Abstract
Pollen hydration is usually tightly regulated and occurs in vivo only when desiccated pollen grains acquire water from the female, thus enabling pollen tube growth. Pollen tubes are easily visualized by staining with decolorized aniline blue, a stain specific for callose. We identified a mutant, raring-to-go, in which pollen grains stained for callose before anther dehiscence. When raring-to-go plants are transferred to high humidity, pollen tubes dramatically elongate within the anther. As early as the bicellular stage, affected pollen grains in raring-to-go plants acquire or retain water within the anther, and precociously germinate. Thus, the requirement for contact with the female is circumvented. We used pollen tetrad analysis to show that raring-to-go is a gametophytic mutation, to our knowledge the first gametophytic mutation in Arabidopsis that affects early events in the pollination pathway. To aid in identifying raring-to-go alleles, we devised a new technique for screening pollen in bulk with decolorized aniline blue. We screened a new M1 mutagenized population and identified several additional mutants with a raring-to-go-like phenotype, demonstrating the usefulness of this technique. Further, we isolated other mutants (gift-wrapped pollen, polka dot pollen, and emotionally fragile pollen) with unexpected patterns of callose staining. We suggest that raring-to-go and these other mutants may help dissect components of the pathway that regulates pollen hydration and pollen tube growth.
In most higher plants, the pollination pathway is initiated when a partially desiccated pollen grain contacts the stigma, acquires water from the stigma, and hydrates. In many plants, pollen hydration is tightly controlled (Heslop-Harrison, 1979). In species exhibiting sporophytic self-incompatibility, the control of water uptake provides a mechanism for preventing self-pollinations (Herrero and Hormaza, 1996). Despite this normally tight regulation of hydration, it is true nonetheless that pollen will also hydrate and grow a pollen tube if placed in a simple medium containing Suc, boric acid, and calcium (Taylor and Hepler, 1997).
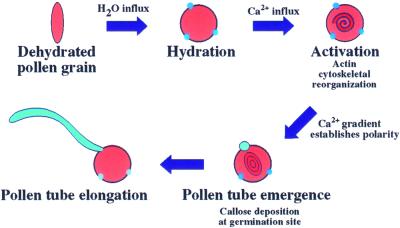
In Arabidopsis, a self-compatible plant, once a pollen grain contacts a stigma papillar cell, the pollen coat, composed of lipids and proteins (Piffanelli et al., 1998; Dickinson and Elleman, 2000), softens into a gelatinous mixture and flows onto the papillar surface. This bridge between the pollen grain and papillar cell, sometimes termed a foot, establishes the route of water flow into the desiccated pollen grain (Elleman et al., 1992). Pollen hydration is complete when the oblong shape of the desiccated pollen grain becomes round. As diagrammed in Figure 1, the pollen grain hydrates and Ca2+ flows into the pollen grain; this influx triggers activation. Activation is characterized by cytoplasmic reorganization within the pollen grain (Heslop-Harrison and Heslop-Harrison, 1992a, 1992b). This reorganization results in the formation of a cytoplasmic gradient of Ca2+ beneath the site of germination; this gradient is critical for polar tip growth (Heslop-Harrison and Heslop-Harrison, 1992a; Franklin-Tong, 1999). Associated with Ca2+ influx, the pollen grain deposits callose, a β-1,3-glucan, at one of the three pores, where the pollen tube will emerge. The pollen tube wall is an extension of the intine, or inner pollen wall, and is composed largely of callose (Schlupmann et al., 1993). The pollen tube extends by tip growth through the pistil to deliver the sperm to the embryo sac.
Figure 1.
Schematic illustrating key features of the pollination pathway: pollen hydration, activation, and pollen tube extension.
Here, we describe an unusual mutant in Arabidopsis, raring-to-go (rtg). Some of the pollen in raring-to-go plants can prematurely hydrate, germinate, and form pollen tubes within the anther. These pollen grains in the raring-to-go mutant thus appear capable of bypassing the need to contact the stigma in order to acquire water, hydrate, and germinate. Although not previously reported in Arabidopsis, pollen germination in the anther is not unknown in the plant kingdom. In fact, a wide diversity of plant species have a breeding system known as cleistogamy, wherein the flowers do not open or open only after fertilization (for review, see Lord, 1981). In a subset of cleistogamous species, all pollen germinates inside the anther. Fertilization mechanisms in such cleistogamous species are diverse. In Lamium amplexicavle, pollen germinates in the anther and the tubes emerge through the anther stomium and grow to the wet stigma (Lord, 1979). In Viola odorata, pollen germinates in unopened anthers and grows through the anther wall to reach the stigma (Mayers and Lord, 1984). An extreme case is seen in several genera of the Malpighiaceae: Here the anthers do not open but pollen tubes can grow through the anther filament tissue and enter the female tissue at the receptacle, completely bypassing the stigma (Anderson, 1980). It is remarkable that most studies on such cleistogamous species have remained descriptive and pollen tube growth within the anther is not genetically understood.
In Arabidopsis, other mutants are known to affect pollen hydration and germination. For example, pollen grains of some eceriferum mutants (Preuss et al., 1993; Hülskamp et al., 1995) fail to hydrate on the stigma; these mutants have defects in the biosynthesis of lipids deposited on the pollen coat. Plants with the grp17-1 mutation lack an oleosin-domain protein in the pollen coat, resulting in delayed pollen hydration on the stigma (Mayfield and Preuss, 2000). In the fiddlehead1 mutant, the leaf epidermis is capable of supporting pollen germination, due to lipid modifications that alter the permeability of the epidermal cuticle (Lolle et al., 1997; Pruitt et al., 2000). The eceriferum, grp17-1, and fiddlehead1 genes act sporophytically. In contrast, we used tetrad analysis to show that the rtg gene acts gametophytically.
We devised a new technique for screening pollen in bulk with histochemical stains, thereby greatly improving the efficiency of finding pollen mutants. To identify additional mutants with precocious pollen tubes, we screened an M1 population by staining with the callose-specific stain decolorized aniline blue. Using this technique, we were able to identify several other rtg-like mutants, as well as novel mutants, including two (gift-wrapped pollen: gwp1, and gwp2) that appear to have pollen tubes within the pollen grain. These mutants should be useful tools for dissecting the roles that gametophyte-encoded proteins play during pollen hydration and pollen tube growth.
RESULTS
Identification of raring-to-go
While screening an M2 population for male gametophytic mutations (Chen and McCormick, 1996), we noticed protrusions from some of the pollen released from anthers of one family. To further characterize this phenotype, pollen from the affected plants were stained with a variety of histochemical stains. Because the protrusions stained with decolorized aniline blue, a stain specific for callose, it seemed probable that these protrusions were pollen tubes. Further examination revealed some elongated pollen tubes within the anthers of these plants. The staining was similar to wild-type germinated pollen stained with aniline blue. Because of the premature pollen germination, we named this mutant raring-to-go. The flowers of raring-to-go plants appear otherwise normal and open normally; the anthers dehisce and shed pollen normally, and the affected plants exhibit full seed set.
