Abstract
Numerous factors involved in general homeostasis are able to modulate ventilation. Classically, this comprises several kind of molecules, including neurotransmitters and steroids that are necessary for fine tuning ventilation under different conditions such as sleep, exercise, and acclimatization to high altitude. Recently, however, we have found that erythropoietin (Epo), the main regulator of red blood cell production, influences both central (brainstem) and peripheral (carotid bodies) respiratory centers when the organism is exposed to hypoxic conditions. Here, we summarize the effect of Epo on the respiratory control in mammals and highlight the potential implication of Epo in the ventilatory acclimatization to high altitude, as well as in the several respiratory sickness and syndromes occurring at low and high altitude. (Part of a multi-author review.)
Keywords: Carotid body, Brainstem respiratory centers, High altitude, Acclimatization, Respiration
Introduction
The classical function of erythropoietin (Epo) in the hematopoietic system is the regulation of red blood cell production, a process that is mediated by its specific cell surface Epo receptor (EpoR). Recently, however, Epo was found to do far more than blood. It was observed that Epo was not only endogenously synthesized in the fetal liver and adult kidney [1, 2], but also in cell lines with neuronal characteristics. Specifically, it was Sasaki and co-authors who, in 1992, detected for the first time the presence of Epo and functional EpoR in the neuron-like cells PC12 and NS6 [3]. Later, we localized Epo and Epo binding sites in specific areas of the mouse brain, including hippocampus and cerebral cortex [4]. More recently, we and others demonstrated in rodents and men that Epo exerts a potent protective function upon experimentally induced brain ischemia [5–10], spinal cord ischemia and trauma [11, 12], and light-induced retinal degeneration [7, 13, 14].
Since the neuroprotective impacts of Epo in neural tissue have been investigated during the last decade, little is known regarding Epo’s physiological function in the brain. Moreover, despite the fact that Epo is a hypoxia-inducible factor, its putative impact on respiratory control has not been considered as a working hypothesis until recently. Thus, the main objective of this review is to summarize the current knowledge on the mammalian regulation of breathing process by Epo. We provide convincing evidence that cerebrally produced Epo is a key factor in the ventilatory response to acute and chronic hypoxia by modulating the respiratory centers in the brainstem. In addition, we also review the evidence that circulating Epo in plasma controls the peripheral carotid bodies, thus establishing the crucial interaction between the erythropoietic system, responsible for increasing the oxygen carrying capacity in blood, and the neural respiratory response, that increases minute ventilation. We finally discuss the putative clinical implications of Epo in breathing diseases and the impact of Epo in the acclimatization and de-acclimatization process to high altitude.
Epo regulates ventilation in hypoxia
Ventilation (VE) is the process by which O2 and CO2 are exchanged by diffusion between the lungs and the environment. The mammalian lungs have the capacity to increase the ventilatory performance by a factor of 20 compared to the resting ventilation. This allows mammals to adapt and colonize extreme environments [15], as well as humans to permanently live at altitudes higher than 4,000 m, and to continuously challenging sports’ limits. Taken together, investigation of factors and molecular mechanism that permit improvement of ventilatory mechanism is a topic of major interest.
Hypoxia may be defined as a relative deficiency in oxygen availability/delivery for maintaining adequate physiological oxygen tensions, and thus results from an imbalance between oxygen demand and supply [16]. As such, a set of genes whose expression is regulated by cellular oxygen tension is activated controlling the corresponding adjustments. The hypoxia-inducible factor-1 (HIF-1) is the most important protein regulating the physiological homeostasis against the reduced oxygen supply [16]. HIF-1 is stabilized under hypoxic conditions conferring selectivity to the hypoxic response. As a major transcription factor, HIF-1 regulates the activation of more than 100 target genes [17, 18], of which Epo is the best known one.
Epo’s impact on the brainstem respiratory centers
During the last decade, we and others have demonstrated that Epo and its receptor (EpoR) are functionally expressed in glial cells and neurons of mice, monkey, and humans, implying that Epo has a much broader field of action than so far recognized. We observed that the amount of Epo in brain dramatically increases after hypoxic exposure of the organism by HIF-1 stabilization [4]. The mechanisms by which Epo exerts its function include several pathways controlling oxygen homeostasis. Epo has been recognized to be involved in the upregulation of oxygen-free radical scavenger enzymes [19–21], avoiding apoptosis by maintaining the expression of Bcl-2 and Bcl-xl [21, 22], preventing glutamate-induced cell death [23, 24] and activating voltage-gated channels [25, 26]. Considering that all these processes are directly or indirectly involved in the ventilatory acclimatization to hypoxia and corresponding morphological and neurochemical regulations in the neural respiratory network, we hypothesized a few years ago that Epo influences the respiratory centers in the brain. To test this hypothesis, we used a transgenic mouse line (tg21) constitutively overexpressing human Epo only in brain [8]. This transgenic mouse model allowed us to test the impact of elevated brain Epo (respiratory center) omitting the influence of elevated plasma Epo that would probably interfere with the peripheral respiratory centers.
Both severe acute hypoxia (6% O2) and chronic hypoxic exposure (3 days at 10% O2) showed a greater ventilatory response in tg21 mice, compared with corresponding wild-type (WT) control animals. Following a bilateral transection of the carotid sinus nerves (chemodenervation) that uncouples the brain from the carotid bodies, tg21 mice responded to severe hypoxia with a sustained ventilation, while chemodenervated WT animals showed life-threatening apneas, and exposure had to be stopped [27]. Because tg21 mice express human Epo only in brain without increasing their Epo plasma level, these results imply that cerebral Epo is a key factor that stimulates ventilation under conditions of reduced oxygen availability. In parallel to these experiments, immunohistochemical analysis revealed that EpoR is expressed in the main brainstem respiratory centers; of note, EpoR was expressed with neurokinin-1 receptors (NK-1R) in neurons present in the pre-Bötzinger complex that are involved in the generation of the respiratory pattern, in the nucleus tractus solitarious (NTS), that relays input from peripheral chemoreceptor to the central respiratory areas, and in catecholaminergic centers in the brainstem that are important modulators of ventilation under hypoxic conditions [27].
