Abstract
The duodenal homeobox-1 protein Pdx-1 is one of the regulators for the transcription of the insulin gene. Pdx-1 is a phosphoprotein, and there is increasing evidence for the regulation of some of its functions by phosphorylation. Here, we asked whether protein kinase CK2 might phosphorylate Pdx-1 and how this phosphorylation could be implicated in the functional regulation of Pdx-1. We used fragments of Pdx-1 as well as phosphorylation mutants for experiments with protein kinase CK2. Transactivation was measured by reporter assays using the insulin promoter. Our data showed that Pdx-1 is phosphorylated by protein kinase CK2 at amino acids thr231 and ser232, and this phosphorylation was implicated in the regulation of the transcription factor activity of Pdx-1. Furthermore, inhibition of protein kinase CK2 by specific inhibitors led to an elevated release of insulin from pancreatic β-cells. Thus, these findings identify CK2 as a novel mediator of the insulin metabolism.
Keywords: Protein kinase, Phosphorylation, Transcription factor, Homeobox protein, Insulin production
Introduction
Glucose homeostasis in mammalian cells is maintained by the concerted action of two hormones, namely insulin and glucagon, which are secreted by the pancreatic β- and α-cells, respectively. On the other hand, an increase in the level of blood glucose stimulates insulin gene transcription and also insulin secretion [10]. Much of the glucose responsiveness is conferred by the transcription factor Pdx-1 (pancreatic and duodenal homeobox-1), also known as IPF-1, IDX-1, STF-1, and IUF-1, which binds to the insulin promoter [1]. Although Pdx-1 has been shown to be crucial for glucose regulation, the exact mechanisms by which glucose modulates the function of Pdx-1 remains to be elucidated.
Mouse Pdx-1 is a protein with 284 amino acids. The N-terminus harbors the transactivation domain. This domain also mediates protein-protein interactions with other transcription factors and transcriptional co-regulators [19, 21]. Pdx-1 also contains a central DNA binding homeodomain, which also harbors a nuclear localization signal [14, 19]. In the middle part of the protein is a proline-rich domain, which seems to be implicated in the heterodimerization with the DNA binding protein PBX-1 [12]. The role of the C-terminus is poorly understood. There are indications that the C-terminus has an inhibitory function, whereas other data show that the C-terminus is required for full transactivation [19]. There is increasing evidence that posttranslational modifications such as sumoylation [15], O-linked glycosylation [9], and phosphorylation [1] might be implicated in the regulation of Pdx-1. It has been shown that Pdx-1 is phosphorylated by PAS (Per-Arnt-Sim) kinase [3], GSK3 (glycogen synthase kinase 3) [5], and DNA-PK (DNA-dependent protein kinase) [16].
Protein kinase CK2 plays multiple roles in the regulation of gene expression and cell proliferation. Here, we analyze whether protein kinase CK2 might also phosphorylate the Pdx-1 protein and whether this phosphorylation could have functional implications for Pdx-1. We found that Pdx-1 was phosphorylated by protein kinase CK2 in the C-terminus at thr231 and ser232. The non-phosphorylated forms of Pdx-1 showed an elevated transcriptional activity indicating that the CK2 phosphorylation of Pdx-1 regulates its transcriptional activity. Inhibition of CK2 kinase activity led to an elevated production of insulin from β-cells of the pancreas.
Materials and methods
Plasmid constructions and site-directed mutagenesis
The full-length mouse Pdx-1 cDNA was subcloned from pACT-Pdx-1 (kindly provided by Dr. Patrick Ziegler, Institut für Medizinische Biochemie und Molekularbiologie, Universität Greifswald) into the BamH1 restriction sites of the pGEX-4T1. The Pdx-1 S232A and/or T231A mutations were introduced into the pGEX4T-1–Pdx-1 vector using a QuikChange site-directed mutagenesis kit (Stratagene, USA) according to the manufacturer’s protocol. BamH1 fragments containing the cDNA encoding the Pdx-1 amino terminus (aa 1–aa 130) and carboxy terminus (aa 131–aa 284) were cloned within the multiple cloning site of a pGEX4T-1 vector. For transient transfection assays in HEK293T cells, the mammalian expression construct was generated by cloning the BamH1 fragment of Pdx-1 from pGEX4T-1 vector directly into BamH1 sites of pcDNA3.1/hygro (−)-Pdx-1. The insulin reporter construct pGEM3ZFM-RIP1-POLYA (kindly provided by [5]) used for luciferase assays contained the rat insulin promoter I (RIP) from residues −410 to +51 cloned upstream of the luciferase gene. pcDNA3.1/Hygro/lacZ vector was used for the expression of β-galactosidase. For transient transfection assay in βTC-3 and MIN6 cells, p3×Flag/CMV-7.1-Pdx-1 constructs were produced by sub-cloning mouse Pdx-1 cDNA from pGEX4T-1–Pdx-1 vectors into BamH1 site of p3×Flag/CMV-7.1 vector. p3×Flag/CMV-7.1-CK2α was generated by cloning the fragment of human CK2α cDNA from pcDNA3.1–CK2α vector directly into Hind III/BamH1 sites of p3×Flag/CMV-7.1 vector. The pcDNA3.1–CK2β vector was produced by cloning human CK2β cDNA from pT7-7–CK2β vector into NheI/BamH1 sites of pcDNA3.1/Hygro(-) vector. Constructs generated by cloning and mutagenesis were confirmed by DNA sequencing.
Expression and purification of bacterially expressed Pdx-1 proteins
Pdx-1 wild type, mutants, and fragments were expressed in E. coli BL21DE3 after induction with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h at 37°C. Pdx-1 was extracted with buffer R1 (100 mM Tris–HCl, pH 7.8, 100 mM NaCl, 10 mM MgCl2, 0.1% Tween 20), purified by affinity chromatography with GSH-Sepharose and eluted with glutathione in R1 buffer. Purified proteins were dialysed in 20 mM Tris-HCl, pH 7.8, 100 mM NaCl, and 5 mM MgCl2 at 4°C overnight.
Purification of the CK2 holoenzyme, CK2α′ and CK2α
Recombinant CK2 holoenzyme cloned in a bicistronic vector kindly provided by C. T. Walsh [24] and CK2α in pT7-7 were expressed in E. coli BL21 (DE3), and the proteins were purified according to the protocol published by [13]. CK2α′ was expressed as a maltose-binding-protein (MBP)-tagged fusion protein and purified by affinity chromatography on amylose resins.
