Abstract
During the last decade, RNA molecules with regulatory functions on gene expression have benefited from a renewed interest. In bacteria, recent high throughput computational and experimental approaches have led to the discovery that 10–20% of all genes code for RNAs with critical regulatory roles in metabolic, physiological and pathogenic processes. The trans-acting RNAs comprise the noncoding RNAs, RNAs with a short open reading frame and antisense RNAs. Many of these RNAs act through binding to their target mRNAs while others modulate protein activity or target DNA. The cis-acting RNAs include regulatory regions of mRNAs that can respond to various signals. These RNAs often provide the missing link between sensing changing conditions in the environment and fine-tuning the subsequent biological responses. Information on their various functions and modes of action has been well documented for gram-negative bacteria. Here, we summarize the current knowledge of regulatory RNAs in gram-positive bacteria.
Keywords: Regulatory RNAs, Small RNAs, Noncoding RNAs, Riboswitches, CRISPR, Gene expression regulation, Virulence, Gram-positive bacteria
Introduction
Until recently, research on the control of gene expression in bacteria had focused mostly on the regulatory functions of proteins. Besides proteins, RNA molecules have emerged lately as key players in gene regulation in adaptive responses [1–3]. Natural antisense RNAs were first discovered as plasmid-encoded elements that are major regulators of plasmid copy number control (i.e., ColE1 and R1 plasmids) [4, 5]. A couple of years later, the first chromosomally encoded regulatory RNA was revealed to act as an antisense RNA and inhibit the translation of an outer membrane porin in Escherichia coli (i.e., micF RNA/OmpF porin) [6, 7]. With the development of genome-wide searches, more than >80 small RNAs were discovered encoded on the chromosome of Escherichia coli [1]. To date, impressive technical advances such as high throughput computational searches [8, 9], deep-sequencing [10] and tiled microarrays [11, 12] have led to the prediction and/or characterization of numerous novel RNAs in various bacteria. Overall 10–20% of genes in bacteria code for RNAs that are likely to have regulatory functions on gene expression. Due to their rather small sizes (from 80 to 500 nucleotides), these RNAs are usually called small RNAs (sRNAs).
Substantial efforts have been made to determine sRNA functions in various physiological responses, identify their primary targets, decipher the mechanisms by which they affect the targets and analyze their participation in more complex regulatory networks in the cell [1–3, 13]. The diverse biological roles of RNA-based elements encompass the regulation of metabolism (e.g., carbohydrate metabolism, metabolite transport, synthesis and degradation), growth processes (e.g., toxicity, biofilm formation), adaptation to stress and varying culture conditions (e.g., temperature, iron limitation, cell density). Moreover, recent evidence shows that regulatory RNAs play key roles in microbial pathogenesis [1, 14–17]. During infection, bacterial pathogens have to quickly react to changing environmental conditions in the host. Subsequent adaptive responses include survival strategies in specific body niches, avoidance of the immune system and systemic toxicity. In the multiple infectious strategies that bacterial pathogens have developed, regulatory RNAs are considered as signal transducers of environmental cues by participating in the precise coordination of gene expression. Several sRNAs have been shown to regulate the production of virulence factors, while others regulate pathogenic traits by adapting the metabolism of the bacteria in response to the host. Recent studies have focused on species-specific sRNAs and their direct implication in virulence. However, a number of regulatory RNAs, such as riboswitches, are conserved across gram-negative and gram-positive bacteria. These elements play critical functions in metabolism and contribute to the maintenance of cell survival and cell integrity, especially during exposure to stress. Bacteria also use regulatory RNAs to interfere with foreign DNA invasion processes, such as lytic bacteriophage infection or plasmid conjugation [18, 19].
Regulatory RNAs employ diverse mechanisms to modulate expression of their targets, functioning primarily at the post-transcriptional level. By using mRNAs as targets, they can affect transcription, translation, RNA stability, maturation and processing [1]. Protein targets can be sequestered away from their biological functions by regulatory RNAs, thus inducing numerous downstream effects [20–22]. When binding to metabolites, tRNAs, proteins and cis-acting regulatory regions of mRNAs can change conformation and affect transcription or translation of the downstream genes [23, 24]. More recently, DNA has been postulated to function as a novel target for regulatory RNAs (i.e., CRISPR family), broadening further the range of RNA-mediated strategies [18, 19].
RNA-based elements in gram-negative bacteria have been extensively reviewed in a number of recently published manuscripts [1–3, 13–17, 20–22]. Except for the class of riboswitches, information on RNA-mediated gene regulation in gram-positive species is rather limited. Here, we provide a comprehensive summary of the information available in the literature on RNAs with regulatory functions in gram-positive bacteria.
A diversity of regulatory RNAs in gram-positive bacteria
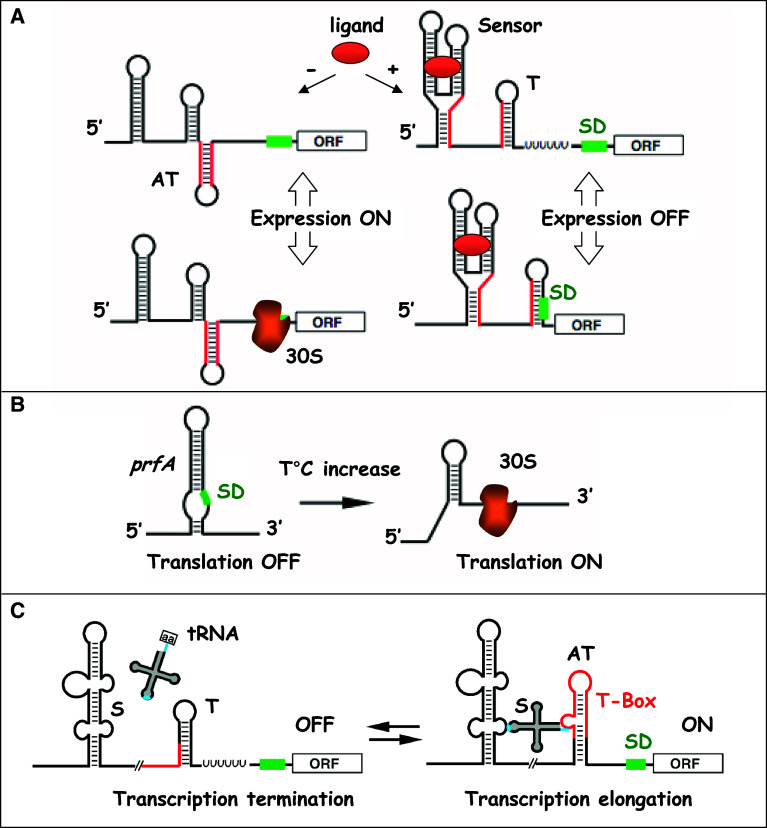
As shown for gram-negative bacteria [1, 3, 17, 23, 24], gram-positive bacteria have developed numerous RNA-dependent regulatory mechanisms to respond to internal and external signals. These regulatory RNAs can be divided into four main classes: (1) The trans-acting sRNAs act as antisense RNAs by basepairing with target mRNAs (Fig. 1). Two different mechanisms have been elucidated for this class of RNAs. In the first case, the sRNA and the target mRNA are encoded on the same DNA locus, but are transcribed in opposite directions. As a result, both RNAs form extended basepairings. In the second case, the sRNA and target mRNAs are encoded on different DNA loci and share only partial basepairing complementarities. These sRNAs can target multiple mRNAs. Some of these sRNAs are bona fide non-coding RNAs (ncRNAs), while others may carry small open reading frames (ORFs). (2) The second class involves RNAs, which can sequester regulatory proteins, thus perturbing their activity. RNAs that are part of ribonucleoprotein complexes and exert cellular housekeeping functions are included in this class. (3) The third class consists of a unique family of small processed RNAs, the CRISPR RNAs, recently described to regulate DNA maintenance. (4) The fourth class comprises the cis-acting regulatory regions of mRNAs, which respond to trans-acting agents [e.g., intracellular concentration of metabolites (riboswitches), uncharged tRNAs (T-boxes) or proteins] or to environmental cues [e.g., temperature (thermosensors)] (Fig. 2).
Fig. 1.
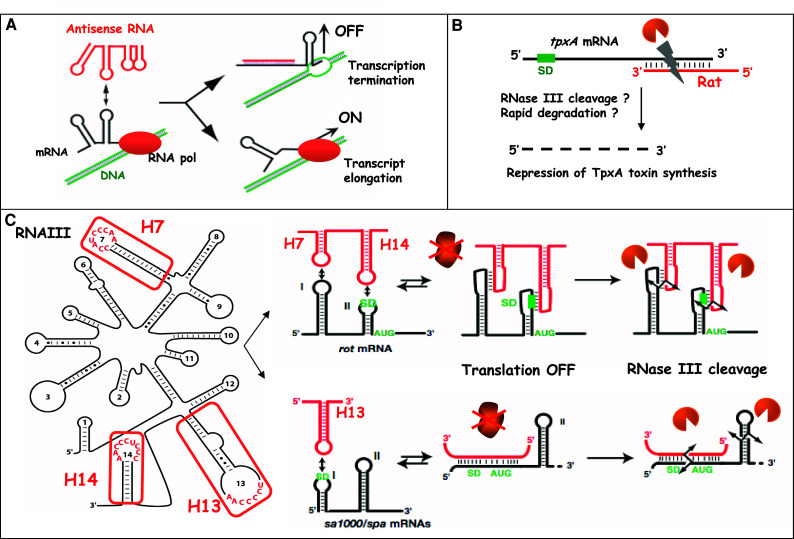
Mechanisms of action of trans-acting antisense sRNAs in gram-positive bacteria. a Schematic drawing of antisense RNA-mediated transcription attenuation in plasmid pT181 (according to [155]). The antisense RNA (red) initiates binding with the mRNA (black) by a loop–loop interaction, which rapidly propagates to form a long duplex. The formation of the duplex stabilizes the formation of a transcription terminator and arrests the elongation of transcription. In the absence of the antisense RNA, the mRNA structure forms an anti-terminator structure allowing the synthesis of RepA protein. b Schematic drawing of the antisense RNA Rat-mediated degradation of txpA mRNA from B. subtilis (according to [43]). The antisense RNA is fully complementary to the 3′ end of the mRNA and induces degradation of the target mRNA. c Structure and mechanism of action of the quorum-sensing RNAIII from S. aureus. The secondary structure of RNAIII is from [52] with the three hairpins 7, 13 and 14. RNAIII uses different hairpin motifs to bind various mRNAs encoding virulence factors (protein A, fibrinogen-binding protein SA1000) and the transcriptional regulatory protein Rot. Formation of RNAIII–mRNA complexes blocks the access of the 30S ribosomal subunit and concomitantly recruits the endoribonuclease III to cleave the repressed mRNAs [27]. SD is for Shine–Dalgarno sequence
Fig. 2.
Mechanisms of action of cis-acting regulatory regions of mRNAs in gram-positive bacteria. a Transcriptional attenuation (top) and translational (below) control mediated by the binding of a ligand. In the absence of ligand, the mRNA forms an anti-terminator hairpin and transcription proceeds through the open reading frame (ORF), or alternatively, the ribosome binding site (RBS) is available for translation initiation. In the presence of ligand, the sensor domain is stabilized by the direct recognition of ligand, causing the formation of a transcription terminator or alternatively a structure that sequesters the RBS. SD is for Shine–Dalgarno sequence. b L. monocytogenes prfA mRNA is regulated by a temperature-dependent mechanism [115]. At low temperature, the mRNA adopts a secondary structure, which blocks access of the 30S ribosomal subunit. At 37°C, the structure melts, allowing access of the 30S subunit and translation of PrfA, a major transcriptional regulator of virulence gene expression. c The T-box family of tRNA-mediated riboswitches. The uncharged tRNA acts as the signal molecule directly sensed by the 5′ untranslated region of the mRNA. When no uncharged tRNA is sensed, a transcription terminator in the T-box of the controlled mRNA is formed, leading to its transcription termination. Upon sensing of the uncharged tRNA, two interactions (blue) with the leader region including the T-box (red) take place. The first one is the pairing of the uncharged tRNA anticodon with the so-called specifier sequence (S) acting as a codon mimic. The second interaction involves pairing of the 3′CCA end of the tRNA with a complementary sequence in the leader region. As a result, an anti-terminator sequence is stabilized, and continued transcription of the downstream controlled ORF is allowed. T is for terminator and AT for anti-terminator of transcription
sRNAs acting at the mRNA level in gram-positive bacteria
Generalities
In gram-positive bacterial pathogens, all reported biological functions of the class of sRNAs are linked to adaptation or virulence processes. Of interest, several sRNA-encoded genes are embedded within pathogenicity islands (e.g., Staphylococcus aureus, [25]). Among the sRNAs encoded on the genome, some are associated with loci expressing two-component regulatory systems (e.g., S. aureus RNAIII, Streptococcus pyogenes fasX) or transcriptional regulators (e.g., S. pyogenes rivX) (Table 1) [26]. In non-pathogenic species such as Bacillus subtilis, sRNAs are suspected to regulate metabolism and sporulation. Most sRNAs acting at the mRNA level are described as ncRNAs. However, two sRNAs are larger in size compared to conventional sRNAs and encode proteins with virulence functions (i.e., S. aureus RNAIII, S. pyogenes pel) (Table 1). The sRNAs that have been investigated to date are expressed mainly in a growth phase-dependent manner, some being under the control of quorum-sensing systems (e.g., S. aureus RNAIII) or sporulation-specific transcriptional factors (e.g., B. subtilis SurA, SurC) (Table 1). Although it is assumed that the sRNAs are part of complex regulatory processes involved in adaptation and pathogenicity, the knowledge of factors, stimuli or environmental conditions being responsible for modulation of sRNA expression is lacking overall. Only a few transcriptional regulators and two-component systems have been reported to control their expression (Table 1).
