Abstract
Mutation of tubulin chaperone E (TBCE) underlies hypoparathyroidism, retardation, and dysmorphism (HRD) syndrome with defective microtubule (MT) cytoskeleton. TBCE/yeast Pac2 comprises CAP-Gly, LRR (leucine-rich region), and UbL (ubiquitin-like) domains. TBCE folds α-tubulin and promotes α/β dimerization. We show that Pac2 functions in MT dynamics: the CAP-Gly domain binds α-tubulin and MTs, and functions in suppression of benomyl sensitivity of pac2Δ mutants. Pac2 binds proteasomes: the LRR binds Rpn1, and the UbL binds Rpn10; the latter interaction mediates Pac2 turnover. The UbL also binds the Skp1-Cdc53-F-box (SCF) ubiquitin ligase complex; these competing interactions for the UbL may impact on MT dynamics. pac2Δ mutants are sensitive to misfolded protein stress. This is suppressed by ectopic PAC2 with both the CAP-Gly and UbL domains being essential. We propose a novel role for Pac2 in the misfolded protein stress response based on its ability to interact with both the MT cytoskeleton and the proteasomes.
Keywords: Pac2, CAP-Gly, Ubiquitin-like domain, Rpn1, Rpn10, Proteasome, TBCE
Introduction
Mutations in tubulin chaperone E, TBCE, cause an autosomal recessive disorder characterized by hypoparathyroidism, growth and mental retardation, and dysmorphism (HRD syndrome, MIM241410). The founder mutation in HRD-affected individuals of the Middle East is an in-frame 12-bp deletion (del52-55) in the third exon of TBCE [1]. Five tubulin-specific chaperones, TBCA–TBCE, are conserved from yeast to mammals [2–4]. TBCB/Saccharomyces cerevisiae Alf1 and TBCE/S. cerevisiae Pac2 bind α-tubulin and promote its assembly with β-tubulin to form the basic α/β-tubulin microtubule (MT) building block [5, 6]. TBCA and TBCD bind β-tubulin, and a supercomplex is formed of TBCC, TBCD, and TBCE together with α- and β-tubulin from which the native α/β-tubulin heterodimers are released by GTP hydrolysis (reviewed in [7]). Cells from HRD-affected individuals show lower MT density at the MT organizing centers and perturbed MT organization. In addition the HRD mutation affects the organization of organelles that require MTs for their trafficking, such as the Golgi and late endosomal compartments [1]. A destabilizing Trp514Gly mutation in mouse Tbce underlies progressive motor neuronopathy (pmn) [8, 9].
The two cofactors involved in folding α-tubulin, TBCB and TBCE/S. cerevisiae Alf1 and Pac2, respectively, have a similar domain organization arranged in opposite orientation. Their functional motifs include: a cytoskeleton-associated protein glycine-rich (CAP-Gly) domain [10], a coiled-coil domain composed of leucine-rich repeats (LRR), and an ubiquitin-like (UbL) domain at the C-terminus in TBCE [11]. The CAP-Gly domain is a highly conserved domain present in a Pfam family of ca. 70 proteins exemplified by the MT-binding proteins, CLIP-170 [12], and the p150Glued subunit of the dynein/dynactin motor complex [13]. Many CAP-Gly domain proteins mediate binding between MTs and other elements of the cytoskeleton or with organelles [12, 14]. The (del52-55) mutation deletes four residues in the CAP-Gly domain including a highly conserved Gly and is adjacent to residues essential for α-tubulin binding by CLIP-170 [1]. No protein-protein interactions have been mapped to the LRR domain of either cofactor, TBCB or TBCE. The UbL domain of TBCB is required for interaction with the Kelch/BTB domain substrate receptor, Gigaxonin, that recruits TBCB to a cullin-RING E3 ubiquitin ligase complex for ubiquitylation and degradation in the proteasome. Mutations in Gigaxonin lead to human giant axonal neuropathy (GAN) characterized by accumulation of TBCB and a diminished MT network [15]. A structural study of the UbL domain of TBCB on which the parallel domain of TBCE was modeled suggests that these two domains could dimerize via electrostatic interactions [16]. This hypothesis is supported by biochemical isolation of a TBCB–TBCE binary complex and of a ternary complex of TBCB and TBCE with α-tubulin after α/β tubulin heterodimer dissocation. These complexes are proposed to escort α-tubulin towards degradation or recycling [17, 18].
Protein ubiquitylation is executed by an enzyme cascade comprising E1 ubiquitin activating and E2 conjugating enzymes and an E3 ubiquitin ligase that recruits the substrate protein. Proteins marked with ubiquitin chains linked via K48 of ubiquitin are targeted for degradation in the proteasome, a complex comprising a 20S catalytic core and usually two 19S regulatory particles (RP), one appended at each end [19, 20]. The 19S RP binds the ubiquitylated substrate, cleaves and releases the ubiquitin chains, and unfolds the substrate for insertion into the catalytic core. Deubiquitylation (DUB) activity is provided by the intrinsic lid Rpn11 subunit [21] or by DUBs that are attached to 19S RP subunits via an ubiquitin-like (UbL) domain, e.g., Ubp6 [22] or Uch37 [23]. Furthermore, the UbL–UbA family of proteins exemplified by yeast Rad23, Dsk2, and Ddi1 bind the 19S RP with their UbL domains and ubiquitin chains with their ubiquitin-associated (UbA) domains. These proteins are proposed to serve as shuttles that deliver ubiquitylated substrates to the proteasome [24–28]. The Rad23 UbL domain is reported to bind the Rpn1 subunit of the 19S RP [29, 30] and Rpn10 [28]; the Dsk2 UbL domain binds Rpn10 [30, 31]; Ddi1 interacts with Rpn1 [26, 32].
Pac2 was found in preparations of proteasomes affinity-purified through a tagged 19S RP subunit [33]. Pac2 shows 30% identity and 54% similarity to human TBCE with conservation of all functional domains. Here we analyzed the interaction of yeast Pac2 and human TBCE with MTs and with the proteasome. pac2Δ mutants are sensitive to benomyl that inhibits MT polymerization [2], and we show that in addition to its established role in folding of α-tubulin, Pac2 interacts with polymerized MTs in vitro via its CAP-Gly domain. The CAP-Gly domain is crucial for suppression of benomyl sensitivity. We found a robust interaction between Pac2 and the proteasome that involves two independent interactions: the LRR domain binds Rpn1, whereas the UbL domain binds Rpn10. Interaction of the UbL domain with Rpn10 is important for Pac2 turnover. The UbL domain also interacts with the SCF ubiquitin ligase complex. The interaction between Pac2 and the proteasome has functional significance as we find that pac2Δ mutants are sensitive to accumulation of misfolded proteins, evidenced as sensitivity to the arginine analog, canavanine. Canavanine sensitivity is suppressed by ectopic full-length Pac2 and requires both the CAP-Gly and UbL domains. Further evidence of a role of Pac2 in the response to accumulation of aberrant proteins is the finding that pac2Δ mutants are sensitive to cadmium. Endoplasmic reticulum (ER)-associated degradation (ERAD) eliminates aberrant proteins from the ER for ubiquitylation and degradation by the proteasome. Cadmium causes ERAD stress through mechanism(s) that are not fully understood. It reacts with thiol groups prevalent in the ER and replaces zinc and iron in metalloproteins, and this may reflect ERAD deficiencies [34]. Similar to the result observed with canavanine, we found that pac2Δ mutants are very sensitive to cadmium and that ectopic expression of full-length PAC2 restored cadmium resistance to wild-type levels. Here too suppression of the sensitivity requires both the MT-interacting CAP-Gly and proteasome-interacting UbL domains of Pac2.
Materials and methods
Yeast strains
Wild-type BY4741 (MAT a ; his3Δ1; leu2Δ0; met15Δ0; ura3Δ0), pac2Δ (BY4741; MATα; his3Δ1; leu2Δ0; met15Δ0; ura3Δ0; yer007w::kanMX4), pdr5Δ (BY4741; MAT a ; his3Δ1; leu2Δ0; met15Δ0; ura3Δ0; yol153c::kanMX4) were purchased from Euroscarf. The rpn10Δ mutant is RJD2478 (leu2-3,-112, his3-11,-15, trp1-1, ura3-1, ade2-1, rpn10::KANMX, MATa) [35]. Yeast strains expressing C-terminal GFP fusions of TUB1 are from [36].
Bacterial strains
Rosetta bacteria [F− ompT hsdS B (rB-mB-) gal dcm lacY1 pRARE (argU, argW, ileX, glyT, leuW, proL) (CmR) (Novagen)] were used for most recombinant protein expression. His-tagged recombinant proteins were expressed in BL21 bacteria [F− ompT, hsdSB(rB-, mB-), dcm, gal, λ(DE3), pLysS (Cmr) (Promega)].
Plasmids
pGAL-GFP-PAC2, pGAL-GFP-PAC2ΔCG, and pGAL-GFP-PAC2ΔUBL were constructed by amplifying each gene product from genomic DNA using primer pairs Pac2BamF (GAATTTCAGAGGATCCATGACTTATGAAATTGGGG) with Pac2BamR (CCAAGATTAACTGGATCCTGTGATTACGATGGGC) to construct pGAL-GFP-PAC2; primers Pac2CGBamF (GCAGGATCCGCATTGTCAAAG) with Pac2BamR for pGAL-GFP-PAC2ΔCG; and primers Pac2BamF with Pac2UbLR (GAATTCTCATGTTTGACAGC) for pGAL-GFP-PAC2ΔUBL. The PCR products were cloned into pGFP-Superglo [37]. The Pac2 LRR domain was amplified with primers Pac2LRR-F (GCTTTGCGAGGATCCGATGTCGCTATATTG) and Pac2LRR-R (CCTTAAATTTGTTATCTGTTGGATCCAAGGATTCAACAG) and cloned into the same vector for expression in yeast. GST, GSTTBCE, and GSTRpn10 were produced from vector pGEX-5X-1 (Amersham Biosciences). pGEX expressing GSTRpn1 was from [29]. HisRpn10 was cloned in pQE30 (QIAGEN), expressed in bacteria, and purified on Ni–NTA [31]. HisPac2LRR was cloned in the pHis-parallel expression vector and purified from inclusion bodies as detailed below.
Transformations
of yeast cells were performed by LiOAc [38].
