Abstract
We previously showed that, in vitro, hyperforin from St. John’s wort (Hypericum perforatum) inhibits 5-lipoxygenase (5-LO), the key enzyme in leukotriene biosynthesis. Here, we demonstrate that hyperforin possesses a novel and unique molecular pharmacological profile as a 5-LO inhibitor with remarkable efficacy in vivo. Hyperforin (4 mg/kg, i.p.) significantly suppressed leukotriene B4 formation in pleural exudates of carrageenan-treated rats associated with potent anti-inflammatory effectiveness. Inhibition of 5-LO by hyperforin, but not by the iron-ligand type 5-LO inhibitor BWA4C or the nonredox-type inhibitor ZM230487, was abolished in the presence of phosphatidylcholine and strongly reduced by mutation (W13A-W75A-W102A) of the 5-LO C2-like domain. Moreover, hyperforin impaired the interaction of 5-LO with coactosin-like protein and abrogated 5-LO nuclear membrane translocation in ionomycin-stimulated neutrophils, processes that are typically mediated via the regulatory 5-LO C2-like domain. Together, hyperforin is a novel type of 5-LO inhibitor apparently acting by interference with the C2-like domain, with high effectiveness in vivo.
Keywords: 5-lipoxygenase, Leukotriene, Inflammation, Neutrophil, C2 domain, Hyperforin, St. John’s wort
Introduction
5-Lipoxygenase (5-LO) is a nonheme iron-containing dioxygenase that catalyzes the formation of leukotriene (LT)A4 from arachidonic acid (AA) (see [1] for review of 5-LO). LTA4 can be enzymatically converted to LTB4 or to the cysteinyl-containing LTC4, D4 and E4. LTs have been implicated in inflammatory and allergic disorders [2, 3], and there is accumulating evidence for a critical role for the 5-LO pathway in cardiovascular diseases and various types of cancer [2–4]. Therefore, blockade of LT receptors and inhibition of the 5-LO pathway are major pharmacological concepts for intervention with LT-associated diseases [4, 5]. Pharmacological 5-LO inhibitors can be categorized into three groups: (1) redox-active compounds that reduce the active-site iron, (2) iron-chelators, and (3) the nonredox-type 5-LO inhibitors which probably interfere with putative fatty acid-binding sites [6]. Unfortunately, despite strong efforts in the development of 5-LO inhibitors, most potential candidates failed due to lack of efficacy in clinical studies or due to severe side effects [7, 8].
Upon adequate cell stimulation, 5-LO is activated by an elevation of the intracellular Ca2+ concentration ([Ca2+]i) and phosphorylations by members of the mitogen-activated protein kinase (MAPK) family, leading to redistribution of 5-LO from a soluble cellular locale to the nuclear membrane, where liberated AA is provided to the enzyme via the 5-LO-activating protein (FLAP). Additional factors stimulating 5-LO activity are Mg2+, ATP, glycerides or phospholipids (PLs), lipid hydroperoxides, interaction with coactosin-like protein (CLP), and presumably with additional proteins [6, 9]. Recently, we found that 5-LO in human neutrophils is regulated by androgens via extracellular signal-regulated kinases, resulting in higher 5-LO product synthesis in females versus males [10]. Previous studies showed that factors influencing the activation of 5-LO such as the redox tone and phosphorylations may have strong impact on the efficacy of nonredox-type 5-LO inhibitors [11–13]. 5-LO consists of a C-terminal catalytic domain that contains the iron, and an N-terminal C2-like β-barrel domain with regulatory functions that binds two Ca2+ ions [14] and targets 5-LO to the nuclear membrane via three tryptophan residues (Trp13, -75, and -102) with selectivity for phosphatidylcholine (PC) [15]. These tryptophan residues are also required for the physical and functional interaction of 5-LO with CLP which supports Ca2+-induced 5-LO activity in the presence of PC [16]. Agents that affect 5-LO product formation by interference with this regulatory C2-like domain are thus far unknown.
The acylphloroglucinol hyperforin, a pharmacologically active constituent of St. John’s wort (Hypericum perforatum), was found to suppress the formation of LTs in vitro by inhibiting 5-LO [17]. Thus, hyperforin blocked 5-LO activity in intact cells and in cell-free systems by a hitherto unrecognized mode of action [17]. Here, we addressed the efficacy of hyperforin to intervene with LT formation and inflammation in vivo, and we investigated the biochemical mechanisms of the inhibition of 5-LO product formation by hyperforin.
Materials and methods
Materials
Hyperforin, ZM230487, and BWA4C were generous gifts by Schwabe (Karlsruhe, Germany), by Dr. R. M. McMillan (Zeneca Pharmaceuticals, Macclesfield, UK), and by Dr. L.G. Garland (Wellcome Research Laboratories), respectively. The GST-CLP fusion protein linked to glutathione-Sepharose 4B beads (20 µl of 50% slurry) was expressed and prepared as described [16]. For animal studies, hyperforin, zileuton, and indomethacin were dissolved in DMSO and diluted with saline achieving a final DMSO concentration of 4%. Materials and sources: AA, glutathione, ionomycin, ionophore A23187, 1-oleoyl-2-acetyl-sn-glycerol (OAG), PC, phosphatidylethanolamine (PE) (Sigma, Deisenhofen, Germany); HPLC solvents (Merck, Darmstadt, Germany); λ-carrageenan type IV isolated from Gigartina aciculaire and Gigartina pistillata and indomethacin (Sigma-Aldrich, Milan, Italy); zileuton (Sequoia Research Products, Oxford, UK); enzyme immunoassay (Cayman Chemical, Inalco, Milan, Italy).
Animals
Male Wistar Han rats (190–200 g; Harlan, Milan, Italy) were housed in a controlled environment and provided with standard rodent chow and water. Animal care complied with Italian regulations on protection of animals used for experimental and other scientific purpose (Ministerial Decree 116192) as well as with the European Economic Community regulations (Official Journal of E.C. L 358/1 12/18/1986).
Carrageenan-induced pleurisy in rats
Hyperforin (4 mg/kg), indomethacin (5 mg/kg); or zileuton (10 mg/kg) were given i.p. 30 min before carrageenan. A group of rats received the vehicle (DMSO, 4%, i.p.) 30 min before carrageenan. Rats were anaesthetized with enflurane 4% mixed with O2, 0.5 l/min and N2O 0.5 l/min and submitted to a skin incision at the level of the left sixth intercostal space. The underlying muscle was dissected, and saline (0.2 ml) or λ-carrageenan type IV 1% (w/v) (0.2 ml) was injected into the pleural cavity. The skin incision was closed with a suture, and the animals were allowed to recover. At 4 h after the injection of carrageenan, the animals were killed by inhalation of CO2. The chest was carefully opened, and the pleural cavity was rinsed with 2 ml saline solution containing heparin (5 U/ml). The exudate and washing solution were removed by aspiration, and the total volume was measured. Any exudate that was contaminated with blood was discarded. The amount of exudate was calculated by subtracting the volume injected (2 ml) from the total volume recovered. Leukocytes in the exudate were resuspended in PBS and counted with an optical light microscope in a Burker’s chamber after vital trypan blue staining.
The amount of LTB4 in the supernatant of centrifuged exudate (800g for 10 min) was assayed by enzyme immunoassay according to manufacturer’s protocol. The results are expressed as nanograms per rat and represent the mean ± S.E. of 10 rats.
Cells and plasmids
Human neutrophils were freshly isolated from leukocyte concentrates obtained at St Markus Hospital, Frankfurt, and at the Blood Center, University Hospital, Tuebingen, Germany. In brief, venous blood was taken from healthy adult donors and subjected to centrifugation at 4,000g for 20 min at 20°C for preparation of leukocyte concentrates. Neutrophils were promptly isolated by dextran sedimentation, centrifugation on Nycoprep cushions (PAA Laboratories, Linz, Austria), and hypotonic lysis of erythrocytes as described previously [18]. Neutrophils (5 × 106 cells/ml; purity >96–97%) were finally resuspended in phosphate-buffered saline pH 7.4 (PBS) plus 1 mg/ml glucose and 1 mM CaCl2 (PGC buffer).
Site-directed mutagenesis of the pT3-5LO plasmid, encoding wild type 5-LO (wt-5-LO), using the QuickChange™ kit from Stratagene yielded the mutated 5-LO plasmid pT3-5LO-W13A-W75A-W102A, referred to as 3W mut-5LO [19]. E. coli MV1190 was transformed with pT3-5LO or 3W mut-5LO plasmids, and recombinant 5-LO proteins were expressed at 27°C as described [14].