Mechanisms of Epo’s impact on the brainstem
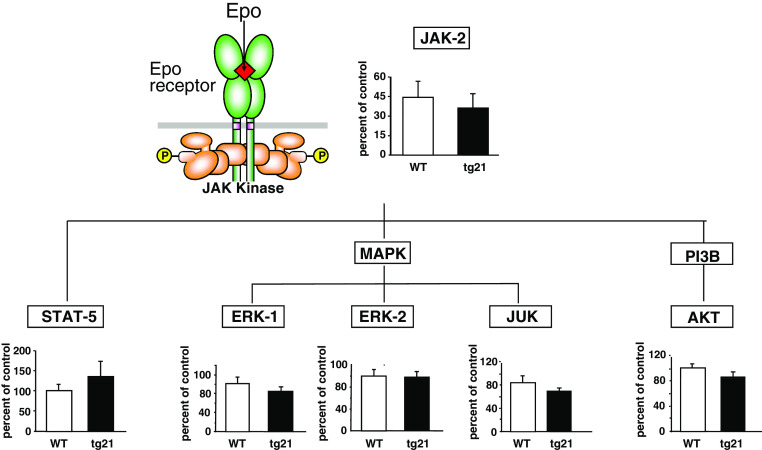
Studies using neuronal-glial cultures and mice revealed that Epo provides neuroprotection action by activating the Janus-tyrosine kinase 2 (JAK-2) that is linked with the EpoR via its Box-1 motif (reviewed in [28]). JAK-2 in turn phosphorylates, thereby activating downstream signaling factors, such as the mitogen-activated protein (MAP) kinases, extracellular-regulated kinase (ERK)-1/-2 [29], phosphatidyl inositol 63 kinase (PI3k)/Akt [29, 30], Jun kinase (JNK) [31, 32], and signal transducers and activators of transcription (SAT)-5 [29, 33] (Fig. 1). With this in mind, we performed western blot analysis to determine whether the Epo signal transduction in tg21 mice was prolonged in comparison to WT animals. We did not observe any differences in the expression of neural JAK-2, MAP kinase, ERK-1/-2, JNK, and AKT pathways, thus showing that the Epo-mediated increased hypoxic ventilation in tg21 mice is not related to higher activation of these molecular neuroprotective signal transduction (note that this result has not been previously published).
Fig. 1.
Transgenic mice overexpressing human Epo only in brain (tg21) does not show activation of Janus tyrosine kinase 2 (JAK-2) or corresponding downstream signal factors such as signal transducers and activators of transcription (STAT-5), extracellular-regulated kinase (ERK-1/-2), jun kinase (JNK), or serin/threonin kinase (AKT)
On the other hand, Epo has been recognized as a potent factor able to modulate release of catecholamines in cells with neuronal characteristics [34–37]. Knowing that catecholamines in brainstem are important factors in the modulation of ventilation upon hypoxic conditions [38–40], we used HPLC to evaluate the noradrenaline content and the tyrosine hydroxylase (TH) activity in tg21 and WT mice. Compared to WT controls, tg21 mice show altered catecholaminergic content in brainstem, higher levels in pons, but lower levels in medulla. These data are in agreement with the report showing that increased hypoxic ventilation—by mean the augmentation of the respiratory frequency—is associated with higher catecholamine level in pontial A5 cell group [41, 42] and with lower catecholamine level in the medullary A1C1 and A2C2 cell groups [43]. Thus, our results suggest that higher Epo level modulates the catecholamine synthesis in brainstem. Once hypoxic, this alteration affects the ventilatory response by increasing the respiratory frequency.
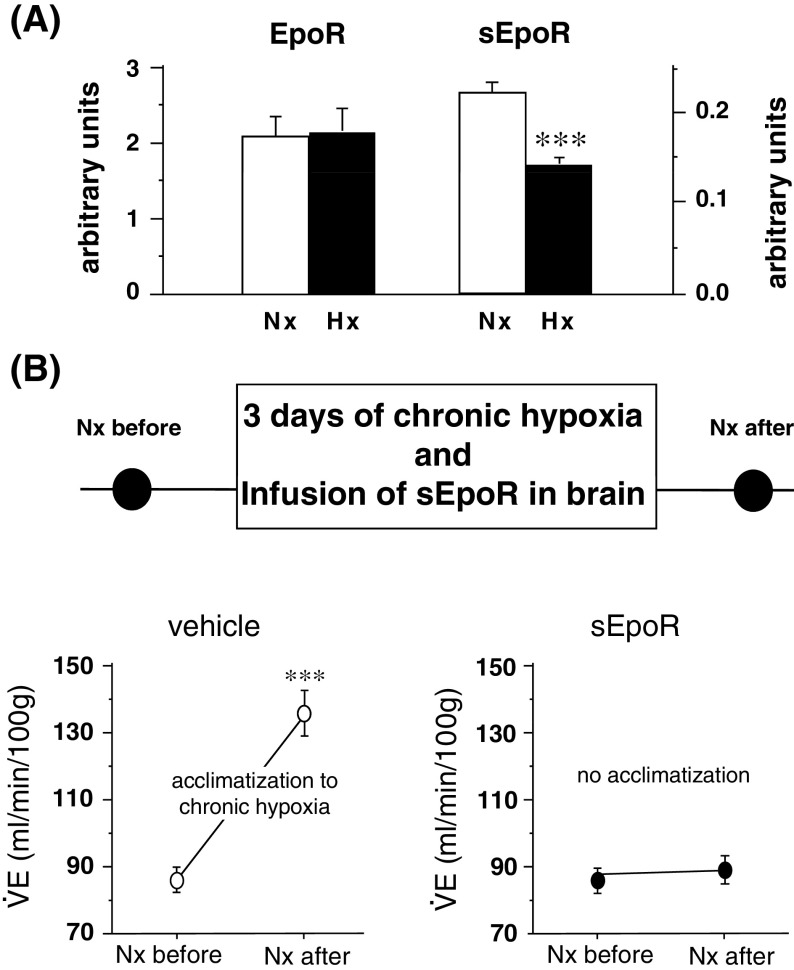
In an attempt to verify our data by using WT mice, we focused the soluble EpoR (sEpoR). In analogy to several other members of the cytokine superfamily type I transmembrane proteins, EpoR is also synthesized in a soluble form that corresponds to the extracellular domain of the complete receptor [44–46]. The sEpoR is synthesized by alternative splicing of EpoR mRNA and secreted to the extracellular fluid. Once there, it binds Epo, thereby sequestering it from the system [47–49]. We observed first that chronic hypoxia produces a drastic downregulation of the sEpoR in the central nervous system of WT mice [49]. In a following step, when sEpoR was chronically infused in the nervous system of mice by a minipump, the process of ventilatory acclimatization to chronic hypoxia (defined as a gradual increase in ventilation to compensate for the low O2 availability) was abolished. In parallel, the neural Epo concentration was decreased by 50% (Fig. 2). These results show that the neural regulation of Epo and its antagonist sEpoR play a critical role in the central nervous system in stabilizing the ventilatory activity and thus ensuring the systemic oxygen delivery under low O2 conditions [49].
