Abstract
In vascular smooth muscle cells, IGF-I stimulates SHPS-1/SHP2/Src complex formation which is required for IGF-I-stimulated cell proliferation. Using SHP2/Src silencing and a Pyk2/Y402F mutant, we showed that Pyk2 was also recruited to the SHPS-1 complex. Pyk2 recruitment to SHPS-1 is mediated via the interaction of Pyk2 Tyr402 and the Src in response to IGF-I. Following Src/Pyk2 association, Src phosphorylates Pyk2 on Tyr881 providing a binding site for Grb2. Cells expressing Pyk2/Y881F showed decreased Grb2 recruitment to SHPS-1 and impaired Shc/Grb2 association. This change led to reduced Erk1/2 (MAP kinase) activation and cell proliferation in response to IGF-I. Our results show that, following its recruitment to the SHPS-1 signaling complex, Pyk2 localizes Grb2 in close proximity to Shc thereby facilitating Shc/Grb2 association which leads to Erk1/2 activation in response to IGF-I. Thus, Pyk2 recruitment to SHPS-1 plays an important role in regulating the IGF-I-stimulated mitogenic response.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-010-0411-x) contains supplementary material, which is available to authorized users.
Keywords: IGF-I, SHPS-1, Pyk2, Grb2, Shc, MAP kinase, Cell proliferation
Introduction
IGF-I has diverse biological actions, including regulation of cellular proliferation, differentiation, migration, and survival [1]. The biological effects of IGF-I are mediated through its receptor tyrosine kinase, which phosphorylates specific substrates to activate downstream signaling [2, 3]. SH2 domain-containing protein tyrosine phosphatase substrate 1 (SHPS-1) is an integral membrane protein that acts as a scaffold for multi-protein signaling complexes that are assembled in response to IGF-I in cells exposed to hyperglycemia. IGF-I stimulates SHPS-1 phosphorylation resulting in localization of SHP2, which then recruits the Src/Shc complex to phosphorylated SHPS-1. This recruitment is necessary for IGF-I-stimulated Shc phosphorylation, Shc/Grb2 association, and subsequent ERK1/2 (MAP kinase) activation as well as cell proliferation [4–6]. Recently, we reported that multiple other proteins are recruited to the SHPS-1 signaling complex and that this molecular scaffold plays a novel role in the regulation of IGF-I-stimulated signal transduction and biological actions [7].
The proline-rich tyrosine kinase 2 (Pyk2), also known as RAFTK, CAK, and CADTK, is a nonreceptor tyrosine kinase that is structurally related to focal adhesion kinase [8, 9]. Pyk2 plays an important role in cellular processes, including cytoskeletal rearrangement, proliferation, migration, inflammatory responses, and apoptosis [10–22]. In response to stimuli, such as Angiotensin II, Pyk2 Tyr402 is autophosphorylated. In some test systems, this autophosphorylation leads to Src tyrosine kinase recruitment [23, 24]. Subsequently, Src phosphorylates Pyk2 on Tyr881 which provides a binding site for Grb2 [8, 25, 26]. Prior studies have suggested that Pyk2 is involved in activation of the Ras/ERK pathway [8, 19, 26–28]. Rocic et al. [19] suggested that the association of Shc, Pyk2, and Grb2 correlates with an increase in the tyrosine phosphorylation of Tyr881. However, none of these studies clearly elucidated the mechanism by which Pyk2 mediated MAP kinase activation.
Our prior studies have shown that truncation of cytoplasmic domain of SHPS-1 significantly impairs IGF-I-stimulated Erk1/2 activation and cell proliferation [4, 5]. IGF-I stimulates SHPS-1/SHP-2/Src/Shc and Shc/Grb2 complex formation which is required for MAP kinase pathway activation. Pyk2 has been shown to associate with SHPS-1 following macrophage activation [29]. However, whether Pyk2 associates with SHPS-1 or if it plays a role in mediating Grb2 recruitment to the SHPS-1 complex in response to IGF-I has not been determined. In the present study, we analyzed the role of the SHP2/Src complex in the recruitment of Pyk2 to SHPS-1. We also investigated the role of Pyk2 in the recruitment of Grb2 to the SHPS-1 signaling complex and determined the molecular domains within each protein that were required for their interaction. By inhibiting their interactions, we were able to determine how they functioned to activate MAP kinase phosphorylation and cell proliferation in response to IGF-I.
Materials and methods
Human IGF-I was a gift from Genentech (South San Francisco, CA). Dulbecco’s modified medium (DMEM) containing 4,500 mg glucose per liter (25 mM) was purchased from GIBCO (Grand Island, NY), and penicillin and streptomycin were purchased from Invitrogen (Carlsbad, CA). Blasticidin was obtained from Invitrogen (San Diego, CA). Antibodies that detected pSrc (Y419) and pPyk2 (Y402) or (Y881) were from Invitrogen (BioSources brand; Camarillo, CA). The anti-β-actin antibody was from Cell Signaling technology (Danvers, MA). Anti-Grb2 (rabbit) and the monoclonal anti-phosphotyrosine antibody (pY99) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The Grb2 monoclonal antibody (mouse) and Pyk2 monoclonal antibody (mouse) were purchased from BD Bioscience (San Diego, CA). The anti-SHP2 and anti-Pyk2 antibodies (rabbit) were purchased from Upstate Cell Signaling Solutions/Millipore (Lake Placid, NY). The anti-Src antibody was purchased from Calbiochem (San Diego, CA). A polyclonal antibody for the extracellular domain of SHPS-1 or SHP2 was generated in our laboratory [7]. Two synthetic peptides (pept.#142: YARAAARQARASPLPPCTPTPPCA and cont.#136: YARAAARQARAVQLYAVVSEE) were prepared as described previously [5]. All other reagents were purchased from Sigma (St. Louis, MO) unless otherwise stated.
Cell culture
VSMCs were prepared from porcine aortas and maintained in culture as described previously [30].
Generation of pLenti expression vectors
The following pLenti expression vectors were generated: pLenti-HA-Pyk2 wild-type (Pyk2/WT), pLenti-HA-Pyk2/Y402F (Pyk2/Y402F), pLenti-HA-Pyk2/Y881F (Pyk2/Y881F), pLenti-HA-SHP2 wild-type (SHP2/WT), pLenti-HA-SHP2/P561/562/565/567/568A (SHP2/5PA), pLenti-HA-p52Shc wild-type (Shc/WT), and pLenti-HA-p52Shc mutant (SHC/3YF: Shc/Y239F/Y240F/Y317F). The full-length Pyk2/WT, Pyk2/Y402F, and Pyk2/Y881F were PCR-amplified using pcDNA-myc-Pyk2/WT, pcDNA-myc-Pyk2/Y402F, and pcDNA-myc-Pyk2/Y881F constructs kindly provided by Drs. H.S. Earp and Lee M. Graves at the University of North Carolina at Chapel Hill, and cloned into the pENTR/D-TOPO Gateway entry vector according to the manufacturer’s instructions (Invitrogen). The forward and reverse primers used to generate the PCR product were: forward primer, 5′-CACC ATG TCC GGG GTG TCT GAG CCC CTG-3′; and reverse primer, 5′-TTA AGC GTA ATC TGG AAC ATC GTA TGG GTA CTC TGC AGG CGG GTG GGC-3′. The forward primer includes an ATG (underlined) start site. The reverse primer contained the sequence encoding HA epitope (underlined) followed by the stop codon (bold).
Using the pENTR-SHP2/WT as template, the proline at positions 561, 562, 565, 567 and 568 were mutated to alanine (SHP2/5PA) by double-stranded mutagenesis. PCR amplification was carried out using forward primer 5′-gagatcagagccctctcGcgGcttgtactGcaacgGcaGcctgtgcagaaatgagagaaga-3′ and reverse primer 5′-tcttctctcatttctgcacaggCtgCcgttgCagtacaagCcgCgagagggctctgatctc-3′ where capitalized bases indicate the substitutions. Point mutations were introduced using standard method (Quick Change; Stratagene, La Jolla, CA). SHC/WT and SHC/3YF were prepared as described previously [4]. After selection of correct clones based on sequencing, the cDNAs encoding the wild-type and mutant proteins were transferred from the entry vector into pLenti6/V5-DEST Gateway vector using the LR Clonase reaction following the manufacturer’s instructions (Invitrogen). Similarly VSMCs expressing these cDNAs were prepared as described previously [4].
Construction of a plasmid containing short hairpin RNA (shRNA) template for SHP2, Src, and Pyk2 silencing
Based on Invitrogen website design tools, sequences containing 21 oligonucleotides located within the proteins of interest were used to construct the shRNA template plasmids. The top strand and bottom strand used to generate the shRNA template plasmid for SHP2 were: top strand, 5′-CACCGCAATGATGGCAAGTCTAAAGCGAACTTTAGACTTGCCATCATTGC-3′ and bottom strand, 5′-AAAAGCAATGATGGCAAGTCTAAAGTTCGCTTTAGACTTGCCATCATTGC-3′; for Pyk2, they were: top strand, 5′-CACCGCTTCTATAGCAACAGCTTCACGAATGAAGCTGTTGCTATAGAAGC-3′ and bottom strand, 5′-AAAAGCTTCTATAGCAACAGCTTCATTCGTGAAGCTGTTGCTATAGAAGC-3′. Each sequence contains 21 oligonucleotides in the shRNA template (underline), the loop sequence (usually in the middle, CGAA) and CACC in the 5′ top strand and AAAA in the bottom strand. The oligonucleotides were annealed to form a double strand and ligated into BLOCK-iTTM U6 RNAi Entry Vector (Cat# K4945-00; Invitrogen). The complete sequences were verified by DNA sequencing. The expression vector was generated using the Gateway LR recombination reaction between the Entry Vector and BLOCK-iTTM Lentiviral RNAi Gateway® Vector (Cat# K4943-00; Invitrogen). Construction of the plasmid containing shRNA template for Src was prepared as described previously [5]. The expression vector of shRNA template for Lac Z was used as a control. After confirmation of the sequences, the plasmid DNAs were prepared and purified using the Plasmid Midi Kit (Promega, Madison, WI) according to the manufacturer’s instructions.