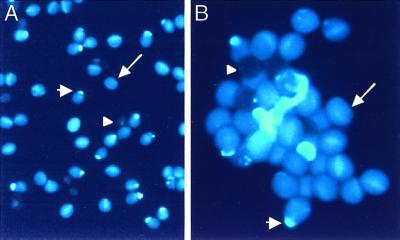
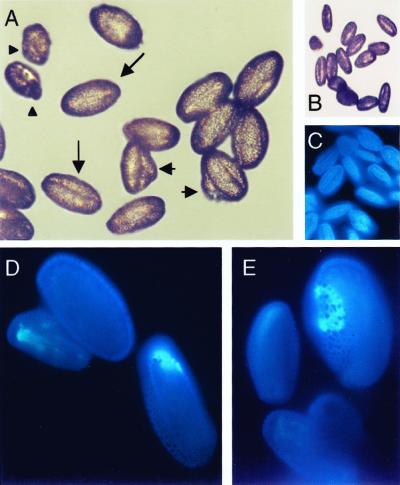
Self and backcross progeny were generated. Figure 2 shows two fields of mature pollen from raring-to-go plants, and illustrates the range of phenotypes: There are pollen grains with pollen tubes, aborted pollen grains, and normal pollen grains. We classified the pollen phenotypes of 40 plants (230 flowers) with the raring-to-go phenotype. Overall, 26% of the pollen grains were aborted, 20% had pollen tubes, and 54% appeared normal. Counts from only 10 of the 230 flowers scored deviated significantly. To illustrate the extremes, one flower had 53% aborted grains, 7% with pollen tubes and 40% normal, whereas a flower on a different plant had 31% aborted grains, 32% with pollen tubes and 38% normal. In each of these 10 cases, the same plant had other flowers that fell within the mean.
Figure 2.
Pollen phenotypes found in rtg/+ plants. A, Field of pollen. B, Higher-magnification of another field. Note the two grains with pollen tubes, three non-stained (aborted) grains, and normal pollen. Representative pollen grains of the three phenotypes show wild-type (long arrow), rtg (short arrow), and aborted (arrowhead). Stained with aniline blue.
raring-to-go Is Gametophytically Expressed
The ratio of 50% normal and 50% affected pollen grains was reminiscent of the ratios obtained with a previously characterized gametophytic mutant, sidecar pollen (Chen and McCormick, 1996); sidecar pollen heterozygotes have approximately 50% normal and 50% affected (aborted or sidecar) pollen grains. By analogy, if the pollen with tubes and the aborted pollen grains can be grouped, then rtg might be a gametophytically expressed mutation, where half the pollen grains in a heterozygote (rtg/+) are not normal.
When raring-to-go plants were crossed as female to wild-type plants, about 50% of the F1 progeny showed the phenotype. Expression of the phenotype in the F1 suggests either that rtg is a dominant, sporophytic mutation or a gametophytic mutation. If rtg is a dominant sporophytic mutation, it might suggest that the anthers of the plant produce a signal that is normally produced by the stigma cells. Gametophytic expression would imply that 50% of the pollen grains initiate a pollination response (i.e. hydrate, germinate, and grow pollen tubes) in the absence of a signal from the stigma.
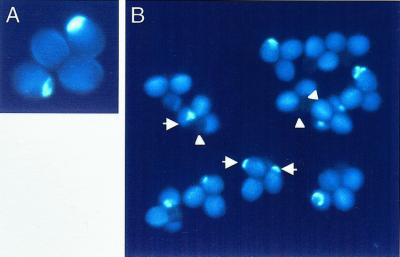
To test whether rtg was a gametophytic mutation, tetrad analysis was performed using quartet1 (qrt1), a sporophytic recessive mutation that keeps all the products of a single meiosis together throughout pollen development (Preuss et al., 1994). If a mutation is sporophytically expressed but has low expressivity, we might expect the numbers of normal and affected pollen resulting from each meiosis to vary. However, if a mutation is gametophytic, the ratio of normal:affected pollen resulting from each meiosis should be 2:2. Table I shows the distribution of pollen phenotypes in the double mutant and, for comparison, the distribution of pollen phenotypes in the scp; qrt1 double mutant (Chen and McCormick, 1996). Because most quartets in the rtg/+; qrt1/qrt1 plants have two normal pollen grains to two affected pollen grains (i.e. either aborted or with a pollen tube; Fig. 3), we conclude that rtg is a gametophytically expressed mutation.
Table I.
Tetrad analysis of rtg
| Plant | Tetrads Scored | Tetrad
|
||||
|---|---|---|---|---|---|---|
| (oooo) | (ooox) | (ooxx) | (oxxx) | (xxxx) | ||
| +/+; qrt1/qrt1a | 434 | 98b | 1.8 | 0 | 0.2 | 0 |
| scp/+; qrt1/qrt1a | 662 | 0 | 0 | 96.5 | 3.5 | 0 |
| rtg/+; qrt1/qrt1 | 464 | 0 | 2.6 | 87.3 | 9.9 | 0.2 |
o, Normal pollen grain. x, Mutant pollen grain.
Percentage of tetrads with a given phenotype.
Figure 3.
rtg is gametophytic. A, Single quartet from rtg/+; qrt1/qrt1 plant illustrates two affected:two normal pollen grains, a distribution expected for a gametophytic mutation. B, Field of mature pollen from an rtg/+; qrt1/qrt1 plant, rtg (short arrow) and aborted pollen (arrowhead) are marked in three quartets. Note the types of combinations possible: two rtg:two normal (center quartet), one rtg, one aborted:two normal (left quartet), and two aborted:two normal (right quartet). Stained with aniline blue.
raring-to-go Pollen Grains Are Affected Beginning at the Bicellular Stage
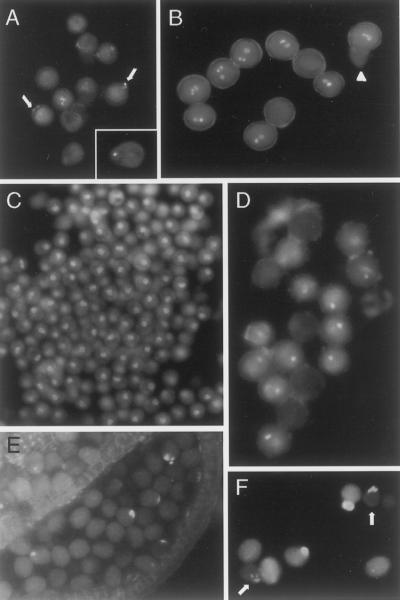
The gametophytic expression of rtg suggests that the occurrence of both aborted pollen and pollen with pollen tubes is due to the same mutation.Therefore, it was of interest to determine how early pollen germination occurred and when aborted pollen grains were first observed. We sorted flower buds by size (Piffanelli et al., 1998) to examine different developmental stages. For each stage, we used sequential staining, first with 4′,6-diamindino-2-phenylindole (DAPI) and then with aniline blue to determine if callose was present. This was necessary because when nuclei were near the aperture or in the growing pollen tube (Fig. 4A), they generally could not be seen after aniline blue staining. And with rare exceptions (Fig. 4A, inset) pollen tube protrusions were generally not visible when stained only with DAPI. Except for the aborted grains, all pollen (>400 scored) showed three nuclei (vegetative and two sperm) when stained only with DAPI (Fig. 4B).
