Abstract
Diapause entails molecular, physiological and morphological remodeling of living animals, culminating in a dormant state characterized by enhanced stress tolerance. Molecular mechanisms driving diapause resemble those responsible for biochemical processes in proliferating cells and include transcriptional, post-transcriptional and post-translational processes. The results are directed gene expression, differential mRNA and protein accumulation and protein modifications, including those that occur in response to changes in cellular redox potential. Biochemical pathways switch, metabolic products change and energy production is adjusted. Changes to biosynthetic activities result for example in the synthesis of molecular chaperones, late embryogenesis abundant (LEA) proteins and protective coverings, all contributing to stress tolerance. The purpose of this review is to consider regulatory and mechanistic strategies that are potentially key to metabolic control and stress tolerance during diapause, while remembering that organisms undergoing diapause are as diverse as the processes itself. Some of the parameters described have well-established roles in diapause, whereas the evidence for others is cursory.
Keywords: Diapause, Gene expression, AMP-activated protein kinase, Molecular chaperone, Stress tolerance, Reactive oxygen/nitrogen species, Apoptosis, LEA proteins
Introduction
Diapause, a developmental program that has existed in metazoans for millions of years [1], is characterized by processes opposite to those of reproductive growth, such as the arrest or slowing of cell division in response to an immediate or anticipated stress, reduction of metabolism and enhancement of stress tolerance, at times to high levels [2–8]. The process of diapause occurs at different genetically established developmental stages, but usually at only one life history stage in any given organism, and it provides adaptive benefits. Two favorable outcomes of diapause are survival of environmental extremes and the synchronization of reproductive activities. Moreover, the reactivation and development of organisms that have lain dormant for periods of time that can extend into years mix temporally separated genotypes, possibly to advantage in ways that would not otherwise occur [2, 9].
Diapause is divided into overlapping stages encompassing induction, preparation, initiation, maintenance and termination, with the latter followed by post-diapause quiescence if environmental conditions are not conducive to growth [3, 6]. The entry into and progression through diapause are mediated by molecular mechanisms similar to those that guide cellular reproductive growth; these include differential gene expression, post-transcriptional events, post-translational protein modifications and protein localization to specific regions within cells and organisms. The ability to execute these events in a coordinated manner implies the function of molecular switches that exert regulatory roles, directing development along the appropriate pathways. Identifying the switches and the extent to which they are shared between organisms are central questions in the study of diapause. Although answers remain elusive, it is known that genes characteristic of reproductive growth are down-regulated during diapause, while other genes, often termed diapause-specific, are up-regulated, and the expression of still others is either not altered or changes intermittently [3, 10–14]. Gene expression and progress through diapause are likely to be influenced by signaling pathways, many of which are receptor responsive and perceive extracellular factors, whether generated by other cells or as a physical signal originating in the environment, the best studied being for Caenorhabditis and Drosophila [8, 15–17]. Internally derived signals including reactive oxygen/nitrogen species (ROS/RNS) like hydrogen peroxide (H2O2) and nitric oxide (NO) may regulate diapause-specific developmental pathways. H2O2 and NO modulate development and physiological activity in many organisms, often in a coordinated manner [18–20], and they support the germination of plant seeds [21, 22], biological structures similar to diapause-specific resting stages of organisms like Artemia franciscana [23].
Arising from the regulatory processes mentioned above are changes from one type of intermediary metabolism to another, metabolic rate depression and the diversion of energy expenditure away from cellular events required for an active lifestyle [7, 10, 24, 25]. For example, energy saved by inhibition of translation and ion pumping, both controlled in part by reversible protein phosphorylation [24, 26, 27], becomes available for the construction of morphological structures such as protective coverings that prevent organismal rupture, collapse during stress and water loss [8, 28, 29]. The synthesis during diapause of molecular chaperones able to inhibit irreversible protein denaturation and apoptosis [3, 4, 10, 23, 30, 31] is empowered by redirection of energy reserves, as is the production of late embryogenesis abundant (LEA) proteins that protect against dehydration [32–37]. Sugars such as trehalose, synthesized in some diapause-destined organisms and thought to act in concert with proteins, shield against drying damage [34].
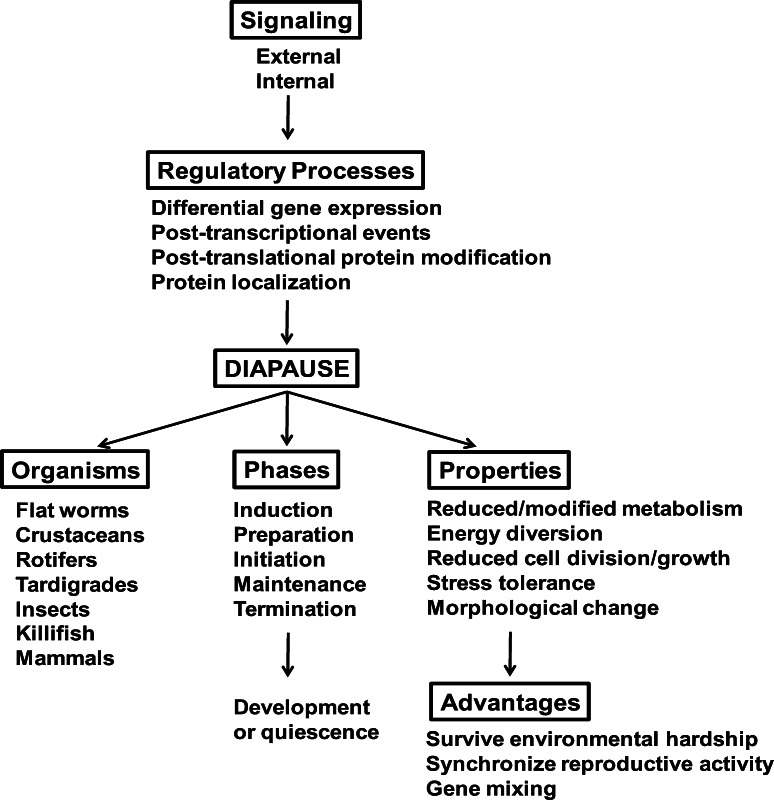
The broad range of mechanistic processes displayed by diapausing organisms, where genes are expressed and energy is utilized to produce properties different from those in growing cells, converges to common outcomes. Respiratory and intermediary metabolism adjust to environmental conditions and the generation of molecules required for survival during diapause, the cell cycle reversibly arrests, and stress tolerance is enhanced to protect cell attributes required for resumption of growth and development when diapause terminates. However, the multiplicity of organisms capable of undergoing diapause and the diversity of developmental programs recognized as diapause dictate that the properties of this developmental pathway will vary from species to species. By way of example, diapause occurs in flat worms where it is known as dauer [8, 10, 15], crustaceans [2, 9, 23, 38, 39], rotifers [14], tardigrades [40–42], insects [3, 6, 7, 13, 16, 17], killifish [43, 44] and mammals, the latter as delayed embryo implantation [4]. In addition, embryonic diapause has been reported for Caenorhabditis elegans [45]. Within these groups diapause manifests in different developmental stages including embryos, pupae, larvae or adults, thus adding to complexity. The degree of dormancy ranges from cessation of development and very low metabolism in the embryonic diapause of A. franciscana [23, 46, 47] and the annual killifish, Austrofundulus limnaeus [26, 48], to relatively mild dormancy where metabolism and growth continue at a lower rate than during reproductive metabolism, as in the larval and adult diapauses of some copepods [9] and insects [3, 7, 13, 16]. The level of stress tolerance achieved during diapause varies, along with the degrees of morphogenesis and behavioral modifications contributing to survival under adverse conditions [8, 16, 23]. The nature and timing of external signals such as day length or temperature, acting alone or in concert, that induce diapause via neurohormonal mechanisms in many organisms differ, as do cues that end dormancy, which in C. elegans requires only return to favorable growth conditions [2, 3, 6, 8, 17]. It is of interest, therefore, to consider diapause properties—those grounded in fact as well as those of a speculative nature—and to identify cell/molecular commonalities that exist in spite of differences at higher organizational planes (Fig. 1). This is done throughout the review with emphasis on metabolic regulation and stress tolerance rather than the equally interesting processes of diapause initiation and termination per se.
Fig. 1.
Diapause. Regulatory processes triggered by external and internal signals occur in many organisms capable of undergoing diapause, a physiological process characterized by overlapping stages. All diapause organisms exhibit one or more properties, in varying degrees of intensity, which enable survival upon exposure to harsh environments and permit the mixing of genes from temporally separated populations. This review demonstrates underlying similarities between diapauses in organisms that may otherwise appear unrelated and that diapause in different organism is often characterized by shared properties expressed in varying intensities
Gene expression during diapause
Differential gene expression during diapause is scrutinized in numerous studies by homology-based identification of mRNAs and proteins on a one-by-one basis. This approach is complemented by the establishment of expressed sequence tag (EST) libraries [49–52] and the application of methodologies permitting multiple-gene analysis in single experiments such as proteomics [12, 39, 53], suppressive subtractive hybridization [11, 14, 38, 54] and microarray analysis [13, 55], these often coupled with PCR-based quantification and probing of northern blots. The result is an expanding list of mRNAs and proteins that increase, decrease or are unchanged during diapause. Some of these approaches, although informative, are limited because only mRNA is examined, and others, although showing the presence of particular proteins, fall short of demonstrating function. Mutational studies, gene knock outs and protein knock down by RNA interference (RNAi) are required to confirm protein functionality. Despite the limitations it is nonetheless very instructive to consider data generated by multi-gene analysis because they comment on earlier studies and suggest avenues for subsequent investigations.
Microarray technology and immunofluorescence were used to explore delayed implantation of mouse embryos [55, 56]. Delayed implantation is a hormone-controlled state of suspended animation where blastocyst cell proliferation and metabolism cease, thereby precluding embryo implantation. Six categories of genes including those involved in cell cycle regulation, metabolism, intracellular signaling, transport into the nucleus, chromatin remodeling and cell adhesion display altered expression upon comparison of diapausing and active mouse embryos. Delayed implantation has similarities to C. elegans dauer, but it potentially differs in the degree of stress tolerance because molecular chaperones are not up-regulated in dormant mouse blastocysts, whereas sHSPs accrue during dauer [57]. The difference reflects the more variable environment experienced by C. elegans as compared to the blastocyst inside an animal.
When preparing for diapause, a process initiated by juvenile hormone reduction, adult females of the northern house mosquito, Culex pipiens L. enhance lipid content by feeding on carbohydrate-rich plant secretions rather than blood [54]. Suppressive subtractive hybridization and probing of northern blots reveal differential regulation of eight gene categories at various stages of C. pipiens diapause. There are similarities to the types of genes expressed in mammalian embryos experiencing delayed implantation such as those modulating metabolism, food use and cell growth regulation. One difference is the slight up-regulation of genes encoding a sHSP and Hsp70, perhaps required for overwintering protection, although neither molecular chaperone is considered a major contributor to C. pipiens diapause. By comparison, stress proteins play an important role in pupal diapause of the flesh fly Sarcophaga crassipalpis [53, 54, 58–60]. Among other stress responses in C. pipiens, a gene encoding the antioxidant selenoprotein, a protein with the capacity to influence lipid peroxidation, is up-regulated. This observation highlights the need to control the redox state of diapause organisms, both as a protective mechanism and in cell signaling.
Drosophilamelanogaster lack a strong diapause response; nonetheless, oogenesis is impeded at pre-vitellogenesis if reproduction is unlikely to be successful, leading to a physiological state termed reproductive diapause [13]. Arrested eggs resume development after several weeks of dormancy or when females return to permissive conditions. Although giving rise to a superficial or light dormancy, reproductive diapause is under hormonal and circadian regulation. The analysis of Drosophila reproductive diapause is complicated by the concurrent expression of genes engaged in early egg development. To facilitate categorization, Baker and Russell [13] proposed that diapause-specific genes are up-regulated within 24 h of diapause induction in Drosophila, and their expression does not change significantly until oogenesis resumption. Genes that change expression when post-diapause vitellogenesis begins may also mediate reproductive diapause. Employing these guiding criteria, microarray analyses established the differential regulation of genes engaged in RNA and protein production during Drosophila reproductive diapause, with TOR signaling pathway members prominent. Hsp23 and Hsp70 mRNA increase, and their protein products could protect proteins from stress-induced irreversible unfolding. Juvenile hormone catabolism is enhanced, perhaps potentiating diapause entry, a process requiring mediation of growth suppression and lipid storage by the insulin signaling pathway. Enzymes of lipid and carbohydrate metabolism are prominent although their activity declines as diapause progresses and animals prepare to over-winter. The buildup of proteolytic enzymes is likely to pave the way for resumption of digestion upon diapause termination. Cuticle structural components are enriched, as are chitin-binding proteins, changes with the capacity to decrease cuticle permeability, prevent water loss and promote survival [13].
Responding to external and internal factors, the parasitic solitary wasp Nasonia vitripennis undergoes maternally induced developmental diapause, with the last larval instar entering dormancy prior to pupation [12]. Non-gel shotgun proteomics revealed the exclusive synthesis of only a few proteins during early Nasonia diapause, but differences were observed in the abundance of proteins concerned with fat and sugar metabolism, results reminiscent of diapause in other invertebrates. The accrual of macromolecules required for protein synthesis and degradation switched, as diapause progressed, to the accumulation of proteins engaged in maintenance. For example, elongation factor 1 and an assortment of ribosomal proteins declined, corroborating reports of protein synthesis and cell growth regression as larvae enter diapause. Reduced histone levels correlate with falling protein synthesis and cell replication, events possibly related to increases in actin and fluctuating proteolytic enzymes, both associated with cell restructuring. Actin changes in C. pipiens were suggested to modulate the cytoskeleton early in diapause [61]. Metabolism adjusts to maintain diapause as cell growth decreases, and in Nasonia this includes the synthesis of glycerol, a cryo-protective molecule. Other protective proteins augmented during Nasonia diapause are ferritin, which may shield against oxidative damage, hinder cell growth by sequestering iron or perform both actions. In addition, Hsp90 dwindles as diapause progresses, while Hsp20, likely to chaperone denaturing proteins, increases.