Immunoprecipitation and immunoblotting
were performed as described in [39]. Proteins were induced from the GAL promoter by overnight growth in minimal medium with 2% galactose. Next morning the culture was diluted 1:3 and grown for a further 1.5 h; 50 ml of logarithmic culture served as the source of a 300-μl extract that had 80 μg/μl protein. Then 200 μl were taken for immunoprecipitation (IP) with the appropriate antibody, and the immunoprecipitate was run in a single lane for Western blotting (WB). Anti-Rpn10 antiserum was used at 1:1,000 for IP and 1:10,000 for WB; anti-Rpn12 was used at 1:1,000 for IP and 1:10,000 for WB; anti-Pre6 was used at 1:1,000 for WB [40]; anti-GFP antibodies from Roche Molecular Biochemicals were used at 1:200 for IP and 1:1,000 for WB, anti-tubulin Yol34/1 antibody (Serotec) was used at 1:1,000 for WB, and rabbit anti-ubiquitin antibodies (Dako) were used at 1:5,000 for WB. Affinity purified rabbit polyclonal antibody to human TBCE was used at 1:200 for WB. Goat anti-mouse, goat anti-rabbit, and goat anti-rat used at 1:1,000 were from Santa Cruz Biotechnology. Protein A-Sepharose was purchased from Amersham and used at 50%; 30 μl was added to each sample.
For TCA precipitation
proteins were precipitated from 300 μl by adding TCA to 10% with 10 min incubation on ice. The pellet was centrifuged at 12,000g for 10 min, and five volumes of cold acetone were added. The protein pellets were harvested and dried. For Western blot analysis proteins were dissolved in 30 μl of sample buffer, and 5 μl of each fraction was separated by SDS-PAGE.
Expression of GST fusion proteins in Rosetta bacteria
Bacteria were transformed by electroporation and the colonies selected on LB-agar plates with 100 μg/ml ampicillin and 34 μg/ml chloramphenicol. A single colony was grown in 1 l of TYx2 with ampicillin and chloramphenicol to an OD600 of 0.6–0.8 (3–5 h) with vigorous agitation at 37°C. Then 0.4 mM of isopropyl-1-thio-ß-d-galactopyranoside (IPTG) was added to induce expression, and the culture was incubated overnight at 20°C. The cells were harvested by centrifugation at 4°C for 10 min at 3,000g. The cell pellet was washed with 20 ml of ice-cold 1× PBS and then resuspended in 3 ml lysis buffer [50 mM Tris-HCl pH 7.5, 150 mM NaCl, 5 mM EDTA, 0.1% NP40, 1:25 of protease inhibitor cocktail (Roche)]. The cells were sonicated on ice six times for 10 s and clarified by centrifugation for 10 min at 12,000g at 4°C. The supernatants containing the GST-fusion proteins were incubated with washed Glutathione-Sepharose 4B beads (Amersham Biosciences) for 1.5 h at 4°C. The samples were washed five times in lysis buffer. The GST-fusion proteins on beads were then stored at −20°C after addition of glycerol to 5%.
Purification of recombinant HisPac2LRR from inclusion bodies
Recombinant HisPac2LRR was expressed in bacteria, and the cells were lysed as above. After centrifugation at 12,000g for 30 min, the pellet was washed twice with DDW. Then 1/100th volume of 1 M NaOH at pH 12 was added and the suspension was sonicated to dissolve the inclusion bodies and incubated at room temperature for 15 min. One-tenth volume of 1 M Tris pH 8.0 was added, and the sample was incubated for 2 h at 4°C followed by overnight dialysis against 100 mM Tris pH 8.0. The sample was centrifuged at 30g for 20 min, and the protein in the supernatant was bound to nickel beads (Qiagen) according to the manufactor’s instructions.
GST in vitro binding assay
Yeast cells were grown overnight till late log phase (OD600 = 0.8) in 2% galactose medium for the GAL-regulated constructs, or in YePD. The cells were harvested by centrifugation at room temperature for 5 min at 4,000 rpm, washed in 50 ml TE, and resuspended in 600 μl lysis buffer. Then 0.5–0.6 mg of glass beads was added, and cells were broken by vigorous vortexing for 25 min at 4°C. The extract was clarified by centrifugation at 12,000 g for 20 min at 4°C, and protein concentration was measured using the Bio-Rad protein reagent; 5–10 mg of protein extract was taken for each GST pull-down in a total volume of 350–400 μl lysis buffer. Then 30–50 μl of 50% Glutathione-Sepharose 4B beads (Amersham) coupled to GST fusion protein was added to each sample and incubated at 4°C for 1–2 h with very mild shaking. The samples were washed six times with lysis buffer with 2.5% Triton-X 100. The pellet was resuspended in 30–50 μl sample buffer ×2, boiled for 5 min, and centrifuged for 3 min at high speed to remove insoluble material. The supernatant was separated on a 12% polyacrylamide SDS gel with protein size standards followed by Western blot analysis.
Microtubule cosedimentation assay
Yeast extracts were prepared in PEM buffer (100 mM PIPES pH 6.8; 1 mM EGTA; 5 mM MgSO4) with glass beads and vortexing as above. Before use the extract was precentrifuged once in a Beckman Airfuge at 10 p.s.i. for 5 min. A 5 mg/ml solution of α/β tubulin heterodimer in PEM buffer was centrifuged in a Beckman Airfuge as above to precipitate tubulin aggregates. Two 20-μl aliquots of supernatant were used in each experiment. One aliquot served for tubulin-binding experiments and was diluted once more into 200 μl PEM buffer and incubated on ice. The other aliquot served to prepare MTs by addition of 5 mM GTP (tubulin-GTP) and incubation at 37°C for 30 min. After incubation, 200 μl stabilizing buffer (1 mM GTP + 0.1 mM taxol in PEM) was added to each 20 μl aliquot of MT; 50 μl of tubulin or MTs was mixed with 50 μl yeast extract, controls were mixed with PEM buffer, and the mixtures were incubated at room temperature for 15 min. The mixtures were layered onto a 60% glycerol solution in PEM buffer and centrifuged at 10 p.s.i. for 5 min. After centrifugation the supernatants were transferred to fresh tubes, and 15 μl sample buffer was added to each pellet. The pellets and 10 μl of each supernatant with 10 μl sample buffer were analyzed by SDS-PAGE followed by Western blotting with anti-tubulin and anti-GFP antibodies.
Glycerol gradients
were performed as in [41]. To each 10–40% glycerol gradient we added 0.7 ml of protein extract and centrifuged the tubes at 25,500 rpm for 22 h using a SW 40 Ti rotor in a Sorvall Discovery 90SE. Eleven 1-ml fractions were collected, and proteins were TCA-precipitated from 200 μl aliquots of each fraction for SDS-PAGE and Western blot analysis. Measurement of protein band intensity was done using ImageJ freeware.
Results
The CAP-Gly domain of human TBCE interacts with yeast α-tubulin
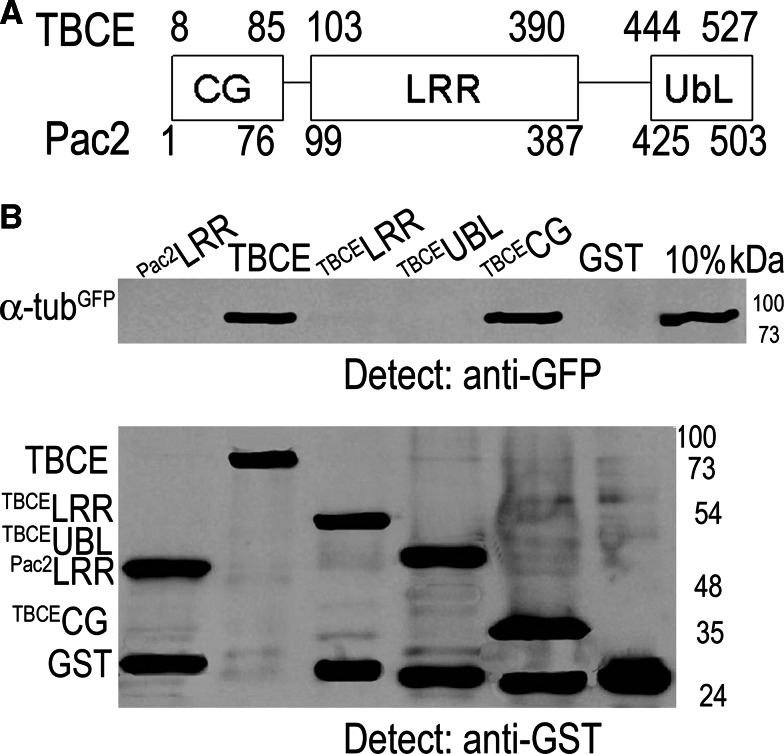
The CAP-Gly domain is predicted to bind α-tubulin, and to map the interaction we incubated full-length TBCE and subclones of the TBCE CAP-Gly, LRR, and UbL domains produced in bacteria with yeast lysate from cells expressing endogenous TUB1-GFP [36]. We also included the yeast Pac2 LRR domain in this experiment. We found an interaction of yeast α-tubulinGFP with both full-length human TBCE and with the subclone of the TBCE CAP-Gly domain. α-tubulinGFP did not interact with the LRR domain of either yeast or human TBCE or with the UbL domain of TBCE (Fig. 1).
Fig. 1.
