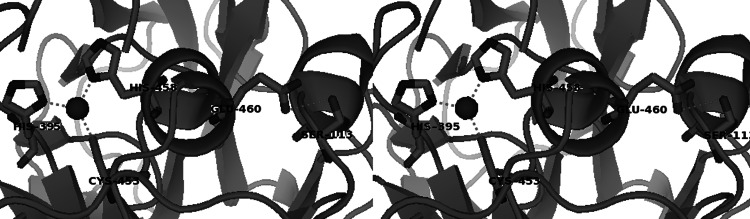Abstract
Laccases (benzenediol:oxygen oxidoreductases, EC 1.10.3.2) are blue multicopper oxidases that catalyze the oxidation of an array of aromatic substrates concomitantly with the reduction of molecular oxygen to water. In fungi, laccases carry out a variety of physiological roles during their life cycle. These enzymes are being increasingly evaluated for a variety of biotechnological applications due to their broad substrate range. In this review, the most recent studies on laccase structural features and catalytic mechanisms along with analyses of their expression are reported and examined with the aim of contributing to the discussion on their structure–function relationships. Attention has also been paid to the properties of enzymes endowed with unique characteristics and to fungal laccase multigene families and their organization.
Keywords: Multicopper oxidases, Laccase structures, Unusual laccases, Laccase gene family, Laccase regulation
Introduction
Laccases (benzenediol:oxygen oxidoreductases, EC 1.10.3.2), representing the largest subgroup of blue multicopper oxidases (MCO), use the distinctive redox ability of copper ions to catalyze the oxidation of a wide range of aromatic substrates concomitantly with the reduction of molecular oxygen to water [1, 2]. Amongst more than 200 kinds of oxidases and oxygenases, only six classes of enzymes are capable of catalyzing this type of oxygen reaction (cytochrome-c oxidase, laccases, l-ascorbate oxidase, ceruloplasmin, bilirubin oxidase, and phenoxazinone synthase).
A laccase was first discovered in the sap of the Japanese lacquer tree Rhus vernicifera [3]. Since then, laccases have also been found in various basidiomycetous and ascomycetous fungi and, thus far, fungal laccases have accounted for the most important group of MCOs with respect to number and extent of characterization. In fungi, laccases carry out a variety of physiological roles including morphogenesis, fungal plant-pathogen/host interaction, stress defense, and lignin degradation [4, 5]. Laccases have been found in nearly all wood-rotting fungi analyzed so far [6] and are almost ubiquitary enzymes as they have been isolated from plants, from some kinds of bacteria, and from insects too [7–13]. In plants, laccases have been found in the wood and cellular walls of herbaceous species [7], where they participate in lignin biosynthesis [8]. Bacterial laccases appear to have a role in morphogenesis [9], in the biosynthesis of the brown spore pigment and in the protection afforded by the spore coat against UV light and hydrogen peroxide, and in copper homeostasis [10]. The main function of the laccase-type proteins in insects is believed to be sclerotization of the cuticle in the epidermis [12].
Laccases are able to catalyze direct oxidation of ortho- and para-diphenols, aminophenols, polyphenols, polyamines, and aryl diamines as well as some inorganic ions [1, 5, 14–17]. They couple the four single-electron oxidations of the reducing substrate to the four electron reductive cleavage of the dioxygen bond, using four Cu atoms distributed against three sites, defined according to their spectroscopic properties [1]. Typical metal content of laccases includes one type-1 (T1) copper (Cu1), and one type-2 (T2) and two type-3 (T3) copper ions (Cu2 and Cu3), with Cu2 and Cu3 arranged in a trinuclear cluster (TNC).
Laccase sequences and structures
The majority of the fungal laccases are extracellular monomeric globular proteins of approximately 60–70 kDa with an acidic isoelectric point (pI) around pH 4.0; they are generally glycosylated, with an extent of glycosylation ranging between 10 and 25% and only in a few cases higher than 30% [18, 19].
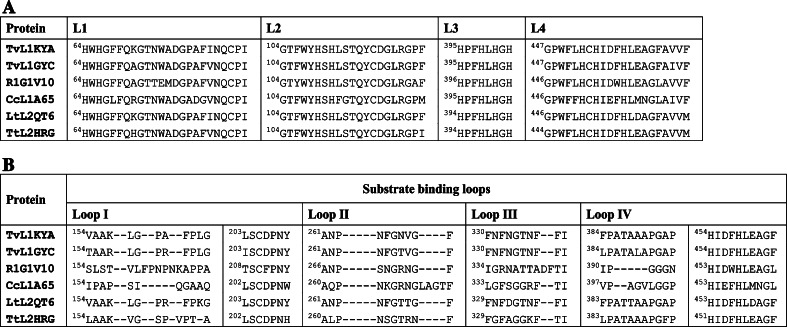
Analysis of the essential sequence features of fungal laccases based on multiple sequence alignments of more than 100 laccases has resulted in the identification of a set of four ungapped sequence regions, L1–L4, useful to identify the laccases and to distinguish them within the broader class of MCOs [20] (Fig. 1a). The 12 amino acid residues acting as the copper ligands are housed within these four identified conserved regions, of which L2 and L4 are in line with the earlier reported copper signature sequences of MCOs, while L1 and L3 are distinctive to the laccases. Furthermore, four loop regions, designated loops I, II, III, and IV and involved in substrate binding, have been identified [21] on the basis of laccase 3D structure superimposition (Fig. 1b).
Fig. 1.
Comparison of the laccase signature sequences (a) and of the substrate binding loops (b) of the laccases with known three-dimensional structure: TvL1KYA from T. versicolor, TvL1GYC from T. versicolor, R1G1V10 from R. lignosus, CcL1A65 from C. cinereus, LtL2QT6 from L. tigrinus, TtL2HRG from T. trogii
A number of 3D structures of basidiomycete laccases have been reported so far. Crystal structures have been solved for the laccases Lac-Cc (CcL1A65) from Coprinus cinereus [22], LccI (TvL1GYC) [23] and LacIIIb (TvL1KYA) from Trametes versicolor [24], RlL (R1G1V10) from Rigidoporus lignosus [25], LtL (LtL2QT6) from Lentinus tigrinus [26], and TtL (TtL2HRG) from Trametes trogii [27]. On the other hand, the only three-dimensional structure so far reported for ascomycete laccases is that of the laccase from Melanocarpus albomyces, whose crystal structures have been solved both for the native enzyme MaL (MaL1GWO) [28] and for the recombinant enzyme rMaL (rMaL2Q9O) expressed in Trichoderma reesei [29]. Crystal structures of bacterial laccases have also been solved [10, 11]. All these laccases exhibit a similar molecular architecture organized in three sequentially arranged cupredoxin-like domains. Each of them has a greek key β-barrel topology, strictly related to that of small copper proteins such as azurin and plastocyanin [30] and common to all the members of the MCOs family, such as ascorbate oxidase [31] and mammalian ceruloplasmin [32]. The Cu1 is located in domain 3, whilst the TNC cluster is embedded between domains 1 and 3 with both domains providing residues for copper coordination. The structure of basidiomycete laccases is stabilized by two disulfide bridges between domains 1 and 3 and between domains 1 and 2, whereas three disulfide bridges were found in the MaL1GWO structure [28].
It is worth noting that the mapping of regions L1–L4 onto the laccases’ three-dimensional structure indicated a specific, more or less C-2 symmetric, protein conformational motif characterizing the active site apparatus of the enzymes [20]. The observed intra-protein homologies between L1 and L3 and between L2 and L4 at both structure and sequence levels suggest that the quasi C-2 symmetric active site conformational motif may have arisen from a structural duplication event.
The T1 copper and the reaction with the reducing substrate
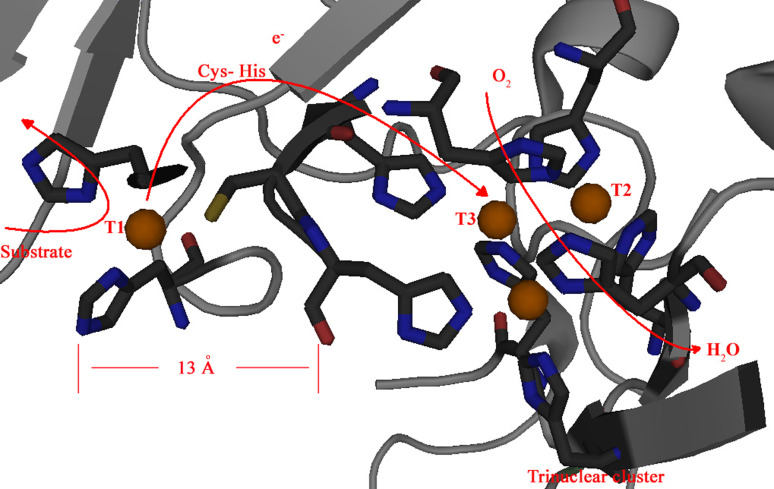
Typically MCOs show a Cu1 tetrahedrically coordinated with an axial fourth ligand, usually a methionine, to complete the first coordination sphere [2]. Vice versa, in laccases Cu1 exhibits a planar triangular coordination with the sulfur atom of a cysteine and with the Nδ1 nitrogen of two histidines. The charge transfer transition SCys → Cu(II) is responsible for an extremely intense absorption band with a molecular absorption coefficient, ε, of 5,300 M−1 cm−1 at 614 nm giving rise to the deep blue color of the enzyme [33]. The Cu1 is the primary electron acceptor site in a laccase catalyzed reaction, where four single-electron oxidations of a reducing substrate occur. The electrons are transferred through the highly conserved His–Cys–His tripeptide to the TNC, where O2 is reduced to water (Fig. 2) [34].
Fig. 2.
The structure of the laccase active site with arrows marking the flow of substrates, electrons (e−) and O2, adapted from [34] with the author’s permission
The reduction of the Cu1 is the rate-limiting step in the catalytic process and involves the Marcus “outer-sphere” mechanism [35]. In this mechanism, ΔE0 between E0 of the Cu1 and that of the reducing substrate determines the electron transfer rate [36]. E0 values of the Cu1 have been determined using potentiometric titrations for a great number of different laccases, and a substantial variation between 420 and 790 mV versus NHE has been found [19, 36–38]. The lack of the fourth axial ligand in laccases is considered as an important factor determining the higher values of E0 displayed by laccases in comparison with the other MCOs [39], as shown by mutagenesis experiments in the Trametes villosa laccase [37] and recently in a bacterial laccase [40]. A major issue still to be fully clarified is how the different laccases modulate their redox potential despite showing a very similar Cu1 coordination geometry.
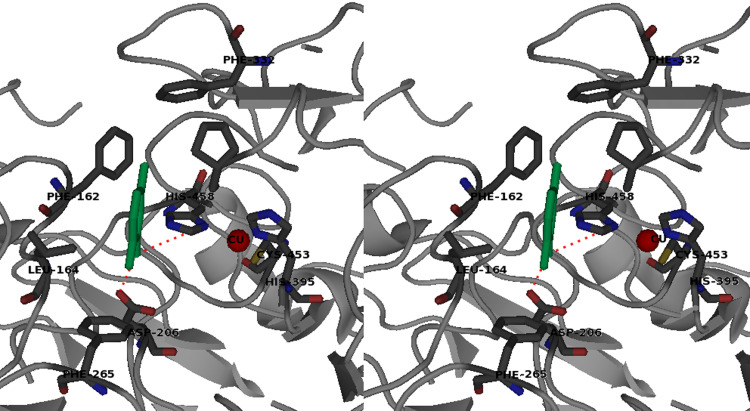
The Cu1 occupies a depression of the enzyme surface that makes possible its role as primary electron acceptor site. The Cu1 cavity is fairly wide, and it can accommodate a large variety of substrates that are not tightly buried in it. The structure of TvL1KYA represents the first high resolution structure of a laccase with an organic reducing substrate, 2,5-xylidine, in the substrate binding cavity [24]. Many hydrophobic protein–ligand interactions were shown to take place involving residues belonging to the four loops delineating the cavity (Fig. 3). Moreover, the residues His458 and Asp206 have also been shown to be important for the interaction between the amino group of the reducing substrate and the enzyme. The ring of the His458, coordinated to the Cu1 and highly conserved in all MCOs, is close to the nitrogen of 2,5-xylidine at a hydrogen bond distance (3.2 Å), suggesting that His458 is the entrance door of the electron during its transfer to Cu1.
Fig. 3.
Stereo view of the active site of TvL1KYA, which binds substrate 2,5-dimethylaniline (in green), elaborated with PyMol from the crystallographic structure
The amino group of 2,5-xylidine is also hydrogen bound to a terminal oxygen of the Asp206 side chain, which is located at the rear wall of the binding site [23, 24, 41]. Asp206 is well conserved among fungal laccases from basidiomycetes, whereas glutamate can be found among ascomycetes. The interaction of Asp206 with the reducing substrate has been shown to have an important role in determining the pH dependence of laccase activity [42, 43]: as the pH increases from 3 to 5, the K M values measured for phenolic substrates decrease, and this can be due to the deprotonation of the Asp206 side chain. At pH 5, the carboxylic function of Asp206 (pKa 3.9) [24] is dissociated, conferring a negative charge to the active site, favoring the interaction with substrates bearing the –OH or –NH2 functionality. The role of Asp206 in reaction with phenolic substrates is neatly supported by recent data acquired with mutants of TvL1KYA [44].