Subtractive hybridization [11] and proteomics [39] were used respectively to study diapause in the crustaceans A. franciscana and A. sinica. Diapausing Artemia embryos enter a profound state of dormancy, and they possess exceptional stress tolerance. At 2 days post-fertilization, the quantities of several mRNAs expand in diapause-destined embryos, but not in embryos developing directly into larvae [11]. This group of mRNAs, whose accumulation was verified by RT–PCR, accommodates a stress-responsive transcription co-factor termed p8, homologues of human tumor suppressor proteins with potential as cell cycle inhibitors, sHSPs and several enzymes, such as glucose-6-phosphate isomerase, cytochrome P450 and chitin-binding proteins, their presence disclosing alterations in cell metabolism. Diapause A. sinica cysts were examined by proteomics-based methodology, but comparisons were not made to other life history stages. A sHSP equivalent to A. franciscana p26 was the major spot on two-dimensional gels containing protein extracts of A. sinica cysts and Hsp70 was present, both presumably required for stress tolerance. Several enzymes responsible for carbohydrate and energy metabolism were identified, and they were proposed to have a role in the resumption of development. Additionally, mediators of protein synthesis like Hsp70, a tRNA synthetase, proteolytic enzymes and cell cycle regulators increased in A. sinica diapause cysts. It is difficult to compare the studies done with Artemia because different methodologies were employed and the embryos were examined at dissimilar stages, but they do open a window on proteins synthesized in organisms experiencing profound dormancy during diapause.
The rotifer Brachionus plicatilis, a zooplanktonic invertebrate, produces resting or diapause eggs that persist for decades and permit survival even though the organism inhabits ephemeral bodies of water [14]. Subtractive hybridization revealed several up-regulated dormancy-associated Brachionus mRNAs with the capacity to protect intracellular macromolecules. mRNAs encoding proteins implicated in anti-oxidant activity and detoxification are highly represented in Brachionus EST libraries. These consist of glutathione S transferases, known to be associated with C. elegans dauer, dismutases that catalyze the formation of H2O2 from superoxide radicals, peroxiredoxins and thioredoxin peroxidase. Hsp70 and sHSPs are found, with the latter highly represented in resting eggs of Brachionus, as is true for Artemia cysts. The molecular chaperones undoubtedly confer macromolecular protection, preventing irreversible stress-induced protein denaturation and contributing to refolding when development resumes. Brachionus synthesizes Group 3 LEA proteins, first found in plants where they enhance resistance to desiccation and stress, and since then observed in Artemia and C. elegans. LEA proteins are discussed in more detail later in the review. Metabolic parameters were considered with interest in genes required for the synthesis of lipids, potentially a major energy source. Several lipoprotein lipase transcripts were noted, and this enzyme may replace vitellogenin in yolk platelets.
Methodologies permitting multiple-gene analysis prove diapause is characterized by the regulation of a plethora of genes varying from species to species. Common themes emerge, frequently echoing those generated by homology-based identification of mRNAs and proteins. Many questions are posed by these findings, and several will be considered, beginning with a speculative consideration of metabolic rate depression, a fundamental diapause characteristic.
Does AMP-activated protein kinase (AMPK) mediate diapause-induced metabolic rate depression and dormancy?
Survival time during diapause and quiescence tends to be proportional to the extent of metabolic depression, making the regulation of metabolic reduction, often accompanied by an increased AMP:ATP ratio, critical to continued existence during dormancy [62–64]. Sensitivity to the intracellular AMP:ATP ratio or energy charge, and a key to control of cell growth and division in response to extracellular signals, is the liver kinase B1-AMP-activated protein kinase (LKB1-AMPK) pathway [65–68]. AMPK was discovered in mammalian cell protein extracts where it functioned as an antagonist of lipid biosynthesis by phosphorylating key enzymes responsible for the manufacture of cholesterol and fatty acids. AMPK is a hetero-trimer composed of the catalytic α-monomer and the regulatory subunits β and γ, products of different, often multi-copy genes. The α-subunits, recognized as serine/threonine kinases, have a highly conserved threonine residue phosphorylated by the up-stream kinase LKB1, a functional tumor suppressor when bound to co-factors STRAD and MO25 [68]. Contrary to what might be expected, LKB1 is not activated directly by AMP even though the AMP:ATP ratio increases in metabolically stressed cells. Instead, AMP binds to Bateman domains in the regulatory γ-subunit, promoting net phosphorylation of the α-subunit by reducing its effectiveness as a phosphatase substrate. The phosphorylated α-subunit is also stimulated allosterically by AMP, which acts synergistically with increased phosphorylation to greatly enhance AMPK, a process requiring only a minor increase in nucleotide concentration. Additionally, the β-subunit has a glycogen-binding domain, potentially allowing response to glycogen levels, an interesting possibility in the context of energy sensing that has yet to be investigated thoroughly [69].
AMPK is triggered by hypoxia and other metabolic stresses that interfere with ATP synthesis. Under energy-limiting circumstances activated AMPK has the capacity to turn on alternative ATP producing catabolic pathways ensuring sufficient energy is available to sustain organisms as overall energy production wanes, a common situation during diapause. Furthermore, as diapause prevails, major energy utilizers like protein synthesis decline upon inhibition of both initiation and elongation [70, 71]. Protein synthesis initiation is limited in some cells by the tumor suppressor tuberous sclerosis complex (TSC2), a target of AMPK. TSC2 decreases phosphorylation of ribosomal S6 kinase (S6 K) and eukaryotic initiation factor 4E binding protein 1 (4EBP1), important regulators of protein synthesis that function via the target of rapamycin (TOR) pathway [70]. In addition, protein synthesis is inhibited by a TOR-independent mechanism where AMPK activates eukaryotic elongation factor 2 (eEF2) kinase, leading to eEF2 phosphorylation and nullification of its activity [71]. Thus, by impeding protein synthesis AMPK eliminates a major cellular energy demand and curbs hypertrophy of non-dividing cells, both effects aiding survival by coordinating energy levels and growth [68, 70]. As just described, AMPK exerts acute regulation within cells by phosphorylating metabolic enzymes and protein synthesis factors. Longer term control is exercised through phosphorylation of p300, a transcriptional co-activator able to modulate the expression of genes participating in metabolic pathways influenced by AMPK [72], or by a combination of transcriptional and post-translational mechanisms acting on discrete cellular processes [68]. For example, AMPK reduces apoptosis induced by energy stress; this is important for organisms undergoing diapause [73].
The potential significance of AMPK-dependent regulatory mechanisms in organisms experiencing reduced metabolism and development due to diapause is obvious, but is there evident for this? Artemia undergo profound metabolic depression during diapause [46, 47, 74], but respiration rates for mitochondria isolated from diapause and metabolically active embryos are similar [74]. Apparently, changes to mitochondria do not explain metabolic rate depression in diapause Artemia embryos, and this leaves the way open for other mechanisms including those mediated by AMPK. Artemia contain AMPK, although it appears not to be phosphorylated in early post-diapause embryos when the AMP:ATP ratio is highest [75, 76]. Phosphorylated AMPK is first detected 4 h post-diapause and increases for 12 h, but it is absent from nauplii and adults. Delegation of phosphorylated (active) AMPK to this narrow timeframe implies promotion of post-diapause development rather than inhibition of metabolism and development in diapause-destined embryos. These results are intriguing, especially because the reported amount of active AMPK in diapause embryos is different from the quantity in embryos immediately post-diapause and yet to initiate development [76]. Setting AMPK in motion at this time, along with restricted distribution of the enzyme mostly to ectoderm nuclei, is a sign AMPK influences gene expression during the differentiation of post-diapause Artemia embryos, rather than exercising metabolic regulation per se.
To my knowledge there are no other reports of AMPK activity in diapausing organisms although a regulatory role is suggested for the enzyme in killifish [26, 48]. As well, quantities of the AMPK-regulated, serine/threonine kinase S6 K in diapause Artemia cysts are lower than those in cysts which have just broken diapause, but the effect of the change is uncertain [77]. S6 K from diapause Artemia embryos is non-phosphorylated, potentially contributing to termination of mitosis and cell division because it is inactive. Support for the idea that AMPK regulates Artemia S6 K phosphorylation is lacking; indeed, experimental evidence points to the contrary (see above). Artemia S6 K is however phosphorylated by 12 h post-diapause, when it may assist in re-initiation of mitosis and cell cycle progression [77].
Molecular chaperons as instruments of diapause maintenance in A. franciscana: a model system for the study of profound dormancy and stress tolerance
Of paramount significance during diapause is the tolerance of environmental and physiological stressors such as temperature extremes, desiccation, ultraviolet radiation, salinity, hypoxia/anoxia and reduced metabolism. Resistance to stress is enhanced several ways with the synthesis of protective proteins especially important. Among these cell guardians are the molecular chaperones and co-chaperones composed of several protein families separated by amino acid sequence, molecular mass and mechanism of action. These consist of Hsp110 [78], Hsp104 [79], Hsp90 [80–82], Hsp70 [78, 83–85], Hsp60 [85, 86] and the sHSPs [78, 87–89]. The molecular chaperones mediate the folding and compartmentalization of nascent proteins, maintain proteins in functional conformations, protect proteins from irreversible denaturation during stress and aid in their refolding, and assist in the destruction of non-salvageable denatured proteins (Table 1).
Table 1.
Primary functions of molecular chaperones
| Chaperone familya | Functions | References |
|---|---|---|
| Hsp110 | Suppress irreversible protein denaturation; nucleotide exchange factor for HSP70; low ATPase activity | [78] |
| Clp/Hsp100 (Hsp104)b | Rescue polypeptides from protein aggregates; facilitate protein refolding or destruction; utilizes ATP | [79] |
| Hsp90c | Promote protein folding, stability, activity and assembly; modulate transcription factors and kinases among other regulatory proteins; influence gene expression and cell signaling; low ATP activity | [80–82] |
| Hsc70/Hsp70 (DnaK) | Folding of nascent and compromised proteins; protein degradation; clathrin uncoating; protein compartmentalization; stress protection; utilizes ATP | [78, 83–85] |
| Hsp60 (GroEL, TRiC/CCT, (chaperonins) | Folding of nascent proteins, especially those with multiple domains and/or enriched in β-strands; facilitate oligomer assembly; utilizes ATP | [85, 86] |
| Small heat shock proteins (sHSPs) | Prevent irreversible protein denaturation during stress; protein refolding and degradation; apoptosis inhibition; cytoskeleton stabilization; no ATPase activity | [78, 87–89] |
aAlthough presented in the table as separate families the molecular chaperones function as networks, ensuring that cells contain the appropriate protein complement
bClp/Hsp100 proteins have not been found in animal cell cytosol [79]
cS-nitrosylation at a highly conserved Cys (597 in humans, 577 in yeast) by NO regulates Hsp90 activity and NO synthesis [80, 82]
Artemia contain several small molecular chaperones (Table 2) and the first to be investigated in detail was p26, initially observed in post-diapause encysted embryos [90, 91]. The p26 protein was subsequently purified to apparent homogeneity and sequenced [92], and its cDNA was cloned and sequenced, revealing a sHSP with a typical α-crystallin domain [93–95]. The Artemia p26 gene has been characterized, as have p26 cDNAs from several Artemia species of diverse geographical locations and habitats, and all are similar in derived amino acid sequence [96]. p26 forms oligomers, either when synthesized in Artemia [90] or in transformed bacteria and transfected mammalian cells [30, 92, 94, 95, 97, 98]. p26 functions as a molecular chaperone in vitro, protecting citrate synthase and Artemia tubulin from heat-induced denaturation, and insulin from reduction-induced aggregation [92, 94, 95, 98, 99], while conferring thermotolerance on first instar nauplii hatching from cysts and transformed bacteria [94, 95, 97, 100]. p26 bestows heat resistance on transfected mammalian cells and limits apoptosis induction by heat, drying/rehydration and staurosporine, by impeding procaspase-3 activation [30].
Table 2.
Diapause-specific Artemia molecular chaperones
| Property | Artemia molecular chaperones | |||
|---|---|---|---|---|
| p26 | ArHsp21 | ArHsp22 | Artemin | |
| Molecular mass (kDa) | 20.8 | 21.1 | 22.4 | 26.0 |
| Amino acid residues | 192 | 181 | 190 | 230 |
| α-Crystallin domain (residue numbers) | +(61–152) | +(70–162) | +(74–168) | – |
| Ferritin homolog | − | − | − | + |
| Oligomerization | + | + | + | + |
| Chaperone activity | + | + | + | + |
| Apoptosis inhibition | + | ND | ND | ND |
| Developmentally regulated | + | + | + | + |
| Stress regulated | − | − | +(Adults only) | − |
| Abundance in cysts | High | ND | ND | High |
(Adults only), expression induced by heat only in adults
+ Property observed; − property not observed; ND not determined
p26 is synthesized only in diapause-destined embryos, appearing at day 3 post-fertilization [100, 101], with p26 mRNA visible a day earlier as determined by probing of Northern blots [100] and quantitative real-time PCR [96]. A very low level of p26 mRNA is observed by PCR in Artemia embryos developing directly into nauplii, but p26 protein is not detected by immuno-probing of Western blots [96]. p26 moves into nuclei upon synthesis in diapause-destined embryos, suggesting the chaperone protects both cytoplasmic and nuclear proteins [93, 100–103]. p26 protein and mRNA disappear during post-diapause development, indicating a diapause-specific function for the protein which is last observed in a sub-group of salt gland nuclei in late instar 2 nauplii [90, 100].
As revealed by suppressive subtractive hybridization two other sHSPs, ArHsp21 and ArHsp22 [11, 104, 105], are synthesized in diapause-destined but not nauplius-destined Artemia embryos. ArHsp21 and ArHsp22 each contain an α-crystallin domain with a highly conserved arginine, a WXDPF amino-terminal motif duplicated in ArHsp22 and a carboxy-terminal I/VXI/V sequence. Quantitative real-time PCR exposed ArHsp21 mRNA in diapause-destined embryos at day 2 post-fertilization followed on day 3 by recognition of its protein product on Western blots, as shown for p26. The appearance of ArHsp22 mRNA and protein was delayed slightly relative to ArHsp21, and neither protein was observed on Western blots containing protein extracts from cyst-derived instar II larvae. ArHsp21 and ArHsp22 oligomerize, whether synthesized in cysts or in transformed bacteria, and they function as molecular chaperones in vitro, preventing heat-induced denaturation of citrate synthase and reduction-induced aggregation of insulin. Of the three Artemia sHSPs, only ArHsp21 fails to migrate into nuclei. ArHsp22 synthesis is induced by thermal stress in adults, but not earlier life history stages, whereas neither p26 nor ArHsp21 responds to heat.