The CAP-Gly domain mediates interaction of TBCE with α-tubulin. a Domain structure of human TBCE and yeast Pac2 showing CAP-Gly (CG), LRR, and UbL domains based on [11]. b Yeast extract from cells with tagged genomic α-tubulinGFP was incubated with GSH beads to which were bound recombinant yeast GSTPac2-LRR domain, human GSTTBCE, GSTTBCE-LRR, GSTTBCE-UbL, GSTTBCE-CG, or GST, produced in bacteria. The bead fraction was analyzed by Western blotting with anti-GFP and anti-GST antibodies; 10% is an aliquot of the yeast extract incubated with the beads
Pac2 interacts with polymerized MTs and may affect MT dynamics
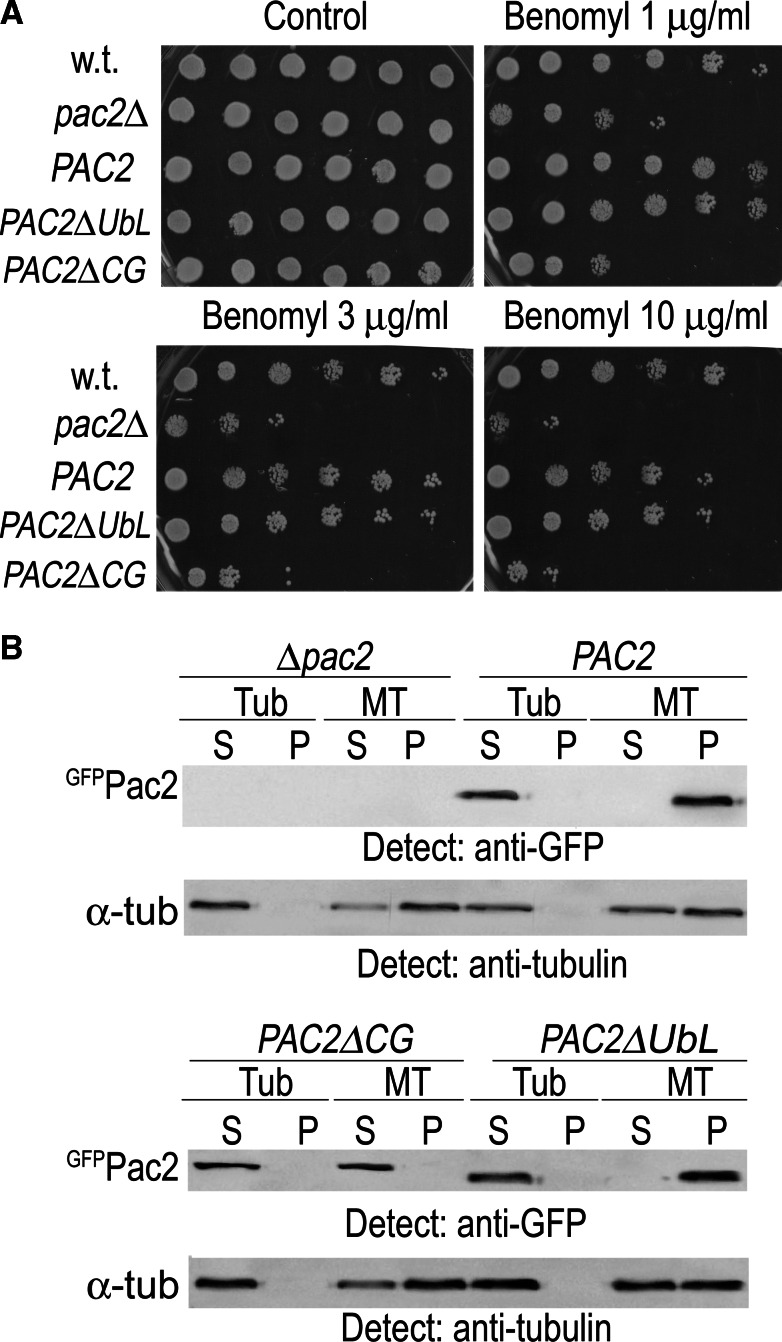
pac2Δ mutants are sensitive to the MT binding agent, benomyl [2], and to test for an effect of Pac2 on MT dynamics we examined suppression of the benomyl sensitivity of pac2Δ mutants by ectopic expression of either full-length GFPPAC2, Pac2 lacking the CAP-Gly domain, GFPPAC2ΔCG, or Pac2 without the UbL domain, GFPPAC2ΔUbL. We found that both full-length GFPPAC2 and PAC2ΔUBL restored wild-type levels of growth in the presence of benomyl, but that deletion of the CAP-Gly domain abrogated suppression of benomyl sensitivity of pac2Δ mutants (Fig. 2a). Furthermore, we tested for direct binding of Pac2 to polymerized MTs using a MT-cosedimentation assay [42]. Tubulin heterodimers were polymerized in vitro and incubated with yeast extracts from pac2Δ cells or from pac2Δ cells that produced GFPPac2, GFPPacΔCG, or GFPPacΔUbL. Controls consisted of these same yeast extracts incubated with tubulin heterodimers. We found that a large proportion of both Pac2 and PacΔUbL cosedimented with the polymerized MTs, but remained in the supernatant when mixed with tubulin heterodimers. In contrast GFPPacΔCG protein was not found in the pellet, only in the supernatant, indicating that Pac2 binds assembled MTs in vitro and that this is dependent on the CAP-Gly domain (Fig. 2b).
Fig. 2.
Pac2 affects MT dynamics. a Transformed pac2Δ mutants expressing pGAL-GFP-PAC2, pGAL-GFP-PAC2ΔUBL, or pGAL-GFP-PAC2ΔCAP-GLY (CG) were induced overnight with galactose and the next morning were observed in the fluorescent microscope to ascertain production of each protein. Serial threefold dilutions of wild-type, pac2Δ mutants, or pac2Δ mutants expressing pGAL-GFP-PAC2, pGAL-GFP-PAC2ΔUBL, or pGAL-GFP-PAC2ΔCG were spotted onto plates without (control) or with 1, 3, or 10 μg/ml benomyl and grown for 48 h prior to documentation. b Tubulin heterodimers (Tub) or microtubules polymerized in vitro (MT) were mixed with yeast extracts from pac2Δ cells or from pac2Δ cells producing GFPPac2, GFPPac2ΔUbL, or GFPPac2ΔCG and spun through a glycerol cushion (“Materials and methods”). The supernatant (S) and pellet (P) fractions were analyzed separately by Western blotting for the presence of GFPPac2, GFPPac2ΔUbL, and GFPPac2ΔCG
Interaction of Pac2 with the proteasome
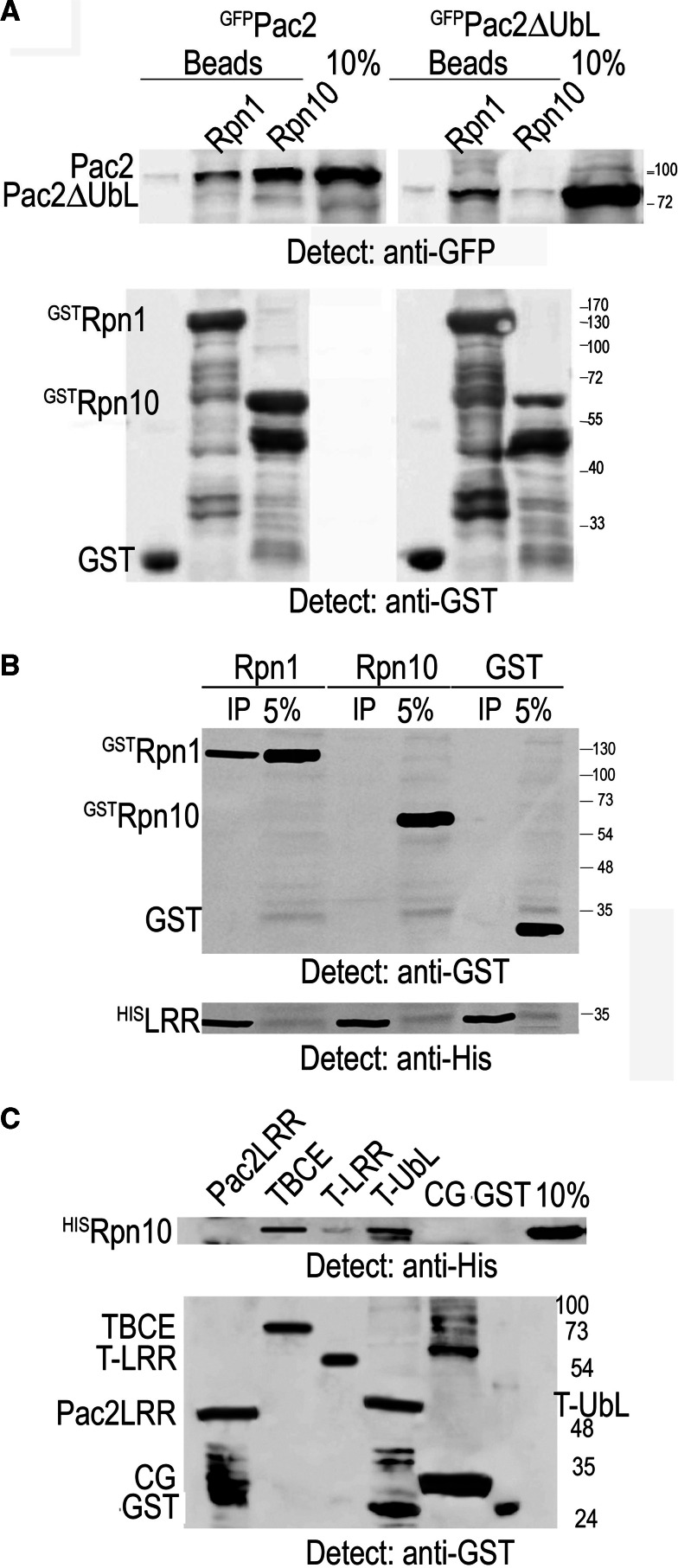
Given that UbL-UbA proteins bind the 19S RP subunits Rpn1 and/or Rpn10 via their UbL domain, we tested whether the UbL domain of Pac2 could be its proteasome-interacting domain. We performed an in vitro experiment in which recombinant yeast 19S RP subunits produced in bacteria, GSTRpn1 or GSTRpn10, were bound to glutathione (GSH) beads and incubated with yeast extracts from pac2Δ mutant cells producing full-length GFPPac2 or GFPPac2ΔUbL. We observed an extremely robust interaction of both GFPPac2 and GFPPac2ΔUbL with GSTRpn1; however, only full-length GFPPac2 interacted with GSTRpn10 (Fig. 3a). This indicates that the UbL domain is not required for interaction with Rpn1 and suggests that it may be the LRR domain that interacts with the proteasome. To test this we expressed in bacteria a recombinant subclone comprising 120 amino acids of the core of the Pac2 LRR (residues 125–255) domain fused to a Histidine tag. HisPac2LRR was bound to nickel beads that were incubated with bacterial lysate from cells that expressed GSTRpn1, GSTRpn10, or control GST peptide. After centrifugation the bead fraction was analyzed by Western blotting with anti-GST antibodies to detect the proteasome subunits followed by anti-His antibodes to show the HisPac2LRR protein on the beads. We observed that only GSTRpn1 bound HisPac2LRR (Fig. 3b), confirming the results of the previous experiment and indicating a direct role for the LRR domain in interaction of Pac2 with the 19S RP.
Fig. 3.
