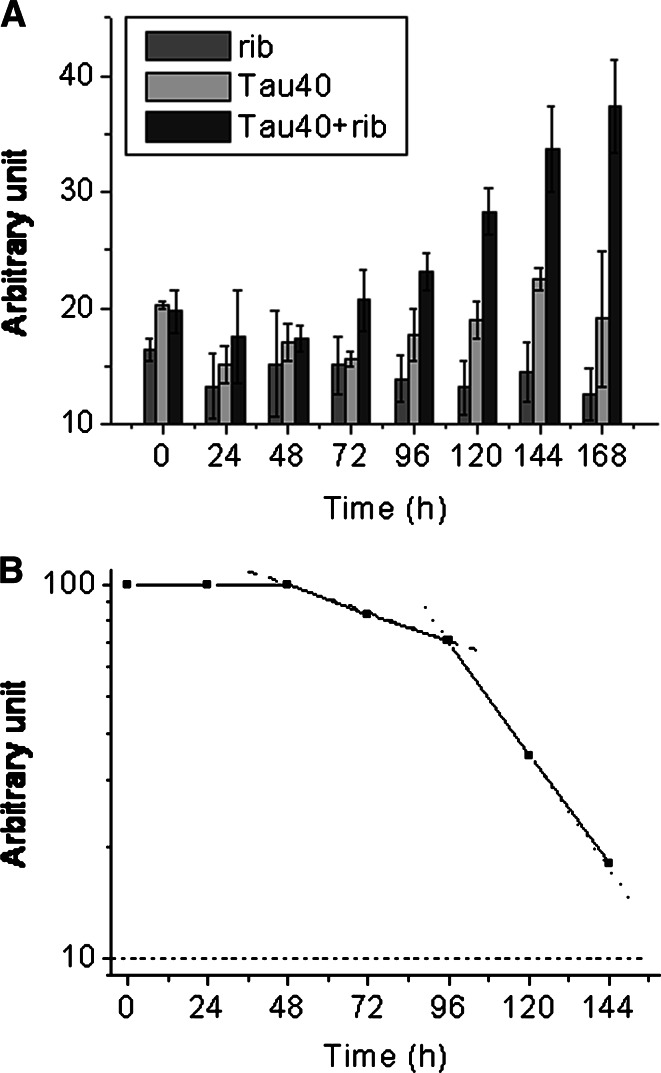
Fructosamine is a common marker for assessing the degree of glycation of a protein [39]. To clarify the time course of the formation of fructosamine in the Tau40 molecule, aliquots were assayed by NBT assay at different incubation times [44]. The absorbance (540 nm) increased markedly with the length of the incubation time of Tau40 with d-ribose (Fig. 2, panel A). The absorption value increased rapidly from the start of incubation and then plateaued. Kinetic studies revealed that absorbance at 540 nm increased in a monophasic time course with a first order rate constant of 6.7 × 10−6 s−1 under these experimental conditions (Fig. 2, panel B), indicating that the NBT assay simply shows the increment in the amount of fructosamine formed in the protein during glycation, but does not indicate protein aggregation.
Fig. 2.
Changes in Tau fructosamine during incubation with d-ribose. NBT was used to detect the formation of fructosamine at different time intervals as described (panel A) [44]. Data were analyzed according to Tsou’s method (panel B) [43]. Conditions for glycation of Tau with d-ribose were the same as those in Fig. 1
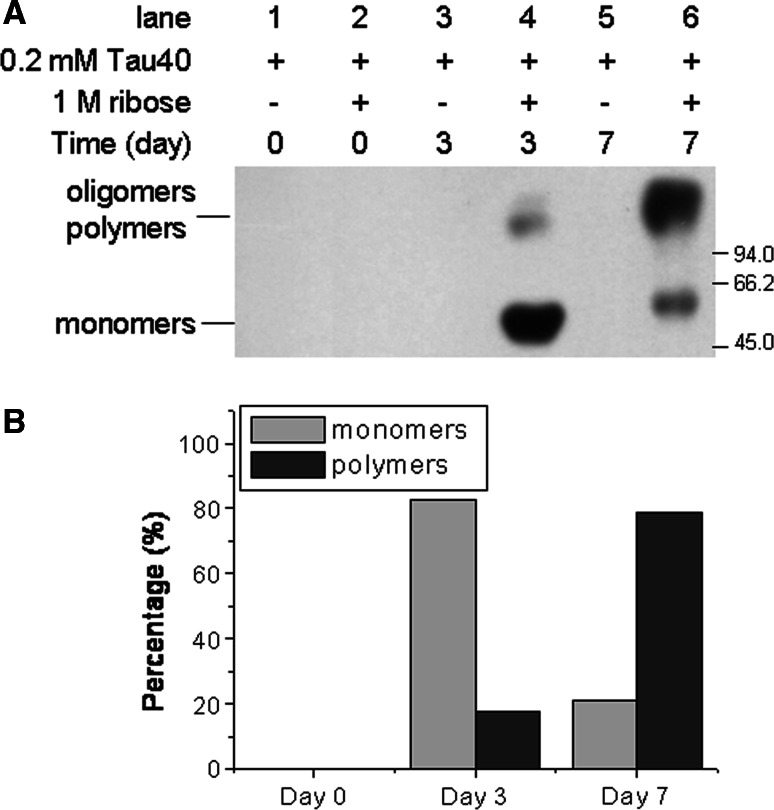
To confirm whether AGEs were formed during the glycation of Tau with d-ribose, a monoclonal antibody (6D12) was used in Western blotting as shown in Fig. 3 (panel A) [45]. Two spots (one for monomers, the other for oligomers and polymers) were detected on the membrane in aliquots from day 3 of the incubation. AGE polymers increased significantly on day 7 (Fig. 3, panel B). However, glycation of Tau with d-glucose showed little AGE formation at this stage under the same conditions (data not shown). This suggests that glycation of Tau protein in the presence of d-ribose results in the formation of AGEs (monomers, oligomers and polymers) at an early stage (within 7 days) of glycation.
Fig. 3.
