Abstract
A 55-year-old man with stage IV lung adenocarcinoma was treated with cisplatin, pemetrexed, nivolumab, and ipilimumab. Approximately 100 days after treatment initiation, he became disoriented and presented to the emergency department with a high fever. Blood tests revealed liver and kidney dysfunctions. Subsequently, the patient developed generalized convulsions that required intensive care. He was clinically diagnosed with cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). Organ damage was gradually controlled with immunosuppressive drugs, including steroids, and the patient was discharged. Successful treatment is rare in patients with CRS, including ICANS, during immune checkpoint inhibitor treatment for solid tumors.
Keywords: immune checkpoint inhibitor, nivolumab, ipilimumab, cytokine release syndrome, immune effector cell-associated neurotoxicity syndrome
Introduction
Immunotherapies have been developed for various cancers, including solid tumors, and they have played a significant role in recent years. However, as the use of these drugs has increased, specific adverse events have become more frequent, and clinicians must manage these events appropriately (1). Cytokine release syndrome (CRS) is a known complication of chimeric antigen receptor T-cell (CAR-T) therapy that can lead to multiple organ damage and a fever. It can also occasionally cause central nervous system abnormalities, leading to a new disease entity known as immune effector cell-associated neurotoxicity syndrome (ICANS).
Although immune checkpoint inhibitors (ICIs) for solid tumors rarely cause CRS, multiple organ failure can lead to fatal outcomes. We herein report a case of severe CRS and ICANS that developed during ICI treatment for lung adenocarcinoma, highlighting the rarity of this type of adverse event during ICI treatment for solid tumors and the importance of its early recognition and appropriate management.
Case Report
A 55-year-old man presented with a 1-month history of persistent hemoptysis and thoracic pain. He was a 35-pack-year smoker. After visiting his local doctor, he underwent chest computed tomography (CT), which revealed a mass shadow in the right lung, micronodular shadow along the pleura, and space-occupying lesion in the liver, suspected of being a metastasis (Fig. 1). Subsequently, the patient was referred to our hospital for a further examination and treatment. The Eastern Cooperative Oncology Group (ECOG) performance status score was 1. A transbronchial biopsy was performed, and the patient was diagnosed with stage IV lung adenocarcinoma.
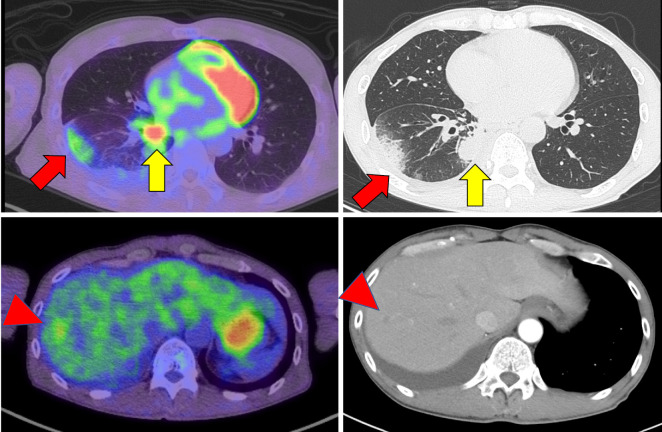
Figure 1.
Computed tomography findings at the time of the lung cancer diagnosis. The nodular shadow in the right lower lobe of the lung (yellow arrows) is thought to be the primary tumor. The peripheral shadow (red arrows) is thought to be a pleural metastasis. Fluorodeoxyglucose-positron emission tomography (FDG-PET) shows a nodular shadow in the liver (red arrowheads).
Given the advanced stage of the patient's lung cancer, we began initial treatment consisting of a combination of cisplatin (75 mg/m2 on day 1, every 3 weeks, up to 2 courses), pemetrexed (500 mg/m2 on day 1, every 3 weeks, up to 2 courses), ipilimumab (1 mg/kg on day 1, every 6 weeks), and nivolumab (360 mg/body on day 1, every 3 weeks). Three weeks after administration, he developed erythema of his extremities [grade 2 according to the Common Terminology Criteria for Adverse Events (CTCAE)]. The patient was referred to a dermatologist who initiated oral prednisone at a dose of 15 mg/day. Based on the dermatologist's assessment, the prednisone dose was later reduced to 2.5 mg/day. Using prednisone to control adverse effects, his erythema improved to grade 1. Therefore, he ultimately received five doses of nivolumab and three doses of ipilimumab.