Xu et al. [42] postulated that the ascending part of the activity-pH profile observed for all laccases towards 2, 6 dimethoxy phenol (DMP) is generated by the redox potential difference between the reducing substrate and the Cu1 of laccase and is favored by a higher pH. As the pKa of DMP is ~9.9, deprotonation of DMP does not occur spontaneously at acidic pHs. The vicinity of a carboxylic residue interacting with the reducing substrate in the active site favors its deprotonation.
Piontek and coworkers [23] have hypothesized a correlation between the coordination distances around the Cu1 ion and its E0: the longer the distance the higher is E0. An elongated Cu–N bond in TvL1GYC could have an effect on the redox potential since the contribution of the free electron pair from the nitrogen to the copper would be decreased, rendering the copper more electron-deficient. This would give rise to a destabilization of the higher oxidation states, thus increasing the copper redox potential. The increase of the Cu–N distance could be a consequence of a hydrogen bond between Ser113 and Glu460, the latter situated in the same helix with His458, thus moving this residue away from Cu1 (Fig. 4). The hypothesis proposed by Piontek and collaborators has been also confirmed by structural observations of Garavaglia and coworkers [25] on R1G1V10, a high E0 enzyme [45]. In this laccase, the residues Glu459 and Ser113 are engaged in a strong hydrogen bond and appear to play the same role suggested in TvL1GYC. R1G1V10 shows the longest value for the Cu1–His distance (2.20 Å). Sequence data reveal that a glutamic acid in the position corresponding to Glu460 and a serine corresponding to Ser113 in TvL1GYC are highly conserved features among high E0 enzymes, as well as in some laccases of ligninolytic fungi with unknown E0 (Fig. 1a).
Fig. 4.
Close up (stereo view) of the Cu1 site of TvL1GYC showing the α-helical segment moved away from Cu1 as a consequence of the H bond between Ser113 and Glu460 (elaborated with PyMol from the crystallographic structure)
In the structure of the laccase TtL2HRG, Matera and coworkers [27] have suggested that the occurrence of two hydrophobic residues Phe460 and Ile452 in the near surroundings of the Cu1 contributes to the high redox potential observed. Furthermore, residue Phe460 is additionally surrounded by a large number of hydrophobic residues that would also contribute to increasing the redox potential of the Cu1.
All together these structural observations indicate that the variations in redox potential of the Cu1 observed among laccases cannot be ascribed to a single structural feature but to a sum of factors such as the Cu1 coordination geometry and the nature of the second sphere residues influencing solvent accessibility, hydrogen bonding, and dielectric anisotropy around the site.
Moreover, along with its redox features, steric hindrance of the substrate largely affects the oxidation proficiency of laccases, as recently demonstrated by Tadesse and coworkers [46], in T. villosa and Myceliophthora thermophila laccases, two enzymes markedly differing in redox potential (0.79 and 0.46 V). The distance between two phenylalanine residues (Phe332 and 265 in TvL1KYA) that mark the entrance to the active site can represent the structural threshold for oxidation of substrates with compatible redox potential (Fig. 3).
The T2/T3 copper cluster and the reaction with the oxygen
The three Cu2/Cu3 ions are arranged in a triangular fashion, as consistently observed in MCOs, and coordinated to a strongly conserved pattern of four His–X–His motifs [2]. Six such histidine residues coordinate the Cu3 copper pair, whereas the Cu2 is coordinated by the remaining two histidine residues. The Cu2 shows a characteristic electron paramagnetic resonance (EPR) spectrum, clearly distinct from that of Cu1, whereas the two Cu3 are antiferromagnetically coupled ions, EPR-silent. Optically, Cu3 absorbs at 330 nm (ε 330 ~ 3,600 M−1 cm−1), whereas Cu2 is practically undetectable [47]. TNC acts in dioxygen binding and reduces the molecular oxygen upon receipt of four electrons forwarded from the mononuclear center Cu1 [1, 2, 22] to the two His coordinating the Cu3a and Cu3b copper ions. A detailed description of the catalytic events taking place at the three copper atoms in the dioxygen reduction site is still the subject of intense debate.
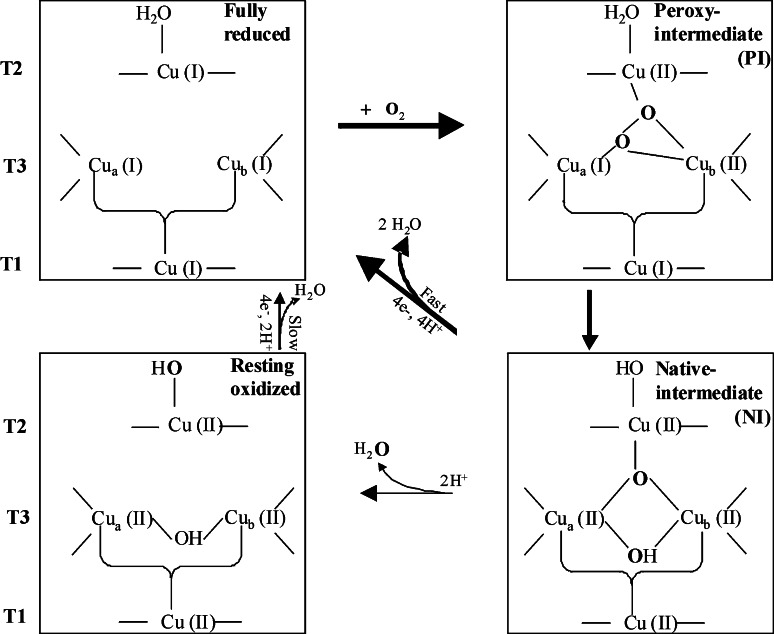
The kinetic and spectral features of copper/dioxygen intermediates in MCOs have been investigated in detail mainly by Solomon’s group [33, 48, 49]. A considerable number of spectroscopic and kinetic studies have been focused on laccases from R. vernicifera [33, 50, 51], and more recently on the laccase-like Fet3p protein from Saccharomyces cerevisiae [52, 53]. The molecular mechanism of O2 reduction to H2O proposed on the basis of spectroscopic studies of O2 intermediates in the MCOs is shown in Fig. 5 [34]. The reaction of the fully reduced enzyme with O2 proceeds via two sequential two-electron steps, generating the peroxy intermediate (PI) and the native intermediate (NI). The first step is rate determining, while the second, involving the 2e− reductive cleavage of the O–O bond, is faster.
Fig. 5.
Mechanism of O2 reduction to water by the MCOs. Broad arrows indicate the steps that take place in the catalytic cycle of the MCOs; thin arrows indicate steps that can be experimentally observed but are not part of the catalytic cycle. The peroxy intermediate is a 2e−-reduced species, and the native intermediate is a 4e−-reduced species. Adapted from [34] with the author’s permission
In the fully reduced form, TNC is coordinatively unsaturated [54]. The anionic charges in the vicinity of the cluster appear to stabilize its high positive charge and tune its coordination unsaturation and redox properties, contributing to the O2 reactivity of the cluster. In particular the presence of a highly conserved anionic residue near the Cu2 (corresponding to D77 in TvL1KYA) plays a critical role in the reaction of the reduced TNC with O2 [34, 54].
NI is a fully oxidized species with O2 fully reduced to water-level products that remain bound to the trinuclear site as µ3-oxo and µ2-hydroxo bridging ligands [33, 49]. The all-bridged structure of NI is consistent with the rapid 4e− reduction of NI to the fully reduced enzyme because the µ3-oxo bridge would allow electron delocalization over the three Cu centers for facile electron transfer (ET) through the cluster [34]. In contrast, the resting form, a fully oxidized form as NI, has the Cu2 isolated from the other atoms of the clusters. In the absence of reducing substrate, the NI decays to the resting form at a rate that is too slow to be relevant in the catalytic cycle, thus the NI appears to be the catalytically fully oxidized form of the enzyme [33]. Indeed the decay of NI proceeds via successive proton-assisted steps and involves large structural rearrangement of the µ3-oxo-bridge ligand from inside to outside the cluster leading to the resting enzyme. In this form, the one remaining O atom of the O2 is bound as OH− to Cu2 outside the cluster, and the two Cu3 centers are bridged by a OH− ligand [49].
The three Cu centers in the TNC are sequentially reduced by reducing substrate via the Cu1. A reasonable mechanism for the reduction of NI has been proposed by Yoon and coworkers [49]. According to the authors, Cu3a is expected to reduce first because the negatively charged residue (D77 in TvL1KYA) near the Cu2 and Cu3b centers significantly lowers the reduction potentials of these Cu centers below that of Cu3a. Then, Cu2 would likely reduce before Cu3b; further reduction of the remaining Cu3b center would be fast via the Cys–His pathway between Cu1 and Cu3 centers and would be accompanied by dissociation of two water molecules from the TNC. On the other hand, reduction of Cu3b before Cu2 would result in protonation of the OH− bridge, leading to the loss of the Cu2–Cu3b electronic coupling for rapid ET to Cu2.
On the basis of X-ray structural studies on the bacterial laccase CotA and other MCOs, Bento and coworkers [55] have suggested a different mechanism for the dioxygen reduction. One of the main differences is linked to the role of the resting state as one of the intermediates within the catalytic cycle. As recently assessed by Ferraroni and coworkers [26] and Matera and coworkers [27], structural data of MCOs obtained to date and acquired using synchrotron radiation are hard to interpret as far as the coordination geometries, the bond distances, and the oxidation states of the Cu1 and Cu2/Cu3 active sites are concerned. Metalloproteins are subjected to progressive reduction by exposure of crystals to high-intensity X-ray synchrotron beams. The X-ray synchrotron radiation reduces copper centers during data collection, and the enzyme undergoes redox cycling. The crystals are exposed to air, the molecular oxygen can be reduced into water molecules, and the catalytic cycle repeated additional times resulting in collection of data generating electron density maps which represent an average of the several catalytic steps. Therefore, a strategy, newly applied to MCOs, was used under aerobic conditions and high pH in order to trap the intermediate states, and the authors observed the progressive reduction of the copper ions. The structure of the two sequential intermediates of the LtL2QT6 in the molecular oxygen reduction pathway was detected—the PI and NI resulting from two- and four-electron reductions of molecular oxygen, respectively [26, 27]. In contrast to what was suggested by spectroscopic studies, the peroxide in the PI appears to bridge only the Cu3 pair and not to connect the reduced Cu2. PI is further reduced in a second step, thus oxidizing the Cu2 and the distant Cu1 centers to generate the NI [26]. An oxo ion bridging the three coppers of the TNC (µ3-oxo bridge) together with an hydroxide ion externally bridging the two Cu3 ions has been modelled in the NI, thus confirming the results of the spectroscopic studies.
As far as Cu3a and Cu3b ions are concerned, they are asymmetrically coordinated to an hydroxide/water moiety in the crystal structures of R1G1V10 [25], LtL2QT6 [26], and TtL2HRG [27]. In R1G1V10 structure [25], the asymmetric binding of the hydroxide ion bridging the Cu3 ions (with the Cu3a–OH bond shorter than the Cu3b–OH bond) has been explained by assuming a reduced oxidation state for Cu2 and Cu3a ions. This asymmetry could have a crucial role in the progression of the dioxygen splitting. In the structures of LtL2QT6 [26] and TtL2HRG [27], the Cu3a ends up closer to the Cu1 site than the Cu3b. According to the theoretical calculations performed by Kyritsis and coworkers [56] for ascorbate oxidase, the Cu1–Cu3a pathway should be up to three times more efficient than the Cu1–Cu3b, thus a differential reduction progress in TNC would result in the observed asymmetries.
Two solvent channels provide access to TNC, located in the interior of the protein structure. The first channel points towards the two Cu3 ions on one side of the TNC allowing the molecular oxygen to enter and to bind to it. The second channel pointing towards the Cu2 ion on the other side of the cluster permits water molecules produced in the O2 reduction to move to the bulk solvent [23, 26, 27, 41].
Role of the C-terminal tail
A C-terminal protruding tail (13–14 amino acids long) has been found in the deduced amino acidic sequences of laccases from the ascomycetes Podospora anserina [57], Neurospora crassa [58], M. albomyces [59], and M. thermophila [60, 61]. This tail is generally cleaved off by proteolysis at a conserved cleavage site to produce the active form of the enzyme. Analysis of the 3D structure of the laccase from the fungus M. albomyces has shown this C-terminal extension as a plug obstructing the solvent channel [28], thus leading to the hypothesis that its cleavage is required to favor the entrance of oxygen and the subsequent exit of water molecules.
When Kiiskinen and Saloheimo [59] studied the expression of MaL1GWO in S. cerevisiae, they suggested that yeast apparatus is not able to process the C terminus correctly, whilst the introduction of a stop codon after the native processing site at the C terminus gives rise to an increase in laccase activity. Furthermore, Bulter and coworkers [60] reported the correct processing of the C terminus of M. thermophila laccase in S. cerevisiae, after the introduction by directed evolution experiments of a Kex2 protease site in the laccase sequence. Thus, it seems to be clear that carboxyl-terminal processing may play a role in the activation of ascomycete enzymes.