Cell culture and transfection
The human embryonic kidney 293T (HEK293T, ATCC CRL 11268) cells were cultured in Dulbecco’s modified Eagle’s medium (GIBCO) containing 5.5 mM glucose, 2 mM glutamine, 10% fetal bovine serum in humidified 5% CO2, and 95% air at 37°C. Transfection of HEK293T cells was performed at more than 50% confluence by a conventional calcium phosphate method. Transfected cells were used within 3 days. The mouse βTC-3 and MIN6 cell lines were maintained in DMEM medium containing 5.5 mM d-glucose and 2 mM glutamine supplemented with 15% (v/v) fetal bovine serum, 100 μM β-mercaptoethanol (β-MSH) in humidified 5% CO2, and 95% air at 37°C. βTC-3 and MIN6 cells were transiently transfected with the Effectene® reagent (Qiagen Inc.) according to the manufacturer's instructions.
Antibodies, preparation of cell extracts and Western blot analysis
Rabbit anti-Pdx-1 polyclonal antibody was purchased from Abcam, UK. Anti-GAPDH polyclonal antibody was from Santa Cruz Biotechnology Inc., USA. Preparation of the cell extracts was as described previously [17]. Signals were developed and visualized by the Lumilight system of Roche Diagnostics, Germany.
In vitro kinase assay
The in vitro kinase assay using the CK2-specific synthetic peptide RRRDDDSDDD was performed as described [17].
In vitro phosphorylation
Bacterially expressed and purified Pdx-1 proteins were mixed with the CK2 holoenzyme, CK2α or CK2α′ in 20 μl kinase buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1 mM DTT). The phosphorylation reaction was started by the addition of 32PγATP. After 30 min at 37°C, the reaction was stopped by the addition of 10 μl 3× sample buffer (6% SDS, 30% glycerol, 15% 2-mercaptoethanol, 0.03% bromophenol blue and 0.195 M Tris–HCl, pH 6.8). Proteins were separated in an SDS-polyacrylamide gel and visualized by autoradiography.
In vivo labeling of cells with 33P-phosphate
12 h post-transfection, 2 × 106 transfected βTC-3 cells were preincubated for 1.5 h with phosphate-free DMEM supplemented with 100 μM β-mercaptoethanol (β-MSH), 2 mM glutamine, and 15% FCS at 37°C/5% CO2, and then cells were labeled for another 5 h with 250 μCi 33P-phosphate (Hartmann Analytic). To inhibit endogenous CK2, labeling with 33P-phosphate was performed in the presence of 15 μM 2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole (DMAT, CALBIOCHEM®). The cells were washed with cold PBS and harvested with lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100) containing protease inhibitors (Complete™, Roche Diagnostics) and phosphatase inhibitors (PhosSTOP, Roche Diagnostics). Lysates were used for immunoprecipitation with agarose conjugated anti-Flag antibodies (ANTI-FLAG M2 Affinity Gel, Sigma-Aldrich). Following SDS-polyacrylamide gel electrophoresis, Pdx-1 protein was transferred to PVDF membrane (Roche Diagnostics) and exposed to a film for autoradiography.
Luciferase assays
HEK293T or βTC-3 cells were seeded in six-well plates and co-transfected with a constant amount of DNA consisting of 1.5 μg luciferase reporter construct, 0.5 μg pcDNA3.1/Hygro/lacZ β-galactosidase expression vector, and 2 μg Pdx-1 expression constructs for HEK293T cells or of 0.15 μg luciferase reporter construct, 0.05 μg pcDNA3.1/Hygro/lacZ β-galactosidase expression vector, and 0.2 μg CK2 encoding vectors. As a control we used an expression vector without insert. For protein expression analysis, cells were harvested 48 h after transfection, and protein lysates were prepared as described above. For luciferase assays, cells were harvested 48 h after transfection with reporter lysis buffer (Promega), and luciferase activities were determined using the luciferase assay kit (Promega) according to the manufacturer’s instructions. To determine the transfection efficiency, β-galactosidase activity was measured by determining the optical density at 405 nm after incubation of cell extracts with o-nitrophenyl-β-d-galactopyranoside (Sigma) at 37°C. Results are expressed either as the ratio of the luciferase activity/β galactosidase activity or as the ratio of the luciferase activity/Pdx-1 protein expression levels. Control cells were transfected with pcDNA3.1/hygro(−)-Pdx-1 wild type, luciferase reporter construct, and lacZ β-galactosidase expression vector. Results are averaged from three independent experiments.
Infection of pancreatic β-cells with a recombinant lentivirus and reporter assay
The lentiviral transfer vector Ins-715luc, encoding a rat insulin promoter I (RIPI) (sequence from −715 to +31)/luciferase gene was a kind gift of Michiyo Amemiya-Kudo from Okinaka Memorial Institute for Medical Research, Tokyo, Japan [2]. The plasmid was cut with PmeI and Bgl II and cloned upstream of the luciferase gene, generating the lentiviral transfer vector pFW-INSluc. The viral particles were produced as described [25] by triple transfection of 293T/17 cells with the gag/pol-rev packaging plasmid, the env plasmid encoding vesicular stomatitis virus glycoprotein and the transfer vector. Cell extracts were prepared with reporter lysis buffer (Promega, Mannheim) and analyzed for luciferase activities.
Determination of insulin in the culture medium of pancreatic β-cells
Confluent Min6 cells were split 1:5 and grown for 2 days until 40–50% confluent in 24-well plates. The cells were then washed twice with Krebs-Ringer bicarbonate HEPES buffer (KRBH) of the following composition: 125 mM NaCl, 5.9 mM KCl, 5.0 mM NaHCO3, 1.2 mM MgCl2, 1.3 mM CaCl2, 25 mM HEPES, pH 7.4, and 4 mM glucose. Bovine serum albumin (0.1%, w/v) was added as an insulin carrier. Next, cells were incubated for 4 h in KRBH supplemented with CK2 inhibitors as indicated. Incubation was stopped by putting the plates on ice, and the supernatants were collected for insulin content assays using a rat/mouse insulin enzyme-linked immunosorbent assay kit (Millipore). When indicated, cells were pre-treated with CK2 inhibitors in complete growth medium for 16 h before assaying for insulin content.