Twenty days after the fifth cycle of nivolumab, the patient was admitted to the emergency room with a persistent fever (39°C) that had lasted for a week. His vital signs on admission were as follows: blood pressure, 80/40 mmHg; heart rate, 120 beats/min; transcutaneous oxygen saturation, 98% in room air; respiratory rate, 18/min; body temperature, 39.0°C; and impaired consciousness (Glasgow Coma Scale E4V4M6). A physical examination revealed an erythematous skin rash in the inguinal region but no abnormal cardiac or respiratory sounds or peripheral edema.
Blood tests revealed significantly elevated D-dimer levels (Table 1) but no evidence of thrombocytopenia, prolonged prothrombin time, or activated partial thromboplastin time. Furthermore, liver enzymes, such as aspartate aminotransferase/alanine aminotransferase (1,672/725 U/L; CTCAE grade 4), creatinine, and blood urea nitrogen levels were elevated, suggesting renal and hepatic dysfunctions. In addition, his C-reactive protein and procalcitonin levels were elevated as well, suggesting a bacterial infection. Serum ferritin levels were markedly elevated. These abnormal values were unexpected, based on regular follow-up blood tests before the fifth nivolumab dose (Table 2). Autoantibodies were measured, and the patient was tested for viral hepatitis and cytomegalovirus; however, the findings were negative. A urinalysis revealed elevated levels of N-acetyl glucosamine, β2-microglobulin, and neutrophil gelatinase-associated lipocalin, indicating renal tubular damage. CT performed on admission showed a significant reduction in tumor size in the right lower lobe of the lung (partial response evaluated by Response Evaluation Criteria in Solid Tumors), but there was also considerable enlargement of both kidneys (Fig. 2).
Table 1.
Laboratory Data on Admission.
| [Complete blood count] | [Biochemistry] | [Arterial blood gas] | |||||||||||
| WBC | 9,600 | /μL | TP | 7.1 | g/dL | pH | 7.4 | ||||||
| Neu | 83.1 | % | Alb | 2.9 | g/dL | pCO2 | 31.8 | Torr | |||||
| Lym | 12.3 | % | T-Bil | 1.39 | mg/dL | pO2 | 84.1 | Torr | |||||
| Mon | 3 | % | D-Bil | 0.63 | mg/dL | HCO3- | 19.2 | mEq/L | |||||
| Eos | 1.3 | % | AST | 1,672 | U/L | ||||||||
| Bas | 0.3 | % | ALT | 725 | U/L | [Urinalysis] | |||||||
| RBC | 422 | ×104/μL | ALP | 1,090 | U/L | Specific gravity | 1.011 | ||||||
| Hb | 13.8 | g/dL | LDH | 7,288 | U/L | pH | 5.5 | ||||||
| Ht | 40 | % | γ-GTP | 481 | U/L | Protein | 1+ | ||||||
| MCV | 90.5 | fL | Na | 135 | mEq/L | U-TP/Cr | 1.35 | g/gCr | |||||
| MCH | 31.2 | pg | K | 4.9 | mEq/L | Glucose | (-) | ||||||
| MCHC | 34.5 | g/dL | Cl | 97 | mEq/L | Ketones | (-) | ||||||
| Plt | 31.5 | ×104/μL | Ca | 8.1 | mg/dL | Blood | 3+ | ||||||
| UA | 16 | mg/dL | Urobilinogen | (±) | |||||||||
| [Coagulation] | BUN | 115.3 | mg/dL | Bilirubin | (-) | ||||||||
| PT | 12 | s | Cre | 10.74 | mg/dL | Leukocytes | (-) | ||||||
| PT-INR | 1.17 | CRP | 8.56 | mg/dL | U-Na | 64 | mEq/L | ||||||
| APTT | 28.4 | s | PCT | 7.57 | ng/mL | U-Cr | 83.2 | mg/dL | |||||
| Fib | 142 | mg/dL | CK | 326 | U/L | U-NAG | 113.7 | U/L | |||||
| D-dimer | 1,440 | μg/mL | CK-MB | 11 | U/L | U-BMG | 0.594 | µg/mL | |||||
| Amy | 102 | U/L | U-NGAL | 2,220 | ng/mL | ||||||||
| BNP | 16.3 | pg/mL | |||||||||||
| TSH | 0.14 | mIU/L | |||||||||||
| FT4 | 1.42 | ng/dL | |||||||||||
| KL-6 | 514 | U/mL | |||||||||||
| Ferritin | 37,673 | ng/mL | |||||||||||
Table 2.