Western blotting of d-ribosylated Tau. Western blotting was carried out by using anti-AGEs monoclonal antibodies (panel A). Samples in lanes were as indicated. Grey densities of the protein bands are illustrated in panel B. Conditions for glycation of Tau with d-ribose were the same as those in Fig. 1
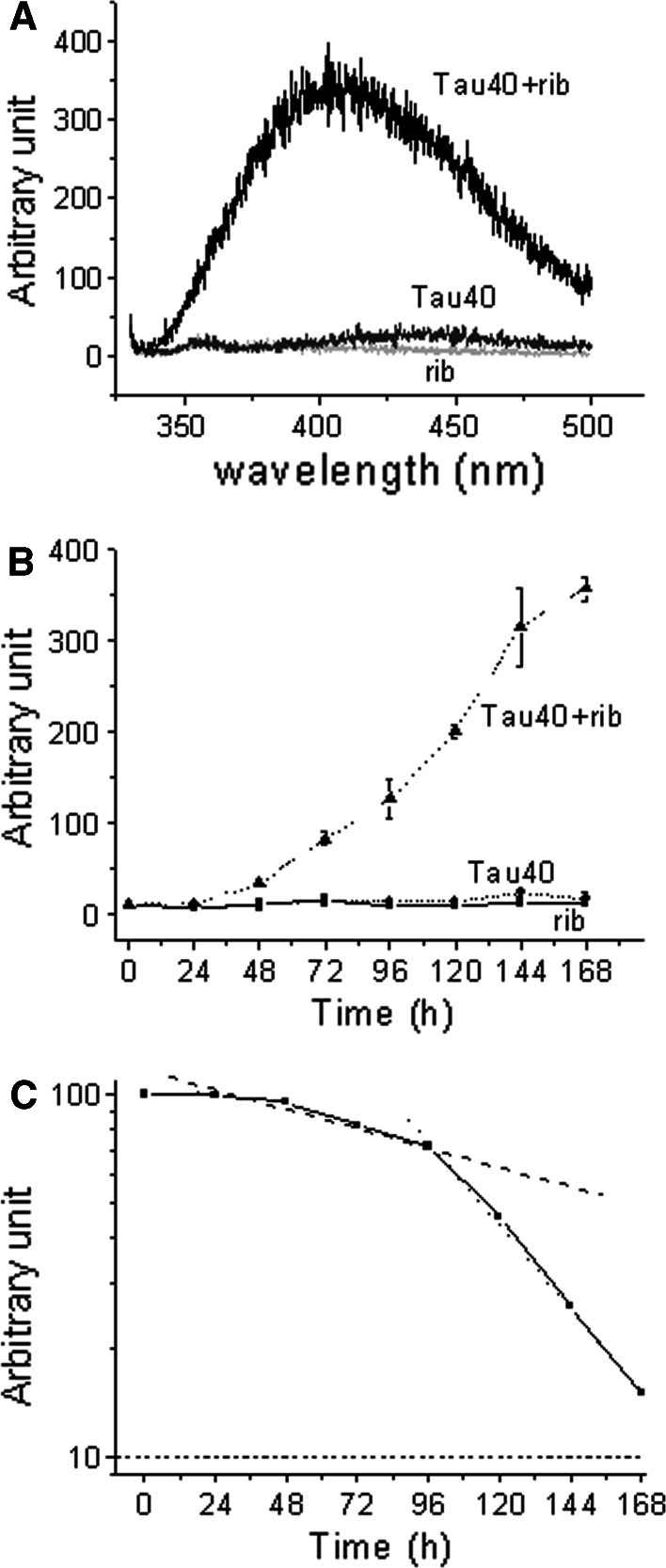
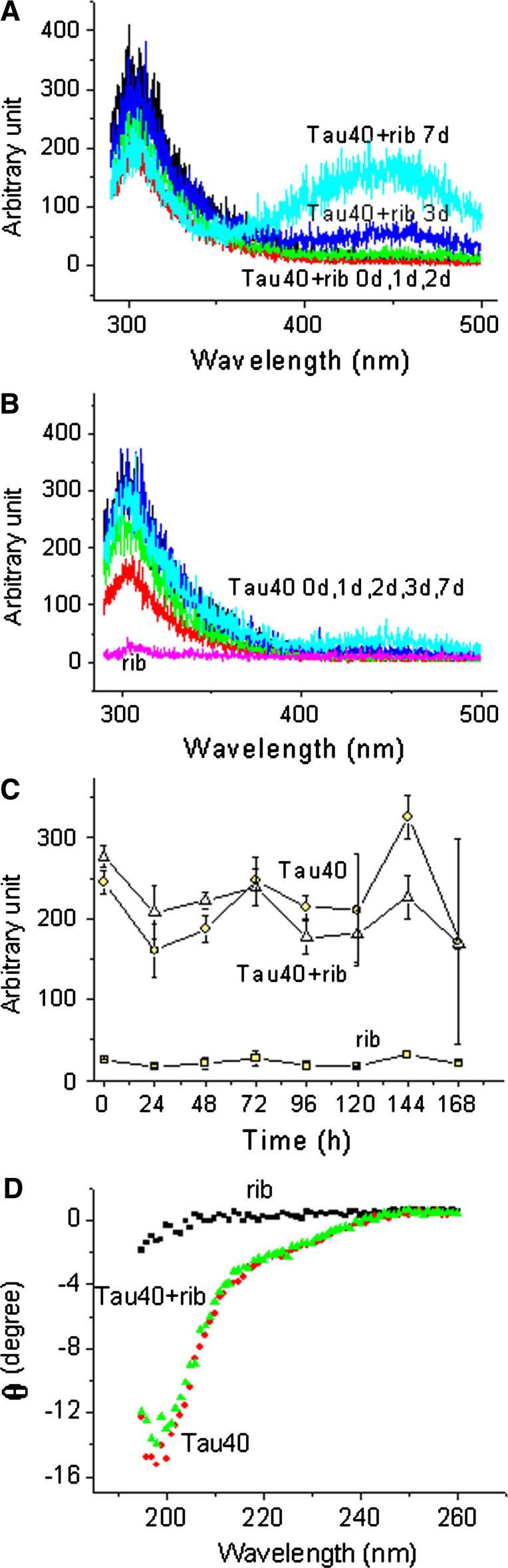
According to Liu and Metzger [26], glycation of a protein produces a new fluorescence derivative (λex 320 nm; λem 410 nm), and thus fluorescence is commonly used to monitor the formation of AGEs. To further clarify the kinetics of the glycation of Tau in the presence of d-ribose, changes in fluorescence were measured as shown in Fig. 4. The fluorescence emission intensity (410 nm) of Tau40 incubated with d-ribose was much stronger than that of Tau40 alone as a control (Fig. 4, panel A). The fluorescence of glycated polymers (collected from PAGE) was markedly stronger than that of glycated monomers (data not shown), suggesting that fluorescence at 410 nm resulted mainly from d-ribosylated polymers.
Fig. 4.
Detection of AGEs by fluorescence. Fluorescence spectra (λex 320 nm) were measured in 20 mM Tris–HCl buffer (pH 7.4) at 25°C (panel A). Changes in maximal fluorescent intensity (λem 410 nm) were monitored (panel B), and analyzed according to Tsou’s method (panel C) [43]. Conditions for glycation of Tau with d-ribose were the same as those in Fig. 1
To confirm that 410 nm emission is related to protein aggregation, the data shown in Fig. 4 (panel B) were analyzed according to Tsou’s method [43]. The kinetic increment of the emission intensity underwent a relaxation period (24 h) and followed a biphasic time course (a slow and a fast phase) (Fig. 4, panel C). First order rate constants obtained from analysis of 410 nm fluorescence emission increments were similar to those obtained by SDS-PAGE analysis of the polymerization of d-ribosylated Tau40 (Fig. 1 and Table 1), indicating that the increase in the emission at 410 nm is related to the aggregation of d-ribosylated Tau protein.