The LRR domain interacts with Rpn1 of the 19S RP. a GSH beads to which recombinant yeast GSTRpn1, GSTRpn10, or GST was bound were incubated with yeast extracts from pac2Δ mutants expressing pGAL-GFP-PAC2 or pGAL-GFP-PAC2ΔUBL. The bead fraction was analyzed by Western blotting with anti-GFP and anti-GST antibodies; 10% is the volume of total cell extract used in the experiment. b Recombinant yeast Pac2 HisLRR domain produced in bacteria was bound to nickel beads and incubated with a bacterial lysate from cells that produced yeast GSTRpn1, GSTRpn10, or GST. The bead fraction was analyzed by Western blotting first with anti-GST and then with anti-His antibodies; 5% is of the bacterial cell lysate. c Recombinant yeast HISRpn10 produced in bacteria was incubated with yeast GSTPac2-LRR domain, full-length human TBCE, or subclones of the TBCE LRR (T-LRR), UbL (T-UbL), or CAP-Gly (CG) domains, or with control GST beads, all produced in bacteria. HISRpn10 that bound TBCE protein was detected with anti-His antibodies; the proteins on the beads are shown using anti-GST antibodies, and 10% is an aliquot of HISRpn10 bacterial lysate incubated with the beads
To follow up our finding that deletion of the Pac2 UbL abrogates interaction with GSTRpn10 in vivo (Fig. 3a), we tested whether Rpn10 may be binding the UbL domain directly. In this experiment we expressed in bacteria recombinant yeast HisRpn10 and subclones of the S. cerevisiae and human TBCE proteins expressed as GST fusions in bacteria. These included the above core Pac2 LRR domain, this time fused to GST, full-length human TBCE, the CAP-Gly domain (residues 8–85), the TBCE-LRR domain in its entirety (residues 103–390), and the TBCE UbL domain (residues 444–527) (Fig. 1a). The GST fusion were bound to GSH beads and incubated with bacterial lysate from cells that expressed HisRpn10. The bead fraction was analyzed by Western blotting using anti-His and anti-GST antibodies sequentially. We found that full-length TBCE and the GSTTBCE UbL domain subclone bound HISRpn10 in vitro (Fig. 3c).
Degradation of Pac2 is dependent on binding of the UbL domain to Rpn10
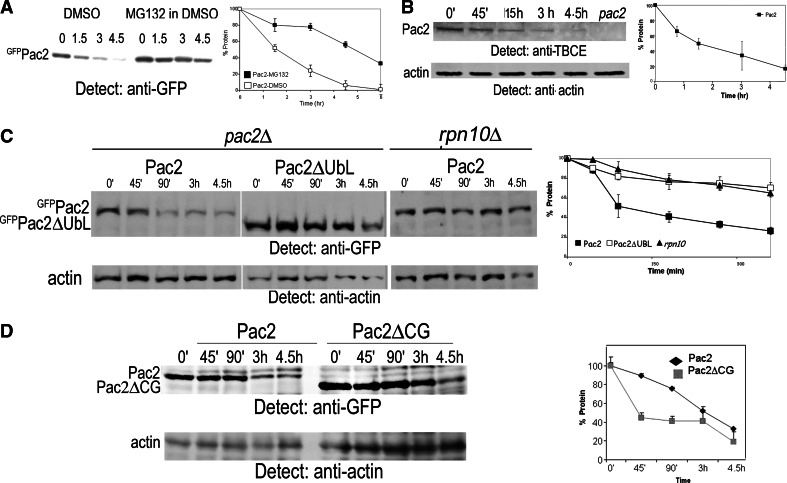
The interaction of the UbL domain with Rpn10 could indicate that Rpn10 serves as a proteasome receptor during degradation of Pac2. Initially, to determine whether Pac2 is a proteasomal substrate, we compared Pac2 half-life in the presence or absence of the proteasome inhibitor, MG132 [43], in pdr5Δ mutants of the ABC transporter to inhibit export of the inhibitor [44]. pGAL-GFP-PAC2 was induced overnight; next morning the cells were diluted 1:3, and after 1 h, at the zero time point 100 μM MG132 was added to the cells together with 3% glucose to repress the GAL promoter and 10 mM cycloheximide (CHX) to inhibit translation. The culture was sampled over 4.5 h. Incubation with MG132 led to stabilization of Pac2, indicating that its degradation proceeds via the proteasome (Fig. 4a). The half-life of endogenous Pac2, assayed using affinity-purified polyclonal antibody to human TBCE, was similar to that determined for plasmid-expressed PAC2 (Fig. 4b).
Fig. 4.
Turnover of Pac2 depends on interaction of its UbL domain with Rpn10. a Pac2 is stabilized in cells treated with the proteasome inhibitor MG132. pGAL-GFP-PAC2 transformed into pdr5Δ mutant cells was induced overnight with 2% galactose. Next morning the cells were diluted 1:3, and after 2 h 100 μl MG132 dissolved in DMSO was added (controls were treated with DMSO), together with 3% glucose to repress transcription and 10 mM CHX to inhibit translation. Equal aliquots of cells were analyzed at the zero time point and at the times indicated above each lane by anti-GFP immunoprecipitation followed by Western blotting. Graph shows protein remaining at each time point. b Half-life of endogenous Pac2 determined using an affinity-purified polyclonal antibody to human TBCE; 10 mM CHX was added at the zero time point, and equal aliquots were analyzed by TCA precipitation and Western blotting at the times indicated. Extract from pac2Δ mutants was used as a control for the specificity of the antibody; anti-actin was used as a loading control. c Pac2 and Pac2ΔUbL turnover in pac2Δ and rpn10Δ cells: pGAL-GFP-PAC2 or pGAL-GFP-PAC2ΔUBL were induced overnight in pac2Δ mutants, and pGAL-GFP-PAC2 was induced overnight in rpn10Δ mutants. At the zero time point, 3% glucose and 10 mM CHX were added, and equal aliquots of cells were TCA-precipitated for Western blotting at the times indicated above the lanes. Actin was used as a loading control. d Pac2 and Pac2ΔCG turnover in pac2Δ mutants: experimental conditions as in c
If Rpn10 is a proteasome receptor for Pac2, deletion of either the Pac2 UbL domain or of Rpn10 through use of a rpn10Δ mutant should cause the protein to be stabilized. We therefore compared the half-life of Pac2ΔUbL with that of full-length Pac2 in wild-type cells and also compared the half-life of full-length Pac2 in pac2Δ and rpn10Δ mutant cells. We found that deletion of the UbL domain leads to stabilization of Pac2ΔUbL in pac2Δ cells. Similarly, full-length Pac2 is stabilized in rpn10Δ mutants (Fig. 4c). The rpn10Δ mutant is competent for protein degradation [35]. Our interpretation is that Rpn10 recruits Pac2 via its UbL domain to the proteasome for degradation. In contrast, when we deleted the Pac2 CAP-Gly domain that binds MTs, we observed that this does not affect the half-life of Pac2 (Fig. 4d).
Pac2 interacts with the SCF complex via its UbL domain
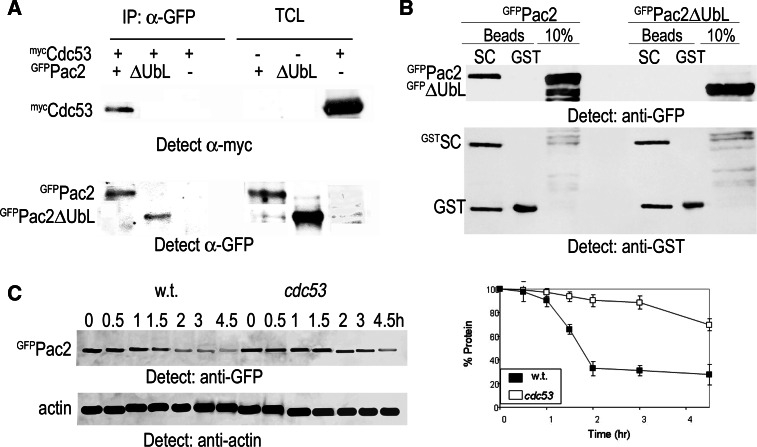
Mammalian TBCB is recruited via its UbL domain by Gigaxonin for ubiquitylation by a cullin-RING ubiquitin ligase (“Introduction”), and here we tested for an interaction between Pac2 and the Skp1-Cdc53/cullin-F-box protein (SCF) complex in vivo. We transformed GFPPac2 or GFPPac2ΔUbL into yeast cells that expressed mycCdc53 and immunoprecipitated cell extracts using anti-GFP antibodies. (The myc tag does not prevent Cdc53 from forming active SCF complexes). We found that Cdc53 coimmunoprecipitates with GFPPac2, but not with GFPPac2ΔUbL (Fig. 5a). To preclude the possibility that GFPPac2ΔUbL may be interacting with endogenous full-length Pac2, we produced GFPPac2 and GFPPac2ΔUbL in pac2Δ mutants and incubated the extracts with GSH beads bound to a yeast GSTSkp1-Cdc53 complex produced in insect cells [45]. The result was similar to that of the coimmunoprecipitation experiment: the interaction of GFPPac2 was robust, whereas there was no interaction with GFPPac2ΔUbL (Fig. 5b). These experiments suggest an additional role for the UbL domain: SCF-mediated ubiquitylation of Pac2. However, when we tested the half-life of Pac2 in cdc53Δ mutants, we found only partial stabilization of the protein, implying that not all Pac2 degradation is dependent on SCF-mediated ubiquitylation (Fig. 5c). Furthermore, by Western blotting with anti-ubiquitin antibodies we found that both GFPPac2 and GFPPac2ΔUbL are ubiquitylated, indicating that the UbL domain is not required for protein ubiquitylation (Fig. 6a). In this experiment each protein was produced in pac2Δ and in rpn10Δ mutants and immunoprecipitated with anti-GFP antibodies and Protein A beads followed by Western blotting with anti-ubiquitin antibodies. Controls were precipitated with Protein A beads alone. In pac2Δ mutants immunoprecipitated GFPPac2 was observed only in the anti-GFP Western blot, and no high MW ubiquitin conjugates were detected with anti-ubiquitin antibodies. In contrast, in rpn10Δ mutants a pronounced smear indicative of high molecular weight ubiquitin conjugates was observed. This smear is specific for ubiquitylated GFPPac2 as it was absent from the controls precipitated with Protein A beads without anti-GFP antibodies. GFPPac2ΔUbL that is stabilized in pac2Δ mutants showed ubiquitylated conjugates in both pac2Δ and rpn10Δ mutants. Thus, the UbL domain is not crucial for ubiquitylation of Pac2, and it is not clear to what extent ubiquitylation of Pac2 can be attributed to SCF activity.
Fig. 5.
The Pac2 UbL domain interacts with the SCF complex. a GFPPac2 and GFPPac2ΔUbL were immunoprecipitated with anti-GFP from yeast extracts made from cells that expressed mycCdc53. The bead fractions were blotted with anti-myc antiserum to detect Cdc53 and with anti-GFP to detect immunoprecipitated GFPPac2. b GSTSkp1-Cdc53 heterodimers (SC) from Sf9 cells on GSH beads or GST peptides were incubated with yeast extracts from pac2Δ mutants expressing pGFP-PAC2 or pGFP-PAC2ΔUBL. Interaction was detected by Western blot with anti-GFP antibodies. c Half-life of Pac2 in wild-type cells and in cdc53 mutants measured at 37°C. Experimental details as in Fig. 4c
Fig. 6.