Laboratory Data before Admission (before Cycle 5 Nivolumab).
| [Complete blood count] | [Biochemistry] | |||||||
| WBC | 7,130 | /μL | TP | 7.2 | g/dL | |||
| Neu | 68.5 | % | Alb | 3.9 | g/dL | |||
| Lym | 18.6 | % | T-Bil | 0.36 | mg/dL | |||
| Mon | 5.8 | % | D-Bil | NA | mg/dL | |||
| Eos | 6.3 | % | AST | 25 | U/L | |||
| Bas | 0.8 | % | ALT | 23 | U/L | |||
| RBC | 425 | ×104/μL | ALP | 124 | U/L | |||
| Hb | 13.9 | g/dL | LDH | 234 | U/L | |||
| Ht | 43.1 | % | γ-GTP | 33 | U/L | |||
| MCV | 101.4 | fL | Na | 135 | mEq/L | |||
| MCH | 32.6 | pg | K | 4.8 | mEq/L | |||
| MCHC | 32.1 | g/dL | Cl | 103 | mEq/L | |||
| Plt | 35.8 | ×104/μL | Ca | 9.7 | mg/dL | |||
| UA | NA | mg/dL | ||||||
| [Coagulation] | BUN | 12.8 | mg/dL | |||||
| PT | NA | s | Cre | 0.88 | mg/dL | |||
| PT-INR | NA | CRP | 0.19 | mg/dL | ||||
| APTT | NA | s | PCT | NA | ng/mL | |||
| Fib | NA | mg/dL | CK | 61 | U/L | |||
| D-dimer | 2.6 | μg/mL | CK-MB | NA | U/L | |||
| Amy | 83 | U/L | ||||||
| BNP | NA | pg/mL | ||||||
| TSH | 0.13 | mIU/L | ||||||
| FT4 | 1.57 | ng/dL | ||||||
| KL-6 | NA | U/mL | ||||||
| Ferritin | NA | ng/mL | ||||||
NA: not assessed
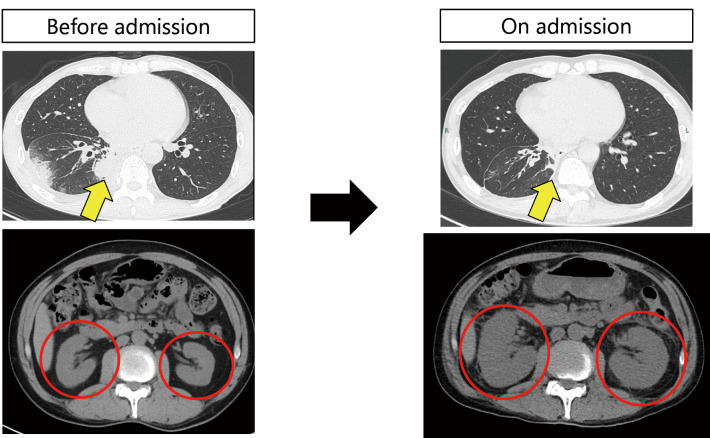
Figure 2.
Computed tomography findings at the time of admission. The nodular shadow in the right lower lobe of the lung (yellow arrows), thought to be the primary tumor, was smaller than in the previous image. The kidneys are markedly enlarged (red circles), but there are no signs of obstructive hydronephrosis.
Based on these findings, we considered bacterial infection-induced septic shock [including disseminated intravascular coagulation (DIC)], severe hepatitis, nephritis due to immune-related adverse events (irAEs), or CRS accompanied by ICANS as potential differential diagnoses. Tumor lysis syndrome was deemed unlikely because the tumor volume was not sufficiently large, and this event occurred long after treatment initiation.
The patient was oliguric because of severe renal dysfunction; therefore, we inserted a catheter to enable dialysis. As we were unable to exclude the possibility of sepsis or irAEs, we initiated treatment with antibiotics (meropenem) and high-dose corticosteroids [methylprednisolone (mPSL) 1 g/day for 3 days]. The level of consciousness was mildly impaired upon admission. After admission, the patient developed generalized convulsions and was transferred to the intensive care unit for tracheal intubation. Brain magnetic resonance imaging (MRI) revealed edematous changes (Fig. 3); however, there were no signs of cancerous meningitis or meningeal irritation, including terminal neck stiffness. Although we considered bacterial or cancerous meningitis, we did not perform lumbar puncture because of coagulopathy and the influence of anticoagulants during dialysis.
