Abstract
Growth hormone-releasing hormone (GHRH) can act as a potent growth factor in various cancers. The mitogenic activity of this neuropeptide is exerted through binding to the pituitary type receptors (GHRH-R) or their splice variants (SV). In the present work, we studied whether this hormone can activate the JAK2/STAT3 pathway which plays a crucial role in cancer cell proliferation and is also linked to carcinogenesis. We transfected HeLa human cervical cancer cells, which are not sensitive to GHRH analogs with the pGHRH-R. Transfected cells responded to the GHRH or its antagonist with an increase or a decrease in proliferation, respectively. These results were confirmed by the expression of proliferating cell nuclear antigen. We then showed that these effects are linked to the activation of the JAK2/STAT3 pathway. Our work demonstrates the activation of JAK/STAT3 pathway by GHRH and sheds further light to the mechanisms of the antitumorogenic action of GHRH antagonists.
Keywords: GHRH, GHRH receptor, JAK2/STAT3
Introduction
Growth hormone-releasing hormone (GHRH) is a hypothalamic neuropeptide that stimulates the secretion of growth hormone (GH) from the anterior pituitary gland upon binding to its receptor (GHRH-R). Pituitary type GHRH receptor (pGHRH-R) is a class II G protein-coupled receptor with seven transmembrane domains and is homologous with the receptors for vasoactive intestinal peptide (VIP), pituitary adenylate cyclase-activating peptide (PACAP), and calcitonin [1]. GHRH-R is activated by GHRH and triggers the mitogen activated protein kinases pathway (MAPKs), which is linked to cell proliferation and differentiation [1, 2]. Both GHRH and GHRH-R are predominantly expressed in the hypothalamo-pituitary system, but they are also found in other normal cells, as well as on many various established cancer cell lines and tumors [3].
The presence of GHRH and its receptors in extra hypothalamic tissues suggests that GHRH also has a local regulatory role. In a recent study, the knocking down of GHRH gene expression in prostate, breast, and lung cancer cell lines resulted in major inhibition of cell growth, which was reversed after treatment of these cells with exogenous GHRH [4].
In order to create new anticancer therapies, one of us (A.V.S.) developed GHRH antagonists which have a strong antiproliferative activity in various experimental cancer models [3]. The inhibitory effect of these analogues is exerted in part by endocrine mechanisms through the suppression of GHRH-evoked release of GH from the pituitary, with a subsequent decrease in serum IGF-I levels [5]. However, direct mechanisms are involved in the main antitumor effects of GHRH antagonists. The likely principal inhibitory pathways of GHRH antagonists appear to be based on the blockade of action of autocrine GHRH in tumors [3]. Recent studies also indicate that GHRH antagonists suppress the proliferation of cancers through the reduction of the reactive oxygen species [6], which are essential for cancer growth and metastasis [7].
The Janus kinases (JAK) are non-receptor tyrosine kinases, which are activated upon cytokine and growth factor binding to their receptors [8]. This leads to the activation of signal transducer and activator of transcription (STAT) proteins such as STAT3, which is a latent nuclear transcription factor [9, 10]. The activation of STAT3 stimulates a wide range of prosurvival, proliferative, and proangiogenic genes [11, 12], increases the tumorogenicity and contributes to increased oxidative phosphorylation in mitochondria [13].
In this study, we investigated whether GHRH can activate the JAK2/STAT3 pathway upon binding to its receptor. In order to address this question we transfected HeLa human cervical carcinoma cells, which do not express GHRH and GHRH-R [14], with pGHRH-R. The transfected cell line responded to GHRH and GHRH antagonists, increasing or decreasing, respectively, their proliferation rate. The effects on proliferation were further supported by the expression of the major proliferative marker PCNA. The expression of PCNA in the transfected cells was enhanced by GHRH and decreased by GHRH antagonist JMR-132. We also showed for the first time that GHRH activates the JAK2/STAT3 pathway upon its binding on GHRH-R. In all these studies non-transfected HeLa cells were used as control. These cells were not influenced by GHRH or its antagonist. Our study supports the concept of a growth factor function of GHRH and sheds further light on the mechanisms of its action.
