Abstract
Over the last ten years there has been growing acceptance that retinal photoreception among mammals extends beyond rods and cones to include a small number of intrinsically photosensitive retinal ganglion cells (ipRGCs). These ipRGCs are capable of responding to light in the absence of rod/cone input thanks to expression of an opsin photopigment called melanopsin. They are specialised for measuring ambient levels of light (irradiance) for a wide variety of so-called non-image-forming light responses. These include synchronisation of circadian clocks to light:dark cycles and the regulation of pupil size, sleep propensity and pineal melatonin production. Here, we provide a review of some of the landmark discoveries in this fast developing field, paying particular emphasis to recent findings and key areas for future investigation.
Keywords: Retina, Ganglion cells, Photoreception, Circadian rhythms, Opsin, Photosensitivity
Introduction
The discovery of melanopsin and inner retinal photoreceptors has its origin in the study of circadian clocks. These endogenous timing mechanisms perform the important task of fine-tuning behaviour and physiology according to the varying demands of the astronomical day, and are a near ubiquitous feature of life on earth. In mammals, circadian oscillators can be found in multiple cell types and tissues, but these are subservient to a dominant circadian clock located in the hypothalamic suprachiasmatic nuclei (SCN) within the brain. A cardinal feature of circadian clocks is that they are self-sustaining, capable of running with a period close to 24 h without any external input. However, in order to be of benefit to the organism, the ‘internal’ representation of time of day must provide an accurate estimate of external time. Circadian clocks achieve this goal by being regularly reset (or entrained) to rhythmic cues in the physical environment. The most reliable external indicator of time of day is the light:dark cycle and, as a result, light is generally regarded as the most powerful entraining signal for the clock.
The sensory requirements of circadian photoentrainment are fundamentally different from those of image-forming vision. Time of day is correlated with ambient light intensity (irradiance), and it is exactly this parameter (integrated over long timescales) that defines the magnitude of circadian clock responses to experimental light stimuli [1–3]. By contrast, classical visual pathways are optimised for spatial and temporal contrast acuity, with rather limited requirement to accurately encode irradiance. Since at least the beginning of the twentieth century, it has been clear that non-mammalian vertebrates respond to this fundamental difference in sensory requirements by having separate photoreceptors for form vision and for irradiance detection. Karl von Frisch first suggested such extra-ocular photoreceptors in his study of skin pigmentation control in minnows [4], and this has been followed by a great body of work describing photoreceptors extrinsic to the retina. These include receptors associated with circadian photoentrainment and other physiological and behavioural responses to environmental irradiance in the central nervous system, skin and peripheral organs of fish, amphibia, reptiles and birds [5–8].
In contrast to the prevalence of extra-ocular photoreceptors in other vertebrate classes, enucleation of the eye in rodents results in a loss of all light detection. This finding implicated retinal photoreceptors as the origin of both image-forming vision and circadian entrainment in mammals [9, 10]. The obvious implication, that the same rod and cone photoreceptors support both tasks, used to be widely accepted but is now seen as outdated. Early indications that this was not the case came from reports that laboratory rodents suffering extensive degeneration of rods and cones retain circadian photoentrainment [11–13]. While it was thought that residual cone photoreceptors in these mice may account for their ability to entrain [14–16], the possibility of an unknown retinal photoreceptor dedicated to circadian entrainment was also raised [11, 12, 15, 17–19]. Case reports of human subjects lacking conscious light perception and yet retaining circadian light responses provided support for this latter possibility [20]. A direct test of this hypothesis came with the generation of transgenic mice lacking detectable rod and cone photoreceptors. These mice retained a variety of irradiance responses including circadian photoentrainment, light-induced suppression of pineal melatonin, and a pupillary light reflex [21–23]. The clear implication that the mammalian eye contains a non-rod non-cone photoreceptor dedicated to measuring ambient light intensity was further supported by descriptions in mice and humans of irradiance responses whose spectral sensitivity did not match that of any known retinal photoreceptor class [23–26].
The first direct description of these non-rod non-cone photoreceptors came with the publication in 2002 of two papers describing a sub-set of retinal ganglion cells with the extraordinary ability of responding to light even in the absence of synaptic inputs [27, 28]. These so-called ‘intrinsically photosensitive retinal ganglion cells’ (ipRGCs) project to the SCN and express melanopsin [27], a member of the opsin family of G-protein-coupled receptors that was initially discovered in the photosensitive dermal melanophores of Xenopus laevis [29]. The subsequent generation of melanopsin knockout mice (Opn4−/−) confirmed that this protein is critical for the intrinsic light response of ipRGCs [30]. Confirmation that this reflects its function as a light absorbing photopigment came from experiments showing light-dependent G-protein activation by melanopsin in vitro [31] or under heterologous expression in a variety of vertebrate cell types [32–34].
The significance of melanopsin photoreception for irradiance responses was initially investigated in melanopsin knockout mice. These animals retain circadian photoentrainment and a pupillary light reflex, but show significant alterations in stimulus:response relationships [30, 35, 36]. It was not until melanopsin knockout was combined with lesions of rod and cone phototransduction that mice lacking photo responsiveness were obtained [37, 38]. This latter result provided the final confirmation that melanopsin retinal ganglion cells represent the non-rod non-cone photoreceptor whose existence was implied by the earlier rodent and human studies.
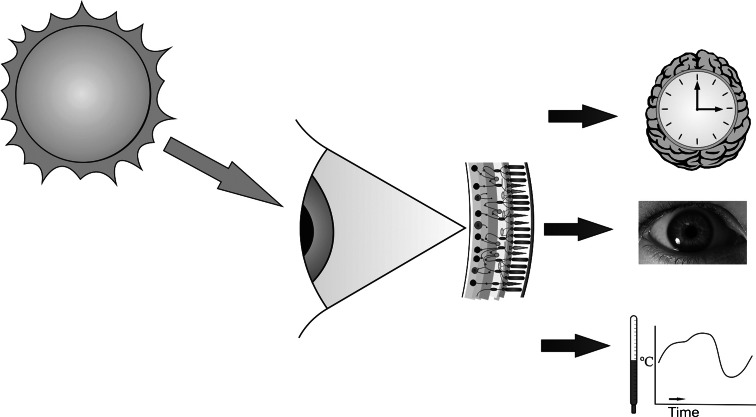
Although the study of ipRGCs has its origins in attempts to explain circadian photoentrainment, it was clear from a relatively early stage that their influence was not restricted to the clock. Mice lacking rods and cones retain a variety of responses to environmental irradiance including pineal melatonin suppression, a pupillary light reflex, inhibition of locomotor activity, and regulation of sleep propensity. In humans, there is evidence that this system regulates body temperature, mood and aspects of arousal and concentration. It seems, therefore, that melanopsin-expressing ipRGCs represent a distinct sensory modality encoding environmental irradiance for a wide variety of physiological and behavioural systems (Fig. 1). Their input to such an array of important biological processes places an imperative to achieve a deeper understanding of their anatomy and physiology, as well as their contribution to our sensory capabilities. Here, we review some of the progress made over the last 5–6 years to address these important questions.
Fig. 1.
A schematic figure of the non-image-forming (NIF) visual system in mammals involving ipRGCs. Light falling on the retina is detected by rod and cone photoreceptors and by the melanopsin phototransduction cascade in ipRGCs. The resultant signals are combined by ipRGCs to send an integral signal of irradiance to the brain. In turn, light information is passed to the brain down the optic nerve, triggering a diverse range of non image-forming responses in addition to normal form vision. NIF responses comprise the entrainment of circadian rhythms including sleep propensity (shown here by an image of a clock superimposed on a brain), the pupil light reflex, regulation of pineal melatonin levels and, in humans, regulation of body temperature and mood
Anatomy of ipRGCs
Since the correlation between expression of melanopsin and intrinsic photosensitivity was first established [27], immunohistochemistry using melanopsin antibodies has been the primary approach to exploring ipRGC morphology. Antibodies against melanopsins from a wide variety of species and recognising both N- and C-terminal epitopes are now available [27, 39–43]. There have been reports of melanopsin immunoreactivity in a subset of cones and in the retinal pigment epithelium [44, 45]. However, at least in mammals, by far the most consistent staining is observed in a small number of retinal ganglion cells whose cell bodies may be found in the ganglion cell or inner nuclear layers [42, 43, 46]. The proportion of ganglion cells immunoreactive for melanopsin varies according to the species under investigation and, to some extent, the antibody used, but never exceeds a few percent. Compared to other ganglion cell classes, they are distinguished by their sparse dendritic arborisation but wide receptive fields [39].