Results
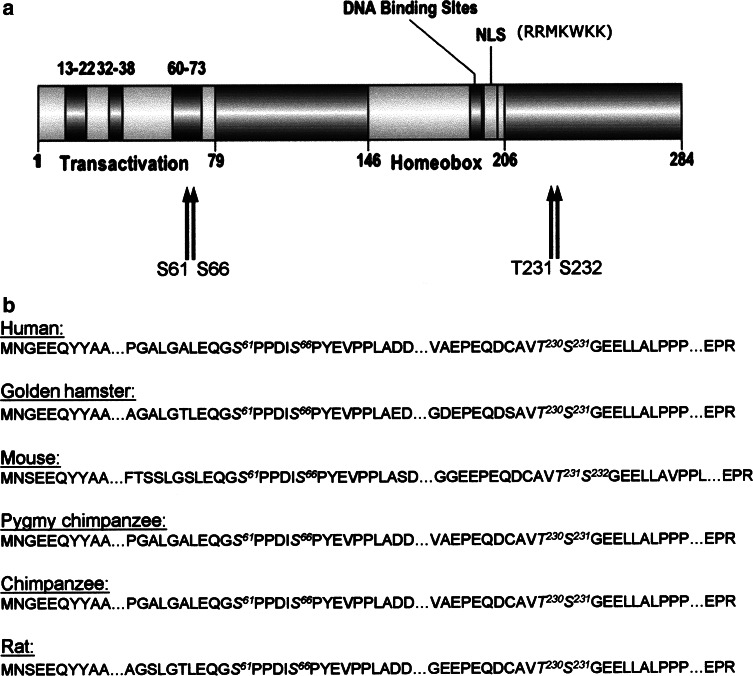
In order to analyze whether Pdx-1 might be a substrate for protein kinase CK2, we first started a computer-based search for consensus sites on the polypeptide chain of Pdx-1. The consensus phosphorylation site for CK2 is characterized by the sequence S/TXXE(D). We found four putative CK2 phosphorylation sites on the polypeptide chain of Pdx-1, which are schematically shown in Fig. 1a. Two putative sites, namely ser61 and ser66, are located in the N-terminal transactivation domain. The two other sites thr231 and ser232 are located in the C-terminus of Pdx-1. An alignment of the amino acid sequences of Pdx-1 from different species (Fig. 1b) revealed that all four sites are highly conserved among different species indicating that these regions may represent functionally relevant domains.
Fig. 1.
Putative CK2 phosphorylation sites on Pdx-1. a Schematic representation of the domain structure of mouse Pdx-1. Transactivation indicates the N-terminal transactivation domain with three highly conserved sub-domains, spanning amino acids 13–22, 32–38, and 60–73. Homeobox signifies the homeodomain, and NLS indicates the nuclear localization signal. Arrows show the putative CK2 phosphorylation sites. b Alignment of Pdx-1 amino acid sequences of different species. Putative consensus sites for CK2 phosphorylation are indicated in italics
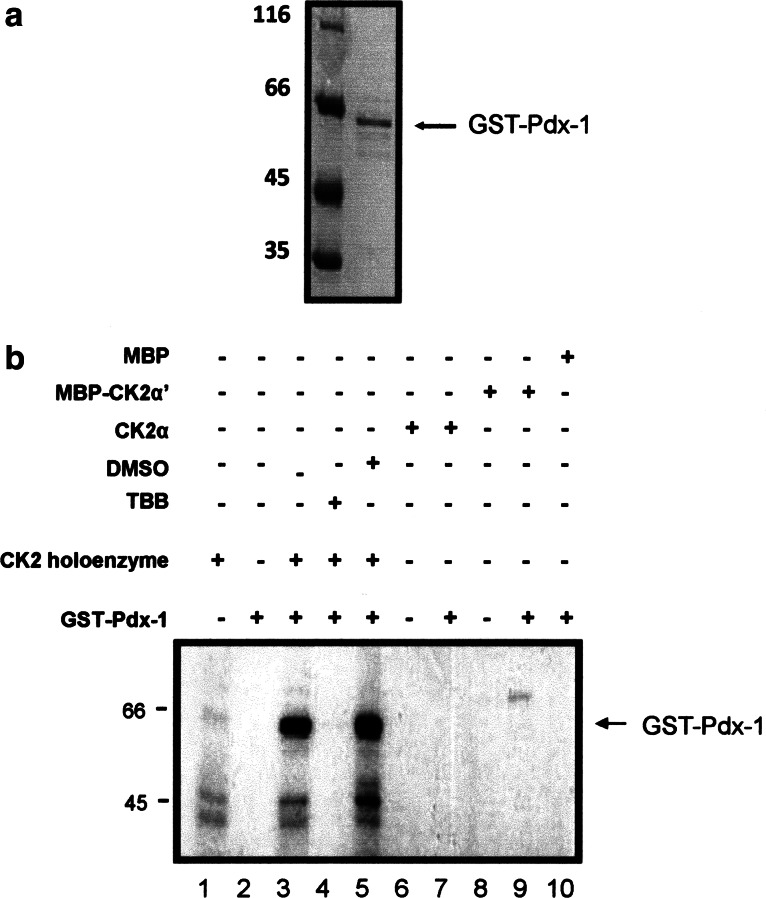
Next, we tested whether bacterially expressed and highly purified Pdx-1 was phosphorylated by protein kinase CK2. Figure 2a shows a Coomassie blue-stained SDS polyacrylamide gel of the Pdx-1 protein, which was used for the following experiments. As shown in Fig. 2b Pdx-1 was phosphorylated by the CK2 holoenzyme (lane 3), whereas there was no signal in the absence of CK2 (lane 2) or in the absence of Pdx-1 (lane 1). Phosphorylation of Pdx-1 by CK2 was inhibited in the presence of 10 μM tetrabromobenzotriazole (TBB) (lane 4), which is an efficient and specific inhibitor of protein kinase CK2 [26]. Protein kinase CK2 is composed of two regulatory β-subunits and two catalytic α- or α′-subunits. Beside the holoenzyme also the free catalytic subunits may phosphorylate a substrate, and therefore we analyzed whether Pdx-1 could also be phosphorylated by the α- or α′-subunit alone. First, the specific activity of the CK2 holoenzyme, CK2α and CK2α′, was normalized to phosphorylate the CK2 specific peptide substrate with the sequence RRRDDDSDDD to the same extent (data not shown). As shown in Fig. 2b neither the α- (lane 7) nor the α′-subunit (lane 9) alone phosphorylated Pdx-1. A weak band in lane 9 may indicate an autophosphorylation of the MBP-CK2α′-subunit.
Fig. 2.