Materials and methods
Peptides and chemicals
GHRH(1–29)NH2 and GHRH antagonist JMR-132 [PhAc0, d-Arg2, Phe (4-C)6, Ala8, Har9, Tyr(Me)10, His11, Abu15, His20, Nle27, d-Arg28, Har29]hGHRH(1–29)NH2, where Abu is α-aminobutyric acid, Agm is agmatine, Har is homoarginine, Nle is norleucine, PhAc is phenylacetyl, and Tyr(Me) is O-methyltyrosine, were synthesized in our laboratory by solid phase methods as previously described [3]. GHRH(1–29)NH2 and GHRH antagonist JMR-132 were dissolved in DMSO and diluted with incubation media. The final concentration of DMSO in medium did not exceed 0.1%.
Cell culture and transfections
HeLa human cervical cancer cell line was obtained from American Type Culture Collection (Manassas, VA, USA) and cultured at 37°C in a humidified 95% air/5% CO2 atmosphere in DMEM supplemented with antibiotics/antimycotics and 10% FBS. The culture media as well as all the cell culture reagents were purchased from GIBCO (Carlsbad, CA). The transfection was performed as described previously [15].
Protein isolation, western Blot assay and quantitative analysis of the immunoblot assay
The protein isolation, the western blot assay and the quantitation of the blots were performed as extensively described [4]. The β-actin signal (1:1,000, sc-47778; Santa Cruz) was used as a loading control unless otherwise stated. The PCNA antibody was obtained from Cell Signaling (#2586), GHRH antibody from Santa Cruz (#10280), the GHRHR antibody from Abcam (ab 28692-100; Cambridge, MA).
Effect of GHRH(1–29)NH2 on the activation of JAK2/STAT3 Pathway
HeLa cells were grown in DMEM with 10% FBS. Before the assay, the media were replaced by the medium free of FBS and the cells were treated with 1 μM GHRH(1–29)NH2 for 5 and 10 min, and 24 h. The cells were then harvested in Cell Lysis Buffer (Sigma) containing protease inhibitor cocktail. The cell lysates (50 μl) were separated according their molecular weight by electrophoresis. The proteins were then transferred onto nitrocellulose membranes and probed for phospho-JAK2 (#3776; Cell Signaling) or phospho-STAT3 (sc-71792; Santa Cruz). The blots were stripped (Restore Plus Western Blot Stripping Buffer, Thermo Scientific, Waltham, MA, USA) and probed for JAK2 (#3230; Cell Signaling) or STAT3 (sc-8019; Santa Cruz), respectively, which were used as loading controls.
Cell proliferation rate assay
The rate of cell proliferation was calculated by seeding 5,000 cells in 24-well plates and, after an incubation for 72 h, counting them under light microscope using the Trypan blue assay. The assay was performed twice in triplicates.
Statistical analysis
The data are expressed as the mean ± SE. Statistical evaluation of the results was performed by the two-tailed Student’s t test. P values shown are expressed against the control group.
Results
HeLa cervical cancer cell line does not express GHRH or GHRH receptors
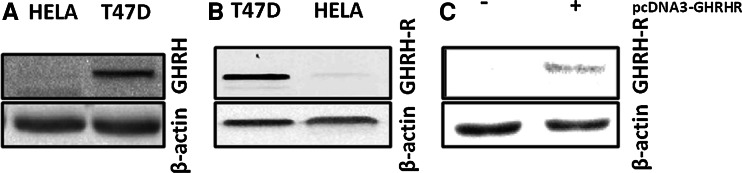
The expression of GHRH and GHRH-R in HeLa cervical cancer cells was examined by western blots. T47D breast cancer cell line which expresses GHRHR or GHRH was used as a positive control. Our results confirm that HeLa cells are negative for the expression of GHRH and GHRH-R (Fig. 1a, b, respectively).