Table 1.
Trans-acting sRNAs in gram-positive bacteria
| Bacterial species/sRNA | Targeta | Target function | Mechanism of action | Other biological function | References |
|---|---|---|---|---|---|
| B. subtilis | |||||
| BS190 | ND | – | ND | ND | [140, 141] |
| BS203 | ND | – | ND | ND | [140, 141] |
| BsrE, BsrF, BsrG, BsrH, BsrI | ND | – | ND | ND | [140, 141] |
| SR1a | ahrC | Transcriptional activator of arginine catabolic operons | Antisense sRNA | Regulated by sugars | [38–40] |
| SurA, SurC, polC-ylxS IGR | ND | – | – | Activated under sporulation-specific conditions | [36, 41] |
| C. perfringens | |||||
| VR RNA | colA | Kappa-toxin | ND | Regulated by the ViR/VirS system | [123, 124] |
| cpd | 2′, 3′-cyclic nucleotide phosphodiesterase alpha-toxin | ND | |||
| plc | Protein tyrosine phosphatase | ND | |||
| ptp | Cystathionine gamma synthase (metB) | ND | |||
| ycgJ–metB–cysK–ygaG | cysteine synthase (cysK) | ND | |||
| L. monocytogenes b | |||||
| LhrA | ND | ND | Binds Hfq | [12, 62, 63] | |
| LhrB | ND | L20 regulatory region | Binds Hfq | [12, 62, 63] | |
| LhrC1-5 | ND | ND | Binds Hfq | [12, 62, 63] | |
| rilA | ND | ND | [37] | ||
| rilB | lmo2104 | Ferrous iron transport protein FeoA | Antisense sRNA | Carries 5 long repeats | [37] |
| lmo2105 | Ferrous iron transport protein FeoB | Antisense sRNA | [37] | ||
| rilC | ND | ND | [37] | ||
| rilD | ND | ND | [37] | ||
| rliE | comEA | Competence factors | Antisense sRNA | [37] | |
| comFA | Competence factors | Antisense sRNA | |||
| lmo0945 | Competence factors | Antisense sRNA | |||
| rilF | ND | ND | [37] | ||
| rilG | ND | ND | [37] | ||
| rliH | ND | ND | [37] | ||
| rliI | lmo1035 | Phosphotransferase system | Antisense sRNA | [37] | |
| sbrA | ND | ND | Activated by Sigma B | [12, 64] | |
| rli22 | ND | ND | ND | [12] | |
| rli23, rli25, rli35 | lmo0172, | Transposase | Antisense homologous sRNAs | ND | [12] |
| 0330, 0828 | [12] | ||||
| rli24, rli26-27 | ND | ND | ND | [12] | |
| rli28, rli50 | ND | Homologous sRNAs | ND | [12] | |
| rli29 | lmo0471 | Antisense sRNA complementary to the 5’UTR | ND | [12] | |
| rli30 | lmo0506 | Antisense sRNA | ND | [12] | |
| rli31, rli32 | ND | ND | ND | [12] | |
| rli33 | ND | Activated by Sigma B | [12] | ||
| rli34, rli36 | ND | ND | ND | [12] | |
| rli37 | ND | Carries 1 ORF 58 aa | ND | [12] | |
| rli39 | ND | Cobalamine riboswitch? (RFAM) | ND | [12] | |
| rli40 | ND | Carries 1 ORF 64 aa | ND | [12] | |
| rli41 | ND | Carries 2 ORF 45 and 35 aa | ND | [12] | |
| rli42 | ND | ND | ND | [12] | |
| rli43 | ND | Carries 1 ORF 35 aa | ND | [12] | |
| rli44 | ND | Carries 1 ORF 28 aa | Repressed by SigmaB | [12] | |
| rli45, rli46 | ND | rli45 antisense to rli46 | sRNA regulatory networks | [12] | |
| rli47 | ND | ND | Activated by Sigma B | [12] | |
| rli48-49 | ND | ND | ND | [12] | |
| S. aureus | |||||
| RNAIIIa | hla | Alpha-hemolysin | Antisense sRNA (translation initiation) | Regulated by the agr system, global effect on virulence factor expression and phenotypes | [51–53] |
| rot | Repressor of toxin | Antisense sRNA (translation initiation, RNase III dependent hydrolysis) | |||
| spa | Protein A | ||||
| sa1000 | Fibrinogen-binding protein | ||||
| lytM | Peptidoglycan hydrolase | c | |||
| Coa | Coagulase | ||||
| sprA | sa2216 | ABC transporter | Possible antisense sRNA | ND | [25] |
| sprA2, sprA3, sprB, sprC, sprD, sprE, speF, sprG, sprFG2, sprFG3 | ND | ND | ND | [25] | |
| rsaOA, rsaOB | ND | ND | ND | [9] | |
| S. pneumoniae | |||||
| csRNA1 | ND | ND | Controlled by CiaR of the CiaRH system | [131] | |
| csRNA2 | ND | ND | |||
| csRNA3 | ND | ND | csRNA4 and 5 involved in stationary phase autolysis | ||
| csRNA4 | ND | ND | |||
| csRNA5 | ND | ND | |||
| S. pyogenes | |||||
| fasX | fbp54 | Fibronectin-binding protein | ND | Regulated by amino-acid starvation, the FasBAC and luxS systems | [125–128] |
| mrp | Fibrinogen-binding protein | ND | |||
| Ska | Streptokinase | ND | |||
| SLS | Streptolysin S | ND | |||
| pel | emm | M protein | ND | Regulated by cell-density mechanism | [129] |
| sic | Streptococcal inhibitor of complement | ND | |||
| SpeB | Cysteine protease | ND | |||
| rivX | emm | M protein | Dependent on RivR, ND | Controlled by the regulator CovR | [130] |
| fbpA | Fibronectin-binding protein | Dependent on RivR, ND | |||
| grm | Gene-regulated by Mga | Dependent on RivR, ND | |||
| Mga | Response regulator | Dependent on RivR, ND | |||
| scpA | C5a peptidase | Dependent on RivR, ND | |||
| scrA | Phosphotransferase system | Dependent on RivR, ND | |||
| speB | Cysteine protease | Dependent on RivR, ND | |||
ahla, rot, spa, sa1000, lytM, coa mRNAs are direct targets of RNAIII; ahrC mRNA is a direct target of SR1
bThe expression of all L. monocytogenes sRNAs changed under various experimental conditions and after growth in the intestinal lumen and blood: the sRNAs (lhrC, rliB, rli24, rli26, rli33, rli37–38, and rli42) were induced in blood; sRNAs (rli28, rli36, rli39, rli45–48) were found enhanced in the intestinal lumen; other sRNAs (rli22, rli27, rli29, rli40) were found modified in blood and intestinal lumen. The expression of all sRNAs is not modified in the mutant ∆hfq strain. Data according to [12], and see supplementary materials
cChevalier, Geissmann, Romby, unpublished data
This table does not include the housekeeping ncRNAs (RNase P, rRNA, tRNA, tmRNA, SRP) and 6S RNA (see text)
Investigation into the mode of action of trans-acting sRNAs in gram-positive bacteria has revealed antisense mechanisms of the sRNAs with their target mRNAs, which leads to either inhibition or enhancement of mRNA translation initiation (e.g., B. subtilis SR1, S. aureus RNAIII) and/or mRNA degradation (e.g., S. aureus RNAIII) [27–33]. The knowledge of protein contribution in these mechanisms is also limited. The role of the Sm-like Hfq protein, which functions as a RNA chaperone in gram-negative bacteria, is dispensable in many gram-positive bacteria, although the protein was found to be important for virulence in some species.
In the following section we will discuss separately the sRNAs encoded on the same locus as the target mRNA (fully complementary to the target mRNA) and the sRNAs encoded in an autonomous locus on the chromosome (partial complementarity with the target mRNAs). Selected examples are described to illustrate the diversity of mechanisms and functions of these regulatory RNAs. Many novel sRNAs have been recently predicted or analyzed in various gram-positive bacteria and are waiting further functional and mechanistic studies (Table 1). It is very likely that many of them might directly target mRNAs through basepairing.
Fully complementary antisense sRNAs control plasmid replication or act as antitoxins
Natural antisense RNA-regulated systems were first described in accessory genetic elements such as plasmids, phages and transposons [34–36]. These antisense RNAs are rather small (around 55–150 nt long), diffusible and untranslated. They form long duplexes with their target mRNA. Several plasmid-encoded antisense RNAs hosted by gram-positive bacteria regulate replication, conjugation or post-segregational killing using various mechanisms, i.e., transcriptional attenuation and translational repression [35]. For example, replication control of the S. aureus rolling-circle-replicating plasmid pT181 [37] and the B. subtilis theta-replicating plasmid pIP501 [38] involves transcription attenuation. In both cases, the plasmid-encoded antisense RNAs bind to the 5′ untranslated region (5′UTR) of repA mRNA encoding the initiator replication protein RepA. This results in the stabilization of an alternative conformation of the mRNA allowing the formation of a terminator structure in the leader region of repA mRNA [39]. In consequence, elongation is arrested, and the expression of the target mRNA is inhibited (Fig. 1a). In contrast, for the S. aureus pSK41 replicon, an antisense RNA was shown to repress indirectly the translation of repA mRNA. This antisense RNA binds the target mRNA far upstream of the ribosome binding site (RBS) and thereby induces the stabilization of a hairpin structure that sequesters the RBS [40]. In Enterococcus faecalis, the par locus of plasmid pAD1, which encodes the toxic protein Fst and an antisense RNA as the antidote, is the first plasmid-killer system identified in gram-positive bacteria [35, 41, 42]. The two genes are transcribed convergently and overlap at the level of the bi-directional terminator. The two RNAs contain two additional short regions of complementarity, which correspond to two distant repeats [35]. These two repeat motifs in the mRNA are located close to the fst RBS and downstream of the fst gene. Interaction between antisense and target RNAs within these two distant complementary regions suffices to block ribosome binding and thus fst translation [41, 42]. It was shown that plasmid-encoded antisense RNAs associate rapidly with the target mRNA, and it is the rate of formation rather than the affinity of duplexes that determines efficient inhibition [34–36].
Several fully complementary antisense RNAs encoded on the chromosome of several gram-positive bacteria have been also described [12, 43, 44]. Some of these chromosomally encoded antisense RNAs repress the synthesis of potentially toxic proteins homologous to plasmid-encoded or mobile element-encoded toxins [13]. In B. subtilis, RatA is described as an antidote sRNA, which is encoded on a cryptic prophage. This RNA affects the accumulation of the overlapping convergently transcribed mRNA, which encodes the toxic peptide TxpA (for toxic nucleotides). RatA basepairs with the 3′ end of the txpA mRNA (Fig. 1b), and experimental data suggest that the duplex triggers target mRNA degradation [43]. Several short and long antisense RNAs have also been recently discovered in L. monocytogenes ([12], see below).
sRNAs regulate metabolism, and sporulation in Bacilli
SR1 was identified as a ncRNA involved in the fine-tuning of arginine catabolism. Expression of SR1 is derepressed under conditions of gluconeogenesis but repressed under glycolytic conditions [31]. The primary target of SR1 is ahrC mRNA, which encodes the transcriptional activator of arginine catabolic operons [28, 29, 31]. SR1 acts as an antisense RNA that interacts with the coding region of ahrC mRNA and prevents translation initiation by changing the structure of the target mRNA in the vicinity of the RBS [29].
Transcriptional profiling using microarrays combined with the use of comparative genomic algorithms revealed a number of additional sRNAs, the expression of which is activated under sporulation-specific conditions [45]. SurA and SurC expression is under the control of the master sporulation regulator Spo0A and the sigma factor Sigma K, respectively. Expression of three other intergenic transcripts from the operon polC-ylxS is induced by the alternative Sigma G and Sigma K factors [45]. Other highly expressed sRNAs were identified in B. subtilis, but their functions remain unknown [46].