In vitro phosphorylation of Pdx-1 by the CK2 holoenzyme and the α- and α′-subunits. a Bacterially expressed and purified GST-Pdx-1 (1.5 μg) was analyzed on a 12.5% SDS-polyacrylamide gel followed by Coomassie blue staining. b In vitro CK2 phosphorylation assay was performed in the presence of GST-Pdx-1 (1.5 μg) wild type by using a purified CK2 holoenzyme (lane 3) or CK2α (lane 7) or MBP-CK2α′ (lane 9) and CK2 holoenzyme in the presence of 10 μM TBB (lane 4). The reaction was carried out in the presence of 32PγATP for 30 min at 37°C. The CK2 holoenzyme (lane 1) or CK2α (lane 6) or MBP-CK2α’ (lane 8) was incubated with 32PγATP in the absence of Pdx-1 as a control. Phosphorylated proteins were separated in a 12.5% SDS polyacrylamide gel, and the gel was subjected to autoradiography
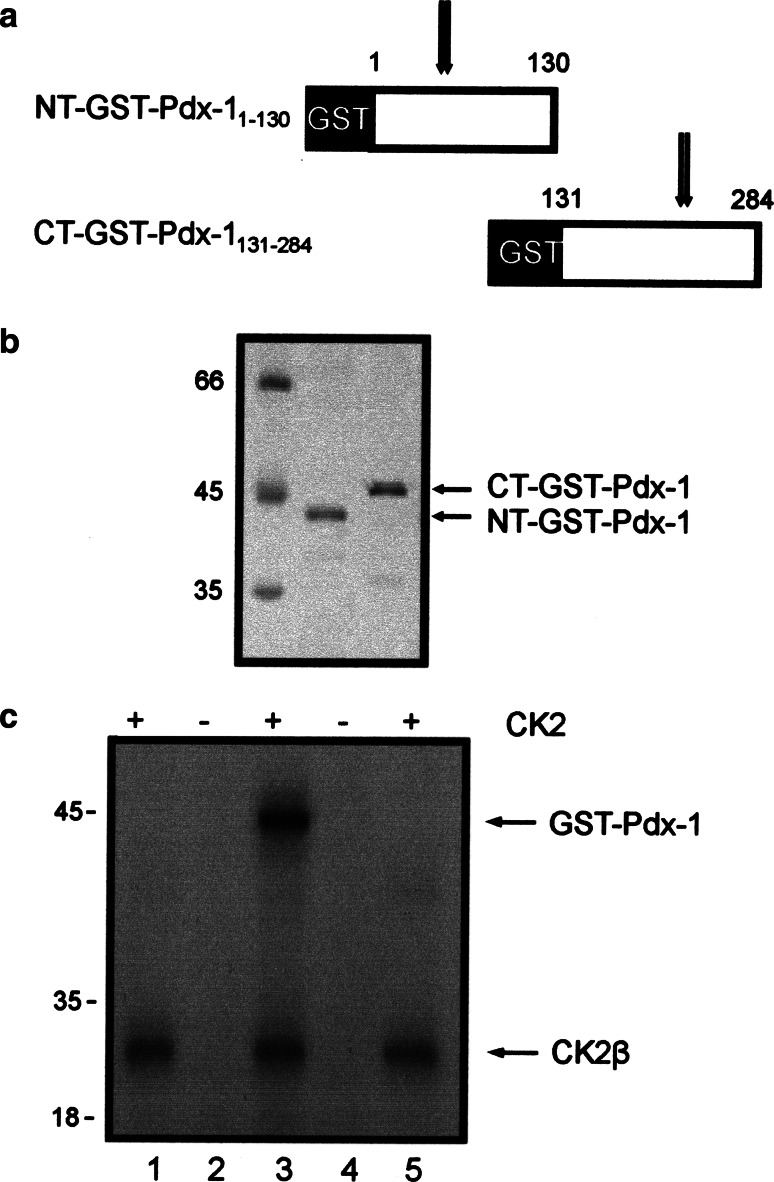
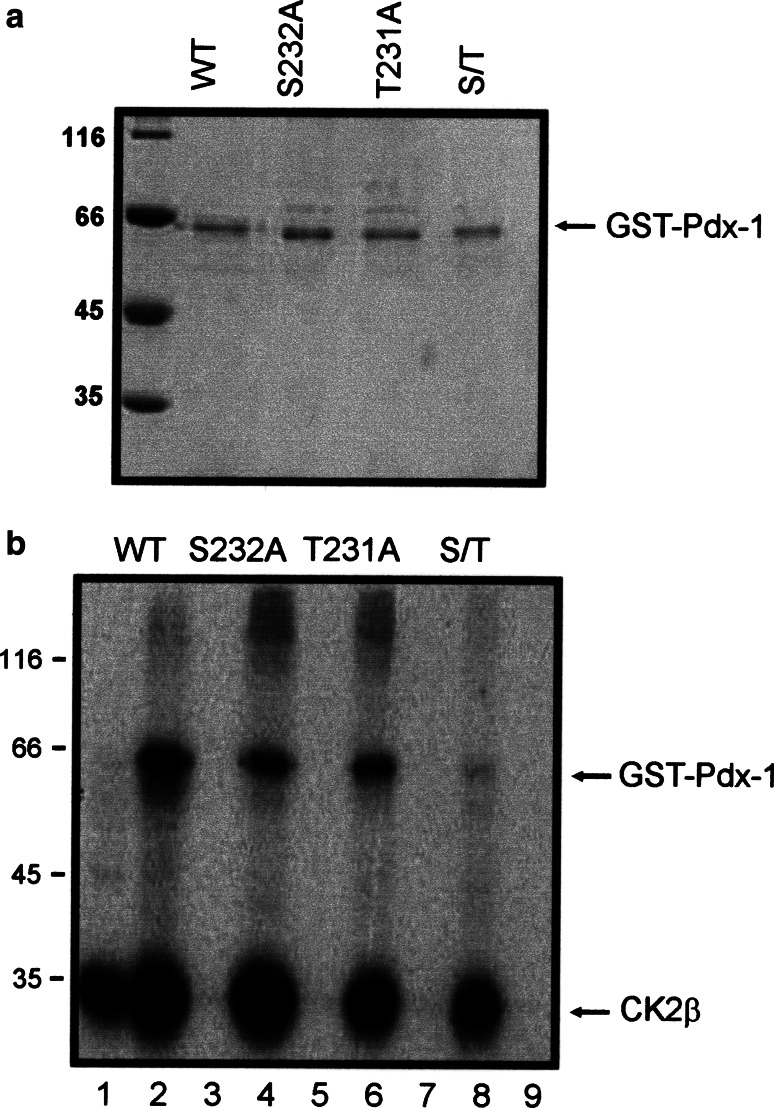
Having shown that Pdx-1 is a substrate for the CK2 holoenzyme, in the next step we wanted to narrow down the phosphorylation sites on the polypeptide chain of Pdx-1. We cloned two recombinant Pdx-1 fragments, one starting at amino acid 1–130 and the other one starting at amino acid 131 until 284 as GST-fusion proteins (Fig. 3a). The N-terminal part contains two putative phosphorylation sites at ser61 and ser66; the C-terminal part contained two sites at thr231 and ser232. Proteins were expressed in bacteria and purified by GSH-Sepharose affinity chromatography. Figure 3b shows a Coomassie blue-stained SDS-polyacrylamide gel with the two Pdx-1 fragments used for the following experiments. Equal amounts of protein were incubated with the CK2 holoenzyme and 32PγATP. Phosphorylated proteins were analyzed on a 12.5% SDS polyacrylamide gel followed by autoradiography. As shown in Fig. 3c, only the C-terminal fragment was phosphorylated by protein kinase CK2. Autophosphorylated CK2β subunit was present in all lanes where the CK2 holoenzyme was used. From this result we conclude that the CK2 phosphorylation sites are located in the C-terminus of Pdx-1, probably at thr231 and/or ser232. In order to further determine whether these two positions were indeed phosphorylated by CK2, we mutated ser232 and thr231 individually to alanine, and we also made a double mutant where both sites were mutated to alanine. Bacterially expressed and purified Pdx-1 mutant proteins as well as Pdx-1 wild type protein were subjected to a kinase reaction with CK2 holoenzyme and 32PγATP. Proteins were analyzed on a 12.5% SDS polyacrylamide gel followed by autoradiography. Wild type Pdx-1 was strongly phosphorylated by CK2, whereas both single mutants were considerably weaker phosphorylated than the wild type Pdx-1 (Fig. 4b). Figure 4a shows that we used equal amounts of Pdx-1 proteins for the phosphorylation reaction. The double mutant was only very weakly phosphorylated if at all. These results show that obviously both sites are phosphorylated.