Figure 4.
Pollen development in rtg/+ plants. A, Tricellular stage, stained with DAPI. Arrows mark nuclei near emerging pollen tube. B, Tricellular stage, stained with DAPI. The two grains whose nuclei are out of the plane of focus have pollen tube protrusions. Arrowhead marks aborted grain. C, Unicellular stage, stained with DAPI. D, Bicellular stage, stained with DAPI. Note that aborted cells lack nuclear staining. E, Bicellular stage, stained with aniline blue. F, Tricellular stage, stained with aniline blue. Arrows mark two aborting grains that lack cytoplasmic fluorescence but have fluorescent protrusions.
If a pollen grain showed some callose deposition, we scored it as affected. The tetrad stage (not shown) and the unicellular stage (Fig. 4C) were equivalent to wild type. Pollen abortion is first evident at the bicellular stage (Fig. 4D), as is the first indication of callose deposition, in some of the grains (Fig. 4E). Furthermore, aborting pollen grains frequently had callose staining (Fig. 4F and data not shown). Callose staining is more extensive at the tricellular stage (Fig. 4, A and B) when protrusions from the pollen surface are obvious (Figs. 2 and 4F).
raring-to-go Pollen Does Not Completely Desiccate and Forms Wide Pollen Tubes
The process of staining with aniline blue unavoidably results in pollen hydration. However, Figure 5A shows that three different pollen phenotypes can be distinguished in pollen from dehiscent anthers of rtg/+ plants, even without aniline blue staining. There were oblong-shaped pollen grains identical to wild-type dehydrated pollen (Fig. 5B), there were some grains that were somewhat rounder in shape and that had bulges, and there were also aborted grains, some with bulges. We suggest that these phenotypes correspond to the three phenotypes we observed in pollen rehydrated in aniline blue.
Figure 5.
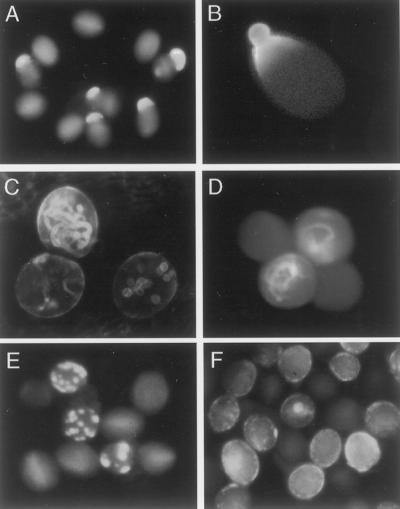
Pollen dimorphism within the anther of rtg/+ plants. A, Pollen released from dehiscent rtg/+ anther. Representative pollen grains of the three phenotypes show wild-type (long arrow), rtg (short arrow), and aborted (arrowhead). B, Pollen released from dehiscent wild-type anther. C, Pollen from wild-type inflorescence after 24 h incubation in HPTS. D and E, Pollen from rtg/+ inflorescence after 24 h incubation in HPTS.
To further test whether there were differences in pollen hydration within the rtg/+ anthers, we adapted an in vitro inflorescence culture (Lardon et al., 1993) to deliver 8-hydroxypyrene-1,3,6-trisulfonic acid (HPTS), a water-soluble, pH-sensitive tracer that fluoresces upon excitation with UV light. HPTS has been used to monitor plasmodesmatal connections in developing meristems (Gisel et al., 1999). Inflorescences from rtg/+ plants and from Columbia-O (Col) wild-type plants were cut from the plant and the cut end placed in a solution of HPTS, at 0.125 μg μL−1. Gisel et al. (1999) used HPTS at 2.5 mg mL−1; we found that level toxic to the inflorescence cultures, the inflorescences stopped growing, and the flowers did not open. We tested serial dilutions in order to identify a concentration of HPTS that would allow anther dehiscence. Pollen grains from dehiscing anthers were gently transferred to slides 24 h after treatment and the pollen examined with a fluorescence microscope. The anthers and other parts of the inflorescence were brightly fluorescent (not shown), indicating efficient transport of the HPTS, via the vascular system and plasmodesmata (Gisel et al., 1999). Pollen within the locule is not connected to the HPTS source via the vascular system or plasmodesmata, and Figure 5C shows that Col wild-type pollen had negligible fluorescence after HPTS treatment. In Figure 5, D and E, representative pollen grains from HPTS-treated rtg/+ anthers are shown. In contrast to wild type, both fluorescent and nonfluorescent pollen grains are always seen when rtg/+ anthers are treated with HPTS. The percentage of HPTS staining pollen grains was equal to or less than the percentage of pollen grains with protrusions.
We suggest that the rtg pollen grains are able to acquire the HPTS from the moisture present within the anther locule, although the wild-type-appearing and the aborted grains in the same anther cannot. Although we did not directly quantitate water content in individual pollen grains, together these results suggest that at dehiscence, water content is higher in the nonaborted rtg pollen grains than it is in normal, oblong-shaped pollen, whether the normal pollen is from the same rtg/+ plant or from wild-type plants.
Pollen Tube Formation in raring-to-go
We used fluorescence microscopy to image pollen tube formation in affected rtg pollen grains. One notable feature of rtg pollen tubes is that they are much wider (Figs. 2, 3, and 6) than wild-type pollen tubes, which are typically one-fifth the diameter of the grain. Sometimes the wide tube does not lengthen and a more normal width pollen tube arises out of the side of the wide rtg tube (not shown).
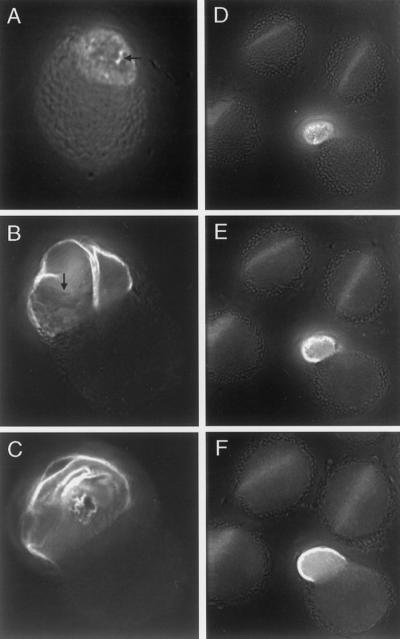
Figure 6.