Fig. 2.
a Western blot analysis shows that Epo and sEpoR are expressed in the mouse brain. b Intracerebral infusion of sEpoR abolishes ventilatory acclimatization to chronic hypoxia. Normoxic minute ventilation was evaluated before and after animals were exposed chronically to hypoxia of 10% O2 during 3 days. After acclimatization, control animals showed large increase of normoxic ventilation. In contrast, this elevation was abolished in sEpoR-treated mice
Taken together, our results imply that endogenous Epo:sEpoR system in the central nervous system play a crucial role in regulating oxygen homeostasis, thereby ultimately contributing to the same goal as Epo in plasma, i.e., increasing overall oxygen delivery capacity.
Plasma Epo and carotid bodies
Because oxygen is able to react fast with several other molecules thereby producing harmful forms of reactive oxygen species, oxygen is not stored in the organism in large amounts. Therefore, oxygen must be continuously supplied to settle the oxygen-dependent enzymatic reactions [50]. As a result, mammals need to be equipped with more than one mechanism to respond to the oxygen reduction in arterial blood. The very first mechanism providing higher level of oxygen consists in the increase of ventilation, which enlarges the overall volume of air and thus increases the level of oxygen in the alveolus. This quick increase of ventilation is controlled by the carotid body. This organ senses the reduced blood oxygenation and activates the chemoreflex pathway. The sensory information is then relayed to brainstem neurons that in turn modulate corresponding ventilatory adjustments. Increasing ventilation is the most important hypoxic response when mammals are acutely exposed to high altitude [51], but it also plays a crucial role in the acclimatization process to chronic hypoxia [52].
Despite being essential, the ventilatory system is not sufficient to ensure adequate oxygen supply to cells and tissues. A second important mechanism increasing the oxygen availability consists in the augmentation of the blood’s oxygen carrying capacity. Upon sustained hypoxemia, Epo synthesis in the adult kidney is accelerated, resulting in increased plasma Epo level [53, 54]. Once in the bone marrow, Epo maintains the availability of erythrocyte progenitor cells, promotes cell division, and increases hemoglobin synthesis culminating in increased hematocrit levels [55, 56]. Despite being complementary, no interaction between the neural control of ventilation and the Epo-mediated elevation of red blood cells has been described so far. Knowing that PC12 cells, a cell line derived from rat pheochromocitoma representing a model of peripheral chemosensitive cells, increase their intracellular calcium concentration, induce membrane depolarization, increase the number of viable cells, and increase the dopamine release and TH activity upon exposure to Epo [25, 34, 36, 37, 57], we suspected that the ventilatory and erythropoietic response crosstalk. We postulated that higher concentration of Epo in plasma stimulates the hypoxic ventilatory response by interacting with carotid body cells, while elevated tissue oxygenation induced by increased ventilation will reduce the hypoxic secretion of Epo in renal cells.
The main peripheral chemoreceptors identified in small mammals are the carotid bodies [58]. These are among the most vascularized organs in the body (five times higher than brain) [58], and are stimulated by the decline of arterial oxygen partial pressure. Considering its embryonic neural origin and its similarity with PC12 cells, we first addressed the question whether EpoR is present in carotid body glomus cells, as it is in brain tissue and neural-like cells. To this end, we performed immunostaining in serial lateral sections of the carotid body bifurcation, and used tyrosine hydroxylase (TH) staining to identify the glomus cells. We detected dense staining of EpoR in the carotid body, apparently localized within islets of chemosensitive cells [27]. This observation implies that circulatory Epo interacts with carotid body cells, probably by binding the EpoR. In a next step, we placed WT animals into the plethysmograph and measured the hypoxic ventilatory response after acute injection of 2,000 U/kg of rhEpo in the tail vein. We observed that Epo-injected mice showed higher respiratory frequency but lower tidal volume than saline-injected controls when exposed to hypoxia. Considering that a glycoprotein such as Epo can hardly cross the blood–brain barrier [59–61], these results suggest that peripheral chemoreceptors can be activated by circulating Epo. It is tempting to speculate that, under hypoxic exposure followed by increased plasma levels, Epo stimulates the response of carotid bodies, thus fine-tuning the neural control of hypoxic ventilation.