It is becoming increasingly clear that melanopsin ganglion cells do not, in fact, form a single homogenous group. In the mouse, at least three anatomically distinct subtypes have been reported. Termed M1–3, these differ mainly in their dendritic stratification (Fig. 2). Ganglion cells in the vertebrate retina receive synaptic input in the inner plexiform layer (IPL), which can be subdivided into anatomically distinct sublaminae. The major distinction is between sublamina a, closest to the rods and cones, in which they make connections with so-called OFF bipolar cells that are excited by decrements in light intensity, and sublamina b, proximal to the major concentration of ganglion cell bodies, characterised by synaptic input from ON bipolars excited by light increments. Dendrites from the so-called M1 cells stratify in sublamina a, those of M2 in sublamina b, while M3 ganglion cells are bistratified with dendrites in both a and b sublaminae [53, 55–57]. One might expect M1 cells therefore to receive mainly OFF signals from the outer retina, but as this appears not to be the case [48–52], the functional significance of this difference in dendritic stratification remains unknown. There are, moreover, other morphological differences between classes. M2 cells have larger soma, larger dendritic fields, more branched/complex arborisation, and greater overall dendritic length than M1 cells [58]. Circumstantial evidence suggests that these classes also differ in their degree of melanopsin expression. Thus, a lac-Z reporter knocked into the melanopsin gene reaches detectable levels of expression only in the M1 class [55, 56], and the intrinsic light response of M2 cells is also smaller and less sensitive than that of M1 [58]. In addition to murines, primates also have separate populations of melanopsin ganglion cells with dendrites either in the inner or outer border of the IPL [43, 59]. However, the degree to which these are correlates of the rodent M1/2 cells remains unclear.
Fig. 2.
A schematic diagram of retinal connections relevant to ipRGCs in the rodent retina. A and B show examples of classical wiring through ON (triggered by increments of light) and OFF (triggered by decrements of light)-cell pathways of the retina, respectively. The typical pathway of vertical transmission flows from photoreceptor cell to bipolar cell to ganglion cell. The inner plexiform layer (IPL) can be subdivided into sublaminae a and b. Bipolar and ganglion cells stratifying in sublamina a are OFF cells, while those stratifying in sublamina b are ON. A cone ON bipolar cell synapses directly onto an ON ganglion cell in sublamina b. Rod bipolar cells are all ON cells but do not contact ganglion cells directly in this classical pathway, but rather synapse onto AII amacrine cells in sublamina b. AII amacrines in turn contact cone ON bipolar cell dendrites through sign-conserving gap junctions, and OFF cone bipolar cells via sign inverting synapses (B). Cone OFF bipolar cells directly synapse onto OFF ganglion cells. Diverse amacrine cell types modulate ON and OFF pathways mainly through inhibitory inputs. ipRGCs can be subdivided into three morphological types (M1–M3). M1 cell dendrites stratify in sublamina a (C), M2 in sublamina b (B), while M3 cells are bistratified in both sublaminae (D). In contrast to normal retinal cell stratification patterns, however, M1 cells stratifying in sublamina a receive strong ON signals [43, 47, 48]. This reflects direct input from cone ON bipolars at the M1 cell body and, anomalously at dendrites in sublamina a [49, 50], as well as direct synaptic contacts with rod bipolars [47]. Rod bipolars may also influence their activity via the classical AII amacrine cell route. Physiological results predict additional input from cone OFF bipolars and inhibitory amacrine cells [51, 52]. Finally, there are direct connections between dopaminergic amacrine cells and presumed M1 cell dendrites in sublamina a, possibly going in both directions [46, 47, 50, 53, 54]. The connections of M2 and M3 cells are currently unknown. Those shown here are predictions based on connectivity of conventional ganglion cells with similar dendritic stratification patterns. Not shown are horizontal cells which modulate information transfer at the OPL. ONL outer nuclear layer, OPL outer plexiform layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer, HC horizontal cell, AC amacrine cell, AC* aII amacrine cell, AC+ dopaminergic amacrine cell, BC, bipolar cell. Blue bipolar cell body, cone ON bipolar; red bipolar cell body, rod bipolar (ON); grey bipolar cell body, cone OFF bipolar
All species examined thus far show melanopsin-positive cells distributed across the retina (with the exception of the primate fovea) with only relatively minor regional differences in concentration [27, 43, 55, 60]. Taken together with their wide dendritic arborisation this means that they form what has been termed a ‘photoreceptive net’ across the retina [39], capturing light from almost all parts of the visual scene. It now also appears that the dendritic fields of neighbouring M1 and M2 cells show significant overlap when filled and traced with neurobiotin, suggesting an overlap of the receptive fields of the two subtypes [58].
Intra-retinal connectivity
Physiological and anatomical studies indicate that excitatory ON and OFF bipolar cells (signalling light increments and decrements respectively), as well as inhibitory amacrine cells, are presynaptic to ipRGCs [47, 51, 57, 59, 61]. Thus, despite their unique ability to respond directly to light, ipRGCs share much of the intra-retinal connectivity of classical retinal ganglion cell classes. However, a number of unusual aspects of ipRGC connectivity have been suggested. Firstly, M1 cells receive strong ON signals from the outer retina, despite having dendrites in the OFF sublamina of the IPL [43, 48]. This has recently been explained by description of a class of ON bipolar which contacts ipRGC dendrites in the OFF sublamina [50]. Secondly, there is anatomical evidence that rod ON bipolars, which normally do not make direct contacts with RGCs, do form synapses with ipRGCs [47] (Fig. 2). Finally, ipRGCs seem capable of exporting information not only onto the brain but also back out to the rest of the retina. Thus, in both humans and mice, there is evidence that melanopsin activity contributes to diurnal/circadian control of cone-based visual pathways [62, 63]. The route by which they exert this influence is currently unknown. There is physiological evidence that ipRGCs make gap junction connections with neighbouring cells in the ganglion cell layer [64]. As the mouse ganglion cell layer contains many amacrine cell bodies, this could allow ipRGCs to influence the major neuromodulator systems of the retina. Alternatively, ipRGCs may provide a more direct regulation of a particular class of amacrine cell that produces dopamine. Dopamine is released from these cells in response to light [65], and modulates many aspects of retinal function [66–70]. A subset of ipRGCs form a discrete plexus with dopaminergic amacrines [46, 50, 53, 54], and receive synaptic input from them. Information may also flow in the opposite direction. Thus, Zhang et al. [54] recently presented evidence that, in the mouse, sustained light-dependent firing of dopaminergic amacrine cells is driven by melanopsin photoreceptors. Consequently, this pathway would be well placed to translate melanopsin activation into modifications in retinal physiology. It should be noted, however, that melanopsin phototransduction does not appear either necessary or sufficient for large-scale dopamine release in the retina [71].
Projections and functions of ipRGCs
Currently, the most comprehensive analysis of central projections for ipRGCs has been from Hattar et al. [55]. That work employed mice in which a tau-lacZ transgene was knocked into the melanopsin locus. The resultant expression of β-galactosidase throughout ipRGC axons enables standard histochemical techniques to be used to trace their passage through the mouse brain. The projection pattern observed was surprisingly widespread and the reader is referred to that paper for a detailed description. In brief, the most intense innervation was in the SCN, with a bilateral projection extending throughout the nucleus. Elsewhere in the hypothalamus, the ventral subparaventricular zone, the lateral hypothalamus and the supraoptic nucleus (SON) all receive ipRGC input, providing a potential route for the direct effects of light on gross physiology and the neuroendocrine axis. ipRGC axons were also found in the ventrolateral preoptic nucleus, consistent with evidence that melanopsin provides photic regulation of sleep propensity [72–74]. Other notable projections were traced to the olivary pretectal nucleus (OPN; the origin of the pupil light reflex) and the intergeniculate leaflet (a known component of the circadian entrainment pathway; [75]).
The major drawback of the tau-lacZ approach to tracing ipRGC projections is that β-galactosidase expression seems to be restricted to the M1 subtype [76]. Thus, the projection pattern described by Hattar et al. [55] is probably incomplete. There are indications that M2 and M3 cells might have quite different projection patterns. Viral tracing techniques suggest that while M1 cells comprise the primary projection to the mouse SCN (80% M1/20% M2), equal numbers of M1 and M2 cells project to the OPN (45% M1/55% M2; [76]). In fact, the relative projection pattern of these two classes suggests a new anatomical sub-division of this nucleus, with M1 axons terminating in a hollow ovoid ‘shell’ surrounding the OPN, and M2 cells innervating the central core.
Another brain region in which the work of Hattar et al. [55] probably underestimates the ipRGC projection is the lateral geniculate. β-galactosidase staining reveals strong innervation of the intergeniculate leaflet but not the neighbouring dorsal lateral geniculate (the major thalamic relay centre for visual information). However, Dacey et al. [43] successfully labelled ipRGCs in the primate using injections of retrograde tracers from the lateral geniculate. Moreover, in our own unpublished work we have observed widespread responses to melanopsin activation in neurons of the lateral geniculate (Brown and Lucas, unpublished observations). It seems reasonable, therefore, to speculate that this brain region receives input from M2/M3 cells. An important area for future study will be to determine what this signal contributes to visual processing.
Recently, fMRI techniques have been applied to exploring irradiance-dependent activity in the human brain. The results reveal fairly widespread changes in activity in sub-cortical and limbic areas [77]. At present, these have not been unambiguously assigned to melanopsin photoreception. However, they are consistent with evidence that this system has widespread effects on attention, mood and perhaps cognitive function.
Physiology of ipRGCs
Historically, a major barrier to a physiological characterisation of the ipRGC light response has been their comparative scarcity within the retinal ganglion cell layer. Data on the number of ipRGCs in the retina depends on the method used to identify them, but is around 1,200–1,600 cells, or ~1.5% of the total ganglion cell population in mice and hamsters [30, 55, 76, 78], and up to 3,000 cells in human and macaque retinae (0.2% of total population; [43]). As a result, their contribution to unbiased samples of physiological activity in the ganglion cell population would be very slight. A number of strategies have been used to overcome this problem.