Deconvolution microscope images of selected pollen grains from rtg/+ plants. A, Optical section of rtg pollen grain 1. Arrow marks rupture in exine where callose stains brightly. B and C, Two different optical sections of rtg pollen grain 2. Arrow marks exine cap. D through F, Optical sections of three wild-type appearing grains and a typical rtg grain. Stained with aniline blue.
Within any flower of a rtg/+ plant, several stages of pollen tube extrusion are usually evident upon aniline blue staining. Pollen grains with only limited callose staining (that we interpret as an early stage) show an annulus ring below the intine surface, consistent with previous observations of callose staining during pollen germination (Heslop-Harrison and Heslop-Harrison, 1992b). The annulus ring is proposed to define the diameter of the pollen tube, and already at this early stage, the annulus ring diameter is enlarged (Fig. 6A) as compared with wild type (not shown). A few localized foci of brightly staining callose at the aperture represent ruptures in the exine where the growing pollen tube will emerge (Fig. 6A). In other pollen grains, one or more finger-like projections are apparent (not shown), and we interpret these as extensions emerging from the foci of callose seen at the earlier stage. These finger-like projections seem to pop off the thin exine overlying the aperture. In pollen grains with slightly more developed tubes, these finger-like projections widen and coalesce and appear to twist out of the pollen grain, as is shown in two optical sections of one such pollen grain (Fig. 6, B and C). Figure 6, D through F, illustrates optical sections of three wild-type-appearing pollen grains, each with negligible callose staining, and one rtg pollen grain with a small tube. We cannot ascertain whether all tubes go through the stages illustrated in Figure 6, A through C, because many tubes (such as the one shown in Fig. 6, D–F), are already developed when the anther is dissected and the pollen is stained with aniline blue.
High Humidity Affects the raring-to-go Phenotype
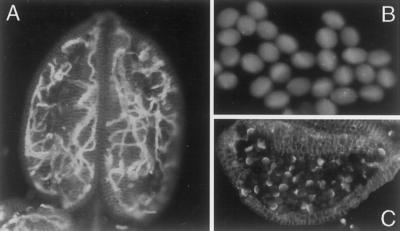
Genetic mapping requires large populations of plants and precise phenotypic scoring. To our knowledge, pollen tubes in Arabidopsis anthers have not been previously reported, and the phenotype of the rtg mutant in the initial F1 and F2 generations was easy to distinguish from wild type. However, because hydration is the first step toward germination, it seemed possible that wild-type plants raised in high humidity might be able to form pollen tubes. Therefore, it was important to assess whether wild-type plants could ever form pollen tubes in the anther. First, pollen from rtg/+ plants was compared with wild-type pollen from Col plants, both grown in standard growth conditions under continuous daylight. The measured relative humidity was 70%. Under these conditions, dehiscent pollen from wild-type Col plants had negligible callose staining, whereas the rtg/+ plants had prominent callose staining (see Fig. 2). Sibling plants, one rtg/+ and one +/+, as well as a wild-type Col plant, were then placed in separate humidity chambers in which the measured relative humidity was >85%. After 2.5 d, pollen was collected from each plant and stained with aniline blue. Figure 7A shows that the rtg/+ plant had long pollen tubes trapped inside the anther. This dramatic phenotype suggests that humidity can certainly enhance the growth of pollen tubes in rtg/+ anthers, and the width of the pollen tubes under these conditions is more like those of wild-type tubes. However, the pollen from the +/+ sibling plant (not shown) and wild-type Col plant (Fig. 7B) still had negligible callose staining. The wild-type Col plant was further maintained at >85% relative humidity. Even after 12 d there was no pollen tube outgrowth or elongation (Fig. 7C), although all the pollen showed some marginal callose deposition within the grain, frequently in a half-moon shape. We suggest that this pattern of callose deposition is due to a stress response.
Figure 7.
Effects of 85% relative humidity on dehiscent pollen. A, Pollen within anther from an rtg/+ plant after 2.5 d. B, Pollen within anther from a wild-type plant after 2.5 d. C, Pollen within anther of a wild-type plant after 12 d. Stained with aniline blue.
Transmission of raring-to-go
We have shown that rtg pollen has a greater content of water upon dehiscence than does wild-type-appearing pollen within the same anther (Fig. 5, C–E), and that under high humidity rtg pollen has the capacity to grow long pollen tubes (Fig. 7A). However, under normal growth conditions rtg does not appear to transmit through the male. When F1 plants with the rtg phenotype (rtg/+) were allowed to self-pollinate, the F2 plants segregated 1:1 for rtg: wild-type, rather than in the expected 3:1 ratio. This F2 ratio suggests either that rtg homozygotes are inviable, or that transmission through one of the parents is impaired. Reciprocal crosses showed that female transmission was normal, but crosses using rtg/+ as the male donor yielded only wild-type progeny. Thus, the name raring-to-go is somewhat of a misnomer and does not reflect success at fertilization. Perhaps the rtg pollen grains that contact the stigma are delayed in elongating their pollen tubes and therefore fail when competing with wild-type pollen.
New Screening Technique Yields rtg-Like Mutants and Other Mutants with Unexpected Patterns of Callose Staining
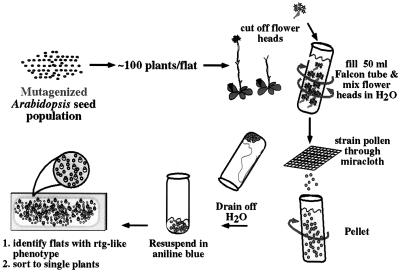
Because raring-to-go was isolated from a nondirected screen, we could not ascertain the frequency of such a phenotype. In addition, we were interested in determining if mutational analysis could be used to dissect the pollination response pathway (Fig. 1). We devised a new technique for screening pollen in bulk (Fig. 8) to aid in identifying raring-to-go alleles or other genes with similar phenotypes. Because we were particularly interested in recovering raring-to-go alleles and raring-to-go is gametophytic, we chose to screen an M1 population instead of an M2 population. By screening an M1 population, we were preferentially selecting for dominant sporophytic or gametophytic mutations. This strategy has been shown previously to be successful in identifying new gametophytic mutations (Chen and McCormick, 1996). From approximately 6,000 M1 plants screened, we identified 52 plants whose pollen stained with aniline blue while still in the anther. Because M1 plants are chimeric for the mutation, each putative mutant was self-pollinated, and 50 M2 seed were planted to test for transmission. We have further characterized 15 lines with distinct and transmitted phenotypes.