Zumarraga and coworkers [61] selected a M. thermophila laccase variant with two consecutive mutations in the C-terminal tail exhibiting an increase in the pI of the C terminus, while keeping constant the pI of the mature protein. The authors assumed that these mutations might contribute to a higher grade of tightness between the C-terminal extension and the main enzymatic core, which would affect the protein folding and therefore the final mature enzyme. Whether a similar role of the C-terminal tail is possible among basidiomycete laccases too is not yet known. Gelo-Pujic and coworkers [62] reported that the redox potential of the TvL1GYC basidiomycete laccase changes when its C terminus is truncated by 11 amino acids, thus suggesting that C-terminal amino acids can also affect the function of fungal laccases from basidiomycetes. An unusual C-terminal extension of 16 amino acids has been found in the POXA1b laccase from Pleurotus ostreatus [63], although a C-terminal processing of two to four amino acids has been observed for both the native and the heterologously expressed protein in Kluyveromyces lactis [64]. The POXA1b C-terminal tail has been found mutated in one of the selected random mutants from directed evolution experiments [65]. The P494T mutation is located in a variable and mobile loop at the C terminus. Molecular dynamic simulations on 3D model structure of this mutant have shown the mutation affects the flexibility of some regions of the protein, thus leading to an improved stability and activity of the enzyme.
Laccase reactivity
Phenols are typical laccase substrates because their redox potentials (ranging from 0.5 to 1.0 V vs. NHE) are low enough to allow electron abstraction by the Cu1. Commonly used phenolic substrates are syringaldazine, DMP and guaiacol; however, laccases are also able to oxidize electron donor substrates such as ABTS [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)] or ferrocyanide [K4Fe(CN)6] [14, 15, 63]. Phenolic substrates are oxidized to phenoxy radicals, which, depending on reaction conditions, can spontaneously polymerize via radical coupling or rearrange themselves leading to quinones (through disproportionation), alkyl–aryl cleavage, Cα oxidation, cleavage of Cα–Cβ bond or aromatic ring [66].
Xu performed a comparative study with several fungal laccases for the oxidation of a series of phenols, anilines, and benzenethiols [67]. The observed catalytic efficiencies were found to be correlated to the structure of the substrate and to the one-electron redox potential difference between the laccase Cu1 and substrate. Garzillo et al. [68] observed an increase in the oxidation rate of different substrates by a laccase from T. trogii when two o- and p-oriented groups were present simultaneously. As reported above, the relative contribution of steric and redox properties of a substrate in determining its susceptibility to laccase oxidation has been recently assessed by Tadesse and coworkers [46].
Because of their high nonspecific oxidation capacity, and because they use readily available molecular oxygen as an electron acceptor, laccases are useful biocatalysts for a wide range of biotechnological applications. Thus, they find potential applications in pulp delignification and biobleaching [69], treatment of industrial plant wastewater [70], enzymatic modification of fibers and dye-bleaching in the textile and dye industries [71], enzymatic removal of phenolic compounds in beverages, fruit juice processing [72], and biosensor and biofuel cell construction [73]. Several recent studies have been focused on dye degradation by laccases. Most of these works used commercially available dyes as model pollutants [74]. Laccase potential in the decolorization of recalcitrant azo dyes, such as those commonly used in the leather industry, has been assessed [75]. A crude laccase mixture preparation from P. ostreatus is able to decolorize the Remazol Brilliant Blue R (RBBR) anthraquinonic dye [76] achieving a maximum of 70% decolorization. Moreover, the same preparation can be re-used several times when immobilized in copper alginate beads [77]. The role of laccase in cytotoxicity reduction during dye decolorization has also been demonstrated [78]. Nevertheless, real industrial effluents usually include mixtures of several dyes, and only limited data are now available on mixed dye degradation. The degradation of a mixture of reactive dyes, simulating a real textile effluent, has been successfully tested in the framework of the SOPHIED EU project (NMP2-CT-2004-505899, 6FP), indicating the possibility of implementing this technique for the treatment of textile-dyeing wastewaters. Real effluents have also been treated and the removal of the mutagenic character of the effluent has been achieved [79].
Laccases might also be useful in synthetic chemistry [80]. For instance, laccase-catalyzed reactions can be used for the polymerization of catechol monomers for polycatechol synthesis [81] or for the production of inert phenolic polymers, such as poly(1-napthol) [82]. These polymers have application in wood composites, fiber bonding, laminates, coatings, and adhesives. A novel system of enzymatic polymerization for the preparation of “artificial urushi” polymeric films (Japanese traditional coating) has been demonstrated, using a laccase-catalyzed cross-linking reaction [83]. Laccases have been used to synthesize dyes [84] and products of pharmaceutical importance, such as the anticancer drug actinocin [85] or the phytoalexin resveratrol [86].
Substrates characterized by high redox potential cannot be oxidized directly by laccases. Nevertheless, laccases are considered to play a major role in the lignin degradation by some white-rot fungi [4]. Suitable compounds, the so-called mediators, acting as intermediate substrates for laccases, enable laccase to indirectly oxidize large molecules and even nonphenolic substrates, such as nonphenolic β-1 lignin model dimer [87, 88]. The first mediator used in the laccase-mediator system (LMS) for pulp delignification was ABTS [88]. Since then about 100 molecules have been tested for their ability to oxidize lignin or lignin models [89, 90]. An effective redox mediator should be a good laccase substrate, its oxidized radical form should have a half-life long enough to permit its diffusion towards the substrate, and possess high oxidation potential to effectively oxidize it. Xu and coworkers [91] have shown that the activity and stability of N–O· radicals seem to be better balanced in comparison with those of phenoxy radicals. Indeed the most effective mediators for lignin degradation have proved to be the N-heterocycle-bearing N–OH groups and in particular 1-hydroxybenzotriazole (HBT).
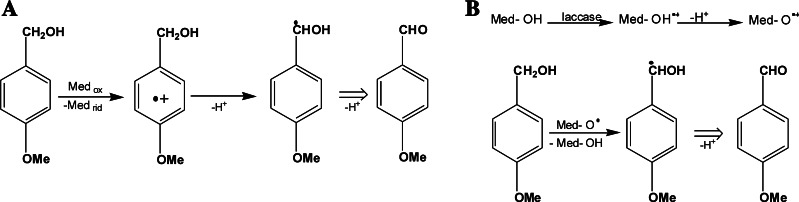
Laccase/mediator-catalyzed oxidations of nonphenolic substrates can proceed via two different mechanisms. ABTS-mediated reactions follow an ET route (Fig. 6a), whereas the >N–OH-type mediators, such as HBT, follow a hydrogen atom transfer (HAT) route by abstraction of a hydrogen atom from the >N–OH-type mediators, producing a >N–O· radical species (Fig. 6b). The former mechanism requires substrates with a low oxidation potential; the latter mechanism requires substrates with relatively weak C–H bonds [92]. The characterization of the radical intermediates (neutral or cationic) formed from both mediators after the enzymatic oxidation has been recently obtained through a multifrequency EPR approach [93].
Fig. 6.
Laccase/mediator-catalyzed oxidations of nonphenolic substrates via ET route (a) and radical HAT route (b), adapted from [92] with the author’s permission
The LMS has been demonstrated to be efficient for degradation of aromatic contaminants [94], paper pulp bleaching [95], pitch control [96], and dye decolorization [97]. An LMS process has been introduced in the textile industry for indigo oxidation on denim garments in industrial laundries—the dye chromophore is split to yield two molecules of uncolored compounds [98].
Utilization of synthetic mediators in industrial processes is hindered by their high cost and the possible generation of toxic species. The availability of low cost and environmentally friendly natural mediators could facilitate their application in white-biotechnological processes. Indeed, the ability of some lignin-derived phenols to mediate laccase decolorization of dyes [99], removal of lignin from paper pulps [100], and oxidation of PAHs [99, 101] has been proved. As a matter of fact, the efficiency of p-coumaric acid as a natural laccase mediator has been higher or similar to that of synthetic mediators in benzo[a]pyrene degradation [101].
Jeon and coworkers [102] compared the mediating capabilities of individual mediators with those of dual-agent mediator cocktails containing ABTS, vanillin, and/or acetovanillone in the oxidation of pentachlorophenol (PCP) by Ganoderma lucidum laccase. Cocktails strongly promoted PCP removal compared to the use of each of the mediators alone. G. lucidum laccase was very prone to react with ABTS rather than vanillin and acetovanillone in the cocktails. Moreover, the presence of the ABTS radical (ABTS+·) and vanillin or acetovanillone significantly enhanced PCP removal concomitantly with ET from vanillin or acetovanillone to ABTS+·. These results strongly suggest that vanillin and acetovanillone mediate the reaction between ABTS and PCP via multiple sequential ET among laccase and its mediators.
Atypical laccases
Laccases with unusual molecular weight
Laccases with regular three domains usually have a molecular mass of 50–70 kDa or larger, whereas a two-domain MCO weighs between 30 and 40 kDa [103]. Two-domain laccases have been isolated from Botrytis cinerea [104] and from fresh fruiting bodies of Tricholoma giganteum [105]. The latter enzyme shows an N-terminal sequence with no homology to those of other mushroom laccases and is able to inhibit HIV-1 reverse transcriptase. Because of their small molecular mass, the two-domain laccases could need to assemble into a quaternary architecture to properly work. However, the reported data suggest that these enzymes work as monomers.
Laccases with quaternary structure
While most laccases are built up and act as monomers, oligomeric laccases are also known. Thus, enzymes exhibiting a homodimeric structure composed of two identical subunits have been isolated. This is the case for T. villosa [106] and Phellinus ribis [107], for the phytopathogenic ascomycetes Rhizoctonia solani [108] and Gaeumannomyces graminis [109], and for the aquatic ascomycete Phoma sp. UHH 5-1-03 [110]. The latter enzyme undergoes a pH-dependent dimerization, with the dimer predominating in a pH range of 5.0–8.0. Homodimeric laccases whose subunits account for two domain laccases have been purified from Pleurotus pulmonarius [18], Pleurotus eryngii [111], and from the mycorrhizal fungus Cantharellus cibarius [112]. These enzymes seem to need dimerization to exploit their function. Moreover, a trimeric arrangement of two-domain chains seems to be essential for some bacterial laccases to ensure a geometry of the active site similar to that of the usual MCOs [113–115]. The quaternary architecture of laccases isolated from the basidiomycetes Agaricus bisporus D621 [116] and Armillaria mellea [117], and from the ascomycetes P. anserina (a tetramer of 390 kDa) [118], Aspergillus nidulans [119], and Monocillium indicum Saxena [120] has been assumed, even if not deeply investigated.
Lac2 from A. bisporus consists of a predominant chain of about 65 kDa and several smaller polypeptides. Perry and coworkers [121] have hypothesized that the enzyme is produced as a dimer of identical polypeptides, one of which is then partially proteolytically cleaved. The heterodimeric laccases POXA3a and POXA3b purified from P. ostreatus [122] are comprised of a large (67 kDa) and a small subunit (18 or 16 kDa), whose heterogeneity is due to the presence or absence of a glycosidic moiety [123]. The POXA3 large subunit is clearly homologous to fungal laccases showing all known consensus sequences involved in copper binding but displaying some peculiarities. The well-conserved Asp206 is replaced by an Arg residue, as already found in the above-mentioned A. bisporus Lac2 [116]. It is worth noting that phylogenetic analysis of laccases revealed that heterodimeric POXA3 and Lac2 fall in the same cluster [124], in contrast to all the other members of the P. ostreatus laccase family [125]. On the other hand, the sequence of the small subunit does not show significant homology with any sequence in data banks, therefore no indication of the function of this subunit can be inferred from its primary structure. A potential role of the POXA3 small subunit in the stabilization of the heterodimer has been assumed [126]. The same role of quaternary structure in stabilizing laccases from T. villosa and R. solani has been briefly mentioned by Xu and coworkers [36]. Moreover, studies on ascorbate oxidase have allowed a stabilizing and functional role to be ascribed to the homodimer formation, as well as a role in driving the final folding to the native conformation [127, 128].
Laccases with unusual spectral properties
Although laccases usually contain four Cu atoms and show a UV absorption peak at around 600 nm, some laccases lacking the Cu1 characteristic absorption spectrum have been isolated. These laccases have been called “yellow” or “white,” although some authors do not regard them as true laccases. However, considering laccases as enzymes able to oxidize polyphenols, methoxy-substituted phenols, aromatic diamines, and a range of other compounds but not tyrosine, both “yellow” and “white” laccases can be defined as true laccases.
Yellow laccases have been purified from the phytopathogenic ascomycete G. graminis (oligomeric) [109] and from the basidiomycetes A. bisporus D621 (oligomeric) [116], Schizophyllum commune [129], Panus tigrinus [130], Phlebia radiata [131], P. ribis (oligomeric) [107], and P. ostreatus D1 [132, 133]. Yellow laccases from P. tigrinus and P. ostreatus D1 are able to oxidize nonphenolic aromatic substrates in the absence of mediators, contrary to their blue counterparts [130, 132, 133]. Leontievsky and colleagues [130] have supposed that yellow laccases are formed as a result of binding of aromatic products of lignin degradation to the blue laccase during fungal growth in solid-state conditions. A consequence of this modification may be the reduction of Cu1 and Cu2 in the active center of the enzyme and the disappearance of the blue color. Apparently, the modifier molecule bound to the apoenzyme of the yellow laccase performs the function of ET mediator in the reaction with nonphenolic substrate.