Retrograde tract tracing of retinal ganglion cells
The original discovery of ipRGCs was made by recording from cells retrogradely traced from the SCN [79–82]. The success of this approach relies upon the fact that the SCN receives predominantly ipRGC innervation [83]. Although it has been used very successfully in a number of studies, this strategy is labour intensive. Moreover, as selective targeting of other ipRGC-recipient nuclei is not always possible, its use has so far been mostly limited to studying those ipRGCs afferent to the SCN.
Identification of light responses in the absence of signals from the outer retina
One of the early confirmations that cells in the mouse ganglion cell layer could be intrinsically photosensitive came from calcium imaging of the ganglion cell layer in rodless + coneless mice [64]. In that work, the ability to survey activity over relatively large areas of the retina, combined with genetic ablation of rod/cone activity, enabled a small number of intrinsically light responsive cells to be identified and studied. Similar approaches employing multi-electrode arrays, and replacing genetic with pharmacological lesions of outer retinal signalling have since been successfully employed in the same way [51, 84, 85].
Viral tracing
An alpha (Bartha) strain of the pseudorabies virus (PRV-Ba), incapable of anterograde transport [86–88], labels retinal ganglion cells afferent to the SCN/OPN when injected into the contralateral eye. This is a fifth order event, following infection of autonomic ganglia innervating the uvea and subsequent retrograde infection of the ipsilateral Edinger-Westphal nucleus (EW), then four bilateral retinorecipient structures (the OPN followed by the IGL, the lateral terminal nucleus—LTN, of the accessory optic system and the SCN; [89]). PRV-Ba has been used by successive authors to investigate aspects of the SCN [89, 90] as well as to identify RGCs of the mammalian retinohypothalamic tract. PRV-Ba constructs have been engineered to express enhanced fluorescent proteins (EGFP/RFP) [53, 78, 91, 92], allowing infected RGCs to be targeted with recording electrodes [49].
Immunopanning
Two groups have successfully purified ipRGCs from rodent retinal homogenates by immunopanning with a melanopsin antibody [93, 94]. These purified ipRGC populations can then be easily isolated for physiological responses.
Genetic
Several mouse lines now exist in which fluorescent proteins are expressed under control of the melanopsin promotor [57, 95, 96]. These fall into two main categories. The first carries a BAC transgene including a fluorescent protein downstream of the melanopsin promotor that has been randomly integrated into the mouse genome [57, 96]. In the second, a Cre recombinase coding sequence has been knocked into the melanopsin locus [95]. Crossing these Opn4 Cre mice with transgenics carrying a ‘floxed’ reporter (a reporter coding sequence driven by a strong promoter upon Cre-mediated excision of a STOP cassette flanked by loxP sites) allows specific expression of the reporter protein in melanopsin-expressing cells. Each type of reporter mouse has its own advantages. Both strategies appear to label not only M1 but also other ipRGC classes, and as they may use fluorescent proteins to do this can be adapted to physiological as well as anatomical analyses. The Cre/loxP system dissociates the strength of reporter expression from the degree of melanopsin promoter activity, as once the STOP cassette has been excised the reporter is driven by its own, much stronger promoter. This could provide a more extensive coverage of the ipRGC population, but is also more liable to yield false positives as a small degree of ‘leaky’ Cre expression could result in strong reporter expression [97], perhaps due to early developmental activity of the promoter in a cells ontogeny. In both cases, it is theoretically possible that the introduction of FP/Cre coding sequences disrupts promoter/enhancer elements sufficiently to produce significant numbers of false positives and/or negatives. In fact, so far these potential limitations appear not to be a big consideration for the melanopsin reporter mice [57, 95, 96], and given their advantages over other methods for identifying ipRGCs, their use looks set to increase.
Light response characteristics in ipRGCs
The basic characteristics of the ipRGC light response have been accepted from the earliest days of their discovery, and fit well with their role as irradiance detectors. Unlike rods and cones, which hyperpolarise in response to light, ipRGCs depolarise, triggering action potentials which propagate to the brain [28, 43, 58, 96, 98]. Although the light intensities required to elicit a change in ipRGC firing rate are well within the physiological range [57], their intrinsic light reaction is significantly less sensitive than either rods or cones [28, 43]. The ipRGC response can also be extremely sluggish, taking several seconds to reach peak under dim light intensities [28]. Brighter stimuli elicit more rapid responses [96, 99], however, but the response latency for the pupillary light reflex in rodless+coneless mice suggests that even at the highest irradiances the melanopsin-mediated response is delayed by ~300 ms compared to that of the conventional photoreceptors [23].
Recently, the origins of these sensory characteristics were addressed in a study describing the response of ipRGCs to absorption of a single photon of light [96], reviewed in [100]. This confirmed the low relative sensitivity of ipRGCs (the photon flux required to elicit a half saturating response is ~104× that of cones [101]), and presented evidence that this is not a reflection of inefficient phototransduction, but rather of very poor photon catch associated with low pigment density. In fact, the membrane current induced by single photon absorption was greater than that reported in rod photoreceptors, and long lasting. The single photon response also recapitulated the sluggish response kinetics reported for ipRGCs, with a time to peak some ~20 times slower than rods and ~100 times slower than cones [101, 102]. As elements of the melanopsin phototransduction cascade become identified it will be important to determine the origin of these response characteristics, e.g. does the relatively large sustained depolarisation of the single photon response reflect high biochemical gain in the phototransduction cascade and/or persistent channel opening?
Diversity in the intrinsic light response has been described in studies of ipRGCs using calcium imaging [64], multi electrode array [85, 103], whole-cell [28, 104] and perforated patch recordings [96]. Only very recently, however, have these varying physiological characteristics been assigned to anatomical subtypes of ipRGCs [58]. Schmidt and Kofuji [58] described 10× higher sensitivity and greater maximal responses in the intrinsic light response of M1 compared to M2 cells, consistent with the suggestion that they express more melanopsin. Differences in intrinsic membrane properties of M1 and M2 cells were also reported, with M2 cells having lower input resistance, a more hyperpolarised resting membrane potential and higher peak and average firing rates. It is tempting to interpret these data in terms of the relative significance of extrinsic (rod/cone) versus intrinsic (melanopsin) signals in defining the firing pattern of M1 and M2 cells, and thus the sensory characteristics of downstream responses. However, this remains unproven.
Phototransduction
Melanopsin is a member of the opsin sub group of G-protein-coupled receptors. In general, opsins bind 11-cis retinaldehyde (or related compounds) in the dark, which acts as an inverse agonist inhibiting G-protein interaction. Light is absorbed by this cofactor, which is isomerised to the all-trans isoform in the process. All-trans retinaldehyde is a receptor agonist and the photoactivated opsin therefore binds its cognate g-protein, setting in train second messenger signalling cascades. There is currently no reason to suspect that melanopsin’s mechanism of action deviates from this basic model.
Across the animal kingdom, there are examples of opsins coupling to G-proteins from Gt, Gq-, Go- and Gs classes (see [105] for review and [106]). Vertebrate rod and cone opsins couple to a Gt-protein (transducin) and trigger a phosphodiesterase-dependent cascade resulting in cyclic nucleotide gated channel closure. The downstream effectors of melanopsin have yet to be proven conclusively. However, most available evidence supports the hypothesis that its cognate G-protein is of the Gq/11 family activating the IP3/DAG pathway and ultimately TRP channels, similar to many invertebrate phototransduction cascades [33, 34, 107–114]. Light triggers an increase in intracellular calcium in ipRGC somata, and calcium responses are highly correlated with action potential firing [64, 93, 115, 116]. In vivo data implicate canonical transient receptor potential (TRP) [110, 111] and/or voltage gated calcium channels [93] as the origin of this calcium increase. Direct evidence for melanopsin interactions with Gq/11 G-proteins is currently limited to heterologous cell expression studies [34]. Melanopsin can also bind other G-proteins under these conditions [31, 32], but there is always the concern of promiscuous G-protein interactions outside of a native cellular environment [117]. Nonetheless, ipRGCs express Gq and G11 G-proteins as well as several phospholipase C isozymes [99] and TRPC channels (TRPC6 and TRPC7; [110, 111]). Moreover, the ipRGC intrinsic photoresponse has been inhibited using pharmacological blockers of phospholipase C [99] and TRPC channels [110, 111].
Signal integration: the triplex retina
Over the last year, three independent studies have confirmed that, at least in mice, cytotoxic lesions of ipRGCs abolish all irradiance responses [95, 118, 119]. These findings indicate that ipRGCs provide a unique sensory modality that cannot be provided by other ganglion cell classes. Nonetheless, while rodless+coneless mice retain a variety of irradiance responses [21–23], so do animals lacking melanopsin [30, 35, 36]. The implication that ipRGCs are capable of supporting irradiance responses either through their own intrinsic photosensitivity or by integrating signals from rods and/or cones is supported by the finding that animals lacking all three photoreceptor classes are truly photo-insensitive and by anatomical and physiological studies showing synaptic input to ipRGCs from the outer retina [28, 43, 47, 48, 51, 52, 57, 59, 61, 118, 119].