Figure 8.
Schematic of screen used to identify rtg-like mutants and other mutants affecting pollination.
We have confirmed seven rtg-like mutants. Six of these look just like raring-to-go (one is shown in Fig. 9A), whereas the seventh, shown in Figure 9B, exhibits a subtle but consistent difference in the width of the pollen tube protrusions. Figure 9 also shows pollen from three other selected M2 families, to illustrate the range of mutants we obtained from this screen. The mutants gift-wrapped pollen1 (Fig. 9C) and gift-wrapped pollen2 (not shown) were so named because some of the grains appear to have ribbons tied in bows within the pollen grain. The ribbons appear to be pollen tubes; optical sections (not shown) through such pollen grains show that the tubes are continuous, although in other pollen grains in these lines the structures staining with aniline blue are less extensive. When pollen from gwp1/+ plants is stained with DAPI, all pollen grains show three stained nuclei, and under bright-field illumination there is no obvious pollen phenotype (not shown). However, like rtg, the gwp1 mutant does not transmit through the male, as shown by reciprocal crosses, and because the F2 population segregates 1:1 (not shown). As we showed for rtg (Fig. 3 and Table I), gwp1 is also gametophytic (Fig. 9D). Of 220 quartets counted in the gwp1/+; qrt1/qrt1 double mutant, 81% had two affected grains and 15% had one affected grain.
Figure 9.
Examples of new mutant lines with aberrant patterns of callose staining. A, rtg like. B, rtg like with subtle difference in tube width. C, gift-wrapped pollen1 (gwp1). D, gwp1/+; qrt1/qrt1. E, polka dot pollen (pdp). F, emotionally fragile pollen (efp). Stained with aniline blue.
Figure 9E shows polka dot pollen, a mutant in which approximately 50% of the pollen shows brightly stained globules of callose. The screen yielded three isolates of pdp. In somatic cells, callose is formed in response to stress. The mutant emotionally fragile pollen (Fig. 9F) was so named because, in the absence of any apparent stress, 50% of the pollen shows a diffuse callose staining. Affected pollen in efp sometimes shows more intense staining near the pollen wall. The screen yielded three isolates of efp.
Mapping
We used simple sequence length polymorphism (SSLP) and cleaved amplified polymorphic sequences (CAPS) markers with a Landsberg erecta (Ler)/Col F2 population of 720 plants to localize rtg to the top arm of chromosome 3, between the CAPS markers cytosolic glyceraldehyde-3-phosphate-dehydrogenase and manganese superoxide dismutase. We have further mapped rtg to a region covered by two bacterial artificial chromosome (BAC) clones. We generated an SSLP from the sequence of one of these BAC clones, and have one recombinant in the population.
In the more recent mutant screen, we identified seven additional mutants whose phenotypes are similar to rtg. Several male gametophytic mutations have reduced (Chen and McCormick, 1996; Park et al., 1998) or no transmission through the male. Therefore, rather than performing allelism tests of the new isolates with the original isolate, these rtg-like mutants and other mutants identified in the screen have been outcrossed to Ler to generate mapping populations and thereby determine if any map to the same region of chromosome 3. So far, one of the rtg-like mutants maps to same region as rtg (no recombinants in 38 plants when the SSLP tightly linked to rtg is assayed), suggesting that we have recovered an rtg allele. If so, sequence analysis of rtg alleles will help in identifying which candidate protein is encoded by rtg, because the annotation of the BAC clones reveals mostly hypothetical proteins of unknown function.
We have roughly mapped gwp1, gwp2, and efp1. The gwp1 mutant is linked to Erecta on chromosome 2 (one recombinant in 56 plants). The gwp2 and efp1 mutants show no linkage to Erecta. Furthermore, gwp2 and efp1 are not alleles of rtg because they independently assort ( approximately 45% recombination) from the SSLP marker that is tightly linked to rtg on chromosome 3.
DISCUSSION
We have identified an unusual pollen mutant. Some of the pollen in raring-to-go prematurely germinates inside the anther at an immature developmental stage, but fails to complete fertilization after dehiscence and subsequent contact with the stigma. Even though pollen tube elongation in raring-to-go occurs more readily at high humidity, these pollen tubes do not appear to grow toward the female, either through the filament or through the open stomium. It is notable that high humidity (>88%) influences directional guidance of growing pollen tubes, at least in Nicotiana alata (Lush et al., 1998). However given our results, rtg is clearly different from some cleistogamous species, where all pollen grains germinate within the anther and fertilization is successful. Nonetheless, because it is defined genetically, rtg may provide insight into mechanisms involved in pollen germination in such cleistogamous species.
The importance of water early in pollination has been demonstrated in cer mutants (Preuss et al., 1993; Hülskamp et al., 1995), which are blocked in germination and all subsequent steps of the pollination pathway as a result of failure to hydrate. We have demonstrated that some pollen grains from rtg/+ plants have a higher water content than wild-type pollen (Fig. 5D, E). The HPTS dye was transferred to the pollen grains in rtg/+ anthers during the 24 h before dehiscence; thus, even if the rtg grains were already hydrated they still were able to acquire more water, while the wild-type and aborted pollen grains within the same anther could not. Pollen germination in rtg/+ anthers can begin at the bicellular stage (Fig. 4), when pollen grains are still in a hydrated state. Therefore, we cannot tell if the rtg mutation allows premature water uptake or if the rtg mutation allows premature germination when the pollen is already somewhat hydrated during pollen maturation.
Pollen tube growth is dependent on an available water source and it is generally accepted that water acts as a solvent to dissolve nutrients needed to sustain pollen tube growth. Pollen tube growth in affected rtg pollen grains is dependent on the availability of water. Under high humidity, pollen tubes dramatically elongate in rtg/+ anthers (Fig. 7A). The loss of water during pollen dehydration and anther dehiscence under normal growth conditions (70% humidity) appears to limit continued pollen tube growth in rtg/+ plants.
Pollen viability in other plants has been shown to be affected by the hydration status during dispersal and by the conditions of rehydration (Crowe et al., 1989; Hoekstra et al., 1992; Heslop-Harrison, 1999). In lily (Lilium longiflorum), pollen is metabolically inactive upon anther dehiscence. Lily pollen removed from the anther 1 d before dehiscence can germinate, although germination frequency is much improved if the pollen is allowed to dry before germination (Lin and Dickinson, 1984). In Arabidopsis, as in most plants, pollen is dehydrated and metabolically inactive upon anther dehiscence, and thus can be viable for long periods (Stanley and Linskens, 1974). Once in contact with the stigma, metabolic activity is restored during hydration. Because some of the pollen grains in rtg plants have germinated and retain water at the time of dehiscence, these pollen grains remain metabolically active and lack a dormancy period before germination. In contrast, in Cucurbita pepo and in several species of Gramineae, pollen is dehisced in a hydrated state and remains metabolically active. In these species, pollen viability is short lived (Dumas et al., 1983; Nepi and Pacini, 1993). Perhaps the affected pollen in rtg/+ plants is similarly dessication intolerant, but this alone cannot explain the aborted pollen in rtg.
