Abstract
Red blood cells (RBC) have emerged as a novel regulatory cell type endowed with bioactivities toward activated human T cells. Herein we show that the RBC bioactivities act on intracellular pathways initiated by T cell receptor (TCR)-dependent and -independent stimuli, including IL-2, IL-15, and the mixture of phorbol dibutyrate and ionomycin. The RBC bioactivities preserve the antioxidant status and are capable of rescuing activated T cells from cell death induced by serum deprivation. They are not mediated by glycosylphosphatidylinositol-linked receptors or sialic acids, and kinetic studies revealed that they hasten the entrance into the cell cycle. By using cyclosporine A (CsA) and rapamycin (Rapa) we show that the RBC bioactivities are calcineurin-dependent. Thus, treatment of T cells with CsA, but not Rapa, impaired RBC bioactivities, and preincubation of RBC with CsA completely abolished their bioactivities. We have demonstrated that RBC carry out bioactivities that are sensitive to CsA.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-009-0119-y) contains supplementary material, which is available to authorized users.
Keywords: Red blood cells, T cells, Cell growth, Survival, Cyclosporine A
Introduction
Red blood cells (RBC) are emerging as a cell type with the capacity to regulate other cells. A number of studies have shown that besides transporting oxygen and carbon dioxide, RBC are endowed with the capacity to regulate biological processes of non-erythroid cells, including vascular endothelial cell contraction [1], platelet aggregation [2, 3], neutrophil apoptosis [4], T cell rolling [5], fibroblast secretion of metalloproteinases [6], B cell responses [7] and IL-12 secretion by dendritic cells [8]. We have reported that human RBC have the capacity to synergize with TCR/CD3-mediated activation signals and enhance T cell growth and proliferation [9]. The RBC-induced T cell proliferation was associated with inhibition of apoptosis and upregulation of cytoprotective proteins and the labile iron pool on dividing T cells [10–12]. Although these studies have unveiled novel regulatory roles for mature circulating RBC, the molecular mechanisms used have remained uncertain. However, recent studies indicate that RBC receptors and released factors may underlie their regulatory role toward non-erythroid cells. Thus, release of nitric oxide by RBC hemoglobin has been proposed to mediate vascular contraction [1]. On the other hand, the RBC-mediated inhibition of IL-12 secretion by dendritic cells depends on interactions between CD47 present on RBC and SIRPα present on dendritic cells [8]. Preliminary studies have shown that interactions between LFA-3/CD58 on RBC and CD2 on T cells might mediate T and B cell responses [7, 13].
Most, if not all, studies examining the role that RBC exert on T cell responses have used polyclonal stimuli, including antibodies against CD3/CD28 receptors and the lectin phytohemagglutinin (PHA) in its different forms [9]. Both CD3 ligation and PHA are known to activate intracellular signaling cascades that lead to early tyrosine phosphorylation events, calcium fluxes and activation of downstream kinases, phosphatases and transcription factors that co-ordinately drive T cells into the cell cycle [14]. However, CD3 ligation and PHA are also associated with the triggering of activation-induced cell death (AICD) or apoptosis [15]. In this context, CD28 ligation activates intracellular signals that surpass some of the apoptotic pathways and promote cell cycle progression [16]. Mitogenic combinations that bypass the TCR/CD3 complex, such as phorbol esters and calcium ionophores, also lead to T cell proliferation by inducing calcium fluxes and activation of genes that regulate cell cycle progression [17]. On the other hand, homeostatic cytokines such as IL-2 and IL-15 are known to induce TCR/CD3-independent proliferation and survival of T cells via Jak/Stat dependent signaling pathways [18]. Both cytokines stimulate T cell proliferation and act as survival factors, but IL-15 can inhibit the IL-2-dependent sensitization to AICD [19]. In this context, RBC have consistently been shown to deliver signals that upregulate pathways leading to cell cycle progression while downplaying apoptotic pathways on CD3/PHA-activated T cells [9]. It remains to be elucidated whether the cell growth and survival bioactivities carried out by RBC are also seen when T cells are activated by external signals that bypass the TCR/CD3 complex.
Among the downstream signaling molecules that are activated as a result of these external signals are calcineurin and the mammalian target of rapamycin (mTOR). Calcineurin is a serine/threonine phosphatase that is activated after receptor-mediated signals that lead to an increase in intracellular calcium levels and regulates the activity of the nuclear factor of activated T cells (NFAT), a transcription factor that upon translocation into the nucleus upregulates the expression of IL-2, which stimulates growth and differentiation of activated T cells [20, 21]. In contrast, mTOR is a serine/threonine kinase involved in the integration of extracellular signals initiated by growth factors and regulates cell growth, proliferation and survival, in part by regulation of the initiation of protein translation [22, 23]. Calcineurin and mTOR are the targets of the immunosuppressive drugs cyclosporine A (CsA) and rapamycin (Rapa), respectively. While CsA binds to endogenous cyclophilin A (CypA), Rapa binds to endogenous FK506 binding protein (FKBP)-12 [24]. These complexes bind and inactivate calcineurin and mTOR, respectively, leading to a decrease in T cell proliferation [25].
In the present study, we report that RBC are carriers of bioactivities that act on intracellular pathways regulating inhibition of oxidative stress and apoptosis and entry into the cell cycle. We show that treatment of RBC with CsA, but not with Rapa or a phosphatidylinositol 3-kinase (PI3K) inhibitor (LY294002), completely blocked their capacity to increase T cell growth, proliferation and survival of activated T cells. These findings will be discussed in the context of recent findings pointing to CypA as a novel cytokine-like factor mediating cell-to-cell communication [26, 27].
Materials and methods
Reagents and antibodies
Phytohaemagglutinin (PHA-P, from Phaseolus vulgaris), antibiotic-antimycotic solution (APS), bovine serum albumin (BSA), propidium iodide (PI), neuraminidase (Neu, from Vibrio cholerae), phosphatidylinositol-specific phospholipase C (PI-PLC, from Bacillus cereus), ionomycin (Ion, from Streptomyces conglobatus), phorbol dibutyrate (PdB) and Triton X-100 were from Sigma-Aldrich (Madrid, Spain). Lymphoprep was from Nycomed (Oslo, Norway). RPMI 1640 GlutaMAX, Hanks Balanced Salt Solution (HBSS) and inactivated Fetal Bovine Serum (FBSi) were obtained from Gibco (Paisley, Scotland). Human rIL-15 and rIL-2 were obtained from R&D Systems (Minneapolis, MI). Rapamycin (Rapa) and Cyclosporin A (CsA) and LY294002 were obtained from Calbiochem (Nottingham, UK). 5-(and -6)-Carboxyfluorescein diacetate succinimidyl ester (CFSE) and Annexin V-Alexa488 were purchased from Molecular Probes (Amsterdam, The Netherlands). Anti-human glycophorin A PE-conjugated (clone AME-1, CD235a-PE) was from ImmunoTools (Friesoythe, Germany). Anti-human CD3-PE, CD4-APC, CD8-PE-Cy5 and rabbit anti-mouse (RAM)-fluorochrome conjugated antibodies were from DAKO (Glostrup, Denmark). Anti-LFA-3/CD58 antibodies (TS2/9) were kindly provided by Dr. Angelo Cardoso (Melvin and Bren Simon Cancer Center, Indiana University School of Medicine, Indianapolis, IN). NHS-sulfo-biotin and biotinylated iodoacetamide (BIAM) were from Pierce (Rockford, USA). Protein-A Sepharose CL-4 beads were form Amersham Biosciences (Pittsburgh, PA).
Cells
Fresh peripheral blood mononuclear cells (PBMC) were obtained from buffy coats after centrifugation over Lymphoprep. PBMCs were washed twice with HBSS and contaminating RBC lysed in red cell lysis solution (RCLS, 10 mM Tris, 150 mM NH4Cl, pH 7.4) for 10 min at 37°C. RBC were collected from the pellet region after Lymphoprep centrifugation, washed twice with HBSS and diluted 1:10 in RPMI supplemented with 1% APS solution and stored at 4°C until use. RBC purity was assessed by flow cytometry and CD235a labeling, which revealed >99.5% CD235a+. Partially purified peripheral blood T lymphocytes (PBL) were obtained after culture of PBMC overnight in RPMI supplemented with 1% APS solution and 5% FBSi. The recovered non-adherent cell suspensions were routinely >85% CD3+ T cells and are referred to as PBL. T cells were enriched by rosetting with sheep RBC (ProBiologica, Lisboa, Portugal), yielding a population with <1% monocytes or B cells. An Epstein-Barr virus transformed B cell line was kindly provided by Dr. Angelo Cardoso and maintained in RPMI 1640 GlutaMAX supplemented with 10% of FBSi and 1% APS solution.
Cell treatments
CFSE labeling
Ten million PBL/T cells were labeled with CFSE at a final concentration of 5uM for 10 min, with occasional mixing, at 37°C. Then, cells were washed twice with PBS/20% FBSi and resuspended in culture media. Analysis of cells immediately following CFSE labeling indicated a labeling efficiency higher than 99%. Rounds of cell divisions were determined by sequential halving of CFSE-fluorescence intensity.