Fig. 3.
In vitro phosphorylation of Pdx-1 fragments by the CK2 holoenzyme. a Schematic representation of GST-tagged Pdx-1 fragments. The GST moiety is shown as a black bar; the Pdx-1 part is shown as a white bar. Numbers above the Pdx-1 part indicate the respective Pdx-1 amino acids according to mouse Pdx-1. Arrows show the localization of putative CK2 phosphorylation sites. b Bacterially expressed and purified Pdx-1N-terminus (1.5 μg) and Pdx-1 C-terminus (1.5 μg) were analyzed on a 12.5% SDS-polyacrylamide gel. The gel was stained by Coomassie blue. c Recombinant Pdx-1 fragments were phosphorylated by CK2 holoenzyme. Phosphorylation was conducted in the presence of GST-Pdx-1 N-terminus (lane 1) or GST-PDX-1 C-terminus (lane 3) with CK2 holoenzyme and 32PγATP. To serve as control, the GST-Pdx-1 N-terminus (lane 2) or the GST-Pdx-1 C-terminus (lane 4) or CK2 holoenzyme (lane 5) alone was also incubated with 32PγATP. Phosphorylated proteins were separated in a 12.5% SDS polyacrylamide gel. Proteins were visualized by autoradiography. The lower band shows the autophosphorylation of CK2β subunit. Molecular weight markers (in KDa) are indicated on the left
Fig. 4.
In vitro phosphorylation of the Pdx-1 wild type and phosphorylation inactive mutants by the CK2 holoenzyme. a Pdx-1 wild type and mutant proteins were analyzed on a 12.5% SDS polyacrylamide gel. Proteins were stained with Coomassie Brilliant Blue. b Pdx-1 is phosphorylated by CK2 on both ser232 and thr231. Bacterially expressed and purified Pdx-1 wild type and alanine mutants were incubated with CK2 holoenzyme in the presence of 32PγATP. Phosphorylated proteins were run on a 12.5% SDS polyacrylamide gel and then subjected to autoradiography. The autoradiogram shows labeled GST-Pdx-1 wild type (lane 2), S232A (lane 4), T231A (lane 6), S232A/T231A (lane 8), and the autophosphorylation of CK2 β subunit. The CK2 holoenzyme (α2β2) (lane 1) or GST-Pdx-1 wild type (lane 3) or S232A (lane 5) or T231A (lane 7) or S232A/T231A (lane 9) protein alone was included as a negative control
In order to analyze the in vivo phosphorylation of Pdx-1 in pancreatic β-cells, we labeled βTC-3 cells, which were transfected with Flag-tagged Pdx-1 with 33P-phosphate in the presence or absence of 15 μM DMAT, which is a rather specific inhibitor of CK2 [22]. Cells were extracted, and Pdx-1 was immunoprecipitated with anti-Flag-tag antibodies. The immunoprecipitate was analyzed on an SDS polyacrylamide gel. Figure 5 lane 2 shows that the phosphorylation of Pdx-1 was much weaker in the presence than in the absence of the CK2 inhibitor. Although Pdx-1 is also phosphorylated by other kinases, this result strongly indicated that CK2 contributes to the phosphorylation of Pdx-1.
Fig. 5.
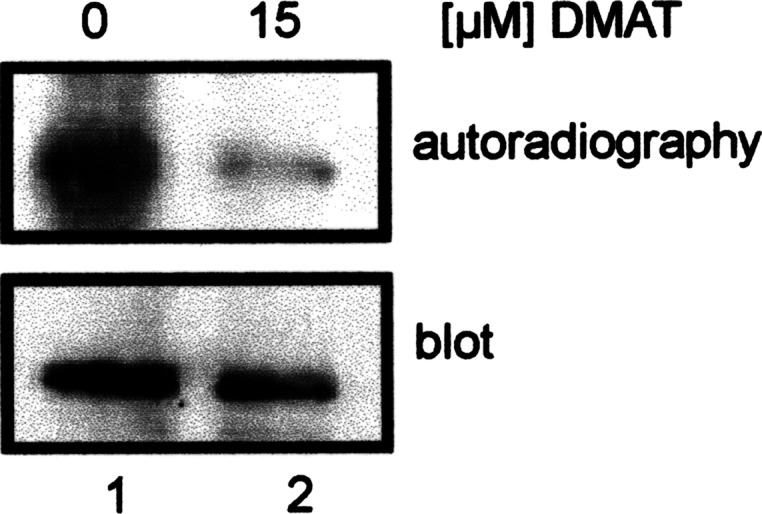
In vivo labeling of βTC-3 cells with 33P-phosphate. βTC-3 cells were labeled with 33P-phosphate for 5 h. Pdx-1 was immunoprecipitated from the cell extract and analyzed on a 12.5% SDS polyacrylamide gel. Phosphate-labeled proteins were identified by autoradiography. For control, the blot was incubated with an Pdx-1 antibody. Protein bands were visualized by the ECL method
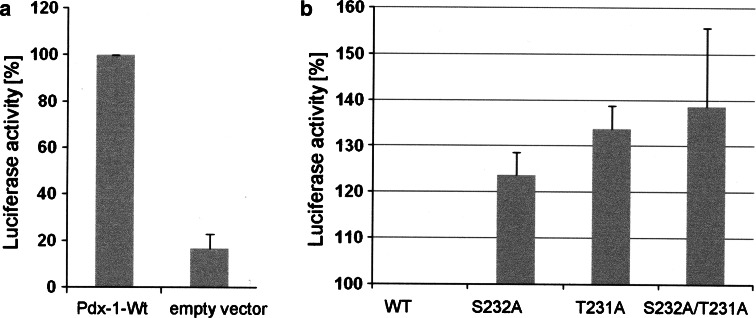
We then asked whether CK2 phosphorylation might have an influence on the transcription factor activity of Pdx-1. To measure the transcriptional activity, we used a luciferase construct whose expression is under the control of the insulin promoter. This construct was transfected into HEK293T cells together with either wild type Pdx-1 or the CK2 phosphorylation mutants. Luciferase activity was measured 48 h after transfection. The results of three independent experiments were shown in Fig. 6. Compared to the control with the empty vector wild type Pdx-1 showed a considerable increase in the luciferase activity. The single mutants S232A and T231A showed an elevated level of transcriptional activity, which was even more pronounced for the double mutant S232A/T231A. The results were normalized for the corresponding proteins expressed after transfection. Thus, CK2 phosphorylation of Pdx-1 modulates its transcriptional activity.
