Abstract
In the present study we demonstrated that neurotoxin MPP+-induced DNA damage is followed by ataxia telangiectasia muted (ATM) activation either in cerebellar granule cells (CGC) or in B65 cell line. In CGC, the selective ATM inhibitor KU-55933 showed neuroprotective effects against MPP+-induced neuronal cell loss and apoptosis, lending support to the key role of ATM in experimental models of Parkinson’s disease. Likewise, we showed that knockdown of ATM levels in neuroblastoma B65 cells using an ATM-specific siRNA attenuates the phosphorylation of retinoblastoma protein without affecting other cell-cycle proteins involved in the G0/G1 cell-cycle phase. Moreover, we demonstrated DNA damage, in human brain samples of PD patients. These findings support a model in which MPP+ leads to ATM activation with a subsequent DNA damage response and activation of pRb. Therefore, this study demonstrates a new link between DNA damage by MPP+ and cell-cycle re-entry through retinoblastoma protein phosphorylation.
Keywords: ATM, MPP+, Cell cycle, Apoptosis, Retinoblastoma protein, B65 neuroblastoma, Parkinson’s disease
Introduction
Parkinson’s disease (PD) is the second most important neurodegenerative disease in the world after Alzheimer’s disease (AD) [1]. However, although enormous efforts have been made to find an effective treatment for the process of neuronal cell loss, there is still no drug therapy currently available to delay the illness. The main reason behind this lack of an effective PD treatment is that the mechanism of neuronal loss is not completely understood.
Consequently, experimental studies have been carried out using specific neurotoxins which reproduce the disease in vivo and in vitro. Among these, the most widely used are various pesticides (rotenone, paraquat), 6-hydroxidopamine and MPTP [2–4]. All these PD neurotoxins have displayed a series of common features regarding the process of neuronal cell death. They induce apoptosis in neuron and neuron-like cell cultures via intrinsic apoptotic pathway, and this process is mediated through the production of reactive oxygen species (ROS) [5]. However, the most widely used neurotoxin is probably MPTP and its metabolite MPP+ because it is a selective complex I inhibitor which reproduces the symptoms of the disease more closely [6].
Although the biochemical pathways involved in the apoptotic process in PD are still not completely understood, several mechanisms have been suggested, including ROS production, the activation of calpains and caspases, the mitochondrial release of apoptosis inducing factor (AIF) and cytochrome c, activation of the c-Jun N-terminal kinase and GSK3β and also the inhibition of prosurvival pathways such as Akt [7–14]. More interestingly, recent studies have suggested that cell-cycle proteins may play a role in the process of neuronal loss in PD [15–17].
In previous reports we demonstrated that apoptosis was induced in cerebellar granule cell (CGC) following treatment with the complex I inhibitor MPP+ [18–20]. Similarly, we showed that this neurotoxin induced cell-cycle re-entry in CGC that may be implicated in neuronal apoptosis. Our hypothesis was based on the evidence that pharmacological CDK inhibitors such as flavopiridol showed neuroprotective and antiapoptotic effects against MPP+ in vitro and also in vivo [16, 19]. Moreover, CDK inhibitors have neuroprotective effects in a wide range of experimental neurodegeneration models, and studies performed using the brains of patients suffering from neurodegenerative diseases such as PD, AD, and Huntington’s disease have demonstrated an increase in the expression of proteins involved in the cell cycle [21–31]. Interestingly, the possible link between cell-cycle re-entry and neuronal apoptosis could be the transcription factor E2F-1 [32, 33]. However, the precise mechanism responsible for orchestrating the process of cell-cycle re-entry in neurons remains unknown. For instance, cell-cycle re-entry is mediated by CDK5, which phosphorylates pRb [34]. Others authors have suggested that the GSK3β enzyme, whether through pRb phosphorylation or E2F-1 regulation, is potentially involved in neuronal cell-cycle control [35–37]. Another possible candidate in this process of cell-cycle re-entry is the ataxia telangiectasia muted (ATM) enzyme, which is activated by DNA damage. Specifically, DNA double-strand breaks activate this enzyme, which then phosphorylates several downstream substrates, including p53. In this context, the evidence demonstrates that DNA damaging agents such as camptothecin and β-amyloid, or the stimulation of N-methyl-d-aspartate receptor induce the activation of ATM, which is associated with a process of cell-cycle re-entry [38–40].
Elsewhere, we have demonstrated that ATM is rapidly activated following treatment with MPP+, although further clarification is still required concerning how ATM favors the process of cell-cycle re-entry [20]. Nevertheless, the role of DNA damage as an upstream initiator of postmitotic neuron apoptosis, or as a consequence of the degeneration is unclear. In this study we examined in detail the role of DNA damage-triggered apoptosis in a neuronal cell culture and in a neuroblastoma cell line. In previous studies it has been demonstrated that CGC are a homogeneous neuronal population highly sensitive to the toxic effects of the mitochondrial toxin MPP+ [2, 6]. Our results indicate that MPP+ induces double-strand breaks (DSB) in DNA, followed by activation of γH2AX. The same effect is seen in brain samples of PD patients and Western-blot samples of B65 neuroblastoma cells. The present research reveals the implication of DNA damage as a key component in the process of cell death mediated by experimental neurotoxins used as models of PD.
Materials and methods
Preparation of cell cultures
Primary cultures of cerebellar granule neurons were prepared from postnatal day 7 Sprague-Dawley rat pups as described elsewhere [18–20]. Cells were dissociated in the presence of trypsin and DNase I and placed in poly-l-lysine (100 μg/ml)-coated dishes at a density of 8 × 105 cells/cm2 in basal Eagle’s medium supplemented with 10% heat-inactivated fetal bovine serum, 0.1 mg/ml gentamicin, 2 mM l-glutamine and 25 mM KCl. Cytosine-d-arabinofuranoside (10 μM) was added to the culture medium 24 h after plating to prevent the replication of non-neuronal cells. The cultures were maintained at 37°C in a humidified incubator with 5% CO2/95% air and left undisturbed until the experiments were performed. All procedures involving animals and their care were approved by the ethics committee of the University of Barcelona, and were conducted in accordance with national (Spanish) laws.
Neuroblastoma B65 is a dopaminergic cell line was purchased from the European Collection of Cell Cultures (ECACC, Salisbury, UK), and is a suitable model for studying the effect of inhibitors of mitochondrial complex I on apoptosis [9]. Cells were placed at 200 cells/mm2 and cultured in DMEM media containing 10% FCS.
Assessment of cell viability
B65 cells were used after 24 h of in vitro culture. CGN were used after 7–8 days in vitro [19]. Drugs: KU-55933, aphidicolin, and 1,5-isoquinonediol (Sigma-Aldrich) were dissolved in dimethyl sulfoxide (DMSO), while dideoxycytidine (Sigma) was dissolved in culture media. These were then added to the neuronal preparation at the precise concentrations, 1 h before addition of MPP+ (200 μM). To assess loss in cell viability, we used the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium] method. MTT was added to the cells at a final concentration of 250 μM and incubated for 1 h, allowing the reduction in MTT to produce a dark blue formazan product. Media were then removed, and cells were dissolved in DMSO. Formazan production was measured by the absorbency change at 595 nm using a microplate reader (BioRad Laboratories, CA, USA). Viability results were expressed as percentages. The absorbance measured from non-treated cells was taken to be 100%.
Lactate dehydrogenase release assay
Cellular injury was quantitatively assessed by measuring the activity of lactate dehydrogenase (LDH) released from damaged cells into the culture medium. CGCs were used after 7–8 days. KU-55933 (10 μM) was added to the medium, at the concentrations indicated, 1 h before the addition of MPP+. The LDH release is proportional to the number of damaged or destroyed cells. Cell death was determined 24 h after MPP+ addition using the LDH-cytotoxicity assay kit (Medical and Biological Laboratories Co., Ltd., Watertown, UK). The increase in LDH activity in the supernatant directly correlates with the amount of formazan formed. Absorbances of the samples were measure at 490 nm using a microplate reader. The results are expresses as percentages of control.
Analysis of DNA fragmentation by flow cytometry
DNA fragmentation was measured by flow cytometric analysis of propidium iodide-stained cellular DNA as described elsewhere [19, 20]. CGC were subsequently stained with 10 μg/ml of propidium iodide and analysis of DNA fragmentation was performed using a Beckman Coulter Epics XL flow cytometer (argon laser, excitation wavelength 488 nm). A minimum of 10,000 events were acquired in list mode while gating the forward and side scatters to exclude propidium iodide-positive cell debris and analyzed in FL-3 for the appearance of the sub-G1 peak.
Detection of condensed nuclei by microscopic cell counting
Propidium iodide staining was also used to evaluate morphologic evidence of apoptosis (e.g., condensed nuclei). After the corresponding treatment (dideoxycytidine, KU-55933, aphidicolin and 1,5-isoquinonediol), cells were fixed in 4% paraformaldehyde PBS solution, pH 7.4, for 1 h at room temperature. After washing with PBS, they were incubated for 3 min with a solution of PI (75 μg/ml) in PBS. Stained cells were visualized under UV illumination using the 20× objective and digitized images were captured. Apoptotic cells resulted in shrunken, brightly fluorescent nuclei showing high fluorescence.
Measurement of cytosolic Ca2+ increase
The increase in intracellular free Ca2+ was determined in CGN grown on glass coverslips (Corning Costar Corp., Acton, MA), using an Mg2+-free, Locke-HEPES buffer (LH–BSA) containing 0.1% BSA, which consisted of 154 mM NaCl, 5.6 mM KCl, 3.6 mM NaHCO3, 1.3 mM CaCl2, 5.6 mM d-glucose, 10 mM HEPES, and 0.1% BSA (pH 7.35). After 9–13 days culture, a coverslip was carefully transferred to a Petri dish containing 3 ml of LH–BSA buffer and 2 μM fura-2 acetoxymethyl ester (Molecular Probes, Eugene, OR) and incubated at 37°C for 1 h in a cell incubator. For fluorescence recording, the coverslip was carefully rinsed in LH–BSA buffer, mounted on a specific holder (coverslip accessory L2250008; PerkinElmer Life and Analytical Sciences, Boston, MA), and placed in a quartz cuvette containing 1.3 ml of LH–BSA buffer. Measurements were made at 37°C with continuous mild stirring in an LS50B PerkinElmer fluorescence spectrometer equipped with a fast-filter accessory for fura-2 fluorescence ratio measurements. Emission data (510 nm) were collected with alternate excitation at 340 and 380 nm and the ratio F340/F380 calculated in real time, using proprietary software (FLWin-Lab 2.0; PerkinElmer).