Generation of virus stocks and establishment of SMCs expressing pLenti construct
293FT cells (Invitrogen) were utilized for generation of virus stocks that were used to transfect VSMC and obtain cells expressing Pyk2/WT, Pyk2/Y402F, Pyk2/Y881F, SHC/WT, SHC/3YF, SHP2/WT, SHP2/5PA, si-SHP2, si-Src, si-Pyk2, and si-LacZ. These cell lines were established using procedures that have been previously described [4, 5, 31, 32]. The expression of the HA-tagged Pyk2/WT, Pyk2/Y402F, Pyk2/Y881F, SHC/WT, SHC/3YF, SHP2/WT or SHP2/5PA protein was detected by immunoblotting with an anti-HA antibody (1:1,000); The effectiveness of shRNA for inhibiting SHP2 or Src or Pyk2 expression was determined by immunoblotting with antibodies that reacted with each protein and comparing the results to SMCs expressing the LacZ shRNA. The immune complexes were detected using either a horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibody and developed with enhanced chemiluminescence following the manufacturer’s instructions (Pierce, Rockford, IL).
Immunoprecipitation, double immunoprecipitations and immunoblotting
Cells were seeded at 2 × 105 cells per 10-cm plate (BD Biosciences, Franklin Lakes, NJ) and grown for 6 days to reach confluence. The cultures were incubated in serum-free DMEM-H for 16–18 h before the addition of IGF-I (50 ng/ml). The cell monolayers were lysed in a modified radioimmunoprecipitation assay (RIPA) buffer in the presence of protein phosphorylation inhibitors [4]. The cell lysates were centrifuged at 14,000g for 10 min at 4°C. The supernatants containing crude membrane and cytosolic proteins were exposed to the following dilutions anti-SHPS-1 (1:500) antibody or other antibodies (as indicated) overnight at 4°C. The immunoprecipitates were immobilized using protein A or protein G beads for 2 h at 4°C and washed three times with the same lysis buffer. The precipitated proteins were eluted in 40 μl of 2× Laemmli sample buffer, boiled for 5 min, and separated on an 8 or 10 or 12% SDS-PAGE, then transferred to Immobilon-P membranes, and probed with an antibody against the protein of interest. The proteins were visualized using enhanced chemiluminescence following the manufacture’s instructions (Pierce). For double immunoprecipitations [33], the first immunoprecipitation was performed with an anti-SHPS-1 antibody or a normal rabbit IgG (control). The beads were resuspended in 30 μl of SDS buffer (20 mM Tris pH 7.5, 50 mM NaCl, 2% SDS) containing 1 mM DTT. The resuspended supernatant was added to cold RIPA buffer (final concentration 0.1% SDS) and incubated overnight, then used for a second immunoprecipitation with an anti-Grb2 antibody or normal rabbit IgG as described above. The resultant supernatant was used for a second immunoprecipitation with an anti-Grb2 antibody or normal rabbit IgG as described above.
Cell proliferation assay
Assessment of SMC proliferation was performed as described previously [34]. Cells were incubated with or without IGF-I (50 ng/ml) in serum-free DMEM-H containing 0.2% platelet-poor plasma for 48 h, and cell number was determined by counting. Each treatment was analyzed in triplicate, and the results represent mean values of three independent experiments.
Statistical analysis
Student’s t test was used to compare differences between treatments. The results that are shown in all experiments are the representative of at least three independent experiments and expressed as the mean ± SE.
Results
IGF-I stimulates Pyk2 recruitment to SHPS-1 and Pyk2 localization is mediated by Src
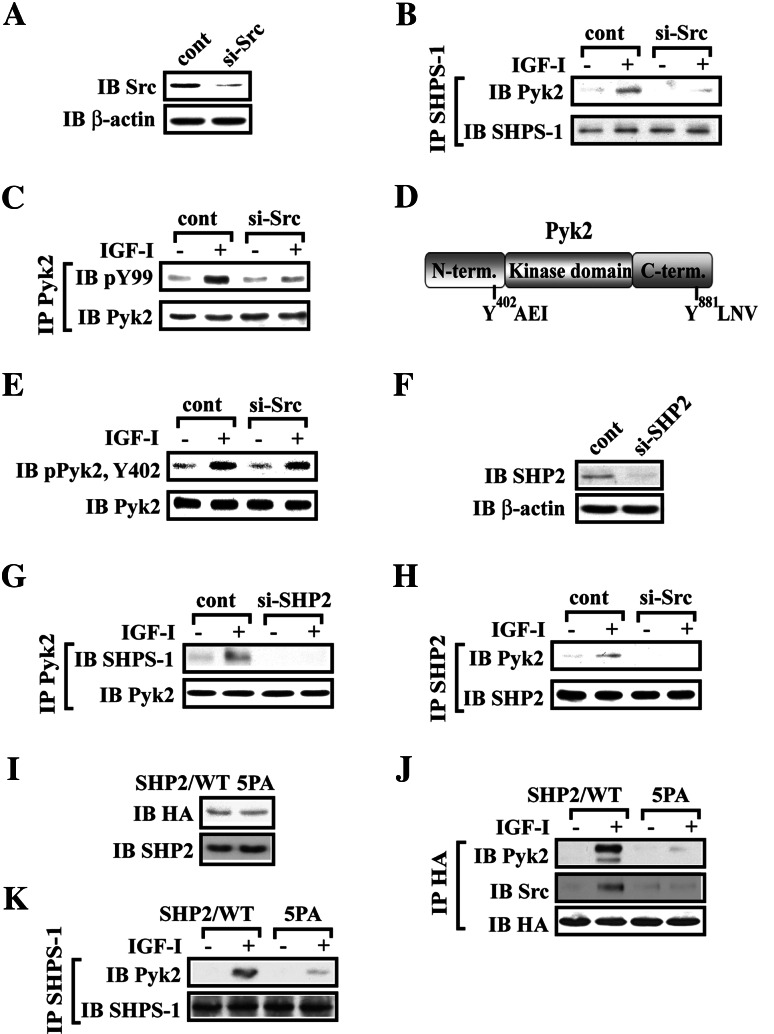
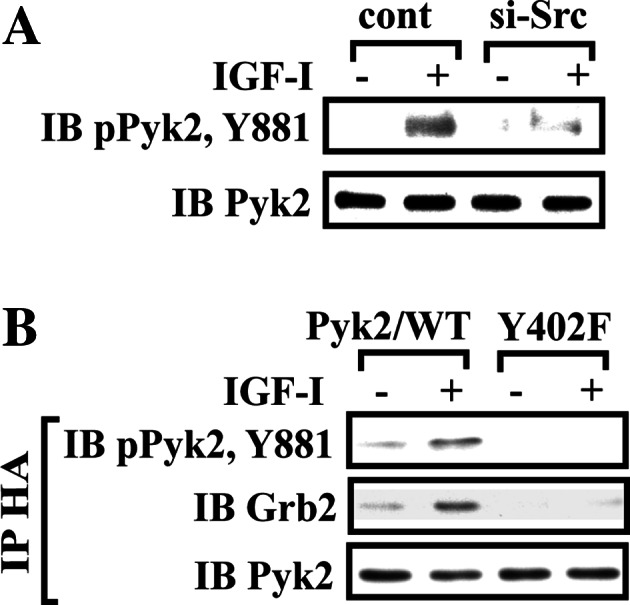
Prior studies have shown that formation of a signaling complex on SHPS-1 is required for IGF-I-stimulated MAP kinase activation in SMCs [4, 5]. Since Pyk2 has been reported to play an essential role in activation of MAP kinase pathway [19, 26–28, 35], we determined whether Pyk2 was recruited to SHPS-1 in response to IGF-I stimulation. IGF-I stimulated a 5.1 ± 1.2 (mean ± SE)-fold increase in SHPS-1/Pyk2 association (Fig. 1a). To investigate the mechanism of Pyk2 recruitment to SHPS-1, we focused on the role of Src kinase since it had been shown to bind to Pyk2 and to be recruited to SHPS-1 in response to IGF-I [5, 23]. IGF-I stimulated a 4.6 ± 1.1-fold increase in Src/Pyk2 association (Fig. 1b). This result was confirmed, by immunoprecipitating Pyk2 and immunoblotting Src or SHPS-1 (Fig. 1c). To determine if Src was absolutely required, we prepared a stable cell line using Src shRNA. Src expression was reduced by 80 ± 11% (Fig. 2a). This resulted in impairment of Pyk2 recruitment to SHPS-1 compared to the control cells following IGF-I stimulation (5.5 ± 1.3 compared to 1.2 ± 0.2-fold, n = 3, p < 0.01) (Fig. 2b). Interestingly, Src knockdown also significantly impaired Pyk2 tyrosine phosphorylation in response to IGF-I (71 ± 9.2% reduction, n = 3, p < 0.01) (Fig. 2c). Pyk2 has several tyrosine phosphorylation sites, and Tyr402 and Tyr881 have been shown the essential for mediating its biological functions (Fig. 2d) [8, 19, 21, 23–25]. IGF-I stimulated Pyk2 Tyr402 phosphorylation in control cells and this response was unchanged when Src was silenced (Fig. 2e). This suggests that IGF-I-stimulated Pyk2 phosphorylation of Tyr402 is Src independent. To confirm the importance of SHPS-1/Src association for Pyk2 recruitment, we focused on SHP2 since it mediates Src recruitment to the SHPS-1 complex [5]. SHP2 knockdown reduced its expression by 84 ± 9% (Fig. 2f). Using these cells, we showed that SHPS-1/Pyk2 association was significantly reduced in response to IGF-I, compared to the control cells (Fig. 2g). More importantly, in control cells, SHP2/Pyk2 association was preserved whereas in Src-silenced cells it was markedly reduced (Fig. 2h). Our previous studies had shown that Src association with SHPS-1 is mediated by SHP2 through an interaction between a polyproline sequence in SHP2 and the SH3 domain in Src following IGF-I stimulation [5]. To exclude the possibility that SHP2 was able to directly recruit Pyk2 to SHSP-1, a mutant in which prolines 561, 562, 565, 567, and 568 in SHP2 were substituted with alanines (SHP2/5PA), was constructed and expressed. The levels of HA and SHP2 were confirmed to be the same when SHP2/WT and SHP2/5PA mutant-expressing cells were compared (Fig. 2i). Consistent with our previous finding [5], the SHP2/5PA mutant had significantly reduced SHP2 association with Src in response to IGF-I compared to SHP2/WT-expressing cells (Fig. 2j). SHP2/Pyk2 association was inhibited significantly in SHP2/5PA mutant-expressing cells compared to SHP2/WT-expressing cells in response to IGF-I (Fig. 2j), whereas IGF-I-stimulated Src Tyr419 (autoactivation) and Pyk2 Tyr402 phosphorylation were not impaired (Electronic supplementary material, ESM, Fig. 1A and 1B). These data suggested that the SH2 domain of SHP2 did not bind to phosphorylated tyrosine sites in Pyk2. More importantly, we confirmed that expression of the SHP2/5PA mutant significantly impaired Pyk2 recruitment to SHPS-1 in response to IGF-I (Fig. 2k). Moreover, disruption of SHP2 and Src association by a cell-permeable peptide (P142), which contained the polypoline-rich region of SHP2, impaired SHP2/Src association in response to IGF-I (ESM, Fig. 2B) [5] and subsequently disrupted Pyk2/SHP2 association whereas a control peptide 136 (P136) had no effect (ESM, Fig. 2A). These results confirm that Pyk2 recruitment to SHPS-1 is mediated by its direct association with Src followed by recruitment of the Src/Pyk2 complex to SHPS-1 via SHP2.