Preparation of homogenates, 100,000×g supernatants and purification of 5-LO proteins
E.coli were lysed by incubation in 50 mM triethanolamine/HCl pH 8.0, 5 mM EDTA, soybean trypsin inhibitor (60 µg/ml), 1 mM phenylmethylsulphonyl fluoride (PMSF) and lysozyme (500 µg/ml), homogenized by sonication (3 × 15 s), and centrifuged at 19,000g for 15 min. Proteins including 5-LO were precipitated with 50% saturated ammonium sulfate during stirring on ice for 60 min. The precipitate was collected by centrifugation (16,000g, 25 min) and the pellet was resuspended in 20 ml PBS containing 1 mM EDTA and 1 mM PMSF. For purification of 5-LO from human neutrophils, 5 × 108 cells were resuspended in 10 ml PBS containing 1 mM EDTA and 1 mM PMSF. After cooling down on ice for 10 min, cells were homogenized by sonication (3 × 10 s).
Whole homogenates of neutrophils or the resuspended 16,000g precipitate from E.coli were centrifuged at 100,000g for 70 min at 4°C, and the 100,000g supernatant (S100) was applied to an ATP-agarose column to partially purify 5-LO as described previously [20]. S100 or purified 5-LOs were immediately used for 5-LO activity assays.
Determination of 5-LO product formation in intact cells
For assays of intact cells, 5 × 106 freshly isolated neutrophils were finally resuspended in 1 ml PGC buffer. After pre-incubation with the indicated amounts of hyperforin at 37°C, 5-LO product formation was started by addition of 1 µM ionomycin plus exogenous AA as indicated. After 10 min at 37°C, the reaction was stopped with 1 ml of methanol and 30 µl of 1 N HCl, 200 ng prostaglandin B1 and 500 µl of PBS were added. Formed 5-LO metabolites were extracted and analyzed by HPLC as described [18]. 5-LO product formation is expressed as ng of 5-LO products per 106 cells which includes LTB4 and its all-trans isomers, 5(S),12(S)-di-hydroxy-6,10-trans-8,14-cis-eicosatetraenoic acid (5(S),12(S)-DiHETE), and 5(S)-hydro(pero)xy-6-trans-8,11,14-cis-eicosatetraenoic acid (5-H(P)ETE). Cysteinyl LTs (LTC4, D4 and E4) were not detected and oxidation products of LTB4 were not determined.
Determination of 5-LO product formation in cell-free systems
Aliquots of whole homogenates or S100, corresponding to 5 × 106 neutrophils, or alternatively 0.5 µg partially purified recombinant 5-LO, were diluted with ice-cold PBS containing 1 mM EDTA, and 1 mM ATP as well as additional proteins, as indicated. Samples were pre-incubated with the test compounds and with additional reagents [e.g., glutathione (GSH), OAG or PLs] as indicated. After 5–10 min at 4°C, samples were pre-warmed for 30 s at 37°C and 2 mM CaCl2 and AA at the indicated concentrations were added to start 5-LO product formation. The reaction was stopped after 10 min at 37°C by addition of 1 ml ice-cold methanol and the formed metabolites were analyzed by HPLC as described for intact cells.
Subcellular localization of 5-LO
Subcellular localization of 5-LO in neutrophils was investigated as described previously [21]. In brief, freshly isolated neutrophils (3 × 107) in 1 ml PGC buffer were pre-treated for 15 min at 37°C with DMSO, hyperforin, ZM230487, or BWA4C as indicated, incubated with 1 µM ionomycin for 10 min and chilled on ice. Nuclear and non-nuclear fractions were obtained after cell lysis by 0.1% NP-40. Aliquots of these fractions were analyzed for 5-LO protein by SDS-PAGE and immunoblotting using anti-5-LO antiserum (AK7, 1551; affinity purified on a 5-LO column).
Indirect immunofluorescence microscopy
Human neutrophils (1.5 × 106 in 500 μl PGC buffer) were incubated at 37°C for 15 min with hyperforin or DMSO (solvent). Cells were then centrifuged at 30g for 1 min onto poly-l-lysine (MW 150,000–300,000; Sigma-Aldrich)-coated glass coverslips in the wells of a 12-well plate, and activated by addition of 1 µM ionomycin for 3 min at 37°C. Cells were fixed in methanol (−20°C, 30 min) and permeabilized in acetone (−20°C, 3 min), followed by two wash steps with PBS. The staining was performed by incubating the coverslips with the anti-5-LO serum (1551, AK-7) for 30 min at RT. The coverslips were then washed five times with PBS, incubated with Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen; diluted 1:300 in PBS) for 10 min at RT in the dark, and washed five times with PBS. The DNA was stained with 0.1 µg/ml diamidino-2-phenylindole (DAPI) in PBS for 3 min at RT in the dark. The coverslips were then washed twice and mounted on glass slides with Mowiol (Calbiochem) containing 2.5% n-propyl gallate (Sigma). The fluorescence was visualized with a Zeiss Axiovert 200 M microscope.
GST-pull down assay
For 5-LO-CLP binding studies in vitro, 20 µg of the GST-CLP fusion protein linked to glutathione-Sepharose 4B beads (20 µl of 50% slurry) was incubated with purified 5-LO protein (5 µg) in the presence of BSA (50 µg) and hyperforin, as indicated, in 200 µl of buffer A (2 mM Tris–Cl pH 8.0, 0.2 mM ATP, 0.2 mM CaCl2, 2 mM MgCl2, 50 mM KCl, 0.5 mM β-mercaptoethanol). After 30 min gentle rotation at RT, beads were washed five times in buffer A (without BSA). Bound proteins were eluted from the beads with 200 µl of elution buffer (10 mM GSH in 50 mM Tris–Cl, pH 8.0), for 60–90 min at RT. Beads were sedimented, and aliquots of the supernatant eluate were mixed with the same volume of SDS loading buffer. After heating at 95°C for 5 min, 5-LO in the eluates was assayed by SDS-PAGE and Western blot.
SDS-PAGE and immunoblotting
Aliquots of nuclear or non-nuclear fractions, or pull-downs (20 µl each) were mixed with 4 µl of glycerol/0.1% bromphenolblue (1:1, vol/vol) and analyzed by SDS-PAGE on a 10% gel. After electroblot to nitrocellulose membrane (Amersham Pharmacia), blocking with 5% non-fat dry milk for 1 h at RT, membranes were washed and incubated with primary antibody against 5-LO (1551, AK 7) overnight at 4°C. The membranes were washed and incubated with 1:1,000 dilution of alkaline phosphatase-conjugated IgGs (Sigma) for 2 h at RT. After washing, proteins were visualized with nitro blue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate (Sigma) in detection buffer (100 mM Tris/HCl, pH 9.5, 100 mM NaCl, 5 mM MgCl2).
Automated docking
Automated molecular docking of compounds was performed using GOLD 2.2., which relies on a genetic algorithm [22]. We used a refined version of our homology model of 5-LO as target [23], where several potential binding pockets were manually selected. GOLD parameter settings for the genetic algorithm were: number of generations = 100,000, population size = 100, selection pressure = 1.1, number of islands = 5, niche size = 2, migrate = 10, mutate = 95, crossover = 95. A 14-Å radius around each binding site center defined the binding pockets. The default Chemscore function was employed for scoring the predicted receptor–ligand complexes. Larger positive score values indicate more favorable receptor–ligand complexes and negative values indicate unfavorable binding modes (non-binding). Each docking run was repeated ten times to obtain average score values and standard deviations. PyMOL was used for visualization of docking poses [24].
Statistics
Statistical evaluation of the data was performed by one-way ANOVA followed by a Bonferroni post-hoc test for multiple comparisons. A P value < 0.05 was considered significant.
