Abstract
Objective
Due to the increasing elderly population and number of dementia patients, the current number of psychiatrists and neurologists remains insufficient to treat dementia in Japan. Therefore, a simple method for accurately performing a dementia diagnosis, including that of primary care physicians, is sought in clinical practice.
Methods
A retrospective study was conducted on patients who made their first visit due to amnesia between October 2020 and October 2022. The sensitivities and specificities of four spatial recognition and planning ability evaluation methods [fox finger imitation test, pentagon-copying test (PCT), cube-copying test (CCT), and clock-drawing test (CDT)] were calculated. The difference between the Mini-mental State Examination (MMSE) scores, as an evaluation of memory and language impairment, and CDT scores were assessed using the Mann-Whitney U test.
Patients
Fifty-one patients with dementia and 6 patients without dementia were examined in this study.
Results
The sensitivity and specificity were 31.4% and 100% for the fox finger imitation tests, 29.4% and 100% for PCT, 62% and 83.3% for CCT, and 72.5% and 100% for CDT, respectively. The sensitivity increased to 78.4% when the CCT and CDT results were combined. Spearman's rank correlation coefficient between the MMSE and CDT scores of the 51 patients with dementia showed a significantly positive correlation (r=0.62, p<0.001). Comparing Alzheimer's disease (AD) and dementia with Lewy bodies (DLB), the difference between the MMSE and CDT scores was significantly greater in patients with DLB.
Conclusion
To quickly screen for dementia, a combination of CCT and CDT is recommended for the highest sensitivity (78.4%). In addition, the difference between the CDT and MMSE scores is considered to be useful for differentiating DLB from AD.
Keywords: Alzheimer's disease, clock-drawing test, cube-copying test, fox finger imitation test, dementia, dementia with Lewy bodies
Introduction
In Japan, the number of dementia patients is currently thought to be 4 million and this number is estimated to increase to approximately 7 million by 2025, comprising approximately 6% of the total Japanese population (1). The rapid increase in the number of patients with dementia is expected to lead to a further increase in social welfare and medical costs. As a result, younger generations will bear a greater financial burden. The total number of neurologists and psychiatrists who diagnose dementia in Japan is approximately 20,000 (2,3). However, neurologists tend to be overloaded with the responsibilities of diagnosing and treating various other diseases, such as stroke, various types of degenerative diseases, headache, and epilepsy. Psychiatrists are also overloaded with the need to diagnose and treat patients with schizophrenia, depression, attention deficit hyperactivity disorder (ADHD), Asperger's syndrome, and addiction-related diseases. Therefore, it is becoming more difficult for psychiatrists and neurologists to find enough time to diagnose dementia.
Therefore, a simple method for diagnosing dementia is required. Although there have been studies examining the sensitivity and specificity of one or two screening tests, to date, no studies have examined the combined use of the four tests in the same dementia population and compared their sensitivities. We herein retrospectively examined the sensitivity of four spatial recognition and planning ability evaluation methods [finger imitation test (4,5), pentagon-copying test (PCT), cube-copying test (CCT) (6), and clock-drawing test (CDT) (7)], along with suggested combinations of memory and language evaluation methods, namely the Mini-Mental State Examination (MMSE) (8).
Materials and Methods
A total of 57 consecutive patients who visited the amnesia consultation outpatient clinic of the Yowa Hospital Dementia Disease Center between October 2020 and October 2022 were retrospectively investigated. The diagnosis of dementia was carefully made by two experienced neurologists, spending approximately 3 hours for each patient, including careful consultation, clinical history, family history, physical examination, neuropsychological examination, electrocardiography, cerebral computed tomography (CT) scans, and blood tests (levels of vitamin B1, vitamin B12, folic acid, zinc, serum iron, ferritin, serum copper, 25(OH) vitamin D, rapid plasma reagin test, thyroid-stimulating hormone, and free thyroxine, in addition to blood counts and biochemical examinations). Considering the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5) published in 2013, a dementia diagnosis was comprehensively made if the patient could not manage self-medication or had difficulty shopping appropriately even when MMSE was 27, referencing the blood chemical data and CT image data.