Fig. 1.
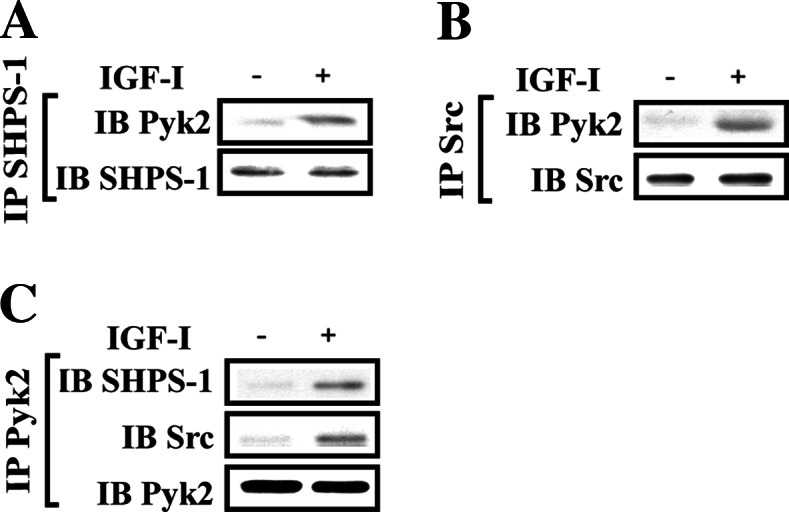
Pyk2 is recruited to the SHPS-1/Src complex in response to IGF-I. a,b Nontransfected cultures were serum starved and then stimulated with IGF-I. The cell lysates were immunoprecipitated (IP) with (a) anti-SHPS-1 antibody or (b) anti-Src antibody followed by immunoblotting for Pyk2. To control for loading, the blot was stripped and reprobed with (a) anti-SHPS-1 or (b) anti-Src antibodies, respectively. c The above cell lysates were immunopreciptated (IP) with anti-Pyk2 and immunoblotted (IB) for SHPS-1 (upper panel) and Src (middle panel). To control for loading, the blot was stripped and reprobed with anti-Pyk2 antibody
Fig. 2.
Src or SHP2 knock-down attenuates Pyk2 recruitment to SHPS-1. a SMCs were transduced with LacZ shRNA (control) or Src shRNA template plasmid and analyzed for Src protein expression. Cell lysates were immunoblotted with anti-Src antibody. The blot was stripped and reprobed with anti-β-actin antibody as a loading control. Quiescent Src knock-down SMCs were stimulated with IGF-I. Cell lysates were immunoprecipitated (IP) with anti-SHPS-1 antibody (b) or anti-Pyk2 antibody (c) followed by immunoblotting with the antibody indicated (upper panel). To control for loading, the blot was stripped and reprobed with the antibody indicated (lower panel). d Diagram of the pyk2 phosporylation sites. The autophosphorylation of Pyk2 at Try402 provides a binding site for Src tyrosine kinase recruitment, and subsequently, Src phosphorylates Pyk2 on Tyr881 which provides a binding site for Grb2. e Autophosphorylation of Pyk2 at Tyr402 in Src knock-down cells. Twenty micrograms of cell lysate was directly immunoblotted for phospho-Pyk2 (Y402). The blot was stripped and reprobed with anti-Pyk2 antibody as a loading control. f SMCs were transduced with LacZ shRNA (control) or SHP2 shRNA template plasmid and analyzed for SHP2 protein expression. Cell lysates were immunoblotted with anti-SHP2 antibody. The blot was stripped and reprobed with anti-β-actin antibody as a loading control. Quiescent SHP2 knock-down or Src knock-down SMCs were stimulated with IGF-I. Cell lysates were immunoprecipitated (IP) with anti-Pyk2 antibody (g) or anti-SHP2 antibody (h) followed by immunoblotting with the antibody indicated (upper panel). To control for loading, the blot was stripped and reprobed with the antibody indicated (lower panel). i SMCs expressing SHP2/WT or SHP2/5PA were lysed. The blot was probed with an anti-HA antibody or anti-SHP2 antibody to detect expression of SHP2/WT or SHP2/5PA. j Quiescent SHP2/WT- or SHP2/5PA-expressing SMCs were stimulated with IGF-I for 5 min. Cell lysates were immunoprecipitated (IP) with anti-HA antibody and immunoblotted (IB) for the protein of interest. To control the loading, the blots were stripped and reprobed with anti-HA antibody. k Quiescent SHP2/WT or SHP2/5PA expressing SMCs were stimulated with IGF-I for 5 min. Cell lysates were immunoprecipitated (IP) with anti-SHPS-1 antibody and immunoblotted (IB) for Pyk2. To control the loading, the blots were stripped and reprobed with anti-SHPS-1 antibody
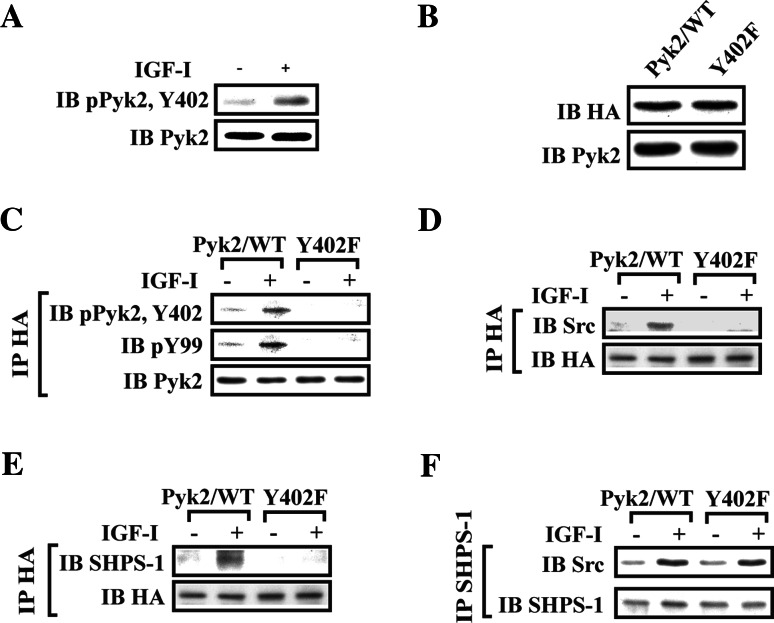
To further investigate the mechanism of Pyk2 recruitment, we focused on Pyk2 Tyr402. Previous studies have shown that this residue is autophosphorylated, creating a binding site for the SH2 domain of Src [23, 26]. Therefore, we determined the effect of IGF-I on Pyk2 Tyr402 phosphorylation. IGF-I stimulated 4.8 ± 0.9-fold increase of Tyr402 phosphorylation (Fig. 3a). To further analyze the role of IGF-I-stimulated Pyk2 Tyr402 phosphorylation in recruitment of Pyk2 to SHPS-1, SMCs expressing wild-type Pyk2 (Pyk2/WT), or a Pyk2 mutant (Pyk2/Y402F) were prepared. The expression levels of Pyk2/WT and the Pyk2/Y402F were comparable (Fig. 3b). The Pyk2/Y402F mutant-expressing cells showed no Tyr402 phosphorylation basally or in response to IGF-I (Fig. 3c). Src/Pyk2 association was significantly reduced in Pyk2/Y402F-expressing cells in response to IGF-I (Fig. 3d). Following IGF-I stimulation, localization of Pyk2 on SHPS-1 was also reduced in Pyk2/Y402F-expressing cells (Fig. 3e). In contrast, IGF-I-stimulated Src recruitment to SHPS-1 was not affected (Fig. 3f), thus demonstrating that IGF-I-stimulated SHPS-1/Src association did not require Src/Pyk2 association. Taken together, these data indicate that Src mediates Pyk2 recruitment to SHPS-1 via its interaction with phosphorylated Tyr402 in Pyk2.
Fig. 3.
