Abstract
Tight junctions control paracellular permeability. Here, we analyzed the impact of residues in the second extracellular loop (ECL2) of mouse claudin-5 on paracellular permeability. Stable expression of claudin-5wild type in MDCK-II cells—but not that of mutants R145A, Y148A, Y158A or E159Q—increased transepithelial electrical resistance and decreased fluorescein permeation. Expression of claudin-5Y148A, Y158A or E159Q enhanced permeability of FITC-dextran10 kDa, which was unchanged in cells expressing claudin-5wild type or claudin-5R145A. In contrast, targeting to tight junctions, strand morphology and tight junction assembly were unchanged. It is concluded that R145 is unessential for trans-interaction of claudin-5, but necessary for tightening against small solutes and ions. The highly conserved residues Y148, Y158 and E159 in ECL2 of claudin-5 contribute to homo- and/or heterophilic trans-interaction between classic claudins and thereby tighten the paracellular space against ions, small and large molecules. These results provide novel insights into the molecular function of tight junctions.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-010-0332-8) contains supplementary material, which is available to authorized users.
Keywords: Claudin, Tight junction, Paracellular permeability, Transmembrane protein, Protein–protein interaction
Introduction
Tight junctions (TJ) are formed between endothelial or epithelial cells. They limit and regulate the paracellular permeation of small ions, solutes and proteins, thus maintaining the homeostasis of different tissue compartments. TJs are mostly located on the apical side of the lateral plasma membrane [1]. They are composed of transmembrane proteins of two opposing cells. Using transmission electron microscopy, TJs appear as fusions of the membranes of two neighboring cells. In freeze-fracture electron microscopic analyses, TJs appear as a continuous network of strands of transmembrane proteins [2].
Claudins, a protein family of about 24 members (subtypes), form the structural backbone of TJ strands. They are able to build up TJ strands under physiological conditions [3]. The various claudin subtypes differ in their permeability properties with respect to the size and charge of small ions or molecules and exhibit tissue-specific expression patterns [4]. For instance, claudin-5 is responsible for tightening the blood-brain barrier against small molecules up to 800 Da [5]. The combination of claudins expressed in a given tissue determines the characteristics of its paracellular tightness [6–8]. The influence of the first extracellular loop (ECL1) of claudins on the paracellular permeability has been studied [4]. For example, Asp65 in the ECL1 has been recently identified as mediating the charge selectivity of the paracellular cation pores that are formed by mouse claudin-2 [9]. For claudin-5, the involvement of the ECL1 in paracellular tightening was also investigated. The exogenous expression of claudin-5 in Madin-Darby canine kidney (MDCK)-II cells expressing other claudin subtypes but not claudin-5 increased the transepithelial electrical resistance (TEER). Alanine substitutions of either of the two conserved cysteine residues in the ECL1 abolished this increase [10].
The impact of the amino acid residues of the second extracellular loop (ECL2) on paracellular sealing has not yet been studied for Cld5 and hardly studied for any other classic claudin [4]. In previous experiments, we investigated the effect of single amino acid substitutions in the ECL2 of Cld5 on homophilic Cld5 interactions in TJ-free cells [11]. Some mutants (for example R145A) showed wild-type-like enrichment of Cld5 in the plasma membrane at contact sites between transfected cells indicating trans-interaction. Other mutants (for example, Y148A, Y158A or E159Q) did not show any enrichment at contact sites, but exhibited homogenous Cld5 distribution in the plasma membrane, indicating block of trans-interaction. The aim of this study was to investigate the impact of the amino acids R145, Y148, Y158 and E159 in the ECL2 of Cld5 on the TJ integrity after stable expression in MDCK-II cells. Only the expression of Cld5wt—but not the expression of any of the Cld5 mutants—increased the paracellular tightness for ions and small solutes. In addition, some mutants disturbed the sealant against large molecules, too. The results demonstrate that the ECL2 is essential for the barrier function of Cld5.
Materials and methods
Antibodies and cell culture
Rabbit anti-Cld1, rabbit anti-occludin, mouse anti-Cld5, mouse anti-GAPDH, goat horseradish peroxidase (HRP)-conjugated anti-rabbit, goat HRP-conjugated anti-mouse, goat Cy3-conjugated anti-mouse and goat 488-conjugated anti-rabbit (Invitrogen. Carlsbad, CA) were used for immunocytochemistry.
Madin-Darby canine kidney (MDCK)-II cells [12] were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 U penicillin and 100 mg/ml streptomycin (Invitrogen. Carlsbad, CA).
Mammalian expression vectors, site-directed mutagenesis and transfection
Vectors for the expression of N-terminally FLAG-tagged mouse Cld5 were based on pEYFP-N1 (Clonetech, Palo Alto, CA). FLAG-Cld5-YFP was generated by amplification of Cld5 using pGTCl-5 (provided by Dr. M. Furuse, Kobe) as template and primers, one encoding the N-terminal FLAG epitope. To generate FLAG-Cld5 without a YFP-tag, site-directed mutagenesis was performed as described in [11] with the introduction of a stop codon 3′ behind the Cld5 open reading frame. The amino acid substitutions R145A, Y148A, Y158A and E159Q were again introduced by site-directed mutagenesis [11]. The amino acid sequence of Cld5-ECL2 wt and mutants is depicted in Fig. 1a. Transfections of MDCK-II cells with FLAG but not YFP tagged Cld5 were performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) or Cell Line Nucleofection® Kit L (Amaxa, Cologne, Germany) according to the recommendations of the supplier. Stable lines were selected by adding 1 mg/ml G418 (Calbiochem, San Diego, CA). To exclude clonal variations, three independent clones with comparable Cld5 expression were initially analyzed, and two of them were used for quantifications for each Cld5 construct.
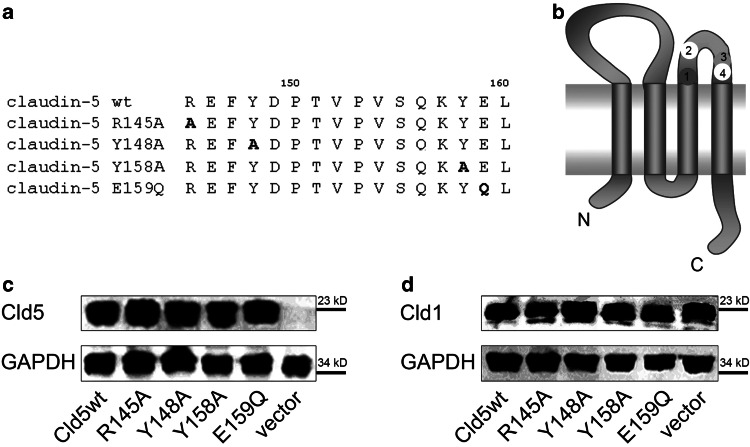
Fig. 1.