Epo modulation of hypoxic ventilation is gender dependent
Several studies on cats and rats pointed out that the carotid bodies are one of the major sites for gender differentiation of ventilatory control in hypoxia [62, 63], but the underlying mechanisms of this hormonal stimulation remain poorly understood. Among the various neuromodulators synthesized by glomus cells under hypoxemia, dopamine is found at high concentration and has been recognized as a potent inhibitory neuromodulator of carotid body chemotransduction [58, 64, 65]. As such, we postulated a few years ago that sexual female hormones can modulate hypoxic ventilation by impacting the carotid body dopaminergic secretion. Indeed, we found that ovarian steroids stimulate ventilation by lowering the peripheral dopaminergic inhibitory drive [51]. These results are in line with several reports showing a better capacity of women and female mammals to adapt to hypoxia [51, 62], and to be less susceptible to a number of hypoxia-associated issues.
Based on the fact that ovarian steroids can influence the expression of hypoxia-inducible genes, such as renal Epo, vascular endothelial growth factor, endotheline 1, nitric oxide synthase, and HIF-1 [66–71], we recently hypothesized that gender-dependent regulation of hypoxic ventilation is mediated by erythropoietin. To test this hypothesis, we used a second transgenic mouse line (tg6) showing high levels of human Epo in brain and plasma, the latter leading to excessive erythrociatosis [8, 72]. Interestingly, despite tg6 mice showing hematocrit values up to 80–90%, we found no differences in minute ventlation between male tg6 and WT mice during exposition to hypoxia. Nevertheless, tg6 mice showed dramatic changes in the ventilatory pattern [73]. In contrast, when hypoxic ventilatory response was evaluated in female tg6 animals, we observed that minute ventilation was dramatically increased in tg6 animals compared to corresponding WT mice [74]. These results suggest that the gender-dependent regulation of hypoxic ventilation imply an interaction between Epo and sexual steroids.
In a next step, based on the notion that an intact blood–brain barrier excludes large glycosylated molecules such as Epo [6, 60, 61], we measured the hypoxic ventilatory response in male and female WT mice following i.v. injection of 2,000 U/kg of rhEpo. This experiment allowed us to study the gender-dependent impact of Epo on carotid bodies without the influence of cerebrally produced Epo. While no differences were observed in male mice during hypoxia, Epo-injected female animals underwent a tremendous increase in hypoxic ventilation [74]. As such, these results suggest that plasma Epo and sex female hormones under hypoxic conditions occurs in the carotid body cells. Note, however, that these date do not discard a putative interaction of cerebral Epo and neurosteroids.
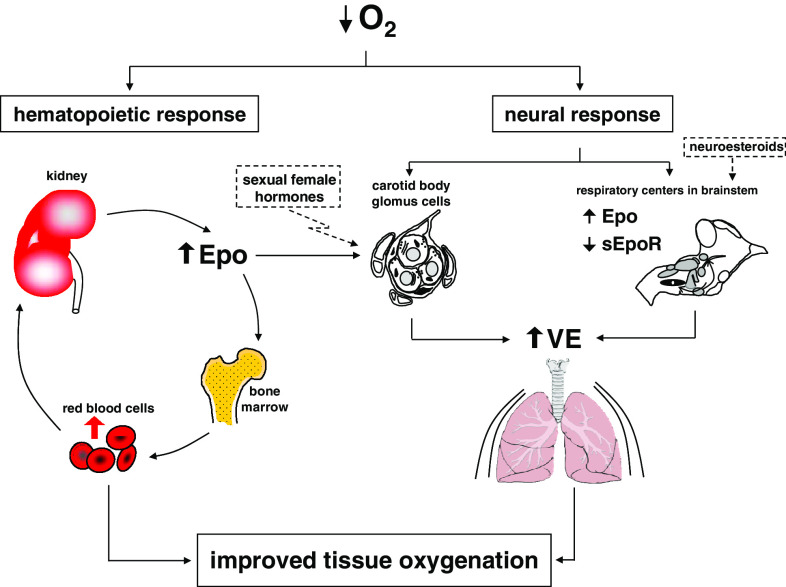
Finally, in an attempt to summarize the impact of central and peripheral Epo on hypoxic ventilation, we proposed a new model for the ventilatory response to hypoxia (Fig. 3).
Fig. 3.
Model of ventilatory response to hypoxia showing the contribution of cerebral and plasma Epo. During the first minutes of hypoxia, carotid bodies sense the drop of arterial oxygen pressure thus leading to a fast response to hypoxia. Longer exposure to hypoxia promotes a higher secretion of Epo by the kidney. An increased level of plasma Epo augments the oxygen carrying capacity (by gradual increase of the hematocrit), but also contributes to the regulation of ventilation (VE) by regulating the activity of the carotid body glomus cells. In parallel, the level of cerebral Epo is increased in brainstem (and decreased the level of sEpoR), thus contributing to the regulation of central ventilation
Clinical implications and future research
The notion that Epo interacts with neural cells is quite recent. Despite this short time, large efforts have been made concerning the pathological implication of Epo as a neuroprotective factor [5–7, 9–14]. On the other hand, little work has been done concerning Epo’s physiologiclal impact. Our work clearly provides convincing evidence that Epo, EpoR, and sEpoR play a crucial role in regulating the systemic oxygen homeostasis in adult mice, by regulating both central (brainstem) and peripheral (carotid bodies) check points of ventilation in hypoxia. The clinical implications of these findings are obvious. We assume that there is an impact of neural Epo:sEpoR in lowlanders suffering from chronic obstructive pulmonary diseases, such as emphysema, chronic bronchitis, and cystic fibrosis, as well as from other types of diseases such as, polycythemia vera, sleep apnea, and renal and liver tumor. Moreover, regarding the increasing population permanently living at high altitude, we postulate that plasma and cerebral Epo are involved in the ventilatory adaptation. At present, we do not know whether high altitude dwellers have a higher concentration of Epo in neural tissue, or whether neural Epo participates in the blunted ventilation observed in acute and chronic mountain sickness.