Pyk2 Tyr402 is essential for Src binding, Pyk2 tyrosine phosphorylation and Pyk2 recruitment to SHPS-1. a IGF-I induces Pyk2 auto-phosphorylation at Tyr402. Cultures were treated with IGF-I for 2 min. The cell lysates were immunoblotted for phospho-Pyk2 (Y402). The blots were stripped and reprobed with anti-Pyk2 antibody as a loading control. b SMCs expressing Pyk2/WT or Pyk2/Y402F were lysed. The blot was probed with an anti-HA or an anti-Pyk2 antibody to detect expression of Pyk2/WT or Pyk2/Y402F. c–f Quiescent Pyk2/WT or Pyk2/Y402F expressing SMCs were stimulated with IGF-I for 2 or 5 min. Cell lysates were immunoprecipitated (IP) with anti-HA antibody or anti-SHPS-1 antibody and immunoblotted (IB) for the protein of interest (upper panels). To control the loading, the blots were stripped and reprobed with anti-Pyk2, anti-HA antibody or an anti-SHPS-1 antibody
Knockdown Pyk2 confirms its essential role in mediating IGF-I-dependent MAP kinase activation and cell proliferation
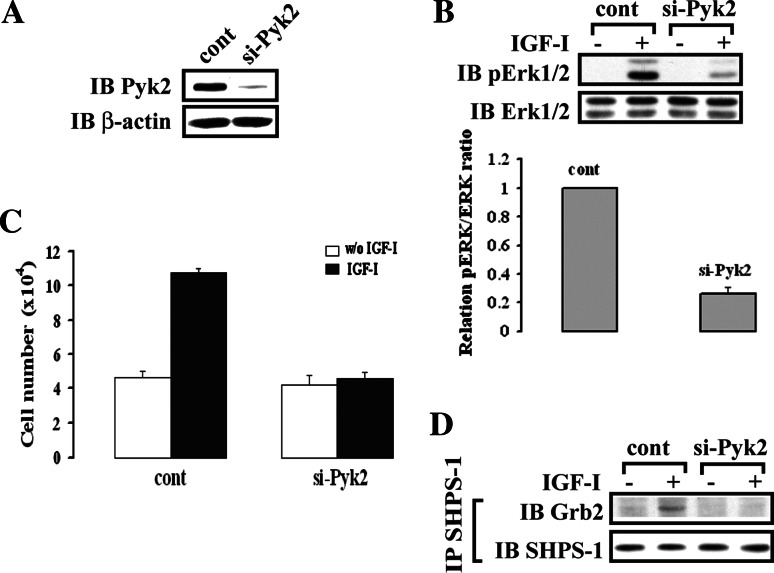
To determine the significance of Pyk2 recruitment to SHPS-1 for IGF-I signaling and growth stimulation, we prepared SMC-expressing Pyk2 shRNA. We achieved an 88 ± 7% reduction in Pyk2 (Fig. 4a). In Pyk2-silenced cells, IGF-I-stimulated Erk1/2 activation was significantly attenuated compared to control cells (74 ± 8.5% reduction; n = 3, p < 0.01) (Fig. 4b). Cell proliferation in response to IGF-I in Pyk2-silenced cells was also significantly reduced (2.3 ± 0.3-fold increase in control cells and a 1.1 ± 0.1-fold change in Pyk2-silenced cells; n = 6, p < 0.01) (Fig. 4c). In contrast, IGF-I-stimulated Src recruitment to SHPS-1 and phosphorylation of Src at Tyr419 (autoactivation) were not impaired (ESM, Fig. 3A and B), demonstrating that Pyk2 is downstream of Src. Previous studies have demonstrated that Grb2 recruitment to the plasma membrane is necessary for Erk1/2 activation, and our studies have shown that factors that mediate Grb2 recruitment to the SHPS-1 complex play a role in regulating the cell proliferation in response to IGF-I. In Pyk2-silenced cells, Grb2 localization on SHPS-1 was significantly impaired in response to IGF-I stimulation compared to control cells (Fig. 4d). This finding suggests that Grb2 recruitment to SHPS-1 is Pyk2-dependent and that Pyk2-mediated Grb2 recruitment to SHPS-1 is required for cell proliferation in response to IGF-I.
Fig. 4.
Pyk2 knock-down attenuates Erk1/2 phosphorylation and cell proliferation in response to IGF-I. a SMCs were transduced with LacZ shRNA (control) or Pyk2 shRNA template plasmid and analyzed for Pyk2 protein expression. Cell lysates were immunoblotted with anti-Pyk2 antibody. The blot was stripped and reprobed with anti-β-actin antibody as a loading control. b Twenty micrograms of cell lysate was used for detection of phospho-Erk1/2. The blot was stripped and reprobed with anti-Erk1/2 antibody as a loading control. The protein levels were quantified using scanning densitometry. The graph shows the mean result from three independent experiments expressed as relative pERK/ERK ratio that was calculated from arbitrary scanning units (b, lower panel). c Proliferation of Pyk2/WT or Pyk2/Y881F mutant cells following IGF-I stimulation. Cell proliferation was determined as described in “Materials and methods”. The results represent a mean value (±SE) of six independent experiments. d Quiescent Pyk2 knock-down SMCs were stimulated with IGF-I. Cell lysates were immunoprecipitated (IP) with anti-SHPS-1 antibody and immunoblotted (IB) for Grb2. To control the loading, the blot was stripped and reprobed with anti-SHPS-1 antibody
Pyk2 mediates Grb2 recruitment to SHPS-1 via Tyr881 phosphorylation, leading to MAP kinase activation and mitogenesis in response to IGF-I
Following Src/Pyk2 association, Src subsequently phosphorylates three other tyrosines on Pyk2 including the Tyr881 in response to IGF-I. Knockdown of Src resulted in a major decrease in Pyk2 tyrosine phosphorylation (Fig. 5a) and cells expressing the Pyk2/Y402F mutant, in which Src/Pyk2 was disrupted also had reduced Tyr881 phosphorylation (1.1 ± 0.1 vs 4.5 ± 0.9-fold; n = 3, p < 0.01) (Fig. 5b). This was accompanied by reduced Pyk2/Grb2 association (Fig. 5b).
Fig. 5.
Src phosphorylates Pyk2 Tyr881 which is essential for subsequent Grb2 association. a Phosphorylation of Pyk2 Tyr881 in Src knock-down cells. Twenty micrograms of cell lysate was directly immunoblotted for phospho-Pyk2 (Y881). The blot was stripped and reprobed with anti-Pyk2 antibody as a loading control. b Quiescent Pyk2/WT- or Pyk2/Y402F-expressing SMCs were stimulated with IGF-I. Cell lysates were immunoprecipitated (IP) with anti-HA antibody and immunoblotted (IB) with an antibody indicated (upper panel). To control the loading, the blots were stripped and reprobed with anti-Pyk2 antibody (lower panel)
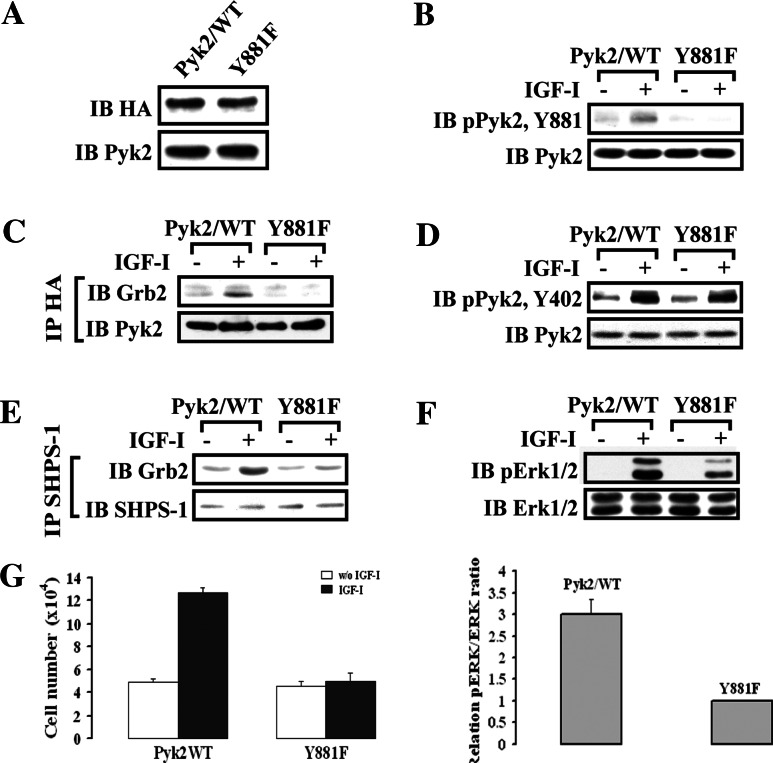
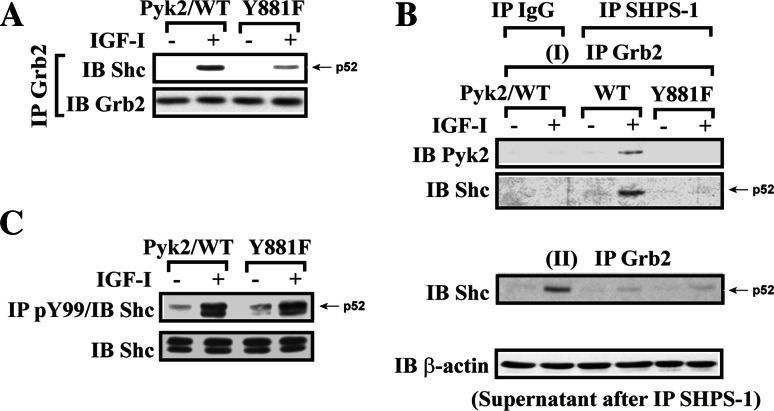
To definitely determine if Pyk2 recruitment of Grb2 played a role in mediating IGF-I-stimulated SMC proliferation, we prepared a Pyk2/Y881F mutant. Comparable expression levels of Pyk2/Y881F mutant and wild-type Pyk2 were confirmed by immunoblotting using anti-HA and anti-Pyk2 antibodies (Fig. 6a). The Pyk2/Y881F mutant showed a complete loss of Tyr881 phosphorylation in response to IGF-I (Fig. 6b), and Pyk2/Grb2 association in response to IGF-I was significantly impaired compared to Pyk2/WT cells (1.0 ± 0.1 vs 4.9 ± 0.8-fold; n = 3, p < 0.01) (Fig. 6c). However, Pyk2 autophosphorylation (Tyr402 phosphorylation) was unchanged (Fig. 6d). Importantly, inhibition of Pyk2/Grb2 association resulted in a major decrease in Grb2 recruitment to SHPS-1 (1.5 ± 0.4 vs 4.6 ± 1.1-fold; n = 3, p < 0.05) (Fig. 6e). IGF-I-stimulated Erk1/2 phosphorylation was significantly reduced (67 ± 11% reduction; n = 3, p < 0.05) compared to the Pyk2/WT cells (Fig. 6f). Additionally IGF-I stimulated a 2.6 ± 0.2-fold increase in cell number in control, Pyk2/WT-expressing cells, but failed to stimulate an increase in Pyk2/Y881F-expressing cells (1.1 ± 0.2-fold increase; n = 6, p < 0.01; see Fig. 6g). These results demonstrate that Pyk2-mediated Grb2 recruitment to SHPS-1 occurs via Src-mediated Tyr881 phosphorylation and that this is required for IGF-I-stimulated MAP kinase activation and cell proliferation.