Sequence and exogenous expression of claudin-5 (Cld5) constructs in stably transfected MDCK-II cells. a Predicted (swissprot) amino acid sequence of the second extracellular loop of mouse Cld5 wild type (wt) and its mutants. Amino acids substituted by site-directed mutagenesis are indicated in bold. b Schematic overview of Cld5 and the positions of the amino acid substitutions in the Cld5 mutants transfected. c Expression of the different Cld5 proteins in stably transfected MDCK-II cell lines. Cld5 was not detected in cells transfected with the vector control. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as loading control. d The expression of the exogenous Cld5 proteins did not influence the expression of endogenous claudin-1 (Cld1). For C and D, Cld5 and Cld1 were detected by Western blot of cell lysates
Immunoblotting
Cell lysis was performed in 50 mM Tris/HCL, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS and complete protease inhibitor cocktail (Roche, Mannheim, Germany). SDS-PAGE and immunoblotting were performed as previously described [13].
Immunocytochemistry
For immunocytochemistry, cells were washed with phosphate-buffered saline (PBS), fixed with acetone (5 min, 4°C), incubated with ethanol and PBS (1 min, 4°C, each), soaked in blocking solution (BS: 1% bovine serum albumin and 0.05% Tween 20 in PBS) for 10 min and then incubated with the first primary antibody in BS for 1 h. After washing (5 × 2 min in BS), samples were incubated with the second primary antibody in BS for 1 h, washed (5 × 2 min in BS), incubated with secondary antibodies for 30 min in BS, and washed (5 × 2 min in BS) and mounted. Cells were examined using an LSM 510 confocal microscope (Zeiss, Jena, Germany) [11].
Measurement of transepithelial electrical resistance (TEER) and permeation coefficients
Stably transfected MDCK-II cells were seeded at a density of 1×105 into 24-well Millipore transwell filters (Schwalbach, Germany). TEER was determined using Endohm electrodes (Millipore, Eschborn, Germany). After stable TEER values had been reached, permeation studies were performed using 40 μg/ml fluorescein (Sigma-Aldrich, Taufkirchen, Germany) or 25 μg/ml 10 kDa fluorescein isothiocyanate dextran (FITC-dextran) in Hank’s buffered salt solution (HBSS, Biochrom, Berlin, Germany) in the apical compartment and pure HBSS in the basal compartment. After 10 min incubation, 100-μl aliquots were removed from the basal compartment, and the fluorescence intensity was measured using a fluorescence plate reader (Tecan, Crailsheim, Germany). Permeation coefficients were calculated as described [14]. Permeation studies with fluorescein have to be done with care since it could be transported by organic anion transporters. However, the suitability of fluorescein as a paracellular tracer for MDCK-II cells has been demonstrated [15, 16]. In addition, the contribution of a potential fluorescein transport by monocarboxylate transporters is very unlikely for the measuring time used [17].
For the measurements of TEER and permeation of tracers, ≥9 filters of two different cell clones were analyzed for each construct.
Freeze-fracture electron microscopy
Transfected MDCK-II cells were grown until confluence, washed with PBS, fixed with 2.5% glutaraldehyde (electron microscopy grade; Sigma-Aldrich, Taufkirchen, Germany) in PBS for 2 h, washed and processed for freeze-fracture electron microscopy as reported [18].
Statistics