This raises the question of what benefit the irradiance response system gains from receiving input from the three different photoreceptor classes. A definitive and comprehensive answer to this question will take some time to arrive. However, the evidence to date is that each receptor contributes to irradiance responses according to its own sensory capabilities and that the inclusion of all three extends the conditions under which irradiance can be accurately encoded. Studies of pupillary responses in rodless+coneless and Opn4−/− mice are consistent with a variety of physiological studies in indicating that melanopsin phototransduction lacks the sensitivity and temporal fidelity of rod and cone pathways [23, 30, 38]. Thus, a simple view in which melanopsin encodes very bright stimuli while rods and cones provide additional sensitivity and increase the temporal bandpass of irradiance detection has gained currency. This model, however, does not readily explain data from other irradiance responses, especially those which may integrate irradiance signals over many tens of minutes or hours [12, 36].
Ontogeny
ipRGCs provide the earliest light detection in mammals. Melanopsin expression is first detected in the mouse retina at embryonic day (E)10.5 [120], following the appearance of retinal ganglion cells at E9. The rodent RHT is present at postnatal day (P)0 [121, 122], and ipRGCs are functional and capable of inducing c-fos within the SCN at least as early as that [115, 121, 123]. By contrast, rod opsin expression is not detectable until P5 [124] and rod/cone responses until P10–12 [125–127]. Rod/cone input to ipRGCs appears also to begin at P11 [57]. Thus, melanopsin photoreception precedes that of the classical visual system. Interestingly, an analogous situation has been noted in chickens, where multi-electrode array recordings measured action potentials from ipRGCs in response to blue light as early as E13 [103]. Pineal photoreception in lampreys [5], fish [128] and amphibians [129] also develops in advance of rods or cones. Such examples of the early development of irradiance detection systems across diverse vertebrate classes suggests that measuring irradiance has far-reaching ecological advantages in early vertebrate life, perhaps in phototaxis as a predator avoidance strategy.
In mice, ipRGCs undergo substantial postnatal changes in cell number, morphology and physiology [115]. Just like the general ganglion cell population [130], ipRGCs are overproduced at birth and subsequently reduced back to their adult numbers [115]. Interestingly, this pruning fails to occur in a mouse model that undergoes photoreceptor degeneration at an early stage of development (complete by the first month of life; CBA/J), indicating that ipRGC pruning may be under photoreceptor regulation [131]. The molecular mechanisms responsible for ipRGC differentiation and for determining their unusual projection pattern are largely unknown. However, recent work suggests that the transcription factor Brn3b is essential for correct axonal structure and path finding in ipRGCs [132].
Melanopsin spectral sensitivity and photobiology
A photoreceptor’s spectral sensitivity function describes its relative responsiveness to light of different wavelengths. It is determined by the absorbance spectrum of the light-absorbing photopigment (which lies at the origin of the photoreceptor light response), adjusted according to any wavelength dependent pre-receptoral filtering by screening pigments/absorptive structures [133]. The first estimates of the spectral sensitivity of the non-rod non-cone photoreceptors that turned out to be ipRGCs came from descriptions of the wavelength dependence of behavioural responses in retinally degenerate mice. Working in rodless (rd/rd) mice Yoshimura and Ebihara [24] described peak sensitivity for circadian entrainment around 480 nm, which was distinct from that of any mouse photoreceptor known at the time. The significance of this finding was not widely appreciated, partly because it was not supported by other work in this genotype [134] and because the possibility that it reflected a polymorphism in cone spectral sensitivity could not be discounted. In 2001, a more detailed action spectrum was described for pupillomotor responses in rodless+coneless (rd/rd cl) mice. It matched the predicted absorbance spectrum of an opsin:vitamin A-based photopigment and again showed peak sensitivity (λmax) around 480 nm [23]. Subsequently, a 480 nm λmax was also reported for rat ipRGCs in in vitro recordings [28], and this is now accepted as an accurate representation of melanopsin’s spectral sensitivity in a variety of mammalian species [43, 135]. There was initially some doubt over whether this extended to humans, as action spectra for suppression of pineal melatonin reported shorter wavelength sensitivity [25, 136], but more recent studies support a 480 nm λmax [135, 137], and it seems likely that, given the challenges of human experimentation, these are all in agreement (Fig. 3).
Fig. 3.
Melanopsin is maximally sensitive to short wavelength ‘blue’ light. The spectral sensitivity profile of melanopsin photoreception in a standard human observer is approximated by the nomogram for an opsin:vitamin A based photopigment with peak sensitivity at 480 nm corrected for likely wavelength-dependent lens absorption. The spectral sensitivity of scotopic (rod-based) and photopic (cone-based) vision (Commission Internationale de l’Eclairage, CIE, luminosity functions) are presented for comparison. A very rough approximation of perceived colour is shown below. Note that although melanopsin sensitivity peaks around 480 nm, it shows good responsiveness well into the green/yellow portion of the spectrum
The most satisfactory way of confirming these estimates of melanopsin spectral sensitivity would be to directly measure its absorbance properties. This has been achieved for rod and cone opsins in vivo by microspectrophotometry and by spectroscopic analysis of in vitro purified opsin protein (see for example [138, 139], and [140] for review). Unfortunately, the low concentration of melanopsin in the retina impacts both of these approaches. As an alternative, heterologous expression methods have been developed to produce melanopsin in cell culture. Success with this approach has been somewhat limited, and the small number of published studies reported only modest yields of light absorbing protein compared to that routinely obtained for e.g. rod opsins [141]. Purified mouse melanopsin also had a λmax (424 nm) quite divergent from the 480 nm pigment from behavioural studies [31]. More success has been reported from non-mammalian melanopsins, with Koyanagi and colleagues reporting λmax of 485 nm for a melanopsin-related pigment from amphioxus [107], and Torii et al. [142] peaks at 476 nm and 484 nm for two chicken melanopsins. These papers notwithstanding, there is still a need for reliable methods for melanopsin production and purification that would allow in vitro spectroscopic, biochemical and perhaps direct structural analysis.
A question that needs to be addressed by more detailed spectroscopic analysis of mammalian melanopsins is that of chromophore recycling. Rod and cone opsins bind only cis isoforms of retinaldehyde, and following light absorption, release all-trans retinaldehyde for regeneration in neighbouring retinal pigment epithelium and, probably, Müller glial cells. An alternative mechanism of bleach recovery is used by invertebrate opsin photopigments. These retain all-trans retinal and use a second photon to regenerate the cis-isoform. Phylogenetic analyses show that melanopsins are more closely related to invertebrate opsins than other vertebrate opsins, and there have subsequently been suggestions that melanopsin also employs such an intrinsic bleach recovery mechanism (see [143] for review). The most comprehensive analysis of this issue has been undertaken for Amphioxus melanopsin which, when reconstituted with 11-cis retinal, was shown to form a stable photoproduct whose spectral sensitivity was shifted to longer (redder) wavelengths. Further light exposure could regenerate a pigment whose spectral sensitivity matched that of the original 11-cis reconstituted melanopsin, supporting the hypothesis that these data reflect light dependent transitions of melanopsin from cis to all-trans retinaldehyde binding states. There is indirect evidence from in vivo [108, 137, 144] (although see also [145]) and in vitro [32, 34] studies that mammalian melanopsins may behave in the same way, although the field awaits more direct confirmation of this hypothesis. Moreover, chromophore extraction from mouse ipRGCs in dark adapted retinas only showed evidence of 11-cis but not all-trans retinaldydehyde. This indicates that irrespective of any intrinsic photocycle, melanopsin employs some independent source of 11-cis retinaldehdye for bleach recovery in vivo [94].
Comparative considerations
Since the initial discovery of melanopsin in Xenopus [29], melanopsin genes have now been described in a variety of vertebrates, including species of fish, reptiles and birds [146–149]. Until recently, it was assumed that these were all orthologues of a single gene. However, the discovery of two melanopsin genes in the chicken turned this thinking on its head [150]. One of these, designated Opn4m, is the true orthologue of mammalian melanopsin, while the other (Opn4x) was found to be orthologous to the original Xenopus melanopsin gene [150]. A re-examination of other species revealed that these two branches of the melanopsin family are widespread in non-mammalian vertebrates, but that Opn4x had been lost due to substantial chromosomal reorganisation early in mammalian evolution [150, 151]. A similar loss of cone opsin genes also occurred in mammalian evolution, and it is tempting to conclude that both events reflect a retrenchment of photosensory capability during the nocturnal phase of our evolution [152]. The consequences for mammalian sensory biology of only having one melanopsin gene remain unknown. There is significant sequence divergence between Opn4m and Opn4x branches of the melanopsin family, and although early data suggest that they do not differ in spectral sensitivity [142], there is scope for them to couple to different signalling cascades or differ in other aspects of their activity.