Fig. 6.
IGF-I-stimulated Pyk2 Tyr881 phosphorylation is essential for Grb2 recruitment to SHPS-1 and leads to Erk1/2 phosphorylation and cell proliferation. a SMCs expressing Pyk2/WT or Pyk2/Y881F were lysed and the lysates immunoblotted for HA or Pyk2 to detect expression of Pyk2/WT or Pyk2/Y881F. b–e Quiescent Pyk2/WT- or Pyk2/Y881F-expressing SMCs were stimulated with IGF-I for 2 or 5 min. Cell lysates were immunoblotted for detection of phospho-Pyk2 (Y881) (b) or phospho-Pyk2 (Y402) (d). The blots were stripped and reprobed with anti-Pyk2 antibody as a loading control. Cell lysates from the same experiment were immunoprecipitated (IP) with anti-HA antibody (c) or anti-SHPS-1 antibody (e) and immunoblotted (IB) with anti-Grb2 antibody. To control the loading, the blots were stripped and reprobed with the antibody indicated (lower panels). f Twenty micrograms of cell lysate from the same experiment was used for detection of phospho-Erk1/2. The blots were stripped and reprobed with anti-Erk1/2 antibody as a loading control. The protein levels were quantified using scanning densitometry. The graph shows the mean result from three independent experiments expressed as relative pERK/ERK ratio that was calculated from arbitrary scanning units (f, lower panel). g Proliferation of Pyk2/WT or Pyk2/Y881F mutant cells following IGF-I stimulation. Cell proliferation was determined as described in “Materials and methods”. The results represent a mean value (±SE) of six independent experiments
Pyk2/Grb2 association is prerequisite for p52Shc/Grb2 association in response to IGF-I
p52Shc/Grb2 association is required for SOS1 recruitment and subsequent MAP kinase activation [36–38]. Since p52Shc is recruited to SHPS-1 and is then phosphorylated by Src resulting in Grb2 localization [4, 5], we wished to determine if the recruitment of Grb2 to Pyk2 was required for p52Shc/Grb2 association. To determine the relationship between Pyk2/Grb2 and p52Shc/Grb2 association, we compared Pyk2/WT and Pyk2/Y881F mutant cells. Initially, we determined that the Pyk2/Y881F substitution led to reduced Grb2 association with p52Shc in response to IGF-I compared to Pyk2/WT-expressing cells (70 ± 12% decreased; n = 3, p < 0.05) (Fig. 7a) whereas IGF-I stimulated p52Shc recruitment to SHPS-1 was not affected (ESM, Fig. 4). This suggests that phosphorylation of Pyk2 Tyr881 is important for p52Shc/Grb2 association. To further confirm the importance of Pyk2/Grb2 association for regulating Grb2 association with the fraction of p52Shc that is localized to the SHPS-1 signaling complex, a double co-immunoprecipitation technique was employed using both cell types. Following immunoprecipitation of SHPS-1, the SHPS-1 immunoprecipitate was resuspended and immunoprecipitated with anti-Grb2 antibody followed by immunoblotting for Pyk2 and p52Shc. The results showed that the association of Pyk2/Grb2 within the SHPS-1 signaling complex was significantly impaired in Pyk2/Y881F mutant-expressing cells, compared to Pyk2/WT-expressing cells (Fig. 7b, upper panel). The association of p52Shc/Grb2 within SHPS-1 signaling complex was also significantly impaired in Pyk2/Y881F mutant cells whereas in Pyk2/WT-expressing cells it was easily detected. The experiment was repeated by immunopreciptating SHPS-1, then conducting a second Co-IP of the supernatant using anti-Grb2 antibody. The results showed that, following depletion of SHPS-1, p52Shc/Grb2 association was decreased (67 ± 13%; n = 3, p < 0.05) and there was no difference between the cells expressing either Pyk2/WT or Pyk2/Y881F (Fig. 7b, bottom panel). As a control, a parallel experiment was conducted using a normal IgG instead of a SHPS-1 antibody. Consistently, in normal IgG immunoprecipitate, no Pyk2/Grb2 or p52Shc/Grb2 association was detected (Fig. 7b, upper panel). In contrast, p52Shc/Grb2 association was detected in the supernatant since the SHPS-1 complex was also present. Given the fact that IGF-I-induced phosphorylation of p52Shc was not impaired in Pyk2/Y881F mutant-expressing cells compared to Pyk2/WT-expressing cells (Fig. 7c), these results suggest that the Pyk2/Grb2 association occurs primarily within the SHPS-1 signaling complex, which localizes the adaptor protein Grb2 in close proximity to p52Shc, thereby allowing the formation of p52Shc/Grb2 association, a critical signaling event for IGF-I-stimulated MAP kinase activation and cell proliferation. To determine the relative importance of Pyk2 and p52Shc for Grb2 recruitment to the SHPS-1 complex, we utilized p52Shc/3YF mutant, which disrupts Shc/Grb2 association [4, 31]. The results showed that most (78.8 ± 14.4%) SHPS-1/Grb2 complex formation was retained in cells expressing p52shc/3YF. IGF-I-stimulated Pyk2 recruitment to SHPS-1 was not affected in p52Shc/3YF-expressing cells (ESM, Fig. 5). These data confirm that Grb2 recruitment to the SHPS-1 complex is primarily mediated by Pyk2.
Fig. 7.
Impairment of Pyk2/Grb2 complex formation leads to decreased Shc/Grb2 association within the SHPS-1 signaling complex in response to IGF-I. a Quiescent Pyk2/WT or Pyk2/Y881F expressing SMCs were stimulated with IGF-I. Cell lysates were immunoprecipitated (IP) with anti-Grb2 antibody and immunoblotted (IB) with anti-Shc antibody. The blot was stripped and reprobed with anti-Grb2 antibody. b Lysates from Pyk2/Wt and Pyk2/Y881F mutant cells were immunoprecipitated first with anti-SHPS-1 antibody or normal rabbit IgG. The double-immunoprecipitation was performed as described in “Materials and methods”. After immunoprecipitation of SHPS-1, (I) the SHPS-1 immunoprecipitate was resuspended and immunoprecipitated with anti-Grb2 antibody and immunoblotted (IB) with anti-Pyk2 or anti-Shc antibody; the resultant supernatants after IP (II) with anti-SHPS-1 antibody or normal IgG were immunoprecipitated with anti-Grb2 antibody and immunoblotted (IB) with anti-Shc antibody. The resultant supernatants from same experiment were also directly immunoblotted for β-actin as a loading control. c Lysates from Pyk2/WT and Pyk2/Y881F mutant cells were immunoprecipitated (IP) with anti-pY99 antibody and immunoblotted (IB) with anti-Shc antibody. Twenty micrograms of cell lysate from the same experiment was used for detection of total Shc protein level as a loading control
Discussion
In response to hyperglycemic stress, SHPS-1 functions as a scaffold protein for mediating IGF-I signaling. The IGF-I receptor directly phosphorylates SHPS-1 leading to SHP2 and Src recruitment. Src phosphorylates p52Shc leading to Grb2 association which activates MAP kinase. This response occurs in both vascular smooth muscle cells (SMCs) and endothelial cells [4–6, 39]. Our prior studies have shown that Grb2 associates with p52Shc when it is bound to the SHPS-1 complex, but they did not determine the mechanism of Grb2 recruitment to SHPS-1. The studies of Eguchi et al. [35] and Rocic et al. [19] have suggested that formation of the Src/Pyk2 complex leads to recruitment of Grb2 in vascular SMCs. Since our studies had shown that Src was associated with SHPS-1, we determined if Pyk2 was also recruited to SHPS-1 and if the SHPS-1-associated Pyk2/Src complex could recruit Grb2.
To determine the mechanism by which Pyk2 was recruited to SHPS-1, we focused on its interaction with Src kinase. Following tyrosine phosphorylation of SHPS-1, only a few signaling proteins, e.g., SHP2 and CTK, bind directly to SHPS-1, whereas several others associate indirectly through these intermediaries [7]. Pyk2 has been shown to associate indirectly with SHPS-1 in macrophages [29], therefore we focused on the identification of an intermediary. The N terminal domain of Pyk2 contains a tyrosine autophosphorylation site, Tyr402, and its phosphorylation increases in response to IGF-I (Fig. 3a) [27]. This residue serves as a binding site for Src family tyrosine kinases [23]. Based on our previous studies [4, 5], we used two approaches (in vitro mutagenesis and shRNA) to show that IGF-I-induced Pyk2 association with SHPS-1 is mediated by the SHP2/Src complex via the direct interaction of Src with Pyk2 Tyr402 (Figs. 2, 3). In addition, our results showed that truncation of cytoplasmic domain of SHPS-1 impaired Pyk2 association with Src but did not affect IGF-I-stimulated autophosphorylation of Pyk2 Tyr402, suggesting that this is independent of SHPS-1 association (data not shown). However, IGF-I stimulation of Pyk2 Tyr402 phosphorylation is required since Pyk2/Src association and Pyk2 recruitment to SHPS-1 are impaired in cells expressing the Pyk2/Y402F mutant.
The underlying mechanism that initiates Pyk2 Tyr402 phosphorylation is unclear. Two proteins, Cbl and dynamin, both bind to the Pyk2/Src complex and regulate Pyk2 Tyr402 phosphorylation [40, 41]. Although some studies have shown that Pyk2 Tyr402 phosphorylation is Src kinase activation-dependent [42, 43], our data support the conclusion of other studies that showed that Tyr402 phosphorylation is Src-independent [27, 44], since Src knockdown had no effect on Pyk2 Tyr402 phosphorylation (Fig. 2e). Overexpression of Pyk2 enhances Src activation while PRNK (dominate negative form of Pyk2), Pyk2−/− mice and Pyk2/Y402F mutant expression show decreased Src activation [28, 45, 46]. Our results support the conclusion that Src is upstream of Pyk2 based on the fact that Pyk2/Y402F mutant and Pyk2 knockdown failed to impair Src activation and SHPS-1/Src association in response to IGF-I. Additionally in Src silenced cells, IGF-I induction of Pyk2 Tyr881 phosphorylation was attenuated even though Pyk2 Tyr402 autophosphorylation was not affected. These results are in agreement with other studies that have shown that Src kinase is upstream of Pyk2 Tyr881 phosphorylation [21, 47–49].