Rapamycin and cyclosporine treatment
One and a half million PBLs (or 15 million RBC) were incubated for 1 h at 37°C, 5% CO2 and 99% humidity in culture media alone (see composition below) or supplemented with 10 ng/ml of rapamycin or 1 μg/ml of cyclosporin, and washed exhaustively prior to their culture in vitro.
Phospholipase C and neuraminidase treatment
Fifteen million RBC were treated with culture media (see composition below) containing 0.1 U of phosphatidylinositol-specific phospholipase or 0.1 U neuraminidase during 1 h in an orbital rotator at 37°C. As a control, RBC were incubated under identical conditions in control media. Afterwards RBC were centrifuged and extensively washed before addition to cultures of activated PBLs and/or T cells.
Culture conditions
PBLs or purified T cells (1.5 × 106) were cultured in 6-well plates or 24-well plates, in a final volume of 5 or 1 ml, respectively, for a maximum of 7 days in an incubator at 37°C, 5% CO2 and 99% humidity. PBLs or T cells were either left unstimulated or stimulated with the following mitogens: (1) 5 μg/ml PHA-P; (2) 5nM PdB + 100 nM Ion; (3) 10 ng/ml of IL-15; (4) 10 ng/ml of IL-2. PBLs and/or T cells were cultured in the absence or presence of autologous RBC at a PBL:RBC ratio of 1:10 for up to 7 days. In some experiments heterologous RBC were also used. Culture medium was RPMI 1640 GlutaMAX supplemented with 1% APS solution and 1% FBSi. In some experiments PBLs and/or T cells were cultured in the absence of serum in RPMI 1640 GlutaMAX supplemented with 1% APS. For immunossupression studies, 1.5 × 106 PBLs were stimulated with 5 μg/ml PHA-P and cultured in the absence or presence of untreated RBC or RBC treated with rapamycin and cyclosporin at a PBL:RBC ratio of 1:10 and cultured in six-well plates as above. At the end of the culture PBL/T cells were harvested, washed and acquired in a FACSCalibur (Becton-Dickinson, Mountain View, CA). For each sample 50,000 events were acquired using FSC/SSC characteristics and analyzed using CellQuest or FlowJo software.
Flow cytometry determinations
Cell stainings were normally performed at 4°C for 30 min in 1X PBS or staining buffer (PBS, 0.2% BSA, 0,1% NaN3) in 96-well round-bottom plates (Greiner bio-one, Frickenhausen, Germany). In cultures that received RBC, erythrocytes were first lysed with RCLS. Irrelevant mAbs were used as negative controls to define background staining. T cell death and survival were determined by two methods: (1) a decrease in cell size according to forward light scatter (FSC)/side light scatter (SSC) parameters; (2) double Annexin V and PI staining using Ca2+-based staining buffer (10mM HEPES/140 mM NaCl/2.5 mM CaCl2). T cell activation and division were studied by two methods: (1) determination of cell size and complexity according to FCS/SSC parameters (blasts); (2) CFSE fluorescence loss.
Biotinylation, immunoprecipitation, SDS/PAGE and Western blot
For the detection of cell surface LFA-3/CD58, 5 × 106 B cell lymphoma cells and 250 × 106 RBC were surface biotinylated as described [10]. Briefly, cells were incubated with 0.5 mg/ml of NHS-sulfo-biotin (Pierce, Rockford, IL) in PBS for 10 min at room temperature followed by four washes in PBS. After washing, labeled cells were lysed in lysis buffer (20 mM Tris pH 7.6, 150 mM NaCl, 1 mM PMSF and 1% Triton X-100) for 30 min on ice. The lysates were centrifuged at 10,000×g to remove cell debris and precleared for 1 h with protein-A Sepharose beads (Amersham Biosciences). Precleared detergent lysates were immunoprecipitated with TS2/9 antibodies followed by Sepharose beads for 2 h at 4°C. Washed immunoprecipitates were boiled in sample buffer and resolved by SDS/PAGE under non-reducing conditions and resolved proteins transferred to nitrocellulose membranes (Whatman, Pittsburgh, PA). Membranes were blocked with 5% non-fat dry milk in TBS-T and then incubated with ExtrAvidin horseradish peroxidase (Sigma-Aldrich). Biotinylated proteins were visualized after exposure to Kodak Biomax MR1 films (Sigma-Aldrich) using the enhanced chemiluminescence (ECL Super Signal West Pico detection reagent). For the detection of proteins containing free thiols, cell lysates of activated T cells in the absence or presence of RBC were labeled with BIAM for 30 min at room temperature. The labeling reaction was quenched by the addition of dithiothreitol (DTT). Afterwards, equivalent aliquots of cell lysates, quantified by the BCA protein assay kit (Pierce), were run in a 10% SDS/PAGE, transferred to a membrane and visualized by ECL, as described above. In parallel, a similar aliquot of cell lysates was run and total proteins visualized by Coomassie brilliant blue R250 (Sigma-Aldrich).
Statistical analysis
Statistical analysis was performed using Excel or GraphPadTM Prism 5 software. Student’s t test was used to evaluate the significance of the differences between group means. Statistical significance was defined as p < 0.05.
Results
RBC have bioactivities that enhance cell growth and division signals initiated by TCR/CD3-independent stimuli
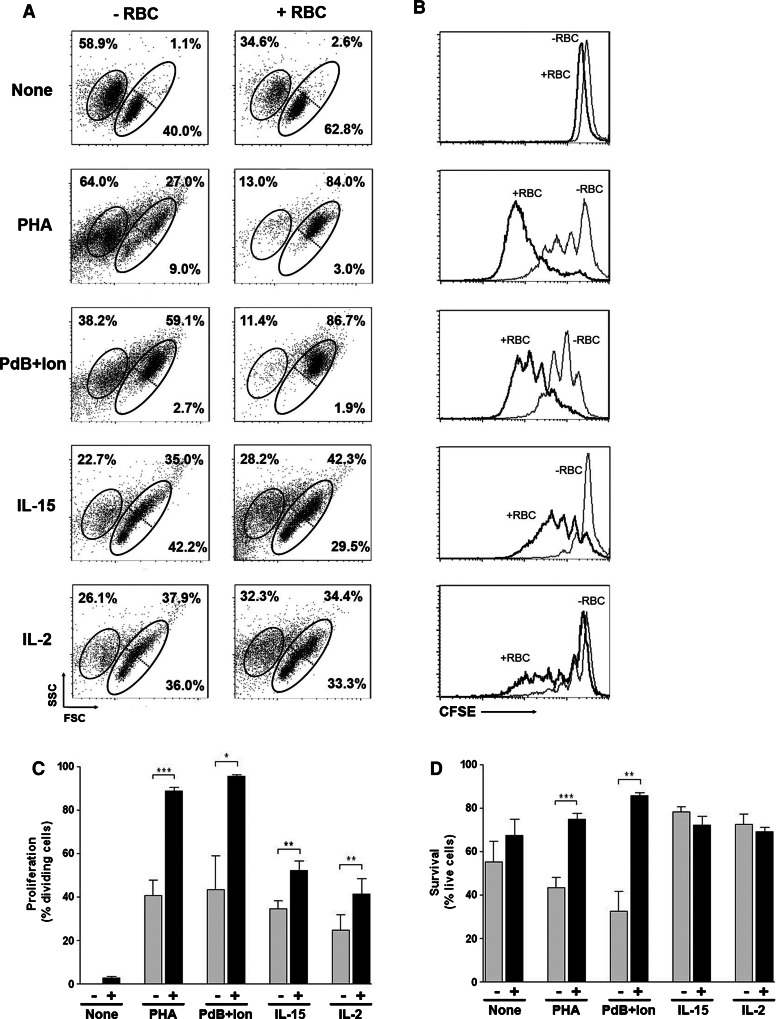
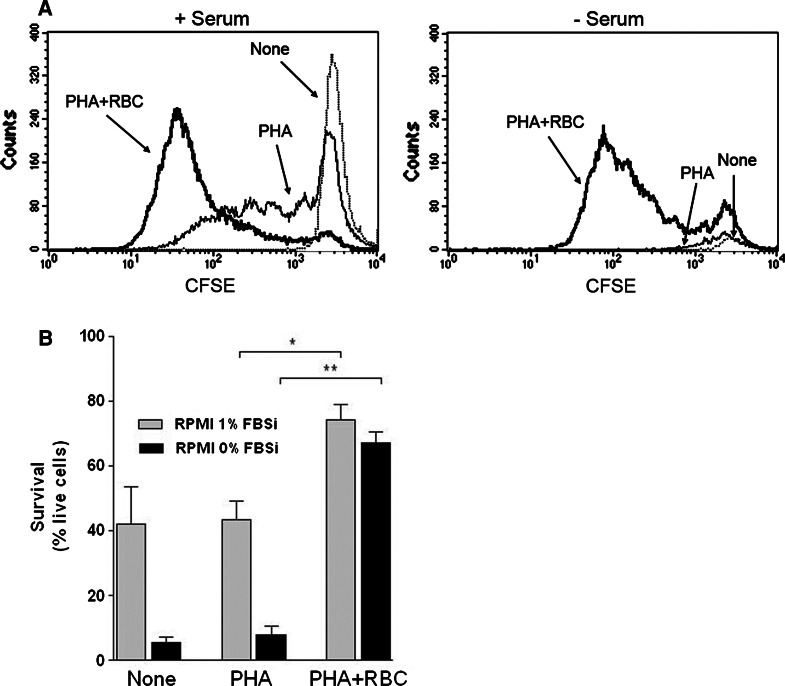