The manufacture of p26, ArHsp21 and ArHsp22 in diapause-destined Artemia embryos, but not in embryos that develop directly into nauplii, indicates their importance to diapause. All three molecular chaperones potentially protect proteins from irreversible denaturation due to energy limitation and environmental stressors. Functional redundancy provides insurance for diapausing embryos, and at the same time each sHSP may perform task(s) not shared by the others. For example, in contrast to ArHsp22 and p26, ArHsp21 appears not to translocate into nuclei, suggesting that only two Artemia sHSPs protect proteins in this organelle. ArHsp22 is induced by heat, making it the only known crustacean sHSP whose synthesis is regulated both developmentally and in response to stress. The latter characteristic suggests an activity for ArHsp22, not shared by p26 and ArHsp21, when Artemia encounter environmental stress. p26 inhibits apoptosis, a function possibly limited to this sHSP, although the effects of ArHsp21 and ArHsp22 on apoptosis have yet to be determined. Artemia embryos synthesize several additional molecular chaperones such as Hsp70, Hsp90 and Hsp110 [39, 49, 103, 106–110], but their roles in diapause are uncertain. By comparison, dehydration-tolerant diapause embryos of the killifish A. limnaeus, which enter a profound dormancy and are resistant to heat, salinity, and anoxia [44], possess a constitutively synthesized Hsp70 with the potential to enhance stress tolerance. Similar possibilities exist for insect molecular chaperones, although insects display diapause characteristics less extreme than those exhibited by Artemia and Austrofundulus.
Molecular chaperones and insect diapause: diverse strategies with related outcomes
Insects enter diapause as embryos, larvae, pupae and adults, with diapause generally restricted to a single developmental period and characterized by varying degrees of dormancy for each species. Diapause in insects entails differential expression of genes, often demonstrated experimentally by mRNA quantification, and this work has cast light on the role of molecular chaperones as protectors of macromolecules and contributors to stress tolerance. To identify trends in the experimental data, the diapause-dependent expression of genes encoding molecular chaperones is considered at progressively more advanced stages of insect development from larvae to adults (Table 3).
Table 3.
Molecular chaperones during insect diapause
| Development stage | Insect | Molecular chaperone | Regulation | References |
|---|---|---|---|---|
| Larva | S. nonagrioides (corn stalk borer) | sHSP | Constant | [111] |
| sHSP | Down | [111] | ||
| Hsc70 | Up | [112] | ||
| Hsp70 | Down | [112] | ||
| Hsp90 | Up | [113] | ||
| O. fuscidentalis (bamboo borer) | Hsc70 | Variable | [114] | |
| Hsp70 | Down | [114] | ||
| Hsp90 | Down | [114] | ||
| C. suppressalia (rice stem borer) | Hsc70 | Constant | [115] | |
| Hsp90 | Up | [115] | ||
| L. sericata (blow fly) | sHSP | Constant | [116] | |
| Hsp70 | Constant | [116] | ||
| Hsp90 | Constant/up | [116] | ||
| N. vitripennis (parasitic solitary wasp) | Hsp90 | Down | [12] | |
| Pupa | S. crassipalpis (flesh fly) | sHSP | Up | [58, 117, 118] |
| sHSP | Up | [60] | ||
| sHSP | Up | [60] | ||
| sHSP | Up | [60] | ||
| Hsp60 | Up | [60] | ||
| Hsp70 | Up | [60, 118, 119] | ||
| Hsp 70 | Up | [60, 118, 119] | ||
| Hsc70 | Constant | [60] | ||
| Hsp90 | Down | [54, 118, 119] | ||
| M. rotundata (solitary bee) | Hsc70 | Constant | [120] | |
| Hsp70 | Up | [120] | ||
| Hsp90 | Constant | [120] | ||
| D. antiqua (onion maggot) | Hsp60 | Up (summer) | [125] | |
| Hsp60 | Up (winter) | [125] | ||
| Hsp70 | Up | [121] | ||
| Hsp90 | Up (summer) | [124] | ||
| Hsp90 | Variable (winter) | [124] | ||
| H. zea (corn earworm) | Hsc70 | Constant | [122] | |
| Hsp70 | Constant | [122] | ||
| Hsp90 | Down | [122] | ||
| H. armigera (cotton bollworm) | Hsp90 | Down | [123] | |
| Adult | C. pipiens (mosquito) | Hsc70 | Down | [129] |
| Hsp70 | Constant | [128] | ||
| D. triauraria (fruit fly) | sHSP | Constant | [131] | |
| sHSP | Constant | [131] | ||
| Hsp70 | Constant | [130] | ||
| Hsp90 | Constant | [131] | ||
| L. decemlineata (Colorado potato beetle) | Hsp70 | Up | [132] | |
| Hsp70 | Constant | [132] |
Up increase in HSP during diapause; down decrease in HSP during diapause; variable HSP increased and decreased during diapause; constant no change in HSP during diapause; constant/up HSP constant for most of diapause and then increased
mRNA and not protein was generally measured
Summer and winter, diapause during the summer and winter seasons, respectively
The accumulation of two sHSP mRNAs varies during larval diapause in the corn stalk borer Sesamia nonagrioides (Lef.), an organism that feeds and moults throughout the period of reduced activity associated with diapause. SnoHsp19.5 mRNA remains constant for the duration of diapause, whereas SnoHsp20.8 mRNA falls off in mid-diapause and expands upon termination, suggesting different functions for these sHSPs [111]. The amount of SnoHsp70 mRNA dips as S. nonagrioides enters diapause, whereas the cognate SnoHsc70 mRNA multiplies in deep diapause [112], as does mRNA encoded by the Hsp83 gene, a homologue of Hsp90 [113]. These observations suggest diapause-related roles for Hsc70 and Hsp90, perhaps in maintaining protein structure and contributing to ecdysone action. The tropical bamboo borer Omphisa fuscidentalis undergoes drought-induced larval diapause within the relatively constant environment of the bamboo culm internode [114]. Hsp70 mRNA drops in O. fuscidentalis fat bodies as diapause initiates and remains depressed into the pupal stage, while Hsc70 mRNA is low for the first 4 months of diapause before experiencing a 12-fold amplification prior to decreasing. The quantity of Hsp90, a molecular chaperone that regulates cell signaling through interaction with metastable proteins [82], is unchanged for much of O. fuscidentalis diapause after going through pre-diapause fluxes, and then it declines before exhibiting transient growth at termination [114]. The authors conclude that O. fuscidentalis Hsp70 is not associated with diapause although Hsp90 and Hsc70, the latter possibly under 20-hydroxyecdysone and juvenile hormone control, are. During larval diapause of the rice stem borer, Chilo suppressalia, in the same family as O. fuscidentalis, Hsp90 but not Hsc70 mRNA builds up [115]. mRNAs encoding Hsp23, Hsp70 and Hsp90 are unchanged in the non-feeding, third instar larval diapause of the blow fly, Lucilia sericata, however Hsp90 transcripts proliferate as diapause terminates, perhaps in response to ecdysteroid [116]. The changes in Hsp90 may reflect increased signaling activity as cells return to pre-diapause activity [82, 116]. In comparison, N. vitripennis Hsp90 protein decays as larval diapause progresses [12].
Diapause is induced in pupae of the flesh fly S. crassipalpis via failure of the brain to promote ecdysteroid production [58]. As made evident by the probing of Northern blots containing RNA from either the entire organism or the brain, transcripts encoding a 23 kDa sHSP (Hsp23) increase significantly upon initiation of flesh fly diapause, remain high throughout this life phase and then decrease as diapause ends [58, 117, 118]. At least three other sHSPs up-regulate during S. crassipalpis diapause [60]. Two different transcripts encoding inducible Hsp70 also rise as flesh flies enter diapause, declining upon termination [60, 118, 119]. Similar results pertain for Hsp70 during the pre-pupal diapause of the solitary bee Megachile rotundata [120] and the summer and winter pupal diapauses of the onion maggot Delia antiqua [121]. Hsc70 mRNA accumulation is however unchanged when S. crassipalpis and M. rotundata pass through diapause and in the corn earworm, Helicoverpa zea, neither Hsp70 nor Hsc70 mRNA is affected by diapause [122].
Hsp90 mRNA ebbs throughout S. crassipalpis diapause, returning to higher levels when development resumes [54, 118, 119]. The drop in S. crassipalpisHsp90 mRNA may be attributed to ecdysone reduction, which leads to the coordinated increase of Hsp23 and Hsp70 mRNA, suggesting the same regulatory mechanisms for these genes. The amount of Hsp90 protein declines in pupal heads from the diapausing cotton bollworm, H. armigera [123], and Hsp90 mRNA is down-regulated in H. zea [122]. In contrast M. rotundataHsp90 mRNA is constant [120], and D. antiquaHsp90 mRNA accumulates gradually during summer diapause, fluctuates throughout winter diapause after an initial pre-diapause boost and regresses at termination [124]. The quantity of D. antiqua mRNA encoding t-complex polypeptide-1 (TCP-1), a subunit of the chaperonin CCT or Hsp60, grows during summer diapause and declines as development resumes, whereas in winter diapause the quantity of mRNA for this protein expands [125]. It was proposed, based on work with other organisms, that Hsp60 contributes to the robust cold hardiness of D. antiqua by interaction with the cytoskeletal elements actin and tubulin [124]. Perhaps Hsp60 has a similar function in S.crassipalpis [60], as well as in A. franciscana as a component of the microtubule proteome [126], and in Daphnia magna where Hsp60 is greater, relative to total protein, in diapause versus subitaneous eggs [127].
Hsp70 mRNA is not augmented in diapausing adults of the mosquito C. pipiens, even though they are more cold and desiccation tolerant than fully active adults [128], and the amount of Hsc70 protein declines in the heads of adult C. pipiens females in early diapause [129]. Nor does Hsp70 mRNA increase in Drosophila triauraria, even though the organism acquires enhanced stress tolerance upon entering reproductive or adult diapause [130]. Moreover, as revealed by RT-PCR, mRNA encoded by D. triaurariaHsp23, Hsp26 and Hsp83, the latter equivalent to Hsp90, do not experience diapause-associated transcriptional regulation [131]. The Colorado potato beetle, Leptinotarsa decemlineata, exhibits differential expression of inducible Hsp70 transcripts, with LdHSP70A expression low during adult diapause and LdHSP70B mRNA not detectable [132].
To summarize, not all classes of molecular chaperones contribute to diapause in each insect species, nor does every type of chaperone function at each diapause stage. Universal participation by chaperones is not required, however, to preserve the notion of their importance during diapause, even in insects which remain relatively active in this phase of their life cycle and have reduced need for protein protection. There are, of course, caveats to this proposal. It is difficult to comment definitively upon the relevance of a particular group of molecular chaperones during diapause until all members of the family are examined, especially if only RNA is analyzed. In spite of this, analysis of mRNA accumulation indicates that diapause induction, maintenance and termination may differently affect the expression of molecular chaperone gene family members. Additionally, the expression of different genes evokes functional heterogeneity among individual stress proteins within and between families, although there is sharing of activities as well. For example, in a general sense, many molecular chaperones protect diapausing insects against environmental and physiological stressors, as shown for flesh fly Hsp70 and Hsp23, but their mechanisms of action and the proteins/processes affected may vary. The sHSPs prevent irreversible aggregation of partially denatured proteins, holding them for renaturation or destruction by other chaperones [78, 88, 89]. Members of the Hsp70/Hsc70 family assist compromised or nascent proteins to attain their native three-dimensional conformation and may work with the sHSPs to this end [85, 89], whereas Hsp60, operating in concert with other chaperones, is likely to mediate the folding of specific proteins such as actin and tubulin [85, 86]. Hsp90, on the other hand, an ATP-requiring dimeric chaperone seldom observed to increase in diapause insects, contributes to cell signaling and gene expression rather than exhibiting a blatant protective role through interaction with denaturing proteins [80, 81]. Decreasing Hsp90 mRNA during diapause reflects reduced need for a chaperone that stabilizes proteins involved in cellular signaling because metabolism is decreasing and dormancy ensues. The molecular chaperones appearing during insect diapause therefore tend to reflect organismal needs, this being a manifestation of changing physiological needs as diapause progresses.
Artemin, a diapause-specific ferritin homologue with chaperone activity
In addition to the more readily recognized HSPs, Artemia cysts contain artemin (Table 2), an abundant protein first shown to resemble ferritin upon sequencing by Edman degradation, but with a high content of cysteine and histidine [133]. cDNA cloning and sequencing confirmed the similarity between artemin and ferritin [134]. Artemin, a heat-tolerant protein, has a monomeric molecular mass of approximately 26 kDa, and it forms oligomers of approximately 600 kDa or 24 subunits [133, 135, 136]. The synthesis of artemin is developmentally regulated, appearing by day 4 post-fertilization in the cytoplasm of diapause-destined Artemia embryos. Artemin disappears during post-diapause growth and is not found in embryos developing directly into nauplii, signifying a role in cyst development and diapause [107, 108].
Unlike ferritin, artemin fails to sequester iron even though it fashions oligomers of equivalent molecular mass [134, 136]. Artemin has a carboxy-terminal amino acid extension not found in ferritin, which is thought to fold inward, filling the space within oligomers that would normally be available to house iron. Five of the six di-iron ferroxidase center residues required for iron storage are also missing from artemin. If artemin does not bind iron, what is its function? Upon heating artemin interacts with RNA, suggesting the protein is an RNA chaperone, a potentially important function during diapause [135]. Artemin purified from transformed bacteria protects citrate synthase from heat-induced denaturation in a concentration-dependent manner, a characteristic surprisingly shared by ferritin and apoferritin, while transfected mammalian cells expressing artemin exhibit enhanced tolerance to heating and oxidative stress [136]. These results, in concert with those from in vitro turbidimetric assays, demonstrate that artemin and ferritin, like p26 and other Artemia sHSPs, are molecular chaperones. As a working hypothesis, artemin arose from ferritin by gene duplication, gradually losing the ability to store iron, but retaining chaperone activity. Accumulation to high levels implies artemin has an important role in diapause-destined Artemia embryos as a molecular chaperone, a sustainable activity because artemin no longer binds metals and would not sequester intracellular iron required by active post-diapause embryos.
Artemin may be unique to Artemia and its close relatives, but ferritin up-regulates in other diapause-destined organisms such as the copepod, Calanus finmarchicus [38], the wasp, N. vitripennis [12] and the rotifer, B. plicatilis [14]. There are many interesting questions regarding diapause-related ferritin functions. Does ferritin possess molecular chaperone activity in vivo, and if so, does it interact with a restricted number of molecular clients because it is present in small amounts even after up-regulation? On another tack, by increasing ferritin the amount of free iron is reduced leading to suppression of cell growth and division, important diapause characteristics. Ferritin scavenges oxygen radicals and by sequestering iron may curb the Fenton reaction, preventing ROS build-up. As a consequence, cellular macromolecules are protected from oxidative damage and intracellular signaling is influenced [12, 14, 34], as considered in the next section of the review.