Of note, appropriate breathing control and optimal oxygen supply during early life is a determinant factor for the normal development of the respiratory control network [75, 76], as well as for several cognitive tasks [77, 78]. Apnoea of the premature, neonatal asphyxia, or respiratory distress syndrome in premature infants are among the most important factors associated with impaired oxygen delivery to the brain [19, 79]. Consequently, the implications of Epo and sEpoR during development are of most interest. In addition, mammalian milk contains substantial concentrations of Epo, suggesting that Epo plays a pleimorphic role in erythropoiesis, neurodevelopment, maturation of the gut, apoptosis, and immunity in breast-fed infants [80–82]. The expression of Epo was also found in the placenta; nevertheless, its role in this tissue remain to be elucidated.
Finally, it is interesting to mention that two different routes of functional adaptation were naturally selected in Tibetan and Andean high altitude natives: Tibetans have developed increased ventilatory capacity compared to Andean Aymaras, but complementary to this, Andeans have generated higher hemoglobin concentrations [83]. Our data fit this observation and provide convincing evidence that Epo links this adaptational mechanism. We hypothesize that cerebral Epo, probably pushing ventilatory adaptation, was the option for lower energetic cost in the evolution of Himalayan people, while renal-synthesized Epo was the better option for the Andean population. We are convinced that these data will contribute to our understanding of respiratory physiology and the way to treat the associated diseases.
Conclusion
Little is known about the physiological implication of Epo in neural-controlled processes, and in addition, this is a very fertile field for investigation. Our work establishing a key implication of Epo in the neural control of hypoxic ventilation, and the link of two complementary systems (ventilatory and erythropoietic) in the process to augment the general oxygen availability upon hypoxia, is very novel and requires more investigation. Keeping in mind that Epo is a safe drug, it will find many more applications in the clinic. However, as only intravenous or subcutaneous drug application is feasible in human patients, systemically applied Epo must cross the blood–brain barrier to also be useful in the central nervous system. In addition, an Epo isoform able to minimize the danger of excessive erythropoiesis that could result from high levels and repeated application of Epo needs to be developed. In other words, a small and new erythropoietic Epo analogue is required.
References
- 1.Fisher JW. Erythropoietin: physiologic, pharmacologic aspects. Proc Soc Exp Biol Med. 1997;216:358–369. doi: 10.3181/00379727-216-44183. [DOI] [PubMed] [Google Scholar]
- 2.Jelkmann W. Erythropoietin: structure, control of production, function. Physiol Rev. 1992;72:449–489. doi: 10.1152/physrev.1992.72.2.449. [DOI] [PubMed] [Google Scholar]
- 3.Sasaki R, Masuda S, Nagao M. Pleiotropic functions and tissue-specific expression of erythropoietin. News Physiol Sci. 2001;16:110–113. doi: 10.1152/physiologyonline.2001.16.3.110. [DOI] [PubMed] [Google Scholar]
- 4.Digicaylioglu M, Bichet S, Marti HH, Wenger RH, Rivas LA, Bauer C, Gassmann M. Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc Natl Acad Sci USA. 1995;92:3717–3720. doi: 10.1073/pnas.92.9.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernaudin M, Marti HH, Roussel S, Divoux D, Nouvelot A, MacKenzie ET, Petit E. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. Erythropoietin crosses the blood–brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gassmann M, Heinicke K, Soliz J, Ogunshola OO. Non-erythroid functions of erythropoietin. Adv Exp Med Biol. 2003;543:323–330. doi: 10.1007/978-1-4419-8997-0_22. [DOI] [PubMed] [Google Scholar]
- 8.Wiessner C, Allegrini PR, Ekatodramis D, Jewell UR, Stallmach T, Gassmann M. Increased cerebral infarct volumes in polyglobulic mice overexpressing erythropoietin. J Cereb Blood Flow Metab. 2001;21:857–864. doi: 10.1097/00004647-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, Rustenbeck HH, Breiter N, Jacob S, Knerlich F, Bohn M, Poser W, Ruther E, Kochen M, Gefeller O, Gleiter C, Wessel TC, De Ryck M, Itri L, Prange H, Cerami A, Brines M, Siren AL. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- 10.Calapai G, Marciano MC, Corica F, Allegra A, Parisi A, Frisina N, Caputi AP, Buemi M. Erythropoietin protects against brain ischemic injury by inhibition of nitric oxide formation. Eur J Pharmacol. 2000;401:349–356. doi: 10.1016/S0014-2999(00)00466-0. [DOI] [PubMed] [Google Scholar]