Fig. 6.
Ser232 and thr231 phosphorylation modulate Pdx-1 transcriptional activity. HEK293T cells seeded in a six-well plate were transfected with 2 μg of empty vector (pcDNA3) or pcDNA3-derived vectors encoding either wild type or mutant Pdx-1, 1.5 μg of RIP luciferase, and 0.5 μg of β-galactosidase by calcium phosphate transfection methods. The cells were lysed, and luciferase activity was measured 48 h after transfection. The data between the control cells (empty vector group) and the Pdx-1 wild type-expressing cells (a) are expressed as the ratio of the RIP luciferase activity/β-galactosidase activity. On the contrary, results between Pdx-1 WT and different mutant-expressing cells (b) are shown as RIP luciferase activity divided by the amount of Pdx-1 proteins expressed in the cells. In both cases, the relative activity in Pdx-1 wild type-expressing cells was set at 100%. The results are the means ± SE of three separate experiments done in triplicate
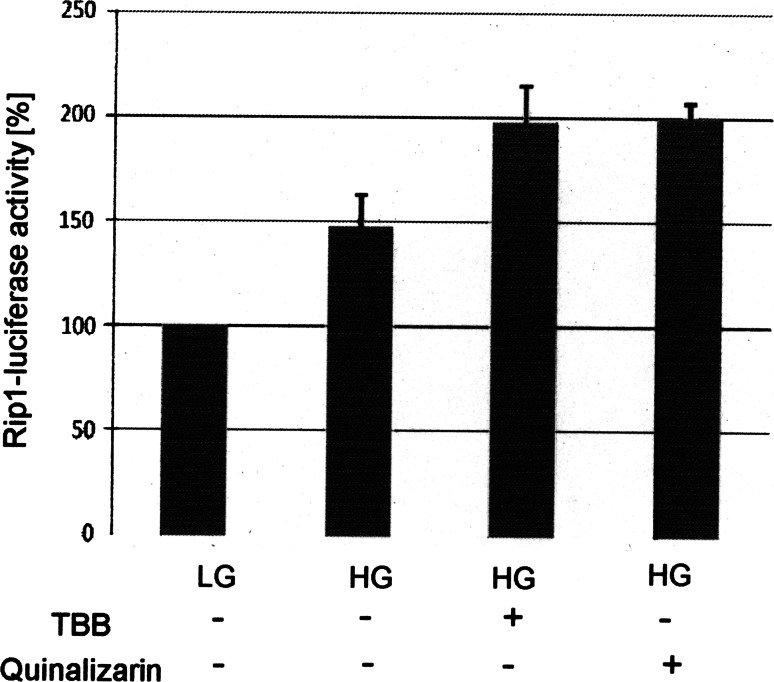
To further prove the influence of the CK2 phosphorylation on the transcription factor activity of Pdx-1, we employed another approach in pancreatic β-cells. We infected Min6 cells with a lentivirus coding for luciferase under the control of the insulin promoter. First, we tested the system by analyzing the insulin promoter activity as a response to elevated glucose concentration. As expected transcription factor activity was elevated by increasing the glucose concentration in the medium from 2 mM glucose (LG) to 25 mM glucose (HG) (Fig. 7). Inhibition of CK2 activity by either TBB [23] or quinalizarin, another CK2-specific inhibitor [7], led to a further increase of the insulin promoter activity. Thus, these results confirm our previous findings obtained with the CK2 phosphorylation mutants of Pdx-1.
Fig. 7.
Effect of CK2 inhibitors on the transcription of the insulin promotor. Min6 cells were infected with a recombinant lentivirus coding for a luciferase construct under the control of the insulin promotor and incubated with 2 mM glucose (LG) or 25 mM glucose (HG) or with HG in the presence of either 50 μM TBB or 50 μM quinalizarin. Luciferase activity measured at LG was set at 100%, and the activity of the other sample was calculated in relation to this value. Standard deviation was calculated from three independent experiments
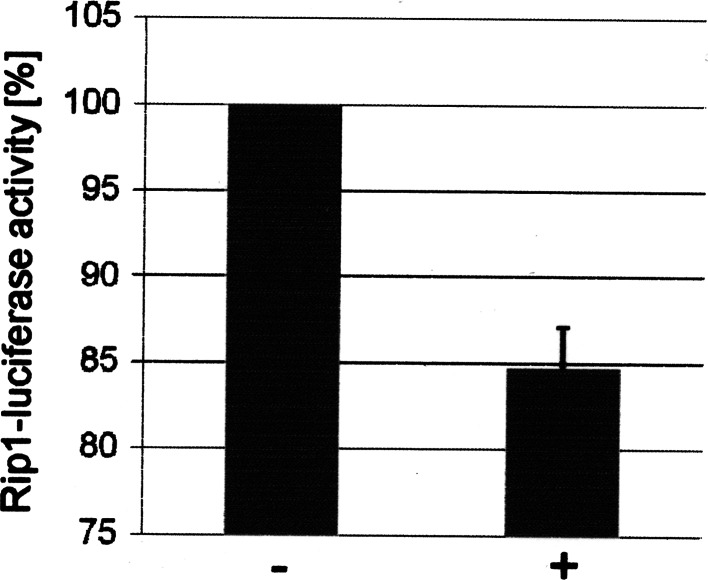
We then analyzed the effect of overexpression of the CK2α and CK2β in β-cells on the transcriptional activity of Pdx-1. βTC-3 cells were transfected with DNA consisting of insulin promotor luciferase reporter construct and with CK2α and CK2β constructs; 48 h after transfection luciferase activity was measured. As a control, we also transfected cells with an empty vector without CK2 inserts. Luciferase activity in the control was set as 100%. As shown in Fig. 8, expression of CK2α and CK2β subunits in β-cells resulted in a considerable decrease of the luciferase activity. This result further supports the notion that phosphorylation of Pdx-1 by CK2 regulates the Pdx-1 transcriptional activity.
Fig. 8.