Our previous studies showed that the presence of RBC in close proximity of peripheral blood lymphocytes (PBLs) that have been activated in vitro with polyclonal stimuli dependent on the expression of a TCR/CD3 complex (e.g., PHA-P, PHA-L, anti-CD3, anti-CD3+ anti-CD28) enhanced proliferation and survival of the activated T cells ([10–12], Fig. 1). To ascertain whether the RBC bioactivities can also influence TCR/CD3-independent signals, PBLs were left unstimulated or stimulated with TCR/CD3-dependent (PHA-P) and TCR/CD3-independent (phorbol + calcium ionophore, IL-15 and IL-2) stimuli in the absence or presence of autologous RBC and proliferation determined by CFSE fluorescence loss using flow cytometry. As expected, PHA stimulation induced an overall increase in cell growth (Fig. 1a) that correlated with reasonable levels of T cell proliferation (Fig. 1b). However, PHA also induced marked levels of activation-induced cell death (Fig. 1a). The presence of RBC, either autologous or heterologous, augmented cell growth and proliferation while markedly inhibiting cell death (Fig. 1a, b, and data not shown). Similar results in cell growth, survival and proliferation were observed when T cells were activated with the combination of PdB and ionomycin, two mitogens that bypass plasma membrane receptors (Fig. 1a, b). RBC had no effect when either mitogen was used alone (see Supplemental Figure 1). IL-15 also induced an overall increase in cell growth (Fig. 1a) that correlated with modest levels of proliferation (Fig. 1b). In contrast to PHA, IL-15 induced high levels of survival (Fig. 1a). As a result, the presence of RBC did not impact on survival but induced a significant increase in the number of blasts and proliferating cells instead (Fig. 1a, b). CD3-labeling at the end of the culture showed that the large majority of lymphocytes that divided in response to IL-15 were CD3+ T cells, and the presence of RBC induced the preferential expansion of CD3+ CD8+ T cells (data not shown). Similar results in cell growth, survival and proliferation were observed when T cells were activated with IL-2 (Fig. 1a, b). Overall, RBC were capable of augmenting the percentage of T cells that entered cell division (Fig. 1c) and also the percentage of cell survival (Fig. 1d) both after TCR-dependent and -independent stimuli.
Fig. 1.
RBC bioactivities strengthen proliferation and survival of dividing T cells. PBLs (1.5 × 106) were labeled with CFSE and cultured either alone or with autologous or heterologous red blood cells (1:10 ratio) in the absence or presence of different stimuli for 7 days, and then harvested, stained and acquired in a FACSCalibur. a Dot plots (FCS vs. SSC) show the percentage of T cell death (left gated population, as determined by positive PI labeling) and survival (right gated population, as determined by negative PI labeling) of PBLs cultured alone (−RBC) or in the presence of autologous RBC (+RBC). Also shown are the percentage of small (lower right gated population) and blast (upper right gated population) cells. b Histograms illustrate the corresponding T cell proliferation levels obtained in the different conditions shown in a, in the absence (−RBC, thin line) or presence (+RBC, thick line) of RBC, as determined by CFSE fluorescence loss. c, d Graphs show the percentage of dividing (c) and surviving (d) T cells (mean ± SEM, n = 4) for the different conditions in the absence (−) or presence (+) of RBC (***p < 0.001; **p < 0.01; *p < 0.05)
RBC bioactivities are independent of GPI-linked receptors and sialic acids
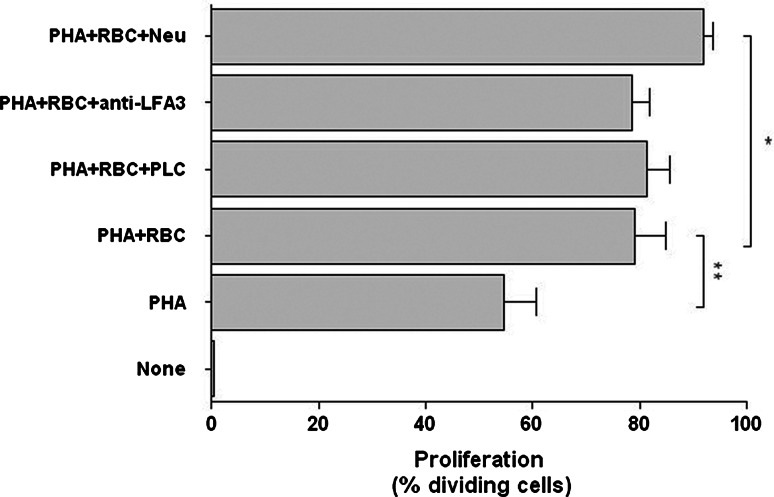
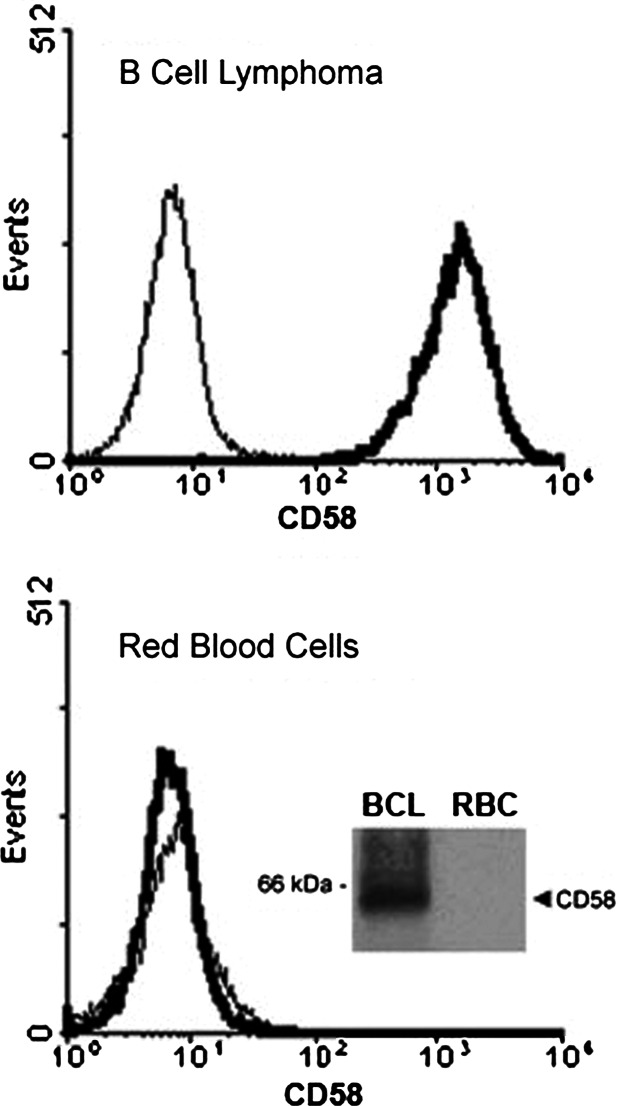
RBC have been reported to express LFA-3/CD58, the ligand for CD2, and previous studies suggested that the enhancing effect that human RBC have on T cell proliferation was mediated by CD58 present on the plasma membrane of RBC [7]. However, neither removal of GPI-linked receptors from the cell surface of RBC by treatment with PLC nor preincubation of RBC with TS2/9 antibodies (that specifically recognize CD58) abolished the RBC bioactivities toward activated T cells (Fig. 2). In view of these results, we investigated the expression of LFA-3/CD58 by freshly isolated human RBC. Surprisingly, flow cytometry and immunoprecipitation studies consistently showed that LFA-3/CD58 cannot be detected on the cell surface of human RBC, or at least it is expressed at levels beyond detection, indicating the unlikelihood of mediating the RBC bioactivities (Fig. 3, see inset). The lack of detection of LFA-3/CD58 on RBC was not an artifact since the TS2/9 antibodies detected LFA-3/CD58 on the cell surface of an immortalized B cell line (Fig. 3, see inset). Next, we examined whether removal of sialic acids from the RBC membranes could influence RBC bioactivities. Sialic acids are the ligands of certain receptors (SIGLEC), some of which are involved in the regulation of activation events in T cells [28]. As shown in Fig. 2, treatment with neuraminidase to remove sialic acids did not abolish RBC bioactivities; rather, neuraminidase-treated RBC significantly increased the percentage of T cells entering division. Sialic acid removal was confirmed by a complete down-modulation in glycophorin A expression (data not shown), which is known to represent more than 80% of the total sialic acid amount of RBC membrane glycoproteins [29].