- 11.Celik M, Gokmen N, Erbayraktar S, Akhisaroglu M, Konakc S, Ulukus C, Genc S, Genc K, Sagiroglu E, Cerami A, Brines M. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci USA. 2002;99:2258–2263. doi: 10.1073/pnas.042693799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorio A, Gokmen N, Erbayraktar S, Yilmaz O, Madaschi L, Cichetti C, Di Giulio AM, Vardar E, Cerami A, Brines M. Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc Natl Acad Sci USA. 2002;99:9450–9455. doi: 10.1073/pnas.142287899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimm C, Wenzel A, Groszer M, Mayser H, Seeliger M, Samardzija M, Bauer C, Gassmann M, Reme CE. Hif-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med. 2002;8:718–724. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- 14.Grimm C, Wenzel A, Stanescu D, Samardzija M, Hotop S, Groszer M, Naash M, Gassmann M, Reme C. Constitutive overexpression of human erythropoietin protects the mouse retina against induced but not inherited retinal degeneration. J Neurosci. 2004;24:5651–5658. doi: 10.1523/JNEUROSCI.1288-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West J. Respiratory physiology. The essentials. Baltimore: Williams & Wilkins; 1995. [Google Scholar]
- 16.Hopfl G, Ogunshola O, Gassmann M. Hifs and tumors—causes and consequences. Am J Physiol. 2004;286:R608–623. doi: 10.1152/ajpregu.00538.2003. [DOI] [PubMed] [Google Scholar]
- 17.Fandrey J, Gorr TA, Gassmann M. Regulating cellular oxygen sensing by hydroxylation. Cardiovasc Res. 2006;71:642–651. doi: 10.1016/j.cardiores.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Yeo EJ, Chun YS, Park JW. New anticancer strategies targeting hif-1. Biochem Pharmacol. 2004;68:1061–1069. doi: 10.1016/j.bcp.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 19.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 20.Nicotera P, Lipton SA. Excitotoxins in neuronal apoptosis and necrosis. J Cereb Blood Flow Metab. 1999;19:583–591. doi: 10.1097/00004647-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Sola A, Rogido M, Lee BH, Genetta T, Wen TC. Erythropoietin after focal cerebral ischemia activates the janus kinase—signal transducer and activator of transcription signaling pathway and improves brain injury in postnatal day 7 rats. Pediatr Res. 2005;57:481–487. doi: 10.1203/01.PDR.0000155760.88664.06. [DOI] [PubMed] [Google Scholar]
- 22.Silva M, Grillot D, Benito A, Richard C, Nunez G, Fernandez-Luna JL. Erythropoietin can promote erythroid progenitor survival by repressing apoptosis through bcl-xl and bcl-2. Blood. 1996;88:1576–1582. [PubMed] [Google Scholar]
- 23.Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1997;76:105–116. doi: 10.1016/S0306-4522(96)00306-5. [DOI] [PubMed] [Google Scholar]
- 24.Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci USA. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Assandri R, Egger M, Gassmann M, Niggli E, Bauer C, Forster I, Gorlach A. Erythropoietin modulates intracellular calcium in a human neuroblastoma cell line. J Physiol. 1999;516:343–352. doi: 10.1111/j.1469-7793.1999.0343v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between jak2 and nf-kappab signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- 27.Soliz J, Joseph V, Soulage C, Becskei C, Vogel J, Pequignot JM, Ogunshola O, Gassmann M. Erythropoietin regulates hypoxic ventilation in mice by interacting with brainstem and carotid bodies. J Physiol. 2005;568:559–571. doi: 10.1113/jphysiol.2005.093328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973–2979. doi: 10.1161/01.CIR.0000039103.58920.1F. [DOI] [PubMed] [Google Scholar]
- 29.Siren AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, Cerami A, Ehrenreich H, Ghezzi P. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chong ZZ, Kang JQ, Maiese K. Erythropoietin: cytoprotection in vascular and neuronal cells. Curr Drug Targets Cardiovasc Haematol Disord. 2003;3:141–154. doi: 10.2174/1568006033481483. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs-Helber SM, Ryan JJ, Sawyer ST. Jnk and p38 are activated by erythropoietin (epo) but are not induced in apoptosis following epo withdrawal in epo-dependent hcd57 cells. Blood. 2000;96:933–940. [PubMed] [Google Scholar]
- 32.Nagata Y, Takahashi N, Davis RJ, Todokoro K. Activation of p38 map kinase and jnk but not erk is required for erythropoietin-induced erythroid differentiation. Blood. 1998;92:1859–1869. [PubMed] [Google Scholar]
- 33.Ihle JN, Witthuhn BA, Quelle FW, Yamamoto K, Silvennoinen O. Signaling through the hematopoietic cytokine receptors. Annu Rev Immunol. 1995;13:369–398. doi: 10.1146/annurev.iy.13.040195.002101. [DOI] [PubMed] [Google Scholar]
- 34.Koshimura K, Murakami Y, Sohmiya M, Tanaka J, Kato Y. Effects of erythropoietin on neuronal activity. J Neurochem. 1999;72:2565–2572. doi: 10.1046/j.1471-4159.1999.0722565.x. [DOI] [PubMed] [Google Scholar]
- 35.Masuda S, Nagao M, Takahata K, Konishi Y, Gallyas F, Jr, Tabira T, Sasaki R. Functional erythropoietin receptor of the cells with neural characteristics. Comparison with receptor properties of erythroid cells. J Biol Chem. 1993;268:11208–11216. [PubMed] [Google Scholar]
- 36.Tanaka J, Koshimura K, Sohmiya M, Murakami Y, Kato Y. Involvement of tetrahydrobiopterin in trophic effect of erythropoietin on pc12 cells. Biochem Biophys Res Commun. 2001;289:358–362. doi: 10.1006/bbrc.2001.6002. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto M, Koshimura K, Kawaguchi M, Sohmiya M, Murakami Y, Kato Y. Stimulating effect of erythropoietin on the release of dopamine and acetylcholine from the rat brain slice. Neurosci Lett. 2000;292:131–133. doi: 10.1016/S0304-3940(00)01441-5. [DOI] [PubMed] [Google Scholar]