Reactive oxygen and nitrogen species: from macromolecule perturbation to cell regulation
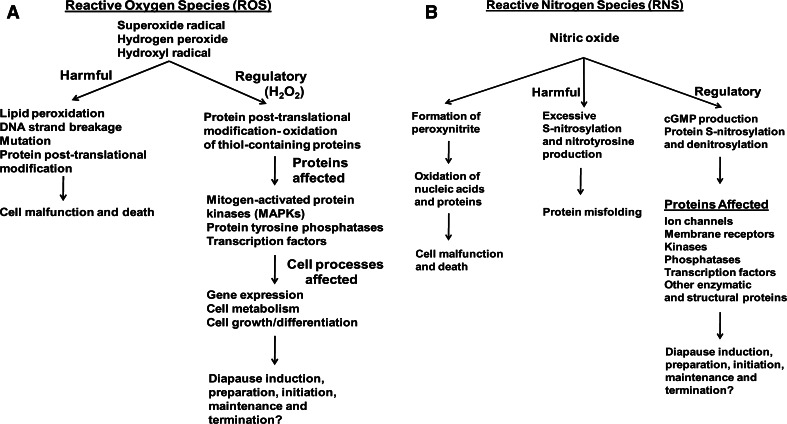
Brachionus plicatilis undergoes profound dormancy, and analysis of EST libraries prepared from this organism indicates an abundance of mRNAs encoding anti-oxidant proteins such as glutathione S transferases, dismutases, peroxiredoxins and thioredoxin peroxidase [14]. Such observations suggest the importance of controlling intracellular redox potential during diapause, but work in this area is limited. Free radicals, highly reactive chemical species possessing an unpaired electron(s), are generated by the loss or gain of free electrons by oxygen, resulting in production of ROS (Fig. 2a) [34] and RNS (Fig. 2b) [137]. Within the family of common biologically significant ROS the superoxide radical is relatively unstable, and it may form H2O2, a non-radical ROS, which if not reduced to H2O by catalase gives rise to the hydroxyl radical via the Fenton reaction [34]. ROSs have long been recognized as purveyors of oxidative damage, with the hydroxyl radical the most destructive. ROS-induced insults include lipid peroxidation, mutations, DNA strand breakage and post-translational modification/inactivation of proteins, changes with the potential to cause cell perturbation and death [138]. The major enzymatic mechanisms that protect cells against excessive ROS, some of which also have a role in signaling (see below), entail interrelated actions by glutathione S transferase, superoxide dismutase, catalase and peroxidases [14, 139, 140], among other effectors. Hsp70 and sHSPs are non-enzymatic modulators of ROS damage, and their synthesis is regulated transcriptionally by heat-induced H2O2 production, suggesting cross-talk between ROS and molecular chaperones [141, 142]. Genes encoding protective proteins may be differentially up-regulated during dormancy and growth in unfavorable conditions [14, 138, 143, 144]. Another major regulator of intracellular ROS accumulation is the abundant tripeptide glutathione (GSH) (l-γ-glutamyl-l-cysteinyl–glycine), a reducing agent with the ability to prevent apoptosis [145]. sHSPs increase reduced glutathione by up-regulating enzymes such as glucose-6-phosphate dehydrogenase, instrumental in the regeneration of NADPH from NADP+, the former conscripted by GSH reductase to reduce glutathione-disulfide (GSSG) to GSH [143–145]. Dangers attributed to ROS increase during seed dehydration and dormancy [22], suggesting protection against oxidative damage is valuable to organisms that experience profound dormancy and dehydration/desiccation as part of diapause, a proposal awaiting further investigation.
Fig. 2.
Regulation of diapause by ROS and RNS. When produced in excess, ROS and RNS damage macromolecules leading to cell malfunction and death. In contrast, both ROS (H2O2) and RNS (NO) act as, or modulate, second messengers resulting in posttranslational modifications which affect protein activity and cell behavior, changes with the potential to influence diapause, although this has yet to be thoroughly investigated at the molecular level
In addition to destructive potential, ROSs function as signaling molecules required for cell growth, division and development. The NADPH oxidases, a family of stress-activated integral membrane proteins, generate H2O2 thought to act as a second messenger [19, 139, 146]. Regulatory levels of H2O2 and superoxide anion are manufactured during respiratory activity, photosynthesis and lipid peroxidation in dry tissues such as seeds [22]. To effect signaling H2O2 oxidizes thiol-containing protein residues, perhaps via protein oxidation relays where peroxidase as primary oxidant receptor transfers oxidation to target proteins [147], ultimately promoting tyrosine phosphorylation and dephosphorylation. Protein tyrosine phosphatases, active in cell metabolism, growth and differentiation are targeted by H2O2, as are mitogen-activated protein kinases (MAPKs) and transcription factors, thereby controlling molecular processes ranging from gene expression to protein posttranslational modification [19, 20, 146, 148]. The consequences of possessing, such a wide range of influences at the molecular level, are many, and as one example H2O2 alleviates seed dormancy and promotes germination by transmitting environmental information within cells [22, 139]. Exposure to H2O2 terminates Artemia cyst diapause [149], but molecular investigations of this process are lacking. In the same context, B. plicatilis requires light for post-diapause development and hatching [150]. Light may promote development by producing ROS in sea water an idea corroborated by H2O2-induced development of B. plicatilis in darkness, perhaps as the result of prostaglandin synthesis due to lipid oxidation [150]. On a different but still beneficial note as far as diapause is concerned, ROS, including H2O2, inhibit caspases and other apoptosis effectors [146]. This is a decided advantage because slowing of cell death, especially upon stress exposure, prolongs the well-being of dormant organisms.
The RNS nitric oxide (NO) (Fig. 2b) is produced in the mitochondrial respiratory chain [137], through the action of NO synthase, which converts l-arginine to l-citrulline [151], via nitrate reductase [152] and as a result of enzymatically mediated nitrite reduction [153, 154]. NO reacts with superoxide to generate peroxynitrite, a potent oxidizer of nucleic acids and proteins [137, 155, 156]. RNS are responsible for S-nitrosylation (S-nitrosation), a reversible protein modification, as well as nitrotyrosine production, a potentially non-reversible change subject to H2O2 modulation [19, 151, 157, 158]. The post-translational S-nitrosylation of proteins entails the covalent interaction of NO with a cysteine thiol group [156, 157, 159, 160], and if excessive due to NO over-production S-nitrosylation causes protein mis-folding. The S-nitrosoglutathione reductase and thioredoxin systems are denitrosylases that protect against too much protein S-nitrosylation, a situation known as nitrosative stress [160].
In a regulatory capacity, NO promotes cyclic 3′,5′-guanosine monophosphate (cGMP) production, a second messenger that impacts ion channel activity, membrane receptors and signaling cascades through the actions of kinases and phosphatases [151, 157]. Posttranslational S-nitrosylation and its reversal or denitrosylation driven by the S-nitrosoglutathione reductase and thioredoxin systems, also affect membrane transporters and receptors, transcription factors, multiple enzymes and structural proteins. As a consequence, physiological processes are regulated in a redox-dependent manner, but with protein specificity as opposed to the global influences exerted by the cell redox state [156, 157, 159, 160]. Protein modification by NO modulates several cellular and organismal processes including the obstruction of apoptosis by caspase S-nitrosylation at the active site Cys [155, 157], this of value to animals undergoing diapause. Moreover, in plant seeds, NO enhances release from dormancy through transcriptional regulation of the gene CYP707A2, which encodes (+)-abscisic acid 8′-hydrolase, an enzyme that degrades abscisic acid (ABA) [161]. Although this is very unlikely to be the mechanism responsible for breakage of diapause-associated dormancy in animals, potential clearly exists for NO and other RNS to control diapause and development through second messenger production, gene regulation and protein posttranslational modification.
Apoptosis during diapause
Apoptosis is a genetically regulated process of programmed cell death induced by energy limitation among other stressors, and characterized by activation of cysteine proteases, or caspases. If organisms are to live through energy deprivation upon exposure to environmental hardship or entry into diapause, apoptosis must be curtailed. In this way cell integrity is maintained, permitting development when diapause terminates and embryos return to favorable growth conditions. In A. franciscana embryos, for example, diapause gives rise to profound reduction in metabolism, arguably to a reversible standstill [47, 162]. During this time, and upon exposure of post-diapause encysted embryos to prolonged anoxia, internal ultrastructure and macromolecules are preserved, indicating inhibition of proteolytic activity and apoptosis [90, 107, 162–164]. These findings are remarkable because energy limitation can stimulate liberation of pro-apoptotic factor(s) via opening of mitochondrial permeability transition pores, activating caspases and causing cell death [165]. However, the Ca++-induced opening of mitochondrial pores commonly observed during apoptosis is lacking in Artemia although their mitochondria contain the main pore structural proteins, and cytochrome c is not released during diapause and anoxia [31, 165–167]. In addition, neither Artemia caspase-9 nor caspase-3 is regulated by cytochrome c, as occurs in mammalian cells, but caspase-9 activity is increased by exogenous Ca++ and decreased by the nucleotides ATP, GTP and ADP, with the latter providing the greatest inhibition [31, 167]. ADP appears not to decline in diapausing Artemia embryos, and this nucleotide may come into play when other apoptosis inhibitory nucleotides are reduced [31].
Apoptosis is controlled by sHSPs the ubiquitously distributed molecular chaperones described earlier. sHSPs inhibit programmed cell death by interacting with β-arrestin [168], p53 [169], pro-apoptotic members of the Bcl-2 family such as Bax and Bcl-Xs [170], cytochrome c [143] and caspases [171, 172]. Notably, the diapause-specific sHSP p26 synthesized by Artemia embryos curtails apoptosis in stably transfected mammalian cells upon exposure to heat, staurosporine and dehydration/rehydration [30]. Inhibition may occur via p26 interaction with pro-apoptotic proteins and/or caspases that have yet to be characterized in Artemia, although binding with cytochrome c appears not to be involved [167]. Apoptosis management potentially benefits cells in energy limited states and represents one way in which Artemia, and perhaps other organisms, survive the physiological stressors, anoxia and diapause.
Water stress tolerance during diapause
Although not an overly common characteristic, some bacteria, plants and animals successfully endure drying or desiccation as part of their normal life cycle, a property often dependent on small molecules such as trehalose and sucrose. These disaccharides are thought to replace water that is hydrogen-bonded to polar residues, enabling proteins and membranes to retain physical properties similar to those of the fully hydrated state and promoting survival during water stress [173–175]. For example, trehalose hydroxyl groups hydrogen bond with membrane phospholipid head groups inhibiting rehydration-induced lipid phase transitions [36, 176]. Sugars also produce a high viscosity cytoplasmic glassy matrix, reducing molecular mobility and protecting embedded organelles [174, 177, 178]. Diapause-destined embryos are the only Artemia life stage to accumulate trehalose, this commencing 2 days post-fertilization and ending with metabolic cessation after cyst release from females [179, 180]. Trehalose may protect Artemia embryos against water stress, serve as an energy reserve for post-diapause development, and as a substrate to generate glycogen and glycerol [46, 177]. The nematode, Aphelenchus avenae, abundantly accumulates trehalose, contributing to its endurance during desiccation-induced dormancy or anhydrobiosis [178]. Larvae of the Antarctic midge, Belgica antarctica, possess several adaptations facilitating survival upon frequent exposure to conditions that cause water loss and one of these is trehalose generation [181]. Trehalose build up is thought to be essential for continued existence of B. antarctica, but this mechanism is not employed by all organisms that tolerate dehydration or cold. For example, resting eggs of the rotifer B. plicatilis are not enriched in mRNA encoding trehalose-6-phosphate, a key enzyme in trehalose synthesis, leaving the role of this disaccharide uncertain even though the organism lives through desiccation [14], nor does trehalose increase in overwintering diapause larvae of the rice stem borer C. suppressalis [182].
Glycerol, possibly arising from glucose and glycogen, functions as a cryoprotectant by reducing freezing temperature, curbing ice crystal growth and shielding against cold-induced macromolecule disruption [3, 127, 181, 182]. Glycerol potentially has a protective role in diapausing organisms. As a case in point, glycerol is more plentiful in dormant as opposed to subitaneous eggs of D. magna where it either protects against stress or represents a substrate suitable for metabolism when growth resumes [127]. Glycerol quantities expand in diapausing larvae and pupae, respectively, of C. suppressalis [115], and S. crassipalpis, reaching a concentration of 110 mM. This level is maintained in diapause pupae although it declines upon diapause termination and the initiation of quiescence [118]. Glycerol also peaks in diapause larvae of C. suppressalis, with maximal accumulation coinciding with the most intense diapause and greatest cold hardiness [182].
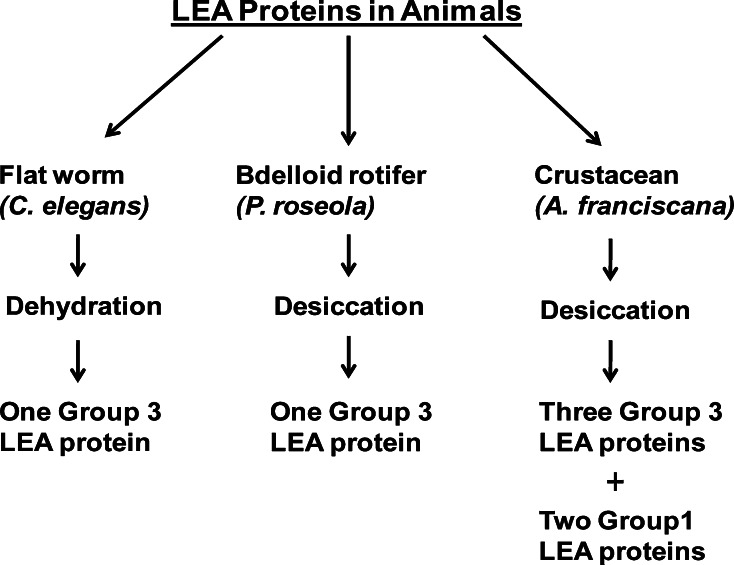
In addition to small molecules, the LEA proteins, first observed in developing cotton seeds [183], augment protection against desiccation and cold [34, 35]. The LEA proteins occur in organisms other than plants including the bacterium, Bacillus subtilis [184], bdelloid rotifers capable of anhydrobiosis [185], and at least two other invertebrates which experience very different diapauses, namely C. elegans [186] and A. franciscana (Fig. 3) [36, 37, 187]. Nematodes successfully withstand desiccation, and exposure of C. elegans dauer larvae to dehydration amplifies accumulation of the LEA protein group 3 mRNA encoded by the gene Ce-lea-1. Partial silencing of Ce-lea-1 diminishes survival during desiccation, showing LEA proteins contribute to drying tolerance [186]. Protection by LEA proteins during water stress is additionally suggested by the amplification of RNAs encoding two highly hydrophilic group 3 LEA proteins in diapause-destined Artemia embryos [188, 189]. The mRNA for a third group 3 LEA protein termed AfrLEA3 is enriched in desiccation-tolerant diapause Artemia embryos. AfrLEA3 is thought to protect mitochondria against water loss, with naturally occurring intra-mitochondrial trehalose having a supplementary role [36, 187]. One 21 kDa, heat stable, group 1 LEA proteins reside in encysted Artemia embryos but not in larvae and adults, the first time these proteins have been observed in animals [37]. The Artemia group 1 LEA proteins protect citrate synthase against drying in vitro, an action boosted significantly by trehalose, but not against heat. Enhancement of LEA protein effects by trehalose was reported previously and may involve stabilization of the sugar-based glassy matrix characteristic of desiccation-tolerant organisms [33–35, 174, 178].