Data are mean ± standard error of the mean (SEM). The Kruskal-Wallis test was used to identify significant differences within experiments, and Dunn’s multiple comparison post-test was used to assess the significance of differences between several experimental groups with p < 0.05 considered as significant. The computer package GraphPad Prism version 3.0 (San Diego, CA) was employed.
Results
The amino acid substitutions R145A, Y148A, Y158A and E159A in the second extracellular loop of claudin-5 do not alter its targeting to tight junctions
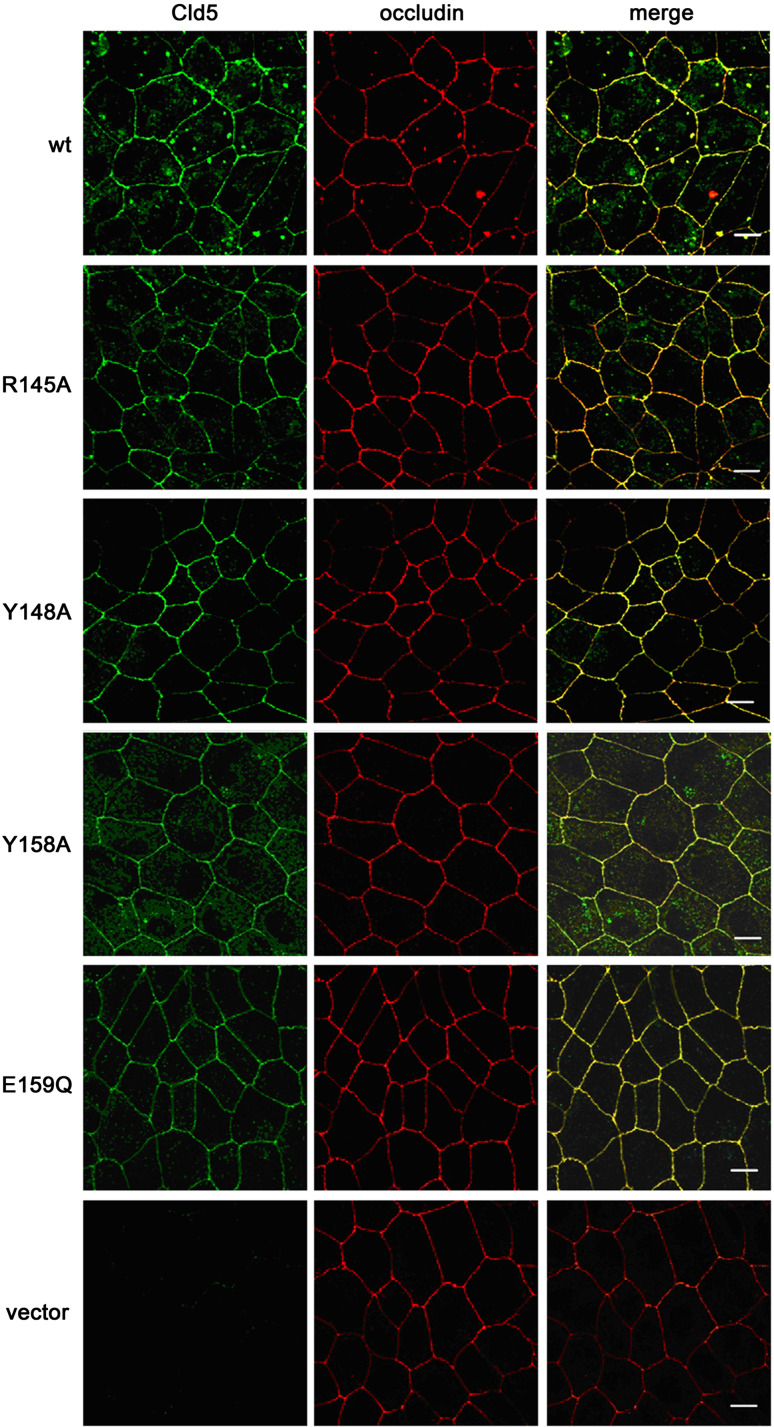
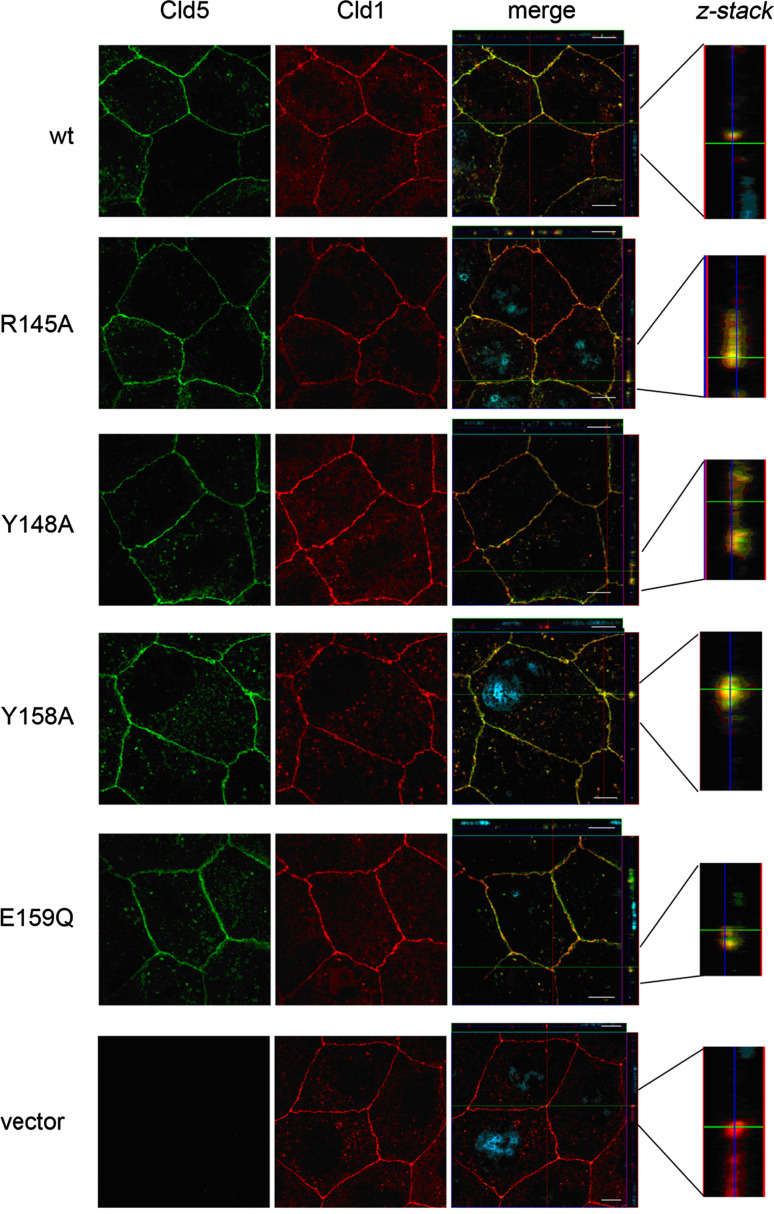
To investigate the impact of the amino acids, residues R145 (1), Y148 (2), Y158 (3) and E159 (4) of Cld5 on the paracellular permeability of epithelial cells expressing endogenous claudins, both Cld5wt and the respective mutants of Cld5 (Fig. 1a and b), were stably transfected into low-resistance MDCK-II cells. All exogenous Cld5 proteins were clearly expressed in the cells, while no Cld5 was detected in MDCK-II cells transfected with the vector control (Figs. 1c, 2, 3). As it has already been demonstrated elsewhere that the expression of exogenous Cld5 does not influence the expression of other endogenous claudins [10, 19], the present analysis was restricted to demonstrating that the expression of endogenous Cld1 did not differ between MDCK-II cells transfected with exogenous Cld5wt or Cld5 mutants (Figs. 1d, 3). Cld5 and mutants were expressed in >95% of the cells used (Figs. 2 and 3). To analyze whether the substitutions affect targeting of Cld5 to TJs, double immunostaining was performed against Cld5 and occludin (Fig. 2), as well as Cld5 and Cld1 (Fig. 3). Cld5wt and all mutants colocalized with both TJ marker proteins, indicating that the substitutions did not change the proper TJ localization of Cld5. In addition, for all constructs similar amounts of Cld5 were detected at the cell-cell contacts (Figs. 2 and 3). These data allowed comparison between Cld5 and its mutants.
Fig. 2.
Colocalization of exogenously expressed Cld5wt, Cld5R145A, Cld5Y148A, Cld5Y158A and Cld5E159Q with the general tight junction marker occludin at cell-cell contacts. The subcellular localization of Cld5 (green) and occludin (red) in stably transfected MDCK-II cells was studied by immunostaining followed by confocal microscopy; bars 10 μm
Fig. 3.
Exogenously expressed Cld5wt, Cld5R145A, Cld5Y148A, Cld5Y158A and Cld5E159Q showed strong colocalization with claudin-1 (Cld1) in the apical part of the lateral plasma membrane. The subcellular localization of Cld5 (green) and Cld1 (red) in stably transfected MDCK-II cells was studied by immunostaining followed by confocal microscopy. XY-Images are shown for Cld5 and Cld1 (large squares). In addition to the XY-images, for the merge, XZ-images are shown on top (flat rectangle), YZ-images on the right and a 5× magnification of the latter in the insert (z-stack). Blue, DAPI staining of the nuclei; bars 10 μm
Amino acid substitutions in the second extracellular loop of claudin-5 affected tight-junction function
The expression of Cld5wt in MDCK-II cells caused a significant, five-fold increase in TEER (507 ± 42 Ω cm²) relative to the vector control (105 ± 3.6 Ω cm²). In contrast, expression of any of the Cld5 mutants failed to alter the TEER significantly compared with vector control (Cld5R145A: 152 ± 10 Ω cm², Cld5Y148A: 107 ± 5.2 Ω cm², Cld5Y158A: 80 ± 7 Ω cm², and Cld5E159Q: 152 ± 11.9 Ω cm²). These results indicated that all amino acid residues of the ECL2 of Cld5 analyzed are essential for the sealing of the paracellular cleft of MDCK-II cells against ions (Fig. 4a).