Perspectives
The decade since the demonstration that mammalian photosensitivity could not be entirely explained by the activity of rods and cones, and since the discovery of melanopsin, has witnessed the birth of a new branch of sensory biology. Few questions regarding melanopsin and ipRGC photoreceptors remain entirely unaddressed (although the factors regulating melanopsin gene expression and ipRGC fate determination are notable exceptions). However, even fewer have received a definitive answer. The coming years should see a more quantitative and analytical understanding of the basic physiology of ipRGCs emerge, alongside full elucidation of its signalling cascade. There is also work to do on melanopsin photobiology: is its spectral sensitivity really invariant between species, and does it have an intrinsic bleach recovery mechanism? A great contribution to this latter effort would be development of heterologous expression and protein purification strategies capable of reliably producing large amounts of melanopsin for spectroscopic, biochemical and ultimately structural investigation. Further insight into the diversity of ipRGCs will come from new reporter mice in which melanopsin-expressing cells can be identified for anatomical and physiological experiments. Thanks to this diversity it seems likely that melanopsin influence will be detected in a wider range of light responses [77], including perhaps in the functioning of the classical image-forming visual system. As challenging as any of these endeavours will be the development of new models of how the ‘triplex’ (rod, cone, melanopsin) retina encodes the light environment. Studies of cell types presynaptic to ipRGCs will be critical to this effort, as will quantitative descriptions of stimulus:response relationships for diverse irradiance dependent behaviours under a variety of lighting conditions.
Acknowledgment
The authors would like to thank the Leverhulme Trust for funding.
References
- 1.Takahashi JS, DeCoursey PJ, Bauman L, Menaker M. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984;308:186–188. doi: 10.1038/308186a0. [DOI] [PubMed] [Google Scholar]
- 2.Foster RG, Helfrich-Forster C. The regulation of circadian clocks by light in fruitflies and mice. Philos Trans R Soc Lond B. 2001;356:1779–1789. doi: 10.1098/rstb.2001.0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson DE, Takahashi JS. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus) J Physiol. 1991;439:115–145. doi: 10.1113/jphysiol.1991.sp018660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Frisch K. Beiträge zur Physiologie der Pigmentellen in der Fischhaut. Pflügers Arch. 1911;138:319–387. [Google Scholar]
- 5.Tamotsu S, Morita Y. Photoreception in pineal organs of larval and adult lampreys, Lampetra japonica . J Comp Physiol A. 1986;159:1–5. doi: 10.1007/BF00612489. [DOI] [PubMed] [Google Scholar]
- 6.Taylor DH. Extra-optic photoreception and compass orientation in larval and adult salamanders (Ambystoma tigrinum) Anim Behav. 1972;20:233–236. doi: 10.1016/s0003-3472(72)80041-1. [DOI] [PubMed] [Google Scholar]
- 7.Underwood H. Extraretinal light receptors can mediate photoperiodic photoreception in male lizard Anolis carolinensis . J Comp Physiol. 1975;99:71–78. [Google Scholar]
- 8.Menaker M, Roberts R, Elliott J, Underwood H. Extraretinal light perception in sparrow, III: eyes do not participate in photoperiodic photoreception. Proc Natl Acad Sci USA. 1970;67:320–325. doi: 10.1073/pnas.67.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson RJ, Zucker I. Photoperiodic control of reproduction in olfactory-bulbectomized rats. Neuroendocrinology. 1981;32:266–271. doi: 10.1159/000123171. [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki S, Goto M, Menaker M. No evidence for extraocular photoreceptors in the circadian system of the Syrian hamster. J Biol Rhythms. 1999;14:197–201. doi: 10.1177/074873099129000605. [DOI] [PubMed] [Google Scholar]
- 11.Ebihara S, Tsuji K. Entrainment of the circadian activity rhythm to the light cycle: effective light intensity for a Zeitgeber in the retinal degenerate C3H mouse and the normal C57BL mouse. Physiol Behav. 1980;24:523–527. doi: 10.1016/0031-9384(80)90246-2. [DOI] [PubMed] [Google Scholar]
- 12.Foster RG, Provencio I, Hudson D, Fiske S, De Grip W, Menaker M. Circadian photoreception in the retinally degenerate mouse (rd/rd) J Comp Physiol A. 1991;169:39–50. doi: 10.1007/BF00198171. [DOI] [PubMed] [Google Scholar]
- 13.Anderson KV, O’Steen WK. Black-white and pattern discrimination in rats without photoreceptors. Exp Neurol. 1972;34:446–454. doi: 10.1016/0014-4886(72)90040-4. [DOI] [PubMed] [Google Scholar]
- 14.Yoshimura T, Ebihara S. Decline of circadian photosensitivity associated with retinal degeneration in CBA/J-rd/rd mice. Brain Res. 1998;779:188–193. doi: 10.1016/s0006-8993(97)01122-0. [DOI] [PubMed] [Google Scholar]
- 15.Argamaso SM, Froehlich AC, McCall MA, Nevo E, Provencio I, Foster RG. Photopigments and circadian systems of vertebrates. Biophys Chem. 1995;56:3–11. doi: 10.1016/0301-4622(95)00009-m. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Fernandez JM, Jimenez AJ, Foster RG. The persistence of cone photoreceptors within the dorsal retina of aged retinally degenerate mice (rd/rd): implications for circadian organization. Neurosci Lett. 1995;187:33–36. doi: 10.1016/0304-3940(95)11330-y. [DOI] [PubMed] [Google Scholar]
- 17.Provencio I, Wong S, Lederman AB, Argamaso SM, Foster RG. Visual and circadian responses to light in aged retinally degenerate mice. Vision Res. 1994;34:1799–1806. doi: 10.1016/0042-6989(94)90304-2. [DOI] [PubMed] [Google Scholar]
- 18.Foster RG, Argamaso S, Coleman S, Colwell CS, Lederman A, Provencio I. Photoreceptors regulating circadian behavior: a mouse model. J Biol Rhythms. 1993;8(Suppl):S17–S23. [PubMed] [Google Scholar]
- 19.Provencio I, Cooper HM, Foster RG. Retinal projections in mice with inherited retinal degeneration: implications for circadian photoentrainment. J Comp Neurol. 1998;395:417–439. doi: 10.1002/(sici)1096-9861(19980615)395:4<417::aid-cne1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Czeisler CA, Shanahan TL, Klerman EB, Martens H, Brotman DJ, Emens JS, Klein T, Rizzo JF., 3rd Suppression of melatonin secretion in some blind patients by exposure to bright light. N Engl J Med. 1995;332:6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- 21.Lucas RJ, Freedman MS, Munoz M, Garcia-Fernandez JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- 22.Freedman MS, Lucas RJ, Soni B, von Schantz M, Munoz M, David-Gray Z, Foster R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 23.Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimura T, Ebihara S. Spectral sensitivity of photoreceptors mediating phase-shifts of circadian rhythms in retinally degenerate CBA/J (rd/rd) and normal CBA/N (+/+)mice. J Comp Physiol A. 1996;178:797–802. doi: 10.1007/BF00225828. [DOI] [PubMed] [Google Scholar]
- 25.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brainard GC, Hanifin JP, Rollag MD, Greeson J, Byrne B, Glickman G, Gerner E, Sanford B. Human melatonin regulation is not mediated by the three cone photopic visual system. J Clin Endocrinol Metab. 2001;86:433–436. doi: 10.1210/jcem.86.1.7277. [DOI] [PubMed] [Google Scholar]
- 27.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 29.Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: an opsin in melanophores, brain, and eye. Proc Natl Acad Sci USA. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- 31.Newman LA, Walker MT, Brown RL, Cronin TW, Robinson PR. Melanopsin forms a functional short-wavelength photopigment. Biochemistry. 2003;42:12734–12738. doi: 10.1021/bi035418z. [DOI] [PubMed] [Google Scholar]
- 32.Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433:741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- 33.Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, Provencio I, Berson DM. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- 34.Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–604. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- 35.Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O’Hara BF. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- 36.Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 37.Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 39.Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature. 2002;415:493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- 40.Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hannibal J, Hindersson P, Knudsen SM, Georg B, Fahrenkrug J. The photopigment melanopsin is exclusively present in pituitary adenylate cyclase-activating polypeptide-containing retinal ganglion cells of the retinohypothalamic tract. J Neurosci. 2002;22:RC191. doi: 10.1523/JNEUROSCI.22-01-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hannibal J, Hindersson P, Ostergaard J, Georg B, Heegaard S, Larsen PJ, Fahrenkrug J. Melanopsin is expressed in PACAP-containing retinal ganglion cells of the human retinohypothalamic tract. Invest Ophthalmol Vis Sci. 2004;45:4202–4209. doi: 10.1167/iovs.04-0313. [DOI] [PubMed] [Google Scholar]