Immunocytochemistry assays
B65 cells were grown on sterile glass slides. After the stimuli, cells were washed twice in PBS and fixed in 4% paraformaldehyde/PBS, pH 7.4 for 1 h at room temperature. Cells were pre-incubated for 30 min with PBS containing 0.3% Triton X-100 and 10% BSA at room temperature. After blocking, cells were incubated overnight at 4°C with a pATM antibody at a 1:100 dilution in PBS containing 0.3% Triton X-100 and 5% BSA. Cells were then washed and incubated with fluorescent secondary antibody, for 2 h at room temperature. An immunosignal analysis was performed using Nikon Eclipse fluorescence microscopy at 20× magnification and digitized images were captured.
Evaluation of DNA synthesis: BrdU labeling
We evaluated cell-cycle progression by monitoring the incorporation of BdrU into the cells cultured in microtiter plates [41]. Following partial denaturation of double-stranded DNA, BdrU was detected immunochemically, which allowed us to count the cells that synthesize DNA. Cells were incubated with various concentrations of aphidicolin, KU-55933 (1–10 μM), aphidicolin (1–50 μM) 24 h before S/K deprivation. Then, apoptotic stimuli were induced in the presence of various concentrations of different compounds. After 24 h, BdrU incorporation was measured using a colorimetric-based detection kit according to the manufacturer’s guidelines (Oncogene, Darmstadt, Germany).
Western-blot analysis
Aliquots of cell homogenate containing 15 μg of protein per sample were analyzed by Western blot [42]. Briefly, samples were placed in sample buffer [0.5 m Tris–HCl, pH 6.8, 10% glycerol, 2% (w = v) SDS, 5% (v = v) 2-β-mercaptoethanol, 0.05% bromophenol blue] and denatured by boiling at 95–100°C for 5 min. Samples were separated by electrophoresis on 10% acrylamide gels. Thereafter, proteins were transferred to PVDF sheets using a transblot apparatus. Membranes were blocked overnight with 5% nonfat milk dissolved in TBS-T buffer (50 mM Tris, 1.5% NaCl, 0.05% Tween 20, pH 7.5). They were then incubated with primary monoclonal antibodies against pγH2AX ser139, cyclin A (1:500; Abcam plc, Cambridge, UK), cyclin D1, p53ser15, tyr15 p-cdc2, pRbser780 (1:500; Cell Signalling Technology, Denvers, MA), total p53, Rb (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA) and β-actin 1:10,000 as a protein loading control was used (Santa Cruz Biotechnology, Santa Cruz, CA). After 12 h of incubation, the blots were washed thoroughly in TBS-T buffer and incubated for 1 h with a peroxidase-conjugated IgG secondary antibody (1:3,000). Immunoreactive protein was visualized using a chemiluminescence-based detection kit. Protein levels were determined by densitometry of the bands using Quantity One®. This software detects the bands obtained by Western blot and gives individual values which are dependent on the light quantification of the corresponding band. Measurements are expressed as arbitrary units. All results were normalized for β-actin.
Human samples
We examined the brains (specifically the gyrus cinguli) of three PD cases and four age-matched controls. Clinically, all PD patients had suffered from parkinsonism for 8–15 years. None of them had suffered from cognitive impairment. The post-mortem delay between death and tissue processing was between 3 and 20 h. Cases with and without clinical neurological disease were processed in parallel. Brain samples were obtained from the Institute of Neuropathology and the University of Barcelona Brain Banks after receiving the informed consent of the patients or their relatives and following the approval of the local ethics committees. At autopsy, one cerebral hemisphere and alternative tangential sections of the brain stem and cerebellum were fixed in 4% buffered formalin, whereas the other hemisphere and remaining sections of the brain stem and cerebellum were frozen on dry ice and stored at −80°C until use (Table 1).
Table 1.
Summary of the cases used in this study
| Sample | Age (years) (mean ± SD) | Gender (M/F) | Postmortem interval (h) (mean ± SD) |
|---|---|---|---|
| Control, n = 4 | 68.0 ± 12.2 | 1/3 | 6.69 ± 2.25 |
| PD, n = 3 | 65.3 ± 12.9 | 3/0 | 6.56 ± 2.44 |
PD Parkinson’s disease, F female, M male
Neuropathological characterization of PD was carried out according to well-established clinical and neuropathological criteria [43]. Following neuropathological examination, patients were categorized as PD (all stage 4). No neurological symptoms or metabolic disorders had occurred in the control cases. Likewise, no abnormalities, including AD-associated changes or vascular disorders, were found in controls. Gel electrophoresis and Western-blot analyses were carried out following procedures described in previous studies (Alvira et al. 2008).
ATM silencing
In the gene-silencing experiments, we used a small interference sequence targeting the boundary between the exon 6 and 7 of the ATM mRNA (sense: GGCUAUUCAGUAUGCCAGAtt; antisense: UCUGGCAUAC UGAAUAGCCtt). To transfect cells with siRNA, we followed the general recommendations of the purchaser. Briefly, the lipid-based agent siPORT™ NeoFX™ was mixed with serum-free medium at a ratio of 5/100 (μl) for each 35-mm well, and the mix was incubated for 10 min at room temperature. Small RNA was then diluted in serum-free medium to a final concentration of 10 nM for each 35-mm well, and 12.5 μl of 2 μM siRNA was diluted with 100 μl of serum-free media. Finally, the newly formed transfection complexes were transferred into the empty wells of the culture plate and mixed with the cell suspension. As a transfection control, both siRNA targeting ATM and pEGFP-C1 plasmid were cotransfected at a molar ratio of 3/1 (respectively) and the percentage of green-fluorescent cells was determined.
Statistical analysis
Data are given as the mean ± SEM of at least three experiments. In all the experiments, the data were analyzed using Student’s t test for two group’s comparisons or with ANOVA followed by the Tukey–Kramer multiple comparisons test. p values lower than 0.05 were considered significant.
Results
KU-55933 inhibits MPP+-induced apoptosis in CGC
Elsewhere we demonstrated that MPP+ was capable of activating the enzyme ATM in CGC (Alvira et al. 2007b). Here, to explore the role of ATM activation in MPP+-induced apoptosis, we used a specific inhibitor of this PI3K signaling pathway, namely KU-55933. Unlike many commonly used inhibitors of the phosphatidylinositol 3′-kinase-related kinase (PIKK) family to which ATM and ATR belong, KU-55933 is considered a relatively specific inhibitor of ATM [44]. It is 100 times more active against ATM than against other PIKK family members [44].
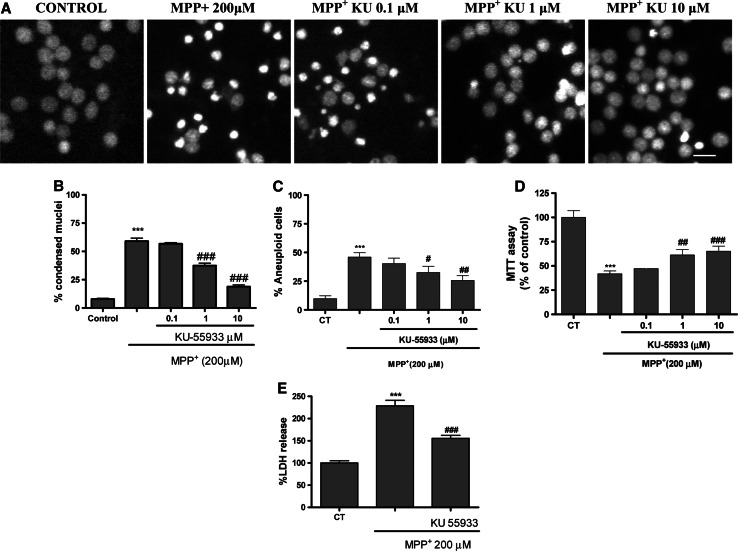
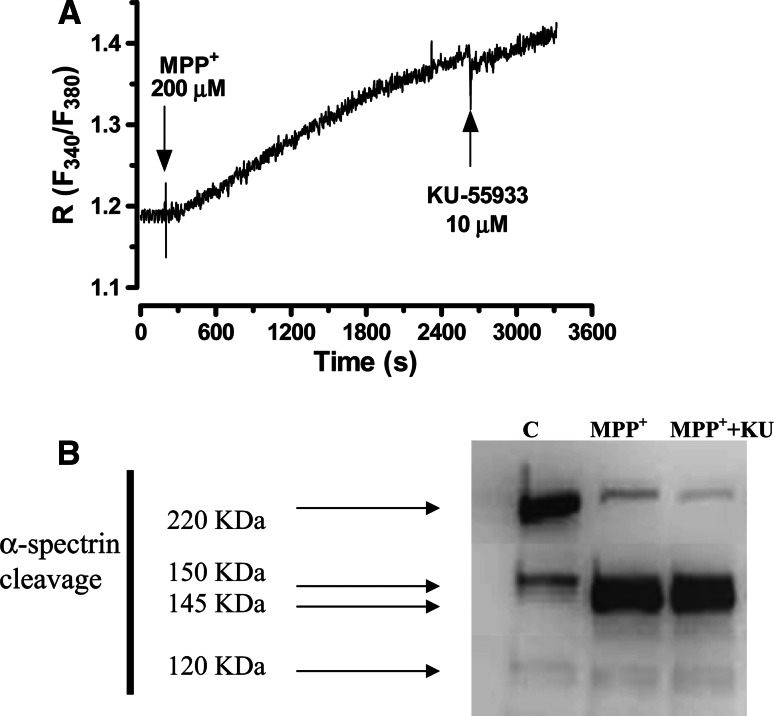
Here, the prior addition of KU-55933 (1–10 μM) to neuronal cell cultures partially prevented the decrease in cell viability measured by the MTT method (Fig. 1d). On the other hand, the neuroprotective effects of 10 μM KU-55933 were demonstrated using the LDH method. Furthermore, KU-55933 inhibited apoptosis measured by nuclear condensed cell counting and DNA fragmentation (Fig. 1a, b). After MPP+ treatment, 55% of CGC showed condensed nuclei and in the presence of KU-55933 (10 μM), the number of condensed nuclei decreased to 18% (p < 0.05) (Fig. 1a). Likewise, KU-55933 prevented MPP+-induced DNA fragmentation measured by flow cytometry (Fig. 1c). On the other hand, KU-55933 did not prevent the strong rise in [Ca2+]I mediated by MPP+ measured with fura-2 dye (Fig. 2a). Previous studies have demonstrated that calpain inhibitors show antiapoptotic effects against MPP+, suggesting that the activation of this cysteine protease constitutes a key apoptotic component of this neurotoxin. In this study, KU-55933 did not prevent α-spectrin proteolysis as measured by a particular antibody which measures specific calpain band 145 kDa, calpain and caspase activity 150-kDa bands and specific caspase band 120 kDa (Fig. 2b).