The Pac2 CAP-Gly and UbL domains are not required for ubiquitylation. a Pac2, Pac2ΔUbL, and Pac2ΔCAP-Gly (CG) were produced in pac2Δ and rpn10Δ mutants and immunoprecipitated with anti-GFP antibodies and Protein A beads (IP) or without antibodies (−). They were analyzed by Western blotting with anti-GFP and anti-ubiquitin antibodies. T is 5% of the extract used. b Ectopic Pac2 and Pac2ΔUbL levels at different stages of the cell cycle. pdr5Δ mutants expressing pGAL-GFP-PAC2 or pGAL-GFP-PAC2ΔUBL were arrested in G1 with α-factor, in S phase with HU, and in prometaphase with nocodazole. Equal aliquots were precipitated with TCA and blotted with anti-GFP and anti-actin antibodies. The ratio of Pac2 and Pac2ΔUbL to the actin loading control is shown in the histogram. c Levels of endogenous Pac2 in wild-type cells were detected with a rabbit polyclonal antibody to human TBCE in cells arrested in different stages of the cell cycle as above. pac2Δ mutants are shown as a control for the specificity of the human antibody
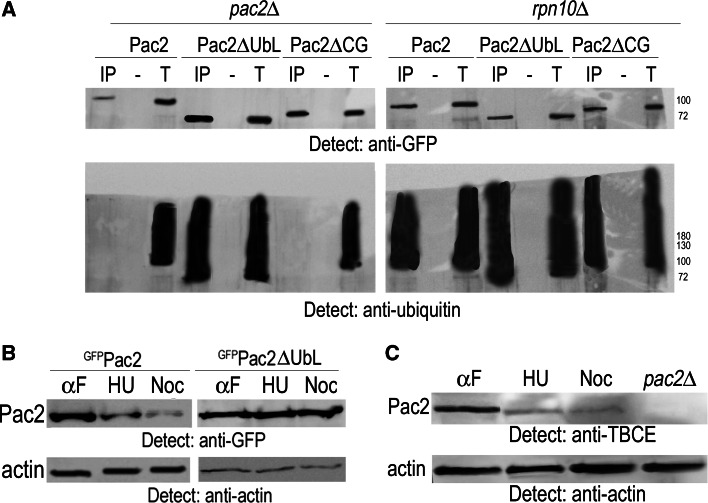
In view of the direct binding of Pac2 to the 19S RP, there is a formal possibility that Pac2 may be degraded without being ubiquitylated. Therefore, to exclude the possibility that the ubiquitylation we observed is of α-tubulin bound to the Pac2 CAP-Gly domain rather than of Pac2 itself, we immunoprecipitated Pac2, Pac2ΔCAP-Gly, and Pac2ΔUbL from pac2Δ and rpn10Δ mutants and analyzed the bead fraction by Western blotting. In pac2Δ mutants all three forms were detected with anti-GFP antibodies, but only Pac2ΔUbL accumulated as an ubiquitylated conjugate. In contrast in the rpn10Δ mutants all three proteins accumulated as ubiquitylated conjugates (Fig. 6a). Ubiquitylation of Pac2ΔCAP-Gly affirms that it is Pac2 itself rather than α-tubulin bound to the CAP-Gly domain that is ubiquitylated. Accumulation of ubiquitylated Pac2ΔUbL in pac2Δ mutants and of all three forms in rpn10Δ mutants emphasizes the importance of the interaction between the Pac2 UbL domain and Rpn10 for recruitment of ubiquitylated Pac2 to the 19S RP for degradation.
Our ability to observe a fraction of GFPPac2 with anti-GFP antibodies that was not detected with antibodies to ubiquitin indicates that not all Pac2 is ubiquitylated at any given time point in a nonsynchronized logarithmic cell population. To check for cell cycle-dependent Pac2 degradation, we synchronized pdr5Δ mutants expressing pGAL-GFP-PAC2 or pGAL-GFP-PAC2ΔUbL in G1 with α-factor, in S phase with hydroxyurea (HU), and in prometaphase with nocodazole. The amount of Pac2 relative to actin was estimated for each culture by Western blotting. We found a considerable variation in the amount of Pac2 with the highest amount present in G1 cells; cells that produced the non-degradable Pac2ΔUbL showed equal amounts of the protein at each phase of the cell cycle (Fig. 6b). This was also observed for endogenous Pac2 (Fig. 6c).
The nature of Pac2 complexes in vivo
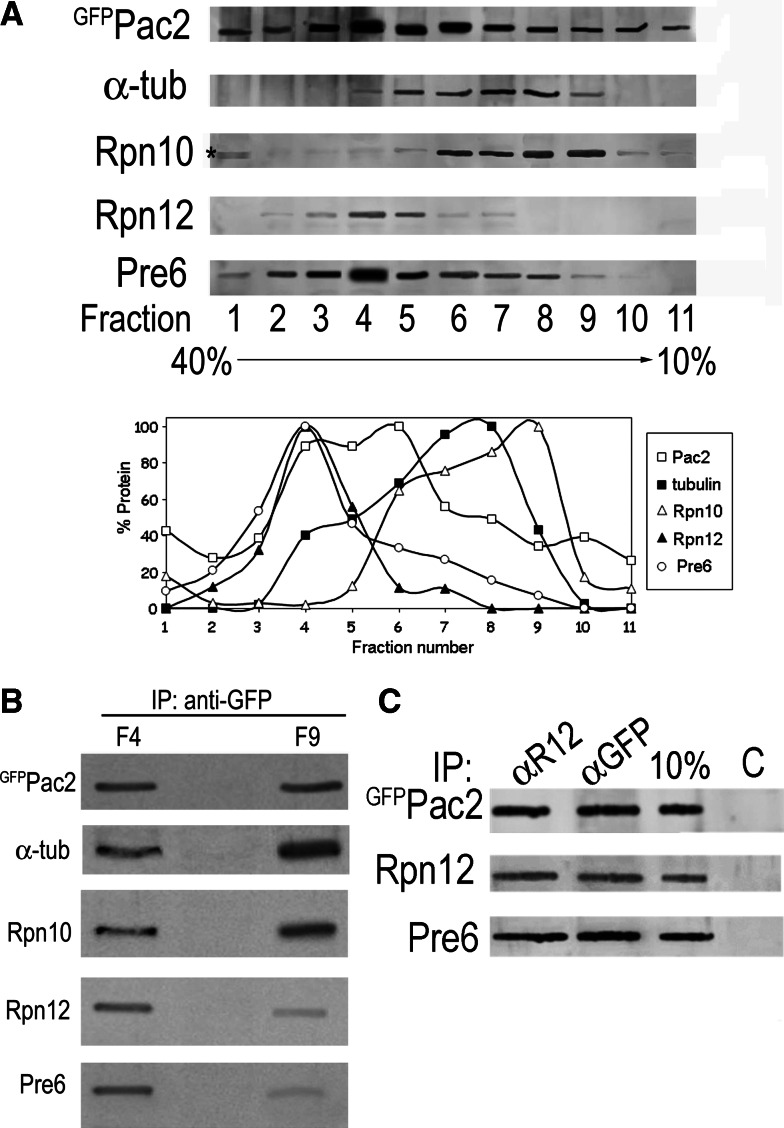
Our experiments above indicate that Pac2 binds both MTs and the proteasome. Therefore, to characterize the complexes formed by Pac2 in vivo we performed 10–40% glycerol gradients of extracts of cells expressing pGAL-GFP-PAC2. The MW of GFPPac2 is ca. 84 kDa, whereas that of the proteasome is ca. 700 kDa. Aliquots of 11 fractions were separated by SDS-PAGE and blotted with antibodies to detect Pac2, α-tubulin, Rpn10, 19S RP (with anti-Rpn12), and 20S CP (with anti-Pre6 antibodies). The presence of both 20S CP and 19S RP in the same fraction is indicative of the assembled 26S proteasomes. We found Pac2 both in the lighter fractions in which tubulin and free extraproteasomal Rpn10 are present, and in the fractions enriched for the 19S and 20S proteasome particles (Fig. 7a). Two fractions were chosen for immunoprecipitation of GFPPac2: Fraction 4 in which stochiometric amounts of the proteasome subunits Rpn12 and Pre6 were present at a high concentration, and Fraction 9 in which we found α-tubulin, Pac2, and Rpn10, but far less Rpn12 or Pre6. Extracts of each fraction were immunoprecipitated with anti-GFP antibodies followed by Western blotting with the above antibodies. Analysis of Fraction 4 showed that Pac2 coimmunoprecipitated with α-tubulin, Rpn10, Rpn12, and Pre6, i.e., 26S proteasomes, whereas immunoprecipitation of GFPPac2 from Fraction 9 led to coimmunoprecipitation of α-tubulin and of Rpn10 with minor traces of 26S proteasome marker proteins (Fig. 7b). This result indicates that the direct interaction between Pac2 and Rpn10 obtained with recombinant proteins (Fig. 3a and c) occurs in vivo. In addition we immunoprecipitated aliquots of Fraction 4 with Protein A beads in the presence of either anti-GFP or anti-Rpn12 antibodies, or without antibodies for controls. We found that when GFPPac2 was immunoprecipitated with anti-GFP antibodies, subunits of both the 19S and 20S proteasome were present in the bead fraction. When the gradient fraction was immunoprecipitated with anti-Rpn12 antibodies, both Pac2 and the 20S particle were present in the bead fraction (Fig. 7c). Thus, Pac2 can be found in two complexes in vivo: one with 26S proteasomes and another with α-tubulin and free extraproteasomal Rpn10.
Fig. 7.