Fig. 3.
LEA proteins in animals. LEA proteins, first observed in plants, have been reported in three animals where they may provide tolerance against water stress. Group 3 LEA proteins are found in all three animals, whereas only A. franciscana exhibits group 1 LEA proteins
Late embryogenesis abundant proteins guard against salt and cold stress, chilling and freezing, and water stress, with activities differing from organism to organism and for one LEA protein versus another [35]. How LEA proteins fulfill diverse roles is uncertain, but functional promiscuity undoubtedly derives in part from their hydrophilicity and limited secondary structure [35, 37, 190]. Stress tolerance is thought to arise in different ways, not all shared by each LEA protein, and various possibilities are summarized in two excellent reviews [34, 35]. LEA proteins could act as “molecular shields,” which decrease interaction between and aggregation of denaturing proteins, and they may generate space filling filaments thereby enabling cells to avert collapse as water content decreases. The hydrophilic character of LEA proteins allows them to sequester ions, preventing oxidative stress when ROS and the metals that influence their production are scavenged. Lastly, LEA proteins have the potential to interact with and fortify membranes, including those of mitochondria, where they reduce oxidative phosphorylation.
Molecular chaperones such as the Artemia sHSP p26 [23] and artemin [136] have the potential to protect against protein damage due to water loss from cells, but this has yet to be tested in embryos. In support of this proposal, however, p26, acting synergistically with trehalose, defends against drying-induced damage in transfected mammalian cells [191], suggesting a similar role for sHSPs in Artemia and other animals where these chaperones accumulate.
Protective coverings—morphological adaptations associated with diapause
Protective coverings, synthesized by organisms undergoing various types of dormancies [9, 28, 40, 41, 150], represent major diapause-related morphological adaptations, but their study is mostly descriptive. A. franciscana cysts are surrounded by a thick, non-cellular, chitinous shell that confers tolerance to ultraviolet radiation, a stress experienced when cysts float on the water or are blown on shore and exposed to sun light [108, 161]. Artemia cyst shell ultrastructure is well described, and this structure has similarities to insect egg shells [189, 192–194]. The multi-layered cyst shell shows species-dependent structural variability, but generally there is an external, darkly pigmented chorion layer consisting of maternally derived cortical and alveolar layers susceptible to hypochlorite degradation. The chorion is separated from the hypochlorite-resistant embryonic cuticle, composed of a fibrous layer containing protein and chitin, and the inner cuticular membrane of embryonic origin by a thin membrane termed the outer cuticular membrane. The intact cyst shell is impermeable to non-volatile substances, a property conferred in part by the outer cuticular membrane, a structure essential to embryo development [192, 195]. Removal of the outermost chorion, a process called dechorionation, opens the way for passage of lipid-soluble molecules into embryos. This observation demonstrates the effect of the chorion on cyst permeability and, importantly, that limited pharmacological approaches can be used to analyze embryo activities [196, 197].
Up-regulated mRNAs in ovisacs of A. parthenogenetica containing embryos destined for diapause have been selected by suppressive subtractive hybridization as a way to examine cyst shell synthesis and function. One of the cDNAs obtained represents a gene (SGEG) expressed specifically in the shell gland and it encodes a pro-protein with a signal sequence of 33 amino acids and a 14 kDa mature protein of 81 residues. Knockdown of the SGEG product by RNAi yields transparent cysts with soft yellow shells containing mal-formed cortical and alveolar layers [29]. Approximately 20% of the abnormal cysts hatched without termination of diapause by drying or cold. Moreover, Artemia cysts with reduced SGEG product are less resistant to drying, osmotic pressure, UV radiation, heat and organic solvents, and hatching success upon diapause termination suffers. The SGRG product is obviously required for cyst integrity because shell structure is compromised and protective properties decrease when the protein dwindles, providing the first molecular description of cyst shell protein function. Lack of the SGEG protein may also cause p26 and artemin decline, which compromises cyst stress tolerance [29]. These results potentially represent an example of coordinated gene regulation where proteins required for different aspects of diapause maintenance are synthesized in concert, maybe in response to a common transcriptional regulator.
Diapause II embryos of A. limnaeus, the most stress-tolerant of three killifish developmental stages to enter diapause, possess the highest known resistance to drying among aquatic vertebrates [28]. Characteristically, water exit from diapause II embryos is high at first and decreases to very low values; the initial water loss, mainly from the perivitelline fluid, almost completely eliminates this space. The enveloping embryo cell layer or chorion has low permeability which assists in limiting water and ion exodus [198]. The biochemical basis of reduced chorion permeability has yet to be determined, but the egg envelope of all A. limnaeus diapause life history stages contains long un-branched protein fibrils similar to amyloid fibers associated with human neurological diseases, including a high content of intermolecular β-sheet. Even though an intact chorion is required it appears this layer is not responsible for the enhanced survival of diapause II embryos. Rather, the perivitelline space is thought to form a hydrophobic barrier or to vitrify, reducing water loss [28] and posing an unusual protective structure very different from the one surrounding encysted Artemia embryos. In another example, proteomic analysis of heads from adult females of C. pipiens revealed that the pupal cuticle protein increased during early diapause and then decreased [129]. The enhanced amount of pupal cuticle protein may provide a harder cuticle, adding to the stress tolerance of overwintering animals.
Conclusions and perspectives
Diapause is an intriguing developmental process occurring in a myriad of species and characterized by properties so diverse that defining the crucial phenotypic attributes of the process is difficult. Even well-acknowledged diapause properties such as dormancy, including cessation of development and metabolic down turn, enhanced stress tolerance and the need for a termination signal before development resumes vary from extreme to non-existent. Diapause therefore represents a continuum of physiological states potentially generated by the differential application of similar molecular mechanisms in one organism versus another, and with this in mind selected regulatory and physiological parameters with the potential to modulate diapause were considered. The intention of the review was to describe unifying cell/molecular properties of diapause, to the extent they exist, with a good example being the role of molecular chaperones in stress resistance. Organisms exhibiting extreme dormancy and stress tolerance during diapause tend to accumulate large amounts of molecular chaperones, while organisms that change little tend to exhibit much smaller quantities of these proteins, if any at all. Other processes with less established roles in diapause such as AMPK-dependent regulation of metabolism and ROS/RNS signaling were also considered, revealing a need for their in depth examination. Such studies, and especially those driven by the application of sophisticated cell/molecular technologies, will bring to light new properties of diapause. Not only is this important from fundamental perspectives related to cell growth, signaling and stress tolerance, but implications exist for agriculture, forestry, aquaculture and medicine. Clearly, there is much to anticipate as the study of diapause expands and the secrets of this physiological process are uncovered.
Acknowledgments
T.H.M. is supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada.
References
- 1.Cohen PA, Knoll AH, Kodner RB. Large spinose microfossils in Ediacaran rocks as resting stages of early animals. Proc Natl Acad Sci USA. 2009;106:6519–6524. doi: 10.1073/pnas.0902322106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hairston NG., Jr Time travelers: what’s timely in diapause research? Arch Hydrobiol Spec Issues Adv Limnol. 1998;52:1–15. [Google Scholar]
- 3.Denlinger DL. Regulation of diapause. Annu Rev Entomol. 2002;47:93–122. doi: 10.1146/annurev.ento.47.091201.145137. [DOI] [PubMed] [Google Scholar]
- 4.Lopes FL, Desmarais JA, Murphy BD. Embryonic diapause and its regulation. Reproduction. 2004;128:669–678. doi: 10.1530/rep.1.00444. [DOI] [PubMed] [Google Scholar]
- 5.MacRae TH. Diapause: diverse states of developmental and metabolic arrest. J Biol Res. 2005;3:3–14. [Google Scholar]
- 6.Koštál V. Eco-physiological phases of insect diapause. J Insect Physiol. 2006;52:113–127. doi: 10.1016/j.jinsphys.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Hahn DA, Denlinger DL. Meeting the energetic demands of insect diapause: nutrient storage and utilization. J Insect Physiol. 2007;53:760–773. doi: 10.1016/j.jinsphys.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Fielenbach N, Antebi A. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 2008;22:2149–2165. doi: 10.1101/gad.1701508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brendonck L, De Meester L. Egg banks in freshwater zooplankton: evolutionary and ecological archives in the sediment. Hydrobiologia. 2003;491:65–84. [Google Scholar]
- 10.Burnell AM, Houthoofd K, O’Hanlon K, Vanfleteren JR. Alternate metabolism during the dauer stage of the nematode Caenorhabditis elegans . Exp Gerentol. 2005;40:850–856. doi: 10.1016/j.exger.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Qiu Z, Tsoi SCM, MacRae TH. Gene expression in diapause-destined embryos of the crustacean, Artemia franciscana . Mech Dev. 2007;124:856–867. doi: 10.1016/j.mod.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Wolschin F, Gadau J. Deciphering proteomic signatures of early diapause in Nasonia . PLoS ONE. 2009;4(7):e6394. doi: 10.1371/journal.pone.0006394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker DA, Russell S (2009) Gene expression during Drosophila melanogaster egg development before and after reproductive diapause. BMC Genomics 10:242. doi:10.1186/1471-2164-10-242 [DOI] [PMC free article] [PubMed]
- 14.Denekamp NY, Thorne MAS, Clark MS, Kube M, Reinhardt R, Lubzens E. Discovering genes associated with dormancy in the monogonont rotifer Brachionus plicatilis . BMC Genomics. 2009;10:108. doi: 10.1186/1471-2164-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumeister R, Schaffitzel E, Hertweck M. Endocrine signaling in Caenorhabditis elegans controls stress response and longevity. J Endocrinol. 2006;190:191–202. doi: 10.1677/joe.1.06856. [DOI] [PubMed] [Google Scholar]
- 16.Allen MJ. What makes a fly enter diapause? Fly. 2007;1:307–310. doi: 10.4161/fly.5532. [DOI] [PubMed] [Google Scholar]
- 17.Emerson KJ, Bradshaw WE, Holzapfel CM. Complications of complexity: integrating environmental, genetic and hormonal control of insect diapause. Trends Genet. 2009;25:217–225. doi: 10.1016/j.tig.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Zhang A, Jiang M, Zhang J, Ding H, Xu S, Hu X, Tan M. Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytol. 2007;175:36–50. doi: 10.1111/j.1469-8137.2007.02071.x. [DOI] [PubMed] [Google Scholar]
- 19.Forman HJ, Fukuto JM, Miller T, Zhang H, Rinna A, Levy S. The chemistry of cell signaling by reactive oxygen and nitrogen species and 4-hydroxynonenal. Arch Biochem Biophys. 2008;477:183–195. doi: 10.1016/j.abb.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Covarrubias L, Hernández-García D, Schnabel D, Salas-Vidal E, Castro-Obregón S. Function of reactive oxygen species during animal development: passive or active? Dev Biol. 2008;320:1–11. doi: 10.1016/j.ydbio.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 21.Oracz K, Bouteau HE-M, Farrant JM, Cooper K, Belghazi M, Job C, Job D, Corbineau F, Bailly C. ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. Plant J. 2007;50:452–465. doi: 10.1111/j.1365-313X.2007.03063.x. [DOI] [PubMed] [Google Scholar]
- 22.Bailly C, El-Maarouf-Bouteau H, Corbineau F. From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. C R Biol. 2008;331:806–814. doi: 10.1016/j.crvi.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 23.MacRae TH. Molecular chaperones, stress resistance and development in Artemia franciscana . Semin Cell Dev Biol. 2003;14:251–258. doi: 10.1016/j.semcdb.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 24.Storey KB, Storey JM. Metabolic rate depression in animals: transcriptional and translational controls. Biol Rev. 2004;79:207–233. doi: 10.1017/s1464793103006195. [DOI] [PubMed] [Google Scholar]
- 25.Storey KB, Storey JM. Tribute to P.L. Lutz: putting life on “pause”—molecular regulation of hypometabolism. J Exp Biol. 2007;210:1700–1714. doi: 10.1242/jeb.02716. [DOI] [PubMed] [Google Scholar]