Fig. 1.
a Representative images of nuclei control sample, MPP+ 200 μM treated and MPP+ in the presence of different concentrations of KU-55933 (0.1–10 μM) respectively (calibration bar 10 μM). bBar chart showing the percentage effects of different concentrations of KU-55933 on MPP+-induced nuclear condensation in CGC. The nuclei were counted on a fluorescence microscope, distinguishing normal nuclei from the condensed ones following the criteria stated in the “Materials and methods”. cBar chart showing the percentage effects of different concentrations of KU-55933 on MPP+-induced DNA fragmentation measured by flow cytometry. d Effects of KU-55933 (0.1–10 μM) on MPP+-induced neuronal cell death. Neuronal survival was determined using the MTT assay as described in “Materials and methods”. eBar chart showing LDH present in the bathing medium 24 h after MPP+ treatment in the presence of KU-55933. Each point is the mean ± SEM of four wells of five to six different cultures. When necessary, statistical analyses were carried out using one-way ANOVA followed by Tukey’s tests: ***p < 0.001 versus control; #p < 0.05; ##p < 0.01; ###p < 0.001 versus MPP+ 200 μM
Fig. 2.
a MPP+-induced calcium increase, measured by fura-2, was not prevented by KU-55933 (10 μM). b Representative Western-blot analysis (from four different Western blots) of α-spectrin degradation products SBPD 150/145, as an indicator of calpain, and SBDP120, as an indicator of caspase-3 activity, in CGC treated with MPP+ 200 μM, for 24 h
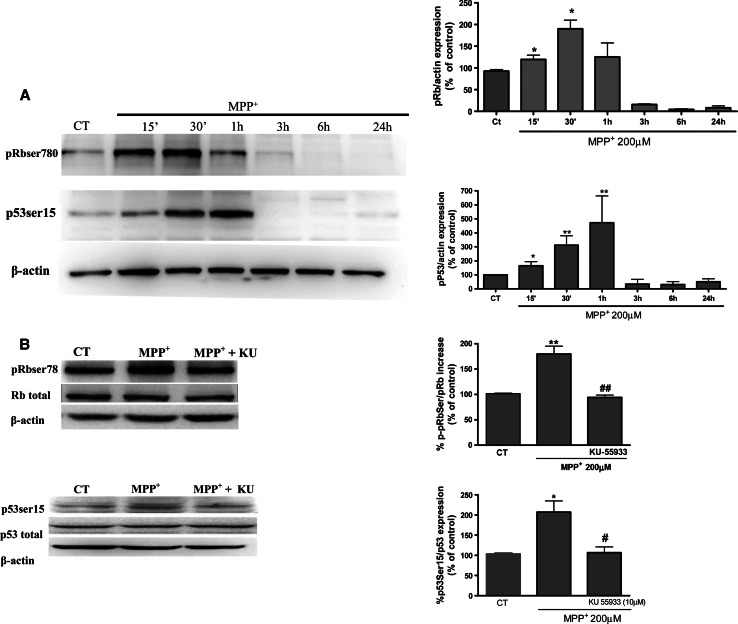
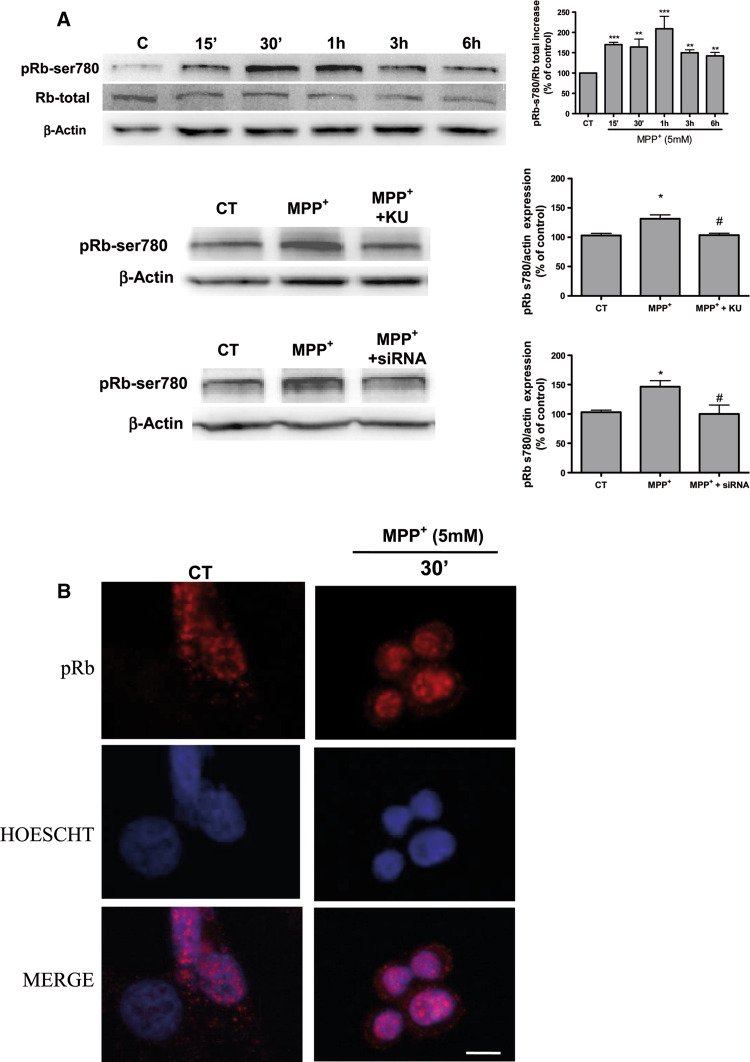
Since KU-55933 showed neuroprotective effects against MPP+ neurotoxicity, the next experiments aimed to evaluate the potential link between DNA damage and cell-cycle re-entry in CGC. Retinoblastoma protein phosphorylation constitutes a key and restrictive checkpoint in the process of cell-cycle re-entry [45]. Here, a time course Western-blot analysis study was carried out in order to evaluate whether retinoblastoma protein is significantly phosphorylated after MPP+ treatment. Thus, as shown in Fig. 3, at 15–30 min of MPP+ treatment (Fig. 2a) pRb was phosphorylated and KU-55933 (10 μM) significantly prevented pRb phosphorylation. Western-blot data clearly demonstrate a rapid phosphorylation in p53ser15 at 15–30 min (Fig. 3a). Likewise KU-55933 inhibits significantly the phosphorylation of p53 and pRb (Fig. 3b).
Fig. 3.
a Representative immunoblots showing the expression of pRbser780 p53-ser15 in CGC after a time course treatment with MPP+. Band intensities were calculated as percentages of the control. b Representative immunoblots showing the expression of pRb-ser780 and p53-ser15 in CGC after a treatment with MPP+ for 30 min alone or in presence of KU-55933 (10 μM). Columns and bars represent the mean ± SEM of four or five separate experiments with four or five different culture preparations (n = 4). Statistical significance was determined by one-way ANOVA followed by Tukey’s tests: *p < 0.05, **p < 0.01, ***p < 0.001 versus control. #p < 0.05; ##p < 0.01; ###p < 0.001 versus KU-55933 treatment
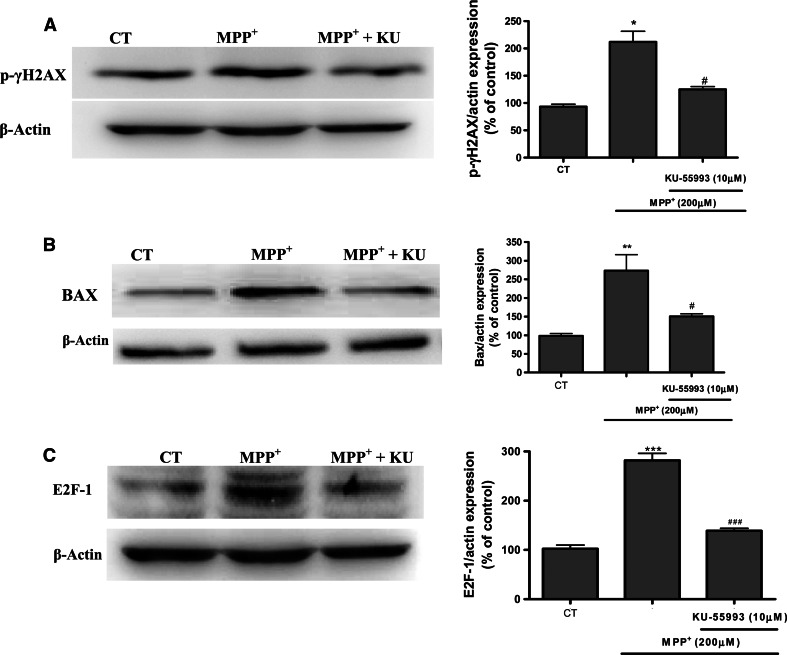
ATM is activated by DNA double-strand breaks; thus, we investigated the induction of DSBs and ATM activation through two direct targets of ATM, p53-ser15 and the expression of phosphorylated histone γ-H2AX at serine 139 (pH2AXser139) (Biton et al. 2006). In response to MPP+ treatment, the γ-H2AX signal was increased at 1 h and treatment with KU-55933 (10 μM) significantly (p < 0.05) suppressed the ser139 phosphorylation (Fig. 4a). The link between DNA damage activation and mitochondrial activation could be BAX induction and mobilization towards the mitochondria, as this facilitates mitochondrial outer membrane permeabilization, which in turn releases the apoptogenic factors that cause apoptotic and non-apoptotic programmed cell death. Western-blot data thus clearly indicates that KU-55933 decreases BAX expression (Fig. 4b).
Fig. 4.
Representative immunoblots showing the expression of p-γH2AX, BAX, and E2F-1 in CGC after a treatment with MPP+ for 1 h in the case of p-γH2AX and 24 h. Band intensities were calculated as percentages of the control. Columns and bars represent the mean ± SEM of four or five separate experiments with four or five different culture preparations (n = 4). Statistical significance was determined by one-way ANOVA followed by Tukey’s tests: *p < 0.05, **p < 0.01, ***p < 0.001 versus control. # p < 0.05; ## p < 0.01; ### p < 0.001 versus KU-55933 treatment
Moreover, since MPP+ induced a process of cell-cycle re-entry, our research showed that 24 h of CGC treatment with MPP+ 200 μM induced an increase in E2F-1 expression that was prevented by KU-55933 (Fig. 4c).