Exclusion criteria were significant visual impairment or hearing loss, hand movement disorders, or an inability to write. All the subjects in this study were right-handed.
MMSE, Hasegawa's Dementia Scale-Revised (HDS-R), fox finger imitation test, PCT, CCT, and CDT were performed. The MMSE, HDS-R, and CCT were performed by three experienced clinical psychologists. All the CDTs were performed by a neurologist (author). In the CDT, patients were instructed to draw a circle as large as possible on a blank A4-sized paper, write 12 numbers in the circle, and draw the hands of a clock at 10:10 on the numbered circle. The CDT was scored by the author and the MMSE was scored by three clinical psychologists.
The fox finger imitation test, which includes three movement processes, was also conducted by the author: 1) show a finger “fox” to the subject for 10 seconds, 2) ask the subject to make the same fox using his/her fingers, then touch the fox's ears, and 3) turn one fox 180 degrees. The success of the test was determined when 2) and 3) were completed correctly.
CDT scoring was performed using Freedman's method, which defines the maximum score at 15 points and includes the following 15 evaluation items (9):
[A] The entire clock (1. An acceptable contour is drawn; 2. The contour is neither too small nor overdrawn).
[B] Written numbers on the clock (3. Write numbers 1 to 12; 4. Arabic number representation; 5. Numbers written in the correct order; 6. Paper not rotated while drawing numbers; 7. Numbers in the correct position; 8. All numbers are located inside the contour).
[C] Hands of clock (9. Clock has 2 hands; 10. Two hands cross each other; 11. There are only two hands; 12. The hour hand is pointed correctly; 13. The minute hand is pointed correctly; 14. The hour hand is shorter than the minute hand; 15. Hands are accurately joined at the clock's center).
The correlation between the MMSE and CDT scores was also examined. The cut-off point for dementia on the CDT test was defined as 12 points, based on the textbook of Freedman (9), which stated that optimal discrimination was found at a cutoff of 12 out of 15 points, as less than 12 provided a sensitivity of 0.78 and a specificity of 0.82.
Statistical analyses: Spearman's rank correlation coefficient was used to analyze the correlation between the groups. Subsequently, the Mann-Whitney U test was used for continuous variables. EZR (Ver.1.52) was used for all statistical analyses (EZR is a free statistical software program with extended functions of R and R commander, which is available on the website of Jichi Medical University Saitama Medical Center, Japan). The significance level was set at less than 5%.
Ethical considerations: Various neuropsychological, blood, and cerebral CT tests were performed as part of outpatient diagnoses, and the use of the resulting data for research purposes was subject to the ethics guidelines of Yowa Hospital. The analysis was performed after approval by the ethical committee of Yowa Hospital. The study was conducted in accordance with the Declaration of Helsinki which was revised in 2013.
Results
Of the 57 subjects, six who were diagnosed as not having dementia were excluded from the sensitivity analyses of the four screening test methods. Instead, six non-dementia subjects were enrolled as normal controls for the specificity evaluation of the four test methods. In the 51 dementia patients, the mean age was 84.1±6.9 years (14 males and 37 females), and the mean years of education was 10.5±2.4 years. Whereas, in the 6 non-dementia patients, the mean age was 87.2±7.6 years (including 2 males and 4 females), and the mean years of education was 11.2±3.1 years. Although the non-dementia patients were slightly older, there were no statistically significant differences in age and years of education between the patients with and without dementia. In addition, no significant gender difference was found in the years of education between males and females. The average MMSE score was 20.1±5.0 for the 51 dementia patients and was 27.7±2.1 for the 6 non-dementia patients.
As shown in Table 1, the 51 dementia subjects included 41 patients with probable Alzheimer's disease (AD), four patients with vascular dementia (VD) according to DSM-5, three patients with probable dementia with Lewy bodies (DLB) according to the diagnostic criteria of the 4th Consensus Report of the DLB Consortium, one patient with probable progressive supranuclear palsy (PSP) according to PSP diagnostic criteria by the Movement Disorder Society (MDS) in 2017, and two patients with unclassifiable dementia (Table 1).
Table 1.