Fig. 1.
Western blot analysis of the expression of a GHRH in T47D human breast and HeLa human cervical cancer cells, b GHRH-R in T47D human breast and HeLa human cervical cancer cells, and c GHRH-R in HeLa human cervical cancer before and after the transfection with pcDNA3-GHRHR vector. Protein levels were normalized to β-actin signal
Expression of GHRH receptor in HeLa pcDNA3-GHRHR cells
The expression of GHRH-R in the HeLa pcDNA3-GHRH-R transfected cells was evaluated by western blot. The results shown in Fig. 1c indicate that the transfected cells express significant amounts of GHRH receptor.
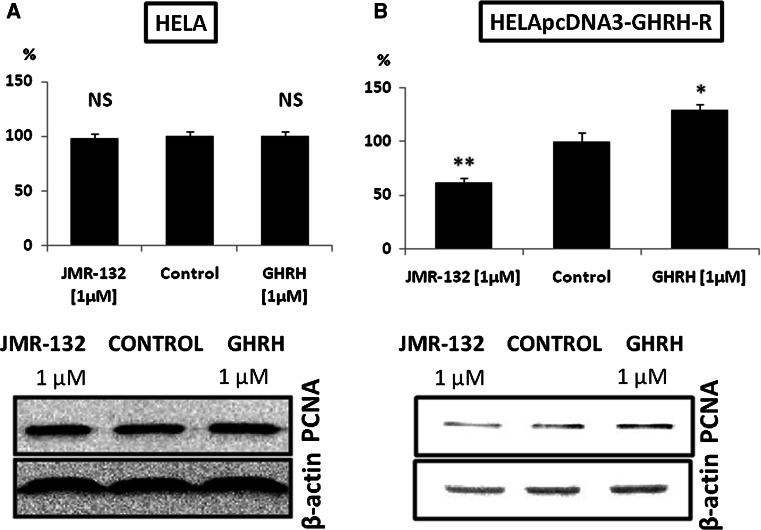
Effect of GHRH(1–29)NH2 and GHRH antagonist JMR-132 on the proliferation rate and the expression of PCNA in the HeLa and HELApcDNA-3-GHRHR cells
HeLa cells transfected with pcDNA3-GHRHR plasmid were treated with 1 μM GHRH (1–29)NH2 or 1 μM GHRH antagonist JMR-132 for 72 h. GHRH increased by 28.9%, while GHRH antagonist reduced the proliferation by 37.9% (Fig. 2b, upper panel). In addition, the expression of PCNA was augmented by 40.1% (RI: 0.999) after the treatment of the transfected cells with GHRH and downregulated by 51.6% (RI: 0.345) after exposure to JMR-132 as compared to the control (RI: 0.713) (Fig. 2b, lower panel). Treatment with GHRH and GHRH antagonist JMR-132 of HeLa cells changed neither the proliferation rate (Fig. 2a, upper panel), nor the expression of PCNA (Fig. 2a, lower panel).
Fig. 2.