- 38.Hilaire G, Viemari JC, Coulon P, Simonneau M, Bevengut M. Modulation of the respiratory rhythm generator by the pontine noradrenergic a5 and a6 groups in rodents. Respir Physiol Neurobiol. 2004;143:187–197. doi: 10.1016/j.resp.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 39.Soulage C, Pascual O, Roux JC, Denavit-Saubie M, Pequignot JM. Chemosensory inputs and neural remodeling in carotid body and brainstem catecholaminergic cells. Adv Exp Med Biol. 2004;551:53–58. doi: 10.1007/0-387-27023-X_9. [DOI] [PubMed] [Google Scholar]
- 40.Soulage C, Perrin D, Cottet-Emard JM, Pequignot JM. A6 noradrenergic cell group modulates the hypoxic ventilatory response. Adv Exp Med Biol. 2003;536:481–487. doi: 10.1007/978-1-4419-9280-2_61. [DOI] [PubMed] [Google Scholar]
- 41.Dick TE, Coles SK. Ventrolateral pons mediates short-term depression of respiratory frequency after brief hypoxia. Respir Physiol. 2000;121:87–100. doi: 10.1016/S0034-5687(00)00121-3. [DOI] [PubMed] [Google Scholar]
- 42.Hilaire G, Duron B. Maturation of the mammalian respiratory system. Physiol Rev. 1999;79:325–360. doi: 10.1152/physrev.1999.79.2.325. [DOI] [PubMed] [Google Scholar]
- 43.Champagnat J, Denavit-Saubie M, Henry JL, Leviel V. Catecholaminergic depressant effects on bulbar respiratory mechanisms. Brain Res. 1979;160:57–68. doi: 10.1016/0006-8993(79)90600-0. [DOI] [PubMed] [Google Scholar]
- 44.Harris KW, Winkelmann JC. Enzyme-linked immunosorbent assay detects a potential soluble form of the erythropoietin receptor in human plasma. Am J Hematol. 1996;52:8–13. doi: 10.1002/(SICI)1096-8652(199605)52:1<8::AID-AJH2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 45.Nagao M, Masuda S, Abe S, Ueda M, Sasaki R. Production and ligand-binding characteristics of the soluble form of murine erythropoietin receptor. Biochem Biophys Res Commun. 1992;188:888–897. doi: 10.1016/0006-291X(92)91139-H. [DOI] [PubMed] [Google Scholar]
- 46.Westphal G, Braun K, Debus J. Detection and quantification of the soluble form of the human erythropoietin receptor (sepor) in the growth medium of tumor cell lines and in the plasma of blood samples. Clin Exp Med. 2002;2:45–52. doi: 10.1007/s102380200006. [DOI] [PubMed] [Google Scholar]
- 47.Baynes RD, Reddy GK, Shih YJ, Skikne BS, Cook JD. Serum form of the erythropoietin receptor identified by a sequence-specific peptide antibody. Blood. 1993;82:2088–2095. [PubMed] [Google Scholar]
- 48.Kuramochi S, Ikawa Y, Todokoro K. Characterization of murine erythropoietin receptor genes. J Mol Biol. 1990;216:567–575. doi: 10.1016/0022-2836(90)90384-X. [DOI] [PubMed] [Google Scholar]
- 49.Soliz J, Gassmann M, Joseph V. Downregulation of soluble erythropoietin receptor in the mouse brain is required for the ventilatory acclimatization to hypoxia. J Physiol. 2007;583:329–336. doi: 10.1113/jphysiol.2007.133454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raymond CB, Collins DM, Bernstein KN, Skwarchuk DE, Vercaigne LM. Erythropoietin-alpha dosage requirements in a provincial hemodialysis population: effect of switching from subcutaneous to intravenous administration. Nephron Clin Pract. 2006;102:c88–92. doi: 10.1159/000089665. [DOI] [PubMed] [Google Scholar]
- 51.Joseph V, Soliz J, Soria R, Pequignot J, Favier R, Spielvogel H, Pequignot JM. Dopaminergic metabolism in carotid bodies and high-altitude acclimatization in female rats. Am J Physiol. 2002;282:R765–773. doi: 10.1152/ajpregu.00398.2001. [DOI] [PubMed] [Google Scholar]
- 52.Kline DD, Yang T, Premkumar DR, Thomas AJ, Prabhakar NR. Blunted respiratory responses to hypoxia in mutant mice deficient in nitric oxide synthase-3. J Appl Physiol. 2000;88:1496–1508. doi: 10.1152/jappl.2000.88.4.1496. [DOI] [PubMed] [Google Scholar]
- 53.Eckardt KU, Kurtz A. Regulation of erythropoietin production. Eur J Clin Invest. 2005;35(Suppl 3):13–19. doi: 10.1111/j.1365-2362.2005.01525.x. [DOI] [PubMed] [Google Scholar]
- 54.Stockmann C, Fandrey J. Hypoxia-induced erythropoietin production. A paradigm for oxygen-regulated gene expression. Clin Exp Pharmacol Physiol. 2006;33:968–979. doi: 10.1111/j.1440-1681.2006.04474.x. [DOI] [PubMed] [Google Scholar]
- 55.Fisher JW. Erythropoietin: physiology and pharmacology update. Exp Biol Med (Maywood) 2003;228:1–14. doi: 10.1177/153537020322800101. [DOI] [PubMed] [Google Scholar]
- 56.Jelkmann W. Effects of erythropoietin on brain function. Curr Pharm Biotechnol. 2005;6:65–79. doi: 10.2174/1389201053167257. [DOI] [PubMed] [Google Scholar]
- 57.Kawakami M, Iwasaki S, Sato K, Takahashi M. Erythropoietin inhibits calcium-induced neurotransmitter release from clonal neuronal cells. Biochem Biophys Res Commun. 2000;279:293–297. doi: 10.1006/bbrc.2000.3926. [DOI] [PubMed] [Google Scholar]
- 58.Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- 59.Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Fratelli M, Latini R, Xie QW, Smart J, Su-Rick CJ, Pobre E, Diaz D, Gomez D, Hand C, Coleman T, Cerami A. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci USA. 2004;101:14907–14912. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marti HH. Function of erythropoietin in the brain. Johnson City, TN: Graham; 2003. [Google Scholar]
- 61.Marti HH. Erythropoietin and the hypoxic brain. J Exp Biol. 2004;207:3233–3242. doi: 10.1242/jeb.01049. [DOI] [PubMed] [Google Scholar]