Overexpression of CK2α and CK2β decreased the transcription of the insulin promotor in pancreatic β-cells. βTC-3 cells were transfected with control or CK2 encoding vectors together with RIP-luciferase and β-galactosidase. Forty-eight hours after transfection luciferase activity was determined. The luciferase activity in the cells transfected with the empty vector (−) was set as 100%, and the activity of cells transfected with CK2α and CK2β (+) was calculated in relation to this 100% value. Standard deviation was calculated from three independent experiments
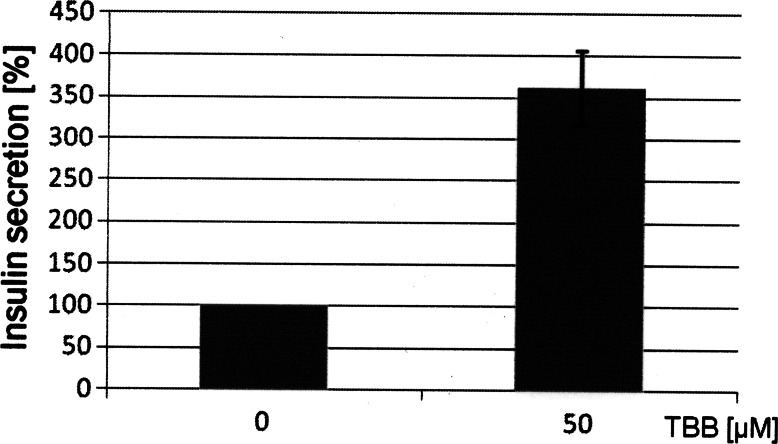
In the next step we analyzed the insulin release from the β-cells in the presence or absence of CK2 inhibitors. Min6 cells were incubated with 50 μM TBB for 20 h. After incubation the supernatant of the cells was analyzed for insulin. As shown in Fig. 9, treatment of cells with TBB led to an elevated level of insulin in the culture medium. The same results were obtained also by using DMAT or quinalizarin as CK2 inhibitors (data not sown). Thus, these results further support our initial findings that CK2 phosphorylation of Pdx-1 is implicated in the regulation of the insulin production in β-cells.
Fig. 9.
Effect of CK2 inhibitors on insulin release from pancreatic β-cells. Min6 cells were treated with TBB or left untreated. The culture medium was collected, and the insulin content was measured by a rat/mouse insulin Elisa Kit (Millipore). The experiment was performed in triplicate, and the data are presented as percentage of insulin release in the absence of inhibitors
Discussion
It has been known for quite some time that the transcription factor Pdx-1 is a phosphoprotein (for review see [1]). Pdx-1 is phosphorylated by DNA-dependent protein kinase (DNA-PK), which is implicated in the repair process of double-strand breaks in DNA [11]. There is strong evidence for an additional role in blocking apoptosis. Furthermore, it has been demonstrated previously that DNA-PK phosphorylates many transcription factors [4, 18]. Although DNA-PK phosphorylates Pdx-1 on threonine 11, which is part of the transactivation domain of Pdx-1, no alteration in the transcriptional activity of Pdx-1 was observed in response to DNA-PK phosphorylation [16]. DNA-PK is a ser/thr kinase composed of two Ku regulatory subunits and a large catalytic subunit. Protein kinase CK2 is also composed of two regulatory β-subunits and two catalytic α- or α′-subunits. Besides, CK2 phosphorylates a number of transcription factors [20], and it plays an important role in preventing apoptosis. Thus, it was an interesting question whether CK2 might also phosphorylate Pdx-1. Here, we show for the first time that Pdx-1 is phosphorylated by protein kinase CK2 at least in vitro. Moreover, in vivo labeling of Pdx-1 in the presence of CK2 inhibitors revealed a considerably weaker phosphorylation of Pdx-1 than in the absence of inhibitors.
The homeodomain-interacting protein kinase 2 (Hipk-2) is another kinase that phosphorylates Pdx-1 within the last 70 amino acids at the C-terminus [6]. Although these phosphorylation sites are far away from the transactivation domain of Pdx-1, Hipk-2 phosphorylation of Pdx-1 increases its transcriptional activity. There are two putative Hipk-2 phosphorylation sites in the C-terminus of Pdx-1, namely thr214 and ser269, which are in the vicinity of the two CK2 phosphorylation sites identified in the present paper. Our data demonstrate that the CK2 phosphorylation of Pdx-1 also regulates its transcriptional activity, although in this case the non-phosphorylatable form showed a higher transcriptional activity. Our results thus strongly support previous observations showing that the C-terminus is also important for full Pdx-1 transactivation potential [19]. In addition also in β-cells of the pancreas, our results give a further confirmation of this effect by observing a considerable increase in the insulin promotor activity after inhibiting CK2 activity. Besides, we see that inhibition of CK2 activity resulted in an elevated secretion of insulin into the culture medium. On the other hand, overexpression of CK2α and CK2β in β-cells resulted in a decrease of the reporter activity.
It has been shown that the mammalian Per-arnt-sim kinase (PASK) activity is stimulated in pancreatic islet β-cells by elevated glucose concentration [8]. Recombinant Pdx-1 was efficiently phosphorylated by purified PASK in vitro on a single site, namely thr152. By using mutants at position 152, which mimic phosphorylation, it was shown that the thr152D and thr152E mutant forms displayed a more cytosolic distribution at both high and low glucose concentrations than wild type or thr152A mutant Pdx-1 [3]. Thus, phosphorylation of Pdx-1 by PASK seems to keep Pdx-1 out of the nucleus. Using either wild type or the two alanine mutants of Pdx-1 at the CK2 phosphorylation site, we found Pdx-1 always in the nucleus (data not shown). Thus, CK2 phosphorylation of Pdx-1 does not result in an altered subcellular localization of Pdx-1. Our present findings suggest CK2 as a novel regulator of the insulin production in pancreatic β cells.
Acknowledgments
Rui Meng is supported by a scholarship for postgraduates from the China Scholarship Council under the supervision of Prof. Dr. Gang Wu from the Cancer Center of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 430023 Wuhan, Hubei, P.R. China. Faizeh Al Quobaili had a stipend from the German academic exchange program DAAD. This study was supported by the program “Das zuckerkranke Kind” from the “Deutsche Diabetes Gesellschaft” and by Homfor T 201 000 378 to Claudia Götz.