Conformational changes of Tau protein during ribosylation
To investigate the conformational changes of Tau protein during glycation, we measured the intrinsic fluorescence of the ribosylated protein by excitation at 280 nm. As shown in Fig. 5 (panel C), intrinsic fluorescence at 310 nm shows a slight change with incubation time. However, the change in fluorescence was not significant compared to that of Tau in the absence of d-ribose as a control. Changes in intrinsic fluorescence represent microenvironmental variations at Tyr residues where solvent molecules collide with the fluorophore and consume the energy of fluorescence [46]. Notably, a new fluorescent derivative appeared around 450 nm from day 3 of the glycation process (Fig. 5, panel A). The appearance of fluorescence at 450 nm also represented the formation of AGEs in Tau polymers. Most interestingly, an energy transfer between Tyr residues and ribosylated fluorescent derivatives (around 450 nm) was observed by excitation at 280 nm. Control Tau in the absence of d-ribose did not show this energy transfer (Fig. 5, panel B), suggesting that the spatial localization of Tyr residues is close to the glycated fluorescent derivative in Tau polymers.
Fig. 5.
Changes in intrinsic fluorescence and CD of Tau during glycation. The intrinsic fluorescence (λex 280 nm) was recorded during glycation from 1 to 7 days (panel A). Tau40 in the absence of d-ribose and d-ribose alone were used as controls (panel B). Changes in the maximal fluorescent intensity (λem 310 nm) were monitored (panel C). The CD spectra of Tau incubated with d-ribose on the third day is shown (panel D). Conditions for glycation of Tau with d-ribose were the same as those in Fig. 1
To detect changes in the secondary structure of Tau40 in the presence of d-ribose, CD spectra of Tau40 were scanned during glycation. However, the spectrum of ribosylated Tau40 showed a similar profile to that of the native protein (Fig. 5, panel D), suggesting that conformational changes in d-ribosylated Tau40 were not at the level of secondary structure.
To investigate differences between glycated Tau40 and native Tau40 we used ThT, a fluorescent dye, to label protein amyloid-like aggregates [47]. ThT fluorescence (λex 450 nm; λem 485 nm) increased with incubation time. Emission intensity increased significantly in the presence of d-ribosylated Tau40 after 3 days of incubation compared with native Tau40 alone as a control (Fig. 6, panel A); the longer the incubation period with d-ribose, the stronger the ThT fluorescence of the glycated protein.
Fig. 6.
ThT-positive aggregation of d-ribosylated Tau. Tau40 was incubated in the presence or absence of d-ribose and aliquots were taken at different time intervals. ThT (final concentration 30 μM) was added to the samples and fluorescence (λex 450 nm; λex 485 nm) was measured as shown in panel A. These fluorescence measurements were analyzed according to Tsou’s method (panel B). Conditions for glycation of Tau with d-ribose were the same as those in Fig. 1
The time course of the increment in ThT fluorescence also underwent a biphasic process after a 48 h relaxation (Fig. 6, panel B). The first order rate constants of the slow and fast phases were not significantly different from those of Tau polymerization (also with a 48-h relaxation) (Table 1), illustrating that the formation of ThT-positive Tau aggregations in the presence of d-ribose appears from 48 h and accelerates after 96 h during glycation under our experimental conditions.
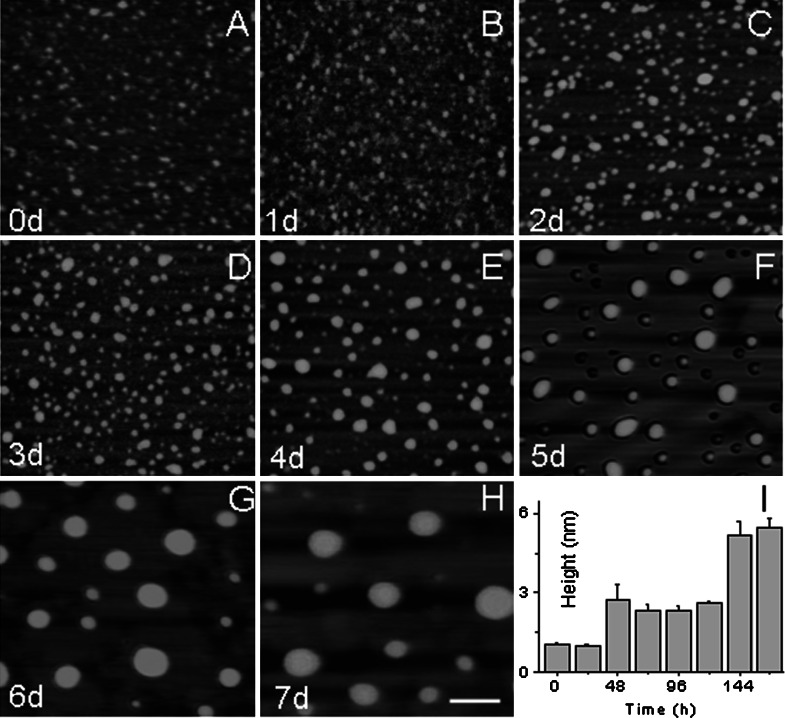
Atomic force microscopy was employed to image glycated Tau aggregations. Globular aggregations of d-ribosylated Tau40 were observed from 2 days of incubation as shown in Fig. 7. The particle size of d-ribose-glycated Tau40 increased significantly as the incubation proceeded. Aggregations remained globular during incubation of Tau protein with d-ribose for 7 days. Ribosylated Tau polymer fibrils were not observed under the experimental conditions used here. These results illustrate that d-ribosylated Tau40 generates soluble ThT-positive aggregates.
Fig. 7.
Atomic force microscopic image of d-ribosylated Tau. Aliquots of Tau40 incubated with d-ribose were observed by AFM (panel A–H). Sizes (nm) of the particles of Tau40 incubated with d-ribose are shown (panel I). Conditions for glycation of Tau with d-ribose were the same as those in Fig. 1. The scale bar equals 100 nm
Toxic effect of d-ribosylated Tau on SHSY5Y cells
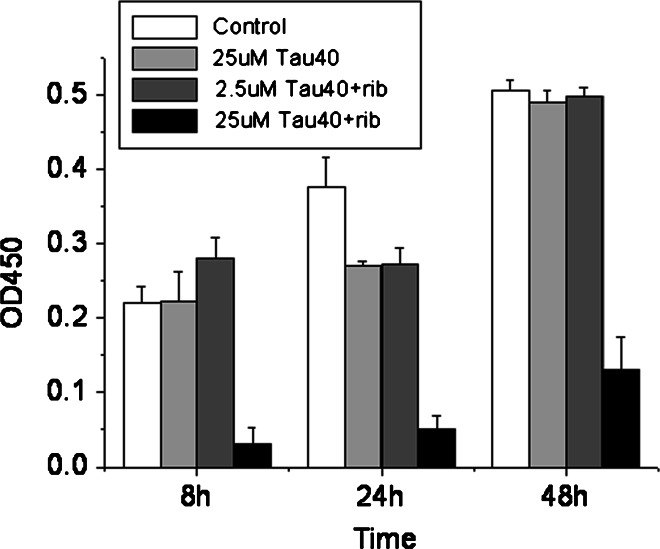
Since glycation of Tau40 with d-ribose induced ThT-positive aggregates, we examined the effect of these aggregates on the viability of SHSY5Y cells using MTT assays, as shown in Fig. 8. The number of viable cells decreased significantly after incubation with d-ribosylated Tau40 at 8, 24 and 48 h, compared with native Tau40 alone as a control. Furthermore, decreases in cell viability showed a concentration-dependent relationship with d-ribosylated Tau40 (2.5 and 25 μM). This indicates that d-ribosylated Tau40 has a high cytotoxicity to SHSY5Y cells.
