Abstract
Defensins are a major family of antimicrobial peptides found throughout the phylogenetic tree. From the spider species: Cupiennius salei, Phoneutria reidyi, Polybetes pythagoricus, Tegenaria atrica, and Meta menardi, defensins belonging to the ‘ancestral’ class of invertebrate defensins were cloned and sequenced. The deduced amino acid sequences contain the characteristic six cysteines of this class of defensins and reveal precursors of 60 or 61 amino acid residues. The mature peptides consist of 37 amino acid residues, showing up to 70% identities with tick and scorpion defensins. In C. salei, defensin mRNA was found to be constitutively expressed in hemocytes, ovaries, subesophageal nerve mass, hepatopancreas, and muscle tissue. This is the first report presenting and comparing antimicrobial peptides belonging to the family of defensins from spiders.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-010-0354-2) contains supplementary material, which is available to authorized users.
Keywords: Spider defensins, Cupiennius salei, Phoneutria reidyi, Polybetes pythagoricus, Tegenaria atrica, Meta menardi, Tissue expression
Introduction
In a world full of microorganisms, many of them pathogenic, defense mechanisms for protection against such pathogens are indispensable. Higher organisms have developed different immune systems and mechanisms to control and eliminate pathogenic invaders from their systems. Arthropods lack the highly sophisticated adaptive immune system of vertebrates. Instead, they have to rely solely on their innate immune system to fight invading microorganisms. The innate immune system consists of cellular as well as humoral responses. Cellular responses include phagocytosis, nodulation, and encapsulation. Humoral responses include proteolytic cascades leading to melanization of invaders or hemolymph coagulation, as well as the production of host defense peptides and killing factors. These killing factors can be reactive oxygen species or reactive nitrogen intermediates—or antimicrobial peptides (AMPs) [1].
AMPs are produced throughout the phylogenetic tree and play a key role in innate immunity [2]. They are highly diverse in sequence and structure, but generally they are small (≤10 kDa), cationic, and amphipathic. Based on structural features, they can be classified into several groups [3]. The most widespread of these groups are the open-ended cyclic cysteine-rich peptides, which have been characterized from plants, invertebrates, and vertebrates. This group can again be divided structurally into three subgroups: (1) peptides adopting β-sheet structures, (2) peptides adopting a β-hairpin-like fold, and (3) peptides exhibiting the cysteine-stabilized αβ (CSαβ) motive. Mammalian defensins belong to the first subgroup, while the second subgroup contains tachyplesin and polyphemusin from horseshoe crabs, androctonin from scorpions, and gomesin from spiders, as well as porcine protegrins and bovine lactoferricin B, and an AMP from the plant Impatiens balsamina. Finally, the third subgroup includes the invertebrate defensins [3–5].
Invertebrate defensins share commonalities on three different levels: sequence identity, 3D-structure, and bioactivity [6]. On the basis of sequence identities of the mature peptides, at least two classes can be distinguished [7]. The larger first class includes defensins from neopteran insects, with sequence identities within this class ranging from 35 to 92%. The second class, which is often referred to as the ‘ancestral’ class, includes defensins from paleopteran insects, arachnids, myriapoda, and mollusks. In this class, identities reach 56–68% [6]. However, the two classes share ≤30% identities, and many invertebrate defensins cannot be classified into either of these two classes [7]. The majority of invertebrate defensins are mainly active against Gram-positive bacteria, with a few exceptions being active against some Gram-negative bacteria and fungi [8]. The structures of some invertebrate defensins and plectasin, a defensin from a fungus, but structurally also belonging to the invertebrate defensins, have been identified, and are all comprised of an α-helix and two antiparallel β-strands stabilized by three or four disulfide bridges, constituting the common CSαβ motive [9–12].
Information on the spider immune system is limited to a few recently published studies on one mygalomorph species, Acanthoscurria gomesiana. Two different AMPs were isolated from naive hemocytes of this spider, gomesin and acanthoscurrin. Gomesin is a small, 18-residue AMP active against Gram-positive and Gram-negative bacteria and fungi, forming two disulphide bridges and adopting a β-hairpin-like structure [13, 14]. Acanthoscurrin is a glycine-rich AMP active against Gram-negative bacteria and yeast [15]. Both are produced constitutively and stored in hemocyte granules, from where they can be released into the hemolymph upon infection. This mechanism is similar to the AMP production in hemimetabolous insects and other invertebrates like horseshoe crabs, shrimps, and mussels [16–18], while AMP production in holometabolous insects is only induced after infection [19, 20].
In contrast to other arachnids, like scorpions and ticks, no AMPs belonging to the group of the invertebrate defensins have so far been reported from spiders, with the exception of an Argiope sp. sequence appearing in a patent (granted patent US 6777592). In this study, we present the sequences of defensins belonging to the ‘ancestral’ class of invertebrate defensins from the five different spider species: Cupiennius salei, Polybetes phytagoricus, Meta menardi, Tegenaria atrica, and Phoneutria reidyi. Furthermore, the tissue distribution and the partial gene-structure of the defensin of Cupiennius salei are presented.