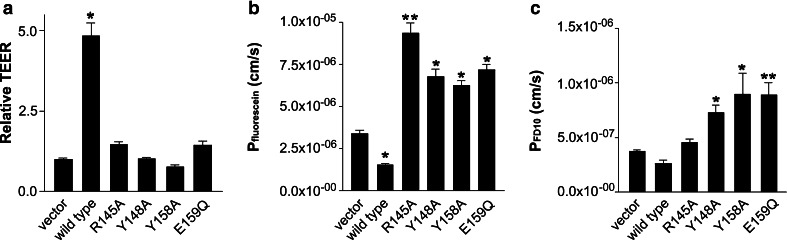
Fig. 4.
Amino acid residues in the second extracellular loop of claudin-5 (Cld5) are necessary for tightening the paracellular cleft. a Cld5wt increased the transepithelial electrical resistance (TEER) after stable transfection into claudin-5-free MDCK-II cells, whereas mutants with the amino acid substitutions R145A, Y148A, Y158A and E159Q in the second extracellular loop of Cld5 did not. b Cld5wt reduced the permeability coefficient of fluorescein (Pfluorescein) after stable transfection in MDCK-II cells, whereas mutants with the amino acid substitutions in the second extracellular loop of Cld5 exhibited higher Pfluorescein compared to the vector control. c The expression of Cld5Y148A, Cld5Y158A and Cld5E159Q increased the permeability coefficient for 10 kDa FITC-dextran (PFD10), whereas that of Cld5wt and Cld5R145A did not. Data are mean ± SEM, n ≥ 9; *p < 0.05, **p < 0.01 vs. vector control
To study whether these amino acid residues of the ECL2 of Cld5 were also involved in the sealing of the paracellular cleft against small molecules, the permeability coefficient of fluorescein (Pfluorescein) was determined for the respective stably transfected MDCK-II cell lines. In cells expressing Cld5wt, the Pfluorescein (1.5 × 10−6 ± 0.1 × 10−6 cm/s) was significantly lower than the vector control by a factor of 2 (3.4 × 10−6 ± 0.2 × 10−6 cm/s). In contrast, expression of Cld5R145A (9.3 × 10−6 ± 0.6 × 10−6 cm/s), Cld5Y148A (6.8 × 10−6 ± 0.4 × 10−6 cm/s), Cld5Y158A (6.3 × 10−6 ± 0.4 × 10−6 cm/s) and Cld5E159Q (7.2 × 10−6 ± 0.4 × 10−6 cm/s) resulted in two- to three-fold increases in the paracellular permeability of MDCK-II cells compared with the control (Fig. 4b). Taken together, the expression of Cld5wt caused sealing of the paracellular cleft against small molecules, whereas the expression of all Cld5 mutants decreased the tightness against small molecules. Hence, the residues in the ECL2 of Cld5 analyzed are critically involved in this tightening.
In addition, it was studied whether the residues in the ECL2 of Cld5 are involved in sealing the paracellular cleft against larger molecules, too. The permeability coefficient of 10 kDa FITC-dextran (PFD10) did not exhibit significant differences between cells expressing Cld5wt (2.6 × 10−7 ± 0.3 × 10−7 cm/s), Cld5R145A (4.5 × 10−7 ± 0.3 × 10−7 cm/s) or the vector control (3.7 × 10−7 ± 0.2 × 10−7 cm/s). However, expression of Cld5Y148A (7.3 × 10−7 ± 0.7 × 10−7 cm/s), Cld5Y158A (9.0 × 10−7 ± 1.9 × 10−7 cm/s, and Cld5E159Q (8.9 × 10−7 ± 1.1 × 10−7 cm/s) increased the PFD10 significantly 2- to 2.4-fold (Fig. 4c). Thus, the expression of Cld5wt and Cld5R145A does not influence the paracellular permeability of MDCK-II cells towards large molecules. In contrast, expression of Cld5Y148A, Cld5Y158A and Cld5E159Q caused an increase in the paracellular permeation of large molecules compared with vector control.
Amino acid substitutions in the second extracellular loop of claudin-5 do not alter the tight-junction strand architecture
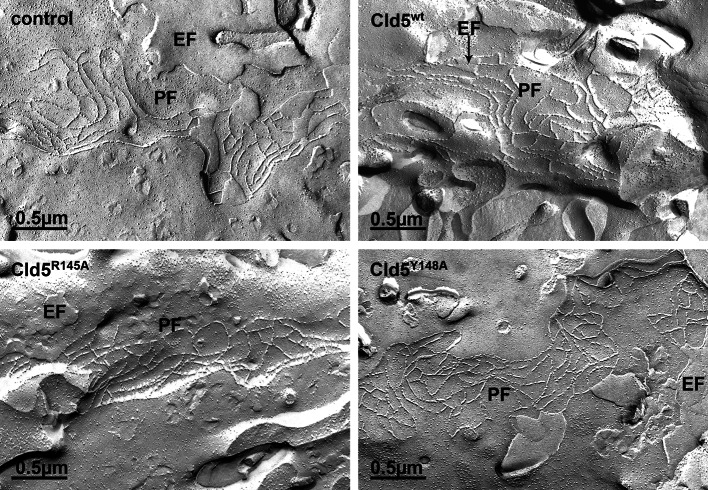
To study whether the increase in paracellular permeability with the expression of the Cld5 mutants was due to changes in TJ strand morphology, freeze-fracture electron microscopy was performed. The MDCK-II cells transfected with vector control showed the typical morphology and protoplasmic fracture face (P-face) association of the strands (Fig. 5). After transfection of Cld5wt, Cld5R145A or Cld5Y148A, no differences were detectable in the morphology of the TJ strands formed by the different cells. The strand networks were still associated with the P-face and exhibited similar density and branching (Fig. 5). Nevertheless, morphology of individual samples within one experimental group may vary. These results indicate that the residues R145 and Y148 in the ECL2 of Cld5 are not required for maintaining the morphology of heteropolymeric TJ strands.
Fig. 5.
Amino acid substitutions in the second extracellular loop of claudin-5 (Cld5) did not alter the morphology of tight junction strands. MDCK-II cells, stably transfected with control vector, Cld5wt, Cld5R145A or Cld5Y148A were fixed and processed for freeze-fracture electron microscopy. In all cases similar network-like intramembranous strands, associated with protoplasmic face (PF) but not with the exoplasmic face (EF), were found
The amino acid substitution Y148A in the second extracellular loop of claudin-5 does not alter disassembly or reassembly of tight junctions
Then, we investigated whether the amino acid substitution Y148A in the ECL2 of Cld5 alters the disassembly or reassembly of TJs. The subcellular localization of Cld5 and Cld1 (serving as an endogenous TJ marker) was therefore studied with MDCK-II cells stably expressing Cld5wt or Cld5Y148A, using a calcium-switch assay. After Ca2+ removal in Cld5wt as well as in Cld5Y148A expressing cells, Cld1 and Cld5 were internalized with comparable kinetics and to a similar extent after Ca2+ removal (Supplementary Figure S1). Likewise, after Ca2+ addition, both in Cld5wt- and Cld5Y148A-expressing cells, Cld5 and Cld1 reappeared in the plasma membrane in a comparable manner and to a similar extent during TJ reassembly upon Ca2+ reintroduction (Supplementary Figure S1). These data indicate that Y148A does not affect the assembly of tight junctions.