Fig. 8.
Cell viability assays. SHSY5Y cells were incubated with d-ribosylated Tau for 8 h and cell viability was assayed with MTT at 24 and 48 h, respectively. (The Tau protein was previously glycated with d-ribose for 3 days.) Tau protein alone and the Tris–HCl buffer were used as controls. Viability of SHSY5Y cells was also assayed in the presence of glycated Tau at the concentrations indicated
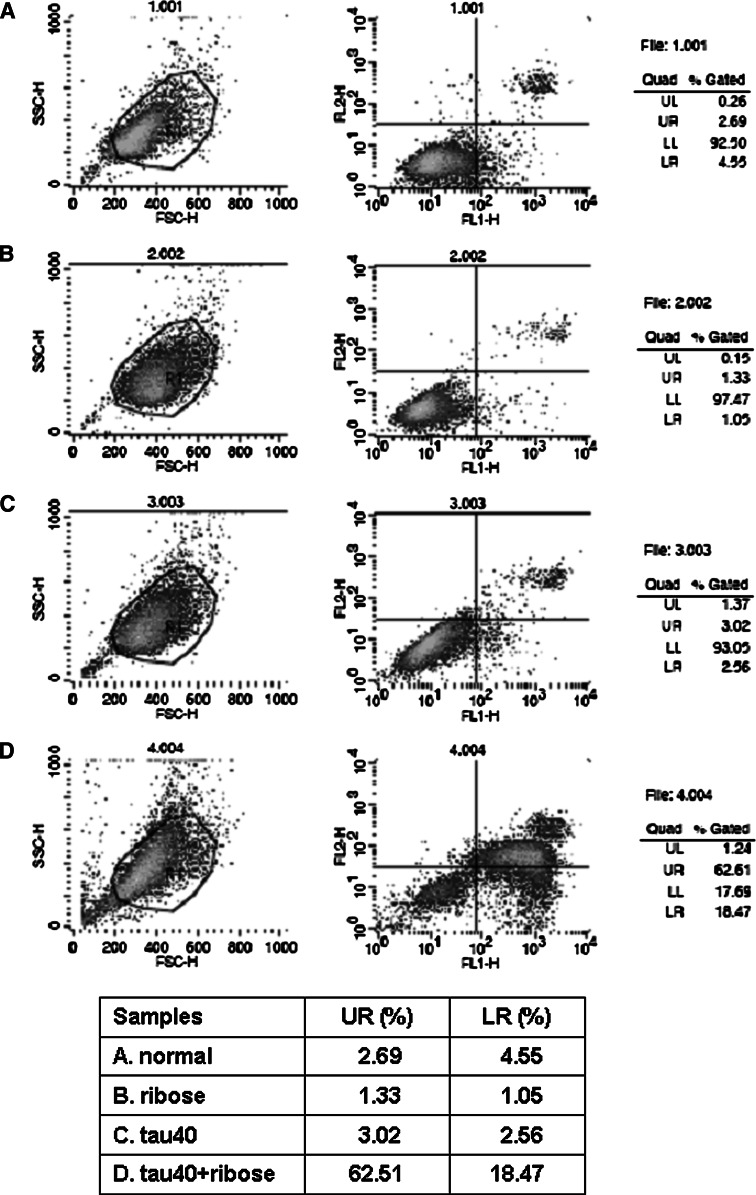
In order to further reveal the effect of glycated Tau40 on cell viability, Annexin V/PI assays were conducted by flow cytometry (Fig. 9). After incubating SHSY5Y cells with glycated Tau40 for 8 h, a significant increase in early apoptosis (LR, 18.47%) and late apoptosis/necrosis (UR, 62.51%) rates were observed (Fig. 9, panel D), while the LR and UR ratios were lower than 5% (LR) and 4% (UR) in Tau alone (panel C), d-ribose-treated (panel B) and untreated cells (panel A) used as controls (Fig. 9). These results demonstrate that d-ribosylated Tau40 was highly toxic to cells, inducing apoptosis and necrosis.
Fig. 9.
d-Ribosylated Tau40 induces SHSY5Y cell apoptosis. SHSY5Y cells were treated with d-ribose-glycated Tau for 8 h, cultured for 24 h (panel D), and subsequently analyzed by flow cytometry. Normal cells used as controls are shown in panel A, and cells incubated with d-ribose and Tau alone are shown in panels B and C
Discussion
Intraneuronal aggregation of Tau protein into filamentous lesions is involved in AD [48]. Posttranslational modifications, including hyperphosphorylation [49], glycation [18, 50], glycosylation [51, 52], ubiquitination [53], polyamination [54, 55], nitration [56], and proteolysis [57], have been shown to promote Tau fibrillization. Glycation is commonly regarded as a cause of disrupted protein function and produces cytotoxic products such as AGEs, leading to medical complications [58, 59]. However, most research groups study glycation of Tau with d -glucose, but not d-ribose. In this work, we show that d-ribose plays a role as an important reducing furanose in the glycation of Tau protein. Our results show that this neuronal protein is vulnerable to glycation with d-ribose and yields globular ThT-positive aggregations with high cytotoxicity to SHSY5Y cells.
d-Ribose glycates Tau with high efficiency
Glycation occurs between the amino groups of a protein, particularly the epsilon amino group of Lys. Arg, His, and Trp also contain reactive groups, which may react with reducing sugars. Such carbohydrate-amino acid reactions, often termed as “Maillard” or “non-enzymatic browning” reactions, result in linkages that are not hydrolyzed by digestive enzymes although the amino acids may still be recovered from the protein by acid hydrolysis [60]. The results from SDS-PAGE suggest that approximately 51 d-ribose units bind to one molecule of Tau protein. As Tau40 contains 44 Lys, 12 His and 14 Arg residues, which can react with d-ribose, our results suggest that most of the available amino acid residues react with d-ribose.
d-Ribose, an efficient glycator, is a naturally occurring pentose monosaccharide and an essential component for energy production in the body. d-Ribose exists in all living cells and in blood, and is present in the human brain [61]. Furthermore, Tau has an unstable conformation with a flexible peptidyl chain. Schweers and colleagues showed that the Tau protein exists in a “worm-like” conformation [62], having the irregular structure of an unfolded protein [63]. Thus, most Lys residues are exposed on the exterior of the Tau molecule because of the hydrophilic nature of the ε-amino group. Thus, the worm-like Tau protein has more opportunities to react with reducing sugars than globular-like proteins, explaining why Tau protein is rapidly glycated in the presence of d-ribose.
Although Tau40 can be glycated with d-glucose in vitro, it is still unclear whether d-glucose is the most important sugar in Tau glycation. It is well known that d-glucose has a lower reducing potential than d-ribose [15, 64]. Necular and Kuret [18] have reported that glycated Tau is not able to promote filament nucleation. Similarly, glycation of alpha-synuclein with d-glucose and d-fructose does not induce amyloidosis products [33]. Our recent data also show that glycation of BSA with d-ribose is much faster than with d-glucose [65]. This is probably due to the low glycation efficiency of d-glucose compared with d-ribose.