Materials and methods
Animals
Mature females of the wandering spider C. salei Keyserling (Ctenidae) and the huntsman spider P. pythagoricus (Sparassidae) were used for the experiments. C. salei were obtained from a permanent breeding line that has been maintained for several years. P. pythagoricus were obtained from the first generation of a newly established breeding line. Armed spiders P. reidyi (Ctenidae) were raised from a cocoon and hemocytes were isolated from male and female spiders. The spiders were housed separately in 2-l glass jars at a room temperature of 23°C and with a light:dark regime of 12:12 h. Twelve female and male house spiders T. atrica (Agelenidae) were collected in buildings in the surroundings of Bern, Switzerland, and 18 European cave spiders M. menardi (Tetragnathidae) of both sexes were collected in a cave near Vallorbe, Switzerland.
Hemolymph collection
For hemolymph collection, spiders were anesthetized with CO2 and the hemolymph was collected by cardiac puncture with a 27G needle on a 1-ml syringe. Collection of the hemolymph was performed in the presence of sodium citrate buffer, pH 4.6, to prevent coagulation [21]. The hemocytes were separated from the plasma by centrifugation at 800g for 10 min at 4°C. Hemocytes were washed once with sodium citrate buffer and again centrifuged at 800g for 10 min at 4°C.
RNA isolation
Total RNA was isolated from hemocytes using the Absolutely RNA® Miniprep Kit (Stratagene, Switzerland) or the RNeasy® Mini Kit (Qiagen, Switzerland). RNA isolation was done following the instructions of the manufacturer in the manual of the kit, including the optional on-column DNA digestion.
cDNA synthesis
3′- and 5′-RACE-ready cDNAs were synthesized from 0.25–1 μg of the isolated total RNA of all spider species using the BD SMART™ RACE cDNA Amplification Kit (BD Biosciences Clontech, France). This system incorporates an anchor sequence (“BD SMART sequence”) either at the 3′- or the 5′- end of the generated cDNA, which can be used as primer binding sites for subsequent RACE PCRs.
3′- and 5′-RACE PCRs
3′- and 5′-RACE PCRs were run with the synthesized cDNAs from the different spider species as templates. For PCR conditions and primers used for these reactions, see Electronic supplementary material (ESM) and Table S1. Combining the results from 3′- and 5′-RACE PCRs yielded the full length cDNA sequences of the defensins.
Isolation of genomic DNA
Genomic DNA was isolated from hemocytes of two adult female C. salei, using the DNeasy® Blood and Tissue Kit (Qiagen). Isolation was carried out following the manufacturer’s protocol for cultured cells. PCRs with 450 ng of this DNA as template and different primer combinations were run with the same cycling conditions: 94°C for 3 min, followed by 45 cycles of 94°C for 30 s, 51°C for 30 s, 72°C for 2 min, and a final elongation at 72°C for 7 min. PCR products were analyzed and compared to cDNA products on a 2% agarose gel containing 0.5 ng ethidium bromide/ml. The PCR products were then purified using the MinElute® PCR Purification Kit.
Cloning and sequencing
Concentrations of purified PCR products were measured on a GeneQuant photometer (Amersham Pharmacia, GE Sweden) and then cloned into pDrive vector using the QIAGEN PCR Cloning Kit, at a vector:insert ratio of 1:8. The products were sequenced using the BigDye® Terminator v3.1 Cycle Sequencing Kit, sequences were separated on an ABI3130XL automated sequencer using POP7 polymer on a 50-cm array and acquired using sequence detection software v.3.0 (all from Applied Biosystems).
Sequence analysis
DNA sequences were translated into protein sequences using the BioEdit v7.0.8 software [22], and putative signal peptides were analyzed using the prediction server SignalP 3.0 [23] (http://www.cbs.dtu.dk/services/SignalP). Amino acid sequences were aligned with previously published sequences from other taxa with Clustal W program version 1.81 [24] (http://www.ch.embnet.org/software/ClustalW.html).
A neighbor-joining (NJ) tree [25] of the aligned amino acid sequences was generated based on Jones-Taylor-Thornton matrix protein distances [26],which were calculated in Phylip version 3.65 [27]. In order to assess the statistical support for individual nodes of the NJ tree, 1,000 bootstrap pseudo-replicates of the protein data were generated and processed with Phylip.
Tissue expression analysis
Different tissues were extracted from CO2-anesthetized C. salei. Total RNA was extracted from these tissues using the RNeasy® Mini Kit (Qiagen). Then, 1 μg of total RNA was reverse-transcribed into cDNA using the BD SMART™ RACE cDNA Amplification Kit. The amount of template used for expression analysis was normalized according to the band intensity values obtained with the ribosomal protein S3A transcript as control. Primers used for this control PCR were Lycosa S3A fwd and Lycosa S3A rev (ESM, Table S1), based on the sequence of Lycosa singoriensis S3A (GenBank EU 247153.1) transcript. The primers used for the expression analysis of C. salei defensin were C.salei sig fwd 1 and C.salei def rev 4 (ESM, Table S1). The PCR program was 3 min at 94°C, followed by 33 cycles of 94°C for 30 s, 44°C for 30 s, 72°C for 30 s, and a final elongation at 72°C for 2 min. PCR products were run on a 2% agarose gel containing 0.5 ng ethidium bromide/ml. Band intensities were quantified using the ImageMaster® VDS 3.0 software (Pharmacia Biotech, Sweden).