Fig. 2.
RBC bioactivities are independent of major RBC membrane proteins. PBLs (1.5 × 106) labeled with CFSE were stimulated with 5 μg/ml of PHA and cultured for 7 days either alone or in the presence of untreated RBC or RBC treated with PLC, anti-LFA-3/CD58 and neuraminidase. Afterward, cells were harvested and acquired in a FACSCalibur. The graph shows the percentage of proliferating PBLs (mean ± SEM, n = 7) as determined by CFSE fluorescence loss in the different conditions (**p < 0.01; *p < 0.05)
Fig. 3.
Human RBC lack expression of LFA-3/CD58. Red blood cells (RBC) and B cell lymphoma (BCL) cells were cell surface labeled with anti-LFA-3/CD58 antibodies (TS2/9) followed by RAM-FITC conjugated antibodies and acquired in a FACSCalibur. Histograms show CD58 expression (thick line) in BCL cells (upper histogram) and RBC (lower histogram). Background staining with RAM-FITC (thin line) is shown. Inset: BCL cells and RBC were surface biotinylated, lysed and LFA-3/CD58 immunoprecipitated with TS2/9 antibodies as described in “Materials and methods.” Aliquots of the immunoprecipitates were resolved in a SDS/PAGE, blotted and visualized by the ECL technique. The band corresponding to LFA-3/CD58 is indicated
RBC bioactivities rescue T cells from activation-induced cell death: correlation with the preservation of thiol levels
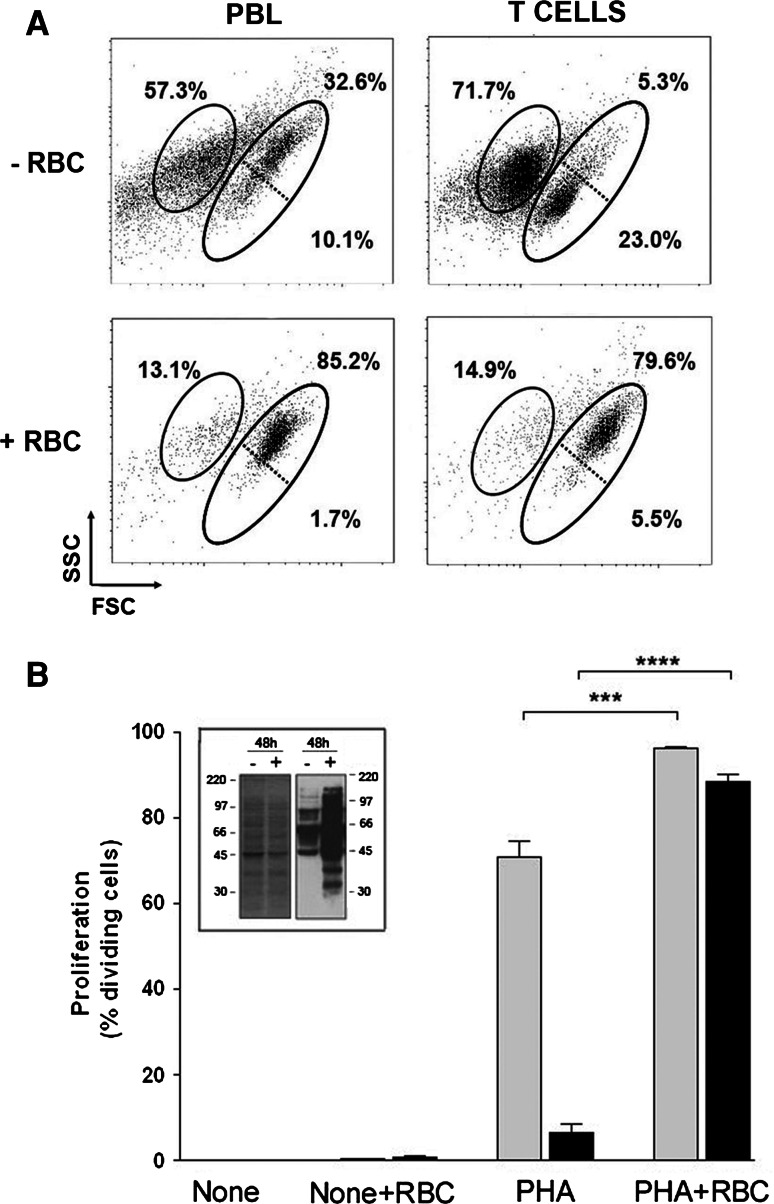
In order to ascertain whether the RBC bioactivities needed accessory cells, we purified T cells, and their cell growth, proliferation and survival rates were examined upon PHA-activation. As shown in Fig. 4a, PHA stimulation of pure T cells induced high levels of cell death without inducing cell growth when compared to PBL preparations which was paralleled by very low levels of proliferation (Fig. 4b). Of note, the presence of RBC in the cultures completely reversed the incapacity of pure T cells to grow and proliferate (Fig. 4). The effect of RBC on reversing the unresponsiveness of pure T cells was of such a magnitude that it resulted in more than a 25-fold increase in cell proliferation when compared to T cells alone. Interestingly, examination of the level of cell surface thiols in dividing T cells in the absence or presence of RBC by using the free-cysteine reagent BIAM showed higher levels in cultures with RBC (Fig. 4b, inset). In order to examine further the strength of the RBC bioactivities, cultures of PBL preparations in the absence of serum were performed. Removal of serum from the culture medium resulted in a complete absence of T cell proliferation (Fig. 5a) mostly because of a strong decrease in cell survival (Fig. 5b). However, the presence of RBC in these cultures allowed T cells to proliferate to levels very similar to those observed in cultures with serum (Fig. 5a), literally rescuing the activated T cells from cell death (Fig. 5b).
Fig. 4.
RBC bioactivities rescue activated T cells from cell death. PBLs or purified T cells (1.5 × 106) were labeled with CFSE, stimulated with 5 μg/ml of PHA and cultured in the absence or presence of RBC for 7 days. Then, cells were harvested and acquired in a FACSCalibur. a Dot plots (FCS vs. SSC) show the percentage of T cell death (left gated population, as determined by positive PI labeling) and survival (right gated population, as determined by negative PI labeling) of PHA-activated PBLs or T cells cultured alone (−RBC) or in the presence of RBC (+RBC). Also shown are the percentage of small (lower right gated population) and blast (upper right gated population) cells. b The graph shows the percentage of proliferating cells (mean ± SEM, n = 6) in cultures of PHA-stimulated PBLs (gray bars) or PHA-stimulated T cells (black bars) in the absence or presence of RBC. The percentage of proliferating cells in unstimulated (none) cultures are included (****p < 0.0001; ***p < 0.001). Inset: Cell lysates of 48-h cultures of PBLs activated with PHA in the absence (−) and presence (+) of RBC were labeled with BIAM as described in “Materials and methods,” and two aliquots of the cell lysates were run on separate SDS/PAGE under non-reducing conditions. One of the gels was stained with Coomassie blue to visualize the total amount of protein (left graph). The other gel was blotted into membranes and thiols-containing proteins visualized by the ECL technique (right graph). Molecular weight markers are indicated
Fig. 5.
RBC bioactivities rescue activated T cells from cell death and enhance proliferation under serum deprivation. PBLs or purified T cells (1.5 × 106) were labeled with CFSE, stimulated with 5 μg/m of PHA in culture media with or without serum and cultured for 7 days in the absence or presence of RBC. Then, cells were harvested and acquired in a FACSCalibur. a Histograms show loss of CFSE fluorescence in cultures of PHA-activated PBLs with (left histogram) or without (right histogram) serum, in the absence (thin line, PHA) or presence (thick line, PHA +RBC) of RBC. Dotted-line (none) shows CFSE fluorescence in unstimulated PBL. b Graph shows the percentage of live cells (mean ± SEM, n = 3) for the different culture conditions in the presence (gray bars) or in the absence (black bars) of serum (**p < 0.01; *p < 0.05)
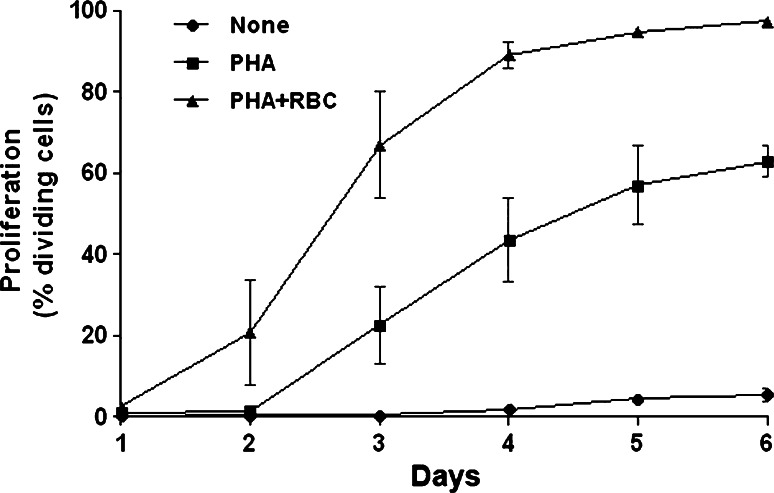
Finally, kinetic studies (1–6 days) of cultures of PHA-activated PBLs revealed that T cell division is only detectable 3 days after the start of the culture (Fig. 6). Thus, the percentage of T cells that divided at least once after 3 days of activation was, on average, about 23% (Fig. 6). Noteworthily, the presence of RBC shortened the time necessary to see the same level of T cell division by 24 h (compare PHA with PHA + RBC in Fig. 6). As a result, the percentage of T cells that divided at least once in cultures with RBC 3 days after activation was threefold higher than in cultures without RBC (67 vs. 23%, Fig. 6). This difference was maintained thereafter. Studies examining the phosphorylation status of intracellular signaling molecules regulating cell survival and proliferation showed that the PI3K/Akt axis may be a target of the RBC bioactivities (see Supplemental Figure 2).