A multifunctional sRNA regulates virulence in S. aureus
The best-studied case of sRNAs controlling bacterial infectious processes is RNAIII from S. aureus, the intracellular effector of the agr (accessory gene regulator) operon. S. aureus has evolved a plethora of sensory systems that turn on/off the expression of virulence genes in response to both environmental and host signals. Complex regulatory networks exist that involve several two-component systems, transcriptional regulatory proteins and regulatory RNAs [47, 48]. Among these systems, the agr operon, which functions as a sensor of the population density, was shown to be required for virulence in animal models [49–51]. The agr operon encodes the two-component system (sensor kinase AgrC and response regulator AgrA) and the quorum-sensing cassette (membrane protease AgrB and secreted auto-inducing peptide AIP). At high cell density, AgrA activates the transcription of its own operon from the P2 promoter and of the regulatory RNAIII from the P3 promoter. RNAIII acts as the intracellular effector of the agr regulon and controls the switch between the early expression of surface proteins and the late expression of exotoxins [33]. Detailed mechanistic studies show that RNAIII acts primarily at the post-transcriptional level and that it targets multiple mRNAs (Table 1). The RNA uses independent structural domains to perform its different tasks [52]. RNAIII is a mRNA that encodes the hemolysin δ along with noncoding regions that carry specific regulatory functions (Fig. 1c). The 5′ domain of RNAIII activates translation of hla mRNA encoding α-hemolysin by competing directly with an intramolecular mRNA structure that sequesters the hla RBS [32, 33]. The 3′ end and the central domain of RNAIII repress the synthesis of major cell surface virulence factors (protein A, coagulase, fibrinogen-binding protein) as well as the transcriptional regulator Rot [27, 30, 53]. Repression of the synthesis of multiple proteins by RNAIII involves a shared mechanism whereby three C-rich hairpin loops of RNAIII bind to the Shine and Dalgarno (SD) sequence of the target mRNAs (Fig. 1c). The RNAIII–mRNA interactions block the access of the ribosome at its loading site and concomitantly recruit the endoribonuclease III, which cleaves the formed complexes [27, 30]. By repressing the synthesis of the regulator Rot, RNAIII also induces many downstream effects and indirectly activates the synthesis of many exotoxins.
Besides the major regulatory RNAIII, several stable RNAs were identified in infectious S. aureus strains. Seven of those were found encoded on pathogenicity islands, which carry important virulence factors [25]. Remarkably, expression of the sRNAs showed large variation among clinical isolates. Based on these observations, it was suggested that these RNAs contribute to S. aureus niche adaptation and pathogenicity [25]. One of the prophage sRNAs, sprA, was shown to form a stable complex with an ABC transporter encoding mRNA [25].
Other numerous stable RNAs that are differentially expressed under stringent, cold and heat shock responses were identified by microarrays in S. aureus [54, 55]. Many of them might originate from protein coding transcripts, while others might be antisense RNAs. Another study based on a high-scale computational approach predicted numerous ncRNA candidates in 932 bacterial strains including 47 novel ncRNA candidates in S. aureus [8, 56]. A more recent phylogenetic profiling study analyzing information on intergenic and coding regions of all available bacterial genomes has shown that five regulatory regions of mRNAs and one novel sRNA are expressed in S. aureus N315 strain [9]. Another group’s evaluation of the intergenic regions of the S. aureus genome using bioinformatics and expression analysis has revealed multiple regulatory regions in the genome and the existence of ten additional sRNAs [57]. The transcription of three sRNAs is activated by the alternative SigmaB factor. Most of these novel sRNAs carry conserved and unpaired C-rich sequences as found in RNAIII. One of these RNAs has been shown to bind to the ribosome binding site of mRNAs encoding metabolic enzymes through its C-rich motif [57]. Thus, the unpaired C-rich motif might be considered as a signature for ncRNAs, which interact with mRNAs in low GC gram-positive bacteria.
Is the RNA chaperone protein Hfq associated with sRNA-mediated regulation in gram-positive bacteria?
In gram-negative bacteria, Hfq is an RNA chaperone that plays a crucial role in sRNA-dependent regulation by either stabilizing the sRNAs or optimizing sRNA/mRNA duplex formation [58, 59]. However, the function of Hfq as a critical co-factor in sRNA-mediated regulatory mechanisms in gram-positive bacteria is unclear. For example, the streptococcal and lactobacillal genomes do not contain any obvious Hfq homologue-encoding gene [60]. In S. aureus, hfq mRNA appears to be weakly transcribed [53], and the function of Hfq in stress response, virulence factor production or metabolism has not yet been determined [61]. Although S. aureus Hfq binds to RNA in vitro, the protein does not stimulate RNAIII-dependent repression of target mRNAs in vivo and is dispensable for RNA–RNA interaction such as RNAIII–spa mRNA [27, 30, 61] and sprA sRNA-mRNA [25]. Furthermore, S. aureus Hfq was reported to not fully complement a hfq-deficient mutant of E. coli [62]. This study also shows that the C-terminal extension of the E. coli Hfq constitutes an RNA interaction surface with specificity for mRNAs. This C-terminal extension is lacking in S. aureus, thus suggesting that the protein has lost some RNA binding properties. In B. subtilis, Hfq is dispensable for the stability of SR1 RNA and the interaction of SR1 RNA with its ahrC mRNA target, but is required for ahrC mRNA translation [28]. Hfq is also not required for the action of the cis-acting antitoxin RatA sRNA on its tpxA mRNA target [43]. In all these cases, the sRNA is able to rapidly form sRNA–mRNA basepairings. In L. monocytogenes, Hfq contributes to tolerance to osmotic and ethanol stress, long-term survival under amino acid-limiting growth conditions and pathogenesis in mice [63, 64]. Transcription of hfq mRNA is weak under rich medium culture conditions, but is induced under stress exposure in a Sigma B-dependent fashion [63, 64]. Hfq was used as bait to fish the sRNAs LhrA, LhrB and LhrC [64, 65]. However, recent work shows that LhrB and LhrA are not bona fide ncRNAs, but originate from mRNA leaders, therefore questioning the role of Hfq for these putative cis-acting RNA elements [12, 65]. Finally, the stability of many newly discovered L. monocytogenes ncRNAs was not affected in a hfq-deficient mutant [12, 44, 65].
Taken together, the lack of hfq homologues in some gram-positive species, the weak expression of hfq in others and the poor evidence for a requirement of Hfq in sRNA stability or sRNA–mRNA interaction cast a direct involvement of Hfq in regard to sRNA-mediated regulation in gram-positive bacteria. Whether other RNA chaperones, helper or helicase proteins substituting for Hfq are required in the basepairing process of sRNAs with their target mRNAs remains to be determined.
RNAs modulating protein activity in gram-positive bacteria
RNAs with protein sequestration activity
Regulatory RNAs also have the property to bind proteins and thereby regulate their activities. This protein sequestration ability was demonstrated in E. coli for CsrB sRNA (targeting the global carbon storage regulatory CsrA protein), GlmY sRNA (targeting YhbJ protein) and 6S RNA (targeting Sigma 70-RNA polymerase) [20–22]. These sRNAs have the common feature to be able to mimic structures of the natural substrates for target proteins and thereby counteract their activity. For example in E. coli, 6S RNA mimics open promoters that bind the Sigma 70-RNA polymerase and thus can titrate the transcriptional factor away from the promoters. Control of gene expression by 6S RNA was shown to occur in response to the shift from exponential to stationary phase of growth, suggesting that 6S RNA is a critical factor in bacterial adaptation processes [22].
In gram-positive bacteria, no protein sequestration mechanism such as CsrB/CsrA and GlmY/YhbJ has been identified yet. However, 6S RNA, which is conserved among bacterial species, is likely to control gene expression in gram-positive bacteria by sequestrating the alternative sigma factor of RNA polymerase as suggested for B. subtilis [66]. In this species, two abundant 6S RNAs are discoordinately expressed during growth, emphasizing the critical role of 6S RNA in bacterial adaptation [66, 67].
RNAs of ribonucleoprotein complexes
The class of protein-binding RNAs includes RNAs that are part of ribonucleoprotein complexes (RNPs). The RNPs have important housekeeping functions. They are involved in protein biogenesis and metabolism and as such are crucial for the maintenance of cell integrity when bacteria are challenged by stresses encountered in their environments.
The bacterial RNase P consists of a single catalytic RNA component associated with a protein subunit. In E. coli, this holoenzyme catalyzes the removal of the 5′ leader sequence of pre-tRNAs to produce the mature 5′ terminus of tRNAs [68–70]. RNase P has been studied in B. subtilis, Clostridium sporogenes and S. aureus [71–73]. Interestingly, an additional function for RNase P was reported in B. subtilis. RNase P cleaves the coenzyme B12 and adenine riboswitches in the absence of metabolite binding. It was proposed that this cleavage might contribute to the stabilization of mRNA under normal conditions [74, 75].
In bacteria, the signal recognition particle (SRP) consists of the 4.5S RNA (also called scRNA for small cytoplasmic RNA) and the Ffh protein [76]. SRP is a universally conserved pathway for targeting polypeptides to the cell membrane via the cotranslational machinery. SRP binds protein signal sequences as they exit the ribosome and targets them to the bacterial membrane. Here the SRP complex binds the membrane-associated receptor FtsY, which releases the signal peptide from the Sec translocon. In gram-positive bacteria, SRP is thought to be the main mechanism for targeting polypeptides. Although SRP is considered an essential cellular component, this pathway is dispensable in Streptococcus where it plays important roles in the secretion of various virulence factors and in biofilm formation [77–81].
Another RNA modulating protein activity is tmRNA, also called ssrA RNA. In bacteria, tmRNA is a specialized RNA with properties of both a tRNA and an mRNA [82–84]. tmRNA bound to the small protein SmpB interacts with the translational ribosomal complexes stalled at the 3′ end of a truncated mRNA and accepts the nascent polypeptide in the same fashion as does the elongator tRNA. Translation is continued on the short mRNA encoded within tmRNA by adding a peptide tag to the truncated protein at its C-terminus. The peptide tag, which contains recognition determinants for intracellular proteases, then targets the tagged protein for rapid degradation. This mechanism, called trans-translation, plays a crucial role in protein quality control pathways. It is involved in cell viability, development, response to stress and virulence when bacteria need to execute large changes in their genetic programs [82]. In this regard in B. subtilis, tmRNA was shown to be required for sporulation events [85, 86].
CRISPR RNAs in gram-positive bacteria
CRISPR RNAs emerged recently as a unique family of regulatory RNAs [87]. They are encoded within the CRISPR loci that are widely present in bacteria and archea. To date CRISPR has been associated with two biological functions: resistance to lytic phages in Streptococcus thermophilus [88] and interference with plasmid conjugation in S. epidermidis [18]. Besides these functions, CRISPR has been considered for a long time as a valuable tool for strain typing and classification, as well as for the evaluation of the historical background of bacterial clones. The CRISPR system has retained recent attention based on certain similarities with the eukaryotic RNAi-driven gene silencing system [1, 87]. Functional CRISPR loci consist of highly variable DNA sequences including a leader sequence followed by a series of repeat-spacer units and of several CRISPR-associated (Cas, Csn) genes. The DNA arrays can contain from 2 to 249 copies of the same repeat. The CRISPR repeats can vary from 24 to 47 base pairs and show significant divergence in sequence among bacteria. However, significant sequence similarity among the repeats is observed within species. The repeats are regularly interspaced with unique spacers of 26–72 base pairs. The spacer sequences are not conserved and often vary among strains of the same species. All sequence spacers studied so far are homologous to DNA from phages or plasmids. The CRISPR-associated genes were predicted to code proteins containing putative RNA- and DNA-binding domains, helicase motifs and endo- and exo-ribonuclease activities.
CRISPR DNA loci are transcribed as full-length RNAs, which are rapidly processed into shorter RNAs (crRNAs, psiRNAs). In E. coli, the CasE protein from the Cascade complex CasA-E was shown to be responsible for the processing event [89]. Of note, in the thermophilic Archea Pyrococcus furiosus, Cas6 functions as a novel endoribonuclease that cleaves specifically CRISPR RNAs within the repeat sequences to release the individual invader targeting RNAs [90]. Based on these data and on the sequence analysis of CRISPR loci, a model for the mode of action of CRISPR RNAs has been proposed. The full-length CRISPR locus is transcribed as a RNA that is further processed by the Cascade complex of CAS proteins into unique single repeat-spacer units called crRNAs [18, 89]. Similar to the RNAi machinery of eukaryotes, CAS proteins would act as essential cofactors to direct basepairings of the crRNA spacer sequences with phage or plasmid target sequences. Although it has been postulated in the past that CRISPR RNAs targeted mRNAs [91], it recently became evident that they target phages (Charpentier, personal communication) and plasmids [18] at the DNA level. Future studies are necessary to decipher the exact molecular mechanism whereby the CRISPR RNAs recognize the extrachromosomal DNA target, the subsequent fate of the target and the exact contribution of the CAS proteins in this process. Efforts are also needed to understand why foreign DNA is preferentially used for integration in the CRISPR loci as well as the mechanisms of spacer recognition, selection and insertion in between the repeats within the DNA region. It is worth noting that so far knowledge available on CRISPR biological function was produced mainly in Streptococcus and Staphylococcus species [18].
Cis-acting RNA elements in gram-positive bacteria
A plethora of cis-acting mRNA elements in gram-positive bacteria
RNA molecules have the extraordinary ability to adopt various structures that can efficiently regulate the expression of genes they encode. This characteristic is largely exploited by the cis-acting RNAs, which are usually located in the 5′ untranslated mRNA leader regions of the regulated genes. They can be recognized by a variety of trans-acting regulators (metabolites, uncharged tRNAs, proteins) but can also sense environmental signals (temperature, divalent ions) without the contribution of any additional regulatory factor [23, 24, 92–98]. As a result of binding to their ligands, the leader regions of mRNAs undergo significant structural changes modifying expression of the transcripts (Fig. 2). The cis-acting mediated regulatory effects include attenuation of transcription, translation initiation and mRNA stability [23, 99, 100]. Globally, this class of RNA elements is involved in cellular metabolism and is expected to be critical in adaptive strategies of bacteria. Some of these elements can also directly affect virulence as exemplified by the pfrA mRNA thermosensor in L. monocytogenes. Overall, more than 70 cis-acting regulatory RNAs were identified in B. subtilis [92, 101, 102]. More recently, 40 functional riboswitches (metabolites, T-boxes, leaders of r-protein operons and PyrR protein-sensing riboswitch) were described in L. monocytogenes [12]. This study also reveals 13 novel regulatory regions of mRNAs. Of interest, two sense the temperature, while expression of most of these RNAs is regulated during infection in the intestinal lumen of mice or in blood [12]. The study also demonstrates that the metabolism of bacteria is submitted to large variations during the infectious process. Such a panel of regulatory RNAs thus offers the advantage for the bacteria to be able to integrate multiple signals to express particular gene products. In the complexity of pathogenic processes, the bacteria need to adapt to numerous changing environments within the host and to coordinate accordingly gene expression at appropriate times and spaces during infection, and these sophisticated RNA-based sensors become critical. From the knowledge available in the literature, gram-positive bacteria seem to rely more on cis-acting RNAs to regulate gene expression than gram-negative bacteria. Furthermore, riboswitches in gram-positive bacteria more frequently utilize transcription attenuation, whereas those in gram-negative bacteria favor translational inhibition [92, 103, 104]. In contrast to trans-acting sRNAs, the cis-acting regulatory RNAs are quite well conserved among firmicutes. Selected typical examples of riboswitches in several gram-positive bacteria are described in the following section.