Results
Hyperforin inhibits LTB4 generation and the inflammatory response in rats in vivo
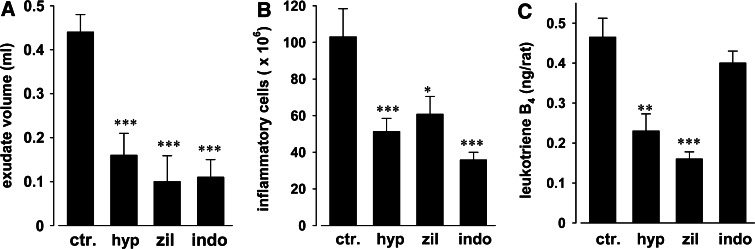
We evaluated the biological relevance of the hyperforin-dependent 5-LO inhibition in vivo by the use of the carrageenan-induced rat pleurisy model. Hyperforin (4 mg/kg, i.p.) significantly inhibited the inflammatory reaction by reducing exudate volume (64%; Fig. 1a), migrating cell number (50%, Fig. 1b) as well as LTB4 generation (50%, Fig. 1c) in the inflammatory exudates 4 h after carrageenan challenge. Zileuton (10 mg/kg, i.p.), a well-recognized 5-LO inhibitor [25], also reduced exudate formation, cell infiltration, and LTB4 levels in the exudates (77, 41, and 65%, respectively) (Fig. 1), and its anti-inflammatory action was not significantly different from that of hyperforin. In contrast, the COX inhibitor indomethacin (5 mg/kg, i.p.), which also reduced exudate volume and number of inflammatory cells to a similar extent as hyperforin, failed to significantly suppress LTB4 levels (Fig. 1).
Fig. 1.
Hyperforin inhibits LTB4 generation in pleural exudates from carrageenan-treated rats. At 30 min before intrapleural injection of carrageenan, rats (n = 10 for each experimental group) were treated i.p. with 4 mg/kg hyperforin (hyp), 10 mg/kg zileuton (zil), 5 mg/kg indomethacin (indo) or vehicle (DMSO 4%, ctr). a Exudate volume, b inflammatory cell accumulation in pleural cavity, and c LTB4 pleural exudate levels were assessed 4 h after carrageenan injection. Data are expressed as mean ± SEM, n = 10. *P < 0.05, **P < 0.01, ***P < 0.001 versus control
Cellular components compromise 5-LO inhibition by hyperforin
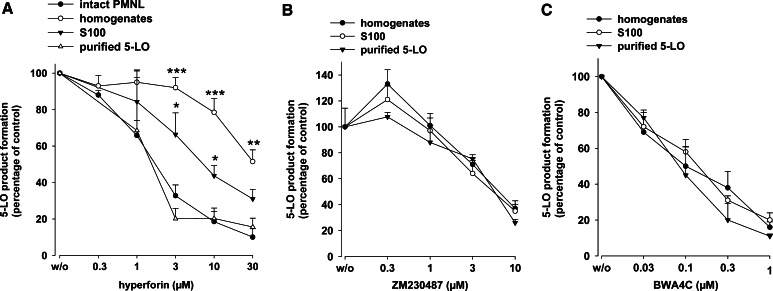
We attempted to investigate the molecular pharmacological profile of hyperforin in more detail using various cell-based and cell-free assays. In agreement with our previous study [17], preincubation (15 min) of freshly isolated neutrophils with hyperforin inhibited 5-LO product synthesis induced by 1 µM ionomycin plus 20 µM of exogenous AA (IC50 = 1.9 µM, Fig. 2a). Exogenous AA was included to circumvent the need of endogenous substrate supply by cPLA2 and, thus, to exclude interference at this level. There was no obvious difference in the efficacy of hyperforin regardless of the presence or absence of exogenous AA. Analysis of the integrity of neutrophils using trypan blue staining and light microscopy indicated no loss of cellular viability during the incubations in the presence of hyperforin. Of interest, the potency of hyperforin for inhibition of 5-LO activity in corresponding cell homogenates was strongly attenuated (IC50 ≈ 30 µM). Removal of the particulate fraction (membranes) by centrifugation (100,000g, 70 min) of cell homogenates, partially restored the potency of hyperforin (IC50 approx. 7 µM; Fig. 2a). The effectiveness of hyperforin was even more improved when partially purified human recombinant 5-LO was analyzed (IC50 = 1.6 µM). Therefore, endogenous cellular components seem to impair the inhibitory effect of hyperforin on 5-LO.
Fig. 2.
Inhibition of 5-LO activity by hyperforin, ZM230487 and BWA4C. For determination of 5-LO product formation in intact cells, freshly isolated neutrophils (5 × 106) were resuspended in 1 ml PGC and hyperforin or vehicle (DMSO) was added at the indicated concentrations. After 15 min at 37°C, cells were stimulated with ionomycin (1 µM) and AA (20 µM), incubated for another 10 min, and 5-LO product formation was determined. For determination of 5-LO product formation in cell-free assays, homogenates and S100, corresponding to 5 × 106 neutrophils, each, or 0.5 µg of partially purified human recombinant 5-LO expressed in E.coli were pre-incubated in 1 ml PG buffer plus 1 mM EDTA with hyperforin (a), ZM230487 (b), or BWA4C (c) at the indicated concentrations or with vehicle (DMSO) at 4°C. ATP (1 mM) was added, and after 5–10 min, samples were prewarmed at 37°C for 30 s, and 5-LO product formation was started by addition of 2 mM CaCl2 and 20 µM AA. After another 10 min, 5-LO product formation was determined. The 100% values correspond to 116 ± 23 (intact cells), 78 ± 16 (homogenates), and 51 ± 12 (S100) ng 5-LO products per 106 cells; for partially purified 5-LO, the 100% value was 5.2 ± 1.1 µmol per mg protein. Values are given as mean + SE, n = 3–5; ***P < 0.001, **P < 0.01, *P < 0.05 versus purified 5-LO at corresponding concentrations of hyperforin
BWA4C and ZM230487, highly selective and potent iron-ligand type and nonredox-type 5-LO inhibitors, respectively, were used for comparison. In contrast to hyperforin, the potency of BWA4C or ZM230487 for inhibition of crude 5-LO in cell-free systems was independent of the assay conditions. Thus, in agreement with our previous findings [12, 13, 26], the IC50 values of the respective inhibitors were almost the same for 5-LO enzyme activity in whole cell homogenates, S100, or for partially purified 5-LO (Fig. 2b, c).
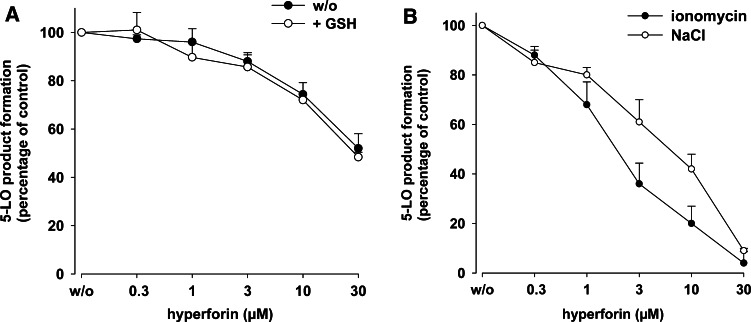
Inhibition of 5-LO by hyperforin is independent of the redox tone and the MAPK pathway
Elevated peroxide levels and 5-LO phosphorylation by MAPK impair the potency of the nonredox-type 5-LO inhibitors ZM230487 and L-739.010 [12, 13], and they require glutathione peroxidase activity for inhibition of 5-LO [13]. To evaluate whether the inhibitory properties of hyperforin resemble those of nonredox-type inhibitors, we investigated if reducing conditions may improve 5-LO inhibition by hyperforin in homogenates. However, in contrast to nonredox-type 5-LO inhibitors [13], no change in the efficacy of hyperforin was apparent when homogenates were supplemented with 1 mM GSH in order to reconstitute glutathione peroxidase activity (Fig. 3a), implying that 5-LO inhibition by hyperforin is not affected by the redox tone. Previous studies also showed that the potency of nonredox-type 5-LO inhibitors was higher in ionomycin-stimulated neutrophils where 5-LO activity is evoked by elevation of [Ca2+]i, as compared to 5-LO activation initiated by cellular stress (e.g., hyperosmotic shock by applying 300 mM NaCl) involving p38MAPK-dependent phosphorylation events [12]. However, the potency of hyperforin is comparable for 5-LO inhibition in neutrophils stimulated by 300 mM NaCl or ionomycin (Fig. 3b). Together, hyperforin does not exhibit the typical properties of nonredox-type 5-LO inhibitors.
Fig. 3.