Prior studies have shown that, following Src association with Pyk2 Tyr402, it phosphorylates several other Pyk2 tyrosine residues, including Tyr881, which is a potential Grb2 binding site [25, 26]. Our findings are consistent with those studies since they showed that vascular SMCs expressing Pyk2/Y881F mutant had significantly impaired Grb2 recruitment to Pyk2 and more importantly to SHPS-1. That this substitution specifically impaired Grb2 recruitment to Pyk2 was demonstrated by showing that both Pyk2 Tyr402 and Src Tyr419 phosphorylation in response to IGF-I were not impaired. Similarly, Src recruitment to SHPS-1- and Src-mediated p52Shc phosphorylation were not inhibited. Therefore, loss of the Tyr881 phosphorylation site has no effect on recruitment of other components to the SHPS-1 signaling complex in response to IGF-I, and it specifically disrupts Grb2/Pyk2 and Grb2/SHPS-1 association.
p52Shc protein phosphorylation has been extensively characterized as a critical step that activates a mitogenic signal through its association with Grb2 [38, 50–52]. IGF-I-dependent mitogenesis requires p52Shc phosphorylation and Grb2/p52Shc association, and this complex is assembled on SHPS-1 [4]. p52Shc phosphorylation is mediated by Src kinase which is activated in response to IGF-I [5]. Tyrosine-phosphorylated Shc forms a complex with Grb2 at the phosphorylated residues 239, 240, and 317 via the SH2 domain of Grb2 [36, 53], then the SH3 domain of Grb2 recruits son of sevenless (SOS) to this complex [37, 54]. This stimulates Ras activation, which leads to stimulation of MAP kinase [36–38, 53, 55–57]. Previous studies [8, 58] have suggested that Pyk2 activates the Ras/MAP kinase pathway by directly binding to Grb2 and recruiting SOS to the Pyk2/Grb2 complex. Blaukat et al. [26] demonstrated that Grb2 binding to tyrosine-phosphorylated p52Shc plays a major role in the Pyk2-induced activation of the Ras/MAP kinase pathway. Rikitake et al. [59] showed that lysophosphatidylcholine stimulates p52Shc association with Pyk2, p52Shc tyrosine phosphorylation, and Grb2 binding to p52Shc. Our current results clearly show that expression of the Pyk2/Y881F mutant not only impaired Pyk2/Grb2 association but it also inhibited p52Shc/Grb2 association, which was stimulated in response to IGF-I, even though p52Shc phosphorylation was not affected. Although both Pyk2 and p52Shc were able to directly bind to Grb2, the amount of p52Shc/Grb2 association that was localized on the SHPS-1 signaling scaffold was significantly reduced by Pyk2/Y881F mutation. In contrast, the amount of Grb2 recruitment to the SHPS-1 complex was decreased by only 21% in SMC expressing the SHC/3YF mutant suggesting that Grb2 recruitment to the SHPS-1 complex is primarily mediated by Pyk2 (ESM, Fig. 5). This conclusion was confirmed by using double co-immunoprecipitation and comparing the amount of Grb2 associated with the SHPS-1 complex in cells expressing the Pyk2/Y881F mutant and Pyk2/WT cells (Fig. 7b, upper panel). Since the Pyk2/Y881F substitution had no effect on Src kinase activation or p52Shc phosphorylation, we conclude that a key function of Pyk2 is to localize Grb2 in close proximity to p52Shc on the SHPS-1 complex, thus leading to enhanced p52Shc/Grb2 complex formation, MAP kinase activation, and cellular proliferation in response to IGF-I.
Grb2 is a modular protein comprised of a central SH2 domain flanked between an N-terminal SH3 domain and a C-terminal SH3 domain. Grb2 associates with SOS through SH3 domain interaction [37]. Previous studies have shown that both Pyk2/Grb2 and p52Shc/Grb2 association require the Grb2 SH2 domain [4, 8, 19, 25, 26, 31]. We did not determine experimentally how the single SH2 domain in Grb2 could confer binding to both Pyk2 and p52Shc simultaneously. One possibility is that Grb2 binds to both Pyk2 and p52Shc as a result of Grb2 dimerization [60]. That the Grb2 dimer could bind to two distinct signaling proteins simultaneously is supported by the work of McDonald et al. [61], who reported that dimerization of Grb2 allows multiple protein interactions. Another possibility is that Grb2 is recruited to Pyk2 initially, and subsequently Pyk2-associated Grb2 is recruited to p52Shc following its tyrosine phosphorylation. Our results show maximal Pyk2/Grb2 association 5 min after IGF-I stimulation, whereas Shc/Grb2 association is still increasing at 10 min. Therefore, this time course of events is consistent with that mechanism.
In summary, in the present study, we have defined the molecular mechanism of Pyk2 recruitment to SHPS-1 in response to IGF-I. We propose a model in which IGF-I stimulates recruitment of Pyk2 to the SHPS-1 signaling complex via a Src/Pyk2 association. Src phosphorylates Pyk2 Tyr881 creating a binding site for Grb2. The role of Pyk2 is to localize Grb2 in close proximity to Shc, facilitating Shc/Grb2 association within the SHPS-1 signaling complex. Since SHPS-1 phosphorylation is activated in SMCs by IGF-I only in response to hyperglycemic stress, the regulated assembly of this complex provides a high level of specificity for the activation of MAPK in response to IGF-I.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1 Effect of SHP2/5PA mutant on Src and Pyk2 phosphorylation. Quiescent SHP2/WT or SHP2/5PA expressing SMCs were stimulated with IGF-I. Twenty micrograms of cell lysate was used for detection of (A) phosphor-Src (Y419) or phosphor-Pyk2 (Y402) and the blot was stripped and reprobed with (A) anti-Src or (B) anti-Pyk2 antibody as a loading control (TIFF 1585 kb)
Supplemental Fig. 2 The effect of SHP2/Src disruption on Pyk2 association with SHP2. (A and B) Confluent cultures were serum starved overnight and incubated with or without the SHP2 cell-permeable peptide (pept. #142, 10 µg/ml) or control peptide (cont. #136) for 1 h followed by IGF-I treatment for 5 min. The cell lysates were immunoprecipitated with anti-SHP2 antibody followed by immunoblotting for (A) Pyk2 or (B) Src. For loading control, the membrane was reprobed with anti-SHP2 antibody (TIFF 1586 kb)
Supplemental Fig. 3 Effect of Pyk2 knock-down on Src phosphorylation and SHPS-1/Src association. Quiescent Pyk2 knock-down SMCs were stimulated with IGF-I. Twenty micrograms of cell lysate was used for detection of phosphor-Src (Y419). The blot was stripped and reprobed with anti-Src antibody as a loading control (Upper panel). Cell lysates from same experiment were immunoprecipitated (IP) with anti-SHPS-1 and immunoblotted (IB) for Src. To control the loading, the blot was stripped and reprobed with anti-SHPS-1 antibody (lower panel) (TIFF 1586 kb)
Supplemental Fig. 4 Effect of Pyk2/Y881F mutant on SHPS-1/SHC association. Quiescent Pyk2/WT or Pyk2/Y881F expressing SMCs were stimulated with IGF-I for 5 min. Cell lysates were immunoprecipitated (IP) with anti-SHC antibody and immunoblotted (IB) for SHPS-1. To control the loading, the blot was stripped and reprobed with anti-SHC antibody (TIFF 1057 kb)
Supplemental Fig. 5 SHPS-1/Grb2 association is mediated by Pyk2. Quiescent SHC/WT or SHC/3YF expressing SMCs were stimulated with IGF-I. Cell lysates were immunoprecipitated (IP) with anti-SHPS-1 antibody and immunoblotted (IB) for Pyk2 or Grb2. To control the loading, the blot was stripped and reprobed with anti-SHPS-1 antibody (TIFF 1233 kb)
Acknowledgments
We thank Dr. Walker H. Busby, Jr. for his help in preparing the SHPS-1 antibody. We thank Drs. H.S. Earp and Lee M Graves (University of North Carolina at Chapel Hill) for providing pcDNA-myc-Pyk2/WT, pcDNA-myc-Pyk2/Y402F, and pcDNA-myc-Pyk2/Y881F constructs. We thank Drs. Laura A. Maile and Lee M Graves for comments. We also thank Ms. Laura Lindsey for her help in preparing the manuscript. This study was supported by a grant HL56850 and AG022331 from the National Institutes of Health.