Pollen viability in rtg is likely compromised by a combination of factors. First, germination of pollen occurs in the anther of rtg/+ plants at an immature developmental stage. In 70% of the angiosperms bicellular pollen is released from the anthers and pollen mitosis II occurs after germination and pollen tube penetration into the female tissue. Arabidopsis normally releases mature tricellular pollen; thus, in Arabidopsis, precocious germination at the bicellular stage (Fig. 4, D and E) disrupts normal development and any crucial steps occurring near the end of pollen maturation may not be completed (Lin and Dickinson, 1984). Second, in rtg/+ plants, germinated pollen is held for some time in the anther prior to dehiscence. In the Arabidopsis mutant ms35 (Dawson et al., 1999), pollen development appears to be normal, but the anthers do not dehisce. Only low seed set is obtained from crosses using pollen dissected from ms35 anthers, suggesting that pollen retained in the anthers loses viability over time. In a similar manner, in the Arabidopsis mutant delayed-dehiscence1, some pollen degeneration is observed in mutant anthers (Sanders et al., 1999). In rtg/+ plants, affected pollen experiences a prolonged period in the anther post-germination and an increasingly limited water supply as the anthers dessicate, which likely slows or stalls pollen tube growth. Taken together, these factors likely account for abortion of some of the affected pollen grains.
Mature pollen can be easily germinated in vitro, and germination and the initial stages of pollen tube growth occur quite rapidly, sometimes within a matter of minutes. This makes it difficult to characterize discrete stages of germination and incipient pollen tube formation in detail. Affected rtg pollen grains have a range of pollen phenotypes during development, which we believe represent different stages of pollen germination, pollen tube formation, and elongation. We have illustrated early pollen tube formation (Fig. 6, A–F) in rtg. Affected rtg pollen grains from rtg/+ plants grown at 70% humidity have pollen tubes that are wider than those of wild type, but when the same plant is grown at high humidity, pollen tube width in affected pollen (Fig. 7A) is comparable to wild type. Because rtg exhibits a range of developmental stages that can be influenced by environmental factors, rtg pollen should be useful in studying a variety of early germination responses. For example, rtg should be useful in examining the dynamics of actin filament organization (Kost et al., 1998; Gibbon et al., 1999).
If rtg is a loss-of-function mutant, it would suggest that the RTG protein is necessary to inhibit pollen hydration or germination, until contact with a receptive stigma in some way overcomes the inhibition. A useful analogy for this scenario is provided by the viviparous (vp) mutants in maize (Zea mays), whose seeds precociously germinate on the ear. vp mutants either do not synthesize, or are insensitive to, the dormancy hormone abscisic acid (McCarty, 1995). To our knowledge there is no evidence that abscisic acid plays a role in preventing pollen germination, but it is certainly possible that some unknown inhibitor fills this role in pollen.
If rtg is a loss-of-function mutation, and there is normally an inhibitor that prevents precocious pollen germination, then how do we explain the phenotypes of efp, pdp, and gwp? In each of these mutants, like in rtg, mature pollen stains for callose within the anther (Fig. 9) and thus each exhibits some aspects of precocious behavior. If rtg is a loss-of-function allele, then some of our new isolates might be alleles of rtg, albeit with quite variant phenotypes. However, we have shown that gwp1, gwp2, efp1, and rtg are not allelic.
In contrast, if rtg is a gain-of-function mutant, it might suggest that the downstream events that normally occur after contact with the stigma are constitutively activated. Perhaps rtg and the other new mutants may define discrete steps in the pollination pathway (Fig. 1). For example, some pollen in emotionally fragile pollen and polka dot pollen can clearly accumulate callose before contact with the female, but perhaps these mutants cannot properly localize callose at the germination pore, a necessary step for subsequent germination. In gift-wrapped pollen plants, callose also accumulates and in some cases pollen tubes appear to form, but polar outgrowth doesn't occur. Perhaps gwp cannot establish or maintain the Ca2+ gradient necessary for the proper orientation of the pollen tube. As we saw with rtg, in each of these mutants the affected pollen grains exhibit a range of phenotypes. For example, in gift-wrapped pollen plants, the percentage of pollen grains containing pollen tube-like structures varies, although gwp plants always have pollen with diffuse, and sometimes abundant, callose deposits. In emotionally fragile pollen plants, callose staining is mainly diffuse, with occasional brighter fluorescence near the surface of some of the affected grains. In polka dot pollen plants, there is variation in the number and size of globules present in affected pollen grains. These variations suggest that gwp, pdp, and efp may also be influenced by environmental factors. Because we screened an M1 population, the pollen phenotypes we identified are either the result of a pollen gametophytic mutation or a sporophytic dominant mutation. We showed that rtg and gwp1 are gametophytic (Figs. 3 and 9D). Because approximately 50% of the pollen grains in the other new mutants are affected, it is likely that most will also be lesions in gametophytically acting genes.
Other screens using histochemical staining have been successful in identifying pollen mutants affected in pollen cell division and cell fate. For example, the sidecar pollen (Chen and McCormick, 1996) and gemini pollen mutants (Park et al., 1998) were identified in screens using DAPI staining. These screens were quite tedious because they required dissection and microscopic examination of individual plants. In contrast, we successfully incorporated a method of pooling pollen, limiting individual plant analysis to a small number of M1 plants. Screens based on segregation distortion have also successfully identified gametophytic mutants (Bonhomme et al., 1998; Howden et al., 1998; Grini et al., 1999), but required subsequent histochemical analysis to identify the particular developmental stage affected. Our screen targeted mutants affected at a discrete stage because we used aniline blue staining as a marker for germination (Figs. 8 and 9). The screen was not exhaustive. Nonetheless, isolation of several rtg-like and novel pollen mutants effectively demonstrates the usefulness of the bulk pollen screening technique. We suggest that bulked pollen screening, used with other histochemical stains, might be useful in identifying other interesting and unexpected cellular phenotypes.
MATERIALS AND METHODS
Plant Materials and Mutant Isolation
The rtg mutant was identified in progeny from a previously generated Col ethyl methanesulfonate-mutagenized population (gift of Bob Fischer, University of California, Berkeley). Pollen was collected and prepared for staining as previously described (Chen and McCormick, 1996) or pollen was released from dehiscent anthers by squashing flowers in a histochemical stain (see below for stains used).