Effects of GSH and cellular stress on 5-LO inhibition by hyperforin. a Effects of GSH. Homogenates, corresponding to 5 × 106 neutrophils, were pre-incubated in 1 ml PG buffer plus 1 mM EDTA with hyperforin or with vehicle (DMSO) at 4°C. ATP (1 mM) and 1 mM GSH was added as indicated, and after 5–10 min, samples were prewarmed at 37°C for 30 s and 5-LO product formation was started by addition of 2 mM CaCl2 and 20 µM AA. After another 10 min, 5-LO product formation was determined. The 100% values correspond to 74 ± 11 and 102 ± 20 ng 5-LO products per 106 cells in the absence and in the presence of GSH, respectively. b Effects of cellular stress. Freshly isolated neutrophils (5 × 106) were resuspended in 1 ml PGC buffer and hyperforin or vehicle (DMSO) was added at the indicated concentrations. After 15 min at 37°C, cells were either preincubated with 300 mM NaCl for 5 min and AA (20 µM) was added, or stimulated with ionomycin (1 µM) together with AA (20 µM). After 10 min incubation, 5-LO product formation was determined. The 100% values correspond to 109 ± 15 (ionomycin) and 54 ± 8 (NaCl) ng 5-LO products per 106 cells. Values are given as mean + SE, n = 3–5
Evaluation of binding of hyperforin to the active site region of 5-LO
Automated molecular docking, using a refined version of a 5-LO homology model [23], was performed in order to probe hyperforin in comparison with BWA4C and ZM230487 in the active site of 5-LO. BWA4C is expected to bind to the active site of 5-LO [27] whereas ZM230487 might bind also to a distinct site [13]. In the active site region, BWA4C and ZM230487 yielded scores of 27 ± 0.1 and 22 ± 2.5, respectively. In contrast, hyperforin obtained a negative average score of −9 ± 1, indicating unfavorable binding to the active site of 5-LO. To estimate this score value of hyperforin, we performed an additional re-docking study with the known X-ray complex of the pregnane X receptor (PXR) and hyperforin (PDB-identifier: 1M13), where hyperforin was successfully re-docked to PXR (K i = 27 nM [28]) with a score of 57 ± 0.5, forming the essential interactions with the target as determined by X-ray crystallography [28].
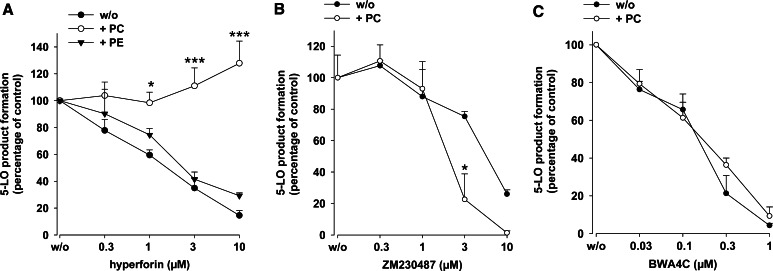
Phospholipids counteract 5-LO inhibition by hyperforin
Since the efficacy of hyperforin was markedly impaired in homogenates where PL-containing membranes are present, we tested whether exogenous addition of PLs to partially purified 5-LO (essentially devoid of PLs [19]) could mimic this effect and, thus, attenuate 5-LO inhibition by hyperforin. In fact, hyperforin (up to 30 µM) failed to block the activity of partially purified 5-LO in the presence of PC (100 µg/ml) at 20 µM AA (Fig. 4a). In agreement with our previous report [17], hyperforin also efficiently blocked 5-LO activity at 5 or 40 µM AA, and again, PC abolished 5-LO inhibition (not shown). Of interest, PE (100 µg/ml), in contrast to PC, did not impair 5-LO inhibition by hyperforin. When ZM230487 or BWA4C were assayed for inhibition of partially purified 5-LO, the presence of PC (100 µg/ml) slightly enhanced the efficacy of ZM230487 (Fig. 4b) and was without effect on 5-LO inhibition by BWA4C (Fig. 4c).
Fig. 4.
Phosphatidylcholine reverses 5-LO inhibition by hyperforin. Partially purified human recombinant 5-LO (0.5 µg) expressed in E.coli was pre-incubated in 1 ml PG buffer plus 1 mM EDTA with hyperforin (a), ZM230487 (b), or BWA4C (c) at the indicated concentrations or with vehicle (DMSO) at 4°C with or without PC or PE (100 µg/ml, each) as given in the figure. ATP (1 mM) was added, and after 5–10 min, samples were pre-warmed at 37°C for 30 s and 5-LO product formation was started by addition of 2 mM CaCl2 and 20 µM AA. After another 10 min, 5-LO product formation was determined. Values are given as mean + SE, n = 3–4; ***P < 0.001, *P < 0.05 versus controls without phospholipids (w/o) at corresponding concentrations of inhibitors
Mutation of the PC-binding tryptophan residues within the 5-LO C2-like domain impairs the potency of hyperforin
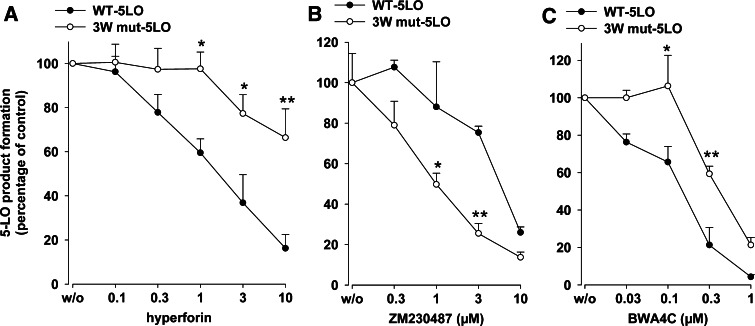
Selective binding of the 5-LO C2-like domain to PC requires three tryptophan residues (Trp13, -75, and -102) located within the C2-like domain [15]. Since PC counteracted the effect of hyperforin, it appeared reasonable that hyperforin may interfere with 5-LO activity via the PL-binding site of 5-LO. Thus, a mutant 5-LO, where these tryptophans had been replaced by alanine (3W mut-5LO), was constructed. The expression levels for both wt- and 3W mut-5LO were comparable, as assessed by Western blotting (not shown). Under our standard assay conditions (0.5 µg 5-LO protein in 1 ml PBS pH 7.4 containing 1 mM EDTA, 1 mM ATP, 2 mM CaCl2, and 20 µM AA) the molecular turnover rates of wt-5LO were 5.5 ± 0.9 s−1 in the absence and 16.0 ± 3.1 s−1 in the presence of PC (10 µg/ml), respectively. For 3W mut-5LO, the activities were reduced and the respective values were 1.67 ± 0.31 and 0.92 ± 0.15 s−1. Hyperforin potently inhibited the activity of wt-5LO with an IC50 of 1.6 µM, but the activity of the 3W mut-5LO was much less sensitive to hyperforin (IC50 > 10 µM, Fig. 5a). Note that the 3W mut-5LO showed significantly higher sensitivity towards inhibition by ZM230487, whereas for BWA4C, a slightly reduced efficacy was obvious, as compared to WT-5LO (Fig. 5a).
Fig. 5.