Fig. 6.
Kinetics of the effect of RBC on T cell proliferation. PBLs (1.5 × 106) were labeled with CFSE, and either left unstimulated (none) or stimulated with 5 μg/ml of PHA and cultured for 6 days in the absence or presence of RBC. Then, cells were harvested at days 1, 2, 3, 4, 5 and 6 and acquired in a FACSCalibur. Graph shows the percentage (mean ± SEM, n = 2) of dividing cells for the different culture conditions along the culture period
RBC bioactivities are differently sensitive to the immunosuppressive drugs rapamycin and cyclosporine A
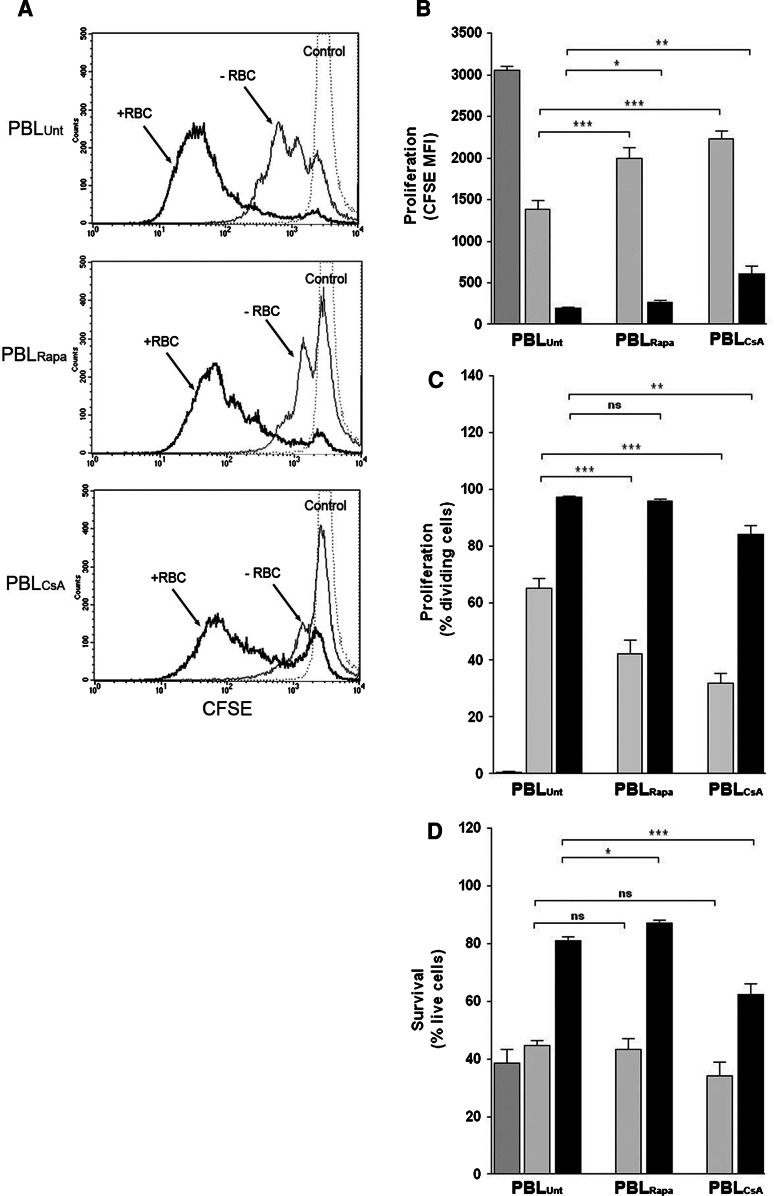
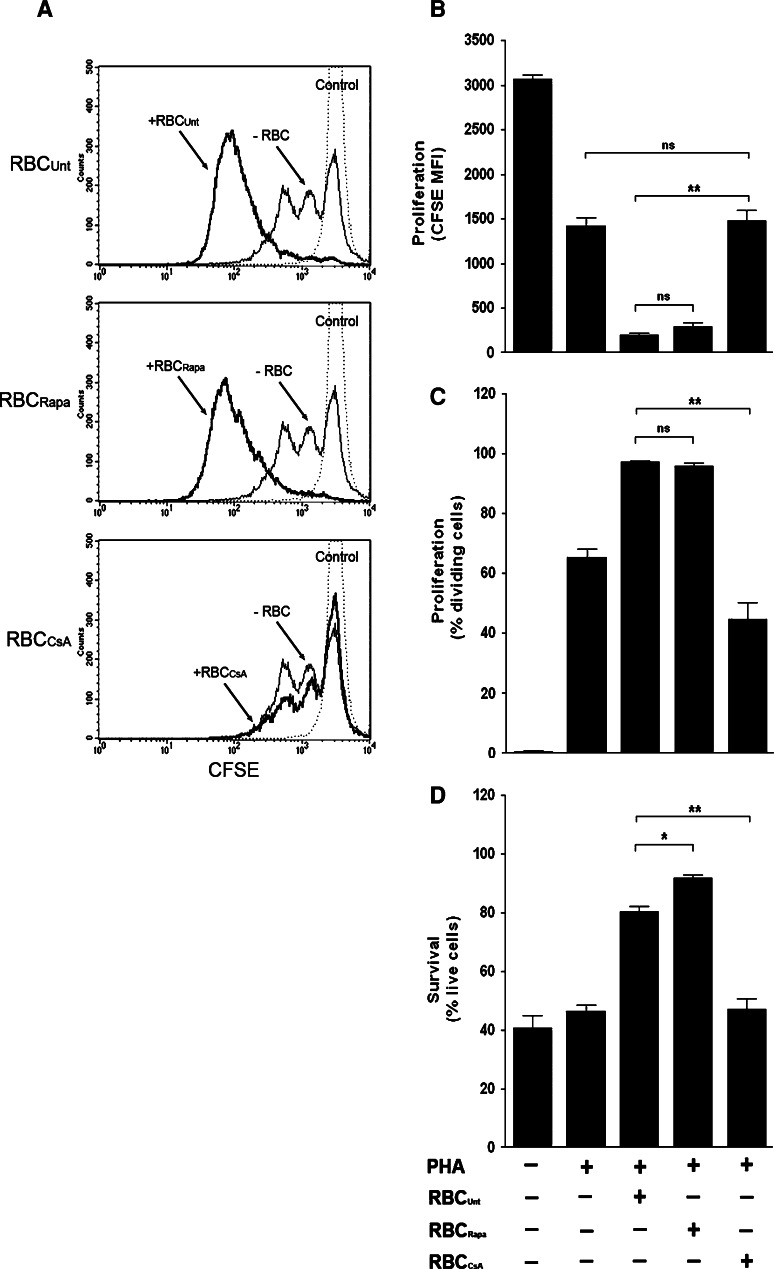
Based on the aforementioned results and in view of the overwhelming effects that RBC exert on T cells that have been activated in vitro, we decided to investigate whether these RBC bioactivities could be abolished or inhibited by drugs commonly used to suppress immune responses, such as cyclosporine (CsA) and rapamycin (Rapa). Preliminary dose-inhibition experiments showed that 10 ng/ml of Rapa and 1ug/ml of CsA induced more than 50% inhibition on T cell proliferation without decreasing cell death (data not shown, see also Fig. 7d), and these were used in all subsequent experiments. As shown in Fig. 7a (thin lines), pre-treatment of PBLs with either drug negatively impacted on the relative extent of T cell proliferation as determined by higher levels of CFSE mean fluorescence intensity. As summarized in Fig. 7b (gray bars), these differences were statistically significant. In addition, Rapa and CsA treatment also negatively affected the percentage of T cells that divided without significantly affecting survival (Fig. 7c, d, gray bars). The addition of RBC to the cultures surpassed the immunosuppressive effects of both drugs differently. Thus, even though the addition of RBC to the cultures of Rapa- and CsA-treated PBLs reversed the inhibition caused by these drugs, as determined by loss of CFSE labeling (Fig. 7a), the effect was less efficient in CsA-treated PBLs (Fig. 7b, black bars). The lesser capacity of RBC to surpass the immunosuppressive effect of CsA was also observed at the level of the percentage of dividing T cells (Fig. 7c, black bars; see also Fig. 7a thick lines) and the level of survival (Fig. 7d, black bars). Then, we wanted to ascertain whether treatment of RBC with these drugs, prior to their addition to the cultures, could have an impact on their bioactivities. As shown in Fig. 8a, Rapa-treated RBC were as efficient as untreated RBC in augmenting the extent of T cell proliferation. In marked contrast, CsA-treated RBC were completely incapable of augmenting T cell proliferation (Fig. 8a). The differential sensitivity of the RBC bioactivities to Rapa and CsA treatment is summarized in Fig. 8b and is corroborated when the percentages of T cells that divided (Fig. 8c), and the percentage of live cells (Fig. 8d) were analyzed. Interestingly, the level of survival observed in cultures with Rapa-treated RBC increased significantly when compared with untreated RBC (Fig. 8d). Addition of either drug directly to the culture media had the same effect as drug pretreatment of RBC (data not shown). The negative impact that the treatment of RBC with CsA has on their bioactivities toward activated T cells is specific since treatment of RBC with Rapa (Fig. 8) or the specific PI3K inhibitor LY294002 (data not shown) had negligible effects on the RBC bioactivities.