The first purification of a white laccase was obtained from the basidiomycete P. ostreatus [134]. POXA1w is a neutral laccase showing a remarkably high stability with respect to both pH and temperature. The enzyme contains only one copper atom/molecule instead of the usual four, along with two zinc atoms and one iron atom in each protein molecule. Other so-called white laccases were purified from Pycnoporous sanguineus [135], from T. hirsuta [136], and from P. radiata BP-11-2 [137]. The analysis of metal ion contents of the laccase from T. hirsuta has shown the presence of copper and manganese in a 3:1 ratio. This laccase is able to oxidize the hydroxy polyaromatic dye alizarin red S and the nonphenolic dye methyl red without mediators.
Other atypical laccases are secreted by the basidiomycete Steccherinum ochraceum strain 1833 [138]. Their absorption band around 600 nm shows values of molar extinction coefficients (ranging from 7,200 to 7,800 M−l cm−1 at 611 nm) higher than those of other laccases and a lower shoulder of absorption at 330 nm. These features can be the result of slightly different coordination geometries of the Cu1 and Cu3 ions. Both isoforms from S. ochraceum 1833 show high optimal temperatures and significant thermostability.
In conclusion, results obtained on these laccases highlight, on the one hand, their ability to oxidize nonphenolic substrates and, on the other hand, their high stability or functionality in extreme conditions. The properties of nonblue laccases give rise to many questions. For example, is it possible to link the peculiarity of these enzymes to an explicit function? If transition metals are simply replacing the positions of copper in laccases, then which ion takes which position? Moreover, if some copper ions of laccases can be replaced with other metals, can copper ions in other MCOs be replaced without losing the function? Nakamura and Go [103] propose that the Cu1 may be replaced with Fe/Mn (losing the blue color) and the Cu3 may be replaced with zinc ions, with the Cu2 remaining.
Laccase gene families
Multiplicity of laccase genes is a common feature in fungi, and the production of several laccase isozymes has been observed in many species. Perry and coworkers [121] described the presence of two laccase genes in the same chromosome of the basidiomycetes A. bisporus, thus reporting the first example of a laccase gene family in fungi. Five distinct laccase genes have been characterized from T. villosa [106] and Trametes sanguinea [139], four from R. solani [108], and three from Trametes sp. I62 [140], Trametes sp. AH28-2 [141], and G. graminis [142]. Laccase gene families have also been described in Pleurotus genera, with four isolated members in P. sajior-caju [143], two in P. eryngii [144], and seven in P. ostreatus [125]. Characterization of laccase gene families has been enhanced by the availability of a growing number of fungal genome sequences. In the Coprinopsis cinerea genome, 17 nonallelic laccase genes were identified and at least 9 of these members are translated into functional laccase products [145]. Likewise, the occurrence of laccase genes in brown-rot fungi has been identified with the discovery of two laccase gene models in the Postia placenta genome [146]. Investigation of the ectomycorrhizal fungus Laccaria bicolor genome disclosed a complex MCO family composed of 11 members, 9 of which correspond to laccases in sensu stricto [147]. The analysis of the recently released P. ostreatus genome has highlighted the presence of previously uncharacterized laccase genes, enriching the panel of laccase genes up to 12 members (unpublished data).
Gene clustering of laccase genes has been observed in many of the above-mentioned examples. The two tandem organized laccase genes from A. bisporus have been mapped 1.5 kb apart [121], and the three genes in R. solani are linked within a 12-kb fragment [108]. A genomic fragment of 150 kb has been shown to contain seven laccase genes in P. ostreatus [125]. The 17 laccase genes of C. cinerea are clustered at seven different loci in the genome [145], whereas the nine laccase-encoding genes from L. bicolor are randomly distributed throughout the genome, except for three clustered genes, suggesting a more complex story of gene duplication events [147]. Based on intron composition and protein similarity, laccase subfamilies have been identified within several gene families [106, 125, 139, 145].
The occurrence of such complex gene families gives rise to a key question: why should a fungus require more than one laccase? A plausible explanation can be put forward considering the variety of different physiological functions proposed for this enzyme during the fungal life cycle. Fungal laccases have been associated with delignification [124], fruiting body formation [148], pigment formation during asexual development [149], pathogenesis [142, 150], and competitor interactions [151]. Laccases of saprophytic and mycorrhizal fungi have also been implicated in soil organic matter cycling [152]. It can be inferred that the paralogous laccase copies within the same species may have specifically evolved to fulfill a variety of targeted functions. The phylogenetic analysis of basidiomycetous laccases further supports this idea, since clustering of the sequences in the neighbor-joining tree was found to reflect, at least in part, the function of the respective enzymes [124].
Laccase gene transcriptional regulation
Synthesis and secretion of laccases are strictly influenced by nutrient levels, culture conditions, and developmental stage as well as by the addition of different inducers to cultural media. The effect of these factors at the level of laccase gene transcription has been demonstrated in many fungal species. In most of the reported examples, laccase expression is regulated by an array of factors, often acting in a synergistic way [153–155].
Metals
Regulation of laccase expression by metals is widespread in fungi. Copper has been shown to regulate transcript levels in T. versicolor [156], C. subvermispora [157], P. ostreatus [158], P. sajor-caju [143], and Trametes pubescens [155]. Cd+2, Ag+, and Mn+2 ions have also been reported as strong modulators of laccase transcription levels [143, 159]. It is worth noting that the same metal can exert opposite effects in different species [160]. In many cases, the observed effect is associated with the presence of putative metal-responsive elements (MRE) in the laccase promoter regions. However, only a few reports go through the molecular mechanisms underlying laccase regulation by metals. In P. ostreatus, the formation of protein complexes on specific MRE elements identified in the promoter regions of two copper-induced laccase genes poxc and poxa1b [158] has been found to occur only in the absence of copper ions. Thus, the involvement of a negative-acting regulatory factor in metal response has been proposed. Moreover, in poxa1b promoter, a GC-rich region homologous to the core binding site for transcription factor Sp1 has been found to decrease the binding affinity of the adjacent MRE, affecting its interactions with fungal protein factors [161].
In silico inspection of some laccase promoters has highlighted the presence of several putative “activation of cup1 expression” (ACE) responsive elements. First described in S. cerevisiae, ACE is the recognition site for the ACE1 copper-responsive transcription factor, which binds its target sequence in a copper-loaded conformation, activating the transcription of several genes [162]. Fungal ACE1-like transcription factors have been isolated in C. subvermispora [163]. It is worth noting that copper induction has been observed for laccase genes whose promoters lack ACE or MRE elements, thus a different mechanism, which might involve the generation of reactive oxygen species, has been hypothesized [156].
Aromatic compounds
Aromatic compounds structurally related to lignin, such as xylidine (XYL), ferulic acid (FA), or veratric acid (VA), are routinely added to fungal cultures to increase laccase production [106, 156, 164]. Laccase induction has been also demonstrated in the presence of dyes, the induction level being highly sensitive to small differences in the chemical structures [165]. Laccase induction by phenolic substances may represent a response developed by fungi against highly reactive aromatic compounds—by catalyzing their polymerization, laccases play a defensive role, reducing the oxidative stress caused by oxygen radicals that originate from these molecules [4]. In many cases, the induction has been found to occur at the transcriptional level, in agreement with the finding of putative xenobiotic response elements (XRE) in the upstream regions of several induced laccase genes, such as those from T. versicolor [156], P. sajor caju [143], P. ostreatus [161], and Trametes sp. AH28-2 [141]. White-rot fungi display a wide diversity in their response to aromatic inducers. Transcription of one laccase gene, lcc1, from T. villosa is induced by the addition of XYL, but a second gene is constitutively expressed under tested conditions [106]. Likewise, lcc1 transcript titres in Volvariella volvacea are differentially tuned by the addition of various aromatic compounds, whereas lcc4 transcription is not affected by these molecules [148]. Laccase induction in various fungi seems to be specific to certain aromatic compounds [141, 143]. In T. versicolor, XYL and HBT effectively activate lcc transcription, whereas no induction is observed in the presence of either VA and FA [156]. In the ligninolytic basidiomycete Trametes sp. I62, nine structural closely related aromatic compounds appear to have different effects on laccase gene expression. The three laccase genes of this fungus are differentially expressed in response to these compounds, with specific induction patterns being observed for each molecule tested [153].
Nitrogen and carbon sources
Laccase activity has also been shown to be dependent on the concentration and nature of carbon and nitrogen sources as well as on their ratio [166]. As far as nitrogen sources are concerned, change in laccase activity in response to nitrogen concentration is a controversial issue, since examples of activity increases have been described under both limiting and nonlimiting conditions [167]. Laccase induction at the transcriptional level has been reported to occur under nonlimiting nitrogen levels in T. versicolor [4] and in the ligninolytic basidiomycete I62 [167]. Expression of lcc1 in T. trogii is preferentially induced by organic nitrogen sources compared with inorganic ones [168]. Individual laccase isoenzyme genes in P. sajor-caju are differentially induced at the transcriptional level by nitrogen sources. Positive regulation by nitrogen has been found to occur in lac2 and lac4, whereas lac1 and lac3 are largely unaffected [143]. The observed N-regulation may be mediated by a NIT2-like protein (NIT2 is involved in N-regulation in N. crassa) since NIT2-binding sites have been found in the upstream region of lac4 in P. sajor-caju and of lcc1 and lcc2 in basidiomycete I62 [167]. Laccase expression has also been found to be subjected to catabolite repression. High glucose levels inhibit laccase transcription in basidiomycete I62 [167], T. pubescens [155], and Trametes sp. AH28-2 [141]. Putative CreA-binding sites have been mapped in the promoter regions of these repressed laccase genes suggesting the existence of a carbon catabolite repressor similar to the CreA isolated in A. nidulans [169].
Fungal growth stages
Several reports support the hypothesis that members of the laccase families may play different roles during the life cycle of the organism [154]. Both lcc1 and lcc2 transcriptions in Trametes sp. I62 are inducible at different growth stages—lcc1 is expressed in early stages of growth and lcc2 in the stationary phase [167]. In A. bisporus and L. edodes growing on solid substrates, laccase expression has been found to be greatest at the fully colonized stage and then declining to very low levels during fruiting [170, 171]. This expression profile is in accordance with a direct involvement of laccase activity in lignin bioconversion, which is expected to be required in the earlier stages of substrate colonization. On the other hand, in V. volvacea a key role in the morphogenesis of its fruiting body has been inferred for lcc1, since laccase expression is induced later in the substrate colonization phase and at the onset stages of fruiting body morphogenesis (primordium formation, pinhead stage) [148]. It has been proposed that laccase could crosslink hyphal walls into coherent aggregates during primordium formation and may continue to act on the hyphal surfaces throughout fruiting body development. A strong correlation between laccase expression and fungal morphogenesis has been determined by transcript analysis of the MCO gene family in the L. bicolor ectomycorrhizal fungus [147]. Transcript profiling has unveiled that L. bicolor laccase genes are differentially expressed as a function of the developmental stage. Some transcripts accumulate in the fruiting bodies, others are believed to play a role in the functioning of fungal symbiotic tissues, and some others are greatest in the free-living mycelium grown on agar medium.
Pathogenesis
Expression studies providing evidence for laccase involvement in pathogenesis have been reported. In the root pathogen G. graminis, lac2 has been found to be expressed only in planta or in the presence of plant homogenate, thus providing the first evidence of a fungal laccase whose transcription depends on the presence of a host [142]. The authors have proposed a specific role for this laccase in plant pathogenesis, suggesting that it could degrade lignin depositions produced by the plant in response to invasion, and/or oxidize/reduce phytoalexins and other toxic compounds.
Laccases have also been described as prominent virulence factors in Cryptococcus neoformans, a pathogenic yeast causing meningoencephalitis [150]. The expression of C. neoformans laccases has been found to be induced in response to reactive oxygen and nitrogen species produced by macrophages as a defense mechanism. Ssa1, a Hsp70 homologue identified in C. neoformans, interacts with a heat shock-factor (HSF) acting as a transcriptional co-activator of fungal laccase in response to the attack encountered in the host [172]. Missal and coworkers [150] have also reported the importance of two C. neoformans laccase isoforms in fungal virulence, suggesting that independent regulatory mechanisms may control the expression of these two genes during nitrosative but not oxidative stress.
Laccases are ancient enzymes with a promising future. This class of enzymes still remains of great relevance both as a model for structure/function relationships and as a green tool in many processes of the biotechnology industries in the near future.
Acknowledgements
This work is supported by grants from the Ministero dell’Università e della Ricerca Scientifica (Progetti di Rilevante Interesse Nazionale, PRIN) and from COST Action FP0602 “Biotechnology for lignocellulose biorefineries” (BIOBIO).