Riboswitch RNAs sensing metabolites
Riboswitches consist of two functional domains [23, 24, 92–98]. The aptamer or the sensor domain is a conserved and structured receptor that is specifically recognized by a defined ligand. The second domain is the expression platform that is responsible for the expression switch of the downstream ORFs as a result of ligand binding. Depending on the positioning of the riboswitch on the mRNA, regulation can occur at the transcriptional or post-transcriptional levels. These regulations lead either to repression or to activation of gene expression. At the transcriptional level, ligand binding can alter the ability of RNA polymerase in the process of transcription elongation through the formation of intrinsic transcription terminator/anti-terminator structures. At the translational level, ligand binding can lead to the formation of structures that either sequester the RBS or render the RBS accessible, thereby affecting the ability to translate the mRNA (Fig. 2a). Riboswitches can also induce site-specific cleavage in the mRNA, inducing its rapid degradation [100]. So far, riboswitches were shown to interact with cofactors [e.g., thiamin pyrophosphate (TPP), flavin mononucleotide (FMN), adenosyl cobalamin, S-adenosyl methionine (SAM)], nucleobases (e.g., adenine, guanine), amino acids (e.g., glycine, lysine), sugar derivatives (e.g., glucosamine-6 phosphate) or divalent ions (Mg2+). The controlled genes are often involved in the biosynthesis and/or transport of the ligands. As a result of this control, the expression of metabolic enzymes or transporters becomes adapted to the intracellular concentration of the metabolite. This metabolite sensing system can be considered as a feedback regulation mechanism allowing the maintenance of physiological levels of the metabolites.
Most of the riboswitches sense one single metabolite. Remarkably, riboswitches arranged in tandem and able to respond to two distinct metabolites exist as well. This is the case of the metE mRNA from Bacillus clausii, which contains in its 5’UTR one riboswitch responding to S-adenosylmethionine and a second one responding to coenzyme B12 [105]. The two ligands can repress independently the tandem riboswitch, thereby exerting a higher degree of control on gene expression.
Another interesting example is the glmS regulatory region that is present in certain gram-positive species, which affects mRNA stability in response to ligand binding [106–109]. This “riboswitch” presents unique characteristics. The structure of the regulatory domain was solved in the absence and in the presence of glucosamine 6-phosphate (GlcN6P), revealing that the ribozyme adopts a pre-formed compact structure in the absence of the metabolite. Therefore, this region does not behave as a classical “riboswitch” [107]. Binding to GlcN6P stimulates a self-cleaving ribozyme activity whereby the ligand itself acts as a coenzyme in the self-cleavage reaction by binding to a pre-folded active site pocket in the RNA. In this reaction, Mg2+ works as a cofactor in ribozyme folding and might be rate-limiting for catalytic activity [110].
RNA elements in 5′UTRs can also function as metal-sensing regulatory RNAs, i.e., “M-box” in B. subtilis [111]. The M-box functions as a divalent metal sensor for the Mg2+-specific regulation of the mgtE mRNA encoding a Mg2+ transport protein. The box appears to utilize a transcription attenuation-like genetic control mechanism through predicted Mg2+-regulated switching between mutually exclusive helices in the RNA. Thus, the M-box constitutes an example whereby control of gene expression by an RNA sensor can be achieved by simply tuning the tertiary structure formation to a particular metal binding affinity.
Recent X-ray structures of aptamer domains of several metabolite-sensing riboswitches have unraveled novel recognition modes [99, 100] and provided a molecular explanation for the selective specificity of the metabolite to bind the RNA sensor. These structures contain various junctions and tertiary contacts between distant domains that generate compact folds and specific clefts for ligand recognition. Some of the metabolites bind to regulatory sequences, while others participate in tertiary interactions. In general, the common feature of these structures is to sequester part of the sequence of the expression platform, leading to a different genetic response (Fig. 2a). Recent single-molecule and NMR analyses of a purine riboswitch show that the mRNA structures fold sequentially and that metabolite binding stabilizes the pre-formed folding of the sensor domain [94, 112]. Furthermore, the rate of RNA transcriptional elongation and the extent of the RNA polymerase pausing were shown to influence the folding of the sensor and the genetic response [113].
Riboswitch RNAs sensing environmental cues
Besides sensing metabolites, RNAs can also respond to environmental cues such as temperature. The RNA thermosensors, also called thermometers, constitute a unique class of structural RNA elements. They do not require any ligand recognition domain, but instead the regulatory function of the RNA element is adjusted in response to a change in temperature. These RNA elements affect expression of the downstream-located genes usually by sequestration of the RBS. In this system, the RBS is sequestered within an inhibitory helix (Fig. 2b). An increase of temperature affects the stability of the helix by melting, which results in an enhanced accessibility of the RBS to the ribosomal initiation complex. These regulatory elements are often used to control the expression of genes involved in cell response to sudden changes in temperature, such as heat-shock genes [114]. Interestingly, this type of regulatory element is also directly involved in virulence. This is the case for the pfrA mRNA thermosensor in L. monocytogenes, which is responsible for expression of the global regulator of virulence factor expression PfrA at 37°C [115] (Fig. 2b).
tRNA-mediated riboswitches
In gram-positive bacteria, the T-box family of riboswitches commonly controls the expression of genes involved in tRNA aminoacylation, amino acid transport and biosynthesis [103, 104]. T-box RNAs are also found in leader regions of mRNAs encoding transcriptional regulators and enzymes involved in quorum sensing [103]. The uncharged tRNA is the signal molecule directly sensed by the T-box mRNA element (Fig. 2c). The 5′UTRs of the controlled mRNAs, including the T-box element, exhibit a conserved set of sequence and structural features, which can fold into two alternative hairpin structures, an intrinsic transcription terminator or a competing transcription anti-terminator. Specificity of regulation is based on pairings between the anticodon of the uncharged tRNA and a codon-mimick, the so-called specifier sequence, located in the sensor domain of the T-box mRNA. A second interaction common to all tRNAs involves the 3′CCA end of the tRNA and a complementary sequence in the T-box leader region. As a result of sensing uncharged tRNA, the anti-terminator structure is stabilized, and continued transcription of the downstream genes is allowed (Fig. 2c). T-box RNA leaders can also control translation initiation. In this case, binding of the uncharged tRNA induces structural rearrangements in the leader region of mRNAs, thus promoting accessibility of the RBS to the ribosome [103, 104]. T-box RNAs are widespread among firmicutes but underrepresented in gram-negative bacteria. Furthermore, T-box RNAs that regulate transcription termination are observed primarily in low GC gram-positive bacteria, while T-box RNAs that control translation initiation predominate in high GC gram-positive and in some gram-negative bacteria [104]. It is intriguing that tandem T-box sequences were also found upstream of biosynthetic genes, thus adding a level of complexity in the fine-tuning of regulation. An example of amino acid metabolism regulated by T-boxes is illustrated below with tryptophan biosynthesis.
Cis-acting RNAs sensing proteins
A number of RNA-binding proteins mediate structural switches within 5′UTRs of mRNAs that can affect either transcriptional termination or translational initiation of the genes located downstream. They are key regulatory players in auto-regulation processes of ribosomal protein synthesis, amino acid metabolism and pyrimidine metabolism. Many of these proteins are often expressed constitutively, and their activity is modulated in response to the regulated effector (metabolite, RNA).
In gram-negative bacteria, the expression of ribosomal proteins is feedback regulated at the level of translation. The regulatory r-proteins have the properties to bind tightly to the rRNAs and to play a role in the assembly process of the ribosome. When the intracellular concentration of the r-protein exceeds that of the rRNA, the free regulatory r-protein (not bound to the ribosome) acts at the mRNA level to repress the synthesis of its whole operon. In many cases, the regulatory r-proteins recognize structurally similar rRNA and mRNA binding sites [116]. The mechanism is based on a competition between the rRNA and the mRNA for binding to the r-protein. The general principle of this autoregulation mechanism was also demonstrated for the B. subtilis rpsD mRNA [117] and infC operon [118], and for the B. stearothermophilus rpsO mRNA [119]. For the B. subtilis infC operon, L20 protein binds to the conserved leader region of the operon mRNA that mimics the 23S rRNA binding site, and thereby stabilizes the formation of a transcriptional terminator that induces premature transcription arrest [118].
In Bacillus species, expression of the pyr genes for the de novo pyrimidine nucleotide biosynthesis is regulated by a transcriptional attenuation mechanism involving the PyrR protein [120]. An RNA segment with conserved sequence and secondary structure located in the 5′UTR of pyr genes can alternatively form transcription anti-terminator/terminator stem-loop structures. Interestingly, PyrR senses the intracellular concentrations of the nucleotide UMP and binds to UMP only when present at high concentrations in the cell. Under these conditions, PyrR binds to a pyr regulatory RNA element in the leader region of the mRNA and induces formation of a transcription terminator. With this mechanism, the expression of downstream genes responsible for biosynthesis of pyrimidines is regulated by both exogenous metabolite (UMP) together with protein PyrR in a coordinated manner. Of interest, recent work shows that transcription of the pyr operon in L. monocytogenes is enhanced during blood infection [12].
Another regulatory protein in Bacillus, which senses the intracellular concentration of a metabolite, is the trp RNA-binding attenuation protein (TRAP) [121]. This protein plays a central role in the control of tryptophan biosynthesis and transport. At high concentrations of tryptophan, the amino acid binds to TRAP and induces allosteric activation of the protein. As a result, TRAP-Trp binds to multiple tryptophan codon repeats in the leader regions of target transcripts. For expression of the trpEDCFBA operon (encoding the tryptophan biosynthetic enzymes), TRAP-trp induces transcriptional termination and blocks translation of the mRNAs that have escaped termination by promoting the formation of a structure that sequesters the trpE RBS. Recent works in B. subtilis show that the transcription elongator factor NusA, a protein that binds to RNA polymerase, stimulates the intrinsic termination and pausing of the polymerase in the trpEDCFBA operon leader [122]. RNA polymerase pauses contribute to the transcription attenuation and translational control mechanisms, presumably by providing time for TRAP-Trp to bind to the nascent trp leader transcript during transcription of the mRNA. One may expect that NusA protein might have a more general role in the regulation mediated by cis-acting mRNA structures. Another degree of complexity is the involvement of a protein called anti-TRAP (AT), which antagonizes the activation of TRAP by tryptophan when the charged tRNATrp levels in the cell decrease [123]. Recent X-ray structures of the AT-TRAP complex reveal that AT binds to TRAP as a trimer and sterically blocks the binding to RNA [124]. In addition, expression of AT is regulated at the transcriptional level by a T-box located in the leader sequence of its own mRNA and by a leader peptide-dependent translational control mechanism [125]. The accumulation of uncharged tRNATrp promotes transcription anti-termination of the at operon and induces translation of the rtpA (encoding AT) coding region. The consequence of AT synthesis is inactivation of TRAP and elevated expression of all genes controlled by TRAP. To insure an adequate supply of charged tRNA for protein synthesis during this process, the uncharged tRNA coordinately increases transcription of the trpS operon encoding the tryptophanyl-tRNA synthetase. Taken together, control of tryptophan metabolism involves interplay of several auto-regulatory systems that sense the free tryptophan levels and the uncharged tRNATrp [121].
Another example of protein-dependent riboswitch is the control of genes for the metabolism of carbohydrates that are taken up by the phosphotransferase system (PTS) in B. subtilis [126]. Four homologous proteins (SacY, SacT, LicT, GlcT) of the BglG/SacY family act as antiterminators of transcription of genes and operons involved in sucrose metabolism (SacY, SacT), oligo-β-glucoside and aryl-β-glucoside metabolism (LicT) and glucose assimilation (GlcT) [127–133]. In the absence of the cognate PTS–sugar, a transcriptional terminator present in the untranslated mRNA leader of the target genes prevents transcriptional elongation. In the presence of the cognate PTS–sugars, the proteins become activated, bind to and stabilize a conserved motif called the ribonucleic antiterminator (RAT) present in the mRNA leader. As a consequence, the formation of an overlapping transcription terminator is prevented and gene expression can take place. Remarkably, the antiterminator proteins can be negatively or positively controlled through phosphorylation [126].