References
- 1.Clemmons DR. Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. Nat Rev Drug Discov. 2007;6:821–833. doi: 10.1038/nrd2359. [DOI] [PubMed] [Google Scholar]
- 2.LeRoith D, Baserga R, Helman L, Roberts CT., Jr Insulin-like growth factors and cancer. Ann Intern Med. 1995;122:54–59. doi: 10.7326/0003-4819-122-1-199501010-00009. [DOI] [PubMed] [Google Scholar]
- 3.Jones JI, Prevette T, Gockerman A, Clemmons DR. Ligand occupancy of the alpha-V-beta3 integrin is necessary for smooth muscle cells to migrate in response to insulin-like growth factor. Proc Natl Acad Sci USA. 1996;93:2482–2487. doi: 10.1073/pnas.93.6.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ling Y, Maile LA, Lieskovska J, Badley-Clarke J, Clemmons DR. Role of SHPS-1 in the regulation of insulin-like growth factor I-stimulated Shc and mitogen-activated protein kinase activation in vascular smooth muscle cells. Mol Biol Cell. 2005;16:3353–3364. doi: 10.1091/mbc.E04-10-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lieskovska J, Ling Y, Badley-Clarke J, Clemmons DR. The role of Src kinase in insulin-like growth factor-dependent mitogenic signaling in vascular smooth muscle cells. J Biol Chem. 2006;281:25041–25053. doi: 10.1074/jbc.M602866200. [DOI] [PubMed] [Google Scholar]
- 6.Maile LA, Capps BE, Ling Y, Xi G, Clemmons DR. Hyperglycemia alters the responsiveness of smooth muscle cells to insulin-like growth factor-I. Endocrinology. 2007;148:2435–2443. doi: 10.1210/en.2006-1440. [DOI] [PubMed] [Google Scholar]
- 7.Shen X, Xi G, Radhakrishnan Y, Clemmons DR. Identification of novel SHPS-1-associated proteins and their roles in regulation of insulin-like growth factor-dependent responses in vascular smooth muscle cells. Mol Cell Proteomics. 2009;8:1539–1551. doi: 10.1074/mcp.M800543-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 9.Yu H, Li X, Marchetto GS, Dy R, Hunter D, Calvo B, Dawson TL, Wilm M, Anderegg RJ, Graves LM, Earp HS. Activation of a novel calcium-dependent protein-tyrosine kinase. Correlation with c-Jun N-terminal kinase but not mitogen-activated protein kinase activation. J Biol Chem. 1996;271:29993–29998. doi: 10.1074/jbc.271.47.29993. [DOI] [PubMed] [Google Scholar]
- 10.Andreev J, Simon JP, Sabatini DD, Kam J, Plowman G, Randazzo PA, Schlessinger J. Identification of a new Pyk2 target protein with Arf-GAP activity. Mol Cell Biol. 1999;19:2338–2350. doi: 10.1128/mcb.19.3.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000;12:123–133. doi: 10.1016/S0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 12.Brinson AE, Harding T, Diliberto PA, He Y, Li X, Hunter D, Herman B, Earp HS, Graves LM. Regulation of a calcium-dependent tyrosine kinase in vascular smooth muscle cells by angiotensin II and platelet-derived growth factor. Dependence on calcium and the actin cytoskeleton. J Biol Chem. 1998;273:1711–1718. doi: 10.1074/jbc.273.3.1711. [DOI] [PubMed] [Google Scholar]
- 13.Duong LT, Lakkakorpi PT, Nakamura I, Machwate M, Nagy RM, Rodan GA. PYK2 in osteoclasts is an adhesion kinase, localized in the sealing zone, activated by ligation of alpha(v)beta3 integrin, and phosphorylated by src kinase. J Clin Invest. 1998;102:881–892. doi: 10.1172/JCI3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuortes M, Melchior M, Han H, Lyon GJ, Nathan C. Role of the tyrosine kinase pyk2 in the integrin-dependent activation of human neutrophils by TNF. J Clin Invest. 1999;104:327–335. doi: 10.1172/JCI6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Dy RC, Cance WG, Graves LM, Earp HS. Interactions between two cytoskeleton-associated tyrosine kinases: calcium-dependent tyrosine kinase and focal adhesion tyrosine kinase. J Biol Chem. 1999;274:8917–8924. doi: 10.1074/jbc.274.13.8917. [DOI] [PubMed] [Google Scholar]
- 16.Lim Y, Lim ST, Tomar A, Gardel M, Bernard-Trifilo JA, Chen XL, Uryu SA, Canete-Soler R, Zhai J, Lin H, Schlaepfer WW, Nalbant P, Bokoch G, Ilic D, Waterman-Storer C, Schlaepfer DD. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J Cell Biol. 2008;180:187–203. doi: 10.1083/jcb.200708194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han H, Fuortes M, Nathan C. Critical role of the carboxyl terminus of proline-rich tyrosine kinase (Pyk2) in the activation of human neutrophils by tumor necrosis factor: separation of signals for the respiratory burst and degranulation. J Exp Med. 2003;197:63–75. doi: 10.1084/jem.20021638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manso AM, Kang SM, Plotnikov SV, Thievessen I, Oh J, Beggs HE, Ross RS. Cardiac fibroblasts require focal adhesion kinase for normal proliferation and migration. Am J Physiol Heart Circ Physiol. 2009;296:H627–H638. doi: 10.1152/ajpheart.00444.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rocic P, Govindarajan G, Sabri A, Lucchesi PA. A role for PYK2 in regulation of ERK1/2 MAP kinases and PI 3-kinase by ANG II in vascular smooth muscle. Am J Physiol Cell Physiol. 2001;280:C90–C99. doi: 10.1152/ajpcell.2001.280.1.C90. [DOI] [PubMed] [Google Scholar]
- 20.Rufanova VA, Alexanian A, Wakatsuki T, Lerner A, Sorokin A. Pyk2 mediates endothelin-1 signaling via p130Cas/BCAR3 cascade and regulates human glomerular mesangial cell adhesion and spreading. J Cell Physiol. 2009;219:45–56. doi: 10.1002/jcp.21649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taniyama Y, Weber DS, Rocic P, Hilenski L, Akers ML, Park J, Hemmings BA, Alexander RW, Griendling KK. Pyk2- and Src-dependent tyrosine phosphorylation of PDK1 regulates focal adhesions. Mol Cell Biol. 2003;23:8019–8029. doi: 10.1128/MCB.23.22.8019-8029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soe NN, Ishida T, Ishida M, Sawano M, Abe K, Miho N, Chayama K, Kihara Y, Yoshizumi M. Nifedipine interferes with migration of vascular smooth muscle cells via inhibition of Pyk2-Src axis. J Atheroscler Thromb. 2009;16:230–238. doi: 10.5551/jat.e422. [DOI] [PubMed] [Google Scholar]
- 23.Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 24.Xiong W, Parsons JT. Induction of apoptosis after expression of PYK2, a tyrosine kinase structurally related to focal adhesion kinase. J Cell Biol. 1997;139:529–539. doi: 10.1083/jcb.139.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Felsch JS, Cachero TG, Peralta EG. Activation of protein tyrosine kinase PYK2 by the m1 muscarinic acetylcholine receptor. Proc Natl Acad Sci USA. 1998;95:5051–5056. doi: 10.1073/pnas.95.9.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blaukat A, Ivankovic-Dikic I, Gronroos E, Dolfi F, Tokiwa G, Vuori K, Dikic I. Adaptor proteins Grb2 and Crk couple Pyk2 with activation of specific mitogen-activated protein kinase cascades. J Biol Chem. 1999;274:14893–14901. doi: 10.1074/jbc.274.21.14893. [DOI] [PubMed] [Google Scholar]
- 27.Sekimoto H, Eipper-Mains J, Pond-Tor S, Boney CM. (alpha)v(beta)3 integrins and Pyk2 mediate insulin-like growth factor I activation of Src and mitogen-activated protein kinase in 3T3-L1 cells. Mol Endocrinol. 2005;19:1859–1867. doi: 10.1210/me.2004-0481. [DOI] [PubMed] [Google Scholar]
- 28.Sun CK, Man K, Ng KT, Ho JW, Lim ZX, Cheng Q, Lo CM, Poon RT, Fan ST. Proline-rich tyrosine kinase 2 (Pyk2) promotes proliferation and invasiveness of hepatocellular carcinoma cells through c-Src/ERK activation. Carcinogenesis. 2008;29:2096–2105. doi: 10.1093/carcin/bgn203. [DOI] [PubMed] [Google Scholar]
- 29.Timms JF, Swanson KD, Marie-Cardine A, Raab M, Rudd CE, Schraven B, Neel BG. SHPS-1 is a scaffold for assembling distinct adhesion-regulated multi-protein complexes in macrophages. Curr Biol. 1999;9:927–930. doi: 10.1016/S0960-9822(99)80401-1. [DOI] [PubMed] [Google Scholar]
- 30.Parker A, Gockerman A, Busby WH, Clemmons DR. Properties of an insulin-like growth factor-binding protein-4 protease that is secreted by smooth muscle cells. Endocrinology. 1995;136:2470–2476. doi: 10.1210/en.136.6.2470. [DOI] [PubMed] [Google Scholar]
- 31.Radhakrishnan Y, Maile LA, Ling Y, Graves LM, Clemmons DR. Insulin-like growth factor-I stimulates Shc-dependent phosphatidylinositol 3-kinase activation via Grb2-associated p85 in vascular smooth muscle cells. J Biol Chem. 2008;283:16320–16331. doi: 10.1074/jbc.M801687200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xi G, Shen X, Clemmons DR. p66shc negatively regulates insulin-like growth factor I signal transduction via inhibition of p52shc binding to Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 leading to impaired growth factor receptor-bound protein-2 membrane recruitment. Mol Endocrinol. 2008;22:2162–2175. doi: 10.1210/me.2008-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen RH, Derynck R. Homomeric interactions between type II transforming growth factor-beta receptors. J Biol Chem. 1994;269:22868–22874. [PubMed] [Google Scholar]
- 34.Nam TJ, Busby WH, Jr, Rees C, Clemmons DR. Thrombospondin and osteopontin bind to insulin-like growth factor (IGF)-binding protein-5 leading to an alteration in IGF-I-stimulated cell growth. Endocrinology. 2000;141:1100–1106. doi: 10.1210/en.141.3.1100. [DOI] [PubMed] [Google Scholar]
- 35.Eguchi S, Iwasaki H, Inagami T, Numaguchi K, Yamakawa T, Motley ED, Owada KM, Marumo F, Hirata Y. Involvement of PYK2 in angiotensin II signaling of vascular smooth muscle cells. Hypertension. 1999;33:201–206. doi: 10.1161/01.hyp.33.1.201. [DOI] [PubMed] [Google Scholar]