- 62.Pequignot JM, Spielvogel H, Caceres E, Rodriguez A, Sempore B, Pequignot J, Favier R. Influence of gender and endogenous sex steroids on catecholaminergic structures involved in physiological adaptation to hypoxia. Pflugers Arch. 1997;433:580–586. doi: 10.1007/s004240050317. [DOI] [PubMed] [Google Scholar]
- 63.Tatsumi K, Pickett CK, Jacoby CR, Weil JV, Moore LG. Role of endogenous female hormones in hypoxic chemosensitivity. J Appl Physiol. 1997;83:1706–1710. doi: 10.1152/jappl.1997.83.5.1706. [DOI] [PubMed] [Google Scholar]
- 64.Iturriaga R, Larrain C, Zapata P. Effects of dopaminergic blockade upon carotid chemosensory activity and its hypoxia-induced excitation. Brain Res. 1994;663:145–154. doi: 10.1016/0006-8993(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 65.Zapata P, Torrealba F. Blockade of dopamine-induced chemosensory inhibition by domperidone. Neurosci Lett. 1984;51:359–364. doi: 10.1016/0304-3940(84)90403-8. [DOI] [PubMed] [Google Scholar]
- 66.Bausero P, Ben-Mahdi M, Mazucatelli J, Bloy C, Perrot-Applanat M. Vascular endothelial growth factor is modulated in vascular muscle cells by estradiol, tamoxifen, and hypoxia. Am J Physiol Heart Circ Physiol. 2000;279:H2033–2042. doi: 10.1152/ajpheart.2000.279.5.H2033. [DOI] [PubMed] [Google Scholar]
- 67.Earley S, Resta TC. Estradiol attenuates hypoxia-induced pulmonary endothelin-1 gene expression. Am J Physiol Lung Cell Mol Physiol. 2002;283:L86–93. doi: 10.1152/ajplung.00476.2001. [DOI] [PubMed] [Google Scholar]
- 68.Mukundan H, Resta TC, Kanagy NL. 17beta-estradiol decreases hypoxic induction of erythropoietin gene expression. Am J Physiol. 2002;283:R496–504. doi: 10.1152/ajpregu.00573.2001. [DOI] [PubMed] [Google Scholar]
- 69.Mukundan H, Resta TC, Kanagy NL. 17-Beta estradiol independently regulates erythropoietin synthesis and nos activity during hypoxia. J Cardiovasc Pharmacol. 2004;43:312–317. doi: 10.1097/00005344-200402000-00023. [DOI] [PubMed] [Google Scholar]
- 70.Shimizu T, Miyamoto A. Progesterone induces the expression of vascular endothelial growth factor (vegf) 120 and flk-1, its receptor, in bovine granulosa cells. Anim Reprod Sci. 2007;102:228–237. doi: 10.1016/j.anireprosci.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 71.Suzuma I, Mandai M, Takagi H, Suzuma K, Otani A, Oh H, Kobayashi K, Honda Y. 17 Beta-estradiol increases vegf receptor-2 and promotes DNA synthesis in retinal microvascular endothelial cells. Invest Ophthalmol Vis Sci. 1999;40:2122–2129. [PubMed] [Google Scholar]
- 72.Ruschitzka FT, Wenger RH, Stallmach T, Quaschning T, de Wit C, Wagner K, Labugger R, Kelm M, Noll G, Rulicke T, Shaw S, Lindberg RL, Rodenwaldt B, Lutz H, Bauer C, Luscher TF, Gassmann M. Nitric oxide prevents cardiovascular disease and determines survival in polyglobulic mice overexpressing erythropoietin. Proc Natl Acad Sci USA. 2000;97:11609–11613. doi: 10.1073/pnas.97.21.11609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soliz J, Soulage C, Hermann DM, Gassmann M. Acute and chronic exposure to hypoxia alters ventilatory pattern but not minute ventilation of mice overexpressing erythropoietin. Am J Physiol. 2007;293:R1702–R1710. doi: 10.1152/ajpregu.00350.2007. [DOI] [PubMed] [Google Scholar]
- 74.Soliz J, Thomsen JJ, Soulage C, Lundby C, Gassmann M. Sex-dependent regulation of hypoxic ventilation in mouse and man is mediated by erythropoietin. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1837–1846. doi: 10.1152/ajpregu.90967.2008. [DOI] [PubMed] [Google Scholar]
- 75.Gaultier C. Development of the control of breathing: implications for sleep-related breathing disorders in infants. Sleep. 2000;23(Suppl 4):S136–139. [PubMed] [Google Scholar]
- 76.Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Attenuation of the hypoxic ventilatory response in adult rats following one month of perinatal hyperoxia. J Physiol. 1996;495:561–571. doi: 10.1113/jphysiol.1996.sp021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Antier D, Zhang BL, Mailliet F, Akoka S, Pourcelot L, Sannajust F. Effects of neonatal focal cerebral hypoxia–ischemia on sleep-waking pattern, ecog power spectra and locomotor activity in the adult rat. Brain Res. 1998;807:29–37. doi: 10.1016/S0006-8993(98)00703-3. [DOI] [PubMed] [Google Scholar]
- 78.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102:616–620. doi: 10.1542/peds.102.3.616. [DOI] [PubMed] [Google Scholar]
- 79.Tuor UI, Del Bigio MR, Chumas PD. Brain damage due to cerebral hypoxia/ischemia in the neonate: pathology and pharmacological modification. Cerebrovasc Brain Metab Rev. 1996;8:159–193. [PubMed] [Google Scholar]
- 80.Juul SE, Yachnis AT, Christensen RD. Tissue distribution of erythropoietin and erythropoietin receptor in the developing human fetus. Early Hum Dev. 1998;52:235–249. doi: 10.1016/S0378-3782(98)00030-9. [DOI] [PubMed] [Google Scholar]
- 81.Juul SE, Zhao Y, Dame JB, Du Y, Hutson AD, Christensen RD. Origin and fate of erythropoietin in human milk. Pediatr Res. 2000;48:660–667. doi: 10.1203/00006450-200011000-00018. [DOI] [PubMed] [Google Scholar]
- 82.Semba RD, Juul SE. Erythropoietin in human milk: physiology and role in infant health. J Hum Lact. 2002;18:252–261. doi: 10.1177/089033440201800307. [DOI] [PubMed] [Google Scholar]
- 83.Beall CM. Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc Natl Acad Sci USA. 2007;104(Suppl 1):8655–8660. doi: 10.1073/pnas.0701985104. [DOI] [PMC free article] [PubMed] [Google Scholar]