References
- 1.Solomon EI, Sundaram UM, Machonkin TE. Multicopper oxidases and oxygenases. Chem Rev. 1996;96:2563–2605. doi: 10.1021/cr950046o. [DOI] [PubMed] [Google Scholar]
- 2.Messerschmidt A. Multi-copper oxidases. Singapore: World Scientific; 1997. [Google Scholar]
- 3.Yoshida H. Chemistry of lacquer (urushi). Part I. J Chem Soc. 1883;43:472–486. [Google Scholar]
- 4.Thurston CF. The structure and function of fungal laccases. Microbiology. 1994;140:19–26. doi: 10.1099/13500872-140-1-19. [DOI] [Google Scholar]
- 5.Gianfreda L, Xu F, Bollag JM. Laccases: a useful group of oxidoreductive enzymes. Bioremediat J. 1999;3:1–26. doi: 10.1080/10889869991219163. [DOI] [Google Scholar]
- 6.Heinzkill M, Messner K. The ligninolytic system of fungi. In: Anke T, editor. Fungal biotechnology. Weinheim: Chapman & Hall; 1997. pp. 213–226. [Google Scholar]
- 7.Bao W, O’Malley DM, Whetten R, Sederoff RR. A laccase associated with lignification in loblolly pine xylem. Science. 1993;260:672–674. doi: 10.1126/science.260.5108.672. [DOI] [PubMed] [Google Scholar]
- 8.Sato Y, Bao W, Sederoff R, Whetten R. Molecular cloning and expression of eight laccase cDNAs in loblolly pine (Pinus taeda) J Plant Res. 2001;114:147–155. doi: 10.1007/PL00013978. [DOI] [Google Scholar]
- 9.Sharma P, Goel R, Capalash N. Bacterial laccases. World J Microbiol Biotechnol. 2007;23:823–832. doi: 10.1007/s11274-006-9305-3. [DOI] [Google Scholar]
- 10.Roberts SA, Weichsel A, Grass G, Thakali K, Hazzard JT, Tollin G, Rensing C, Montfort WR. Crystal structure and electron transfer kinetics of CueO, a multicopper oxidase required for copper homeostasis in Escherichia coli . Proc Natl Acad Sci USA. 2002;99:2766–2771. doi: 10.1073/pnas.052710499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enguita FJ, Martins LO, Henriques AO, Carrondo MA. Crystal structure of a bacterial endospore coat component. A laccase with enhanced thermostability properties. J Biol Chem. 2003;278:19416–19425. doi: 10.1074/jbc.M301251200. [DOI] [PubMed] [Google Scholar]
- 12.Dittmer NT, Suderman RJ, Jiang H, Zhu YC, Gorman MJ, Kramer KJ, Kanost MR. Characterization of cDNAs encoding putative laccase-like multicopper oxidases and developmental expression in the tobacco hornworm, Manduca sexta, and the malaria mosquito, Anopheles gambiae . Insect Biochem Mol Biol. 2004;34:29–41. doi: 10.1016/j.ibmb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Parkinson NM, Conyers CM, Keen JN, MacNicoll AD, Smith I, Weaver RJ. cDNAs encoding large venom proteins from the parasitoid wasp Pimpla hypochondriaca identified by random sequence analysis. Comp Biochem Physiol C Toxicol Pharmacol. 2003;134:513–520. doi: 10.1016/S1532-0456(03)00041-3. [DOI] [PubMed] [Google Scholar]
- 14.Yaropolov AI, Skorobogat’ko OV, Vartanov SS, Varfolomeev SD. Laccase: properties, catalytic mechanism, and applicability. Appl Biochem Biotechnol. 1994;49:257–280. doi: 10.1007/BF02783061. [DOI] [Google Scholar]
- 15.Sakurai T. Anaerobic reactions of Rhus vernicifera laccase and its type-2 copper-depleted derivatives with hexacyanoferrate(II) Biochem J. 1992;284:681–685. doi: 10.1042/bj2840681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofer C, Schlosser D. Novel enzymatic oxidation of Mn2+ to Mn3+ catalyzed by a fungal laccase. FEBS Lett. 1999;451:186–190. doi: 10.1016/S0014-5793(99)00566-9. [DOI] [PubMed] [Google Scholar]
- 17.Schlosser D, Hofer C. Laccase-catalyzed oxidation of Mn2+ in the presence of natural Mn3+ chelators as a novel source of extracellular H2O2 production and its impact on manganese peroxidase. Appl Environ Microbiol. 2002;68:3514–3521. doi: 10.1128/AEM.68.7.3514-3521.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Souza CGM, Peralta RM. Purification and characterization of the main laccase produced by the white-rot fungus Pleurotus pulmonarius on wheat bran solid state medium. J Basic Microbiol. 2003;43:278–286. doi: 10.1002/jobm.200390031. [DOI] [PubMed] [Google Scholar]
- 19.Shleev SV, Morozova O, Nikitina O, Gorshina ES, Rusinova T, Serezhenkov VA, Burbaev DS, Gazaryan IG, Yaropolov AI. Comparison of physico-chemical characteristics of four laccases from different basidiomycetes. Biochimie. 2004;86:693–703. doi: 10.1016/j.biochi.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Kumar SVS, Phale PS, Durani S, Wangikar PP. Combined sequence and structure analysis of the fungal laccase family. Biotechnol Bioeng. 2003;83:386–394. doi: 10.1002/bit.10681. [DOI] [PubMed] [Google Scholar]
- 21.Larrondo LF, Salas L, Melo F, Vicuna R, Cullen D. A novel extracellular multicopper oxidase from Phanerochaete chrysosporium with ferroxidase activity. Appl Environ Microbiol. 2003;69:6257–6263. doi: 10.1128/AEM.69.10.6257-6263.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ducros V, Brzozowski AM, Wilson KS, Brown SH, Østergaard P, Schneider P, Yaver DS, Pedersen AH, Davies GJ. Crystal structure of the type-2 Cu depleted laccase from Coprinus cinereus at 2.2 Å resolution. Nat Struct Biol. 1998;5:310–316. doi: 10.1038/nsb0498-310. [DOI] [PubMed] [Google Scholar]
- 23.Piontek K, Antorini M, Choinowski T. Crystal structure of a laccase from the fungus Trametes versicolor at 1.90-Å resolution containing a full complement of coppers. J Biol Chem. 2002;277:37663–37669. doi: 10.1074/jbc.M204571200. [DOI] [PubMed] [Google Scholar]
- 24.Bertrand T, Jolivalt C, Briozzo P, Caminade E, Joly N, Madzak C, Mougin C. Crystal structure of a four-copper laccase complexed with an arylamine: insights into substrate recognition and correlation with kinetics. Biochemistry. 2002;41:7325–7333. doi: 10.1021/bi0201318. [DOI] [PubMed] [Google Scholar]
- 25.Garavaglia S, Cambria MT, Miglio M, Ragusa S, Iacobazzi V, Palmieri F, D’Ambrosio C, Scaloni A, Rizzi M. The structure of Rigidoporus lignosus laccase containing a full complement of copper ions, reveals an asymmetrical arrangement for the T3 copper pair. J Mol Biol. 2004;342:1519–1531. doi: 10.1016/j.jmb.2004.07.100. [DOI] [PubMed] [Google Scholar]
- 26.Ferraroni M, Myasoedova NM, Schmatchenko V, Leontievsky AA, Golovleva LA, Scozzafava A, Briganti F. Crystal structure of a blue laccase from Lentinus tigrinus: evidences for intermediates in the molecular oxygen reductive splitting by multicopper oxidases. BMC Struct Biol. 2007;7:60–72. doi: 10.1186/1472-6807-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matera I, Gullotto A, Tilli S, Ferraroni M, Scozzafava A, Briganti F. Crystal structure of the blue multicopper oxidase from the white-rot fungus Trametes trogii complexed with p-toluate . Inorg Chim Acta. 2008;361:4129–4137. doi: 10.1016/j.ica.2008.03.091. [DOI] [Google Scholar]
- 28.Hakulinen N, Kiiskinen LL, Kruus K, Saloheimo M, Paananen A, Koivula A, Rouvinen J. Crystal structure of a laccase from Melanocarpus albomyces with an intact trinuclear copper site. Nat Struct Biol. 2002;9:601–605. doi: 10.1038/nsb823. [DOI] [PubMed] [Google Scholar]
- 29.Hakulinen N, Andberg M, Kallio J, Koivula A, Kruus K, Rouvinen J. A near atomic resolution structure of a Melanocarpus albomyces laccase. J Struc Biol. 2008;162:29–39. doi: 10.1016/j.jsb.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Murphy MEP, Lindley PF, Adman ET. Structural comparison of cupredoxin domains: domain recycling to construct proteins with novel functions. Protein Sci. 1997;6:761–770. doi: 10.1002/pro.5560060402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messerschmidt A, Ladenstein R, Huber R, Bolognesi M, Avigliano L, Petruzzelli R, Rossi A, Finazzi Agrò A. Refined crystal structure of ascorbate oxidase at 1.9 Å resolution. J Mol Biol. 1992;224:179–205. doi: 10.1016/0022-2836(92)90583-6. [DOI] [PubMed] [Google Scholar]
- 32.Zaitsev I, Zaitsev V, Card G, Moshkov K, Bax B, Ralph A, Lindley P. The nature of the copper centres in human ceruloplasmin. J Biol Inorg Chem. 1996;1:15–23. doi: 10.1007/s007750050018. [DOI] [Google Scholar]
- 33.Lee SK, George SD, Antholine WE, Hedman B, Hodgson KO, Solomon EI. Nature of the intermediate formed in the reduction of O2 to H2O at the trinuclear copper cluster active site in native laccase. J Am Chem Soc. 2002;124:6180–6193. doi: 10.1021/ja0114052. [DOI] [PubMed] [Google Scholar]
- 34.Solomon EI, Augustine AJ, Yoon J. O2 reduction to H2O by the multicopper oxidases. Dalton Trans. 2008;30:3921–3932. doi: 10.1039/b800799c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcus RA, Sutin N. Electron transfers in chemistry and biology. Biochim Biophys Acta. 1985;811:265–322. [Google Scholar]
- 36.Xu F, Shin W, Brown SH, Wahleithner JA, Sundaram UM, Solomon EI. A study of a series of recombinant fungal laccases and birilubin oxidase that exhibit significant differences in redox potential, substrate specificity and stability. Biochim Biophys Acta. 1996;1292:303–311. doi: 10.1016/0167-4838(95)00210-3. [DOI] [PubMed] [Google Scholar]
- 37.Xu F, Palmer AE, Yaver DS, Berka RM, Gambetta GA, Brown SH, Solomon EI. Targeted mutations in a Trametes villosa laccase, axial perturbations of the T1 copper. J Biol Chem. 1999;274:12372–12375. doi: 10.1074/jbc.274.18.12372. [DOI] [PubMed] [Google Scholar]
- 38.Klonowska A, Gaudin C, Fournel A, Asso M, Le Petit J, Giorgi M, Tron T. Characterization of a low redox potential laccase from the basidiomycete C30 . Eur J Biochem. 2002;269:6119–6125. doi: 10.1046/j.1432-1033.2002.03324.x. [DOI] [PubMed] [Google Scholar]
- 39.Gray HB, Malmstrom BG, Williams RJ. Copper coordination in blue proteins. J Biol Inorg Chem. 2000;5:551–559. doi: 10.1007/s007750000146. [DOI] [PubMed] [Google Scholar]
- 40.Durao P, Bento I, Fernandes AT, Melo EP, Lindley PF, Martins LO. Perturbation of the T1 copper site in CotA-laccase from Bacillus subtilis: structural, biochemical, enzymatic and stability studies. J Biol Inorg Chem. 2006;11:514–526. doi: 10.1007/s00775-006-0102-0. [DOI] [PubMed] [Google Scholar]
- 41.Enguita FJ, Marcal D, Martins LO, Grenha R, Henriques AO, Lindley PF, Carrondo MA. Substrate and dioxygen binding to the endospore coat laccase from Bacillus subtilis . J Biol Chem. 2004;279:23472–23476. doi: 10.1074/jbc.M314000200. [DOI] [PubMed] [Google Scholar]
- 42.Xu F. Effects of redox potential and hydroxide inhibition on the pH activity profile of fungal laccases. J Biol Chem. 1997;272:924–928. doi: 10.1074/jbc.272.2.924. [DOI] [PubMed] [Google Scholar]
- 43.Xu F, Berka RM, Wahleithner JA, Nelson BA, Shuster JR, Brown SH, Palmer AE, Solomon EI. Site-directed mutations in fungal laccase: effect on redox potential, activity and pH profile. Biochem J. 1998;334:63–70. doi: 10.1042/bj3340063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madzak C, Mimmi MC, Caminade E, Brault A, Baumberger S, Briozzo P, Mougin C, Jolivalt C. Shifting the optimal pH of activity for a laccase from the fungus Trametes versicolor by structure-based mutagenesis. Protein Eng Des Sel. 2006;19:77–84. doi: 10.1093/protein/gzj004. [DOI] [PubMed] [Google Scholar]
- 45.Bonomo RP, Boudet AM, Cozzolino R, Rizzarelli E, Santoro AM, Sterjiades R, Zappala R. A comparative study of two isoforms of laccase secreted by the “white-rot” fungus Rigidoporus lignosus, exhibiting significant structural and functional differences. J Inorg Biochem. 1998;71:205–211. doi: 10.1016/S0162-0134(98)10057-0. [DOI] [PubMed] [Google Scholar]
- 46.Tadesse MA, D’Annibale A, Galli C, Gentilia P, Sergia F. An assessment of the relative contributions of redox and steric issues to laccase specificity towards putative substrates. Org Biomol Chem. 2008;6:868–878. doi: 10.1039/b716002j. [DOI] [PubMed] [Google Scholar]