Results
Hemolymph, hemocytes and RNA yields
Hemolymph yields from the different spiders differed according to their body size. Average yield for C. salei was ~300 μl/spider, for P. pythagoricus ~200 μl/spider, for P. reidyi ~270 μl/spider, and for T. atrica ~35 μl/spider. Amounts for M. menardi were in the range of ~10 μl/spider. The average yields of hemocytes isolated from these hemolymphs were ~1.3×107 cells/spider for C. salei and P. pythagoricus, and ~5×106 cells/spider for T. atrica, an average hemocyte weighing about 1 ng. The amounts of isolated RNA were 29.5 μg from the pooled hemocytes of 11 C. salei, 3.3 μg from the pooled hemocytes of 14 P. pythagoricus, 4.8 μg from the pooled hemocytes of 14 P. reidyi, 6.6 μg from the pooled hemocytes of 12 T. atrica, and 6.8 μg from the pooled hemocytes of 18 M. menardi.
C. salei defensin sequence characterization, tissue expression and partial gene organization
The N-terminal sequence of putative defensin from C. salei hemocyte cDNA, including a signal peptide, and the 5′-UTR were obtained by 5′- RACE PCR using primers designed based on a spider defensin sequence (patent US 6777592) and its similarities with scorpion and tick defensins. Based on this sequence, specific primers were designed and used in 3′-RACE PCR, resulting in the C-terminal sequence of the putative defensin, followed by the 3′-UTR including a polyadenylation signal and the poly-A tail. Combining the sequences of the two RACE PCRs yielded the full-length transcript sequence of 356 nucleotides. This sequence contains a 5′-UTR of 43 bp, followed by an ORF of 186 bp and a 3′-UTR of 102 bp, and finally the poly-A tail (Fig. 1). The deduced amino acid sequence of the ORF starts with a signal peptide of 24 amino acid residues, the cleavage site for the signal peptidase most likely being located after the alanine residue preceding the glycine in position 25, as predicted by SignalP 3.0 software. The signal peptide is followed by the putative mature defensin of 37 amino acid residues. The sequence contains the six conserved cysteines of invertebrate defensins.
Fig. 1.
C. salei defensin cDNA sequence. The deduced amino acid sequence is presented under the nucleotide sequence. The mature peptide sequence is underlined, the nucleotide sequence of the mature peptide is in bold. Asterisks mark the stop codon. The polyadenylation signal is in bold italics
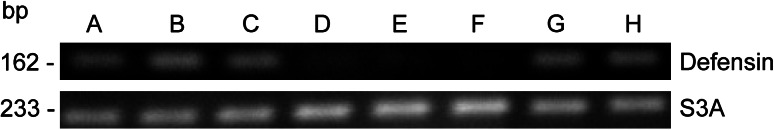
The expression of defensin mRNA in various tissues of C. salei was determined by semi-quantitative RT–PCR, using tissue cDNAs normalized against the ribosomal protein S3A transcript. Expression of defensin mRNA was detected in hemocytes, ovaries, subesophageal nerve mass, hepatopancreas, and muscle tissue (Fig. 2). Defensin-RNA expression in all the tissues mentioned was about the same, while only a very weak expression was detected in the venom glands, silk glands and the heart tube.
Fig. 2.
RT-PCR analysis of defensin-gene expression in different tissues. Expression was assayed in ovaries (A), subesophageal nerve mass (B), hepatopancreas (C), venom glands (D), heart (E), silk glands (F), hemocytes (G), and muscle (H)
Using different specific primer combinations with primers located in the 5′-UTR, the signal peptide, mature peptide, and the 3′-UTR, genomic PCR was performed. Only PCR products starting from the beginning of the putative mature peptide could be obtained. These products had the same size as products obtained from cDNA. Sequencing revealed that there are no introns in that region of the gene (ESM, Fig. S1). We failed to obtain the gene sequence upstream of the mature peptide despite trying a number of different primer combinations, PCR conditions and additives, and polymerases, which all worked with cDNA. This might suggest that there is at least one intron in the gene upstream of the mature peptide in the signal peptide sequence which seems to be difficult to amplify.
P. pythagoricus,T. atrica, P. reidyi and M. menardi defensin sequence characterization
With different primers, the cDNAs of the different spider species were examined closely for defensin sequences by 3′- and 5′-RACE PCRs. Combining the results from the RACE PCRs yielded the full mRNA defensin sequences from P. pythagoricus, T. atrica, and P. reidyi (Fig. 3a–c). Due to lack of material from M. menardi, only 3′-RACE PCRs were run, yielding only the C-terminal part of a defensin (Fig. 3d), but this C-terminal part clearly groups with the C-terminal parts of the other defensins (Fig. 4). The ORFs of all defensin mRNAs are coding for putative signal peptides of 24 amino acid residues followed directly by the mature peptides of 37 amino acid residues. The only exception to this is the T. atrica signal peptide, which consists of only 23 amino acid residues instead of 24. The mRNA characterizations of all identified defensins are summarized in Table 1, showing that beside the mentioned exception, differences are only found in the lengths of the 5′- and 3′- UTRs.