MMSE and CDT Scores of 51 Dementia Patients by Disease Types and 6 Normal Controls.
| AD | VD | DLB | Unclassifiable dementia | PSP | Non-dementia | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n=41) | (n=4) | (n=3) | (n=2) | (n=1) | (n=6) | |||||||
| Age (years) (mean±SD) | 85.1±6.0 | 84.3±5.7 | 85.3±7.1 | 74±1.4 | 62 | 87.2±7.6 | ||||||
| Sex (male/female) | 12/29 | 0/4 | 0/3 | 1/1 | 1/0 | 2/4 | ||||||
| MMSE scores (mean±2 SD) | 19.8±5.3 | 19.0±2.8 | 21.3±0.6 | 22±2.8 | 30 | 27.7±2.1 | ||||||
| CDT scores (mean±2 SD) | 9.0±4.1 | 7.5±2.7 | 5.8±2.3 | 4.8±3.2 | 13.5 | 13.8±1.2 |
MMSE: mini-mental-state examination, CDT: clock-drawing test, AD: Alzheimer’s disease, VD: vascular dementia, DLB: dementia with Lewy bodies, PSP: progressive supranuclear palsy
The sensitivities and specificities of the four types of 4 spatial recognition and planning ability tests were 31.4% (51 patients) and 100% for the fox finger imitation tests, 29.4% (51 patients) and 100% for PCT, 62% (50 patients) and 83.3% for CCT, and 72.5% (51 patients) and 100% for CDT, respectively (Table 2). The sensitivity increased to 78.4% when the CCT and CDT results were combined. For the specificity evaluation, one of the six patients diagnosed with non-dementia failed to successfully draw a cube in the CCT test, although the patient succeeded in the other three tests. Five of the five non-dementia patients succeeded in all the four tests.
Table 2.
Comparison of Sensitivity and Specificity of Four Qualitative Tests.
| Fox finger imitation test | Pentagon-copying test | Cube-copying test (CCT) | Clock-drawing test (CDT) | ||
|---|---|---|---|---|---|
| Dementia | Test positive | 16 | 15 | 31 | 37 |
| Test negative | 35 | 36 | 19 | 14 | |
| Total patients | 51 | 51 | 50 | 51 | |
| Sensitivity | 31.4% | 29.4% | 62.0% | 72.5% | |
| Non-dementia | Test positive | 0 | 0 | 1 | 0 |
| Test negative | 6 | 6 | 5 | 6 | |
| Total patients | 6 | 6 | 6 | 6 | |
| Specificity | 100% | 100% | 83.3% | 100% |
The sensitivity became the highest (78.4%) when CCT and CDT results were combined. The sensitivity was calculated referring results of 6 non-dementia patients.
Spearman's rank correlation coefficient between the MMSE and CDT scores of the 51 patients with dementia showed a significantly positive correlation (r=0.62, p<0.001). Comparing AD and DLB, the difference between MMSE and CDT scores was significantly greater in the patients with DLB.
As shown in Fig. 1, CDT and MMSE scores of 51 patients with dementia showed a significant positive correlation (r=0.62, p<0.001) (Fig. 1). Comparing the differences between the MMSE and CDT scores in AD and DLB patients, DLB patients showed a significantly greater difference (Fig. 2).
Figure 1.
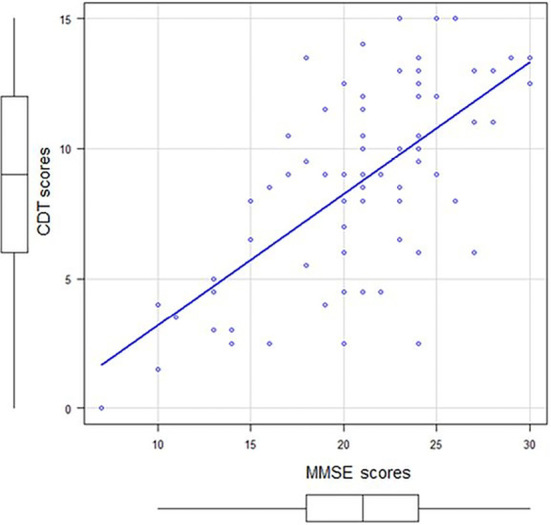
The clock-drawing test (CDT) score relative to the mini-mental-state examination (MMSE) score of 51 dementia patients. A significant positive correlation (r=0.62, p<0.001) was noted between the CDT and MMSE scores.