- 43.Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 44.Dkhissi-Benyahya O, Rieux C, Hut RA, Cooper HM. Immunohistochemical evidence of a melanopsin cone in human retina. Invest Ophthalmol Vis Sci. 2006;47:1636–1641. doi: 10.1167/iovs.05-1459. [DOI] [PubMed] [Google Scholar]
- 45.Peirson SN, Bovee-Geurts PH, Lupi D, Jeffery G, DeGrip WJ, Foster RG. Expression of the candidate circadian photopigment melanopsin (Opn4) in the mouse retinal pigment epithelium. Brain Res Mol Brain Res. 2004;123:132–135. doi: 10.1016/j.molbrainres.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Vugler AA, Redgrave P, Semo M, Lawrence J, Greenwood J, Coffey PJ. Dopamine neurones form a discrete plexus with melanopsin cells in normal and degenerating retina. Exp Neurol. 2007;205:26–35. doi: 10.1016/j.expneurol.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 47.Ostergaard J, Hannibal J, Fahrenkrug J. Synaptic contact between melanopsin-containing retinal ganglion cells and rod bipolar cells. Invest Ophthalmol Vis Sci. 2007;48:3812–3820. doi: 10.1167/iovs.06-1322. [DOI] [PubMed] [Google Scholar]
- 48.Pickard GE, Baver SB, Ogilvie MD, Sollars PJ. Light-induced fos expression in intrinsically photosensitive retinal ganglion cells in melanopsin knockout (opn4) mice. PLoS ONE. 2009;4:e4984. doi: 10.1371/journal.pone.0004984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dumitrescu ON, Pucci FG, Wong F, Berson DM. Ectopic ON bipolar cell synapses in the OFF inner plexiform layer. Contacts with dopaminergic amacrine cells and melanopsin ganglion cells. J Comp Neurol. 2009;517:226–244. doi: 10.1002/cne.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoshi H, Liu WL, Massey SC, Mills SL. ON inputs to the OFF layer: bipolar cells that break the stratification rules of the retina. J Neurosci. 2009;29:8875–8883. doi: 10.1523/JNEUROSCI.0912-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong KY, Dunn FA, Graham DM, Berson DM. Synaptic influences on rat ganglion-cell photoreceptors. J Physiol. 2007;582:279–296. doi: 10.1113/jphysiol.2007.133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perez-Leon JA, Warren EJ, Allen CN, Robinson DW, Lane Brown R. Synaptic inputs to retinal ganglion cells that set the circadian clock. Eur J Neurosci. 2006;24:1117–1123. doi: 10.1111/j.1460-9568.2006.04999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viney TJ, Balint K, Hillier D, Siegert S, Boldogkoi Z, Enquist LW, Meister M, Cepko CL, Roska B. Local retinal circuits of melanopsin-containing ganglion cells identified by transsynaptic viral tracing. Curr Biol. 2007;17:981–988. doi: 10.1016/j.cub.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 54.Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, McMahon DG. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc Natl Acad Sci USA. 2008;105:14181–14186. doi: 10.1073/pnas.0803893105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baver SB, Pickard GE, Sollars PJ, Pickard GE. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci. 2008;27:1763–1770. doi: 10.1111/j.1460-9568.2008.06149.x. [DOI] [PubMed] [Google Scholar]
- 57.Schmidt TM, Taniguchi K, Kofuji P. Intrinsic and extrinsic light responses in melanopsin-expressing ganglion cells during mouse development. J Neurophysiol. 2008;100:371–384. doi: 10.1152/jn.00062.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidt TM, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J Neurosci. 2009;29:476–482. doi: 10.1523/JNEUROSCI.4117-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jusuf PR, Lee SC, Hannibal J, Grunert U. Characterization and synaptic connectivity of melanopsin-containing ganglion cells in the primate retina. Eur J Neurosci. 2007;26:2906–2921. doi: 10.1111/j.1460-9568.2007.05924.x. [DOI] [PubMed] [Google Scholar]
- 60.Semo M, Munoz Llamosas M, Foster RG, Jeffery G. Melanopsin (Opn4) positive cells in the cat retina are randomly distributed across the ganglion cell layer. Vis Neurosci. 2005;22:111–116. doi: 10.1017/S0952523805001069. [DOI] [PubMed] [Google Scholar]
- 61.Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol. 2003;460:380–393. doi: 10.1002/cne.10652. [DOI] [PubMed] [Google Scholar]
- 62.Barnard AR, Hattar S, Hankins MW, Lucas RJ. Melanopsin regulates visual processing in the mouse retina. Curr Biol. 2006;16:389–395. doi: 10.1016/j.cub.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 63.Hankins MW, Lucas RJ. The primary visual pathway in humans is regulated according to long-term light exposure through the action of a nonclassical photopigment. Curr Biol. 2002;12:191–198. doi: 10.1016/s0960-9822(02)00659-0. [DOI] [PubMed] [Google Scholar]
- 64.Sekaran S, Foster RG, Lucas RJ, Hankins MW. Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Curr Biol. 2003;13:1290–1298. doi: 10.1016/s0960-9822(03)00510-4. [DOI] [PubMed] [Google Scholar]
- 65.Iuvone PM, Galli CL, Garrison-Gund CK, Neff NH. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science. 1978;202:901–902. doi: 10.1126/science.30997. [DOI] [PubMed] [Google Scholar]
- 66.Bjelke B, Goldstein M, Tinner B, Andersson C, Sesack SR, Steinbusch HW, Lew JY, He X, Watson S, Tengroth B, Fuxe K. Dopaminergic transmission in the rat retina: evidence for volume transmission. J Chem Neuroanat. 1996;12:37–50. doi: 10.1016/s0891-0618(96)00176-7. [DOI] [PubMed] [Google Scholar]
- 67.Lasater EM, Dowling JE. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci USA. 1985;82:3025–3029. doi: 10.1073/pnas.82.9.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hayashida Y, Ishida AT. Dopamine receptor activation can reduce voltage-gated Na+ current by modulating both entry into and recovery from inactivation. J Neurophysiol. 2004;92:3134–3141. doi: 10.1152/jn.00526.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ichinose T, Lukasiewicz PD. Ambient light regulates sodium channel activity to dynamically control retinal signaling. J Neurosci. 2007;27:4756–4764. doi: 10.1523/JNEUROSCI.0183-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nir I, Harrison JM, Haque R, Low MJ, Grandy DK, Rubinstein M, Iuvone PM. Dysfunctional light-evoked regulation of cAMP in photoreceptors and abnormal retinal adaptation in mice lacking dopamine D4 receptors. J Neurosci. 2002;22:2063–2073. doi: 10.1523/JNEUROSCI.22-06-02063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cameron MA, Pozdeyev N, Vugler AA, Cooper H, Iuvone PM, Lucas RJ. Light regulation of retinal dopamine that is independent of melanopsin phototransduction. Eur J Neurosci. 2009;29:761–767. doi: 10.1111/j.1460-9568.2009.06631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsai JW, Hannibal J, Hagiwara G, Colas D, Ruppert E, Ruby NF, Heller HC, Franken P, Bourgin P. Melanopsin as a sleep modulator: circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4(−/−) mice. PLoS Biol. 2009;7:e1000125. doi: 10.1371/journal.pbio.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lupi D, Oster H, Thompson S, Foster RG. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci. 2008;11:1068–1073. doi: 10.1038/nn.2179. [DOI] [PubMed] [Google Scholar]
- 74.Altimus CM, Guler AD, Villa KL, McNeill DS, Legates TA, Hattar S. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc Natl Acad Sci USA. 2008;105:19998–20003. doi: 10.1073/pnas.0808312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harrington ME. The ventral lateral geniculate nucleus and the intergeniculate leaflet: interrelated structures in the visual and circadian systems. Neurosci Biobehav Rev. 1997;21:705–727. doi: 10.1016/s0149-7634(96)00019-x. [DOI] [PubMed] [Google Scholar]
- 76.Baver SB, Pickard GE, Sollars PJ. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci. 2008;27:1763–1770. doi: 10.1111/j.1460-9568.2008.06149.x. [DOI] [PubMed] [Google Scholar]
- 77.Vandewalle G, Schmidt C, Albouy G, Sterpenich V, Darsaud A, Rauchs G, Berken PY, Balteau E, Degueldre C, Luxen A, Maquet P, Dijk DJ. Brain responses to violet, blue, and green monochromatic light exposures in humans: prominent role of blue light and the brainstem. PLoS ONE. 2007;2:e1247. doi: 10.1371/journal.pone.0001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sollars PJ, Smeraski CA, Kaufman JD, Ogilvie MD, Provencio I, Pickard GE. Melanopsin and non-melanopsin expressing retinal ganglion cells innervate the hypothalamic suprachiasmatic nucleus. Vis Neurosci. 2003;20:601–610. doi: 10.1017/s0952523803206027. [DOI] [PubMed] [Google Scholar]