Fig. 3.
Spider defensin cDNA sequences. The deduced amino acid sequences are presented under the nucleotide sequences. The mature peptide sequences are underlined, the nucleotide sequence of the mature peptides are in bold. Asterisks mark the stop codons. The polyadenylation signals are in bold italics. a P. pythagoricus defensin, b T. atrica defensin, c P. reidyi defensin, d partial M. menardi defensin
Fig. 4.
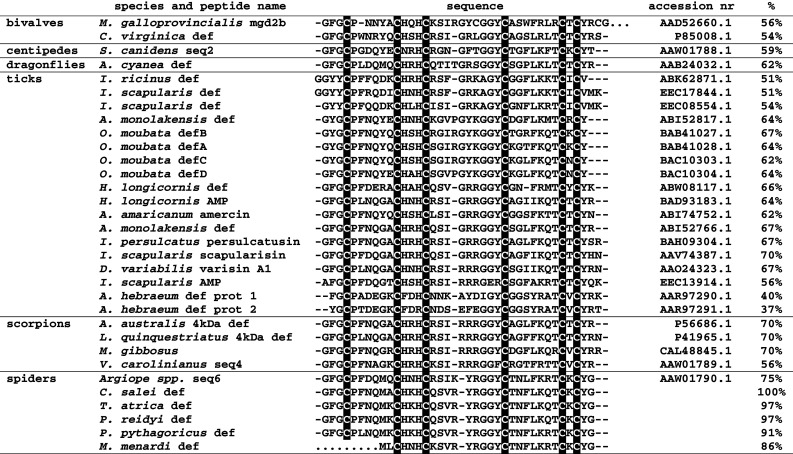
Alignment of spider defensins with defensins belonging to the ‘ancestral’ group of invertebrate defensins. The conserved cysteine residues are highlighted in black. The characteristic cysteine bonding pattern of arthropod defensins is C1-C4, C2-C5, C3-C6 (C1-C5, C2-C6, C3-C7, C4-C8 for mgd2b, which possesses two additional cysteines). The mgd2b sequence is shown incomplete, the M. menardi sequence was not completely solved (indicated by the dots at the end and the beginning of the sequences). % identities are given compared to C. salei defensin
Table 1.
Defensin mRNA characterization
| Spider species | 5′- UTR | ORF | 3′- UTR | Full length mRNAa | |
|---|---|---|---|---|---|
| Signal peptide | Mature peptide | ||||
| C. salei | 43 | 72 | 111 | 105 | 356 |
| P. pythagoricus | 57 | 72 | 111 | 221 | 487 |
| T. atrica | 55 | 69 | 111 | 138 | 398 |
| P. reidyi | 76 | 72 | 111 | 136 | 420 |
| M. menardi b | >88 | 66 | >179 | ||
All numbers are presented as base-pairs (bp)
aFull length mRNA is calculated including a polyA-tail of 25 adenines
bThe M. menardi sequence was not solved completely due to lack of material
All five indentified putative spider defensins contain the six conserved cysteines of the arthropod defensins and align with the members of the ‘ancestral’ class of invertebrate defensins (Fig. 4). Among the mature spider defensins, including the Argiope sequence from the patent, similarities were 72–84% at the nucleotide level and 75–100% at the amino acid level. Similarities for the signal peptide were 21–83% at the nucleotide level and 52–79% at the amino acid level (data not shown).
Discussion
We report here for the first time the cloning of AMPs belonging to the ancestral class of invertebrate defensins from different spider species.
With primers designed from known invertebrate defensins, we searched for defensin sequences in C. salei hemocyte cDNA because secretion of AMPs by hemocytes is a major defense mechanism in spiders [28]. To investigate whether the identified C. salei defensin is expressed exclusively in hemocytes or whether other tissues are also involved in the production of this AMP, cDNA from different tissues was analyzed with specific primers for defensin expression. C. salei defensin was found to be constitutively expressed in numerous tissues in uninfected spiders, namely hemocytes, ovaries, subesophageal nerve mass, hepatopancreas, and muscle tissue (Fig. 2). This suggests that defensin plays an important role in host defense not only in the hemolymph but also in the different tissues. In contrast to the C. salei defensin, gomesin and acanthoscurrin, two structurally different AMPs isolated from spider hemocytes, are exclusively expressed in hemocytes [15, 29]. In venom glands, silk glands, and the heart tube, only a very weak defensin expression could be observed, possibly originating from hemocytes infiltrating these tissues. The proposed absence of defensin expression in the heart tube tissue is justified by the fact that nearly all circulating mature hemocytes were removed by cardiac puncture before tissue extraction. In the case of the venom glands, these glands are protected by the presence of different highly cationic and α-helical cytolytic acting peptides [30], which may play a dual role in the venom, being involved in prey paralysis and killing [31] as well as protecting the venom glands against microbial invaders. The silk glands must be protected by different AMPs, or a different expression regulation, possibly inducing defensin expression only after infection. Finally, we can not exclude that the defensin signals detected in tissues other than hemocytes partially originate from hemocytes invading these tissues, as hemocytes are able to migrate to infection sites [28]. But the comparable signal intensities in hemocytes and these tissues make this possibility rather unlikely, as only a very small subset of the cells of a certain tissue would be hemocytes, leading to a weaker signal than from pure hemocytes.