Figure 2.
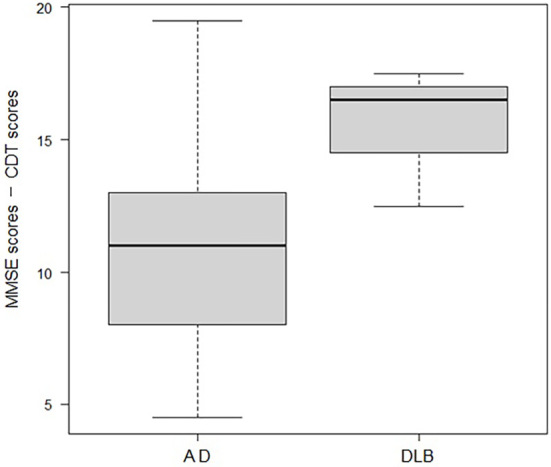
Subtraction of the clock-drawing test (CDT) scores from the mini-mental-state examination (MMSE) scores. Subtraction of the CDT scores from the MMSE scores in the Alzheimer’s disease (AD) group (n=41) and dementia with Lewy bodies (DLB) group (n=3) resulted in significantly higher values in the DLB group. When the Mann-Whitney U test was performed, the minimum score for each: 4.5 points for AD; 12.5 points, DLB, the first quartile: 8.0 points for AD; 14.5 points, DLB; the median: 11 points for AD; 16.5 points, DLB; the third quartile: 13 points for AD; 17 points, DLB; the maximum value: 19.5 points for AD; 17.5 points, DLB, and p-value was 0.03, thus indicating significantly higher values with DLB.
Discussion
In the present study, four types of tests were performed on the same patient group to evaluate spatial recognition and planning ability. The author's institution diagnoses dementia by spending approximately three hours based on several evaluation items, including the DSM-5 and the four tests. In other words, the sensitivities and specificities of the four simple test methods were retrospectively assessed after the diagnosis of dementia and the types of dementia were confirmed.
Yamaguchi et al. reported a low sensitivity of 11.4% for patients with dementia, although the sensitivity was 80.7% when a pigeon imitation test was conducted (4). The fox finger imitation test in the present study also had a low sensitivity of 31.4%.
An optimum dementia screening test battery for primary care physicians has been and is still being debated, namely, simple test methods with high sensitivity and specificity in a short time using a pen and paper. In 1975, Folstein et al. conceived the MMSE (8). Although PCT was included at the end of the MMSE, MMSE is to mainly evaluate a language cognitive function. In 2000, Borson et al. conceived the Mini-Cog for screening dementia (10). In the Mini-Cog test, some items such as the ability to recall 3 words after responding to several questions would be equally evaluated even when the examiner changes; however, evaluations of a success or failure of CDT may vary depending on the examiner. According to the searched Cochrane Review of 2021, three Mini-Cog clinical test results were reported, of which the sensitivity and specificity varied: 1) sensitivity of 67% and specificity of 87%, 2) sensitivity of 60% and specificity of 65%, and 3) sensitivity of 87% and specificity of 100% (11).
Maruta et al. reported that the sensitivity of PCT was low at 29.4%; however, when pre-trained artifical intelligence (AI) models (fine-tuned GoogLeNet CNN) were used to distinguish the difference between pentagons drawn by dementia patients and those drawn by non-dementia patients, AI was able to improve both the sensitivity and specificity with a high level of accuracy, namely approximately 79 to 93% (12). Administering PCT and CCT to patients with Parkinson's disease dementia, Alty JE reported that the sensitivity of PCT was 26% and CCT was 74%, showing that CCT was superior to PCT in sensitivity (13). This finding is consistent with the results of the present study.