Pac2 complexes in vivo. a Western analysis of 11 40–10% glycerol gradient fractions blotted with anti-GFP antibodies to detect GFPPac2 (MW 89 KDa); and with anti-α-tubulin (MW 50 KDa); with anti-Rpn10 (MW 30 KDa) and anti-Rpn12 (MW 32 KDa) to detect the 19S RP; and with anti-Pre6 (MW 28.5 KDa) antibodies to detect the 20S CP. Graphs show relative amount of each protein in the different fractions. b Comparison of the Pac2 complexes from Fraction 4 and Fraction 9. GFPPac2 was immunoprecipitated with anti-GFP antibodies, and the bead fraction was separated by SDS-PAGE for Western blotting with anti-GFP, anti-α-tubulin, anti-Rpn10, anti-Rpn12, and anti-Pre6 antibodies. c Immunoprecipitation of aliquots of Fraction 4 with anti-Rpn12 (αR12 lane) to immunoprecipitate the 19S RP or anti-GFP antibodies (αGFP lane) to immunoprecipitate GFPPac2. Components of the complex that co-immunoprecipitated in each fraction were detected by Western blotting with the antibodies used in Fig. 8a. Controls (C) were Protein A beads without antibody; 10% is a TCA precipitation of an aliquot of the gradient fraction
pac2Δ mutants are sensitive to canavanine and cadmium stress
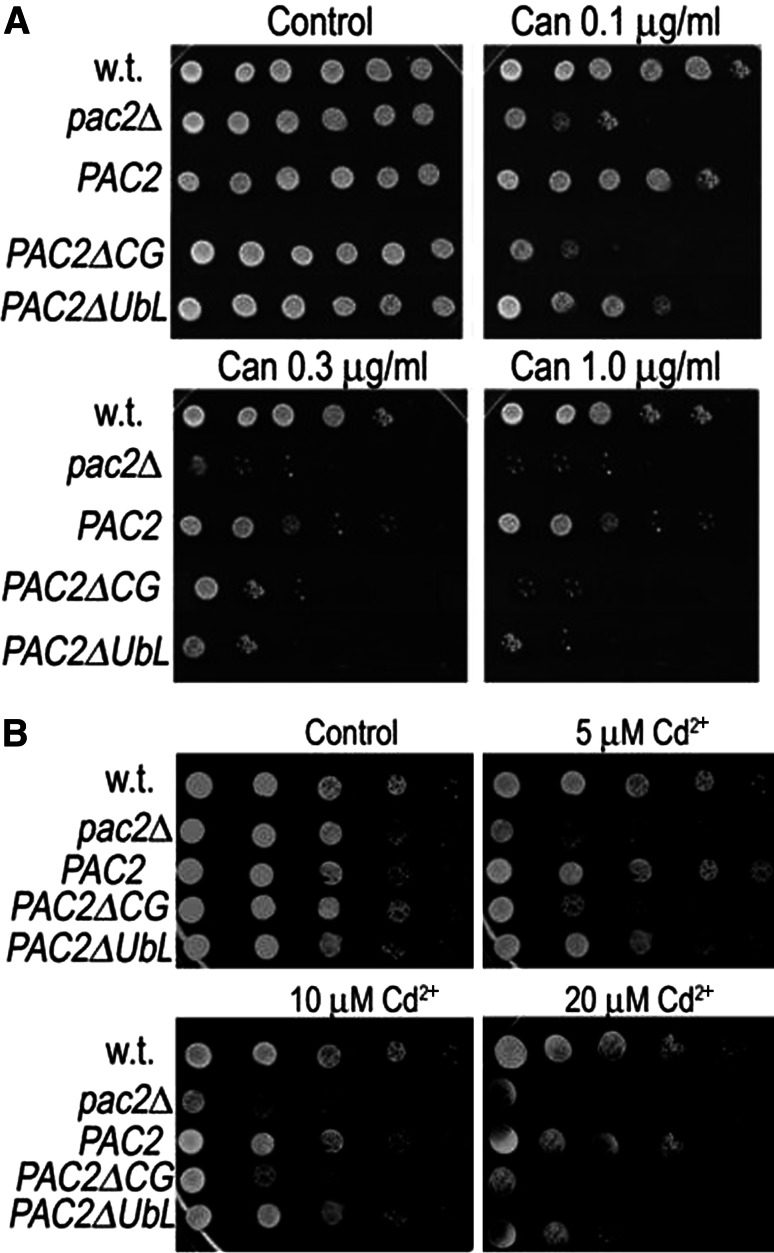
Our experiments demonstrate interactions with the proteasome for two different domains of Pac2. The Pac2 LRR domain binds Rpn1, whereas the UbL domain binds Rpn10, and this mediates Pac2 turnover. To determine the functional significance of these interactions, we first tested the sensitivity of pac2Δ mutants to misfolded protein stress using the arginine analog, canavanine. Incorporation of canavanine into proteins affects their folding, and accumulation of a high level of misfolded proteins leads to stress on the proteasome. Threefold serial dilutions of cells were plated in the presence of increasing concentrations of canavanine. We found that compared with wild-type cells, pac2Δ mutants are very sensitive to canavanine and that ectopic expression of full-length PAC2 restored canavanine resistance to wild-type levels. The Pac2 CAP-Gly domain was crucial for suppression of the canavanine sensitivity even at very low canavanine concentrations, whereas both the CAP-Gly and the UbL domains were essential at the higher concentrations (Fig. 8a). Similar to canavanine sensitivity, we found that pac2Δ mutants are very sensitive to cadmium, which leads to ERAD deficiencies [34] (and references therein). Ectopic expression of full-length PAC2 restored cadmium resistance to wild-type levels. Here too the CAP-Gly domain was crucial for suppression of cadmium sensitivity at every dose tested, whereas the UbL domain became essential at the highest concentration of cadmium (Fig. 8b).
Fig. 8.
pac2Δ mutants are sensitive to canavanine and to cadmium stresses. a Suppression of canavanine sensitivity of pac2Δ mutants by ectopic PAC2. Serial threefold dilutions of wild-type, pac2Δ mutants, or pac2Δ mutants expressing pGAL-GFP-PAC2, pGAL-GFP-PAC2ΔCAP-Gly (CG), or pGAL-GFP-PAC2ΔUbL were plated on cells with different concentrations of canavanine as indicated above the plates. They were photographed after 48 h at 30oC. b Suppression of cadmium sensitivity of pac2Δ mutants by ectopic PAC2. Cells were spotted as above onto plates with different concentrations of cadmium chloride as indicated above the plates
Discussion
The sensitivity of pac2Δ mutants to the MT-binding drug, benomyl, has been known for some time [2], and here we show that restoration of growth in the presence of benomyl depends on the presence of the CAP-Gly domain, the domain shown by our analysis to interact with α-tubulin and with in vitro polymerized taxol-stabilized MTs. In this respect Pac2 is similar to Alf1, the mammalian TBCB ortholog, that interacts with α-tubulin via its CAP-Gly domain and associates with MTs in vivo [5]. Mammalian TBCE has been implicated in MT dynamics through regulation of tubulin heterodimer dissociation [17]; in this context it acts in a heterodimer with TBCB [18]. Similarly, in Drosophila TBCE promotes MT network formation at neuromuscular synapses [46]. Likewise, the C. elegans ortholog of Pac2, K07H8.1, has a role in MT dynamics enhancing lethality of spindle assembly checkpoint mutants, MAD1-3, that is attributable to accelerated chromosome loss [47].
Our experimental results confirm the interaction of Pac2 with the proteasome reported by [33]. However, in contrast to the family of UbL-UbA proteins that bind the 19S RP via interaction of their UbL domains with the 19S RP [25–27], we demonstrate two different interactions, each mediated by a separate domain of the protein. The Pac2/TBCE LRR domain binds Rpn1, whereas the Pac2 UbL domain binds Rpn10. The importance of the interaction between the LRR domain and the proteasome was confirmed by the recent discovery of a new case of HRD syndrome in which the TBCE protein lacks the founder CAP-Gly mutation, but is deleted for the N-terminal half of the LRR domain (R. Parvari, in preparation). The interaction between the Pac2 UbL domain and Rpn10 is essential for turnover of Pac2 and possibly of α-tubulin that binds its CAP-Gly domain. Rpn10 binds K48 ubiquitin chains in vitro [48] and recruits ubiquitylated substrates to the proteasome [49, 50]. Rpn10 may also fulfill a facilitator role, e.g., in degradation of Sic1 in the presence of Rad23 [35]. Rpn10 also interacts with the UbL domain of Dsk2 [30], and extraproteasomal Rpn10 may regulate degradation of substrates that reach the proteasome via Dsk2 [30, 31]. In contrast to UbL-UbA shuttle proteins that are protected from degradation by their UbA domain [51], Pac2 itself is a proteasome substrate being stabilized in the presence of the proteasome inhibitor, MG132. In the absence of interaction between the Pac2 UbL and Rpn10, there is little deubiquitylation of Pac2. Pac2ΔUbL is highly ubiquitylated but is not degraded; similarly full-length Pac2 accumulates as a high MW ubiquitylated conjugate in rpn10Δ mutants. We propose that deubiquitylation of Pac2 may involve a proteasome-associated deubiquitylating enzyme whose access to Pac2 is dependent on interaction of its UbL domain with Rpn10. The association of Pac2 with the 19S RP via its LRR domain and Rpn1 does not promote Pac2 deubiquitylation.
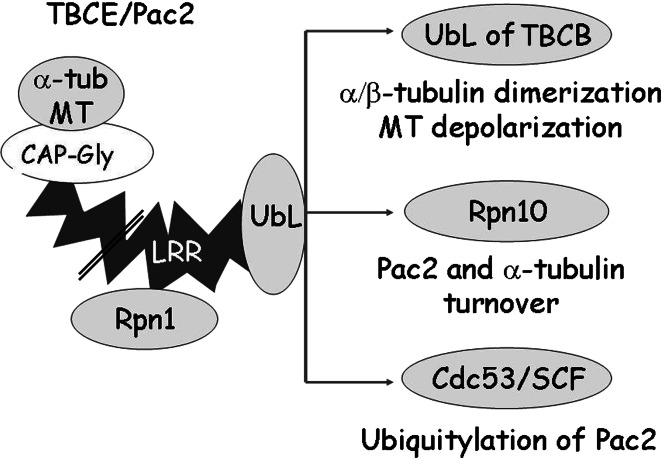
The amount of Pac2 protein varies during the cell cycle, and not all the protein undergoes ubiquitylation, suggesting that ubiquitylation of Pac2 is regulated. A possible mechanism could be through the proposed interaction between the UbL domains of TBCB and TBCE [16, 18]. Dimerization of the two α-tubulin cofactors could serve a regulatory function by determining the proportion of cellular Pac2 that is involved in MT polymerization, that interacts with the SCF, or that is degraded through interaction with Rpn10; this could also determine whether the α-tubulin bound to TBCE is recycled or degraded (Fig. 9).
Fig. 9.
Interactions of the Pac2 protein domains. Left Pac2/TBCE protein domains. The CAP-Gly domain interacts with α-tubulin and MTs; the LRR domain interacts with Rpn1; the UbL domain: a dimerizes with the UbL of TBCB [16, 18] and affects MT dynamics; b interacts with Rpn10 and this determines turnover of Pac2 (present study), and of α-tubulin[52]; c interacts with the SCF ubiquitin ligase complex core subunits, Skp1-Cdc53 (present study). We propose that by engaging in these different interactions the UbL domain could fulfill a regulatory role in MT dynamics. The LRR domain is not drawn to scale (double line)
The UbL domain of mammalian TBCB serves for recruitment to a cullin-RING ubiquitin ligase complex, and in the absence of the substrate receptor, Gigaxonin, TBCB accumulates in the cells [15]. Similarly, we find that the Pac2 UbL domain binds the SCF core subunits, Skp1-Cdc53. However, in contrast to mammalian TBCB, deletion of the Pac2 UbL domain does not abrogate Pac2 ubiquitylation indicating the involvement of an additional as yet unidentified E3.