References
- 1.Al-Quobaili F, Montenarh M. Pancreatic duodenal homeobox factor-1 and diabetes mellitus type 2. Int J Mol Med. 2008;21:399–404. [PubMed] [Google Scholar]
- 2.Amemiya-Kudo M, Oka J, Ide T, Matsuzaka T, Sone H, Yoshikawa T, Yahagi N, Ishibashi S, Osuga J, Yamada N, Murase T, Shimano H. Sterol regulatory element-binding proteins activate insulin gene promoter directly and indirectly through synergy with BETA2/E47. J Biol Chem. 2005;280:34577–34589. doi: 10.1074/jbc.M506718200. [DOI] [PubMed] [Google Scholar]
- 3.An R, daSilva XG, Hao HX, Semplici F, Rutter J, Rutter GA. Regulation by Per-Arnt-Sim (PAS) kinase of pancreatic duodenal homeobox-1 nuclear import in pancreatic beta-cells. Biochem Soc Trans. 2006;34:791–793. doi: 10.1042/BST0340791. [DOI] [PubMed] [Google Scholar]
- 4.Bannister AJ, Gottlieb TM, Kouzarides T, Jackson SP. c-Jun is phosphorylated by the DNA-dependent protein kinase in vitro; definition of the minimal kinase recognition motif. Nucleic Acids Res. 1993;21:1289–1295. doi: 10.1093/nar/21.5.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boucher MJ, Selander L, Carlsson L, Edlund H. Phosphorylation marks IPF1/PDX1 protein for degradation by glycogen synthase kinase 3-dependent mechanisms. J Biol Chem. 2006;281:6395–6403. doi: 10.1074/jbc.M511597200. [DOI] [PubMed] [Google Scholar]
- 6.Boucher MJ, Simoneau M, Edlund H. The homeodomain-interacting protein kinase 2 regulates insulin promoter factor-1/pancreatic duodenal homeobox-1 transcriptional activity. Endocrinology. 2009;150:87–97. doi: 10.1210/en.2007-0865. [DOI] [PubMed] [Google Scholar]
- 7.Cozza G, Mazzorana M, Papinutto E, Bain J, Elliott M, Di MG, Gianoncelli A, Pagano MA, Sarno S, Ruzzene M, Battistutta R, Meggio F, Moro S, Zagotto G, Pinna LA. Quinalizarin as a potent, selective and cell-permeable inhibitor of protein kinase CK2. Biochem J. 2009;421:387–395. doi: 10.1042/BJ20090069. [DOI] [PubMed] [Google Scholar]
- 8.da Silva X, Rutter J, Rutter GA. Involvement of Per-Arnt-Sim (PAS) kinase in the stimulation of preproinsulin and pancreatic duodenum homeobox 1 gene expression by glucose. Proc Natl Acad Sci USA. 2004;101:8319–8324. doi: 10.1073/pnas.0307737101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Y, Miyazaki J, Hart GW. The transcription factor PDX-1 is post-translationally modified by O-linked N-acetylglucosamine and this modification is correlated with its DNA binding activity and insulin secretion in min6 beta-cells. Arch Biochem Biophys. 2003;415:155–163. doi: 10.1016/S0003-9861(03)00234-0. [DOI] [PubMed] [Google Scholar]
- 10.Goodison S, Kenna S, Ashcroft SJ. Control of insulin gene expression by glucose. Biochem J. 1992;285(Pt 2):563–568. doi: 10.1042/bj2850563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottlieb TM, Jackson SP. Protein kinases and DNA damage. Trends Biochem Sci. 1994;19:500–503. doi: 10.1016/0968-0004(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 12.Goudet G, Delhalle S, Biemar F, Martial JA, Peers B. Functional and cooperative interactions between the homeodomain PDX1, Pbx, and Prep1 factors on the somatostatin promoter. J Biol Chem. 1999;274:4067–4073. doi: 10.1074/jbc.274.7.4067. [DOI] [PubMed] [Google Scholar]
- 13.Grankowski N, Boldyreff B, Issinger O-G. Isolation and characterization of recombinant human casein kinase II subunits α and β from bacteria. Eur J Biochem. 1991;198:25–30. doi: 10.1111/j.1432-1033.1991.tb15982.x. [DOI] [PubMed] [Google Scholar]
- 14.Hessabi B, Ziegler P, Schmidt I, Hessabi C, Walther R. The nuclear localization signal (NLS) of PDX-1 is part of the homeodomain and represents a novel type of NLS. Eur J Biochem. 1999;263:170–177. doi: 10.1046/j.1432-1327.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- 15.Kishi A, Nakamura T, Nishio Y, Maegawa H, Kashiwagi A. Sumoylation of Pdx1 is associated with its nuclear localization and insulin gene activation. Am J Physiol Endocrinol Metab. 2003;284:E830–E840. doi: 10.1152/ajpendo.00390.2002. [DOI] [PubMed] [Google Scholar]
- 16.Lebrun P, Montminy MR, Van OE. Regulation of the pancreatic duodenal homeobox-1 protein by DNA-dependent protein kinase. J Biol Chem. 2005;280:38203–38210. doi: 10.1074/jbc.M504842200. [DOI] [PubMed] [Google Scholar]
- 17.Lehnert S, Götz C, Montenarh M. Protein kinase CK2 interacts with the splicing factor hPrp3p. Oncogene. 2008;27:2390–2400. doi: 10.1038/sj.onc.1210882. [DOI] [PubMed] [Google Scholar]
- 18.Liu L, Kwak YT, Bex F, Garcia-Martinez LF, Li XH, Meek K, Lane WS, Gaynor RB. DNA-dependent protein kinase phosphorylation of IkappaB alpha and IkappaB beta regulates NF-kappaB DNA binding properties. Mol Cell Biol. 1998;18:4221–4234. doi: 10.1128/mcb.18.7.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu M, Miller C, Habener JF. Functional regions of the homeodomain protein IDX-1 required for transactivation of the rat somatostatin gene. Endocrinology. 1996;137:2959–2967. doi: 10.1210/en.137.7.2959. [DOI] [PubMed] [Google Scholar]
- 20.Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 21.Mosley AL, Ozcan S. The pancreatic duodenal homeobox-1 protein (Pdx-1) interacts with histone deacetylases Hdac-1 and Hdac-2 on low levels of glucose. J Biol Chem. 2004;279:54241–54247. doi: 10.1074/jbc.M410379200. [DOI] [PubMed] [Google Scholar]
- 22.Pagano MA, Meggio F, Ruzzene M, Andrzejewska M, Kazimierczuk Z, Pinna LA. 2-Dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole: a novel powerful and selective inhibitor of protein kinase CK2. Biochem Biophys Res Commun. 2004;321:1040–1044. doi: 10.1016/j.bbrc.2004.07.067. [DOI] [PubMed] [Google Scholar]
- 23.Sarno S, Reddy H, Meggio F, Ruzzene M, Davies SP, Donella-Deana A, Shugar D, Pinna LA. Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 (‘casein kinase-2’) FEBS Lett. 2001;496:44–48. doi: 10.1016/S0014-5793(01)02404-8. [DOI] [PubMed] [Google Scholar]
- 24.Shi Y, Brown ED, Walsh CT. Expression of recombinant human casein kinase II and recombinant heat shock protein 90 in Escherichia coli and characterization of their interactions. Proc Natl Acad Sci USA. 1994;91:2767–2771. doi: 10.1073/pnas.91.7.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stefano L, Al SJ, Rossler OG, Vinson C, Thiel G. Up-regulation of tyrosine hydroxylase gene transcription by tetradecanoylphorbol acetate is mediated by the transcription factors Ets-like protein-1 (Elk-1) and Egr-1. J Neurochem. 2006;97:92–104. doi: 10.1111/j.1471-4159.2006.03749.x. [DOI] [PubMed] [Google Scholar]
- 26.Zien P, Duncan JS, Skierski J, Bretner M, Litchfield DW, Shugar D. Tetrabromobenzotriazole (TBBt) and tetrabromobenzimidazole (TBBz) as selective inhibitors of protein kinase CK2: evaluation of their effects on cells and different molecular forms of human CK2. Biochim Biophys Acta. 2005;1754:271–280. doi: 10.1016/j.bbapap.2005.07.039. [DOI] [PubMed] [Google Scholar]