Cis-acting RNAs sensing antibiotics
Translational attenuation by site-specific ribosome stalling is a mechanism used for the control of expression of antibiotic resistance genes such as cat (chloramphenicol acetyl transferase) and erm (methyltransferase) [134–136]. In the case of erythromycin resistance gene control, the inducible ermC gene in B. subtilis is preceded by a short ORF encoding a regulatory leader peptide, ErmCL. In the absence of an inducing antibiotic, the RBS of ermC is sequestered in an mRNA secondary structure and ermC expression is repressed. Sub-inhibitory concentrations of an inducer (erythromycin or similar macrolides) cause the ribosome to stall at the ermCL ORF, triggering a conformational change in the mRNA that releases the ermC RBS sequestration and activates expression of the methylase gene. A similar mechanism was proposed to occur for S. aureus ermA whose product confers resistance to the macrolide-lincosamide-streptogramin B family of antibiotics [137].
Other RNAs with regulatory functions in gram-positive bacteria
Many novel chromosomally encoded RNAs have been recently identified in the gram-positive Bacillus, Clostridium, Listeria, Staphylococcus and Streptococcus (Table 1). For most of these RNAs described to date, their direct target(s) and mechanism(s) of action remain to be experimentally validated. However, many have been indirectly or directly associated with adaptation of bacteria to the host and to pathogenesis. Because these RNAs are expected to act in trans, the nomenclature of sRNAs is applied here. A brief overview is given for several bacterial species.
VR-RNA regulates virulence in Clostridia
VR-RNA is a sRNA, the expression of which is positively regulated by the two-component VirR/VirS system [138]. VR-RNA is responsible for the transcriptional regulation of the toxin-encoding genes colA and plc, and thus functions as a mediator of the regulatory information originating from the VirR/VirS system. The 3’-region of the VR-RNA molecule was shown to act as the positive effector in regulation, but the exact mechanism of action remains to be addressed [138, 139].
sRNAs regulate virulence gene expression in Streptococcus
In S. pyogenes, three sRNAs have been described: ncRNA (fasX RNA), a protein coding RNA (pel RNA) and a putatively processed RNA (rivX RNA). All participate in the control of virulence factor expression; however, the regulatory mechanisms used by these RNAs have not been determined yet. fasX RNA is part of an operon encoding two histidine kinases (FasBC) and one response regulator (FasA) [140]. In a M49 serotype, the fasBCA regulon, via the effector fasX RNA, negatively controls the expression of two matrix protein-binding adhesins (fibronectin-binding protein FBP54 and fibrinogen-binding protein MRP) at the transcriptional level and has a positive influence on the activities of two secreted virulence factors (streptokinase and streptolysin S) [140]. Whether fasX RNA regulates synthesis of a transcriptional regulator or binds to a regulatory protein, thereby inducing indirect effects at the transcriptional level remains to be addressed. Interestingly, fasX positively controls the interaction of S. pyogenes with epithelial laryngeal cells and bacterial aggressiveness in terms of host cell response [141]. Its expression is under the influence of amino acid starvation in a M49 serotype and regulated by the luxS/AI2 signalling system in a M19 serotype [142, 143]. The second sRNA, pel RNA of the pleiotropic effect locus functions as a regulator of virulence factor expression at both transcriptional (emm encoding the surface-exposed virulence M protein and sic encoding a streptococcal inhibitor of the complement system) and post-transcriptional levels (SpeB encoding a cysteine protease) in a M1 strain [144]. These regulations however are indirect, and the primary targets are not known. The pel locus comprises sagA encoding Streptolysin S, one of the two hemolysins of S. pyogenes. Similar to S. aureus RNAIII, pel RNA displays bi-functionality, being an effector of virulence factor expression and encoding at the same time a hemolysin. More recently, a study revealed rivX RNA is co-expressed with the downstream gene encoding the response regulator RivR (RALP4) [145]. rivX RNA together with RivR was shown to positively affect expression of virulence genes that are activated by the global transcriptional regulon Mga [145]. Data suggest that rivX RNA is a processed form of the rivRX cotranscript and may act as a trans-acting sRNA [145]. Lately, a genome-wide computational and experimental search for novel sRNAs in S. pyogenes led to the prediction and validation of a number of candidates encoded within the intergenic regions of the genome (Charpentier, personal communication). In S. pneumoniae, five sRNAs sharing a high degree of similarity among each other were recently identified. The so-called csRNAs are expressed under control of the response regulator CiaR of the two-component system, CiaRH. Two of these sRNAs, csRNA4 and csRNA5, are involved in stationary-phase autolysis [146].
Multiple regulatory RNAs in L. monocytogenes
As mentioned above, in L. monocytogenes, the protein Hfq contributes to stress tolerance and virulence. This suggested that RNA-dependent regulation might be associated with these phenotypes [64]. By using coimmunoprecipitation, three sRNAs conserved among Listeria species were identified (LhrA, LhrB, LhrC). The stability of these sRNAs is Hfq-dependent. LhrB appears to be produced by a transcriptional attenuation mechanism and was recently annotated as the regulatory region of the L20 r-protein operon. LhrC is present in five copies within the L. monocytogenes genome. The expression of the three sRNAs is dependent on growth phase, and all of them are expressed in L. monocytogenes multiplying within mammalian cells [63]. Using in silico approaches, nine additional sRNAs were identified [44]. In contrast to the previous study, the stability of these sRNAs seems to be Hfq-independent. Among these sRNAs, five were found only in the pathogenic strains. Three of these sRNAs act as antisense RNAs: (1) RliD is complementary to pnp mRNA encoding the 3′-5′ exoribonuclease polynucleotide phosphorylase; (2) RliH is complementary to a mRNA encoding a putative regulator of transcription; (3) RliE acts as antisense RNA of comC mRNA and forms stable complexes with several mRNAs encoding proteins that are part of a complex similar to the competence machinery of B. subtilis. All of these ncRNAs overlap the RBS of the mRNAs, suggesting that they would regulate translation. Of interest, RliB carries five repeats of 29 nucleotides reminiscent of a CRISPR RNA except that none of the genes in the vicinity of rliB shows significant homology with the cas genes. More recently, transcription of the entire genome of L. monocytogenes was probed using tiling arrays from wild-type and mutant strains grown in rich medium or purified from infected cells and mice [12]. In addition to 21 previously known sRNAs, 29 additional ones were characterized. All of them have a size between 77 and 534 nucleotides, and three contain a small ORF. Although the expression of none of the sRNAs seems to be dependent on Hfq or the major transcriptional regulator of virulence PrfA, the synthesis of three sRNAs was significantly induced by the stress–response alternative Sigma B factor. One of these RNAs (SbrA) was already characterized [65]. Remarkably, major changes in the expression pattern of many sRNAs were observed in the intestinal lumen or in blood after infection of mice [12]. For instance, expression of the five LhrC and of RliB is strongly enhanced after blood infection. Prediction of basepairings of the Rli sRNAs with mRNAs provided some ideas on their functions and suggest that many of the sRNAs would target mRNAs involved in metabolism or mRNAs encoding virulence factors such as the internalins. Several sRNAs could potentially interact with other sRNAs [12]. Two of the sRNAs (rli38 and rliB) affect virulence in different ways. The expression of Rli38 is strongly induced in blood and in response to oxidative stress, and the rli38 deletion mutant is attenuated when bacteria are orally inoculated in mice. In contrast, the rliB deletion mutant strain colonizes the liver faster than the wild-type strain. Therefore, this study highlights the crucial role of sRNAs in adaptation of the bacteria to the host and during the infection process [12]. Besides sRNAs, tiling arrays experimentally validated 40 cis-acting regulatory regions of mRNAs, which respond to metabolites, uncharged tRNAs or regulatory proteins. Of relevance, long RNAs corresponding to 5′-UTRs of mRNAs were revealed for known virulence genes and genes that encode putatively virulence-associated surface proteins [12, 147]. Three long antisense RNAs complementary to more than one ORF were identified, and several RNAs corresponding to long 5′ or 3′UTRs of mRNAs and partially overlapping with mRNAs produced from convergent genes were also shown to be expressed. Such a long RNA, the expression of which is induced by Sigma B, was proposed to regulate flagellum biosynthesis [12]. In addition to the coordinated global transcriptional changes observed during the infection process, this study further emphasizes the importance of RNA-dependent regulation in L. monocytogenes virulence.
Concluding remarks and perspectives
In recent years, major findings have been made in the field of regulatory RNAs in gram-negative bacteria. In gram-positive bacteria, while many riboswitches have been discovered and studied, the information on the modes of action of trans-acting sRNAs is often missing. This picture may change considerably given the fact that in silico and experimental genome-wide approaches have allowed the identification of a battery of regulatory RNA candidates in several gram-positive species. Recent data show that some of the identified RNAs play critical functions in adaptive responses of the bacteria and in pathogenesis events and thus constitute key players in the signaling networks of gene expression control. It is very likely that other newly revealed RNAs interfere as well with these biological processes.
Interestingly several regulatory signals on mRNAs (e.g., metabolite-sensing ribosowitches, leader regions of r-protein operon mRNAs) are conserved in the two distant bacteria, E. coli and B. subtilis, which have been proposed to have diverged about 109 years ago. These conserved regulatory signals reflect primitive regulatory mechanisms. On the other hand, these bacteria have retained/evolved different strategies for regulation. For instance, RNA-dependent regulation in firmicutes provides a genetic response primarily focused at the transcriptional termination/anti-termination level, while in gram-negative bacteria gene expression is regulated at the translational level (e.g., metabolite-sensing ribosowitches, uncharged tRNAs, proteins). Firmicutes also use different RNA-based strategies to regulate the expression of similar genes. For example, in low GC gram-positive bacteria, glmS expression is regulated by a cis-acting regulatory element, whereas in enterobacteriae, a regulatory cascade, involving two trans-acting sRNAs, leads to the activation of glmS mRNA translation [21]. Gram-positive and gram-negative bacteria utilize distinct machineries for mRNA degradation and different strategies to initiate translation initiation that might contribute to explaining why the bacteria use different RNA-based regulatory mechanisms. In gram-positive bacteria, many mRNAs carry strong SD sequences, and the ribosomal 30S subunit lacks the ribosomal protein S1. Furthermore, stalled ribosomes can have a long-distance effect on mRNA stability in gram-positive bacteria. This protection can be the consequence of the mRNA decay by RNase J1, a 5′-3′ exoribonuclease, which does not exist in E. coli [148–150]. In addition regulation by sRNAs in gram-positive bacteria can regulate translation, but degradation is not always a consequence as in gram-negative bacteria. The RNA chaperone Hfq does not seem to play a major role in the action of sRNAs on their target mRNAs in gram-positive bacteria, and a system analogous to the RNase E–Hfq–sRNA complex has not been identified yet. Whether the sRNAs associate with other trans-acting proteins to exert their function remains to be determined. Another point of interest is how do sRNAs recognize their targets? Are there some specific recognition signatures on sRNAs that target mRNAs, like the C-rich motif of S. aureus RNAIII, and in Listeria and B. subtilis sRNAs? Do the sRNAs target the coding sequence and the 3′UTR of target mRNAs? The current data accumulating from high-throughput genome analyses (e.g., deep-sequencing, tiling arrays, biocomputational tools) should expand not only the number, but also the diversity of regulatory RNAs. What is the proportion of trans-acting sRNAs versus cis-acting regulatory elements? What are the roles of the class of long RNAs? Would they be involved in RNA quality control (processing and maturation events, elimination of non-translated mRNAs)? Recently, in E. coli and Salmonella, a novel RNA-based regulatory mechanism has been identified whereby a mRNA mimics a mRNA target and serves as a sRNA trap to induce the degradation of a constitutively expressed sRNA [149, 150]. Therefore, a mRNA can be potentially regarded as a target of sRNA, but also as a trans-acting regulator of sRNA [151, 152]. In L. monocytogenes and S. aureus, several sRNAs could also potentially interact with other sRNAs [12]. In addition to classical transcriptional control, it might be possible that RNA-mediated degradation of sRNA is also playing an important role in gram-positive bacteria. How widespread are multifunctional RNAs like S. aureus RNAIII, which is a mRNA and acts as an antisense RNA? What are the mechanisms used by the CRISPR RNAs to target extrachromosomal DNA? Are there some RNAs that would be involved in control of genome maintenance? Like viruses, phytopathogenic bacteria have evolved strategies to suppress or counteract cellular RNA silencing to cause disease in the host plant [153]. There is increasing evidence that endogenous plant small RNAs have broad regulatory functions in response to various bacteria. Whether pathogenic bacteria derived sRNAs would be produced/secreted within the host cell awaits further demonstration [154].
The diversity of RNA-mediated regulatory systems described so far in gram-positive bacteria might predict that additional regulatory mechanisms are still to be expected. Future research is therefore necessary to identify the exact mechanisms by which regulatory RNAs, in particular the trans-acting sRNAs, act on their targets. This information will help our understanding of the regulatory circuits involved in bacterial adaptation and virulence. One of the next challenges in gram-positive regulatory RNA biology will be to understand how regulation by RNAs occurs at a single cell level within a population of cells to coordinately regulate gene expression. In their environments, bacteria are forming heterogeneous populations of cells that communicate with each other and respond distinctly to specific external signals. In this context, it is predicted that regulatory RNAs are expressed at specific times and in defined spaces. Future development of mechanistic and cell biology studies will be necessary to obtain knowledge of the spatial and temporal dynamics of RNA-mediated regulation in the cell.