The tissue expression of some tick defensins shows similarities to the expression of C. salei defensin, being expressed in different tissues [32–35], while other tick defensins seem to be tissue-specific [36–38]. In mollusks, which also express defensins belonging to the invertebrate defensin group, some of the defensins are also expressed in a single tissue [39], while others are found in several tissues [40, 41].
Differences in defensins can be found not only in their tissue expression profiles but also in their gene structure. While we could not solve the full gene structure of the C. salei defensin, we were able to show that the sequence of the mature peptide and the 3′-UTR do not contain any introns (ESM, Fig. S1). This is in agreement with the defensins from ticks, scorpions, and mollusks, as well as defensin genes from insects. The gene structure upstream of the mature peptide could not be identified with our methods, whereas the same conditions worked out well with cDNA. This finding hints at one or more, difficult to PCR, intron(s) upstream of the mature peptide. Introns upstream of the mature peptide can be found in defensin genes of scorpions and mollusks, as well as in some ticks [6, 7]. These tick genes also exhibit an additional intron between the propetide and the mature peptide, whereas other tick defensins have a more simple gene structure, not containing any introns in the whole gene [32]. Insect defensins also exhibit different gene structures [6, 7]. Our results concerning C. salei hint at a similar defensin gene structure as found in scorpions and mollusks [6, 42, 43], with most likely a single intron localized in the C-terminal part of the signal peptide.
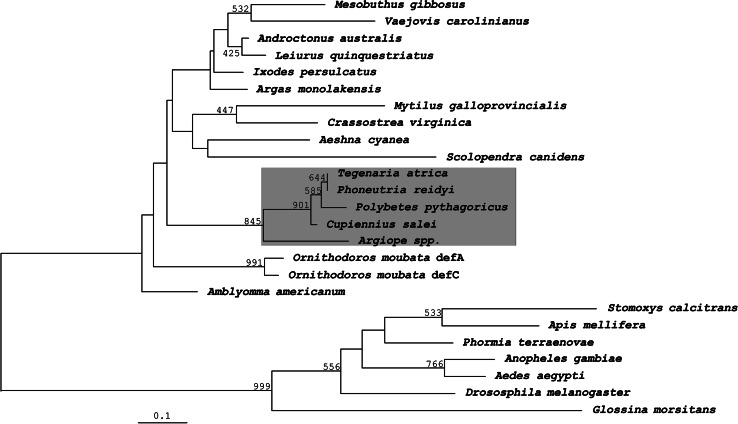
In a phylogenetic tree, invertebrate defensins are split into two distinct classes, one consisting of defensins from neopteran insects, the other being the ‘ancestral’ class with defensins from ticks, scorpions, centipedes, dragonflies and mollusks. The spider defensins clustered together with the ‘ancestral’ class (Fig. 5), with similarities to defensins from neopteran insects being only 18–40%. Among the different spiders, the similarities of the mature putative defensins were from 72 to 84% at the nucleotide level and 75–100% at the peptide level, the defensins from T. atrica and P. reidyi being identical at the peptide level (Fig. 4). Interestingly, the defensins from C. salei and P. reidyi do not cluster together as close as expected based on the current view on the phylogenetic relationship, but this is in agreement with an analysis of mitochondrial 16S rDNA of different spider species where the authors suggest a revision of the family Ctenidae [44]. The greatest similarities with the spider defensins were found in scorpions and ticks, both also belonging to chelicerates. Compared to scorpions, similarities for the mature defensins were between 30 and 66% on the nucleotide and between 51 and 70% on the peptide level, while for ticks the similarities ranged from 9 to 70% on the nucleotide and from 34 to 70% on the peptide level (Fig. 4), the minimal values being due to a fairly high diversity within the tick defensins. The similarities to the mature defensins of the phylogenetically more distant dragonfly Aeshna cyanea and the centipede Scolopendra canidens are lower, but still range between 55 and 64% for A. cyanea, and between 55 and 59% for S. canidens. Finally, for mollusks, the phylogenetically most distant invertebrates expressing defensins belonging to the ‘ancestral’ class, the similarities for the mature defensins were between 7 and 64% on the nucleotide and between 31 and 56% on the peptide level. It is worth mentioning that the incomplete M. menardi defensin sequence, missing the highly conserved N-terminus (Fig. 4), is always responsible for the lowest similarity. If this sequence was excluded, the minimal values would be even higher. Interestingly, no AMP belonging to the group of invertebrate defensins was discovered in an EST cDNA library from hemocytes of the mygalomorph spider A. gomesiana [45], while defensins isolated from plants and fungi sometimes show quite high similarities to the defensins of the ‘ancestral’ class, implying a possible common ancestor of these defensins. Plectasin, isolated from the fungus Pseudoplectania nigrella [12], and defensins isolated from the white spruce Picea glauca (CS419493.1) and the fungus Aspergillus terreus (XM_001209108.1) exhibit high similarities to the mature spider defensins on the peptide level, ranging from 43 to 64%.