- 47.Torres J, Svistunenko D, Karlsson B, Cooper CE, Wilson MT. Fast reduction of a copper center in laccase by nitric oxide and formation of a peroxide intermediate. J Am Chem Soc. 2002;124:963–967. doi: 10.1021/ja016107j. [DOI] [PubMed] [Google Scholar]
- 48.Yoon J, Solomon EI. Electronic structure of the peroxy intermediate and its correlation to the native intermediate in the multicopper oxidases: insights into the reductive cleavage of the O–O bond. J Am Chem Soc. 2007;129:13127–13136. doi: 10.1021/ja073947a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoon J, Liboiron BD, Sarangi R, Hodgson KO, Hedman B, Solomon EI. The two oxidized forms of the trinuclear Cu cluster in the multicopper oxidases and mechanism for the decay of the native intermediate. Proc Natl Acad Sci USA. 2007;104:13609–13614. doi: 10.1073/pnas.0705137104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palmer AE, Lee SK, Solomon EI. Decay of the peroxide intermediate in laccase: reductive cleavage of the O–O bond. J Am Chem Soc. 2001;123:6591–6599. doi: 10.1021/ja010365z. [DOI] [PubMed] [Google Scholar]
- 51.Zoppellaro G, Sakurai T, Huang H. A novel mixed valence form of Rhus vernicifera laccase and its reaction with dioxygen to give a peroxide intermediate bound to the trinuclear center. J Biochem. 2001;129:949–953. doi: 10.1093/oxfordjournals.jbchem.a002942. [DOI] [PubMed] [Google Scholar]
- 52.Augustine AJ, Quintanar L, Stoj CS, Kosman DJ, Solomon EI. Spectroscopic and kinetic studies of perturbed trinuclear copper clusters: the role of protons in reductive cleavage of the O–O bond in the multicopper oxidase Fet3p. J Am Chem Soc. 2007;129:13118–13126. doi: 10.1021/ja073905m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Augustine AJ, Kragh ME, Sarangi R, Fujii S, Liboiron BD, Stoj CS, Kosman DJ, Hodgson KO, Hedman B, Solomon EI. Spectroscopic studies of perturbed T1 Cu sites in the multicopper oxidases Saccharomyces cerevisiae Fet3p and Rhus vernicifera laccase: allosteric coupling between the T1 and trinuclear Cu sites. Biochemistry. 2008;47:2036–2045. doi: 10.1021/bi7020052. [DOI] [PubMed] [Google Scholar]
- 54.Quintanar L, Yoon J, Aznar CP, Palmer AE, Andersson KK, Britt RD, Solomon EI. Spectroscopic and electronic structure studies of the trinuclear Cu cluster active site of the multicopper oxidase laccase: nature of its coordination unsaturation. J Am Chem Soc. 2005;127:13832–13845. doi: 10.1021/ja0421405. [DOI] [PubMed] [Google Scholar]
- 55.Bento I, Martins LO, Lopes GG, Carrondo MA, Lindley PF. Dioxygen reduction by multi-copper oxidases; a structural perspective. Dalton Trans. 2005;21:3507–3513. doi: 10.1039/b504806k. [DOI] [PubMed] [Google Scholar]
- 56.Kyritsis P, Messerschmidt A, Huber R, Salmon GA, Sykes AG. Pulse-radiolysis studies on the oxidized form of the multicopper enzyme ascorbate oxidase—evidence for 2 intramolecular electron-transfer steps. Dalton Trans. 1993;5:731–735. [Google Scholar]
- 57.Fernandez Larrea J, Stahl U. Isolation and characterization of a laccase gene from Podospora anserina . Mol Gen Genet. 1996;252:539–551. doi: 10.1007/BF02172400. [DOI] [PubMed] [Google Scholar]
- 58.Germann UA, Muller G, Hunziker PE, Lerch K. Characterization of 2 allelic forms of Neurospora crassa laccase amino-terminal and carboxyl-terminal processing of a precursor. J Biol Chem. 1988;263:885–896. [PubMed] [Google Scholar]
- 59.Kiiskinen LL, Saloheimo M. Molecular cloning and expression in Saccharomyces cerevisiae of a laccase gene from the ascomycete Melanocarpus albomyces . Appl Environ Microb. 2004;70:137–144. doi: 10.1128/AEM.70.1.137-144.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bulter T, Alcalde M, Sieber V, Meinhold P, Schlachtbauer C, Arnold FH. Functional expression of a fungal laccase in Saccharomyces cerevisiae by directed evolution. Appl Environ Microb. 2003;69:987–995. doi: 10.1128/AEM.69.2.987-995.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zumárraga M, Camarero S, Shleev S, Martínez-Arias A, Ballesteros A, Plou FJ, Alcalde M. Altering the laccase functionality by in vivo assembly of mutant libraries with different mutational spectra. Proteins. 2008;71:250–260. doi: 10.1002/prot.21699. [DOI] [PubMed] [Google Scholar]
- 62.Gelo-Pujic M, Kim HH, Butlin NG, Palmore GT. Electrochemical studies of a truncated laccase produced in Pichia pastoris . Appl Environ Microbiol. 1999;65:5515–5521. doi: 10.1128/aem.65.12.5515-5521.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giardina P, Palmieri G, Scaloni A, Fontanella B, Faraco V, Cennamo G, Sannia G. Protein and gene structure of a blue laccase from Pleurotus ostreatus . Biochem J. 1999;341:655–663. doi: 10.1042/0264-6021:3410655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Piscitelli A, Giardina P, Mazzoni C, Sannia G. Recombinant expression of Pleurotus ostreatus laccases in Kluyveromyces lactis and Saccharomyces cerevisiae . Appl Microbiol Biotechnol. 2005;69:428–439. doi: 10.1007/s00253-005-0004-z. [DOI] [PubMed] [Google Scholar]
- 65.Festa G, Autore F, Fraternali F, Giardina P, Sannia G. Development of new laccases by directed evolution: functional and computational analyses. Proteins. 2007;72:25–34. doi: 10.1002/prot.21889. [DOI] [PubMed] [Google Scholar]
- 66.Kawai S, Umezawa T, Higuchi T. Degradation mechanisms of phenolic beta-1 lignin substructure model compounds by laccase of Coriolus versicolor . Arch Biochem Biophys. 1988;262:99–110. doi: 10.1016/0003-9861(88)90172-5. [DOI] [PubMed] [Google Scholar]
- 67.Xu F. Oxidation of phenols, anilines, and benzenethiols by fungal laccases: correlation between activity and redox potentials as well as halide inhibition. Biochem. 1996;35:7608–7614. doi: 10.1021/bi952971a. [DOI] [PubMed] [Google Scholar]
- 68.Garzillo AM, Colao MC, Caruso C, Caporale C, Celletti D, Buonocore V. Laccase from the white-rot fungus Trametes trogii . Appl Microbiol Biotechnol. 1998;49:545–551. doi: 10.1007/s002530051211. [DOI] [PubMed] [Google Scholar]
- 69.Bajpai P. Application of enzymes in the pulp and paper industry. Biotechnol Prog. 1999;15:147–157. doi: 10.1021/bp990013k. [DOI] [PubMed] [Google Scholar]
- 70.Durán N, Esposito E. Potential applications of oxidative enzymes and phenoloxidase-like compounds in wastewater and soil treatment: a review. Appl Catal B Environ. 2000;28:83–99. doi: 10.1016/S0926-3373(00)00168-5. [DOI] [Google Scholar]
- 71.Abadulla E, Tzanov T, Costa S, Robra KH, Cavaco-Paulo A, Gübitz GM. Decolorization and detoxification of textile dyes with a laccase from Trametes hirsuta . Appl Environ Microbiol. 2000;66:3357–3362. doi: 10.1128/AEM.66.8.3357-3362.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Minussi R, Pastore GM, Duran N. Potential applications of laccase in the food industry. Trends Food Sci Technol. 2002;13:205–216. doi: 10.1016/S0924-2244(02)00155-3. [DOI] [Google Scholar]
- 73.Amir L, Tam TK, Pita M, Meijler MM, Alfonta L, Katz E. Biofuel cell controlled by enzyme logic systems. J Am Chem Soc. 2009;131:826–832. doi: 10.1021/ja8076704. [DOI] [PubMed] [Google Scholar]
- 74.Pereira L, Coelho AV, Viegas CA, Santos MM, Robalo MP, Martins LO. Enzymatic biotransformation of the azo dye Sudan Orange G with bacterial CotA-laccase. J Biotechnol. 2009;139:68–77. doi: 10.1016/j.jbiotec.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 75.Couto SR. Decolouration of industrial azo dyes by crude laccase from Trametes hirsuta . J Hazard Mater. 2007;148:768–770. doi: 10.1016/j.jhazmat.2007.06.123. [DOI] [PubMed] [Google Scholar]
- 76.Palmieri G, Cennamo G, Sannia G. Remazol Brilliant Blue R decolourisation by the fungus Pleurotus ostreatus and its oxidative enzymatic system. Enzyme Microb Technol. 2005;36:17–24. doi: 10.1016/j.enzmictec.2004.03.026. [DOI] [Google Scholar]
- 77.Palmieri G, Giardina P, Sannia G. Laccase-mediated Remazol Brilliant Blue R decolorization in a fixed-bed bioreactor. Biotechnol Prog. 2005;21:1436–1441. doi: 10.1021/bp050140i. [DOI] [PubMed] [Google Scholar]
- 78.Vanhulle S, Trovaslet M, Enaud E, Lucas M, Sonveaux M, Decock C, Onderwater R, Schneider YJ, Corbisier AM. Cytotoxicity and genotoxicity evolution during decolourisation of dyes by white rot fungi. World J Microbiol Biotechnol. 2008;24:337–344. doi: 10.1007/s11274-007-9475-7. [DOI] [Google Scholar]
- 79.Vanhulle S, Trovaslet M, Enaud E, Lucas M, Taghavi S, Van Der Lelie D, Van Aken B, Foret M, Onderwater R, Wesenberg D, Agathos S, Schneider YJ, Corbisier AM. Decolourisation, cytotoxicity and genotoxicity reduction during a combined ozonation/fungal treatment of dye contaminated wastewater. Environ Sci Technol. 2008;42:584–589. doi: 10.1021/es071300k. [DOI] [PubMed] [Google Scholar]
- 80.Kunamneni A, Camarero S, García-Burgos C, Plou FJ, Ballesteros A, Alcalde M. Engineering and applications of fungal laccases for organic synthesis. Microb Cell Fact. 2008;7:32–49. doi: 10.1186/1475-2859-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aktaş N, Tanyolaç A. Reaction conditions for laccase catalyzed polymerization of catechol. Bioresour Technol. 2003;87:209–214. doi: 10.1016/S0960-8524(02)00254-7. [DOI] [PubMed] [Google Scholar]
- 82.Ceylan H, Kubilay S, Aktas N, Sahiner N. An approach for prediction of optimum reaction conditions for laccase-catalyzed bio-transformation of 1-naphthol by response surface methodology (RSM) Bioresour Technol. 2008;99:2025–2031. doi: 10.1016/j.biortech.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 83.Ikeda R, Tanaka H, Oyabu H, Uyama H, Kobayashi S. Preparation of artificial urushi via an environmentally benign process. Bull Chem Soc Jpn. 2001;74:1067–1073. doi: 10.1246/bcsj.74.1067. [DOI] [Google Scholar]
- 84.Bruyneel F, Enaud E, Billottet L, Vanhulle S, Marchand-Brynaert J. Regioselective synthesis of 3-hydroxyorthanilic acid and its biotransformation with laccase into a novel phenoxazinone dye. Eur J Org Chem. 2008;1:72–79. doi: 10.1002/ejoc.200700865. [DOI] [Google Scholar]
- 85.Ossiadacz J, Al-Adhami AJH, Bajraszewska D, Fischer P, Peczynska-Czoch W. On the use of Trametes versicolor laccase for the conversion of 4-methyl-3-hydroxyanthranilic acid to actinocin chromophore. J Biotechnol. 1999;72:141–149. doi: 10.1016/S0168-1656(99)00100-5. [DOI] [Google Scholar]
- 86.Nicotra S, Cramarossa MR, Mucci A, Pagnoni UM, Riva S, Forti L. Biotransformation of resveratrol: synthesis of trans-dehydrodimers catalyzed by laccases from Myceliophtora thermophyla and from Trametes pubescens . Tetrahedron. 2004;60:595–600. doi: 10.1016/j.tet.2003.10.117. [DOI] [Google Scholar]
- 87.Bourbonnais R, Paice MG, Freiermuth B, Bodie E, Borneman S. Reactivities of various mediators and laccases with kraft pulp and lignin model compounds. Appl Environ Microbiol. 1997;63:4627–4632. doi: 10.1128/aem.63.12.4627-4632.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bourbonnais R, Paice MG. Oxidation of nonphenolic substrates—an expanded role for laccase in lignin biodegradation. FEBS Lett. 1990;267:99–102. doi: 10.1016/0014-5793(90)80298-W. [DOI] [PubMed] [Google Scholar]
- 89.Barreca AM, Fabbrini M, Galli C, Gentili P, Ljunggren S. Laccase-mediated oxidation of lignin model for improved delignification procedures. J Mol Cat B Enzym. 2003;26:105–110. doi: 10.1016/j.molcatb.2003.08.001. [DOI] [Google Scholar]