Evaluation of additional DNA repair signals after MPP+ treatment
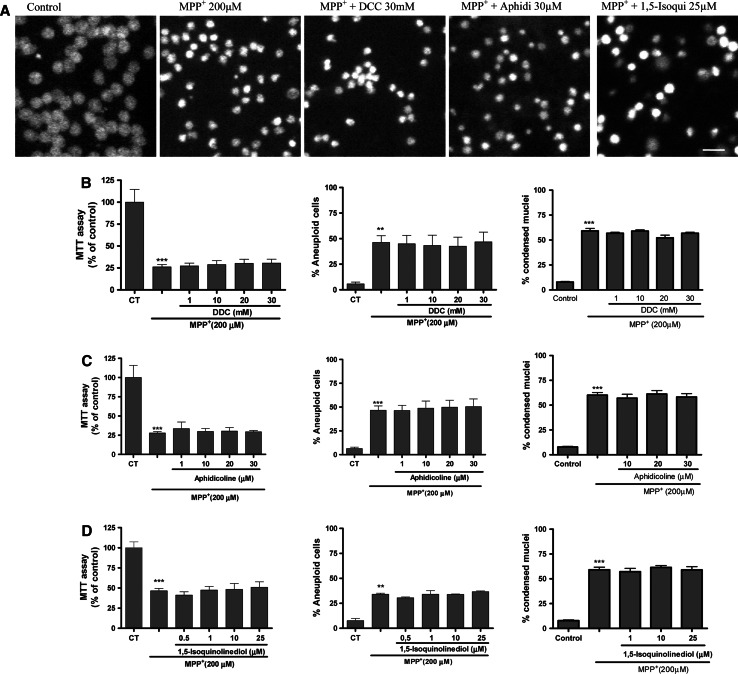
In an attempt to relate the process of neuronal cell-cycle re-entry and apoptotic cell death loss, it has been hypothesized that the polymerase-β enzyme is implicated in this pathway [46–48]. In this study, we evaluated the expression of DNA polymerase β and δ, and studied the effects of dideoxycytidine (DCC), an inhibitor of polymerase β activity and aphidicolin (Aphi), a drug which inhibits DNA synthesis on MPP+-induced CGC cell death. Both drugs were unable to prevent the process of neuronal loss mediated by MPP+ (200 μM) (Fig. 5a–c). To explore the implication of PARP-1 in MPP+-induced CGC cell death we used a selective PARP-1 inhibitor 1,5-isoquinonediol (Iso) (1–25 μM). This compound did not attenuate loss of cell viability or apoptosis mediated by MPP+ (Fig. 5a, d). Therefore, this data is consistent with previous studies using mice lacking the PARP-1 protein, where neuronal cultures were not protected from MPP+ toxicity [50]. All these data suggest that MPP+-induced cell death is not dependent on DNA replication.
Fig. 5.
Evaluation of neuroprotective properties of DDC, aphidicolin and 1,5-isoquinonediol on MPP+-induced neurotoxicity in CGC. The bar chart shows the effects of the three different (compounds) tested on 200 μM MPP+-induced toxicity in CGNs exposed for 24 h. Viability is based on MTT assays, apoptotic cells are based on flow cytometry analyses and condensed nuclei counting by propidium iodide staining under fluorescence illumination. The nuclei were counted on a fluorescence microscope, distinguishing normal nuclei from the condensed ones following the criteria stated in “Materials and methods”. Each point is the mean ± SEM of four wells of five to six different cultures. Statistical significance was determined by one-way ANOVA followed by Tukey’s tests: **p < 0.01 and ***p < 0.001 versus control
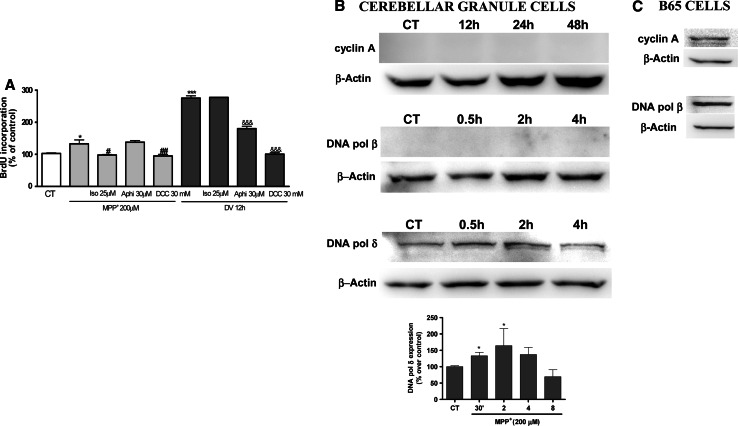
To confirm this hypothesis, we performed experiments of bromodeoxyuridine incorporation, a marker of DNA synthesis (Fig. 6a). In addition, S/K withdrawal was used as positive control of BrdU incorporation, as we have demonstrated in previous studies [41]. Thus MPP+ 200 μM stimulated BrdU incorporation, indicating DNA synthesis in CGC. This action was totally prevented in the presence of Iso 25 μM and DCC 30 mM. However, Aphi 30 μM did not prevent BrdU incorporation in CGC. On the other hand, the same compounds in another experimental model of apoptosis and cell-cycle entry such as S/K withdrawal showed a distinct effect on the incorporation of BrdU (Fig. 6a), for example Aphi 30 μM significantly prevents BrdU incorporation and DCC 30 mM as well.
Fig. 6.
aBar chart showing the quantitation of the percentage of BdrU incorporation into DNA induced by MPP+ and 24 h S/K deprivation (positive control) on CGC in the absence or presence of various concentrations of Iso (25 μM), Aphi (30 μM), DCC 30 mM. Each point is the mean ± SEM of three to four cultures, carried out in duplicate. The statistical analysis used was non-parametric ANOVA followed by Tukey’s test: *p < 0.05 and ***p < 0.001 versus MPP+ and S/K deprivation and #p < 0.05; ##p < 0.01 versus treatment Iso and DCC and &&&p < 0.01 versus treatment Aphi and DCC. b Western-blot analysis of the levels of cyclin A, DNA polymerase β, and DNA polymerase δ in CGC after treatment with MPP+ 200 μM. Cultures were treated with MPP+ 200 μM for different times. At the end of the treatments, cells were lysed and the cell lysates were subjected to immunoblot analysis with an antibody directed against cyclin A, DNA polymerase β and DNA polymerase δ (see “Materials and methods”). c Western-blot analysis of cycling A and DNA polymerase β in B65 cells. Changes in the band intensities were calculated as percentages of the control band intensity. Columns and bars represent the mean ± SEM of three or four separate experiments with four different culture preparations (n = 4). Statistical significance was determined by one-way ANOVA followed by Tukey’s tests: *p < 0.05 versus control
However, although MPP+-treated CGC retain the ability to reactivate the cell cycle and induce an increase in the expression of proteins involved in G0/G1 phase, they rarely synthesize cell-cycle proteins involved in the S phase. Consistent with this hypothesis, Western-blot data confirmed that CGC treated with MPP+ did not induce the expression of proteins involved in the S phase, such as cyclin A, cyclin B (data not shown), or DNA polymerase β. The increase in DNA polymerase δ could be due to the process of DNA damage (Fig. 6b). In order to verify that the antibodies recognized cell-cycle proteins (specifically cyclin A and polymerases), Western blots were carried out on B65 cells (Fig. 6c). This, together with our previous data, strongly suggests that MPP+ induces a process of cell-cycle activation. However, this process would not appear to progress towards DNA synthesis because an increase in the expression of proteins of the S phase was not observed.
MPP+-induced activation of the ATM pathway in B65 neuroblastoma cells
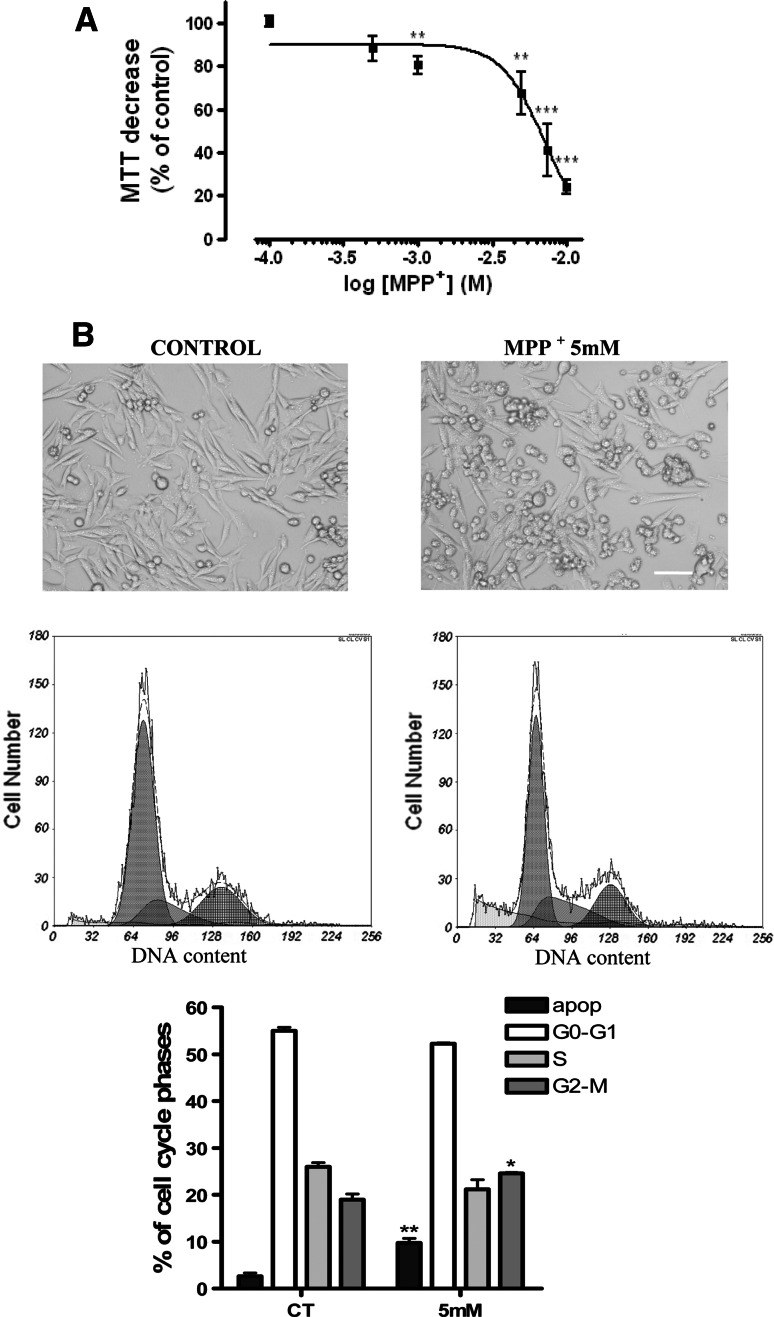
In order to confirm that activation of the ATM pathway may constitute an important step in the process of MPP+-induced apoptosis, we evaluated the effects of this neurotoxin on ATM activation in the B65 neuroblastoma cell line. As shown in Fig. 7a, MPP+ significantly decreased cell viability, and this effect was dose-dependent at concentrations up to 10 mM. Thus, we chose 5 mM as an acute cell model of neurotoxicity in the following experiments on B65 cells (Fig. 7a, b). At this concentration, MPP+ induced changes in the cell cycle, as has been demonstrated in previous studies and here we also demonstrated apoptosis (Fig. 7b).