Inhibition of 3W mut-5LO activity by hyperforin. Human recombinant wt-5LO or mutated 3W mut-5LO (0.5 µg each) were expressed in E.coli, partially purified and pre-incubated in 1 ml PG buffer plus 1 mM EDTA with hyperforin, ZM230487, or BWA4C at the indicated concentrations or with vehicle (DMSO) at 4°C. ATP (1 mM) was added, and after 5–10 min, samples were prewarmed at 37°C for 30 s and 5-LO product formation was started by addition of 2 mM CaCl2 and 20 µM AA. After another 10 min, 5-LO product formation was determined. Values are given as mean + SE, n = 3–4; **P < 0.01, *P < 0.05 versus WT-5LO at corresponding concentrations of inhibitors
1-Oleoyl-2-acetylglycerol impairs 5-LO inhibition by hyperforin
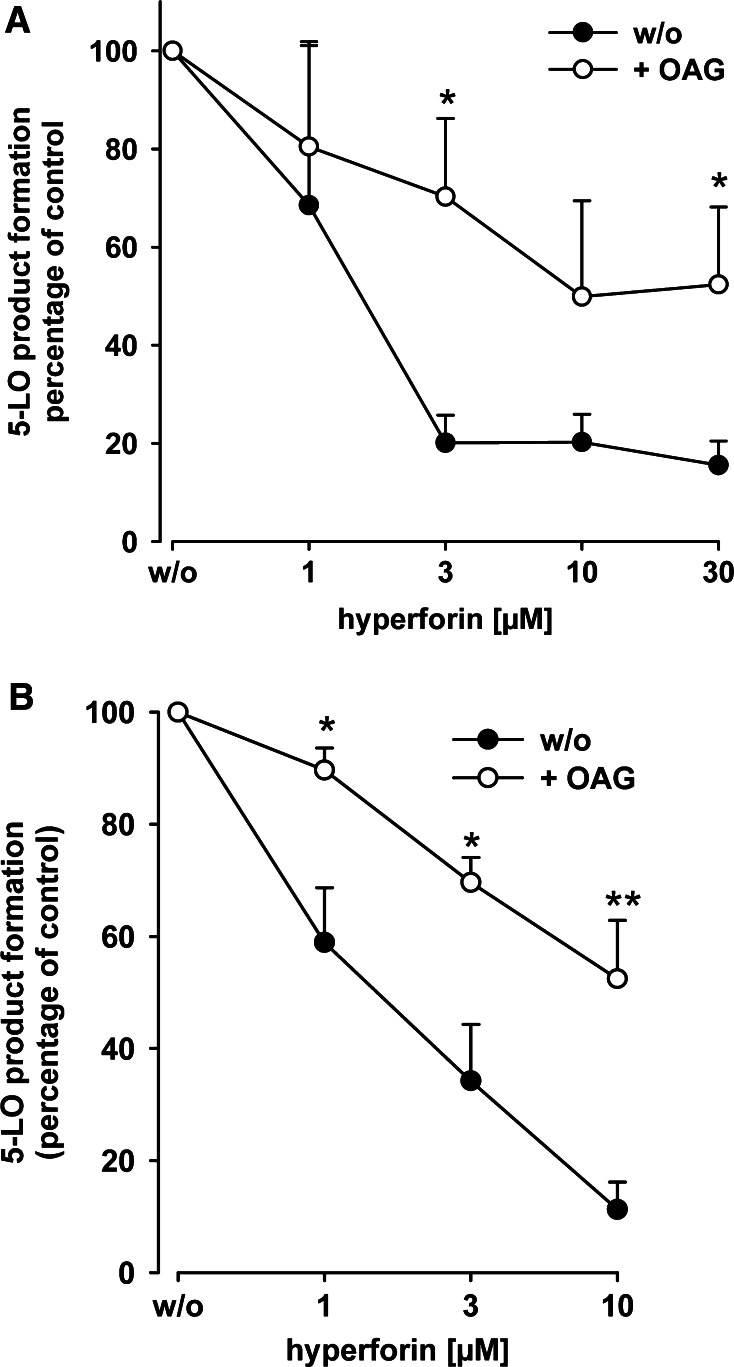
We found that OAG induces 5-LO product formation in intact neutrophils [29], apparently by stimulating 5-LO activity via the PL-binding site within the C2-like domain [19]. As shown in Fig. 6, OAG antagonized inhibition of partially purified 5-LO by hyperforin (Fig. 6a), but also in intact neutrophils, and the potency of hyperforin was reduced by OAG (Fig. 6b; Table 1). On the other hand, OAG did not markedly affect the efficacy of BWA4C or ZM230487 (Table 1).
Fig. 6.
1-Oleoyl-2-acetyl-sn-glycerol (OAG) reduces the potency of hyperforin to inhibit 5-LO activity. a Effects of OAG on hyperforin-mediated 5-LO inhibition in intact neutrophils. Freshly isolated neutrophils (5 × 106) were resuspended in 1 ml PGC and pre-incubated with hyperforin (or DMSO as vehicle) for 15 min at 37°C at the indicated concentrations. OAG (30 µM) or solvent (DMSO, negative control) was added and, after another 3 min, neutrophils were challenged by ionomycin (1 µM) and AA (20 µM). The reaction was stopped after another 10 min and 5-LO product formation was determined. The 100% values correspond to 103 ± 6 (ionomycin) and 142 ± 31 (ionomycin + OAG) ng 5-LO products per 106 cells. b Partially purified human recombinant 5-LO (0.5 µg) expressed in E.coli was pre-incubated in 1 ml PG buffer plus 1 mM EDTA with hyperforin at the indicated concentrations or with vehicle at 4°C with or without 30 µM OAG. ATP (1 mM) was added, and, after 5–10 min, samples were prewarmed at 37°C for 30 s and 5-LO product formation was started by addition of 2 mM CaCl2 and 20 µM AA. After 10 min, 5-LO product formation was determined. The 100% values correspond to 5.3 ± 0.6 (w/o) and 4.4 ± 0.3 (+ OAG) µmol 5-LO products per mg protein. Values are given as mean + SE, n = 5; **P < 0.01, *P < 0.05 versus controls without OAG (w/o) at corresponding concentrations of hyperforin
Table 1.
Influence of 1-oleoyl-2-acetyl-sn-glycerol (OAG) on the efficacy of hyperforin, BWA4C, and ZM230487. Intact neutrophils and partially purified 5-LO were preincubated with the compounds and 5-LO products were analyzed as described in the legend of Fig. 6
| Compound | IC50 (µM) (intact cells) | IC50 (µM) (cell-free) | ||
|---|---|---|---|---|
| −OAG | +OAG | −OAG | +OAG | |
| Hyperforin | 1.8 | ≥10 | 1.4 | ≥10 |
| BWA4C | 0.09 | 0.14 | 0.05 | 0.06 |
| ZM230487 | 0.018 | 0.025 | 7.4 | 6.9 |
IC50 values were calculated by nonlinear regression using SigmaPlot 9.0 (Systat Software, San Jose, USA). Values are given as means, n = 3–5
Hyperforin interrupts the interaction of 5-LO and CLP
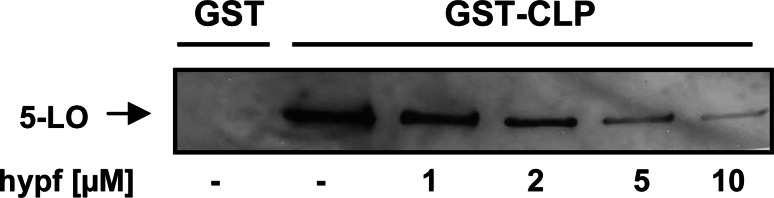
The functional interaction between CLP and 5-LO requires Trp13,- 75, and -102 within the 5-LO C2-like domain [16]. We tested whether hyperforin could interrupt the interaction between 5-LO and CLP using a pull-down assay [16]. As found before [16, 30], 5-LO was bound to GST-CLP (attached to GSH-sepharose beads) but not to GST (Fig. 7). Inclusion of hyperforin reduced the amounts of precipitated 5-LO starting at approx. 1–3 µM hyperforin, indicating that the compound displaced 5-LO from the immobilized CLP. We previously showed that CLP together with a low concentration of PC (2.5–25 µg/ml) upregulated LT production [16]. Here, we analyzed whether hyperforin could suppress the CLP-mediated enhancement of LTA4 synthesis (expressed as LTB4 isomers) more efficiently than 5-H(P)ETE formation. In the absence of both CLP and PC, hyperforin (10 µM) gave 83% inhibition of 5-LO total product formation (Table 2; compare Fig. 2). In the presence of low PC (25 µg/ml), this was reduced to 36% inhibition, and hyperforin reduced LTA4 and 5-HPETE synthesis quite similarly (Table 2). However, in the presence of both PC and CLP, the effect of CLP to upregulate LTA4 synthesis was more efficiently inhibited by hyperforin as compared to 5-H(P)ETE synthesis or 5-LO total product formation.
Fig. 7.
Hyperforin interrupts the interaction between 5-LO and CLP. Purified human recombinant 5-LO (5 µg) was incubated with 20 µg of either GST (neg. control) or GST-CLP fusion protein linked to glutathione-Sepharose 4B beads in presence of BSA (50 µg) and hyperforin, as indicated. After 30 min incubation, washing, and elution of the proteins with the same volume of SDS loading buffer, 5-LO was assayed by SDS-PAGE and Western blot. Similar results were obtained in three additional independent experiments
Table 2.