Fig. 5.
Phylogenetic (NJ) tree of invertebrate defensins. The NJ tree was constructed based on amino acid sequences. Bootstrap values based on 1,000 pseudoreplicates are indicated at the left of the respective nodes. Only values >400 are shown. Spider defensins are highlighted in grey. Only complete sequences were used in the alignment, M. menardi partial sequence was excluded. The accession numbers of the ‘ancestral’ class are the same as in Fig. 4. The accession numbers of the ‘insect’ class are: S. calcitrans: O16137; A. mellifera: C55392; P. terraenovae: S12558; A. gambiae: Q17027; A. aegypti: P81603; D. melanogaster: P36192; G. morsitans: AAL34112
This is to the best of our knowledge the first report on defensins cloned from different spiders. A first MS-based screening of HPLC separated hemocyte lysats of C. salei for defensin failed, so all data presented are for a putative peptide. In the tick I. scapularis, a defensin sequence was also only identified on mRNA level, but not detected on peptide level [32]. It might be that the C. salei defensin is constitutively expressed only in very low, and on peptide level not detectable, amounts in the identified tissues, with the expression being up-regulated upon infection. This would be in contrast to other spider AMPs, which are expressed constitutively in detectable amounts and stored in hemocyte granules [14, 15]. These AMPs are then released into the hemolymph upon infection, but their tissue expression is limited to hemocytes. Since the tissue expression profile of defensin is so different from that of the other spider AMPs, the expression regulation and/or activation might also be different. Research to identify C. salei defensin in the hemolymph/hemocytes on the peptide level and its regulation is still ongoing. In addition, the complete gene structure is still under investigation, as it might help to shed light on the evolution of invertebrate defensins.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1 Partial C. salei defensin gene structure. PCR products of hemocyte cDNA and genomic DNA with defensin-specific primers. The 50-bp molecular weight marker is marked with MW; band sizes are in bp. (TIFF 4753 kb)
Acknowledgments
We thank Dr. C. Kropf for kindly providing M. menardi, Prof. Dr. J. Schaller for MS measurements, Dr. D. Destoumieux-Garzón and Dr. H. Murray for critical comments on the manuscript, and the Swiss National Science Foundation for funding.
References
- 1.Jiravanichpaisal P, Lee BL, Söderhäll K. Cell-mediated immunity in arthropods: hematopoiesis, coagulation, melanization and opsonization. Immunobiology. 2006;211:213–236. doi: 10.1016/j.imbio.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 3.Bulet P, Stöcklin R, Menin L. Anti-microbial peptides: from invertebrates to vertebrates. Immunol Rev. 2004;198:169–184. doi: 10.1111/j.0105-2896.2004.0124.x. [DOI] [PubMed] [Google Scholar]
- 4.Hwang PM, Zhou N, Shan X, Arrowsmith CH, Vogel HJ. Three-dimensional solution structure of lactoferricin B, an antimicrobial peptide derived from bovine lactoferrin. Biochemistry. 1998;37:4288–4298. doi: 10.1021/bi972323m. [DOI] [PubMed] [Google Scholar]
- 5.Patel SU, Osborn R, Rees S, Thornton JM. Structural studies of Impatiens balsamina antimicrobial protein (Ib-AMP1) Biochemistry. 1998;37:983–990. doi: 10.1021/bi971747d. [DOI] [PubMed] [Google Scholar]
- 6.Froy O, Gurevitz M. Arthropod and mollusk defensins-evolution by exon-shuffling. Trends Genet. 2003;19:684–687. doi: 10.1016/j.tig.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez de la Vega RC, Possani LD. On the evolution of invertebrate defensins. Trends Genet. 2005;21:330–332. doi: 10.1016/j.tig.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Wong JA, Xia L, Ng TB. A review of defensins of diverse origins. Curr Prot Pept Sci. 2007;5:446–459. doi: 10.2174/138920307782411446. [DOI] [PubMed] [Google Scholar]
- 9.Cornet B, Bonmatin JM, Hetru C, Hoffmann JA, Ptak M, Vovelle F. Refined three-dimensional solution structure of insect defensin A. Structure. 1995;3:435–448. doi: 10.1016/S0969-2126(01)00177-0. [DOI] [PubMed] [Google Scholar]
- 10.Hanzawa H, Shimada I, Kuzuhara T, Komano H, Kohda D, Inagaki F, Natori S, Arata Y. 1H nuclear magnetic resonance study of the solution conformation of an antibacterial protein, sapecin. FEBS Lett. 1990;269:413–420. doi: 10.1016/0014-5793(90)81206-4. [DOI] [PubMed] [Google Scholar]