As mentioned above, the far-UV CD spectra (Fig. 5, panel D) showed little change in the secondary structure of Tau protein. Similar results have been observed during glycation of albumin in that the far-UV CD of the glycated protein was unchanged compared with albumin alone as a control [29]. Furthermore, the intrinsic fluorescence of Tau did not markedly change during the initial incubation with d-ribose, suggesting there was no distinct conformational change in the protein molecule. It is interesting, however, that a new fluorescence around 450 nm appears during glycation on excitation at 280 nm. According to Matiacevich and Buera [23], this most likely represents the formation of AGEs.
Fluorescence at 410 nm is related to polymerization of d-ribosylated Tau protein
Fluorescence at 410 nm appeared during the incubation of Tau protein with d-ribose (Fig. 4). This fluorescence is thought to be an indicator for AGEs resulting from glycation [23–26]. Here, however, we suggest that this fluorescence can also act as a probe for AGE-polymerization of Tau as (1) formation of the 410 nm fluorescent derivative follows the appearance of glycated Tau polymers as shown by SDS-PAGE (Fig. 1), (2) the kinetic increase in the fluorescence is a biphasic process (with a slow and a fast phase) similar to that of polymerization of the glycated protein on SDS-PAGE, and the first order rate constants are not significantly different (Table 1), and (3) the kinetic increase in the fluorescence is unlike that of the NBT assay to show the formation of fructosamine but similar to polymerization during glycation.
With respect to energy transfer between the intrinsic fluorophore (Tyr residue) and the glycated fluorescent derivative (Fig. 5, panel A), we detected a new fluorescence around 450 nm by excitation at 280 nm. The intrinsic fluorescence of Tyr residues around 310 nm may be absorbed by the glycated fluorescent derivative to emit around 450 nm. This evidence suggests that glycated fluorescent derivatives have close in situ associations with Tyr residues in Tau polymers.
Glycation of Tau protein with d-ribose promotes ThT-positive aggregation
According to previous reports, it is still unclear whether glycation with d-glucose can promote Tau protein to form ThT-positive aggregates [18]. This work, however, has revealed that d-ribose was able to glycate Tau40 quickly and to promote aggregation of glycated products into ThT-positive aggregates (Fig. 6, panel A). In particular, measurements of first order rate constants showed that formation of ThT-positive aggregations was rapid from day 5 of glycation, and paralleled glycated Tau protein polymerization. Under AFM, ThT-positive aggregates looked like globules on the mica surface, and fibrils could not be observed on the mica surface under the experimental conditions (Fig. 7). That is to say, glycation with d-ribose induces Tau protein to polymerize and form globular ThT-positive aggregations.
Advanced glycation end-products are thought to play an important role in AD by oxidation of Tau [16, 66]. AGE formation has detrimental effects on the structure and function of affected proteins [15]. Accumulation of AGEs has been implicated in normal aging and in the pathogenesis of diabetes-associated complications and AD. In the case of d-ribosylated Tau, the highly cytotoxic glycated products appear to be resulted from the globular aggregations of AGEs. Anti-AGE monoclonal antibodies recognized both glycated monomers and glycated polymers; that is, AGEs could be monomers, oligomers and polymers of glycated Tau. Cytotoxicity of glycated Tau was marked under the experimental conditions (Fig. 8), suggesting that ThT-positive aggregation of Tau protein has high cytotoxicity in addition to that of glycated Tau monomers.
Transition of d-ribosylated Tau from monomer to oligomer and polymer
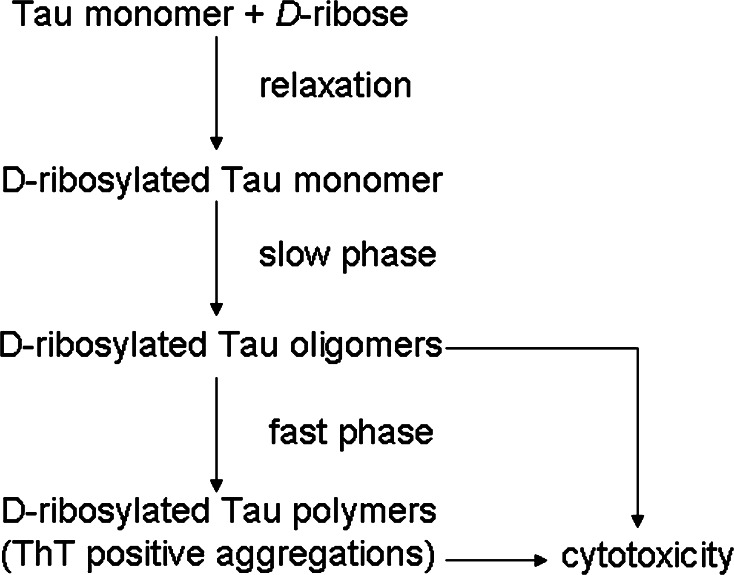
Glycation is a non-enzymatic posttranslational modification that involves a covalent linkage between a sugar and an amino group of a protein molecule forming a ketoamine [67]. Subsequent oxidation, fragmentation and/or crosslinking of ketoamines leads to the production of AGEs [68, 69]. Here, we present a brief summary of the glycation procedure of Tau protein in the presence of d-ribose (Fig. 10). d-Ribose initially reacts with Tau protein undergoing Schiff’s base reactions between the sugar and the protein, followed by conversion to ketoamines through Amadori rearrangement, resulting in a marked increase in the absorbance at 540 nm of the NBT reagent (a monophasic process). Second, the glycated Tau protein starts to oligomerize in a relatively slow procedure after a relaxation period (1–2 days). Finally, the glycated protein polymerizes further and forms ThT-positive aggregations through a relatively fast process. In short, the formation of Tau ThT-positive aggregations (polymerization) is fast, after the relatively slow oligomerization of d-ribosylated protein.
Fig. 10.
A putative scheme for polymerization of d-ribosylated Tau protein and its conversion to ThT-positive aggregations
d-Ribosylated Tau has high cytotoxicity
In this laboratory, we have studied glycated BSA in the presence of d-ribose and d-glucose. The cytotoxicity of d-ribosylated BSA is much higher than that of d-glucosylated BSA under the same conditions. We have also obtained similar results for the glycation of alpha-synuclein, an important protein involved in Parkinson’s disease (data not shown).
This work showed d-ribosylated Tau40 was able to inhibit growth of SHSY5Y cells significantly (Fig. 8). The dose-dependent cytotoxic manner observed revealed that glycated Tau40 may disturb neural cell metabolism and viability. Results of the flow cytometric analysis of Annexin V/PI support this viewpoint and showed that apoptosis and necrosis of SHSY5Y cells was induced by d-ribosylated Tau40 (Fig. 9). Nie and colleagues have reported that globular aggregates of Tau possess cytotoxicity [34]. Similarly, soluble ThT-positive aggregates of glycated Tau induced by d-ribose also have the ability to promote SHSY5Y cell death. It is known that Tau protein aggregation in NFTs in AD has cytotoxicity. d-Ribosylated Tau aggregation may be useful as a molecular model for simulating protein misfolding in vitro. In the light of high cytotoxicity, it appears that glycation may play an important role in Tau pathological processes.
Advanced glycation end-product-recombinant Tau generated reactive oxygen intermediates and induced oxidant stress when introduced into the cytoplasm of SHSY5Y neuroblastoma cells [66]. Our unpublished data show that apoptosis induced by d-ribosylated BSA is involved in oxidative stress [70]. It appears that the oxidative signaling pathway is at least involved in the cell death induced by the d-ribosylated Tau protein.