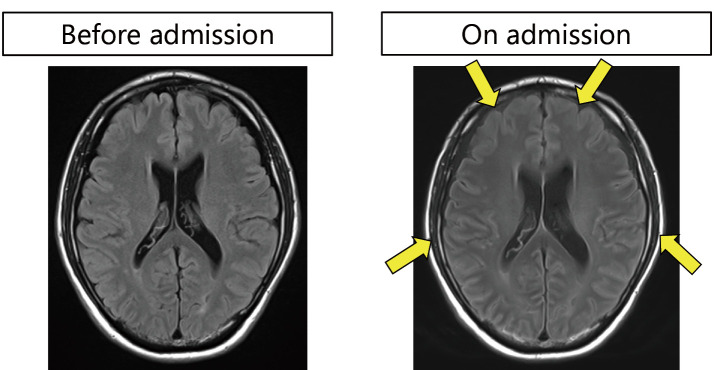
Figure 3.
Magnetic resonance imaging on admission. The cerebral sulcus is narrowed (yellow arrows), reflecting brain swelling.
After the initiation of steroid treatment, the patient's abnormal blood test results rapidly improved (Fig. 4). However, the fever persisted, and multiple blood cultures yielded no abnormal bacteriological findings. Because the patient was considered to have CRS accompanied by ICANS induced by ICI treatment, further immunosuppressive treatment was needed. Therefore, intravenous cyclophosphamide and immunoglobulin were administered in combination with steroids. In addition, we considered the possibility of a bacterial infection that was not susceptible to meropenem. Therefore, we added daptomycin to the patient's treatment regimen.
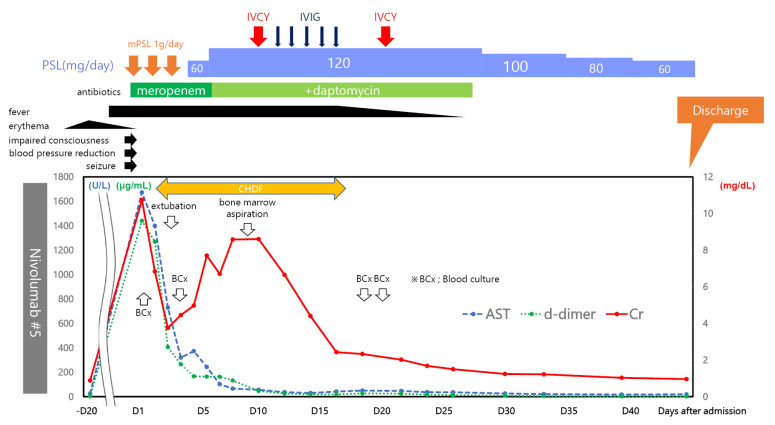
Figure 4.
Timeline of the patient’s clinical course and laboratory values. The patient developed a skin rash and fever after the fifth nivolumab dose. Sepsis or some form of immune-related adverse event (irAE) was suspected on the day of the emergency room visit based on laboratory abnormalities. Blood cultures were obtained, and treatment with meropenem and 1 g of methylprednisolone was initiated. On the day of admission, the patient’s consciousness was clouded, and he developed generalized convulsions; therefore, he was intubated, and mechanical ventilation was initiated. He was also hypotensive; therefore, he was treated with vasopressors; within one day, his blood pressure recovered to within the normal range. However, his renal function was poor; therefore, continuous hemodiafiltration (CHDF) was continued until his renal function recovered. We attempted to decrease the prednisone dose, but his temperature remained elevated; therefore, we increased the steroid dose from 1 to 2 mg/kg and administered daptomycin in combination with cyclophosphamide and intravenous immunoglobulin. With multidisciplinary treatment, the patient’s liver and kidney functions slowly improved, and he was weaned off dialysis. The patient was discharged 47 days after admission and continued to receive prednisone (1 mg/kg/day) after discharge.
These treatments were then continued. The patient was successfully weaned off dialysis, and his fever gradually decreased. Subsequently, we gradually reduced the steroid dosage and confirmed that the patient's blood test results were mostly within the respective normal ranges. He was discharged 47 days after admission (Fig. 4).