- 79.Pickard GE. The afferent connections of the suprachiasmatic nucleus of the golden hamster with emphasis on the retinohypothalamic projection. J Comp Neurol. 1982;211:65–83. doi: 10.1002/cne.902110107. [DOI] [PubMed] [Google Scholar]
- 80.Balkema GW, Drager UC. Origins of uncrossed retinofugal projections in normal and hypopigmented mice. Vis Neurosci. 1990;4:595–604. doi: 10.1017/s0952523800005794. [DOI] [PubMed] [Google Scholar]
- 81.Murakami DM, Miller JD, Fuller CA. The retinohypothalamic tract in the cat: retinal ganglion cell morphology and pattern of projection. Brain Res. 1989;482:283–296. doi: 10.1016/0006-8993(89)91191-8. [DOI] [PubMed] [Google Scholar]
- 82.Pickard GE. Morphological characteristics of retinal ganglion cells projecting to the suprachiasmatic nucleus: a horseradish peroxidase study. Brain Res. 1980;183:458–465. doi: 10.1016/0006-8993(80)90481-3. [DOI] [PubMed] [Google Scholar]
- 83.Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- 84.Semo M, Lupi D, Peirson SN, Butler JN, Foster RG. Light-induced c-fos in melanopsin retinal ganglion cells of young and aged rodless/coneless (rd/rd cl) mice. Eur J Neurosci. 2003;18:3007–3017. doi: 10.1111/j.1460-9568.2003.03061.x. [DOI] [PubMed] [Google Scholar]
- 85.Tu DC, Zhang D, Demas J, Slutsky EB, Provencio I, Holy TE, Van Gelder RN. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 86.Brideau AD, Eldridge MG, Enquist LW. Directional transneuronal infection by pseudorabies virus is dependent on an acidic internalization motif in the Us9 cytoplasmic tail. J Virol. 2000;74:4549–4561. doi: 10.1128/jvi.74.10.4549-4561.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Husak PJ, Kuo T, Enquist LW. Pseudorabies virus membrane proteins gI and gE facilitate anterograde spread of infection in projection-specific neurons in the rat. J Virol. 2000;74:10975–10983. doi: 10.1128/jvi.74.23.10975-10983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tomishima MJ, Enquist LW. A conserved alpha-herpesvirus protein necessary for axonal localization of viral membrane proteins. J Cell Biol. 2001;154:741–752. doi: 10.1083/jcb.200011146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pickard GE, Smeraski CA, Tomlinson CC, Banfield BW, Kaufman J, Wilcox CL, Enquist LW, Sollars PJ. Intravitreal injection of the attenuated pseudorabies virus PRV Bartha results in infection of the hamster suprachiasmatic nucleus only by retrograde transsynaptic transport via autonomic circuits. J Neurosci. 2002;22:2701–2710. doi: 10.1523/JNEUROSCI.22-07-02701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smeraski CA, Sollars PJ, Ogilvie MD, Enquist LW, Pickard GE. Suprachiasmatic nucleus input to autonomic circuits identified by retrograde transsynaptic transport of pseudorabies virus from the eye. J Comp Neurol. 2004;471:298–313. doi: 10.1002/cne.20030. [DOI] [PubMed] [Google Scholar]
- 91.Smith BN, Banfield BW, Smeraski CA, Wilcox CL, Dudek FE, Enquist LW, Pickard GE. Pseudorabies virus expressing enhanced green fluorescent protein: A tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits. Proc Natl Acad Sci USA. 2000;97:9264–9269. doi: 10.1073/pnas.97.16.9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Glatzer NR, Derbenev AV, Banfield BW, Smith BN. Endomorphin-1 modulates intrinsic inhibition in the dorsal vagal complex. J Neurophysiol. 2007;98:1591–1599. doi: 10.1152/jn.00336.2007. [DOI] [PubMed] [Google Scholar]
- 93.Hartwick AT, Bramley JR, Yu J, Stevens KT, Allen CN, Baldridge WH, Sollars PJ, Pickard GE. Light-evoked calcium responses of isolated melanopsin-expressing retinal ganglion cells. J Neurosci. 2007;27:13468–13480. doi: 10.1523/JNEUROSCI.3626-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Walker MT, Brown RL, Cronin TW, Robinson PR. Photochemistry of retinal chromophore in mouse melanopsin. Proc Natl Acad Sci USA. 2008;105:8861–8865. doi: 10.1073/pnas.0711397105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hatori M, Le H, Vollmers C, Keding SR, Tanaka N, Buch T, Waisman A, Schmedt C, Jegla T, Panda S. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS ONE. 2008;3:e2451. doi: 10.1371/journal.pone.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Do MT, Kang SH, Xue T, Zhong H, Liao HW, Bergles DE, Yau KW. Photon capture and signalling by melanopsin retinal ganglion cells. Nature. 2009;457:281–287. doi: 10.1038/nature07682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rossant J, McMahon A. “Cre”-ating mouse mutants-a meeting review on conditional mouse genetics. Genes Dev. 1999;13:142–145. doi: 10.1101/gad.13.2.142. [DOI] [PubMed] [Google Scholar]
- 98.Warren EJ, Allen CN, Brown RL, Robinson DW. Intrinsic light responses of retinal ganglion cells projecting to the circadian system. Eur J Neurosci. 2003;17:1727–1735. doi: 10.1046/j.1460-9568.2003.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Graham DM, Wong KY, Shapiro P, Frederick C, Pattabiraman K, Berson DM. Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J Neurophysiol. 2008;99:2522–2532. doi: 10.1152/jn.01066.2007. [DOI] [PubMed] [Google Scholar]
- 100.Brown TM, Lucas RJ. Melanopsin phototransduction: great excitement over a poor catch. Curr Biol. 2009;19:R256–R257. doi: 10.1016/j.cub.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 101.Nikonov SS, Kholodenko R, Lem J, Pugh EN., Jr Physiological features of the S- and M-cone photoreceptors of wild-type mice from single-cell recordings. J Gen Physiol. 2006;127:359–374. doi: 10.1085/jgp.200609490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Raport CJ, Lem J, Makino C, Chen CK, Fitch CL, Hobson A, Baylor D, Simon MI, Hurley JB. Downregulation of cGMP phosphodiesterase induced by expression of GTPase-deficient cone transducin in mouse rod photoreceptors. Invest Ophthalmol Vis Sci. 1994;35:2932–2947. [PubMed] [Google Scholar]
- 103.Neumann T, Ziegler C, Blau A. Multielectrode array recordings reveal physiological diversity of intrinsically photosensitive retinal ganglion cells in the chick embryo. Brain Res. 2008;1207:120–127. doi: 10.1016/j.brainres.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 104.O’Brien BJ, Isayama T, Richardson R, Berson DM. Intrinsic physiological properties of cat retinal ganglion cells. J Physiol. 2002;538:787–802. doi: 10.1113/jphysiol.2001.013009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Terakita A. The opsins. Genome Biol. 2005;6:213. doi: 10.1186/gb-2005-6-3-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koyanagi M, Takano K, Tsukamoto H, Ohtsu K, Tokunaga F, Terakita A. Jellyfish vision starts with cAMP signaling mediated by opsin-G(s) cascade. Proc Natl Acad Sci USA. 2008;105:15576–15580. doi: 10.1073/pnas.0806215105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koyanagi M, Kubokawa K, Tsukamoto H, Shichida Y, Terakita A. Cephalochordate melanopsin: evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr Biol. 2005;15:1065–1069. doi: 10.1016/j.cub.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 108.Fu Y, Zhong H, Wang MH, Luo DG, Liao HW, Maeda H, Hattar S, Frishman LJ, Yau KW. Intrinsically photosensitive retinal ganglion cells detect light with a vitamin A-based photopigment, melanopsin. Proc Natl Acad Sci USA. 2005;102:10339–10344. doi: 10.1073/pnas.0501866102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Isoldi MC, Rollag MD, Castrucci AM, Provencio I. Rhabdomeric phototransduction initiated by the vertebrate photopigment melanopsin. Proc Natl Acad Sci USA. 2005;102:1217–1221. doi: 10.1073/pnas.0409252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sekaran S, Lall GS, Ralphs KL, Wolstenholme AJ, Lucas RJ, Foster RG, Hankins MW. 2-Aminoethoxydiphenylborane is an acute inhibitor of directly photosensitive retinal ganglion cell activity in vitro and in vivo. J Neurosci. 2007;27:3981–3986. doi: 10.1523/JNEUROSCI.4716-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Warren EJ, Allen CN, Brown RL, Robinson DW. The light-activated signaling pathway in SCN-projecting rat retinal ganglion cells. Eur J Neurosci. 2006;23:2477–2487. doi: 10.1111/j.1460-9568.2006.04777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Contin MA, Verra DM, Salvador GA, Ilincheta MG, Giusto NM, Guido ME (2009) Intrinsically photoreceptive retinal ganglion cells: involvement of a phosphoinositide cycle in the phototransduction cascade. IOVS Abstract, 5033/D709
- 113.Contin MA, Verra DM, Guido ME. An invertebrate-like phototransduction cascade mediates light detection in the chicken retinal ganglion cells. FASEB J. 2006;20:2648–2650. doi: 10.1096/fj.06-6133fje. [DOI] [PubMed] [Google Scholar]
- 114.Berson DM. Phototransduction in ganglion-cell photoreceptors. Pflügers Arch. 2007;454:849–855. doi: 10.1007/s00424-007-0242-2. [DOI] [PubMed] [Google Scholar]