Fig. 7.
RBC bioactivities differently overcome immunossupression induced by Rapa and CsA. PBLs (1.5 × 106) were incubated with culture media Rapa (10 ng/ml) and CsA (1ug/ml) for 1 h at 37°C and extensively washed. Then, PBLs were labeled with CFSE and left either unstimulated or stimulated with 5 μg/ml of PHA and cultured in the absence or presence of RBC for 7 days. Afterwards, cells were harvested, stained and acquired in a FACSCalibur. a Histograms show proliferation levels of activated PBLs, as determined by CFSE fluorescence loss, of either PBLs that were untreated (PBLUnt) and pre-treated with Rapa (PBLRapa) or CsA (PBLCsA), and cultured in the absence (−RBC, thin line) or presence of RBC (+RBC, thick line). CFSE levels in unstimulated PBLs (control, dotted-line) are also shown. b–d The graphs show the corresponding percentage (mean ± SEM, n = 9) of proliferating T cells (b), the percentage of dividing T cells (c) and the percentage of survival (d) in the different culture conditions in the absence (gray bars) or presence (black bars) of RBC. Proliferation and survival values in unstimulated PBLs are indicated in the first-left bar (***p < 0.001; **p < 0.01; *p < 0.05, ns not significant)
Fig. 8.
RBC bioactivities are abolished by treatment CsA. RBC (15 × 106) were incubated with culture media alone or containing either Rapa (10 ng/ml) or CsA (1 µg/ml) for 1 h at 37°C and extensively washed. Then, PBLs were labeled with CFSE and left either unstimulated or stimulated with 5 μg/ml of PHA and cultured in the absence or presence of RBC for 7 days. Afterwards, cells were harvested, stained and acquired in a FACSCalibur. a Histograms show proliferation levels of activated PBLs, as determined by CFSE fluorescence loss, in the absence of RBC (−RBC) or in the presence of RBC that were either left untreated (RBCUnt) or treated with Rapa (RBCRapa) or CsA (RBCCsA). CFSE levels in unstimulated PBLs (control, dotted-line) are also shown. b–d The graphs show the corresponding percentage (mean ± SEM, n = 6) of proliferating T cells (b), the percentage of dividing T cells (c) and the percentage of survival (d) in the indicated different culture conditions (**p < 0.01; *p < 0.05, ns not significant)
Discussion
We have previously shown that RBC can enhance cell proliferation and inhibit apoptosis of human T cells that have been activated in vitro with polyclonal stimuli, such as phytohemagglutinin or CD3 triggering either alone or in combination with CD28 [11, 12]. By using different approaches we showed that the RBC effect is closely linked with the upregulation of cytoprotective proteins heme oxygenase 1 and ferritin and downregulation of the prooxidant state generated after activation [10]. As a result, we proposed that the RBC is a cell type endowed with bioactivities capable of regulating T cell growth and survival of normal human T cells [9].
In the present study we extend these findings by showing that bioactivities carried out by RBC act on intracellular pathways initiated by TCR-dependent and TCR-independent stimuli, including IL-15 and the mixture of phorbol dibutyrate and a calcium ionophore. IL-15 is a gamma-common cytokine with a wide range of biological effects on cells of the immunological system. Regarding T cells, we have shown the induction of activation and proliferation of CD8+ T cells and contribution to T cell homeostasis [18]. The interaction of IL-15 with its receptor triggers activation signals different from the ones triggered by the TCR, namely the activation of the JAK/STAT family proteins whose combined action leads to the subsequent activation of the MEK/ERK and PI3K/Akt pathways, which regulate T cell survival and proliferation [30]. RBC increased IL-15-induced T cell growth and proliferation while leaving survival levels unchanged, suggesting that the RBC bioactivities are able to intersect in a positive manner with the intracellular pathways activated by IL-15, namely pathways related with cell growth and entry into the cell cycle. This assumption is supported by similar results obtained with IL-2, another gamma-common cytokine that activates similar activation pathways. The lack of an effect on survival of RBC on IL-15 stimulated T cells is not surprising since IL-15 is already known to markedly increase T cell survival. The combination of PdB and ionomycin, though bypassing TCR-proximal events, initiates a cascade of downstream events that are very similar to the ones initiated by TCR-mediated signals and include activation of serine/threonine protein kinase C and the serine/threonine phosphatase calcineurin. Of note, the presence of RBC increased cell growth, proliferation and survival of PdB + ion activated T cells to levels similar to those seen in PHA-activated T cells. These results strongly suggest that RBC bioactivities act on downstream signaling regulators, such as calcineurin, responsible for the initiation of transcription of genes related with the control of proliferation, such as IL-2, and not on isolated early pathways that are activated when PdB and ionomycin are used alone.
We have also demonstrated that the RBC bioactivities are not mediated by RBC receptors, are exerted directly over T cells and are independent of serum factors. Our previous studies suggested that RBC surface receptors proposed to mediate the effect of RBC were not involved [11, 12]. In this study we have extended these results by showing that neither GPI-linked receptors nor sialic acids present at the plasma membrane of RBC are involved in the enhancement of T cell growth, survival and proliferation, pointing to a different mechanism of action. It is worth mentioning that in our studies we could not detect expression of LFA-3/CD58 in human RBC. However, the possibility that the levels of LFA-3/CD58 on the surface of human RBC are below the detection limits of these sensitive techniques cannot be ruled out. In any case, these levels of expression would be inappropriate in terms of the delivery of co-stimulatory signals to activated T cells and most likely do not play an important role in the RBC effect. These data together with previous results indicating that the RBC effect could be observed even when RBC are added to cultures of activated T cells 1–2 days after the initial stimulation suggest a lack of involvement of RBC co-stimulatory signals during the early stages of T cell activation [11]. Importantly, RBC were also capable of driving unresponsive pure T cells activated with PHA to increase cell size and proliferate, reinforcing the notion that RBC bioactivities act directly on the activated T cells without the need of accessory cells. Moreover, we have shown that this effect is associated with the preservation of the antioxidant state of the activated T cells, namely the level of cell surface thiols. These results are in agreement with our previous results showing that RBC are capable of reducing the intracellular production of oxygen and nitrogen radicals in activated T cells [12]. The possible role of NF-kB, a transcription factor regulated by oxidative stress, in mediating the RBC effect remains to be determined. NF-kB is known to block apoptotic pathways that in turn will favor cell division [31]. The pro-survival bioactivities of RBC are of such magnitude that they were able to rescue activated T cells from cell death induced by serum deprivation and allow the activated T cells to enter multiple cycles of cell division. Besides stressing the importance of the bioactivities carried out by RBC, these results indicate that the RBC bioactivities do not need any serum factor to augment survival and proliferation of activated T cells. Based on the kinetic studies, we concluded that the RBC bioactivities are not exerted during the immediate hours following T cell activation but later on, perhaps after 18–24 h when key cell cycle regulators have already been activated. As a result there is a hastened entry of activated T cells into the cell cycle in cultures with RBC in comparison with cultures without, namely at days 2 and 3 after activation, and our results point to the PI3K/Akt axis as a putative target of the RBC bioactivities. Akt is thought to be an upstream regulator of NF-kB, reinforcing the possible involvement of this transcription factor on the RBC effect [32].