- 26.Podrabsky JE, Hand SC. Depression of protein synthesis during diapause in embryos of the annual killifish Austrofundulus limnaeus . Physiol Biochem Zool. 2000;73:799–808. doi: 10.1086/318106. [DOI] [PubMed] [Google Scholar]
- 27.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 28.Podrabsky JE, Carpenter JF, Hand SC. Survival of water stress in annual fish embryos: dehydration avoidance and egg envelope amyloid fibers. Am J Physiol Regul Integr Comp Physiol. 2001;280:R123–R131. doi: 10.1152/ajpregu.2001.280.1.R123. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y-L, Zhao Y, Dai Z-M, Chen H-M, Yang W-J. Formation of diapause cyst shell in brine shrimp, Artemia parthenogenetica, and its resistance role in environmental stresses. J Biol Chem. 2009;284:16931–16938. doi: 10.1074/jbc.M109.004051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villeneuve TS, Ma X, Sun Y, Oulton MM, Oliver AE, MacRae TH. Inhibition of apoptosis by p26: implications for small heat shock protein function during Artemia development. Cell Stress Chaperones. 2006;11:71–80. doi: 10.1379/CSC-154R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menze MA, Hand SC. Caspase activity during cell stasis: avoidance of apoptosis in an invertebrate extremophile. Artemia franciscana . Am J Physiol Regul Integr Comp Physiol. 2007;292:R2039–R2047. doi: 10.1152/ajpregu.00659.2006. [DOI] [PubMed] [Google Scholar]
- 32.Bartels D. Molecular mechanisms of desiccation tolerance in plants. In: Storey KB, editor. Molecular mechanisms of metabolic arrest: life in limbo. Oxford: BIOS Scientific Publishers Ltd.; 2001. pp. 187–196. [Google Scholar]
- 33.Goyal K, Walton LJ, Tunnacliffe A. LEA proteins prevent protein aggregation due to water loss. Biochem J. 2005;388:151–157. doi: 10.1042/BJ20041931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berjak P. Unifying perspectives of some mechanisms basic to desiccation tolerance across life forms. Seed Sci Res. 2006;16:1–15. [Google Scholar]
- 35.Tunnacliffe A, Wise MJ. The continuing conundrum of the LEA proteins. Naturwissenschaften. 2007;94:791–812. doi: 10.1007/s00114-007-0254-y. [DOI] [PubMed] [Google Scholar]
- 36.Menze MA, Boswell L, Toner M, Hand SC. Occurrence of mitochondria-targeted late embryogenesis abundant (LEA) gene in animals increases organelle resistance to water stress. J Biol Chem. 2009;284:10714–10719. doi: 10.1074/jbc.C900001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharon MA, Kozarova A, Clegg JS, Vacratsis PO, Warner AH. Characterization of a group 1 late embryogenesis abundant protein in encysted embryos of the brine shrimp Artemia franciscana . Biochem Cell Biol. 2009;87:415–430. doi: 10.1139/o09-001. [DOI] [PubMed] [Google Scholar]
- 38.Tarrant AM, Baumgartner MF, Verslycke T, Johnson CL. Differential gene expression in diapausing and active Calanus finmarchicus (Copepoda) Mar Ecol Prog Ser. 2008;355:193–207. [Google Scholar]
- 39.Zhou Q, Wu C, Dong B, Liu F, Xiang J. The encysted dormant embryo proteome of Artemia sinica . Mar Biotechnol. 2008;10:438–446. doi: 10.1007/s10126-007-9079-0. [DOI] [PubMed] [Google Scholar]
- 40.Guidetti R, Boschini D, Rebecchi L, Bertolani R. Encystment processes and the “Matrioshka-like stage” in a moss-dwelling and in a limnic species of eutardigrades (Tardigrada) Hydrobiologia. 2006;558:9–21. [Google Scholar]
- 41.Guidetti R, Boschini D, Altiero T, Bertolani R, Rebecchi L. Diapause in tardigrades: a study of factors involved in encystment. J Exp Biol. 2008;211:2296–2302. doi: 10.1242/jeb.015131. [DOI] [PubMed] [Google Scholar]
- 42.Förster F, Liang C, Shkumatov A, Beisser D, Engelmann JC, Schnölzer M, Frohme M, Müller T, Schill RO, Dandekar T. Tardigrade workbench: comparing stress-related proteins, sequence-similar and functional protein clusters as well as RNA elements in tardigrades. BMC Genomics. 2009;10:469. doi: 10.1186/1471-2164-10-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Podrabsky JE, Lopez JP, Fan TWM, Higashi R, Somero GN. Extreme anoxia tolerance in embryos of the annual killifish Austrofundulus limnaeus: insights from a metabolomics analysis. J Exp Biol. 2007;210:2253–2266. doi: 10.1242/jeb.005116. [DOI] [PubMed] [Google Scholar]
- 44.Podrabsky JE, Somero GN. An inducible 70 kDa-class heat shock protein is constitutively expressed during early development and diapause in the annual killifish Austrofundulus limnaeus . Cell Stress Chaperones. 2007;12:199–204. doi: 10.1379/CSC-280.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller DL, Roth MB. C. elegans are protected from lethal hypoxia by an embryonic diapause. Curr Biol. 2009;19:1233–1237. doi: 10.1016/j.cub.2009.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drinkwater LE, Clegg JS. Experimental biology of cyst diapause. In: Browne RA, Sorgeloos P, Trotman CAN, editors. Artemia biology. Boca Raton: CRC Press, Inc.; 1991. pp. 93–117. [Google Scholar]
- 47.Clegg JS, Drinkwater LE, Sorgeloos P. The metabolic status of diapause embryos of Artemia franciscana (SFB) Physiol Zool. 1996;69:49–66. [Google Scholar]
- 48.Podrabsky JE, Hand SC. The bioenergetics of embryonic diapause in an annual killifish, Austrofundulus limnaeus . J Exp Biol. 1999;202:2567–2580. doi: 10.1242/jeb.202.19.2567. [DOI] [PubMed] [Google Scholar]
- 49.Chen T, Reith ME, Ross NW, MacRae TH. Expressed sequence tag (EST)-based characterization of gene regulation in Artemia larvae. Invert Reprod Dev. 2003;44:33–44. [Google Scholar]
- 50.Li Y-P, Xia R-X, Wang H, Li X-S, Liu Y-Q, Wei Z-J, Lu C, Xiang Z-H. Construction of a full-length cDNA library from Chinese oak silkworm pupa and identification of a KK-42-binding protein gene in relation to pupa-diapause termination. Int J Biol Sci. 2009;5:451–457. doi: 10.7150/ijbs.5.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hahn DA, Ragland GJ, Shoemaker DW, Denlinger DL. Gene discovery using massively parallel pyrosequencing to develop ESTs for the flesh fly Sarcophaga crassipalpis . BMC Genomics. 2009;10:234. doi: 10.1186/1471-2164-10-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu X, Song S, Wang Q, Qin F, Liu K, Zhang X, Hu S, Zhao Y. Analysis and comparison of a set of expressed sequence tags of the parthenogenetic water flea Daphnia carinata . Mol Genet Genomics. 2009;282:197–203. doi: 10.1007/s00438-009-0459-1. [DOI] [PubMed] [Google Scholar]
- 53.Li AQ, Popova-Butler A, Dean DH, Denlinger DL. Proteomics of the flesh fly brain reveals an abundance of upregulated heat shock proteins during pupal diapause. J Insect Physiol. 2007;53:385–391. doi: 10.1016/j.jinsphys.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 54.Robich RM, Rinehart JP, Kitchen LJ, Denlinger DL. Diapause-specific gene expression in the northern house mosquito, Culex pipiens L., identified by suppressive subtractive hybridization. J Insect Physiol. 2007;53:235–2435. doi: 10.1016/j.jinsphys.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamatani T, Daikoku T, Wang H, Matsumoto H, Carter MG, Ko MSH, Dey SK. Global gene expression analysis identifies molecular pathways distinguishing blastocyst dormancy and activation. Proc Natl Acad Sci USA. 2004;101:10326–10331. doi: 10.1073/pnas.0402597101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hondo E, Stewart CL. Profiling gene expression in growth-arrested mouse embryos in diapause. Genome Biol. 2004;6:202. doi: 10.1186/gb-2004-6-1-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McElwee JJ, Schuster E, Blanc E, Thomas JH, Gems D. Shared transcriptional signature in Caenorhabditis elegans dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J Biol Chem. 2004;279:44533–44543. doi: 10.1074/jbc.M406207200. [DOI] [PubMed] [Google Scholar]
- 58.Flannagan RD, Tammariello SP, Joplin KH, Cikra-Ireland RA, Yocum GD, Denlinger DL. Diapause-specific gene expression in pupae of the flesh fly Sarcophaga crassipalpis . Proc Natl Acad Sci USA. 1998;95:5616–5620. doi: 10.1073/pnas.95.10.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rinehart JP, Yocum GD, Denlinger DL. Developmental upregulation of inducible hsp70 transcripts, but not the cognate form, during pupal diapause in the flesh fly, Sarcophaga crassipalpis . Insect Biochem Mol Biol. 2000;30:515–521. doi: 10.1016/s0965-1748(00)00021-7. [DOI] [PubMed] [Google Scholar]
- 60.Rinehart JP, Li A, Yocum GD, Robich RM, Hayward SAL, Denlinger DL. Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proc Natl Acad Sci USA. 2007;104:11130–11137. doi: 10.1073/pnas.0703538104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim M, Robich RM, Rinehart JP, Denlinger DL. Upregulation of two actin genes and redistribution of actin during diapause and cold stress in the northern house mosquito, Culex pipiens . J Insect Physiol. 2006;52:1226–1233. doi: 10.1016/j.jinsphys.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hand SC, Hardewig I. Downregulation of cellular metabolism during environmental stress: mechanisms and implications. Annu Rev Physiol. 1996;58:539–563. doi: 10.1146/annurev.ph.58.030196.002543. [DOI] [PubMed] [Google Scholar]
- 63.Hand SC. Quiescence in Artemia franciscana embryos: reversible arrest of metabolism and gene expression at low oxygen levels. J Exp Biol. 1998;201:1233–1242. doi: 10.1242/jeb.201.8.1233. [DOI] [PubMed] [Google Scholar]
- 64.Menze MA, Clavenna MJ, Hand SC. Depression of cell metabolism and proliferation by membrane-permeable and-impermeable modulators: role for AMP-to-ATP ratio. Am J Physiol Regul Integr Comp Physiol. 2005;288:R501–R510. doi: 10.1152/ajpregu.00490.2004. [DOI] [PubMed] [Google Scholar]
- 65.Shackelford DB, Shaw RJ. The LBK1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hardie DG. New roles for the LKB1 → AMPK pathway. Curr Opin Cell Biol. 2005;17:167–173. doi: 10.1016/j.ceb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 67.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 68.Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes. 2008;32:S7–S12. doi: 10.1038/ijo.2008.116. [DOI] [PubMed] [Google Scholar]
- 69.McBride A, Hardie DG. AMP-activated protein kinase–a sensor of glycogen as well as AMP and ADP? Acta Physiol. 2009;196:99–113. doi: 10.1111/j.1748-1716.2009.01975.x. [DOI] [PubMed] [Google Scholar]
- 70.Inoki K, Zhu T, Guan K-L. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 71.Horman S, Browne GJ, Krause U, Patel JV, Vertommen D, Bertrand L, Lavoinne A, Hue L, Proud CG, Rider MH. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- 72.Yang W, Hong YH, Shen X-Q, Frankowski C, Camp HS, Leff T. Regulation of transcription by AMP-activated protein kinase. Phosphorylation of p300 blocks its interaction with nuclear receptors. J Biol Chem. 2001;276:38341–38344. doi: 10.1074/jbc.C100316200. [DOI] [PubMed] [Google Scholar]
- 73.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reynolds JA, Hand SC. Differences in isolated mitochondria are insufficient to account for respiratory depression during diapause in Artemia franciscana embryos. Physiol Biochem Zool. 2004;77:366–377. doi: 10.1086/420950. [DOI] [PubMed] [Google Scholar]
- 75.Zhu X-J, Feng C-Z, Dai Z-M, Zhang R-C, Yang W-J. AMPK alpha subunit gene characterization in Artemia and expression during development and in response to stress. Stress. 2007;10:53–63. doi: 10.1080/10253890601130773. [DOI] [PubMed] [Google Scholar]
- 76.Zhu X-J, Dai J-Q, Tan X, Zhao Y, Yang W-J (2009) Activation of an AMP-activated protein kinase is involved in post-diapause development of Artemia franciscana encysted embryos. BMC Develop Biol 9:21. http://www.biomedcentral.com/1471-213X/9/21 [DOI] [PMC free article] [PubMed]
- 77.Dai J-Q, Zhu X-J, Liu F-Q, Xiang J-H, Nagasawa H, Yang W-J. Involvement of p90 ribosomal S6 kinase in termination of cell cycle arrest during development of Artemia-encysted embryos. J Biol Chem. 2008;283:1705–1712. doi: 10.1074/jbc.M707853200. [DOI] [PubMed] [Google Scholar]
- 78.Vos MJ, Hageman J, Carra S, Kampinga HH. Structural and functional diversities between members of the human HSPB, HSPH, HSPA, and DNAJ chaperone families. Biochemistry. 2008;47:7001–7011. doi: 10.1021/bi800639z. [DOI] [PubMed] [Google Scholar]
- 79.Bösl B, Grimminger V, Walter S. The molecular chaperone Hsp104—a molecular machine for protein disaggregation. J Struct Biol. 2006;156:139–148. doi: 10.1016/j.jsb.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 80.Wandinger SK, Richter K, Buchner J. The Hsp90 chaperone machinery. J Biol Chem. 2008;283:18473–18477. doi: 10.1074/jbc.R800007200. [DOI] [PubMed] [Google Scholar]
- 81.Hahn J-S. The Hsp90 chaperone machinery: from structure to drug development. BMB Rep. 2009;42:623–630. doi: 10.5483/bmbrep.2009.42.10.623. [DOI] [PubMed] [Google Scholar]
- 82.Retzlaff M, Stahl M, Eberl HC, Lagleder S, Beck J, Kessler H, Buchner J. Hsp90 is regulated by a switch point in the C-terminal domain. EMBO Rep. 2009;10:1147–1153. doi: 10.1038/embor.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Daugaard M, Rohde M, Jäättelä M. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 84.Meimaridou E, Gooljar SB, Chapple JP. From hatching to dispatching: the multiple cellular roles of the Hsp70 molecular chaperone machinery. J Mol Endocrinol. 2009;42:1–9. doi: 10.1677/JME-08-0116. [DOI] [PubMed] [Google Scholar]
- 85.Hartl FU, Hayer-Hartl M. Converging concepts of protein folding in vitro and in vivo. Nat Struct Mol Biol. 2009;16:574–581. doi: 10.1038/nsmb.1591. [DOI] [PubMed] [Google Scholar]