Discussion
To obtain novel insights into the molecular mechanism of paracellular tightening by claudins, we have previously demonstrated that defined amino acid residues within the ECL2 of the transmembrane TJ protein Cld5 are essential for its homophilic trans-interaction between two opposing cells [11, 36]. However, it was not clear if these residues contribute to the paracellular tightening mediated by epi-/endothelial tight junctions consisting of several transmembrane and associated proteins. To address this issue in this study, we stably expressed mutants of claudin-5 in MDCK-II cells that contain endogenous claudins and form functional TJs [20, 21]. We demonstrated that residues within the ECL2 of claudin-5 are essential for paracellular tightening.
The effect of amino acid substitutions (human pathogenic as well as designed mutations) in claudins on paracellular permeability has been analyzed for the ECL1 of Cld2 [9], Cld4 and Cld15 [21], Cld5 [10], Cld7 [22] and Cld16 [23]. These studies indicate that the ECL1 is mainly responsible for paracellular tightening, pore formation and charge selectivity. However, the contribution of the ECL2 has been neglected so far.
To obtain better mechanistic insights into the role of the ECL2 of Cld5 in paracellular barrier formation, we have previously analyzed the effect of systematic single amino acid substitutions in the ECL2 on plasma membrane targeting, homophilic cis- and trans-interaction as well as formation of tight junction strands [11]. By expressing mutants of claudin-5 in TJ- and claudin-free HEK cells, we identified residues in the ECL2 that are specifically involved in folding or in strand formation and in trans- but not cis-interaction. The single amino acid substitutions F147A, Y148A, Q156A, Y158A and E159Q strongly inhibited trans-interaction and strand formation [11]. Due to the lack of polarity and additional TJ machinery, claudin-transfected HEK cells could not be used for the measurement of paracellular permeability. In contrast, MDCK-II cells are a well-established model to study the latter [10]. For this reason, stably transfected MDCK-II lines expressing Cld5wt, Cld5R145A, Cld5Y148A, Cld5Y158A and Cld5E159Q were generated to analyze the impact of the ECL2 on paracellular sealing.
Exogenous claudin-5 improves the paracellular tightness of MDCK-II cells
The expression of Cld5wt in MDCK-II cells caused a fivefold increase in TEER and halved the permeability coefficient for fluorescein (376 Da) compared to the control. In contrast, only a slight but not significant reduction was found for the permeability coefficient of 10 kDa-FITC-dextran. These results are consistent with earlier reports obtained in MDCK-II cells [10], Caco-2 cells [19], bEND3 brain endothelial cells [24] and knock-out mice [5]. This verified that Cld5 tightens the junctions against small ions and small molecules, but does not significantly enhance the barrier against large molecules that is formed by other endogenous TJ proteins [4].
The residues R145, Y148, Y158 and E159 in the ECL2 are not necessary for incorporation of claudin-5 into preexisting tight junctions and the polymerization of heteropolymeric claudin strands
In addition to MDCK-II lines that express Cld5wt, lines that express the previously characterized ECL2 mutants Cld5R145A, Cld5Y148A, Cld5Y158A and Cld5E159Q [11] have been generated. In all cell clones analyzed, the Cld5 constructs were clearly expressed, as judged by Western blot and immunostaining. A comparable staining indicates similar amounts of Cld5 at TJs for all constructs. After expression of Cld5wt or Cld5 mutants, no change in the expression of endogenous Cld1 was detected. This is consistent with earlier reports showing no change in expression of endogenous Clds, including Cld2, after Cld5 transfection of MDCK-II [10], MDCK-C7 or Caco-2 cells [19]. Similarly, overexpression of claudin-7 also does not affect expression and localization of endogenous Cld1, -3, -4 and -7 in LLC-PK1 cells [25]. On the other hand, replacement of endogenous claudin-2 in MDCK-II is possible by exogenous claudin-8 [26]. Hence, it is not a general phenomenon for all claudins that overexpression does not change expression of endogenous claudins.
In TJ-free HEK cells, Cld5wt and Cld5R145A but not Cld5Y148A, Cld5Y158A and Cld5E159Q showed enrichment at cell-cell contacts demonstrating a lack of trans-interaction for the latter [11]. In contrast, in our MDCK cells Cld5wt and all mutants were strongly enriched at cell-cell contacts where they co-localized with occludin and claudin-1 in the apical part of the lateral plasma membrane as known for the TJ proteins in polarized epithelial cells. These data indicate that the residues R145, Y148, Y158 and E159 in the ECL2 of Cld5 are not involved in protein interactions that are necessary for incorporation of exogenous claudin-5 into endogenous tight junctions. This has been expected since the substitutions in Cld5-ECL2 may not affect binding to ZO-1 [27] and cis-interaction with endogenous claudins [11]. Furthermore, Ca2+ switch assays [13] indicated that none of the substitutions affected TJ dis- or reassembly. Finally, the expression of either Cld5wt, Cld5R145A or Cld5Y148A (the latter as an example of those substitutions that inhibit trans-interaction, Y148A, Y158A and E159Q) did not change the morphology of the intramembranous TJ strands. Taken together, these data demonstrate that the analyzed substitutions in the ECL2 of Cld5 do not affect the formation of heteropolymeric strands consisting of endogenous claudins and exogenous Cld5.
Amino acid substitutions in the second extracellular loop affect the barrier properties of claudin-5
The effect of mutations in claudins on paracellular permeability has been analyzed in several studies [22, 28]. For example, it is well known that Cld2 forms paracellular cation pores and that the residue Asp65 (mouse Cld2) in the ECL1 is involved in pore formation and charge selectivity [9]. However, the molecular structure of this pore is not known. Compared to pore formation, even less is known about the mechanism that leads to the formation of tight paracellular barriers, e.g., by Cld1, Cld3, Cld4 and Cld5 [4]. It has been shown that C54, C64, L50 and Trp51 in the ECL1 of CLd5 are involved in paracellular barrier formation against small molecules [10]. However, distinct molecular interactions have not been addressed, and the ECL2 has been largely neglected. In our approach, we performed permeability measurements with MDCK-II cells transfected with claudin-5-ECL2 mutants of which the interaction properties had been characterized before by cellular reconstitution of TJ strands in TJ-free cells [11, 12]. Interestingly, each substitution in the ECL2 analyzed (R145A, Y148A, Y158A, E159Q) impeded the Cld5-induced increase in TEER. In addition, they showed a dominant negative effect on the permeability of small molecules, since Pfluorescein was not reduced, but greatly increased, compared to the control. There is no evidence that transcellular transport of fluorescein contributes to the observed changes in Pfluorescein. In addition to other reports [15, 16], it is particularly unlikely that the point mutants with very specific effects on trans-interaction [11] should influence transcellular transport of fluorescein in the opposite way than Cld5wt.