For CDT, Shulman conducted a search and analyzed Medline and Psycho-info from 1983 to 1998 for articles that examined the sensitivity and specificity of the CDT when used for dementia screening purposes, its correlation with the MMSE, and other tests (7). He reported that the results were good, with an average sensitivity of 85% and specificity of 85% for the CDT, and high correlations with the MMSE and other cognitive tests, generally exceeding r=0.5. These data support the results of the present study. In 2022, Costa et al. reported that CDT was the most useful tool for detecting dementia, followed by CCT (14). They applied three test methods: 1) overlapping infinity loops (similar to drawing double pentagons), 2) wire cube (cube copying test), and 3) CDT on 112 subjects, including 51 normal subjects, 21 patients with a subjective cognitive impairment, 24 patients with MCI, and 16 patients with dementia. The fact that their report was published last year suggests that an optimum combination of dementia screening tests is an old and new question in clinical practice.
There have been reports distinguishing between dementia and non-dementia patients using an AI analysis (15,16). Our data (using only pen and paper) were also as highly sensitive as the AI data. The analysis of the CDT results using AI is certainly able to judge many subjects with high accuracy. However, there is one question regarding a CDT diagnosis using AI: “Is AI software readily available for primary care physicians?”. I speculate that it is still difficult to make AI diagnostics widely available to primary care physicians at a reasonable cost.
Researchers have developed many different CDT modalities and scoring methods. Freedman's method is known as a quantitative scoring method (9) and Rouleau's classification is known as a qualitative scoring method (17). However, although CDT is useful for screening dementia patients, it is not as popular as MMSE or HDS-R, which mainly evaluate memory and language impairment.
There are two reasons why Japan does not endorse the use of CDT. First, the medical fee for MMSE and HDS-R is currently 800 yen, respectively, while the CDT is not counted as a medical fee. Furthermore, the CDT was omitted from comprehensive cognitive function tests in driver's license verification for elderly drivers in Japan. It is regrettable that the CDT was eliminated, even though the CDT has the highest sensitivity in screening dementia patients (7). A clear and quick CDT scoring method should be widespread among examiners in Japan.
Many previous studies regarding CDT have shown a high positive correlation with the MMSE (7), which is used worldwide as a dementia screening test. Our study also showed a significant positive correlation between the CDT and MMSE results, suggesting that the quantitative evaluation of CDT using Freedman's method is valid as a cognitive function screening test.
The present study was associated with two limitations. The first limitation is the small number of subjects, especially the control subjects, which included only six non-dementia patients. Our institution admits only one patient per week for dementia diagnosis with outpatient forgetfulness consultation, and the yearly number of diagnoses is approximately 50. The specificities of the four tests would be higher if a larger number of healthy subjects were included. The second limitation, as previously pointed out by Yamaguchi et al., is that the fox finger imitation test has a low sensitivity for screening dementia patients (4). To the best of my knowledge, only one study in Japan has compared the fox finger and pigeon finger imitation tests (5). In the present study, 25 of the 51 patients who underwent the fox finger imitation test also underwent the pigeon finger imitation test. Overall, the sensitivity of the pigeon finger imitation test was 44%, which was close to that reported by Ishioka et al. (43.8%, n=16). They also reported that the female dementia patient group had a significantly higher failure rate in the pigeon-finger imitation test (5), but the present results showed no statistically significant sex difference. Ishioka et al. also reported that DLB had a higher success rate in the pigeon-finger imitation test than AD; however, the present study results showed that DLB had a significantly higher failure rate (p=0.0274, Fisher's exact test). Further studies are necessary to determine the reasons for the contradictory results between Ishioka et al. and the present study. Our hospital is currently conducting a follow-up pigeon finger imitation test instead of a fox finger imitation test (4).
In summary, dementia can be detected with a sensitivity of 78.4% using the CDT and CCT, which are spatial recognition and planning ability evaluation tests. In addition, a comparison of each dementia disease type shows that DLB has a larger difference between MMSE and CDT values than does AD. A simple subtraction of the CDT score from the MMSE score may provide a clue to differentiate between AD and DLB. With reference to the detailed medical history of each patient, dementia may be readily screened and diagnosed by primary care physicians. Further studies with larger numbers of subjects are required to verify this conclusion.
The author states that he has no Conflict of Interest (COI).
Acknowledgement
The author would like to thank Dr. Ken Watanabe, Chairman of the Tottori Medical Association, for his valuable advice on the present study.