- 11.Yang YS, Mitta G, Chavanieu A, Calas B, Sanchez JF, Roch P, Aumelas A. Solution structure and activity of the synthetic four-disulfide bond Mediterranean mussel defensin (MGD-1) Biochemistry. 2000;39:14436–14447. doi: 10.1021/bi0011835. [DOI] [PubMed] [Google Scholar]
- 12.Mygind PH, Fischer RL, Schnorr KM, Hansen MT, Sonksen CP, Ludvigsen S, Raventos D, Buskov S, Christensen B, De Maria L, Taboureau O, Yaver D, Elvig-Jorgensen SG, Sorensen MV, Christensen BE, Kjaerulff S, Frimodt-Moller N, Lehrer RI, Zasloff M, Kristensen HH. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature. 2005;437:975–980. doi: 10.1038/nature04051. [DOI] [PubMed] [Google Scholar]
- 13.Mandard N, Bulet P, Caille A, Daffre S, Vovelle F. The solution structure of gomesin, an antimicrobial cysteine-rich peptide from the spider. Eur J Biochem. 2002;269:1190–1198. doi: 10.1046/j.0014-2956.2002.02760.x. [DOI] [PubMed] [Google Scholar]
- 14.Silva PI, Jr, Daffre S, Bulet P. Isolation and characterization of gomesin, an 18-residue cysteine-rich defense peptide from the spider Acanthoscurria gomesiana hemocytes with sequence similarities to horseshoe crab antimicrobial peptides of the tachyplesin family. J Biol Chem. 2000;275:33464–33470. doi: 10.1074/jbc.M001491200. [DOI] [PubMed] [Google Scholar]
- 15.Lorenzini DM, da Silva PI, Fogaça AC, Jr, Bulet P, Daffre S. Acanthoscurrin: a novel glycine-rich antimicrobial peptide constitutively expressed in the hemocytes of the spider Acanthoscurria gomesiana . Dev Comp Immunol. 2003;27:781–791. doi: 10.1016/S0145-305X(03)00058-2. [DOI] [PubMed] [Google Scholar]
- 16.Bachère E, Gueguen Y, Gonzalez M, de Lorgeril J, Garnier J, Romestand B. Insights into the anti-microbial defense of marine invertebrates: the penaeid shrimps and the oyster Crassostrea gigas . Immunol Rev. 2004;198:149–168. doi: 10.1111/j.0105-2896.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- 17.Mitta G, Vandenbulcke F, Noel T, Romestand B, Beauvillain JC, Salzet M, Roch P. Differential distribution and defence involvement of antimicrobial peptides in mussel. J Cell Sci. 2000;113:2759–2769. doi: 10.1242/jcs.113.15.2759. [DOI] [PubMed] [Google Scholar]
- 18.Shigenaga T, Muta T, Toh Y, Tokunaga F, Iwanaga S. Antimicrobial tachyplesin peptide precursor. cDNA cloning and cellular localization in the horseshoe crab (Tachypleus tridentatus) J Biol Chem. 1990;265:21350–21354. [PubMed] [Google Scholar]
- 19.Gillespie JP, Kanost MR, Trenczek T. Biological mediators of insect immunity. Annu Rev Entomol. 1997;42:611–643. doi: 10.1146/annurev.ento.42.1.611. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann JA, Reichhart JM. Drosophila innate immunity: an evolutionary perspective. Nat Immunol. 2002;3:121–126. doi: 10.1038/ni0202-121. [DOI] [PubMed] [Google Scholar]
- 21.Söderhäll K, Smith VJ. Separation of the haemocyte populations of Carcinus maenas and other marine decapods, and prophenoloxidase distribution. Dev Comp Immunol. 1983;7:229–239. doi: 10.1016/0145-305X(83)90004-6. [DOI] [PubMed] [Google Scholar]
- 22.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 23.Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 24.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 26.Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 27.Felsenstein J. PHYLIP, phylogeny inference package (Version 3.2) Cladistics. 1989;5:164–166. [Google Scholar]
- 28.Fukuzawa AH, Vellutini BC, Lorenzini DM, Silva PI, Jr, Mortara RA, da Silva JM, Daffre S. The role of hemocytes in the immunity of the spider Acanthoscurria gomesiana . Dev Comp Immunol. 2008;32:716–725. doi: 10.1016/j.dci.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Lorenzini DM, Fukuzawa AH, da Silva PI, Machado-Santelli G, Jr, Bijovsky AT, Daffre S. Molecular cloning, expression analysis and cellular localization of gomesin, an anti-microbial peptide from hemocytes of the spider Acanthoscurria gomesiana . Insect Biochem Mol Biol. 2003;33:1011–1016. doi: 10.1016/S0965-1748(03)00115-2. [DOI] [PubMed] [Google Scholar]
- 30.Kuhn-Nentwig L, Müller J, Schaller J, Walz A, Dathe M, Nentwig W. Cupiennin 1, a new family of highly basic antimicrobial peptides in the venom of the spider Cupiennius salei (Ctenidae) J Biol Chem. 2002;277:11208–11216. doi: 10.1074/jbc.M111099200. [DOI] [PubMed] [Google Scholar]