- 90.Li K, Xu F, Eriksson KE. Comparison of fungal laccases and redox mediators in oxidation of a nonphenolic lignin model compound. Appl Environ Microbiol. 1999;65:2654–2660. doi: 10.1128/aem.65.6.2654-2660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu F, Kulys JJ, Duke K, Li K, Krikstopaitis K, Deussen HJW, Abbate E, Galinyte V, Schneider P. Redox chemistry in laccase-catalyzed oxidation of N-hydroxy compounds. Appl Environ Microbiol. 2000;66:2052–2056. doi: 10.1128/AEM.66.5.2052-2056.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fabbrini M, Galli C, Gentili P. Comparing the catalytic efficiency of some mediators of laccase. J Mol Cat B Enzym. 2002;16:231–240. doi: 10.1016/S1381-1177(01)00067-4. [DOI] [Google Scholar]
- 93.Brogioni B, Biglino D, Sinicropi A, Reijerse EJ, Giardina P, Sannia G, Lubitz W, Basosi R, Pogni R. Characterization of radical intermediates in laccase-mediator systems. A multifrequency EPR, ENDOR and DFT/PCM investigation. Phys Chem Chem Phys. 2008;10:7284–7292. doi: 10.1039/b812096j. [DOI] [PubMed] [Google Scholar]
- 94.Collins PJ, Kotterman MJJ, Field JA, Dobson ADW. Oxidation of anthracene and benzo[a]pyrene by laccases from Trametes versicolor . Appl Environ Microbiol. 1996;62:4563–4567. doi: 10.1128/aem.62.12.4563-4567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Camarero S, García O, Vidal T, Colom J, del Río JC, Gutiérrez A, Gras JM, Monje R, Martínez MJ, Martínez T. Efficient bleaching of non-wood high-quality paper pulp using laccase-mediator system. Enzyme Microbiol Technol. 2004;35:113–120. doi: 10.1016/j.enzmictec.2003.10.019. [DOI] [Google Scholar]
- 96.Gutiérrez A, del Río JC, Ibarra D, Rencoret J, Romero J, Speranza M, Camarero S, Martínez MJ, Martínez AT. Enzymatic removal of free and conjugated sterols forming pitch deposits in environmentally sound bleaching of eucalypt paper pulp. Environ Sci Technol. 2006;40:3416–3422. doi: 10.1021/es052547p. [DOI] [PubMed] [Google Scholar]
- 97.Hu MR, Chao YP, Zhang GQ, Xue ZQ, Qian S. Laccase-mediator system in the decolorization of different types of recalcitrant dyes. J Ind Microbiol Biotechnol. 2009;36:45–51. doi: 10.1007/s10295-008-0471-1. [DOI] [PubMed] [Google Scholar]
- 98.Galante YM, Formantici C. Enzyme applications in detergency and in manufacturing industries. Curr Org Chem. 2003;7:1399–1422. doi: 10.2174/1385272033486468. [DOI] [Google Scholar]
- 99.Camarero S, Cañas AI, Nousiainen P, Record E, Lomascolo A, Martínez MJ, Martínez AT. P-hydroxycinnamic acids as natural mediators for laccase oxidation of recalcitrant compounds. Environ Sci Technol. 2008;42:6703–6709. doi: 10.1021/es8008979. [DOI] [PubMed] [Google Scholar]
- 100.Camarero S, Ibarra D, Martínez AT, Romero J, Gutiérrez A, del Río JC. Paper pulp delignification using laccase and natural mediators. Enzyme Microb Technol. 2007;40:1264–1271. doi: 10.1016/j.enzmictec.2006.09.016. [DOI] [Google Scholar]
- 101.Cañas A, Alcalde M, Plou FJ, Martínez MJ, Martínez AT, Camarero S. Transformation of polycyclic aromatic hydrocarbons by laccase is strongly enhanced by phenolic compounds present in soil. Environ Sci Technol. 2007;41:2964–2971. doi: 10.1021/es062328j. [DOI] [PubMed] [Google Scholar]
- 102.Jeon JR, Murugesan K, Kim YM, Kim EJ, Chang YS. Synergistic effect of laccase mediators on pentachlorophenol removal by Ganoderma lucidum laccase. Appl Microbiol Biotechnol. 2008;81:783–790. doi: 10.1007/s00253-008-1753-2. [DOI] [PubMed] [Google Scholar]
- 103.Nakamura K, Go N. Function and molecular evolution of multicopper blue proteins. Cell Mol Life Sci. 2005;62:2050–2066. doi: 10.1007/s00018-004-5076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pezet R. Purification and characterization of a 32-kDa laccase-like stilbene oxidase produced by Botrytis cinerea Pers.:Fr . FEMS Microbiol Lett. 1998;167:203–208. doi: 10.1111/j.1574-6968.1998.tb13229.x. [DOI] [Google Scholar]
- 105.Wang HX, Ng TB. Purification of a novel low molecular-mass laccase with HIV-1 reverse transcriptase inhibitory activity from the mushroom Tricholoma giganteum . Biochem Biophys Res Commun. 2004;315:450–454. doi: 10.1016/j.bbrc.2004.01.064. [DOI] [PubMed] [Google Scholar]
- 106.Yaver DS, Xu F, Golightly EJ, Brown KM, Brown SH, Rey MW, Schneider P, Halkier T, Mondorf K, Dalboge H. Purification, characterization, molecular cloning, and expression of two laccase genes from the white rot basidiomycete Trametes villosa . Appl Environ Microbiol. 1996;62:834–841. doi: 10.1128/aem.62.3.834-841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Min KL, Kim YH, Kim YW, Jung HS, Hah YC. Characterization of a novel laccase produced by the wood-rotting fungus Phellinus ribis . Arch Biochem Biophys. 2001;392:279–286. doi: 10.1006/abbi.2001.2459. [DOI] [PubMed] [Google Scholar]
- 108.Wahleithner JA, Xu F, Brown KM, Brown SH, Golightly EJ, Halkier T, Kauppinen S, Pederson A, Schneider P. The identification and characterization of four laccases from the plant pathogenic fungus Rhizoctonia solani . Curr Genet. 1996;29:395–403. doi: 10.1007/BF02208621. [DOI] [PubMed] [Google Scholar]
- 109.Edens WA, Goins TQ, Dooley D, Henson JM. Purification and characterization of a secreted laccase of Gaeumannomyces graminis var. tritici . Appl Environ Microbiol. 1999;65:3071–3074. doi: 10.1128/aem.65.7.3071-3074.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Junghanns C, Pecyna MJ, Böhm D, Jehmlich N, Martin C, von Bergen M, Schauer F, Hofrichter M, Schlosser D (2009) Biochemical and molecular genetic characterisation of a novel laccase produced by the aquatic ascomycete Phoma sp. UHH 5-1-03. Appl Microbiol Biotechnol. doi:10.1007/s00253-009-2028-2 [DOI] [PubMed]
- 111.Wang HX, Ng TB. Purification of a laccase from fruiting bodies of the mushroom Pleurotus eryngii . Appl Microbiol Biotechnol. 2006;69:521–525. doi: 10.1007/s00253-005-0086-7. [DOI] [PubMed] [Google Scholar]
- 112.Ng TB, Wang HX. A homodimeric laccase with unique characteristics from the yellow mushroom Cantharellus cibarius . Biochem Biophys Res Commun. 2004;313:37–41. doi: 10.1016/j.bbrc.2003.11.087. [DOI] [PubMed] [Google Scholar]
- 113.Lawton TJ, Sayavedra-Soto LA, Arp DJ, Rosenzweig AC. Crystal structure of a two-domain multicopper oxidase: implications for the evolution of multicopper blue proteins. J Biol Chem. 2009;284:10174–10180. doi: 10.1074/jbc.M900179200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Skálová T, Dohnálek J, Østergaard LH, Østergaard PR, Kolenko P, Dušková J, Štepánková A, Hašek J. The structure of the small laccase from Streptomyces coelicolor reveals a link between laccases and nitrite reductases. J Mol Biol. 2009;385:1165–1178. doi: 10.1016/j.jmb.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 115.Komori H, Miyazaki K, Higuchi Y. X-ray structure of a two-domain type laccase: a missing link in the evolution of multi-copper proteins. FEBS Lett. 2009;583:1189–1195. doi: 10.1016/j.febslet.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 116.Wood DA. Production, purification and properties of extracellular laccase of Agaricus bisporus . J Gen Microbiol. 1980;117:327–338. [Google Scholar]
- 117.Curir P, Thurston CF, Daquila F, Pasini C, Marchesini A. Characterization of a laccase secreted by Armillaria mellea pathogenic for Genista . Plant Physiol Biochem. 1997;35:147–153. [Google Scholar]
- 118.Minuth W, Klischies M, Esser K. The phenoloxidases of the ascomycete Podospora anserina. Structural differences between laccases of high and low molecular weight. Eur J Biochem. 1978;90:73–82. doi: 10.1111/j.1432-1033.1978.tb12576.x. [DOI] [PubMed] [Google Scholar]
- 119.Kurtz MB, Champe SP. Purification and characterization of the conidial laccase of Aspergillus nidulans . J Bacteriol. 1982;151:1338–1345. doi: 10.1128/jb.151.3.1338-1345.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thakker GD, Evans CS, Rao KK. Purification and characterization of laccase from Monocillium indicum Saxena . Appl Microbiol Biotechnol. 1992;37:321–323. doi: 10.1007/BF00210986. [DOI] [Google Scholar]
- 121.Perry CR, Matcham SE, Wood DA, Thurston CF. The structure of laccase protein and its synthesis by the commercial mushroom Agaricus bisporus . J Gen Microbiol. 1993;139:171–178. doi: 10.1099/00221287-139-1-171. [DOI] [PubMed] [Google Scholar]
- 122.Palmieri G, Cennamo G, Faraco V, Amoresano A, Sannia G, Giardina P. Atypical laccase isoenzymes from copper supplemented Pleurotus ostreatus cultures. Enzyme Microb Technol. 2003;33:220–230. doi: 10.1016/S0141-0229(03)00117-0. [DOI] [Google Scholar]
- 123.Giardina P, Autore F, Faraco V, Festa G, Palmieri G, Piscitelli A, Sannia G. Structural characterization of heterodimeric laccases from Pleurotus ostreatus . Appl Microbiol Biotechnol. 2007;75:1293–1300. doi: 10.1007/s00253-007-0954-4. [DOI] [PubMed] [Google Scholar]
- 124.Hoegger PJ, Kilaru S, James TY, Thacker JR, Kues U. Phylogenetic comparison and classification of laccase and related multicopper oxidase protein sequences. FEBS J. 2006;273:2308–2326. doi: 10.1111/j.1742-4658.2006.05247.x. [DOI] [PubMed] [Google Scholar]
- 125.Pezzella C, Autore F, Giardina P, Piscitelli A, Sannia G, Faraco V. The Pleurotus ostreatus laccase multi-gene family: isolation and heterologous expression of new family members. Curr Genet. 2009;55:45–57. doi: 10.1007/s00294-008-0221-y. [DOI] [PubMed] [Google Scholar]
- 126.Faraco V, Ercole C, Festa G, Giardina P, Piscitelli A, Sannia G. Heterologous expression of heterodimeric laccase from Pleurotus ostreatus in Kluyveromyces lactis . Appl Microbiol Biotechnol. 2008;77:1329–1335. doi: 10.1007/s00253-007-1265-5. [DOI] [PubMed] [Google Scholar]
- 127.Mei G, Di Venere A, Buganza M, Vecchini P, Rosato N, Finazzi-Agrò A. Role of quaternary structure in the stability of dimeric proteins: the case of ascorbate oxidase. Biochemistry. 1997;36:10917–10922. doi: 10.1021/bi970614p. [DOI] [PubMed] [Google Scholar]
- 128.Nicolai E, Di Venere A, Rosato N, Rossi A, Finazzi Agrò A, Mei G. Physico-chemical properties of molten dimer ascorbate oxidase. FEBS J. 2006;273:5194–5204. doi: 10.1111/j.1742-4658.2006.05515.x. [DOI] [PubMed] [Google Scholar]
- 129.Vries OMH, Kooistra WHCF, Wessels GH. Formation of an extracellular laccase by Schizophyllum commune dikaryon. J Gen Microbiol. 1986;132:2817–2826. [Google Scholar]
- 130.Leontievsky A, Myasoedova N, Pozdnyakova N, Golovleva L. “Yellow” laccase of Panus tigrinus oxidizes nonphenolic substrates without electron-transfer mediators. FEBS Lett. 1997;413:446–448. doi: 10.1016/S0014-5793(97)00953-8. [DOI] [PubMed] [Google Scholar]
- 131.Leontievsky AA, Vares T, Lankinen P, Shergill JK, Pozdnyakova NN, Myasoedova NM, Kalkkinen N, Golovleva LA, Cammack R, Thurston CF, Hatakka A. Blue and yellow laccases of ligninolytic fungi. FEMS Microbiol Lett. 1997;156:9–14. doi: 10.1016/S0378-1097(97)00393-5. [DOI] [PubMed] [Google Scholar]
- 132.Pozdnyakova NN, Rodakiewicz-Nowak J, Turkovskaya OV. Catalytic properties of yellow laccase from Pleurotus ostreatus D1 . J Mol Catal B. 2004;30:19–24. doi: 10.1016/j.molcatb.2004.03.005. [DOI] [Google Scholar]
- 133.Pozdnyakova NN, Turkovskaya OV, Yudina EN, Rodakiewicz-Nowak Ya. Yellow laccase from the fungus Pleurotus ostreatus D1: purification and characterization. Appl Biochem Microbiol. 2006;42:56–61. doi: 10.1134/S000368380601008X. [DOI] [PubMed] [Google Scholar]