Glycated Tau protein has been found in human brain, especially in PHFs
It has been reported that glycated Tau40 is found in the brain of patients with AD [15, 16]. Many research groups have studied the relationship between glycation of Tau protein and the formation of PHF-Tau [15, 18, 50] and have found that Tau is glycated in PHF-Tau. Furthermore, glycated Tau induces lipid peroxidation in vivo and results in lesions within cells [71]. This suggests that glycation plays a role in stabilizing the PHF aggregations that are related to tangle formation in AD [15, 16, 21]. The monoclonal antibody against AGEs is commonly used to detect the glycated tau protein [65]. Previous studies show the antibody also recognizes both ribosylated and glucosylated products [65]. It is difficult to clarify whether the bound sugar is d-ribose or d-glucose using the anti-AGEs antibody. Thus, ribosylation of Tau is worth investigation.
The suggestion that d-ribosylated Tau may exist in brain is based on these observations: (1) non-enzymatically catalytic ribosylation occurs spontaneously with a relatively high speed [65]; (2) d-ribose exists in vivo such as cytoplasm and CSF [30]; (3) glycated Tau has been found in PHFs of Alzheimer’s patients [15, 66]; (4) the cytotoxicity of d-ribosylated protein is much higher than that of d-glucosylated protein; and (5) the intracellular protein was ribosylated when d-ribose was added to medium for cell culture (our unpublished data). This suggests that Tau is glycated in the presence of d-ribose.
In conclusion, d-ribosylated Tau can generate ThT-positive aggregates. These aggregates may induce SHSY5Y cell death by oxidative stress. Since Tau glycated with d-ribose has a similar character of pathological aggregation and high cytotoxicity, it could be used as an in vitro model for research to identify drugs that are valuable for disease treatment, such as the “anti-glycation” treatment for AD [72].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Xinyong Chen (Laboratory of Biophysics and Surface Analysis, School of Pharmacy, The University of Nottingham, Nottingham, NG7 2RD, UK) for his processing of AFM imaging. This project was supported by the following grants: NSFB-06J11, NSFC-30621004, 973-project-2006CB500703, and CAS-KSCX2-YW-R-119.
References
- 1.Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drechsel DN, Hyman AA, Cobb MH, Kirschner MW. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell. 1992;3:1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friede RL, Ho KC. The relation of axonal transport of mitochondria with microtubules and other axoplasmic organelles. J Physiol. 1977;265:507–519. doi: 10.1113/jphysiol.1977.sp011727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binder LI, Frankfurter A, Rebhun LI. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985;101:1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khatoon S, Grundke-Iqbal I, Iqbal K. Levels of normal and abnormally phosphorylated tau in different cellular and regional compartments of Alzheimer disease and control brains. FEBS Lett. 1994;351:80–84. doi: 10.1016/0014-5793(94)00829-9. [DOI] [PubMed] [Google Scholar]
- 6.Papasozomenos SC, Binder LI. Phosphorylation determines two distinct species of Tau in the central nervous system. Cell Motil Cytoskeleton. 1987;8:210–226. doi: 10.1002/cm.970080303. [DOI] [PubMed] [Google Scholar]
- 7.Wei Y, Qu MH, Wang XS, Chen L, Wang DL, Liu Y, Hua Q, He RQ. Binding to the minor groove of the double-strand, tau protein prevents DNA from damage by peroxidation. PLoS ONE. 2008;3:e2600. doi: 10.1371/journal.pone.0002600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindwall G, Cole RD. Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem. 1984;259:5301–5305. [PubMed] [Google Scholar]
- 9.Iqbal K, Grundke-Iqbal I, Zaidi T, Merz PA, Wen GY, Shaikh SS, Wisniewski HM, Alafuzoff I, Winblad B. Defective brain microtubule assembly in Alzheimer’s disease. Lancet. 1986;2:421–426. doi: 10.1016/S0140-6736(86)92134-3. [DOI] [PubMed] [Google Scholar]
- 10.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084–6089. [PubMed] [Google Scholar]
- 12.Smith MA, Taneda S, Richey PL, Miyata S, Yan SD, Stern D, Sayre LM, Monnier VM, Perry G. Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc Natl Acad Sci USA. 1994;91:5710–5714. doi: 10.1073/pnas.91.12.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horie K, Miyata T, Yasuda T, Takeda A, Yasuda Y, Maeda K, Sobue G, Kurokawa K. Immunohistochemical localization of advanced glycation end products, pentosidine, and carboxymethyllysine in lipofuscin pigments of Alzheimer’s disease and aged neurons. Biochem Biophys Res Commun. 1997;236:327–332. doi: 10.1006/bbrc.1997.6944. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki N, Fukatsu R, Tsuzuki K, Hayashi Y, Yoshida T, Fujii N, Koike T, Wakayama I, Yanagihara R, Garruto R, Amano N, Makita Z. Advanced glycation end products in Alzheimer’s disease and other neurodegenerative diseases. Am J Pathol. 1998;153:1149–1155. doi: 10.1016/S0002-9440(10)65659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko LW, Ko EC, Nacharaju P, Liu WK, Chang E, Kenessey A, Yen SH. An immunochemical study on tau glycation in paired helical filaments. Brain Res. 1999;830:301–313. doi: 10.1016/S0006-8993(99)01415-8. [DOI] [PubMed] [Google Scholar]
- 16.Yan SD, Yan SF, Chen X, Fu J, Chen M, Kuppusamy P, Smith MA, Perry G, Godman GC, Nawroth P. Non-enzymatically glycated tau in Alzheimer’s disease induces neuronal oxidant stress resulting in cytokine gene expression and release of amyloid beta-peptide. Nat Med. 1995;1:693–699. doi: 10.1038/nm0795-693. [DOI] [PubMed] [Google Scholar]
- 17.Kuhla B, Haase C, Flach K, Luth HJ, Arendt T, Munch G. Effect of pseudophosphorylation and cross-linking by lipid peroxidation and advanced glycation end product precursors on tau aggregation and filament formation. J Biol Chem. 2007;282:6984–6991. doi: 10.1074/jbc.M609521200. [DOI] [PubMed] [Google Scholar]
- 18.Necula M, Kuret J. Pseudophosphorylation and glycation of tau protein enhance but do not trigger fibrillization in vitro. J Biol Chem. 2004;279:49694–49703. doi: 10.1074/jbc.M405527200. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez C, Farias G, Maccioni RB. Modification of tau to an Alzheimer’s type protein interferes with its interaction with microtubules. Cell Mol Biol (Noisy-le-grand) 1998;44:1117–1127. [PubMed] [Google Scholar]