Acknowledgments
We are grateful to Brian Jester for critical reading of the manuscript and helpful comments, and we thank members of our teams for stimulating discussions. This work was supported by the European Community FP6 project no. BacRNA-018618 (E.C. and P.R.), the Austrian Science Fund (FWF, project nos. P17238-B09 and W1207-B09) (E.C.), the Austrian Research Promotion Agency (FFG, project no. 812138-SCK/KUG) (E.C.), the Theodor Körner Fonds (E.C.), Umeå University (E.C.), the Swedish Research Council (VR) (E.C.), the CNRS “Centre National de la Recherche Scientifique” (P.R.) and the ANR “Agence Nationale pour la Recherche” (ANR05-MIIM-034-01, ANR09-Blan-436938).
References
- 1.Waters LS, Storz G. Regulatory RNAs in bacteria. Cell. 2009;136:615–628. doi: 10.1016/j.cell.2009.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogel J, Wagner EG. Target identification of small noncoding RNAs in bacteria. Curr Opin Microbiol. 2007;10:262–270. doi: 10.1016/j.mib.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Gottesman S. Micros for microbes: non-coding regulatory RNAs in bacteria. Trends Genet. 2005;21:399–404. doi: 10.1016/j.tig.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Tomizawa J, Itoh T, Selzer G, Som T. Inhibition of ColE1 RNA primer formation by a plasmid-specified small RNA. Proc Natl Acad Sci USA. 1981;78:1421–1425. doi: 10.1073/pnas.78.3.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stougaard P, Molin S, Nordstrom K. RNAs involved in copy-number control and incompatibility of plasmid R1. Proc Natl Acad Sci USA. 1981;78:6008–6012. doi: 10.1073/pnas.78.10.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen J, Forst SA, Zhao K, Inouye M, Delihas N. The function of micF RNA micF RNA is a major factor in the thermal regulation of OmpF protein in Escherichia coli . J Biol Chem. 1989;264:17961–17970. [PubMed] [Google Scholar]
- 7.Delihas N. Antisense micF RNA and 5′-UTR of the target ompF RNA: phylogenetic conservation of primary and secondary structures. Nucleic Acids Symp Ser. 1997;36:33–35. [PubMed] [Google Scholar]
- 8.Livny J, Teonadi H, Livny M, Waldor MK. High-throughput, kingdom-wide prediction and annotation of bacterial non-coding RNAs. PLoS ONE. 2008;3:e3197. doi: 10.1371/journal.pone.0003197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchais A, Naville M, Bohn C, Bouloc P, Gautheret D. Single-pass classification of all noncoding sequences in a bacterial genome using phylogenetic profiles. Genome Res. 2009;19:1084–1092. doi: 10.1101/gr.089714.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sittka A, Lucchini S, Papenfort K, Sharma CM, Rolle K, Binnewies TT, Hinton JC, Vogel J. Deep sequencing analysis of small noncoding RNA and mRNA targets of the global post-transcriptional regulator, Hfq. PLoS Genet. 2008;4:e1000163. doi: 10.1371/journal.pgen.1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landt SG, Abeliuk E, McGrath PT, Lesley JA, McAdams HH, Shapiro L. Small non-coding RNAs in Caulobacter crescentus . Mol Microbiol. 2008;68:600–614. doi: 10.1111/j.1365-2958.2008.06172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toledo-Arana A, Dussurget O, Nikitas G, Sesto N, Guet-Revillet H, Balestrino D, Loh E, Gripenland J, Tiensuu T, Vaitkevicius K, Barthelemy M, Vergassola M, Nahori MA, Soubigou G, Regnault B, Coppee JY, Lecuit M, Johansson J, Cossart P. The Listeria transcriptional landscape from saprophytism to virulence. Nature. 2009;459:950–956. doi: 10.1038/nature08080. [DOI] [PubMed] [Google Scholar]
- 13.Fozo EM, Hemm MR, Storz G. Small toxic proteins and the antisense RNAs that repress them. Microbiol Mol Biol Rev. 2008;72:579–589. doi: 10.1128/MMBR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romby P, Vandenesch F, Wagner EG. The role of RNAs in the regulation of virulence-gene expression. Curr Opin Microbiol. 2006;9:229–236. doi: 10.1016/j.mib.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Toledo-Arana A, Repoila F, Cossart P. Small noncoding RNAs controlling pathogenesis. Curr Opin Microbiol. 2007;10:182–188. doi: 10.1016/j.mib.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Bejerano-Sagie M, Xavier KB. The role of small RNAs in quorum sensing. Curr Opin Microbiol. 2007;10:189–198. doi: 10.1016/j.mib.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Vogel J. A rough guide to the non-coding RNA world of Salmonella . Mol Microbiol. 2009;71:1–11. doi: 10.1111/j.1365-2958.2008.06505.x. [DOI] [PubMed] [Google Scholar]
- 18.Marraffini LA, Sontheimer EJ. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science. 2008;322:1843–1845. doi: 10.1126/science.1165771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marraffini LA, Sontheimer EJ. Invasive DNA, Chopped and in the CRISPR. Structure. 2009;17:786–788. doi: 10.1016/j.str.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babitzke P, Romeo T. CsrB sRNA family: sequestration of RNA-binding regulatory proteins. Curr Opin Microbiol. 2007;10:156–163. doi: 10.1016/j.mib.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Gorke B, Vogel J. Noncoding RNA control of the making and breaking of sugars. Genes Dev. 2008;22:2914–2925. doi: 10.1101/gad.1717808. [DOI] [PubMed] [Google Scholar]
- 22.Wassarman KM. 6S RNA: a small RNA regulator of transcription. Curr Opin Microbiol. 2007;10:164–168. doi: 10.1016/j.mib.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Coppins RL, Hall KB, Groisman EA. The intricate world of riboswitches. Curr Opin Microbiol. 2007;10:176–181. doi: 10.1016/j.mib.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henkin TM. RNA-dependent RNA switches in bacteria. Methods Mol Biol. 2009;540:207–214. doi: 10.1007/978-1-59745-558-9_15. [DOI] [PubMed] [Google Scholar]
- 25.Pichon C, Felden B. Small RNA genes expressed from Staphylococcus aureus genomic and pathogenicity islands with specific expression among pathogenic strains. Proc Natl Acad Sci USA. 2005;102:14249–14254. doi: 10.1073/pnas.0503838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valverde C, Haas D. Small RNAs controlled by two-component systems. Adv Exp Med Biol. 2008;631:54–79. doi: 10.1007/978-0-387-78885-2_5. [DOI] [PubMed] [Google Scholar]
- 27.Boisset S, Geissmann T, Huntzinger E, Fechter P, Bendridi N, Possedko M, Chevalier C, Helfer AC, Benito Y, Jacquier A, Gaspin C, Vandenesch F, Romby P. Staphylococcus aureus RNAIII coordinately represses the synthesis of virulence factors and the transcription regulator Rot by an antisense mechanism. Genes Dev. 2007;21:1353–1366. doi: 10.1101/gad.423507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heidrich N, Chinali A, Gerth U, Brantl S. The small untranslated RNA SR1 from the Bacillus subtilis genome is involved in the regulation of arginine catabolism. Mol Microbiol. 2006;62:520–536. doi: 10.1111/j.1365-2958.2006.05384.x. [DOI] [PubMed] [Google Scholar]
- 29.Heidrich N, Moll I, Brantl S. In vitro analysis of the interaction between the small RNA SR1 and its primary target ahrC mRNA. Nucleic Acids Res. 2007;35:4331–4346. doi: 10.1093/nar/gkm439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huntzinger E, Boisset S, Saveanu C, Benito Y, Geissmann T, Namane A, Lina G, Etienne J, Ehresmann B, Ehresmann C, Jacquier A, Vandenesch F, Romby P. Staphylococcus aureus RNAIII and the endoribonuclease III coordinately regulate spa gene expression. EMBO J. 2005;24:824–835. doi: 10.1038/sj.emboj.7600572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Licht A, Preis S, Brantl S. Implication of CcpN in the regulation of a novel untranslated RNA (SR1) in Bacillus subtilis . Mol Microbiol. 2005;58:189–206. doi: 10.1111/j.1365-2958.2005.04810.x. [DOI] [PubMed] [Google Scholar]
- 32.Morfeldt E, Taylor D, von Gabain A, Arvidson S. Activation of alpha-toxin translation in Staphylococcus aureus by the trans-encoded antisense RNA, RNAIII. EMBO J. 1995;14:4569–4577. doi: 10.1002/j.1460-2075.1995.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagner EG, Altuvia S, Romby P. Antisense RNAs in bacteria and their genetic elements. Adv Genet. 2002;46:361–398. doi: 10.1016/s0065-2660(02)46013-0. [DOI] [PubMed] [Google Scholar]
- 35.Weaver KE. Emerging plasmid-encoded antisense RNA regulated systems. Curr Opin Microbiol. 2007;10:110–116. doi: 10.1016/j.mib.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Brantl S. Regulatory mechanisms employed by cis-encoded antisense RNAs. Curr Opin Microbiol. 2007;10:102–109. doi: 10.1016/j.mib.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Novick RP, Iordanescu S, Projan SJ, Kornblum J, Edelman I. pT181 plasmid replication is regulated by a countertranscript-driven transcriptional attenuator. Cell. 1989;59:395–404. doi: 10.1016/0092-8674(89)90300-0. [DOI] [PubMed] [Google Scholar]
- 38.Brantl S, Birch-Hirschfeld E, Behnke D. RepR protein expression on plasmid pIP501 is controlled by an antisense RNA-mediated transcription attenuation mechanism. J Bacteriol. 1993;175:4052–4061. doi: 10.1128/jb.175.13.4052-4061.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brantl S, Wagner EG. Antisense RNA-mediated transcriptional attenuation occurs faster than stable antisense/target RNA pairing: an in vitro study of plasmid pIP501. EMBO J. 1994;13:3599–3607. doi: 10.1002/j.1460-2075.1994.tb06667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwong SM, Skurray RA, Firth N. Replication control of staphylococcal multiresistance plasmid pSK41: an antisense RNA mediates dual-level regulation of Rep expression. J Bacteriol. 2006;188:4404–4412. doi: 10.1128/JB.00030-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenfield TJ, Ehli E, Kirshenmann T, Franch T, Gerdes K, Weaver KE. The antisense RNA of the par locus of pAD1 regulates the expression of a 33-amino-acid toxic peptide by an unusual mechanism. Mol Microbiol. 2000;37:652–660. doi: 10.1046/j.1365-2958.2000.02035.x. [DOI] [PubMed] [Google Scholar]
- 42.Greenfield TJ, Weaver KE. Antisense RNA regulation of the pAD1 par post-segregational killing system requires interaction at the 5’ and 3’ ends of the RNAs. Mol Microbiol. 2000;37:661–670. doi: 10.1046/j.1365-2958.2000.02034.x. [DOI] [PubMed] [Google Scholar]
- 43.Silvaggi JM, Perkins JB, Losick R. Small untranslated RNA antitoxin in Bacillus subtilis . J Bacteriol. 2005;187:6641–6650. doi: 10.1128/JB.187.19.6641-6650.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mandin P, Repoila F, Vergassola M, Geissmann T, Cossart P. Identification of new noncoding RNAs in Listeria monocytogenes and prediction of mRNA targets. Nucleic Acids Res. 2007;35:962–974. doi: 10.1093/nar/gkl1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silvaggi JM, Perkins JB, Losick R. Genes for small, noncoding RNAs under sporulation control in Bacillus subtilis . J Bacteriol. 2006;188:532–541. doi: 10.1128/JB.188.2.532-541.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saito S, Kakeshita H, Nakamura K. Novel small RNA-encoding genes in the intergenic regions of Bacillus subtilis . Gene. 2009;428:2–8. doi: 10.1016/j.gene.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 47.Novick RP. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol. 2003;48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 48.Novick RP, Geisinger E. Quorum sensing in staphylococci. Annu Rev Genet. 2008;42:541–564. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 49.Cheung AL, Eberhardt KJ, Chung E, Yeaman MR, Sullam PM, Ramos M, Bayer AS. Diminished virulence of a sar−/agr− mutant of Staphylococcus aureus in the rabbit model of endocarditis. J Clin Invest. 1994;94:1815–1822. doi: 10.1172/JCI117530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gillaspy AF, Hickmon SG, Skinner RA, Thomas JR, Nelson CL, Smeltzer MS. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect Immun. 1995;63:3373–3380. doi: 10.1128/iai.63.9.3373-3380.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Novick RP, Muir TW. Virulence gene regulation by peptides in staphylococci and other Gram-positive bacteria. Curr Opin Microbiol. 1999;2:40–45. doi: 10.1016/s1369-5274(99)80007-1. [DOI] [PubMed] [Google Scholar]
- 52.Benito Y, Kolb FA, Romby P, Lina G, Etienne J, Vandenesch F. Probing the structure of RNAIII, the Staphylococcus aureus agr regulatory RNA, and identification of the RNA domain involved in repression of protein A expression. RNA. 2000;6:668–679. doi: 10.1017/s1355838200992550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geisinger E, Adhikari RP, Jin R, Ross HF, Novick RP. Inhibition of rot translation by RNAIII, a key feature of agr function. Mol Microbiol. 2006;61:1038–1048. doi: 10.1111/j.1365-2958.2006.05292.x. [DOI] [PubMed] [Google Scholar]