In contrast to the substitution R145A that does not strongly affect trans-interaction [11], the substitutions Y148A, Y158A and E159Q that inhibit trans-interaction also showed a dominant negative effect on the permeability of large molecules, since P10 kDa FITC-dextran has been strongly increased compared to the control. These findings demonstrate that, in the ECL2 of Cld5, residues Y148, Y158 and E159 are necessary for tightening against ions as well as against small and large molecules. In contrast, R145 is involved in the tightening against ions and small molecules, only.
We previously demonstrated that the substitutions R145A, Y148A and E159Q, in contrast to others (e.g., K157A, V152A), do not influence, and Y158A only slightly influences, the plasma membrane targeting of Cld5 [11]. This indicates that these substitutions do not change the conformation of Cld5. In addition, R145A, Y148A and E159A did not affect the homophilic cis-interaction of Cld5. In contrast, Y148, Y158 and E159, but not R145, are involved in homophilic trans-interaction and thereby in the formation of homopolymeric Cld5 strands. Molecular modeling indicated that Y148 and Y158 together with F147A from two opposing Cld5 molecules form an aromatic binding core triggering the trans-interaction [11]. According to this model, the residues R145, E159 and the earlier described Q156 point to additional interaction mechanism(s). However, the respective binding residues are not identified. Hence, they could interact with any loop in the neighbourhood, which could be an ECL2 or an ECL1 of another claudin molecule or the ECL1 of the same claudin-5 molecule. It is likely, but not proven, that both loops cooperate in tightening the paracellular space.
Interestingly, the residues, necessary for trans-interaction, are highly conserved within the classic claudins [4] and are likely to be involved in heterophilic trans-interaction, too. Heterophilic interaction has been reported for different claudin pairs, including Cld5-Cld3, Cld1-Cld3, Cld2-Cld3, Cld3-Cld4 [6, 29, 30] and Cld16-Cld19 [31, 32]. Hence, it is likely that in MDCK-II cells endogenously expressed claudins (claudin-1, -2, -3, -4, -7; [20]) and exogenous Cld5 form homophilic as well as heterophilic claudin-claudin interactions. However, the precise pattern of heterophilic cis- and trans-interactions still has to be identified.
Model of claudin-based barrier formation
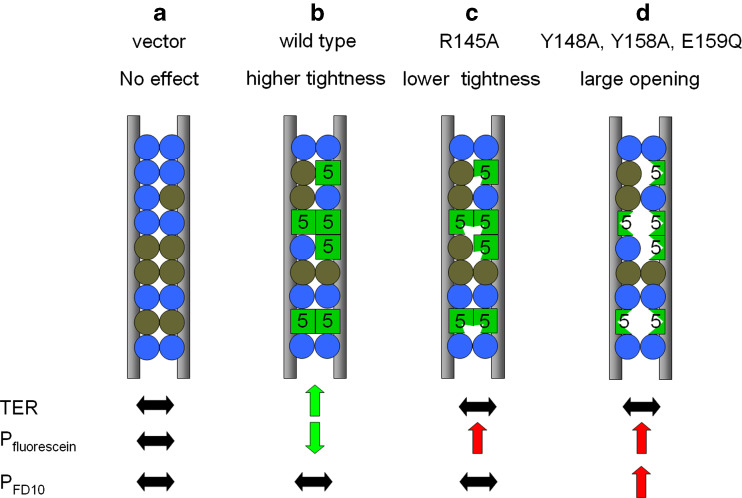
Taken together, we propose the following model of claudin-based barrier formation (Fig. 6):
Fig. 6.
Scheme of the claudin-5-based tightening of the paracellular cleft between two plasma membranes (gray). The additional expression of Cld5wild type (green square) in MDCK-II cells that contain endogenous claudins (blue and olive circles) caused an increase in the transepithelial electrical resistance (TER) and a decrease in paracellular permeation of small molecules (Pfluorescein), without influencing the paracellular permeation of large molecules (PFD10). Removal of the basic side chain R145 of Cld5 (indicated by the slot) does not block trans-interaction but diminishes the tightening against small ions (no increase in TER) and elevated the paracellular permeation of small molecules (increase in PFluorescein). However, Cld5R145A had no effect on the paracellular permeation of large molecules (no change in PFD10). This demonstrates that R145 is essential for the tightening function of Cld5 against small ions and small but not large molecules. Blocking the trans-interaction of Cld5 by the substitutions Y148A, Y158A or E159Q (indicated by the gaps) does not block the formation of heteropolymeric strands but results in clefts that reduce the tightening against small ions (increase in TER), small and even large molecules (increase in PFluorescein and PFD10)
In non-transfected MDCK-II cells, endogenous claudins (e.g., Cld1, -2, -3, -4, -7) copolymerize in heteropolymeric intramembranous TJ strands [6] that form an effective barrier against large and, to a lesser extent, against small molecules (Fig. 6a). Small ions are able to permeate—due to the pore formation mediated by claudin-2 [33, 34]. Due to heterophilic cis- and/or trans-interactions, exogenously expressed Cld5 is incorporated in the heteropolymeric strands. This tightens the barrier against small ions and small molecules (Fig. 6b). This might be due to a higher packing density or an altered molecular organization of the claudins in the TJ. The side chain of R145 seems to be directly involved in this tightening, and if it is missing, then the seal is slightly loosened (Fig. 6c). The substitutions Y148A, Y158A and E159Q do not inhibit the homo- and heterophilic cis-interactions of Cld5, but specifically inhibit its homo- and heterophilic trans-interactions. In contrast, trans-interactions between endogenous claudins are not affected (Fig. 6d). The resulting partial inhibition of trans-interaction is not sufficient to block heteropolymerization of the claudins, but causes larger clefts at the position within the polymer where Cld5 molecules are located. According to the model, these clefts are responsible for the increase in paracellular permeability of small and large molecules. All substitutions reduce TER relative to Cld5wt, but not below the level of the vector control. This is due to the leakiness for ions caused by ion pores that are formed by endogenous Cld2 in MDCK-II.