a Effect of 1 μM GHRH (1–29)NH2 and 1 μM GHRH antagonist JMR-132 on the proliferation rate (upper panel) and the expression of PCNA (lower panel) in the HeLa cervical carcinoma cells. NS Not significant. PCNA protein levels were normalized to β-actin signal. The results are representative of two independent experiments. b Effect of 1 μM GHRH (1–29)NH2 and 1 μM GHRH antagonist JMR-132 on the proliferation rate (upper panel) and the expression of PCNA (lower panel) in the HeLa cells transfected with pcDNA3-GHRH-R vector. *P < 0.05 versus control, **P < 0.01 versus control. PCNA protein levels were normalized to β-actin signal. The results are representative of two independent experiments
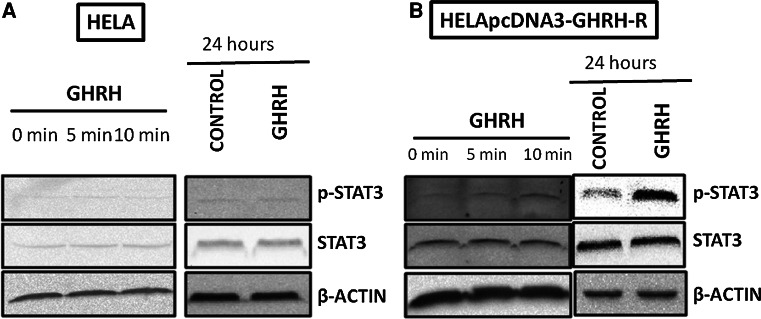
Effects of GHRH (1–29)NH2 on phosphorylation of STAT3 in HeLa and HeLa-pcDNA3-GHRHR cells
We examined the activation of STAT3 protein after incubating the transfected cells with GHRH for 5 and 10 min, and 24 h. The phosphorylation of STAT3 was increased in all these experiments with the relative intensity reaching 0.256, 0.376, and 1.269, respectively. The RIs of the controls were 0.189, 0.189, and 0.378, respectively. The results are shown in Fig. 3b. The non-transfected HeLa cells were not influenced by the treatment with GHRH(1–29)NH2 (Fig. 3a).
Fig. 3.
Activation of STAT3 in HeLa cells (a) and in HeLa-pcDNA3-GHRH-R cells (b) by 1 μΜ GHRH after 0, 5, or 10 min, and after 24 h. Protein levels were normalized to STAT3 signal (loading control). The figure is representative of two independent experiments
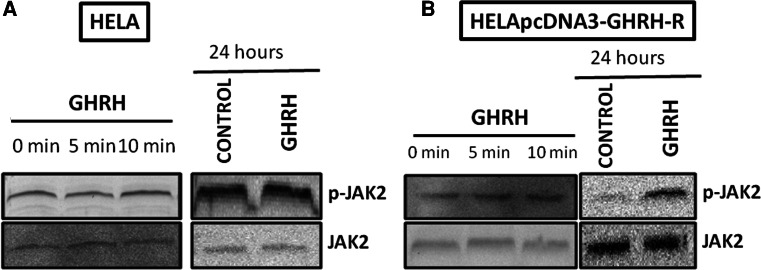
Effects of GHRH(1–29)NH2 on phosphorylation of JAK2 in HeLa and HeLa-pcDNA3-GHRHR cells
The effect of GHRH treatment on the phosphorylation of JAK2 was evaluated after 5 and 10 min, and 24 h. GHRH activated the JAK2 after 5 and 10 min, and 24 h with the relative intensity being 1.027, 1.147, and 0.549, respectively. The R.Is of the controls were 0.703, 0.703, and 0.197, respectively. The results are shown in Fig. 4b. No changes in the levels of phosphorylated JAK2 occurred after the treatment with GHRH(1–29)NH2 in HELA cells not expressing GHRH and GHRH-R (Fig. 4a).
Fig. 4.
Activation of JAK2 in HeLa cells (a) and in HeLa-pcDNA3-GHRH-R cells (b) by 1 μΜ GHRH after 0, 5, or 10 min, and after 24 h. Protein levels were normalized to JAK2 signal (loading control). The figure is representative of two independent experiments
Discussion
GHRH antagonists have been previously shown to exert strong antitumor effects in various xenografted human cancer lines in vivo. These peptides antagonize the mitogenic signals of autocrine and paracrine GHRH, which has been previously shown to be a crucial growth factor in cancer survival and progression [5]. Cancer cells in which the GHRH gene expression is knocked down show a suppression of proliferation rate [4].
In order to further investigate the intracellular pathways which are involved in the mediation of the mitogenic GHRH signals in cancers, we employed HeLa human cervical cancer cell line, which does not express GHRHR, and we transfected this receptor to these cells. After demonstrating that the transfected cells expressed significant amount of this receptor, we evaluated their proliferation after treatment with GHRH and its antagonist.