- 134.Palmieri G, Giardina P, Bianco C, Scaloni A, Capasso A, Sannia G. A novel white laccase from Pleurotus ostreatus . J Biol Chem. 1997;272:31301–31307. doi: 10.1074/jbc.272.50.31301. [DOI] [PubMed] [Google Scholar]
- 135.Litthauer D, Jansen van Vuuren M, van Tonder A, Wolfaardt FW. Purification and kinetics of a thermostable laccase from Pycnoporus sanguineus (SCC 108) Enzyme MicrobTechnol. 2007;40:563–568. doi: 10.1016/j.enzmictec.2006.05.011. [DOI] [Google Scholar]
- 136.Haibo Z, Yinglong Z, Feng H, Peiji G, Jiachuan C. Purification and characterization of a thermostable laccase with unique oxidative characteristics from Trametes hirsuta . Biotechnol Lett. 2009;31:837–843. doi: 10.1007/s10529-009-9945-0. [DOI] [PubMed] [Google Scholar]
- 137.Kaneko S, Cheng M, Murai H, Takenaka S, Murakami S, Aoki K. Purification and characterization of an extracellular laccase from Phlebia radiata strain BP-11-2 that decolorizes fungal melanin. Biosci Biotechnol Biochem. 2009;73:939–942. doi: 10.1271/bbb.80740. [DOI] [PubMed] [Google Scholar]
- 138.Chernykh A, Myasoedova N, Kolomytseva M, Ferraroni M, Briganti F, Scozzafava A, Golovleva L. Laccase isoforms with unusual properties from the basidiomycete Steccherinum ochraceum strain 1833. J Appl Microbiol. 2008;105:2065–2075. doi: 10.1111/j.1365-2672.2008.03924.x. [DOI] [PubMed] [Google Scholar]
- 139.Hoshida H, Nakao M, Kanazawa H, Kubo K, Hakukawa T, Morimasa K, Akada R, Nishizawa Y. Isolation of five laccase gene sequences from the white-rot fungus Trametes sanguinea by PCR, and cloning, characterization and expression of the laccase cDNA in yeasts. J Biosci Bioeng. 2001;92:372–380. doi: 10.1263/jbb.92.372. [DOI] [PubMed] [Google Scholar]
- 140.Mansur M, Suárez T, Fernández-Larrea JB, Brizuela MA, González AE. Identification of a laccase gene family in the new lignin-degrading basidiomycete CECT 20197. Appl Environ Microbiol. 1997;63:2637–2646. doi: 10.1128/aem.63.7.2637-2646.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xiao YZ, Hong YZ, Li JF, Hang J, Tong PG, Fang W, Zhou CZ. Cloning of novel laccase isozyme genes from Trametes sp. AH28–2 and analyses of their differential expression. Appl Microbiol Biotechnol. 2006;71:493–501. doi: 10.1007/s00253-005-0188-2. [DOI] [PubMed] [Google Scholar]
- 142.Litvintseva AP, Henson JM. Cloning, characterization, and transcription of three laccase genes from Gaeumannomyces graminis var. tritici, the take-all fungus. Appl Environ Microbiol. 2002;68:1305–1311. doi: 10.1128/AEM.68.3.1305-1311.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Soden DM, Dobson AD. Differential regulation of laccase gene expression in Pleurotus sajor-caju . Microbiology. 2001;147:1755–1763. doi: 10.1099/00221287-147-7-1755. [DOI] [PubMed] [Google Scholar]
- 144.Rodríguez E, Ruiz-Dueñas FJ, Kooistra R, Ram A, Martínez AT, Martínez MJ. Isolation of two laccase genes from the white-rot fungus Pleurotus eryngii and heterologous expression of the pel3 encoded protein. J Biotechnol. 2008;134:9–19. doi: 10.1016/j.jbiotec.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 145.Kilaru S, Hoegger PJ, Kües U. The laccase multi-gene family in Coprinopsis cinerea has seventeen different members that divide into two distinct subfamilies. Curr Genet. 2006;50:45–60. doi: 10.1007/s00294-006-0074-1. [DOI] [PubMed] [Google Scholar]
- 146.Martinez D, Challacombe J, Morgenstern I, Hibbett D, Schmoll M, Kubicek CP, Ferreira P, Ruiz-Duenas FJ, Martinez AT, Kersten P, Hammel KE, Vanden Wymelenberg A, Gaskell J, Lindquist E, Sabat G, Bondurant SS, Larrondo LF, Canessa P, Vicuna R, Yadav J, Doddapaneni H, Subramanian V, Pisabarro AG, Lavín JL, Oguiza JA, Master E, Henrissat B, Coutinho PM, Harris P, Magnuson JK, Baker SE, Bruno K, Kenealy W, Hoegger PJ, Kües U, Ramaiya P, Lucas S, Salamov A, Shapiro H, Tu H, Chee CL, Misra M, Xie G, Teter S, Yaver D, James T, Mokrejs M, Pospisek M, Grigoriev IV, Brettin T, Rokhsar D, Berka R, Cullen D. Genome, transcriptome, and secretome analysis of wood decay fungus Postia placenta supports unique mechanisms of lignocellulose conversion. Proc Natl Acad Sci USA. 2009;106:1954–1959. doi: 10.1073/pnas.0809575106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Courty PE, Hoegger PJ, Kilaru S, Kohler A, Buée M, Garbaye J, Martin F, Kües U. Phylogenetic analysis, genomic organization, and expression analysis of multi-copper oxidases in the ectomycorrhizal basidiomycete Laccaria bicolor . New Phytol. 2009;182:736–750. doi: 10.1111/j.1469-8137.2009.02774.x. [DOI] [PubMed] [Google Scholar]
- 148.Chen S, Ge W, Buswell JA. Molecular cloning of a new laccase from the edible straw mushroom Volvariella volvacea: possible involvement in fruit body development. FEMS Microbiol Lett. 2004;30:171–176. doi: 10.1016/S0378-1097(03)00878-4. [DOI] [PubMed] [Google Scholar]
- 149.Tsai HF, Wheeler MH, Chang YC, Kwon-Chung KJ. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus . J Bacteriol. 1999;181:6469–6477. doi: 10.1128/jb.181.20.6469-6477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Missal TA, Moran JM, Corbett JA, Lodge JK. Distinct stress responses of two functional laccases in Cryptococcus neoformans are revealed in the absence of the thiol-specific antioxidant Tsa1. Eukaryot Cell. 2005;4:202–208. doi: 10.1128/EC.4.1.202-208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Iakovlev A, Stenlid J. Spatiotemporal patterns of laccase activity in interacting mycelia of wood-decaying basidiomycete fungi. Microb Ecol. 2000;39:236–245. doi: 10.1007/s002480000022. [DOI] [PubMed] [Google Scholar]
- 152.Luis P, Kellner H, Zimdars B, Langer U, Martin F, Buscot F. Patchiness and spatial distribution of laccase genes of ectomycorrhizal, saprotrophic, and unknown basidiomycetes in the upper horizons of a mixed forest cambisol. Microb Ecol. 2005;50:570–579. doi: 10.1007/s00248-005-5047-2. [DOI] [PubMed] [Google Scholar]
- 153.Terrón MC, González T, Carbajo JM, Yagüe S, Arana-Cuenca A, Téllez A, Dobson AD, González AE. Structural close-related aromatic compounds have different effects on laccase activity and on lcc gene expression in the ligninolytic fungus Trametes sp. I-62. Fungal Genet Biol. 2004;41:954–962. doi: 10.1016/j.fgb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 154.Solé M, Kellner H, Brock S, Buscot F, Schlosser D. Extracellular laccase activity and transcript levels of putative laccase genes during removal of the xenoestrogen technical nonylphenol by the aquatic hyphomycete Clavariopsis aquatica . FEMS Microbiol Lett. 2008;288:47–54. doi: 10.1111/j.1574-6968.2008.01333.x. [DOI] [PubMed] [Google Scholar]
- 155.Galhaup C, Goller S, Peterbauer CK, Strauss J, Haltrich D. Characterization of the major laccase isoenzyme from Trametes pubescens and regulation of its synthesis by metal ions. Microbiology. 2002;148:2159–2169. doi: 10.1099/00221287-148-7-2159. [DOI] [PubMed] [Google Scholar]
- 156.Collins PJ, Dobson A. Regulation of laccase gene transcription in Trametes versicolor . Appl Environ Microbiol. 1997;63:3444–3450. doi: 10.1128/aem.63.9.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Karahanian E, Corsini G, Lobos S, Vicuña R. Structure and expression of a laccase gene from the ligninolytic basidiomycete Ceriporiopsis subvermispora . Biochim Biophys Acta. 1998;26:65–74. doi: 10.1016/s0167-4781(98)00197-3. [DOI] [PubMed] [Google Scholar]
- 158.Palmieri G, Giardina P, Bianco C, Fontanella B, Sannia G. Copper induction of laccase isoenzymes in the ligninolytic fungus Pleurotus ostreatus . Appl Environ Microbiol. 2000;66:920–924. doi: 10.1128/AEM.66.3.920-924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Baldrian P, Gabriel J. Copper and cadmium increase laccase activity in Pleurotus ostreatus . FEMS Microbiol Lett. 2002;206:69–74. doi: 10.1111/j.1574-6968.2002.tb10988.x. [DOI] [PubMed] [Google Scholar]
- 160.Manubens A, Canessa P, Folch C, Avila M, Salas L, Vicuña R. Manganese affects the production of laccase in the basidiomycete Ceriporiopsis subvermispora . FEMS Microbiol Lett. 2007;275:139–145. doi: 10.1111/j.1574-6968.2007.00874.x. [DOI] [PubMed] [Google Scholar]
- 161.Faraco V, Giardina P, Sannia G. Metal-responsive elements in Pleurotus ostreatus laccase gene promoters. Microbiology. 2003;149:2155–2162. doi: 10.1099/mic.0.26360-0. [DOI] [PubMed] [Google Scholar]
- 162.Buchman C, Scroch P, Welch J, Fogel S, Karin M. The CUP2 gene product, regulator of yeast metallothionein expression, is a copper-activated DNA-binding protein. Mol Cell Biol. 1989;9:4091–4095. doi: 10.1128/mcb.9.9.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Alvarez JM, Canessa P, Mancilla RA, Polanco R, Santibáñez PA, Vicuña R. Expression of genes encoding laccase and manganese-dependent peroxidase in the fungus Ceriporiopsis subvermispora is mediated by an ACE1-like copper-fist transcription factor. Fungal Genet Biol. 2009;46:104–111. doi: 10.1016/j.fgb.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 164.Giatti Marques De Souza C, Tychanowicz GK, Farani De Souza D, Peralta RM. Production of laccase isoforms by Pleurotus pulmonarius in response to presence of phenolic and aromatic compounds. J Basic Microbiol. 2004;44:129–136. doi: 10.1002/jobm.200310365. [DOI] [PubMed] [Google Scholar]
- 165.Vanhulle S, Enaud E, Trovaslet M, Nouaimeh N, Bols CM, Keshavarz T, Tron T, Sannia G, Corbisier AM. Overlap of laccases/cellobiose dehydrogenase activities during the decolourisation of anthraquinonic dyes with close chemical structures by Pycnoporus strains. Enzyme Microb Technol. 2007;40:1723–1731. doi: 10.1016/j.enzmictec.2006.10.033. [DOI] [Google Scholar]
- 166.Eggert C, Temp U, Eriksson KE. The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl Environ Microbiol. 1996;62:1151–1158. doi: 10.1128/aem.62.4.1151-1158.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mansur M, Suárez T, González AE. Differential gene expression in the laccase gene family from basidiomycete I-62 (CECT 20197) Appl Environ Microbiol. 1998;64:771–774. doi: 10.1128/aem.64.2.771-774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Colao MC, Garzillo AM, Buonocore V, Schiesser A, Ruzzi M. Primary structure and transcription analysis of a laccase-encoding gene from the basidiomycete Trametes trogii . Appl Microbiol Biotechnol. 2003;63:153–158. doi: 10.1007/s00253-003-1429-x. [DOI] [PubMed] [Google Scholar]
- 169.Strauss J, Horvath HK, Abdallah BM, Kindermann J, Mach RL, Kubicek CP. The function of CreA, the carbon catabolite repressor of Aspergillus nidulans, is regulated at the transcriptional and post-transcriptional level. Mol Microbiol. 1999;32:169–178. doi: 10.1046/j.1365-2958.1999.01341.x. [DOI] [PubMed] [Google Scholar]
- 170.Ohga S, Smith M, Thurston CF, Wood DA. Transcriptional regulation of laccase and cellulase genes in the mycelium of Agaricus bisporus during fruit body development on a solid substrate. Mycol Res. 1999;103:1557–1560. doi: 10.1017/S0953756299008692. [DOI] [Google Scholar]
- 171.Ohga S, Royse DJ. Transcriptional regulation of laccase and cellulose genes during growth and fruiting of Lentinula edodes on supplemented sawdust. FEMS Microbiol Lett. 2001;201:111–115. doi: 10.1111/j.1574-6968.2001.tb10741.x. [DOI] [PubMed] [Google Scholar]
- 172.Zhang S, Hacham M, Panepinto J, Hu G, Shin S, Zhu X, Williamson PR. The Hsp70 member, Ssa1, acts as a DNA-binding transcriptional co-activator of laccase in Cryptococcus neoformans . Mol Microbiol. 2006;62:1090–1101. doi: 10.1111/j.1365-2958.2006.05422.x. [DOI] [PubMed] [Google Scholar]