- 54.Anderson KL, Roberts C, Disz T, Vonstein V, Hwang K, Overbeek R, Olson PD, Projan SJ, Dunman PM. Characterization of the Staphylococcus aureus heat shock, cold shock, stringent, and SOS responses and their effects on log-phase mRNA turnover. J Bacteriol. 2006;188:6739–6756. doi: 10.1128/JB.00609-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roberts C, Anderson KL, Murphy E, Projan SJ, Mounts W, Hurlburt B, Smeltzer M, Overbeek R, Disz T, Dunman PM. Characterizing the effect of the Staphylococcus aureus virulence factor regulator, SarA, on log-phase mRNA half-lives. J Bacteriol. 2006;188:2593–2603. doi: 10.1128/JB.188.7.2593-2603.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Livny J, Brencic A, Lory S, Waldor MK. Identification of 17 Pseudomonas aeruginosa sRNAs and prediction of sRNA-encoding genes in 10 diverse pathogens using the bioinformatic tool sRNAPredict2. Nucleic Acids Res. 2006;34:3484–3493. doi: 10.1093/nar/gkl453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geissmann T, Chevalier C, Cross MJ, Boisset S, Fechter P, Noirot C, Schrenzel J, Francois P, Vandenesch F, Gaspin C, Romby P (2009) A search for small noncoding RNAs in Staphylococcus aureus reveals a conserved motif for regulation. Nucleic Acids Res (in press) [DOI] [PMC free article] [PubMed]
- 58.Aiba H. Mechanism of RNA silencing by Hfq-binding small RNAs. Curr Opin Microbiol. 2007;10:134–139. doi: 10.1016/j.mib.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 59.Brennan RG, Link TM. Hfq structure, function and ligand binding. Curr Opin Microbiol. 2007;10:125–133. doi: 10.1016/j.mib.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 60.Valentin-Hansen P, Eriksen M, Udesen C. The bacterial Sm-like protein Hfq: a key player in RNA transactions. Mol Microbiol. 2004;51:1525–1533. doi: 10.1111/j.1365-2958.2003.03935.x. [DOI] [PubMed] [Google Scholar]
- 61.Bohn C, Rigoulay C, Bouloc P. No detectable effect of RNA-binding protein Hfq absence in Staphylococcus aureus . BMC Microbiol. 2007;7:10. doi: 10.1186/1471-2180-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vecerek B, Rajkowitsch L, Sonnleitner E, Schroeder R, Blasi U. The C-terminal domain of Escherichia coli Hfq is required for regulation. Nucleic Acids Res. 2008;36:133–143. doi: 10.1093/nar/gkm985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Christiansen JK, Nielsen JS, Ebersbach T, Valentin-Hansen P, Sogaard-Andersen L, Kallipolitis BH. Identification of small Hfq-binding RNAs in Listeria monocytogenes . RNA. 2006;12:1383–1396. doi: 10.1261/rna.49706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Christiansen JK, Larsen MH, Ingmer H, Sogaard-Andersen L, Kallipolitis BH. The RNA-binding protein Hfq of Listeria monocytogenes: role in stress tolerance and virulence. J Bacteriol. 2004;186:3355–3362. doi: 10.1128/JB.186.11.3355-3362.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nielsen JS, Olsen AS, Bonde M, Valentin-Hansen P, Kallipolitis BH. Identification of a sigma B-dependent small noncoding RNA in Listeria monocytogenes . J Bacteriol. 2008;190:6264–6270. doi: 10.1128/JB.00740-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trotochaud AE, Wassarman KM. A highly conserved 6S RNA structure is required for regulation of transcription. Nat Struct Mol Biol. 2005;12:313–319. doi: 10.1038/nsmb917. [DOI] [PubMed] [Google Scholar]
- 67.Barrick JE, Sudarsan N, Weinberg Z, Ruzzo WL, Breaker RR. 6S RNA is a widespread regulator of eubacterial RNA polymerase that resembles an open promoter. RNA. 2005;11:774–784. doi: 10.1261/rna.7286705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hartmann RK, Gossringer M, Spath B, Fischer S, Marchfelder A. The making of tRNAs and more—RNase P and tRNase Z. Prog Mol Biol Transl Sci. 2009;85:319–368. doi: 10.1016/S0079-6603(08)00808-8. [DOI] [PubMed] [Google Scholar]
- 69.Altman S. A view of RNase P. Mol Biosyst. 2007;3:604–607. doi: 10.1039/b707850c. [DOI] [PubMed] [Google Scholar]
- 70.Marvin MC, Engelke DR. RNase P: increased versatility through protein complexity? RNA Biol. 2009;6:40–42. doi: 10.4161/rna.6.1.7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wegscheid B, Hartmann RK. In vivo and in vitro investigation of bacterial type B RNase P interaction with tRNA 3′-CCA. Nucleic Acids Res. 2007;35:2060–2073. doi: 10.1093/nar/gkm005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roselli DM, Marsh TL. Purification and characterization of RNase P from Clostridium sporogenes . Mol Microbiol. 1990;4:1393–1400. doi: 10.1111/j.1365-2958.1990.tb00718.x. [DOI] [PubMed] [Google Scholar]
- 73.Spitzfaden C, Nicholson N, Jones JJ, Guth S, Lehr R, Prescott CD, Hegg LA, Eggleston DS. The structure of ribonuclease P protein from Staphylococcus aureus reveals a unique binding site for single-stranded RNA. J Mol Biol. 2000;295:105–115. doi: 10.1006/jmbi.1999.3341. [DOI] [PubMed] [Google Scholar]
- 74.Altman S, Wesolowski D, Guerrier-Takada C, Li Y. RNase P cleaves transient structures in some riboswitches. Proc Natl Acad Sci USA. 2005;102:11284–11289. doi: 10.1073/pnas.0505271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seif E, Altman S. RNase P cleaves the adenine riboswitch and stabilizes pbuE mRNA in Bacillus subtilis . RNA. 2008;14:1237–1243. doi: 10.1261/rna.833408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keenan RJ, Freymann DM, Stroud RM, Walter P. The signal recognition particle. Annu Rev Biochem. 2001;70:755–775. doi: 10.1146/annurev.biochem.70.1.755. [DOI] [PubMed] [Google Scholar]
- 77.Crowley PJ, Svensater G, Snoep JL, Bleiweis AS, Brady LJ. An ffh mutant of Streptococcus mutans is viable and able to physiologically adapt to low pH in continuous culture. FEMS Microbiol Lett. 2004;234:315–324. doi: 10.1016/j.femsle.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 78.Hasona A, Zuobi-Hasona K, Crowley PJ, Abranches J, Ruelf MA, Bleiweis AS, Brady LJ. Membrane composition changes and physiological adaptation by Streptococcus mutans signal recognition particle pathway mutants. J Bacteriol. 2007;189:1219–1230. doi: 10.1128/JB.01146-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hasona A, Crowley PJ, Levesque CM, Mair RW, Cvitkovitch DG, Bleiweis AS, Brady LJ. Streptococcal viability and diminished stress tolerance in mutants lacking the signal recognition particle pathway or YidC2. Proc Natl Acad Sci USA. 2005;102:17466–17471. doi: 10.1073/pnas.0508778102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosch JW, Vega LA, Beyer JM, Lin A, Caparon MG. The signal recognition particle pathway is required for virulence in Streptococcus pyogenes . Infect Immun. 2008;76:2612–2619. doi: 10.1128/IAI.00239-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kremer BH, van der Kraan M, Crowley PJ, Hamilton IR, Brady LJ, Bleiweis AS. Characterization of the sat operon in Streptococcus mutans: evidence for a role of Ffh in acid tolerance. J Bacteriol. 2001;183:2543–2552. doi: 10.1128/JB.183.8.2543-2552.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Keiler KC. Biology of trans-translation. Annu Rev Microbiol. 2008;62:133–151. doi: 10.1146/annurev.micro.62.081307.162948. [DOI] [PubMed] [Google Scholar]
- 83.Moore SD, Sauer RT. The tmRNA system for translational surveillance and ribosome rescue. Annu Rev Biochem. 2007;76:101–124. doi: 10.1146/annurev.biochem.75.103004.142733. [DOI] [PubMed] [Google Scholar]
- 84.Saguy M, Gillet R, Metzinger L, Felden B. tmRNA and associated ligands: a puzzling relationship. Biochimie. 2005;87:897–903. doi: 10.1016/j.biochi.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 85.Abe T, Sakaki K, Fujihara A, Ujiie H, Ushida C, Himeno H, Sato T, Muto A. tmRNA-dependent trans-translation is required for sporulation in Bacillus subtilis . Mol Microbiol. 2008;69:1491–1498. doi: 10.1111/j.1365-2958.2008.06381.x. [DOI] [PubMed] [Google Scholar]
- 86.Wiegert T, Schumann W. SsrA-mediated tagging in Bacillus subtilis . J Bacteriol. 2001;183:3885–3889. doi: 10.1128/JB.183.13.3885-3889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sorek R, Kunin V, Hugenholtz P. CRISPR—a widespread system that provides acquired resistance against phages in bacteria and archaea. Nat Rev Microbiol. 2008;6:181–186. doi: 10.1038/nrmicro1793. [DOI] [PubMed] [Google Scholar]
- 88.Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- 89.Brouns SJ, Jore MM, Lundgren M, Westra ER, Slijkhuis RJ, Snijders AP, Dickman MJ, Makarova KS, Koonin EV, van der Oost J. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science. 2008;321:960–964. doi: 10.1126/science.1159689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carte J, Wang R, Li H, Terns RM, Terns MP. Cas6 is an endoribonuclease that generates guide RNAs for invader defense in prokaryotes. Genes Dev. 2008;22:3489–3496. doi: 10.1101/gad.1742908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Makarova KS, Grishin NV, Shabalina SA, Wolf YI, Koonin EV. A putative RNA-interference-based immune system in prokaryotes: computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action. Biol Direct. 2006;1:7. doi: 10.1186/1745-6150-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barrick JE, Breaker RR. The distributions, mechanisms, and structures of metabolite-binding riboswitches. Genome Biol. 2007;8:R239. doi: 10.1186/gb-2007-8-11-r239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dambach MD, Winkler WC. Expanding roles for metabolite-sensing regulatory RNAs. Curr Opin Microbiol. 2009;12:161–169. doi: 10.1016/j.mib.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Greenleaf WJ, Frieda KL, Foster DA, Woodside MT, Block SM. Direct observation of hierarchical folding in single riboswitch aptamers. Science. 2008;319:630–633. doi: 10.1126/science.1151298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nudler E, Mironov AS. The riboswitch control of bacterial metabolism. Trends Biochem Sci. 2004;29:11–17. doi: 10.1016/j.tibs.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 96.Nudler E. Flipping riboswitches. Cell. 2006;126:19–22. doi: 10.1016/j.cell.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 97.Soukup JK, Soukup GA. Riboswitches exert genetic control through metabolite-induced conformational change. Curr Opin Struct Biol. 2004;14:344–349. doi: 10.1016/j.sbi.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 98.Vitreschak AG, Rodionov DA, Mironov AA, Gelfand MS. Riboswitches: the oldest mechanism for the regulation of gene expression? Trends Genet. 2004;20:44–50. doi: 10.1016/j.tig.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 99.Serganov A, Patel DJ. Towards deciphering the principles underlying an mRNA recognition code. Curr Opin Struct Biol. 2008;18:120–129. doi: 10.1016/j.sbi.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Serganov A. The long and the short of riboswitches. Curr Opin Struct Biol. 2009;19:251–259. doi: 10.1016/j.sbi.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weinberg Z, Barrick JE, Yao Z, Roth A, Kim JN, Gore J, Wang JX, Lee ER, Block KF, Sudarsan N, Neph S, Tompa M, Ruzzo WL, Breaker RR. Identification of 22 candidate structured RNAs in bacteria using the CMfinder comparative genomics pipeline. Nucleic Acids Res. 2007;35:4809–4819. doi: 10.1093/nar/gkm487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Winkler WC, Breaker RR. Regulation of bacterial gene expression by riboswitches. Annu Rev Microbiol. 2005;59:487–517. doi: 10.1146/annurev.micro.59.030804.121336. [DOI] [PubMed] [Google Scholar]
- 103.Gutierrez-Preciado A, Henkin TM, Grundy FJ, Yanofsky C, Merino E. Biochemical features and functional implications of the RNA-based T-box regulatory mechanism. Microbiol Mol Biol Rev. 2009;73:36–61. doi: 10.1128/MMBR.00026-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vitreschak AG, Mironov AA, Lyubetsky VA, Gelfand MS. Comparative genomic analysis of T-box regulatory systems in bacteria. RNA. 2008;14:717–735. doi: 10.1261/rna.819308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sudarsan N, Hammond MC, Block KF, Welz R, Barrick JE, Roth A, Breaker RR. Tandem riboswitch architectures exhibit complex gene control functions. Science. 2006;314:300–304. doi: 10.1126/science.1130716. [DOI] [PubMed] [Google Scholar]