References
- 1.Ministry of Health, Labour and Welfare, Japan. Comprehensive strategy for promotion of dementia measures, New Orange Plan (Ninchisho Shisaku Suishin Sogo Senryaku, Shin Orange Plan) [Internet]. [Updated 2017 Jul; cited 2023 Jun 30]. Available from: https://www.mhlw.go.jp/file/06-Seisakujouhou-12300000-Roukenkyoku/kaitei_orangeplan.pdf (in Japanese).
- 2.Ministry of Health, Labour and Welfare, Japan. Overview of statistics on physicians, dentists, and pharmacists, 2020. (Reiwa 2-nen Ishi, Shikaishi, Yakuzaishi Toukei no Gaikyo) [Internet]. [cited 2023 May 28]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/ishi/20/index.html (in Japanese).
- 3.Ministry of Health, Labour and Welfare, Japan. Outline of dementia policy promotion (Ninchisyo Shisaku Suishin Taiko Nitsuite) [Internet]. 2020 [cited 2023 May 28]. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000076236_00002.html (in Japanese).
- 4. Yamaguchi H, Maki Y, Yamagami T. Yamaguchi fox-pigeon imitation test: a rapid test for dementia. Dement Geriatr Cogn Disord 29: 254-258, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Ishioka M, Sugawara N, Kaneda A, et al. The Yamaguchi fox/pigeon-imitation test, a brief cognitive performance rating tool, in a community-dwelling population: normative data for Japanese subjects - a preliminary study. Neuropsychiatr Dis Treat 10: 1721-1725, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maeshima S, Osawa A, Maeshima E, et al. Usefulness of a cube-copying tests in outpatients with dementia. Brain Inj 18: 889-898, 2004. [DOI] [PubMed] [Google Scholar]
- 7. Shulman KI. Clock-drawing: is it the ideal cognitive screening test? Int J Geriatr Psychiatry 15: 548-561, 2000. [DOI] [PubMed] [Google Scholar]
- 8. Folstein MF, Folstein SE. “Mini-mental state": A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189-198, 1975. [DOI] [PubMed] [Google Scholar]
- 9.Freedman M, Leach L, Kaplan E, Winocur G, Shulman K, Delis DC. Clock Drawing: A Neuropsychological Analysis. Oxford University Press, New York, 1994: 42. [Google Scholar]
- 10. Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The Mini-Cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 15: 1021-1027, 2000. [DOI] [PubMed] [Google Scholar]
- 11. Chan CC, Fage BA, Burton JK, et al. Mini-Cog for the detection of dementia within a secondary care setting. Cochrane Database Syst Rev 7: CD011414, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maruta J, Uchida K, Kurozumi H, et al. Deep convolutional neural networks for automated scoring of pentagon copying test results. Sci Rep 12: 9881, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alty JE, Cosgrove J, Jamieson S, Smith SL, Possin KL. Which figure copy test is more sensitive for cognitive impairment in Parkinson's disease: wire cube or interlocking pentagons? Clin Neurol Neurosurg 139: 244-246, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Costa S, George R, MacDonald J, Wang X, Alty J. Diagnostic accuracy of the overlapping infinity loops, wire cube, and clock drawing tests in subjective cognitive decline, mild cognitive impairment and dementia. Geriatrics (Basel) 7: 72, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Masuo A, Ito Y, Kanaiwa T, Naito K, Sakuma T, Kato S. Dementia screening based on SVM using qualitative drawing error of clock drawing test. Annu Int Conf IEEE Eng Med Biol Soc 2022: 4484-4487, 2022. [DOI] [PubMed] [Google Scholar]
- 16. Sato K, Niimi Y, Mano T, Iwata A, Iwatsubo T. Automated evaluation of conventional clock-drawing test using deep neural network: potential as a mass screening tool to detect individuals with cognitive decline. Front Neurol 13: 896403, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rouleau I, Salmon DP, Butters N, Kennedy C, McGuire K. Quantitative and qualitative analyses of clock drawings in Alzheimer's and Huntington's disease. Brain Cogn 18: 70-87, 1992. [DOI] [PubMed] [Google Scholar]