- 36.Gotoh N, Toyoda M, Shibuya M. Tyrosine phosphorylation sites at amino acids 239 and 240 of Shc are involved in epidermal growth factor-induced mitogenic signaling that is distinct from Ras/mitogen-activated protein kinase activation. Mol Cell Biol. 1997;17:1824–1831. doi: 10.1128/mcb.17.4.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li N, Batzer A, Daly R, Yajnik V, Skolnik E, Chardin P, Bar-Sagi D, Margolis B, Schlessinger J. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature. 1993;363:85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- 38.Sasaoka T, Ishiki M, Wada T, Hori H, Hirai H, Haruta T, Ishihara H, Kobayashi M. Tyrosine phosphorylation-dependent and -independent role of Shc in the regulation of IGF-1-induced mitogenesis and glycogen synthesis. Endocrinology. 2001;142:5226–5235. doi: 10.1210/en.142.12.5226. [DOI] [PubMed] [Google Scholar]
- 39.Xi G, Shen X, Clemmons DR. p66shc Inhibits insulin-like growth factor-I signaling via direct binding to Src through its polyproline and Src homology 2 domains, resulting in impairment of Src kinase activation. J Biol Chem. 2010;285:6937–6951. doi: 10.1074/jbc.M109.069872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanjay A, Houghton A, Neff L, DiDomenico E, Bardelay C, Antoine E, Levy J, Gailit J, Bowtell D, Horne WC, Baron R. Cbl associates with Pyk2 and Src to regulate Src kinase activity, alpha(v)beta(3) integrin-mediated signaling, cell adhesion, and osteoclast motility. J Cell Biol. 2001;152:181–195. doi: 10.1083/jcb.152.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruzzaniti A, Neff L, Sandoval A, Du L, Horne WC, Baron R. Dynamin reduces Pyk2 Y402 phosphorylation and SRC binding in osteoclasts. Mol Cell Biol. 2009;29:3644–3656. doi: 10.1128/MCB.00851-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bayer AL, Heidkamp MC, Howes AL, Heller Brown J, Byron KL, Samarel AM. Protein kinase C epsilon-dependent activation of proline-rich tyrosine kinase 2 in neonatal rat ventricular myocytes. J Mol Cell Cardiol. 2003;35:1121–1133. doi: 10.1016/S0022-2828(03)00228-1. [DOI] [PubMed] [Google Scholar]
- 43.Sorokin A, Kozlowski P, Graves L, Philip A. Protein-tyrosine kinase Pyk2 mediates endothelin-induced p38 MAPK activation in glomerular mesangial cells. J Biol Chem. 2001;276:21521–21528. doi: 10.1074/jbc.M008869200. [DOI] [PubMed] [Google Scholar]
- 44.Wu SS, Jacamo RO, Vong SK, Rozengurt E. Differential regulation of Pyk2 phosphorylation at Tyr-402 and Tyr-580 in intestinal epithelial cells: roles of calcium, Src, Rho kinase, and the cytoskeleton. Cell Signal. 2006;18:1932–1940. doi: 10.1016/j.cellsig.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 45.Melendez J, Turner C, Avraham H, Steinberg SF, Schaefer E, Sussman MA. Cardiomyocyte apoptosis triggered by RAFTK/pyk2 via Src kinase is antagonized by paxillin. J Biol Chem. 2004;279:53516–53523. doi: 10.1074/jbc.M408475200. [DOI] [PubMed] [Google Scholar]
- 46.Gil-Henn H, Destaing O, Sims NA, Aoki K, Alles N, Neff L, Sanjay A, Bruzzaniti A, De Camilli P, Baron R, Schlessinger J. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2(−/−) mice. J Cell Biol. 2007;178:1053–1064. doi: 10.1083/jcb.200701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andreev J, Galisteo ML, Kranenburg O, Logan SK, Chiu ES, Okigaki M, Cary LA, Moolenaar WH, Schlessinger J. Src and Pyk2 mediate G-protein-coupled receptor activation of epidermal growth factor receptor (EGFR) but are not required for coupling to the mitogen-activated protein (MAP) kinase signaling cascade. J Biol Chem. 2001;276:20130–20135. doi: 10.1074/jbc.M102307200. [DOI] [PubMed] [Google Scholar]
- 48.Lakkakorpi PT, Bett AJ, Lipfert L, Rodan GA, Duong le T. PYK2 autophosphorylation, but not kinase activity, is necessary for adhesion-induced association with c-Src, osteoclast spreading, and bone resorption. J Biol Chem. 2003;278:11502–11512. doi: 10.1074/jbc.M206579200. [DOI] [PubMed] [Google Scholar]
- 49.Park SY, Avraham HK, Avraham S. RAFTK/Pyk2 activation is mediated by trans-acting autophosphorylation in a Src-independent manner. J Biol Chem. 2004;279:33315–33322. doi: 10.1074/jbc.M313527200. [DOI] [PubMed] [Google Scholar]
- 50.Boney CM, Gruppuso PA, Faris RA, Frackelton AR., Jr The critical role of Shc in insulin-like growth factor-I-mediated mitogenesis and differentiation in 3T3-L1 preadipocytes. Mol Endocrinol. 2000;14:805–813. doi: 10.1210/me.14.6.805. [DOI] [PubMed] [Google Scholar]
- 51.Chow JC, Condorelli G, Smith RJ. Insulin-like growth factor-I receptor internalization regulates signaling via the Shc/mitogen-activated protein kinase pathway, but not the insulin receptor substrate-1 pathway. J Biol Chem. 1998;273:4672–4680. doi: 10.1074/jbc.273.8.4672. [DOI] [PubMed] [Google Scholar]
- 52.Kim B, Cheng HL, Margolis B, Feldman EL. Insulin receptor substrate 2 and Shc play different roles in insulin-like growth factor I signaling. J Biol Chem. 1998;273:34543–34550. doi: 10.1074/jbc.273.51.34543. [DOI] [PubMed] [Google Scholar]
- 53.Ishihara H, Sasaoka T, Wada T, Ishiki M, Haruta T, Usui I, Iwata M, Takano A, Uno T, Ueno E, Kobayashi M. Relative involvement of Shc tyrosine 239/240 and tyrosine 317 on insulin induced mitogenic signaling in rat1 fibroblasts expressing insulin receptors. Biochem Biophys Res Commun. 1998;252:139–144. doi: 10.1006/bbrc.1998.9621. [DOI] [PubMed] [Google Scholar]
- 54.Chardin P, Camonis JH, Gale NW, van Aelst L, Schlessinger J, Wigler MH, Bar-Sagi D. Human Sos1: a guanine nucleotide exchange factor for Ras that binds to GRB2. Science. 1993;260:1338–1343. doi: 10.1126/science.8493579. [DOI] [PubMed] [Google Scholar]
- 55.Salcini AE, McGlade J, Pelicci G, Nicoletti I, Pawson T, Pelicci PG. Formation of Shc-Grb2 complexes is necessary to induce neoplastic transformation by overexpression of Shc proteins. Oncogene. 1994;9:2827–2836. [PubMed] [Google Scholar]
- 56.Marshall CJ. Signal transduction. Hot lips and phosphorylation of protein kinases. Nature. 1994;367:686. doi: 10.1038/367686a0. [DOI] [PubMed] [Google Scholar]
- 57.Patrussi L, Savino MT, Pellegrini M, Paccani SR, Migliaccio E, Plyte S, Lanfrancone L, Pelicci PG, Baldari CT. Cooperation and selectivity of the two Grb2 binding sites of p52Shc in T-cell antigen receptor signaling to Ras family GTPases and Myc-dependent survival. Oncogene. 2005;24:2218–2228. doi: 10.1038/sj.onc.1208384. [DOI] [PubMed] [Google Scholar]
- 58.Blaukat A, Pizard A, Rajerison RM, Alhenc-Gelas F, Muller-Esterl W, Dikic I. Activation of mitogen-activated protein kinase by the bradykinin B2 receptor is independent of receptor phosphorylation and phosphorylation-triggered internalization. FEBS Lett. 1999;451:337–341. doi: 10.1016/S0014-5793(99)00613-4. [DOI] [PubMed] [Google Scholar]
- 59.Rikitake Y, Kawashima S, Takahashi T, Ueyama T, Ishido S, Inoue N, Hirata K, Yokoyama M. Regulation of tyrosine phosphorylation of PYK2 in vascular endothelial cells by lysophosphatidylcholine. Am J Physiol Heart Circ Physiol. 2001;281:H266–H274. doi: 10.1152/ajpheart.2001.281.1.H266. [DOI] [PubMed] [Google Scholar]
- 60.Chardin P, Cussac D, Maignan S, Ducruix A. The Grb2 adaptor. FEBS Lett. 1995;369:47–51. doi: 10.1016/0014-5793(95)00578-W. [DOI] [PubMed] [Google Scholar]
- 61.McDonald CB, Seldeen KL, Deegan BJ, Lewis MS, Farooq A. Grb2 adaptor undergoes conformational change upon dimerization. Arch Biochem Biophys. 2008;475:25–35. doi: 10.1016/j.abb.2008.04.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1 Effect of SHP2/5PA mutant on Src and Pyk2 phosphorylation. Quiescent SHP2/WT or SHP2/5PA expressing SMCs were stimulated with IGF-I. Twenty micrograms of cell lysate was used for detection of (A) phosphor-Src (Y419) or phosphor-Pyk2 (Y402) and the blot was stripped and reprobed with (A) anti-Src or (B) anti-Pyk2 antibody as a loading control (TIFF 1585 kb)
Supplemental Fig. 2 The effect of SHP2/Src disruption on Pyk2 association with SHP2. (A and B) Confluent cultures were serum starved overnight and incubated with or without the SHP2 cell-permeable peptide (pept. #142, 10 µg/ml) or control peptide (cont. #136) for 1 h followed by IGF-I treatment for 5 min. The cell lysates were immunoprecipitated with anti-SHP2 antibody followed by immunoblotting for (A) Pyk2 or (B) Src. For loading control, the membrane was reprobed with anti-SHP2 antibody (TIFF 1586 kb)
Supplemental Fig. 3 Effect of Pyk2 knock-down on Src phosphorylation and SHPS-1/Src association. Quiescent Pyk2 knock-down SMCs were stimulated with IGF-I. Twenty micrograms of cell lysate was used for detection of phosphor-Src (Y419). The blot was stripped and reprobed with anti-Src antibody as a loading control (Upper panel). Cell lysates from same experiment were immunoprecipitated (IP) with anti-SHPS-1 and immunoblotted (IB) for Src. To control the loading, the blot was stripped and reprobed with anti-SHPS-1 antibody (lower panel) (TIFF 1586 kb)
Supplemental Fig. 4 Effect of Pyk2/Y881F mutant on SHPS-1/SHC association. Quiescent Pyk2/WT or Pyk2/Y881F expressing SMCs were stimulated with IGF-I for 5 min. Cell lysates were immunoprecipitated (IP) with anti-SHC antibody and immunoblotted (IB) for SHPS-1. To control the loading, the blot was stripped and reprobed with anti-SHC antibody (TIFF 1057 kb)
Supplemental Fig. 5 SHPS-1/Grb2 association is mediated by Pyk2. Quiescent SHC/WT or SHC/3YF expressing SMCs were stimulated with IGF-I. Cell lysates were immunoprecipitated (IP) with anti-SHPS-1 antibody and immunoblotted (IB) for Pyk2 or Grb2. To control the loading, the blot was stripped and reprobed with anti-SHPS-1 antibody (TIFF 1233 kb)