Effects of hyperforin on the 5-LO product profile. Partially purified human recombinant 5-LO (1 µg) was assayed for product formation from 100 µM AA with (1 µg/ml) or without CLP, with (25 µg/ml) or without PC, and in the presence or absence of 10 µM hyperforin. Values (µmol 5-LO products per mg protein) are given as means, n = 3. LTs, all-trans isomers of LTB4
| Condition | 5-LO product formation (μmol/mg) | |||||
|---|---|---|---|---|---|---|
| − | + hyperforin | % inhibition | ||||
| LTs | 5-H(P)ETE | LTs | 5-H(P)ETE | LTs | 5-H(P)ETE | |
| − | 0.5 | 3.5 | – | 0.7 | – | 80% |
| 83% (total prod.) | ||||||
| +CLP | – | 13 | – | 5.9 | – | 55% |
| +PC | 3.5 | 16.2 | 1.9 | 10.8 | 46% | 33% |
| 36% (total prod.) | ||||||
| +CLP + PC | 7.6 | 11.2 | 2.1 | 6.0 | 72% | 46% |
| 57% (total prod.) | ||||||
Hyperforin blocks the translocation of 5-LO to the nuclear membrane in activated neutrophils
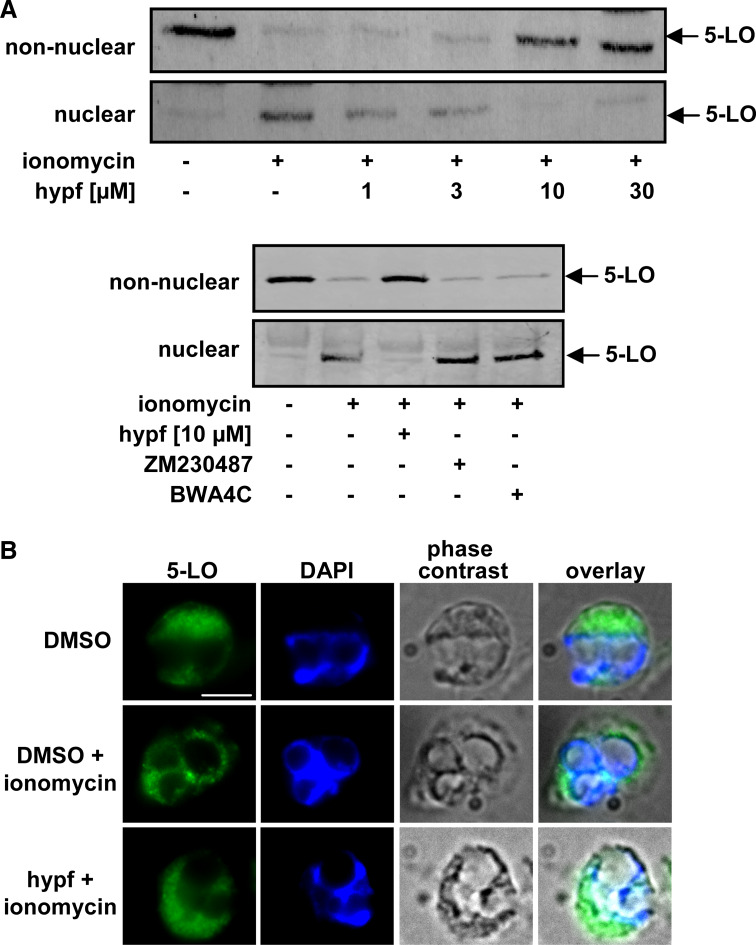
Upon neutrophil activation, 5-LO can associate with a nuclear membrane-bound compartment, which is governed by the PC-selective binding of the C2-like domain of 5-LO [6, 15]. We determined whether hyperforin interferes with subcellular redistribution of 5-LO in ionomycin-stimulated neutrophils. The subcellular localization of 5-LO was assessed by subcellular fractionation using mild detergent lysis (0.1% NP-40) and immunodetection in nuclear and non-nuclear fractions. Ionomycin caused accumulation of 5-LO in the nuclear fraction (see also [21]), with a concomitant loss in the non-nuclear fraction. Hyperforin concentration-dependently attenuated the ionomycin-induced redistribution of 5-LO, with significant effects at 1 µM (Fig. 8a). BWA4C or ZM230487 did not significantly affect 5-LO translocation (Fig. 8a). Similar results for 5-LO redistribution were obtained by indirect IF microscopy (Fig. 8b). Thus, in resting neutrophils, 5-LO was distributed within the cytosol, but following ionophore, 5-LO accumulated around the nucleus, which was clearly prevented by hyperforin.
Fig. 8.
Hyperforin inhibits nuclear translocation of 5-LO. a Subcellular fractionation and immunodetection of 5-LO. Freshly isolated neutrophils (3 × 107 in 1 ml PGC buffer) were preincubated with hyperforin at the indicated concentrations, BWA4C (1 µM), ZM230487 (10 µM) or with vehicle (DMSO) for 15 min at 37°C. Then, 1 µM ionomycin or vehicle (DMSO, unstimulated cells) was added to the samples and incubated for another 5 min at 37°C. 5-LO was detected in nuclear and non-nuclear fractions by immunoblotting after subcellular fractionation. Similar results were obtained in three additional independent experiments. b Indirect immunofluorescence microscopy. Neutrophils were pre-incubated with 10 µM hyperforin or DMSO (vehicle) as indicated, centrifuged onto poly-l-lysine-coated glass coverslips, and activated by ionomycin (1 µM). After 3 min, cells were fixed, permeabilized, and incubated with anti-5-LO serum (1551, AK-7). After addition of Alexa Fluor 488 goat anti-rabbit IgG, the fluorescence was analyzed. Single staining for 5-LO (green), DNA-stain (diamidino-2-phenylindole, DAPI, blue), phase contrast, and merged pictures are shown. Scale bar 5 µm. The slides shown are representatives out of three similar samples
Discussion
We showed that hyperforin inhibits 5-LO product synthesis in vitro and in vivo, associated with potent anti-inflammatory activity. Hyperforin directly inhibits 5-LO catalysis, but also interrupts regulatory functions of the C2-like domain such as the stimulatory interaction with CLP and 5-LO subcellular trafficking. We suggest that the 5-LO inhibitory action of hyperforin involves the C2-like domain, supported by the following findings: (1) PC and OAG (that both interact at the 5-LO C2-like domain [15, 19]), but not PE (that fails in this respect) reverse 5-LO inhibition by hyperforin; (2) the 3W mut-5LO lacking three tryptophan residues that are essential for functionality of the 5-LO C2-like domain [15] is hardly susceptible to hyperforin; and (3) hyperforin interrupts the binding between 5-LO and CLP in vitro and prevents 5-LO nuclear membrane association in neutrophils, processes that are both conferred by the C2-like domain [15, 16, 31]. Comparative studies with the nonredox-type 5-LO inhibitor ZM230487 and the iron-ligand BWA4C support the unique inhibitory profile of hyperforin on 5-LO product formation and translocation.
The biological relevance of hyperforin-dependent 5-LO inhibition is clearly demonstrated by the carrageenan-induced pleurisy in rats, a suitable model for assessing the anti-inflammatory efficacy of 5-LO inhibitors [32, 33]. Administration (i.p.) of 4 mg/kg of hyperforin to rats potently reduced carrageenan-induced pleural exudate formation and inflammatory cell infiltration to a similar extent as the well-recognized 5-LO inhibitor zileuton (10 mg/kg) or the cyclooxygenase inhibitor indomethacin (5 mg/kg). LTB4 formation was comparably blocked by hyperforin and zileuton, whereas indomethacin failed to reduce LTB4 levels, as expected. This indicates that suppression of LTB4 by hyperforin is not an unspecific effect due to general impairment of the inflammatory response but instead is directly related to interference with 5-LO product formation.
5-LO inhibitors are essentially grouped into redox-active compounds reducing the active-site iron, chelators of the iron, and nonredox-type 5-LO inhibitors that act at fatty acid-binding sites of 5-LO in a competitive fashion [6, 7]. There is no support for an iron-chelating capacity of hyperforin [17], and direct reducing or radical scavenging properties of hyperforin could not be demonstrated [34, 35]. Also, due to the lipophilic acid character of hyperforin, a competitive mode of action appeared reasonable, but previous studies indicate an uncompetitive inhibition of 5-LO [17], and the potency of hyperforin was independent on the redox tone and the phosphorylation state, which typically modulates the efficacy of nonredox-type 5-LO inhibitors [12, 13].