The transfected cells showed an increased proliferation rate and became sensitive to GHRH and GHRH antagonist JMR-132. These results were further confirmed with the expression of proliferating cell nuclear antigen. The expression of this marker was increased in the transfected cells treated with GHRH. In contrast, JMR-132 suppressed the expression of this marker.
Our results are in accord with previous findings in our and other laboratories, which show that the expression of this G-protein-coupled receptor in cancer cells stimulates their proliferation rate and increases the expression of major proliferative markers [15]. In addition, the binding of the GHRH to its receptor results to the activation of the MAPKs ERK1/2 [16, 17].
In this study, we also examined whether GHRH-R can activate the JAK2/STAT3 pathway. The importance of this pathway in cancer growth is underlined by the fact that the activated STAT3 can function as an oncogene [10, 18]. This transcription factor regulates cell proliferation and survival, induces cellular transformation, and enhances tumorigenicity [10]. In addition, elevated expression of phosphorylated STAT3 in HeLa cells is linked to increased expression of antiapoptotic genes [19]. Furthermore, JAK/STAT3 inhibition induces apoptosis in HeLa cells and decreases the proliferation rate [19].
Our results show for the first time that GHRH can activate the JAK2/STAT3 pathway, which is strongly involved in the progression of cancers and which is a target of novel anticancer therapy [11, 19–22].
Recent studies show that the activation of JAK2 and STAT3 transcription factors is also strongly linked with the regulation of the metabolism of the reactive oxygen and nitrogen species [21, 23, 24]. These species are involved in the generation of various diseases related to the deregulation of the redox status of the cells, such as diabetes and its complications as well as the neurodegenerative diseases [25–29].
In conclusion, we demonstrate for the first time that GHRH can stimulates the JAK2 and STAT3 proteins upon specific binding to GHRH-R. Our work strongly supports the role of GHRH as a growth factor and underlines the importance of GHRH antagonists as anticancer agents.
Acknowledgments
The work described in this paper was supported by the Medical Research Service of the Veterans Affairs Department, University of Miami Miller School of Medicine, Departments of Pathology and Medicine, Division of Hematology/Oncology, the South Florida Veterans Affairs Foundation for Research and Education (A.V.S.).
Contributor Information
Andrew V. Schally, Phone: +1-305-5753477, FAX: +1-305-5757279, Email: Andrew.Schally@va.gov
Nektarios Barabutis, Phone: +1-305-5753477, FAX: +1-305-5757279, Email: nbarabutis@gmail.com.
References
- 1.Mayo KE, Miller T, DeAlmeida V, Godfrey P, Zheng J, Cunha SR. Regulation of the pituitary somatotroph cell by GHRH and its receptor. Recent Prog Horm Res. 2000;55:237–266. [PubMed] [Google Scholar]
- 2.Barabutis N, Siejka A, Schally AV, Block NL, Cai R, Varga JL (2009) Activation of mitogen activated protein kinases by a splice variant of growth hormone releasing hormone receptor. J Mol Endocrinol (in press). doi:10.1677/JME-09-0121 [DOI] [PubMed]
- 3.Schally AV, Varga JL, Engel JB. Antagonists of growth-hormone-releasing hormone: an emerging new therapy for cancer. Nat Clin Pract Endocrinol Metab. 2008;4:33–43. doi: 10.1038/ncpendmet0677. [DOI] [PubMed] [Google Scholar]
- 4.Barabutis N, Schally AV. Knocking down gene expression for growth hormone-releasing hormone inhibits proliferation of human cancer cell lines. Br J Cancer. 2008;98:1790–1796. doi: 10.1038/sj.bjc.6604386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiaris H, Schally AV, Kalofoutis A. Extrapituitary effects of the growth hormone-releasing hormone. Vitam Horm. 2005;70:1–24. doi: 10.1016/S0083-6729(05)70001-7. [DOI] [PubMed] [Google Scholar]