Immunoprecipitation of Pac2 from light glycerol gradient fractions in which α-tubulin and Rpn10 are present indicates the presence of a trimeric complex that does not include stochiometric amounts of the 26S subunits. α-tubulin is among the polyubiquitylated proteins that accumulate in rpn10Δ mutants, indicating a role for Rpn10 in its turnover [52]. However, our results do not allow us to determine whether α-tubulin reaches the proteasome as part of a trimeric complex formed initially with the extraproteasomal Rpn10. Rpn10 is cleaved by caspases during apoptosis in mammalian cells [53], and our results imply that this would impact on the MT cytoskeleton. The new interactions we identify for the UbL imply a regulatory role for the UbL domain and a new facet in the regulation of MT dynamics.
pac2Δ mutants are sensitive to canavanine that leads to misfolded protein stress on the proteasome, indicating that the interaction between Pac2 and the proteasome is functionally significant. Canavanine sensitivity of pac2Δ mutants is suppressed by ectopic expression of PAC2, and both the Pac2 CAP-Gly and UbL domains are required for this. Similarly, we report that pac2Δ mutants are sensitive to cadmium, which leads to ERAD deficiency [34] (and references therein). Here too both the MT-interacting CAP-Gly and the proteasome-interacting UbL domains are required to suppress cadmium sensitivity, particularly at the higher concentrations.
Proteasomes are reported to localize to the centrosomes of cells where they are biochemically active, and when cells accumulate misfolded proteins or are treated with a proteasomal inhibitor, there is an increase in centrosome-associated proteasomes [54, 55]. Cyclin B [56] and the cell cycle regulated kinase, Nek2 [57], are two substrates shown to undergo degradation at the centrosome. In addition the UbL-domain E3 Parkin binds MTs [58] and is recruited to the centrosome in response to inhibition of the proteasome [59], further implying a role for MTs and MT-binding proteins in mediating activity of the ubiquitin-proteasome system at the centrosome. The mechanisms that recruit proteins to the centrosomes are poorly understood, and minus-end-directed MT motors such as cytoplasmic dynein have been implicated in their transport [60]. Moreover, a recent report indicates that proteasome subunits of both the 19S and 20S particles are found in association with MTs in Drosophila embryos [61]. The interaction of Pac2 with both the proteasome and with MTs, the benomyl sensitivity of pac2Δ mutants, and the requirement for both its CAP-Gly and its UbL domains to withstand misfolded protein stress lead us to hypothesize that Pac2 may have a role in subcellular localization of proteasomes.
Acknowledgments
We dedicate this article to the memory of the late Mark Rubinstein. This work was supported by the German-Israel Foundation for Scientific Research and Development (GIF), FP5 EU contract HPRN-CT-2002-00238, the Israel Cancer Research Fund (ICRF), and the Israel Cancer Association (to D. Raveh); by the Israel-US Bi-National grant no. 2003141, the Israel Science Foundation grant no. 1043/09 (to L. Gheber) and Israel-US Bi-National grant no. 2005525 (to R. Parvari), and a grant to D. Raveh and R. Parvari from the National Institute of Biotechnology, Negev. We thank Professors Michael Glickman and Michal Shapira for many useful discussions. We thank Professor Dorota Skowyra for crucial reagents and for useful discussions.
References
- 1.Parvari R, Hershkovitz E, Grossman N, Gorodischer R, Loeys B, Zecic A, Mortier G, Gregory S, Sharony R, Kambouris M, Sakati N, Meyer BF, Al Aqeel AI, Al Humaidan AK, Al Zanhrani F, Al Swaid A, Al Othman J, Diaz GA, Weiner R, Khan KT, Gordon R, Gelb BD. Mutation of TBCE causes hypoparathyroidism-retardation-dysmorphism and autosomal recessive Kenny-Caffey syndrome. Nat Genet. 2002;32:448–452. doi: 10.1038/ng1012. [DOI] [PubMed] [Google Scholar]
- 2.Hoyt MA, Macke JP, Roberts BT, Geiser JR. Saccharomyces cerevisiae PAC2 functions with CIN1, 2 and 4 in a pathway leading to normal microtubule stability. Genetics. 1997;146:849–857. doi: 10.1093/genetics/146.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radcliffe PA, Hirata D, Vardy L, Toda T. Functional dissection and hierarchy of tubulin-folding cofactor homologues in fission yeast. Mol Biol Cell. 1999;10:2987–3001. doi: 10.1091/mbc.10.9.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian G, Lewis SA, Feierbach B, Stearns T, Rommelaere H, Ampe C, Cowan NJ. Tubulin subunits exist in an activated conformational state generated and maintained by protein cofactors. J Cell Biol. 1997;138:821–832. doi: 10.1083/jcb.138.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feierbach B, Nogales E, Downing KH, Stearns T. Alf1p, a CLIP-170 domain-containing protein, is functionally and physically associated with alpha-tubulin. J Cell Biol. 1999;144:113–124. doi: 10.1083/jcb.144.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowan NJ, Lewis SA. Type II chaperonins, prefoldin, and the tubulin-specific chaperones. Adv Protein Chem. 2001;59:73–104. doi: 10.1016/S0065-3233(01)59003-8. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Fanarraga M, Avila J, Guasch A, Coll M, Zabala JC. Review: postchaperonin tubulin folding cofactors and their role in microtubule dynamics. J Struct Biol. 2001;135:219–229. doi: 10.1006/jsbi.2001.4386. [DOI] [PubMed] [Google Scholar]
- 8.Bommel H, Xie G, Rossoll W, Wiese S, Jablonka S, Boehm T, Sendtner M. Missense mutation in the tubulin-specific chaperone E (Tbce) gene in the mouse mutant progressive motor neuronopathy, a model of human motoneuron disease. J Cell Biol. 2002;159:563–569. doi: 10.1083/jcb.200208001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin N, Jaubert J, Gounon P, Salido E, Haase G, Szatanik M, Guenet JL. A missense mutation in Tbce causes progressive motor neuronopathy in mice. Nat Genet. 2002;32:443–447. doi: 10.1038/ng1016. [DOI] [PubMed] [Google Scholar]
- 10.Riehemann K, Sorg C. Sequence homologies between four cytoskeleton-associated proteins. Trends Biochem Sci. 1993;18:82–83. doi: 10.1016/0968-0004(93)90159-K. [DOI] [PubMed] [Google Scholar]
- 11.Grynberg M, Jaroszewski L, Godzik A. Domain analysis of the tubulin cofactor system: a model for tubulin folding and dimerization. BMC Bioinformatics. 2003;4:46–53. doi: 10.1186/1471-2105-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheel J, Pierre P, Rickard JE, Diamantopoulos GS, Valetti C, van der Goot FG, Haner M, Aebi U, Kreis TE. Purification and analysis of authentic CLIP-170 and recombinant fragments. J Biol Chem. 1999;274:25883–25891. doi: 10.1074/jbc.274.36.25883. [DOI] [PubMed] [Google Scholar]
- 13.Culver-Hanlon TL, Lex SA, Stephens AD, Quintyne NJ, King SJ. A microtubule-binding domain in dynactin increases dynein processivity by skating along microtubules. Nat Cell Biol. 2006;8:264–270. doi: 10.1038/ncb1370. [DOI] [PubMed] [Google Scholar]
- 14.Rickard JE, Kreis TE. CLIPs for organelle-microtubule interactions. Trends Cell Biol. 1996;6:178–183. doi: 10.1016/0962-8924(96)10017-9. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Ding J, Allen E, Zhu P, Zhang L, Vogel H, Yang Y. Gigaxonin interacts with tubulin folding cofactor B and controls its degradation through the ubiquitin-proteasome pathway. Curr Biol. 2005;15:2050–2055. doi: 10.1016/j.cub.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 16.Lytle BL, Peterson FC, Qiu SH, Luo M, Zhao Q, Markley JL, Volkman BF. Solution structure of a ubiquitin-like domain from tubulin-binding cofactor B. J Biol Chem. 2004;279:46787–46793. doi: 10.1074/jbc.M409422200. [DOI] [PubMed] [Google Scholar]
- 17.Kortazar D, Carranza G, Bellido J, Villegas JC, Fanarraga ML, Zabala JC. Native tubulin-folding cofactor E purified from baculovirus-infected Sf9 cells dissociates tubulin dimers. Protein Expr Purif. 2006;30:30. doi: 10.1016/j.pep.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Kortazar D, Fanarraga ML, Carranza G, Bellido J, Villegas JC, Avila J, Zabala JC. Role of cofactors B (TBCB) and E (TBCE) in tubulin heterodimer dissociation. Exp Cell Res. 2007;313:425–436. doi: 10.1016/j.yexcr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Glickman MH, Raveh D. Proteasome plasticity. FEBS Lett. 2005;579:3214–3223. doi: 10.1016/j.febslet.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 20.Pickart CM, Cohen RE. Proteasomes and their kin: proteases in the machine age. Nat Rev Mol Cell Biol. 2004;5:177–187. doi: 10.1038/nrm1336. [DOI] [PubMed] [Google Scholar]
- 21.Verma R, Aravind L, Oania R, McDonald WH, Yates JR, 3rd, Koonin EV, Deshaies RJ. Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science. 2002;298:611–615. doi: 10.1126/science.1075898. [DOI] [PubMed] [Google Scholar]
- 22.Guterman A, Glickman MH. Complementary roles for Rpn11 and Ubp6 in deubiquitination and proteolysis by the proteasome. J Biol Chem. 2004;279:1729–1738. doi: 10.1074/jbc.M307050200. [DOI] [PubMed] [Google Scholar]
- 23.Husnjak K, Elsasser S, Zhang N, Chen X, Randles L, Shi Y, Hofmann K, Walters KJ, Finley D, Dikic I. Proteasome subunit Rpn13 is a novel ubiquitin receptor. Nature. 2008;453:481–488. doi: 10.1038/nature06926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartmann-Petersen R, Gordon C. Protein degradation: recognition of ubiquitinylated substrates. Curr Biol. 2004;14:R754–R756. doi: 10.1016/j.cub.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Elsasser S, Finley D. Delivery of ubiquitinated substrates to protein-unfolding machines. Nat Cell Biol. 2005;7:742–749. doi: 10.1038/ncb0805-742. [DOI] [PubMed] [Google Scholar]