Fig. 7.
a MPP+ decrease cell viability in B65 neuroblastoma cells measured by the MTT method (see “Materials and methods”). b Representative phase-contrast images of the effects of 5 mM MPP+ (calibration bar 10 μM) and typical flow cytometric cell-cycle representation with the quantification of different cell-cycle phases. Statistical significance was determined by one-way ANOVA followed by Tukey’s tests: *p < 0.05 and **p < 0.01 and versus control
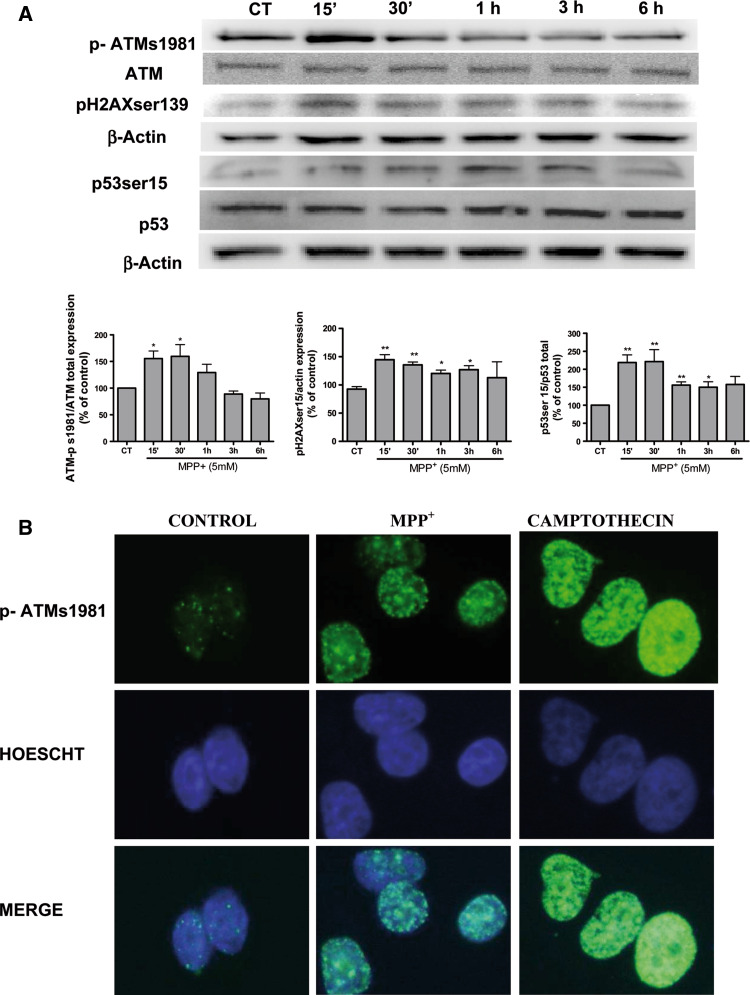
Next, we studied the expression of proteins involved in DNA damage after treating B65 cells with 5 mM MPP+. Western-blot analysis revealed a significant (p < 0.05) and rapid increase in p-ATM ser1981 (Fig. 8a). This finding was also confirmed by immunohistochemistry studies and 10 μM camptothecin was used as positive control of DNA damage (Fig. 8b). We therefore examined the activation and/or expression of proteins involved in this pathway upon DNA damaged, downstream targets of ATM, specifically analyzing p-γH2AX and p53ser15. Interestingly, both markers of DNA damage were rapidly expressed, at 15 min (Fig. 8a). Therefore, our data lend support to a process of ATM activation in this neuroblastoma cell line.
Fig. 8.
a Representative immunoblots showing the expression of p-ATMs1981, p-γH2AX, and p53ser15 in B65 cells after a time course treatment with 5 mM MPP+. Band intensities were calculated as percentages of the control. Columns and bars represent the mean ± SEM of five separate experiments with four or five different culture preparations (n = 4). Statistical significance was determined by one-way ANOVA followed by Tukey’s tests: *p < 0.05, **p < 0.01, ***p < 0.001 versus control. b Representative (from four different experiments with similar results) fluorescence photomicrographs showing effects of 5 mM MPP+ on nuclear p-ATMs1981 expression. Camptothecin 10 μM was used as control DNA damaging agent
ATM regulates the phosphorylation of retinoblastoma protein
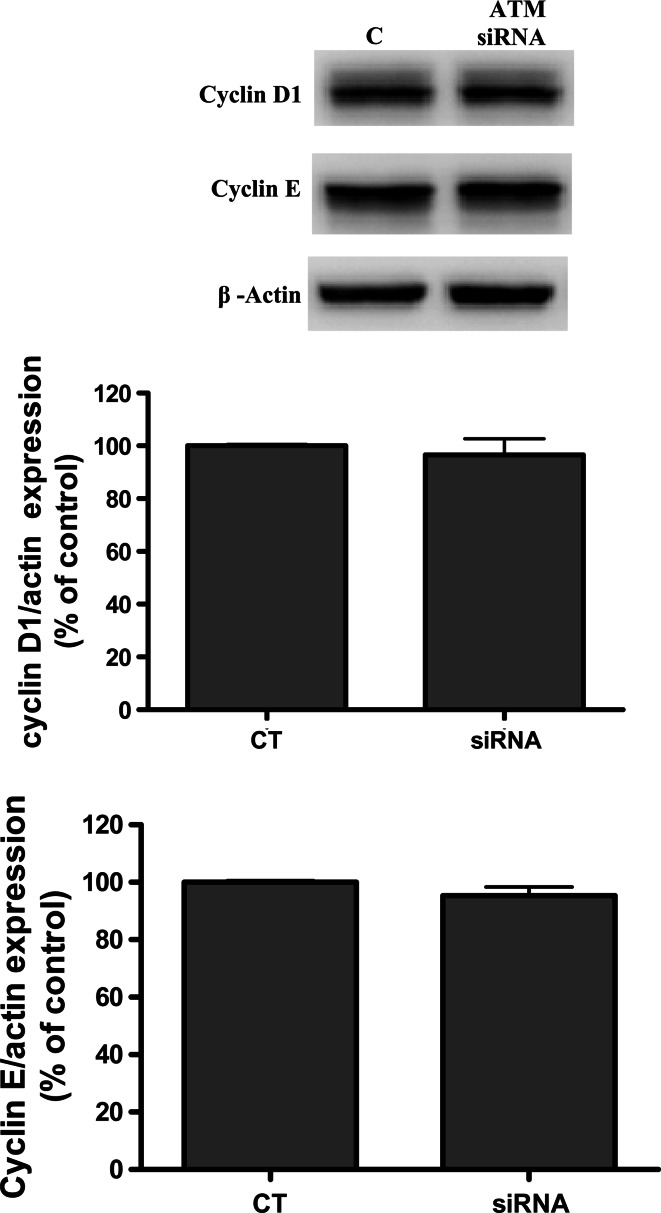
Following the CGC findings, we used Western-blot analysis to study time-course dependent phosphorylation in B65 neuroblastoma cells. Densitometry Western-blot data indicated a significant increase at 15 min of Rb phosphorylation, which was confirmed by immunocytochemistry assays (9A and 9B). To study the hypothesis of a link between ATM activation by DSBs and retinoblastoma protein phosphorylation, we established a knocked down cellular ATM using RNA interference in neuroblastoma B65 cells. Western-blot analysis revealed that treating B65 cells with 5 mM of MPP+ in the presence of a siRNA for ATM and KU-55933 severely decreased the levels of pRB (Fig. 9a). Moreover, we evaluated the expression of G0/G1 cell-cycle proteins such as cyclin D and E following treatment with the siRNA for ATM and no changes in protein expression were found (Figs. 10, 11).
Fig. 9.
a Representative immunoblots showing the expression of pRbser780 in B65 cells after a time-course treatment with MPP+. Band intensities were calculated as percentages of the control. b Representative immunoblots showing the expression of pRbser780 in B65 after a treatment with MPP+ for 30 min alone or in presence of KU-55933 (10 μM) or siRNA against ATM. Columns and bars represent the mean ± SEM of four or five separate experiments with four or five different culture preparations (n = 4). Statistical significance was determined by one-way ANOVA followed by Tukey’s tests: *p < 0.05, **p < 0.01, ***p < 0.001 versus control. #p < 0.05 versus KU-55933 or siRNA treatment
Fig. 10.
Representative immunoblots showing the expression of cyclin D1 and cyclin E in B65 after a treatment with siRNA against ATM MPP+ for 24 h
Fig. 11.
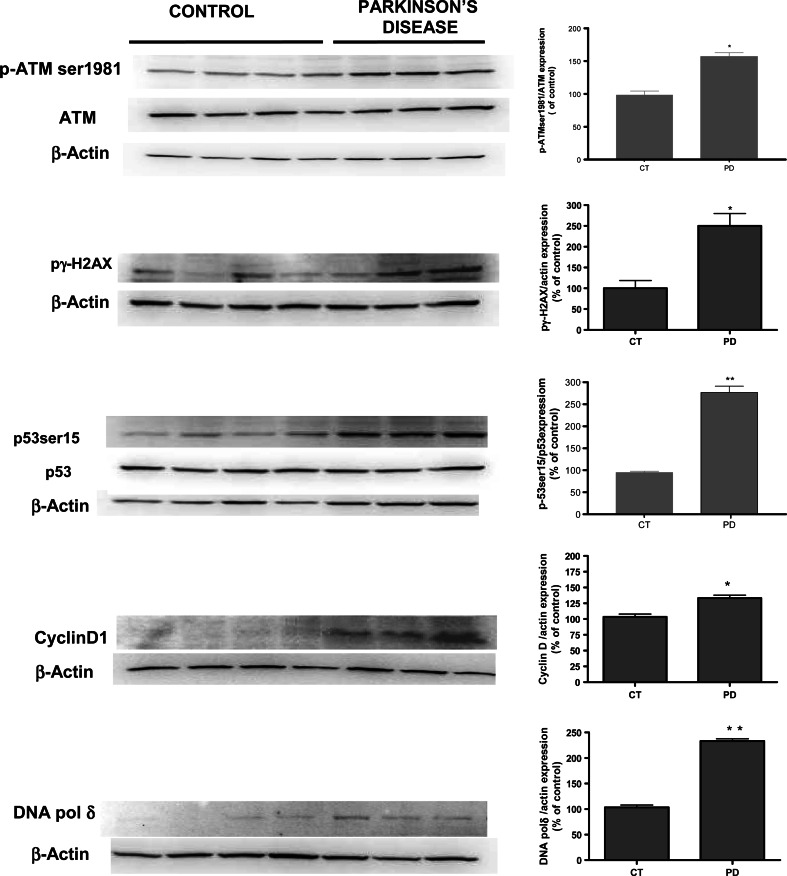
Immunoblots showing the expression of p-ATMser1981, p53ser15, p-γH2AX, cyclin D1, and DNA polymerase δ human cyrus ginguli Parkinson samples and matched controls. Band intensities were calculated as percentages of the control. Columns and bars represent the mean ± SEM of four control samples and three PD brain samples. Statistical significance was determined by Student’s t test: *p < 0.05, **p < 0.01 versus control
Protein analysis of DNA damage in human PD patients
Our experimental data strongly indicate the importance of DNA damage in apoptosis mediated by MPP+ in cell cultures. Finally, we also examined the implication of this pathway in human samples of cyrus ginguli from PD patients. Western-blot data revealed that human PD samples showed a significant increase in p-ATMser1981 (p < 0.05) compared to control samples. Furthermore, downstream targets of ATM, such as p-γH2AX and p53ser15, were also significantly up-regulated. The present results showed a significantly higher expression of all these proteins in PD patients than in age-matched controls (Fig. 12). Likewise, and consistent with previous studies, cyclin D was over-expressed in human brain samples and DNA pol δ was also significantly increased.