Discussion
CRS is an acute systemic inflammatory syndrome characterized by a fever and multiple organ dysfunction and is often triggered by immunotherapy. It is the most frequent adverse event associated with CAR-T cell therapy (2). CRS is diagnosed based on the clinical presentation, which consists of a high fever (≥38.0°C) with or without variable degrees of hypotension, hypoxia, and/or other end-organ dysfunction that develops within hours to days after immunotherapy. There are no clear diagnostic criteria for CRS, and the differential diagnosis between CRS and sepsis is crucial. Neurotoxicity associated with CAR-T cell therapy, known as ICANS, develops in approximately 20-70% of treated patients (3). ICANS is characterized by confusion, delirium, aphasia, motor dysfunction, and somnolence. In patients with moderate-to-severe ICANS, edematous changes are detected on brain MRI. MRI findings other than cerebral edema include hyperintensities in the periventricular or subcortical white matter, occasional external and extreme capsule involvement on symmetrical T2-weighted or fluid-attenuated inversion-recovery (FLAIR), and edema in the thalami, pons, and medulla on hyperintense T2-weighted or FLAIR signals (4). In severe cases, life-threatening events, such as convulsions and coma, may occur.
CRS is infrequent in patients with solid tumors. According to the World Health Organization database, 80,700 adverse events related to ICIs were reported in January 2020, among which only 58 cases of CRS were reported (5). However, the diagnosis can be challenging and can be missed if not strongly suspected, as in this case. Furthermore, we found no reports of neurological symptoms (convulsions and disorientation) associated with CRS during ICI treatment of solid tumors, as in this case. CRS associated with ICANS usually occurs during the early stages of CAR-T cell therapy (6). In contrast, CRS associated with ICI treatment tends to have a later onset of 1-18 weeks after treatment initiation (5). Therefore, it is plausible that ICANS during ICI treatment could occur several weeks after ICI treatment initiation. The difference in the timing of the onset may be because the magnitude of immune cell (T-cell) activation after CAR-T therapy is stronger than that after ICI therapy (7). In the present case, irAE-related encephalitis could not be ruled out. However, the patient displayed impaired consciousness and seizures, accompanied by severe CRS. He also showed cerebral edematous changes in the brain on MRI, which have been reported in severe ICANS (4,8). In contrast, cerebral edema has not been reported as a typical change in irAE-related encephalitis (9). Therefore, we considered that the patient had developed ICANS accompanied by CRS.
In this case, we encountered great difficulties in diagnosing CRS complicated by ICANS. Sepsis is the primary differential diagnosis. Sepsis causes multiple organ failure and coagulopathy, as was observed in this case. A cerebrospinal fluid analysis could not be performed; however, various bacterial tests yielded negative results. In addition, the blood tests performed at the time of consultation showed abnormal laboratory values only for D-dimer, which did not meet the diagnostic criteria for DIC. Furthermore, the patient's condition improved after treatment with immunosuppressive drugs, including steroids, which was inconsistent with the clinical course of multi-organ failure due to sepsis.
The most likely primary diagnoses were hepatitis, nephritis, and encephalopathy secondary to irAEs. Although these could have been complications, coagulopathy, characterized only by elevated D-dimer levels, cannot be attributed solely to irAEs. Tumor lysis syndrome, characterized by damage to multiple organs after anticancer therapy, was unlikely in this case because the patient had been treated for a solid tumor, and the tumor volume was not extremely large. Distinguishing between CRS and hemophagocytic syndrome is often challenging. However, in the present case, a bone marrow biopsy was performed, and hemophagocytic syndrome was ruled out under the supervision of a hematologist. Although the loss of consciousness could have been associated with the fever or environmentally induced delirium, the occurrence of generalized convulsions combined with mild cerebral edema observed on head MRI suggests that the clinical manifestations were caused by ICANS.
CRS management depends on its cause and severity. Milder cases require symptomatic management only. Patients with more severe CRS require intensive therapy. Glucocorticoid administration is beneficial. Treatment for CRS often includes dexamethasone, hydrocortisone, and mPSL. Similarly, the primary treatment for irAEs is prednisolone (PSL) or mPSL at 1-2 mg/kg. In Japan, mPSL 500-1,000 mg/day for 3 days followed by PSL maintenance therapy is the standard treatment for severe (grade 3-4) irAEs (10,11). For severe CRS caused by CAR-T cell therapy, initial treatment with tocilizumab plus glucocorticoids may be better than treatment with either agent alone (12).