- 31.Kuhn-Nentwig L, Trachsel C, Nentwig W. Spider venom and hemolymph-derived cytolytic and antimicrobial peptides. In: Howl J, Jones S, editors. Bioactive Peptides. Boca Raton: CRC Press; 2009. pp. 447–464. [Google Scholar]
- 32.Hynes WL, Ceraul SM, Todd SM, Seguin KC, Sonenshine DE. A defensin-like gene expressed in the black-legged tick, Ixodes scapularis . Med Vet Entomol. 2005;19:339–344. doi: 10.1111/j.1365-2915.2005.00579.x. [DOI] [PubMed] [Google Scholar]
- 33.Nakajima Y, van Naters-Yasui A, Taylor D, Yamakawa M. Antibacterial peptide defensin is involved in midgut immunity of the soft tick, Ornithodoros moubata . Insect Mol Biol. 2002;11:611–618. doi: 10.1046/j.1365-2583.2002.00372.x. [DOI] [PubMed] [Google Scholar]
- 34.Fogaça AC, Lorenzini DM, Kaku LM, Esteves E, Bulet P, Daffre S. Cysteine-rich antimicrobial peptides of the cattle tick Boophilus microplus: isolation, structural characterization and tissue expression profile. Dev Comp Immunol. 2004;28:191–200. doi: 10.1016/j.dci.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Todd SM, Sonenshine DE, Hynes WL. Tissue and life-stage distribution of a defensin gene in the Lone Star tick, Amblyomma americanum . Med Vet Entomol. 2007;21:141–147. doi: 10.1111/j.1365-2915.2007.00682.x. [DOI] [PubMed] [Google Scholar]
- 36.Ceraul SM, Dreher-Lesnick SM, Gillespie JJ, Rahman MS, Azad AF. New tick defensin isoform and antimicrobial gene expression in response to Rickettsia montanensis challenge. Infect Immun. 2007;75:1973–1983. doi: 10.1128/IAI.01815-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou J, Liao M, Ueda M, Gong H, Xuan X, Fujisaki K. Sequence characterization and expression patterns of two defensin-like antimicrobial peptides from the tick Haemaphysalis longicornis . Peptides. 2007;28:1304–1310. doi: 10.1016/j.peptides.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 38.Saito Y, Konnai S, Yamada S, Imamura S, Nishikado H, Ito T, Onuma M, Ohashi K. Identification and characterization of antimicrobial peptide, defensin, in the taiga tick, Ixodes persulcatus . Insect Mol Biol. 2009;18:531–539. doi: 10.1111/j.1365-2583.2009.00897.x. [DOI] [PubMed] [Google Scholar]
- 39.Mitta G, Vandenbulcke F, Hubert F, Roch P. Mussel defensins are synthesised and processed in granulocytes then released into the plasma after bacterial challenge. J Cell Sci. 1999;112:4233–4242. doi: 10.1242/jcs.112.23.4233. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez M, Gueguen Y, Desserre G, de Lorgeril J, Romestand B, Bachère E. Molecular characterization of two isoforms of defensin from hemocytes of the oyster Crassostrea gigas . Dev Comp Immunol. 2007;31:332–339. doi: 10.1016/j.dci.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Gueguen Y, Herpin A, Aumelas A, Garnier J, Fievet J, Escoubas JM, Bulet P, Gonzalez M, Lelong C, Favrel P, Bachère E. Characterization of a defensin from the oyster Crassostrea gigas. Recombinant production, folding, solution structure, antimicrobial activities, and gene expression. J Biol Chem. 2006;281:313–323. doi: 10.1074/jbc.M510850200. [DOI] [PubMed] [Google Scholar]
- 42.Mitta G, Hubert F, Dyrynda EA, Boudry P, Roch P. Mytilin B and MGD2, two antimicrobial peptides of marine mussels: gene structure and expression analysis. Dev Comp Immunol. 2000;24:381–393. doi: 10.1016/S0145-305X(99)00084-1. [DOI] [PubMed] [Google Scholar]
- 43.Rodríguez de la Vega RC, García BI, D’Ambrosio C, Diego-García E, Scaloni A, Possani LD. Antimicrobial peptide induction in the haemolymph of the Mexican scorpion Centruroides limpidus limpidus in response to septic injury. Cell Mol Life Sci. 2004;61:1507–1519. doi: 10.1007/s00018-004-4096-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huber KC, Haider TS, Müller MW, Huber BA, Schweyen RJ, Barth FG. DNA-sequence data indicates the polyphyly of the family Ctenidae (Araneae) J Arachnol. 1993;21:194–201. [Google Scholar]
- 45.Lorenzini DM, da Silva PI, Soares MB, Jr, Arruda P, Setubal J, Daffre S. Discovery of immune-related genes expressed in hemocytes of the tarantula spider Acanthoscurria gomesiana . Dev Comp Immunol. 2006;30:545–556. doi: 10.1016/j.dci.2005.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Partial C. salei defensin gene structure. PCR products of hemocyte cDNA and genomic DNA with defensin-specific primers. The 50-bp molecular weight marker is marked with MW; band sizes are in bp. (TIFF 4753 kb)