Fig. 12.
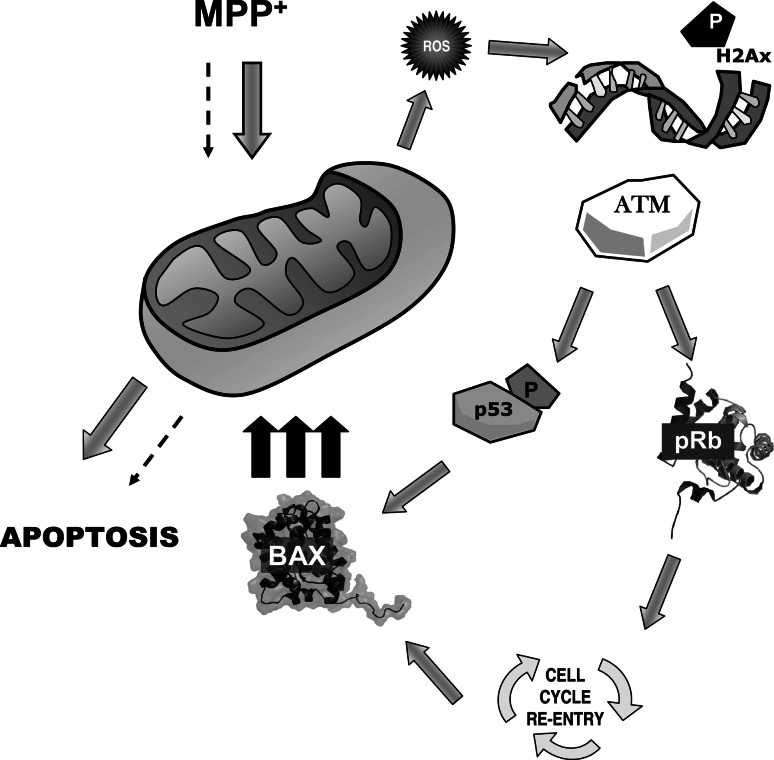
Intracellular pathways involved in MPP+-induced cell death in CGC cells. MPP+ induces an early mitochondrial alteration with an ROS increase which is not sufficient to induce apoptosis in cells. However, mitochondrial alteration (complex I inhibition) generated ROS, which induced DNA damage with an activation of enzymes involved in DNA repair such as ATM. Subsequently, ATM could activate downstream targets such as p53 or, as we suggest in the present manuscript, the modulation of pRb phosphorylation and regulate cell-cycle proteins implicated in the G1/S checkpoint, thus driving CGC cell-cycle re-entry or BAX induction. When BAX is over-expressed, it migrates toward the mitochondria and favors the apoptotic process via the release of pro-apoptotic proteins
Discussion
The results of the present study demonstrate the following:
Pharmacological inhibition of ATM enhances neuronal cell viability after MPP+ treatment.
Our findings further suggest that ATM activation is involved in cell-cycle regulation in neuronal and neuroblastoma cells, probably via regulation of retinoblastoma protein phosphorylation.
Moreover, in human PD samples, the DNA damage pathway is also activated and could contribute to the neurodegenerative process in PD. To our knowledge, this is the first study where the activation of ATM in human PD has been suggested.
Over the last decade, it has been shown that neurons from patients with AD over-express proteins involved in the cellular cell cycle as a part of the apoptotic process [46–51]. In this context, it has been suggested that re-entry in the cell cycle, together with oxidative stress production, constitutes a component of the apoptotic cascade in the process of neuronal cell death through the expression of the transcription factor E2F-1 [25, 52–58]. Currently, the mechanism responsible for cell-cycle activation is unknown and its place in the apoptotic route, prior to the activation of the intrinsic (mitochondrial) programmed cell-death pathway, still requires clarification.
Elsewhere, we reported that MPP+ increased intracellular ROS production, expression in cell-cycle proteins, and DNA damage, specifically ATM activation [20]. In the present study, using the well-characterized ATM inhibitor KU-55933, we have demonstrated that neuronal cell loss and apoptosis mediated by MPP+ was attenuated, thus identifying the important role of ATM in neuronal apoptosis. Moreover, KU-55933 did not modify either intracellular calcium increase or calpain activation. Thus, in this model we observed that both cell-cycle re-entry and double-strand DNA breaks are strongly associated. Likewise, Kruman and co-workers [39] demonstrated that DNA synthesis was associated with the activation of ATM, and that this favors the apoptotic process in neuronal cell cultures after a process of DNA damage. Alternatively, it has been proposed that cell-cycle re-entry in neurons may reflect a requirement for activation of the DNA repair machinery [40]. Here, we propose that cell-cycle activation in postmitotic neurons is an important step because it contributes to apoptosis induced by DNA damage, since KU-55933 attenuates the expression of proapoptotic proteins.
On the other hand, our results suggest that MPP+ induced a process of DNA damage in postmitotic neurons, which favors an activation of G0/G1 phase. However, this process probably does not progress towards S and G2/M phase as no expression of proteins involved in these cell-cycle phases, such as cyclin A or cyclin B, was observed [39]. Moreover, we also investigated whether the process of DNA synthesis occurs through the expression of DNA polymerases, specifically polymerase β and polymerase δ [40, 46]. Western-blot analysis revealed that polymerase β was not expressed following treatment with MPP+ and although a significant increase in polymerase δ was observed at 2 h, we would suggest that the transient expression of this enzyme was probably related to DNA repair. Using a pharmacological approach with DCC (inhibitor of polymerase β) and aphidicolin (an inhibitor of polymerases and therefore S phase inhibition), we did not find an increase in cell viability or prevention of neuronal apoptosis [30]. However, all these compounds were able to affect differently the process of DNA synthesis measured by BrdU incorporation. Notably, MPP+ causes an increase in the synthesis of DNA which is inferior to that produced by other apoptotic stimuli such as deprivation of S/K. Also not all alike compounds affect DNA synthesis. This result can be interpreted as the process of DNA synthesis and therefore entry into the cell cycle is different in the two processes. This is logical, since, deprivation of S/K causes apoptosis independent of p53 expression whereas MPP+ causes apoptosis through p53 [32].
This implies that MPP+-induced cell death is not dependent on DNA replication, and that blockade of DNA synthesis per se is not sufficient to account for the protective actions of G1/S blockers and CDK inhibitors, as we have shown in previous studies [19]. Likewise, we ruled out the hypothesis of a prominent role for the PARP-1 enzyme in the CGC apoptotic cascade, because exposure to the selective pharmacological inhibitor 1,5-isoquinolinediol did not yield any protective effect. Moreover, this data is consistent with previous studies on the absence of neuroprotection in CGC neuronal cultures of mice lacking the PARP-1 enzyme against MPP+ [7, 57]. However, in a recent study [58], the administration of MPTP increased PARP-1 activity in a mouse model of PD, suggesting the implication of DNA damage in the MPTP model of neurodegeneration.
We then delineated the potential molecular mechanisms of DNA damage-induced apoptosis upstream and downstream of p53. For this purpose, we used the neuroblastoma cell line B65, a rat dopaminergic cell line. Consistent with the results for CGC, we can confirm that MPP+ induced a DSB with rapid activation of ATM, and using Western-blot analysis, we observed that MPP+ induced activation of p-γH2AXser139 as well as rapid activation of p53ser15 at 15 min of MPP+ addition to cell cultures. Accordingly, we demonstrated that MPP+ induced DNA damage and that DSB constitutes an early signal in the apoptotic route of this neurotoxin. Given the potential role of ATM in MPP+ neurotoxicity, experiments using a siRNA strategy were carried out to evaluate the potential downstream targets of ATM, which would enable us to explain how DNA favors the process of cell-cycle re-entry. Results suggest that when ATM is significantly inhibited, the expression of pRb by Western-blot analysis was also inhibited and other cyclins and CDKs involved in G0/G1 cell phase were not affected. These observations led us to hypothesize that when DNA damage occurs and ATM is activated, this enzyme is involved in pRbser780 phosphorylation. To our knowledge, this is the first time that this new route has been proposed in order to explain the process of MPP+-induced apoptosis. Similarly, the presence of the pharmacological inhibitor KU-55933 in both cell preparations prevented pRb phosphorylation mediated by MPP+. It is well known that retinoblastoma protein phosphorylation constitutes a restrictive point in the process of cell-cycle re-entry. This process is mediated by cyclins, specifically cyclin D, and can be phosphorylated by E, favoring the release of E2F-1 and cell-cycle progression or apoptosis [59–63]. However, recent studies have reported that Rb may also be phosphorylated by other enzymes such as CDK5, GSK3β, and p38 [34, 42, 56, 63]. Our data revealed that ATM could be involved in pRb phosphorylation and furthermore that Rb phosphorylation was abolished in the presence of KU-55933 and siRNA specific against ATM.
The next question that we wished to address was whether DNA damage and the subsequent activation of the ATM pathway were also activated in PD patients. Protein lysates from the brains of PD patients yielded significant detectable levels of p-γH2AXser139, pATM, and p53ser15 compared to age-matched controls. These results provide evidence of the presence of this activated pathway in human PD.
In conclusion, our data indicate that MPP+ induces the activation of the DNA damage response pathway by mediating DNA breakage. Once ATM has been activated, it phosphorylates different substrates, initiating a neuronal signaling cascade which in turn activates other proteins such as H2AX and p53 [64–66]. It is well known that p53, through BAX, favors the apoptotic process and neurodegeneration. In addition, we have demonstrated a new target regulated by ATM, the retinoblastoma protein. This new finding may partially explain a new pathway connecting the process of DNA damage with cell-cycle re-entry (Fig. 12). Therefore, we propose a mechanism of neuronal cell-cycle re-entry, DNA damage and apoptosis mediated by the neurotoxin MPP+, where initially MPP+ induces a first stimulus (inhibition of complex I), which is probably insufficient to induce an apoptotic process. Then, MPP+ generates mitochondrial ROS production, favoring the process of DNA damage and ATM activation. This enzyme is capable of activating the process of re-entry in the cell cycle which initiates the apoptotic process via E2F-1, or ATM may activate p53. Both signals could come together to induce an increase in BAX expression, which would induce a mitochondrial over-stimulation responsible for neuronal cell apoptosis. In conclusion, we believe that oxidative stress and abortive cell-cycle re-entry occurs in PD (the “two hit hypothesis”) as well as in AD [50]. Finally, the activation of the DNA damage response pathway in human PD samples could contribute to neuronal loss and have potential therapeutic implications in PD.