From these studies we can conclude that two main bioactivities can be distinguished in RBC: (1) survival bioactivities responsible for inhibiting pathways related with apoptosis and (2) cell growth bioactivities responsible for potentiating pathways related with progression of T cells into the cell cycle. The combined action of these bioactivities is capable of rescuing activated T cells from cell death induced by serum deprivation. In this context, by using the immunosuppressive drugs rapamycin and cyclosporine A, we have provided evidence that the RBC bioactivities appear to use the calcineurin and mTOR pathways differently. Thus, we have shown that the RBC bioactivities were able to revert very efficiently the immunosuppressive effect caused by Rapa, but not by CsA when PBLs were pre-treated with these drugs. While Rapa is known to bind to a family of proteins known as FK506-binding proteins (FKBP), more specifically to FKBP12, CsA binds to a family of proteins called cyclophilins, namely to cyclophilin A [22, 24]. While the Rapa-FKBP complex binds and inhibits the kinase mTOR, the CsA-CypA complex binds and inhibits the phosphatase calcineurin [24]. The capacity of RBC to efficiently counteract the inhibition caused by Rapa but not by CsA has, in our opinion, several interpretations. First, the peptidyl-prolyl-isomerases FKBP12 and CypA are part of the RBC proteome and compete for the binding of the drugs present within the pre-incubated T cells releasing the inhibitory effect and allowing the RBC bioactivities to exert their function. Although FKBP12 and CypA are present in RBC [33, 34], we think this hypothesis unlikely. Second, different amounts of CsA-CypA and Rapa-FKBP12 complexes within the T cells used in our assays might underlie the differences observed. This hypothesis is not unlikely if we consider early studies indicating differences in the amount of CypA among lymphocyte populations [35]. Third, the RBC bioactivities are capable by themselves to overcome efficiently the inhibition caused by Rapa but not by CsA, through the activation of downstream signaling pathways capable of driving T cells into the cell cycle. These pathways may be independent of mTOR activity but not from upstream pathways, such as those resulting from calcineurin activity, therefore justifying the differences observed. In this context, a recent study has shown that the inhibitory capacity of Rapa in leukemia cells can be overcome by IL-7-mediated signaling, most likely through the alternative activation of the p70 S6 kinase [36]. The RBC bioactivities may act in a similar manner. Nevertheless, the two latter hypotheses are not mutually exclusive.
Considering that the Rapa and CsA ligands are also present in RBC [33, 34], we wanted to ascertain whether treatment of RBC with these drugs had any effect on their bioactivities. Most interestingly, pretreatment of RBC with CsA, but not Rapa, completely blocked the bioactivities of RBC. Similar results were obtained when the drugs were added directly to cultures, mimicking an in vivo drug administration. To our knowledge, these are novel results regarding the biology of RBC, and they unveil a novel relationship between RBC and the immunosuppressive drug CsA. The fact that the bioactivities carried out by RBC are also exerted with heterologous RBC (unpublished data) raises important issues in the context of blood transfusions administered to patients undergoing immunossupression [9]. In this context, the complete block of the RBC bioactivities described herein by CsA and not Rapa raises interesting questions. First, both drug ligands are present in the RBC, but only CsA interferes with the RBC bioactivities that regulate T cell growth, survival and proliferation, pointing to a specific inhibitory effect. Indeed, pretreatment of RBC with LY294002, an inhibitor of PI3K lacking an endogenous RBC ligand, had no effect on the RBC bioactivities. All these results suggest the possible involvement of CypA, the CsA ligand, as being responsible for the RBC bioactivities. In this context, recent studies point to CypA as a novel growth factor involved in cell–cell communication that is secreted under inflammatory conditions by several types of leucocytes and endothelial cells [26, 27]. Unfortunately, we found that recombinant human CypA per se is incapable of augmenting T cell growth, survival and proliferation as intact RBC do (unpublished observations). The possibility that CypA involvement could be related to its isomerase activity within the RBC or to the regulation of the secretion of bioactive factors present within the RBC remains to be elucidated.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1. RBC enhance proliferation of PBL activated by the combination of PdB and Ionomycin. PBL (1.5x106) were labelled with CFSE and cultured either alone or with red blood cells (1:10 ratio) in the absence or presence of the different stimuli for 7 days. Then, cells were harvested, stained and acquired in a FACSCalibur. Graph shows the percentage of dividing PBL (mean±SEM, n=4) in the different culture conditions. P values are shown (*, p<0.05) (TIFF 110 kb)
Supplemental Figure 2. RBC hasten T cell proliferation and activates the Akt/PKB pathway. PBL (1.5x106) were labelled with CFSE and cultured for 3 days as indicated in the legend of Figure 6. Then, cells were harvested at days 1, 2 and 3 and acquired in a FACSCalibur. Graph shows the percentage (mean±SEM) of dividing cells for the different culture conditions along the culture period. Inset, PBL (1.5x106) were stimulated with 5 μg/mL of PHA overnight, harvested and washed out to remove PHA. Activated T cells were recultured without stimulus for an additional 48 hours. Then, cells were harvested and incubated at 37°C in culture medium alone or with RBC (1:10 ratio) for 1, 30 and 60 minutes as indicated. After lysis of RBC, T cells were immediately spun down and lysed in cell lysis buffer containing a protease and phosphatase inhibitor cocktail (Sigma-Aldrich). Cell lysates were quantified using the BCA. protein assay and aliquots corresponding to 25 μg of protein were resolved by 10% SDS-PAGE. After transfer of proteins to nitrocelulose membranes, phosphorylation of PKB was determined by using anti-phospho-Akt/PKB (Ser473) followed by rabbit anti-mouse horseradish peroxidase-conjugated antibodies (Cell Signalling Technology, USA) and ECL. Levels of total Akt/PKB were determined after membrane stripping and re-probing with anti-Akt/PKB polyclonal antibodies. Levels of phosphorylated and total Akt/PKB are indicated (TIFF 527 kb)
Acknowledgments
We thank the Instituto Português do Sangue (IPS, Porto, Portugal) for providing the buffy coats used in this study. This work was funded by grant APCL2006-30.1.AP/MJ from Associação Portuguesa Contra a Leucemia (APCL, Portugal) and is part of the PhD thesis of RFA. RFA was supported by a PhD fellowship from Fundação para a Ciência e a Tecnologia (SFRH/BD/24524/2005).
References
- 1.Pawloski JR, Hess DT, Stamler JS. Export by red blood cells of nitric oxide bioactivity. Nature. 2001;409:622–626. doi: 10.1038/35054560. [DOI] [PubMed] [Google Scholar]
- 2.Megson IL, Sogo N, Mazzei FA, Butler AR, Walton JC, Webb DJ. Inhibition of human platelet aggregation by a novel S-nitrosothiol is abolished by haemoglobin and red blood cells in vitro: implications for anti-thrombotic therapy. Br J Pharmacol. 2000;131:1391–1398. doi: 10.1038/sj.bjp.0703731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valles J, Santos MT, Aznar J, Martinez M, Moscardo A, Pinon M, Broekman MJ, Marcus AJ. Platelet–erythrocyte interactions enhance alpha(IIb)beta(3) integrin receptor activation and P-selectin expression during platelet recruitment: down-regulation by aspirin ex vivo. Blood. 2002;99:3978–3984. doi: 10.1182/blood.V99.11.3978. [DOI] [PubMed] [Google Scholar]
- 4.Aoshiba K, Yasui S, Hayashi M, Tamaoki J, Nagai A. Red blood cells inhibit apoptosis of human neutrophils. Blood. 1999;93:4006–4010. [PubMed] [Google Scholar]
- 5.Melder RJ, Yuan J, Munn LL, Jain RK. Erythrocytes enhance lymphocyte rolling and arrest in vivo. Microvasc Res. 2000;59:316–322. doi: 10.1006/mvre.1999.2223. [DOI] [PubMed] [Google Scholar]
- 6.Fredriksson K, Liu XD, Lundahl J, Klominek J, Rennard SI, Skold CM. Red blood cells increase secretion of matrix metalloproteinases from human lung fibroblasts in vitro. Am J Physiol Lung Cell Mol Physiol. 2006;290:L326–L333. doi: 10.1152/ajplung.00057.2005. [DOI] [PubMed] [Google Scholar]
- 7.Virella G, Rugeles MT, Hyman B, La Via M, Goust JM, Frankis M, Bierer BE. The interaction of CD2 with its LFA-3 ligand expressed by autologous erythrocytes results in enhancement of B cell responses. Cell Immunol. 1988;116:308–319. doi: 10.1016/0008-8749(88)90233-X. [DOI] [PubMed] [Google Scholar]