We used epithelial MDCK-II cells as an established TJ-containing but Cld5-free model system [10] to analyze mechanistic aspects of TJ formation. Cld5 is mainly expressed in endothelial cells. In the blood-brain barrier, it is likely to be coexpressed with Cld3, Cld12 and maybe Cld1 as well as other potential claudins [4]. We propose that the mechanism of homophilic and heterophilic Cld5 trans-interactions (e.g., Cld5 and Cld3) is similar for epithelial and endothelial cells. In addition, we showed that the substitution Y148A of Cld5, in zebrafish Cld5a, blocked its function in a cerebral barrier consisting of epithelial cells during the development of the zebrafish embryo [35]. Hence, the identified molecular determinants are of relevance in vivo, too.
In conclusion, we have identified molecular mechanisms by which the ECL2 of claudin-5 essentially contributes to the paracellular tightening against ions, small and large molecules. These sealing properties of the ECL2 are assumed to be largely shared by the classic group of claudin proteins, as most of the ECL residues discussed in this paper are highly conserved [4].
Electronic supplementary material
Fig. S1. The amino acid substitution Y148A in claudin-5 does not affect disassembly nor reassembly of tight junctions. (A) MDCK-II cells, stably transfected with Cld5wt or Cld5Y148A were cultured until confluent before standard medium was replaced by medium containing 2mM EGTA for Ca2+-delpetion. The subcellular localization of endogenous Cld1 (green), exogenous Cld5 (red) and the nuclei (blue) were detected before, 0.5, 1, 2 and 3 h after removal of Ca2+ by immunostaining and subsequent confocal microscopy. With increasing time of Ca2+-depletion the detected amount of Cld5 and Cld1 in the plasma membrane decreased and that in intracellular compartments increased for Cld5wt- as well as for Cld5Y148A-expressing cells. At no time point internalization of Cld5 or Cld1 was more pronounced for Cld5Y148A- than for Cld5wt-expressing cells (B) The subcellular localization of endogenous Cld1 (green), exogenous Cld5 (red) and nuclei (blue) were detected at 0, 0.25, 0.5, 1, 2, 3 and 4 h after media exchange to Ca2+ containing standard medium. Between Cld5wt- and Cld5Y148A-expressing cells, no difference in the reappearance at the plasma membrane was detectable for either Cld1 or Cld5. (JPEG 3.61 MB)
Fig. S1. The amino acid substitution Y148A in claudin-5 does not affect disassembly nor reassembly of tight junctions. (A) MDCK-II cells, stably transfected with Cld5wt or Cld5Y148A were cultured until confluent before standard medium was replaced by medium containing 2mM EGTA for Ca2+-delpetion. The subcellular localization of endogenous Cld1 (green), exogenous Cld5 (red) and the nuclei (blue) were detected before, 0.5, 1, 2 and 3 h after removal of Ca2+ by immunostaining and subsequent confocal microscopy. With increasing time of Ca2+-depletion the detected amount of Cld5 and Cld1 in the plasma membrane decreased and that in intracellular compartments increased for Cld5wt- as well as for Cld5Y148A-expressing cells. At no time point internalization of Cld5 or Cld1 was more pronounced for Cld5Y148A- than for Cld5wt-expressing cells (B) The subcellular localization of endogenous Cld1 (green), exogenous Cld5 (red) and nuclei (blue) were detected at 0, 0.25, 0.5, 1, 2, 3 and 4 h after media exchange to Ca2+ containing standard medium. Between Cld5wt- and Cld5Y148A-expressing cells, no difference in the reappearance at the plasma membrane was detectable for either Cld1 or Cld5. (JPEG 4.70 MB)
Acknowledgments
We thank Barbara Eilemann for technical assistance. We gratefully acknowledge the help of Ria Knittel in freeze-fracturing. This work was funded by DFG BL308/7-3, 7-4 and PI 837/2-1.
Abbreviations
- Cld
Claudin
- Da
Dalton
- DMEM
Dulbecco’s modified Eagle’s medium
- ECL
Extracellular loop
- FITC
Fluorescein isothiocyanate
- HBSS
Hank’s buffered salt solution
- HRP
Horseradish peroxidase
- MDCK
Madin-Darby canine kidney
- PBS
Phosphate buffered saline
- Pfluorescein
Permeability coefficient of fluorescein
- PFD10
Permeability coefficient of 10 kDa FITC-dextran
- SEM
Standard error of the mean
- TEER
Transepithelial electrical resistance
- TJ
Tight junctions
Footnotes
C. Piehl and J. Piontek contributed equally to this work.
References
- 1.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Staehelin LA. Further observations on the fine structure of freeze-cleaved tight junctions. J Cell Sci. 1973;13:763–786. doi: 10.1242/jcs.13.3.763. [DOI] [PubMed] [Google Scholar]
- 3.Furuse M, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta. 2008;1778:631–645. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol. 1999;147:891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 8.Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol. 2003;285:F1078–F1084. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- 9.Yu AS, Cheng MH, Angelow S, Gunzel D, Kanzawa SA, Schneeberger EE, Fromm M, Coalson RD. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J Gen Physiol. 2009;133:111–127. doi: 10.1085/jgp.200810154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen H, Watry DD, Marcondes MC, Fox HS. Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Mol Cell Biol. 2004;24:8408–8417. doi: 10.1128/MCB.24.19.8408-8417.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piontek J, Winkler L, Wolburg H, Muller SL, Zuleger N, Piehl C, Wiesner B, Krause G, Blasig IE. Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J. 2008;22:146–158. doi: 10.1096/fj.07-8319com. [DOI] [PubMed] [Google Scholar]
- 12.Blasig IE, Winkler L, Lassowski B, Mueller SL, Zuleger N, Krause E, Krause G, Gast K, Kolbe M, Piontek J. On the self-association potential of transmembrane tight junction proteins. Cell Mol Life Sci. 2006;63:505–514. doi: 10.1007/s00018-005-5472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andreeva AY, Krause E, Muller EC, Blasig IE, Utepbergenov DI. Protein kinase C regulates the phosphorylation and cellular localization of occludin. J Biol Chem. 2001;276:38480–38486. doi: 10.1074/jbc.M104923200. [DOI] [PubMed] [Google Scholar]
- 14.Gaillard PJ, Voorwinden LH, Nielsen JL, Ivanov A, Atsumi R, Engman H, Ringbom C, de Boer AG, Breimer DD. Establishment and functional characterization of an in vitro model of the blood-brain barrier, comprising a co-culture of brain capillary endothelial cells and astrocytes. Eur J Pharm Sci. 2001;12:215–222. doi: 10.1016/S0928-0987(00)00123-8. [DOI] [PubMed] [Google Scholar]