To screen for new mutants, 0.2 g (approximately 10,000 seeds) of Col were ethyl methanesulfonate mutagenized and sown on germination medium (Chen and McCormick, 1996). Seedlings were transplanted and each flat was scored for pollen phenotypes by staining bulk-collected pollen (Kulikauskas and McCormick, 1997). Arabidopsis plants have a terminal inflorescence and side branches. Because only the terminal inflorescences are cut off to pool the pollen, the side branch inflorescences were checked to identify the affected plant.
For humidity treatments, plants were placed in a chamber constructed from two magenta boxes, one inverted on top of the other, allowing air flow only at the point where the boxes join. The relative humidity inside these chambers was >85%.
Inflorescences from rtg/+ and wild-type Col plants were cultured using a system developed for Brassica napus flowers (Lardon et al., 1993). An opening was made in the lid of each well of a 24-well tissue culture plate (Corning) by inserting a hot needle. The peduncles of the inflorescences were inserted through the hole in the lid and incubated in HPTS (Molecular Probes, Eugene, OR) at 0.125 μg μL−1 in water. The lid of the culture plate was covered with aluminum foil to prevent photodegradation. Flowers that had already undergone anthesis were removed prior to culture.
Microscopy
DAPI (Molecular Probes) at 1 μg mL−1 was used to stain nuclei and decolorized 0.1% (w/v) aniline blue was used to stain callose (Regan and Moffatt, 1990). Immersion oil 518 n (Zeiss, Thornwood, NY) was used for observation of dry pollen and HPTS-treated pollen. An Axiophot compound microscope (Zeiss) was used for fluorescent and light microscopy. Images were photographed using Ektachrome 160T color slide film (Kodak, Rochester, NY). Some images were acquired with a deconvolution microscope (Applied Precision, Issaguah, WA).
Genetic Analysis
To perform pollen tetrad analysis, rtg/+ plants (Col) or gwp1/+ plants (Col) were crossed as females to homozygous Ler quartet1 (Preuss et al., 1994). Those F1 progeny whose pollen stained with aniline blue were selfed, and the F2 progeny were scored to identify the rtg/+; qrt1/qrt1 or gwp1/+; qrt1/qrt1 double mutants.
Mapping
Heterozygous plants in Col background were crossed as females to wild-type Ler. F1 progeny were scored and several F1 plants exhibiting pollen staining with aniline blue were self-pollinated to obtain an F2. Genomic DNA was isolated from F2 progeny using the method of Edwards et al. (1991). The mutations were mapped using PCR-based markers SSLP and CAPS as previously described (Chen and McCormick, 1996).
ACKNOWLEDGMENTS
We thank Vincent Vanoosthuyse for telling us about the usefulness of HPTS for pollen hydration, Jean-Louis Magnard for advice on inflorescence culture, and Paul Herzmark and Henry Bourne for assistance with and use of the deconvolution microscope. For help with the mutant screen and mapping, we thank the University of California (Berkeley) Undergraduate Research Opportunity Program students Ed Chow, Kitty Cheung, Cria Gregory, Jon Mallen-St. Claire, Nikkisha Faulcon, Elizabeth Story, and Marin Academy high school students Katie Augustyn and Jennifer Cribbs. We thank Paul Herzmark, William Brown, and members of our lab for discussions and comments on this manuscript.
Footnotes
This work was supported by the U.S. Department of Agriculture-Current Research Information System (grant no. 5335–21000–011–00D).
LITERATURE CITED
- Anderson WR. Cryptic self-fertilization in the Malpighiaceae. Science. 1980;207:892–893. doi: 10.1126/science.207.4433.892. [DOI] [PubMed] [Google Scholar]
- Bonhomme S, Horlow C, Vezon D, de Laissardiere, Guyon A, Férault M, Marchand M, Bechtold N, Pelletier G. T-DNA mediated disruption of essential gametophytic genes in Arabidopsis is unexpectedly rare and cannot be inferred from segregation distortion alone. Mol Gen Genet. 1998;260:444–452. doi: 10.1007/s004380050915. [DOI] [PubMed] [Google Scholar]
- Chen Y-CS, McCormick S. sidecar pollen, an Arabidopsis thaliana male gametophytic mutant with aberrant cell divisions during pollen development. Development. 1996;122:3243–3253. doi: 10.1242/dev.122.10.3243. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Hoekstra FA, Crowe LM. Membrane phase transitions are responsible for imbibitional damage in dry pollen. Proc Natl Acad Sci USA. 1989;86:520–523. doi: 10.1073/pnas.86.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson J, Sozen E, Vizir I, Van Waeyenberge S, Wilson ZA, Mulligan BJ. Characterization and genetic mapping of a mutation (ms35) which prevents anther dehiscence in Arabidopsis thaliana by affecting secondary wall thickening in the endothecium. New Phytol. 1999;144:213–222. [Google Scholar]
- Dickinson HG, Elleman CJ. Pollen coatings: chimeric genetics and new functions. Sex Plant Reprod. 2000;12:302–309. [Google Scholar]
- Dumas C, Duplan J-C, Said C, Soulier J-P. 1H nuclear magnetic resonance to correlate water content and pollen viability. In: Mulcahy DL, Ottaviano E, editors. Pollen Biology and Implications in Plant Breeding. New York: Elsevier; 1983. pp. 15–20. [Google Scholar]
- Edwards K, Johnstone C, Thompson C. A simple and rapid method for the preparation of plant genomic DNA and PCR analysis. Nuc Acids Res. 1991;19:1349. doi: 10.1093/nar/19.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleman CJ, Franklin-Tong V, Dickinson HG. Pollination in species with dry stigmas: the nature of the early stigmatic response and the pathway taken by pollen tubes. New Phytol. 1992;121:413–424. doi: 10.1111/j.1469-8137.1992.tb02941.x. [DOI] [PubMed] [Google Scholar]
- Franklin-Tong VE. Signaling and the modulation of pollen tube growth. Plant Cell. 1999;11:727–738. doi: 10.1105/tpc.11.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon BC, Kovar DR, Staiger CJ. Latrunculin B has different effects on pollen germination and tube growth. Plant Cell. 1999;11:2349–2363. doi: 10.1105/tpc.11.12.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisel A, Barella S, Hempel FD, Zambryski PC. Temporal and spatial regulation of symplastic trafficking during development in Arabidopsis thaliana apices. Development. 1999;126:1879–1889. doi: 10.1242/dev.126.9.1879. [DOI] [PubMed] [Google Scholar]