- 86.Yam AY, Xia Y, Lin H-TJ, Burlingame A, Gerstein M, Frydman J. Defining the TRiC/CCT interactome links chaperonin function to stabilization of newly made proteins with complex topologies. Nat Struct Mol Biol. 2008;15:1255–1262. doi: 10.1038/nsmb.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Laksanalamai P, Robb FT. Small heat shock proteins from extremophiles: a review. Extremophiles. 2004;8:1–11. doi: 10.1007/s00792-003-0362-3. [DOI] [PubMed] [Google Scholar]
- 88.Sun Y, MacRae TH. Small heat shock proteins: molecular structure and chaperone function. Cell Mol Life Sci. 2005;62:2460–2476. doi: 10.1007/s00018-005-5190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mchaourab HS, Godar JA, Stewart PL. Structure and mechanism of protein stability sensors: chaperone activity of small heat shock proteins. Biochemistry. 2009;48:3828–3837. doi: 10.1021/bi900212j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clegg JS, Jackson SA, Warner AH. Extensive intracellular translocations of a major protein accompany anoxia in embryos of Artemia franciscana . Exp Cell Res. 1994;212:77–83. doi: 10.1006/excr.1994.1120. [DOI] [PubMed] [Google Scholar]
- 91.Clegg JS, Jackson SA, Liang P, MacRae TH. Nuclear-cytoplasmic translocations of protein p26 during aerobic-anoxic transitions in embryos of Artemia franciscana . Exp Cell Res. 1995;219:1–7. doi: 10.1006/excr.1995.1197. [DOI] [PubMed] [Google Scholar]
- 92.Liang P, Amons R, MacRae TH, Clegg JS. Purification, structure and in vitro molecular-chaperone activity of Artemia p26, a small heat-shock/α-crystallin protein. Eur J Biochem. 1997;243:225–232. doi: 10.1111/j.1432-1033.1997.0225a.x. [DOI] [PubMed] [Google Scholar]
- 93.Liang P, Amons R, Clegg JS, MacRae TH. Molecular characterization of a small heat shock/α-crystallin protein in encysted Artemia embryos. J Biol Chem. 1997;272:19051–19058. doi: 10.1074/jbc.272.30.19051. [DOI] [PubMed] [Google Scholar]
- 94.Sun Y, MacRae TH. Characterization of novel sequence motifs within N- and C-terminal extensions of p26, a small heat shock protein from Artemia franciscana . FEBS J. 2005;272:5230–5243. doi: 10.1111/j.1742-4658.2005.04920.x. [DOI] [PubMed] [Google Scholar]
- 95.Sun Y, Bojikova-Fournier S, MacRae TH. Structural and functional roles for β-strand 7 in the α-crystallin domain of p26, a polydisperse small heat shock protein from Artemia franciscana . FEBS J. 2006;273:1020–1034. doi: 10.1111/j.1742-4658.2006.05129.x. [DOI] [PubMed] [Google Scholar]
- 96.Qiu Z, Bossier P, Wang X, Bojikova-Fournier S, MacRae TH. Diversity, structure, and expression of the gene for p26, a small heat shock protein from Artemia . Genomics. 2006;88:230–240. doi: 10.1016/j.ygeno.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 97.Crack JA, Mansour M, Sun Y, MacRae TH. Functional analysis of a small heat shock/α-crystallin protein from Artemia franciscana . Eur J Biochem. 2002;269:933–942. doi: 10.1046/j.0014-2956.2001.02726.x. [DOI] [PubMed] [Google Scholar]
- 98.Sun Y, Mansour M, Crack JA, Gass GL, MacRae TH. Oligomerization, chaperone activity, and nuclear localization of p26, a small heat shock protein from Artemia franciscana . J Biol Chem. 2004;279:39999–40006. doi: 10.1074/jbc.M406999200. [DOI] [PubMed] [Google Scholar]
- 99.Day RM, Gupta JS, MacRae TH. A small heat shock/α-crystallin protein from encysted Artemia embryos suppresses tubulin denaturation. Cell Stress Chaperones. 2003;8:183–193. doi: 10.1379/1466-1268(2003)008<0183:ashcpf>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liang P, MacRae TH. The synthesis of a small heat shock/α-crystallin protein in Artemia and its relationship to stress tolerance during development. Dev Biol. 1999;207:445–456. doi: 10.1006/dbio.1998.9138. [DOI] [PubMed] [Google Scholar]
- 101.Jackson SA, Clegg JS. Ontogeny of low molecular weight stress protein p26 during early development of the brine shrimp, Artemia franciscana . Dev Growth Differ. 1996;38:153–160. doi: 10.1046/j.1440-169X.1996.t01-1-00004.x. [DOI] [PubMed] [Google Scholar]
- 102.Willsie JK, Clegg JS. Nuclear p26, a small heat shock/α-crystallin protein, and its relationship to stress resistance in Artemia franciscana embryos. J Exp Biol. 2001;204:2339–2350. doi: 10.1242/jeb.204.13.2339. [DOI] [PubMed] [Google Scholar]
- 103.Willsie JK, Clegg JS. Small heat shock protein p26 associates with nuclear lamins and HSP70 in nuclei and nuclear matrix fractions from stressed cells. J Cell Biochem. 2002;84:601–614. [PubMed] [Google Scholar]
- 104.Qiu Z, MacRae TH. ArHsp21, a developmentally regulated small heat-shock protein synthesized in diapausing embryos of Artemia franciscana . Biochem J. 2008;411:605–611. doi: 10.1042/BJ20071472. [DOI] [PubMed] [Google Scholar]
- 105.Qiu Z, MacRae TH. ArHsp22, a developmentally regulated small heat shock protein produced in diapause-destined Artemia embryos, is stress inducible in adults. FEBS J. 2008;275:3556–3566. doi: 10.1111/j.1742-4658.2008.06501.x. [DOI] [PubMed] [Google Scholar]
- 106.McLennan AG, Miller D. A biological role for the heat shock response in crustaceans. J Therm Biol. 1990;15:61–66. [Google Scholar]
- 107.Clegg JS, Jackson SA, Popov VI. Long-term anoxia in encysted embryos of the crustacean, Artemia franciscana: viability, ultrastructure, and stress proteins. Cell Tissue Res. 2000;301:433–446. doi: 10.1007/s004410000249. [DOI] [PubMed] [Google Scholar]
- 108.Tanguay JA, Reyes RC, Clegg JS. Habitat diversity and adaptation to environmental stress in encysted embryos of the crustacean Artemia . J Biosci. 2004;29:489–501. doi: 10.1007/BF02712121. [DOI] [PubMed] [Google Scholar]
- 109.Clegg JS, Campagna V. Comparisons of stress proteins and soluble carbohydrate in encysted embryos of Artemia franciscana and two species of Parartemia . Comp Biochem Physiol B. 2006;145:119–125. doi: 10.1016/j.cbpb.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 110.Wang W, Meng B, Chen W, Ge X, Liu S, Yu J. A proteomic study on postdiapaused embryonic development of brine shrimp (Artemia franciscana) Proteomics. 2007;7:3580–3591. doi: 10.1002/pmic.200700259. [DOI] [PubMed] [Google Scholar]
- 111.Gkouvitsas T, Kontogiannatos D, Kourti A. Differential expression of two small Hsps during diapause in the corn stalk borer Sesamia nonagrioides (Lef.) J Insect Physiol. 2008;54:1503–1510. doi: 10.1016/j.jinsphys.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 112.Gkouvitsas T, Kontogiannatos D, Kourti A. Cognate Hsp70 gene is induced during deep larval diapause in the moth Sesamia nonagrioides . Insect Mol Biol. 2009;18:253–264. doi: 10.1111/j.1365-2583.2009.00866.x. [DOI] [PubMed] [Google Scholar]
- 113.Gkouvitsas T, Kontogiannatos D, Kourti A. Expression of the Hsp83 gene in response to diapause and thermal stress in the moth Sesamia nonagrioides . Insect Mol Biol. 2009;18:759–768. doi: 10.1111/j.1365-2583.2009.00922.x. [DOI] [PubMed] [Google Scholar]
- 114.Tungjitwitayakul J, Tatun N, Singtripop T, Sakurai S. Characteristic expression of three heat shock-responsive genes during larval diapause in the bamboo borer Omphisa fuscidentalis . Zool Sci. 2008;25:321–333. doi: 10.2108/zsj.25.321. [DOI] [PubMed] [Google Scholar]
- 115.Sonoda S, Fukumoto K, Izumi Y, Yoshida H, Tsumuki H. Cloning of heat shock protein genes (hsp90 and hsc70) and their expression during larval diapause and cold tolerance acquisition in the rice stem borer, Chilo suppressalis Walker. Arch Insect Biochem Physiol. 2006;63:36–47. doi: 10.1002/arch.20138. [DOI] [PubMed] [Google Scholar]
- 116.Tachibana S-I, Numata H, Goto SG. Gene expression of heat-shock proteins (Hsp23, Hsp70 and Hsp90) during and after larval diapause in the blow fly Lucilia sericata . J Insect Physiol. 2005;51:641–647. doi: 10.1016/j.jinsphys.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 117.Yocum GD, Joplin KH, Denlinger DL. Upregulation of a 23 kDa small heat shock protein transcript during pupal diapause in the flesh fly, Sarcophaga crassipalpis . Insect Biochem Mol Biol. 1998;28:677–682. doi: 10.1016/s0965-1748(98)00046-0. [DOI] [PubMed] [Google Scholar]
- 118.Hayward SAL, Pavlides SC, Tammariello SP, Rinehart JP, Denlinger DL. Temporal expression patterns of diapause-associated genes in flesh fly pupae from the onset of diapause through post-diapause quiescence. J Insect Physiol. 2005;51:631–640. doi: 10.1016/j.jinsphys.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 119.Rinehart JP, Denlinger D. Heat-shock protein 90 is down-regulated during pupal diapause in the flesh fly, Sarcophaga crassipalpis, but remains responsive to thermal stress. Insect Mol Biol. 2000;9:641–645. doi: 10.1046/j.1365-2583.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- 120.Yocum GD, Kemp WP, Bosch J, Knoblett JN. Temporal variation in overwintering gene expression and respiration in the solitary bee Megachile rotundata . J Insect Physiol. 2005;51:621–629. doi: 10.1016/j.jinsphys.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 121.Chen B, Kayukawa T, Monteiro A, Ishikawa Y. Cloning and characterization of the HSP70 gene, and its expression in response to diapauses and thermal stress in the onion maggot, Delia antiqua . J Biochem Mol Biol. 2006;39:749–758. doi: 10.5483/bmbrep.2006.39.6.749. [DOI] [PubMed] [Google Scholar]
- 122.Zhang Q, Denlinger DL (2009) Molecular characterization of heat shock protein 90, 70 and 70 cognate cDNAs and their expression patterns during thermal stress and pupal diapause in the corn earworm. J Insect Physiol. doi:10.1016/j.jinsphys.2009.09.013 [DOI] [PubMed]
- 123.Chen Ma W, Wang X, Niu C, Lei C (2009) Analysis of pupal head proteome and its alteration in diapausing pupae of Helicoverpa armigera. J Insect Physiol. doi:10.1016/j.jinsphys.2009.10.008 [DOI] [PubMed]
- 124.Chen B, Kayukawa T, Monteiro A, Ishikawa Y. The expression of the Hsp90 gene in response to winter and summer diapauses and thermal-stress in the onion maggot, Delia antiqua . Insect Mol Biol. 2005;14:697–702. doi: 10.1111/j.1365-2583.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- 125.Kayukawa T, Chen B, Miyazaki S, Itoyama K, Shinoda T, Ishikawa Y. Expression of mRNA for the t-complex polypeptide-1, a subunit of chaperonin CCT, is upregulated in association with increased cold hardiness in Delia antiqua . Cell Stress Chaperones. 2005;10:204–210. doi: 10.1379/CSC-106R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.O’Connell PA, Pinto DM, Chisholm KA, MacRae TH. Characterization of the microtubule proteome during post-diapause development of Artemia franciscana . Biochim Biophys Acta. 2006;1764:920–928. doi: 10.1016/j.bbapap.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 127.Pauwels K, Stoks R, Verbiest A, De Meester L. Biochemical adaptation for dormancy in subitaneous and dormant eggs of Daphnia magna . Hydrobiologia. 2007;594:91–96. [Google Scholar]
- 128.Rinehart JP, Robich RM, Denlinger DL. Enhanced cold and desiccation tolerance in diapausing adults of Culex pipiens, and a role for Hsp70 in response to cold shock but not as a component of the diapause program. J Med Entomol. 2006;43:713–722. doi: 10.1603/0022-2585(2006)43[713:ECADTI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 129.Li A, Denlinger DL. Pupal cuticle protein is abundant during early adult diapause in the mosquito Culex pipiens . J Med Entomol. 2009;46:1382–1386. doi: 10.1603/033.046.0618. [DOI] [PubMed] [Google Scholar]
- 130.Goto SG, Yoshida KM, Kimura MT. Accumulation of Hsp70 mRNA under environmental stresses in diapausing and nondiapausing adults of Drosophila triauraria . J Insect Physiol. 1998;44:1009–1015. doi: 10.1016/s0022-1910(97)00143-1. [DOI] [PubMed] [Google Scholar]
- 131.Goto SG, Kimura MT. Heat-shock-responsive genes are not involved in the adult diapause of Drosophila triauraria . Gene. 2004;326:117–122. doi: 10.1016/j.gene.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 132.Yocum GD. Differential expression of two HSP70 transcripts in response to cold shock, thermoperiod, and adult diapause in the Colorado potato beetle. J Insect Physiol. 2001;47:1139–1145. doi: 10.1016/s0022-1910(01)00095-6. [DOI] [PubMed] [Google Scholar]
- 133.De Graaf J, Amons R, Möller W. The primary structure of artemin from Artemia cysts. Eur J Biochem. 1990;193:737–750. doi: 10.1111/j.1432-1033.1990.tb19394.x. [DOI] [PubMed] [Google Scholar]
- 134.Chen T, Amons R, Clegg JS, Warner AH, MacRae TH. Molecular characterization of artemin and ferritin from Artemia franciscana . Eur J Biochem. 2003;270:137–145. doi: 10.1046/j.1432-1033.2003.03373.x. [DOI] [PubMed] [Google Scholar]
- 135.Warner AH, Brunet RT, MacRae TH, Clegg JS. Artemin is an RNA-binding protein with high thermal stability and potential RNA chaperone activity. Arch Biochem Biophys. 2004;424:189–200. doi: 10.1016/j.abb.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 136.Chen T, Villeneuve TS, Garant K, Amons R, MacRae TH. Functional characterization of artemin, a ferritin homolog synthesized in Artemia embryos during encystment and diapause. FEBS J. 2007;274:1093–1101. doi: 10.1111/j.1742-4658.2007.05659.x. [DOI] [PubMed] [Google Scholar]
- 137.Poyton RO, Ball KA, Castello P. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol Metabol. 2009;20:332–340. doi: 10.1016/j.tem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 138.Lopez-Martinez G, Elnitsky MA, Benoit JB, Lee RE, Jr, Denlinger DL. High resistance to oxidative damage in the Antarctic midge Belgica antarctica, and developmentally linked expression of genes encoding superoxide dismutase, catalase and heat shock proteins. Insect Biochem Mol Biol. 2008;38:796–804. doi: 10.1016/j.ibmb.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 139.Oracz K, El-Maarouf-Bouteau H, Kranner I, Bogatek R, Corbineau F, Bailly C. The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signaling during germination. Plant Physiol. 2009;150:494–505. doi: 10.1104/pp.109.138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cox AG, Winterbourn CC, Hampton MB. Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem J. 2010;425:313–325. doi: 10.1042/BJ20091541. [DOI] [PubMed] [Google Scholar]