- 15.Krug SM, Fromm M, Gunzel D. Two-path impedance spectroscopy for measuring paracellular and transcellular epithelial resistance. Biophys J. 2009;97:2202–2211. doi: 10.1016/j.bpj.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krug SM, Amasheh S, Richter JF, Milatz S, Gunzel D, Westphal JK, Huber O, Schulzke JD, Fromm M. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol Biol Cell. 2009;20:3713–3724. doi: 10.1091/mbc.E09-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konishi Y, Hagiwara K, Shimizu M. Transepithelial transport of fluorescein in Caco-2 cell monolayers and use of such transport in in vitro evaluation of phenolic acid availability. Biosci Biotechnol Biochem. 2002;66:2449–2457. doi: 10.1271/bbb.66.2449. [DOI] [PubMed] [Google Scholar]
- 18.Mack AF, Wolburg H. Growing axons in fish optic nerve are accompanied by astrocytes interconnected by tight junctions. Brain Res. 2006;1103:25–31. doi: 10.1016/j.brainres.2006.04.135. [DOI] [PubMed] [Google Scholar]
- 19.Amasheh S, Schmidt T, Mahn M, Florian P, Mankertz J, Tavalali S, Gitter AH, Schulzke JD, Fromm M. Contribution of claudin-5 to barrier properties in tight junctions of epithelial cells. Cell Tissue Res. 2005;321:89–96. doi: 10.1007/s00441-005-1101-0. [DOI] [PubMed] [Google Scholar]
- 20.Hou J, Gomes AS, Paul DL, Goodenough DA. Study of claudin function by RNA interference. J Biol Chem. 2006;281:36117–36123. doi: 10.1074/jbc.M608853200. [DOI] [PubMed] [Google Scholar]
- 21.Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol. 2002;283:C142–C147. doi: 10.1152/ajpcell.00038.2002. [DOI] [PubMed] [Google Scholar]
- 22.Alexandre MD, Jeansonne BG, Renegar RH, Tatum R, Chen YH. The first extracellular domain of claudin-7 affects paracellular Cl- permeability. Biochem Biophys Res Commun. 2007;357:87–91. doi: 10.1016/j.bbrc.2007.03.078. [DOI] [PubMed] [Google Scholar]
- 23.Hou J, Paul DL, Goodenough DA. Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci. 2005;118:5109–5118. doi: 10.1242/jcs.02631. [DOI] [PubMed] [Google Scholar]
- 24.Koto T, Takubo K, Ishida S, Shinoda H, Inoue M, Tsubota K, Okada Y, Ikeda E. Hypoxia disrupts the barrier function of neural blood vessels through changes in the expression of claudin-5 in endothelial cells. Am J Pathol. 2007;170:1389–1397. doi: 10.2353/ajpath.2007.060693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexandre MD, Lu Q, Chen YH. Overexpression of claudin-7 decreases the paracellular Cl- conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. J Cell Sci. 2005;118:2683–2693. doi: 10.1242/jcs.02406. [DOI] [PubMed] [Google Scholar]
- 26.Angelow S, Schneeberger EE, Yu AS. Claudin-8 expression in renal epithelial cells augments the paracellular barrier by replacing endogenous claudin-2. J Membr Biol. 2007;215:147–159. doi: 10.1007/s00232-007-9014-3. [DOI] [PubMed] [Google Scholar]
- 27.Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angelow S, Ahlstrom R, Yu AS. Biology of Claudins. Am J Physiol Renal Physiol. 2008;295:F867–F876. doi: 10.1152/ajprenal.90264.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1166–L1178. doi: 10.1152/ajplung.00182.2003. [DOI] [PubMed] [Google Scholar]
- 30.Daugherty BL, Ward C, Smith T, Ritzenthaler JD, Koval M. Regulation of heterotypic claudin compatibility. J Biol Chem. 2007;282:30005–30013. doi: 10.1074/jbc.M703547200. [DOI] [PubMed] [Google Scholar]
- 31.Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest. 2008;118:619–628. doi: 10.1172/JCI33970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci USA. 2009;106:15350–15355. doi: 10.1073/pnas.0907724106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 34.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Piontek J, Wolburg H, Piehl C, Liss M, Otten C, Christ A, Willnow TE, Blasig IE, Abdelilah-Seyfried S. Establishment of a neuroepithelial barrier by Claudin5a is essential for zebrafish brain ventricular lumen expansion. Proc Natl Acad Sci USA. 2010;107:1425–1430. doi: 10.1073/pnas.0911996107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winkler L, Gehring C, Wenzel A, Müller SL, Piehl C, Krause G, Blasig IE, Piontek J. Molecular determinants of the interaction between Clostridium perfringens enterotoxin fragments and claudin-3. J Biol Chem. 2009;284:18863–18872. doi: 10.1074/jbc.M109.008623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. The amino acid substitution Y148A in claudin-5 does not affect disassembly nor reassembly of tight junctions. (A) MDCK-II cells, stably transfected with Cld5wt or Cld5Y148A were cultured until confluent before standard medium was replaced by medium containing 2mM EGTA for Ca2+-delpetion. The subcellular localization of endogenous Cld1 (green), exogenous Cld5 (red) and the nuclei (blue) were detected before, 0.5, 1, 2 and 3 h after removal of Ca2+ by immunostaining and subsequent confocal microscopy. With increasing time of Ca2+-depletion the detected amount of Cld5 and Cld1 in the plasma membrane decreased and that in intracellular compartments increased for Cld5wt- as well as for Cld5Y148A-expressing cells. At no time point internalization of Cld5 or Cld1 was more pronounced for Cld5Y148A- than for Cld5wt-expressing cells (B) The subcellular localization of endogenous Cld1 (green), exogenous Cld5 (red) and nuclei (blue) were detected at 0, 0.25, 0.5, 1, 2, 3 and 4 h after media exchange to Ca2+ containing standard medium. Between Cld5wt- and Cld5Y148A-expressing cells, no difference in the reappearance at the plasma membrane was detectable for either Cld1 or Cld5. (JPEG 3.61 MB)
Fig. S1. The amino acid substitution Y148A in claudin-5 does not affect disassembly nor reassembly of tight junctions. (A) MDCK-II cells, stably transfected with Cld5wt or Cld5Y148A were cultured until confluent before standard medium was replaced by medium containing 2mM EGTA for Ca2+-delpetion. The subcellular localization of endogenous Cld1 (green), exogenous Cld5 (red) and the nuclei (blue) were detected before, 0.5, 1, 2 and 3 h after removal of Ca2+ by immunostaining and subsequent confocal microscopy. With increasing time of Ca2+-depletion the detected amount of Cld5 and Cld1 in the plasma membrane decreased and that in intracellular compartments increased for Cld5wt- as well as for Cld5Y148A-expressing cells. At no time point internalization of Cld5 or Cld1 was more pronounced for Cld5Y148A- than for Cld5wt-expressing cells (B) The subcellular localization of endogenous Cld1 (green), exogenous Cld5 (red) and nuclei (blue) were detected at 0, 0.25, 0.5, 1, 2, 3 and 4 h after media exchange to Ca2+ containing standard medium. Between Cld5wt- and Cld5Y148A-expressing cells, no difference in the reappearance at the plasma membrane was detectable for either Cld1 or Cld5. (JPEG 4.70 MB)