The inhibition of isolated 5-LO by hyperforin suggests a direct interaction with the 5-LO enzyme. Attempts in order to demonstrate a selective physical binding between hyperforin and 5-LO or 5-LO C2-like-domain [using different methodologies, including surface plasmon resonance spectroscopy (BIAcore), affinity chromatography, and isothermal titration calorimetry] failed, due to the unspecific binding pattern of the lipophilic hyperforin (not shown). Based on the automated molecular docking studies, binding of hyperforin to the active site region of 5-LO is unlikely, whereas BWA4C and ZM230487 seemingly interfere with this site. We like to emphasize that any docking experiment is error-prone, and using a homology-based protein model in conjunction with a flexible ligand like hyperforin easily leads to purely speculative results and, thus, these are only suggestive. On the other hand, hyperforin could act via the PL-binding site, which is located within the C2-like domain. Thus, hyperforin potently inhibited partially purified 5-LO, but lost its efficacy when cellular membrane components were included in crude 5-LO incubations. Such dependence on the incubation composition (homogenates, S100, and partially purified 5-LO) has not been reported before for any 5-LO inhibitor, and was also not observed here for BWA4C or ZM230487. Also, PC specifically attenuated 5-LO inhibition by hyperforin, but not by BWA4C or ZM230487, and we conclude that (phospho)lipids might be responsible for the detrimental effects in homogenates and S100. 5-LO catalytic activity is stimulated by PC or membranes [36], but not by PE and other PLs [36], and PC vesicles bind to 5-LO [37]. The C2-like domain mediates association of 5-LO with the nuclear membrane and selectively binds PC, which is conferred by Trp-13, -75, and -102, whereas other PLs including PE bound with considerably lower affinity [15]. Ca2+ induces binding of 5-LO to PC and to a minor extent also to PE, but only PC conferred 5-LO activity [37]. Along these lines, PC, but not PE, could interfere with 5-LO inhibition by hyperforin. This selective effect of PC indicates that reversal of 5-LO inhibition was not simply an unspecific partitioning of hyperforin into a newly formed lipid phase, thus reducing its free/available concentration. Also, diglycerides (i.e., OAG) may act at this regulatory site, thereby stimulating 5-LO activity [19, 29]. Accordingly, OAG reduced the potency of hyperforin in vitro as well as in intact neutrophils, but did not affect 5-LO inhibition by BWA4C and ZM230487. Replacement of Trp-13, -75 and -102 by alanine in the isolated C2-like domain led to reduced affinity to PC vesicles [15]. The same mutations in intact 5-LO reduced the susceptibility to hyperforin suggesting that hyperforin requires these tryptophan residues to efficiently inhibit 5-LO. In contrast, the potency of BWA4C was not different for wt- or 3W mut-5LO. Moreover, ZM230487 was even more effective for the 3W mut-5LO versus wt-5-LO, and PC also promoted the inhibitory action of ZM230487 for wt-5-LO, which cannot be readily explained. Taken together, one may conclude that the C2-like domain not only regulates 5-LO activity but can also determine the molecular pharmacology of the enzyme.
Another regulatory property of the C2-like domain is the interaction with CLP, which also requires Trp-13, -75, and -102 [16]. CLP may serve as a scaffold for Ca2+-induced 5-LO activity in the presence of PC and may function in a complex together with 5-LO and membranes to increase the capacity of cellular 5-LO product synthesis [16]. Hyperforin abolished the interaction between 5-LO and CLP at concentrations that inhibited 5-LO product synthesis. Moreover, hyperforin blocked the functional interaction between CLP and 5-LO, visualized by the inhibition of the stimulatory effect of CLP on LTA4 synthesis.
It is remarkable that hyperforin (but not BWA4C or ZM230487) prevented nuclear membrane-association upon stimulation with ionomycin, a process required for substantial 5-LO product synthesis [10, 21]. It was shown that the C2-domain mediates nuclear membrane translocation [31], where 5-LO may bind to PC in a Ca2+-dependent manner [37], involving Trp-13, -75, and -102 [15]. Note that hyperforin failed to block Ca2+ mobilization in neutrophils evoked by ionomycin [35], thus excluding the possibility that suppressed 5-LO translocation is simply due to repressed Ca2+ mobilization. We rather conclude that hyperforin prevents membrane-binding by interruption of PC/5-LO interactions.
Collectively, we propose hyperforin as novel type of 5-LO inhibitor that affects LT biosynthesis by functional interference with the putative CLP/PL-binding site within the C2-like domain of 5-LO. This interference not only results in a direct inhibitory effect on 5-LO catalysis, but also interrupts the interaction of 5-LO with CLP and with membranes that both support 5-LO product formation in the cell. Although many 5-LO inhibitors have been developed in order to treat LT-related diseases [8], novel molecular strategies for intervention with 5-LO activity are certainly needed for steps forward in anti-LT therapy. The effectiveness of hyperforin as a novel inhibitor of LT synthesis is strengthened by its in vivo action as demonstrated by the reduced LTB4 generation in rat pleural exudates associated with an anti-inflammatory action, comparable to zileuton. Along these lines, hyperforin was previously demonstrated to reduce acute neutrophil recruitment and to enhance resolution in a murine pulmonary bleomycin-induced inflammation model, significantly reducing consequent fibrosis [38]. Targeting of the functionality of the C2-like domain of 5-LO may represent a novel pharmacological approach for future 5-LO inhibitor developments.
Acknowledgments
We thank Sven George and Bianca Jazzar for expert technical assistance. The study was supported by the Deutsche Forschungsgemeinschaft, the Beilstein-Institut zur Förderung der chemischen Wissenschaften, the Swedish Research Council, 03X-217, and the European Union (EICOSANOX, LSHM-CT-2004-005033. Disclaimer: The report reflects only the author’s views and the Community is not liable for any use that may be made of the information herein). C.P. received a Carl-Zeiss stipend.
References
- 1.Rådmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem Sci. 2007;32:332–341. doi: 10.1016/j.tibs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 3.Peters-Golden M, Henderson WR., Jr Leukotrienes. N Engl J Med. 2007;357:1841–1854. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 4.Werz O, Steinhilber D. Therapeutic options for 5-lipoxygenase inhibitors. Pharmacol Ther. 2006;112:701–718. doi: 10.1016/j.pharmthera.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Capra V, Ambrosio M, Riccioni G, Rovati GE. Cysteinyl-leukotriene receptor antagonists: present situation and future opportunities. Curr Med Chem. 2006;13:3213–3226. doi: 10.2174/092986706778742963. [DOI] [PubMed] [Google Scholar]
- 6.Werz O. 5-Lipoxygenase: cellular biology and molecular pharmacology. Curr Drug Targets Inflamm Allergy. 2002;1:23–44. doi: 10.2174/1568010023344959. [DOI] [PubMed] [Google Scholar]
- 7.Ford-Hutchinson AW, Gresser M, Young RN. 5-Lipoxygenase. Annu Rev Biochem. 1994;63:383–417. doi: 10.1146/annurev.bi.63.070194.002123. [DOI] [PubMed] [Google Scholar]
- 8.Werz O, Steinhilber D. Pharmacological intervention with 5-lipoxygenase: new insights and novel compounds. Expert Opin Ther Pat. 2005;15:505–519. doi: 10.1517/13543776.15.5.505. [DOI] [Google Scholar]