- Grini PE, Schnittger A, Schwarz H, Zimmermann I, Schwab B, Jürgens G, Hülskamp M. Isolation of ethyl methanesulfonate-induced gametophytic mutants in Arabidopsis thaliana by a segregation distortion assay using the multimarker chromosome 1. Genetics. 1999;151:859–863. doi: 10.1093/genetics/151.2.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero M, Hormaza JI. Pistil strategies for controlling pollen tube growth. Sex Plant Reprod. 1996;9:343–347. [Google Scholar]
- Heslop-Harrison J. An interpretation of the hydrodynamics of pollen. Am J Bot. 1979;66:737–743. [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y. Germination of monocolpate angiosperm pollen: effects of inhibitory factors and the Ca2+-channel blocker nifedipine. Ann Bot. 1992a;69:395–403. [Google Scholar]
- Heslop-Harrison JS. Aspects of the cell biology of pollination and wide hybridization. In: Cresti M, Cai G, Moscatelli A, editors. Fertilization in Higher Plants: Molecular and Cytological Aspects. Berlin: Springer-Verlag; 1999. pp. 73–112. [Google Scholar]
- Heslop-Harrison Y, Heslop-Harrison J. Germination of monocolpate angiosperm pollen: evolution of the actin cytoskeleton and wall during hydration, activation and tube emergence. Ann Bot. 1992b;69:385–394. [Google Scholar]
- Hoekstra F, Crowe JH, Crowe LM, van Bilsen DGJL. Membrane behavior and stress tolerance in pollen. In: Ottaviano E, Mulcahy DL, Sari Gorla M, Bergamini Mulcahy G, editors. Angiosperm Pollen and Ovules. New York: Springer-Verlag; 1992. pp. 177–186. [Google Scholar]
- Howden R, Park SK, Moore JM, Orme J, Grossniklaus U, Twell D. Selection of T-DNA-tagged male and female gametophytic mutants by segregation distortion in Arabidopsis. Genetics. 1998;149:621–631. doi: 10.1093/genetics/149.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hülskamp M, Kopczak SD, Horejsi TF, Kihl BK, Pruitt RE. Identification of genes required for pollen-stigma recognition in Arabidopsis thaliana. Plant J. 1995;8:703–714. doi: 10.1046/j.1365-313x.1995.08050703.x. [DOI] [PubMed] [Google Scholar]
- Kost B, Spielhofer P, Chua NH. A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J. 1998;16:393–401. doi: 10.1046/j.1365-313x.1998.00304.x. [DOI] [PubMed] [Google Scholar]
- Kulikauskas R, McCormick S. Identification of the tobacco and Arabidopsis homologues of the pollen-expressed LAT59 gene of tomato. Plant Mol Biol. 1997;34:809–814. doi: 10.1023/a:1005856531693. [DOI] [PubMed] [Google Scholar]
- Lardon A, Triboi-Blondel AM, Dumas C. A model for studying pollination and pod development in Brassica napus: the culture of isolated flowers. Sex Plant Reprod. 1993;6:52–56. [Google Scholar]
- Lin J-J, Dickinson DB. Ability of pollen to germinate prior to anthesis and effect of desiccation on germination. Plant Physiol. 1984;74:746–748. doi: 10.1104/pp.74.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolle SJ, Berlyn GP, Engstrom EM, Krolikowski KA, Reiter W-D, Pruitt RE. Developmental regulation of cell interactions in the Arabidopsis fiddlehead-1 mutant: a role for the epidermal cell wall and cuticle. Dev Biol. 1997;189:311–321. doi: 10.1006/dbio.1997.8671. [DOI] [PubMed] [Google Scholar]
- Lord EM. The development of cleistogamous and chasmogamous flowers in Lamium amplexicaule (Labiatae): an example of heteroblastic inflorescence development. Bot Gaz. 1979;140:39–50. [Google Scholar]
- Lord EM. Cleistogamy: a tool for the study of floral morphogenesis, function and evolution. Bot Rev. 1981;47:421–449. [Google Scholar]
- Lush WM, Grieser F, Wolters-Arts M. Directional guidance of Nicotiana alata pollen tubes in vitro and on the stigma. Plant Physiol. 1998;118:733–741. doi: 10.1104/pp.118.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayers AM, Lord EM. Comparative flower development in the cleistogamous species Viola odorata: III. A histological study. Bot Gaz. 1984;145:83–91. [Google Scholar]
- Mayfield JA, Preuss D. Rapid initiation of Arabidopsis pollination requires the oleosin-domain protein GRP17. Nat Cell Biol. 2000;2:128–130. doi: 10.1038/35000084. [DOI] [PubMed] [Google Scholar]
- McCarty DR. Genetic control and integration of maturation and germination pathways in seed development. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:71–93. [Google Scholar]
- Nepi M, Pacini E. Pollination, pollen viability and pistil receptivity in Cucurbita pepo. Ann Bot. 1993;72:526–536. [Google Scholar]
- Park SK, Howden R, Twell D. The Arabidopsis thaliana gametophytic mutation gemini pollen1 disrupts microspore polarity, division asymmetry and pollen cell fate. Development. 1998;125:3789–3799. doi: 10.1242/dev.125.19.3789. [DOI] [PubMed] [Google Scholar]
- Piffanelli P, Ross JHE, Murphy DJ. Biogenesis and function of the lipidic structures of pollen grains. Sex Plant Reprod. 1998;11:65–80. [Google Scholar]
- Preuss D, Lemieux B, Yen G, Davis RW. A conditional sterile mutation eliminates surface components from Arabidopsis pollen and disrupts cell signaling during fertilization. Genes Dev. 1993;7:974–985. doi: 10.1101/gad.7.6.974. [DOI] [PubMed] [Google Scholar]
- Preuss D, Rhee SY, Davis RW. Tetrad analysis possible in Arabidopsis with mutation of the QUARTET (QRT) genes. Science. 1994;264:1458–1460. doi: 10.1126/science.8197459. [DOI] [PubMed] [Google Scholar]
- Pruitt RE, Vielle-Calzada J-P, Ploense SE, Grossniklaus U, Lolle SJ. FIDDLEHEAD, a gene required to suppress epidermal cell interactions in Arabidopsis, encodes a putative lipid biosynthetic enzyme. Proc Natl Acad Sci USA. 2000;97:1311–1316. doi: 10.1073/pnas.97.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan SM, Moffatt BA. Cytochemical analysis of pollen development in wild-type Arabidopsis and a male sterile mutant. Plant Cell. 1990;2:877–889. doi: 10.1105/tpc.2.9.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders PM, Bui AQ, Weterings K, McIntire KN, Hsu Y-C, Lee PY, Truong MT, Beals TP, Goldberg RB. Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod. 1999;11:297–322. [Google Scholar]
- Schlupmann H, Bacic A, Read SM. A novel callose synthase from pollen tubes of Nicotiana. Planta. 1993;191:470–481. [Google Scholar]
- Stanley RG, Linskens HF. Pollen: Biology, Biochemistry and Management. Berlin: Springer; 1974. [Google Scholar]
- Taylor LP, Hepler PK. Pollen germination and tube growth. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:461–491. doi: 10.1146/annurev.arplant.48.1.461. [DOI] [PubMed] [Google Scholar]