Acknowledgments
This study was supported by grants from Spain’s “Ministerio de Educación y Ciencia” SAF2009-13093 (MP), SAF2008-05143-C03-1 (J.J.), the “Fondo de Investigación Sanitaria”, and the “Instituto de Salud Carlos III” PI080400 and PS09/01789 (FEDER FOUNDS). 610RT0405 from Programa Iberoamericano de Ciencia y Tecnologia para el Desarrollo (CYTED). We would like to thank the “Generalitat de Catalunya” for supporting the research groups (2009/SGR00853) and the “Fundació la Marató TV3” (063230). Ester Verdaguer holds a “Beatriu de Pinós” postdoctoral contract, awarded by the “Generalitat de Catalunya”. We wish to thank the Language Assessment Service of the University of Barcelona for revising the manuscript.
References
- 1.Gupta A, Dawson VL, Dawson TM. What causes cell death in Parkinson’s disease? Ann Neurol. 2008;64(Suppl 2):S3–S15. doi: 10.1002/ana.21573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.González-Polo RA, Soler G, Alvarez A, Fabregat I, Fuentes JM. Vitamin E blocks early events induced by 1-methyl-4-phenylpyridinium (MPP +) in cerebellar granule cells. J Neurochem. 2003;84:305–315. doi: 10.1046/j.1471-4159.2003.01520.x. [DOI] [PubMed] [Google Scholar]
- 3.Smith PD, Crocker SJ, Jackson-Lewis V, Jordan-Sciutto KL, Hayley S, Mount MP, O’Hare MJ, Callaghan S, Slack RS, Przedborski S, Anisman H, Park DS. Cyclin-dependent kinase 5 is a mediator of dopaminergic neuron loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci USA. 2003;100:13650–13655. doi: 10.1073/pnas.2232515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nair VD. Activation of p53 signaling initiates apoptotic death in a cellular model of Parkinson’s disease. Apoptosis. 2006;11:955–966. doi: 10.1007/s10495-006-6316-3. [DOI] [PubMed] [Google Scholar]
- 5.Tretter L, Sipos I, Adam-Vizi V. Initiation of neuronal damage by complex I deficiency and oxidative stress in Parkinson’s disease. Neurochem Res. 2004;29:569–577. doi: 10.1023/B:NERE.0000014827.94562.4b. [DOI] [PubMed] [Google Scholar]
- 6.González-Polo RA, Soler G, Fuentes JM. MPP + : mechanism for its toxicity in cerebellar granule cells. Mol Neurobiol. 2004;30:253–264. doi: 10.1385/MN:30:3:253. [DOI] [PubMed] [Google Scholar]
- 7.Leist M, Volbracht C, Fava E, Nicotera P. 1-Methyl-4-phenylpyridinium induces autocrine excitotoxicity, protease activation, and neuronal apoptosis. Mol Pharmacol. 1998;54(5):789–801. doi: 10.1124/mol.54.5.789. [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Ma C, Mao Z, Li M. JNK inhibition as a potential strategy in treating Parkinson’s disease. Drug News Perspect. 2004;17:646–654. doi: 10.1358/dnp.2004.17.10.873916. [DOI] [PubMed] [Google Scholar]
- 9.Chu CT, Zhu JH, Cao G, Signore A, Wang S, Chen J. Apoptosis inducing factor mediates caspase-independent 1-methyl-4-phenylpyridinium toxicity in dopaminergic cells. J Neurochem. 2005;94:1685–1895. doi: 10.1111/j.1471-4159.2005.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith PD, Mount MP, Shree R, Callaghan S, Slack RS, Anisman H, Vincent I, Wang X, Mao Z, Park DS. Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2. J Neurosci. 2006;26:440–447. doi: 10.1523/JNEUROSCI.2875-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nunomura A, Moreira PI, Lee HG, Zhu X, Castellani RJ, Smith MA, Perry G. Neuronal death and survival under oxidative stress in Alzheimer and Parkinson diseases. CNS Neurol Disord Drug Targets. 2007;6:411–423. doi: 10.2174/187152707783399201. [DOI] [PubMed] [Google Scholar]
- 12.Nakaso K, Ito S, Nakashima K. Caffeine activates the PI3 K/Akt pathway and prevents apoptotic cell death in a Parkinson’s disease model of SH-SY5Y cells. Neurosci Lett. 2008;432:146–150. doi: 10.1016/j.neulet.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 13.Petit-Paitel A, Brau F, Cazareth J, Chabry J. Involvement of cytosolic and mitochondrial GSK-3beta in mitochondrial dysfunction and neuronal cell death of MPTP/MPP-treated neurons. PLoS One. 2009;4:e5491. doi: 10.1371/journal.pone.0005491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan J, Xiao Q, Sheng CY, Hong Z, Yang HQ, Wang G, Ding JQ, Chen SD. Blockade of the translocation and activation of c-Jun N-terminal kinase 3 (JNK3) attenuates dopaminergic neuronal damage in mouse model of Parkinson’s disease. Neurochem Int. 2009;54:418–425. doi: 10.1016/j.neuint.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Smith PD, O’Hare MJ, Park DS. CDKs: taking on a role as mediators of dopaminergic loss in Parkinson’s disease. Trends Mol Med. 2004;10:445–451. doi: 10.1016/j.molmed.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Höglinger GU, Breunig JJ, Depboylu C, Rouaux C, Michel PP, Alvarez-Fischer D, Boutillier AL, Degregori J, Oertel WH, Rakic P, Hirsch EC, Hunot S. The pRb/E2F cell-cycle pathway mediates cell death in Parkinson’s disease. Proc Natl Acad Sci USA. 2007;104:3585–3590. doi: 10.1073/pnas.0611671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alvira D, Ferrer I, Gutierrez-Cuesta J, Garcia-Castro B, Pallàs M, Camins A. Activation of the calpain/cdk5/p25 pathway in the girus cinguli in Parkinson’s disease. Parkinsonism Relat Disord. 2008;14:309–313. doi: 10.1016/j.parkreldis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Alvira D, Tajes M, Verdaguer E, Acuña-Castroviejo D, Folch J, Camins A, Pallas M. Inhibition of the cdk5/p25 fragment formation may explain the antiapoptotic effects of melatonin in an experimental model of Parkinson’s disease. J Pineal Res. 2006;40:251–258. doi: 10.1111/j.1600-079X.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 19.Alvira D, Tajes M, Verdaguer E, de Arriba SG, Allgaier C, Matute C, Trullas R, Jiménez A, Pallàs M, Camins A. Inhibition of cyclin-dependent kinases is neuroprotective in 1-methyl-4-phenylpyridinium-induced apoptosis in neurons. Neuroscience. 2007;146:350–365. doi: 10.1016/j.neuroscience.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 20.Alvira D, Yeste-Velasco M, Folch J, Casadesús G, Smith MA, Pallàs M, Camins A. Neuroprotective effects of caffeine against complex I inhibition-induced apoptosis are mediated by inhibition of the Atm/p53/E2F–1 path in cerebellar granule neurons. J Neurosci Res. 2007;85:3079–3088. doi: 10.1002/jnr.21427. [DOI] [PubMed] [Google Scholar]
- 21.McShea A, Harris PL, Webster KR, Wahl AF, Smith MA. Abnormal expression of the cell cycle regulators P16 and CDK4 in Alzheimer’s disease. Am J Pathol. 1997;150:1933–1939. [PMC free article] [PubMed] [Google Scholar]
- 22.Raina AK, Zhu X, Rottkamp CA, Monteiro M, Takeda A, Smith MA. Cyclin’ toward dementia: cell cycle abnormalities and abortive oncogenesis in Alzheimer disease. J Neurosci Res. 2000;61:128–133. doi: 10.1002/1097-4547(20000715)61:2<128::AID-JNR2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 23.Jordan-Sciutto KL, Dorsey R, Chalovich EM, Hammond RR, Achim CL. Expression patterns of retinoblastoma protein in Parkinson disease. J Neuropathol Exp Neurol. 2003;62:68–74. doi: 10.1093/jnen/62.1.68. [DOI] [PubMed] [Google Scholar]
- 24.Lim AC, Qi RZ. Cyclin-dependent kinases in neural development and degeneration. J Alzheimers Dis. 2003;5:329–335. doi: 10.3233/jad-2003-5409. [DOI] [PubMed] [Google Scholar]
- 25.Krantic S, Mechawar N, Reix S, Quirion R. Molecular basis of programmed cell death involved in neurodegeneration. Trends Neurosci. 2005;28:670–676. doi: 10.1016/j.tins.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Nagy Z. The dysregulation of the cell cycle and the diagnosis of Alzheimer’s disease. Biochim Biophys Acta. 2007;1772:402–408. doi: 10.1016/j.bbadis.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Nagy Z. The last neuronal division: a unifying hypothesis for the pathogenesis of Alzheimer’s disease. J Cell Mol Med. 2005;9:531–541. doi: 10.1111/j.1582-4934.2005.tb00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becker EB, Bonni A. Cell cycle regulation of neuronal apoptosis in development and disease. Prog Neurobiol. 2004;72:1–25. doi: 10.1016/j.pneurobio.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Pelegrí C, Duran-Vilaregut J, del Valle J, Crespo-Biel N, Ferrer I, Pallàs M, Camins A, Vilaplana J. Cell cycle activation in striatal neurons from Huntington’s disease patients and rats treated with 3-nitropropionic acid. Int J Dev Neurosci. 2008;26:665–671. doi: 10.1016/j.ijdevneu.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Copani A, Guccione S, Giurato L, Caraci F, Calafiore M, Sortino MA, Nicoletti F. The cell cycle molecules behind neurodegeneration in Alzheimer’s disease: perspectives for drug development. Curr Med Chem. 2008;15:2420–2432. doi: 10.2174/092986708785909030. [DOI] [PubMed] [Google Scholar]
- 31.Iyirhiaro GO, Brust TB, Rashidian J, Galehdar Z, Osman A, Phillips M, Slack RS, Macvicar BA, Park DS. Delayed combinatorial treatment with flavopiridol and minocycline provides longer term protection for neuronal soma but not dendrites following global ischemia. J Neurochem. 2008;105:703–713. doi: 10.1111/j.1471-4159.2007.05166.x. [DOI] [PubMed] [Google Scholar]