- 26.Kaplun L, Tzirkin R, Bakhrat A, Shabek N, Ivantsiv Y, Raveh D. The DNA damage-inducible UbL–UbA protein Ddi1 participates in Mec1-mediated degradation of Ho endonuclease. Mol Cell Biol. 2005;25:5355–5362. doi: 10.1128/MCB.25.13.5355-5362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivantsiv Y, Kaplun L, Tzirkin-Goldin R, Shabek N, Raveh D. Turnover of SCFUfo1 complexes requires the UbL–UbA motif protein, Ddi1. Mol Cell Biol. 2006;26:1579–1588. doi: 10.1128/MCB.26.5.1579-1588.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goh AM, Walters KJ, Elsasser S, Verma R, Deshaies RJ, Finley D, Howley PM. Components of the ubiquitin-proteasome pathway compete for surfaces on Rad23 family proteins. BMC Biochem. 2008;9:4. doi: 10.1186/1471-2091-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elsasser S, Gali RR, Schwickart M, Larsen CN, Leggett DS, Muller B, Feng MT, Tubing F, Dittmar GA, Finley D. Proteasome subunit Rpn1 binds ubiquitin-like protein domains. Nat Cell Biol. 2002;4:725–730. doi: 10.1038/ncb845. [DOI] [PubMed] [Google Scholar]
- 30.Ishii T, Funakoshi M, Kobayashi H. Yeast Pth2 is a UBL domain-binding protein that participates in the ubiquitin-proteasome pathway. EMBO J. 2006;25:5492–5503. doi: 10.1038/sj.emboj.7601418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matiuhin Y, Kirkpatrick DS, Ziv I, Kim W, Dakshinamurthy A, Kleifeld O, Gygi SP, Reis N, Glickman MH. Extraproteasomal Rpn10 restricts access of the polyubiquitin-binding protein Dsk2 to proteasome. Mol Cell. 2008;32:415–425. doi: 10.1016/j.molcel.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saeki Y, Saitoh A, Toh-e A, Yokosawa H. Ubiquitin-like proteins and Rpn10 play cooperative roles in ubiquitin-dependent proteolysis. Biochem Biophys Res Commun. 2002;293:986–992. doi: 10.1016/S0006-291X(02)00340-6. [DOI] [PubMed] [Google Scholar]
- 33.Verma R, Chen S, Feldman R, Schieltz D, Yates J, Dohmen J, Deshaies RJ. Proteasomal proteomics: identification of nucleotide-sensitive proteasome-interacting proteins by mass spectrometric analysis of affinity-purified proteasomes. Mol Biol Cell. 2000;11:3425–3439. doi: 10.1091/mbc.11.10.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipson C, Alalouf G, Bajorek M, Rabinovich E, Atir-Lande A, Glickman M, Bar-Nun S. A proteasomal ATPase contributes to dislocation of endoplasmic reticulum-associated degradation (ERAD) substrates. J Biol Chem. 2008;283:7166–7175. doi: 10.1074/jbc.M705893200. [DOI] [PubMed] [Google Scholar]
- 35.Verma R, Oania R, Graumann J, Deshaies RJ. Multiubiquitin chain receptors define a layer of substrate selectivity in the ubiquitin-proteasome system. Cell. 2004;118:99–110. doi: 10.1016/j.cell.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O’Shea EK. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- 37.Solsbacher J, Maurer P, Bischoff FR, Schlenstedt G. Cse1p is involved in export of yeast importin alpha from the nucleus. Mol Cell Biol. 1998;18:6805–6815. doi: 10.1128/mcb.18.11.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams A, Gottschling DE, Kaiser CA, Stearns T. Methods in Yeast Genetics. NY: CSHL Press; 1997. [Google Scholar]
- 39.Kaplun L, Ivantsiv Y, Bakhrat A, Raveh D. DNA damage response-mediated degradation of Ho endonuclease via the ubiquitin system involves its nuclear export. J Biol Chem. 2003;278:48727–48734. doi: 10.1074/jbc.M308671200. [DOI] [PubMed] [Google Scholar]
- 40.Babbitt SE, Kiss A, Deffenbaugh AE, Chang YH, Bailly E, Erdjument-Bromage H, Tempst P, Buranda T, Sklar LA, Baumler J, Gogol E, Skowyra D. ATP hydrolysis-dependent disassembly of the 26S proteasome is part of the catalytic cycle. Cell. 2005;121:553–565. doi: 10.1016/j.cell.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 41.Tone Y, Tanahashi N, Tanaka K, Fujimuro M, Yokosawa H, Toh-e A. Nob1p, a new essential protein, associates with the 26S proteasome of growing saccharomyces cerevisiae cells. Gene. 2000;243:37–45. doi: 10.1016/S0378-1119(99)00566-1. [DOI] [PubMed] [Google Scholar]
- 42.Huang TG, Hackney DD. Drosophila kinesin minimal motor domain expressed in Escherichia coli. Purification and kinetic characterization. J Biol Chem. 1994;269:16493–16501. [PubMed] [Google Scholar]
- 43.Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/S0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- 44.Ravid T, Hochstrasser M. Autoregulation of an E2 enzyme by ubiquitin-chain assembly on its catalytic residue. Nat Cell Biol. 2007;9:422–427. doi: 10.1038/ncb1558. [DOI] [PubMed] [Google Scholar]
- 45.Skowyra D, Koepp DM, Kamura T, Conrad MN, Conaway RC, Conaway JW, Elledge SJ, Harper JW. Reconstitution of G1 cyclin ubiquitination with complexes containing SCFGrr1 and Rbx1. Science. 1999;284:662–665. doi: 10.1126/science.284.5414.662. [DOI] [PubMed] [Google Scholar]
- 46.Jin S, Pan L, Liu Z, Wang Q, Xu Z, Zhang YQ. Drosophila Tubulin-specific chaperone E functions at neuromuscular synapses and is required for microtubule network formation. Development. 2009;136:1571–1581. doi: 10.1242/dev.029983. [DOI] [PubMed] [Google Scholar]
- 47.Tarailo M, Tarailo S, Rose AM. Synthetic lethal interactions identify phenotypic “interologs” of the spindle assembly checkpoint components. Genetics. 2007;177:2525–2530. doi: 10.1534/genetics.107.080408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raasi S, Orlov I, Fleming KG, Pickart CM. Binding of polyubiquitin chains to ubiquitin-associated (UBA) domains of HHR23A. J Mol Biol. 2004;341:1367–1379. doi: 10.1016/j.jmb.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 49.van Nocker S, Sadis S, Rubin DM, Glickman M, Fu H, Coux O, Wefes I, Finley D, Vierstra RD. The multiubiquitin-chain-binding protein Mcb1 is a component of the 26S proteasome in Saccharomyces cerevisiae and plays a nonessential, substrate-specific role in protein turnover. Mol Cell Biol. 1996;16:6020–6028. doi: 10.1128/mcb.16.11.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu H, Sadis S, Rubin DM, Glickman M, van Nocker S, Finley D, Vierstra RD. Multiubiquitin chain binding and protein degradation are mediated by distinct domains within the 26 S proteasome subunit Mcb1. J Biol Chem. 1998;273:1970–1981. doi: 10.1074/jbc.273.4.1970. [DOI] [PubMed] [Google Scholar]
- 51.Heessen S, Masucci MG, Dantuma NP. The UBA2 domain functions as an intrinsic stabilization signal that protects Rad23 from proteasomal degradation. Mol Cell. 2005;18:225–235. doi: 10.1016/j.molcel.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 52.Mayor T, Lipford JR, Graumann J, Smith GT, Deshaies RJ. Analysis of polyubiquitin conjugates reveals that the rpn10 substrate receptor contributes to the turnover of multiple proteasome targets. Mol Cell Proteomics. 2005;4:741–751. doi: 10.1074/mcp.M400220-MCP200. [DOI] [PubMed] [Google Scholar]
- 53.Sun XM, Butterworth M, MacFarlane M, Dubiel W, Ciechanover A, Cohen GM. Caspase activation inhibits proteasome function during apoptosis. Mol Cell. 2004;14:81–93. doi: 10.1016/S1097-2765(04)00156-X. [DOI] [PubMed] [Google Scholar]
- 54.Wigley WC, Fabunmi RP, Lee MG, Marino CR, Muallem S, DeMartino GN, Thomas PJ. Dynamic association of proteasomal machinery with the centrosome. J Cell Biol. 1999;145:481–490. doi: 10.1083/jcb.145.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fabunmi RP, Wigley WC, Thomas PJ, DeMartino GN. Activity and regulation of the centrosome-associated proteasome. J Biol Chem. 2000;275:409–413. doi: 10.1074/jbc.275.1.409. [DOI] [PubMed] [Google Scholar]
- 56.Wakefield JG, Huang JY, Raff JW. Centrosomes have a role in regulating the destruction of cyclin B in early Drosophila embryos. Curr Biol. 2000;10:1367–1370. doi: 10.1016/S0960-9822(00)00776-4. [DOI] [PubMed] [Google Scholar]
- 57.Hames RS, Crookes RE, Straatman KR, Merdes A, Hayes MJ, Faragher AJ, Fry AM. Dynamic recruitment of Nek2 kinase to the centrosome involves microtubules, PCM-1, and localized proteasomal degradation. Mol Biol Cell. 2005;16:1711–1724. doi: 10.1091/mbc.E04-08-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang F, Jiang Q, Zhao J, Ren Y, Sutton MD, Feng J. Parkin stabilizes microtubules through strong binding mediated by three independent domains. J Biol Chem. 2005;280:17154–17162. doi: 10.1074/jbc.M500843200. [DOI] [PubMed] [Google Scholar]
- 59.Zhao J, Ren Y, Jiang Q, Feng J. Parkin is recruited to the centrosome in response to inhibition of proteasomes. J Cell Sci. 2003;116:4011–4019. doi: 10.1242/jcs.00700. [DOI] [PubMed] [Google Scholar]
- 60.Pfister KK, Shah PR, Hummerich H, Russ A, Cotton J, Annuar AA, King SM, Fisher EM. Genetic analysis of the cytoplasmic dynein subunit families. PLoS Genet. 2006;2:e1. doi: 10.1371/journal.pgen.0020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hughes JR, Meireles AM, Fisher KH, Garcia A, Antrobus PR, Wainman A, Zitzmann N, Deane C, Ohkura H, Wakefield JG. A microtubule interactome: complexes with roles in cell cycle and mitosis. PLoS Biol. 2008;6:e98. doi: 10.1371/journal.pbio.0060098. [DOI] [PMC free article] [PubMed] [Google Scholar]