- 141.Scarpeci TE, Zanor MI, Valle EM. Investigating the role of plant heat shock proteins during oxidative stress. Plant Signal Behav. 2008;3:856–857. doi: 10.4161/psb.3.10.6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Scarpeci TE, Zanor MI, Carrillo N, Mueller-Roeber B, Valle EM. Generation of superoxide anion in chloroplasts of Arabidopsis thaliana during active photosynthesis: a focus on rapidly induced genes. Plant Mol Biol. 2008;66:361–378. doi: 10.1007/s11103-007-9274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Arrigo A-P (2007) The cellular “networking” of mammalian Hsp27 and its functions in the control of protein folding, redox state and apoptosis. In: Csermely P, Vígh L (eds) Molecular aspects of the stress response: chaperones, membranes and networks, Landes Bioscience and Springer Science + Business Media, pp 14–26 [DOI] [PubMed]
- 144.Hartwig K, Heidler T, Moch J, Daniel H, Wenzel U. Feeding a ROS-generator to Caenorhabditis elegans leads to increased expression of small heat shock protein HSP-16.2 and hormeis. Genes Nutr. 2009;4:59–67. doi: 10.1007/s12263-009-0113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Franco R, Cidlowski JA. Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ. 2009;16:1303–1314. doi: 10.1038/cdd.2009.107. [DOI] [PubMed] [Google Scholar]
- 146.Groeger G, Quiney C, Cotter TG. Hydrogen peroxide as a cell-survival signaling molecule. Antiox Redox Signal. 2009;11:2655–2671. doi: 10.1089/ars.2009.2728. [DOI] [PubMed] [Google Scholar]
- 147.Gutscher M, Sobotta MC, Wabnitz GH, Ballikaya S, Meyer AJ, Samstag Y, Dick TP. Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. J Biol Chem. 2009;284:31532–31540. doi: 10.1074/jbc.M109.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Rad Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 149.Van Stappen G, Lavens P, Sorgeloos P. Effects of hydrogen peroxide treatment in Artemia cysts of different geographical origin. Arch Hydrobiol Spec Issues Adv Limnol. 1998;52:281–296. [Google Scholar]
- 150.Hagiwara A, Hoshi N, Kawahara F, Tominaga K, Hirayama K. Resting eggs of the marine rotifer Brachionus plicatilis Müller: development, and effect of irradiation on hatching. Hydrobiologia. 1995;313(314):223–229. [Google Scholar]
- 151.Villalobo A. Nitric oxide and cell proliferation. FEBS J. 2006;273:2329–2344. doi: 10.1111/j.1742-4658.2006.05250.x. [DOI] [PubMed] [Google Scholar]
- 152.Kolbert Z, Ortega L, Erdei L. Involvement of nitrate reductase (NR) in osmotic stress-induced NO generation of Arabidopsis thaliana L. roots. J Plant Physiol. 2010;167:77–80. doi: 10.1016/j.jplph.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 153.Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006;45:113–122. doi: 10.1111/j.1365-313X.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- 154.Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I. Nitric oxide, stomatal closure, and abiotic stress. J Exp Bot. 2008;59:165–176. doi: 10.1093/jxb/erm293. [DOI] [PubMed] [Google Scholar]
- 155.Maejima Y, Adachi S, Morikawa K, Ito H, Isobe M. Nitric oxide inhibits myocardial apoptosis by preventing caspase-3 activity via S-nitrosylation. J Mol Cell Cardiol. 2005;38:163–174. doi: 10.1016/j.yjmcc.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 156.Nakamura T, Lipton SA. Emerging roles of S-nitrosylation in protein misfolding and neurodegenerative diseases. Antiox Redox Signal. 2008;10:87–101. doi: 10.1089/ars.2007.1858. [DOI] [PubMed] [Google Scholar]
- 157.Hess DT, Matsumoto A, Kim S-O, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 158.Hancock J, Desikan R, Harrison J, Bright J, Hooley R, Neill S. Doing the unexpected: proteins involved in hydrogen peroxide perception. J Exp Bot. 2006;57:1711–1718. doi: 10.1093/jxb/erj180. [DOI] [PubMed] [Google Scholar]
- 159.López-Sánchez LM, Muntané J, de la Mata M, Rodríguez-Ariza A. Unraveling the S-nitrosoproteome: tools and strategies. Proteomics. 2009;9:808–818. doi: 10.1002/pmic.200800546. [DOI] [PubMed] [Google Scholar]
- 160.Benhar M, Forrester MT, Stamler JS. Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat Rev Mol Cell Biol. 2009;10:721–732. doi: 10.1038/nrm2764. [DOI] [PubMed] [Google Scholar]
- 161.Liu Y, Shi L, Ye N, Liu R, Jia W, Zhang J. Nitric oxide-induced rapid decrease of abscisic acid concentration is required in breaking seed dormancy in Arabidopsis. New Phytol. 2009;183:1030–1042. doi: 10.1111/j.1469-8137.2009.02899.x. [DOI] [PubMed] [Google Scholar]
- 162.Clegg JS, Jackson SA. The metabolic status of quiescent and diapause embryos of Artemia franciscana (Kellogg) Arch Hydrobiol Spec Issues Adv Limnol. 1998;52:425–439. [Google Scholar]
- 163.Clegg JS. Embryos of Artemia franciscana survive four years of continuous anoxia: the case for complete metabolic rate depression. J Exp Biol. 1997;200:467–475. doi: 10.1242/jeb.200.3.467. [DOI] [PubMed] [Google Scholar]
- 164.Clegg JS. Protein stability in Artemia embryos during prolonged anoxia. Biol Bull. 2007;212:74–81. doi: 10.2307/25066582. [DOI] [PubMed] [Google Scholar]
- 165.Menze MA, Fortner G, Nag S, Hand SC (2010) Mechanisms of apoptosis in Crustacea: what conditions induce versus suppress cell death? Apoptosis. doi:10:1007/s10495-009-0443-6 [DOI] [PMC free article] [PubMed]
- 166.Menze MA, Hutchinson K, Laborde SM, Hand SC. Mitochondrial permeability transition in the crustacean Artemia franciscana: absence of a calcium-regulated pore in the face of profound calcium storage. Am J Physiol Regul Integr Comp Physiol. 2005;289:R68–R76. doi: 10.1152/ajpregu.00844.2004. [DOI] [PubMed] [Google Scholar]
- 167.Hand SC, Menze MA. Mitochondria in energy-limited states: mechanisms that blunt the signaling of cell death. J Exp Biol. 2008;211:1829–1840. doi: 10.1242/jeb.000299. [DOI] [PubMed] [Google Scholar]
- 168.Rojanathammanee L, Harmon EB, Grisanti LA, Govitrapong P, Ebadi M, Grove BD, Miyagi M, Porter JE. The 27-kDa heat shock protein confers cytoprotective effects through a β2-adrenergic receptor agonist-initiated complex with β-arrestin. Mol Pharmacol. 2009;75:855–865. doi: 10.1124/mol.108.053397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu S, Li J, Tao Y, Xiao X. Small heat shock protein αB-crystallin binds to p53 to sequester its translocation to mitochondria during hydrogen peroxide-induced apoptosis. Biochem Biophys Res Commun. 2007;354:109–114. doi: 10.1016/j.bbrc.2006.12.152. [DOI] [PubMed] [Google Scholar]
- 170.Mao Y-W, Liu J-P, Xiang H, Li DW-C. Human αA- and αB-crystallins bind to Bax and Bcl-Xs to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11:521–526. doi: 10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]
- 171.Voss OH, Batra S, Kolattukudy SJ, Gonzalez-Mejia ME, Smith JB, Doseff AI. Binding of caspase-3 prodomain to heat shock protein 27 regulates monocyte apoptosis by inhibiting caspase-3 proteolytic activity. J Biol Chem. 2007;282:25088–25099. doi: 10.1074/jbc.M701740200. [DOI] [PubMed] [Google Scholar]
- 172.Shin J-H, Kim S-W, Lim C-M, Jeong J-Y, Piao C-S, Lee J-K. αB-crystallin suppresses stress-induced astrocyte apoptosis by inhibiting caspase-3 activation. Neurosci Res. 2009;64:355–361. doi: 10.1016/j.neures.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 173.Clegg JS. Cryptobiosis—a peculiar state of biological organization. Comp Biochem Physiol B. 2001;128:613–624. doi: 10.1016/s1096-4959(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 174.Wolkers WF, Tablin F, Crowe JH. From anhydrobiosis to freeze-drying of eukaryotic cells. Comp Biochem Physiol A. 2002;131:535–543. doi: 10.1016/s1095-6433(01)00505-0. [DOI] [PubMed] [Google Scholar]
- 175.Crowe JH. Trehalose as a “chemical chaperone”: fact and fantasy. Adv Exp Med Biol. 2007;594:143–158. doi: 10.1007/978-0-387-39975-1_13. [DOI] [PubMed] [Google Scholar]
- 176.Crowe JH, Clegg JS, Crowe LM. Anhydrobiosis: the water replacement hypothesis. In: Reid DS, editor. The properties of water in foods ISOPOW 6. New York: Blackie Academic and Professional; 1998. pp. 440–455. [Google Scholar]
- 177.Clegg JS, Trotman CNA. Physiological and biochemical aspects of Artemia ecology. In: ThJ Abatzopoulos, Beardmore JA, Clegg JS, Sorgeloos P., editors. Artemia basic and applied biology. The Netherlands: Kluwer; 2002. pp. 129–170. [Google Scholar]
- 178.Browne J, Tunnacliffe A, Burnell A. Plant desiccation gene found in a nematode. Nature. 2002;416:38. doi: 10.1038/416038a. [DOI] [PubMed] [Google Scholar]
- 179.Clegg JS. The origin of trehalose and its significance during the formation of encysted dormant embryos of Artemia salina . Comp Biochem Physiol A. 1965;14:135–143. doi: 10.1016/0010-406x(65)90014-9. [DOI] [PubMed] [Google Scholar]
- 180.Clegg JS, Willsie JK, Jackson SA. Adaptive significance of a small heat shock/α-crystallin protein (p26) in encysted embryos of the brine shrimp, Artemia franciscana . Am Zool. 1999;39:836–847. [Google Scholar]
- 181.Benoit JB, Lopez-Martinez G, MR Michaud, Elnitsky MA, Lee RE, Jr, Denlinger DL. Mechanisms to reduce dehydration stress in larvae of the Antarctic midge, Belgica antarctica . J Insect Physiol. 2007;53:656–667. doi: 10.1016/j.jinsphys.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 182.Atapour M, Moharramipour S. Changes in cold hardiness, supercooling capacity, and major cryoprotectants in overwintering larvae of Chilo suppressalis (Lepidoptera: Pyralidae) Physiol Ecol. 2009;38:260–265. doi: 10.1603/022.038.0132. [DOI] [PubMed] [Google Scholar]
- 183.Galau GA, Hughes DW, Dure L., III Abscisic acid induction of cloned cotton late embryogenesis-abundant (Lea) mRNAs. Plant Mol Biol. 1986;7:155–170. doi: 10.1007/BF00021327. [DOI] [PubMed] [Google Scholar]
- 184.Stacy RAP, Aalen RB. Identification of sequence homology between the internal hydrophilic repeated motifs of Group 1 late-embryogenesis-abundant proteins in plants and hydrophilic repeats of the general stress protein GsiB of Bacillus subtilis . Planta. 1998;206:476–478. doi: 10.1007/s004250050424. [DOI] [PubMed] [Google Scholar]
- 185.Tunnacliffe A, Lapinski J, McGee B. A putative LEA protein, but no trehalose, is present in anhydrobiotic bdelloid rotifers. Hydrobiologia. 2005;546:315–321. [Google Scholar]
- 186.Gal TZ, Glazer I, Koltai H. An LEA group 3 family member is involved in survival of C. elegans during exposure to stress. FEBS Lett. 2004;577:21–26. doi: 10.1016/j.febslet.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 187.Menze MA, Hand SC. How do animal mitochondria tolerate water stress? Commun Integr Biol. 2009;2:428–430. doi: 10.4161/cib.2.5.9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hand SC, Jones D, Menze MA, Witt TL. Life without water: expression of plant LEA genes by an anhydrobiotic arthropod. J Exp Zool. 2007;307A:62–66. doi: 10.1002/jez.a.343. [DOI] [PubMed] [Google Scholar]
- 189.Wang S, Sun S. Comparative observations on the cyst shells of seven Artemia strains from China. Microsc Res Tech. 2007;70:663–670. doi: 10.1002/jemt.20451. [DOI] [PubMed] [Google Scholar]
- 190.Tompa P, Szász C, Buday L. Structural disorder throws new light on moonlighting. Trends Biochem Sci. 2005;30:484–489. doi: 10.1016/j.tibs.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 191.Ma X, Jamil K, MacRae TH, Clegg JS, Russell JM, Villeneuve TS, Euloth M, Sun Y, Crowe JH, Tablin F, Oliver AE. A small stress protein acts synergistically with trehalose to confer desiccation tolerance on mammalian cells. Cryobiology. 2005;51:15–28. doi: 10.1016/j.cryobiol.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 192.Morris JE, Afzelius BA. The structure of the shell and outer membranes in encysted Artemia salina embryos during cryptobiosis and development. J Ultrastruct Res. 1967;20:244–259. doi: 10.1016/s0022-5320(67)90285-7. [DOI] [PubMed] [Google Scholar]
- 193.Anderson E, Lochhead JH, Lochhead MS, Huebner E. The origin and structure of the tertiary envelope in thick-shelled eggs of the brine shrimp, Artemia . J Ultrastruct Res. 1970;32:497–525. doi: 10.1016/s0022-5320(70)80025-9. [DOI] [PubMed] [Google Scholar]
- 194.Rosowski J, Belk D, Gouthro MA, Lee KW. Ultrastructure of the cyst shell and underlying membranes of the brine shrimp Artemia franciscana Kellogg (Anostraca) during postencystic development, emergence, and hatching. J Shellfish Res. 1997;16:233–249. [Google Scholar]
- 195.De Chaffoy D, De Maeyer-Criel G, Kondo M. On the permeability and formation of the embryonic cuticle during development in vivo and in vitro of Artemia salina embryos. Differentiation. 1978;12:99–109. [Google Scholar]
- 196.Covi JA, Hand SC. V-ATPase expression during development of Artemia franciscana embryos: potential role for proton gradients in anoxia signaling. J Exp Biol. 2005;208:2783–2798. doi: 10.1242/jeb.01680. [DOI] [PubMed] [Google Scholar]
- 197.Covi JA, Hand SC. Energizing an invertebrate embryo: bafilomycin-dependent respiration and the metabolic cost of proton pumping by the V-ATPase. Physiol Biochem Zool. 2007;80:422–432. doi: 10.1086/518344. [DOI] [PubMed] [Google Scholar]
- 198.Machado BE, Podrabsky JE. Salinity tolerance in diapausing embryos of the annual killifish Austrofundulus limnaeus is supported by exceptionally low water and ion permeability. J Comp Physiol B. 2007;177:809–820. doi: 10.1007/s00360-007-0177-0. [DOI] [PubMed] [Google Scholar]