Although steroids remain the primary treatment for CRS resulting from irAEs in solid tumors, tocilizumab is underutilized owing to diagnostic difficulties (13). However, some reports suggest relatively positive outcomes in young patients, women, and those undergoing long-term treatment, highlighting the importance of making an appropriate diagnosis and performing therapeutic intervention (5,13).
The specific dose of glucocorticoids and the optimal therapeutic regimen for CRS and ICANS after ICI treatment are yet to be established. However, most protocols for CRS and ICANS after CAR-T cell therapy suggest using dexamethasone at a starting dose of 10 mg every 6 to 12 h, with a plan to taper over 2 to 5 days (14). Early intervention with tocilizumab in patients with both CRS and ICANS may reduce the severity of ICANS, although it is not widely used, so additional studies are warranted (15).
It is important to be aware of the possibility of CRS during ICI treatment of solid tumors. Furthermore, because the initial symptoms of CRS are similar to those of sepsis, the possibility of CRS should be considered when treating patients with suspected sepsis as well. As immunocomplex therapies continue to be developed, it is important to be aware of the possibility of CRS and monitor patients accordingly.
This case had two limitations. First, we were unable to measure the levels of inflammatory cytokines, including interleukin (IL)-6 and interferon (IFN)-γ, which are released from activated T-cells and have been reported to cause CRS. Although these are not essential for the diagnosis of CRS, their measurement can provide additional diagnostic information (10). However, IL-6 and IFN-γ are known to be markedly elevated in inflammatory conditions such as sepsis, so they may not be useful for differentiating CRS from sepsis. Based on the exclusion criteria listed above, CRS was not aggressively suspected at first. Second, we were unable to test the patient's cerebrospinal fluid because of the risk of complications due to coagulation abnormalities, intensive care, and the use of anticoagulants for continuous dialysis, so we had to rely on MRI and clinical findings to rule out cancer or bacterial meningitis.
Despite these limitations, we believe that our data are sufficient to diagnose CRS complicated by ICANS during ICI treatment for lung cancer. We consider this case worthy of reporting because of its complexity.
Written informed consent was obtained from the patient for the publication of this case report and accompanying images.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank the patient for allowing us to publish the details of this case.
References
- 1. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 4: 1721-1728, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Santomasso BD, Nastoupil LJ, Adkins S, et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO guideline. J Clin Oncol 39: 3978-3992, 2021. [DOI] [PubMed] [Google Scholar]
- 3. Holtzman NG, Xie H, Bentzen S, et al. Immune effector cell-associated neurotoxicity syndrome after chimeric antigen receptor T-cell therapy for lymphoma: predictive biomarkers and clinical outcomes. Neuro Oncol 23: 112-121, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Groot PM, Arevalo O, Shah K, et al. Imaging primer on chimeric antigen receptor T-cell therapy for radiologists. Radiographics 42: 176-194, 2022. [DOI] [PubMed] [Google Scholar]
- 5. Ceschi A, Noseda R, Palin K, Verhamme K. Immune checkpoint inhibitor-related cytokine release syndrome: analysis of WHO global pharmacovigilance database. Fron Pharmacol 11: 557, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev 34: 45-55, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yomota M, Mirokuji K, Sakaguchi M, et al. Cytokine release syndrome induced by immune-checkpoint inhibitor therapy for non-small-cell lung cancer. Intern Med 60: 3459-3462, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guha-Thakurta N, Wierda WG. Cerebral edema secondary to chimeric antigen receptor T-cell immunotherapy. Neurology 91: 843, 2018. [DOI] [PubMed] [Google Scholar]
- 9. Gao Y, Pan J, Shen D, et al. Immune checkpoint inhibitor associated autoimmune encephalitis, rare and novel topic of neuroimmunology: a case report and review of the literature. Brain Sci 12: 773, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer 6: 56, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aldea M, Orillard E, Mansi L, et al. How to manage patients with corticosteroids in oncology in the era of immunotherapy? Eur J Cancer 141: 239-251, 2020. [DOI] [PubMed] [Google Scholar]
- 12. Frey NV, Porter DL. Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program 2016: 567-572, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ohira J, Kawamoto M, Sugino Y, Kohara N. A case report of fulminant cytokine release syndrome complicated by dermatomyositis after the combination therapy with immune checkpoint inhibitors. Medicine 99: e19741, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neelapu SS. Managing the toxicities of CAR T-cell therapy. Hematol Oncol 37: 48-52, 2019. [DOI] [PubMed] [Google Scholar]
- 15. Gust J, Hay KA, Hanafi LA, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov 7: 1404-1419, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]