- 106.Roth A, Nahvi A, Lee M, Jona I, Breaker RR. Characteristics of the glmS ribozyme suggest only structural roles for divalent metal ions. RNA. 2006;12:607–619. doi: 10.1261/rna.2266506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Klein DJ, Ferre-D’Amare AR. Structural basis of glmS ribozyme activation by glucosamine-6-phosphate. Science. 2006;313:1752–1756. doi: 10.1126/science.1129666. [DOI] [PubMed] [Google Scholar]
- 108.Hampel KJ, Tinsley MM. Evidence for preorganization of the glmS ribozyme ligand binding pocket. Biochemistry. 2006;45:7861–7871. doi: 10.1021/bi060337z. [DOI] [PubMed] [Google Scholar]
- 109.Winkler WC, Nahvi A, Roth A, Collins JA, Breaker RR. Control of gene expression by a natural metabolite-responsive ribozyme. Nature. 2004;428:281–286. doi: 10.1038/nature02362. [DOI] [PubMed] [Google Scholar]
- 110.Brooks KM, Hampel KJ. A rate-limiting conformational step in the catalytic pathway of the glmS ribozyme. Biochemistry. 2009;48:5669–5678. doi: 10.1021/bi900183r. [DOI] [PubMed] [Google Scholar]
- 111.Dann CE, 3rd, Wakeman CA, Sieling CL, Baker SC, Irnov I, Winkler WC. Structure and mechanism of a metal-sensing regulatory RNA. Cell. 2007;130:878–892. doi: 10.1016/j.cell.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 112.Buck J, Furtig B, Noeske J, Wohnert J, Schwalbe H. Time-resolved NMR methods resolving ligand-induced RNA folding at atomic resolution. Proc Natl Acad Sci USA. 2007;104:15699–15704. doi: 10.1073/pnas.0703182104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wickiser JK, Cheah MT, Breaker RR, Crothers DM. The kinetics of ligand binding by an adenine-sensing riboswitch. Biochemistry. 2005;44:13404–13414. doi: 10.1021/bi051008u. [DOI] [PubMed] [Google Scholar]
- 114.Morita MT, Tanaka Y, Kodama TS, Kyogoku Y, Yanagi H, Yura T. Translational induction of heat shock transcription factor sigma32: evidence for a built-in RNA thermosensor. Genes Dev. 1999;13:655–665. doi: 10.1101/gad.13.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Johansson J, Mandin P, Renzoni A, Chiaruttini C, Springer M, Cossart P. An RNA thermosensor controls expression of virulence genes in Listeria monocytogenes . Cell. 2002;110:551–561. doi: 10.1016/s0092-8674(02)00905-4. [DOI] [PubMed] [Google Scholar]
- 116.Romby P, Springer M (2007) Translational control in biology and medicine. In: Hershey J, Sonenberg N, Matthews M (eds) Cold Spring Harbor Laboratory Press, NY, pp 807–832
- 117.Grundy FJ, Henkin TM. Characterization of the Bacillus subtilis rpsD regulatory target site. J Bacteriol. 1992;174:6763–6770. doi: 10.1128/jb.174.21.6763-6770.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Choonee N, Even S, Zig L, Putzer H. Ribosomal protein L20 controls expression of the Bacillus subtilis infC operon via a transcription attenuation mechanism. Nucleic Acids Res. 2007;35:1578–1588. doi: 10.1093/nar/gkm011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Scott LG, Williamson JR. The binding interface between Bacillus stearothermophilus ribosomal protein S15 and its 5′-translational operator mRNA. J Mol Biol. 2005;351:280–290. doi: 10.1016/j.jmb.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 120.Turnbough CL, Jr, Switzer RL. Regulation of pyrimidine biosynthetic gene expression in bacteria: repression without repressors. Microbiol Mol Biol Rev. 2008;72:266–300. doi: 10.1128/MMBR.00001-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Merino E, Jensen RA, Yanofsky C. Evolution of bacterial trp operons and their regulation. Curr Opin Microbiol. 2008;11:78–86. doi: 10.1016/j.mib.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yakhnin AV, Yakhnin H, Babitzke P. Function of the Bacillus subtilis transcription elongation factor NusG in hairpin-dependent RNA polymerase pausing in the trp leader. Proc Natl Acad Sci USA. 2008;105:16131–16136. doi: 10.1073/pnas.0808842105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Valbuzzi A, Gollnick P, Babitzke P, Yanofsky C. The anti-trp RNA-binding attenuation protein (Anti-TRAP), AT, recognizes the tryptophan-activated RNA binding domain of the TRAP regulatory protein. J Biol Chem. 2002;277:10608–10613. doi: 10.1074/jbc.M111813200. [DOI] [PubMed] [Google Scholar]
- 124.Watanabe M, Heddle JG, Kikuchi K, Unzai S, Akashi S, Park SY, Tame JR. The nature of the TRAP–Anti-TRAP complex. Proc Natl Acad Sci USA. 2009;106:2176–2181. doi: 10.1073/pnas.0801032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cruz-Vera LR, Gong M, Yanofsky C. Physiological effects of anti-TRAP protein activity and tRNA(Trp) charging on trp operon expression in Bacillus subtilis . J Bacteriol. 2008;190:1937–1945. doi: 10.1128/JB.01820-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fujita Y. Carbon catabolite control of the metabolic network in Bacillus subtilis . Biosci Biotechnol Biochem. 2009;73:245–259. doi: 10.1271/bbb.80479. [DOI] [PubMed] [Google Scholar]
- 127.Crutz AM, Steinmetz M, Aymerich S, Richter R, Le Coq D. Induction of levansucrase in Bacillus subtilis: an antitermination mechanism negatively controlled by the phosphotransferase system. J Bacteriol. 1990;172:1043–1050. doi: 10.1128/jb.172.2.1043-1050.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Debarbouille M, Arnaud M, Fouet A, Klier A, Rapoport G. The sacT gene regulating the sacPA operon in Bacillus subtilis shares strong homology with transcriptional antiterminators. J Bacteriol. 1990;172:3966–3973. doi: 10.1128/jb.172.7.3966-3973.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Langbein I, Bachem S, Stulke J. Specific interaction of the RNA-binding domain of the Bacillus subtilis transcriptional antiterminator GlcT with its RNA target, RAT . J Mol Biol. 1999;293:795–805. doi: 10.1006/jmbi.1999.3176. [DOI] [PubMed] [Google Scholar]
- 130.Schilling O, Langbein I, Muller M, Schmalisch MH, Stulke J. A protein-dependent riboswitch controlling ptsGHI operon expression in Bacillus subtilis: RNA structure rather than sequence provides interaction specificity. Nucleic Acids Res. 2004;32:2853–2864. doi: 10.1093/nar/gkh611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schnetz K, Stulke J, Gertz S, Kruger S, Krieg M, Hecker M, Rak B. LicT, a Bacillus subtilis transcriptional antiterminator protein of the BglG family. J Bacteriol. 1996;178:1971–1979. doi: 10.1128/jb.178.7.1971-1979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stulke J, Martin-Verstraete I, Zagorec M, Rose M, Klier A, Rapoport G. Induction of the Bacillus subtilis ptsGHI operon by glucose is controlled by a novel antiterminator, GlcT. Mol Microbiol. 1997;25:65–78. doi: 10.1046/j.1365-2958.1997.4351797.x. [DOI] [PubMed] [Google Scholar]
- 133.Tortosa P, Le Coq D. A ribonucleic antiterminator sequence (RAT) and a distant palindrome are both involved in sucrose induction of the Bacillus subtilis sacXY regulatory operon. Microbiology. 1995;141(Pt 11):2921–2927. doi: 10.1099/13500872-141-11-2921. [DOI] [PubMed] [Google Scholar]
- 134.Lovett PS, Rogers EJ. Ribosome regulation by the nascent peptide. Microbiol Rev. 1996;60:366–385. doi: 10.1128/mr.60.2.366-385.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vazquez-Laslop N, Thum C, Mankin AS. Molecular mechanism of drug-dependent ribosome stalling. Mol Cell. 2008;30:190–202. doi: 10.1016/j.molcel.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 136.Yao S, Blaustein JB, Bechhofer DH. Erythromycin-induced ribosome stalling and RNase J1-mediated mRNA processing in Bacillus subtilis . Mol Microbiol. 2008;69:1439–1449. doi: 10.1111/j.1365-2958.2008.06370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sandler P, Weisblum B. Erythromycin-induced ribosome stall in the ermA leader: a barricade to 5′-to-3′ nucleolytic cleavage of the ermA transcript. J Bacteriol. 1989;171:6680–6688. doi: 10.1128/jb.171.12.6680-6688.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ohtani K, Kawsar HI, Okumura K, Hayashi H, Shimizu T. The VirR/VirS regulatory cascade affects transcription of plasmid-encoded putative virulence genes in Clostridium perfringens strain 13. FEMS Microbiol Lett. 2003;222:137–141. doi: 10.1016/S0378-1097(03)00255-6. [DOI] [PubMed] [Google Scholar]
- 139.Shimizu T, Yaguchi H, Ohtani K, Banu S, Hayashi H. Clostridial VirR/VirS regulon involves a regulatory RNA molecule for expression of toxins. Mol Microbiol. 2002;43:257–265. doi: 10.1046/j.1365-2958.2002.02743.x. [DOI] [PubMed] [Google Scholar]
- 140.Kreikemeyer B, Boyle MD, Buttaro BA, Heinemann M, Podbielski A. Group A streptococcal growth phase-associated virulence factor regulation by a novel operon (Fas) with homologies to two-component-type regulators requires a small RNA molecule. Mol Microbiol. 2001;39:392–406. doi: 10.1046/j.1365-2958.2001.02226.x. [DOI] [PubMed] [Google Scholar]
- 141.Klenk M, Koczan D, Guthke R, Nakata M, Thiesen HJ, Podbielski A, Kreikemeyer B. Global epithelial cell transcriptional responses reveal Streptococcus pyogenes Fas regulator activity association with bacterial aggressiveness. Cell Microbiol. 2005;7:1237–1250. doi: 10.1111/j.1462-5822.2005.00548.x. [DOI] [PubMed] [Google Scholar]
- 142.Siller M, Janapatla RP, Pirzada ZA, Hassler C, Zinkl D, Charpentier E. Functional analysis of the group A streptococcal luxS/AI-2 system in metabolism, adaptation to stress and interaction with host cells. BMC Microbiol. 2008;8:188. doi: 10.1186/1471-2180-8-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Steiner K, Malke H. relA—Independent amino acid starvation response network of Streptococcus pyogenes. J Bacteriol. 2001;183:7354–7364. doi: 10.1128/JB.183.24.7354-7364.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mangold M, Siller M, Roppenser B, Vlaminckx BJ, Penfound TA, Klein R, Novak R, Novick RP, Charpentier E. Synthesis of group A streptococcal virulence factors is controlled by a regulatory RNA molecule. Mol Microbiol. 2004;53:1515–1527. doi: 10.1111/j.1365-2958.2004.04222.x. [DOI] [PubMed] [Google Scholar]
- 145.Roberts SA, Scott JR. RivR and the small RNA RivX: the missing links between the CovR regulatory cascade and the Mga regulon. Mol Microbiol. 2007;66(6):1506–1522. doi: 10.1111/j.1365-2958.2007.06015.x. [DOI] [PubMed] [Google Scholar]
- 146.Halfmann A, Kovacs M, Hakenbeck R, Bruckner R. Identification of the genes directly controlled by the response regulator CiaR in Streptococcus pneumoniae: five out of 15 promoters drive expression of small non-coding RNAs. Mol Microbiol. 2007;66:110–126. doi: 10.1111/j.1365-2958.2007.05900.x. [DOI] [PubMed] [Google Scholar]
- 147.Loh E, Gripenland J, Johansson J. Control of Listeria monocytogenes virulence by 5′-untranslated RNA. Trends Microbiol. 2006;14:294–298. doi: 10.1016/j.tim.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 148.Daou-Chabo R, Mathy N, Benard L, Condon C. Ribosomes initiating translation of the hbs mRNA protect it from 5′-to-3′ exoribonucleolytic degradation by RNase J1. Mol Microbiol. 2009;71:1538–1550. doi: 10.1111/j.1365-2958.2009.06620.x. [DOI] [PubMed] [Google Scholar]
- 149.Condon C. Maturation and degradation of RNA in bacteria. Curr Opin Microbiol. 2007;10:271–278. doi: 10.1016/j.mib.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 150.Dreyfus M. Killer and protective ribosomes. Prog Mol Biol Transl Sci. 2009;85:423–466. doi: 10.1016/S0079-6603(08)00811-8. [DOI] [PubMed] [Google Scholar]
- 151.Figueroa-Bossi N, Valentini M, Malleret L, Bossi L. Caught at its own game: regulatory small RNA inactivated by an inducible transcript mimicking its target. Genes Dev. 2009;23:000–000. doi: 10.1101/gad.541609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Overgaard M, Johansen J, Moller-Jensen J, Valentin-Hansen P. Switching off small RNA regulation with trap-mRNA. Mol Microbiol. 2009;73:790–800. doi: 10.1111/j.1365-2958.2009.06807.x. [DOI] [PubMed] [Google Scholar]
- 153.Navarro L, Jay F, Nomura K, He SY, Voinnet O. Suppression of the microRNA pathway by bacterial effector proteins. Science. 2008;321:964–967. doi: 10.1126/science.1159505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ruiz-Ferrer V, Voinnet O. Roles of plant small RNAs in biotic stress responses. Annu Rev Plant Biol. 2009;60:485–510. doi: 10.1146/annurev.arplant.043008.092111. [DOI] [PubMed] [Google Scholar]
- 155.Brantl S, Wagner EG. Antisense RNA-mediated transcriptional attenuation: an in vitro study of plasmid pT181. Mol Microbiol. 2000;35:1469–1482. doi: 10.1046/j.1365-2958.2000.01813.x. [DOI] [PubMed] [Google Scholar]