- 6.Barabutis N, Schally AV. Antioxidant activity of growth hormone-releasing hormone antagonists in LNCaP human prostate cancer line. Proc Natl Acad Sci USA. 2008;105:20470–20475. doi: 10.1073/pnas.0811209106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 8.Yamaoka K, Saharinen P, Pesu M, Holt VE, 3rd, Silvennoinen O, O’Shea JJ. The Janus kinases (Jaks) Genome Biol. 2004;5:253. doi: 10.1186/gb-2004-5-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 10.Yu H, Jove R. The STATs of cancer—new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 11.Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reich, N.C. (2009). STAT3 revs up the powerhouse. Sci Signal 2, pe61 [DOI] [PubMed]
- 13.Gough DJ, Corlett A, Schlessinger K, Wegrzyn J, Larner AC, Levy DE. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science. 2009;324:1713–1716. doi: 10.1126/science.1171721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeitler P, Siriwardana G. Stimulation of mitogen-activated protein kinase pathway in rat somatotrophs by growth hormone-releasing hormone. Endocrine. 2000;12:257–264. doi: 10.1385/ENDO:12:3:257. [DOI] [PubMed] [Google Scholar]
- 15.Barabutis N, Tsellou E, Schally AV, Kouloheri S, Kalofoutis A, Kiaris H. Stimulation of proliferation of MCF-7 breast cancer cells by a transfected splice variant of growth hormone-releasing hormone receptor. Proc Natl Acad Sci USA. 2007;104:5575–5579. doi: 10.1073/pnas.0700407104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pombo CM, Zalvide J, Gaylinn BD, Dieguez C. Growth hormone-releasing hormone stimulates mitogen-activated protein kinase. Endocrinology. 2000;141:2113–2119. doi: 10.1210/en.141.6.2113. [DOI] [PubMed] [Google Scholar]
- 17.Siriwardana G, Bradford A, Coy D, Zeitler P. Autocrine/paracrine regulation of breast cancer cell proliferation by growth hormone releasing hormone via Ras, Raf, and mitogen-activated protein kinase. Mol Endocrinol. 2006;20:2010–2019. doi: 10.1210/me.2005-0001. [DOI] [PubMed] [Google Scholar]
- 18.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/S0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 19.Chen CL, Hsieh FC, Lieblein JC, Brown J, Chan C, Wallace JA, Cheng G, Hall BM, Lin J. Stat3 activation in human endometrial and cervical cancers. Br J Cancer. 2007;96:591–599. doi: 10.1038/sj.bjc.6603597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cross JV, Templeton DJ. Regulation of signal transduction through protein cysteine oxidation. Antioxid Redox Signal. 2006;8:1819–1827. doi: 10.1089/ars.2006.8.1819. [DOI] [PubMed] [Google Scholar]
- 22.Heimberger AB, Priebe W. Small molecular inhibitors of p-STAT3: novel agents for treatment of primary and metastatic CNS cancers. Recent Pat CNS Drug Discov. 2008;3:179–188. doi: 10.2174/157488908786242489. [DOI] [PubMed] [Google Scholar]
- 23.Maziere C, Maziere JC. Activation of transcription factors and gene expression by oxidized low-density lipoprotein. Free Radic Biol Med. 2009;46:127–137. doi: 10.1016/j.freeradbiomed.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 24.Zong CS, Zeng L, Jiang Y, Sadowski HB, Wang LH. Stat3 plays an important role in oncogenic Ros- and insulin-like growth factor I receptor-induced anchorage-independent growth. J Biol Chem. 1998;273:28065–28072. doi: 10.1074/jbc.273.43.28065. [DOI] [PubMed] [Google Scholar]
- 25.Finkel T. Cell biology: a clean energy programme. Nature. 2006;444:151–152. doi: 10.1038/444151a. [DOI] [PubMed] [Google Scholar]
- 26.Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448:767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- 27.Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10:175–176. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Magalhaes JP, Church GM. Cells discover fire: employing reactive oxygen species in development and consequences for aging. Exp Gerontol. 2006;41:1–10. doi: 10.1016/j.exger.2005.09.002. [DOI] [PubMed] [Google Scholar]