- 20.Ledesma MD, Bonay P, Colaco C, Avila J. Analysis of microtubule-associated protein tau glycation in paired helical filaments. J Biol Chem. 1994;269:21614–21619. [PubMed] [Google Scholar]
- 21.Nacharaju P, Ko L, Yen SH. Characterization of in vitro glycation sites of tau. J Neurochem. 1997;69:1709–1719. doi: 10.1046/j.1471-4159.1997.69041709.x. [DOI] [PubMed] [Google Scholar]
- 22.Nelson DL, Cox MM. Lehninger principle of biochemistry. 3. New York: Worth; 2004. pp. 297–324. [Google Scholar]
- 23.Matiacevich SB, Buera MP. A critical evaluation of fluorescence as a potential marker for the Maillard reaction. Food Chem. 2006;95:423–430. doi: 10.1016/j.foodchem.2005.01.027. [DOI] [Google Scholar]
- 24.Moreaux V, Birlouez-Aragon I. Degradation of tryptophan in heated ß-lactoglobulin-lactose mixtures is associated with intense Maillard reaction. J Agric Food Chem. 1997;45:1905–1910. doi: 10.1021/jf9605005. [DOI] [Google Scholar]
- 25.Ferrer E, Alegria A, Farre R, Clemente G, Calvo C. Fluorescence, browning index, and color in infant formulas during storage. J Agric Food Chem. 2005;53:4911–4917. doi: 10.1021/jf0403585. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Metzger LE. Application of fluorescence spectroscopy for monitoring changes in nonfat dry milk during storage. J Dairy Sci. 2007;90:24–37. doi: 10.3168/jds.S0022-0302(07)72605-X. [DOI] [PubMed] [Google Scholar]
- 27.Garlick RL, Mazer JS, Higgins PJ, Bunn HF. Characterization of glycosylated hemoglobins. Relevance to monitoring of diabetic control and analysis of other proteins. J Clin Invest. 1983;71:1062–1072. doi: 10.1172/JCI110856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaklai N, Garlick RL, Bunn HF. Nonenzymatic glycosylation of human serum albumin alters its conformation and function. J Biol Chem. 1984;259:3812–3817. [PubMed] [Google Scholar]
- 29.Mendez DL, Jensen RA, McElroy LA, Pena JM, Esquerra RM. The effect of non-enzymatic glycation on the unfolding of human serum albumin. Arch Biochem Biophys. 2005;444:92–99. doi: 10.1016/j.abb.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 30.James PE, Lang D, Tufnell-Barret T, Milsom AB, Frenneaux MP. Vasorelaxation by red blood cells and impairment in diabetes: reduced nitric oxide and oxygen delivery by glycated hemoglobin. Circ Res. 2004;94:976–983. doi: 10.1161/01.RES.0000122044.21787.01. [DOI] [PubMed] [Google Scholar]
- 31.He RQ, Yang MD, Zheng X, Zhou JX. Isolation and some properties of glycated d-glyceraldehyde-3-phosphate dehydrogenase from rabbit muscle. Biochem J. 1995;309(Pt 1):133–139. doi: 10.1042/bj3090133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He RQ, Li YG, Wu XQ, Li L. Inactivation and conformation changes of the glycated and non-glycated d-glyceraldehyde-3-phosphate dehydrogenase during guanidine-HCl denaturation. Biochim Biophys Acta. 1995;1253:47–56. doi: 10.1016/0167-4838(95)00145-k. [DOI] [PubMed] [Google Scholar]
- 33.Sheng Z, Liu Y, Chen L, He R. Nonenzymatic glycation of α-synuclein and changes in its conformation. Prog Biochem Biophys. 2008;35:1202–1208. [Google Scholar]
- 34.Nie CL, Wei Y, Chen X, Liu YY, Dui W, Liu Y, Davies MC, Tendler SJ, He RG. Formaldehyde at low concentration induces protein tau into globular amyloid-like aggregates in vitro and in vivo. PLoS ONE. 2007;2:e629. doi: 10.1371/journal.pone.0000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crowther RA, Olesen OF, Smith MJ, Jakes R, Goedert M. Assembly of Alzheimer-like filaments from full-length tau protein. FEBS Lett. 1994;337:135–138. doi: 10.1016/0014-5793(94)80260-2. [DOI] [PubMed] [Google Scholar]
- 36.Hua Q, He RQ. Tau could protect DNA double helix structure. Biochim Biophys Acta. 2003;1645:205–211. doi: 10.1016/s1570-9639(02)00538-1. [DOI] [PubMed] [Google Scholar]
- 37.Morton RE, Evans TA. Modification of the bicinchoninic acid protein assay to eliminate lipid interference in determining lipoprotein protein content. Anal Biochem. 1992;204:332–334. doi: 10.1016/0003-2697(92)90248-6. [DOI] [PubMed] [Google Scholar]
- 38.Baker JR, Metcalf PA, Johnson RN, Newman D, Rietz P. Use of protein-based standards in automated colorimetric determinations of fructosamine in serum. Clin Chem. 1985;31:1550–1554. [PubMed] [Google Scholar]
- 39.Baker JR, Zyzak DV, Thorpe SR, Baynes JW. Mechanism of fructosamine assay: evidence against role of superoxide as intermediate in nitroblue tetrazolium reduction. Clin Chem. 1993;39:2460–2465. [PubMed] [Google Scholar]
- 40.Coussons PJ, Jacoby J, McKay A, Kelly SM, Price NC, Hunt JV. Glucose modification of human serum albumin: a structural study. Free Radic Biol Med. 1997;22:1217–1227. doi: 10.1016/S0891-5849(96)00557-6. [DOI] [PubMed] [Google Scholar]
- 41.Nie CL, Wang XS, Liu Y, Perrett S, He RQ. Amyloid-like aggregates of neuronal tau induced by formaldehyde promote apoptosis of neuronal cells. BMC Neurosci. 2007;8:9. doi: 10.1186/1471-2202-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayo L, Stein R. Characterization of LPS and interferon-gamma triggered activation-induced cell death in N9 and primary microglial cells: induction of the mitochondrial gateway by nitric oxide. Cell Death Differ. 2007;14:183–186. doi: 10.1038/sj.cdd.4401989. [DOI] [PubMed] [Google Scholar]
- 43.Tsou CL. Folding of the nascent peptide chain into a biologically active protein. Biochemistry. 1988;27:1809–1812. doi: 10.1021/bi00406a001. [DOI] [PubMed] [Google Scholar]
- 44.Xu YJ, Wu XQ, Liu W, Lin XH, Chen JW, He RQ. A convenient assay of glycoserum by nitroblue tetrazolium with iodoacetamide. Clin Chim Acta. 2002;325:127–131. doi: 10.1016/S0009-8981(02)00277-2. [DOI] [PubMed] [Google Scholar]
- 45.Ikeda K, Higashi T, Sano H, Jinnouchi Y, Yoshida M, Araki T, Ueda S, Horiuchi S. N (epsilon)-(carboxymethyl)lysine protein adduct is a major immunological epitope in proteins modified with advanced glycation end products of the Maillard reaction. Biochemistry. 1996;35:8075–8083. doi: 10.1021/bi9530550. [DOI] [PubMed] [Google Scholar]
- 46.Swamy MJ, Surolia A. Studies on the tryptophan residues of soybean agglutinin. Involvement in saccharide binding. Biosci Rep. 1989;9:189–198. doi: 10.1007/BF01115995. [DOI] [PubMed] [Google Scholar]