- 115.Sekaran S, Lupi D, Jones SL, Sheely CJ, Hattar S, Yau KW, Lucas RJ, Foster RG, Hankins MW. Melanopsin-dependent photoreception provides earliest light detection in the mammalian retina. Curr Biol. 2005;15:1099–1107. doi: 10.1016/j.cub.2005.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kumbalasiri T, Rollag MD, Isoldi MC, Castrucci AM, Provencio I. Melanopsin triggers the release of internal calcium stores in response to light. Photochem Photobiol. 2007;83:273–279. doi: 10.1562/2006-07-11-RA-964. [DOI] [PubMed] [Google Scholar]
- 117.Hermans E. Biochemical and pharmacological control of the multiplicity of coupling at G-protein-coupled receptors. Pharmacol Ther. 2003;99:25–44. doi: 10.1016/s0163-7258(03)00051-2. [DOI] [PubMed] [Google Scholar]
- 118.Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goz D, Studholme K, Lappi DA, Rollag MD, Provencio I, Morin LP. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS ONE. 2008;3:e3153. doi: 10.1371/journal.pone.0003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tarttelin EE, Bellingham J, Bibb LC, Foster RG, Hankins MW, Gregory-Evans K, Gregory-Evans CY, Wells DJ, Lucas RJ. Expression of opsin genes early in ocular development of humans and mice. Exp Eye Res. 2003;76:393–396. doi: 10.1016/s0014-4835(02)00300-7. [DOI] [PubMed] [Google Scholar]
- 121.Hannibal J, Fahrenkrug J. Melanopsin containing retinal ganglion cells are light responsive from birth. Neuroreport. 2004;15:2317–2320. doi: 10.1097/00001756-200410250-00003. [DOI] [PubMed] [Google Scholar]
- 122.Speh JC, Moore RY. Retinohypothalamic tract development in the hamster and rat. Brain Res Dev Brain Res. 1993;76:171–181. doi: 10.1016/0165-3806(93)90205-o. [DOI] [PubMed] [Google Scholar]
- 123.Lupi D, Sekaran S, Jones SL, Hankins MW, Foster RG. Light-evoked FOS induction within the suprachiasmatic nuclei (SCN) of melanopsin knockout (Opn4−/−) mice: a developmental study. Chronobiol Int. 2006;23:167–179. doi: 10.1080/07420520500545870. [DOI] [PubMed] [Google Scholar]
- 124.Bibb LC, Holt JK, Tarttelin EE, Hodges MD, Gregory-Evans K, Rutherford A, Lucas RJ, Sowden JC, Gregory-Evans CY. Temporal and spatial expression patterns of the CRX transcription factor and its downstream targets. Critical differences during human and mouse eye development. Hum Mol Genet. 2001;10:1571–1579. doi: 10.1093/hmg/10.15.1571. [DOI] [PubMed] [Google Scholar]
- 125.Dowling JE, Sidman RL. Inherited retinal dystrophy in the rat. J Cell Biol. 1962;14:73–109. doi: 10.1083/jcb.14.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fulton AB, Graves AL. Background adaptation in developing rat retina: an electroretinographic study. Vision Res. 1980;20:819–826. doi: 10.1016/0042-6989(80)90062-0. [DOI] [PubMed] [Google Scholar]
- 127.Bakall B, Marmorstein LY, Hoppe G, Peachey NS, Wadelius C, Marmorstein AD. Expression and localization of bestrophin during normal mouse development. Invest Ophthalmol Vis Sci. 2003;44:3622–3628. doi: 10.1167/iovs.03-0030. [DOI] [PubMed] [Google Scholar]
- 128.Omura Y, Oguri M. Early development of the pineal photoreceptors prior to the retinal differentiation in the embryonic rainbow-trout, Oncorhynchus mykiss (Teleostei) Arch Histol Cytol. 1993;56:283–291. doi: 10.1679/aohc.56.283. [DOI] [PubMed] [Google Scholar]
- 129.Roberts A. Pineal eye and behavior in Xenopus tadpoles. Nature. 1978;273:774–775. doi: 10.1038/273774a0. [DOI] [PubMed] [Google Scholar]
- 130.Young RW. Cell death during differentiation of the retina in the mouse. J Comp Neurol. 1984;229:362–373. doi: 10.1002/cne.902290307. [DOI] [PubMed] [Google Scholar]
- 131.Ruggiero L, Allen CN, Lane Brown R, Robinson DW. The development of melanopsin-containing retinal ganglion cells in mice with early retinal degeneration. Eur J Neurosci. 2009;29:359–367. doi: 10.1111/j.1460-9568.2008.06589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Badea TC, Cahill H, Ecker J, Hattar S, Nathans J. Distinct roles of transcription factors brn3a and brn3b in controlling the development, morphology, and function of retinal ganglion cells. Neuron. 2009;61:852–864. doi: 10.1016/j.neuron.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lythgoe JN. The ecology of vision. Oxford: Clarendon; 1979. [Google Scholar]
- 134.Provencio I, Foster RG. Circadian rhythms in mice can be regulated by photoreceptors with cone-like characteristics. Brain Res. 1995;694:183–190. doi: 10.1016/0006-8993(95)00694-l. [DOI] [PubMed] [Google Scholar]
- 135.Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47:946–954. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mure LS, Cornut PL, Rieux C, Drouyer E, Denis P, Gronfier C, Cooper HM. Melanopsin bistability: a fly’s eye technology in the human retina. PLoS One. 2009;4:e5991. doi: 10.1371/journal.pone.0005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lem J, Krasnoperova NV, Calvert PD, Kosaras B, Cameron DA, Nicolo M, Makino CL, Sidman RL. Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc Natl Acad Sci USA. 1999;96:736–741. doi: 10.1073/pnas.96.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Johnson RL, Grant KB, Zankel TC, Boehm MF, Merbs SL, Nathans J, Nakanishi K. Cloning and expression of goldfish opsin sequences. Biochemistry. 1993;32:208–214. doi: 10.1021/bi00052a027. [DOI] [PubMed] [Google Scholar]
- 140.Yokoyama S. Molecular evolution of vertebrate visual pigments. Prog Retin Eye Res. 2000;19:385–419. doi: 10.1016/s1350-9462(00)00002-1. [DOI] [PubMed] [Google Scholar]
- 141.Reeves PJ, Thurmond RL, Khorana HG. Structure and function in rhodopsin: high level expression of a synthetic bovine opsin gene and its mutants in stable mammalian cell lines. Proc Natl Acad Sci USA. 1996;93:11487–11492. doi: 10.1073/pnas.93.21.11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Torii M, Kojima D, Okano T, Nakamura A, Terakita A, Shichida Y, Wada A, Fukada Y. Two isoforms of chicken melanopsins show blue light sensitivity. FEBS Lett. 2007;581:5327–5331. doi: 10.1016/j.febslet.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 143.Lucas RJ. Chromophore regeneration: melanopsin does its own thing. Proc Natl Acad Sci USA. 2006;103:10153–10154. doi: 10.1073/pnas.0603955103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mure LS, Rieux C, Hattar S, Cooper HM. Melanopsin-dependent nonvisual responses: evidence for photopigment bistability in vivo. J Biol Rhythms. 2007;22:411–424. doi: 10.1177/0748730407306043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mawad K, Van Gelder RN. Absence of long-wavelength photic potentiation of murine intrinsically photosensitive retinal ganglion cell firing in vitro. J Biol Rhythms. 2008;23:387–391. doi: 10.1177/0748730408323063. [DOI] [PubMed] [Google Scholar]
- 146.Bellingham J, Whitmore D, Philp AR, Wells DJ, Foster RG. Zebrafish melanopsin: isolation, tissue localisation and phylogenetic position. Brain Res Mol Brain Res. 2002;107:128–136. doi: 10.1016/s0169-328x(02)00454-0. [DOI] [PubMed] [Google Scholar]
- 147.Frigato E, Vallone D, Bertolucci C, Foulkes NS. Isolation and characterization of melanopsin and pinopsin expression within photoreceptive sites of reptiles. Naturwissenschaften. 2006;93:379–385. doi: 10.1007/s00114-006-0119-9. [DOI] [PubMed] [Google Scholar]
- 148.Chaurasia SS, Rollag MD, Jiang G, Hayes WP, Haque R, Natesan A, Zatz M, Tosini G, Liu C, Korf HW, Iuvone PM, Provencio I. Molecular cloning, localization and circadian expression of chicken melanopsin (Opn4): differential regulation of expression in pineal and retinal cell types. J Neurochem. 2005;92:158–170. doi: 10.1111/j.1471-4159.2004.02874.x. [DOI] [PubMed] [Google Scholar]
- 149.Drivenes O, Soviknes AM, Ebbesson LO, Fjose A, Seo HC, Helvik JV. Isolation and characterization of two teleost melanopsin genes and their differential expression within the inner retina and brain. J Comp Neurol. 2003;456:84–93. doi: 10.1002/cne.10523. [DOI] [PubMed] [Google Scholar]
- 150.Bellingham J, Chaurasia SS, Melyan Z, Liu C, Cameron MA, Tarttelin EE, Iuvone PM, Hankins MW, Tosini G, Lucas RJ. Evolution of melanopsin photoreceptors: discovery and characterization of a new melanopsin in nonmammalian vertebrates. PLoS Biol. 2006;4:e254. doi: 10.1371/journal.pbio.0040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pires SS, Shand J, Bellingham J, Arrese C, Turton M, Peirson S, Foster RG, Halford S. Isolation and characterization of melanopsin (Opn4) from the Australian marsupial Sminthopsis crassicaudata (fat-tailed dunnart) Proc Biol Sci. 2007;274:2791–2799. doi: 10.1098/rspb.2007.0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Menaker M, Tosini G. The evolution of vertebrate circadian systems. In: Honma K, Honma S, editors. Sixth Sapporo symposium on biological rhythms: circadian organization and oscillatory coupling. Sapporo: Hokkaido University Press; 1996. pp. 39–52. [Google Scholar]