- 32.Camins A, Verdaguer E, Folch J, Beas-Zarate C, Canudas AM, Pallàs M. Inhibition of ataxia telangiectasia-p53–E2F-1 pathway in neurons as a target for the prevention of neuronal apoptosis. Curr Drug Metab. 2007;8:709–715. doi: 10.2174/138920007782109814. [DOI] [PubMed] [Google Scholar]
- 33.Chong ZZ, Li F, Maiese K. Attempted cell cycle induction in post-mitotic neurons occurs in early and late apoptotic programs through Rb, E2F1, and caspase 3. Curr Neurovasc Res. 2006;3:25–39. doi: 10.2174/156720206775541741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamdane M, Bretteville A, Sambo AV, Schindowski K, Bégard S, Delacourte A, Bertrand P, Buée L. p25/Cdk5-mediated retinoblastoma phosphorylation is an early event in neuronal cell death. J Cell Sci. 2005;118:1291–1298. doi: 10.1242/jcs.01724. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Qu D, Morris EJ, O’Hare MJ, Callaghan SM, Slack RS, Geller HM, Park DS. The Chk1/Cdc25A pathway as activators of the cell cycle in neuronal death induced by camptothecin. J Neurosci. 2006;26:8819–8828. doi: 10.1523/JNEUROSCI.2593-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.García-Alvarez G, Ventura V, Ros O, Aligué R, Gil J, Tauler A. Glycogen synthase kinase-3beta binds to E2F1 and regulates its transcriptional activity. Biochim Biophys Acta. 2007;1773:375–382. doi: 10.1016/j.bbamcr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Espada L, Udapudi B, Podlesniy P, Fabregat I, Espinet C, Tauler A. Apoptotic action of E2F1 requires glycogen synthase kinase 3-beta activity in PC12 cells. J Neurochem. 2007;102:2020–2028. doi: 10.1111/j.1471-4159.2007.04686.x. [DOI] [PubMed] [Google Scholar]
- 38.Macleod MR, Ramage L, McGregor A, Seckl JR. Reduced NMDA-induced apoptosis in neurons lacking ataxia telangiectasia mutated protein. Neuroreport. 2003;14:215–217. doi: 10.1097/00001756-200302100-00011. [DOI] [PubMed] [Google Scholar]
- 39.Kruman II, Wersto RP, Cardozo-Pelaez F, Smilenov L, Chan SL, Chrest FJ, Emokpae R, Jr, Gorospe M, Mattson MP. Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron. 2004;41:549–561. doi: 10.1016/S0896-6273(04)00017-0. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz EI, Smilenov LB, Price MA, Osredkar T, Baker RA, Ghosh S, Shi FD, Vollmer TL, Lencinas A, Stearns DM, Gorospe M, Kruman II. Cell cycle activation in postmitotic neurons is essential for DNA repair. Cell Cycle. 2007;6:318–329. doi: 10.4161/cc.6.3.3752. [DOI] [PubMed] [Google Scholar]
- 41.Verdaguer E, Jordá EG, Canudas AM, Jiménez A, Pubill D, Escubedo E, Camarasa J, Pallàs M, Camins A. Antiapoptotic effects of roscovitine in cerebellar granule cells deprived of serum and potassium: a cell cycle-related mechanism. Neurochem Int. 2004;44:251–261. doi: 10.1016/S0197-0186(03)00147-5. [DOI] [PubMed] [Google Scholar]
- 42.Yeste-Velasco M, Folch J, Pallàs M, Camins A. The p38(MAPK) signaling pathway regulates neuronal apoptosis through the phosphorylation of the retinoblastoma protein. Neurochem Int. 2009;54:99–105. doi: 10.1016/j.neuint.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Braak H, Del Tredici K, Rüb U, Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 44.Hickson I, Zhao Y, Richardson CJ, Green SJ, Martin NM, Orr AI, Reaper PM, Jackson SP, Curtin NJ, Smith GC. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 2004;64:9152–9159. doi: 10.1158/0008-5472.CAN-04-2727. [DOI] [PubMed] [Google Scholar]
- 45.Thakur A, Siedlak SL, James SL, Bonda DJ, Rao A, Webber KM, Camins A, Pallàs M, Casadesus G, Lee HG, Bowser R, Raina AK, Perry G, Smith MA, Zhu X. Retinoblastoma protein phosphorylation at multiple sites is associated with neurofibrillary pathology in Alzheimer disease. Int J Clin Exp Pathol. 2008;1:134–146. [PMC free article] [PubMed] [Google Scholar]
- 46.Copani A, Caraci F, Hoozemans JJ, Calafiore M, Sortino MA, Nicoletti F. The nature of the cell cycle in neurons: focus on a “non-canonical” pathway of DNA replication causally related to death. Biochim Biophys Acta. 2007;1772:409–412. doi: 10.1016/j.bbadis.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 47.Copani A, Condorelli F, Caruso A, Vancheri C, Sala A, Giuffrida Stella AM, Canonico PL, Nicoletti F, Sortino MA. Mitotic signaling by beta-amyloid causes neuronal death. FASEB J. 1999;13:2225–2234. [PubMed] [Google Scholar]
- 48.Copani A, Uberti D, Sortino MA, Bruno V, Nicoletti F, Memo M. Activation of cell-cycle-associated proteins in neuronal death: a mandatory or dispensable path? Trends Neurosci. 2001;24:25–31. doi: 10.1016/S0166-2236(00)01663-5. [DOI] [PubMed] [Google Scholar]
- 49.Leist M, Single B, Künstle G, Volbracht C, Hentze H, Nicotera P. Apoptosis in the absence of poly-(ADP-ribose) polymerase. Biochem Biophys Res Commun. 1997;233:518–522. doi: 10.1006/bbrc.1997.6491. [DOI] [PubMed] [Google Scholar]
- 50.Zhu X, Lee HG, Perry G, Smith MA. Alzheimer disease, the two-hit hypothesis: an update. Biochim Biophys Acta. 2007;1772:494–502. doi: 10.1016/j.bbadis.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 51.Zhu X, Raina AK, Boux H, Simmons ZL, Takeda A, Smith MA. Activation of oncogenic pathways in degenerating neurons in Alzheimer disease. Int J Dev Neurosci. 2000;18:433–437. doi: 10.1016/S0736-5748(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 52.Klein JA, Ackerman SL. Oxidative stress, cell cycle, and neurodegeneration. J Clin Invest. 2003;111:785–793. doi: 10.1172/JCI18182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Langley B, Ratan RR. Oxidative stress-induced death in the nervous system: cell cycle dependent or independent? J Neurosci Res. 2004;77:621–629. doi: 10.1002/jnr.20210. [DOI] [PubMed] [Google Scholar]
- 54.Staropoli JF. Tumorigenesis and neurodegeneration: two sides of the same coin? Bioessays. 2008;30:719–727. doi: 10.1002/bies.20784. [DOI] [PubMed] [Google Scholar]
- 55.Webber KM, Raina AK, Marlatt MW, Zhu X, Prat MI, Morelli L, Casadesus G, Perry G, Smith MA. The cell cycle in Alzheimer disease: a unique target for neuropharmacology. Mech Ageing Dev. 2005;126:1019–1025. doi: 10.1016/j.mad.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 56.Camins A, Verdaguer E, Folch J, Canudas AM, Pallàs M. The role of CDK5/P25 formation/inhibition in neurodegeneration. Drug News Perspect. 2006;19:453–460. doi: 10.1358/dnp.2006.19.8.1043961. [DOI] [PubMed] [Google Scholar]
- 57.Qu D, Rashidian J, Mount MP, Aleyasin H, Parsanejad M, Lira A, Haque E, Zhang Y, Callaghan S, Daigle M, Rousseaux MW, Slack RS, Albert PR, Vincent I, Woulfe JM, Park DS. Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson’s disease. Neuron. 2007;55:37–52. doi: 10.1016/j.neuron.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 58.Hoang T, Choi DK, Nagai M, Wu DC, Nagata T, Prou D, Wilson GL, Vila M, Jackson-Lewis V, Dawson VL, Dawson TM, Chesselet MF, Przedborski S. Neuronal NOS and cyclooxygenase-2 contribute to DNA damage in a mouse model of Parkinson disease. Free Radic Biol Med. 2009;47:1049–1056. doi: 10.1016/j.freeradbiomed.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee HG, Casadesus G, Zhu X, Castellani RJ, McShea A, Perry G, Petersen RB, Bajic V, Smith MA. Cell cycle re-entry mediated neurodegeneration and its treatment role in the pathogenesis of Alzheimer’s disease. Neurochem Int. 2009;54:84–88. doi: 10.1016/j.neuint.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim D, Tsai LH. Linking cell cycle reentry and DNA damage in neurodegeneration. Ann NY Acad Sci. 2009;1170:674–679. doi: 10.1111/j.1749-6632.2009.04105.x. [DOI] [PubMed] [Google Scholar]
- 61.Herrup K, Neve R, Ackerman SL, Copani A. Divide and die: cell cycle events as triggers of nerve cell death. J Neurosci. 2004;24:9232–9239. doi: 10.1523/JNEUROSCI.3347-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Panickar KS, Nonner D, White MG, Barrett JN. Overexpression of Cdk5 or non-phosphorylatable retinoblastoma protein protects septal neurons from oxygen-glucose deprivation. Neurochem Res. 2008;33:1852–1858. doi: 10.1007/s11064-008-9647-3. [DOI] [PubMed] [Google Scholar]
- 63.Yeste-Velasco M, Folch J, Trullàs R, Abad MA, Enguita M, Pallàs M, Camins A. Glycogen synthase kinase-3 is involved in the regulation of the cell cycle in cerebellar granule cells. Neuropharmacology. 2007;53:295–307. doi: 10.1016/j.neuropharm.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 64.Keramaris E, Hirao A, Slack RS, Mak TW, Park DS. Ataxia telangiectasia-mutated protein can regulate p53 and neuronal death independent of Chk2 in response to DNA damage. J Biol Chem. 2003;278:37782–37789. doi: 10.1074/jbc.M304049200. [DOI] [PubMed] [Google Scholar]
- 65.Biton S, Dar I, Mittelman L, Pereg Y, Barzilai A, Shiloh Y. Nuclear ataxia-telangiectasia mutated (ATM) mediates the cellular response to DNA double strand breaks in human neuron-like cells. J Biol Chem. 2006;281:17482–17491. doi: 10.1074/jbc.M601895200. [DOI] [PubMed] [Google Scholar]
- 66.Martin LJ, Liu Z, Pipino J, Chestnut B, Landek MA. Molecular regulation of DNA damage-induced apoptosis in neurons of cerebral cortex. Cereb Cortex. 2009;19:1273–1293. doi: 10.1093/cercor/bhn167. [DOI] [PMC free article] [PubMed] [Google Scholar]