- 9.Rådmark O, Samuelsson B. Regulation of 5-lipoxygenase enzyme activity. Biochem Biophys Res Commun. 2005;338:102–110. doi: 10.1016/j.bbrc.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Pergola C, Dodt G, Rossi A, Neunhoeffer E, Lawrenz B, Northoff H, Samuelsson B, Rådmark O, Sautebin L, Werz O. ERK-mediated regulation of leukotriene biosynthesis by androgens: a molecular basis for gender differences in inflammation and asthma. Proc Natl Acad Sci USA. 2008;105:19881–19886. doi: 10.1073/pnas.0809120105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer L, Steinhilber D, Werz O. Molecular pharmacological profile of the nonredox-type 5-lipoxygenase inhibitor CJ-13, 610. Br J Pharmacol. 2004;142:861–868. doi: 10.1038/sj.bjp.0705860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer L, Szellas D, Rådmark O, Steinhilber D, Werz O. Phosphorylation- and stimulus-dependent inhibition of cellular 5-lipoxygenase activity by nonredox-type inhibitors. FASEB J. 2003;17:949–951. doi: 10.1096/fj.03-0205com. [DOI] [PubMed] [Google Scholar]
- 13.Werz O, Szellas D, Henseler M, Steinhilber D. Nonredox 5-lipoxygenase inhibitors require glutathione peroxidase for efficient inhibition of 5-lipoxygenase activity. Mol Pharmacol. 1998;54:445–451. doi: 10.1124/mol.54.2.445. [DOI] [PubMed] [Google Scholar]
- 14.Hammarberg T, Provost P, Persson B, Rådmark O. The N-terminal domain of 5-lipoxygenase binds calcium and mediates calcium stimulation of enzyme activity. J Biol Chem. 2000;275:38787–38793. doi: 10.1074/jbc.M006136200. [DOI] [PubMed] [Google Scholar]
- 15.Kulkarni S, Das S, Funk CD, Murray D, Cho W. A molecular basis of specific subcellular localization of the C2-like domain of 5-lipoxygenase. J Biol Chem. 2002;277:13167–13174. doi: 10.1074/jbc.M112393200. [DOI] [PubMed] [Google Scholar]
- 16.Rakonjac M, Fischer L, Provost P, Werz O, Steinhilber D, Samuelsson B, Rådmark O. Coactosin-like protein supports 5-lipoxygenase enzyme activity and up-regulates leukotriene A4 production. Proc Natl Acad Sci USA. 2006;103:13150–13155. doi: 10.1073/pnas.0605150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albert D, Zundorf I, Dingermann T, Muller WE, Steinhilber D, Werz O. Hyperforin is a dual inhibitor of cyclooxygenase-1 and 5-lipoxygenase. Biochem Pharmacol. 2002;64:1767–1775. doi: 10.1016/S0006-2952(02)01387-4. [DOI] [PubMed] [Google Scholar]
- 18.Werz O, Burkert E, Samuelsson B, Rådmark O, Steinhilber D. Activation of 5-lipoxygenase by cell stress is calcium independent in human polymorphonuclear leukocytes. Blood. 2002;99:1044–1052. doi: 10.1182/blood.V99.3.1044. [DOI] [PubMed] [Google Scholar]
- 19.Hornig C, Albert D, Fischer L, Hornig M, Rådmark O, Steinhilber D, Werz O. 1-Oleoyl-2-acetylglycerol stimulates 5-lipoxygenase activity via a putative (phospho) lipid binding site within the N-terminal C2-like domain. J Biol Chem. 2005;280:26913–26921. doi: 10.1074/jbc.M500068200. [DOI] [PubMed] [Google Scholar]
- 20.Burkert E, Arnold C, Hammarberg T, Rådmark O, Steinhilber D, Werz O. The C2-like beta-barrel domain mediates the Ca2+-dependent resistance of 5-lipoxygenase activity against inhibition by glutathione peroxidase-1. J Biol Chem. 2003;278:42846–42853. doi: 10.1074/jbc.M302471200. [DOI] [PubMed] [Google Scholar]
- 21.Werz O, Klemm J, Samuelsson B, Rådmark O. Phorbol ester up-regulates capacities for nuclear translocation and phosphorylation of 5-lipoxygenase in Mono Mac 6 cells and human polymorphonuclear leukocytes. Blood. 2001;97:2487–2495. doi: 10.1182/blood.V97.8.2487. [DOI] [PubMed] [Google Scholar]
- 22.Jones G, Willett P, Glen RC. Molecular recognition of receptor sites using a genetic algorithm with a description of desolvation. J Mol Biol. 1995;245:43–53. doi: 10.1016/S0022-2836(95)80037-9. [DOI] [PubMed] [Google Scholar]
- 23.Werz O, Tretiakova I, Michel A, Ulke-Lemee A, Hornig M, Franke L, Schneider G, Samuelsson B, Rådmark O, Steinhilber D. Caspase-mediated degradation of human 5-lipoxygenase in B lymphocytic cells. Proc Natl Acad Sci USA. 2005;102:13164–13169. doi: 10.1073/pnas.0505991102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeLano WL (2002) The PyMOL molecular graphics system (2002). DeLano Scientific, San Carlos, CA, USA. http://www.pymol.org
- 25.Carter GW, Young PR, Albert DH, Bouska J, Dyer R, Bell RL, Summers JB, Brooks DW. 5-Lipoxygenase inhibitory activity of zileuton. J Pharmacol Exp Ther. 1991;256:929–937. [PubMed] [Google Scholar]
- 26.Werz O, Schneider N, Brungs M, Sailer ER, Safayhi H, Ammon HPT, Steinhilber D. A test system for leukotriene synthesis inhibitors based on the in vitro differentiation of the human leukemic cell lines HL-60 and Mono Mac 6. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:441–445. doi: 10.1007/PL00005074. [DOI] [PubMed] [Google Scholar]
- 27.Tateson JE, Randall RW, Reynolds CH, Jackson WP, Bhattacherjee P, Salmon JA, Garland LG. Selective inhibition of arachidonate 5-lipoxygenase by novel acetohydroxamic acids: biochemical assessment in vitro and ex vivo. Br J Pharmacol. 1988;94:528–539. doi: 10.1111/j.1476-5381.1988.tb11557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, Collins JL, Kliewer SA. St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci USA. 2000;97:7500–7502. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albert D, Buerkert E, Steinhilber D, Werz O. Induction of 5-lipoxygenase activation in polymorphonuclear leukocytes by 1-oleoyl-2-acetylglycerol. Biochim Biophys Acta. 2003;1631:85–93. doi: 10.1016/s1388-1981(02)00359-1. [DOI] [PubMed] [Google Scholar]
- 30.Provost P, Doucet J, Hammarberg T, Gerisch G, Samuelsson B, Rådmark O. 5-Lipoxygenase interacts with coactosin-like protein. J Biol Chem. 2001;276:16520–16527. doi: 10.1074/jbc.M011205200. [DOI] [PubMed] [Google Scholar]
- 31.Chen XS, Funk CD. The N-terminal “beta-barrel” domain of 5-lipoxygenase is essential for nuclear membrane translocation. J Biol Chem. 2001;276:811–818. doi: 10.1074/jbc.M008203200. [DOI] [PubMed] [Google Scholar]
- 32.Rao TS, Currie JL, Shaffer AF, Isakson PC. Evaluation of 5-lipoxygenase inhibitors, zileuton, A-78773 and ICI-D-2138 in an ionophore (A-23187)-induced pleural inflammation model in the rat. Life Sci. 1993;53:PL147–PL152. doi: 10.1016/0024-3205(93)90253-Y. [DOI] [PubMed] [Google Scholar]
- 33.Cuzzocrea S, Rossi A, Serraino I, Mazzon E, Di Paola R, Dugo L, Genovese T, Calabro B, Caputi AP, Sautebin L. 5-Lipoxygenase knockout mice exhibit a resistance to pleurisy and lung injury caused by carrageenan. J Leukoc Biol. 2003;73:739–746. doi: 10.1189/jlb.1002477. [DOI] [PubMed] [Google Scholar]
- 34.Heilmann J, Winkelmann K, Sticher O. Studies on the antioxidative activity of phloroglucinol derivatives isolated from hypericum species. Planta Med. 2003;69:202–206. doi: 10.1055/s-2003-38477. [DOI] [PubMed] [Google Scholar]
- 35.Feisst C, Werz O. Suppression of receptor-mediated Ca2+ mobilization and functional leukocyte responses by hyperforin. Biochem Pharmacol. 2004;67:1531–1539. doi: 10.1016/j.bcp.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 36.Puustinen T, Scheffer MM, Samuelsson B. Regulation of the human leukocyte 5-lipoxygenase: stimulation by micromolar calcium levels and phosphatidylcholine vesicles. Biochim Biophys Acta. 1988;960:261–267. doi: 10.1016/0005-2760(88)90033-1. [DOI] [PubMed] [Google Scholar]
- 37.Noguchi M, Miyano M, Matsumoto T, Noma M. Human 5-lipoxygenase associates with phosphatidylcholine liposomes and modulates LTA(4) synthetase activity. Biochim Biophys Acta. 1994;1215:300–306. doi: 10.1016/0005-2760(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 38.Dell’Aica I, Niero R, Piazza F, Cabrelle A, Sartor L, Colalto Brunetta E, Lorusso G, Benelli R, Albini A, Calabrese F, Agostini C, Garbisa S. Hyperforin blocks neutrophil activation of matrix metalloproteinase-9, motility and recruitment, and restrains inflammation-triggered angiogenesis and lung fibrosis. J Pharmacol Exp Ther. 2007;321:492–500. doi: 10.1124/jpet.106.116459. [DOI] [PubMed] [Google Scholar]