- 8.Schakel K, Schäkel K, von Kietzell M, Hänsel A, Ebling A, Schulze L, Haase M, Semmler C, Sarfati M, Barclay AN, Randolph GJ, Meurer M, Rieber EP. Human 6-sulfo LacNAc-expressing dendritic cells are principal producers of early interleukin-12 and are controlled by erythrocytes. Immunity. 2006;24:767–777. doi: 10.1016/j.immuni.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Arosa FA, Pereira CF, Fonseca AM. Red blood cells as modulators of T cell growth and survival. Curr Pharm Des. 2004;10:191–201. doi: 10.2174/1381612043453432. [DOI] [PubMed] [Google Scholar]
- 10.Fonseca AM, Pereira CF, Porto G, Arosa FA. Red blood cells upregulate cytoprotective proteins and the labile iron pool in dividing human T cells despite a reduction in oxidative stress. Free Radic Biol Med. 2003;35:1404–1416. doi: 10.1016/j.freeradbiomed.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Fonseca AM, Pereira CF, Porto G, Arosa FA. Red blood cells promote survival and cell cycle progression of human peripheral blood T cells independently of CD58/LFA-3 and heme compounds. Cell Immunol. 2003;224:17–28. doi: 10.1016/S0008-8749(03)00170-9. [DOI] [PubMed] [Google Scholar]
- 12.Fonseca AM, Porto G, Uchida K, Arosa FA. Red blood cells inhibit activation-induced cell death and oxidative stress in human peripheral blood T lymphocytes. Blood. 2001;97:3152–3160. doi: 10.1182/blood.V97.10.3152. [DOI] [PubMed] [Google Scholar]
- 13.Plunkett ML, Sanders ME, Selvaraj P, Dustin ML, Springer TA. Rosetting of activated human T lymphocytes with autologous erythrocytes. Definition of the receptor and ligand molecules as CD2 and lymphocyte function-associated antigen 3 (LFA-3) J Exp Med. 1987;165:664–676. doi: 10.1084/jem.165.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whisler RL, Newhouse YG, Bagenstose SE. Age-related reductions in the activation of mitogen-activated protein kinases p44mapk/ERK1 and p42mapk/ERK2 in human T cells stimulated via ligation of the T cell receptor complex. Cell Immunol. 1996;168:201–210. doi: 10.1006/cimm.1996.0067. [DOI] [PubMed] [Google Scholar]
- 15.Wesselborg S, Janssen O, Kabelitz D. Induction of activation-driven death (apoptosis) in activated but not resting peripheral blood T cells. J Immunol. 1993;150:4338–4345. [PubMed] [Google Scholar]
- 16.Appleman LJ, van Puijenbroek AA, Shu KM, Nadler LM, Boussiotis VA. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signalling pathway in primary human T cells. J Immunol. 2002;168:2729–2736. doi: 10.4049/jimmunol.168.6.2729. [DOI] [PubMed] [Google Scholar]
- 17.Kumagai N, Benedict SH, Mills GB, Gelfand EW. Requirements for the simultaneous presence of phorbol esters and calcium ionophores in the expression of human T lymphocyte proliferation-related genes. J Immunol. 1987;139:1393–1399. [PubMed] [Google Scholar]
- 18.Alves NL, Hooibrink B, Arosa FA, van Lier RA. IL-15 induces antigen-independent expansion and differentiation of human naive CD8+ T cells in vitro. Blood. 2003;102:2541–2546. doi: 10.1182/blood-2003-01-0183. [DOI] [PubMed] [Google Scholar]
- 19.Brenner D, Krammer PH, Arnold R. Concepts of activated T cell death. Crit Rev Oncol Hematol. 2008;66:52–64. doi: 10.1016/j.critrevonc.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Lewis RS. Calcium signalling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita M, Katsumata M, Iwashima M, Kimura M, Shimizu C, Kamata T, Shin T, Seki N, Suzuki S, Taniguchi M, Nakayama T. T cell receptor-induced calcineurin activation regulates T helper type 2 cell development by modifying the interleukin 4 receptor signalling complex. J Exp Med. 2000;191:1869–1879. doi: 10.1084/jem.191.11.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 23.Mills RE, Jameson JM. T cell dependence on mTOR signalling. Cell Cycle. 2009;8:545–548. doi: 10.4161/cc.8.4.7625. [DOI] [PubMed] [Google Scholar]
- 24.Galat A. Peptidylprolyl cis/trans isomerases (immunophilins): biological diversity—targets—functions. Curr Top Med Chem. 2003;3:1315–1347. doi: 10.2174/1568026033451862. [DOI] [PubMed] [Google Scholar]
- 25.Weischer M, Rocken M, Berneburg M. Calcineurin inhibitors and rapamycin: cancer protection or promotion? Exp Dermatol. 2007;16:385–393. doi: 10.1111/j.1600-0625.2007.00555.x. [DOI] [PubMed] [Google Scholar]
- 26.Arora K, Gwinn WM, Bower MA, Watson A, Okwumabua I, MacDonald HR, Bukrinsky MI, Constant SL. Extracellular cyclophilins contribute to the regulation of inflammatory responses. J Immunol. 2005;175:517–522. doi: 10.4049/jimmunol.175.1.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin ZG, Melaragno MG, Liao DF, Yan C, Haendeler J, Suh YA, Lambeth JD, Berk BC. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res. 2000;87:789–796. doi: 10.1161/01.res.87.9.789. [DOI] [PubMed] [Google Scholar]
- 28.Crocker PR. Siglecs: sialic-acid-binding immunoglobulin-like lectins in cell–cell interactions and signalling. Curr Opin Struct Biol. 2002;12:609–615. doi: 10.1016/S0959-440X(02)00375-5. [DOI] [PubMed] [Google Scholar]
- 29.Bratosin D, Mazurier J, Tissier JP, Estaquier J, Huart JJ, Ameisen JC, Aminoff D, Montreuil J. Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. A review. Biochimie. 1998;80:173–195. doi: 10.1016/S0300-9084(98)80024-2. [DOI] [PubMed] [Google Scholar]
- 30.Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–280. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Hildeman DA, Mitchell T, Kappler J, Marrack P. T cell apoptosis and reactive oxygen species. J Clin Invest. 2003;111:575–581. doi: 10.1172/JCI18007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song J, Lei FT, Xiong X, Haque R. Intracellular signals of T cell costimulation. Cell Mol Immunol. 2008;5:239–247. doi: 10.1038/cmi.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunningham EB. An inositolphosphate-binding immunophilin, IPBP12. Blood. 1999;94:2778–2789. [PubMed] [Google Scholar]
- 34.Pasini EM, Kirkegaard M, Mortensen P, Lutz HU, Thomas AW, Mann M. In-depth analysis of the membrane and cytosolic proteome of red blood cells. Blood. 2006;108:791–801. doi: 10.1182/blood-2005-11-007799. [DOI] [PubMed] [Google Scholar]
- 35.Sarris AH, Harding MW, Jiang TR, Aftab D, Handschumacher RE. Immunofluorescent localization and immunochemical determination of cyclophilin-A with specific rabbit antisera. Transplantation. 1992;54:904–910. doi: 10.1097/00007890-199211000-00026. [DOI] [PubMed] [Google Scholar]
- 36.Brown VI, Fang J, Alcorn K, Barr R, Kim JM, Wasserman R, Grupp SA. Rapamycin is active against B-precursor leukemia in vitro and in vivo, an effect that is modulated by IL-7-mediated signalling. Proc Natl Acad Sci USA. 2003;100:15113–15118. doi: 10.1073/pnas.2436348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. RBC enhance proliferation of PBL activated by the combination of PdB and Ionomycin. PBL (1.5x106) were labelled with CFSE and cultured either alone or with red blood cells (1:10 ratio) in the absence or presence of the different stimuli for 7 days. Then, cells were harvested, stained and acquired in a FACSCalibur. Graph shows the percentage of dividing PBL (mean±SEM, n=4) in the different culture conditions. P values are shown (*, p<0.05) (TIFF 110 kb)
Supplemental Figure 2. RBC hasten T cell proliferation and activates the Akt/PKB pathway. PBL (1.5x106) were labelled with CFSE and cultured for 3 days as indicated in the legend of Figure 6. Then, cells were harvested at days 1, 2 and 3 and acquired in a FACSCalibur. Graph shows the percentage (mean±SEM) of dividing cells for the different culture conditions along the culture period. Inset, PBL (1.5x106) were stimulated with 5 μg/mL of PHA overnight, harvested and washed out to remove PHA. Activated T cells were recultured without stimulus for an additional 48 hours. Then, cells were harvested and incubated at 37°C in culture medium alone or with RBC (1:10 ratio) for 1, 30 and 60 minutes as indicated. After lysis of RBC, T cells were immediately spun down and lysed in cell lysis buffer containing a protease and phosphatase inhibitor cocktail (Sigma-Aldrich). Cell lysates were quantified using the BCA. protein assay and aliquots corresponding to 25 μg of protein were resolved by 10% SDS-PAGE. After transfer of proteins to nitrocelulose membranes, phosphorylation of PKB was determined by using anti-phospho-Akt/PKB (Ser473) followed by rabbit anti-mouse horseradish peroxidase-conjugated antibodies (Cell Signalling Technology, USA) and ECL. Levels of total Akt/PKB were determined after membrane stripping and re-probing with anti-Akt/PKB polyclonal antibodies. Levels of phosphorylated and total Akt/PKB are indicated (TIFF 527 kb)