- 47.Naiki H, Higuchi K, Hosokawa M, Takeda T. Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavin T1. Anal Biochem. 1989;177:244–249. doi: 10.1016/0003-2697(89)90046-8. [DOI] [PubMed] [Google Scholar]
- 48.Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33:95–130. doi: 10.1016/S0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 49.Gong CX, Liu F, Grundke-Iqbal I, Iqbal K. Post-translational modifications of tau protein in Alzheimer’s disease. J Neural Transm. 2005;112:813–838. doi: 10.1007/s00702-004-0221-0. [DOI] [PubMed] [Google Scholar]
- 50.Harrington CR, Colaco CA. Alzheimer’s disease. A glycation connection. Nature. 1994;370:247–248. doi: 10.1038/370247a0. [DOI] [PubMed] [Google Scholar]
- 51.Wang JZ, Grundke-Iqbal I, Iqbal K. Glycosylation of microtubule-associated protein tau: an abnormal posttranslational modification in Alzheimer’s disease. Nat Med. 1996;2:871–875. doi: 10.1038/nm0896-871. [DOI] [PubMed] [Google Scholar]
- 52.Robertson LA, Moya KL, Breen KC. The potential role of tau protein O-glycosylation in Alzheimer’s disease. J Alzheimers Dis. 2004;6:489–495. doi: 10.3233/jad-2004-6505. [DOI] [PubMed] [Google Scholar]
- 53.Bancher C, Grundke-Iqbal I, Iqbal K, Fried VA, Smith HT, Wisniewski HM. Abnormal phosphorylation of tau precedes ubiquitination in neurofibrillary pathology of Alzheimer disease. Brain Res. 1991;539:11–18. doi: 10.1016/0006-8993(91)90681-K. [DOI] [PubMed] [Google Scholar]
- 54.Murthy SN, Wilson JH, Lukas TJ, Kuret J, Lorand L. Cross-linking sites of the human tau protein, probed by reactions with human transglutaminase. J Neurochem. 1998;71:2607–2614. doi: 10.1046/j.1471-4159.1998.71062607.x. [DOI] [PubMed] [Google Scholar]
- 55.Tucholski J, Kuret J, Johnson GV. Tau is modified by tissue transglutaminase in situ: possible functional and metabolic effects of polyamination. J Neurochem. 1999;73:1871–1880. [PubMed] [Google Scholar]
- 56.Horiguchi T, Uryu K, Giasson BI, Ischiropoulos H, LightFoot R, Bellmann C, Richter-Landsberg C, Lee VM, Trojanowski JQ. Nitration of tau protein is linked to neurodegeneration in tauopathies. Am J Pathol. 2003;163:1021–1031. doi: 10.1016/S0002-9440(10)63462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang JZ, Gong CX, Zaidi T, Grundke-Iqbal I, Iqbal K. Dephosphorylation of Alzheimer paired helical filaments by protein phosphatase-2A and -2B. J Biol Chem. 1995;270:4854–4860. doi: 10.1074/jbc.270.9.4854. [DOI] [PubMed] [Google Scholar]
- 58.Hasegawa Y, Suehiro A, Higasa S, Namba M, Kakishita E. Enhancing effect of advanced glycation end products on serotonin-induced platelet aggregation in patients with diabetes mellitus. Thromb Res. 2002;107:319–323. doi: 10.1016/S0049-3848(02)00348-1. [DOI] [PubMed] [Google Scholar]
- 59.Koga K, Yamagishi S, Okamoto T, Inagaki Y, Amano S, Takeuchi M, Makita Z. Serum levels of glucose-derived advanced glycation end products are associated with the severity of diabetic retinopathy in type 2 diabetic patients without renal dysfunction. Int J Clin Pharmacol Res. 2002;22:13–17. [PubMed] [Google Scholar]
- 60.Scott ML, Nesheim MC, Young RJ. Nutrition of the chicken. Ithaca: Scott; 1982. pp. 100–102. [Google Scholar]
- 61.Pliml W, von Arnim T, Stablein A, Hofmann H, Zimmer HG, Erdmann E. Effects of ribose on exercise-induced ischaemia in stable coronary artery disease. Lancet. 1992;340:507–510. doi: 10.1016/0140-6736(92)91709-H. [DOI] [PubMed] [Google Scholar]
- 62.Schweers O, Schonbrunn-Hanebeck E, Marx A, Mandelkow E. Structural studies of tau protein and Alzheimer paired helical filaments show no evidence for beta-structure. J Biol Chem. 1994;269:24290–24297. [PubMed] [Google Scholar]
- 63.Rosenberg KJ, Ross JL, Feinstein HE, Feinstein SC, Israelachvili J. Complementary dimerization of microtubule-associated tau protein: implications for microtubule bundling and tau-mediated pathogenesis. Proc Natl Acad Sci USA. 2008;105:7445–7450. doi: 10.1073/pnas.0802036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grandhee SK, Monnier VM. Mechanism of formation of the Maillard protein cross-link pentosidine. Glucose, fructose, and ascorbate as pentosidine precursors. J Biol Chem. 1991;266:11649–11653. [PubMed] [Google Scholar]
- 65.Wei Y, Chen L, Chen J, Ge L, He RQ. Rapid glycation with d-ribose induces globular amyloid-like aggregations of BSA with high cytotoxicity to SH-SY5Y cells. BMC Cell Biol. 2009;10:10. doi: 10.1186/1471-2121-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yan SD, Chen X, Schmidt AM, Brett J, Godman G, Zou YS, Scott CW, Caputo C, Frappier T, Smith MA. Glycated tau protein in Alzheimer disease: a mechanism for induction of oxidant stress. Proc Natl Acad Sci USA. 1994;91:7787–7791. doi: 10.1073/pnas.91.16.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Day JF, Thorpe SR, Baynes JW. Nonenzymatically glucosylated albumin. In vitro preparation and isolation from normal human serum. J Biol Chem. 1979;254:595–597. [PubMed] [Google Scholar]
- 68.Degenhardt TP, Thorpe SR, Baynes JW. Chemical modification of proteins by methylglyoxal. Cell Mol Biol (Noisy-le-grand) 1998;44:1139–1145. [PubMed] [Google Scholar]
- 69.McCance DR, Dyer DG, Dunn JA, Bailie KE, Thorpe SR, Baynes JW, Lyons TJ. Maillard reaction products and their relation to complications in insulin-dependent diabetes mellitus. J Clin Invest. 1993;91:2470–2478. doi: 10.1172/JCI116482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alikhani M, Maclellan CM, Raptis M, Vora S, Trackman PC, Graves DT. Advanced glycation end products induce apoptosis in fibroblasts through activation of ROS, MAP kinases, and the FOXO1 transcription factor. Am J Physiol Cell Physiol. 2007;292:C850–C856. doi: 10.1152/ajpcell.00356.2006. [DOI] [PubMed] [Google Scholar]
- 71.Rofina JE, Singh K, Skoumalova-Vesela A, van Ederen AM, van Asten AJ, Wilhelm J, Gruys E. Histochemical accumulation of oxidative damage products is associated with Alzheimer-like pathology in the canine. Amyloid. 2004;11:90–100. doi: 10.1080/13506120412331285779. [DOI] [PubMed] [Google Scholar]
- 72.Munch G, Kuhla B, Luth HJ, Arendt T, Robinson SR. Anti-AGEing defences against Alzheimer’s disease. Biochem Soc Trans. 2003;31:1397–1399. doi: 10.1042/BST0311397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.